Abstract
Background
A large number of studies have shown a close relationship between ADORA2A and the pathological mechanism of schizophrenia. However, to our knowledge, there has been no studies examining the association between the ADORA2A gene and schizophrenia in Chinese Han population.
Purpose
The objective of this study was to examine the relationship between adenosine A2A receptor (ADORA2A) single nucleotide polymorphisms and schizophrenia in the North Chinese Han population.
Patients and methods
We detected ADORA2A single nucleotide polymorphisms (SNPs) using polymerase chain reaction-restriction fragment length polymorphism analyses and summarized our results using SPSS statistical software and Haploview in schizophrenia case group (n=398) and healthy control group (n=535).
Results
The frequency of the CC homozygote genotype of SNP rs2298383T/C were significantly higher in the case than the control group (p=0.005, OR=1.712, 95% CI=1.172–2.502). After linkage disequilibrium analysis, SNPs rs5996696A/C and rs2298383T/C displayed strong linkage disequilibrium. We found that the frequencies of haplotypes TA (χ2=6.268, p=0.0123) and CA (χ2=7.012, p=0.0081) were significantly higher in the case group than in the control group.
Conclusion
In conclusion, SNPs in the ADORA2A gene may be associated with schizophrenia in the northern Chinese Han population.
Keywords: ADORA2A, gene polymorphisms, schizophrenia
Introduction
Schizophrenia is a complex and serious mental disorder which has a strong hereditary tendency. Patients mainly manifest with abnormal behavior and cognition syndrome. Recent studies have viewed schizophrenia as a developmental disorder,1 which might be related to the disruption of brain development caused by genetic defects and environmental conditions.2,3 In their review, Coyle et al4 suggested that cortical atrophy caused by loss of cortical synaptic connectivity on pyramidal neurons might be the initial onset mechanism driven by inactivation of one or more risk genes. Schizophrenia has a prevalence of about 1% and not only brings extreme suffering and loss of personal potential to the patients themselves, but also places a heavy burden on their families and on society.5 Through detecting and integrating the gene risk factors involved in schizophrenia, we can achieve a better understanding of its pathogenesis, offer a more effective prevention strategy and provide patients with an earlier more accurate diagnosis and more personalized treatment.6
The adenosine receptor refers to a G protein-coupled receptor which includes the A1, A2a, A2b and A3 subtypes in mammals.7 Adenosine receptors are widely distributed in various tissues and organs, and almost all affect the physiological function of the human body.8 In the central nervous system, A1 and A2A receptors are highly expressed and participate in a number of important processes such as neuron development,9 the sleep/wake cycle,10 learning and memory,11,12 movement function and drug addiction. The adenosine A2A receptor (ADORA2A) has gained wide attention because of its complex functions which can promote the release of presynaptic neurotransmitter and increase postsynaptic excitability.13 ADORA2A has a high affinity for endogenous adenosine and binds the Gs protein which is a key step in the activation of the adenosine cyclase-adenosine cyclophosphate-protein kinase A pathway.
ADORA2A is mainly expressed in intermediate spinous neurons of the corpus striatum and pallidum in the central nervous system and can form a functional heteromeric complex with the dopamine D2 and D3 receptor and the metabotropic glutamate receptor mGluR5, which plays key roles in regulating and integrating neurotransmission.14 In addition, ADORA2A and mGLu5 receptors in the corpus striatum have synergistic functions, regulating GABA (gamma-aminobutyric acid) neurons mediated by D2 receptors, patients with schizophrenia, the impairment of compensation function of NMDA-GABA pathway may be the main cause of positive symptoms.15 Recent studies demonstrated that dysfunction of ADORA2A in astrocytes can influence the activity of GLT-1 (glial glutamate transporter 1) and further cause glutamine disturbance which is related to schizophrenic etiology.16 In the basal ganglia, hippocampus and corpus striatum, ADORA2A can promote the inflow of Ca2+ through L-type Ca2+ ion channels and the outflow of glutamate from the endoplasmic reticulum into extracellular space where it produces excitotoxic effects, cell death caused by intracellular calcium overload may be one of the pathological mechanisms of schizophrenia.17 The latest study found that ADORA2A plays a key role in regulating brain activity, especially glutamine levels after brain damage, by regulating excitatory amino acid transporters in the cerebral vascular endothelium.18
The clinical effectiveness of schizophrenia therapy can be improved by increasing extracellular adenosine levels with agents such as dipyridamole and allopurinol.19 The A1 and A2 receptors blurred appropriate coding of information salience in neuronal pathways which is thought to be the core pathological feature in the development of schizophrenia endotypes.19 Moscoso-Castro et al illustrated the effect of adenosine defects on cognitive processes and suggested a new therapeutic strategy to combat the cognitive defects caused by schizophrenia.20 Clinical studies also found that, after drug treatment of schizophrenia patients, the expression of ADORA2A were significantly increased.21 Later studies found that about 50% of schizophrenia patients demonstrated a reduction in ADORA2A at the translational level and an increase in the percentage of 5-methylcytosine in the 5´untranslated region, which suggested the potential of using ADORA2A in the corpus striatum as a biochemical indicator of a schizophrenia subtype accompanied by an altered motor phenotype.22 A large number of studies thus show a close relationship between ADORA2A and the pathological mechanism of schizophrenia.
There have been no studies to our knowledge examining the association between the ADORA2A gene and schizophrenia in Chinese Han population. Therefore, in this study, we used polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) analysis to detect ADORA2A single nucleotide polymorphisms (SNPs) and analyzed the relationship between ADORA2A genetic variants and the occurrence of schizophrenia in North Chinese Han subjects.
Subjects and methods
Subjects
Schizophrenia case group
In our study, all of the participants in the case group were chosen from inpatients in six hospitals in Liaoning Province, including 251 males and 147 females with an age range of 18–64 years.
Diagnosis criteria: All of the patients were diagnosed in accordance with the DSM-IV schizophrenia diagnosis criteria and were diagnosed by at least two associate senior or higher psychiatrists. Patients with other mental diseases or serious physiological diseases were excluded.
Healthy control group
The control group consisted of 535 healthy subjects contemporaneous with the case group with no mental or serious physiological diseases, including 259 males and 276 females with an age range of 18–58 years. All of the participants were obtained from students, staff and volunteers recruited in health examinations in the same region as the case group. There was neither genetic relationship nor significant age difference between the members of case group and control group.
Principles of ethical review
This study was conducted in accordance with the Declaration of Helsinki. All of the participants were informed of the experimental method, objective, experimental benefits, risks and their rights before the study and signed informed consent forms granting permission to use their data. This study was approved by the Ethics Committee of China Medical University (Shenyang, Liaoning, China).
Methods
Our study was a case-control study, in which, rs5996696 and rs2298383 were selected based on a large number of literatures. The single nucleotide polymorphism of rs2298383 was closely related to caffeine sensitivity and anxiety,23,24 and may be a causative mutation site. In recent years, studies have shown that it is also associated with general psychopathological symptoms,25 while rs5996696 polymorphism is closely related to the risk of Parkinson’s disease,26,27 we used PCR-RFLP to detect ADORA2A gene polymorphisms.
Detection of ADORA2A gene SNPs
This study was a case-control study. Polymorphism typing experiments were conducted according to our previous research methodologies.28 All of the participants provided 2 ml of peripheral vein blood voluntarily, using EDTA for anticoagulation and extracting DNA through the KI method. PCR-RFLP was used to analyze the genotype of single nucleotide polymorphisms (SNPs) at two sites in the gene for ADORA2, rs5996696 and rs2298383. Genomic DNA was amplified with the following primers, designed by the primer design software Primer Premier 5.0 (http://www.premierbiosoft.com/primerdesign/) and produced by bio-company (China, XiaMen): rs5996696 sense, GAGGGAAAGTTGGGGTCTGT and antisense, TGGACCTCTGCCCGTTAAAT; the PCR fragment was 328-bp long. rs2298383 sense, CCGCACTCAGGAGCAGA and antisense, CCCAAAGGCACGAAGC; the PCR fragment was 647-bp long. The PCR reaction mixture (25 μl) contained 1 μl of DNA, dNTP (0.2 mM of each), sense and antisense primers (100 pmol of each) and 10× PCR buffer (2.5 μl) containing 100 mM Tris–HCl (pH 8.3), 500 mM KCl, 15 mM MgCl2 and Taq (0.725 U; Takara, China, DaLian). PCR was performed in a Techne DNA Thermal Cycler (Biometra, ABI, USA) with 5 min of denaturation at 94 °C followed by 30 cycles with denaturation for 30 s at 94 °C, annealing for 60 s at 61 °C (rs5996696) or 59.2 °C (rs2298383) and extension for 1 min at 72 °C, followed by a final 10-min extension at 72 °C. We then digested 10 μl of PCR product with restriction enzymes purchased from New England Biolabs (China,BeiJing). rs5996696 amplifications were digested with the restriction enzyme MscI (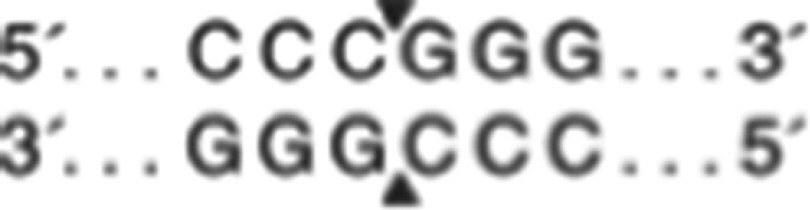 ) at 37 °C, and rs2298383 amplifications were digested with the restriction enzyme SmaI (
) at 37 °C, and rs2298383 amplifications were digested with the restriction enzyme SmaI (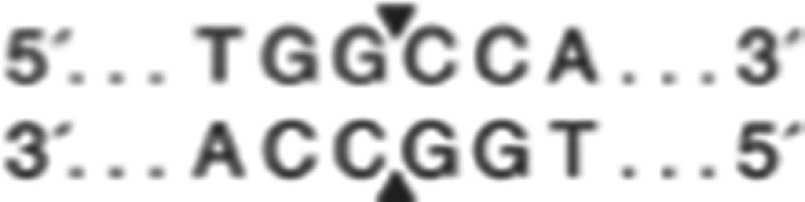 ) at 25 °C. The restriction digest products were analyzed by electrophoresis on a 2.0% agarose gel using Genefounder™ (Bio-V, Xiamen, China) to observe DNA bands that indicated gene types (wild-type homozygous genotypes, mutant homozygous genotypes and heterozygous genotypes).
) at 25 °C. The restriction digest products were analyzed by electrophoresis on a 2.0% agarose gel using Genefounder™ (Bio-V, Xiamen, China) to observe DNA bands that indicated gene types (wild-type homozygous genotypes, mutant homozygous genotypes and heterozygous genotypes).
Statistical analysis
Data are presented as means ± SD or as percent frequency. Calculation of allele frequencies was based on the genotype of subjects. Hardy-Weinberg equilibrium analysis was also performed. SPSS software (IBM, Armonk, NY, USA) was used to perform logistic regression after controlling gender and to calculate the value of p and the odds ratio (OR); significance was defined as p<0.05 and we apply Bonferroni correction for multiple testing. We used Haploview 4.2 software (https://www.broadinstitute.org/haploview/haploview) to conduct linkage disequilibrium analysis and haplotype detection and analysis.
Results
Hardy-Weinberg equilibrium
Hardy-Weinberg equilibrium analysis of the two loci is shown in Table S1. For rs5996696, the control group (χ2=0.452, p=0.461) and the case group (χ2=0.076, p=0.783) did not deviate from the Hardy-Weinberg equilibrium. For rs2298383, the control group (χ2=4.073, p=0.044) deviated from the Hardy-Weinberg equilibrium, while the case group (χ2=0.641, p=0.432) did not deviate from the Hardy-Weinberg equilibrium. The samples of the two groups for rs5996696 and rs2298383 were the same, through comparative analysis; the reason the control group for rs2298383 deviated from the Hardy-Weinberg equilibrium may be due to other factors which will be explained in the Discussion. Therefore, our control samples are considered to be representative of the general population.
Table S1.
Hardy-Weinberg equilibrium of ADORA2A single nucleotide polymorphisms (SNP) sites
| SNPs | group | Gene type (frequency) | χ2 | p |
|---|---|---|---|---|
| rs5996696 | Control group | CC:5 (0.9%) | 0.452 | 0.461 |
| AC:79 (14.8%) | ||||
| AA:451 (84.3%) | ||||
| Case group | CC:3 (0.8%) | 0.076 | 0.783 | |
| AC:58 (14.7%) | ||||
| AA:334 (84.5%) | ||||
| rs2298383 | Control group | CC:93 (17.4%) | 4.073 | 0.044* |
| TC:286 (53.7%) | ||||
| TT:154 (28.9%) | ||||
| Case group | CC:105 (26.4%) | 0.641 | 0.432 | |
| TC:191 (48.0%) | ||||
| TT:102 (25.6%) |
Note: *P<0.05 (indicates statistical significance).
Association between ADORA2A single nucleotide polymorphisms and schizophrenia
We conducted genotype analysis to distinguish between wild-type, homozygous mutant and heterozygous mutant genotypes, regarding the less-frequent genotype between the two kinds of homozygous genotypes to be a mutant genotype. Shown in Table 1, The rs5996696A/C genotypes had no significant differences after Additive, dominance or recessive model analysis. About rs2298383T/C, the Additive model was significantly different (p=0.005; OR=1.712). The dominance model displayed the distribution of the CC genotype (p=0.001; OR=1.753) which was significantly different between the case and control group, well the recessive model analysis showed no significant difference.
Table 1.
rs5996696A/C and rs2298383T/C genotype frequency distribution comparisons
| Groups | Genotype, n (%) | Models | Adjusted p-Value | Adjusted OR (95%CI) | |||
|---|---|---|---|---|---|---|---|
| AA | AC | CC | |||||
| rs5996696A/C | Control group | 451 (84.3) | 79 (14.8) | 5 (0.9) | Additive | 0.983 | 0.984 (0.230,4213) |
| Case group | 334 (84.5) | 58 (14.7) | 3 (0.8) | Dominant | 0.976 | 0.978 (0.229,4.178) | |
| Recessive | 0.899 | 0.977 (0.679,1.405) | |||||
| rs2298383T/C | Control group | 154 (28.9) | 286 (53.7) | 93 (17.4) | Additive | 0.005* | 1.712 (1.172,2.502) |
| Case group | 102 (25.6) | 191 (48.0) | 105 (26.4) | Dominant | 0.001* | 1.753 (1.273,2.415) | |
| Recessive | 0.366 | 0.872 (0.649,1.173) | |||||
Notes: Adjusted OR was obtained on logistic regression after controlling gender. Additive model: AA vs CC; Dominant model: AA/AC vs CC; Recessive model: CC/AC vs AA. *p<0.0083 (Bonferroni correction: a’=a/k, where a’ is the corrected significance level for multiple comparison, a is the significance level (0.05), and k is the number of independent significance tests (2*3=6), a’=0.05/6).
Linkage disequilibrium analysis
Linkage disequilibrium refers to non-random associations between different alleles in a group, expressed by D´ and r2. D´ ranges from 0 to 1; the closer to 1, the higher the level of linkage disequilibrium. In the linkage disequilibrium analysis of the two loci (rs5996696 and rs2298383), D´=0.943 and r2=0.09, which demonstrates that these two loci show strong linkage disequilibrium (shown in Figure 1).
Figure 1.

Linkage disequilibrium of the two loci.
Haplotype analysis
In the case and control groups, we conducted further haplotype analysis of the two SNPs (rs5996696 A/C, rs2298383 T/C) when LDs (linkage disequilibrium) between the case group and control group were apparent; results are shown in Table 2. In all groups, when the frequency was less than 1%, the haplotype was rejected and we did not study it. Results show that the frequency of the TA haplotype (χ2=6.268; p=0.0123) and of the CA haplotype (χ2=7.012; p=0.0081) was significantly different between the case and control group.
Table 2.
Haplotype distribution of the two single nucleotide polymorphisms between the case group and control group
| Haplotype | Total frequency | Case group-control group ratio | χ2 | p |
|---|---|---|---|---|
| TA | 0.528 | 0.977:1.237 | 6.268 | 0.0123* |
| CA | 0.390 | 0.738:0.572 | 7.012 | 0.0081* |
| CC | 0.080 | 0.087:0.086 | 0.006 | 0.9365 |
Note: *P<0.05 (indicates statistical significance).
Discussion
The ADORA2A gene is located on chromosome 22q11.2/22q11.2-13.1 and encodes a protein of 410 amino acids.9 A large number of studies has confirmed that the ADORA2A gene is related to various mental disorders including depression,29 anxiety30 and schizophrenia.16,21,22
The mutation of ADORA2A gene was found to dictate individual differences in sleep awareness and sensitivity to caffeine.31 This study also found that ADORA2A gene polymorphisms affected mental alertness under a state of sleep deprivation. A large sample from a Brazilian population also demonstrated that the ADORA2A gene polymorphism influenced the intake amount of caffeine and objective sleep parameters.32 Another study of ADORA2A gene polymorphisms indicated that, despite the presence of the C-T allele in rs5751876, interestingly enough, subjects with the C allele seemed more sensitive to sleep disorder induced by caffeine than subjects with the T allele,33 while subjects with the T allele were more likely to display anxiety.24 A study at Germany’s Wiltzburg University also confirmed this finding, in particular, females TT homozygotes were more sensitive to anxiety mood.34 The rs2298383 in gene intron 1a and rs4822492 in the 3’-flanking sequence were in complete linkage disequilibrium, as subjects with both of the CC genotypes at this site had higher anxiety scores than subjects with either the rs2298383TT or the rs4822492GG.23
Deckert and his research team examined the 405C/T and 1083C/T silent mutations and the Gly-430-Ser receptor mutation, conducting large sample case-control studies that found that ADORA2A did not work in schizophrenia.35,36 They also observed that drug treatments to schizophrenia patients increased ADORA2A in a dose-dependent manner, which demonstrated that ADORA2A played a key role in the molecular effects of anti-schizophrenic drugs.37 Luu et al in Taiwan chose 412 participants to conduct an ADORA2A c.1083T>C gene polymorphism study, and they found no obvious differences in the frequencies of the allele or the genotype, and concluded that there was no close relationship between the ADORA2A gene itself and schizophrenia.38 Hong et al enrolled 227 schizophrenia patients in China to conduct an ADORA2 1976T/C gene polymorphism study that yielded no positive result.39
By using the case control method, we compared the genotypes and their frequency distribution between the case group and the control group. The frequency of the CC homozygote genotype in the rs2298383T/C case group was increased, indicating this genotype might be a risk factor for schizophrenia. There was no significant difference in rs5996696A/C between the two groups. Based on linkage disequilibrium analysis and haplotype analyses, we found that rs2298383T/C and rs5996696A/C showed strong linkage disequilibrium, and that the percentage of the CA haplotype in the case group was much larger than in the control group. Therefore, we propose that the CA haplotype might also be a potential risk factor for the onset of schizophrenia. In contrast, the percentage of the TA haplotype in the case group was much smaller than in the control group and can be viewed as a potential protective factor against schizophrenia.
We believe that the reason for these different results may be due to the choice of loci or to differences in regional ethnic populations sampled in the studies.rs2298383T/C is situated in intron 1a, and there are no studies to our knowledge about the involvement of this locus in the etiology of schizophrenia at present, but one 2008 study showed that it was related to anxiety caused by caffeine.23 Hohoff et al found that the mutation in rs2298383T/C was related to different anxiety-related personality scores, displayed functional potential and might be the real mutation induction site.24 In 2016, a comprehensive research study examined the ADORA2A gene polymorphism in non-acute schizophrenia patients who had received long-term antipsychotic treatment and found a relationship between the rs2298383T/C gene polymorphism and psychopathological symptoms.25 These studies further confirmed our results, particularly in the case group of CC homozygous genotypes containing rs2298383T/C polymorphism, which might be a risk factor for schizophrenia. As for the rs5996696A/C mutation, a 2011 epidemiological and genetic association study of Parkinson’s disease in the US (PEGASUS), using large sample case-control analysis, proposed that this gene polymorphism was related to the onset risk of Parkinson’s disease.26 However, in the same year, a Korean study failed to find any relationship between the rs2298383T/C gene polymorphism and the onset risk for Parkinson’s disease in a Korean population.27 Other than the above studies, to our knowledge there have been no studies which related the rs2298383T/C gene polymorphism to schizophrenia or other mental disorders. Our study found no direct relationship between the rs5996696A/C polymorphism and onset risk of schizophrenia in the Northern Han population. However, through linkage disequilibrium with rs2298383T/C, the rs5996696A/C polymorphism demonstrated an indirect relationship with schizophrenia. In summary, we conclude that the mutation of ADORA2A is a potential risk factor for schizophrenia because of its influence on dopamine receptors and its function in regulating the release of glutamine. The hypothesis that rs2398383 may be the real mutation induction site will require further research.
Our study leaves some other issues to address. We calculated the Hardy-Weinberg equilibrium of rs2298383 with control group at p<0.05, which may be due to a small sample size. Insufficient sample size is one of our research limitations, we will include more samples in future studies. To verify the superposition effect of gender on gene polymorphisms also will be the subject of our next, more-detailed and in-depth research direction.
In summary, we found a significant association between schizophrenia and the rs2298383T/C polymorphism of the ADORA2A gene previous studies have not found this link. The hypothesis that rs2398383 may be the real mutation induction site will require further research.
Supplementary material
Acknowledgments
This study was funded by Dr. Yinglin Huang (XZR20160010) of CMU young teachers foundation.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Aparicio-Prat E, Arnan C, Sala I, Bosch N, Guigo R, Johnson R. Erratum to: ‘DECKO: single-oligo, dual-CRISPR deletion of genomic elements including long non-coding RNAs’. BMC Genomics. 2016;17:215. doi: 10.1186/s12864-016-3328-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Modai S, Shomron N. Molecular risk factors for schizophrenia. Trends Mol Med. 2016;22(3):242–253. doi: 10.1016/j.molmed.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 3.Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet. 2016;388(10039):86–97. doi: 10.1016/S0140-6736(15)01121-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coyle JT, Balu DT, Puhl MD, Konopaske GT. History of the concept of disconnectivity in schizophrenia. Harv Rev Psychiatry. 2016;24(2):80–86. doi: 10.1097/HRP.0000000000000102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirayama-Kurogi M, Takizawa Y, Kunii Y, et al. Downregulation of GNA13-ERK network in prefrontal cortex of schizophrenia brain identified by combined focused and targeted quantitative proteomics. J Proteomics. 2017;158:31–42. doi: 10.1016/j.jprot.2017.02.009 [DOI] [PubMed] [Google Scholar]
- 6.Zwicker A, Denovan-Wright EM, Uher R. Gene-environment interplay in the etiology of psychosis. Psychol Med. 2018;48(12):1925–1936. doi: 10.1017/S003329171700383X [DOI] [PubMed] [Google Scholar]
- 7.Coelho JE, Alves P, Canas PM, et al. Overexpression of adenosine A2A receptors in rats: effects on depression, locomotion, and anxiety. Front Psychiatry. 2014;5:67. doi: 10.3389/fpsyt.2014.00067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peleli M, Fredholm BB, Sobrevia L, Carlstrom M. Pharmacological targeting of adenosine receptor signaling. Mol Aspects Med. 2017;55:4–8. doi: 10.1016/j.mam.2016.12.002 [DOI] [PubMed] [Google Scholar]
- 9.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50(3):413–492. [PubMed] [Google Scholar]
- 10.Reichert CF, Maire M, Schmidt C, Cajochen C. Sleep-wake regulation and its impact on working memory performance: the role of adenosine. Biology (Basel). 2016;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costenla AR, Diogenes MJ, Canas PM, et al. Enhanced role of adenosine A(2A) receptors in the modulation of LTP in the rat hippocampus upon ageing. Eur J Neurosci. 2011;34(1):12–21. doi: 10.1111/j.1460-9568.2011.07719.x [DOI] [PubMed] [Google Scholar]
- 12.Lepiarczyk M, Kaluza Z, Bielawska A, Czarnomysy R, Gornowicz A, Bielawski K. Cytotoxic activity of octahydropyrazin[2,1-a:5,4-a’]diisoquinoline derivatives in human breast cancer cells. Arch Pharm Res. 2015;38(5):628–641. doi: 10.1007/s12272-014-0444-z [DOI] [PubMed] [Google Scholar]
- 13.Stockwell J, Jakova E, Cayabyab FS. Adenosine A1 and A2A receptors in the brain: current research and their role in neurodegeneration. Molecules. 2017;22:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen JF, Eltzschig HK, Fredholm BB. Adenosine receptors as drug targets–what are the challenges? Nat Rev Drug Discov. 2013;12(4):265–286. doi: 10.1038/nrd3955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beggiato S, Tomasini MC, Borelli AC, et al. Functional role of striatal A2A, D2, and mGlu5 receptor interactions in regulating striatopallidal GABA neuronal transmission. J Neurochem. 2016;138(2):254–264. doi: 10.1111/jnc.13652 [DOI] [PubMed] [Google Scholar]
- 16.Matos M, Shen HY, Augusto E, et al. Deletion of adenosine A2A receptors from astrocytes disrupts glutamate homeostasis leading to psychomotor and cognitive impairment: relevance to schizophrenia. Biol Psychiatry. 2015;78(11):763–774. doi: 10.1016/j.biopsych.2015.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayu S, Makiko S, Daisuke N, et al. ADORA2A polymorphism predisposes children to encephalopathy with febrile status epilepticus. Neurology. 2013;80(17):1571. doi: 10.1212/WNL.0b013e31828f18d8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bai W, Li P, Ning YL, et al. Adenosine A2A receptor inhibition restores the normal transport of endothelial glutamate transporters in the brain. Biochem Biophys Res Commun. 2018;498(4):795–802. doi: 10.1016/j.bbrc.2018.03.060 [DOI] [PubMed] [Google Scholar]
- 19.Fukushima K, Fukushima N, Ejima K, et al. Left atrial appendage flow velocity and time from P-wave onset to tissue Doppler-derived A’ predict atrial fibrillation recurrence after radiofrequency catheter ablation. Echocardiography. 2015;32(7):1101–1108. doi: 10.1111/echo.12823 [DOI] [PubMed] [Google Scholar]
- 20.Moscoso-Castro M, Lopez-Cano M, Gracia-Rubio I, Ciruela F, Valverde O. Cognitive impairments associated with alterations in synaptic proteins induced by the genetic loss of adenosine A2A receptors in mice. Neuropharmacology. 2017;126:48–57. doi: 10.1016/j.neuropharm.2017.08.027 [DOI] [PubMed] [Google Scholar]
- 21.Zhang J, Abdallah CG, Wang J, et al. Upregulation of adenosine A2A receptors induced by atypical antipsychotics and its correlation with sensory gating in schizophrenia patients. Psychiatry Res. 2012;200(2–3):126–132. doi: 10.1016/j.psychres.2012.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Villar-Menendez I, Diaz-Sanchez S, Blanch M, et al. Reduced striatal adenosine A2A receptor levels define a molecular subgroup in schizophrenia. J Psychiatr Res. 2014;51:49–59. doi: 10.1016/j.jpsychires.2013.12.013 [DOI] [PubMed] [Google Scholar]
- 23.Childs E, Hohoff C, Deckert J, Xu K, Badner J, de Wit H. Association between ADORA2A and DRD2 polymorphisms and caffeine-induced anxiety. Neuropsychopharmacology. 2008;33(12):2791–2800. doi: 10.1038/npp.2008.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hohoff C, Mullings EL, Heatherley SV, et al. Adenosine A(2A) receptor gene: evidence for association of risk variants with panic disorder and anxious personality. J Psychiatr Res. 2010;44(14):930–937. doi: 10.1016/j.jpsychires.2010.02.006 [DOI] [PubMed] [Google Scholar]
- 25.Turcin A, Dolzan V, Porcelli S, Serretti A, Plesnicar BK. Adenosine hypothesis of antipsychotic drugs revisited: pharmacogenomics variation in nonacute schizophrenia. OMICS. 2016;20(5):283–289. doi: 10.1089/omi.2016.0003 [DOI] [PubMed] [Google Scholar]
- 26.Popat RA, Van Den Eeden SK, Tanner CM, et al. Coffee, ADORA2A, and CYP1A2: the caffeine connection in Parkinson’s disease. Eur J Neurol. 2011;18(5):756–765. doi: 10.1111/j.1468-1331.2011.03353.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park JA, Pak JJ, Kim J, et al. Adenosine A2A receptor polymorphisms in Korean patients with systemic sclerosis. Int J Immunopathol Pharmacol. 2011;24(2):505–508. doi: 10.1177/039463201102400224 [DOI] [PubMed] [Google Scholar]
- 28.Yinglin H, Jingying L, Lijuan W, et al. Association between a casein kinase 1 ε gene polymorphism and schizophrenia in a Chinese Han population. J Mol Neurosci. 2012;47(3):470–474. doi: 10.1007/s12031-012-9729-1 [DOI] [PubMed] [Google Scholar]
- 29.Oliveira S, Ardais AP, Bastos CR, et al. Impact of Genetic Variations in ADORA2A Gene on Depression and Symptoms: A Cross-sectional Population-based Study. Purinergic Signalling 2018. doi: 10.1007/s11302-018-9635-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiu GS,Darmody PT, Walsh JP, et al. Adenosine through the A2A adenosine receptor increases IL-1β in the brain contributing to anxiety. Brain Behav Immun. 2014;41:218–231. doi: 10.1016/j.bbi.2014.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bodenmann S, Hohoff C, Freitag C, et al. Polymorphisms of ADORA2A modulate psychomotor vigilance and the effects of caffeine on neurobehavioural performance and sleep EEG after sleep deprivation. Br J Pharmacol. 2012;165(6):1904–1913. doi: 10.1111/j.1476-5381.2011.01689.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nunes RA, Mazzotti DR, Hirotsu C, Andersen ML, Tufik S, Bittencourt L. The association between caffeine consumption and objective sleep variables is dependent on ADORA2A c.1083T>C genotypes. Sleep Med. 2017;30:210–215. doi: 10.1016/j.sleep.2016.06.038 [DOI] [PubMed] [Google Scholar]
- 33.Retey JV, Adam M, Khatami R, et al. A genetic variation in the adenosine A2A receptor gene (ADORA2A) contributes to individual sensitivity to caffeine effects on sleep. Clin Pharmacol Ther. 2007;81(5):692–698. doi: 10.1038/sj.clpt.6100102 [DOI] [PubMed] [Google Scholar]
- 34.Gajewska A, Blumenthal TD, Winter B, et al. Effects of ADORA2A gene variation and caffeine on prepulse inhibition: a multi-level risk model of anxiety. Prog Neuropsychopharmacol Biol Psychiatry. 2013;40:115–121. doi: 10.1016/j.pnpbp.2012.08.008 [DOI] [PubMed] [Google Scholar]
- 35.Deckert J, Nothen MM, Bryant SP, et al. Mapping of the human adenosine A2a receptor gene: relationship to potential schizophrenia loci on chromosome 22q and exclusion from the CATCH 22 region. Hum Genet. 1997;99(3):326–328. [DOI] [PubMed] [Google Scholar]
- 36.Deckert J, Nothen MM, Rietschel M, et al. Human adenosine A2a receptor (A2aAR) gene: systematic mutation screening in patients with schizophrenia. J Neural Transm (Vienna). 1996;103(12):1447–1455. doi: 10.1007/BF01271259 [DOI] [PubMed] [Google Scholar]
- 37.Alsene K, Deckert J, Sand P, de Wit H. Association between A2a receptor gene polymorphisms and caffeine-induced anxiety. Neuropsychopharmacology. 2003;28(9):1694–1702. doi: 10.1038/sj.npp.1300232 [DOI] [PubMed] [Google Scholar]
- 38.Luu SU, Liao HM, Hung TW, et al. Mutation analysis of adenosine A2a receptor gene and interaction study with dopamine D2 receptor gene in schizophrenia. Psychiatr Genet. 2008;18(1):43. doi: 10.1097/YPG.0b013e3281b1173c [DOI] [PubMed] [Google Scholar]
- 39.Hong CJ, Liu HC, Liu TY, Liao DL, Tsai SJ. Association studies of the adenosine A2a receptor (1976T>C) genetic polymorphism in Parkinson’s disease and schizophrenia. J Neural Transm (Vienna). 2005;112(11):1503–1510. doi: 10.1007/s00702-005-0286-4 [DOI] [PubMed] [Google Scholar]


