Abstract
Background
Idiopathic thrombocytopenic purpura (ITP) is a common hematologic disorder caused by immune‐mediated thrombocytopenia. The magnitude of the maternal‐fetal risk of ITP during pregnancy is controversial. Labour management of pregnant women with ITP remains controversial. Management of ITP during pregnancy is complex because of the disparity between maternal and fetal platelet counts.
Objectives
To assess the effectiveness and safety of corticosteroids, intravenous immunoglobulin, vinca alkaloids, danazol, dapsone, and any other types of pharmacological treatments for the treatment of idiopathic thrombocytopenic purpura during pregnancy.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register (February 2009), LILACS (1982 to 8 February 2009), ClinicalTrials.gov (8 February 2009), Current Controlled Trials (16 February 2009), Google Scholar (16 February 2009) and ongoing and unpublished trials cited in the reference lists of relevant articles.
Selection criteria
Randomised controlled trials (RCTs) on any medical treatments for idiopathic thrombocytopenia purpura during pregnancy.
Data collection and analysis
Two review authors independently evaluated methodological quality and extracted trial data. Any disagreement was resolved by discussion or by consulting a third review author.
Main results
This review included one RCT in which 38 women (41 pregnancies) were randomised, with only 26 women (28 pregnancies) being analysed.
This RCT comparing the effect of betamethasone (1.5 mg/day) with no medication found no statistically significant difference in neonatal thrombocytopenia (risk ratio (RR) 1.12, 95% confidence interval (CI) 0.62 to 2.05) and neonatal bleeding (RR 1.00, 95% CI 0.24 to 4.13). Review authors conducted an intention‐to‐treat analysis which showed similar findings: RR 1.18, 95% CI 0.57 to 2.45 and RR 1.05, 95% CI 0.24 to 4.61, respectively. Maternal death, perinatal mortality, postpartum haemorrhage and neonatal intracranial haemorrhage were not studied by this RCT.
Authors' conclusions
Current evidence indicates that compared to no medication, betamethasone did not reduce the risk of neonatal thrombocytopenia and neonatal bleeding in ITP during pregnancy. There is insufficient evidence to support the use of betamethasone for treating ITP. This Cohrane review does not provide evidence about other medical treatments for ITP during pregnancy. This systematic review also identifies the need for well‐designed, adequately powered randomised clinical trials for this medical condition during pregnancy. Unless randomised clinical trials provide evidence of a treatment effect and the trade off between potential benefits and harms are established, policy‐makers, clinicians, and academics should not use betamethasone for ITP in pregnant women. Any future trials on medical treatments for treating ITP during pregnancy should test a variety of important maternal, neonatal or both outcome measures, including maternal death, perinatal mortality, postpartum haemorrhage and neonatal intracranial haemorrhage.
Plain language summary
Drug therapy for treating idiopathic thrombocytopenic purpura during pregnancy
Idiopathic thrombocytopenic purpura (ITP) is an immune‐mediated hematologic disorder caused by a low blood platelet count (thrombocytopenia). Antiplatelet antibodies act against the platelets resulting in platelet destruction by the spleen. In adults, the clinical features of ITP often have an insidious onset and are highly variable, ranging from no symptoms, mild bruising, to mucosal bleeding, and skin discolorations. Management of ITP during pregnancy is complex because of large differences between maternal and fetal platelet counts. The circulating antibodies can cross the placenta and cause a neonatal passive immune thrombocytopenia that may increase the risk of cerebral haemorrhage in the newborn infant. For this reason, it seems reasonable that cesarean section delivery is safer for the infant than vaginal delivery yet the mode of delivery may not affect the rate of haemorrhage. Many different pharmacological interventions are used for treating this medical disorder and treatment for ITP in pregnant women is not standardised. Some of these drugs have potential side effects for pregnant women and some can cause fetal malformation.
Current evidence from one randomised controlled trial indicates that betamethasone does not reduce the risk of neonatal thrombocytopenia and neonatal bleeding in ITP during pregnancy when compared to no medication. We could not identify evidence on other medical treatments for ITP during pregnancy.
This review included one controlled trial in which 38 women (41 pregnancies) were randomised, with only 26 women (28 pregnancies) being analysed. There was also a severe imbalance between comparison groups. Giving the mother betamethasone (1.5 mg/day) did not result in a difference in the neonatal platelet count at birth and at the first week of life. The study reported that the maternal platelet count of peripheral blood did not change significantly during the study period for both the betamethasone and no treatment groups. Maternal postpartum haemorrhage and neonatal intracranial haemorrhage were not studied. Nor were maternal clinical and pregnancy outcomes reported. The researchers used no treatment in the control group, which may have increased the risk of performance bias in the trial.
Background
Idiopathic thrombocytopenic purpura (ITP) is a common hematologic disorder caused by immune‐mediated thrombocytopenia (Cines 2005; Harrington 1951; Harrington 1953). The incidence of ITP during pregnancy ranges between 1 in 1000 to 1 in 10,000 (Sukenik‐Halevy 2008) and 1 in 5500 (Perruca 2003). However, ITP is a diagnosis of exclusion, there is no pathognomonic symptom or sign of the disease (Sukenik‐Halevy 2008). Therefore, ITP presents particular challenges in its definition and management.
Approximately 7% to 8% of pregnant women have thrombocytopenia (Burrows 1990). Thrombocytopenia is defined as a low peripheral blood platelet count, less than 150 x 109/l. ITP causes thrombocytopenia through antiplatelet antibodies which act against platelet antigens, resulting in platelet destruction by the spleen (Woods 1984a; Woods 1984b). In adults, the clinical features of ITP often have an insidious onset, and are highly variable ranging from patients who are commonly asymptomatic or those with mild bruising, to mucosal bleeding (BCSHGH 2003), ecchymosis and petechiae (Martí‐Carvajal 2003). However, bleeding symptoms are uncommon unless the ITP is severe (platelet count < 30 x 109/l) (George 1998). Circulating antiplatelet antibodies can cross the placenta and cause a neonatal passive immune thrombocytopenia (Cines 1982; Yamada 1999).
The magnitude of the maternal‐fetal risk of ITP during pregnancy is controversial. Some claim that the level of risk is low (Kalish 2001; Nisaratanaporn 2006; Suri 2006; Webert 2003), whereas others believe it to be high (Chedraui 2003; el Hajoui 2003; Tampakoudis 1995). For the mother, a differential diagnosis of ITP or gestational thrombocytopenia may be generally of little clinical importance, because cases where diagnosis is unclear involve mild thrombocytopenia that is not threatening to maternal health (Capogna 2007), and maternal morbidity and mortality is low (Kryc 1983). Conversely, for the fetus, differential diagnosis is clinically important ‐ mild maternal ITP may cause thrombocytopenia in the fetus, whereas gestational thrombocytopenia does not (Capogna 2007). Fetal thrombocytopenia does not correlate with maternal platelet count, levels of maternal platelet antibodies, or the mother’s response to therapy (Sukenik‐Halevy 2008), and idiopathic thrombocytopenic purpura‐related haemorrhagic events occur after birth (Sukenik‐Halevy 2008).
There are two clinical guidelines for treating ITP in children, adults, non‐pregnant and pregnant women (BCSHGH 2003; George 1996). These guidelines have been reviewed by Gernsheimer 2007. However, both point out a paucity of randomised controlled trials as a base for recommending approaches to treatment.
Historically, medical (pharmacological) approaches to treating ITP have been divided into conventional therapy (first‐line) and conventional second‐line treatment approaches, the first group includes prednisone (BCSHGH 2003; Dufault 1957; Heys 1966; McGee 2002). Several observational studies point out that corticosteroids are beneficial for treating ITP during pregnancy (Wang 2004). However, there are side effects for pregnant women on long‐term steroids (McGee 2002; Yildirim 2006). The second group includes high‐dose steroids (Widmer 1999), high‐dose intravenous immunoglobulin (Widmer 1999), intravenous anti‐D, vinca alkaloids (Gross 1995), danazol, immunosuppressive agents including azathioprine and cyclophosphamide, combination chemotherapy, and dapsone (Avilés‐Miranda 1983; BCSHGH 2003; Byrne 1997; Gibson 1989; Kimura 2001; Lush 2000). More recently, thrombopoietin receptor agonists and drugs to stimulate platelet production (romiplostim, eltrombopag) have been reported as effective treatments for ITP (Bussel 2007; Kutter 2008; Stasi 2008). However, many of these cannot be used during pregnancy because they are teratogenic agents.
Labour management of pregnant women with ITP remains controversial (Cook 1991). Bhatla 1994 has indicated that the obstetric management should be individualised and not based on platelet count alone. Management of ITP during pregnancy is complex because of the disparity between maternal and fetal platelet counts (Suri 2006). Although neonatal thrombocytopenia may increase the risk of cerebral haemorrhage in the newborn infant, and it seems reasonable that cesarean section delivery is safer for the infant than vaginal delivery, there are no data to support this hypothesis (Capogna 2007; Devendra 2002). The mode of delivery may not affect the rate of intracranial haemorrhage in thrombocytopenic newborns (Cook 1991). Many studies suggest that cesarean section should be based on obstetric indications (Borna 2002; Devendra 2002; Suri 2006; Won 2005).
ITP during pregnancy needs the close collaboration of a medical team that should include a hematologist, an obstetrician, an anaesthesiologist, and a neonatologist (Ali 2003; Nisaratanaporn 2006).
This review assessed the evidence for the clinical effectiveness of medical treatments, including the safety of their use, for ITP during pregnancy.
Objectives
To assess the effectiveness and safety of corticosteroids, intravenous immunoglobulin, vinca alkaloids, danazol, dapsone, and any other types of pharmacological treatments for treating of idiopathic thrombocytopenic purpura during pregnancy.
Methods
Criteria for considering studies for this review
Types of studies
We considered randomised clinical trials (RCTs), irrespective of their publication status (we considered trials published in abstract form or letters), language or country.
We included RCTs with the following study designs: parallel and crossover. If crossover trials were identified, then only data from the first intervention period were included.
Quasi‐randomised studies and prospective observational studies were excluded for evaluating clinical effectiveness. However, these studies were considered for reports on adverse events.
Types of participants
Pregnant women with idiopathic thrombocytopenic purpura irrespective of age, or setting.
Types of interventions
Different non‐surgical treatments including corticosteroids, intravenous immunoglobulin, vinca alkaloids, danazol, dapsone, and any other types of pharmacological treatments for treating of idiopathic thrombocytopenic purpura during pregnancy.
We did not include any surgical approach.
Types of outcome measures
Primary
Maternal death defined as "death of a woman while pregnant or within 42 days of termination of pregnancy, irrespective of the duration and the site of pregnancy, from any cause related to or aggravated by the pregnancy or its management but not from accidental or incidental causes" (Porta 2008).
Perinatal mortality defined as death during "time limited to the period between 28 weeks' gestation and one week postnatally" (Porta 2008).
Postpartum haemorrhage defined as blood loss of 500 mL or more in the first 24 hours postpartum.
Neonatal intracranial haemorrhage.
Secondary
Preterm delivery.
Spontaneous miscarriage.
Cesarean section.
Maternal and neonatal platelet count of peripheral blood.
Neonatal thrombocytopenia.
Neonatal bleeding episodes.
Safety
Gestational diabetes mellitus or impaired glucose tolerance.
Hypertensive disorders complicating pregnancy.
Maternal infection.
Neonatal infection.
Haemolytic anaemia (maternal).
Search methods for identification of studies
Electronic searches
We contacted the Trials Search Co‐ordinator to search the Cochrane Pregnancy and Childbirth Group’s Trials Register (February 2009).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL and MEDLINE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
In addition, we searched LILACS (1982 to 8 February 2009), ClinicalTrials.gov (8 February 2009), Current Controlled Trials (16 February 2009) and Google Scholar (16 February 2009).
See Appendix 1, Appendix 2, Appendix 3 and Appendix 4 for the search strategies used.
We did not apply any language restrictions.
Data collection and analysis
Selection of studies
Two authors (Arturo Martí‐Carvajal (AMC) and Gabriella Comunián (GC)) independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We resolved any disagreement through discussion and we consulted with Guiomar Peña‐Marti (GPM).
Data extraction and management
Data extraction was carried out by two review authors (AMC and GC) using a pre‐designed data extraction form that contains publication details, patient population, randomisation, allocation concealment, details of blinding measures, description of interventions and results (Zavala 2006). We resolved discrepancies through discussion. Data were entered into Review Manager software (RevMan 2008) and checked for accuracy.
Assessment of risk of bias in included studies
Three review authors independently assessed risk of bias using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). Any disagreement was resolved by discussion.
We examined the adequacy of the methods used to generate the allocation sequence; the concealment of allocation; and the level of blinding (clinician, participant or outcome assessor). For each trial the authors classified the risk of bias as high, moderate or low. Overall, we considered trials to be at low risk of bias if allocation concealment and blinding of participants were adequate.
(1) Sequence generation (checking for possible selection bias)
For each included study, we described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
adequate (any truly random process, e.g. random number table; computer random number generator);
inadequate (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear.
(2) Allocation concealment (checking for possible selection bias)
For each included study, we described the method used to conceal the allocation sequence in sufficient detail and determine whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
adequate (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
inadequate (open random allocation; unsealed or non‐opaque envelopes; alternation; date of birth);
unclear.
(3) Blinding (checking for possible performance bias)
For each included study, we described the method used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. Studies were judged at low risk of bias if they were blinded, or if we judge that the lack of blinding could not have affected the results. Blinding was assessed separately for different outcomes or classes of outcomes.
We assessed the methods as:
adequate, inadequate or unclear for participants;
adequate, inadequate or unclear for personnel;
adequate, inadequate or unclear for outcome assessors.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. We assessed methods as:
adequate;
inadequate;
unclear.
We also evaluated the risk of attrition bias, as estimated by the percentage of participants lost to follow up. For future updates, we will exclude studies with total attrition more than 30%, or where the difference between groups exceeds 10%, or both, from the meta‐analysis, but include them in the review.
(5) Selective reporting bias
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
adequate (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
inadequate (where not all the study pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so cannot be used; study failed to include results of a key outcome that would have been expected to have been reported);
unclear.
(6) Other sources of bias
We described for each included study any important concerns we had about other possible sources of bias. For example, was there a potential source of bias related to the specific study design? Was the trial stopped early due to some data‐dependent process? Was there extreme baseline imbalance? Has the study been claimed to be fraudulent?
We assessed whether each study was free of other problems that could put it at risk of bias:
yes;
no;
unclear.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2008). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. For future updates, we will explore the impact of the level of bias through Sensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For future updates, we will use the mean difference if outcomes are measured in the same way between trials. We will use the standardised mean difference to combine trials that measure the same outcome, but use different methods.
Dealing with missing data
For included studies, levels of attrition were noted. For the future updates, the impact of including studies with high levels of missing data in the overall assessment of treatment effect will be explored by using sensitivity analysis.
For all outcomes analyses that were carried out on an intention‐to‐treat (ITT) basis, we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes are known to be missing. We did not contact the author of Christiaens 1990. We did ITT analysis by using the imputation method (worse‐case scenario versus best‐case scenario).
Assessment of heterogeneity
This review did not include meta‐analysis. For future updates, we will use the I² statistic to measure heterogeneity among the trials in each analysis. If we identify substantial heterogeneity (I² > 50%) we will explore it by prespecified subgroup analysis.
Assessment of reporting biases
Where we suspect reporting bias (see 'Selective reporting bias' above), we will attempt to contact study authors asking them to provide missing outcome data. Where this is not possible, and the missing data are thought to introduce serious bias, the impact of including such studies in the overall assessment of results will be explored by Sensitivity analysis.
For future updates, we will also attempt to assess whether the review is subject to publication bias by using a funnel plot.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2008). Due to this Cochrane review containing only one randomised controlled trial (Christiaens 1990), we did not pool data. In subsequent updates, we will use fixed‐effect inverse variance meta‐analysis for combining data where trials are examining the same intervention, and the trials’ populations and methods are judged sufficiently similar. Where we suspect clinical or methodological heterogeneity between studies sufficient to suggest that treatment effects may differ between trials we will use random‐effects meta‐analysis.
If substantial heterogeneity is identified in a fixed‐effect meta‐analysis this will be noted and the analysis repeated using a random‐effects method.
We will exclude from analyses data from trials or outcomes that are at high risk of bias, for example, those with high levels of missing data or a large number of participants analysed in the wrong group.
Subgroup analysis and investigation of heterogeneity
In subsequent updates of this review, when sufficient data are available, we plan to carry out the following subgroup analyses:
pregnant women receiving steroids at doses greater than 15 mg/day;
splenectomy prior to pregnancy;
refractory idiopathic thrombocytopenic purpura;
type of corticosteroid;
type of medical treatment.
The following outcomes will be used in subgroup analysis:
maternal death;
perinatal mortality;
postpartum haemorrhage;
neonatal intracranial haemorrhage.
For fixed‐effect meta‐analyses we will conduct planned subgroup analyses classifying whole trials by interaction tests as described by Deeks 2001. For random‐effects meta‐analyses we will assess differences between subgroups by inspection of the subgroups’ confidence intervals; non‐overlapping confidence intervals indicate a statistically significant difference in treatment effect between the subgroups.
Sensitivity analysis
In subsequent updates we also plan to conduct a sensitivity analysis comparing the results using all studies and using only those of high methodological quality.
Results
Description of studies
Results of the search
Three publications were identified as potentially eligible for inclusion in this review.
Included studies
This review included one randomised controlled trial (RCT) (Christiaens 1990) in which 38 women (41 pregnancies) were randomised, with only 26 women (28 pregnancies) being analysed, see 'Characteristics of included studies'.
Excluded studies
Two publications (Gill 2002; Lee 2002) were narrative reviews and were therefore excluded, see 'Characteristics of excluded studies'.
Risk of bias in included studies
The risk of bias of the included study (Christiaens 1990) is summarised in Figure 1 and Figure 2. We classified it as a trial with high risk of bias due to it having 'unclear' allocation concealment. Furthermore, this RCT has a high attrition rate (31.7%).
1.
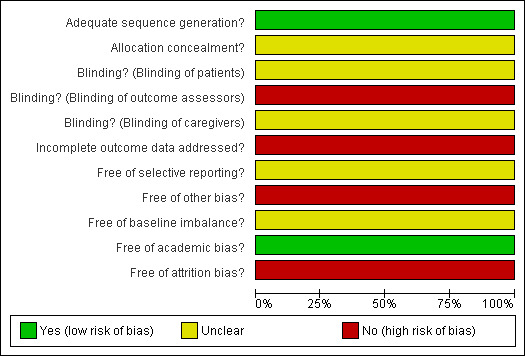
Methodological quality graph for Christiaens 1990.
2.
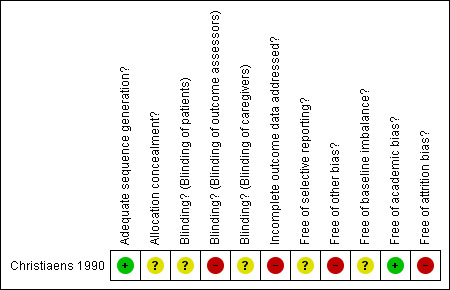
Methodological quality summary: review authors' judgements about each methodological quality item for Christiaens 1990.
Effects of interventions
Results are based on one RCT (28 pregnancies/ 28 newborns).
Primary
Christiaens 1990 did not study the following outcomes: maternal death, perinatal mortality, postpartum haemorrhage and neonatal intracranial haemorrhage.
Secondary
Christiaens 1990 did not study the following outcomes: preterm delivery, spontaneous miscarriage and cesarean section.
Maternal and neonatal platelet count of peripheral blood
The maternal platelet count of peripheral blood for both groups in the Christiaens 1990 study, "...did not change significantly during the study period". However, those counts were not reported.
For neonatal platelet count at birth (median), there was no significant differences between the betamethasone group (140 x 109/l (range 49 to 298 x 109/l) and the group receiving no treatment (196 x 109/l (range 26 to 289 x 109/l).
For neonatal platelet count at first week of life (median), there was no significant differences between the betamethasone group (121 x 109/l (range 15 to 248 x 109/l) and the group receiving no treatment (120 x 109/l (range 17 to 275 x 109/l).
Neonatal thrombocytopenia
There was no significant difference in overall (17 events), severe (4 events) and mild (13 events) of neonatal thrombocytopenia incidence between the betamethasone and the no treatment groups (risk ratio (RR) 1.12, 95% confidence interval (CI) 0.62 to 2.05, P = 0.70; RR 1.00, 95% CI 0.16 to 6.14, P =1.00; RR 1.17, 95% CI 0.52 to 2.60, P = 0.71), respectively.
We performed an intention‐to‐treat analysis including 13 pregnancies that were excluded by Christiaens 1990. We assumed that all missing participants did not experience neonatal thrombocytopenia.
There was no significant difference in overall (17 events), severe (four events) and mild (13 events) of neonatal thrombocytopenia incidence between betamethasone and the no treatment groups (RR 1.18, 95% CI 0.57 to 2.45; RR 1.05, 95% CI 0.16 to 6.76; RR 1.22, 95% CI 0.50 to 3.02), respectively. SeeAnalysis 4.1.
4.1. Analysis.
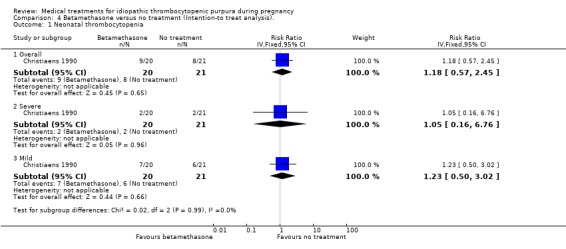
Comparison 4 Betamethasone versus no treatment (Intention‐to treat analysis)., Outcome 1 Neonatal thrombocytopenia.
Sensitivity analyses of neonatal thrombocytopenia to account for the 13 excluded pregnancies showed discrepancy on this outcome. According to Christiaens 1990 et al, the RRs for the worst‐case scenario (all participants with missing outcomes in the betamethasone group had 'neonatal thrombocytopenia', and all those with missing outcomes in the no treatment group had 'normal' outcomes) and the best‐case scenario (all participants with missing outcomes in the betamethasone group had 'normal' outcomes, and all those with missing outcomes in the no treatment group had 'neonatal thrombocytopenia') were: RR 1.97 (95% CI 1.08 to 3.59) for overall neonatal thrombocytopenia and RR 0.63 (95% CI 0.36 to 1.10) overall neonatal thrombocytopenia, respectively. (SeeAnalysis 2.1 for worst‐case scenario and Analysis 5.1 for best‐case scenario).
2.1. Analysis.
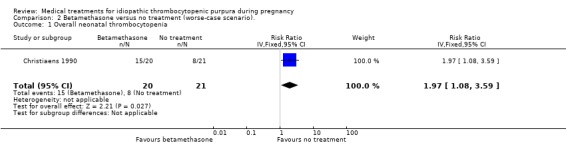
Comparison 2 Betamethasone versus no treatment (worse‐case scenario)., Outcome 1 Overall neonatal thrombocytopenia.
5.1. Analysis.
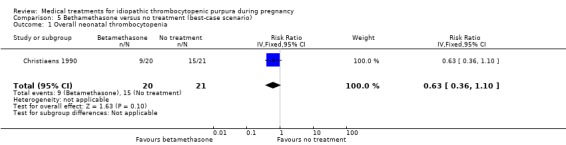
Comparison 5 Bethamethasone versus no treatment (best‐case scenario), Outcome 1 Overall neonatal thrombocytopenia.
Neonatal bleeding episodes
Betamethasone versus no treatment (one RCT; 28 pregnancies, six events)
There was no significant difference in incidence of neonatal bleeding episodes between the betamethasone and no treatment control group (RR 1.00, 95% CI 0.24 to 4.13).
We conducted an intention‐to‐treat analysis including 13 pregnancies that were excluded by Christiaens 1990. We assumed that all missing participants did not experience neonatal bleeding episodes.
Betamethasone versus no treatment (one RCT; 41 pregnancies, six events)
There was no significant difference in incidence of neonatal bleeding episodes between the betamethasone and no treatment control group (RR 1.05, 95% CI 0.24 to 4.61). SeeAnalysis 4.2.
4.2. Analysis.
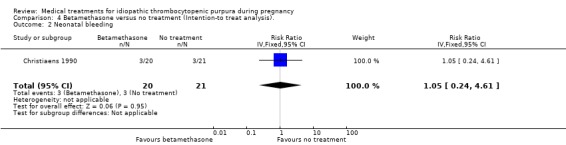
Comparison 4 Betamethasone versus no treatment (Intention‐to treat analysis)., Outcome 2 Neonatal bleeding.
Sensitivity analyses of neonatal bleeding episodes to account for the thirteen excluded pregnancies showed inconsistency on this outcome. According to Christiaens 1990 et al, the RRs for the worst‐case scenario (all participants with missing outcomes in the betamethasone group had 'neonatal bleeding episodes', and all those with missing outcomes in the no treatment control group had 'normal' outcomes) was RR 3.15 (95% CI 0.99 to 9.99) for neonatal bleeding. (SeeAnalysis 2.2 for worst‐case scenario). The best‐case scenario (all participants with missing outcomes in the betamethasone group had 'normal' outcomes, and all those with missing outcomes in the no treatment control group had 'neonatal bleeding episodes') shows RR 0.32 (95% CI 0.10 to 0.98) (See Analysis 3.1).
2.2. Analysis.
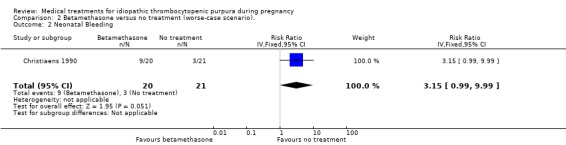
Comparison 2 Betamethasone versus no treatment (worse‐case scenario)., Outcome 2 Neonatal Bleeding.
3.1. Analysis.
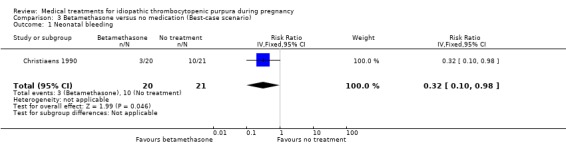
Comparison 3 Betamethasone versus no medication (Best‐case scenario), Outcome 1 Neonatal bleeding.
Safety
Gestational diabetes mellitus or impaired glucose tolerance, hypertensive disorders complicating pregnancy, maternal infection, neonatal infection and haemolytic anaemia (maternal) were not reported by Christiaens 1990.
Discussion
Summary of main results
This review of medical treatments idiopathic thrombocytopenia purpura (ITP) during pregnancy found one randomised controlled trial, and its critical appraisal does not support the use of betamethasone for treating ITP during pregnancy. We did not find significant differences on incidence of neonatal thrombocytopenia and bleeding neonatal outcomes in pregnant women with ITP treated with betamethasone versus no treatment. Main clinical outcomes such as maternal death, perinatal mortality, postpartum haemorrhage and intracranial haemorrhage neonatal were not evaluated by Christiaens 1990. The relative effect of betamethasone versus control remains unclear. Christiaens 1990 was singularly directed at neonatal outcomes rather than outcomes related to pregnant women.
Overall completeness and applicability of evidence
Only one RCT was found by this review and it failed to detect statistically significant differences between the groups.
Quality of the evidence
The only included RCT evaluated one surrogate marker (neonatal thrombocytopenia) and clinical outcome (neonatal bleeding). However, the researchers excluded maternal clinical outcomes. Additionally, the RCT was underpowered because of attrition. Therefore, these issues affected or distorted the internal validity of Christiaens 1990. The selected comparison group was another factor that could have affected the results of this RCT (Appel 2006). The researchers used 'no treatment' as a control group and this may have increased the risk of performance bias. Also, in the Christiaens 1990 trial, sample size calculations were not determined a priori and there was a high level of attrition bias.
Potential biases in the review process
The main limitation of this Cochrane review is the paucity of evidence in pregnant women suffering from ITP. Publication bias is an important factor that affects the validity of systematic reviews and can modify the quality of the evidence. However, we did an exhaustive search which included many clinical trial registries, but were unable to find any registered completed trials that were unpublished.
Agreements and disagreements with other studies or reviews
There are no other reviews or studies to compare with this Cochrane review.
Authors' conclusions
Implications for practice.
Based on one trial stating that pregnant women suffering from idiopathic thrombocytopenia purpura (ITP) treated with betamethasone did not have a better prognosis compared to those who did not receive treatment, the question of a possibly beneficial or deleterious effect of betamethasone remains open. This conclusion is based on one randomised controlled trial (RCT) (38 women randomised, but only 26 women (28 pregnancies) analysed)) with a high risk of bias. There is, therefore, insufficient evidence to support the use of betamethasone for treating ITP during pregnancy. Consequently, policy‐makers, clinicians, and academics should not yet recommend this drug for use in those patients. This Cochrane review does not provide evidence about other medical interventions for treating ITP during pregnancy.
Implications for research.
This systematic review has identified the need for well‐designed, adequately powered RCTs to assess the benefits and harms of medical treatments for treating idiopathic thrombocytopenic purpura in pregnant women. The trials should include clinical outcomes such as maternal death, perinatal mortality, postpartum haemorrhage and neonatal intracranial haemorrhage. Such RCTs should be reported according to the CONSORT statement for improving the quality of reporting of efficacy and harms in clinical research.
What's new
| Date | Event | Description |
|---|---|---|
| 16 May 2012 | Amended | Contact details updated. |
Acknowledgements
As part of the pre‐publication editorial process, this review has been commented on by two peers (an editor and referee who is external to the editorial team), and the Group’s Statistical Adviser.
We want to express our gratitude to Dr David L Streiner, his suggestions improved the quality of this review and our knowledge of systematic reviews.
We want to express our gratitude to Dr. Zarko Alferivic, peer reviewers for both the protocol and review, and Mrs Janet Wale. Their comments and suggestions have improved the quality of this Cochrane review.
We wish to express our gratitude to Cochrane Pregnancy and Childbirth group for their support in completing this review.
Appendices
Appendix 1. LILACS search strategy
Date: 1982 to 8 February 2009.
1) ((Pt ENSAYO CONTROLADO ALEATORIO OR Pt ENSAYO CLINICO CONTROLADO OR Mh ENSAYOS CONTROLADOS ALEATORIOS OR Mh DISTRIBUCIÓN ALEATORIA OR Mh METODO DOBLE CIEGO OR Mh METODO SIMPLECIEGO OR Pt ESTUDIO MULTICÉNTRICO) or ((tw ensaio or tw ensayo or tw trial) and (tw azar or tw acaso or tw placebo or tw control$ or tw aleat$ or tw random$ or (tw duplo and tw cego) or (tw doble and tw ciego) or (tw double and tw blind)) and tw clinic$)) AND NOT ((Ct ANIMALES OR Mh ANIMALES OR Ct CONEJOS OR Ct RATÓN OR MH Ratas OR MH Primates OR MH Perros OR MH Conejos OR MH Porcinos) AND NOT (Ct HUMANO AND Ct ANIMALES)) [Palavras]
2) idiopathic thrombocytopen$ purpura
3) pregnan$
4) 1 and 2 and 3
Result: no relevant studies.
Appendix 2. Clinical trials.gov search strategy
Date: 8 February 2009.
(thrombocytopenic purpura OR thrombocytopaenic purpura) AND (pregnancy OR pregnant women)
Result: no relevant studies.
Appendix 3. Current Controlled Trials search strategy
Date: 16 February 2009.
thrombocytopenic! AND purpura AND pregnan%
Appendix 4. Google scholar search strategy
Date: 16 February 2009.
idiopathic thrombocytopenic purpura and pregnancy
Data and analyses
Comparison 1. Betamethasone versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Neonatal thrombocytopenia | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Overall | 1 | 28 | Risk Ratio (IV, Fixed, 95% CI) | 1.13 [0.62, 2.05] |
| 1.2 Severe | 1 | 28 | Risk Ratio (IV, Fixed, 95% CI) | 1.0 [0.16, 6.14] |
| 1.3 Mild | 1 | 28 | Risk Ratio (IV, Fixed, 95% CI) | 1.17 [0.52, 2.60] |
| 2 Neonatal bleeding | 1 | 28 | Risk Ratio (IV, Fixed, 95% CI) | 1.0 [0.24, 4.13] |
1.1. Analysis.
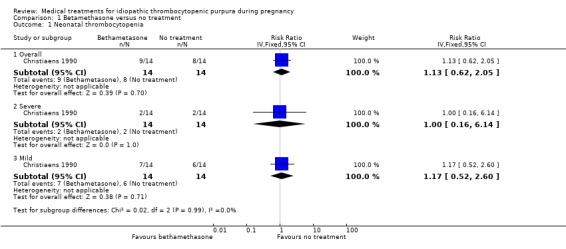
Comparison 1 Betamethasone versus no treatment, Outcome 1 Neonatal thrombocytopenia.
1.2. Analysis.
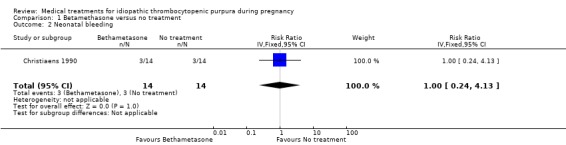
Comparison 1 Betamethasone versus no treatment, Outcome 2 Neonatal bleeding.
Comparison 2. Betamethasone versus no treatment (worse‐case scenario).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall neonatal thrombocytopenia | 1 | 41 | Risk Ratio (IV, Fixed, 95% CI) | 1.97 [1.08, 3.59] |
| 2 Neonatal Bleeding | 1 | 41 | Risk Ratio (IV, Fixed, 95% CI) | 3.15 [0.99, 9.99] |
Comparison 3. Betamethasone versus no medication (Best‐case scenario).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Neonatal bleeding | 1 | 41 | Risk Ratio (IV, Fixed, 95% CI) | 0.32 [0.10, 0.98] |
Comparison 4. Betamethasone versus no treatment (Intention‐to treat analysis).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Neonatal thrombocytopenia | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Overall | 1 | 41 | Risk Ratio (IV, Fixed, 95% CI) | 1.18 [0.57, 2.45] |
| 1.2 Severe | 1 | 41 | Risk Ratio (IV, Fixed, 95% CI) | 1.05 [0.16, 6.76] |
| 1.3 Mild | 1 | 41 | Risk Ratio (IV, Fixed, 95% CI) | 1.23 [0.50, 3.02] |
| 2 Neonatal bleeding | 1 | 41 | Risk Ratio (IV, Fixed, 95% CI) | 1.05 [0.24, 4.61] |
Comparison 5. Bethamethasone versus no treatment (best‐case scenario).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall neonatal thrombocytopenia | 1 | 41 | Risk Ratio (IV, Fixed, 95% CI) | 0.63 [0.36, 1.10] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Christiaens 1990.
| Methods | Randomised controlled trial. Open label.
Study type: multicenter study (34 hospitals). National: The Netherlands.
Study phase: III.
Study design: parallel.
Phase of perinatal period at entry: pregnancy (< 36 weeks' gestation).
Randomisation: by computer‐generated table of random numbers allocation. It was conducted before 36 weeks' gestation.
Allocation concealment: not described.
Blinding: pediatricians who assessed the babies were not masked to the treatment. Analysis results: per‐protocol analysis. Follow‐up period: from "day 259 of pregnancy till delivery". Lost post‐randomisation: 13 (32%) (not included the final analysis).
|
|
| Participants | Recruited participants: 59 women (64 pregnancies). Number randomised: 38 women (41 pregnancies). Number analysed: 26 women (28 pregnancies). Mean (SD) age of experimental group (n = 14): 29 (5) years. Mean (SD) age of experimental group (n = 14): 29 (6) years. Inclusion criteria: pregnant women with idiopathic thrombocytopenic purpura (thrombocytopenia (maternal platelet count < 150 x 109/l) on more than 1 occasion, the presence of a normal or increased number of megakaryocytes in bone marrow, and the absence of other diseases, of splenomegaly, of antinuclear antibodies and of the use of drugs known to induce thrombocytopenia). Exclusion criteria: pregnant women already using corticosteroids. |
|
| Interventions | Bethametasone: 0.5 mg thrice/day (1.5 mg/day) by the first 2 weeks and 0.5 mg twice/day/ during the third week. Control: no treatment. | |
| Outcomes | Primary:
|
|
| Notes | Funding/support: no special funding required. 8 pregnant women were included with normal platelet counts with diagnosis of ITP made before the current pregnancy and who had had a splenectomy. Trial period: March 1984 to March 1987. Reasons for non‐admitted participants during recruitment period: use of corticosteroids (10 participants), concomitant disease (7 participants) and refused to participate (6 participants). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Adequate: quote: "...according to a computer‐generated table of random numbers". |
| Allocation concealment? | Unclear risk | Insufficient information to permit judgement of ‘yes’ or ‘no’. |
| Blinding? Blinding of patients | Unclear risk | Insufficient information to permit judgement of ‘yes’ or ‘no’. |
| Blinding? Blinding of outcome assessors | High risk | Inadequate. Comment: treatment received by mother were known by paediatricians who assessed newborns. |
| Blinding? Blinding of caregivers | Unclear risk | Insufficient information to permit judgement of ‘yes’ or ‘no’. |
| Incomplete outcome data addressed? All outcomes | High risk | Inadequate. Comment: study excluded 13 pregnancies (32%). |
| Free of selective reporting? | Unclear risk | Unclear. Comments: study reported data on the planned outcomes. However, this RCT did not include relevant clinical outcome: maternal death, perinatal mortality, postpartum haemorrhage and intracranial haemorrhage neonatal. |
| Free of other bias? | High risk | Inadequate. There is a chance of bias due to inadequate sample size for estimating a real effect. This trial may be affected by early stopping bias: |
| Free of baseline imbalance? | Unclear risk | Inadequate. Comments: it only shows the baseline characteristics of the analysed participants. |
| Free of academic bias? | Low risk | Adequate.
No previous published RCTs of the same intervention. No previous published RCTs of the same medical condition. This RCT reports a retrospective study which used prednisone and suggested a beneficial effect on the neonatal platelet count. |
| Free of attrition bias? | High risk | Inadequate. Comment: there were 32% (13/41) post‐randomisation drop‐outs. |
ITP: idiopathic thrombocytopenia purpura RCT: randomised controlled trial SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
Contributions of authors
Arturo Martí‐Carvajal (AMC) wrote the first draft of the review with subsequent input from Guiomar Peña‐Martí and Gabriella Comunián‐Carrasco. AMC is guarantor for the review.
Sources of support
Internal sources
Universidad de Carabobo, Venezuela.
External sources
Iberoamerican Cochrane Center, Spain.
Declarations of interest
In 2004 Arturo Martí‐Carvajal was employed by Eli Lilly to run a four hour workshop on 'How to critically appraise clinical trials on osteoporosis and how to teach this'. This activity was not related to his work with The Cochrane Collaboration or any Cochrane review.
In 2007 Arturo Martí‐Carvajal was employed by Merck to run a four hour workshop 'How to critically appraise clinical trials and how to teach this'. This activity was not related to his work with The Cochrane Collaboration or any Cochrane review.
Guiomar Peña‐Martí and Gabriella Comunián‐Carrasco: None known.
Edited (no change to conclusions)
References
References to studies included in this review
Christiaens 1990 {published data only}
- Christiaens GCML, Nieuwenhuis HK, Dem Borne AEGK, Ouwehand WH, Helmerhorst FM, Dalen CM, et al. Idiopathic thrombocytopenic purpura in pregnancy: a randomized trial on the effect of antenatal low dose corticosteroids on neonatal platelet count. British Journal of Obstetrics and Gynaecology 1990; Vol. 97:893‐8. [MEDLINE: ] [DOI] [PubMed]
References to studies excluded from this review
Gill 2002 {published data only}
- Gill KK, Kelton JG. Management of idiopathic thrombocytopenic purpura in pregnancy. Seminars in Hematology 2000;37:275‐89. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lee 2002 {published data only}
- Lee LH. Idiopathic thrombocytopenia in pregnancy. Annals of the Academy of Medicine, Singapore 2002;31:335‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Additional references
Ali 2003
- Ali R, Ozkalemkas F, Ozçelik T, Ozkocaman V, Ozan U, Kimya Y, et al. Idiopathic thrombocytopenic purpura in pregnancy: a single institutional experience with maternal and neonatal outcomes. Annals of Hematology 2003;82(6):348‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Appel 2006
- Appel LJ. A primer on the design, conduct, and interpretation of clinical trials. Clinical Journal of the American Society of Nephrology 2006;1(6):1360‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Avilés‐Miranda 1983
- Avilés‐Miranda A, Ambríz Fernández R, Niz‐Ramos J, García‐Alonso A, Luna‐Olsen E, Sinco‐Angeles A, et al. Idiopathic thrombocytopenic purpura and pregnancy. Use of corticosteroids for the prevention of neonatal purpura. Revista de Investigación Clínica 1983;35:111‐4. [MEDLINE: ] [PubMed] [Google Scholar]
BCSHGH 2003
- British Committee for Standards in Haematology General Haematology Task Force. Guidelines for the investigation and management of idiopathic thrombocytopenic purpura in adults, children and in pregnancy. British Journal of Haematology 2003;120:574‐96. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bhatla 1994
- Bhatla N, Buckshee K, Bhargava VL, Takkar D, Malhotra OP. Pregnancy with idiopathic thrombocytopenic purpura. Journal of the Association of Physicians of India 1994;42:105‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Borna 2002
- Borna S, Borna H, Khazardoost S. Maternal and neonatal outcomes in pregnant women with immune thrombocytopenic purpura. Archives of Iranian Medicine 2006;9:115‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Burrows 1990
- Burrows RF, Kelton JG. Thrombocytopenia at delivery: a prospective survey of 6715 deliveries. American Journal of Obstetrics and Gynecology 1990;162:731‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bussel 2007
- Bussel JB, Cheng G, Saleh MN, Psaila B, Kovaleva L, Meddeb B, et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. New England Journal of Medicine 2007;357:2237‐47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Byrne 1997
- Byrne JD, Incerpi MH, Goodwin TM. Idiopathic thrombocytopenic purpura in pregnancy treated with pulsed high‐dose oral dexamethasone. American Journal of Obstetrics and Gynecology 1997;177:468‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Capogna 2007
- Capogna G. Maternal and perinatal outcome in idiopathic thrombocytopenic purpura (ITP) with pregnancy. Obstetric Anesthesia Digest 2007;27:158‐9. [1536‐5395 ] [Google Scholar]
Chedraui 2003
- Chedraui PA, Hidalgo LA, San Miguel G. Fatal intracranial hemorrhage in a pregnant patient with autoimmune thrombocytopenic purpura. Journal of Perinatal Medicine 2003;31:526‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cines 1982
- Cines DB, Dusak B, Tomaski A, Mennuti M, Schreiber AD. Immune thrombocytopenic purpura and pregnancy. New England Journal of Medicine 1982;306:826‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cines 2005
- Cines DB, McMillan R. Management of adult idiopathic thrombocytopenic purpura. Annual Review of Medicine 2005;56:425‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cook 1991
- Cook RL, Miller RC, Katz VL, Cefalo RC. Immune thrombocytopenic purpura in pregnancy: a reappraisal of management. Obstetrics & Gynecology 1991;78:578‐83. [MEDLINE: ] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic reviews in health care: meta‐analysis in context. London: BMJ Books, 2001. [Google Scholar]
Devendra 2002
- Devendra K, Koh LP. Pregnancy in women with idiopathic thrombocytopaenic purpura. Annals of the Academy of Medicine, Singapore 2002;31:276‐80. [MEDLINE: ] [PubMed] [Google Scholar]
Dufault 1957
- Dufault FX Jr, Kaye BM. Idiopathic thrombocytopenic purpura and pregnancy: report of a case treated with cortisone. Obstetrics & Gynecology 1957;9:228‐30. [MEDLINE: ] [PubMed] [Google Scholar]
el Hajoui 2003
- Hajoui S, Nabil S, Khachani M, Kaddioui S, Alami MH, Bezad R, et al. Idiopathic thrombopenic purpura and pregnancy. La Tunisie Mèdicale 2003;81:213‐6. [MEDLINE: ] [PubMed] [Google Scholar]
George 1996
- George JN, Woolf SH, Raskob GE, Wasser JS, Aledort LM, Ballem PJ, et al. Idiopathic thrombocytopenic purpura: a practice guideline developed by explicit methods for the American Society of Hematology. Blood 1996;88:3‐40. [MEDLINE: ] [PubMed] [Google Scholar]
George 1998
- George JN, Raskob GE. Idiopathic thrombocytopenic purpura: a concise summary of the pathophysiology and diagnosis in children and adults. Seminars in Hematology 1998;35(1 Suppl 1):5‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Gernsheimer 2007
- Gernsheimer T, McCraeb KR. Immune thrombocytopenic purpura in pregnancy. Current Opinion in Hematology 2007;14:574‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gibson 1989
- Gibson J, Laird PP, Joshua DE, Child A, Harris J, Kronenberg H. Very high dose intravenous gammaglobulin in thrombocytopenia of pregnancy. Australian and New Zealand Journal of Medicine 1989;19:151‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gross 1995
- Gross Z, Rodriguez JJ, Stalnaker BL. Vincristine for refractory autoimmune thrombocytopenic purpura in pregnancy. A case report. Journal of Reproductive Medicine 1995;40(10):739‐42. [MEDLINE: ] [PubMed] [Google Scholar]
Harrington 1951
- Harrington WJ, Minnich V, Hollingsworth JW, Moore CV. Demonstration of a thrombocytopenic factor in the blood of patients with thrombocytopenic purpura. Journal of Laboratory and Clinical Medicine 1951;38:1‐10. [MEDLINE: ] [PubMed] [Google Scholar]
Harrington 1953
- Harrington WJ, Sprague CC, Minnich V, Moore CV, Aulvin RC, Dubach R. Immunologic mechanisms in idiopathic and neonatal thrombocytopenic purpura. Annals of Internal Medicine 1953;38:433‐69. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Heys 1966
- Heys RF. Steroid therapy for idiopathic thrombocytopenic purpura during pregnancy. Obstetrics & Gynecology 1966;28:532‐42. [MEDLINE: ] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Kalish 2001
- Kalish, RB, Christiaens GCML, Skupski DW, Bussel JB. Patterns of progression of immune thrombocytopenia purpura during pregnancy. American Journal of Obstetrics and Gynecology 2001;185(Suppl 6):S181. [Google Scholar]
Kimura 2001
- Kimura S, Kuroda J, Akaogi T, Hayashi H, Ogino Y, Kobayashi Y, et al. Treatment of steroid‐resistant idiopathic thrombocytopenic purpura in pregnancy with repeated high‐dose intravenous immunoglobulin. Haematologia 2001;31:263‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kryc 1983
- Kryc JJ, Corrigan JJ Jr. Idiopathic thrombocytopenic purpura during pregnancy. A pediatric viewpoint. American Journal of Pediatric Hematology/Oncology 1983;5(1):21‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Kutter 2008
- Kuter DJ, Bussel JB, Lyons RM, Pullarkat V, Gernsheimer TB, Senecal FM, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double‐blind randomised controlled trial. Lancet 2008;371:395‐403. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lush 2000
- Lush R, Iland H, Peat B, Young G. Successful use of dapsone in refractory pregnancy‐associated idiopathic thrombocytopenic purpura. Australian and New Zealand Journal of Medicine 2000;30:105‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Martí‐Carvajal 2003
- Martí‐Carvajal A. El Paciente con...Semiología y Clínica Hematológica. Valencia, Venezuela: Universidad de Carabobo, 2003. [Google Scholar]
McGee 2002
- McGee DC. Steroid use during pregnancy. Journal of Perinatal and Neonatal Nursing 2002;16:26‐39. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nisaratanaporn 2006
- Nisaratanaporn S, Sukcharoen N. Outcome of idiopathic thrombocytopenic purpura in pregnancy in King Chulalongkorn Memorial Hospital. Journal of the Medical Association of Thailand 2006;89(Suppl 4):S70‐S75. [MEDLINE: ] [PubMed] [Google Scholar]
Perruca 2003
- Perruca PE, Munoz MP, Liendo PF, Ricci AP, Pérez CC, Domínguez CC, et al. Experience and management of idiopathic thrombocytopenic purpura during pregnancy [Experiencia y manejo del purpura trombocitopenico idiopatico durante el embarazo]. Revista Chilena de Obstetricia y Ginecología 2003;68(4):293‐9. [ISSN 0717‐7526] [Google Scholar]
Porta 2008
- Porta M, editor. A dictionary of epidemiology. New York: Oxford University Press, 2008. [ISBN 978‐0‐19‐531449‐6] [Google Scholar]
RevMan 2008 [Computer program]
- The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen, The Nordic Cochrane Centre: The Cochrane Collaboration, 2008.
Stasi 2008
- Stasi R, Evangelista ML, Amadori S. Novel thrombopoietic agents: a review of their use in idiopathic thrombocytopenic purpura. Drugs 2008;68:901‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sukenik‐Halevy 2008
- Sukenik‐Halevy R, Ellis MH, Fejgin MD. Management of immune thrombocytopenic purpura in pregnancy. Obstetrical and Gynecological Survey 2008;63:182‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Suri 2006
- Suri V, Aggarwal N, Saxena S, Malhotra P, Varma S. Maternal and perinatal outcome in idiopathic thrombocytopenic purpura (ITP) with pregnancy. Acta Obstetricia et Gynecologica Scandinavica 2006;85:1430‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tampakoudis 1995
- Tampakoudis P, Billi H, Tantanassis T, Kalachanis I, Garipidou B, Sinakos Z, et al. Pregnancy and labor in idiopathic thrombocytopenic purpura. Geburtshilfe und Frauenheilkunde 1995;55:583‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wang 2004
- Wang Q, Nie LL. Clinical analysis of 92 cases of pregnancy with idiopathic thrombocytopenic purpura. Zhonghua Fu Chan Ke Za Zhi. 2004;39:729‐32. [MEDLINE: ] [PubMed] [Google Scholar]
Webert 2003
- Webert KE, Mittal R, Sigouin C, Heddle NM, Kelton JG. A retrospective 11‐year analysis of obstetric patients with idiopathic thrombocytopenic purpura. Blood 2003;102:4306‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Widmer 1999
- Widmer A, Brühwiler H, Krause M, Lüscher KP. Severe autoimmune thrombocytopenia in pregnancy. Zeitschrift für Geburtshilfe und Neonatologie 1999;203:258‐60. [MEDLINE: ] [PubMed] [Google Scholar]
Won 2005
- Won YW, Moon W, Yun YS, Oh HS, Choi JH, Lee YY, et al. Clinical aspects of pregnancy and delivery in patients with chronic idiopathic thrombocytopenic purpura (ITP). Korean Journal of Internal Medicine 2005;20:129‐34. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Woods 1984a
- Woods VL Jr, Kurata Y, Montgomery RR, Tani P, Mason D, Oh EH, et al. Autoantibodies against platelet glycoprotein Ib in patients with chronic immune thrombocytopenic purpura. Blood 1984;164:156‐60. [MEDLINE: ] [PubMed] [Google Scholar]
Woods 1984b
- Woods VL Jr, Oh EH, Mason D, McMillan R. Autoantibodies against the platelet glycoprotein IIb/IIIa complex in patients with chronic ITP. Blood 1984;63:368‐75. [MEDLINE: ] [PubMed] [Google Scholar]
Yamada 1999
- Yamada H, Kato EH, Kobashi G, Kishida T, Ebina Y, Kaneuchi M, et al. Passive immune thrombocytopenia in neonates of mothers with idiopathic thrombocytopenic purpura: incidence and risk factors. Seminars in Thrombosis and Hemostasis 1999;25:491‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yildirim 2006
- Yildirim Y, Tinar S, Oner RS, Kaya B, Toz E. Gestational diabetes mellitus in patients receiving long‐term corticosteroid therapy during pregnancy. Journal of Perinatal Medicine 2006;34:280‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zavala 2006
- Zavala D, Martí‐Carvajal A, Peña‐Martí G, Comunián G. Data extraction sheet to help manage the characteristics of included studies in Cochraen reviews. (www.cochrane.fcs.edu.ve/hrs). Valencia‐Venezuela: Universidad de Carabobo, 2006.


