Abstract
Context/Objective
To evaluate the impact of long-term nitrofurantoin for UTI prophylaxis in veterans with SCI.
Design
Matched pairs study.
Setting
Veterans cared for at VA facilities from 10/1/2012-9/30/2013.
Participants
Veterans.
Interventions
n/a.
Outcomes measures
UTI, positive urine cultures, resistant cultures.
Methods
Cases receiving long-term nitrofurantoin (≥90 days supply) were matched to controls by facility. Controls were patients who did not receive long-term nitrofurantoin with a history of ≥3 positive urine cultures and at least one diagnosis of UTI or asymptomatic bacteriuria in the previous year.
Results
122 SCI cases were identified and matched to 196 controls. After adjusting for differences in baseline demographic characteristics, UTIs were less frequent in cases (OR = 0.60 [95% CI 0.44-0.72]). Cases had a greater mean number of days between positive urine cultures as compared to controls (<0.0001). Cases were more likely to have isolates resistant to nitrofurantoin (P ≤ 0.0001); however, the frequency of multi-drug resistant organisms isolated from the urine was not significantly different.
Conclusions
Long-term prescription of nitrofurantoin may reduce UTIs in veterans with SCI and there is no evidence that it promotes multi-drug resistance. Future prospective studies should be conducted prior to incorporating routine use of long-term nitrofurantoin into clinical care.
Keywords: Nitrofurantoin, Spinal cord injuries, Urinary tract infections, Antibiotic prophylaxis, Outcome assessment, Practice patterns, Physicians
Introduction
Over 250,000 people in the United States are living with traumatic spinal cord injury (SCI) with approximately 12,000 new injuries annually.1 Patients with SCI have an increased risk of infection secondary to frequent healthcare exposure, chronic wounds and other comorbidities, and frequent use of invasive devices.2–5 Patients with SCI frequently have impaired bladder function due to neurogenic bladder and structural and physiologic factors that alter the dynamics of voiding.6 This impaired bladder function necessitates bladder drainage by indwelling or intermittent catheterization, which, in turn, introduces bacteria into the bladder and increases the risk of urinary tract infection (UTI).3,7 Nearly half of all patients with SCI experience a UTI annually, making UTIs the most common infection in SCI.2,6,8–10
Nearly all patients who use indwelling bladder drainage devices will have bacterial colonization of the bladder (bacteriuria).11 Bacteriuria is also prevalent in patients with neurogenic bladder. In the absence of signs or symptoms indicating infection, antimicrobial treatment of bacteriuria in patients with neurogenic bladder is not routinely recommended.11 Despite this, inappropriate antimicrobial treatment of bacteriuria is common. This is particularly an issue in long-term care and contributes to the problem of bacterial resistance, antibiotic-associated adverse events, and Clostridium difficile infection.6–7,12 However, distinguishing between bacteriuria and UTI in patients with SCI can be difficult because these patients may not exhibit classic UTI symptoms of dysuria, urinary urgency/frequency, and pelvic pain.6,11
Studies have investigated the possible benefit of long-term use of antibiotics to prevent UTIs in patients requiring long-term bladder drainage. A systematic review of antibiotic prophylaxis in SCI patients did not find evidence of benefit in reducing UTIs and identified a two-fold increase in antibiotic resistant bacteria in those receiving prophylaxis.13 A recent Cochrane review identified inconsistent findings about the effect of antibiotic prophylaxis in preventing UTIs in adult and pediatric patients with long-term bladder drainage and emphasized that data were sparse.14 This suggests an ongoing need for further studies that evaluate the benefit of antibiotic prophylaxis to prevent recurrent UTIs in high-risk subgroups (i.e. SCI) and to focus on specific antibiotics with a low likelihood of developing resistance.
Nitrofurantoin is approved for the treatment of acute, uncomplicated UTI and is active against common uropathogens, such as Escherichia coli and other Enterobacteriaceae.15 These organisms are commonly isolated from urine cultures in veterans with SCI.16,17 Although resistance to nitrofurantoin in urinary isolates has been reported, studies demonstrate the continued susceptibility despite increasing resistance to other first- and second-line therapies (e.g. fluoroquinolones).18–23 Most studies on the efficacy of nitrofurantoin for the prevention of UTIs in the SCI population were conducted thirty years ago and were limited by different methods used to diagnose UTI, small sample sizes and varying study durations.19,24–25 A recent prospective, observational study in SCI found a significant reduction in bacteriuria and UTI when alternating cefixime and nitrofurantoin.26
The Department of Veterans Affairs (VA) is the largest system of care for patients with SCI in the nation, providing expertise in treating new and longstanding injuries.27 In this study, our objective was to describe the use of long-term nitrofurantoin in a large SCI cohort receiving care in VA. We further evaluated the effect of nitrofurantoin on positive urine cultures, UTI symptoms, and resistance.
Methods
Study design and population
This was a matched pairs study of veterans with SCI ≥ 18 years of age cared for at VA facilities from 10/1/2012-9/30/2013. Veterans with multiple sclerosis, amyotrophic lateral sclerosis, and Guillain-Barre syndrome were excluded because the VA SCI system of care has historically focused on individuals with stable non-progressive spinal cord neurological deficits. Our primary analysis consisted of pairs defined as “cases” and “controls”. Cases were defined as veterans with SCI receiving long-term nitrofurantoin, defined as ≥90 days of continuous nitrofurantoin prescribed at least daily over the one-year study period. The case group was matched to control patients with SCI who had not received long-term nitrofurantoin treatment and had a history of recurrent positive urine cultures, defined as ≥3 positive urine cultures and at least one occurrence of UTI or asymptomatic bacteriuria (based on ICD-9 codes, defined below) between 10/1/2011-9/30/2012 (12-months prior to the study year).6,28 Cases and controls were matched by facility. Matching with replacement was allowed at facilities where unique controls were exhausted for a specific year. To assess any differences in the duration of nitrofurantoin, we conducted an unmatched subanalysis of the case group stratified by nitrofurantoin duration. The Case1 group was defined as SCI patients continuously receiving daily nitrofurantoin over the one-year study period (for 365 days). The Case1 group was compared to patients who had a history of at least 90 continuous days (90-364 days) of nitrofurantoin (Case2 group). Patients were excluded if they were prescribed other antibiotics for UTI prophylaxis or were deceased at any time during the study period.
Clinical data collection
Culture results and clinical and demographic data were collected from national VA datasets that included the VA Corporate Data Warehouse (CDW) and VA Spinal Cord Dysfunction (SCD) registry. CDW is a relational database that includes information about all medical encounters in the VA. These data are updated nightly with medical record information from the local VA facility. The SCD registry includes information on injury characteristics of veterans with SCI.
Clinical outcomes of asymptomatic bacteriuria and UTI occurring during the study year were extracted from the CDW using ICD-9s for asymptomatic bacteriuria (791.9), acute cystitis (595.0), unspecified cystitis (595.9), acute pyelonephritis (590.1x), other pyelonephritis (590.8x), chronic pyelonephritis (590.0), infection of kidney (590.9), infection and inflammatory reaction due to urinary catheter (996.64), urinary tract infectious disease (599.0), and bladder catheter infection (996.64). These ICD-9 codes were selected based on clinical expertise and ongoing performance improvement projects to increase evidence-based treatment of urine cultures. All ICD-9 codes for UTI diagnosis were confirmed for accuracy by review of the electronic health record to assess UTI symptoms within 72 hours of a positive urine culture (referred to as “UTI” herein). Positive cultures where no symptoms were documented were defined as asymptomatic bacteriuria. All positive and negative urine cultures during the study period were reviewed for organism isolated and antibiotic sensitivities (for positive cultures). Electronic health record review was conducted by a single investigator (AC) based on pre-determined rules for defining a symptomatic UTI vs asymptomatic bacteriuria occurring during the study year. Symptomatic UTI was defined as a positive urine culture (defined as 105 colony forming units) and symptoms consistent with the National Institute on Disability and Rehabilitation Research Consensus Statement (fatigue, fever/chills, new leukocytosis, onset of or increased spasticity, dysuria, urinary incontinence).6 For patients with multiple positive urine cultures during the study period, assessment of UTI symptoms was performed for the first positive culture associated with symptoms. Chart reviews were also used to identify bladder management strategy at time of culture and nitrofurantoin indication (treatment of a UTI vs prophylaxis).
Multi-drug resistant organisms were defined as intermediate or resistant to ≥3 of the following antimicrobial groups: 1st-4th generation cephalosporins, aminoglycosides, fluoroquinolones, carbapenems, cephamycins, folate pathway inhibitors, glycylcyclines, antipseudomonal penicillins, β–lactam/β-lactamase inhibitors, penicillins, phenicols, monobactams, polymyxins, and tetracyclines.29 Carbapenem-resistant Enterobacteriaceae (CRE) were defined as gram-negative bacteria nonsusceptible (intermediate or resistant) to carbapenems. Intrinsic resistance was not considered in the definition of CRE. A modified Charlson comorbidity index30 was calculated for all patients using clinical data collected from the year prior to the study period (10/1/2011-9/30/2012).
Statistical analysis
For the matched analyses (comparing all cases to controls), McNemar’s test was used for categorical data, paired t-tests were used for continuous, parametric data, and Wilcoxon-signed rank sum tests were used for continuous, non-parametric data. For the unmatched comparison (comparing the Case1 to the Case2 group), chi-square test, unpaired t-test, and Wilcoxon-Mann-Whitney tests were used for continuous, non-parametric data and were applied as appropriate. Fisher’s exact test was used for frequencies <5 occurrences in the unmatched analysis. Bonferroni correction was applied to adjust for multiple comparisons with a P value of <0.025 considered statistically significant. Conditional logistic regression analysis was applied to adjust for additional demographic variables identified as significant in the univariate analysis for the categorical outcomes of positive urine culture, UTI, and antimicrobial resistance (MDRO; nitrofurantoin resistance). Mixed models were similarly applied for the continuous outcome of days between positive urine cultures. A backward selection process was used to determine covariates for inclusion in the models. Variables with a P value <0.3 remained in the model. SAS Version 9.3 (SAS, Inc, Cary, NC) was used for all statistical analyses.
Results
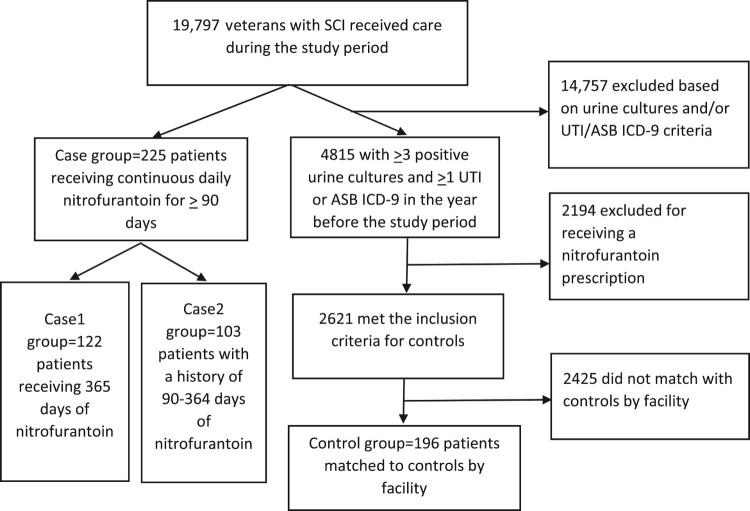
There were 19,797 veterans with SCI receiving VA care during the study period. The selection of cases and controls is illustrated in Figure 1.
Figure 1.
Selection of the case and control groups.
Two hundred and twenty-five veterans with SCI met the inclusion criteria for the case group. Of these patients, 122 (29.0% of the study sample) received long-term nitrofurantoin for the entirety of 10/1/2012-9/30/2013 (Case1 group) and 103 (25.7%) had a history of between 90 and 364 days of nitrofurantoin during the study period (Case2 group). Seventy-five (49.3%) of 152 facilities had at least one patient receiving long-term nitrofurantoin; 19 facilities comprised 54.1% of the long-term cases, and no single facility accounted for more than 6% of prescribing. One hundred and ninety-six controls were matched to cases by facility and year, had a history of ≥3 positive urine cultures and ≥1 ICD-9 code for UTI or asymptomatic bacteriuria in the previous year and had not received long-term nitrofurantoin.
Group comparisons
Baseline demographic and clinical characteristics were similar between the groups (Table 1) with a few exceptions. As compared to the case group, controls were more likely to be non-white (24.5% controls vs. 11.1% cases; P < 0.0001), use suprapubic or indwelling catheters (50.5% vs. 40.9%; P = 0.02), and have a higher median Charlson Index (3.5 vs. 2.6, P < 0.0001). There was no difference in the nitrofurantoin dose prescribed in the Case1 group (continuous nitrofurantoin prophylaxis) or Case2 group (history of nitrofurantoin prophylaxis), but condom or intermittent catheters were more likely in the Case1 group (P < 0.02).
Table 1. Demographics and clinical characteristics of case and control groups.
| All Casesa N = 225 |
Controlb N = 196 |
Casesa vs. Controlb P-Value |
Case1c N = 122 |
Case2d N = 103 |
Case1c vs. Case2d P-Value |
|
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age, Mean ± SD (range) | 60.5 ± 12.4 (21-92) | 61.1 ± 12.2 (22-88) | 0.90 | 61.1 ± 11.1 (25-84) | 59.7 ± 13.8 (21-92) | 0.39 |
| Sex, male | 208 (92.4%) | 187 (95.4%) | 0.57 | 116 (95.1%) | 92 (89.3%) | 0.09 |
| Race | ||||||
| White | 193 (85.8%) | 140 (71.4%) | 105 (86.1%) | 88 (85.4%) | ||
| Non-white | 25 (11.1%) | 48 (24.5%) | 12 (9.8%) | 13 (12.6%) | ||
| Unknown | 7 (3.1%) | 8 (4.1%) | <0.0001 | 5 (4.1%) | 2 (1.9%) | 0.53 |
| Hispanic (%) | 4 (1.8%) | 6 (3.1%) | 0.53 | 2 (1.6%) | 2 (1.9%) | 1.0 |
| Charlson index, Mean ± SD (range) | 2.6 ± 1.6 (0-9) | 3.5 ± 2.2 (0-12) | <0.0001 | 2.7 ± 1.7 (0-9) | 2.5 ± 1.5 (0-8) | 0.37 |
| Characteristics of spinal cord injury | ||||||
| Age at injury, Mean ± SD (range) | 36.3 ± 15.5 (11-88) | 38.7 ± 15.0 (19-74) | 0.12 | 35.9 ± 14.5 (11-74) | 36.7 ± 16.5 (18-88) | 0.74 |
| Duration of injury, Mean ± SD (range) | 24.4 ± 14.2 (1-61) | 22.0 ± 13.9 (1-59) | 0.28 | 24.9 ± 14.2 (1-61) | 23.9 ± 14.4 (2-57) | 0.62 |
| Level of injury | ||||||
| Tetraplegia | 130 (57.8%) | 113 (57.7%) | 0.57 | 65 (53.3%) | 65 (63.1%) | 0.13 |
| Paraplegia | 57 (25.3%) | 29 (14.8%) | 0.80 | 34 (27.9%) | 23 (22.3%) | 0.42 |
| Undocumented | 38 (16.9%) | 54 (27.6%) | 0.02 | 23 (18.9%) | 15 (14.6%) | 0.18 |
| Nitrofurantoin dose | ||||||
| 50 mg daily | 29 (12.9%) | --- | --- | 14 (11.5%) | 15 (14.6%) | 0.52 |
| 100 mg daily | 192 (85.3%) | --- | 105 (86.1%) | 87 (84.5%) | ||
| Othere | 4 (1.8%) | --- | 3 (2.5%) | 1 (1.0%) | ||
| Urinary catheter | ||||||
| Condom or intermittent | 87 (38.7%) | 64 (32.7%) | <0.02 | 52 (42.6%) | 35 (34.0%) | 0.17 |
| Suprapubic or urethral | 92 (40.9%) | 99 (50.5%) | 44 (36.1%) | 48 (46.6%) | ||
| Otherf | 46 (20.4%) | 33 (16.8%) | 26 (21.3%) | 20 (19.4%) |
aCases = Veterans with SCI receiving nitrofurantoin daily for ≥ 90 days.
bControls = Veterans with SCI who had not received prophylactic treatment and had a history of recurrent positive urine cultures (≥ 3 positive urine cultures the year prior to the study period); cases were matched to control by facility and year.
cCase1 group = Veterans with SCI continuously receiving daily nitrofurantoin for UTI prophylaxis over the one-year study period.
dCase2 group = Veterans with SCI with a history of at least 90 continuous days of nitrofurantoin for UTI prophylaxis.
eOne patient with 50 mg twice daily, one patient with 100 mg twice daily, and two patients with 50 mg four times daily.
fUndocumented or ileal conduit (4 patients Case2, 1 patient control).
Treatment outcomes
There were no significant differences in the frequency or types of UTI symptoms between the case and control groups (Table 2). In the Case2 group, 44.7% had at least one occurrence of UTI symptoms compared to 29.5% patients in Case1 (P = 0.02).
Table 2. Urinary tract infection symptoms in case-case-control groups.
| All Casesa N = 225 | Controlb N = 196 | Casesa vs. Controlb P-Value | Case1c N = 122 | Case2d N = 103 | Case1c vs. Case2d P-Value | |
|---|---|---|---|---|---|---|
| Matched analysis | Unmatched analysis | |||||
| Unique patients with UTI signs/symptomse | 82 (36.4%) | 111 (56.6%) | 0.75 | 36 (29.5%) | 46 (44.7%) | 0.02 |
| Dysuria | 13 (15.9%)f | 18 (16.2%) | 0.58 | 6 (16.7%) | 7 (15.2%) | 0.55 |
| Frequency | 4 (4.9%) | 9 (8.1%) | 0.78 | 1 (2.8%) | 3 (6.5%) | 0.33 |
| Urgency | 3 (3.7%) | 2 (1.8%) | 0.65 | 1 (2.8%) | 2 (4.3%) | 0.59 |
| Suprapubic pain | 2 (2.4%) | 3 (2.7%) | 0.65 | 1 (2.8%) | 1 (2.2%) | 0.59 |
| Hematuria | 15 (18.3%) | 10 (9.0%) | 0.16 | 10 (27.8%) | 5 (10.9%) | 0.32 |
| Flank pain | 2 (2.4%) | 4 (3.6%) | 0.41 | 1 (2.8%) | 1 (2.2%) | 1.0 |
| Spasms | 17 (20.7%) | 11 (9.9%) | 0.13 | 7 (19.4%) | 10 (21.7%) | 0.26 |
| Rigors | 11 (13.4%) | 24 (21.6%) | 0.40 | 4 (11.1%) | 7 (15.2%) | 0.35 |
| Fever | 23 (28.0%) | 35 (31.5%) | 1.0 | 12 (33.3%) | 11 (23.9%) | 0.84 |
| Altered mental status | 8 (9.8%) | 16 (14.4%) | 0.39 | 2 (5.6%) | 6 (13.0%) | 0.15 |
| Malaise | 7 (8.5%) | 8 (7.2%) | 0.44 | 2 (5.6%) | 5 (10.9%) | 0.25 |
| Nausea | 8 (9.8%) | 10 (9.0%) | 0.64 | 4 (11.1%) | 4 (8.7%) | 0.98 |
| Lethargy | 11 (13.4%) | 13 (11.7%) | 1.0 | 6 (16.7%) | 5 (10.9%) | 1.0 |
| Pelvic pain | 12 (14.6%) | 3 (2.7%) | 0.02 | 5 (13.9%) | 7 (15.2%) | 0.47 |
| Vomiting | 8 (9.8%) | 5 (4.5%) | 0.41 | 3 (8.3%) | 5 (10.9%) | 0.37 |
aCases = Veterans with SCI receiving nitrofurantoin daily for ≥ 90 days.
bControls = Veterans with SCI who had not received prophylactic treatment and had a history of recurrent positive urine cultures (≥ 3 positive urine cultures the year prior to the study period); cases were matched to control by facility and year.
cCase1 group = Veterans with SCI continuously receiving daily nitrofurantoin for UTI prophylaxis over the one-year study period.
dCase2 group = Veterans with SCI with a history of at least 90 continuous days of nitrofurantoin for UTI prophylaxis.
ePatients may have reported multiple symptoms during UTI infection.
fAll Percents for the specific symptoms were calculated based on the number of patients in that group with documented signs/symptoms.
There were no differences in the number of urine cultures collected, but significant differences were identified between groups for specific microbiologic outcomes and healthcare utilization (Table 3). The control group had a higher proportion of positive urine cultures as compared to cases (P < 0.0001); no differences were identified between the case groups (P = 0.26). There was no difference in culture location for cases (34.2% of cultures were outpatient) and controls (30.0% outpatient; P = 0.08), but the Case2 group was significantly more likely to have a culture location of inpatient or long-term care as compared to the Case1 group (57.7% vs 42.3%; P < 0.0001). The average number of days between positive urine cultures was significantly shorter in the control group as compared to cases (96.7 vs 139.6 days, respectively; P < 0.0001), and in the Case2 group as compared to the Case1 group (125.3 vs 158.7 days; P < 0.0001). Of the isolated organisms, E coli was identified in urine cultures most frequently in cases and controls, followed by Klebsiella and Enterococci. There were no differences in the frequency of isolates for cases and controls. However, Enterococci was isolated significantly more often in the Case2 as compared to the Case1 group (28.7% vs. 9.1% respectively; P < 0.001) and Proteus spp. was more frequent in the Case1 as compared to the Case2 group (21.9% vs. 14.6%; P = 0.02). Of the positive cultures, there was a non-significant trend toward a greater proportion of MDROs isolated in the controls as compared to the cases (43.8% vs. 33.3% of isolates were MDRO; P = 0.04). There was no significant difference in the proportion of isolates defined as MDROs between the Case1 and Case2 groups (37.3% vs. 30.3% of isolates were MDRO; P = 0.08). Nitrofurantoin-resistant isolates were more frequent in the Case1 group compared to the Case2 group (75.6% vs. 52.5% of isolates; P < 0.0001), with the control group having the lowest proportion of NF-resistant isolates (36.6%, P < 0.0001).
Table 3. Microbiologic and healthcare utilization outcomes by case control groups.
| All Casesa N = 225 | Controlb N = 196 | Casesa vs. Controlb P-Value |
Case1c N = 122 | Case2d N = 103 | Case1c vs. Case2d P-Value |
|
|---|---|---|---|---|---|---|
| Average # of cultures/patient, Mean ± SD (range) | 2.05 ± 1.37 (1-7) |
1.93 ± 1.40 1-8) |
0.36 | 1.96 ± 1.4 (1-7) |
2.13 ± 1.32 (1-7) |
0.19 |
| Positive urine culture, # cultures (%)e | 387 (64.5%) | 407 (70.8%) | <0.0001 | 206 (64.6%) | 181 (64.4%) | 0.26 |
| Days between positive cultures, Mean ± SD (range) | 139.6± 111.3 (31-526) |
96.7± 78.8 (31-509) |
<0.0001 | 158.7± 123.5 (32-526) |
125.3± 99.1 (31-455) |
<0.0001 |
| Organisms isolated | ||||||
| Enterococci sp., # organisms (%)f | 112 (20.1%) | 129 (19.2%) | 0.82 | 22 (9.1%) | 90 (28.7%) | <0.0001 |
| E. coli, # organisms (%) | 135 (24.3%) | 208 (31.0%) | 0.38 | 66 (27.3%) | 69 (22.0%) | 0.13 |
| Klebsiella sp., # organisms (%) | 120 (21.6%) | 112 (16.7%) | 0.82 | 59 (24.4%) | 61 (19.4%) | 0.14 |
| Proteus sp., # organisms (%) | 99 (17.8%) | 84 (12.5%) | 0.25 | 53 (21.9%) | 46 (14.6%) | 0.02 |
| Pseudomonas sp., # organisms (%) | 90 (16.2%) | 138 (20.6%) | 0.25 | 42 (17.4%) | 48 (15.3%) | 0.46 |
| Resistance profiles | ||||||
| MDRO, # organisms (%) | 194 (33.3%) | 308 (43.8%) | 0.04 | 95 (37.3%) | 99 (30.3%) | 0.08 |
| CRE, # organisms (%) | 2 (0.6%) | 3 (0.7%) | 1.0 | 0 (0%) | 2 (1.1%) | 0.49 |
| Nitrofurantoin resistant, # organisms (%) | 166 (63.4%) | 105 (36.6%) | <0.0001 | 93 (75.6%) | 73 (52.5%) | 0.0001 |
| Health care utilization | ||||||
| Outpatient encounters in FY2013, Mean ± SD (range) | 53.2 ± 44.9 (2-300) |
89.7 ± 67.6 (2-355) |
<0.0001 | 43.8 ± 37.5 (2-215) |
64.5 ± 50.2 (4-300) |
0.007 |
| Patients with admission in FY2013 (%) | 90 (40.0%) | 131 (66.8%) | <0.0001 | 40 (32.8%) | 50 (48.5%) | 0.02 |
aCases = Veterans with SCI receiving nitrofurantoin daily for ≥ 90 days.
bControls = Veterans with SCI who had not received prophylactic treatment and had a history of recurrent positive urine cultures (≥ 3 positive urine cultures the year prior to the study period); cases were matched to control by facility and year.
cCase1 group = Veterans with SCI continuously receiving daily nitrofurantoin for UTI prophylaxis over the one-year study period.
dCase2 group = Veterans with SCI with a history of at least 90 continuous days of nitrofurantoin for UTI prophylaxis.
eTotal number of urine cultures in demographic.
fTotal number of organisms in demographic.
Cases had significantly fewer outpatient encounters as compared to controls (53.2 vs. 89.7; P < 0.0001). The average number of outpatient encounters was also lower for the Case1 group compared to the Case2 group (43.8 vs. 64.5, P = 0.007). Sixty-seven percent of controls had at least one hospitalization during the study period, compared to 40.0% of cases (P < 0.0001). A lower proportion of the Case1 group also had a hospitalization (32.8%) as compared to the Case2 group (48.5%; P = 0.02).
Regression analyses adjusted for matching and baseline differences between cases and controls for Charlson score, race, and bladder management strategy for each outcome (listed in Table 4). After adjusting for these baseline differences, UTIs were significantly less frequent in the cases (Table 4). Cases also had fewer positive urine cultures and a greater number of days between positive urine cultures as compared to controls. Other models showed no difference in the odds of multi-drug resistant organisms between cases and controls. However, cases continued to have an increased odds of nitrofurantoin resistance as compared to controls.
Table 4. Conditional logistic regression analyses results for the binary outcomes.
| Outcome | Adjusted OR (95% CI) |
|---|---|
| UTI signs/symptoms1 | 0.51 (0.32-0.81) |
| Positive urine culture2 | 0.43 (0.24-0.79) |
| MDRO1 | 1.03 (0.66-1.6) |
| Nitrofurantoin resistance3 | 11.1 (3.9-31.9) |
1No demographic variables remained in the model after backwards selection.
2Adjusted for patient race and urinary catheter (Charlson was not significant in the model).
3Adjusted for urinary catheter (patient race and Charlson were not significant in the model).
Discussion
UTIs are the most common infections in SCI and represent a substantial burden. Effective preventative strategies to decrease UTI risk in high-risk populations with chronic catheters are urgently needed. This study found a decrease in UTIs over the one-year study period when nitrofurantoin was used long-term. Long-term prescribing of nitrofurantoin did not broadly increase frequency of MDRO urine isolates, but there was a significant increase in nitrofurantoin resistance. An increase of MDROs at other sites (i.e. fecal carriage) cannot be ruled out as we only assessed urine isolates over a short timeframe (one year). Since nitrofurantoin primarily concentrates in the urine,31 its impact at other sites may be minimal. There was a significant increase in time between positive urine cultures when patients were receiving nitrofurantoin. This suggests that long-term prescribing may decrease urine culture collection and, thus, the identification of bacteriuria. Antibiotics are frequently overprescribed for bacteriuria in asymptomatic patients and decreasing urine culture collection has been shown to decrease inappropriate antibiotic prescribing.32 Patients receiving nitrofurantoin also had fewer inpatient and outpatient encounters overall over a course of one year. It is important to note these were all healthcare encounters and not just related to a diagnosis of UTI. The rationale for this may be secondary to patient-reported urinary signs and symptoms both related and unrelated to infection (e.g. foul smelling urine) as a reason for encounters. Additionally, routine collection of urine cultures (appropriate and inappropriate), identification of asymptomatic bacteriuria, and inappropriate diagnosis of UTI may result in hospitalization and subsequent outpatient follow-up. Prophylaxis may indirectly prevent healthcare utilization by decreasing patient and provider concern because a UTI is assumed to be unlikely when nitrofurantoin is prescribed for a prolonged duration.
Literature on the long-term use of nitrofurantoin is scarce, with conflicting evidence regarding the benefit in prevention of UTI. Kuhlemeier et al. concluded that prophylactic doses of ascorbic acid or antibacterials (including nitrofurantoin) had no benefit in SCI patients without indwelling catheters.24 However, a prospective study by Lindan et al. reported a reduction in bacteriuria accompanied by pyuria in the bladder of hospitalized patients with SCI receiving low dose nitrofurantoin, even in the presence of Foley catheters.25 However, no difference was observed for “clinical infection” characterized as bacteriuria with fever and leukocytosis.25 Anderson, et al. similarly identified a significant decrease in bacteriuria in SCI patients undergoing intermittent catheterization who received daily low-dose NF.19 However, these studies were limited in their small sample size, few study sites, short duration of injury, and lack of longitudinal data. In addition, in earlier studies “significant” bacteriuria was deemed active infection and may have caused an over-diagnosis of UTI. While our study was retrospective, it evaluated a large population of chronically injured SCI patients nationally and was able to evaluate diagnoses, medication use, urine cultures and health care utilization. While previous studies focused on bacteriuria, we also collected symptoms to identify the presence of a UTI. As compared to previous reports, our study better reflects differences in UTI diagnosis over a prolonged time period and total health care consumption. Additionally, much has been published which has contributed to our understanding of asymptomatic bacteriuria, UTIs, and CAUTIs since these previous studies were reported in the 1980s, including updated UTI/CAUTI diagnosis and treatment guidelines. However, recent guidelines specific to SCI are lacking.
Importantly, there are several limitations to this study. ICD-9s were used to identify the cohort. ICD-9 coding is heavily determined based on provider documentation and omission of important documentation could have resulted in over- or under-representation of UTIs/symptoms. In addition, there may have been coding errors and variability in coding done by trained medical coders and providers. However, ICD-9s were not used to identify the occurrence of UTIs in the study year (UTIs were verified by chart review). Therefore, some UTIs may have been missed if an ICD-9 was not documented. In addition, symptoms had to be documented in order to meet our definition of UTI. Thus, the frequency of UTI may be higher across groups. Although we did adjust for differences in baseline outcomes for the primary study outcomes with regression analyses (including Charlson, bladder management), there may be unmeasured factors influencing these results. This includes not accounting for the baseline frequency of UTI and/or positive cultures, method of urine culture collection, type of neurogenic bladder, history of bladder, kidney stones, or vesicoureteral reflux, concomitant invasive procedures (e.g. botulinum injections) and inclusion of the ICD-9 for asymptomatic bacteriuria. However, our administrative databases indicate that this ICD-9 is infrequently used in the SCI population (2 veterans with SCI in a cohort of 19,797 in a 1-year period). Our criteria for control group selection likely represents patients where the provider suspected or confirmed a UTI diagnosis in the year prior to our study period. While these criteria may have overrepresented UTIs in controls, controls without any criteria for positive urine cultures/UTIs may have underrepresented UTIs identifying a larger difference in the UTI outcome. For the treatment groups, no information could be obtained regarding duration of prophylactic nitrofurantoin prior to the study or non-VA medication use. Finally, veterans may not be representative of the SCI population in the private sector. However, our use of the veteran population and VA data allowed us to sample a large group of SCI patients across all levels of care (inpatient, outpatient, long-term care, specialty SCI centers, non-specialty centers).
The long-term use of nitrofurantoin was relatively infrequent (∼1% of all SCI veterans). The nitrofurantoin macrocrystal formulation is the only formulation indicated for prophylaxis. We included all nitrofurantoin formulations (i.e. mono/macrocrystal and macrocrystal) prescribed as the drug files between facilities were not standardized. Finally, we did not record data on nitrofurantoin adverse reactions. Prior literature suggests that nitrofurantoin is associated with an overall low rate of adverse reactions,33 but this is an important factor when considering long-term nitrofurantoin. Reported adverse drug reactions with long-term usage include peripheral neuropathy and hepatic disorders (hepatitis, hepatic necrosis). The study timeframe may not be sufficient to identify the impact of long-term use of an antibiotic on the frequency of MDRO. Finally, prescribing of long-term nitrofurantoin may be based on the practices of select providers or influenced based on patient panel (caring for local patients vs geographically distant patients with limited access to a SCI specialty care) or provider assignment to specific care settings (e.g. long-term care). To account for this, matching was based on facility since practice patterns are more likely to be similar within a facility as compared to between facilities. Unfortunately, the use of administrative data cannot determine differences in provider patient panels or assignment to specific care settings.
Despite these limitations, our study provides an important initial evaluation of long-term nitrofurantoin prescribing in SCI patients that can inform a future prospective study. Given the retrospective study design and short duration, recommending the routine prescribing of long-term nitrofurantoin in SCI is premature. Prior to incorporating long-term nitrofurantoin in the treatment of recurrent UTIs, future prospective trials with a randomized, blinded design are necessary.
Conclusion
Long-term nitrofurantoin may be effective in the prevention of UTIs in SCI with recurrent UTIs, but it is currently prescribed for long-term use infrequently (∼1%) in veterans with SCI. While we observed a significant decrease in occurrence of UTI in patients receiving long-term nitrofurantoin without a significant increase in MDRO urine isolates, the nature of the retrospective study design and unmeasured factors may have influenced our results. Thus, these results should be confirmed with a prospective study design with random treatment assignment before long-term nitrofurantoin is routinely incorporated into clinical care.
Funding Statement
This work was supported by Veterans Health Administration, Office of Research and Development, Rehabilitation Research and Development Service SPIRE Award (B-1583-P), and Health Services Research and Development Service Post-Doctoral Fellowship Award (TPR 42-005).
Acknowledgements
The authors wish to thank Dr. Beverly Gonzalez for statistical expertise and manuscript review. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs of the United States government.
Disclaimer statements
Contributors None.
Conflicts of interest The authors have no conflicts of interest to declare.
Ethics approval None.
References
- 1.NINDS Spinal Cord Injury: Hope Through Research. July 2013. http://www.ninds.nih.gov/disorders/sci/detail_sci.htm?css=print. Accessed October 12, 2014.
- 2.Whiteneck GG, Charlifue SW, Frankel HL, Fraser MH, Gardner BP, Gerhart KA, et al. . Mortality, morbidity, and psychosocial outcomes of persons SCI more than 20 years ago. Paraplegia 1992;30(9):617-30. [DOI] [PubMed] [Google Scholar]
- 3.Vickrey BG, Shekelle P, Morton S, Clark K, Pathak M, Kamberg C.. Prevention and management of UTIs in paralyzed persons. Evid Rep Technol Assess (Summ) 1999;(6):1-3. [PMC free article] [PubMed] [Google Scholar]
- 4.Evans CT, Rogers TJ, Chin AM, Johnson S, Smith B, Weaver FM, et al. . Antibiotic prescribing trends in the emergency department for Veterans with SCI/D 2002-2007. J Spinal Cord Med 2013;36(5):492-8. doi: 10.1179/2045772312Y.0000000076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mylotte JM, Graham R, Kahler L, Young L, Goodnough S.. Epidemiology of nosocomial infection and resistant organisms in patients admitted for the first time to an acute rehabilitation unit. Clin Infect Dis 2000;30(3):425-32. doi: 10.1086/313708 [DOI] [PubMed] [Google Scholar]
- 6.The prevention and management of urinary tract infections among people with spinal cord injuries: National Institute on Disability and Rehabilitation Research Consensus Statement: January 27-29. 1992 J Am Paraplegia Soc 1992;15(3):194-204. doi: 10.1080/01952307.1992.11735873 [DOI] [PubMed] [Google Scholar]
- 7.Esclarin De Ruz A, Garcia Leoni E, Herruzo Cabrera R.. Epidemiology and risk factors for urinary tract infection in patients with spinal cord injury. J Urol 2000;164(4):1285–9. [PubMed] [Google Scholar]
- 8.Noreau L, Proulx P, Gagnon L, Drolet M, Laramée MT.. Secondary impairments after SCI: a population-based study. Am J Phys Med Rehabil 2000;79(6):526-35. doi: 10.1097/00002060-200011000-00009 [DOI] [PubMed] [Google Scholar]
- 9.Evans CT, Levela SL, Weaver FM, Priebe M, Sandford P, Niemiec P, et al. . Epidemiology of hospital-acquired infections in veterans with SCI/D. Infect Control Hosp Epi 2008;29(3):234-42. doi: 10.1086/527509 [DOI] [PubMed] [Google Scholar]
- 10.Charlifue SW, Weitzenkamp DA, Whiteneck GG.. Longitudinal outcomes in SCI: aging, secondary conditions, and well-being. Arch Phys Med Rehabil.1999;80(11):1429-34. doi: 10.1016/S0003-9993(99)90254-X [DOI] [PubMed] [Google Scholar]
- 11.Hooton TM, Bradley SF, Cardenas DD, Colgan R, Geerlings SE, Rice JC, et al. . Diagnosis, Prevention, and Treatment of Catheter-Associated Urinary Tract Infection in Adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. [DOI] [PubMed]
- 12.Phillips CD, Adepoju O, Stone N, Moudouni DK, Nwaiwu O, Zhao H, et al. . Asymptomatic bacteriuria, antibiotic use, and suspected urinary tract infections in four nursing homes. BMC Geriatr 2012;12:73. doi: 10.1186/1471-2318-12-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morton SC, Shekelle PG, Adams JL, Bennett C, Dobkin BH, Montgomerie J, et al. . Antimicrobial prophylaxis for urinary tract infection in persons with spinal cord dysfunction. Arch Phys Med Rehabil 2002;83(1):129-38. doi: 10.1053/apmr.2002.26605 [DOI] [PubMed] [Google Scholar]
- 14.Niël-Weise BS, van den Broek PJ, da Silva EM, Silva LA.. Urinary catheter policies for long-term bladder drainage. Cochrane Database Syst Rev 2012;(8):CD004201. [DOI] [PubMed] [Google Scholar]
- 15.Macrobid®[package insert]. Cincinnati, OH: Procter and Gamble Pharmaceuticals, Inc.; Oct 2007.
- 16.Fitzpatrick MA, Suda KJ, Safdar N, Burns SP, Jones MM, Poggensee, et al. . Changes in bacterial epidemiology and antibiotic resistance among veterans with spinal cord injury/disorder over the past 9 years. J Spinal Cord Med. 2017;41:199-207. doi: 10.1080/10790268.2017.1281373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suda KJ, Patel UC, Sabzwari R, Cao L, Ramanathan S, Hill JN, Evans CT.. Bacterial susceptibility patterns in patients with spinal cord injury and disorder (SCI/D): an opportunity for customized stewardship tools. Spinal Cord. 2016;54:1001-9. doi: 10.1038/sc.2016.38 [DOI] [PubMed] [Google Scholar]
- 18.Sanchez GV, Master RN, Karlowsky JA, Bordon JM.. In vitro antimicrobial resistance of urinary Escherichia coli isolates among U.S. outpatients from 2000 to 2010. Antimicrob Agents Chemother 2012;56(4):2181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson RU. Prophylaxis of bacteriuria during intermittent catheterization of the acute neurogenic bladder. J Urol 1980;123(3):364-6. doi: 10.1016/S0022-5347(17)55938-8 [DOI] [PubMed] [Google Scholar]
- 20.Kashanian J, Hakimian P, Blute M Jr., Wong J, Khanna H, Wise G, et al. . Nitrofurantoin: the return of an old friend in the wake of growing resistance. BJU Int 2008;102(11):1634-7. doi: 10.1111/j.1464-410X.2008.07809.x [DOI] [PubMed] [Google Scholar]
- 21.Brumfitt W, Hamilton-Miller JM.. Efficacy and safety profile of long-term nitrofurantoin in urinary infections: 18 years’ experience. J Antimicrob Chemother 1998;42(3):363-71. doi: 10.1093/jac/42.3.363 [DOI] [PubMed] [Google Scholar]
- 22.Karlowsky JA, Kelly LJ, Thornsberry C, Jones ME, Sahm DF.. Trends in antimicrobial resistance among urinary tract infection isolates of Escherichia coli from female outpatients in the United States. Antimicrob Agents Chemother 2002;46(8):2540-5. doi: 10.1128/AAC.46.8.2540-2545.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biering-Sørensen F, Høiby N, Nordenbo A, Ravnborg M, Bruun B, Rahm V.. Ciprofloxacin as prophylaxis for urinary tract infection: prospective, randomized, cross-over, placebo controlled study in patients with spinal cord lesion. J Urol 1994;151(1):105-8. doi: 10.1016/S0022-5347(17)34882-6 [DOI] [PubMed] [Google Scholar]
- 24.Kuhlemeier KV, Stover SL, Lloyd LK.. Prophylactic antibacterial therapy for preventing urinary tract infections in spinal cord injury patients. J Urol 1985;134(3):514-6. doi: 10.1016/S0022-5347(17)47268-5 [DOI] [PubMed] [Google Scholar]
- 25.Lindan R, Joiner E.. A prospective study of the efficacy of low dose nitrofurantoin in preventing urinary tract infections in spinal cord injury patients, with comments on the role of pseudomonas. Paraplegia 1984;22(2):61-5. [DOI] [PubMed] [Google Scholar]
- 26.Salomon J, Denys P, Merle C, Chartier-Kastler E, Perronne C, Gaillard JL, et al. . Prevention of urinary tract infection in spinal cord-injured patients: safety and efficacy of a weekly oral cyclic antibiotic (WOCA) programme with a 2 year follow-up--an observational prospective study. J Antimicrob Chemother 2006;57(4):784-8. doi: 10.1093/jac/dkl010 [DOI] [PubMed] [Google Scholar]
- 27.Spinal Cord Injuries and Disorders System of Care U.S. Department of Veterans Affairs. 10 Jun. 2016. http://www.sci.va.gov/. Accessed July 23, 2016.
- 28.Cardenas DD, Hooton TM.. Urinary Tract Infection in Persons with Spinal Cord Injury. Arch Phys Med Rehabil 1995;76(3):272-80. doi: 10.1016/S0003-9993(95)80615-6 [DOI] [PubMed] [Google Scholar]
- 29.Clinical and Laboratory Standards Institute (CLSI) Performance Standards for Antimicrobial Susceptibility Testing; Twenty-third Informational Supplement M100-S23. CLSI: Wayne, PA, USA; 2013. [Google Scholar]
- 30.Charlson ME, Pompei P, Ales KL, MacKenzie CR.. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40(5):373-83. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 31.Conklin JD. The pharmacokinetics of nitrofurantoin and its related bioavailability. Antibiot Chemother (1971) 1978;25:233-52. doi: 10.1159/000401065 [DOI] [PubMed] [Google Scholar]
- 32.Trautner BW, Grigoryan L, Petersen NJ, Hysong S, Cadena J, Patterson JE, et al. . Effectiveness of an Antimicrobial Stewardship Approach for Urinary Catheter-Associated Asymptomatic Bacteriuria. JAMA Intern Med 2015;175(7):1120-7. doi: 10.1001/jamainternmed.2015.1878 [DOI] [PubMed] [Google Scholar]
- 33.Shamna M, Dilip C, Ajmal M, Linu Mohan P, Shinu C, Jafer CP, et al. . A prospective study on Adverse Drug Reactions of antibiotics in a tertiary care hospital. Saudi Pharm J 2014;22(4):303-8. doi: 10.1016/j.jsps.2013.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]