Abstract
Background
Using hypofractionation (fewer, larger doses of daily radiation) to treat localized prostate cancer may improve convenience and resource use. For hypofractionation to be feasible, it must be at least as effective for cancer‐related outcomes and have comparable toxicity and quality of life outcomes as conventionally fractionated radiation therapy.
Objectives
To assess the effects of hypofractionated external beam radiation therapy compared to conventionally fractionated external beam radiation therapy for men with clinically localized prostate cancer.
Search methods
We searched CENTRAL, MEDLINE (Ovid), Embase (Ovid) and trials registries from 1946 to 15 March 2019 with reference checking, citation searching and contact with study authors. Searches were not limited by language or publication status. We reran all searches within three months (15th March 2019) prior to publication.
Selection criteria
Randomized controlled comparisons which included men with clinically localized prostate adenocarcinoma where hypofractionated radiation therapy (external beam radiation therapy) to the prostate using hypofractionation (greater than 2 Gy per fraction) compared with conventionally fractionated radiation therapy to the prostate delivered using standard fractionation (1.8 Gy to 2 Gy per fraction).
Data collection and analysis
We used standard Cochrane methodology. Two authors independently assessed trial quality and extracted data. We used Review Manager 5 for data analysis and meta‐analysis. We used the inverse variance method and random‐effects model for data synthesis of time‐to‐event data with hazard ratios (HR) and 95% confidence intervals (CI) reported. For dichotomous data, we used the Mantel‐Haenzel method and random‐effects model to present risk ratios (RR) and 95% CI. We used GRADE to assess evidence quality for each outcome.
Main results
We included 10 studies with 8278 men in our analysis comparing hypofractionation with conventional fractionation to treat prostate cancer.
Primary outcomes
Hypofractionation may result in little or no difference in prostate cancer‐specific survival [PC‐SS] (HR 1.00, 95% CI 0.72 to 1.39; studies = 8, participants = 7946; median follow‐up 72 months; low‐certainty evidence). For men in the intermediate‐risk group undergoing conventional fractionation this corresponds to 976 per 1000 men alive after 6 years and 0 more (44 fewer to 18 more) alive per 1000 men undergoing hypofractionation.
We are uncertain about the effect of hypofractionation on late radiation therapy gastrointestinal (GI) toxicity (RR 1.10, 95% CI 0.68 to 1.78; studies = 4, participants = 3843; very low‐certainty evidence).
Hypofractionation probably results in little or no difference to late radiation therapy genitourinary (GU) toxicity (RR 1.05, 95% CI 0.93 to 1.18; studies = 4, participants = 3843; moderate‐certainty evidence). This corresponds to 262 per 1000 late GU radiation therapy toxicity events with conventional fractionation and 13 more (18 fewer to 47 more) per 1000 men when undergoing hypofractionation.
Secondary outcomes
Hypofractionation results in little or no difference in overall survival (HR 0.94, 95% CI 0.83 to 1.07; 10 studies, 8243 participants; high‐certainty evidence). For men in the intermediate‐risk group undergoing conventional fractionation this corresponds to 869 per 1000 men alive after 6 years and 17 fewer (54 fewer to 17 more) participants alive per 1000 men when undergoing hypofractionation.
Hypofractionation may result in little to no difference in metastasis‐free survival (HR 1.07, 95% CI 0.65 to 1.76; 5 studies, 4985 participants; low‐certainty evidence). This corresponds to 981 men per 1000 men metastasis‐free at 6 years when undergoing conventional fractionation and 5 more (58 fewer to 19 more) metastasis‐free per 1000 when undergoing hypofractionation.
Hypofractionation likely results in a small, possibly unimportant reduction in biochemical recurrence‐free survival based on Phoenix criteria (HR 0.88, 95% CI 0.68 to 1.13; studies = 5, participants = 2889; median follow‐up 90 months to 108 months; moderate‐certainty evidence). In men of the intermediate‐risk group, this corresponds to 804 biochemical‐recurrence free men per 1000 participants at six years with conventional fractionation and 42 fewer (134 fewer to 37 more) recurrence‐free men per 1000 participants with hypofractionation
Hypofractionation likely results in little to no difference to acute GU radiation therapy toxicity (RR 1.03, 95% CI 0.95 to 1.11; 4 studies, 4174 participants at 12 to 18 weeks' follow‐up; moderate‐certainty evidence). This corresponds to 360 episodes of toxicity per 1000 participants with conventional fractionation and 11 more (18 fewer to 40 more) per 1000 when undergoing hypofractionation.
Authors' conclusions
These findings suggest that moderate hypofractionation (up to a fraction size of 3.4 Gy) results in similar oncologic outcomes in terms of disease‐specific, metastasis‐free and overall survival. There appears to be little to no increase in both acute and late toxicity.
Plain language summary
Use of shorter radiation treatments for prostate cancer
Review question
We asked if giving radiation therapy (cancer treatment with high energy x‐rays) for localized prostate cancer in fewer fractions (treatment visits for radiation) and shorter overall treatment time with a larger dose (more than 2 Gray) given each day, works as well as the usual (conventional) number of fractions (1.8 Gray per day to 2 Gray per day) for cancer control and had similar side effects.
Background
Using fewer fractions, with a larger dose given at each visit is possibly better for treating prostate cancer with radiation. Radiation therapy for prostate cancer can cause bladder and bowel side effects, and affect sexual function. If using larger doses for each treatment, with fewer treatments overall (called hypofractionation), works as well for cancer control, and the side effects and effects on certainty of life are about the same, then hypofractionation may benefit men with prostate cancer contained within the prostate (localized) who are treated with radiation therapy. If cancer control is as good, and the side effects about the same, then using fewer (but larger dose) radiation treatments may be more convenient for men with prostate cancer, use fewer resources and save money.
Study characteristics
This evidence is current to 15 March 2019. The men studied were ages 64 years and over and had prostate cancer limited to the pelvis.
Key results
We studied the use of fewer, but larger doses of radiation to treat 8278 men with prostate cancer. We found 10 studies.
We found that using hypofractionation may result in similar risk of dying from prostate cancer (low‐certainty evidence) but are uncertain how it affects late bowel side effects (very low‐certainty evidence). It probably results in similar rates of late bladder side effects (moderate‐certainty evidence).
Using hypofractionation results in similar overall survival (high‐certainty evidence) and may be similar for metastasis‐free survival (low‐certainty evidence). Acute bladder side effects may be similar (moderate‐certainty evidence).
Summary of findings
Summary of findings for the main comparison. Altered fraction schedules compared to conventional fractionation for clinically localized prostate cancer.
| Altered fraction schedules compared to conventional fractionation for clinically localized prostate cancer | |||||
| Patient or population: clinically localized prostate cancer Setting:hospitals and cancer centers Intervention: altered fraction schedules Comparison: conventional fractionation | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with conventional fractionation | Risk difference with altered fraction schedules | ||||
|
Prostate cancer‐specific survival Follow‐up: median 60–108 months |
7946 (8 RCTs) | ⊕⊕⊝⊝ Lowa,b | HR 1.00 (0.72 to 1.39) | Lowc | |
| 996 per 1000 | 0 more per 1000 (15 fewer to 4 more) | ||||
| Intermediated | |||||
| 976 per 1000 | 0 more per 1000 (44 fewer to 18 more) | ||||
| Highe | |||||
| 962 per 1000 | 0 more per 1000 (57 fewer to 27 more) | ||||
|
Late gastrointestinal RT toxicity
≥ Grade II RTOG/EORTC Follow‐up: median 60 months |
3843 (4 RCTs) | ⊕⊝⊝⊝ Very low1a,f,g | RR 1.10 (0.68 to 1.78) | Study population | |
| 109 per 1000h | 11 more per 1000 (35 fewer to 85 more) | ||||
|
Late genitourinary RT toxicity
≥ Grade II RTOG/EORTC Follow‐up: median 60 months |
3843 (4 RCTs) | ⊕⊕⊕⊝ Moderatei | RR 1.05 (0.93 to 1.18) | Study population | |
| 262 per 1000h | 13 more per 1000 (13 fewer to 47 more) | ||||
|
Overall survival Follow‐up: median 12–108 months |
8243 (10 RCTs) | ⊕⊕⊕⊕ High | HR 0.94 (0.83 to 1.07) | Lowc | |
| 905 per 1000 | 14 fewer per 1000 (47 fewer to 14 more) | ||||
| Intermediated | |||||
| 869 per 1000 | 17 fewer per 1000 (54 fewer to 17 more) | ||||
| Highe | |||||
| 851 per 1000 | 18 fewer per 1000 (57 fewer to 19 more) | ||||
|
Metastasis‐free survival Follow‐up: median 68.4–100.5 months |
4985 (5 RCTs) | ⊕⊕⊝⊝ Lowa,b | HR 1.07 (0.65 to 1.76) | Study populationj | |
| 981 per 1000 | 5 more per 1000 (58 fewer to 19 more) | ||||
|
Biochemical relapse‐free survival Follow‐up: median 90–108 months |
2889 (5 RCTs) | ⊕⊕⊕⊝ Moderatea,k,l | HR 0.88 (0.68 to 1.13) | Lowc | |
| 907 per 1000 | 31 fewer per 1000 (106 fewer to 25 more) | ||||
| Intermediated | |||||
| 804 per 1000 | 42 fewer per 1000 (134 fewer to 37 more) | ||||
| Acute GU RT toxicity assessed with: ≥ Grade II RTOG/EORTC | 4174 (4 RCTs) | ⊕⊕⊕⊝ Moderatea | RR 1.03 (0.95 to 1.11) | Study populationh | |
| 360 per 1000 | 9 more per 1000 (15 fewer to 34 more) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; EORTIC: European Organisation for Research and Treatment of Cancer; HR: hazard ratio; RCT: randomized controlled trial; RR: risk ratio; RT: radiation therapy; RTOG: Radiation Therapy Oncology Group. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded for study limitations (lack of blinding with risk of performance of detection bias). bDowngraded one level for imprecision because there were fewer than 300 events. cLee 2016 was used for control event rate: a contemporary study that used highly conformal radiation therapy with image guidance in a low‐risk population. dPROFIT 2016 was used for control event rate: a contemporary study that used highly conformal radiation therapy with image guidance in an intermediate‐risk population. eHYPRO Dutch 2016 was used for control event rate: a contemporary study that used highly conformal radiation therapy with image guidance in a population that had 74% of participants with high‐risk prostate cancer. fDowngraded one level because there may have been moderate heterogeneity (I2 = 76%). gDowngraded one level for imprecision; although it met optimum information size, the 95% confidence interval included both clinically meaningful and clinically insignificant harms. hPROFIT 2016 was used for control event rate: a contemporary source of prospectively collected toxicity data that used highly conformal radiation therapy with image guidance in an intermediate‐risk population. iDowngraded one level for study limitations (lack of blinding with risk of performance of detection bias) and attrition bias. jControl event rate was derived from the included studies for this outcome. kAlthough there may have been meaningful heterogeneity (P = 0.09, I2 = 55%), this could be explained by excluding the two studies for which biochemical relapse‐free survival was not a compound endpoint (P = 0.36, I2 = 0%). For the other studies, biochemical relapse‐free survival was a compound endpoint, incorporating prostate‐specific antigen failure, deaths and salvage therapy. lDowngraded for study limitations (attrition bias).
Background
The use of hypofractionated external beam radiation therapy (EBRT) regimens for prostate cancer has become an area of interest, due to better understanding of the radiobiology of prostate cancer. Hypofractionated EBRT could potentially improve therapeutic outcome through the use of large‐sized daily fractions (Fowler 2001; Fowler 2005). Hypofractionation also offers a reduction in the number of fractions and thus the total treatment duration. This results in a reduction in treatment cost and increased convenience for patients. While conventional fractionation radiation regimens usually employ fractions of 1.8 Gy to 2.0 Gy daily, hypofractionation refers to the delivery of the radiation therapy (RT) dose in a smaller number of treatments than would be used in a traditional dosing scheme. Therefore, the daily fraction size is larger than that given in standard fractionation. Hypofractionated EBRT for prostate cancer has been used clinically for a number of years, particularly in the UK (Collins 1991).
Toxicity to normal tissues is an important consideration in prostate EBRT as the prostate gland lies in close proximity to the rectum, bladder and neurovascular bundles. In radiobiology, the α/β ratio (defined as the dose at which killing of a cell by linear (α) and quadratic (β) components are equal) is used to quantify the fractionation sensitivity of both normal tissues and tumors. It is a theoretical measure of a tissue's predicted response to a dose of radiation, relative to the size of the dose delivered per fraction. The α/β ratio of prostate cancer may be as low as 1.2 Gy, in contrast with higher values of about 10 Gy for many other tumor types (Bentzen 2005; Brenner 1999; Brenner 2002; Daşu 2012; Duchesne 1999; Fowler 2001; Leborgne 2012; Vogelius 2013). Higher α/β ratios mean that tumor response is less dependent on the amount of radiation administered with each fraction, and therefore that a lower radiation dose per treatment can typically be used, in order to limit toxicity to normal tissues. Conversely, if the α/β ratio for prostate cancer is lower than that of the nearby normal tissues, then a therapeutic advantage can be gained by using fewer and larger fractions to improve efficacy in terms of tumor control (Fowler 2005).
Quality of life is an important issue when making treatment decisions for men with prostate cancer (Penson 2003; Potosky 2004). Concerns have been raised as to the possibility of an increase in acute and late toxicities with these hypofractionated schedules, which may adversely affect quality of life after RT (Kupelian 2001). However, a limited but growing number of hypofractionation trials in prostate cancer have reported acceptable short‐term toxicities and biochemical control, although most have insufficient follow‐up to be sure of the long‐term safety and efficacy of this approach. This review will critically appraise the entire body of evidence to include the most recent trials. If current and future data affirm the efficacy and safety of hypofractionated prostate EBRT, the adoption of such regimens as a standard of care could profoundly influence the future management of clinically localized prostate cancer.
Description of the condition
Prostate cancer is the second most common cause of cancer in men worldwide. In 2018, an estimated 1.6 million new cases of prostate cancer will be diagnosed (19% of all new cancer diagnoses in men) (Siegel 2018), and there are predictions that by 2030 the number of new cases will almost have doubled (Bray 2012). Prostate cancer incidence rates are highest in Australia and New Zealand, followed by Northern Europe (GLOBOCAN 2018). It is estimated that the lifetime risk of being diagnosed with prostate cancer for men living in the US is 11%, with the risk of dying from prostate cancer at 2.5% (NCI).
EBRT is considered a standard treatment for clinically localized prostate cancer, with cure rates similar to those achieved with surgery (radical prostatectomy) (Wolff 2015). Clinically localized prostate cancer is defined as cancer confined to the prostate gland. Using the American Joint Committee on Cancer (AJCC) nomenclature, these tumors are clinical stage T1c (normal digital rectal exam [DRE]), T2 (abnormal DRE but no evidence of disease beyond the prostate gland), T3 (disease extending through the prostate capsule or with seminal vesicle invasion, or both), N0 to Nx (no evidence of spread to regional lymph nodes or regional lymph nodes were not assessed) and M0 (no evidence of metastatic spread) (AJCC 2010). EBRT may be chosen as a treatment option due to patient preference, medical comorbidities precluding surgical management or adverse effect profile.
Description of the intervention
Standard intervention
EBRT is a non‐surgical curative treatment modality for clinically localized prostate cancer, using megavoltage (high‐energy) x‐rays. Typically, EBRT is delivered to a total dose of 70 Gy to 78 Gy in daily fractions (episodes of treatment requiring attendance at a radiation oncology department). The conventional fraction size is 1.8 Gy to 2 Gy, given five days per week, requiring a total of 35 to 39 fractions. This is called conventional fractionation. Curative EBRT for prostate cancer can require daily attendance for close to eight weeks. This can be onerous for the patient, requiring significant time spent on commuting to the treatment facility, and interruption to work and family life. Curative treatments using conventional fractionation are time‐consuming and resource‐intensive which may lengthen delays for other patients.
The quality of the RT delivered is important (and confirmed by compliance with rigorous quality assurance [QA]). Delivery of high‐quality RT is associated with improvements in both local control and survival. In head and neck cancer, delivery of poor‐quality RT (plan not compliant with QA requirements) is associated with a 20% decrease in survival and a 29% decrease in locoregional control (Peters 2010).
Experimental intervention
If EBRT is delivered in larger fraction sizes (greater than 2 Gy per fraction), this is called hypofractionation. When hypofractionation is used, courses of EBRT are shorter, with fewer fractions and participant attendances required. This approach has been validated in the treatment of early breast cancer (START A 2008; START B 2008; Whelan 2002), and in the palliative treatment of lung cancer (Lester 2006), and bone metastases (McQuay 1999; Nielsen 1998; Price 1986). Hypofractionated EBRT must be shown to achieve similar tumor control to conventional EBRT without increased toxicity before it can become an acceptable approach for men with clinically localized prostate cancer.
The aim of hypofractionated EBRT for men with prostate cancer is to deliver a tumoricidal dose in fewer fractions, without increasing toxicity (especially rectal, urethral and bladder toxicity).
Acute effects of radiation therapy
Acute effects (early adverse effects of RT) are complications or side effects that occur within three months after completing treatment. The cells of early responding tissues (with short cell cycle times measured in days, e.g. the intestinal mucosa) express toxicity quickly. Tissues that are particularly susceptible to early effects, which influence treatment tolerability for men with prostate cancer, include the rectum (expressed as rectal urgency and frequency) and bladder (expressed as frequency and urgency). Cells with short cell cycle times have a significant linear component to their cell survival curves, and total dose rather than fraction size determines the severity of early effects. Acute effects are less dependent on fraction size than late effects. Thus, for these cells, we expect similar rates or severity of acute toxicity when comparing hypofractionation to conventional fractionation regimens, provided both deliver the same total dose over the same treatment duration. However, the severity of early toxicity can also be dependent on the dose density of RT. This means that a similar total dose delivered over a much shorter treatment duration can result in more severe toxicities to the early reacting tissues surrounding the prostate. This severe early toxicity may predispose to the development of subsequent late toxicity, called 'consequential' late toxicity (Dörr 2001).
Late effects of radiation therapy
Late effects of RT are complications present or persisting three months (90 days) or more after the end of treatment. Late effects (side effects seen in tissues with long cell cycle times) are more sensitive to the increase in fraction size. When prostate cancer is treated with EBRT, the tissues particularly susceptible to late effects include the rectum, bladder and urethra. Unwanted late effects in these tissues can include rectal ulceration, urethral stricture or bladder contracture. Hypofractionation may result in increased late and long‐term effects of radiation on these tissues, thus lowering the therapeutic ratio (Brenner 1999).
How the intervention might work
Normal tissues usually have low α/β ratios, which is consistent with a greater capacity for repair between fractions. This results in greater relative sparing with small fraction sizes than for tumors, with their typically higher α/β ratios. However, tumors with low α/β ratios are also more sensitive to fraction size, so fraction sizes more than 2.0 Gy may offer a therapeutic advantage in terms of increased tumor control. Thus, the potential radiobiologic advantage for hypofractionated prostate EBRT is related to the estimated range of the α/β ratio of prostate cancer.
The α/β ratio of prostate cancer is estimated to be between 1.4 Gy and 1.86 Gy (Bentzen 2005; Brenner 1999; Brenner 2002; Daşu 2012; Duchesne 1999; Fowler 2001; Leborgne 2012; Miralbell 2012; Proust‐Lima 2011; Vogelius 2013). This suggests that prostate cancer may be more sensitive to fraction size than the late‐responding organs at risk (OAR), as the α/β ratio for late complications in the rectum or bladder is estimated to be about 3 Gy (Heemsbergen 2006; Tucker 2011). As such, hypofractionated prostate EBRT is of increasing interest due to this potential improvement in the therapeutic ratio.
There are other potential advantages to hypofractionation. These include the participant‐related benefits of decreased overall treatment time, increased convenience and the treatment facility benefits of increased participant capacity because less machine time is used treating prostate cancer patients.
However, concerns have been raised as to the efficacy (tumor control) and the safety (acute and late toxicity) of these hypofractionated schedules.
Why it is important to do this review
The optimum fraction size for the treatment of clinically localized prostate cancer with EBRT is unknown; a systematic review and meta‐analysis may answer this question. One systematic review with a search date of 2012 identified 16 randomized controlled trials (RCTs), which included hypofractionated prostate EBRT (Zaorsky 2013). Their meta‐analysis used surrogate outcomes as primary endpoints, but these outcomes have little relevance for consumers and clinicians. Another systematic review of prostate EBRT with a search date of 2011 found four RCTs examining fraction size in prostate cancer, but analyzed cohort studies and RCTs together (Bannuru 2011).
Our systematic review and meta‐analysis used a comprehensive search strategy, rigorous systematic review methodology, focused on RCTs, patient‐important outcomes and used GRADE to rate the evidence certainty. This review includes both disease and self‐reported outcomes of hypofractionation, and assessed the quality of RT delivered. If shorter EBRT courses can provide equivalent outcomes in men, this approach may reduce healthcare costs and medical personnel workload through the more efficient use of radiation services, and may improve participant experience and convenience with a more expedient treatment.
Objectives
To assess the effects of hypofractionated external beam radiation therapy compared to conventionally fractionated external beam radiation therapy for men with clinically localized prostate cancer.
Methods
Criteria for considering studies for this review
Types of studies
We only considered randomized controlled comparisons for inclusion. We excluded cluster‐randomized trials and cross‐over trials. Studies were included regardless of publication language or publication status.
Types of participants
Men with histologically confirmed, clinically localized prostate adenocarcinoma (AJCC T1 to T3N0M0) (AJCC 2010). Biopsies and transurethral resection of the prostate (TURP) were permitted, but we excluded men who had a radical prostatectomy. We included studies that included subsets of relevant participants if the data for the relevant subsets were reported separately, but we only included the data for the relevant subsets.
Types of interventions
We investigated the comparison of hypofractionated versus conventionally fractionated RT.
Intervention: hypofractionated EBRT to the prostate plus or minus the seminal vesicles using hypofractionation (greater than 2 Gy per fraction)
Comparator: conventionally fractionated EBRT to the prostate plus or minus the seminal vesicles delivered using standard fractionation (1.8 Gy to 2 Gy per fraction).
EBRT could be given using intensity‐modulated radiation therapy (IMRT) (high‐precision, highly conformal RT delivered by linear accelerator, advanced arc therapy, tomotherapy or novel techniques) or conformal radiation therapy (3DCRT) (planned using computerized tomography to increase precision and conformity) or other novel RT techniques, provided that the same technique was used in each arm. The use of image‐guided radiation therapy (IGRT) (using fiducial markers or other techniques) was permitted as long as this was equally applied to each arm. The use of techniques to reduce acute and late toxicity (e.g. bladder and bowel protocols to reduce positional variability of OAR) were permitted, as long as these were equally applied to both arms.
Concomitant interventions (such as androgen deprivation, chemotherapy or other therapies) had to be the same in both the intervention and comparator groups to establish fair comparisons.
The dose prescribed and the prescription point or volume were to be clearly described (ICRU 1999).
If a trial included multiple arms, we planned to include any arm that met the inclusion criteria in the review.
Exclusion criteria: we excluded studies using brachytherapy or protons. RT to the pelvic nodes was not permitted
The minimum duration of the intervention was the length of the shortest hypofractionated RT treatment course over which the intervention was conducted. Minimum duration of follow‐up was five years for cancer‐related outcomes, one month for acute RT toxicity and three months for late RT toxicity outcomes.
Types of outcome measures
We did not exclude trials because one or several of our primary or secondary outcome measures were not reported in the publication. If none of our primary or secondary outcomes were reported, we did not include this trial but provided some basic information in an additional table.
Primary outcomes
Prostate cancer‐specific survival [PC‐SS] measured from randomization date to date of prostate cancer death.
Late gastrointestinal (GI) radiation therapy toxicity (occurring or lasting more than 90 days after RT is completed) Grade II Radiation Therapy Oncology Group/European Organisation for Research and Treatment of Cancer (RTOG/EORTC) or greater toxicity.
Late gastrointestinal (GU) radiation therapy toxicity (occurring or lasting more than 90 days after RT is completed) Grade II RTOG/EORTC RT or greater toxicity.
Secondary outcomes
Overall survival measured from randomization date to date of death.
Metastasis‐free survival measured from randomization date to date of diagnosis of metastatic disease.
Biochemical relapse‐free survival (BR‐FS) assessed using the Phoenix definition of a rise of 2 ng/mL or more above the prostate‐specific antigen (PSA) nadir after EBRT with or without hormonal therapy (Roach 2006).
Acute radiation therapy gastrointestinal toxicity. Acute effects of RT were those effects occurring during and within 90 days of starting RT. We used individual protocol‐based definitions.
Acute radiation therapy genitourinary (GU) toxicity. Acute effects of RT were those effects occurring during and within 90 days of starting RT. We used individual protocol‐based definitions.
Late radiation‐induced malignancy
Health‐related quality of life (using trial‐specific instruments) at five years and 10 years.
Method and timing of outcome measurement
Inclusion criteria required a minimum of 60 months' follow‐up from baseline. If multiple measures were available for a particular outcome, we extracted the measures closest to 60 months and 120 months for cancer‐related outcomes. For late RT toxicity, we extracted the measure closest to 60 months. For acute RT toxicity, we extracted the measure closest to three months. For quality of life measures, we extracted the measure closest to 60 months.
Outcomes for 'Summary of findings' table
PC‐SS.
Late radiation therapy toxicity (specifically late gastrointestinal toxicity).
Overall survival.
Metastasis‐free survival.
Biochemical relapse‐free survival.
Acute radiation therapy GU toxicity.
Health‐related quality of life.
Search methods for identification of studies
We performed comprehensive searches from database inception and did not limit the searches by language or publication status. We reran all searches within three months prior to publication and screened the results for eligible studies. If we detected additional relevant key words during any electronic or other searches, we modified the electronic search strategies to incorporate these terms and would document the changes to the search strategy.
Electronic searches
We searched the Cochrane Library (see Appendix 1 for search strategy), which is composed of several databases including the Cochrane Central Register of Controlled Trials (CENTRAL), Database of Abstracts of Reviews of Effects (DARE) and Health Technology Assessment (HTA) database to 2 April 2018. We conducted a comprehensive search of MEDLINE (Ovid; see Appendix 2 for search strategy) from 1974 to 15 March 2019, and Embase (Ovid; see Appendix 3 for search strategy) from 1946 to 15 March 2019.
We searched the PubMed database (www.ncbi.nlm.nih.gov/pubmed) using the MEDLINE search strategy; however, we limited the search to non‐MEDLINE records (by adding NOT MEDLINE[sb] to the search line). We searched the Latin American and Caribbean Health Sciences Literature Database (LILACS; lilacs.bvsalud.org/en/) using the search strategy described in Appendix 4 (search date 15 March 2019). We searched several grey literature databases (www.opengrey.eu/; www.greylit.org/; www.oclc.org/oaister.en.html) using terms based on the MEDLINE search strategy.
We searched the following trials registries.
International Standard Randomized Controlled Trial Number (ISRCTN) Register to 15 March 2019 (www.controlled‐trials.com/isrctn/; see Appendix 5 for search strategy).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal to 15 March 2019 (apps.who.int/trialsearch/; see Appendix 6 for search strategy).
EORTC to 15 March 2019 (www.eortc.be; see Appendix 7 for search strategy).
National Cancer Institute (NCI) Clinical Trials Search Form to 15 March 2019 (www.cancer.gov/clinicaltrials/search; see Appendix 8 for search strategy).
Australian New Zealand Clinical Trials Registry to 15 March 2019 (ANZCTR; www.anzctr.org.au/; see Appendix 9 for search strategy).
We also searched the National Guideline Clearinghouse (www.guideline.gov; see Appendix 10 for search strategy) to 3 April 2018.
Searching other resources
Handsearching
We searched conference proceedings to identify eligible trials from:
European Society for Radiotherapy and Oncology (ESTRO) (www‐clinicalkey‐com‐au.ezproxy.library.uq.edu.au/#!/browse/journal/01678140/latest) (searched to 2018);
American Urological Association (AUA) (www‐clinicalkey‐com‐au.ezproxy.library.uq.edu.au/#!/browse/journal/00225347/latest);
European Association of Urology (EAU).
We handsearched from 2008 to 2018.
We tried to identify other potentially eligible trials or ancillary publications by searching the reference lists of included trials, systematic reviews, meta‐analyses and health technology assessment reports. We contacted authors of included trials to identify additional information on the retrieved trials and trials that we may have missed.
Data collection and analysis
Selection of studies
Two review authors (MLJ, BH) independently scanned the abstract, title, or both, of every record we retrieved in the literature searches, to determine which trials to assess further. We obtained the full‐text of all potentially relevant records (we removed duplicate studies using Covidence). We resolved any disagreements through consensus or by recourse to a third review author (MJ). If we could not resolve a disagreement, we planned to categorize the trial as a 'study awaiting classification' and contact the trial authors for clarification. The Characteristics of excluded studies table documented reasons for the exclusion of studies which might have reasonably be expected to be included. We presented an adapted PRISMA flow diagram to show the process of trial selection (Liberati 2009).
Data extraction and management
For trials that fulfilled our inclusion criteria, two review authors (MLJ, BH) independently extracted key participant and intervention characteristics. We resolved any disagreements by discussion or, if required, by consultation with a third review author (MJ).
We provide information about potentially relevant ongoing trials, including the trial identifier in the Characteristics of ongoing studies table.
We requested the protocol for each included trial and reported primary, secondary and other outcomes in comparison with data in publications in a joint appendix if it was received.
Dealing with duplicate and companion publications
In the event of duplicate publications, companion documents or multiple reports of a primary trial, we maximized the information yield by collating all available data and used the most complete data set aggregated across all known publications. We listed duplicate publications, companion documents, multiple reports of a primary trial and trial documents of included trials (such as trial registry information) as secondary references under the study ID of the included trial. Furthermore, we listed duplicate publications, companion documents, multiple reports of a trial and trial documents of excluded trials (such as trial registry information) as secondary references under the study ID of the excluded trial.
Data from clinical trial registers
In case data of included trials are available as study results in clinical trial registers such as ClinicalTrials.gov or similar sources, we made full use of this information and extracted data. If there was also a full publication of the trial, we collated and critically appraised all available data. If an included trial was marked as a completed study in a clinical trial register but no additional information was available, we would have added this trial to the Characteristics of studies awaiting classification table.
We constructed and piloted a data extraction form for two studies. Three review authors (MJ, BH, FS) independently performed data extraction, with disagreements resolved by discussion. We entered data into Review Manager 5 for analysis (Review Manager 2014). Where data were limited, we requested further information from the authors of the original studies. For each study, we aimed to collect and report the following details in the Characteristics of included studies table:
study design;
inclusion and exclusion criteria for the study;
setting;
accrual dates;
number of participants in each study and in each intervention/comparator group;
median age and range;
stage;
radiation dose;
dose per fraction;
number of fractions;
QA procedures performed (to investigate the quality of radiation delivered);
type of image guidance used;
use of androgen deprivation;
details of outcomes relevant to this review that were assessed in the study, including how measured, the times at which they were measured and any subgroups relevant to this review that were assessed for the outcomes;
study funding sources;
declarations of interest for study authors.
We converted the radiation doses to the equivalent dose in 2 Gy fractions (EQD2) (Maciejewski 1986; Withers 1983), using the formula: EQD2 = D (d + (α/β)/(2 + α/β), where D = total dose, d = dose per fraction and α/β = Gy (Joiner 1997). This was to facilitate comparison of radiation doses given at a different dose per fraction. No time correction factor was used.
Two studies reported data for biochemical relapse as first event data, which meant we were unable to report these outcomes (HYPRO Dutch 2016; Lukka NCIC 2005). One study reported data for distant metastases as first event data, which meant we were unable to report these outcomes (Lukka NCIC 2005).
We used the methods according to a spreadsheet developed by Matthew Sydes (Parmar 1998; Tierney 2007) to derive log HR and standard error (SE) where necessary.
We used method three (where HR and 95% CI available)(Tierney 2007) to derive Log HR and SE:
metastasis‐free survival (Fox Chase 2013).
We used method four (where HR number of events in each arm are available and randomization is 1:1)(Tierney 2007) to derive log HR and SE for:
overall survival (Lukka NCIC 2005);
PC‐SS (Lukka NCIC 2005);
metastasis‐free survival (Arcangeli 2010).
We used method 11 (where data for curve with numbers at risk are available) (Williamson 2002) to derive log HR and SE for:
overall survival (Arcangeli 2010; Fox Chase 2013; Yeoh 2011);
PC‐SS (Fox Chase 2013).
We derived log HR and SE by using the Review Manager 5 calculator for (Review Manager 2014):
overall survival (CHHiP 2016; HYPRO Dutch 2016; Norkus 2009);
BR‐FS (Arcangeli 2010; Yeoh 2011);
distant metastasis‐free survival (CHHiP 2016);
PC‐SS (HYPRO Dutch 2016).
We used method 9 (where P value, total number of events and number randomized were available)(Tierney 2007) to derive Log HR and SE for:
overall survival (MDACC 2014);
BR‐FS (MDACC 2014).
Information about potentially relevant studies (including the study identifier) is provided in the Characteristics of ongoing studies table.
Assessment of risk of bias in included studies
Two review authors (MJ, BH) independently assessed the risk of bias of each included trial. We resolved any disagreements by consensus or by consultation with a third review author (ML). In case of disagreement, we consulted the rest of the group and made a judgment based on consensus. If adequate information was not available from trial authors, trial protocols or both, we contacted trial authors for missing data on risk of bias items.
We used the Cochrane 'Risk of bias' assessment tool and judged 'Risk of bias' criteria as having low, high or unclear risk (Higgins 2011). We evaluated individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions according to the criteria and associated categorizations contained therein (Higgins 2011).
We assessed the following domains for risk of bias.
Random sequence generation (selection bias)
For each included trial we described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
Low risk of bias: the trial authors achieved sequence generation using computer‐generated random numbers or a random numbers table. Drawing of lots, tossing a coin, shuffling cards or envelopes, and throwing dice were deemed adequate if an independent person performed this who was not otherwise involved in the trial. We considered the use of the minimization technique as equivalent to being random.
Unclear risk of bias: insufficient information about the sequence generation process.
High risk of bias: the sequence generation method was non‐random or quasi‐random (e.g. sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number; allocation by judgment of the clinician; allocation by preference of the participant; allocation based on the results of a laboratory test or a series of tests; or allocation by availability of the intervention).
Allocation concealment (selection bias due to inadequate concealment of allocation prior to assignment)
We described for each included trial the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of or during recruitment, or changed after assignment.
Low risk of bias: central allocation (including telephone, interactive voice‐recorder, web‐based and pharmacy‐controlled randomization); sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes.
Unclear risk of bias: insufficient information about the allocation concealment.
High risk of bias: using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards; alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
We also evaluated trial baseline data to incorporate assessment of baseline imbalance into the risk of bias judgment for selection bias (Corbett 2014). Chance imbalances may also affect judgments on the risk of attrition bias. In case of unadjusted analyses, we distinguished between studies we rated as at low risk of bias on the basis of both randomization methods and baseline similarity, and studies we rated at low risk of bias on the basis of baseline similarity alone (Corbett 2014). We reclassified judgments of unclear, low or high risk of selection bias as specified in Appendix 2.
Blinding of participants and personnel (performance bias due to knowledge of the allocated interventions by participants and personnel during the trial) (for subjective and objective outcomes)
We evaluated the risk of detection bias separately for each outcome (Hróbjartsson 2013). We noted whether endpoints were self‐reported, investigator‐assessed or adjudicated outcome measures (see below).
Low risk of bias: blinding of participants and key study personnel was ensured, and it was unlikely that the blinding could have been broken; no blinding or incomplete blinding, but we judged that the outcome was unlikely to have been influenced by lack of blinding.
Unclear risk of bias: insufficient information about the blinding of participants and study personnel; the trial does not address this outcome.
High risk of bias: no blinding or incomplete blinding, and the outcome was likely to have been influenced by lack of blinding; blinding of trial participants and key personnel attempted, but likely that the blinding could have been broken, and the outcome was likely to be influenced by lack of blinding.
Risk of performance bias by outcome
PC‐SS: this investigator‐assessed outcome was not at risk of performance bias in the absence of blinding.
Late GI RT and GU RT toxicity: these self‐assessed or investigator‐assessed outcomes were at risk of performance bias if, for example, trial participants were seen more frequently (in the knowledge that they were having experimental treatment), bias could have been introduced in the absence of blinding.
Overall survival: we felt this investigator‐assessed outcome was not at risk of performance bias.
Distant metastases‐free survival (DM‐FS): this outcome was at risk of performance bias, for example, if trial participants had more frequent investigations (in the knowledge that they were having experimental treatment), bias could have been introduced in the absence of blinding.
BR‐FS: this investigator‐assessed outcome was at risk of performance bias, for example, if trial participants had more frequent investigations (in the knowledge that they were having experimental treatment), bias could have been introduced in the absence of blinding.
Acute GI RT and GU RT toxicity: these self‐assessed or investigator‐assessed outcomes were at risk of performance bias if, for example, trial participants were seen more frequently (in the knowledge that they were having experimental treatment), bias could have been introduced in the absence of blinding.
Second malignancy: this investigator‐assessed outcome was not felt to be at risk of performance bias because it was an objective outcome and likely to have been assessed by clinicians other than the investigators after a long time delay (e.g. 10 to 15 years).
Quality of life: this investigator‐assessed or self‐assessed outcome was at risk of performance bias.
We judged the following outcomes to be similarly susceptible to performance bias and rated them in one group.
Late GI RT toxicity.
Late GU RT toxicity.
Metastasis‐free survival.
Biochemical recurrence‐free survival.
Quality of life.
We judged the following outcomes as not susceptible to performance bias and rated them in one group.
PC‐SS.
Overall survival.
Secondary malignancy.
Blinding of outcome assessment (detection bias due to knowledge of the allocated interventions by outcome assessment) (for subjective and objective outcomes)
We evaluated the risk of detection bias separately for each outcome (Hróbjartsson 2013). We noted whether endpoints were self‐reported, investigator‐assessed or adjudicated outcome measures (see below).
Low risk of bias: blinding of outcome assessment was ensured, and it was unlikely that the blinding could have been broken; no blinding of outcome assessment, but we judged that the outcome measurement was unlikely to have been influenced by lack of blinding.
Unclear risk of bias: insufficient information about the blinding of outcome assessors; the trial did not address this outcome.
High risk of bias: no blinding of outcome assessment, and the outcome measurement was likely to have been influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement was likely to be influenced by lack of blinding.
We judged the following outcomes to be susceptible to detection bias, thereby making blinding of outcome assessors important.
PC‐SS.
Late GI RT toxicity and late GU RT toxicity.
Metastasis‐free survival.
Biochemical recurrence‐free survival.
Quality of life.
Given that risk of judgments for these outcomes were the same, we reported them in one group.
We judged the following outcomes as not to susceptible to detection bias.
Overall survival.
Secondary malignancy.
Incomplete outcome data (attrition bias due to amount, nature or handling of incomplete outcome data)
For each included trial and or each outcome, we described the completeness of data, including attrition and exclusions from the analyses. We stated whether the trial reported attrition and exclusions, and the number of participants included in the analysis at each stage (compared with the number of randomized participants per intervention/comparator groups). We noted if the trial reported the reasons for attrition or exclusion and whether missing data were balanced across groups or were related to outcomes. We considered the implications of missing outcome data per outcome such as high dropout rates (e.g. above 15%) or disparate attrition rates (e.g. difference of 10% or more between trial arms) where it was applicable.
Low risk of bias: no missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to introduce bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk is not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (mean difference [MD] or standardized mean difference [SMD]) among missing outcomes was not enough to have a clinically relevant impact on observed effect size; appropriate methods, such as multiple imputation, were used to handle missing data.
Unclear risk of bias: insufficient information to assess whether missing data in combination with the method used to handle missing data were likely to induce bias; the trial did not address this outcome.
High risk of bias: reason for missing outcome data were likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (MD or SMD) among missing outcomes enough to induce clinically relevant bias in observed effect size; 'as‐treated' or similar analysis done with substantial departure of the intervention received from that assigned at randomization; potentially inappropriate application of simple imputation.
Selective reporting (reporting bias due to selective outcome reporting)
We assessed outcome reporting bias by integrating the results of the appendix 'Matrix of trial endpoints (publications and trial documents)' (Boutron 2014; Jones 2015; Mattieu 2009), with those of the appendix 'High risk of outcome reporting bias according to ORBIT classification' (Kirkham 2010). This analysis formed the basis for the judgment of selective reporting.
Low risk of bias: the trial protocol was available and all of the trial's prespecified (primary and secondary) outcomes that were of interest in the review were reported in the prespecified way; the study protocol was unavailable, but it was clear that the published reports included all expected outcomes (ORBIT classification).
Unclear risk of bias: insufficient information about selective reporting.
High risk of bias: not all of the trial's prespecified primary outcomes were reported; one or more primary outcomes were reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not prespecified; one or more reported primary outcomes were not prespecified (unless clear justification for their reporting was provided, such as an unexpected adverse effect); one or more outcomes of interest in the Cochrane Review were reported incompletely so that we could not enter them in a meta‐analysis; the trial report failed to include results for a key outcome that we would expect to have been reported for such a trial (ORBIT classification).
Other sources of bias
Low risk of bias: the trial appeared free of other sources of bias.
Unclear risk of bias: there was insufficient information to assess whether an important risk of bias existed; insufficient rationale or evidence that an identified problem introduced bias.
High risk of bias: the trial had a potential source of bias related to the specific trial design used; the trial has been claimed to have been fraudulent or the trial had some other serious problem.
Summary assessment of risk of bias
Risk of bias for a trial across outcomes: some risk of bias domains such as selection bias (sequence generation and allocation sequence concealment), affect the risk of bias across all outcome measures in a trial. In case of high risk of selection bias, all endpoints investigated in the associated trial would be marked as high risk. Otherwise, we would not have performed a summary assessment of the risk of bias across all outcomes for a trial.
Risk of bias for an outcome within a trial and across domains: we assessed the risk of bias for an outcome measure by including all entries relevant to that outcome (i.e. both trial‐level entries and outcome‐specific entries). We considered low risk of bias to denote a low risk of bias for all key domains, unclear risk to denote an unclear risk of bias for one or more key domains and high risk to denote a high risk of bias for one or more key domains.
Risk of bias for an outcome across trials and across domains: these were our main summary assessments that we incorporated into our judgments about the certainty of evidence in the 'Summary of finding' table. We defined outcomes as low risk of bias when most information came from trials at low risk of bias, unclear risk when most information came from trials at low or unclear risk of bias and high risk when a sufficient proportion of information came from trials at high risk of bias.
We present a 'Risk of bias' summary figure (Figure 1).
1.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
We distinguished between self‐reported, investigator‐assessed and adjudicated outcome measures.
We accepted the following outcomes as self‐reported:
health‐related quality of life as reported by participants.
We accepted the following outcomes as investigator‐assessed:
PC‐SS, overall survival, metastasis‐free survival and BR‐FS as measured by trial personnel;
adverse events: acute and late GU and GI toxicity as measured by study personnel.
Measures of treatment effect
Dichotomous data
When at least two included trials were available for a comparison and a given outcome, we tried to express dichotomous data as a risk ratio (RR) with 95% confidence interval (CI) (Deeks 2002).
Continuous data
For continuous outcomes measured on the same scale (e.g. weight loss in kilograms), we estimated the intervention effect using the MD with 95% CI. For continuous outcomes measuring the same underlying concept (e.g. health‐related quality of life) but using different measurement scales, we calculated the SMD with 95% CI (Deeks 2002).
Time‐to‐event data
We expressed time‐to‐event data as hazard ratio (HR) with 95% CI (Cox 1972; Cox 2001). If time‐to‐event data were not available in, or possible to derive from, study reports, and were not available after consultation with study authors, we planned to present the RR with 95% CI as discussed above (at 10 years).
Individual participant data analysis was not performed.
Unit of analysis issues
We explicitly excluded cluster‐randomized and cross‐over trials so the unit of analysis was the individual man. If more than one comparison from the same trial was eligible for inclusion in the same meta‐analysis, we combined groups to create a single pair‐wise comparison.
Dealing with missing data
We performed an intention‐to‐treat analysis. When data were missing, we attempted to obtain these data by contacting the study authors. We did not impute missing data.
Assessment of heterogeneity
In the event of substantial clinical or methodologic heterogeneity, we planned not to report trial results as the pooled effect estimate in a meta‐analysis.
We identified heterogeneity (inconsistency) by visually inspecting the forest plots and by using a standard Chi² test with a significance level of α = 0.1. In view of the low power of this test, we also considered the I² statistic (Higgins 2002; Higgins 2003), which quantifies inconsistency across trials to assess the impact of heterogeneity on the meta‐analysis. An I² statistic of 75% or greater indicated a considerable level of heterogeneity (Higgins 2011).
Where we did identify heterogeneity, we rechecked our data, considered whether meta‐analysis was appropriate and attempted to explore the reasons for it by examining individual trial and subgroup characteristics. BR‐FS was a compound endpoint, with different events contributing data in different studies (see Table 2), we explored this by (post‐hoc) excluding those studies where PSA failure was the only event contributing to that outcome (Arcangeli 2010; Yeoh 2011).
1. Outcomes.
| Study | Acute RT toxicity scale used | Late RT toxicity scale used | PSA failure definition | Events contributing to biochemical relapse endpoint | Self‐reported outcomes (PRO) | Sexual function | Quality of life | Follow‐up (median) |
| Arcangeli 2010 | RTOG/EORTCa | LENT‐SOMA | Phoenixb | PSA rise | Not reported | Not reported | — | 108 months |
| CHHiP 2016 | RTOG/EORTCc | RTOG/EORTCc | Phoenix | PSA failure, LR, DM | PROd | Not reported | UCLA‐PCI, EPICe, FACT‐P EPIC‐50 was used for bowel and urinary domains EPIC‐26 for sexual and hormonal domains. For all quality of life instruments, scores range from 0 to 100, and higher was better |
62.4 months |
| Fox Chase 2013 | 4‐point scale, detailed, but not referenced | LENT‐SOMA | Phoenixb | PSA rise, LR, DM | PRO | Not reported | Self‐reported: EPICe, IPSSf, EQ5Dg assessed at baseline, and 12, 24, 36, 48 and 60 months | 69 months |
| HYPRO Dutch 2016 | RTOG/EORTC | RTOG/EORTC | Phoenixc | PSA riseh, LR, DM, salvage AD | PROi | Not reported | IIIEFj used at baseline, and 6, 12, 24 and 36 months. EORTC ‐QLQ‐PR25k used | 60 months |
| Lee 2016 | NCI CTCAE Maximum toxicity | RTOG/EORTC | Phoenixc DFS |
Death without recurrence, PSA rise, salvage AD, DM | PRO | Not reported | EPICe was used, with assessments at baseline, 6 months and 12 months after randomization | 60 months |
| Lukka NCIC 2005 | NCIC Grade III‐IV | NCIC Grade III‐IV | ASTROl BCDF |
PSA rise, LR, DM, salvage AD, PC death | Not reported | Not reported | — | 68 months |
| MDACC 2014 | Not reported | Modified EORTC/RTOGm | Phoenix | PSA rise, salvage AD | PROn | Not reported | Urinary, sexual and bowel function assessed at baseline, 2, 3, 4 and 5 yearsn | 102 months |
| Norkus 2009 | Scale not reported | Scale not reported | ASTROl | PSA rise | Not reported | Not reported | — | 12 months |
| PROFIT 2016 | RTOG/EORTCa | RTOG/EORTCa | ASTROl Phoenixb |
PSA rise, LR, DM, salvage AD, death any cause | PRO | Not reported | EPICe, AUA at baseline, and 24 and 48 months | 72 months |
| Yeoh 2011 | Not reported | Modified LENT‐SOMAo | Phoenixb ASTROl |
PSA rise | Not reported | EORTCk | — | 90 monthsp |
AD: androgen deprivation; ASTRO: American Society for Radiation Oncology; AUA: American Urological Association; BCDF: biochemical or clinical disease failure, or both; DFS: disease‐free survival; DM: distant metastases; EORTC: European Organisation for Research and Treatment of Cancer; EPIC: Expanded Prostate Cancer Index Composite; EQ5D: EuroQoL 5‐dimension; FACT‐P: Functional Assessment of Cancer Therapy – Prostate (Esper 1997); IIIEF: International Index of Erectile Function; IPSS: International Prostate Symptom Score; LENT‐SOMA: Late Effects Normal Tissue Task Force‐Subjective, Objective, Management, Analytic system (Pavy 1995); LR: local recurrence; NCI CTCAE: National Cancer Institute Common Toxicity Criteria for Adverse Events version 3 (Table 10); NCIC: National Institute of Cancer Canada toxicity 5‐point scale; PC: prostate cancer; PRO: participant‐reported outcome; PSA: prostate‐specific antigen; QLQ‐PR25: Quality of Life Questionnaire – Prostate Cancer Module; RT: radiation therapy; RTOG: Radiation Therapy Oncology Group; UCLA‐PCI: University of California, Los Angeles Prostate Cancer Index (Litwin 1998). aRTOG/EORTC RT toxicity scoring scale (Cox 1995). bPhoenix definition of biochemical failure: PSA nadir plus 2 (Roach 2006). PSA measured at three monthly follow‐up visits for first two years, six monthly for years three to five, then annually to 10 years. cAssessed weekly during RT; weeks 10, 12 and 18 for acute toxicity; then at 26 weeks and every six months for five years for late toxicity. dIn CHHiP 2016, the question: "Overall, how much of a problem have your bowels been for you in the last 4/52?" was asked. A seven‐item bowel bother was assessed. The bowel domain summary (5‐point scale) is reported, those with small, moderate or severe bowel bother (Grade II or more) (Table 3). CHHiP 2016: quality of life scales changed during the study, because better instruments became available. Initially, UCLA‐PCI was used from trial initiation to early 2009 (Litwin 1998). The UCLA‐PCI included 36‐item Short Form (SF‐36) and FACT‐P (Esper 1997). From March 2009, the EPIC and SF‐12 (Ware 1996) replaced UCLA‐PCI. EPIC‐50 was used for bowel and urinary domains and EPIC‐26 for sexual and hormonal domains. eEPIC and 12‐item Short Form 12 (Ware 1996). The tool is scored from 0 to 100 (with higher scores being better, a significant change is 0.5 standard deviations and four domains are assessed: bowel, urinary, sexual and hormonal. fIPSS measures urinary obstructive symptoms: 0 = no symptoms, 35 = maximum (Barry 1992). It also incorporates a 1 to 6 visual analogue scale: 0 = 'delighted' with current state of urinary symptoms. gEuroQoL 5‐dimension (EQD5) questionnaire covers five dimensions (mobility, self‐care, usual activities, pain/discomfort, anxiety/depression), also incorporates visual analog scale: the two scores are transformed into a utility score where 0 = 'worst health state' and 1 = 'best health state'. hPSA failure was reported as "first event" data, so we could not include it (HYPRO Dutch 2016). iLate RTOG/EORTC gastrointestinal and genitourinary toxicity events were counted if noted in clinical record, participant self‐assessments or both (HYPRO Dutch 2016). jIIEF although not formally validated in men who have RT or radical prostatectomy for PC, is the most commonly used validated tool for assessment of erectile function (Rosen 1997). HYPRO Dutch 2016 used it to assess the following sexual domains in 671/820 men enrolled: erectile function (in hormone‐naive men), orgasmic function, sexual desire, intercourse and overall satisfaction. The minimally important clinical difference for erectile function was 4. In HYPRO Dutch 2016, 322/820 men completed the IIEF at baseline and at least one other time‐point enrolled . kEORTC scale measures toxicity and functional subscales (van Andel 2008). For all quality of life instruments, scores range from 0 to 100, and higher score is better for functional outcomes and lower is better for toxicity outcomes. Quality of life was assessed at baseline, and six, 12, 24, 36, 48 and 60 months. Change from baseline of five points was considered relevant clinically. Non‐inferiority was set at 8%, i.e. the incidence of clinically relevant deterioration in the hypofractionation group will be no worse than 8% more than the incidence in the conventional arm. lASTRO definition of biochemical failure: three consecutive PSA rises (Cox 1997). mModified RTOG/EORTC scoring system (see Table 9) (Cox 1995). n185 men in MDACC 2014 were eligible for PROs, they did not differ from the remainder of men randomized in the study, and completion of the PRO questionnaire was similar at each time‐point. Self‐reported urinary, bowel and sexual function were assessed at baseline (links.lww.com/AJCO/A138) and at two, three, four and five years (links.lww.com/AJCO/A140). oModified LENT‐SOMA (see Table 11). p Participants reviewed at first month after RT, three‐month intervals for two years, then six‐month intervals for three years, then annually thereafter.
10. Bowel 'bother'.
| Score | Description |
| 0 | No bother |
| I | Very small bother |
| II | Small bother |
| III | Moderate bother |
| IV | Big bother |
Assessment of reporting biases
Where we included 10 or more trials that investigated a particular outcome, we used funnel plots to assess small‐trial effects. Several explanations may have accounted for funnel plot asymmetry, including true heterogeneity of effect with respect to trial size, poor methodologic design (and hence bias of small trials) and publication bias. Therefore, we interpreted results carefully (Sterne 2011). We attempted to obtain the study protocols, in order to assess for selective outcome reporting bias.
Data synthesis
We used Mantel‐Haenszel (M‐H) methods to calculate pooled data for dichotomous data (if participants, interventions, comparisons and outcomes were judged to be sufficiently similar to ensure an answer that was clinically meaningful). Unless good evidence showed homogeneous effects across trials, we primarily summarized low risk of bias data using a random‐effects model (Wood 2008). We interpreted random‐effects meta‐analyses with due consideration to the whole distribution of effects, ideally by presenting a prediction interval (Higgins 2009). A prediction interval specifies a predicted range for the true treatment effect in an individual trial (Riley 2011). For rare events such as event rates below 1%, we planned to use Peto's odds ratio (OR) method, provided that there was no substantial imbalance between intervention and comparator group sizes and intervention effects were not exceptionally large. In addition, we performed statistical analyses according to the statistical guidelines presented in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011 from the University College, Cork protocol) (Greenland 1985; Mantel 1959).
Where time‐to‐event data were available, we calculated the log rank statistic (O‐E) and its variance using an Excel spreadsheet developed by Matthew Sydes (Cancer Division) in collaboration with the Meta‐analysis Group of the Medical Research Council Clinical Trials Unit, London (Tierney 2007). We derived the log HR and used the Peto fixed‐effect model in Review Manager 5 to pool the data, when appropriate (Deeks 2011; Review Manager 2014).
Certainty of the evidence
We presented the overall certainty of the evidence for each outcome according to the GRADE approach, which takes into account issues related to internal validity (risk of bias, inconsistency, imprecision, publication bias) and external validity (directness of results). Two review authors (MJ, BH) independently rated the certainty of evidence for each outcome. We present a summary of the evidence in Table 1. This provides key information about the best estimate of the magnitude of the effect, in relative terms and as absolute differences, for each relevant comparison of alternative management strategies, numbers of participants and trials addressing each important outcome, and rating of overall confidence in effect estimates for each outcome. We created the 'Summary of findings' table based on the methods described in the Cochrane Handbook for Systematic Reviews of Interventions by means of Review Manager 5's table editor (Review Manager 2014). We used the GRADEpro Guideline Development Tool (GDT) software (GRADEpro GDT). We presented results for the outcomes as described in the Types of outcome measures section. If meta‐analysis was not possible, we presented the results in a narrative format in the 'Summary of findings' table. We justified all decisions to downgrade the certainty of studies using footnotes, and we made comments to aid the reader's understanding of the Cochrane Review where necessary.
Subgroup analysis and investigation of heterogeneity
We expected the following characteristics to introduce clinical heterogeneity, and we planned to carry out the following subgroup analyses including investigation of interactions.
Risk stratification of primary disease (based on clinical stage, Gleason score and PSA; where possible examining the effect of the intervention in each of the relevant risk categories: very low, low, intermediate, high, very high). Category definitions were as follows: low risk: clinical stage T1c or T2a, PSA 10 ng/mL and Gleason score 6 or less; intermediate risk: clinical stage T2b, PSA 10 ng/mL to 20 ng/mL or Gleason score 7; high risk: clinical stage T2c, PSA 20 ng/mL or Gleason score 8 to 10 (D'Amico 1998), although this was not possible.
-
RT dose, according to:
EQD2 74 Gy or greater in conventional arm (which reflects current practice);
EQD2 less than 74 Gy in conventional arm (Table 4).
Difference in EQD2 between the RT doses delivered in the two study arms (which allowed us to distinguish the effect of hypofractionation from dose escalation, given that we expected that the studies we found may have had an element of dose escalation in addition to hypofractionation), according to:
2. EQD2 dose comparison (α/β 1.93).
| Study | Hypofractionation | Dose per fraction |
EQD2 (α/β = 1.93 prostate cancer) |
Conventional | Dose per fraction |
EQD2 (α/β = 1.93 prostate cancer) |
| Arcangeli 2010 | 62 Gy/20 fractions | 3.1 | 79.49 | 80 Gy/40 fractions | 2 | 80 |
| CHHiP 2016 | 60 Gy/20 fractions 57 Gy/19 fractions |
3 | 75.38 71.62 |
74 Gy/37 fractions | 2 | 74 |
| Fox Chase 2013 | 70.2 Gy/26 fractions | 2.7 | 82.80 | 76 Gy/38 fractions | 2 | 76 |
| HYPRO Dutch 2016 | 64.6 Gy/19 fractions | 3.4 | 87.79 | 78 Gy/39 fractions | 2 | 78 |
| Lee 2016 | 70 Gy/28 fractions | 2.5 | 78.97 | 73.8 Gy/41 fractions | 1.8 | 69.58 |
| Lukka NCIC 2005 | 52.5 Gy/20 fractions | 2.6 | 60.00 | 66 Gy/33 fractions | 2 | 66 |
| MDACC 2014 | 72 Gy/30 fractions | 2.4 | 79.38 | 75.6 Gy/42 fractions | 1.8 | 71.28 |
| Norkus 2009 | 57 Gy/17 fractions | 3.35 | 78.55 | 74 Gy/37 fractions | 2 | 74 |
| PROFIT 2016 | 60 Gy/20 fractions | 3 | 75.38 | 78 Gy/39 fractions | 2 | 78 |
| Yeoh 2011 | 55 Gy/20 fractions | 2.75 | 63.69 | 64 Gy/32 fractions | 2 | 64 |
EQD2: equivalent dose in 2 Gy fractions.
difference in EQD2 greater than 4 Gy (which represents dose escalation in addition to hypofractionation);
difference in EQD2 4 Gy or less (which represents hypofractionation without dose escalation).
Quality of delivered radiation (based on performance of QA of delivered radiation, i.e. using performance of QA as a surrogate for the quality of the RT delivered), according to:
performance of QA;
lack of performance of QA.
RT technique: highly conformal RT techniques allow both dose escalation and reduce dose to OARs. Reduction in dose to normal tissues reduces both acute and late radiation toxicity, according to:
use of 3DCRT;
highly conformal RT (IMRT or volumetric modulated arc therapy [VMAT]).
-
Androgen deprivation: post‐hoc analysis performed in response to peer reviewer input)
use of androgen deprivation
no androgen deprivation.
Sensitivity analysis
We performed sensitivity analyses to explore the influence of the following factors on effect sizes by restricting analysis to the following.
Study age (excluding those studies that commenced accrual prior to 2002).
Study quality (excluding studies at high risk of bias for that outcome).
Duration of follow‐up (excluding studies with follow‐up of less than 10 years, measured from time of randomization to outcome assessment).
For the calculation of EQD2, we used an α/β ratio for prostate cancer of 1.93 Gy (Vogelius 2013), in the assumption that a shorter total treatment time would impact on tumor control. We also performed sensitivity analysis based on an α/β value of 0.58 Gy (see Table 5), and 4.14 Gy (see Table 6), selected based on the 95% CI of estimated α/β derived from four randomized phase III studies and one non‐randomized study on hypofractionated prostate RT (Vogelius 2013).
3. EQD2 (α/β 0.58).
| Study | Hypofractionation | Dose per fraction |
EQD2 (α/β = 0.58 prostate cancer) |
Conventional | Dose per fraction |
EQD2 α/β = 0.58 prostate cancer |
| Arcangeli 2010 | 62 Gy/20 fractions | 3.1 | 88.23 | 80 Gy/40 fractions | 2 | 80 |
| CHHiP 2016 | 60 Gy/20 fractions 57 Gy/19 fractions |
3 | 83.08 78.92 |
74 Gy/37 fractions | 2 | 74 |
| Fox Chase 2013 | 70.2 Gy/26 fractions | 2.7 | 89.1 | 76 Gy/38 fractions | 2 | 76 |
| HYPRO Dutch 2016 | 64.6 Gy/19 fractions | 3.4 | 99.38 | 78 Gy/39 fractions | 2 | 78 |
| Lee 2016 | 70 Gy/28 fractions | 2.5 | 83.46 | 73.8 Gy/41 fractions | 1.8 | 68.12 |
| Lukka NCIC 2005 | 52.5 Gy/20 fractions | 2.6 | 64 | 66 Gy/33 fractions | 2 | 66 |
| MDACC 2014 | 72 Gy/30 fractions | 2.4 | 83.08 | 75.6 Gy/42 fractions | 1.8 | 69.78 |
| Norkus 2009 | 57 Gy/17 fractions | 3.35 | 70.62 | 74 Gy/37 fractions | 2 | 74 |
| PROFIT 2016 | 60 Gy/20 fractions | 3 | 83.08 | 78 Gy/39 fractions | 2 | 78 |
| Yeoh 2011 | 55 Gy/20 fractions | 2.75 | 73.23 | 64 Gy/32 fractions | 2 | 64 |
EQD2: equivalent dose in 2 Gy fractions.
4. EQD2 (α/β 4.14).
| Study | Hypofractionation |
Dose per fraction |
EQD2 (α/β = 4.14 prostate cancer) |
Conventional |
Dose per fraction |
EQD2 α/β = 4.14 prostate cancer |
| Arcangeli 2010 | 62 Gy/20 fractions | 3.1 | 73.18 | 80 Gy/40 fractions | 2 | 80 |
| CHHiP 2016 | 60 Gy/20 fractions 57 Gy/19 fractions |
3 | 69.84 66.34 |
74 Gy/37 fractions | 2 | 74 |
| HYPRO Dutch 2016 | 64.6 Gy/19 fractions | 3.4 | 79.43 | 78 Gy/39 fractions | 2 | 78 |
| Fox Chase 2013 | 70.2 Gy/26 fractions | 2.7 | 78.26 | 76 Gy/38 fractions | 2 | 76 |
| Lee 2016 | 70 Gy/28 fractions | 2.5 | 75.74 | 73.8 Gy/41 fractions | 1.8 | 71.38 |
| Lukka NCIC 2005 | 52.5 Gy/20 fractions | 2.6 | 57.11 | 66 Gy/33 fractions | 2 | 66 |
| MDACC 2014 | 72 Gy/30 fractions | 2.4 | 76.72 | 75.6 Gy/42 fractions | 1.8 | 73.12 |
| Norkus 2009 | 57 Gy/17 fractions | 3.35 | 68.06 | 74 Gy/37 fractions | 2 | 74 |
| PROFIT 2016 | 60 Gy/20 fractions | 3 | 69.84 | 78 Gy/39 fractions | 2 | 78 |
| Yeoh 2011 | 55 Gy/20 fractions | 2.75 | 63.34 | 64 Gy/32 fractions | 2 | 64 |
EQD2: equivalent dose in 2 Gy fractions.
We tested the robustness of results by repeating the analyses using different measures of effect size (RR, OR, etc.) and different statistical models (fixed‐effect and random‐effects models).
'Summary of findings' table
We used GRADEpro GDT to create a 'Summary of findings' table following the Cochrane methods and recommendations in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünneman 2011). We used the GRADE approach to evaluate the certainty of the evidence (GRADE Working Group 2004). One author (BH) initially applied the GRADE system and then two authors (BH and MJ) jointly agreed on the decisions made with respect to downgrading in the 'Summary of findings' table, with discussion of the decisions to reach consensus. Our decisions were informed by (but not limited to) the following principles. If a study contributing more than 30% of weight to an outcome was at high risk of bias for domains relevant to that outcome, we downgraded. If there was evidence of unexplained heterogeneity (P less than 0.1 and I2 greater than 30%), we downgraded for inconsistency. If studies did not directly evaluate the intervention, we downgraded for indirectness. We downgraded for imprecision if there were fewer than 300 events, if optimum information size (OIS) was not met or if the 95% CI did not exclude 0.75 to 1.25 (this did not exclude clinically insignificant benefits or harms) (Ryan 2016). Because we believed our search had identified all potentially relevant studies, we did not downgrade for publication bias. The assumed control risk used in Table 1 was the median control risk in the studies contributing to the comparison.
The review only included RCTs, and we reported four evidence certainty levels: high, moderate, low and very low, with our rationale detailed in the 'Summary of findings' table.
Results
Description of studies
Results of the search
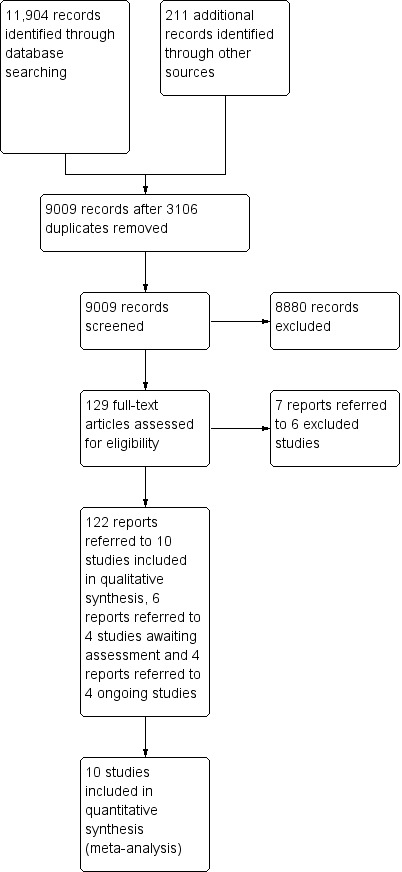
We screened 12,115 records and removed 3106 duplicate publications. We screened the titles and abstracts of 9009 and excluded irrelevant 8880 publications. We examined 129 full‐text articles and excluded six studies (seven reports) with reasons (Characteristics of excluded studies table). This version of the review included 10 studies (104 reports). Four studies await classification (Characteristics of studies awaiting classification table). Four reports referred to four ongoing studies (Characteristics of ongoing studies table; Figure 2).
2.
Study flow diagram.
Included studies
Study population
We studied 8278 men (ages 64 years or greater) enrolled in 10 studies. All men had localized prostate cancer and median follow‐up ranged from 12 months to 120 months (see Table 7 and Characteristics of included studies table for details).
5. Participants.
| Study | n | Age (years) | NCCN risk classification | PSA (ng/mL)1 | Gleason score |
| Arcangeli 2010 | 168 | Median 75 | Intermediate 42% High risk 15% |
< 6–7: 97% | 5–7: 75% |
| CHHiP 2016 | 3216 | 69 | "Intermediate (73%) and high risk" (12%) | Median PSA 10 | 5–7: 96% |
| Fox Chase 2013 | 303 | Mean 66 | "Intermediate (66%) and high risk" (33–35%) | < 10: 64% | 6–7: 81% |
| HYPRO Dutch 2016 | 820 | 70 | "Intermediate (26–27%) and high risk" (74–74%) | median 14 | 7–9: 68% |
| Lee 2016 | 1115 | 84% 60 + years | Low | 4–9: 80% | 5–6: 99.5% |
| Lukka NCIC 2005 | 936 | Mean 70 | Not reported | Mean 10.5 | 5–7: 82% |
| MDACC 2014 | 206 | Median 67 | Low risk 28% Intermediate risk 71% High risk 1% |
≤ 10: 90% | 7: 65% |
| Norkus 2009 | 91 | Median 64 | Not reported | < 10: 100% | ≤ 6: 97% |
| PROFIT 2016 | 1206 | Median 71 | Intermediate risk | ≤ 10: 68% | 7: 90.6% |
| Yeoh 2011 | 217 | Median 69 | Study predates risk stratification | Mean 13 | Not reported |
n: number of participants; PSA: prostate‐specific antigen.
Interventions
Seven studies used highly conformal radiation therapy, six used IGRT and two reported some form of motion management. Four studies described normal QA to evaluate delivered RT. EQD2 was within plus or minus 5% in both study arms in six studies. There was an element of dose escalation in addition to hypofractionation in six studies and five studies delivered RT doses less than 78 Gy. Five studies used androgen deprivation. Studies using fraction sizes greater than 2 Gy were eligible, the included studies used fraction sizes ranging between 2.35 Gy and 3.4 Gy (see Table 8 and Characteristics of included studies table for details).
6. Interventions.
| Study | Technique | IGRT | Motion control | RT QA | Dose | Androgen deprivation (in both study arms) | RT volume | CTV to PTV margins |
| Arcangeli 2010 | 3DCRT and IMRT (center dependent) | 2/3 centers used either ultrasound or fiducial markers | Not reported | Not reported | EQD2 ± 5% in both study arms | 100% | CTV = prostate + seminal vesicles | 1.0 isotropically (0.6 cm posteriorly) |
| CHHiP 2016 | IMRT | Permitted, but not required | Not reported | National QA program | EQD2 ± 5% in both study arms | 100% | Prostate + seminal vesicles | 5–10 mm (depending on OARs) |
| Fox Chase 2013 | IMRT | Ultrasound | Empty rectum, moderately full bladder | Not reported | Dose escalation in hypofractionated arm | 45% | Risk adapteda | Hypofractionated arm: 7 mm with 3 mm posteriorly conventional fractionation: 8 mm with 5 mm posteriorly |
| HYPRO Dutch 2016 | IMRT 95% | Not reported | Not reported | Not reported | Dose escalation in hypofractionated arm | 66% | Risk adaptedb | CTV + 3–10 mm = PTV. Boost margins 0 mm towards the posteriorly, 3–5 mm in other directionsc |
| Lee 2016 | IMRT 79% | Yes (fiducial markers, ultrasound, cone beam CT) | Not reported | Central QA describedd | Dose escalation in hypofractionated arm | Nil | Prostate | 4–10 mm margin |
| Lukka NCIC 2005 | 3DCRT | Not reported | Reported | Real‐time QA for first 30 participants from each center, if OK, then random spot checks on 20% of plans | Dose hypofractionated arm < conventional arm Dose in both arms < 78 Gy |
Nil | Prostate | 15 mm margin |
| MDACC 2014 | IMRT | Fiducial markers or ultrasound | Full bladder | Not reported | Dose escalation in hypofractionated arm | 24% | Prostate + seminal vesicles | 10–15 mm all dimensions except 4–8 mm posteriorly |
| Norkus 2009 | 3DCRT | Skin marks, weekly portal images | Not reported | Not reported | Dose escalation in hypofractionated arm | Nil | Prostate + base of seminal vesicles | 8–10 mm all dimensions |
| PROFIT 2016 | IMRT (3DCRT permitted if OAR constraints met) | Daily image guidance (fiducial markers, cone beam CT or ultrasound) | Not reported | Real‐time central QA for all cases prior to fraction 3 | EQD2 ± 5% in both study arms | Nil | Risk adaptede | 10 mm with 7 mm posteriorly |
| Yeoh 2011 | 156/217 (72%) 2DRT 61/217 (28%) 3DCRT |
Not reported | Not reported | Not reported | EQD2 ± 5% in both study arms Dose in both arms < 78 Gy |
Nil | Prostate | 15 mm margin |
2DRT: two‐dimensional radiation therapy; 3DCRT: three‐dimensional radiation therapy; CT: computer tomography; CTV: clinical target volume; EQD2: equivalent dose in 2 Gy fractions; IMRT: intensity‐modulated radiation therapy; OAR: organs at risk: planning target volume; QA: quality assurance; RT: radiation therapy. aFox Chase 2013: CTV1 = at least 50% of the seminal vesicles (all gross disease extending to the seminal vesicles received the full dose), in addition to the prostate and any extraprostatic extension. CTV2 = distal seminal vesicles. CTV3 = pelvic lymph nodes (periprostatic, periseminal vesicle, external iliac, obturator and internal iliac lymph nodes). Pelvic nodes treated in high‐risk men (17/154) in hypofractionated arm, (32/153) in conventional arm. bHYPRO Dutch 2016: prostate alone treated to 64.6 Gy in 19 fractions (hypofractionated group) or 79 Gy in 39 fractions (conventional group). Prostate + boost to seminal vesicle (35 fractions of 2 Gy up to 70 Gy) or 39 fractions of 1.85 Gy (standard fractionation group), or a dose of 16 fractions of 3.4 Gy or 19 fractions of 3.04 Gy (hypofractionation group). Prostate + seminal vesicle treated to 64.6 Gy in 19 fractions (hypofractionated group) or 79 Gy in 39 fractions (conventional group). cCTV to PTV margin depended on the setup verification and correction strategy used in each participating institute. This boost could either be delivered sequentially or simultaneously integrated depending on the institute's preference. dQuality assurance: "All RT plans were submitted as digital DICOM files to the Image guided Therapy Quality Assurance Center for central quality assurance review. CT scans, target volumes, organ‐at‐risk contours, radiation dose distributions, dose volume histograms, and dose statistics were reviewed for compliance with protocol guidelines (Lee 2016)". ePROFIT 2016: CTV = either prostate alone (if nodal involvement risk < 155 according to Partin's nomogram; [Partin 1997]) or prostate + base of seminal vesicles (if nodal risk according to Partin's nomogram was 15% or greater).
Outcomes
Median follow‐up ranged from 12 months to 120 months (see Table 2 and Characteristics of included studies for details). A variety of scales were used to report both acute and late RT toxicity. Four studies reported acute RT toxicity using the RTOG/EORTC scoring system (Cox 1995) (Table 9), two studies used the NCI scoring system (NCI 2006), and four studies did not describe the scoring system (Table 10). Five studies reported late RT toxicity using the RTOG/EORTC scoring system (Cox 1995), one study used the NCI scoring system (NCI 2006), three studies used the Late Effects Normal Tissue Task Force‐Subjective, Objective, Management, Analytic (LENT‐SOMA) system (Pavy 1995) (Table 11), and one study reported no late RT toxicity (see Table 2).
7. RTOG/EORTC scale.
| Organ tissue | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
| Lower gastrointestinal (modified) (Lukka NCIC 2005) | Excess bowel movements twice baseline Slight rectal discharge or blood |
≤ 2 antidiarrheals/week ≥ 2 coagulations for bleeding Occasional steroids for ulceration Occasional dilation Intermittent use of incontinence pads Regular non‐narcotic or occasional narcotic for pain |
> 2 antidiarrheals/day ≥ 1 blood transfusion or > 2 coagulations for bleeding Steroids per enema Hyperbaric oxygen for ulceration Regular dilation Persistent use of incontinence pads Regular narcotic for pain |
Dysfunction requiring surgery Perforation Life‐threatening bleeding |
| Lower gastrointestinal | Mild diarrhea Mild cramping Bowel movement 5 times daily Slight rectal discharge or bleeding |
Moderate diarrhea and colic Bowel movement > 5 times daily Excessive rectal mucus or intermittent bleeding |
Obstruction or bleeding requiring surgery | Necrosis/perforation Fistula |
| Urinary (modified) (Lukka NCIC 2005) | Nocturia twice baseline Microscopic hematuria Light mucosal atrophy and minor telangiectasia |
Moderate‐frequency nocturia > twice baseline Generalized telangiectasia Intermittent macroscopic hematuria Occasional blood transfusions ≤ 2 coagulations Regular non‐narcotics for pain |
Severe frequency and dysuria Nocturia more frequent than once every hour Reduction in bladder capacity (150 mL) Frequent hematuria Frequent transfusions > 1 coagulation for hematuria Regular narcotic for pain |
Severe hemorrhagic cystitis Ulceration Requirement for urinary diversion or cystectomy, or both |
| Urinary | Slight epithelial atrophy Minor telangiectasia (microscopic hematuria) |
Moderate urinary frequency Generalized telangiectasia Intermittent macroscopic hematuria |
Severe urinary frequency and dysuria Severe generalized telangiectasia (often with petechiae) Frequent hematuria Reduction in bladder capacity (< 150 mL) |
Necrosis/contracted bladder (capacity < 100 mL) Severe hemorrhagic cystitis |
EORTC: European Organisation for Research and Treatment of Cancer; RTOG: Radiation Therapy Oncology Group.
8. NCIC CTCAE Toxicity scoring.
| Symptom | Grade I | Grade II | Grade III | Grade IV | Grade V |
| Gastrointestinal | |||||
| Anorexia | — | — | — | — | — |
| Diarrhea | Increase of < 4 stools per day over baseline Increase in ostomy output |
Increase of 4–6 stools per day over baseline Moderate increase in ostomy output compared to baseline |
Increase of 7 stools per day over baseline Incontinence Hospitalization indicated Severe increase in ostomy output compared to baseline |
Life‐threatening consequences Urgent intervention indicated |
Death |
| Gastrointestinal bleeding | Mild symptoms Intervention not indicated |
Moderate symptoms Medical intervention or minor cauterization indicated |
Transfusion, radiologic, endoscopic or elective operative intervention indicated | Life‐threatening consequences Urgent intervention indicated |
Death |
| Nausea | Loss of appetite without alteration in eating habits | Oral intake decreased without significant weight loss, dehydration or malnutrition | Inadequate oral caloric or fluid intake; tube feeding, TPN, or hospitalization indicated | — | — |
| Pain/cramping | Mild pain | Moderate pain Limiting instrumental ADL |
Severe pain Limiting self‐care ADL |
— | — |
| Vomiting | 1–2 episodes (separated by 5 minutes) in 24 hours | 3–5 episodes (separated by 5 minutes) in 24 hours | ≥ 6 episodes (separated by 5 minutes) in 24 hours Tube feeding, TPN or hospitalization indicated |
Life‐threatening consequences Urgent intervention indicated |
Death |
| Bladder changes | |||||
| Cystitis | Microscopic hematuria Minimal increase in frequency, urgency, dysuria or nocturia New‐onset incontinence |
Moderate hematuria Moderate increase in frequency, urgency, dysuria, nocturia or incontinence Urinary catheter placement or bladder irrigation indicated Limiting instrumental ADL |
Gross hematuria Transfusion, IV medications or hospitalization indicated Elective endoscopic, radiologic or operative intervention indicated |
Life‐threatening consequences Urgent radiologic or operative intervention indicated |
Death |
| Fistula | — | Non‐invasive intervention indicated Urinary or suprapubic catheter placement indicated |
Limiting self‐care ADL Elective radiologic, endoscopic or operative intervention indicated Permanent urinary diversion indicated |
Life‐threatening consequences Urgent radiologic or operative intervention indicated |
Death |
| Genitourinary pain | Mild pain | Moderate pain Limiting instrumental ADL |
Severe pain Limiting self‐care ADL |
— | — |
| Hematuria | Asymptomatic Clinical or diagnostic observations only Intervention not indicated |
Symptomatic Urinary catheter or bladder irrigation indicated Limiting instrumental ADL |
Gross hematuria Transfusion, IV medications or hospitalization indicated Elective endoscopic, radiologic or operative intervention indicated Limiting self‐care ADL |
Life‐threatening consequences Urgent radiologic or operative intervention indicated |
— |
| Ureteric obstruction | Asymptomatic Clinical or diagnostic observations only |
Symptomatic but no hydronephrosis, sepsis or renal dysfunction Urethral dilation, urinary or suprapubic catheter indicated |
Symptomatic and altered organ function (e.g. hydronephrosis or renal dysfunction) Elective radiologic, endoscopic or operative intervention indicated |
Life‐threatening consequences Urgent intervention indicated |
— |
ADL: activities of daily living; IV: intravenous; NCIC CTCAE: National Cancer Information Center Common Terminology Criteria for Adverse Events; TPN: total parenteral nutrition.
9. Modified LENT‐SOMA radiation therapy late effects.
| Toxicity | Grade I | Grade II | Grade III | Grade IV |
| Genitourinary | Nocturia twice baseline or non‐narcotic medication (e.g. α‐blocker) once per day increase over baseline Microscopic hematuria Light mucosal atrophy and minor telangiectasia Dysuria not requiring medication Incontinence or dribbling not requiring sanitary pad (over baseline) |
Frequency ≤ every hour requiring α‐blocker > once per day Nocturia > twice baseline Generalized telangiectasias Macroscopic hematuria requiring ≤ 2 cauterization Dysuria requiring medication (non‐narcotic > once per day or narcotic for pain ≥ 1 per day over baseline) ≤ 2 dilations Foley or self‐catheterization for ≤ 2 weeks' incontinence requiring ≤ 2 pads (over baseline) |
Frequency > 1 every hour or dysuria requiring narcotic > once per day Nocturia > 1 per hour Reduction in bladder capacity (150 cm3) ≥ 1 blood transfusions or > 2 cauterizations for bleeding Narcotic use > once per day Hyperbaric oxygen Foley or self‐catheterization > 2 weeks TURP or > 2 dilations Incontinence requiring > 2 pads (over baseline) |
Gross hematuria requiring > 1 blooded transfusion Severe hemorrhagic cystitis or ulceration requiring urinary diversion with or without cystectomy |
LENT‐SOMA: Late Effects Normal Tissue Task Force‐Subjective, Objective, Management, Analytic; TURP: transurethral resection of the prostate.
Excluded studies
We excluded six studies for the following reasons: use of protons (NCT01230866), failure to compare fraction size (MRC RT01), use of pelvic nodal irradiation (NCT01444820; Norkus 2013; NCT02300389), and comparison of two different hypofractionated regimens (NCT01794403).
Risk of bias in included studies
See 'Risk of bias' tables for included studies and Figure 1.
Allocation
Sequence generation
Six studies were at low risk of bias for sequence generation and four were at unclear risk of bias.
Allocation concealment
Six studies were at low risk of bias for allocation concealment and four were at unclear risk of bias.
Blinding
Blinding of participants and personnel
Subjective outcomes
Seven studies were at unclear risk of bias for lack of blinding and three were at high risk of bias.
Objective outcomes
Ten studies were at low risk of bias for objective outcomes.
Blinding of outcome assessment
Subjective outcomes
Two studies were at low risk for assessment of subjective outcomes, five were at unclear risk of bias and three were at high risk of bias.
Objective outcomes
Ten studies were at low risk of bias for assessment of objective outcomes.
Incomplete outcome data
One study was at high risk of bias for incomplete outcome data, eight were at low risk of bias and one was at unclear risk of bias.
Selective reporting
Four studies were at low risk of reporting bias and six at unclear risk of bias.
Other potential sources of bias
The 10 included studies were at low risk of other sources of bias. One study stopped early because of a change in RT technique, "implementation of three dimensional conventional radiation therapy" (Yeoh 2011). Two studies changed the primary endpoint (Lee 2016; Lukka NCIC 2005). In Lee 2016, early stopping occurred on the basis of prespecified interim analyses, so this was at low risk of bias. Lukka NCIC 2005 changed the primary outcome measure prior to unblinding as the Study Safety Monitoring Board requested changing the definition of PSA failure from American Society of Therapeutic Radiation Oncology to Phoenix; we deemed this domain at low risk of bias.
Risk of bias summarized for each outcome
See 'Risk of bias' tables for included studies and Figure 1.
Time to death from prostate cancer
Eight studies contributed data for this outcome (Arcangeli 2010; CHHiP 2016; Fox Chase 2013; HYPRO Dutch 2016; Lee 2016; Lukka NCIC 2005; PROFIT 2016; Yeoh 2011).
Performance bias: Arcangeli 2010; CHHiP 2016; and HYPRO Dutch 2016 were at risk of performance bias, given the lack of blinding participants and personnel to the intervention, but both Arcangeli 2010 and CHHiP 2016 blinded outcome assessors for cause of death. The single study unblinded for this outcome accounted for 20% of the weight (HYPRO Dutch 2016). The remaining studies were at unclear risk of bias for lack of information (Fox Chase 2013; Lee 2016; Lukka NCIC 2005; PROFIT 2016; Yeoh 2011). No study reported the details of on‐study care and permissible cointerventions participants received.
Detection bias: in two studies, masked investigators ascertained cause of death, so this domain was at low risk of bias (Arcangeli 2010; CHHiP 2016). In HYPRO Dutch 2016, there was no blinding, so this outcome was at high risk of detection bias. Two studies (Lee 2016; Lukka NCIC 2005) were deemed at low risk of detection bias. The remaining studies did not mention blinding, so were at unclear risk of bias. Attrition bias was not considered at high risk of bias for this outcome.
Late toxicity
Late RTOG radiation therapy gastrointestinal toxicity
Four studies contributed data for late RTOG GI RT toxicity (CHHiP 2016; HYPRO Dutch 2016; MDACC 2014; PROFIT 2016),
Performance bias: CHHiP 2016 and HYPRO Dutch 2016 were at risk of performance bias, given the lack of blinding of participants and personnel to the intervention and accounted for 52% of the weight. The other studies contributing data for this outcome were at unclear risk of bias (MDACC 2014; PROFIT 2016). No study reported the details of on‐study care and permissible cointerventions participants received.
Detection bias: two studies were at high risk of bias for lack of blinding for this subjective outcome (CHHiP 2016; HYPRO Dutch 2016). CHHiP 2016 was at high risk of attrition bias for late RTOG GI RT toxicity. The other studies were at unclear risk of bias.
Late RTOG radiation therapy genitourinary toxicity
Four studies contributed data for late RTOG GU RT toxicity (Arcangeli 2010; CHHiP 2016; MDACC 2014; PROFIT 2016),
Performance bias: CHHiP 2016 and HYPRO Dutch 2016 were at risk of performance bias, given the lack of blinding participants and personnel to the intervention and accounted for 52% of the weight. The other studies contributing data for this outcome were at unclear risk of bias (MDACC 2014; PROFIT 2016). No study reported the details of on‐study care and permissible cointerventions participants received.
Detection bias: two studies were at high risk of bias for lack of blinding for this subjective outcome (CHHiP 2016; HYPRO Dutch 2016).
Attrition bias: risk of attrition bias was high for CHHiP 2016 and low for the other four studies.
Time to death from any cause
Performance bias: Arcangeli 2010; CHHiP 2016; and HYPRO Dutch 2016 were at risk of performance bias, and for lack of blinding participants and personnel to the intervention. The other studies contributing data for this outcome were at unclear risk of bias (Fox Chase 2013; Lee 2016; Lukka NCIC 2005; MDACC 2014; Norkus 2009; PROFIT 2016; Yeoh 2011). No study reported the details of on‐study care and permissible cointerventions participants received.
Detection bias: all 10 studies that contributed data for this objective outcome were at low or unclear risk of bias for sequence generation and allocation concealment. Risk of attrition bias was low in eight studies contributing data for this outcome, unclear in one study and the single study at high risk of attrition bias contributed 5.5% to the study weight (Yeoh 2011).
Time to metastasis
Performance bias: Arcangeli 2010 and CHHiP 2016 were at risk of performance bias, given the lack of blinding participants and personnel to the intervention. The other studies contributing data for this outcome were at unclear risk of bias (Fox Chase 2013; Lee 2016; MDACC 2014). No study reported the details of on‐study care and permissible cointerventions participants received.
Detection bias: two of the five (Arcangeli 2010; CHHiP 2016; Fox Chase 2013; Lee 2016; MDACC 2014) studies that contributed data for time to metastasis were at high risk of bias for lack of blinding for this subjective outcome and accounted for 77% of study weight (Arcangeli 2010; CHHiP 2016). Risk of detection bias was unclear for two studies (Fox Chase 2013; MDACC 2014). All studies contributing to this outcome reported on 100% of participants, so risk of attrition bias was low.
Time to biochemical relapse
Performance bias: Arcangeli 2010 was at risk of performance bias for lack of blinding participants and personnel to the intervention and accounted for 15% of study weight. Lee 2016; MDACC 2014; and PROFIT 2016 were at unclear risk of bias. Yeoh 2011, which accounted for 23% of study weight was at high risk of attrition bias. No study reported the details of on‐study care and permissible cointerventions participants received.
Detection bias: in two of the five studies contributing data to this outcome BR‐FS was not a compound endpoint, only PSA failure events contributed and PSA failure was assessed with a prespecified protocol, so this domain was at low risk of bias (see Characteristics of included studies table). It was unclear whether there was blinding in the other studies (Lee 2016; MDACC 2014; PROFIT 2016). Yeoh 2011 was at high risk of attrition bias for this outcome, and accounted for 23% of study weight.
Acute toxicity EORTC/RTOG gastrointestinal and gastrointestinal toxicity
Performance bias: Arcangeli 2010; CHHiP 2016; and HYPRO Dutch 2016 were at risk of performance bias for lack of blinding participants and personnel to the intervention. The other study contributing data for this outcome was at unclear risk of bias (PROFIT 2016). No study reported the details of on‐study care and permissible cointerventions participants received.
Detection bias: three of the four studies contributing data to this subjective outcome were at high risk of bias because of a lack of blinding (Arcangeli 2010; CHHiP 2016; HYPRO Dutch 2016); the other was at unclear risk of bias for lack of information (PROFIT 2016). The studies contributing to this outcome were at low risk of attrition bias.
Quality of life
Performance bias: CHHiP 2016 was at risk of performance bias for lack of blinding participants and personnel to the intervention. The other studies contributing data for this outcome were at unclear risk of bias (Fox Chase 2013; MDACC 2014; PROFIT 2016). No study reported the details of on‐study care and permissible cointerventions participants received.
Detection bias: one study did not mask the participants and personnel (CHHiP 2016), so was at high risk of bias and two studies were at unclear risk of bias for lack of information (Lee 2016; PROFIT 2016).
Effects of interventions
See: Table 1
Primary outcomes
PC‐SS
Hypofractionation may result in little or no difference in PC‐SS (HR 1.00, 95% CI 0.72 to 1.39; studies = 8, participants = 7946; median follow‐up 72 months; low‐certainty evidence; Analysis 1.1). For men in the intermediate‐risk group undergoing conventional fractionation this corresponds to 976 per 1000 men alive after 6 years and 0 more (44 fewer to 18 more) alive per 1000 men undergoing hypofractionation (Table 1). We downgraded the certainty of evidence by one level for study limitations (lack of blinding with concerns over performance and detection bias) and imprecision,
1.1. Analysis.
Comparison 1 Hypofraction versus conventional fractionation, Outcome 1 Prostate cancer‐specific survival (PC‐SS).
Late gastrointestinal radiation therapy toxicity
We are uncertain about the effect of hypofractionation on late EORTC/RTOG (Cox 1995) GI toxicity at 60 months (RR 1.10, 95% CI 0.68 to 1.78; studies = 4; participants = 3843; very low‐certainty evidence; Analysis 1.7). There was evidence of heterogeneity (P = 0.008; I2 = 75%). When a fixed‐effect model was used the HR was 0.97 (95% CI 0.8 to 1.17). This corresponds to 109 toxicity events per 1000 participants and 11 more (35 fewer to 85 more), with hypofractionation compared with conventional fractionation at median follow‐up of 72 months in an intermediate‐risk population (Table 1). We downgraded the certainty of the evidence by three levels for inconsistency, lack of blinding and imprecision.
1.7. Analysis.
Comparison 1 Hypofraction versus conventional fractionation, Outcome 7 ≥ Grade II late gastrointestinal (GI) Radiation Therapy Oncology Group/European Organisation for Research and Treatment of Cancer (RTOG/EORTC) toxicity at 60 months.
Late genitourinary radiation therapy toxicity
Hypofractionation probably results in little to no difference in late GU toxicity at 60 months (RR 1.05, 95% CI 0.93 to 1.18; studies = 4; participants = 3843; moderate‐certainty evidence; Analysis 1.11). This corresponds to 262 toxicity events per 1000 participants and 13 more (18 fewer to 47 more) with hypofractionation compared with conventional fractionation (Table 1). We downgraded the certainty of the evidence by one level for study limitations (lack of blinding with risk of performance of detection bias) and attrition bias.
1.11. Analysis.
Comparison 1 Hypofraction versus conventional fractionation, Outcome 11 Late genitourinary (GU) ≥ Grade II RTOG/EORTC toxicity at 60 months.
Secondary outcomes
Overall survival
Hypofractionation results in little or no difference in overall survival (HR 0.94, 95% CI 0.83 to 1.07; studies = 10, participants = 8243; high‐certainty evidence; Analysis 1.15). For men in the intermediate‐risk group undergoing conventional fractionation this corresponds to 869 per 1000 men alive after six years and 17 fewer (54 fewer to 17 more) alive per 1000 men when undergoing hypofractionation (Table 1).
1.15. Analysis.
Comparison 1 Hypofraction versus conventional fractionation, Outcome 15 Overall survival.
Metastasis‐free survival
Hypofractionation may result in little to no difference to metastasis‐free survival (HR 1.07, 95% CI 0.65 to 1.76; studies = 5, participants = 4985; median follow‐up 62.4 months to 96.5 months; low‐certainty evidence; Analysis 1.16). This corresponds to 19 metastatic events per 1000 participants and five more (58 fewer to 19 more) with hypofractionation compared to conventional fractionation. We downgraded the certainty of the evidence by two levels for imprecision and detection bias.
1.16. Analysis.
Comparison 1 Hypofraction versus conventional fractionation, Outcome 16 Metastasis‐free survival.
Biochemical relapse‐free survival
Hypofractionation likely results in a small possibly unimportant reduction in BR‐FS based on Phoenix criteria (HR 0.88, 95% CI 0.68 to 1.13; studies = 5, participants = 2889; median follow‐up 90 months to 108 months; moderate‐certainty evidence; Analysis 1.17). In men of the intermediate‐risk group, this corresponds to 804 biochemical‐recurrence free per 1000 participants at six years with conventional fractionation and 42 fewer (134 fewer to 37 more) recurrence‐free per 1000 participants with hypofractionation (Table 1). We downgraded the certainty of the evidence by one level for study limitations and imprecision.
1.17. Analysis.
Comparison 1 Hypofraction versus conventional fractionation, Outcome 17 Biochemical relapse‐free survival Phoenix.
There was moderate heterogeneity (P = 0.14; I2 = 43%). When the studies where PSA failure was the only event contributing to the outcome were excluded (Arcangeli 2010; Yeoh 2011), the HR was 0.90 (95% CI 0.75 to 1.08); this appeared to explain the source of heterogeneity (P = 0.53; I2 = 0%).
Acute radiation therapy gastrointestinal toxicity (Grade II acute RTOG/EORTC or greater)
Hypofractionation probably increases acute GI RT toxicity slightly (RR 1.45, 95% CI 1.19 to 1.75; studies = 4; participants = 4174; at 12 weeks' to 18 weeks' follow‐up; moderate‐certainty evidence; Analysis 1.18). We found there may be some heterogeneity (P = 0.17; I2 = 41%). This corresponds to 306 episodes of toxicity per 1000 participants with hypofractionation and 47 more (20 more to 87 more) compared with conventional fractionation in an intermediate‐risk population at median follow‐up of 72 months. We downgraded the certainty of the evidence by one level for lack of blinding and imprecision.
1.18. Analysis.
Comparison 1 Hypofraction versus conventional fractionation, Outcome 18 Acute GI ≥ Grade II RTOG/EORTC.
Acute radiation therapy genitourinary toxicity (Grade II acute RTOG/EORTC or greater)
Hypofractionation likely results in little or no difference to acute GU RT toxicity (RR 1.03, 95% CI 0.95 to 1.11; studies = 4; participants = 4174; at 12 weeks' to 18 weeks' follow‐up; moderate‐certainty evidence; Analysis 1.19). This corresponds to 360 episodes of toxicity per 1000 participants and 11 more (18 fewer to 40 more) with hypofractionation compared with conventional fractionation. We downgraded the certainty of the evidence by one level for risk of bias.
1.19. Analysis.
Comparison 1 Hypofraction versus conventional fractionation, Outcome 19 Acute GU ≥ Grade II RTOG/EORTC toxicity.
Late radiation‐induced malignancy
We found no data with respect to second malignancy.
Health‐related quality of life
Global quality of life
EuroQoL 5‐dimension (EQ5D) (303 assessable men in one study) did not differ between treatment arms in Fox Chase 2013 (see Table 2).
Self‐reported bowel quality of life
Self‐reported bowel function (185 participants, one study) did not differ between the two study arms at baseline or five years (P < 0.01; figure from text; MDACC 2014) (see Table 2).
Self‐reported bowel 'bother'
We were able to report on 1258 men in one study (CHHiP 2016). In CHHiP 2016, 2100/2163 men participated in the quality of life substudy. These included 1404 men in the hypofractionation arm (698 received 60 Gy, 706 received 57 Gy) and 696 in the conventional arm. One thousand four hundred forty‐four men provided data at 24 months post‐RT. Hypofractionation probably results in little or no difference in self‐reported bowel‐related quality of life (RR 1.14, 95% CI 0.84 to 1.56; studies = 1, participants = 1258; Analysis 1.21). The 95% CI included clinically insignificant benefits and clinically meaningful harms (CHHiP 2016). The evidence certainty for this outcome was low, being downgraded for imprecision and attrition bias. In PROFIT 2016, there was no difference in questionnaire completion rates between study arms. Both Expanded Prostate Cancer Index Composite (EPIC) and 12‐item Short Form (SF‐12) had statistically significant decline over time, but no difference between arms (no P value reported). AUA Symptom Index was stable over time, with no difference between study arms. There was no difference in EPIC Bowel Score between treatment arms in Fox Chase 2013.
1.21. Analysis.
Comparison 1 Hypofraction versus conventional fractionation, Outcome 21 HRQoL: ≥ bowel 'bother'.
At three years, the MD in GI symptoms was 2.03% in favor of conventional fractionation (90% CI –6.18% to 10.23%) (HYPRO Dutch 2016), so non‐inferiority could not be demonstrated for hypofractionation. To demonstrate non‐inferiority required the incidence of clinically relevant deterioration in the hypofractionation group to be no worse than 8% more than the incidence in the conventional arm (figures from text) (Table 2).
Sexual quality of life
We were able to report on the 962/1092 men who participated in the quality of life substudy (Lee 2016). At six months, there were no differences in EPIC scoring, and at 12 months the men in the hypofractionated arm had a larger (but not clinically significant) decline in bowel score. There was no difference between EPIC sexual scores by treatment arm in Fox Chase 2013.
Self‐reported sexual function
Self‐reported sexual function (studies = 1, participants = 185) did not differ between the two study arms at baseline or five years (P < 0.01; figure from text; MDACC 2014) (see Table 2). Erectile dysfunction (assessed in hormone‐naive men who had full or partial erectile function at baseline) was "comparable in both study arms" (quotation from study text) (RR 0.88, 95% CI 0.55 to 1.40; studies = 1, participants = 120; HYPRO Dutch 2016; see Table 2). Orgasmic function was higher in the hypofractionated arm at 36 months (P = 0.043; figure from text; HYPRO Dutch 2016).
Sexual function was non‐inferior at three years (MD –10.48%, 90% CI –24.88% to –3.91%; HYPRO Dutch 2016). To demonstrate non‐inferiority required the incidence of clinically relevant deterioration in the hypofractionation group to be no worse than 8% more than the incidence in the conventional arm (figures from text) (Table 2).
Self‐reported sexual 'bother' at 60 months
We studied 1084 men in one study (CHHiP 2016). We found that hypofractionation probably makes little or no difference to self‐reported sexual 'bother', although we could not exclude either clinically insignificant benefits or clinically insignificant harms (RR 1.00, 95% CI 0.88 to 1.12). Evidence certainty for this outcome was moderate, being downgraded for attrition bias.
Doctor‐reported sexual 'bother'
We studied 1416 men in one study (CHHiP 2016). We found hypofractionation makes little or no difference to doctor‐reported sexual 'bother', and excluded clinically significant benefits and harms (RR 0.97, 95% CI 0.90 to 1.05).
Self‐reported bladder quality of life
Self‐reported bladder function (studies = 1, participants = 185) did not differ between the two study arms at baseline or five years (P < 0.01; figure from text; MDACC 2014) (see Table 2).
At three years, the MD in GU symptoms was 0.49% in favor of conventional fractionation (90% CI –7.20% to 8.18%; HYPRO Dutch 2016), so non‐inferiority could not be demonstrated for hypofractionation. To demonstrate non‐inferiority required the incidence of clinically relevant deterioration in the hypofractionation group to be no worse than 8% more than the incidence in the conventional arm (figures from text) (Table 2).
Self‐reported late bladder 'bother'
Grade II or greater bladder 'bother' at five years was not different between the study arms in CHHiP 2016 (9.6% [60 Gy regimen] and 7.9% [57 Gy regimen] in the hypofractionated arms and 6.5% in the conventional arm) (P = 0.74 [60 Gy regimen] and P = 0.83 ][57 Gy regimen]). There was no difference between EPIC Urinary scores (irritative/obstructive or incontinence) by treatment arm in Fox Chase 2013. International Prostate Symptom Score (IPSS) score and IPSS Quality of Life (Barry 1992) did not differ between treatment arms in Fox Chase 2013 (see Table 2).
Subgroup analyses
Risk stratification
We were not able to do subgroup analyses according to risk group stratification as planned
Radiation therapy dose 74 Gy or greater versus less than 74 Gy in conventional arm (which reflects current practice)
Using α/β = 1.93, for prostate‐specific survival, there was no evidence of a subgroup effect with hypofractionation when the two subgroups were formally compared (Chi2 = 2.17, P = 0.14, I2 = 54%; Analysis 1.3). PC‐SS with dose 74 Gy or greater had an HR of 1.17 (95% CI 0.79 to 1.73) and with a dose less than 74 Gy had an HR of 0.67 (95% CI 0.36 to 1.25).
1.3. Analysis.
Comparison 1 Hypofraction versus conventional fractionation, Outcome 3 PC‐SS subgroup analysis (SGA) by dose 74 Gy or greater control arm.
For late GI RT toxicity (Grade II EORTC/RTOG or greater), no studies included in the comparison had a dose less than 74 Gy in the conventional arm.
For late GU RT toxicity (Grade II EORTC/RTOG or greater), no studies included in the comparison had a dose less than 74 Gy in the conventional arm.
Difference in EQD2 between the radiation therapy doses delivered in the two study arms (EQD2 difference less than 4 Gy versus 4 Gy or greater) chosen to explore any effect of dose escalation
For prostate‐specific survival, there was no evidence of a subgroup effect when the two subgroups were formally compared (Chi2 = 0.36, P = 0.55, I2 = 0%; Analysis 1.4). Dose difference less than 4 Gy had an HR of 1.11 (95% CI 0.66 to 1.87) and 4 Gy or greater had an HR of 0.90 (95% CI 0.56 to 1.43).
1.4. Analysis.
Comparison 1 Hypofraction versus conventional fractionation, Outcome 4 PC‐SS < 4 Gy equivalent dose in 2 Gy fractions (EQD2) vs ≥ 4 Gy.
For late GI RT toxicity (Grade II RTOG/EORTC or greater), there was no evidence of a subgroup effect when the two groups were formally compared (Chi2 = 0.36, P = 0.55, I2 = 0%; Analysis 1.8). Dose difference less than 4 Gy had an HR of 0.94 (95% CI 0.38 to 2.34) and 4 Gy or greater had an HR of 1.30 (95% CI 0.79 to 2.14).
1.8. Analysis.
Comparison 1 Hypofraction versus conventional fractionation, Outcome 8 Late GI RT toxicity by dose ≥ 4 Gy difference between arms.
For late GU RT toxicity (Grade II RTOG/EORTC or greater), there was no evidence of a subgroup effect when the two groups were formally compared (Chi2 = 0.24, P = 0.63, I2 = 0%; Analysis 1.12). Dose difference less than 4 Gy had an HR 1.24 (95% CI 0.71 to 2.18) and 4 Gy or greater had an HR of 1.08 (95% CI 0.93 to 1.25).
1.12. Analysis.
Comparison 1 Hypofraction versus conventional fractionation, Outcome 12 Late GU RT toxicity by dose ≥ 4 Gy difference between arms.
Quality of delivered radiation
For PC‐SS, there was no evidence of a subgroup effect with hypofractionation when the two subgroups were formally compared (Chi2 = 0.89, P = 0.35, I2 = 0%; Analysis 1.6). RT QA process had an HR of 0.88 (95% CI 0.57 to 1.34) and no RT QA process had an HR of 1.22 (95% CI 0.72 to 2.07).
1.6. Analysis.
Comparison 1 Hypofraction versus conventional fractionation, Outcome 6 PC‐SS quality assurance (QA) versus no QA.
For late GI RTOG/EORTC RT toxicity, there was no evidence of a subgroup effect when the two groups were formally compared (Chi2 = 1.44, P = 0.23, I2 = 30.5%; Analysis 1.10). RT QA process had an HR of 0.94 (95% CI 0.38 to 2.34) and no RT QA process had an HR of 2.18 (95% CI 0.78 to 6.05).
1.10. Analysis.
Comparison 1 Hypofraction versus conventional fractionation, Outcome 10 Late GI RT toxicity by QA vs no QA.
For late GU RTOG/EORTC RT toxicity, there was no evidence of a subgroup effect when the two groups were formally compared (Chi2 = 0.66, P = 0.42, I2 = 0%; Analysis 1.14). RT QA process had an HR of 1.00 (95% CI 0.82 to 1.23) and no RT QA process had an HR of 1.7 (95% CI 0.48 to 6.04).
1.14. Analysis.
Comparison 1 Hypofraction versus conventional fractionation, Outcome 14 Late GU RT toxicity by QA vs no QA.
Radiation therapy technique (highly conformal radiation therapy versus 3DCRT)
There was no evidence of a subgroup effect on PC‐SS with hypofractionation when the two subgroups were formally compared (test for subgroup interaction: Chi2 = 0.01, P = 0.93, I2 = 0%; Analysis 1.2). PC‐SS with highly conformal radiation therapy use had an HR of 1.03 (95% CI 0.69 to 1.50) versus with 3DCRT use had an HR of 0.99 (95% CI 0.42 to 2.32).
1.2. Analysis.
Comparison 1 Hypofraction versus conventional fractionation, Outcome 2 PC‐SS 3‐dimensional conformal radiation therapy (3DCRT) vs intensity‐modulated radiation therapy (IMRT).
For late GI RT toxicity (Grade II EORTC/RTOG or greater), all studies included in the comparison used highly conformal radiation therapy.
For late GU RT toxicity (Grade II EORTC/RTOG or greater), all studies included in the comparison used highly conformal radiation therapy.
Androgen deprivation in more than 50% of participants in both study arms (for efficacy outcomes only)
For prostate‐specific survival, there was no evidence of a subgroup effect with hypofractionation when the two subgroups were formally compared (Chi2 = 2.4, P = 0.12, I2 = 58.3%; Analysis 1.5). Use of androgen deprivation had an HR of 1.29 (95% CI 0.81 to 2.04) and no androgen deprivation had an HR of 0.76 (95% CI 0.47 to 1.23).
1.5. Analysis.
Comparison 1 Hypofraction versus conventional fractionation, Outcome 5 PC‐SS androgen deprivation (AD) versus no AD.
For late GI RT toxicity (Grade II RTOG/EORTC or greater), there was evidence of a subgroup effect when the two groups were formally compared (Chi2 = 9.95, P = 0.002, I2 = 89%; Analysis 1.9). Androgen deprivation had an RR of 1.22 (95% CI 0.96 to 1.56) and no androgen deprivation had an RR of 0.64 (95% CI 0.46 to 0.88).
1.9. Analysis.
Comparison 1 Hypofraction versus conventional fractionation, Outcome 9 SGA ≥ Grade II late GI RTOG/EORTC toxicity AD vs no AD.
For late GU RT toxicity (Grade II RTOG/EORTC or greater), there was no evidence of a subgroup effect when the two groups were formally compared (Chi2 = 0.50, P = 0.48, I2 = 0%; Analysis 1.13). Androgen deprivation had an RR of 1.14 (95% CI 0.85 to 1.52) and no androgen deprivation had an RR of 1.00 (95% CI 0.81 to 1.23).
1.13. Analysis.
Comparison 1 Hypofraction versus conventional fractionation, Outcome 13 SGA late GU ≥ Grade II RTOG/EORTC toxicity: AD vs no AD.
Sensitivity analysis
Study age (excluding studies that commenced accrual before 2002)
For the outcome of prostate‐specific survival: our findings were robust to this sensitivity analysis when the one study that commenced accrual before 2002 was excluded (Lukka NCIC 2005) (HR 1.09, 95% CI 0.75 to 1.59), there was no evidence of heterogeneity (P = 0.66, I2 = 0%).
For the outcome Grade II late RTOG/EORTC or greater GI toxicity at 60 months, no studies met the criteria for this sensitivity analysis.
For the outcome Grade II late RTOG/EORTC or greater GU toxicity at 60 months, no studies met the criteria for this sensitivity analysis.
Study quality (excluding studies at high risk of bias for that outcome)
No study contributing to this outcome met the criteria for this sensitivity analysis.
Duration of follow‐up (excluding studies with follow‐up of less than 10 years, measured from time of randomization to outcome assessment)
All included studies had fewer than 10 years' follow‐up.
By altering the α/β ratio for the subgroup analysis dose 74 Gy or greater versus less than 74 Gy in conventional arm (chosen to reflect current practice)
Using α/β = 4.14 for PC‐SS, there was no evidence of an effect with hypofractionation when the two subgroups were formally compared (Chi2 = 2.17, P = 0.14, I2 = 54%). PC‐SS with dose 74 Gy or greater had an HR of 1.17 (95% CI 0.79 to 1.73) and dose less than 74 Gy had an HR of 0.67 (95% CI 0.36 to 1.25).
Using α/β = 0.58 for PC‐SS, there was no evidence of an effect with hypofractionation when the two subgroups were formally compared (Chi2 = 2.17, P = 0.14, I2 = 54%). PC‐SS with dose 74 Gy or greater had an HR of 1.17 (95% CI 0.79 to 1.73) and dose less than 74 Gy had an HR of 0.67 (95% CI 0.36 to 1.25).
By altering the α/β ratio for the subgroup analysis difference in EQD2 between the radiation therapy doses delivered in the two study arms (EQD2 difference less than 4 Gy versus 4 Gy or greater) chosen to explore any effect of dose escalation
Using α/β = 4.14 for PC‐SS, there was no evidence of an effect with hypofractionation when the two subgroups were formally compared (Chi2 = 0.00, P = 0.97, I2 = 0%). PC‐SS with dose 74 Gy or greater had an HR of 0.99 (95% CI 0.66 to 1.49) and dose less than 74 Gy had an HR of 1.01 (95% CI 0.55 to 1.84).
Using α/β = 0.58 for PC‐SS, there was no evidence of an effect with hypofractionation when the two subgroups were formally compared (Chi2 = 1.2, P = 0.27, I2 = 16.8%). PC‐SS with dose 74 Gy or greater had an HR of 1.14 (95% CI 0.77 to 1.68) and dose less than 74 Gy had an HR of 0.73 (95% CI 0.36 to 1.47).
We tested how robust our findings were by using both random‐effects and fixed‐effect models to analyze outcomes other than time‐to‐event data. For the outcome of late GI RT toxicity (Grade II EORTC/RTOG or greater), there was an effect (HR 0.97, 95% CI 0.8 to 1.17). Changing the model did not affect other outcomes.
Discussion
Summary of main results
With hypofractionation for localized prostate cancer we found: low‐certainty evidence that PC‐SS is similar, very low‐certainty evidence that late GI RT toxicity is probably similar, moderate‐certainty evidence that late GU RT toxicity is similar, high‐certainty evidence that overall survival is similar and low‐certainty evidence that metastasis‐free survival is similar. We found moderate‐certainty evidence that BR‐FS is probably slightly increased with hypofractionation. We found moderate‐certainty evidence that hypofractionation probably increases acute GU RT toxicity slightly while it likely results in little to no difference in acute GI toxicity. Hypofractionation probably results in little to no difference in quality of life.
Overall completeness and applicability of evidence
We found no evidence of indirectness; the studies included all directly evaluated our review question.
The participants included reflected those seen in the clinic. The studies included a mixture of men with low‐, intermediate‐ and high‐risk disease (NCCN 2014), with the exception of Lee 2016, which was limited to low‐risk men (see Table 7). We were unable to report the time‐to‐event outcomes by risk subgroup.
Most (73.6%) men studied received highly conformal radiation therapy, which is the standard of care for men treated radically with RT for prostate cancer. highly conformal radiation therapy (paired with image guidance) allows safe dose escalation, while minimizing both acute and late GI RT and GU RT toxicity (Dearnaley 2014; Hou 2015; Peeters 2005; Peeters 2006).
BR‐FS was a compound outcome in some studies (see Table 2); contributing events included PSA failure, deaths and initiation of androgen deprivation. Fox Chase 2013 reported a compound endpoint which included clinical and biochemical failure, Lee 2016 reported disease‐free survival (which included local progression, distant metastases, biochemical relapse or death any cause) and HYPRO Dutch 2016 reported relapse‐free survival (events comprised clinical or biochemical progression, distant metastases or commencement of androgen deprivation).
Some studies reported cumulative toxicity of a certain grade, while others reported maximum toxicity reported, which did not allow combination of the results. Some studies used the ASTRO 1997 definition to report biochemical failure, but we chose to use the Phoenix definition of biochemical failure because it is a better predictor for distant metastases, PC‐SS and overall survival (Abramowitz 2008).
The long natural history of prostate cancer necessitates prolonged observation to observe events. Competing causes of death in older men make PC‐SS important to evaluate the effectiveness of prostate cancer therapies. The relatively short duration of follow‐up in the included studies (median 69 months) may have contributed to imprecision for the CIs around the point estimates for PC‐SS and DM‐FS. Biochemical relapse can precede clinical manifestations of prostate cancer relapse after dose‐escalated RT for localized prostate cancer by more than 10 years (Zumsteg 2015). Late RT toxicity can increase with time, so rates may increase with longer follow‐up duration. With longer follow‐up duration, we may see more precision for these outcomes in updates of this review. More effective salvage therapies may postpone or reduce prostate cancer deaths.
Dose‐escalated RT (74 Gy or more) with or without androgen deprivation is the standard of care for external beam treatment of localized intermediate‐ and high‐risk prostate cancer. Most (6/8) studies contributing to cancer outcomes used dose‐escalated RT. Two studies used doses less than 74 Gy, so do not represent current practice (Lukka NCIC 2005; Yeoh 2011). Dose escalation improves BR‐FS, but not PC‐SS or OS (Michalski 2013). The doses used in the included studies in this review were isoeffective between study arms, so demonstration of equivalent safety and toxicity (both acute and late) was required. The assumption of isoeffectiveness is based on radiobiologic modeling of the α/β ratio for prostate cancer.
Studies using more than 2 Gy per fraction were eligible for inclusion in this review. Fraction size in included studies ranged from 2.35 Gy to 3.4 Gy, this could be considered moderate hypofractionation. The conclusions are not valid for fraction sizes larger than 3.4 Gy.
A QA process decreased late RTOG/ERTC GI toxicity (Analysis 1.10). Dose escalation without a careful QA process can be associated with increased late GI RT toxicity.
Quality of the evidence
The certainty of evidence ranged from high (overall survival) to very low (late GI toxicity). We frequently downgraded for study limitations due to lack of blinding and the risk of performance and detection bias and imprecision related to study size, event rate and potentially insufficient length of follow‐up. This lack of precision may be addressed in updates of this review. We also downgraded for unexplained inconsistency.
Potential biases in the review process
We believe we have identified all the relevant studies for inclusion in this analysis; despite our best efforts, we may have missed studies in particular foreign language and unpublished studies.
For the elderly men in 5/10 included studies treated with androgen deprivation, the presence of prolonged testosterone suppression might have biased the outcome of biochemical relapse if assessed prematurely. We performed a post‐hoc subgroup analysis of the effect of androgen deprivation (based on editorial advice), considering it a potential effect‐modifier but found no suggestion of a subgroup effect.
We explored the heterogeneity we detected for the outcome of acute RT toxicity (Grade II EORTC/RTOG or greater) by excluding HYPRO Dutch 2016, and we recognize that this post‐hoc analysis may be a potential source of bias. However, it is a biologically plausible explanation for the potential heterogeneity.
Studies contributing data for the outcome of BR‐FS had different events contributing data for the outcome (see Table 2). We included all studies in the analysis, but detected what we thought was meaningful heterogeneity, which we explained by excluding the two studies which only contributed PSA failure events to their endpoint of biochemical relapse. This decision was post‐hoc, and done to explain our findings, but may be a potential source of bias.
Agreements and disagreements with other studies or reviews
We found five systematic reviews addressing the topic.
Bannuru 2011 (search date 2007 to 2011) included only English language studies, and included Fox Chase 2013; Lukka NCIC 2005; Norkus 2009; and Yeoh 2011. The evidence was not systematically reviewed, so the search was not comprehensive. The search date was not recent (2001). No evidence quality assessment was performed. They reported "no significant difference" in overall survival, freedom from biochemical relapse, GI toxicity and GU toxicity for the comparison of hypofractionation versus conventional fractionation of RT for prostate cancer.
Koontz 2015 (search date January 1990 to June 2014) included English language studies and performed a systematic search. They included Arcangeli 2010; CHHiP 2016; Fox Chase 2013; Lukka NCIC 2005; MDACC 2014; and Yeoh 2011. They concluded that there was evidence to support the safety of the technique of hypofractionated RT for localized prostate cancer, but that efficacy data were lacking, because follow‐up duration was inadequate.
Zaorsky 2013 (search date 1970 to 2012) searched MEDLINE, PubMed and conference proceedings, but excluded non‐English language studies. The search date was not recent. They included five RCTs (Arcangeli 2010; Fox Chase 2013; Lukka NCIC 2005; MDACC 2014; Yeoh 2011). They concluded that there was no clear evidence that hypofractionated RT for localized PC was not associated with either improved outcomes or reduced toxicity, and that its use be limited to clinical trials.
Sun 2014 (search date 2014) systematically searched and reviewed the literature, they included six RCTs (Arcangeli 2010; Fox Chase 2013; Lukka NCIC 2005; MDACC 2014; Norkus 2009; Yeoh 2011), three cohort studies and three retrospective case controlled studies. They included non‐randomized data. They reported that hypofractionation improved BR‐FS in localized prostate cancer.
Datta 2017 (search date not stated to 30 March 2017) included English language studies with five years' follow‐up. The authors included nine studies (Arcangeli 2010; CHHiP 2016; Fox Chase 2013; HYPRO Dutch 2016; Lee 2016; Lukka NCIC 2005; MDACC 2014; PROFIT 2016; Yeoh 2011). The evidence was systematically reviewed and the search was systematic. The authors reported to Grade II or greater acute and late RT toxicity, amalgamating data reported using different scoring systems (RTOG/EORTC, LENT‐SOMA and NCI) without explaining how this was accounted for. They concluded they found "non‐significant" differences for PC‐SS, PS and biochemical failure (reported graphically in text, no effect size measures or 95% CI reported). Acute GI RT toxicity was increased with hypofractionation (OR 1.68, CI not reported). For acute GI RT toxicity, and late GI and GU RT toxicity differences were "non‐significant" (no effect sizes or CI reported). They concluded that "hypofractionation provides similar therapeutic outcomes to conventional fractionation except for a significantly greater risk of acute GI toxicity".
Arcangeli 2018 (search date not stated to 30 December 2017) included English language studies reporting biochemical failure and greater than Grade II toxicity with five years' follow‐up. The review included nine studies (Arcangeli 2010; CHHiP 2016; Fox Chase 2013; HYPRO Dutch 2016; Lee 2016; Lukka NCIC 2005; MDACC 2014; PROFIT 2016; Yeoh 2011). They did not perform quality assessment. They amalgamated data reported using different scoring systems (RTOG/EORTC, LENT‐SOMA and NCI) without explaining how this was accounted for. They concluded that moderate hypofractionation was associated with equivalent results in freedom from biochemical relapse (risk difference (RD) 0.93, 95% CI 0.84 to1.04), late GI toxicity (RD 0.96, 95% CI 0.77 to 1.25) and late GU toxicity (RD 1.06, 95% CI 0.90 to 1.26).
These reviews were conducted with less methodologic rigor than the Cochrane methodology we used, no systematic review assessed evidence certainty on a per outcome basis, the search dates were not current and two limited their search to studies published in English (Bannuru 2011; Zaorsky 2013). One review included non‐randomized data (Sun 2014). No other systematic review defined what effect size was considered clinically meaningful for these outcomes and presented no absolute effects. Since publication of these reviews, new studies (HYPRO Dutch 2016; Lee 2016; PROFIT 2016) and updated results (Arcangeli 2010; Fox Chase 2013; MDACC 2014) have become available to contribute to the evidence with respect to this question.
Joint guidelines produced by American Society of Clinical Oncology (ASCO) and American Society for Therapeutic Radiation Oncology (ASTRO) support the use of hypofractionation for prostate cancer stating that "moderately hypofractionated radiation therapy should be offered to patients who choose EBRT for treatment of prostate cancer" and "hypofractionated radiation therapy provides important potential advantages in cost and convenience for patients" (Morgan 2018). Guidelines from the European Association of Urology (EAU), ESTRO and International Society of Geriatric Oncology (SIOG) support the use of moderate hypofractionation as safe and effective, but note that long‐term data are still lacking (Mottett 2017).
Authors' conclusions
Implications for practice.
Moderate hypofractionation (up to a fraction size of 3.4 Gy) when compared to standard fractionation for prostate cancer appears to have minimal effect on cancer‐related outcomes, can be delivered with similar late gastrointestinal and genitourinary toxicity but comes at the cost of a likely slight increase in acute gastrointestinal toxicity and without detriment to quality of life.
Implications for research.
Updating data in published randomized controlled trials with longer follow‐up will likely add precision to these findings.
What's new
| Date | Event | Description |
|---|---|---|
| 10 October 2019 | Amended | Added Plain Language Summary title, "Use of shorter radiation treatments for prostate cancer", in Plain language summary section. No other changes. |
Acknowledgements
Daniel Francis for writing the search strategy. Adrienne See for assisting with running the search strategy and for checking data extraction.
Wai Wai Lui and Catherine Haden for assistance with literature searching.
The Cochrane Urology Group, in particular Alea Miller.
Peer reviewers: M Flentje, D Böhmer and T Wiegel.
Appendices
Appendix 1. The Cochrane Library search strategy
#1 MeSH descriptor: [Prostatic Neoplasms] explode all trees
#2 MeSH descriptor: [Prostatic Intraepithelial Neoplasia] explode all trees
#3 (prostat*):ti,ab,kw near/3 (cancer* or carcinoma* or malignan* or tumour* or tumour* or neoplas* or intraepithelial or adeno*):ti,ab,kw
#4 #1 or #2 or #3
#5 MeSH descriptor: [Dose Fractionation] explode all trees
#6 MeSH descriptor: [Dose‐Response Relationship, Radiation] explode all trees
#7 (radio* or radiat*);ti,ab,kw near/2 (dos* or regimen* or schedul*):ti,ab,kw
#8 (hypofraction* or hypo‐fraction* or fraction* or superfraction* or super‐fraction* or multifraction* or multi‐fraction* or hypofractio* or hypo‐fraction*):ti,ab,kw
#9 #5 or #6 or #7 or #8
#10 #4 and #9
Appendix 2. MEDLINE (Ovid) search strategy
prostatic neoplasms/
prostatic intraepithelial neoplasia/
prostat* adj3 (cancer* or carcinoma* or malignan* or tumo?r* or neoplas* or intraepithelial or adeno*).mp
or/1‐3
dose fractionation/
dose response relationship, radiation/
radiotherapy dosage/
(radiotherapy* or radiat*).tw adj2 (dose or dosage or regimen* or schedule*).tw
hypofractionat*.mp
fraction*.mp
superfract*.mp
multifraction*.mp
hypo‐fraction*.mp
super‐fraction*.tw
multi‐fraction*.tw
hyperfraction*.tw
or/5‐16
randomized controlled trial.pt
controlled clinical trial.pt
randomized.ab
placebo.ab
drug therapy.fs
randomly.ab
trial.ab
groups.ab
18 or 19 or 20 or 21 or 22 or 23 or 24 or 25
exp animals/ not humans.sh
26 not 27
4 and 17 and 28
Appendix 3. Embase (Ovid) search strategy
prostate tumor/
prostatic intraepithelial neoplasia/
prostat* adj3 (cancer* or carcinoma* or malignan* or tumo?r* or neoplas* or intraepithelial or adeno*).mp
or/1‐3
(radiotherapy* or radiat*).tw adj2 (dose or dosage or regimen* or schedule*).tw
radiation response/
radiation dose fractionation/
hypofractionat*.mp
fraction*.mp
superfract*.mp
multifraction*.mp
hypo‐fraction*.mp
superfraction*.mp
multi‐fraction*hyperfraction*.tw
or/5‐14
random*
Factorial*
crossover*
cross over*
placebo*
doubl*adj blind*
singl* adj blind*
assign*
allocate*
volunteer*
crossover‐procedure/
double‐blind procedure/
randomized controlled trial/
single‐blind procedure/
or/16‐29
4 and 16 and 31
Appendix 4. LILACS search strategy
(MH:"prostatic neoplasms" OR (prostat$ AND cancer$) OR (prostat$ AND carcinoma) OR (prostat$ AND malignan$) OR (prostat$ AND tumo$) OR (prostat$ AND neoplasm$) or (prostat$ AND intraepithelial) OR (prostat$ AND adeno$)) AND (MH:"dose fractionation" OR MH:"Dose‐Response Relationship, Radiation" OR (radio$ AND dos$) OR (radio$ near regimen$) OR (radio$ near schedule$) OR (radia$ near dos$) OR (radia$ near regimen$) OR (radia$ near schedule$) OR hypofraction$ OR fraction$ OR superfract$ OR multifract$ OF hypo‐fraction$ OR super‐fraction$ OR multi‐fraction$ OR hyperfraction$) AND ((PT:"randomized controlled trial" OR PT:"controlled clinical trial" OR PT:"multicenter study" OR MH:"randomized controlled trials as topic" OR MH:"controlled clinical trials as topic" OR MH:"multicenter studies as topic" OR MH:"random allocation" OR MH:"double‐blind method" OR MH:"single‐blind method") OR ((ensaio$ OR ensayo$ OR trial$) AND (azar OR acaso OR placebo OR control$ OR aleat$ OR random$ OR enmascarado$ OR simpleciego OR ((simple$ OR single OR duplo$ OR doble$ OR double$) AND (cego OR ciego OR blind OR mask))) AND clinic$)) AND NOT (MH:animals OR MH:rabbits OR MH:rats OR MH:primates OR MH:dogs OR MH:cats OR MH:swine OR PT:"in vitro")
Appendix 5. ISRCTN Register search strategy
prostate AND cancer AND (radiation OR radiotherapy)
Appendix 6. WHO ICTRP search portal search strategy
Advanced search (with Recruitment set at ALL):
Search 1.
Condition field: prostate
Intervention field: fraction size AND radiation
Search 2.
Condition field: adenocarcinoma AND prostate
Intervention field: radiation
Search 3.
Condition field: adenocarcinoma AND prostate
Intervention field: irradiation
Search 4.
Condition: prostate
Intervention field: irradiation
Search 5.
Condition: prostate
Intervention: hypofractionated radiation
Appendix 7. EORTC search strategy
prostate
Appendix 8. NCI Clinical Trials Search Form search strategy
Cancer type/ condition: prostate cancer
Treatment/ intervention: radiation therapy AND radiotherapy
Appendix 9. ANZCTR search strategy
Search terms: prostate
Intervention: radiation or radiotherapy
Appendix 10. National Guideline Clearinghouse search strategy
Disease or condition: prostate cancer
Appendix 11. Trial identification data list
| Study | ISRTCTN | NCT |
| Arcangeli 2010 | ISRCTN97182923 | NCT00392535 |
| CHHiP 2016 | ISRCTN00392535 | — |
| Fox Chase 2013 | — | NCT00062309 |
| HYPRO Dutch 2016 | ISRCTN85138529 | — |
| Lee 2016 | — | NCT00331773 |
| Lukka NCIC 2005 | — | NCT00667888 |
| MDACC 2014 | — | NCT00667888 |
| Norkus 2009 | — | — |
| PROFIT 2016 | ISRCTN43853433 | NCT00304759 |
| Yeoh 2011 | — | — |
Data and analyses
Comparison 1. Hypofraction versus conventional fractionation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Prostate cancer‐specific survival (PC‐SS) | 8 | 7946 | Hazard Ratio (Random, 95% CI) | 1.00 [0.72, 1.39] |
| 2 PC‐SS 3‐dimensional conformal radiation therapy (3DCRT) vs intensity‐modulated radiation therapy (IMRT) | 8 | Hazard Ratio (Random, 95% CI) | 1.00 [0.72, 1.39] | |
| 2.1 3DCRT | 3 | Hazard Ratio (Random, 95% CI) | 0.99 [0.42, 2.32] | |
| 2.2 IMRT | 5 | Hazard Ratio (Random, 95% CI) | 1.03 [0.69, 1.56] | |
| 3 PC‐SS subgroup analysis (SGA) by dose 74 Gy or greater control arm | 8 | Hazard Ratio (Random, 95% CI) | 1.00 [0.72, 1.39] | |
| 3.1 > 74 Gy | 5 | Hazard Ratio (Random, 95% CI) | 1.17 [0.79, 1.73] | |
| 3.2 ≤ 74 Gy | 3 | Hazard Ratio (Random, 95% CI) | 0.67 [0.36, 1.25] | |
| 4 PC‐SS < 4 Gy equivalent dose in 2 Gy fractions (EQD2) vs ≥ 4 Gy | 8 | Hazard Ratio (Random, 95% CI) | 1.00 [0.72, 1.39] | |
| 4.1 EQD2 difference < 4Gy | 4 | Hazard Ratio (Random, 95% CI) | 1.11 [0.66, 1.87] | |
| 4.2 EQD2 difference ≥ 4 Gy | 4 | Hazard Ratio (Random, 95% CI) | 0.90 [0.56, 1.43] | |
| 5 PC‐SS androgen deprivation (AD) versus no AD | 8 | Hazard Ratio (Random, 95% CI) | 1.00 [0.72, 1.39] | |
| 5.1 AD | 3 | Hazard Ratio (Random, 95% CI) | 1.29 [0.81, 2.04] | |
| 5.2 No AD | 5 | Hazard Ratio (Random, 95% CI) | 0.76 [0.47, 1.23] | |
| 6 PC‐SS quality assurance (QA) versus no QA | 8 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 6.1 Radiation therapy (RT) QA process described | 4 | Hazard Ratio (Random, 95% CI) | 0.88 [0.57, 1.34] | |
| 6.2 No RT QA process reported | 4 | Hazard Ratio (Random, 95% CI) | 1.22 [0.72, 2.07] | |
| 7 ≥ Grade II late gastrointestinal (GI) Radiation Therapy Oncology Group/European Organisation for Research and Treatment of Cancer (RTOG/EORTC) toxicity at 60 months | 4 | 3843 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.68, 1.78] |
| 8 Late GI RT toxicity by dose ≥ 4 Gy difference between arms | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 EQD2 differs > 4 Gy between study arms | 2 | 985 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.79, 2.14] |
| 8.2 EQD2 differs by < 4 Gy between arms | 2 | 2858 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.38, 2.34] |
| 9 SGA ≥ Grade II late GI RTOG/EORTC toxicity AD vs no AD | 4 | 3843 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.68, 1.78] |
| 9.1 AD | 3 | 2637 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.96, 1.56] |
| 9.2 No AD | 1 | 1206 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.46, 0.88] |
| 10 Late GI RT toxicity by QA vs no QA | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 QA process reported | 2 | 2858 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.38, 2.34] |
| 10.2 No QA process reported | 1 | 203 | Risk Ratio (M‐H, Random, 95% CI) | 2.18 [0.78, 6.05] |
| 11 Late genitourinary (GU) ≥ Grade II RTOG/EORTC toxicity at 60 months | 4 | 3843 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.93, 1.18] |
| 12 Late GU RT toxicity by dose ≥ 4 Gy difference between arms | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 12.1 EQD2 differs by > 4 Gy between study arms | 2 | 985 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.93, 1.25] |
| 12.2 EQD2 differs by < 4 Gy between study arms | 3 | 3026 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.71, 2.18] |
| 13 SGA late GU ≥ Grade II RTOG/EORTC toxicity: AD vs no AD | 5 | 4011 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.93, 1.22] |
| 13.1 AD | 4 | 2805 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.85, 1.52] |
| 13.2 No AD | 1 | 1206 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.81, 1.23] |
| 14 Late GU RT toxicity by QA vs no QA | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14.1 QA process described | 2 | 2858 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.82, 1.23] |
| 14.2 No QA process described | 2 | 371 | Risk Ratio (M‐H, Random, 95% CI) | 1.70 [0.48, 6.04] |
| 15 Overall survival | 10 | 8243 | Hazard Ratio (Random, 95% CI) | 0.94 [0.83, 1.07] |
| 16 Metastasis‐free survival | 5 | 4985 | Hazard Ratio (Random, 95% CI) | 1.07 [0.65, 1.76] |
| 17 Biochemical relapse‐free survival Phoenix | 5 | 2889 | Hazard Ratio (Random, 95% CI) | 0.88 [0.68, 1.13] |
| 18 Acute GI ≥ Grade II RTOG/EORTC | 4 | 4174 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [1.19, 1.75] |
| 19 Acute GU ≥ Grade II RTOG/EORTC toxicity | 4 | 4174 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.95, 1.11] |
| 20 Health‐related quality of life (HRQoL): ≥ Grade II sexual 'bother' (participant reported) at 60 months | 1 | 1084 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.88, 1.12] |
| 21 HRQoL: ≥ bowel 'bother' | 1 | 1258 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.84, 1.56] |
| 22 HRQoL: ≥ Grade II sexual 'bother' (doctor reported) at 60 months | 1 | 1416 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.90, 1.05] |
| 23 HRQoL: erectile function | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.55, 1.40] |
| 24 PC‐SS sensitivity analysis (SA) dose ≥ 74 Gy conventional (α/β 4.14) | 8 | Hazard Ratio (Random, 95% CI) | 1.00 [0.72, 1.39] | |
| 24.1 ≥ 74 Gy | 5 | Hazard Ratio (Random, 95% CI) | 1.17 [0.79, 1.73] | |
| 24.2 < 74 Gy | 3 | Hazard Ratio (Random, 95% CI) | 0.67 [0.36, 1.25] | |
| 25 PC‐SS SA dose control arm ≥ 74 Gy (α/β 0.58) | 8 | Hazard Ratio (Random, 95% CI) | 1.00 [0.72, 1.39] | |
| 25.1 Dose ≥ 74 Gy | 5 | Hazard Ratio (Random, 95% CI) | 1.17 [0.79, 1.73] | |
| 25.2 Dose < 74 Gy | 3 | Hazard Ratio (Random, 95% CI) | 0.67 [0.36, 1.25] | |
| 26 PC‐SS SA < 4 Gy EQD2 vs ≥ 4 Gy (α/β 4.14) to 4 Gy | 8 | Hazard Ratio (Random, 95% CI) | 1.00 [0.72, 1.39] | |
| 26.1 EQD2 difference ≥ 4 Gy | 5 | Hazard Ratio (Random, 95% CI) | 0.99 [0.66, 1.49] | |
| 26.2 EQD2 difference < 4 Gy | 3 | Hazard Ratio (Random, 95% CI) | 1.01 [0.55, 1.84] | |
| 27 PC‐SS SA EQD2 < 4 Gy vs ≥ 4 Gy (α/β 0.58)) | 7 | Hazard Ratio (Random, 95% CI) | 1.03 [0.73, 1.44] | |
| 27.1 EQD2 difference ≥ 4 Gy | 6 | Hazard Ratio (Random, 95% CI) | 1.14 [0.77, 1.68] | |
| 27.2 EQD2 difference < 4 Gy | 1 | Hazard Ratio (Random, 95% CI) | 0.73 [0.36, 1.47] |
1.20. Analysis.
Comparison 1 Hypofraction versus conventional fractionation, Outcome 20 Health‐related quality of life (HRQoL): ≥ Grade II sexual 'bother' (participant reported) at 60 months.
1.22. Analysis.
Comparison 1 Hypofraction versus conventional fractionation, Outcome 22 HRQoL: ≥ Grade II sexual 'bother' (doctor reported) at 60 months.
1.23. Analysis.
Comparison 1 Hypofraction versus conventional fractionation, Outcome 23 HRQoL: erectile function.
1.24. Analysis.
Comparison 1 Hypofraction versus conventional fractionation, Outcome 24 PC‐SS sensitivity analysis (SA) dose ≥ 74 Gy conventional (α/β 4.14).
1.25. Analysis.
Comparison 1 Hypofraction versus conventional fractionation, Outcome 25 PC‐SS SA dose control arm ≥ 74 Gy (α/β 0.58).
1.26. Analysis.
Comparison 1 Hypofraction versus conventional fractionation, Outcome 26 PC‐SS SA < 4 Gy EQD2 vs ≥ 4 Gy (α/β 4.14) to 4 Gy.
1.27. Analysis.
Comparison 1 Hypofraction versus conventional fractionation, Outcome 27 PC‐SS SA EQD2 < 4 Gy vs ≥ 4 Gy (α/β 0.58)).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Arcangeli 2010.
| Methods |
Parallel RCT Setting: cancer center, Italy Dates: January 2003–December 200 Follow‐up: median 96.5 months (range 6.2–130.8 months) Randomization ratio: 1:1 Superiority design: no confidence interval specified |
|
| Participants | 168 men Inclusion criteria: diagnosis < 6 months ago, written consent, availability for follow‐up Exclusion criteria: PSA > 100 ng/mL, no DM, contraindications to 9 months AD, previous pelvic RT, prostate surgery other than TURP, ulcerative colitis, WHO performance > 2, nodes > 1 cm on CT Diagnostic criteria: histologically confirmed adenocarcinoma prostate |
|
| Interventions |
Number of study centers: 1 Run‐in period: not stated Extension period: no Experimental arm: 83/83 participants Conventional arm: 85/85 participants Complex interventions: Experimental arm: 62 Gy in 20 fractions over 4 weeks Conventional arm: 80 Gy in 40 fractions over 8 weeks |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Complete outcome measures reported: yes |
|
| Funding sources | Not stated | |
| Declarations of interest | Not stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "assigned to the study group with a balanced randomisation method using a computer program based on a pseudo‐random routine in C‐language", page 1044. Study endpoints and randomization procedures, paragraph 4. Comment: we deemed at low risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described, so we deemed this domain at unclear risk of bias. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk |
Quote from publication: "No blinding was done", page 1014. Study endpoints and randomisation procedures, paragraph 5. Comment: lack of blinding likely a source of bias. |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk |
Quote from publication: "No blinding was done", page 1014. Study endpoints and randomisation procedures, paragraph 5. Comment: lack of blinding likely a source of bias. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk |
Quote from publication: "No blinding was done", page 1014. Study endpoints and randomisation procedures, paragraph 5. Participants were monitored weekly during RT then at 3‐month intervals for 3 years and twice per year thereafter. At each visit, PSA determination and a history were performed". Comment:
|
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk |
Quote from publication: "no blinding was done", page 1014. Study endpoints and randomisation procedures, paragraph 5. Comment: we deemed this domain at low risk of bias for OS. |
| Incomplete outcome data (attrition bias) | Low risk |
Quote from publication: "No patients were lost to follow up", page 1015.
|
| Selective reporting (reporting bias) | Unclear risk | Outcomes specified in Methods: BCDF, acute and late GI and GU toxicity Outcomes reported in paper: BCDF, acute and late GI and GU toxicity Comment: we did not have access to the study protocol. We deemed this domain at unclear risk of bias. |
| Other bias | Low risk | We found no evidence of other sources of bias. |
CHHiP 2016.
| Methods |
Parallel RCT Setting: cancer centers, UK Dates: Aug 2002–June 2011 Follow‐up: median 62.4 months Randomization ratio: 1:1:1 Non‐inferiority design: 1‐sided confidence interval |
|
| Participants | 3216 men Inclusion criteria: > 16 years, T1B–T3A N0 M0 prostate cancer, PSA < 30 ng/mL, estimated risk of lymph‐node involvement < 30% (prior to 2006, when 454 men had been recruited, PSA < 40 ng/mL and risk of node involvement < 30% were eligible), WHO performance status 0–1. Exclusion criteria: T3 + Gleason score ≥ 8, life expectancy < 10 years, previous pelvic RT, previous androgen suppression, another active malignancy in the past 5 years (other than cutaneous basal‐cell carcinoma), comorbid conditions precluding radical RT, full anticoagulation treatment or hip prosthesis. Diagnostic criteria: histologically confirmed prostate cancer |
|
| Interventions |
Number of study centers: 71 Run‐in period: not stated Extension period: no Experimental arm: 2151/2151 participants Conventional arm: 1065/1065 participants Complex interventions: Hypofractionation arm: Group 1 (n = 1074): 60 Gy in 20 fractions in 4.0 weeks (3 Gy per fraction) Group 2 (n = 1077): 57 Gy in 19 fractions in 3.8 weeks (3 Gy per fraction) Conventional arm (n = 1065): 74 Gy in 37 fractions (2 Gy per fraction) over 7.4 weeks (see Table 8) |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Complete outcome measures reported: yes |
|
| Funding sources | Institute of Cancer Research UK | |
| Declarations of interest | CP reported grants and personal fees from Bayer, personal fees from Janssen and personal fees from BNIT. JS reported personal fees from Janssen‐Cilag Limited and personal fees from Bayer. DD reported grants from Cancer Research UK, during the conduct of the study. EH reported grants from Cancer Research UK, during the conduct of the study; and a grant from Accuracy Inc. to the Institute of Cancer Research to support independent statistical analysis of a phase 3 trial of stereotactic body RT in prostate cancer, outside the submitted work. VK reported advisory and educational fees and non‐financial support from Astellas, educational fees from Bayer, non‐financial educational support from Janssen, advisory and educational fees and non‐financial support from Ipsen, and educational fees from Tolmar. All other authors declared no competing interests. | |
| Notes | All men received short‐course androgen suppression (3–6 months) before and during RT (optional for men with low‐risk disease). Treatment technique: IMTRT (integrated simultaneous boost used) AD: all had 3–6 months AD Pelvic nodes not treated Margins: 59 Gy PTV 1.0 cm isotropically (0.5 cm posteriorly), 74 Gy PTV, margin 0.5 cm isotropically, with 0 cm posterior margin Target volumes covered by 95% isodose IGRT portal imaging films daily A national quality assurance program was used in CHHiP 2016. Note: this is a 3‐phase study. Phase 1: n = 150 designed to evaluate and exclude unacceptable toxicity Phase 2: n = 300 to refine estimates of acute and late toxicity, this data was necessary to gain approval for phase 3 Phase 3: n = 1713 Total n = 3216 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "Computer‐generated random permuted blocks were used, with block sizes of six and nine", page 3, 2016. Randomization and masking. Comment: we deemed at low risk of bias |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "Randomisation was done centrally via telephone calls to the ICR‐CTSU [Institute of Cancer Research Clinical Trials and Statistics Unit]", page 3, 2016. Randomization and masking. Comment: we deemed this at low risk of bias. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk |
Quote from publication: "it was not possible to mask patients or clinicians to treatment allocation", page 3, 2016. Randomization and masking. Comment: we deemed this domain at high risk of bias. |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk |
Quote from publication: "it was not possible to mask patients or clinicians to treatment allocation", page 3, 2016. Randomization and masking. Comment: we deemed this domain at low of bias because of the nature of the outcome.
|
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk |
Quote from publication: "Treatment allocation was not masked and, because of the trial's size, assessors could not be blinded to toxicity or clinical assessments", page 44. Randomization and masking. Comment:
The following outcomes were at high risk of bias for lack of blinding, physicians were not blinded, both participant and physician‐reported outcomes were combined.
The following outcomes were at high risk of bias for lack of blinding.
|
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk |
Quote from publication: "Neither treatment allocation nor clinical assessment were masked because sham radiotherapy was not given", page 1606. Randomization and masking. Comment: although outcome assessors for objective outcomes were not blinded, we deemed it was not a source of bias for OS |
| Incomplete outcome data (attrition bias) | Low risk | Attrition and exclusions were clearly described by study arm, with reasons given (see Figure 1, page 45), so we deemed this at low risk of bias. 3216/3216 men enrolled were reported on for efficacy. They gave details of how many men had a complete set of follow‐up forms for toxicity reporting, so we deemed this at low risk of bias. Number of participants reported on by outcome
Quote from publication: "Acute toxicity data was collected for the first 2163 … patients. When the sample size was increased, it was felt that sufficient data had been collected on acute toxicity to all robust conclusions to be drawn about acute toxicity", Dearnaley 2016, page 4.
Self‐reported bowel bother: 1258/3163 assessable participants (40%). Deemed at high risk of attrition bias. Self‐reported sexual bother: 1084/3216 assessable participants (34%). Deemed at high risk of attrition bias. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes specified in methods: time to biochemical or clinical failure, disease‐free survival, OS, DM, starting AD, acute and late GI and GU toxicity (participant and physician reported), and quality of life. All the outcomes specified were reported. We did not have access to the study protocol. We deemed this domain at unclear risk of bias. |
| Other bias | Low risk | We did not identify other sources of bias. |
Fox Chase 2013.
| Methods |
Parallel RCT Setting: cancer center, US Dates: June 2002–May 2006 Follow‐up: median 68.4 months Randomization ratio: 1: 1 Superiority design CCT |
|
| Participants | 303 men Inclusion criteria: 1 of the following: PSA > 10 ng/mL, Gleason score > 6, ≥ T2b disease, ≥ 3 cores involved with Gleason score ≥ 5 Exclusion criteria: no other medical condition that would preclude study participation; no other active malignancy within the past 5 years except non‐metastatic skin cancer or early‐stage chronic lymphocytic leukemia (well‐differentiated small cell lymphocytic leukemia); fertile men must use effective contraception; no more than 4 months of prior AD therapy; no prior pelvic RT; no prior or planned radical prostate surgery Diagnostic criteria: histologically confirmed prostate cancer |
|
| Interventions |
Number of study centers: 1 Run‐in period: not stated Extension period: no Experimental arm: 151/154 participants Conventional arm: 152/153 participants Complex interventions: Hypofractionation: 70.2 Gy in 26 fractions Conventional: 76 Gy in 38 fractions |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Complete outcome measures reported: yes |
|
| Funding sources | National Cancer Institute Grants No. CA101984‐01 and CA‐00692 and Florida Bankhead Coley Grant No. 09BW11 | |
| Declarations of interest | Consultant or Advisory Role: Alan Pollack, GE Healthcare, Calypso; Robert G Uzzo, WILEX; Mark K Buyyounouski, Augmenix, GE Healthcare, Honoraria: Alan Pollack, Varian Medical Systems, Accuray, Siemens Healthcare Research Funding: Alan Pollack, Varian Medical Systems, Siemens Healthcare; Benjamin Movsas, Varian Medical Systems, Philips Healthcare; Mark K Buyyounouski, Varian Medical Systems Patents: Mark K Buyyounouski, Amersys, UpToDate | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "pre‐populated block randomization sheet was used for assignment by the office of protocol research", page 3861. Participants, paragraph 2. Comment: did not give clear details with respect to the process of sequence generation, although it did suggest there was use of a randomization sequence, so we deemed this domain at unclear risk of bias. |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "a pre‐populated block randomisation sheet was used for assignment by the office of protocol research", page 3861. Participants, paragraph 2. Comment: suggests that the process of randomization was controlled by the office of protocol research, so we deemed this domain at low risk of bias. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Not mentioned, probably not done; we deemed this domain at unclear risk of bias. |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Not mentioned, probably not done, we deemed this domain at unclear risk of bias.
|
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | There was no mention of blinding, probably not done. This made the assessment of subjective outcomes (e.g. toxicity) at unclear risk of bias, even though a validated scoring tool was used.
There was no information with respect to the timing of assessments (both clinical and PSA testing).
|
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Not mentioned, probably not done, we deemed this domain at low risk of bias.
|
| Incomplete outcome data (attrition bias) | Low risk | Exclusions and attrition were detailed by arm, with reasons, and 303/307 men randomized were reported on so we deemed this domain at low risk of bias. See Figure 1, page 3861 of report. Number of participants reported on by outcome PC‐SS: 307/307 Late EORTC/RTOG GI RT toxicity: not reported Late EORTC/RTOG GU RT toxicity: not reported OS: 307/307 DM‐FS: 307/307 BR‐FS: not reported Acute EORTC/RTOG GI RT toxicity: not reported Acute EORTC/RTOG GU RT toxicity: not reported Second malignancy:not reported Quality of life: not reported |
| Selective reporting (reporting bias) | Unclear risk | Outcomes specified: BCDF (events comprised local failure, regional or DM, PSA failure), acute and late GI and GU toxicity. Outcomes reported: BCDF, local and distant failure, PC‐SS, OS, acute and late GI and GU toxicity. We did not have access to the study protocol. We deemed this domain at unclear risk of bias. |
| Other bias | Low risk | We found no evidence of other sources of bias. |
HYPRO Dutch 2016.
| Methods |
Parallel RCT Setting: Netherlands cancer centers Dates: March 2007–December 2020 Follow‐up: median 60 months Randomization ratio: 1:1 Non‐inferiority design: powered to detect a 10% absolute increase in relapse‐free survival |
|
| Participants | n = 820 Inclusion criteria: intermediate‐risk and high‐risk men with prostate cancer, ages 44–85 years, histologically confirmed stage T1b–T4 NX–0 MX–0, ≤ PSA 60 ng/mL, WHO PS 0–2. When PSA < 20 ng/mL or a Gleason score < 8, men could be included without a work‐up for metastases. Exclusion criteria: previous pelvis irradiation, radical prostatectomy, evidence of pelvic nodal disease (determined by CT of pelvis), presence of DM (determined by bone scintigraphy), and low‐risk men (stage T1b–T2a, Gleason score ≤ 6, PSA ≤ 10 ng/mL). Diagnostic criteria: histologically confirmed prostate adenocarcinoma |
|
| Interventions |
Number of study centers: 7 Run‐in period: not stated Extension period: no Experimental arm: 403/410 participants Conventional arm: 392/410 participants Complex interventions: see Table 8 |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Complete outcome measures reported: yes (R‐FS, acute and late toxicity reported) |
|
| Funding sources | Dutch Cancer Society (grant No CKTO 2006‐08) | |
| Declarations of interest | Quote: "We declare no competing interests", page 474, paragraph 3. | |
| Notes | AD: each center followed own adjuvant AD protocol, which was equally applied to both study arms. Risk stratification based on Partin Table CKTO‐2006‐08 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "Patients were randomly assigned (1:1) to open‐label treatment groups with standard fractionation or hypofractionation, applying a minimisation procedure. There was a random element in the randomisation and it ensured overall balance and within each stratum of the stratification factors (i.e., treatment centre and risk group)", page 466. Randomization and masking, paragraph 1. Comment: as this was not completely described, we judged this domain at unclear risk of bias. |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "Patients were assigned via a web‐based application (done by the Clinical Trials Center, Erasmus MC Cancer Institute, Rotterdam) and the assigned treatment group was sent immediately via fax, telephone, or email to the local investigator", page 466. Randomization and masking, paragraph 1. Comment: this was a remote concealed process, so we deemed this at low risk of bias. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk |
Quote from publication: "The local investigators were treating physicians, so they were not masked to treatment". Randomization, page 466. Randomization and masking, paragraph 1. Comment: participants and personnel were not blinded, so judged this domain at high risk of bias.
|
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk |
Quote from publication: "The local investigators were treating physicians, so they were not masked to treatment", page 466. Randomization and masking, paragraph 1. Comment: participants and personnel were not blinded, we deemed this domain at low risk of bias.
|
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk |
Quote from publication: "The local investigators were treating physicians, so they were not masked to treatment", randomization page 466. Randomization and masking, paragraph 1. Quote from publication: "If we suspected tumour recurrence or metastases based on complaints or clinical signs, we did transrectal ultrasound of the prostate, pelvic CT, and bone scintigraphy, at the discretion of the treating physician". Comment: blinding was not done and likely to be a source of bias, so we deemed this domain at high risk of bias.
|
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk |
Quote from publication: "The local investigators were treating physicians, so they were not masked to treatment". Randomization page 466. Randomization and masking, paragraph 1. We did not feel objective outcomes were at risk of bias.
|
| Incomplete outcome data (attrition bias) | Low risk | See Figure 1, page 4 in study text. The exclusions and attrition were detailed by study arm, with reasons given. Acute toxicity was reported in 794/820 men randomized, late toxicity was reported in 782/820 men randomized and efficacy was reported in 804/820 men randomized so we deemed this domain at low risk of bias. Number of participants reported on by outcome
EORTC QLQ‐PR25 was assessed in 206/356 evaluable men in the hypofractionated arm and 208/341 evaluable men in the conventional arm at baseline and 3 years. This domain was deemed at low risk of bias. |
| Selective reporting (reporting bias) | Low risk | Outcomes specified: R‐FS (events comprised biochemical relapse, clinical relapse, locoregional or distant relapse, or start of hormone therapy), acute and late GI and GU toxicity, quality of life and erectile function. Outcomes reported: R‐FS, OS (post‐hoc), acute and late GI and GU toxicity. Comment: although quality of life and erectile function were not reported, it is likely that this will be reported in future publications, so we deemed this domain at low risk of bias. We did not have access to the study protocol. |
| Other bias | Low risk | We found no evidence of other sources of bias. |
Lee 2016.
| Methods |
Parallel RCT Setting: community‐based and tertiary medical sites that were members of the RTOG, US Dates: April 2006–December 2009 Follow‐up: median 69.6 months Randomization ratio: 1:1 Non‐inferiority design: 1‐sided confidence interval |
|
| Participants | 1115 men Inclusion criteria: men ages ≥ 18 years, T1b‐T2cN0M0, Gleason score 2–6 and PSA < 10 ng/mL, Zubrod PS 2 Exclusion criteria: another invasive cancer, prior orchidectomy, chemotherapy, RT, cryosurgery or definitive therapy for prostate cancer, AD Diagnostic criteria: low‐risk prostate cancer |
|
| Interventions |
Number of study centers: not reported Run‐in period: not stated Extension period: no Experimental arm: 542/558 participants Conventional arm: 550/557 participants Complex interventions: Conventional arm: 70 Gy in 28 fractions Experimental arm: 73.8 Gy in 41 fractions (see Table 8) |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Complete outcome measures reported: yes |
|
| Funding sources | National Cancer Institute Grants No: U10CA21661, U10CA37422, CA81647, U10CA180868, U10CA180822 | |
| Declarations of interest | Not stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "randomly assigned by using the permuted block method", page 2326. Random assignment, paragraph 1. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described, so we deemed this domain at unclear risk of bias. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Not mentioned, we deemed this domain at unclear risk of bias.
|
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Not mentioned, we deemed this domain at low risk of bias
|
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk |
Quote from publication: "History and physical examination, assessment of adverse events … were performed every 3 months for the first 2 years, every 6 months for the next 3 years", page 2326. Participant assessment and endpoints, paragraph 1. Comment: there was no mention of blinding for assessment of subjective outcomes, probably not done, so we deemed this domain at unclear risk of bias. Quote from publication: "Death was attributed to prostate cancer if certified primarily as such, disease progressed on salvage androgen suppression, or death resulted from an adverse effect of therapy", page 2326. Participant assessment and endpoints, paragraph 1. PC‐SS: comment: although this was not blinded, the authors made the decision process clear, which we thought made assessment of this subjective outcome PC‐SS at low risk of bias.
Quote from publication: "History and physical examination, assessment of adverse events, and PSA measurement were performed every 3 months for the first 2 years, every 6 months for the next 3 years", page 2326. Participant assessment and endpoints, paragraph 1. Comment: although there was a prespecified regimen for PSA testing, this was a compound endpoint (including DM, salvage AD and death without recurrence) (see Table 2), which made this outcome at unclear risk of bias.
|
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Blinding of outcome assessment was not mentioned, but we deemed this at low risk of bias
|
| Incomplete outcome data (attrition bias) | Low risk | Clear details provided (page 2327, Figure 1) with respect to attrition, so we deemed this at low risk of bias. Number of participants reported on by outcome PC‐SS: 1092/1092: low risk of bias. Late EORTC/RTOG GI RT toxicity: not reported in usable form Late EORTC/RTOG GU RT toxicity: not reported in usable form OS: 1092/1092: low risk of bias. DM‐FS: 1092/1092: low risk of bias. BR‐FS: 1092/1092: low risk of bias. Acute EORTC/RTOG GI RT toxicity: not reported in usable form Acute EORTC/RTOG GU RT toxicity: not reported in usable form Second malignancy: not reported Quality of life: sexual quality of life 962/1092 (88%): low risk of bias. |
| Selective reporting (reporting bias) | Low risk | Outcome specified: D‐FS (events comprised local progression, DM, OS, PC‐SS, time to progression, time to PSA failure, adverse events) Outcomes reported: D‐FS, local progression, DM, OS, PC‐SS, adverse events). We did not have access to the study protocol. We deemed this domain at low risk of bias. |
| Other bias | Low risk |
Quote from publication: "At the third interim analysis, the Data Monitoring Committee recommended that results of the trial be disclosed. On the basis of the event information at that time (185 [78%] of 238 events required for definitive analysis), stopping to reject the null hypothesis of inferiority required a test statistic P value of < .011, and this condition was satisfied", page 2326. Statistical methods, paragraph 1. Comment: although early stopping occurred, this was on the basis of prespecified interim analyses, so was deemed at low risk of bias. |
Lukka NCIC 2005.
| Methods |
Parallel RCT Setting: tertiary cancer centers, Canada Dates: median 5.7 years (range 4.5–8.3 years) Follow‐up: March 1995–December 1998 Randomization ratio: 1:1 Superiority design: 1‐sided confidence interval |
|
| Participants | 936 men Inclusion criteria: T1–2 prostate cancer Exclusion criteria: PSA > 40 ng/ml; previous therapy for prostate carcinoma (other than biopsy or TURP); AD; prior or active malignancy other than non‐melanoma skin cancer, colon cancer or thyroid cancer treated a minimum of 5 years before the trial and presumed cured; simulated volume exceeding 1000 mL; previous pelvic RT; presence of inflammatory bowel disease; diagnosis of serious non‐malignant disease that would preclude RT or surgical biopsy; geographically inaccessible for follow‐up; a psychiatric or addictive disorder that would preclude obtaining informed consent or adherence to protocol; inability to commence RT within 26 weeks of the date of last prostatic biopsy; and failure to provide informed consent to participate in the clinical trial. Diagnostic criteria: adenocarcinoma prostate |
|
| Interventions |
Number of study centers: 16 Run‐in period: not stated Extension period: no Experimental arm: 466/466 participants Conventional arm: 470/470 participants Complex interventions: Experimental arm: 52.5 Gy in 20 fractions over 4 weeks Conventional arm: 66 Gy in 33 fractions over 6.5 weeks |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Complete outcome measures reported: yes |
|
| Funding sources | National Cancer Centre Ontario and the National Cancer Institute of Canada Clinical Trials Group | |
| Declarations of interest | Quote: "The authors indicated no potential conflicts of interest", page 6137, paragraph 8. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "Patients were assigned to one of two treatment regimens according to a central computer‐generated randomization schedule within", page 6133. Treatment regimens, paragraph 1. Comment: we deemed this at low risk of bias, because a computer‐generated randomization schedule was used. |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "a central computer‐generated randomisation schedule … ", page 6133. Treatment regimens, paragraph 1. Comment: we deemed this at low risk of bias, because allocation was managed centrally; therefore, we thought it was concealed. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Not mentioned, we deemed this domain at unclear risk of bias
|
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Not mentioned, we deemed this domain at low risk of bias
|
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk |
Quote from publication: "Blinded assessment was used to verify the occurrence of biochemical or clinical failure and to determine the earliest date of failure", page 6133. Outcomes, paragraph 1. Comment: we deemed these outcomes at low risk of bias because of this blinding
Quote from publication: "The investigating clinician or clinical trials nurse assessed radiation toxicity using the standardised National Cancer Institute of Canada toxicity scale", page 6134. Outcomes, paragraph 3. Comment: there is no mention of blinding of outcome assessors, it was probably not done, though there was a prespecified follow‐up schedule, these outcomes were deemed at unclear risk of bias.
|
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Blinding not mentioned, deemed at low risk of bias
|
| Incomplete outcome data (attrition bias) | Low risk |
Quote from publication: "During the study, seven patients did not receive radiation (five patients in the long arm and two patients in the short arm …)", page 6134. Study population, paragraph 2. Comment: there is no mention of exclusions or attrition, but in the tables, all participants randomized were included. All men randomized were included in the analysis so we deemed this at low risk of bias. Number of participants reported on by outcome PC‐SS: 936/936 Late EORTC/RTOG GI RT toxicity: not reported in usable form Late EORTC/RTOG GU RT toxicity: not reported in usable form OS: 936/936 DM‐FS: not reported in usable form BR‐FS: not reported in usable form Acute EORTC/RTOG GI RT toxicity: not reported in usable form Acute EORTC/RTOG GU RT toxicity: not reported in usable form Second malignancy: not reported Quality of life: not assessed |
| Selective reporting (reporting bias) | Low risk | Outcomes specified: BCF, radiation toxicity (GI and GU) Outcomes reported: BCF, OS, prostate cancer deaths, prostate biopsy at 2 years, radiation toxicity We did not have access to the study protocol. We deemed this domain at low risk of bias. |
| Other bias | Low risk |
Quote from publication: "Although the trial was originally designed with biopsy positivity at 2 years after randomization as the primary outcome, the emerging literature suggested that the combination of BCF was the optimal measure of efficacy … Therefore, before the study completion and data un‐blinding, an amendment was issued and approved by the study Steering Committee (September 14, 2001) to change the primary outcome to BCF. The protocol modification was then distributed to all participating clinical centres", page 6133. Outcomes, paragraph 1. Comment: although there was a change made in the primary outcome measure, this was done prior to unblinding, so we did not consider it a source of bias. |
MDACC 2014.
| Methods |
Parallel RCT Dates: January 2001–January 2010 Setting: cancer center, US Follow‐up: median 100.8 months Randomization ratio: 1:1 Superiority design |
|
| Participants | 206 men Inclusion criteria: good PS (Zubrod < 2), clinical stage (c) T1b‐T3b disease (1992 AJCC staging system), PSA 20 ng/mL, Gleason score < 10, and no clinical, radiographic, or pathologic evidence of nodal or bone metastasis. A bone scan within 3 months of signing the protocol consent or starting androgen ablation was required for men with PSA > 10 ng/mL or cT3 disease, and a CT scan of the pelvis was required for men with cT3 disease. Men with cT3 disease were required to have Gleason score < 8 and pretreatment PSA 10 ng/mL. Men with Gleason score 8 or 9 disease were required to have cT1/T2 disease and pretreatment PSA 10 ng/mL. Exclusion criteria: palpable stage cT3c (AJCC 1992: seminal vesicle involvement) or cT4 disease, men with a history of prior pelvic RT, men who received > 4 months of hormone ablation therapy, men with prior or planned radical prostate surgery, and men with concurrent active malignancy other than non‐metastatic skin cancer or early‐stage chronic lymphocytic leukemia. Diagnostic criteria: biopsy‐confirmed prostate adenocarcinoma |
|
| Interventions |
Number of study centers: 1 Run‐in period: not stated Extension period: no Experimental arm: 102/111 participants Conventional arm: 104/111 participants Complex interventions: hypofractionation: 72 Gy in 30 fractions Conventional: 75.6 Gy in 42 fractions (see Table 8) |
|
| Outcomes | Late GI and GU toxicity and dosimetric parameters Primary outcomes
Secondary outcomes
Complete outcome measures reported: yes |
|
| Funding sources | Not stated | |
| Declarations of interest | Not stated | |
| Notes | Technique: highly conformal radiation therapy IGRT: daily prostate localization for treatment alignment was primarily performed with B‐mode acquisition and targeting ultrasound; daily kV imaging with fiducial alignment was permitted in the later years of the trial. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "that randomized men…", page 1075. Methods, paragraph 1. Comment: no details given with respect to sequence generation, so deemed at unclear risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | No details given regarding allocation concealment, so we deemed this at unclear risk of bias. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Not mentioned, probably not done, so we deemed this at unclear risk of bias
|
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | No mention of blinding, we deemed this at low risk of bias
|
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk |
Quote from publication: "Physician‐reported toxicity was prospectively evaluated during treatment and at each follow‐up visit. After the completion of radiation therapy, according to the protocol, the men were seen at least every 6 months for the first 2 years and then annually thereafter. Late genitourinary (GU) and gastrointestinal (GI) toxicity, starting 90 days after the completion of radiation therapy, was analysed in this study with the use of modified Radiation Therapy Oncology Group (RTOG) toxicity grading (Table E1, available online at www.redjournal.org) … The self‐reported baseline American Urologic Association‐International Prostate Symptom Score (AUA‐IPSS) … recorded at consultation was obtained from the patients' charts", page 1076. Toxicity evaluation. Comment: blinding of participants and personnel not mentioned, probably not done. The toxicity was scored by unblinded physicians, so, despite the prespecified assessment schedule and the use of a validated scoring system modified RTOG toxicity grading, as well as the use of self‐reported symptom scores, meant we deemed this at unclear risk of bias. The following outcomes were deemed at unclear risk of bias
|
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Blinding not mentioned, but
|
| Incomplete outcome data (attrition bias) | Low risk | Attrition and exclusions detailed by study arm, with reasons given (Figure 1, page 1077), but the final efficacy report (in abstract form) did not report the number of men analyzed, we deemed this domain at unclear risk of bias. Number of participants reported on by outcome: PC‐SS: not reported in usable form Late EORTC/RTOG GI RT toxicity: reported in 206/206 participants, low risk of attrition bias Late EORTC/RTOG GU RT toxicity: reported in 206/206 participants, low risk of attrition bias OS: reported in 104/111 (94%) randomized participants in experimental arm and 102/111 (92%) randomized participants in conventional arm DM‐FS: reported in 206/206 participants BR‐FS: reported in 104/111 (94%) in experimental arm and 102/111 (92%) in conventional arm Acute EORTC/RTOG GI RT toxicity: not reported in usable form Acute EORTC/RTOG GU RT toxicity: not reported in usable form Second malignancy: not reported Quality of life: self‐reported bowel quality of life: 185/206 (89%), low risk of attrition bias self‐reported bladder quality of life: 185/206 (89%), low risk of attrition bias self‐reported sexual quality of life: 185/206 (89%), low risk of attrition bias |
| Selective reporting (reporting bias) | Unclear risk | Outcomes specified: physician reported acute and late radiation GU and GI toxicity, dosimetric evaluation. Outcomes reported: late GI and GU toxicity and dosimetric correlation with toxicity, failure (biochemical, clinical and initiation of AD), and OS. We did not have access to the study protocol. We deemed this domain at unclear risk of bias. |
| Other bias | Low risk | We did not identify other sources of bias, so judged this domain at low risk of bias. |
Norkus 2009.
| Methods |
Parallel RCT Setting: tertiary cancer center Lithuania Dates: 2004 to not reported Follow‐up: minimum 12 months Randomization ratio: 1:1 CCT |
|
| Participants | 91 men Inclusion criteria: men with localized prostate cancer (T1–3A), low‐to‐intermediate risk with risk of seminal vesicle or pelvic lymph node (or both) involvement of < 15% (Partin's nomograms and Roach formula). Exclusion criteria: neoadjuvant AD therapy or surgical castration prior to surgery Diagnostic criteria: not stated |
|
| Interventions |
Number of study centers: 1 Run‐in period: not stated Extension period: no Experimental arm: 47/47 participants Conventional arm: 44/44 participants Complex interventions: Experimental arm: 57 Gy in 17 fractions over 3.5 weeks +4 fractions of 4.5 Gy Conventional arm: 74 Gy in 37 fractions over 7.5 weeks (see Table 8) |
|
| Outcomes |
Primary outcome
Secondary outcomes
Assessed weekly for 12 weeks from start of treatment for acute toxicity, with PSA tested every 3 months during the first year, then every 6 months thereafter. Complete outcome measures reported: yes (FFBR reported at 12 months' follow‐up)/no other outcomes reported |
|
| Funding sources | Not stated | |
| Declarations of interest | Not stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "The protocol was designed to randomize patients … ", page 470. Material and methods, paragraph 1. Comment: this domain deemed at unclear risk of bias because no details provided. |
| Allocation concealment (selection bias) | Unclear risk | No details provided with respect to allocation concealment, therefore, this domain deemed at unclear risk of bias. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Not mentioned, probably not done. We deemed this domain at unclear risk of bias
|
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Not mentioned, probably not done. We deemed this domain at unclear risk of bias.
|
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk |
Quote from publication: "Patients were evaluated weekly for 12 weeks starting from the beginning of irradiation. PSA tests were performed every 3 months during the first year after irradiation and every 6 months later. Descriptive statistics were used to characterize the patient age, disease stage", page 470. Materials and methods, paragraph 4. Comment: although there was no mention of blinding, the prespecified protocol for PSA testing would mean this domain was at low risk of bias. PC‐SS was deemed at low risk of bias (not a compound endpoint) (see Table 2). No details given with respect to blinding of outcome assessors for the subjective outcomes of acute and late toxicity. No details given about the method used to score toxicity. We deemed the following outcomes at unclear risk of bias.
|
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Blinding not mentioned, deemed at low risk of bias
|
| Incomplete outcome data (attrition bias) | Unclear risk | There was no information provided about attrition, and it was unclear if this was an interim report for an ongoing study, or the included 91 men represented the entire cohort. We deemed this domain at unclear risk of bias. Number of participants reported on by outcome PC‐SS: not reported in usable form Late EORTC/RTOG GI RT toxicity: not reported in usable form Late EORTC/RTOG GU RT toxicity: not reported in usable form OS: reported in 91/91 participants DM‐FS: not reported in usable form BR‐FS: not reported in usable form Acute EORTC/RTOG GI RT toxicity: not reported in usable form Acute EORTC/RTOG GU RT toxicity: not reported in usable form Second malignancy: not reported Quality of life: not reported in usable form |
| Selective reporting (reporting bias) | Unclear risk |
Quote from publication: "preliminary report", page 469. Abstract The outcomes detained in the paper comprise: OS, FFBR, PSA response, acute and late toxicity. Outcomes reported (at 12 months' follow‐up): deaths, biochemical relapse. Comment: as this is a preliminary report, we deemed this domain at unclear risk of bias. |
| Other bias | Low risk | We found no evidence of other sources of bias. |
PROFIT 2016.
| Methods |
Parallel RCT Setting: cancer centers in Australia, Canada and France Dates: May 2006–November 2011 Follow‐up: median 6.0 years (range 4.5–10.0 years) Randomization ratio: 1:1 Non‐inferiority design: 1‐sided confidence interval |
|
| Participants | 1206 men Inclusion criteria: T1–2a, Gleason score < 6, PSA 10.1–20.0 ng/mL; T2b‐c Gleason < 6, PSA ≤ 20.0 ng/mL; T1–2, Gleason 7, PSA ≤ 20.0 ng/mL (intermediate risk) Exclusion criteria: metastatic disease to lymph nodes, bone or lung; prostate cancer diagnosed > 6 months prior to randomization, previous prostate cancer therapy (biopsy and transurethral resection permitted), > 12 weeks AD, any other active malignancy, RT plan did not meet dose constraints, previous pelvic RT and inflammatory bowel disease Diagnostic criteria: histologic diagnosis of carcinoma of the prostate within 6 months of entry |
|
| Interventions |
Number of study centers: 27 in Canada, Australia and France Run‐in period: not stated Extension period: no Experimental arm: 608/608 participants Conventional arm: 598/598 participants Complex interventions:
|
|
| Outcomes |
Primary outcomes
Secondary outcomes
Complete outcome measures reported: yes |
|
| Funding sources | Canadian Institutes for Health Research | |
| Declarations of interest | Not stated | |
| Notes | highly conformal radiation therapy, non‐inferiority study. Quality assurance: real‐time radiation quality assurance was performed. No AD was used. MCT‐78776 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "a computer‐generated randomization schedule assigned patients to either …", page 2, paragraph 4. Comment: we deemed this domain at low risk of bias. |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "Randomization was performed centrally through the Ontario Clinical Oncology Group coordinating centre …", 2107, page 2, paragraph 5. Comment: we deemed this domain at low risk of bias. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Not mentioned, probably not done
|
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Not mentioned, probably not done, deemed at low risk of bias
|
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk |
Quote from publication: "Patients were followed according to a prescribed schedule. Toxicity assessments were performed weekly during RT followed by telephone assessments to week 14 and again at follow‐up visits scheduled every 6 months after random assignment; PSAs were collected starting with the 6‐month post‐random assignment visit; and health‐related quality of life (HRQoL) assessments were performed at baseline, 24 months, and 48 months after random assignment". Comment: blinding not mentioned, probably not done.
|
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Blinding not mentioned, but not the lack of blinding was not thought a source of bias for
|
| Incomplete outcome data (attrition bias) | Low risk | 1206/1206 men randomized were reported on, so we deemed this domain at low risk of bias. Number of participants reported on by outcome PC‐SS: 1206/1206 Late EORTC/RTOG GI RT toxicity: 1206/1206, low risk of bias Late EORTC/RTOG GU RT toxicity: 1206/1206, low risk of bias OS: 1206/1206, low risk of bias DM‐FS: not reported in usable form BR‐FS: 1206/1206 Acute EORTC/RTOG GI RT toxicity: 1206/1206, low risk of bias Acute EORTC/RTOG GU RT toxicity: 1206/1206, low risk of bias Second malignancy: not reported Quality of life: not reported in usable form |
| Selective reporting (reporting bias) | Low risk |
Outcomes specified: biochemical or clinical failure (composite endpoint comprised of BCF, local or distant recurrence, death from any cause), acute and late toxicity, quality of life, OS Outcomes reported: BCF, OS, acute and late GI and GU toxicity and quality of life. We deemed this domain at low risk of bias. |
| Other bias | Low risk | The study steering committee requested that definition of PSA failure be changed from ASTRO to Phoenix, this request was made without knowledge of the outcome data (since almost all contemporary prostate hypofractionation studies have used the Phoenix definition) and the necessity for a change in PSA failure definition had been foreshadowed in the original protocol. We deemed this domain at low risk of bias. |
Yeoh 2011.
| Methods |
Parallel RCT Setting: tertiary cancer center, Australia Dates: July 1996–August 2003 Follow‐up: median 90 months (range 3–138 months) Randomization ratio: 1:1 Superiority design CCT |
|
| Participants |
217 men Inclusion criteria: T1–2N0M0 prostate cancer Exclusion criteria: PSA > 80 ng/mL, AD Diagnostic criteria: not stated |
|
| Interventions |
Number of study centers: 1 Run‐in period: not stated Extension period: no Experimental arm: 108/108 participants Conventional arm: 109/109 participants Complex interventions:
|
|
| Outcomes |
Primary outcomes
Secondary outcomes
Complete outcome measures reported: yes (late GI/GU toxicity, FFBR, OS) |
|
| Funding sources | Not stated | |
| Declarations of interest | Not stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "Patient randomization was done using blocked computer generated numbers", page 1272, 2011. Comment: we deemed this at low risk of bias |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "randomization was … administered by data managers", page 1272, 2011. Comment: we deemed this concealed, so at low risk of bias |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Blinding not mentioned, probably not done, we deemed this domain at unclear risk of bias.
|
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Blinding not mentioned, probably not done, we deemed this domain at low risk of bias.
|
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | There was no mention of blinding of outcome assessors for subjective outcomes (toxicity), even though there was a validated scoring tool used, we considered this domain at unclear risk of bias.
|
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Although there was no mention of blinding, and it was probably not done, the prespecified timing of PSA measurement would make the assessment of biochemical relapse not subject to bias, so this we deemed at low risk of bias.
|
| Incomplete outcome data (attrition bias) | High risk | Number of participants reported on by outcome PC‐SS: not reported Late EORTC/RTOG GI RT toxicity: not reported using this scale Late EORTC/RTOG GU RT toxicity: not reported using this scale Quote from publication: "Of the 217 patients, 101 had been lost to follow‐up and 65 had < 5 years of follow‐up, mainly because advancing age and ill health associated with medical co‐morbidities and domicile distant from the centres equipped with the resources for proper evaluation of their disease prevented travel", page 1273, 2011. Comment: we deemed this domain at high risk of bias. OS: reported in 116/217 randomized participants DM‐FS: not reported Quote from publication: "2 year PSA data available for 208 (96%) of patients … after 2 years, 76 patients had elected to attend elsewhere and 35 patients had died (31 of these died of causes unrelated to prostate cancer … 5‐year PSA … were available in 96 of the 217 patients)", page 1076. Results, paragraph 1. BR‐FS: reported in 96/217 randomized participants Acute EORTC/RTOG GI RT toxicity: not reported Acute EORTC/RTOG GU RT toxicity: not reported Second malignancy: not reported Quality of life: not reported |
| Selective reporting (reporting bias) | Unclear risk | Outcomes specified: acute and late GI and GU toxicity Outcomes reported: acute and late GI and GU toxicity, biochemical failure, sites of clinical relapse and OS. We did not have access to the study protocol. We deemed this domain at unclear risk of bias. |
| Other bias | Low risk | The study stopped early. Quote from publication: "because of full implementation of 3D CRT at the institution", page 1073, paragraph 3. Comment: we did not consider this a source of other bias. |
3DCRT: three‐dimensional conformal radiation therapy; AD: androgen deprivation; AJCC: American Joint Committee on Cancer; BCDF: biochemical or clinical disease failure, or both; BCF: biochemical or clinical failure; BR‐FS: biochemical relapse‐free survival; CCT: controlled clinical trial; CT: computer tomography; D‐FS: disease‐free survival; DM: distant metastases; DM‐FS: distant metastases‐free survival; EORTC: European Organisation for Research and Treatment of Cancer; EPIC: Expanded Prostate Cancer Index Composite; FACT‐P: Functional Assessment of Cancer Therapy – Prostate (Esper 1997); FFBR: freedom from biochemical relapse; GI: gastrointestinal; GU: genitourinary; IGRT: image‐guided radiation therapy; IMRT: intensity‐modulated radiation therapy; M‐FS: metastasis‐free survival; n: number of participants; OS: overall survival; PC‐SS: PC‐SS; PS: performance scale; PSA: prostate‐specific antigen; PTV: planning target volume; R‐FS: relapse‐free survival; RCT: randomized controlled trial; RT: radiation therapy; RTOG: Radiation Therapy Oncology Group; TURP: transurethral resection of the prostate; UCLA‐PCI: University of California, Los Angeles Prostate Cancer Index (Litwin 1998); WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| MRC RT01 | Not a comparison of fraction size |
| NCT01230866 | Protons used: ineligible intervention |
| NCT01444820 | Pelvic nodal irradiation used: ineligible intervention |
| NCT01794403 | Compared 2 different hypofractionated regimens |
| NCT02303327 | Conventional arm used brachytherapy: ineligible intervention |
| Norkus 2013 | Pelvic nodal irradiation used: ineligible intervention |
Characteristics of studies awaiting assessment [ordered by study ID]
El‐Ghamrawi 2015.
| Methods | Randomized controlled trial |
| Participants | 20 men with prostate cancer |
| Interventions | Hypofraction: 65–67.5 Gy in 25 fractions over 5 weeks Conventional: 74–78 Gy in 2 Gy/fraction |
| Outcomes |
|
| Notes | Dates: July 2012–December 2013 |
Felix 2012.
| Methods |
Parallel RCT Setting: Mexico Dates: January 2008–June 2011 Follow‐up: median 17 months (range 6–42 months) Randomization ratio: 1: 1 CCT: design not specified |
| Participants | 202 men Inclusion criteria
Exclusion criteria: no details provided Diagnostic criteria: prostate cancer |
| Interventions |
Number of study centers: 1 Run‐in period: not stated Extension period: not stated Complex interventions Hypofractionation arm: 65.75 Gy in 25 fractions Conventional arm: 73.8 Gy in 41 fractions |
| Outcomes |
Primary outcomes
Secondary outcomes: not stated Complete outcome measures reported: yes |
| Notes |
ISRCTN45905321.
| Methods | Phase III randomized open multicenter trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions | Hypofractionated: RT given working‐days with 7 fractions of 6.1 Gy, i.e. total 42.7 Gy. Total treatment time 15–19 days. Treatment given every other weekday, always including 2 weekends. Conventional: radiation therapy given daily (5 days/week) with 39 fractions of 2.0 Gy, i.e. total 78.0 Gy. Total treatment time 53–55 days. Maximum allowed treatment days 65. |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Notes | Dates: 1 July 2005–30 June 2015 Funder: Nordic Cancer Union (Nordiska Cancerunionens [NCU]) (Sweden) |
Spagnoletti 2011.
| Methods |
Parallel RCT Setting: Italy Dates: September 2008–July 2009 Follow‐up: median 25 months Randomization ratio: 1:1 |
| Participants | 40 men Inclusion criteria: cTl‐T2N0M0 prostate cancer Exclusion criteria: not reported Diagnostic criteria: not reported |
| Interventions |
Number of study centers: not stated Run‐in period: not stated Experimental: number of participants not reported (in abstract form) Conventional: number of participants not reported (in abstract form) Complex interventions: Hypofractionation: 72–78 Gy in 36–39 fractions within 7–8 weeks Conventional: 64.8–70.2 Gy in 24–26 fractions within 4‐6 weeks |
| Outcomes |
Primary outcomes Acute and late RTOG GI and GU toxicity Complete outcome measures reported: yes (acute and late RTOG GI and GU toxicity) |
| Notes | Technique: 3DCRT High‐risk men had two months AD Risk stratification based on Partin table |
3DCRT: three‐dimensional conformal radiation therapy; AD: androgen deprivation; CCT: controlled clinical trial; GI: gastrointestinal; GU: genitourinary; PSA: prostate‐specific antigen; RCT: randomized controlled trial; RT: radiation therapy; TNM; tumor, node and metastasis; UICC: Union for International Cancer Control; WHO: World Health Organization.
Characteristics of ongoing studies [ordered by study ID]
NCT01411332.
| Trial name or title | A phase III trial of hypofractionated external beam image‐guided highly targeted radiotherapy: the HEIGHT trial |
| Methods | RCT |
| Participants | Men ages 35–85 years No healthy volunteers Inclusion criteria
Exclusion criteria
|
| Interventions | Hypofractionation: hypofractionated targeted highly conformal radiation therapy Dose escalation to the dynamic contrast enhanced MRI‐defined dominant region(s) by dose painting at 2.35 Gy per fraction, while the rest of the CTV receives 2.0 Gy a fraction to 76 Gy. The hypofractionated targeted boost region will receive an absolute dose of 89.3 Gy. Assuming an α/β ratio of 3.0, this would be equivalent to 95.5 Gy in 2.0 Gy fractions. Conventional: standard highly conformal radiation therapy Total dose of 80 Gy will be delivered in 40 fractions to the CTV. |
| Outcomes | Primary outcomes
Secondary outcome
|
| Starting date | May 2011 |
| Contact information | apollack@med.miami.edu |
| Notes | HEIGHT Alan Pollack, University of Miami |
NCT01584258.
| Trial name or title | International randomized study of laparoscopic prostatectomy vs stereotactic body radiotherapy (SBRT) and conventionally fractionated radiotherapy vs SBRT for early stage organ‐confined prostate cancer |
| Methods | Phase III RCT |
| Participants | Inclusion criteria: all the following criteria are mandatory for inclusion.
Exclusion criteria: 1 of the following criteria is sufficient for exclusion:
|
| Interventions | Comparison: 1) laparoscopic prostatectomy vs SBRT 2) conventionally fractionated RT vs prostate SBRT Participants for whom surgery is not considered or who refuse surgery will be randomized to either conventionally fractionated RT delivered to 78 Gy in 2 Gy fractions or SBRT delivered with 36.25 Gy in 5 fractions. |
| Outcomes | Primary outcomes
Secondary outcomes:
|
| Starting date | April 2012 |
| Contact information | Pace‐icrctsu@icr.ac.uk |
| Notes | PACE Peter Ostler, MD Mount Vernon Cancer Centre, UK |
NCT02300389.
| Trial name or title | Randomized, multi‐center clinical trial comparing hypofractionated radiotherapy boost to conventionally Fractionated in a high risk group of prostate cancer patients (HYPOPROST) |
| Methods | RCT |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Hypofractionated: radiation: hypofractionated highly conformal radiation therapy boost RT. All participants are irradiated to 46 Gy in 2 Gy fractions to the whole pelvis, seminal vesicles and prostate gland (phase I) and then the boost dose is limited to the prostate gland with some part of seminal vesicles with hypofractionated dose of 7.5 Gy in 2 fractions (phase II) to a total dose of 61 Gy. Additionally all participants receive neoadjuvant AD therapy (3–4 months prior starting RT) and during RT and during the follow‐up to 24 months. Conventional: radiation: conventional fractionated IMRT boost RT. All participants are irradiated to 46 Gy in 2 Gy fractions to the whole pelvis, seminal vesicles and prostate gland (phase I) and then the boost dose is limited to the prostate gland with some part of seminal vesicles with conventional fractionated dose of 2 Gy in 15 fractions (phase II) to the total dose of 76 Gy. Additionally all participants received neoadjuvant AD therapy (3–4 months prior starting RT) and during RT and during the follow‐up to 24 months. |
| Outcomes | Primary outcomes
Secondary outcomes
|
| Starting date | December 2011 |
| Contact information | Piotr Milecki |
| Notes |
NCT02934685.
| Trial name or title | A phase III randomized study of hypofractionated image‐guided volumetric modulated arc radiotherapy (IG‐VMAT) versus conventionally fractionated IG‐VMAT in patients with localized prostate cancer |
| Methods | RCT |
| Participants | Men ages 50–79 years Inclusion criteria
Exclusion criteria
|
| Interventions | Hypofractionation: 70 Gy in 28 fractions over 5.6 weeks Conventional: 80 Gy in 40 fractions over 8 weeks |
| Outcomes | Primary outcome
Secondary outcomes
|
| Starting date | 2016 |
| Contact information | Gaofing Li, Director of Radiotherapy Department, Beijing Hospital; drzhongqiuzi@163.com |
| Notes |
AD: androgen deprivation; AJCC: American Joint Committee on Cancer; CSS: cause‐specific survival; CT: computer tomography; CTCAE: National Cancer Institute Common Toxicity Criteria for Adverse Events; CTV: clinical target volume; DWI: diffusion weighted imaging; ECOG: Eastern Cooperative Oncology Group; IMRT: intensity‐modulated radiation therapy; KPS: Karnofsky Performance Status; LFT: liver function test; MRI: magnetic resonance imaging; NCI: National Cancer Institute; OS: overall survival; PSA: prostate‐specific antigen; RCT: randomized controlled trial; RT: radiation therapy; SBRT: stereotactic body radiation therapy; SD: standard deviation; TURP: transurethral resection of the prostate.
Differences between protocol and review
We made the following changes from the published protocol (Soh 2015).
We initially specified sexual dysfunction as part of the second primary outcome, but have reported it as part of the quality of life endpoint.
We chose late gastrointestinal toxicity as an outcome for the 'Summary of findings' table as this is the late toxicity of greatest clinical relevance to both men with prostate cancer treated with radiation therapy and clinicians.
For greater clarity around the definition of the event of interest, the time to event outcomes have been renamed to time‐to‐death from prostate cancer, time to death from any cause, time to metastases and time to biochemical relapse.
We performed post‐hoc subgroup analysis based on the use of androgen deprivation (in response to peer reviewer suggestion), we added post‐hoc subgroup analysis based on radiation therapy technique.
We changed our secondary outcome measures from late gastrointestinal and genitourinary radiation therapy toxicity and second malignancy to late gastrointestinal radiation therapy toxicity, our third primary outcome measure to late genitourinary radiation therapy toxicity and reported late genitourinary and second malignancy as secondary outcomes.
We had initially included costs as a secondary outcome but deleted this outcome from the review based on guidance by the editorial group.
We did not handsearch American Society of Clinical Oncology (ASCO) and American Society for Radiation Oncology (ASTRO) as they are included in Embase.
We changed the title (in response to editorial suggestion) from Altered radiation fractionation schedules for clinically localized prostate cancer.
We re‐named the outcome from disease‐specific survival to prostate cancer‐specific survival.
Contributions of authors
BH: edited the ’Background’ and ’Methods’ and provided methodologic support, general advice, led the data analysis and secured funding.
MLJ: provided general and methodologic advice and wrote the 'Background" and "Methods".
TD: provided clinical input.
FS: conceived and designed the review, and assisted with the ’Background’ and ’Methods’.
MJ: conceived and designed the review, and wrote the ’Background’ and ’Methods’.
Sources of support
Internal sources
-
Princess Alexandra Cancer Collaborative Group, Australia.
Financial support for a data manager and handsearching was provided
External sources
No sources of support supplied
Declarations of interest
BH: received financial support from the Princess Alexandra Cancer Collaborative Group (PAH CCG), which funded a research assistant for handsearching and data management.
MLJ: none.
TD: none.
FYS: none.
MJ: none.
Edited (no change to conclusions)
References
References to studies included in this review
Arcangeli 2010 {published data only}
- Arcangeli G, Fowler J, Gomellini S, Arcangeli S, Saracino B, Petrongari M, et al. Acute and late toxicity in a randomized trial of conventional versus hypofractionated three‐dimensional conformal radiotherapy for prostate cancer. International Journal of Radiation Oncology, Biology, Physics 2011;79(4):1013‐21. [DOI] [PubMed] [Google Scholar]
- Arcangeli G, Fowler J, Gomellini S, Arcangeli S, Saracino B, Petrongari MG, et al. A phase III randomized study of high‐dose conventional versus hypofractionated radiotherapy in patients with high‐risk prostate cancer. International Journal of Radiation Oncology, Biology, Physics 2009;75(3):S79. [Google Scholar]
- Arcangeli G, Saracino B, Arcangeli S, Gomellini S, Petrongari MG, Sanguinetti G, et al. Moderate hypofractionation in high‐risk, organ‐confined prostate cancer: final results of a phase III randomised controlled trial. Journal of Clinical Oncology 2017;35(17):1891‐7. [DOI: 10.1200/JCO.2016.70.4189] [DOI] [PubMed] [Google Scholar]
- Arcangeli G, Saracino B, Gomellini S, Petrongari M, Arcangeli G, Sentinelli S, et al. A prospective phase III randomized trial of hypofractionation versus conventional fractionation in patients with high‐risk prostate cancer. International Journal of Radiation Oncology, Biology, Physics 2010;78(1):11‐8. [DOI] [PubMed] [Google Scholar]
- Arcangeli S, Strigari L, Gomellini S, Saracino B, Petrongari M, Pinnaro P, et al. Updated results and pattern of failure in a randomized hypofractionation trial for high‐risk prostate cancer. International Journal of Radiation Oncology, Biology, Physics 2012;84(3):S147. [DOI] [PubMed] [Google Scholar]
- Arcangeli S, Strigari L, Gomellini S, Saracino B, Petrongari MG, Pinnaro P, et al. Updated results and patterns of failure in a randomized hypofractionation trial for high‐risk prostate cancer. International Journal of Radiation Oncology, Biology, Physics 2012;84(5):1172‐8. [DOI] [PubMed] [Google Scholar]
- Arcangeli S, Strigari L, Soete G, Meelleer G, Gomellini S, Fonteyne V, et al. Clinical and dosimetric predictors of acute toxicity after a 4‐week hypofractionated external beam radiotherapy regimen for prostate cancer: results from a multicentric prospective trial. International Journal of Radiation Oncology, Biology, Physics 2009;73(1):39‐45. [DOI] [PubMed] [Google Scholar]
- Giordano C, Ferraro AM, Saracino B, Petrongari MG, Arcangeli G, Landoni V, et al. Hypofractionated versus conventionally fractionated 3DCRT for high‐risk prostate cancer: updated results of a randomized trial. Anticancer Research 2015;35(6):3615‐3740. [Google Scholar]
- Landoni V, Arcidiacono F, Saracino BM, Farneti A, Petrongari MG, Sanguineti G. Macroscopic hematuria after conventional or hypofractionated radiation therapy: results from a prospective phase 3 study. International Journal of Radiation Oncology, Biology, Physics 2015;93:S200. [DOI] [PubMed] [Google Scholar]
- Marzi S, Saracino B, Petrongari MG, Arcangeli S, Gomellini S, Arcangeli G, et al. Modeling of alpha/beta for late rectal toxicity from a randomized phase II study: conventional versus hypofractionated scheme for localized prostate cancer. Journal of Experimental Clinical Cancer Research 2009;28(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguineti G, Arcidiacono F, Landoni V, Saracino BM, Farneti A, Arcangeli S, et al. Macroscopic hematuria after conventional or hypofractionated radiation therapy: results from a prospective phase 3 study. International Journal of Radiation Oncology, Biology, Physics 2016;96(2):304‐12. [DOI: 10.1016/j.ijrobp.2016.05.017] [DOI] [PubMed] [Google Scholar]
- Saracino B, Arcangeli G, Strigari L, Petrongari M, Gomellini S, Giordano C. Hypo versus conventionally fractionated 3DCRT for high risk prostate cancer: updated results of a randomized trial. International Journal of Radiation Oncology, Biology. Physics 2014;90(1):S53. [Google Scholar]
- Strigari L, Arcangeli G, Arcangeli G, Benassi M. Mathematical model for evaluating incidence of acute rectal toxicity during conventional or hypofractionated radiotherapy courses for prostate cancer. International Journal of Radiation Oncology, Biology, Physics 2009;73(5):1454‐60. [DOI] [PubMed] [Google Scholar]
CHHiP 2016 {published data only}
- CHHiP. Correction to Lancet Oncol 2016; 17: 1055. Lancet Oncology 2016;17(8):e321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHHiP Trial Management Committee. Conventional or hypofractionated high dose intensity modulated radiotherapy for prostate cancer protocol version 9.2. www.thelancet.com/cms/10.1016/S1470‐2045(11)70293‐5/attachment/69c8416a‐a567‐43a2‐ab17‐5926fc5cc8b6/mmc1.pdf (accessed 10 March 2019).
- Dearnaley D, Griffin CL, Syndikus I, Scrase C, Thomas S, Naismith O. IGRT for prostate cancer: results from the CHHiP IGRT phase II sub‐study. Radiotherapy and Oncology 2014;19(1):S59. [Google Scholar]
- Dearnaley D, Syndikus E, Mossop H, Khoo V, BIrtle A, Bloomfield D, et al. Conventional versus hypo‐fractionated high‐dose intensity modulated radiotherapy for prostate cancer: 5‐year outcomes of the randomised, non‐inferiority, phase 3 CHHiP trial. Lancet Oncology 2016;17(8):1047‐60. [DOI: 10.2016/S1470-2045(16)30102-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearnaley D, Syndikus I, Mossop H, Birtle A, Bloomfield D, Cruickshank C, et al. 5 year outcomes of a phase III randomised trial of conventional or hypofractionated high dose intensity modulated radiotherapy for prostate cancer (CRUK/06/016): report from the CHHiP Trial Investigators Group. European Journal of Oncology 2015;51:S712. [Google Scholar]
- Dearnaley D, Syndikus I, Mossop H, Khoo V, Birtle A, Bloomfield D, et al. on behalf of the CHHiP Investigators. Supplement to conventional versus hypofractionated high‐dose intensity‐modulated radiotherapy for prostate cancer: 5‐year outcomes of the randomised, non‐inferiority, phase 3 CHHiP trial. Lancet Oncology 2016;17(8):1047‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearnaley D, Syndikus I, Sumo G, Bidmead M, Bloomfield D, Clark C, et al. (ISRCTN47772397) The early toxicity of escalated versus standard dose conformal radiotherapy with neo‐adjuvant androgen suppression for patients with localised prostate cancer: results from the MRC RT01 trial. Radiotherapy and Oncology 2007;83(1):31‐41. [DOI] [PubMed] [Google Scholar]
- Dearnaley D, Syndikus I, Sumo G, Bidmead M, Bloomfield D, Clark C, et al. Conventional versus hypofractionated high‐dose intensity‐modulated radiotherapy for prostate cancer: preliminary safety results from the CHHiP randomised controlled trial. Lancet Oncology 2012;13(1):43‐54. [DOI] [PubMed] [Google Scholar]
- Dearnaley DP, Hall E, Lawrence D, Huddart RA, Eeles R, Nutting CM, et al. Phase III pilot study of dose escalation using conformal radiotherapy in prostate cancer: PSA control and side effects. British Journal of Cancer 2005;92(3):488‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearnaley DP, Naismith O, Sumo G. A dosimetry comparison of forward‐ and inverse‐planned IMRT for prostate cancer in the CHHiP trial. Radiotherapy and Oncology 2011;99 S1:S585. [Google Scholar]
- Dearnaley on behalf of the CHHiP Trial Management Group. CHHiP (Conventional of Hypofractionated High‐dose intensity‐modulated radiotherapy for Prostate cancer) (CRUK/06/016). International Journal of Radiation Oncology, Biology, Physics 2016;96:S2. [Google Scholar]
- ISRCTN97182923. A randomised phase III multi‐centre trial of Conventional or Hypofractionated High dose Intensity modulated radiotherapy for Prostate cancer. www.isrctn.com/ISRCTN97182923 (first received 9 September 2005). [DOI 10.1186/ISRCTN97182923]
- Khoo VS, Dearnaley DP. Question of dose, fractionation and technique: ingredients for testing hypofractionation in prostate cancer – the CHHiP trial. Clinical Oncology (Royal College of Radiologists (Great Britain)) 2008;20(1):12‐4. [DOI] [PubMed] [Google Scholar]
- NCT00392535. Conventional or Hypofractionated High Dose Intensity Modulated Radiotherapy for Prostate Cancer: CHHIP. clinicaltrials.gov/ct2/show/NCT00392535 (first received 26 October 2006).
- Naismith O, Dearnaley DP, Hall E. A survey of the benefits to RT processes and techniques of participating in the CHHiP trial. Radiotherapy and Oncology 2011;99 S1:S585. [Google Scholar]
- Staffurth J, Haviland J, Wilkins A, Syndikus I, Khoo V, Bloomfield D, et al. Impact of prostate cancer hypofractionation on patient reported outcomes: baseline to 5 years change in the CHHIP trial. International Journal of Radiation Oncology, Biology, Physics 2018;102:S1. [Google Scholar]
- Wilkins A, Mossop H, Griffin C, Dearnaley D, Hall E, on behalf of the CHHiP Trial Management Group. Patient reported outcomes of overall bowel and urinary bother in the CHHiP trial (CRUK: 8262/A7257). Radiotherapy and Oncology 2015;115:S355. [Google Scholar]
- Wilkins A, Mossop H, Syndikus I, Khoo V, Bloomfield D, Parker C, et al. Hypofractionated radiotherapy versus conventionally fractionated radiotherapy for patients with intermediate‐risk localised prostate cancer: 2‐year patient‐reported outcomes of the randomised, non‐inferiority, phase 3 CHHiP trial. Lancet Oncology 2015;16(16):1605‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins AC, Gusterson B, Szijgyarto Z, Haviland J, Griffin C, Stuttle C, et al. Ki67 is an independent predictor of recurrence in the largest randomized trial of 3 radiation fractionation schedules in localized prostate cancer. www.ncbi.nlm.nih.gov/m/pubmed/29559283/ (accessed 8 April 2018). [DOI: 10.1016/j.ijrobp.2018.01.072] [DOI] [PMC free article] [PubMed]
- Wilson JM, Dearnaley DP, Syndikus I, Khoo V, Birtle A, Bloomfield D, et al. The efficacy and safety of conventional and hypofractionated high‐dose radiation therapy for prostate cancer in an elderly population: a subgroup analysis of the CHHiP trial. International Journal of Radiation Oncology, Biology, Physics 2018;100(5):1179‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fox Chase 2013 {published data only}
- Avkshtol V, Li T, Hallman MA, Greenberg R, Price RA, Uzzo RG, et al. 10‐year update of a randomized prospective trial of conventional versus hypofractionated radiation therapy for localized prostate cancer. International Journal of Radiation Oncology, Biology, Physics 2018;102:S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean‐Pierre P, Stoyanova R, Penedo F, Antoni M, Abramowitz M, Buyyounouski M, et al. Treatment‐related side effects and quality of life among prostate cancer patients treated with conventional versus hypofractionated intensity mediated radiotherapy: a phase iii hypofractionation trial. International Journal of Radiation Oncology, Biology, Physics 2011;2:S667. [Google Scholar]
- Konski AA, Buyyounouski MK, Stoyanova R, Movsas B, Uzzo R, Horwitz EM, et al. Quality adjusted survival comparing standard versus hypofractionated radiation therapy in the treatment of prostate cancer. International Journal of Radiation Oncology, Biology, Physics 2015;93(3):S118‐9. [Google Scholar]
- NCT00062309. A phase III intensity modulated radiotherapy dose escalation trial for prostate cancer using hypofractionation. clinicaltrials.gov/ct2/show/NCT00062309 (first received 6 June 2003).
- Pollack A, Bae K, Khor LY, Hammond E, Al‐Saleem T Li T, et al. Stability of tumor biomarkers in archival tissue from men treated with radiotherapy for prostate cancer: an analysis of RTOG 92‐02 and Fox Chase randomized trials. International Journal of Radiation Oncology Biology, Physics. 2006; Vol. 66 3S:S365‐S366.
- Pollack A, Buyyounouski TM, Horwitz E, Price R, Feigenberg S, Konski A, et al. Hypofractionation for prostate cancer: interim results of a randomized trial. International Journal of Radiation Oncology, Biology, Physics 2009;75(3):S81. [Google Scholar]
- Pollack A, Hanlon AL, Horwitz EM, Feigenberg SJ, Konksi AA, Movsas B, et al. Dosimetry and preliminary acute toxicity in the first 100 men treated for prostate cancer on a randomized hypofractionation dose escalation trial. International Journal of Radiation Oncology, Biology, Physics 2006;64(2):518‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack A, Walker G, Buyyounouski M, Horwitz E, Price R, Feigenberg S, et al. Five year results of a randomized external beam radiotherapy hypofractionation trial for prostate cancer. International Journal of Radiation Oncology, Biology, Physics 2011;81(2):S1. [Google Scholar]
- Pollack A, Walker G, Horwitz EM, Price R, Feigenberg S, Konski AA, et al. Randomized trial of hypofractionated external‐beam radiotherapy for prostate cancer. Journal of Clinical Oncology 2013;31(3):3860‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack H, Walker G, Horwitz EM, Price K, Feigenberg S, Konski AA, et al. Reply to M.J. Brenner et al and I.R. Vogelius et al. Journal of Clinical Oncology 2014; Vol. 32, issue 17:1853‐4. [DOI] [PubMed]
- Shaikh T, Ruth K, Devarajan K, Zaorsky NG, Hallman MA, Sobczak ML, et al. Dosimetric and clinical predictors of long‐term toxicity in patients undergoing hypofractionated prostate radiation therapy: results from a randomized phase 3 trial. International Journal of Radiation Oncology, Biology, Physics 2016;96:S123. [Google Scholar]
- Shaikh T, Tianyu L, Handorf EA, Johnson ME, Wang LS, Hallman MA, et al. Long‐term patient‐reported outcomes from a phase 3 randomised prospective trial of conventional versus hypofractionated radiation therapy for localized prostate cancer. International Journal of Radiation Oncology, Biology, Physics 2017;97(4):722‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoyanova R, Walker G, Sandler K, Khor L, Horwitz E, Buyyounouski M, et al. Determinants of prostate biopsy positivity 2 years after radiation therapy for men with prostate cancer treated on a randomized trial. International Journal of Radiation Oncology, Biology, Physics 2012;84(3):S60. [Google Scholar]
- Turaka A, Zhu F, Buyyounouski MK, Horwitz D, Watkins‐Bruner D, Konski A, Pollack A. Conventional versus hypofractionated IMRT: results of late GI and GU toxicity and quality of life from a phase III trial. International Journal of Radiation Oncology, Biology, Physics 2010;78:S62. [Google Scholar]
HYPRO Dutch 2016 {unpublished data only}
- Aluwini S, Pos F, Schimmel E, Krol S, Toorn PP, Jager H, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): late toxicity results from a randomised, non‐inferiority, phase 3 trial. Lancet Oncology 2016;17(4):464‐74. [DOI] [PubMed] [Google Scholar]
- Aluwini S, Pos F, Schimmel E, Lin E, Krol S, Toorn PP, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): acute toxicity results from a randomised non‐inferiority phase 3 trial. Lancet Oncology 2015;16(3):274‐83. [DOI] [PubMed] [Google Scholar]
- Aluwini S, Pos F, Lin E, Schimmel E, Krol A, Toorn P, et al. Acute toxicity of the randomized phase III Dutch Hypofractionation trial (HYPRO) for prostate cancer. Radiotherapy and Oncology 2012;103:S84‐5. [Google Scholar]
- Heemsbergen W, Incrocci I, Vens C, Witte M, Aluwini S, Pos F, et al. More acute proctitis symptoms with hypofractionation (3.4Gy) than 2 Gy fractions. Radiotherapy and Oncology 2016;119 S1:S155‐6. [Google Scholar]
- Heemsbergen W, Wortel R, Pos F, Smeenk R, Kro S, Aluwini S, et al. Patient‐reported outcome in the prostate HYPRO trial: gastrointestinal toxicity. Radiotherapy and Oncology 2017;123:S60. [Google Scholar]
- ISRCTN85138529. HYpofractionated irradiation for PROstate cancer: a randomised multicentre phase III study. www.isrctn.com/ISRCTN85138529 (first received 22 November 2006). [DOI: 10.1186/ISRCTN85138529] [DOI]
- Incrocci I, Wortel RC, Alemayehu WG, Aluwini S, Schimmel E, Krol S. Sexual function after hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: results from the randomized phase III HYPRO trial. Journal of Sexual Medicine 2016;13(11):1695‐703. [DOI] [PubMed] [Google Scholar]
- Incrocci L, Wortel RC, Alemayehu WG, Aluwini S, Schimmel E, Krol S, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): final efficacy results from a randomised, multicentre, open‐label, phase 3 trial. Lancet Oncology 2016;17(8):1061‐9. [DOI: 10.1016/S1470-2045(16)30070-5] [DOI] [PubMed] [Google Scholar]
- Incrocci L, Wortel RC, Aluwini S, Schimmel E, Krol AD, Toorn PP. Hypofractionated versus conventionally fractionated radiation therapy for prostate cancer: five‐year oncologic outcomes of the Dutch randomized phase 3 HYPRO trial. International Journal of Radiation Oncology, Biology, Physics 2016;94:1. [Google Scholar]
- Incrocci L, Wortel RC, Aluwini S, Schimmel E, Krol AD, Toorn PP, et al. Hypofractionated versus conventionally fractionated radiation therapy for prostate cancer: five‐year oncologic outcomes of the Dutch randomized phase 3 HYPRO trial. International Journal of Radiation Oncology, Biology, Physics 2015;94(1):1‐2. [Google Scholar]
- Incrocci L, Wortel RC, Aluwini S, Schimmel E, Krol DG, Toorn PP. Hypofractionated versus conventionally fractionated radiation therapy for prostate cancer: five‐year oncologic outcomes of the Dutch randomized phase 3 HYPRO trial. International Journal of Radiation Oncology, Biology, Physics 2015;94 S1:LBA2. [Google Scholar]
- Pos F, Incrocci L, Wortel R, Heide U, Lebesque J, Aluwini S, et al. Anal dose reduction for radiotherapy of prostate cancer does not lead to less rectal incontinence. Radiotherapy and Oncology 2016;S:157. [Google Scholar]
- Pos F, Wortel RC, Heemsbergen WD, Oomen‐de Hoop E, Incrocci L. Health‐related quality of life from the prostate hypofractionation (HYPRO) trial. Radiotherapy and Oncology 2018;127 s1:s26‐s27. [DOI] [PubMed] [Google Scholar]
- Sharfo AW, Dirkx ML, Bijman RG, Schillemans W, Breedveld S, Aluwini S, et al. Late toxicity in HYPRO randomized trial analyzed by automated planning and intrinsic NTCP modelling. Radiotherapy and Oncology 2017;123:S126. [DOI] [PubMed] [Google Scholar]
- Sharfo AW, Maarten LP, Dirkx RG, Schilemans W, Breedveld S, Aluwini S, et al. Late toxicity in the randomized multicenter HYPRO trial for prostate cancer analyzed with automated treatment planning. Radiotherapy and Oncology 2018;128(2):2349‐56. [DOI] [PubMed] [Google Scholar]
- Witte M, Heemsbergen W, Pos F, Vens C, Aluwini S, Incrocci L. Linear‐quadratic modelling of acute rectum toxicity in a prostate hypo‐fractionation trial. Radiotherapy and Oncology 2016;119 S1:S119. [Google Scholar]
- Witte M, Pos F, Incrocci L, Heemsbergen W. Relating dose outside the prostate with freedom from failure in the Dutch HYPRO trial. Radiotherapy and Oncology 2018;127(S1):S258. [Google Scholar]
- Witte M, Pos F, Incrocci L, Heemsbergen W. Relating dose outside the prostate with freedom from failure in the Dutch HYPRO trial. Radiotherapy and Oncology 2018;128:S258. [Google Scholar]
- Wortel R, Incrocci L. Sexual function after hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: results of the prospective, randomized phase 3 randomized phase 3 HYPRO trial. Journal of Urology 2016 4S;195:e626‐7. [DOI] [PubMed] [Google Scholar]
- Wortel R, Incrocci L, Aluwini S, Schimmel E, Krol S, Toorn P, et al. Hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5‐year oncologic outcomes of the Dutch randomized phase 3 HYPRO trial. American Journal of Urology 2016:e142‐e143.
- Wortel RC, Floris JP, Heemsbergen WD, Incrocci L. Sexual function after hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: results from the randomized phase III HYPRO trial. Journal of Sexual Medicine 2016;13:1695‐703. [DOI] [PubMed] [Google Scholar]
- Wortel RC, Heemsbergen WD, Smeenk RJ, Witte MG, SDG, Pos FJ. Local protocol variations for image guided radiation therapy in the multicenter Dutch hypofractionation (HYPRO) trial: impact of rectal balloon and MRI delineation on anorectal dose and gastrointestinal toxicity levels. International Journal of Radiation, Oncology, Physics 2017;99(5):1243‐52. [DOI] [PubMed] [Google Scholar]
- Wortel RC, Incrocci L, Pos FJ, Smeenk RJ, Krol AD, Aluwini S. Patient‐reported outcomes from the phase III prostate HYPRO trial: urinary toxicity. Radiotherapy and Oncology 2017;123:S295. [Google Scholar]
- Wortel RC, Incrocci L, Pos FJ, Heide UA, Lebesque V, Aluwini S. Late side effects after image guided intensity modulated radiation therapy compared to 3D‐conformal radiation therapy for prostate cancer: results from 2 prospective cohorts. International Journal of Radiation Oncology, Biology, Physics 2016;95(2):680‐99. [DOI] [PubMed] [Google Scholar]
- Wortel RC, Oomen‐de Hoop E, Heemsbergen WD, Pos FJ, Incrocci L. Moderate hypofractionation in intermediate and high‐risk, localized prostate cancer: health‐related quality of life from the randomized, phase 3 HYPRO trial. International Journal of Radiation Oncology, Biology, Physics 2019;103(4):823‐33. [DOI] [PubMed] [Google Scholar]
- Wortel RC, Pos FJ, Heemsbergen WD, Incrocci L. Sexual function after hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: results from the randomized phase III HYPRO trial. Journal of Sexual Medicine 2016;13:1695‐703. [DOI] [PubMed] [Google Scholar]
Lee 2016 {unpublished data only}
- Lee RL, Dignam JL, Amin MB, Bruner DW, Low D, Swanson GP, et al. Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low‐risk prostate cancer. Journal of Clinical Oncology 2016;34(20):2325‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RW, Dignam JJ, Amin MB, Bruner DW, Low D, Swanson GP, et al. NRD Oncology RTOG 0415: a randomized phase III non‐inferiority study comparing two fractionation schedules in men with low‐risk prostate cancer. International Journal of Radiation Oncology, Biology, Physics 2016;94 1S:S3. [Google Scholar]
- NCT00331773. A phase III randomized study of hypofractionated 3D‐CRT/MRT versus conventionally fractionated 3D‐CRT/MRT in patients with favorable‐risk prostate cancer. clinicaltrials.gov/ct2/show/NCT00331773 (first received 31 May 2006).
- Watkins Bruner D, Pugh SL, Lee WR, Dignam JJ, Low D, Swanson GP, et al. NRG Oncology/RTOG 0415, Phase 3 noninferiority study comparing 2 fractionation schedules in patients with low‐risk prostate cancer: prostate‐specific quality of life results. International Journal of Radiation Oncology, Biology, Physics 2016;96(2S):S2‐3. [Google Scholar]
Lukka NCIC 2005 {published data only}
- Lukka H, Hayter C, Julian JA, Warde P, Morris WJ, Gospodarowicz M, et al. Randomized trial comparing two fractionation schedules for patients with localized prostate cancer. Journal of Clinical Oncology 2005;23(25):6132‐8. [DOI] [PubMed] [Google Scholar]
- Lukka H, Hayter C, Warde P, Morris J, Julian J, Gospodarowicz M, et al. on behalf of Investigators. A randomized trial comparing two fractionation schedules for patients with localized prostate cancer. International Journal of Radiation Oncology, Biology, Physics 2003;57(2):S126. [DOI] [PubMed] [Google Scholar]
- NCT00201916. A randomized trial of a shorter radiation fractionation schedule for the treatment of localized prostate cancer. clinicaltrials.gov/ct2/show/NCT00201916 (first received 20 September 2005).
MDACC 2014 {published data only}
- Hoffman K, Voong KR, Levy LB, Pugh TJ, Munsell MF, Choi S, et al. Randomized trial of hypofractionated dose‐escalated IMRT vs conventionally fractionated IMRT for localized prostate cancer. International Journal of Radiation Oncology, Biology, Physics 2016;96 2S:S32. [Google Scholar]
- Hoffman KE, Skinner H, Pugh TJ, Voong KR, Levy LB, Choi S, et al. Patient‐reported urinary, bowel, and sexual function after hypofractionated intensity‐modulated radiation therapy for prostate cancer results from a randomized trial. American Journal of Clinical Oncology 2018;41:558‐67. [DOI] [PubMed] [Google Scholar]
- Hoffman KE, Skinner H, Pugh TJ, Voong KR, Levy LB, Choi S, et al. Patient‐reported urinary, bowel, and sexual function after hypofractionated intensity‐modulated radiation therapy for prostate cancer: results from a randomized trial. journals.lww.com/amjclinicaloncology/Abstract/publishahead/Patient_reported_Urinary,_Bowel,_and_Sexual.99020.aspx (accessed 24 May 2017). [DOI: 10.1097/COC.0000000000000325] [DOI] [PubMed]
- Hoffman KE, Voong KR, Levy LB, Allen PK, Choi S, Schlembach PJ, et al. Randomized trial of hypofractionated, dose‐escalated, intensity‐modulated radiation therapy (IMRT) versus conventionally fractionated IMRT for localized prostate cancer. Journal of Clinical Oncology 2018;36(29):2943‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman KE, Voong KR, Pugh TJ, Skinner H, Levy LB Takair V, et al. Risk of late toxicity in men receiving dose‐escalated hypo‐fractionated intensity modulated prostate radiation therapy: results from a randomized trial. International Journal of Radiation Oncology, Biology, Physics 2014;88(5):1074‐84. [DOI] [PubMed] [Google Scholar]
- Kuban DA, Levy LB, Cheung MR, Lee AK, Choi S, Frank S, et al. Long‐term failure patterns and survival in a randomized dose‐escalation trial for prostate cancer. Who dies of disease?. International Journal of Radiation Oncology, Biology, Physics 2011;79(5):1310‐7. [DOI] [PubMed] [Google Scholar]
- Kuban DA, Nogueras‐Gonzalez GM, Hamblin L, Lee AK, Choi S, Frank SJ, et al. Preliminary report of a randomized dose escalation trial for prostate cancer using hypofractionation. International Journal of Radiation Oncology, Biology, Physics 2010;78(3):S58‐9. [Google Scholar]
- NCT00667888. A phase III intensity radiation dose escalation for prostate cancer using hypofractionation. clinicaltrials.gov/ct2/show/NCT00667888 (first received 28 April 2008).
- Skinner HD, Hoffman KE, Levy LB, Lee AK, Frank SJ, Choi S, et al. Risk of late toxicity in men receiving dose‐escalated hypofractionated intensity modulated prostate radiation therapy: results from a randomized trial. International Journal of Radiation Oncology, Biology, Physics 2014;88(5):1074‐84. [DOI] [PubMed] [Google Scholar]
- Skinner HD, Hoffman KE, Levy LB, Lee AK, Frank SK, Choi S, et al. Toxicity and dosimetric parameters related to conventional versus dose‐escalated, hypofractionated radiation in prostate cancer. International Journal of Radiation Oncology, Biology, Physics 2012;84(3S):S147. [Google Scholar]
- Voong KR, Lal LS, Kuban DA, Pugh TJ, Swint M, Godby J, et al. Long‐term economic value of hypofractionated prostate radiation: secondary analysis of a randomized trial. Advances in Radiation Oncology 2017;2(3):249‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Norkus 2009 {published data only}
- Norkus D, Miller A, Kurtinaitis J, Haverkamp U, Popov S, Prott FJ. A randomized trial comparing hypofractionated and conventionally fractionated three‐dimensional external‐beam radiotherapy for localized prostate adenocarcinoma: a report on acute toxicity. Strahlentherapie Onkologie 2009;185(11):715‐21. [DOI: 10.1007/s00066-009-1982-z] [DOI] [PubMed] [Google Scholar]
- Norkus D, Miller A, Plieskiene A, Janulionis E, Valuka KV. A randomized trial comparing hypofractionated and conventionally fractionated three‐dimensional conformal external beam radiotherapy for localized prostate adenocarcinoma: a report on the first‐year biochemical response. Medicina (Kaunas) 2009;45(6):469‐75. [PubMed] [Google Scholar]
- Norkus D, Valuckas KP, Miller A, Plieskienė A, Kurtinaitis J. Safety investigation of hypofractionated prostate gland radiotherapy [Neišplitusio priešinės liaukos vėžio hipofrakcionuoto spindulinio gydymo saugumo tyrimas]. Medicina 2005;41(12):1035. [PubMed] [Google Scholar]
PROFIT 2016 {unpublished data only}
- Catton C, on behalf of the PROFIT trial investigators. A randomized trial of a shorter radiation fractionation schedule for the treatment of localized prostate cancer. International Journal of Radiation Oncology, Biology, Physics 2016;96(2S):32‐3. [Abstract #5003] [Google Scholar]
- Catton CN, Lukka H, Gu CS, Martin J, Supiot S, Chung PW, et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. Journal of Clinical Oncology 2017;35(17):1884‐90. [DOI: 10.1200/JCO.2016.71.7397] [DOI] [PubMed] [Google Scholar]
- Healy B, Kron T, Frantzis J, Thompson K, Murry R, Catton C, et al. Multi‐centre Australian dosimetric inter‐comparison of prostate IMRT delivery for the OCOG PROFIT trial. Journal of Medical Imaging and Radiation Therapy 2009;53 S1:A64. [Google Scholar]
- ISRCTN43853433. A randomised trial of a shorter radiation fraction schedule for the treatment of localised prostate cancer. www.isrctn.com/ISRCTN43853433 (first received 5 November 2007). [DOI: 10.1186/ISRCTN43853433] [DOI]
- MIddleton M, Frantzis J, Healy B, Jones M, Murry R, Kron T, et al. Successful implementation of image‐guided radiation therapy quality assurance implementation in the Trans Tasman Radiation Oncology Group 08.01 PROFIT Study. International Journal of Radiation Oncology, Biology, Physics 2011;81(5):1576‐81. [DOI] [PubMed] [Google Scholar]
- Martin J, Frantzis J, Chung P, Langah I, Craind M, Cornes D, et al. Prostate radiotherapy clinical trial quality assurance: how real should real time review be? (a TROG‐OCOG intergroup project). Radiotherapy and Oncology 2013;107:333‐8. [DOI] [PubMed] [Google Scholar]
- Martin J, Tai KH, Turner S, Tang C, Eade T, Nasser L, et al. A randomised trial of a shorter schedule for the treatment of localised prostate cancer (PC): profit – an OCOG/TROG intergroup study. Journal of Medical Imaging and Radiation therapy 2016;60 1S:S23. [Google Scholar]
- NCT00304759. A randomized trial of a shorter radiation fractionation schedule for the treatment of localized prostate cancer. clinicaltrials.gov/ct2/show/record/NCT00304759 (first received 20 March 2006).
Yeoh 2011 {published data only}
- Yeoh EE, Botten RJ, Butters J, Matteo AC, Holloway RH, Fowler J. Anorectal dysfunction increases with time following radiation therapy for carcinoma of the prostate. International Journal of Radiation Oncology, Biology, Physics 2004;99(2):361‐9. [DOI] [PubMed] [Google Scholar]
- Yeoh EE, Botten RJ, Butters J, Matteo AC, Holloway RH, Fowler J. Hypofractionated versus conventionally fractionated radiotherapy for prostate carcinoma: final results of phase III randomized trial. International Journal of Radiation Oncology, Biology, Physics 2011;81(5):1271‐8. [DOI] [PubMed] [Google Scholar]
- Yeoh EE, Fraser RJ, McGowan RE, Botten RJ, Matteo AC, Roos DE, et al. Evidence for efficacy without increased toxicity of hypofractionated radiotherapy for prostate carcinoma: early results of a Phase III randomized trial. International Journal of Radiation Oncology, Biology, Physics 2003;55(4):943‐55. [DOI] [PubMed] [Google Scholar]
- Yeoh EE, Holloway RH, Fraser RJ, Botten RJ, Matteo AC, Butters J, et al. Hypofractionated versus conventionally fractionated radiation therapy for prostate carcinoma: updated results of a phase III randomized trial. International Journal of Radiation Oncology, Biology, Physics 2006;66(4):1072‐83. [DOI] [PubMed] [Google Scholar]
- Yeoh EK, Holloway RH, Fraser RJ, Botten R, Matteo A, Moore JW. Anorectal function after three‐ versus two‐dimensional radiation therapy for carcinoma of the prostate. International Journal of Radiation Oncology, Biology, Physics 2009;73(1):46‐52. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
MRC RT01 {published data only}
- Dearnaley DP, Sydes AM, Langley RE, Graham JD, Huddart RA, Syndikus I, et al. The early toxicity of escalated versus standard dose conformal radiotherapy with neo‐adjuvant androgen suppression for patients with localised prostate cancer: results from the MRC RT01 trial (ISCTN47772397). Radiotherapy and Oncology 2007;83:31‐41. [DOI] [PubMed] [Google Scholar]
- Dearnaley PD, Hall PD, Lawrence D, Huddart RA, Elles R, Nutting CM, et al. Phase III pilot study of dose escalation using conformal radiotherapy in prostate cancer: PSA control and side effects. British Journal of Cancer 2005;92:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT01230866 {unpublished data only}
- NCT01230866. A phase III prospective randomized trial of standard‐fractionation vs. hypo‐fractionation with proton radiation therapy for low risk adenocarcinoma of the prostate. clinicaltrials.gov/ct2/show/NCT01230866 (first received 29 October 2010).
NCT01444820 {published data only}
- NCT01444820. Phase III study of hypofractionated, dose escalation radiotherapy for high risk adenocarcinoma of the prostate, using 3D‐CRT or intensity‐modulated radiotherapy. clinicaltrials.gov/ct2/show/NCT01444820 (first received 3 October 2011).
NCT01794403 {published data only}
- Pollack A, Abramowitz M. Radiation hypofractionation via extended versus accelerated therapy (HEAT) for prostate cancer (HEAT). clinicaltrials.gov/ct2/show/NCT01794403 (first received 18 February 2013).
NCT02303327 {unpublished data only}
- NCT02303327. Phase III study of hypofractionated, dose escalation radiotherapy vs. conventional pelvic radiation therapy followed by HDR brachy boost for high risk adenocarcinoma of the prostate. clinicaltrials.gov/ct2/show/NCT02303327 (first received 27 November 2014).
Norkus 2013 {published data only}
- Norkus D, Karklelyte A, Engels B, Vermessen H, Griskevicius R, Ridder M, et al. A randomized hypofractionation dose escalation trial for high risk prostate cancer patients: interim analysis of acute toxicity and quality of life in 124 patients. Radiation Oncology 2013;8(206):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
El‐Ghamrawi 2015 {published data only}
- El‐Ghamrawi K, El‐Haddad M, Hanna S, Kali A, Amal M. Hypofractionated simultaneous integrated boost (SIB) versus conventional fractionation in localized prostate cancer: a randomized pilot study. Gulf Journal of Oncology 2015;1(18):44‐53. [PubMed] [Google Scholar]
Felix 2012 {unpublished data only}
- Felix AL, Huerta J, Calva A, Reyes J, Ceja F. Morbidity results in a prospective randomized trial of hypofractionation versus standard fractionation for prostate cancer using conformal radiation therapy. International Journal of Radiation Oncology, Biology, Physics 2012;84(3S):379. [Google Scholar]
ISRCTN45905321 {unpublished data only}
- Gunnlaugsson A, Kiellén E, Hagberg, O, Thellenberg‐Kalsson C, Widmark A, Nilsson P. Change in prostate volume during extreme hypo‐fractionation analysed with MRI. Radiotherapy and Oncology 2014;9:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN45965321. Phase III study of HYPOfractionated radiotherapy of intermediate risk localised prostate cancer. www.isrctn.com/ISRCTN45905321 (first received 9 December 2008).
- Widmark A, Gunnlaugsson A, Beckman L, Thellenberg‐Karlsson C, Hoyer M, Lagerlund M, et al. Extreme hypofractionation versus conventionally fractionated radiotherapy for intermediate risk prostate cancer: early toxicity results from the Scandinavian randomized phase III trial "HYPO‐RT‐PC". International Journal of Radiation Oncology, Biology, Physics 2016;96(5):938. [Google Scholar]
- Widmark A, Gunnlaugsson A, Beckman L, Thellenberg‐Karlsson C, Hoyer M, Lagerlund M, et al. Ultrahypofractionation for prostate cancer: outcome from the Scandinavian phase 3 HYPO‐RT‐PC trial. Radiotherapy and Oncology 2018;128:S314. [Google Scholar]
Spagnoletti 2011 {unpublished data only}
- Spagnoletti G, Marchese R, Rignanese R, Leo AM, Verile V, Plotino G, et al. Hypofractionation versus conventionally fractionated radiation therapy for prostate cancer: late toxicity. International Journal of Radiation Oncology, Biology, Physics 2011;31(5):1887‐8. [Google Scholar]
- Spagnoletti G, Rignanese R, Cocco G, Oriolo V, Piserchia M, Enfasiet M, et al. Biochemical control after radiation therapy for prostate cancer: hypofractionation versus conventional fractionation. Anticancer Research 2013;33(5):2307‐2308. [Google Scholar]
- Spagnoletti G, Rignanese R, Verile V, Plotino G, Oriolo V, Bove G. Hypofractionation versus conventionally fractionated radiation therapy for prostate cancer: first results. International Journal of Radiation Oncology, Biology, Physics 2010;30(4):1472‐3. [Google Scholar]
References to ongoing studies
NCT01411332 {published data only}
- NCT01411332. A phase III trial of hypofractionated external beam image‐guided highly targeted radiotherapy: the HEIGHT trial. www.clinicaltrials.gov/ct2/show/NCT01411332 (first received 8 August 2011).
NCT01584258 {published data only}
- NCT01584258. International randomised study of laparoscopic prostatectomy vs stereotactic body radiotherapy (SBRT) and conventionally fractionated radiotherapy vs SBRT for early stage organ‐confined prostate cancer. www.clinicaltrials.gov/ct2/show/NCT01584258 (first received 24 April 2012).
NCT02300389 {unpublished data only}
- NCT02300389. Randomized, multi‐center clinical trial comparing hypofractionated radiotherapy boost to conventionally fractionated in a high risk group of prostate cancer patients. clinicaltrials.gov/ct2/show/NCT02300389 (first received 24 November 2014).
NCT02934685 {unpublished data only}
- NCT02934685. A phase III randomized study of hypofractionated image‐guided volumetric modulated arc radiotherapy (IG‐VMAT) versus conventionally fractionated IG‐VMAT in patients with localized prostate cancer. clinicaltrials.gov/ct2/show/NCT02934685 (first received 17 October 2016).
Additional references
Abramowitz 2008
- Abramowitz MC, Li T, Buyyounouski MK, Ross E, Uzzo RG, Pollack A, et al. The Phoenix definition of biochemical failure predicts for overall survival in patients with prostate cancer. Cancer 2008;12(1):55‐60. [DOI] [PubMed] [Google Scholar]
AJCC 2010
- American Joint Committee on Cancer. AJCC Cancer Staging Manual. 7th Edition. New York (NY): Springer, 2010. [Google Scholar]
Arcangeli 2018
- Arcangeli G, Arcangeli S, Pinzic V, Benassid M, Benassie M, Strigarie L. Optimal scheduling of hypofractionated radiotherapy for localized prostate cancer: a systematic review and metaanalysis of randomized clinical trials. Cancer Treatment Reviews 2018;70:22‐9. [DOI] [PubMed] [Google Scholar]
ASTRO 1997
- American Society for Therapeutic Radiology and Oncology Consensus Panel. Consensus statement: guidelines for PSA following radiation therapy. International Journal of Radiation Oncology, Biology, Physics 1997;37:1035‐41. [PubMed] [Google Scholar]
Bannuru 2011
- Bannuru RR, Dvorak T, Obadan N, Yu WW, Patel K, Chung M, et al. Comparative evaluation of radiation treatments for clinically localized prostate cancer: an updated systematic review. Annals of Internal Medicine 2011;155(3):171‐8. [DOI] [PubMed] [Google Scholar]
Barry 1992
- Barry MH, Fowler FJ Jr, O'Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, et al. The American Urological Association index for benign prostatic hyperplasia: the measurement committee of the American Urological Association. Journal of Urology 1992;148(5):1549‐57. [DOI] [PubMed] [Google Scholar]
Bentzen 2005
- Bentzen SM, Ritter MA. The α/β ratio for prostate cancer: what is it, really?. Radiotherapy and Oncology 2005;76(1):1‐3. [DOI] [PubMed] [Google Scholar]
Boutron 2014
- Boutron I, Altman DG, Hopewell S, Vera‐Badillo F, Tannock I, Ravaud P. Impact of spin in the abstracts of articles reporting results of randomized controlled trials in the field of cancer: the SPIIN randomized controlled trial. Journal of Clinical Oncology 2014;32:4120‐6. [DOI] [PubMed] [Google Scholar]
Bray 2012
- Bray F, Jemal A, Grey N, Ferlay J, Forman D. Global cancer transitions according to the Human Development Index (2008‐2030): a population‐based study. Lancet Oncology 2012;13(8):790‐801. [DOI] [PubMed] [Google Scholar]
Brenner 1999
- Brenner DJ, Hall EJ. Fractionation and protraction for radiotherapy of prostate carcinoma. International Journal of Radiation Oncology, Biology, Physics 1999;43(5):1095‐101. [DOI] [PubMed] [Google Scholar]
Brenner 2002
- Brenner DJ, Martinez AA, Edmundson GK, Mitchell C, Thames HD, Armour EP. Direct evidence that prostate tumors show high sensitivity to fractionation (low α/β ratio), similar to late‐responding normal tissue. International Journal of Radiation Oncology, Biology, Physics 2002;52(1):6‐13. [DOI] [PubMed] [Google Scholar]
Collins 1991
- Collins CD, Lloyd‐Davies RW, Swan AV. Radical external beam radiotherapy for localised carcinoma of the prostate using a hypofractionation technique. Clinical Oncology (Royal College of Radiologists (Great Britain)) 1991;3(3):127‐32. [DOI] [PubMed] [Google Scholar]
Corbett 2014
- Corbett MS, Higgins JP, Woolacott NF. Assessing baseline imbalance in randomised trials: implications for the Cochrane risk of bias tool. Research Synthesis Methods 2014;5:79‐85. [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation. Covidence. Version accessed 6 June 2017. Melbourne, Australia: Veritas Health Innovation.
Cox 1972
- Cox DR. Regression models and life‐tables. Journal of the Royal Statistical Society. Series B, Statistical Methodology 1972;34(2):187‐220. [Google Scholar]
Cox 1995
- Cox JD, Statz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organisation for Research and Treatment of Cancer (EORTC). International Journal of Radiation Oncology, Biology, Physics 1995;31:1341‐6. [DOI] [PubMed] [Google Scholar]
Cox 1997
- Cox J, Grignon D, Kaplan R, Parsons JT, Schelhammer PF. Concensus statement: guidelines for PSA following radiation therapy. International Journal of Radiation Oncology, Biology, Physics 1997;37(5):1035‐41. [PubMed] [Google Scholar]
Cox 2001
- Cox DR, Oakes D. Analysis of Survival Data. London (UK): Chapman and Hall, 2001. [Google Scholar]
D'Amico 1998
- D'Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 1998;280:969. [DOI] [PubMed] [Google Scholar]
Datta 2017
- Datta NR, Stutz E, Rogers S, Bodis S. Conventional versus hypofractionated radiation therapy for localized or locally advanced prostate cancer: a systematic review and meta‐analysis along with therapeutic implications. International Journal of Radiation Oncology, Biology, Physics 2017;99(3):573‐89. [DOI] [PubMed] [Google Scholar]
Daşu 2012
- Daşu A, Toma‐Daşu I. Prostate alpha/beta revisited – an analysis of clinical results from 14168 patients. Acta Oncologica (Stockholm, Sweden) 2012;51(8):963‐74. [DOI] [PubMed] [Google Scholar]
Dearnaley 2014
- Dearnaley DP, Jovic G, Syndikus I, Khoo V, Cowan RA, Graham JD, et al. Escalated‐dose versus control‐dose conformal radiotherapy for prostate cancer: long‐term results from the MRC RT01 randomised controlled trial. Lancet Oncology 2014;15(4):464‐73. [DOI] [PubMed] [Google Scholar]
Deeks 2002
- Deeks JJ. Issues in the selection of a summary statistic for meta‐analysis of clinical trials with binary outcomes. Statistics in Medicine 2002;21(11):1575‐600. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Duchesne 1999
- Duchesne GM, Peters LJ. What is the α/β ratio for prostate cancer? Rationale for hypofractionated high‐dose‐rate brachytherapy. International Journal of Radiation Oncology, Biology, Physics 1999;44(4):747‐8. [DOI] [PubMed] [Google Scholar]
Dörr 2001
- Dörr W, Hendry JH. Consequential late effects in normal tissues. Radiotherapy and Oncology 2001;61(3):223‐31. [PUBMED: 11730991] [DOI] [PubMed] [Google Scholar]
Esper 1997
- Esper P, Mo F, Chodak G, Sinner M, Cella D, Pienta KJ. Measuring quality of life in men with prostate cancer using the functional assessment of cancer‐therapy‐prostate instrument. Urology 1997;50:920‐8. [DOI] [PubMed] [Google Scholar]
Fowler 2001
- Fowler J, Chappell R, Ritter M. Is α/β for prostate tumors really low?. International Journal of Radiation Oncology, Biology, Physics 2001;50(4):1021‐31. [DOI] [PubMed] [Google Scholar]
Fowler 2005
- Fowler JF. The radiobiology of prostate cancer including new aspects of fractionated radiotherapy. Acta Oncologica (Stockholm, Sweden) 2005;44(3):265‐76. [DOI] [PubMed] [Google Scholar]
GLOBOCAN 2018
- International Agency for Research on Cancer. 27 – Prostate cancer fact sheet. gco.iarc.fr/today/data/factsheets/cancers/27‐Prostate‐fact‐sheet.pdf (accessed 23 February 2010).
GRADE Working Group 2004
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ (Clinical Research Ed.) 2004;328:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 12 May 2016. Hamilton (ON): McMaster University (developed by Evidence Prime).
Greenland 1985
- Greenland S, Robins JM. Estimation of a common effect parameter from sparse follow‐up data. Biometrics 1985;41(1):55‐68. [PubMed] [Google Scholar]
Heemsbergen 2006
- Heemsbergen WD, Peeters ST, Koper PC, Hoogeman MS, Lebesque JV. Acute and late gastrointestinal toxicity after radiotherapy in prostate cancer patients: consequential late damage. International Journal of Radiation Oncology, Biology, Physics 2006;66(1):3‐10. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ (Clinical Research Ed.) 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JP, Thompson SG, Spiegelhalter DJ. A re‐evaluation of random‐effects meta‐analysis. Journal of the Royal Statistical Society. Series A, (Statistics in Society) 2009;172(1):137‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hou 2015
- Hou Z, Li G, Bai S. High dose versus conventional dose in external‐beam radiotherapy of prostate cancer: a meta‐analysis of long‐term follow‐up. Journal of Cancer Research Clinical Oncology 2015;141:1063‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hróbjartsson 2013
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and nonblinded assessors. Canadian Medical Association Journal 2013;185(4):E201‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
ICRU 1999
- International Commission on Radiation Units and Measurements. ICRU report 62: prescribing, recording, and reporting photon beam therapy (supplement to ICRU report 50). Bethesda (MD): International Commission on Radiation Units and Measurements, 1999. [Google Scholar]
Joiner 1997
- Joiner MC, Kogel AJ. The linear‐quadratic approach to fractionation and calculation of isoeffect relationships. Basic Clinical Radiobiology. 1st Edition. New York (NY): Oxford University Press, 1997:106‐12. [Google Scholar]
Jones 2015
- Jones CW, Keil LG, Holland WC, Caughey MC, Platts‐Mills TF. Comparison of registered and published outcomes in randomized controlled trials: a systematic review. BMC Medicine 2015;13:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirkham 2010
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [DOI] [PubMed] [Google Scholar]
Koontz 2015
- Koontz BF, Bossi A, Cozzarini C, Wiegel T, D'Amico A. A systematic review of hypofractionation for primary management of prostate cancer. European Urology 2015;68:683‐91. [DOI] [PubMed] [Google Scholar]
Kupelian 2001
- Kupelian PA, Reddy CA, Klein EA, Willoughby TR. Short‐course intensity‐modulated radiotherapy (70 Gy at 2.5 Gy per fraction) for localized prostate cancer: preliminary results on late toxicity and quality of life. International Journal of Radiation Oncology, Biology, Physics 2001;51(4):988‐93. [DOI] [PubMed] [Google Scholar]
Leborgne 2012
- Leborgne F, Fowler J, Leborgne JH, Mezzera J. Later outcomes and alpha/beta estimate from hypofractionated conformal three‐dimensional radiotherapy versus standard fractionation for localized prostate cancer. International Journal of Radiation Oncology, Biology, Physics 2012;82(3):1200‐7. [DOI] [PubMed] [Google Scholar]
Lester 2006
- Lester JF, Macbeth F, Toy E, Coles B. Palliative radiotherapy regimens for non‐small cell lung cancer. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD002143.pub2] [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic and meta‐analyses of studies that evaluate interventions: explanation and elaboration. PLoS Medicine 2009;6(7):1‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Litwin 1998
- Litwin MS, Hays RD, Fink A, Ganz PA Leake B, Brooke RH. The UCLA Prostate Cancer Index: development, reliability, and validity of a health‐related quality of life measure. Medical Care 1998;36:1002‐12. [DOI] [PubMed] [Google Scholar]
Maciejewski 1986
- Maciejewski B, Taylor JM, Withers HR. Alpha/beta value and the importance of size of dose per fraction for late complications of the supraglottic larynx. Radiotherapy and Oncology 1986;7:323‐6. [DOI] [PubMed] [Google Scholar]
Mantel 1959
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute 1959;22(4):719‐48. [PubMed] [Google Scholar]
Mattieu 2009
- Mathieu S, Boutron I, Moher D, Altman DG, Ravaud P. Comparison of registered and published primary outcomes in randomized controlled trials. JAMA 2009;302:977‐84. [DOI] [PubMed] [Google Scholar]
McQuay 1999
- McQuay HJ, Collins S, Carroll D, Moore RA. Radiotherapy for the palliation of painful bone metastases. Cochrane Database of Systematic Reviews 1999, Issue 3. [DOI: 10.1002/14651858.CD001793] [DOI] [PubMed] [Google Scholar]
Michalski 2013
- Michalski JM, Yan Y, Watkins‐Bruner D, Cosch WR, Winter K, Galvin JM, et al. Preliminary toxicity analysis of 3‐dimensional conformal radiation therapy versus intensity modulated radiation therapy on the high‐dose arm of the Radiation Therapy Oncology Group 0126 prostate cancer trial. International Journal of Radiation Oncology, Biology, Physics 2013;87(5):932‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miralbell 2012
- Miralbell R, Roberts SA, Zubizarreta E, Hendry JH. Dose‐fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional data‐sets: α/β = 1.4 (0.9–2.2) Gy. International Journal of Radiation Oncology, Biology, Physics 2012;82(1):e17‐24. [PUBMED: 21324610] [DOI] [PubMed] [Google Scholar]
Morgan 2018
- Morgan SC, Hoffman K, Loblaw A, Buyyounouski MK, Caroline Patton S, Barocas D, et al. Hypofractionated radiation therapy for localized prostate cancer: executive summary of an ASTRO, ASCO, and AUA evidence‐based guideline, 2018. Practical Radiation Oncology 2018; Vol. 8, issue 6:354‐60. [DOI] [PubMed]
Mottett 2017
- Mottett N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, Santos M, et al. EAU‐ESTRO‐SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. European Urology 2017;71(4):618‐29. [DOI] [PubMed] [Google Scholar]
NCCN 2014
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN Guidelines®): prostate cancer. Version 1.2015. www.nccn.org (accessed 23 November 2014).
NCI
- National Cancer Institute. Cancer statistical facts: prostate cancer. seer.cancer.gov/statfacts/html/prost.html (accessed 4 February 2019).
NCI 2006
- National Cancer Institute. Common Terminology Criteria for Adverse Events v3.0 (CTCAE). ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf (accessed 30 September 2016).
Nielsen 1998
- Nielsen OS, Bentzen SM, Sandberg E, Gadeberg CC, Timothy AR. Randomized trial of single dose versus fractionated palliative radiotherapy of bone metastases. Radiotherapy and Oncology 1998;47(3):233‐40. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Partin 1997
- Partin AW, Kattan MW, Subong EN, Walsh PC, Wojno KJ, Oesterling JE, et al. Combination of prostate‐specific antigen, clinical stage, and Gleason score to predict pathological stage of localized prostate cancer. A multiple institutional update. JAMA 1997;277:1445‐51. [PubMed] [Google Scholar]
Pavy 1995
- Pavy JJ, Denekamp J, Letschert J, Littbrand B, Mornex F, Bernier J, et al. Late effects toxicity scoring: the SOMA scale. International Journal of Radiation Oncology, Biology, Physics 1995;31(5):1043‐7. [DOI] [PubMed] [Google Scholar]
Peeters 2005
- Peeters ST, Heemsbergen WD, Putten WL, Slot A, Tabak H, Mens JW, et al. Acute and late complications after radiotherapy for prostate cancer: results of a multicenter randomized trial comparing 68 Gy of radiotherapy with 78 Gy. International Journal of Radiation Oncology, Biology, Physics 2005;61(4):1019‐34. [DOI] [PubMed] [Google Scholar]
Peeters 2006
- Peeters ST, Heemsbergen WD, Koper PC, Putten WL, Slot A, Dielwart MF, et al. Dose‐response in radiotherapy for localised prostate cancer treated with 3D conformal radiotherapy. Journal of Clinical Oncology 2006;24:1990‐6. [DOI] [PubMed] [Google Scholar]
Penson 2003
- Penson DF, Litwin MS. Quality of life after treatment for prostate cancer. Current Urology Reports 2003;4(3):185‐95. [PUBMED: 12756081] [DOI] [PubMed] [Google Scholar]
Peters 2010
- Peters LJ, O'Sullivan B, Giralt J, Fitzgerald TJ, Trotti A, Bernier J, et al. Critical impact of radiotherapy protocol compliance and quality in the treatment of advanced head and neck cancer: results from TROG 02.02. Journal of Clinical Oncology 2010;28(18):2996‐3001. [DOI] [PubMed] [Google Scholar]
Potosky 2004
- Potosky AL, Davis WW, Hoffman RM, Stanford JL, Stephenson RA, Penson DF, et al. Five‐year outcomes after prostatectomy or radiotherapy for prostate cancer: the prostate cancer outcomes study. Journal of the National Cancer Institute 2004;96(18):1358‐67. [PUBMED: 15367568] [DOI] [PubMed] [Google Scholar]
Price 1986
- Price P, Hoskin PJ, Easton D, Austin D, Palmer SG, Yarnold JR. Prospective randomised trial of single and multifraction radiotherapy schedules in the treatment of painful bony metastases. Radiotherapy and Oncology 1986;6(4):247‐55. [DOI] [PubMed] [Google Scholar]
Proust‐Lima 2011
- Proust‐Lima C, Taylor JM, Sécher S, Sandler H, Kestin L, Pickles T, et al. Confirmation of a low α/β ratio for prostate cancer treated by external beam radiation therapy alone using a post‐treatment repeated‐measures model for PSA dynamics. International Journal of Radiation Oncology, Biology, Physics 2011;79(1):195‐201. [PUBMED: 20381268] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riley 2011
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta‐analyses. BMJ 2011;342:d549. [DOI] [PubMed] [Google Scholar]
Roach 2006
- Roach M 3rd, Hanks G, Thames H Jr, Schellhammer P, Shipley WU, Sokol GH, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG‐ASTRO Phoenix Consensus Conference. International Journal of Radiation Oncology, Biology, Physics 2006;65(4):965‐74. [DOI] [PubMed] [Google Scholar]
Rosen 1997
- Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The International Index of Erectile Function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology 1997;49:822‐30. [DOI] [PubMed] [Google Scholar]
Ryan 2016
- Ryan R, Synnot A, Hill S. Describing results: Cochrane Consumers and Communication Group, 2016. cccrg.cochrane.org/author‐resources. Version 1.0 (accessed prior to 7 July 2019).
Schünneman 2011
- Schünneman HJ, Oxman AD, Vist GE, Higgins JP, Deeks JT, Glaziou P, et al. Interpretation of results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Siegel 2018
- Siegel RL, Miller KD, Jemal A. Cancer statistics 2018. CA Cancer Clinical Journal 2018;768:7‐30. [DOI] [PubMed] [Google Scholar]
START A 2008
- The START Trialists' Group. The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet Oncology 2008;9(4):331‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
START B 2008
- The START Trialists' Group. The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet 2008;371(9618):1098‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
Sun 2014
- Sun L, Zhu S, Zhao Y, Zhang H, Shang Z, Jiang N, et al. Who benefits from hypofractionated radiotherapy for clinically localized prostate cancer: evidence from meta‐analysis. Tumor Biology 2014;35:9911‐8. [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tucker 2011
- Tucker SL, Thames HD, Michalski JM, Bosch WR, Mohan R, Winter K, et al. Estimation of α/β for late rectal toxicity based on RTOG 94‐06. International Journal of Radiation Oncology, Biology, Physics 2011;81(2):600‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Andel 2008
- Andel G, Bottomley A, Fossa SD. An international field study of the health‐related quality of life of patients with prostate cancer. European Journal of Cancer 2008;44:2418‐24. [DOI] [PubMed] [Google Scholar]
Vogelius 2013
- Vogelius IR, Bentzen SM. Meta‐analysis of the alpha/beta ratio for prostate cancer in the presence of an overall time factor: bad news, good news, or no news?. International Journal of Radiation Oncology, Biology, Physics 2013;85(1):89‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ware 1996
- Ware J, Kosinski M, Keller SD. A 12‐item short‐form health survey: construction of scales and preliminary tests of reliability and validity. Medical Care 1996;34:220‐33. [DOI] [PubMed] [Google Scholar]
Whelan 2002
- Whelan T, MacKenzie R, Julian J, Levine M, Shelley W, Grimard L, et al. Randomized trial of breast irradiation schedules after lumpectomy for women with lymph node‐negative breast cancer. Journal of the National Cancer Institute 2002;94(15):1143‐50. [DOI] [PubMed] [Google Scholar]
Williamson 2002
- Williamson PR, Smith CT, Hutton JL, Marson AG. Aggregate data meta‐analysis with time‐to‐event outcomes. Statistics in Medicine 2002;21(22):3337‐51. [DOI] [PubMed] [Google Scholar]
Withers 1983
- Withers HR, Thames HD Jr, Peters LJ. A new isoeffect curve for change in dose per fraction. Radiotherapy and Oncology 1983;1(2):187‐91. [DOI] [PubMed] [Google Scholar]
Wolff 2015
- Wolff RF, Ryder S, Bossi A, Briganti A, Crook J, Henry A, et al. A systematic review of randomised controlled trials of radiotherapy for localised prostate cancer. European Journal of Cancer 2015;51(16):2345‐67. [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zaorsky 2013
- Zaorsky NG, Ohri N, Showalter TN, Dicker AP, Den RB. Systematic review of hypofractionated radiation therapy for prostate cancer. Cancer Treatment Reviews 2013;39(7):728‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zumsteg 2015
- Zumsteg ZS, Spratt DE, Romesser PB, Pei Z, Zhang Z, Polkinghorn W, et al. The natural history and predictors of outcome following biochemical relapse in the dose escalation era for prostate cancer patients undergoing definitive external beam radiotherapy. European Urology 2015;67(6):1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Soh 2015
- Soh FY, James ML, Hickey BE, Daly T, Jeffery M, See AM, et al. Altered radiation fractionation schedules for clinically localised and locally advanced prostate cancer. Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD011462] [DOI] [Google Scholar]


