Abstract
Background
Oxytocin and prostaglandin are hormones responsible for uterine contraction during the third stage of labour. Receptors in the uterine muscles are stimulated by exogenous or endogenous oxytocin leading to uterine contractions. Nipple stimulation or breastfeeding are stimuli that can lead to the secretion of oxytocin and consequent uterine contractions. Consequently, uterine contractions can reduce bleeding during the third stage of labour.
Objectives
To investigate the effects of breastfeeding or nipple stimulation on postpartum haemorrhage (PPH) during the third stage of labour.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (15 July 2015) and reference lists of retrieved studies.
Selection criteria
Randomised and quasi‐randomised controlled trials comparing breast stimulation, breastfeeding or suckling for PPH in the third stage of labour were selected for this review.
Data collection and analysis
Two review authors independently assessed studies for inclusion in terms of risk of bias and independently extracted data. Disagreements were resolved by a third review author.
Main results
We included four trials (4608 women), but only two studies contributed data to the review's analyses (n = 4472). The studies contributing data were assessed as of high risk of bias overall. One of these studies was cluster‐randomised and conducted in a low‐income country and the other study was carried out in a high‐income country. All four included studies assessed blood loss in the third stage of labour. Birth attendants estimated blood loss in two trials. The third trial assessed the hematocrit level on the second day postpartum to determine the effect of the bleeding. The fourth study measured PPH ≥ 500 mL.
Nipple stimulation versus no treatment
One study (4385 women) compared the effect of suckling versus no treatment. Blood loss was not measured in 114 women (59 in control group and 55 in suckling group). After excluding twin pregnancies, stillbirths and neonatal deaths, the main analyses for this trial were performed on 4227 vaginal deliveries. In terms of maternal death or severe morbidity, one maternal death occurred in the suckling group due to retained placenta (risk ratio (RR) 3.03, 95% confidence interval (CI) 0.12 to 74.26; one study, participants = 4227; very low quality evidence); severe morbidity was not mentioned. Severe PPH (≥ 1000 mL) was not reported in this study.
The incidence of PPH (≥ 500 mL) was similar in the suckling and no treatment groups (RR 0.95, 95% CI 0.77 to 1.16; one study, participants = 4227; moderate quality). There were no group differences between nipple stimulation and no treatment regarding blood loss in the third stage of labour (mean difference (MD) 2.00, 95% CI ‐7.39 to 11.39; one study, participants = 4227; low quality). The rates of retained placenta were similar (RR 1.01, 95% CI 0.14 to 7.16; one study, participants = 4227; very low quality evidence), as were perinatal deaths (RR 1.06, 95% CI 0.57 to 1.98; one study, participants = 4271; low quality), and maternal readmission to hospital (RR 1.01, 95% CI 0.14 to 7.16; one study, participants = 4227; very low quality). We downgraded the evidence for this comparison for risk of bias concerns in the one included trial (inappropriate analyses for cluster design) and for imprecision (wide CIs crossing the line of no difference and, for some outcomes, few events).
Many maternal secondary outcomes (including side effects) were not reported. Similarly, most neonatal secondary outcomes were not reported.
Nipple stimulation versus oxytocin
Another study compared the effect of nipple stimulation (via a breast pump) with oxytocin. Eighty‐seven women were recruited but only 85 women were analysed. Severe PPH ≥ 1000 mL and maternal death or severe morbidity were not reported.
There was no clear effect of nipple stimulation on blood loss (MD 15.00, 95% CI ‐24.50 to 54.50; one study, participants = 85; low quality evidence), or on postnatal anaemia compared to the oxytocin group (MD ‐0.40, 95% CI ‐2.22 to 1.42; one study, participants = 85; low quality evidence). We downgraded evidence for this comparison due to risk of bias concerns in the one included trial (alternate allocation) and for imprecision (wide CIs crossing the line of no difference and small sample size).
Many maternal secondary outcomes (including side effects) were not reported, and none of this review's neonatal secondary outcomes were reported.
Authors' conclusions
None of the included studies reported one of this review's primary outcomes: severe PPH ≥ 1000 mL. Only one study reported on maternal death or severe morbidity. There were limited secondary outcome data for maternal outcomes and very few secondary outcome data for neonatal outcomes.
There was no clear differences between nipple stimulation (suckling) versus no treatment in relation to maternal death, the incidence of PPH (≥ 500 mL), blood loss in the third stage of labour, retained placenta, perinatal deaths or maternal readmission to hospital. Whilst these data are based on a single study with a reasonable sample size, the quality of these data are mostly low or very low.
There is insufficient evidence to evaluate the effect of nipple stimulation for reducing postpartum haemorrhage during the third stage of labour and more evidence from high‐quality studies is needed. Further high‐quality studies should recruit adequate sample sizes, assess the impact of nipple stimulation compared to uterotonic agents such as syntometrine and oxytocin, and report on important outcomes such as those listed in this review.
Plain language summary
Breastfeeding the newborn or nipple stimulation for reducing postpartum haemorrhage in the third stage of labour
What is the issue?
After a baby's birth, the placenta is delivered. The time between delivery of the baby and the placenta is called the third stage of labour. Excessive blood loss during this stage can endanger the mother's life. Several different methods are used to decrease the amount of bleeding in the third stage of labour. Nipple stimulation either manually, using a breast pump or by encouraging the baby to suckle is one method to reduce postpartum bleeding. It can be used immediately after childbirth to increase the secretion of the hormone called ‘oxytocin’. When oxytocin is released it causes uterine contractions, which in turn can lead to reduction of postpartum bleeding.
Why is this important?
Most maternal deaths in developing countries related to childbirth are due to postpartum bleeding. This is preventable. This review investigated the effects of breastfeeding and nipple stimulation on bleeding during the third stage of labour.
What evidence did we find?
We searched for evidence on 15 July 2015 and included four randomised controlled studies with 4608, women but only two of the studies had useable data. We assessed both studies contributing outcome data as being at a high risk of bias. One study compared the effect of the baby suckling immediately after birth with no intervention. Another study compared nipple stimulation (with a breast pump) versus oxytocin injection. Neither study reported postpartum haemorrhage. Side effects of the treatments were not reported. Similarly, there was limited information on other consequences for women and their babies.
When comparing nipple stimulation (suckling) with no breastfeeding, there were no clear differences in terms of the number of maternal deaths. The incidence of severe maternal illness was not reported. One woman in the suckling group died as a result of a retained placenta. Blood loss greater than or equal to 500 mL, retained placenta, perinatal deaths and maternal readmission to hospital were not clearly different between those who breast fed and those who did not. While these data were based on a single study with a reasonable sample size (4227 women), the results were mostly of low or very low quality due to concerns related to data analysis and study methodology.
Our comparison of nipple stimulation (using a breast pump) versus oxytocin included one small study involving 85 women only. There was no clear difference between the groups in relation to blood loss or postnatal anaemia. These results were of low quality due to our concerns about the way the trial was conducted and its small size.
What does this mean?
There is insufficient evidence to evaluate the effectiveness of nipple stimulation for reducing bleeding during the third stage of labour and more evidence from high‐quality studies is warranted. Future randomised clinical trials, with sufficient sample sizes should assess the impact of nipple stimulation in comparison to agents that stimulate the uterus such as syntometrine or oxytocin alone and report on important outcomes such as those listed in this review.
Summary of findings
Background
Description of the condition
Excessive blood loss during childbirth and the consequential morbidity and mortality is a serious problem. Postpartum haemorrhage (PPH) is the most common cause of maternal morbidity and mortality worldwide, especially in developing countries. It is estimated that nearly 800 women die every day, mostly from preventable causes related to pregnancy and childbirth, of which, 99% of these deaths occur in developing countries (WHO 2012). Although obstetric care has altered in an attempt to decrease blood loss, PPH is the leading cause of maternal mortality worldwide with a prevalence rate of approximately 6% (Carroli 2008). The high mortality rate is due to uncontrolled blood loss, and rapid onset of PPH. Mild to moderate PPH is defined as vaginal bleeding in excess of 500 mL (WHO 2007), while severe PPH is defined as vaginal bleeding more than 1000 mL during the first 24 hours after birth (WHO 2007). PPH is also associated with long‐term morbidity due to anaemia, blood transfusion, renal failure, coagulation deficiencies and hysterectomy. Other surgical procedures that reduce blood flow to the uterus and the subsequent consequence of infertility may be considered as late complications of PPH, although it is difficult to quantify the burden of these outcomes.
The common cause of PPH is uterine atony. Other causes of PPH that are less common include; damage to the cervix or vagina or perineum, retained fragments of placenta, and coagulopathy disorders. It has been suggested that uterine atony is the main cause (75% to 90%) of immediate PPH (Koh 2009). Compared to other complications, PPH can quickly become a critical condition and can be life‐threatening if a woman does not receive proper prevention and treatment (Khushk 2002).
Several factors can impact upon PPH rates including whether the bleeding rate is measured, how it is measured, and how the third stage of labour/separation of the placenta and membrane from the uterus is managed. The impact of blood loss at birth on woman's health is highly variable depending on the amount and rate of blood loss, the general health status of the woman, haemoglobin (Hb) level and coagulation system status (Razvi 2008). Blood type and cross‐match test should be done for women at high risk for PPH. According to the California Maternal Quality Care Collaboration (CMQCC 2010), women who have placenta praevia, placenta accreta, a haematocrit < 30%, active bleeding at time of admission to hospital and those with platelets < 100, 000/mL are at high risk for PPH. The following criteria have been considered for women at moderate risk for PPH: women who have a history of prior caesarean section, multiple gestation, > four previous vaginal births, women who have chorioamnionitis, a history of previous PPH, uterine fibroids, large baby and body mass index > 35 kg/m2. Women at low risk for PPH include: women with a singleton pregnancy, women with ≤ four previous vaginal births, no known bleeding disorder, no previous uterine incision, and no history of PPH (CMQCC 2010).
The third stage of labour is defined as the time between the birth of the baby and the expulsion of the placenta. After the baby is born, contraction of the uterus continues and the size of the uterus is markedly decreased. This reduction results in early separation of the placenta (Arenson 2007). According to Brandt 1933, the compressed placenta causes pressure on the deciduas sinuses. These sinuses are closed by powerful contractions of the myometrium. The sinuses are filled with blood and eventually burst. Blood from the ruptured sinuses causes the rupture of the thin layer of deciduas basalis, which then leads to the separation of the placenta (Brandt 1933).
Uterine contractions are stimulated by both electrical and hormonal mediators. The most important hormones in the third stage of labour are oxytocin and prostaglandin (Gimpl 2001). There are receptors for oxytocin in the myometrium. The secretion of oxytocin is pulsatile and the pulses of oxytocin secretion increase during labour (Fuchs 1991), leading to a surge of oxytocin at birth. Blood loss at postpartum predominantly originates from the placental bed; this source has been addressed in comparative trials of active versus expectant management. Uterine spiral arteries in the placenta bed are bared in their muscular layer and this is one of the physiological adaptations of pregnancy. Occlusion of these vessels, therefore, depends on uterine contraction to compress them as they run among the uterine smooth muscle fibres (Ridley 2002).
Management of the third stage of labour to reduce PPH
Maternal mortality has decreased by 45% based on the Millennium development goals, but still 800 women die every day because of preventable causes, mostly in the developing countries (WHO 2014). There are three approaches to the clinical management of the third stage including expectant, active and mixed management.
The expectant management of the third stage of labour is the observation of signs of placenta separation by the health provider and subsequently the delivery of the placenta spontaneously, or with the aid of gravity or nipple stimulation (Begley 2015). Expectant management is also known as conservative or physiological management and is commonly performed in some Northern European countries, America and Canada, with a higher usage in some developing countries (Festin 2003). A Cochrane systematic review including seven studies and 8247 women compared active versus expectant versus mixed management of labour. Results showed that for women who are at mixed levels of risk of bleeding, active management could reduce the risk of primary haemorrhage (risk ratio (RR) 0.34, 95% confidence interval (CI) 0.14 to 0.87), maternal haemoglobin (Hb) less than 9 g/dL after birth (RR 0.50, 95% CI 0.30 to 0.83). In the subgroup of women at low risk of postpartum bleeding, they found similar findings, however the number of adverse effects was increased in the active compared to the expectant management of third stage of labour (Begley 2015).
Active management of labour has three components: administration of uterotonic agents within one minute of birth, controlled cord traction, and uterine massage after expulsion of the placenta, as appropriate (ICM/FIGO 2006). More recently, the component of early cord clamping has been modified in some guidelines (Gülmezoglu 2012). Although active management of the third stage of labour has been widely recommended, a report from 10 countries showed that the rate of use varies from 0% to 98% (Festin 2003). Reduction of the rate of PPH is applicable using oxytocin injection or misoprostol as an important component of active management of the third stage of labour, even when there is no access to a skilled birth attendant (Aflaifel 2012).
Mixed management of the third stage of labour involves a combination of two active and expectant managements of labour, but not necessarily containing all the components of active and expectant management of the third stage of labour (Begley 2015). It is worth noting that extensive literature on the management of PPH has been published (Elbourne 1995; Gyte 1994; Maughan 2006; McDonald 2007; Prendiville 1989; Prendiville 1996; Soltani 2008).
Description of the intervention
Nipple stimulation in labour has been reported in the medical literature since the eighteenth century but in the last 20 years has fallen out of favour due to emerging synthetic medicines to control PPH. Both suckling and stimulation of the nipple is thought to cause the release of endogenous oxytocin from the posterior pituitary gland and has been used historically to augment and induce labour (Coad 2011). The oxytocin level increases during nipple stimulation, which can result in a short burst of oxytocin during contractions (Christensson 1989). The cholinergic and α‐adrenergic fibres act as stimuli inputs while the β‐adrenergic fibres act as an inhibitor for oxytocin release (Dewey 2001). Stimulation can be achieved in various ways including breast massage, rolling the nipples and utilising a breast pump. Although in the small body of literature there is no standard definition of terms or technique of nipple stimulation, all forms of stimulation have produced better results than the controls (Hatjis 1989). Nipple stimulation in the third trimester of pregnancy in women who are suitable candidates for contraction stress test could significantly increase the level of oxytocin in plasma (Finley 1986). Bilateral nipple stimulation commencing immediately after delivery and continued for 15 minutes, produced evidence of increased intrauterine pressure; however, this effect was less than the intrauterine pressure achieved with syntometrine injection (Irons 1994).
How the intervention might work
The role of suckling and nipple stimulation in the prevention of acute third stage haemorrhage has not been established. Any agent that can increase the release of oxytocin and/or the number of oxytocin receptors may cause uterine contraction and reduce blood loss (Gimpl 2001). Synthetic agents have been used in induction, augmentation of labour, and reduction of bleeding during the third stage of labour. However, synthetic oxytocin used for labour augmentation is sometimes associated with uterine atony, which can be due to the non‐pulsatile nature of synthetic agents (Phaneuf 1998). Oxytocin in moderate doses can cause slow, regular contractions of uterine muscles with relaxation in between, while high doses of oxytocin may cause sustained tonic contractions that can be dangerous (POPPHI 2008).
Studies have confirmed the effect of nipple stimulation on the oxytocin level of pregnant women, especially in term pregnancy (Christensson 1989). Nipple stimulation can excite the hypothalamic/pituitary axis. Nipple stimulation causes the release of the oxytocin, which stimulates the uterine smooth muscle, especially in late pregnancy, at the delivery time and immediately postpartum (Alexandrova 1980). In a randomised controlled trial to evaluate the effect of suckling on PPH, the results showed that the frequency of PPH and the mean blood loss were not significantly different between the suckling and the control group (Bullough 1989).
Why it is important to do this review
This review was undertaken because of the need to determine if nipple stimulation or breastfeeding is effective in the prevention of PPH compared with no intervention or active management/medication protocols. It is important to assess the impact of nipple stimulation or breastfeeding on both the mother and the baby. We believe that this review is highly relevant to families and clinicians. On the other hand, in many places, especially in low‐income countries, birth attendants use expectant management of third stage of labour due to lack of training and/or lack of injectable uterotonic agents. Two traditional methods of PPH prevention are nipple stimulation and breastfeeding immediately after the birth of the baby and before removal of the placenta. Moreover, self‐administered therapies or prevention techniques (such as, nipple stimulation) are important; both in terms of finding a cost‐effective intervention and promoting an intervention that can be conducted at home where there is no birth attendant present.
Objectives
The aim of this systematic review is to examine the impact of breastfeeding or nipple stimulation on reducing postpartum haemorrhage during the third stage of labour.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised and quasi‐randomised controlled trials of breastfeeding/nipple stimulation for preventing postpartum haemorrhage (PPH) in the third stage of labour. Cluster‐randomised controlled trials were eligible for inclusion, but trials using a cross‐over design were not eligible for inclusion. All studies presented only as abstracts were also considered.
Types of participants
All women who had normal vaginal delivery during third stage of labour.
Types of interventions
Nipple stimulation was defined as nipple stimulation or suckling by the baby immediately after the birth and before the delivery of the placenta. The primary comparisons were as follows.
Nipple stimulation versus no treatment
Nipple stimulation versus oxytocin
Nipple stimulation versus ergometrine
Nipple stimulation versus ergometrine + oxytocin
Nipple stimulation versus prostaglandins
Nipple stimulation versus oxytocin agonists
Nipple stimulation versus uterine massage (before or after placental delivery)
Nipple stimulation + uterotonic versus nothing
Nipple stimulation + uterotonic versus uterotonic alone
Types of outcome measures
Primary outcomes
Severe PPH (measured or estimated blood loss of 1000 mL or more, or as defined by the trial authors)
Maternal death or severe morbidity (including major surgery, organ failure, hyperpyrexia, intensive care unit admission, hysterectomy, compression sutures, artery ligations, or as defined by trial authors), reported together or separately
Secondary outcomes
Maternal
Manual removal of the placenta
Blood transfusion
PPH (measured or estimated blood loss of ≥ 500 mL, or as defined by the trial authors) (not pre‐specified)
Use of therapeutic uterotonic
Additional treatment for PPH (uterine tamponade, X‐Ray, embolisation)
Amount of blood loss (measured or estimated blood loss) (not pre‐specified)
Incidence of retained placenta (not pre‐specified)
Side effects reported either individually or as a composite where appropriate; e.g. vomiting, nausea, elevation of diastolic blood pressure, shivering, headache, chest pain, shortness of breath, pyrexia, diarrhoea
Postnatal anaemia (defined by trial authors, as a reduction of haemoglobin (Hb))
Thromboembolic events
Cost
Maternal satisfaction
Maternal readmission to hospital (or transfer to hospital for home births)
Neonatal
Admission to neonatal intensive care unit
Frequency of infants with jaundice requiring phototherapy
Frequency of infants not breast fed at discharge
Neonatal/infant anaemia up to four to six months post birth (outcomes reported separately, Hb, haematocrit)
Infant re‐admission to hospital
Perinatal morbidity (including respiratory distress, hypoxia, and as defined by the trial authors)
Perinatal mortality
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (15 July 2015).
For full search methods used to populate the Pregnancy and Childbirth Group’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth Group in The Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly, the Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth Group review topic (or topics), and is then added to the Register. The Trials Search Co‐ordinator searches the Register for each review using this topic number rather than keywords. This results in a more specific search set which has been fully accounted for in the relevant review sections (Included, Excluded, Awaiting Classification or Ongoing).
Searching other resources
We searched the reference lists of retrieved studies for additional relevant references.
We did not apply any language or date restrictions.
Data collection and analysis
We used the method for data collection and analysis on the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
Two review authors independently assessed all eligible trials for potential inclusion. Any disagreement through this process was resolved by a third review author.
Data extraction and management
We designed a form to extract data. For eligible studies, at least two review authors Parvin Abedi (PA) and Jasmine Lee (JL) extracted the data independently using a standard form. Any discrepancies were resolved by discussion with the third review author, Shayesteh Jahanfar (SJ). We entered data into the Review Manager software (RevMan 2014) and checked for accuracy.
Assessment of risk of bias in included studies
Two review authors (PA and JL) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor (SJ). In cases where that judgment was confirmed as a high risk of bias, we attempted to contact the authors to find out if they blinded the personnel and outcomes assessors. If data were unobtainable, and there was a serious bias from the missing and uncompleted data, we planned to assess the effect of such studies in the overall assessment of results by sensitivity analysis. We used the following criteria in the assessment of bias.
(1) Random sequence generation (checking for possible selection bias)
We assessed the quality of the randomisation method in each included trial that they utilized to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as follows:
low risk of bias (any truly random process, random number table; computer random number generator);
high risk of bias (any non‐random process, odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We assessed for each included study the method used to conceal allocation to interventions prior to assignment and whether intervention allocation could have been foreseen in advance of, during recruitment or changed after assignment.
We assessed the methods as follows:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described the methods used for each included study, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding were unlikely to affect the results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as follows:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described the methods used for each included study, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we re‐included missing data in the analyses that we undertook . No studies required re‐analysis with the original allocated treatment group being restored to their correct groups.
We assessed methods of studies as follows:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups), or less than 10% attrition at any stage;
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis performed with substantial departure of intervention received from that assigned at randomisation) or more than 20% attrition;
unclear risk of bias.
We planned that for studies with an unclear attrition level, or more than 20% missing data, we would undertake sensitivity analysis.
(5) Selective reporting (checking for reporting bias)
We described for each included study the methods by which we investigated the possibility of selective outcome reporting bias and we assessed reporting method as follows:
low risk of bias (where it was clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review had been reported);
high risk of bias (where not all the study’s pre‐specified outcomes had reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We assessed and described for each included study any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there was risk of other bias.
(7) Overall risk of bias
We made explicit judgments about whether studies are at high risk of bias, according to the criteria given in the Cochrane Handbook(Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we consider it was likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses.
Assessment of the quality of the evidence using the GRADE approach
We assessed the quality of the evidence using the GRADE approach as outlined in the GRADE handbook relating to the following outcomes for the main comparisons nipple stimulation (suckling) versus no treatment and nipple stimulation (breast pump) versus oxytocin.
Severe PPH (> 1000 mL or as defined by authors) or PPH (> 500 mL)
Maternal death or severe morbidity
Amount of blood loss
Retained placenta
Perinatal deaths
Maternal readmission to hospital (or admissions for home births)
Postnatal anaemia
We used the GRADEpro Guideline Development Tool to import data from Review Manager 5.3 (RevMan 2014) in order to create ’Summary of findings’ tables. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
The statistical analysis was performed using the Review Manager Software (RevMan 2014).
Dichotomous data
For dichotomous data, we presented results as a summary risk ratio with 95% confidence intervals.
Continuous data
We planned to use the mean difference for continuous data if outcomes were measured in the same way between trials and the standardised mean difference to combine trials that measured the same outcome but used different methods. As we did not combine trials in the meta‐analysis, we used the mean difference. In one study (Irons 1994), only the mean of blood loss was reported without standard deviation or error. We could not find any contact information from the author to get the correct information. Therefore, the estimation of effect was not possible.
Ordinal data
We did not include ordinal data in the review. If in future updates we find relevant ordinal data, we will incorporate these as follows: longer ordinal scales will be analysed in meta‐analyses as continuous data, whilst shorter ordinal scales will be changed into dichotomous data by combining adjacent categories together. The latter will be considered appropriate if an established, defensible cut‐point is available.
Unit of analysis issues
Cluster‐randomised trials
We included one cluster‐randomised trial in this review (Bullough 1989). Trial authors analysed the outcome data as if the trial were individually randomised. The report provides no details of adjustments made for clustering, and the study is not identified as a cluster trial. However, by randomising birth attendants instead of pregnant women, the trial is clearly of cluster‐design. The birth attendant creates a cluster with the women under her care. We have included data from Bullough 1989 in our meta‐analyses, without performing adjustments with an intracluster correlation co‐efficient (ICC). It is therefore reasonable to assume that the confidence intervals associated with treatment effects will be underestimated. For Bullough 1989, there are no statistically significant effects reported for any outcome included in our review. We have noted that all results from the Bullough 1989 trial should be considered at high risk of bias.
If in future updates we have additional cluster‐randomised trials, we will include these trials in the analyses along with individually‐randomised trials. We will adjust their sample sizes or standard errors using the methods described in the Handbook [Section 16.3.4 or 16.3.6] using an estimate of the ICC derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity or subgroup analysis to investigate the effects of the randomisation unit.
Cross‐over trials
Cross‐over trials were not eligible for inclusion in this review.
Multi‐armed studies
We included two multiple‐armed studies in this review (Badhwar 1991; Irons 1994). All meta‐analyses address pair‐wise comparisons, so there are three separate issues to consider when faced with a study with more than two intervention groups. Authors must:
determine which intervention groups are relevant to the systematic review;
determine which intervention groups are relevant to a particular meta‐analysis;
determine how the study is to be included in the meta‐analysis if more than two groups are relevant.
When including trials with more than two arms, we planned to split trials or combine trial arms in order to make pair‐wise comparisons that are relevant to our review. For example, if Irons 1994 or Badhwar 1991 had reported usable data, we would have created pair‐wise comparisons of nipple stimulation versus no treatment or nipple stimulation versus oxytocin.
Dealing with missing data
For included studies, we noticed levels of attrition. We planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis. However, because of the small number of studies, this task was not possible.
For all outcomes, we planned to carry out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing. If losses to follow‐up were greater than 20%, or where trial authors excluded participants at a level greater than 15% and for reasons that affected the outcomes, we marked the trial as of high risk of bias for missing data.
Incomplete data were defined as the proportion of participants for whom no outcome data were obtained and reported in a 'Risk of bias' table. We used available case analysis to include data on only those participants whose results were known, using as a denominator the total number of women who had data recorded for the specific outcome under study. Variation in the degree of missing data across studies was considered as a potential source of heterogeneity.
In future updates, we may also perform imputation to deal with incomplete data. For imputing dichotomous outcome data, we will assume that all missing participants experienced the event (e.g. perinatal mortality).
Assessment of heterogeneity
No data are pooled in meta‐analysis in this review.
We planned to assess statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics. We regard heterogeneity as substantial if an I² is greater than 30% and either the T² is greater than zero, or there is a low P value (less than 0.10) in the Chi² test for heterogeneity. If in future updates we find substantial heterogeneity, we will attempt to identify its source. Clinical heterogeneity could be investigated in terms of a specific intervention or population and only pooling the studies judged sufficiently clinically similar.
To investigate statistical heterogeneity, we will visually inspect the forest plot and exclude the most extreme effect estimate. Studies with an extreme effect estimate or the greatest weight will be removed individually and in a sequential manner to investigate their impact on the overall effect estimate. We will then conduct a subgroup analysis considering "year of publication", "sample size", and "type of intervention". Finally, we will use a random‐effects model to obtain the overall effect estimate.
Assessment of reporting biases
There were too few studies included in the review to investigate reporting bias. In future updates of this review, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We performed statistical analysis using the Review Manager software (RevMan 2014) but data were not pooled in meta‐analysis.
In future updates we will use fixed‐effect meta‐analysis for combining data where it is reasonable to assume that studies are estimating the same underlying treatment effect; that is, where trials are examining the same intervention, and the trials’ populations and methods are judged sufficiently similar. If there is clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if substantial statistical heterogeneity is detected, we will use random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials would consider clinically meaningful. The random‐effects summary will be treated as the average range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we will not combine trials.
If we use random‐effects analyses, the results will be presented as the average treatment effect with 95% confidence intervals, and the estimates of T² and I².
Subgroup analysis and investigation of heterogeneity
Due to the small number of studies included in the review, we did not conduct planned subgroup analysis. In future updates, if the inclusion of more studies creates substantial heterogeneity for the review's primary outcomes, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful, and if it is, we will use random‐effects analysis to produce it.
We plan to carry out the following subgroup analyses.
Nulliparous versus multiparous women
Manual nipple stimulation versus infant breastfeeding/suckling
Birth attended by skilled birth attendants versus those not attended by skilled birth attendants
Low‐income versus high‐income setting
Full‐term versus preterm birth
We will assess subgroup differences by interaction tests available within RevMan (RevMan 2014). We will report the results of subgroup analyses quoting the χ2 statistic and P value, and the interaction test I² value. Subgroup analysis based on quality of studies (low versus high quality) and study design (randomised versus quasi‐randomised) will also be conducted.
Sensitivity analysis
In future updates, if we have sufficient studies to investigate aspects of trial quality and risks of bias, we will perform the following sensitivity analyses. If we question the eligibility of some studies in the meta‐analysis because they are not fully reported and of high risk of bias, we will perform a sensitivity analysis that would involve two steps. First, we will include all studies in the meta‐analysis. Second, we will run analyses with studies of low risk of selection bias only, to see if this restriction alters the overall effect. Sensitivity analysis will be restricted to the review's primary outcomes.
Results
Description of studies
Results of the search
See: Figure 1
1.
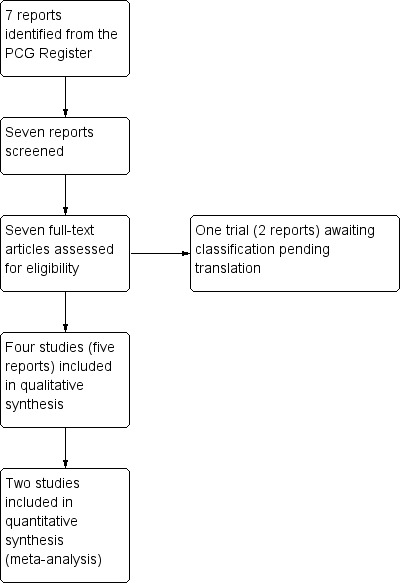
Study flow diagram.
The search of the Cochrane Pregnancy and Childbirth Group's Trials Register retrieved seven reports relating to five trials (See: Figure 1). Four trials (five reports) were included, though only two contributed data to the review's meta‐analyses. One trial (two reports) (Narenji 2012, is published in Persian and is listed within Studies awaiting classification pending translation.
Included studies
Four studies were included for this review involving 4608 women (Badhwar 1991; Bullough 1989; Irons 1994; Kim 1986). Only two trials contributed data to the review (Bullough 1989; Kim 1986). Eligible studies were conducted in India (Badhwar 1991), South Tyneside in the UK (Irons 1994), Malawi (Bullough 1989), and in New York in the USA (Kim 1986). All studies, except for that of Bullough 1989 took place in a hospital setting. All studies were conducted on healthy pregnant women who expected a normal vaginal delivery, apart from approximately 24% of the women in Kim 1986. Most women in three trials were at low risk of bleeding in the third stage of labour. The protocols in the four studies were different. Bullough 1989 compared the effect of suckling on postpartum bleeding with a no intervention control group. Irons 1994 enrolled four groups, comparing nipple stimulation, routine syntometrine injections, ergometrine maleate injections, and a no intervention control group. Kim 1986, recruited women in two groups, nipple stimulation and intravenous infusion of oxytocin after delivery. Badhwar 1991 compared three groups: nipple stimulation, methyl ergometrine, and no treatment.
The study by Bullough 1989 (n=4385 randomised) after exclusions encouraged early suckling as soon as the cord was cut on 4227 women who gave birth by traditional birth attendants (TBAs). They compared early suckling after birth in the intervention group (n = 2104) with no intervention (standard care) in the control group (n = 2123) and assessed the amount of blood loss and frequency of postpartum haemorrhage (PPH). If the baby did not suckle immediately, repeated attempts were made. In Malawi culture, standard care involves relatives looking after the newborn immediately, allowing the mother to rest. Randomisation was performed by the TBAs rather than women to reduce the rate of contamination. In the suckling group, TBAs held the baby under the mother's breast immediately after birth and the baby was dried and wrapped. The mean time to first suckling was 7.25 minutes (range 3.5 to 15 minutes). In this study, PPH was defined as loss of blood ≥ 500 mL during the third stage of labour or within the first 24 hours after delivery. Blood was collected in transparent plastic jugs in units of 100 mL. If the TBAs observed any PPH or a retained placenta, they referred these patients to the selected hospital. In these hospitals, staff co‐operated by maintaining records of any information regarding blood loss and a retained placenta.
In Irons 1994 (n=16 randomised), after exclusions 14 women were allocated to one of the following groups for reduction of bleeding in the third stage of labour; (i) routine intramuscular ergometrine maleate 500 µg, (ii) oxytocin 5 IU (international units) (syntometrine) injection with delivery of the anterior shoulder (n = 3), or (iii) bilateral nipple stimulation (n = 6), or no treatment (n = 5). Nipple stimulation was conducted by compressing the nipple between two fingers intermittently, mimicking the action and frequency of suckling for 15 minutes. All deliveries were conducted by midwives in the hospital. The blood loss in the third stage of labour was reported in mL.
Kim 1986 (n = 87 randomised) after exclusions compared the duration of the third stage of labour, physician estimation of blood loss and reduction of haematocrit level on the second day postpartum between two groups including nipple stimulation (n = 32) and a group who received 20 IU intravenous oxytocin after delivery of the placenta (n = 53). Nipple stimulation was performed using a breast shield that was placed on one of the nipples and a pump with a negative pressure of 250 mmHg was run for five minutes immediately after delivery. Overall, two nipples were stimulated for 20 minutes. The control group received 20 IU of an oxytocin infusion after delivery of the placenta. Vaginal deliveries were conducted by midwives. The amount of blood loss in the third stage of labour was estimated by physicians and the reduction in the haematocrit on the second day of postpartum was used as an objective scale for blood loss.
Badhwar 1991 was reported in abstract form only, so there is very little detail about the study, apart from the sample size (n = 120 randomised) and the three arms: bilateral manual nipple stimulation, methyl ergometrine and no treatment.
Excluded studies
There are no excluded studies.
Risk of bias in included studies
We considered the overall risk of bias to be high in two studies (Bullough 1989; Kim 1986) and unclear in two studies (Badhwar 1991; Irons 1994). Please see Figure 2 for specific concerns.
2.

Methodological quality summary: review authors' judgements about each risk of bias item for each included study.
Allocation
No included trial described the generation of a random sequence; all trials were assessed as unclear for sequence generation.
We identified one trial as at high risk of bias due to inadequate allocation concealment (Kim 1986); trialists assigned even digits (hospital registration number) to the study intervention arm (nipple stimulation) and odd digits to the control group. Participants and personnel could have known the allocation simply by looking at the last digit of the registration number. Irons 1994 used sealed envelopes, and Bullough 1989 used central randomisation; we assessed both methods as of low risk of bias. Badhwar 1991 did not describe allocation concealment and was assessed as of unclear risk.
Blinding
Blinding of participants by personnel is not usually possible for the trials relevant to our review. We identified two studies with high risk of bias for blinding of staff and participants (Irons 1994; Kim 1986); both of these same trials had separate outcomes assessors, but it was unclear how much these researchers knew about the trials or patients. Blinding of outcomes assessors was then unclear. One study, Badhwar 1991 was of unclear risk due to lack of information about blinding of staff, participants and outcomes assessors. We assessed Bullough 1989 as of low risk of bias because TBAs were specifically not told of their participation in the trial. Presumably the women under their care would not have been aware of trial participation, either.
Incomplete outcome data
We considered the study by Bullough 1989 to be at high risk of attrition bias because 10 out of 33 TBAs (30.3%) in the suckling group and nine out of 35 TBAs (25.7%) in the control group were excluded from the analysis because of concerns about birth attendant training and data quality. Each individual attendant cared for up to 100 women, and it is likely that the outcomes for these women differed from those women whose attendants remained in the study.
Two studies (Irons 1994; Kim 1986) were classified as of low risk bias for attrition. From the participants in the Irons 1994 study, only two were dropped of the 16 initially recruited mothers and 14 successfully completed the study. In the study by Kim 1986, a total of 87 patients were recruited, from which 32 were study cases and 53 were controls. Badhwar 1991 was assessed as of unclear risk due to lack of information about attrition.
Selective reporting
We identified that the study by Bullough 1989, Irons 1994 and Kim 1986 had a low risk of bias for selective reporting. Badhwar 1991 was assessed as of unclear risk due to insufficient information.
Other potential sources of bias
Bullough 1989 was assessed to be at high risk of bias due to several factors: we were unsure of the effects of the problems with losing large numbers of clusters due to inadequate training of birth attendants and known problems with the quality of data collected. Further, we considered this trial to be of high risk of bias due to incorrect analysis methods, because no adjustments were made for cluster design. We found no additional sources of bias beyond those stated above in Irons 1994 or Kim 1986 (low risk of bias), and Badhwar 1991 was of unclear risk due to lack of information.
Effects of interventions
Summary of findings for the main comparison. Summary of findings ‐ Nipple stimulation (suckling) versus no treatment (comparison 1).
| Nipple Stimulation (suckling) versus no treatment | ||||||
| Patient or population: nipple stimulation (suckling) for preventing postpartum haemorrhage in the third stage of labour. Setting: Malawi. Intervention: nipple stimulation (suckling). Early suckling encouraged as soon as the cord was cut on 4227 women who gave birth by traditional birth attendants. Comparison: no treatment. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect RR (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no treatment | Risk with Nipple stimulation | |||||
| Maternal death | Anticipated effects not possible to calculate with 0 events in control arm. | RR 3.03 (0.12 to 74.26) | 4227 (1 RCT) | ⊕ VERY LOW 1, 2 | The review primary outcomes of severe PPH (> 1000 mL) or maternal serious morbidity were not reported in any trial in this comparison. | |
| Frequency of PPH ≥ 500 mL | Study population | RR 0.95 (0.77 to 1.16) | 4227 (1 RCT) | ⊕⊕⊕ MODERATE 1 | ||
| 84 per 1000 | 80 per 1000 (65 to 97) | |||||
| Amount of blood loss | Anticipated effects not calculated for continuous variables. | The mean amount of blood loss in the intervention group was 2.00 mL more (7.39 fewer to 11.39 more) |
4227 (1 RCT) | ⊕⊕ LOW 1 3 | ||
| Retained placenta | Study population | RR 1.01 (0.14 to 7.16) | 4227 (1 RCT) | ⊕ VERY LOW 1, 2 | ||
| 1 per 1000 | 1 per 1000 (0 to 7) | |||||
| Perinatal death | Study population | RR 1.06 (0.57 to 1.98) | 4271 (1 RCT) | ⊕⊕ LOW 1, 3 | ||
| 9 per 1000 | 9 per 1000 (5 to 18) | |||||
| Maternal admission to hospital | Study population | RR 1.01 (0.14 to 7.16) | 4227 (1 RCT) | ⊕ VERY LOW 1 2 | ||
| 1 per 1000 | 1 per 1000 (0 to 7) | |||||
| Moderate | ||||||
| 1 per 1000 | 1 per 1000 (0 to 6) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 One study with risk of bias concerns, including analysis inappropriate for cluster‐study design.
2 Wide confidence interval crossing the line of no effect and few events.
3 Wide confidence interval crossing the line of no difference.
Summary of findings 2. Summary of findings ‐ Nipple stimulation (breast pump) versus oxytocin.
| Nipple stimulation (breast pump) versus Oxytocin | ||||
| Patient or population: a total of 87 primigravida women were enrolled, 85 completed the study: 32 were study cases and 53 were controls.28% (study group) and 22% (controls) of pregnant women were considered to be of high risk. Setting: hospital setting in New York, USA. Intervention: nipple stimulation in this study was performed using a breast shield that was placed on one of the nipples and a pump with a negative pressure of 250 mmHg was run for 5 minutes immediately after delivery. Overall, 2 nipples were stimulated for 20 minutes. Comparison: women received an infusion of 20 IU of oxytocin after delivery of the placenta, with an average of 1.2 to 3 units given in the 20 minutes following delivery. | ||||
| Outcomes | Relative effect MD (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments |
| Amount blood loss | The mean amount blood loss in the intervention group was 15.00 mL more (24.5 fewer to 54.5 more) | 85 (1 RCT) | ⊕⊕ LOW 1, 2 | The review primary outcomes of severe PPH (>1000 mL) or maternal death or serious morbidity were not reported in any trial in this comparison. |
| Postnatal anaemia | The mean postnatal anaemia in the intervention group was 0.40 mL fewer (2.22 fewer to 1.42 more) | 85 (1 RCT) | ⊕⊕ LOW 1, 2 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: mean difference | ||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||
1 One study with risk of bias concerns, including alternate allocation by hospital number.
2 Wide confidence interval crossing the line of no difference and small sample size.
This review included four studies involving 4606 women but two studies (Badhwar 1991; Irons 1994, involving a total of 134 women) did not contribute any data towards our analyses.
None of the studies measured the review's primary outcome of severe PPH (measured or estimated blood loss of 1000 mL or more, or, as defined by the trial authors).
Nipple stimulation versus no treatment ‐ comparison 1
One study (Bullough 1989), contributed data towards this comparison and in that study, early suckling was used as a form of nipple stimulation. Another study (Irons 1994) (which was a three‐arm trial) reported on one of this review's secondary outcomes (amount of blood loss) but data were not presented in a usable form for inclusion in our meta‐analysis. In the Irons 1994 study, nipple stimulation was manual, bilateral nipple stimulation, starting immediately after delivery.
Primary outcomes
Severe PPH (> 1000 mL)
This outcome was not reported in the included studies.
Maternal death or severe maternal morbidity
In the Bullough 1989 study, one maternal death occurred ‐ this was in the suckling group with a risk ratio (RR) 3.03, 95% confidence interval (CI) 0.12 to 74.26; one study, participants = 4227 (Analysis 1.1).
1.1. Analysis.
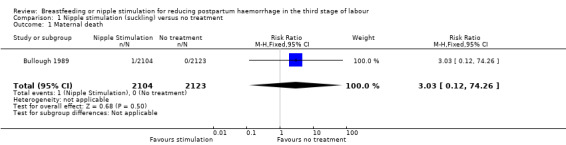
Comparison 1 Nipple stimulation (suckling) versus no treatment, Outcome 1 Maternal death.
Severe maternal morbidity was not reported in this trial.
Secondary outcomes
PPH of ≥ 500 mL
The frequency of PPH was not reduced in the nipple stimulation group compared to the control group (RR 0.95, 95% CI 0.77 to 1.16; one study, participants = 4227) (Analysis 1.2).
1.2. Analysis.
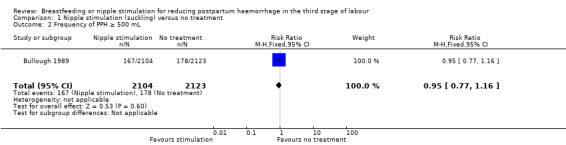
Comparison 1 Nipple stimulation (suckling) versus no treatment, Outcome 2 Frequency of PPH ≥ 500 mL.
Amount of blood loss
The mean postpartum blood loss in Bullough 1989 was 258 mL and 256 mL in the nipple stimulation (suckling) and control groups, respectively and did not show any clear difference between groups (mean difference (MD) 2.00 mL, 95% CI ‐7.39 to 11.39; one study, participants = 4227) (Analysis 1.3).
1.3. Analysis.

Comparison 1 Nipple stimulation (suckling) versus no treatment, Outcome 3 Amount of blood loss.
Data from Irons 1994 were not included in the data and analysis due to lack of standard deviation; the mean blood loss was 166 mL in the nipple stimulation group (n = 6) and 257 mL in the control group (n = 5).
Retained placenta
There was no difference in the incidence of retained placenta in the Bullough 1989 study. Four cases of retained placenta occurred, two in the suckling group and two in the control group (RR 1.01, 95% CI 0.14 to 7.16; one study, participants = 4227) (Analysis 1.4).
1.4. Analysis.
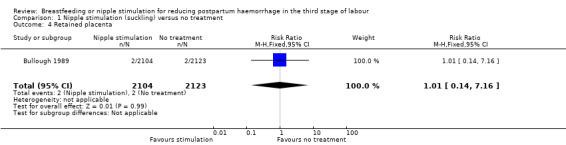
Comparison 1 Nipple stimulation (suckling) versus no treatment, Outcome 4 Retained placenta.
Perinatal death
There were no clear differences in rate of perinatal death between the suckling and control group (RR 1.06, 95% CI 0.57 to 1.98; one study, participants = 4271) (Analysis 1.5).
1.5. Analysis.

Comparison 1 Nipple stimulation (suckling) versus no treatment, Outcome 5 Perinatal death.
Maternal admission to hospital
Equal numbers of women were admitted to hospital in the suckling and control groups (RR 1.01, 95% CI 0.14 to 7.16; one study, participants = 4227) (Analysis 1.6).
1.6. Analysis.
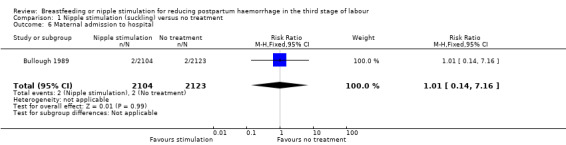
Comparison 1 Nipple stimulation (suckling) versus no treatment, Outcome 6 Maternal admission to hospital.
Secondary outcomes not reported
A number of maternal secondary outcomes of this review were not reported: blood transfusion; use of therapeutic uterotonic; additional treatment for PPH; side effects, postnatal anaemia; thromboembolic events; cost; maternal satisfaction. Similarly, the majority of neonatal outcomes were not reported: admission to neonatal intensive care unit; jaundice requiring phototherapy; not breast fed at discharge; neonatal/infant anaemia up to four to six months of age; infant readmission to hospital; perinatal morbidity.
Nipple stimulation (breast pump) versus oxytocin ‐ comparison 2
There was only one study under this comparison, Kim 1986, which enrolled 32 women in the nipple stimulation group and 53 women in the oxytocin group. Nipple stimulation in this study was performed using a breast shield that was placed on one of the nipples and a pump with a negative pressure of 250 mmHg was run for five minutes immediately after delivery. Overall, two nipples were stimulated for 20 minutes.
Primary outcomes
Severe PPH (> 1000 mL)
This outcome was not reported.
Maternal death or severe maternal morbidity
These outcomes were not reported.
Secondary outcomes
Amount of blood loss
The results showed that there was no clear difference between nipple stimulation and oxytocin in mean measured blood loss (MD 15.00 mL, 95% CI ‐24.50 to 54.50; one study, participants = 85)) (Analysis 2.1).
2.1. Analysis.
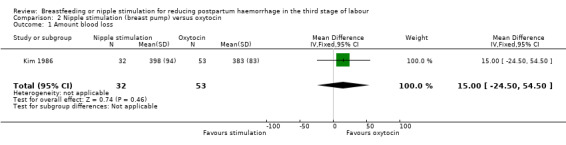
Comparison 2 Nipple stimulation (breast pump) versus oxytocin, Outcome 1 Amount blood loss.
Postnatal anaemia
There was no clear difference in postnatal haematocrit between the two groups (MD ‐0.40, 95% CI ‐2.22 to 1.42; one study, participants = 85) (Analysis 2.2).
2.2. Analysis.
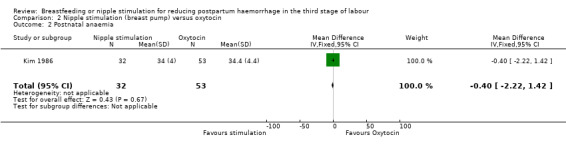
Comparison 2 Nipple stimulation (breast pump) versus oxytocin, Outcome 2 Postnatal anaemia.
Secondary outcomes not reported
A number of this review's maternal and neonatal outcomes were not reported under this comparison. For the mother: manual removal of the placenta; blood transfusion; PPH (≥ 500 mL); use of therapeutic uterotonic; additional treatment for PPH; incidence of retained placenta; side effects; thromboembolic events; cost; maternal satisfaction; maternal readmission to hospital. For the baby: admission to neonatal intensive care; jaundice requiring phototherapy; not breast fed at discharge; neonatal/infant anaemia up to four to six months of age; infant readmission to hospital; perinatal morbidity; perinatal mortality.
Nipple stimulation versus ergometrine and oxytocin (syntometrine) ‐ no analysis possible
One included study is relevant to this comparison, but the trial did not contribute any usable data to the review. Therefore these results for this trial are presented below in narrative form only, taken directly from the published report.
Irons 1994 recruited participants in three groups; nipple stimulation versus syntometrine and no treatment (n = 6; n = 3 and n = 5, respectively). Nipple stimulation was manual, bilateral nipple stimulation, starting immediately after delivery. Blood loss was reported in mL, but there were no standard deviations associated with the means and the sample sizes were very small, so we did not use the data (166 mL nipple stimulation; 83 mL syntometrine and 257 mL no treatment).
Primary outcomes
Severe PPH (> 1000 mL)
This outcome was not reported.
Maternal death or severe maternal morbidity
This outcome was not reported.
Secondary outcomes
Mean blood loss
The mean blood loss in the study was 166 mL in the nipple stimulation group (n = 6) and 83 mL in the syntometrine group (n = 3). Due to the low number of participants (n = 9) and no reported standard deviation or standard error, these data were not included in the data and analysis.
Secondary outcomes not reported
A number this review's maternal and neonatal outcomes were not reported. For the mother: manual removal of the placenta; blood transfusion; PPH (≥ 500 mL); use of therapeutic uterotonic; additional treatment for PPH; incidence of retained placenta; side effects; thromboembolic events; cost; maternal satisfaction; postnatal anaemia; maternal readmission to hospital. For the baby: admission to neonatal intensive care; jaundice requiring phototherapy; not breast fed at discharge; neonatal/infant anaemia up to four to six months of age; infant readmission to hospital; perinatal morbidity; perinatal mortality.
Discussion
The aim of this review was to investigate the effect of breastfeeding or nipple stimulation for reducing postpartum haemorrhage (PPH).
We found no trials that compared other types of interventions that could potentially be considered in this review, such as nipple stimulation versus prostaglandins, nipple stimulation versus oxytocin agonists, nipple stimulation versus uterine massage (before or after placental delivery), nipple stimulation and uterotonic versus nothing, or nipple stimulation and uterotonic versus uterotonic alone.
This review consists of four trials comprising of 4608 women. All trials had high or unclear risks of bias.Two of the trials were conducted in a hospital setting and two at home. Severe PPH (> 1000 mL) was not reported in any of the included studies. Just one trial reported perinatal death (Bullough 1989). All women enrolled in the included trials were considered at low risk for postpartum bleeding, apart from approximately 26% of women recruited to Kim 1986. There was only one maternal death that occurred in the suckling group of Bullough 1989. The fixed‐effect analyses revealed no clear effects of nipple stimulation on bleeding in the third stage of labour, PPH, retained placenta, perinatal deaths, maternal admission to hospital or postpartum anaemia.
Summary of main results
1. Nipple stimulation (suckling) versus no treatment
One trial contributed to this comparison (Bullough 1989). In this review we did not find any clear difference between nipple stimulation and the control groups regarding blood loss in the third stage of labour and PPH. The frequency of PPH (≥ 500 mL) did not show any clear reduction in the nipple stimulation group compared to the control group. One maternal death in the suckling group occurred following placenta retention. The wide confidence intervals (CI) (95% CI 0.12 to 74.38) indicate that the number of participants was not sufficient to reach a definitive conclusion regarding maternal deaths. The incidence of retained placenta was not different between the nipple stimulation and control groups (two in nipple stimulation and two in the control groups). Groups were also very similar for perinatal deaths.
2 Nipple stimulation (breast pump) versus oxytocin
In this review, one trial (Kim 1986) studied the effect of nipple stimulation (with a breast pump) versus oxytocin. Results showed that there was no clear difference in mean blood loss in the third stage of labour between nipple stimulation and oxytocin. In this study, nipple stimulation was performed using a pump with negative pressure. The findings suggest that in low‐income countries with insufficient facilities, nipple stimulation may be a good alternative to oxytocin. The clinical and laboratory evidence also show that the haematocrit in the second day postpartum was not different between nipple stimulation and oxytocin groups, confirming that nipple stimulation may induce uterine contraction and early retraction of the uterus after birth.
3 Nipple stimulation versus syntometrine
Blood loss during the third stage of labour, for nipple stimulation and ergometrine groups, was studied by Irons 1994. The peak placental venous pressure was measured after delivery of the baby. The blood loss in the nipple stimulation group was reported as being reduced compared to the control group, but the blood loss was greater than in the syntometrine group. The authors concluded that nipple stimulation is a good alternative for women in low‐income countries, when parenteral oxytocin is not available. However, because the standard deviations of the data were not reported, and they used a very low sample size, drawing a definitive conclusion from this study was not possible.
Overall completeness and applicability of evidence
One trial compared the effect of suckling on blood loss in the third stage of labour with a control group. This study was conducted at home and in a low‐income country using traditional birth attendants (TBAs) for the delivery (n = 4227) (Bullough 1989). Women who were recruited to this study were at low risk for bleeding and if complications arose they were transferred to a hospital. Two other studies were conducted in hospital settings in high‐income countries (USA ‐ Kim 1986; and UK ‐ Irons 1994); the meta‐analysis was only applicable for one of these studies (Kim 1986). A fourth trial was reported in abstract form only with few details and no usable data (Badhwar 1991). As only two trials (one in low‐income and one in high‐income countries) compared nipple stimulation with a control or oxytocin group, definite conclusions cannot be drawn on the effect of nipple stimulation on bleeding in the third stage of labour, or PPH.
Quality of the evidence
The methodological quality was assessed as mixed among the included studies, with all studies contributing data at high risk of bias due to at least one domain. Overall, the risk of bias was high in two trials (Bullough 1989; Kim 1986) and unclear in two trials (Badhwar 1991; Irons 1994).
We categorised two trials as ‘high quality’ in terms of adequate blinding of personnel and participants (Bullough 1989), and appropriate allocation concealment in sealed envelopes (Irons 1994). Due to alternate allocation Kim 1986 was assessed as of low quality for allocation concealment. Two further studies were assessed as low quality for lack of blinding of personnel and participants (Irons 1994; Kim 1986). No blinding was performed for the assessment of blood loss in the third stage of labour by midwives or physicians, because blinding was not possible. Only one study used another index of blood loss (haematocrit in the second day postpartum) for the amount of blood loss (Kim 1986). Two studies (Bullough 1989; Irons 1994) enrolled women considered to be at low risk for bleeding.
We assessed several outcomes for two comparisons according to the GRADE methodology (see Table 1; Table 2). The quality of the evidence was largely of low and very low quality for both comparisons, due to risk of bias concerns with both of the contributing trials and imprecise estimates of effect. For the comparison of nipple stimulation versus no treatment, the outcome of PPH was assessed as of moderate quality (downgraded for risk of bias). Blood loss was assessed as of low quality (downgraded for risk of bias and imprecision), and maternal deaths, retained placenta, perinatal deaths and maternal admission to hospital were all graded as of very low quality (downgraded for risk of bias, imprecision and few events). For the comparison of nipple stimulation with oxytocin, evidence for both blood loss and postnatal anaemia was assessed as of low quality, due to risk of bias concerns in the contributing trial (alternate allocation) and imprecision (wide confidence intervals (CIs) crossing the line of no difference, and small sample size).
Regarding adherence to protocol, in the study by Bullough 1989, TBAs were taught to put the baby to the mother's breast after delivery, as soon as the cord was cut and the baby was dried and wrapped. Suckling took place before placenta delivery in 889 cases, both before and after in 575 cases, only after placenta delivery in 507 cases, and not at all in 32 cases. With a total intervention group of 2140, compliance with the designated intervention was approximately 70%. There was no change in the protocol in the study by Irons 1994.
Potential biases in the review process
We have followed Cochrane standard methods to reduce the potential for bias in the review process. We conducted a search without language restrictions. Further, two review authors independently assessed trial reports for eligibility. Data extraction was double‐checked, and two review authors independently assessed trials for risks of bias. Remaining biases in this review should be minimal.
Agreements and disagreements with other studies or reviews
This is the first systematic review conducted to examine the effect of nipple stimulation on blood loss in the third stage of labour. Only a paucity of studies have been published in this area of investigation. Myometrium contraction in response to endogenous oxytocin has been documented in other studies (Kavanagh 2005; Mashini 1987). One study (Mashini 1987) compared the effect of nipple stimulation with oxytocin infusion on inducing contractions during contraction stress tests. It was apparent that regular uterine activity occurred more rapidly but at a lower level in the nipple stimulation group (P < 0.05). The researchers concluded that nipple stimulation and exogenous oxytocin may produce different effects on uterine contraction. In addition, a Cochrane review (Kavanagh 2005), showed that breast stimulation promotes uterine contractions and reduces postpartum haemorrhage in women with post‐term pregnancy (0.7% versus 6%; risk ratio (RR) 0.16; 95% confidence interval (CI) 0.03 to 0.87). However, it was not recommended to use this method in high‐risk women.
The study by Irons 1994, compared the effect of nipple stimulation with syntometrine using an objective method for measuring residual placental venous pressure (RPVP).They cannulated the cord with a 15‐gauge cannula within 30 seconds after delivery and RPVP was measured and compared in three study groups. Results showed that the nipple stimulation was effective as syntometrine in increasing the RPVP (103 versus 111.6 mmHg).
A study conducted by Christensson 1989 examined nipple stimulation (30 minutes) in 10 women who were in their 38th to 39th week of pregnancy. The rates of uterine contractions, fetal heart rate and blood levels of oxytocin were measured. Results showed that in nine out of 10 women, uterine contractions had commenced and one woman showed signs of uterine hyperactivity. Oxytocin levels increased significantly during nipple stimulation. Furthermore, one of the reasons the World Health Organization recommends early breastfeeding, within one hour of birth, is to promote the uterine activity and to reduce the risk of heavy bleeding (UNICEF 2002; WHO 1998).
Authors' conclusions
Implications for practice.
This review aimed to assess the effect of breastfeeding or nipple stimulation for reducing postpartum haemorrhage (PPH). None of the included studies reported severe PPH (blood loss greater or equal to 1000 mL). In this review there was no clear effects of either breastfeeding or nipple stimulation on blood loss in the third stage of labour or PPH (PPH greater than or equal to 500 mL). Overall, there is insufficient evidence to evaluate the effect of nipple stimulation for reducing bleeding during the third stage of labour and more evidence from high‐quality studies is needed.
In the context of the studies in this review, low‐quality evidence from one small study (involving data from 85 women) suggests that nipple stimulation may be comparable with oxytocin in terms of blood loss in the third stage of labour and also postpartum anaemia. Therefore, this evidence should be interpreted with caution since it is based on one trial; there is a need for more studies in this area to clarify this.
According to national statistics in the USA for 2006‐2010, the maternal mortality ratio is 16.0 per 100,000 live births, with 11.4% of these deaths due to hemorrhage (Creanga 2015). According to World Health Organization statistics, 25% of maternal deaths in developing countries are caused by PPH (Andolina 1999). An average blood loss of 500 mL does not appear to put the lives of mothers at risk. Three trials in this review showed the average blood loss was less than 500 mL (from 258 ± 163 mL (Bullough 1989), to 398 ± 94 mL (Kim 1986). Only a small percentage of women in this review showed postpartum blood loss of > 500 mL (7.9% in the suckling group and 8.4% in the control group) (Bullough 1989). It seems that in a hospital setting with adequate access to emergency care, healthy women can tolerate an average blood loss of 500 mL. Therefore, in women with a low risk for bleeding, breastfeeding or nipple stimulation may be a safe alternative to reduce possible blood loss but this needs to be evaluated in further high‐quality studies.
Implications for research.
There is insufficient evidence to evaluate the effect of breastfeeding/nipple stimulation for reducing bleeding during the third stage of labour and more evidence from high‐quality studies is needed. Further high‐quality studies should involve adequate sample sizes, assess the impact of nipple stimulation compared to uterotonic agents such as syntometrine and oxytocin, and report on important outcomes such as those listed in this review.
Future studies should utilise more objective methods to estimate blood loss such as measuring the levels of haemoglobin (Hb) and haematocrit. In the context of this review, almost all included women were at a low risk for bleeding in the third stage of labour. Women with a high risk for bleeding should not be considered for research on nipple stimulation and blood loss during labour. Research should be conducted in a quiet and private environment for the mother to stimulate her nipples or to breast‐feed. Furthermore, women who receive uterotonic agents for induction or augmentation of labour should not be considered for nipple stimulation studies. Future studies should also examine the effects of nipple stimulation versus physiological management of the third stage of labour. For example, comparing the effect of nipple stimulation with an active management intervention during the third stage of labour is recommended. Also further studies are needed to look at the difference between nipple stimulation or breastfeeding on primary PPH (within the first 24 hours) and secondary PPH (after 24 hours up to six to 12 weeks post‐delivery.
What's new
| Date | Event | Description |
|---|---|---|
| 28 January 2016 | Amended | Corrected typographic error in the main title. |
Acknowledgements
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Data and analyses
Comparison 1. Nipple stimulation (suckling) versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal death | 1 | 4227 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.12, 74.26] |
| 2 Frequency of PPH ≥ 500 mL | 1 | 4227 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.77, 1.16] |
| 3 Amount of blood loss | 1 | 4227 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐7.39, 11.39] |
| 4 Retained placenta | 1 | 4227 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.14, 7.16] |
| 5 Perinatal death | 1 | 4271 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.57, 1.98] |
| 6 Maternal admission to hospital | 1 | 4227 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.14, 7.16] |
Comparison 2. Nipple stimulation (breast pump) versus oxytocin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Amount blood loss | 1 | 85 | Mean Difference (IV, Fixed, 95% CI) | 15.0 [‐24.50, 54.50] |
| 2 Postnatal anaemia | 1 | 85 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐2.22, 1.42] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Badhwar 1991.
| Methods | Randomised trial in Delhi, India. | |
| Participants | 120 pregnant women. | |
| Interventions | Arm 1: no treatment. Arm 2: bilateral digital nipple stimulation. Arm 3: IV methyl ergometrine. |
|
| Outcomes | Duration of and blood loss during the 3rd stage of labour. Authors did not provide any definite definition of PPH. | |
| Notes | This study was reported in abstract form only, and no numerical data were provided. Authors state that nipple stimulation led to a decrease in blood loss. We have not been able to contact the authors of this trial for further clarification or data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data not reported. |
| Selective reporting (reporting bias) | Unclear risk | Data not reported. |
| Other bias | Unclear risk | Trial reported in abstract form only. |
Bullough 1989.
| Methods | Cluster‐randomised controlled trial with 2 arms, Malawi. | |
| Participants | TBAs were randomised in the trial and not pregnant women. 23 TBAs (2184 deliveries) were allocated to the early suckling group and 26 TBAs (2201 deliveries) to the control group. The main analysis was carried out on 4227 singleton vaginal deliveries (2123 control and 2104 early suckling). | |
| Interventions | Arm 1: early suckling (breastfeeding immediately after birth) Arm 2: standard care of pregnant women. In Malawi culture, standard care involves relatives looking after the newborn immediately, allowing the mother to rest. All TBAs were given training before the trial but none were made aware of the trial. Only TBAs in the intervention arm were educated about the importance of early suckling and if initiating this practice with women in their care. |
|
| Outcomes | The frequency of PPH defined by authors as blood loss > 500 mL. The mean blood loss. The frequency of retained placenta. |
|
| Notes | The unit of randomisation in this trial was the provider rather than pregnant women; this fact will produce a clustering‐effect. No adjustments were made to the data to account for possible similarities among women receiving care from the same birth attendant, either in the original trial report or in this review's analysis. Therefore, it is reasonable to assume that the confidence intervals associated with treatment effects have been underestimated. Women with vaginal tears were not excluded, but twin pregnancies were. Neonatal deaths were not excluded from the main analysis, but stillbirths were. We have added stillbirths and neonatal deaths from Table 2 to create the perinatal deaths outcome. For PPH, twin births and stillbirths are analysed separately in the trial report; we have not included these data in our review's analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomisation of TBAs to suckling or control groups was carried out by member of the Malawi Ministry of Health within districts and after stratification for the number of deliveries attended (more or less than 30 per month) and for distance from a telephone or health centre (less than 5 km, or 5 km and further)." Random sequence generation not described. |
| Allocation concealment (selection bias) | Low risk | Randomisation was done centrally, as above. All women attending a single TBA would have had the same intervention (either early suckling or standard care). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants: "Randomisation into early suckling and control groups was by TBAs rather than by women to reduce likelihood of contamination of the treatment group by knowledge of the intervention reaching women awaiting delivery”. Personnel: “The TBAs were not informed that they were taking part in the trials”. Comment: the trial participants and the TBAs were not aware that they were involved in a randomised study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quotes: “The TBAs were not informed that they were taking part in the trial”. “TBAs were taught that the reason for this practice (early suckling) was to keep baby warm or promotes bonding.” “a record collection form was designed with drawings to represent the information required” (record keeping – quality control). Comments: TBA as outcome assessors of end points were not aware of the trial; however, because most TBAs had minimal education, trialists used methods to ensure quality control. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 10 out of 33 (30.3%) of the TBAs in the suckling group and 9 out of 35 (25.7%) in the control group were excluded from the analysis due to problems with birth attendant training and with quality control (for example, some TBAs measured amniotic fluid along with blood for the 'blood loss' outcome). Of 4385 deliveries from the remaining TBAs, blood loss measurements were missing for 114 (59 in control group and 55 in suckling group). There are discrepancies in the numbers for frequency of PPH between the table on p. 524 and the paragraph on p. 524. We have used the larger numbers from the paragraph. The relative risk is similar with both sets of numbers. |
| Selective reporting (reporting bias) | Low risk | All outcomes are reported. |
| Other bias | High risk | Observer bias: TBAs had little formal education. Trialists excluded data from several TBAs due to concerns about the accuracy of their data collection. TBAs were only partly successful at achieving early suckling, with suckling before the delivery of the placenta in just 1464/2104 women. Analysis bias: methods used in the trial analysis were not appropriate for cluster‐randomised trial design. |
Irons 1994.
| Methods | Randomised controlled trial with 3 arms, Tyneside UK. | |
| Participants | 16 women were randomised from the antenatal clinic at 34‐38 weeks of gestation. | |
| Interventions | Arm 1: nipple stimulation (n = 6). Manual, bilateral nipple stimulation for 15 minutes, starting immediately after delivery. Arm 2: routine intramuscular ergometrine 500 µg, and oxytocin 5 unit injection with delivery of the anterior shoulder (n = 3). Arm 3: no treatment (n = 5). |
|
| Outcomes | The frequency of contractions. The peak placental venous pressure during contractions (mmHg). The amount of blood loss (mL). The amount of blood loss as PPH was not clearly defined by authors. Blood loss was reported as a mean only, without a standard deviation. |
|
| Notes | This trial was set up to study the effects of nipple stimulation on uterine pressure, not on PPH. Exclusions included: PPH or retained placenta in a previous pregnancy; multiple pregnancy; prolonged labour (over 16 hours); anaemia; oxytocin infusion or operative delivery. 2 women in the control group had not delivered the placenta by 30 minutes and were given Syntometrin. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Randomisation took place during 2nd stage of labor..” “The participants were allocated into three type of management”. Comment: method of generation sequence not clearly specified. |
| Allocation concealment (selection bias) | Low risk | Quote: “Instructions were contained in sealed envelope, stratify parity and blocked in group of 6”. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the intervention, blinding of the participants and personnel was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not clear whether or not outcomes assessors were independent and unaware of the 3 different treatments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 of 16 initially recruited were excluded due to failed cannulation. |
| Selective reporting (reporting bias) | Low risk | Trialists reported outcomes related to uterine activity only. |
| Other bias | Low risk | None noted. |
Kim 1986.
| Methods | Randomised controlled trial, New York, USA. | |
| Participants | A total of 87 primigravida women were enrolled, 85 completed the study: 32 were study cases and 53 were controls.28% (study group) and 22% (controls) of pregnant women were considered to be of high risk. | |
| Interventions | Arm 1: nipple stimulation using breast shield and a pump with negative pressure of 250 mmHg for 5 minutes in each breast. Breast stimulation lasted for a total of 20 minutes starting immediately after delivery. Arm 2: women received an infusion of 20 IU of oxytocin after delivery of the placenta, with an average of 1.2‐3 units given in the 20 minutes following delivery. |
|
| Outcomes | Blood loss and haematocrit. The amount of blood loss considered as PPH was not defined by authors. | |
| Notes | Baseline characteristics comparable. Third stage of labour longer in the study group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “the subject were randomised by the last digit of their last hospital admission numbers”. Comment: hospital registration number is usually generated by computer. |
| Allocation concealment (selection bias) | High risk | Quote: “the even digits (hospital registration number) were assigned to the study (nipple stimulation) and odd digits to the control group”. Comment: the allocation could be known by looking at the last digit of the registration number. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the intervention, blinding of the participants and personnel was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The amount of blood loss was estimated by physicians; there was no information regarding the specialty of physicians or their knowledge of the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Total number of patients recruited were 87; 32 were study cases and 53 were controls. Data are present for all women recruited. |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes are reported. |
| Other bias | Low risk | None noted. |
IU: international units IV: intravenous PPH: postpartum haemorrhage TBA: traditional birth attendants
Characteristics of studies awaiting assessment [ordered by study ID]
Narenji 2012.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | This trial report is written in Persian and is awaiting translation. |
Differences between protocol and review
In this review we used the GRADE approach to assess the quality of the body of evidence ‐ this was not pre‐specified in our published protocol (Abedi 2013).
We have also edited the review title from 'Nipple stimulation or breastfeeding for preventing postpartum haemorrhage in the third stage of labour' to 'Breasfeeding or nipple stimulation for reducing postpartum haemorrhage in the third stage of labour'.
We have clarified our primary outcome of maternal death and severe maternal morbidity to say that these outcomes may be reported as a composite or individually.
We added the following new secondary outcomes at the review stage.
PPH (measured or estimated blood loss of ≥ 500 mL, or as defined by the trial authors)
Amount of blood loss (measured or estimated blood loss)
Incidence of retained placenta
We have edited some secondary outcomes.
'Readmission to hospital for mother' has been changed to 'Maternal readmission to hospital (or transfer to hospital for home births)'
'Jaundice requiring phototherapy' has been rephrased to 'Freqency of infants with jaundice requiring phototherapy'
'Not breast fed at discharge' has been rephrased to 'Frequency of infants not breast fed at discharge'
'Re‐admission to hospital for baby' has been rephrased to 'Infant re‐admission to hospital'
'Perinatal morbidity' has been changed to 'Perinatal morbidity (including respiratory distress, hypoxia, and as defined by the trial authors)'
We have removed some secondary outcomes: Maternal death; individual components of severe morbidity.
We have removed the proposed subgroup that explored a difference in comparator (placebo/expectant comparator versus active/oxytocin comparator).
Contributions of authors
Parvin Abedi and Jasmine Lee were responsible for completing data extraction forms. Parvin Abedi drafted the results, discussion and conclusion. Results were prepared under the supervision of Shayesteh Jahanfar. Farideh Namvar, Jasmine Lee and Shayesteh Jahanfar edited the results, discussion and conclusion sections.
Declarations of interest
Parvin Abedi: none known
Shayesteh Jahanfar: none known
Farideh Namvar: none known
Jasmine Lee: none known
Edited (no change to conclusions)
References
References to studies included in this review
Badhwar 1991 {published data only}
- Badhwar L, Singh K, Sethi N, Gupta I, Aggarwal N. The value of nipple stimulation in the management of third stage of labour. International Journal of Gynecology and Obstetrics 1991;36 Suppl:16. [Google Scholar]
Bullough 1989 {published data only}
- Bullough CHW. Breast suckling or nipple stimulation: prevention of postpartum haemorrhage?. European Journal of Obstetrics & Gynecology and Reproductive Biology 1996;69:39. [Google Scholar]
- Bullough CHW, Msuku RS, Karonde L. Early suckling and postpartum haemorrhage: controlled trial in deliveries by traditional birth attendants. Lancet 1989;2:522‐5. [DOI] [PubMed] [Google Scholar]
Irons 1994 {published data only}
- Irons DW, Sriskandabalan P, Bullough CHW. A simple alternative to parenteral oxytocics for the third stage of labor. International Journal of Gynecology & Obstetrics 1994;46:15‐8. [DOI] [PubMed] [Google Scholar]
Kim 1986 {published data only}
- Kim YM, Tejani N, Chayen B, Verma UL. Management of the third stage of labor with nipple stimulation. Journal of Reproductive Medicine 1986;31:1033‐4. [PubMed] [Google Scholar]
References to studies awaiting assessment
Narenji 2012 {published data only}
- Narenji F. Comparison the effect of intramuscular injection of oxytocin and nipple stimulation on the third stage of delivery length and bleeding. IRCT Iranian Registry of Clinical Trials (www.irct.ir) [accessed 16 July 2015] 2012.
- Narenji F, Bahmani Z, Mirzaie R. Comparison of the effect of oxytocin IM injection and nipple stimulation on bleeding and length of third stage of deliver. Complementary Medicine Journal of Faculty of Nursing & Midwifery 2012; Vol. 2, issue 1:119‐25.
Additional references
Aflaifel 2012
- Aflaifel N, Weeks AD. Active management of the third stage of labour, oxytocin is all you need. BMJ 2012;345:1‐2. [DOI: 10.1136/bmj.e4546] [DOI] [PubMed] [Google Scholar]
Alexandrova 1980
- Alexandrova M, Soloff MS. Oxytocin receptors and parturition. I. Control of oxytocin receptor concentration in the rat myometrium at term. Endocrinology 1980;106(3):730‐5. [DOI] [PubMed] [Google Scholar]
Andolina 1999
- Andolina K, Daly S, Roberts N, Tolosa J, Wapner R. Objective measurement of blood loss at delivery: is it more than a guess?. American Journal of Obstetrics and Gynecology 1999;180:S69. [Google Scholar]
Arenson 2007
- Arenson J, Drake P. Maternal and Newborn Health. Jones & Bartlett Learning, 2006. [Google Scholar]
Begley 2015
- Begley CM, Gyte GM, Declan D, McGuire W, Weeks A. Active versus expectant management for women in the third stage of labour. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD007412.pub4] [DOI] [PubMed] [Google Scholar]
Brandt 1933
- Brandt M. The mechanism and management of the third stage of labor. American Journal of Obstetrics and Gynecology 1933;25:662‐7. [Google Scholar]
Carroli 2008
- Carroli G, Cuesta C, Abalos E, Gulmezoglu AM. Epidemiology of postpartum haemorrhage: a systematic review. Best Practice & Obstetrics and Gynecology 2008;22(6):999‐1012. [DOI: 10.1016/j.bpobgyn.2008.08.004] [DOI] [PubMed] [Google Scholar]
Christensson 1989
- Christensson K, Nilsson BA, Stock S, Matthiesen AS, Uvnäs‐Moberg K. Effect of nipple stimulation on uterine activity and on plasma levels of oxytocin in full term, healthy, pregnant women. Acta Obstetricia et Gynecologica Scandinavica 1989;68(3):205‐10. [DOI] [PubMed] [Google Scholar]
CMQCC 2010
- Maternity Quality Care Collaborative. OB Hemorrhage Care Guidelines: Checklist Format V.1.4. Retrieved from http://www.cmqcc.org/resources/ob_hemorrhage/ob_hemorrhage_care_guidelines_checklist_flowchart_tablechart_v1_4 2010.
Coad 2011
- Coad J, Melvyn D. Anatomy and Physiology for Midwives. 3rd Edition. Churchill Livingstone, 2011. [Google Scholar]
Creanga 2015
- Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Callaghan WM. Pregnancy‐Related Mortality in the United States, 2006–2010. Obstetrics and Gynecology 2015;125:5‐12. [DOI: 10.1097/AOG.0000000000000564] [DOI] [PubMed] [Google Scholar]
Dewey 2001
- Dewey KG. Maternal and fetal stress are associated with impaired lactogenesis in human. Journal of Nutrition 2001;131(11):3012S‐5S. [DOI] [PubMed] [Google Scholar]
Elbourne 1995
- Elbourne D. Care in the third stage of labour. In: Robinson S, Thomson AM editor(s). Midwives, Research and Childbirth. Vol. 4, London: Chapman & Hall, 1995:192‐207. [Google Scholar]
Festin 2003
- Festin MR, Lumbiganon P, Tolosa JE, Finney KA, Ba‐Thike K, Chipato T, et al. International survey on variations in practice of the management of the third stage of labour. Bulletin of the World Health Organization 2003;81(4):286‐91. [PMC free article] [PubMed] [Google Scholar]
Finley 1986
- Finley BE, Amico J, Castillo M, Seitchik J. Oxytocin and prolactin responses associated with nipple stimulation contraction stress tests. Obstetrics and Gynecology 1986;67(6):836‐9. [DOI] [PubMed] [Google Scholar]
Fuchs 1991
- Fuchs AR, Romero R, Keefe D, Parra M, Oyarzun E, Behnke E. Oxytocin secretion and human parturition: pulse frequency and duration increase during spontaneous labor in women. American Journal of Obstetrics and Gynecology 1991;165(5 Pt 1):1515‐23. [DOI] [PubMed] [Google Scholar]
Gimpl 2001
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function and regulation. Physiological Reviews 2001;81(2):629‐83. [DOI] [PubMed] [Google Scholar]
Gyte 1994
- Gyte GM. Evaluation of the meta‐analyses on the effects, on both mother and baby, of the various components of active’ management of the third stage of labour. Midwifery 1994;10(4):183‐99. [DOI] [PubMed] [Google Scholar]
Gülmezoglu 2012
- Gülmezoglu AM, Lumbiganon P, Landoulsi S, Widmer M, Abdel‐Aleem H, Festin M, et al. Active management of the third stage of labour with and without controlled cord traction: a randomised, controlled, non‐inferiority trial. Lancet 2012;379(9827):1721‐7. [DOI: 10.1016/S0140-6736(12)60206-2] [DOI] [PubMed] [Google Scholar]
Hatjis 1989
- Hatjis CG, Morris M, Rose JC, Kofinas AD, Penry M, Swain M. Oxytocin, vasopressin, and prolactin response associated with nipple stimulation. Southern Medical Journal 1989;82(2):193‐6. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
ICM/FIGO 2006
- ICM/FIGO. Prevention and treatment of postpartum haemorrhage. http://www.figo.org/projects/prevent/PPH 2006.
Kavanagh 2005
- Kavanagh J, Kelly AJ, Thomas J. Breast stimulation for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD003392.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khushk 2002
- Khushk IA, Baig LA. Maternal mortality in Pakistan: no room for complacency. Journal of College of Physicians and Surgeons Pakistan 2002;12:511‐2. [Google Scholar]
Koh 2009
- Koh E, Devendra K, Tan LK. B‐Lynch suture for the treatment of uterine atony. Singapore Medical Journal 2009;50(7):693‐7. [PubMed] [Google Scholar]
Mashini 1987
- Mashini IS, Devoe LD, McKenzie JS, Hadi HA, Sherline DM. Comparison of uterine activity induced by nipple stimulation and oxytocin. Obstetrics and Gynecology 1987;69:74‐8. [PubMed] [Google Scholar]
Maughan 2006
- Maughan KL, Heim SW, Galazka SS. Preventing postpartum hemorrhage: managing the third stage of labor. American Family Physician 2006;73(6):1025‐8. [PubMed] [Google Scholar]
McDonald 2007
- McDonald S. Management of the third stage of labor. Journal of Midwifery and Women’s Health 2007;52(3):254‐61. [DOI] [PubMed] [Google Scholar]
Phaneuf 1998
- Phaneuf S, Asbóth G, Carrasco MP, Liñares BR, Kimura T, Harris A, et al. Desensitisation of oxytocin receptors in human myometrium. Human Reproduction Update 1998;4(5):625‐33. [DOI] [PubMed] [Google Scholar]
POPPHI 2008
- Prevention of Postpartum Hemorrhage Initiative (POPPHI). Fact sheets: Uterotonic drugs for the prevention and treatment of postpartum hemorrhagia. Seattle: PATH. http://www.path.org/publications/files/MCHN_popphi_pph_fs_uterotonic.pdf (accessed 2013) 2008.
Prendiville 1989
- Prendiville WJ, Elbourne DR. Care during the third stage of labour. Effective Care in Pregnancy and Childbirth. Oxford: Oxford University Press, 1989:1145‐69. [Google Scholar]
Prendiville 1996
- Prendiville WJ. The prevention of post partum haemorrhage: optimising routine management of the third stage of labour. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1996;69(1):19‐24. [DOI] [PubMed] [Google Scholar]
Razvi 2008
- Razvi K, Chua S, Arulkumaran S, Ratnam SS. A comparison between visual estimation and laboratory determination of blood loss during the third stage of labour. Australian and New Zealand Journal of Obstetrics and Gynaecology 2008;36(2):152‐4. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ridley 2002
- Ridley S, Smith G, Batchelor A. Core Cases in Critical Care. Cambridge University Press, 2002. [Google Scholar]
Soltani 2008
- Soltani H. Global implications of evidence based practice: management of the third stage of labour. Midwifery 2008;24:138‐42. [DOI] [PubMed] [Google Scholar]
UNICEF 2002
- Anonymous. Facts for Life. 3rd Edition. New York: United Nations Children's Fund/UNICEF, 2002. [Google Scholar]
WHO 1998
- World Health Organization. Evidence for the Ten Steps to Successful Breastfeeding. Geneva: WHO, 1998. [Google Scholar]
WHO 2007
- World Health Organization. WHO Recommendations for the Prevention of Postpartum Haemorrhage. Geneva: WHO, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2012
- World Health Organization. Maternal Mortality. Geneva: WHO, 2012. [Google Scholar]
WHO 2014
- World Health Organization. Maternal mortality fact sheet No. 348. (http://apps.who.int/iris/bitstream/10665/112318/1/WHO_RHR_14.06_eng.pdf?ua=1, accessed 5 February 2015 2015.
References to other published versions of this review
Abedi 2013
- Abedi P, Jahanfar S, Namvar F. Nipple stimulation or breastfeeding for preventing postpartum haemorrhage in the third stage of labour. Cochrane Database of Systematic Reviews 2013, Issue 11. [DOI: 10.1002/14651858.CD010845] [DOI] [PMC free article] [PubMed] [Google Scholar]


