Abstract
Background
Blood loss during liver resection is considered one of the most important factors affecting the peri‐operative outcomes of patients undergoing liver resection.
Objectives
To determine the benefits and harms of cardiopulmonary interventions to decrease blood loss and to decrease allogeneic blood transfusion requirements in patients undergoing liver resections.
Search methods
We searched the Cochrane Hepato‐Biliary Group Controlled Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, and Science Citation Index Expanded until January 2012 to identify randomised trials.
Selection criteria
We included all randomised clinical trials comparing various cardiopulmonary interventions aimed at decreasing blood loss and allogeneic blood transfusion requirements in patients undergoing liver resection. Trials were included irrespective of whether they included major or minor liver resections of normal or cirrhotic livers, vascular occlusion was used or not, and irrespective of the reason for liver resection.
Data collection and analysis
Two authors independently identified trials for inclusion and independently extracted data. We analysed the data with both the fixed‐effect and the random‐effects models using RevMan Analysis. For each outcome we calculated the risk ratio (RR), mean difference (MD), or standardised mean difference (SMD) with 95% confidence intervals (CI) based on intention‐to‐treat analysis or available case analysis. For dichotomous outcomes with only one trial included under the outcome, we performed the Fisher's exact test.
Main results
Ten trials involving 617 patients satisfied the inclusion criteria. The interventions included low central venous pressure (CVP), autologous blood donation, haemodilution, haemodilution with controlled hypotension, and hypoventilation. Only one or two trials were included under most comparisons. All trials had a high risk of bias. There was no significant difference in the peri‐operative mortality in any of the comparisons: low CVP versus no intervention (3 trials, 0/88 (0%) patients in the low CVP group versus 1/89 (1.1%) patients in the no intervention group); autologous blood donation versus no intervention (1 trial, 0/40 (0%) versus 0/39 (0%)); haemodilution versus no intervention (2 trials, 1/73 (1.4%) versus 3/77 (3.9%) in one of these trials); haemodilution with controlled hypotension versus no intervention (1 trial, 0/10 (0%) versus 0/10 (0%)); haemodilution with bovine haemoglobin (HBOC‐201) versus haemodilution with hydroxy ethyl starch (HES) (1 trial, 1/6 (16.7%) versus 0/6 (0%)); hypoventilation versus no intervention (1 trial, 0/40 (0%) versus 0/39 (0%)). None of the trials reported long‐term survival or quality of life. The risk ratio of requiring allogeneic blood transfusion was significantly lower in the haemodilution versus no intervention groups (3 trials, 16/115 (weighted proportion = 14.2%) versus 41/118 (34.7%), RR 0.41 (95% CI 0.25 to 0.66), P = 0.0003); and for haemodilution with controlled hypotension versus no intervention (1 trial, 0/10 (0%) versus 10/10 (100%), P < 0.0001). There were no significant differences in the allogeneic transfusion requirements in the other comparisons which reported this outcome, such as low CVP versus no intervention, autologous blood donation versus control, and hypoventilation versus no intervention.
Authors' conclusions
None of the interventions seemed to decrease peri‐operative morbidity or offer any long‐term survival benefit. Haemodilution shows promise in the reduction of blood transfusion requirements in liver resection surgery. However, there is a high risk of type I (erroneously concluding that an intervention is beneficial when it is actually not beneficial) and type II errors (erroneously concluding that an intervention is not beneficial when it is actually beneficial) because of the few trials included, the small sample size in each trial, and the high risk of bias in the trials. Further randomised clinical trials with low risk of bias and random errors that assess clinically important outcomes such as peri‐operative mortality are necessary to assess any cardiopulmonary interventions aimed at decreasing blood loss and blood transfusion requirements in patients undergoing liver resections. Trials need to be designed to assess the effect of a combination of different interventions in liver resections.
Keywords: Humans; Blood Loss, Surgical; Blood Loss, Surgical/prevention & control; Blood Transfusion; Blood Transfusion/statistics & numerical data; Blood Transfusion, Autologous; Blood Transfusion, Autologous/methods; Central Venous Pressure; Central Venous Pressure/physiology; Hemodilution; Hemodilution/methods; Hepatectomy; Hepatectomy/methods; Hypotension, Controlled; Randomized Controlled Trials as Topic; Respiration
Plain language summary
Haemodilution shows promise in decreasing blood loss and blood transfusion requirements during liver resection
Blood loss during liver resection (partial removal of liver) is one of the important factors affecting the post‐operative complications experienced by patients. Allogeneic blood transfusion (using blood donated by a different individual) is associated with increased morbidity and lower survival in patients with liver cancer. This systematic review was aimed at determining whether any cardiopulmonary intervention (interventions that change the circulation or breathing during surgery) decreased blood loss or decreased allogeneic blood transfusion requirements in patients undergoing liver resections. This review included 10 trials with 617 patients. All trials had high risk of bias (with the possibility of overestimating the benefits and underestimating the harms of the treatment) and play of chance ('random error'). The interventions included low central venous pressure (CVP; lowering the pressure in the major veins), autologous blood donation (using the patient's own blood obtained prior to liver resection), haemodilution (replacing blood with other fluids), haemodilution with controlled hypotension (lowering the blood pressure in addition to diluting the blood), and hypoventilation (decreasing the rate of artificial breathing). They were compared with controls not receiving the interventions. There were no differences in the number of deaths or complications due to surgery in any of the comparisons. Long‐term survival was not reported in any of the trials. Fewer patients required transfusion of blood donated by others when haemodilution or haemodilution with controlled hypotension were compared with a control group. The other comparisons did not decrease the transfusion requirements. However, there is a high risk of type I errors (erroneously concluding that an intervention is beneficial when it is actually not beneficial) and type II errors (erroneously concluding that an intervention is not beneficial when it is actually beneficial) because of the few trials included and the small sample size in each trial, as well as the inherent risk of bias (systematic errors which can result in overestimation of the benefits and underestimation of the harms of the intervention). Haemodilution showed promise in the reduction of blood transfusion requirements in patients undergoing liver resections. Further randomised clinical trials with low risk of bias (systematic errors) and low risk of play of chance (random errors) which assess clinically important outcomes (such as death and complications due to the operation) are necessary to assess cardiopulmonary interventions aimed at decreasing blood loss in liver resections. Trials need to be designed to assess the effect of a combination of different interventions during liver resections.
Summary of findings
Summary of findings for the main comparison. Cardiopulmonary interventions to decrease blood loss and blood transfusion requirements for liver resection.
| Cardiopulmonary intervention versus control for liver resection | ||||||
| Patient or population: patients with liver resection. Settings: secondary or tertiary care. Intervention: intervention versus control. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Intervention versus control | |||||
| Serious adverse events ‐ Haemodilution versus control. | Study population | |||||
| 217 per 1000 | 219 per 1000 (124 to 391) | Rate ratio 1.01 (0.57 to 1.8) | 208 (2 studies) | ⊕⊝⊝⊝ very low1,2,3,4,5 | ||
| Number requiring allogeneic blood transfusion ‐ Low central venous pressure (CVP) versus control. | Study population | RR 0.66 (0.42 to 1.03) | 177 (3 studies) | ⊕⊝⊝⊝ very low1,3,4,5 | ||
| 303 per 1000 | 200 per 1000 (127 to 312) | |||||
| Number requiring allogeneic blood transfusion ‐ Haemodilution versus control. | Study population | RR 0.41 (0.25 to 0.66) | 233 (3 studies) | |||
| 347 per 1000 | 142 per 1000 (87 to 229) | |||||
| Red cell transfusion ‐ Low CVP versus control. | The mean red cell transfusion ‐ low CVP versus control in the intervention groups was 0.31 standard deviations lower (0.65 lower to 0.03 higher) | 135 (2 studies) | ⊕⊝⊝⊝ very low1,5,6,7 | SMD ‐0.31 (‐0.65 to 0.03) | ||
| Red cell transfusion ‐ Haemodilution versus control. | The mean red cell transfusion ‐ haemodilution versus control in the intervention groups was 0.33 standard deviations lower (0.63 to 0.03 lower) | 180 (3 studies) | ⊕⊝⊝⊝ very low1,5,6 | SMD ‐0.33 (‐0.63 to ‐0.03) | ||
| *The basis for the assumed risk was the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Trials were of high risk of bias. 2 The seriousness of the outcomes was based on the study authors' judgement. 3 The confidence intervals overlap 1 and 0.75 or 1.25, or both. 4 Fewer than 300 events (in both groups). 5 There were few trials to assess whether there was any publication bias. 6 The I‐squared value was high. 7 There were less than 400 patients in both the groups together and the confidence intervals overlap 0 and ‐0.25 and/or +0.25.
Background
Elective liver resection is performed mainly for benign and malignant liver tumours (Belghiti 1993). The malignant tumours may arise primarily within the liver (hepatocellular carcinoma and cholangiocarcinoma) or may be metastases from malignancies of other organs (Belghiti 1993; Chouker 2004). More than 2100 elective liver resections are performed annually in the United Kingdom alone (HES 2011). About 11,000 liver resections are performed in the USA (Asiyanbola 2008). Colorectal cancer is the third most common cancer in the world with approximately 1.2 million people developing colorectal cancer each year (IARC 2010). Many are cured by resection of the primary cancer but 50% to 60% develop liver metastases (CLM) (Garden 2006). In 20% to 30% the metastases are confined to the liver and patients are suitable for liver resection. Liver resection is the only curative option for patients with colorectal liver metastases, with a 5 year survival of over 40% (Garden 2006).
Hepatocellular carcinoma (HCC) is one of the most common cancers, with a worldwide annual incidence of 750,000 new cases (IARC 2010). The majority develop in cirrhotic livers (Llovet 2005). Liver transplantation and liver resection are the main curative treatments (Llovet 2005). Of patients presenting with HCC about 5% are suitable for liver resection (Chen 2006). Survival following surgery depends on the stage of cancer and the severity of the underlying chronic liver disease. Early stage disease (cancers < 5 cm) have 5 year survival around 50% whereas those patients with more advanced disease have a 5 year survival of around 30% (Chen 2006). Screening programmes should lead to a diagnosis at an earlier stage where surgery is feasible and is associated with a better outcome.
The liver is subdivided into eight Couinaud segments (Strasberg 2000), which can be removed individually or by right hemi‐hepatectomy (Couinaud segments 5 to 8), left hemi‐hepatectomy (segments 2 to 4), right trisectionectomy (segments 4 to 8), or left trisectionectomy (segments 2 to 5 and 8 ± 1) (Strasberg 2000). Although every liver resection is considered major surgery, only resection of three or more segments is considered a major liver resection (Belghiti 1993).
Blood loss during liver resection is one of the important factors affecting the peri‐operative outcomes of patients (Shimada 1998; Yoshimura 2004; Ibrahim 2006). Blood loss and peri‐operative blood transfusion requirements also affect the long‐term survival after liver resection for cancers (Poon 2001; Gomez 2008). Various methods have been attempted to reduce the blood loss during liver resection. These include lowering the central venous pressure (CVP) (Wang 2006), hypoventilation (Hasegawa 2002), applying topical haemostatic agents (Frilling 2005), and occluding the blood flow to the liver (Gurusamy 2009a).
Allogeneic blood transfusion (transfusion of blood donated by a blood donor) is associated with increased morbidity (Shinozuka 2000) and lower survival in patients with primary liver cancer (Kitagawa 2001) compared with autologous blood transfusion (patient's own blood is collected and re‐infused) because of the possible immunosuppressive effect of donor blood (Shinozuka 2000). Various methods of autologous blood transfusion have been attempted in liver resection and include pre‐operative autologous blood donation or peri‐operative autologous blood donation (PABD) (Shinozuka 2000; Kitagawa 2001), intra‐operative blood salvage (Hashimoto 2007), and normovolemic haemodilution (Matot 2002).
We have addressed the role of vascular occlusion in liver resections in a Cochrane review (Gurusamy 2009a), the role of topical haemostatic agents is being addressed in another Cochrane review (Gurusamy 2012), and the role of pharmacological interventions in another Cochrane review (Gurusamy 2009b). This review is an update of a previous review on the role of cardiopulmonary interventions in decreasing blood loss or decreasing allogeneic blood transfusion requirements during liver resections (Gurusamy 2009).
Objectives
To determine the benefits and harms of cardiopulmonary interventions to decrease blood loss and to decrease allogeneic blood transfusion requirements in patients undergoing liver resections.
Methods
Criteria for considering studies for this review
Types of studies
We considered for inclusion all randomised clinical trials irrespective of language, blinding, publication status, or sample size.
Quasi‐randomised studies (where the method of allocating participants to a treatment are not strictly random, for example, by date of birth, hospital record number, alternation) were not included regarding assessment of benefit but were to be considered for inclusion regarding assessment of harms. This was because the trials with poor methodological quality overestimate the beneficial intervention effects (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008).
Types of participants
Patients undergoing liver resection irrespective of aetiology, being major or minor liver resections of normal or cirrhotic liver, method of vascular occlusion, and the use of topical haemostatic agents.
Types of interventions
We included any cardiopulmonary intervention aimed at reducing operative blood loss or peri‐operative allogeneic blood transfusion requirements during liver resection compared with no intervention, placebo, or another intervention aimed at reducing blood loss during liver resection or at decreasing allogeneic blood transfusion requirements during liver resections. We included interventions such as lowering the central venous pressure (Wang 2006) and hypoventilation (Hasegawa 2002).
Co‐interventions were allowed if carried out equally in the trial groups.
Types of outcome measures
Primary outcomes
-
Mortality:
peri‐operative mortality;
long‐term survival.
Serious adverse events. Adverse events were defined as any untoward medical occurrence not necessarily having a causal relationship with the treatment but resulting in a dose reduction or discontinuation of treatment (ICH‐GCP 1997). Serious adverse events were defined as any event that would increase mortality; was life‐threatening; required inpatient hospitalisation; resulted in a persistent or significant disability; or any important medical event which might have jeopardised the patient or required intervention to prevent it.
Quality of life.
Secondary outcomes
-
Transfusion requirements
-
Whole blood or red cell allogeneic transfusion (ie, transfusion of blood donated by others to the patient:
number of patients requiring whole blood or red cell allogenic transfusion,
overall mean number of units or volume of allogenic whole blood or red cell transfused;
Fresh frozen plasma.
Platelets.
-
Operative blood loss.
Operating time.
Hospital stay.
Search methods for identification of studies
We searched the Cochrane Hepato‐Biliary Group Controlled Trials Register (Gluud 2011), the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, and Science Citation Index Expanded (Royle 2003). We have given the search strategies in Appendix 1 with the time span of the searches until January 2012. We also searched the references of the identified trials to identify further relevant trials.
Data collection and analysis
Trial selection and extraction of data
Two authors (KG and JV, JL, or BO) independently of each other identified the trials for inclusion. We also listed the excluded studies with the reasons for their exclusion.
Two authors (KG and JV, JL, or BO) independently extracted the following data.
Year and language of publication.
Country in which the trial was conducted.
Year of conduct of the trial.
Inclusion and exclusion criteria.
Number of major and minor liver resections.
Number of cirrhotic patients.
Method of vascular occlusion.
Use of topical haemostatic agents.
Outcomes (mentioned above).
Risk of bias (described below).
Clarification on any unclear or missing information was sought by contacting the authors of the individual trials. If there was any doubt whether the trial reports shared the same participants, completely or partially (by identifying common authors and centres). the authors of the trials were contacted to clarify whether the trial report had been duplicated.
We resolved any differences in opinion through discussion or arbitration by the third author (BRD).
Assessment of risk of bias
Two authors (KG and JV, JL, or BO) independently assessed the risk of bias of the trials, without masking of the trial names. We followed the instructions given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and the Cochrane Hepato‐Biliary Group Module (Gluud 2011). According to empirical evidence (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008), the following risk of bias components were extracted from each trial.
Allocation sequence generation
Low risk of bias: sequence generation was achieved using computer random number generation or a random number table. Drawing lots, tossing a coin, shuffling cards, and throwing dice were adequate if performed by an independent adjudicator.
Uncertain risk of bias: the trial was described as randomised, but the method of sequence generation was not specified.
High risk of bias: the sequence generation method was not, or may not have been, random. Quasi‐randomised studies, those using dates, names, or admittance numbers in order to allocate patients, were inadequate and were excluded for the assessment of benefits but not for assessing harms.
Allocation concealment
Low risk of bias: allocation was controlled by a central and independent randomisation unit; sequentially numbered, opaque and sealed envelopes; or similar so that intervention allocations could not have been foreseen, in advance of or during enrolment.
Uncertain risk of bias: the trial was described as randomised but the method used to conceal the allocation was not described so that intervention allocations may have been foreseen in advance of or during enrolment.
High risk of bias: if the allocation sequence was known to the investigators who assigned participants, or if the study was quasi‐randomised. Quasi‐randomised studies were excluded for the assessment of benefits but not for assessing harms.
Blinding of participants and personnel
Low risk of bias: blinding was performed adequately or the outcome measurement was not likely to be influenced by lack of blinding.
Uncertain risk of bias: there was insufficient information to assess whether the type of blinding used was likely to induce bias in the estimate of effect.
High risk of bias: no blinding or incomplete blinding and the outcome or the outcome measurement was likely to be influenced by lack of blinding.
Blinding of outcome assessors
Low risk of bias: blinding was performed adequately or the outcome measurement was not likely to be influenced by lack of blinding.
Uncertain risk of bias: there was insufficient information to assess whether the type of blinding used was likely to induce bias in the estimate of effect.
High risk of bias: no blinding or incomplete blinding, and the outcome or the outcome measurement was likely to be influenced by lack of blinding.
Incomplete outcome data
Low risk of bias: the underlying reasons for missing data were unlikely to make treatment effects depart from plausible values, or proper methods were employed to handle missing data.
Uncertain risk of bias: there was insufficient information to assess whether the missing data mechanism in combination with the method used to handle missing data were likely to induce bias in the estimate of effect.
High risk of bias: the crude estimate of effects (eg, complete case estimate) would clearly be biased due to the underlying reasons for missing data, and the methods used to handle missing data were unsatisfactory.
Selective outcome reporting
Low risk of bias: pre‐defined, or clinically relevant and reasonably expected outcomes (such as mortality and morbidity) were reported on.
Uncertain risk of bias: not all pre‐defined or clinically relevant and reasonably expected outcomes (such as mortality and morbidity) were reported on or were not reported fully, or it was unclear whether data on these outcomes were recorded or not.
High risk of bias: one or more clinically relevant and reasonably expected outcomes were not reported on; data on these outcomes were likely to have been recorded.
Vested interest bias
Low risk of bias: if the trial was conducted by a party without any vested interests in the outcome of the trial.
Uncertain risk of bias: if it was not clear if the trial was conducted by a party with vested interest in the outcome of the trial.
High risk of bias: if the trial was conducted by a party with vested interests in the outcome of the trial.
We considered trials to have a low risk of bias if we assessed all the above domains as being low risk of bias. In all other cases, the trials were considered to have high risk of bias.
Statistical methods
We performed the meta‐analyses according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and the Cochrane Hepato‐Biliary Group Module (Gluud 2011). We used the software package Review Manager 5 (RevMan 2011). For dichotomous variables, we calculated the risk ratio (RR) with 95% confidence interval (CI) if there were two or more trials for an outcome. If there was only trial included under the comparison, we performed Fisher's exact test using StatsDirect 2.7; we have reported the proportion of patients with the outcome in each group and the P value for the comparison between the groups. For continuous variables, we calculated the mean difference (MD) or standardised mean difference (SMD) (for outcomes such as transfusion requirements where the requirements may be reported as units or as volume in millilitres) with 95% confidence interval. For count outcomes, such as serious adverse events, the rate ratio was calculated. For all outcomes a P value of less than 0.05 was considered statistically significant even if this included only one trial. We used a random‐effects model (DerSimonian 1986) and a fixed‐effect model (DeMets 1987) for meta‐analysis in the presence of two or more trials included under each of the outcomes. In the case of discrepancy between the two models, we have reported both results; otherwise we have reported the results of the fixed‐effect model. Heterogeneity was explored by the Chi2 test with significance set at P ≤ 0.10, and the quantity of heterogeneity was measured by the I2 statistic (Higgins 2002) set at 30% (Higgins 2011). We have highlighted the primary outcomes where the heterogeneity was more than 30%.
The analysis was performed on an intention‐to‐treat basis (Newell 1992) whenever possible using the best‐best, best‐worst, worst‐worst, and worst‐best scenarios (best‐best indicates the missing patients had favourable outcomes in the intervention and control; best‐worst indicated the missing patients had favourable outcomes in the intervention and unfavourable outcomes in the control; and so on). Otherwise, we adopted the 'available‐case analysis' (Higgins 2011). We did not impute any data for the post‐randomisation drop‐outs for any of the continuous outcomes. We had planned to perform a sensitivity analysis with and without empirical continuity correction factors (Sweeting 2004) using StatsDirect 2.7 in case there were 'zero‐event' trials in statistically significant outcomes. We also reported the results using risk difference if they were different from the results of risk ratio.
Imputation
We imputed the standard deviation from P values according to the instructions given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and used the median for the meta‐analysis when the mean was not available. If it was not possible to calculate the standard deviation from the P value or confidence intervals, we imputed the standard deviation as the highest standard deviation noted for that group under that outcome. If the mean and standard deviation for blood transfusion was given only for patients who required transfusion, we calculated the mean and standard deviation for the entire group by using the methods for combining groups that is suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). While this decision was made a priori, we have stated this to provide clarity.
Summary of findings table
Although we planned to report all the primary outcomes and blood transfusion requirements in the summary of findings table (GradePro 3.6), we have reported only serious adverse events and blood transfusion requirements for comparisons in which two or more trials were included; the other outcomes did not have two or more trials for any comparison (Table 1).
Subgroup analysis
We intended to perform the following subgroup analyses:
trials with low risk of bias compared to trials with high risk of bias;
major or minor liver resection;
cirrhotic or non‐cirrhotic liver;
different methods of autologous blood transfusion.
As all the trials had a high risk of bias and few trials were included under each outcome, we were not able to perform any subgroup analysis.
Bias exploration
We planned to use a funnel plot to explore bias (Egger 1997; Macaskill 2001) and to use asymmetry in the funnel plot of trial size against treatment effect to assess this bias. We also planned to perform the linear regression approach described by Egger 1997 to determine the funnel plot asymmetry. However, we performed neither of these because of the few trials included under each outcome.
Results
Description of studies
We identified a total of 1651 references through electronic searches of the Cochrane Hepato‐Biliary Group Controlled Trials Register and the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (n = 484), MEDLINE (n = 369), EMBASE (n = 438), and Science Citation Index Expanded (n = 360). We excluded 533 duplicates and 1105 clearly irrelevant references through reading the abstracts. Thirteen references were retrieved for further assessment. No reference was identified through scanning reference lists of the identified randomised trials. We excluded one reference for the reason listed under the table 'Characteristics of excluded studies'. Although one trial included patients undergoing liver resection, patients who required allogeneic transfusion and those who did not undergo any blood transfusion were excluded (Kostopanagiotou 2007). The review authors felt that this trial should not be included for any of the outcomes because such a trial is unlikely to provide any useful information on the outcomes included in this review. So, this trial was also excluded. Ten completed randomised trials described in 11 publications fulfilled the inclusion criteria and could provide data for the review. One trial had three arms and provided data for two comparisons (Yao 2006) (see section on haemodilution versus control). The remaining trials were two‐armed trials. The reference flow is shown in Figure 1. Details about the patients, interventions, reasons for post‐randomisation drop‐outs, and the methodological quality of the trials are shown in the table 'Characteristics of included studies'.
1.
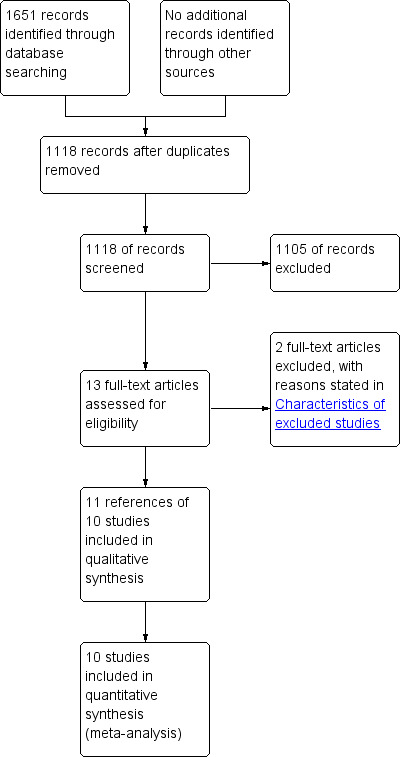
Study flow diagram.
Low central venous pressure (CVP) versus control
A total of 177 patients who underwent liver resection were randomised in three trials (El‐Kharboutly 2004; Wang 2006; Kato 2008) to low CVP (n = 88) versus control (n = 89). The number of participants in each trial was 40 (El‐Kharboutly 2004), 52 (Wang 2006), and 85 (Kato 2008). Two patients were excluded after randomisation in one trial (Wang 2006). The proportion of females and the mean age of participants in the trials that reported this were 30% (El‐Kharboutly 2004; Wang 2006) and 56.8 years (El‐Kharboutly 2004; Wang 2006; Kato 2008) respectively. The proportion of major liver resections in the two trials that stated this (El‐Kharboutly 2004; Wang 2006) was 37.8%. The proportion of cirrhotic livers in the two trials that stated this (El‐Kharboutly 2004; Wang 2006) was 60%.
Autologous blood donation versus control
A total of 80 adult living liver donors were randomised in one trial (Hashimoto 2007) to autologous blood donation (n = 40) versus control (n = 40). One patient from the control group who did not undergo surgery because of an asthmatic attack was excluded post‐randomisation from the analysis. Data were available for the remaining 79 patients. In the remaining patients, 38% were females. The median age in the two groups were 30 years and 37 years respectively. Seventy‐seven patients (97.5%) underwent major resection. All the patients had normal livers.
Haemodilution versus control
A total of 238 patients undergoing elective liver resection were randomised in three trials (Matot 2002; Jarnagin 2008; Guo 2010) to haemodilution (n = 117) or control (n = 121). The number of participants in each trial was 78 (Matot 2002), 130 (Jarnagin 2008), and 30 (Guo 2010). Five other patients were excluded post‐randomisation in one trial (Jarnagin 2008). The proportion of females was 48.7%. The mean age of the patients was 55.9 years. In one trial, all patients (n = 78) in both groups underwent major liver resection (Matot 2002). In another trial 85.4% of the 130 patients underwent major liver resection (Jarnagin 2008). The proportion of patients who underwent major liver resection was not stated in the third trial (Guo 2010). The two groups were evenly matched for the number of major liver resections in the two trials that gave this information (Matot 2002; Jarnagin 2008). None of the trials stated the proportion of cirrhotic livers.
A total of 30 patients undergoing liver resection were randomised to haemodilution with controlled hypotension (n =10), haemodilution (n =10), and to control (n = 10) (Yao 2006). The proportion of females was 46.7%. The mean age, proportion of major liver resections, and the proportion of cirrhotic livers were not stated in this trial.
We included 20 patients (haemodilution group and control group) for this comparison and considered haemodilution with controlled hypotension group as a separate intervention. So, we performed another comparison of haemodilution with controlled hypotension versus control.
Haemodilution with controlled hypotension versus control
The details of this group are stated in the previous comparison.
Haemodilution with bovine haemoglobin (HBOC‐201) versus hydroxy ethyl starch (HES)
A total of 12 patients who underwent elective liver resections were randomised in one trial (Standl 1998) to HBOC‐201 (n = 6) versus HES (n = 6). The proportion of females and the mean age of participants in the trials were 50% and 59 years respectively. The proportion of major liver resections was 41.7%. All the patients had normal livers.
Hypoventilation versus control
A total of 80 patients who underwent liver resections for removal of tumours were randomised in one trial (Hasegawa 2002) to hypoventilation (n = 40) versus control (n = 40). One patient from the control group who did not undergo liver resection was excluded from analysis after randomisation. The sex ratio was not stated. In the patients who underwent liver resection, the mean age was 65 years. The proportion of major liver resections was 32.9%. The proportion of cirrhotic livers was 44.3%.
Risk of bias in included studies
The sequence generation was adequate in four (40%) trials (Matot 2002; Hashimoto 2007; Jarnagin 2008; Kato 2008). The allocation concealment was adequate in two trials (20%) (Hashimoto 2007; Kato 2008). None of the trials achieved patient, healthcare provider, and outcome assessor blinding. Three trials were free from incomplete data outcome bias (30%) (Hasegawa 2002; Hashimoto 2007; Kato 2008). None of the trials were free from selective outcome reporting. Two trials were free from vested interest bias (10%) (Hashimoto 2007; Jarnagin 2008).
All the trials were considered to be at high risk of bias.
The risk of bias is summarised in Figure 2 and Figure 3.
2.
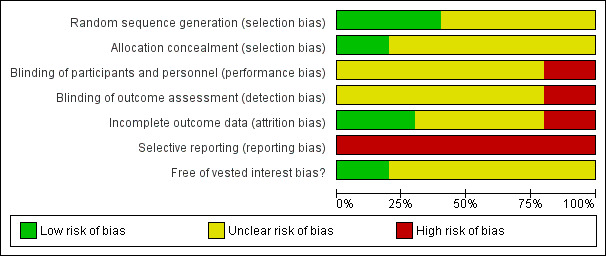
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.
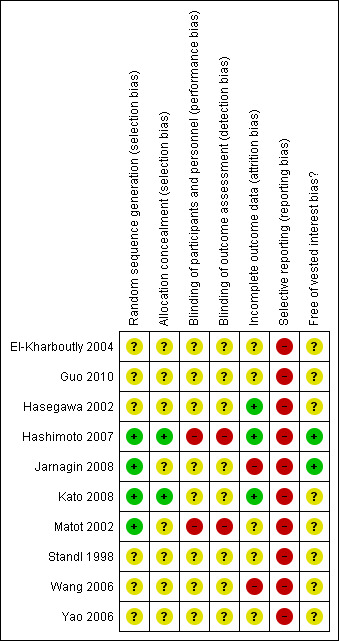
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Effects of interventions
See: Table 1
The summary measures used were risk ratio (RR), rate ratio (RaR), mean difference (MD), or standardised mean difference (SMD). The 95% confidence intervals (95% CI) are also stated.
Primary outcomes
Mortality
Peri‐operative mortality
There was no significant difference in the peri‐operative mortality in any of the comparisons (Analysis 1.1). There was no significant difference in the peri‐operative mortality in any of the comparisons: low CVP versus no intervention (3 trials (El‐Kharboutly 2004; Wang 2006; Kato 2008), no deaths in two trials (El‐Kharboutly 2004; Kato 2008) including 63 patients in the low CVP group and 62 patients in the no intervention group, 0/25 (0%) versus 1/27 (3.7%) in the third trial (Wang 2006), P = 1.000); autologous blood donation versus no intervention (1 trial (Hashimoto 2007), 0/40 (0%) versus 0/39 (0%)); haemodilution versus no intervention (2 trials (Yao 2006; Jarnagin 2008), no death in one trial (Yao 2006) including 10 patients in the haemodilution group and 10 patients in the no intervention group, 1/63 (1.4%) versus 3/67 (3.9%) in the second trial (Jarnagin 2008), P = 0.620); haemodilution with controlled hypotension versus no intervention (1 trial (Yao 2006), 0/10 (0%) versus 0/10 (0%)); haemodilution with bovine haemoglobin (HBOC‐201) versus haemodilution with hydroxy ethyl starch (HES) (1 trial (Standl 1998), 1/6 (16.7%) versus 0/6 (0%), P = 1.000); hypoventilation versus no intervention (1 trial (Hasegawa 2002), 0/40 (0%) versus 0/39 (0%)).
1.1. Analysis.
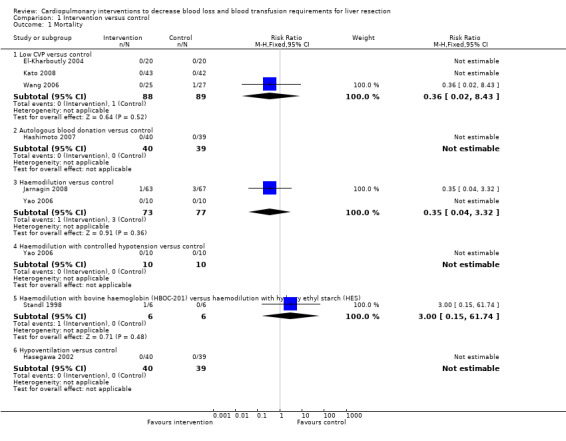
Comparison 1 Intervention versus control, Outcome 1 Mortality.
Long‐term survival
None of the trials reported long‐term survival.
Serious adverse events
There was no significant difference in the serious adverse events in any of the comparisons (Analysis 1.2). This was reported as major complications or serious complications in three trials (Standl 1998; Hashimoto 2007; Jarnagin 2008). There was no significant difference in the serious peri‐operative morbidity in any of the comparisons: autologous blood donation versus no intervention (1 trial (Hashimoto 2007), 1/40 (0.025 events per patient) versus 2/39 (0.051 events per patient), rate ratio 0.49 (95% CI 0.04 to 5.32), P = 0.56); haemodilution versus no intervention (2 trials (Matot 2002; Jarnagin 2008), 22/102 (weighted number of events per patient = 0.217) versus 23/106 (0.216 events per patient), rate ratio 1.01 (95% CI 0.57 to 1.80), P = 0.98); haemodilution with bovine haemoglobin (HBOC‐201) versus haemodilution with hydroxy ethyl starch (HES) (1 trial (Standl 1998), 1/6 (0.167 events per patient) versus 2/6 (0.333 events per patient), rate ratio 0.50 (95% CI 0.05 to 5.48), P = 0.57); hypoventilation versus no intervention (1 trial (Hasegawa 2002), 2/40 (0.050 events per patient) versus 1/39 (0.026 events per patient), rate ratio 1.95 (95% CI 0.18 to 21.35), P = 0.58).
1.2. Analysis.
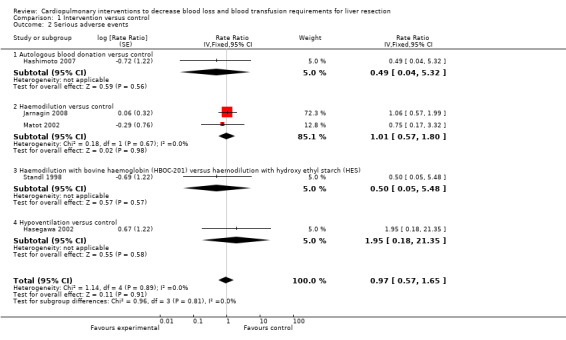
Comparison 1 Intervention versus control, Outcome 2 Serious adverse events.
All complications were listed in four trials (Hasegawa 2002; Matot 2002; El‐Kharboutly 2004; Wang 2006) (Analysis 1.3). However, the severity or treatment of these complications could not be identified for most of the complications. In two of these trials there were some serious complications (Hasegawa 2002; Matot 2002). Such serious complications were included in the outcome serious complications (Analysis 1.2). Thus these two trials featured in serious adverse events and adverse events of unknown severity with differing rate ratios (Analysis 1.2; Analysis 1.3). There was no significant difference in the rate of adverse events (severity unknown) in any of the comparisons (Analysis 1.3).
1.3. Analysis.
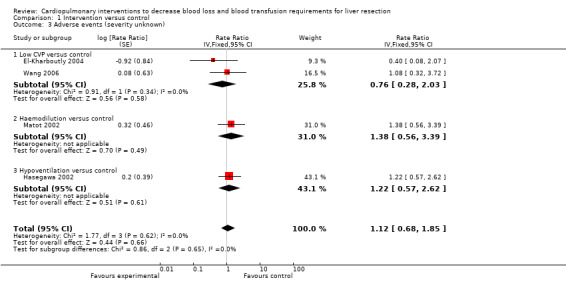
Comparison 1 Intervention versus control, Outcome 3 Adverse events (severity unknown).
Quality of life
This outcome was not reported in any of the trials.
Secondary outcomes
Transfusion requirements
See Analysis 1.4; Analysis 1.5; Analysis 1.6. Fresh frozen plasma requirements were reported only in the comparison of low CVP with control. None of the trials reported platelet transfusion in sufficient detail to be included in this review.
1.4. Analysis.
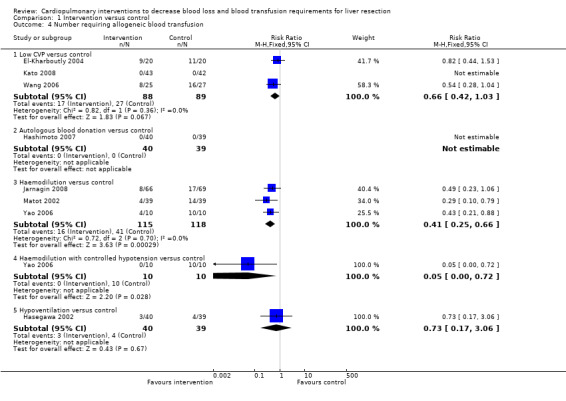
Comparison 1 Intervention versus control, Outcome 4 Number requiring allogeneic blood transfusion.
1.5. Analysis.
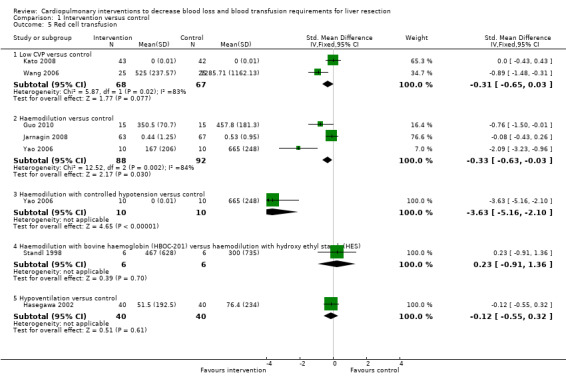
Comparison 1 Intervention versus control, Outcome 5 Red cell transfusion.
1.6. Analysis.
Comparison 1 Intervention versus control, Outcome 6 Fresh frozen plasma.
| Fresh frozen plasma | ||||
|---|---|---|---|---|
| Study | Mean (standard deviation) ml (intervention; 25 patients) | Mean (standard deviation) ml (control; 25 patients) | Mean difference (95% confidence intervals) | P‐value |
| Low central venous pressure versus control | ||||
| Wang 2006 | 437.5 (250.36) | 1057.14 (658.33) | ‐619.64 ml (‐895.73, ‐343.55) | < 0.001 |
Low CVP versus control
Three trials were included (El‐Kharboutly 2004; Wang 2006; Kato 2008). There was no significant difference in the proportion of patients requiring allogeneic blood transfusion between the groups (RR 0.72; 95% CI 0.45 to 1.14) (19.3% low CVP versus 28.1% control). In one of the trials two patients were excluded from the control group because of peri‐operative death and the procedure being abandoned because of unclear tumour demarcation. There were no significant differences in the results when adopting an intention‐to‐treat analysis based on best‐best, best‐worst, worst‐worst, and worst‐best scenarios (Gluud 2011; Higgins 2011). There was no significant difference in red cell transfusion between the groups in the two trials that reported this outcome (Wang 2006; Kato 2008) (SMD ‐0.31; 95% CI ‐0.65 to 0.03). The I2 measure of heterogeneity was 83%. The need for fresh frozen plasma (FFP containing clotting factors) was significantly lower in the low CVP group than in the control group (MD ‐619.64 ml; 95% CI ‐895.73 to ‐343.55) in the only trial that reported this outcome (Wang 2006).
Autologous blood donation versus control
One trial was included (Hashimoto 2007). None of the patients required allogeneic blood transfusion in this trial.
Haemodilution versus control
Four trials were included (Matot 2002; Yao 2006; Jarnagin 2008; Guo 2010). The number of patients requiring allogeneic blood transfusion was significantly lower in the haemodilution group than in the control group (RR 0.41; 95% CI 0.25 to 0.66) (14.3% haemodilution versus 35.3% control). There was no significant change in the results by adopting an intention‐to‐treat analysis based on best‐best, best‐worst, worst‐worst, and worst‐best scenarios (Gluud 2011; Higgins 2011). The amount of allogenic red cell transfusion was significantly lower in the haemodilution group than in the control group (SMD ‐0.33; 95% CI ‐0.63 to ‐0.03) (Yao 2006; Jarnagin 2008; Guo 2010). The I2 measure of heterogeneity was 84%.
Haemodilution with controlled hypotension versus control
One trial was included (Yao 2006). The number of patients requiring allogeneic blood transfusion was lower in the intervention group than in the control group (0% haemodilution with controlled hypotension versus 100% control; Fisher's exact test P < 0.0001). The mean allogenic red cell transfusion volume was also lower in the intervention group than in the control group (MD ‐665.00 ml; 95% CI ‐818.71 to ‐511.29).
Haemodilution with bovine haemoglobin (HBOC‐201) versus hydroxy ethyl starch (HES)
One trial was included (Standl 1998). The number of patients requiring transfusion was not stated in the trial. There was no significant difference in the amount of allogenic red cell transfusion between the groups (MD 167.00 ml; 95% CI ‐606.55 to 940.55).
Hypoventilation versus control
One trial was included (Hasegawa 2002). There was no significant difference in the number of patients requiring allogeneic blood transfusion (7.5% hypoventilation versus 10.3% control; Fisher's exact test P = 0.7119) or amount of blood transfused between the groups (MD ‐24.90 ml; 95% CI ‐118.80 to 69.00).
Blood loss
See Analysis 1.7.
1.7. Analysis.
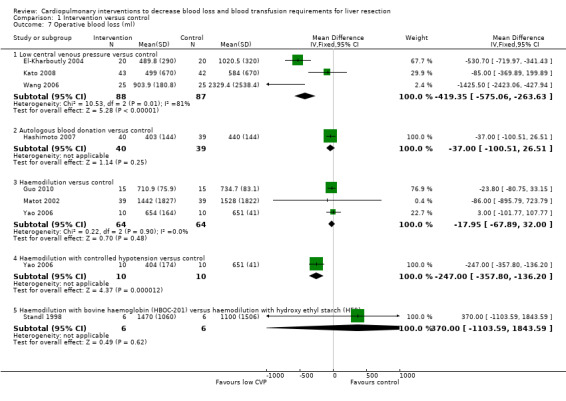
Comparison 1 Intervention versus control, Outcome 7 Operative blood loss (ml).
Low CVP versus control
Three trials were included (El‐Kharboutly 2004; Wang 2006; Kato 2008). The operative blood loss was statistically significantly lower in the low CVP group than in the control group (MD ‐419.35 ml; 95% CI ‐575.06 to ‐263.63) (El‐Kharboutly 2004; Wang 2006; Kato 2008).
Autologous blood donation versus control
One trial was included (Hashimoto 2007). There was no significant difference in the operative blood loss between the groups (MD ‐37.00 ml; 95% CI ‐100.51 to 26.51).
Haemodilution versus control
Four trials were included (Matot 2002; Yao 2006; Jarnagin 2008; Guo 2010). There was no significant difference in the operative blood loss between the groups (MD ‐17.95 ml; 95% CI ‐67.89 to 32.00) in the three trials that reported this outcome (Matot 2002; Yao 2006; Guo 2010).
Haemodilution with controlled hypotension versus control
One trial was included (Yao 2006). The operative blood loss was significantly lower in the intervention group than in the control group (MD ‐247.00 ml; 95% CI ‐357.80 to ‐136.20).
Haemodilution with bovine haemoglobin (HBOC‐201) versus hydroxy ethyl starch (HES)
One trial was included (Standl 1998). There was no significant difference in the operative blood loss between the groups (MD 370.00 ml; 95% CI ‐1103.59 to 1843.59).
Hypoventilation versus control
One trial was included (Hasegawa 2002). This trial did not report the operative blood loss.
Operating time
The operating time was significantly less in the intervention group than control in the following comparisons: low CVP versus control (El‐Kharboutly 2004; Wang 2006) (MD ‐24.69 minutes; 95% CI ‐44.28 to ‐5.09); and haemodilution versus control (Matot 2002; Jarnagin 2008) (MD ‐28.86 minutes; 95% CI ‐57.37 to ‐0.35). There was no significant difference in the operating time between the intervention and control groups in the comparison of haemodilution with bovine haemoglobin (HBOC‐201) versus hydroxy ethyl starch (HES). The operating time was not reported in the following comparisons: autologous blood donation versus control; and hypoventilation versus control (Analysis 1.8).
1.8. Analysis.
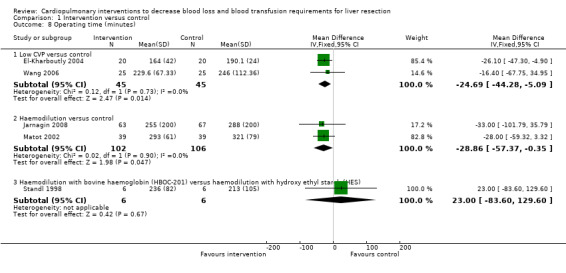
Comparison 1 Intervention versus control, Outcome 8 Operating time (minutes).
Hospital stay
The hospital stay was significantly lower in the intervention group than the control group in one comparison (low CVP versus control) (Wang 2006; Kato 2008) (MD ‐4.53 days; 95% CI ‐7.38 to ‐1.68). There was no significant difference in the hospital stay between the intervention and control groups in the following comparisons: haemodilution versus control; and haemodilution with bovine haemoglobin (HBOC‐201) versus hydroxy ethyl starch (HES). The hospital stay was not reported in the remaining comparisons (Analysis 1.9).
1.9. Analysis.
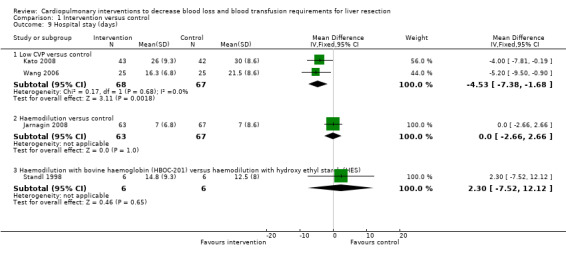
Comparison 1 Intervention versus control, Outcome 9 Hospital stay (days).
Variations in statistical analysis
There were no changes in results by adopting the random‐effects model in any of the comparisons with more than one trial. There was no change in results by calculating the risk difference for any of the dichotomous outcomes.
Subgroup analysis
We did not perform any subgroup analysis because of the few trials included under each category in this review.
Exploration of bias
We did not perform the funnel plot or the linear regression approach described by Egger 1997 to determine the funnel plot asymmetry because of the few trials included under each outcome.
Discussion
In this review, the safety and efficacy of cardiopulmonary interventions in reducing blood loss and allogeneic blood transfusion requirements have been evaluated. There was no significant difference in the mortality or morbidity between the intervention groups and controls. However, none of the trials were powered to identify differences in mortality or morbidity. The choice of which morbidity to report and which morbidity not to report varies from one report to another. Thus, it is not possible to make conclusions on the safety of these interventions from the present trials.
Low CVP reduces blood loss and fresh frozen plasma requirements but not red cell transfusion requirements. Although the CVP is reduced by venodilation, reduction of perfusion to important organs due to hypotension is avoided by using dopamine. There was no evidence of any complications resulting because of reduced CVP and prolonged hypotension but, as stated above, the trials were not designed to detect this. A reduced CVP may decrease the hepatic venous pressure resulting in a decrease in the blood loss. However, this has not translated into a reduction in the red cell transfusion requirement.
Autologous blood donation does not decrease operative blood loss. The main advantage of autologous blood donation is avoiding allogeneic blood transfusion. The only trial, which assessed autologous blood transfusion (Hashimoto 2007) reported that none of the patients in either group required allogeneic blood transfusion. Thus the benefit of autologous blood transfusion in decreasing allogeneic blood transfusion is not clear.
Haemodilution does not significantly decrease the operative blood loss. However, it has the potential to decrease the allogeneic blood transfusion as blood withdrawn as a part of haemodilution (that is, autologous blood donation used in conjunction with haemodilution) can be used first if necessary. Another reason is the loss of diluted blood rather than blood with high haematocrit resulting in the loss of fewer red blood cells although the volume lost is the same. The benefit appears to be greater if haemodilution is combined with controlled hypotension.
Hypoventilation was assessed as a method of decreasing blood loss because of its role in decreasing the CVP. In the only trial that assessed this intervention, hypoventilation was carried out only during the clamping phase of the intermittent portal triad clamping (PTC). While the mean CVP was lower during the clamping phase, the mean CVP during the entire operative procedure was the same between the hypoventilation group and the control group. The hepatic venous pressure increases to normal levels during the unclamping phase (Hasegawa 2002). This might be the reason for the apparent lack of benefit in hypoventilation.
The decreased operating time in some of the interventions, that is, low CVP versus control (El‐Kharboutly 2004; Wang 2006) and haemodilution versus control (Matot 2002; Jarnagin 2008) may be due to the quicker haemostasis achieved as the groups were matched for major and minor liver resections in the majority of cases. This may benefit the patient and also decrease the costs.
Most of the trials employed intermittent vascular occlusion. It is not clear whether the beneficial effects of the interventions will be increased or decreased in situations where vascular occlusion is not employed. Furthermore, the effect of a combination of interventions has to be assessed using adequately powered factorial trials.
However, there is a high risk of type I (erroneously concluding that an intervention is beneficial when it is actually not beneficial) and type II errors (erroneously concluding that an intervention is not beneficial when it is actually beneficial) because of the few trials included and the small sample size in each trial. Furthermore, the risks of type I errors are increased due to the many risks of bias (Wood 2008).
Authors' conclusions
Implications for practice.
None of the interventions seem to decrease peri‐operative morbidity or offer any long‐term survival benefit. Haemodilution shows promise in the reduction of blood loss and blood transfusion requirements, but it needs to be assessed in further trials.
Implications for research.
Randomised clinical trials with low risk of systematic errors and random errors are necessary to assess these cardiopulmonary interventions in patients undergoing liver resections. Trials need to be designed (factorial design) to assess the effect of a combination of different interventions in patients undergoing liver resections. Trials need to be conducted and reported according to the CONSORT statement (www.consort‐statement.org) (Moher 2001; Boutron 2008).
What's new
| Date | Event | Description |
|---|---|---|
| 2 February 2012 | New search has been performed | One new trial was identified and included in the review. The outcomes and risk of bias table were modified following the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). |
| 2 February 2012 | New citation required but conclusions have not changed | Adding data from one trial (Guo 2010) did not change the conclusions of the review published earlier (Gurusamy 2009). |
Notes
This is one of the two reviews written based on the protocol Non‐surgical interventions to decrease blood loss and blood transfusion requirements for liver resection' (Gurusamy 2008). This protocol was split into two reviews because of the comments from Editors.
Acknowledgements
To Bujar Osmani, Macedonia, who partially extracted the data for the review. To TC Mahendran, Chennai, India, who was the first author's first surgical teacher. To the Cochrane Hepato‐Biliary Group for the support that they have provided.
Peer Reviewers: Ingmar Königsrainer, Germany; Tahany Awad, Denmark. Contact Editor: Frederic Keus, the Netherlands.
Appendices
Appendix 1. Search strategies
| Database | Period of search | Search strategy used |
| The Cochrane Hepato‐Biliary Group Controlled Trials Register | January 2012. | (Blood loss OR bleeding OR hemorrhage OR haemorrhage OR hemorrhages OR haemorrhages OR hemostasis OR haemostasis OR transfusion) AND (((liver OR hepatic OR hepato) AND (resection OR segmentectomy)) OR hepatectomy) |
| Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library | Issue 1, 2012 | #1 Blood loss OR bleeding OR hemorrhage OR haemorrhage OR hemorrhages OR haemorrhages OR hemostasis OR haemostasis OR transfusion #2 MeSH descriptor Hemorrhage explode all trees #3 MeSH descriptor Blood Transfusion explode all trees #4 (#1 OR #2 OR #3) #5 liver OR hepatic OR hepato #6 MeSH descriptor Liver explode all trees #7 (#5 OR #6) #8 resection OR segmentectomy #9 (#7 AND #8) #10 hepatectomy #11 MeSH descriptor Hepatectomy explode all trees #12 (#9 OR #10 OR #11) #13 (#4 AND #12) |
| MEDLINE (Pubmed) | January 1947 to January 2012. | (Blood loss OR bleeding OR hemorrhage OR haemorrhage OR hemorrhages OR haemorrhages OR hemostasis OR haemostasis OR transfusion OR "Hemorrhage"[Mesh] OR "Blood Transfusion"[Mesh]) AND (((liver OR hepatic OR hepato OR "liver"[MeSH]) AND (resection OR segmentectomy)) OR hepatectomy OR "hepatectomy"[MeSH]) AND (((randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR ("clinical trial" [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw])) OR (placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp]) NOT (animals [mh] NOT human [mh])))) |
| EMBASE (Ovid SP) | January 1980 to January 2012. | 1 exp CROSSOVER PROCEDURE/ 2 exp DOUBLE BLIND PROCEDURE/ 3 exp SINGLE BLIND PROCEDURE/ 4 exp RANDOMIZED CONTROLLED TRIAL/ 5 (((RANDOM* or FACTORIAL* or CROSSOVER* or CROSS) and OVER*) or PLACEBO* or (DOUBL* and BLIND*) or (SINGL* and BLIND*) or ASSIGN* or ALLOCAT* or VOLUNTEER*).af. 6 1 or 2 or 3 or 4 or 5 7 exp BLEEDING/ 8 exp Blood Transfusion/ 9 (Blood loss or bleeding or hemorrhage or haemorrhage or hemorrhages or haemorrhages or hemostasis or haemostasis or transfusion).af. 10 8 or 7 or 9 11 (liver or hepatic or hepato).af. 12 (segmentectomy or resection).af. 13 11 and 12 14 hepatectomy.af. 15 exp Liver Resection/ 16 13 or 15 or 14 17 6 and 16 and 10 |
| Science Citation Index Expanded (http://portal.isiknowledge.com) | January 1970 to January 2012. | #1 TS=(Blood loss OR bleeding OR hemorrhage OR haemorrhage OR hemorrhages OR haemorrhages OR hemostasis OR haemostasis OR transfusion) #2 TS=(((liver OR hepatic OR hepato) AND (resection OR segmentectomy)) OR hepatectomy) #3 TS=(random* OR blind* OR placebo* OR meta‐analysis) #4 #3 AND #2 AND #1 |
Data and analyses
Comparison 1. Intervention versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Low CVP versus control | 3 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.43] |
| 1.2 Autologous blood donation versus control | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Haemodilution versus control | 2 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.04, 3.32] |
| 1.4 Haemodilution with controlled hypotension versus control | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.5 Haemodilution with bovine haemoglobin (HBOC‐201) versus haemodilution with hydroxy ethyl starch (HES) | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.15, 61.74] |
| 1.6 Hypoventilation versus control | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Serious adverse events | 5 | Rate Ratio (Fixed, 95% CI) | 0.97 [0.57, 1.65] | |
| 2.1 Autologous blood donation versus control | 1 | Rate Ratio (Fixed, 95% CI) | 0.49 [0.04, 5.32] | |
| 2.2 Haemodilution versus control | 2 | Rate Ratio (Fixed, 95% CI) | 1.01 [0.57, 1.80] | |
| 2.3 Haemodilution with bovine haemoglobin (HBOC‐201) versus haemodilution with hydroxy ethyl starch (HES) | 1 | Rate Ratio (Fixed, 95% CI) | 0.50 [0.05, 5.48] | |
| 2.4 Hypoventilation versus control | 1 | Rate Ratio (Fixed, 95% CI) | 1.95 [0.18, 21.35] | |
| 3 Adverse events (severity unknown) | 4 | Rate Ratio (Fixed, 95% CI) | 1.12 [0.68, 1.85] | |
| 3.1 Low CVP versus control | 2 | Rate Ratio (Fixed, 95% CI) | 0.76 [0.28, 2.03] | |
| 3.2 Haemodilution versus control | 1 | Rate Ratio (Fixed, 95% CI) | 1.38 [0.56, 3.39] | |
| 3.3 Hypoventilation versus control | 1 | Rate Ratio (Fixed, 95% CI) | 1.22 [0.57, 2.62] | |
| 4 Number requiring allogeneic blood transfusion | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Low CVP versus control | 3 | 177 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.42, 1.03] |
| 4.2 Autologous blood donation versus control | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Haemodilution versus control | 3 | 233 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.25, 0.66] |
| 4.4 Haemodilution with controlled hypotension versus control | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.72] |
| 4.5 Hypoventilation versus control | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.17, 3.06] |
| 5 Red cell transfusion | 7 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Low CVP versus control | 2 | 135 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.31 [‐0.65, 0.03] |
| 5.2 Haemodilution versus control | 3 | 180 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐0.63, ‐0.03] |
| 5.3 Haemodilution with controlled hypotension versus control | 1 | 20 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐3.63 [‐5.16, ‐2.10] |
| 5.4 Haemodilution with bovine haemoglobin (HBOC‐201) versus haemodilution with hydroxy ethyl starch (HES) | 1 | 12 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.23 [‐0.91, 1.36] |
| 5.5 Hypoventilation versus control | 1 | 80 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.55, 0.32] |
| 6 Fresh frozen plasma | Other data | No numeric data | ||
| 6.1 Low central venous pressure versus control | Other data | No numeric data | ||
| 7 Operative blood loss (ml) | 8 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Low central venous pressure versus control | 3 | 175 | Mean Difference (IV, Fixed, 95% CI) | ‐419.35 [‐575.06, ‐263.63] |
| 7.2 Autologous blood donation versus control | 1 | 79 | Mean Difference (IV, Fixed, 95% CI) | ‐37.0 [‐100.51, 26.51] |
| 7.3 Haemodilution versus control | 3 | 128 | Mean Difference (IV, Fixed, 95% CI) | ‐17.95 [‐67.89, 32.00] |
| 7.4 Haemodilution with controlled hypotension versus control | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐245.00 [‐357.80, ‐136.20] |
| 7.5 Haemodilution with bovine haemoglobin (HBOC‐201) versus haemodilution with hydroxy ethyl starch (HES) | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | 370.0 [‐1103.59, 1843.59] |
| 8 Operating time (minutes) | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Low CVP versus control | 2 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐24.69 [‐44.28, ‐5.09] |
| 8.2 Haemodilution versus control | 2 | 208 | Mean Difference (IV, Fixed, 95% CI) | ‐28.86 [‐57.37, ‐0.35] |
| 8.3 Haemodilution with bovine haemoglobin (HBOC‐201) versus haemodilution with hydroxy ethyl starch (HES) | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | 23.0 [‐83.60, 129.60] |
| 9 Hospital stay (days) | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 Low CVP versus control | 2 | 135 | Mean Difference (IV, Fixed, 95% CI) | ‐4.53 [‐7.38, ‐1.68] |
| 9.2 Haemodilution versus control | 1 | 130 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐2.66, 2.66] |
| 9.3 Haemodilution with bovine haemoglobin (HBOC‐201) versus haemodilution with hydroxy ethyl starch (HES) | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | 2.30 [‐7.52, 12.12] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
El‐Kharboutly 2004.
| Methods | Randomised clinical trial. | |
| Participants | Country: Egypt.
Number randomised: 40.
Post‐randomisation drop‐out: not stated.
Mean age: 51.1 years.
Females: 17 (42.5%).
Major liver resections: 25 (62.5%).
Cirrhotic livers: 40 (100%). Inclusion criteria: Elective liver resection. Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to two groups. Group 1: low CVP (n = 20). Group 2: control (n = 20). Further details of intervention: CVP maintained at 0 to 4 mm Hg using nitroglycerine infusion Sytemic hypotension was avoided Urine output was maintained at > 0.5 ml/Kg Other details: Vascular occlusion: intermittent PTC. Method of parenchymal transection: not stated. Management of raw surface: sutures, argon beam and infra‐red heating. Other co‐interventions to decrease blood loss: none reported. |
|
| Outcomes | The outcome measures were mortality, transfusion requirements, peri‐operative morbidity, operating time, blood loss, and liver function tests. | |
| Notes | Attempts to contact the authors in November 2008 were unsuccessful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were randomly (closed envelope method) divided into two groups...." Comment: It is not clear whether the authors used opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes such as liver failure were not reported. |
| Free of vested interest bias? | Unclear risk | Comment: This information was not available. |
Guo 2010.
| Methods | Randomised clinical trial. | |
| Participants | Country: China.
Sample size: 30.
Post‐randomisation drop‐out(s): not stated.
Mean age: 65 years.
Females: 8 (26.7%).
Major hepatic resection: not stated.
Cirrhosis: not stated. Inclusion criteria: 1. Patients undergoing liver resection for cancers 2. American Society of Anesthesiologists (ASA) physical status I‐II 3. Aged 60 to 70 years 4. Weighing 45 to 74 kg. Exclusion criteria: 1. Severe dysfunction of liver, kidney, or coagulation system 2. Severe pulmonary or cardiovascular diseases 3. Anticoagulation medication in the previous 2 weeks 4. Pre‐operative haematocrit > 35% and haemoglobin > 120 g/L. |
|
| Interventions | The patients were randomised to the following groups.
Group 1: acute normovolemic dilution (n = 15).
Group 2: no intervention (n = 15). Further details of the intervention: a volume of blood calculated based on the estimated blood volume, actual haematocrit, and ideal haemotocrit was collected and replaced by equal volume of hydroxy ethyl starch; the blood collected was transfused as appropriate. Other details: Vascular occlusion: not stated. Method of parenchymal transection: not stated. Management of raw surface: not stated. Other co‐interventions to decrease blood loss: not stated. |
|
| Outcomes | The outcomes reported were blood loss and transfusion requirements. | |
| Notes | Attempts were made to contact the author in January 2012. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Control group did not receive hemodilution during operation". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes were not reported. |
| Free of vested interest bias? | Unclear risk | Comment: This information was not available. |
Hasegawa 2002.
| Methods | Randomised clinical trial. | |
| Participants | Country: Japan.
Number randomised: 80.
Post‐randomisation drop‐out: 1 (1.3%) (see notes).
Mean age: 65 years.
Females: not stated.
Major liver resections: 26 (32.9%).
Cirrhotic livers: 35 (44.3%). Inclusion criteria: Liver resection for the removal of tumours. Exclusion criteria: Severe pulmonary dysfunction (< 70% vital capacity or FEV1/FVC < 60%). |
|
| Interventions | Participants were randomly assigned to two groups. Group 1: hypoventilation (n = 40) (see notes). Group 2: control (n = 40). Further details of intervention and control: Intervention: hypoventilation (4 ml/kg tidal volume; respiratory rate 15/min) only during clamping. Control: Ventilation at 10 ml/kg tidal volume; respiratory rate 10/min. Other details Vascular occlusion: intermittent PTC. Method of parenchymal transection: clamp crush or CUSA. Management of raw surface: not stated. Other co‐interventions to decrease blood loss: none reported. |
|
| Outcomes | The outcome measures were mortality, transfusion requirements, peri‐operative morbidity, hospital stay, blood loss, and liver function tests. | |
| Notes | One patient from intervention group who did not undergo liver resection was excluded from analysis. Attempts to contact the authors in November 2008 were unsuccessful. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: There was one post‐randomisation drop‐out. This patient did not undergo liver resection. This was not due to the treatment effect. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes such as liver failure were not reported. |
| Free of vested interest bias? | Unclear risk | Comment: This information was not available. |
Hashimoto 2007.
| Methods | Randomised clinical trial. | |
| Participants | Country: Japan.
Number randomised: 80.
Post‐randomisation drop‐outs: 1 (1.3%) (see notes).
Median age: 30 and 37 years in the two groups.
Females: 30 (38.0%).
Major liver resections: 77 (97.5%).
Cirrhotic livers: 0 (0%). Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to two groups. Group 1: autologous blood donation (n = 40). Group 2: control (n = 39) (See notes). Further details of intervention: Whole blood equal to 0.7% of body weight was withdrawn before start of hepatic parenchymal division and stored for re‐transfusion after graft procurement. Other details: Vascular occlusion: intermittent PTC. Method of parenchymal transection: CUSA. Management of raw surface: not stated. Other co‐interventions to decrease blood loss: none reported. |
|
| Outcomes | The outcome measures were mortality, transfusion requirements, peri‐operative morbidity, hospital stay, blood loss and liver function tests. | |
| Notes | One patient from control group in whom the surgery was cancelled because of asthmatic attack was excluded from analysis. Authors replied to questions related to mortality and transfusion requirements in November 2008. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The participants were randomly assigned in the operating room to either the blood salvage group (BS group) or a control group using a minimization method with 3 stratifying factors: age....." |
| Allocation concealment (selection bias) | Low risk | Quote: "The participants were randomly assigned in the operating room to either the blood salvage group (BS group) or a control group using a minimization method with 3 stratifying factors: age....." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "the surgical team and participants were blinded to the data throughout the study period." Comment: The anaesthetists were not blinded to the groups. This could have resulted in different managements for the two groups. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "the surgical team and participants were blinded to the data throughout the study period." Comment: The anaesthetists were not blinded to the groups. This could have resulted in different managements for the two groups. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: There was one post‐randomisation drop‐out. This patient did not undergo surgery and none of the outcomes could be measured or reported. This was not due to the treatment effect. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes such as liver failure were not reported. |
| Free of vested interest bias? | Low risk | Quote: "Supported by a grant from the Kanae Foundation for Life & Socio‐medical Science, a grant from the Public Trust Surgery Research Fund, a grant from the Japanese Clinical Oncology Fund, a grant from the Public Trust Haraguchi Memorial Cancer Research Fund in Japan, and a Grant‐in‐Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (No. 18790955)." |
Jarnagin 2008.
| Methods | Randomised clinical trial. | |
| Participants | Country: USA.
Number randomised: 135.
Post‐randomisation drop‐outs: 5 (3.7%).
Mean age: 53.5 years.
Females: 61 (46.9%).
Major liver resections: 111 (85.4%).
Cirrhotic livers: not stated. Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Participants were randomly assigned to two groups. Group 1: haemodilution (n = 63). Group 2: control (n = 67). Further details of intervention: haemodilution by withdrawing blood and replacing it with colloids to a target haemoglobin of 8 gm/dl. Other details: Vascular occlusion: intermittent PTC. Method of parenchymal transection:not stated. Management of raw surface: not stated. Other co‐interventions to decrease blood loss: low CVP in both groups. |
|
| Outcomes | The outcomes reported were mortality, transfusion requirements, peri‐operative morbidity, operating time, and hospital stay. | |
| Notes | There were 3 drop‐outs in ANH group and 2 in standard. The reason for drop‐outs were resection not performed in 2, resection smaller than required by the study in 2 and inability to accurately determine blood loss in 1 (author replies). Authors replied to questions related to methodological quality in November 2008. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The random sequence was computer generated" (author replies). |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Allocation was concealed by sealed envelopes" (author replies). Comment: It is not clear whether the authors used opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: There were 5 post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes such as liver failure were not reported. |
| Free of vested interest bias? | Low risk | Quote: "The authors thank ... Robert Wittes, MD, Physician‐in‐Chief, Memorial Hospital, for providing financial support." |
Kato 2008.
| Methods | Randomised clinical trial. | |
| Participants | Country: Japan.
Number randomised: 85.
Post‐randomisation drop‐outs: 0.
Mean age: 66 years.
Females: not stated.
Major liver resections: not stated.
Cirrhotic livers: not stated. Inclusion criteria: Liver resection. |
|
| Interventions | Participants were randomly assigned to two groups. Group 1: low CVP (n = 20). Group 2: control (n = 20). Further details of intervention: CVP was lowered by clamping the infra‐hepatic inferior vena cava. Other details: Vascular occlusion: intermittent PTC. Method of parenchymal transection: CUSA. Management of raw surface: diathermy, sutures, fibrin glue. Other co‐interventions to decrease blood loss: none reported. |
|
| Outcomes | The outcomes reported were mortality, transfusion requirements, and hospital stay. | |
| Notes | Attempts to contact the authors in November 2008 were unsuccessful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eighty‐five patients who underwent hepatic resection....randomly assigned to an IVC clamping or an IVC non‐clamping group by the minimization method with stratified factors of age, .." |
| Allocation concealment (selection bias) | Low risk | Quote: "Eighty‐five patients who underwent hepatic resection....randomly assigned to an IVC clamping or an IVC non‐clamping group by the minimization method with stratified factors of age, .." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: There were no post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes such as liver failure were not reported. |
| Free of vested interest bias? | Unclear risk | Unclear. |
Matot 2002.
| Methods | Randomised clinical trial. | |
| Participants | Country: Israel.
Number randomised: 78.
Post‐randomisation drop‐outs: not stated.
Mean age: 56.5 years.
Females: 47 (60.3%).
Major liver resections: 78 (100%).
Cirrhotic livers: not stated Inclusion criteria:
Exclusion criteria: Severe liver dysfunction. |
|
| Interventions | Participants were randomly assigned to two groups. Group 1: haemodilution (n = 39). Group 2: control (n = 39). Further details of intervention: haemodilution by withdrawing blood and replacing it with colloids to a target hematocrit of 24%. Other details: Vascular occlusion: not stated. Method of parenchymal transection: not stated. Management of raw surface: not stated. Other co‐interventions to decrease blood loss: none reported. |
|
| Outcomes | The outcome measures were transfusion requirements, peri‐operative morbidity, operating time, blood loss, and liver function tests. | |
| Notes | Attempts to contact the authors in November 2008 were unsuccessful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "On admission to the operating room, patients who met inclusion criteria were randomly assigned (random numbers) to one of two groups..." |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The anaesthesiologist making decisions regarding transfusion was not blinded to patient group assignment." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The anaesthesiologist making decisions regarding transfusion was not blinded to patient group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes such as liver failure were not reported. |
| Free of vested interest bias? | Unclear risk | Comment: This information was not available. |
Standl 1998.
| Methods | Randomised clinical trial. | |
| Participants | Country: Germany.
Number randomised: 12.
Post‐randomisation drop‐outs: not stated.
Mean age: 59 years.
Females: 6 (50.0%).
Major liver resections: 5 (41.7%).
Cirrhotic livers: 0 (0%). Inclusion criteria: Elective liver resection. Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to two groups. Group 1: HBOC‐201 (n = 6). Group 2: control (n = 6). Further details of intervention and control: Pre‐operative haemodilution was performed after induction of anaesthesia (1 litre autologous blood donation followed by 2 litres of RL) followed by intervention or control within 30 minutes. Other details: Vascular occlusion: not stated. Method of parenchymal transection: not stated. Management of raw surface: not stated. Other co‐interventions to decrease blood loss: none reported. |
|
| Outcomes | The outcome measures were mortality, transfusion requirements, peri‐operative morbidity, operating time, hospital stay, blood loss, and liver function tests. | |
| Notes | Attempts to contact the authors in November 2008 were unsuccessful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes such as liver failure were not reported. |
| Free of vested interest bias? | Unclear risk | Comment: This information was not available. |
Wang 2006.
| Methods | Randomised clinical trial. | |
| Participants | Country: China.
Number randomised: 52
Post‐randomisation drop‐outs: 2 (3.8%) (see notes).
Mean age: 45.7 years.
Females: 10 (20%).
Major liver resections: 17 (34%).
Cirrhotic livers: 29 (58%). Inclusion criteria: Liver resection. |
|
| Interventions | Participants were randomly assigned to two groups. Group 1: low CVP (n = 25). Group 2: control (n = 27). Further details of intervention: CVP maintained at 2 to 4 mm Hg using nitroglycerine infusion, furosemide and by limiting volume of infusion. Systolic blood pressure was maintained at > 90 mm Hg using dopamine infusion. Trendelenburg's position (15 degree tilt) was used to avoid the risk of air embolism. Other details: Vascular occlusion: no vascular occlusion for cirrhotic livers; PTC or selective vascular occlusion for non‐cirrhotic livers. Method of parenchymal transection: blunt division. Management of raw surface: ligatures and fibrin glue. Other co‐interventions to decrease blood loss: none reported. |
|
| Outcomes | The outcome measures were mortality, transfusion requirements, peri‐operative morbidity, operating time, hospital stay, blood loss, and liver function tests. | |
| Notes | Two patients from control group in whom per‐operative death occurred (n = 1) and in whom the procedure was abandoned because of unclear tumour demarcation (n = 1) were excluded from analysis. Attempts to contact the authors in November 2008 were unsuccessful. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "By the sealed envelope method, the patients were blindly randomised into LCVP group....". Comment: It is not clear whether the authors used opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 2 patients were excluded post‐randomisation. This could be related to the treatment effect. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes such as liver failure were not reported. |
| Free of vested interest bias? | Unclear risk | Comment: This information was not available. |
Yao 2006.
| Methods | Randomised clinical trial. | |
| Participants | Country: China.
Number randomised: 30.
Post‐randomisation drop‐outs: not stated.
Mean age: not stated.
Females: 14 (46.7%).
Major liver resections: not stated.
Cirrhotic livers: not stated. Inclusion criteria:
|
|
| Interventions | Participants were randomly assigned to three groups. Group 1: acute normovolemic haemodilution with hypotension (n = 10). Group 2: acute normovolemic haemodilution without hypotension (n = 10). Group 3: control (n = 10). Further details of intervention: Group 1: dilution with crystalloid solution (10ml/kg), autologous blood donation (replaced with 6% HES), and reduced the MAP to 70% with 0.01% sodium nitroprusside (1 mcg/kg/min). Group 2: dilution with crystalloid solution (10ml/kg) and autologous blood donation (replaced with 6% HES). Group 3: control. Other details: Vascular occlusion: not stated. Method of parenchymal transection: not stated. Management of raw surface: not stated. Other co‐interventions to decrease blood loss: none reported. |
|
| Outcomes | The outcomes reported were mortality, transfusion requirements, and operative blood loss. | |
| Notes | Attempts to contact the authors in December 2008 were unsuccessful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Selective reporting (reporting bias) | High risk | Comment: Important outcomes such as liver failure were not reported. |
| Free of vested interest bias? | Unclear risk | Comment: This information was not available. |
CUSA = cavitron ultrasonic surgical aspirator CVP = central venous pressure MAP = mean arterial pressure PTC = portal triad clamping
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bower 2011 | No separate data were available for patients undergoing liver resection. |
| Kostopanagiotou 2007 | This trial compared plasma fibronectin levels in liver resection patients who underwent pre‐operative autologous blood donation and controls. Patients who required allogenic blood transfusion and who did not require any transfusion were excluded from analysis. The review authors felt that none of the information in this trial will be useful for the purposes of this review. |
Differences between protocol and review
This is one of the two reviews written based on the protocol 'Non‐surgical interventions to decrease blood loss and blood transfusion requirements for liver resection'. This protocol was split into two reviews because of the comments from editors.
The outcomes are divided into primary and secondary outcomes.
For dichotomous outcomes with only one trial included under the comparison, we performed the Fisher's exact test.
Differences between the previously published review and this updated review version
The searches were updated. One trial was added (Guo 2010). The outcomes and bias risk were modified according to the version 5.1.0 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Contributions of authors
KS Gurusamy wrote the review and assessed the trials for inclusion and extracted data on included trials. J Li is the co‐author of the review and independently assessed the trials for inclusion and extracted data on included trials. J Vaughan identified new trials for the update, extracted the data for the new trials, and obtained the data for serious adverse events for all the trials. D Sharma and BR Davidson critically commented on the review and provided advice for improving the review.
Sources of support
Internal sources
University College London, UK.
External sources
National Insititute of Health Research, UK.
Declarations of interest
None known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
El‐Kharboutly 2004 {published data only}
- El‐Kharboutly WS, El‐Wahab MA. The role of adoption of low central venous pressure in hepatic resection with pringle manoeuvre in reducing blood loss and improving operative outcome. Egyptian Journal of Anaesthesia 2004;20(4):369‐76. [Google Scholar]
Guo 2010 {published data only}
- Guo JR, Yu J, Jin XJ, Du JM, Guo W, Yuan XH. Effects of acute normovolemic hemodilution on perioperative coagulation and fibrinolysis in elderly patients undergoing hepatic carcinectomy. Chinese Medical Sciences Journal 2010;25(3):146‐50. [DOI] [PubMed] [Google Scholar]
Hasegawa 2002 {published data only}
- Hasegawa K, Takayama T, Orii R, Sano K, Sugawara Y, Imamura H, et al. Effect of hypoventilation on bleeding during hepatic resection: a randomized controlled trial. Archives of Surgery 2002;137(3):311‐5. [DOI] [PubMed] [Google Scholar]
Hashimoto 2007 {published data only}
- Hashimoto T, Kokudo N, Orii R, Seyama Y, Sano K, Imamura H, et al. Intraoperative blood salvage during liver resection: A randomized controlled trial. Annals of Surgery 2007;245(5):686‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jarnagin 2008 {published data only}
- Jarnagin WR, Gonen M, Maithel SK, Fong Y, D'Angelica MI, Dematteo RP, et al. A prospective randomized trial of acute normovolemic hemodilution compared to standard intraoperative management in patients undergoing major hepatic resection. Annals of Surgery 2008;248(3):360‐9. [PUBMED: 18791356] [DOI] [PubMed] [Google Scholar]
Kato 2008 {published data only}
- Kato M, Kubota K, Kita J, Shimoda M, Rokkaku K, Sawada T. Effect of infra‐hepatic inferior vena cava clamping on bleeding during hepatic dissection: A prospective, randomized, controlled study. World Journal of Surgery 2008;32(6):1082‐7. [DOI] [PubMed] [Google Scholar]
Matot 2002 {published data only}
- Matot I, Scheinin O, Jurim O, Eid A. Effectiveness of acute normovolemic hemodilution to minimize allogeneic blood transfusion in major liver resections. Anesthesiology 2002;97(4):794‐800. [DOI] [PubMed] [Google Scholar]
Standl 1998 {published data only}
- Standl T, Burmeister MA, Horn EP, Wilhelm S, Knoefel WT, Schulte am Esch J. Bovine haemoglobin‐based oxygen carrier for patients undergoing haemodilution before liver resection. British Journal of Anaesthesia 1998;80(2):189‐94. [DOI] [PubMed] [Google Scholar]
- Standl T, Wilhelm S, Horn EP, Burmeister M, Gundlach M, Schulte am Esch J. [Preoperative hemodilution with bovine hemoglobin. Acute hemodynamic effects in liver surgery patients]. Der Anaesthesist 1997;46(9):763‐70. [DOI] [PubMed] [Google Scholar]
Wang 2006 {published data only}
- Wang WD, Liang LJ, Huang XQ, Yin XY. Low central venous pressure reduces blood loss in hepatectomy. World Journal of Gastroenterology 2006;12(6):935‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yao 2006 {published data only}
- Yao XH, Wang B, Xiao ZK, Zhou P, Chen CY, Qing ZH. [Acute normovolemic hemodilution combined with controlled hypotension in patients undergoing liver tumorectomy]. Journal of Southern Medical University 2006;26(6):828‐30. [PubMed] [Google Scholar]
References to studies excluded from this review
Bower 2011 {published data only}
- Bower MR, Ellis SF, Scoggins CR, McMasters KM, Martin RC. Phase II comparison study of intraoperative autotransfusion for major oncologic procedures. Annals of Surgical Oncology 2011;18(1):166‐73. [DOI] [PubMed] [Google Scholar]
Kostopanagiotou 2007 {published data only}
- Kostopanagiotou G, Pandazi A, Matsota P, Arkadopoulos N, Dalamanga N, Politou M, et al. Effect of packed red blood cells transfusion on plasma fibronectin during liver resection. Transfusion Medicine 2007;17(2):115‐8. [DOI] [PubMed] [Google Scholar]
Additional references
Asiyanbola 2008
- Asiyanbola B, Chang D, Gleisner AL, Nathan H, Choti MA, Schulick RD, et al. Operative mortality after hepatic resection: Are literature‐based rates broadly applicable?. Journal of Gastrointestinal Surgery 2008;12(5):842‐51. [DOI] [PubMed] [Google Scholar]
Belghiti 1993
- Belghiti J, Kabbej M, Sauvanet A, Vilgrain V, Panis Y, Fekete F. Drainage after elective hepatic resection. A randomized trial. Annals of Surgery 1993;218(6):748‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boutron 2008
- Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P. Methods and processes of the CONSORT Group: example of an extension for trials assessing nonpharmacologic treatments. Annals of Internal Medicine 2008;148(4):W60‐6. [PUBMED: 18283201] [DOI] [PubMed] [Google Scholar]
Chen 2006
- Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Annals of Surgery 2006;243(3):321‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chouker 2004
- Chouker A, Schachtner T, Schauer R, Dugas M, Lohe F, Martignoni A, et al. Effects of Pringle manoeuvre and ischaemic preconditioning on haemodynamic stability in patients undergoing elective hepatectomy: a randomized trial. British Journal of Anaesthesia 2004;93(2):204‐11. [DOI] [PubMed] [Google Scholar]
DeMets 1987
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey SG, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed) 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frilling 2005
- Frilling A, Stavrou GA, Mischinger HJ, Hemptinne B, Rokkjaer M, Klempnauer J, et al. Effectiveness of a new carrier‐bound fibrin sealant versus argon beamer as haemostatic agent during liver resection: a randomised prospective trial. Langenbecks Archives of Surgery 2005;390(2):114‐20. [DOI] [PubMed] [Google Scholar]
Garden 2006
- Garden OJ, Rees M, Poston GJ, Mirza D, Saunders M, Ledermann J, et al. Guidelines for resection of colorectal cancer liver metastases. Gut 2006;55 Suppl 3:iii1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gluud 2011
- Gluud C, Nikolova D, Klingenberg SL, Alexakis N, Als‐Nielsen B, Colli A, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2011, Issue 12. Art. No.: LIVER.
Gomez 2008
- Gomez D, Morris‐Stiff G, Wyatt J, Toogood GJ, Lodge JP, Prasad KR. Surgical technique and systemic inflammation influences long‐term disease‐free survival following hepatic resection for colorectal metastasis. Journal of Surgical Oncology 2008;98(5):371‐6. [PUBMED: 18646038] [DOI] [PubMed] [Google Scholar]
GradePro 3.6 [Computer program]
- Brozek JL, Oxman A, Schünemann HJ. GRADEProfiler. Version 3.6. GRADE Working Group, 2011.
Gurusamy 2008
- Gurusamy KS, Osmani B, Sharma D, Davidson BR. Non‐surgical interventions to decrease blood loss and blood transfusion requirements for liver resection. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD007338] [DOI] [Google Scholar]
Gurusamy 2009a
- Gurusamy KS, Kumar Y, Sharma D, Davidson BR. Vascular occlusion for elective liver resections. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD006409.pub2] [DOI] [PubMed] [Google Scholar]
Gurusamy 2009b
- Gurusamy KS, Li J, Sharma D, Davidson BR. Pharmacological interventions to decrease blood loss and blood transfusion requirements for liver resection. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD008085] [DOI] [PubMed] [Google Scholar]
Gurusamy 2012
- Gurusamy KS, Davidson BR. Topical haemostatic agents in liver resection. Cochrane Database of Systematic Reviews (under preparation). [Google Scholar]
HES 2011
- Hospital Episode Statistics. Main procedures and interventions: 3 character 2010‐11. http://www.hesonline.nhs.uk/Ease/servlet/ContentServer?siteID=1937&categoryID=215 (accessed 03 February 2012).
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
IARC 2010
- International Agency for Research on Cancer (World Health Organization). GLOBOCAN 2008. Cancer incidence and mortality worldwide in 2008. http://globocan.iarc.fr/ 2010 (accessed on 27th August 2011).
Ibrahim 2006
- Ibrahim S, Chen CL, Lin CC, Yang CH, Wang CC, Wang SH, et al. Intraoperative blood loss is a risk factor for complications in donors after living donor hepatectomy. Liver Transplantation 2006;12(6):950‐7. [DOI] [PubMed] [Google Scholar]
ICH‐GCP 1997
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice1997 CFR & ICH Guidelines. Vol. 1, PA 19063‐2043, USA: Barnett International/PAREXEL, 1997. [Google Scholar]
Kitagawa 2001
- Kitagawa K, Taniguchi H, Mugitani T, Koh T, Obayashi T, Kunishima S, et al. Safety and advantage of perioperative autologous blood transfusion in hepatic resection for hepatocellular carcinoma. Anticancer Research 2001;21(5):3663‐7. [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Llovet 2005
- Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Seminars in Liver Disease 2005;25(2):181‐200. [DOI] [PubMed] [Google Scholar]
Macaskill 2001
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20(4):641‐54. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomised trials. Lancet 2001;357(9263):1191‐4. [PUBMED: 11323066] [PubMed] [Google Scholar]
Newell 1992
- Newell DJ. Intention‐to‐treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837‐41. [DOI] [PubMed] [Google Scholar]
Poon 2001
- Poon RT, Fan ST, Lo CM, Ng IO, Liu CL, Lam CM, et al. Improving survival results after resection of hepatocellular carcinoma: a prospective study of 377 patients over 10 years. Annals of Surgery 2001;234(1):63‐70. [PUBMED: 11420484] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Shimada 1998
- Shimada M, Takenaka K, Fujiwara Y, Gion T, Shirabe K, Yanaga K, et al. Risk factors linked to postoperative morbidity in patients with hepatocellular carcinoma. The British Journal of Surgery 1998;85(2):195‐8. [DOI] [PubMed] [Google Scholar]
Shinozuka 2000
- Shinozuka N, Koyama I, Arai T, Numajiri Y, Watanabe T, Nagashima N, et al. Autologous blood transfusion in patients with hepatocellular carcinoma undergoing hepatectomy. American Journal of Surgery 2000;179(1):42‐5. [DOI] [PubMed] [Google Scholar]
StatsDirect 2.7 [Computer program]
- StatsDirect Ltd. StatsDirect Statistical software. Version 2.7.2. StatsDirect Ltd, 2008.
Strasberg 2000
- Strasberg SM, Belghiti J, Clavien PA, Gadzijev E, Garden JO, Lau WY, et al. The Brisbane 2000 terminology of liver anatomy and resections. HPB Surgery 2000;2(3):333‐9. [Google Scholar]
Sweeting 2004
- Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta‐analysis of sparse data. Statistics in Medicine 2004;23(9):1351‐75. [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical Research Ed.) 2008;336(7644):601‐5. [PUBMED: 18316340] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yoshimura 2004
- Yoshimura Y, Kubo S, Shirata K, Hirohashi K, Tanaka H, Shuto T, et al. Risk factors for postoperative delirium after liver resection for hepatocellular carcinoma. World Journal of Surgery 2004;28(10):982‐6. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Gurusamy 2009
- Gurusamy KS, Li J, Sharma D, Davidson BR. Cardiopulmonary interventions to decrease blood loss and blood transfusion requirements for liver resection.. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD007338.pub2] [DOI] [PubMed] [Google Scholar]


