Summary
Extracellular vesicle (EV) exchange is emerging as a novel method of communication at the maternal–fetal interface. The presence of the EVs has been demonstrated in the preimplantation embryo culture medium from different species, such as bovines, porcines and humans. Preimplantation embryo‐derived EVs have been shown to carry molecules potentially able to modulate the local endometrial immune system. The non‐classical major histocompatibility complex (MHC) class I molecule human leucocyte antigen (HLA)‐G, the immunomodulatory molecule progesterone‐induced blocking factor and some regulatory miRNAs species are contained in embryo‐derived EV cargo. The implanted syncytiotrophoblasts are also well known to secrete EVs, with microvesicles exerting a mainly proinflammatory effect while exosomes in general mediate local immunotolerance. This review focuses on the current knowledge on the potential role of EVs released by the embryo in the first weeks of pregnancy on the maternal immune cells. Collectively, the data warrant further exploration of the dialogue between the mother and the embryo via EVs.
Keywords: extracellular vesicles, embryo, HLA‐G, maternal–fetal interface, trophoblast
Extracellular vesicles: characterization and roles
In the last century, the development of electron microscopy (TEM) allowed the discovery of spherical particles released by some blood cells. For a long time, these vesicles were thought to be artefacts resulting from cell damage, but some studies showed that all cells, from bacteria to plants and animal cells, seem to release extracellular vesicles (EVs) into their environment 1.
In recent decades, the scientific community focused on the central role of EVs in intracellular communication and it is becoming increasingly evident that they have specific functions in signalling, waste management, coagulation and inflammation. There are several populations of cell‐derived vesicles differing in size, composition, origin and functions, but they are all enclosed by a membrane of a phospholipid bilayer sharing some features with their origin cells. They are usually divided into different classes, of which the most important are exosomes, microvesicles and apoptotic bodies 2.
Exosomes are the smallest (diameter 50–150 nm) and the most studied. They are generated by exocytosis of multi‐vesicular bodies (MVBs), which are intracellular endosomal bodies made up of single outer membranes that gather multiple intraluminal vesicles. There are two different types of MVBs, one involved in the degradative pathway towards lysosomes and the other in the exocytosis or recycling pathway 3, 4. On the surface of most exosomes there is an evolutionarily conserved set of proteins such as tetraspanins CD9 or CD63, Alix, and Tsg101, but also specific proteins that reflect their cellular source. Recent studies indicate that the vesiculation process depends on three different cell features: the membrane lipid content, the transversal asymmetry and the lateral heterogeneity 5, 6, 7. Exosomes have been isolated from urine, blood, cerebrospinal fluid, synovial fluid, bronchial lavage fluid and embryo spent medium 7.
Microvesicles (MVs) are heterogeneous extracellular vesicles (diameter 100–1000 nm). generated by the releasing of outward detachment of portions of plasma membrane. This typical secretion process confers to MVs many characteristics in common with the source cells 8. Nevertheless, their biogenesis is only a partially known process. Flotillin‐2, selectins, integrins and CD40 are considered MV markers 9.
Apoptotic bodies are the largest vesicles (diameter 1–5 μm) generated directly by fragmentation of the plasma membrane of those cells undergoing the controlled process of apoptosis. One remarkable resemblance with the other EVs is to have an outer membrane particularly enriched in phosphatidylserine. Annexin, C3b and thrombospondin seem to be the only available markers 10. Both exosomes and MVs may contain different biological compounds, such as lipids, proteins and nucleic acids (DNA, RNA, miRNA), depending on their targets and the tissues of origin. Apoptotic bodies can carry cell organelles, proteins, DNA fragments and histones deriving directly from the intracellular environment, thus playing a central role into cellular waste management.
In recent years, several functions have been attributed to EVs, as they seem to take part in the angiogenesis process, coagulation, cell survival and proliferation, with implications in tumorigenesis and infections 11, 12, 13.
In the context of the immune and inflammatory response, EVs appear to be involved in the communication between innate and adaptive immune cells. Their modulation occurs in order to stimulate or suppress the response depending on the receptors carried by the EV membrane and the chemical mediators in their content, such as cytokines and chemokines. The enhancement of the inflammatory signals can take place through interleukin (IL)‐1 carried by EVs, which are typically released from monocytes or through platelet‐activating factor (PAF, 1‐o‐alkyl‐2‐acetyl‐sn‐glyc‐ero‐3‐phosphocholine), IL‐1β and caspase‐1 from EVs detached from platelets, macrophages and dendritic cells (DC). Similarly, mast cells can excrete EVs with a proinflammatory role favouring the maturation and migration of immune cells such as DC, while EVs released by neutrophils are more probably associated with suppression signals 14. Exosomes can even carry preformed antigen–peptide/major histocompatibility complexes (pMHCs) directly from peripheral infected or neoplastic cells to antigen‐presenting cells (APCs). This mechanism is known as MHC cross‐dressing, and leads to the activation of APCs 15. The proinflammatory mediators contained in EVs can be transported not only to other immune cells but also to other cell types, including fibroblasts, adipose tissue cells, smooth muscle cells, neurones, endothelium and several organ cells leading to changes in protein expression 16. Conversely, exosomes can also mediate an immune suppression in both physiological and pathological reactions. The inclusion of Fas ligand (FasL) into exosomes, produced by T cells or infected cells, triggers apoptosis of several target cells orchestrating cell death during immune regulations.
Recently, the emerging interest in EVs has contributed to the setting of different online databases, related to their biological characteristics such as ExoCarta, EVpedia, Vesiclepedia and miRandola, and even a dedicated journal (Journal of Extracellular Vesicles) 17, 18.
Preimplantation embryo‐derived EVs: characterization and roles at the implantation sites
The involvement of the immune system during the early stages of pregnancy is well described in the literature. Several immune cells are recruited to the placental bed to secure a successful pregnancy 19. CD56brightCD16−natural killer (NK) cells are mainly present in human endometrium, and tend to increase from 20% of local lymphocytes in the proliferative phase to 40–50% in the secretory phase to 70–80% in early pregnancy decidua. Decidual NK cells have been shown to express killer immunoglobulin receptors (KIR), CD94/NKG2A and human inhibitory receptors immunoglobulin (Ig)‐like transcript 2 (ILT2), which are receptors for human leucocyte antigen (HLA)‐C, HLA‐E and HLA‐G on trophoblast cells. The interaction of HLA‐G on trophoblast and ILT2 expressed by uterine NK cells is thought to increase the secretion of inflammatory and proangiogenic factors, while the interaction between HLA‐E on trophoblast and its receptor CD94/NKG2A on uterine NK cells might prevent the lysis of tissue cells 19. The uterine immune milieu favouring pregnancy can also benefit from the dialogue between DC and decidual NK cells. Upon in‐vivo expansion of DC during early gestation, NK cells secrete increased levels of IL‐10, which contribute to restrain potentially harmful DC immunogenic activation. Reciprocally, DC were shown to play an important role in NK cell homeostasis by enhancing proliferation and survival 20, 21.
Uterine APCs play central roles in vascular remodelling in early pregnancy through the release of angiogenetic factors and display immunosuppressive properties. They express inhibitory receptors such as ILT2 and ILT4 for HLA‐G expressed on trophoblasts which lead to the stimulation of anti‐inflammatory cytokines and tolerance to the trophoblast. Moreover, they are involved in the differentiation of endometrial NK cells into activated NK cells through the production of IL‐15 and can favour the release of interferon (IFN)‐γ by NK cells via DC‐specific intercellular adhesion molecule (ICAM)‐grabbing non‐integrin (DC‐SIGN)/ICAM‐3. The release of IFN‐γ by NK cells can, in turn, stimulate indoleamine dioxygenase (IDO) production in APCs that is toxic to T cells and favours regulatory T cell (Treg) cell induction. Natural Treg cells are the major population of decidual Treg cells, and a series of molecules expressed or secreted by Treg cells may directly contribute to their local suppressive functions, such as the up‐regulation of IDO expression in APCs and DC. Increased levels of immunosuppressive CD4+HLA‐G+T cells have also been found in decidua in healthy pregnancy 19.
As early as in the peri‐implantation period, the embryo communicates with the endometrium releasing factors able to modulate these decidual immune cells 22, 23. Among these, soluble HLA‐G, found in the embryonic secretome 24, 25 is believed to represent a major player in the initiation of immunotolerance for the invading blastocyst into the decidual tissues. Early human embryo also produces human chorionic gonadotrophin (hCG), which promotes the generation of Treg cells in the uterus 26. Analysis of embryo secretome also identified preimplantation factor (PIF) in spent media that exhibits a direct autotrophic effect, modulating maternal immune system and increasing endometrial receptivity 27. Therefore, communication of the preimplantation embryo with the endometrial immune system is thought to occur via a mechanism of signal conveyance that has not yet been comprehensively understood. The involvement of the EVs in the intercellular communication processes makes them excellent candidates for the role of communication facilitators between the embryo and the endometrium.
The presence of the EVs has been demonstrated in the embryo culture medium from different species, such as bovines 28, porcines 29, mice 30 and humans 7. The diameter of EVs found in the embryo spent media is quite different among these species, as shown in Table 1. Regardless of the species of origin, EVs released from preimplantation embryos present tetraspannins CD9 and CD63 on their surface and, for both humans and porcines, are enriched in mRNAs of pluripotency genes such as OCT4 and NANOG. The hypothesis that these EVs may favour the embryo implantation process is supported by experimental evidence showing that dye‐labelled human embryo‐derived EVs can be taken up by in‐vitro‐cultured human primary endometrial epithelial and stromal cells 7. Similarly, in sheep, in‐vivo intrauterine instillation of labelled conceptus‐derived EVs lead to their uptake in the luminal epithelium 31. There is also evidence supporting the idea that preimplantation embryo‐derived EVs can modulate the local endometrial immune system.
Table 1.
Size of embryo‐derived extracellular vesicles (EVs) among different species
| Reference | Species | EVs size (average ±SD) | EVs size range (nm) | ||
|---|---|---|---|---|---|
| Mellisho et al. 26 | *Bovine | IVF‐CB | 107.3 ± 12.9 nm (days 7–9) | 30–385 | |
| IVF‐NCB | 122.0 ± 28.7 nm (days 7–9) | ||||
| PA‐CB | 107.0 ± 13.3 nm (days 7–9) | ||||
| PA‐NCB | 104.5 ± 18.4 nm (days 7–9) | ||||
| Kim et al. 28 | Mouse | 87.1 ± 5.8 nm (outgrowth embryo) | 20–225 | ||
| Saadeldin et al. 27 | Porcine | <40 nm in early‐stage embryos | <120 nm in late‐stage embryos | 30–120 | |
| Giacomini et al. 7 | Human | 80.4 ± 2.16 nm (days 1–3) | 77.0 ± 3.16 nm (days 3–5) | 50–200 | |
Bovine embryos were produced by in‐vitro fertilization (IVF) or parthenogenetic activation (PA), competent blastocysts (CB); non‐competent blastocysts (NCB). SD = standard deviation.
EVs and HLA‐G
We have recently demonstrated that HLA‐G, reported to be present in human preimplantation embryo secretome 32, 33, was indeed associated with embryo‐derived EVs 7 (Fig. 1). HLA‐G can modulate the activity not only of decidual NK cells but also of macrophages, T cells and B cells. HLA‐G is a well‐established inhibitory ligand of NK cells, acting via KIR or other receptors 19, 34. Upon interaction with KIR2DL4, HLA‐G induces the secretion of cytokines and growth factors characteristic of decidual NK cells and required for successful vascular remodelling and pregnancy maintenance 35. The cross‐talk between HLA‐G‐expressing trophoblasts and myeloid cells in the decidua might favour the generation of tolerogenic DCs, including decidual DC‐10. Decidual DC‐10 express HLA‐G and can interact with either decidual NK cells or macrophages via ILT2 and promote their proangiogenic effects 36, 37. Decidual macrophages have been found to produce proinflammatory cytokines in response to HLA‐G binding to ILT2. A fraction of CD4+ and CD8+ T cells also express surface ILT2, which suppresses T cell proliferation upon interacting with HLA‐G 35. A similar inhibitory effect can be obtained consequently to the acquisition of HLA‐G, possibly through trogocytosis by activated CD4+ T cells. HLA‐G molecules have been detected by our group 7 in EVs isolated from media conditioned by IVF day 3 (D3) and day 5 (D5) human embryos, but not in EV‐depleted spent culture media from the same samples. HLA‐G‐mediated immune suppression by intercellular transfer mechanisms such as trogocytosis or EVs has gained considerable attention in recent years 38, suggesting that the effects of HLA‐G‐mediated maternal–fetal tolerance may be favoured by HLA‐G‐bearing EVs released by embryos.
Figure 1.
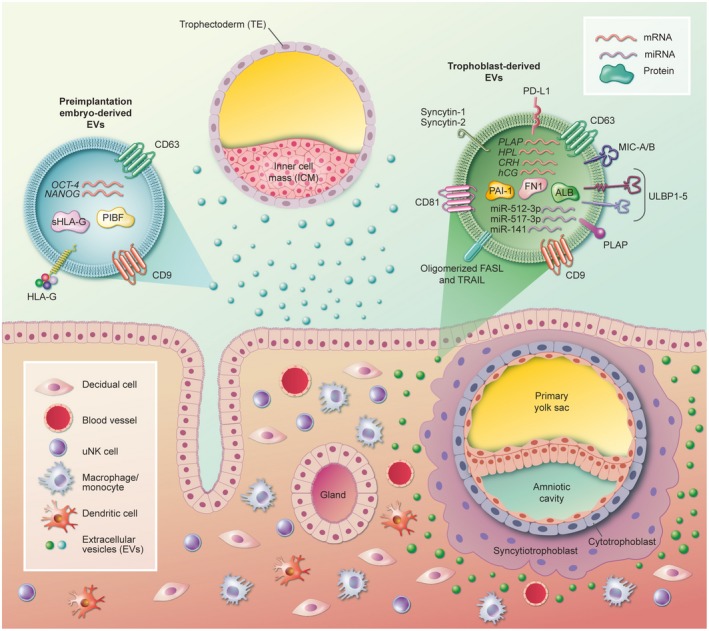
Cargos of extracellular vesicles released by the preimplantation embryo and by trophoblasts with reported effects on the local maternal immune cells. PIBF = progesterone‐induced blocking factor; ALB = albumin; ULBP1‐5 = UL‐16 binding proteins 1‐5; PLAP = placental alkaline phosphatase; PD‐L1 = programmed death‐ligand 1; MIC‐A/B = major histocompatibility complex (MHC) class I‐related protein; PAI‐1 = plasminogen activator inhibitor‐1; hCG = human chorionic gonadotrophin; CRH = corticotrophin‐releasing hormone; HPL = human placental lactogen; FN1 = fibronectin‐1.
EVs and progesterone‐induced blocking factor
Embryo‐derived EVs have been also detected at the embryo–maternal interface in mouse implantation sites 23. Pallinger and collaborators demonstrated that these EVs can transport progesterone‐induced blocking factor (PIBF) 39. The PIBF full‐length form has been suggested to be able to modulate the invasiveness of both the trophoblast and malignant tumours 40, 41 while the shorter form seems to act mainly by regulating NK cell activity. During normal pregnancy, decidual NK cells exert a low cytotoxic activity despite the abundant presence of cytotoxic molecules, such as perforin, in their cytoplasmic granules 23. Interestingly, PIBF is thought to control this cytolytic effect by inhibiting degranulation and perforin liberation from NK cells 42. Although this effect was observed in peripheral blood NK cells, it is also assumed to act locally. The identification of PIBF in embryo‐derived EVs through immuno‐electron microscopy supports the hypothesis that its function may be carried out via EV signalling.
According to Pallinger and collaborators, mouse embryo‐derived PIBF‐carrying EVs can adhere to the surface of both CD4+ and CD8+ peripheral T cells inducing IL‐10 production. The abrogation of this effect by pretreatment of the EVs with an anti‐PIBF antibody confirms the existence of a communication between the embryo and the maternal immune system via EVs 39. Large vesicles rather than exosomes seemed to mediate this action (Fig. 1).
EVs and miRNAs
The porcine embryo‐derived EV cargo also contains a variety of miRNA species with different targets on both epithelial and stromal cells. The target genes of these miRNAs are thought to mediate cellular activities such as adhesion and migration, suggesting that embryos could potentially modify the endometrial function 43. miRNAs have also been identified in culture media of human blastocysts and were detected in biopsied trophectoderm cells, suggesting that they are released from blastocysts 44, 45. Although proof that these miRNAs derive from EVs is still lacking, it may be presumed that these embryo‐derived miRNAs could be secreted via EVs. Interestingly in this regard, EVs isolated from human co‐culture supernatant of endometrial epithelial cells and cryopreserved D5 blastocysts revealed significant alterations in miRNA expression in relation to the age of the blastocyst donors 46. Eighteen EV‐bound miRNAs displayed altered levels in presence of blastocysts from advanced maternal‐age subjects. Among these, microRNA‐155 (miR‐155) plays a role in mediating an appropriate inflammatory response within the endometrial environment. Primary miR‐155 and miR‐155‐5p expression is greatly enhanced by antigen receptor stimulation of T and B cells 47, 48 or by Toll‐like receptor (TLR) agonist stimulation of DC and macrophages 49, 50, 51, 52, 53, 54. miR‐155 is also critical to Treg cell expansion and differentiation 55, 56. As EVs were analysed in a spent media of a co‐culture, it is not possible to determine if the miRNA‐155‐carrying EVs derived from the embryo or from the endometrial cells, but these observations could suggest the involvement of the immune system in early embryo–maternal communication through EVs.
Trophoblast‐derived extracellular vesicles: characterization and roles at the maternal–fetal interface
Shedding of trophoblast‐derived microparticles into the maternal circulation is a well‐known phenomenon, first described in 1893. It is therefore not surprising that several studies focused on the characterization of trophoblast‐derived EVs and their effect on target cells, as well as on their change in number and composition in different pathological conditions (Fig. 1).
The placenta is composed of cytotrophoblasts, multi‐nucleated syncytiotrophoblasts (ST) and the uterine artery‐invading extravillous cytotrophoblasts (EVT). Extracellular vescicles are mainly secreted by ST and EVT which is in direct contact with the maternal circulation.
Several proteomic studies have investigated the composition of placenta‐derived exosomes and MVs. In a meta‐analysis published in 2017, Familari et al. 57 summarizing the results of six different studies, showed that only three proteins were consistently found in all studies, namely albumin, fibronectin‐1 (FN1) and plasminogen activator inhibitor‐1 (PAI‐1). The variability of the results obtained can be ascribed to differences in isolation techniques, placental age and to the interindividual genetic difference. Of these, the gestational age of the placenta may have the greatest impact, considering that the number of secreted EVs, as well as the number of different proteins contained, increased with gestational age 58. A recent study has analysed the protein content of both exosomes and MVs from first‐trimester human placenta, showing that most of the proteins are shared between different EV types, with remarkable exceptions in proteins involved in cellular proliferation 59. Moreover, EVs seem to express ‘eat me’ signals such as annexin V and calreticulin, which enable them to be taken up by target cells (Fig. 1).
Several types of nucleic acids have also been identified in EVs. Deep sequencing analysis revealed that most RNAs are fewer than 500 nucleotides 60, 61, 62, the majority being miRNAs and then mRNAs. Corticotrophin‐releasing hormone (CRH), hCG, placental alkaline phosphatase (PLAP) and human placental lactogen (HPL) mRNAs have been identified in trophoblast‐derived EVs, but their functionality in vivo has yet to be confirmed 57, 63, 64.
Trophoblast MVs and maternal immune system
Placental microvesicles seem to have a more proinflammatory effect, stimulating the release of cytokines such as tumour necrosis factor (TNF)‐α, IL‐18, IL‐12 and IFN‐γ from circulating leucocytes 65, as well as the activation of circulating monocytes 66. Moreover, in presence of activated neutrophils, trophoblast‐derived MVs stimulate the production of neutrophil extracellular traps (NETs). There are, however, some studies showing that placental MVs may have an immunomodulatory rather than a proinflammatory effect. On B cells and monocytes, a down‐regulatory effect of MVs on IFN‐inducible protein 10 (IP‐10) expression would contribute to the skewing of the immune system towards a T helper type 2 (Th2) phenotype 67. Another line of research has focused on the role syncytin‐1 in MVs. Syncytin‐1 and ‐2 are endogenous retroviral proteins essential for the trophoblast cell‐to‐cell fusion process enabling placentation. Syncytin‐1 has also been found on MVs, and is thought to favour the fusion of the MV membrane with the target cell. In addition, trophoblast‐derived MVs containing syncytin‐1 were shown to activate peripheral blood mononuclear cells (PBMC) while dampening their response to lipopolysaccharides (LPS) 68.
Trophoblast exosomes and the maternal immune system
Placental exosomes are characterized in general by an immune‐suppressive action. While absent on the ST cell surface, FasL and TNF‐related apoptosis‐inducing ligand (TRAIL) were found in the endosomal compartment of ST and exclusively secreted in exosomes, mediating the apoptosis of T cells at the level of the placental barrier and offering local immunotolerance 69, 70. Moreover, placental exosomes express programmed death ligand 1 (PD‐L1), as well as other members of the B7 family which mediate T cell suppression through alterations in the CD3‐zeta and Janus kinase 3 (JAK‐3) pathways 71. Interestingly, while syncytin‐1‐bearing MVs have been shown to exert proinflammatory effects, syncytin‐1‐bearing exosomes can suppress the production of TNF‐α, IFN‐γ and C‐X‐C motif chemokine (CXCL)‐10 by PBMC, presumably mediating the Th1–Th2 cytokine shift 72. Finally, the NKG2D receptor expression on NK, CD8 and gamma delta cells was shown to be down‐regulated by treatment with vesicles containing MHC class I‐related protein (MIC‐A MIC‐B) and UL‐16 binding proteins (ULBP1‐5), resulting in a decreased activity of the NK cell population 73.
Exosomes are also able to modulate gene transcription in target cells through C19MC miRNAs. miR‐512‐3p and miR‐517‐3p, secreted by trophoblast cells in exosomes, transfer viral resistance to other non‐trophoblastic cells in a paracrine manner. When internalized by T and NK cells, miR‐517a‐3p is able to down‐regulate PRKG1 gene and consequently inhibits their activation and proliferation via the nitric oxide/cyclic guanosine monophosphate (cGMP) signalling pathway 74. Another miRNA, miR‐141, when present in EVs, supresses T cell proliferation in vitro, possibly further contributing to the mechanisms of maternal tolerance 75.
In contrast to these anti‐inflammatory effects, in the first phases of pregnancy trophoblast exosomes also seem to play an active role in promoting monocyte migration and macrophage activation in the decidua, creating a supportive environment for placentation. This effect is thought to be mainly mediated by fibronectin present in exosomes being able to increase the release of proinflammatory cytokines such as IL‐1β, IL‐6, serpin‐E1, granulocyte–monocyte colony‐stimulating factor (GM‐CSF) and TNF‐α 76.
Effects of placental EVs from pre‐eclamptic pregnancies on the maternal immune system
The involvement of EVs in the aetiology of pre‐eclamptic pregnancies has been supposed based on both qualitative as well as quantitative molecular differences in placental EVs observed in pre‐eclampsia 77. Compared to normal first‐term placentae, placental EVs from pre‐eclamptic women tend to exacerbate the maternal immune response to LPS, which is normally reduced during gestation 78. A possible explanation for these findings might be the reduction of both syncytin‐1 and ‐2 proteins in MVs that, inter alia, take part in dampening PBMC secretion of IL‐1β, TNF‐α, monocyte chemoattractant protein 1 and IL‐6 68. This might be a contributory factor to the exaggerated inflammatory milieu and the poor placentation that histologically characterizes the disease 79, 80.
Early pregnancy is associated with infiltration of the decidua by N2‐like neutrophils characterized by immunosuppressive and pro‐angiogenetic capabilities 81. Neutrophil contribution to the aetiology of pre‐eclampsia and its complications is clear, and trophoblast EVs seem to be involved in this phenomenon by activating classical N1‐like neutrophils. Indeed, in pre‐eclampsia, placental EVs induce the increase of superoxide production by neutrophils and trigger the production of NETs with relative prothrombotic effects, which are hallmarks of pre‐eclampsia 82, 83. Endoglin‐containing EVs are also increased in pre‐eclampsia 84. It cannot be excluded that these EVs may be responsible, at least in part, of the impaired Treg induction within the decidua, working as inhibitors of transforming growth factor (TGF)‐β 85, with the consequently unsuccessful Th1–Th2 shifting. Finally, galectin‐13, a key regulator of immune homeostasis by inducing T cell death and inflammation, has been shown to be increased in the cargo of EVs from pre‐eclamptic pregnancies 77.
Conclusions
Immune system components are major regulators of the success of embryo implantation and pregnancy establishment. Some of these regulatory functions on the immune cells may be carried out through the local release of small EVs loaded with bioactive molecules that act in a paracrine manner in adjacent cells. This overview has summarized the evidence provided so far on the potential effects exerted by EVs of embryonic origin on the local immune components. However, the precise function of EVs in pregnancy is not fully understood. So far, whether EVs will serve as biomarkers for diagnosing or assessing some dysfunctions of pregnancy or to encapsulate small molecule drugs as therapeutics remains completely unclear.
Disclosure
The authors declare no conflicts of interest.
OTHER ARTICLES PUBLISHED IN THIS REVIEW SERIES
The immunology of the fetal‐placental unit comes of age. Clinical and Experimental Immunology 2019, 198: 11–14.
The role of neutrophil activation in determining the outcome of pregnancy and modulation by hormones and/or cytokines. Clinical and Experimental Immunology 2019, 198: 24–36.
Overview of procalcitonin in pregnancy and in pre‐eclampsia. Clinical and Experimental Immunology 2019, 198: 37–46.
Influence of maternal microbiota during pregnancy on infant immunity. Clinical and Experimental Immunology 2019, 198: 47–56.
References
- 1. Deatheragea BL, Cooksona BT. Membrane vesicle release in bacteria, eukaryotes, and archaea: a conserved yet underappreciated aspect of microbial life. Infect Immun 2012; 80:1948–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Raposo G, Stoorvoge W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 2013; 200:373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mathivanan S, Simpson RJ. ExoCarta: a compendium of exosomal proteins and RNA. Proteomics 2009; 9:4997–5000. [DOI] [PubMed] [Google Scholar]
- 4. Wollert T, Hurley JH. Molecular mechanism of multivesicular body biogenesis by ESCRT complexes. Nature 2010; 464:864–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Verderio C, Gabrielli M, Giussani P. Role of sphingolipids in the biogenesis and biological activity of extracellular vesicles. J Lipid Res 2018; 59:1325–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bretscher MS. Phosphatidyl‐ethanolamine: differential labelling in intact cells and cell ghosts of human erythrocytes by a membrane‐impermeable reagent. J Mol Biol 1972; 71:523–8. [DOI] [PubMed] [Google Scholar]
- 7. Giacomini E, Vago R, Sanchez AM et al Secretome of in vitro cultured human embryos contains extracellular vesicles that are uptaken by the maternal side. Sci Rep 2017; 7:5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. György B, Szabó TG, Pásztói M et al Membrane vesicles, current state‐of‐the‐art: emerging role of extracellular vesicles. Cell Mol Life Sci 2011; 68:2667–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jayachandran M, Miller VM, Heit JA, Owen WG. Methodology for isolation, identification and characterization of microvesicles in peripheral blood. J Immunol Methods 2012; 375:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Akers JC, Gonda D, Kim R, Carter BS, Chen CC. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus‐like vesicles, and apoptotic bodies. J Neurooncol 2013; 113:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shen B, Fan Y, Wu N, Gould SJ. Biogenesis of the posterior pole is mediated by the exosome/microvesicle protein‐sorting pathway. J Biol Chem 2011; 286:44162–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Muralidharan‐Chari V, Clancy JW, Sedgwick A, D’Souza‐Schorey C. Microvesicles: mediators of extracellular communication during cancer progression. J Cell Sci 2010; 123:1603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barteneva NS, Maltsev N, Vorobjev IA. Microvesicles and intercellular communication in the context of parasitism. Front Cell Infect Microbiol 2013; 3:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Groot Kormelink T, Mol S, de Jong EC, Wauben MHM. The role of extracellular vesicles when innate meets adaptive. Semin Immunopathol 2018; 40:439–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zeng F, Morelli AE. Extracellular vesicle‐mediated MHC cross‐dressing in immune homeostasis, transplantation, infectious diseases, and cancer. Semin Immunopathol 2018; 40:477–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van Hezel ME, Nieuwland R, van Bruggen R, Juffermans NP. The ability of extracellular vesicles to induce a pro‐inflammatory host response. Int J Mol Sci 2017; 18:1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim DK, Kang B, Kim OY et al EVpedia: an integrated database of high‐throughput data for systemic analyses of extracellular vesicles. J Extracell Vesicles 2013; 2:20384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kalra H, Simpson RJ, Ji H et al Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLOS Biol 2012; 10:e1001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu S, Diao L, Huang C, Li Y, Zeng Y, Kwak‐Kim JYH. The role of decidual immune cells on human pregnancy. J Reprod Immunol 2017; 124:44–53. [DOI] [PubMed] [Google Scholar]
- 20. Blois SM, Freitag N, Tirado‐González I et al NK cell‐derived IL‐10 is critical for DC–NK cell dialogue at the maternal–fetal interface. Sci Rep 2017; 7:2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Viganò P, Gaffuri B, Somigliana E, Infantino M, Vignali M, Di Blasio AM. Interleukin‐10 is produced by human uterine natural killer cells but does not affect their production of interferon‐gamma. Mol Hum Reprod 2001; 7:971–7. [DOI] [PubMed] [Google Scholar]
- 22. Herrler A, von Rango U, Beier HM. Embryo–maternal signalling: how the embryo starts talking to its mother to accomplish implantation. Reprod Biomed Online 2003; 6:244–56. [DOI] [PubMed] [Google Scholar]
- 23. Szekeres‐Bartho J. The role of progesterone in feto–maternal immunological cross talk. Med Princ Pract 2018; 27:301–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jurisicova A, Casper RF, MacLusky NJ, Mills GB, Librach CL. HLA‐G expression during preimplantation human embryo development. Proc Natl Acad Sci USA 1996; 93:161–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rizzo R, Stignani M, Melchiorri L, Baricordi OR. Possible role of human leukocyte antigen‐G molecules in human oocyte/embryo secretome. Hum Immunol 2009; 70:970–5. [DOI] [PubMed] [Google Scholar]
- 26. Schumacher A. Human chorionic gonadotropin as a pivotal endocrine immune regulator initiating and preserving fetal tolerance. Int J Mol Sci 2017; 18:2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Roussev RG, Coulam CB, Kaider BD, Yarkoni M, Leavis PC, Barnea ER. Embryonic origin of preimplantation factor (PIF): biological activity and partial characterization. Mol Hum Reprod 1996; 2:883–7. [DOI] [PubMed] [Google Scholar]
- 28. Mellisho EA, Velásquez AE, Nuñez MJ, et al Identification and characteristics of extracellular vesicles from bovine blastocysts produced in vitro . PLOS ONE 2017; 12:e0178306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Saadeldin IM, Kim SJ, Choi Y, Lee BC. Improvement of cloned embryos development by co‐culturing with parthenotes: a possible role of exosomes/microvesicles for embryos paracrine communication. Cell Reprogram 2014; 16:223–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim J, Lee J, Lee TB, Jun JH. Embryotrophic effects of extracellular vesicles derived from outgrowth embryos in pre‐ and peri‐implantation embryonic development in mice. Mol Reprod Dev 2019; 86:187–96. B. [DOI] [PubMed] [Google Scholar]
- 31. Burns GW, Brooks KE, Spencer TE. Extracellular vesicles originate from the conceptus and uterus during early pregnancy in sheep. Biol Reprod 2016; 94:56. [DOI] [PubMed] [Google Scholar]
- 32. Noci I, Fuzzi B, Rizzo R et al Embryonic soluble HLA‐G as a marker of developmental potential in embryos. Hum Reprod 2005; 20:138–46. [DOI] [PubMed] [Google Scholar]
- 33. Rizzo R, Vercammen M, Van De Velde H, Horn PA, Rebmann V. The importance of HLA‐G expression in embryos, trophoblast cells, and embryonic stem cells. Cell Mol Life Sci 2011; 68:341–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vento‐Tormo R, Efremova M, Botting RA et al Single‐cell reconstruction of the early maternal–fetal interface in humans. Nature 2018; 563:347–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ferreira LMR, Meissner TB, Tilburgs T, Strominger JL. HLA‐G: at the interface of maternal–fetal tolerance. Trends Immunol 2017; 38:272–86. [DOI] [PubMed] [Google Scholar]
- 36. Amodio G, Sales de Albuquerque R, Gregori S. New insights into HLA‐G mediated tolerance. Tissue Antigens 2014; 84:255–63. [DOI] [PubMed] [Google Scholar]
- 37. Gregori S, Amodio G, Quattrone F, Panina‐Bordignon P. HLA‐G orchestrates the early interaction of human trophoblasts with the maternal niche. Front Immunol 2015; 6:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lin A, Yan WH. Heterogeneity of HLA‐G expression in cancers: facing the challenges. Front Immunol 2018; 9:2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pallinger E, Bognar Z, Bogdan A, Csabai T, Abraham H, Szekeres‐Bartho J. PIBF+ extracellular vesicles from mouse embryos affect IL‐10 production by CD8+cells. Sci Rep 2018; 8:4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Miko E, Halasz M, Jericevic‐Mula B et al Progesterone‐induced blocking factor (PIBF) and trophoblast invasiveness. J Reprod Immunol 2011; 90:50–7. [DOI] [PubMed] [Google Scholar]
- 41. Halasz M, Polgar B, Berta G, Czimbalek L, Szekeres‐Bartho J. Progesterone‐induced blocking factor differentially regulates trophoblast and tumor invasion by altering matrix metalloproteinase activity. Cell Mol Life Sci 2013; 70:4617–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Faust Z, Laskarin G, Rukavina D, Szekeres‐Bartho J. Progesterone‐induced blocking factor inhibits degranulation of natural killer cells. Am J Reprod Immunol 1999; 42:71–5. [PubMed] [Google Scholar]
- 43. Bidarimath M, Khalaj K, Kridli RT, Kan FWK, Koti M, Tayade C. Extracellular vesicle mediated intercellular communication at the porcine maternal–fetal interface: a new paradigm for conceptus‐endometrial cross‐talk. Sci Rep 2017; 7:40476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Capalbo A, Ubaldi FM, Cimadomo D, et al MicroRNAs in spent blastocyst culture medium are derived from trophectoderm cells and can be explored for human embryo reproductive competence assessment. Fertil Steril 2016; 105:225–35. [DOI] [PubMed] [Google Scholar]
- 45. Cuman C, Van Sinderen M, Gantier MP et al Human blastocyst secreted microRNA regulate endometrial epithelial cell adhesion. EBioMedicine 2015; 2:1528–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Parks JC, McCallie BR, Patton AL et al The impact of infertility diagnosis on embryo–endometrial dialogue. Reproduction 2018; 155:543–52. [DOI] [PubMed] [Google Scholar]
- 47. Elton TS, Selemon H, Elton SM, Parinandi NL. Regulation of the MIR155 host gene in physiological and pathological processes. Gene 2013; 532:1–12. [DOI] [PubMed] [Google Scholar]
- 48. Banerjee A, Schambach F, Dejong CS, Hammond SM, Reiner SL. Micro‐RNA‐155 inhibits IFN‐γ signaling in CD4+ T cells. Eur J Immunol 2010; 40:225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ceppi M, Pereira PM, Dunand‐Sauthier I et al MicroRNA‐155 modulates the interleukin‐1 signaling pathway in activated human monocyte‐derived dendritic cells. Proc Natl Acad Sci USA 2009; 106:2735–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cremer TJ, Ravneberg DH, Clay CD et al MiR‐155 induction by F. novicida but not the virulent F. tularensis results in SHIP down‐regulation and enhanced pro‐inflammatory cytokine response. PLOS ONE 2009; 4:e8508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mao CP, He L, Tsai C et al In vivo microRNA‐155 expression influences antigen‐specific T cell‐mediated immune responses generated by DNA vaccination. Cell Biosci 2011; 1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. O’Connell RM, Taganov KD, Boldin MP, Cheng G, Baltimore D. MicroRNA‐155 is induced during the macrophage inflammatory response. Proc Natl Acad Sci USA 2007; 104:1604–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Taganov KD, Boldin MP, Chang K‐J, Baltimore D. NF‐kappaB‐dependent induction of microRNA miR‐146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA 2006; 103:12481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tili E, Michaille J‐J, Cimino A et al Modulation of miR‐155 and miR‐125b levels following lipopolysaccharide/TNF‐α stimulation and their possible roles in regulating the response to endotoxin shock. J Immunol 2007; 179:5082–9. [DOI] [PubMed] [Google Scholar]
- 55. Cobb BS, Hertweck A, Smith J et al A role for Dicer in immune regulation. J Exp Med 2006; 203:2519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lu LF, Thai TH, Calado DP et al Foxp3‐dependent MicroRNA155 confers competitive fitness to regulatory T cells by targeting SOCS1 protein. Immunity 2009; 30:80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Familari M, Cronqvist T, Masoumi Z, Hansson SR. Placenta‐derived extracellular vesicles: their cargo and possible functions. Reprod Fertil Dev 2017; 29:433–47. [DOI] [PubMed] [Google Scholar]
- 58. Salomon C, Torres MJ, Kobayashi M et al A gestational profile of placental exosomes in maternal plasma and their effects on endothelial cell migration. PLOS ONE 2014; 9:e98667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tong M, Kleffmann T, Pradhan S et al Proteomic characterization of macro‐, micro‐ and nano‐extracellular vesicles derived from the same first trimester placenta: relevance for feto–maternal communication. Hum Reprod 2016; 31:687–99. [DOI] [PubMed] [Google Scholar]
- 60. Bellingham SA, Coleman BM, Hill AF. Small RNA deep sequencing reveals a distinct miRNA signature released in exosomes from prion‐infected neuronal cells. Nucleic Acids Res 2012; 40:10937–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Li M, Zeringer E, Barta T, Schageman J, Cheng A, Vlassov AV. Analysis of the RNA content of the exosomes derived from blood serum and urine and its potential as biomarkers. Phil Trans R Soc B Biol Sci 2014; 369:20130502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nolte’T Hoen ENM, Buermans HPJ, Waasdorp M, Stoorvogel W, Wauben MHM, ’T Hoen, PAC . Deep sequencing of RNA from immune cell‐derived vesicles uncovers the selective incorporation of small non‐coding RNA biotypes with potential regulatory functions. Nucleic Acids Res 2012; 40:9272–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gupta AK, Holzgreve W, Huppertz B, Malek A, Schneider H, Hahn S. Detection of fetal DNA and RNA in placenta‐derived syncytiotrophoblast microparticles generated in vitro . Clin Chem 2004; 50:2187–90. [DOI] [PubMed] [Google Scholar]
- 64. Kumpel B, King MJ, Sooranna S et al Phenotype and mRNA expression of syncytiotrophoblast microparticles isolated from human placenta. Ann NY Acad Sci 2008; 1137:144–7. [DOI] [PubMed] [Google Scholar]
- 65. Germain SJ, Sacks GP, Soorana SR, Sargent IL, Redman CW. Systemic inflammatory priming in normal pregnancy and preeclampsia: The role of circulating syncytiotrophoblast microparticles. J Immunol 2007; 178:5949–56. [DOI] [PubMed] [Google Scholar]
- 66. Messerli M, May K, Hansson SR et al Feto‐maternal interactions in pregnancies: placental microparticles activate peripheral blood monocytes. Placenta 2010; 31:106–12. [DOI] [PubMed] [Google Scholar]
- 67. Southcombe J, Tannetta D, Redman C, Sargen I. The immunomodulatory role of syncytiotrophoblast microvesicles. PLOS ONE 2011; 6:e20245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Holder BS, Tower CL, Forbes K, Mulla MJ, Aplin JD, Abrahams VM. Immune cell activation by trophoblast‐derived microvesicles is mediated by syncytin 1. Immunology 2012; 136:184–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Stenqvist A‐C, Nagaeva O, Baranov V, Mincheva‐Nilsson L. Exosomes secreted by human placenta carry functional Fas ligand and TRAIL molecules and convey apoptosis in activated immune cells, suggesting exosome‐mediated immune privilege of the fetus. J Immunol 2013; 191:5515–23. [DOI] [PubMed] [Google Scholar]
- 70. Frängsmyr L, Baranov V, Nagaeva O, Stendahl U, Kjellberg L, Mincheva‐Nilsson L. Cytoplasmic microvesicular form of Fas ligand in human early placenta: switching the tissue immune privilege hypothesis from cellular to vesicular level. Mol Hum Reprod 2005; 11:35–41. [DOI] [PubMed] [Google Scholar]
- 71. Sabapatha A, Gercel‐Taylor C, Taylor DD. Specific isolation of placenta‐derived exosomes from the circulation of pregnant women and their immunoregulatory consequences. Am J Reprod Immunol 2006; 56:345–55. [DOI] [PubMed] [Google Scholar]
- 72. Tolosa JM, Schjenken JE, Clifton VL et al The endogenous retroviral envelope protein syncytin‐1 inhibits LPS/PHA‐stimulated cytokine responses in human blood and is sorted into placental exosomes. Placenta 2012; 33:933–41. [DOI] [PubMed] [Google Scholar]
- 73. Hedlund M, Stenqvist A‐C, Nagaeva O et al Human placenta expresses and secretes NKG2D ligands via exosomes that down‐modulate the cognate receptor expression: evidence for immunosuppressive function. J Immunol 2009; 183:340–51. [DOI] [PubMed] [Google Scholar]
- 74. Kambe S, Yoshitake H, Yuge K et al Human exosomal placenta‐associated miR‐517a‐3p modulates the expression of PRKG1 mRNA in Jurkat cells. Biol Reprod 2014; 91:129. [DOI] [PubMed] [Google Scholar]
- 75. Ospina‐Prieto S, Chaiwangyen W, Herrmann J et al MicroRNA‐141 is upregulated in preeclamptic placentae and regulates trophoblast invasion and intercellular communication. Transl Res 2016; 172:61–72. [DOI] [PubMed] [Google Scholar]
- 76. Atay S, Gercel‐Taylor C, Kesimer M, Taylor DD. Morphologic and proteomic characterization of exosomes released by cultured extravillous trophoblast cells. Exp Cell Res 2011; 317:1192–202. [DOI] [PubMed] [Google Scholar]
- 77. Chiarello DI, Salsoso R, Toledo F, Mate A, Vázquez CM, Sobrevia L. Foetoplacental communication via extracellular vesicles in normal pregnancy and preeclampsia. Mol Aspects Med 2018; 60:69–80. [DOI] [PubMed] [Google Scholar]
- 78. Holder BS, Tower CL, Jones CJP, Aplin JD, Abrahams VM. Heightened pro‐inflammatory effect of preeclamptic placental microvesicles on peripheral blood immune cells in humans. Biol Reprod 2012; 86:103. [DOI] [PubMed] [Google Scholar]
- 79. Vargas A, Zhou S, Et́hier‐Chiasson M et al Syncytin proteins incorporated in placenta exosomes are important for cell uptake and show variation in abundance in serum exosomes from patients with preeclampsia. FASEB J 2014; 28:3703–19. [DOI] [PubMed] [Google Scholar]
- 80. Liang C‐Y, Wang L‐J, Chen C‐P, Chen L‐F, Chen Y‐H, Chen H. GCM1 regulation of the expression of syncytin 2 and its cognate receptor MFSD2A in human placenta. Biol Reprod 2010; 83:387–95. [DOI] [PubMed] [Google Scholar]
- 81. Amsalem H, Kwan M, Hazan A et al Identification of a novel neutrophil population: proangiogenic granulocytes in second‐trimester human decidua. J Immunol 2014; 193:3070–9. [DOI] [PubMed] [Google Scholar]
- 82. Tannetta D, Masliukaite I, Vatish M, Redman C, Sargent I. Update of syncytiotrophoblast derived extracellular vesicles in normal pregnancy and preeclampsia. J Reprod Immunol 2017; 119:98–106. [DOI] [PubMed] [Google Scholar]
- 83. Gupta AK, Hasler P, Holzgreve W, Gebhardt S, Hahn S. Induction of neutrophil extracellular DNA lattices by placental microparticles and IL‐8 and their presence in preeclampsia. Hum Immunol 2005; 66:1146–54. [DOI] [PubMed] [Google Scholar]
- 84. Tannetta D, Dragovic R, Alyahyaei Z, Southcombe J. Extracellular vesicles and reproduction‐promotion of successful pregnancy. Cell Mol Immunol 2014; 11:548–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hsu P, Santner‐Nanan B, Dahlstrom JE et al Altered decidual DC‐SIGN+ antigen‐presenting cells and impaired regulatory T‐cell induction in preeclampsia. Am J Pathol 2012; 181:2149–60. [DOI] [PubMed] [Google Scholar]


