Abstract
Disruption of neuronal Ca2+ homeostasis plays a well-established role in cell death in a number of neurodegenerative disorders. Recent evidence suggests that proteolysis of the type 1 inositol 1,4,5-trisphosphate receptor (InsP R1), a Ca2+ release channel on the endoplasmic reticulum, generates a dysregulated channel, which may contribute to aberrant Ca2+ signaling and neurodegeneration in disease states. However, the specific effects of InsP3R1 proteolysis on neuronal Ca2+ homeostasis are unknown, as are the functional contributions of this pathway to neuronal death. This study evaluates the consequences of calpain-mediated InsP3R1 proteolysis on neuronal Ca2+ signaling and survival using adeno-associated viruses to express a recombinant cleaved form of the channel (capn-InsP3R1) in rat primary cortical neurons. Here, we demonstrate that expression of capn-InsP3R1 in cortical cultures reduced cellular viability. This effect was associated with increased resting cytoplasmic Ca2+ concentration ([Ca2+]i), increased [Ca2+]i response to glutamate, and enhanced sensitivity to excitotoxic stimuli. Together, our results demonstrate that InsP3R1 proteolysis disrupts neuronal Ca2+ homeostasis, and potentially acts as a feed-forward pathway to initiate or execute neuronal death.
Keywords: calcium; calpain; endoplasmic reticulum; excitotoxicity; inositol 1,4,5-trisphosphate receptor; neuron
Spatial and temporal regulation of cytoplasmic free Ca2+ concentration ([Ca2+]i) plays a vital role in neuronal signaling, and dysregulation of [Ca2+]i can trigger cell death (Berridge etal. 2000, 2003; Orrenius etal. 2003; Mattson 2007). Under physiologic conditions, changes in neuronal [Ca2+]i are tightly controlled by Ca2+ pumps, Ca2+-binding proteins, and intracellular organelles. The endoplasmic reticulum (ER), the primary Ca2+ storage organelle in neurons, is capable of both Ca2+ sequestration and release. Proper ER Ca2+ regulatory function is essential to neuronal [Ca2+]i buffering, signaling, and survival (Mattson et al. 2000; Bardo et al. 2006; Stutzmann and Mattson 2011). A ubiquitous mechanism for Ca2+ signaling in neurons is Ca2+ release from the ER lumen through the inositol 1,4,5-trisphosphate receptor (InsP3R) (Foskett et al. 2007). Of the three identified InsP3R genes, type 1 InsP3R (InsP3R1) is the most abundant isoform expressed in brain (Sharp et al. 1993). Physiologic InsP3R1-mediated Ca2+ signaling in neurons is important for gene expression, membrane excitability, and synaptic plasticity (Bardo et al.2006; Stutzmann and Mattson 2011). InsP3R1 dysfunction has been implicated in a number of neurodegenerative disease states as a mechanism for impaired ER Ca2+ buffering, disruption of Ca2+ homeostasis, and neuronal death (Verkhratsky and Toescu 2003; Foskett 2010). In most of these cases, InsP3R1 dysfunction has been associated with allosteric modulation of the channel (Foskett et al. 2007). However, increasing evidence suggests that proteolytic modification of InsP3R1 can also dramatically disrupt channel physiology (Assefa et al. 2004; Nakayama et al. 2004; Verbert et al. 2008; Kopil et al. 2011), and may potentially contribute to neurodegeneration.
InsP3R1 is a target for caspase-3 and calpain-mediated proteolysis, which generate similarly sized ~95 kDa carboxyl-terminal fragments (Hirota et al. 1999; Kopil et al. 2011). Both caspase and calpain-mediated proteolysis of InsP3R1 remove the amino-terminal ligand-binding domain and a large portion of the coupling domain, leaving the pore-forming transmembrane domains intact. Studies examining the functional properties of the caspase-derived carboxyl-terminal InsP3R1 fragment demonstrated increased ER Ca2+ leak, and corresponding decreased ER [Ca2+] ([Ca2+]ER), and buffering capacity in cell lines expressing the recombinant channel (Assefa et al. 2004; Nakayama et al. 2004; Verbert et al. 2008). These effects were mediated by InsP3-independent Ca2+ release through caspase-cleaved InsP3R1, and expression of the truncated channel increased cellular sensitivity to apoptotic stimuli (Assefa et al. 2004). Our laboratory recently identified the calpain-cleavage site for InsP3R1 and determined that the calpain-derived carboxyl-terminal channel fragment displayed InsP3-independent gating and constitutive channel activity (Kopil et al. 2011). When expressed in cell lines, calpain-cleaved InsP3R1 decreased [Ca2+]ER via increased ER Ca2+ leak (Kopil et al. 2011). Although no study to date has investigated the effects of caspase- or calpain-cleaved InsP3R1 on neuronal Ca2+ homeostasis, these observations suggest that proteolysis of InsP3R1 may transform spatial and temporal regulation of neuronal [Ca2+]i and potentially signal cell death. Consistent with this hypothesis, we previously identified calpain-cleaved InsP3R1 in selectively vulnerable cerebellar Purkinje cells at both early and late times following ischemic brain injury (Kopil et al. 2011).
To define the role of InsP3R1 proteolysis in neuronal Ca2+ homeostasis and neurodegeneration, we examined responses of primary cortical neurons to expression of recombinant calpain-cleaved InsP3R1. Expression of the cleaved channel reduces viability of cortical cultures at baseline. Using single-cell Ca2+ imaging, we determined that calpain-cleaved InsP3R1 increases resting neuronal [Ca2+]i and leads to greater rises in [Ca2+]i in response to physiologic stimuli. Calpain-cleaved InsP3R1 also sensitized cultures to excitotoxic injury. Together, these data suggest a specific role for proteolyzed InsP3R1-mediated Ca2+ signaling in the molecular injury cascade in neurons, which has important therapeutic implications for neurodegenerative disorders associated with Ca2+ dysregulation.
Materials and methods
Materials
Unless otherwise noted, all chemicals were purchased from (Sigma-Aldrich, St Louis, MO USA).
Antibodies
Rabbit polyclonal antibody targeted against the 20 carboxyl-terminal residues of rat InsP3R1 was generated using previously described methods (Joseph and Samanta 1993; Kopil et al. 2011) (Covance ImmunoTechnologies, Denver, PA, USA). Rabbit polyclonal antibody to microtubule-associated protein 2 (MAP2) was a gift from Dr. Virginia Lee (University of Pennsylvania). Mouse monoclonal antibody to BiP/GRP78 was purchased from BD Biosciences. Mouse monoclonal antibody to α-spectrin (Ab1622) was purchased from Chemicon, Temecula, CA, USA. Alexa Fluor 488- and Alexa Fluor 568-conjugated secondary antibodies used for immunofluorescence were purchased from Invitrogen, Carlsbad, CA, USA. Horseradish peroxidase-linked secondary antibodies used for immunoblotting were purchased from Perkin Elmer, Waltham, MA, USA.
Rat primary mixed cortical cultures
Primary dissociated cultures were prepared from embryonic rat cortices as described previously (Cummings et al. 1996). Briefly, E19 embryos from pregnant female Sprague–Dawley rats (Charles River Laboratories Inc., Wilmington, MA, USA) were removed, the brains extracted, and cortices dissected. Cortices were trypsinized in Dulbecco’s minimum essential medium (Whittaker Bioproducts, Walkersville, MD, USA) at 4°C for 20 min. Cells were triturated in media consisting of Dulbecco’s minimum essential medium supplemented with 10% bovine calf serum (HyClone, Logan, UT, USA), 10% Ham’s F12 with glutamine (Whittaker Bioproducts), and 50 U/mL penicillin–streptomycin. Dissociated cortical cultures were plated on poly-l-lysine-coated 35-mm dishes (4.8 × 105 cells/well; Western blot), 12 mm glass coverslips in 24-well plates (8 × 104 cells/well; immunofluorescence), or 20 × 50-mm glass coverslips in four-well plates(3.2 × 106 cells/well; single-cell Ca2+ imaging). Dissociated cells were grown in serum-free Neurobasal medium (Gibco, Rockville, MD, USA) supplemented by B27 (Gibco) and cultured at 37°C in a humidified 5% CO2 incubator. Mitotic inhibitors and antibiotics were not used. This study was approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
Constructs and site-directed mutagenesis
The transgene for rat calpain-cleaved InsP3R1 (capn-InsP3R1; Fig. 1a) was previously generated (Kopil et al. 2011). To construct the pore-inactivating D2550A mutation (capn-InsP3R1D2550A), site-directed mutagenesis was performed with Quik-Change (Stratagene) using primers (5′–GGCGGAGTAGGAGCT GTGCTCAGGAAG–3′ and 5′–CTTCCTGAGCACAGCTCCTAC TCCGCC–3′ (codon change is shown in boldface type and underlined)) and capn-InsP3R1 as a template. The mutation was confirmed by DNA sequencing. For transduction of primary neurons, both capn-InsP3R1 and capn-InsP3R1 D2550A were subcloned into a plasmid containing the adeno-associated viral (AAV) inverted terminal repeats and polyA sequence for viral vector generation.
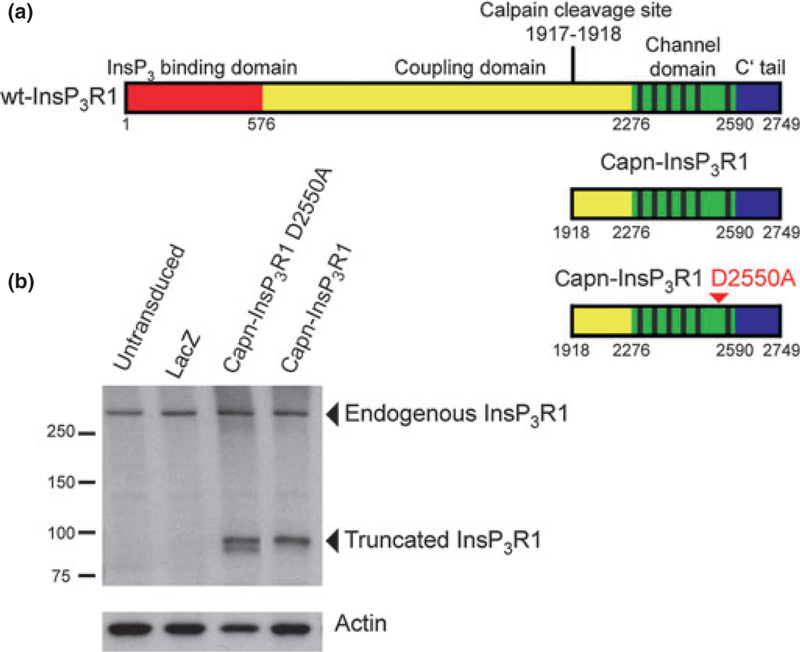
Fig. 1.
Schematic representation and expression of recombinant InsP3R1 constructs in primary neurons. (a) Protein domain structure of wt-InsP3R1 (top), calpain-cleaved InsP3R1 recombinant (middle), and calpain-cleaved InsP3R1 with the D2550A point mutation (bottom; arrowhead). Residues numbered according to rat type 1 SI+, SII+, SIII-sequence (protein accession NP_001007236.1). (b) Western blot analysis of whole-cell lysates from untransduced rat primary cortical cultures and primary cortical cultures transduced with AAV 2/1 expressing lacZ, capn-InsP3R1 D2550A, or capn-InsP3R1 at 1 week post-transduction (14 DIV). Carboxyl-terminal InsP3R1 antibody was used to detect endogenous and recombinant truncated rat InsP3R1. Antibody against actin was used as a loading control.
Viral vector generation
Recombinant AAV vectors were generated by the University of Pennsylvania Vector core as previously described (Fisher et al. 1997). Briefly, AAV vectors were prepared by triple transfection of HEK293 cells and purified by cesium chloride gradient sedimentation. AAV 2/1 vectors were designed to express capn-InsP3R1 (lots V2049 and V2451), capn-InsP3R1 D2550A (lots V2521 and V2601), or lacZ (lot V0861). All transgenes were expressed under the control of the cytomegalovirus promoter.
AAV 2/1 Vector transduction of primary neurons
Rat primary cortical cultures were transduced at 7 days in vitro (DIV) by adding AAV 2/1 vector in a vehicle of phosphate-buffered saline and 10% glycerol directly to the culture media. Cultures were transduced with 7.5 × 1010 genome copies (GC) per mL of media. All cell viability, biochemical, and functional assays were performed 1 week later (14 DIV).
Cell viability assays
Transduced cultures were fixed with 4% paraformaldehyde, immunolabeled for the neuronal marker MAP2 with a goat anti-rabbit secondary antibody (Alexa Fluor 568), and counter-stained with 4,6-diamidino-2-phenylindole nuclear label (Vector Laboratories, Burlingame, CA, USA). Five random 100× images were taken from each 12-mm coverslip. The number of surviving MAP2-labeled neurons per coverslip was estimated by blinded counting of all captured fields. For each transduction condition, 8–12 coverslips were counted from four independent experiments. To control for variability in culture health between experiments, the percentage of viable cells was determined by normalizing to the average cell count from lacZ-transduced cultures for each independent experiment and the average of the normalized experiments is shown. Reported SEM and error bars reflect propagated error through normalization.
Cell viability was independently measured by staining cultures with calcein-AM and propidium iodide (PI; Invitrogen). Live cultures were incubated with 3 μM calcein-AM and 5 μM PI in Dulbecco’s phosphate-buffered saline (DPBS; Gibco) for 30 min at 37°C and 5% CO2. Cultures were rinsed with DPBS and immediately imaged. Five random 100× images were taken from each 12-mm coverslip. The number of surviving, calcein-AM-positive, PI-negative cells per coverslip was estimated by blinded counting of all captured fields. For each transduction condition, 6–14 coverslips were counted from three independent experiments. The number of viable cells in each transduction condition was normalized to lacZ controls as described above.
Western blotting of primary neuron cultures
Media was removed from transduced cultures and replaced with DPBS at 4°C. Cells were harvested by scraping, and resuspended in homogenization buffer (50 mM Tris, 150 mM NaCl, 2 mM EGTA) with a protease inhibitor cocktail and sonicated. Lysates were treated with sodium dodecyl sulfate loading buffer, boiled, and analyzed on sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels.
Western blots were visualized using enhanced chemiluminescence supplies purchased from Perkin Elmer. Protein and polypeptide densities were quantified using computer densitometry (ImageJ, US National Institutes of Health, Bethesda, MD, USA) and normalized to lacZ controls. Error bars reflect propagated error through normalization.
Single-cell Ca2+ imaging
Cortical cultures were plated onto glass coverslips (Warner Instruments, Hamden, CT, USA) and transduced with AAV 2/1 at 7 DIV. One week following transduction, cells on coverslips were secured in a perfusion chamber, and mounted on the stage of an inverted microscope (Nikon Eclipse TE2000; Nikon Inc., Melville, NY, USA). Cells were loaded with Fura-2-AM (2.5 μM; Molecular Probes, Eugene, OR, USA) for 45 min at 37°C and 5% CO2 in Ca2+-containing extracellular solution (in mM: 120 NaCl, 4 KCl, 20 HEPES, 15 Glucose, 2 CaCl2, 1 MgCl2, pH 7.3). Fura-2 was alternately illuminated at 340/380 nm, and fluorescence intensity filtered at 510 nm. Data were collected and recorded as described previously (White et al. 2005; Cheung et al. 2008). Cells were perfused with 2 mM Ca2+ extracellular solution to establish baseline [Ca2+]i before (S)-3,5-dihydroxyphenylglycine (DHPG; 10 μM), glutamate (10 μM), thapsigargin (Tg; 1 μM), or KCl (50 mM) was applied in the extracellular solution. At the end of the experiment, Mn2+ was used to quench Fura-2 fluorescence (Ca2+-containing extracellular solution supplemented with 10 mM MnCl2 and 10 μM ionomycin). The remaining background fluorescence following Mn2+ quench was subtracted during analysis. Glial cells were identified by their morphology and ability accumulate high levels of Fura-2 compared with neurons (Ikegaya et al. 2005), and were excluded from analysis. Experiments using DHPG, glutamate alone, glutamate plus thapsigargin, and KCl were performed on different weeks, in cultures from different platings. Within each experiment using DHPG, glutamate alone, glutamate plus thapsigargin, or KCl, however, the studied lacZ-, capn-InsP3R1 D2550A-, and capn-InsP3R1-transduced cultures were derived from the same plating. To compare between experiments using glutamate alone and glutamate plus thapsigargin, the percent of maximum [Ca2+]i was determined by normalizing to the average peak [Ca2+]i of capn-InsP3 R1-transduced cultures from each plating. Reported SEM and error bars reflect propagated error through normalization.
Changes in [Ca2+]i are presented as changes in fluorescence ratio. Dye calibration was achieved by applying experimentally determined constants to the equation: [Ca2+] = Kd β(R–Rmin)/(Rmax–R). Macros used for analysis were custom macros written for IGOR Pro (WaveMetrics, Portland, OR, USA).
Glutamate injury characterization
HEPES-buffered saline (HBS; in mM: 145 NaCl, 3 KCl, 10 HEPES, 8 Glucose, 2 CaCl2, 1 MgCl2, pH 7.4) with or without l-glutamic acid was added to the media of transduced cortical cultures at 14 DIV. Twenty-four hours after HBS or glutamate application, cultures were fixed with 4% paraformaldehyde, immunolabeled for MAP2, and counter-stained with 4,6-diamidino-2-phenylindole. Five random 100× images were taken from each 12-mm coverslip. The number of surviving MAP2-labeled neurons per coverslip was estimated by blinded counting of all captured fields. For each transduction condition, six to eight coverslips were counted from three independent experiments. To control for variability in culture health between experiments and differences in baseline toxicity, the percent of viable cells at each glutamate dose was determined by normalizing within transduction conditions to the average cell count from HBS-treated cultures. Data were normalized within independent experiments and the average of the normalized experiments is shown. Reported SEM and error bars reflect propagated error through normalization. Dose-response curves were calculated by fitting plotted mean data points using the Hill equation and holding the base and max at 0 and 100, respectively. Reported error for LD50 values represents error of the fit.
Data analysis and statistics
Data are presented as the mean ± SEM, and statistical significance of differences between the means was assessed using either unpaired t-tests or ANOVA for repeated measures using Barlett’s test for equal variances and a Bonferroni correction. Differences between means were accepted as statistically significant at the 95% level (p < 0.05).
Results
Expression of recombinant InsP3R1 constructs inprimary cortical neurons
Previously, we showed that expression of a recombinant form of rat InsP3R1 corresponding to the stable carboxyl-terminal fragment generated by calpain proteolysis (capn-InsP3R1; Δ1–1917; Fig. 1a) altered [Ca2+]i homeostasis in immortalized cell lines. In this study, we examined the functional consequences of capn-InsP3R1 expression in primary neurons. To achieve high transduction efficiency of primary cortical cultures, we utilized AAV 2/1-mediated gene delivery of capn-InsP3R1, which transduces over 90% of cells in primary neuron cultures (Royo et al. 2008; Bevers et al. 2009). As a control for InsP3-independent Ca2+ release through the truncated channel, we generated AAV 2/1 vector expressing capn-InsP3R1 with a D2550A point mutation in the pore-forming region of the channel (capn-InsP3R1 D2550A; Fig. 1a). This point mutation eliminates Ca2+ permeability of wild-type (wt) InsP3R1 (Boehning and Joseph 2000; Boehning et al. 2001; Cardenas et al. 2010). Because of the genome size of AAV, viral packaging capacity of transgenes is limited (Dong et al. 1996), and we were unable to generate vectors expressing full-length InsP3R1 with or without the D2550A mutation. We used AAV 2/1 expressing lacZ to control for effects of viral transduction.
To examine expression of InsP3R1 constructs in neurons, we stably transduced cortical cultures with AAV 2/1 expressing capn-InsP3R1, capn-InsP3R1 D2550A, or lacZ at 7 DIV and harvested cells 1 week later (14 DIV). Western blotting of whole-cell lysates using a carboxyl-terminal InsP3R1 antibody demonstrated expression of endogenous wt-InsP3R1 in all samples (Fig. 1b). In lysates from cultures transduced with capn-InsP3R1 and capn-InsP3R1 D2550A, we also detected the 95 kDa form of the channels, confirming expression of recombinant InsP3R1 constructs.
Capn-InsP3R1 reduces neuronal viability
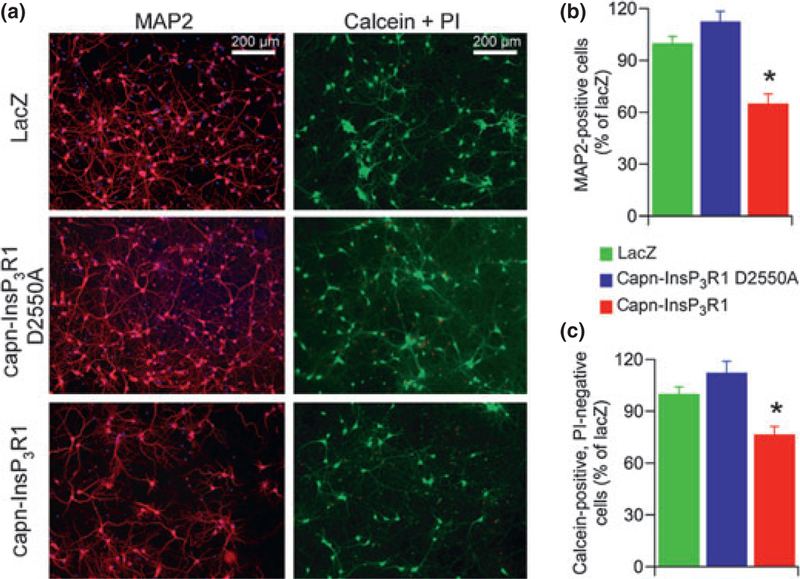
To determine the effects of capn-InsP3R1 on neuronal viability, we transduced 7 DIV cultures with capn-InsP3R1, capn-InsP3R1 D2550A, or lacZ control vectors. One week post-transduction (14 DIV), we immunostained cultures for MAP2 (Fig. 2a, left) and quantified the number of surviving neurons (Fig. 2b). Expression of capn-InsP3R1 resulted in significant decrease in the percentage of viable neurons compared with expression of capn-InsP3R1 D2550A or lacZ (capn-InsP3R1 = 65.3 ± 5.5%, capn-InsP3R1 D2550A = 112.7 ± 5.8%, lacZ = 100.0 ± 3.8%; p < 0.001). To confirm the capn-InsP3R1 toxicity phenotype, we incubated transduced cultures with calcein-AM and PI to identify living cells and dead cells, respectively (Fig. 2a, right). A significant decrease in percentage of surviving cells was again observed in cultures transduced with capn-InsP3R1 compared with controls (capn-InsP3R1 = 76.7 ± 4.5%, capn-InsP3R1 D2550A = 112.5 ±6.5%, lacZ = 100.0 ± 4.0%; p < 0.001; Fig. 2c). Interestingly, the percentage of viable cells in capn-InsP3R1-transduced cultures determined by calcein-AM and PI staining was significantly higher than survival quantified by counts of MAP2-labeled cells (p < 0.05). This likely reflects the mixed constituents of the cortical cultures, which contain ~10% glia (data not shown) that are identified using calcein-AM but not MAP2, suggesting that glial viability is not altered by capn-InsP3R1. Together, these results suggest that expression of capn-InsP3R1 specifically reduces neuronal viability.
Fig. 2.
Primary cortical neuron viability is reduced by expression of capn-InsP3R1. Rat primary cortical cultures were transduced with adeno-associated virus 2/1 expressing lacZ, capn-InsP3R1 D2550A, or capn-InsP3R1 (7 DIV). (a) One week following transduction (14 DIV), cultures were stained for microtubule-associated protein 2 (MAP2) (red) and nuclei (blue; left panel), or incubated with calcein-AM (green) and propidium iodide (PI) (red; right panel). Representative 100· epifluorescence images captured for counting are shown. (b) Percent neuronal survival as determined by counts of MAP2-reactive cells (one-way ANOVA; *p < 0.001). (c) Percent cell survival as determined by counts of calcein-AM positive, PI-negative cells (unpaired t-tests with unequal variance; *p < 0.001). Expression of capn-InsP3R1 resulted in a significant decrease in percent viable neurons compared with lacZ and capn-InsP3R1 D2550A as determined by both assays.
We hypothesized that expression of capn-InsP3R1 reduced neuronal viability by disrupting intracellular Ca2+ homeostasis. The proteolyzed channel, which has InsP3-independent gating and increases the ER Ca2+ leak (Kopil et al. 2011), could potentially trigger neurodegeneration through either ER Ca2+ depletion and induction of ER stress, or by increased [Ca2+]i and feed-forward activation of proteases. To examine the mechanism responsible for capn-InsP3R1 reductions in neuronal viability, we performed Western blotting of whole-cell lysates from transduced cultures. Using an antibody against the stress-inducible ER chaperone protein BiP (Figure S1a), we did not detect any difference in activation of the ER stress pathway between cultures (Figure S1b). Nor did we detect differences in calpain or caspase-3 protease activation using an antibody against a shared substrate, α-spectrin (Figure S1c and d). Absence of Western blot evidence of increased ER stress or protease activation with expression of capn-InsP3R1 does not conclusively imply that neither pathologic cascade plays a causal role in capn-InsP3R1-induced neuronal death. However, if either pathway is responsible for executing cell death in capn-InsP3R1-transduced cultures, Western blotting of neuronal lysates is not sensitive enough to detect it at the times examined here.
Capn-InsP3R1 does not deplete intracellular Ca2+ stores
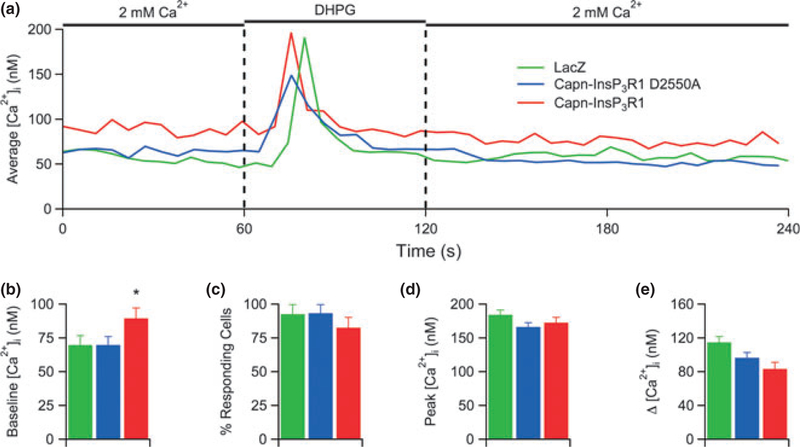
Previous data from our laboratory indicated that capn-InsP3R1 functions as a Ca2+ leak channel in the ER (Kopil et al. 2011). To examine whether this leak was sufficient to deplete intracellular Ca2+ stores in neurons, we utilized single-cell Ca2+ imaging to measure InsP3-induced changes in [Ca2+]i. We monitored somatic [Ca2+]i using Fura-2 in primary cortical cultures transduced with capn-InsP3R1, capn-InsP3R1 D2550A, or lacZ (Fig. 3a). To stimulate Ca2+ release from InsP3-sensitive stores, we applied saturating concentrations of the Group I metabotropic glutamate receptor agonist DHPG (10 lM). Expression of capn-InsP3R1 significantly increased resting [Ca2+]i in neurons (90 ± 3.3 nM) compared with capn-InsP3R1 D2550A (70 ± 1.8 nM) and lacZ controls (70 ± 1.6 nM; p < 0.001; Fig. 3b), supporting previous evidence that capn-InsP3R1 leaks Ca2+ from the ER. Application of DHPG elicited responses in a majority of neurons from all transduced cultures (Fig. 3c). Despite the apparent basal Ca2+ leak through the cleaved channel, expression of capn-InsP3R1 did not affect the DHPG-induced peak [Ca2+]i in neurons (Fig. 3d) or the maximum change in [Ca2+]i from baseline to peak (Fig. 3e). These data demonstrate that expression of the leaky channel, while sufficient to increase resting [Ca2+]i, does not deplete [Ca2+]ER.
Fig. 3.
Capn-InsP3R1 does not deplete InsP3-sensitive stores. (a) Representative single cell [Ca2+]i responses to (S)-3,5-dihydroxyphenylglycine (DHPG) in Fura-2-loaded primary cortical neurons (14 DIV) transduced with lacZ, capn-InsP3R1 D2550A, or capn-InsP3R1. Total number of single-cell Ca2+ responses analyzed in these experiments was 219, 246, and 172 for lacZ-, capn-InsP3R1 D2550A-, and capn-InsP3R1-transduced cultures, respectively. (b) Summary of average resting [Ca2+]i in neurons from cultures used for DHPG Ca2+ imaging experiments. Cultures expressing capn-InsP3R1 demonstrate increased baseline [Ca2+]i (unpaired t-tests with unequal variance; *p < 0.001). (c) Percentage of cells in transduced cortical cultures that exhibited > 10% increase in [Ca2+]i in response to DHPG. (d) Summary of average peak [Ca2+]i responses elicited by DHPG. (e) Summary of average change in [Ca2+]i from resting to peak [Ca2+]i. Despite significant differences in resting [Ca2+]i in these experiments, no statistical differences were observed in average change in [Ca2+]i.
Decreased ER Ca2+ buffering in neurons expressing capn-insP3R1
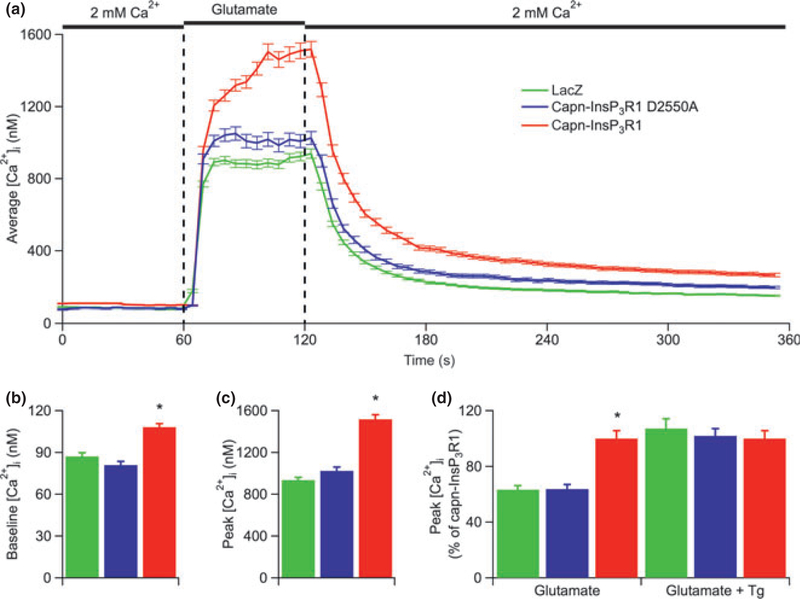
To examine the effects of calpain-cleaved InsP3R1 on neuronal Ca2+ homeostasis more broadly, we measured changes in [Ca2+]i in response to acute glutamate (10 μM) application (Fig. 4a), which activates both ionotropic and metabotropic glutamate receptors (Michaelis 1998). Consistent with results from Ca2+ imaging experiments above, resting [Ca2+]i was significantly increased in neurons expressing capn-InsP3R1 compared with capn-InsP3R1 D2550A- and lacZ-expressing cells (p < 0.001; Fig. 4b). In addition, capn-InsP3R1 significantly increased the glutamate-induced peak [Ca2+]i (1,520 ± 43 nM) compared with capn-InsP3R1 D2550A (1,030 ± 36 nM) and lacZ controls (940 ± 25 nM; p < 0.001; Fig. 4c). Stimulation of an alternate pathway for Ca2+ influx also resulted in a significantly increased peak [Ca2+]i in cultures transduced with capn-InsP3R1 (50 mM KCl; Figure S2). This result suggests that the increased glutamate-induced [Ca2+]i in capn-InsP3R1-transduced neurons is not dependent on increased glutamate receptor expression. Instead, the increased [Ca2+]i rise is likely because of an impaired ability of the ER to buffer Ca2+ influx from the extracellular space. To test this hypothesis, we measured changes in [Ca2+]i in response to acute glutamate (10 μM) application following a 3-min pre-incubation with Tg (1 μM) to inhibit the sarco-endoplasmic reticulum Ca2+-ATPase. Blocking ER Ca2+ uptake eliminated the difference in the glutamate-induced peak [Ca2+]i between neurons expressing capn-InsP3R1 and control cultures expressing lacZ or capn-InsP3R1 D2550A (Fig. 4d). Together, these data indicate that capn-InsP3R1 disrupts normal intracellular Ca2+ homeostasis and impairs the ability of the ER to buffer physiologic rises in [Ca2+]i.
Fig. 4.
Neurons expressing capn-InsP3R1 have increased glutamate-induced rises in [Ca2+]i. (a) Averaged single-cell [Ca2+]i responses to glutamate in Fura-2-loaded primary cortical neurons (14 DIV) transduced with lacZ, capn-InsP3R1 D2550A, or capn-InsP3R1. Total number of single-cell Ca2+ responses analyzed in these experiments was 491, 271, and 396 for lacZ-, capn-InsP3R1 D2550A-, and capn-InsP3R1-transduced cultures, respectively. (b) Summary of average resting [Ca2+]i in neurons from cultures used for glutamate Ca2+ imaging experiments. Expression of capn-InsP3R1 significantly increased resting [Ca2+]i in neurons (108.2 ± 2.6 nM) compared with capn-InsP3R1 D2550A (81.0 ± 2.5 nM) and lacZ controls(87.3 ± 2.6 nM; unpaired t-tests with unequal variance; *p < 0.001).(c) Summary of average peak [Ca2+]i responses elicited by glutamate shows an increased maximum [Ca2+]i achieved in capn-InsP3R1-expressing cells (unpaired t-tests with unequal variance; *p < 0.001).(d) Summary of average peak [Ca2+]i elicited by glutamate in the absence or presence of Tg (normalized data for glutamate alone (left) are the same as absolute data shown Fig. 4c). Total number of single-cell Ca2+ responses analyzed in experiments with Tg was 182, 209, and 146 for lacZ-, capn-InsP3R1 D2550A-, and capn-InsP3R1-transduced cultures, respectively. Tg eliminated the capn-InsP3R1-associated glutamate-induced enhanced peak [Ca2+]i (lacZ, 1,357 ± 66 nM; capn-InsP3R1 D2550a, 1,276 ± 44 nM; capn-InsP3R1, 1,288 ± 54 nM).
Capn-InsP3R1 increases sensitivity to glutamate-induced neurotoxicity
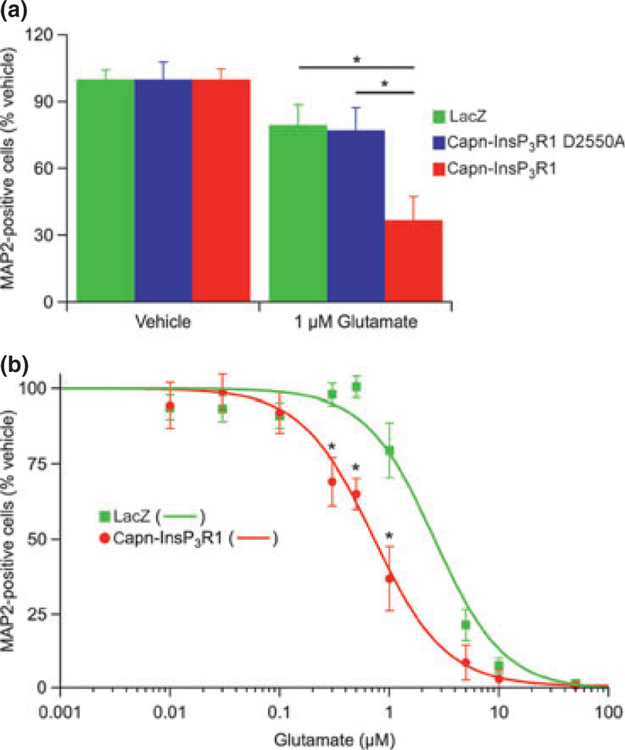
To determine whether the observed capn-InsP3R1-induced impairment of ER Ca2+ buffering increased neuronal susceptibility to excitotoxic injury, we exposed transduced 14 DIV primary cortical cultures to 1 μM glutamate or HBS vehicle. We selected this dose from initial studies performed in untransduced cultures, where treatment with 1 μM glutamate resulted in negligible injury (Figure S3). After incubation in glutamate for 24 h, the cultures were immunostained for MAP2 to quantify the number of surviving neurons (Fig. 5a). To account for baseline differences in viability between capn-InsP3R1- and control-transduced cultures, the percentage of MAP2-positive neurons in glutamate-injured cultures was normalized to vehicle-treated cultures transduced with the same vector. Exposure to 1 lM glutamate resulted in significant loss of neurons in all cultures (p < 0.05). Of note, cultures transduced with capn-InsP3R1 were particularly susceptible (survival: capn-InsP3R1 = 36.9 ± 10.6%, capn-InsP3R1 D2550A = 77.2 ±10.1%, lacZ = 79.5 ± 9.2%; p < 0.001). This result indicates that capn-InsP3R1 increases neuronal susceptibility to excitotoxic injury.
Fig. 5.
Expression of capn-InsP3R1 increases neuronal susceptibility to glutamate-mediated injury. (a) Transduced primary cortical cultures (14 DIV) were exposed to 1 μM glutamate or HEPES-buffered saline vehicle. Twenty-four hours later, cultures were stained for microtubule-associated protein 2 (MAP2) and percent neuronal survival quantified relative to vehicle-treated cultures. Expression of capn-InsP3R1 resulted in a significant decrease in percentage of MAP2-positive cells following glutamate injury compared with lacZ and capn-InsP3R1 D2550A (one-way ANOVA; *p < 0.01). (b) Glutamate dose-response curves for lacZ and capn-InsP3R1-transduced cultures (data at 1 μM glutamate are the same as shown in Fig. 5a). Expression of capn-InsP3R1 significantly decreased percent MAP2-positive cells following treatment with 0.3, 0.5, and 1 lM glutamate compared with lacZ (unpaired t tests; *p < 0.01) and resulted in a shift in the glutamate dose-response curve. LD50 for glutamate in neurons expressing capn-InsP3R1 (0.75 ± 0.19 μM) is reduced compared with neurons expressing lacZ (2.61 ± 0.78 μM).
To further examine the enhanced vulnerability to glutamate-mediated injury in capn-InsP3R1-transduced cultures, we treated primary cortical neurons with a wide range of glutamate doses (0.01–50 μM). As we did not observe differences between neuronal survival in lacZ- and capn-InsP3R1 D2550A-transduced cultures at baseline or following glutamate injury, we chose a single control (lacZ) for these experiments. Transduced primary cortical neurons were incubated with glutamate or HBS vehicle and the number of surviving neurons was quantified 24 h later by MAP2 staining (Fig. 5b). Glutamate concentrations ≥ 0.3 μM induced significant loss of neurons in cultures transduced with capn-InsP3R1 (p < 0.01), whereas lacZ-transduced cultures were only sensitive at concentrations ≥ 1 μM. To facilitate comparison across doses, we fit data using a Hill equation to produce normalized dose-response curves. Capn-InsP3R1 expression resulted in a leftward shift of the glutamate dose-response curve compared with lacZ control. These results suggest that the proteolyzed channel increases neuronal sensitivity to glutamate-mediated injury and decreases the threshold for excitotoxicity.
Discussion
While several studies have examined the effects of InsP3R1 proteolysis on cellular Ca2+ homeostasis (Assefa et al. 2004; Nakayama et al. 2004; Verbert et al. 2008; Kopil et al. 2011), the current study presents the first functional investigation of calpain-cleaved InsP3R1 in neurons. We have demonstrated that capn-InsP3R1 disrupts [Ca2+]i homeostasis and decreases neuronal viability. Impaired ER Ca2+ buffering caused by the leaky channel likely accounts for the increased sensitivity to excitotoxic injury observed in primary cortical neurons expressing capn-InsP3R1. Together, these results suggest that InsP3R1 proteolysis is a novel, possible feed-forward pathway for executing neuronal cell death.
Capn-InsP3R1 is sufficient to signal neuronal death in culture
Previous studies examining caspase- and calpain-cleaved InsP3R1 in cell lines did not report effects of the dysregulated channel on baseline cell viability (Assefa et al. 2004; Nakayama et al. 2004; Verbert et al. 2008; Kopil et al. 2011). This is likely because of the relatively low open probability (Po) of the cleaved channel at resting [Ca2+]i (Kopil et al. 2011). Unlike cell lines, however, primary neurons in culture form complex networks with spontaneous activity and neurotransmission, leading to transient increases in [Ca2+]i (Murphy et al. 1992; Maeda et al. 1995). Thus, we hypothesized that expression of capn-InsP3R1 in neurons would be sufficient to signal cell death through disruption of neuronal Ca2+ homeostasis. To examine the effect of capn-InsP3R1 on neuronal viability, we employed AAV-mediated gene delivery to achieve high transduction efficiency in primary cortical cultures. We determined that expression of capn-InsP3R1 indeed significantly decreased neuronal viability at baseline (Fig. 2). However, capn-InsP3R1 expression did not lead to complete cell loss, as the majority (~65%) of capn-InsP3R1-transduced neurons were viable, at least at the time studied. This likely explains why we were unable to determine the mechanism(s) for capn-InsP3R1-induced cell death using Western blotting (Figure S1). The moderate effect of capn-InsP3R1 on baseline neuron viability may suggest gene expression heterogeneity within the population of neurons in primary cortical cultures. Identifying gene expression differences, and understanding their potential interactions with capn-InsP3R1 that modulate neuronal death requires additional investigation.
Studying capn-InsP3R1 in neurons using AAV-mediated gene delivery has some limitations. Foremost, the limited packaging capacity of AAV prohibits viral vector expression of full-length InsP3R1 (Dong et al. 1996). As an alternative control, we expressed capn-InsP3R1 with a point mutation in the putative pore selectivity filter between transmembrane domains 5 and 6. In wt-InsP3R1, replacing the aspartate at residue 2550 with alanine (D2550A) results in a channel that is still able to bind InsP3 and gates normally, but lacks Ca2+ permeability and does not have Ca2+-release activity (Boehning and Joseph 2000; Boehning et al. 2001; Cardenas et al. 2010). The capn-InsP3R1 D2550A mutant channel is expected to have reduced Ca2+-release activity similar to the mutant full-length channel, but with InsP3-independent gating, similar to capn-InsP3R1. Thus, the structure and function of capn-InsP3R1 D2550A serve as a specific control for the effects of InsP3-independent Ca2+ release through capn-InsP3R1.
Studying the Ca2+-permeable and impermeable capn-InsP3R1 in neurons may be complicated by heteroligomerization of the recombinant truncated channels with endogenous InsP3R1. At 14 DIV, we observed expression of each 95 kDa InsP3R1 construct at an approximately 1 : 1 ratio with endogenous 260 kDa InsP3R1 (Fig. 1b; densitometry analysis not shown). Additional studies are required to fully elucidate the stochiometry and functional properties of the different heteroligomeric channels. The single-cell Ca2+ imaging results may provide some initial insights into the function of heteroligomeric channels. Specifically, we did not observe a reduction in the percentage of cells with InsP3-induced Ca2+ release in capn-InsP3R1 D2550A-transduced cultures (Fig. 3c). Nor did we observe a reduction in Ca2+ released from InsP3-sensitive stores (Fig 3d and e). Assuming that recombinant InsP3R1 subunits oligomerize with endogenous InsP3R in transduced neurons, we conclude that expression of the D2550A mutant under the conditions of our experiments did not exert a dominant negative effect on Ca2+ permeability. This is consistent with theoretical models of a similar mutation in ryanodine receptors, where mutating a negatively charged residue in the selectivity filter of an individual subunit resulted in only a partial decrease in Ca2+ conductance (Gao et al. 2000; Wang et al. 2005). Similarly, there is no expectation of a strong dominant negative effect of capn-InsP3R1 on the InsP3 requirement for channel gating. This is based on evidence that binding of four InsP3 molecules is not required for channel gating. Instead, binding of each InsP3 molecule increases the likelihood that the channel will open (Mak et al. 2003; Foskett et al. 2007; Mak et al. 2007), although it is unknown if the absence of some InsP3 binding sites in a heteroligomeric channel of truncated and full-length InsP3R subunits affects the sensitivity to activation by InsP3. By studying the effects of stable capn-InsP3R1 expression in neurons with endogenous InsP3R, we are perhaps more closely modeling a physiologically relevant pathologic process of limited channel proteolysis.
Neuronal Ca2+ homeostasis is disrupted by capn-InsP3R1
How does InsP3-independent Ca2+ release through capn-InsP3R1 affect neuronal Ca2+ homeostasis? Using single-cell imaging, we observed normal InsP3-induced Ca2+ release in capn-InsP3R1-transduced cortical neurons through activation of mGluR1 and mGluR5 receptors (Fig. 3b, d and e) (Conn and Pin 1997; Fagni et al. 2000). This result is consistent with previous reports showing that expression of caspase- or calpain-cleaved InsP3R1 does not deplete [Ca2+]ER, even though it increases the rate of ER Ca2+ leak (Assefa et al. 2004; Verbert et al. 2008; Kopil et al. 2011). This increased ER Ca2+ leak in neurons expressing capn-InsP3R1 was manifested as elevated resting [Ca2+]i (Figs 3b and 4b, Figure S2b). While the resting [Ca2+]i was variable across experiments performed on cortical cultures from different embryos and platings, the ~20 nM increase in [Ca2+]i in capn-InsP3R1-transduced neurons versus controls remained consistent. This elevated resting [Ca2+]i suggests that capn-InsP3R1 generates an ER Ca2+ leak that cannot be fully compensated for by other Ca2+ transport mechanisms. This is in contrast to previous data from Neuro-2A cells, where we did not detect an effect of capn-InsP3R1 on resting [Ca2+]i (Kopil et al. 2011). The elevated basal [Ca2+]i observed here could be a cell-type specific effect, or a consequence of chronic capn-InsP3R1 expression, as our previous study examined changes in [Ca2+]i at 6 h post-transfection. In either case, sustained elevations in resting [Ca2+]i may explain the decreased neuronal viability in primary cortical cultures expressing capn-InsP3R1.
The apparent increased ER Ca2+ leak through capn-InsP3R1 resulted in more pronounced changes in neuronal [Ca2+]i in response to Ca2+ influx. Expression of capn-InsP3R1 significantly increased the peak [Ca2+]i response to glutamate (Fig. 4c). This likely reflects a decreased capacity of normal ER Ca2+ buffering in neurons expressing capn-InsP3R1, as the significant difference in peak [Ca2+]i between capn-InsP3R1 and controls was eliminated by inhibiting ER Ca2+ uptake with Tg (Fig. 4d). The kinetics of the glutamate Ca2+ responses were also altered in capn-InsP3R1-transduced cultures. [Ca2+]i in control neurons rapidly reached a plateau after the initial rise, whereas [Ca2+]i in capn-InsP3R1-transduced neurons continued to rise until glutamate was removed from the bath (Fig. 4a). Interestingly, average peak [Ca2+]i in capn-InsP3R1-expressing neurons approached 2 lM, which is optimal [Ca2+]i for wt-InsP3R1 and capn-InsP3R1 activation (Foskett et al. 2007; Kopil et al. 2011). We expect that increased channel Po of capn-InsP3R1 at this higher [Ca2+] compromises ER Ca2+ buffering, and provides a potential mechanism for increased sensitivity to glutamate injury (Figure 5) through irreversible disruption of neuronal Ca2+ homeostasis.
It is important to note that we measured somatic [Ca2+]i. As neuronal dendritic and synaptic structure is complex, with heterogeneous distributions of ER and InsP3R (Bardo et al. 2006; Stutzmann and Mattson 2011), capn-InsP3R1 may exert varying influences in these cellular compartments. Understanding the spatial complexity and functional consequences of capn-InsP3R1-mediated Ca2+ signaling in dendrites and synapses warrants future investigation.
Role of calpain-cleavage of InsP3R1 in neurodegeneration
Sustained pathologic rises in [Ca2+]i are a key component of both apoptotic and necrotic cell death (Berridge et al. 2000; Orrenius et al. 2003; Mattson 2007). Therefore, we hypothesized that the dramatic glutamate-induced rise in [Ca2+]i in capn-InsP3R1-expressing primary cortical neurons would increase their sensitivity to excitotoxic injury. We tested this by exposing transduced cultures to glutamate rather than NMDA, as expression of pathogenic NMDA receptor subunits is developmentally regulated in primary neurons (O’Donnell et al. 2006), and glutamate produced a more consistent, dose-dependent injury in untransduced 14 DIV cultures (data not shown). As predicted, expression of capn-InsP3R1 significantly decreased the percentage of surviving neurons over a range of glutamate concentrations (Fig. 5). Enhanced glutamate-induced cell death is likely precipitated by Ca2+-induced Ca2+ release through capn-InsP3R1 in addition to decreased basal ER Ca2+ buffering in these cells. Together, these impairments of neuronal Ca2+ homeostasis increase sensitivity to excitatory stimuli, leading to [Ca2+]i overload. The downstream effects of this [Ca2+]i overload may signal cell death through either necrotic or apoptotic pathways. Sustained elevations of [Ca2+]i could directly lead to pathologic activation of calpains and calpain-mediated cell death (Goll et al., 2003). Alternatively, [Ca2+]i overload could enhance mitochondrial Ca2+ uptake, triggering mitochondrial permeability transition and caspase-mediated apoptosis (Orrenius et al. 2003; Taylor et al. 2008). Future mechanistic studies are required to clarify the precise pathologic pathway(s) activated by capn-InsP3R1-mediated Ca2+ signaling. Current available data suggest that calpain proteolysis of InsP3R1 in neurons acts to activate and accelerate cell death pathways after injury through [Ca2+]i overload. Interestingly, chronic activation and Ca2+ release through InsP3R1 can signal ubiquitination and subsequent channel degradation by the proteasome (Alzayady and Wojcikiewicz 2005; Bhanumathy et al. 2006). However, calpain proteolysis of the channel removes all of the potential polyubiquitination sites within the InsP3R1-coupling domain (Bhanumathy et al. 2006; Sliter et al. 2008), which are required for proteasomal degradation. Thus, calpain cleavage of InsP3R1 may be critical to executing cell death by removing activity-dependent regulation of the channel, thereby causing Ca2+ overload.
Conclusions
In summary, our results indicate that expression of calpain-cleaved InsP R1 impairs neuronal ER Ca2+ buffering, leading to increased sensitivity to excitotoxic stimuli and neurodegeneration in primary neuron cultures. As an early part of the molecular injury cascade (Kopil et al. 2011), calpain proteolysis of InsP3R1 could contribute a feed-forward pathway to accelerate neuronal death through [Ca2+]i overload. Thus, inhibiting Ca2+ release through calpain-cleaved InsP3R1 is a potentially important therapeutic strategy for intervention in disorders associated with calpain activity and disruption of Ca2+ homeostasis.
Supplementary Material
Figure S1. Activation of ER stress markers and proteases in transduced primary cortical neurons.
Figure S2. Neurons expressing capn-InsP3R1 have increased rises in[Ca2+]i in response to depolarization.
Figure S3. Characterization of glutamate injury model.
Acknowledgements
We thank Margaret Maronski for the preparation of cortical cultures. We thank Dr. Virginia Lee for supplying antibody. We thank Dr. Shu-Jen Chen, Dr. Arbansjit Sandhu, and the University of Pennsylvania Vector Core for technical expertise in cloning and viral vector generation. We also thank Dr. Don-On Daniel Mak for IGOR macros and helpful discussions, and Dr. Horia Vais for comments on the manuscript. This work was supported by National Institutes of Health grants NS039481 (RWN), NS069951 (RWN), NS071828 (CMK), MH059937 (JKF), and GM065830 (JKF).
Abbreviations used:
- [Ca2+]i
cytoplasmic free Ca2+ concentration
- AAV
adeno-associated virus
- capn-InsP3R1,
calpain-cleaved InsP3R1
- DIV
days in vitro
- ER
endoplasmic reticulum
- InsP3R1
type 1 InsP3 receptor
- MAP2
microtubule-associated protein 2
- PI
propidium iodide
- Tg
thapsigargin
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Supporting information
Additional supporting information may be found in the online version of this article:
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
References
- Alzayady KJ and Wojcikiewicz RJ (2005) The role of Ca2+ in triggering inositol 1,4,5-trisphosphate receptor ubiquitination. Biochem. J 392, 601–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assefa Z, Bultynck G, Szlufcik K, Nadif Kasri N, Vermassen E, Goris J, Missiaen L, Callewaert G, Parys JB and De Smedt H (2004) Caspase-3-induced truncation of type 1 inositol trisphosphate receptor accelerates apoptotic cell death and induces inositol trisphosphate-independent calcium release during apoptosis.J. Biol. Chem 279, 43227–43236. [DOI] [PubMed] [Google Scholar]
- Bardo S, Cavazzini MG and Emptage N (2006) The role of the endoplasmic reticulum Ca2+ store in the plasticity of central neurons. Trends Pharmacol. Sci 27, 78–84. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P and Bootman MD (2000) The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol 1, 11–21. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD and Roderick HL (2003) Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol 4, 517–529. [DOI] [PubMed] [Google Scholar]
- Bevers MB, Lawrence E, Maronski M, Starr N, Amesquita M and Neumar RW (2009) Knockdown of m-calpain increases survival of primary hippocampal neurons following NMDA excitotoxicity.J. Neurochem 108, 1237–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhanumathy CD, Nakao SK and Joseph SK (2006) Mechanism of proteasomal degradation of inositol trisphosphate receptors in CHO-K1 cells. J. Biol. Chem 281, 3722–3730. [DOI] [PubMed] [Google Scholar]
- Boehning D and Joseph SK (2000) Functional properties of recombinant type I and type III inositol 1, 4,5-trisphosphate receptor isoforms expressed in COS-7 cells. J. Biol. Chem 275, 21492–21499. [DOI] [PubMed] [Google Scholar]
- Boehning D, Mak DO, Foskett JK and Joseph SK (2001) Molecular determinants of ion permeation and selectivity in inositol 1,4,5-trisphosphate receptor Ca2+ channels. J. Biol. Chem 276, 13509–13512. [DOI] [PubMed] [Google Scholar]
- Cardenas C, Miller RA, Smith I et al. (2010) Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell 142, 270–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung KH, Shineman D, Muller M, Cardenas C, Mei L, Yang J, Tomita T, Iwatsubo T, Lee VM and Foskett JK (2008) Mechanism of Ca2+ disruption in Alzheimer’s disease by presenilin regulation of InsP3 receptor channel gating. Neuron 58, 871–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ and Pin JP (1997) Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol 37, 205–237. [DOI] [PubMed] [Google Scholar]
- Cummings DD, Wilcox KS and Dichter MA (1996) Calcium-dependent paired-pulse facilitation of miniature EPSC frequency accompanies depression of EPSCs at hippocampal synapses in culture. J. Neurosci 16, 5312–5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong JY, Fan PD and Frizzell RA (1996) Quantitative analysis of the packaging capacity of recombinant adeno-associated virus. Hum. Gene Ther 7, 2101–2112. [DOI] [PubMed] [Google Scholar]
- Fagni L, Chavis P, Ango F and Bockaert J (2000) Complex interactions between mGluRs, intracellular Ca2+ stores and ion channels in neurons. Trends Neurosci 23, 80–88. [DOI] [PubMed] [Google Scholar]
- Fisher KJ, Jooss K, Alston J, Yang Y, Haecker SE, High K, Pathak R, Raper SE and Wilson JM (1997) Recombinant adeno-associated virus for muscle directed gene therapy. Nat. Med 3, 306–312. [DOI] [PubMed] [Google Scholar]
- Foskett JK (2010) Inositol trisphosphate receptor Ca2+ release channels in neurological diseases. Pflugers Arch 460, 481–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foskett JK, White C, Cheung KH and Mak DO (2007) Inositol trisphosphate receptor Ca2+ release channels. Physiol. Rev 87, 593–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Balshaw D, Xu L, Tripathy A, Xin C and Meissner G (2000) Evidence for a role of the lumenal M3–M4 loop in skeletal muscle Ca(2+) release channel (ryanodine receptor) activity and conductance. Biophys. J 79, 828–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goll DE, Thompson VF, Li H, Wei W and Cong J (2003) The calpain system. Physiol. Rev 83, 731–801. [DOI] [PubMed] [Google Scholar]
- Hirota J, Furuichi T and Mikoshiba K (1999) Inositol 1,4,5-trisphosphate receptor type 1 is a substrate for caspase-3 and is cleaved during apoptosis in a caspase-3-dependent manner. J. Biol. Chem 274, 34433–34437. [DOI] [PubMed] [Google Scholar]
- Ikegaya Y, Le Bon-Jego M and Yuste R (2005) Large-scale imaging of cortical network activity with calcium indicators. Neurosci. Res 52, 132–138. [DOI] [PubMed] [Google Scholar]
- Joseph SK and Samanta S (1993) Detergent solubility of the inositol trisphosphate receptor in rat brain membranes. Evidence for association of the receptor with ankyrin. J. Biol. Chem 268, 6477–6486. [PubMed] [Google Scholar]
- Kopil CM, Vais H, Cheung KH, Siebert AP, Mak DO, Foskett JK and Neumar RW (2011) Calpain-cleaved type 1 inositol 1,4,5-trisphosphate receptor (InsP(3)R1) has InsP(3)-independent gating and disrupts intracellular Ca(2+) homeostasis. J. Biol. Chem 286, 35998–36010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda E, Robinson HP and Kawana A (1995) The mechanisms of generation and propagation of synchronized bursting in developing networks of cortical neurons. J. Neurosci 15, 6834–6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak DO, McBride SM and Foskett JK (2003) Spontaneous channel activity of the inositol 1,4,5-trisphosphate (InsP3) receptor (InsP3R). Application of allosteric modeling to calcium and InsP3 regulation of InsP3R single-channel gating. J. Gen. Physiol 122, 583–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak DO, Pearson JE, Loong KP, Datta S, Fernandez-Mongil M and Foskett JK (2007) Rapid ligand-regulated gating kinetics of single inositol 1,4,5-trisphosphate receptor Ca2+ release channels. EMBO Rep 8, 1044–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP (2007) Calcium and neurodegeneration. Aging Cell 6,337–350. [DOI] [PubMed] [Google Scholar]
- Mattson MP, LaFerla FM, Chan SL, Leissring MA, Shepel PN and Geiger JD (2000) Calcium signaling in the ER: its role in neuronal plasticity and neurodegenerative disorders. Trends Neurosci 23, 222–229. [DOI] [PubMed] [Google Scholar]
- Michaelis EK (1998) Molecular biology of glutamate receptors in the central nervous system and their role in excitotoxicity, oxidative stress and aging. Prog. Neurobiol 54, 369–415. [DOI] [PubMed] [Google Scholar]
- Murphy TH, Blatter LA, Wier WG and Baraban JM (1992) Spontaneous synchronous synaptic calcium transients in cultured cortical neurons. J. Neurosci 12, 4834–4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama T, Hattori M, Uchida K, Nakamura T, Tateishi Y, Bannai H, Iwai M, Michikawa T, Inoue T and Mikoshiba K (2004) The regulatory domain of the inositol 1,4,5-trisphosphate receptor is necessary to keep the channel domain closed: possible physiological significance of specific cleavage by caspase 3. Biochem. J 377, 299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell LA, Agrawal A, Jordan-Sciutto KL, Dichter MA, Lynch DR and Kolson DL (2006) Human immunodeficiency virus (HIV)-induced neurotoxicity: roles for the NMDA receptor subtypes. J. Neurosci 26, 981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrenius S, Zhivotovsky B and Nicotera P (2003) Regulation of cell death: the calcium-apoptosis link. Nat. Rev. Mol. Cell Biol 4, 552–565. [DOI] [PubMed] [Google Scholar]
- Royo NC, Vandenberghe LH, Ma JY, Hauspurg A, Yu L, Maronski M, Johnston J, Dichter MA, Wilson JM and Watson DJ (2008) Specific AAV serotypes stably transduce primary hippocampal and cortical cultures with high efficiency and low toxicity. Brain Res 1190, 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp AH, McPherson PS, Dawson TM, Aoki C, Campbell KP and Snyder SH (1993) Differential immunohistochemical localization of inositol 1,4,5-trisphosphate- and ryanodine-sensitive Ca2+ release channels in rat brain. J. Neurosci 13, 3051–3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliter DA, Kubota K, Kirkpatrick DS, Alzayady KJ, Gygi SP and Wojcikiewicz RJ (2008) Mass spectrometric analysis of type 1 inositol 1,4,5-trisphosphate receptor ubiquitination. J. Biol. Chem 283, 35319–35328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutzmann GE and Mattson MP (2011) Endoplasmic reticulum Ca(2+) handling in excitable cells in health and disease. Pharmacol. Rev 63, 700–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RC, Cullen SP and Martin SJ (2008) Apoptosis: controlled demolition at the cellular level. Nat. Rev. Mol. Cell Biol 9, 231–241. [DOI] [PubMed] [Google Scholar]
- Verbert L, Lee B, Kocks SL, Assefa Z, Parys JB, Missiaen L, Callewaert G, Fissore RA, De Smedt H and Bultynck G (2008) Caspase-3-truncated type 1 inositol 1,4,5-trisphosphate receptor enhances intracellular Ca2+ leak and disturbs Ca2+ signalling. Biol. Cell 100, 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A and Toescu EC (2003) Endoplasmic reticulum Ca(2+) homeostasis and neuronal death. J. Cell Mol. Med 7, 351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Xu L, Pasek DA, Gillespie D and Meissner G (2005) Probing the role of negatively charged amino acid residues in ion permeation of skeletal muscle ryanodine receptor. Biophys. J 89, 256–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White C, Li C, Yang J, Petrenko NB, Madesh M, Thompson CB and Foskett JK (2005) The endoplasmic reticulum gateway to apoptosis by Bcl-X(L) modulation of the InsP3R. Nat. Cell Biol 7, 1021–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Activation of ER stress markers and proteases in transduced primary cortical neurons.
Figure S2. Neurons expressing capn-InsP3R1 have increased rises in[Ca2+]i in response to depolarization.
Figure S3. Characterization of glutamate injury model.