Abstract
Background
Oxidative stress has been proposed as a key factor involved in the development of pre‐eclampsia. Supplementing women with antioxidants during pregnancy may help to counteract oxidative stress and thereby prevent or delay the onset of pre‐eclampsia.
Objectives
To determine the effectiveness and safety of any antioxidant supplementation during pregnancy and the risk of developing pre‐eclampsia and its related complications.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (May 2007), the Cochrane Central Register of Controlled Trials (The Cochrane Library 2006, Issue 3), MEDLINE (1950 to October 2007) and Current Contents (1998 to August 2004).
Selection criteria
All randomised trials comparing one or more antioxidants with either placebo or no antioxidants during pregnancy for the prevention of pre‐eclampsia, and trials comparing one or more antioxidants with another, or with other interventions.
Data collection and analysis
Two review authors independently assessed trials for inclusion and trial quality and extracted data.
Main results
Ten trials, involving 6533 women, were included in this review, five trials were rated high quality. For the majority of trials, the antioxidant assessed was combined vitamin C and E therapy. There was no significant difference between antioxidant and control groups for the relative risk (RR) of pre‐eclampsia (RR 0.73, 95% confidence intervals (CI) 0.51 to 1.06; nine trials, 5446 women) or any other primary outcome: severe pre‐eclampsia (RR 1.25, 95% CI 0.89 to 1.76; two trials, 2495 women), preterm birth (before 37 weeks) (RR 1.10, 95% CI 0.99 to 1.22; five trials, 5198 women), small‐for‐gestational‐age infants (RR 0.83, 95% CI 0.62 to 1.11; five trials, 5271 babies) or any baby death (RR 1.12, 95% CI 0.81 to 1.53; four trials, 5144 babies). Women allocated antioxidants were more likely to self‐report abdominal pain late in pregnancy (RR 1.61, 95% CI 1.11 to 2.34; one trial, 1745 women), require antihypertensive therapy (RR 1.77, 95% CI 1.22 to 2.57; two trials, 4272 women) and require an antenatal hospital admission for hypertension (RR 1.54, 95% CI 1.00 to 2.39; one trial, 1877 women). However, for the latter two outcomes, this was not clearly reflected in an increase in any other hypertensive complications.
Authors' conclusions
Evidence from this review does not support routine antioxidant supplementation during pregnancy to reduce the risk of pre‐eclampsia and other serious complications in pregnancy.
Keywords: Female; Humans; Infant, Newborn; Pregnancy; Antioxidants; Antioxidants/therapeutic use; Infant, Small for Gestational Age; Oxidative Stress; Pre‐Eclampsia; Pre‐Eclampsia/prevention & control; Pregnancy Outcome; Premature Birth; Premature Birth/etiology; Randomized Controlled Trials as Topic
Plain language summary
Antioxidants for preventing pre‐eclampsia
Pre‐eclampsia can occur during pregnancy when women have high blood pressure and protein in their urine. In some cases, it can lead to poor growth for the baby and premature birth. There can also be serious complications for the woman, sometimes affecting the liver, kidneys, brain or blood clotting system. Both mother and baby are at risk of mortality. A possible contributing factor to the development of pre‐eclampsia may be the presence of excessive amounts of chemicals called 'free radicals'. Antioxidants, such as vitamin C, vitamin E, selenium and lycopene, can neutralize free radicals. The review covered 10 trials, involving 6533 women, and looked at several antioxidants. Overall the review found no reduction in pre‐eclampsia, high blood pressure or preterm birth with the use of antioxidant supplements. When antioxidants were assessed separately, there were insufficient data to be clear about whether there was any benefit or not, except for vitamin C and E. The current evidence does not support the use of antioxidants to reduce the risk of pre‐eclampsia or other complications in pregnancy, but there are trials still in progress.
Background
Hypertensive disorders in pregnancy remain a leading cause of maternal death worldwide (HMSO 1998; NHMRC 1998). The majority of deaths occur in the developing world. Pre‐eclampsia, defined as hypertension complicated with proteinuria (Gifford 2000), is a multiorgan disease, affecting the liver, kidneys, brain and blood clotting system. Severe pre‐eclampsia often results in life threatening complications such as eclampsia (fitting) and the HELLP syndrome, which involves haemolysis, elevated liver enzymes and low platelets; however, these complications are rare. Symptoms of the HELLP syndrome include pain in the upper right part of the abdomen, fatigue, a general feeling of being unwell (malaise), nausea, vomiting and increased fluid in the body tissues. Pre‐eclampsia also poses serious health risks for the baby. Pre‐eclampsia is implicated in 12% of cases of intrauterine growth restriction (Kramer 2000) and is a known antecedent in up to 19% of preterm births. Small‐for‐gestational‐age survivors are at risk of later health problems, including poor growth in childhood (McCowan 1999) and in adult life, an increased risk of hypertension and diabetes (Barker 1993). Preterm birth is the leading cause of early neonatal death and infant mortality. Preterm survivors are at risk of serious morbidity such as chronic lung disease and long‐term neurological disability (Donoghue 2000).
Antioxidants are loosely defined as "any substance that, when present in low concentrations compared to that of an oxidisable substrate, significantly delays or inhibits oxidation of that substrate" (Diplock 1998). Antioxidants protect proteins and enzymes from oxidation and destruction by free radicals, and help to maintain cellular membrane integrity. Antioxidants can be categorised as either free radical scavengers that trap or decompose existing free radicals, or cellular and extracellular enzymes that inhibit peroxidase reactions involved in the production of free radicals (Diplock 1998). Free radical scavengers include vitamin C (ascorbate), vitamin E (tocopherols), carotenoids and glutathione. Antioxidant enzymes include glutathione peroxidase, superoxide dismutase and catalase, which are dependent on the presence of co‐factors such as selenium, zinc and iron. While antioxidant enzymes are important for intracellular defences, non‐enzymatic antioxidants are the major defence mechanism in the extracellular compartment.
The exact cause of pre‐eclampsia is unknown. However, a key factor in the development of pre‐eclampsia is inadequate cytotrophoblast invasion of the spiral arteries in the uterus leading to faulty implantation and development of the placenta (Roberts 1993). One consequence of this abnormal placental development may be reduced placental perfusion, and consequent reduction in blood flow through the placenta. Various hypotheses have been put forward to support interventions that might delay or reverse this process. These include antiplatelet agents and calcium supplements, topics which are covered by other Cochrane reviews (Hofmeyr 2006; Knight 2007). More recently, the observation that women with pre‐eclampsia have decreased plasma and placental concentrations of antioxidants (Hubel 1997; Wang 1996) has led to the proposal that placental underperfusion may mediate a state of oxidative stress. This includes overproduction of highly reactive oxygen molecules or free radicals, depleting the body's stores of antioxidants. Oxidative stress, coupled with an exaggerated inflammatory response, may result in the release of maternal factors that result in inappropriate endothelial cell activation and endothelial cell damage. Endothelial cells line the inside surfaces of blood vessels and their impairment results in the clinical signs of pre‐eclampsia, such as hypertension and proteinuria. A woman's risk of, and response to, oxidative stress depends on various factors. These include the propensity for small dense low density lipoproteins, hyperhomocysteinaemia, a genetically determined poor resistance to oxidative stress, and a dietary deficiency of antioxidants (Roberts 2001). Supplementing women with antioxidants may increase their resistance to oxidative stress, and hence could limit the systemic and uteroplacental endothelial damage seen in pre‐eclampsia. Accordingly, antioxidants have been proposed as potential prophylaxes against pre‐eclampsia.
There is limited evidence about the safety of giving antioxidants to women during pregnancy. In non‐pregnant people, there have been inconsistent findings about the benefits and harms of antioxidants. For example, the Heart Protection Study, in which 20,536 high‐risk adults took antioxidant vitamins C, E and beta‐carotene or matching placebo for five years, concluded these vitamins appeared to be safe (HPS 2002). Controlled clinical trials of vitamin E supplementation in adults have also failed to demonstrate any adverse effects (Bendich 1993). Similarly, the only consistent side‐effect reported from controlled clinical trials of high doses of vitamin C is diarrhoea (Bendich 1997). Of concern, however, a recent systematic review of trials evaluating vitamin E supplementation demonstrated harmful effects associated with supplementation (Miller 2005). The review included information for 135,967 men and non‐pregnant women supplemented with vitamin E in a range of dosages (16.5 to 2000 international units (IU) per day). An increase in all cause mortality was seen in individuals supplemented with 400 or more IU vitamin E per day for at least one year. This review encompassed trials of vitamin E supplementation in individuals either at risk of or with established cardiovascular disease, with Alzheimer's or early onset Parkinson's disease, institutionalised elderly individuals as well as the general population. The authors cautioned that the results cannot reliably be generalised to healthy adults including pregnant women, and for those individuals supplemented with lower dosages or short‐term vitamin E supplementation (less than one year). While these findings cannot be generalised to healthy pregnant women, they do highlight the need for controlled evaluation of vitamin E and other antioxidant supplementation in pregnancy.
Furthermore, while water‐soluble antioxidants such as vitamin C may be easily excreted, lipid soluble antioxidants such as vitamin E and beta‐carotene may accumulate in the body, and in very high doses may have a toxic effect. Many antioxidant preparations, particularly antioxidant vitamins, are available in over the counter preparations, highlighting the possibility for self‐medication. The need to demonstrate safety, as well as effectiveness, during pregnancy is particularly important when antioxidants are used in high doses and are readily available. We need reliable evidence about short‐term and long‐term safety for both mother and child before antioxidants are introduced into routine antenatal care.
Antioxidants, particularly antioxidant vitamins, are relatively inexpensive to produce and are readily available. For this reason, antioxidants may be of particular importance for women in low‐income countries, who carry the greatest burden of morbidity and mortality associated with pre‐eclampsia. The aims of this review are (i) to identify randomised trials evaluating antioxidants for the prevention of pre‐eclampsia and (ii) to investigate the benefits and hazards of using antioxidants to prevent pre‐eclampsia.
Objectives
To determine the effectiveness and safety of any antioxidant supplementation during pregnancy on the risk of:
pre‐eclampsia;
small‐for‐gestational‐age infants;
baby death;
maternal and neonatal morbidity;
long‐term development of the child;
side‐effects and adverse events.
Methods
Criteria for considering studies for this review
Types of studies
All randomised trials comparing one or more antioxidants with either placebo or no antioxidants during pregnancy for the prevention of pre‐eclampsia, and trials comparing one or more antioxidants with another, or with other interventions. Trials were included if the primary aim of the study was to prevent pre‐eclampsia or if the primary aim was otherwise but pre‐eclampsia was reported by the authors.
The previous version of this review included quasi‐randomised studies; however, our protocol stated these would be excluded when sufficient data from adequately randomised trials became available. Such data became available for this version of the review, with the inclusion of two trials involving 4272 women.
Types of participants
Pregnant women considered to be at low, moderate or high risk of developing pre‐eclampsia. Women with established pre‐eclampsia were excluded. Women were classified into subgroups based on: (1) their level of risk at trial entry of developing pre‐eclampsia: (a) high risk, defined as women having one or more of the following: previous severe pre‐eclampsia, diabetes, chronic hypertension, renal disease or autoimmune disease; (b) moderate/low risk, defined as women who do not meet the criteria for high risk and have any of the following risk factors, in particular first pregnancy, a mild rise in blood pressure and no proteinuria, positive roll‐over test, abnormal uterine artery Doppler scan, multiple pregnancy, a family history of pre‐eclampsia, maternal age less than 20 and known thrombophilia.
When the risk was unclear or unspecified women were classified as moderate/low risk.
(2) Gestation at randomisation: less than 12 weeks' gestation, 12 to 20 weeks' gestation, 21 to 28 weeks' gestation and greater than 28 weeks' gestation. In the previous version of this review, participants were subgrouped on gestation at randomisation of less than 20 weeks' gestation or greater than or equal to 20 weeks' gestation. This subgroup has been amended to allow an assessment of the effects of antioxidant supplementation in the first trimester and the impact on timing of disease progression and severity, particularly as symptoms of pre‐eclampsia usually occur after 20 weeks' gestation.
Types of interventions
Comparisons of any antioxidant/s (any dosage regimen) with either placebo or no antioxidant/s.
Comparisons of one or more antioxidant with other antioxidant/s.
Comparisons of antioxidant/s with other interventions.
Comparisons of one or more antioxidants with other agents compared with placebo or no antioxidant/s, other antioxidants or other interventions.
Trials were classified into subgroups based on:
type of antioxidant(s);
dose of antioxidant/s (above, within or below accepted pharmacologic range);
antioxidant intake before trial entry, where applicable.
All types of antioxidants, including enzymes that inhibit or retard the production of oxidative substances or free radical scavengers that interact with free radicals, were included. In the previous version of the review the type of antioxidant was classified as either vitamin, mineral or non‐vitamin antioxidants. In this review, all included trials except one (Han 1994) assessed vitamin antioxidants, therefore the subgroup analyses based on antioxidant type classify trials according to the type of antioxidant vitamin(s) assessed, and whether the antioxidant(s) was given alone or with other agents.
Types of outcome measures
Primary outcomes
Pre‐eclampsia: onset before or after 34 weeks, variously defined by the authors.
Severe pre‐eclampsia; including HELLP (haemolysis elevated liver enzymes and low platelets) syndrome and imminent eclampsia.
Preterm birth, very preterm birth and extremely preterm birth: less than 37 complete weeks' gestation, less than 33 completed weeks' gestation or less than 27 completed weeks' gestation.
Small‐for‐gestational‐age infants: whenever possible, defined as less than the third centile, otherwise the most extreme centile reported.
Baby death (miscarriage, stillbirth, neonatal or infant death).
Secondary outcomes
For the mother:
death up to six weeks postpartum; gestational hypertension; severe hypertension; use of antihypertensives; elective delivery (induction of labour or elective caesarean section); caesarean section (emergency plus elective); bleeding episodes (such as abruption of the placenta, antepartum haemorrhage, postpartum haemorrhage, need for transfusion); measures of serious maternal morbidity (including eclampsia, liver failure, renal failure, disseminated intravascular coagulation, stroke); and maternal views of care.
For the child
Gestational age at birth; birthweight; Apgar score less than seven at five minutes; Apgar score less than four at five minutes; respiratory distress syndrome; chronic lung disease; bleeding episodes (such as intraventricular haemorrhage, periventricular leukomalacia); bacterial sepsis; necrotising enterocolitis; retinopathy of prematurity; disability during childhood (such as cerebral palsy, intellectual disability, hearing disability and visual impairment); and poor childhood growth.
For mother and child
Side‐effects and adverse effects of antioxidants sufficient to stop supplementation; side‐effects and adverse effects of supplementation not sufficient to stop supplementation. Use of health service resources for the woman Antenatal hospital admission; visits to day care units; use of intensive care; ventilation and dialysis.
Use of health service resources for the infant Admission to special care/intensive care nursery; use of mechanical ventilation; length of stay in hospital; as well as developmental and special needs after discharge.
Economic outcomes
Costs to health service resources: short term and long term for both mother and baby; costs to the woman, her family, and society associated with the interventions.
We added the following outcomes to this updated version of the review: gestational hypertension, use of antihypertensives, miscarriage, extremely preterm birth (before 27 weeks' completed gestation), Apgar score less than seven at five minutes and economic outcomes. We included data for these additional outcomes to ensure that all outcomes specified in the pre‐eclampsia generic protocol (Meher 2005) are reported in this review.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register by contacting the Trials Search Co‐ordinator (May 2007).
The Cochrane Pregnancy and Childbirth Group's Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
monthly searches of MEDLINE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness search of a further 36 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL and MEDLINE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the 'Search strategies for identification of studies' section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are given a code (or codes) depending on the topic. The codes are linked to review topics. The Trials Search Co‐ordinator searches the register for each review using these codes rather than keywords.
In addition, we searched the Cochrane Central Register of Controlled Trials (The Cochrane Library 2006, Issue 3) using the terms pregnan*, preeclamp*, pre‐eclamp*, antioxidant*.
We also searched MEDLINE (1950 to October 2007) and Current Contents (1998 to August 2004) using the strategy:
("antioxidants"[TIAB] NOT Medline[SB]) OR "antioxidants"[MeSH Terms] OR "antioxidants"[Pharmacological Action] OR Antioxidant[Text Word]
"ascorbic acid"[MeSH Terms] OR Ascorbic‐Acid[Text Word] OR ("ascorbic acid"[TIAB] NOT Medline[SB]) OR vitamin C[Text Word]
"vitamin e"[MeSH Terms] OR Vitamin‐E[Text Word] OR "alpha‐tocopherol"[MeSH Terms] OR alpha‐tocopherol[Text Word]
"beta carotene"[MeSH Terms] OR Beta‐carotene[Text Word]
"selenium"[MeSH Terms] OR Selenium[Text Word]
"glutathione peroxidase"[MeSH Terms] OR Glutathione peroxidase[Text Word]
"superoxide dismutase"[MeSH Terms] OR superoxide dismutase[Text Word]
"catalase"[MeSH Terms] OR catalase[Text Word]
"pregnancy"[MeSH Terms] OR pregnancy[Text Word]
"pre‐eclampsia"[MeSH Terms] OR ("pre‐eclampsia"[TIAB] NOT Medline[SB]) OR preeclampsia[Text Word]
"pregnancy complications"[MeSH Terms] OR Pregnancy complications[Text Word]
#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8
#12 AND (#9 OR #10 OR #11)
"controlled clinical trial"[Publication Type] OR "controlled clinical trials"[MeSH Terms] OR "controlled clinical trial"[Text Word]
"randomized controlled trial"[Publication Type] OR "randomized controlled trials"[MeSH Terms] OR "randomized controlled trials"[Text Word]
#14 OR #15
#13 AND #16
We did not apply any language restrictions.
Data collection and analysis
Two review authors assessed potentially eligible trials for their suitability for inclusion in the review. Decisions regarding inclusion were made separately and results compared. Where review authors were associated with potentially eligible trials (Poston 2006; Rumbold 2006), decisions regarding suitability for inclusion in the review were made by a review author not involved in the trial and by an independent assessor (Philippa Middleton). Any disagreement was resolved through discussion. Data were extracted by two authors (neither involved with the individual trial) using an agreed format, and again discrepancies resolved through discussion. Data were double‐entered and checked.
We assessed the validity of each included trial according to the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2006). Trials were assessed with a grade allocated to each trial on the basis of allocation concealment: A (adequate), B (unclear) or C (inadequate). Where the method of allocation concealment was unclear, attempts were made to contact authors to provide further details.
Blinding, completeness of follow up and use of placebo were assessed for each outcome using the following criteria: A. double blind, neither investigator nor participant knew or were likely to guess the allocated treatment; B. single blind, either the investigator or the participant knew the allocation. Or, the trial is described as double blind, but side‐effects of one or other treatment mean that it is likely that for a significant proportion (at least 20%) of participants the allocation could be correctly identified; C. no blinding, both investigator and participant knew (or were likely to guess) the allocated treatment; D. unclear.
For completeness of follow up: A. less than 3% of participants excluded; B. 3% to 9.9% of participants excluded; C. 10% to 19.9% of participants excluded; D. excluded: if not possible to present the data by all participants analysed or if more than 20% of participants excluded.
For use of placebo control: A. placebo controlled; B. unclear whether placebo controlled; C. no placebo control.
Statistical analyses were carried out using the Review Manager software (RevMan 2003), with results presented as summary relative risks. Where there were missing data for outcomes (either due to exclusions or losses to follow up),an available case analysis was performed. Tests of heterogeneity between trials were applied to assess the significance of any differences between trials (I2 greater than or equal to 50%), and possible causes of any heterogeneity were explored. Summary relative risks were calculated using a fixed‐effect model. If heterogeneity was detected, subgroup analyses for the primary outcomes were performed by risk of pre‐eclampsia, gestational age at trial entry, type of antioxidant used, antioxidant dosage and prior dietary intake of antioxidants. Heterogeneity that was not explained by subgroup analyses was modelled using random‐effects analysis.
Subgroup analyses based on antioxidant dosage involved classifying trials according to whether dose was above, within or below accepted pharmacological range. For vitamin C, maximum circulating concentrations of vitamin C in the body have been show to result from taking vitamin C in doses ranging from 400 mg (Levine 2001) to 1000 mg (Levine 1996). For vitamin E, an oral dose of 400 international units has been shown to have antioxidant effects (Devaraj 1997). However, for the other antioxidants assessed in this review, little is currently known about effective pharmacological doses.
All included trials were included in the initial analyses and sensitivity analyses carried out to explore the effect of trial quality. This involved analysis based on an 'A' rating of allocation concealment, blinding of assessment of outcome and placebo control. The results of high‐quality studies were compared with overall results for all included trials.
Our prespecified subgroup analyses for the primary outcomes were based on comparing: (a) women who are at low/moderate or high risk of pre‐eclampsia; (b) women's gestation at randomisation (less than 12 weeks' gestation, 12 to 20 weeks' gestation, 21 to 28 weeks' gestation, greater than 28 weeks' gestation or other); (c) the type of antioxidant supplement(s) (vitamin C and E alone, vitamin C and E with other agents, vitamin C alone, lycopene, red palm oil, selenium); (d) the dosage of the antioxidant supplement(s) (above, within or below accepted pharmacological range); (e) women who have low or adequate dietary antioxidant(s) intake (where applicable) before trial entry.
If antioxidants are effective, the subgroup analyses will determine which of these agents are best, what is the ideal dose, and to compare antioxidants with other interventions.
The planned subgroup analyses aimed to explore the impact of differences in characteristics of women involved and in the antioxidant treatment assessed. However, for many of the subgroups there was either insufficient information in the trial publications to allow data extraction for discrete subgroups (for example, specific gestational age categories) or it was unclear whether information that would enable classification into subgroups was collected (such as women's dietary intake of antioxidants). For future updates of this review we plan to write to the trialists who conducted the studies in this review. In doing so, we will request further information to allow data extraction for the subgroups specified in this review and request any additional data that have been collected but not already published about outcomes of interest to this review (such as maternal side‐effects), to ensure there is complete capture of the data about antioxidants.
Results
Description of studies
See tables 'Characteristics of included studies' and 'Characteristics of excluded studies' for details of individual studies.
Ten trials, involving 6533 women, were included in the review (Beazley 2002; Chappell 1999; Han 1994; Mahdy 2004; Merchant 2005; Poston 2006; Rivas 2000; Rumbold 2006; Sharma 2003; Steyn 2002).
Participants
Half the women (3307, 52%) in this review were at moderate/low risk of developing pre‐eclampsia at trial entry. Five reports stated that women at "high or increased risk of pre‐eclampsia" were enrolled (Beazley 2002; Chappell 1999; Han 1994; Poston 2006; Rivas 2000). Of these, four studies listed their criteria for determining risk status, and the criteria varied considerably. They included: previous pre‐eclampsia, chronic hypertension, insulin requiring diabetes mellitus or multiple gestation (Beazley 2002); abnormal uterine artery doppler at 18 to 22 weeks' gestation or a history in the preceding pregnancy of pre‐eclampsia necessitating delivery before 37 weeks' gestation, eclampsia or the HELLP (haemolysis elevated liver enzymes and low platelets) syndrome (Chappell 1999); pre‐eclampsia in the pregnancy preceding the index pregnancy, requiring delivery before 37 completed weeks' gestation; diagnosis of HELLP syndrome in any previous pregnancy at any stage of gestation; eclampsia in any previous pregnancy at any stage of gestation; essential hypertension requiring medication, currently or previously; maternal diastolic blood pressure of 90 mmHg or more before 20 weeks' gestation in the current pregnancy; type 1 or 2 diabetes, requiring insulin or oral hypoglycaemic therapy before the pregnancy; antiphospholipid syndrome; chronic renal disease; multiple pregnancy; abnormal uterine artery doppler waveform (18 to 22 weeks' gestation) or primiparity with a body‐mass index of 30 kg/m2 or more at first antenatal visit (Poston 2006); and nulliparity, previous pre‐eclampsia, obesity, hypertension, less than 20 years old, diabetes, nephropathy, mean arterial pressure above of 85 mmHg, positive roll‐over test, black race, family history of hypertension or pre‐eclampsia, twin pregnancy and poor socioeconomic conditions (Rivas 2000). These studies are grouped as moderate/high risk in this review, as they included high‐ and moderate‐risk women. Nevertheless, most women actually recruited to these studies were probably high risk, as 16% in the control group developed pre‐eclampsia, compared with 6% for the studies enrolling moderate/low‐risk women. However, the reported incidence of pre‐eclampsia may be influenced by differences in the definitions of pre‐eclampsia used in each study.
Two trials (351 women) recruited women exclusively between 12 and 20 weeks' gestation, including between 14 to 20 weeks' (Beazley 2002) and between 16 to 20 weeks' (Sharma 2003). The remaining studies all recruited women both before and after 20 weeks' gestation, including between 12 to 27 weeks' gestation (Merchant 2005), 14 to 21+6 weeks' gestation (Poston 2006; Rumbold 2006) and 16 to 22 weeks' gestation (Chappell 1999), while other studies merely stated below 16 weeks' gestation (Mahdy 2004), below 26 weeks' gestation (Steyn 2002) or below 29 weeks' gestation (Rivas 2000). One trial merely reported women were recruited "during late pregnancy" (Han 1994). No trials recruited women prior to 12 weeks' gestation. There are insufficient data to assess the effects of antioxidants in the prespecified subgroups based on gestation at trial entry (less than 12 weeks, 12 to 20 weeks, 21 to 28 weeks, greater than 28 weeks); none of the included trials reported results by gestation at trial entry that would enable classification into these subgroups. In this review, the gestational age subgroups reported are the gestational age categories reported by individual trials.
Interventions
One or more vitamins were the antioxidants evaluated in nine trials (6212 women). One small study (100 women) evaluated the mineral selenium. The interventions assessed were vitamin C and E (Beazley 2002; Chappell 1999; Poston 2006; Rumbold 2006), vitamin C alone (Steyn 2002), vitamin C and E plus fish oil and aspirin (Rivas 2000), multivitamin containing vitamin C and E (Merchant 2005), lycopene (Sharma 2003), red palm oil (Mahdy 2004) and selenium (Han 1994). For vitamin C, the daily dosages varied from 500 mg to 1000 mg. For vitamin E, all trials except one (Merchant 2005) used a daily dosage of 400 international units. For selenium the daily dose was 100 μg and for lycopene 4 mg. For the one trial assessing red palm oil, no information about dosage was provided.
Outcomes
Definition of pre‐eclampsia
Eight of the trials (5346 women) reported pre‐eclampsia as an outcome (Beazley 2002; Chappell 1999; Mahdy 2004; Poston 2006; Rivas 2000; Rumbold 2006; Sharma 2003; Steyn 2002), although four of these did not say how they defined pre‐eclampsia (Beazley 2002; Mahdy 2004; Rivas 2000; Steyn 2002). Two studies did not report pre‐eclampsia. One study (109 women) reported pregnancy‐induced hypertension and proteinuria (Han 1994), we have used the data for proteinuria in this review, on the assumption that it probably represents pre‐eclampsia. The other trial (1078 women) reported hypertension occurring at any time in pregnancy but no proteinuria or other symptoms of pre‐eclampsia (Merchant 2005). We have included data for this trial in the gestational hypertension outcome.
Three trials (Chappell 1999; Poston 2006; Sharma 2003) defined pre‐eclampsia according to the definitions specified by the International Society for the Study of Hypertension in Pregnancy (two recordings of diastolic blood pressure of 90 mmHg or above at least four hours apart plus proteinuria defined as excretion of 300 mg or more in 24 hours or two readings of 2+ or higher on dipstick analysis). One trial (Rumbold 2006) defined pre‐eclampsia according to the definition specified by the Australian Society for the Study of Hypertension in Pregnancy (two occasions of systolic blood pressure of at least 140 mmHg or diastolic blood pressure of at least 90 mmHg, or both, and one or more of: proteinuria, renal insufficiency, liver disease, neurological problems, haematological disturbances or fetal growth restriction). No trials reported the timing of onset of pre‐eclampsia (either prior to or after 34 weeks' gestation), two trials (Beazley 2002; Poston 2006) reported severe pre‐eclampsia.
Other outcomes
Five trials reported preterm birth (Beazley 2002; Chappell 1999; Poston 2006; Rumbold 2006; Steyn 2002) and small‐for‐gestational‐age infants as birthweight less than the 10th centile (Beazley 2002; Chappell 1999; Poston 2006; Rumbold 2006; Sharma 2003). Four trials reported any baby death (Chappell 1999; Poston 2006; Rumbold 2006; Steyn 2002), including miscarriage, stillbirth or neonatal death. One trial (Beazley 2002) reported "there were no pregnancy losses before 20 weeks' gestation", but provided no information about either stillbirth or neonatal death. No trials reported infant death (death after 28 days of life).
Secondary outcomes
Data for the secondary outcomes were poorly reported. For many outcomes, data were reported by one or two trials only. No trials reported maternal views of care or views about the acceptability of the intervention. Similarly, no trials reported bacterial sepsis in the infant, disability during childhood or poor childhood growth.
Excluded trials
Twenty‐two studies were excluded from the review. Six studies were excluded as women involved had established pre‐eclampsia at trial entry (Anthony 1996; Gulmezoglu 1997; Morrison 1984; Roes 2006; Sawhney 2000; Sikkema 2002); four studies were excluded as the interventions assessed were not considered to have direct antioxidant properties (Ferguson 1955; Herrera 1993; Marya 1987; Theobald 1937); three studies were non‐randomised or quasi‐randomised (Bolisetty 2002; People's League 1942; Powers 2000); seven studies did not report any clinically meaningful outcomes or pre‐eclampsia (Borna 2005; Casanueva 2005; Dijkhuizen 2004; Pawlowicz 2000; Pressman 2003; Radhika 2003; Thomson 2001) and two studies (Chaudhuri 1969; West 1999) had greater than 20% losses to follow up.
Risk of bias in included studies
Five trials (Chappell 1999; Poston 2006; Rumbold 2006; Sharma 2003; Steyn 2002) fulfilled all of the criteria for a high‐quality trial, that is they were rated 'A' for allocation concealment; women, caregivers and research staff were blinded to treatment allocation; had less than 3% of participants excluded and were placebo controlled. Five trials were not rated high quality (Beazley 2002; Han 1994; Mahdy 2004; Merchant 2005; Rivas 2000); these trials were excluded from the sensitivity analyses.
Randomisation and allocation concealment
Formal randomisation was reported in five trials by use of third‐party randomisation (Chappell 1999; Poston 2006; Rumbold 2006; Sharma 2003; Steyn 2002), that is women were allocated to each group either by an individual not directly involved in the research or via telephone or computer allocation. The degree of allocation concealment for these five trials was therefore adequate. For the other five trials (Beazley 2002; Han 1994; Mahdy 2004; Merchant 2005; Rivas 2000), the degree of allocation concealment was unclear, as no information was provided about the methods of randomisation and allocation concealment.
Blinding
Six trials explicitly stated that women, caregivers and researchers were blinded to treatment allocations (Chappell 1999; Merchant 2005; Poston 2006; Rumbold 2006; Sharma 2003; Steyn 2002). Two trials stated "double‐blind" in the text (Beazley 2002; Mahdy 2004) while the other trial used the term "triple‐blind" in the text (Rivas 2000). The degree of blinding, if any, was unclear for one trial (Han 1994).
Completeness of follow up
Three trials reported outcomes for all randomised women according to treatment allocation (Chappell 1999; Rumbold 2006; Sharma 2003) and another four did not mention any losses to follow up (Han 1994; Mahdy 2004; Rivas 2000; Steyn 2002). In the three remaining trials, losses to follow up were less than 1% (Poston 2006), 8% (Beazley 2002) and 19% (Merchant 2005).
Use of placebo
All trials used a placebo control.
Effects of interventions
Ten trials, involving 6533 women, compared any antioxidant supplementation with placebo (Beazley 2002; Chappell 1999; Han 1994; Mahdy 2004; Merchant 2005; Poston 2006; Rivas 2000; Rumbold 2006; Sharma 2003; Steyn 2002). In view of the heterogeneity in results for pre‐eclampsia, small‐for‐gestational‐age infant, gestational hypertension, bleeding episodes, gestational age at birth, birthweight, necrotising enterocolitis and respiratory distress syndrome, a random‐effects model was used for these outcomes.
Primary outcomes
Pre‐eclampsia
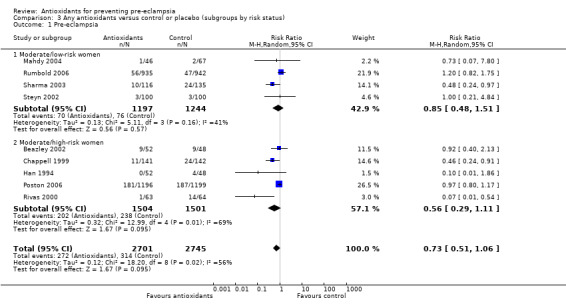
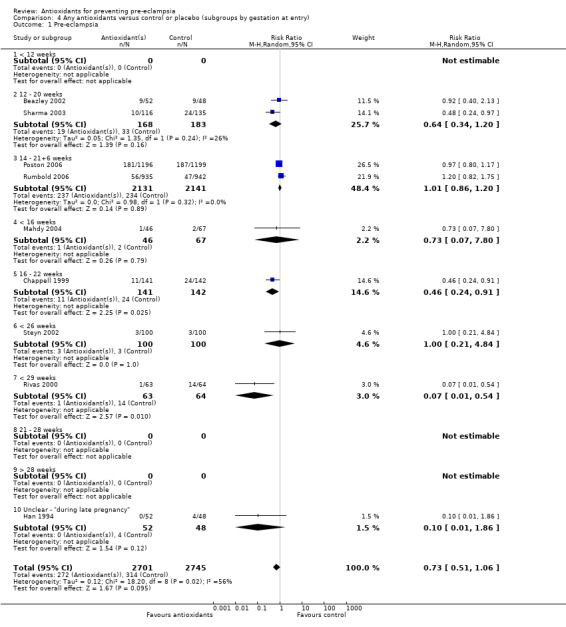
There was no clear difference in the relative risk (RR) of pre‐eclampsia between antioxidant supplemented and control groups using a random effects model (RR 0.73, 95% confidence intervals (CI) 0.51 to 1.06; nine trials, 5446 women) or a fixed effects model (RR 0.88, 95% CI 0.75 to 1.02). This result was not significantly different in the sensitivity analysis restricted to high‐quality studies (RR 0.82, 95% CI 0.58 to 1.16; five trials, 5006 women). Similarly, there were no clear differences between groups for moderate/low‐risk women (RR 0.85, 95% CI 0.48 to 1.51; four trials, 2441 women) or moderate/high‐risk women (RR 0.56, 95% CI 0.29 to 1.11; five trials, 3005 women). There are insufficient data to assess the effects of antioxidants in subgroups based on gestation at trial entry (less than 12 weeks, 12 to 20 weeks, 21 to 28 weeks, greater than 28 weeks); most trials included women in some or all of these gestational age subgroups, but did not report results by gestation at trial entry. The two largest trials (4272 women) recruited women between 14 and 21+6 weeks' gestation, and showed there was no clear difference in the RR of pre‐eclampsia between treatment groups (RR 1.01, 95% CI 0.86 to 1.20). The other subgroups by gestational age at trial entry should be interpreted with caution, as the numbers are small.
There was no clear difference between the groups for women allocated vitamin C and E alone (RR 0.92, 95% CI 0.68 to 1.25; four trials, 4655 women). All of the other subgroups by type of antioxidant should be interpreted with caution, as the numbers are small. Nevertheless, the RR of pre‐eclampsia was reduced for women allocated lycopene (RR 0.48, 95% CI 0.24 to 0.97; one trial, 251 women) and for women allocated vitamin C and E combined with aspirin and fish oil (RR 0.07, 95% CI 0.01 to 0.54; one trial, 127 women) rather than placebo. There were no clear differences between the treatment groups for women allocated vitamin C alone (RR 1.00, 95% CI 0.21 to 4.84; one trial, 200 women), red palm oil (RR 0.73, 95% CI 0.07 to 7.80; one trial, 113 women) or selenium (RR 0.10, 95% CI 0.01 to 1.86; one trial, 100 women). There were insufficient data to assess the possible impact of antioxidant dose or dietary intake of antioxidants at trial entry. All of the included trials used a daily dose of vitamin C considered to be within pharmacological range. For vitamin E, only one trial used a daily dosage considered to be below pharmacological range (Merchant 2005), however, the only outcome reported by this trial was gestational hypertension. Only one trial (Rumbold 2006) (1877 women) reported information about women's dietary intake at trial entry, where approximately 94% of women in both groups had an adequate intake of vitamin C, and 43% of women in both groups had an adequate intake of vitamin E at trial entry. Therefore, subgroup analyses based on antioxidant dose and dietary intake were not undertaken.
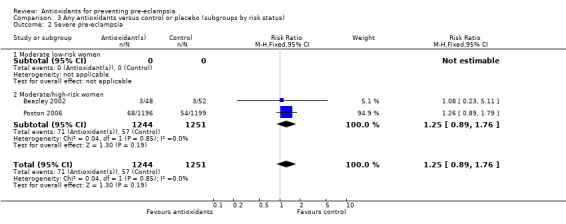
Severe pre‐eclampsia
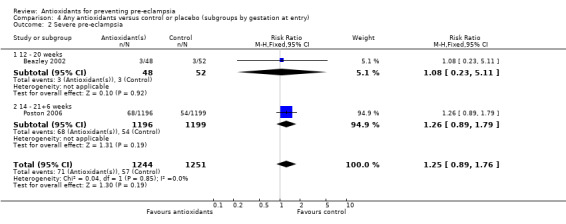
There was no clear difference in the RR of severe pre‐eclampsia (RR 1.25, 95% CI 0.89 to 1.76; two trials, 2495 women) between antioxidant and control groups. Sensitivity analysis based on quality included one trial (2395 women), the RR of severe pre‐eclampsia for women allocated antioxidants was 1.26 (95% CI 0.89 to 1.79). Only two trials reported on severe pre‐eclampsia, therefore the effect of antioxidants in subgroups is unclear; no data were available for women at moderate/low risk or for women allocated antioxidants other than vitamin C and E alone.
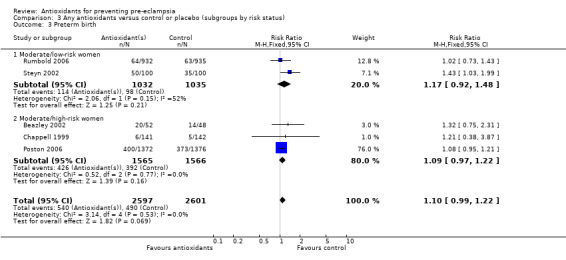
Preterm birth
Antioxidants were associated with a small increase in the RR of any preterm birth (RR 1.10, 95% CI 0.99 to 1.22; five trials, 5198 women), although this finding did not reach statistical significance (P = 0.07). There was no significant difference between treatment groups for the RR of very preterm birth (less than 34 weeks' gestation) (RR 1.19, 95% CI 0.98 to 1.45; two trials, 4651 women) or extremely preterm birth (less than 28 weeks' gestation) (RR 1.07, 95% CI 0.55 to 2.06; two trials, 2077 women) between antioxidant supplemented and control groups. Similarly, there was no significant difference in the RR of any preterm birth in the sensitivity analysis restricted to high‐quality studies (RR 1.10, 95% CI 0.98 to 1.22; four trials, 5098 women) or in the subgroup analyses for moderate/low‐risk women (RR 1.17, 95% CI 0.92 to 1.48; two trials, 2067 women) or moderate/high‐risk women (RR 1.09, 95% CI 0.97 to 1.22; three trials, 3131 women).
There was no significant difference in the RR of preterm birth between antioxidant and control groups for any of the subgroups based on gestation at trial entry. The only exception was an apparent increase in the RR for women enrolled at "less than 26 weeks' gestation" (RR 1.43, 95% CI 1.03 to 1.99; one trial, 200 women). Data for this subgroup should be interpreted with caution as they are based on one small trial (200 women). There was no clear difference between treatment groups for women allocated vitamin C and E alone (RR 1.08, 95% CI 0.96 to 1.20; four trials, 4998 women). However, the RR of preterm birth was increased for women allocated vitamin C alone (RR 1.43, 95% CI 1.03 to 1.99; one trial, 200 women); this is same trial that enrolled women at less than 26 weeks' gestation. The data should be interpreted with caution as the numbers are small. No data were available for women allocated antioxidants other than vitamin C and E or vitamin C alone.
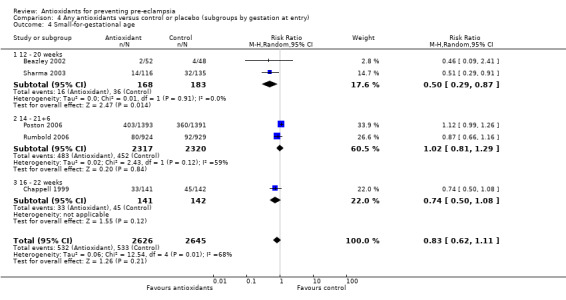
Small‐for‐gestational‐age infants
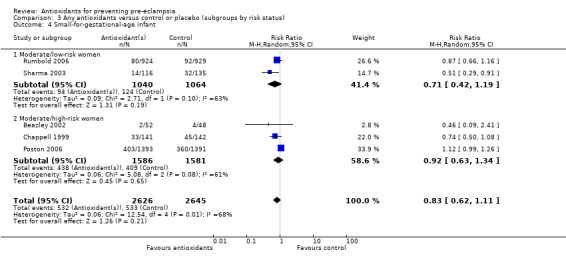
There was no clear difference between antioxidant and control groups for the RR of having a small‐for‐gestational‐age infant (RR 0.83, 95% CI 0.62 to 1.11; five trials, 5271 babies). Similarly, there was no clear difference in risk in the sensitivity analyses restricted to high‐quality studies (RR 0.84, 95% CI 0.63 to 1.13; four trials, 5171 babies) or in the subgroup analyses for moderate/low‐risk women (RR 0.71, 95% CI 0.42 to 1.19; two trials, 2104 babies) or moderate/high‐risk women (RR 0.92, 95% CI 0.63 to 1.34; three trials, 3167 babies).
For women recruited between 12 and 20 weeks' gestation the RR of the baby being small‐for‐gestational age was reduced for women allocated antioxidants (RR 0.50, 95% CI 0.29 to 0.87; two trials, 351 babies). However, these data should be interpreted with caution as the numbers are small. There was no clear difference between the treatment groups in the RR of small‐for‐gestational‐age infants for any other gestational age subgroups. There was no clear difference between treatment groups for women allocated vitamin C and E alone (RR 0.93, 95% CI 0.73 to 1.19; four trials, 5020 babies); however, the risk of small‐for‐gestational‐age infants was reduced for women allocated lycopene (RR 0.51, 95% CI 0.29 to 0.91; one trial, 251 babies). Again, these data should be interpreted with caution as the numbers are small. No data were available for women allocated antioxidants other than vitamin C and E or lycopene.
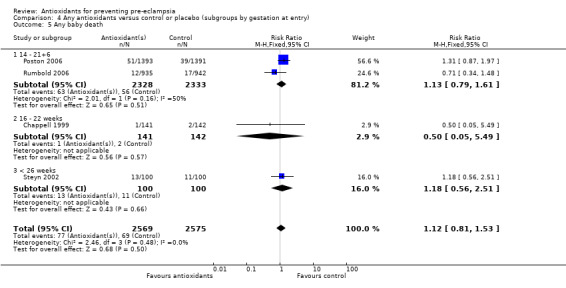
Baby death
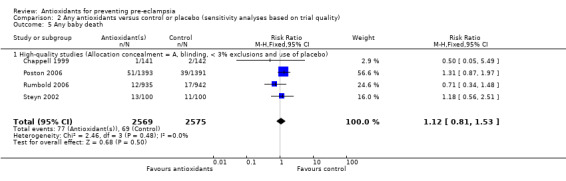
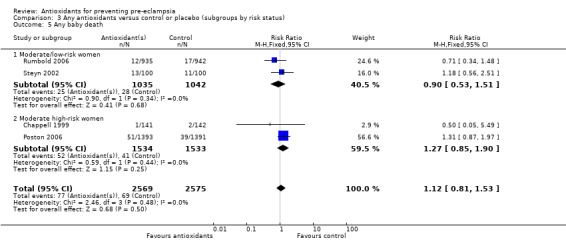
There was no clear difference between antioxidant and control groups in the RR of any baby death (RR 1.12, 95% CI 0.81 to 1.53; four trials, 5144 babies), or when any baby death was subgrouped on timing of death, including miscarriage or stillbirth (RR 1.32, 95% CI 0.92 to 1.90; four trials, 5144 babies) or neonatal death (RR 0.59, 95% CI 0.28 to 1.23; three trials, 4748 babies).
All of the trials reporting on baby death were rated high quality. There was no clear difference in the RR of baby death for moderate/low‐risk women (RR 0.90, 95% CI 0.53 to 1.51; two trials, 2077 babies) or moderate/high‐risk women (RR 1.27, 95% CI 0.85 to 1.90; two trials, 3067 babies). Similarly, there was no clear difference in the RR of baby death for any of the gestational age subgroups or the subgroups based on antioxidant type; however, no data were available for women allocated antioxidants other than vitamin C and E or vitamin C alone.
Other outcomes
One trial (Rumbold 2006) reported there were no maternal deaths in either the antioxidant or control group (1877 women) and another trial (Poston 2006) reported two maternal deaths (one in each group), however both deaths were unrelated to pre‐eclampsia. There were no clear differences between groups for the RR of gestational hypertension (RR 0.89, 95% CI 0.62 to 1.26; seven trials, 5817 women) or severe hypertension (RR 1.39, 95% CI 0.85 to 2.30; two trials, 4272 women); however, women allocated antioxidants were more likely to require antihypertensive therapy (RR 1.77, 95% CI 1.22 to 2.57; two trials, 4272 women). There were no clear differences between groups for any other outcomes including elective delivery (RR 1.11, 95% CI 0.99 to 1.25; two trials, 2077 women); caesarean section (RR 1.02, 95% CI 0.87 to 1.18; one trial, 1877 women); bleeding episodes (RR 1.28, 95% CI 0.35 to 4.68; three trials, 2360 women); serious maternal morbidity (RR 1.22, 95% CI 0.39 to 3.81; three trials, 4523 women); gestational age at birth (weighted mean difference (WMD) 0.26 weeks, 95% CI ‐0.84 to 1.36; three trials, 3135 women); birthweight (WMD 14.60 grams, 95% CI ‐61.99 to 91.18; five trials, 5089 women); Apgar score at five minutes: low (less than seven) (RR 1.63, 95% CI 0.92 to 2.87; one trial, 2784 infants), very low (less than four) (RR 0.92, 95% CI 0.41 to 2.07; two trials, 4651 infants); respiratory distress syndrome (RR 0.48, 95% CI 0.08 to 2.85; two trials, 4567 women); chronic lung disease (RR 0.20, 95% CI 0.02 to 1.72; one trial, 1853 infants); neonatal bleeding episodes (RR 0.61, 95% CI 0.29 to 1.29; two trials, 4637 infants); necrotising enterocolitis (RR 1.10, 95% CI 0.09 to 13.15; two trials, 4567 women) or retinopathy of prematurity (RR 0.88, 95% CI 0.31 to 2.50; two trials, 4567 infants).
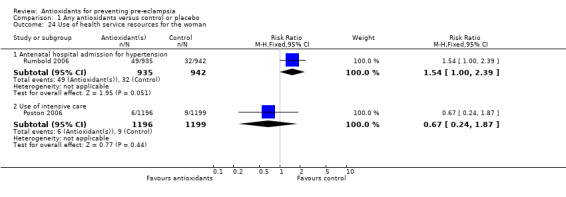
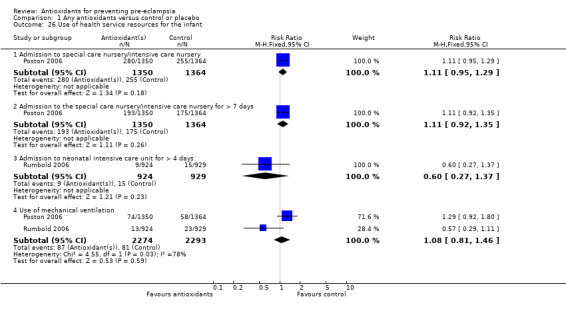
Only one trial (Rumbold 2006) reported maternal side‐effects. Women allocated antioxidants in this trial were more likely to self‐report abdominal pain late in pregnancy (RR 1.61, 95% CI 1.11 to 2.34; one trial, 1745 women); however, the trial reported there were no differences in any other self‐reported side‐effects between antioxidant and control groups. Use of health service resources for women or infants was variously reported by three trials (Poston 2006; Rumbold 2006; Steyn 2002). There was no overall effect on the use of health service resources for either the woman or infant except for antenatal hospital admission for hypertension. Women allocated antioxidants were more likely to require an antenatal hospital admission for hypertension (RR 1.54, 95% CI 1.00 to 2.39; one trial, 1877 women).
Heterogeneity
To explore potential sources of heterogeneity we undertook prespecified sensitivity and subgroup analyses investigating the impact of: methodological variation; clinical variation, including differences in women's baseline risk of pre‐eclampsia and dietary intake of antioxidants at trial entry; and variation in treatment characteristics, including duration of antioxidant therapy (related to gestational age at trial entry), and the type and dosage of antioxidant assessed.
Differences in the quality of trials included in this review are unlikely to be a major source of heterogeneity; there was no difference in the effect of antioxidants in the sensitivity analysis restricted to high‐quality trials. Furthermore, none of the prespecified subgroups explored in this review clearly explain the heterogeneity. There was no significant difference in the effects of antioxidants for women at moderate/low risk or moderate/high risk of pre‐eclampsia. For the other subgroup analyses, there were insufficient data to adequately explore the effects of antioxidants in all subgroups. Nevertheless, differences in the type of antioxidant assessed may have contributed to the heterogeneity, as the magnitude of the effect of antioxidants was greater for women allocated lycopene and for women allocated vitamin C and E combined with aspirin and fish oil, compared with women allocated vitamin C and E alone.
The possible factors contributing to heterogeneity may become clearer when the results of currently ongoing trials are available.
Discussion
Uncertainty remains about the effects of antioxidants for the prevention of pre‐eclampsia and its related complications. Routine use of antioxidant supplements in pregnancy should only be considered if antioxidants are shown to be associated with substantive benefits for the mother or baby, or both; none have been consistently demonstrated in this review. The point estimate for the effect of antioxidant supplements on pre‐eclampsia was a 27% reduction in the relative risk, however, the 95% confidence interval for the true effect ranged from a 49% reduction to a 6% increase, when compared with the control group. There was no clear difference between antioxidant and control groups for the risk of other perinatal complications, including small‐for‐gestational‐age infants and baby death.
For many outcomes, including pre‐eclampsia, the effects of antioxidants were not consistent across trials, and there was significant statistical heterogeneity. Differences in the type of antioxidant assessed may be one source of this heterogeneity, as the magnitude of effect of antioxidants on the relative risk of pre‐eclampsia was much greater for women allocated lycopene (52% reduction) and for women allocated vitamin C and E combined with aspirin and fish oil (93% reduction) compared with women allocated vitamin C and E alone (8% reduction). However, the subgroup data on heterogeneity related to antioxidant type should be interpreted with caution. The number of women included in the subgroups for types of antioxidants other than combined vitamin C and E was small, resulting in the potential to be misled by bias and random error. Much more information is needed to assess the effects of antioxidants other than vitamin C and E on the risk of pre‐eclampsia and other complications.
The majority of data in this review are contributed to by two large trials assessing combined vitamin C and E therapy (Poston 2006; Rumbold 2006), therefore it is likely that differences between these studies are contributing to the heterogeneity. There are clear differences between these studies in participant characteristics; one trial enrolled nulliparous women with a singleton pregnancy (Rumbold 2006), the other enrolled women at increased risk of pre‐eclampsia based on a range of risk factors (Poston 2006). Subgroup analyses compared the effects of antioxidants for moderate/low‐risk women and moderate/high‐risk women, and found no overall difference between these subgroups. However, in the moderate/high‐risk group, the incidence of pre‐eclampsia in control groups ranged from 8% to 22%, suggesting a range of maternal risk. It is possible that the effects of antioxidants are different for women with specific risk factors, and this may also contribute to the heterogeneity. However, there is insufficient detail in the trial publications to extract data for specific risk factors, and therefore further assess the effects of antioxidants for women at different baseline risk. Undertaking a review based on data from individual women may help to determine if the effects of antioxidants are different for women at different baseline risk.
The heterogeneity found may also be related to study size; the majority of the small studies included in this review had positive results. Therefore, there may be publication bias, where small studies that failed to show an effect for antioxidants have not being published. When the effect size reported by trials is plotted against the trial sample size, the resulting graph (funnel plot) for the risk of pre‐eclampsia is asymmetric (Figure 1), suggesting that data from small negative trials are missing. It is also possible that differences between trials in women's risk status at trial entry and the type of antioxidant assessed are contributing to funnel plot asymmetry. However, caution must be taken when interpreting the funnel plot asymmetry. Our assessment of publication bias was not prespecified and post‐hoc investigations are more susceptible to bias.
1.
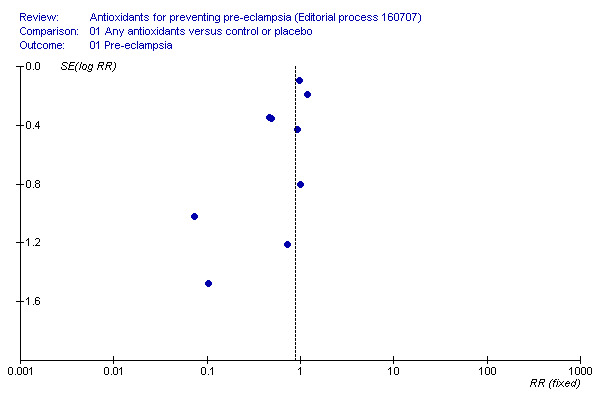
Funnel plot of the effects of antioxidants for preventing pre‐eclampsia
Antioxidants were associated with an increased risk of antihypertensive therapy and antenatal admission for hypertension. Although these findings raise concern about the safety of antioxidants in pregnancy, they were not clearly reflected in an increase in the risk of other hypertensive complications in pregnancy including pre‐eclampsia, gestational hypertension or in the use of health services such as intensive care. It is therefore possible that these results reflect reporting bias, as only two studies reported on use of antihypertensives and only one study reported specifically on hospital admission for hypertension.
Antioxidants also appeared to be associated with a small increase in the risk of preterm birth, although this result is strongly influenced by one small trial evaluating vitamin C alone for women already at high risk of giving birth preterm (Steyn 2002). The effect is not statistically significant for studies evaluating vitamin C and E alone. There was no significant difference in the relative risk of preterm birth for other types of antioxidants, and importantly, there was no increase in adverse neonatal outcomes associated with any type of antioxidants. Nevertheless, further evidence is needed to clearly demonstrate that antioxidants do more good than harm.
Women allocated antioxidants were also more likely to self‐report abdominal pain in late pregnancy, although this outcome was only reported by one trial (Rumbold 2006). We are unaware of any other studies reporting an association between antioxidants and abdominal pain. Although this potential side‐effect did not stop women taking antioxidant supplements, these findings highlight the need to assess potential side‐effects and adverse effects in trials. None of the included trials reported on the acceptability of the intervention to women. Data were poorly reported for many of the secondary outcomes, particularly substantive measures of morbidity for the baby. From the available data, there were no significant differences between groups for serious infant outcomes, including respiratory distress syndrome, necrotising enterocolitis, neonatal bleeding episodes and retinopathy of prematurity, although the direction of effect for most neonatal outcomes favoured antioxidants.
Results of the current ongoing trials will help to clarify whether antioxidant supplementation overall results in benefit or harm, and whether the effects of antioxidants are mediated by specific maternal risk factors and the type, dosage and timing of antioxidant supplementation.
Authors' conclusions
Implications for practice.
Evidence from this review does not support routine antioxidant supplementation during pregnancy to reduce the risk of pre‐eclampsia and other serious complications in pregnancy.
Implications for research.
Further large trials should not be initiated until the results of currently ongoing trials (see below) become available. Data from these studies should clarify whether or not antioxidants have any impact on pre‐eclampsia and its complications. They should also provide information about potential side‐effects and adverse events associated with antioxidants. Although most of the ongoing trials are evaluating the combination of vitamin C and E, trials of other antioxidant agents should not be initiated until there has been an opportunity to assess the combined results of existing and currently ongoing trials.
To date, no trials have reported on long‐term outcome, for either the women or the children. If antioxidants are shown to have an overall beneficial effect in the short term, such long‐term follow up will become a priority, Where possible, currently recruiting trials should collect information that would facilitate such follow up, should it become necessary.
If, when all the currently ongoing trials have reported their results, it appears that antioxidants might be worthwhile for at least some women, undertaking a review based on data from individual women may help to determine who is most likely to benefit.
The ongoing trials are in: the United Kingdom (DAPIT: david.mccance@royalhospitals.n‐i.nhs.uk); the United States of America (CAPPS: rsijmr@mwri.magee.edu); Brazil (salvio_freire@uol.com.br) and India, Peru, South Africa and Vietnam (VIP: WHO component; merialdim@who.int or lucilla.poston@kcl.ac.uk); and Canada (INTAPP: william.fraser@ogy.ulaval.ca), however, the INTAPP trial has stopped recruiting women.
What's new
| Date | Event | Description |
|---|---|---|
| 16 May 2012 | Amended | Contact details updated. |
History
Protocol first published: Issue 2, 2003 Review first published: Issue 4, 2005
| Date | Event | Description |
|---|---|---|
| 4 February 2008 | Amended | Converted to new review format. |
| 26 October 2007 | New citation required and conclusions have changed | The conclusions have changed: the evidence from this review does not support routine antioxidant supplementation during pregnancy to reduce the risk of pre‐eclampsia and other serious complications in pregnancy. |
| 26 October 2007 | New search has been performed | Search updated in May 2007. We identified 15 new trials. Four have been included (Mahdy 2004; Merchant 2005; Poston 2006; Rumbold 2006); three are awaiting assessment (Kubik 2004; Negro 2007; Rumiris 2006) and eight have been excluded (Borna 2005; Casanueva 2005; Dijkhuizen 2004; Radhika 2003; Roes 2006; Theobald 1937; Thomson 2001; West 1999). We moved one study from the included to the excluded studies (People's League 1942) because we now exclude quasi‐random studies ‐ see differences between protocol and review. |
Acknowledgements
We thank Philippa Middleton for assisting with the data extraction and for her advice and comments regarding the format of the review. We thank Associate Professor Parul Christian and Dr Anwar Merchant for providing additional unpublished information about their trials.
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), one or more members of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
Data and analyses
Comparison 1. Any antioxidants versus control or placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pre‐eclampsia | 9 | 5446 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.51, 1.06] |
| 2 Severe pre‐eclampsia | 2 | 2495 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.89, 1.76] |
| 3 Preterm birth | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 < 37 weeks | 5 | 5198 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.99, 1.22] |
| 3.2 < 34 weeks | 2 | 4651 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.98, 1.45] |
| 3.3 < 28 weeks | 2 | 2077 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.55, 2.06] |
| 4 Small‐for‐gestational age | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 < 10th centile | 5 | 5271 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.62, 1.11] |
| 4.2 < 5th centile | 1 | 2784 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.98, 1.32] |
| 4.3 < 3rd centile | 1 | 1853 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.38, 1.08] |
| 5 Any baby death | 4 | 5144 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.81, 1.53] |
| 6 Any baby death (subgrouped by timing of death) | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Miscarriage or stillbirth | 4 | 5144 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.92, 1.90] |
| 6.2 Neonatal death | 3 | 4748 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.28, 1.23] |
| 7 Maternal death | 2 | 4272 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.06, 16.01] |
| 8 Gestational hypertension | 7 | 5817 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.62, 1.26] |
| 9 Severe hypertension | 2 | 4272 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.85, 2.30] |
| 10 Use of antiypertensives | 2 | 4272 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [1.22, 2.57] |
| 10.1 Any antihypertensives | 1 | 1877 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.03, 2.69] |
| 10.2 Intravenous antihypertensives | 1 | 2395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.07, 3.53] |
| 11 Elective delivery (induction of labour or elective caesarean section) | 2 | 2077 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.99, 1.25] |
| 12 Caesarean section (emergency plus elective) | 1 | 1877 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.87, 1.18] |
| 13 Bleeding episodes (including placental abruption, APH, PPH, need for transfusion) | 3 | 2360 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.35, 4.68] |
| 14 Serious maternal morbidity (including eclampsia, liver and renal failure, DIC, stroke) | 3 | 4523 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.39, 3.81] |
| 15 Gestational age at birth | 3 | 3135 | Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.84, 1.36] |
| 16 Birthweight | 5 | 5089 | Mean Difference (IV, Random, 95% CI) | 14.60 [‐61.99, 91.18] |
| 17 Apgar score at 5 minutes | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 Low (< 7) | 1 | 2784 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [0.92, 2.87] |
| 17.2 Very low (< 4) | 2 | 4651 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.41, 2.07] |
| 18 Respiratory distress syndrome | 2 | 4567 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.08, 2.85] |
| 19 Chronic lung disease | 1 | 1853 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.02, 1.72] |
| 20 Neonatal bleeding episodes (intraventricular haemorrhage and periventricular leukomalacia) | 2 | 4637 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.29, 1.29] |
| 21 Necrotising enterocolitis | 2 | 4567 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.09, 13.15] |
| 22 Retinopathy of prematurity | 2 | 4567 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.31, 2.50] |
| 23 Side‐effects not sufficient to stop supplementation | 1 | 1745 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [1.11, 2.34] |
| 23.1 Self‐reported abdominal pain in late pregnancy | 1 | 1745 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [1.11, 2.34] |
| 24 Use of health service resources for the woman | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 24.1 Antenatal hospital admission for hypertension | 1 | 1877 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [1.00, 2.39] |
| 24.2 Use of intensive care | 1 | 2395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.24, 1.87] |
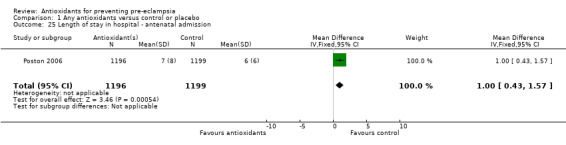
| 25 Length of stay in hospital ‐ antenatal admission | 1 | 2395 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [0.43, 1.57] |
| 26 Use of health service resources for the infant | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 26.1 Admission to special care nursery/intensive care nursery | 1 | 2714 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.95, 1.29] |
| 26.2 Admission to the special care nursery/intensive care nursery for > 7 days | 1 | 2714 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.92, 1.35] |
| 26.3 Admission to neonatal intensive care unit for > 4 days | 1 | 1853 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.27, 1.37] |
| 26.4 Use of mechanical ventilation | 2 | 4567 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.81, 1.46] |
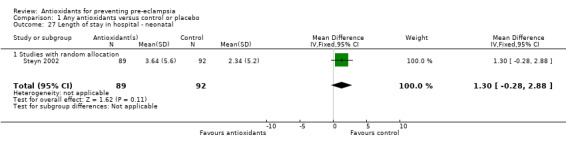
| 27 Length of stay in hospital ‐ neonatal | 1 | 181 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [‐0.28, 2.88] |
| 27.1 Studies with random allocation | 1 | 181 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [‐0.28, 2.88] |
1.1. Analysis.
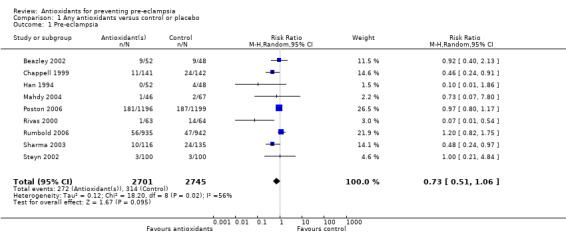
Comparison 1 Any antioxidants versus control or placebo, Outcome 1 Pre‐eclampsia.
1.2. Analysis.
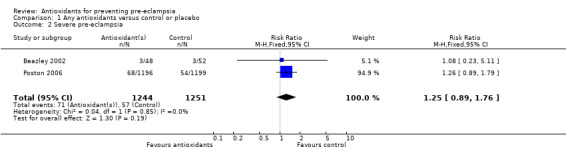
Comparison 1 Any antioxidants versus control or placebo, Outcome 2 Severe pre‐eclampsia.
1.3. Analysis.
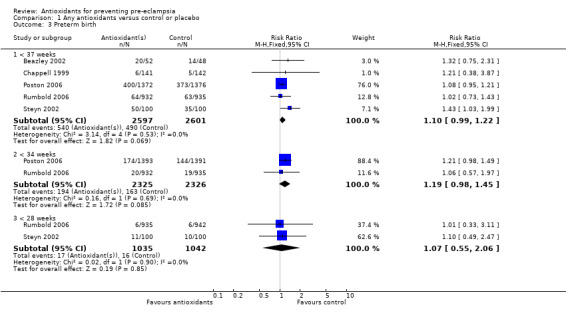
Comparison 1 Any antioxidants versus control or placebo, Outcome 3 Preterm birth.
1.4. Analysis.
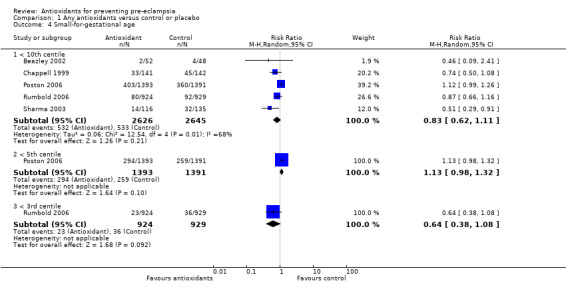
Comparison 1 Any antioxidants versus control or placebo, Outcome 4 Small‐for‐gestational age.
1.5. Analysis.
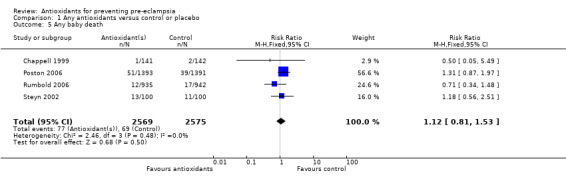
Comparison 1 Any antioxidants versus control or placebo, Outcome 5 Any baby death.
1.6. Analysis.
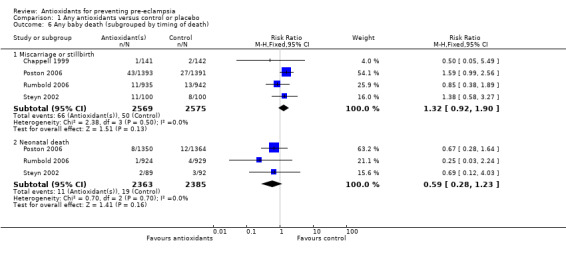
Comparison 1 Any antioxidants versus control or placebo, Outcome 6 Any baby death (subgrouped by timing of death).
1.7. Analysis.
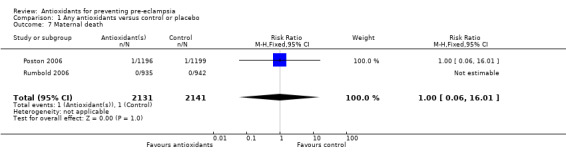
Comparison 1 Any antioxidants versus control or placebo, Outcome 7 Maternal death.
1.8. Analysis.
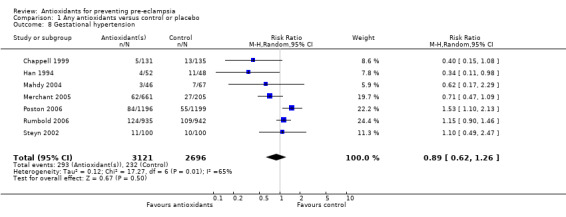
Comparison 1 Any antioxidants versus control or placebo, Outcome 8 Gestational hypertension.
1.9. Analysis.
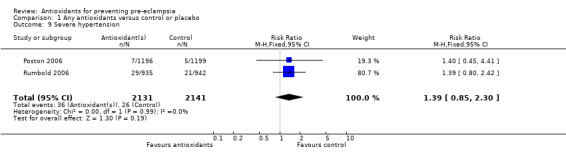
Comparison 1 Any antioxidants versus control or placebo, Outcome 9 Severe hypertension.
1.10. Analysis.
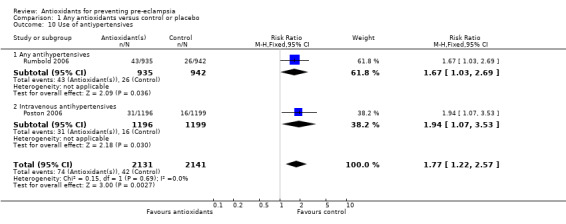
Comparison 1 Any antioxidants versus control or placebo, Outcome 10 Use of antiypertensives.
1.11. Analysis.
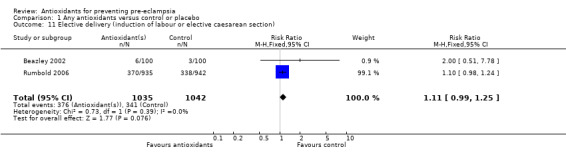
Comparison 1 Any antioxidants versus control or placebo, Outcome 11 Elective delivery (induction of labour or elective caesarean section).
1.12. Analysis.
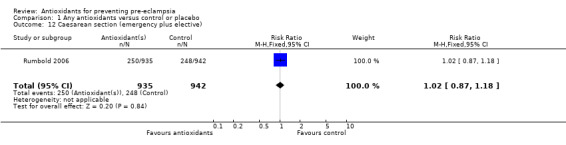
Comparison 1 Any antioxidants versus control or placebo, Outcome 12 Caesarean section (emergency plus elective).
1.13. Analysis.
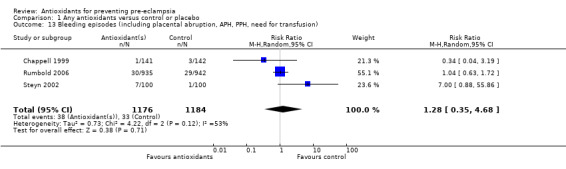
Comparison 1 Any antioxidants versus control or placebo, Outcome 13 Bleeding episodes (including placental abruption, APH, PPH, need for transfusion).
1.14. Analysis.
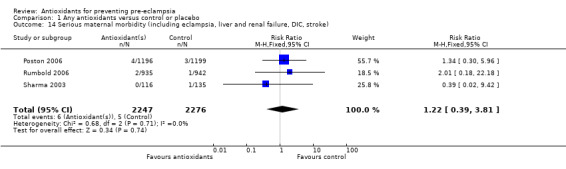
Comparison 1 Any antioxidants versus control or placebo, Outcome 14 Serious maternal morbidity (including eclampsia, liver and renal failure, DIC, stroke).
1.15. Analysis.
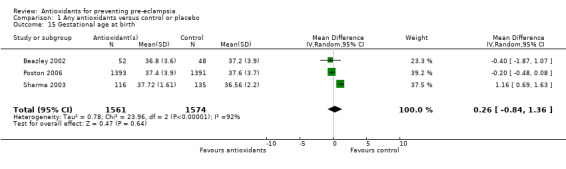
Comparison 1 Any antioxidants versus control or placebo, Outcome 15 Gestational age at birth.
1.16. Analysis.
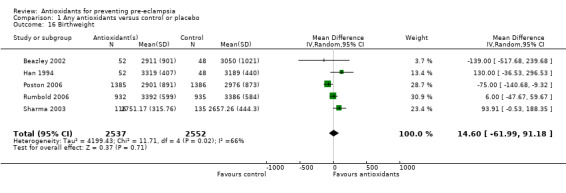
Comparison 1 Any antioxidants versus control or placebo, Outcome 16 Birthweight.
1.17. Analysis.
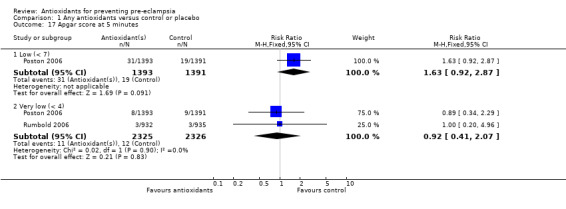
Comparison 1 Any antioxidants versus control or placebo, Outcome 17 Apgar score at 5 minutes.
1.18. Analysis.
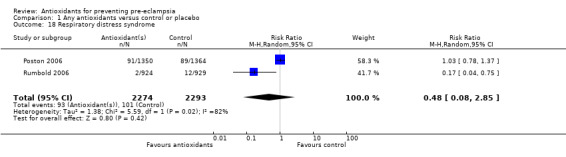
Comparison 1 Any antioxidants versus control or placebo, Outcome 18 Respiratory distress syndrome.
1.19. Analysis.
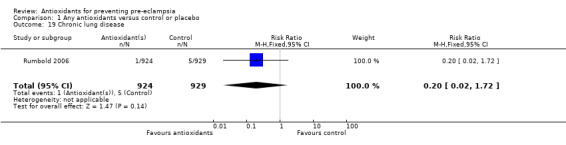
Comparison 1 Any antioxidants versus control or placebo, Outcome 19 Chronic lung disease.
1.20. Analysis.
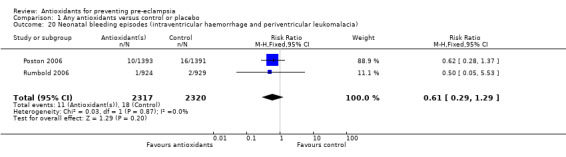
Comparison 1 Any antioxidants versus control or placebo, Outcome 20 Neonatal bleeding episodes (intraventricular haemorrhage and periventricular leukomalacia).
1.21. Analysis.
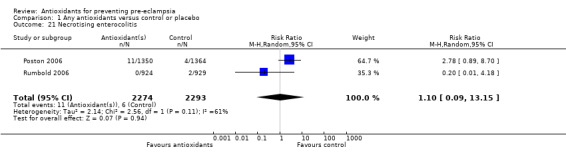
Comparison 1 Any antioxidants versus control or placebo, Outcome 21 Necrotising enterocolitis.
1.22. Analysis.
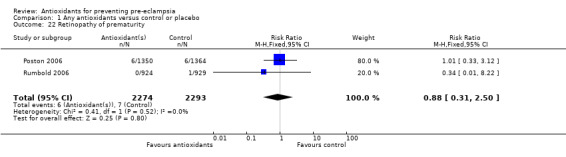
Comparison 1 Any antioxidants versus control or placebo, Outcome 22 Retinopathy of prematurity.
1.23. Analysis.
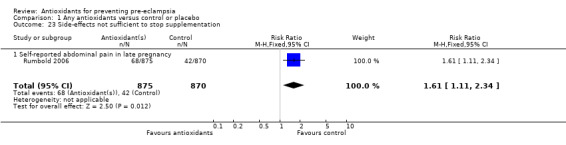
Comparison 1 Any antioxidants versus control or placebo, Outcome 23 Side‐effects not sufficient to stop supplementation.
1.24. Analysis.
Comparison 1 Any antioxidants versus control or placebo, Outcome 24 Use of health service resources for the woman.
1.25. Analysis.
Comparison 1 Any antioxidants versus control or placebo, Outcome 25 Length of stay in hospital ‐ antenatal admission.
1.26. Analysis.
Comparison 1 Any antioxidants versus control or placebo, Outcome 26 Use of health service resources for the infant.
1.27. Analysis.
Comparison 1 Any antioxidants versus control or placebo, Outcome 27 Length of stay in hospital ‐ neonatal.
Comparison 2. Any antioxidants versus control or placebo (sensitivity analyses based on trial quality).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
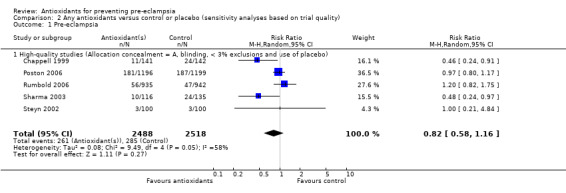
| 1 Pre‐eclampsia | 5 | 5006 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.58, 1.16] |
| 1.1 High‐quality studies (Allocation concealment = A, blinding, < 3% exclusions and use of placebo) | 5 | 5006 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.58, 1.16] |
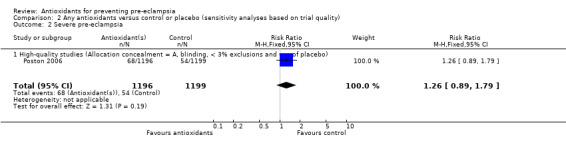
| 2 Severe pre‐eclampsia | 1 | 2395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.89, 1.79] |
| 2.1 High‐quality studies (Allocation concealment = A, blinding, < 3% exclusions and use of placebo) | 1 | 2395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.89, 1.79] |
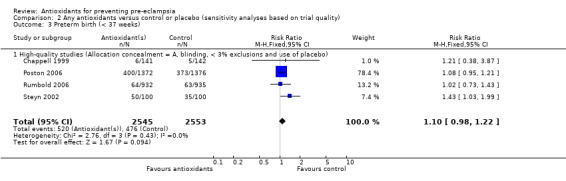
| 3 Preterm birth (< 37 weeks) | 4 | 5098 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.98, 1.22] |
| 3.1 High‐quality studies (Allocation concealment = A, blinding, < 3% exclusions and use of placebo) | 4 | 5098 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.98, 1.22] |
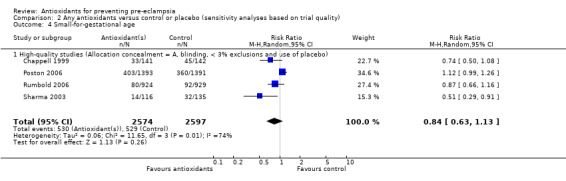
| 4 Small‐for‐gestational age | 4 | 5171 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.63, 1.13] |
| 4.1 High‐quality studies (Allocation concealment = A, blinding, < 3% exclusions and use of placebo) | 4 | 5171 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.63, 1.13] |
| 5 Any baby death | 4 | 5144 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.81, 1.53] |
| 5.1 High‐quality studies (Allocation concealment = A, blinding, < 3% exclusions and use of placebo) | 4 | 5144 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.81, 1.53] |
2.1. Analysis.
Comparison 2 Any antioxidants versus control or placebo (sensitivity analyses based on trial quality), Outcome 1 Pre‐eclampsia.
2.2. Analysis.
Comparison 2 Any antioxidants versus control or placebo (sensitivity analyses based on trial quality), Outcome 2 Severe pre‐eclampsia.
2.3. Analysis.
Comparison 2 Any antioxidants versus control or placebo (sensitivity analyses based on trial quality), Outcome 3 Preterm birth (< 37 weeks).
2.4. Analysis.
Comparison 2 Any antioxidants versus control or placebo (sensitivity analyses based on trial quality), Outcome 4 Small‐for‐gestational age.
2.5. Analysis.
Comparison 2 Any antioxidants versus control or placebo (sensitivity analyses based on trial quality), Outcome 5 Any baby death.
Comparison 3. Any antioxidants versus control or placebo (subgroups by risk status).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pre‐eclampsia | 9 | 5446 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.51, 1.06] |
| 1.1 Moderate/low‐risk women | 4 | 2441 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.48, 1.51] |
| 1.2 Moderate/high‐risk women | 5 | 3005 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.29, 1.11] |
| 2 Severe pre‐eclampsia | 2 | 2495 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.89, 1.76] |
| 2.1 Moderate low‐risk women | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Moderate/high‐risk women | 2 | 2495 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.89, 1.76] |
| 3 Preterm birth | 5 | 5198 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.99, 1.22] |
| 3.1 Moderate/low‐risk women | 2 | 2067 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.92, 1.48] |
| 3.2 Moderate/high‐risk women | 3 | 3131 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.97, 1.22] |
| 4 Small‐for‐gestational‐age infant | 5 | 5271 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.62, 1.11] |
| 4.1 Moderate/low‐risk women | 2 | 2104 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.42, 1.19] |
| 4.2 Moderate/high‐risk women | 3 | 3167 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.63, 1.34] |
| 5 Any baby death | 4 | 5144 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.81, 1.53] |
| 5.1 Moderate/low‐risk women | 2 | 2077 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.53, 1.51] |
| 5.2 Moderate high‐risk women | 2 | 3067 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.85, 1.90] |
3.1. Analysis.
Comparison 3 Any antioxidants versus control or placebo (subgroups by risk status), Outcome 1 Pre‐eclampsia.
3.2. Analysis.
Comparison 3 Any antioxidants versus control or placebo (subgroups by risk status), Outcome 2 Severe pre‐eclampsia.
3.3. Analysis.
Comparison 3 Any antioxidants versus control or placebo (subgroups by risk status), Outcome 3 Preterm birth.
3.4. Analysis.
Comparison 3 Any antioxidants versus control or placebo (subgroups by risk status), Outcome 4 Small‐for‐gestational‐age infant.
3.5. Analysis.
Comparison 3 Any antioxidants versus control or placebo (subgroups by risk status), Outcome 5 Any baby death.
Comparison 4. Any antioxidants versus control or placebo (subgroups by gestation at entry).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pre‐eclampsia | 9 | 5446 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.51, 1.06] |
| 1.1 < 12 weeks | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 12 ‐ 20 weeks | 2 | 351 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.34, 1.20] |
| 1.3 14 ‐ 21+6 weeks | 2 | 4272 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.86, 1.20] |
| 1.4 < 16 weeks | 1 | 113 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.07, 7.80] |
| 1.5 16 ‐ 22 weeks | 1 | 283 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.24, 0.91] |
| 1.6 < 26 weeks | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.21, 4.84] |
| 1.7 < 29 weeks | 1 | 127 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.01, 0.54] |
| 1.8 21 ‐ 28 weeks | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.9 > 28 weeks | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.10 Unclear ‐ "during late pregnancy" | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.01, 1.86] |
| 2 Severe pre‐eclampsia | 2 | 2495 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.89, 1.76] |
| 2.1 12 ‐ 20 weeks | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.23, 5.11] |
| 2.2 14 ‐ 21+6 weeks | 1 | 2395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.89, 1.79] |
| 3 Preterm birth | 5 | 5198 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.99, 1.22] |
| 3.1 12 ‐ 20 weeks | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.75, 2.31] |
| 3.2 14 ‐ 21+6 | 2 | 4615 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.95, 1.20] |
| 3.3 16 ‐ 22 weeks | 1 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.38, 3.87] |
| 3.4 < 26 weeks | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [1.03, 1.99] |
| 4 Small‐for‐gestational age | 5 | 5271 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.62, 1.11] |
| 4.1 12 ‐ 20 weeks | 2 | 351 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.29, 0.87] |
| 4.2 14 ‐ 21+6 | 2 | 4637 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.81, 1.29] |
| 4.3 16 ‐ 22 weeks | 1 | 283 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.50, 1.08] |
| 5 Any baby death | 4 | 5144 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.81, 1.53] |
| 5.1 14 ‐ 21+6 | 2 | 4661 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.79, 1.61] |
| 5.2 16 ‐ 22 weeks | 1 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.05, 5.49] |
| 5.3 < 26 weeks | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.56, 2.51] |
4.1. Analysis.
Comparison 4 Any antioxidants versus control or placebo (subgroups by gestation at entry), Outcome 1 Pre‐eclampsia.
4.2. Analysis.
Comparison 4 Any antioxidants versus control or placebo (subgroups by gestation at entry), Outcome 2 Severe pre‐eclampsia.
4.3. Analysis.
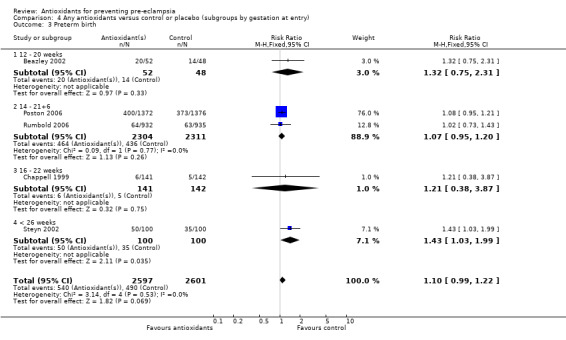
Comparison 4 Any antioxidants versus control or placebo (subgroups by gestation at entry), Outcome 3 Preterm birth.
4.4. Analysis.
Comparison 4 Any antioxidants versus control or placebo (subgroups by gestation at entry), Outcome 4 Small‐for‐gestational age.
4.5. Analysis.
Comparison 4 Any antioxidants versus control or placebo (subgroups by gestation at entry), Outcome 5 Any baby death.
Comparison 5. Any antioxidants versus control or placebo (subgroups by antioxidant type).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pre‐eclampsia | 9 | 5446 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.51, 1.06] |
| 1.1 Vitamin C and E alone | 4 | 4655 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.68, 1.25] |
| 1.2 Vitamin C and E with other agents | 1 | 127 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.01, 0.54] |
| 1.3 Vitamin C alone | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.21, 4.84] |
| 1.4 Lycopene | 1 | 251 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.24, 0.97] |
| 1.5 Red palm oil | 1 | 113 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.07, 7.80] |
| 1.6 Selenium | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.01, 1.86] |
| 2 Severe pre‐eclampsia | 2 | 2495 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.89, 1.76] |
| 2.1 Vitamin C and E alone | 2 | 2495 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.89, 1.76] |
| 3 Preterm birth | 5 | 5198 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.99, 1.22] |
| 3.1 Vitamin C and E alone | 4 | 4998 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.96, 1.20] |
| 3.2 Vitamin C and E with other agents | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Vitamin C alone | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [1.03, 1.99] |
| 3.4 Lycopene | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.5 Red palm oil | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.6 Selenium | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Small‐for‐gestational age | 5 | 5271 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.62, 1.11] |
| 4.1 Vitamin C and E alone | 4 | 5020 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.73, 1.19] |
| 4.2 Vitamin C and E with other agents | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Vitamin C alone | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 Lycopene | 1 | 251 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.29, 0.91] |
| 4.5 Red palm oil | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.6 Selenium | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Any baby death | 4 | 5144 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.81, 1.53] |
| 5.1 Vitamin C and E alone | 3 | 4944 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.78, 1.57] |
| 5.2 Vitamin C and E with other agents | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Vitamin C alone | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.56, 2.51] |
| 5.4 Lycopene | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.5 Red palm oil | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.6 Selenium | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
5.1. Analysis.
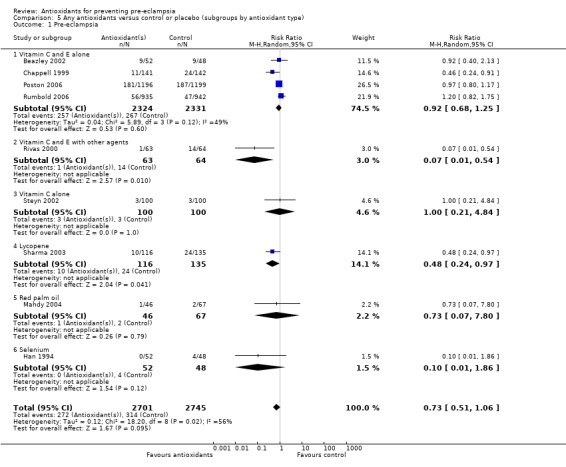
Comparison 5 Any antioxidants versus control or placebo (subgroups by antioxidant type), Outcome 1 Pre‐eclampsia.
5.2. Analysis.
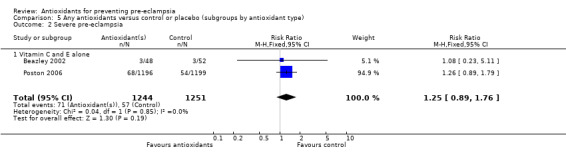
Comparison 5 Any antioxidants versus control or placebo (subgroups by antioxidant type), Outcome 2 Severe pre‐eclampsia.
5.3. Analysis.
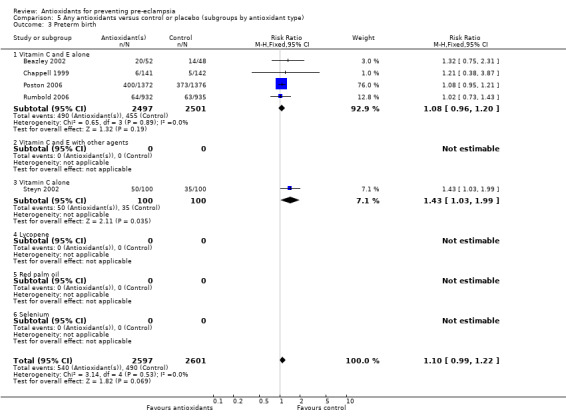
Comparison 5 Any antioxidants versus control or placebo (subgroups by antioxidant type), Outcome 3 Preterm birth.
5.4. Analysis.
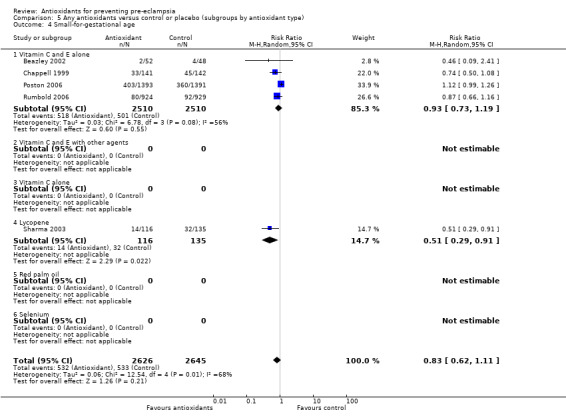
Comparison 5 Any antioxidants versus control or placebo (subgroups by antioxidant type), Outcome 4 Small‐for‐gestational age.
5.5. Analysis.
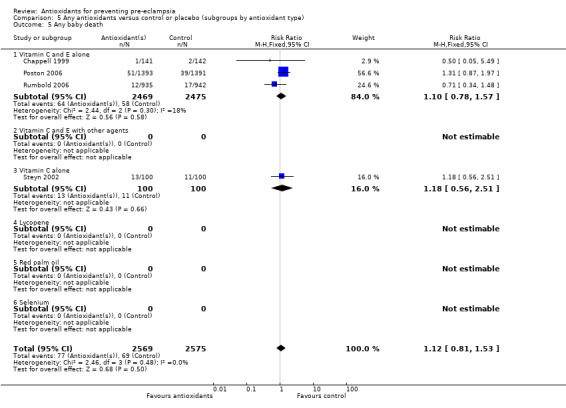
Comparison 5 Any antioxidants versus control or placebo (subgroups by antioxidant type), Outcome 5 Any baby death.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Beazley 2002.
| Methods | Treatment allocation: unclear, no methodological details given, women were "randomised". Blinding of outcome assessment: "double blind" stated. Documentation of exclusion: 9 (8%) women were lost to follow up. Use of placebo control: placebo control. |
|
| Participants | 109 women between 14‐20 weeks' gestation and at "high risk of PE" including those with previous pre‐eclampsia, chronic hypertension, insulin requiring diabetes mellitus and multifetal gestation. Nil exclusion criteria stated. | |
| Interventions | Antioxidant: 1000 mg vitamin C and 400 IU vitamin E daily (n = 54). Control: placebo, no further details (n = 55). | |
| Outcomes | 1. PE (not defined). 2. GA at delivery (mean, SD). 3. Preterm birth (< 37 weeks). 4. Birthweight (mean, SD). 5. Birthweight < 10 centile. 6. TAS and 8‐IP. | |
| Notes | Criteria for subgroups: risk of PE: mixed, by review criteria included both high and moderate/low risk. GA at trial entry: 12‐20 weeks' gestation. Antioxidant intake before trial entry: unclear. Sample size calculation: none reported. Compliance: no details of women's compliance were given. Location: United States of America. Timeframe: unclear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment | Unclear risk | B ‐ Unclear |
Chappell 1999.
| Methods | Treatment allocation: a computer‐generated randomisation list using blocks of 10 was given to the hospital pharmacy departments. Researchers allocated the next available number to participants and women collected the trial tablets from the pharmacy department. Blinding of outcome assessment: double blind; women, caregivers and researchers were blinded to the treatment allocation until recruitment, data collection and laboratory analyses were complete. Documentation of exclusion: pregnancy outcome data were reported for all women randomised (n = 283). Use of placebo control: placebo control. |
|
| Participants | 283 women between 16‐22 weeks' gestation with an abnormal Doppler waveform in either uterine artery at 18‐22 weeks' gestation or a history in the preceding pregnancy of pre‐eclampsia necessitating delivery before 37 weeks' gestation, eclampsia or HELLP syndrome. Exclusion criteria: heparin or warfarin treatment, abnormal fetal‐anomaly scan or multiple pregnancy. 1512 women underwent Doppler screening, 273 women had abnormal waveforms and of these, 242 women consented to the study. An additional 41 women who had a history of pre‐eclampsia consented. 283 women were randomised, 72 women had normal Doppler scans at 24 weeks' gestation and 24 women did not return for a second scan and these women were withdrawn. A further 27 women withdrew from the trial after 24 weeks' gestation for various reasons. In total, 160 women completed the trial protocol until delivery (n = 81 vitamin C and E group, n = 79 placebo group). | |
| Interventions | Antioxidant: 1000 mg vitamin C and 400 IU vitamin E daily (n = 141). Control: placebo containing microcrystalline cellulose and soya bean oil, identical in appearance to the vitamin C tablets and vitamin E capsules. | |
| Outcomes | 1. Ratio of PAI‐1 to PAI‐2. 2. Incidence of PE (defined according to ISSHP guidelines). 3. Placental abruption. 4. Spontaneous preterm delivery (< 37 weeks). 5. Intrauterine death. 6. Small‐for‐gestational‐age infants (on or below the 10th centile). 7. Mean systolic and diastolic blood pressure before delivery. 8. GA at delivery (median, IQR). 9. Birthweight (median, IQR). 10. Birthweight centile (median, IQR). 11. Mean plasma ascorbic acid and alpha‐tocopherol concentrations during gestation. 12. Biochemical indices of oxidative stress and placental function. | |
| Notes | Risk of PE: mixed, by review criteria included both high and moderate/low‐risk women. GA at trial entry: mixed. Between 16‐22 weeks' gestation. Antioxidant intake before trial entry: unclear, not assessed. Sample size calculation: the study had 80% power to detect a 30% reduction in PAI‐1. Compliance: not specifically reported. "Within the treated group, plasma ascorbic acid concentration increased by 32% from baseline values and plasma alpha‐tocopherol increased by 54%." Location: London, United Kingdom. Timeframe: unclear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment | Low risk | A ‐ Adequate |
Han 1994.
| Methods | Treatment allocation: women were "divided into two groups randomly". No other methodological details given. Blinding of outcome assessment: unclear, single blinded, but no information given on blinding of carers or outcome assessors. Documentation of exclusion: none stated. Use of placebo control: placebo stated but no details given. |
|
| Participants | 100 women with "high‐risk factors of PIH". No other information was provided about the participants, nil exclusion criteria stated. | |
| Interventions | Antioxidant: 100 ug/d selenium, given as a "natural dietetic liquid containing 100 ug/d selenium for 6‐8 weeks during late pregnancy" (n = 52). Control: placebo, given in "the same manner" (n = 48). | |
| Outcomes | 1. Change in maternal and umbilical blood selenium level. 2. Change in systolic and diastolic blood pressure. 3. Incidence of PIH (not defined). 4. Incidence of gestational oedema. 5. Incidence of proteinuria. 6. Birthweight. 7. Side‐effects as measured by changes in the blood and urine routine analysis and liver and renal function. | |
| Notes | Risk of PE: unclear, therefore moderate/low risk. Author's state women were at high risk but no details given. GA at trial entry: unclear. Women supplemented "during late pregnancy". Antioxidant intake before trial entry: unclear, no information provided. Blood selenium level was assessed in women prior to starting the trial. Sample size calculation: none reported. Compliance: unclear, no information provided. Location: China. Timeframe: unclear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment | Unclear risk | B ‐ Unclear |
Mahdy 2004.
| Methods | Treatment allocation: unclear, "randomised double‐blind placebo controlled clinical trial" stated. No other methodological details given. Blinding of outcome assessment: "double‐blind" stated but no information given on blinding of carers or outcome assessors. Documentation of exclusion: none stated. Use of placebo control: placebo stated but no details given. |
|
| Participants | 113 primigravid women in early mid trimester (before 16 weeks' gestation). No other information was provided about the participants, nil exclusion criteria stated. | |
| Interventions | Antioxidant: red palm oil which includes a tocotrienol‐rich fraction (TRF). No other information including dosage was provided (n = 46). Control: placebo, no other information given (n = 67). | |
| Outcomes | 1. PIH (not defined). 2. PE (not defined). | |
| Notes | Risk of PE: moderate/low risk. GA at trial entry: mixed. Women supplemented before 16 weeks' gestation. Antioxidant intake before trial entry: unclear, no information provided. Sample size calculation: none reported. Compliance: unclear, no information provided. Location: unclear. Timeframe: unclear. Published in abstract form only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment | Unclear risk | B ‐ Unclear |
Merchant 2005.
| Methods | Treatment allocation: unclear, "randomisation followed a 2x2 factorial design" stated. No other methodological details given. Blinding of outcome assessment: "double‐blind" stated; the participant, tablet dispenser and study physicians were blinded. Documentation of exclusion: 123 (11.4%) women were excluded from the analysis, of which 41 had hypertension at trial entry. Data were not available for a further 89 (8.2%) women. Use of placebo control: placebo stated but no details given. |
|
| Participants | 1078 pregnant women between 12 and 27 weeks' gestation, who were HIV‐1 infected without WHO‐defined stage IV disease, resident of Dar‐es‐Salaam, Tanzania and intending to remain in the city for the duration of the pregnancy and 1 year thereafter. | |
| Interventions | There were 4 treatment arms. Antioxidant: daily multivitamin which contained 500 mg vitamin C, 30 mg vitamin E, 20 mg thiamin, 20 mg riboflavin, 20 mg B‐6, 50 micrograms b‐12, 0.8 mg folic acid) or daily multivitamin plus vitamin A (30 mg beta‐carotene + 5000 IU preformed vitamin A) or vitamin A alone (30 mg beta‐carotene + 5000 IU preformed vitamin A). Control: placebo. | |
| Outcomes | 1. Hypertension in pregnancy, defined as systolic blood pressure >= 140 mmHg or diastolic blood pressure >= 90 mmHg at any time in pregnancy (women hypertensive at randomisation were excluded from the analysis). | |
| Notes | Risk of PE: moderate/low‐risk women. GA at trial entry: mixed. Between 12‐27 weeks' gestation. Antioxidant intake before trial entry: unclear, not assessed. Sample size calculation: a sample size calculation for the risk of hypertension in pregnancy was not reported. Compliance: based on counts of returned pills from the mean compliance in all treatment groups was 91% (median 96%). Location: Tanzania. Timeframe: April 1995‐1997. The primary aim of the study was to assess the effect of vitamin supplementation on adverse birth outcomes and mother‐to‐child transmission of HIV. Assessing hypertension in pregnancy was a secondary aim of the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment | Unclear risk | B ‐ Unclear |
Poston 2006.
| Methods | Treatment allocation: computer‐generated randomisation list blocked by centre in groups of 10 to 12 individuals (mean size 6.7) was given to a third party. Midwives randomised eligible women online, women were allocated a locally stored treatment pack with a centre‐specific and participant‐specific number. Blinding of outcome assessment: double blind; women, caregivers and researchers were blinded to the treatment allocation until after completion of the study. Documentation of exclusion: 2404 women were randomised, data for 2395 women (99.6%) were analysed. Use of placebo control: placebo control. |
|
| Participants | 2395 women with a gestational age between 14+0 ‐ 21+6 weeks and one or more of: pre‐eclampsia in the pregnancy preceding the index pregnancy, requiring delivery before 37 completed weeks' gestation; diagnosis of HELLP syndrome in any previous pregnancy at any stage of gestation; eclampsia in any previous pregnancy at any stage of gestation; essential hypertension requiring medication, currently or previously; maternal diastolic blood pressure of 90 mmHg or more before 20 weeks' gestation in the current pregnancy; type 1 or 2 diabetes, requiring insulin or oral hypoglycaemic therapy before the pregnancy; antiphospholipid syndrome; chronic renal disease; multiple pregnancy; abnormal uterine artery Doppler waveform (18‐22 weeks' gestation) or primiparity with a body‐mass index of 30 kg/m2 or more at first antenatal visit. Exclusion criteria: women unable or unwilling to give written informed consent, being treated with warfarin or taking vitamin supplements that contained a dose of vitamin C of 200 mg or more or vitamin E of 40 IU or more daily. | |
| Interventions | Antioxidant: 1000 mg vitamin C and 400 IU vitamin E daily (n = 1196). Control: placebo tablets containing microcrystalline cellulose and capsules containing sunflower seed oil, identical in appearance and taste to the vitamin C tablets and vitamin E capsules (n = 1199). | |
| Outcomes | 1. Incidence and severity of PE (defined according to ISSHP guidelines).
2. Incidence and severity of gestational hypertension.
3. Low birthweight (< 2.5 kg).
4. Small‐for‐gestational age (reported as < 5th centile and < 10th centile).
5. Preterm birth (< 37 weeks' gestation and < 34 weeks' gestation).
6. Gestational age at delivery.
7. Use of healthcare resources.
8. Longitudinal biochemical indices. The trial had a range of other prespecified maternal and neonatal outcomes. |
|
| Notes | Risk of PE: mixed, by review criteria included both high and moderate/low‐risk women. GA at trial entry: mixed. Between 14‐21+6 weeks' gestation. Antioxidant intake before trial entry: unclear, not assessed. Sample size calculation: the study had 90% power to detect a 30% relative reduction in pre‐eclampsia, 25% reduction in low birthweight, and a 30% reduction in SGA. Compliance: based on counts of returned pills from 2070 women, 80% of these women took at least 50% of their tables, 65% took 80% or more, 32% took all their tablets and 6% took none. There we no great differences in compliance between groups. Location: United Kingdom. Timeframe: 6th August 2003‐27th June 2005. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment | Low risk | A ‐ Adequate |
Rivas 2000.
| Methods | Treatment allocation: unclear, women were "randomly divided into 2 subgroups". Blinding of outcome assessment: "triple blind" stated. No other details given. Documentation of exclusion: none stated. Use of placebo control: placebo control. |
|
| Participants | 127 women less than 29 weeks' gestation and with "high risk for PE", including nulliparity, previous PE, obesity, hypertension, less than 20 years old, diabetes, nephropathy, mean arterial pressure above of 85 mmHg, positive roll‐over test, black race, family history of hypertension or PE, twin pregnancy and poor socioeconomic conditions. Exclusion criteria: unclear, none stated. | |
| Interventions | Antioxidant: 500 mg vitamin C and 400 IU vitamin E per day, in addition to 1 g fish oil 3 times a day and 100 mg aspirin 3 times a week (n = 63). Control: placebo, given "at the same posology and presentation" (n = 64). | |
| Outcomes | 1. PE (not defined). 2. The authors report that "no serious maternal and neonatal side‐effects of treatment occurred in either group", no other details were given. | |
| Notes | Risk of PE: mixed, by review criteria included both high and moderate/low‐risk women. GA at trial entry: mixed. Women were < 29 weeks' gestation. Antioxidant intake before trial entry: unclear. Sample size calculation: none stated. Compliance: unclear, no information provided. Location: Venezuela. Timeframe: unclear. Published in abstract format only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment | Unclear risk | B ‐ Unclear |
Rumbold 2006.
| Methods | Treatment allocation: randomisation was performed through a central telephone randomisation service, the randomisation schedule was stratified by collaborating centre and gestational age (< 18 weeks versus 18 weeks or more). Treatment packs were prepared by a researcher not involved in recruitment or clinical care, packs were labelled with a unique study number and contained 4 sealed opaque white plastic bottles of either vitamin C and vitamin E or placebo tablets. Blinding of outcome assessment: double blind; women, caregivers and researchers were blinded to the treatment allocation until after completion of the study. Documentation of exclusion: 1877 were randomised, there were no losses to follow up. For the 4 pairs of twins in the study, outcomes for 1 randomly selected infant in each twin pair were chosen, outcomes for the other 4 infants were excluded from the analyses. Use of placebo control: placebo control. |
|
| Participants | 1877 nulliparous women with a singleton pregnancy between 14+0 and 21+6 weeks' gestation, with normal blood pressure at first measurement in pregnancy and again at trial entry. Exclusion criteria: known multiple pregnancy, known potential lethal fetal anomaly, known thrombophilia, chronic renal failure, antihypertensive therapy, or specific contraindications to vitamin C or E therapy such as haemochromatosis or anticoagulant therapy. Women were advised not to take any other supplements although a multivitamin that provided a daily intake of no more than 200 mg vitamin C or 50 IU vitamin was permitted. | |
| Interventions | Antioxidant: 1000 mg vitamin C and 400 IU vitamin E daily (n = 935). Control: placebo tablets containing microcrystalline cellulose identical in appearance and taste to the vitamin C and E tablets (n = 942). | |
| Outcomes | 1. Death or serious outcomes in the infant (a composite measure of prespecified fetal and neonatal outcomes).
2. Pre‐eclampsia (defined according to ASSHP criteria).
3. Small‐for‐gestational age (< 10th centile). The trial also included a range of prespecified maternal and neonatal outcomes, including preterm birth reported as < 37 weeks' gestation, < 34 weeks' gestation and < 28 weeks' gestation. |
|
| Notes | Risk of PE: moderate/low‐risk women. GA at trial entry: mixed. Between 14‐21+6 weeks' gestation. Antioxidant intake before trial entry: at trial entry 94% of women in both groups had an adequate intake of vitamin C, 43% of women in both groups had an adequate intake of vitamin E. Sample size calculation: the study had 80% power to detect a reduction in the risk of death or serious outcomes in the infants from 6.5% to 3.7%, and a reduction in the risk of pre‐eclampsia from 10.0 to 6.3%. Compliance: based on verbal recall from women, 66.9% of women in the vitamin group took 80% or more of their tables, compared with 69.9% in the placebo group. Location: Australia. Timeframe: December 2001 to January 2005. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment | Low risk | A ‐ Adequate |
Sharma 2003.
| Methods | Treatment allocation: third‐party randomisation using numbered opaque envelopes made up from computer‐generated numbers. Blinding of outcome assessment: women, caregivers, research staff and outcome assessors were blinded to the treatment allocation. Documentation of exclusion: none. Use of placebo control: placebo control. |
|
| Participants | 251 primigravidas between 16‐20 weeks with no medical complication such as renal disease, primary hypertension, cardiovascular disease, diabetes or connective tissue disease. Exclusion criteria: unclear, none stated. | |
| Interventions | Antioxidant: 2 mg lycopene twice daily (4 mg daily total) until delivery (n = 116). Control: placebo, similar looking tablets of soya bean oil and bees wax (n = 135). | |
| Outcomes | 1. PE (defined according to ISSHP guidelines). 2. Eclampsia. 3. Mean diastolic blood pressure. 4. Intrauterine growth restriction (weight below the 10th centile for gestational age). 5. Mean fetal weight. 6. Mean gestation. | |
| Notes | Risk of PE: moderate/low risk. GA at trial entry: 12‐20 weeks' gestation. Antioxidant intake before trial entry: not assessed. Sample size calculation: stated as "considering the incidence of pre‐eclampsia to be 7‐10% in primigravidas, and taking 5% as error, a sample size of approximately 124 women in each group was calculated". The trial planned to enrol 300 women (150 in each group) however staffing limitations resulted in the trial being stopped after 251 women were recruited, leaving 116 women and 135 in each group. Compliance: unclear, compliance was assessed by pill counts, but no other information provided. Location: New Delhi, India. Timeframe: unclear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment | Low risk | A ‐ Adequate |
Steyn 2002.
| Methods | Randomisation: third‐party randomisation, Roche Pharmaceuticals supplied numbered containers with either vitamin C or placebo tablets, and they retained the code until completion of the study. Blinding of outcome assessment: "double blind" stated, Roche Pharmaceuticals retained the code until completion of the study. Documentation of exclusion: none reported. Use of placebo control: placebo control. |
|
| Participants | 200 women less than 26 weeks' gestation and with a history of a previous mid‐trimester abortion (spontaneous expulsion of the uterine contents between 13 and 26 weeks' gestation) or previous preterm labour (spontaneous onset of labour and delivery before 37 weeks' gestation). Exclusion criteria: women with previous preterm labour due to iatrogenic causes, such as previous induction of labour before term for severe pre‐eclampsia, or women with multiple pregnancies, proven cervical incompetence or other known reasons for preterm labour were excluded. 203 consecutive women were invited to participate in the study, of which 200 consented. | |
| Interventions | Antioxidant: 250 mg vitamin C twice daily (500 mg daily total) until 34 weeks' gestation (n = 100). Control: "exact matching" placebo, no other details given (n = 100). Women attended for an antenatal visit every 2 weeks until 34 weeks' gestation, and at each visit women were tested for bacterial vaginosis. Women who tested positive for Mycoplasma hominis at trial entry were treated with 500 mg erythromycin 6 hourly for 7 days, from 22 weeks' gestation up until 32 weeks' gestation. | |
| Outcomes | 1. Preterm labour, defined as delivery before 37 completed weeks and subdivided into delivery < 34 weeks' gestation and < 28 weeks' gestation. 2. Gestational age at delivery (median and range). 3. Birthweight (median and range). 4. Miscarriage. 5. Intrauterine death. 6. Early and late neonatal death. 7. Duration of neonatal hospitalisation. 8. Antepartum haemorrhage (including placental abruption). 9. PE. 10. Hypertension (not defined). 11. Induction of labour. 12. Bacterial vaginosis. 13. Leucocyte vitamin C levels at trial entry. | |
| Notes | Risk of PE: moderate/low risk, by review criteria. GA at trial entry: mixed. Women were below 26 weeks' gestation. Antioxidant intake before trial entry: 11 women (5.5%) had a vitamin C intake less than 67% of the RDI (70 mg), as assessed by a food frequency questionnaire. Sample size calculation: none indicated. The results presented are from an interim analysis performed by an independent panel after 100 women in each group had delivered, which indicated "very few differences between the 2 groups. Further recruitment will not have resulted in obtaining a significant difference". Compliance: no information provided. Location: South Africa. Timeframe: unclear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment | Low risk | A ‐ Adequate |
ASSHP: Australian Society for the Study of Hypertension in Pregnancy GA: gestational age HELLP: haemolysis, elevated liver enzymes and low platelets IP: isoprostane IQR: interquartile range ISSHP: International Society for the Study of Hypertension in Pregnancy IU: international units PAI‐1: plasminogen activator inhibitor‐1 PAI‐2: plasminogen activator inhibitor‐2 PE: pre‐eclampsia PIH: pregnancy‐induced hypertension RDI: recommended daily intake SD: standard deviation SGA: small‐for‐gestational age TAS: total antioxidant status WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anthony 1996 | Study design: randomised controlled trial. Intervention: vitamin E supplementation (dose not stated). Participants: 73 women undergoing conservative antenatal management of pre‐eclampsia. Outcomes: duration of conservative management, indications for delivery, renal function, protein excretion and platelet levels. Published as an abstract only. |
| Bolisetty 2002 | Study design: pilot case control, not randomised. Intervention: daily 20 mg beta‐carotene, 167.8 mg vitamin E and 1000 mg vitamin C versus control. Participants: 5 women at risk of preterm delivery between 30 and 36 weeks were supplemented and 7 women of comparable gestation acted as controls. Outcomes: biochemical assessments of oxidative stress and maternal plasma concentrations of beta‐carotene, vitamin E and vitamin C. |
| Borna 2005 | Study design: randomised controlled trial. Intervention: daily 500 mg vitamin C and 400 IU vitamin E or placebo. Participants: 60 women with a preterm premature rupture of membranes, a singleton pregnancy and between 26 and 34 weeks' gestation. Outcomes: chorioamnionitis, latency until delivery, gestational age at delivery, mode of delivery, birthweight, early neonatal sepsis, respiratory distress syndrome, neonatal death and postpartum endometritis. |
| Casanueva 2005 | Study design: randomised controlled trial. Intervention: daily 100 mg vitamin C or placebo. Participants: 120 women with no acute or chronic diseases, < 20 weeks' gestation, with a singleton pregnancy, no consumption of vitamin supplements and providing informed written consent. Outcomes: premature rupture of the chorioamniotic membranes and preterm labour. |
| Chaudhuri 1969 | Study design: quasi‐randomised controlled trial. Intervention: multivitamin containing vitamin C versus control (no supplement). 32% of participants were excluded from the analyses. Participants: women attending the antenatal clinic who were below 24 weeks' gestation. Outcomes: toxaemia (any, moderate and severe). |
| Dijkhuizen 2004 | Study design: randomised controlled trial. Intervention: daily 4.5 mg beta‐carotene, 30 mg zinc or both or placebo. All women received daily 30 mg iron and 0.4 mg folic acid. Participants: 230 women at < 20 weeks' gestation. Twin pregnancies or congenital abnormalities that interfered with growth, development, or metabolism were excluded. Outcomes: micronutrient status at 1 and 6 months postpartum, preterm birth, birthweight, postnatal infant anthropometric measures. |
| Ferguson 1955 | Study design: quasi‐randomised controlled trial. Intervention: daily 1 g methionine, 20 mg thiamine chloride, 10 mg riboflavin and 100 mg niacinamide or placebo. Methionine is not considered to act directly as an antioxidant. Participants: 436 women attending a 'Toxaemia Clinic', at various stages in their pregnancy. 38% of women were excluded. Outcomes: pre‐eclampsia, superimposed pre‐eclampsia, weight gain during pregnancy, eclampsia, abortions, stillbirths, neonatal deaths, birthweight < 2500 grams and < 2300 grams. |
| Gulmezoglu 1997 | Study design: randomised controlled trial. Intervention: daily 800 IU vitamin E, 1000 mg vitamin C and 200 mg allopurinol, or placebo. Participants: 56 women with severe pre‐eclampsia diagnosed between 24 and 32 weeks' gestation at trial entry. Outcomes: prolongation of pregnancy, biochemical assessment of oxidative stress and antioxidants, maternal complications, side‐effects, infant outcomes and haematologic and renal parameters. |
| Herrera 1993 | Study design: randomised controlled trial. Intervention: nutritional supplementation with soy proteins, linoleic acid and calcium or placebo. These interventions are not considered to be antioxidants. Participants: 74 normotensive women at 28‐29 weeks' gestation with either mean blood pressure of 80 mmHg or higher and a positive roll‐over test. Outcomes: PIH, pre‐eclampsia, gestational age at birth, caesarean section and fetal weight. |
| Marya 1987 | Study design: randomised controlled trial. Intervention: calcium (375 mg/day) and vitamin D (1200 IU/day) versus control. Calcium and vitamin D are not considered to be antioxidants. Participants: 400 women between 20‐24 weeks' gestation. Outcomes: systolic and diastolic blood pressure, incidence of toxaemia. |
| Morrison 1984 | Study design: randomised controlled trial. Intervention: random allocation to the treatment or normal diet, where the treatment diet involved additional linoleic acid. Participants: 34 primigravid women with PIH. Outcomes: diastolic blood pressure, birthweight, the "development of complications in PIH". Published in abstract form only. |
| Pawlowicz 2000 | Study design: randomised controlled trial. Intervention: anthocyanins derived from chokeberry or placebo. Participants: 105 pregnant women with intrauterine growth restriction of idiopathic and pre‐eclamptic origin on the turn of the second trimester. Outcomes: serum concentration of autoantibodies to oxidized low density lipoproteins. No clinically relevant outcomes reported. |
| People's League 1942 | Study design: quasi‐randomised controlled trial. Intervention: daily multivitamin preparation containing 100 mg vitamin C, ferrous iron 0.26 g, calcium 0.26 g, minute quantities of iodine, manganese and copper, adsorbate of vitamin B1 containing all factors of the B complex and halibut liver oil 0.36 g containing vitamin A (52,000 IU per g) and vitamin D (2500 IU per g) or control (no placebo). Participants: 5021 women <= 24 weeks' gestation, attending the antenatal clinics and in 'good health'. Outcomes: toxaemia (hypertension only, albuminuria with or without hypertension, or hypertension with albuminuria), maternal sepsis, length of gestation, stillbirth, birthweight, neonatal mortality and breastfeeding. |
| Powers 2000 | Not a trial. This paper involves comment on the trial by Chappell and colleagues (Chappell 1999). |
| Pressman 2003 | Study design: randomised controlled trial. Intervention: 500 mg vitamin C and 400 IU vitamin E daily. Participants: pregnant women at 35 weeks' gestation. Outcomes: maternal plasma concentrations and amniotic fluid concentrations of vitamin C and E. No relevant clinical outcomes reported. |
| Radhika 2003 | Study design: randomised controlled trial. Intervention: daily sachets containing red palm oil (approximately 2400 micrograms beta‐carotene) or placebo. Participants: 170 women between 16 and 24 weeks' gestation. Outcomes: maternal and neonatal vitamin A status, maternal anaemia, maternal weight gain, birthweight, low birthweight (< 2.5 kg), gestational age at birth and preterm birth. |
| Roes 2006 | Study design: randomised controlled trial. Intervention: 3, 600 mg oral N‐acetylcysteine tablets every 8 hours or matching placebo until delivery. Participants: 38 women with severe pre‐eclampsia or HELLP syndrome, or both, a singleton pregnancy and between 25 and 33 weeks' gestation. Outcome: treatment to delivery interval, biochemical assessment of glutathione and parameters of oxidative stress. |
| Sawhney 2000 | Study design: randomised controlled trial. Intervention: vitamin E supplementation (dose not reported) or no treatment. Participants: 60 women with established pre‐eclampsia. Outcomes: lipid peroxide levels, alpha‐tocopherol levels and pregnancy continuation > 14 days. Published in abstract form only. |
| Sikkema 2002 | Study design: randomised controlled trial. Intervention: single dose of 2 grams vitamin C or placebo. Participants: 18 women with established pre‐eclampsia. Outcome: flow mediated vasodilation. Published in abstract form only. |
| Theobald 1937 | Study design: quasi‐randomised trial. Intervention: daily 20 g calcium lactate, 11,000 IU vitamin A and 450 units vitamin D or control (no placebo). Participants: 100 'apparently healthy' women < 24 weeks' gestation. Outcomes: toxaemia (hypertension only, hypertension and albuminuria, albuminuria only) and 'other symptoms' of pregnancy. |
| Thomson 2001 | Study design: unclear, randomisation not stated. Intervention: daily 50 micrograms selenium (L‐selenomethionine) or placebo during pregnancy and postpartum. Participants: 35 women in the 'earliest stages of pregnancy' plus 17 non‐pregnant women who received the selenium, serving as 'positive controls'. Outcomes: measures of selenium and iodine status, clearance and excretion; no pregnancy outcomes reported. |
| West 1999 | Study design: randomised controlled trial. Intervention: weekly oral vitamin A (7000 micrograms retinol equivalents) or beta‐carotene (42 mg, or 7000 microgram retinol equivalents) or placebo given prior to, during and after pregnancy. Participants: 44,646 women, of whom 20,119 became pregnant, 22,819 times. Data about 'symptoms of illness' in pregnancy available for 15,832 women. Outcome: pregnancy‐related mortality and a range of 'symptoms of illness' in pregnancy. Pre‐eclampsia/eclampsia was reported but defined as "any swelling of hands/face or convulsions" at either < 12, 12‐28, > 28 weeks' gestation or postpartum. Blood pressure was assessed in a subset of women, for this subset, pre‐eclampsia defined as systolic blood pressure >= 140 mmHg or diastolic blood pressure >= 90 mmHg plus any swelling of hands/face or convulsions. Data are reported for the subset of women assessed for pre‐eclampsia after 20 weeks' gestation (472/15,832, 2.99%). The rate of pre‐eclampsia in each treatment group was: 7/185 (3.78%), beta‐carotene group; 3/152 (1.97%), vitamin A group and 6/135 (4.44%), placebo group. Reason for exclusion: missing data for > 20% of participants. |
HELLP: haemolysis, elevated liver enzymes and low platelets IU: international units PIH: pregnancy induced hypertension
Characteristics of ongoing studies [ordered by study ID]
DAPIT 2004.
| Trial name or title | The Diabetes and Pre‐eclampsia Intervention Trial (DAPIT). Clinical Trial Identifier: ISRCTN27214045 |
| Methods | |
| Participants | Inclusion criteria: type 1 diabetes preceding pregnancy; age >= 16 years; between 8 and 22 weeks' gestation; date of last menstrual period certain or ultrasound estimation from 6‐22 weeks of gestational age is available, or both; singleton pregnancy. Exclusion criteria: ingestion of preparations containing vitamin C > 500 mg/day or vitamin E > 200 IU/day; participation in another study which may interfere with DAPIT; participation in DAPIT during a previous pregnancy, where DAPIT trial medication was taken within the last 6 months; any notation of drug abuse: cocaine, LSD, heroin, marijuana, inhaled solvents/gases; warfarin therapy. Planned sample size: 945 women. |
| Interventions | Daily 1000 mg vitamin C and 400 IU vitamin E or placebo. |
| Outcomes | Primary outcome: incidence of pre‐eclampsia. Secondary outcomes: endothelial activation, birthweight centile. |
| Starting date | April 2003. |
| Contact information | Dr David R McCance Consultant Physician/Honorary Senior Lecturer, Regional Centre for Endocrinology and Diabetes, Royal Victoria Hospital, Belfast BT12 6BA. E‐mail: david.mccance@royalhospitals.n‐i.nhs.uk |
| Notes |
INTAPP 2005.
| Trial name or title | International Trial of Antioxidants for the Prevention of Preeclampsia (INTAPP). |
| Methods | |
| Participants | Two stratum's of participants: 1. nulliparous women without additional risk factors. 2. nulliparous women with at least 1 of the risk factors: diabetes, chronic hypertension, obesity, multiple pregnancy, and for multiparous women, a past history of pre‐eclampsia. Planned sample size: 13,334 (stratum 1) and 3760 (stratum 2). |
| Interventions | Daily 1000 mg vitamin C and 400 IU vitamin E or placebo. |
| Outcomes | Frequency of pre‐eclampsia, gestational hypertension with adverse conditions, intrauterine growth restriction, preterm birth. |
| Starting date | January 2004. On April 10th 2006, the INTAPP Steering Committee made a decision to stop the trial. At the time of this decision, 2647 women had been recruited into the trial. The last INTAPP participant delivered in October 2006, the trial results are expected to be available in mid‐2007. |
| Contact information | Prof W Fraser Department of Obstetrics and Gynaecology, Laval University, Quebec, Canada. E‐mail: william.fraser@ogy.ulaval.ca |
| Notes |
NICHD 2004.
| Trial name or title | Antioxidant therapy to prevent preeclampsia. Clinical Trial Identifier: NCT00097110 |
| Methods | |
| Participants | Inclusion criteria: gestational age between 12+0‐19+6 weeks; chronic hypertension or past history of pre‐eclampsia/eclampsia, and attendance at a participating hospital. Exclusion criteria: planned delivery elsewhere; multifetal gestation; allergy to vitamin C or vitamin E; requirement for aspirin or anticoagulant medication; proteinuria = 2+ on dipstick urine test, or proteinuria = 1+ on dipstick and = 300 mg/24 hours; pre‐pregnancy diabetes mellitus; known fetal anomaly incompatible with life; prior participation in the study; unwillingness to take the study medication. Planned sample size: 734 women. |
| Interventions | Daily 1000 mg vitamin C and 400 IU vitamin E or placebo until delivery. |
| Outcomes | Primary outcome: incidence of pre‐eclampsia. Secondary outcomes: severity of pre‐eclampsia; incidence of gestational hypertension or pre‐eclampsia; frequency of abruptio placentae; incidence of preterm birth; small‐for‐gestational‐age infant or low birthweight infants; biomarker level correlation with pre‐eclampsia. |
| Starting date | Enrolment: July 2003‐October 2007. Last follow up: April 2007. Data entry closure: July 2007. |
| Contact information | Dr JA Spinnato University of Cincinnati, The United States of America. Dr Salvio Freire, Federal University of Pernambuco, Recife, Pernambuco. E‐mail: salvio_freire@uol.com.br |
| Notes |
NICHD MFMU Network.
| Trial name or title | Combined Antioxidants and Preeclampsia Prediction Studies (CAPPS). |
| Methods | |
| Participants | Inclusion criteria: gestational age 9+0‐16+6 weeks, singleton pregnancy, nulliparous, BP < 135/85 mmHg, no antihypertensive medication/diuretics, proteinuria 0 or trace, no vitamin C or E > amount in prenatal vitamins and with informed consent. Planned sample size: 10,000 women. |
| Interventions | Daily 1000 mg vitamin C and 400 IU vitamin E or placebo until delivery. |
| Outcomes | Primary outcomes: BP > 160/110 mmHg or BP > 140/90 mmHg > 20 weeks' and one of: 1. SGOT (AST) > 100 U/L. 2. Platelets < 100,000/mm3. 3. Creatinine > 1.5 mg/dL. 4. Eclampsia. 5. Fetal/neonatal death. 6. SGA < 3rd centile. 7. Preterm delivery < 32 weeks. |
| Starting date | Enrolment: May 2003‐April 2005. Data collection: May 2003‐March 2006. Closeout/final analysis: April 2006‐November 2006. |
| Contact information | Prof JM Roberts Director, Magee‐Women's Research Institute Professor and Vice Chair (Research) of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh. E‐mail: rsijmr@mwri.magee.edu |
| Notes |
VIP WHO Component.
| Trial name or title | Vitamins in Preeclampsia (VIP): a multicentre randomised clinical trial of vitamin C and E supplementation in pregnancy for the prevention of pre‐eclampsia (India, Peru, Vietnam). Clinical Trial Identifier: ISRCTN86677348 |
| Methods | |
| Participants | Inclusion criteria:
gestational age between 14+0 ‐ 21+6 weeks with 1 or more of the following risk factors: chronic hypertension (diastolic blood pressure (BP) > 90 mmHg); pre‐gestational diabetes; arterial, venous or small vessel thrombosis in any organ tissue; unexplained death of morphologically normal fetus at or beyond 10 weeks' gestation; premature births before 34 weeks due to pre‐eclampsia, eclampsia or severe placental insufficiency; unexplained consecutive spontaneous abortions before 10 weeks; chronic renal disease; multiple pregnancy; past history of pre‐eclampsia, eclampsia or HELLP syndrome (haemolysis, elevated liver enzyme levels and low platelet count). Exclusion criteria: inability to give informed consent; women taking supplements containing > 200 mg vitamin C or > 50 IU vitamin E daily dose; women taking warfarin. Planned sample size: 1700 women. |
| Interventions | Daily 1000 mg vitamin C and 400 IU vitamin E or placebo until delivery. |
| Outcomes | Incidence of pre‐eclampsia, birthweight centile (< 5th). |
| Starting date | Unclear. Expected to be completed in 2006. |
| Contact information | Dr M Merialdi
20, Avenue Appia,
Geneva‐27, CH 1211, Switzerland
E‐mail: merialdim@who.int In conjunction with: Prof L Poston Director of Research Division of Reproductive Health, Endocrinology and Development (King's College London) and Prof A Shennan, Maternal and Fetal Research Unit 10th Floor North Wing St Thomas' Hospital London SE1 7EH E‐mail: lucilla.poston@kcl.ac.uk or andrew.shennan@kcl.ac.uk |
| Notes |
AST: aspartate aminotransferase BP: blood pressure HELLP: haemolysis, elevated liver enzymes and low platelets IU: international units SGA: small‐for‐gestational age SGOT: serum glutamic‐oxaloacetic transaminase SLE: systemic lupus erythematosus
Differences between protocol and review
We excluded quasi‐random studies. The previous version of this review included quasi‐random studies; we planned to exclude quasi‐random studies from future updates of the review, when sufficient data became available from large randomised controlled trials. We added the following new outcomes: gestational hypertension, use of antihypertensives, miscarriage, extremely preterm birth (less than 27 completed weeks' gestation), Apgar score less than seven at five minutes and economic outcomes. These outcomes were added to ensure all outcomes specified in the pre‐eclampsia generic protocol are reported in this review.
Contributions of authors
Alice Rumbold and Lelia Duley developed and wrote the protocol. Caroline Crowther and Ross Haslam commented on and revised the various drafts of the protocol during its development. Alice Rumbold and Caroline Crowther extracted the data. Alice Rumbold wrote the first draft of the review. Lelia Duley, Caroline Crowther and Ross Haslam commented on various versions of the review.
For the 2007 update, Alice Rumbold, Caroline Crowther and Lelia Duley extracted the data. Alice Rumbold wrote the first draft of the updated review. Lelia Duley, Caroline Crowther and Ross Haslam commented on various versions of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
Discipline of Obstetrics and Gynaecology, The University of Adelaide, Australia.
Medical Research Council, UK.
Declarations of interest
Caroline Crowther and Ross Haslam were chief investigators for the Australian Collaborative Trial of Supplements with vitamin C and vitamin E for the prevention of pre‐eclampsia (ACTS). Alice Rumbold was the PhD student involved with this trial (ACTS). Lelia Duley was a member of the steering committee for the Vitamins in Pregnancy (VIP) trial, which assessed vitamins C and E for prevention of pre‐eclampsia.
Edited (no change to conclusions)
References
References to studies included in this review
Beazley 2002 {published data only}
- Beazley D, Ahokas R, Livingston J, Griggs M, Scroggs M, Sibai B. Effects of vitamin c and e supplementation on total antioxidant status (tas) and 8‐isoprostane (ip) levels in women at high risk for preeclampsia [abstract]. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S76. [DOI] [PubMed] [Google Scholar]
- Beazley D, Ahokas R, Livingston J, Griggs M, Sibai BM. Vitamin C and E supplementation in women at high risk for preeclampsia: a double‐blind, placebo controlled trial. American Journal of Obstetrics and Gynecology 2005;192:520‐1. [DOI] [PubMed] [Google Scholar]
- Beazley D, Livingston J, Kao L, Sibai B. Vitamin c and e supplementation in women at high risk for preeclampsia: a double‐blind placebo controlled trial [abstract]. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S216. [DOI] [PubMed] [Google Scholar]
Chappell 1999 {published data only}
- Chappell L, Seed P, Briley A, Kelly F, Lee R, Hunt B, et al. Effect of antioxidants on the occurrence of pre‐eclampsia in women at increased risk: a randomised controlled trial. Lancet 1999;354:810‐6. [DOI] [PubMed] [Google Scholar]
- Chappell LC, Seed PT, Kelly FJ, Briley A, Hunt BJ, Charnock‐Jones DS, et al. Vitamin c and e supplementation in women at risk of preeclampsia is associated with changes in indices of oxidative stress and placental function. American Journal of Obstetrics and Gynecology 2002;187(3):777‐84. [DOI] [PubMed] [Google Scholar]
Han 1994 {published data only}
- Han L, Zhou SM. Selenium supplement in the prevention of pregnancy induced hypertension. Chinese Medical Journal 1994;107(11):870‐1. [PubMed] [Google Scholar]
Mahdy 2004 {published data only}
- Mahdy ZA, Siraj HH, Azwar MH, Wahab MA, Khaza'ai H, Mutalib MSA, et al. The role of palm oil vitamin E in the prevention of pregnancy‐induced hypertension [abstract]. Hypertension in Pregnancy 2004;23(Suppl 1):67. [Google Scholar]
Merchant 2005 {published data only}
- Merchant AT, Msamanga G, Villamor E, Saathoff E, O'Brien M, Hertzmark E, et al. Multivitamin supplementation of hiv‐positive women during pregnancy reduces hypertension. Journal of Nutrition 2005;135(7):1776‐81. [DOI] [PubMed] [Google Scholar]
Poston 2006 {published data only}
- Briley AL, Poston L, Shennan AH. Vitamins C and E and the prevention of pre‐eclampsia. New England Journal of Medicine 2006;355(10):1065‐6. [PubMed] [Google Scholar]
- Poston L, Briley AL, Seed PT, Kelly FJ, Shennan AH, for the Vitamins in Pre‐eclampsia (VIP) Trial Consortium. Vitamin C and vitamin E in pregnant women at risk for pre‐eclampsia (VIP trial): randomised placebo‐controlled trial. Lancet 2006;367(9517):1145‐54. [DOI] [PubMed] [Google Scholar]
- Shennan AH, Briley AL, Seed PT, Kelly FJ, Poston L. A randomised controlled trial (RCT) of vitamin C and E supplementation in high risk women to prevent pre‐eclampsia (the vip trial; on behalf of the vip trial consortium) [abstract]. Journal of Obstetrics and Gynaecology 2006;26(1):S18‐S20. [Google Scholar]
Rivas 2000 {published data only}
- Rivas‐Echeverria CA, Echeverria Y, Molina L, Novoa D. Synergic use of aspirin, fish oil and vitamins C and E for the prevention of preeclampsia [abstract]. Hypertension in Pregnancy 2000;19(Suppl 1):30. [Google Scholar]
Rumbold 2006 {published data only}
- Crowther CA, Rumbold AR, Robinson JS. Vitamins C and E and the prevention of pre‐eclampsia. New England Journal of Medicine 2006;355(10):1066. [Google Scholar]
- Rumbold AR, Crowther CA, Haslam RR, Dekker GA, Robinson JS. Australian collaborative trial of supplements (ACTS) with vitamin C and E for the prevention of pre‐eclampsia ‐ a randomised controlled trial. Perinatal Society of Australia and New Zealand 10th Annual Congress; 2006 April 3‐6; Perth, Australia. 2006:180.
- Rumbold AR, Crowther CA, Haslam RR, Dekker GA, Robinson JS, for the ACTS Study Group. Vitamins C and E and the risks of preeclampsia and perinatal complications. New England Journal of Medicine 2006;354(17):1796‐806. [DOI] [PubMed] [Google Scholar]
Sharma 2003 {published data only}
- Sharma JB, Kumar A, Kumar A, Malhotra M, Arora R, Prasad S, et al. Effect of lycopene on pre‐eclampsia and intra‐uterine growth retardation in primigravidas. International Journal of Gynecology & Obstetrics 2003;81(3):257‐62. [DOI] [PubMed] [Google Scholar]
Steyn 2002 {published data only}
- Steyn P, Odendaal H, Schoeman J, Grove D. Vitamin c, pre‐eclampsia and preterm labour: a randomized, placebo‐controlled, double blind trial [abstract]. Hypertension in Pregnancy 2002;21(Suppl 1):43. [Google Scholar]
- Steyn PS, Odendaal HJ, Schoeman J, Stander C, Fanie N, Grove D. A randomised, double‐blind placebo‐controlled trial of ascorbic acid supplementation for the prevention of preterm labour. Journal of Obstetrics and Gynaecology 2003;23(2):150‐5. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anthony 1996 {published data only}
- Anthony J, Smith P, Mantel G, Johanson R. A randomised controlled trial of vitamin E supplementation in conservatively managed pre‐eclamptic patients. 10th World Congress of the International Society for the Study of Hypertension in Pregnancy; 1996 August 4‐8; Washington, Seattle, USA. 1996:193.
Bolisetty 2002 {published data only}
- Bolisetty S, Naidoo, Lei K, Koh T, Watson D, Whitehal J. Antenatal supplementation of antioxidant vitamins to reduce the oxdiative stress at delivery ‐ a pilot study. Early Human Development 2002;67:47‐53. [DOI] [PubMed] [Google Scholar]
Borna 2005 {published data only}
- Borna S, Borna H, Daneshbodie B. Vitamins C and E in the latency period in women with preterm premature rupture of membranes. International Journal of Gynecology & Obstetrics 2005;90:16‐20. [DOI] [PubMed] [Google Scholar]
Casanueva 2005 {published data only}
- Casanueva E, Ripoll C, Tolentino M, Morales RM, Pfeffer F, Vichis P, et al. Vitamin C supplementation to prevent premature rupture of the choroamniotic membranes: a randomized trial. American Journal of Clinical Nutrition 2005;81(4):859‐63. [DOI] [PubMed] [Google Scholar]
Chaudhuri 1969 {published data only}
- Chaudhuri SK. Effect of nutrient supplementation on the incidence of toxaemia of pregnancy. Journal of Obstetrics and Gynaecology of India 1969;19:156‐61. [Google Scholar]
- Chaudhuri SK. Role of nutrition in the etiology of toxemia of pregnancy. American Journal of Obstetrics and Gynecology 1971;110:46‐8. [DOI] [PubMed] [Google Scholar]
Dijkhuizen 2004 {published data only}
- Dijkhuizen MA, Wieringa FT, West CE, Muhilal. Zinc plus beta‐carotene supplementation of pregnant women is superior to beta‐carotene supplementation alone in improving vitamin A status in both mothers and infants. American Journal of Clinical Nutrition 2004;80(5):1299‐307. [DOI] [PubMed] [Google Scholar]
Ferguson 1955 {published data only}
- Ferguson JH. Methionine‐vitamin B therapy. Effect of a supplement of methionine and vitamin B on pregnancy and prematurity. Obstetrics & Gynecology 1955;6:221‐7. [PubMed] [Google Scholar]
Gulmezoglu 1997 {published data only}
- Gulmezoglu AM, Hofmeyr GJ, Oosthuisen MM. Antioxidants in the treatment of severe pre‐eclampsia: an explanatory randomised controlled trial. British Journal of Obstetrics and Gynaecology 1997;104(6):689‐96. [DOI] [PubMed] [Google Scholar]
Herrera 1993 {published data only}
- Herrerra JA. Nutritional factors and rest reduce pregnancy‐induced hypertension and pre‐eclampsia in positive roll‐over test primigravidas. International Journal of Gynecology & Obstetrics 1993;41:31‐5. [DOI] [PubMed] [Google Scholar]
Marya 1987 {published data only}
- Marya RK, Rathee S, Manrow M. Effect of calcium and vitamin D supplementation on toxaemia of pregnancy. Gynecologic and Obstetric Investigation 1987;24:38‐42. [DOI] [PubMed] [Google Scholar]
Morrison 1984 {published data only}
- Morrison RA, O'Brien PMS, Micklewright A. The effect of dietary supplementation with linoleic acid on the development of pregnancy induced hypertension. 4th World Congress of the International Society for the study of Hypertension in Pregnancy; 1984 June 18‐21; Amsterdam, The Netherlands. 1996:P48.
Pawlowicz 2000 {published data only}
- Pawlowicz P, Wilczynski J, Stachowiak G, Hincz P. Administration of natural anthocyanins derived from chokeberry retardation of idiopathic and preeclamptic origin. Influence on metabolism of plasma oxidized lipoproteins: the role of autoantibodies to oxidized low density lipoproteins [Zastosowanie naturalnych antocyjanow pochodzacych z soku aronii czarnoowocowej (aronia melanocarpa) w leczeniu idiopatycznego oraz powiklanego preeclampsja wewnatrzmacicznego zahamowania wzrostu plodu. Wplyw na metabolizm utlenowanych lipoprotein osocza‐‐rola prezeciwcial anty‐o‐LDL (olab)]. Ginekologia Polska 2000;71(8):848‐53. [PubMed] [Google Scholar]
People's League 1942 {published data only}
- People's League of Health. Nutrition of expectant and nursing mothers: interim report. Lancet 1942;2:10‐2. [Google Scholar]
- People's League of Health. The nutrition of expectant and nursing mothers in relation to maternal and infant mortality and morbidity. Journal of Obstetrics and Gynaecology of the British Empire 1946;53:498‐509. [DOI] [PubMed] [Google Scholar]
Powers 2000 {published data only}
- Powers H, Fraser R, Gibson AT. Antioxidants and pre‐eclampsia [letter; comment]. Lancet 2000;355(9197):64‐5. [DOI] [PubMed] [Google Scholar]
Pressman 2003 {published data only}
- Pressman EK, Cavanaugh JL, Mingione M, Norkus EP, Woods JR. Effects of maternal antioxidant supplementation on maternal and fetal antioxidant levels: a randomized, double‐blind study. American Journal of Obstetrics and Gynecology 2003;189(6):1720‐5. [DOI] [PubMed] [Google Scholar]
Radhika 2003 {published data only}
- Radhika MS, Bhaskaram P, Balakrishna N, Ramalakshmi BA. Red palm oil supplementation: a feasible diet‐based approach to improve the vitamin A status of pregnant women and their infants. Food and Nutrition Bulletin 2003;24(2):208‐17. [DOI] [PubMed] [Google Scholar]
Roes 2006 {published data only}
- Roes EM, Raijmakers MT, Boo NT, Zusterzeel PL, Merkus HM, Peters WH, et al. Oral N‐acetylcysteine administration does not stabilise the process of established preeclampsia. European Journal of Obstetrics & Gynecology and Reproductive Biology 2006;127(1):61‐7. [DOI] [PubMed] [Google Scholar]
Sawhney 2000 {published data only}
- Sawhney H, Singhal S, Vasishta K, Majumbar S. Effect of vitamin e supplementation on lipid peroxide levels in preeclampsia [abstract]. XVI FIGO World Congress of Obstetrics & Gynecology; 2000 Sept 3‐8; Washington DC, USA (Book 5). 2000:31.
Sikkema 2002 {published data only}
- Sikkema J, Franx A, Bruinse H. A double blinded randomised trial on the effect of a single dose of vitamin c on endothelium dependent vasodilatation in preeclampsia [abstract]. Hypertension in Pregnancy 2002;21(Suppl 1):46. [Google Scholar]
Theobald 1937 {published data only}
- Theobald GW, Camb MD. Effect of calcium and vitamins a and d on incidence of pregnancy toxaemia. Lancet 1937;2:1397‐9. [Google Scholar]
Thomson 2001 {published data only}
- Thomson CD, Packer MA, Butler JA, Duffield AJ, O'Donaghue KL, Whanger PD. Urinary selenium and iodine during pregnancy and lactation. Journal of Trace Elements in Medicine & Biology 2001;14(4):210‐7. [DOI] [PubMed] [Google Scholar]
West 1999 {published data only}
- West KPJ, Katz J, Khatry SK, LeClerq SC, Pradhan EK, Shrestha SR, et al. Double blind, cluster randomised trial of low dose supplementation with vitamin a or beta carotene on mortality related to pregnancy in nepal. The nnips‐2 study group [see comments]. BMJ 1999;318(7183):570‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Kubik 2004 {published data only}
- Kubik P, Kowalska B, Laskowska‐Klita T, Chelchowska M, Leibschang J. Effect of vitamin‐mineral supplementation on the status of some microelements in pregnant women. Przeglad Lekarski 2004;61(7):764‐8. [PubMed] [Google Scholar]
Negro 2007 {published data only}
- Negro R, Greco G, Mangieri T, Pezzarossa A, Dazzi D, Hassan H. The influence of selenium supplementation on postpartum thyroid status in pregnant women with thyroid peroxidase autoantibodies. Journal of Clinical Endocrinology & Metabolism 2007;92(4):1263‐8. [DOI] [PubMed] [Google Scholar]
Rumiris 2006 {published data only}
- Rumiris D, Purwosunu Y, Wibowo N, Farina A, Sekizawa A. Lower rate of preeclampsia after antioxidant supplementation in pregnant women with low antioxidant status. Hypertension in Pregnancy 2006;25(3):241‐53. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
DAPIT 2004 {published data only}
- Holmes VA, Young IS, Maresh MJA, Pearson DWM, Walker JD, McCance DR, et al. The diabetes and pre‐eclampsia intervention trial. International Journal of Gynecology & Obstetrics 2004;87:66‐71. [DOI] [PubMed] [Google Scholar]
INTAPP 2005 {published data only}
- Fraser W, Xiong X, Poston L, Shennan A. Bi‐national randomized controlled trial of vitamin c and e supplementation of pregnancy [abstract]. Hypertension in Pregnancy 2002;21(Suppl 1):43. [Google Scholar]
- Fraser WD. International trial of antioxidant for the prevention of preeclampsia. http://controlled‐trials.com (accessed 29 November 2005).
- Fraser WD, Audibert F, Bujold E, Leduc L, Xu H, Boulvain M, Julien P. The vitamin E debate: implications for ongoing trials of pre‐eclampsia prevention. BJOG: an international journal of obstetrics and gynaecology 2005;112:684‐88. [DOI] [PubMed] [Google Scholar]
NICHD 2004 {published data only}
- Spinnato JA, Freire S, Sibai BM, Myatt L. Antioxidant therapy to prevent preeclampsia. http://www.clinicaltrials.gov/ct/show/NCT00097110?order=1 (accessed 2005).
NICHD MFMU Network {unpublished data only}
- NICHD MFMU Network. Combined antioxidants and preeclampsia prediction studies. http://www.bsc.gwu.edu/mfmu/projects/capps.cgi (accessed 2004).
VIP WHO Component {published data only}
- Merialdi M. Vitamins in Preeclampsia (VIP): a multicentre randomized clinical trial of vitamin C and E supplementation in pregnancy for the prevention of pre‐eclampsia (India, Peru, Viet Nam). http://www.controlled‐trials.com/isrctn/trial/|/0/86677348.html (accessed 2005).
Additional references
Barker 1993
- Barker DJP, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet 1993;341:938‐41. [DOI] [PubMed] [Google Scholar]
Bendich 1993
- Bendich A, Machlin LJ. The safety of oral intake of vitamin E: data from clincial studies from 1986‐1991. In: Packer L, Fuchs J editor(s). Vitamin E in health and disease. New York: Marcel Dekker, 1993:411‐6. [Google Scholar]
Bendich 1997
- Bendich A. Vitamin C safety in humans. In: Packer L, Fuchs K editor(s). Vitamin C in health and disease. New York: Marcel Dekker, 1997:367‐9. [Google Scholar]
Devaraj 1997
- Devaraj S, Adams‐Huet B, Fuller CJ, Jialal I. Dose‐response comparison of RR‐alpha‐tocopherol and all‐racemic alpha‐tocopherol on LDL oxidation. Arteriosclerosis, Thrombosis and Vascular Biology 1997;17:2273‐9. [DOI] [PubMed] [Google Scholar]
Diplock 1998
- Diplock AT, Charleux, JL, Crozier‐Wili G, Kok FJ, Rice‐Evans C, Roberfroid M, et al. Functional food science and defence against reactive oxidative species. British Journal of Nutrition 1998;80:S77‐S112. [DOI] [PubMed] [Google Scholar]
Donoghue 2000
- Donoghue D, Cust A. Report of the Australian and New Zealand Neonatal Network 1998. AIHW National Perinatal Statistics Unit, Sydney 2000.
Gifford 2000
- Gifford RW Jr, August PA, Cunningham G, Green LA, Lindhemier MD, McNellis D, et al. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. American Journal of Obstetrics and Gynecology 2000;183:S1‐S22. [PubMed] [Google Scholar]
Higgins 2006
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6 [updated September 2006]. In: The Cochrane Library, Issue 4, 2006. Chichester, UK: John Wiley & Sons, Ltd.
HMSO 1998
- HMSO. Report on confidential enquiries into maternal deaths in the United Kingdom 1994‐1996. London: HMSO, 1998. [Google Scholar]
Hofmeyr 2006
- Hofmeyr GJ, Atallah AN, Duley L. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database of Systematic Reviews 2006, Issue 3. [Art. No.: CD001059. DOI: 10.1002/14651858.CD001059.pub2] [DOI] [PubMed] [Google Scholar]
HPS 2002
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20 536 high‐risk individuals: a randomised placebo‐controlled trial. Lancet 2002;360:23‐33. [Google Scholar]
Hubel 1997
- Hubel CA, Kagan VE, Kisin ER, McLaughlin MK, Roberts JM. Increased ascorbate radical formation and ascorbate depletion in plasma from women with preeclampsia: implications for oxidative stress. Free Radical Biology & Medicine 1997;23:597‐609. [DOI] [PubMed] [Google Scholar]
Knight 2007
- Knight M, Duley L, Henderson‐Smart DJ, King JF. Antiplatelet agents for preventing and treating pre‐eclampsia. Cochrane Database of Systematic Reviews 2007, Issue 2. [Art. No.: CD000492. DOI: 10.1002/14651858.CD000492.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kramer 2000
- Kramer MS, Seguin L, Lydon J, Goulet L. Socio‐economic disparities in pregnancy outcome: why do the poor fare so poorly?. Paediatric and Perinatal Epidemiology 2000;14:194‐210. [DOI] [PubMed] [Google Scholar]
Levine 1996
- Levine M, Conry‐Cantilena C, Wang Y, Welch RW, Washko PW, Dhariwal KR, et al. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proceedings of the National Academy of Science of the United States of America 1996;93(8):3704‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Levine 2001
- Levine M, Wang Y, Padayatty SJ, Morrow J. A new recommended dietary allowance of vitamin C for healthy young women. Proceedings of the National Academy of Science of the United States of America 2001;98(17):9842‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCowan 1999
- McCowan L, Harding J, Barker S, Ford C. Perinatal predictors of growth at six months in small for gestational age babies. Early Human Development 1999;56(2‐3):205‐16. [DOI] [PubMed] [Google Scholar]
Meher 2005
- Meher S, Duley L, Prevention of Pre‐eclampsia Cochrane Review authors. Interventions for preventing pre‐eclampsia and its consequences: generic protocol. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD005301] [DOI] [Google Scholar]
Miller 2005
- Miller ER 3rd, Pastor‐Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta‐analysis: high‐dosage vitamin E supplementation may increase all‐cause mortality. Annals of Internal Medicine 2005;142(1):37‐46. [DOI] [PubMed] [Google Scholar]
NHMRC 1998
- NHMRC. Report on Maternal Deaths in Australia 1991‐1993. NHMRC, 1998. [Google Scholar]
RevMan 2003 [Computer program]
- The Cochrane Collaboration. Review Manager (RevMan). Version 4.2.7 for Windows. Oxford, England: The Cochrane Collaboration, 2003.
Roberts 1993
- Roberts JM, Redman CWG. Pre‐eclampsia: more than pregnancy‐induced hypertension. Lancet 1993;341:1447‐51. [DOI] [PubMed] [Google Scholar]
Roberts 2001
- Roberts JM, Cooper DW. Pathogenesis and genetics of pre‐eclampsia. Lancet 2001;357:53‐6. [DOI] [PubMed] [Google Scholar]
Wang 1996
- Wang Y, Walsh SW. Antioxidant activities and mRNA expression of superoxide dismutase, catalase, and glutathione peroxidase in normal and preeclamptic pregnancies. Journal of the Society for Gynecological Investigation 1996;3:179‐84. [PubMed] [Google Scholar]
References to other published versions of this review
Rumbold 2005
- Rumbold A, Duley L, Crowther C, Haslam R. Antioxidants for preventing pre‐eclampsia. Cochrane Database of Systematic Reviews 2005, Issue 4. [Art. No.: CD004227. DOI: 10.1002/14651858.CD004227.pub2] [DOI] [PubMed] [Google Scholar]


