Abstract
The standard membrane-feeding assay (SMFA) is a functional assay that has been used to inform the development of transmission-blocking vaccines (TBV) against Plasmodium falciparum malaria. For Pfs230, a lead target antigen for TBV development, a few studies have tested either a single anti- Pfs230 polyclonal or monoclonal antibody (one antibody per study) at serial dilutions and showed a dose-dependent response. Further, there have been reports that the SMFA activity of anti-Pfs230 polyclonal and monoclonal antibodies were enhanced in the presence of complement. However, no analysis has been performed with multiple samples, and the impact of anti-Pfs230 antibody titers, IgG subclass profile and avidity were evaluated together in relation to transmission-reducing activity (TRA) by SMFA. In this report, a total of 39 unique anti-Pfs230 IgGs from five different mouse immunization studies were assessed for their ELISA units (EU), IgG2/IgG1 ratio and avidity by ELISA, and the functionality (% transmission-reducing activity, %TRA) by SMFA. The mice were immunized with Pfs230 alone, Pfs230 conjugated to CRM197, or a mixture of unconjugated Pfs230 and CRM197 proteins using Alhydrogel or Montanide ISA720 adjuvants. In all studies, the Pfs230 antigen was from the same source. There was a significant correlation between EU and %TRA (p < 0.0001 by a Spearman rank test) for the anti-Pfs230 IgGs. Notably, multiple linear regression analyses showed that both IgG2/IgG1 ratio and avidity significantly affected %TRA (p = 0.003 to p = 0.014, depending on the models) after adjusting for EU. The results suggest that in addition to antibody titers, IgG2/IgG1 ratio and avidity should each be evaluated to predict the biological activity of anti-Pfs230 antibodies for future vaccine development.
Keywords: Plasmodium falciparum, Pfs230, Transmission-blocking vaccine, Standard membrane-feeding assay, IgG subclass, Avidity
1. Introduction
Implementation of multiple anti-malarial control measures, such as insecticide-treated nets, rapid diagnosis, and antimalarial drugs, has successfully reduced the morbidity and mortality of malaria in the last 15–20 years [1]. However, there were still an estimated 216 million cases and 445,000 deaths related to malaria in 2016, and the progress has stalled very recently [1]. A transmission-blocking vaccine (TBV), which targets the biological bottleneck of the malaria lifecycle, can be one of the novel control measures to achieve malaria eradication [2,3]. In the field, the numbers of oocysts per mosquito are very low, usually <5–10; therefore, an intervention needs to kill only a few parasites to prevent new transmission. TBVs are designed to induce antibodies in human hosts against sexual stage malaria antigens or to antigens found in the mosquito vector, and these antibodies inhibit parasite development in the mosquito when they ingest parasites.
The standard membrane-feeding assay (SMFA) has been utilized to determine the biological activity of TBV-induced antibodies [2,3]. In this assay, a mixture of cultured Plasmodium falciparum gametocytes and test antibodies are fed to Anopheles mosquitoes through a membrane-feeding apparatus, and the mosquitoes are dissected approximately one week later to enumerate oocysts in the midgut. A similar assay, the direct membranefeeding assay (DMFA), where blood from a malaria-infected individual is used as the source of gametocytes instead of cultured parasites, has also been employed for TBV development. While both assays are considered “gold standard” assays, they are very labor intensive and low-throughput assays. Therefore, if a less labor-intensive and higher-throughput assay could be used as a surrogate to down-select samples for SMFA and/or DMFA testing, it could accelerate vaccine development. In the case of Pfs25, one of the most studied TBV candidates, we have shown that anti- Pfs25 antibody levels measured by the enzyme-linked immunosorbent assay (ELISA) directly correlated with SMFA activity, at least when the same Pfs25 recombinant protein was used as immunogen with various adjuvants in mouse, rabbit, monkey and human [4,5]. In addition, our recent monkey study has shown for the first time that the avidity of anti-Pfs25 antibodies associates with SMFA activity after adjusting for antibody level [6]. However, similar correlations have not yet been evaluated for other TBV candidates using multiple samples.
Disruption of the Pfs230 gene in P. falciparum parasites resulted in >90% reduction in oocyst numbers per mosquito compared to that in wild type parasites [7], suggesting there is no compensatory mechanism for Pfs230. In addition, a study with the rodent malaria parasite P. berghei indicated that the Pfs230 ortholog P230 played an important role in male gamete fertility [8]. Vaccine formulations with recombinant Pfs230 proteins, which were produced using a variety of expression systems [9–15], and virus vectored vaccines [16,17] could elicit functional antibodies in mouse or rabbit immunization studies. In addition, one human phase 1 trial with a Pfs230-based vaccine has been completed (ClinicalTrials.gov Identifier: ), and another phase 1 trial has been underway (). Throughout this report, the term “functional” antibody means that antibody prevents oocyst formation in mosquitoes measured by a SMFA and/or a DMFA; but it does not necessarily indicate the antibody inhibits the biological function of Pfs230 in a live parasite.
In several studies, anti-Pfs230 monoclonal antibody (mAb) [18,19] and anti-Pfs230 polyclonal antibodies (pAb) [12, 13, 15−17] were tested by SMFA at serial dilutions and showed dose-dependent responses (i.e., higher concentration of antibodies showed higher functional activity). As is the case with Pfs25, the results suggest that % inhibition in SMFA may correlate with anti-Pfs230 ELISA titers. On the other hand, in contrast to Pfs25, these studies have shown the effect of complement on functional activity for anti-Pfs230 antibodies. Anti-Pfs230 mAb only showed significant inhibitions in the presence of complement [18,19], and anti-Pfs230 pAb displayed higher inhibition in the presence of complement, while complement is not an absolute requirement [11,12,15]. The results indicate the IgG subclass profile might also correlate with the functional activity of anti-Pfs230 antibodies, as in general, mouse IgG2 and IgG3 antibodies fix human complement better than IgG1 [20].
In this report, 39 unique anti-Pfs230 pAbs from five different mouse immunization studies were assessed for their antibody levels (ELISA units, EU), IgG2/IgG1 ratio and avidity by ELISA, and the functionality (% transmission-reducing activity, %TRA) by SMFA. Similar to anti-Pfs25 pAb, there was a significant correlation between EU and %TRA for the anti-Pfs230 pAb. Furthermore, IgG2/ IgG1 ratio and avidity also significantly affected functionality after adjusting for EU. The results indicate that not only total antibody titers, but also IgG2/IgG1 ratio and avidity should be evaluated in future studies as possible predictors of functional activity of anti- Pfs230 antibodies.
2. Materials and methods
2.1. Preparations of Pfs230C1 recombinant protein, CRM197 and Pfs230-CRM197 conjugates
The expression and characterization of Pfs230C1 protein have been published elsewhere [15]. In brief, the N-terminal region of the Pfs230 molecule (amino acids 443–731) with a mutation at the potential N-glycosylation site (N585Q) was expressed using a baculovirus system. CRM197, a genetically detoxified diphtheria toxin, was produced by Fina Biosolutions LLC (Rockville, MD) using an Escherichia coli expression system [21]. Pfs230-CRM197 conjugates were also prepared by Fina Biosolutions LLC following methods described by Jones et al. [22]. Briefly, the thiol-modified Pfs230C1 and maleimide-modified CRM197 were mixed and resulting Pfs230C1-CRM conjugates were purified by size-exclusion chromatography. The conjugated proteins had an average molecular weight of approximately 300 kDa and contained 59–68% (by weight) of Pfs230C1 as confirmed by amino acid analysis.
2.2. Generation of mouse anti-Pfs230Cl antiserum and total IgG purification
To generate anti-Pfs230C1 antisera, five independent mouse immunization studies were conducted (Table 1). Studies #1−3 were performed at the Laboratory of Malaria and Vector Research (LMVR) of the National Institute of Allergy and Infectious Disease (NIAID), NIH in compliance with the Animal Welfare Act regulations in the Guide for Care and Use of Laboratory Animals and reviewed and approved by NIAID’s Animal Care and Use Committee (LMVR10E). Studies #4 and 5 were conducted by Noble Life Sciences, Inc. (NLS; Woodbine, MD) under an approved NLS IACUC protocol. NLS is an AAALACi accredited, USDA compliant and OLAW assured contract research organization.
Table 1.
| Study # | Antigen | Dose [μg]c | Adjuvant | # of IgGd |
|---|---|---|---|---|
| 1 | Pfs230C1 or Pfs230C1-CRMe | 0.5, 5 or 16.5 | Alumf or ISAg | 4 |
| 2 | Pfs230C1 or Pfs230C1-CRM | 0.02, 0.1, 0.5 or 2.5 | Alum | 6 h |
| 3 | Pfs230C1 | 0.2, 1, 5 or 15 | Alum or ISA | 10 |
| 4 | Pfs230C1, Pfs230C1-CRM or “Pfs230C1 & CRM”i | 0.5, 1.5 or 5 | Alum | 9 |
| 5 | Pfs230C1 or Pfs230C1-CRM | 0.15, 0.5, 1 or 5 | Alum | 10 |
For all immunization studies, CD1 mice (n = 10 per group) were immunized intramuscularly on days 0 and 21 and blood samples were collected on day 42.
Study #1–3 were conducted at LMVR/NIAID, and Study #4 & 5 were conducted at Noble Life Sciences.
Dose of Pfs230C1 protein, not total protein amount.
Number of test IgGs (exclude control IgG) generated for SMFA testing.
Pfs230C1 conjugated with CRM197.
Alhydrogel (Brenntag).
Montanide ISA720 (Seppic Inc).
One IgG sample, which showed 100% inhibition was excluded from linear regression analysis.
A simple mixture of Pfs230C1 and CRM197 proteins at the time of vaccine formulation, no conjugation.
For all studies, a group of ten CD1 mice were immunized at the indicated doses of Pfs230C1 indicated in Table 1 and Table S1, with either Alhydrogel (Alum; Brenntag, Reading, PA) or Montanide ISA720 (ISA; Seppic, Fairfield, NJ) adjuvant on days 0 and 21 by intramuscular injection, and the serum samples were collected on day 42. For each group (n = 10 mice per group), an equal volume of serum from each mouse (regardless of its antibody level) was used to create the group’s serum pool. A total of 39 anti- Pfs230C1 antiserum pools were generated, then total IgG was purified using a protein G column (GE Healthcare, Pittsburgh, PA) according to the manufacturer’s instructions, and adjusted to a final concentration of 4–6 mg/ml in phosphate buffered saline (PBS).
2.3. ELISA
The basic methodology of regular (total IgG) ELISA has been published elsewhere [23]. The IgG subclass ELISA was performed as described previously [15]. The avidity of each IgG sample was determined as described before [6]. In brief, all IgGs samples were diluted to 2 ELISA units. After 2 h of the primary antibody incubation, varying concentrations of urea (from 0 to 8 M) were added for 15 min to determine the avidity. While the same methodology was utilized, the concentration of urea resulting in 50% of the original EU (IC50) was calculated in this study, instead of IC20 in the previous study. For all ELISA, Pfs230C1 protein was used as the coating antigen. While all IgG subclasses (IgG1, 2a, 2b, 2c and 3) were measured in individual purified IgG samples, IgG3 responses were negative for all. Therefore, IgG3 data were not presented in this paper.
2.4. SMFA
The standardized methodology for performing the SMFA has been described previously [24]. Briefly, 16–18 day old gametocyte cultures of the P. falciparum NF54 line were mixed with test IgGs at 0.75 mg/ml, and the final mixture was immediately fed to ~50 female Anopheles stephensi mosquitoes through a membranefeeding apparatus. All feeding experiments were performed with human complement (i.e., with 31% v/v of non-heat inactivated human serum). Mosquitoes were kept for eight days and dissected (n = 20 per group) to enumerate the oocysts in the midgut. Only midguts from mosquitoes with any eggs in their ovaries at the time of dissection were analyzed, as egg development is a marker for having taken a blood meal. The human serum and red blood cells used for the gametocyte cultures and feeding experiments were purchased from Interstate Blood Bank (Memphis, TN).
2.5. Statistical analysis
The ratio of IgG2/IgG1 was calculated for each IgG sample as the sum of the OD values (IgG2a + IgG2b + IgG2c) divided by the OD value of IgG1.
As indicators of functional activity, two readouts are used in the analyses: %TRA and Log of mean oocyst ratio (LMR). Percent TRA=100 × {1 − (mean number of oocysts in the test)/(mean number of oocysts in the control)}, and LMR = Log10 {(mean number of oocysts in the control)/(mean number of oocysts in the test)}. Therefore, LMR is also expressed as; Log10 {100/(100 − % TRA)}.
The level of anti-Pfs230C1 EU, IgG2/IgG1 ratio or avidity (IC50) among four groups were compared by a Kruskal-Wallis test, followed by Dunn’s multiple comparison tests. The correlation between 2 of the 3 factors (EU, IgG2/IgG1 ratio, or avidity), or the correlation between %TRA and each of the 3 factors was assessed by a Spearman rank test. For a linear regression analysis, anti- Pfs230C1 EU (square root scale), ratio of IgG2/IgG1, avidity, “adjuvant” (Alum or ISA) and “antigen” (Pfs230C1, Pfs230C1-CRM197, or a mixture of Pfs230C1 and CRM197) were included either solely (a single linear regression analysis) or combinations (a multiple linear regression) as the explanatory variables. There was 1 out of 39 IgGs that showed 100 %TRA, and the data was excluded from the linear regression analyses as before [25], because 100 %TRA was the upper plateau level of dose-response.
All statistical tests were performed in JMP13 (SAS Institute, Cary, NC) or Prism 7 (GraphPad Software, La Jolla, CA), and p- values <0.05 were considered significant.
3. Results and discussions
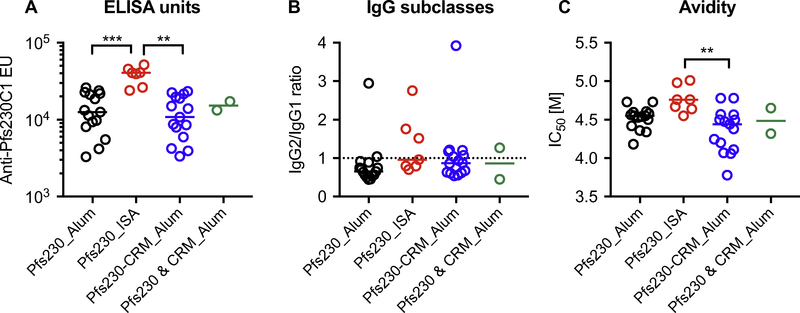
A total of 39 groups of mice (n = 10 per group) were immunized in five independent studies with three Pfs230C1-based immunogens (Pfs230C1 alone, Pfs230C1-CRM197 conjugate, and a simple mixture of Pfs230C1 and CRM197) and two adjuvants (Alum or ISA). The overall immunization study designs are seen in Table 1, and details of each of the 39 groups are also presented in Table S1. For each group, a pooled serum was generated for IgG purification, and then anti-Pfs230C1 ELISA units (EU), IgG2/IgG1 ratio and avidity of the purified IgGs were determined using the recombinant Pfs230C1 protein (without CRM197 conjugation) as an ELISA plate coating antigen, regardless of immunogens. All analyses (both ELISA and SMFA) shown in this manuscript were performed with the purified IgG data (one IgG per group of ten mice). The resulting total IgGs displayed various ranges of antibody level (3300−51,770 EU in 1 mg/mL of total IgG), IgG2/IgG1 ratio (0.445–3.921) and avidity (3.78–5.01 M) (Fig. 1). While the differences among four groups (i.e., Pfs230_Alum, Pfs230_ISA, Pfs230-CRM_Alum, and Pfs230 & CRM_Alum groups shown in Fig. 1 ) did not always reach significance, overall, total IgG samples in Pfs230_ISA category showed higher EU, IgG2/IgG1 ratio, and avidity. It is important to note that the comparisons were performed to display the characteristics of IgG samples, normalized to a single test concentration of 750 μg/mL, in SMFA, not for immunogenicity comparisons (e.g., whether or not CRM-conjugation vaccines induced higher antibody levels than unconjugated vaccines), because differing doses of Pfs230 antigens were used for different immunization groups across the various studies. For example, in Study #1, groups of mice were immunized with 5 and 0.5 μg of CRM-conjugated vaccines (Alum), 5 μg for unconjugated vaccine with Alum, or 15 μg for unconjugated vaccine with ISA 720 adjuvant (Table S1), and all 4 IgG data are presented in Fig. 1.
Fig. 1. Characteristics of anti-Pfs230 IgGs.
Anti-Pfs230C1 ELISA units (EU) per 1 mg/mL of purified total IgG (A), IgG2/IgG1 ratio (B) and avidity (C) were determined for each IgG sample, and the data are grouped by the combinations of immunogen and adjuvant used for immunization. Individual and median values are shown. Pfs230_Alum, Pfs230C1 alone on Alhydrogel (Alum), n = 15 IgG samples; Pfs230_ISA, Pfs230C1 alone in Montanide ISA720 (ISA), n = 7; Pfs230-CRM_Alum, conjugated Pfs230C1 and CRM197 proteins on Alum, n = 15; Pfs230 & CRM_Alum, a simple mixture of Pfs230C1 and CRM197 proteins was adjuvanted with Alum, n = 2. The difference among 4 groups was significant for ELISA units, EU (p = 0.001 by a Kruskal-Wallis test) and avidity (p = 0.007), but not IgG2/IgG1 ratio (p = 0.108). Significant differences between two groups (by Dunn’s multiple comparison tests) are shown; ***, p < 0.0001; **, p < 0.001. The dotted line (B) shows an IgG2/IgG1 ratio of 1.
We first assessed the correlation between each of the three immunological readouts and % TRA separately. There was a significant correlation between anti-Pfs230C1 EU and avidity; p = 0.016 and rs = 0.382 (95% Confidence Interval, 95%CI, 0.065–0.629) by a Spearman rank test. On the other hand, the other correlations were not significant (p = 0.282 for anti-Pfs230C1 EU vs. IgG2/IgG1 ratio; p = 0.875 for IgG2/IgG1 ratio vs. avidity).
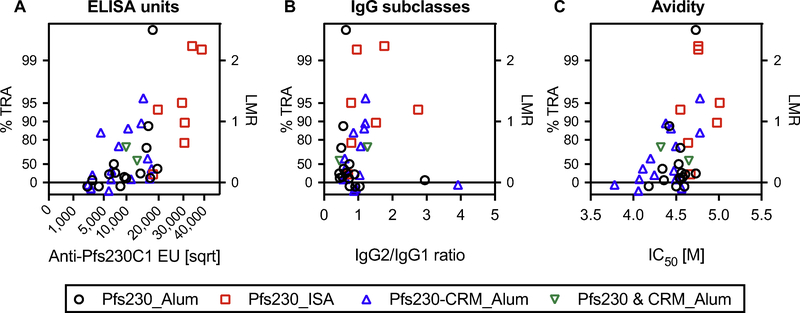
The 39 pAb samples were tested by SMFA at 750 μg/mL in the presence of human complement. While several of them were evaluated in two or three independent assays, to avoid a bias from the repeats, one % TRA value per pAb (the result of first SMFA) was included in the analyses. As with the anti-Pfs25 antibodies [4,5], there was a significant correlation between %TRA and anti-Pfs230C1 EU; p< 0.0001 and rs = 0.688 (95%CI, 0.468−0.827) by a Spearman rank test (Fig. 2A). The correlation between %TRA and IgG2/IgG1 ratio was not significant (p = 0.308, Fig. 2B), whilst the correlation with avidity was significant; p = 0.003 and rs = 0.463 (95%CI, 0.164–0.685) (Fig. 2C). However, the significant correlation between %TRA and avidity should be interpreted with caution. Avidity level showed a positive correlation with anti-Pfs230C1 EU (IgGs with higher EU also showed higher avidity), therefore there could be confounder effects of EU on both avidity and %TRA.
Fig. 2. Functional activity of anti-Pfs230 IgGs judged by SMFA.
A total of 39 IgGs were tested at 750 μg/mL with complement. Anti-Pfs230C1 EU in a feeder (on a square root scale, sqrt) (A), IgG2/IgG1 ratio (B) or avidity (C) is shown on the x-axis, and the ratio of mean oocyst (mean oocyst density in control divided by mean in test) is plotted on a log scale (log of mean oocyst ratio, LMR, right side of y-axis). The associated % inhibition (%TRA) value is shown on the left side of the y-axis. The tested IgGs are grouped as in Fig. 1. One total IgG (in Pfs230_Alum category) which showed 100% TRA (LMR of infinity) in SMFA is assigned the value of LMR = 2.5 (%TRA = 99.7) for graphical presentation purposes.
To determine the effect of various factors together, multiple linear regressions were performed next (Table 2). Previous studies [13, 25] with different antibodies (rodent anti-Pfs25, anti-Pfs230, anti-Pfs48/45 antibodies, and human antibodies induced by natural infection) have shown that when the dose of antibody is plotted on a square root scale against LMR, SMFA data can be approximated to a linear regression for each antibody. Therefore, LMR was used as a response variable, instead of %TRA, in the following analyses. In the first model, all 5 factors (anti-Pfs230C1 EU, IgG2/ IgG1 ratio, avidity, “adjuvant” and “antigen”) were included as explanatory variables (Model #1). EU, IgG2/IgG1 ratio, and avidity significantly associated with LMR after adjusting the other factors, but adjuvant and antigen did not. Since adjuvant and antigen did not significantly correlate with LMR, the next multiple linear regression was performed with only 3 significant factors (Model #2). The overall fit to the linear model was R2 = 0.647, and EU had the most significant impact on LMR (p< 0.0001), then IgG2/ IgG1 ratio (p = 0.0027), followed by avidity (p = 0.0137). On the other hand, when only EU factor was included in a linear model (Model #3), the overall fit decreased to R2 = 0.499. Finally, the impact of each of the other two factors was also evaluated using the same linear model. As expected from the results of Spearman rank tests, IgG2/IgG1 alone did not correlate with the SMFA activity (p = 0.3457, Model #4), and avidity alone only weakly associated with the functional activity (p = 0.0014, but overall fit was R2 = 0.248, Model #5). Taken together, anti-Pfs230C1 EU had the strongest influence on the functional activity, however, IgG2/IgG1 ratio and avidity also significantly affected the SMFA results after adjusting for anti-Pfs230C1 EU.
Table 2.
Single or multiple linear regressions for SMFA activitya.
| Model # | Explanatory variablesb |
Overall model fit (R2) | ||||
|---|---|---|---|---|---|---|
| ELISA unitsc | IgG2/1 ratiod | aviditye | adjuvantf | antigeng | ||
| 1 | <0.0001 | 0.0026 | 0.0026 | 0.5207 | 0.1221 | 0.700 |
| 2 | <0.0001 | 0.0027 | 0.0137 | n.i.h | n.i. | 0.647 |
| 3 | <0.0001 | n.i. | n.i. | n.i. | n.i. | 0.499 |
| 4 | n.i. | 0.3457 | n.i. | n.i. | n.i. | 0.025 |
| 5 | n.i. | n.i. | 0.0014 | n.i. | n.i. | 0.248 |
Response variable is functional activity measured by SMFA: LMR, Log of mean oocyst ratio between control and test IgGs. One IgG sample which showed 100% inhibition was excluded from the analyses.
p-value of each variable in each model is shown.
Square-root-transformed anti-Pfs230C1 ELISA units.
Sum of the OD values (IgG2a + IgG2b + IgG2c) divided by the OD value of IgG1.
IC50 [M].
Alhydrogel or Montanide ISA720.
Pfs230C1, Pfs230C1 conjugated with CRM197, or a simple mixture of Pfs230C1 and CRM197 proteins.
n.i.; The variable is not included in the model.
While not the main objective of this report, to evaluate the effect of CRM-conjugation, we paired two groups (with or without CRM conjugation) of mice which were immunized with the same dose of Pfs230 antigens and same adjuvant (Alum) in the same study (e.g., sample #1 and #3 in Study #1, sample #2 and #4 in Study #2). A total of twelve such pairs from four studies were compared for their %TRA results in SMFA (Fig. S1 ). Excluding eight pairs with insignificant and unreliable TRA (i.e. both paired IgGs showed <50 %TRA where error of assay is too high [24,25]), all other four pairs displayed higher %TRA in groups received CRM-conjugated vaccines (Fig S1). The result suggested that CRM197 conjugation improved the functionality of induced antibodies, while an additional experiment is required to perform a statistical analysis.
When a single mAb or pAb sample is tested at serial dilutions, as performed in most previous studies, the antibody has a fixed IgG2/ IgG1 ratio and avidity, therefore, the impact of those factors on functional activity cannot be evaluated. Using multiple different samples from five independent mouse studies, this report has shown for the first time that the IgG subclass profile had significant impact on functional activity after adjusting antibody titers for any of anti-TBV pAbs. In addition, avidity of antibodies also significantly associates with SMFA activity after adjusting the other two. In vaccine studies, either in animals or humans, antibody titers are almost always evaluated. However, IgG subclass profile and avidity are not necessarily assessed in all studies. This study suggests that if a vaccine formulation (e.g., different adjuvant) and/or an immunization strategy (e.g., dose and regimen) can induce more IgG2 and/or higher avidity index antibodies, the elicited pAbs could show higher functional activities, even if the antibody titers measured by ELISA are unchanged. In this study, pAbs against a single source of Pfs230 recombinant protein were evaluated in mice. It will be interesting to investigate whether pAbs elicited with different Pfs230 recombinant proteins (or other immunization strategy) show the same SMFA activity at the same ELISA units, IgG2/IgG1 ratio, and avidity (measured against a reference Pfs230 protein) and whether the similar associations hold in other species, especially in humans. If it is confirmed, researchers can use more high-throughput ELISA-type assays for lead identification/optimization, formulation optimization and/or dose/regi-men optimization. As shown in Table 2, the overall fit for Model #2 was 0.647, indicating that other factors, which were not determined in this study (e.g., fine specificity of antibodies, mosquito factors), could also affect SMFA results. This study has shown the importance of measuring not only total antibody titers, but also IgG2/IgG1 ratio and avidity, to predict the biological activity of antibodies in future studies with Pfs230-based vaccines.
Supplementary Material
Acknowledgements
We would like to thank Drs. Andrew Lees and Morgane Ollivault-Shiflett at Fina Biosolutions LLC for producing the CRM197 and the Pfs230C1-CRM197 conjugated proteins.
Funding
The study was supported by PATH’s Malaria Vaccine Initiative, and the intramural program of the National Institute of Allergy and Infectious Disease/NIH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- DMFA
direct membrane-feeding assay
- EU
ELISA units
- LMR
log of mean oocyst ratio
- mAb
monoclonal antibody
- pAb
polyclonal antibody
- SMFA
standard membrane-feeding assay
- TBV
transmission-blocking vaccine
- %TRA
% transmission-reducing activity
Footnotes
Conflict of interest statement
The authors have no conflict of interest to declare.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2019.02.071.
References
- [1].Organization WH. World malaria report, 2017. http://wwwwhoint/malaria/publications/world-malaria-report-2017/report/en/[Accessed 13 November 2018].
- [2].Wu Y, Sinden RE, Churcher TS, Tsuboi T, Yusibov V. Development of malaria transmission-blocking vaccines: from concept to product. Adv Parasitol 2015;89:109–52. [DOI] [PubMed] [Google Scholar]
- [3].Nikolaeva D, Draper SJ, Biswas S. Toward the development of effective transmission-blocking vaccines for malaria. Exp Rev Vaccines 2015:1–28. [DOI] [PubMed] [Google Scholar]
- [4].Miura K, Keister DB, Muratova OV, Sattabongkot J, Long CA, Saul A. Transmission-blocking activity induced by malaria vaccine candidates Pfs25/ Pvs25 is a direct and predictable function of antibody titer. Malaria J 2007;6:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cheru L, Wu Y, Diouf A, Moretz SE, Muratova OV, Song G, et al. The IC(50) of anti-Pfs25 antibody in membrane-feeding assay varies among species. Vaccine 2010;28:4423–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Thompson EA, Ols S, Miura K, Rausch K, Narum DL, Spangberg M, et al. TLR- adjuvanted nanoparticle vaccines differentially influence the quality and longevity of responses to malaria antigen Pfs25. JCI Insight 2018;3(10): e120692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Eksi S, Czesny B, van Gemert GJ, Sauerwein RW, Eling W, Williamson KC. Malaria transmission-blocking antigen, Pfs230, mediates human red blood cell binding to exflagellating male parasites and oocyst production. Mol Microbiol 2006;61:991–8. [DOI] [PubMed] [Google Scholar]
- [8].van Dijk MR, van Schaijk BC, Khan SM, van Dooren MW, Ramesar J, Kaczanowski S, et al. Three members of the 6-cys protein family of Plasmodium play a role in gamete fertility. PLoS Pathog 2010;6:e1000853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Williamson KC, Keister DB, Muratova O, Kaslow DC. Recombinant Pfs230, a Plasmodium falciparum gametocyte protein, induces antisera that reduce the infectivity of Plasmodium falciparum to mosquitoes. Mol Biochem Parasitol 1995;75:33–42. [DOI] [PubMed] [Google Scholar]
- [10].Bustamante PJ, Woodruff DC, Oh J, Keister DB, Muratova O, Williamson KC. Differential ability of specific regions of Plasmodium falciparum sexual-stage antigen, Pfs230, to induce malaria transmission-blocking immunity. Parasite Immunol 2000;22:373–80. [DOI] [PubMed] [Google Scholar]
- [11].Farrance CE, Rhee A, Jones RM, Musiychuk K, Shamloul M, Sharma S, et al. A Plant-produced Pfs230 vaccine candidate blocks transmission of Plasmodium falciparum. Clin Vaccine Immunol 2011;18:1351–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tachibana M, Wu Y, Iriko H, Muratova O, Macdonald NJ, Sattabongkot J, et al. N-terminal pro-domain of Pfs230 synthesized using cell-free system is sufficient to induce the complement dependent malaria transmission-blocking activity. Clin Vaccine Immunol 2011;18:1343–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Miura K, Takashima E, Deng B, Tullo G, Diouf A, Moretz SE, et al. Functional comparison of Plasmodium falciparum transmission-blocking vaccine candidates by the standard membrane-feeding assay. Infect Immun 2013;81:4377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].MacDonald NJ, Nguyen V, Shimp R, Reiter K, Herrera R, Burkhardt M, et al. Structural and immunological characterization of recombinant 6-cysteine domains of the Plasmodium falciparum sexual stage protein Pfs230.JBiol Chem 2016;291:19913–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lee SM, Wu CK, Plieskatt JL, Miura K, Hickey JM, King CR. An N-terminal Pfs230 domain produced in baculovirus as a biological active transmission-blocking vaccine candidate. Clin Vaccine Immunol 2017;24(10):e00140–e217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kapulu MC, Da DF, Miura K, Li Y, Blagborough AM, Churcher TS, et al. Comparative assessment of transmission-blocking vaccine candidates against Plasmodium falciparum. Sci Rep 2015;5:11193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Menon V, Kapulu MC, Taylor I, Jewell K, Li Y, Hill F, et al. Assessment of antibodies induced by multivalent transmission-blocking malaria vaccines. Front Immunol 2017;8:1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Quakyi IA, Carter R, Rener J, Kumar N, Good MF, Miller LH. The 230-kDa gamete surface protein of Plasmodium falciparum is also a target for transmission-blocking antibodies. J Immunol 1987;139:4213–7. [PubMed] [Google Scholar]
- [19].Roeffen W, Geeraedts F, Eling W, Beckers P, Wizel B, Kumar N, et al. Transmission blockade of Plasmodium falciparum malaria by anti-Pfs230- specific antibodies is isotype dependent. Infect Immun 1995;63:467–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Collins AM. IgG subclass co-expression brings harmony to the quartet model of murine IgG function. Immunol Cell Biol 2016;94:949–54. [DOI] [PubMed] [Google Scholar]
- [21].Hickey JM, Toprani VM, Kaur K, Mishra RPN, Goel A, Oganesyan N, et al. Analytical comparability assessments of 5 recombinant CRM197 proteins from different manufacturers and expression systems. J Pharm Sci 2018;107:1806–19. [DOI] [PubMed] [Google Scholar]
- [22].Jones DS, Rowe CG, Chen B, Reiter K, Rausch KM, Narum DL, et al. A method for producing protein nanoparticles with applications in vaccines. PLoS One 2016;11:e0138761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Miura K, Orcutt AC, Muratova OV, Miller LH, Saul A, Long CA. Development and characterization of a standardized ELISA including a reference serum on each plate to detect antibodies induced by experimental malaria vaccines. Vaccine 2008;26:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Miura K, Deng B, Tullo G, Diouf A, Moretz SE, Locke E, et al. Qualification of standard membrane-feeding assay with Plasmodium falciparum malaria and potential improvements for future assays. PLoS One 2013;8:e57909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Miura K, Stone WJ, Koolen KM, Deng B, Zhou L, van Gemert GJ, et al. An interlaboratory comparison of standard membrane-feeding assays for evaluation of malaria transmission-blocking vaccines. Malar J 2016;15:463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.