Abstract
Background
Vitamin K deficiency can cause bleeding in an infant in the first weeks of life. This is known as Hemorrhagic Disease of the Newborn (HDN). HDN is divided into three categories: early, classic and late HDN. Early HDN occurs within 24 hours post partum and falls outside the scope of this review. Classic HDN occurs on days one to seven; common bleeding sites are gastrointestinal, cutaneous, nasal and from a circumcision. Late HDN occurs from week 2‐12; the most common bleeding sites are intracranial, cutaneous, and gastrointestinal.
Vitamin K is commonly given prophylactically after birth for the prevention of HDN, but the preferred route is uncertain.
Objectives
To review the evidence from randomized trials in order to determine the effectiveness of vitamin K prophylaxis in the prevention of classic and late HDN. Main questions are: Is one dose of vitamin K, given after birth, able to significantly reduce the incidence of classic and late HDN? Is there a significant difference between the oral route and the intramuscular route in preventing classic and late HDN? Are multiple oral doses of vitamin K, given after birth, able to significantly reduce the incidence of classic and late HDN?
Search methods
The standard search strategy of the Cochrane Neonatal Review Group was used.
Selection criteria
All trials using random or quasi‐random patient allocation, in which methods of vitamin K prophylaxis in infants were compared to each other, placebo or no treatment, were included.
Data collection and analysis
Data were extracted independently by each author and were analysed with the standard methods of the Cochrane Collaboration and its Neonatal Review Group, using relative risk, risk difference and weighted mean difference.
Main results
Two eligible randomized trials, each comparing a single dose of intramuscular vitamin K with placebo or nothing, assessed effect on clinical bleeding. One dose of vitamin K reduced clinical bleeding at 1‐7 days, including bleeding after circumcision, and improved biochemical indices of coagulation status. Eleven additional eligible randomized trials compared either a single oral dose of vitamin K with placebo or nothing, a single oral with a single intramuscular dose of vitamin K, or three oral doses with a single intramuscular dose. None of these trials assessed clinical bleeding. Oral vitamin K improved biochemical indices of coagulation status at 1‐7 days. There was no evidence of a difference between the oral and intramuscular route in effects on biochemical indices of coagulation status. A single oral compared with a single intramuscular dose resulted in lower plasma vitamin K levels at two weeks and one month, whereas a 3‐dose oral schedule resulted in higher plasma vitamin K levels at two weeks and at two months than did a single intramuscular dose.
Authors' conclusions
A single dose (1.0 mg) of intramuscular vitamin K after birth is effective in the prevention of classic HDN. Either intramuscular or oral (1.0 mg) vitamin K prophylaxis improves biochemical indices of coagulation status at 1‐7 days. Neither intramuscular nor oral vitamin K has been tested in randomized trials with respect to effect on late HDN. Oral vitamin K, either single or multiple dose, has not been tested in randomized trials for its effect on either classic or late HDN.
Plain language summary
Prophylactic vitamin K for vitamin K deficiency bleeding in neonates
Vitamin K injection can prevent hemorrhagic disease of the newborn. Vitamin K helps the blood to clot but the body's capacity to store it is very low. Hemorrhagic disease of the newborn (HDN) is caused by a deficiency of Vitamin K in newborns and results in life‐threatening bleeding in an infant in the first hours to months of life. Classic HDN occurs on days one to seven and late HDN occurs from week two to 12. Some Vitamin K comes from the placenta but it is not always enough. The review of randomized trials found that a single injection of Vitamin K prevents classic HDN.
Background
Vitamin K deficiency can cause bleeding in an infant in the first hours to months of life. This is known as Hemorrhagic Disease of the Newborn (HDN) or Vitamin K Deficiency Bleeding (VKDB). The diagnosis is based on bleeding in an infant with a prolonged prothrombin time with a normal fibrinogen level and platelet count. A rapid correction of the prothrombin time and/or cessation of bleeding after vitamin K administration are confirmative.
Sutor 1999 have recently suggested that the term "Hemorrhagic Disease of the Newborn" (HDN) should be replaced by "Vitamin K Deficiency Bleeding" (VKDB), as neonatal bleeding is often not due to vitamin K deficiency and VKDB may occur after the 4‐week neonatal period. However, VKDB may occur at any age, and in this review we use the term HDN to include bleeding in neonates and infants up to 4 months of age due to inadequate activity of vitamin K‐dependent coagulation factors (II, VII, IX, X), correctable by vitamin K replacement.
HDN is divided into three categories: early, classic and late HDN. Early HDN occurs within 24 hours after birth, cannot be prevented by postnatal vitamin K prophylaxis and falls outside the scope of this review. Classic HDN occurs on days one to seven. Common bleeding sites are gastrointestinal, cutaneous, nasal and from a circumcision (Zipursky 1999). Late HDN occurs from week 2‐12. The most common bleeding sites in this latter condition are intracranial, cutaneous, and gastrointestinal (Hathaway 1991, Cornelissen 1997, von Kries 1998). The estimated incidence of late HDN in Europe is approximately five to seven per 100,000 live births (von Kries 1998, Cornelissen 1997).
HDN is caused by low plasma levels of vitamin K‐dependent clotting factors. In the newborn the plasma concentrations of these factors are normally 30‐60% of those of adults. They gradually reach adult values by six weeks of age. Administration of vitamin K immediately after birth prevents a further drop in these factors (Lane 1985, Zipursky 1999). Although placental transfer of vitamin K does occur, this is not always adequate. Administering vitamin K to mothers before birth does not seem to be a useful method to prevent deficiency in infants (Shearer 1992).
Vitamin K is necessary for synthesis of coagulation factors II (prothrombin), VII, IX and X in the liver. In the absence of vitamin K, the liver will synthesize inactive precursor proteins known as PIVKA's (proteins induced by the absence of vitamin K). Because vitamin K is fat‐soluble, it can only be absorbed from the intestine in the presence of bile salts. The body's capacity to store vitamin K is very low and the half life of the vitamin K‐ dependent coagulation factors is short (Zipursky 1999). Because of this, deficiency can occur quickly if the intake is not sufficient.
In nature there are two types of vitamin K: vitamin K1 or phylloquinone, which is found in foodstuffs like green vegetables, vegetable oils, and dairy products, and K2 or menaquinone, which is synthesized by the intestinal flora and only absorbed in small amounts. Vitamin K3 or menadione is a synthetic, water‐soluble vitamin, which is no longer used as a prophylaxis in most parts of the world, because of its potential to cause hemolytic anemia with jaundice. The only source of vitamin K in newborns is food because the bowel is still sterile and there is no synthesis of K2 by bacterial flora. The amount of vitamin K ingested differs with the way the child is fed. Breast milk contains lower amounts of vitamin K than modern formula milk or cow's milk and the risk of developing vitamin K deficiency is higher for the breast‐fed infant (Haroon 1982).
Trials that investigate HDN prophylaxis using vitamin K often measure biochemical outcomes rather than clinical ones, because of the low incidence of vitamin K deficiency bleeding in neonates and infants. The International Normalised Ratio, INR, compares the blood coagulation status of an individual to that of the normal population; thus, an INR >1 indicates that coagulation is slower than in the control group. The detection of PIVKA serves commonly as a biochemical outcome, with a high PIVKA level indicating vitamin K deficiency. The prothrombin time is dependent on several clotting factors, several of which are vitamin K dependent; thus, vitamin K deficiency results in a prolonged prothrombin time. The prothrombin index compares the prothrombin time of a subject to the normal prothrombin time. Prothrombin (antigen) is a measure for the serum level of prothrombin. Echis factor II and the ratio of factor II/ Echis factor II are outcomes that are rarely used. This method substitutes Echis carinatus snake venom for thromboplastin. A ratio of factor II/ Echis factor II is used to denote the amount of active prothrombin/ total prothrombin. When vitamin K is adequate, the ratio equals one. When vitamin K is deficient the ratio is <1 (O'Connor 1986).
In different parts of the world, various methods of vitamin K prophylaxis are practiced. The benefits of oral prophylaxis are that it is easy and non‐invasive and it can also be administered by midwives. The main disadvantages are that absorption is not certain and can be adversely affected by vomiting or regurgitation. If multiple doses are prescribed, compliance can be a problem (Croucher 1994). Intramuscular prophylaxis is more invasive than oral prophylaxis and can cause pain and a muscular hematoma at the site of the injection.
Since Golding et al. reported an increased risk of developing childhood cancer after parenteral vitamin K prophylaxis (Golding 1990, Golding 1992) this has been a cause of concern. After the reports by Golding et al. there have been several studies of this relationship. Recently Ross and Davies (Ross 2000) reviewed the evidence in this field. They found no randomized or quasi randomized evidence with regard to the association between parenteral vitamin K prophylaxis and cancer in childhood. Ten case‐control studies were identified, of which seven found no relationship and three found a weak relationship of neonatal administration of intramuscular or intravenous vitamin K with the risk of solid childhood tumors or leukemia. Taken as a whole, these studies do not establish a causal relationship between these routes of administration and an increased risk of cancer.
One systematic review of prophylactic vitamin K for newborn infants was published in 1996 (Brousson 1996). The authors studied both the randomized and non‐randomized evidence. They concluded that administering vitamin K intramuscularly to newborns is effective and safe, and that an oral regimen of three doses of 1 to 2 mg, the first given at the first feeding, the second at 2 to 4 weeks and the third at 8 weeks, may be an acceptable alternative but needs further testing in large clinical trials.
Objectives
Primary objective: To review the evidence from randomized trials in order to determine the effectiveness of vitamin K prophylaxis in the prevention of classic and late HDN. Main questions are: Is one dose of vitamin K, given after birth, able to significantly reduce the incidence of classic and late HDN? Is there a significant difference between the oral route and the intramuscular route in preventing classic and late HDN? Are multiple oral doses of vitamin K , given after birth, able to significantly reduce the incidence of classic and late HDN?
Secondary objectives: Subgroup analyses were to be carried out in breast fed and formula fed infants, attempting to answer the same questions as in the primary objective.
Methods
Criteria for considering studies for this review
Types of studies
All trials using random or quasi‐random patient allocation were included.
Types of participants
Studies that enrolled term and preterm infants were included.
Types of interventions
Different methods of vitamin K prophylaxis given to neonates within the first hours of life. All preparations ( i.e. vitamin K1, K2, K3 and mixed micellar vitamin K), routes and dose schedules ( i.e. single oral, single intramuscular, or multiple oral, or no vitamin K prophylaxis) were included.
Types of outcome measures
Primary clinical outcome measures were: 1. Classic HDN 2. Late HDN
Classic HDN was considered to be spontaneous bleeding or bleeding following circumcision after the first 24 hours but within the first week of life in previously healthy infants. Late HDN was considered to be spontaneous bleeding or bleeding following circumcision after the first week but within the first three months of life in previously healthy infants.
HDN was only considered an outcome of the study if the authors specifically mentioned this as a prespecified outcome and the infants were actively assessed for bleeding.
Secondary outcome measures included laboratory values and potential adverse effects of vitamin K prophylaxis. 1. Plasma Vitamin K1 2. PIVKA II levels 3. Activity of clotting factors II, VII and X 4. Prothrombin time and index 5. Partial thromboplastin time 6. Prothrombin (antigen) 7. Echis factor II 8. Ratio factor II/ Echis II 9. Plasma bilirubin levels 10. Muscular hematoma
Search methods for identification of studies
The standard search strategy of the Cochrane Neonatal Review Group was used. This included searches of the Oxford Database of Perinatal Trials (up to Jan. 2000), Cochrane Controlled Trials Register (2000 issue 1), MEDLINE (1966‐Jan 2000), previous reviews including cross references and abstracts. Disagreement was settled by consensus.
Data collection and analysis
Standard methods of the Cochrane Collaboration and its Neonatal Review Group were used. The methodological quality of each trial was reviewed independently by both authors.
Each author extracted the data separately. Data were compared and differences resolved. The standard methods of the Neonatal Review Group to synthesize data using relative risk (RR), risk difference (RD), and weighted mean difference (WMD) were used.
Results
Description of studies
We found 17 trials in our search; 13 studies were included after assessment (Bakhshi 1996, Cornelissen 1992, Greer 1998, Hathaway 1991, Jorgensen 1991, Malik 1992, Maurage 1995, Motohara 1985, O'Connor 1986, Sharma 1995, Sutherland 1967, Ulusahin 1996 and Vietti 1960). The details of each of these studies are given in the tables 'Characteristics of Included Studies' and 'References'. The trials by Hogenbirk 1993 and Schubiger 1993 are still awaiting assessment, because the data cannot be analysed as given in the studies. The authors will be contacted for extra data. We excluded two studies from this review (Arteaga‐Vizcaino 95 and Felbo 1965) because of uncertainty regarding method of allocation or poor methodological quality respectively. The exact reasons for exclusion of these studies are given in the table 'Characteristics of Excluded Studies'.
Types of participants: Almost all authors stated that they included in the trial only term infants or infants born without complications. Only Vietti 1960 does not mention this. Eight studies (Bakhshi 1996, Cornelissen 1992, Greer 1998, Malik 1992, Maurage 1995, O'Connor 1986, Sharma 1995, Ulusahin 1996) included only infants that were fully breast fed. In Motohara 1985 the infants were mainly breast fed. Sutherland 1967 studied both formula and breast fed infants. Only Jorgensen 1991 and Vietti 1960 did not mention the method of feeding.
Interventions: Seven studies (Bakhshi 1996, Cornelissen 1992, Jorgensen 1991, Malik 1992, Maurage 1995, Sharma 1995 and Ulusahin 1996) compared one dose of oral vitamin K with one dose of intramuscular vitamin K. The oral and intramuscular doses ranged from 1 mg to 5 mg; all doses were given within 12 hours post partum. One study (Greer 1998) compared three oral doses of 2 mg vitamin K1, given at birth, at 7 days and at 30 days with one dose of intramuscular vitamin K (1 mg). Four studies (Malik 1992, Motohara 1985, O'Connor 1986 and Sharma 1995) compared one dose of oral vitamin K with nothing or placebo; four studies (Malik 1992Sharma 1995, Sutherland 1967 and Vietti 1960) compared one dose of intramuscular vitamin K with placebo or nothing.
Five studies (Cornelissen 1992, Jorgensen 1991, Maurage 1995, O'Connor 1986, Ulusahin 1996) used vitamin K1; one study (Greer 1998) used mixed micellar vitamin K1; one study (Motohara 1985) used vitamin K2; five studies (Bakhshi 1996, Malik 1992, Sharma 1995, Sutherland 1967 and Vietti 1960) used the water soluble vitamin K3.
Outcome: Most of the studies mentioned that there were no cases of bleeding among infants included in the trial, without mentioning HDN as a prespecified outcome. In the present review HDN was only considered an outcome of the study if the authors specifically mentioned that the infants were assessed for bleeding. None of the studies had the power to detect clinically significant differences in incidence of HDN between treatment groups. The results in all these studies concern changes in prothrombin times, coagulation factors, plasma vitamin K levels and PIVKA II levels. A subsequent search for studies that relate these laboratory measurements to subsequent clinical outcomes produced no relevant studies. No randomized or quasi randomized evidence with regard to the association between parenteral vitamin K prophylaxis and cancer in childhood was found. Also we found no RCT's on the side effects of vitamin K prophylaxis, i.e. high plasma bilirubin levels or muscular hematomas. It was not possible to perform subgroup analyses on breast and bottle fed infants, since practically all the trials used bottle feeding as an exclusion criterion.
Risk of bias in included studies
Randomization: All 13 studies included in the review claimed to have used a random method of allocation to treatment. However, nine of them (Bakhshi 1996, Cornelissen 1992, Malik 1992, Maurage 1995, Motohara 1985, O'Connor 1986, Sharma 1995, and Ulusahin 1996) did not state how randomization had taken place.
Greer 1998 randomized the infants by computer allocation in blocks of four. Jorgensen 1991 used randomized envelopes. Sutherland 1967 used coded preparations to randomize the patients. Vietti 1960 conducted a "quasi‐random" study, treating infants depending on their day of birth. This could have influenced the results of the trial if inclusion in the study could have been influenced by the physician's knowledge of the child's date of birth.
Differences in groups at entry: Overall there were few significant differences between treatment and control groups after randomization in terms of birth weights, gestational ages, Apgar scores or the male/female ratio in the studies. In O'Connor 1986 the average birth weight of the oral group was significantly higher than in the group with no treatment. In Ulusahin 1996 the male/ female ratio was significantly higher in the oral group.
Blinding of intervention: It would have been technically and ethically difficult to use blinding of intervention when comparing an oral with an intramuscular regime, because it would involve injecting neonates with a placebo. The only studies that did blind the intervention were Sutherland 1967 and Vietti 1960. These studies compared intramuscular regimes with placebo or no intervention.
Blinding of outcome measurement: Cornelissen 1992, Greer 1998, Sutherland 1967 and Vietti 1960 did blind the outcome measurement. The other studies did not state whether the measurement of outcomes was blinded. Sutherland 1967 and Vietti 1960 were the only two trials with clinical outcomes.
Exclusions after randomization: There were many exclusions after randomization and losses to follow‐up. For exact data on this, see table, Characteristics of included studies.
Effects of interventions
1. Is one dose of vitamin K, given after birth, able to significantly reduce the incidence of classic and late HDN?
Contrast: intramuscular vitamin K vs. placebo or nothing We found five trials investigating this contrast: Malik 1992, Ulusahin 1996, Sharma 1995, Sutherland 1967, and Vietti 1960.
Primary outcomes: HDN/ VKDB The two trials with this outcome are Sutherland 1967 and Vietti 1960. Sutherland 1967 investigated all cases of bleeding at one to seven days and found a significant difference favoring prophylactic vitamin K, RR 0.73 (0.56, 0.96), RD ‐0.02 (‐0.04, 0.00). Vietti 1960 investigated bleeding after circumcision and found a significant difference favoring prophylactic vitamin K, RR 0.18 (0.08, 0.42), RD ‐0.11 (‐0.16, ‐0.07). A meta‐analysis of the data was not possible because these trials used different outcomes. Both trials support an effect of intramuscular vitamin K in preventing classic HDN.
Secondary outcomes: PIVKA II present The two trials with this outcome are Sharma 1995 and Ulusahin 1996. The pooled results of these trials show a significant reduction in the detection of PIVKA II at one to seven days favoring prophylactic vitamin K, RR 0.43 (0.26, 0.71), RD ‐0.49 (‐0.70, ‐0.28). Only Ulusahin 1996 analysed the infants at one month; none of the infants were PIVKA II positive.
Prothrombin index (% of normal) The only trial with this outcome was Malik 1992. Infants were analysed at one to seven days. The trial showed a significantly higher prothrombin index in the prophylactic vitamin K group, mean difference 14.4% (7.5, 21.3)
Contrast: Oral vitamin K vs. placebo or nothing
Primary outcomes: None of the trials specifically investigated the occurrence of HDN/ VKDB.
Secondary outcomes: PIVKA II present The three trials with this outcome are Motohara 1985, Sharma 1995, and Ulusahin 1996.
The pooled results of these trials show a significant reduction in the detection of PIVKA II at three days favoring prophylactic vitamin K, RR 0.40 (0.26, 0.61), RD ‐0.44 (‐0.60, ‐0.29). Motohara 1985 analysed the infants at five days as well and found a significant difference favoring prophylactic vitamin K, RR 0.22 (0.08, 0.56), RD ‐0.42 (‐0.59, ‐0.24). Only Ulusahin 1996 analysed the infants at one month; none of the infants were PIVKA II positive.
Prothrombin time (sec.) Only O'Connor 1986 analysed this outcome (at three days). This trial found a significant difference favoring the treatment group, mean difference ‐2.50 sec (‐4.26, ‐0.74).
Echis factor II Only O'Connor 1986 analysed this outcome (at three days). No significant difference between the groups was found.
Ratio factor II/ Echis II Only O'Connor 1986 analysed this outcome (at three days). This trial found a significant increase in the ratio in favor of the treatment group, mean difference 0.37 (0.06, 0.68).
Prothrombin index (% of normal) The only trial with this outcome was Malik 1992. Infants were analysed at one to seven days. The trial found a significantly higher prothrombin index in the prophylactic vitamin K group, mean difference 12.1% (4.7, 19.5)
2. Is there a significant difference between the oral route and the intramuscular route in preventing classic and late HDN?
Contrast: Single oral dose vs. intramuscular vitamin K
Primary outcomes: None of the trials specifically investigated the occurrence of HDN/ VKDB.
Secondary outcomes: PIVKA II present The four trials with this outcome are Bakhshi 1996, Cornelissen 1992, Sharma 1995, and Ulusahin 1996. At one to seven days the infants were analysed by Bakhshi 1996, Sharma 1995, and Ulusahin 1996. Neither the individual studies nor the pooled results showed significant differences. Only Cornelissen 1992 analysed the infants at two weeks; none of the infants were PIVKA II positive. At one month the infants were analysed in Cornelissen 1992 and Ulusahin 1996. Neither the individual studies nor the pooled results showed significant differences. At three months the infants were analysed in Cornelissen 1992. No significant difference was found.
PIVKA II levels (unit not mentioned) Only Jorgensen 1991 analysed this outcome (at one to seven days). No significant difference was found.
Plasma vitamin K1 (ng/ml) The two trials with this outcome are Cornelissen 1992 and Maurage 1995. At one to seven days only Maurage 1995 analysed this outcome. No significant difference between the groups was found. At two weeks only Cornelissen 1992 analysed this outcome. This trial showed a significant difference, with higher plasma levels in the intramuscular group, mean difference ‐0.79 ng/ml (‐1.02, ‐0.56). (Note: A one hundred fold difference was found in the values shown by Maurage 1995 at 1‐7 days compared to Cornelissen 1992 at two weeks. We cannot explain this large difference).
At one month both trials analysed this outcome. The pooled results of these trials showed a significant difference, with higher plasma levels in the intramuscular group, mean difference ‐0.23 ng/ml (‐0.30, ‐0.16).
Prolonged prothrombin time (>1.5x normal) Only Bakhshi 1996 analysed this outcome (at one to seven days). No infants had a prolonged prothrombin time.
Prothrombin (antigen) Only Jorgensen 1991 analysed this outcome (at one to seven days). No significant difference was found.
Prothrombin index Only Malik 1992 analysed this outcome (at one to seven days). No significant difference was found.
Combined activity of coagulation factors II, VII and X Only Jorgensen 1991 analysed this outcome (at one to seven days). No significant difference was found.
3. Are multiple oral doses of vitamin K , given after birth, able to significantly reduce the incidence of classic and late HDN?
Contrast: Three oral doses vs. intramuscular vitamin K
Primary outcomes: The only trial investigating this contrast is Greer 1998; this trial did not specifically investigate the occurrence of HDN/ VKDB.
Secondary outcomes: Plasma Vitamin K1 (ng/ml) At two weeks Greer 1998 found a significantly higher level in the oral group, mean difference 0.80 ng/ml (0.34, 1.27). At one month no significant difference was found. At three months Greer 1998 found a significantly higher level in the oral group, mean difference 0.30 ng/ml (0.10, 0.50).
INR No significant differences were found at two weeks, one month or three months.
Analyses that could not be done. We found no trials that investigated the side effects from the different types of vitamin K prophylaxis. Because of this we could not perform the planned analyses on serum bilirubin levels and muscular hematomas. Practically all the infants in the investigated studies were stated to be breast fed. Jorgensen 1991 did not state the method by which the infants were fed. We could not investigate the influence of feeding method on the effects of various forms of vitamin K administration. Because practically all infants in the included trials were term infants, the results may not be applicable to preterm infants.
Discussion
We retrieved two types of trial from the search. Two trials dealt with clinical outcomes, but mostly the trials dealt with surrogate, biochemical outcomes i.e. PIVKA II and plasma concentration of vitamin K. Overall, methodological quality was poor: randomisation procedures were often not described or inappropriate, and loss to follow up was high.
Some of the trials used different types of vitamin K (K1, K2, K3, and mixed micellar K1). We did not do subgroup analyses based on type of vitamin K. In a systematic literature search, we could find no evidence concerning the relation of type of vitamin K to its effectiveness in preventing HDN.
Some of the vitamin K doses compared in the trials are much higher than those commonly used in current practice. We found no evidence that a higher dose of vitamin K will improve coagulation status more than the commonly used dose of 1 mg. Also, we found no evidence that higher than physiological vitamin K plasma levels are better than physiological levels.
Because of the rarity of HDN, it would take an extremely large sample size to create significant results if clinical outcomes had been chosen in these trials. The follow‐up would also be a problem, because to identify all the cases of classic and late HDN, the patients would have to be observed for at least three months. Although a relationship between the surrogate outcomes and HDN is expected on biological grounds, no evidence could be found in the literature that proved this relationship.
We retrieved only one trial comparing a multiple oral dose regimen to a single intramuscular dose of vitamin K; the outcomes studied were Vitamin K1 levels and INRs. As the correlation of vitamin K levels with the child's coagulation status is not known, and the coagulation status does not necessarily correlate with clinical outcomes, we cannot conclude that a single oral dose is as good as a single intramuscular dose. Although the randomised evidence summarized in this review shows no evidence of a difference in this respect, it cannot be deduced that there is evidence of no difference.
In the absence of evidence from randomized trials on the effect of vitamin K, intramuscular or oral, on late HDN, evidence from observational studies of large cohorts can be turned to. We did not do a systematic review of such studies but we note that several descriptive studies have been published on the relationship of method of vitamin K prophylaxis and HDN or VKDB that had large sample sizes and dealt with clinical outcomes. Many of these studies report observational comparisons of different multiple oral dose regimens, either with each other or with intramuscular vitamin K. For example, in The Netherlands, all healthy, breastfed infants receive l mg of oral vitamin K directly after birth and then 25 mcg orally daily from week 1 to week 13. This scheme is believed to be safe and efficient (Cornelissen 1993), but there are no randomized controlled trials to support this.
Cornelissen 1997 summarized the findings of over 2,000,000 infants that had been followed in different countries and after different methods of prophylaxis. This summary found that the 95% confidence interval of the total incidence of late VKDB of groups of children that received 1 mg of intramuscular vitamin K overlapped with that of the group that received 1 mg of oral vitamin K directly after birth and then 25 mcg orally daily from week 1 to week 13. This could indicate that the latter scheme of prophylaxis is as effective as intramuscular prophylaxis. But since these studies were not randomized controlled trials, their validity can be doubted. For example, the infants were born in different countries and the participating countries observed the infants over different time periods between the 1960's and the 1990's. It is not known how this could have affected the results.
Recent observational studies describe the occurrence of classic and late VKDB after a new regimen was introduced (Schubiger 1999, Wariyara 2000, Cornelissen 1993). Since clinical decisions must be made on the basis of the best available evidence, current local recommendations on routine administration of vitamin K to newborns are based on these observational studies. It is no surprise that these recommendations differ in regard to the route of administration, and/or number of repeated oral doses.
In the absence of randomized evidence, the recent reviews by Brousson 1996, Cornelissen 1997, Ross 2000, von Kries 1999a, and Wariyara 2000 provide important information on which to base clinical decisions. Some of these reviews conclude that almost all cases of late VKDB are preventable with intramuscular phytomenadione prophylaxis administered once at birth, whereas a single oral dose given at birth may be less effective (Brousson 1996, von Kries 1999). Repeated oral phytomenadione doses given to breast‐fed infants either weekly (1 mg) or daily (25 mcg) seem to be as effective as intramuscular phytomenadione prophylaxis (Schubiger 1999). A daily supplement would mimic what happens to the bottle fed baby because all formula milks are artificially fortified (Wariyara 2000).
Maternal prophylaxis during breast feeding to enhance the content in breastmilk provides an alternative, rather more complex, strategy. So far, there is one randomized trial. This found that in exclusively breastfed infants who receive intramuscular phylloquinone at birth, the vitamin K status as measured by plasma phylloquinone and des‐gamma‐carboxy‐prothrombin concentrations is improved by maternal oral supplements of 5 mg/d phylloquinone through the first 12 weeks of life (Greer 1999). The efficacy of three oral two mg doses using the new mixed micellar preparation ('Konakion MM') remains to be established.
The method of prophylaxis that is chosen will depend not only on efficacy but on other factors as well. Cost will be an important factor in the decision for some countries. Another factor is the possibility for follow‐up if repeated oral doses are to be given. This is especially important when advising daily vitamin K supplements for the first three months of life. Another issue is the acceptability of performing an invasive procedure on a neonate if it has not been proven absolutely necessary.
Authors' conclusions
Implications for practice.
A single dose (1.0 mg) of intramuscular vitamin K after birth is effective in the prevention of classic HDN. Either intramuscular or oral (1.0 mg) vitamin K prophylaxis improves biochemical indices of coagulation status at 1‐7 days. Neither intramuscular nor oral vitamin K has been tested in randomized trials with respect to effect on late HDN. Oral vitamin K, either single or multiple dose, has not been tested in randomized trials for its effect on either classic or late HDN. Different methods of prophylaxis have been tested in randomized trials for their effect on coagulation status and vitamin K levels, but these outcomes are of uncertain clinical importance. When single doses of oral and intramuscular vitamin K are compared, the only difference found is lower plasma vitamin K levels in the oral group at two weeks. Despite this, there is no evidence of a difference in indices of coagulation status. When three doses of oral vitamin K are compared to a single dose of intramuscular vitamin K, the plasma vitamin K levels are higher in the oral group at two weeks and two months, but, again, there is no evidence of a difference in coagulation status.
Implications for research.
There have been several trials done on this topic. Practically all of them measured surrogate outcomes. It does not seem necessary to do more studies on these outcomes comparing oral with intramuscular administration since clinical outcomes provide stronger evidence. A randomized controlled trial comparing multiple oral doses of vitamin K with a single intramuscular dose and assessing clinical outcomes could provide important evidence on the effects of this method of prophylaxis, since it might be an alternative for the more invasive intramuscular prophylaxis. The outcomes of such a trial would have to be classic and late HDN and, because the method in which the infants are fed can affect the results, this will have to be taken into account. HDN is rare and to acquire significant results, the sample size of a trial would have to be extremely large, up to 500,000 participants, making the likelihood of such a trial ever being done seem extremely remote. A systematic review is needed of all observational studies of acceptable quality which examine the effects of different methods of vitamin K prophylaxis on classic and late HDN.
What's new
| Date | Event | Description |
|---|---|---|
| 16 October 2008 | Amended | Converted to new review format. |
Acknowledgements
Dr. Jack Sinclair Dr. Stuart Logan Dr. Michael Bracken Dr. Jeffrey Horbar The Dutch Cochrane Centre
Data and analyses
Comparison 1. IM vitamin K vs. placebo or nothing.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HDN/ VKDB | 1 | 3338 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.56, 0.96] |
| 1.1 At 1‐7 days | 1 | 3338 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.56, 0.96] |
| 2 Bleeding after circumcision | 1 | 470 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.08, 0.42] |
| 3 PIVKA II present | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 At 1‐7 days | 2 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.26, 0.71] |
| 3.2 At 1 month | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Prothrombin index (% of normal) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 14.40 [7.47, 21.33] |
| 4.1 At 1‐7 days | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 14.40 [7.47, 21.33] |
1.1. Analysis.
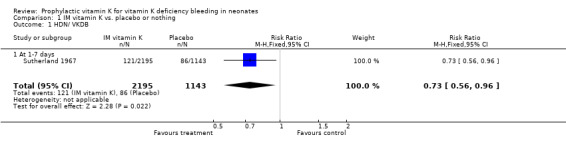
Comparison 1 IM vitamin K vs. placebo or nothing, Outcome 1 HDN/ VKDB.
1.2. Analysis.
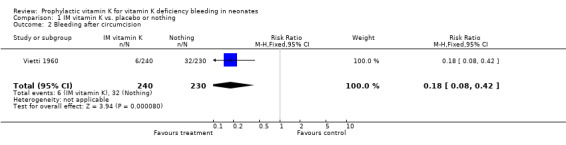
Comparison 1 IM vitamin K vs. placebo or nothing, Outcome 2 Bleeding after circumcision.
1.3. Analysis.
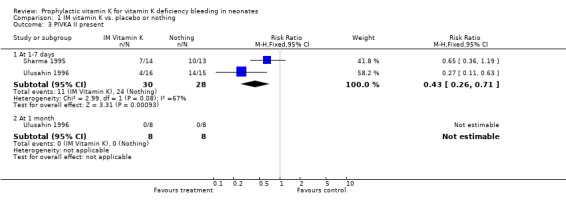
Comparison 1 IM vitamin K vs. placebo or nothing, Outcome 3 PIVKA II present.
1.4. Analysis.
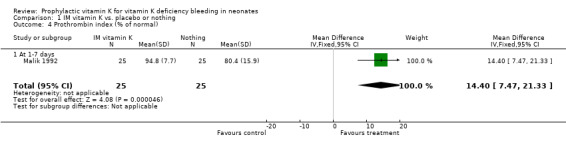
Comparison 1 IM vitamin K vs. placebo or nothing, Outcome 4 Prothrombin index (% of normal).
Comparison 2. Oral (single dose) vitamin K vs. placebo or nothing.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PIVKA II present | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 At 3 days | 3 | 118 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.26, 0.61] |
| 1.2 At 5 days | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.08, 0.56] |
| 1.3 At 1 month | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Prothrombin time (seconds) | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐2.5 [‐4.26, ‐0.74] |
| 2.1 At 3 days | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | ‐2.5 [‐4.26, ‐0.74] |
| 3 Echis factor II | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.01, 0.23] |
| 3.1 At 3 days | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.01, 0.23] |
| 4 Ratio factor II/ Echis II | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 0.37 [0.06, 0.68] |
| 4.1 At 3 days | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 0.37 [0.06, 0.68] |
| 5 Prothrombin index (% of normal) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 12.10 [4.70, 19.50] |
| 5.1 At 1‐7 days | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 12.10 [4.70, 19.50] |
2.1. Analysis.
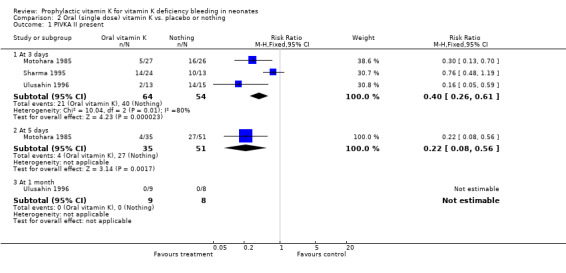
Comparison 2 Oral (single dose) vitamin K vs. placebo or nothing, Outcome 1 PIVKA II present.
2.2. Analysis.
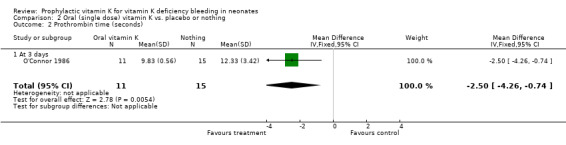
Comparison 2 Oral (single dose) vitamin K vs. placebo or nothing, Outcome 2 Prothrombin time (seconds).
2.3. Analysis.
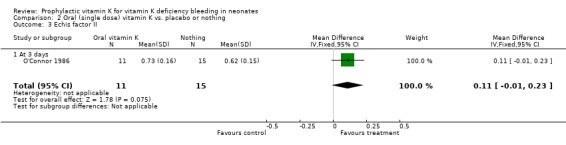
Comparison 2 Oral (single dose) vitamin K vs. placebo or nothing, Outcome 3 Echis factor II.
2.4. Analysis.
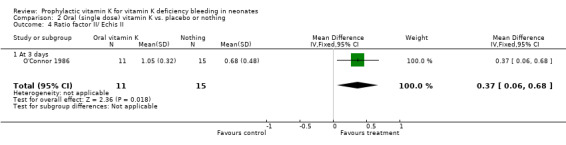
Comparison 2 Oral (single dose) vitamin K vs. placebo or nothing, Outcome 4 Ratio factor II/ Echis II.
2.5. Analysis.
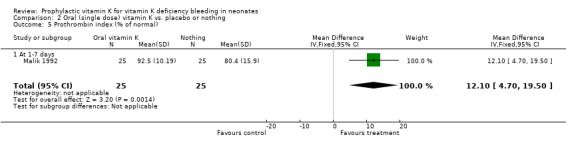
Comparison 2 Oral (single dose) vitamin K vs. placebo or nothing, Outcome 5 Prothrombin index (% of normal).
Comparison 3. Oral (single dose) vs. intramuscular vitamin K.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PIVKA II present | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 At 1‐7 days | 3 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.60, 1.76] |
| 1.2 At 2 weeks | 1 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 At 1 month | 2 | 279 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.82 [0.30, 26.78] |
| 1.4 At 3 months | 1 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.31, 2.11] |
| 2 PIVKA II levels | 1 | 220 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.00, 0.00] |
| 2.1 At 1‐7 days | 1 | 220 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.00, 0.00] |
| 3 Plasma vitamin K1 (ng/ml) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 At 1‐7 days | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | 36.89 [‐48.21, 121.99] |
| 3.2 At 2 weeks | 1 | 138 | Mean Difference (IV, Fixed, 95% CI) | ‐0.79 [‐1.02, ‐0.56] |
| 3.3 At 1 month | 2 | 200 | Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.30, ‐0.16] |
| 3.4 At 3 months | 1 | 119 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.12, 0.00] |
| 4 Prolonged prothrombin time (>1.5 normal) | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 At 1‐7 days | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Prothrombin antigen | 1 | 259 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.04, 0.04] |
| 5.1 At 1‐7 days | 1 | 259 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.04, 0.04] |
| 6 Prothrombin index (% of normal) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐2.30 [‐7.31, 2.71] |
| 6.1 At 1‐7 days | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐2.30 [‐7.31, 2.71] |
| 7 Coagulation factors II+ VII+ X (fraction of normal adult values) | 1 | 269 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.03, 0.05] |
| 7.1 At 1‐7 days | 1 | 269 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.03, 0.05] |
3.1. Analysis.
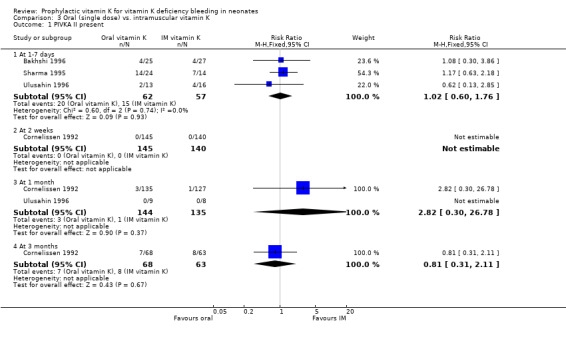
Comparison 3 Oral (single dose) vs. intramuscular vitamin K, Outcome 1 PIVKA II present.
3.2. Analysis.
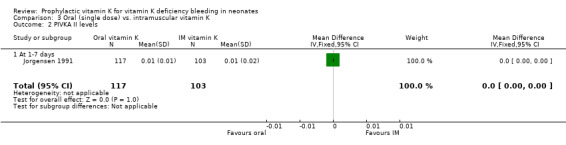
Comparison 3 Oral (single dose) vs. intramuscular vitamin K, Outcome 2 PIVKA II levels.
3.3. Analysis.
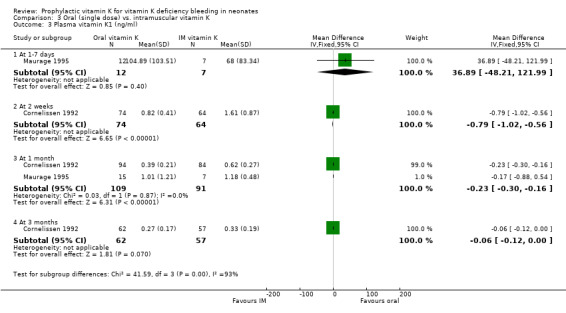
Comparison 3 Oral (single dose) vs. intramuscular vitamin K, Outcome 3 Plasma vitamin K1 (ng/ml).
3.4. Analysis.
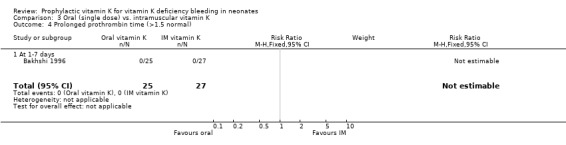
Comparison 3 Oral (single dose) vs. intramuscular vitamin K, Outcome 4 Prolonged prothrombin time (>1.5 normal).
3.5. Analysis.
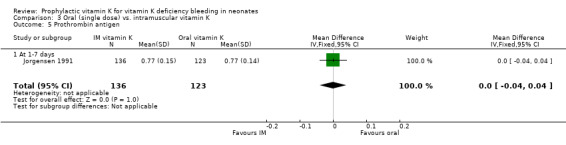
Comparison 3 Oral (single dose) vs. intramuscular vitamin K, Outcome 5 Prothrombin antigen.
3.6. Analysis.
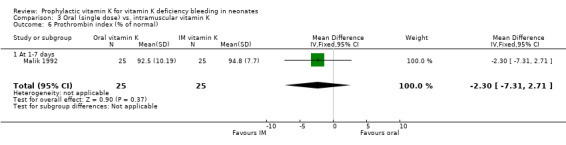
Comparison 3 Oral (single dose) vs. intramuscular vitamin K, Outcome 6 Prothrombin index (% of normal).
3.7. Analysis.
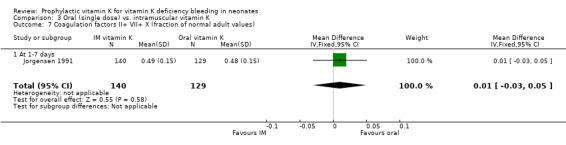
Comparison 3 Oral (single dose) vs. intramuscular vitamin K, Outcome 7 Coagulation factors II+ VII+ X (fraction of normal adult values).
Comparison 4. Oral (3 doses mixed micellar) vs. intramuscular vitamin K.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Plasma vitamin K1 (ng/ml) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 At 2 weeks | 1 | 135 | Mean Difference (IV, Fixed, 95% CI) | 0.8 [0.33, 1.27] |
| 1.2 At 1 month | 1 | 129 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.19, 0.19] |
| 1.3 At 2 months | 1 | 129 | Mean Difference (IV, Fixed, 95% CI) | 0.3 [0.10, 0.50] |
| 2 INR | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 At 2 weeks | 1 | 139 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.09, 0.11] |
| 2.2 At 1 month | 1 | 137 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.03, 0.07] |
| 2.3 At 2 months | 1 | 131 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.06, 0.04] |
4.1. Analysis.
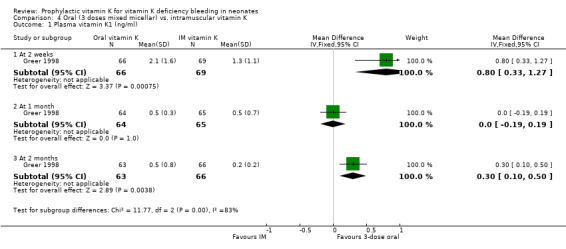
Comparison 4 Oral (3 doses mixed micellar) vs. intramuscular vitamin K, Outcome 1 Plasma vitamin K1 (ng/ml).
4.2. Analysis.
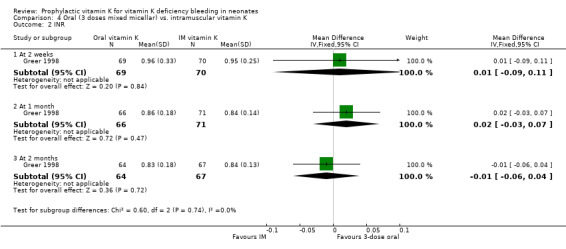
Comparison 4 Oral (3 doses mixed micellar) vs. intramuscular vitamin K, Outcome 2 INR.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bakhshi 1996.
| Methods | Randomisation: Block Single center. Blinding of outcome measurement: not stated Complete follow‐up: yes Two (7.5%) infants in the oral group were excluded for the use of top milk; no infants were excluded in the IM group. | |
| Participants | 54 infants were randomized, 27 in each group. They were exclusively breast fed, term infants with normal Apgar scores. Exclusion criteria: congenital malformations, IV fluids to infant, treatment of mother with drugs including phenobarbitone, anti‐coagulants and anti‐tubercular agents, maternal hypertension. Mean BW in kg (SD), oral group: 2.9 (0.2). IM group: 2.8 (0.3) Mean GA in wk (SD), oral group: 38.8 (1.0), IM group: 38.7 (1.3) | |
| Interventions | 2.0 mg of oral vitamin K3 (n=27) or 1.0 mg of IM vitamin K3 (n=27) both given within four hours after birth | |
| Outcomes | PIVKA‐II presence (>0.10 ‐ >0.13 AU/ml or > 2ng/ ml) and prolonged prothrombin time (>1.5 X normal) at 72 +/‐ 12 hours | |
| Notes | Water soluble vitamin K was used in this study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Cornelissen 1992.
| Methods | Randomisation: method not stated Single center and home births Blinding of outcome measurement: yes Infants were excluded because of cessation of breast feeding and sampling errors: at one month 10 infants in the oral group and 13 infants in the IM group, at three months 77 infants in the oral group and 77 in the IM group had been excluded. At two weeks 20 (12.1%) infants in the oral group and 26 (15.7%) in the IM group had been excluded or lost for follow‐up for the PIVKA analysis, at one month this was 30 (18.2%) and 39 (23.5%) infants, respectively, and at three months 97 (58.8%) and 103 (62.5%) infants, respectively. At two weeks 91 (55.2%) infants in the oral group and 102 (61.4%) in the IM group had been excluded or lost to follow‐up for the analysis of vitamin K levels, at one month this was 71 (43.0%) and 82 (49.4%) infants, respectively and at three months 103 (62.4%) and 109 (65.7%) infants, respectively. | |
| Participants | 331 infants, delivered spontaneously and vaginally, were randomized, 166 to the IM group and 165 to the oral group. Inclusion criteria: Intention to breast feed, GA at least 37 wk, BW over the 2.3 percentile, Apgar score at least seven at five minutes, healthy mother. Exclusions: treatment of mother with antibiotics, anti‐coagulants or anti‐epileptics. Cessation of breast feeding. Mean BW in kg (SD), oral group: 3.424 (0.439). IM group: 3.417 (0.440) Mean GA in wk (SD), oral group: 39.8 (1.3), IM group: 40.1 (1.2) | |
| Interventions | 1 mg oral vitamin K1 (Konakion, Hoffman‐La Roche) (n=165) or 1 mg IM vitamin K1 (Konakion, Hoffman‐La Roche) (n=166) | |
| Outcomes | Plasma vitamin K1 levels (pg/ml) and PIVKA‐II presence (>0.10 ‐ >0.13 AU/ml or > 2ng/ ml) at two weeks, one month and three months. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Greer 1998.
| Methods | Randomisation: computer randomised in blocks of four Multi center Blinding of outcome measurement: yes Seven infants in the oral group and one in the IM group dropped out of the study before two weeks; 14 other infants dropped out before the measurements at two months had taken place. At two weeks 13 (16.5%) infants in the oral group and eight (10.4%) in the IM group were not analysed for plasma vitamin K, at one month this was 15 (19.0%) and 12 (15.6%) infants, respectively and at two months 16 (20.3%) and 11 (13.9%) infants, respectively. At two weeks 10 (12.7%) infants in the oral group and seven (9.1%) in the IM group had not been analysed for the INR, at one month this was 13 (16.5%) and six (7.8%) infants, respectively and at two months 15 (19.0%) and 10 (13.0%) infants, respectively. | |
| Participants | 156 infants were randomized, 79 to the oral group and 77 to the IM group. The infants were fully breast fed during the study. Inclusion criteria: GA: 37‐42 weeks, BW: >2,500 g, singleton, mother intending to breastfeed. Apgar score seven or greater at five min, informed consent. Exclusion criteria: major congenital or malabsorptive disorders, anticonvulsant therapy during pregnancy Mean BW in kg (SD), oral group: 3.6 (0.6), IM group: 3.5 (0.4) | |
| Interventions | Three doses of 2 mg oral vitamin K1 (Konakion MM) at birth (n=79), at seven days and at 30 days or one dose of 1 mg IM vitamin K1 (Konakion MM) at birth (n=77) | |
| Outcomes | Plasma vitamin K1 levels (ng/ml) and prothrombin time (INR) at 14, 30, and 56 days | |
| Notes | Mixed micellar vitamin K was used in this study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Hathaway 1991.
| Methods | Randomisation: method not stated Multi center Blinding of outcome measurement: no Complete follow‐up: not stated Post‐randomisation exclusions: not stated | |
| Participants | 66 term, healthy, breast fed infants were analyzed, no information on number of infants randomized | |
| Interventions | 2 mg oral vitamin K1 (Konakion, Roche) or 5 mg oral vitamin K1 (Konakion, Roche) or 1 mg IM vitamin K1 (Konakion, Roche), all given within four hours after birth | |
| Outcomes | Plasma vitamin K1 levels (ng/ml) and noncarboxylated prothrombin (mcg/ml) levels at four weeks | |
| Notes | Data from this study could not be included in the data tables since no standard deviations were given. There was a group of untreated control subjects; this group, however, was not randomized and therefore was excluded from the review. The group that received 5 mg of oral vitamin K was also excluded, because it was not possible to insert both oral groups in the tables and a dose of 2 mg is more commonly used. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Jorgensen 1991.
| Methods | Randomisation: by envelopes Single center Blinding of outcome measurement: not stated One infant was excluded in the IM group because the mother had taken vitamin K during her pregnancy. Five others were lost for follow‐up in this group (4%). In the oral group one infant was lost for follow‐up (0.7%) | |
| Participants | 300 infants were randomized, 150 in each group Inclusion criteria: GA at least 35 completed weeks Exclusion criteria: ambulatory delivery, treatment of mother with anti‐coagulants, anti‐epileptics, anti‐tubercular agents, maternal vitamin K intake during pregnancy. Mean BW in kg (SD), oral group: 3.404 (0.519). IM group: 3.382(0.518) Median GA in wk (range), oral group: 40 (35‐43), IM group:40 (35‐42) | |
| Interventions | 1 mg oral vitamin K1 (Konakion, Roche) (n=150) or 1 mg IM vitamin K1 (Konakion, Roche) (n=150) , both given within one hour after birth | |
| Outcomes | Plasma PIVKA‐II levels, combined activity of clotting factors II, VII and X (as fraction of normal adult values), and prothrombin (antigen) at 48‐72 hours | |
| Notes | No units were given for PIVKA levels or prothrombin antigen | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Malik 1992.
| Methods | Randomisation: method not stated Multi center Blinding of outcome measurement: not stated Complete follow‐up: not stated Post‐randomisation exclusions: not stated | |
| Participants | 100 exclusively breast fed infants with birth weights above 2.5 kg were randomized, 25 in each of the four groups. Groups had comparable characteristics | |
| Interventions | 1 mg IM vitamin K3 (menadione sodium bisulphite) (n=25) or 0.5 mg IM vitamin K3 (n=25) or 1 mg oral vitamin K3 (n=25) , all given immediately after birth, or nothing (n=25) | |
| Outcomes | Prothrombin index (% of normal) at 36‐72 hours | |
| Notes | Water soluble vitamin K was used in this study. The group that received 0.5 mg of IM vitamin K was excluded, because it was not possible to enter both IM groups into the data tables and a dose of 1.0 mg IM is more commonly used. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Maurage 1995.
| Methods | Randomisation: method not stated Single center Blinding of outcome measurement: not stated Eight infants were excluded due to cessation of breast feeding, two due to failure of the laboratory analysis and one due to the use of antibiotics. It is not clear if these exclusions took place before or after randomization. Another ten infants dropped out before the analysis at one month; it is not stated in which groups these infants had been randomized. Thus it is not possible to calculate the percentages of infants by group that were excluded or lost for follow‐up. | |
| Participants | 50 full term, exclusively breast fed infants were randomized, seven in the 5mg oral group, 15 in the 3 mg oral group and eight in the IM group. 20 were excluded because of cessation of breast feeding, technical problems, treatment of infant with antibiotics or lack of parental permission Mean BW in kg (SD), oral group: 3.688 (0.1199). MM oral group: 3.215 (0.469), IM group: 3.557 (0.3427) Mean GA in wk (SD), oral group: 39.6 (0.49), MM oral group: 39.6 (0.81), IM group: 39.4 (1.05) | |
| Interventions | 5 mg oral vitamin K1 (cremophor) (n=7) or 3 mg oral vitamin K1 (Mixed‐micellar) (n=15) or 1.5 mg IM vitamin K1 (Mixed‐micellar) (n=8) | |
| Outcomes | Plasma vitamin K1 levels (ng/ml) and prothrombin activity at 12‐36 hours and at 25‐31 days | |
| Notes | The group that received 5 mg of vitamin K orally was not entered in the data tables, because both oral groups could not be entered in the tables and the 3 mg is closer to common practice. The author did not state the SD's of the prothrombin activity. Therefore, these data could not be included in the data tables. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Motohara 1985.
| Methods | Randomisation: method not stated Single center Blinding of outcome measurement: not stated Twenty six (50%) infants in the no treatment group and 25 (49%) in the oral vitamin K group were not included in the three‐day analysis; at five days this was zero (0%) and 16 (31.2%) infants, respectively. The reasons for this are not stated. | |
| Participants | 102 full‐term infants weighing more than 2,800 g. were randomized, 51 in each group. The infants were mainly breast fed. | |
| Interventions | 5 mg of oral vitamin K2 (Menatetrenone Keytwo syrup, Eisai co. ltd.) (n=51) within 6‐12 hours after birth or nothing (n=51) | |
| Outcomes | PIVKA‐II levels (AU/ml) at three and five days | |
| Notes | Vitamin K2 was used in this study. We converted the data from this study from PIVKA II levels to PIVKA II present (>0.10 ‐ >0.13 AU/ml or > 2ng/ ml) or not present to be able to compare it to the other studies. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
O'Connor 1986.
| Methods | Randomisation: method not stated Single center and home births Blinding of outcome measurement: not stated Three (16.7%) infants were excluded in the no‐intervention group and eight (42%) in the oral group mostly due to unsuccessful venepuncture. | |
| Participants | 37 children born from uncomplicated pregnancies were randomized, 18 in the oral group and 19 in the no treatment group. Mean BW in kg, oral group: 3.9077, group without treatment: 3.5041 | |
| Interventions | 2 mg oral vitamin K1 (Aquamephyton; Merck Sharp & Dohme) within two hours after birth (n=18) or nothing (n=19) | |
| Outcomes | PT (sec.), Echis factor II and ratio Echis factor II/ Echis II (abnormal when <1) at 24‐84 hours. | |
| Notes | Another group was included in this study. The participants in this group however were not randomized. The average birth weight of the oral group was significantly higher. No unit was given for Echis factor II | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Sharma 1995.
| Methods | Randomisation: method not stated Single center Blinding of outcome measurement: not stated Eleven (44%) infants in the IM group, one (4%) in the oral group and 13 (52%) in the no‐treatment group were lost for follow up because of early discharge of the infants or technical problems. | |
| Participants | 75 exclusively breast fed, appropriately grown, term infants with normal Apgar scores were randomized, 25 in each of the three groups. Exclusion criteria: perinatal complications, treatment of mother with drugs including aspirin, luminal etc., maternal hypertension. Mean BW in kg (SD), oral group: 2.77 (2.1). IM group: 2.85 (0.31), control group: 2.84 (0.33) Mean GA in wk (SD), oral group: 38.8 (1.0), IM group: 38.7 (1.3) | |
| Interventions | 1 mg of IM vitamin K3 (n=25) or 2 mg of oral vitamin K3 (n=25) both within one hour after birth or nothing (n=25) | |
| Outcomes | PIVKA‐II plasma presence (>0.10 ‐ >0.13 AU/ml or > 2ng/ ml) at 72‐78 hours | |
| Notes | Injectable water soluble vitamin K was also given orally | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Sutherland 1967.
| Methods | Randomisation: by coded preparations Single center Blinding of outcome measurement: yes Complete follow‐up: yes Post‐randomisation exclusions: not stated | |
| Participants | 3,338 full term infants were randomized, both breast and formula fed, 1,143 in the placebo group, 1,132 in the 100 mcg IM group and 1,063 in the 5mg IM group. | |
| Interventions | 100 mcg vitamin K3 IM (n=1,132) or 5 mg of vitamin K3 IM (n=1,063) or a placebo (0.9% saline) (n=1,143) | |
| Outcomes | Any, moderate, and severe bleeding, up to approximately 82‐110 hours. | |
| Notes | Water soluble vitamin K was used in this study. In this review the group that received 100 mcg of vitamin K and the group that received 5 mg of vitamin K were grouped together for practical reasons. Bleeding was reported by different individuals, nurses and doctors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Ulusahin 1996.
| Methods | Randomisation: method not stated Single center Blinding of outcome measurement: not stated No infants were stated to be excluded or lost for follow‐up at three days; four (30.8%) infants in the oral group, eight (50%) in the IM group and seven (46.7%) in the control group were lost for follow up at one month because the mother did not return with the infant. | |
| Participants | 44 healthy, term infants with Apgar scores greater than six at five minutes were randomized, 13 in the oral group, 16 in the IM group and 15 in the no treatment group. The infants were born to mothers with uneventful pregnancies. Mothers fully intended to breast feed. Exclusion criteria: treatment of mother with vitamin K1, anti‐coagulants, anti‐epileptic or anti‐tubercular agents. Mean GA in wk (SD) oral group: 39.92 (1.04), IM group: 39.19 (1.05), placebo group: 39.13 (1.13) | |
| Interventions | 2 mg oral vitamin K1 (Konakion, Hoffman‐La Roche) (n=13) or 1 mg IM vitamin K1 (Konakion, Hoffman‐La Roche) (n=16) both within six hours after birth or nothing (n=15) | |
| Outcomes | PIVKA‐II presence (>0.10 ‐ >0.13 AU/ml or > 2ng/ ml) at three days and one month | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Vietti 1960.
| Methods | Allocation: by day of birth Single center Blinding of outcome measurement: yes Complete follow‐up: not stated Post‐randomisation exclusions: not stated | |
| Participants | 470 male infants were analysed after a circumcision was performed on the mother's request. No information on number initially entered. | |
| Interventions | 5 mg of IM vitamin K3 or nothing | |
| Outcomes | Bleeding after circumcision | |
| Notes | Water soluble vitamin K was used in this study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arteaga‐Vizcaino 95 | The groups in this study were either breast fed or formula fed. These groups were then each divided into 2 subgroups which received either oral or no vitamin K. The authors of the study are being contacted to clarify the method of allocation, both to feeding group and to vitamin K. |
| Felbo 1965 | This trial allocated infants to vitamin K or nothing on an alternating month schedule. The outcome was clinical bleeding, but the individual reporting the bleeding was aware of whether the infant had received vitamin K or not. This trial was judged to be very prone to bias and was excluded for this reason. |
Characteristics of studies awaiting assessment [ordered by study ID]
Hogenbirk 1993.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes |
Schubiger 1993.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes |
Sources of support
Internal sources
The Dutch Cochrane Centre, Netherlands.
Dept of Pediatrics, Emma Childrens' Hospital A.M.C. Amsterdam, Netherlands.
External sources
No sources of support supplied
Declarations of interest
None
Edited (no change to conclusions)
References
References to studies included in this review
Bakhshi 1996 {published data only}
- Bakhshi S, Deorari AK, Roy S, et al. Prevention of subclinical vitamin K deficiency based on PIVKA‐II levels: oral versus intramuscular route. Indian Pediatrics 1996;33:1040‐3. [MEDLINE: ] [PubMed] [Google Scholar]
Cornelissen 1992 {published data only}
- Cornelissen EAM, Kolée LAA, Abreu RA, et al. Effects of oral and intramuscular vitamin K prophylaxis on vitamin K1, PIVKA‐II, and clotting factors in breast fed infants. Archives of Disease in Childhood 1992;67:1250‐4. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Greer 1998 {published data only}
- Greer FR, Marshall SP, Severson RR, et al. A new mixed micellar preparation for oral vitamin K prophylaxis: randomised controlled comparison with an intramuscular formulation in breast fed infants. Archives of Disease in Childhood 1998;79:300‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hathaway 1991 {published data only}
- Hathaway WE, Isarangkura PB, Mahasandana C, et al. Comparison of oral and parenteral vitamin K prophylaxis for prevention of late hemorrhagic disease of the newborn. Journal of Pediatrics 1991;119:461‐4. [DOI] [PubMed] [Google Scholar]
Jorgensen 1991 {published data only}
- Jorgensen FS, Felding P, Vinther S, Andersen GE. Vitamin K to neonates. Peroral versus intramuscular administration. Acta Paediatrica Scandinavica 1991;80:304‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Malik 1992 {published data only}
- Malik S, Udani RH, Bichile SK, et al. Comparative study of oral versus injectable vitamin K in neonates. Indian Pediatrics 1992;29:857‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Maurage 1995 {published data only}
- Maurage C, Dalloul C, Moussa F, et al. Efficacite de l’administration orale d’une solution micellaire de vitamine K en periode neonatale. Arch Pediatr 1995;2:328‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Motohara 1985 {published data only}
- Motohara K, Endo F, Matsuda I. Effect of vitamin K administration on acarboxy prothrombin (PIVKA‐II) levels in newborns. Lancet 1985;2:242‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
O'Connor 1986 {published data only}
- O’Connor ME, Addiego JE. Use of oral vitamin K1 to prevent hemorrhagic disease of the newborn infant. Journal of Pediatrics 1986;108:616‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sharma 1995 {published data only}
- Sharma RK, Marwaha N, Kumar P, Narang A. Effect of oral water soluble vitamin K on PIVKA‐II levels in newborns. Indian Pediatrics 1995;32:863‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Sutherland 1967 {published data only}
- Sutherland JM, Glueck HI, Gleser G. Hemorrhagic disease of the newborn: breast feeding as a necessary factor in the pathogenesis. American Journal of Diseases of Children 1967;113:524‐33. [DOI] [PubMed] [Google Scholar]
Ulusahin 1996 {published data only}
- Ulusahin N, Arsan S, Ertogan F. Effects of oral and intramuscular vitamin K prophylaxis on PIVKA‐II assay parameters in breastfed infants in Turkey. Turkish Journal of Pediatrics 1996;38:295‐300. [MEDLINE: ] [PubMed] [Google Scholar]
Vietti 1960 {published data only}
- Vietti TJ, Murphy TP, James JA, Pritchard JA. Observations on the prophylactic use of vitamin K in the newborn infant. Journal of Pediatrics 1960;56:343‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Arteaga‐Vizcaino 95 {published data only}
- Arteaga‐Vizcaino M, Torres‐Espina M, Redondo L, et al. Efecto de la administracion de la vitamin K sobre la actividad de los factores II, VII y X en neonatos sanos. Invest Clin 1995;36:83‐93. [PubMed] [Google Scholar]
Felbo 1965 {published data only}
- Felbo M, Hauge Kristensen K, Melchior JC. Significance of treating newborn infants with vitamin K I. A clinical study. Annales Paediatriae Fenniae 1965;11:175‐81. [PubMed] [Google Scholar]
References to studies awaiting assessment
Hogenbirk 1993 {published data only}
- Hogenbirk K, Peters M, Bouman P, et al. The effect of formula versus breast feeding and exogenous vitamin K1 supplementation on circulating levels of vitamin K1 and vitamin K‐dependent clotting factors in newborns. European Journal of Pediatrics 1993;152:72‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schubiger 1993 {published data only}
- Schubiger G, Tönz O, Grüter J, Shearer MJ. Vitamin K1 concentration in breast‐fed neonates after oral or intramuscular administration of a single dose of a new mixed‐micellar preparation of phylloquinone. Journal of Pediatric Gastroenterology and Nutrition 1993;16:435‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Additional references
Brousson 1996
- Brousson MA, Klein MC. Controversies surrounding the administration of vitamin K to newborns: a review. CMAJ 1996;154:307‐15. [PMC free article] [PubMed] [Google Scholar]
Cornelissen 1993
- Cornelissen EA, Kollee LA, Lith TG, et al. Evaluation of a daily dose of 25 micrograms vitamin K1 to prevent vitamin K deficiency in breast‐fed infants. Journal of Pediatric Gastroenterology and Nutrition 1993;16:301‐5. [DOI] [PubMed] [Google Scholar]
Cornelissen 1997
- Cornelissen M, Kries R, Loughnan P, Schubiger G. Prevention of vitamin K deficiency bleeding: efficacy of different multiple oral dose schedules of vitamin K. European Journal of Pediatrics 1997;156:126‐30. [DOI] [PubMed] [Google Scholar]
Croucher 1994
- Croucher C, Azzopardi D. Compliance with recommendations for giving vitamin K to newborn infants. BMJ 1994;308:894‐5. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Golding 1990
- Golding J, Paterson M, Kinlen LJ. Factors associated with childhood cancer in a national cohort study. British Journal of Cancer 1990;62:304‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Golding 1992
- Golding J, Greenwood R, Birmingham K, Mott M. Childhood cancer, intramuscular vitamin K, and pethidine given during labour. BMJ 1992;305:341‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Greer 1999
- Greer FR. Vitamin K status of lactating mothers and their infants. Acta Paediatrica. Supplement 1999;88(430):95‐103. [DOI] [PubMed] [Google Scholar]
Haroon 1982
- Haroon Y, Shearer MJ, Rahim S, et al. The content of phylloquinone (vitamin K1) in human milk, cows' milk and infant formula foods determined by high‐performance liquid chromatography. Journal of Nutrition 1982;112:1105‐7. [DOI] [PubMed] [Google Scholar]
Lane 1985
- Lane PA, Hathaway WE. Vitamin K in infancy. Journal of Pediatrics 1985;106:351‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ross 2000
- Ross JA, Davies SM. Vitamin K prophylaxis and childhood cancer. Med Pediatr Oncol 2000;34:434‐7. [DOI] [PubMed] [Google Scholar]
Schubiger 1999
- Schubiger G, Stocker C, Banziger O, et al. Oral vitamin K1 prophylaxis for newborns with a new mixed‐micellar preparation of phylloquinone: 3 years experience in Switzerland. European Journal of Pediatrics 1999;158:599‐602. [DOI] [PubMed] [Google Scholar]
Shearer 1992
- Shearer MJ. Vitamin K metabolism and nutriture. Blood Reviews 1992;6:92‐104. [DOI] [PubMed] [Google Scholar]
Shearer 1995
- Shearer MJ. Vitamin K. Lancet 1995;345:229‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sutor 1999
- Sutor AH, Kries R, Cornelissen EA, et al. Vitamin K deficiency bleeding (VKDB) in infancy. ISTH Pediatric/Perinatal Subcommittee. International Society on Thrombosis and Haemostasis. Thrombosis and Haemostasis 1999;81:456‐61. [PubMed] [Google Scholar]
von Kries 1998
- Kries R. Neonatal vitamin K prophylaxis: the Gordian knot still awaits untying. BMJ 1998;316:161‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
von Kries 1999
- Kries R. Oral versus intramuscular phytomenadione: safety and efficacy compared. Drug Safety 1999;21:1‐6. [DOI] [PubMed] [Google Scholar]
von Kries 1999a
- Kries R, Hachmeister A, Gobel U. Can 3 oral 2 mg doses of vitamin K effectively prevent late vitamin K deficiency bleeding?. European Journal of Pediatrics 1999;158(Suppl 3):S183‐6. [DOI] [PubMed] [Google Scholar]
Wariyara 2000
- Wariyara U, Hiltona S, Paganb J, et al. Six years' experience of prophylactic oral vitamin K. Archives of Disease in Childhood. Fetal and Neonatal Edition 2000;82:F64‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zipursky 1999
- Zipursky A. Prevention of vitamin K deficiency bleeding in newborns. British Journal of Haematology 1999;104:430‐7. [DOI] [PubMed] [Google Scholar]


