Abstract
Background
Epilepsy is a common neurological condition that affects almost 0.5% to 1% of the population. Nearly 30% of people with epilepsy are resistant to currently available drugs. Tiagabine is one of the newer antiepileptic drugs; its effects as an adjunct (add‐on) to standard drugs are assessed in this review.
Objectives
To evaluate the effects of add‐on treatment with tiagabine on seizures, adverse effects, cognition and quality of life for people with drug‐resistant localisation‐related seizures.
Search methods
This is an updated version of the original Cochrane review published in 2012 (Issue 5). We searched the Cochrane Epilepsy Group Specialised Register (November 2013), the Cochrane Central Register of Controlled Trials (CENTRAL, 2013, Issue 10) and MEDLINE (1946 to November 2013). No language restrictions were imposed. We also contacted the manufacturers of tiagabine and experts in the field to seek any ongoing or unpublished studies.
Selection criteria
Randomised placebo‐controlled add‐on trials of people of any age with localisation‐related seizures in which an adequate method of concealment of randomisation was used were included. The studies could be double‐blind, single‐blind or unblinded and of parallel or cross‐over design. They had to have a minimum treatment period of eight weeks. Trials using an active drug control group were also included.
Data collection and analysis
Two review authors independently selected trials for inclusion and extracted data. Disagreements were resolved by discussion. Outcomes investigated included 50% or greater reduction in seizure frequency, treatment withdrawal, adverse effects, effects on cognition and quality of life. The primary analyses were performed by intention‐to‐treat. Worst‐case and best‐case analyses were calculated for seizure outcomes. Dose response was evaluated in regression models. Risk of bias in each study was assessed by two review authors using the Cochrane 'Risk of bias' tool.
Main results
Four parallel‐group and two cross‐over group trials were included. The overall risk ratio (RR) with 95% confidence intervals (CIs) for a 50% or greater reduction in seizure frequency (tiagabine vs placebo) was 3.16 (95% CI 1.97 to 5.07). Because of differences in response rates among trials, regression models were unable to provide reliable estimates of response to individual doses. The RR for treatment withdrawal was 1.81 (95% CI 1.25 to 2.62). The 99% CIs for the adverse effects of dizziness, fatigue, nervousness and tremor did not include unity, indicating that they are significantly associated with tiagabine. For cognitive and quality of life outcomes, the limited available data suggested no significant effects on cognition and mood and adjustment. Two of the five studies were judged as having low risk of bias, three studies unclear risk of bias and one study high risk of bias. Overall study quality was rated as high using the GRADE approach.
Authors' conclusions
Tiagabine reduces seizure frequency but is associated with some adverse effects when used as an add‐on treatment for people with drug‐resistant localisation‐related seizures.
Keywords: Humans; Drug Resistance; Anticonvulsants; Anticonvulsants/adverse effects; Anticonvulsants/therapeutic use; Cognition; Cognition/drug effects; Epilepsies, Partial; Epilepsies, Partial/drug therapy; Nipecotic Acids; Nipecotic Acids/adverse effects; Nipecotic Acids/therapeutic use; Randomized Controlled Trials as Topic; Tiagabine
Tiagabine add‐on for drug‐resistant partial epilepsy
Epilepsy is a disorder in which recurrent seizures are caused by abnormal electrical discharges from the brain. Most seizures can be controlled by a single antiepileptic drug (AED); this approach is known as monotherapy. Unfortunately, some people require more than one antiepileptic drug to control their seizures, especially if these originate from one area of the brain (partial epilepsy), instead of being generalised.
Tiagabine is a newer antiepileptic drug that has been used as an additional AED to monotherapy. This review looks at the evidence about how effective tiagabine is in reducing seizures, as well as the side effects that may be associated with its use.
A search of databases was carried out on 11 November 2013. Six trials were found that included 900 people with partial epilepsy. These trials were all randomised controlled trials (RCTs) that compared the antiepileptic drug topiramate versus a placebo drug or a different AED for a period of up to 24 weeks. By taking all evidence from the trials into account, the review found that tiagabine is effective when used with other drugs to reduce the number of seizures in drug‐resistant partial epilepsy. However, adding tiagabine to the usual treatment is associated with an increase in side effects such as dizziness, fatigue, nervousness and tremor.
Trials were assessed with regards to bias and quality, and overall, the quality of evidence for the outcome of seizure reduction was rated as high. The trials included in this review did not examine the long‐term effects of topiramate as an add‐on treatment. Future research is needed to determine how this drug performs in comparison with other newer antiepileptic drugs.
Summary of findings
Summary of findings for the main comparison.
Tiagabine versus placebo control—50% or greater reduction in seizure frequency for drug‐resistant partial epilepsy
| Tiagabine versus placebo control—50% or greater reduction in seizure frequency for drug‐resistant partial epilepsy | ||||||
| Patient or population: patients with drug‐resistant partial epilepsy Settings: hospital outpatient setting Intervention: tiagabine versus placebo control—50% or greater reduction in seizure frequency | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Tiagabine versus placebo control—50% or greater reduction in seizure frequency | |||||
| Intention‐to‐treat analysis | Study population | RR 3.16 (1.97 to 5.07) | 769 (three studies) | ⊕⊕⊕⊕ high | All three trials found significant improvement in the tiagabine add‐on group compared with the placebo group | |
| 69 per 1000 | 218 per 1000 (136 to 350) | |||||
| Moderate | ||||||
| 65 per 1000 | 205 per 1000 (128 to 330) | |||||
| Worst‐case scenario | Study population | RR 2.7 (1.75 to 4.19) | 769 (three studies) | ⊕⊕⊕⊕ high | When participants with missing data were treated as non‐responders, tiagabine add‐on was still significantly more effective in reducing seizures than placebo | |
| 80 per 1000 | 216 per 1000 (140 to 335) | |||||
| Moderate | ||||||
| 65 per 1000 | 176 per 1000 (114 to 272) | |||||
| Best‐case scenario | Study population | RR 3.32 (2.08 to 5.32) | 769 (three studies) | ⊕⊕⊕⊕ high | The overall effect was increased slightly when participants with missing data were treated as responders to tiagabine | |
| 69 per 1000 | 229 per 1000 (144 to 368) | |||||
| Moderate | ||||||
| 65 per 1000 | 216 per 1000 (135 to 346) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Background
Description of the condition
Epilepsy is a common neurological condition, with an estimated incidence of 50 per 100,000 and a prevalence of five to 10 per 1000 in the developed world (Sander 1996). Between 2% and 3% of the population will be given a diagnosis of epilepsy at some time in their lives (Hauser 1993); most will go into remission. However, up to 30% will continue to have seizures (i.e. become drug‐resistant) despite treatment with adequate doses of antiepileptic drugs (AEDs) (Cockerell 1995) and often are treated with combinations of AEDs. No definition of 'drug‐resistant' has been internationally accepted, and for the purposes of this review, people will be considered drug‐resistant if they have failed to respond to a minimum of two AEDs given as monotherapy. Most of these drug‐resistant individuals have partial‐onset seizures, which are divided into three types: simple partial, complex partial and secondary generalised tonic‐clonic seizures (Commission 1989).
Description of the intervention
Over the past 10 to 15 years, renewed interest has been seen in the development of new AEDs, as standard drugs (e.g. carbamazepine, phenytoin, valproate) do not leave all patients seizure‐free, and they are not without adverse effects. In the first instance, new AEDs are tested in RCTs as add‐on treatment for people with drug‐resistant partial epilepsy. Because they have demonstrated a therapeutic effect in these trials, new AEDs tend to be licensed for add‐on use before monotherapy trials have been undertaken in which new AEDs are compared with standard AEDs.
How the intervention might work
Tiagabine, one of these newer drugs, exerts its antiepileptic effect by inhibiting presynaptic and glial uptake of gamma‐aminobutyric acid (GABA) (Ostergaard 1995).
Why it is important to do this review
This review is part of a series of reviews conducted to investigate the newer AEDs. An initial tiagabine review reported its effects on seizure frequency and adverse effects (Marson 1996; Marson 1997). In this initial review, the most common adverse effects were investigated. However, one concern is that AEDs may impair cognitive abilities, and in this Cochrane review, we have included outcomes assessing cognitive effects. In addition, we have chosen to include quality of life outcomes to assess the global impact of this drug on well‐being. In this review, we assess the effects of tiagabine on seizures, adverse effects, cognition and quality of life when used as add‐on treatment for people with drug‐resistant localisation‐related seizures.
Objectives
To evaluate the effects of add‐on treatment with tiagabine on seizures, adverse effects, cognition and quality of life for people with drug‐resistant localisation‐related seizures.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials in which an adequate method of concealment of randomisation was used (e.g. allocation of sequentially sealed packages of medication, sealed opaque envelopes, telephone randomisation).
Double‐blind, single‐blind or unblinded trials.
Placebo‐controlled or active drug control group.
Parallel‐group or cross‐over studies.
Minimum treatment period of eight weeks.
Types of participants
People of any age with drug‐resistant localisation‐related seizures (i.e. experiencing simple partial, complex partial or secondary generalised tonic‐clonic seizures).
Types of interventions
The active treatment group received treatment with tiagabine, in addition to conventional antiepileptic drug treatment; the control group received matched placebo or a different add‐on AED, in addition to conventional antiepileptic drug treatment.
Types of outcome measures
Primary outcomes
50% or greater reduction in seizure frequency
The proportion of people with a 50% or greater reduction in seizure frequency during the treatment period compared with the prerandomisation baseline period was chosen as the primary outcome. This outcome was chosen as it is commonly reported in this type of study and can be calculated for studies that do not report this outcome, provided that baseline seizure data were recorded.
Secondary outcomes
Treatment withdrawal
The proportion of people who have treatment withdrawn during the course of the treatment period was used as a measure of global effectiveness. Treatment is likely to be withdrawn because of adverse effects, lack of efficacy or a combination of the two, and this is an outcome to which individuals make a direct contribution. In trials of short duration, it is likely that adverse effects will be the most common reason for withdrawal.
Adverse effects
1. The proportion of people experiencing any of the following five adverse effects, considered by the review authors to be common and important adverse effects of antiepileptic drugs. (a) Ataxia. (b) Dizziness. (c) Fatigue. (d) Nausea. (e) Somnolence.
2. The proportion of people experiencing the five most common adverse effects if different from 1. above.
Cognitive effects
At present, no consensus has been reached on which instruments should be used to assess the effects of AEDs on cognition. As a result, assessment of cognitive effects has been approached in a heterogeneous way (Cochrane 1998). In view of this difficulty, in the first instance we tabulated results but made no attempt to combine results in a meta‐analysis.
Quality of life
Once again, no consensus has been reached on which instruments should be used to assess quality of life (Baker 2000); in the first instance we tabulated results but made no attempt to combine results in a meta‐analysis.
Search methods for identification of studies
Electronic searches
This review is an update of a Cochrane review published in 2012. We searched the following databases and applied no language restrictions.
The Cochrane Epilepsy Group Specialised Register (11 November 2013).
The Cochrane Central Register of Controlled Trials (CENTRAL, 2013, Issue 10), using the search strategy outlined in Appendix 1.
MEDLINE (Ovid) (1946 to 11 November 2013), using the strategy outlined in Appendix 2.
Searching other resources
We reviewed the reference lists of retrieved studies to search for additional reports of relevant studies.
We contacted Sanofi‐Synthelabo (makers of tiagabine) and experts in the field to ask for information about unpublished or ongoing studies.
Data collection and analysis
Selection of studies
Two review authors (JP and AGM) independently assessed trials for inclusion. Disagreements were resolved by mutual discussion. The same two review authors extracted data and assessed risk of bias, with disagreements again resolved by discussion.
Data extraction and management
The following information was extracted for each trial using a data extraction form.
Trial design
Methods of sequence generation and randomisation concealment.
Method of blinding.
Whether any people had been excluded from reported analyses.
Duration of baseline period.
Duration of treatment period.
Dose(s) of tiagabine tested.
Participant demographic information
Total number of people allocated to each treatment group.
Age/sex of participants.
Number with localisation‐related/generalised seizures.
Seizure frequency during baseline period.
Number of background drugs.
All trials found so far have been sponsored by Sanofi‐Synthelabo, which confirmed the following information.
Method of randomisation.
Total number randomly assigned to each group.
Number of people in each group achieving a 50% or greater reduction in seizure frequency per treatment group.
Number of people having treatment withdrawn post randomisation per treatment group.
-
For those excluded:
the reason for exclusion;
whether any of those excluded completed the treatment phase;
whether any of those excluded had a 50% or greater reduction in seizure frequency during the treatment phase.
Outcomes
The number of people experiencing each outcome was recorded per randomly assigned group.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias for each trial using the Cochrane 'Risk of bias table', as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2010). Disagreements were discussed and resolved. Included studies were rated as adequate, inadequate or unclear on six domains applicable to randomised controlled trials: randomisation method, allocation concealment, blinding methods, incomplete outcome data, selective outcome reporting and other sources of bias. 'Summary of findings' tables were created for which the GRADE approach to assessing quality of evidence was employed.
Measures of treatment effect
The primary outcome of seizure reduction was presented as a risk ratio. Secondary outcomes, including seizure freedom, treatment withdrawal and adverse effects, were presented as risk ratios.
Unit of analysis issues
For continuous outcome data (i.e. cognition and quality of life), it was expected that different measures may be used for these outcomes. If this was found to be the case, the standardised mean difference would be employed to present these data if this was deemed appropriate and if the data were available.
Dealing with missing data
Missing data were sought from the study authors. We carried out intention‐to‐treat, best‐case and worst‐case analyses on the primary outcome to account for missing data. All analyses are presented in the main report.
Assessment of heterogeneity
Clinical heterogeneity was assessed by comparing the distribution of important individual participant factors among trials (e.g. age, seizure type, duration of epilepsy, number of AEDs taken at the time of randomisation) and trial factors (e.g. randomisation concealment, blinding, losses to follow‐up). Statistical heterogeneity was examined using a Chi2 test and the I2 statistic for heterogeneity; provided no significant heterogeneity was present (P > 0.10), we employed a fixed‐effect model. In the event that heterogeneity was found, a random‐effects model analysis was planned using the inverse variance method.
Assessment of reporting biases
All protocols were requested from study authors to enable a comparison of outcomes of interest. Outcome reporting bias will be investigated using the ORBIT matrix system (Kirkham 2010). Examination of funnel plots was planned; however, this was not undertaken because of the small number of trials included.
Data synthesis
A fixed‐effect model meta‐analysis was employed to synthesise the data. We expected to carry out the following analyses.
Intervention group versus controls on seizure reduction.
Intervention group versus controls on treatment withdrawal.
Intervention group versus controls on adverse effects.
Intervention group versus controls on cognitive effects.
Intervention group versus controls on quality of life.
Each comparison was to be stratified by type of control group, that is, placebo or active control, and by study characteristics to ensure the appropriate combination of study data.
Our preferred estimator was the Mantel‐Haenszel risk ratio (RR). For the outcomes of 50% or greater reduction in seizure frequency and treatment withdrawal, we used 95% confidence intervals (CIs). For individual adverse effects, we used 99% CIs to make an allowance for multiple testing.
Our analyses included all participants in the treatment group to which they had been allocated. For the efficacy outcome (50% or greater reduction in seizure frequency), we undertook three analyses.
Primary (intention‐to‐treat (ITT)) analysis: Participants not completing follow‐up or with inadequate seizure data were assumed non‐responders. To test the effect of this assumption, we undertook the following sensitivity analyses. Analysis was done by ITT when this information was reported by the included studies.
Worst‐case analysis: Participants not completing follow‐up or with inadequate seizure data were assumed to be non‐responders in the calcium antagonist group and responders in the placebo group.
Best‐case analysis: Participants not completing follow‐up or with inadequate seizure data were assumed to be responders in the calcium antagonist group and non‐responders in the placebo group.
Dose regression analysis
Dose‐response relationships were examined using logistic regression in the framework of generalised linear models (McCullagh 1989) and the package GLIM. For these models, a binary variable was defined with value 0 if the response was less than 50% and with value 1 otherwise. Models included an indicator variable for trials. To examine the effect of dose, the following were considered as possible explanatory variables: dose levels, dose as a continuous variable and logarithmic transformation of dose. Interactions between dose and trials were also considered. Odds ratios and response rates were calculated.
Cognitive and quality of life data
As stated under 'Outcomes' in the first instance, data for these outcomes were tabulated, but no attempt was made to undertake a meta‐analysis. We found that trials had not used similar outcome measures, so we did not undertake a meta‐analysis, as it was deemed inappropriate to do so.
Subgroup analysis and investigation of heterogeneity
Subgroup analysis was undertaken for adverse effects. We intended to investigate heterogeneity using sensitivity analysis if deemed appropriate.
Sensitivity analysis
We also intended to carry out sensitivity analysis if peculiarities were found between study quality, characteristics of participants, interventions and outcomes.
Results
Description of studies
Results of the search
The search (November 2011 to November 2013) revealed 10 records identified from the databases outlined in Electronic searches. Seven records remained after duplicates (three) were removed, and all were screened for inclusion in the review. All seven were excluded at this point because of irrelevance; therefore, no new studies are included in this review update. A total of six studies are included in this review, three of which were included in meta‐analyses. See Figure 1 for a diagram of study flow.
Figure 1.
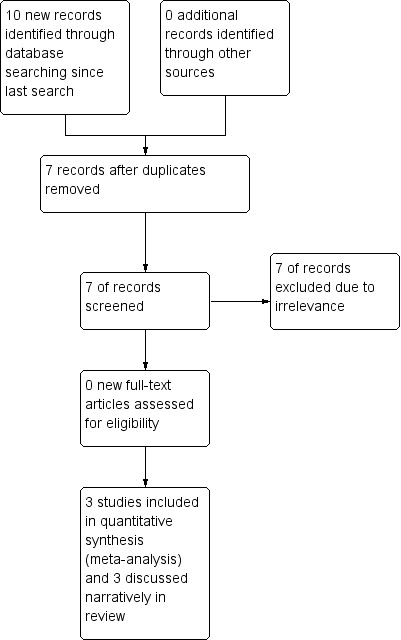
Study flow diagram.
Included studies
We found three parallel‐group studies and two cross‐over studies comparing tiagabine versus placebo for people with drug‐resistant localisation‐related seizures (Crawford 2001; Kalviainen 1998; Richens 1995; Sachdeo 1997; Uthman 1998). All trials were initially sponsored by Novo Nordisk as part of a prelicensing programme, but the drug patent is now owned by Sanofi‐Synthelabo. People were excluded from these studies if they had a history of non‐epileptic attacks; any active progressive disease of the central nervous system (e.g. brain tumour); any significant illness within the previous three months; any medical or neurological disorder requiring frequent changes in medication or dosage; abnormal laboratory findings that were not attributable to their concomitant AEDs; a history of drug abuse or addiction (including alcohol) or poor compliance with past medication or medical advice. Women who were pregnant or at risk of pregnancy and those who were lactating were also excluded. Valproate monotherapy was not allowed in two studies (Sachdeo 1997; Uthman 1998). We found one further parallel study (Fritz 2005), which examined tiagabine and topiramate in participants with refractory epilepsy. This study was previously excluded from the review because a placebo control group was not included. It has now been included as a head‐to‐head trial.
The parallel trial (Kalviainen 1998) had a 12‐week prerandomisation baseline period, and people who had eight or more localisation‐related seizures during this period were eligible to be randomly assigned. The treatment period lasted 22 weeks, and 154 individuals were randomly assigned—77 to placebo and 77 to 30 mg tiagabine per day. Data on cognitive and quality of life effects on a subset of 43 people were reported separately (Kalviainen 1996).
Another parallel trial (Fritz 2005) had an eight‐week screening period, a titration phase of three months and a three‐month maintenance phase. Twenty‐one participants were randomly assigned to tiagabine (final dose at least 20 mg/d) and 20 were randomly assigned to topiramate treatment (final dose at least 200 mg/d). This study reported seizure outcome, cognitive outcome and quality of life effects.
Sachdeo 1997, a parallel trial, included an eight‐week prerandomisation baseline period, and people who had eight or more seizures during this period were eligible to be randomly assigned. The treatment period lasted 12 weeks, and 318 individuals were randomly assigned—107 to placebo and 211 to 32 mg tiagabine per day. Of those randomly assigned to tiagabine, 106 were allocated 16 mg twice a day and 105 were allocated 8 mg four times a day. No effects on cognition or quality of life were reported for this study.
The parallel trial (Uthman 1998) had a 12‐week prerandomisation baseline period, and people who had eight or more localisation‐related seizures during this period were eligible to be randomly assigned. The treatment period lasted 20 weeks, and 297 individuals were randomly assigned—91 to placebo, 61 to 16 mg, 88 to 32 mg and 57 to 56 mg tiagabine per day. Data on cognitive and quality of life effects were reported separately for a subset of 162 individuals (Dodrill 1997).
The cross‐over trials ( Crawford 2001; Richens 1995) were similar in design, using what has been called a response conditional design. People who had six or more seizures in the eight weeks before the study were entered into a 12‐week screening phase. During the screening phase, all participants were given tiagabine, the dose of which was titrated up with a target dose of 52 mg/d in Richens 1995 and 64 mg/d in Crawford 2001. Participants with a reduction in seizure frequency of 25% or more during the screening phase were eligible to be randomly assigned (hence response conditional). Eligible individuals were randomly assigned into a two‐by‐two cross‐over trial and to a sequence of placebo‐tiagabine or tiagabine‐placebo. Treatment periods lasted seven weeks and the cross‐over periods three weeks. Richens 1995 screened 94 individuals, 46 of whom were randomly assigned; Crawford 2001 screened 88 individuals, 44 of whom were randomly assigned. The reports for these two studies contain insufficient information to allow planned analyses using data from the first treatment period. For the cohort recruited in Richens 1995, the cognitive effects of tiagabine were reported separately for a subset of 22 individuals (Sveinbjornsdottir 1994).
In total, the studies assessing seizure outcomes and treatment withdrawal randomly assigned 900 individuals. Studies assessing adverse effects randomly assigned 859 participants. Data on cognitive effects were available for 251 individuals, and data on quality of life were available for 229 individuals. Further details are provided in the Characteristics of included studies,
Excluded studies
Four studies (Arroyo 2005; Bauer 1995; Gustavson 1997; Uldall 1995) were excluded from the previous review. See Characteristics of excluded studies tables for reasons for exclusion.
Risk of bias in included studies
Overall, two studies (Kalviainen 1998; Sachdeo 1997) were judged to be at low risk of bias, three studies (Crawford 2001; Richens 1995; Uthman 1998) at unclear risk of bias and one study (Fritz 2005) at high risk of bias. See Figure 2 for a visual representation of the risk of bias in all included studies. For the outcome of 50% reduction in seizure frequency, the three trials that contributed to the meta‐analysis were judged overall to be at low risk of bias.
Figure 2.
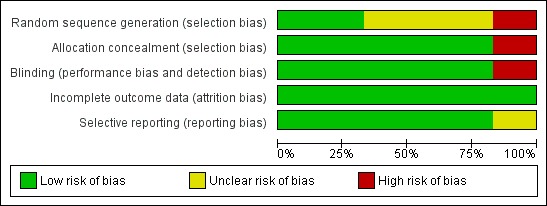
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
All included studies randomly assigned participants to treatment arms. Of the six trials included, only two used computer‐generated sequences to allocate participants (Fritz 2005; Kalviainen 1998). The other studies did not provide details on how their sequence allocation was generated. All but Fritz 2005 used sequentially allocated sealed packages to conceal group allocation. Fritz 2005 used no methods of concealment. Overall for sequence generation, three studies were rated as unclear, two as low and two as high risk of bias. For allocation concealment, five studies were rated as low and one as high risk of bias.
Blinding
All studies reported that they were double‐blinded, except for Fritz 2005, which was an open‐label study and thus used no blinding techniques. In the four other included studies, identical packaging and medication were used to maintain blinding. No specific details regarding who was blinded were provided within the papers (i.e. participants, study personnel or outcome assessors). Overall for blinding, five studies were rated as low and one as high risk of bias.
Incomplete outcome data
All studies reported attrition rates and reasons for dropout. An ITT analysis was used by all studies to deal with these missing data. Overall for incomplete outcome data, six studies were rated as low risk of bias.
Selective reporting
Most of the studies detailed outcomes within the methods of the paper and reported the data; however, no protocols were available to permit detailed assessment of this. Fritz 2005 selected a subset of randomly assigned participants and reported data for this subset only on baseline measures. Overall for selective reporting, five studies were rated as low and one as unclear risk of bias.
Effects of interventions
See: Table 1
As already outlined in the description of studies, we found four parallel‐group and two cross‐over studies. The cross‐over studies used a response conditional design in which participants were given tiagabine during a screening period, and those with a 25% or greater reduction in seizure frequency were eligible to be randomly assigned. The people randomly assigned therefore are highly selected and are biased towards a response to tiagabine. We have chosen to report separately the effects on seizure frequency from the parallel and cross‐over trials. In addition, reports of the cross‐over studies do not provide detailed adverse effect data or data on treatment withdrawal, and only the parallel studies contributed results for these outcomes. The parallel study that examined tiagabine and topiramate was assessed separately (Fritz 2005).
Tiagabine versus placebo control
50% or greater reduction in seizure frequency: parallel trials
A Chi2 test for heterogeneity for a response to tiagabine indicated no significant heterogeneity between trials (ITT analysis: Chi2 = 0.81, df = 2, P value 0.67; worst‐case scenario: Chi2 = 0.41, df = 2, P = 0.81; best‐case scenario: Chi2 = 1.09, df = 2, P value 0.58). The overall risk ratio for a response to tiagabine is 3.16 (95% CI 1.97 to 5.07), indicating that participants are significantly more likely to respond to tiagabine than to placebo. The risk ratios for worst‐case and best‐case scenarios are 2.70 (95% CI 1.75 to 4.19) and 3.32 (95% CI 2.08 to 5.32), respectively (Figure 3).
Figure 3.

Forest plot of comparison: 1 Tiagabine versus placebo control—50% or greater reduction in seizure frequency, outcome: 1.1 Intention‐to‐treat analysis.
Dose regression analysis
Intention‐to‐treat
Because of differences in response rates in individual trials, it is not possible to give valid estimates of precise responses to individual doses. Because of differences in response rates among trials, the model with the best summary of the log odds includes a contrast between Kalviainen 1998, Uthman 1998 and Sachdeo 1997. After adjustments for trial effects, this model includes two contrasting dose groups: one group containing placebo and 16 mg, and the other 30, 32 and 56 mg tiagabine per day. The reduction in deviance due to dose at these two levels with adjustment for trial effects is 40.6 on 1 df (P value 0.001). Upon contrast of trials included, the residual deviance is 4.8 on 5 df.
The estimated odds ratio relative to placebo is 3.67 (95% CI 2.30 to 5.86). The odds ratio for trials two and three versus trial one is 1.65 (95% CI 1.11 to 2.44).
Actual and estimated response rates are given in Table 6. Although we are unable to give precise overall estimates for the proportion responding to individual doses, strong evidence suggests an effect for daily doses of 30 to 56 mg or more. As an indication of the possible effects, we can say that, with placebo rates in the range of 6% to 10%, at least an additional 13%, and possibly 20%, of people similar to those in these trials would experience a 50% or greater reduction in seizure frequency when taking a dose of 30 mg tiagabine or more per day.
Table 1.
Percentage of 50% responders (95% CI), intention‐to‐treat regression analysis
| Trial | Placebo, proportion | 16 mg/d | 30 to 32 mg/d | 56 mg/d |
| Uthman 1998 actual | 4.4 | 8.2 | 19.3 | 18.1 |
| Kalviainen 1998 actual | 6.5 | 14.3 | ||
| Uthman 1998 and Kalviainen 1998 fitted | 6.3 (4.1 to 9.4) | 6.3 (4.1 to 9.4) | 19.7 (15.2 to 25.0) | 19.7 (15.2 to 25.0) |
| Sachdeo 1997 actual | 10.2 | 28.6 | ||
| Sachdeo 1997 fitted | 9.8 (6.4 to 14.9) | 28.7 (23.3 to 34.9) |
Best‐case and worst‐case scenarios
The best‐case results are very similar to the ITT results. After adjustments for trial effects, contrast between placebo and 16 mg and 30, 32 or 56 mg gives the best summary of the log odds. The reduction in deviance due to dose, with adjustments for trial effects, is 47.7 on 1 df (P value 0.001). With contrast of trials included, the residual deviance is 6.7 on 5 df.
The estimated odds ratio relative to placebo is 4.03 (95% CI 1.97 to 8.25). The odds ratio for trials two and three versus trial one is 1.51 (95% CI 0.98 to 2.33). Actual and estimated response rates are given in Table 7.
Table 2.
Percentage of 50% responders (95% CI), best‐case regression analysis
| Trial | Placebo | 16 mg/day | 30 to 32 mg/day | 56 mg/day |
| Uthman 1998 actual | 4.4 | 8.2 | 21.6 | 31.6 |
| Kalviainen 1998 actual | 6.5 | 14.3 | ||
| Uthman 1998 and Kalviainen 1998 fitted | 6.2 (4.9 to 9.3) | 6.2 (4.9 to 9.3) | 21.5 (16.9 to 27.1) | 21.5 (16.9 to 27.1) |
| Sachdeo 1997 actual | 9.3 | 29.4 | ||
| Sachdeo 1997 fitted | 9.2 (5.9 to 14.0) | 29.5 (24.0 to 35.6) |
The worst‐case results are also very similar to the ITT results. After adjustments for trial effects, contrast between placebo and 16 mg and 30, 32 or 56 mg gives the best summary of the log odds. The reduction in deviance due to dose, with adjustments for trial effects, is 35.4 on 1 df (P value 0.001). With contrast of trials included, the residual deviance is 4.5 on 5 df.
The estimated odds ratio relative to placebo is 3.22 (95% CI 2.05 to 5.05). The odds ratio for trials two and three versus trial one is 1.70 (95% CI 1.15 to 2.50). Actual and estimated response rates are given in Table 8.
Table 3.
Percentage of 50% responders (95% CI), worst‐case regression analysis
| Trial | Placebo | 16 mg/d | 30 to 32 mg/d | 56 mg/d |
| Uthman 1998 actual | 5.5 | 8.2 | 19.3 | 28.1 |
| Kalviainen 1998 actual | 6.5 | 14.3 | ||
| Uthman 1998 and Kalviainen 1998 fitted | 7.0 (4.7 to 10.2) | 7.0 (4.7 to 10.2) | 19.4 (15.0 to 24.7) | 19.4 (15.0 to 24.7) |
| Sachdeo 1997 actual | 12.1 | 28.6 | ||
| Sachdeo 1997 fitted | 11.3 (7.5 to 16.6) | 29.0 (23.6 to 35.1) |
50% or greater reduction in seizure frequency: cross‐over trials
Reports of the cross‐over trials contain insufficient data from the first treatment period to allow the analyses that were planned. Reported results from the two cross‐over trials therefore have been summarised below.
Of the 46 people randomly assigned in Richens 1995, 11 (24%) had a 50% reduction in seizure frequency in the tiagabine versus placebo phase. Of the 44 people randomly assigned in Crawford 2001, 12 (27%) had a 50% reduction in seizure frequency in the tiagabine versus placebo phase. Pooling these data, weighted according to the inverse variance, gives an estimate of the proportion of responders of 0.25 (95% CI 0.16 to 0.34). The proportion of responders is higher than that seen in the parallel‐group trials; this is not surprising given that people in the cross‐over trials had to have an apparent response to tiagabine before they were randomly assigned.
Treatment withdrawal
Treatment withdrawal data were available only for the parallel trials. A Chi2 test for heterogeneity suggests no significant statistical heterogeneity (Chi2 = 1.75, df = 2, P value 0.42). The overall risk ratio for discontinuation for any reason is 1.81 (95% CI 1.25 to 2.62), indicating that people are significantly more likely to withdraw from tiagabine than from placebo (Analysis 2.1).
Analysis 2.1.
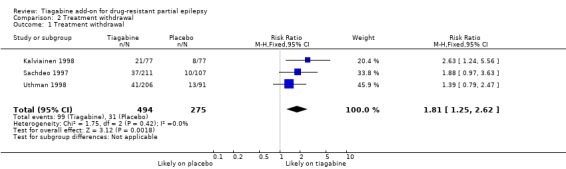
Comparison 2 Treatment withdrawal, Outcome 1 Treatment withdrawal.
Adverse effects
Results for the parallel and cross‐over trials are reported separately. For the parallel trials, in addition to the five adverse effects specified in the protocol for this review, headache, infection, nervousness and tremor were amongst the five most commonly reported adverse effects. A Chi2 test for heterogeneity for adverse effects indicated no significant heterogeneity between trials. The 99% CIs around RR estimates for the following did not cross unity, indicating that they are significantly associated with tiagabine: dizziness 1.69 (99% CI 1.31 to 2.51); fatigue 1.38 (99% CI 0.89 to 2.14); nervousness 10.65 (99% CI 0.78 to 146.08) and tremor 4.56 (99% CI 1.00 to 20.94). The 99% CIs for the following adverse effects did not cross unity and therefore were not significantly associated with tiagabine: ataxia 1.24 (99% CI 0.34 to 4.55); nausea 1.24 (99% CI 0.69 to 2.22); somnolence 1.18 (99% CI 0.76 to 1.83); headache 1.15 (99% CI 0.48 to 2.79) and infection 1.00 (99% CI 0.36 to 2.76) (Analysis 3.1; Analysis 3.2; Analysis 3.3; Analysis 3.4; Analysis 3.5; Analysis 3.6; Analysis 3.7; Analysis 3.8; Analysis 3.9).
Analysis 3.1.
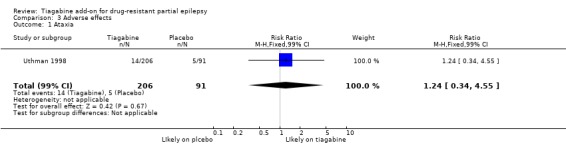
Comparison 3 Adverse effects, Outcome 1 Ataxia.
Analysis 3.2.
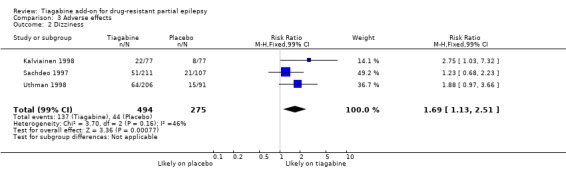
Comparison 3 Adverse effects, Outcome 2 Dizziness.
Analysis 3.3.
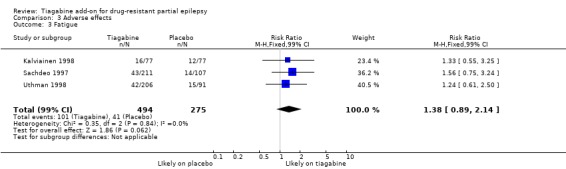
Comparison 3 Adverse effects, Outcome 3 Fatigue.
Analysis 3.4.
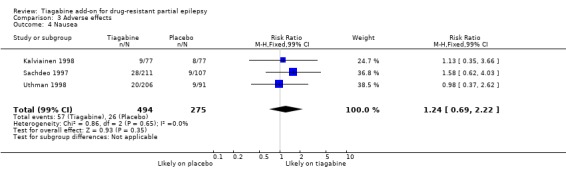
Comparison 3 Adverse effects, Outcome 4 Nausea.
Analysis 3.5.

Comparison 3 Adverse effects, Outcome 5 Somnolence.
Analysis 3.6.
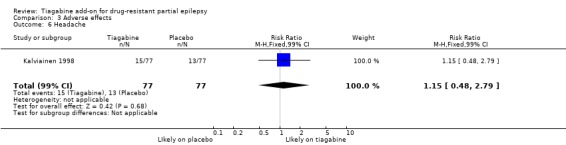
Comparison 3 Adverse effects, Outcome 6 Headache.
Analysis 3.7.

Comparison 3 Adverse effects, Outcome 7 Infection.
Analysis 3.8.

Comparison 3 Adverse effects, Outcome 8 Nervousness.
Analysis 3.9.
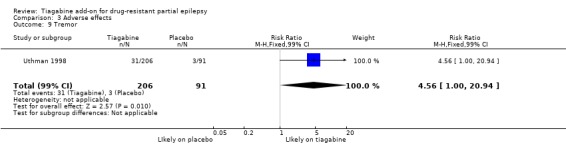
Comparison 3 Adverse effects, Outcome 9 Tremor.
For the cross‐over trials, Crawford 2001 reported that eight people experienced adverse effects while taking tiagabine and 10 people reported adverse effects while taking placebo. While taking tiagabine, two people reported dizziness and another two incoordination. While taking placebo, three people reported accidental injury. All other adverse events were reported by one individual only. Richens 1995 did not report a detailed breakdown of adverse events occurring with tiagabine or placebo.
Cognitive effects
Reviewing the cognitive effects of tiagabine is complicated by lack of uniformity of approach in the included studies (Dodrill 1997; Kalviainen 1996; Sveinbjornsdottir 1994). In total, these studies used 24 neuropsychological tests, only two of which—the Stroop test and the Rey auditory verbal learning test—were used in all three studies that reported cognitive effects. Three tests were used by two of the three studies, and the remaining 19 tests were used in just one study. Even when the same test had been used in two or more studies, it was not already clear that the same aspects of the test had been used. The tests used in the individual studies are outlined in Table 9.
Table 4.
Neuropsychological tests used
| Study | Test |
| Dodrill 1997 | Stroop test |
| Rey auditory verbal learning test | |
| Controlled oral word association test | |
| Lafayette grooved pegboard | |
| Benton visual retention test | |
| Symbol digit modalities test | |
| Wonderlic personnel test | |
| Digit cancellation | |
| Fritz 2005 | Edinburgh inventory |
| Mehrfachwahl‐Wortschatz‐Intelligenztest (MWT‐B) | |
| Kurztest zur cerebralen Insuffizienz (c.I. test) | |
| Trailmaking test | |
| Weiner test system | |
| HAWIE‐R (verbal memory) | |
| Corsi block test | |
| Verbal learning and memory test (VLMT) | |
| Diagnosticum fur cerebralschaden (DSC‐R) (visual memory) | |
| Boston naming test | |
| Word fluency test (LPS) | |
| Token test | |
| Kalviainen 1996 | Stroop test |
| Rey auditory verbal learning test | |
| Controlled oral word association test | |
| Modified finger tapping test | |
| Binary choice reaction | |
| Full‐scale I.Q. (WAIS) | |
| Logical prose, story A from the Wechsler memory scale | |
| Forward digit span | |
| Corsi block span | |
| Alternating S‐task | |
| Letter cancellation task | |
| The WMS visual reproduction subtest | |
| Auditory and visual reaction times | |
| Sveinbjornsdottir 1994 | Stroop test |
| Rey auditory verbal learning test | |
| Binary choice reaction | |
| Modified finger tapping | |
| Semantic processing | |
| Information processing speed | |
| Bimanual hand movements | |
| Simple reaction time | |
| Tapping rate | |
| Verbal memory |
No statistically significant differences were noted at the 0.01 level of confidence for any test, and for only three tests statistically significant differences were seen at the 0.05 level (Table 10). Evidence therefore is insufficient to conclude that tiagabine as an add‐on treatment has an effect on cognition.
Table 5.
Neuropsychological tests with statistically significant differences
| Test | Study | Result (P) | Treatment favoured |
| Benton visual retention test, form F | Dodrill 1997 | 0.049 | Placebo |
| Benton visual retention test, form G | Dodrill 1997 | 0.051 | Placebo |
| Symbol digit modalities test | Dodrill 1997 | 0.051 | Placebo |
Quality of life measures
Two reports addressed quality of life outcomes (Dodrill 1997; Kalviainen 1996). Both report effects on mood and adjustment but used different tests to assess them (Table 11). Neither study found a significant difference between tiagabine and placebo; hence evidence is insufficient to conclude that tiagabine has an effect on quality of life.
Table 6.
Tests of mood and adjustment
| Study | Test | Domain |
| Dodrill 1997 | Profile of Mood States (POMS) | Tension‐anxiety |
| Depression‐dejection | ||
| Anger‐hostility | ||
| Vigour‐activity | ||
| Fatigue‐inertia | ||
| Confusion‐bewilderment | ||
| Total mood disturbance | ||
| Washington Psychosocial Seizure Inventory (WPSI) | Family background | |
| Emotional adjustment | ||
| Interpersonal adjustment | ||
| Vocational adjustment | ||
| Financial status | ||
| Adjustment to seizures | ||
| Medicine and medical management | ||
| Overall functioning | ||
| Mood Rating Scale | Average score | |
| Fritz 2005 | Befindlichkeits‐Skala (BFS) | Dysphoria |
| Beck Depression Inventory | Depression | |
| Self‐rating Anxiety Scale (SAS) | Anxiety | |
| QOLIE‐31 | Health‐related quality of life | |
| Sveinbjornsdottir 1994 | The Mood Adjective Check List (MACL) | Depression |
| Anxiety | ||
| Fatigue | ||
| Activity | ||
| Aggression | ||
| Rating Scale Adapted from Brooks and McKinlay | Individual's behaviour |
Tiagabine versus topiramate
Only Fritz 2005 compared tiagabine versus an active control drug (topiramate); therefore pooling of data was not undertaken for this comparison.
50% or greater reduction in seizure frequency
Within this study, no significant differences were noted between the two add‐on drugs (RR 0.54, 95% CI 0.19 to 1.58). According to the study authors' analysis of 37 participants, three participants (one tiagabine, two topiramate) became seizure‐free; 11 participants (four tiagabine, seven topiramate) reduced their seizure frequency by 50% or more (Analysis 4.1).
Analysis 4.1.
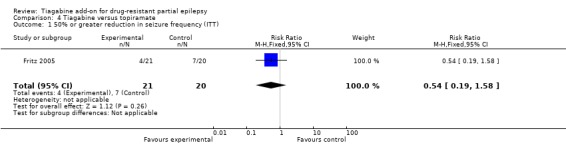
Comparison 4 Tiagabine versus topiramate, Outcome 1 50% or greater reduction in seizure frequency (ITT).
Treatment withdrawal
No significant differences were found between tiagabine and topiramate upon withdrawal from the study (RR 1.43, 95% CI 0.74 to 2.74) (Analysis 4.2).
Analysis 4.2.
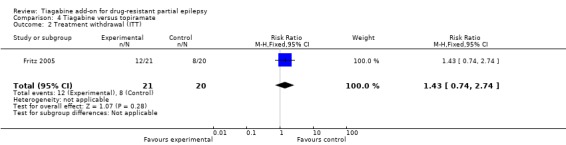
Comparison 4 Tiagabine versus topiramate, Outcome 2 Treatment withdrawal (ITT).
Cognitive effects
For this outcome, authors of this study did not compare the two add‐on antiepileptic drugs. Both add‐on drugs were examined over separate time points: baseline/post‐titration phase and post‐titration/maintenance phase. Within the first evaluation, baseline/post‐titration, significant deteriorations were found within the topiramate group in phonematic verbal fluency (P value 0.001), semantic verbal fluency (P value 0.006), language comprehension (P value 0.002), working memory (P < 0.05) and visual block tapping (P value 0.032).. A significant deterioration in verbal memory was also found in the tiagabine group (P value 0.039).
Within the post‐titration/maintenance phase, one significant improvement was found in mental flexibility in the topiramate group (P value 0.045). No changes were found in the tiagabine group for any of the cognitive outcomes.
Quality of life measures
Again, authors did not use appropriate analysis; therefore, we are unable to compare the two add‐on antiepileptic drugs. Within the baseline/post‐titration phase, significantly more complaints about medication were reported within the tiagabine group (P value 0.048). Participants taking topiramate reported significantly higher depression scores (P value 0.011), lower cognitive functioning (P value 0.024) and increased medication adverse effects (P value 0.008).
For the post‐titration/maintenance phase evaluation, the tiagabine group was significantly more fatigued/had less energy (P value 0.025). No other differences were found from post‐titration to maintenance.
Discussion
Summary of main results
All trials included in this review were sponsored by Sanofi‐Synthelabo as part of a drug licensing programme. Five of the six included trials used adequate methods of concealment of randomisation; however, only two of the trials were rated as low risk of bias for the way the randomisation sequence was generated. All trials except one were double‐blinded and used identical packaging and medication to maintain this; however, little detail was reported about whether specific study personnel and outcome assessors were blinded. All studies were rated as low of bias for missing data and how this was dealt with because intention‐to‐treat analyses were used. We had no access to the protocols for any of the studies; therefore selective outcome reporting bias is unclear for each of the studies. Limitations of two of the trials include the use of a response conditional design, the aim of which was to select and randomly assign a group of people likely to respond favourably to tiagabine. In theory, this will maximise the effects observed and may lead to the efficiency of requiring the recruitment of fewer individuals into trials. This is not a process that mimics clinical practice, and the results of these studies are difficult to translate into everyday clinical practice. The length of the treatment period was another limitation of all included trials: seven weeks in the cross‐over studies and 12 to 22 weeks in the parallel studies. Clinical practice will be better informed by trials of longer duration, especially for a chronic condition such as epilepsy. Despite these limitations, this review provides strong evidence that tiagabine reduces seizure frequency for people with drug‐resistant localisation‐related seizures. Because of differences in response rates among trials, regression models were unable to give valid estimates of the proportion of participants responding to specific doses of tiagabine. We can say however that for people similar to those recruited into the trials reviewed, compared with placebo, we might expect at least an additional 13%, and possibly 20%, of people to experience a 50% or greater reduction in seizure frequency when taking a dose of 30 mg tiagabine or more per day.
Results for the outcome of treatment withdrawal show that tiagabine is more likely to be withdrawn than placebo. In trials of relatively short duration, such as those reviewed here, this is likely to represent problems with tolerability rather than poor seizure control. Of the adverse effects investigated, dizziness, nervousness and tremor were significantly more likely to occur with tiagabine than with placebo.
Because of lack of uniformity in the approach used to test for cognitive effects and quality of life, as well as the relatively small numbers of participants tested, evidence is insufficient to conclude whether tiagabine has an effect on these outcomes.
Given that only one trial included in this review compared add‐on tiagabine versus another add‐on antiepileptic drug, the results from this study must be interpreted with caution. Authors of this study compared the two drugs only for two of the outcomes reported. Given the increasing number of licensed antiepileptic drugs, this is an important issue that must be addressed in trials that compare one drug versus another. We should emphasise that the results of this review do not provide information about the effects of tiagabine when used as monotherapy, and that the results apply only to localisation‐related seizures.
Quality of the evidence
Of the six included studies, two studies were rated individually as low risk of bias, three as unclear because of the method of sequence generation used and one as high. For all studies included in the meta‐analysis for the primary outcome, the overall risk of bias was rated as low, and the evidence was considered to be methodologically sound. The GRADE approach was employed to rate the level of evidence for the primary outcome. This is presented in a 'Summary of findings' table.
Authors' conclusions
Add‐on tiagabine reduces the frequency of seizures for people with drug‐resistant localisation‐related seizures. Doses between 30 and 56 mg per day are likely to reduce the frequency of seizures by 50% or more for between 13% and 20% of people. Doses higher than 56 mg per day were not tested in the trials included in this review.
To further evaluate the place of tiagabine in the armamentarium of available AEDs, further studies are required to address the following.
Long‐term effects of add‐on tiagabine.
How tiagabine compares with other add‐on treatments in drug‐resistant partial epilepsy.
Role of tiagabine in childhood and generalised epilepsies.
-
How tiagabine compares with standard antiepileptic drugs such as:
tiagabine as monotherapy in partial epilepsy; and
tiagabine as monotherapy in generalised epilepsy.
Economic aspects of tiagabine therapy.
Acknowledgements
We acknowledge Joao Pereira for contributing towards the original review.
Appendices
Appendix 1. CENTRAL search strategy
#1MeSH descriptor Epilepsy explode all trees #2MeSH descriptor Seizures explode all trees #3epilep* or seizure* or convulsion* #4(#1 OR #2 OR #3) #5(tiagabine or gabitril) #6(#4 AND #5)
Appendix 2. MEDLINE search strategy
For the June 2010 and December 2011 update of this review, the following search strategy was used. It is based on the Cochrane Highly Sensitive Search Strategy for identifying randomized trials published in Lefebvre 2009.
1. randomized controlled trial.pt.
2. controlled clinical trial.pt.
3. randomized.ab.
4. placebo.ab.
5. clinical trials as topic.sh.
6. randomly.ab.
7. trial.ti.
8. 1 or 2 or 3 or 4 or 5 or 6 or 7
9. exp animals/ not humans.sh.
10. 8 not 9
11. exp Epilepsy/
12. Seizures/
13. (epilep$ or seizure$ or convuls$).tw.
14. 11 or 12 or 13
15. (tiagabine or gabitril).tw.
16. 10 and 14 and 15
Earlier versions of this review employed the following search strategy. It was combined with phases 1 and 2 of the earlier Cochrane highly sensitive search strategy for MEDLINE as set out in Appendix 5b of the Cochrane Handbook for Systematic Reviews of Interventions (version 4.2.4, updated March 2005) (Higgins 2010).
1. tiagabine.tw. 2. exp epilepsy/ OR epilep$.tw. 3. exp seizures/ OR seizure$.tw. 4. convulsion$.tw. 5. 2 OR 3 OR 4 6. 1 AND 5
Data and analyses
Comparison 1.
Tiagabine versus placebo control—50% or greater reduction in seizure frequency
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Intention‐to‐treat analysis | 3 | 769 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.16 [1.97, 5.07] |
| 2 Worst‐case scenario | 3 | 769 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.70 [1.75, 4.19] |
| 3 Best‐case scenario | 3 | 769 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.32 [2.08, 5.32] |
Analysis 1.1.
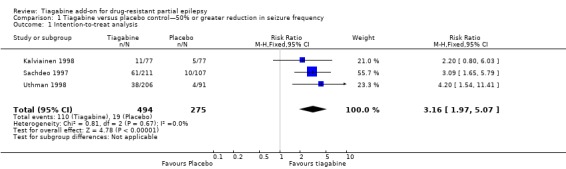
Comparison 1 Tiagabine versus placebo control—50% or greater reduction in seizure frequency, Outcome 1 Intention‐to‐treat analysis.
Analysis 1.2.
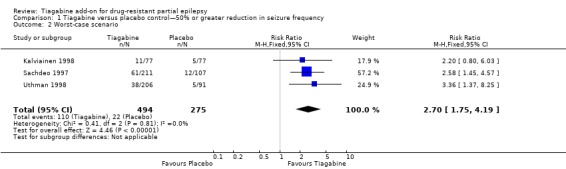
Comparison 1 Tiagabine versus placebo control—50% or greater reduction in seizure frequency, Outcome 2 Worst‐case scenario.
Analysis 1.3.
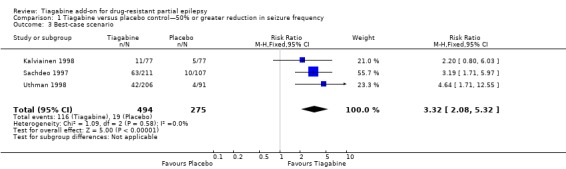
Comparison 1 Tiagabine versus placebo control—50% or greater reduction in seizure frequency, Outcome 3 Best‐case scenario.
Comparison 2.
Treatment withdrawal
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment withdrawal | 3 | 769 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [1.25, 2.62] |
Comparison 3.
Adverse effects
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Ataxia | 1 | 297 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.24 [0.34, 4.55] |
| 2 Dizziness | 3 | 769 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.69 [1.13, 2.51] |
| 3 Fatigue | 3 | 769 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.38 [0.89, 2.14] |
| 4 Nausea | 3 | 769 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.24 [0.69, 2.22] |
| 5 Somnolence | 3 | 769 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.18 [0.76, 1.83] |
| 6 Headache | 1 | 154 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.15 [0.48, 2.79] |
| 7 Infection | 1 | 154 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.0 [0.36, 2.76] |
| 8 Nervousness | 1 | 318 | Risk Ratio (M‐H, Fixed, 99% CI) | 10.65 [0.78, 146.08] |
| 9 Tremor | 1 | 297 | Risk Ratio (M‐H, Fixed, 99% CI) | 4.56 [1.00, 20.94] |
Comparison 4.
Tiagabine versus topiramate
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 50% or greater reduction in seizure frequency (ITT) | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.19, 1.58] |
| 2 Treatment withdrawal (ITT) | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.74, 2.74] |
What's new
| Date | Event | Description |
|---|---|---|
| 11 November 2013 | New citation required but conclusions have not changed | Conclusions remain unchanged. |
| 11 November 2013 | New search has been performed | Searches updated 11 November 2013; no new trials identified. |
History
Protocol first published: Issue 1, 2000 Review first published: Issue 3, 2002
| Date | Event | Description |
|---|---|---|
| 15 December 2011 | New citation required but conclusions have not changed | Searches updated 15 December 2011; no new trials identified. |
| 11 June 2010 | New search has been performed | Searches updated 11 June 2010; no new trials identified. |
| 21 August 2008 | Amended | Converted to new review format. |
| 15 February 2008 | New search has been performed | Searches updated 15 February 2008; no new trials identified. |
Differences between protocol and review
According to the original protocol, only trials using a placebo control group would be included within the review if they met all other inclusion criteria. We have decided to extend this to include any other studies with active add‐on drug used as a control group versus tiagabine.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised, double‐blind, placebo‐controlled, cross‐over study, based on a response‐dependent design. Two treatment arms: one TGB, one PCB. Participants randomly assigned to one of two sequences. TGB started during screening phase at 12 mg/d QID. Seven‐week double‐blind treatment period during which participants continued on TGB or crossed over to PCB arm | |
| Participants | Multi‐centre study (five centres: two in UK, two in The Netherlands and one in Denmark) 44 participants with drug‐resistant partial epilepsy were randomly assigned (30 male), aged 18 to 53 years Participants already taking one to three background AEDs Median baseline seizure frequency = 2.7/wk. | |
| Interventions | Group one: PCB Group two: TGB (optimal dose 64 mg/d) Mean daily dose for all randomly assigned participants was 46 mg during the double‐blind phase |
|
| Outcomes |
|
|
| Notes | From the 44 people randomly assigned to the double‐blind phase, seven were excluded from the intent‐to‐treat analysis. All participants were evaluated for adverse events | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants randomly assigned in a 1:1 ratio at each centre to one of two sequences. No further details on how the sequences were generated |
| Allocation concealment (selection bias) | Low risk | Sequentially allocated sealed packages used |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Used identical packaging and medication |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates reported and intention‐to‐treat analysis employed |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in methods section were reported in text; however, no protocol is available for comparison of outcomes |
| Methods | Open‐label, head‐to‐head controlled, parallel study. Two treatment arms: one TGB, one TPM. Participants were randomly assigned using non‐random component Duration of screening period: eight weeks, followed by a titration phase of three months and a maintenance period of three months | |
| Participants | No information regarding study sites or countries 41 participants with drug‐resistant partial epilepsy randomly assigned. Baseline data reported for only 30 participants who completed the whole study Participants already taking one to three background AEDs |
|
| Interventions | Group one: TGB at least 20 mg/d; mean 32 mg/d Group two: TPM at least 200 mg/d; mean 335 mg/d |
|
| Outcomes |
|
|
| Notes | Non‐accurate baseline data reported. Four participants excluded from ITT analysis for seizure reduction outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Non‐randomisation component employed in the sequence generation process. Sequence generated by odd versus even number of week in which participant was seen |
| Allocation concealment (selection bias) | High risk | No concealment used |
| Blinding (performance bias and detection bias) All outcomes | High risk | Study was open‐label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates reported and intention‐to‐treat analysis employed |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported in methods section were reported in text; however, no protocol was available for comparison of outcomes. Participant characteristics reported for only 30 participants who completed the whole study of the 41 randomly assigned |
| Methods | Randomised, double‐blind, placebo‐controlled, two‐treatment parallel‐group study. Two treatment arms: one PCB, one TGB. Participants randomly assigned using computer‐generated sequence. Treatment period: 22 weeks (six‐week run in period, 12‐week fixed‐dose period, four‐week termination period) | |
| Participants | Multi‐centre study (11 centres in Europe—one each in Denmark and Sweden, two in Finland and seven in UK 154 participants with drug‐resistant partial epilepsy were randomly assigned (90 male), aged 17 to 71 years 77 were randomly assigned to placebo and 77 to 30 mg/d tiagabine Participants already taking one to three background AEDs Median four‐weekly baseline seizure frequency: placebo = 10.5, tiagabine = 12.2 Cognitive and quality of life effects were assessed on a subset of 43 individuals | |
| Interventions | Group one: PCB Group two: TGB 30 mg/d TDS |
|
| Outcomes |
|
|
| Notes | No participants were excluded from analysis. 29 people withdrew from the study: 21 receiving tiagabine and eight receiving placebo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a computer‐generated allocation sequence |
| Allocation concealment (selection bias) | Low risk | Used sequentially allocated sealed packages |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Used identical packaging and medication |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attriition rates reported and intention‐to‐treat analysis employed |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in methods section were reported in text; however, no protocol available for comparison of outcomes |
| Methods | Randomised, double‐blind, placebo‐controlled, cross‐over study based on a response‐dependent design. Two treatment arms: one PCB, one TGB. Participants randomly assigned using 1:1 ratio. Treatment period: 23 weeks (three‐week run‐in period, seven‐week assessment period, three‐week cross‐over period, seven‐week assessment period, three‐week termination period) | |
| Participants | Multi‐centre study (five centres in UK and Denmark) 94 participants with drug‐resistant partial epilepsy were randomly assigned (61% male), aged 19 to 71 years 25 participants were randomly assigned to placebo‐tiagabine sequence and 21 to tiagabine‐placebo Participants already taking one to three AEDs Median complex partial seizure rate per four weeks = six Cognitive effects were reported for a subset of 22 individuals | |
| Interventions | Group one: PCB Group two: TGB 52 mg/d QID Mean daily dose was 33.4 mg (range 12 to 52 mg) |
|
| Outcomes |
|
|
| Notes | From the 46 people randomly assigned to the double‐blind phase, seven failed to complete the study. Of these, only four were excluded from the intent‐to‐treat analysis. All participants were evaluated for adverse events. The characteristics of people ineligible for the double‐blind phase were similar to those of people qualifying, with regard to epilepsy history, seizure frequency and concurrent treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 1:1 ratio employed at each centre. No further details of sequence generation |
| Allocation concealment (selection bias) | Low risk | Used sequentially allocated sealed packages |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Used identical packaging and medication |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates reported and intention‐to‐treat analysis employed |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in methods section were reported in text; however, no protocol was available for comparison of outcomes |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group study. Three treatment arms: one PLC, two TGB. Participants randomly assigned using ratio 1:1:1 in blocks of six Treatment periods = 12 weeks | |
| Participants | Multi‐centre US study (26 centres) 318 participants (178 male) aged 12 to 71 years with drug‐resistant partial epilepsy were randomly assigned 107 to PCB, 106 to TGB 16 mg BID, 105 to TGB 8 mg QID Valproate allowed in combination with an enzyme‐inducing drug but not as monotherapy Median baseline four‐weekly complex partial seizures; frequency during baseline was as follows: PCB = 8.4; TGB 16 mg BID = 8.4; TGB 8 mg QID = 7.9 | |
| Interventions | Add‐on placebo, TGB 16 mg BID or TGB 8 mg QID | |
| Outcomes |
|
|
| Notes | From the 318 people randomly assigned to the double‐blind phase, four were excluded from the intention‐to‐treat analyses. All 318 people were evaluated for adverse events. 47 people withdrew from the study: 10 receiving PLC; 16 receiving TGB 16 mg BID; 21 receiving TGB 8 mg QID | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used ratio of 1:1:1 in blocks of six per study centre |
| Allocation concealment (selection bias) | Low risk | Used sequentially allocated sealed packages |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Used identical packaging and medication |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates reported and intention‐to‐treat analysis employed |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in methods section were reported in text; however no protocol was available for comparison of outcomes |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group study. Four treatment arms: one PCB, three TGB. Participants randomly assigned using ratio of 3:2:3:2. Treatment period: 20 weeks (four‐week titration phase, 12‐week fixed‐dose treatment period, four‐week tapering period) | |
| Participants | Multi‐centre US study (21 centres) 297 participants with drug‐resistant partial epilepsy were randomly assigned (172 male), aged 12 to 77 years Participants already taking one to three AEDs, valproate allowed in combination with an enzyme‐inducing drug but not as monotherapy Median four‐weekly baseline complex partial seizure frequency was: PCB = 7.4; TGB 16 mg = 8.5; TGB 32 mg = 9.6; TGB 56 mg = 9.1 Cognitive effects were reported for a subset of 162 individuals | |
| Interventions | Group one: PCB Group two: TGB 16 mg/d QID Group three: TGB 32 mg/d QID Group 4: TGB 56 mg/d QID |
|
| Outcomes |
|
|
| Notes | From the 297 people randomly assigned to the double‐blind phase, five were excluded from the intention‐to‐treat analyses because no double‐blind assessments were done or their centres lacked participants in all treatment groups. All 297 people were evaluated for adverse events 54 people withdrew from the study: 13 receiving PCB; six receiving 16 mg TGB; 18 receiving 32 mg TGB and 17 receiving 56 mg TGB | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Ratio of 3:2:3:2 was used, no further details of sequence generation reported in text |
| Allocation concealment (selection bias) | Low risk | Used sequentially allocated sealed packages |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Used identical packaging and medication |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates reported and intention‐to‐treat analysis employed |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in methods section were reported in text; however no protocol was available for comparison of outcomes |
Abbreviations
- AED = antiepileptic drug.
- BID = twice daily.
- ITT = intention‐to‐treat analysis.
- PCB = placebo.
- QID = four times daily.
- TGB = tiagabine.
- TPB = topiramate.
- TID = three times daily.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arroyo 2005 | This is a non‐placebo controlled study |
| Bauer 1995 | This is an open add‐on study of tiagabine in treatment of people with resistant partial and/or secondarily generalised seizures. In this study, the results reported were part of an ongoing multi‐national, multi‐centre trial. It is not randomised |
| Gustavson 1997 | This is an open‐label, single‐dose study that was designed to examine the pharmacokinetics of tiagabine in children with epilepsy. It is not randomised |
| Uldall 1995 | This is a single‐blind study of safety, tolerability and preliminary efficacy of tiagabine as adjunctive treatment of children with epilepsy. It is not randomised |
Contributions of authors
Jennifer Pulman updated the protocol and carried out the review with Anthony G Marson. JP and AGM independently assessed trials for inclusion and assessed studies for risk of bias. The original dose regression analysis was undertaken by Jane L Hutton.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
- This review presents independent research commissioned by the National Institute for Health Research (NIHR). The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Declarations of interest
Prof Marson has received payment for speaking and for attending conferences by Sanofi‐Synthelabo, the current manufacturers of tiagabine, and GSK. In addition, a consortium of pharmaceutical companies (GSK, EISAI, UCB Pharma) funded the National Audit of Seizure Management in Hospitals (NASH) through grants paid to University of Liverpool.
Jennifer Pulman and Jane L Hutton have no conflicts of interest.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
- Crawford PM, Meinardi H, Brown S, Rentmeester TW, Pedersen PC, Lassen LC. Tiagabine: efficacy and safety in adjunctive treatment of partial seizures. Epilepsia 2001;42(4):531‐8. [DOI] [PubMed] [Google Scholar]
- Fritz N, Glogau S, Hoffmann J, Rademacher M, Elger CE, Helmstaedter C. Efficacy and cognitive side effects of tiagabine and topiramate in patients with epilepsy. Epilepsy & Behavior 2005;6(3):373‐81. [DOI] [PubMed] [Google Scholar]
- Kalviainen R, Aikia M, Mervaala E, Saukkonen AM, Pitkanen A, Riekkinen PJ Sr. Long‐term cognitive and EEG effects of tiagabine in drug‐resistant partial epilepsy. Epilepsy Research 1996;25(3):291‐7. [DOI] [PubMed] [Google Scholar]; Kalviainen R, Brodie MJ, Duncan J, Chadwick D, Edwards D, Lyby K. A double‐blind, placebo‐controlled trial of tiagabine given three‐times daily as add‐on therapy for refractory partial seizures. Epilepsy Research 1998;30:31‐40. [DOI] [PubMed] [Google Scholar]
- Richens A, Chadwick DW, Duncan JS, Dam M, Gram L, Mikkelsen M, et al. Adjunctive treatment of partial seizures with tiagabine: a placebo‐controlled trial. Epilepsy Research 1995;21(1):37‐42. [MEDLINE: ; CN‐00015195 ‐ CCTR] [DOI] [PubMed] [Google Scholar]; Sveinbjornsdottir S, Sander JW, Patsalos PN, Upton D, Thompson PJ, Duncan JS. Neuropsychological effects of tiagabine, a potential new antiepileptic drug. Seizure 1994;3(1):29‐35. [DOI] [PubMed] [Google Scholar]
- Sachdeo RC, Leroy RF, Krauss GL, Drake ME Jr, Green PM, Leppik IE, et al. Tiagabine therapy for complex partial seizures: a dose‐frequency study. Archives of Neurology 1997;54:595‐601. [DOI] [PubMed] [Google Scholar]
- Dodrill CB, Arnett JL, Sommerville KW, Shu V. Cognitive and quality of life effects of differing dosages of tiagabine in epilepsy. Neurology 1997;48(4):1025‐31. [DOI] [PubMed] [Google Scholar]; Uthman BM, Rowan AJ, Ahmann PA, Leppik IE, Schachter SC, Sommerville KW, et al. Tiagabine for complex partial seizures: a randomized, add‐on, dose‐response trial. Archives of Neurology 1998;55:56‐62. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Arroyo S, Bootham BR, Brodie MJ, Duncan JS, Duncan R, Nieto M. A randomised open‐label study of tiagabine given two or three times daily in refractory epilepsy. Seizure 2005;14(2):81‐4. [DOI] [PubMed] [Google Scholar]
- Bauer J, Stawowy B, Lenders T, Bettig U, Elger CE. Efficacy and tolerability of tiagabine: results of an add‐on study in patients with refractory partial seizures. Journal of Epilepsy 1995;8(1):83‐6. [Google Scholar]
- Gustavson LE, Boellner SW, Granneman GR, Qian JX, Guenther HJ, El‐Shourbagy T, et al. A single‐dose study to define tiagabine pharmacokinetics in pediatric patients with complex partial seizures. Neurology 1997;48(4):1032‐7. [DOI] [PubMed] [Google Scholar]
- Uldall P, Bulteau C, Pedersen SA, Dulac O, Meinild H, Lassen LC. Single‐blind study of safety, tolerability and preliminary efficacy of tiagabine as adjunctive treatment of children with epilepsy. Epilepsia 1995;36 Suppl(3):S147‐S148. [Google Scholar]
Additional references
- Baker GA, Hesdon M, Marson AG. Quality of life and behavioural outcome measures in randomized controlled trials of antiepileptic drugs: a systematic review of reporting standards. Epilepsia 2000;41(11):1357‐63. [DOI] [PubMed] [Google Scholar]
- Cochrane HC, Baker GA, Chadwick DW. Neuropsychological outcomes in randomized controlled trials of antiepileptic drugs: a systematic review of methodology and reporting standards. Epilepsia 1998;39(10):1088‐97. [DOI] [PubMed] [Google Scholar]
- Cockerell OC, Johnson AL, Sander JW, Hart YM, Shorvon SD. Remission of epilepsy: results from the national general practice study of epilepsy. Lancet 1995;346:140‐4. [DOI] [PubMed] [Google Scholar]
- Commission on Classification and Terminology of the International League Against Epilepsy. Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia 1989;30(4):389‐99. [DOI] [PubMed] [Google Scholar]
- Dodrill CB, Arnett JL, Sommerville KW, Shu V. Cognitive and quality of life effects of differing dosages of tiagabine in epilepsy. Neurology 1997;48(4):1025‐31. [DOI] [PubMed] [Google Scholar]
- Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935‐1984. Epilepsia 1993;34(3):453‐68. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Chichester, UK: John Wiley & Sons, Ltd, 2010. [Google Scholar]
- Kalviainen R, Aikia M, Mervaala E, Saukkonen AM, Pitkanen A, Riekkinen PJ Sr. Long‐term cognitive and EEG effects of tiagabine in drug‐resistant partial epilepsy. Epilepsy Research 1996;25(3):291‐7. [DOI] [PubMed] [Google Scholar]
- Kirkham J, Dwan K, Altman D, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. British Medical Journal 2010;340:365. [DOI] [PubMed] [Google Scholar]
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2009. www.cochrane‐handbook.org.
- Marson AG, Kadir ZA, Chadwick DW. New antiepileptic drugs: a systematic review of their efficacy and tolerability. BMJ 1996;313(7066):1169‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marson AG, Kadir ZA, Hutton JL, Chadwick DW. The new antiepileptic drugs: a systematic review of their efficacy and tolerability. Epilepsia 1997;38(8):859‐80. [DOI] [PubMed] [Google Scholar]
- McCullagh P, Nelder JA. Generalized Linear Models. 2nd Edition. London: Chapman and Hall, 1989. [Google Scholar]
- Ostergaard LH, Gram L, Dam M. Tiagabine. In: Levy RH, Mattson RH, Medlrum BS editor(s). Mechanisms of Action in Antiepileptic Drugs. 4th Edition. New York: Raven Press, 1995. [Google Scholar]
- Sander JW, Shorvon SD. Epidemiology of the epilepsies. Journal of Neurology, Neurosurgery, & Psychiatry 1996;61(5):433‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sveinbjornsdottir S, Sander JW, Patsalos PN, Upton D, Thompson PJ, Duncan JS. Neuropsychological effects of tiagabine, a potential new antiepileptic drug. Seizure 1994;3(1):29‐35. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Pulman J, Hutton J, Marson A. Tiagabine add‐on for drug‐resistant partial epilepsy. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD001908.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


