Summary
Type 1 diabetes (T1D) results from autoimmune destruction of insulin‐producing beta cells in pancreatic islets. Various immune cell populations are involved in disease development and natural course. However, to our knowledge, so far there are no comprehensive comparative investigations of all main immune cell populations and their most important subsets at the onset of disease. Therefore, in the current study, we analyzed 51 peripheral blood immune cell populations in 22 young T1D patients and in 25 age‐matched controls using a comprehensive polychromatic flow cytometry panel developed for whole blood by the COST Action no. BM0907 ENTIRE (European Network for Translational Immunology Research and Education: From Immunomonitoring to Personalized Immunotherapy) consortium. We found that in T1D patients, frequencies and absolute counts of natural killer (NK) cells, dendritic cells (DC) and T cells, as well as their respective subsets, were significantly altered compared to controls. Further, we observed that changes in several cell populations (e.g. CD14+CD16+ non‐classical monocytes, plasmablasts) were dependent on the age of the patient. In addition to age‐related changes, we also found that alterations in immune cell patterns were associated with parameters such as the presence of ketoacidosis and C‐peptide serum levels. Our study provides a foundation for future studies investigating different cell lineages and their role in T1D and illustrates the value of polychromatic flow cytometry for evaluating all main peripheral immune cells and their subsets in whole blood samples.
Keywords: autoimmunity, cell surface molecules, diabetes, pancreas
Introduction
Type 1 diabetes (T1D) is an immune‐mediated multi‐factorial disease characterized by the destruction of insulin‐producing beta cells in pancreatic islets, which results in almost complete insulin deficit. T1D is one of the most common chronic diseases among children 1, and is caused by the interplay of environmental, immunological and genetic factors 2. Among several environmental factors, enteroviruses have a potential role 3. A major part in genetic susceptibility is determined by the human leukocyte antigen (HLA) class II, primarily by the HLA‐DR/DQ region‐encoded determinants 4. Among various important metabolic measures, blood C‐peptide levels (a marker of the degree of pancreatic destruction) and diabetic ketoacidosis (DKA) are most critical in the characterization of T1D.
Although T1D has been widely studied, disease immunopathogenesis still remains not fully understood. Many studies suggest that the development of T1D is associated with changes in various blood immune cell types, both in innate and adaptive compartments 5, 6, 7, 8, 9, 10. Most such studies, however, have mainly examined selected immune cell subsets or only main immune cell populations without deeper analysis of their minor subsets. In the current study, we aimed to analyze a wide spectrum of fundamentally important peripheral blood immune cells at the same time with the approach to provide a comprehensive comparative overview of these cell subsets in T1D patients. Thus, we examined the phenotypical characteristics of following cell populations and their subsets: T cells, T regulatory cells (Treg), B cells, natural killer (NK) cells, dendritic cells (DC) and monocytes (Table 1). In addition, we paid special attention to a subpopulation of 6‐sulfo LacNAc (slan)‐expressing proinflammatory subset of myeloid DCs. This subpopulation has been studied in other autoimmune conditions where their important role has been shown 11, 12, and they may also have a substantial function in the pathogenesis of T1D. In addition, we analyzed whether the cell profile might be associated with clinical and age‐related differences in T1D patients.
Table 1.
Marker combinations used for identification of cell subtypes in T cell, regulatory T cell (Treg), B cell, dendritic cell (DC), natural killer (NK) cell and monocyte compartments
| T cell panel | Treg panel | ||
|---|---|---|---|
| CD3+ T cells | CD3+ | CD4+ T regulatory cells (Treg) | CD3+CD4+CD25+CD127– |
| CD4+ T helper (Th) cells | CD3+CD4+ | Memory Treg | CD3+CD4+CD25+CD127–CD45R0+CD194+ |
| CD4+ naive Th cells | CD3+CD4+CD45RA+CD197+ | HLA–DR+ Treg | CD3+CD4+CD25+CD127–CD45R0+CD194+HLA–DR+ |
| CD4+ TEMRA | CD3+CD4+CD45RA+CD197– | NK cell, DC and monocyte panel | |
| CD4+ effector memory (EM) Th cells | CD3+CD4+CD45RA–CD197– | NK cells | CD3–CD19–CD20–CD14–CD56+ |
| EM Th1 | CD3+CD4+CD45RA–CD197–CD183+CD196– | CD56++CD16– (CD56brightCD16–) regulatory NK cells | CD3–CD19–CD20–CD14–CD56++CD16– |
| EM Th2 | CD3+CD4+CD45RA–CD197–CD183–CD196– | CD56++CD16+ (CD5brightCD16+) intermediate NK cells | CD3–CD19–CD20–CD14–CD56++CD16+ |
| EM Th17 | CD3+CD4+CD45RA–CD197–CD183–CD196+ | CD56+CD16– (CD56dimCD16–) NK | CD3–CD19–CD20–CD14–CD56+CD16– |
| CD4+ central memory (CM) Th cells | CD3+CD4+CD45RA–CD197+ | CD56+CD16+ (CD56dimCD16+) effector NK cells | CD3–CD19–CD20–CD14–CD56+CD16+ |
| CM Th1 | CD3+CD4+CD45RA–CD197+CD183+CD196– | Dendritic cells (DC) | CD3–CD19–CD20–CD14–CD56–HLA–DR+ |
| CM Th2 | CD3+CD4+CD45RA–CD197+CD183–CD196– | Plasmacytoid DC | CD3–CD19–CD20–CD14–CD56–HLA–DR+CD123+CD11c– |
| CM Th17 | CD3+CD4+CD45RA–CD197+CD183–CD196+ | Myeloid DC (mDC) | CD3–CD19–CD20–CD14–CD56–HLA–DR+CD123–CD11c+ |
| I activated CD4+ T cells | CD4+HLA–DR+CD38– | slan– CD16– mDC | CD3–CD19–CD20–CD14–CD56–HLA–DR+CD123–CD11c+slan–CD16– |
| II activated CD4+ T cells | CD4+HLA–DR+CD38+ | slan– CD16+ mDC | CD3–CD19–CD20–CD14–CD56–HLA–DR+CD123–CD11c+slan–CD16+ |
| Non‐activated CD4+ T cells | CD4+HLA–DR–CD38– | slan+ CD16+ mDC | CD3–CD19–CD20–CD14–CD56–HLA–DR+CD123–CD11c+slan+CD16+ |
| III activated CD4+ T cells | CD4+HLA–DR–CD38+ | Monocytes | CD3–CD19–CD20–CD14+ |
| CD8+ cytotoxic lymphocyte (CTL) T cells | CD3+CD8+ | CD14+CD16– classical monocytes | CD3–CD19–CD20–CD14+CD16– |
| CD8+ naive CTL | CD3+CD8+CD45RA+CD197+ | CD14+CD16+ non–classical monocytes | CD3–CD19–CD20–CD14+CD16+ |
| CD8+ TEMRA CTL | CD3+CD8+CD45RA+CD197– | B cell panel | |
| CD8+ EM CTL | CD3+CD8+CD45RA–CD197– | CD19+ B cells | CD3–CD19+ |
| CD8+ CM CTL | CD3+CD8+CD45RA–CD197+ | Switched memory B cells (SM B cell) | CD3–CD19+IgD–CD27+ |
| I activated CD8+ T cells | CD8+HLA–DR+CD38– | Plasmablasts | CD3–CD19+IgD–CD27+CD38+CD20– |
| II activated CD8+ T cells | CD8+HLA–DR+CD38+ | Preswitch memory B cells (PSM B cell) | CD3–CD19+IgD+CD27+ |
| Non‐activated CD8+ T cells | CD8+HLA–DR–CD38– | Exhausted memory B cells | CD3–CD19+IgD–CD27– |
| III activated CD8+ T cells | CD8+HLA–DR–CD38+ | Naive B cells | CD3–CD19+IgD+CD27– |
| CD4+CD8+ double‐positive T cells | CD3+CD4+CD8+ | Transitional B cells | CD3–CD19+IgD+CD27–CD38+CD24+ |
| CD4–CD8– double‐negative T cells | CD3+CD4–CD8– | ||
TEMRA = T cell effector memory subset that expresses CD45RA; HLA‐DR = human leukocyte antigen DR, slan = 6‐sulfo LacNac.
Materials and methods
Study population
We investigated 22 young recent‐onset T1D patients (aged 5·0–21·0 years, mean age = 11·6 years, 13 female) and 25 healthy individuals (aged 4·5–22·0 years, mean age = 12·5, 13 female) (Table 2). Patients were recruited from the Tartu University Hospital from 2014 to 2017. Diagnosis of T1D was based on internationally accepted diagnostic criteria 13. In all but one patient, venous blood samples were obtained 0–16 days following diagnosis, and all patients were on insulin treatment at the time of blood collection. Random C‐peptide values of patients ranged from 0·1 nmol/l to 0·9 nmol/l; (mean = 0·3 nmol/l; reference range = 0·37–1·47 nmol/l). Because the natural history of T1D may depend on the age at onset of the studied individuals, we opted to divide patients into two groups: (1) patients aged under 11 years and (2) patients aged 11 years and older.
Table 2.
Summarized characteristics of study participants
| Controls (n = 25) | T1D patients (n = 22) | |
|---|---|---|
| Age (years) | 12.5 (4.5–22.0)* | 11.6 (5.0–21.0)* |
| Female (%) | 52% (13/25) | 59% (13/22) |
| Islet autoantibodies (AAB) | ||
| GADA | 4% (1/25) | 73% (16/22) |
| ZnT8A | 0% (0/25) | 64% (14/22) |
| IA2A | 0% (0/25) | 82% (18/22) |
| Positive for 1 AAB | 4% (1/25) | 23% (5/22) |
| Positive for 2 AAB | 0% (0/25) | 36% (8/22) |
| Positive for 3 AAB | 0% (0/25) | 41% (9/22) |
| Enterovirus (EV) antibodies | ||
| EV IgA | 32% (8/23) | 36% (8/22) |
| EV IgG | 56% (14/23) | 27% (6/22) |
| EV IgA/IgG | 68% (17/23) | 55% (12/22) |
| HLA genotypes | ||
| High risk | 0% (0/25) | 46% (10/22) |
| Moderately increased risk | 0% (0/25) | 18% (4/22) |
| Slightly increased risk | 24% (6/25) | 9% (2/22) |
| Neutral | 36% (9/25) | 18% (4/22) |
| Slightly decreased risk | 40% (10/25) | 9% (2/22) |
| Strongly decreased risk | 0% (0/25) | 0% (0/22) |
| Metabolic data | ||
| Ketoacidosis | n.a. | 32% (7/22) |
Percentages or mean values and range from minimum to maximum (*). Ig = immunoglobulin; HLA = human leukocyte antigen; n.a. = not available; GADA = glutamic acid decarboxylase 65 autoantibody, IA‐2A = protein tyrosine phophatase autoantibody, ZnT8A = zinc transporter 8 autoantibody .
The control group consisted of non‐diabetic volunteers and children who visited Tartu University Hospital with minor surgical problems. All controls had normal blood glucose or/and glycated hemoglobin (HbA1c). Glucose, HbA1c and C‐peptide were measured by the United Laboratories of the Tartu University Hospital, according to their routine protocol. All patients and controls, or their parents and/or guardians if needed, signed a written consent form before participation. The study was approved by the Research Ethics Committee of the University of Tartu (Protocols 163/T‐6 from 24.09.2007 and 242/M‐8 from 17.11.2014).
HLA class II status and diabetes‐associated autoantibodies
HLA class II status and presence of diabetes‐associated autoantibodies (AAB) were determined in all controls and T1D patients (Table 2).
HLA
HLA‐DR/DQ genotypes were determined using polymerase chain reaction (PCR)‐based lanthanide‐labeled oligonucleotide hybridization and time‐resolved fluorometry, as described previously 4. On the basis of HLA‐DRB1–DQA1–DQB1 haplotype combinations, six groups were formed: strongly increased, moderately increased, slightly increased, neutral, slightly decreased and strongly decreased risk for T1D development (Table 2).
Islet autoantibodies
Detection of islet AAB against 65‐kDa glutamic acid decarboxylase (GAD), protein tyrosine phosphatase (IA‐2), and zinc transporter 8 (ZnT8) was performed according to the manufacturer's instructions using commercial enzyme‐linked immunosorbent assay (ELISA) kits (RSR Ltd, Cardiff, UK). The following cut‐off levels were used: ≥5 U/ml for GADA; ≥15 U/ml for ZnT8A; and ≥15 U/ml (until May 2015) or ≥7·5 U/ml (after May 2015) or for IA‐2A. All tests had a specificity of 96–99% and sensitivity of 64–86%, as confirmed by the Islet Autoantibody Standardization Program (IASP) in 2012 and 2015.
Polychromatic multi‐parameter flow cytometry
Fresh heparinized whole blood was separated into100‐μl aliquots for four staining panels: (1) T cells; (2) Treg cells; (3) B cells; and (4) DC, NK cells and monocytes. For all panels, blood was incubated with the appropriate fluorochrome‐conjugated monoclonal antibodies for 30 min at room temperature (RT) in the dark. After incubation, red blood cells were lysed using 2 ml ×1 BD Lysing Solution (BD Biosciences, San Jose, CA, USA). Prepared samples were stored at RT in the dark until analysis (within 6 h of preparation), and 300 000 events were recorded for each sample as a rule.
Flow cytometric immunophenotyping was performed on LSR Fortessa (BD Biosciences), and flow cytometry data were analyzed with FACS Diva software (BD Biosciences) according to the gating strategy (Fig. 1) from COST‐ENTIRE HIP‐C version 3.3 protocol. In total, 51 immune cell subsets data were collected by staining panels, including the following main cell populations: (1) CD4+ T helper (Th), Th1, Th2, Th17, CD8+ cytotoxic T lymphocyte (CTL), naive, central memory (CM), effector memory (EM), effector memory RA (TEMRA – T cell effector memory subset that expresses CD45RA) and activated and non‐activated CD4+ and CD8+ T cells; (2) Treg cells, memory Treg cells and activated HLA‐DR+ Treg cells; (3) CD19+ B cells, naive B cells, transitional cells, preswitch memory B cells (PSM), class‐switched memory B cells (SM), plasmablasts and exhausted memory B cells; (4) DC, plasmacytoid DC (pDC), myeloid DCs (mDC), slan– CD16‐ mDC, slan– CD16+ mDC, slan+ CD16+ mDC, NK cells, CD56+CD16– NK, CD56++CD16– NK, CD56++CD16+ NK, CD56+CD16+ NK, monocytes and CD14+CD16– classical and CD14+CD16+ non‐classical monocytes (Table 1).
Figure 1.
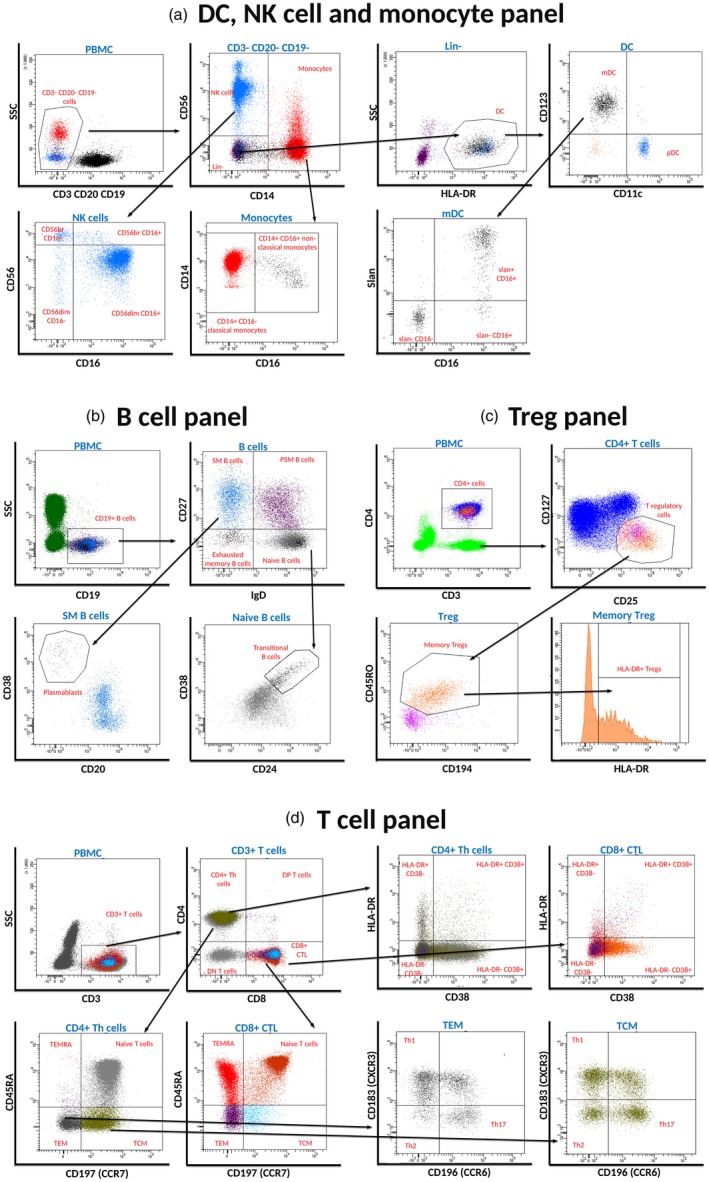
Delineation of peripheral blood immune cells. For all staining panels, cells were first gated upon forward‐scatter (FSC)‐height versus FSC‐area for doublet discrimination, followed by gating on side‐scatter (SSC)‐area versus CD45 for distinguishing peripheral blood mononuclear cells (PBMCs). (a) Gating strategy for dendritic cell (DC), natural killer (NK) cell and monocyte panel; (b) gating strategy for B cell panel; (c) gating strategy for regulatory T cell (Treg) panel; and (d) gating strategy for T cell panel.
Identification of the aforementioned subsets based on differential expression of cell surface markers shown in Tables 1 and 3. Table 3 also displays the list of fluorochromes and antibody clones used. For the determination of absolute cell numbers, BD Multitest™ 6‐color TBNK reagent (measures T, B and NK cells, as well as CD4 and CD8 subpopulations of T cells; BD Biosciences) and TruCount tubes (BD Biosciences), that consist of the known number of beads, were used. An absolute number of cells in 1 μl of blood was calculated by comparing cellular events to bead events.
Table 3.
List of fluorochromes, cell surface markers and respective clones of monoclonal antibodies used for the identification of cell subtypes in T cell, regulatory T cell (Treg), B cell, dendritic cell (DC), natural killer (NK) cell and monocyte compartments
| Fluorochromes | T cell panel | Treg panel | B cell panel | DC/NK cell/monocyte panel | ||||
|---|---|---|---|---|---|---|---|---|
| Marker | Clone | Marker | Clone | Marker | Clone | Marker | Clone | |
| FITC | CD24* | ML5 | Slan**** | DD‐1 | ||||
| AF488 | CD183 (CXCR3)** | G025H7 | ||||||
| PerCP‐Cy5.5 | CD4* | RPA‐T4 | CD4* | RPA‐T4 | CD19* | HIB19 | CD123* | 7G3 |
| PE | CD197 (CCR7)* | 150503 | CD25* | M‐A251 | CD56*** | MY31 | ||
| PE‐Cy7 | CD45RA* | HI100 | CD194 (CCR4)* | 1G1 | IgD** | IA6‐2 | CD11c* | B‐LY6 |
| APC | CD38* | HIT2 | CD38* | HIT2 | CD16* | B73.1 | ||
| AF647 | CD127* | hIL‐7R‐M21 | ||||||
| AF700 | CD45** | HI30 | CD45** | HI30 | CD45** | HI30 | CD45** | HI30 |
| APC‐H7 | CD8* | SK1 | CD45RO* | UCHL1 | CD20* | L27 | CD3*, CD19*, CD20* | SK7, HIB19, L27 |
| V450 | CD3* | UCHT1 | CD3* | UCHT1 | CD14* | MoP9 | ||
| BV421 | CD27** | O323 | ||||||
| V500 | HLA‐DR* | L243(G46‐6) | HLA‐DR* | L243(G46‐6) | CD3* | UCHT1 | HLA‐DR* | L243(G46‐6) |
| BV605 | CD196 (CCR6)** | G034E3 | ||||||
Reagent manufacturer:
Becton Dickinson (BD);
BioLegend;
Beckman Coulter;
Miltenyi Biotec. FITC = fluorescein isothiocyanate; PerCp = peridinin chlorophyll; PE‐Cy7 = phycoerythrin‐cyanin 7; HLA‐DR = human leukocyte antigen DR. APC = allophycocyanin.
Statistical analysis
Statistical analysis was performed in r version 2.15.0 (R Core Team, 2012) and GraphPad Prism version 5. Mann–Whitney U‐tests were performed to compare absolute cell counts and frequencies between patients and controls. Cell frequencies were calculated as a percentage of the number of peripheral blood mononuclear cells (PBMC). Associations between cell counts/frequencies and C‐peptide levels were assessed using Spearman's rank correlation tests. Due to the exploratory nature of the study, we did not adjust for multiple comparisons and results were considered statistically significant when P < 0.05.
Results
Demographic and medical data in T1D patients and healthy controls
T1D patient age and sex did not differ significantly from controls. All patients were positive for at least one AAB, and IA2A was the most prevalent (82%), followed by GADA (73%) and ZnT8A (64%). The combination of three AABs (41%) was the most prevalent AAB pattern for T1D patients, with only 23% of patients having one AAB. One individual from the control group was positive for islet AAB (4%); however, because this male control had low‐titer GADA (6 U/ml) and no autoimmune or GADA‐associated neurological diseases, the patient was not excluded from analysis (Table 2). Patients positive for one diabetes‐specific AAB had higher frequencies and absolute counts of activated (HLA‐DR+ CD38+) CD8+ T cells (P = 0·03; 1·7 versus 0·8%; P = 0·03; 33 versus 19·5 cells/μl) than those patients positive for two or more AAB. At the diagnosis of T1D, DKA was present in 32% of patients.
Most patients (64%) were classified having HLA haplotypes, suggesting a strongly or moderately increased risk for T1D, whereas these haplotypes were absent in the control group (Table 2). Based on HLA‐DR/DQ genotypes, T1D patients were divided into two groups: (1) patients with strongly increased risk for T1D (HLA‐DR4/3 positives); and (2) patients with other HLA combinations. Patients with strongly increased risk of T1D had lower frequencies of non‐activated (HLA‐DR– CD38–) CD4+ T cells (P = 0·04; 8·7 versus 10·3%) and higher frequencies of activated (HLA‐DR– CD38+) CD8+ T cells (P = 0·04; 7·7 versus 5·5%) compared to the group of patients with other HLA combinations.
Enterovirus (EV) immunoglobulin (Ig)A‐ and/or IgG‐positive T1D patients did not differ significantly from controls. When EV IgA‐ and/or IgG‐positive and ‐negative patients were compared, we found that within the T cell compartment a limited number of subsets exhibited changes associated with the presence of EV antibodies. EV IgA‐ and/or IgG‐positive patients had lower frequencies and lower absolute numbers of activated (HLA‐DR+ CD38+) CD4+ T cells (P = 0·02; 0·52 versus 0·88%; P = 0·01; 13 versus 22 cells/µl), as well as higher frequencies of non‐activated (HLA‐DR– CD38–) CD8+ T cells (P = 0·01; 10·8 versus 6·4%) compared to EV IgA‐/IgG‐negative patients.
Differences in immune cell subsets between T1D patients and controls
We compared cell frequencies (% of PBMC) and median values of absolute numbers of immune cells (cells/μl) in patients and controls among all 51 immune cell populations (Figs 2, 3, 4, 5 and Supporting information, Tables 1, 2, 3). Absolute cell counts in T1D patients that differed significantly from controls and within the patient's subgroups are shown in Supporting information, Figs 1, 2, 3, 4. In general, the differences found that between groups either cell numbers or frequencies overlap.
Figure 2.
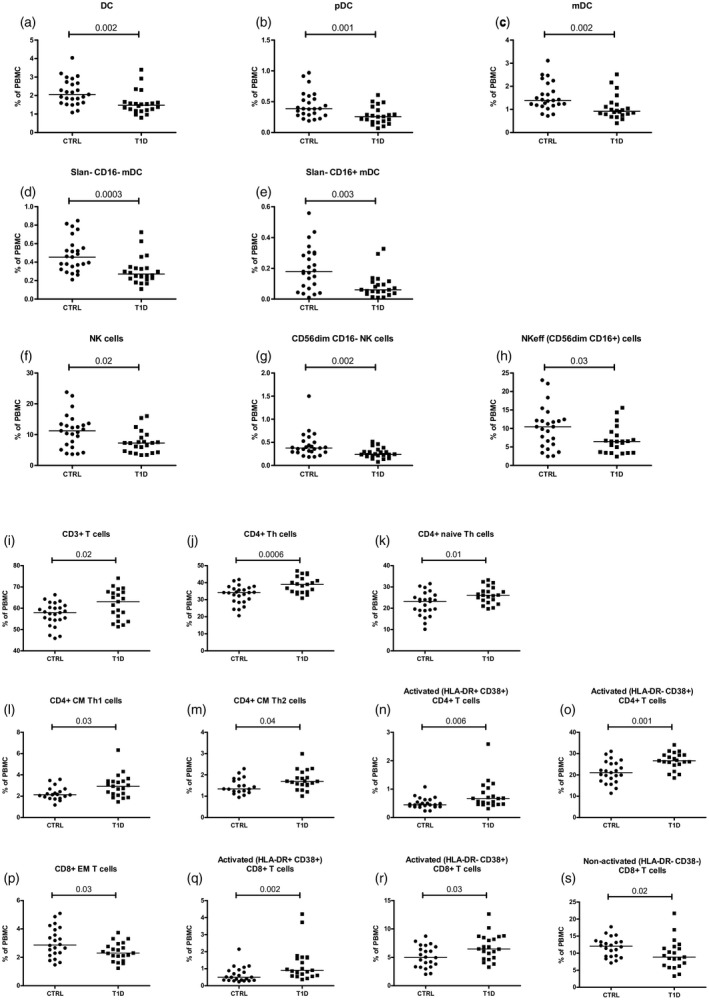
The following immune cell frequencies in type 1 diabetes (T1D) patients differed significantly from controls (CTRL): (a–e) dendritic cell (DC) compartment; (f–h) natural killer (NK) cells and its subsets; and (i–s) many subsets in T cell compartment. Each data point represents an individual subject. Horizontal lines indicate median values of cell frequencies; P‐values ≤ 0.05 were considered statistically significant in the Mann–Whitney U‐test.
Figure 3.
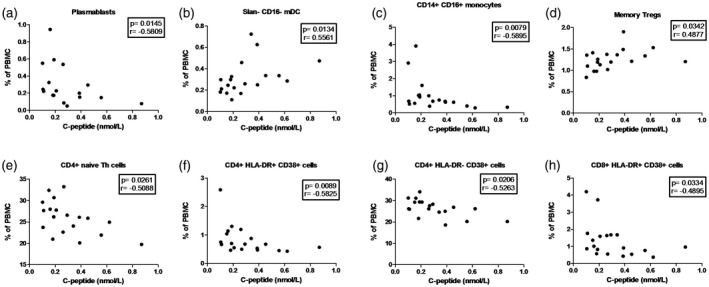
Correlation between immune cell frequencies and serum C‐peptide (nmol/l) values in type 1 diabetes (T1D) patients. Statistically significant negative or positive correlation were detected in: (a) plasmablasts; (b) slan– CD16– myeloid dendritic cells (mDC); (c) CD14+CD16+ non‐classical monocytes; (d) memory regulatory T cells (Tregs); (e) CD4+ naive T helper (Th) cells; (f,g) activated [human leukocyte antigen DR (HLA‐DR)+ CD38+ and HLA‐DR‐ CD38+] CD4+ T cells; and (h) activated (HLA‐DR+ CD38+) CD8+ T cells. Each data point represents an individual subject. P‐values ≤ 0.05 were considered statistically significant in Spearman's rank correlation test.
Figure 4.
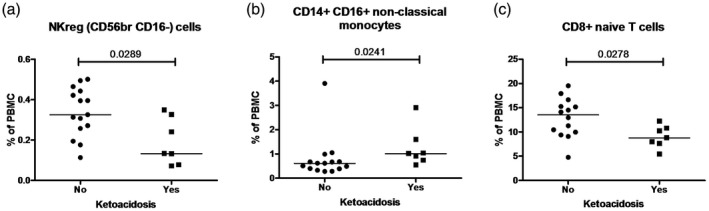
Significant differences in immune cell frequencies between type 1 diabetes (T1D) patients with and without ketoacidosis in: (a) natural killer (NK)reg (CD56brightCD16–) cells; (b) CD14+CD16+ non‐classical monocytes; and (c) CD8+ naive T cells. Each data point represents an individual subject. Horizontal lines indicate median values of cell frequencies. P‐values ≤ 0.05 were considered statistically significant in the Mann–Whitney U‐test.
Figure 5.
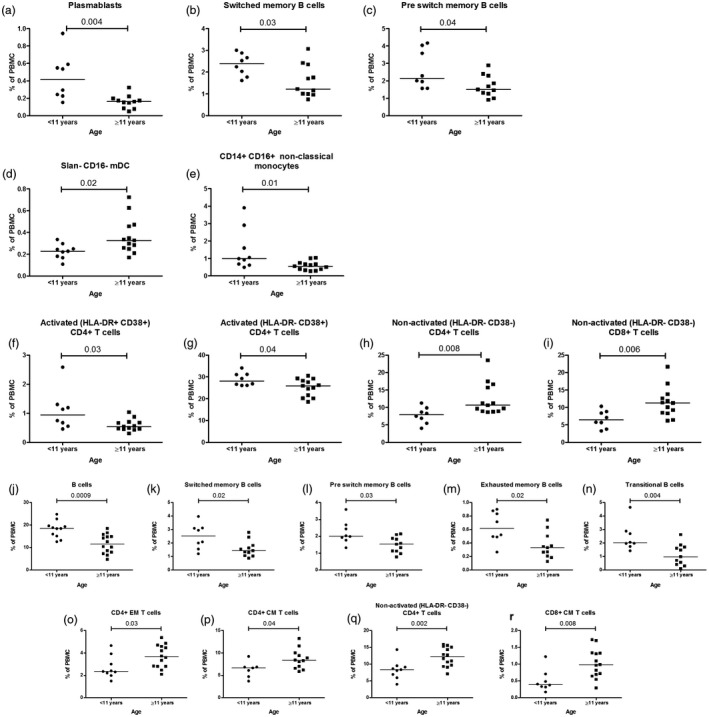
Comparison of immune cell frequencies in type 1 diabetes (T1D) patients (a–i) and controls (j–r) aged under 11 years and aged 11 years and older. Younger T1D patients (<11 years) differed significantly in older (≥11 years) T1D patients in (a–c) B cell compartment; (d) slan– CD16– myeloid dendritic cells (mDC); (e) CD14+CD16+ non‐classical monocytes; (f–i) T cell subsets. Younger controls (<11 years) differed significantly compared to older (≥11 years) controls in (j–n) B cell compartment and (o–r) T cell compartment. Horizontal lines indicate median values of cell frequencies, and each data point represents an individual subject. P‐values ≤ 0.05 were considered statistically significant in the Mann–Whitney U‐test.
T1D patients had lower frequency of DC and decreased levels of its subsets: pDC, mDC, slan– CD16– mDC and slan– CD16+ mDC) (Fig. 2a–e) compared to controls. Similarly, patients had lower NK cell values and a decreased proportion of cells in NK cell subsets: effector NK (NKeff) (CD56dimCD16+) cells and CD56dimCD16– NK cells compared to controls (Fig. 2f–h). On the contrary, in the T cell panel patients showed a higher proportion of CD3+, CD4+, CD4+ naive, CD4+ CM Th1, CD4+ CM Th2, activated (HLA‐DR+ CD38+ and HLA‐DR– CD38+) CD4+ T cells, activated (HLA‐DR+ CD38+ and HLA‐DR– CD38+) CD8+ T cells and lower levels of CD8+ effector memory (EM) and non‐activated (HLA‐DR– CD38–) CD8+ T cells compared to controls (Fig. 2i–s).
Correlations between cell numbers and C‐peptide values in T1D patients
C‐peptide values correlated positively with slan– CD16– mDC and memory Treg cell frequencies (Fig. 3b,d). However, C‐peptide correlated negatively with the following subsets: plasmablasts, CD14+CD16+ non‐classical monocytes, CD4+ naive Th cells, activated (HLA‐DR+ CD38+ and HLA‐DR– CD38+) CD4+ and activated (HLA‐DR+ CD38+) CD8+ T cells (Fig. 3a,c,e–h).
Associations between immune cells and diabetic ketoacidosis
Comparison of flow cytometry data between patients with and without diabetic ketoacidosis (DKA) showed statistically significant differences between several subsets. T1D patients with DKA had higher frequencies of CD14+CD16+ non‐classical monocytes and lower frequencies of NKreg (CD56bright CD16–) cells and CD8+ naive T cells compared to T1D patients without DKA (Fig. 4a–c).
Age‐dependent differences in immune cell counts and frequencies in T1D patients
Younger T1D children (<11 years) had more plasmablasts, switched memory B cells and preswitch memory B cells; CD14+CD16+ non‐classical monocytes and activated (HLA‐DR+CD38+ and HLA‐DR+ CD38–) CD4+ T cells compared to older T1D patients (≥11 years) (Fig. 5a–c,e–g). Patients diagnosed at older age (≥11 years) had a higher proportion of slan– CD16– mDC, non‐activated (HLA‐DR– CD38–) CD4+ and CD8+ T cells compared to younger children (Fig. 5d,h,i). The same age groups were also compared among controls, and younger controls had significantly higher frequencies of B cells and various subsets, including switched memory B cells (SM), preswitch memory (PSM) B cells, exhausted memory B cells, and transitional B cells compared to older controls (Fig. 5j–n). In the T cell compartment, younger controls had lower frequencies of CD4+ EM and CM T cells, non‐activated (HLA‐DR CD38–) CD4+ T cells and CD8+ CM T cells (Fig. 5o–r) compared to controls in the older age group. SM and PSM memory B cells and non‐activated (HLA‐DR– CD38–) CD4+ T cells differed significantly across ages within the control group and the T1D group, and therefore these changes appear to be age‐dependent and not T1D‐specific.
Discussion
T1D has been classically described as a T cell‐mediated disease 2; however, an increasing number of studies have shown the importance of other immune cells in disease pathogenesis 6, 8, 9, 14. In the current study, we examined changes in a wide spectrum of immune cells in children with newly diagnosed T1D using unseparated PBMCs staining panels and HIP‐C version 3.3 protocol developed by the COST‐ENTIRE consortium. We found that NK, DC and T cell compartments and their subsets in T1D patients differed significantly from healthy controls (Fig. 2a–s and Supporting information, Fig. 1a–m). However, T1D patients’ B cells, T regulatory cells and monocytes did not differ compared to healthy controls. Thus, our study supports the results of previous investigations, which suggest substantial changes in cell populations responsible for both innate and adaptive immunity in T1D patients.
In the present study, NK cells were subdivided into four subsets based on the expression of CD56 and CD16. The current literature deals mainly with two main subsets of NK cells: (1) CD56dimCD16+ are mature cells forming the majority of NK cells in peripheral blood; and (2) CD56brightCD16– cells are considered to be immature NK cells 15. These cells have also been termed NKeff cells and NKreg cells, respectively 16. There is also an intermediate subset characterized as CD56brightCD16+ 15, and some studies suggest a fourth population of NK cells, which express CD56 but lack CD16 (CD56dimCD16–) 17. Although some authors doubt the existence of the latter NK subpopulation 18, we are confident that all four NK subpopulations could be determined. Our ability to determine all four subpopulations may have been dependent on our use of fresh whole blood, enabling us to identify CD56dimCD16– cells. However, the exact role of this latter subset in normal physiological conditions, as well as in autoimmune diseases, remains to be defined. Therefore the significance of lower frequencies and/or decrease of absolute numbers (Fig. 2f–h and Supporting information, Fig. 1g–j) of NK cells and its subsets (NKreg CD56brightCD16–, NKeff CD56dimCD16+, CD56dimCD16–) in our study and in others 8, 14 warrants further investigation. One possible explanation for the lower NK count in T1D patients might be related to decreased responsiveness to interleukin (IL)‐2 and IL‐15 stimulation 8 which, in combination with impaired cell numbers in the NK cell compartment, may cause increased susceptibility to certain viruses (e.g. enterovirus) 16.
We have shown the decrease of myeloid and plasmacytoid DC in T1D that is in keeping with previous studies 9, 19. Although our flow cytometry panel did not enable us to measure conventional DC (mDC) subtypes cDC1 and cDC2 20, we characterized these cells by the expression of CD16 and slan, a carbohydrate modification of P‐selectin glycoprotein ligand 1 (PSGL‐1) that has been studied in the context of different immune‐mediated diseases 12. To our knowledge, this is the first study investigating the role of slan+ DC in T1D. Although the frequency of slan‐expressing cells in T1D patients did not differ from controls, controls exhibited a higher slan+ CD16+ absolute count compared to T1D patients (Supporting information, Fig. 1f). Despite the fact that no other differences between patient subgroups and controls were found, further investigations into whether the slan+ DC subset may be a predictor for the development of T1D complications, e.g. slan‐expressing monocytes are in nephropathy in lupus erythematosus 21, is needed.
Increases either in total counts or percentages of different T cell subsets in T1D have been demonstrated by several authors 22, 23, 24, 25. The character of changes varies between studies and is due largely to variability in patient populations and methods selection. We were particularly interested in counting activated T cells. In addition to HLA‐DR, CD38 expression reflects the activation of both CD4+ and CD8+ T cells, although so far a variety of other CD38 functions in immune and non‐immune cells have been described 26. Therefore, the increase in activated CD8+ (HLA‐DR– CD38+) and (HLA‐DR+ CD38+) cells in T1D (Fig. 2q,r and Supporting information, Fig. 1l) may deserve special attention as a sign of active cytotoxicity. Although the antigen‐specificity of these T cells remains unknown, both autoantigen‐ and virus‐specific CD8+ cells could be seen 27.
B cells play an essential role in adaptive immunity because of the production of antibodies, as well as their ability to act as antigen‐presenting cells (APCs) and initiate the specific immune response. B cells also have regulatory and effector functions mediated by cytokines and chemokines 28. In contrast to some previous studies 6, 29, in our study the B cell compartment in T1D patients did not differ from controls. However, in one study focusing on patients with long‐standing T1D, T1D patients did not differ from controls in most B cell subpopulations, but the expression of maturation‐associated markers was impaired in T1D patients 30. This may indicate that the B cell compartment is largely involved in the T1D pathogenesis through functional defects. Interestingly, in the current study, B cell counts and proportions in younger controls differed from older controls; however, B cells in younger and older T1D patients tend to have similar results in both age groups (Supporting information, Tables S2 and S5).
Here we found that C‐peptide levels were correlated inversely with frequencies of plasmablasts, CD14+CD16+ non‐classical monocytes, CD4+ naive Th cells, activated (HLA‐DR+ CD38+ and HLA‐DR– CD38+) CD4+ T cells and activated (HLA‐DR+ CD38+) CD8+ T cells (Fig. 3a,c,e–h and Supporting information, Fig. 2). These results indicate that changes in these cell populations may be associated with more extensive destruction of β cells. In addition, C‐peptide correlated positively with slan– CD16– mDC and memory Treg cells, reflecting the positive impact of these cells in the natural history of T1D (Fig. 3b,d). Furthermore, it has also been shown that children with higher C‐peptide levels at diagnosis have longer periods of remission (i.e. honeymoon phase) 25.
Our finding of DKA being present at diagnosis in a third of patients is in line with previous reports 2. DKA is a serious clinical problem in newly diagnosed childhood T1D, and our results here show that T1D patients with DKA exhibit significant changes in peripheral blood immune cells. Our patients with DKA had a higher frequency and/or an absolute number of plasmablasts, CD14+CD16+ non‐classical monocytes compared to T1D patients without DKA (Fig. 4b and Supporting information, Fig. 3a,b). Also, the absolute number of both activated (HLA‐DR+ CD38+ and HLA‐DR+ CD38–) CD4+ and CD8+ T cells was increased in these patients (Supporting information, Fig. 3d–g). Patients without DKA showed a higher frequency for NKreg (CD56brightCD16–) cells and CD8+ naive T cells (Fig. 4a,c). Our results and findings from others suggest increased activation of the immune system in T1D patients with DKA. Previous investigations of others suggest that DKA patients have higher leukocyte, monocyte and neutrophil counts and elevated hs‐C‐reactive protein (CRP), IL‐6 and IL‐10 levels compared to controls 31, 32. Further, ketoacidosis may trigger T cell activation, therefore the elevated levels of activated (HLA‐DR+ CD38+ and HLA‐DR+ CD38–) CD4+ and CD8+ cells 33 (Supporting information, Fig. 3d–g) in the current DKA patients provide evidence for this idea. However, a more severe manifestation of T1D might occur as a consequence of more extensive activation of a variety of immune cell compartments, leading to a higher risk of long‐term diabetic complications, as DKA at diagnosis of T1D is associated with poor long‐term glycemic control 34.
As T1D immunopathogenesis may differ between younger and older patient groups 35, 36, 37, and because the biggest increase in T1D incidence has been observed among young children 2, we divided the patients into two groups: patients aged under 11 years and those aged 11 years and older. In a comparison of these two groups, we found that younger patients had increased values of plasmablasts, CD14+CD16+ non‐classical monocytes and activated (HLA‐DR+ CD38+ and HLA‐DR+ CD38–) CD4+ T cells compared to patients with an older age at onset (Fig. 5a,e–g and Supporting information, Fig. 4a–c). However, older children had higher values of slan– CD16– mDC and non‐activated (HLA‐DR– CD38–) CD4+ and CD8+ T cells compared to younger children (Fig. 5d,h,i and Supporting information, Fig. 4d,e). These findings, along with other similar studies 38, 39, 40, 41, suggest the presence of a more prominent proinflammatory profile of immune cells in children with a younger age at onset of T1D than those diagnosed at older ages. In addition, our previous study suggests that the timing by which immunological markers are investigated following the onset of T1D is critical in understanding disease development 42. Our present study further supports this idea, as we detected significant changes in various cell subtypes within the first weeks following T1D diagnosis. It is possible that persistent metabolic pressure in the patients’ organism might hide some immunological changes that are evident at the disease onset.
It is important to note that by using monoclonal antibodies in four panels, we were able to discriminate 51 immune cell populations in 400 μl of patients’ whole blood. Despite the fact that flow cytometry operations require substantial time and experience to perform accurately, this study provides evidence of the usefulness of such an approach in the characterization of immune cell subsets in the context of T1D and other immune‐mediated disorders. Importantly, our approach does not limit the characterization of novel clinically important cell subpopulations if additional reagent combinations are determined.
The main strength of our study consists in a wide range of investigated immune cell subsets. However, we acknowledge that it could also be considered as a limitation, as we performed multiple comparisons. One option for correcting for multiple testing is Bonferroni adjustment, which decreases the risk of type I errors and would guarantee that our data would not include potential false positive results. At the same time, there is a great probability that with this approach we would also miss many potential associations (true findings), which are worthy of further study to confirm their exact role in T1D. Also, one of the assumptions of Bonferroni correction is that individual tests should be independent, but the distribution of immune cell subsets are often related to each other. Altogether, as our study is somewhat exploratory rather than confirmative, we decided similarly to other authors 43, 44, 45 not to correct for multiple comparisons because it increases the risk of type II errors 46.Taken together, our findings show evidence of the prominent changes seen in nearly all peripheral blood immune cell compartments and demonstrate increased immune activation in patients with T1D. Also, our results suggest that alterations in immune cell patterns in T1D patients may be related to parameters such as age, ketoacidosis and serum C‐peptide levels, but further investigation into these relationships is needed.
Disclosures
The authors have no conflicts of interest to declare.
Author contributions
A. O. performed experiments and analyzed the results. A. P. and V. T. supervised the sample collecting; R. U. and T. G. designed and supervised the research, and wrote the paper with A. O. All authors read and approved the final manuscript.
Supporting information
Fig. S1. Absolute cell (cells/μl) counts in T1D patients differed significantly in the following cell subsets compared to controls (CTRL): (a‐f) DC and its subpopulations; (g‐j) NK cell subsets; and (k‐m) T cell compartment. Each point represents an individual subject. Horizontal lines indicate median values of cell numbers. P values ≤ 0.05 were considered statistically significant in the Mann‐Whitney test.
Fig. S2. Absolute count (cells/μl) of CD14+ CD16+ non‐classical monocytes in T1D patients correlated positively with serum C‐peptide (nmol/L) values. P values ≤ 0.05 were considered statistically significant in the Spearman Rank Correlation test.
Fig. S3. Immune cell subtype absolute counts (cells/μl) in T1D patients with ketoacidosis differed significantly from T1D patients without ketoacidosis. T1D patients with ketoacidosis had a higher absolute number than T1D patients without ketoacidosis in following cell subsets: (a) plasmablasts; (b) CD14+ CD16+ monocytes; and (c‐g) many subsets in the T cell compartment. Each data point represents an individual subject. Horizontal lines indicate median values of cell numbers. P values ≤ 0.05 were considered statistically significant in the Mann‐Whitney test.
Fig. S4. Comparison of immune cell absolute counts (cells/μl) in T1D patients under 11 years of age and 11 years of age and older. The number of (a) plasmablasts; (b) CD14+ CD16+ non‐classical monocytes; (c) activated (HLA‐DR+ CD38+) CD4+ T cells; and (d,e) non‐activated (HLA‐DR‐ CD38‐) CD4+ and CD8+ T cells differed between age groups. Horizontal lines indicate median values of cell numbers. P values ≤ 0.05 were considered statistically significant in the Mann‐Whitney test.
Table S1. Summary table of NK cells, DC, monocytes, and their subsets in T1D patients and healthy controls separated into two age groups: under 11 years of age and 11 years of age and older. Median cell counts (cells/ml) are displayed. P values ≤ 0.05 were considered statistically significant in Mann‐Whitney test and are marked in blue.
Table S2. Summary table of B cells and its subsets in T1D patients and healthy controls (CTRL) separated into two age groups: under 11 years of age and 11 years of age and older. Median cell counts (cells/ml) are displayed. P values ≤ 0.05 were considered statistically significant in Mann‐Whitney test and are marked in blue.
Table S3. Summary table of T cells and Tregs, and their subsets in T1D patients and healthy controls (CTRL) separated into two age groups: under 11 years of age and 11 years of age and older. Median cell counts (cells/ml) are displayed. P values ≤ 0.05 were considered statistically significant in Mann‐Whitney test and are marked in blue.
Table S4. Summary table of NK cells, DC, monocytes, and their subsets in T1D patients and healthy controls (CTRL) separated into two age groups: under 11 years of age and 11 years of age and older. Median % are displayed. P values ≤ 0.05 were considered statistically significant in Mann‐Whitney test and are marked in blue.
Table S5. Summary table of B cells and its subsets in T1D patients and healthy controls (CTRL) separated into two age groups: under 11 years of age and 11 years of age and older. Median % are displayed. P values ≤ 0.05 were considered statistically significant in Mann‐Whitney test and are marked in blue.
Table S6. Summary table of T cells and Tregs, and their subsets in T1D patients and healthy controls (CTRL) separated into two age groups: under 11 years of age and 11 years of age and older. Median % are displayed. P values ≤ 0.05 were considered statistically significant in Mann‐Whitney test and are marked in blue.
Acknowledgments
This study was supported by the Estonian Research Council, Estonian Ministry of Education and Research (grant IUT20‐43) and by the European Union Regional Development Fund (project no. 3.2.0101.08‐0008). The authors acknowledge the help of Professor Jorma Ilonen (University of Turku, Finland) in measuring the HLA genotypes. In addition, the support of Dr Karin Varik, Dr Ingrid Reppo and Dr Olga Gusseva in the collection of study materials from the diabetes patients is greatly appreciated.
References
- 1. Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet 2014; 383:69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. DiMeglio LA, Evans‐Molina C, Oram RA. Type 1 diabetes. Lancet 2018; 391:2449–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Borchers AT, Uibo R, Gershwin ME. The geoepidemiology of type 1 diabetes. Autoimmun Rev 2010; 9:A355–65. [DOI] [PubMed] [Google Scholar]
- 4. Ilonen J, Kiviniemi M, Lempainen J et al Genetic susceptibility to type 1 diabetes in childhood ‐ estimation of HLA class II associated disease risk and class II effect in various phases of islet autoimmunity: HLA class II effects in T1D. Pediatr Diabetes 2016; 17:8–16. [DOI] [PubMed] [Google Scholar]
- 5. Chen X, Makala LHC, Jin Y et al Type 1 diabetes patients have significantly lower frequency of plasmacytoid dendritic cells in the peripheral blood. Clin Immunol 2008; 129:413–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Deng C, Xiang Y, Tan T et al Altered peripheral B‐lymphocyte subsets in type 1 diabetes and latent autoimmune diabetes in adults. Diabetes Care 2016; 39:434–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nieminen JK, Vakkila J, Salo HM et al Altered phenotype of peripheral blood dendritic cells in pediatric type 1 diabetes. Diabetes Care 2012; 35:2303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Qin H, Lee I‐F, Panagiotopoulos C et al Natural killer cells from children with type 1 diabetes have defects in NKG2D‐dependent function and signaling. Diabetes 2011; 60:857–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vuckovic S, Withers G, Harris M et al Decreased blood dendritic cell counts in type 1 diabetic children. Clin Immunol 2007; 123:281–8. [DOI] [PubMed] [Google Scholar]
- 10. Thompson WS, Pekalski ML, Simons HZ et al Multi‐parametric flow cytometric and genetic investigation of the peripheral B cell compartment in human type 1 diabetes. Clin Exp Immunol 2014; 177:571–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thomas K, Dietze K, Wehner R et al Accumulation and therapeutic modulation of 6‐sulfo LacNAc + dendritic cells in multiple sclerosis. Neurol Neuroimmunol Neuroinflam 2014; 1:e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Döbel T, Schäkel K. The role of human 6‐sulfo LacNAc dendritic cells (slanDCs) in autoimmunity and tumor diseases. J Dtsch Dermatol Ges J Ger Soc Dermatol JDDG 2014; 12:874–80. [DOI] [PubMed] [Google Scholar]
- 13. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. Available at: http://care.diabetesjournals.org/content/26/suppl_1/s5.long (accessed 12 November 2018) [Google Scholar]
- 14. Zhang Y, Wang H, Lou X et al Decreased percentage of NKG2D+NK cells in patients with incident onset of Type 1 Diabetes. Clin Exp Pharmacol Physiol 2017; 44:180–90. [DOI] [PubMed] [Google Scholar]
- 15. Béziat V, Duffy D, Quoc SN et al CD56brightCD16+ NK cells: a functional intermediate stage of NK cell differentiation. J Immunol 2011; 186:6753–61. [DOI] [PubMed] [Google Scholar]
- 16. Fraker C, Bayer AL. The expanding role of natural killer cells in type 1 diabetes and immunotherapy. Curr Diab Rep 2016; 16 Available at: http://link.springer.com/10.1007/s11892-016-0806-7 (accessed 14 December 2018). [DOI] [PubMed] [Google Scholar]
- 17. Takahashi E, Kuranaga N, Satoh K et al Induction of CD16+CD56bright NK cells with antitumour cytotoxicity not only from CD16–CD56bright NK cells but also from CD1–CD56dim NK cells. Scand J Immunol 2007; 65:126–38. [DOI] [PubMed] [Google Scholar]
- 18. Lugthart G, van Ostaijen‐ten Dam MM, van Tol MJD, Lankester AC, Schilham MW. CD56dimCD16–NK cell phenotype can be induced by cryopreservation. Blood 2015; 125:1842–3. [DOI] [PubMed] [Google Scholar]
- 19. Wilkinson A. Type 1 diabetic children and siblings share a decrease in dendritic cell and monocyte numbers but are differentiated by expansion of CD4+T cells expressing IL‐17. J Clin Cell Immunol 2011; Available at: https://www.omicsonline.org/type-1-diabetic-children-and-siblings-share-a-decrease-in-dendritic-cell-2155-9899.S2-001.php?axml:id=2281 (accessed 30 August 2018). [Google Scholar]
- 20. Mair F, Prlic M. OMIP‐044: 28‐color immunophenotyping of the human dendritic cell compartment. Cytometry A 2018; 93:402–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Olaru F, Döbel T, Lonsdorf AS et al Intracapillary immune complexes recruit and activate slan‐expressing CD16+ monocytes in human lupus nephritis. JCI Insight 2018; 3 Available at: https://insight.jci.org/articles/view/96492 (accessed 12 November 2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Matteucci E, Ghimenti M, Di Beo S, Giampietro O. Altered proportions of naïve, central memory and terminally differentiated central memory subsets among CD4+ and CD8+ T cells expressing CD26 in patients with Type 1 diabetes. J Clin Immunol 2011; 31:977–84. [DOI] [PubMed] [Google Scholar]
- 23. Menart‐Houtermans B, Rütter R, Nowotny B et al Leukocyte profiles differ between Type 1 and Type 2 diabetes and are associated with metabolic phenotypes: results from the German Diabetes Study (GDS). Diabetes Care 2014; 37:2326–33. Available at: http://care.diabetesjournals.org/content/37/8/2326.long (accessed 14 December 2018). [DOI] [PubMed] [Google Scholar]
- 24. Bian ML, Haigh O, Munster D, Harris M, Cotterill A, Miles JJ, Vuckovic S. Reactivated CD4+ Tm cells of T1D patients and siblings display an exaggerated effector phenotype with heightened sensitivity to activation-induced cell death. Diabetes. 2015; 64(6):2161–2171. [DOI] [PubMed] [Google Scholar]
- 25. Fitas AL, Martins C, Borrego LM et al Immune cell and cytokine patterns in children with type 1 diabetes mellitus undergoing a remission phase: a longitudinal study. Pediatr Diabetes 2018; 19:963–71. [DOI] [PubMed] [Google Scholar]
- 26. Quarona V, Zaccarello G, Chillemi A, Brunetti E, Singh VK, Ferrero E, et al. CD38 and CD157: A long journey from activation markers to multifunctional molecules. Cytometry B Clin Cytom. 2013. Jul 1; 84B(4):207–17. [DOI] [PubMed] [Google Scholar]
- 27. Laban S, Suwandi JS, van Unen V et al Heterogeneity of circulating CD8 T‐cells specific to islet, neo‐antigen and virus in patients with type 1 diabetes mellitus. PLOS ONE 2018; 13:e0200818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lund FE. Cytokine‐producing B lymphocytes‐key regulators of immunity. Curr Opin Immunol 2008; 20:332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Parackova Z, Klocperk A, Rataj M et al Alteration of B cell subsets and the receptor for B cell activating factor (BAFF) in paediatric patients with type 1 diabetes. Immunol Lett 2017; 189:94–100. [DOI] [PubMed] [Google Scholar]
- 30. Hanley P, Sutter JA, Goodman NG et al Circulating B cells in type 1 diabetics exhibit fewer maturation‐associated phenotypes. Clin Immunol 2017; 183:336–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xu W, Wu H, Ma S et al Correlation between peripheral white blood cell counts and hyperglycemic emergencies. Int J Med Sci 2013; 10:758–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Karavanaki K, Kakleas K, Georga S et al Plasma high sensitivity C‐reactive protein and its relationship with cytokine levels in children with newly diagnosed type 1 diabetes and ketoacidosis. Clin Biochem 2012; 45:1383–8. [DOI] [PubMed] [Google Scholar]
- 33. Kitabchi Abbas E, Stentz Frankie B, Umpierrez Guillermo E. Diabetic ketoacidosis induces in vivo activation of human T-lymphocytes. Biochemical and Biophysical Research Communications. 2004; 315:404–407. [DOI] [PubMed] [Google Scholar]
- 34. Duca LM, Wang B, Rewers M, Rewers A. Diabetic ketoacidosis at diagnosis of Type 1 diabetes predicts poor long‐term glycemic control. Diabetes Care 2017; 40:1249–55. [DOI] [PubMed] [Google Scholar]
- 35. Merger SR, Leslie RD, Boehm BO. The broad clinical phenotype of Type 1 diabetes at presentation. Diabet Med 2013; 30:170–8. [DOI] [PubMed] [Google Scholar]
- 36. Panarina M, Kisand K, Alnek K, Heilman K, Peet A, Uibo R. Interferon and interferon‐inducible gene activation in patients with Type 1 diabetes. Scand J Immunol 2014; 80:283–92. [DOI] [PubMed] [Google Scholar]
- 37. Pruul K, Kisand K, Alnek K et al Differences in B7 and CD28 family gene expression in the peripheral blood between newly diagnosed young‐onset and adult‐onset type 1 diabetes patients. Mol Cell Endocrinol 2015; 5:265–71. [DOI] [PubMed] [Google Scholar]
- 38. Espino‐Paisan L, de la Calle H, Fernández‐Arquero M et al A polymorphism in PTPN2 gene is associated with an earlier onset of type 1 diabetes. Immunogenetics 2011; 63:255–8. [DOI] [PubMed] [Google Scholar]
- 39. Leete P, Willcox A, Krogvold L et al Differential insulitic profiles determine the extent of β‐cell destruction and the age at onset of Type 1 diabetes. Diabetes 2016; 65:1362–9. [DOI] [PubMed] [Google Scholar]
- 40. Pehlić M, Vrkić D, Škrabić V et al IL12RB2 gene is associated with the age of Type 1 diabetes onset in Croatian family trios. PLOS ONE 2012; 7:e49133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Steck AK, Johnson K, Barriga KJ et al Age of islet autoantibody appearance and mean levels of insulin, but not GAD or IA‐2 autoantibodies, predict age of diagnosis of Type 1 diabetes: diabetes autoimmunity study in the young. Diabetes Care 2011; 34:1397–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Alnek K, Kisand K, Heilman K, Peet A, Varik K, Uibo R. Increased blood levels of growth factors, proinflammatory cytokines, and Th17 cytokines in patients with newly diagnosed Type 1 diabetes. PLOS ONE 2015; 10:e0142976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Becking K, Haarman BCM, Grosse L et al The circulating levels of CD4+ T helper cells are higher in bipolar disorder as compared to major depressive disorder. J Neuroimmunol 2018; 319:28–36. [DOI] [PubMed] [Google Scholar]
- 44. Herold KC, Gitelman SE, Ehlers MR et al Teplizumab (Anti‐CD3 mAb) treatment preserves C‐peptide responses in patients with new‐onset Type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes 2013; 62:3766–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thieme C, Schlickeiser S, Metzner S, Dames C, Pleyer U. Immune mediator profile in aqueous humor differs in patients with primary acquired ocular toxoplasmosis and recurrent acute ocular toxoplasmosis. Mediat Inflamm 2019; 2019:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Perneger TV. What's wrong with Bonferroni adjustments. BMJ 1998; 316:1236–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Absolute cell (cells/μl) counts in T1D patients differed significantly in the following cell subsets compared to controls (CTRL): (a‐f) DC and its subpopulations; (g‐j) NK cell subsets; and (k‐m) T cell compartment. Each point represents an individual subject. Horizontal lines indicate median values of cell numbers. P values ≤ 0.05 were considered statistically significant in the Mann‐Whitney test.
Fig. S2. Absolute count (cells/μl) of CD14+ CD16+ non‐classical monocytes in T1D patients correlated positively with serum C‐peptide (nmol/L) values. P values ≤ 0.05 were considered statistically significant in the Spearman Rank Correlation test.
Fig. S3. Immune cell subtype absolute counts (cells/μl) in T1D patients with ketoacidosis differed significantly from T1D patients without ketoacidosis. T1D patients with ketoacidosis had a higher absolute number than T1D patients without ketoacidosis in following cell subsets: (a) plasmablasts; (b) CD14+ CD16+ monocytes; and (c‐g) many subsets in the T cell compartment. Each data point represents an individual subject. Horizontal lines indicate median values of cell numbers. P values ≤ 0.05 were considered statistically significant in the Mann‐Whitney test.
Fig. S4. Comparison of immune cell absolute counts (cells/μl) in T1D patients under 11 years of age and 11 years of age and older. The number of (a) plasmablasts; (b) CD14+ CD16+ non‐classical monocytes; (c) activated (HLA‐DR+ CD38+) CD4+ T cells; and (d,e) non‐activated (HLA‐DR‐ CD38‐) CD4+ and CD8+ T cells differed between age groups. Horizontal lines indicate median values of cell numbers. P values ≤ 0.05 were considered statistically significant in the Mann‐Whitney test.
Table S1. Summary table of NK cells, DC, monocytes, and their subsets in T1D patients and healthy controls separated into two age groups: under 11 years of age and 11 years of age and older. Median cell counts (cells/ml) are displayed. P values ≤ 0.05 were considered statistically significant in Mann‐Whitney test and are marked in blue.
Table S2. Summary table of B cells and its subsets in T1D patients and healthy controls (CTRL) separated into two age groups: under 11 years of age and 11 years of age and older. Median cell counts (cells/ml) are displayed. P values ≤ 0.05 were considered statistically significant in Mann‐Whitney test and are marked in blue.
Table S3. Summary table of T cells and Tregs, and their subsets in T1D patients and healthy controls (CTRL) separated into two age groups: under 11 years of age and 11 years of age and older. Median cell counts (cells/ml) are displayed. P values ≤ 0.05 were considered statistically significant in Mann‐Whitney test and are marked in blue.
Table S4. Summary table of NK cells, DC, monocytes, and their subsets in T1D patients and healthy controls (CTRL) separated into two age groups: under 11 years of age and 11 years of age and older. Median % are displayed. P values ≤ 0.05 were considered statistically significant in Mann‐Whitney test and are marked in blue.
Table S5. Summary table of B cells and its subsets in T1D patients and healthy controls (CTRL) separated into two age groups: under 11 years of age and 11 years of age and older. Median % are displayed. P values ≤ 0.05 were considered statistically significant in Mann‐Whitney test and are marked in blue.
Table S6. Summary table of T cells and Tregs, and their subsets in T1D patients and healthy controls (CTRL) separated into two age groups: under 11 years of age and 11 years of age and older. Median % are displayed. P values ≤ 0.05 were considered statistically significant in Mann‐Whitney test and are marked in blue.


