Abstract
Background
Hospital‐acquired infection is a frequent adverse event in patient care; it can lead to longer stays in the intensive care unit (ICU), additional medical complications, permanent disability or death. Whilst all hospital‐based patients are susceptible to infections, prevalence is particularly high in the ICU, where people who are critically ill have suppressed immunity and are subject to increased invasive monitoring. People who are mechanically‐ventilated are at infection risk due to tracheostomy and reintubation and use of multiple central venous catheters, where lines and tubes may act as vectors for the transmission of bacteria and may increase bloodstream infections and ventilator‐associated pneumonia (VAP). Chlorhexidine is a low‐cost product, widely used as a disinfectant and antiseptic, which may be used to bathe people who are critically ill with the aim of killing bacteria and reducing the spread of hospital‐acquired infections.
Objectives
To assess the effects of chlorhexidine bathing on the number of hospital‐acquired infections in people who are critically ill.
Search methods
In December 2018 we searched the Cochrane Wounds Specialised Register; the Cochrane Central Register of Controlled Trials (CENTRAL); Ovid MEDLINE; Ovid Embase and EBSCO CINAHL Plus. We also searched clinical trial registries for ongoing and unpublished studies, and checked reference lists of relevant included studies as well as reviews, meta‐analyses and health technology reports to identify additional studies. There were no restrictions with respect to language, date of publication or study setting.
Selection criteria
We included randomised controlled trials (RCTs) that compared chlorhexidine bathing with soap‐and‐water bathing of patients in the ICU.
Data collection and analysis
Two review authors independently assessed study eligibility, extracted data and undertook risk of bias and GRADE assessment of the certainty of the evidence .
Main results
We included eight studies in this review. Four RCTs included a total of 1537 individually randomised participants, and four cluster‐randomised cross‐over studies included 23 randomised ICUs with 22,935 participants. We identified one study awaiting classification, for which we were unable to assess eligibility.
The studies compared bathing using 2% chlorhexidine‐impregnated washcloths or dilute solutions of 4% chlorhexidine versus soap‐and‐water bathing or bathing with non‐antimicrobial washcloths.
Eight studies reported data for participants who had a hospital‐acquired infection during the ICU stay. We are uncertain whether using chlorhexidine for bathing of critically ill people reduces the rate of hospital‐acquired infection, because the certainty of the evidence is very low (rate difference 1.70, 95% confidence interval (CI) 0.12 to 3.29; 21,924 participants). Six studies reported mortality (in hospital, in the ICU, and at 48 hours). We cannot be sure whether using chlorhexidine for bathing of critically‐ill people reduces mortality, because the certainty of the evidence is very low (odds ratio 0.87, 95% CI 0.76 to 0.99; 15,798 participants). Six studies reported length of stay in the ICU. We noted that individual studies found no evidence of a difference in length of stay; we did not conduct meta‐analysis because data were skewed. It is not clear whether using chlorhexidine for bathing of critically ill people reduced length of stay in the ICU, because the certainty of the evidence is very low. Seven studies reported skin reactions as an adverse event, and five of these reported skin reactions which were thought to be attributable to the bathing solution. Data in these studies were reported inconsistently and we were unable to conduct meta‐analysis; we cannot tell whether using chlorhexidine for bathing of critically ill people reduced adverse events, because the certainty of the evidence is very low.
We used the GRADE approach to downgrade the certainty of the evidence of each outcome to very low. For all outcomes, we downgraded evidence because of study limitations (most studies had a high risk of performance bias, and we noted high risks of other bias in some studies). We downgraded evidence due to indirectness, because some participants in studies may have had hospital‐acquired infections before recruitment. We noted that one small study had a large influence on the effect for hospital‐acquired infections, and we assessed decisions made in analysis of some cluster‐randomised cross‐over studies on the effect for hospital‐acquired infections and for mortality; we downgraded the evidence for these outcomes due to inconsistency. We also downgraded the evidence on length of stay in the ICU, because of imprecision. Data for adverse events were limited by few events and so we downgraded for imprecision.
Authors' conclusions
Due to the very low‐certainty evidence available, it is not clear whether bathing with chlorhexidine reduces hospital‐acquired infections, mortality, or length of stay in the ICU, or whether the use of chlorhexidine results in more skin reactions.
Plain language summary
Bathing critically ill patients with chlorhexidine to prevent hospital‐acquired infections
What is the aim of this review?
The aim of this review was to find out whether people who are critically ill in hospital should be bathed with the antiseptic chlorhexidine, in order to prevent them from developing infections. Researchers from Cochrane collected and analysed all relevant studies to answer this question and found eight relevant randomised trials. Randomised trials are medical studies where people are chosen at random to receive different treatments. This study design provides the most reliable evidence on whether treatments have a relationship with desired or undesired health outcomes.
Key messages
This review assesses whether using chlorhexidine (instead of soap and water) to bathe patients in an intensive care unit (ICU), or a high‐dependency or critical care unit reduces the number of hospital‐acquired infections. The evidence available from the studies we analysed was very low quality, meaning that we cannot be certain whether bathing with chlorhexidine reduces the likelihood of critically‐ill patients developing an infection, or dying. We are also uncertain whether bathing critically ill patients with chlorhexidine shortens the length of time people spend in hospital, or lowers their risk of developing skin reactions.
What was studied in the review?
People who are critically ill (in an ICU, or a high‐dependency or critical care unit) often catch infections during their time in hospital. These infections can lead to longer hospital stays, additional medical complications, permanent disability or even death. Patients in ICUs are particularly vulnerable to infections because the body's ability to fight infection is reduced by illness or trauma. Surgical tubes and lines (for example to help with feeding or breathing) may enable bacteria to enter the body. Chlorhexidine is a low‐cost product which is used as an antiseptic and disinfectant in hospitals.
What are the main results of the review?
In December 2018 we searched for studies looking at the use of chlorhexidine for bathing critically ill patients. We found eight studies dating from 2005 to 2018, involving a total of 24,472 people across more than 20 ICUs. Seven studies included people who were adults, and one study included only children. All studies included both males and females. All studies compared bathing with chlorhexidine versus bathing with soap and water or non‐antimicrobial washcloths. Four studies received funding from independent funders (government organisations, or from hospital or university departments) or reported no external funding, and four studies received funding from companies that manufactured chlorhexidine products.
The evidence from all eight studies combined is not sufficient to allow us to be certain whether patients bathed in chlorhexidine are less likely to catch an infection during their stay in the ICU. We are also uncertain whether patients bathed in chlorhexidine are less likely to die, because the certainty of the evidence from the six studies that reported on this is very low. We did not pool the evidence from the six studies that reported how long patients had stayed in the ICU, because the results differed widely. We are also uncertain whether patients bathed in chlorhexidine are likely to be in the ICU for less time, because the certainty of the evidence is very low. Reports from five studies provided different evidence about whether chlorhexidine led to more or less skin reactions; we are uncertain whether patients bathed in chlorhexidine are likely to have more or less skin reactions, because the certainty of the evidence is very low.
Quality of evidence
Most studies did not use methods to conceal the type of bathing solution that staff were using, which increases the risk that staff may have treated patients differently depending on whether patients were in the chlorhexidine study group or the soap‐and‐water study group. Participants in some studies may have already caught an infection before the start of the study and we were concerned that this might have affected our results. We also noticed wide differences in some results, and some outcomes had few reported events. These were reasons to judge the quality of the evidence to be very low.
How up to date is this review?
We searched for studies that had been published up to December 2018.
Summary of findings
Summary of findings for the main comparison. Bathing of the critically ill with chlorhexidine versus bathing with soap and water or non‐antimicrobial washcloths for the prevention of hospital acquired infections.
| Bathing of the critically ill with chlorhexidine versus bathing with soap and water or non‐antimicrobial washcloths for the prevention of hospital acquired infections | ||||||
| Population: people who are critically ill Settings: ICUs in France, Italy, Thailand, and USA; studies included single‐centre or multicentre settings Intervention: bathing with a solution of chlorhexidine versus bathing with a solution of soap and water or non‐antimicrobial washcloths | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk with soap and water bathing | Assumed risk with chlorhexidine bathing | |||||
|
Hospital‐acquired infections Data collected during ICU stay |
Study population | Rate difference 1.70 (0.12 to 3.29) | 21,924 (8 studies) | ⊕⊝⊝⊝ Very lowa | We are uncertain whether using chlorhexidine for bathing of critically‐ill people reduced the rate of hospital‐acquired infection. We used data from cluster‐randomised cross‐over studies in which appropriate adjustments were made for study design. We calculated rate difference using generic inverse variance in order to account for studies that reported data as number of events or rates. |
|
| 9.5 infections per 1000 patient days | 7.8 infections per 1000 patient days (6.2 to 9.4) | |||||
|
Mortality Data collected (where reported) in hospital, in the ICU, and at 48 hours |
Study population | OR 0.87 (0.76 to 0.99) | 15,798 (6 studies) | ⊕⊝⊝⊝ Very lowb | We are uncertain whether using chlorhexidine for bathing of critically‐ill people reduced mortality. We used standard errors imputed using an estimated design effect for 2 cluster‐randomised cross‐over studies. We calculated OR using generic inverse variance. |
|
| 9.7 deaths per 100 patients | 8.5 deaths per 100 patients (7.6 to 9.6) | |||||
| Length of stay in the ICU | Study population | Not estimable | 18,570 (6 studies) |
⊕⊝⊝⊝ Very lowc | We are uncertain whether using chlorhexidine for bathing of critically‐ill people reduced length of stay in the ICU. We did not conduct meta‐analysis because data were skewed. We noted no evidence of any difference in effect in each study. |
|
| 7 days (median) | Not estimable | |||||
|
Adverse effects: skin reactions. Reported as attributable to chlorhexidine or soap and water. Data collected during ICU stay |
Of participants bathed with chlorhexidine, 1 study reported 5 mild skin reaction, 1 study reported 1 mild skin reaction, 1 study reported 12 skin reactions, and 1 study reported 21 skin reactions. Comparative data for the control was not clearly reported in 2 studies and 1 study reported 23 skin reactions, respectively. In 1 multi‐armed study, 6 participants in 2 chlorhexidine groups and 6 participants in 2 control groups had skin reactions | Not estimable | 6365 (5 studies) |
⊕⊝⊝⊝ Very lowd | We are uncertain whether using chlorhexidine for bathing of critically‐ill people reduced adverse events. We did not combine data due to insufficient information from study authors or incomparable data. Two additional studies reported skin reactions but believed that these were not attributable to the bathing solution. |
|
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; ICU: intensive care unit | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
aDowngraded by three levels: one level for study limitations (high risk of performance bias in most studies, high risks of other bias in individual studies); one level for inconsistency (sensitivity analysis showed that one small study had a large influence on result, and use of an alternative design effect in one cluster‐randomised cross‐over study changed the effect); one level for indirectness (participants in some studies may have had infections before randomisation) bDowngraded by three levels: one level for study limitations (high risk of performance bias in most studies, high risks of other bias in individual studies); one level for inconsistency (sensitivity analysis showed that use of an alternative design effect in two cluster‐randomised cross‐over studies changed the effect); one level for indirectness (participants in some studies may have had infections before randomisation) c Downgraded by three levels: one level for study limitations (high risk of performance bias in most studies, high risks of other bias in individual studies; one level for imprecision (visual inspection of data showed skewed data); one level for indirectness (participants in some studies may have had infections before randomisation) d Downgraded by three levels: one level for study limitations (high risk of performance bias in most studies, high risks of other bias in individual studies); one level for imprecision (events are very few); one level for indirectness (participants in some studies may have had infections before randomisation)
Background
Description of the condition
Hospital‐acquired infection is one of the most frequent types of adverse event to affect patient care, and can lead not only to discomfort and increased length of stay in hospital, but also to permanent disability and even death. The prevalence of such infections varies internationally, and there are limited data from low‐income countries, where the rates are greater than in high‐income countries. Examples of prevalence include up to 6% of patients in the UK (Health Protection Agency 2016), and 4% of patients in the USA (Magill 2014), whilst reports of prevalence in settings with limited resources vary, for example 5.4% in Mongolia (Ider 2010), 14.5% in Tunisia (Mahjoub 2015) and 19.1% in Albania (Faria 2007).
Whilst all people staying in hospital are susceptible to infections, prevalence in the intensive care unit (ICU) is particularly high. A one‐day prospective, multi‐centre, international study reported 51% of adult patients were classified as infected, and the rate of infection increased to more than 70% for people whose ICU stay was seven days or longer (Vincent 2009). Patients in ICUs are critically ill; they have suppressed immunity as a result of trauma, injury or blood loss (or a combination of these), which increases their susceptibility to infection (Volk 2002). In addition, people who are mechanically‐ventilated in the ICU are at risk due to tracheostomy, reintubation and the use of multiple central venous catheters (Ibrahim 2001), where lines and tubes may act as a vector for the transmission of bacteria and lead to ventilator‐associated pneumonia (VAP).
Common pathogens in hospital‐acquired infection include Staphylococcus aureus, Clostridium difficile and Enterococci; and the overuse of broad spectrum antibiotics has promoted bacteria which are drug‐resistant and difficult to treat (Bereket 2012). Methicillin‐resistant S. aureus (MRSA) causes a range of infections including abscesses, surgical site infections, gastroenteritis, pneumonia, urinary tract infections and endocarditis. It is transmitted by direct contact with an infected person or their environment (or both), and colonises the skin or nostrils. Similarly transmitted, vancomycin‐resistant Enterococci (VRE) leads to urinary tract infections, skin/wound infections, and intra‐abdominal infections. C. difficile causes diarrhoea following administration of antibiotics, and is transmitted through the faecal‐oral route by an infected person or environment (Kelly 2012).
In 2009, Vincent and colleagues reported the most common sites of infection in the ICU as the respiratory tract, abdominal, bloodstream and renal/urinary tract, with respiratory tract infections representing 63.5% of these (Vincent 2009). Healthcare packages and guidelines are now being established to reduce hospital‐acquired infections and subsequent morbidity and mortality rates, for example, a 'central line bundle' of care is being used to try to reduce central line‐associated bloodstream infections (CLABSI), which includes interventions such as education programmes for personnel, hand hygiene and daily review of the need for catheters (Sacks 2014).
Description of the intervention
Chlorhexidine is a biocide on the World Health Organization's List of Essential Medicines (WHO 2017). It has a broad spectrum of action, destabilising the cell walls of gram positive and gram negative bacteria and fungi (Puig Silla 2008; WHO 2011). It can kill most bacteria within 30 seconds of contact (Genuit 2001). In binding to proteins in human tissue, such as skin and mucous membranes, chlorhexidine can also have a slow‐release action, with prolonged activity up to 48 hours after the initial application (Hibbard 2005), and this residual antibacterial activity suggests that organisms that come into contact after chlorhexidine use may not be able to grow (Wade 1991). Chlorhexidine is known to be effective against organisms present in hospital‐acquired infections including S. aureus and Enterococcus (McDonnell 1999).
Chlorhexidine is widely used as a disinfectant and antiseptic in applications such as oral hygiene mouthwashes, hand disinfectants, wound cleansers and preoperative skin preparation (McDonnell 1999). Concentrations range from 0.004% to 4%, in alcohol or aqueous pharmaceutical solution, and it is available in these different dose forms as gels, lotions, solutions, and liquids, and in pads, dressings and sponges.
Chlorhexidine is a low‐cost product. Cochrane systematic reviews have demonstrated that it is effective in particular situations, for example in the reduction of neonatal mortality when used for skin and umbilical cord care in the community setting (Sinha 2015), and in the reduction in rates of ventilator‐acquired pneumonia when used in dental hygiene care of people in the ICU (Shi 2013).
How the intervention might work
People in ICUs are subject to increased invasive monitoring by healthcare personnel. They may be mechanically‐ventilated, have central venous catheters, arterial lines, intravenous catheters, urinary catheters and/or chest tubes, as well as having wounds (both surgical and trauma). All these factors increase the risk of transmission of infection in people who also have reduced immunity (Inweregbu 2005).
Using an antibacterial solution that disinfects the whole skin area during bathing of part or all of the body, may quickly begin to kill existing bacteria. However, chlorhexidine may also form a 'protective coating' to further reduce the risk of hospital‐acquired infections, such as VAP, CLABSI, catheter‐related blood stream infections (CRBSI) and catheter‐associated urinary tract infection (CAUTI) in this high‐risk population.
Although chlorhexidine is known to be a low‐risk skin irritant, the risk of irritation, such as contact dermatitis, may differ between chlorhexidine products with differing concentrations (Calogiuri 2013; McDonnell 1999).
Why it is important to do this review
Hospital‐acquired infections are estimated to lead to 37,000 deaths in Europe, with additional financial burdens (for example through prolonged hospital stay) of EUR 7 billion a year, and up to 99,000 annual deaths in the USA and costs of USD 6.5 billion (WHO 2011).
Morbidity and mortality related to such infections is preventable. People in the ICU are inevitably at high risk, and establishing strategies to reduce rates of infection (in this case, establishing the effectiveness of bathing with a suitable solution) would be beneficial to healthcare systems worldwide, improving outcomes for people who stay in hospital and reducing the length of hospital and ICU stay.
As yet, there are no reports of chlorhexidine‐resistant bacteria. However, chlorhexidine is a widely used product and there are reports of reduced susceptibility of MRSA to chlorhexidine (Horner 2012).
It is important to assess the potential benefits and harms of chlorhexidine for bathing people who stay in the ICU.
Objectives
To assess the effects of chlorhexidine bathing on the number of hospital‐acquired infections in people who are critically ill.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs). We included both parallel and cross‐over designs, as well as cluster and non‐cluster designs. We only included cross‐over designs if data was available for the participants or clusters randomised to the initial treatment group.
Types of participants
We included adult and child participants with any condition that required admission to the intensive care unit (ICU). We included admission to high‐dependency or critical care units or other hospital wards specifically designed to cater for people who are critically ill. We did not include studies of neonates.
We had intended to exclude studies in which participants were diagnosed with a hospital‐acquired infection prior to randomisation, but we found that this was not clearly reported in studies. We therefore noted how this was reported in each included study and considered it during the 'Risk of bias' assessment. See Differences between protocol and review.
Types of interventions
We included studies that compared bathing with a solution of chlorhexidine by any means (e.g. impregnated washcloths or chlorhexidine gel) to bathing using an alternative solution (e.g. soap and water) or no bathing. We defined bathing as the washing of all body areas either at the bedside (e.g. wipe with an impregnated cloth) or in a bath or shower; we excluded studies in which only one body area was washed with a solution of chlorhexidine. We included studies of bathing interventions at different frequencies, for example daily washing or weekly washing.
Types of outcome measures
Our primary interest was whether bathing with chlorhexidine reduced the risk of any hospital‐acquired infection and we therefore recorded the number of participants who acquired an infection since the introduction of the intervention. We included data that were collected from appropriate clinical evaluation of symptoms, or physical signs of infection, or laboratory test results. We collected mortality data from any cause. We collected data for the length of stay in the ICU as number of days. We recorded the number of participants who had any reaction that may be attributable to the intervention or comparison (to include known adverse effects such as skin irritation, rash, contact dermatitis, redness, blistering, swelling of face, hands or feet, or difficulty breathing).
Primary outcomes
Hospital‐acquired infections, including bloodstream infections; central‐line associated bloodstream infections; ventilator‐associated pneumonia; catheter‐associated urinary tract infections; multidrug‐resistant organisms (MDROs), e.g. Methicillin‐resistant Staphylococcus aureus (MRSA), vancomycin‐resistant Enterococci (VRE).
Secondary outcomes
Mortality.
Length of stay in the ICU.
Adverse effects, including skin irritation, or responses such as swelling of face, hands or feet, or breathing difficulties (as defined by the study authors).
Search methods for identification of studies
Electronic searches
We searched the following electronic databases to identify reports of RCTs:
The Cochrane Wounds Specialised Register (searched 10 December 2018);
The Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 11) in the Cochrane Library (searched 10 December 2018);
Ovid MEDLINE (1946 to 10 December 2018);
Ovid Embase (1974 to 10 December 2018);
EBSCO CINAHL Plus (Cumulative Index to Nursing and Allied Health Literature; 1937 to 10 December 2018).
The search strategies for the Cochrane Wounds Specialised Register, CENTRAL, Ovid MEDLINE, Ovid Embase and EBSCO CINAHL Plus can be found in Appendix 1. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) (Lefebvre 2011). We combined the Embase search with the Ovid Embase filter terms developed by the UK Cochrane Centre (Lefebvre 2011). We combined the CINAHL Plus searches with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN 2018). There were no restrictions with respect to language, date of publication or study setting.
We also searched the following clinical trial registries:
ClinicalTrials.gov (www.ClinicalTrials.gov/) (searched 10 December 2018)(Appendix 2);
World Health Organization (WHO) International Clinical Trials Registry Platform (www.who.int/ictrp/search/en/) (searched 10 December 2018)(Appendix 3).
Searching other resources
We carried out backward citation searching of key reviews identified from the searches. We carried out forward citation searching of included studies.
We also carried out grey literature searching through 'Opengrey' (www.opengrey.eu/).
Data collection and analysis
We carried out data collection and analysis according to the methods stated in the published protocol (Lewis 2016), which were based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Two review authors (Sharon Lewis (SL) and Oliver Schofield‐Robinson (OSR)) independently carried out all initial data collection and analysis, before comparing results and reaching consensus. A third author was available to resolve conflicts if required. An additional author (Sarah Rhodes (SR)) was introduced after data extraction to help incorporate cluster‐randomised cross‐over trials into the analysis.
Selection of studies
We used reference management software to collate the results of the searches and to remove duplicates (Endnote 2011).
Two review authors (SL and OSR) used Covidence 2017 software to screen the results of the search from the titles and abstracts and identify any potentially relevant studies from this information alone. Two review authors (SL and OSR) sourced the full texts of all those potentially relevant studies and considered whether they met the inclusion criteria (see Criteria for considering studies for this review). We planned to include abstracts at this stage if they contained sufficient information and relevant results that included denominator figures for each intervention/comparison group.
We recorded the number of papers retrieved at each stage and reported this using a PRISMA flow chart (Liberati 2009). We collected brief details of closely related but excluded papers.
Data extraction and management
Two review authors (SL and OSR) used Covidence 2017 to extract data from individual studies. We extracted the following information.
Methods: type of study design; setting; dates of study; funding sources.
Participants: number of participants randomised to each group; baseline characteristics (including Acute Physiology and Chronic Health Evaluation II (APACHE II) scores).
Interventions: details of intervention and comparison, including concentration of chlorhexidine.
Outcomes: review outcomes measured and reported by study authors.
Outcome data: results of outcome measures.
We considered the applicability of information from individual studies and generalisability of the data to our intended study population (i.e. the potential for indirectness in our review).
There were multiple publications of some studies. In this case, we created a composite data set from all the eligible publications.
Assessment of risk of bias in included studies
We assessed study quality, study limitations and the extent of potential bias using the Cochrane 'Risk of bias' tool (Higgins 2017). See Appendix 4. We considered the following domains.
Sequence generation (selection bias);
Allocation concealment (selection bias);
Blinding of participants, personnel and outcome assessors (performance and detection bias);
Incomplete outcome data (attrition bias);
Selective outcome reporting (reporting bias);
Other potential risks of bias: use of concomitant methods to reduce infection.
We anticipated that there would be a risk of performance bias in the methodology of the studies included in this review, and we noted any methods used by study authors to minimise this risk. We expected that robust study methodology would include blinding of outcome assessors as some outcomes could be measured at a later stage and by personnel not involved with the bathing routine. We anticipated that different hospital units were likely to follow different practices for infection prevention and control in addition to bathing, e.g. use of antiseptic or antibiotic‐coated catheters. We collected available data of any additional infection prevention strategies and noted whether these were likely to be equivalent between groups.
For cluster‐randomised cross‐over study designs, we referred to particular guidance on assessing risk of bias in cluster‐randomised studies and in cross‐over studies (Higgins 2011) (see Appendix 5). In particular, we assessed: recruitment bias; loss of clusters; baseline imbalances between clusters; and whether analysis was appropriate for the cluster design.
For each domain, we judged whether study authors had made sufficient attempts to reduce bias. We made our judgements using one of three measures (low risk, high risk, unclear). We recorded this in 'Risk of bias' tables and present a summary 'Risk of bias' figures.
Measures of treatment effect
We recorded the number of hospital‐acquired infections as rate differences; this was a change from the original protocol (see Differences between protocol and review). Mortality was recorded as dichotomous data in order to calculate odds ratios (OR), and we reported the number of adverse events as dichotomous data.
We recorded length of stay as continuous data.
Unit of analysis issues
We identified one study which had a 2x2 factorial design (Camus 2005). Only one arm included chlorhexidine and we selected this intervention arm (which also included mupirocin as a treatment agent) and compared it to the group with no active agent. We did not include any multi‐armed studies comparing more than one type of chlorhexidine bathing.
In this review, we encountered studies that were randomised by cluster and also included a cross‐over design. For studies that used analysis methods to take account of both the clustering effect and the cross‐over design, we extracted appropriately adjusted standard errors (SEs) for meta‐analysis using the generic inverse‐variance method. For studies in which appropriate adjusted SEs were not reported, we applied appropriate adjustment using an estimate of the design effect for each study (Higgins 2011).
The standard formulae to calculate the design effect of cluster‐randomised studies only takes into account the effect of clustering (which we would expect to increase the SE), but not the effect of the cross‐over design (which we would expect to reduce the SE) (Higgins 2011). We aimed to estimate the square root of the design effect for cluster‐randomised cross‐over studies as (unadjusted SE)/(adjusted SE) when we could obtain crude SEs and SEs that adjusted for clustering and cross‐over design. This estimation method assumes that the design effect is consistent across each outcome in the same study; therefore, when this estimation method was used, we interpreted the results with caution.
Please see Differences between protocol and review for details of changes to this section.
Dealing with missing data
We contacted study authors to clarify missing data. We used available reported data if necessary, rather than imputing values.
Assessment of heterogeneity
We assessed whether there was evidence of inconsistency within our results through consideration of heterogeneity. We assessed clinical heterogeneity by comparing similarities between the participants, interventions and outcomes in the included studies. We assessed statistical heterogeneity by calculation of the Chi2 (with an associated P value) or I2 measure (with an associated percentage). We used the following values as a guide to interpretation: I2 at 0% to 40% is not considered important, 30% to 60% suggests moderate heterogeneity, 50% to 90% suggests substantial heterogeneity, and 75% to 100% represents considerable heterogeneity (Higgins 2011). When assessing heterogeneity, we also considered the point estimates and the overlap of confidence intervals (CIs). If the CIs overlapped then we considered the results to be more consistent. However, it is possible for combined studies to show a large consistent effect but with significant heterogeneity. We therefore interpreted heterogeneity with caution (Guyatt 2011b).
Assessment of reporting biases
We attempted to source published protocols for each of our included studies using clinical trial registers. We compared published protocols with published study results, to assess the risk of selective reporting bias.
We did not have sufficient studies, i.e. more than 10 (Sterne 2017), to generate a funnel plot to assess the risk of publication bias in the review. An asymmetric funnel plot may indicate the publication of only positive results (Egger 1997).
Data synthesis
We completed meta‐analysis for outcomes where comparable effect measures were available from more than one study, and where measures of heterogeneity indicated that pooling of results was appropriate.
For hospital‐acquired infections, we analysed rate differences by entering the rate difference and the associated SE into the generic inverse variance function in Review Manager 2014. This method accounted for the inclusion of cluster‐randomised cross‐over studies. For mortality, we used generic inverse variance to calculate the log OR, which also accounted for the inclusion of cluster‐randomised cross‐over studies. We used a random‐effects model in all analyses to account for the anticipated differences in illness severity or participant conditions. For length of stay in the ICU we planned to use mean difference, and for adverse events we planned to use the OR. See Differences between protocol and review.
We calculated CIs at 95% and used a P value of 0.05 or less to judge whether a result was statistically significant.
We considered whether there was imprecision in the results of analyses by assessing the CI around an effects measure; a wide CI would suggest a higher level of imprecision in the results. A small number of studies may also reduce the precision (Guyatt 2011a).
Subgroup analysis and investigation of heterogeneity
We did not identify sufficient studies to explore differences between them using subgroup analysis. If there had been more than 10 studies (Deeks 2017), we would have conducted subgroup analyses for the following:
illness severity (e.g. based on APACHE II scores);
age of participants (e.g. infants, adults, older adults);
invasive device use (e.g. intravascular devices, mechanical ventilation, feeding lines).
Sensitivity analysis
We explored the potential effects of decisions made as part of the review process as follows:
we excluded all studies that we judged to be at high or unclear risk of selection bias;
we excluded studies in which participant outcome data were missing, for which we used available reported data;
we conducted meta‐analysis using the alternate meta‐analytic effects model (fixed‐effect versus random‐effects).
We compared effect estimates from the analysis of our primary outcome with effect estimates calculated during the above sensitivity analyses. We reported differences that altered our interpretation of the effect.
In addition to planned sensitivity analyses, we considered the effect of including cluster‐randomised cross‐over study designs in the review. We imputed more conservative SEs using standard adjustment for clustering, but ignored the effect of the cross‐over design. See Differences between protocol and review.
'Summary of findings' tables
The GRADE approach incorporates assessment of indirectness, study limitations, inconsistency, publication bias and imprecision (GRADE 2013). We used the assessments made during our analysis to inform the GRADE process (see Data extraction and management, Assessment of risk of bias in included studies, Assessment of heterogeneity, Assessment of reporting biases and Data synthesis, respectively). This approach gives an overall measure of how confident we can be that our estimate of effect is correct (Guyatt 2008).
We used the principles of the GRADE system to give an overall assessment of the evidence relating to each of the following outcomes:
hospital‐acquired infections;
mortality;
length of stay;
adverse event: skin irritation.
Two authors (SL and OSR) independently used the GRADEpro Guideline Development Tool software to create a 'Summary of findings' table (GRADEpro 2015). We assessed the evidence for limitations, inconsistency, indirectness, publication bias and imprecision using the following ratings of certainty: high; moderate; low and very low. We reached consensus and resolved disagreements through informal discussion, with a third review author available if further consultation had been required.
Results
Description of studies
Results of the search
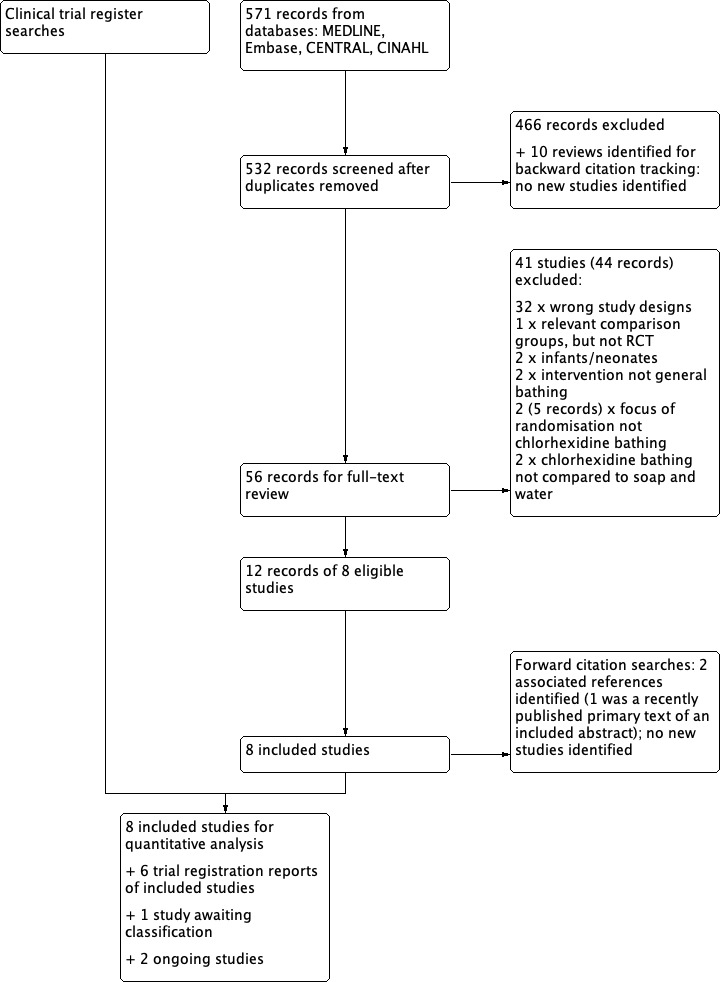
We screened 532 titles and abstracts from database searches, and sourced the full text of 56 potentially eligible studies. Of these, we identified 12 records of 8 studies that were eligible for inclusion in our review. There were multiple publications of some studies and we combined these into eight unique studies.
We identified ten reviews from the database searches (Afonso 2013; Afonso 2016; Chen 2015; Choi 2015; Derde 2012; Frost 2016; Huang 2016; Kim 2016; O'Horo 2012; Shah 2016). We carried out backward citation searching on these and did not identify any additional studies for inclusion. We carried out forward citation tracking on our eight included studies using Google Scholar and Web of Science, and identified no additional studies eligible for inclusion.
We also carried out searches of clinical trial registers and identified clinical trial reports for seven of our included studies. From this search, we found one completed study without published results, and two ongoing studies. We carried out a grey literature search and found no studies that matched our criteria. See Figure 1.
1.
Flow diagram
Included studies
See Characteristics of included studies.
Types of studies
We included eight studies (Bleasdale 2007; Boonyasiri 2016; Camus 2005; Climo 2013; Milstone 2013; Noto 2015; Pallotto 2018; Swan 2016). Four studies were randomised controlled trials (RCTs) and included 1537 individually randomised participants (Boonyasiri 2016; Camus 2005; Pallotto 2018; Swan 2016); four studies were cluster‐randomised cross‐over studies with the ICU as the unit of randomisation, and they included 23 randomised ICUs with 22,935 participants (Bleasdale 2007; Climo 2013; Milstone 2013; Noto 2015).
Types of participants and setting
Four studies were conducted within a single centre (Bleasdale 2007; Noto 2015; Pallotto 2018; Swan 2016), and four were conducted in multiple centres (Boonyasiri 2016; Camus 2005; Climo 2013; Milstone 2013). The ICUs in which the studies were conducted were general, medical, surgical, trauma, neurological, cardiac care, and respiratory care. All studies included adult participants except Milstone 2013, which included only paediatric participants.
Five studies did not report whether any participants had a hospital‐acquired infection at enrolment (Bleasdale 2007; Climo 2013; Milstone 2013; Noto 2015; Pallotto 2018). Two studies reported that some participants had infections prior to randomisation (Camus 2005; Swan 2016); we have reported the number of infections with the respective study baseline characteristics (Characteristics of included studies) One study did not report hospital‐acquired infections at baseline but reported multi‐drug‐resistant bacteria colonisation, which was balanced between groups (Boonyasiri 2016).
Types of interventions and comparisons
Five studies compared daily bathing using 2% chlorhexidine‐impregnated washcloths, with daily or twice daily soap‐and‐water bathing or bathing with non‐antimicrobial washcloths (Bleasdale 2007; Boonyasiri 2016; Climo 2013; Milstone 2013; Noto 2015). One study compared alternate‐day bathing using washcloths submerged in a solution of 4% chlorhexidine, diluted with warm water to 2%, with soap‐and‐water bathing or bathing with washcloths (Swan 2016). One study compared once‐daily bathing with 4% chlorhexidine using washcloths followed by water rinsing (Pallotto 2018). Another study used 4% chlorhexidine at a 12‐hourly rate, compared with liquid soap; there were no further details of dilution or bathing methods in this study (Camus 2005). Camus and colleagues employed a 2 x 2 factorial design in which chlorhexidine was combined with mupirocin to form one intervention, which was compared with another intervention group (polymyxin and tobramycin) and two control groups (Camus 2005); we included data for the chlorhexidine and mupirocin group, compared to a control group that did not have any active intervention. Impregnated washcloths were pre‐manufactured by pharmaceutical companies in four studies (Bleasdale 2007; Climo 2013; Milstone 2013; Noto 2015) and prepared by the hospital pharmacy in one study (Boonyasiri 2016); this information was not reported in one study (Pallotto 2018).
Outcomes
We collected data for hospital‐acquired infections from eight studies (Bleasdale 2007; Boonyasiri 2016; Camus 2005; Climo 2013; Milstone 2013Noto 2015; Pallotto 2018; Swan 2016). Six studies reported mortality data (Boonyasiri 2016; Camus 2005; Milstone 2013; Noto 2015; Pallotto 2018; Swan 2016), and six studies reported the length of stay in ICU (Boonyasiri 2016; Camus 2005; Climo 2013; Noto 2015; Pallotto 2018; Swan 2016). Adverse effects of skin irritation were reported in seven studies (Bleasdale 2007; Boonyasiri 2016; Camus 2005; Climo 2013; Milstone 2013; Pallotto 2018; Swan 2016). Other adverse effects were not reported.
Funding sources
Three studies received institutional funding (Boonyasiri 2016; Noto 2015; Swan 2016) and one study reported that no external funding was received (Pallotto 2018); and four studies reported full or partial funding from companies which manufacture chlorhexidine products (Bleasdale 2007; Camus 2005; Milstone 2013; Noto 2015).
Excluded studies
We excluded 41 (44 reports) studies at the stage of full‐text review (see Figure 1). We excluded 32 studies (with 32 reports) that were the wrong study design (i.e. editorials, letters/comments, reviews, and study designs that were not RCTs. See Appendix 6). We did not report details of these 32 studies in the review. In addition, we excluded nine RCTs (with 12 reports) and we report details of these key studies in Characteristics of excluded studies. Two studies had used chlorhexidine bathing with newborn infants and we believed that these were not comparable with studies of a general ICU population (Cunha 2008; Sankar 2009). One study randomised participants specifically for bathing of the perineal area to prevent catheter‐associated urinary tract infections (CAUTIs), and was not comparable with studies of general bathing (Choi 2012). One study compared solutions used to cleanse the periurethral area prior to urinary catheter placement (Duzkaya 2017). One study randomised participants to receive screening for Methicillin‐resistant Staphylococcus aureus (MRSA) and only administered chlorhexidine bathing to those within the intervention group who were MRSA‐positive (Camus 2011). We excluded one study which was both an interrupted‐time series and an RCT, however the focus of randomisation was on screening, rather than chlorhexidine use (Derde 2014); we identified two associated conference abstract references for this study. One RCT included a relevant intervention group of chlorhexidine bathing, however the comparison was screening, isolation and decolonization strategies, not soap‐and‐water bathing or no bathing (Huang 2013; we identified one associated reference to this study. Another study compared two different methods of chlorhexidine bathing, and did not employ comparison groups of soap‐and‐water bathing or no bathing (Dean 2011). One study compared chlorhexidine bathing with soap and water, in a prospective cross‐over study, but it was not randomised (Lowe 2017). See Characteristics of excluded studies.
Studies awaiting classification
We identified one study that was registered with a clinical trial register and described as having completed participant recruitment (ChiCTR‐TRC‐13004164). We have been unable to source a report of this study and have contacted the authors to request information. We are awaiting any relevant information. See Characteristics of studies awaiting classification.
Ongoing studies
We identified two ongoing studies (IRCT2017030932293N1; NCT02870062). Both RCTs include use of daily chlorhexidine bathing with adults in the ICU. The anticipated recruitment is 80 participants (IRCT2017030932293N1), and 40 participants (NCT02870062).
Risk of bias in included studies
For a summary of the 'Risk of bias' assessments, see Figure 2 and Figure 3.
2.
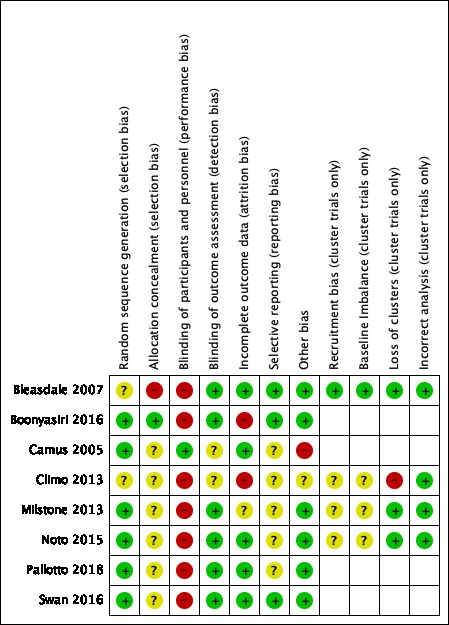
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
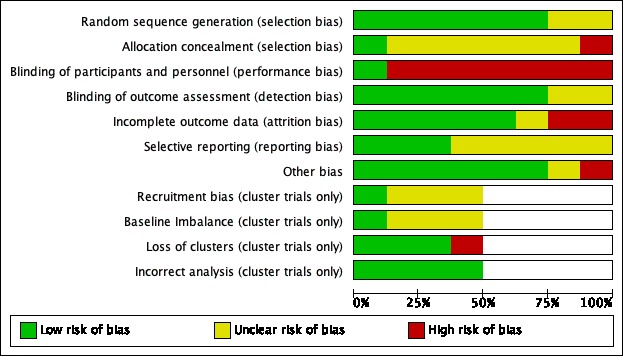
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
All eight included studies were described as randomised trials. We judged four RCTs to be at low risk of selection bias (Boonyasiri 2016; Camus 2005; Pallotto 2018; Swan 2016): all had reported adequate methods of randomisation. For the cluster‐randomised cross‐over studies, each study used a separate ICU for each cluster, and randomisation was completed at cluster level. Two studies reported adequate methods of randomisation; we judged these studies to have low risk of bias (Milstone 2013; Noto 2015). We could not be certain of the risk of bias in two studies because methods were not described (Bleasdale 2007; Climo 2013).
One study reported adequate methods to conceal allocation (Boonyasiri 2016). Four studies reported no methods to conceal allocation and we assessed the risk of bias as unclear (Camus 2005; Climo 2013; Noto 2015; Pallotto 2018). Two studies did not provide adequate information for allocation concealment and we judged these to also have an unclear risk of bias (Milstone 2013; Swan 2016). One study had only two clusters; we believed that allocation concealment was not feasible and the risk of bias was high (Bleasdale 2007).
Blinding
Blinding of participants and hospital personnel was not undertaken in seven studies and we judged these to have a high risk of performance bias (Bleasdale 2007; Boonyasiri 2016; Climo 2013; Milstone 2013; Noto 2015; Pallotto 2018; Swan 2016). Only one study described adequate methods to blind both the participants and the personnel to the intervention (Camus 2005), and we judged this to have a low risk of performance bias. Six studies reported that outcome assessors were blinded to group allocation (Bleasdale 2007; Boonyasiri 2016; Milstone 2013; Noto 2015; Pallotto 2018; Swan 2016). Two studies did not provide sufficient detail of whether outcome assessors were blinded and we judged the detection bias as representing an unclear risk (Camus 2005; Climo 2013).
Incomplete outcome data
We assessed studies that reported no losses or few losses as having low risk of attrition bias (Bleasdale 2007; Camus 2005; Noto 2015; Pallotto 2018; Swan 2016). One study had a large number of losses in one group because of lack of consent; study authors used an intention‐to‐treat (ITT) analysis and reported that this was comparable to a per‐protocol analysis(Milstone 2013); we assessed this study as having unclear risk of attrition bias. We noted a large of number of losses in Boonyasiri 2016, which may have influenced the results of this study. In Climo 2013, study authors reported no losses and had used an ITT analysis but we noted discrepancies in the reported number of randomised participants. We judged two studies to have a high risk of attrition bias (Boonyasiri 2016; Climo 2013).
Selective reporting
Five studies were prospectively registered with clinical trial registers (Bleasdale 2007; Boonyasiri 2016; Climo 2013; Milstone 2013; Swan 2016); three of these had reported outcomes in the final report which matched those in the clinical trial register documents and we judged these studies to have low risk of reporting bias (Bleasdale 2007; Boonyasiri 2016; Swan 2016). Two had inconsistencies between outcomes listed in the clinical trial register documents and the final report, and we were unclear if this introduced bias (Climo 2013; Milstone 2013). Two studies were retrospectively registered with a clinical trial register (Noto 2015; Pallotto 2018), and we were unable to identify clinical trial registration for the remaining study (Camus 2005). It was therefore not feasible to judge any risks of reporting bias for these studies.
Other potential sources of bias
We identified no additional sources of bias in six studies (Bleasdale 2007; Boonyasiri 2016; Milstone 2013; Noto 2015; Pallotto 2018; Swan 2016). We noted a lack of wash‐out period in Climo 2013, but we judged that the study investigators had addressed this risk effectively. We judged the study by Camus and colleagues to have a high risk of bias because the chlorhexidine group also included treatment with mupirocin, which was not given to the participants in the control group (Camus 2005). We also noted that more participants in the control group in this study had a hospital‐acquired infection prior to randomisation, which introduced a high risk of bias.
Recruitment bias (cluster trials only)
We judged the risk of recruitment bias to be unclear in three cluster‐randomised cross‐over studies (Climo 2013; Milstone 2013; Noto 2015); some or all of the clusters in these studies were within the same hospital, which could influence recruitment to a particular ICU according to the current bathing regime. In Bleasdale 2007, the clusters were geographically separate which reduced this risk of recruitment bias; we judged this study to be at low risk.
Baseline imbalances (cluster trials only)
We judged one study to have low risk of bias because characteristics were reported, and were comparable, for each cluster (Bleasdale 2007). Three studies did not report baseline characteristics for each cluster, or we noted some differences between characteristics, and judged these to have an unclear risk of bias (Climo 2013; Milstone 2013; Noto 2015).
Loss of clusters (cluster trials only)
One study reported a loss of clusters (one unit withdrew, and two units were withdrawn from analysis by the study investigators because of low compliance with the protocol), and we judged this to introduce high risk of bias (Climo 2013).
Incorrect analysis (cluster trials only)
All cluster‐randomised cross‐over studies used appropriate analysis to account for the study design, and we judged them to have a low risk of bias for this domain (Bleasdale 2007; Climo 2013; Milstone 2013; Noto 2015).
Effects of interventions
See: Table 1
We found data from eight studies, with a total of 24,472 participants, that compared bathing with a solution of chlorhexidine versus bathing with a solution of soap and water or non‐antimicrobial washcloths. Study authors measured data for our primary outcome (hospital‐acquired infections) and our secondary outcomes (mortality, and length of stay). We contacted study authors to provide clarification on missing data, and we included these data in the analysis where appropriate.
Chlorhexidine bathing versus bathing with soap and water or non‐antimicrobial washcloths (seven studies; 24,023 participants)
Primary outcome: hospital‐acquired infections
All studies collected and reported hospital‐acquired infections during the intensive care unit (ICU) stay, and in the analysis we used data for bloodstream infections (BSI) (Bleasdale 2007; Climo 2013); hospital‐acquired infections (Camus 2005); central line‐associated bloodstream infections (CLABSI) (Milstone 2013); and composite infections of ventilator‐associated pneumonia (VAP), CLABSI, and catheter‐associated urinary tract infection (CAUTI) (Boonyasiri 2016); CLABSI, CAUTI, VAP, and clostridium difficile (Noto 2015); composite infections of BSI, CLABSI, urinary tract infection (UTI), CAUTI, and VAP (Pallotto 2018); and CAUTI, VAP, surgical site infection (SSI) and BSI (Swan 2016). Rates of bacteraemia were also reported in Milstone 2013; we did not include these data in analysis.
Details of the rate data, event data, and analysis process for this outcome are included in Appendix 7 and Appendix 8.
Despite a rate difference which indicated fewer hospital‐acquired infections with chlorhexidine use, we are unsure whether using chlorhexidine for bathing critically ill people reduces hospital‐acquired infections because the certainty of the evidence is very low (rate difference 1.70, 95% confidence interval (CI) 0.12 to 3.29; 21, 924 participants). See Analysis 1.1.
1.1. Analysis.
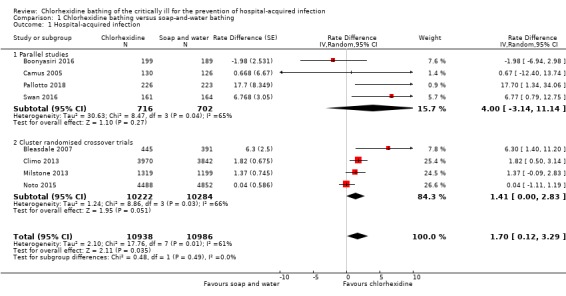
Comparison 1 Chlorhexidine bathing versus soap‐and‐water bathing, Outcome 1 Hospital‐acquired infection.
We noted that one small study had a large influence on the rate difference for this outcome (Bleasdale 2007). We explored this in a sensitivity analysis, and we also explored the effect of using alternative design effects for one cluster‐randomised cross‐over study (Milstone 2013). See 'sensitivity analysis' below. Because of the results of the sensitivity analysis, we used the GRADE approach to downgrade the certainty of the evidence by one level for inconsistency. Most studies had a high risk of performance bias because personnel were aware of which product they were using to bathe participants, and we were concerned by other high risks of bias in individual studies; we downgraded by one level for study limitations. Participants in some studies may have had infections before randomisation; we downgraded by one level for indirectness. See Table 1.
Secondary outcome: mortality
Six studies collected and reported data for mortality (Boonyasiri 2016; Camus 2005; Milstone 2013; Noto 2015; Pallotto 2018; Swan 2016). One study had excluded participants who died within 48 hours of randomisation (Boonyasiri 2016); we included these participants in the mortality data. Time points for data collection in other studies were: in‐hospital mortality (Noto 2015; Swan 2016), and in‐ICU mortality (Camus 2005; Pallotto 2018). The remaining study did not report a time point for data collection (Milstone 2013).
We analysed data for RCTs and cluster‐randomised cross‐over studies with generic inverse variance, and used standard errors imputed using an estimated design effect for two cluster‐randomised cross‐over studies (Milstone 2013; Noto 2015). We reported event data and details of the analysis process for these studies in Appendix 9.
It is not clear whether using chlorhexidine for bathing critically ill people reduces mortality because the certainty of the evidence is very low (OR 0.87, 95% CI 0.76 to 0.99; 15,798 participants). See Analysis 1.2.
1.2. Analysis.
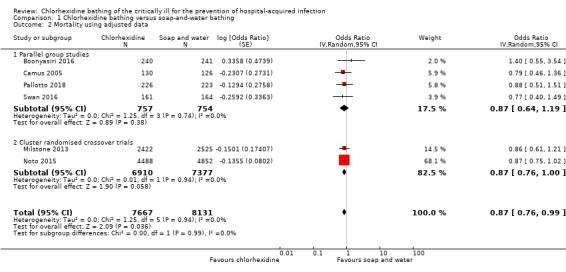
Comparison 1 Chlorhexidine bathing versus soap‐and‐water bathing, Outcome 2 Mortality using adjusted data.
In a sensitivity analysis, we explored the effect of analysis decisions for the inclusion of two cluster‐randomised cross‐over studies (Milstone 2013; Noto 2015). Consequently, we believe that the effect for mortality should be interpreted cautiously, and we used the GRADE approach to downgrade the certainty of the evidence by one level for inconsistency. Most studies had a high risk of performance bias because personnel were aware of which product they were using to bathe participants, and we were concerned by other high risks of bias in individual studies; we downgraded by one level for study limitations. Participants in some studies may have had infections before randomisation; we downgraded by one level for indirectness. See Table 1.
Secondary outcome: length of stay in the intensive care unit
Six studies collected and reported length of stay in the ICU (Boonyasiri 2016; Camus 2005; Climo 2013; Noto 2015; Pallotto 2018; Swan 2016).
We noted from visual inspection of the data,that reported ranges, SDs, and CIs in these studies were skewed; we decided it was not appropriate to combine data in analysis because of this. Individual study data are reported in Table 3. We noted no evidence of any difference in length of stay in the ICU according to whether participants were bathed with chlorhexidine or soap and water.
1. Data for length of stay.
| Study ID | Study design | Data reported by study authors | Chorhexidine group | Control group | Inference |
| Boonyasiri 2016 | Parallel | Median (range) length of ICU stay, in days | 9 (3 to 212) | 10 (3 to 136) | P = 0.42 |
| Camus 2005 | Parallel | Median (range) length of ICU stay, in days | 15 (3 to 132) | 16 (3 to 83) | "Not significantly different" |
| Climo 2013 | Cluster‐randomised cross‐over | Mean length of ICU stay, in days | 6.4 | 6.4 | P = 0.53 (unadjusted) |
| Noto 2015 | Cluster‐randomised cross‐over | Mean (95% CI) length of ICU stay, in days | 2.56 (1.24 to 5.09) | 2.39 (1.21 to 4.95) | Difference (95% CI) = 0.169 (‐0.01 to 0.321) (unadjusted using Mann‐Whitney U) |
| Pallotto 2018 | Parallel | Median (IQR) length of ICU stay, in days | 4 (2 to 8) | 4 (2 to 7) | P > 0.05 |
| Swan 2016 | Parallel | Mean (SD) length of SICU stay, in days | 7.2 (± 11.4) | 7.0 (± 8.6) | P = 0.89 |
CI: confidence interval; ICU: intensive care unit; SD: standard deviation; SICU: surgical intensive care unit
It is unclear whether using chlorhexidine for bathing critically ill people reduces the length of stay in the ICU because the certainty of the evidence is very low.
We used the GRADE approach to downgrade the evidence by one level for imprecision because of skewed data reported by study authors. Most studies had a high risk of performance bias because personnel were aware of which product they were using to bathe participants, and we were concerned by other high risks of bias in individual studies: we downgraded by one level for study limitations. Participants in some studies may have had infections before randomisation; we downgraded by one level for indirectness. See Table 1.
Secondary outcome: adverse effects
Seven studies reported participants who had skin irritation (Bleasdale 2007; Boonyasiri 2016; Camus 2005; Climo 2013; Milstone 2013; Pallotto 2018; Swan 2016). Two studies reported adverse events of skin irritation but perceived these as not attributable to bathing (Bleasdale 2007; Climo 2013); we have reported these data in Characteristics of included studies.
It was not possible to combine data in meta‐analysis for the remaining four studies. One study reported five participants with a mild skin reaction attributable to chlorhexidine (Boonyasiri 2016), but did not report whether data were collected for the control group. Another study reported 12 participants with a skin reaction attributable to chlorhexidine (Milstone 2013); skin reactions for the control group in this study were not reported according to whether they were attributable to the control. In Pallotto 2018, one participant who was bathed with chlorhexidine had a mild skin reaction and chlorhexidine was discontinued in this participant.
In Camus 2005, six participants in the control group had a skin reaction and six participants who had used chlorhexidine had a skin reaction. However, study authors had not reported how many of these were in the chlorhexidine with mupirocin group, which we had used as the intervention in the review. In Swan 2016, there were 21 participants who were bathed with chlorhexidine, and 23 participants in the control group, who had skin reactions that were perceived as possibly or probably related to bathing.
We used the GRADE approach to downgrade the certainty of evidence for adverse events to very low. We downgraded by one level for study limitations; we judged some studies to have a high risk of performance bias, and some studies had high risks of other bias. Participants in some studies may have had infections before randomisation; we downgraded by one level for indirectness. We found few adverse events and we downgraded by one level for imprecision. See Table 1.
Sensitivity analysis
1. Risk of bias
We assessed five of the eight studies included in our primary outcome to have unclear or high risk of selection bias (Bleasdale 2007; Camus 2005; Climo 2013; Milstone 2013; Noto 2015).
Hospital‐acquired infection
Analysis using only the remaining three parallel design studies showed little or no difference in infections according to bathing regime (rate difference 5.12, 95% CI ‐3.83 to 14.06).
Mortality
Analysis using only the remaining three parallel design studies did not alter interpretation of the effect.
2. Missing outcome data
All study authors reported losses and provided reasons. Four studies had reported the data as intention‐to‐treat and we had used these data in our meta‐analyses (Camus 2005; Climo 2013; Milstone 2013; Noto 2015). The remaining studies had reported data only for those who were not lost during follow‐up and we removed these studies from each of our meta‐analyses. This did not alter direction or interpretation of the results.
3. Effects model for meta‐analysis
When all available studies were included in the primary analysis, the conclusions remained the same regardless of whether a fixed‐effect or random‐effects model was used in meta‐analysis.
4. Study design
Hospital‐acquired infection
We included one cluster‐randomised cross‐over trial with only two clusters in the primary analysis (Bleasdale 2007). In our sensitivity analysis, we removed this study and found that the rate difference was reduced to indicate little or no difference in infections according to bathing regime (rate difference 1.26, 95% CI ‐.21 to 2.72).
We included one study in which we imputed a SE using an estimated design effect (Milstone 2013). In our sensitivity analysis, we re‐analysed the data by imputing an extremely conservative design effect of 5.06 (obtained by ignoring the cross‐over effect and using the formula DE = 1 + (M ‐ 1) ICC with ICC = 0.05 and M = 495, where DE = design effect, M = mean cluster size, ICC = intracluster correlation coefficient). We found that the rate difference was reduced in the sensitivity analysis, to indicate little or no difference in infections according to bathing regime when an extremely conservative design effect was used (rate difference 1.97, 95% CI ‐0.06 to 4.00).
Mortality
We included two cluster‐randomised cross‐over studies and used SE imputed using an estimated design effect (Milstone 2013; Noto 2015). In the sensitivity analysis, we re‐analysed the data by imputing an extremely conservative design effect. We found little or no difference in mortality between different bathing regimes when extreme conservative design effects were used (OR 0.87, 95% CI 0.69 to 1.20).
Discussion
Summary of main results
We identified eight studies: four randomised controlled trials (RCTs), which included 1537 randomised participants; and four cluster‐randomised cross‐over studies, which included 23 randomised intensive care units (ICUs) with a total of 22,935 participants. We identified one study awaiting classification, which was listed as completed in a clinical trial register but was not published.
Eight studies reported data for participants who had a hospital‐acquired infection during their stay in the intensive care unit (ICU). Although the effect estimate showed fewer hospital‐acquired infections with chlorhexidine bathing of critically ill people, the certainty of the evidence is very low. Six studies reported mortality (in hospital, in the ICU, and at 48 hours). Although the effect estimate showed reduced mortality with chlorhexidine bathing of critically ill people, the certainty of the evidence is very low. Six studies reported length of stay in the ICU. We noted that individual studies found no evidence of a difference in length of stay, and we did not conduct meta‐analysis because data were skewed. We are uncertain whether using chlorhexidine for bathing of critically ill people reduced length of stay in the ICU because the certainty of the evidence is very low. Seven studies reported skin reactions as an adverse event, and five of these reported skin reactions which were thought to be attributable to the bathing solution. In these studies, data for skin irritation were reported inconsistently and we were unable to conduct meta‐analysis; we are uncertain whether using chlorhexidine for bathing of critically ill people reduced adverse events, because the certainty of the evidence is very low. No other adverse events were reported in studies.
Overall completeness and applicability of evidence
We conducted a thorough search, including forward citation tracking of included studies, backward citation tracking of relevant reviews, and searches of grey literature. Included studies all compared chlorhexidine bathing with soap‐and‐water bathing or bathing with non‐microbial washcloths, and included participants who were critically ill. We noted that participants in two studies had hospital‐acquired infections before randomisation (Camus 2005; Swan 2016), and study authors in five studies did not report whether participants had hospital‐acquired infections at baseline (Bleasdale 2007; Climo 2013; Milstone 2013; Noto 2015; Pallotto 2018). We believe that this introduces indirectness, and reduces the applicability of the evidence for this review. Studies were published from 2005 to 2018, with five studies based in the USA, one in France, one in Italy, and one in Thailand.
Quality of the evidence
We used the GRADE approach to judge the evidence for each outcome to be of very low quality.
We considered study limitations identified during the 'Risk of bias' assessment. We noted some inconsistency in reporting between studies such that it was not possible to effectively judge all domains for each study. It was feasible to design a study so that personnel could be masked to the treatment allocation, yet only one study had effectively blinded personnel to the intervention and control, leading to a high risk of performance of bias across studies. Six studies did, however, make an effort to blind outcome assessors. We noted high participant attrition in some studies. We judged four studies with a cluster‐randomised design to have an unclear risk of bias; these studies may have had differences at the unit‐level of randomisation (i.e. between randomised ICUs). We noted differences in one study in which the chlorhexidine group received an additional treatment. We downgraded our assessment of the quality of the evidence, due to study limitations.
We noted some inconsistencies between results, and we used sensitivity analyses to explore this. We found that one small study had a large influence on the effect for hospital‐acquired infections, and we found that decisions taken when estimating a design effect for some cluster‐randomised cross‐over studies may also have influenced results; therefore, we downgraded the evidence due to inconsistency. We also noted imprecision in individual study data for length of stay in the ICU, which were skewed. We were unable to explore potential differences between study participants (for example differences in illness severity or differences between adult and paediatric participants) because we had insufficient studies to conduct subgroup analysis. Whilst study participants were mostly applicable to our review question, we noted some indirectness because participants in some studies may have had hospital‐acquired infections before randomisation. Because studies reported few adverse events, we downgraded our assessment of the evidence for this outcome because of imprecision. We were unable to assess the risk of publication bias because of lack of available data for this review.
Potential biases in the review process
We included four cluster‐randomised cross‐over studies in this review. We believe that this is an appropriate design for the study of infection practices. However, we did not anticipate this study design during preparation of the protocol, and so the methods used to analyse data from these studies were decided post‐hoc. We used data reported by study authors if they were appropriately adjusted for both the clustering effect and the cross‐over design; when we used estimation methods to calculate a design effect for the cluster studies, we assessed these decisions in sensitivity analysis.
We also used sensitivity analysis to explore the decisions to use a random‐effects model for meta‐analysis, and to use data reported only for participants who were not lost to follow‐up in three of our included studies, neither of which influenced interpretation of our results. There were insufficient studies to explore risks of selection bias in our analyses. We did not attempt to consider other factors that may have impacted on our data. The decision of which treatment and control group to include in one study meant that participants in the chlorhexidine group for this study were also treated with mupirocin, which the control group did not receive (Camus 2005); this may have acted as a confounder for these data, which we did not explore. We included three studies in which it was noted that some participants had a hospital‐acquired infection and we did not assess whether this influenced our results, nor did we explore the impact of two large multi‐centre studies on our data (Milstone 2013; Noto 2015).
We conducted the review according to the protocol, with two reviewers independently assessing studies for eligibility, extracting data and carrying out the 'Risk of bias' assessment.
Agreements and disagreements with other studies or reviews
There have been several systematic reviews that have assessed the effect of chlorhexidine bathing on the critically ill. Reviews have previously concluded that chlorhexidine bathing reduces risk of infection in the ICU (Chen 2015; Choi 2015; Huang 2016; Kim 2016; O'Horo 2012). These reviews have collected event data for specific infections (bloodstream infections, central line‐associated bloodstream infections, ventilator‐associated pneumonia, Methicillin‐resistant Staphylococcus aureus (MRSA) and vancomycin‐resistant Enterococci) rather than a composite outcome for the number of participants with any hospital‐acquired infection.
Most notably, these systematic reviews include both RCTs and non‐randomised study designs; and one review noted that this effect was not consistent when non‐randomised studies were excluded from analysis (Chen 2015). A review that had only included RCTs, did not exclude Huang 2013; this study had a large sample size but did not compare chlorhexidine with soap and water. It is possible that the results of our analyses are dependent on our restriction to RCTs.
Authors' conclusions
Implications for practice.
It is not clear whether bathing with chlorhexidine reduces hospital‐acquired infections, mortality or length of stay in the intensive care unit, or whether chlorhexidine use results in more skin reactions, because the certainty of the evidence is very low. One study is awaiting classification and two studies are ongoing; we do not know if inclusion of these studies in future updates of this Cochrane Review will increase our certainty in the results of the review.
Implications for research.
Additional research is needed to evaluate whether chlorhexidine bathing may reduce hospital‐acquired infections in the intensive care unit. We recommend that studies are sufficiently powered and methodologically robust, and that attention is paid to reduce the risk of performance bias through blinding of personnel. Cluster‐randomised studies and cross‐over trials would benefit from reporting data in more detail, including important parameters such as the intracluster correlation coefficient and interperiod correlation. Some consensus on the reporting of hospital‐acquired infection rates, for example through the adoption of a core outcome set for trials of infection prevention, would also be helpful.
History
Protocol first published: Issue 6, 2016 Review first published: Issue 8, 2019
| Date | Event | Description |
|---|---|---|
| 30 March 2020 | Amended | Minor edit to 'Summary of Findings' table. |
Acknowledgements
The authors would like to acknowledge the contributions of peer referees who commented on the protocol, the review or both: Zipporah Iheozor‐Ejiofor, Julie Bruce, Sharon Van Wicklin, Mahmoud Alkhatib, Amanda Roberts and Gill Norman and to thank Megan Prictor and Jessica Sharp who copy‐edited the protocol and the review respectively. The authors would also like to thank Phil Alderson, Andrew Butler and David Evans for their work on the protocol.
Appendices
Appendix 1. Search strategies
Cochrane Wounds Specialised Register
1 MESH DESCRIPTOR Chlorhexidine EXPLODE ALL AND INREGISTER
2 chlorhexidine or CDG AND INREGISTER
3 #1 OR #2
4 MESH DESCRIPTOR Intensive Care Units EXPLODE ALL AND INREGISTER
5 MESH DESCRIPTOR Critical Care EXPLODE ALL AND INREGISTER
6 MESH DESCRIPTOR Critical Illness EXPLODE ALL AND INREGISTER
7 MESH DESCRIPTOR Critical Care Nursing EXPLODE ALL AND INREGISTER
8 (critical* near3 ill*) AND INREGISTER
9 (intensive care or ICU or intensive therapy or intensive treatment or ITU or (high* next dependen*) or HDU or critical care or CCU) AND INREGISTER
10 #4 OR #5 OR #6 OR #7 OR #8 OR #9
11 #10 AND #3
The Cochrane Central Register of Controlled Clinical Trials (CENTRAL) via the Cochrane Register of Studies (CRS‐Web)
1 MESH DESCRIPTOR Chlorhexidine EXPLODE ALL AND CENTRAL:TARGET
2 chlorhexidine or CDG AND CENTRAL:TARGET
3 #1 OR #2
4 MESH DESCRIPTOR Intensive Care Units EXPLODE ALL AND CENTRAL:TARGET
5 MESH DESCRIPTOR Critical Care EXPLODE ALL AND CENTRAL:TARGET
6 MESH DESCRIPTOR Critical Illness EXPLODE ALL AND CENTRAL:TARGET
7 MESH DESCRIPTOR Critical Care Nursing EXPLODE ALL AND CENTRAL:TARGET
8 (critical* near3 ill*) AND CENTRAL:TARGET
9 (intensive care or ICU or intensive therapy or intensive treatment or ITU or (high* next dependen*) or HDU or critical care or CCU) AND CENTRAL:TARGET
10 #4 OR #5 OR #6 OR #7 OR #8 OR #9
11 #3 AND #10
Ovid MEDLINE
1 exp Chlorhexidine/
2 (Chlorhexidine or CHG).tw.
3 or/1‐2
4 exp Intensive Care Units/
5 exp Critical Care/
6 exp Critical Illness/
7 exp Critical Care Nursing/
8 (critical* adj3 ill*).tw.
9 (intensive care or ICU or intensive therapy or intensive treatment or ITU or high* dependen* or HDU or critical care or CCU).tw.
10 or/4‐9
11 and/3,10
12 randomised controlled trial.pt.
13 controlled clinical trial.pt.
14 randomi?ed.ab.
15 placebo.ab.
16 clinical trials as topic.sh.
17 randomly.ab.
18 trial.ti.
19 or/12‐18
20 exp animals/ not humans.sh.
21 19 not 20
22 11 and 21
Ovid Embase
1 exp chlorhexidine/
2 exp chlorhexidine gluconate/
3 (Chlorhexidine or CDG).tw.
4 or/1‐3
5 exp intensive care unit/
6 intensive care/
7 exp critical illness/
8 exp critically ill patient/
9 (critical* adj3 ill*).tw.
10 (intensive care or ICU or intensive therapy or intensive treatment or ITU or high* dependen* or HDU or critical care or CCU).tw.
11 or/5‐10
12 and/4,11
13 Randomized controlled trials/
14 Single‐Blind Method/
15 Double‐Blind Method/
16 Crossover Procedure/
17 (random* or factorial* or crossover* or cross over* or cross‐over* or placebo* or assign* or allocat* or volunteer*).ti,ab.
18 (doubl* adj blind*).ti,ab.
19 (singl* adj blind*).ti,ab.
20 or/13‐19
21 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
22 human/ or human cell/
23 and/21‐22
24 21 not 23
25 20 not 24
26 12 and 25
EBSCO CINAHL Plus
S25 S11 AND S24
S24 S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23
S23 TI allocat* random* or AB allocat* random*
S22 MH "Quantitative Studies"
S21 TI placebo* or AB placebo*
S20 MH "Placebos"
S19 TI random* allocat* or AB random* allocat*
S18 MH "Random Assignment"
S17 TI randomi?ed control* trial* or AB randomi?ed control* trial*
S16 AB ( singl* or doubl* or trebl* or tripl* ) and AB ( blind* or mask* )
S15 TI ( singl* or doubl* or trebl* or tripl* ) and TI ( blind* or mask* )
S14 TI clinic* N1 trial* or AB clinic* N1 trial*
S13 PT Clinical trial
S12 MH "Clinical Trials+"
S11 S3 AND S10
S10 S4 OR S5 OR S6 OR S7 OR S8 OR S9
S9 TI (intensive care or ICU or intensive therapy or intensive treatment or ITU or high* dependen* or HDU or critical care or CCU) OR AB (intensive care or ICU or intensive therapy or intensive treatment or ITU or high* dependen* or HDU or critical care or CCU)
S8 TI (critical* N3 ill*) OR AB (critical* N3 ill*)
S7 (MH "Critically Ill Patients")
S6 (MH "Critical Illness")
S5 (MH "Critical Care+")
S4 (MH "Intensive Care Units+")
S3 S1 OR S2
S2 TI (Chlorhexidine or CHG) OR AB (Chlorhexidine or CHG)
S1 (MH "Chlorhexidine")
Appendix 2. US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov) search strategy
cholorhexidine AND (ICU OR SICU OR MICU)
Appendix 3. WHO International Clinical Trials Registry search strategy
chlorhexidine AND ICU OR SICU OR MICU
Appendix 4. Assessment of risk of bias (individually randomised controlled trials)
1. Was the allocation sequence randomly generated?
Low risk of bias: the investigators describe a random component in the sequence generation process such as: referring to a random number table; using a computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots.
High risk of bias: the investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number.
Unclear: insufficient information about the sequence generation process provided to permit a judgement of low or high risk of bias.
2. Was the treatment allocation adequately concealed?
Low risk of bias: participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially‐numbered drug containers of identical appearance; sequentially‐numbered, opaque, sealed envelopes.
High risk of bias: participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: use of an open random allocation schedule (e.g. a list of random numbers); assignment envelopes without appropriate safeguards (e.g. envelopes were unsealed, non‐opaque, or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear: insufficient information provided to permit a judgement of low or high risk of bias. This is usually the case if the method of concealment is not described, or not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed.
3. Blinding ‐ was knowledge of the allocated interventions adequately prevented during the study?
Low risk of bias: any one of the following.
No blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding.
Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others unlikely to introduce bias.
High risk of bias: any one of the following.
No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding.
Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, and the non‐blinding of others likely to introduce bias.
Unclear: either of the following.
Insufficient information to permit judgement of low or high risk of bias.
The study did not address this outcome.
4. Were incomplete outcome data adequately addressed?
Low risk of bias: any one of the following.
No missing outcome data.
Reasons for missing outcome data are unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias).
Missing outcome data are balanced in numbers across intervention groups, with similar reasons for missing data across groups.
For dichotomous outcome data, the proportion of missing outcomes compared with the observed event risk is not enough to have a clinically relevant impact on the intervention effect estimate.
For continuous outcome data, a plausible effect size (difference in means or standardised difference in means) among missing outcomes is not enough to have a clinically relevant impact on the observed effect size.
Missing data have been imputed using appropriate methods.
High risk of bias: any one of the following.
Reason for missing outcome data are likely to be related to the true outcome, with either an imbalance in numbers or reasons for missing data across intervention groups.
For dichotomous outcome data, the proportion of missing outcomes compared with the observed event risk is enough to induce clinically relevant bias in the intervention effect estimate.
For continuous outcome data, a plausible effect size (difference in means or standardised difference in means) among missing outcomes is enough to induce a clinically relevant bias in the observed effect size.
'As‐treated' analysis done with a substantial departure of the intervention received from that assigned at randomisation.
Potentially inappropriate application of simple imputation.
Unclear: either of the following.
Insufficient reporting of attrition/exclusions to permit a judgement of low or high risk of bias (e.g. number randomised not stated, no reasons for missing data provided).
The study did not address this outcome.
5. Are reports of the study free of suggestion of selective outcome reporting?
Low risk of bias: either of the following.
The study protocol is available and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way.
The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon).
High risk of bias: any one of the following.
Not all of the study’s prespecified primary outcomes have been reported.
One or more primary outcomes is/are reported using measurements, analysis methods, or subsets of the data (e.g. subscales) that were not prespecified.
One or more reported primary outcomes was/were not prespecified (unless clear justification for their reporting is provided, such as an unexpected adverse effect).
One or more outcomes of interest in the review is/are reported incompletely so that they cannot be entered in a meta‐analysis.
The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear: insufficient information provided to permit a judgement of low or high risk of bias. It is likely that the majority of studies will fall into this category.
6. Other sources of potential bias
Low risk of bias: the study appears to be free of other sources of bias.
High risk of bias: there is at least one important risk of bias. For example, the study:
had a potential source of bias related to the specific study design used; or
has been claimed to have been fraudulent; or
had some other problem.
Unclear: there may be a risk of bias, but there is either:
insufficient information to assess whether an important risk of bias exists; or
insufficient rationale or evidence that an identified problem will introduce bias.
Appendix 5. Assessment of risk of bias (cluster randomised controlled trials)
In cluster‐randomised trials, particular biases to consider include: (i) recruitment bias; (ii) baseline imbalance; (iii) loss of clusters; (iv) incorrect analysis; and (v) comparability with individually randomised trials.
(i) Recruitment bias can occur when individuals are recruited to the trial after the clusters have been randomised, as the knowledge of whether each cluster is an ‘intervention’ or ‘control’ cluster could affect the types of participants recruited.
(ii) Cluster‐randomised trials often randomise all clusters at once, so lack of concealment of an allocation sequence should not usually be an issue. However, because small numbers of clusters are randomised, there is a possibility of chance baseline imbalance between the randomised groups, in terms of either the clusters or the individuals. Although not a form of bias as such, the risk of baseline differences can be reduced by using stratified or pair‐matched randomisation of clusters. Reporting of the baseline comparability of clusters, or statistical adjustment for baseline characteristics, can help reduce concern about the effects of baseline imbalance.
(iii) Occasionally, complete clusters are lost from a trial, and have to be omitted from the analysis. Just as for missing outcome data in individually randomised trials, this may lead to bias. In addition, missing outcomes for individuals within clusters may also lead to a risk of bias in cluster‐randomised trials.
(iv) Many cluster‐randomised trials are analysed by incorrect statistical methods, not taking the clustering into account. Such analyses create a ‘unit of analysis error’ and produce over‐precise results (the standard error of the estimated intervention effect is too small) and P values that are too small. They do not lead to biased estimates of effect. However, if they remain uncorrected, they will receive too much weight in a meta‐analysis.
(v) In a meta‐analysis including both cluster and individually randomised trials, or including cluster‐randomised trials with different types of clusters, possible differences between the intervention effects being estimated need to be considered. For example, in a vaccine trial of infectious diseases, a vaccine applied to all individuals in a community would be expected to be more effective than if the vaccine was applied to only half of the people. Another example is provided by a Cochrane Review of hip protectors. The cluster trials showed large positive effect, whereas individually randomised trials did not show any clear benefit. One possibility is that there was a ‘herd effect’ in the cluster‐randomised trials (which were often performed in nursing homes, where compliance with using the protectors may have been enhanced). In general, such ‘contamination’ would lead to underestimates of effect. Thus, if an intervention effect is still demonstrated despite contamination in those trials that were not cluster‐randomised, a confident conclusion about the presence of an effect can be drawn. However, the size of the effect is likely to be underestimated. Contamination and ‘herd effects’ may be different for different types of cluster.
Appendix 6. References for excluded references (wrong study design)
Abad CL, Pulia MS, Krupp A, Safdar N. Reducing transmission of methicillin‐resistant staphylococcus aureus and vancomycin‐resistant enterococcus in the ICU: an update on prevention and infection control practices. Journal of Clinical Outcomes Management 2014;21(5):218‐32
Agrafiotis M, Siempos II, Falagas ME. Frequency, prevention, outcome and treatment of ventilator‐associated tracheobronchitis: systematic review and meta‐analysis. Respiratory Medicine 2010;104(3):325‐36
Akhtar Saadia R. Should ICU patients be bathed daily with chlorhexidine? Hospital Medicine Alert 2011;6(2):10‐1
Alexander S, Nieder M, Zerr DM, Fisher BT, Dvorak CC, Sung L. Prevention of bacterial infection in pediatric oncology: what do we know, what can we learn? Pediatric Blood & Cancer 2012;59(1):16‐20
Anderson DJ, Podgorny K, Berrios‐Torres SI, Bratzler DW, Dellinger EP, Greene L, et al. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infection Control and Hospital Epidemiology 2014;35(SUPPL2):605‐627
Bassetti M, Poulakou G, Timsit J‐F. Focus on antimicrobial use in the era of increasing antimicrobial resistance in ICU. Intensive Care Medicine 2016;42(6):955‐8
Biffi R, Pozzi S. Chlorhexidine‐gluconate compared with povidone‐iodine solution for disinfection of vascular catheter sites: assessment of the results of a meta‐analysis. Rivista Italiana di Nutrizione Parenterale ed Enterale 2003;21(4):159‐63
BMJ. Screening for MRSA and isolating carriers doesn't reduce ICU infections. BMJ 2013;346:f3581
BMJ. Chlorhexidine bathing cuts infections on ICU. BMJ 2013;346:f876
Brun‐Buisson C. Controlling endemic multi‐resistant bacteria: lessons from recent studies. Journal des Anti‐Infectieux 2014;16(3):144‐53
Buckley MS, Dzierba AL, Smithburger PL, McAllen KJ, Jordan CJ, Kane‐Gill SL. Chlorhexidine for the prevention of ventilator associated pneumonia in critically ill adults. Journal of Infection Prevention 2013;14(5):162‐9
Camargo LFA, Marra AR, Silva CV, Laselva CR, Moura Junior DF, Cal RGG, et al. Low compliance with alcohol gel compared with chlorhexidine for hand hygiene in ICU patients: results of an alcohol gel implementation program. Brazilian Journal of Infectious Diseases 2009;13(5):330‐4
Cassir N, Thomas G, Hraiech S, Brunet J, Fournier P‐E, Scola B, et al. Chlorhexidine daily bathing: impact on health care‐associated infections caused by gram‐negative bacteria. American Journal of Infection Control 2015;43(6):640‐3
Choi E, Park J. Effect of daily bathing with chlorhexidine on hospital‐acquired bloodstream infection in critically ill patients: a meta‐analysis of randomised controlled trials. Critical Care 2015;19(SUPPL 1):S28‐S29
Chopra V, Saint S. Vascular catheter infections: time to get technical. Lancet 2015;386(10008):2034‐6
Daneman N, Sarwar S, Fowler RA, Cuthbertson BH, on behalf of SuDDICUCanadian Study Group. Effect of selective decontamination on antimicrobial resistance in intensive care units: a systematic review and meta‐analysis. Lancet Infectious Diseases 2013;13(4):328‐41
Dean R, Dillworth J, Phillips M. Assessment of daily bathing protocols: a comparison of chlorhexidine solution and chlorhexidine impregnated cloths. Critical Care Medicine 2011 Vol. 39, No. 12 (Suppl.):142
Derde LP, Dautzenberg MJ, Van Duijn PJ, Brun‐Buisson C, Bonten MJ, on behalf of MOSAR research consortium. Tracking antibiotic‐resistance in gram positive cocci: colonisation and transmission of resistant bacteria in 13 European intensive care units. Clinical Microbiology and Infection 2011;17(SUPPL 4):S58
Doebbeling BN, Stanley GL, Sheetz CT, Pfaller MA, Houston AK, Annis L, et al. Comparative efficacy of alternative hand‐washing agents in reducing nosocomial infections in intensive care units. New England Journal of Medicine 1992;327(2):88‐93
Edgeworth JD. Has decolonization played a central role in the decline in UK methicillin‐resistant staphylococcus aureus transmission? A focus on evidence from intensive care. Journal of Antimicrobial Chemotherapy 2011;66(SUPPL. 2):ii41‐ii47
Gastmeier P. Evidence‐based infection control in the ICU (except catheters). Current Opinion in Critical Care 2007;13(5):557‐62
Joram N, De Saint Blanquat L, Stamm D, Launay E, Guen CG‐L. Healthcare‐associated infection prevention in pediatric intensive care units: a review. European Journal of Clinical Microbiology & Infectious Diseases 2012;31(10):2481‐90
Jull A. Bed baths to beat bacteria. Nursing Review (1173‐8014) 2013;13(3):14
Lowe CF, Lloyd‐Smith E, Sidhu B, Ritchie G, Sharma A, Jang W, et al. Reduction in hospital‐associated methicillin‐resistant staphylococcus aureus and vancomycin‐resistant enterococcus with daily chlorhexidine gluconate bathing for medical inpatients. American Journal of Infection Control 2017;45(3):255‐9
Morgan DJ. Choosing between methods to prevent methicillin‐resistant staphylococcus aureus in ICUs. Critical Care Medicine 2015;43(2):496‐7
Nelson MU, Bizzarro MJ, Dembry LM, Baltimore RS, Gallagher PG. One size does not fit all: why universal decolonization strategies to prevent methicillin‐resistant staphylococcus aureus colonization and infection in adult intensive care units may be inappropriate for neonatal intensive care units. Journal of Perinatology 2014;34(9):653‐5
Pittet D, Angus DC. Daily chlorhexidine bathing for critically ill patients: a note of caution. JAMA 2015;313(4):365‐6
Raines K, Rosen K. The effect of chlorhexidine bathing on rates of nosocomial Infections among the critically ill population: an analysis of current clinical research and recommendations for practice. Dimensions of Critical Care Nursing 2016;35(2):84‐91
Seyman D, Oztoprak N, Berk H, Kizilates F, Emek M. Weekly chlorhexidine douche: does it reduce healthcare‐associated bloodstream infections? Scandinavian Journal of Infectious Diseases 2014;46(10):697‐703
Vernon MO, Hayden MK, Trick WE, Hayes RA, Blom DW, Weinstein RA. Chlorhexidine gluconate to cleanse patients in a medical intensive care unit: the effectiveness of source control to reduce the bioburden of vancomycin‐resistant enterococci. Archives of Internal Medicine 2006;166(3):306‐12
Wang EW, Layon AJ. Chlorhexidine gluconate use to prevent hospital acquired infections: a useful tool, not a panacea. Annals of Translational Medicine 2017;5(1):14
Warren DK, Prager M, Munigala S, Wallace MA, Kennedy CR, Bommarito KM, et al. Prevalence of qacA/B genes and mupirocin resistance among methicillin‐resistant staphylococcus aureus (MRSA) isolates in the setting of chlorhexidine bathing without mupirocin. Infection Control and Hospital Epidemiology 2016;37(5):590‐7
Appendix 7. Statistical analysis details: hospital‐acquired infections
| Study ID | Study design | Analysis reported by study author | Definition of outcome used in the review for ‘hospital acquired infection’ | Data reported by study authors | Manipulation by review authors |
| Bleasdale 2007 | Cluster‐randomised cross‐over trial with 2 clusters and 836 participants | Multivariate models that include a term for geographical unit | BSI | Rate (per 1000 patient days) 10.4 vs 4.1, 95% CI for rate difference 1.2 to 11 | SE for rate difference calculated from CI |
| Boonyasiri 2016 | Parallel group trial of 481 participants | Individual incidence | VAP, CLABSI, and CAUTI | 28 infections during 3284 patient days vs 29 infections during 2759 patient days (adding up infections and using mean ICU stay to calculate patient days) | Rate difference and associated SE calculated using section 9.4.8 Higgins 2011 |
| Camus 2005 | 2x2 factorial trial with 256 participants in two relevant arms | Number of infections and patient days | Acquired infections | 87 infections during 1961 patient days vs 87 infections during 1991 patient days | Rate difference and associated SE calculated using section 9.4.8 Higgins 2011 |
| Climo 2013 | Cluster‐randomised cross‐over trial with 9 clusters and 7727 participants | GEE (according to SAP) | Hospital acquired BSI | Rate (per 1000 patient days) 6.60 vs 4.78, P = 0.007 | P value used to calculate Z‐value, and rate difference/ Z gives estimate of SE |
| Milstone 2013 | Cluster‐randomised cross‐over trial with 10 clusters and 4947 participants | Poisson regression adjusted for cluster and time | CLABSI | Rate (per 1000 patient days) 3 vs 1.63, rate ratio 0.52 (95% CI 0.26 to 1.08), adjusted CI 0.25 to 1.08 | Unadjusted and adjusted CI used to calculate SE for log rate ratio: from these design effect = 1.032 applied to inflate SE of rate difference |
| Noto 2015 | Cluster‐randomised cross‐over trial with 5 clusters and 9340 participants | Supplementary materials present group level analysis of clusters | Composite outcome including CLABSI, CAUTI, VAP and Clostridium difficile | Rate (per 1000 patient days) 3.35 vs 3.31, 95% CI for rate difference (‐1.19 to 1.11) | SE for rate difference calculated from CI |
| Pallotto 2018 | Parallel group trial of 449 participants | Number of infections per patient days | Composite outcome including BSI, CLABSI, UTI, CAUTI, and VAP | Rate (per 1000 patient days) 40.9 vs 23.2, P = 0.034 | P value used to calculate Z‐value, and rate difference/ Z gives estimate of SE |
| Swan 2016 | Parallel group trial of 350 participants | Hazard ratios and risk difference | Composite outcome of CAUTI, VAP, SSI, BSI | 35 infections during 2416 patient days vs 18 infections during 2332 patient days (supplementary digital content) | Rate difference and associated SE calculated using section 9.4.8 Higgins 2011 |
BSI: blood stream infection; CAUTI: catheter associated urinary tract infection; CI: confidence interval; CLABSI: central line associated blood stream infection; GEE: general estimating equation; ICU: intensive care unit; SAP: statistical analysis plan; SE: standard error; UTI: urinary tract infection; VAP: ventilator‐acquired pneumonia
Appendix 8. Analysis of rate differences: hospital‐acquired infections
| Study ID | Rate difference (control – treatment) | 95% CI | SE |
| Bleasdale 2007 | 6.3 | 1.2 to 11 | 2.5 |
| Boonyasiri 2016 | ‐1.984 | 2.53 | |
| Camus 2005 | 0.668 | 6.68 | |
| Climo 2013 | 1.82 | 0.43 to 3.13 | 0.675 |
| Milstone 2013 | 1.37 | ‐0.24 to 2.25 | 0.756 |
| Noto 2015 | 0.04 | ‐1.11 to 1.19 | 0.586 |
| Pallotto 2018 | 17.7 | 1.34 to 34.06 | 8.349 |
| Swan 2016 | 6.768 | 3.050 |
CI: confidence interval; SE: standard error
Appendix 9. Statistical analysis details: mortality
| Study ID | Data reported by study authors | Manipulation by review authors |
| Milstone 2013 | 88/2525 vs 73/2422 with unadjusted RD ‐0.48(95% CI ‐01.47 to 0.51) | Design effect = 1.032 calculated for primary outcome. This was applied to inflate standard error of log OR |
| Noto 2015 | 449/4852 vs 367/4488 with unadjusted CIl for RD ‐1.07(95% CI‐2.22 to 0.07) | Adjusted and unadjusted CI presented for RD for primary outcome: these were used to estimate design effect = 1.092 which was applied to inflate SE of log OR |
CI: confidence interval; OR: odds ratio; RD: risk difference; SE: standard error
Data and analyses
Comparison 1. Chlorhexidine bathing versus soap‐and‐water bathing.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hospital‐acquired infection | 8 | 21924 | Rate Difference (Random, 95% CI) | 1.70 [0.12, 3.29] |
| 1.1 Parallel studies | 4 | 1418 | Rate Difference (Random, 95% CI) | 4.00 [‐3.14, 11.14] |
| 1.2 Cluster randomised crossover trials | 4 | 20506 | Rate Difference (Random, 95% CI) | 1.41 [‐0.00, 2.83] |
| 2 Mortality using adjusted data | 6 | 15798 | Odds Ratio (Random, 95% CI) | 0.87 [0.76, 0.99] |
| 2.1 Parallel group studies | 4 | 1511 | Odds Ratio (Random, 95% CI) | 0.87 [0.64, 1.19] |
| 2.2 Cluster randomised crossover trials | 2 | 14287 | Odds Ratio (Random, 95% CI) | 0.87 [0.76, 1.00] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bleasdale 2007.
| Methods | Cluster‐randomised cross‐over study; single centre Setting: 2 MICUs, USA Unit of randomisation: MICU 28‐week initial phase followed by alternative bathing routine for 24 weeks 2‐week washout period |
|
| Participants |
Total number of randomised participants: 836 Inclusion criteria: all people who attended one of 2 MICUs Exclusion criteria: not reported Number of participants with an HAI before randomisation: not reported Baseline characteristics Chlorhexidine gluconate (n = 391; 3 excluded due to skin rash, but use of ITT, therefore number analysed = 391)
Soap and water (n = 445; no losses)
|
|
| Interventions | Chlorhexidine gluconate (2%)
Soap and water
Concurrent decolonisation strategies: use of sterile catheter insertion policy (without CHG coating), full barrier drapes and insertion site disinfection with CHG |
|
| Outcomes | Number of primary bloodstream infections, culture negative sepsis, laboratory confirmed bloodstream infections, nosocomial infections Notes
|
|
| Notes |
Funding/declarations of interest: funded by Sage Products (manufacturer of intervention) and by the Centers for Disease Control and Prevention Cooperative Agreement. Funding sources had no role in study design or conduct, data collection, management and interpretation of results Study dates: June 2005 to June 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | MICU was randomly selected; methods of randomisation were not reported |
| Allocation concealment (selection bias) | High risk | Only two units of randomisation, therefore not feasible to conceal allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Hospital personnel were unblinded to intervention groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Some outcome assessors were unblinded but consensus was reached with four investigators and potential bias in the results investigated with computer software |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study authors report a loss of three participants due to skin reactions. We included these data in the review for adverse events. |
| Selective reporting (reporting bias) | Low risk | Prospective clinical trial registration, NCT00130221. Outcomes reported according to clinical trial register documents |
| Other bias | Low risk | Appropriate washout period was used for cross‐over design |
| Recruitment bias (cluster trials only) | Low risk | MICUs were geographically separate |
| Baseline Imbalance (cluster trials only) | Low risk | Baseline characteristics appeared comparable between each cluster |
| Loss of clusters (cluster trials only) | Low risk | No loss of clusters |
| Incorrect analysis (cluster trials only) | Low risk | Appropriate analysis was used to account for study design |
Boonyasiri 2016.
| Methods | RCT; multicentre; parallel design Setting: 4 ICUs (to include MICU, respiratory care unit and cardiac care units), Thailand |
|
| Participants |
Total number of randomised participants: 481 Inclusion criteria: aged ≥ 18 years with expected stay in MICU > 48 hours, swab samples collected within 48 hours Exclusion criteria: allergic to chlorhexidine, extensive skin lesions, unable to receive routine bathing Number of participants with an HAI before randomisation: HAIs not reported but study authors report prevalence of MDR bacterial colonization at enrolment, not broken down by number of participant but balanced between groups Baseline characteristics Chlorhexidine gluconate (n = 240 randomised; 51 losses; number analysed = 189)
Soap and water (n = 241 randomised; 42 losses; number analysed = 199)
|
|
| Interventions | Chlorhexidine gluconate (2%)
Soap and water
Concurrent decolonisation strategies: standard hygiene practices with adherence during study period reported at 70% |
|
| Outcomes | Favourable events, target MDR bacteria colonisation‐free time, hospital‐acquired infections (VAP, CLABSI, CAUTI), length of ICU stay, length of hospital stay, adverse skin reactions Notes
|
|
| Notes |
Funding/declarations of interest: Health Systems Research and Development Project, Siriraj Hospital; Health Systems Research Institute; Thai Health Promotion Foundation; International Development Research Centre Study dates: December 2013 to January 2015 Notes
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was generated by a computer using a block size of 4 |
| Allocation concealment (selection bias) | Low risk | Use of sequentially numbered, sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study is described as open‐label and study authors do not report methods used to blind personnel to intervention groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome assessed by laboratory workers who were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Large number of participants not included in analysis |
| Selective reporting (reporting bias) | Low risk | Prospective clinical trial registration (NCT01989416). Limited information in clinical trials register documents. Primary outcome is reported according to these documents, other outcomes are not included in documents |
| Other bias | Low risk | No other sources of bias identified |
Camus 2005.
| Methods | RCT; multicentre; parallel design Setting: 3 MICUs, university‐affiliated hospitals, France |
|
| Participants |
Total number of randomised participants: 257 Inclusion criteria: aged > 18 years, intubated for < 48 hours, likely to require mechanical ventilation for > 48 hours Exclusion criteria: pregnant women, those with a SAPS II of > 80, life expectancy of < 48 hours resulting from brain death or a palliative treatment, a polymorphonuclear count of < 500 cells/mm³, severe diarrhoea, and anyone who had received either a prior decontamination regimen or was participating in another ongoing clinical trial Number of participants with an HAI before randomisation: 31 chlorhexidine gluconate group; 43 soap‐and‐water group Baseline characteristics Chlorhexidine gluconate (n = 130; no losses)
Soap and water (n = 127; 1 loss; number analysed = 126)
|
|
| Interventions | Chlorhexidine gluconate (4%)
Soap and water
Concurrent decolonisation strategies: standard hygiene methods and isolation procedures |
|
| Outcomes | Number of hospital‐acquired infections (reported for 1 infection and multiple infections), probability of freedom from acquired infection, incidence rates of total and device‐related infections, ICU mortality rate, adverse events (to include skin allergy), length of stay in ICU (reported as median and range) Notes
|
|
| Notes |
Funding/declarations of interest: supported in part by the Programme Hospitalier de Recherche Clinique, and GlaxoSmithKline. Some study drugs provided by AstraZeneca Study dates: April 1996 to October 1998 Notes
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomised using a computer‐generated list. |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Attempts to blind personnel are described: antibiotic placebo (gelatin) was in an opaque vial supplied by the pharmacy; non‐antiseptic liquid soap was dyed and perfumed to mimic appearance of CHG |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss of 1 participant, unlikely to affect results |
| Selective reporting (reporting bias) | Unclear risk | Clinical trial registration or prepublished protocol not reported. Not feasible to judge risk of selective reporting bias |
| Other bias | High risk | Intervention group also includes treatment with mupirocin. Control group does not include mupirocin. This may act as a confounder in the results |
Climo 2013.
| Methods | Cluster‐randomised cross‐over trial; multicentre Setting: 8 ICUs (medical, surgical, coronary care and cardiac surgery) and 1 bone marrow transplantation unit in 6 hospitals, USA Unit of randomisation: ICU 6‐month bathing regime followed by alternate bathing regime for 6 months No washout period. However, any new infections and MDRO acquisitions for 2 days after cross‐over were assigned to the first bathing regime |
|
| Participants |
Total number of randomised participants: inconsistently reported in paper. We have used number of randomised participants as 7812 (study abstract reports 7727 participants) Inclusion criteria: all people admitted to study units during study interval (information taken from prospective clinical trial register report) Exclusion criteria: known allergies to CHG or other ingredients in the product; burns that include a high percentage of disrupted body surface area, if undergoing lumbar punctures or contact with meninges, large open skin wounds, children aged < 18 years (information taken from prospective clinical trial register report) Number of participants with an HAI before randomisation: not reported Baseline characteristics Chlorhexidine gluconate (n = 3970 randomised; losses not reported, ITT analysis)
Soap and water (n = 3842 randomised, losses not reported, ITT analysis)
|
|
| Interventions | Chlorhexidine gluconate (2%)
Soap and water
|
|
| Outcomes | Rate of infection (MRSA, VRE and hospital‐acquired bloodstream infections), adverse effects (to include skin reactions), length of stay Notes
|
|
| Notes |
Funding/declarations of interest: co‐operative programme grant from Centers for Disease Control and Prevention, and Sage Products (manufacturers of intervention and control products). Authors have received various grants from pharmaceutical companies, all declared in study report. Sage Products provided technical and education support but were not involved in any study design, data analysis or preparation of the manuscript. Study dates: August 2007 to February 2009 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised (each cluster was randomly assigned); no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Investigators and clinical staff were aware of treatment groups |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details of whether personnel who assessed HAIs were blinded. Unblinded nursing personnel assessed participants for skin reactions |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No losses reported and study authors report that all randomised participants were analysed as intention‐to‐treat. However, some discrepancies in number of participants throughout the paper; the study authors note that there were 7727 participants in ITT analysis, yet elsewhere they report that 7812 participants were treated and analysed. |
| Selective reporting (reporting bias) | Unclear risk | Prospective clinical trial registration NCT00502476. Online protocol linked to the full report in the New England Journal of Medicine, which appears to be prospectively prepared. Skin reactions is not listed as an outcome in the protocol but is reported in the full text. |
| Other bias | Unclear risk | Study authors reported no wash‐out period. However, infections and MDRO acquisitions were monitored for 2 days after transition and any new infections were assigned to the previous treatment. |
| Recruitment bias (cluster trials only) | Unclear risk | Each cluster was a separate ICU, in 6 hospitals, but we could not be certain that there was no risk of recruitment bias to a particular ICU (particularly when more than one cluster/ICU was located in the same hospital). |
| Baseline Imbalance (cluster trials only) | Unclear risk | We were unable to assess whether clusters were comparable. |
| Loss of clusters (cluster trials only) | High risk | Study reports that 12 units were initially randomised, however, one unit withdrew from the study and two units were withdrawn from analysis because of low compliance with the study protocol. |
| Incorrect analysis (cluster trials only) | Low risk | Appropriate analysis used for study design |
Milstone 2013.
| Methods | Cluster‐randomised cross‐over trial; multi‐centre Setting: 10 paediatric ICUs (medical, surgical and cardiac) in 5 hospitals, USA Unit of randomisation: ICU 6‐month bathing regime followed by alternate bathing regime for 6 months 2‐week washout |
|
| Participants |
Total number of randomised participants: 4947 Inclusion criteria: children admitted to any of the included ICUs with an anticipated stay of > 48 hours Exclusion criteria: children younger than 2 months old, had an indwelling epidural or lumbar drain, severe skin diseases or burns, or had an allergy to chlorhexidine Number of participants with an HAI before randomisation: not reported Baseline characteristics Chlorhexidine gluconate (n = 2422 randomised; 875 losses after randomisation but ITT analysis used)
Soap and water (n = 2525 randomised, no losses reported)
|
|
| Interventions | Chlorhexidine gluconate (2%)
Soap and water
|
|
| Outcomes | Events of bacteraemia, primary CLABSI, rates of surgical‐site infections, incidence of methicillin‐resistant staphylococcus aureus and vancomycin‐resistant enterococci, skin rashes, mortality Notes
|
|
| Notes |
Funding/declarations of interest: grant from Sage Products (manufacturers of intervention). Additional funding from National Institutes for Health and National Center for Research Resources. Others have received grant support from Sage Products, as well as other pharmaceutical companies. Sage Products had no role in study design, data collection, data analysis, data interpretation or writing of the report Study dates: February 2008 to September 2010 Notes
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generator (Microsoft Excel 2007) to assign ICUs to treatment or control |
| Allocation concealment (selection bias) | Unclear risk | Assignment concealed until unit agreed to participate, with no additional details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked trial: after assignment, hospital personnel were informed as to the regime. It is unclear if participants remained blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors for HAIs were masked (assessment of laboratory data). Clinical care teams assessed skin reactions, unclear if they were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | A large number of participants in the treatment group were not included in the per‐protocol analysis due to lack of consent. An ITT analysis was completed. Study authors report per‐protocol analysis data and ITT analysis which are comparable |
| Selective reporting (reporting bias) | Unclear risk | Prospective clinical trial registration (NCT00549393). Skin reactions and additional HAIs not listed as secondary outcomes in trial registration report |
| Other bias | Low risk | Appropriate washout period for cross‐over study design |
| Recruitment bias (cluster trials only) | Unclear risk | Clusters were separate ICUs, in 5 different hospitals, but we could not be certain that there was no risk of recruitment bias to a particular ICU (particularly when more than one cluster/ICU was located in the same hospital). |
| Baseline Imbalance (cluster trials only) | Unclear risk | Study authors reported baseline characteristics of each unit, and we noted some differences in characteristics (e.g. people in the bone marrow transplant unit had a longer mean length of stay); we could not be certain whether this would influence overall results |
| Loss of clusters (cluster trials only) | Low risk | No loss of clusters |
| Incorrect analysis (cluster trials only) | Low risk | Appropriate analysis used to account for study design |
Noto 2015.
| Methods | Cluster‐randomised crossover trial; single centre Setting: 5 ICUs (cardiovascular, medial, neurological, surgical, trauma) at a tertiary care medical centre, USA Unit of randomisation: ICUs within a single centre Alternating 10‐week intervention phase and 10‐week control phase. Two phases of each trial arm |
|
| Participants |
Total number of randomised participants: 9340 Inclusion criteria: all those admitted to various adult ICUs within the tertiary centre Exclusion criteria: allergy to chlorhexidine, burns or toxic epidermal necrolysis, Stevens‐Johnson syndrome, or the treating physician advised against it. Those admitted during a washout period were also excluded from primary analysis Number of participants with an HAI before randomisation: not reported Baseline characteristics Chlorhexidine gluconate (n = 4488; no losses)
Soap and water (n = 4852 randomised; no losses)
|
|
| Interventions | Chlorhexidine gluconate (2%)
Soap and water
Concurrent decolonisation strategies: cardiovascular ICU used chlorhexidine for preoperative cleaning as routine regardless of assignment to treatment/control arm. Contact precautions were followed for any person known to be colonised or infected with MDROs |
|
| Outcomes | Number of hospital‐acquired infections (to include CLABSI, CAUTI, VAP, C‐difficile infection), rates of each infection, hospital and ICU length of stay, rates of clinical cultures positive for multi‐drug‐resistant organisms, blood culture contamination, healthcare associated bloodstream infections, rates of primary outcome by ICU Notes
|
|
| Notes |
Funding/declarations of interest: grants from National Institute of Health and through the Vanderbilt Institute for Clinical and Translational Research. Sponsors had no role in design and conduct of the study. Study dates: July 2012 to July 2013 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Cluster‐randomised cross‐over study design: "Each unit was randomised to a bathing sequence by generating 5 numbers from 1 to 2 at random using software available at www.randomizer.org." Appropriate study design to assess the intervention |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants or staff who ran the units or applied the treatments |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Infection control personnel responsible for assessing infection outcomes were blinded to treatment assignments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants excluded if they were admitted during washout period. Some protocol violations for which study authors had conducted sensitivity analyses. No participant loss and data reported as ITT |
| Selective reporting (reporting bias) | Unclear risk | Study was retrospectively registered with a clinical trial register (NCT02033187). It was not feasible to assess risk of selective reporting bias |
| Other bias | Low risk | No other sources of bias identified |
| Recruitment bias (cluster trials only) | Unclear risk | Clusters were separate units, but we could not be certain that there was no risk of recruitment bias to a particular ICU because ICU clusters were located in the same hospital |
| Baseline Imbalance (cluster trials only) | Unclear risk | Baseline characteristics appeared comparable between intervention and control group but were not reported by cluster and therefore we could not be certain of comparability between clusters |
| Loss of clusters (cluster trials only) | Low risk | No loss of clusters |
| Incorrect analysis (cluster trials only) | Low risk | Appropriate analysis used for study design |
Pallotto 2018.
| Methods | RCT; single centre; parallel design Setting: ICU and postoperative cardiosurgical ICU, Italy |
|
| Participants |
Total number of randomised participants: 449 Inclusion criteria: ICU stay for at least one night, aged > 18 years Exclusion criteria: known allergy to chlorhexidine: admission diagnosis of burns, toxic epidermal necrolysis or Stevens‐Johnson syndrome; pregnancy; aged < 18 years Numbers of participants with an HAI before randomisation: not reported Baseline characteristics Chlorhexidine gluconate (n = 226; no losses; number analysed = 226)
Soap and water (n = 223; no losses; number analysed = 223)
|
|
| Interventions | Chlorhexidine gluconate (4%)
Soap and water
"This infection control intervention was the only one in progress during the study period" |
|
| Outcomes | Incidence of HAI (cumulative incidence of BSI, CLABSI, UTI, CAUTI, and VAP); incidence of VAP, BSI, CLABSI, UTI, CAUTI; time until infection; adverse events; mortality; length of ICU stay | |
| Notes |
Funding/declarations of interest: no external funding, and authors declared no conflicts of interest Study dates: August 2015 to April 2016 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of a computer‐generated randomization list |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Personnel were unblinded to intervention groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded to intervention groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses |
| Selective reporting (reporting bias) | Unclear risk | Retrospective registration with clinical trial registration (in August 2018) ‐ NCT03639363. Not feasible to assess risk of selective reporting bias using these documents |
| Other bias | Low risk | No other sources of bias identified |
Swan 2016.
| Methods | RCT; single centre; parallel design Setting: SICU, 24‐bed unit, USA |
|
| Participants |
Total number of randomised participants: 350 Inclusion criteria: aged ≥ 18 years with anticipated SICU stay ≥ 48 hours Exclusion criteria: Braden Scale for Predicting Pressure Sore Risk score < 9, pregnancy, skin irritation that precluded chlorhexidine bathing, chlorhexidine allergy, or SICU stay > 48 hours prior to screening Participants who had HAI before randomisation: BSIs: 4 CHG group; 3 control group. VAP: 3 CHG group; 3 control group. SSIs: 3 CHG group; 9 control group. CAUTIs: 9 CHG group; 13 control group (data taken from supplementary appendix provided with online full study report) Baseline Characteristics Chlorhexidine gluconate (n = 175; 13 losses, number analysed = 161)
Soap and water (n = 175; 11 losses, number analysed = 164)
|
|
| Interventions | Chlorhexidine gluconate (2%)
Soap and water
Concurrent decolonisation strategies: washbasins discarded after each use |
|
| Outcomes | Number of participants with at least one hospital‐acquired infection (also reported number with one infection and number with 2 infections), data for specific infections (CAUTI, VAP, SSI, primary bloodstream infections), length of stay in SICU, hospital stay, in‐hospital mortality, mortality during study period, adverse skin occurrences Notes
|
|
| Notes |
Funding/declarations of interest: Funded by intramural grant from Houston Methodist Research Institute, US. Funder had no role in study design, data collection and analysis Study dates: July 2012 to May 2013 Notes
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was completed using Microsoft Excel 2007 |
| Allocation concealment (selection bias) | Unclear risk | "Investigators opened folders only after a patient was enrolled and study numbers were assigned in order. Folder contents were not visible prior to opening" Unclear if this method is sufficient to ensure allocation is concealed. Folders are not described as sealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Patients and bedside clinicians were aware of treatment‐group assignment" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators who determined efficacy and safety outcomes were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11 participants in the control group and 13 participants in the CHG group were excluded after randomisation as they had been previously enrolled. One participant withdrew from the CHG group before treatment started. Reasons are justifiable and do not increase risk of bias |
| Selective reporting (reporting bias) | Low risk | Prospective clinical trial registration NCT01640925. Outcomes all reported according to registration report |
| Other bias | Low risk | No other sources of bias identified |
APACHE II: Acute Physiology and Chronic Health Evaluation II; BSI: blood‐stream infection; CAUTI: catheter‐associated urinary tract infection; CDC: Centers for Disease Control and Prevention; CHG: chlorhexidine; CLABSI: central line‐associated blood stream infection; GCS: Glasgow coma score; HAI: hospital‐acquired infection; ICU: intensive care unit; IQR; interquartile range; MDR: multi‐drug resistant; MDRO: multi‐drug resistant organism; M/F: male/female; MICU: medical intensive care unit; MRSA: methicillin‐resistant staphylococcus aureus; n: number of participants; NHSN: National Healthcare Safety Network; RCT: randomised controlled trial; SAPS II: Simplifed Acute Physiology Score II; SD: standard deviation; SICU: surgical intensive care unit; SOFA: Sequential Organ Failure Assessment; SSI: surgical site infection; VAP: ventilator‐associated pneumonia; VRE: vancomycin‐resistant enterococci
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Camus 2011 | RCT, adult participants in ICU, intervention group received repeated MRSA screening, contact and droplet isolation precautions if at risk of MRSA at ICU admission and for MRSA‐positive people, and decontamination with nasal mupirocin and chlorhexidine body wash for MRSA‐positive people. Ineligible intervention |
| Choi 2012 | RCT, participants in ICU, use of chlorhexidine bathing specifically in perineal area to prevent CAUTIs |
| Cunha 2008 | RCT, bathing with chlorhexidine or control of neonates for their first bath. Not critically ill |
| Dean 2011 | RCT, participants in ICU, bathing with chlorhexidine solution compared with bathing using chlorhexidine‐impregnated wash‐cloths. No eligible comparison group |
| Derde 2014 | Interrupted time‐series study with all participants receiving chlorhexidine bathing and then randomised to receive a MRSA and VRE screening procedure |
| Duzkaya 2017 | RCT, participants in a paediatric ICU, periurethral cleansing with chlorhexidine or povidone‐iodine or water prior to placement of urinary catheter |
| Huang 2013 | RCT, participants in ICU, treatment groups were: MRSA screening and isolation; targeted decolonisation with chlorhexidine and mupirocin of MRSA‐carriers; universal decolonisation with chlorhexidine and mupirocin of all participants. No control group described as bathing with soap and water or receiving no bathing |
| Lowe 2017 | Prospective non‐randomised cross‐over study, participants in ICU, bathing routine with chlorhexidine for seven months then bathing routine with soap and water |
| Sankar 2009 | RCT, preterm low‐weight infants wiped once with chlorhexidine or saline or nothing within 3 hours of birth |
CAUTI: catheter‐associated urinary tract infection; ICU: intensive care unit; MRSA: methicillin‐resistant staphylococcus aureus; RCT: randomised controlled trial; VRE: vancomycin‐resistant enterococci
Characteristics of studies awaiting assessment [ordered by study ID]
ChiCTR‐TRC‐13004164.
| Methods | RCT, parallel design |
| Participants | Inclusion criteria: participants more than 18 years of age with an expected length of stay more than 48 hours in the ICU, written informed consent obtained from the family of the participants, basic screening of colonising bacteria obtained during the first 2 hours after admission to the ICU |
| Interventions | Daily body bathing with chlorhexidine versus daily body bathing with soap and water and routine care |
| Outcomes | Nosocomial infections in the ICU, ICU‐attributable blood stream infections |
| Notes | Registered in clinical trial register. Described recruitment as completed but we have not been able to source a published full‐text of this report. We have contacted study authors by email to request details |
ICU: intensive care unit; RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
IRCT2017030932293N1.
| Trial name or title | Chlorhexidine effect on skin colonization |
| Methods | RCT, parallel design |
| Participants |
Target number of randomised participants: 80 Inclusion criteria: people with multiple trauma, 20 to 60 years of age, hospitalised for ≥ 48 hours Exclusion criteria: people had an infectious disease or diabetes, pregnancy, discharge or transfer to another ward < 48 hours |
| Interventions | Daily body bathing with 2% chlorhexidine. Control not described |
| Outcomes | Pathogen colonisation (in culture), type of micro‐organisms (in culture), drug resistance (using antibiotic sensitivity test), length of hospital stay |
| Starting date | April 2017 |
| Contact information | Hamed Sarani; Khatm‐al‐anbia Hospital; sarani@zaums.ac.ir |
| Notes |
NCT02870062.
| Trial name or title | Impact of daily bathing with chlorhexidine in the critical patient |
| Methods | RCT, parallel design |
| Participants |
Target number of randomised participants: 40 Inclusion criteria: people admitted to MICU, < 48 hours in the MICU, ≥ 18 years of age Exclusion criteria: people with burns > 20% body surface, pregnancy, recorded allergy history to chlorhexidine |
| Interventions | Daily bathing with 2% chlorhexidine wipes, plus oral spray with 0.2% chlorhexidine chlorhydrate and 0.12% chlorhexidine shampoo versus bathing with wipes, oral spray, and shampoo not containing chlorhexidine. Control products have same labels and same smell as intervention products |
| Outcomes | Pathogen colonisation (in culture); HAIs; pathogen clonal relationship, biofilm index, and susceptibility to chlorhexidine |
| Starting date | August 2016 |
| Contact information | Michel Fernando Martinez‐Resendez, Universidad Autonoma de Nuevo Leon |
| Notes |
HAI: hospital‐acquired infection; MICU: medical intensive care unit; RCT: randomised controlled trial
Differences between protocol and review
We made the following changes from the protocol (Lewis 2016).
New author: we added an additional author to the review (Sarah Rhodes).
Criteria for considering studies in this review: we did not exclude studies in which participants were diagnosed with a hospital‐acquired infection (HAI) prior to randomisation. This was not reported in four studies (Bleasdale 2007; Climo 2013; Milstone 2013; Noto 2015), such that we could not be certain whether participants in these studies had been monitored for an HAI at enrolment; and two included studies reported a small number of participants with some infections at baseline (Camus 2005; Swan 2016). We included all studies, but collected baseline data on HAI as reported by study authors. However, we believe this indicated indirectness, and we downgraded the certainty of the evidence for this reason. We excluded studies of neonates because these participants have a different set of critical care needs. We specified the exclusion of studies in which only one body part was bathed; our intention was to look at the effect of chlorhexidine when used for bathing of all body areas.
The original protocol stated 'For studies with a cross‐over design, we will only include data from the first intervention period, i.e. before cross‐over to the alternative treatment'. We did not anticipate that we would identify any cluster‐randomised crossover trials; however it was felt that the cluster‐randomised cross‐over trial was a valid design to answer the research question, given that the same participants were unlikely to be included in both the intervention and control period. We decided post‐hoc to include, where possible, both periods of cluster‐randomised cross‐over trials, using methods described in Unit of analysis issues.
Data synthesis: in the protocol we stated that the primary outcome (hospital‐acquired infections) would be analysed using odds ratios. Most of the trials reported infections using number of events and rates rather than number of people having at least one infection; this included several cluster‐randomised cross‐over trials that analysed infections using rate differences with appropriately adjusted confidence intervals. In order to utilise data from as many trials as possible, and to incorporate the cluster‐randomised cross‐over trials, we chose to use rate differences as the summary statistic for the primary outcome.
Sensitivity analysis: because of the inclusion of cluster‐randomised cross‐over studies, and subsequent changes to analysis of data, we used sensitivity analysis to assess the inclusion of such studies. We expanded the sensitivity analysis to include analysis of a secondary outcome (mortality) because we also included cluster‐randomised cross‐over studies in this analysis.
Contributions of authors
Sharon R Lewis: conceived, designed and coordinated the review; extracted data; checked the quality of data extraction; analysed or interpreted data; undertook and checked quality assessment; produced the first draft of the review; contributed to writing and editing the review; and wrote to study authors/experts/companies.
Oliver J Schofield‐Robinson: extracted data; checked the quality of data extraction; analysed or interpreted data; undertook and checked quality assessment; produced the first draft of the review; and wrote to study authors/experts/companies.
Sarah Rhodes: analysed or interpreted data; performed statistical analysis; checked the quality of the statistical analysis; produced the first draft of the review; and contributed to writing or editing the review.
Andrew Smith: checked the quality of data extraction; checked quality assessment; produced the first draft of the review; advised on the review; secured funding; approved the final review prior to submission; and is a guarantor of the review.
Contributions of the editorial base
Joan Webster (Editor): edited the protocol; advised on methodology, interpretation and content; approved the final protocol prior to submission.
Jo Dumville (Coordinating Editor): edited the review; advised on methodology, interpretation and content; approved the final review prior to submission.
Gill Rizzello (Managing Editor): coordinated the editorial process; advised on content; edited the protocol and the review.
Reetu Child, Naomi Shaw and Sophie Bishop (Information Specialists): designed the search strategy, ran the search and edited the search methods section.
Ursula Gonthier (Editorial Assistant): edited the Plain language summary and reference sections of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Wounds. The views and opinions expressed are those of the authors and not necessarily those of the NIHR, NHS or the Department of Health and Social Care, UK.
NIHR Cochrane Programme Grant 13/89/16 'Back to normal': speed and quality of recovery after surgery, major injury and critical care, UK.
-
National Institute for Health Research Collaboration for Leadership in Applied Research and Care (NIHR CLAHRC) Greater Manchester, UK.
Sarah Rhodes was partly funded by the NIHR CLAHRC Greater Manchester. The funder had no role in the design of the studies, data collection and analysis, decision to publish, or preparation of the manuscript. However, the review may be considered to be affiliated to the work of the NIHR CLAHRC Greater Manchester. The views expressed herein are those of the authors and not necessarily those of the NHS, NIHR or the Department of Health.
Declarations of interest
Sharon R Lewis: my work on this review is funded by the National Insitute for Health Research (NIHR) Cochrane programme grant 13/89/16 ('Back to normal': speed and quality of recovery after surgery, major injury and critical care).
Oliver J Schofield‐Robinson: my work on this review is funded by the National Insitute for Health Research (NIHR) Cochrane programme grant 13/89/16 ('Back to normal': speed and quality of recovery after surgery, major injury and critical care).
Sarah Rhodes: my salary is funded by the NIHR through three different grants, partly to provide statistical support to the CLAHRC Wounds Group.
Andrew F Smith: NIHR Cochrane Collaboration programme grant for programme of reviews in perioperative care.
Edited (no change to conclusions)
References
References to studies included in this review
Bleasdale 2007 {published data only}
- Bleasdale SC, Trick WE, Gonzalez IM, Lyles RD, Hayden MK, Weinstein RA. Effectiveness of chlorhexidine bathing to reduce catheter‐associated bloodstream infections in medical intensive care unit patients. Archives of Internal Medicine 2007;167(19):2073‐9. [PUBMED: 17954801] [DOI] [PubMed] [Google Scholar]
Boonyasiri 2016 {published data only}
- Boonyasiri A, Thaisiam P, Permpikul C, Judaeng T, Suiwongsa B, Apiradeewajeset N, et al. Effectiveness of chlorhexidine wipes for the prevention of multidrug‐resistant bacterial colonization and hospital‐acquired infections in intensive care unit patients: a randomized trial in Thailand. Infection Control and Hospital Epidemiology 2016;37(3):245‐53. [PUBMED: 26894621] [DOI] [PubMed] [Google Scholar]
Camus 2005 {published data only}
- Camus C, Bellissant E, Sebille V, Perrotin D, Garo B, Legras A, et al. Prevention of acquired infections in intubated patients with the combination of two decontamination regimens. Critical Care Medicine 2005;33(2):307‐14. [PUBMED: 15699832] [DOI] [PubMed] [Google Scholar]
- Camus C, Sebille V, Legras A, Garo B, Renault A, Corre P, et al. Mupirocin/chlorhexidine to prevent methicillin‐resistant Staphylococcus aureus infections: post hoc analysis of a placebo‐controlled, randomized trial using mupirocin/chlorhexidine and polymyxin/tobramycin for the prevention of acquired infections in intubated patients. Infection 2014;42(3):493‐502. [PUBMED: 24464791] [DOI] [PubMed] [Google Scholar]
Climo 2013 {published data only}
- Climo MW, Yokoe DS, Warren DK, Perl TM, Bolon M, Herwaldt LA, et al. Effect of daily chlorhexidine bathing on hospital‐acquired infection. New England Journal of Medicine 2013;368(6):533‐42. [PUBMED: 23388005] [DOI] [PMC free article] [PubMed] [Google Scholar]
Milstone 2013 {published data only}
- Milstone AM, Elward A, Song X, Zerr DM, Orscheln R, Speck K, et al. Daily chlorhexidine bathing to reduce bacteraemia in critically ill children: a multicentre, cluster‐randomised, crossover trial. Lancet 2013;381(9872):1099‐1106. [PUBMED: 23363666] [DOI] [PMC free article] [PubMed] [Google Scholar]
Noto 2015 {published data only}
- Noto M, Domenico H, Talbot T, Byrne D, Wheeler A. Healthcare‐associated infections and chlorhexidine bathing ‐ a pragmatic cluster‐randomized trial. Critical Care Medicine 2014;42(12):A1478‐9. [Abstract 493] [Google Scholar]
- Noto MJ, Domenico HJ, Byrne DW, Talbot T, Rice TW, Bernard GR, et al. Chlorhexidine bathing and health care‐associated infections: a randomized clinical trial. Journal of the American Medical Association 2015;313(4):369‐78. [PUBMED: 25602496] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pallotto 2018 {published data only}
- Pallotto C, Fiorio M, Angelis V, Ripoli A, Franciosini E, Quondam Girolamo L, et al. Daily bathing with 4% chlorhexidine gluconate in intensive care settings: a randomized controlled trial. Clinical Microbiology and Infection 2018 Sept 26 [Epub ahead of print]. [DOI: 10.1016/j.cmi.2018.09.012; PUBMED: 30267930] [DOI] [PubMed]
Swan 2016 {published data only}
- Bui L, Badawi N, Swan J, Bersamin J. Preliminary results of a randomized controlled trial comparing the incidence of nosocomial infections with chlorhexidine bathing versus standard bathing in the surgical intensive care unit. Journal of the American Pharmacists Association 2013;53(2):e82‐e83. [Google Scholar]
- NCT01640925. Randomized controlled trial of 2% chlorhexidine bathing on nosocomial infections in the surgical intensive care unit. clinicaltrials.gov/show/NCT01640925 (first received 16 July 2012).
- Swan J, Bui L, Pham V, Shirkey B, Graviss E, Hai S, et al. RCT of chlorhexidine vs. soap & water bathing for prevention of hospital‐acquired infections in SICU. Critical Care Medicine 2014;42(12 (Suppl 1)):A1369‐70. [Google Scholar]
- Swan JT, Ashton CM, Bui LN, Pham VP, Shirkey BA, Blackshear JE, et al. Effect of chlorhexidine bathing every other day on prevention of hospital‐acquired infections in the surgical ICU: a single‐center, randomized controlled trial. Critical Care Medicine 2016;44(10):1822‐32. [PUBMED: 27428384] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Camus 2011 {published data only}
- Camus C, Bellissant E, Legras A, Renault A, Gacouin A, Lavoué S, et al. Randomized comparison of 2 protocols to prevent acquisition of methicillin‐resistant Staphylococcus aureus: results of a 2‐center study involving 500 patients. Infection Control and Hospital Epidemiology 2011;32(11):1064‐72. [PUBMED: 22011532] [DOI] [PubMed] [Google Scholar]
Choi 2012 {published data only}
- Choi JS, Yeon JH. Effects of perineal care in preventing catheter associated urinary tract infections (CAUTI) in intensive care units (ICU). Journal of Korean Academy of Fundamentals of Nursing 2012;19(2):223‐32. [Google Scholar]
Cunha 2008 {published data only}
- Cunha ML, Procianoy RS, Franceschini DT, Oliveira LL, Ballin A, Livshiz V, et al. Effect of the first bath with chlorhexidine on skin colonization with Staphylococcus aureus in normal healthy term newborns. Scandinavian Journal of Infectious Diseases 2008;40(8):615‐20. [PUBMED: 18979599] [DOI] [PubMed] [Google Scholar]
Dean 2011 {published data only}
- Dean R, Dillworth J, Phillips M. Assessment of daily bathing protocols: a comparison of chlorhexidine solution and chlorhexidine impregnated cloths. Critical Care Medicine 2011;39(12 (Suppl)):142. [Google Scholar]
Derde 2014 {published data only}
- Derde LP, Cooper BS, Brun‐Buisson C, Bonten MJ. Reducing acquisition of resistant bacteria in intensive cares: a European cluster randomised trial. Clinical Microbiology and Infection 2012;18(Suppl 3):712. [Google Scholar]
- Derde LP, Cooper BS, Goossens H, Malhotra‐Kumar S, Willems RJ, Gniadkowski M, et al. Interventions to reduce colonisation and transmission of antimicrobial‐resistant bacteria in intensive care units: an interrupted time series study and cluster randomised trial. Lancet Infectious Diseases 2014;14(1):31‐9. [PUBMED: 24161233] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derde LP, Dautzenberg MJ, Duijn PJ, Brun‐Buisson C, Bonten MJ. Antimicrobial resistance in ICU‐acquired bacteraemias in 13 European intensive care units. Clinical Microbiology and Infection 2011;17(Suppl 4):S423. [Poster 1490] [Google Scholar]
Duzkaya 2017 {published data only}
- Duzkaya DS, Uysal G, Bozkurt G, Yakut T, Citak A. Povidone‐iodine, 0.05% chlorhexidine gluconate, or water for periurethral cleaning before indwelling urinary catheterization in a pediatric intensive care: a randomized controlled trial. Journal of Wound, Ostomy, and Continence Nursing 2017;44(1):84‐8. [PUBMED: 27824737] [DOI] [PubMed] [Google Scholar]
Huang 2013 {published data only}
- Hayden MK, Lolans K, Haffenreffer K, Avery TR, Kleinman K, Li H, et al. Chlorhexidine and mupirocin susceptibility of methicillin‐resistant staphylococcus aureus isolates in the REDUCE‐MRSA trial. Journal of Clinical Microbiology 2016;54(11):2735‐42. [PUBMED: 27558180] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SS, Septimus E, Kleinman K, Moody J, Hickok J, Avery TR, et al. Targeted versus universal decolonization to prevent ICU infection. The New England Journal of Medicine 2013;368(24):2255‐65. [PUBMED: 23718152] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lowe 2017 {published data only}
- Lowe CF, Lloyd‐Smith E, Sidhu B, Ritchie G, Sharma A, Jang W, et al. Reduction in hospital‐associated methicillin‐resistant Staphylococcus aureus and vancomycin‐resistant Enterococcus with daily chlorhexidine gluconate bathing for medical inpatients. American Journal of Infection Control 2017;45(3):255‐9. [PUBMED: 27938986] [DOI] [PubMed] [Google Scholar]
Sankar 2009 {published data only}
- Sankar MJ, Paul VK, Kapil A, Kalaivani M, Agarwal R, Darmstadt GL, et al. Does skin cleansing with chlorhexidine affect skin condition, temperature and colonization in hospitalized preterm low birth weight infants? A randomized clinical trial. Journal of Perinatology 2009;29(12):795‐801. [PUBMED: 19710679] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
ChiCTR‐TRC‐13004164 {published data only}
- ChiCTR‐TRC‐13004164. The efficacy of daily chlorhexidine body bathing for reducing nosocomial infections in intensive care units. www.chictr.org.cn/showproj.aspx?proj=5404 (first received 13 December 2013).
References to ongoing studies
IRCT2017030932293N1 {published data only}
- IRCT2017030932293N1. Chlorhexidine effect on skin colonization [The effect of daily 2% chlorhexidine bathing, on colonization of the skin of patients admitted to Khatam's hospital ICU of the Zahedan University of Medical Sciences in 2017]. apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2017030932293N1 (first received 23 March 2017).
NCT02870062 {published data only}
- NCT02870062. Impact of daily bathing with chlorhexidine in the critical patient [Impact of daily bathing with chlorhexidine in the critical patient: colonization and environment]. clinicaltrials.gov/ct2/show/NCT02870062 (first received 7 August 2016).
Additional references
Afonso 2013
- Afonso E, Llauradó M, Gallart E. The value of chlorhexidine gluconate wipes and prepacked washcloths to prevent the spread of pathogens: a systematic review. Australian Critical Care 2013;26(4):158‐66. [PUBMED: 23827390] [DOI] [PubMed] [Google Scholar]
Afonso 2016
- Afonso E, Blot K, Blot S. Prevention of hospital‐acquired bloodstream infections through chlorhexidine gluconate‐impregnated washcloth bathing in intensive care units: a systematic review and meta‐analysis of randomised crossover trials. Euro Surveillance 2016;21(46):30400. [PUBMED: 27918269] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bereket 2012
- Bereket W, Hemalatha K, Getenet B, Wondwossen T, Solomon A, Zeynudin A, et al. Update on bacterial nosocomial infections. European Review for Medical and Pharmacological Sciences 2012;16(8):1039‐44. [PUBMED: 22913154] [PubMed] [Google Scholar]
Calogiuri 2013
- Calogiuri GF, Leo E, Trautmann A, Nettis E, Ferrannini A, Vacca A. Chlorhexidine hypersensitivity: a critical and updated review. The Journal of Allergy and Therapy 2013;4(141):2. [Google Scholar]
Chen 2015
- Chen W, Cao Q, Li S, Li H, Zhang W. Impact of daily bathing with chlorhexidine gluconate on ventilator associated pneumonia in intensive care units: a meta‐analysis. Journal of Thoracic Disease 2015;7(4):746‐53. [PUBMED: 25973242] [DOI] [PMC free article] [PubMed] [Google Scholar]
Choi 2015
- Choi EY, Park D‐A, Kim HJ, Park J. Efficacy of chlorhexidine bathing for reducing healthcare associated bloodstream infections: a meta‐analysis. Annals of Intensive Care 2015;5(1):31. [PUBMED: 26445950] [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence 2017 [Computer program]
- Veritas Health Innovation. Covidence. Version accessed 18 October 2017. Melbourne, Australia: Veritas Health Innovation, 2017.
Deeks 2017
- Deeks JJ, Higgins JP, Altman DG, editor(s) onbehalfoftheCSMG. Chapter 9: Analysing data and undertaking metaanalyses. In: Higgins JP, Churchill R, Chandler J, Cumpston MS editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Derde 2012
- Derde LP, Dautzenberg MJ, Bonten MJ. Chlorhexidine body washing to control antimicrobial‐resistant bacteria in intensive care units: a systematic review. Intensive Care Medicine 2012;38(6):931‐9. [PUBMED: 22527065] [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
Endnote 2011 [Computer program]
- Thomson Reuters. Endnote. Version X5. New York: Thomson Reuters, 2011.
Faria 2007
Frost 2016
- Frost SA, Alogso MC, Metcalfe L, Lynch JM, Hunt L, Sanghavi R, et al. Chlorhexidine bathing and health care‐associated infections among adult intensive care patients: a systematic review and meta‐analysis. Critical Care 2016;20(1):379. [PUBMED: 27876075] [DOI] [PMC free article] [PubMed] [Google Scholar]
Genuit 2001
- Genuit T, Bochicchio G, Napolitano LM, McCarter RJ, Roghman MC. Prophylactic chlorhexidine oral rinse decreases ventilator‐associated pneumonia in surgical ICU patients. Surgical Infections 2001;2(1):5‐18. [PUBMED: 12594876] [DOI] [PubMed] [Google Scholar]
GRADE 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
GRADEpro 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 9 October 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schünemann HJ. What is 'quality of evidence' and why is it important to clinicians?. BMJ 2008;336(7651):995‐8. [PUBMED: 18456631] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence ‐ imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence ‐ inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [PUBMED: 21803546] [DOI] [PubMed] [Google Scholar]
Health Protection Agency 2016
- Health Protection Agency. Healthcare associated infections (HAI): point prevalence survey, England. Updated August 2016. www.gov.uk/government/publications/healthcare‐associated‐infections‐hcai‐point‐prevalence‐survey‐england (accessed 24 September 2018).
Hibbard 2005
- Hibbard JS. Analyses comparing the antimicrobial activity and safety of current antiseptic agents: a review. Journal of Infusion Nursing 2005;28(3):194‐207. [PUBMED: 15912075] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2017
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Horner 2012
- Horner C, Mawer D, Wilcox M. Reduced susceptibility to chlorhexidine in staphylococci: is it increasing and does it matter?. Journal of Antimicrobial Chemotherapy 2012;67(11):2547‐59. [PUBMED: 22833635] [DOI] [PubMed] [Google Scholar]
Huang 2016
- Huang HP, Chen B, Wang HY, He M. The efficacy of daily chlorhexidine bathing for preventing healthcare‐associated infections in adult intensive care units. The Korean Journal of Internal Medicine 2016;31:1159‐70. [PUBMED: 27048258] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ibrahim 2001
- Ibrahim EH, Tracy L, Hill C, Fraser VJ, Kollef MH. The occurrence of ventilator‐associated pneumonia in a community hospital: risk factors and clinical outcomes. Chest 2001;120(2):555‐61. [PUBMED: 11502658] [DOI] [PubMed] [Google Scholar]
Ider 2010
Inweregbu 2005
- Inweregbu K, Dave J, Pittard A. Nosocomial infections. Continuing Education in Anaesthesia, Critical Care and Pain 2005;5(1):14‐7. [Google Scholar]
Kelly 2012
- Kelly KN, Monson JR. Hospital‐acquired infections. Surgery 2012;30(12):640‐4. [Google Scholar]
Kim 2016
- Kim HY, Lee WK, Na S, Roh YH, Shin CS, Kim J. The effects of chlorhexidine gluconate bathing on health care‐associated infection in intensive care units: a meta‐analysis. Journal of Critical Care 2016;32:126‐37. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [PUBMED: 19622552] [DOI] [PMC free article] [PubMed] [Google Scholar]
Magill 2014
- Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, et al. Multistate point‐prevalence survey of health care‐associated infections. New England Journal of Medicine 2014;370(13):1198‐208. [PUBMED: 24670166] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mahjoub 2015
- Mahjoub M, Bouafia N, Bannour W, Masmoudi T, Bouriga R, Hellali R, et al. Healthcare‐associated infections in a Tunisian university hospital: from analysis to action. Pan African Medical Journal 2015;20:197. [PUBMED: 26113928] [DOI] [PMC free article] [PubMed] [Google Scholar]
McDonnell 1999
- McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clinical Microbiology Reviews 1999;12(1):147‐79. [PUBMED: 9880479] [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Horo 2012
- O'Horo JC, Silva GL, Munoz‐Price S, Safdar N. The efficacy of daily bathing with chlorhexidine for reducing healthcare‐associated bloodstream infections: a meta‐analysis. Infection Control and Hospital Epidemiology 2012;33(3):257‐67. [PUBMED: 22314063] [DOI] [PubMed] [Google Scholar]
Puig Silla 2008
- Puig Silla M, Montiel Company JM, Almerich Silla JM. Use of chlorhexidine varnishes in preventing and treating periodontal disease: a review of the literature. Medicina Oral, Patologia Oral y Cirugia Bucal 2008;13(4):E257‐60. [PUBMED: 18379452] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sacks 2014
- Sacks GD, Diggs BS, Hadjizacharia P, Green D, Salim A, Malinoski DJ. Reducing the rate of catheter‐associated bloodstream infections in a surgical intensive care unit using the Institute for Healthcare Improvement Central Line Bundle. American Journal of Surgery 2014;207(6):817‐23. [PUBMED: 24576582] [DOI] [PubMed] [Google Scholar]
Shah 2016
- Shah HN, Schwartz JL, Luna G, Cullen DL. Bathing with 2% chlorhexidine gluconate: evidence and costs associated with central line‐associated bloodstream infections. Critical Care Nursing Quarterly 2016;39(1):42‐50. [PUBMED: 26633158] [DOI] [PubMed] [Google Scholar]
Shi 2013
- Shi Z, Xie H, Wang P, Zhang Q, Wu Y, Chen E, et al. Oral hygiene care for critically ill patients to prevent ventilator‐associated pneumonia. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD008367.pub2] [DOI] [PubMed] [Google Scholar]
SIGN 2018
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters. www.sign.ac.uk/search‐filters.html (accessed 24 September 2018).
Sinha 2015
- Sinha A, Sazawal S, Pradhan A, Ramji S, Opiyo N. Chlorhexidine skin or cord care for prevention of mortality and infections in neonates. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD007835.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2017
- Sterne JA, Egger M, Moher D, Boutron I, editor(s). Chapter 10: Addressing reporting biases. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Vincent 2009
- Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, et al. International study of the prevalence and outcomes of infection in intensive care units. Journal of the American Medical Association 2009;302(21):2323‐9. [PUBMED: 19952319] [DOI] [PubMed] [Google Scholar]
Volk 2002
- Volk HD. Immunodepression in the surgical patient and increased susceptibility to infection. Critical Care 2002;6(4):279‐81. [PUBMED: 12225595] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wade 1991
- Wade JJ, Casewell MW. The evaluation of residual antimicrobial activity on hands and its clinical relevance. The Journal of Hospital Infection 1991;18(Suppl B):23‐8. [PUBMED: 1679443] [DOI] [PubMed] [Google Scholar]
WHO 2011
- World Health Organization (WHO). Report on the burden of endemic health care‐associated infection worldwide. 2011. apps.who.int/iris/bitstream/10665/80135/1/9789241501507_eng.pdf (accessed 24 September 2018).
WHO 2017
- World Health Organization (WHO). WHO model lists of essential medicines. Amended August 2017. www.who.int/medicines/publications/essentialmedicines/en/ (accessed 24 September 2018).
References to other published versions of this review
Lewis 2016
- Lewis SR, Butler AR, Evans DJ, Alderson P, Smith AF. Chlorhexidine bathing of the critically ill for the prevention of hospital‐acquired infection. Cochrane Database of Systematic Reviews 2016, Issue 6. [DOI: 10.1002/14651858.CD012248] [DOI] [PMC free article] [PubMed] [Google Scholar]


