Abstract
Background
Digital health is poised to transform health care and redefine personalized health. As Internet and mobile phone usage increases, as technology develops new ways to collect data, and as clinical guidelines change, all areas of medicine face new challenges and opportunities. Inflammatory bowel disease (IBD) is one of many chronic diseases that may benefit from these advances in digital health. This review intends to lay a foundation for clinicians and technologists to understand future directions and opportunities together.
Objective
This review covers mobile health apps that have been used in IBD, how they have fit into a clinical care framework, and the challenges that clinicians and technologists face in approaching future opportunities.
Methods
We searched PubMed, Scopus, and ClinicalTrials.gov to identify mobile apps that have been studied and were published in the literature from January 1, 2010, to April 19, 2019. The search terms were (“mobile health” OR “eHealth” OR “digital health” OR “smart phone” OR “mobile app” OR “mobile applications” OR “mHealth” OR “smartphones”) AND (“IBD” OR “Inflammatory bowel disease” OR “Crohn's Disease” (CD) OR “Ulcerative Colitis” (UC) OR “UC” OR “CD”), followed by further analysis of citations from the results. We searched the Apple iTunes app store to identify a limited selection of commercial apps to include for discussion.
Results
A total of 68 articles met the inclusion criteria. A total of 11 digital health apps were identified in the literature and 4 commercial apps were selected to be described in this review. While most apps have some educational component, the majority of apps focus on eliciting patient-reported outcomes related to disease activity, and a few are for treatment management. Significant benefits have been seen in trials relating to education, quality of life, quality of care, treatment adherence, and medication management. No studies have reported a negative impact on any of the above. There are mixed results in terms of effects on office visits and follow-up.
Conclusions
While studies have shown that digital health can fit into, complement, and improve the standard clinical care of patients with IBD, there is a need for further validation and improvement, from both a clinical and patient perspective. Exploring new research methods, like microrandomized trials, may allow for more implementation of technology and rapid advancement of knowledge. New technologies that can objectively and seamlessly capture remote data, as well as complement the clinical shift from symptom-based to inflammation-based care, will help the clinical and health technology communities to understand the full potential of digital health in the care of IBD and other chronic illnesses.
Keywords: digital health, mHealth, mobile health, mobile technology, smartphone, eHealth, review, inflammatory bowel disease, Crohn’s disease, ulcerative colitis
Introduction
Digital health technologies—tools leveraging mobile phones, tablets, Web platforms, and wearables to improve health outcomes—are rapidly changing the practice of medicine and redefining approaches to health care. By the end of 2018, 67% of the global population (5.1 billion people) subscribed to mobile Internet services, a number expected to increase to 71% (5.8 billion people) by 2025 [1]. In 2013, the Pew Research Center showed that 72% of Internet users in the United States searched for health information the previous year, with 35% admitting to using the Internet to try to determine their own or someone else’s medical condition [2].
More specifically, Makovsky’s Pulse of Online Health in 2015 found that 66% of the US population reported interest in using mobile apps to manage their health [3]. Accordingly, in 2017 over 325,000 mobile health-related apps were commercially available for download, a 25% increase from 2016 [4]. Unfortunately, reviews of these commercially available health apps frequently lack an evidence base or adherence to guidelines, with very few going through clinical trials [5,6]. Moreover, clinical research has struggled to study and define clinical guidelines for the new data collected [7-9], even with exciting opportunities to engage hard-to-reach populations and provide innovative care [10]. Over 50% of individuals in developed countries have at least one chronic disease [11], and about one-fourth of them experience limitations in their activities of daily living [12].
One such chronic disease, inflammatory bowel disease (IBD), which is composed of Crohn’s disease (CD) and ulcerative colitis (UC), is well-situated for technological intervention. By engaging, educating, and monitoring patients, technology can help us understand and improve care in a disease that presents uniquely in each individual. IBD has a 0.5% prevalence for both CD and UC in the western world [13], a number expected to increase globally, with specific spikes in certain populations [14]. IBD is relapsing and remitting in nature, with disease exacerbations (ie, flares) being a key driver of the acute need for medical care [15] and having a negative impact on quality of life (QoL) [16]. Treatment is unique to each patient’s circumstance, including self-monitoring and behavioral interventions [17,18]. Some long-term intestinal complications of CD include strictures, fistulas, and abscesses [19,20]; some long-term intestinal complications of UC are perforation, colitis, colonic strictures, and colorectal cancer [20-23].
The Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) recommendations and the CALM trial have sparked significant changes in clinical management, suggesting that resolution of symptoms alone is not a sufficient outcome; objective evidence, such as fecal calprotectin (FC) or C-reactive protein, is also necessary to guide clinical decisions [24,25]. Using the objective marker, FC, and patient symptoms to guide therapeutic decisions, 46% of patients in the CALM trial reached mucosal healing after 48 weeks compared to 30% of control subjects [25]. As a result, the goals of treatment are evolving from symptom-based to inflammation-based (ie, symptom and biomarker) control to slow or even reverse disease progression. Due to this shift, patients and providers are challenged to find new ways to engage in monitoring, set goals for treatment, and discuss longitudinal care. Digital health can help close these gaps.
Prior reviews have discussed both clinically tested and commercially available digital health interventions in IBD by listing out apps or discussing the various kinds of technological interventions [26-29]. This review includes some new digital health apps and will focus on how they have fit into clinical care. By highlighting areas where they have been tested and shown promise in the clinical flow, this review builds the foundation for clinicians and technologists contemplating new apps in IBD.
Methods
Scoping Review
Scoping reviews embrace systematic discovery and selection of literature to elucidate and summarize the depth and breadth of a field of interest. One of the benefits of creating this review is to allow commentary regarding potential gaps or areas for innovation. Given this background, we believed a scoping review was most appropriate, using the most current guidelines to (1) identify the research question, (2) identify relevant studies, (3) select relevant studies, (4) chart the data, and (5) collate and summarize the results [30,31].
Development of Research Questions
The research questions were as follows: What digital health apps have been described in the published literature in the setting of IBD? How have these apps complemented or been used in current clinical practice?
Identifying Apps Discussed in Published Literature
The literature was reviewed using PubMed, Scopus, and ClinicalTrials.gov for articles or trials published from January 1, 2010, through April 19, 2019; published articles and trials were accessed on April 19, 2019. The search terms were “mobile health” OR “eHealth” OR “digital health” OR “smart phone” OR “mobile app” OR “mobile applications” OR “mHealth” OR “smartphones” AND “IBD” OR “Inflammatory bowel disease” OR “Crohn's Disease” OR “Ulcerative Colitis” OR “UC” OR “CD”. Only English-language articles were included. The Scopus search included titles, abstracts, and keywords. The PubMed search included the entire paper and excluded “UC” and “CD” in the search terms. The ClinicalTrials.gov search included studies containing these terms. All papers were reviewed by the first author of this paper (ALY), with consultation from other authors when necessary.
Identifying Commercially Available Apps
The iTunes iOS app store was reviewed to find a selection of commercial apps for the purpose of discussion and recognition of the large consumer market that patients face. As the review of commercial apps was not intended to be exhaustive, Google Play and other app stores were not explored. The search terms “inflammatory bowel disease,” “crohn’s disease,” “ulcerative colitis,” and “colitis” were used to identify potential apps; the iTunes iOS app store was last accessed on April 30, 2019. Considering the desired length and scope of this review, a restricted number of apps were included. These were selected based on top search hits in the app store, which reflect commercial apps that have historically been used most [29].
Article Selection
The titles and abstracts of all hits were reviewed. Articles were included if they explored the use of a digital health intervention in the care of IBD, or CD or UC specifically. Citations of included articles were then also screened for additional relevant articles or apps. Articles were excluded if they did not involve IBD, CD, or UC. They were excluded if they discussed teleconferencing or video chatting as the sole intervention, as this was decided to be out of the scope of the review.
Data Charting
The articles were organized according to the apps that they described. Each app was assessed for how it addressed any of five identified areas of clinical care: education, monitoring, treatment, follow-up, and patient satisfaction.
Collation and Summary
We summarize and present the relevant features of the apps by breaking them into the five areas of clinical care identified, providing a framework for each of these areas. The goal of the scoping review was to summarize the depth and breadth of digital health apps that have been used in IBD clinical care in order to provide a foundation for discussion for physicians and technologists to pursue future opportunities.
Results
Overview
The Scopus search yielded 227 hits. The PubMed search returned 168 hits. Search of ClinicalTrials.gov returned 12 hits. This totaled 407 hits from the three databases. A total of 39 duplicates were identified. The titles and abstracts of the remaining 368 articles were reviewed; 68 were identified to meet the inclusion criteria and were examined fully, after which 28 articles were selected (see Figure 1). Exploring citations revealed further information to be included. In total, 11 digital health apps and 4 commercial apps were included.
Figure 1.
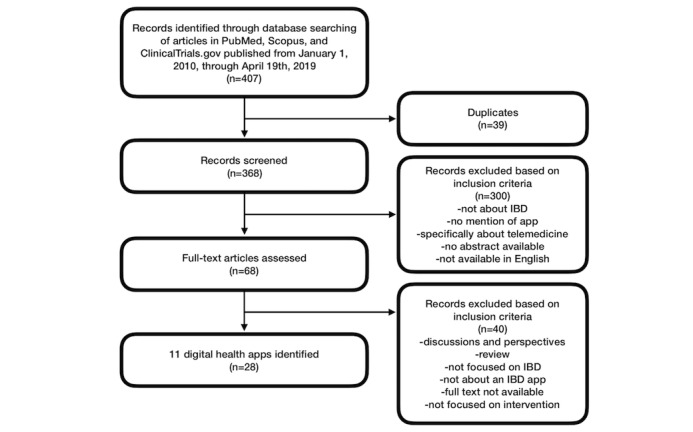
Selection process for articles about inflammatory bowel disease (IBD) digital health apps.
The five areas of clinical care discussed are education, monitoring, treatment, follow-up, and patient satisfaction. The sections below provide an overview of each area, a review of the clinically studied apps, and a review of a small selection of commercially available apps. An overview of each clinically studied app and the trials’ significant findings can be found in Table 1 [26,32-55]. An overview of each commercial app can be found in Textbox 1 [56-59]. An overview of the features of each app as related to clinical care can be found in Tables 2 and 3.
Table 1.
Overview of clinically studied apps.
| App (sample size) | Feature highlights | Significant findings from trials |
| Constant Care (n=333, n=95, and n=17) [32-35] |
|
|
| Young Constant Care (n=50) [36,55] |
|
|
| HealthPROMISE (n=320) [37-41] |
|
|
| IBD-live (n=84) [42,43] |
|
|
| myIBDcoach (n=909) [44,45] |
|
|
| Telemonitoring of Crohn’s Disease and Ulcerative Colitis (TECCU) (n=63) [46,47] |
|
|
| TELE-IBD (n=348 and n=219) [48-50] |
|
|
| TrueColours UC (n=66) [51] |
|
|
| UC HAT/HAT (n=25 and n=47) [52,53] |
|
|
| UCLA eIBD (n=194 UCm and n=217 CDn) [26,54] |
|
|
aSCCAI: Simple Clinical Colitis Activity Index.
bFC: fecal calprotectin.
cQoL: quality of life.
dSIBDQ: Short Inflammatory Bowel Disease Questionnaire.
eEQ-5D: EuroQol-5 Dimension questionnaire.
fQoC: quality of care.
gIBD: inflammatory bowel disease.
hHBI: Harvey Bradshaw Index.
iCCKNOW: Crohn’s and Colitis Knowledge Score.
J5-ASA: 5-aminosalicylate.
kUC HAT: home automated telemanagement in ulcerative colitis.
lHAT: home automated telemanagement.
mCD: Crohn’s disease.
nUC: ulcerative colitis.
oUCLA: University of California, Los Angeles.
pPRO: patient-reported outcome.
qmHI: Mobile Health Index.
An overview of commercial apps and their key features.
GI Monitor [56]
Log symptoms, meals, bowel habits, medications, and mood
Tries to help users make insights into behavior and symptoms
Data can be exported to be shared with physicians
ibd.care [57]
Website with basics of inflammatory bowel disease (IBD), tips for talking with a care team, advice about lifestyle, and insurance tips
Parallel site for physicians to learn about insurance and IBD management
Created by PRIME, a recognized provider of continuing medical education, reviewed by the Academy of Managed Care Pharmacy, and supported by the Case Management Society of America
myIBD [58]
Mobile app built to assist pediatric patient transition to adult care
Learn module with videos, articles, and short quizzes
Journey module for input of features related to disease history: diagnosis, physicians, medications, allergies, major events, goals, symptoms, etc
Access to disease activity questionnaires that can be saved
Roadmap of the illness can be shared with new physicians
Oshi [59]
Shares wellness and symptom scores and insights related to associations with flares
Tracks symptoms and behavioral information (eg, exercise) and can sync to fitness devices
Learn component has articles, IBD-friendly recipes, and personal stories
Ask component provides a space to message Oshi health professionals
Table 2.
An overview of the features of each clinically studied app as related to clinical care.
| App (sample size) | Clinical care features | ||||
|
|
Educate | Monitor | Treatment | Follow-up | Patient sentiments |
| Constant Care (n=333, n=95, and n=17) [32-35] |
|
|
|
|
|
| Young Constant Care (n=50) [36,55] |
|
|
|
|
|
| HealthPROMISE (n=320) [37-41] |
|
|
|
|
|
| IBD-live (n=84) [42,43] |
|
|
|
|
|
| myIBDcoach (n=909) [44,45] |
|
|
|
|
|
| Telemonitoring of Crohn’s Disease and Ulcerative Colitis (TECCU) (n=63) [46,47] |
|
|
|
|
|
| TELE-IBD (n=348 and n=219) [48-50] |
|
|
|
|
|
| TrueColours UC (n=66) [51] |
|
|
|
|
|
| UC HAT/HAT (n=25 and n=47) [52,53] |
|
|
|
|
|
| UCLA eIBD (n=194 UC, 217 CD) [26,54] |
|
|
|
|
|
aSCCAI: Simple Clinical Colitis Activity Index.
bHBI: Harvey Bradshaw Index.
cFC: fecal calprotectin.
dN/A: not applicable.
ePUCAI: Pediatric Ulcerative Colitis Activity Index.
fPCDAI: Pediatric Crohn’s Disease Activity Index.
gSIBDQ: Short Inflammatory Bowel Disease Questionnaire.
hEQ-5D: EuroQol-5 Dimension questionnaire.
iQoC: quality of care.
jEMR: electronic medical record.
kIBD: inflammatory bowel disease.
lMIAH: monitor IBD at home.
mCD: Crohn’s disease.
nUC: ulcerative colitis.
o5-ASA: 5-aminosalicylate.
pUC HAT: home automated telemanagement in ulcerative colitis.
qHAT: home automated telemanagement.
rPRO: patient-reported outcome.
Table 3.
An overview of the features of each commercial app as related to clinical care.
| App | Clinical care features | ||
|
|
Educate | Monitor | Follow-up |
| GI Monitor [56] |
|
|
|
| ibd.care [57] |
|
|
|
| myIBD [58] |
|
|
|
| Oshi [59] |
|
|
|
aN/A: not applicable.
bIBD: inflammatory bowel disease.
cSIBDQ: Short Inflammatory Bowel Disease Questionnaire.
dSCCAI: Simple Clinical Colitis Activity Index.
eHBI: Harvey Bradshaw Index.
fPHQ-9: 9-item Patient Health Questionnaire.
Education
After initial diagnosis, patients may learn more about their disease through interactions with their health team, materials provided by clinical providers, discussions with family or friends, or support groups, but the Internet is also a common source of information. Some physicians encourage patients to use curated websites as sources of information. Patient education benefits QoL and continuity of care, reduces patient anxiety and complications from illness, and increases treatment adherence [60]. After diagnosis, up to 86% of individuals diagnosed with a chronic condition will turn to the Internet for information [61], but it is generally accepted that IBD information on the Internet may be too hard to read and have inaccuracies [62-65].
The app myIBDcoach utilizes interactive eLearning (electronic learning) modules about medications, adherence, smoking cessation, nutrition, symptom management, fatigue, work productivity, anxiety, and depression [44]. In addition to eLearning modules, Constant Care gives a disease-specific lecture during the onboarding process and provides access to an eHealth nurse and educational video clips. Constant Care researchers report that this has been valuable in empowering IBD patients to perform individualized, self-administered therapy [35]. At the end of 12 months, a study of 333 participants showed significant improvement in general knowledge about IBD (P<.001) and medications (P=.001) in the Danish cohort of the study compared to controls, as measured by the Crohn’s and Colitis Knowledge Score (CCKNOW); however, the same effects were not observed in the Irish cohort [32,66]. Home automated telemanagement in ulcerative colitis (UC HAT) developed its own curriculum based on materials from the Crohn’s and Colitis Foundation of America (CCFA), providing educational packages with each weekly check-in [52]. Telemonitoring of Crohn’s Disease and Ulcerative Colitis (TECCU) produced its own educational and preventative materials that were incorporated and available on the platform [46].
TELE-IBD used a text message-based curriculum to send educational information at various frequencies, providing tips related to both general (ie, vaccinations and screenings) and specific (ie, IBD medication side effects) health information. In a study of 219 participants, patients were randomized to receive either TELE-IBD messages at different frequencies or standard of care. Results measured by CCKNOW showed significant improvement in patients receiving messages every other week as compared to controls (P=.03) and greater changes in scores in participants with lower baselines (P<.01); however, after adjusting for race, site, and baseline, researchers found no significant changes between control and TELE-IBD groups [48-50].
Of the commercial apps, myIBD, though mainly built for transition of care between providers, contains a robust educational component with videos, short articles on many related topics, and quizzes to assess knowledge [58]. The app ibd.care is created by PRIME, a recognized provider of continuing medical education, reviewed by the Academy of Managed Care Pharmacy, and supported by the Case Management Society of America. This app covers a broad base of information about IBD, including basics of disease, treatment, tips for talking with your care team, navigating insurance, and how to align lifestyle with IBD goals. The site similarly aims to educate health care providers about managing patients and navigating insurance [57]. Many apps direct patients to the CCFA, where a large breadth of curated IBD information is available [20].
The apps approach education through learning modules, videos, and automated text messages covering a wide array of IBD topics. Besides the TELE-IBD study, these apps have acted as a repository for educational materials to be accessed by patients when needed or when the platforms are used, but the materials are not delivered proactively.
Although education is a common component across almost all apps, there is minimal assessment of the information quality or the value that the information provides to patients. It would be interesting to assess whether patients using these apps rely less on unverified sources of information and, as a result, experience improvements in treatment adherence, QoL, anxiety, and complications. With the varieties of technology available, technologists may be able to engage with physicians to create more interactive and engaging educational materials that increase health literacy and empower patients in managing their care.
Monitoring
Generally speaking, monitoring a patient’s symptoms occurs at discrete time points at certain frequencies (ie, they can vary from every few months to every year) through outpatient office visits, when physicians and patients discuss symptoms experienced since a prior visit. The subjective reports from patients and laboratory data may both be collected at these time points to monitor the state of a patient’s disease. Understanding the symptoms that patients experience is a foundational component of clinical care, but physicians are not always effective at collecting this information [67]. One study observed that health care providers frequently misinterpret reported symptoms, leading to differences in how the patient and the physician perceive a patient’s current state [68]. As a further challenge, the concordance between a patient’s memory of experience and actual experience of gastrointestinal (GI) symptoms has been observed to be generally poor [69]. The insufficient identification and management of IBD flares is one reason that many patients have poor outcomes [49].
Using a Web-based monitoring package, Constant Care maps symptoms reported through the Simple Clinical Colitis Activity Index (SCCAI) and Harvey Bradshaw Index (HBI) questionnaires, along with fecal calprotectin (FC) measurements, to a “traffic light” color system, which illustrates disease activity based on a total inflammatory burden score. Patients with recent flares take the surveys daily or weekly until they enter the green zone (ie, remission). Patients already in the green zone report symptoms monthly. Studies involving Constant Care have shown improved QoL, shorter flares, and decreased acute care and office visits [26,28,32].
HealthPROMISE tracks patient symptoms and QoL biweekly using the Short Inflammatory Bowel Disease Questionnaire (SIBDQ) and the EuroQol-5 Dimension questionnaire (EQ-5D), respectively. Providers can view the data on a Web-based dashboard integrated with this provider’s electronic medical record (EMR). Due to the integration, researchers believe that in-person office visits can focus more on quality of care (QoC) as opposed to eliciting symptom history, allowing for more meaningful goal-focused discussions [37]. In their trial with 320 participants, the results showed fatigue and tension as the most important drivers of QoL [41], with QoL having significantly improved for study patients as compared to controls after 575 days of follow-up [39].
IBD-live is a Web-based app comprised of three modules that were tested on adolescents. The first module focuses on monitoring using a flarometer, where patients report disease activity through the Pediatric Ulcerative Colitis Activity Index (PUCAI) or the Pediatric Crohn’s Disease Activity Index (PCDAI) and send an FC sample to track their status, as mapped to a “traffic light” system. Patients receive email reminders to report into the module. In the study, the 84 users of the platform had no difference in experiencing flares (33% vs 34% in controls) but showed some improvement in QoL (+1.32 vs -0.32 in controls, measured using the IMPACT-III questionnaire, P=.27) [42,43].
In TECCU, the 21 people in the intervention group were monitored via an IBD-modified version of NOMHADhome, a technological system designed for managing chronically ill patients and accessible on computers, tablets, or mobile phones. Patients reported symptoms by answering questions sent via text message and accessed questionnaires on the platform to monitor disease activity, adverse effects, and medication adherence. Monitoring frequency varied depending on the therapy plan for each patient, ranging from every week to every 4 weeks. Measurement of disease activity (HBI for CD or SCCAI for UC), QoL (Inflammatory Bowel Disease Questionnaire 9), and productivity and activity impairment (Work Productivity and Activity Impairment questionnaire) were done at the beginning and the end of the 24-week study. Researchers observed greater improvement in disease activity in the TECCU group compared to standard care, but the results did not reach statistical significance. QoL and social impairment improved significantly in all groups in the study, with no significant differences between them [46,47].
In a study with 47 participants, UC HAT used a symptom diary to elicit information on weekly symptoms, medications, side effects, and weight. The study did not find significant improvement in disease activity or QoL over standard care [52]. The app myIBDcoach has a monthly monitoring module to collect information about disease activity as measured with the Monitor IBD at Home questionnaire, medication use, treatment adherence, treatment satisfaction, and side effects. In a study with 909 participants using myIBDcoach, there was no significant difference in disease activity [44]. UCLA eIBD developed two specific, four-question, patient-reported outcome (PRO) questionnaires, named the Mobile Health Index (mHI), that are integrated into the EMR and used to create a patient value quotient. A study of 194 UC and 217 CD patients compared their mHI to standard UC and CD clinical disease activity measures, showing significant correlation for each (P<.001). Both had a strong inverse correlation with QoL as measured by the SIBDQ (P<.001) [29,54]. TrueColours UC emails users to report symptoms every day through the SCCAI and to report on QoL every 2 weeks through the EQ-5D. Combined with a monthly FC test, users tracked disease activity using the system’s “traffic light” severity tracker [51].
In the commercial market, symptom diaries represent about 57% of the IBD-related apps available for English speakers on the Google Play and Apple app stores [29]. Apps like Oshi, GI Monitor, and myIBD have various methods to track symptoms, bowel movements, medications, meals, emotional state (ie, stress and anxiety), sleep or fatigue, and physical activity. In return, these apps graphically represent the data to help users track and potentially discover associations between symptoms and reported information [56,58,59].
All but two of the apps included in this review have some approach to collecting PROs. Most apps going through trials tend to collect data at discrete, weekly, biweekly, or monthly time points, using standard clinical questionnaires for IBD. Conversely, commercial apps tend to aim for daily data entry and ask patients to report more granular data (eg, specific symptoms, activities, and emotions). No studies have reported decreases in QoL as a result of app use, and many—Constant Care, HealthPROMISE, and TECCU—have reported improved QoL.
Treatment
IBD management, for both UC and CD, includes the following: induction therapy and maintenance therapy. The goal is first to control inflammation quickly and then to sustain that control over time. Symptoms, side effects, laboratory data, and imaging inform the choice of therapy. Clinical treatment guidelines provide algorithms for physicians to follow based on patient symptoms, laboratory data, and prior treatments. The recommended treatments can vary greatly depending on these factors, and the treatments may not consider unique patient treatment preferences or individual characteristics [17,18].
Constant Care can provide recommendations for medication management based on reported symptoms. In one trial with Constant Care, patients with UC who reported acute symptoms indicative of a flare received recommendations to initiate 5-aminosalicylate (5-ASA) for a certain duration. Based on their own prior experience and current treatment guidelines, patients could also select additional treatments. In this trial with 333 participants, 100% of patients in the treatment group received 5-ASA in response to a flare as compared to 10% of control patients. Researchers attributed this benefit to the patient’s ability to better recognize and understand the correct treatment for a flare [32,35]. Constant Care has also been studied in the context of down-titration of mesalazine therapy in mild-to-moderate UC patients in a trial with 95 participants. Patients were encouraged to decrease or maintain their dose or reach out to their care team based on the “traffic light” system. Results showed a significant increase in adherence to mesalazine (Visual Analog Scale 88 vs 100, Medical Adherence Rating Scale 23 vs 24, P<.001) in the group from baseline to the end of the study; results also showed significantly improved QoL (P<.001) as measured by the SIBDQ and 12-Item Short Form Health Survey (SF-12) from baseline to the end [33]. A third trial with 17 participants used Constant Care to determine the timing of infliximab maintenance therapy for CD, using weekly entries converted to the “traffic light” system. At the end of the trial, only 10% of individuals received therapy at 8-week intervals (ie, standard of care), 39% received treatment at shorter intervals, and 50% received treatment at longer intervals [34].
A similar randomized controlled trial of 50 patients used Young Constant Care—an adapted version of Constant Care—in a pediatric population. Based on symptom reporting and FC, patients reaching the red level or 2 weeks of the yellow level received an infusion of infliximab (ie, standard maintenance care). Otherwise, patients were allowed a maximum of 12 weeks between infusions. Using these methods, researchers observed a significant increase in the mean treatment interval (9.5 weeks vs 6.9 weeks, P<.001) and no change in QoL or levels of antibodies between treatment and controls who received standard care [36].
In TECCU, the responses provided through the platform were used to create individually tiered alerts and action plans. Patients received recommendations to adjust medication or visit their physician based on the alert level matching their reported symptoms. At the end of the trial, the HBI for CD or the SCCAI for UC were combined with partial Mayo scores from face-to-face visits to assess remission status. After 24 weeks, the 21 patients in the intervention group had no significant change in remission in UC (odds ratio [OR] 0.12, P=.19) or CD (OR 0.11, P=.13) or in mean improvement in measured FC (-0.90 mg/g, P=.11) [46,47].
In HealthPROMISE, patients have reported feeling that their care decision making has been more equitable and have experienced improved health outcomes (QoC increased +19% more than controls, P<.01) [40]. TrueColours UC used the data collected during its study of 66 participants to create a flare algorithm to help predict the need for escalation in therapy at outpatient visits and observed 95% accuracy of this algorithm [26].
Current evidence about the role of apps in IBD treatment comes largely from four different studies involving the Web-based platform Constant Care. Suggesting the inception or alteration of a medication through the use of IBD digital health apps has been viable. Certain studies have shown significantly increased QoL and have explored individualizing treatment timing. None of these studies have reported worsening QoL, QoC, flares, or treatment outcomes in participants.
The uses of Constant Care to titrate treatment for flares or maintenance therapy are exciting and have been recognized as an early step in the pursuit of pharmacokinetic monitoring [70]. One single treatment of a biologic therapy like infliximab can cost thousands of dollars and require an entire day in an infusion center [71]. The prospect of individualizing treatments according to when they are needed could benefit patients (ie, reduced medication exposure and time lost from daily life) as well as the health care system (ie, cost avoidance, fewer visits, and fewer side effects). Further studies are needed to validate this potential in IBD digital health apps.
Follow-Up
After diagnosis, patients with chronic diseases will regularly visit their physician to share updates about symptoms and side effects. Unexpected or worsening symptoms may warrant scheduling additional visits to alter or add therapies. Severe changes may require visits to the emergency room or hospitalizations for immediate care. Higher numbers of outpatient visits can serve as a significant protective factor against IBD-related hospitalization in the following year [72], but nonadherence to medication is common in IBD and reported to be in the range of 40%-60%, with related adverse economic and clinical implications [73]. Appropriate continuity of medical care is associated with higher patient satisfaction, fewer hospitalizations, fewer emergency room visits, and improvement in receiving preventative services [74].
During testing of the Constant Care app, researchers observed an increase in the amount of contacts over the phone and through email, but a reduction in visits to the emergency department and the same number of hospitalizations [28]. The Constant Care trial also noted improved adherence to medication during flares (73% vs 42%, P=.005) [32]. UC HAT had a messaging option to a nurse coordinator through their Web portal but showed no changes in adherence and did not assess visits or hospitalizations [52]. Over 12 months, the trial of the myIBDcoach system showed a significant reduction in both visits to the gastroenterologist (1.55 vs 2.34 average visits over a year, P<.001) and telephone calls to the gastroenterologist (0.58 vs 0.84 average calls over a year, P<.001) compared to controls; however, there was no change in visits (0.29 vs 0.36, P=.17) or telephone calls (0.70 vs 0.74, P=.45) to the nurse. Users of the system had fewer hospitalizations (P=.046) and higher medication adherence, as measured by the Morisky Medication Adherence Scale (P<.001) [44].
In TECCU, patients could use the platform to send messages to their teams and receive direction about when to follow up or adjust medications. Researchers observed a significant increase in medication adherence in all groups in the trial, with significantly higher increase in the patients in the app group as measured by the Morisky-Green index (OR 0.0001). Researchers noted lower numbers of outpatient visits and telephone calls in the intervention group compared to controls [46,47].
Users of the IBD-live platform were triaged to different follow-up plans depending on their reported symptoms. Patients considered low risk would report symptoms again in 3 months, those at intermediate risk would report again in 1 month, and those at high risk would be directed to contact their physician. Patients were also able to contact their local health team through the platform. Among the 84 users of the Web-based system, there were significantly fewer face-to-face follow-ups as compared to controls (3.6 visits vs 4.3 in controls, P<.001) [42,43].
Among the commercial apps, MyIBD, aims to support the transition from a pediatric to an adult gastroenterologist. Problems with transition from pediatric to adult care can lead to treatment nonadherence, increased disease severity, and undue emotional and financial stress for patients [75]. Data show that 79% of adult gastroenterologists report inadequate preparation of adolescents coming from pediatric care [76]. The MyIBD app provides a My Journey module where patients can record all aspects of their own health record and grant access to new physicians they meet in their care [58]. Oshi has a feature where users can give the app permission to contact their physician if reported symptoms may indicate worsening disease [59].
The above apps provide another way for patients to interact with their care, whether through direct messaging or the ability to view information about their disease. Medication adherence is generally improved in the studied app users. These users also benefit from decreases in acute care and outpatient visits, although results are mixed about changes in telephone contact with providers. No studies reported increased hospitalizations or emergency visits or decreased medication adherence.
Patient Satisfaction
There is no globally agreed-upon formulation for patient satisfaction [77], and determinants of patient satisfaction vary across different studies, providing little explanation for the influencers of satisfaction [78-80]. One review of mobile health in managing digestive diseases determined that patient satisfaction ranged from 74% to 100% in the reviewed studies, with compliance ranging from 25.7% to 100% [81]. IBD patients want to be involved in decision making, with many reporting the desire for equitable collaboration with their physician [82]. In one set of focus groups, IBD patients reported a lack of understanding of how well their disease was being controlled, including a feeling that QoL was not discussed in many visits with their physicians. The patients reported an overuse of jargon and felt a lack of tangible goals or goal-setting in their care [83].
At the end of the largest Constant Care trial, 88.8% of individuals said the system was feasible and wanted to continue using it [32]. In another trial, 100% of individuals who finished the study reported being satisfied [33]. In a third trial, users reported high satisfaction with Web programs, education, and impact of the program on their disease [34]. The app myIBDcoach noted that 94% of users continued using the platform at the 1-year follow-up but saw no significant difference in satisfaction, as measured by a Visual Analog Scale in Web platform patients versus those receiving standard of care [44]. For UC HAT, only 14 of the 25 (56%) participants completed the study [52]; in the following version, home automated telemanagement (HAT), 86% reported that using the system did not interfere with their daily routines, 91% would consider using it in the future, and 91% were adherent to using the platform for 6 months [53]. In TELE-IBD, a posttrial qualitative study of both adherent and nonadherent patients identified benefits of understanding disease, monitoring symptoms, and feeling connected to their health provider. This study also noted that many participants had trouble remembering details of their action plans and that there were mixed results regarding timing, repetition, and technical aspects of the platform [84]. HealthPROMISE continued to have 75% adherence after 6 months of follow-up [37,41]. Participants of the Young Constant Care trial completed 74% of total desired survey entries [55]. In TECCU, patients in the control and intervention groups reported significant increases in satisfaction from baseline, as measured by an adapted version of the Client Satisfaction Questionnaire. Patients and providers also reported no perceived privacy breaches and minimal technical issues [46,47]. In IBD-live, 96% of users reported the platform to be time-saving, 71% wished to continue, and highly compliant patients averaged €360 in annual savings [43].
Patients must remain the center of iteration and development. App adherence in the above studies usually means that users continue to use the app over the time frame stated, but researchers have not described the quantity of use and its possible relationship with patient progress. Although adherence has generally been adequate and satisfaction generally positive, studies have described little beyond these simple measures: a shortcoming observed across much of digital health [85]. As in the TELE-IBD posttrial study, narrative feedback will be important in improving specifics related to apps (ie, design and function) and answering larger questions about the perceived shortcomings of interventions. Clinical guidelines are able to advise specific treatments and medications but struggle to account for specific patient characteristics, such as treatment preference, access to care, childbearing interest, age, treatment history, etc. This can lead to disagreements or misunderstandings between patients and physicians. Digital health may be able to empower patients to better understand some of this context and help providers be more aware of patient preferences.
Discussion
Principal Findings
As in many chronic diseases, multiple influences—genetics, medications, behavior, social network, environment, psychological factors, and social determinants—play a role in the course of IBD, greatly increasing the number of variables and potential interventions available for study [8,86-88]. In such a large dimensional context, a randomized controlled trial (RCT) could assess whether an intervention, on average, has some effect, but it is unlikely to determine which of the components led to the observed effect. As seen in this review, adherence, QoL, QoC, and knowledge, among others, were valuable outcomes measured in the studies, but determining which components of each app contributed to these measurements poses a difficult challenge.
Given the breadth of variables and opportunities possible with digital health, RCTs have some limits. Microrandomized trials may be an interesting method to explore moving forward, as they randomly assign intervention options at relevant decision points. This allows for assessment of the effects of each intervention, including when and for whom it causes effects, as well as the examination of factors influencing these effects [89]. This may be a path to better understanding effects of individual components of increasingly complicated digital health apps.
New technologies have the potential to change the care of IBD. One such example is augGI, a technology company whose aim is to improve the management of chronic gut disorders. The company augGI is developing technology that uses computer vision and deep learning to characterize stool specimens from just an image. In particular, they are focusing on measuring stool consistency to better characterize motility changes [90]. Another area is the iteration on symptom monitoring. Many of the reviewed apps use written surveys converted into mobile text versions. Making these questionnaires visual, adaptive, or more specific to individuals could make the data more valuable and individually meaningful, as have been used in other areas [91-93]. On the horizon, toilets may be capable of collecting various data on urine and stool, making some data available more frequently [94-96]. Finally, apps for patients to assess FC at home (ie, IBDoc or Calpro) have been developed and have been positively validated, in general [28,97-99].
As promising as these technologies are, they bring to the forefront the lack of clinical guidelines for what types of new data should be collected and the appropriate frequency of data collection. In the CALM trial, FC was measured at 12-week intervals, already an increase in frequency from the norm. But what if FC is regularly measured every week or every day? As these questions arise, it is vital to utilize datasets that may already exist [100] and find new sets that lead to meaningful, cost-effective guidance for patients. Technologists will be challenged to design devices that elicit and present data streams with clinical relevance. Researchers will be challenged to build clinical guidelines and frameworks for translating these data streams into patient recommendations in real time as the data become available. One prospective study is exploring this dilemma using Fitbits to passively collect daily steps, heart rate, and sleep data and to determine if this data can help predict elevation in biomarkers. One of their early findings has shown that decreased physical activity, as measured by steps the week before, has occurred prior to the finding of active disease (P<.001) [101].
The clinical care of IBD is shifting from symptom-based to inflammation-based management. As digital health evolves, it becomes hard to ignore its potential to contribute to this shift. Digital health can help engage patients to track both the visible (ie, symptoms they experience) and invisible (ie, FC or other biomarkers) markers of disease. What a patient feels symptomatically is not fully descriptive of their disease state. Clinicians and technologists alike must pursue other lab or digitally trackable biomarkers to better describe the disease state. In tracking these, patients can receive treatment early in the course of flares, thereby reducing the need for acute care and the risk of long-term complications. Longitudinal assessment of FC or other markers will be able to not only reshape treatment but also allow for a more interactive, goal-focused dialogue between patients and providers.
Summary
This review discussed the role of studied IBD digital health apps and a small sample of commercial apps in clinical care. Significant benefits have been observed in education, QoL, QoC, treatment adherence, and medication management with the use of some apps. While digital health technologies have shown an ability to fit into, complement, and improve the standard clinical care of patients with IBD, research to further validate these findings from both a clinical and patient perspective is needed. As technologies change, research must expand to define new norms for using the different kinds of data that can be collected and integrated into clinical care. As the clinical management paradigm changes from symptom-based to inflammation-based care, it is an important time for all groups involved—patients, clinicians, technologists, insurers, etc—to discuss and explore new opportunities to use digital health to improve understanding of disease, patient experience, and patient care.
Acknowledgments
We would like to acknowledge DE and EJS as the senior coauthors of this paper. We give a special thanks to the Small Data Lab at Cornell Tech as well as to those who supported the conception, discussion, and revision of the paper.
Abbreviations
- 5-ASA
5-aminosalicylate
- CCFA
Crohn’s and Colitis Foundation of America
- CCKNOW
Crohn’s and Colitis Knowledge Score
- CD
Crohn’s disease
- EMR
electronic medical record
- EQ-5D
EuroQol-5 Dimension questionnaire
- FC
fecal calprotectin
- GI
gastrointestinal
- HAT
home automated telemanagement
- HBI
Harvey Bradshaw Index
- IBD
inflammatory bowel disease
- mHI
Mobile Health Index
- MIAH
monitor IBD at home
- N/A
not applicable
- OR
odds ratio
- PCDAI
Pediatric Crohn’s Disease Activity Index
- PHQ-9
9-item Patient Health Questionnaire
- PRO
patient-reported outcome
- PUCAI
Pediatric Ulcerative Colitis Activity Index
- QoC
quality of care
- QoL
quality of life
- RCT
randomized controlled trial
- SCCAI
Simple Clinical Colitis Activity Index
- SF-12
12-Item Short Form Health Survey
- SIBDQ
Short Inflammatory Bowel Disease Questionnaire
- STRIDE
Selecting Therapeutic Targets in Inflammatory Bowel Disease
- TECCU
Telemonitoring of Crohn’s Disease and Ulcerative Colitis
- UC
ulcerative colitis
- UC HAT
home automated telemanagement in ulcerative colitis
- UCLA
University of California, Los Angeles
Footnotes
Conflicts of Interest: DH is cofounder and CEO of augGI Technologies Inc. EJS receives research funding from the CCFA and the UCSF-CCFA Clinical Research Alliance and also consults with the CCFA. The remaining authors have no conflicts of interest to declare.
References
- 1.The Mobile Economy: 2019. London, UK: GSM Association; 2019. [2019-07-29]. https://www.gsmaintelligence.com/research/?file=b9a6e6202ee1d5f787cfebb95d3639c5&download . [Google Scholar]
- 2.Fox S, Duggan M. Pew Research Center. Washington, DC: Pew Internet & American Life Project; 2013. Jan 15, [2019-07-29]. Health online 2013 https://www.pewinternet.org/2013/01/15/health-online-2013/ [Google Scholar]
- 3.Makovsky. 2015. Feb 24, [2019-04-30]. Fifth annual "Pulse of Online Health" Survey finds 66% of Americans eager to leverage digital tools to manage personal health http://www.makovsky.com/news/fifth-annual-pulse-of-online-health-survey-2/
- 4.mHealth App Economics 2017: Current Status and Future Trends in Mobile Health. Berlin, Germany: Research2Guidance; 2017. Nov, [2019-04-30]. 325,000 mobile health apps available in 2017: Android now the leading mHealth platform https://research2guidance.com/325000-mobile-health-apps-available-in-2017/ [Google Scholar]
- 5.Con D, Jackson B, Gray K, De Cruz P. eHealth for inflammatory bowel disease self-management: The patient perspective. Scand J Gastroenterol. 2017 Sep;52(9):973–980. doi: 10.1080/00365521.2017.1333625. [DOI] [PubMed] [Google Scholar]
- 6.Foster KR, Callans DJ. Smartphone apps meet evidence-based medicine: The future of medicine may (or may not) be in your smartphone. IEEE Pulse. 2017;8(6):34–39. doi: 10.1109/MPUL.2017.2750783. [DOI] [PubMed] [Google Scholar]
- 7.Murray E, Hekler EB, Andersson G, Collins LM, Doherty A, Hollis C, Rivera DE, West R, Wyatt JC. Evaluating digital health interventions: Key questions and approaches. Am J Prev Med. 2016 Nov;51(5):843–851. doi: 10.1016/j.amepre.2016.06.008. http://europepmc.org/abstract/MED/27745684 .S0749-3797(16)30229-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patrick K, Hekler EB, Estrin D, Mohr DC, Riper H, Crane D, Godino J, Riley WT. The pace of technologic change: Implications for digital health behavior intervention research. Am J Prev Med. 2016 Nov;51(5):816–824. doi: 10.1016/j.amepre.2016.05.001.S0749-3797(16)30138-6 [DOI] [PubMed] [Google Scholar]
- 9.Bradway M, Ribu L, Hartvigsen G, Årsand E. The evolution of clinicians’ preparedness for mHealth use (2013-2017) and current barriers. Proceedings from the 16th Scandinavian Conference on Health Informatics 2018; 16th Scandinavian Conference on Health Informatics 2018; August 28-29, 2018; Aalborg, Denmark. Linköping, Sweden: Linköping University Electronic Press; 2018. pp. 45–50. http://www.ep.liu.se/ecp/151/008/ecp18151008.pdf . [Google Scholar]
- 10.Kane JM. Technology-based interventions in health care. Epidemiol Psychiatr Sci. 2014 Dec;23(4):323–326. doi: 10.1017/S2045796014000444.S2045796014000444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ward BW, Schiller JS. Prevalence of multiple chronic conditions among US adults: Estimates from the National Health Interview Survey, 2010. Prev Chronic Dis. 2013 Apr 25;10:e65. doi: 10.5888/pcd10.120203. https://www.cdc.gov/pcd/issues/2013/12_0203.htm .E65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burttorff C, Ruder R, Bauman M. Multiple Chronic Conditions in the United States. Santa Monica, CA: The RAND Corporation; 2017. [2019-04-30]. https://sbgg.org.br//informativos/29-06-17/1497877975_1_Chronic_Conditions.pdf . [Google Scholar]
- 13.Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012 Jan;142(1):46–54.e42; quiz e30. doi: 10.1053/j.gastro.2011.10.001.S0016-5085(11)01378-3 [DOI] [PubMed] [Google Scholar]
- 14.Shivashankar R, Tremaine WJ, Harmsen WS, Loftus EV. Incidence and prevalence of Crohn's disease and ulcerative colitis in Olmsted County, Minnesota from 1970 through 2010. Clin Gastroenterol Hepatol. 2017 Jun;15(6):857–863. doi: 10.1016/j.cgh.2016.10.039. http://europepmc.org/abstract/MED/27856364 .S1542-3565(16)31055-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feagins LA, Iqbal R, Spechler SJ. Case-control study of factors that trigger inflammatory bowel disease flares. World J Gastroenterol. 2014 Apr 21;20(15):4329–4334. doi: 10.3748/wjg.v20.i15.4329. http://www.wjgnet.com/1007-9327/full/v20/i15/4329.htm . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernklev T, Jahnsen J, Lygren I, Henriksen M, Vatn M, Moum B. Health-related quality of life in patients with inflammatory bowel disease measured with the short form-36: Psychometric assessments and a comparison with general population norms. Inflamm Bowel Dis. 2005 Oct;11(10):909–918. doi: 10.1097/01.mib.0000179467.01748.99.00054725-200510000-00007 [DOI] [PubMed] [Google Scholar]
- 17.Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG Clinical Guideline: Ulcerative colitis in adults. Am J Gastroenterol. 2019 Mar;114(3):384–413. doi: 10.14309/ajg.0000000000000152.00000434-201903000-00010 [DOI] [PubMed] [Google Scholar]
- 18.Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG Clinical Guideline: Management of Crohn's disease in adults. Am J Gastroenterol. 2018 Apr;113(4):481–517. doi: 10.1038/ajg.2018.27. [DOI] [PubMed] [Google Scholar]
- 19.Peyrin-Biroulet L, Loftus EV, Colombel J, Sandborn WJ. Long-term complications, extraintestinal manifestations, and mortality in adult Crohn's disease in population-based cohorts. Inflamm Bowel Dis. 2011 Jan;17(1):471–478. doi: 10.1002/ibd.21417. [DOI] [PubMed] [Google Scholar]
- 20.Crohn's & Colitis Foundation. [2019-04-30]. https://www.crohnscolitisfoundation.org/resources/
- 21.De Dombal FT, Watts JM, Watkinson G, Goligher JC. Local complications of ulcerative colitis: Stricture, pseudopolyposis, and carcinoma of colon and rectum. Br Med J. 1966 Jun 11;1(5501):1442–1447. doi: 10.1136/bmj.1.5501.1442. http://europepmc.org/abstract/MED/5933046 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lutgens MW, van Oijen MG, van der Heijden GJ, Vleggaar FP, Siersema PD, Oldenburg B. Declining risk of colorectal cancer in inflammatory bowel disease: An updated meta-analysis of population-based cohort studies. Inflamm Bowel Dis. 2013;19(4):789–799. doi: 10.1097/MIB.0b013e31828029c0. [DOI] [PubMed] [Google Scholar]
- 23.Viladomiu M, Kivolowitz C, Abdulhamid A, Dogan B, Victorio D, Castellanos JG, Woo V, Teng F, Tran NL, Sczesnak A, Chai C, Kim M, Diehl GE, Ajami NJ, Petrosino JF, Zhou XK, Schwartzman S, Mandl LA, Abramowitz M, Jacob V, Bosworth B, Steinlauf A, Scherl EJ, Wu HJ, Simpson KW, Longman RS. IgA-coated E. coli enriched in Crohn's disease spondyloarthritis promote T17-dependent inflammation. Sci Transl Med. 2017 Feb 08;9(376):1–27. doi: 10.1126/scitranslmed.aaf9655. http://europepmc.org/abstract/MED/28179509 .9/376/eaaf9655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peyrin-Biroulet L, Sandborn W, Sands BE, Reinisch W, Bemelman W, Bryant RV, D'Haens G, Dotan I, Dubinsky M, Feagan B, Fiorino G, Gearry R, Krishnareddy S, Lakatos PL, Loftus EV, Marteau P, Munkholm P, Murdoch TB, Ordás I, Panaccione R, Riddell RH, Ruel J, Rubin DT, Samaan M, Siegel CA, Silverberg MS, Stoker J, Schreiber S, Travis S, Van Assche G, Danese S, Panes J, Bouguen G, O'Donnell S, Pariente B, Winer S, Hanauer S, Colombel J. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): Determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015 Sep;110(9):1324–1338. doi: 10.1038/ajg.2015.233.ajg2015233 [DOI] [PubMed] [Google Scholar]
- 25.Colombel J, Panaccione R, Bossuyt P, Lukas M, Baert F, Vaňásek T, Danalioglu A, Novacek G, Armuzzi A, Hébuterne X, Travis S, Danese S, Reinisch W, Sandborn WJ, Rutgeerts P, Hommes D, Schreiber S, Neimark E, Huang B, Zhou Q, Mendez P, Petersson J, Wallace K, Robinson AM, Thakkar RB, D'Haens G. Effect of tight control management on Crohn's disease (CALM): A multicentre, randomised, controlled phase 3 trial. Lancet. 2018 Dec 23;390(10114):2779–2789. doi: 10.1016/S0140-6736(17)32641-7.S0140-6736(17)32641-7 [DOI] [PubMed] [Google Scholar]
- 26.Walsh A, Travis S. What's app? Electronic health technology in inflammatory bowel disease. Intest Res. 2018 Jul;16(3):366–373. doi: 10.5217/ir.2018.16.3.366. https://irjournal.org/journal/view.php?doi=10.5217/ir.2018.16.3.366 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atreja A, Otobo E, Ramireddy K, Deorocki A. Remote patient monitoring in IBD: Current state and future directions. Curr Gastroenterol Rep. 2018 Mar 07;20(2):6. doi: 10.1007/s11894-018-0611-3.10.1007/s11894-018-0611-3 [DOI] [PubMed] [Google Scholar]
- 28.Patil SA, Cross RK. Current landscape of telemedicine practice in inflammatory bowel disease. Inflamm Bowel Dis. 2018 Sep;24(9):1910–1917. doi: 10.1093/ibd/izy113.4989997 [DOI] [PubMed] [Google Scholar]
- 29.Kelso M, Feagins LA. Can smartphones help deliver smarter care for patients with inflammatory bowel disease? Inflamm Bowel Dis. 2018 Jun 08;24(7):1453–1459. doi: 10.1093/ibd/izy162.5032595 [DOI] [PubMed] [Google Scholar]
- 30.Arksey H, O'Malley L. Scoping studies: Towards a methodological framework. Int J Soc Res Methodol. 2005 Feb;8(1):19–32. doi: 10.1080/1364557032000119616. [DOI] [Google Scholar]
- 31.Levac D, Colquhoun H, O'Brien KK. Scoping studies: Advancing the methodology. Implement Sci. 2010 Sep 20;5:69. doi: 10.1186/1748-5908-5-69. https://implementationscience.biomedcentral.com/articles/10.1186/1748-5908-5-69 .1748-5908-5-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elkjaer M, Shuhaibar M, Burisch J, Bailey Y, Scherfig H, Laugesen B, Avnstrøm S, Langholz E, O'Morain C, Lynge E, Munkholm P. E-health empowers patients with ulcerative colitis: A randomised controlled trial of the Web-guided 'Constant-care' approach. Gut. 2010 Dec;59(12):1652–1661. doi: 10.1136/gut.2010.220160.59/12/1652 [DOI] [PubMed] [Google Scholar]
- 33.Pedersen N, Thielsen P, Martinsen L, Bennedsen M, Haaber A, Langholz E, Végh Z, Duricova D, Jess T, Bell S, Burisch J, Munkholm P. eHealth: Individualization of mesalazine treatment through a self-managed Web-based solution in mild-to-moderate ulcerative colitis. Inflamm Bowel Dis. 2014 Dec;20(12):2276–2285. doi: 10.1097/MIB.0000000000000199. [DOI] [PubMed] [Google Scholar]
- 34.Pedersen N, Elkjaer M, Duricova D, Burisch J, Dobrzanski C, Andersen NN, Jess T, Bendtsen F, Langholz E, Leotta S, Knudsen T, Thorsgaard N, Munkholm P. eHealth: Individualisation of infliximab treatment and disease course via a self-managed Web-based solution in Crohn’s disease. Aliment Pharmacol Ther. 2012 Nov;36(9):840–849. doi: 10.1111/apt.12043. doi: 10.1111/apt.12043. [DOI] [PubMed] [Google Scholar]
- 35.Ankersen DV, Carlsen K, Marker D, Munkholm P, Burisch J. Using eHealth strategies in delivering dietary and other therapies in patients with irritable bowel syndrome and inflammatory bowel disease. J Gastroenterol Hepatol. 2017 Mar;32 Suppl 1:27–31. doi: 10.1111/jgh.13691. [DOI] [PubMed] [Google Scholar]
- 36.Carlsen K, Jakobsen C, Houen G, Kallemose T, Paerregaard A, Riis LB, Munkholm P, Wewer V. Self-managed eHealth disease monitoring in children and adolescents with inflammatory bowel disease: A randomized controlled trial. Inflamm Bowel Dis. 2017 Mar;23(3):357–365. doi: 10.1097/MIB.0000000000001026.00054725-201703000-00004 [DOI] [PubMed] [Google Scholar]
- 37.Atreja A, Khan S, Rogers JD, Otobo E, Patel NP, Ullman T, Colombel JF, Moore S, Sands BE, HealthPROMISE Consortium Group Impact of the mobile HealthPROMISE platform on the quality of care and quality of life in patients with inflammatory bowel disease: Study protocol of a pragmatic randomized controlled trial. JMIR Res Protoc. 2015 Feb 18;4(1):e23. doi: 10.2196/resprot.4042. http://www.researchprotocols.org/2015/1/e23/ v4i1e23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Atreja A, Khan S, Rogers J, Ullman A, Grinspan A, Itzkowitz S, Maser E, Cohen B, Colombel JF, Sands B. Impact of real-world home-based remote monitoring on quality of care and quality of life in inflammatory bowel disease patients: One-year results of pragmatic randomized trial. J Crohns Colitis. 2017 Feb 01;11(Suppl 1):S362–S363. doi: 10.1093/ecco-jcc/jjx002.678. doi: 10.1093/ecco-jcc/jjx002.678. [DOI] [Google Scholar]
- 39.Atreja A, Otobo E, Szigethy E, Kohli A, Shroff H, Chang H, Rogers J, Ullman T, Cohen B, Itzkowitz S, Sands B. Improved quality of care and quality of life for IBD patients using mobile-based remote monitoring platform: A randomized control trial. Inflamm Bowel Dis. 2018 Feb;24(Suppl 1):S21–S22. doi: 10.1093/ibd/izy019.063. [DOI] [Google Scholar]
- 40.Atreja A, Szigethy E, Otobo E, Chang HL, Keefer L, Rogers J, Kohli A, Ullman TA, Marion JF, Cohen BL, Maser E, Itzkowitz S, Colombel JF, Sands BE. Improved quality of care and quality of life for IBD patients using the HealthPROMISE app: A randomized, control trial. Gastroenterology. 2018 May;154(6):S-6. doi: 10.1016/s0016-5085(18)30506-7. [DOI] [Google Scholar]
- 41.Atreja A, Otobo E, Chang H, Keefer L, Rogers J, Ullman T, Ramireddy K, Marion J, Deorocki A, Berde E, Zlatopolsky R, Kohli A, Choksi P, Itzkowitz S, Colombel J, Sands B. HealthPROMISE: Utilization of patient-reported outcomes to measure quality of life in inflammatory bowel disease. iProc. 2017 Sep 22;3(1):e28. doi: 10.2196/iproc.8452. [DOI] [Google Scholar]
- 42.Heida A, Dijkstra A, Muller Kobold A, Rossen JW, Kindermann A, Kokke F, de Meij T, Norbruis O, Weersma RK, Wessels M, Hummel T, Escher J, van Wering H, Hendriks D, Mearin L, Groen H, Verkade HJ, van Rheenen PF. Efficacy of home telemonitoring versus conventional follow-up: A randomized controlled trial among teenagers with inflammatory bowel disease. J Crohns Colitis. 2018 Mar 28;12(4):432–441. doi: 10.1093/ecco-jcc/jjx169.4710333 [DOI] [PubMed] [Google Scholar]
- 43.Dijkstra A, Heida A, van Rheenen PF. Exploring the challenges of implementing a Web-based telemonitoring strategy for teenagers with inflammatory bowel disease: Empirical case study. J Med Internet Res. 2019 Mar 29;21(3):e11761. doi: 10.2196/11761. http://www.jmir.org/2019/3/e11761/ v21i3e11761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Jong MJ, van der Meulen-de Jong AE, Romberg-Camps MJ, Becx MC, Maljaars JP, Cilissen M, van Bodegraven AA, Mahmmod N, Markus T, Hameeteman WM, Dijkstra G, Masclee AA, Boonen A, Winkens B, van Tubergen A, Jonkers DM, Pierik MJ. Telemedicine for management of inflammatory bowel disease (myIBDcoach): A pragmatic, multicentre, randomised controlled trial. Lancet. 2017 Sep 02;390(10098):959–968. doi: 10.1016/S0140-6736(17)31327-2.S0140-6736(17)31327-2 [DOI] [PubMed] [Google Scholar]
- 45.de Jong M, van der Meulen-de Jong A, Romberg-Camps M, Degens J, Becx M, Markus T, Tomlow H, Cilissen M, Ipenburg N, Verwey M, Colautti-Duijsens L, Hameeteman W, Masclee A, Jonkers D, Pierik M. Development and feasibility study of a telemedicine tool for all patients with IBD: MyIBDcoach. Inflamm Bowel Dis. 2017 Apr;23(4):485–493. doi: 10.1097/MIB.0000000000001034. [DOI] [PubMed] [Google Scholar]
- 46.Aguas M, Del Hoyo J, Faubel R, Muñoz D, Domínguez D, Bastida G, Navarro B, Barrios A, Valdivieso B, Correcher M, Nos P. A Web-based telemanagement system for patients with complex inflammatory bowel disease: Protocol for a randomized controlled clinical trial. JMIR Res Protoc. 2018 Dec 21;7(12):e190. doi: 10.2196/resprot.9639. http://www.researchprotocols.org/2018/12/e190/ v7i12e190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Del Hoyo J, Nos P, Faubel R, Muñoz D, Domínguez D, Bastida G, Valdivieso B, Correcher M, Aguas M. A Web-based telemanagement system for improving disease activity and quality of life in patients with complex inflammatory bowel disease: Pilot randomized controlled trial. J Med Internet Res. 2018 Nov 27;20(11):e11602. doi: 10.2196/11602. http://www.jmir.org/2018/11/e11602/ v20i11e11602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cross RK, Jambaulikar G, Langenberg P, Tracy JK, Collins JF, Katz J, Regueiro M, Schwartz DA, Quinn CC. TELEmedicine for patients with Inflammatory Bowel Disease (TELE-IBD): Design and implementation of randomized clinical trial. Contemp Clin Trials. 2015 May;42:132–144. doi: 10.1016/j.cct.2015.03.006.S1551-7144(15)00061-0 [DOI] [PubMed] [Google Scholar]
- 49.Cross RK, Langenberg P, Regueiro M, Schwartz DA, Tracy JK, Collins JF, Katz J, Ghazi L, Patil SA, Quezada SM, Beaulieu D, Horst SN, Russman K, Riaz M, Jambaulikar G, Sivasailam B, Quinn CC. A randomized controlled trial of TELEmedicine for patients with Inflammatory Bowel Disease (TELE-IBD) Am J Gastroenterol. 2019 Mar;114(3):472–482. doi: 10.1038/s41395-018-0272-8.10.1038/s41395-018-0272-8 [DOI] [PubMed] [Google Scholar]
- 50.Abutaleb A, Buchwald A, Chudy-Onwugaje K, Langenberg P, Regueiro M, Schwartz DA, Tracy JK, Ghazi L, Patil SA, Quezada SM, Russman KM, Quinn CC, Jambaulikar G, Beaulieu DB, Horst S, Cross RK. Inflammatory bowel disease telemedicine clinical trial: Impact of educational text messages on disease-specific knowledge over 1 year. Inflamm Bowel Dis. 2018 Sep 15;24(10):2191–2197. doi: 10.1093/ibd/izy149.4999387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walsh A. TrueColours: Real Time Data Collection in Patients With Ulcerative Colitis [doctoral thesis] Oxford, UK: University of Oxford; 2017. [2019-07-29]. https://ora.ox.ac.uk/objects/uuid:a4ab55af-5364-4fa4-86ca-e84b917d7f70/download_file?file_format=pdf&safe_filename=DPhil%2BWALSH%2BTRUECOLOURS%2BFEB%2B2018.pdf&type_of_work=Thesis . [Google Scholar]
- 52.Cross RK, Cheevers N, Rustgi A, Langenberg P, Finkelstein J. Randomized, controlled trial of home telemanagement in patients with ulcerative colitis (UC HAT) Inflamm Bowel Dis. 2012 Jun;18(6):1018–1025. doi: 10.1002/ibd.21795. http://europepmc.org/abstract/MED/21688350 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cross RK, Finkelstein J. Feasibility and acceptance of a home telemanagement system in patients with inflammatory bowel disease: A 6-month pilot study. Dig Dis Sci. 2007 Feb;52(2):357–364. doi: 10.1007/s10620-006-9523-4. [DOI] [PubMed] [Google Scholar]
- 54.van Deen WK, van der Meulen-de Jong AE, Parekh NK, Muyshondt Y, Kane E, Eimers L, Inserra EK, Zand A, DiNicola CA, Bhatia S, Choi JM, Ha C, van Oijen MG, Esrailian E, Hommes DW. Remote monitoring of IBD disease activity using the mobile Health Index (mHI): A validation study. Gastroenterology. 2015 Apr;148(4 Suppl 1):S-446. doi: 10.1016/s0016-5085(15)31501-8. [DOI] [Google Scholar]
- 55.Carlsen K, Houen G, Jakobsen C, Kallemose T, Paerregaard A, Riis LB, Munkholm P, Wewer V. Individualized infliximab treatment guided by patient-managed eHealth in children and adolescents with inflammatory bowel disease. Inflamm Bowel Dis. 2017 Sep;23(9):1473–1482. doi: 10.1097/MIB.0000000000001170. [DOI] [PubMed] [Google Scholar]
- 56.Apple App Store. [2019-07-29]. GI Monitor by Vertical Health, LLC https://itunes.apple.com/us/app/gi-monitor/id315539155?mt=8.
- 57.ibd.care. Fort Lauderdale, FL: PRIME Education; [2019-05-02]. https://ibd.care/ [Google Scholar]
- 58.Apple App Store. [2019-04-30]. MyIBD https://itunes.apple.com/us/app/myibd/id1383119954?mt=8.
- 59.Apple App Store. [2019-04-30]. Oshi: IBD Tracker & Magazine by Oshi Health, Inc https://itunes.apple.com/us/app/oshi-ibd-tracker-magazine/id1371752119?mt=8.
- 60.Bastable SB. Essentials Of Patient Education. 2nd edition. Burlington, MA: Jones & Bartlett Learning; 2017. [Google Scholar]
- 61.Fox S. Pew Research Center. Washington, DC: Pew Internet & American Life Project; 2007. Oct 08, [2019-04-30]. E-patients with a disability or chronic disease https://www.pewinternet.org/2007/10/08/e-patients-with-a-disability-or-chronic-disease/ [Google Scholar]
- 62.Promislow S, Walker JR, Taheri M, Bernstein CN. How well does the Internet answer patients' questions about inflammatory bowel disease? Can J Gastroenterol. 2010 Nov;24(11):671–677. doi: 10.1155/2010/957264. http://europepmc.org/abstract/MED/21157582 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Siegel CA. Embracing the Internet for progress in shared decision-making. Inflamm Bowel Dis. 2007 Dec;13(12):1579–1580. doi: 10.1002/ibd.20259. [DOI] [PubMed] [Google Scholar]
- 64.Azer SA, AlOlayan TI, AlGhamdi MA, AlSanea MA. Inflammatory bowel disease: An evaluation of health information on the Internet. World J Gastroenterol. 2017 Mar 07;23(9):1676–1696. doi: 10.3748/wjg.v23.i9.1676. http://www.wjgnet.com/1007-9327/full/v23/i9/1676.htm . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Mierlo T, Fournier R, Fedorak R. Don't forget the doctor: Gastroenterologists' preferences on the development of mHealth tools for inflammatory bowel disease. JMIR Mhealth Uhealth. 2015 Jan 21;3(1):e5. doi: 10.2196/mhealth.3987. http://mhealth.jmir.org/2015/1/e5/ v3i1e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eaden JA, Abrams K, Mayberry JF. The Crohn's and Colitis Knowledge Score: A test for measuring patient knowledge in inflammatory bowel disease. Am J Gastroenterol. 1999 Dec;94(12):3560–3566. doi: 10.1111/j.1572-0241.1999.01536.x.S000292709900595X [DOI] [PubMed] [Google Scholar]
- 67.Fung CH, Hays RD. Prospects and challenges in using patient-reported outcomes in clinical practice. Qual Life Res. 2008 Dec;17(10):1297–1302. doi: 10.1007/s11136-008-9379-5. http://europepmc.org/abstract/MED/18709564 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pittet V, Maillard MH, Simonson T, Fournier N, Rogler G, Michetti P. Differences in outcomes reported by patients with inflammatory bowel diseases vs their health care professionals. Clin Gastroenterol Hepatol. 2018 Nov 22;:1. doi: 10.1016/j.cgh.2018.11.029.S1542-3565(18)31272-2 [DOI] [Google Scholar]
- 69.Jones MP, Walter S, Faresjö Å, Grodzinsky E, Kjellström L, Viktorsson L, Talley NJ, Agreus L, Andreasson A. Gastrointestinal recall questionnaires compare poorly with prospective patient diaries for gastrointestinal symptoms: Data from population and primary health centre samples. Eur J Gastroenterol Hepatol. 2019 Feb;31(2):163–169. doi: 10.1097/MEG.0000000000001296. [DOI] [PubMed] [Google Scholar]
- 70.Dreesen E, Gils A. Pharmacodynamic monitoring of biological therapies in chronic inflammatory diseases. Ther Drug Monit. 2019 Apr;41(2):131–141. doi: 10.1097/FTD.0000000000000571.00007691-201904000-00005 [DOI] [PubMed] [Google Scholar]
- 71.Yokomizo L, Limketkai B, Park KT. Cost-effectiveness of adalimumab, infliximab or vedolizumab as first-line biological therapy in moderate-to-severe ulcerative colitis. BMJ Open Gastroenterol. 2016;3(1):e000093. doi: 10.1136/bmjgast-2016-000093. http://europepmc.org/abstract/MED/27195130 .bmjgast-2016-000093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Limsrivilai J, Stidham RW, Govani SM, Waljee AK, Huang W, Higgins PD. Factors that predict high health care utilization and costs for patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2017 Mar;15(3):385–392.e2. doi: 10.1016/j.cgh.2016.09.012. http://europepmc.org/abstract/MED/27645518 .S1542-3565(16)30669-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lakatos PL. Prevalence, predictors, and clinical consequences of medical adherence in IBD: How to improve it? World J Gastroenterol. 2009 Sep 14;15(34):4234–4239. doi: 10.3748/wjg.15.4234. http://www.wjgnet.com/1007-9327/full/v15/i34/4234.htm . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cabana MD, Jee SH. Does continuity of care improve patient outcomes? J Fam Pract. 2004 Dec;53(12):974–980.jfp_1204_5312i [PubMed] [Google Scholar]
- 75.Gray WN, Resmini AR, Baker KD, Holbrook E, Morgan PJ, Ryan J, Saeed SA, Denson LA, Hommel KA. Concerns, barriers, and recommendations to improve transition from pediatric to adult IBD care: Perspectives of patients, parents, and health professionals. Inflamm Bowel Dis. 2015 Jul;21(7):1641–1651. doi: 10.1097/MIB.0000000000000419. [DOI] [PubMed] [Google Scholar]
- 76.Sebastian S, Jenkins H, McCartney S, Ahmad T, Arnott I, Croft N, Russell R, Lindsay JO. The requirements and barriers to successful transition of adolescents with inflammatory bowel disease: Differing perceptions from a survey of adult and paediatric gastroenterologists. J Crohns Colitis. 2012 Sep;6(8):830–844. doi: 10.1016/j.crohns.2012.01.010.S1873-9946(12)00016-5 [DOI] [PubMed] [Google Scholar]
- 77.Batbaatar E, Dorjdagva J, Luvsannyam A, Savino MM, Amenta P. Determinants of patient satisfaction: A systematic review. Perspect Public Health. 2017 Mar;137(2):89–101. doi: 10.1177/1757913916634136.1757913916634136 [DOI] [PubMed] [Google Scholar]
- 78.Naidu A. Factors affecting patient satisfaction and healthcare quality. Int J Health Care Qual Assur. 2009;22(4):366–381. doi: 10.1108/09526860910964834. [DOI] [PubMed] [Google Scholar]
- 79.Vranceanu A, Ring D. Factors associated with patient satisfaction. J Hand Surg Am. 2011 Sep;36(9):1504–1508. doi: 10.1016/j.jhsa.2011.06.001.S0363-5023(11)00703-9 [DOI] [PubMed] [Google Scholar]
- 80.Sitzia J, Wood N. Patient satisfaction: A review of issues and concepts. Soc Sci Med. 1997 Dec;45(12):1829–1843. doi: 10.1016/s0277-9536(97)00128-7.S0277953697001287 [DOI] [PubMed] [Google Scholar]
- 81.Helsel BC, Williams JE, Lawson K, Liang J, Markowitz J. Telemedicine and mobile health technology are effective in the management of digestive diseases: A systematic review. Dig Dis Sci. 2018 Jun;63(6):1392–1408. doi: 10.1007/s10620-018-5054-z.10.1007/s10620-018-5054-z [DOI] [PubMed] [Google Scholar]
- 82.Baars JE, Markus T, Kuipers EJ, van der Woude CJ. Patients' preferences regarding shared decision-making in the treatment of inflammatory bowel disease: Results from a patient-empowerment study. Digestion. 2010;81(2):113–119. doi: 10.1159/000253862.000253862 [DOI] [PubMed] [Google Scholar]
- 83.Khan S, Dasrath F, Farghaly S, Otobo E, Riaz MS, Rogers J, Castillo A, Atreja A, Health PROMISE Consortium Group Unmet communication and information needs for patients with IBD: Implications for mobile health technology. Br J Med Med Res. 2016;12(3):12119. doi: 10.9734/BJMMR/2016/21884. http://europepmc.org/abstract/MED/28217532 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Quinn CC, Chard S, Roth EG, Eckert JK, Russman KM, Cross RK. The Telemedicine for patients with Inflammatory Bowel Disease (TELE-IBD) clinical trial: Qualitative assessment of participants' perceptions. J Med Internet Res. 2019 Jun 03;21(6):e14165. doi: 10.2196/14165. http://www.jmir.org/2019/6/e14165/ v21i6e14165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maramba I, Chatterjee A, Newman C. Methods of usability testing in the development of eHealth applications: A scoping review. Int J Med Inform. 2019 Jun;126:95–104. doi: 10.1016/j.ijmedinf.2019.03.018.S1386-5056(18)31318-2 [DOI] [PubMed] [Google Scholar]
- 86.Loddo I, Romano C. Inflammatory bowel disease: Genetics, epigenetics, and pathogenesis. Front Immunol. 2015;6:551. doi: 10.3389/fimmu.2015.00551. doi: 10.3389/fimmu.2015.00551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gerbarg PL, Jacob VE, Stevens L, Bosworth BP, Chabouni F, DeFilippis EM, Warren R, Trivellas M, Patel PV, Webb CD, Harbus MD, Christos PJ, Brown RP, Scherl EJ. The effect of breathing, movement, and meditation on psychological and physical symptoms and inflammatory biomarkers in inflammatory bowel disease: A randomized controlled trial. Inflamm Bowel Dis. 2015 Dec;21(12):2886–2896. doi: 10.1097/MIB.0000000000000568. [DOI] [PubMed] [Google Scholar]
- 88.Matsuoka K, Kanai T. The gut microbiota and inflammatory bowel disease. Semin Immunopathol. 2015 Jan;37(1):47–55. doi: 10.1007/s00281-014-0454-4. http://europepmc.org/abstract/MED/25420450 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Klasnja P, Hekler EB, Shiffman S, Boruvka A, Almirall D, Tewari A, Murphy SA. Microrandomized trials: An experimental design for developing just-in-time adaptive interventions. Health Psychol. 2015 Dec;34S:1220–1228. doi: 10.1037/hea0000305. http://europepmc.org/abstract/MED/26651463 .2015-56045-003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.auggi. augGI Technologies Inc; [2019-04-30]. http://www.auggi.ai/ [Google Scholar]
- 91.Selter A, Tsangouri C, Ali SB, Freed D, Vatchinsky A, Kizer J, Sahuguet A, Vojta D, Vad V, Pollak JP, Estrin D. An mHealth app for self-management of chronic lower back pain (Limbr): Pilot study. JMIR Mhealth Uhealth. 2018 Sep 17;6(9):e179. doi: 10.2196/mhealth.8256. http://mhealth.jmir.org/2018/9/e179/ v6i9e179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang L, Freed D, Wu A, Wu J, Pollak J, Estrin D. Your activities of daily living (YADL): An image-based survey technique for patients with arthritis. Proceedings of the 10th European Alliance for Innovation (EAI) International Conference on Pervasive Computing Technologies for Healthcare; 10th European Alliance for Innovation (EAI) International Conference on Pervasive Computing Technologies for Healthcare; May 16-19, 2016; Cancun, Mexico. 2016. May 16, pp. 41–44. [Google Scholar]
- 93.Pollak J, Adams P, Gay G. PAM: A photographic affect meter for frequent, in situ measurement of affect. Proceedings of the SIGCHI Conference on Human Factors in Computing Systems; SIGCHI Conference on Human Factors in Computing Systems; May 7-12, 2011; Vancouver, BC. 2011. May 07, pp. 725–734. [Google Scholar]
- 94.Ranjitkar P. Toilet lab: Diagnostic tests on smart toilets? Clin Chem. 2018 Jun 28;64(7):1128–1129. doi: 10.1373/clinchem.2018.286567. [DOI] [PubMed] [Google Scholar]
- 95.Wald C. Diagnostics: A flow of information. Nature. 2017 Nov 08;551(7679):S48–S50. doi: 10.1038/551S48a.551S48a [DOI] [PubMed] [Google Scholar]
- 96.Alm E, Turgeman Y, Ratti C. WIRED. 2014. Mar 21, [2019-04-30]. 'Smart toilets and sewer sensors are coming' https://www.wired.co.uk/article/yaniv-j-turgeman .
- 97.Haisma S, Galaurchi A, Almahwzi S, Adekanmi Balogun JA, Muller Kobold AC, van Rheenen PF. Head-to-head comparison of three stool calprotectin tests for home use. PLoS One. 2019;14(4):e0214751. doi: 10.1371/journal.pone.0214751. http://dx.plos.org/10.1371/journal.pone.0214751 .PONE-D-18-33982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Heida A, Knol M, Kobold AM, Bootsman J, Dijkstra G, van Rheenen PF. Agreement between home-based measurement of stool calprotectin and ELISA results for monitoring inflammatory bowel disease activity. Clin Gastroenterol Hepatol. 2017 Nov;15(11):1742–1749.e2. doi: 10.1016/j.cgh.2017.06.007. https://linkinghub.elsevier.com/retrieve/pii/S1542-3565(17)30712-7 .S1542-3565(17)30712-7 [DOI] [PubMed] [Google Scholar]
- 99.Vinding KK, Elsberg H, Thorkilgaard T, Belard E, Pedersen N, Elkjaer M, Marker D, Carlsen K, Burisch J, Munkholm P. Fecal calprotectin measured by patients at home using smartphones: A new clinical tool in monitoring patients with inflammatory bowel disease. Inflamm Bowel Dis. 2016 Feb;22(2):336–344. doi: 10.1097/MIB.0000000000000619. [DOI] [PubMed] [Google Scholar]
- 100.Chung AE, Sandler RS, Long MD, Ahrens S, Burris JL, Martin CF, Anton K, Robb A, Caruso TP, Jaeger EL, Chen W, Clark M, Myers K, Dobes A, Kappelman MD. Harnessing person-generated health data to accelerate patient-centered outcomes research: The Crohn's and Colitis Foundation of America PCORnet Patient Powered Research Network (CCFA Partners) J Am Med Inform Assoc. 2016 May;23(3):485–490. doi: 10.1093/jamia/ocv191. http://europepmc.org/abstract/MED/26911821 .ocv191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sossenheimer P, Yvellez O, Andersen MJ, Pearl TA, Jurdi KE, Rubin DB, Mayampurath A, Rubin DT. Wearable devices can predict disease activity in inflammatory bowel disease patients. Gastroenterology. 2019 May;156(6):S-111. doi: 10.1016/S0016-5085(19)37069-6. doi: 10.1016/S0016-5085(19)37069-6. [DOI] [Google Scholar]


