Abstract
Objective
To determine whether dual energy x-ray absorptiometry (DXA) compared to magnetic resonance imaging (MRI) may accurately quantify trunk lean mass (LM) after chronic spinal cord injury (SCI) and to investigate the relationships between trunk LM, visceral adiposity, trunk fat mass and basal metabolic rate (BMR).
Design
Cross-sectional design and correlational analysis.
Setting
Research setting in a medical center.
Participants
Twenty-two men with motor complete paraplegia (n = 14; T4-T11) and tetraplegia (n = 8; C5-C7) were recruited as part of a clinical trial.
Interventions
Not applicable.
Outcome Measures
Trunk and android LM were measured using DXA. The volume of six trunk muscle groups were then measured using MRI to quantify trunk LM-MRI. Subcutaneous and visceral adipose tissue (VAT) cross-sectional areas were also measured using MRI. After overnight fast, BMR was evaluated using indirect calorimetry.
Results
Trunk LM-DXA (24 ± 3.3 kg) and android LM-DXA (3.6 ± 0.7 kg) overestimated (P < 0.0001) trunk LM-MRI (1.7 ± 0.5 kg). Trunk LM-MRI = 0.088* log (trunk LM-DXA)-0.415; r2=0.29, SEE= 0.44 kg, P = 0.007. Trunk LM-MRI = 1.53* android LM-DXA + 0.126; r2=0.26, SEE= 0.21 kg, P = 0.018. Percentage trunk LM-MRI was inversely related to VAT (r=–0.79, P < 0.0001) and trunk fat mass (r=–0.83, P < 0.001). Only trunk LM-DXA was related to BMR (r = 0.61, P = 0.002). Persons with tetraplegia have 13% smaller trunk muscle cross-sectional areas (P = 0.036) compared to those with paraplegia.
Conclusions
Trunk LM-DXA and android LM-DXA overestimated trunk LM-MRI. Percentage trunk LM-MRI, but not LM-DXA, was inversely related to trunk central adiposity. The findings highlight the importance of exercising trunk LM to attenuate cardio-metabolic disorders after SCI.
Keywords: Trunk lean mass, DXA, MRI, Body composition, Spinal cord injury
Introduction
Skeletal muscle size has been shown to be tightly related to several health-related medical consequences and an independent risk factor for mortality in several epidemiological studies.1,2–3 In persons with spinal cord injury (SCI), most studies have focused on evaluating the size and the composition of the lower extremity muscles with less attention given to the trunk muscles. Trunk lean mass (LM) represents ∼34–38% of the total body mass in persons with SCI and able-bodied controls, respectively.4 They are essential for maintaining an upright position of the spine, breathing, reaching, dressing, postural balance and transfering.5,6 The prevalence of low back pain has approached 49% after SCI.7,8 Respiratory insufficiency is prevalent, with 51% of high thoracic (T1–T6) and 28% of T7–T12 SCI individuals suffering serious respiratory complications.9 Those with tetraplegia are at high risk of developing respiratory insufficiency partially because of paralysis of abdominal muscles.9,10 This evidence highlights the significance of trunk LM for persons with SCI.
Advancement in imaging technology allows for a comprehensive evaluation of skeletal muscle size and composition.11–14 The use of magnetic resonance imaging (MRI) provides insights on skeletal muscle atrophy as well as infiltration of ectopic adipose tissue including intramuscular fat and visceral adipose tissue (VAT).11,12 MRI is considered the gold standard technique and has been used to measure trunk skeletal muscle cross-sectional area (CSA) at the level of the iliac crest (L4-L5) in obese and lean individuals.15,16 However, the use of MRI is costly, time consuming and requires special expertise in image analysis. Unlike MRI, dual energy x-ray absorptiometry (DXA) may be a more widely available and cheaper alternative for evaluation of trunk LM. Trunk LM-DXA was significantly 9 and 2% lower in persons with tetraplegia and paraplegia, respectively, compared to able-bodied controls.13 However, DXA imaging cannot accurately separate the actual magnitude of trunk muscle CSAs from the internal organs.13,14 This may likely mask the real effects of trunk LM (i.e. muscle only) on central adiposity and cardio-metabolic outcomes after SCI. The increase in VAT has detrimental effects on lipid and carbohydrate profile after SCI.17 However, the effects of trunk LM on parameters of central adiposity and cardio-metabolic profile has yet to be determined. Another problem is that compared to healthy controls, muscle mass in fat-free mass is overestimated by 15% when using DXA for persons with SCI.18 Therefore, the use of DXA against the gold standard MRI may validate the measurements of trunk or android LM (starting at L4-L5).19 The android region of interest is defined with a rectangle that starts at the iliac crest with a height representing 20% of the total distance from the top of iliac crest to the base of the skull.19 Moreover, accurate assessment of trunk LM may serve as an initial step towards determining the robust changes in trunk LM following rehabilitation interventions, similar to electrical stimulation.
A SCI dramatically declines the basal metabolic rate (BMR), which accounts for 70% of total energy expenditure.20,21 The decline in BMR has been estimated to be 14-27% lower than able-bodied controls.21 The tight association between fat-free mass and BMR has been previously documented.20–22 Loss in lean mass has been shown to be a pre-determining factor for reduced BMR following SCI. Because trunk fat-free mass represents 51% of total body mass,14 it is fair to hypothesize that trunk LM may explain a considerable variance in BMR following SCI. Decrease in both LM and BMR is accompanied with concomitant increases in fat mass and central adiposity as characterized by accumulation of VAT.12,13,17,19,23 This further leads to high prevalence of obesity in this population with increases in cardio-metabolic disorders and socio-economic burden.24
The primary purpose of the current work was 1) to accurately quantify trunk or android LM (i.e. muscle only) using DXA compared to the gold-standard MRI and to establish accurate prediction equations to quantify trunk LM using DXA 2) to establish the relationships between trunk LM and parameters of central adiposity and BMR in persons with SCI. We hypothesized that the magnitude of trunk or android LM-DXA would overestimate trunk LM-MRI. Considering the tight associations between muscle size, body composition and BMR, we anticipated that trunk LM would be associated with trunk fat mass, VAT and BMR.
Materials and methods
Participants
Twenty-two men with chronic (1-year post-injury) motor complete SCI volunteered to participate in a clinical trial (NCT01652040). The levels of injury ranged from C5 to T11 and were classified according to the American Spinal Injury Association (ASIA) impairment scale (AIS) A or B. A detailed study protocol of the parent clinical trial with all the inclusion and exclusion criteria was recently published.25 Briefly, men ages 18–50 were included if they had motor complete SCI (AIS A or B) level C5-L2 and were over one year post injury. Exclusion criteria included cardiovascular disease, uncontrolled type II diabetes or pressure sores greater than stage II. All participants received and signed an informed consent that was approved by local ethics committee. Participant characteristics are summarized in Table 1. All the study measurements were executed within 24 hours from arrival to the laboratory starting with DXA, MRI and followed with BMR after 10–12 hours overnight fast.
Table 1. Baseline participant characteristics. Values are presented as mean ± standard deviation (SD).
| Characteristics | Total
participants (n = 22) |
Paraplegics (n = 14) |
Tetraplegics (n = 8) |
|---|---|---|---|
| Age (years) | 36 ± 10 | 35 ± 9 | 38 ± 12 |
| Ethnicity | African American (n = 8) White (n = 14) |
African American (n = 6) White (n = 8) |
African American (n = 2) White (n = 6) |
| Weight (kg) | 78 ± 13 | 80 ± 13 | 75 ± 14 |
| Height (cm) | 178 ± 6 | 178 ± 7 | 180 ± 5 |
| BMI (kg/m2) | 25 ± 4 | 25 ± 3 | 23 ± 4 |
| Single Neurological Level (SNL) | C5-T11 | T4-T11 | C5-C7 |
| TSI (years) | 8 ± 8 | 8 ± 8 | 8 ± 7 |
| AIS classification | A (n = 16) B (n = 6) |
A (n = 11) B (n = 3) |
A (n = 5) B (n = 3) |
TSI, time since injury; AIS, American Spinal Injury Association Impairment Scale; n, number.
Body weight and height
After physical examination, participants were weighed on a wheelchair scale (Tanita, Arlington Heights, IL). The wheelchair was weighed empty and was subtracted from the total weight.14,23 Height was measured in a supine position by placing a board at the soles of the feet to ensure dorsiflexion using a Harpenden Stadiometer to the nearest 0.1 cm.
Dual-energy x-ray absorptiometry (DXA)
DXA was used to assess trunk regional body composition into fat mass, LM (i.e. excluding bone tissues) and fat-free mass compartments after a lower extremity elevation for at least 20 minutes. LM-DXA was evaluated at the android and the trunk regions to overlap with the trunk LM-MRI. Whole body scans were taken to assess body composition using a Lunar Prodigy Advance scanner (Lunar DPX, Madison, WI, USA). Lines were placed by a computer program delineating different anatomical regions and were manually adjusted by a DXA trained researcher.14,22,23 Whole trunk fat mass and regional trunk %fat mass and fat-free mass were calculated after accounting for bone mass.
Magnetic resonance imaging (MRI)
MRI scans were obtained with a 1.5 T scanner (General Electric Signa scanner, Milwaukee, WI, USA). T1-weighted images, fast spin-echo sequence: axial in-phase/out-phase, repetition time: 140 ms, echo time: 4.3 (in-phase), 2 ms (out-phase), field of view: 42 cm, matrix size: 256 × 256, number of excitations: 1, and acquisition time: 2 minutes using an abdominal coil were captured.12,17 Transverse images with a slice thickness of 0.8 cm and inter-slice space gap of 1.2 cm were captured from the xiphoid process to the femoral heads. Participants were asked to inhale deeply and to hold their breath for 20 seconds to prevent breathing artifact. Knees and feet were strapped to establish a neutral supine position of the lower extremity to prevent potential muscle spasms during the scan.12,17
Trunk muscle and VAT CSA
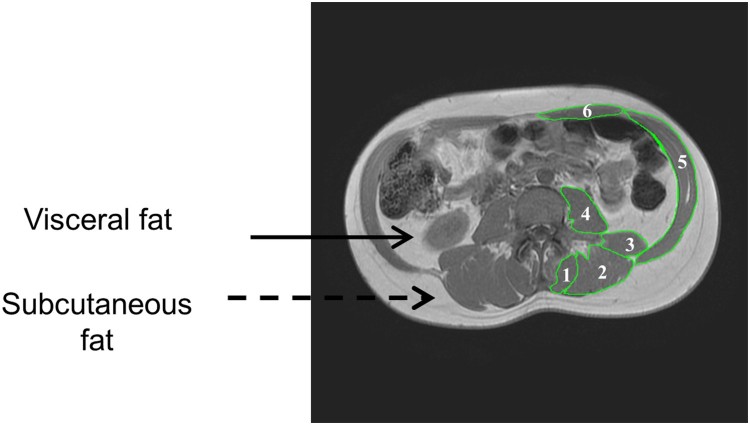
All images were analyzed using specialized imaging software for MRI analysis (Win Vessel 2, Ronald Meyer, MSU, MI, USA). The technician manually traced regions of interest for quantification of VAT, SAT and total trunk CSAs (Figure 1). The ratio of VAT:SAT CSA was calculated as an index of increasing VAT relative to trunk adipose tissue. MRI analysis was done in a blinded fashion to ensure internal validity of the study. The coefficient of variability of measuring muscle, total trunk, SAT and VAT CSAs was less than 3, 0.3, 1.2 and 2.1%, respectively. Six to seven multi-axial images were traced and analyzed including back extensor (BE; 1, 2 and 3), erector spinae (ES; 2), multifidus (MF; 3), iliopsoas (IP; 4), external and internal obliques (EIO; 5), and rectus abdominis (RA; 6; Figure 1). The muscle CSA for the 6 muscle groups was the average of right and left sides (Table 2). The anatomical location of the images was consistent across participants and extended proximally to include the upper lobes of the two kidneys and distally to the image before the two iliac crests. Anatomical selection was based on the images in which the boundaries of the six muscle groups were visually distinguished. Percent (%) trunk muscle CSA was calculated as each individual muscle CSA divided by the total trunk CSA (Figure 1). %Trunk LM-MRI= (Trunk LM-MRI/whole trunk CSA) *100. Whole trunk CSA is defined as the entire area including SAT, muscles, VAT, internal organs and bone. This allowed us to control for the variance in trunk size among our SCI participants. SAT is defined as the region between the dermal layer and the outside boundaries of the trunk muscles (Figure 1).
Figure 1.
Representative magnetic resonance images of an individual with SCI. The distribution of subcutaneous and visceral adipose tissue. Trunk muscles: back extensor (BE; 1, 2, and 3), erector spinae (ES; 2), multifidus (MF; 3), iliopsoas (IP; 4), external and internal obliques (EIO; 5), and rectus abdominis (RA; 6) were traced and quantified.
Table 2. Trunk muscle CSA (cm²) and %trunk muscle (muscle CSA/ total trunk CSA) by muscle group and by participant group. Data are presented as mean ± standard deviation (SD). The “total” column shows the mean of the sum of six muscle groups ± SD. *P < 0.05, Para vs. Tetra.
| Muscle group | Total participants CSA (cm2) (n = 22) |
Paraplegic Total muscle CSA (cm2) (n = 14) |
Tetraplegic Total muscle CSA (cm2) (n = 8) |
Paraplegic %Trunk muscle CSA |
Tetraplegic %Trunk muscle CSA |
|---|---|---|---|---|---|
| BE | 20.2 ± 4.4 | 21.2 ± 5.2# | 18.5 ± 1.9 | 3.5 ± 1.2%# | 3.0 ± 0.6% |
| ES | 13.9 ± 3.3 | 14.7 ± 3.9* | 12.6 ± 1.3 | 2.4 ± 0.9%# | 2.0 ± 0.5% |
| MF | 5.8 ± 1.5 | 6.1 ± 1.7 | 5.5 ± 1.1 | 1.0 ± 0.3% | 0.8 ± 0.2% |
| QL | 4.9 ± 1.6 | 5.3 ± 1.6 | 4.4 ± 1.6 | 0.9 ± 0.3%# | 0.7 ± 0.2% |
| IP | 8.1 ± 2.9 | 8.8 ± 3.0# | 7.0 ± 2.5 | 1.5 ± 0.8%# | 1.1 ± 0.5% |
| RA | 6.1 ± 2.1 | 6.0 ± 2.6 | 6.2 ± 1.0 | 1.0 ± 0.5%# | 1.0 ± 0.3% |
| EIO | 19.4 ± 5.7 | 20.5 ± 6.6 | 17.5 ± 3.4 | 3.3 ± 1.0% | 2.8 ± 0.8% |
| Sum | 58.2 ± 11.3 | 61.3 ± 12.6* | 53.2 ± 6.6 | 10 ± 2.9% | 8.4 ± 1.9% |
BE, back extensors; ES, erector spinae; MF, multifidus; QL, quadratus lumborum; IP, iliopsoas; RA, rectus abdominis; EIO, external and internal obliques.
*Statistical significance in muscle CSA between paraplegic and tetraplegic with P < 0.05.
#Trends towards statistical significance with P= 0.05-0.09.
Sum is equal to the 6 individual muscle groups [ES+MF+QL+IP+RA+EIO].
%Trunk muscle is equal to the individual trunk muscle CSA divided by the whole trunk CSA*100.
Trunk lean mass (LM)-MRI
Muscle volumes were calculated as the respective muscle CSA (cm2) x (inter-slice distance (1.2 cm) + slice thickness (0.8 cm)) for 6–7 slices. Trunk LM-MRI (kg) was acquired by multiplying the total volume by 1.1 g/cm3 for muscle density.26 Trunk LM-MRI was then used as a reference to evaluate the accuracy of trunk and android LM-DXA (i.e, muscle only excluding internal organs).
Basal metabolic rate (BMR)
After overnight fast for 10–12 hours, a BMR measurement was performed with indirect calorimetery.22,23,27 Participants were kept in a dark room in a supine position for 20 minutes to attain a resting state during which BMR was measured by using a canopy that covers the whole head and portable COSMED K4b2c.27 BMR (kcal/day) and respiratory exchange ratio were then recorded.
Statistical analysis
Shapiro-Wilk tests were used to test for normality of individual muscle groups, LM-DXA, trunk fat mass, VAT and BMR. Bland-Altman analysis (mean of measurement difference ± 2 SD) was used to determine the agreement between LM-MRI and LM-DXA. Linear regression analysis was used to determine whether LM-DXA predicts LM-MRI and whether both variables (LM-DXA or LM-MRI) predict BMR. Standard error of the estimate was used to evaluate the accuracy of prediction equations for using LM-DXA to estimate LM-MRI. Independent t-tests were used to analyze the differences in LM-DXA or LM-MRI as well as the differences in muscle CSAs between persons with paraplegia and tetraplegia. Pearson’s correlations were used to investigate the relationships between trunk muscle CSAs or LM-DXA and age, BMI, VAT, % trunk regional fat mass, and trunk fat mass (kg). All data are recorded as mean ± standard deviation (SD). Statistical significance was set at P < 0.05 and analysis was performed using IBM-SPSS (version 24.0, SPSS, Chicago, IL, USA).
Results
The physical characteristics of the 22 participants (14 paraplegia and 8 tetraplegia) are presented in Table 1. Data were normally distributed according to Shapiro-Wilk tests except for android LM-DXA data that were log-transformed before conducting any statistical analysis. Table 2 listed the differences in muscle CSAs between persons with paraplegia and tetraplegia. The sum of the six muscle group CSAs was 13% significantly smaller in tetraplegics compared to persons with paraplegia (61.3 ± 12.6 cm2 vs. 53.2 ± 6.6 cm2; P = 0.036). There were trends for greater muscle CSAs of back extensors (P = 0.05), erector spinae (P = 0.049) and iliopsoas (P = 0.08) in paraplegics compared to tetraplegics (Table 2).
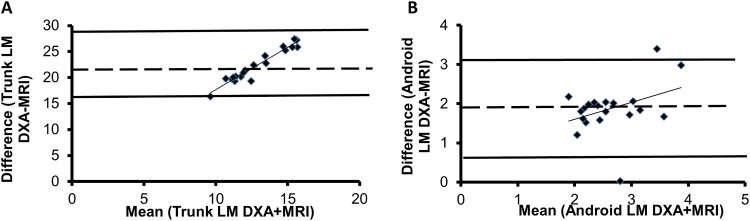
1. Agreement between Trunk LM measured by MRI and. DXA
Android LM-DXA (3.6 ± 0.7 kg) and trunk LM-DXA (24 ± 3.3 kg) overestimated trunk LM-MRI (1.7 ± 0.5 kg; P < 0.0001). The Bland-Altman analysis showed that mean difference between trunk LM-DXA and trunk LM-MRI was 22.2 kg, the SD of the differences was 3.1 kg. The 95% confidence interval (mean ± 2SD) ranged from 16.1–28.4 kg (Figure 2A). The Bland-Altman analysis showed that the mean difference between android LM-DXA and trunk LM-MRI was 1.9 kg, the SD of the differences was 0.6 kg. The 95% confidence interval (mean ± 2SD) ranged from 0.60–3.14 kg (Figure 2B).
Figure 2.
Bland-Altman plots between (A) trunk LM-DXA and LM-MRI as well as (B) android LM-DXA and LM-MRI. The dashed line represents the average of mean differences between the two measurements, with the solid lines representing the 95% confidence intervals (mean ± 2*standard deviations).
2. Prediction of trunk LM-MRI from DXA
Linear regression analysis between trunk LM-MRI and both trunk or android LM-DXA revealed the following equations: (Trunk LM-MRI = 0.088* log (trunk LM-DXA) –0.415; r2=0.29, SEE= 0.44 kg, P = 0.007) or (Trunk LM-MRI = 1.53* android LM-DXA + 0.126; r2=0.26, SEE= 0.21 kg, P = 0.018). The regression equations indicated that trunk LM-DXA and android LM-DXA can explain 29% and 26%, of the variance in trunk LM-MRI, respectively. Linear regression analysis between BMR and trunk LM-DXA revealed the following equation: (BMR = 34.0* trunk LM-DXA + 738.8; r2=0.37, SEE= 144 kcal/day, P = 0.002). The regression equation indicates that trunk lean mass can explain 37% of the variance in BMR.
3. Significance of measuring trunk muscle LM to metabolic and body composition
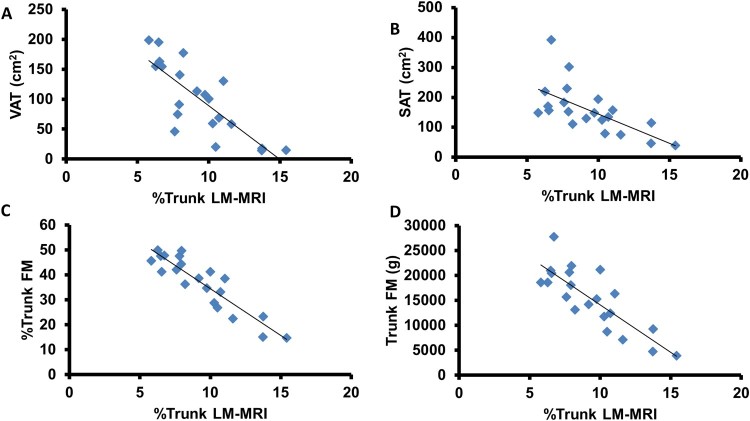
Trunk LM-MRI adjusted to whole trunk CSA (%trunk LM-MRI) was inversely related to VAT (r=–0.79, P < 0.0001; Figure 3A), SAT (r=–0.64, P = 0.002; Figure 3B), %trunk FM (r=–0.91, P < 0.0001; Figure 3C), and trunk FM (r=–0.83, P < 0.0001; Figure 3D). These relationships remained significant when partial correlations were run to account for age. Trunk LM-MRI was not related to VAT, SAT, %trunk FM, or trunk FM.
Figure 3.
Relationship between %trunk LM-MRI and parameters of central adiposity. %trunk LM-MRI was strongly inversely related to (A) visceral adipose tissue (VAT; cm2), (B) subcutaneous adipose tissue (SAT; cm2), (C) % trunk fat mass (FM), and (D) trunk fat mass (g).
Trunk LM-DXA was not related to VAT or SAT, %trunk regional FM, or trunk FM. Trunk LM-MRI was not related to trunk LM-DXA or android LM-DXA. There were no significant relationships between android LM-DXA and VAT, SAT, trunk regional %FM, trunk FM, or BMR.
Discussion
The primary findings suggest that both trunk LM-DXA and android LM-DXA overestimate trunk LM-MRI. Trunk LM-MRI represents only 7% and 48% of trunk LM-DXA and android LM-DXA, respectively. The developed prediction equations may be used to accurately quantify both trunk LM-DXA and android LM-DXA in persons with SCI. %trunk LM-MRI was inversely related to VAT, SAT, %trunk fat mass, and trunk fat mass. Trunk LM-DXA predicted 37% of the variance in BMR. Finally, persons with tetraplegia have significantly 13% smaller trunk muscle CSAs compared to those with paraplegia.
There is scarce evidence regarding the changes in trunk muscles compared to lower extremity muscles after SCI. Trunk fat-free mass represents 49–51% compared to only 27% of leg fat-free mass of the whole body fat-free mass and almost 1/3 of the total body mass.4,14 Trunk muscles provide postural balance and support as wells as assistance in respiratory functions and other activities such as transferring and dressing.5–9 In the current study, trunk LM-DXA overestimated trunk LM-MRI by almost 10 fold. This is in accordance with other studies that showed a similar difference in an able-bodied population.28 The overestimation is attributed to the fact that trunk LM-DXA may include other internal organs, connective tissue, and vessels in the quantification of LM-DXA. Based on the current findings, future software algorithms may be available to estimate of trunk LM-DXA (i.e. muscle only) separated from other internal organs using the developed prediction equations. Trunk LM-DXA predicted 37% of BMR, but not android LM-DXA or trunk LM-MRI, which suggests that trunk LM-MRI may play a minor role compared to the internal organs in estimating BMR. Based on these findings, one may speculate that preventing trunk muscle atrophy is an effective strategy to decrease VAT and subsequently maintain the vitality of other internal organs (liver, spleen, intestines, etc.), which is likely to help in increasing BMR after SCI. Therefore, an increase in trunk muscle mass may defend against accumulation of VAT and decreased BMR.
Multi-axial slices (6–7 images per subject) were analyzed starting at the iliac crest (L4-l5) and moving proximally for 7.2 or 8.4 cm overlapping with the android LM-DXA and lower trunk LM-DXA. Others have used a single transverse slice at L4-L5 when studying trunk muscle CSAs of lean or obese individuals.15,16 These findings demonstrate the importance of analyzing multiple MRI slices when quantifying trunk muscle CSA in persons with SCI. In the current work, our measurement of the back extensor CSA was 23–29% greater than what was previously reported by our group.29 The discrepancy may be explained by the number of slices analyzed between both studies; 9–12 slices were analyzed in the earlier study compared to only 6–7 slices in the current study.29 Another factor is the difference in ethnic background;30 both white and African American were included in the current study compared to only white in the initial study. Another factor is failure to account for intramuscular fat in the initial study,29 which accounts for ∼5% of an individual’s muscle CSA (data not shown). %Trunk LM-MRI was inversely related to VAT, SAT and trunk FM. These are important relationships that were not observed with LM-DXA. Tahan et al. found an inverse relationship between age and abdominal muscle thickness in an able-bodied population using ultrasonography.31 Spungen et al. found a decline in % trunk LM with increasing age in persons with SCI.4 Collectively, these findings may suggest the significance of establishing rehabilitation interventions that can attenuate trunk muscle atrophy. The accurate quantification of trunk LM will allow for comparison of different rehabilitation interventions in order to determine the most effective way to increase lean mass. If future studies determine that the prediction equation is an accurate way to predict LM-MRI this may overcome the cost and time limitations of taking multiple MRI images and analysis.
Clinical implications of the current findings
Cardio-metabolic dysfunction is considered an independent risk factor for all-cause mortality and is associated with dyslipidemia, insulin resistance and impaired glucose tolerance after SCI.32 Detrimental loss in total body and regional lean mass is characterized by mirror increase in fat mass with concomitant decrease in BMR.4,20,21,23 This is likely to expose survivors with SCI to obesity associated metabolic syndrome.24 Furthermore, increase in VAT has been tightly associated with impaired metabolic profile after SCI.17 These cluster of events are likely to impact the quality of life and impose economic burden after SCI. We have previously shown that 8–12 weeks of neuromuscular electrical stimulation resistance training is accompanied with decrease in intramuscular fat,33,34 a depot of ectopic adipose tissue that has been linked to 70% of impaired glucose tolerance after SCI.35 It is yet to be determined whether a similar training program may lead to trunk muscle hypertrophy with decrease in VAT.
Limitations
The current study is limited by small sample size and this is primarily because of difficulty in recruitment of persons with motor complete SCI and the cost associated with conducting MRI and DXA. A larger sample size is needed to validate the equation used to determine trunk LM-MRI from DXA. The study is restricted to men with SCI as no women were recruited to participate in the trial which may limit the generalizability of the current findings. Only men were recruited as a part of a clinical trial investigating the effects of testosterone replacement therapy and evoked resistance training in persons with motor complete SCI.25 We have limited the study to C5-L2 level of injuries, as persons with injury above C5 are more dependent on others in feeding, which may influence their body composition assessment in unpredictable manner. We have included those with L2 level of injury as long as they do not experience lower motor neuron injury that leads to flaccid paralysis. Finally, the lack of age or weight matched able-bodied controls limits the ability to determine how trunk muscle size differs between SCI and able-bodied individuals. Budgetary procurement limited the ability to include a matched able-bodied control group. Finally, the study was not powered to measure trunk LM and the current data can be used as preliminary data to power future studies.
Conclusion
Trunk or android LM-DXA overestimated the trunk LM-MRI by 22 kg or 1.8 kg, respectively. The findings may suggest that SCI may lead to 13% smaller trunk muscle CSA in persons with tetraplegia compared to those with paraplegia. Increase in trunk LM (i.e trunk muscle CSA) is negatively associated with parameters of central adiposity including VAT, SAT, and trunk fat mass. Finally, BMR is positively related to trunk LM-DXA but not trunk LM-MRI. The findings should be considered as an initial step towards establishing additional rehabilitation interventions that may increase trunk LM and attenuate the decline in cardio-metabolic health after SCI.
Acknowledgements
We would like to thank all the study participants for their time and effort. We would also like to thank Hunter Holmes McGuire Research Institute and Spinal Cord Injury Services and Disorders for providing the environment to conduct clinical human research trials. We would like to thank the Radiology Department and MRI technicians for providing the time and effort in collecting the scans.
Abbreviations
List of Abbreviations
- AIS:
American spinal injury association impairment scale
- BE:
back extensors
- BMR:
basal metabolic rate
- BMI:
body mass index
- CSA:
cross-sectional area
- DXA:
dual-energy x-ray absorptiometry
- EIO:
external and internal obliques
- ES:
erector spinae
- IP:
iliopsoas
- LM:
lean mass
- LM-DXA:
trunk lean mass measured by DXA
- LM-MRI:
trunk lean mass measured by MRI
- MF:
multifidus
- MRI:
magnetic resonance imaging
- QL:
quadratus lumborum
- RA:
rectus abdominis
- SAT:
subcutaneous adipose tissue
- SCI:
spinal cord injury
- VAT:
visceral adipose tissue
Disclaimer statements
Contributors None.
Funding The work is supported by the Department of Veteran Affairs, Office of Rehabilitation Research and Development Service, Award # B7867-W.
Conflicts of interest None.
Ethics approval None.
ORCID
Ashraf S. Gorgey http://orcid.org/0000-0002-9157-6034
Supplies
a. PW-630U; Tanita Corporation of America, Inc, 2625 South Clearbook Dr, Arlington Heights, IL 60005.
b. Lunar Prodigy Advance Dual-Energy X-Ray Absorptiometry (DXA) scanner; Genral Electric, PO Box 7550, Madison, WI 53707-7550.
c. Dietary intake data were collected and analyzed using Nutrition Data System for Research software version 2014, developed by the Nutrition Coordinating Center (NCC), University of Minnesota, Minneapolis, MN, 55455.
d. Cosmed K4b2- COSMED USA, Inc. 2211 N Elston Ave Ste 305, Chicago, IL 60614
e. IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp.
References
- 1.Janssen I, Heymsfield SB, Wang ZM, Ross R.. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol (1985). 2000;89(1):81–8. doi: 10.1152/jappl.2000.89.1.81 [DOI] [PubMed] [Google Scholar]
- 2.Therkelsen KE, Pedley A, Speliotes EK, Massaro JM, Murabito J, Hoffmann U, Fox CS.. Intramuscular fat and associations with metabolic risk factors in the Framingham Heart Study. Arterioscler Thromb Vasc Biol 2013;33(4):863–70. doi: 10.1161/ATVBAHA.112.301009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moon SS. Low skeletal muscle mass is associated with insulin resistance, diabetes, and metabolic syndrome in the Korean population: the Korea National Health and Nutrition Examination Survey (KNHANES) 2009-2010. Endocr J 2014;61(1):61–70. doi: 10.1507/endocrj.EJ13-0244 [DOI] [PubMed] [Google Scholar]
- 4.Spungen AM, Adkins RH, Stewart CA, Wang J, Pierson RN, Waters RL, Bauman WA.. Factors influencing body composition in persons with spinal cord injury: a cross-sectional study. J Appl Physiol 2003;95:2398–407. doi: 10.1152/japplphysiol.00729.2002 [DOI] [PubMed] [Google Scholar]
- 5.Chen CL, Yeung KT, Bih LI, Wang CH, Chen MI, Chien JC.. The relationship between sitting stability and functional performance in patients with paraplegia. Arch Phys Med Rehabil 2003;84(9):1276–81. doi: 10.1016/S0003-9993(03)00200-4 [DOI] [PubMed] [Google Scholar]
- 6.Granata KP, Wilson SE.. Trunk posture and spinal stability. Clin Biomech (Bristol, Avon). 2001;16(8):650–9. [DOI] [PubMed] [Google Scholar]
- 7.Shin DC, Song CH.. Relationship of trunk muscle atrophy and provocation position in patients with chronic low back pain. Phys Thera Rehabil Sci 2012;1:28–32. [Google Scholar]
- 8.Danneels LA, Vanderstraeten GG, Cambier DC, Witvrouw EE, De Cuyper HJ.. CT imaging of trunk muscles in chronic low back pain patients and healthy control subjects. Eur Spine J 2000;9(4):266–72. doi: 10.1007/s005860000190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cotton BA, Pryor JP, Chinwalla I, Wiebe DJ, Reilly PM, Schwab C, William MD.. Respiratory Complications and Mortality Risk Associated with Thoracic Spine Injury. Journal of Trauma-Injury Infection & Critical Care 2005;59:400–9 [DOI] [PubMed] [Google Scholar]
- 10.Veeravagu A, Jiang B, Rincon F, Maltenfort M, Jallo J, Ratliff JK.. Acute respiratory distress syndrome and acute lung injury in patients with vertebral column fracture(s) and spinal cord injury: a nationwide inpatient sample study. Spinal Cord 2013;51:461–5. doi: 10.1038/sc.2013.16 [DOI] [PubMed] [Google Scholar]
- 11.Gorgey AS, Dudley GA.. Skeletal muscle atrophy and increased intramuscular fat after incomplete spinal cord injury. Spinal Cord 2007;45:304–9. doi: 10.1038/sj.sc.3101968 [DOI] [PubMed] [Google Scholar]
- 12.Gorgey AS, Mather KJ, Poach HJ, Gater DR.. Influence of motor complete spinal cord injury on visceral and subcutaneous adipose tissue measured by multi-axial magnetic resonance imaging. J Spinal Cord Med 2011;34:99–109. doi: 10.1179/107902610X12911165975106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorgey AS, Dolbow DR, Dolbow JD, Khalil RK, Castillo C, Gater DR.. Effects of spinal cord injury on body composition and metabolic profile - part I. J Spinal Cord Med 2014;37(6):693–702. doi: 10.1179/2045772314Y.0000000245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorgey AS, Dolbow DR, Gater DR.. A model of prediction and cross-validation of fat-free mass in men with motor complete spinal cord injury. Arch Phys Med Rehabil 2012;93:1240–5. doi: 10.1016/j.apmr.2012.02.027 [DOI] [PubMed] [Google Scholar]
- 15.Gille O, de Sèze MP, Guérin P, Jolivet E, Vital JM, Skalli W.. Reliability of magnetic resonance imaging measurements of the cross-sectional area of the muscle contractile and non-contractile components. Surg Radiol Anat 2011;33(8):735–41. doi: 10.1007/s00276-011-0843-5 [DOI] [PubMed] [Google Scholar]
- 16.Wood S, Pearsall DJ, Ross R, Reid JG.. Trunk muscle parameters determined from MRI for lean to obese males. Clin Biomech (Bristol, Avon) 1996;11(3):139–144. doi: 10.1016/0268-0033(95)00018-6 [DOI] [PubMed] [Google Scholar]
- 17.Gorgey AS, Mather KJ, Dr Gater. Central Adiposity Associations to Carbohydrate and Lipid Metabolism in Individuals with Complete Motor Spinal Cord Injury. Metabolism 2011;60(6):843–51. doi: 10.1016/j.metabol.2010.08.002 [DOI] [PubMed] [Google Scholar]
- 18.Modlesky CM, Bickel CS, Slade JM, Meyer RA, Cureton KJ, Dudley GA.. Assessment of skeletal muscle mass in men with spinal cord injury using dual-energy X-ray absorptiometry and magnetic resonance imaging. J Appl Physiol (1985) 2004;96(2):561–5. [DOI] [PubMed] [Google Scholar]
- 19.Cirnigliaro CM, LaFountaine MF, Dengel DR, Bosch TA, Emmons RR, Kirshblum SC, Sauer S, Asselin P, Spungen AM, Bauman WA.. Visceral adiposity in persons with chronic spinal cord injury determined by dual energy X-ray absorptiometry. Obesity (Silver Spring) 2015;23(9):1811–7. doi: 10.1002/oby.21194 [DOI] [PubMed] [Google Scholar]
- 20.Nevin AN, Steenson J, Vivanti A, Hickman IJ.. Investigation of measured and predicted resting energy needs in adults after spinal cord injury: a systematic review. Spinal Cord 2016;54(4):248–53. doi: 10.1038/sc.2015.193 [DOI] [PubMed] [Google Scholar]
- 21.Buchholz AC, McGillivray CF, Pencharz PB.. Differences in resting metabolic rate between paraplegic and able-bodied subjects are explained by differences in body composition. Am J Clin Nutr 2003;77(2):371–8. doi: 10.1093/ajcn/77.2.371 [DOI] [PubMed] [Google Scholar]
- 22.Gorgey AS, Chiodo AE, Zemper ED, Hornyak JE, Rodriguez GM, Gater DR.. Relationship of Spasticity to Soft Tissue Body Composition and the Metabolic Profile in Persons With Chronic Motor Complete Spinal Cord Injury. J Spinal Cord Med 2010;33(1):6–15. doi: 10.1080/10790268.2010.11689669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorgey AS, Gater DR.. Regional and relative adiposity patterns in relation to carbohydrate and lipid metabolism in men with spinal cord injury. Appl Physiol Nutr Metab 2011;36(1):107–14. doi: 10.1139/H10-091 [DOI] [PubMed] [Google Scholar]
- 24.Gater D. Pathophysiology of Obesity After Spinal Cord Injury. Top Spinal Cord Inj Rehabil 2007;12(4):20–34. doi: 10.1310/sci1204-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gorgey AS, Khalil RE, Gill R, O'Brien LC, Lavis T, Castillo T, Cifu DX, Savas J, Khan R, Cardozo C, Lesnefsky EJ, Gater DR, Adler RA.. Effects of Testosterone and Evoked Resistance Exercise after Spinal Cord Injury (TEREX-SCI): study protocol for a randomised controlled trial. BMJ Open 2017;7(4): e014125. doi: 10.1136/bmjopen-2016-014125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brozek J, Grande F, Anderson JT, Keys A.. Densitometric analysis of body composition: Revision of some quantitative assumptions. Ann NY Acad Sci 1963;110:113–40. doi: 10.1111/j.1749-6632.1963.tb17079.x [DOI] [PubMed] [Google Scholar]
- 27.Gorgey AS, Caudill C, Sistrun S, Khalil RE, Gill R, Castillo T, Lavis T, Gater DR.. Frequency of Dietary Recalls, Nutritional Assessment, and Body Composition Assessment in Men with Chronic Spinal Cord Injury. Arch Phys Med Rehabil 2015;96(9):1646–53. doi: 10.1016/j.apmr.2015.05.013 [DOI] [PubMed] [Google Scholar]
- 28.Lee S, Kuk JL.. Changes in fat and skeletal muscle with exercise training in obese adolescents: comparison of whole-body MRI and dual energy X-ray absorptiometry. Obesity (Silver Spring) 2013;21(10):2063–71. doi: 10.1002/oby.20448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorgey AS, Dolbow DR, Cifu DX, Gater DR.. Neuromuscular electrical stimulation attenuates thigh skeletal muscles atrophy but not trunk muscles after spinal cord injury. J Electromyogr Kinesiol 2013;23(4):977–84. doi: 10.1016/j.jelekin.2013.04.007 [DOI] [PubMed] [Google Scholar]
- 30.Silva AM, Shen W, Heo M, Gallagher D, Wang Z, Sardinha LB, Heymsfield SB.. Ethnicity-related skeletal muscle differences across the lifespan. Am J Hum Biol 2010;22(1):76–82. doi: 10.1002/ajhb.20956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tahan N, Khademi-Kalantari K, Mohseni-Bandpei MA, Mikaili S, Baghban AA, Jaberzadeh S.. Measurement of superficial and deep abdominal muscle thickness: an ultrasonography study. J Physiol Anthropol 2016;35(1):17. doi: 10.1186/s40101-016-0106-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bauman WA, Spungen AM.. Coronary heart disease in individuals with spinal cord injury: assessment of risk factors. Spinal Cord 2008;46(7):466–76. doi: 10.1038/sj.sc.3102161 [DOI] [PubMed] [Google Scholar]
- 33.Gorgey AS, Mather KJ, Cupp HR, Gater DR.. Effects of resistance training on adiposity and metabolism after spinal cord injury. Med Sci Sports Exerc 2012;44(1):165–74. doi: 10.1249/MSS.0b013e31822672aa [DOI] [PubMed] [Google Scholar]
- 34.Gorgey AS, Lester RM, Wade RC, Khalil RE, Khan RK, Anderson ML, Castillo T.. A feasibility pilot using telehealth videoconference monitoring of home-based NMES resistance training in persons with spinal cord injury. Spinal Cord Ser Cases 2017;29(3):17039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elder CP, Apple DF, Bickel CS, Meyer RA, Dudley GA.. Intramuscular fat and glucose tolerance after spinal cord injury – a cross-sectional study. Spinal Cord 2004;42:711–6. doi: 10.1038/sj.sc.3101652 [DOI] [PubMed] [Google Scholar]