Abstract
Background
Despite efforts to increase the uptake of prevention of mother to child transmission of HIV (PMTCT) services, coverage is still lower than desired in developing countries. A lack of male partner involvement in PMTCT services is a major barrier for women to access these services.
Objectives
To evaluate the impact of interventions which aim to enhance male involvement to increase women’s uptake of PMTCT interventions in developing countries.
Search methods
We searched the following databases from the year 2000 to November 2011: Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, PsycINFO, the WHO Global Health Library, ClinicalTrials.gov, Current Controlled Trials, AEGIS, CROI, IAS, IAC web sites.
Selection criteria
We included randomised controlled trials (RCTs), cluster‐randomised controlled trials, quasi‐randomised controlled trials, controlled before and after studies and interrupted time series studies assessing interventions to increase male involvement for improvement of uptake PMTCT services in low‐ and middle‐income countries..
Data collection and analysis
Two reviewers independently searched, screened, assessed study quality and extracted data. A third reviewer resolved any disagreement.
Main results
Only one study met the inclusion criteria, an RCT conducted in Tanzania between May 2003 and October 2004. Women in the intervention group (n=760) received a letter for their male partners, which invited them to return together to receive Couple Voluntary Counselling and Testing (CVCT) for HIV. Women in the control group (n=761) received individual HIV VCT during their first ANC visit and then usual care. The percentages of women who received HIV VCT and collected their results were 48%, 45% and 39% in the intervention group and 93%, 78% and 71% in the control group (p <0,001). Only 33% of women in the intervention group returned with their male partners and only 47% of them went through the whole CVCT process. The proportion of women who received HIV prophylaxis at delivery was not different between the two arms (27% in the intervention and 22% in the control group). The study had a high risk of bias.
Authors' conclusions
We found only one eligible study that assessed the effectiveness of male involvement in improving women’s uptake of PMTCT services, which only focused on one part of the perinatal PMTCT cascade. We urgently need more rigorously designed studies assessing the impact of male engagement interventions on women’s uptake of PMTCT services to know if this intervention can contribute to improve uptake of PMTCT services and reduce vertical transmission of HIV in children.
Keywords: Female; Humans; Male; Sex Factors; Developing Countries; HIV Infections; HIV Infections/transmission; Infectious Disease Transmission, Vertical; Infectious Disease Transmission, Vertical/prevention & control; Program Evaluation; Randomized Controlled Trials as Topic; Spouses; Tanzania
Increasing male involvement to improve women’s uptake of interventions to reduce the mother to child transmission of HIV
During the past ten years, national governments and international agencies have strengthened the implementation of PMTCT programmes. However, the majority of women still do not access these services. In 2010 there were 390,000 new HIV infections in children, 90% of which were infected through vertical transmission. Research has shown that fear of violence or abandonment by male partners, cultural gender rules and disparate decision making power for women are among the main reasons that women do not access PMTCT services. Thus interventions should focus on promoting gender equality and improving male awareness and engagement in their families’ health in order to improve uptake of PMTCT services. We aimed to assess the effectiveness of male involvement interventions on women’s uptake of PMTCT services in developing countries.
We undertook a comprehensive search to identify relevant studies. We found 3,072 references, but only one study that met our criteria. The study was performed in 2003‐2004 in Tanzania. Pregnant women in the intervention group were provided with a letter inviting their male partners to accompany them to their next visit, in which they were offered voluntary HIV counselling and testing (VCT) together or separately. Women in the control group received the VCT individually during their first visit. The proportions of women that received VCT and collected their HIV test results were significantly lower in the intervention group than in the control group. Most of the women in the intervention group did not return to the clinic for the subsequent visit and most of those that returned accompanied refused to receive VCT together with their male partners. The invitation letter had a negative impact on the PMTCT uptake in that setting. We urgently need more studies assessing different interventions to improve male engagement in PMTCT to identify the most successful approach for women to safely access health care for their own health and to deliver HIV negative children.
Background
In 2001 members of the United Nations General Assembly Special Session (UNGASS 2001) committed to reduce the number of children living with HIV by 50% in 2010. In order to achieve this goal, an estimated 80% of pregnant women receiving antenatal care (ANC) would need access to HIV counselling and testing and to the PMTCT services (UNGASS 2001).
During the past ten years, national governments and international agencies have strengthened the implementation of PMTCT programmes to achieve this UNGASS target. As a result, the proportion of pregnant women tested for HIV and provided with antiretroviral (ARV) prophylaxis during pregnancy in low‐ and middle‐income countries in 2009 increased from 7% and 15% in 2005 to 26% and 53% respectively (WHO/UNICEF/UNAIDS 2010). The number of new HIV infections in children decreased from around 500,000 in 2001 to 390,000 in 2010 UNAIDS 2011b. However, the gap between the achieved coverage and the UNGASS target is still substantial. Even where infrastructure and services are in place, the majority of women are not accessing the complete cascade of PMTCT services.
In many settings, particularly in sub‐Saharan Africa, traditional gender roles confer power to men to make decisions related to women’s health issues, such as the participation in PMTCT and HIV care and treatment programs, including HIV testing, follow up appointments, and uptake and adherence to ARV regimens (WHO 2009). In some countries 75% of women report that their male partners make health decisions related to the woman's health (UNICEF 2007). In 2006 the United Nations General Assembly Declarations of Commitment on HIV/AIDS highlighted the need to tackle gender inequality as an essential way to halt and reverse the HIV epidemic (UNAIDS 2006).
Women’s decision‐making regarding HIV testing
Research from different countries has found that male partners, either directly or indirectly, have a tremendous impact on women’s uptake of HIV testing in the context of PMTCT programmes. The findings were that women’s perception of their husband’s approval of their taking an HIV test was the strongest predictor of women’s willingness to accept an HIV test (Bajunirwe 2005). Opposition from male partners was one of the major factors to which low HIV counselling and testing uptake, as well as failure to return for HIV test results, can be attributed in some contexts (Sarker 2007).
In one Ugandan programme, 31% of women refused HIV testing at ANC, 82% of them because they needed the permission of their male partner and 54% due to the fear of the male partner’s reaction in case of a positive HIV test result (Dahl 2008). A comprehensive review of international studies examining individual‐level factors influencing pregnant women’s HIV testing uptake identified women’s fear of negative reactions from their male partners as key in women’s individual level reasoning for declining HIV testing (Maman 2008).
Disclosure of HIV status to male partners is considered important for ensuring that individuals are able to access a range of services, including prevention of vertical HIV transmission services and HIV treatment and care (Kebaabetswe 2007). However, a World Health Organisation (WHO) study revealed that between 16‐86% of women in developing countries choose not to disclose their HIV status to their male partners (Medley 2004). In an evaluation of studies conducted by WHO that looked at violence as an outcome of HIV status disclosure for women who chose to disclose, the highest rates of disclosure‐related violence were reported among women in ANC (WHO Dept Gender 2003). Fear of the negative consequences of disclosure (i.e. violence, abandonment, loss of economic support) is a major barrier to uptake of HIV testing in women and especially pregnant women (Kilewo 2001; Sarker 2007; Wang 2006). In 2006, WHO strongly recommended that tailored strategies for different contexts should be implemented in order to reduce the barriers that women meet during HIV counselling and testing and to protect them from violent reactions due to HIV disclosure (WHO, Dept Gender 2006).
Uptake and adherence to ARV Prophylaxis and Treatment
A substantial number of studies suggest that gender‐ specific barriers impede women’s ability to adhere to services for PMTCT and HIV treatment and care. Women may need permission from their male partners to travel to access services, including safe delivery. Implementation of safe delivery practices is part of the prevention of the perinatal HIV transmission; however, only 62% of deliveries in developing countries are assisted by skilled birth attendants (WHO 2008). Some women might not have the autonomy in their households to decide where they give birth. A recent study from rural Uganda involving more than 10,000 women reported that the male partner’s decision influenced not only the possibility for women to participate in the PMTCT programmes but also to deliver at a health facility (Mbonye 2010).
Male partner support is also a key factor in supporting safe infant feeding. The choice of an infant feeding option can be the basis of discrimination. In many societies not breastfeeding implies that a woman has a HIV positive status. In many contexts replacement milk is not affordable to mothers without the financial support of their male partner. The discrimination that can result from straying from social norms and the cost of infant feeding can lead to a significant gap between women’s intentions to protect their children from HIV and the feasibility of a feeding method. Choosing and adhering to an infant feeding method can be difficult for women without their male partner’s support (de Paoli 2004; Eide 2006;Leshabari 2007).
Yet currently, most awareness and implementation efforts related to HIV prevention and care are directed heavily towards women, thereby disregarding the cultural norms that often inform women’s decision‐making regarding these issues (Peacock 2009, HIggens 2010, Remien 2009, Dworkin 2009). As a result, men often lack the information or understanding of their role in relation to preventing HIV, family planning, and helping their families to access HIV prevention, testing, care and treatment services. In qualitative research assessing barriers to male involvement in PMTCT services, men expressed cultural perspectives on gender roles and the belief that PMTCT services are something related only to women as key barriers to their participation (Reece 2010; Theuring 2009).
Results from evaluations and documentation of programme experiences attest to the importance of constructively engaging men to address gender inequality and the impact of such interventions on making women less vulnerable to HIV.
When male partners attend ANC with their female partners during pregnancy, and are tested for HIV at the same time, women are more likely to accept HIV testing (Sherr 2010). There is also a higher uptake of ARV prophylaxis and better adherence to the suggested infant feeding option when male partners participate in PMTCT services (Farquhar 2004; Msuya 2008).
Why it is important to do this review
To achieve elimination of mother to child transmission of HIV (MTCT), at least 80% of pregnant women must have access to PMTCT services (UNAIDS 2009).
One of the key obstacles to women’s access to PMTCT services is the lack of full male partner engagement, which is strongly related to gender inequalities, and lackof understanding among men of health interventions for the health of men and women, present in many countries. Lack of evidence on the way to address this issue in the design and provision of PMTCT services severely limits the efforts of Ministries of Health, HIV policy‐makers and implementers to maximize the effectiveness of PMTCT programmes.
In this review we assess the effectiveness of interventions aiming to increase engagement of male partners to support their women and families in uptake of PMTCT services. Our findings will assist Ministries of Health, HIV policy makers and international organizations in focusing funding and efforts to reach the virtual elimination of the MTCT of HIV.
Objectives
To evaluate the impact of interventions which aim to enhance male involvement to increase women’s uptake of PMTCT interventions in developing countries.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), quasi–randomised controlled trials (q‐RCTs), cluster‐randomised trials (c‐RCTs), controlled before‐and‐after studies (CBA), and interrupted time series studies (ITS) with at least three time points before and after the intervention. We included q‐RTCs, c‐RCTs, CBA and ITS because of the scarcity of RCTs on the topic, based on the findings from a preliminary literature search.
We considered studies which were performed in low‐ and middle‐ income countries according the World Bank definition (World Bank 2010). We included studies which were performed after the year 2000, the year of the publication and release of the first guidelines from WHO on the use of the antiretroviral treatment (ART) for low‐ and middle‐income countries for PMTCT programmes (WHO Dept HIV/AIDS 2007).
Studies had to focus on male involvement interventions aimed to improve women’s uptake of PMTCT services.
Types of participants
We included studies focusing on men in relationships with pregnant women attending ANC in developing countries. Participants were pregnant women attending ANC and their male partners. We excluded studies focusing only on pregnant women, on interventions targeting all community members or only adult male relatives of pregnant women other than their male partners.
Types of interventions
We considered interventions aiming to improve all degrees of male partner involvement: from the participation in HIV counselling and testing to more constructive engagement of men to support their women and families through the pregnancy, delivery, and postpartum follow up and care.
We focused on two prongs of a comprehensive PMTCT programme as defined by WHO; Prong 3: Prevention of transmission from HIV‐positive women to their infants and Prong 4: Provision of treatment, care and support to HIV‐positive women and their children and families (UNAIDS 2011a).
Therefore, we included studies on interventions which focus on male involvement with the scope of:
‐ improving the identification of HIV positive pregnant women at ANC,
‐ the provision of ARV prophylaxis or treatment during pregnancy,
‐ the acceptance of safe delivery practices and hospital delivery,
‐ delivery and the post‐partum period for women and infants,
‐ the implementation of the recommended infant feeding option for the context,
‐ infant testing and follow up and the enrollment of women in PMTCT and HIV care and treatment programmes,
‐ the enrolment of male partners and their infants into HIV care and treatment services (WHO 2010; WHO 2011).
Eligible interventions included counselling programmes, health worker training, male peer groups, information campaigns, strategies at the community level, and changes in service delivery at health facilities to improve the male participation.
The comparison was the absence of any specific intervention aimed at male partners; the usual practice of the PMTCT programme.
Types of outcome measures
Studies had to provide quantitative data to be included. Studies providing only information on knowledge, attitudes, and acceptability of hypothetical interventions were not considered.
Primary outcomes
HIV incidence among infants
Proportion of pregnant women who accept HIV testing at ANC
Proportion of HIV positive pregnant women who receive ARV prophylaxis or treatment at ANC
Proportion of mothers who adhere to a selected infant feeding option, either exclusive breastfeeding for 6 months or infant formula
Proportion of HIV‐exposed infants born who are tested for HIV
Proportion of women at ANC identified as living with HIV who are enrolled in HIV care and treatment services
Proportion of infants diagnosed with HIV who are enrolled in HIV care and treatment services
Proportion of identified male partners of pregnant women living with HIV who are enrolled in HIV care and treatment services
Secondary outcomes
Proportion of hospital deliveries among women living with HIV
Proportion of infants born alive to women living with HIV who receive ARV prophylaxis
Proportion of male partner of pregnant women who receive HIV counselling
Proportion of male partners of pregnant women who receive HIV testing
Proportion of women living with HIV who disclose their HIV status to their male partners
Impact of male participation on the health service settings
Attitude of health workers to male participation
Male perspective and experience with ANC services
Search methods for identification of studies
We performed a comprehensive search to identify all relevant studies (published or unpublished) on our topic. We applied the Cochrane HIV/AIDS Group's validated strategies to identify relevant citations. We searched for studies published since the year 2000 regardless of the language of publication.
We combined the following search terms (searched in text/title/abstract/keyword):
Participant: Sexual partners, sexual partner, spouses, spouse, spousal, men, male, males, husband, husbands.
Intervention: PMTCT, MTCT, PPTCT, pPTCT, Prevention mother to child transmission, Prevention‐ mother‐to‐child‐transmission, Mother‐to‐child‐transmission, mother to child transmission, Maternal fetal infection, maternal‐fetal‐infection, Transmission Maternal‐Fetal‐Infection, Transmission Maternal Fetal Infection, Transmission Vertical Infection.
HIV: HIV Infections, HIV, HIV‐1, HIV‐2, human immunedeficiency virus, human immune‐deficiency virus, human immunodeficiency virus, human immuno‐deficiency virus acquired immunodeficiency syndrome, acquired immunedeficiency syndrome, acquired immuno‐deficiency syndrome, acquired immune‐deficiency syndrome.
Study type: randomized controlled trial, controlled clinical trial, random allocation, randomly, trial, clinical trial, comparative study, control, pre‐post controlled designs, comparison group, comparative study, evaluation study, multicenter study.
We added to the above terms the following subject headings: “Infectious Disease Transmission, Vertical”, “HIV infections”, “HIV”, “Spouse”, and “Husband”.
In order to increase sensitivity of the search strategy, we did not include specific terms to describe specific male involvement interventions.
Electronic searches
We searched the following databases:
Cochrane Central Register of Controlled Trials (CENTRAL)
MEDLINE
EMBASE
PsycINFO
The WHO Global Health Library (http://www.globalhealthlibrary.net), to obtain reference from the African Index Medicus (AIM), Index Medicus for the Eastern Mediterranean Region (EMRO), Latin America and the Caribbean (LILACS), Index Medicus for the South‐East Asia Region (IMSEAR), Western Pacific Region Index Medicus (WPRIM)
We searched the following ongoing trial websites:
ClinicalTrials.gov
Current Controlled Trials (http://www.controlled‐trials.com/mrct/)
We also searched the AEGIS database for relevant conference abstracts from the Conference on Retroviruses and Opportunistic Infections (CROI) up to 2008, from the International AIDS Conference (IAC) up to 2008, and for the HIV Pathogenesis (IAS) up to 2009. In addition, we searched the CROI, IAS, IAC web sites for conference abstracts up to 2011.
Searching other resources
We screened the reference lists of all selected articles to identify additional articles. Authors of conference proceedings were contacted as well as authors of relevant studies for additional information on potentially relevant studies.
Data collection and analysis
Selection of studies
We merged the results of the searches and removed duplicates. Subsequently, two reviewers independently selected relevant studies by screening titles and abstracts. Full texts of articles selected through the first screening were reviewed to assure that they meet the eligibility criteria. The selection of each reviewer was compared and discussed to reach a consensus. If disagreements persisted after this comparison, they were resolved by the involvement of the third reviewer. A flow chart was designed to present the study selection process.
Data extraction and management
Two reviewers independently extracted data of the included studies using a standardized data extraction form. We collected data on: citation, study design and method, inclusion criteria, participant’s characteristics, settings, type of intervention, outcome measures, outcome results and additional information.
The two reviewers compared the extracted data and any discrepancy was discussed to reach a consensus. When disagreement still persisted after the discussion, a third reviewer was involved.
Assessment of risk of bias in included studies
Two reviewers independently performed the risk of bias assessment of the included studies. Any disagreement was discussed to reach a consensus, if necessary, with the involvement of a third reviewer. RCTs were assessed using the Cochrane Collaboration’s Risk of Bias tool (Higgins 2011). We used a template to assess the following domains: sequence generation, allocation concealment, blinding, incomplete outcome data and selective outcome reporting and other issues. We assessed the risk of bias for each domain as low risk, high risk or unclear (which indicate uncertainty on the extent of the risk) and used RevMan for presenting the results.
Dealing with missing data
The author of the study was contacted in order to obtain additional information on the methodology of the study followed during the course of the study.
Data synthesis
We performed a narrative description of outcomes presented in the included study.
Results
Description of studies
Results of the search
The search yielded 3,072 citations. The search strategy is presented in the Appendix. After removing duplicates and screening titles and abstracts, we selected ten records reporting on seven completed studies. For three of the seven studies we found two records each and two records reporting on one on‐going trial. We did not find any relevant conference proceedings.
Through the full text screening we excluded eight records that were related to six studies. A PRISMA flow‐diagram is presented in Figure 1.
Figure 1.
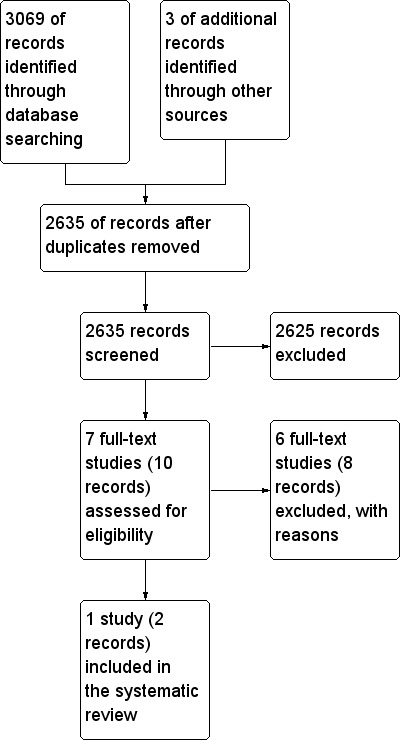
PRISMA flow diagram.
Included studies
We found one study that met all the inclusion criteria, an RCT for which we found two records, the protocol registration and a journal article (See characteristics of included studies). The study was conducted at three urban ANC clinics in Tanzania between May 2003 and October 2004. A total of 1521 women were recruited, including 760 in the intervention group and 761 in the control group. In addition, 254 male partners of the pregnant women in the intervention group agreed to participate. Participants were married pregnant women presenting for the first ANC visit within the first six months of pregnancy. Women were considered married if they were involved in a legal marriage, a traditional marriage or if they had been living with the same male partner, father of the unborn baby, for at least two years.
Women willing to participate were randomised to the intervention group, Couple Voluntary Counselling and Testing (CVCT), or the control group, Individual Voluntary Counselling and Testing (IVCT). Women in the intervention group received an invitation letter addressed to their male partner and written by a medical officer. In the letter the male partner was asked to accompany his wife to ANC to discuss health issues related to his female partner's pregnancy and the health of the baby. Women did not receive HIV counselling and testing during the first ANC visit and were asked to return even if their male partner refused to accompany them. Couples that returned to the clinic together could receive the HIV counselling and testing together as couple, if they both consented, or individually. In order for couples to complete CVCT, they had to receive each step (counselling, testing, and result) as a couple. Depending on the testing results, all couples were counselled on safe sex practices. In addition, if the couple or the woman was HIV positive, these couples were counselled on PMTCT, the father’s role in supporting safe infant feeding (either formula or exclusive breastfeeding), ARV therapy and self‐care. Women in the control group received individual HIV counselling and testing during their first ANC visit and then usual care.
HIV testing was performed with an HIV rapid test so that the result was available the same day. All HIV positive women were provided with ARV prophylaxis that consisted of nevirapine at delivery for both the mother and the infant.
Women were asked to fill out a baseline questionnaire and a follow up questionnaire three months after the delivery due date. The questionnaire included general demographic data and data on knowledge of HIV, experience of domestic violence, use of nevirapine and use of protection during sexual intercourse. Data on VCT were collected through clinical records books and VCT forms routinely used at the clinics.
The primary outcomes were the proportion of couples and women that received each component of VCT and, within the women included in the intervention group, the percentage of women who accepted CVCT and the proportion of women who crossed over to IVCT. Couples received complete CVCT if they received counselling, HIV testing and HIV results together.
Secondary outcomes were the proportion of women who received nevirapine at delivery, the use of condoms during sexual intercourse and the proportion of cases of domestic violence.
Excluded studies
Six studies were excluded during the full text screening (Byamugisha 2011; Ditekemena 2011; Farquhar 2004; Kakimoto 2007; Mohlala 2011; Msuya 2008). Records reporting protocol registration were available for two of these studies (Byamugisha 2011; Mohlala 2011). Reasons for the exclusion of these studies are presented in the Characteristics of excluded studies table. Three studies were excluded because of their study design (Farquhar 2004; Kakimoto 2007; Msuya 2008) while the other three studies were excluded since they focused on the male testing without presenting data on uptake of PMTCT services (Byamugisha 2011; Ditekemena 2011; Mohlala 2011).
On‐going study
We identified two records reporting on one relevant ongoing trial (Peltzer 2011). It is a double‐blind cluster‐RCT performed at 12 ANC clinics in community health centres in South Africa. The aim of the study was to increase male participation in the PMTCT services through male participation in a couples' session on risk reduction. This was intended to increase women's and male partners’ uptake of HIV counselling and testing and improve adherence to perinatal PMTCT services and ARV therapy for HIV positive individuals. Only pregnant women who accepted HIV counselling and testing and whose male partners were willing to participate were included.
Six clinics were randomised to the control group to provide the usual PMTCT intervention package, including a video on health education, HIV counselling and testing to all pregnant women, ARV therapy to all HIV positive pregnant women and advising all pregnant women to have their male partners tested for HIV in accordance with the South African PMTCT guidelines. The other six clinics were randomised to provide a PartnerPlus intervention. Recruited couples allocated to the intervention clinics received four group sessions aimed at changing cognitive behavioural regarding HIV prevention and transmission, sexual negotiation and risk reduction. Outcomes assessed were the rate of maternal and infant adherence to each component of the perinatal PMTCT cascade and the proportion of men that completed HIV counselling and testing. The study ran for one year and its anticipated completion is May 2012. Data from this study will be considered for inclusion in the update of the present review when available.
Risk of bias in included studies
The included RCT had a high risk of selection bias due to the randomisation process, which consisted of assigning women to the intervention or the control group sequentially as they arrived at the clinics. It was not possible to blind the participants or the health workers. However, the lack of blindness did not affect the study results and therefore the risk of performance bias was low. There was no way to blind either the participants or the staff since the intervention implied the presence of the male partner in one of the two groups. The risk of detection bias was unclear for the main outcomes and low for the secondary outcomes reported by the author. The baseline and follow up questionnaires were administered by a blinded assessor, but it was not clear whether the main outcomes were also assessed by a blinded researcher. In addition we consider the study to have a low risk of attrition bias. The risk of reporting bias was unclear since the protocol was published after the study was completed.
Effects of interventions
The study examined only some of the outcomes we aimed to assess in this review. The outcomes only focused on HIV counselling, HIV testing, result collection and ARV prophylaxis at delivery. No data on infants' follow up (testing and breastfeeding), HIV testing of family members and HIV care for identified HIV positive women were provided.
The proportions of women that received HIV counselling, HIV testing and collected their HIV test result were significantly lower in the intervention group than in the control group. As intention to treat analysis, only 48% of women in the intervention groups received counselling versus 93% in the control group (RR 0.52; 95% CI 0.48 to 0.56, p <0,001).The proportion of women who accepted HIV testing in the intervention group was 45% and 78% in the control group (RR 0.55; 95% CI 0.50 to 0.60, p <0,001); while 39% and 71% of the total recruited women respectively collected their result (RR 0.55; 95% CI 0.49 to 0.61, p <0,001).
Of the 760 women who were recruited in the intervention group 33% (n= 254) returned to clinics accompanied by their male partner, 15% (n=115) returned unaccompanied and 51% (n=391) did not return at all. Among women that returned with their male partners, 63% (n=161) accepted couple HIV counselling, 53% (n 135) accepted couple HIV testing and 47% (n 119) accepted to receive their HIV results together. Thus, only 16% of all pregnant women in the intervention group completed all of the components of CVCT.
HIV prevalence among women tested was 10% in both the groups; 91% (N 31/34) of the HIV positive women in the CVCT group and 85% (N 50/59) in the control group completed the follow up. According to ITT analysis the proportion of women and infants who received nevirapine at delivery was not different between the two arms (27% in the intervention group and 22% in the control group). Of the HIV positive women who received CVCT and returned for follow up, 55% received nevirapine at delivery. However, the difference was not statistically significant because of the small sample size.
According to the information collected through the questionnaires, women randomised to CVCT that accepted to receive VCT as a couple were significantly younger, with lower parity, less proportionally of Muslim faith and less likely to have ever experienced domestic violence compared to women recruited in the same group that refused to receive the intervention as a couple.
Discussion
Summary of main results
There was no evidence in our review of an effective method to increase women’s uptake of PMTCT services through an intervention focused on male involvement in PMTCT services. The existing evidence demonstrates low uptake of PMTCT services and that lack of male involvement is a key obstacle to women’s access and uptake. However, we did not identify an example of a documented, rigorously evaluated intervention successful at increasing women’s uptake of PMTCT services through male involvement in PMTCT programmes.
Moreover, the only study we identified that assessed the impact of male involvement on women’s uptake of PMTCT services shows this intervention having a negative impact on the health of these women. The male involvement intervention in this study reduced not only pregnant women’s uptake of HIV testing, but also their use of the ANC services.
Overall completeness and applicability of evidence
We performed an exhaustive search of an extensive range of databases. Moreover we considered as eligible all Cochrane eligible experimental study designs. Nevertheless we only found one trial that assessed the impact of the male involvement on women’s uptake of PMTCT services. Additionally, this study only partially addressed the question as it focused only on the effect of male involvement on women’s uptake of HIV testing and perinatal ARV prophylaxis. The RCT had a high risk of bias overall mainly due to the lack of a proper randomisation process.
This study was performed in Tanzania and evaluated the effectiveness of an invitation letter inviting male partners to accompany women to ANC. It evaluated the acceptance of HIV counselling and testing by male partners that accepted the invitation and their pregnant partners’ use of ANC.
The proportion of women who returned to attend the second ANC visit was significantly lower in the intervention group compared to the control group. This is exacerbated by the fact that in the study setting most women attend the second ANC visit. Therefore the invitation letters actually had a negative effect, acting as a barrier at the use of health services rather than having the intended positive impact. In addition among women in the intervention group who returned to clinics, a lower proportion accepted VCT both individually or in a couple. This was despite the fact that the couple had the option to refuse to HIV counselling and testing as a couple, altogether or they could receive it individually.
The study did not allow us to draw any conclusions on the remaining PMTCT components because of the small number of women in both groups who were followed through the delivery.
The results of the included study suggest that postponing VCT for pregnant women in order to provide the opportunity for male partners’ participation and provide CVCT is not a recommended approach. Moreover, the male invitation letter, although being a general letter without sensitive topics, was not effective in improving women’s access to and uptake of PMTCT services in the study context.
Potential biases in the review process
This review has a number of strengths. We rigorously followed the Cochrane methodology, performed a comprehensive search and included all Cochrane accepted experimental study design. We decided not to use any specific terms to define the male involvement in order to increase sensitivity of the search.
Agreements and disagreements with other studies or reviews
To the best of our knowledge, this is the first review focused on the impact of male involvement interventions on women’s uptake of PMTCT services. We found scarce evidence that did not demonstrate a beneficial effect of male involvement on women’s uptake of PMTCT services. However, existing qualitative literature (Dahl 2008; Maman 2008; Reece 2010; Theuring 2009) indicates that male involvement could increase effectiveness of PMTCT services. More research to define and assess the effectiveness of male involvement on women’s uptake of PMTCT services is needed.
Authors' conclusions
The use of an invitation letter to involve male partners, although without any sensitive words, did not improve the uptake of PMTCT services among pregnant women. In contrast it reduced the number of women who attended a second ANC visit and those who agreed to receive VCT. Two of the excluded studies also assessed the effect of an invitation letter sent to male partners of pregnant women. However those studies only focused on the impact of the letter on male attendance and not the impact on women’s uptake of PMTCT services.
We believe that to increase women’s uptake of PMTCT services, it is necessary to have, not only interventions that aim to promote VCT among male partners, but broader interventions to transform the harmful gender norms, attitudes and behaviours inherent in communities that make women and girls more vulnerable to HIV and serve as obstacles to their uptake of family planning, HIV prevention, testing, care and treatment services. Given that existing evidence demonstrates that low male involvement is a key obstacle to women’s access and uptake of PMTCT services, we believe that gender transformative work in communities should also involve increasing awareness among males on the positive role that they can play in protecting their families from HIV and helping them access family planning and HIV prevention, care and treatment services.
While tremendous investment has been made to improve access to PMTCT services in developing countries, most efforts have only focused on women and perpetuate the idea that PMTCT only concerns women. A broader perspective moving beyond "a mother focused intervention" to "a couple focused intervention” has been proposed as a way to overcome the obstacle linked to the low use of the PMTCT programme, however the included RCT which implemented a couple strategy did not prove to be effective. While many NGOs and organizations are engaged in gender transformative interventions on the ground, there is a paucity of rigorous analysis to evaluate this work. Most of the studies to evaluate this work consist of surveys, qualitative studies or descriptive studies that do not provide the quantitative data that we assessed in this review and do not reach the quality design that allow them to reach solid conclusions.
Many efforts have been made during the past ten years to prevent mother‐to‐child transmission of HIV through PMTCT programmes in developing countries. Researchers and implementers identified low male involvement as a key obstacle to women’s access and uptake of PMTCT services. For this reason, it is highly relevant to rigorously analyse the most effective way to engage men to increase women’s access and uptake of PMTCT services. The evidence on successful approaches to do this is lacking.
Acknowledgements
We would like to express our gratitude to Joy Oliver from the Cochrane HIV/AIDS group and Tim J A Reeves from Medicine Central Library, Imperial College London for helping us develop the search strategy. We are also very grateful to Tara Horvath and the rest of the Cochrane HIV/AIDS group for their support and guidance.
Appendices
Appendix 1. AEGIS search strategy
(randomised controlled trial OR controlled clinical trial OR comparative study OR randomised OR random allocation OR cluster OR random* OR trial OR groups OR control*) AND (HIV infection or HIV infections or HIV or HIV 1 or HIV 2) AND (MTCT OR PMTCT OR pPTCT OR mother‐to‐child transmission OR parent‐to‐child transmission OR maternal‐to‐child transmission OR vertical transmission OR vertical infectious diseases transmission OR vertical infectious transmission) AND (Spouse OR Husband OR male OR men OR sexual partner OR spousal)
Appendix 2. CENTRAL search strategy
ID Search
#1 MeSH descriptor HIV explode all trees
#2 MeSH descriptor HIV Infections explode all trees
#3 (randomized controlled trial or controlled clinical trial or comparative study):ti,ab,kw or (randomized controlled trial or controlled clinical trial or comparative study):pt
#4 (random* OR randomly OR control* OR cluster OR groups OR placebo):ti,ab,kw
#5 MeSH descriptor Cluster Analysis explode all trees
#6 MeSH descriptor Random Allocation explode all trees
#7 (human immuno‐deficiency virus OR human immunodeficiency virus OR human immunedeficiency virus OR human immune‐deficiency virus OR ((human immun*) and (deficiency virus)) OR acquired immunodeficiency syndrome OR acquired immunedeficiency syndrome OR acquired immuno‐deficiency syndrome OR acquired immune‐deficiency syndrome OR ((acquired immun*) and (deficiency syndrome))):ti,ab,kw
#8 (HIV infection OR HIV OR HIV 1 OR HIV 2 OR HIV infections):ti,ab,kw
#9 MeSH descriptor Infectious Disease Transmission, Vertical explode all trees
#10 (MTCT OR PMTCT OR pPTCT):ti,ab,kw
#11 (mother‐to‐child transmission or parent‐to‐child transmission or maternal‐to‐child transmission or vertical transmission or vertical infectious diseases transmission or vertical infectious transmission or maternal‐fetal infection transmission or fetomaternal infection transmission):ti,ab,kw
#12 MeSH descriptor Spouses explode all trees
#13 (Spouse or Spouses or Husband or husbands or males or male or men or sexual partner or sexual partners spousal or spousals):ti,ab,kw
#14 (#3 OR #4 OR #5 OR #6)
#15 (#1 OR #2 OR #7 OR #8)
#16 (#9 OR #10 OR #11)
#17 (#12 OR #13)
#18 (#14 AND #15 AND #16 AND #17)
#19 (#18), from 2000 to 2011
Appendix 3. Clinicaltrials.gov search strategy
(mtct OR pmtct OR pptct OR (mother‐to‐child AND transmission) OR (parent‐to‐child AND transmission) OR (maternal‐to‐child AND transmission) OR (vertical AND transmission) OR (maternal‐fetal AND transmission)) AND (Spouse OR Spouses OR Husband OR husbands OR males OR male OR (sexual AND partner) OR (sexual AND partners)) | Interventional Studies | HIV | received from 01/01/2000 to 10/04/2011
Appendix 4. Current Controlled Trials Search strategy
(mtct OR pmtct OR pptct OR (mother‐to‐child AND transmission) OR (parent‐to‐child AND transmission) OR (maternal‐to‐child AND transmission) OR (vertical AND transmission))
Appendix 5. EMBASE search strategy
1 exp vertical transmission/
2 mother‐to‐child transmission.ab. or mother‐to‐child transmission.kw. or mother‐to‐child transmission.ti.
3 parent‐to‐child transmission.ab. or parent‐to‐child transmission.kw. or parent‐to‐child transmission.ti.
4 PMTCT.ab. or PMTCT.kw. or PMTCT.ti.
5 pPTCT.ab. or pPTCT.kw. or pPTCT.ti.
6 MTCT.ab. or MTCT.kw. or MTCT.ti.
7 maternal‐to‐child transmission.ab. or maternal‐to‐child transmission.kw. or maternal‐to‐child transmission.ti.
8 vertical transmission.ab. or vertical transmission.kw. or vertical transmission.ti.
9 vertical infectious diseases transmission.ab. or vertical infectious diseases transmission.kw. or vertical infectious diseases transmission.ti.
10 maternal‐fetal infection transmission.ab. or maternal‐fetal infection transmission.kw. or maternal‐fetal infection transmission.ti.
11 vertical infectious transmission.ab. or vertical infectious transmission.ti. or vertical infectious transmission.kw.
12 fetomaternal infection transmission.ab. or fetomaternal infection transmission.ti. or fetomaternal infection transmission.kw.
13 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12
14 exp human immunodeficiency virus/ or exp human immunodeficiency virus 1/ or exp human immunodeficiency virus 2/ or exp human immunodeficiency virus 1 infection/ or exp human immunodeficiency virus 2 infection/ or exp human immunodeficiency virus infection/
15 HIV.ab. or HIV.kw. or HIV.ti. or HIV1.ab. or HIV1.kw. or HIV1.ti. or HIV2.ab. or HIV2.kw. or HIV2.ti.
16 human immunodeficiency virus.ab. or human immunodeficiency virus.kw. or human immunodeficiency virus.ti.
17 human immunedeficiency virus.ab. or human immunedeficiency virus.kw. or human immunedeficiency virus.ti.
18 human immuno‐deficiency virus.ab. or human immuno‐deficiency virus.kw. or human immuno‐deficiency virus.ti.
19 human immune‐deficiency virus.ab. or human immune‐deficiency virus.kw. or human immune‐deficiency virus.ti.
20 (human immun$ and deficiency virus).ab. or (human immun$ and deficiency virus).kw. or (human immun$ and deficiency virus).ti.
21 acquired immunodeficiency syndrome.ab. or acquired immunodeficiency syndrome.kw. or acquired immunodeficiency syndrome.ti.
22 acquired immunedeficiency syndrome.ab. or acquired immunedeficiency syndrome.kw. or acquired immunedeficiency syndrome.ti.
23 acquired immune‐deficiency syndrome.ab. or acquired immune‐deficiency syndrome.kw. or acquired immune‐deficiency syndrome.ti.
24 acquired immuno‐deficiency syndrome.ab. or acquired immuno‐deficiency syndrome.kw. or acquired immuno‐deficiency syndrome.ti.
25 (acquired immun$ and deficiency syndrome).ab. or (acquired immun$ and deficiency syndrome).kw. or (acquired immun$ and deficiency syndrome).ti.
26 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25
27 exp spouse/
28 exp male/
29 sexual partner.ab. or sexual partner.kw. or sexual partner.ti.
30 sexual partners.ab. or sexual partners.kw. or sexual partners.ti.
31 spouse.ab. or spouse.kw. or spouse.ti.
32 spouses.ab. or spouses.kw. or spouses.ti.
33 spousal.ab. or spousal.kw. or spousal.ti.
34 husband.ab. or husband.kw. or husband.ti.
35 husbands.ab. or husbands.kw. or husbands.ti.
36 men.ab. or men.kw. or men.ti.
37 male.ab. or male.kw. or male.ti.
38 males.ab. or males.kw. or males.ti.
39 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38
40 13 and 26 and 39
41 limit 40 to (embase and yr="2000 ‐Current")
Appendix 6. MEDLINE search strategy
1 exp Infectious Disease Transmission, Vertical/
2 (MTCT or PMTCT or pPTCT).ab. and (MTCT or PMTCT or pPTCT).ti. and (MTCT or PMTCT or pPTCT).af.
3 (MTCT or PMTCT or pPTCT).ab. or (MTCT or PMTCT or pPTCT).ti.
4 mother‐to‐child transmission.ab. or mother‐to‐child transmission.ti.
5 parent‐to‐child transmission.ab. or parent‐to‐child transmission.ti.
6 maternal‐to‐child transmission.ab. or maternal‐to‐child transmission.ti.
7 vertical transmission.ab. or vertical transmission.ti.
8 vertical infectious diseases transmission.ab. or vertical infectious diseases transmission.ti.
9 vertical infectious transmission.ab. or vertical infectious transmission.ti.
10 maternal‐fetal infection transmission.ab. or maternal‐fetal infection transmission.ti.
11 fetomaternal infection transmission.ab. or fetomaternal infection transmission.ti.
12 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11
13 exp hiv infections/ or exp acquired immunodeficiency syndrome/
14 exp HIV/ or exp HIV‐2/ or exp HIV‐1/
15 (HIV infection or HIV infections or HIV or HIV‐1 or HIV1 or HIV‐2 or HIV2 or HIV 1 or HIV 2).ab. or (HIV infection or HIV infections or HIV or HIV‐1 or HIV1 or HIV‐2 or HIV2 or HIV 1 or HIV 2).ti.
16 ((((human immuno‐deficiency virus or human immunodeficiency virus or human immunedeficiency virus or human immune‐deficiency virus or human immun$) and deficiency virus) or acquired immunodeficiency syndrome or acquired immunedeficiency syndrome or acquired immuno‐deficiency syndrome or acquired immune‐deficiency syndrome or acquired immun$) and deficiency syndrome).ab. or ((((human immuno‐deficiency virus or human immunodeficiency virus or human immunedeficiency virus or human immune‐deficiency virus or human immun$) and deficiency virus) or acquired immunodeficiency syndrome or acquired immunedeficiency syndrome or acquired immuno‐deficiency syndrome or acquired immune‐deficiency syndrome or acquired immun$) and deficiency syndrome).ti.
17 13 or 14 or 15 or 16
18 Spouses/
19 exp sexual partners/ or exp spouses/
20 Male/
21 (Spouse or Spouses or Husband or husbands or male or men or sexual partner or spousal or males or sexual partners).ab. or (Spouse or Spouses or Husband or husbands or male or men or sexual partner or spousal or males or sexual partners).ti.
22 18 or 19 or 20 or 21
23 (randomized controlled trial or controlled clinical trial or comparative study).pt.
24 randomized.ab. or randomized.ti.
25 placebo.ab. or placebo.ti.
26 Random$.ab. or random$.ti.
27 randomly.ab. or randomly.ti.
28 cluster.ab. or cluster.ti.
29 groups.ab. or groups.ti.
30 trial.ab. or trial.ti.
31 exp Random Allocation/
32 exp cluster analysis/
33 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32
34 12 and 17 and 22
35 limit 34 to yr="2000 ‐Current"
36 33 and 34
37 limit 36 to yr="2000 ‐Current"
Appendix 7. PsycINFO search strategy
1 exp HIV/
2 (HIV infection or HIV infections or HIV or HIV 1 or HIV 2).ti. or (HIV infection or HIV infections or HIV or HIV 1 or HIV 2).ab. or (HIV infection or HIV infections or HIV or HIV 1 or HIV 2).hw.
3 ((((human immuno‐deficiency virus or human immunodeficiency virus or human immunedeficiency virus or human immune‐deficiency virus or human immun$) and deficiency virus) or acquired immunodeficiency syndrome or acquired immunedeficiency syndrome or acquired immuno‐deficiency syndrome or acquired immune‐deficiency syndrome or acquired immun$) and deficiency syndrome).ti. or ((((human immuno‐deficiency virus or human immunodeficiency virus or human immunedeficiency virus or human immune‐deficiency virus or human immun$) and deficiency virus) or acquired immunodeficiency syndrome or acquired immunedeficiency syndrome or acquired immuno‐deficiency syndrome or acquired immune‐deficiency syndrome or acquired immun$) and deficiency syndrome).ab. or ((((human immuno‐deficiency virus or human immunodeficiency virus or human immunedeficiency virus or human immune‐deficiency virus or human immun$) and deficiency virus) or acquired immunodeficiency syndrome or acquired immunedeficiency syndrome or acquired immuno‐deficiency syndrome or acquired immune‐deficiency syndrome or acquired immun$) and deficiency syndrome).hw.
4 exp Disease Transmission/
5 (MTCT or PMTCT or pPTCT).ti. or (MTCT or PMTCT or pPTCT).ab. or (MTCT or PMTCT or pPTCT).hw.
6 (mother‐to‐child transmission or parent‐to‐child transmission or maternal‐to‐child transmission or vertical transmission or vertical infectious diseases transmission or vertical infectious transmission or maternal‐fetal infection transmission or fetomaternal infection transmission).ti. or (mother‐to‐child transmission or parent‐to‐child transmission or maternal‐to‐child transmission or vertical transmission or vertical infectious diseases transmission or vertical infectious transmission or maternal‐fetal infection transmission or fetomaternal infection transmission).ab. or (mother‐to‐child transmission or parent‐to‐child transmission or maternal‐to‐child transmission or vertical transmission or vertical infectious diseases transmission or vertical infectious transmission or maternal‐fetal infection transmission or fetomaternal infection transmission).hw.
7 exp spouses/
8 (Spouse or Spouses or Husband or male or men or sexual partner or spousal or husbands or males or sexual partners or spousals).ti. or (Spouse or Spouses or Husband or male or men or sexual partner or spousal or husbands or males or sexual partners or spousals).ab. or (Spouse or Spouses or Husband or male or men or sexual partner or spousal or husbands or males or sexual partners).hw.
9 1 or 2 or 3
10 4 or 5 or 6
11 7 or 8
12 9 and 10 and 11
13 limit 12 to yr="2000 ‐ 2011"
Appendix 8. WHO Global Health Library search strategy
(HIV infection or HIV infections or HIV or HIV1 or HIV2) AND (MTCT or PMTCT or pPTCT OR mother‐to‐child transmission or parent‐to‐child transmission or maternal‐to‐child transmission or vertical transmission or vertical infectious diseases transmission or vertical infectious transmission or maternal‐fetal infection transmission or fetomaternal infection transmission) AND (Spouse or Spouses or Husband or male or men or sexual partner or spousal or husbands or males or sexual partners or spousals)
History
Protocol first published: Issue 11, 2011 Review first published: Issue 10, 2012
| Date | Event | Description |
|---|---|---|
| 9 May 2012 | Amended | ready for submission |
Differences between protocol and review
There are a number of differences between the protocol and the present review. These differences are mainly due to the fact that only one study met the inclusion criteria, thus they are mostly in the data analysis section.
Search strategy: due to the small number of RCTs performed on the present topic we included not only RCTs but also cluster‐RCT, CCCT, CBA and ITS. For this reason we did not apply the Cochrane Highly Sensitive Search Strategy to identify reports of RCT.
Assessment of risk of bias in included studies: we did not find any eligible studies with study designs other than RCT, so using other tools to assess risk of bias (EPOC 2009), as previously stated in the protocol, was not necessary.
Data analysis: if we found more than one study meeting our criteria we would have presented a summary of the treatment effect by study design, type of intervention and setting. Moreover, we would have analysed the outcomes using RevMan and performed a meta‐analysis for the primary dichotomous outcomes presented by RCT studies if appropriate according to the homogeneity of interventions, settings and characteristic of the measurement of the studies. We would have tested heterogeneity by using the I2 statistic with significance set at >50% and the chi‐squared statistic with significance set at P<0.10. Possible sources of heterogeneity would have evaluated by sensitivity analysis (Higgins 2003) and a random‐effect would have used if heterogeneity was detected.
Reporting bias: we would have used funnel plots and tests for funnel plot asymmetry to assess the presence of reporting bias.
Data synthesis: studies would been presented by narrative description if the use of meta‐analysis or a summary of results for studies design others than RCTs would been inappropriate. In this case the studies would be organized according the type of intervention and income countries classification.
Subgroup analysis and investigation of heterogeneity: we would have conducted subgroups analyses for low and middle income countries, type of intervention and setting of intervention.
Assessment of quality of evidence across studies: we would have used the GRADE approach to evaluate the aggregate quality of the evidence (Higgins 2011) for the primary outcomes.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | RCT conducted at three urban ANC clinics in Tanzania between May 2003 and October 2004. | |
| Participants | 1521 women were recruited: 760 in the intervention (CVCT) group; 761 in the control (IVCT) group. In addition 254 male partners of the pregnant women in the CVCT group agreed to participate. Participants were married pregnant women presenting for the first ANC visit during the first six months of their pregnancy and their male partner for the intervention group. Women were considered as being married if they were involved in a legal marriage, a traditional marriage or if they have been living with the same actual male partner, father of the unborn baby, for at least two years. |
|
| Interventions | Intervention: Couple Voluntary Counselling and Testing (CVCT). Pregnant women attending their first ANC visit received an invitation letter, written by the medical officer, addressed to their male partners. The husband was asked to accompany his wife to the clinic for discussing health issues related to the pregnancy and the health of the baby. The women did not receive counselling and testing during that first visit and were asked to come back even if the husband refused to accompany her. Couple that return to the clinic together could receive each step of counselling and testing process together. If only one of the couple consented to VCT he/she received IVCT. If the couple consented to receive VCT together but not to collect their results together they received IVCT. Control group: Individual Voluntary Counselling and Testing (IVCT). Pregnant women attending their first ANC visit received IVCT during their first ANC visit and received usual care. All HIV positive women were provided with ARV prophylaxis that consisted of Nevirapine at delivery for both, mother and infant. |
|
| Outcomes | The primary outcomes were the proportion of couple and women receiving each step of the VCT and, within the women included in the intervention group the percentage of women who accepted the CVCT and the proportion of women who cross over to IVCT. A couple was considered as receiving complete CVCT if they received counselling, testing and result together. Secondary outcomes were the proportion of women who received Nevirapine at delivery, the use of condom during sexual intercourse and the proportion of cases of domestic violence. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quoted: "All consented women were randomised to either CVCT or IVCT (every other one, sequentially)." Not Randomized |
| Allocation concealment (selection bias) | High risk | Quoted: "All consented women were randomised to either CVCT or IVCT (every other one, sequentially)." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quoted: "Women randomised to the control arm were then consented to receive IVCT immediately" . However the knowledge of the randomisation was unlikely to affect the results |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quantitative Outcomes: not stated. Qualitative data (not included in the outcomes of the present review) quoted "2004, after consent a follow‐up questionnaire was administered by an interviewer blinded to the woman’s study": Low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing baseline demographics data for 0,5% of all the participants. |
| Selective reporting (reporting bias) | Unclear risk | All the outcomes presented in the protocol were also presented in the paper. However the protocol has been published after the study was concluded |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Byamugisha 2011 | This study was conducted at an ANC clinic at Mbale Regional Referral Hospital, Uganda. Women received a written invitation letter for their male partners to attend HIV counselling and testing. However, the study did not present data on women’s uptake of the PMTCT services. |
| Ditekemena 2011 | This RCT evaluated the acceptance of HIV counselling and testing by male partners of pregnant women attending a maternity unit in Kinshasa, Democratic Republic of Congo (DRC). The study did not present data on women uptake of the PMTCT services. |
| Farquhar 2004 | This descriptive study presented data on the uptake of HIV counselling, HIV testing, Nevirapine prophylaxis among pregnant women attending an ANC clinic in Nairobi, Kenya after the involvement of their male partners in the PMTCT programme. |
| Kakimoto 2007 | This descriptive study explored the uptake of PMTCT services among pregnant women before and after the inclusion of male partners a mother class in Phnom Penh, Cambodia. |
| Mohlala 2011 | This RCT assessed the effectiveness of an invitation letter for pregnant women to give to their male partners on uptake of HIV counselling and testing in Khayelitsha, South Africa. The study did not present data on women’s uptake of PMTCT services. |
| Msuya 2008 | This was a cohort study that describes the uptake of HIV voluntary counselling and testing (VCT) among male partners of pregnant women followed at two primary healthcare clinics in urban, Moshi, Tanzania. It also presented women’s uptake of perinatal PMTCT services. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | CT01448512. Promoting Male Involvement to Improve Prevention of Mother to Child Transmission (PMTCT) Uptake and Reduce ANC Infection. |
| Methods | Double Blind Cluster RCT, performed at 12 ANC clinics in South Africa |
| Participants | Pregnant women who accepted HIV counselling and testing and whose male partners were willing to participate were included |
| Interventions | Intervention: six clinics provided a PartnerPlus intervention, which included four group sessions for the recruited couples and provision of cognitive behavioural skills on HIV prevention and transmission, sexual negotiation and risk reduction. Control: six clinics provided usual care; it is implied that this included offering HIV counselling and testing, ARV therapy in accordance with the South African National PMTCT Guidelines to all pregnant women and the advice to have their male partner tested. Participating couples also received a video on health education. |
| Outcomes | Primary Outcome: number of ANC visits by the mother and father, assessed at baseline and three time points (one month post intervention to 32 weeks to 3 days post‐partum) Secondary Outcome:· Rate of adherence to HIV counselling by women, their male partners and infants· Adherence of women and infants to the ARV prophylaxis· Infant HIV testing (by using PCRI at 6 weeks of ageRate of condom use from baseline to one month to 3 days post‐partum. |
| Starting date | September 2010 |
| Contact information | Responsible party D.Jones, University of Miami, USA, |
| Notes | Estimated study completion date: May 2012 |
Contributions of authors
SB and LTC conceived the idea for the review. SB wrote the protocol with assistance from EG and LTC. SB, and MvV performed the searches, SB, EG, MvV and LTC performed the screening and data extraction. JC and AM helped to revise the protocol. SB led the review process.
Sources of support
Internal sources
-
The Department of Primary Care and Public Health, Imperial College London, UK.
The review received a partial financial contribution from The Department of Primary Care and Public Health, Imperial College London. The Department of Primary Care & Public Health at Imperial College is grateful for support from the NIHR Collaboration for Leadership in Applied Health Research & Care (CLAHRC) Scheme, the NIHR Biomedical Research Centre scheme, and the Imperial Centre for Patient Safety and Service Quality.
The Elizabeth Glaser Pediatric AIDS Foundation, Global Technical Policy Unit, USA.
External sources
No sources of support supplied
Declarations of interest
None to declare
New
References
References to studies included in this review
- Becker S, Mlay R, Schwandt HM, Lyamuya E. Comparing couples' and individual voluntary counseling and testing for HIV at antenatal clinics in Tanzania: a randomized trial. AIDS & Behavior 2010;14(3):558‐566. [DOI] [PubMed] [Google Scholar]; NCT00631384. Experimental Design of Couple Counseling and Testing in Antenatal Clinics in Dar es Salaam, Tanzania (CVCT). http://clinicaltrials.gov/show/NCT00631384.
References to studies excluded from this review
- Byamugisha R, Astrom AN, Ndeezi G, Karamagi CA, Tylleskar T, Tumwine JK. Male partner antenatal attendance and HIV testing in eastern Uganda: a randomized facility‐based intervention trial. Journal of the International AIDS Society 2011;14(1):43‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]; NCT01144234. Male Involvement in Antenatal Care and the Prevention Programme of Mother‐to‐child Transmission of HIV in Uganda (InvolveMaleUg). http://clinicaltrials.gov/show/NCT01144234.
- Ditekemena J, Matendo R, Koole O, Colebunders R, Kashamuka M, Tshefu A, Kilese N, Nanlele D, Ryder R. Male partner voluntary counselling and testing associated with the antenatal services in Kinshasa, Democratic Republic of Congo: A randomized controlled trial. International Journal of STD and AIDS 2011;22(3):165‐170. [DOI] [PubMed] [Google Scholar]
- Farquhar C, Kiarie JN, Richardson BA, Kabura MN, John FN, Nduati RW, et al. Antenatal couple counseling increases uptake of interventions to prevent HIV‐1 transmission. Journal of Acquired Immune Deficiency Syndromes 2004;37(5):1620‐6. [PUBMED: 15577420] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakimoto K, Kanal K, Mukoyama Y, Chheng TV, Chou TL, Sedtha C. Influence of the involvement of partners in the mother class with voluntary confidential counselling and testing acceptance for prevention of mother to child transmission of HIV programme (PMTCT programme) in Cambodia. AIDS Care 2007;19(3):381‐384. [DOI] [PubMed] [Google Scholar]
- Mohlala BK, Boily MC, Gregson S. The forgotten half of the equation: randomized controlled trial of a male invitation to attend couple voluntary counselling and testing. AIDS 2011;25(12):1535‐1541. [DOI] [PMC free article] [PubMed] [Google Scholar]; NCT00405990. Male Partner Involvement in the Prevention of MTCT of HIV. http://clinicaltrials.gov/show/NCT00405990.
- Msuya SE, Mbizvo EM, Hussain A, Uriyo J, Sam NE, Stray‐Pedersen B. Low male partner participation in antenatal HIV counselling and testing in northern Tanzania: implications for preventive programs. AIDS Care 2008;20(6):700‐9. [PUBMED: 18576172] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
- NCT01448512. Promoting Male Involvement to Improve Prevention of Mother to Child Transmission (PMTCT) Uptake and Reduce Antenatal Infection. http://clinicaltrials.gov/show/NCT01448512. ; Peltzer K, Jones D, Weiss SM, Shikwane E. Promoting male involvement to improve PMTCT uptake and reduce antenatal HIV infection: a cluster randomized controlled trial protocol. BMC Public Health 2011;11(778‐787). [PUBMED: 21985332] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
- Bajunirwe F, Muzoora M. Barriers to the implementation of programs for the prevention of mother‐to‐child transmission of HIV: a cross‐sectional survey in rural and urban Uganda. AIDS Research and Therapy 2005;2:10. [PUBMED: 16255776] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl V, Mellhammar L, Bajunirwe F, Bjorkman P. Acceptance of HIV testing among women attending antenatal care in south‐western Uganda: risk factors and reasons for test refusal. AIDS Care 2008;20(6):746‐52. [PUBMED: 18576178] [DOI] [PubMed] [Google Scholar]
- Paoli M, Manongi R, Klepp KI. Are infant feeding options that are recommended for mothers with HIV acceptable, feasible, affordable, sustainable and safe? Pregnant women's perspectives. Public Health Nutrition 2004;7(5):611‐9. [PUBMED: 15251051] [DOI] [PubMed] [Google Scholar]
- Eide M, Myhre M, Lindbaek M, Sundby J, Arimi P, Thior I. Social consequences of HIV‐positive women's participation in prevention of mother‐to‐child transmission programmes. Patient Education and Counseling 2006;60(2):146‐51. [PUBMED: 16442457] [DOI] [PubMed] [Google Scholar]
- Cochrane Effective Practice and Organisation of Care Group. Risk of Bias. http://epoc.cochrane.org/epoc‐resources‐review‐authors2009.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. British Medical Journal (Clinical Research Ed.) 2003;327(7414):557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.2011.
- Kebaabetswe PM. Barriers to participation in the prevention of mother‐to‐child HIV transmission program in Gaborone, Botswana a qualitative approach. AIDS Care 2007;19(3):355‐60. [PUBMED: 17453569] [DOI] [PubMed] [Google Scholar]
- Kilewo C, Massawe A, Lyamuya E, Semali I, Kalokola F, Urassa E, et al. HIV counseling and testing of pregnant women in sub‐Saharan Africa: experiences from a study on prevention of mother‐to‐child HIV‐1 transmission in Dar es Salaam, Tanzania. Journal of Acquired Immune Deficiency Syndromes 2001;28(5):458‐62. [PUBMED: 11744835] [DOI] [PubMed] [Google Scholar]
- Leshabari SC, Blystad A, Moland KM. Difficult choices: infant feeding experiences of HIV‐positive mothers in northern Tanzania. Sahara J 2007;4(1):544‐55. [PUBMED: 18040533] [DOI] [PubMed] [Google Scholar]
- Maman S, Groves A, King E, Pierce M, Wyckoff S. HIV Testing During Pregnancy. A Literature and Policy Review. http://www.aidslex.org/site_documents/TP‐0018E.pdf2008.
- Mbonye AK, Hansen KS, Wamono F, Magnussen P. Barriers to prevention of mother‐to‐child transmission of HIV services in Uganda. Journal of Biosocial Science 2010;42(2):271‐83. [PUBMED: 19895727] [DOI] [PubMed] [Google Scholar]
- Medley A, Garcia‐Moreno C, McGill S, Maman S. Rates, barriers and outcomes of HIV serostatus disclosure among women in developing countries: implications for prevention of mother‐to‐child transmission programmes. Bulletin of the World Health Organization 2004;82(4):299‐307. [PUBMED: 15259260] [PMC free article] [PubMed] [Google Scholar]
- Peacock D, Stemple L, Sawires S, Coates TJ. Men, HIV/AIDS, and human rights. Journal of Acquired Immune Deficiency Syndromes 2009;51 Suppl 3:S119‐25. [PUBMED: 19553779] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reece M, Hollub A, Nangami M, Lane K. Assessing male spousal engagement with prevention of mother‐to‐child transmission (pMTCT) programs in western Kenya. AIDS Care 2010;22(6):743‐50. [PUBMED: 20461572] [DOI] [PubMed] [Google Scholar]
- Sarker M, Sanou A, Snow R, Ganame J, Gondos A. Determinants of HIV counselling and testing participation in a prevention of mother‐to‐child transmission programme in rural Burkina Faso. Tropical Medicine and International Health 2007;12(12):1475‐83. [PUBMED: 18076555] [DOI] [PubMed] [Google Scholar]
- Sherr L. Fathers and HIV: considerations for families. Journal of the International AIDS Society 2010;13 Suppl 2:S4. [PUBMED: 20573286] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theuring S, Mbezi P, Luvanda H, Jordan‐Harder B, Kunz A, Harms G. Male involvement in PMTCT services in Mbeya Region, Tanzania. AIDS and Behavior 2009;13 Suppl 1:92‐102. [PUBMED: 19308720] [DOI] [PubMed] [Google Scholar]
- Joint United Nations Programme on HIV/AIDS. Resolution adopted by the General Assembly at the Sixtieth Session: Political declaration on HIV/AIDS. http://www.un.org/ga/aidsmeeting2006/documents.html2006.
- Joint United Nations Programme on HIV/AIDS. UNAIDS calls for a virtual elimination of mother to child transmission of HIV by 2015. http://data.unaids.org/pub/PressRelease/2009/20090521_pr_priorityareas_en.pdf.2009.
- Joint United Nations Programme on HIV/AIDS. Global report: UNAIDS report on the global AIDS epidemic 2010. www.unaids.org/documents/20101123_GlobalReport_em.pdf2010.
- UNAIDS. Global Plan Towards the Elimination of New HIV Infections among Children by 2015 and Keeping Their Mothers Alive. Geneva: UNAIDS2011a.
- UNAIDS. World AIDS Day Report. Geneva: UNAIDS2011b.
- United Nations General Assembly Special Session. Declaration of Commitment on HIV/AIDS. http://data.unaids.org/publications/irc‐pub03/aidsdeclaration_en.pdf2001.
- United Nations Children’s Fund (UNICEF). The state of the world’s children 2007: Women and children, the double dividend of gender equality. http://www.unicef.org/sowc07/2007.
- Wang A, Wang L, Su S. Gender inequalities, and the use of prevention of mother‐to‐child transmission of HIV services, in Xingjiang, China. AIDS 2006 ‐ XVI International AIDS Conference: Abstract no. CDD0053. 2006. [Google Scholar]
- World Health Organization, Regional Office for Africa. Skilled birth attendants. http://www.who.int/making_pregnancy_safer/events/2008/mdg5/factsheet_sba.pdf2008.
- World Health Organization. Integrating gender into HIV/AIDS programmes in the health sector: tool to improve responsiveness to women’s needs. http://www.who.int/gender/documents/gender_hiv/en/2009. [PubMed]
- World Health Organization. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: recommendations for a public health approach – 2010 version. http://whqlibdoc.who.int/publications/2010/9789241599818_eng.pdf2010. [PubMed]
- World Health Organization. Kesho Bora Study. Preventing mother‐to‐child transmission of HIV during breastfeeding Information. http://www.who.int/reproductivehealth/publications/rtis/keshobora/en/index.html.2011.
- World Health Organization Department of Gender, Women and Health, WHO Cluster of Family and Community Health. Gender Dimensions of HIV Status Disclosure to Sexual Partners:Rates, Barriers and Outcomes A Review Paper. http://www.who.int/gender/documents/women_and_girls/9241590734/en/2003.
- World Health Organization, Department of HIV/AIDS. Prevention of Mother‐To‐Child Transmission (PMTCT). Briefing Note.. http://www.who.int/hiv/pub/toolkits/PMTCT%20HIV%20Dept%20brief%20Oct%2007.pdf2007.
- World Health Organization, Department of Gender, Women and Health, Department of HIV/AIDS. Addressing violence against women in HIV testing and counselling: A meeting report. http://whqlibdoc.who.int/publications/2006/9241594594_eng.pdf2006.
- World Health Organization, United Nations Children’s Fund, Joint United Nations Programme on HIV/AIDS. Towards universal access: scaling up priority HIV/AIDS interventions in the health sector. Progress report 2010. http://www.who.int/hiv/pub/2010progressreport/report/en/index.html2010.
- World Bank. The Wolrd bank. Country Classification. http://go.worldbank.org/AJGKUS0E802010.
References to other published versions of this review
- Dworkin SL, Kambou SD, Sutherland C, Moalla K, Kapoor A. Gendered empowerment and HIV prevention: policy and programmatic pathways to success in the MENA region.. J Acquir Immune Defic Syndr 2009 Jul 1;51 Suppl 3(S111‐8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins, J, Hoffman, S, Dworkin, S.L. Rethinking gender, heterosexual men, and women's vulnerability to HIV. American Journal of Public Health. 2010;100:435‐435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remien RH, Chowdhury J, Mokhbat JE, Soliman C, Adawy ME, El‐Sadr W. Gender and care: access to HIV testing, care, and treatment. J Acquir Immune Defic Syndr. 2009 Jul 1;51 Suppl 3:S106‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]


