Abstract
Background
Obesity is closely related to type 2 diabetes and long‐term weight reduction is an important part of the care delivered to obese persons with diabetes.
Objectives
To assess the efficacy of pharmacotherapy for weight loss in adults with type 2 diabetes.
Search methods
Computerized searches were performed of MEDLINE, EMBASE, Web of Science and other electronic bibliographic databases, supplemented with hand searches of reference lists and selected journals.
Selection criteria
Randomized, controlled trials were included where pharmacotherapy was used as the primary strategy for weight loss among adults with type 2 diabetes. Published and unpublished literature in any language and with any study design was included.
Data collection and analysis
Two reviewers abstracted data and the quality of included studies was evaluated by assessing potential attrition, as well as selection and measurement bias, and a Jadad score was obtained. Effects were combined using a random effects model.
Main results
A sufficient number of studies were available for a quantitative synthesis for fluoxetine, orlistat, and sibutramine. Twenty two randomized controlled trials were included in the review, with a total of 296 participants for fluoxitine, 2036 for orlistat, and 1047 for sibutramine. Pharmacotherapy produced modest reductions in weight for fluoxetine (5.1 kg (95% confidence interval [CI], 3.3 ‐ 6.9) at 24 to 26 weeks follow up; orlistat 2.0 kg (CI, 1.3 ‐ 2.8) at 12 to 57 weeks follow‐up, and sibutramine 5.1 kg (CI, 3.2 ‐ 7.0) at 12 to 52 weeks follow‐up. Glycated hemoglobin also modestly and significantly reduced for fluoxetine and orlistat. Gastrointestinal side effects were common with orlistat; tremor, somnolence and sweating with fluoxetine; and palpitations with sibutramine. Some studies, using a variety of study designs, were available on other drugs and a significant decrease in weight was noted in three studies of mazindol, one of phenmetrazine, two of phentermine. No studies were identified that fit inclusion criteria for pseudoephedrine, ephedra, sertraline, yohimbine, amphetamine or its derivatives, bupropion, topiramate, benzocaine, threachlorocitric acid, sertraline, and bromocriptine.
Authors' conclusions
Fluoxetine, orlistat, and sibutramine can achieve statistically significant weight loss over 12 to 57 weeks. The magnitude of weight loss is modest, however, and the long‐term health benefits remain unclear. The safety of sibutramine is uncertain. There is a paucity of data on other drugs for weight loss or control in persons with type 2 diabetes.
Plain language summary
Pharmacotherapy for weight loss in adults with type 2 diabetes mellitus
Obesity is closely related to type 2 diabetes and weight reduction is an important part of the care delivered to obese persons with diabetes. This review of drugs for weight loss among adults with type 2 diabetes revealed weight loss of between 2.0 and 5.1 kg for fluoxetine, orlistat and sibutramine at follow‐up of up to 57 weeks. The long‐term effects remain uncertain. Adverse events were common in all three drugs: gastrointestinal side effects with orlistat; tremor, somnolence, and sweating with fluoxetine; and palpitations with sibutramine. There were few studies examining other drugs used for weight loss in populations with diabetes.
Background
Description of the condition
Diabetes mellitus is a metabolic disorder resulting from a defect in insulin secretion, insulin action, or both. A consequence of this defect is chronic hyperglycaemia (i.e., elevated levels of plasma glucose) with disturbances of carbohydrate, fat, and protein metabolism. Long‐term complications of diabetes mellitus include retinopathy, nephropathy, and neuropathy, and the risk of cardiovascular disease increases over time. For a detailed overview of diabetes mellitus, please see under 'Additional information' in the information on the Metabolic and Endocrine Disorders Group on the Cochrane Library (see 'About the Cochrane Collaboration', 'Collaborative Review Groups‐CRGs'). For an explanation of methodological terms, see the main Glossary on The Cochrane Library.
The prevalence of both obesity and diabetes continues to increase. Obesity rates have risen threefold since 1980 in North America, parts of Europe, the Middle East, the Pacific Islands, Australasia, and China. More than one billion adults worldwide are overweight (body mass index (BMI (kg/m²) ≥ 25); at least 300 million of them are obese (BMI ≥ 30) (WHO 2002; WHO 2003). In the developed world, recent survey data (Flegal 2002) indicate that 65% of American adults are overweight and 31% obese (BM I≥ 30) (NHLBI 1998). The prevalence of diabetes is also rising, with worldwide prevalence estimated at 4.0% in 1995, but expected to rise to 5.4% by 2025 (King 1998). An estimated 135 million adults had diabetes in 1995, a number expected to rise to 300 million by 2025; this represents increases of 42% in developed countries and 170% in developing countries (King 1998). In the United States, the prevalence of diabetes increased 49% from 1990 to 2000 (U.S. DHHS 2002b). Of U.S. adults over age 20, 8.6% have diabetes, of whom one‐third are undiagnosed (U.S. DHHS 2002).
Both obesity and weight gain are major risk factors for diabetes (Maggio 1997; Pi‐Sunyer 1993) and every 1‐kg increase in weight (self‐reported) is associated with a 9% relative increase in the prevalence of diabetes (Mokdad 2000). Eighty to ninety percent of persons with type 2 diabetes are overweight (Wing 2000) and obesity worsens the metabolic and physiologic abnormalities associated with diabetes, particularly hyperglycemia, hyperlipidemia, and hypertension (Maggio 1997).
Description of the intervention
Weight loss is one cornerstone of diabetes care for overweight persons, as it improves insulin sensitivity and glycemic control (Pi‐Sunyer 2000), and moderate, intentional weight loss is associated with reduced mortality (Williamson 2000). Among persons with diabetes, weight loss improves lipid profiles by decreasing triglycerides and low‐density lipoprotein (LDL) cholesterol levels, and weight loss improves blood pressure (Maggio 1997), mental health, and quality of life (Wing 1987; Wing 1991). These benefits are clinically meaningful only if weight loss is sustained over time, however (Wing 1985). The findings of a reduced incidence of hypertension and diabetes in populations with impaired glucose tolerance or obesity that maintained weight loss over extended periods provide indirect evidence of this benefit (DPP 2002; HT Trials 1997; Tuomilehto 2001).
Dietary and behavioral treatment for weight loss can produce an average loss of 8% of initial body weight over 3 to 12 months (NHLBI 1998), but it is difficult to define effective weight control measures for the long term in general populations (NHLBI 1998; O'Meara 1998). The majority of obese patients regain most of the weight initially lost in successful interventions (Maggio 1997; Wing 1985; Wadden 1989). Studies suggest that persons with diabetes lose less weight than persons without diabetes and regain their weight more rapidly, although the mechanisms responsible are unclear and the validity of this observation has not been systematically examined (Wing 2000). Obese or overweight persons with diabetes may face additional barriers than non‐diabetic persons trying to achieve weight loss. The use of insulin to achieve glycemic control may produce weight gain. The complex treatment regimens for diabetes, hypertension, and hyperlipidemia all complicate behavioral change aimed at weight reduction. In addition, Wing has noted that obese persons with diabetes who present for treatment are older and sicker than persons without diabetes (Wing 1985).
Obesity may be viewed as a chronic disease (NIH 1985); Greenway (Greenway 1999) suggests that obesity should therefore be treated as such and that optimal management may require long‐term pharmacotherapy. In patients who have failed behavioral therapy, adjunct treatment with drugs may help them reduce or maintain their weight while improving other parameters of health, including glycemic control and lipid profiles. Numerous anti‐obesity agents have been used for weight loss in general populations as well as in persons with diabetes (Yanovski 2002). These drugs act through a variety of mechanisms, including centrally acting appetite suppression (e.g., sibutramine and phentermine), increased energy expenditure (e.g., ephedrine and caffeine), and nutrient partitioning via decreased food absorption from the gastrointestinal tract (e.g., orlistat). Anti‐obesity drugs may be available over‐the‐counter or by prescription. Some drugs with other specific clinical indications are associated with weight loss (e.g., metformin), and many drugs are used for weight loss although they are not approved for that indication (i.e., off‐label usage, e.g., fluoxetine).
Because obese and overweight adults with type 2 diabetes benefit from weight loss but may have more difficulty losing weight than persons without diabetes, we need to define the scope of our knowledge about the efficacy of pharmacologic interventions for losing weight or preventing weight gain in these populations. We must determine which, if any, drugs are effective in obese and overweight persons, particularly in the long term, and we must define the nature and incidence of side effects. In addition, we must define areas of uncertainty where further research is needed.
Why it is important to do this review
We have identified four recent reviews of anti‐obesity pharmacotherapy for type 2 diabetes. Scheen and Lefebvre (Scheen 2000) and Scheen and Ernest (Scheen 2002) discussed the effects of anti‐obesity drugs on weight loss, glycemic control, and cardiovascular risk profile for obese persons with type 2 diabetes. Greenway 1999 reviewed the use of a broad range of anti‐obesity agents among persons with diabetes. Hauner 1999 discussed both the impact of antidiabetic agents on weight and the effect of weight management drugs on glycemic control in obese diabetic patients.
None of these articles was a systematic review, involved quantitative syntheses, or assessed the quality of individual studies. In addition, none examined a broad range of outcomes, such as morbidity, mortality, and quality of life. Thus, to date we have not located any quality systematic reviews on the efficacy of drugs for weight loss or weight maintenance in overweight and obese adults with type 2 diabetes.
Objectives
To assess the efficacy of pharmacotherapy for weight loss and the maintenance of weight loss in adults with type 2 diabetes.
Primary research question
What drugs are effective in achieving or maintaining weight loss in overweight and obese adults with type 2 diabetes?
Secondary research questions
What additional interventions are delivered with drug therapy and how do they affect outcomes?
What side effects/complications of the drugs are reported?
How does the follow‐up interval relate to outcomes?
What are the effects of the weight loss interventions on glycemic control, blood pressure, and lipid profiles?
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials only were included in the review of efficacy as these minimize the potential effects of bias on our results. All study designs, however, were included in the review of adverse events: those with a contemporaneous comparison group (randomized controlled trial, non‐randomized trial, or observational study with a concurrent comparison group), or a pre‐versus‐post design, a cross‐sectional design, case‐control studies, or a case series. We recognize the potential for bias from confounding and secular trends in studies without randomization, but because observational studies yield important information on adverse events related to treatment, particularly on rare, long‐term side effects (Elphick 2002), we searched for, and synthesized in narrative form, the available observational data on side effects. In our protocol we indicated that we would include additional comparative study designs if we had found an insufficient number of randomized, controlled trials. Since we identified a sufficient number of randomized trials, we only included this study design in the review for efficacy.
Length of follow‐up and timing of outcomes measurement
We included studies of any duration and length of follow‐up. We defined follow‐up from the time of randomization (or for studies without randomization, from the time of entrance into the study) until the last outcomes measurement. We recognize that long‐term outcomes are of paramount importance, but examination of the efficacy of pharmacotherapy in the short term also has value. For example, if weight loss can be demonstrated with drugs in the short term, pharmacotherapy may be combined with behavioral interventions for long‐term weight control. In addition, an exploration of the relationships between the population and intervention characteristics and the efficacy of these drugs in the short‐term may provide insights into how to achieve longer‐term success.
Full text and abstracts
Both full‐text publications and abstracts are included in this review. Because it is more difficult to assess the quality of abstracts, data from these publications was analyzed both separately and combined with full‐text publications.
Publication status
We examined published data only, as we had no success in obtaining unpublished data from private or public sector sources.
Types of participants
Age
Studies included adults aged 18 years or older with type 2 diabetes. If the type of diabetes was not specified, studies were included if they involved adults with diabetes, with or without insulin treatment. Persons labelled with "NIDDM" were assumed to have type 2 diabetes. Studies involving only "IDDM" participants were excluded unless there was information to indicate that they have type 2 disease (e.g., concurrent use of oral hypoglycemic agents and insulin). Studies that include participants without diabetes were also included if there were outcome data on the subpopulation with diabetes.
Type 2 diabetes
The acceptable diagnostic criteria for diabetes includes those described by the standards of the National Diabetes Data Group (Data Group 1979), the World Health Organization (WHO Committee 1980; WHO Committee 1985; Alberti 1998), or the American Diabetes Association (Expert Committee). If the diagnostic criteria were not given in the study, the authors' statement of the diagnosis of diabetes among participants was accepted.
Overweight or obese
Participants were overweight as defined in the study; there was no minimum weight or BMI at baseline.
Types of interventions
Any drug therapy delivered for the primary purpose of losing and/or controlling weight was included. Studies that combined pharmacotherapy with other weight loss strategies, including behavioral, educational, lifestyle (diet and exercise), or surgical interventions, were included. Both prescription and over‐the‐counter medications were included. Drugs that were not approved for weight loss, but which were used for the primary purpose of weight loss were included (i.e., off‐label usage of the drug, e.g., fluoxetine).
The drugs examined included:
Centrally acting appetite suppressants:
Amphetamine/dextroamphetamine;
Bupropion;
Diethylpropion;
Fluoxetine;
Mazindol;
Methamphetamine/benzphetamine;
Phenmetrazine/Phendimetrazine;
Phentermine;
Sibutramine;
Topiramate;
Yohimbine.
Peripheral effect on appetite:
Benzocaine.
Nutrient partitioning:
Orlistat/tetrahydrolipstatin;
Treacholorocitric acid.
Increase thermogenesis:
Ephedra alkaloids;
Caffeine.
Combined drug therapy:
Ephedrine and caffeine.
Comparison groups:
Studies that involved a comparison group with a different intervention were included regardless of the nature of the comparison intervention. We included studies with a range of comparison groups as we wanted to determine which interventions were more effective than others.
The comparison group could receive:
placebo;
no intervention;
usual care;
Any other weight loss intervention: behavioral strategy, dietary program, physical activity program, surgery, other.
Types of outcome measures
Primary outcomes
Weight or BMI must be measured at both baseline and follow‐up in order for the study to be included in this review.
weight and body fat distribution: weight (kg), BMI (kg/m²);
drug‐related morbidity: severe (necessitating withdrawal) or minor;
quality of life.
Secondary outcomes
glycemic control: glycated hemoglobin, fasting blood sugar;
serum lipids;
blood pressure;
non drug‐related morbidity;
mortality.
Exclusion criteria
study populations with binge eating or other eating disorders were excluded from this review.
drugs withdrawn from market in the U.S. were excluded, including fenfluramine, dexfenfluramine, and phenylpropanolamine.
investigational drugs, defined as those drugs not yet approved for use in the U.S., were excluded (e.g., leptin, beta‐2 agonists such as BRL‐26830A).
herbal supplements, including ginseng and a number of other herbal supplements that are not regulated by the United States Food and Drug Administration, were excluded.
drugs that may produce weight loss but whose primary purpose is another clinical indication were excluded. These include metformin, acarbose, and benfluorex, all of which may produce weight loss but are used primarily for glycemic control. We recognize that the clinical indications for these drugs may change and that in the future they may be regarded as drugs whose primary purpose includes weight loss.
Search methods for identification of studies
Electronic searches
A number of electronic databases were screened for potentially relevant titles and abstracts. There were no language restrictions on our searches. Conference proceedings and abstracts were included in the review but not in the primary pooled analysis, because they had insufficient detail to evaluate the intervention and the quality of the study. These are summarized in narrative form and presented as potentially important studies that may appear in future in the literature. Dissertations were excluded, as they were difficult to locate in full text.
The following electronic databases were searched between the date in parentheses and June 30, 2004.
The Cochrane Library (Issue 3, 2003), including Cochrane Controlled Trials Register, DARE, CRG specialized registers;
MEDLINE (1966) (includes Healthstar);
EMBASE (1974);
CINAHL (1982);
Web of Science (1981);
Biosis (1980);
International Pharmaceutical Abstracts (1970).
For the detailed MEDLINE search strategy see under Appendix 1. The search strategy was improved with minor modifications, from the protocol.
Other searches are available upon request.
Searching other resources
The following journals, believed to be of high topic relevance, were hand searched from 1980 to February 2003: Diabetes Care; International Journal of Obesity and Related Metabolic Disorders (prior to 1992 this journal was the International Journal of Obesity);Obesity Research (journal commenced in 1993);American Journal of Clinical Nutrition;Journal of the American Dietetic Association.
Potential missing and unpublished studies were sought by contacting experts in the field and authors of relevant identified studies as well as drug manufacturers. The reference lists of all relevant review articles and of the studies included in the review were reviewed. The National Heart, Lung, and Blood Institute 1998 review (NHLBI 1998) and a review by the University of York, National Health Centre for Reviews and Dissemination (York CRD 1997) were examined for relevant citations.
Data collection and analysis
Selection of studies
Search results for MEDLINE and CINAHL were examined by two authors (SLN and XZ) and the remaining databases by one author (SLN). Potentially relevant full‐text articles were then reviewed for inclusion (SLN); if there was uncertainty about inclusion, a second author (AA) reviewed the paper and consensus was achieved. Due to resource constraints and the need for efficiency, only SLN reviewed the full text for potential inclusion (this is a change from the protocol). AA provided secondary review and consensus was achieved for studies when SLN had any uncertainty. After consensus was reached between AA and SN, XZ screened all included papers to confirm inclusion.
Data extraction and management
For studies that fulfilled inclusion criteria, two reviewers abstracted the relevant data using a standardized template. Extraction was not blinded, as there is no evidence that such blinding decreases bias in conducting systematic reviews and meta‐analyses (Berlin 1997; Irwig 1994). We attempted to contact study authors for missing data or when we needed clarification of the data presented. For continuous outcomes we extracted for each study group the baseline sample size, pre‐ and post‐intervention mean, and a measure of dispersion (SD [standard deviation], standard error of the mean (SEM), or 95% confidence interval) for the intervention and comparison groups. If the post‐intervention measures of dispersion were not available, they were assumed to be the same as the pre‐intervention measures. When necessary, mean and SD were approximated from figures using an image scanner to optimize resolution. For dichotomous variables (e.g., mortality) the number of participants, person‐years, and the number of events were extracted for each study group.
Assessment of risk of bias in included studies
Internal validity
Internal validity was assessed by two reviewers for each study. For randomized controlled trials (RCTs), the component assessment method of Cochrane was used (Clarke 2003) as well as the Jadad score (Jaded 1998). For the former method, the following quality criteria were assessed as "met" or "unmet": 1. Minimisation of selection bias: a) Was the randomization procedure adequate? b) Was the allocation concealment adequate? 2. Minimisation of performance bias: Were the participants and those administering the treatment blind to the intervention? 3. Minimisation of attrition bias: a) Were withdrawals and dropouts completely described? b) Was analysis by intention‐to‐treat? 4. Minimisation of detection bias: Were outcome assessors blind to the intervention?
The risk of bias was assessed as low (A) (all criteria were met), moderate (B) (one or more criteria were only partly met), or high (C) (one or more criteria were not met).
For studies that were not RCTs, the comparability of groups at baseline and attrition were noted. Studies were not excluded because of poor quality; where data were sufficient the effect of potentially biasing factors on outcomes was examined.
Other design issues
In addition to the above‐mentioned components of the assessment of internal validity, we noted whether the study used an intention‐to‐treat analysis and whether the last‐outcome measurement was carried forward (LOCF) to subsequent follow‐up measurements.
Assessment of heterogeneity
Data were pooled using the random effects model and using the DerSimonian and Laird formula for calculating between‐study variance (DerSimonian 1954). Each study was weighted by the inverse of the study variance. Heterogeneity between trial results was tested using a standard chi‐square test (Cochran 1954) with a significance level of alpha = 0.1 in view of the low power of such tests. When we found heterogeneity we tried to explain it by examining individual study characteristics and those of subgroups of the main body of evidence. When heterogeneity was thought to be too great to meaningfully pool the results quantitatively, the results are presented in a narrative fashion.
Assessment of reporting biases
Funnel plots were used in exploratory data analysis to assess for the potential existence of small sample bias. An asymmetrical funnel plot, however, has several explanations, including true heterogeneity of effect with respect to study size, poor methodological design of small studies (Sterne 2001; Tang 2000; Thornton 2000), and publication bias. Thus, we did not place undue emphasis on this tool.
Data synthesis
Statistical pooling
Where data from RCTs were thought to be sufficiently homogeneous with respect to interventions and outcomes, we calculated pooled effect sizes. For continuous variables reported on the same scale we calculated weighted mean differences. The absolute differences in outcome between each follow‐up and the baseline measure for the intervention and comparison study group (ΔI and ΔC) were calculated and inserted in Review Manager Software (Review Manager 4.2). When the estimate of variance of (ΔI) and (ΔC) was not given, it was calculated from the outcome measures in each study group using the formula Vpre+ Vpost ‐ 2r(SDpre*SDpost), where Vpre is the variance of the mean baseline outcome, Vpost is the variance of the mean follow‐up outcome, r is the correlation between the baseline and follow‐up values, and SDpre and SDpost are the standard deviations of the baseline and follow‐up groups, respectively. Since most studies do not report r, and its true value is unknown, data are presented with r = 0.75, and a sensitivity analysis was performed as described below.
Regression analyses
We performed a meta‐regression to determine whether various study‐level characteristics affect weight change and GHb. The meta‐regression was also weighted by the inverse of the variance of (ΔI ‐ ΔC). Interaction terms were examined for all models. The study‐level variables examined in the meta‐regression model included follow‐up interval, the number of contacts between the care provider and participants, and the percentage attrition in the intervention group. SAS was used to perform the meta‐regression (version 8.01, SAS Institute Inc., Cary, NC).
Subgroup analysis and investigation of heterogeneity
We planned analyses by the following subgroups if there was a significant change in weight and the amount of data would allow meaningful analyses:
overweight (25.0 ≤ BMI ≤ 30.0), obese (BMI >30.0), normal weight (BMI <25.0);
age: young (<40 years), middle‐aged (40 to 65 years), old (>65 years);
treatment: on insulin, oral agents, diet only;
sex;
race / ethnicity;
time frame over which the intervention was delivered.
Sensitivity analysis
Analyses were planned to examine the effect of internal validity on study results, using the categories of low, moderate, and high risk of bias. Sensitivity analyses were also used to compare the fixed and random effects model and using different values of the correlation between pre‐ and post‐ measures (0.25, 0.5, and 1.0).
Results
Description of studies
Figure 1 presents the review flow diagram. No studies were identified that fit inclusion criteria for amphetamine and its derivatives, benzocaine, bromocriptine, bupropion, ephedrine, ephedra, pseudoephedrine, sertraline, threachlorocitric acid, topiramate, and yohimbine. Data were sufficient to perform a quantitative analysis of fluoxetine, orlistat, and sibutramine, and thus this review focuses initially on these three drugs. We then provide narrative summaries of the results for cimetidine, diethylpropion, mazindol, phenmetrazine, and phentermine.
1.
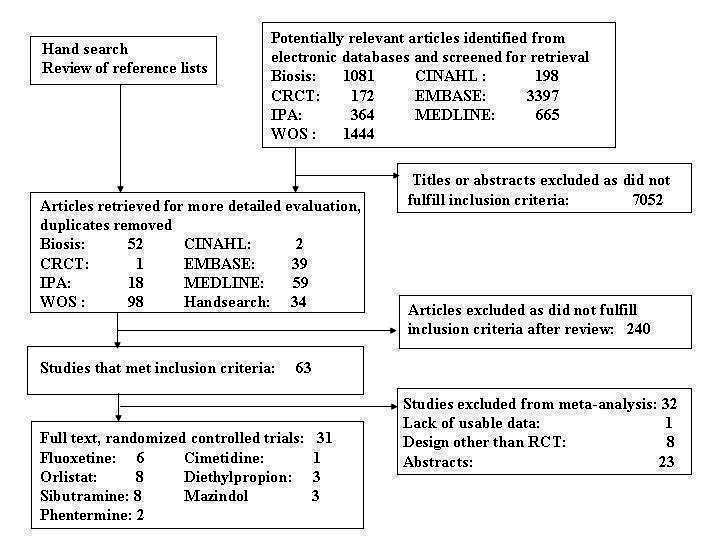
Study flow diagram CRCT, Cochrane Controlled Trials Register IPA, International Pharmaceutical Abstracts WOS, Web of Science
Fluoxetine, orlistat, and sibutramine
Characteristics of the 22 eligible RCTs examining fluoxetine, orlistat, and sibutramine are shown in Appendix 3, Appendix 4, Appendix 7, Appendix 9 and Appendix 12. These studies included 296 participants who received fluoxetine, 2036 orlistat, and 1047, sibutramine. Follow‐up intervals ranged from 8 to 52 weeks for fluoxetine, 12 to 57 weeks for orlistat, and 12 to 52 weeks for sibutramine. Most studies used a run‐in period lasting 1‐5 weeks, where a placebo was given and dietary counselling started. Generally the duration of drug treatment was the same as the follow‐up interval, although in three studies weight change was recorded from the beginning of the run‐in period (Hollander 1998; Hanefeld 2002; Lindgarde 2000). Only one study (Gray 1992) examined weight maintenance after discontinuation of the study drug. Study participants' mean age was between 48 and 66 years across studies and somewhat more than half were female. There were insufficient data to draw conclusions from the funnel plots.
Mean weight of the control group at baseline was 95 kg (SD 18.5 kg) for fluoxetine, 95.9 kg (11.1 kg) for orlistat, and 97 kg (17.3 kg) for sibutramine. BMI was presented in only 14 of the studies (range 31 to 37). Participants generally had poor glycemic control by current treatment standards (ADA 2003). Most studies excluded patients who were taking insulin, although two studies examined insulin‐using subjects exclusively (Gray 1992; Kelley 2002). Drug dosages were very consistent among studies, except for one study of sibutramine that used a twice‐daily dosage regime (Gokcel 2001). All studies examined continuous therapy. All except one study of fluoxetine (O'Kane 1994) involved a dietary intervention for both the treatment and control groups, and the comparison groups all received a placebo. Average contacts ranged from 2 to 18, an average of 1.1 per month. Attrition during the run‐in period ranged from 1.5% to 22% in the studies where it was reported (Hollander 1998; Lindgarde 2000; Gray 1992; Finer 2000; Hanefeld 2002) In three studies (Hanefeld 2002; Hollander 1998; Daubresse 1996; Daubresse 1996) participants were randomized only if they had high rates of compliance for visits or pill consumption during the run‐in period.
Risk of bias in included studies
Fluoxetine, orlistat and sibutramine
The sampling frame and subject recruitment methods were rarely described. Only two studies described the randomization process (Zelissen 1992; Redmon 2003) and one discussed allocation concealment (Redmon 2003). In 18 of the 22 trials the drug's manufacturer supported the study. Attrition varied considerably; for the intervention group it ranged from 0% to 49%; for the control group from 0% to 52%. In seven of 20 studies where attrition rates were reported, the control group had a higher rate than did the intervention group, including four of seven studies of orlistat (Hollander 1998, Kelley 2002; Miles 2002). Most studies were described as double‐blinded (16 of 22), but none reported exactly which two parties were blinded. One study was open label (Tankova 2003). In nine studies LOCF measures were used in the event of attrition (Kelley 2002; Kutnowski 1992; Miles 2002; Fujioka 2000; Serrano‐Rios 2001; Hanefeld 2002; Redmon 2003; Kaukua 2004; Bloch 2003). Most studies reported using intention‐to‐treat methods of analysis, but several excluded participants for protocol violation (Hanefeld 2002; Fujioka 2000; Kutnowski 1992; Miles 2002; Kelley 2002; Wang 2003), noncompliance (Hanefeld 2002; Miles 2002; Hollander 1998; Lindgarde 2000), or treatment failure (Miles 2002). A study examining sibutramine (Halpern 2003) fit our inclusion criteria, but because of numerous inconsistencies noted in the presentation of data in the paper, this study was not included in the meta‐analysis.
Effects of interventions
Fluoxetine, orlistat, and sibutramine
Change in weight (kg) and GHb (%) for full‐text studies of fluoxetine, orlistat, and sibutramine are shown in Figure 2 and Figure 3,and the meta‐analysis results are presented in Appendix 15, Appendix 17, and Appendix 20. A summary of pooled effects for fluoxetine is found in Appendix 27, and for orlistat and sibutramine in Appendix 28. Weight loss ranged from 10.5 kg for sibutramine at 26 weeks follow‐up (95% CI, 7.6 ‐ 13.4) (Gokcel 2001) to 1.4 kg for fluoxitine at 8 weeks of follow‐up (95% CI, 0.2 to 2.6) (Kutnowski 1992). The pooled effects were the following: orlistat over all follow‐up periods demonstrated a loss of 2.0 kg (95% CI, 1.3 to 2.8); sibutramine over all follow‐up periods produced a loss of 5.1 kg (95% CI, 3.2 to 7.0); loss for fluoxitine at 8 to 16 weeks was 3.4 kg (95% CI, 1.7 to 5.2), 24 to 26 weeks was 5.1 kg (95% CI, 3.3 to 6.9), and one study examining fluoxetine at 52 weeks produced a loss of 5.8 kg (95% CI, 0.8 to 10.8).
2.
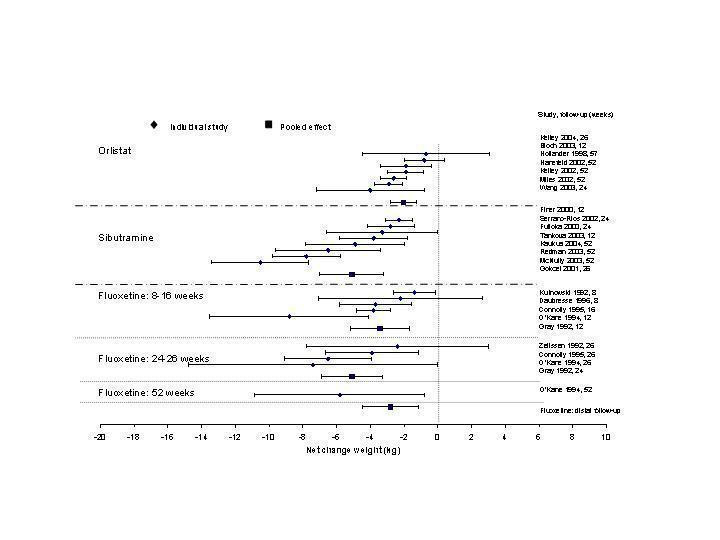
Net change in weight (kg) Pooled estimates are represented by boxes.
3.
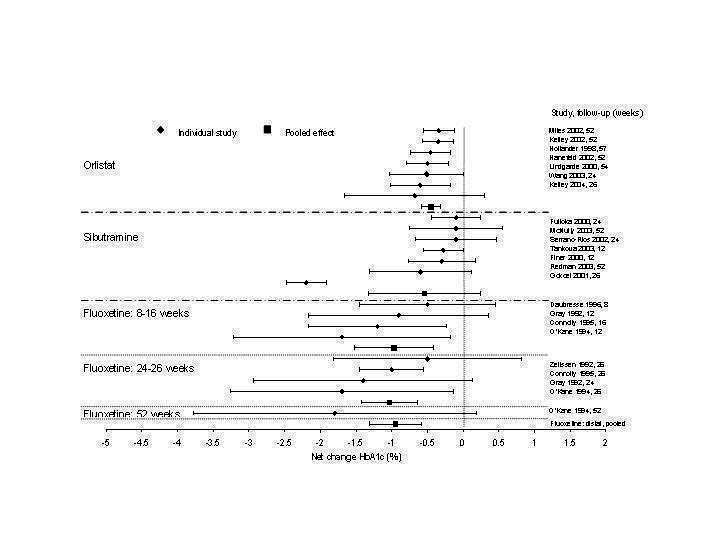
Net change in hemoglobin A1c (Hb A1c) (%) Pooled estimates are represented by boxes.
Reduction of GHb ranged from 2.2% for sibutramine at 26 weeks follow‐up (Gokcel 2001) to 0.1% for three studies of sibutramine at follow‐up intervals of 24 and 52 weeks (Fujioka 2000; McNulty 2003; Serrano‐Rios 2002). Gokcel and colleagues used a dose of sibutramine of 10 mg twice daily, unlike other studies which used 15 or 20 mg in a single daily dose. One study published only as an abstract utilized up to 30 mg as a single daily dosage (Vargas 1994). The pooled reduction for GHb was 0.5% (95% CI, 0.3 to 0.6) for orlistat (follow‐up between 24 and 57 weeks); sibutramine 0.5% (95% CI, ‐0.2 to 1.3) (follow‐up 12 to 52 weeks); fluoxetine 1.0% (95% CI, 0.4 to 1.5) at 8 to 16 weeks, 1.0% (95% CI, 0.6 to 1.4) at 24‐26 weeks, and one study with a follow‐up of 52 weeks demonstrated a reduction of 1.8% (95% CI, ‐0.2 to 3.8) (O'Kane 1994).
Several studies examined more than one follow‐up interval (O'Kane 1994; Gray 1992; Connolly 1995); these results are shown in Figure 2 and Figure 3. Fluoxetine had sufficient studies to allow stratification by treatment duration. Weight loss was slightly greater with longer follow‐up, but differences were small and only one study examined fluoxetine for longer than 30 weeks of treatment (O'Kane 1994). We identified 22 studies published only as abstracts for fluoxitine (four total, all RCTs), orlistat (14 total, 9 RCTs), and sibutramine (four total, three RCTs) that fulfilled our inclusion criteria: six for fluoxetine, 20 for orlistat, and seven for sibutramine. Pooled effects obtained by combining abstracts of RCTs and full‐text RCTs did not produce significant changes in the direction or significance of results of the full‐text studies only.
We performed a sensitivity analysis making two different assumptions about the behavior of intervention group dropouts. After excluding studies that used last outcome carried forward data‐reporting techniques, we had three sibutramine, two orlistat, and five fluoxetine studies remaining. We therefore performed the sensitivity analysis only for fluoxetine. When we assumed that none of the dropouts had a weight change, the pooled reduction in weight was 3.0 kg (95% CI, 1.4 to 4.6), and when we assumed that dropouts had weight changes equivalent to those of the control group, the pooled reduction was virtually identical 3.0 kg (95% CI, 1.5 to 4.6). These estimates did not differ a great deal from the pooled estimate of 4.0 kg (95% CI, 2.0 ‐ 5.9) for the five studies eligible for this analysis (O'Kane 1994; Zelissen 1992; Daubresse 1996; Connolly 1995; Gray 1992).
Using the between‐group change for each study, we performed a meta‐regression to investigate potential interactions of weight loss with study‐level variables, including follow‐up interval, number of contacts between care provider and participant, and percentage attrition in the intervention group. None of the interactions was significant.
Because all but one study involved a dietary intervention combined with the drug or placebo, we could not investigate whether the addition of a lifestyle or behavioral intervention added to the efficacy of a drug. Nor did we have enough studies in different strata to examine the relationship between patient characteristics (e.g., age, race, baseline weight, GHb) and change in weight.
We attempted to explore the relationship between the score for risk of bias and change in weight, but we did not have enough studies to stratify into categories (A, B and C) for each drug. We examined the relationship between risk of bias and weight change in a regression model, however, and found no significant interaction.
We did not have sufficient data to perform subgroup analyses for weight, age, sex, diabetes treatment, race / ethnicity, or duration of treatment. In particular, we were not able to examine the effect of metformin or acarbose on weight loss, as only two full‐text studies reported participants using metformin (Fujioka 2000; Gokcel 2001), and none using acarbose.
Since we had statistical heterogeneity for our pooled estimates of weight change ,we present the random effects model in our summary pooled effects. Both fixed and random effects models are presented in Appendix 21, Appendix 22, Appendix 23, Appendix 24, Appendix 25, and Appendix 26. These tables also contain the pooled effects using different values of the correlation between pre‐ and post‐ measures (0.25, 0.5, and 1.0); in no case was there a significant change in the results compared to using a value of 0.75.
Sibutramine studies showed significant heterogeneity for both weight (Chi‐squared test for heterogeneity, P < 0.0001) and GHb (p < 0.0001). The study by Gokcel and colleagues (Gokcel 2001) utilized twice daily dosing for sibutramine and had more marked improvements in both weight and GHb. The pooled effect excluding this study was a reduction in weight of 4.3 kg (95% CI 2.7 to 6.0) and in GHb of 0.2% (95% CI 0.4 to 0.04). Heterogeneity remained significant for weight (p < 0.0001), but was no longer significant for GHb (p = 0.84)
There were few data available for fluoxetine on other outcomes (Appendix 15). Orlistat was associated with statistically significant improvements in total cholesterol, LDL, and triglycerides, that were sustained at 52 weeks follow‐up. Several studies examined the effects of sibutramine on blood pressure (Finer 2000; Fujioka 2000; McNulty 2003; Serrano‐Rios 2002; Redmon 2003; Kaukua 2004) and lipids (Fujioka 2000; Gokcel 2001; McNulty 2003; Serrano‐Rios 2002; Kaukua 2004; Redmon 2003), and a decrease in systolic blood pressure of 0.8 mm Hg, 95% CI, 0.02 to 1.65) and in triglycerides (0.3 mmol/L (95% CI 0.04 to 0.50)).
Adverse events for fluoxetine, orlistat, and sibutramine are summarized in Appendix 2. Adverse events were common in all three drugs, both in the intervention and control groups. Rates of gastrointestinal side effects with orlistat were about 20 percentage points higher in the treatment groups than in control groups. Tremor, somnolence, and sweating were common with fluoxetine, and palpitations with sibutramine. We included a study by Bach and colleagues (Bach 1999) which did not fulfil our inclusion criteria as no weight outcomes were presented, but the study examined cardiac value dysfunction among persons with diabetes using sibutramine, and we felt it was important to include this study in our narrative presentation of adverse events.
Narrative synthesis of other drugs
There were studies in the literature examining the efficacy of five other drugs for weight loss in adults with type 2 diabetes: cimetidine, diethylproprion, mazindol, phenmetrazine, and phentermine (Appendix 5, Appendix 6, Appendix 8, Appendix 10; Appendix 11, Appendix 13, Appendix 14, Appendix 16, Appendix 18, Appendix 19). There were insufficient data on these drugs for quantitative syntheses, therefore the results will be described in a narrative fashion.
One study examined the efficacy of cimetidine for weight loss in a double blind RCT (Stoa‐Birketvedt 1998) with 12 weeks of treatment. They noted a nonsignificant decrease in weight of 3.7 kg associated with a small improvement in glycemic control. Side effects included diarrhoea (10%), and one patient each with arthralgia, abdominal pain, and vomiting. No other literature was located on cimetidine that fit our inclusion criteria.
Three RCTs (Bratusch‐Marrian1979; Silverstone 1966; Williams 1968) and two pre versus post design studies (Montenero 1964; Hendon 1962) examined the efficacy of diethylpropion for weight loss. These were mostly older studies with sample size between 40 and 58 and follow‐up from 8 to 40 weeks. Weight change was the only outcome examined in these studies, and 2 RCTs demonstrated significant weight loss of 1.6 kg (95% CI 0.2 to 3.0) (Bratusch‐Marrian1979), 8.8 kg (95% CI 6.9 to 10.7) (Hendon 1962). In a pre versus post design study, Montenero and colleagues (Montenero 1964) demonstrated a loss of 5.3 kg (95% CI 4.1 to 6.4). Side effects were noted in two studies: dry mouth (13%) (Silverstone 1966) and headache and nausea (rate not given) (Hendon 1962).
Mazindol was examined in three full‐text RCTs (Bandisode 1975; Crommelin 1974; Slama 1978), and one abstract (Boshell 1974), as well as one study of uncertain design (Sanders 1976), one pre versus post design study (Dolecek 1976), and one cohort study with a comparison group (Felt 1977). These are all studies from the 1970's, with sample sizes ranging from 10 to 64, and follow‐up between 6 and 12 weeks. Significant weight loss was noted in three studies: Sanders and colleagues (Sanders 1976) 3.3 kg (95% CI 2.5 to 4.1), Slama et al. (Slama 1978) 12.5 kg (95% CI 5.5 to 19.5), and Boshell et al. 1.9 kg (Boshell 1974) (95% CI 3.1 to 0.8). Three other studies also demonstrated favorable changes in weight (Dolecek 1976; Bandisode 1975; Crommelin 1974; Felt 1977). None of these studies noted significant changes in fasting blood sugar. Constipation was not infrequent (Felt 1977; Dolecek 1976); other side effects included dry mouth (Crommelin 1974; Dolecek 1976), nervousness or the sensation of stimulation (Dolecek 1976; Bandisode 1975; Sanders 1976), and headache (Sanders 1976; Felt 1977; Bandisode 1975).
Phenmetrazine was only examined in one small study (Buckle 1966) which compared participants taking phenmetrazine hydrochloride to those taking a combination of phenmetrazine theoclate and phenbutrazate hydrochloride. This was a cross‐over study, and the results were presented for both groups combined. A significant decrease in weight was noted (2.9 kg (95% CI 2.3 to 3.6)) and no other outcomes were measured. Side effects included dizziness (20%), abdominal discomfort and nausea (15%), and dry mouth (5%).
Two studies examined phentermine (Campbell 1977; Gershberg 1977). It was unclear whether an abstract (Gershberg 1972) overlapped with the full text paper (Gershberg 1977). Follow‐up intervals were 16 to 26 weeks and a weight loss of 3.8 kg (95% CI 2.3 to 5.3) (Campbell 1977) and 5.7 kg (95% CI, 1.9 ‐ 7.9) were noted. Small, favorable changes in fasting blood sugar (p > 0.05), total cholesterol (P < 0.05), triglycerides (p < 0.05), and blood pressure (p > 0.05) were noted. Irritability and insomnia were noted in the first week of treatment in one study (Gershberg 1977), and dry mouth and a minor sleep disturbance in the other (Campbell 1977).
Discussion
This meta‐analysis provides evidence that fluoxetine, orlistat and sibutramine can achieve modest but statistically significant short‐term weight loss when used as a primary weight reduction strategy among adults with type 2 diabetes. Since treatment duration was up to 57 weeks for these three drugs, the long‐term effects of these drugs on weight and health outcomes in persons with type 2 diabetes remain uncertain. Across studies, participants were middle aged, were for the most part not using insulin, and were in moderately poor glycemic control. BMI was infrequently reported, making it difficult to characterize the degree of overweight of participants. Since study populations might be highly selected and run‐in periods eliminated noncompliant participants in some studies, our findings should be considered generalizable only to similar populations and not, for example, to the elderly.
There were few studies examining other drugs used for weight loss in populations with diabetes. Significant weight loss was seen in a small number of studies examining mazindol, phenmetrazine, and phentermine.
Weight loss from pharmacotherapy in nondiabetic populations is generally also modest, ranging from 2 kg to 10 kg; weight is usually regained after discontinuation of the drug; and generally there is no difference between treatment and placebo groups several months after treatment ends (National Task Force). The rather small reductions in weight noted in the current review may reflect the difficulty persons with diabetes have in losing weight (Wing 2000). Greenway (Greenway 1999) compared weight loss with orlistat and sibutramine in populations with and without diabetes and noted that weight loss was 52% and 69% greater for the subjects without diabetes.
This review has important limitations. Publication bias is possible in weight loss intervention studies (Allison 1996) and pharmacotherapy trials, which are often sponsored and financed by drug manufacturers. We attempted to obtain unpublished studies from the manufacturers of each of the included drugs as well as from researchers in this field, but received no data. We tried to minimize language bias by not excluding studies based on language of publication. Published drug trials funded by for‐profit organizations have been shown to have more positive conclusions about the drug than studies funded by nonprofit organizations (Als‐Nielson 2003). Although the causes for this association are not known, possible explanations include biased interpretation of trial results and reporting. The quality of individual studies in this review was fairly consistent and common deficiencies were noted. Methods for concealing allocation (Clarke 2003) were described in only one study, and randomization method was described in only two. Most studies were described as double‐blind, but it was unclear which two parties were blinded. As Devereaux and colleagues have discussed (Devereaux 2002), the term double‐blind can have various definitions and interpretations among clinicians and researchers. Blinding may be difficult due to drug specific adverse events, for example, gastrointestinal side effects with orlistat. The reported data were too homogeneous to explore the effects of allocation concealment and blinding on outcomes. The quality of descriptive information on study population, setting, and the intervention was generally adequate. Sampling frame and the method of recruitment and selection of participants were rarely described, however, making it difficult to conclude from individual and pooled studies, to whom the interventions can be applied.
Attrition is an important issue in weight‐loss studies because selective loss to follow‐up has been demonstrated; higher attrition occurs among those who do not achieve a weight‐loss goal (Kaplan 1987). Attrition was often very significant in the control group, particularly for orlistat, perhaps because control participants became unblinded due to fewer gastrointestinal adverse events and had weight loss expectations that were not being fulfilled. Last‐outcome‐carried‐forward data were presented in a number of studies, which could have variable effects on measured outcomes depending on when the participant dropped out. If drug treatment was effective and the participant dropped out early after achieving minimal weight loss, final outcomes would be biased toward the null effect. If participants dropped out after 4 to 6 months in the longer follow‐up studies, however, their departure weight might have been lower than it would have been had they completed the study, as other researchers have noted that weight loss with pharmacotherapy tends to plateau at 6 months (Goldstein 1994a; National Task Force). We had to exclude from our sensitivity analysis of the effect of attrition in intervention groups, studies that used Last‐outcome‐carried‐forward techniques. Ideally, researchers would provide complete data on all subjects, including last measured weight and time and reason for attrition, particularly in studies of longer duration. The sensitivity analysis for fluoxetine demonstrates that with conservative assumptions for weight loss in the intervention dropouts, weight loss is smaller but remains statistically significant.
Orlistat, sibutramine and fluoxetine were generally well tolerated, and produced a low incidence of serious adverse events. Participants who took orlistat noted a high incidence of minor gastrointestinal side effects, as would be expected from the drug's mechanism. The use of orlistat has been associated with lower levels of fat soluble vitamins and supplementation (O'Meara 1998), although this was only evident in one study in this review (Hollander 1998). A variety of minor gastrointestinal and other side effects were noted with fluoxetine, a selective serotonin reuptake inhibitor (Yanovski 2002) and no cases were reported of withdrawal due to major adverse events.
Sibutramine produced palpitations and a nonsignificant increase in pulse rate consistent with its mechanism as a reuptake inhibitor of serotonin, norepinephrine, and dopamine (Yanovski 2002). Palpitations led to withdrawal from one study in two of 69 patients (Serrano‐Rios 2002). Major adverse cardiovascular events were not noted and rates of rhythm disturbances were similar in the intervention and control groups (Finer 2000). We found no significant blood pressure increase with sibutramine, however, only four studies reported this outcome (Serrano‐Rios 2002; Finer 2000; Fujioka 2000; McNulty 2003). Concerns have been raised about the safety of sibutramine after review of post‐marketing data (Wolfe 2002). Health Canada and a number of European countries are reviewing the safety of sibutramine, and Italy temporarily suspended marketing of the drug in March 2002 after adverse events (tachycardia, hypertension, arrhythmias) and two deaths were associated with use of the drug (Health Canada).
In nondiabetic populations, comprehensive, intensive group behavioral programs without pharmacotherapy produce mean losses of 8 kg to 10 kg at six months with a regain of 30% to 35% of weight loss at one year; 50% of participants have returned to baseline weight at 3 to 5 years (Kramer 1989; Wadden 2000). Brown and colleagues reviewed the effectiveness of weight‐loss interventions in persons with diabetes (Brown 1996), and noted that dietary interventions produced a weight loss of 9 kg and behavioral programs 3 kg, but few studies examined outcomes beyond six months. Pharmacotherapy in persons with diabetes thus appears to be no more efficacious than behavioral therapy at 1 year. Padwal 2004 recently reviewed the efficacy of long‐term pharmacotherapy for weight loss among general populations, including persons with diabetes. They noted a pooled weight change at one year follow‐up of ‐2.7 kg (95% CI 2.3 to 3.1) (11 studies) for orlistat, and 4.3 kg (95% CI 3.6 to 4.9) (five studies) for sibutramine. Similar results were observed in weight maintenance trials at up to tow year follow‐up.
Authors' conclusions
Implications for practice.
Although the weight loss demonstrated in this review is small, evidence in general populations suggests that modest loss may have health benefits. There are positive associations between weight loss and blood pressure, blood glucose, and serum lipid levels over a range of weight loss (Anderson 2001). Although few of our studies examined blood pressure, the magnitude of weight loss demonstrated in this review is equivalent to weight changes that have been efficacious in managing and preventing hypertension in high‐risk individuals over 2 to 4 years (Valdez 2002).
Fluoxetine and orlistat had statistically significant effects on GHb. The reduction of 1.0% for fluoxetine at 8 to 26 week follow‐up was sustained at 52 weeks in one study (1.8%) (O'Kane 1994). This reduction in GHb is encouraging given that the magnitude achieved was similar to that in the United Kingdom Prospective Diabetes Study (UKPDS 1998) and in the Diabetes Control and Complications Trial (DCCT 1993), where 1% absolute reductions in HbA1c resulted in significant reductions in microvascular complications from diabetes.
Orlistat was associated with statistically significant improvements in total cholesterol, LDL, and triglycerides, that were sustained at 52 weeks follow‐up. These changes in lipid levels have been noted by others (NHLBI 1998) and although modest improvements, they correspond to changes associated with a decrease in the incidence of ischemic heart disease (Law 1994). It remains unclear whether the improved glycemic control and lipid levels noted in this review could be maintained over the long‐term to influence the risk of complications as demonstrated in large trials.
The populations in the studies reviewed were generally self‐ or researcher‐selected, and often noncompliant patients were excluded from analyses. Therefore the efficacy of these drugs as delivered in a real‐world setting, will likely be less than that noted in these studies.
Concerns have been raised about adverse cardiovascular effects of sibutramine. Since this review was confined to populations with diabetes, we were not able to present a lot of information on adverse effects. Since persons with diabetes are at particularly high risk of cardiovascular events, the safety of sibutramine is of critical importance in this population, particularly if this drug is used in the long‐term.
Implications for research.
No studies in this review examined the efficacy of pharmacotherapy combined with a comprehensive lifestyle or behavioral‐modification program. In general populations, drugs have been combined with various lifestyle interventions, but most trials include relatively weak lifestyle programs, perhaps in part to better reveal the medication effects (Bray 1999a). There is some evidence that adding a lifestyle intervention improved treatment with pharmacotherapy in general obese populations (Craighead 1981; Wadden 2001a). Because moderate physical activity (Lee 1999) and improved lipid levels (Law 1994) can reduce the risk of cardiovascular disease independent of weight change, combined interventions can likely achieve improved health outcomes.
It is clear that obesity in persons with diabetes must be treated aggressively in the long‐term, as one would treat any other cardiovascular disease risk factor. Various potential approaches need to be examined in the future. Although pharmacotherapy has been used in nondiabetic populations for treatment lasting longer than one year (Hauner 1999), further research is needed with long‐term follow‐up of large populations with diabetes. More data are needed on health outcomes such as cardiovascular events, in addition to risk factors. Populations with broad ranges of BMI, age, and ethnicity need to be studied. Research is needed on the efficacy and safety of over‐the‐counter drugs that persons with diabetes are using for weight loss, and additional research is also needed on other drugs that appear promising in populations without diabetes. Goldstein has suggested that a targeted approach may be useful and that further research is needed to identify subsets of patients who can safely achieve and maintain long‐term weight loss with initial pharmacotherapy (Goldstein 1994a). Several years ago, Blackburn 1987 suggested an incremental approach with repeated goal‐setting for small amounts of weight loss; perhaps intermittent pharmacotherapy could be used with this approach. Future research must address reporting deficiencies noted in this literature, particularly descriptions of the sampling frame, methods of participant recruitment, and details of accompanying dietary interventions. Ideally, an analysis of individual patient data should be performed to examine relationships between weight loss and patient‐level characteristics such as age and initial weight.
Further work is needed to examine whether the combination of lifestyle modification and pharmacotherapy improves the efficacy of drug therapy (Phelan 2002), whether such combinations are synergistic or additive (Phelan 2002), and what dosage schedules and sequencing of the two interventions are optimal. The incidence of adverse events must be carefully monitored over the long term in diabetic populations, which already have multiple risk factors for major cardiovascular and neurologic events. The advancement of research in these areas will help reduce cardiovascular disease risk factors and events for persons with type 2 diabetes.
Feedback
Clarification about references, 27 February 2009
Summary
I could not find this reference in Diabetes ‐ on that date, page and volume is another paper. A pub med search did not reveal the true source of this: Guy‐Grand B, Valensi P, Joubert JM, Eschwege E, Amouyel P, Fagnani F. Modelisation of the 10‐year incidence reduction of coronary events in obese Type 2 diabetes patients treated with Orlistat. Diabetes 2002;51:1938. Can you help me find the correct link? I have just sent a request stating that one of the articles had an incorrect link. On continuing to go through the references I have found another problem: Hanefeld M, Platon J, Sachse G. Orlistat promotes weight loss and improves glycaemic control in overweight patients with type 2 diabetes. Diabetologia 2001;44:889 ‐ the link goes to another article altogether. THIRD reference with incorrect link and not found at journal web site/pubmed or any other place: Hawkins F, Duran S, Vilardell E, Soriguer F, Cabezas J, Escobar F, Milalles JM, Faure E, Bellido D, Herrera JL, Serrano‐Rios M, Tebar J, Freijane J, Armero F. Orlistat promotes glucemia control and other cardiovascular risk factors lowering in obese patients with type 2 diabetes. Randomised clinical trial. Diabetologia 2000;43:658. I am now questioning both my own searching but seriously worried about this paper .......
Reply
Thank you for picking up our errors. Abstract numbers were confused with page numbers. The correct citations are:
Guy‐Grand et al: Diabetes 2002; vol 51 (suppl 2): page A471
Hanefeld et al: Diabetologia 2001; vol 44 (suppl 1): page A231
Hawkins et al: Diabetologia 2000; vol 43 (suppl); page 171
Contributors
Comments made by Martin Dawes, occupation doctor (martin.dawes@mcgill.ca).
Susan Norris replied to the comments on behalf of the review authors for the review.
What's new
| Date | Event | Description |
|---|---|---|
| 15 May 2009 | Feedback has been incorporated | Clarification about references |
Acknowledgements
The authors wish to thank Nathalie Bousader MD, Florence J. Dallo MPH, and Rolanda Watkins MPH for assistance with abstracting data from studies. Jan Stansell MSc, Karla Bergerhoff MD, and Tamara Brown MS were invaluable in their assistance devising and running search strategies.
Appendices
Appendix 1. Search strategy
| ELECTRONIC SEARCHES: Unless otherwise stated, search terms were free text terms; exp = exploded MeSH: Medical subject heading (Medline medical index term); the dollar sign ($) stands for any character(s); the question mark (?) = to substitute for one or no characters; tw = text word; pt = publication type; sh = MeSH: Medical subject heading (Medline medical index term); adj = adjacency. 1. exp Drug Therapy/ 2. exp Drug Combinations/ 3. exp Anti‐Obesity Agents/ 4. exp MAZINDOL/ 5. exp YOHIMBINE/ 6. exp AMPHETAMINE/ 7. exp BUPROPION/ 8. exp BENZOCAINE/ 9. exp EPHEDRINE/ 10. exp CAFFEINE/tu [Therapeutic Use] 11. exp BROMOCRIPTINE/tu [Therapeutic Use] 12. exp SERTRALINE/tu [Therapeutic Use] 13. drug therap$.tw. 14. drug treatment$.tw. 15. drug combination$.tw. 16. appetite suppressant$.tw. 17. appetite depressant$.tw. 18. appetite inhibitor$.tw. 19. appetite reducing.tw. 20. anorectic agent$.tw. 21. anorectic drug$.tw. 22. anorectic compound$.tw. 23. anorectic treatment$.tw. 24. anti‐obesity agent$.tw. 25. anti‐obesity drug$.tw. 26. anorexiant agent$.tw. 27. anorexiant drug$.tw. 28. anorexic drug$.tw. 29. anorexigenetic drug$.tw. 30. anorexigenic agent$.tw. 31. phentermin$.tw. 32. phenmetrazin$.tw. 33. phendimetrazin$.tw. 34. diethylpropion$.tw. 35. mazindol$.tw. 36. yohimbin$.tw. 37. amphetamin$.tw. 38. metamphetamin$.tw. 39. benzphetamin$.tw. 40. bupropion$.tw. 41. topiramat$.tw. 42. benzocain$.tw. 43. orlistat.tw. 44. tetrahydrolipstatin$.tw. 45. cimetidin$.tw. 46. ephedrin$.tw. 47. caffein$.tw. 48. bromocriptin$.tw. 49. sertralin$.tw. 50. prozac.tw. 51. tagamet.tw. 52. meridia.tw. 53. sanorex.tw. 54. xenical.tw. 55. zoloft.tw. 56. threochlorocitric acid.tw. 57. sibutramin$.tw. 58. fluoxetin$.tw. 59. or/1‐58 60. exp diabetes mellitus, non‐insulin‐dependent/ 61. exp insulin resistance/ 62. impaired glucose toleranc$.tw. 63. glucose intoleranc$.tw. 64. insulin$ resistanc$.tw. 65. exp obesity in diabetes/ 66. (obes$ adj diabet$).tw. 67. (MODY or NIDDM).tw. 68. (non insulin$ depend$ or noninsulin$ depend$ or noninsulin?depend$ or non 69. insulin?depend$).tw. 70. ((typ$ 2 or typ$ II) adj diabet$).tw. 71. ((keto?resist$ or non?keto$) adj diabet$).tw. 72. ((adult$ or matur$ or late or slow or stabl$) adj diabet$).tw. 73. (insulin$ defic$ adj relativ$).tw. 74. pluri?metabolic$ syndrom$.tw. 75. or/60‐74 76. exp diabetes insipidus/ 77. diabet$ insipidus.tw. 78. 76 or 77 79. 74 not 78 80. Obesity/ 81. exp Weight Gain/ 82. exp Weight Loss/ 83. body mass index/ 84. (overweight or over weight).tw. 85. adipos$.tw. 86. fat overload syndrom$.tw. 87. (overeat or over eat).tw. 88. (overfeed or over feed).tw. 89. weight cycling.tw. 90. weight reduc$.tw. 91. weight losing.tw. 92. weight maint$.tw. 93. weight decreas$.tw. 94. weight watch$.tw. 95. weight control$.tw. 96. obes$.tw. 97. weight gain.tw. 98. weight loss.tw. 99. body mass index.tw. 100. weight chang$.tw. 101. weight losing.tw. 102. exp Pickwickian Syndrome/ 103. exp Prader‐Willi Syndrome/ 104. binge eating disorder$.tw. 105. or/80‐104 106. 59 and 79 and 105 |
Appendix 2. Adverse effects
| Adverse events | Orlistat | Sibutramine | Fluoxetine |
| Gastrointestinal | Minor GI events: range 65% to 80% I, 27% to 62% C , most mild to moderate, transient (Hollander 1998, Lindgarde 2000, Kelley 2002, Miles 2002, Shi 2001, Halpern 2003, Hanefeld 2002, Kelley 2004) 34% GI effects (Allie 2004) | Minor Constipation: 9% to 55% I, 6% to 8% C(Gokcel 2001, Fujioka 2000, Serrano‐Rios 2002, Chaisson 1989); 4% (Tankova 2003) | Minor Various: NSD between I and C(Connolly 1995) Nausea: range 15% to 35% I, 6% to 20% C(Daubresse 1996, Kutnowski 1992, Chaisson 1989) Diarrhea: 6% I, 2% C (p>0.05)(Daubresse 1996); 8% I, 4% C (p>0.05)(Gray 1992) Anorexia: 12% I, 3% C (p<0.05)(Chaisson 1989) Nausea, vomiting, diarrhea: 66% I, 60% C(O'Kane 1994) |
| Cardiovascular | Major Rhythm disturbances: NSD between groups(Finer 1994) Chest pain not suggestive of angina: 7% (2/27)(Sircar 2001) Palpitations (moderate to severe): 41% I, 29% C(Serrano‐rios 2002) Minor Increased pulse rate: mean 2.4 beats/minute I (p>0.05)(Serrano‐Rios 2002); mean 6 beats/minute I (p<0.01) (McNulty 2003) Increased systolic blood pressure (4 mmHg) and diastolic blood pressure (3 mmHg) in 15mg qd group; systolic blood pressure >=10 mmHG higher at endpont than baseline in 36% and 29% of patients receiving 15 and 20 mg (MuNulty 2003) Palpitations: 7.4% I(Chaisson 1989) | ||
| Neurologic | Minor Headache: 22% to 32% I, 40% C(Finer 2000, Sircir 2001) Dizziness: 9 to 14% I, 5% to 13% C(Finer 2000, Sircir 2001) Anxiety: 9% I, 0% C(Serrano‐Rios 2002) Sleeplessness: 7% (Tankova 2003) | Minor Tremor: 5% to 15% I, 0% to 3% C(Daubresse 1996, Kutnowski 1992, Chaisson 1989, Wise 1989) Somnolence: 11% to 22% I, 4% to 7% C(Daubresse 1996, Chaisson 1989) Headache: 13% I, 8% C(Gray 1992) Asthenia: 37% I, 20% C (p>0.05)(Chaisson 1989) Sweating: 28% I, 11% C (p<0.05)(Chaisson 1989) Abnormal dreams: 12% I, 4% C (p<0.05)(Chaisson 1989) Sweating, somnolence, nausea, tremor, anorexia: I > C(no statistics) (Goldstein 1992) | |
| Withdrawal due to adverse effects | Minor Various: 13% I, 8% C (Kelley 2002); 10% I, 5% C (p<0.05)(Miles 2002) Deterioration in glycemic control: 15% I, 28% C(Kelley 2002) GI: 4.3% I, 1.2% C(Hollander 1998); 2.6% I, 0.5% C(Lindgarde 2000); 4.7% I, 2.9% C(Halpern 2003); 0.3% I(Shi 2001); 13% I, NR for C (Kelley 2004) 22% I (Allie 2004) | Major Palpitations: 3% I, 0% C(Serrano‐Rios 2002) Hypertension: 3% (one patient) developed (Gokcel 2001) Minor Insomnia, nervousness: 6% (Redmon 2003) Dizziness, insomnia, or diarrhea: 7% I(Finer 2000) Chest pain not suggestive of angina: 4% I(Sircar 2001) Dizziness, hyperglycemia, nausea: 3% I(Fukuika 2000) | Major Chest pain: 8% I, 0% C (p>0.05)(Gray 1992) Minor GI: 22%(O'Kane 1994) Nausea, lethargy, or excessive sweating: 20%(Connolly 1995) Unspecified: 1% to 9% I, 1% to 2% C (Daubresse 1996, Kutnowski 1992) Connolly 1995) |
| Other | Minor Hypoglycemia: 7% to 17% I, 3% to 10% C(Kelley 2002, Miles 2002, Hanfeld 2002) No gallstones, no renal stones(Hollander 1998) Normal plasma concentrations vitamin A,D,E, beta‐carotene(Hollander 1998) Decrease in vitamin E and beta‐carotene concentrations in I vs C (p<0.001)(Hollander 1998) No significant difference in adverse events I and C (p=0.75)(Serrano‐rios 2001) | Major Serious AE: 6% I, 1% C (1/ 5 in I possibly drug‐related (somnolence, dizziness, confusion)) (Fujioka 2000) Minor Dry mouth: 38% I, NR C(Gokcel 2001); 23% I, 11% C(Finer 2000); "common"(McNulty 2003); reported in Redmon 2003 (no data); 6% (Tankova 2003) Infection (not specified): 18% to 26% I, 2% to 24% C(Finer 2000, Fujioka 2000) Increased platelet count and increased serum sodium in I (Serrano‐Rios 2002) AE unspecified: 61% I, 52% C(Serrano‐Rios 2002) | Minor Infections: 50% I, 55% C(Breum 1995); NSD between groups(Connolly 1995) Decreased libido: 13% I, 0% C (p=0.07)(Gray 1992) |
Appendix 3. Characteristics of eligible studies for meta‐analysis
| Study | Number | Follow‐up (weeks) | Age (years) | Sex (%female) | Weight* kg) | GHb* (%) | Diet only** (%) | Using insulin (%) | Diet |
| Fluoxetine Connolly 1995 Daubresse 1996 Gray 1992 Kutnowski 1992 O'Kane 1994 Zelissen 1992 | 30 82 48 97 19 20 | 16 8 24 9 52 26 | 66 52 NR 51 57 51 | 38 NR 54 47 68 69 | 85.1(12.0) 90.9(16.4) 107.3(24.5) 92.3(16.7) 97.8(NR) 106.1(25.0) | 8.7(2.5) 8.6(3.3) 10.2(3.0) NR 8.8(NR) 9.0(1.6) | 100 40 0 NR 37 NR | 0 0 100 0 0 0 | Low calorie Low calorie 1200 Kcal/d Low calorie Usual Low calorie |
| 296 Total | 8‐52 range | 54 mean | 51.23 mean | 94.9 (18.5) mean(SD) | 9.1 (3.0) mean(SD) | ||||
| Orlistat Bloch 2003 Hollander 1998 Kelley 2002 Kelley 2004 Hanefeld 2002 Lindgarde 2000 Miles 2002 Wang 2003 | 76 322 550 39 383 99 504 63 | 12 57 52 26 52 54 52 24 | 56 55 58 51 56 54 53 41 | 83 49 56 67 51 64 48 48 | 87.5(17.9) 99.6(14.5) 102.0(1.0) 102 (16.9) 98.4(18.5) NR 102.1(1.1) 83(9) | NR 8.5(1.0) 9.0(0.1) 8.1(1.2) 8.6(1.2) 10.0(NR) 8.9(1.0) 8.2(1.2) | 79 0 0 NR NR NR 0 0 | 13 0 100 0 0 NR 0 0 | 30% fat 500‐600 Kcal/d deficit or low fat 500‐600 Kcal/d deficit 500‐600 Kcal/d deficit 600 Kcal/d deficit 500‐600 Kcal/d deficit + behavioral modification 500‐600 Kcal/d deficit NR |
| 2036 | 12‐57 | 53 | 58.3 | 95.9 (11.1) | 8.8 (0.9) | ||||
| Sibutramine Finer 2000 Fujioka 2000 Gokcel 2001 Kaukua 2004 McNulty 2003 Redmon 2003 Serrano‐Rios 2002 Tankova 2003 | 91 175 60 236 195 61 134 95 | 12 24 26 52 52 52 24 13 | 54 54 48 54 49 54 54 46 | 53 41 100 70 56 46 68 54 | 82.5 (NR) 98.2 (14.6) 95.5 (14.2) 100.8(17.4) 100.7(20.8) 112.4(21.0) 94.2 (19.9) 91.7(8.8) | 9.2 (1.3) 8.3 (1.2) 9.8 (0.1) NR 9.7(0.3) 8.2 (1.1) 9.5 (2.1) NR | 14 17 0 100 0 NR 0 30 | 24 0 NR 0 0 0 0 0 | 500‐600 Kcal/d deficit or low fat 500‐600 Kcal/d deficit or low fat Low calorie 700 Kcal/d deficit Standard diet advice 500‐1000 Kcal/d deficit; some meal replacem. Low calorie Low calorie |
| d, day Ghb< glycated hemoglobin NR, not reported SD, standard deviation | 1047 | 12‐52 * Weight and glycated hemoglobin (GHb) for control group at baseline ** % of the study population treated with diet only | 52 | 61 | 97.0 (17.3) | 9.3 (1.3) |
Appendix 4. Characteristics of eligible studies for meta‐analysis (Cont.)
| Study | Drug dosage | Int. attrition(%) | Control attrition(%) |
| Fluoxetine Connolly 1995 Daubresse 1996 Gray 1992 Kutnowski 1992 O'Kane 1994 Zelissen 1992 | 60 mg qd 60 mg qd 60 mg qd 60 mg qd 60 mg qd 60 mg qd | 27.0 20.5 33.0 14.9 22.0 0.0 | 15 13.9 17.0 10.0 10.0 0.0 |
| 20.1 (0‐33.0) mean(range) | 12.1 (0‐17.0) mean(range) | ||
| Orlistat Bloch 2003 Hollander 1998 Kelley 2002 Kelley 2004 Hanefeld 2002 Lindgarde 2000 Miles 2002 Wang 2003 | 120 mg tid 120 mg tid 120 mg tid 120 mg tid 120 mg tid 120 mg tid 120 mg tid 120 mg bid‐tid | 6.7 14.7 49.0 34,6 33.0 NR 35.0 3.2 | 22.4 27.7 52.0 15.4 29.2 NR 44.0 0 |
| 25.2 (3.2‐49.0) | 27.2 (0‐52.0) | ||
| Sibutramine Finer 2000 Fujioka 2000 Gokcel 2001 Kaukua 2004 McNulty 2003 Redmon 2003 Serrano‐Rios 2002 Tankova 2003 | 15 mg qd 5‐10 mg qd 10 mg bid 15 mg qd 15 or 20 mg qd 10‐15 mg qd 15 mg qd 10‐15 mg qd | 9.0 33.0 3.0 8.0 24.6 10.0 23.2 NR | 9.0 29.0 17.0 11.0 28.1 6.9 12.0 NR |
| bid, twice daily Int, intervention NR, not reported qd, daily | 15.8 (3.0‐33.0) mean(range) | 16.0 (6.9‐29.0) mean(range) |
Appendix 5. Characteristics of included studies: Cimetidine
| Study ID | Methods | Participants | Intervention | Outcomes | Notes |
| Stoa‐Birketvedt 1988Multiple pub: No | Study design: RCTRandomization procedure: Randomized according to BMI; details unclearAllocation concealment: UnclearFollow‐up: 12w | Country: NorwaySetting: Hospital clinicNumber: 62Age: 48YSex: 33%FMedications: 49% on oral agentsBL wt: I 103.9, C 102.0BL BMI: I 33.8, C 34.0BL GHb: NR | Drug: CimetidineDosage: 400mg tidDuration: 12wDiet: Usual diet and activityComparison: Placebo + usual diet and activity | Weight: YesBMI: Yes>5% loss (%):FBS: YesGHb: YesCholesterol: YesLDL:HDL: YesTG: YesSBP: YesDBP: YesSide effects: Yes; 10% diarrhea, 5% each of abdominal pain, vomiting and arthralgia | Funding: Norwegian Research council, The Novo Nordic Foundation, The Norwegian Diabetes AssociationAbstract/full text: FTLOCF: NRITT: Yes, with attritionAttrition: 19%Blinding: Double blindBlinding assessor: UnclearBL comparable: YesJadad Score: 1,1,1,BRisk of bias: B |
| A, abstract; BMI, body mass index (kg/m2); C, comparison group; CHO, carbohydrate; F, female; FBS, fasting blood sugar; d, day; | FT, full text; GHb, glycated hemoglobin; I, intervention group; ITT, intention to treat; LOCF, last outcome carried forward; NA, not applicable; NR, not reported; qd, daily; RCT, randomized, controlled trial; y, year; w, weeks |
Appendix 6. Characteristics of included studies: Diethylpropion
| Study ID | Methods | Participants | Intervention | Outcomes | Notes |
| Bratusch‐Marrain 1979Multiple pub: No | Study design: RCTRandomization procedure: Random number tablesAllocation concealment: AdequateFollow‐up: 8w | Country: AustriaSetting: UnclearNumber: 40Age: 50Sex: 66%FMedications: NRBL wt: I 80.3, C 93.9BL BMI: I 30.8, C 41.7BL GHb: NR | Drug: DiethylpropionDosage: 75mg qdDuration: 8wDiet: NRComparison: Placebo | Weight: YesBMI:>5% loss (%):FBS:GHb:Cholesterol:LDL:HDL:TG:SBP:DBP:Side effects: | Funding: NRAbstract/full text: FTLOCF: NR ITT: Yes, with attritionAttrition: 20%Blinding: Double‐blindBlinding assessor: YesBL comparable: YesJadad score: 1,1,1,BRisk of bias: B |
| Hendon 1962Multiple pub:No | Study design: Pre‐versus‐postRandomization procedure: NAAllocation concealment: NAFollow‐up: 2 to 19m | Country: USASetting: academic endocrine clinicNumber: 40Age: 51ySex: NRMedications: NoneBL wt: 85BL BMI: NRBL GHb: NR | Drug: DiethylpropionDosage: 25‐75mg tidDuration: 40wDiet: noneComparison: NA | Weight: YesBMI:>5% loss (%):FBS:GHb:Cholesterol:LDL:HDL:TG:SBP:DBP:Side effects: YesHeadache, lightheaded, nausea; no incidence given | Funding: NRAbstract/full text: FTLOCF: NRITT: Yes, with attritionAttrition: 25%Blinding assessor: NoBL comparable: NAJadad score: NARisk of bias: NA |
| Montenero 1964ItalianMultiple pub: No | Study design: Two study groups; pre‐versus‐postRandomization procedure: NAAllocation concealment: NAFollow‐up: 20‐240d | Country: ItalySetting: NRNumber: 50Age: 54Sex: 65%FMedications: 17% insulin; 67% oral agentsBL wt: I 97 , C 92 BL BMI: NRBL GHb: NR | Drug: DiethylpropionDosage: 2‐3qd (dosage not specified)Duration: 20‐240dDiet: 1000‐1800kcal/dComparison: Both groups got same diet and dosage diethylpropion; group A was on hypoglycemic agents, group B was diet controlled | Weight: YesBMI:>5% loss (%):FBS: YesGHb:Cholesterol:LDL:HDL:TG:SBP:DBP:Side effects: Yes; per Pina: 4/50 quit for SE, including general malaise, epigastric disturbance, and dermatitis. No untoward effects in person with HT and CVD; normal LFT and renal function | Funding: NRAbstract/full text: FTLOCF: NRITT: Yes, with attritionAttrition: 8%Blinding assessor: NRBL comparable: NRJadad score: NARisk of bias: NA |
| Silverstone 1966Multiple pub: No | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 26w | Country: EnglandNumber: 50Age: 56Sex: 80%FMedications: 56% diet only; no insulinBL wt: I 84.4, C 89.4BL BMI: NRBL GHb: NR | Drug: DiethylpropionDosage: 75mg qd; 40% 3w on, 3w off; 60% 5w on, 5w off Duration: 26wDiet: 1000kcal/dComparison: Placebo + diet | Weight: YesBMI:>5% loss (%):FBS:GHb:Cholesterol:LDL:HDL:TG:SBP:DBP:Side effects: Yes; dry mouth in 2/15 pts | Funding: Merrell‐National Laboratories, Ltd. supplied drugAbstract/full text: FTLOCF: NRITT: Yes, with attritionAttrition: 20%Blinding: Double‐blindBlinding assessor: YesBL comparable: NRJadad score: 1,1,1,BRisk of bias: B |
| Williams 1968Multiple pub: No | Study design: RCTRandomization procedure: random number tableAllocation concealment: adequateFollow‐up: 8w | Country: EnglandSetting: UnclearNumber: 63Age: 58Sex: 89%FMedications: NoneBL wt: NRBL BMI: NRBL GHb: NR | Drug: DiethylpropionDosage: 75mg qdDuration: 8wDiet: Low fatComparison: Placebo + diet | Weight: YesBMI:>5% loss (%):FBS:GHb:Cholesterol:LDL:HDL:TG:SBP:DBP:Side effects: Yes; no SE on drug; one with placebo | Funding: John Wyeth and BrotherAbstract/full text: FTLOCF: NoITT: Yes, with attritionAttrition: 22%Blinding: Double‐blindBlinding assessor: NRBL comparable: NRJadad score: 2,1,1,ARisk of bias: B |
Appendix 7. Characteristics of included studies: Fluoxetine
| Study ID | Methods | Participants | Intervention | Outcomes | Notes |
| Chaisson J‐L 1989Multiple pub: No | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 36w | Country: CanadaSetting: NRNumber: 278Age: 52ySex: NRMedications: NRBL wt: 100.5BL BMI: 37BL GHb: I 7.4, C 7.3 | Drug: FluoxetineDosage: 60mg qdDuration: 36wDiet: Dietary counselingComparison: Placebo | Weight: YesBMI:>5% loss (%):FBS: YesGHb: YesCholesterol:LDL:HDL:TG:SBP:DBP:Side effects: Yes | Funding: NRAbstract/full text: ALOCF: NRITT: NRAttrition: NRBlinding: Double‐blindBlinding assessor: UnclearBL comparable: UnclearJadad score: 1,1,0,BRisk of bias: C |
| Connolly VM 1994 | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 26w | Country: ScotlandSetting: Diabetic clinicNumber: 30Age: 66Sex: 38%FMedications: Diet onlyBL wt: I 92.0, C 85.1BL BMI: I 32.0, C 31.5BL GHb: I 8.0, C 8.7 | Drug: FluoxetineDosage: 60mg qdDuration: 26wDiet: 1200‐1600 kcal/d, 50% CHOComparison: Placebo + diet | Weight: YesBMI: Yes>5% loss (%):FBS: YesGHb: YesCholesterol:LDL:HDL:TG:SBP:DBP:Side effects: Yes | Funding: Lilly Industries, Ltd.Abstract/full text: FTLOCF: NRITT: Yes, with attritionAttrition: 20%Blinding: Double‐blindBlinding assessor: UnclearBL comparable: UnclearJadad score: 1,1,0,BRisk of bias: C |
| Daubresse J‐C 1996Multiple pub: No | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 8w | Country: BelgiumSetting: Community hospital clinicNumber: 82Age: 52ySex: NRMedications:BL wt: I 93, C 90.9 BL BMI: I 34.5, C 34.0BL GHb: I 8.5, C 8.6 | Drug: FluoxetineDosage: 60mg qdDuration: 8wDiet: Low calorie Comparison: Placebo + diet | Weight: YesBMI: Yes>5% loss (%):FBS: YesGHb: YesCholesterol: YesLDL:HDL:TG: YesSBP:DBP:Side effects: Yes | Funding: NRAbstract/full text: FTLOCF: NRITT: Yes, with attritionAttrition: 17%Blinding: Double‐blindBlinding assessor: UnclearBL comparable: NRJadad score: 1,1,1,BRisk of bias: B |
| Goldstein 1992Multiple pub: Goldstein 1991 | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 36w | Country: USASetting: NRNumber: 278Age: NRSex: NRMedications: NRBL wt: 100BL BMI: NRBL GHb: I 7.4, C 7.2 | Drug: FluoxetineDosage: 60mg qdDuration: 36wDiet: Low calorieComparison: Placebo + diet | Weight: YesBMI:>5% loss (%):FBS: YesGHb: YesCholesterol:LDL:HDL:TG:SBP:DBP:Side effects: | Funding: Lilly LaboratoriesAbstract/full text: ALOCF: NRITT: NRAttrition: NRBlinding: Double‐blindBlinding assessor: UnclearBL comparable: NRJadad score: 1,1,0,BRisk of bias: C |
| Gray 1992aMultiple pub: Gray 1992b | Study design: RCT Randomization procedure:NRAllocation concealment:UnclearFollow‐up: 24w | Country: USASetting: Single, university clinicNumber: 48Age: 55Sex: I 67% F, C 42% F Medications: InsulinBL wt: I 106, C 107BL BMI: I 38, C 39.0BL GHb: I 10.5, C 10.2 | Drug: FluoxetineDosage: 60mgqdDuration: 24wDiet: 1200 kcal/d American Diabetes Association diet Comparison: Placebo + diet | Weight: YesBMI:>5% loss (%):FBS: YesGHb: YesCholesterol:LDL:HDL:TG:SBP:DBP:Side effects: Yes | Funding: NRAbstract/full text: FT LOCF: Performed but data NRITT: Yes, with attritionAttrition: 25%Blinding: Double‐blindBlinding assessor: UnclearBL comparable: YesJadad score: 1,1,1,BRisk of bias: B |
| Kutnowski 1990Multiple pub: Appears to be a different population from Kutnowski 1992 and Daubresse 1996 | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 8w | Country: BelgiumSetting: Multicenter, no detailsNumber: 134Age: NRSex: 66%FMedications: NR; NIDDM and IGT patients combinedBL wt: NRBL BMI: I 34.1, C 34.1 BL GHb: NR | Drug: Fluoxetine Dosage: 60mg qdDuration: 8wDiet: 1400kcal/dComparison: Placebo + diet | Weight:BMI: Yes>5% loss (%):FBS: YesGHb: YesCholesterol: YesLDL:HDL:TG: YesSBP:DBP:Side effects: | Funding: YesAbstract/full text: ALOCF: YesITT: CompleteAttrition: 14.2%Blinding: Double‐blindBlinding assessor: UnclearBL comparable: YesJadad score: 1,1,1,BRisk of bias: B |
| Kutnowski 1992Multiple pub: Unclear if overlap with Kutnowski 1990 abstract | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 9w | Country: BelgiumSetting: Multicenter; details UnclearNumber: 97Age: 51Sex: 47%FMedications:BL wt: I 91.0, C 92.3BL BMI: I 34.4, C 34.3BL GHb: NR | Drug: FluoxetineDosage: 60mg qdDuration: 9wDiet: Low calorie Comparison: Placebo + diet | Weight:BMI: Yes>5% loss (%):FBS: YesGHb: YesCholesterol:LDL: YesHDL:TG: YesSBP:DBP:Side effects: | Funding: Eli LillyAbstract/full text: FTLOCF: YesITT: CompleteAttrition: 12.4%Blinding: Double‐blindBlinding assessor: NRBL comparable: YesJadad score: 1,1,1,BRisk of bias: B |
| O'Kane 1993 | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 52w | Country: United KingdomSetting: Diabetic clinicNumber: 19Age: 57Sex: 68%FMedications: 37% diet only; 63% on oral agents; no insulinBL wt: I 97.5, C 97.8BL BMI: I 36.8, C 35.8BL GHb: I 9.7, C 9.2 | Drug: FluoxetineDosage: 60mg qdDuration: 52wDiet: Usual Comparison: Placebo | Weight: YesBMI: >5% loss (%):FBS: YesGHb: YesCholesterol: YesLDL: HDL:TG: YesSBP:DBP:Side effects: Yes | Funding: Lilly Industries LtdAbstract/full text: FTLOCF: NRITT: Yes, with attritionAttrition: 16%Blinding: Double‐blindBlinding assessor: NRBL comparable: NRJadad score: 1,1,1,BRisk of bias: B |
| Wise 1989Multiple pub: No | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 12w | Country: UKSetting: NRNumber: 190Age: 51ySex: 73%FMedications: NRBL wt: 96BL BMI: 35BL GHb: 9.6 | Drug: FluoxetineDosage: NRDuration: 12wDiet: NRComparison: Placebo | Weight: YesBMI:>5% loss (%):FBS: YesGHb: YesCholesterol:LDL:HDL:TG:SBP:DBP:Side effects: Yes | Funding: Lilly Research Centre, Surrey, UKAbstract/full text: ALOCF: NRITT: NRAttrition: NRBlinding: Double‐blindBlinding assessor: UnclearBL comparable: NROther: Demographic data is combined group of persons with type 2 diabetes and IGT; GHb results are for people with diabetes onlyJadad score: 1,1,0,BRisk of bias: C |
| Zelissen PMJMultiple pub:No | Study design: RCTRandomization procedure: Computer‐generated sequence numberingAllocation concealment: UnclearFollow‐up: 26w | Country: The NetherlandsSetting: Single, hospital clinicNumber: 20Age: 50Sex: 60%FMedications: None or oral agentBL wt: I 97, C 106 BL BMI: >=29BL GHb: I 9.6, C 9.1 | Drug: FluoxetineDosage: 60mg qdDuration: 26wDiet: 1000kcal/dComparison: Placebo + diet | Weight: YesBMI:>5% loss (%): YesFBS: YesGHb: YesCholesterol:LDL:HDL:TG:SBP:DBP:Side effects: | Funding: Eli Lilly, Nieuwegein, The Netherlands, supplied fluoxetineAbstract/full text: FTLOCF: NRITT: CompleteAttrition: 0%Blinding: NRBlinding assessor: NRBL comparable: NRJadad score: 2,0,1,BRisk of bias: B |
Appendix 8. Characteristics of included studies: Mazindol
| Study ID | Methods | Participants | Intervention | Outcomes | Notes |
| Bandisode 1975Multiple pub:No | Study design: RCTRandomization procedure: AdequateAllocation concealment: YesFollow‐up: 12w | Country: USASetting: NRNumber: 64Age: 50ySex: 72%FMedications: No insulinBL wt: 95BL BMI: NRBL GHb: NR | Drug: MazindolDosage: 2mg qdDuration: 12wDiet: 5‐19 kcal/pound body weight, depending on activity levelsComparison: Placebo + diet | Weight: YesBMI:>5% loss (%):FBS:GHb:Cholesterol: YesLDL:HDL:TG:SBP: YesDBP: YesSide effects: Yes; 1/64 pts each with drowsiness, headache, nervousness (2), dizziness, flushed face, | Funding: NRAbstract/full text: FTLOCF: NRITT: Yes (with attrition)Attrition: I 38%, C 28%Blinding: Double‐blindBlinding assessor: UnclearBL comparable: NRJadad score: 2,1,1,ARisk of bias: A |
| Boshell 1974Multiple pub: No | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 12w | Country: USASetting: NRNumber: 64Age: NRSex: NRMedications: None, diet only controlBL wt:BL BMI:BL GHb: | Drug: MazindolDosage: 2mg qdDuration: 12wDiet: 5‐10kcal/pound, depending on activity levelComparison: Diet + placebo | Weight: YesBMI:>5% loss (%):FBS:GHb:Cholesterol:LDL:HDL:TG:SBP:DBP:Side effects: | Funding: NRAbstract/full text: A LOCF: NRITT: Yes (with attrition)Attrition: I 41%, C 25%Blinding: Double‐blindBlinding assessor: NRBL comparable: NROther: 2 patients excluded due to nonadherence to treatment scheduleJadad score: 1,1,1,BRisk of bias: B |
| Crommelin 1974Multiple pub: No | Study design: RCT Randomization procedure: NRAllocation concealment: NRFollow‐up: 12w | Country: USASetting: Private practiceNumber: 10Age: Approximately 50Sex: Predominantly femaleMedications: NRBL wt: 85.0BL BMI: NRBL GHb: NR | Drug: MazindolDosage: 1mg tidDuration: 12wDiet: Individual diet, no detailsComparison: Placebo + diet | Weight: YesBMI:>5% loss (%):FBS:GHb:Cholesterol:LDL:HDL:TG:SBP:DBP:Side effects: Yes; lightheadedness, dry mouth, vertigo; increased pulse rate noted with I group, not quantified. | Funding: NRAbstract/full text: FTLOCF: NRITT: Yes, with attritionAttrition: 10%Blinding: Double‐blindBlinding assessor: UnclearBL comparable: YesJadad score: 1,1,1,BRisk of bias: B |
| Dolocek 1976Multiple pub: No | Study design: Pre‐versus‐postRandomization procedure: NAAllocation concealment: NAFollow‐up: 2m | Country: CzechoslovakiaSetting: NRNumber: 32Age: Sex: 78%FMedications: 38% oral agents, 31% insulinBL wt: 97.3BL BMI: NRBL GHb: NR | Drug: MazindolDosage: 2mg qd at lunchDuration: 2mDiet: 150g CHOComparison: NA | Weight: YesBMI:>5% loss (%):FBS: YesGHb:Cholesterol: YesLDL:HDL:TG:SBP:DBP:Side effects: Yes; constipation most frequent, also dry mouth, initial anxiety and palpitations | Funding: NRAbstract/full text: FTLOCF: NRITT: NAAttrition: 6%Blinding: NABlinding assessor: NoBL comparable: NRJadad score: NARisk of bias: NA |
| Felt 1977Multiple pub: No | Study design: Cohort with comparison groupRandomization procedure: NAAllocation concealment: NAFollow‐up: 12w | Country: CzechoslovakiaSetting: NRNumber: 24Age: 47ySex: 83%FMedications: 50% diet only, 50% oral agentBL wt:BL BMI:BL GHb: | Drug: MazindolDosage: 1mg bidDuration: 12wDiet: NRComparison: 20 healthy women with normal weight | Weight: YesBMI:>5% loss (%):FBS: YesGHb:Cholesterol: YesLDL:HDL:TG:SBP:DBP:Side effects: Yes; constipation most common, rare headache, insomnia, dizziness | Funding: NRAbstract/full text: FTLOCF: NRITT: NAAttrition: NRBlinding: NoBlinding assessor: NoBL comparable: NAJadad score: NARisk of bias: NA |
| Sanders 1976Multiple pub: No | Study design: Two groups, unclear if randomized; cross‐over q6wRandomization procedure: NRAllocation concealment: NRFollow‐up: 6w | Country: AustraliaSetting: NRNumber: 18Age: 40‐65Sex: 80%FMedications: 11% diet, 61% oral agents, 28% insulinBL wt: NRBL BMI: NRBL GHb: NR | Drug: MazindolDosage: 2mg qdDuration: 6wDiet: Dietary advice for 8w before onset of drug treatmentComparison: Placebo | Weight: YesBMI:>5% loss (%):FBS: YesGHb:Cholesterol:LDL:HDL:TG:SBP:DBP:Side effects: Yes; "stimulation", headache | Funding: NRAbstract/full text: FTLOCF: NRITT: Yes, with attritionAttrition: 17%Blinding: Double‐blindBlinding assessor: UnclearBL comparable: BLJadad score: NARisk of bias: B |
| Slama 1978Multiple pub: No | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 12w | Country: FranceSetting: NRNumber: 46Age: 48ySex: 38%FMedications: Diet onlyBL wt: I 84.9, C 81.0BL BMI: NRBL GHb: NR | Drug: MazindolDosage: 2mg qdDuration: 12wDiet: 1000kcal/dComparison: Diet + placebo | Weight: YesBMI:>5% loss (%):FBS: YesGHb:Cholesterol: YesLDL:HDL:TG: YesSBP:DBP:Side effects: | Funding: NRAbstract/full text: FT LOCF: NRITT: Yes, with attritionAttrition: 20%Blinding: Double‐blindBlinding assessor: UnclearBL comparable: NRJadad score: 1,1,0,BRisk of bias: B |
Appendix 9. Characteristics of included studies: Orlistat
| Study ID | Methods | Participants | Outcomes | Intervention | NOtes |
| Allie 2004 Multiple pub: No | Study design: Pre vs post, retrospectiveRandomization procedure: NAAllocation concealment: NAFollow‐up: 26 weeks | Country: UDASetting: Endocrinology clinicNumber: 23Age: 53Sex: NRMedications: NRBL wt: 118.0(2.5)BL BMI: 40.5(7.0)BL GHb: 7.9(1.6) | Drug: Orlistat Dosage: 120mg tidDuration: 13 to 26 weeksDiet: NRComparison: NA | Weight: YBMI: Y>5% loss (%): YFBS: GHb: YCholesterol: YLDL: YHDL: YTG: YSBP: YDBP: YSide effects: Y | Funding: Abstract/full text: FTLOCF: NAITT: NAAttrition: NA (retrospective)Blinding: NA Blinding pt: No Blinding assessor: NABlinding provider: NoBL comparable: NA |
| Bloch 2003 Multiple pub: No | Study design: RCTRandomization procedure: Central random number listAllocation concealment: AdequateFollow‐up: 12 weeks | Country: BrazilSetting: Hypertension clinicNumber: 204 total; 76 analyzed with diabetesAge: 56 yearsSex: 83% overallMedications: I: 68% oral agents, 8% insulin; C: 63% oral agents and 18% insulinBL wt: I 91.5, C 87.5BL BMI: I 36.6, C 35.4BL GHb: NRNote: Demographic information was given only for whole study group (39% with diabetes), including persons with diabetes and those without. | Drug: Orlistat Dosage: 120mg tidDuration: 12 weeksDiet: Low calorie diet, 30% fat; advised to increase activityComparison: Diet and activity as for intervention group | Weight: Y BMI: >5% loss (%): Y FBS: Y GHb: Y Cholesterol: Y LDL: HDL: Y TG: Y SBP: Y DBP: Side effects: Y | Funding: University Hospital Abstract/full text: FTLOCF: YesITT: YesAttrition: 31% overallBlinding: NRBlinding pt: No Blinding assessor: NRBlinding provider: NRBL comparable: Yes |
| Bonnici 2002Multiple pub: No | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 24w | Country: South AfricaSetting: Multicenter trial; no detailsNumber: 284Age: NRSex: NRMedications: Metformin and/or sulfonylureaBL wt: NRBL BMI: NRBL GHb: NR | Drug: Orlistat Dosage: 120mg tidDuration: 24wDiet: 600kcal/d deficitComparison: Placebo + diet | Weight: YesBMI:>5% loss (%): YesFBS: YesGHb: YesCholesterol:LDL: YesHDL:TG:SBP:DBP:Side effects: | Funding: NRAbstract/full text: ALOCF: NRITT: NRAttrition: NR Blinding: Double‐blindBlinding assessor: UnclearBL comparable: NRJadad score: 1,1,0,BRisk of bias: C |
| Deerochanawong 2001Multiple pub: No | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 24w | Country: NRSetting: NRNumber: 252Age: NRSex: NRMedications: No insulin or acarboseBL wt: I 77, C 77BL BMI: NRBL GHb: NR | Drug: OrlistatDosage: 120mg tidDuration: 24wDiet: 600kcal/d deficitComparison: Placebo + diet | Weight: YESBMI:>5% loss (%): YesFBS: YesGHb: YesCholesterol:LDL:HDL:TG:SBP:DBP:Side effects: | Funding: NRAbstract/full text: ALOCF: NRITT: UnclearAttrition: NRBlinding: Double‐blindBlinding assessor: UnclearBL comparable: NRJadad score: 1,1,0,BRisk of bias: B |
| Dimitrov 2001Multiple pub:No | Study design: Pre‐versus‐postRandomization procedure: NAAllocation concealment: NAFollow‐up: 3m | Country: BulgariaSetting: Academic medical clinicNumber: 12Age: NRSex: NRMedications: NRBL wt: 103.6BL BMI: NRBL GHb: NR | Drug: OrlistatDosage: 120mg tidDuration: 3mDiet: NRComparison: Nondiabetic, obese persons | Weight: YesBMI:>5% loss (%):FBS:GHb:Cholesterol: YesLDL: YesHDL:TG:SBP:DBP:Side effects: | Funding: NRAbstract/full text: ALOCF: NRITT: NAAttrition: NRBlinding: NABlinding assessor: NoBL comparable: NAJadad score: NARisk of bias: NA |
| Guy‐Grand 2001aMultiple pub: Guy‐Grande 2002bGuy‐Grand 2002 | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 26w | Country: FranceSetting: Multicenter, details NRNumber: 193Age: 52Sex: NRMedications: Oral hypoglycemic agentsBL wt: NRBL BMI: 33.7BL GHb: 7.7 | Drug: OrlistatDosage: 120mg tidDuration: 26wDiet: low calorieComparison: Placebo + diet | Weight: YesBMI:>5% loss (%):FBS: YesGHb: YesCholesterol:LDL:HDL:TG:SBP:DBP:Side effects: | Funding: NRAbstract/full text: ALOCF: NRITT: UnclearAttrition: NRBlinding: Double‐blindBlinding assessor: UnclearBL comparable: YesJadad score: 1,1,1,BRisk of bias: C |
| Halpern 2003Halpern 2001 (abstract) | Study design: Multicenter RCTRandomization procedure: Randomization list generated by sponsorAllocation concealment: UnclearFollow‐up: 26w | Country: Latin AmericaSetting: NRNumber: 338Age: 51Sex: 69%FMedications: No insulin or acarboseBL wt: 89.6BL BMI: 34.6BL GHb: 8.4% | Drug: OrlistatDosage: 120mg tidDuration: 24wDiet: 600kcal/d deficit; caloric content: 30% fat, 50% CHO, 20% proteinComparison: Placebo + diet | Weight: YesBMI:>5% loss (%): YesFBS: YesGHb: YesCholesterol: YesLDL: YesHDL: YesTG: YesSBP: YesDBP: YesSide effects: Yes | Funding: F. Hoffman‐La roche (Basel, Switzerland)Abstract/full text: FT LOCF: YesITT: No; 5 patients withdrawn (no reason stated) after at least one follow‐up measurement; some patients withdrawn for 'noncompliance'Attrition: 18.4%Blinding: Double‐blindBlinding assessor: UnclearBL comparable: YesOther: Must have >60% compliance with placebo during 2w lead‐in to enter studyJadad score: 1,1,0,BRisk of bias: C |
| Hanefeld 2002Multiple pub: Hanefeld 2001 (abstract) | Study design: RCT, multicenterRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 52w | Country: GermanySetting: Outpatient clinicsNumber: 383Age: 51%FSex: 56yMedications: Diet or sulphonurea; no insulinBL wt: I 98.4, C 99.4BL BMI: I 33.7, C 34.5BL GHb: I 8.6, C 8.6 | Drug: OrlistatDosage: 120mg tidDuration: 48wDiet: 600kcal/d deficit Comparison: Diet + Placebo | Weight: YesBMI: Yes>5% loss (%): YesFBS: YesGHb: YesCholesterol: YesLDL:HDL: YesTG: YesSBP: YesDBP: YesSide effects: Yes | Funding: Hoffman‐La Roche AGAbstract/full text: FTLOCF: NRITT: No; some patients withdrawn for failure to complyAttrition: 31%Blinding: Double‐blindBlinding assessor: UnclearBL comparable: NROther: 22% of study population were not randomized after lead‐in period as did not comply with study processesJadad score: 1,1,1,B Risk of bias: C |
| Hawkins 2000Multiple pub: No | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 6m | Country: NRSetting: Multicenter trial, details unclearNumber: 307Age: NRSex: NRMedications: NRBL wt: NRBL BMI: >27BL GHb: NR | Drug: OrlistatDosage: 120mg tidDuration: 24wDiet: HypocaloricComparison: Placebo + diet | Weight: YesBMI:>5% loss (%):FBS: YesGHb: YesCholesterol: YesLDL: YesHDL:TG:SBP: YesDBP: YesSide effects: | Funding: NRAbstract/full text: ALOCF: NRITT: Yes, with attrition Attrition: 2.5%Blinding: Double‐blindBlinding assessor: UnclearBL comparable: NRJadad score: 1,1,0,BRisk of bias: C |
| Hollander 1998a Multiple pub: Hollander 1997, 1998, 1999 | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 57w | Country: USASetting: multicenter, academic medical centersNumber: 322Age: 55Sex: 49%FMedications: Oral sulfonureaBL wt: I 99.7, C 99.6 BL BMI: I 34.0, C 34.5BL GHb: I 8.2, C 8.5 | Drug: OrlistatDosage: 120mg tidDuration: 52wDiet: 500kcal/d deficitComparison: Placebo + diet | Weight: YesBMI:>5% loss (%): YesFBS: YesGHb: YesCholesterol: YesLDL: YesHDL: YesTG: YesSBP:DBP:Side effects: Yes | Funding: Hoffman‐LaRocheAbstract/full text: FTLOCF: NRITT: Yes, with attritionAttrition: 21%Blinding: Double‐blindBlinding assessor: UnclearBL comparable: YesJadad score: 1,1,1,BRisk of bias: C |
| Hollander 2001Multiple pub: No | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 1y | Country: USASetting: NRNumber: 503Age: NRSex: NRMedications: MetforminBL wt: NRBL BMI: >28BL GHb: NR | Drug: OrlistatDosage: 120mg tidDuration: 1yDiet: Mildly reduced caloric Comparison: Placebo + diet | Weight: YesBMI:>5% loss (%): YesFBS: YesGHb: YesCholesterol: LDL: YesHDL: YesTG: YesSBP: YesDBP: YesSide effects: | Funding: NRAbstract/full text: ALOCF: YesITT: CompleteAttrition: NRBlinding: Double‐blindBlinding assessor: UnclearBL comparable: UnclearJadad score: 1,1,0,BRisk of bias: C |
| Kelley 2004 Multiple pub: No | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 26 weeks | Country: USA Setting: Academic center; community recruitmentNumber: 39Age: 51Sex: 67Medications: Oral agents or diet; oral agents withdrawn 1 month prior to interventionBL wt: I 99, C 102BL BMI: I 34.0, C 35.9BL GHb: I 8.1, C7.8 | Drug: Orlistat Dosage: 120mg tidDuration: 3 monthsDiet: 500 calorie deficit; <=30% fat; activity encouragedComparison: 500 calorie deficit; <=30% fat; activity encouraged | Weight: YBMI: Y>5% loss (%): FBS: YGHb: YCholesterol: YLDL: YHDL: YTG:SBP:DBP:Side effects: Y | Funding: Roche laboratoriesAbstract/full text: FTLOCF: NoITT: PartialAttrition: 25%Blinding: Double blindBlinding pt: YBlinding assessor: UnclearBlinding provider: UnclearBL comparable: Y |
| Kelley 2002Multiple pub: Kelley 2001Bray 2001 | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 52w | Country: USASetting: Multicenter; academic medical centersNumber: 550Age: 58Sex: 57%FMedications: Insulin +/‐ oral agent (excluding thazolidindiones)BL wt: I 101.8, C 102.0 BL BMI: I 35.6, C 35.8BL GHb: I 9.0, C 9.0 | Drug: OrlistatDosage: 120mg bidDuration: 52wDiet: 500kcal/d deficitComparison: Placebo + diet | Weight: YesBMI:>5% loss (%): YesFBS: YesGHb: YesCholesterol: YesLDL: YesHDL: YesTG: YesSBP: YesDBP: YesSide effects: Yes | Funding: Hoffman‐LaRocheAbstract/full text: FTLOCF: YesITT: CompleteAttrition: 52%Blinding: Double‐blindBlinding assessor: UnclearBL comparable: YesJadad score: 1,1,1,BRisk of bias: B |
| Kelly 1997Multiple pub: No | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 57w | Country: USASetting: MulticenterNumber: 322Age: NRSex: NRMedications: SulfonureasBL wt: NRBL BMI: NRBL GHb: NR | Drug: OrlistatDosage: 120mg tidDuration: 52wDiet: 500kcal/d deficitComparison: Placebo + diet | Weight: YesBMI:>5% loss (%):FBS: YesGHb: YesCholesterol: YesLDL: YesHDL: TG: YesSBP:DBP:Side effects: Yes | Funding: Hoffman‐LaRocheAbstract/full text: ALOCF: NRITT: Yes, with attritionAttrition: I 15%, C 28%Blinding: Double‐blindBlinding assessor: UnclearBL comparable: NRJadad score: 1,1,0,BRisk of bias: B |
| Le Roux 2001Multiple pub: No | Study design: Pre‐versus‐postRandomization procedure: NAAllocation concealment: NAFollow‐up: 6m | Country: EnglandSetting: NRNumber: 7Age: NRSex: NRMedications: NRBL wt: NRBL BMI: 40.2BL GHb: 8.7 | Drug: OrlistatDosage: 120mg tidDuration: 6mDiet: UnclearComparison: NA | Weight:BMI: Yes>5% loss (%):FBS:GHb: YesCholesterol: YesLDL: YesHDL:TG: YesSBP:DBP:Side effects: | Funding: NRAbstract/full text: ALOCF: NAITT: NAAttrition: NRBlinding: NABlinding assessor: NoBL comparable: NAJadad score: NARisk of bias: NA |
| Lindgarde 2000Multiple pub: No | Study design: RCT; 26% of total study population had type 2 diabetesRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 54w | Country: SwedenSetting: 33 primary care centersNumber: 99Age: 54y (whole population)Sex: 64% (whole population)Medications: NRBL wt: NR for diabetic populationBL BMI: NR for diabetic populationBL GHb: I 8.7, C 10.0 | Drug: OrlistatDosage: 120mg tidDuration: 52wDiet: 600kcal/d deficitComparison: Placebo + diet | Weight: YesBMI:>5% loss (%): YesFBS: YesGHb: YesCholesterol:LDL:HDL:TG:SBP:DBP:Side effects: Yes | Funding: Roche AB, Stockholm, SwedenAbstract/full text: FTLOCF: NRITT: Yes, with attritionAttrition: 14%Blinding: Double‐blindBlinding assessor: UnclearBL comparable: Yes (for whole population)Jadad score: 1,1,1,B Risk of bias: B |
| Martin SF 2001 Multiple pub: No | Study design: Cohort with comparison groupRandomization procedure: NAAllocation concealment: NAFollow‐up: 6m | Country: Northern IrelandSetting: Obesity clinicNumber: 55Age: NRSex: 51%FMedications: NRBL wt: I: 102.8, C 101.1BL BMI: NRBL GHb: I 37.8, C 42 | Drug: OrlistatDosage: NRDuration: 26wDiet: Dietary adviceComparison: No orlistat | Weight: YesBMI:>5% loss (%): YesFBS:GHb:Cholesterol:LDL:HDL:TG:SBP:DBP:Side effects: | Funding: NRAbstract/full text: A LOCF: NoITT: Yes, with attritionAttrition: 59%Blinding: NRBlinding assessor: NRBL comparable: NoOther: Intervention group was persons who lost >‐2kg in 4w lead‐in periodJadad score: NARisk of bias: C |
| Mendoza‐Guadarrama 2000Multiple pub: No | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 26w | Country: MexicoSetting: obesity clinicNumber: 30Age: 51Sex: 60%FMedications: NRBL wt: NRBL BMI: I 31.3, C 30.6BL GHb: NR | Drug: OrlistatDosage: 120mg tidDuration: 26wDiet: 500kcal/d deficitComparison: Placebo + diet | Weight: BMI: Yes>5% loss (%):FBS:GHb:Cholesterol:LDL:HDL:TG:SBP:DBP:Side effects: | Funding: NRAbstract/full text: ALOCF: NRITT: UnclearAttrition: NRBlinding: Double‐blindBlinding assessor: UnclearBL comparable: NRJadad score: 1,1,0,BRisk of bias: C |
| Miles 2002Multiple pub: Miles 2001 | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 52w | Country: USASetting: Multicenter; UnclearNumber: 505Age: 53ySex: 48%FMedications: Metformin +/‐ sulfonureaBL wt: I 101.1, C 102.1BL BMI: I 35.2, C 35.6BL GHb: I 8.8, C 8.9 | Drug: OrlistatDosage: 120mg tidDuration: 52wDiet: 500kcal/d deficitComparison: Placebo + diet | Weight:BMI:>5% loss (%):FBS:GHb:Cholesterol:LDL:HDL:TG:SBP:DBP:Side effects: | Funding: Hoffman‐LarocheAbstract/full text: FTLOCF: YesITT: CompleteAttrition: 40%Blinding: Double‐blindBlinding assessor: UnclearBL comparable: YesJadad score: 1,1,1,BRisk of bias: B |
| Segal 2000Multiple pub: No | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 52w | Country: USASetting: NRNumber: 245Age: NRSex: NRMedications: Oral sulfonureasBL wt: NRBL BMI: NRBL GHb: NR | Drug: OrlistatDosage: 120mg tidDuration: 52wDiet: low calorieComparison: Placebo; unclear if dietary intervention | Weight: YesBMI:>5% loss (%):FBS:GHb:Cholesterol:LDL:HDL:TG:SBP:DBP:Side effects: | Funding: Hoffman La Roche, NJ, USAAbstract/full text: ALOCF: NRITT: NRAttrition: NRBlinding: Double‐blindBlinding assessor: UnclearBL comparable: NRJadad score: 1,1,0,BRisk of bias: C |
| Serrano‐Rios 2001Multiple pub:No | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 26w | Country: SpainSetting: Multicenter; no other detailsNumber: 237Age: NRSex: NR Medications: Sulfonureas and/or metforminBL wt: NRBL BMI: >27BL GHb: NR | Drug: OrlistatDosage: 120mg tidDuration: 24wDiet: HypocaloricComparison: Placebo + diet | Weight: YesBMI: Yes>5% loss (%): YesFBS: YesGHb: YesCholesterol: LDL:HDL:TG:SBP: YesDBP: YesSide effects: Yes | Funding: NRAbstract/full text: ALOCF: NRITT: NRAttrition: NRBlinding: Double‐blindBlinding assessor: UnclearBL comparable: NRJadad score: 1,1,0,BRisk of bias: C |
| Tong 2002 Multiple pub: Sea 2002 ; unclear if related to Chan 2001 and Sea 2001 | Study design: Pre‐versus‐postRandomization procedure: NAAllocation concealment: NAFollow‐up: 26w | Country: ChinaSetting: NRNumber: 27Age: 36Sex: 61%FMedications: NRBL wt: 93.2BL BMI: 34.2BL GHb: 8.5 | Drug: OrlistatDosage: 120mg tidDuration: 26wDiet: NoneComparison: NA | Weight: YesBMI: Yes>5% loss (%):FBS: YesGHb: YesCholesterol: YesLDL: YesHDL: YesTG: YesSBP: YesDBP: YesSide effects: Yes | Funding: NRAbstract/full text: FTLOCF: NRITT: NAAttrition: NRBlinding: NABlinding assessor: NABL comparable: NAJadad score: NARisk of bias: NA |
| Vesari 2000Multiple pub: No | Study design: Pre‐versus‐postRandomization procedure: NAAllocation concealment: UnclearFollow‐up: 45d | Country: NRSetting: NRNumber: 21Age: 55ySex: 80%FMedications: 48% on oral agentsBL wt: NRBL BMI: 36.3BL GHb: NR | Drug: OrlistatDosage: 120mg bid to tidDuration: 45dDiet: 1500kcal/dComparison: NA | Weight:BMI: Yes>5% loss (%):FBS: YesGHb: YesCholesterol: YesLDL: YesHDL: YesTG: YesSBP:DBP:Side effects: | Funding: NRAbstract/full text: ALOCF: NRITT: NAAttrition: NRBlinding: NABlinding assessor: NoBL comparable: NAJadad score: NARisk of bias: NA |
| Wang 2003 Multiple pub: No | Study design: RCTRandomization procedure: Randomization tableAllocation concealment: Unclear Follow‐up: 24w | Country: China Setting: ClinicNumber: 63Age: 41Sex: 47.6Medications: 100% oral agentsBL wt: I 85.0, C 83.0BL BMI: I 30.0, C 31.0 BL GHb: I 8.3, C 8.2 | Drug: Orlistat Dosage: 120mg bid to tidDuration: 24wDiet: NRComparison: Placebo + diet | Weight: YBMI: Y>5% loss (%): YFBS: YGHb: YCholesterol: YLDL: YHDL: YTG: YSBP: YDBP: YSide effects: NR | Funding: NRAbstract/full text: FTLOCF: NRITT: 2 patients withdrawn (no reason stated)Attrition: 3.2%Blinding: NRBlinding pt: YesBlinding assessor: UnclearBlinding provider: UnclearBL comparable: yes |
| Zaletel 2002Multiple pub:No | Study design: Pre‐versus‐postRandomization procedure: NAAllocation concealment: NAFollow‐up: Unclear; second phase was 6m | Country: SloveniaSetting: UnclearNumber: 31Age: 54Sex: 58Medications: NRBL wt: NRBL BMI: 38.1BL GHb: NR | Drug: OrlistatDosage: 120mg tidDuration: UnclearDiet: UnclearComparison: NA | Weight: YesBMI:>5% loss (%):FBS: YesGHb: YesCholesterol: YesLDL:HDL:TG: YesSBP: YesDBP:Side effects: | Funding: NRAbstract/full text: ALOCF: NAITT: NAAttrition: 6%Blinding: NABlinding assessor: NoBL comparable: NAJadad score: NARisk of bias: NA |
Appendix 10. Characteristics of included studies: Phenmetrazine
| Study ID | Methods | Participants | Intervention | Outcomes | Notes |
| Buckle 1966Multiple pub: No | Study design: Cross‐over study comparing phenmetrazine hydrochloride with phenmetrazine hydrochloride plus phenbutrazate hydrochloride Randomization procedure: NR Allocation concealment: Unclear Follow‐up: 8w | Country: UKSetting: Hospital diabetes clinicNumber: 22Age: 58 from table 1Sex: 80%FMedications: NRBL wt: 78BL BMI: NRBL GHb: NR | Drug: PhenmetrazineDosage: 25mg tidDuration: 8w (until first cross‐over)Diet: 1000 kcal/d Comparison: Filon® [phenmetrazine theoclate 30mg and phenbutrazate hydrochloride 20mg] tid with 1000 kcal/d diet | Weight: YesBMI:>5% loss (%):FBS:GHb:Cholesterol:LDL:HDL:TG:SBP:DBP:Side effects: Yes; dizziness (20%), abdominal discomfort and nausea (15%, and dry mouth 5%) | Funding: NRAbstract/full text: FTLOCF: NRITT: Yes, with attritionAttrition: 9%Blinding: Double‐blindBlinding assessor: UnclearBL comparable: NRJadad score: 1,1,0,BRisk of bias: B |
Appendix 11. Characteristics of included studies: Phentermine
| Study ID | Methods | Participants | Intervention | Outcomes | Notes |
| Campbell 1977Multiple pub: No | Study design: RCTRandomization procedure: adequateAllocation concealment: adequateFollow‐up: 26w | Country: ScotlandSetting: Community clinicNumber: 66Age: NRSex: NRMedications: 12% insulin; 44% oral treatmentBL wt: NRBL BMI: NRBL GHb: NR | Drug: PhentermineDosage: 30mg qdDuration: 26wDiet: NoneComparison: Placebo | Weight: YesBMI:>5% loss (%):FBS:GHb:Cholesterol:LDL:HDL:TG:SBP:DBP:Side effects: Yes; dry mouth and initial sleep disturbance | Funding: Riker Laboratories supplied the drugAbstract/full text: FTLOCF: NRITT: Yes, with attritionAttrition: 7%Blinding: Double‐blindBlinding assessor: UnclearBL comparable: NRJadad score:2,1,1,ARisk of bias: B |
| Gershberg 1972Multiple pub: Unclear if Gershberg 1977 is overlapping population | Study design: Unclear; 2 parallel groupsRandomization procedure: NRAllocation concealment: NRFollow‐up: 16w | Country: USASetting: NRNumber: 12Age: NRSex: NRMedications: NRBL wt: ave 143% ideal body weightBL BMI: NRBL GHb: NR | Drug: PhentermineDosage: NRDuration: 16wDiet: 1000kal/dComparison: Placebo + diet | Weight: YesBMI:>5% loss (%):FBS: YesCholesterol: YesLDL:HDL:TG: YesSBP:DBP:Side effects: | Funding: NRAbstract/full text: ALOCF: NRITT: NRAttrition: NRBlinding: Double‐blindBlinding assessor: UnclearBL comparable: NRJadad score:0,1,0,BRisk of bias: C |
| Gershberg 1977Multiple pub: Unclear if Gershberg 1972 is overlapping population | Study design: RCTRandomization procedure: UnclearAllocation concealment: NRFollow‐up: 16w | Country: USASetting: UnclearNumber: 22Age: NRSex: 64%FMedications: No insulinBL wt: I 85.0, C 84.1BL BMI: NRBL GHb: NR | Drug: PhentermineDosage: 30mg qdDuration: 16wDiet: 1000kcal/dComparison: Placebo + diet | Weight: YesBMI:>5% loss (%):FBS: YesGHb: Cholesterol: YesLDL:HDL:TG: YesSBP: YesDBP: YesSide effects: Yes; 3 pts complained of irritability and insomnia in the first week of RX; then subsided | Funding: NRAbstract/full text: FTLOCF: YesITT: CompleteAttrition: 9%Blinding: Double‐blindBlinding assessor: UnclearBL comparable: YesJadad score:1,1,1,BRisk of bias: B |
Appendix 12. Characteristics of included studies: Sibutramine
| Study ID | Methods | Participants | Intervention | Outcomes | Notes |
| Bach 1999Multiple pub: No | Study design: RCT; some Pre‐versus‐post without comparison group Randomization procedure: NR Allocation concealment: Unclear Follow‐up: 32w Note: This study did not fit inclusion criteria as did not present weight outcomes, however it presented adverse event data among persons with diabetes, and is therefore presented here. | Country: UKSetting: Multicenter; details unclearNumber: 210Age: 54Sex: 59Medications: None (diet only)BL wt: NRBL BMI: NRBL GHb: NR | Drug: SibutramineDosage: 15‐20mg qdDuration: 32wDiet: NRComparison: Placebo | Weight:BMI:>5% loss (%):FBS:GHb:Cholesterol:LDL:HDL:TG:SBP:DBP:Side effects: Yes | Funding: Knoll Pharmaceutical Co.,US and UKAbstract/full text: FTLOCF: NRITT: Yes, with attritionAttrition: 11%Blinding: Double‐blindBlinding assessor: UnclearBL comparable: YesJadad score: 1,1,1,BRisk of bias: B |
| Finer 2000Multiple pub: No | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 12w | Country: UKSetting: Two hospital‐based diabetes clinicsNumber: 91Age: 54Sex: 53%Medications: 14% diet only; 24% insulinBL wt: I 84.6, C 82.5BL BMI: I 30.6, C 31.0BL GHb: 9.5 | Drug: SibutramineDosage: 15mg qdDuration: 12wDiet: 500kcal/d deficitComparison: Placebo + diet | Weight: YesBMI: Yes>5% loss (%):FBS:GHb: YesCholesterol:LDL:HDL:TG:SBP:DBP:Side effects: Yes | Funding: Knoll Pharmaceutical Co.Abstract/full text: FTLOCF: NRITT: Yes, with attritionAttrition: 9%Blinding: Double‐blindBlinding assessor: UnclearBL comparable: YesJadad score: 1,1,1,BRisk of bias: B |
| Fujioka 2000Multiple pub: No | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 24 | Country: USASetting: Multicenter; medical centersNumber: 175Age: 54Sex: 41%FMedications: Sulfonurea, metformin or diet onlyBL wt: 99.3(1) 98.2 CBL BMI: 34.1(1) 33.8 CBL GHb: 8.4 (1) 8.3 C | Drug: SibutramineDosage: 5‐ 20mg qd Duration: 24Diet: 500kcal/d deficitComparison: Placebo + diet | Weight: YesBMI: Yes>5% loss (%): YesFBS: YesGHb: YesCholesterol: YesLDL: YesHDL: YesTG: YesSBP: YesDBP: YesSide effects: Yes | Funding: Knoll Pharmaceutical Co., USAAbstract/full text: FTLOCF: YesITT: PartialAttrition: 31%Blinding: Double‐blindBlinding assessor: YesBL comparable: YesJadad score: 1,1,1,BRisk of bias: B |
| Gokcel 2001Multiple pub: No | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 26w | Country: TurkeySetting: Academic medical cetnerNumber: 60Age: 48Sex: 100%FMedications: Sulfonurea and metforminBL wt: 95.6(1) 95.5©BL BMI: 39.3(1) 37.4©BL GHb: 10.0 (I) 9.8© | Drug: SibutramineDosage: 10mg bidDuration: 26wDiet: Low calorieComparison: Placebo + diet | Weight: YesBMI:>5% loss (%): YesFBS: YesGHb: YesCholesterol: YesLDL: YesHDL: YesTG: YesSBP: DBP: Side effects: Yes | Funding: NRAbstract/full text: FTLOCF: NRITT: Yes, with attritionAttrition: 10%Blinding: Double‐blindBlinding assessor: UnclearBL comparable: Similar (no statistics)Jadad score: 1,1,1,BRisk of bias: B |
| Griffiths 1995Multiple pub: Griffiths 1995a | Study design: Two parallel groups, unclear if randomizedRandomization procedure: UnclearAllocation concealment: UnclearFollow‐up: 12w | Country: USASetting: NRNumber: 83Age: NRSex: NRMedications: NRBL wt: NRBL BMI: NRBL GHb: NR | Drug: SibutramineDosage: 15mg qdDuration: 12wDiet: NRComparison: Placebo | Weight: YesBMI:>5% loss (%):FBS: YesGHb: YesCholesterol: YesLDL:HDL:TG: YesSBP:DBP:Side effects: | Funding: NRAbstract/full text: ALOCF: NRITT: NRAttrition: NRBlinding: Double‐blindBlinding assessor: UnclearBL comparable: NRJadad: 0,1,0,BRisk of bias: C |
| Kaukua JK 2004 | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 1 year | Country: FinlandSetting: Finnish primary medical care centersNumber: 236Age: 54 Sex: 70%F (calculated weighted)Medications: Diet only BL wt: I 100.8, C 98.1BL BMI: I 35.7, C 35.6 BL GHb: NR | Drug: SibutamineDosage: 15 mg qdDuration: 1 yearDiet: 700 Kcal/d deficit diet Comparison: Placebo and 700 Kcal/d deficit diet | Weight: YBMI: >5% loss (%): FBS: GHb: Y Cholesterol: LDL: HDL: TG: SBP: YDBP: Y Side effects: | Funding: Knoll Laboratories Abstract/full text: FTLOCF: Y ITT: Participants could be withdrawn for protocol violation; numbers unclear Attrition: 8%Blinding: Double blind Blinding assessor: UnclearBL comparable: NRJadad Score: 1,2,0,BQuality category: C |
| McNulty SJ 2003 | Study design: RCTRandomization procedure: UnclearAllocation concealment: UnclearFollow‐up: 52w | Country: Multicenter: England, Canada, France, BelgiumSetting: NRNumber: 195Age: 49Sex: 56%FMedications: MetforminBL wt: 103.3BL BMI: 36.3BL GHb: 9.6 | Drug: SibutramineDosage: 15 or 20 mg qdDuration: 52wDiet: Standard dietary adviceComparison: Dietary advice + placebo | Weight: YesBMI: Yes>5% loss (%):FBS: YesGHb: YesCholesterol: YesLDL: YesHDL: YesTG: YesSBP: YesDBP: YesSide effects: Yes | Funding: Abbott Laboratories Abstract/full text: FTLOCF: NRITT: NRAttrition: 26%Blinding: Double‐blind Blinding assessor: UnclearBL comparable: YesJadad score : 1,1,0,BRisk of bias: C |
| Peirce 1999 | Study design: RCTRandomization procedure: NRAllocation concealment: Unclear Follow‐up: 12w | Country: USASetting: NRNumber: 35Age: 18‐60ySex: NRMedications: Diet onlyBL wt: NRBL BMI: 28‐40BL GHb: NR | Drug: SibutramineDosage: 15mg qdDuration: 12wDiet: Dietary adviceComparison: Placebo | Weight: YesBMI:>5% loss (%):FBS:GHb: Yes Cholesterol:LDL:HDL:TG:SBP:DBP:Side effects: | Funding: Knoll Pharmaceutical Co. Abstract/full text: A LOCF: NR ITT: NR Attrition: NR Blinding: Double‐blind Blinding assessor: NR BL comparable: NR Jadad score: 1,1,0,B Risk of bias: C |
| Redmon JB 2003 | Study design: RCTRandomization procedure: Random allocation schedule provided by the study statisticianAllocation concealment: AdequateFollow‐up: 1 year | Country: USASetting: Academic medical centerNumber: 61Age: 54 Sex: 46%FMedications: No insulinBL wt: I 109.1, C 112.4 BL BMI: I 37.8, C 38.6 BL GHb: I 8.1, C 8.2 | Drug: SibutamineDosage: 10‐15mg dailyDuration: 1 yearDiet: 500‐1000 kcal/d deficit diet with some meal replacements; physical activity counseling and prescriptionComparison: 500‐1000 kcal/d deficit diet; physical activity counseling and prescription | Weight: YBMI: Y >5% loss (%): Y FBS: YGHb: YCholesterol: YLDL: YHDL: YTG: YSBP: Y. DBP: Y. Side effects: Y | Funding: Abbott laboratories and Slim Fast Nutrition InstituteAbstract/full text: FTLOCF: YLOCF: YITT: ReportedAttrition: 8%Blinding: NRBlinding assessor: NRBL comparable: YJadad Score: 1,0,1,B Quality category: C |
| Rissanen A 1999 | Study design: RCTRandomization procedure: NRAllocation concealment: Unclear Follow‐up: 52w | Country: FinlandSetting: NRNumber: 236Age: 18‐60ySex: NRMedications: Diet onlyBL wt: NRBL BMI: >28BL GHb: NR | Drug: SibutramineDosage: 15mg qdDuration: 52wDiet: 700 kcal/d deficit dietComparison: Placebo + 700 kcal/d deficit diet | Weight: YesBMI:>5% loss (%): YesFBS:GHb: Yes Cholesterol: LDL:HDL: YesTG: YesSBP:DBP:Side effects: | Funding: NR Abstract/full text: A LOCF: NR ITT: NR Attrition: 11% Blinding: Double‐blind Blinding assessor: NR BL comparable: NR Jadad score: 1,1,0,B Risk of bias: C |
| Serrano‐Rios 2002Multiple pub: No | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 24w | Country: Europe Setting: Multicenter Number: 134 Age: 53.6 Sex: 58%F Medications: Sulfonylurea BL wt: I 92.0, C 94.2 BL BMI: NR BL GHb: I 9.0, C 9.5 | Drug: SibutramineDosage: 15mg qdDuration: 24wDiet: Low calorieComparison: Placebo + diet | Weight:BMI:>5% loss (%):FBS:GHb:Cholesterol:LDL:HDL:TG:SBP:DBP:Side effects: | Funding: Knoll Pharmaceutical Co., UKAbstract/full text: FTLOCF: YesITT: CompleteAttrition: 18%Blinding: Double‐blindBlinding assessor: UnclearBL comparable: YesJadad score: 1,1,1,BRisk of bias: B |
| Sircar 2001Multiple pub: No | Study design: Pre‐versus‐postRandomization procedure: NAAllocation concealment: NAFollow‐up: 12w | Country: IndiaSetting: UnclearNumber: 27Age: 44.7Sex: 89%Medications: NRBL wt: 75.4BL BMI: 32.1BL GHb: 9.6 | Drug: SibutramineDosage: 10‐15mg qdDuration: 12wDiet: Prescribed; Unclear typeComparison: NA | Weight: YesBMI:>5% loss (%):FBS:GHb: YesCholesterol:LDL:HDL:TG:SBP:DBP:Side effects: Yes | Funding: Knoll Pharmaceutical, IndiaAbstract/full text: FTLOCF: NoITT: NAAttrition: 12.5%Blinding: NABlinding assessor: NoBL comparable: NAJadad score: NARisk of bias: NA |
| Tankova T 2003 | Study design: RCT Randomization procedure: NR Allocation concealment: Unclear Follow‐up: 3 months | Country: BulgariaSetting: Clinical Center of Endocrinology and Gerontology, Medical University‐SofiaNumber: 95Age: 45.8 Sex: 53.7 % female Medications: 70% oral agents, 30% dietBL wt: I 95.3, C 91.7 BL BMI: I 33.9, C 34.2 BL GHb: I 7.4, C 7.3 | Drug: SibutamineDosage: 10 mg qd for first month; average daily dosage over 3 months 12.7 mg qdDuration: 3 monthsDiet: Low calorie dietComparison: Low calorie diet | Weight: YBMI: NR >5% loss (%): FBS: GHb: YCholesterol: YLDL: HDL: TG: Y SBP: YDBP: Side effects: Y | Funding: NRAbstract/full text: FTLOCF: NITT: Y Attrition: NR Blinding: Open‐labelBlinding assessor: NRBL comparable: YJadad Score: 1,0,0,B Quality category: C |
| Vargas 1994Multiple pub:No | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 12w | Country: USASetting: NRNumber: 18Age: NRSex: NRMedications: BRBL wt: NRBL BMI: NRBL GHb: NR | Drug: SibutramineDosage: 20‐30mg qdDuration: 12wDiet: NRComparison: Placebo | Weight: YesBMI:>5% loss (%):FBS: YesGHb:Cholesterol:LDL:HDL:TG:SBP:DBP:Side effects: | Funding: NRAbstract/full text: ALOCF: NRITT: NRAttrition: NRBlinding: Double‐blindBlinding provider: UnclearBL comparable: NRJadad score: 1,1,0,BRisk of bias: C |
Appendix 13. Outcomes: Cimetidine
| Study | Weight | Glycemic Control | Lipids | Blood pressure |
| Stoa‐Birketvedt 1998 Study design: RCT Follow‐up interval: 12 weeks C, control group I, intervention group SE, standard error RCT, randomized controlled trial SBP, systolic blood pressure DBP, diastolic blood pressure | 1. Weight change (kg) Delta I (SE): ‐5 (0.5) Delta C (SE): ‐1.3 (0.2) Delta (I‐C) (SE): ‐3.7 (2.0) 2. BMI (kg/m2) Delta I (SE): ‐1.6 (0.5) Delta C (SE): ‐0.4 (0.6) Delta (I‐C) (SE): ‐1.2 (0.8) 3. % of weight loss Delta I (SE): ‐4.8 (0.5) Delta C (SE): ‐1.3 (0.2) Delta (I‐C) (SE): ‐3.5 (0.5) | 1. GHb (%) Delta I (SE): ‐0.5 (0.2) Delta C (SE): ‐0.3 (0.2) Delta (I‐C) (SE): ‐0.2 (0.3) 2. Fasting blood sugar (mml/L) Delta I (SE): ‐1.3 (0.4) Delta C (SE): ‐0.5 (0.4) Delta (I‐C) (SE): ‐0.8 (0.5) | 1. Total cholesterol (mmol/L) Delta I (SE): ‐0.1 (0.2) Delta C (SE): ‐0.3 (0.2) Delta (I‐C) (SE): 0.2 (0.2) 2. HDL cholesterol (mmol/L) Delta I (SE): 0.1 (0.0) Delta C (SE): ‐0.1 (0.0) Delta (I‐C) (SE): 0.2 (0.1) 3. Triglycerides (mmol/L) Delta I (SE): ‐0.5 (0.2) Delta C (SE): 0 (0.4) Delta (I‐C) (SE): ‐0.5 (0.4) | 1. SBP Delta I (SE): ‐6.9 (2.6) Delta C (SE): ‐7.0 (2.7) Delta (I‐C) (SE): 0.1 (2.7) 2. DBP Delta I (SE): ‐6.0 (1.5) Delta C (SE): ‐3.0 (1.0) Delta (I‐C) (SE): ‐3.0 (1.0) |
Appendix 14. Outcomes: Diethylproprion
| Study | Weight | Glycemic control | Lipids | Blood pressure |
| Williams 1968 Study design: RCT Follow‐up interval: 8 weeks | 1. Weight change (kg) Delta I (SE): ‐5.0 (0.4) Delta C (SE): ‐3.7 (0.6) Delta (I‐C) (SE): ‐1.3 (0.7) | |||
| Silverstone 1966 Study design: RCT Follow‐up interval: 26 weeks | 1. Weight change (kg) Delta I (SE): ‐5.0 (0.6) Delta C (SE): ‐3.5 (1.9) Delta (I‐C) (SE): ‐1.5 (2.0) 2. % of weight loss Delta I (SE): ‐5.9 (0.8) Delta C (SE): ‐3.9 (2.1) Delta (I‐C) (SE): ‐2.0 (2.3) | |||
| Bratusch‐Marrain 1979 Study design: RCT Follow‐up interval: 8 weeks | 1. Weight change (kg) Delta I (SE): ‐3.9 (0.4) Delta C (SE): ‐3.0 (0.5) Delta (I‐C) (SE): ‐0.9 (0.6) 2. % of weight loss Delta I (SE): ‐4.9 (0.5) Delta C (SE): ‐3.3 (0.5) Delta (I‐C) (SE): ‐1.6 (0.7) | |||
| Hendon 1962 Study design: Pre vs post Follow‐up interval: 40 weeks | 1. Weight change (kg) Delta I (SE): ‐8.8 (1.0) | |||
| Mentenero 1964 Study design: Pre vs post Follow‐up interval: 20‐240 days | 1. Weight change (kg) Group 1 Delta I (SE): ‐5.3 (0.6) Group 2 Delta I (SE): ‐4.6 (0.9) 2. % of weight loss Group 1 Delta I (SE): ‐5.2 (2.1) Group 2 Delta I (SE): ‐3.9 (3.2) |
Appendix 15. Outcomes and pooled effects: Fluoxetine
| Study | Weight | Glycemic control | Lipids | Blood pressure |
| Gray 1992 Study design: RCT Follow‐up interval: 18 weeks | 1. Weight change (kg) Delta I (SE): ‐10 (1.6) Delta C (SE): ‐1.2 (1.8) Delta (I‐C) (SE): ‐8.8 (2.4) 2. % of weight loss Delta I (SE): ‐9.5 (1.5) Delta C (SE): ‐1.1 (1.7) Delta (I‐C) (SE): ‐8.4 (2.3) | 1. GHb (%) Delta I (SE): ‐1.7 (0.5) Delta C (SE): ‐0.8 (0.4) Delta (I‐C) (SE): ‐0.9 (0.6) 2. Fasting blood sugar (mml/L) Delta I (SE): ‐0.9 (0.6) Delta C (SE): ‐3.0 (0.8) Delta (I‐C) (SE): 2.1 (1.0) | ||
| Goldstein 1992 Study design: RCT Follow‐up interval: 36 weeks | 1. Weight change (kg) Delta I (SE): ‐2.9 (0.6) Delta C (SE): ‐0.8 (0.6) Delta (I‐C) (SE): ‐2.0 (0.8) 2. % of weight loss Delta I (SE): ‐2.9 (0.6) Delta C (SE): ‐0.8 (0.6) Delta (I‐C) (SE): ‐2.0 (0.8) | 1. GHb (%) Delta I (SE): ‐0.5 (0.2) Delta C (SE): 0.3 (0.2) Delta (I‐C) (SE): ‐0.8 (0.3) 2. Fasting blood sugar (mml/L) Delta I (SE): ‐2.1 (0.5) Delta C (SE): ‐0.8 (0.5) Delta (I‐C) (SE): ‐1.3 (0.7) | ||
| Chiasson 1989 Study design: RCT Follow‐up interval: 36 weeks | 1. Weight change (kg) Delta I (SE): ‐2.9 (0.6) Delta C (SE): ‐0.8 (0.6) Delta (I‐C) (SE): ‐2.1 (0.8) 2. % of weight loss Delta I (SE): ‐2.9 (0.6) Delta C (SE): ‐0.8 (0.6) Delta (I‐C) (SE): ‐2.1 (0.8) | 1. GHb (%) Delta I (SE): ‐0.5 (0.2) Delta C (SE): 0.2 (0.2) Delta (I‐C) (SE): ‐0.7 (0.2) 2. Fasting blood sugar (mml/L) Delta I (SE): ‐2.1 (0.1) Delta C (SE): ‐0.9 (0.1) Delta (I‐C) (SE): ‐1.2 (0.1) | ||
| Wise 1989 Study design: RCT Follow‐up interval: 12 weeks | 1. Weight change (kg) Delta I (SE): ‐3.9 (0.6) Delta C (SE): ‐1.1 (0.6) Delta (I‐C) (SE): ‐2.9 (0.8) 2. % of weight loss Delta I (SE): ‐4.1 (0.6) Delta C (SE): ‐1.1 (0.6) Delta (I‐C) (SE): ‐3.0 (0.9) | 1. GHb (%) Delta I (SE): ‐1.0 (0.2) Delta C (SE): ‐0.3 (0.2) Delta (I‐C) (SE): ‐0.7 (0.3) 2. Fasting blood sugar (mml/L) Delta I (SE): ‐1.8 (0.3) Delta C (SE): ‐0.4 (0.3) Delta (I‐C) (SE): ‐1.5 (0.5) | ||
| Daubresse 1996 Study design: RCT Follow‐up interval: 8 weeks | Weight change (kg) Delta I (SE): ‐3.1 (1.8) Delta C (SE): ‐0.9 (1.7) Delta (I‐C) (SE): ‐2.2 (2.5) 2. % of weight loss Delta I (SE): ‐3.3 (1.9) Delta C (SE): ‐1.0 (1.9) Delta (I‐C) (SE): ‐2.3 (2.7) | 1. GHb (%) Delta I (SE): ‐0.8 (0.3) Delta C (SE): ‐0.3 (0.4) Delta (I‐C) (SE): ‐0.5 (0.5) 2. Fasting blood sugar (mml/L) Delta I (SE): ‐1.7 (0.5) Delta C (SE): ‐0.0 (0.4) Delta (I‐C) (SE): ‐1.7 (0.6) | 1. Total cholesterol (mmol/L) Delta I (SE): 0.1 (0.1) Delta C (SE): 0.1 (0.1) Delta (I‐C) (SE): 0 (0.2) 2. HDL cholesterol (mmol/L) Delta I (SE): 0.0 (0.0) Delta C (SE): ‐0.0 (0.0) Delta (I‐C) (SE): 0.0 (0.0) 3. Triglycerides (mmol/L) Delta I (SE): ‐0.4 (0.2) Delta C (SE): 0.1 (0.3) Delta (I‐C) (SE): ‐0.5 (0.4) | |
| O'Kane 1993 Study design: RCT Follow‐up interval: 52 weeks | 1. Weight change (kg) Delta I (SE): ‐4.3 (2.0) Delta C (SE): 1.5 (1.7) Delta (I‐C) (SE): ‐5.8 (2.6) 2. % of weight loss Delta I (SE): ‐4.4 (2.0) Delta C (SE): 1.5 (1.7) Delta (I‐C) (SE): ‐5.9 (2.6) | 1. GHb (%) Delta I (SE): ‐0.8 (0.6) Delta C (SE): 1.0 (0.8) Delta (I‐C) (SE): ‐1.8 (1.0) 2. Fasting blood sugar (mml/L) Delta I (SE): ‐0.3 (0.6) Delta C (SE): 0.5 (0.6) Delta (I‐C) (SE): ‐0.8 (0.9) | 1. Total cholesterol (mmol/L) Delta I (SE): 0.4 (0.3) Delta C (SE): ‐0.1 (0.3) Delta (I‐C) (SE): 0.5 (0.4) 2. Triglycerides (mmol/L) Delta I (SE): ‐0.3 (0.1) Delta C (SE): 0.2 (0.3) Delta (I‐C) (SE): ‐0.5 (0.3) | |
| Connolly 1995 Study design: RCT Follow‐up interval: 26 weeks | 1. Weight change (kg) Delta I (SE): ‐3.9 (1.3) Delta C (SE): 0 (0.4) Delta (I‐C) (SE): ‐3.9 (1.4) 2. % of weight loss Delta I (SE): ‐4.2 (1.5) Delta C (SE): 0 (0.5) Delta (I‐C) (SE): ‐4.2 (1.5) | 1. GHb (%) Delta I (SE): ‐0.9 (0.1) Delta C (SE): 0.1 (0.2) Delta (I‐C) (SE): ‐1.0 (0.2) 2. Fasting blood sugar (mml/L) Delta I (SE): ‐0.8 (1.1) Delta C (SE): 1.2 (0.5) Delta (I‐C) (SE): ‐2.0 (1.1) | ||
| Kutnowski 1992 Study design: RCT Follow‐up interval: 8 weeks | 1. Weight change (kg) Delta I (SE): ‐2.6 (0.5) Delta C (SE): ‐1.2 (0.4) Delta (I‐C) (SE): ‐1.4 (0.6) 2. BMI Delta I (SE): ‐1.0 (0.2) Delta C (SE): ‐0.4 (0.2) Delta (I‐C) (SE): ‐0.5 (0.2 3. % of weight loss Delta I (SE): ‐2.8 (0.5) Delta C (SE): ‐1.3 (0.5) Delta (I‐C) (SE): ‐1.5 (0.7) | 1. Fasting blood sugar (mml/L) Delta I (SE): ‐2.2 (0.5) Delta C (SE): ‐0.5 (0.4) Delta (I‐C) (SE): ‐1.6 (0.6) | ||
| Zelissen 1992 Study design: RCT Follow‐up interval: 26 weeks | 1. Weight change (kg) Delta I (SE): ‐2.5 (2.4) Delta C (SE): ‐0.1 (1.3) Delta (I‐C) (SE): ‐2.4 (2.8) 2. % of weight loss Delta I (SE): ‐2.5 (2.5) Delta C (SE): ‐0.1 (1.2) Delta (I‐C) (SE): ‐2.5 (2.8) | 1. GHb (%) Delta I (SE): ‐0.5 (0.5) Delta C (SE): 0 (0.4) Delta (I‐C) (SE): ‐0.5 (0.7) 2. Fasting blood sugar (mml/L) Delta I (SE): ‐0.5 (1.0) Delta C (SE): 0.2 (0.7) Delta (I‐C) (SE): ‐0.7 (1.3) | ||
| Pooled effects (Follow‐up: 8‐16 w) Outcomes S=Number of studies N=Number of participants pooled effects (95% CI) | 1. Weight loss (kg) S=5 N=192 ‐3.4 [‐5.2, ‐1.7] 2. BMI kg/m2) S=1 N=47 ‐0.5 [‐1.0, ‐0.1] | 1. GHb (%) S=4 N=145 ‐1.0 [‐1.5, ‐0.4] 2. Fasting glucose (mmol/L) S=5 N=192 ‐0.9 [‐2.1, 0.4] | 1. Total cholesterol (mmol/L) S=2 N=85 ‐0.1 [‐0.3, 0.2] 2. HDL cholesterol (mmol/L) S=1 N=68 0.0 [‐0.1, 0.1] 3. Triglycerides (mmol/L) S=2 N=85 ‐0.5 [‐01.1, 0.1] | |
| Pooled effects (Follow‐up: 24‐30w) Outcomes S=Number of studies N=Number of participants pooled effects (95% CI) | 1. Weight loss (kg) S=4 N=97 ‐5.1 [‐6.9, ‐3.3] 2. Percent weight loss S=1 N=20 ‐2.5 [‐7.9, 3.0] | 1. GHb (%) S=4 N=97 ‐1.0 [‐1.4, ‐0.6] 2. Fasting glucose (mmol/L) S=4 N=97 ‐0.9 [‐2.0, 0.2] | 1. Total cholesterol (mmol/L) S=1 N=17 0.1 [‐0.4, 0.6] 2. Triglycerides (mmol/L) S=1 N=17 ‐0.2 [‐1.0, 0.7] | |
| Pooled effects (Follow‐up: 52w) Outcomes S=Number of studies N=Number of participants pooled effects (95% CI) | 1. Weight loss (kg) S=1 N=17 ‐5.8 [‐10.8, ‐0.8] | 1. GHb (%) S=1 N=17 ‐1.8 [‐3.8, 0.2] 2. Fasting glucose (mmol/L) S=1 N=17 ‐0.8 [‐2.5, 0.9] | . Total cholesterol (mmol/L) S=1 N=17 0.5 [‐0.3, 1.3] 2. Triglycerides (mmol/L) S=1 N=17 ‐0.5 [‐1.2, 0.2] | |
| Pedrinola 1996 Study design: Pre vs post Follow‐up interval: 34 weeks | 1. Weight change (kg) Delta I (SE): ‐6.2 (1.7) 2. BMI Delta I (SE): ‐2.3 (0.5) | 1. Total cholesterol (mmol/L) Delta I (SE): ‐1.9 (0.2) 2. HDL cholesterol (mmol/L) Delta I (SE): 0.1 (0.1) 3. Triglycerides (mmol/L) Delta I (SE): ‐0.7 (0.1) |
Appendix 16. Outcomes: Mazindol
| Study | Weight | Glycemic control | Lipids | Blood pressure |
| Sanders 1976 Study design: RCT Follow‐up interval: 6 weeks | 1. Weight change (kg) Delta I (SE): ‐4.2 (0.4) Delta C (SE): ‐0.9 (0.2) Delta (I‐C) (SE): ‐3.3 (0.4) | 1. Fasting blood sugar (mml/L) Delta I (SE): ‐2.3 (0.2) Delta C (SE): ‐2.0 (0.2) Delta (I‐C) (SE): ‐0.3 (0.3) | ||
| Slama 1978 Study design: RCT Follow‐up interval: 12 weeks | 1. Weight change (kg) Delta I (SE): ‐13.5 (2.9) Delta C (SE): ‐4.2 (2.1) Delta (I‐C) (SE): ‐9.3 (1.8) 2. % of weight loss Delta I (SE): ‐22.3 (2.9) Delta C (SE): ‐9.8 (2.1) Delta (I‐C) (SE): ‐12.5 (3.6) | 1. Fasting blood sugar (mml/L) Delta I (SE): ‐0.3 (0.6) Delta C (SE): ‐0.4 (0.7) Delta (I‐C) (SE): 0.1 (0.9) | 1. Total cholesterol (mmol/L) Delta I (SE): ‐1.1 (0.3) Delta C (SE): ‐0.3 (0.2) Delta (I‐C) (SE): ‐0.8 (0.4) 2. Triglycerides (mmol/L) Delta I (SE): ‐0.4 (0.1) Delta C (SE): ‐0.9 (0.3) Delta (I‐C) (SE): 0.5 (0.3) | |
| Bandisode 1975 Study design: RCT Follow‐up interval: 12 weeks | 1. Weight change (kg) Delta I (SE): ‐5.0 (0.9) Delta C (SE): ‐3.6 (0.7) Delta (I‐C) (SE): ‐1.4 (1.2) | 1. Total cholesterol (mmol/L) Delta I (SE): ‐0.8 (0.4) Delta C (SE): 0.1 (0.3) Delta (I‐C) (SE): ‐0.8 (0.5) | ||
| Boshell 1974 Study design: RCT Follow‐up interval: 12 weeks | 1. Weight change (kg) Delta I (SE): ‐5.4 (0.4) Delta C (SE): ‐3.4 (0.4) Delta (I‐C) (SE): ‐1.9 (0.6) | |||
| Crommelin 1974 Study design: RCT Follow‐up interval: 12 weeks | 1. Weight change (kg) Delta I (SE): ‐4.4 (NR) Delta C (SE): ‐2.5 (NR) Delta (I‐C) (SE): ‐2.0 (NR) | |||
| Felt 1977 Study design: NR Follow‐up interval: 12 weeks | 1. Weight change (kg) Delta I (SE): ‐9.0 (2.6) Delta C (SE): ‐6.0 (2.5) Delta (I‐C) (SE): ‐3.0 (3.6) | 1. Fasting blood sugar (mml/L) Delta I (SE): ‐1.4 (0.7) Delta C (SE): ‐0.9 (0.7) Delta (I‐C) (SE): ‐0.5 (1.0) | 1. Total cholesterol (mmol/L) Delta I (SE): ‐0.2 (0.1) Delta C (SE): 0.3 (0.1) Delta (I‐C) (SE): ‐0.4 (0.2) | |
| Dolecek 1976 Study design: Pre vs post Follow‐up interval: 8 weeks | 1. Weight change (kg) Delta I (SE): ‐7.7 (NR) 2. % of weight loss Delta I (SE): ‐7.8 (NR) | 1. Fasting blood sugar (mml/L) Delta I (SE): ‐0.5 (0.5) | 1. Total cholesterol (mmol/L) Delta I (SE): ‐0.5 (0.5) |
Appendix 17. Outcomes and pooled effects: Orlistat
| Study | Weight | Glycemic control | Lipids | Blood pressure |
| Bloch 2003 Study design: RCT Follow‐up interval: 12 weeks | 1. Weight change (kg) Delta I (SE): ‐2.3 (0.5) Delta C (SE): ‐1.5 (0.4) Delta (I‐C) (SE): ‐0.8 (0.6) 2. Waist circumference (cm) Delta I (SE): ‐2.1 (0.5) Delta C (SE): ‐2.5 (0.5) Delta (I‐C) (SE): 0.4 (0.7) | 1. Fasting blood sugar (mml/L) Delta I (SE): ‐1.6 (0.5) Delta C (SE): ‐0.1 (0.5) Delta (I‐C) (SE): ‐1.6 (0.7) | 1. Total cholesterol (mmol/L) Delta I (SE): ‐0.9 (0.2) Delta C (SE): ‐0.4 (0.2) Delta (I‐C) (SE): ‐0.5 (0.3) 2. HDL cholesterol (mmol/L) Delta I (SE): 0.0 (0.0) Delta C (SE): 0.0 (0.0) Delta (I‐C) (SE): 0.0 (0.0) 3. Triglycerides (mmol/L) Delta I (SE): ‐0.5 (0.2) Delta C (SE): ‐0.4 (0.2) Delta (I‐C) (SE): ‐0.1 (0.2) | 1. SBP Delta I (SE): ‐17.8 (4.2) Delta C (SE): ‐4.5 (2.5) Delta (I‐C) (SE): ‐13.3 (4.8) 2. DBP Delta I (SE): ‐11.5 (2.5) Delta C (SE): ‐1.6 (2.0) Delta (I‐C) (SE): ‐9.9 (3.2) |
| Hanefeld 2002 Study design: RCT Follow‐up interval: 52 weeks | 1. Weight change (kg) Delta I (SE): ‐5.3 (0.4) Delta C (SE): ‐3.4 (0.4) Delta (I‐C) (SE): ‐1.9 (0.5) 2. % of weight loss Delta I (SE): ‐5.4 (0.4) Delta C (SE): ‐3.6 (0.4) Delta (I‐C) (SE): ‐1.8 (0.6) | 1. GHb (%) Delta I (SE): ‐0.9 (0.1) Delta C (SE): ‐0.4 (0.1) Delta (I‐C) (SE): ‐0.5 (0.2) 2. Fasting blood sugar (mml/L) Delta I (SE): ‐1.6 (0.2) Delta C (SE): ‐0.8 (0.2) Delta (I‐C) (SE): ‐0.8 (0.3) | 1. Total cholesterol (mmol/L) Delta I (SE): ‐0.1 (0.2) Delta C (SE): 0.1 (0.2) Delta (I‐C) (SE): ‐0.2 (0.3) 2. LDL cholesterol (mmol/L) Delta I (SE): ‐0.1 (0.1) Delta C (SE): 0.2 (0.1) Delta (I‐C) (SE): ‐0.3 (0.2) | |
| Hollander 1998 Study design: RCT Follow‐up interval: 12 weeks | 1. Weight change (kg) Delta I (SE): ‐6.2 (0.5) Delta C (SE): ‐4.3 (0.6) Delta (I‐C) (SE): ‐1.9 (0.8) 2. % of weight loss Delta I (SE): ‐6.2 (0.5) Delta C (SE): ‐4.3 (0.5) Delta (I‐C) (SE): ‐1.9 (0.7) | 1. GHb (%) Delta I (SE): ‐0.3 (0.1) Delta C (SE): 0.2 (0.1) Delta (I‐C) (SE): ‐0.5 (0.1) 2. Fasting blood sugar (mml/L) Delta I (SE): ‐0.0 (0.1) Delta C (SE): 0.5 (0.0) Delta (I‐C) (SE): ‐0.6 (0.1) | 1. Total cholesterol (mmol/L) Delta I (SE): ‐0.1 (0.1) Delta C (SE): 0.4 (0.1) Delta (I‐C) (SE): ‐0.5 (0.1) 2. LDL cholesterol (mmol/L) Delta I (SE): ‐0.1 (0.1) Delta C (SE): 0.2 (0.1) Delta (I‐C) (SE): ‐0.4 (0.1) 3. HDL cholesterol (mmol/L) Delta I (SE): 0.1 (0.0) Delta C (SE): 0.1 (0.0) Delta (I‐C) (SE): ‐0.0 (0.0) 4. Triglycerides (mmol/L) Delta I (SE): ‐0.0 (0.1) Delta C (SE): 0.2 (0.2) Delta (I‐C) (SE): ‐0.2 (0.2) | |
| Kelley 2004Study design: RCTFollow‐up interval: 26 weeks | 1. weight loss (kg) Delta I (SE): ‐10.1 (1.4) Delta C (SE): ‐9.4 (1.3) Delta (I‐C) (SE): ‐0.7 (1.9) 1. BMI (kg/m2) Delta I (SE): ‐3.6 (0.5) Delta C (SE): ‐3.3 (0.4) Delta (I‐C) (SE): ‐0.3 (0.6) | 1. GHb (%) Delta I (SE): ‐1.7 (0.3) Delta C (SE): ‐1.0 (0.4) Delta (I‐C) (SE): ‐0.7 (0.5) 2. Fasting blood sugar (mml/L) Delta I (SE): ‐3.4 (0.5) Delta C (SE): ‐1.8 (0.4) Delta (I‐C) (SE): ‐1.7 (0.7) | 1. LDL cholesterol (mmol/L) Delta I (SE): ‐0.5 (0.1) Delta C (SE): 0.1 (0.1) Delta (I‐C) (SE): ‐0.6 (0.1) 2. HDL cholesterol (mmol/L) Delta I (SE): ‐0.1 (0.1) Delta C (SE): 0.1 (0.1) Delta (I‐C) (SE): ‐0.2 (0.1) 3. Triglycerides (mmol/L) Delta I (SE): ‐0.7 (0.3) Delta C (SE): ‐0.5 (0.1) Delta (I‐C) (SE): ‐0.2 (0.3) | 1. SBP Delta I (SE): ‐3.0 (2.0) Delta C (SE): ‐4.0 (2.0) Delta (I‐C) (SE): 1.0 (2.8) 2. DBP Delta I (SE): ‐6.0 (2.0) Delta C (SE): ‐5.0 (2.0) Delta (I‐C) (SE): ‐1.0 (2.8) |
| Kelley 2002 Study design: RCT Follow‐up interval: 52 weeks | 1. Weight change (kg) Delta I (SE): ‐3.9 (0.3) Delta C (SE): ‐1.3 (0.3) Delta (I‐C) (SE): ‐2.6 (0.4) 2. % of weight loss Delta I (SE): ‐3.8 (0.3) Delta C (SE): ‐1.2 (0.3) Delta (I‐C) (SE): ‐2.5 (0.4) | 1. GHb (%) Delta I (SE): ‐0.6 (0.1) Delta C (SE): ‐0.3 (0.1) Delta (I‐C) (SE): ‐0.4 (0.1) 2. Fasting blood sugar (mml/L) Delta I (SE): ‐1.6 (0.3) Delta C (SE): ‐1.1 (0.3) Delta (I‐C) (SE): ‐0.6 (0.4) | 1. Total cholesterol (mmol/L) Delta I (SE): ‐0.3 (0.1) Delta C (SE): 0.1 (0.1) Delta (I‐C) (SE): ‐0.4 (0.1) 2. LDL cholesterol (mmol/L) Delta I (SE): ‐0.4 (0.1) Delta C (SE): ‐0.1 (0.1) Delta (I‐C) (SE): ‐0.3 (0.1) 3. HDL cholesterol (mmol/L) Delta I (SE): 0.0 (0.0) Delta C (SE): 0.1 (0.0) Delta (I‐C) (SE): ‐0.0 (0.0) 4. Triglycerides (mmol/L) Delta I (SE): 0.2 (0.2) Delta C (SE): 0.3 (0.1) Delta (I‐C) (SE): ‐0.1 (0.2) | 1. SBP Delta I (SE): ‐1.2 (1.0) Delta C (SE): ‐0.9 (1.0) Delta (I‐C) (SE): ‐0.3 (1.4) 2. DBP Delta I (SE): ‐2.3 (0.7) Delta C (SE): ‐1.0 (0.5) Delta (I‐C) (SE): ‐1.3 (0.9) |
| Lindgarde 2000 Study design: RCT Follow‐up interval: 54 weeks | 1. % of weight loss Delta I (SE): ‐5.4 (0.7) Delta C (SE): ‐3.5 (0.7) Delta (I‐C) (SE): ‐1.9 (1.0) | 1. GHb (%) Delta I (SE): ‐0.7 (0.2) Delta C (SE): ‐0.1 (0.2) Delta (I‐C) (SE): ‐0.5 (0.3) 2. Fasting blood sugar (mml/L) Delta I (SE): ‐1.6 (0.4) Delta C (SE): ‐0.3 (0.4) Delta (I‐C) (SE): ‐1.4 (0.6) | ||
| Miles 2002 Study design: RCT Follow‐up interval: 52 weeks | 1. Weight change (kg) Delta I (SE): ‐4.7 (0.3) Delta C (SE): ‐1.8 (0.3) Delta (I‐C) (SE): ‐2.9 (0.4) 2. % of weight loss Delta I (SE): ‐4.6 (0.3) Delta C (SE): ‐1.7 (0.2) Delta (I‐C) (SE): ‐2.9 (0.4) | 1. GHb (%) Delta I (SE): ‐0.8 (0.1) Delta C (SE): ‐0.4 (0.1) Delta (I‐C) (SE): ‐0.4 (0.1) 2. Fasting blood sugar (mml/L) Delta I (SE): ‐2 (0.2) Delta C (SE): ‐0.7 (0.2) Delta (I‐C) (SE): ‐1.3 (0.3) | 1. Total cholesterol (mmol/L) Delta I (SE): ‐0.3 (0.0) Delta C (SE): 0.1 (0.1) Delta (I‐C) (SE): ‐0.3 (0.1) 2. LDL cholesterol (mmol/L) Delta I (SE): ‐0.3 (0.0) Delta C (SE): ‐0.1 (0.1) Delta (I‐C) (SE): ‐0.2 (0.1) 3. HDL cholesterol (mmol/L) Delta I (SE): 0.1 (0.0) Delta C (SE): 0.1 (0.0) Delta (I‐C) (SE): ‐0.0 (0.0) 4. Triglycerides (mmol/L) Delta I (SE): ‐0.3 (0.1) Delta C (SE): 0.0 (0.1) Delta (I‐C) (SE): ‐0.3 (0.1) | 1. SBP Delta I (SE): ‐2.1 (0.8) Delta C (SE): ‐0.4 (0.9) Delta (I‐C) (SE): ‐1.7 (1.2) |
| Wang 2003Study design: RCTFollow‐up itnerval: 24 weeks | 1. Weight change (kg) Delta I (SE): ‐7.0 (1.2) Delta C (SE): ‐3.0 (1.1) Delta (I‐C) (SE): ‐4.0 (1.6) 2. BMI (kg/m2) Delta I (SE): ‐2.0 (0.4) Delta C (SE): ‐1.0 (0.4) Delta (I‐C) (SE): ‐1.0 (0.5) 3. Waist circumference (cm) Delta I (SE): ‐7.0 (1.0) Delta C (SE): ‐3.0 (1.1) Delta (I‐C) (SE): ‐4.0 (1.5) | 1. GHb (%) Delta I (SE): ‐1.1 (0.2) Delta C (SE): ‐0.5 (0.2) Delta (I‐C) (SE): ‐0.6 (0.2) 2. Fasting blood sugar (mml/L) Delta I (SE): ‐0.5 (0.2) Delta C (SE): ‐0.2 (0.2) Delta (I‐C) (SE): ‐0.3 (0.2) | 1. Total cholesterol (mmol/L) Delta I (SE): ‐1.3 (0.3) Delta C (SE): ‐0.8 (0.3) Delta (I‐C) (SE): ‐0.5 (0.4) 2. LDL cholesterol (mmol/L) Delta I (SE): ‐0.3 (0.2) Delta C (SE): ‐0.2 (0.2) Delta (I‐C) (SE): ‐0.1 (0.3) 3. HDL cholesterol (mmol/L) Delta I (SE): 0.1 (0.1) Delta C (SE): 0.1 (0.1) Delta (I‐C) (SE): 0.0 (0.1) 4. Triglycerides (mmol/L) Delta I (SE): ‐0.6 (0.1) Delta C (SE): ‐0.3 (0.1) Delta (I‐C) (SE): ‐0.3 (0.2) | 1. SBP Delta I (SE): ‐12.0 (1.7) Delta C (SE): ‐5.3 (1.4) Delta (I‐C) (SE): ‐6.7 (2.2) 2. DBP Delta I (SE): ‐7.5 (0.6) Delta C (SE): ‐1.5 (0.6) Delta (I‐C) (SE): ‐6.0 (0.9) |
| Bonnici 2002 Study design: RCT Follow‐up interval: 24 weeks | 1. % of weight loss Delta I (SE): ‐3.8 (0.5) Delta C (SE): ‐1.2 (0.5) Delta (I‐C) (SE): ‐2.6 (0.7) | 1. GHb (%) Delta I (SE): ‐1.0 (0.3) Delta C (SE): 0.5 (0.3) Delta (I‐C) (SE): ‐1.5 (0.5) 2. Fasting blood sugar (mml/L) Delta I (SE): ‐1.4 (0.2) Delta C (SE): ‐0.4 (0.2) Delta (I‐C) (SE): ‐1.0 (0.3) | 1. LDL cholesterol (mmol/L) Delta I (SE): ‐0.1 (0.1) Delta C (SE): 0.2 (0.1) Delta (I‐C) (SE): ‐0.3 (0.1) | |
| Segal 2000 Study design: RCT Follow‐up interval: 54 weeks | 1.% of weight loss Delta I (SE): ‐6.3 (0.5) Delta C (SE): ‐4.2 (0.6) Delta (I‐C) (SE): ‐2.1 (0.8) | |||
| Hawkins 2000 Study design: RCT Follow‐up interval: 26 weeks | 1. Weight change (kg) Delta I (SE): ‐5.4 (0.4) Delta C (SE): ‐2.7 (0.4) Delta (I‐C) (SE): ‐2.7 (0.5) 2. % of weight loss Delta I (SE): ‐5.5 (0.4) Delta C (SE): ‐2.6 (0.4) Delta (I‐C) (SE): ‐2.9 (0.6) | 1. GHb (%) Delta I (SE): ‐0.9 (0.1) Delta C (SE): ‐0.4 (0.1) Delta (I‐C) (SE): ‐0.5 (0.2) 2. Fasting blood sugar (mml/L) Delta I (SE): ‐1.5 (0.3) Delta C (SE): 0 (0.3) Delta (I‐C) (SE): ‐1.5 (0.5) | ||
| Hollander 2001 Study design: RCT Follow‐up interval: 52 weeks | 1. % of weight loss Delta I (SE): ‐4.6 (0.3) Delta C (SE): ‐1.7 (0.3) Delta (I‐C) (SE): ‐2.9 (0.4) | |||
| Kelley 1997 Study design: RCT Follow‐up interval: 52 weeks | 1. % of weight loss Delta I (SE): ‐6.2 (0.4) Delta C (SE): ‐4.3 (0.5) Delta (I‐C) (SE): ‐1.9 (0.7) | 1. GHb (%) Delta I (SE): ‐0.2 (0.1) Delta C (SE): 0.3 (0.1) Delta (I‐C) (SE): ‐0.5 (0.2) 2. Fasting blood sugar (mml/L) Delta I (SE): ‐0.0 (0.1) Delta C (SE): 0.5 (0.1) Delta (I‐C) (SE): ‐0.6 (0.2) | ||
| Guy‐Grand 2002 Study design: RCT Follow‐up interval: 26 weeks | 1. Weight change (kg) Delta I (SE): ‐3.9 (0.4) Delta C (SE): ‐1.3 (0.3) Delta (I‐C) (SE): ‐2.6 (0.4) | 1. GHb (%) Delta I (SE): ‐0.5 (0.1) Delta C (SE): ‐0.2 (0.1) Delta (I‐C) (SE): ‐0.4 (0.1) 2. Fasting blood sugar (mml/L) Delta I (SE): ‐1.4 (0.2) Delta C (SE): ‐0.5 (0.2) Delta (I‐C) (SE): ‐0.9 (0.3) | ||
| Mendoza‐Guadarrama 2000 Study design: RCT Follow‐up interval: 26 weeks | 1. BMI (kg/m2) Delta I (SE): ‐0.4 (0.1) Delta C (SE): ‐0.2 (0.1) Delta (I‐C) (SE): ‐0.2 (0.1) | |||
| Shi 2001 Study design: RCT Follow‐up interval: 26 weeks | 1. GHb (%) Delta I (SE): ‐0.7 (0.2) Delta C (SE): ‐0.2 (0.2) Delta (I‐C) (SE): ‐0.7 (0.3) 2. Fasting blood sugar (mml/L) Delta I (SE): ‐2.1 (0.3) Delta C (SE): ‐1.0 (0.3) Delta (I‐C) (SE): ‐1.1 (0.4) | |||
| Serrano‐Rios 2001 Study design: RCT Follow‐up interval: 26 weeks | 1. % of weight loss Delta I (SE): ‐4.2 (0.7) Delta C (SE): ‐1.0 (0.7) Delta (I‐C) (SE): ‐3.2 (1.0) | 1. GHb (%) Delta I (SE): ‐0.9 (0.1) Delta C (SE): ‐0.4 (0.1) Delta (I‐C) (SE): ‐0.5 (0.2) | 1. SBP Delta I (SE): ‐3.4 (1.2) Delta C (SE): 1.4 (1.2) Delta (I‐C) (SE): ‐4.8 (1.7) 2. DBP Delta I (SE): ‐2.2 (0.7) Delta C (SE): 0.8 (0.9) Delta (I‐C) (SE): ‐3.0 (1.2) | |
| Deerochanawong 2001 Study design: RCT Follow‐up interval: 12 weeks | 1. Weight change (kg) Delta I (SE): ‐2.6 (0.2) Delta C (SE): ‐1.4 (0.2) Delta (I‐C) (SE): ‐1.2 (0.3) | 1. GHb (%) Delta I (SE): ‐0.9 (0.1) Delta C (SE): ‐0.6 (0.1) Delta (I‐C) (SE): ‐0.3 (0.1) 2. Fasting blood sugar (mml/L) Delta I (SE): ‐1.7 (0.2) Delta C (SE): ‐1.0 (0.2) Delta (I‐C) (SE): ‐0.8 (0.3) | ||
| Halpern 2003 Study design: RCT Follow‐up interval: 26 weeks | 1. Weight change (kg) Delta I (SE): ‐4.2 (0.2) Delta C (SE): ‐2.6 (1.5) Delta (I‐C) (SE): ‐1.7 (1.5) 2. % of weight loss Delta I (SE): ‐4.7 (0.5) Delta C (SE): ‐3.0 (1.3) Delta (I‐C) (SE): ‐1.7 (1.4) | 1. GHb (%) Delta I (SE): ‐0.6 (0.2) Delta C (SE): ‐0.2 (0.1) Delta (I‐C) (SE): ‐0.4 (0.2) 2. Fasting blood sugar (mml/L) Delta I (SE): ‐1.0 (0.3) Delta C (SE): ‐0.0 (0.3) Delta (I‐C) (SE): ‐1.0 (0.5) | 1. Total cholesterol (mmol/L) Delta I (SE): ‐0.4 (0.0) Delta C (SE): ‐0.0 (0.0) Delta (I‐C) (SE): ‐0.4 (0.0) 2. LDL cholesterol (mmol/L) Delta I (SE): ‐0.3 (0.0) Delta C (SE): 0.0 (0.0) Delta (I‐C) (SE): ‐0.3 (0.0) 3. HDL cholesterol (mmol/L) Delta I (SE): ‐0.0 (0.0) Delta C (SE): 0.0 (0.0) Delta (I‐C) (SE): ‐0.0 (0.0) 4. Triglycerides (mmol/L) Delta I (SE): ‐0.2 (0.0) Delta C (SE): ‐0.1 (0.0) Delta (I‐C) (SE): ‐0.1 (0.0) | |
| Pooled effects (Full Text) Outcomes S=Number of studies N=Number of participants pooled effects (95% CI) * not including Halpern 2003 | 1. Weight loss (kg) S=7 N=1363 ‐2.0 [‐2.8, ‐1.3] 2. Percent weight loss S=4 N=1008 ‐2.3 [‐3.0, ‐1.7] 3. % participants with weight loss >5% S=5 N=1273 21.4 [15.2, 27.6] 4. BMI (kg/m2) S=2 N=100 ‐0.7 [‐1.5, 0.1] 5. Waist circumference (cm) S=6 N=1111 ‐1.8 [‐3.0, ‐0.7] | 1. GHb (%) S=7 N=1373 ‐0.5 [‐0.6, ‐0.3] 2. Fasting glucose (mmol/L) S=8 N=1449 ‐0.8 [‐1.1, ‐0.5] | 1. Total cholesterol (mmol/L) S=6 N=1324 ‐0.4 [‐0.5, ‐0.3] 2. LDL cholesterol (mmol/L) S=6 N=1287 ‐0.3 [‐0.4, ‐0.2] 3. HDL cholesterol (mmol/L) S=5 N=994 ‐0.0 [‐0.1, 0.0] 4. Triglycerides (mmol/L) S=6 N=994 ‐0.2 [‐0.4, ‐0.1] | 1. SBP (mmHg) S=5 N=740 ‐3.0 [‐6.3, 0.3] 2. DBP (mmHg) S=4 N=441 ‐4.2 [‐7.8, ‐0.6] |
| Pooled effects (Full Text+Abstract) Outcomes S=Number of studies N=Number of participants pooled effects (95% CI) * not including Halpern 2003 (FT), but Halpern 2001 (abstract) | 1. Weight loss (kg) S=10 N=2045 ‐2.1 [‐2.7, ‐1.6] 2. Percent weight loss S=11 N=3171 ‐2.4 [‐2.8, ‐2.1] 3. % participants with weight loss >5% S=11 N=3209 19.7 [15.8, 23.7] 4. BMI (kg/m2) S=3 N=130 ‐0.3 [‐0.6, 0.1] 5. Waist circumference (cm) S=8 N=1647 ‐1.7 [‐2.5, ‐0.9] | 1. GHb (%) S=14 N=3236 ‐0.4 [‐0.5, ‐0.3] 2. Fasting glucose (mmol/L) S=14 N=3075 ‐0.8 [‐1.0, ‐0.6] | 1. Total cholesterol (mmol/L) S=6 N=1324 ‐0.4 [‐0.5, ‐0.3] 2. LDL cholesterol (mmol/L) S=7 N=1571 ‐0.3 [‐0.4 ‐0.2] 3. HDL cholesterol (mmol/L) S=5 N=994 ‐0.0 [‐0.0, ‐0.0] 4. Triglycerides (mmol/L) S=6 N=994 ‐0.2 [‐0.4 ‐0.1 | 1. SBP (mmHg) S=6 N=977 ‐3.2 [‐5.9, ‐0.5] 2. DBP (mmHg) S=5 N=678 ‐3.9[‐6.5, ‐1.2 |
| Allie 2004 Study design: pre vs post Follow‐up interval: 12‐26 weeks | 1. Weight change (kg) Delta I (SE): ‐6.0 (3.6) 2. BMI (kg/m2) Delta I (SE): ‐2.0 (1.1) | 1. GHb (%) Delta I (SE): ‐0.4 (0.2) | 1. Total cholesterol (mmol/L) Delta I (SE): ‐0.7 (0.2 2. LDL cholesterol (mmol/L) Delta I (SE): ‐0.5 (0.1) 3. HDL cholesterol (mmol/L) Delta I (SE): ‐0.1 (0.1) 4. Triglycerides (mmol/L) Delta I (SE): ‐0.5 (0.4) | 1. SBP Delta I (SE): ‐4.0 (1.6) 2. DBP Delta I (SE): ‐2.0 (0.9) |
Appendix 18. Outcomes: Phenmetrazine
| Study | Weight | Glycemic control | Lipids | Blood pressure |
| Buckle 1966 Study design: RCT, cross‐over design, only phenmetrazine group reported (comparison group received Filon(R) (phenmetrazine theoclate and phenbutrazate) Follow‐up interval: 8 weeks | 1. Weight change (kg) Delta I (SE): ‐2.9 (0.3) 2. % of weight loss Delta I (SE): ‐3.8(0.4) |
Appendix 19. Outcomes: Phentermine
| Study | Weight | Glycemic control | Lipids | Blood pressure |
| Campbell 1977 Study design: RCT Follow‐up interval: 12 weeks | 1. Weight change (kg) Delta I (SE): ‐5.2 (0.5) Delta C (SE): ‐1.4 (0.6) Delta (I‐C) (SE): ‐3.8 (0.8) | |||
| Gershberg 1977 Study design: RCT Follow‐up interval: 16 weeks | 1. Weight change (kg) Delta I (SE): ‐7.8 (1.1) Delta C (SE): ‐2.9 (1.1) Delta (I‐C) (SE): ‐4.9 (1.5) 2. % of weight loss Delta I (SE): ‐9.2(1.3) Delta C (SE): ‐3.5 (1.3) Delta (I‐C) (SE): ‐5.7 (1.8) | 1. Fasting blood sugar (mml/L) Delta I (SE): ‐0.5 (0.7) Delta C (SE): 0.7 (0.5) Delta (I‐C) (SE): ‐1.2 (0.9) | 1. Total cholesterol (mmol/L) Delta I (SE): ‐1.2 (0.5) Delta C (SE): 0.4 (0.5) Delta (I‐C) (SE): ‐1.6(0.7) 2. Triglycerides (mmol/L) Delta I (SE): ‐0.4 (0.2) Delta C (SE): 0.2 (0.1) Delta (I‐C) (SE): ‐0.6 (0.2) | 1. SBP Delta I (SE): ‐9.5 (4.1) Delta C (SE): ‐4.1 (3.6) Delta (I‐C) (SE): ‐5.4 (5.4) 2. DBP Delta I (SE): ‐8.6 (1.9) Delta C (SE): ‐7.3 (2.4) Delta (I‐C) (SE): ‐1.3 (3.1) |
Appendix 20. Outcomes and pooled effects: Sibutramine
| Study | Weight | Glycemic control | Lipids | Blood pressure |
| Vargas 1994 Study design: RCT Follow‐up interval: 12 weeks | 1. Weight change (kg) Delta I (SE): ‐2.7 (0.9) Delta C (SE): ‐0.5 (0.9) Delta (I‐C) (SE): ‐2.2 (1.3) | |||
| Rissanen 1999 Study design: RCT Follow‐up interval: 12 weeks | 1. % of weight loss Delta I (SE): ‐7.3 (1.1) Delta C (SE): ‐2.4 (1.1) Delta (I‐C) (SE): ‐4.9 (1.5) | |||
| Gokcel 2001 Study design: RCT Follow‐up interval: 26 weeks | 1. Weight change (kg) Delta I (SE): ‐9.6 (1.4) Delta C (SE): 0.9 (0.5) Delta (I‐C) (SE): ‐10.5 (1.5) 2. BMI (kg/m2) Delta I (SE): ‐3.9 (0.5) Delta C (SE): 0.4 (0.2) Delta (I‐C) (SE): ‐4.3 (0.6) 3. % of weight loss Delta I (SE): ‐10.1 (1.4) Delta C (SE): 0.9 (0.6) Delta (I‐C) (SE): ‐11.0(1.5) | 1. GHb (%) Delta I (SE): ‐2.7 (0.1) Delta C (SE): ‐0.5 (0.1) Delta (I‐C) (SE): ‐2.2 (0.1) 2. Fasting blood sugar (mml/L) Delta I (SE): ‐6.9 (0.5) Delta C (SE): ‐0.9 (0.2) Delta (I‐C) (SE): ‐6.1 (0.5) | 1. Total cholesterol (mmol/L) Delta I (SE): ‐0.7 (0.1) Delta C (SE): ‐0.2 (0.1) Delta (I‐C) (SE): ‐0.5 (0.2) 2. LDL cholesterol (mmol/L) Delta I (SE): ‐0.5 (0.1) Delta C (SE): ‐0.3 (0.1) Delta (I‐C) (SE): ‐0.2 (0.2) 3. HDL cholesterol (mmol/L) Delta I (SE): ‐0.0 (0.4) Delta C (SE): 0 (0.0) Delta (I‐C) (SE): ‐0.0 (0.4) 4. Triglycerides (mmol/L) Delta I (SE): ‐0.5 (0.2) Delta C (SE): 0 (0.2) Delta (I‐C) (SE): ‐0.5 (0.2) | |
| Finer 2000 Study design: RCT Follow‐up interval: 12 weeks | 1. Weight change (kg) Delta I (SE): ‐2.4 (0.3) Delta C (SE): ‐0.1 (0.3) Delta (I‐C) (SE): ‐2.3 (0.4) 2. BMI (kg/m2) Delta I (SE): ‐0.9 (0.2) Delta C (SE): ‐0.1 (0.2) Delta (I‐C) (SE): ‐0.8 (0.2) 3. % of weight loss Delta I (SE): ‐2.8 (0.4) Delta C (SE): ‐0.1 (0.3) Delta (I‐C) (SE): ‐2.7 (0.5) | 1. GHb (%) Delta I (SE): ‐0.3 (0.2) Delta C (SE): 0 (0.2) Delta (I‐C) (SE): ‐0.3 (0.2) | 1. SBP Delta I (SE): ‐0.2 (0.5) Delta C (SE): ‐0.1 (0.4) Delta (I‐C) (SE): ‐0.1 (0.6) | |
| Fujioka 2000 Study design: RCT Follow‐up interval: 24 weeks | 1. Weight change (kg) Delta I (SE): ‐3.7 (1.2) Delta C (SE): ‐0.4 (1.2) Delta (I‐C) (SE): ‐3.3 (1.7) 2. BMI (kg/m2) Delta I (SE): ‐1.3 (0.2) Delta C (SE): ‐0.2 (0.1) Delta (I‐C) (SE): ‐1.1 (0.2) 3. % of weight loss Delta I (SE): ‐3.8 (1.2) Delta C (SE): ‐0.5 (1.2) Delta (I‐C) (SE): ‐3.3 (1.7) | 1. GHb (%) Delta I (SE): 0.2 (0.1) Delta C (SE): 0.3 (0.1) Delta (I‐C) (SE): ‐0.1 (0.2) 2. Fasting blood sugar (mml/L) Delta I (SE): 0.6 (0.3) Delta C (SE): 0.4 (0.3) Delta (I‐C) (SE): 0.2 (0.4) | 1. Total cholesterol (mmol/L) Delta I (SE): 0.1 (0.1) Delta C (SE): 0.1 (0.1) Delta (I‐C) (SE): 0.0 (0.1) 2. LDL cholesterol (mmol/L) Delta I (SE): 0.1 (0.1) Delta C (SE): ‐0.1 (0.1) Delta (I‐C) (SE): 0.2 (0.1) 3. HDL cholesterol (mmol/L) Delta I (SE): 0.1 (0.0) Delta C (SE): 0.1 (0.0) Delta (I‐C) (SE): 0.1 (0.0) 4. Triglycerides (mmol/L) Delta I (SE): ‐0.3 (0.1) Delta C (SE): 0.2 (0.1) Delta (I‐C) (SE): ‐0.5 (0.2) | 1. SBP Delta I (SE): 3.9 (1.6) Delta C (SE): 2.4 (1.8) Delta (I‐C) (SE): 1.5 (2.4) 2. DBP Delta I (SE): 2.6 (1.1) Delta C (SE): 1.4 (1.1) Delta (I‐C) (SE): 1.2 (1.6) |
| Serrano‐Rios 2002 Study design: RCT Follow‐up interval: 24 weeks | 1. Weight change (kg) Delta I (SE): ‐4.5 (0.5) Delta C (SE): ‐1.7 (0.5) Delta (I‐C) (SE): ‐2.8 (0.7) 2. BMI (kg/m2) Delta I (SE): ‐1.9 (0.2) Delta C (SE): ‐0.6 (0.2) Delta (I‐C) (SE): ‐1.3 (0.3) 3. % of weight loss Delta I (SE): ‐4.9 (0.5) Delta C (SE): ‐1.8 (0.5) Delta (I‐C) (SE): ‐3.1 (0.8) | 1. GHb (%) Delta I (SE): ‐0.8 (0.2) Delta C (SE): ‐0.7 (0.2) Delta (I‐C) (SE): ‐0.1 (0.3) 2. Fasting blood sugar (mml/L) Delta I (SE): ‐0.8 (0.3) Delta C (SE): ‐0.3 (0.4) Delta (I‐C) (SE): ‐0.5 (0.5) | 1. Total cholesterol (mmol/L) Delta I (SE): ‐0.1 (0.1) Delta C (SE): 0 (0.1) Delta (I‐C) (SE): ‐0.1 (0.1) 2. LDL cholesterol (mmol/L) Delta I (SE): 0.1 (0.1) Delta C (SE): 0 (0) Delta (I‐C) (SE): 0.1 (0.1) 3. HDL cholesterol (mmol/L) Delta I (SE): 0.1 (0) Delta C (SE): 0 (0) Delta (I‐C) (SE): 0.1 (0) 4. Triglycerides (mmol/L) Delta I (SE): ‐0.2 (0.1) Delta C (SE): ‐0.2 (0.1) Delta (I‐C) (SE): 0 (0.5) | 1. SBP Delta I (SE): ‐1.1 (0.3) Delta C (SE): 0.5 (0.3) Delta (I‐C) (SE): ‐1.6 (0.5) |
| Kaukua 2004 Study design: RCT Follow‐up interval: 52 weeks | 1. Weight change (kg) Delta I (SE): ‐7.1 (1.0) Delta C (SE): ‐2.6 (1.0) Delta (I‐C) (SE): ‐4.5 (1.4) 2. % of weight loss Delta I (SE): ‐7.3 (1.1) Delta C (SE): ‐2.4 (1.1) Delta (I‐C) (SE): ‐4.9 (1.5) | 1. SBP Delta I (SE): 4.1 (1.4) Delta C (SE): 3.6 (1.4) Delta (I‐C) (SE): 0.5 (2.0) 2. DBP Delta I (SE): 1.7 (0.7) Delta C (SE): ‐0.2 (0.7) Delta (I‐C) (SE): 1.9 (1.0) | ||
| McNulty 2003 Study design: RCT Follow‐up interval: 52 weeks | 1. Weight change (kg) Delta I (SE): ‐8.0 (0.9) Delta C (SE): ‐0.2 (0.5) Delta (I‐C) (SE): ‐7.8 (1.0) 2. BMI (kg/m2) Delta I (SE): ‐2.9 (0.7) Delta C (SE): ‐0.3 (0.7) Delta (I‐C) (SE): ‐2.6 (0.3) | 1. GHb (%) Delta I (SE): ‐0.3 (0.2) Delta C (SE): ‐0.2 (0.2) Delta (I‐C) (SE): ‐0.1 (0.3) 2. Fasting blood sugar (mml/L) Delta I (SE): ‐0.1 (0.3) Delta C (SE): 0.2 (0.5) Delta (I‐C) (SE): ‐0.3 (0.4) | 1. Total cholesterol (mmol/L) Delta I (SE): 0 (0.1) Delta C (SE): ‐0.2 (0.1) Delta (I‐C) (SE): 0.2 (0.1) 2. LDL cholesterol (mmol/L) Delta I (SE): 0.1 (0.1) Delta C (SE): ‐0.2 (0.1) Delta (I‐C) (SE): 0.1 (0.1) 3. HDL cholesterol (mmol/L) Delta I (SE): 0.1 (0.0) Delta C (SE): 0 (0.1) Delta (I‐C) (SE): 0.1 (0.0) 4. Triglycerides (mmol/L) Delta I (SE): ‐0.2 (0.2) Delta C (SE): 0.1 (0.1) Delta (I‐C) (SE): ‐0.3 (0.2) | 1. SBP Delta I (SE): ‐1.5 (2.0) Delta C (SE): ‐0.2(2.0) Delta (I‐C) (SE): ‐1.3 (1.3) 2. DBP Delta I (SE): 0.4 (1.0) Delta C (SE): 0.5 (1.1) Delta (I‐C) (SE): ‐0.1 (1.8) |
| Redmon 2003 Study design: RCT Follow‐up interval: 52 weeks | 1. Weight change (kg) Delta I (SE): ‐7.3 (1.3) Delta C (SE): ‐0.8 (0.9) Delta (I‐C) (SE): ‐6.5 (1.6) 2. BMI (kg/m2) Delta I (SE): ‐2.6 (0.5) Delta C (SE): ‐0.3 (0.3) Delta (I‐C) (SE): ‐2.3 (0.6) | 1. GHb (%) Delta I (SE): ‐0.6 (0.3) Delta C (SE): 0.0 (0.2) Delta (I‐C) (SE): ‐0.6 (0.4) 2. Fasting blood sugar (mml/L) Delta I (SE): ‐0.7 (0.5) Delta C (SE): ‐0.6 (0.5) Delta (I‐C) (SE): ‐0.1 (0.7) | 1. Total cholesterol (mmol/L) Delta I (SE): ‐0.4 (0.2) Delta C (SE): ‐0.4 (0.2) Delta (I‐C) (SE): 0.0 (0.3) 2. LDL cholesterol (mmol/L) Delta I (SE): ‐0.3 (0.1) Delta C (SE): ‐0.3 (0.2) Delta (I‐C) (SE): 0.0 (0.2) 3. HDL cholesterol (mmol/L) Delta I (SE): 0.1 (0.0) Delta C (SE): 0.0(0.0) Delta (I‐C) (SE): 0.1 (0.0) 4. Triglycerides (mmol/L) Delta I (SE): ‐0.5 (0.3) Delta C (SE): 0.1 (0.2) Delta (I‐C) (SE): ‐0.6 (0.3) | 1. SBP Delta I (SE): ‐6.0 (3.0) Delta C (SE): ‐6.0(2.0) Delta (I‐C) (SE): 0.0 (3.6) 2. DBP Delta I (SE): ‐3.0 (1.0) Delta C (SE): ‐6.0 (2.0) Delta (I‐C) (SE): 3.0 (2.2) |
| Tankova 2003Study design: RCTFollow‐up interval: 13 weeks | 1. Weight change (kg) Delta I (SE): ‐6.5 (0.9) Delta C (SE): ‐2.7 (0.9) Delta (I‐C) (SE): ‐3.8 (1.3) 2. % of weight loss Delta I (SE): ‐6.8 (0.7) Delta C (SE): ‐2.9 (0.7) Delta (I‐C) (SE): ‐3.9 (1.0) 3. Waist circumference (cm) Delta I (SE) ‐8.4 (1.0) Delta C (SE): ‐1.9 (1.3) Delta (I‐C) (SE): ‐6.5 (1.7) | 1. GHb (%) Delta I (SE): ‐0.3 (0.1) Delta C (SE): ‐0.1 (0.1) Delta (I‐C) (SE): ‐0.2 (0.1) | 1. Total cholesterol (mmol/L) Delta I (SE): ‐0.4 (0.1) Delta C (SE): ‐0.2 (0.1) Delta (I‐C) (SE): ‐0.2 (0.2) 2. LDL cholesterol (mmol/L) Delta I (SE): ‐0.5 (0.1) Delta C (SE): ‐0.1 (0.1) Delta (I‐C) (SE): ‐0.4 (0.2) 3. HDL cholesterol (mmol/L) Delta I (SE): 0.1 (0.0) Delta C (SE): 0.0(0.0) Delta (I‐C) (SE): 0.1 (0.0) 4. Triglycerides (mmol/L) Delta I (SE): ‐0.1 (0.1) Delta C (SE): ‐0.1 (0.1) Delta (I‐C) (SE): 0.0 (0.1) | |
| Pooled effects (Full Text) Outcomes S=Number of studies N=Number of participants pooled effects (95% CI) | 1. Weight loss (kg) S=8 N=845 ‐5.1 [‐7.0, ‐3.2] 2. Percent weight loss S=3 N=426 ‐4.0 [‐5.5, ‐2.6] 3. % participants with weight loss >5% S=2 N=204 21.2 [12.5, 29.8] 4. BMI kg/m2) S=6 N=517 ‐1.9 [‐2.6, ‐1.1] 5. Waist circumference (cm) S=5 N=475 ‐4.7 [‐7.4, ‐2.0] | 1. GHb (%) S=7 N=612 ‐0.5 [‐1.3, 0.2] 2. Fasting glucose (mmol/L) S=5 N=434 ‐1.4 [‐3.7, 1.0] | 1. Total cholesterol (mmol/L) S=6 N=529 ‐0.1 [‐0.4, 0.2] 2. LDL cholesterol (mmol/L) S=5 N=529 ‐0.1 [‐0.3, 0.2] 3. HDL cholesterol (mmol/L) S=5 N=419 0.1 [0.0, 0.1] 4. Triglycerides (mmol/L) S=6 N=529 ‐0.3 [‐0.5, 0.0] | 1. SBP (mmHg) S=6 N=673 ‐0.8 [‐1.7, ‐0.0] 2. DBP (mmHg) S=4 N=480 1.4 [0.1, 2.8] |
| Pooled effects (Full Text+Abstract) Outcomes S=Number of studies N=Number of participants pooled effects (95% CI) (Full Text+Abstract) | 1. Weight loss (kg) S=9 N=863 ‐4.8 [‐6.5, ‐3.0] 2. Percent weight loss S=4 N=662 ‐4.2 [‐5.5, ‐2.9] 3. % participants with weight loss >5% S=3 N=440 25.9 [13.3, 38.5] 4. BMI kg/m2) S=6 N=517 ‐1.9 [‐2.6, ‐1.1] 5. Waist circumference (cm) S=5 N=475 ‐4.7 [‐7.4, ‐2.0] | 1. GHb (%) S=7 N=612 ‐0.5 [‐1.3, 0.2] 2. Fasting glucose (mmol/L) S=5 N=434 ‐1.4 [‐3.7, 1.0] | 1. Total cholesterol (mmol/L) S=6 N=529 ‐0.1 [‐0.4, 0.2] 2. LDL cholesterol (mmol/L) S=5 N=529 ‐0.1 [‐0.3, 0.2] 3. HDL cholesterol (mmol/L) S=5 N=419 0.1 [0.0, 0.1] 4. Triglycerides (mmol/L) S=6 N=529 ‐0.3 [‐0.5, ‐0.0] | 1. SBP (mmHg) S=6 N=673 ‐0.8 [‐1.7, 0.0] 2. DBP (mmHg) S=4 N=480 1.4 [0.1, 2.8] |
| Sircar 2001 Study design: pre vs post Follow‐up interval: 12 weeks | 1. Weight change (kg) Delta I (SE): ‐4.2 (1.5) 2. BMI (kg/m2) Delta I (SE): ‐1.6 (0.6) | 1. GHb (%) Delta I (SE): ‐0.5 (0.2) 2. Fasting blood sugar (mml/L) Delta I (SE): ‐0.2 (0.5) |
Appendix 21. Weighted mean differences in weight (kg) for fluoxetine versus placebo
| Fluoxetine | rho = 0.25 | rho = 0.5 | rho = 0.75 | rho = 1.0 |
| 8‐16 weeks (88 treated vs 104 controls) fixed effects model | ‐3.9 (95% CI ‐3.0 to ‐4.8) | ‐3.9 (95% CI ‐3.0 to ‐4.8) | ‐3.0 (95% CI ‐2.3 to ‐3.8) | ‐1.9 (95% CI ‐1.5 to ‐2.2) |
| Heterogeneity (q, p value) | q = 4.64, p = 0.33 | q = 4.85, p = 0.3 | q = 75. 58, p = 0.004 | q = 30.6, p = 0.00001 |
| Random effects model | ‐4.0 (95% CI ‐2.8 to ‐5.3) | ‐4.0 (95% CI ‐2.7 to ‐5.3) | ‐3.4 (95% CI ‐1.7 to ‐5.2) | ‐3.6 (95% CI ‐1.5 to ‐5.7) |
| Heterogeneity (q, p value) | q = 4.64, p = 0.33 | q = 4.85, p = 0.3 | q = 15.58, p = 0.004 | q = 30.6, p = 0.00001 |
| 24‐26 weeks (45 treated vs 52 controls) Fixed effects model | ‐5.1 (95% CI ‐3.4 to ‐6.8) | ‐5.1 (95% CI ‐3.4 to ‐6.8) | ‐5.1 (95% CI ‐3.4 to ‐6.8) | ‐5.1 (95% CI ‐3.4 to ‐6.8) |
| Heterogeneity (q, p value) | q = 3.19, p = 0.36 | q = 3.19, p = 0.36 | q = 3.19, p = 0.36 | q = 3.19, p = 0.36 |
| Random effects model | ‐5.1 (95% CI ‐3.3 to ‐6.9) | ‐5.1 (95% CI ‐3.3 to ‐6.9) | ‐5.1 (95% CI ‐3.3 to ‐6.9) | ‐5.1 (95% CI ‐3.3 to ‐6.9) |
| Heterogeneity (q, p value) CI, confidence interval | q = 3.19, p = 0.36 | q = 3.19, p = 0.36 | q = 1.26, p = 0.74 | q = 3.19, p = 0.36 |
Appendix 22. Weighted mean differences in GHb for fluoxetine versus placebo
| Fluoxetine | rho = 0.25 | rho = 0.5 | rho = 0.75 | rho = 1.0 |
| 8‐16 weeks (66 treated vs 79 controls) Fixed effects model | ‐1.1 (95% CI ‐0.5 to ‐1.7) | ‐1.1 (95% CI ‐0.5 to ‐1.7) | ‐1.0 (95% CI ‐0.4 to ‐1.5) | ‐1.2 (95% CI ‐0.5 to ‐1.9) |
| Heterogeneity (q, p value) | q = 1.31, p = 0.73 | q = 1.57, p = 0.67 | q = 2.05, p = 0.56 | q = 0.63, p = 0.73 |
| Random effects model | ‐1.1 (95% CI ‐0.5 to ‐1.7) | ‐1.1 (95% CI ‐0.5 to ‐1.7) | ‐1.0 (95% CI ‐0.4 to ‐1.5) | ‐1.2 (95% CI ‐0.5 to ‐1.9) |
| Heterogeneity (q, p value) | q = 1.31, p = 0.73 | q = 1.57, p = 0.67 | q = 2.05, p = 0.56 | q = 0.63, p = 0.73 |
| 24‐26 weeks (45 treated vs 52 controls) Fixed effects model | ‐1.1 (95% CI ‐0.7 to ‐1.5) | ‐1.1 (95% CI ‐0.7 to ‐1.5) | ‐1.0 (95% CI ‐0.6 to ‐1.4) | ‐0.7 (95% CI ‐0.5 to ‐0.9) |
| Heterogeneity (q, p value) | q = 1.15, p = 0.77 | q = 1.26, p = 0.74 | q = 1.58, p = 0.66 | q = 5.99, p = 0.11 |
| Random effects model | ‐1.1 (95% CI ‐0.7 to ‐1.5) | ‐1.1 (95% CI ‐0.7 to ‐1.5) | ‐1.0 (95% CI ‐0.6 to ‐1.4) | ‐0.9 (95% CI ‐0.4 to ‐1.3) |
| Heterogeneity (q, p value) | q = 1.15, p = 0.77 | q = 1.26, p = 0.74 | q = 1.58, p = 0.66 | q = 5.99, p = 0.11 |
Appendix 23. Weighted mean differences in weight (kg) for orlistat versus placebo
| Orlistat | rho = 0.25 | rho = 0.5 | rho = 0.75 | rho = 1.0 |
| 52‐57 weeks (710 treated vs 653 controls) Fixed effects model | ‐2.1 (95% CI ‐1.6 to ‐2.6) | ‐2.1 (95% CI ‐1.6 to ‐2.6) | ‐2.1 (95% CI ‐1.6 to ‐2.6) | ‐2.1 (95% CI ‐1.5 to ‐2.6) |
| Heterogeneity (q, p value) | q = 9.57, p = 0.14 | q = 9.8, p = 0.13 | q = 10.48, p = 0.11 | q = 9.11, p = 0.1 |
| Random effects model | ‐2.0 (95% CI ‐1.2 to ‐2.7) | ‐2.0 (95% CI ‐1.2 to ‐2.8) | ‐2.0 (95% CI ‐1.3 to ‐2.8) | ‐1.9 (95% CI ‐1.1 to ‐2.7) |
| Heterogeneity (q, p value) | q = 9.6, p = 0.14 | q = 9.8, p = 0.13 | q = 10.48, p = 0.11 | q = 9.11, p = 0.1 |
Appendix 24. Weighted mean differences in GHb for orlistat versus placebo
| Orlistat | rho = 0.25 | rho = 0.5 | rho = 0.75 | rho = 1.0 |
| 52‐57 weeks (718 treated vs 655 controls) Fixed effects model | ‐0.4 (95% CI ‐0.3 to ‐0.6) | ‐0.4 (95% CI ‐0.3 to ‐0.6) | ‐0.5 (95% CI ‐0.3 to ‐0.6) | ‐0.4 (95% CI ‐0.3 to ‐0.6) |
| Heterogeneity (q, p value) | q = 1.48, p = 0.96 | q = 1.58, p = 0.95 | q = 1.85, p = 0.93 | q = 1.27, p = 0.94 |
| Random effects model | ‐0.4 (95% CI ‐0.3 to ‐0.6) | ‐0.4 (95% CI ‐0.3 to ‐0.6) | ‐0.5 (95% CI ‐0.3 to ‐0.6) | ‐0.4 (95% CI ‐0.3 to ‐0.6) |
| Heterogeneity (q, p value) | q = 1.48, p = 0.96 | q = 1.58, p = 0.95 | q = 1.85, p = 0.93 | q = 1.27, p = 0.94 |
Appendix 25. Weighted mean differences in weight (kg) for sbutramine versus placebo
| Sibutramine | rho = 0.25 | rho = 0.5 | rho = 0.75 | rho = 1.0 |
| 12‐26 weeks (431 treated vs 414 controls) Fixed effects model | ‐3.6 (95% CI ‐3.0 to ‐4.2) | ‐3.6 (95% CI ‐3.0 to ‐4.2) | ‐3.6 (95% CI ‐3.0 to ‐4.2) | ‐3.7 (95% CI ‐3.3 to ‐4.1) |
| Heterogeneity (q, p value) | q = 54.34, p = 0.00001 | q = 54.35, p = 0.00001 | q = 54.37, p = 0.00001 | q = 54.61, p = 0.00001 |
| Random effects model | ‐5.2 (95% CI ‐3.1 to ‐7.2) | ‐5.2 (95% CI ‐3.1 to ‐7.2) | ‐5.1 (95% CI ‐3.2 to ‐7.0) | ‐5.0 (95% CI ‐3.5 to ‐6.4) |
| Heterogeneity (q, p value) | q = 54.34, p = 0.00001 | q = 54.35, p = 0.00001 | q = 54.37, p = 0.00001 | q = 54.61, p = 0.00001 |
Appendix 26. Weighted mean differences in GHb for sibutramine versus placebo
| Sibutramine | rho = 0.25 | rho = 0.5 | rho = 0.75 | rho = 1.0 |
| 12‐26 weeks (314 treated vs 298 controls) Fixed effects model | ‐1.0 (95% CI ‐0.8 to ‐1.1) | ‐0.9 (95% CI ‐0.7 to ‐1.1) | ‐0.8 (95% CI ‐0.7 to ‐1.0) | ‐0.3 (95% CI ‐0.3 to ‐0.4) |
| Heterogeneity (q, p value) | q = 127.27, p = 0.00001 | q = 130.77, p = 0.00001 | q = 138.79, p = 0.00001 | q = 183.1, p = 0.00001 |
| Random effects model | ‐0.5 (95% CI 0.3 to ‐1.4) | ‐0.5 (95% CI 0.3 to ‐1.4) | ‐0.5 (95% CI 0.2 to ‐1.3) | ‐0.5 (95% CI 0.1 to ‐1.2) |
| Heterogeneity (q, p value) | q = 127.27, p = 0.00001 | q = 130.77, p = 0.00001 | q = 138.79, p = 0.00001 | q = 183.1, p = 0.00001 |
Appendix 27. Meta‐analysis results, fluoxitine
| Outcome | Follow‐up 8‐16w | Follow‐up 24‐26w | ||||||
| No. of studies | N | Point estimate | 95% CI | No. of studies | N | Point estimate | 95% CI | |
| Weight (kg) | 5 | 192 | ‐3.4 | (‐5.2, ‐1.7) | 4 | 97 | ‐5.1 | (‐6.90, ‐3.26) |
| percent weight loss | 1 | 20 | ‐2.5 | (‐7.9, 3.0) | ||||
| % participants with weight loss >5% | ||||||||
| BMI (kg/m2) | 1 | 47 | ‐0.5 | (‐1.0, ‐0.1) | ||||
| Waist circumference (cm) | ||||||||
| GHb (%) | 4 | 145 | ‐1.0 | (‐1.5, ‐0.4) | 4 | 97 | ‐1.0 | (‐1.4, ‐0.6) |
| Fasting glucose (mmol/l) | 5 | 192 | ‐0.9 | (‐2.1, 0.4) | 4 | 97 | ‐0.9 | (‐2.0, 0.2) |
| SBP (mm Hg) | ||||||||
| DBP (mm Hg) | ||||||||
| Total cholesterol (mmol/l) | 2 | 85 | ‐0.1 | (‐0.3, 0.2) | 1 | 17 | 0.1 | (‐0.4, 0.6) |
| LDL cholesterol (mmol/l) | ||||||||
| HDL cholesterol (mmol/l) | 1 | 68 | 0 | (‐0.1, 0.1) | ||||
| Triglycerides (mmol/l) | 2 | 85 | ‐0.5 | (‐1.1, 0.1) | 1 | 17 | ‐0.2 | (‐1.0, 0.7) |
Appendix 28. Meta‐analysis results, orlistat and sibutramine
| Outcome | Orlistat | Sibutramine | ||||||
| No. of studies | N | Point estimate | 95% CI | No. of studies | N | Point estimate | 95% CI | |
| Weight (kg) | 7 | 1363 | ‐2.0 | (‐2.8, ‐1.3) | 8 | 845 | ‐5.1 | (‐7.0, ‐3.2) |
| percent weight loss | 4 | 1008 | ‐2.3 | (‐3.0, ‐1.7) | 3 | 426 | ‐4.0 | (‐5.5, ‐2.6) |
| % participants with weight loss >5% | 5 | 1273 | 21.4 | (15.2, 27.6) | 2 | 204 | 21.2 | (12.5, 29.8) |
| BMI (kg/m2) | 2 | 100 | ‐0.7 | (‐1.5, 0.1) | 6 | 517 | ‐1.9 | (‐2.6, ‐1.1) |
| Waist circumference (cm) | 6 | 1111 | ‐1.8 | (‐3.0, ‐0.7) | 5 | 475 | ‐4.7 | (‐7.4, ‐2.0) |
| GHb (%) | 7 | 1373 | ‐0.5 | (‐0.6, ‐0.3) | 7 | 612 | ‐0.5 | (‐1.3, 0.2) |
| Fasting glucose (mmol/l) | 8 | 1449 | ‐0.8 | (‐1.1, ‐0.5) | 5 | 434 | ‐1.4 | (‐3.7, 1.0) |
| SBP (mm Hg) | 5 | 740 | ‐3.0 | (‐6.3, 0.3) | 6 | 673 | ‐0.8 | (‐1.7, 0.0) |
| DBP (mm Hg) | 4 | 441 | ‐4.2 | (‐7.8, ‐0.6) | 4 | 480 | 1.4 | (0.1, 2.8) |
| Total cholesterol (mmol/l) | 6 | 1324 | ‐0.4 | (‐0.5, ‐0.3) | 6 | 529 | ‐0.1 | (‐0.4, 0.2) |
| LDL cholesterol (mmol/l) | 6 | 1287 | ‐0.3 | (‐0.4, ‐0.2) | 5 | 529 | ‐0.1 | (‐0.3, 0.2) |
| HDL cholesterol (mmol/l) | 5 | 994 | 0 | (‐0.1, 0.0) | 5 | 419 | 0.1 | (0.0, 0.1) |
| Triglycerides (mmol/l) | 6 | 994 | ‐0.2 | (‐0.4, ‐0.1) | 6 | 529 | ‐0.3 | (‐0.5, 0.0) |
Data and analyses
Comparison 1. Drug therapy versus placebo for Fluoxetine (FT: 1‐7, fixed model; 8‐14, random mode; 8‐16 wks. rho=0.75).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight (kg) | 5 | 192 | Mean Difference (IV, Fixed, 95% CI) | ‐3.04 [‐3.75, ‐2.33] |
| 2 BMI | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | ‐0.52 [‐0.96, ‐0.08] |
| 3 GHb | 4 | 145 | Mean Difference (IV, Fixed, 95% CI) | ‐0.97 [‐1.52, ‐0.41] |
| 4 Fasting glucose | 5 | 192 | Mean Difference (IV, Fixed, 95% CI) | ‐1.05 [‐1.71, ‐0.40] |
| 5 Total cholesterol | 2 | 85 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.33, 0.24] |
| 6 HDL cholesterol | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.05, 0.11] |
| 7 Triglycerides | 2 | 85 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐1.11, 0.11] |
| 8 Weight (kg) | 5 | 192 | Mean Difference (IV, Random, 95% CI) | ‐3.43 [‐5.20, ‐1.66] |
| 9 BMI | 1 | 47 | Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.96, ‐0.08] |
| 10 GHb | 4 | 145 | Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐1.52, ‐0.41] |
| 11 Fasting glucose | 5 | 192 | Mean Difference (IV, Random, 95% CI) | ‐0.85 [‐2.07, 0.38] |
| 12 Total cholesterol | 2 | 85 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.33, 0.24] |
| 13 HDL cholesterol | 1 | 68 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.05, 0.11] |
| 14 Triglycerides | 2 | 85 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐1.11, 0.11] |
1.1. Analysis.
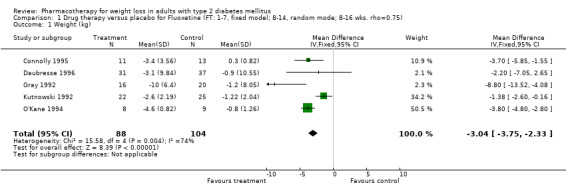
Comparison 1 Drug therapy versus placebo for Fluoxetine (FT: 1‐7, fixed model; 8‐14, random mode; 8‐16 wks. rho=0.75), Outcome 1 Weight (kg).
1.2. Analysis.
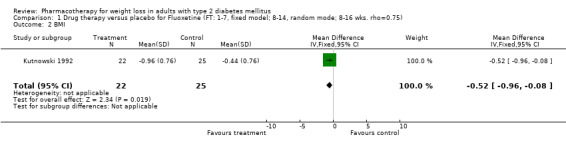
Comparison 1 Drug therapy versus placebo for Fluoxetine (FT: 1‐7, fixed model; 8‐14, random mode; 8‐16 wks. rho=0.75), Outcome 2 BMI.
1.3. Analysis.
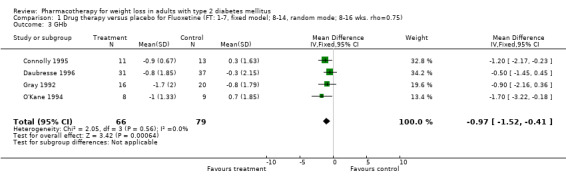
Comparison 1 Drug therapy versus placebo for Fluoxetine (FT: 1‐7, fixed model; 8‐14, random mode; 8‐16 wks. rho=0.75), Outcome 3 GHb.
1.4. Analysis.
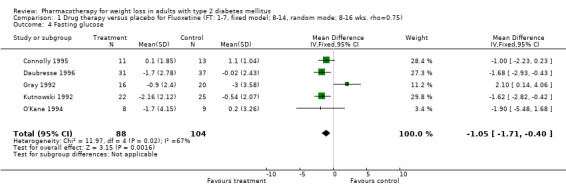
Comparison 1 Drug therapy versus placebo for Fluoxetine (FT: 1‐7, fixed model; 8‐14, random mode; 8‐16 wks. rho=0.75), Outcome 4 Fasting glucose.
1.5. Analysis.
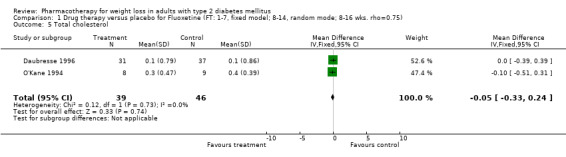
Comparison 1 Drug therapy versus placebo for Fluoxetine (FT: 1‐7, fixed model; 8‐14, random mode; 8‐16 wks. rho=0.75), Outcome 5 Total cholesterol.
1.6. Analysis.
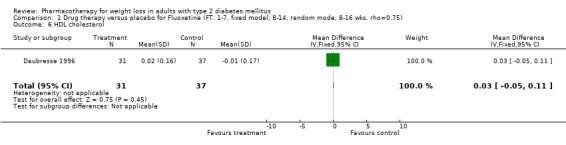
Comparison 1 Drug therapy versus placebo for Fluoxetine (FT: 1‐7, fixed model; 8‐14, random mode; 8‐16 wks. rho=0.75), Outcome 6 HDL cholesterol.
1.7. Analysis.
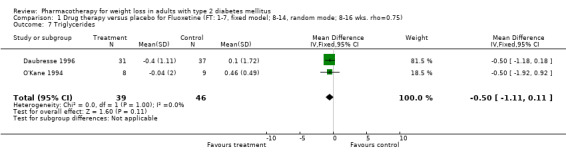
Comparison 1 Drug therapy versus placebo for Fluoxetine (FT: 1‐7, fixed model; 8‐14, random mode; 8‐16 wks. rho=0.75), Outcome 7 Triglycerides.
1.8. Analysis.
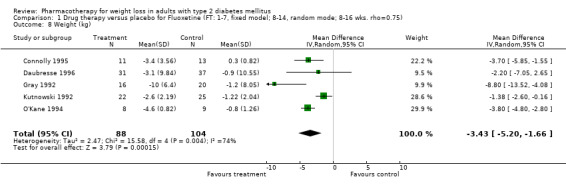
Comparison 1 Drug therapy versus placebo for Fluoxetine (FT: 1‐7, fixed model; 8‐14, random mode; 8‐16 wks. rho=0.75), Outcome 8 Weight (kg).
1.9. Analysis.
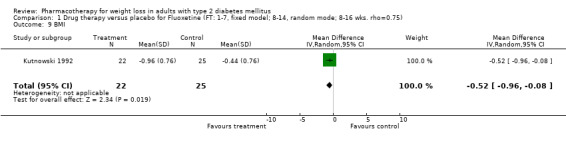
Comparison 1 Drug therapy versus placebo for Fluoxetine (FT: 1‐7, fixed model; 8‐14, random mode; 8‐16 wks. rho=0.75), Outcome 9 BMI.
1.10. Analysis.
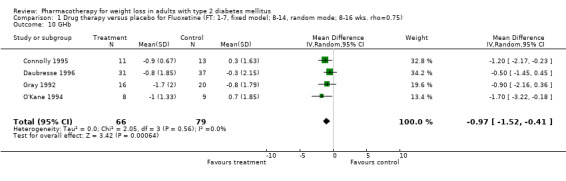
Comparison 1 Drug therapy versus placebo for Fluoxetine (FT: 1‐7, fixed model; 8‐14, random mode; 8‐16 wks. rho=0.75), Outcome 10 GHb.
1.11. Analysis.
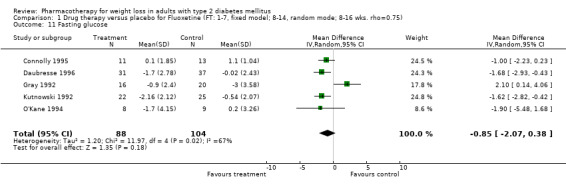
Comparison 1 Drug therapy versus placebo for Fluoxetine (FT: 1‐7, fixed model; 8‐14, random mode; 8‐16 wks. rho=0.75), Outcome 11 Fasting glucose.
1.12. Analysis.
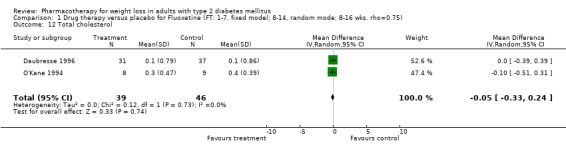
Comparison 1 Drug therapy versus placebo for Fluoxetine (FT: 1‐7, fixed model; 8‐14, random mode; 8‐16 wks. rho=0.75), Outcome 12 Total cholesterol.
1.13. Analysis.
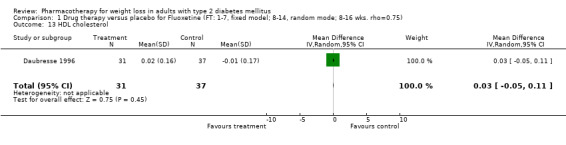
Comparison 1 Drug therapy versus placebo for Fluoxetine (FT: 1‐7, fixed model; 8‐14, random mode; 8‐16 wks. rho=0.75), Outcome 13 HDL cholesterol.
1.14. Analysis.
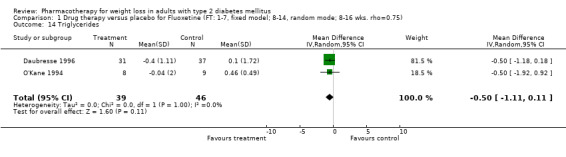
Comparison 1 Drug therapy versus placebo for Fluoxetine (FT: 1‐7, fixed model; 8‐14, random mode; 8‐16 wks. rho=0.75), Outcome 14 Triglycerides.
Comparison 2. Drug therapy versus placebo for Fluoxetine (FT: 1‐6, fixed model; 7‐12, random model; 24‐26 wks. rho=0.75).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight (kg) | 4 | 97 | Mean Difference (IV, Fixed, 95% CI) | ‐5.10 [‐6.83, ‐3.37] |
| 2 Percent weight loss | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐2.48 [‐7.94, 2.98] |
| 3 GHb | 4 | 97 | Mean Difference (IV, Fixed, 95% CI) | ‐1.03 [‐1.42, ‐0.63] |
| 4 Fasting glucose | 4 | 97 | Mean Difference (IV, Fixed, 95% CI) | ‐0.87 [‐1.96, 0.22] |
| 5 Total cholesterol | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.40, 0.60] |
| 6 Triglycerides | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | ‐0.16 [‐1.02, 0.70] |
| 7 Weight (kg) random | 4 | 97 | Mean Difference (IV, Random, 95% CI) | ‐5.08 [‐6.90, ‐3.26] |
| 8 Percent weight loss | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐2.48 [‐7.94, 2.98] |
| 9 GHb | 4 | 97 | Mean Difference (IV, Random, 95% CI) | ‐1.03 [‐1.42, ‐0.63] |
| 10 Fasting glucose | 4 | 97 | Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐1.96, 0.22] |
| 11 Total cholesterol | 1 | 17 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.40, 0.60] |
| 12 Triglycerides | 1 | 17 | Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐1.02, 0.70] |
2.1. Analysis.
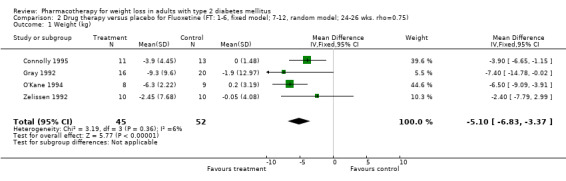
Comparison 2 Drug therapy versus placebo for Fluoxetine (FT: 1‐6, fixed model; 7‐12, random model; 24‐26 wks. rho=0.75), Outcome 1 Weight (kg).
2.2. Analysis.
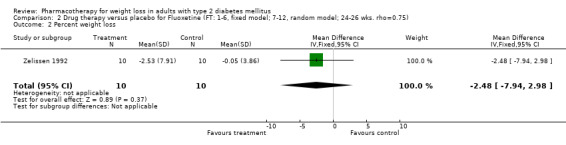
Comparison 2 Drug therapy versus placebo for Fluoxetine (FT: 1‐6, fixed model; 7‐12, random model; 24‐26 wks. rho=0.75), Outcome 2 Percent weight loss.
2.3. Analysis.
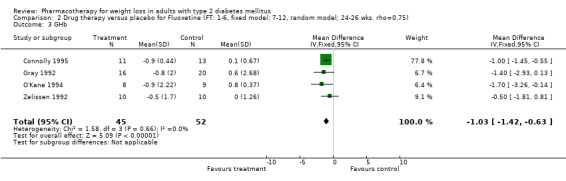
Comparison 2 Drug therapy versus placebo for Fluoxetine (FT: 1‐6, fixed model; 7‐12, random model; 24‐26 wks. rho=0.75), Outcome 3 GHb.
2.4. Analysis.
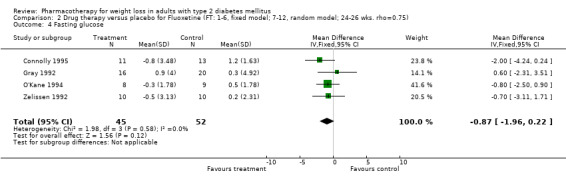
Comparison 2 Drug therapy versus placebo for Fluoxetine (FT: 1‐6, fixed model; 7‐12, random model; 24‐26 wks. rho=0.75), Outcome 4 Fasting glucose.
2.5. Analysis.
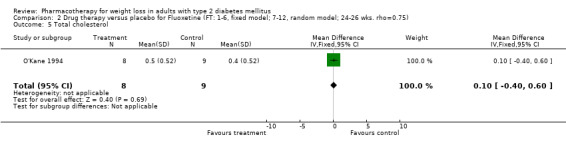
Comparison 2 Drug therapy versus placebo for Fluoxetine (FT: 1‐6, fixed model; 7‐12, random model; 24‐26 wks. rho=0.75), Outcome 5 Total cholesterol.
2.6. Analysis.
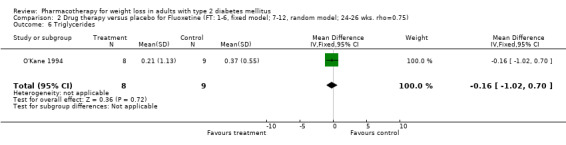
Comparison 2 Drug therapy versus placebo for Fluoxetine (FT: 1‐6, fixed model; 7‐12, random model; 24‐26 wks. rho=0.75), Outcome 6 Triglycerides.
2.7. Analysis.
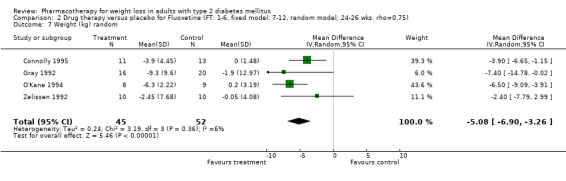
Comparison 2 Drug therapy versus placebo for Fluoxetine (FT: 1‐6, fixed model; 7‐12, random model; 24‐26 wks. rho=0.75), Outcome 7 Weight (kg) random.
2.8. Analysis.
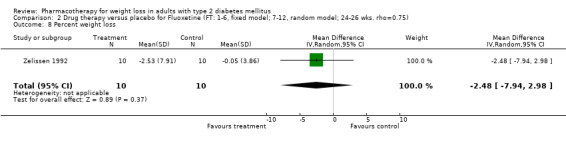
Comparison 2 Drug therapy versus placebo for Fluoxetine (FT: 1‐6, fixed model; 7‐12, random model; 24‐26 wks. rho=0.75), Outcome 8 Percent weight loss.
2.9. Analysis.
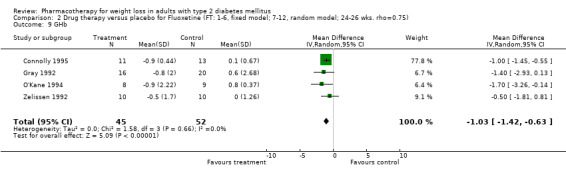
Comparison 2 Drug therapy versus placebo for Fluoxetine (FT: 1‐6, fixed model; 7‐12, random model; 24‐26 wks. rho=0.75), Outcome 9 GHb.
2.10. Analysis.
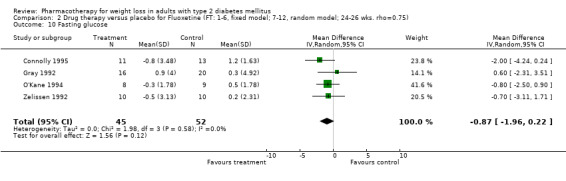
Comparison 2 Drug therapy versus placebo for Fluoxetine (FT: 1‐6, fixed model; 7‐12, random model; 24‐26 wks. rho=0.75), Outcome 10 Fasting glucose.
2.11. Analysis.
Comparison 2 Drug therapy versus placebo for Fluoxetine (FT: 1‐6, fixed model; 7‐12, random model; 24‐26 wks. rho=0.75), Outcome 11 Total cholesterol.
2.12. Analysis.
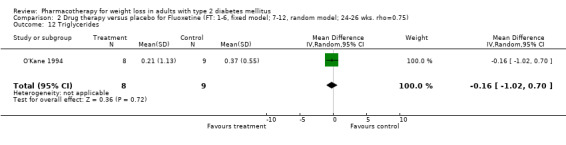
Comparison 2 Drug therapy versus placebo for Fluoxetine (FT: 1‐6, fixed model; 7‐12, random model; 24‐26 wks. rho=0.75), Outcome 12 Triglycerides.
Comparison 3. Drug therapy versus placebo for Fluoxetine (FT: 1‐13, fixed model; 14‐23, random model; 52 wks. rho=0.75).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight (kg) | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | ‐5.8 [‐10.84, ‐0.76] |
| 2 Percent weight loss | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 % with wt loss > 5% | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 BMI | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Waist circumference | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 GHb | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | ‐1.8 [‐3.78, 0.18] |
| 7 Fasting glucose | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | ‐0.8 [‐2.50, 0.90] |
| 8 SBP | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 DBP | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Total cholesterol | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.31, 1.31] |
| 11 LDL cholesterol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 HDL cholesterol | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Triglycerides | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐1.15, 0.15] |
| 14 Weight (kg) random | 1 | 17 | Mean Difference (IV, Random, 95% CI) | ‐5.8 [‐10.84, ‐0.76] |
| 15 Percent weight loss | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 BMI | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 GHb | 1 | 17 | Mean Difference (IV, Random, 95% CI) | ‐1.8 [‐3.78, 0.18] |
| 18 Fasting glucose | 1 | 17 | Mean Difference (IV, Random, 95% CI) | ‐0.8 [‐2.50, 0.90] |
| 19 SBP | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20 DBP | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Total cholesterol | 1 | 17 | Mean Difference (IV, Random, 95% CI) | 0.5 [‐0.31, 1.31] |
| 22 HDL cholesterol | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Triglycerides | 1 | 17 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐1.15, 0.15] |
3.1. Analysis.
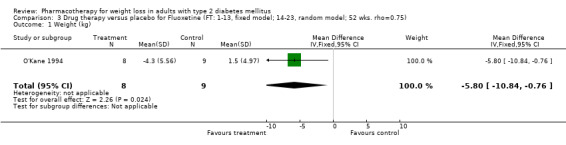
Comparison 3 Drug therapy versus placebo for Fluoxetine (FT: 1‐13, fixed model; 14‐23, random model; 52 wks. rho=0.75), Outcome 1 Weight (kg).
3.6. Analysis.
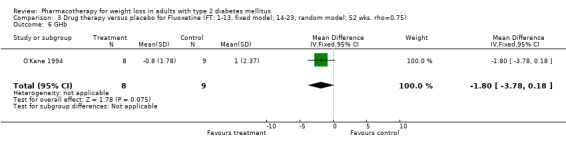
Comparison 3 Drug therapy versus placebo for Fluoxetine (FT: 1‐13, fixed model; 14‐23, random model; 52 wks. rho=0.75), Outcome 6 GHb.
3.7. Analysis.
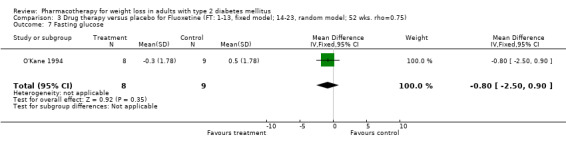
Comparison 3 Drug therapy versus placebo for Fluoxetine (FT: 1‐13, fixed model; 14‐23, random model; 52 wks. rho=0.75), Outcome 7 Fasting glucose.
3.10. Analysis.
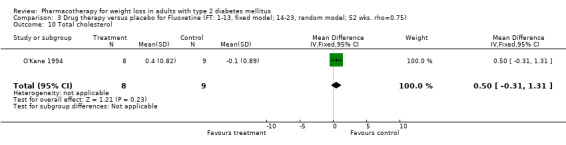
Comparison 3 Drug therapy versus placebo for Fluoxetine (FT: 1‐13, fixed model; 14‐23, random model; 52 wks. rho=0.75), Outcome 10 Total cholesterol.
3.13. Analysis.
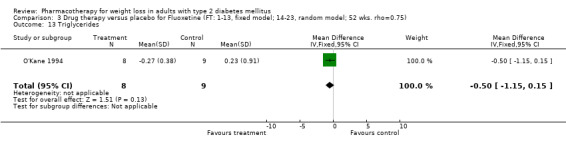
Comparison 3 Drug therapy versus placebo for Fluoxetine (FT: 1‐13, fixed model; 14‐23, random model; 52 wks. rho=0.75), Outcome 13 Triglycerides.
3.14. Analysis.
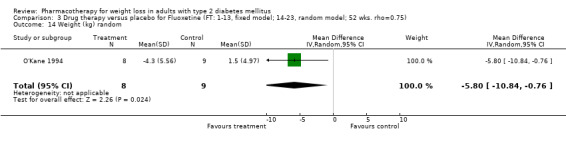
Comparison 3 Drug therapy versus placebo for Fluoxetine (FT: 1‐13, fixed model; 14‐23, random model; 52 wks. rho=0.75), Outcome 14 Weight (kg) random.
3.17. Analysis.
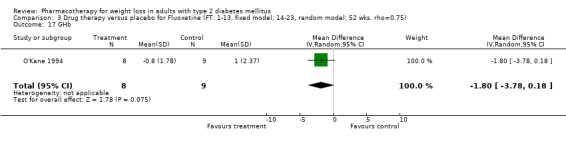
Comparison 3 Drug therapy versus placebo for Fluoxetine (FT: 1‐13, fixed model; 14‐23, random model; 52 wks. rho=0.75), Outcome 17 GHb.
3.18. Analysis.
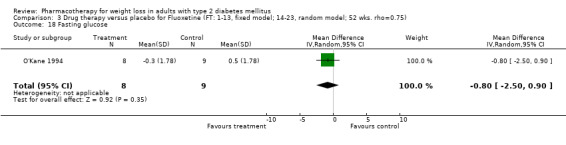
Comparison 3 Drug therapy versus placebo for Fluoxetine (FT: 1‐13, fixed model; 14‐23, random model; 52 wks. rho=0.75), Outcome 18 Fasting glucose.
3.21. Analysis.
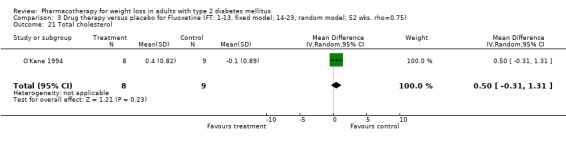
Comparison 3 Drug therapy versus placebo for Fluoxetine (FT: 1‐13, fixed model; 14‐23, random model; 52 wks. rho=0.75), Outcome 21 Total cholesterol.
3.23. Analysis.
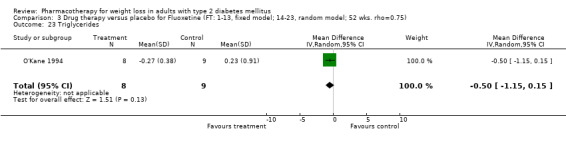
Comparison 3 Drug therapy versus placebo for Fluoxetine (FT: 1‐13, fixed model; 14‐23, random model; 52 wks. rho=0.75), Outcome 23 Triglycerides.
Comparison 4. Drug therapy versus placebo for Fluoxetine (SA dropout weight=C loss; RE; FT, LOCFremoved).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 weight loss (kg) | 5 | 200 | Mean Difference (IV, Random, 95% CI) | ‐3.03 [‐4.59, ‐1.47] |
4.1. Analysis.
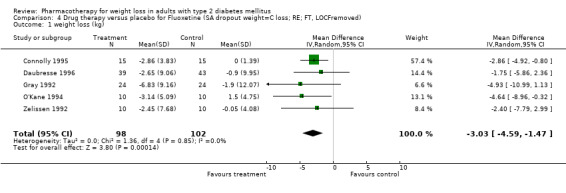
Comparison 4 Drug therapy versus placebo for Fluoxetine (SA dropout weight=C loss; RE; FT, LOCFremoved), Outcome 1 weight loss (kg).
Comparison 5. Drug therapy versus placebo for Fluoxetine (SA dropout weight=0 loss; RE; FT, LOCFremoved).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 weight loss (kg) | 5 | 200 | Mean Difference (IV, Random, 95% CI) | ‐3.00 [‐4.56, ‐1.44] |
5.1. Analysis.
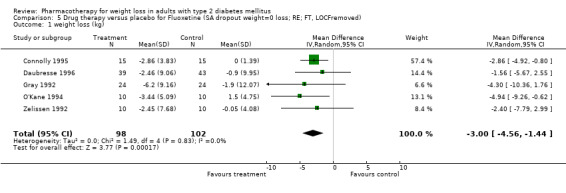
Comparison 5 Drug therapy versus placebo for Fluoxetine (SA dropout weight=0 loss; RE; FT, LOCFremoved), Outcome 1 weight loss (kg).
Comparison 6. Drug therapy vs placebo Fluoxetine (SA FT: LOCF removed).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight (kg) random | 5 | 165 | Mean Difference (IV, Random, 95% CI) | ‐3.96 [‐5.89, ‐2.02] |
6.1. Analysis.
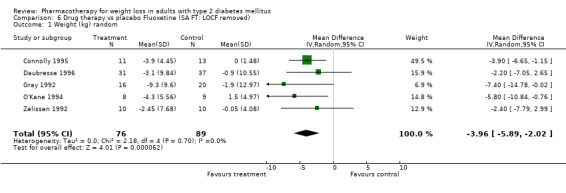
Comparison 6 Drug therapy vs placebo Fluoxetine (SA FT: LOCF removed), Outcome 1 Weight (kg) random.
Comparison 7. Drug therapy versus placebo for Orlistat (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight (kg) | 7 | 1363 | Mean Difference (IV, Fixed, 95% CI) | ‐2.12 [‐2.63, ‐1.60] |
| 2 Percent weight loss | 4 | 1008 | Mean Difference (IV, Fixed, 95% CI) | ‐2.43 [‐2.95, ‐1.90] |
| 3 % with wt loss > 5% | 5 | 1273 | Mean Difference (IV, Fixed, 95% CI) | 21.39 [15.16, 27.62] |
| 4 BMI | 2 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐0.71 [‐1.52, 0.10] |
| 5 Waist circumference | 6 | 1111 | Mean Difference (IV, Fixed, 95% CI) | ‐1.02 [‐1.33, ‐0.70] |
| 6 GHb | 7 | 1373 | Mean Difference (IV, Fixed, 95% CI) | ‐0.45 [‐0.58, ‐0.31] |
| 7 Fasting glucose | 8 | 1449 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐0.89, ‐0.51] |
| 8 SBP | 5 | 740 | Mean Difference (IV, Fixed, 95% CI) | ‐2.19 [‐3.84, ‐0.55] |
| 9 DBP | 4 | 441 | Mean Difference (IV, Fixed, 95% CI) | ‐3.94 [‐5.18, ‐2.71] |
| 10 Total cholesterol | 6 | 1324 | Mean Difference (IV, Fixed, 95% CI) | ‐0.41 [‐0.52, ‐0.30] |
| 11 LDL cholesterol | 6 | 1287 | Mean Difference (IV, Fixed, 95% CI) | ‐0.32 [‐0.42, ‐0.23] |
| 12 HDL cholesterol | 6 | 994 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.05, 0.00] |
| 13 Triglycerides | 6 | 994 | Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.40, ‐0.05] |
| 14 Weight (kg) | 7 | 1363 | Mean Difference (IV, Random, 95% CI) | ‐2.03 [‐2.82, ‐1.25] |
| 15 Percent weight loss | 4 | 1008 | Mean Difference (IV, Random, 95% CI) | ‐2.34 [‐2.97, ‐1.70] |
| 16 % with wt loss > 5% | 5 | 1273 | Mean Difference (IV, Random, 95% CI) | 21.39 [15.16, 27.62] |
| 17 BMI | 2 | 100 | Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐1.52, 0.10] |
| 18 Waist circumference | 6 | 1111 | Mean Difference (IV, Random, 95% CI) | ‐1.84 [‐2.99, ‐0.68] |
| 19 GHb | 7 | 1373 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.58, ‐0.31] |
| 20 Fasting glucose | 8 | 1449 | Mean Difference (IV, Random, 95% CI) | ‐0.82 [‐1.14, ‐0.50] |
| 21 SBP | 5 | 740 | Mean Difference (IV, Random, 95% CI) | ‐2.99 [‐6.29, 0.32] |
| 22 DBP | 4 | 441 | Mean Difference (IV, Random, 95% CI) | ‐4.21 [‐7.82, ‐0.61] |
| 23 Total cholesterol | 6 | 1324 | Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.52, ‐0.30] |
| 24 LDL cholesterol | 6 | 1287 | Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.43, ‐0.21] |
| 25 HDL cholesterol | 6 | 994 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.05, 0.00] |
| 26 Triglycerides | 6 | 994 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.40, ‐0.05] |
7.1. Analysis.
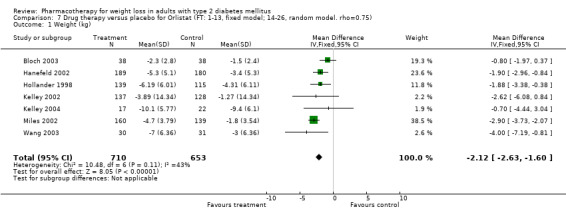
Comparison 7 Drug therapy versus placebo for Orlistat (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 1 Weight (kg).
7.2. Analysis.
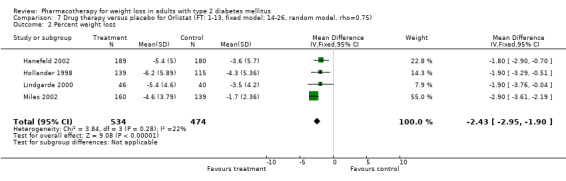
Comparison 7 Drug therapy versus placebo for Orlistat (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 2 Percent weight loss.
7.3. Analysis.
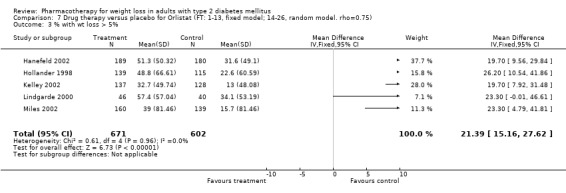
Comparison 7 Drug therapy versus placebo for Orlistat (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 3 % with wt loss > 5%.
7.4. Analysis.
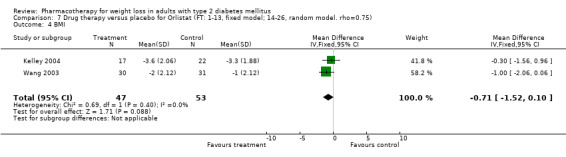
Comparison 7 Drug therapy versus placebo for Orlistat (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 4 BMI.
7.5. Analysis.
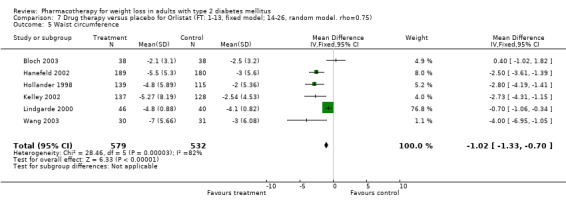
Comparison 7 Drug therapy versus placebo for Orlistat (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 5 Waist circumference.
7.6. Analysis.
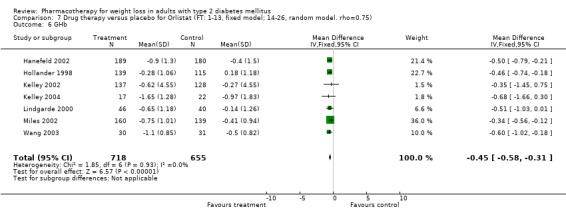
Comparison 7 Drug therapy versus placebo for Orlistat (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 6 GHb.
7.7. Analysis.
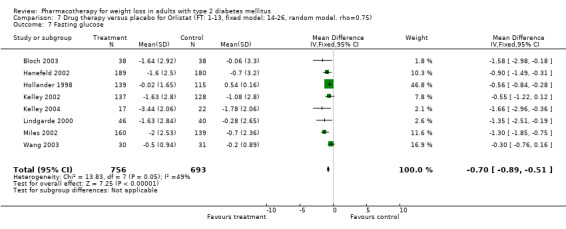
Comparison 7 Drug therapy versus placebo for Orlistat (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 7 Fasting glucose.
7.8. Analysis.
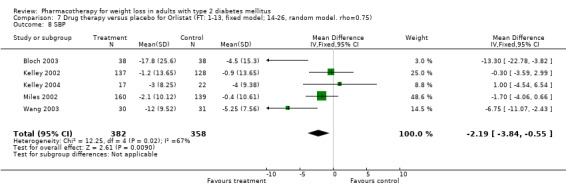
Comparison 7 Drug therapy versus placebo for Orlistat (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 8 SBP.
7.9. Analysis.
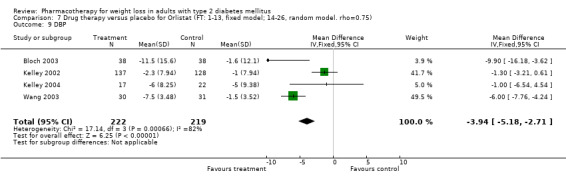
Comparison 7 Drug therapy versus placebo for Orlistat (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 9 DBP.
7.10. Analysis.
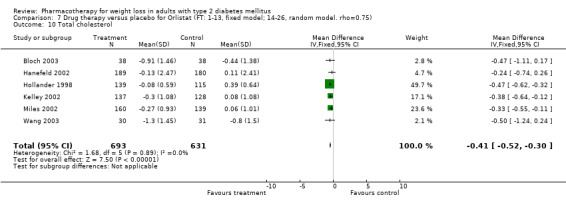
Comparison 7 Drug therapy versus placebo for Orlistat (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 10 Total cholesterol.
7.11. Analysis.
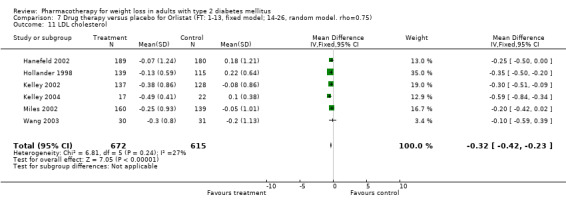
Comparison 7 Drug therapy versus placebo for Orlistat (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 11 LDL cholesterol.
7.12. Analysis.
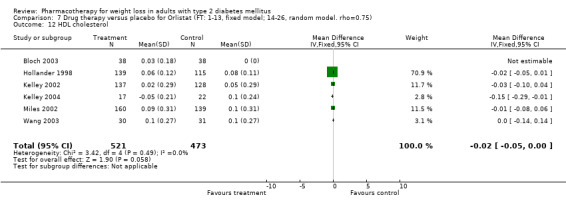
Comparison 7 Drug therapy versus placebo for Orlistat (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 12 HDL cholesterol.
7.13. Analysis.
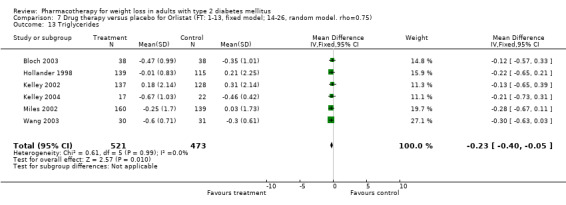
Comparison 7 Drug therapy versus placebo for Orlistat (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 13 Triglycerides.
7.14. Analysis.
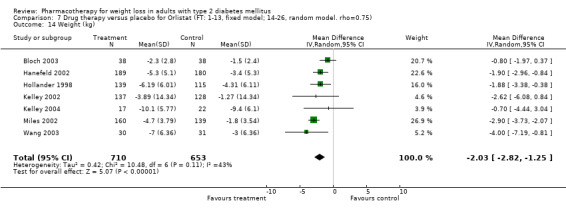
Comparison 7 Drug therapy versus placebo for Orlistat (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 14 Weight (kg).
7.15. Analysis.
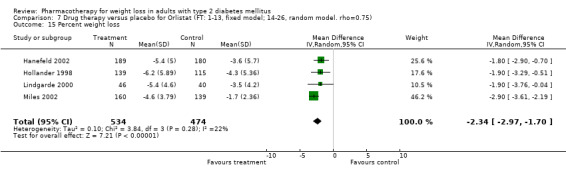
Comparison 7 Drug therapy versus placebo for Orlistat (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 15 Percent weight loss.
7.16. Analysis.
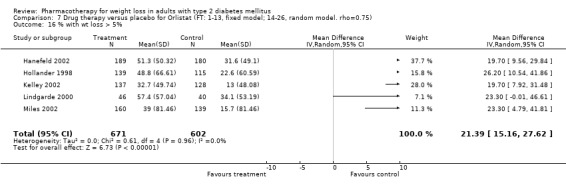
Comparison 7 Drug therapy versus placebo for Orlistat (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 16 % with wt loss > 5%.
7.17. Analysis.
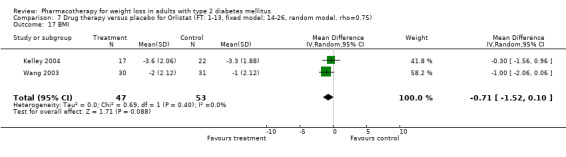
Comparison 7 Drug therapy versus placebo for Orlistat (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 17 BMI.
7.18. Analysis.
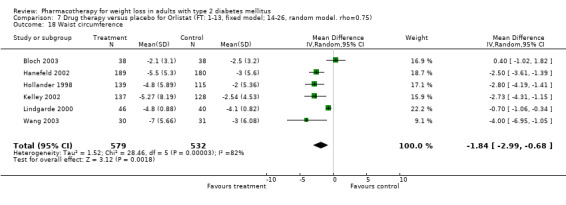
Comparison 7 Drug therapy versus placebo for Orlistat (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 18 Waist circumference.
7.19. Analysis.
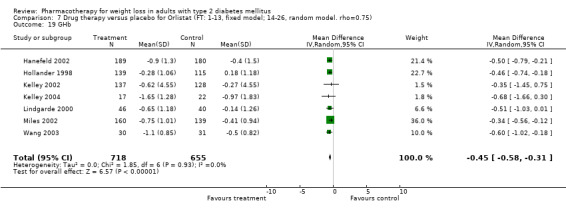
Comparison 7 Drug therapy versus placebo for Orlistat (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 19 GHb.
7.20. Analysis.
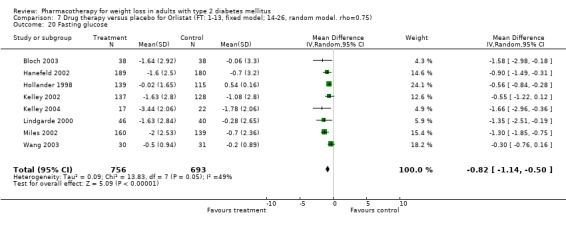
Comparison 7 Drug therapy versus placebo for Orlistat (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 20 Fasting glucose.
7.21. Analysis.
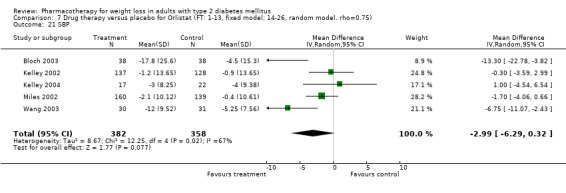
Comparison 7 Drug therapy versus placebo for Orlistat (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 21 SBP.
7.22. Analysis.
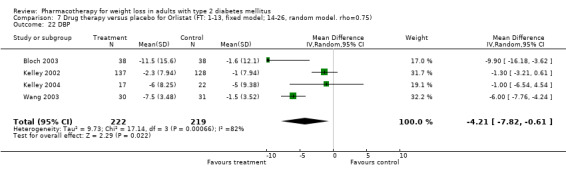
Comparison 7 Drug therapy versus placebo for Orlistat (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 22 DBP.
7.23. Analysis.
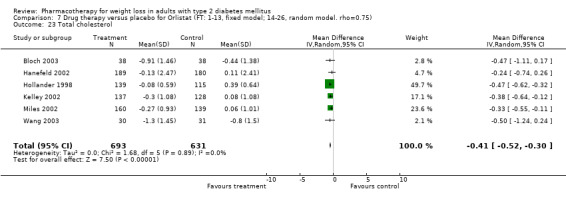
Comparison 7 Drug therapy versus placebo for Orlistat (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 23 Total cholesterol.
7.24. Analysis.
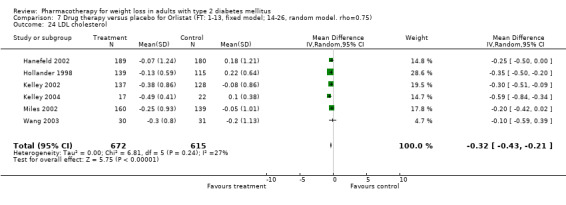
Comparison 7 Drug therapy versus placebo for Orlistat (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 24 LDL cholesterol.
7.25. Analysis.
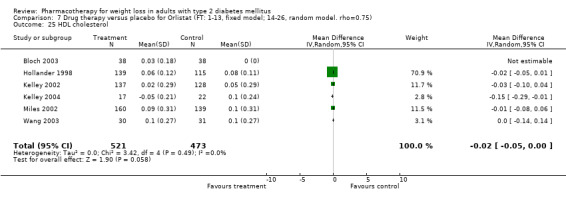
Comparison 7 Drug therapy versus placebo for Orlistat (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 25 HDL cholesterol.
7.26. Analysis.
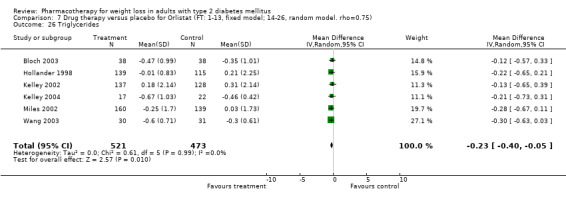
Comparison 7 Drug therapy versus placebo for Orlistat (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 26 Triglycerides.
Comparison 8. Drug therapy versus placebo for Orlistat (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight (kg) | 10 | 2045 | Mean Difference (IV, Fixed, 95% CI) | ‐2.08 [‐2.39, ‐1.77] |
| 2 Percent weight loss | 10 | 2833 | Mean Difference (IV, Fixed, 95% CI) | ‐2.55 [‐2.91, ‐2.20] |
| 3 % with wt loss > 5% | 10 | 2871 | Mean Difference (IV, Fixed, 95% CI) | 21.59 [17.08, 26.09] |
| 4 BMI | 3 | 130 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.33, ‐0.06] |
| 5 Waist circumference | 8 | 1647 | Mean Difference (IV, Fixed, 95% CI) | ‐1.13 [‐1.42, ‐0.85] |
| 6 GHb | 13 | 2898 | Mean Difference (IV, Fixed, 95% CI) | ‐0.42 [‐0.50, ‐0.34] |
| 7 Fasting glucose | 13 | 2737 | Mean Difference (IV, Fixed, 95% CI) | ‐0.73 [‐0.88, ‐0.58] |
| 8 SBP | 6 | 977 | Mean Difference (IV, Fixed, 95% CI) | ‐2.54 [‐3.95, ‐1.13] |
| 9 DBP | 5 | 678 | Mean Difference (IV, Fixed, 95% CI) | ‐3.54 [‐4.59, ‐2.49] |
| 10 Total cholesterol | 6 | 1324 | Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐0.47, ‐0.30] |
| 11 LDL cholesterol | 7 | 1571 | Mean Difference (IV, Fixed, 95% CI) | ‐0.29 [‐0.36, ‐0.22] |
| 12 HDL cholesterol | 6 | 994 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.04, ‐0.01] |
| 13 Triglycerides | 6 | 994 | Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐0.38, ‐0.09] |
| 14 Weight (kg) | 10 | 2045 | Mean Difference (IV, Random, 95% CI) | ‐2.12 [‐2.67, ‐1.57] |
| 15 Percent weight loss | 10 | 2833 | Mean Difference (IV, Random, 95% CI) | ‐2.55 [‐2.91, ‐2.20] |
| 16 % with wt loss > 5% | 10 | 2871 | Mean Difference (IV, Random, 95% CI) | 21.59 [17.08, 26.09] |
| 17 BMI | 3 | 130 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.56, 0.06] |
| 18 Waist circumference | 8 | 1647 | Mean Difference (IV, Random, 95% CI) | ‐1.71 [‐2.48, ‐0.94] |
| 19 GHb | 13 | 2898 | Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.50, ‐0.34] |
| 20 Fasting glucose | 13 | 2737 | Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐1.01, ‐0.60] |
| 21 SBP | 6 | 977 | Mean Difference (IV, Random, 95% CI) | ‐3.22 [‐5.93, ‐0.51] |
| 22 DBP | 5 | 678 | Mean Difference (IV, Random, 95% CI) | ‐3.85 [‐6.53, ‐1.18] |
| 23 Total cholesterol | 6 | 1324 | Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.47, ‐0.30] |
| 24 LDL cholesterol | 7 | 1571 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.38, ‐0.21] |
| 25 HDL cholesterol | 6 | 994 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.04, ‐0.01] |
| 26 Triglycerides | 6 | 994 | Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.38, ‐0.09] |
8.1. Analysis.
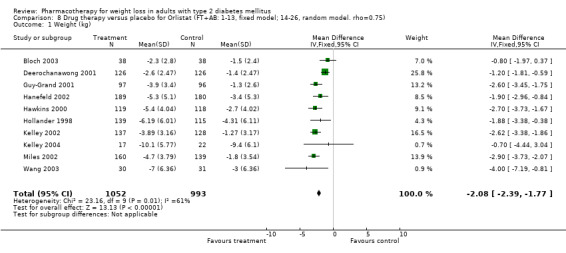
Comparison 8 Drug therapy versus placebo for Orlistat (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 1 Weight (kg).
8.2. Analysis.
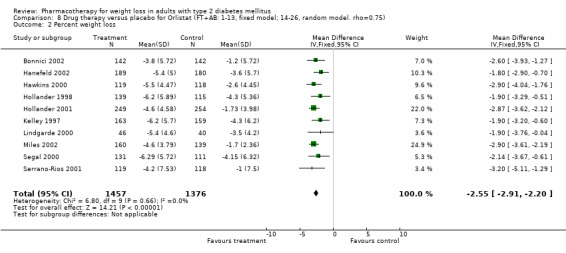
Comparison 8 Drug therapy versus placebo for Orlistat (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 2 Percent weight loss.
8.3. Analysis.
Comparison 8 Drug therapy versus placebo for Orlistat (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 3 % with wt loss > 5%.
8.4. Analysis.
Comparison 8 Drug therapy versus placebo for Orlistat (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 4 BMI.
8.5. Analysis.
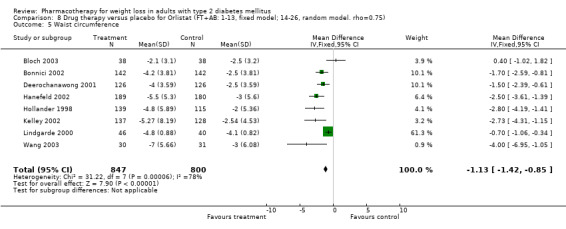
Comparison 8 Drug therapy versus placebo for Orlistat (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 5 Waist circumference.
8.6. Analysis.
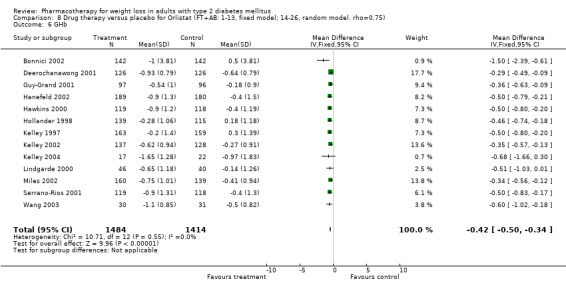
Comparison 8 Drug therapy versus placebo for Orlistat (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 6 GHb.
8.7. Analysis.
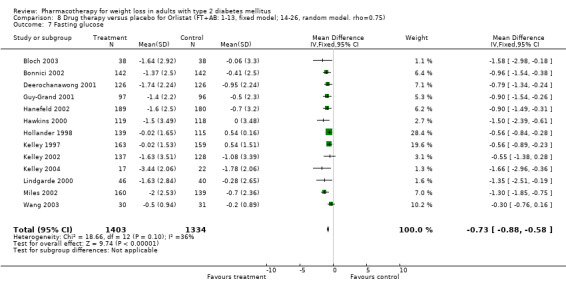
Comparison 8 Drug therapy versus placebo for Orlistat (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 7 Fasting glucose.
8.8. Analysis.
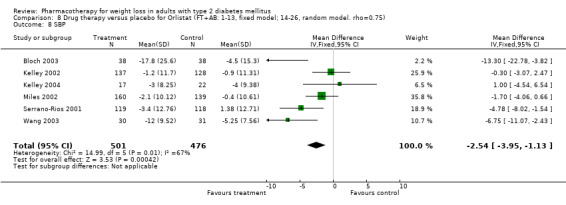
Comparison 8 Drug therapy versus placebo for Orlistat (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 8 SBP.
8.9. Analysis.
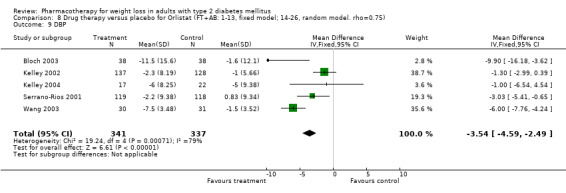
Comparison 8 Drug therapy versus placebo for Orlistat (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 9 DBP.
8.10. Analysis.
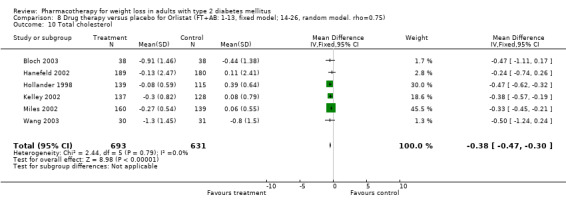
Comparison 8 Drug therapy versus placebo for Orlistat (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 10 Total cholesterol.
8.11. Analysis.
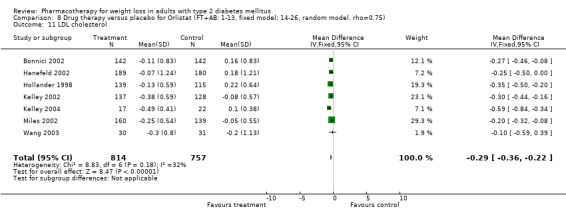
Comparison 8 Drug therapy versus placebo for Orlistat (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 11 LDL cholesterol.
8.12. Analysis.
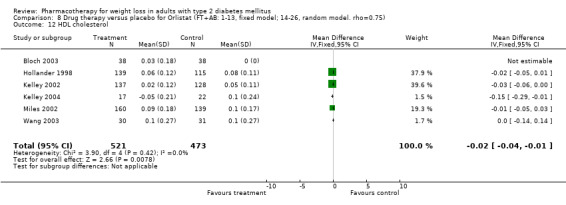
Comparison 8 Drug therapy versus placebo for Orlistat (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 12 HDL cholesterol.
8.13. Analysis.
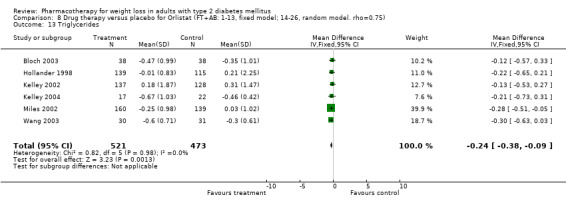
Comparison 8 Drug therapy versus placebo for Orlistat (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 13 Triglycerides.
8.14. Analysis.
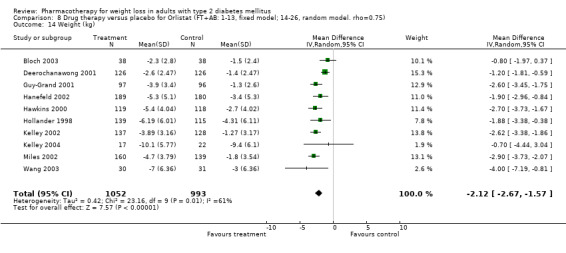
Comparison 8 Drug therapy versus placebo for Orlistat (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 14 Weight (kg).
8.15. Analysis.
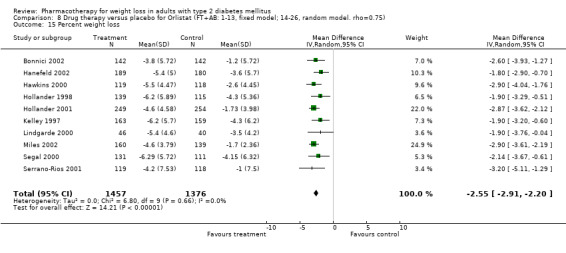
Comparison 8 Drug therapy versus placebo for Orlistat (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 15 Percent weight loss.
8.16. Analysis.
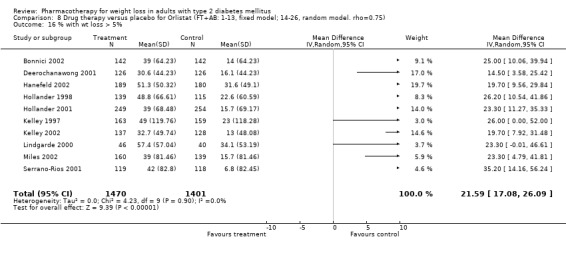
Comparison 8 Drug therapy versus placebo for Orlistat (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 16 % with wt loss > 5%.
8.17. Analysis.
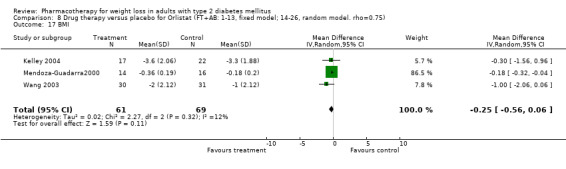
Comparison 8 Drug therapy versus placebo for Orlistat (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 17 BMI.
8.18. Analysis.
Comparison 8 Drug therapy versus placebo for Orlistat (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 18 Waist circumference.
8.19. Analysis.
Comparison 8 Drug therapy versus placebo for Orlistat (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 19 GHb.
8.20. Analysis.
Comparison 8 Drug therapy versus placebo for Orlistat (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 20 Fasting glucose.
8.21. Analysis.
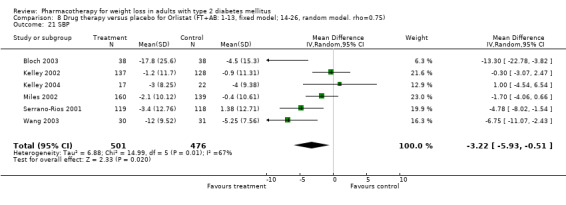
Comparison 8 Drug therapy versus placebo for Orlistat (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 21 SBP.
8.22. Analysis.
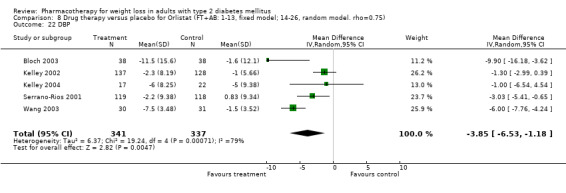
Comparison 8 Drug therapy versus placebo for Orlistat (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 22 DBP.
8.23. Analysis.
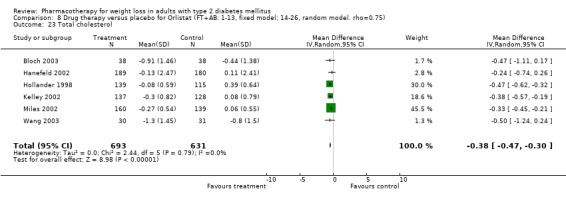
Comparison 8 Drug therapy versus placebo for Orlistat (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 23 Total cholesterol.
8.24. Analysis.
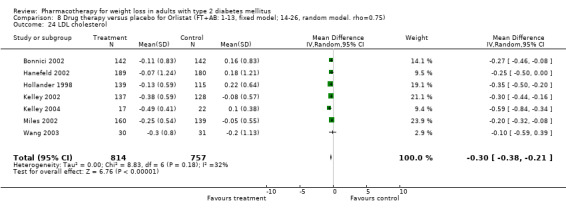
Comparison 8 Drug therapy versus placebo for Orlistat (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 24 LDL cholesterol.
8.25. Analysis.
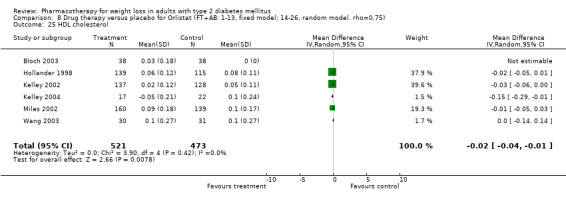
Comparison 8 Drug therapy versus placebo for Orlistat (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 25 HDL cholesterol.
8.26. Analysis.
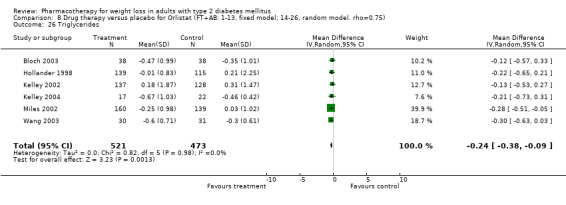
Comparison 8 Drug therapy versus placebo for Orlistat (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 26 Triglycerides.
Comparison 9. Drug therapy versus placebo for Sibutramine (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight (kg) | 8 | 845 | Mean Difference (IV, Fixed, 95% CI) | ‐3.60 [‐4.18, ‐3.01] |
| 2 Percent weight loss | 3 | 426 | Mean Difference (IV, Fixed, 95% CI) | ‐4.03 [‐5.52, ‐2.55] |
| 3 % with wt loss > 5% | 2 | 204 | Mean Difference (IV, Fixed, 95% CI) | 21.16 [12.48, 29.83] |
| 4 BMI | 6 | 517 | Mean Difference (IV, Fixed, 95% CI) | ‐1.29 [‐1.53, ‐1.04] |
| 5 Waist circumference | 5 | 475 | Mean Difference (IV, Fixed, 95% CI) | ‐4.13 [‐5.16, ‐3.10] |
| 6 GHb | 7 | 612 | Mean Difference (IV, Fixed, 95% CI) | ‐0.82 [‐0.97, ‐0.66] |
| 7 Fasting glucose | 5 | 434 | Mean Difference (IV, Fixed, 95% CI) | ‐1.27 [‐1.73, ‐0.82] |
| 8 SBP | 6 | 673 | Mean Difference (IV, Fixed, 95% CI) | ‐0.91 [‐1.61, ‐0.20] |
| 9 DBP | 4 | 480 | Mean Difference (IV, Fixed, 95% CI) | 1.43 [0.06, 2.79] |
| 10 Total cholesterol | 6 | 529 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.12, 0.09] |
| 11 LDL cholesterol | 6 | 529 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.11, 0.11] |
| 12 HDL cholesterol | 5 | 419 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [0.03, 0.11] |
| 13 Triglycerides | 6 | 529 | Mean Difference (IV, Fixed, 95% CI) | ‐0.22 [‐0.35, ‐0.08] |
| 14 Weight (kg) | 8 | 845 | Mean Difference (IV, Random, 95% CI) | ‐5.10 [‐5.00, ‐3.20] |
| 15 Percent weight loss | 3 | 426 | Mean Difference (IV, Random, 95% CI) | ‐4.03 [‐5.52, ‐2.55] |
| 16 % with wt loss > 5% | 2 | 204 | Mean Difference (IV, Random, 95% CI) | 21.16 [12.48, 29.83] |
| 17 BMI | 6 | 517 | Mean Difference (IV, Random, 95% CI) | ‐1.87 [‐2.64, ‐1.10] |
| 18 Waist circumference | 5 | 475 | Mean Difference (IV, Random, 95% CI) | ‐4.68 [‐7.36, ‐1.99] |
| 19 GHb | 7 | 612 | Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐1.32, 0.24] |
| 20 Fasting glucose | 5 | 434 | Mean Difference (IV, Random, 95% CI) | ‐1.35 [‐3.68, 0.99] |
| 21 SBP | 6 | 673 | Mean Difference (IV, Random, 95% CI) | ‐0.84 [‐1.65, ‐0.02] |
| 22 DBP | 4 | 480 | Mean Difference (IV, Random, 95% CI) | 1.43 [0.06, 2.79] |
| 23 Total cholesterol | 6 | 529 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.37, 0.15] |
| 24 LDL cholesterol | 6 | 529 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.31, 0.16] |
| 25 HDL cholesterol | 5 | 419 | Mean Difference (IV, Random, 95% CI) | 0.07 [0.03, 0.11] |
| 26 Triglycerides | 6 | 529 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.50, ‐0.04] |
9.1. Analysis.
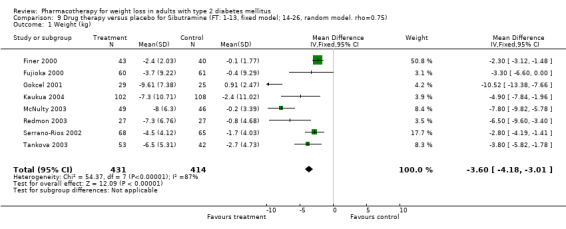
Comparison 9 Drug therapy versus placebo for Sibutramine (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 1 Weight (kg).
9.2. Analysis.
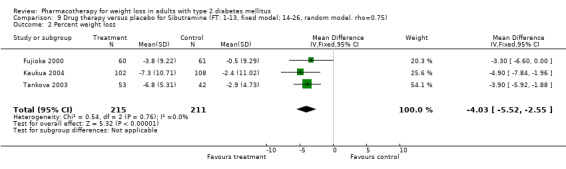
Comparison 9 Drug therapy versus placebo for Sibutramine (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 2 Percent weight loss.
9.3. Analysis.
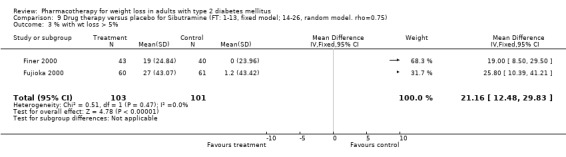
Comparison 9 Drug therapy versus placebo for Sibutramine (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 3 % with wt loss > 5%.
9.4. Analysis.
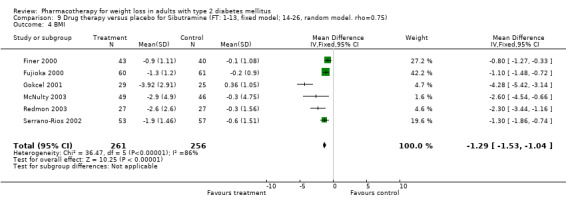
Comparison 9 Drug therapy versus placebo for Sibutramine (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 4 BMI.
9.5. Analysis.
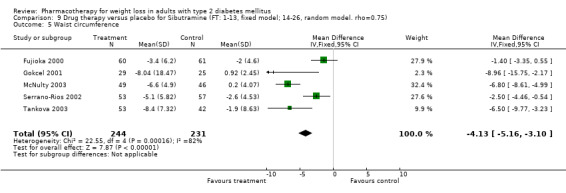
Comparison 9 Drug therapy versus placebo for Sibutramine (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 5 Waist circumference.
9.6. Analysis.
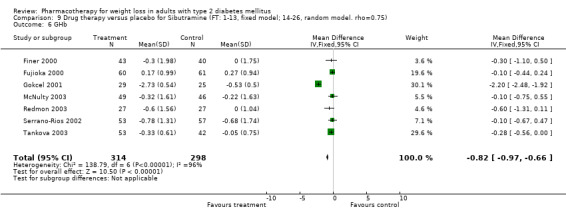
Comparison 9 Drug therapy versus placebo for Sibutramine (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 6 GHb.
9.7. Analysis.
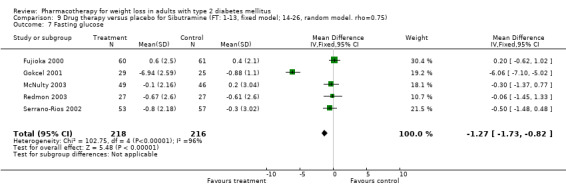
Comparison 9 Drug therapy versus placebo for Sibutramine (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 7 Fasting glucose.
9.8. Analysis.
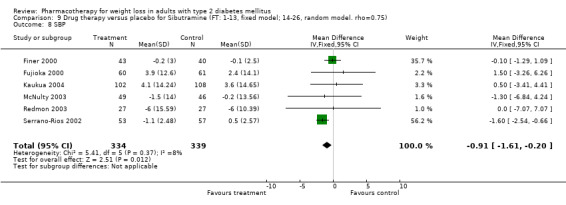
Comparison 9 Drug therapy versus placebo for Sibutramine (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 8 SBP.
9.9. Analysis.
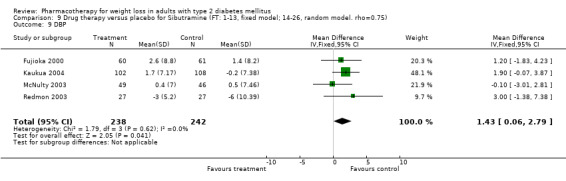
Comparison 9 Drug therapy versus placebo for Sibutramine (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 9 DBP.
9.10. Analysis.
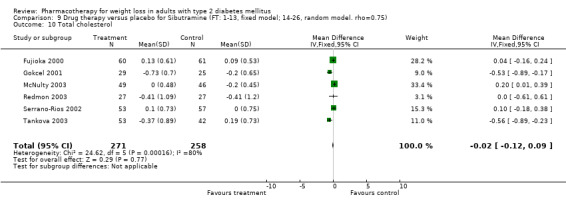
Comparison 9 Drug therapy versus placebo for Sibutramine (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 10 Total cholesterol.
9.11. Analysis.
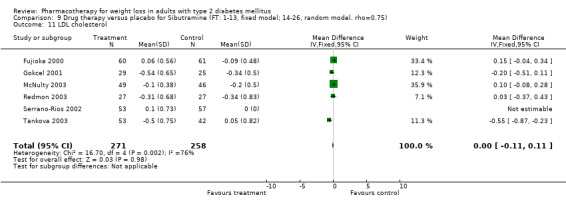
Comparison 9 Drug therapy versus placebo for Sibutramine (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 11 LDL cholesterol.
9.12. Analysis.
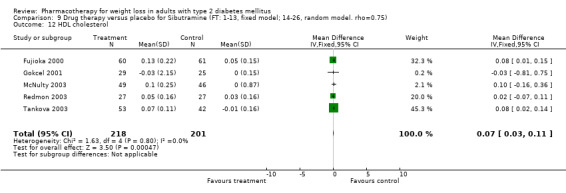
Comparison 9 Drug therapy versus placebo for Sibutramine (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 12 HDL cholesterol.
9.13. Analysis.
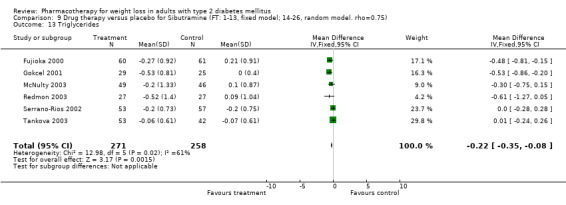
Comparison 9 Drug therapy versus placebo for Sibutramine (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 13 Triglycerides.
9.14. Analysis.
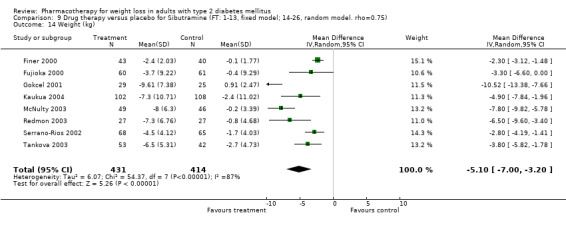
Comparison 9 Drug therapy versus placebo for Sibutramine (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 14 Weight (kg).
9.15. Analysis.
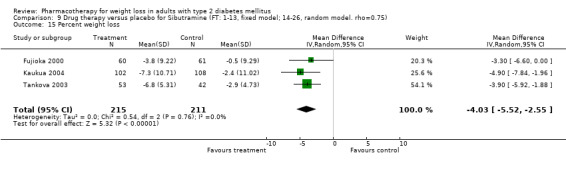
Comparison 9 Drug therapy versus placebo for Sibutramine (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 15 Percent weight loss.
9.16. Analysis.
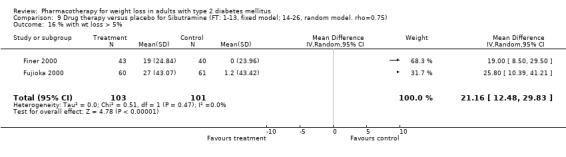
Comparison 9 Drug therapy versus placebo for Sibutramine (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 16 % with wt loss > 5%.
9.17. Analysis.
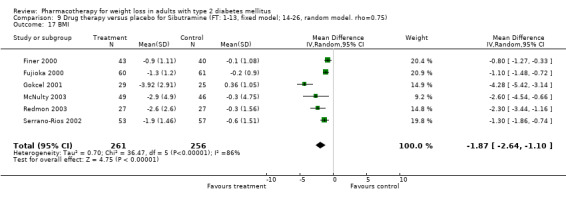
Comparison 9 Drug therapy versus placebo for Sibutramine (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 17 BMI.
9.18. Analysis.
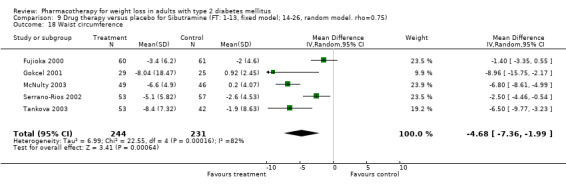
Comparison 9 Drug therapy versus placebo for Sibutramine (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 18 Waist circumference.
9.19. Analysis.
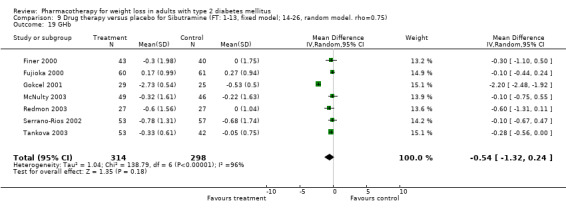
Comparison 9 Drug therapy versus placebo for Sibutramine (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 19 GHb.
9.20. Analysis.
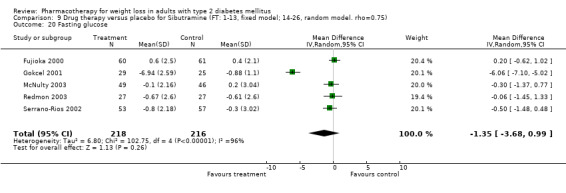
Comparison 9 Drug therapy versus placebo for Sibutramine (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 20 Fasting glucose.
9.21. Analysis.
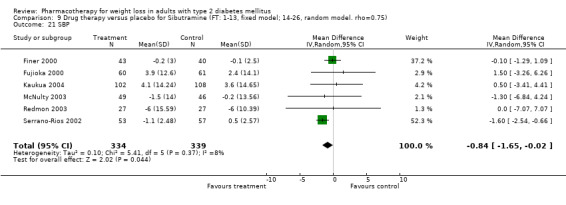
Comparison 9 Drug therapy versus placebo for Sibutramine (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 21 SBP.
9.22. Analysis.
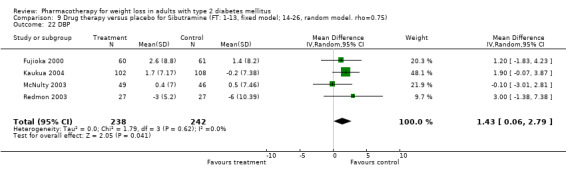
Comparison 9 Drug therapy versus placebo for Sibutramine (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 22 DBP.
9.23. Analysis.
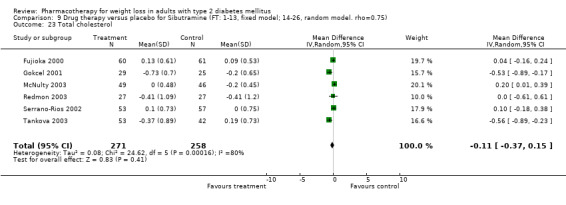
Comparison 9 Drug therapy versus placebo for Sibutramine (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 23 Total cholesterol.
9.24. Analysis.
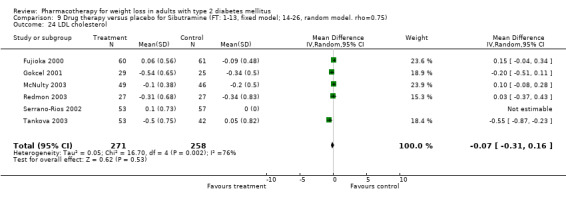
Comparison 9 Drug therapy versus placebo for Sibutramine (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 24 LDL cholesterol.
9.25. Analysis.
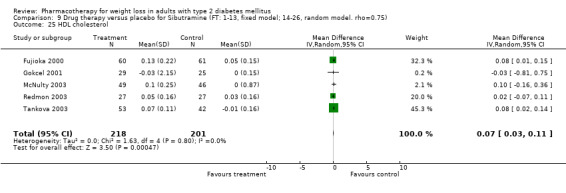
Comparison 9 Drug therapy versus placebo for Sibutramine (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 25 HDL cholesterol.
9.26. Analysis.
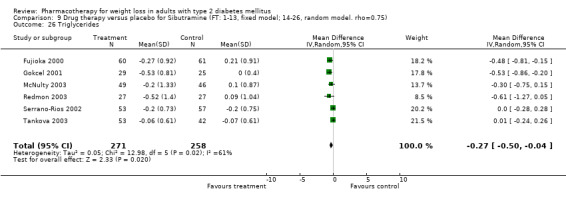
Comparison 9 Drug therapy versus placebo for Sibutramine (FT: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 26 Triglycerides.
Comparison 10. Drug therapy versus placebo for Sibutramine (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight (kg) | 9 | 863 | Mean Difference (IV, Fixed, 95% CI) | ‐3.53 [‐4.10, ‐2.96] |
| 2 Percent weight loss | 4 | 662 | Mean Difference (IV, Fixed, 95% CI) | ‐4.21 [‐5.54, ‐2.88] |
| 3 % with wt loss > 5% | 3 | 440 | Mean Difference (IV, Fixed, 95% CI) | 23.41 [15.10, 31.71] |
| 4 BMI | 6 | 517 | Mean Difference (IV, Fixed, 95% CI) | ‐1.29 [‐1.53, ‐1.04] |
| 5 Waist circumference | 5 | 475 | Mean Difference (IV, Fixed, 95% CI) | ‐4.13 [‐5.16, ‐3.10] |
| 6 GHb | 7 | 612 | Mean Difference (IV, Fixed, 95% CI) | ‐0.82 [‐0.97, ‐0.66] |
| 7 Fasting glucose | 5 | 434 | Mean Difference (IV, Fixed, 95% CI) | ‐1.27 [‐1.73, ‐0.82] |
| 8 SBP | 6 | 673 | Mean Difference (IV, Fixed, 95% CI) | ‐0.91 [‐1.61, ‐0.20] |
| 9 DBP | 4 | 480 | Mean Difference (IV, Fixed, 95% CI) | 1.43 [0.06, 2.79] |
| 10 Total cholesterol | 6 | 529 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.12, 0.09] |
| 11 LDL cholesterol | 6 | 529 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.11, 0.11] |
| 12 HDL cholesterol | 5 | 419 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [0.03, 0.11] |
| 13 Triglycerides | 6 | 529 | Mean Difference (IV, Fixed, 95% CI) | ‐0.22 [‐0.35, ‐0.08] |
| 14 Weight (kg) | 9 | 863 | Mean Difference (IV, Random, 95% CI) | ‐4.77 [‐6.50, ‐3.04] |
| 15 Percent weight loss | 4 | 662 | Mean Difference (IV, Random, 95% CI) | ‐4.21 [‐5.54, ‐2.88] |
| 16 % with wt loss > 5% | 3 | 440 | Mean Difference (IV, Random, 95% CI) | 25.86 [13.25, 38.47] |
| 17 BMI | 6 | 517 | Mean Difference (IV, Random, 95% CI) | ‐1.87 [‐2.64, ‐1.10] |
| 18 Waist circumference | 5 | 475 | Mean Difference (IV, Random, 95% CI) | ‐4.68 [‐7.36, ‐1.99] |
| 19 GHb | 7 | 612 | Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐1.32, 0.24] |
| 20 Fasting glucose | 5 | 434 | Mean Difference (IV, Random, 95% CI) | ‐1.35 [‐3.68, 0.99] |
| 21 SBP | 6 | 673 | Mean Difference (IV, Random, 95% CI) | ‐0.84 [‐1.65, ‐0.02] |
| 22 DBP | 4 | 480 | Mean Difference (IV, Random, 95% CI) | 1.43 [0.06, 2.79] |
| 23 Total cholesterol | 6 | 529 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.37, 0.15] |
| 24 LDL cholesterol | 6 | 529 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.31, 0.16] |
| 25 HDL cholesterol | 5 | 419 | Mean Difference (IV, Random, 95% CI) | 0.07 [0.03, 0.11] |
| 26 Triglycerides | 6 | 529 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.50, ‐0.04] |
10.1. Analysis.
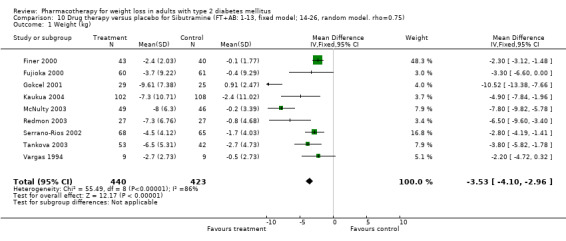
Comparison 10 Drug therapy versus placebo for Sibutramine (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 1 Weight (kg).
10.2. Analysis.
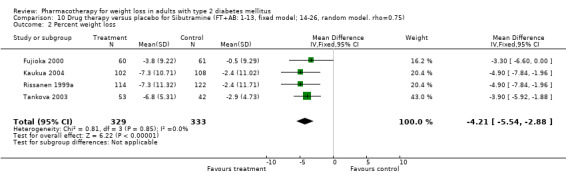
Comparison 10 Drug therapy versus placebo for Sibutramine (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 2 Percent weight loss.
10.3. Analysis.
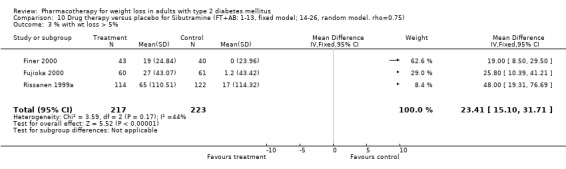
Comparison 10 Drug therapy versus placebo for Sibutramine (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 3 % with wt loss > 5%.
10.4. Analysis.
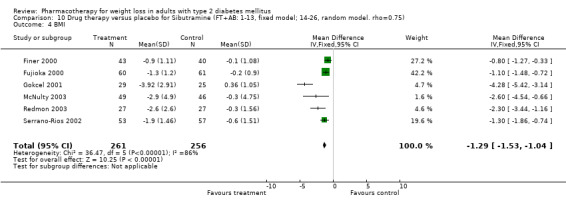
Comparison 10 Drug therapy versus placebo for Sibutramine (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 4 BMI.
10.5. Analysis.
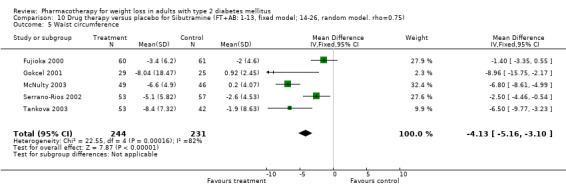
Comparison 10 Drug therapy versus placebo for Sibutramine (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 5 Waist circumference.
10.6. Analysis.
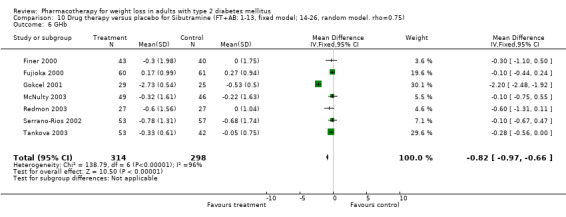
Comparison 10 Drug therapy versus placebo for Sibutramine (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 6 GHb.
10.7. Analysis.
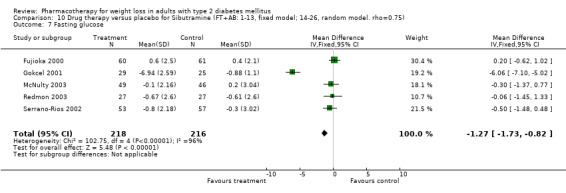
Comparison 10 Drug therapy versus placebo for Sibutramine (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 7 Fasting glucose.
10.8. Analysis.
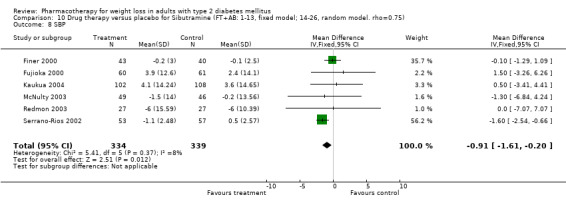
Comparison 10 Drug therapy versus placebo for Sibutramine (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 8 SBP.
10.9. Analysis.
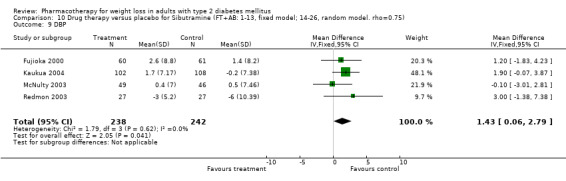
Comparison 10 Drug therapy versus placebo for Sibutramine (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 9 DBP.
10.10. Analysis.
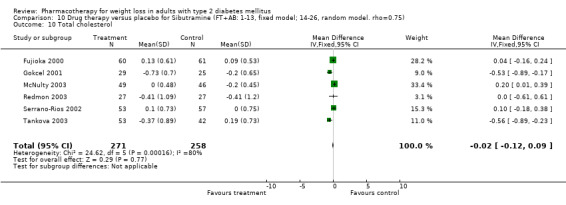
Comparison 10 Drug therapy versus placebo for Sibutramine (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 10 Total cholesterol.
10.11. Analysis.
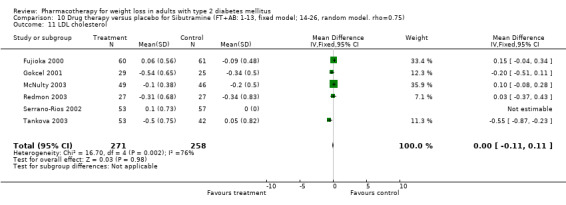
Comparison 10 Drug therapy versus placebo for Sibutramine (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 11 LDL cholesterol.
10.12. Analysis.
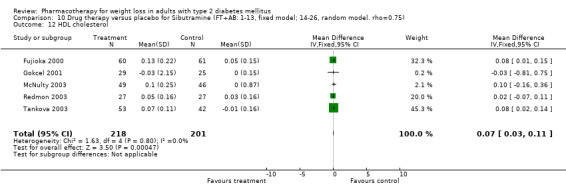
Comparison 10 Drug therapy versus placebo for Sibutramine (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 12 HDL cholesterol.
10.13. Analysis.
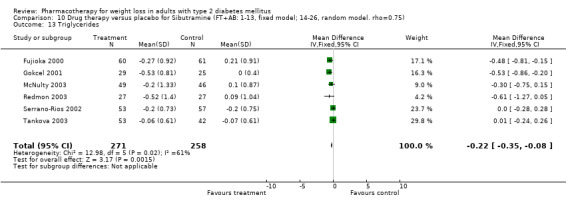
Comparison 10 Drug therapy versus placebo for Sibutramine (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 13 Triglycerides.
10.14. Analysis.
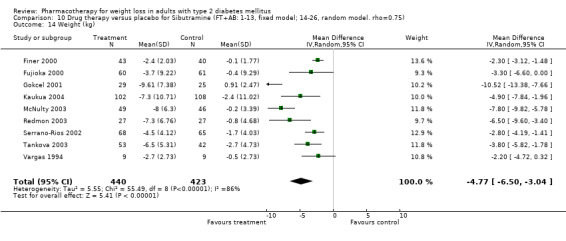
Comparison 10 Drug therapy versus placebo for Sibutramine (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 14 Weight (kg).
10.15. Analysis.
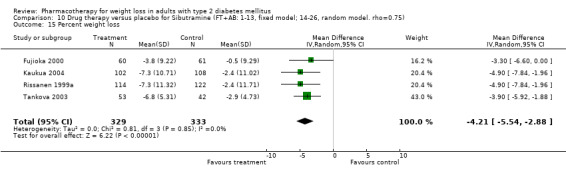
Comparison 10 Drug therapy versus placebo for Sibutramine (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 15 Percent weight loss.
10.16. Analysis.
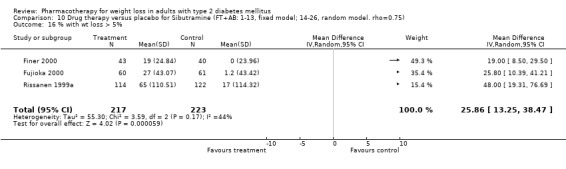
Comparison 10 Drug therapy versus placebo for Sibutramine (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 16 % with wt loss > 5%.
10.17. Analysis.
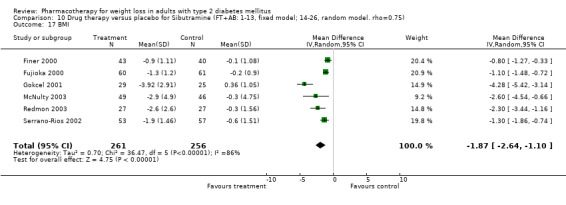
Comparison 10 Drug therapy versus placebo for Sibutramine (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 17 BMI.
10.18. Analysis.
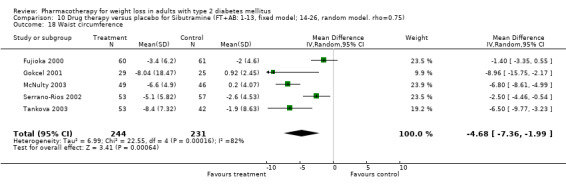
Comparison 10 Drug therapy versus placebo for Sibutramine (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 18 Waist circumference.
10.19. Analysis.
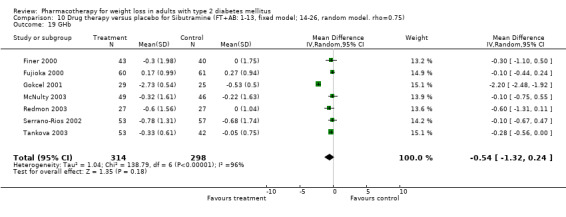
Comparison 10 Drug therapy versus placebo for Sibutramine (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 19 GHb.
10.20. Analysis.
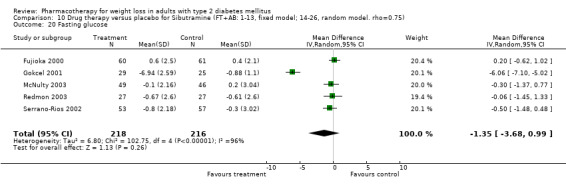
Comparison 10 Drug therapy versus placebo for Sibutramine (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 20 Fasting glucose.
10.21. Analysis.
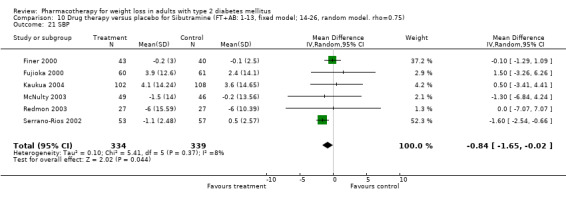
Comparison 10 Drug therapy versus placebo for Sibutramine (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 21 SBP.
10.22. Analysis.
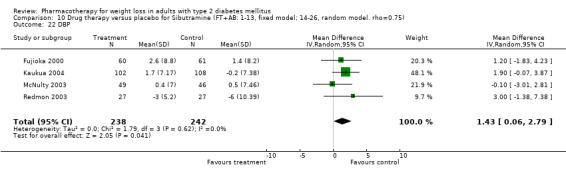
Comparison 10 Drug therapy versus placebo for Sibutramine (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 22 DBP.
10.23. Analysis.
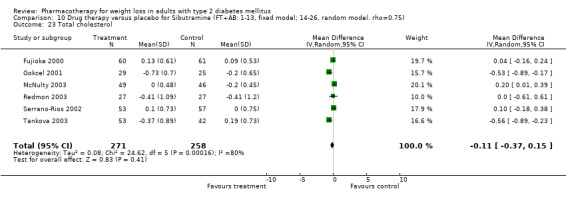
Comparison 10 Drug therapy versus placebo for Sibutramine (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 23 Total cholesterol.
10.24. Analysis.
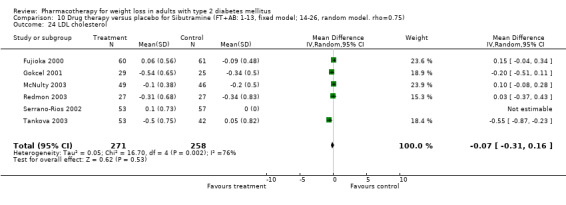
Comparison 10 Drug therapy versus placebo for Sibutramine (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 24 LDL cholesterol.
10.25. Analysis.
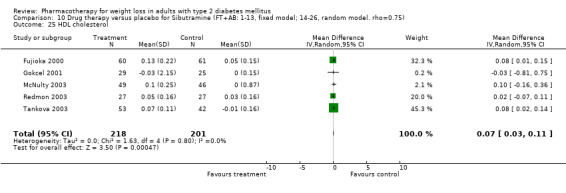
Comparison 10 Drug therapy versus placebo for Sibutramine (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 25 HDL cholesterol.
10.26. Analysis.
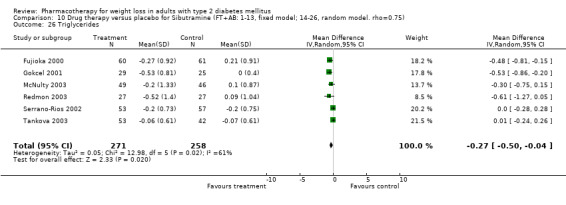
Comparison 10 Drug therapy versus placebo for Sibutramine (FT+AB: 1‐13, fixed model; 14‐26, random model. rho=0.75), Outcome 26 Triglycerides.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Allie 2004.
| Methods | Study design: Pre vs post, retrospectiveRandomization procedure: NAAllocation concealment: NAFollow‐up: 26 weeks | |
| Participants | Country: USA Setting: Endocrinology clinic Number: 23 Age: 53 Sex: NR Medications: NR BL wt: 118.0(2.5) BL BMI: 40.5(7.0) BL GHb: 7.9(1.6) | |
| Interventions | Drug: Orlistat Dosage: 120mg tidDuration: 13 to 26 weeksDiet: NRComparison: NA | |
| Outcomes | Weight: YBMI: Y>5% loss (%): YFBS: GHb: YCholesterol: YLDL: YHDL: YTG: YSBP: YDBP: YSide effects: Y | |
| Notes | Funding: Abstract/full text: FTLOCF: NAITT: NAAttrition: NA (retrospective)Blinding: NA Blinding pt: No Blinding assessor: NABlinding provider: NoBL comparable: NA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Bach 1999.
| Methods | Study design: RCT; some Pre‐versus‐post without comparison group Randomization procedure: NR Allocation concealment: Unclear Follow‐up: 32w Note: This study did not fit inclusion criteria as did not present weight outcomes, however it presented adverse event data among persons with diabetes, and is therefore presented here. | |
| Participants | Country: UKSetting: Multicenter; details unclearNumber: 210Age: 54Sex: 59Medications: None (diet only)BL wt: NRBL BMI: NRBL GHb: NR | |
| Interventions | Drug: Sibutramine dosage: 15‐20mg qdDuration: 32wDiet: NRComparison: Placebo | |
| Outcomes | Weight:BMI:>5% loss (%):FBS:GHb:Cholesterol:LDL:HDL:TG:SBP:DBP:Side effects: Yes | |
| Notes | Funding: Knoll Pharmaceutical Co.,US and UKAbstract/full text: FTLOCF: NRITT: Yes, with attritionAttrition: 11%Blinding: Double‐blindBlinding assessor: UnclearBL comparable: YesJadad score: 1,1,1,BRisk of bias: B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Bandisode 1975.
| Methods | Study design: RCTRandomization procedure: AdequateAllocation concealment: YesFollow‐up: 12w | |
| Participants | Country: USASetting: NRNumber: 64Age: 50ySex: 72%FMedications: No insulinBL wt: 95BL BMI: NRBL GHb: NR | |
| Interventions | Drug: MazindolDosage: 2mg qdDuration: 12wDiet: 5‐19 kcal/pound body weight, depending on activity levelsComparison: Placebo + diet | |
| Outcomes | Weight: YesBMI:>5% loss (%):FBS:GHb:Cholesterol: YesLDL:HDL:TG:SBP: YesDBP: YesSide effects: Yes; 1/64 pts each with drowsiness, headache, nervousness (2), dizziness, flushed face, | |
| Notes | Funding: NRAbstract/full text: FTLOCF: NRITT: Yes (with attrition)Attrition: I 38%, C 28%Blinding: Double‐blindBlinding assessor: UnclearBL comparable: NRJadad score: 2,1,1,ARisk of bias: A | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Bloch 2003.
| Methods | Study design: RCTRandomization procedure: Central random number listAllocation concealment: AdequateFollow‐up: 12 weeks | |
| Participants | Country: BrazilSetting: Hypertension clinicNumber: 204 total; 76 analyzed with diabetesAge: 56 yearsSex: 83% overallMedications: I: 68% oral agents, 8% insulin; C: 63% oral agents and 18% insulinBL wt: I 91.5, C 87.5BL BMI: I 36.6, C 35.4BL GHb: NRNote: Demographic information was given only for whole study group (39% with diabetes), including persons with diabetes and those without. | |
| Interventions | Drug: Orlistat Dosage: 120mg tidDuration: 12 weeksDiet: Low calorie diet, 30% fat; advised to increase activityComparison: Diet and activity as for intervention group | |
| Outcomes | Weight: YBMI:>5% loss (%): YFBS: YGHb: YCholesterol:LDL: YHDL:TG:SBP:DBP:Side effects: Y | |
| Notes | Funding: University Hospital Abstract/full text: FTLOCF: YesITT: YesAttrition: 31% overallBlinding: NRBlinding pt: No Blinding assessor: NRBlinding provider: NRBL comparable: Yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Bonnici 2002.
| Methods | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 24w | |
| Participants | Country: South AfricaSetting: Multicenter trial; no detailsNumber: 284Age: NRSex: NRMedications: Metformin and/or sulfonylureaBL wt: NRBL BMI: NRBL GHb: NR | |
| Interventions | Drug: Orlistat Dosage: 120mg tidDuration: 24wDiet: 600kcal/d deficitComparison: Placebo + diet | |
| Outcomes | Weight: YesBMI:>5% loss (%): YesFBS: YesGHb: YesCholesterol:LDL: YesHDL:TG:SBP:DBP:Side effects: | |
| Notes | Funding: NRAbstract/full text: ALOCF: NRITT: NRAttrition: NR Blinding: Double‐blindBlinding assessor: UnclearBL comparable: NRJadad score: 1,1,0,BRisk of bias: C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Boshell 1974.
| Methods | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 12w | |
| Participants | Country: USASetting: NRNumber: 64Age: NRSex: NRMedications: None, diet only controlBL wt:BL BMI:BL GHb: | |
| Interventions | Drug: MazindolDosage: 2mg qdDuration: 12wDiet: 5‐10kcal/pound, depending on activity levelComparison: Diet + placebo | |
| Outcomes | Weight: YesBMI:>5% loss (%):FBS:GHb:Cholesterol:LDL:HDL:TG:SBP:DBP:Side effects: | |
| Notes | Funding: NRAbstract/full text: A LOCF: NRITT: Yes (with attrition)Attrition: I 41%, C 25%Blinding: Double‐blindBlinding assessor: NRBL comparable: NROther: 2 patients excluded due to nonadherence to treatment scheduleJadad score: 1,1,1,BRisk of bias: B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Bratusch‐Marrian1979.
| Methods | Study design: RCTRandomization procedure: Random number tablesAllocation concealment: AdequateFollow‐up: 8w | |
| Participants | Country: AustriaSetting: UnclearNumber: 40Age: 50Sex: 66%FMedications: NRBL wt: I 80.3, C 93.9BL BMI: I 30.8, C 41.7BL GHb: NR | |
| Interventions | Drug: DiethylpropionDosage: 75mg qdDuration: 8wDiet: NRComparison: Placebo | |
| Outcomes | Weight: YesBMI:>5% loss (%):FBS:GHb:Cholesterol:LDL:HDL:TG:SBP:DBP:Side effects: | |
| Notes | Funding: NRAbstract/full text: FTLOCF: NR ITT: Yes, with attritionAttrition: 20%Blinding: Double‐blindBlinding assessor: YesBL comparable: YesJadad score: 1,1,1,BRisk of bias: B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Buckle 1966.
| Methods | Study design: Cross‐over study comparing phenmetrazine hydrochloride with phenmetrazine hydrochloride plus phenbutrazate hydrochloride Randomization procedure: NR Allocation concealment: Unclear Follow‐up: 8w | |
| Participants | Country: UKSetting: Hospital diabetes clinicNumber: 22Age: 58 from table 1Sex: 80%FMedications: NRBL wt: 78BL BMI: NRBL GHb: NR | |
| Interventions | Drug: PhenmetrazineDosage: 25mg tidDuration: 8w (until first cross‐over)Diet: 1000 kcal/d Comparison: Filon® [phenmetrazine theoclate 30mg and phenbutrazate hydrochloride 20mg] tid with 1000 kcal/d diet | |
| Outcomes | Weight: YesBMI:>5% loss (%):FBS:GHb:Cholesterol:LDL:HDL:TG:SBP:DBP:Side effects: Yes; dizziness (20%), abdominal discomfort and nausea (15%, and dry mouth 5%) | |
| Notes | Funding: NRAbstract/full text: FTLOCF: NRITT: Yes, with attritionAttrition: 9%Blinding: Double‐blindBlinding assessor: UnclearBL comparable: NRJadad score: 1,1,0,BRisk of bias: B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Campbell 1977.
| Methods | Study design: RCTRandomization procedure: adequateAllocation concealment: adequateFollow‐up: 26w | |
| Participants | Country: ScotlandSetting: Community clinicNumber: 66Age: NRSex: NRMedications: 12% insulin; 44% oral treatmentBL wt: NRBL BMI: NRBL GHb: NR | |
| Interventions | Drug: PhentermineDosage: 30mg qdDuration: 26wDiet: NoneComparison: Placebo | |
| Outcomes | Weight: YesBMI:>5% loss (%):FBS:GHb:Cholesterol:LDL:HDL:TG:SBP:DBP:Side effects: Yes; dry mouth and initial sleep disturbance | |
| Notes | Funding: Riker Laboratories supplied the drugAbstract/full text: FTLOCF: NRITT: Yes, with attritionAttrition: 7%Blinding: Double‐blindBlinding assessor: UnclearBL comparable: NRJadad score:2,1,1,ARisk of bias: B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Chiasson 1989.
| Methods | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 36w | |
| Participants | Country: CanadaSetting: NRNumber: 278Age: 52ySex: NRMedications: NRBL wt: 100.5BL BMI: 37BL GHb: I 7.4, C 7.3 | |
| Interventions | Drug: FluoxetineDosage: 60mg qdDuration: 36wDiet: Dietary counselingComparison: Placebo | |
| Outcomes | Weight: YesBMI:>5% loss (%):FBS: YesGHb: YesCholesterol:LDL:HDL:TG:SBP:DBP:Side effects: Yes | |
| Notes | Funding: NRAbstract/full text: ALOCF: NRITT: NRAttrition: NRBlinding: Double‐blindBlinding assessor: UnclearBL comparable: UnclearJadad score: 1,1,0,BRisk of bias: C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Connolly 1995.
| Methods | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 26w | |
| Participants | Country: ScotlandSetting: Diabetic clinicNumber: 30Age: 66Sex: 38%FMedications: Diet onlyBL wt: I 92.0, C 85.1BL BMI: I 32.0, C 31.5BL GHb: I 8.0, C 8.7 | |
| Interventions | Drug: FluoxetineDosage: 60mg qdDuration: 26wDiet: 1200‐1600 kcal/d, 50% CHOComparison: Placebo + diet | |
| Outcomes | Weight: YesBMI: Yes>5% loss (%):FBS: YesGHb: YesCholesterol:LDL:HDL:TG:SBP:DBP:Side effects: Yes | |
| Notes | Funding: Lilly Industries, Ltd.Abstract/full text: FTLOCF: NRITT: Yes, with attritionAttrition: 20%Blinding: Double‐blindBlinding assessor: UnclearBL comparable: UnclearJadad score: 1,1,0,BRisk of bias: C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Crommelin 1974.
| Methods | Study design: RCT Randomization procedure: NRAllocation concealment: NRFollow‐up: 12w | |
| Participants | Country: USASetting: Private practiceNumber: 10Age: Approximately 50Sex: Predominantly femaleMedications: NRBL wt: 85.0BL BMI: NRBL GHb: NR | |
| Interventions | Drug: MazindolDosage: 1mg tidDuration: 12wDiet: Individual diet, no detailsComparison: Placebo + diet | |
| Outcomes | Weight: YesBMI:>5% loss (%):FBS:GHb:Cholesterol:LDL:HDL:TG:SBP:DBP:Side effects: Yes; lightheadedness, dry mouth, vertigo; increased pulse rate noted with I group, not quantified. | |
| Notes | Funding: NRAbstract/full text: FTLOCF: NRITT: Yes, with attritionAttrition: 10%Blinding: Double‐blindBlinding assessor: UnclearBL comparable: YesJadad score: 1,1,1,BRisk of bias: B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Daubresse 1996.
| Methods | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 8w | |
| Participants | Country: BelgiumSetting: Community hospital clinicNumber: 82Age: 52ySex: NRMedications:BL wt: I 93, C 90.9 BL BMI: I 34.5, C 34.0BL GHb: I 8.5, C 8.6 | |
| Interventions | Drug: FluoxetineDosage: 60mg qdDuration: 8wDiet: Low calorie Comparison: Placebo + diet | |
| Outcomes | Weight: YesBMI: Yes>5% loss (%):FBS: YesGHb: YesCholesterol: YesLDL:HDL:TG: YesSBP:DBP:Side effects: Yes | |
| Notes | Funding: NRAbstract/full text: FTLOCF: NRITT: Yes, with attritionAttrition: 17%Blinding: Double‐blindBlinding assessor: UnclearBL comparable: NRJadad score: 1,1,1,BRisk of bias: B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Deerochanawong 2001.
| Methods | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 24w | |
| Participants | Country: NRSetting: NRNumber: 252Age: NRSex: NRMedications: No insulin or acarboseBL wt: I 77, C 77BL BMI: NRBL GHb: NR | |
| Interventions | Drug: OrlistatDosage: 120mg tidDuration: 24wDiet: 600kcal/d deficitComparison: Placebo + diet | |
| Outcomes | Weight: YESBMI:>5% loss (%): YesFBS: YesGHb: YesCholesterol:LDL:HDL:TG:SBP:DBP:Side effects: | |
| Notes | Funding: NRAbstract/full text: ALOCF: NRITT: UnclearAttrition: NRBlinding: Double‐blindBlinding assessor: UnclearBL comparable: NRJadad score: 1,1,0,BRisk of bias: B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Dimitrov 2001.
| Methods | Study design: Pre‐versus‐postRandomization procedure: NAAllocation concealment: NAFollow‐up: 3m | |
| Participants | Country: BulgariaSetting: Academic medical clinicNumber: 12Age: NRSex: NRMedications: NRBL wt: 103.6BL BMI: NRBL GHb: NR | |
| Interventions | Drug: OrlistatDosage: 120mg tidDuration: 3mDiet: NRComparison: Nondiabetic, obese persons | |
| Outcomes | Weight: YesBMI:>5% loss (%):FBS:GHb:Cholesterol: YesLDL: YesHDL:TG:SBP:DBP:Side effects: | |
| Notes | Funding: NRAbstract/full text: ALOCF: NRITT: NAAttrition: NRBlinding: NABlinding assessor: NoBL comparable: NAJadad score: NARisk of bias: NA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Dolecek 1976.
| Methods | Study design: Pre‐versus‐postRandomization procedure: NAAllocation concealment: NAFollow‐up: 2m | |
| Participants | Country: CzechoslovakiaSetting: NRNumber: 32Age: Sex: 78%FMedications: 38% oral agents, 31% insulinBL wt: 97.3BL BMI: NRBL GHb: NR | |
| Interventions | Drug: MazindolDosage: 2mg qd at lunchDuration: 2mDiet: 150g CHOComparison: NA | |
| Outcomes | Weight: YesBMI:>5% loss (%):FBS: YesGHb:Cholesterol: YesLDL:HDL:TG:SBP:DBP:Side effects: Yes; constipation most frequent, also dry mouth, initial anxiety and palpitations | |
| Notes | Funding: NRAbstract/full text: FTLOCF: NRITT: NAAttrition: 6%Blinding: NABlinding assessor: NoBL comparable: NRJadad score: NARisk of bias: NA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Felt 1977.
| Methods | Study design: Cohort with comparison groupRandomization procedure: NAAllocation concealment: NAFollow‐up: 12w | |
| Participants | Country: CzechoslovakiaSetting: NRNumber: 24Age: 47ySex: 83%FMedications: 50% diet only, 50% oral agentBL wt:BL BMI:BL GHb: | |
| Interventions | Drug: MazindolDosage: 1mg bidDuration: 12wDiet: NRComparison: 20 healthy women with normal weight | |
| Outcomes | Weight: YesBMI:>5% loss (%):FBS: YesGHb:Cholesterol: YesLDL:HDL:TG:SBP:DBP:Side effects: Yes; constipation most common, rare headache, insomnia, dizziness | |
| Notes | Funding: NRAbstract/full text: FTLOCF: NRITT: NAAttrition: NRBlinding: NoBlinding assessor: NoBL comparable: NAJadad score: NARisk of bias: NA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Finer 2000.
| Methods | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 12w | |
| Participants | Country: UKSetting: Two hospital‐based diabetes clinicsNumber: 91Age: 54Sex: 53%Medications: 14% diet only; 24% insulinBL wt: I 84.6, C 82.5BL BMI: I 30.6, C 31.0BL GHb: 9.5 | |
| Interventions | Drug: SibutramineDosage: 15mg qdDuration: 12wDiet: 500kcal/d deficitComparison: Placebo + diet | |
| Outcomes | Weight: YesBMI: Yes>5% loss (%):FBS:GHb: YesCholesterol:LDL:HDL:TG:SBP:DBP:Side effects: Yes | |
| Notes | Funding: Knoll Pharmaceutical Co.Abstract/full text: FTLOCF: NRITT: Yes, with attritionAttrition: 9%Blinding: Double‐blindBlinding assessor: UnclearBL comparable: YesJadad score: 1,1,1,BRisk of bias: B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Fujioka 2000.
| Methods | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 24 | |
| Participants | Country: USASetting: Multicenter; medical centersNumber: 175Age: 54Sex: 41%FMedications: Sulfonurea, metformin or diet onlyBL wt: 99.3(1) 98.2 CBL BMI: 34.1(1) 33.8 CBL GHb: 8.4 (1) 8.3 C | |
| Interventions | Drug: SibutramineDosage: 5‐ 20mg qd Duration: 24Diet: 500kcal/d deficitComparison: Placebo + diet | |
| Outcomes | Weight: YesBMI: Yes>5% loss (%): YesFBS: YesGHb: YesCholesterol: YesLDL: YesHDL: YesTG: YesSBP: YesDBP: YesSide effects: Yes | |
| Notes | Funding: Knoll Pharmaceutical Co., USAAbstract/full text: FTLOCF: YesITT: PartialAttrition: 31%Blinding: Double‐blindBlinding assessor: YesBL comparable: YesJadad score: 1,1,1,BRisk of bias: B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Gershberg 1972.
| Methods | Study design: Unclear; 2 parallel groupsRandomization procedure: NRAllocation concealment: NRFollow‐up: 16w | |
| Participants | Country: USASetting: NRNumber: 12Age: NRSex: NRMedications: NRBL wt: ave 143% ideal body weightBL BMI: NRBL GHb: NR | |
| Interventions | Drug: PhentermineDosage: NRDuration: 16wDiet: 1000kal/dComparison: Placebo + diet | |
| Outcomes | Weight: YesBMI:>5% loss (%):FBS: YesCholesterol: YesLDL:HDL:TG: YesSBP:DBP:Side effects: | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Gershberg 1977.
| Methods | Study design: RCTRandomization procedure: UnclearAllocation concealment: NRFollow‐up: 16w | |
| Participants | Country: USASetting: UnclearNumber: 22Age: NRSex: 64%FMedications: No insulinBL wt: I 85.0, C 84.1BL BMI: NRBL GHb: NR | |
| Interventions | Drug: PhentermineDosage: 30mg qdDuration: 16wDiet: 1000kcal/dComparison: Placebo + diet | |
| Outcomes | Weight: YesBMI:>5% loss (%):FBS: YesGHb: Cholesterol: YesLDL:HDL:TG: YesSBP: YesDBP: YesSide effects: Yes; 3 pts complained of irritability and insomnia in the first week of RX; then subsided | |
| Notes | Funding: NRAbstract/full text: FTLOCF: YesITT: CompleteAttrition: 9%Blinding: Double‐blindBlinding assessor: UnclearBL comparable: YesJadad score:1,1,1,BRisk of bias: B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Gokcel 2001.
| Methods | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 26w | |
| Participants | Country: TurkeySetting: Academic medical cetnerNumber: 60Age: 48Sex: 100%FMedications: Sulfonurea and metforminBL wt: 95.6(1) 95.5©BL BMI: 39.3(1) 37.4©BL GHb: 10.0 (I) 9.8© | |
| Interventions | Drug: SibutramineDosage: 10mg bidDuration: 26wDiet: Low calorieComparison: Placebo + diet | |
| Outcomes | Weight: YesBMI:>5% loss (%): YesFBS: YesGHb: YesCholesterol: YesLDL: YesHDL: YesTG: YesSBP: DBP: Side effects: Yes | |
| Notes | Funding: NRAbstract/full text: FTLOCF: NRITT: Yes, with attritionAttrition: 10%Blinding: Double‐blindBlinding assessor: UnclearBL comparable: Similar (no statistics)Jadad score: 1,1,1,BRisk of bias: B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Goldstein 1992.
| Methods | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 36w | |
| Participants | Country: USASetting: NRNumber: 278Age: NRSex: NRMedications: NRBL wt: 100BL BMI: NRBL GHb: I 7.4, C 7.2 | |
| Interventions | Drug: FluoxetineDosage: 60mg qdDuration: 36wDiet: Low calorieComparison: Placebo + diet | |
| Outcomes | Weight: YesBMI:>5% loss (%):FBS: YesGHb: YesCholesterol:LDL:HDL:TG:SBP:DBP:Side effects: | |
| Notes | Funding: Lilly LaboratoriesAbstract/full text: ALOCF: NRITT: NRAttrition: NRBlinding: Double‐blindBlinding assessor: UnclearBL comparable: NRJadad score: 1,1,0,BRisk of bias: C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Goldstein 1995.
| Methods | Companion abstract to Gray 1992 and 1991 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Gray 1992.
| Methods | Study design: RCT Randomization procedure:NRAllocation concealment:UnclearFollow‐up: 24w | |
| Participants | Country: USASetting: Single, university clinicNumber: 48Age: 55Sex: I 67% F, C 42% F Medications: InsulinBL wt: I 106, C 107BL BMI: I 38, C 39.0BL GHb: I 10.5, C 10.2 | |
| Interventions | Drug: FluoxetineDosage: 60mgqdDuration: 24wDiet: 1200 kcal/d American Diabetes Association diet Comparison: Placebo + diet | |
| Outcomes | Weight: YesBMI:>5% loss (%):FBS: YesGHb: YesCholesterol:LDL:HDL:TG:SBP:DBP:Side effects: Yes | |
| Notes | Funding: NRAbstract/full text: FT LOCF: Performed but data NRITT: Yes, with attritionAttrition: 25%Blinding: Double‐blindBlinding assessor: UnclearBL comparable: YesJadad score: 1,1,1,BRisk of bias: B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Griffiths 1995.
| Methods | Study design: Two parallel groups, unclear if randomizedRandomization procedure: UnclearAllocation concealment: UnclearFollow‐up: 12w | |
| Participants | Country: USASetting: NRNumber: 83Age: NRSex: NRMedications: NRBL wt: NRBL BMI: NRBL GHb: NR | |
| Interventions | Drug: SibutramineDosage: 15mg qdDuration: 12wDiet: NRComparison: Placebo | |
| Outcomes | Weight: YesBMI:>5% loss (%):FBS: YesGHb: YesCholesterol: YesLDL:HDL:TG: YesSBP:DBP:Side effects: | |
| Notes | Funding: NRAbstract/full text: ALOCF: NRITT: NRAttrition: NRBlinding: Double‐blindBlinding assessor: UnclearBL comparable: NRJadad: 0,1,0,BRisk of bias: C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Guy‐Grand 2001.
| Methods | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 26w | |
| Participants | Country: FranceSetting: Multicenter, details NRNumber: 193Age: 52Sex: NRMedications: Oral hypoglycemic agentsBL wt: NRBL BMI: 33.7BL GHb: 7.7 | |
| Interventions | Drug: OrlistatDosage: 120mg tidDuration: 26wDiet: low calorieComparison: Placebo + diet | |
| Outcomes | Weight: YesBMI:>5% loss (%):FBS: YesGHb: YesCholesterol:LDL:HDL:TG:SBP:DBP:Side effects: | |
| Notes | Funding: NRAbstract/full text: ALOCF: NRITT: UnclearAttrition: NRBlinding: Double‐blindBlinding assessor: UnclearBL comparable: YesJadad score: 1,1,1,BRisk of bias: C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Halpern 2003.
| Methods | Study design: Multicenter RCTRandomization procedure: Randomization list generated by sponsorAllocation concealment: UnclearFollow‐up: 26w | |
| Participants | Country: Latin AmericaSetting: NRNumber: 338Age: 51Sex: 69%FMedications: No insulin or acarboseBL wt: 89.6BL BMI: 34.6BL GHb: 8.4% | |
| Interventions | Drug: OrlistatDosage: 120mg tidDuration: 24wDiet: 600kcal/d deficit; caloric content: 30% fat, 50% CHO, 20% proteinComparison: Placebo + diet | |
| Outcomes | Weight: YesBMI:>5% loss (%): YesFBS: YesGHb: YesCholesterol: YesLDL: YesHDL: YesTG: YesSBP: YesDBP: YesSide effects: Yes | |
| Notes | Funding: F. Hoffman‐La roche (Basel, Switzerland)Abstract/full text: FT LOCF: YesITT: No; 5 patients withdrawn (no reason stated) after at least one follow‐up measurement; some patients withdrawn for 'noncompliance'Attrition: 18.4%Blinding: Double‐blindBlinding assessor: UnclearBL comparable: YesOther: Must have >60% compliance with placebo during 2w lead‐in to enter studyJadad score: 1,1,0,BRisk of bias: C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Hanefeld 2002.
| Methods | Study design: RCT, multicenterRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 52w | |
| Participants | Country: GermanySetting: Outpatient clinicsNumber: 383Age: 51%FSex: 56yMedications: Diet or sulphonurea; no insulinBL wt: I 98.4, C 99.4BL BMI: I 33.7, C 34.5BL GHb: I 8.6, C 8.6 | |
| Interventions | Drug: OrlistatDosage: 120mg tidDuration: 48wDiet: 600kcal/d deficit Comparison: Diet + Placebo | |
| Outcomes | Weight: YesBMI: Yes>5% loss (%): YesFBS: YesGHb: YesCholesterol: YesLDL:HDL: YesTG: YesSBP: YesDBP: YesSide effects: Yes | |
| Notes | Funding: Hoffman‐La Roche AGAbstract/full text: FTLOCF: NRITT: No; some patients withdrawn for failure to complyAttrition: 31%Blinding: Double‐blindBlinding assessor: UnclearBL comparable: NROther: 22% of study population were not randomized after lead‐in period as did not comply with study processesJadad score: 1,1,1,B Risk of bias: C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Hawkins 2000.
| Methods | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 6m | |
| Participants | Country: NRSetting: Multicenter trial, details unclearNumber: 307Age: NRSex: NRMedications: NRBL wt: NRBL BMI: >27BL GHb: NR | |
| Interventions | Drug: OrlistatDosage: 120mg tidDuration: 24wDiet: HypocaloricComparison: Placebo + diet | |
| Outcomes | Weight: YesBMI:>5% loss (%):FBS: YesGHb: YesCholesterol: YesLDL: YesHDL:TG:SBP: YesDBP: YesSide effects: | |
| Notes | Funding: NRAbstract/full text: ALOCF: NRITT: Yes, with attrition Attrition: 2.5%Blinding: Double‐blindBlinding assessor: UnclearBL comparable: NRJadad score: 1,1,0,BRisk of bias: C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Hendon 1962.
| Methods | Study design: Pre‐versus‐postRandomization procedure: NAAllocation concealment: NAFollow‐up: 2 to 19m | |
| Participants | Country: USASetting: academic endocrine clinicNumber: 40Age: 51ySex: NRMedications: NoneBL wt: 85BL BMI: NRBL GHb: NR | |
| Interventions | Drug: DiethylpropionDosage: 25‐75mg tidDuration: 40wDiet: noneComparison: NA | |
| Outcomes | Weight: YesBMI:>5% loss (%):FBS:GHb:Cholesterol:LDL:HDL:TG:SBP:DBP:Side effects: YesHeadache, lightheaded, nausea; no incidence given | |
| Notes | Funding: NRAbstract/full text: FTLOCF: NRITT: Yes, with attritionAttrition: 25%Blinding assessor: NoBL comparable: NAJadad score: NARisk of bias: NA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Hollander 1998.
| Methods | Companion abstract to Hollander 1998a | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Hollander 2001.
| Methods | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 1y | |
| Participants | Country: USASetting: NRNumber: 503Age: NRSex: NRMedications: MetforminBL wt: NRBL BMI: >28BL GHb: NR | |
| Interventions | Drug: OrlistatDosage: 120mg tidDuration: 1yDiet: Mildly reduced caloric Comparison: Placebo + diet | |
| Outcomes | Weight: YesBMI:>5% loss (%): YesFBS: YesGHb: YesCholesterol: LDL: YesHDL: YesTG: YesSBP: YesDBP: YesSide effects: | |
| Notes | Funding: NRAbstract/full text: ALOCF: YesITT: CompleteAttrition: NRBlinding: Double‐blindBlinding assessor: UnclearBL comparable: UnclearJadad score: 1,1,0,BRisk of bias: C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kaukua 2004.
| Methods | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 1 year | |
| Participants | Country: FinlandSetting: Finnish primary medical care centersNumber: 236Age: 54 Sex: 70%F (calculated weighted)Medications: Diet only BL wt: I 100.8, C 98.1BL BMI: I 35.7, C 35.6 BL GHb: NR | |
| Interventions | Drug: SibutamineDosage: 15 mg qdDuration: 1 yearDiet: 700 Kcal/d deficit diet Comparison: Placebo and 700 Kcal/d deficit diet | |
| Outcomes | Weight: YBMI: >5% loss (%): FBS: GHb: Y Cholesterol: LDL: HDL: TG: SBP: YDBP: Y Side effects: | |
| Notes | Funding: Knoll Laboratories Abstract/full text: FTLOCF: Y ITT: Participants could be withdrawn for protocol violation; numbers unclear Attrition: 8%Blinding: Double blind Blinding assessor: UnclearBL comparable: NRJadad Score: 1,2,0,BQuality category: C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kelley 1997.
| Methods | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 57w | |
| Participants | Country: USASetting: MulticenterNumber: 322Age: NRSex: NRMedications: SulfonureasBL wt: NRBL BMI: NRBL GHb: NR | |
| Interventions | Drug: OrlistatDosage: 120mg tidDuration: 52wDiet: 500kcal/d deficitComparison: Placebo + diet | |
| Outcomes | Weight: YesBMI:>5% loss (%):FBS: YesGHb: YesCholesterol: YesLDL: YesHDL: TG: YesSBP:DBP:Side effects: Yes | |
| Notes | Funding: Hoffman‐LaRocheAbstract/full text: ALOCF: NRITT: Yes, with attritionAttrition: I 15%, C 28%Blinding: Double‐blindBlinding assessor: UnclearBL comparable: NRJadad score: 1,1,0,BRisk of bias: B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kelley 2002.
| Methods | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 52w | |
| Participants | Country: USASetting: Multicenter; academic medical centersNumber: 550Age: 58Sex: 57%FMedications: Insulin +/‐ oral agent (excluding thazolidindiones)BL wt: I 101.8, C 102.0 BL BMI: I 35.6, C 35.8BL GHb: I 9.0, C 9.0 | |
| Interventions | Drug: OrlistatDosage: 120mg bidDuration: 52wDiet: 500kcal/d deficitComparison: Placebo + diet | |
| Outcomes | Weight: YesBMI:>5% loss (%): YesFBS: YesGHb: YesCholesterol: YesLDL: YesHDL: YesTG: YesSBP: YesDBP: YesSide effects: Yes | |
| Notes | Funding: Hoffman‐LaRocheAbstract/full text: FTLOCF: YesITT: CompleteAttrition: 52%Blinding: Double‐blindBlinding assessor: UnclearBL comparable: YesJadad score: 1,1,1,BRisk of bias: B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kelley 2004.
| Methods | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 26 weeks | |
| Participants | Country: USA Setting: Academic center; community recruitmentNumber: 39Age: 51Sex: 67Medications: Oral agents or diet; oral agents withdrawn 1 month prior to interventionBL wt: I 99, C 102BL BMI: I 34.0, C 35.9BL GHb: I 8.1, C7.8 | |
| Interventions | Drug: Orlistat Dosage: 120mg tidDuration: 3 monthsDiet: 500 calorie deficit; <=30% fat; activity encouragedComparison: 500 calorie deficit; <=30% fat; activity encouraged | |
| Outcomes | Weight: YBMI: Y>5% loss (%): FBS: YGHb: YCholesterol: YLDL: YHDL: YTG:SBP:DBP:Side effects: Y | |
| Notes | Funding: Roche Laboratories Abstract/full text: FT LOCF: No ITT: Partial Attrition: 25% Blinding: Double blind Blinding pt: Y Blinding assessor: Unclear Blinding provider: Unclear BL comparable: Y | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kutnowski 1990.
| Methods | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 8w | |
| Participants | Country: BelgiumSetting: Multicenter, no detailsNumber: 134Age: NRSex: 66%FMedications: NR; NIDDM and IGT patients combinedBL wt: NRBL BMI: I 34.1, C 34.1 BL GHb: NR | |
| Interventions | Drug: Fluoxetine Dosage: 60mg qdDuration: 8wDiet: 1400kcal/dComparison: Placebo + diet | |
| Outcomes | Weight:BMI: Yes>5% loss (%):FBS: YesGHb: YesCholesterol: YesLDL:HDL:TG: YesSBP:DBP:Side effects: | |
| Notes | Funding: YesAbstract/full text: ALOCF: YesITT: CompleteAttrition: 14.2%Blinding: Double‐blindBlinding assessor: UnclearBL comparable: YesJadad score: 1,1,1,BRisk of bias: B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kutnowski 1992.
| Methods | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 9w | |
| Participants | Country: BelgiumSetting: Multicenter; details UnclearNumber: 97Age: 51Sex: 47%FMedications:BL wt: I 91.0, C 92.3BL BMI: I 34.4, C 34.3BL GHb: NR | |
| Interventions | Drug: FluoxetineDosage: 60mg qdDuration: 9wDiet: Low calorie Comparison: Placebo + diet | |
| Outcomes | Weight:BMI: Yes>5% loss (%):FBS: YesGHb: YesCholesterol:LDL: YesHDL:TG: YesSBP:DBP:Side effects: | |
| Notes | Funding: Eli LillyAbstract/full text: FTLOCF: YesITT: CompleteAttrition: 12.4%Blinding: Double‐blindBlinding assessor: NRBL comparable: YesJadad score: 1,1,1,BRisk of bias: B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
le Roux 2001.
| Methods | Study design: Pre‐versus‐postRandomization procedure: NAAllocation concealment: NAFollow‐up: 6m | |
| Participants | Country: EnglandSetting: NRNumber: 7Age: NRSex: NRMedications: NRBL wt: NRBL BMI: 40.2BL GHb: 8.7 | |
| Interventions | Drug: OrlistatDosage: 120mg tidDuration: 6mDiet: UnclearComparison: NA | |
| Outcomes | Weight:BMI: Yes>5% loss (%):FBS:GHb: YesCholesterol: YesLDL: YesHDL:TG: YesSBP:DBP:Side effects: | |
| Notes | Funding: NRAbstract/full text: ALOCF: NAITT: NAAttrition: NRBlinding: NABlinding assessor: NoBL comparable: NAJadad score: NARisk of bias: NA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Lindgarde 2000.
| Methods | Study design: RCT; 26% of total study population had type 2 diabetesRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 54w | |
| Participants | Country: SwedenSetting: 33 primary care centersNumber: 99Age: 54y (whole population)Sex: 64% (whole population)Medications: NRBL wt: NR for diabetic populationBL BMI: NR for diabetic populationBL GHb: I 8.7, C 10.0 | |
| Interventions | Drug: OrlistatDosage: 120mg tidDuration: 52wDiet: 600kcal/d deficitComparison: Placebo + diet | |
| Outcomes | Weight: YesBMI:>5% loss (%): YesFBS: YesGHb: YesCholesterol:LDL:HDL:TG:SBP:DBP:Side effects: Yes | |
| Notes | Funding: Roche AB, Stockholm, SwedenAbstract/full text: FTLOCF: NRITT: Yes, with attritionAttrition: 14%Blinding: Double‐blindBlinding assessor: UnclearBL comparable: Yes (for whole population)Jadad score: 1,1,1,B Risk of bias: B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Martin 2001.
| Methods | Study design: Cohort with comparison groupRandomization procedure: NAAllocation concealment: NAFollow‐up: 6m | |
| Participants | Country: Northern IrelandSetting: Obesity clinicNumber: 55Age: NRSex: 51%FMedications: NRBL wt: I: 102.8, C 101.1BL BMI: NRBL GHb: I 37.8, C 42 | |
| Interventions | Drug: OrlistatDosage: NRDuration: 26wDiet: Dietary adviceComparison: No orlistat | |
| Outcomes | Weight: YesBMI:>5% loss (%): YesFBS:GHb:Cholesterol:LDL:HDL:TG:SBP:DBP:Side effects: | |
| Notes | Funding: NRAbstract/full text: A LOCF: NoITT: Yes, with attritionAttrition: 59%Blinding: NRBlinding assessor: NRBL comparable: NoOther: Intervention group was persons who lost >‐2kg in 4w lead‐in periodJadad score: NARisk of bias: C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
McNulty 2003.
| Methods | Study design: RCTRandomization procedure: UnclearAllocation concealment: UnclearFollow‐up: 52w | |
| Participants | Country: Multicenter: England, Canada, France, BelgiumSetting: NRNumber: 195Age: 49Sex: 56%FMedications: MetforminBL wt: 103.3BL BMI: 36.3BL GHb: 9.6 | |
| Interventions | Drug: SibutramineDosage: 15 or 20 mg qdDuration: 52wDiet: Standard dietary adviceComparison: Dietary advice + placebo | |
| Outcomes | Weight: YesBMI: Yes>5% loss (%):FBS: YesGHb: YesCholesterol: YesLDL: YesHDL: YesTG: YesSBP: YesDBP: YesSide effects: Yes | |
| Notes | Funding: Abbott Laboratories Abstract/full text: FTLOCF: NRITT: NRAttrition: 26%Blinding: Double‐blind Blinding assessor: UnclearBL comparable: YesJadad score : 1,1,0,BRisk of bias: C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Mendoza‐Guadarra2000.
| Methods | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 26w | |
| Participants | Country: MexicoSetting: obesity clinicNumber: 30Age: 51Sex: 60%FMedications: NRBL wt: NRBL BMI: I 31.3, C 30.6BL GHb: NR | |
| Interventions | Drug: OrlistatDosage: 120mg tidDuration: 26wDiet: 500kcal/d deficitComparison: Placebo + diet | |
| Outcomes | Weight: BMI: Yes>5% loss (%):FBS:GHb:Cholesterol:LDL:HDL:TG:SBP:DBP:Side effects: | |
| Notes | Funding: NRAbstract/full text: ALOCF: NRITT: UnclearAttrition: NRBlinding: Double‐blindBlinding assessor: UnclearBL comparable: NRJadad score: 1,1,0,BRisk of bias: C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Miles 2002.
| Methods | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 52w | |
| Participants | Country: USASetting: Multicenter; UnclearNumber: 505Age: 53ySex: 48%FMedications: Metformin +/‐ sulfonureaBL wt: I 101.1, C 102.1BL BMI: I 35.2, C 35.6BL GHb: I 8.8, C 8.9 | |
| Interventions | Drug: OrlistatDosage: 120mg tidDuration: 52wDiet: 500kcal/d deficitComparison: Placebo + diet | |
| Outcomes | Weight:BMI:>5% loss (%):FBS:GHb:Cholesterol:LDL:HDL:TG:SBP:DBP:Side effects: | |
| Notes | Funding: Hoffman‐LarocheAbstract/full text: FTLOCF: YesITT: CompleteAttrition: 40%Blinding: Double‐blindBlinding assessor: UnclearBL comparable: YesJadad score: 1,1,1,BRisk of bias: B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Montenero 1964.
| Methods | Study design: Two study groups; pre‐versus‐postRandomization procedure: NAAllocation concealment: NAFollow‐up: 20‐240d | |
| Participants | Country: ItalySetting: NRNumber: 50Age: 54Sex: 65%FMedications: 17% insulin; 67% oral agentsBL wt: I 97 , C 92 BL BMI: NRBL GHb: NR | |
| Interventions | Drug: DiethylpropionDosage: 2‐3qd (dosage not specified)Duration: 20‐240dDiet: 1000‐1800kcal/dComparison: Both groups got same diet and dosage diethylpropion; group A was on hypoglycemic agents, group B was diet controlled | |
| Outcomes | Weight: YesBMI:>5% loss (%):FBS: YesGHb:Cholesterol:LDL:HDL:TG:SBP:DBP:Side effects: Yes; per Pina: 4/50 quit for SE, including general malaise, epigastric disturbance, and dermatitis. No untoward effects in person with HT and CVD; normal LFT and renal function | |
| Notes | Funding: NRAbstract/full text: FTLOCF: NRITT: Yes, with attritionAttrition: 8%Blinding assessor: NRBL comparable: NRJadad score: NARisk of bias: NA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
O'Kane 1994.
| Methods | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 52w | |
| Participants | Country: United KingdomSetting: Diabetic clinicNumber: 19Age: 57Sex: 68%FMedications: 37% diet only; 63% on oral agents; no insulinBL wt: I 97.5, C 97.8BL BMI: I 36.8, C 35.8BL GHb: I 9.7, C 9.2 | |
| Interventions | Drug: FluoxetineDosage: 60mg qdDuration: 52wDiet: Usual Comparison: Placebo | |
| Outcomes | Weight: YesBMI: >5% loss (%):FBS: YesGHb: YesCholesterol: YesLDL: HDL:TG: YesSBP:DBP:Side effects: Yes | |
| Notes | Funding: Lilly Industries LtdAbstract/full text: FTLOCF: NRITT: Yes, with attritionAttrition: 16%Blinding: Double‐blindBlinding assessor: NRBL comparable: NRJadad score: 1,1,1,BRisk of bias: B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Peirce 1999.
| Methods | Study design: RCTRandomization procedure: NRAllocation concealment: Unclear Follow‐up: 12w | |
| Participants | Country: USASetting: NRNumber: 35Age: 18‐60ySex: NRMedications: Diet onlyBL wt: NRBL BMI: 28‐40BL GHb: NR | |
| Interventions | Drug: SibutramineDosage: 15mg qdDuration: 12wDiet: Dietary adviceComparison: Placebo | |
| Outcomes | Weight: YesBMI:>5% loss (%):FBS:GHb: Yes Cholesterol:LDL:HDL:TG:SBP:DBP:Side effects: | |
| Notes | Funding: Knoll Pharmaceutical Co. Abstract/full text: A LOCF: NR ITT: NR Attrition: NR Blinding: Double‐blind Blinding assessor: NR BL comparable: NR Jadad score: 1,1,0,B Risk of bias: C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Redmon 2003.
| Methods | Study design: RCTRandomization procedure: Random allocation schedule provided by the study statisticianAllocation concealment: AdequateFollow‐up: 1 year | |
| Participants | Country: USASetting: Academic medical centerNumber: 61Age: 54 Sex: 46%FMedications: No insulinBL wt: I 109.1, C 112.4 BL BMI: I 37.8, C 38.6 BL GHb: I 8.1, C 8.2 | |
| Interventions | Drug: SibutamineDosage: 10‐15mg dailyDuration: 1 yearDiet: 500‐1000 kcal/d deficit diet with some meal replacements; physical activity counseling and prescriptionComparison: 500‐1000 kcal/d deficit diet; physical activity counseling and prescription | |
| Outcomes | Weight: YBMI: Y >5% loss (%): Y FBS: YGHb: YCholesterol: YLDL: YHDL: YTG: YSBP: Y. DBP: Y. Side effects: Y | |
| Notes | Funding: Abbott laboratories and Slim Fast Nutrition InstituteAbstract/full text: FTLOCF: YLOCF: YITT: ReportedAttrition: 8%Blinding: NRBlinding assessor: NRBL comparable: YJadad Score: 1,0,1,B Quality category: C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Rissanen 1999a.
| Methods | Study design: RCTRandomization procedure: NRAllocation concealment: Unclear Follow‐up: 52w | |
| Participants | Country: FinlandSetting: NRNumber: 236Age: 18‐60ySex: NRMedications: Diet onlyBL wt: NRBL BMI: >28BL GHb: NR | |
| Interventions | Drug: SibutramineDosage: 15mg qdDuration: 52wDiet: 700 kcal/d deficit dietComparison: Placebo + 700 kcal/d deficit diet | |
| Outcomes | Weight: YesBMI:>5% loss (%): YesFBS:GHb: Yes Cholesterol: LDL:HDL: YesTG: YesSBP:DBP:Side effects: | |
| Notes | Funding: NR Abstract/full text: A LOCF: NR ITT: NR Attrition: 11% Blinding: Double‐blind Blinding assessor: NR BL comparable: NR Jadad score: 1,1,0,B Risk of bias: C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Sanders 1976.
| Methods | Study design: Two groups, unclear if randomized; cross‐over q6wRandomization procedure: NRAllocation concealment: NRFollow‐up: 6w | |
| Participants | Country: AustraliaSetting: NRNumber: 18Age: 40‐65Sex: 80%FMedications: 11% diet, 61% oral agents, 28% insulinBL wt: NRBL BMI: NRBL GHb: NR | |
| Interventions | Drug: MazindolDosage: 2mg qdDuration: 6wDiet: Dietary advice for 8w before onset of drug treatmentComparison: Placebo | |
| Outcomes | Weight: YesBMI:>5% loss (%):FBS: YesGHb:Cholesterol:LDL:HDL:TG:SBP:DBP:Side effects: Yes; "stimulation", headache | |
| Notes | Funding: NRAbstract/full text: FTLOCF: NRITT: Yes, with attritionAttrition: 17%Blinding: Double‐blindBlinding assessor: UnclearBL comparable: BLJadad score: NARisk of bias: B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Segal 2000.
| Methods | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 52w | |
| Participants | Country: USASetting: NRNumber: 245Age: NRSex: NRMedications: Oral sulfonureasBL wt: NRBL BMI: NRBL GHb: NR | |
| Interventions | Drug: OrlistatDosage: 120mg tidDuration: 52wDiet: low calorieComparison: Placebo; unclear if dietary intervention | |
| Outcomes | Weight: YesBMI:>5% loss (%):FBS:GHb:Cholesterol:LDL:HDL:TG:SBP:DBP:Side effects: | |
| Notes | Funding: Hoffman La Roche, NJ, USAAbstract/full text: ALOCF: NRITT: NRAttrition: NRBlinding: Double‐blindBlinding assessor: UnclearBL comparable: NRJadad score: 1,1,0,BRisk of bias: C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Serrano‐Rios 2001.
| Methods | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 26w | |
| Participants | Country: SpainSetting: Multicenter; no other detailsNumber: 237Age: NRSex: NR Medications: Sulfonureas and/or metforminBL wt: NRBL BMI: >27BL GHb: NR | |
| Interventions | Drug: OrlistatDosage: 120mg tidDuration: 24wDiet: HypocaloricComparison: Placebo + diet | |
| Outcomes | Weight: YesBMI: Yes>5% loss (%): YesFBS: YesGHb: YesCholesterol: LDL:HDL:TG:SBP: YesDBP: YesSide effects: Yes | |
| Notes | Funding: NRAbstract/full text: ALOCF: NRITT: NRAttrition: NRBlinding: Double‐blindBlinding assessor: UnclearBL comparable: NRJadad score: 1,1,0,BRisk of bias: C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Serrano‐Rios 2002.
| Methods | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 24w | |
| Participants | Country: Europe Setting: Multicenter Number: 134 Age: 53.6 Sex: 58%F Medications: Sulfonylurea BL wt: I 92.0, C 94.2 BL BMI: NR BL GHb: I 9.0, C 9.5 | |
| Interventions | Drug: SibutramineDosage: 15mg qdDuration: 24wDiet: Low calorieComparison: Placebo + diet | |
| Outcomes | Weight:BMI:>5% loss (%):FBS:GHb:Cholesterol:LDL:HDL:TG:SBP:DBP:Side effects: | |
| Notes | Funding: Knoll Pharmaceutical Co., UKAbstract/full text: FTLOCF: YesITT: CompleteAttrition: 18%Blinding: Double‐blindBlinding assessor: UnclearBL comparable: YesJadad score: 1,1,1,BRisk of bias: B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Silverstone 1966.
| Methods | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 26w | |
| Participants | Country: EnglandNumber: 50Age: 56Sex: 80%FMedications: 56% diet only; no insulinBL wt: I 84.4, C 89.4BL BMI: NRBL GHb: NR | |
| Interventions | Drug: DiethylpropionDosage: 75mg qd; 40% 3w on, 3w off; 60% 5w on, 5w off Duration: 26wDiet: 1000kcal/dComparison: Placebo + diet | |
| Outcomes | Weight: YesBMI:>5% loss (%):FBS:GHb:Cholesterol:LDL:HDL:TG:SBP:DBP:Side effects: Yes; dry mouth in 2/15 pts | |
| Notes | Funding: Merrell‐National Laboratories, Ltd. supplied drugAbstract/full text: FTLOCF: NRITT: Yes, with attritionAttrition: 20%Blinding: Double‐blindBlinding assessor: YesBL comparable: NRJadad score: 1,1,1,BRisk of bias: B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Sircar 2001.
| Methods | Sircar 2001Multiple pub: No | |
| Participants | Study design: Pre‐versus‐postRandomization procedure: NAAllocation concealment: NAFollow‐up: 12w | |
| Interventions | Country: IndiaSetting: UnclearNumber: 27Age: 44.7Sex: 89%Medications: NRBL wt: 75.4BL BMI: 32.1BL GHb: 9.6 | |
| Outcomes | Drug: SibutramineDosage: 10‐15mg qdDuration: 12wDiet: Prescribed; Unclear typeComparison: NA | |
| Notes | Weight: YesBMI:>5% loss (%):FBS:GHb: YesCholesterol:LDL:HDL:TG:SBP:DBP:Side effects: Yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Slama 1978.
| Methods | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 12w | |
| Participants | Country: FranceSetting: NRNumber: 46Age: 48ySex: 38%FMedications: Diet onlyBL wt: I 84.9, C 81.0BL BMI: NRBL GHb: NR | |
| Interventions | Drug: MazindolDosage: 2mg qdDuration: 12wDiet: 1000kcal/dComparison: Diet + placebo | |
| Outcomes | Weight: YesBMI:>5% loss (%):FBS: YesGHb:Cholesterol: YesLDL:HDL:TG: YesSBP:DBP:Side effects: | |
| Notes | Funding: NRAbstract/full text: FT LOCF: NRITT: Yes, with attritionAttrition: 20%Blinding: Double‐blindBlinding assessor: UnclearBL comparable: NRJadad score: 1,1,0,BRisk of bias: B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Stoa‐Birketvedt 1998.
| Methods | Study design: RCTRandomization procedure: Randomized according to BMI; details unclearAllocation concealment: UnclearFollow‐up: 12w | |
| Participants | Country: NorwaySetting: Hospital clinicNumber: 62Age: 48YSex: 33%FMedications: 49% on oral agentsBL wt: I 103.9, C 102.0BL BMI: I 33.8, C 34.0BL GHb: NR | |
| Interventions | Drug: CimetidineDosage: 400mg tidDuration: 12wDiet: Usual diet and activityComparison: Placebo + usual diet and activity | |
| Outcomes | Weight: YesBMI: Yes>5% loss (%):FBS: YesGHb: YesCholesterol: YesLDL:HDL: YesTG: YesSBP: YesDBP: YesSide effects: Yes; 10% diarrhea, 5% each of abdominal pain, vomiting and arthralgia | |
| Notes | Funding: Norwegian Research council, The Novo Nordic Foundation, The Norwegian Diabetes AssociationAbstract/full text: FTLOCF: NRITT: Yes, with attritionAttrition: 19%Blinding: Double blindBlinding assessor: UnclearBL comparable: YesJadad Score: 1,1,1,BRisk of bias: B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Tankova 2003.
| Methods | Study design: RCT Randomization procedure: NR Allocation concealment: Unclear Follow‐up: 3 months | |
| Participants | Country: BulgariaSetting: Clinical Center of Endocrinology and Gerontology, Medical University‐SofiaNumber: 95Age: 45.8 Sex: 53.7 % female Medications: 70% oral agents, 30% dietBL wt: I 95.3, C 91.7 BL BMI: I 33.9, C 34.2 BL GHb: I 7.4, C 7.3 | |
| Interventions | Drug: SibutamineDosage: 10 mg qd for first month; average daily dosage over 3 months 12.7 mg qdDuration: 3 monthsDiet: Low calorie dietComparison: Low calorie diet | |
| Outcomes | Weight: YBMI: NR >5% loss (%): FBS: GHb: YCholesterol: YLDL: HDL: TG: Y SBP: YDBP: Side effects: Y | |
| Notes | Funding: NRAbstract/full text: FTLOCF: NITT: Y Attrition: NR Blinding: Open‐labelBlinding assessor: NRBL comparable: YJadad Score: 1,0,0,B Quality category: C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Tong 2002.
| Methods | Study design: Pre‐versus‐postRandomization procedure: NAAllocation concealment: NAFollow‐up: 26w | |
| Participants | Country: ChinaSetting: NRNumber: 27Age: 36Sex: 61%FMedications: NRBL wt: 93.2BL BMI: 34.2BL GHb: 8.5 | |
| Interventions | Drug: OrlistatDosage: 120mg tidDuration: 26wDiet: NoneComparison: NA | |
| Outcomes | Weight: YesBMI: Yes>5% loss (%):FBS: YesGHb: YesCholesterol: YesLDL: YesHDL: YesTG: YesSBP: YesDBP: YesSide effects: Yes | |
| Notes | Funding: NRAbstract/full text: FTLOCF: NRITT: NAAttrition: NRBlinding: NABlinding assessor: NABL comparable: NAJadad score: NARisk of bias: NA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Vargas 1994.
| Methods | Vargas 1994Multiple pub:No | |
| Participants | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 12w | |
| Interventions | Country: USASetting: NRNumber: 18Age: NRSex: NRMedications: BRBL wt: NRBL BMI: NRBL GHb: NR | |
| Outcomes | Drug: SibutramineDosage: 20‐30mg qdDuration: 12wDiet: NRComparison: Placebo | |
| Notes | Weight: YesBMI:>5% loss (%):FBS: YesGHb:Cholesterol:LDL:HDL:TG:SBP:DBP:Side effects: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Versari 2000.
| Methods | Study design: Pre‐versus‐postRandomization procedure: NAAllocation concealment: UnclearFollow‐up: 45d | |
| Participants | Country: NRSetting: NRNumber: 21Age: 55ySex: 80%FMedications: 48% on oral agentsBL wt: NRBL BMI: 36.3BL GHb: NR | |
| Interventions | Drug: OrlistatDosage: 120mg bid to tidDuration: 45dDiet: 1500kcal/dComparison: NA | |
| Outcomes | Weight:BMI: Yes>5% loss (%):FBS: YesGHb: YesCholesterol: YesLDL: YesHDL: YesTG: YesSBP:DBP:Side effects: | |
| Notes | Funding: NRAbstract/full text: ALOCF: NRITT: NAAttrition: NRBlinding: NABlinding assessor: NoBL comparable: NAJadad score: NARisk of bias: NA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Wang 2003.
| Methods | Study design: RCTRandomization procedure: Randomization tableAllocation concealment: Unclear Follow‐up: 24w | |
| Participants | Country: China Setting: ClinicNumber: 63Age: 41Sex: 47.6Medications: 100% oral agentsBL wt: I 85.0, C 83.0BL BMI: I 30.0, C 31.0 BL GHb: I 8.3, C 8.2 | |
| Interventions | Drug: Orlistat Dosage: 120mg bid to tidDuration: 24wDiet: NRComparison: Placebo + diet | |
| Outcomes | Weight: YBMI: Y>5% loss (%): YFBS: YGHb: YCholesterol: YLDL: YHDL: YTG: YSBP: YDBP: YSide effects: NR | |
| Notes | Funding: NRAbstract/full text: FTLOCF: NRITT: 2 patients withdrawn (no reason stated)Attrition: 3.2%Blinding: NRBlinding pt: YesBlinding assessor: UnclearBlinding provider: UnclearBL comparable: yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Williams 1968.
| Methods | Study design: RCTRandomization procedure: random number tableAllocation concealment: adequateFollow‐up: 8w | |
| Participants | Country: EnglandSetting: UnclearNumber: 63Age: 58Sex: 89%FMedications: NoneBL wt: NRBL BMI: NRBL GHb: NR | |
| Interventions | Drug: DiethylpropionDosage: 75mg qdDuration: 8wDiet: Low fatComparison: Placebo + diet | |
| Outcomes | Weight: YesBMI:>5% loss (%):FBS:GHb:Cholesterol:LDL:HDL:TG:SBP:DBP:Side effects: Yes; no SE on drug; one with placebo | |
| Notes | Funding: John Wyeth and BrotherAbstract/full text: FTLOCF: NoITT: Yes, with attritionAttrition: 22%Blinding: Double‐blindBlinding assessor: NRBL comparable: NRJadad score: 2,1,1,ARisk of bias: B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Wise 1989.
| Methods | Study design: RCTRandomization procedure: NRAllocation concealment: UnclearFollow‐up: 12w | |
| Participants | Country: UKSetting: NRNumber: 190Age: 51ySex: 73%FMedications: NRBL wt: 96BL BMI: 35BL GHb: 9.6 | |
| Interventions | Drug: FluoxetineDosage: NRDuration: 12wDiet: NRComparison: Placebo | |
| Outcomes | Weight: YesBMI:>5% loss (%):FBS: YesGHb: YesCholesterol:LDL:HDL:TG:SBP:DBP:Side effects: Yes | |
| Notes | Funding: Lilly Research Centre, Surrey, UKAbstract/full text: ALOCF: NRITT: NRAttrition: NRBlinding: Double‐blindBlinding assessor: UnclearBL comparable: NROther: Demographic data is combined group of persons with type 2 diabetes and IGT; GHb results are for people with diabetes onlyJadad score: 1,1,0,BRisk of bias: C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Zaletel 2002.
| Methods | Study design: Pre‐versus‐postRandomization procedure: NAAllocation concealment: NAFollow‐up: Unclear; second phase was 6m | |
| Participants | Country: SloveniaSetting: UnclearNumber: 31Age: 54Sex: 58Medications: NRBL wt: NRBL BMI: 38.1BL GHb: NR | |
| Interventions | Drug: OrlistatDosage: 120mg tidDuration: UnclearDiet: UnclearComparison: NA | |
| Outcomes | Weight: YesBMI:>5% loss (%):FBS: YesGHb: YesCholesterol: YesLDL:HDL:TG: YesSBP: YesDBP:Side effects: | |
| Notes | Funding: NRAbstract/full text: ALOCF: NAITT: NAAttrition: 6%Blinding: NABlinding assessor: NoBL comparable: NAJadad score: NARisk of bias: NA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Zelissen 1992.
| Methods | Study design: RCTRandomization procedure: Computer‐generated sequence numberingAllocation concealment: UnclearFollow‐up: 26w | |
| Participants | Country: The NetherlandsSetting: Single, hospital clinicNumber: 20Age: 50Sex: 60%FMedications: None or oral agentBL wt: I 97, C 106 BL BMI: >=29BL GHb: I 9.6, C 9.1 | |
| Interventions | Drug: FluoxetineDosage: 60mg qdDuration: 26wDiet: 1000kcal/dComparison: Placebo + diet | |
| Outcomes | Weight: YesBMI:>5% loss (%): YesFBS: YesGHb: YesCholesterol:LDL:HDL:TG:SBP:DBP:Side effects: | |
| Notes | Funding: Eli Lilly, Nieuwegein, The Netherlands, supplied fluoxetineAbstract/full text: FTLOCF: NRITT: CompleteAttrition: 0%Blinding: NRBlinding assessor: NRBL comparable: NRJadad score: 2,0,1,BRisk of bias: B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Abbreviations A, abstract; BMI, body mass index (kg/m2); C, comparison group; CHO, carbohydrate; F, female; FBS, fasting blood sugar; d, day; FT, full text; GHb, glycated hemoglobin; I, intervention group; ITT, intention to treat; LOCF, last outcome carried forward; NA, not applicable; NR, not reported; qd, daily; RCT, randomized, controlled trial; y, year; w, weeks;
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anchors 1997 | No diabetes population |
| Apfelbaum 1999 | No diabetes population |
| Astrup 1985 | No diabetes population |
| Astrup 1992 | No diabetes population |
| Boneva 2002 | No weight outcomes for diabetes subgroup |
| Bowen 2000 | No diabetes population |
| Bray 1996 | No diabetes population |
| Bray 1999 | No diabetes population |
| Breum 1995 | IGT and type 2 diabetes; can't separate the two populations |
| Broom 2001 | No diabetes subgroup |
| Chengappa 2001 | Goal is not weight loss |
| Conte 1973 | No diabetes population |
| Daly 1993 | No diabetes population |
| Darga 1991 | Only 2 persons with diabetes |
| Davison 1999 | No diabetes subpopulation outcome data |
| Derby 1999 | No diabetes population |
| Drent 1995 | No diabetes population |
| Duncan 1960 | No diabetes population |
| Edmonds 1983 | Goal is treatment of diabetic neuropathic edema, not weight loss |
| Egart 1979 | No subgroup analysis |
| Enzi 1976 | No diabetes population |
| Fanghanel 2000 | No diabetes population |
| Faria 2001 | No diabetes subgroup |
| Fava 1999 | Not a weight loss study and no diabetes subgroup |
| Fernandez‐Soto 1995 | No diabetes population |
| Finer 2000e | No diabetes population |
| Generali 2001 | Review |
| Gokcel 2002a | No diabetes population |
| Gokcel 2002b | Only 10% with diabetes; no subgroup analysis |
| Goldstein 1993 | No diabetes population |
| Goldstein 1994 | No diabetes population |
| Greenway 1999e | No diabetes population |
| Hadler 1967 | No diabetes population |
| Haller 2000 | Review |
| Hanefeld 2002b | No weight outcomes |
| Hanotin 1998 | No diabetes population |
| Hansen 2001 | No diabetes population |
| Hauptman 1992 | No diabetes population |
| Hauptman 2000 | No diabetes population |
| Heal 1998 | No diabetes patients |
| Heath 1999 | Duplicate abstract with Rissanen |
| Heymsfield 2000 | Meta‐analysis; no primary data |
| Hill 1999 | No diabetes population |
| Hollenbeck 1987 | No weight loss drug |
| Inoue 1992 | No diabetes population |
| Inoue 1995 | No diabetes population |
| Jacob 2002 | No weight outcomes |
| James 1997 | No diabetes population |
| James 2000 | No diabetes population |
| Jones 1995 | No diabetes‐specific data |
| Langlois 1974 | No diabetes population |
| Lee 1999a | IGT population only; no diabetes population |
| Lee 1999b | IGT population; no diabetes population |
| Lustman 2000 | Goal is not weight loss |
| Maetzel 2002 | No weight outcomes, is an economic study |
| Maheux 1997 | Goal is not weight loss |
| Malchow‐Moller 1981 | No diabetes population |
| Marcus 1990 | No diabetes population |
| McLaughlin 2001 | No diabetes population |
| McMahon 2000 | No diabetes population |
| Meier 1992 | Goal is to decrease body fat, not weight loss |
| Michelson 1999 | No diabetes subgroup analysis |
| Miles 2001 | No weight outcomes |
| Miles 2002b | No weight outcomes |
| Pasquali 1987 | No diabetes population |
| Pedrinola 1996 | No diabetes population |
| Pijl 2000 | Goal is to decrease body fat, not weight loss |
| Rasmussen 1993 | No diabetes population |
| Rissanen 1999b | No weight outcomes |
| Rissanen 2000a | No weight outcomes |
| Rissanen 2000b | No weight outcomes |
| Rolls 1998 | No diabetes population |
| Rosenfalck 2002 | No diabetes population |
| Samsa 2001 | No diabetes specific data |
| Sax 1991 | No diabetes population |
| Seagle 1998 | No diabetes population |
| Seedat 1974 | No diabetes population |
| Shi 2001 | No weight results for diabetes subgroup |
| Sirtori 1971 | No diabetes population |
| Sjostrom 1998 | No weight results for diabetes subgroup |
| Steel 1973 | No diabetes subpopulation |
| Stoa‐Birketvedt 1993 | No diabetes population |
| Tan 2002 | Goal not weight loss; 8 hour follow‐up only |
| Thompson 1998 | Not a weight loss drug |
| Toft‐Nielsen 1999 | Not a weight loss study |
| Toplak 1998 | No diabetes population |
| Torgerson 2001 | No outcomes |
| Toubro 1993 | No diabetes population |
| Van Gaal 1998a | No diabetes population |
| Van Gaal 1998b | No diabetes population |
| Vanloon 1992 | Goal not weight loss |
| Vernace 1974 | No diabetes population |
| Wadden 1995 | No diabetes population |
| Wadden 1997 | No diabetes population |
| Wadden 2001 | No diabetes population |
| Walker 1977 | No diabetes population |
| Wasada 2000 | Goal is to decrease body fat, not weight loss |
| Wilding 1998 | No weight outcomes |
| Wilding 1999 | No diabetes population |
| Wilding 2001 | No diabetes subgroup |
| Williams 1981 | No diabetes subgroup |
| Wilson 1960 | No diabetes subgroup |
| Wirth 2001 | No population with diabetes |
| Woodhouse 1975 | Experimental drug (AN448); no diabetes population |
| Yoshida 1994 | No diabetes populaion |
| Zavoral 1998 | No weight outcomes for diabetes subgroup |
| Ziegler 1971 | Formula diet; not a weight loss drug |
Contributions of authors
SUSAN L. NORRIS: Conceiving the review, designing the review, coordinating the review, data collection for the review (including developing the search strategy, screening search results, screening retrieved papers, appraising quality of papers, abstracting data from papers, writing to study authors for additional information), data management, analysis of data, interpretation of the data (providing a methodological and clinical perspective), writing the review.
XUANPING ZHANG: Coodinating the review, data collection for the review (including screening search results, organizing retrieval of papers, screening retrieved papers, appraising quality of papers, abstracting data from papers, writing to study authors for additional information), data management, analysis of data, interpretation of the data (providing a methodological) , writing the review.
ALISON AVENELL: Conceiving the review, designing the review, data collection for the review (including screening retrieved papers, appraising quality of papers, analysis of data, interpretation of the data (providing a methodological and clinical perspective), writing the review.
EDWARD GREGG: Designing the review, analysis of data, interpretation of the data (providing a methodological, epidemiologic, and public health), writing the review.
CHRISTOPHER H. SCHMID: Designing the review, analysis of data, interpretation of the data (providing a methodological and statistical perspective), writing the review.
JOSEPH LAU: Designing the review, analysis of data, interpretation of the data (providing a methodological and clinical perspective), writing the review.
Sources of support
Internal sources
Centers for Disease Control and Prevention, USA.
External sources
No sources of support supplied
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Allie 2004 {published data only}
- Allie EC, Kane MP, Busch RS, Bakst G, Hamilton RA. Orlistat in Obese Patients with Type 2 Diabetes: A Retrospective Assessment of Weight Loss and Metabolic Effects. Hospital Pharmacy 2004;39(1):37‐42. [Google Scholar]
Bach 1999 {published data only}
- Bach DS, Rissanen AM, Mendel CM, Shepherd G, Weinstein SP, Kelly F, Seaton TB, Patel B, Pekkarinen TA, Armstrong F. Absence of cardiac valve dysfunction in obese patients treated with sibutramine. Obesity Research 1999;7:363‐9. [DOI] [PubMed] [Google Scholar]
Bandisode 1975 {published data only}
- Bandisode MS, Boshell BR. Double‐blind clinical evaluation of mazindol (42‐548) in obese diabetics. Current Therapeutic Research, Clinical and Experimental 1975;18(6):816‐24. [PubMed] [Google Scholar]
Bloch 2003 {published data only}
- Bloch KV, Salles GF, Muxfeldt ES, Rocha NA. Orlistat in hypertensive overweight/obese patients: Results of a randomized clinical trial. Journal of Hypertension 21, (11):2159‐65. [DOI] [PubMed] [Google Scholar]
Bonnici 2002 {published data only}
- Bonnici F. Effect of orlistat on glycemic control and body weight in overweight or obese South African patients with type 2 diabetes. Diabetes 2002;51(Suppl 2):1692. [Google Scholar]
Boshell 1974 {published data only}
- Boshell B. The efficacy and safety of Mazindol in patients with diabetes mellitus. The First International Congress Association for the Study of Obesity 1974:172. [Google Scholar]
Bratusch‐Marrian1979 {published data only}
- Bratusch‐Marrain P, Dudczak R, Waldhausl W. Weight reduction in obese diabetics: a double‐blind study of diethylpropionate. Wiener Klinische Wochenschrift 1979;91(13):455‐8. [PubMed] [Google Scholar]
Buckle 1966 {published data only}
- Buckle RM, Silverstone JT. Weight control in obese diabetics. A comparetive trial of Filon and Phenmetrazine. British Journal of Clinical Practice 1966;20:363‐5. [PubMed] [Google Scholar]
Campbell 1977 {published data only}
- Campbell CJ, Bhalla IP, Steel JM, Duncan LJ. A controlled trial of phentermine in obese diabetic patients. Practitioner 1977;218(1308):851‐5. [PubMed] [Google Scholar]
Chiasson 1989 {published data only}
- Chiasson JL, Lau DCW, Leiter LA, Tildesley HD, Birmingham CL, Ekoe JM, et al. Fluoxetine has potential in obese NIDDM ‐ Multicenter Canadian trial. Diabetes 1989;38(Suppl 2):A154. [Google Scholar]
Connolly 1995 {published data only}
- Connolly VM, Gallagher A, Kesson CM. A study of fluoxetine in obese elderly patients with type 2 diabetes. Diabetic Medicine 1995;12:416‐8. [DOI] [PubMed] [Google Scholar]
Crommelin 1974 {published data only}
- Crommelin RM. Noramphetamine, anorectic medication for obese diabetic patients: controlled and open investigations of mazindol. Clinical Medicine 1974;81:20‐4. [Google Scholar]
Daubresse 1996 {published data only}
- Daubresse JC, Kolanowski J, Krzentowski G, Kutnowski M, Scheen A, Gaal L. Usefulness of fluoxitine in obese non‐insulin‐dependent diabetics: a multicenter study. Obesity Research 1996;4:3912‐396. [DOI] [PubMed] [Google Scholar]
Deerochanawong 2001 {published data only}
- Deerchanawong C. Effect of treatment with orlistat in overweight or obese Thai patients with type 2 diabetes. Diabetes 2001;50(Suppl 2):A433. [Google Scholar]
Dimitrov 2001 {published data only}
- Dimitrov D, Koeva L, Kovatcheva T, Rousseva T. Effect of orlistat on insulin resistance, cardiovascular risk factors and serum leptin levels in obese type 2 diabetic patients. International Journal of Obesity 2001:S116. [Google Scholar]
Dolecek 1976 {published data only}
- Dolecek R, Reil P, Skarpova O, Zavada M. Mazindol in the treatment of obese diabetic patients. Vnitrni Lekarstvi 1976;22:798‐804. [PubMed] [Google Scholar]
Felt 1977 {published data only}
- Felt V, Nedvidkova J. Mazindol in the treatment of obesity in diabetics. Casopis Lekaru Ceskych 1976;116:1214‐7. [PubMed] [Google Scholar]
Finer 2000 {published data only}
- Finer N, Bloom SR, Frost GS, Banks LM, Griffiths J. Sibutramine is effective for weight loss and diabetic control in obesity with type 2 diabetes; a randomised, double‐blind, placebo‐controlled study. Diabetes, Obesity Metabolism 2000;2:105‐12. [DOI] [PubMed] [Google Scholar]
Fujioka 2000 {published data only}
- Fujioka K, Seaton TB, Rowe E, Jelinek CA, Raskin P, Lebovitz HE, Weinstein SP, The Sibutramine/Diabetes Clinical Study Group. Weight loss with sibutramine improves glcaemic control and other metabolic parameters in ovese patients with type 2 diabetes mellitus. Diabetes, Obesity and Metabolism 2000;2:175‐87. [DOI] [PubMed] [Google Scholar]
Gershberg 1972 {published data only}
- Gershberg H, Hulse M, Million M. The effect of a low calorie diet and an anorectic agent on body weight and on serum insulin, cholesterol, and triglyceride levels obese diabetics. Diabetes 1972;21(Suppl):21. [Google Scholar]
Gershberg 1977 {published data only}
- Gershberg H, Kane R, Hulse M, Pengsen E. Effects of diet and an anorectic drug (phentermine resin) in obese diabetics. Current Therapeutic Research 1977;22:814‐20. [Google Scholar]
Gokcel 2001 {published data only}
- Gokcel A, Karakose H, Ertorer EM, Tanaci N, Tutuncu NB, Guvener N. Effects of sibutramine in obese female subjects with type 2 diabetes and poor blood glucose control. Diabetes Care 2001;24(11):1957‐60. [DOI] [PubMed] [Google Scholar]
Goldstein 1992 {published data only}
- Goldstein DJ, Rampey AH, Bray GA, Gray D. Fluoxetine treatment of obese patients with noninsulin dependent diabetes‐mellitus. Clinical Research 1991;39(3):A767. [Google Scholar]
- Goldstein DJ, Rampey AH, Potvin JH, Fludzinski LA. Fluoxetine in obese patients with noninsulin‐dependent diabetes mellitus. Clinical Research 1992;40(2):A240. [Google Scholar]
Goldstein 1995 {published data only}
- Goldstein DJ, Sayler ME, Rampey AH, Roback PJ. Fluoxetine therapy in obese patients with noninsulin‐dependent diabetes mellitus. Clinical Pharmacology & Therapeutics 1995;57(2):200. [Google Scholar]
Gray 1992 {published data only}
- Gray DS, Fujioka K, Devine W, Bray GA. A randomized double‐blind clinical trial of fluoxetine in obese diabetics. International Journal of Obesity 1992;16(Suppl):167‐72. [PubMed] [Google Scholar]
- Gray DS, Fujioka K, Devine W, Bray GA. Fluoxetine treatment of the obese diabetic. International Journal of Obesity 1992;16:193‐8. [PubMed] [Google Scholar]
Griffiths 1995 {published data only}
- Griffiths J, Brynes AE, Frost G, Bloom SR, Finer N, Jones SP, Romanec FM. Sibutramine in the treatment of overweight non‐insulin‐dependent diabetics. International Journal of Obesity 1995;19(Suppl2):S41. [Google Scholar]
- Griffiths J, Bloom SR, Finer N, et al. Body composition changes following weight loss induced by sibutramine. International Journal of Obesity 1995;19 (suppl 2):144. [Google Scholar]
Guy‐Grand 2001 {published data only}
- Guy‐Grand B, Gin H, Valensi P, Crouin P, Eschwege E. Differential weight loss in orlistat treated obese and overweight patients with various comorbidities. International Journal of Obesity 2001;S93. [Google Scholar]
- Guy‐Grand B, Valensi P, Drouin P, Gin H, Eschwege E. Improvement of metabolic control in obese type 2 diabetic patients treated with orlistat for 6 months. Diabetes 2001;50 (Suppl 2)(A436). [Google Scholar]
- Guy‐Grand B, Valensi P, Joubert JM, Eschwege E, Amouyel P, Fagnani F. Modelisation of the 10‐year incidence reduction of coronary events in obese Type 2 diabetes patients treated with Orlistat. Diabetes 2002;51(Suppl 2):A471. [Google Scholar]
Halpern 2003 {published data only}
- Halpern A. Latin‐American multicentric study with orlistat in overweight or obese patients with type 2 diabetes. Diabetes 2001;50(Suppl 2):A437. [Google Scholar]
- Halpern A, Mancini MC, Suplicy H, Zanella MT, Repetto G, Gross J, Jadzinsky M, Barranco J, Aschner P, Ramirez L, Matos AG. Latin‐American trial of orlistat for weight loss and improvement in glycaemic profile in obese diabetic patients. Diabetes, Obesity and Metabolism 2003;5:180‐8. [DOI] [PubMed] [Google Scholar]
Hanefeld 2002 {published data only}
- Hanefeld M, Platon J, Sachse G. Orlistat promotes weight loss and improves glycaemic control in overweight patients with type 2 diabetes. Diabetologia 2001;44(Suppl 1):A231. [Google Scholar]
- Hanefeld M, Sachse G. The effects of orlistat on body weight and glycaemic control in overweight patients with type 2 diabetes: a randomized, placebo‐controlled trial. Diabetes, Obesity and Metabolism 2002;4:415‐23. [DOI] [PubMed] [Google Scholar]
Hawkins 2000 {published data only}
- Hawkins F, Duran S, Vilardell E, Soriguer F, Cabezas J, Escobar F, Milalles JM, Faure E, Bellido D, Herrera JL, Serrano‐Rios M, Tebar J, Freijane J, Armero F. Orlistat promotes glucemia control and other cardiovascular risk factors lowering in obese patients with type 2 diabetes. Randomised clinical trial. Diabetologia 2000;43(Suppl):171. [Google Scholar]
Hendon 1962 {published data only}
- Hendon JR, Urbach S. Use of diethylpropion in obese diabetic persons. Metabolism 1962;11:337‐41. [PubMed] [Google Scholar]
Hollander 1998 {published data only}
- Hollander P. Orlistat enhances weight loss in obese patients with diabetes. American Family Physician 1997;56(2):566. [Google Scholar]
- Hollander P, Lucas C, Hauptman J, Boldrin MN, Segal KR. Orlistat reduces body weight and cardiovascular disease risk factors in obese men and women with type 2 diabetes. Diabetes 1999;48:1356. [Google Scholar]
- Hollander P, Lucas C, Segal KR. Orlistat (xenical (R)) reduces cardiovascular disease risk factors in obese patients with type 2 diabetes. Diabetologia 1998;41:492. [Google Scholar]
- Hollander PA, Elbein SC, Hirsch IB, Kelley D, McGill J, Taylor T, et al. Role of orlistat in the treatment of obese patients with type 2 diabetes. A 1‐year randomized double‐blind study. Diabetes Care 1998;21(8):1288‐94. [DOI] [PubMed] [Google Scholar]
Hollander 2001 {published data only}
- Hollander P, Miles JM. Effects of orlistat in obese metformin‐treated patients with type 2 diabetes: attainment of treatment goals. International Journal of Obesity 2001;25(Suppl 2):S92. [Google Scholar]
Kaukua 2004 {published data only}
- Kaukua JK, Pekkarinen TA, Rissanen AM. Health‐related quality of life in a randomised placebo‐controlled trial of sibutramine in obese patients with type II diabetes. International Journal of Obesity 2004;28(4):600‐5. [DOI] [PubMed] [Google Scholar]
Kelley 1997 {published data only}
- Kelley D. A one‐year study of weight loss and glycemic control in type 2 diabetics following orlistat treatment. Obesity Research 1997;5(Suppl 1):21S. [Google Scholar]
Kelley 2002 {published data only}
- Bray GA, Pi‐Sunyer FX, Hollander P, Hollander P, Kelley DE. Effect of orlistat in overweight patients with diabetes receiving insulin therapy. Diabetes 2001;50(Suppl 2):A107. [Google Scholar]
- Kelley DE. Impact of orlistat on fasting hyperglycemia in obese patients with type 2 diabetes mellitus. Diabetes 2001;50(Suppl 2):A439. [Google Scholar]
- Kelley DE, Bray GA, Pi‐Sunyer FX, Klein S, Hill J, Miles J, Hollander P. Clinical efficacy of orlistat therapy in overweight and obese patients with insulin‐treated type 2 diabetes. Diabetes Care 2002;25:1033‐41. [DOI] [PubMed] [Google Scholar]
Kelley 2004 {published data only}
- Kelley DE, Kuller LH, McKolanis TM, Harper P, Kalhan S. Effects of moderate weight loss and orlistat on insulin resistance, regional adiposity, and fatty acids in type 2 diabetes. Diabetes Care 2004;27(1):33‐40. [DOI] [PubMed] [Google Scholar]
Kutnowski 1990 {published data only}
- Kutnowski M, Daubresse JC, Friedman H, Kolanowski M, Krzentowski G, Scheen A, Gaal L, Chadenas D, Fossati P, Grandmottet P, Matthews D. Eight weeks fluoxitine therapy in obese patients with impaired glucose tolerance. International Journal of Obersity 1990;14(Suppl):48. [Google Scholar]
Kutnowski 1992 {published data only}
- Kutnowski M, Daubresse JC, Friedman H, Kolanowski J, Krzentowski G, Scheen A, et al. Fluoxetine therapy in obese diabetic and glucose intolerant patients. International Journal of Obesity 1992;16(Suppl):S6. [PubMed] [Google Scholar]
le Roux 2001 {published data only}
- Roux CW, Alaghband‐Zadeh J, Suttard J, Frost G, Laycock JF. Biochemical markers of patients with type 2 diabetes and orlistat induced weight loss. International Journal of Obesity 2001;25(Suppl):S83. [Google Scholar]
Lindgarde 2000 {published data only}
- Lindgarde F. The effect of orlistat on body weight and coronary heart disease risk profile in obese patients: The Swedish Multimorbidity Study. Journal of Internal Medicine 2000;248(3):245‐54. [DOI] [PubMed] [Google Scholar]
Martin 2001 {published data only}
- Martin SJ, Maguire IE, Irwin A, Archbold GPR. Weight loss in type 2 diabetics treatd with orlistat. International Journal of Obesity 2001;25:S113. [Google Scholar]
McNulty 2003 {published data only}
- McNulty SJ, Ur E, Williams G. A randomized trial of sibutramine in the management of obese type 2 diabetic patients treated with metformin. Diabetes Care 2003;26(1):125‐31. [DOI] [PubMed] [Google Scholar]
Mendoza‐Guadarra2000 {published data only}
- Mendoza‐Guadarrama LG, Lopez‐Alvarenga JC, Castillo‐Martinez L, Gallegos J, Portocarrero L, Garcia‐Garcia R, Roiz‐Simancas M, Gonzalez‐Barranco J. Orlistat reduces visceral fat independent of weight changes in obese diabetics type 2. International Journal of Obesity 2000;24((Suppl 1)):S167. [Google Scholar]
Miles 2002 {published data only}
- MIles JM, Leiter L, Hollander P, Wadden T, Anderson JW, Doyle M, Foreyt J, Aronne L, Klein S. Effect of orlistat in overweight and obese patients with type 2 diabetes treated with metformin. Diabetes Care 2002;25:1123‐8. [DOI] [PubMed] [Google Scholar]
- Miles JM, Aronne LJ, Hollander P, Klein S. Effect of orlistat in overweight and obese type 2 diabetes patients treated with metformin. Diabetes 2001;50(Suppl 2):A442‐3. [DOI] [PubMed] [Google Scholar]
Montenero 1964 {published data only}
- Montenero P, Colletti A. Experience on the therapeutic use of an anorexic substance in obese diabetes. Minerva Medica 1964;55:2800‐5. [PubMed] [Google Scholar]
O'Kane 1994 {published data only}
- O'Kane M, Wiles PG, Wales JK. Fluoxetine in the treatment of obese type 2 diabetic patients. Diabetic Medicine 1994;11:105‐10. [DOI] [PubMed] [Google Scholar]
Peirce 1999 {published data only}
- Peirce NS, Tattersall RB, Stubbs TA, Macdonald IA. The effect of sibutramine on weight loss and glucose metabolism in obese patients with type 2 diabetes mellitus. British Journal of Clincial Pharmacology 1999;48:880P. [Google Scholar]
Redmon 2003 {published data only}
- Redmon JB, Raatz SK, Reck KP, Swanson JE, Bantle JP. One‐year outcome of a combination of weight loss therapies for subjects with type 2 diabetes ‐ A radomized trial. Diabetes Care 2003;26(9):2505‐11. [DOI] [PubMed] [Google Scholar]
Rissanen 1999a {published data only}
- Rissanen A, Pekkarinen, T, Heinanen T, Saltevo J, Taskinen MR. Weight loss with sibutramine in obese patients with type 2 diabetes: a double‐blind, placebo‐controlled study. Obesity Research 1999;7(Suppl 1):93S. [Google Scholar]
Sanders 1976 {published data only}
- Sanders M, Breidahl H. The effect of an anorectic agent (Mazindol) on control of obese diabetics. Medical Journal of Australia 1976;2(15):576‐7. [DOI] [PubMed] [Google Scholar]
Segal 2000 {published data only}
- Segal KR, Wilson PW, Lucas C, Hauptman J. Impact of orlistat‐induced weight loss on cardiovascular risk estimate in diabetic and non‐diabetic subjects. Circulation 2000;102(18):4078. [Google Scholar]
Serrano‐Rios 2001 {published data only}
- Serrano‐Rios M, Armero F, Genis M. Orlistat efficacy on weight loss in overweight or obese patients with type 2 diabetes mellitus. Diabetes 2001;50(Suppl 1):A131. [Google Scholar]
Serrano‐Rios 2002 {published data only}
- Serrano‐Rios M, Meichionda N, Moreno‐Carretero E. Role of sibutramine in the treatment of obese type 2 diabetic patients receiving sulphonylurea therapy. Diabetic Medicine 2002;19(2):119‐24. [DOI] [PubMed] [Google Scholar]
Silverstone 1966 {published data only}
- Silverstone JT, Buckle RM. Obesity in diabetes. Some considerations on treatment. American Journal of Clinical Nutrition 1966;19(3):158‐62. [DOI] [PubMed] [Google Scholar]
Sircar 2001 {published data only}
- Sircar AR, Kumar A, Lal M. Clinical evaluation of sibutramine in obese type 2 diabetic patients refractory to dietary management. Journal of Association Physicians India 2001;49:885‐8. [PubMed] [Google Scholar]
Slama 1978 {published data only}
- Slama G, Selmi A, Hautecouverture M, Tchobroutsky G. Double‐blind clinical trial of mazindol on weight loss, blood glucose, plasma insulin and serum lipids in overweight diabetic patients. Diabetes and metabolism 1978;4:193‐9. [PubMed] [Google Scholar]
Stoa‐Birketvedt 1998 {published data only}
- Stoa‐Birketvedt G, Paus PN, Ganss R, Ingebretsen OC, Florholmen J. Cimetidine reduces weight and improves metabolic control in overweight patients with type 2 diabetes. International Journal of Obesity 1998;22(11):1041‐5. [DOI] [PubMed] [Google Scholar]
Tankova 2003 {published data only}
- Tankova T, Dakovska G, Lazarova M, Dakovska L, Kirilov G, Koev D. Sibutramine in the treatment of obesity in type 2 diabetic patients. [Bulgarian]. Endocrinologia 2003;8(4):257‐65. [DOI] [PubMed] [Google Scholar]
Tong 2002 {published data only}
- Chan JC, Tong PC, Lee ZSK, Ko GTC, Sea MMM, M RC, So W‐Y, Chan W‐B, Ozaki R, Chow C‐C, Critchley JAJH, Cockram CS. Effect of orlistat on cardiovascular risk factors and insulin sensitivity in young obese Chinese type 2 diabetic patients. Diabetes 2001;50(Suppl 2):A108. [Google Scholar]
- Sea M‐M, Chong A, Tong PC, Ko GT, Chow CC, Critchley JA, Woo J, Tomlinson B, Cockram CS, Chan JC. The effect of orlistat on body composition in obese diabetic and non‐diabetic chinese adults. Diabetes 2001;50(Suppl 2):A130. [Google Scholar]
- Sea MM, Tong PC, Chow CC, Woo J, Tomlinson B, Cockram CS, Chan JC. A pilot study to examine the efficacy of orlistat and lifestyle modification in Chinese obese subjects with or without Type 2 diabetes mellitus. Diabetes 2002;51(Suppl):A609. [Google Scholar]
- Tong PCY, Lee ZSK, Sea MM, Chow CC, Ko GTC, Chan WB, So WY, Ma RCW, Ozaki R, Woo J, Cockram CS, Chan JCN. The effect of orlistat‐induced weight loss, without concomitant hypocaloric diet, on cardiovascular risk factors and insulin sensitivity in young obese Chinese subjects with or without type 2 diabetes. Archives of Internal Medicine 2002;162:2428‐35. [DOI] [PubMed] [Google Scholar]
Vargas 1994 {published data only}
- Vargas R, McMahon FG, Jain AK. Effects of Sibutramine. Clinical Pharmacology and Therapeutics 1994;55(2):188. [Google Scholar]
Versari 2000 {published data only}
- Versari G, Cuttica CM, Falivene MR, Devoto GL, Boletto N, Ferrari B, Corsi L. Orlistat in obese type 2 diabetes mellitus: metabolic effects of a short term treatment. Journal of Endocrinological Investigation 2000;Suppl:46. [Google Scholar]
Wang 2003 {published data only}
- Wang Y, Liu C, Liu Y. Orlistat for adjutant treatment of fatty type 2 diabetes mellitus in 32 patients. Chinese Journal of New Drugs 2003;22(11):651‐3. [Google Scholar]
Williams 1968 {published data only}
- Williams J. Trial of a long‐acting preparation of diethylpropion in obese diabetics. Practitioner 1968;200(197):411‐4. [PubMed] [Google Scholar]
Wise 1989 {published data only}
- Wise SD. Fluoxetine, efficacy and safety in treatment of obese type‐2 (non‐insulin‐dependent) diabetes. Diabetologia 1989;32(7):A557. [Google Scholar]
Zaletel 2002 {published data only}
- Zaletel J, Janez A, Kocijancic A. Highly educative programme added upon treatment with orlistat in type 2 diabetic patients. International Journal of Obesity 2002;26(Suppl):S153. [Google Scholar]
Zelissen 1992 {published data only}
- Zelissen PMJ, Koppeschaar HPF, Thijssen JHH, Erkelens DW. Growth hormone secretion in obese patients with non‐insulin dependent diabetes mellitus: effect of weight reduction and of fluoxetine treatment. Diabetes, Nutrition, and Metabolism 1992;5(2):131‐5. [Google Scholar]
References to studies excluded from this review
Anchors 1997 {published data only}
- Anchors M. Fluoxetine is a safer alternative to fenfluramine in the medical treatment of obesity. Archives of Internal Medicine 1997;157(11):1270. [PubMed] [Google Scholar]
Apfelbaum 1999 {published data only}
- Apfelbaum M, Vague P, Ziegler O, Hanotin C, Thomas F, Leutenegger E. Long‐term maintenance of weight loss after a very‐low‐calorie diet: A randomized blinded trial of the efficacy and tolerability of sibutramine. American Journal of Medicine 1999;106:179‐84. [DOI] [PubMed] [Google Scholar]
Astrup 1985 {published data only}
- Astrup A, Lundsgaard C, Madsen J, Christenen NJ. Enhanced thermogenic responsiveness during chronic ephedrine treatment in man. American Journal of Clinical Nutrition 1985;42:83‐94. [DOI] [PubMed] [Google Scholar]
Astrup 1992 {published data only}
- Astrup A, Breum L, Toubro S, Hein P, Quaade F. The effect and safety of an ephedrine/caffeine compound compared to ephedrine, caffeine and placebo in obese subjects on an energy‐restricted diet: A double blind trial. International Journal of Obesity 1992;16:269‐77. [PubMed] [Google Scholar]
Boneva 2002 {published data only}
- Boneva Ah, Christov VI. Reductil (sibutramine hydrochloride) effect in treating obese patients. Endocrinologia 2002;7:49‐55. [Google Scholar]
Bowen 2000 {published data only}
- Bowen RL. Addition of orlistat to long term phentermine treatment for obesity. Obesity Research 2000;8(1):118. [Google Scholar]
Bray 1996 {published data only}
- Bray GA, Ryan DH, Gordon D, Heidingsfelder S, Cerise F, Wilson K. A double‐blind randomized placebo‐controlled trial of sibutramine. Obesity Research 1996;4:263‐70. [DOI] [PubMed] [Google Scholar]
Bray 1999 {published data only}
- Bray GA, Blackburn GL, Ferguson JM, Greenway FL, Jain AK, Mendels CM, Ryan DH, Schwartz SL, Scheinbaum Ml, Seaton TB. Sibutramine produces dose‐related weight loss. Obesity Research 1999;7(2):189‐98. [DOI] [PubMed] [Google Scholar]
Breum 1995 {published data only}
- Breum L, Bjerre U, Bak JF, Jacobsen S, Astrup A. Long‐term effects of fluoxetine on glycemic control in obese patients with non‐insulin‐dependent diabetes mellitus or glucose intolerance: influence on muscle glycogen synthase and insulin receptor kinase activity. Metabolism: Clinical & Experimental 1995;44(12):1570‐6. [DOI] [PubMed] [Google Scholar]
Broom 2001 {published data only}
- Broom I, on behalf of the UK Multimorbidities Study Group. Randomised trial of the effect of orlistat on body weight and CVD risk profile in overweight and obese patients with co‐morbidities. International Journal of Obesity 2001;25(Suppl 2):S106. [Google Scholar]
Chengappa 2001 {published data only}
- Chengappa KNR, Levine J, Rathore D, Parepally H, Atzert R. Long‐term effects of topiramate on bipolar mood instability, weight change and glycemic control: a case‐series. European Psychiatry 2001;16(3):186‐90. [DOI] [PubMed] [Google Scholar]
Conte 1973 {published data only}
- Conte A. Evaluation of Sanorex‐a new appetitie suppressant. Journal of Obese Bariatric Medicine 1973;2:104‐7. [Google Scholar]
Daly 1993 {published data only}
- Daly PA, Krieger DR, Dulloo AG, Young JB, Landsberg L. Ephedrine, caffeine and aspirin: safety and efficacy for treatment of human obesity. International Journal of Obesity 1993;17(Suppl 1):S73‐8. [PubMed] [Google Scholar]
Darga 1991 {published data only}
- Darga LL, Carroll‐Michals L, Botsford SJ, Lucas CP. Fluoxetine's effect on weight loss in obese subjects. American Journal of Clinical Nutrition 1991;54:321‐5. [DOI] [PubMed] [Google Scholar]
Davison 1999 {published data only}
- Davison MH, Hauptman J, DiGirolamo M, Foreyt JP, Halsted CH, Heber D, Heimburger DC, Lucas CP, Robbins DC, Chung J, Heymsfield SB. Weight control and risk factor reduction in obese subjects treated for 2 years with Orlistat: a randomized controlled trial. Journal of the American Medical Association 1999;281(3):235‐42. [DOI] [PubMed] [Google Scholar]
Derby 1999 {published data only}
- Derby LE, Myers MW, Jick H. Use of dexfenfluramine, fenfluramine and phentermine and the risk of stroke. British Journal of Clinical Pharmacology 1999;47(5):565‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Drent 1995 {published data only}
- Drent ML, Larsson I, William‐Olsson T, Quaade F, Czubayko F, Bergmann K, et al. Orlistat (Ro 18‐0647), a lipase inhibitor, in the treatment of human obesity: a multiple dose study. International Journal of Obesity 1995;19:221‐6. [PubMed] [Google Scholar]
Duncan 1960 {published data only}
- Duncan LJP, Rose K, Meiklejohn AP. Phenmetrazine hydrochloride and methylcellulose in the treatment of 'refractory' obesity. Lancet 1960;1:1262‐5. [DOI] [PubMed] [Google Scholar]
Edmonds 1983 {published data only}
- Edmonds ME, Archer AG, Watkins PJ. Ephedrine: new treatment for diabetic neuropathic edema. Lancet 1983;1(Mar. 12):548‐51. [DOI] [PubMed] [Google Scholar]
Egart 1979 {published data only}
- Egart FM, Korotkova VD. Use of teronak for the treatment of obesity with and without diabetes mellitus. Problemy Endokrinologii 1979;26(2):16‐20. [PubMed] [Google Scholar]
Enzi 1976 {published data only}
- Enzi G, Baritussio A, Marchiori E, Crerpaldi G. Short‐term and long‐term clinical evaluation of a non‐amphetaminic anorexiant (mazindol) in the treatment of obesity. The Journal of International Medical Research 1976;4:305‐18. [DOI] [PubMed] [Google Scholar]
Fanghanel 2000 {published data only}
- Fanghanel G. A clinical trial of the use of Sibutramine for the treatment of patients suffering essential obesity. International Journal of Obesity 2000;24:144‐50. [DOI] [PubMed] [Google Scholar]
Faria 2001 {published data only}
- Faria AN. Sibutramine reduces glucose intolerance in centrally obese hypertensive patients. International Journal of Obesity 2001;25(Suppl 2):S115. [Google Scholar]
Fava 1999 {published data only}
- Fava M, Rosenbaum J, Judge R, Hoog S, Millard D, Koke S. Fluoxetine versus sertraline and paroxetine in major depression: long‐term changes in weight. Biological Psychiatry 1999;45(Suppl):S74. [Google Scholar]
Fernandez‐Soto 1995 {published data only}
- Fernandez‐Soto ML, Gonzalez‐Jimenez A, Barredo‐Acedo F, Luna del Castillo JD, Escobar‐Jimenez F. Comparison of fluoxetine and placebo in the treatment of obesity. Annals of Nutrition and Metabolism 1995;39:159‐63. [DOI] [PubMed] [Google Scholar]
Finer 2000e {published data only}
- Finer N, James WP, Kopelman PG, Lean ME, Williams G. One‐year treatment of obesity: a randomized, double‐blind, placebo‐controlled multicentre study of orlistat, a gastrointestinal lipase inhibitor. International Journal of Obesity 2000;24:396‐13. [DOI] [PubMed] [Google Scholar]
Generali 2001 {published data only}
- Generali J, Cada DJ. Cimetidine: Weight loss. Hospital Pharmacy 2001;36(3):313‐8. [Google Scholar]
Gokcel 2002a {published data only}
- Gokcel A, Gumurdulu Y, Karakose H, Karademir BM, Anarat R. Effects of sibutramine in non‐dieting obese women. Journal of Endocrinological Investigation 2002;25(2):101‐5. [DOI] [PubMed] [Google Scholar]
Gokcel 2002b {published data only}
- Gokcel A, Gumurdulu Y, Karakose H, Melek EE, Tanaci N, BascilTutuncu N, Guvener N. Evaluation of the safety and efficacy of sibutramine, orlistat and metformin in the treatment of obesity. Diabetes Obesity and Metabolism 2002;4(1):49‐55. [DOI] [PubMed] [Google Scholar]
Goldstein 1993 {published data only}
- Goldstein DJ, Rampey AH Jr, Dornseif BE, Levine LR, Potvin JH, Fludzinski LA. Fluoxitine: a randomized clinical trial in the maintenance of weight loss. Obesity Research 1993;1(2):92‐8. [DOI] [PubMed] [Google Scholar]
Goldstein 1994 {published data only}
- Goldstein DJ, Rampey AHJ, Enas GG, Potvin JH, Fludzinski LA, Levine LR. Fluoxetine: a randomized clinical trial in the treatment of obesity. International Journal of Obesity 1994;18:129‐35. [PubMed] [Google Scholar]
Greenway 1999e {published data only}
- Greenway F, Heber D, Raum W, Morales S. Double‐blind, randomized, placebo‐controlled clinical trials with non‐prescription medications for the treatment of obesity. Obesity Research 1999;7:370‐8. [DOI] [PubMed] [Google Scholar]
Hadler 1967 {published data only}
- Hadler AJ. Weight reduction with phenmetrazine and chlorphentermine a double‐blind study. Current Therapeutic Research, Clinical and Experimental 1967;9(11):563‐9. [PubMed] [Google Scholar]
Haller 2000 {published data only}
- Haller CA. Adverse cardiovascular and central nervous system events associated with dietary supplements containing ephedra alkaloids. New England Journal of Medicine 2000;343(25):1833‐8. [DOI] [PubMed] [Google Scholar]
Hanefeld 2002b {published data only}
- Hanefeld M. Effect of orlistat on post‐prandial glucose levels in overweight or obese patients with type 2 diabetes. Diabetes 2002;51:404. [Google Scholar]
Hanotin 1998 {published data only}
- Hanotin C, Thomas F, Jones SP, Leutenegger E, Drouin P. A comparison of sibutramine and dexfenfluramine in the treatment of obesity. Obesity Research 1998;6:285‐91. [DOI] [PubMed] [Google Scholar]
Hansen 2001 {published data only}
- Hansen DL, Astrup A, Toubro S, Finer N, Kopelman P, Hilsted J, Rossner S, Saris WHM, Gaal LF, James WPT, Goulder M. Predictors of weight loss and maintenance during 2 years of treatment by sibutramine in obesity, results from the European multi‐centre STORM trial. International Journal of Obesity 2001;25:496‐501. [DOI] [PubMed] [Google Scholar]
Hauptman 1992 {published data only}
- Hauptman JB, Jeunet FS, Hartmann D. Initial studies in humans with the novel gastointestinal lipase inhibitor Ro 18‐0647. American Journal of Clinical Nutrition 1992;55(Suppl):S309‐13. [DOI] [PubMed] [Google Scholar]
Hauptman 2000 {published data only}
- Hauptman JB, Lucas C, Boldrin MN, Collins H, Segal KR. Orlistat in the long‐term treatment of obesity in primary care settings. Archives of Family Medicine 2000;9:160‐7. [DOI] [PubMed] [Google Scholar]
Heal 1998 {published data only}
- Heal DJ, Cheetham SC, Prow MR, Martin KF, Buckett WR. A comparison of the effects on central 5‐HT function of sibutramine hydrochloride and other weight‐modifying agents. British Journal of Pharmacology 1998;125:301‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heath 1999 {published data only}
- Heath MJ, Chong E, Weinstein SP, Seaton TB. Sibutramine enhances weight loss and improves glycemic control and plasma lipid profile in obese patients with type 2 diabetes mellitus. Diabetes 1999;48:1346. [Google Scholar]
Heymsfield 2000 {published data only}
- Heymsfield SB, Segal KR, Hauptman J, Lucas CP, Boldrin MN, Rissanen A, et al. Effects of weight loss with orlistat on glucose tolerance and progression to type 2 diabetes in obese adults. Archives of Internal Medicine 2000;160(9):1321‐6. [DOI] [PubMed] [Google Scholar]
Hill 1999 {published data only}
- Hill JO. Orlistat, a lipase inhibitor, for weight maintenance after conventional dieting: a 1‐y study. American Journal of Clinical Nutrition 1999;69:1108‐16. [DOI] [PubMed] [Google Scholar]
Hollenbeck 1987 {published data only}
- Hollenbeck CB, Reaven GM. Treatment of patients with non‐insulin‐dependent diabetes mellitus: diabetic control and insulin secretion and action after different treatment modalities. Diabetic Medicine 1987;4(4):311‐6. [DOI] [PubMed] [Google Scholar]
Inoue 1992 {published data only}
- Inoue S, Egawa M, Satoh S, Saito M, Suzuki H, Kumahara Y, et al. Clinical and basic aspects of an anorexiant, mazindol, as an antiobesity agent in Japan. American Journal of Clinical Nutrition 1992;55(Suppl 1):S199‐202. [DOI] [PubMed] [Google Scholar]
Inoue 1995 {published data only}
- Inoue S. Clinical studies with mazindol. Obesity Research 1995;3(Suppl):S549‐52. [DOI] [PubMed] [Google Scholar]
Jacob 2002 {published data only}
- Jacob S, Gomis R, Miles JM. Effect of Orlistat on glycemic control in patients on or near maximal doses of oral anti‐diabetic (OAD) medications. Diabetes 2002;51(6):1693‐P. [Google Scholar]
James 1997 {published data only}
- James W, Avenel A, Broom J, Whitehead J. A one year trial to assess the value of orlistat in the management of obesity. International Journal of Obesity 1997;21(Suppl):S24‐30. [PubMed] [Google Scholar]
James 2000 {published data only}
- James WPT, Astrup A, Finer N, Hilsted J, Kopelman P, Rossner S, Saris WHM, Gaal LF, for the STORM Study Group. Effect of sibutramine on weight maintenance after weight loss: a randomised trial. Lancet 2000;256:2119‐25. [DOI] [PubMed] [Google Scholar]
Jones 1995 {published data only}
- Jones S, Smith I, Kelly F, Gray J. Long term weight loss with sibutramine. International Journal of Obesity 1995;19:41. [Google Scholar]
Langlois 1974 {published data only}
- Langlois KJ, Forbes JA, Bell GW, Grant GF Jr. A double‐blind clinical evaluation of the safety and efficacy of phentermine hydrochloride (Fastin) in the treatment of exogenous obesity. Current Therapeutic Research, Clinical and Experimental 1974;16(4):289‐96. [PubMed] [Google Scholar]
Lee 1999a {published data only}
- Lee A, Eddings E. Therapeutic comparison of metformin and fluoxetine alone and in combination in obese subjects with impaired glucose tolerance. Diabetes 1999;48(Suppl 1):A307. [Google Scholar]
Lee 1999b {published data only}
- Lee A. Use of metformin and fluoxetine combination in obese subjects with glucose intolerance. The FASEB Journal 1999;13(4 part 1):A268. [Google Scholar]
Lustman 2000 {published data only}
- Lustman PJ, Freedland KE, Griffith LS, Clouse RE. Fluoxetine for depression in diabetes: a randomized double‐blind placebo‐controlled trial. Diabetes Care 2000;23(5):618‐23. [DOI] [PubMed] [Google Scholar]
Maetzel 2002 {published data only}
- Maetzel A, Ruof J, Covington MT, Anne W. Cost‐effectiveness of treatment of overweight and obese diabetic patients with Orlistat. Diabetes 2002;51:1122. [Google Scholar]
Maheux 1997 {published data only}
- Maheux P, Ducros F, Bourque J, Garon J, Chiasson JL. Fluoxetine improves insulin sensitivity in obese patients with non‐insulin‐dependent diabetes mellitus independently of weight loss. International Journal of Obesity 1997;21(2):97‐102. [DOI] [PubMed] [Google Scholar]
Malchow‐Moller 1981 {published data only}
- Malchow‐Moller A, Larsen S, Hey H, Stokholm KH, Juhl E, Quaade F. Ephedrine as an anorectic: The story of the "Elsinore pill". International Journal of Obesity 1981;5:183‐7. [PubMed] [Google Scholar]
Marcus 1990 {published data only}
- Marcus MD, Wing RR, Ewing L, Kern E, McDermott M, Gooding W. A double‐blind, placebo‐controlled trial of fluoxetine plus behavior modification in the treatment of obese binge‐eaters and non‐binge‐eaters. American Journal of Psychiatry 1990;147:876‐81. [DOI] [PubMed] [Google Scholar]
McLaughlin 2001 {published data only}
- McLaughlin T, Abbasi F, Lamendola C, Kim HS, Reaven GM. Metabolic changes following sibutramine‐assisted weight loss in obese individuals: role of plasma free fatty acids in the insulin resistance of obesity. Metabolism, Clinical and Experimental 2001;50(7):819‐24. [DOI] [PubMed] [Google Scholar]
McMahon 2000 {published data only}
- McMahon FG, Fujioka K, Singh BN, Mendel CM, Rowe E, Rolston K, Johnson F, Mooradian AD, et al. Efficacy and safety of sibutramine in obese white and African American patients with hypertension ‐ A 1‐year, double‐blind, placebo‐controlled, multicenter trial. Archives of Internal Medicine 2000;160(14):2185‐91. [DOI] [PubMed] [Google Scholar]
Meier 1992 {published data only}
- Meier AH, Cincotta AH, Lovell WC. Timed bromocriptine administration reduces body fat stores in obese subjects and hyperglycemia in type II diabetics. Experientia 1992;48(3):248‐53. [DOI] [PubMed] [Google Scholar]
Michelson 1999 {published data only}
- Michelson D, Amsterdam JD, Quitkin FM, Reimherr FW, Rosenbaum JF, Zajecka J, Sundell KL, Kim Y, Beasley CM. Changes in weight during a 1‐year trial of fluoxetine. American Journal of Psychiatry 1999;156(8):1170‐6. [DOI] [PubMed] [Google Scholar]
Miles 2001 {published data only}
- Miles JM, Aronne LJ, Hollander P, Klein S. Effect of orlistat in overweight and obese type 2 diabetes patients treated with metformin. Diabetes 2001;50(Suppl 1):A442‐3. [DOI] [PubMed] [Google Scholar]
Miles 2002b {published data only}
- Miles JM, Halpern A. Effect of orlistat on the need for concomitant anti‐diabetic medication in overweight and obese patients with type 2 diabetes. Diabetes 2002;51(Suppl 2):A475. [Google Scholar]
Pasquali 1987 {published data only}
- Pasquali R, Cesari MP, Melchionda N, Stefanini C, Raitano A, Labo G. Does ephedrine promote weight loss in low‐energy‐adapted obese women?. International Journal of Obesity 1987;11:163‐8. [PubMed] [Google Scholar]
Pedrinola 1996 {published data only}
- Pedrinola F, Sztejnsznajd C, Lima N, Halpern A, Medeiros‐Neto G. The addition of dexfenfluramine to fluoxetine in the treatment of obesity: a randomized clinical trial. Obesity Research 1996;4:549‐54. [DOI] [PubMed] [Google Scholar]
Pijl 2000 {published data only}
- Pijl H, Ohashi S, Matsuda M, Miyazaki Y, Mahankali A, Kumar V, Pipek R, Iozzo P, Lancaster JL, Cincotta AH, DeFronzo RA. Bromocriptine: a novel approach to the treatment of type 2 diabetes. Diabetes Care 2000;23:1154‐61. [DOI] [PubMed] [Google Scholar]
Rasmussen 1993 {published data only}
- Rasmussen MH, Anderson T, Breum L, Gotzsche PC, Hilsted J. Cimetidine suspension as adjuvant to energy restricted diet in treating obesity. British Medical Journal 1993;306:1093‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rissanen 1999b {published data only}
- Rissanen A, Pekkarinen T, Heinanen T, Saltevo J, Taskinen MR. Weight loss with sibutramine in obese patients with type 2 diabetes: a double‐blind, placebo‐controlled study. Obesity Research 1999;7(Suppl 1):S93. [Google Scholar]
Rissanen 2000a {published data only}
- Rissanen A, Finer N, Fujioka K. Sibutramine‐induced weight loss improves lipid profile in obese type 2 diabetics: Results of 3 placebo‐controlled, randomized trials. Diabetes 2000;49(Suppl 1):A270. [Google Scholar]
Rissanen 2000b {published data only}
- Rissanen A, Taskinen MR. Weight loss on sibutramine treatment for 12 months improves lipid profile in obese type 2 diabetic patients. Diabetologia 2000;443:657. [Google Scholar]
Rolls 1998 {published data only}
- Rolls BJ, Shide DJ, Thorwart ML, Ulbrecht JS. Sibutramine reduces food intake in non‐dieting women with obesity. Obesity Research 1998;6:1‐11. [DOI] [PubMed] [Google Scholar]
Rosenfalck 2002 {published data only}
- Rosenfalck AM, Hendel H, Rasmussen MH, Almdal T, Anderson T, Hilsted J, Madsbad S. Minor long‐term changes in weight have beneficial effects on insulin sensitivity and beta‐cell function in obese subjects. Diabetes Obesity and Metabolism 2002;4(1):19‐28. [DOI] [PubMed] [Google Scholar]
Samsa 2001 {published data only}
- Samsa GP, Kolotkin RL, Williams GR, Nguyen MH, Mendel CM. Effect of moderate weight loss on health‐related quality of life: An analysis of combined data from 4 randomized trials of sibutramine vs placebo. American Journal of Managed Care 2001;7(9):875‐83. [PubMed] [Google Scholar]
Sax 1991 {published data only}
- Sax L. Yohimbine does not affect fat distribution in men. International Journal of Obesity 1991;15:561‐5. [PubMed] [Google Scholar]
Seagle 1998 {published data only}
- Seagle HM, Bessesen DH, Hill JO. Effects of sibutramine on resting metabolic rate and weight loss in overweight women. Obesity Research 1998;6:115‐21. [DOI] [PubMed] [Google Scholar]
Seedat 1974 {published data only}
- Seedat YK, Reddy J. Diethylpropion hydrochloride (Tenuate Dospan) in combination with hypotensive agents in the treatment of obesity associated with hypertension. Current Therapeutic Research, Clinical and Experimental 1974;16(5):398‐413. [PubMed] [Google Scholar]
Shi 2001 {published data only}
- Shi YF, Zhu JR. Effect of orlistat on weight loss and glycemic control in overeight Chinese patients with type 2 diabetes. Diabetes 2001;50(Suppl 2):A101. [Google Scholar]
Sirtori 1971 {published data only}
- Sirtori C, Hurwitz A, Azarnoff DL. Hyperinsulinemia secondary to chronic administration of mazindol and d‐amphetamine. The American Journal of Medical Science 1971;261:341‐9. [DOI] [PubMed] [Google Scholar]
Sjostrom 1998 {published data only}
- Sjostrom L, Rissanen A, Andersen T, Boldrin M, Golay A, Koppeschaar HPF, Krempf M. Randomised placebo‐controlled trail of orlistat for weight losss and prevention of weight regain in obese patients; European Multicentre Orlistat Study Group. Lancet 1998;352:167‐72. [DOI] [PubMed] [Google Scholar]
Steel 1973 {published data only}
- Steel JM, Munro JF, Duncan LJ. A comparative trial of different regimens of fenfluramine and phentermine in obesity. Practitioner 1973;211:232‐6. [PubMed] [Google Scholar]
Stoa‐Birketvedt 1993 {published data only}
- Stoa‐Birketvedt G. Effect of cimetidine suspension on appetite and weight in overweight subjects. British Medical Journal 1993;306:1091‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tan 2002 {published data only}
- Tan KC, Tso AW, Tam SC, Pang RW, Lam KS. Acute effect of orlistat on post‐prandial lipaemia and free fatty acids in overweight patients with Type 2 diabetes mellitus. Diabetic Medicine 2002;19:944‐8. [DOI] [PubMed] [Google Scholar]
Thompson 1998 {published data only}
- Thompson RG, Pearson L, Schoenfeld SL, Kolterman OG, and the Pramlintide in Type 2 Diabetes Group. Pramlintide, a synthetic analog of human amylin, improves the metabolic profile of patients with type 2 diabetes using insulin. Diabetes Care 1998;21:987‐93. [DOI] [PubMed] [Google Scholar]
Toft‐Nielsen 1999 {published data only}
- Toft‐Nielsen MB, Madsbad S, Holst JJ. Continuous subcutaneous infusion of glucagon‐like peptide 1 lowers plasma glucose and reduces appetite in type 2 diabetic patients. Diabetes Care 1999;22(7):1137‐43. [DOI] [PubMed] [Google Scholar]
Toplak 1998 {published data only}
- Toplak H, Marhardt K. The reduction of overweight and the improvement of metabolic parameters with the lipase inhibitor orlistat: Preliminary results [German]. Acta Medica Austriaca 1998;25(4‐5):142‐5. [PubMed] [Google Scholar]
Torgerson 2001 {published data only}
- Torgerson JS, Arlinger K, Kappi M, Sjostrom L. Principles for enhanced recruitment of subjects in a large clinical trial. the XENDOS (XENical in the prevention of Diabetes in Obese Subjects) study experience. Controlled Clinical Trials 2001;22(5):515‐25. [DOI] [PubMed] [Google Scholar]
Toubro 1993 {published data only}
- Toubro S, Astrup A, Breum L, Quaade F. The acute and chronic effects of ephedrine/caffeine mixtures on energy expenditure and glucose metabolism in humans. International Journal of Obesity 1993;17(Suppl 3):S73‐7. [PubMed] [Google Scholar]
Van Gaal 1998a {published data only}
- Gaal LF, Broom JI, Enzi G, Toplak H. Efficacy and tolerability of orlistat in the treatment of obesity ‐ A 6‐month dose‐ranging study. European Journal of Clinical Pharmacology 1998;54:125‐32. [DOI] [PubMed] [Google Scholar]
Van Gaal 1998b {published data only}
- Gaal LF, and the STORM study group. Sibutramine trial of obesity reduction and maintenance. Effects on risk factors. International Journal of Obesity 1998;22(Suppl 3):S272. [Google Scholar]
Vanloon 1992 {published data only}
- Vanloon BJP, Radder JK, Frolich M, Krans HMJ, Zwinderman AH, Meinders AE. Fluoxetine increases insulin action in obese type‐II (non‐insulin‐dependent) diabetic‐patients. International Journal of Obesity 1992;16(Suppl):S55‐61. [PubMed] [Google Scholar]
Vernace 1974 {published data only}
- Vernace BJ. Controlled comparative investigation of mazindol, D‐amphetamine, and placebo. Obesity/Bariatric Medicine 1974;3:124‐9. [Google Scholar]
Wadden 1995 {published data only}
- Wadden TA, Bartlett SJ, Foster GD, Greenstein RA, Wingate BJ, Stunkard AJ, Letizia KA. Sertraline and relapse prevention training following treatment by very‐low‐calorie diet: A controlled clinical trial. Obesity Research 1995;3:549‐57. [DOI] [PubMed] [Google Scholar]
Wadden 1997 {published data only}
- Wadden TA, Berkowitz RI, Vogt RA, Steen SN, Stunkard AJ, Foster GD. Lifestyle modification in the pharmacologic treatment of obesity: a pilot investigation of a potential primary care approach. Obesity Research 1997;5:218‐26. [DOI] [PubMed] [Google Scholar]
Wadden 2001 {published data only}
- Wadden TA, Berkowitz RI, Sarwer DB, Prus‐Wisniewski R, Steinberg C. Benefits of lifestyle modification in the pharmacologic treatment of obesity: a randomized trial. Archives of Internal Medicine 2001;161:218‐7. [DOI] [PubMed] [Google Scholar]
Walker 1977 {published data only}
- Walker BR, Ballard IM, Gold JA. A multicentre atudy comparing mazindol and placebo in obese patients. Journal of International Medical Research 1977;5(2):85‐90. [DOI] [PubMed] [Google Scholar]
Wasada 2000 {published data only}
- Wasada T, Kawahara R, Iwamoto Y. Lack of evidence for bromocriptine effect on glucose tolerance, insulin resistance, and body fat stores in obese type 2 diabetic patients. Diabetes Care 2000;23:1039‐40. [DOI] [PubMed] [Google Scholar]
Wilding 1998 {published data only}
- Wilding JPH, Stolshek B. Obesity with orlistat (Xenical) helps to prevent deterioration in glucose tolerance. Diabetologia 1998;41(Suppl 1):A126. [Google Scholar]
Wilding 1999 {published data only}
- Wilding JPH. Orlistat‐induced weight loss improves insulin resistance in obese patients. Diabetologia 1999;42(Suppl 1):A215. [Google Scholar]
Wilding 2001 {published data only}
- Wilding JPH. Early response to orlistat treatment predicts long‐term success in overweight and obese patients with co‐morbidities. International Journal of Obesity 2001;25(Suppll 2):S108. [Google Scholar]
Williams 1981 {published data only}
- Williams RA, Foulsham BM. Weight reduction in osteoarthritis using phentermine. Practitioner 1981;225:231‐2. [PubMed] [Google Scholar]
Wilson 1960 {published data only}
- Wilson R, Long C. A clinical evaluation of Tenuate‐ a new anti‐appetite compound. Journal of Irish Medical Association 1960;46:86‐8. [PubMed] [Google Scholar]
Wirth 2001 {published data only}
- Wirth A, Krause J. Long‐term weight losss with sibutramine: a randomized, controlled trial. Journal of American Medical Association 2001;286(11):1331‐9. [DOI] [PubMed] [Google Scholar]
Woodhouse 1975 {published data only}
- Woodhouse SP, Nye ER, Anderson K, Rawlings J. A double‐blind controlled trial of a new anorectic agent AN448. The New Zealand Medical Journal 1975;81:546‐9. [PubMed] [Google Scholar]
Yoshida 1994 {published data only}
- Yoshida T, Sakane N, Umekawa T, Yoshioka K, Kondo M, Wakabayashi Y. Usefulness of mazinol in combined diet therapy consisting of low‐calorie diet and optifast in severely obese women. International Journal of Clinical Pharmacology 1994;104:125‐32. [PubMed] [Google Scholar]
Zavoral 1998 {published data only}
- Zavoral JH. Treatment with orlistat reduces cardiovascular risk in obese patients. Journal of Hypertension 1998;16:2013‐7. [DOI] [PubMed] [Google Scholar]
Ziegler 1971 {published data only}
- Ziegler A. Therapy of obesity with "Redukal". Das Deutsche Gesundheitswesen 1971;26(27):1247‐51. [PubMed] [Google Scholar]
Additional references
ADA 2003
- American Diabetes Association. Standards of medical care for patients with diabetes mellitus. Diabetes Care 2003;26(suppl 1):S33‐S50. [DOI] [PubMed] [Google Scholar]
Alberti 1998
- Alberti KM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part I: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetic Medicine 1998;15:539‐53. [DOI] [PubMed] [Google Scholar]
Allison 1996
- Allison DB, Faith MS, Gorman BS. Publication bias in obesity treatment trials?. International Journal of Obesity 1996;20:931‐7. [PubMed] [Google Scholar]
Als‐Nielson 2003
- Als‐Nielson B, Chen W, Gluud C, Kjaergard LL. Association of funding and conclusions in randomized drug trials; a reflection of treatment effect or adverse events?. Journal of the American Medical Association 2003;290:921‐8. [DOI] [PubMed] [Google Scholar]
Anderson 2001
- Anderson JW, Konz EC. Obesity and disease management: effects of weight loss on comorbid conditions. Obesity Research 2001;9(suppl 4):326S‐34S. [DOI] [PubMed] [Google Scholar]
Berlin 1997
- Berlin JA. Does blinding of readers affect the results of meta‐analyses? Results of a randomized trial. Lancet 1997;350(9072):185‐6. [DOI] [PubMed] [Google Scholar]
Blackburn 1987
- Blackburn GL, Kanders BS. Medical evaluation of the obese patient with cardiovascular disease. American Journal of Cardiology 1987;60:55G‐8G. [DOI] [PubMed] [Google Scholar]
Bray 1999a
- Bray GA, Greenway FL. Current and potential drugs for treatment of obesity. Endocrinology Reviews 1999;20:805‐75. [DOI] [PubMed] [Google Scholar]
Brown 1996
- Brown SA, Upchurch S, Anding R, Winter M, Ramirez G. Promoting weight loss in type II diabetes. Diabetes Care 1996;19:613‐24. [DOI] [PubMed] [Google Scholar]
Brownell 1987
- Brownell KD, Jeffery RW. Improving long‐term weight loss: pushing the limits of treatment. Behavioral Therapy 1987;18:353‐74. [Google Scholar]
Clarke 2003
- Clarke M, Oxman AD. Cochrane Reviewers' Handbook 4.2.0 (updated March 2003). Oxford: The Cochrane Library, 2003. [Google Scholar]
Cochran 1954
- Cochran WG. The combination of estimates from different experiments. Biometrics 1954;10:101‐129. [Google Scholar]
Craighead 1981
- Craighead LW, Stunkard AJ, O'Brein RM. Behavior therapy and pharmacotherapy for obesity. Archives of General Psychiatry 1981;38:763‐8. [DOI] [PubMed] [Google Scholar]
Data Group 1979
- National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039‐57. [DOI] [PubMed] [Google Scholar]
DCCT 1993
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. New England Journal of Medicine 1993;329:977‐86. [DOI] [PubMed] [Google Scholar]
DerSimonian 1954
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1954;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Devereaux 2002
- Devereaux PJ, Manns BJ, Ghali WA, Quan H, Lacchetti C, Montori VM, Bhandari M, Guyatt GH. Physican interpretations and textbook definitions of blinding terminology in randomized controlled trials. Journal of the American Medical Association 2002;285:2000‐3. [DOI] [PubMed] [Google Scholar]
DPP 2002
- Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. New England Journal of Medicine 2002;346:393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elphick 2002
- Elphick HE, Tan A, Ashby D, Smyth RL. Systematic reviews and lifelong diseases. British Medical Journal 2002;325:381‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Expert Committee
- The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2002;25(suppl 1):S5‐S20. [DOI] [PubMed] [Google Scholar]
Flegal 2002
- Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among U.S. adults, 1999‐2000. Journal of American Medical Association 2002;288:1723‐7. [DOI] [PubMed] [Google Scholar]
Goldstein 1994a
- Goldstein DJ, Potvin JH. Long‐term weight loss: the effect of pharmacologic agents. American Journal of Clinical Nutrition 1994;60(5):647‐57. [DOI] [PubMed] [Google Scholar]
Greenway 1999
- Greenway F. Obesity medications and the treatment of type 2 diabetes. Diabetes Technology & Therapeutics 1999;1(3):277‐87. [DOI] [PubMed] [Google Scholar]
Hauner 1999
- Hauner H. The impact of pharmacotherapy on weight management in type 2 diabetes. International Journal of Obesity & Related Metabolic Disorders 1999;23(suppl 7):S12‐S17. [DOI] [PubMed] [Google Scholar]
Health Canada
- Health Canada. Advisory: Health Canada investigates safety of MERIDIA (sibutramine). Health Canada Online 3‐27‐2002. Jan 10, 2003.
HT Trials 1997
- The Trials of Hypertension Prevention Collaborative Research Group. Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high‐normal blood pressure. Archives of Internal Medicine 1997;157:657‐67. [PubMed] [Google Scholar]
Irwig 1994
- Irwig L, Toteson A, Gatsonis C, Lau J, Colditz G, Chalmers TC, Mosteller F. Guidelines for meta‐analyses evaluating diagnostic tests. Annals of Internal Medicine 1994;120:667‐76. [DOI] [PubMed] [Google Scholar]
Jaded 1998
- Jadad A. Assessment the quality of RCTs: why, what, how, and by whom?. Randomised Controlled Trials. London: British Medical Journal Books, 1998. [Google Scholar]
Kaplan 1987
- Kaplan RM, Atkins CJ. Selective attrition causes overestimates of treatment effects in studies of weight loss. Addictive Behavior 1987;12:297‐302. [DOI] [PubMed] [Google Scholar]
King 1998
- King H, Aubert RE, Herman WH. Global Burden of diabetes, 1995‐2025: prevalence, numerical estimates, and projections. Diabetes Care 1998;21:1414‐31. [DOI] [PubMed] [Google Scholar]
Kramer 1989
- Kramer FM, Jeffery RW, Forster JL, Snell MK. Long‐term follow‐up of behavioral treatment for obesity: patterns of weight regain in men and women. International Journal of Obesity 1989;13:123‐36. [PubMed] [Google Scholar]
Law 1994
- Law MR, Walk NJ, Thompson SG. By how much and how quickly does reduction in serum cholesterol concentration lower risk of ischaemic heart disease?. British Medical Journal 1994;308:367‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 1999
- Lee CD, Blair SN, Jackson AS. Cardiorespiratory fitness, body composition, and all‐cause and cardiovascular disease mortality in men. American Journal of Clinical Nutrition 1999;69:373‐80. [DOI] [PubMed] [Google Scholar]
Maggio 1997
- Maggio CA, Pi‐Sunyer FX. The prevention and treatment of obesity. Application to type 2 diabetes. Diabetes Care 1997;20(11):1744‐66. [DOI] [PubMed] [Google Scholar]
Mokdad 2000
- Mokdad A, Ford E, Bowman B, Nelson D, Engelgau M, Vinicor F, Marks JS. Diabetes trends in the U.S.: 1990‐1998. Diabetes Care 2000;23(9):1278‐83. [DOI] [PubMed] [Google Scholar]
Mokdad 2001
- Mokad AH, Bowman BA, Ford ES, Vinicor F, Marks J, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. Journal of the American Medical Association 2001;286(10):1195‐7. [DOI] [PubMed] [Google Scholar]
National Task Force
- National Task Force on the Prevention and Treatment of Obesity. Long‐term pharmacotherapy in the management of obesity. Journal of the American Medical Association 1996;276:1907‐15. [DOI] [PubMed] [Google Scholar]
NHLBI 1998
- National Heart, Lung and Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overwieght and obesity in adults: the evidence report. Bethesda, MD: National Institutes of Health, 1998. [Google Scholar]
NIH 1985
- National Institutes of Health. Health implications of obesity: National Institutes of Health Consensus Development Conference Statement. Annals of Internal Medicine 1985;1034:1073‐77. [PubMed] [Google Scholar]
O'Meara 1998
- O'Meara S, Glenny A‐M, Sheldon T, Melville A, Wilson C. Systematic review of the effectiveness of interventions used in the management of obesity. Journal of Human Nutrition and Dietetics 1998;11:203‐6. [Google Scholar]
Padwal 2004
- R Padwal, SK Li, DCW Lau. Long‐term pharmacotherapy for obesity and overweight Long‐term pharmacotherapy for obesity and overweight. The Cochrane Database of Systematic Reviews 2004, Issue Issue 4. [Google Scholar]
Phelan 2002
- Phelan S, Wadden TA. Combining behavioral and pharmacological treatments for obesity. Obesity Research 2002;10(6):560‐74. [DOI] [PubMed] [Google Scholar]
Pi‐Sunyer 1993
- Pi‐Sunyer FX. Medical hazards of obesity. Annals of Internal Medicine 1993;119:655‐60. [DOI] [PubMed] [Google Scholar]
Pi‐Sunyer 2000
- Pi‐Sunyer FX. Weight loss and mortality in type 2 diabetes. Diabetes Care 2000;23:1451‐2. [DOI] [PubMed] [Google Scholar]
Scheen 2000
- Scheen AJ, Lefebvre PJ. Antiobesity pharmacotherapy in the management of type 2 diabetes. Diabetes and Metabolism Research Reviews 2000;16(2):114‐24. [DOI] [PubMed] [Google Scholar]
Scheen 2002
- Scheen AJ, Ernest P. New antiobesity agents in type 2 diabetes: Overview of clinical trials with sibutramine and orlistat. Diabetes & Metabolism (Paris) 2002;28:437‐45. [PubMed] [Google Scholar]
Sterne 2001
- Sterne JAC, Egger M, Davey Smith G. Investigating and dealing with publication and other biases. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care; Meta‐analysis in Context. Second Edition. London: BMJ Publishing, 2001:189‐208. [Google Scholar]
Tang 2000
- Tang JL, Liu JLY. Misleading funnel plot for detection of bias in meta‐analysis. Journal of Clinical Epidemiology 2000;53:477‐84. [DOI] [PubMed] [Google Scholar]
Task Force 2000
- Task Force on Community Preventive Services. Introducing the Guide to Community Preventive Services: methods, first recommendations and expert commentary. American Journal of Preventive Medicine 2000;18(suppl 1):1‐142. [Google Scholar]
Thornton 2000
- Thornton A, Lee P. Publication bias in meta‐analysis: its causes and consequences. Journal of Clinical Epidemiology 2000;53:207‐16. [DOI] [PubMed] [Google Scholar]
Tuomilehto 2001
- Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne‐Parikka P, Keinanen‐Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M, Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. New England Journal of Medicine 2001;344:1343‐50. [DOI] [PubMed] [Google Scholar]
U.S. DHHS 1997
- U. S. Department of Health and Human Services. Cardiac valvulopathy associated with exposure to fenfluramine and dexfenfluramine: U.S. Department of Health and Human Services Interim Public Health Recommendations. Morbidity and Mortality Weekly Report 1997;46:1061‐6. [PubMed] [Google Scholar]
U.S. DHHS 2002
- U. S. Department of Health and Human Services, CDC, Atlanta GA. National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2000. 2002://www.cdc.gov/diabetes/pubs/factsheet.htm (Accessed Oct. 2, 2003).
U.S. DHHS 2002b
- U.S. Department of Health and Human Services. Diabetes: disabling, deadly and on the rise, 2002. At‐A‐Glance. Atlanta, GA: National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention. [Google Scholar]
UKPDS 1998
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). The Lancet 1998;352:837‐53. [PubMed] [Google Scholar]
Valdez 2002
- Valdez R, Gregg EW, Williamson DF. Effects of weight loss on morbidity and mortality. In: Fairburn CG, Brownell KD editor(s). Eating Disorders and Obesity. The Guilford Press, 2002:490‐4. [Google Scholar]
Wadden 1989
- Wadden TA, Sternberg JA, Letizia KA, Stunkard AJ, Foster GD. Treatment of obesity by very low calorie diet, behavior therapy, and their combination: a five‐year perspective. International Journal of Obesity 1989;13:39‐46. [PubMed] [Google Scholar]
Wadden 2000
- WaddenTA, Foster GD. Behavioral treatment of obesity. Medical Clinics of North America 2000;85:441‐61. [DOI] [PubMed] [Google Scholar]
Wadden 2001a
- Wadden TA, Berkowitz RI, Sarwer DB, Prus‐Wisniewski R, Steinberg C. Benefits of lifestyle modification in the pharmacologic treatment of obesity: a randomized trial. Archives of Internal Medicine 2001;161:218‐27. [DOI] [PubMed] [Google Scholar]
WHO 2002
- World Health Organization. The global strategy on diet, physical activity and health. http://www.who.int/hpr/NPH/docs/gs_global_strategy_general.pdf 2002 (Accessed Sept. 15, 2003). [Google Scholar]
WHO 2003
- World Health Organization. Obesity and overweight. http://www.who.int/hpr/NPH/docs/gs_obesity.pdf 2003 (Accessed Sept. 15, 2003). [Google Scholar]
WHO Committee 1980
- WHO Expert Committee on Diabetes Mellitus. World Health Organization Technical Report. World Health Organization Technical Report Series. Vol. 646, Geneva: World Health Organization, 1980:1‐80. [PubMed] [Google Scholar]
WHO Committee 1985
- WHO Expert Committee on Diabetes Mellitus. World Health Organization Technical Report.. World Health Organization Technical Report Series 727. Vol. 727, Geneva: World Health Organization, 1985. [Google Scholar]
Williamson 2000
- Williamson DF, Thompson TJ, Thun M, Flanders D, Pamuk E, Byers T. Intentional weight loss and mortality among overweight individuals with diabetes. Diabetes Care 2000;23:1499‐1504. [DOI] [PubMed] [Google Scholar]
Wing 1985
- Wing R, Epstein L, Nowalk M, Koeske R, Hagg S. Behavior change, weight loss, and physiological improvements in type II diabetic patients. Journal of Consulting and Clinical Psychology 1985;53(1):111‐22. [DOI] [PubMed] [Google Scholar]
Wing 1987
- Wing RR, Koeske R, Epstein LH, Nowalk MP, Gooding W, Becker D. Long‐term effects of modest weight loss in type II diabetic patients. Archives of Internal Medicine 1987;147:1749‐53. [PubMed] [Google Scholar]
Wing 1991
- Wing RR, Marcus MD, Salata R, Epstein LH, Miaskiewicz S, Blair E. Effects of a very‐low‐calorie diet on long‐term glycemic control in obese type 2 diabetic subjects. Archives of Internal Medicine 1991;151:1334‐40. [PubMed] [Google Scholar]
Wing 1992
- Wing RR. Behavioral treatment of severe obesity. American Journal of Clinical Nutrition 1992;55:S545‐51. [DOI] [PubMed] [Google Scholar]
Wing 2000
- Wing RR. Weight loss in the management of type 2 diabetes. In: Gerstein HC, Haynes RB editor(s). Evidence‐Based Diabetes Care. Ontario, Canada: B.C. Decker, Inc, 2000:252‐76. [Google Scholar]
Wing 2001
- Wing RR, Gorin A, Tate D. Strategies for changing eating and exercise behavior. In: Bowman BA, Russell RM editor(s). Present knoweldge in nutrition. Eighth. Washington, DC: ILSI Press, 2001:650‐60. [Google Scholar]
Wolfe 2002
- Wolfe S, Sasich LD, Barbehenn E. Letter from Public Citizen to Tommy Thompson, Secretary DHHS. Public Citizen Website 2002 (Accessed March 19, 2003).
Yanovski 2002
- Yanovski SZ, Yanovski JA. Obesity. New England Journal of Medicine 2002;346:591‐602. [DOI] [PubMed] [Google Scholar]
York CRD 1997
- The University of York Centre for Reviews and Dissemination. The prevention and treatment of obesity. Effective Health Care Bulletin. London: The Royal Society of Medicine Press Ltd. [Google Scholar]


