Abstract
PURPOSE:
Optical coherence tomography (OCT) has revealed many details of retinal disease that were not available with older imaging technologies. In eyes of adults >60 years old with normal maculas as determined by color fundus photography (CFP) and a validated grading system, we screened for pathology using OCT. We also tested visual functions to assess potential impact of the observed pathologies on patients.
DESIGN:
cross-sectional study
PARTICIPANTS:
This study recruited persons from primary ophthalmology care clinics.
METHODS:
CFP were assessed by the 9-step AREDS scale. OCT macular volumes of participants at step 1 on the AREDS scale, considered normal, were reviewed by a retina specialist masked to other participant characteristics. Participants were tested for 6 different cone- and rod-mediated visual functions.
MAIN OUTCOME MEASURES:
Percentage of participants with disorders detected upon OCT review; visual function measures.
RESULTS:
In 138 of 984 eyes (14%) considered normal by CFP, pathology was detectable by OCT, with 8.4% having vitreomacular interface disorders. Among the low-prevalence disorders found, 5 eyes (0.5%) had macular telangiectasia type 2. Relative to eyes lacking detectable chorioretinal pathology, eyes with any pathology were associated with poorer low-luminance visual acuity and rod-mediated dark adaptation. In eyes with epiretinal membranes, the largest single entity identified (n=61, 6.2%), significantly worse visual functions were best-corrected visual acuity (p=0.0444), low luminance visual acuity (0.0151), and light sensitivity (central 3° and 9°, p=0.0035 and 0.0097 respectively).
CONCLUSIONS:
Macular pathology with functional visual implications not identified by clinical examination or CFP is detectable with OCT. Vitreomacular interface disorders are often visually significant and treatable conditions that are visible by OCT but are easily missed on CFP and clinical examination. Another such condition best seen on OCT is macular telangiectasia type 2, an untreatable disorder for which a clinical trial is in progress. OCT has a potential role in primary eye care clinics to screen for retinal pathology, especially in eyes with decreased visual acuity and otherwise normal examination.
Keywords: Optical coherence tomography, fundus grading, vitreomacular interface disorders, epiretinal membranes, macular telangiectasia type 2, cone-mediated vision, rod-mediated dark adaptation
Précis
In 14.0% of 984 eyes of adults ≥60 years, considered normal by color fundus photography, retinal pathology affecting visual function was detected by optical coherence tomography; 8.4% involved vitreomacular interface disorders, and 6.2%, epiretinal membranes.
Introduction
Clinical examination by ophthalmoscopy and color fundus photography (CFP) have long been considered acceptable methods to differentiate normal and abnormal retinas. Large epidemiology studies1,2 use standardized CFP and validated grading systems to discover pathology in populations. Vitreomacular interface disorders, including epiretinal membrane (ERM), vitreomacular traction syndrome, lamellar hole, and full-thickness macular hole are common disorders3–5 that may be difficult to detect with CFP and ophthalmoscopy. Several epidemiologic studies using CFP to screen for ERM reported population prevalence ranging from 6.0 to 28.9%6–10 and 5-year cumulative incidence11 of 1.5% and 3.8% for severe and mild forms, respectively. ERMs are thin sheets of avascular fibrous tissue containing cells (astrocytes, fibroblasts, hyalocytes) and extracellular matrix (vitreous collagen and fibrous long spacing collagen) that can develop on the macular surface12 preferentially in older adults.6,7
Advances in commercial optical coherence tomography (OCT) have made subcellular-level detail routinely available in the clinic, impacting all aspects of retinal disease diagnosis and management. These improvements include signal averaging and point-of-capture quality control to ensure visibility of retinal fine structure, eye-tracking for precise longitudinal follow-up, and integration with multiple imaging techniques. OCT is recommended as a base modality for clinical trials for agents targeting age-related macular degeneration (AMD).13 With axial resolution of 4 μm in some clinical systems, OCT reveals ERM as a thin reflective line at the vitreomacular interface.4 This advance allows a staging system incorporating disruption of inner retinal layers,14 improved surgical management,15,16 and estimates of ERM prevalence in populations.17,18
In a large cohort of older adults considered normal by ophthalmoscopy in a primary eye care clinic, we recently demonstrated that approximately 1/3 of patients had early AMD,19 as determined by the 9-step AREDS scale for CFP. In the current study, we examined the normal eyes in our cohort to ask whether OCT would reveal further pathology and report the percentages of participants with the observed disorders. We additionally assessed the impact of this pathology on different aspects of cone- and rod-mediated vision in the macula, which has both cone-dominated and rod-dominated subregions.20 We focus our assessment on vitreomacular interface disorders, especially ERMs, as they were the most prevalent in our cohort.
Methods
Participant data was sourced from the baseline cohort for the Alabama Study on Early Age-Related Macular Degeneration (ALSTAR),21 a prospective study of older adults ≥ age 60 years in normal macular health at baseline designed to understand the relationship between delayed rod-mediated dark adaptation and the incident development of early AMD three years later. Enrollees provided written informed consent after the nature and purpose of the study were described. The study followed the tenets of the Declaration of Helsinki and was approved by institutional review at UAB. Participants were recruited from two primary care ophthalmology practices in the Callahan Eye Hospital at UAB. One or both eyes of participants were required to meet criteria for normal macular health as determined by 3-field digital stereo-CFP (Carl Zeiss Meditec 450+, Dublin, CA) evaluated by an experienced grader masked to other study variables (intra-observer agreement κ =0.88, inter-observer agreement κ =0.75). Eyes receiving a grade of 1 in the AREDS 9-step classification system22 were defined as normal. Exclusion criteria were no previous diagnoses of glaucoma, retina and optic nerve conditions, corneal disease, brain injury, or neurological or psychiatric conditions as revealed by the medical record or self-report. Demographic characteristics (age, sex, race/ethnicity) were obtained through participant interview. Intraocular lens (IOL) status was confirmed through chart review.
We acquired spectral-domain OCT volumes of all maculas (Spectralis HRA + OCT, Heidelberg Engineering, Heidelberg, Germany). B-scans (n=73) were horizontally oriented and centered over the fovea in a 20° × 15° (5.7 × 4.2 mm) area. Automatic Real-Time averaging was 8–18, and quality was 20–47 dB. A fellowship-trained retina specialist (J.N.C), masked to all participant characteristics except OCT, evaluated OCT volumes. Each B-scan for each eye was assessed for specific pathologies commonly encountered in a retina practice, which were then annotated in the database. The review checklist included (and was not limited to) ERM, non-neovascular AMD (definite or questionable), (any) drusen, neovascular AMD (definite or questionable), vitreomacular traction, lamellar holes, macular telangiectasia type 2, microaneurysms, macular schisis, pachychoroid spectrum disorders including central serous retinopathy, choroidal nevi, staphylomas, pattern dystrophies, chorioretinal scars, full thickness macular holes, peripapillary choroidal neovascularization, cystic changes, cystoid macular edema, post-internal limiting membrane peel appearance, non-arteritic anterior ischemic optic neuropathy, retinal thinning, and non-specific RPE changes. This list of pathologies was created prior to the OCT review, and the OCT grader (J.N.C.) looked for each of these disorders in every OCT.
Vitreomacular interface disorders were assessed throughout the dataset by the single reviewer. Those ERMs that were eccentrically located, involved only part of the macula, or distorted retinal architecture negligibly were not scored. Vitreomacular adhesion alone without any disruption of the inner retina was graded as normal. Attachment of the vitreous in the macula with any distortion of the foveal architecture was graded as vitreomacular traction. In all cases of vitreomacular traction the cause was the abnormal adherence of the posterior hyaloid to the inner retina. ERM was graded separately.
The following visual function tests were administered for each eye, as described.23 Best-corrected visual acuity for each eye was assessed via the Electronic Visual Acuity tester24 (EVA; JAEB Center, Tampa FL) under photopic conditions expressed (logarithm of the minimum angle of resolution (logMAR)). Low luminance visual acuity was also assessed using the EVA for each eye with participants viewing letters througha 2.0 log unit neutral density filter that reduced luminance to 1 cd/m2. 25 Visual acuity under low luminance was defined by the increase in logMAR under conditions of low light compared to bright light. Contrast sensitivity was estimated by the Pelli-Robson chart26 (Precision Vision, La Salle, IL) under photopic conditions and scored letter-by-letter method.27 Light sensitivity for each eye was assessed using the Humphrey Field Analyzer (Carl Zeiss Meditec, Dublin, CA, 24–2 SITA standard protocol). To estimate sensitivity in the central 3° and 9° of the macula, respectively, sensitivity fo r targets in that region were averaged and expressed in decibels (dB). Rod-mediated dark adaptation (RMDA) was measured psychophysically (AdaptDx, MacuLogix, Hummelstown, PA)21,28,29 in one eye only after dilation, i.e., the eye with better best-corrected visual acuity, because of time constraints in the study visit. The procedure began with a photo-bleach exposure to a flash (0.25 ms duration, 58,000 scotopic cd/m2 s intensity; equivalent ~83% bleach) while the participant was focused on the fixation light. The photo-bleach flash subtended 4° and was centered at 5° on the inferior vertical meridian (i.e. superior to the fovea on the retina) which was also the position of the test target. Threshold measurement for a 2° diameter cir cular target of 500 nm wavelength (green) light began 15 seconds after bleach offset, with participants pressing a button when a flashing target first became visible. Log thresholds were expressed as sensitivity in dB units as a function of time after bleach offset. Dark adaptation speed is defined by the rod intercept time (RIT),29 the duration in minutes required for sensitivity to recover to a criterion value of 5.0 × 10−3 scotopic cd/m2, in the latter half of the second component of RMDA.30
For the analysis, demographic and IOL status of the sample are reported at the eye level. The prevalence of various types of incidental findings based on the OCT pathology review was computed. Visual function variables were categorized into better or worse vision to make interpretations easier. Dichotomous categories were: best-corrected visual acuity, logMAR: ≤0.00 (20/20 or better), >0.00 (worse than 20/20); low luminance acuity, logMAR: ≤0.30 (better), >0.30 (worse); low luminance deficit: ≤0.30 (better), >0.30 (worse); contrast sensitivity, log sensitivity: ≥1.65 (better), <1.65 (worse); rod-mediated dark adaptation, rod intercept time: <12.3 minutes (better), ≥12.3 minutes (worse); light sensitivity in central 3°, log sensi tivity: ≥30 dB (better), <30 dB (worse); light sensitivity in central 9°, log sensitivity: ≥30 dB (better), <30 dB (worse). To examine the association between visual function and the odds of having any pathology, logistic regression models using generalized estimating equations (GEE) were used to generate odds ratios (OR), both unadjusted and adjusted for age and intraocular lens status, with corresponding 95% confidence intervals (CI). GEE models, unadjusted and adjusted, were also used to assess visual function and the odds of having a vitreomacular interface disorder and the odds of having an epiretinal membrane. The level of significance was 0.05.
Results
The sample included 984 eyes, described as normal on CFP, from 558 persons. Table 1 indicates that 96.5% of eyes were from persons in their 60s or 70s, with the balance in their 80s (mean age 68.7 years, standard deviation 5.7). OCT review (Table 2) indicated that 14.0% (n = 138) had incidental findings. ERM was the most common finding (n=61, 6.2%). Other low prevalence lesions were also noted (Table 2), including OCT findings suggestive of non-neovascular age-related macular degeneration (20), vitreomacular traction (15), or lamellar hole (12) in 1–2% of eyes, and non-specific RPE changes in 0.6% of eyes. Less than 0.5% of eyes had macular telangiectasia type 2 (n=5), microaneurysm (5), macular schisis (4), pachychoroid (4), choroidal nevus (3), staphyloma (3), and drusen (2). Conditions occurring in only a single eye (0.1%), including cystic changes, cystoid macular edema, post-internal limiting membrane peel appearance, non-arteritic ischemic optic neuropathy, and retinal thinning, were listed under “other pathology” in Table 2. One eye had both ERM and vitreomacular traction, with epiretinal proliferation overlying the macula and anomalous attachment of the posterior hyaloid with traction. A total of 8.4% of eyes had vitreomacular interface disorders, specifically ERM, vitreomacular traction syndrome, and lamellar macular holes. Eyes with these latter findings, regardless of type, tended to be older and pseudophakic compared to eyes without pathologies.
Table 1.
Demographic and IOL characteristics of 984 eyes from the ALSTAR cohort study in normal macular health per grading of color fundus photographs
| Age, years | |
| 60–69 | 622 (63.2) |
| 70–79 | 328 (33.3) |
| 80–89 | 34 (3.5) |
| Gender | |
| Men | 332 (33.7) |
| Women | 652 (66.3) |
| Race | |
| White, non-Hispanic | 932 (94.7) |
| African American | 43 (4.4) |
| Other | 9 (0.9) |
| IOL status | |
| Phakic | 706 (71.8) |
| Pseudophakic | 278 (28.2) |
N (%)
Table 2.
Summary of OCT pathologies found in eyes in normal macular health per grading of color fundus photographs (n=984)
| Overall | |
| No pathology1 | 846 (86.0) |
| Any pathology | 138 (14.0) |
| Freauency of specific pathologies | |
| Epiretinal membrane2 (ERM) | 61 (6.2) |
| Non-neovascular AMD | 20 (2.0) |
| Vitreomacular traction2 | 15 (1.5) |
| Lamellar hole2 | 12 (1.2) |
| Non-specific RPE changes | 6 (0.6) |
| Macular telangiectasia type 2 | 5 (0.5) |
| Microaneurysm | 5 (0.5) |
| Other pathology3 | 5 (0.5) |
| Macular schisis | 4 (0.4) |
| Pachychoroid | 4 (0.4) |
| Choroidal nevus | 3 (0.3) |
| Staphyloma | 3 (0.3) |
| Drusen | 2 (0.2) |
N (%)
Any pathology included all those under the subheading “Frequency of specific pathologies.”
Pathologies which are vitreomacular interface disorders.
Other pathology included one eye each (prevalence =1 (0.1) with the following lesions: cystic changes, cystoid macular edema, post-internal limiting membrane peel appearance, non-arteritic anterior ischemic optic neuropathy, and retinal thinning.
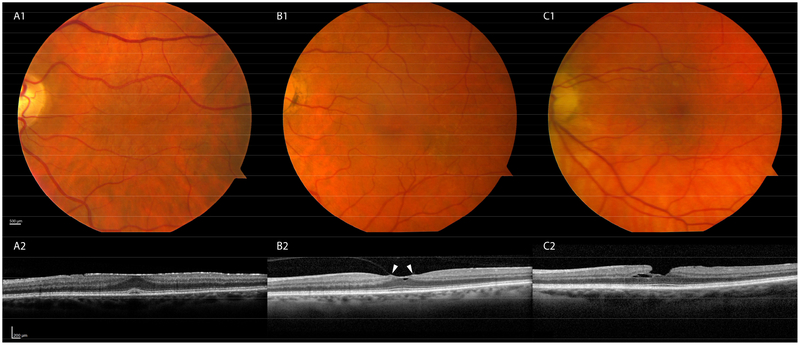
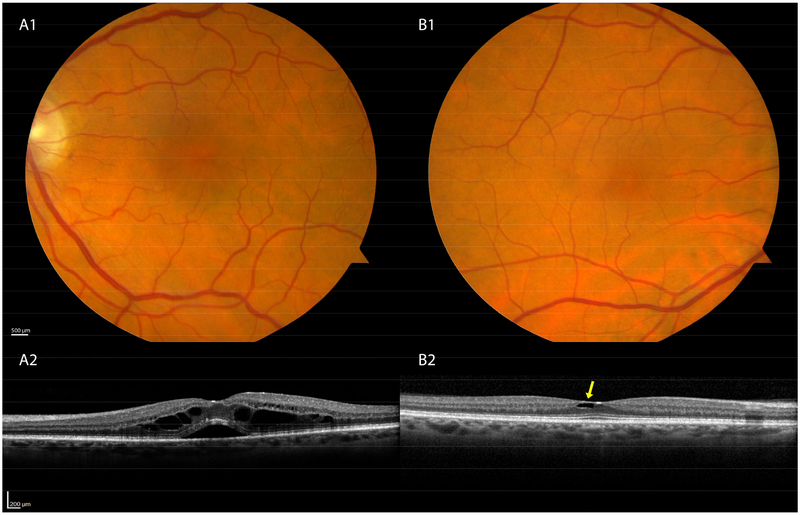
Figures 1 and 2 show examples of eyes with pathologies that were readily detectable by review of macular OCT volumes, yet were not seen either during prior review of CFP or by dilated ophthalmoscopy in a primary care eye clinic. An example of a mild, yet likely clinically significant ERM (Stage 2 of Govetto et al14) is shown in Figure 1A. Other vitreomacular interface disorders included vitreomacular traction syndrome (Figure 1B) and a lamellar hole (Figure 1C). Additional examples include cystoid macular edema, possibly of the Irvine-Gass variety or secondary to a retinal vein occlusion (Figure 2A), and macular telangiectasia 2, evidenced by ILM draping and scaphoid-shaped areas of intraretinal hyporeflectivity (Figure 2B).
Figure 1: Commonly seen disorders of the vitreomacular interface.
Fundus photographs are unremarkable (A1, B1,C1); SD-OCT reveals pathologies (A2,B2,C2). A2. Epiretinal membrane: A hyperreflective sheet overlies the retina. A foveal pit is not discernible but retinal layers are maintained (Stage 214) B2. Vitreomacular traction: Hyaloid attachment to the retina (white arrows) with distortion. C2. Lamellar hole: Loss of the inner retina in the parafovea.
Figure 2: Less common disorders seen in OCT screening of normal eyes.
A1–2. Intraretinal fluid and subretinal fluid most likely represents post-operative cystoid macular edema (Irvine-Gass). B1–2. Eye with Macular Telangiectasia Type 2 has architectural cavitation with draping of the overlying internal limiting membrane. Fundus photographs are unremarkable.
Table 3 shows impairment in cone- and rod-mediated visual functions and their association with eyes having any pathology (n=138) versus eyes lacking pathology. Considering associations adjusted for age and IOL status, visual functions that were significantly worse in eyes with any pathology were low luminance visual acuity (p=0.0306) and rod-mediated dark adaptation (p=0.0297). Best corrected visual acuity (p=0.1154), contrast sensitivity (p=0.2354), and light sensitivity in the central 3° and 9° (p=0.1242 and 0.1646, respectively) were worse in eyes with any pathology, but did not reach statistical significance after adjusting for confounders of age and IOL status. Low luminance deficit was not associated with having any pathology (p=0.8618).
Table 3.
Odds ratios (OR) and 95% confidence intervals (95% CI) for having any pathology1 comparing visual function in eyes in normal macular health per grading of color fundus photographs
| Any pathology (N=138) | No pathology (N=846) | Crude OR (95%CI) | Crude OR p-value | Adjusted2 OR (95% CI) | Adjusted OR p-value | |
|---|---|---|---|---|---|---|
| Best-corrected visual acuity, logMAR | ||||||
| ≤0.00 (20/20 or better) | 51 (37.0) | 400 (47.3) | Ref. | Ref. | ||
| >0.00 (worse than 20/20) | 87 (63.0) | 446 (52.7) | 1.53 (1.02, 2.30) | 0.0407 | 1.39 (0.92, 2.11) | 0.1154 |
| Low luminance acuity, logMAR | ||||||
| ≤0.30 (better) | 40 (29.0) | 351 (41.5) | Ref. | Ref. | ||
| >0.30 (worse) | 98 (71.0) | 495 (58.5) | 1.74 (1.15, 2.62) | 0.0085 | 1.58 (1.04, 2.40) | 0.0306 |
| Low luminance deficit | ||||||
| ≤0.30 (better) | 72 (52.2) | 443 (52.4) | Ref. | Ref. | ||
| >0.30 (worse) | 66 (47.8) | 403 (47.6) | 1.01 (0.68, 1.48) | 0.9693 | 1.04 (0.70, 1.53) | 0.8618 |
| Contrast sensitivity, log sensitivity | ||||||
| ≥1.65 (better) | 51 (37.0) | 399 (47.2) | Ref. | Ref. | ||
| <1.65 (worse) | 87 (63.0) | 447 (52.8) | 1.52 (1.04, 2.24) | 0.0327 | 1.27 (0.86, 1.89) | 0.2354 |
| Rod-mediated dark adaptation, rod intercept time3 | ||||||
| <12.3 minutes (better) | 36 (61.0) | 298 (80.0) | Ref. | Ref. | ||
| ≥12.3 minutes (worse) | 23 (39.0) | 74 (19.89) | 2.57 (1.44, 4.60) | 0.0015 | 1.98 (1.07, 3.67) | 0.0297 |
| Light sensitivity in central 3°, log sensitivity | ||||||
| ≥30 dB (better) | 107 (77.5) | 726 (85.8) | Ref. | Ref. | ||
| <30 dB (worse) | 31 (22.5) | 120 (14.2) | 1.75 (1.06, 2.91) | 0.0301 | 1.51 (0.89, 2.54) | 0.1242 |
| Light sensitivity in central 9°, log sensitivity | ||||||
| ≥30 dB (better) | 82 (59.4) | 597 (70.6) | Ref. | Ref. | ||
| <30 dB (worse) | 56 (40.6) | 249 (29.4) | 1.64 (1.10, 2.44) | 0.0156 | 1.35 (0.88, 2.05) | 0.1646 |
N (%)
Any pathologies included: epiretinal membrane, vitreomacular traction disorder, non-neovascular AMD, lamellar hole, macular telangiectasia type 2, microaneurysm, macular schisis, pachychoroid, choroidal nevus, staphyloma, cystic changes, cystoid macular edema, post-Internal limiting membrane peel appearance, non-arteritic anterior ischemic optic neuropathy, and retinal thinning
Adjusted for age and IOL status
N=431 for dark adaptation eye
The analysis was repeated using only those eyes with vitreomacular interface disorders (N=83), since these were the most prevalent incidental findings. These disorders were associated with poorer light sensitivity in the central 3° (p=0.0316) and such eyes tend to have worse low luminance visual acuity that did not reach statistical significance (p=0.0512) (Table 4).
Table 4.
Odds ratios (OR) and 95% confidence intervals (95% CI) for having a vitreomacular interface disorder1 (VID) comparing visual function in eyes in normal macular health per grading of color fundus photographs
| VID (N=83) | No pathology (N=846) | Crude OR (95%CI) | Crude OR p-value | Adjusted2 OR (95% CI) | Adjusted OR p-value | |
|---|---|---|---|---|---|---|
| Best-corrected visual acuity, logMAR | ||||||
| ≤0.00 (20/20 or better) | 30 (36.1) | 400 (47.3) | Ref. | Ref. | ||
| >0.00 (worse than 20/20) | 53 (63.9) | 446 (52.7) | 1.58 (0.95, 2.63) | 0.0752 | 1.42 (0.85, 2.38) | 0.1860 |
| Low luminance acuity, logMAR | ||||||
| ≤0.30 (better) | 23 (27.7) | 351 (41.5) | Ref. | Ref. | ||
| >0.30 (worse) | 60 (72.3) | 495 (58.5) | 1.85 (1.13, 3.04) | 0.0149 | 1.64 (1.00, 2.70) | 0.0512 |
| Low luminance deficit | ||||||
| ≤0.30 (better) | 45 (54.2) | 443 (52.4) | Ref. | Ref. | ||
| >0.30 (worse) | 38 (45.8) | 403 (47.6) | 0.93 (0.58, 1.50) | 0.7607 | 0.96 (0.59, 1.56) | 0.8752 |
| Contrast sensitivity, log sensitivity | ||||||
| ≥1.65 (better) | 29 (34.9) | 399 (47.2) | Ref. | Ref. | ||
| <1.65 (worse) | 54 (65.1) | 447 (52.8) | 1.66 (1.05, 2.62) | 0.0290 | 1.34 (0.84, 2.13) | 0.2272 |
| Rod-mediated dark adaptation, rod intercept time3 | ||||||
| <12.3 minutes (better) | 23 (63.9) | 298 (80.0) | Ref. | Ref. | ||
| ≥12.3 minutes (worse) | 13 (36.11) | 74 (19.89) | 2.28 (1.10, 4.71) | 0.0264 | 1.57 (0.73, 3.40) | 0.2512 |
| Light sensitivity in central 3°, log sensitivity | ||||||
| ≥30 dB (better) | 60 (72.3) | 726 (85.8) | Ref. | Ref. | ||
| <30 dB (worse) | 23 (27.7) | 120 (14.2) | 2.32 (1.30, 4.14) | 0.0044 | 1.94 (1.06, 3.54) | 0.0316 |
| Light sensitivity in central 9°, log sensitivity | ||||||
| ≥30 dB (better) | 46 (55.4) | 597 (70.6) | Ref. | Ref. | ||
| <30 dB (worse) | 37 (44.6) | 249 (29.4) | 1.93 (1.19, 3.12) | 0.0076 | 1.53 (0.93, 2.53) | 0.0974 |
N (%)
VID included: epiretinal membrane, vitreomacular traction, and lamellar hole;
Adjusted for age and IOL status;
N=408 for dark adaptation eye
ERM was the most common incidental finding of a single entity (n=61), and visual functions in eyes with ERM were compared to eyes lacking pathology (Table 5). Considering adjusted associations, significantly worse functions were best-corrected visual acuity (p=0.0444), low luminance visual acuity (0.0151), and light sensitivity (central 3° and 9°, p=0.0035 and 0.0097 respectivel y).
Table 5.
Odds ratios (OR) and 95% confidence intervals (95% CI) for having an epiretinal membrane (ERM) comparing visual function in eyes in normal macular health per grading of color fundus photographs
| ERM (N=61) | No pathology (N=846) | Crude OR (95%CI) | Crude OR p-value | Adjusted1 OR (95% CI) | Adjusted OR p-value | |
|---|---|---|---|---|---|---|
| Best-corrected visual acuity, logMAR | ||||||
| ≤0.00 (20/20 or better) | 19 (31.2) | 400 (47.3) | Ref. | Ref. | ||
| >0.00 (worse than 20/20) | 42 (68.9) | 446 (52.7) | 1.98 (1.07, 3.67) | 0.0288 | 1.88 (1.02, 3.47) | 0.0444 |
| Low luminance acuity, logMAR | ||||||
| ≤0.30 (better) | 15 (24.6) | 351 (41.5) | Ref. | Ref. | ||
| >0.30 (worse) | 46 (75.4) | 495 (58.5) | 2.17 (1.21, 3.92) | 0.0097 | 2.07 (1.15, 3.72) | 0.0151 |
| Low luminance deficit | ||||||
| ≤0.30 (better) | 34 (55.7) | 443 (52.4) | Ref. | Ref. | ||
| >0.30 (worse) | 27 (44.3) | 403 (47.6) | 0.87 (0.50, 1.52) | 0.6305 | 0.91 (0.52, 1.58) | 0.7290 |
| Contrast sensitivity, log sensitivity | ||||||
| ≥1.65 (better) | 20 (32.8) | 399 (47.2) | Ref. | Ref. | ||
| <1.65 (worse) | 41 (67.2) | 447 (52.8) | 1.83 (1.08, 3.12) | 0.0253 | 1.55 (0.90, 2.65) | 0.1120 |
| Rod-mediated dark adaptation, rod intercept time2 | ||||||
| <12.3 minutes (better) | 15 (65.2) | 298 (80.0) | Ref. | Ref. | ||
| ≥12.3 minutes (worse) | 8 (34.8) | 74 (19.89) | 0.98 (0.88, 5.26) | 0.0941 | 1.62 (0.63, 4.16) | 0.3142 |
| Light sensitivity in central 3°, log sensitivity | ||||||
| ≥30 dB (better) | 41 (67.2) | 726 (85.8) | Ref. | Ref. | ||
| <30 dB (worse) | 20 (32.8) | 120 (14.2) | 2.95 (1.57, 5.53) | 0.0007 | 2.62 (1.37, 4.99) | 0.0035 |
| Light sensitivity in central 9°, log sensitivity | ||||||
| ≥30 dB (better) | 30 (49.2) | 597 (70.6) | Ref. | Ref. | ||
| <30 dB (worse) | 31 (50.8) | 249 (29.4) | 2.48 (1.43, 4.30) | 0.0013 | 2.10 (1.20, 3.70) | 0.0097 |
N (%)
Adjusted for age and IOL status
N=395 for dark adaptation eye
Discussion
Fourteen percent of eyes (n=138) determined to be normal by clinical ophthalmoscopy and by standardized CFP were found to have some pathology detectable by OCT. Combining eyes with ERM, vitreomacular traction syndrome, and lamellar holes, 8.4% of study eyes were found to have vitreomacular interface disorders. We focus discussion on ERM, because it was the most frequent finding of a single entity (6.2%) and on macular telangiectasia 2, an untreatable disorder for which a clinical trial is now in progress (NCT03316300).
Previous comparisons of ophthalmoscopy or CFP with OCT for detecting ERM reported clinic populations with diseases, whereas we compared color and OCT scans in normal eyes. Current data agree that ERMs are more readily detectable by OCT than by CFP or clinical examination, in conditions such as cataract31,32 and uveitis,33 although differences vary widely (3% vs ~40%). ERMs can be difficult to detect by ophthalmoscopy even by highly skilled examiners. Most ERMs are detected by experienced examiners using a slit lamp and fundus lens. CFP does not provide dynamic light reflections or the same degree of stereopsis available to an examiner at the slit lamp.
Our observed 6.2% per-eye prevalence of ERM (9.1% per-person) are comparable with many estimates from population-based samples, despite our participants being recruited from primary eye care, having normal maculas per CFP, and being subject to exclusion criteria. Most per-person prevalence estimates for ERM in adults fall ≤12% for CFP6,7,9,10,34 (but see8) and per-eye prevalence estimates by OCT are 6.0–7.7%17,18. Our ERM prevalence estimates differ substantially from others (0.99% in older adults, by CFP and telemedicine35 and 38.2%−43.4% in patients with intermediate AMD and controls, respectively, by OCT).36 Variability among studies is due to differences in age ranges, ethnicities, entry criteria, definition of ERM, and detection technology.
Our results on cone- and rod-mediated vision expands upon prior studies that assessed only photopic acuity in eyes with ERM.14,33,36 Over time, visual acuity can sharply decline in association with severe retinal distortion due to ERM.14 Because the human macula contains a central cone-dominated fovea, surrounded by an annulus of rod-dominated perifovea,20,37,38 tests of rod- as well as cone-mediated vision are warranted in assessing macular health.39 Among ERM eyes in our cohort, after age-adjustment, some visual functions were significantly although modestly impaired. These effects were less discernible in larger patient groups with mixed etiologies (any pathology, Table 3; all vitreomacular interface disorders, Table 4). The visual tasks most affected by ERM (best-corrected visual acuity and photopic light sensitivity) were driven by cone photoreceptors or mixed rod-cone inputs (low-luminance visual acuity). The non-association of low-luminance deficit and contrast sensitivity with ERM may be due to the different neurophysiologic mechanisms underlying these tasks.39,40 Interestingly rod-mediated dark adaptation was not significantly affected by ERM. In aging and AMD, performance on this task declines early and preferentially,28,41–43 due to a dependence on retinoid supply from the choroid.30 Cones are additionally sustained by Müller cells,44–46 which also participate in tractional disorders of the fovea.47 It is also possible that severe ERMs might have impacted rod function more markedly.
Another disorder revealed by our survey was macular telangiectasia type 2 (MacTel2), found in 0.5% of eyes (and persons). Macular telangiectasia type 2 is a bilateral disorder that degrades foveal and perifoveal vision, with characteristic vascular changes, outer retinal atrophy, and decreased xanthophyll pigment.48,49 The OCT signs shown in Figure 2 are typical for early non-proliferative stages.48,50 Prevalence in our cohort is higher than those reported for population-based studies using CFP51–53 and for a retina clinic-based study using OCT.50 Large observational studies of MacTel2 have documented loss of perifoveal light sensitivity, decreased photopic visual acuity, expansion of existing scotomas, and appearance of new ones.54 A clinical trial of an encapsulated cell-based delivery of ciliary neurotrophic growth factor for MacTel2 is in progress (NCT03316300). If trial outcomes are positive, prompt referral of patients at early disease stages like our participants may be warranted.
Strengths of our study include the large sample of eyes, availability of both CFP and high-quality OCT, fundus grading by an accepted system, unbiased review by a retina specialist masked to other participant characteristics, and the availability of data for both cone- and rod-mediated visual tasks. Limitations include non-availability to the specialist of information found in a typical clinical setting, (i.e., other imaging modalities, patient history, functional information such as visual acuity and the status of metamorphopsia), lack of a second grader, monocular RMDA testing only, lack of band thicknesses and other OCT metrics, and unknown generalizability to patients of other races and ethnicities, and to diseases other than ERM.
Despite these limitations, our results have several implications. First, OCT might replace or complement CFP for screening eyes in clinical trials and observational studies and assist in assessing older patients in the clinic for purposes such as unexplained vision loss.31,32 Second, primary eye care providers should be advised to maintain a high index of suspicion for vitreomacular interface disorders in older patients, especially those with unexplained visual decline or symptoms. Such patients should be referred for evaluation by a specialist with appropriate instrumentation and expertise. Thus, although 14% of the eyes in our study were identified as abnormal by OCT, not all necessarily require referral from primary care to a retinal specialist. Those with symptoms along with pathology identified by OCT screening (and functional visual implications beyond visual acuity) may benefit from evaluation by a specialist.
Towards that end, OCT image capture, transmittal, and automated review via deep learning approaches55,56,57 may help improve diagnostic accuracy, reduce patient travel and waiting time, and optimize specialist efficiency by referring only patients needing advanced care.58 The involvement of Müller cells in tractional disorders47 and MacTel259 suggest that 2-wavelength autofluorescence and fluorescence lifetime imaging60–63 may be of additional help to the retina specialist evaluating these referrals. Interestingly, our functional results for eyes with any pathology (Table 3) also suggest that these imaging modalities may help characterize early macular pathology in patients with findings on OCT but normal best-corrected visual acuity. Future studies incorporating OCT, visual function testing, and the above imaging technologies may shed more light on this interesting patient group.
In conclusion, in 989 eyes of patients aged 60 and older with normal clinical examinations and CFPs, we found that 8.4% had vitreomacular interface disorders on OCT, of which 6.2% were ERMs, and these disorders affected visual performance, possibly even before visual acuity in some cases. We suggest that retina specialists communicate with primary care providers about appropriate use of OCT in detecting vitreoretinal surface disorders. We also suggest that the referral process be streamlined with new technology, and that studies involving OCT-based multimodal imaging and visual function tests be considered to detect early and currently subclinical disease in “normal” eyes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosure
CAC receives research support from Heidelberg Engineering (Heidelberg Germany), Genentech (South San Francisco CA USA / Hoffmann-LaRoche (Switzerland)
R01AG04212, R01EY027948, R01EY029595; EyeSight Foundation of Alabama; Dorsett Davis Discovery Fund; Alfreda J. Schueler Trust; Research to Prevent Blindness
References
- 1.Smith W, Assink J, Klein R, et al. Risk factors for age-related macular degeneration. Pooled findings from three continents. Ophthalmology. 2001;108:697–704. [DOI] [PubMed] [Google Scholar]
- 2.Joachim N, Colijn JM, Kifley A, et al. Five-year progression of unilateral age-related macular degeneration to bilateral involvement: the Three Continent AMD Consortium report. Br J Ophthalmol. 2017;101(9):1185–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duker JS, Kaiser PK, Binder S, et al. The International Vitreomacular Traction Study Group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology. 2013;120(12):2611–2619. [DOI] [PubMed] [Google Scholar]
- 4.Stevenson W, Prospero Ponce CM, Agarwal DR, Gelman R, Christoforidis JB. Epiretinal membrane: optical coherence tomography-based diagnosis and classification. Clin Ophthalmol. 2016;10:527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gattoussi S, Buitendijk GHS, Peto T, et al. The European Eye Epidemiology spectral-domain optical coherence tomography classification of macular diseases for epidemiological studies. Acta Ophthalmol. 2018. [DOI] [PubMed] [Google Scholar]
- 6.Klein R, Klein BE, Wang Q, Moss SE. The epidemiology of epiretinal membranes. Transactions of the American Ophthalmological Society. 1994;92:403–425; discussion 425–430. [PMC free article] [PubMed] [Google Scholar]
- 7.Mitchell P, Smith W, Chey T, Wang JJ, Chang A. Prevalence and associations of epiretinal membranes. The Blue Mountains Eye Study, Australia. Ophthalmology. 1997;104(6):1033–1040. [DOI] [PubMed] [Google Scholar]
- 8.Ng CH, Cheung N, Wang JJ, et al. Prevalence and risk factors for epiretinal membranes in a multi-ethnic United States population. Ophthalmology. 2011;118(4):694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim JM, Lee H, Shin JP, et al. Epiretinal membrane: prevalence and risk factors from the Korea National Health and Nutrition Examination Survey, 2008 through 2012. Korean J Ophthalmol. 2017;31(6):514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheung N, Tan SP, Lee SY, et al. Prevalence and risk factors for epiretinal membrane: the Singapore Epidemiology of Eye Disease study. Br J Ophthalmol. 2017;101(3):371–376. [DOI] [PubMed] [Google Scholar]
- 11.Fraser-Bell S, Guzowski M, Rochtchina E, Wang JJ, Mitchell P. Five-year cumulative incidence and progression of epiretinal membranes: the Blue Mountains Eye Study. Ophthalmology. 2003;110(1):34–40. [DOI] [PubMed] [Google Scholar]
- 12.Parolini B, Schumann RG, Cereda MG, Haritoglou C, Pertile G. Lamellar macular hole: a clinicopathologic correlation of surgically excised epiretinal membranes. Invest Ophthalmol Vis Sci. 2011;52(12):9074–9083. [DOI] [PubMed] [Google Scholar]
- 13.Holz FG, Sadda S, Staurenghi G, et al. Imaging protocols for clinical studies in age-related macular degeneration – recommendations from Classification of Atrophy (CAM) Consensus Meeting. Ophthalmology. 2017;124(4):464–478. [DOI] [PubMed] [Google Scholar]
- 14.Govetto A, Lalane RA 3rd, Sarraf D, Figueroa MS, Hubschman JP. Insights into epiretinal membranes: presence of ectopic inner foveal layers and a new optical coherence tomography staging scheme. Am J Ophthalmol. 2017;175:99–113. [DOI] [PubMed] [Google Scholar]
- 15.Koizumi H, Spaide RF, Fisher YL, Freund KB, Klancnik JM Jr., Yannuzzi LA. Three-dimensional evaluation of vitreomacular traction and epiretinal membrane using spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;145(3):509–517. [DOI] [PubMed] [Google Scholar]
- 16.Ehlers JP, Khan M, Petkovsek D, et al. Outcomes of intraoperative OCT-assisted epiretinal membrane surgery from the PIONEER Study. Ophthalmol Retina. 2018;2(4):263–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye H, Zhang Q, Liu X, et al. Prevalence and associations of epiretinal membrane in an elderly urban Chinese population in China: the Jiangning Eye Study. Br J Ophthalmol. 2015;99(12):1594–1597. [DOI] [PubMed] [Google Scholar]
- 18.You QS, Peng XY, Xu L, Chen CX, Wang YX, Jonas JB. Myopic maculopathy imaged by optical coherence tomography: the Beijing Eye Study. Ophthalmology. 2014;121(1):220–224. [DOI] [PubMed] [Google Scholar]
- 19.Neely DC, Bray KJ, Huisingh CE, Clark ME, McGwin G Jr., Owsley C. Prevalence of undiagnosed age-related macular degeneration in primary eye care. JAMA Ophthalmol. 2017;135(6):570–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Curcio CA, Sloan KR, Kalina RE, Hendrickson AE. Human photoreceptor topography. J Comp Neurol. 1990;292(4):497–523. [DOI] [PubMed] [Google Scholar]
- 21.Owsley C, McGwin G Jr., Clark ME, et al. Delayed rod-mediated dark adaptation is a functional biomarker for incident early age-related macular degeneration. Ophthalmology. 2016;123(2):344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS Report No. 17. Arch Ophthalmol. 2005;123(11):1484–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Owsley C, Huisingh C, Clark ME, Jackson GR, McGwin G Jr. Comparison of visual function in older eyes in the earliest stages of age-related macular degeneration to those in normal macular health. Curr Eye Res. 2016;41(2):266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beck RW, Moke PS, Turpin AH, et al. A computerized method of visual acuity testing: adaptation of the early treatment of diabetic retinopathy study testing protocol. Am J Ophthalmol. 2003;135(2):194–205. [DOI] [PubMed] [Google Scholar]
- 25.Sunness JS, Rubin GS, Applegate CA, et al. Visual function abnormalities and prognosis in eyes with age-related geographic atrophy of the macula and good visual acuity. Ophthalmology. 1997;104(10):1677–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pelli DG, Robson JG, Wilkins AJ. The design of a new letter chart for measuring contrast sensitivity. Clin Vision Sci. 1988;2:197–199. [Google Scholar]
- 27.Elliott DB, Bullimore MA, Bailey IL. Improving the reliability of the Pelli-Robson contrast sensitivity test. Clin Vision Sci. 1991;6:471–475. [Google Scholar]
- 28.Owsley C, Huisingh C, Jackson GR, et al. Associations between abnormal rod-mediated dark adaptation and health and functioning in older adults in normal macular health. Invest Ophthalmol Vis Sci. 2014;55(8):4776–4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson GR, Edwards JG. A short-duration dark adaptation protocol for assessment of age-related maculopathy. J Ocul Biol Dis Infor. 2008;1(1):7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamb TD, Pugh EN Jr. Dark adaptation and the retinoid cycle of vision. Prog Retin Eye Res. 2004;23(3):307–380. [DOI] [PubMed] [Google Scholar]
- 31.Milani P, Raimondi G, Morale D, Scialdone A. Biomicroscopy versus optical coherence tomography screening of epiretinal membranes in patients undergoing cataract surgery. Retina. 2012;32(5):897–904. [DOI] [PubMed] [Google Scholar]
- 32.Moreira Neto CA, Moreira Junior CA, Moreira AT. Optical coherence tomography in patients undergoing cataract surgery. Arq Bras Oftalmol. 2015;78(4):241–245. [DOI] [PubMed] [Google Scholar]
- 33.Nicholson BP, Zhou M, Rostamizadeh M, et al. Epidemiology of epiretinal membrane in a large cohort of patients with uveitis. Ophthalmology. 2014;121(12):2393–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miyazaki M, Nakamura H, Kubo M, et al. Prevalence and risk factors for epiretinal membranes in a Japanese population: the Hisayama study. Graefes Arch Clin Exp Ophthalmol. 2003;241(8):642–646. [DOI] [PubMed] [Google Scholar]
- 35.De Bats F, Vannier Nitenberg C, Fantino B, Denis P, Kodjikian L. Age-related macular degeneration screening using a nonmydriatic digital color fundus camera and telemedicine. Ophthalmologica. 2014;231(3):172–176. [DOI] [PubMed] [Google Scholar]
- 36.Leuschen JN, Schuman SG, Winter KP, et al. Spectral-domain optical coherence tomography characteristics of intermediate age-related macular degeneration. Ophthalmology. 2013;120(1):140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Polyak SL. The Retina. Chicago: University of Chicago; 1941. [Google Scholar]
- 38.Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs--an extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98(5 Suppl):786–806. [PubMed] [Google Scholar]
- 39.Owsley C. Vision and Aging. Annu Rev Vis Sci. 2016;2:255–271. [DOI] [PubMed] [Google Scholar]
- 40.Sunness JS, Rubin GS, Broman A, Applegate CA, Bressler NM, Hawkins BS. Low luminance visual dysfunction as a predictor of subsequent visual acuity loss from geographic atrophy in age-related macular degeneration. Ophthalmology. 2008;115(9):1480–1488, 1488 e1481–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Owsley C, Jackson GR, Cideciyan AV, et al. Psychophysical evidence for rod vulnerability in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2000;41(1):267–273. [PubMed] [Google Scholar]
- 42.Owsley C, Clark ME, Huisingh CE, Curcio CA, McGwin G Jr. Visual function in older eyes in normal macular health: association with incident early age-related macular degeneration 3 years later. Invest Ophthalmol Vis Sci. 2016;57(4):1782–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cocce KJ, Stinnett SS, Luhmann UFO, et al. Visual function metrics in early and intermediate dry age-related macular degeneration for use as clinical trial endpoints. Am J Ophthalmol. 2018;189:127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mata NL, Radu RA, Clemmons RC, Travis GH. Isomerization and oxidation of vitamin A in cone-dominant retinas: a novel pathway for visual-pigment regeneration in daylight. Neuron. 2002;36(1):69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burris C, Klug K, Ngo IT, Sterling P, Schein S. How Muller glial cells in macaque fovea coat and isolate the synaptic terminals of cone photoreceptors. J Comp Neurol. 2002;453(1):100–111. [DOI] [PubMed] [Google Scholar]
- 46.Wang JS, Kefalov VJ. The cone-specific visual cycle. Prog Retin Eye Res. 2011;30(2):115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Govetto A, Bhavsar KV, Virgili G, et al. Tractional abnormalities of the central foveal bouquet in epiretinal membranes: clinical spectrum and pathophysiological perspectives. Am J Ophthalmol. 2017;184:167–180. [DOI] [PubMed] [Google Scholar]
- 48.Yannuzzi LA, Bardal AM, Freund KB, Chen KJ, Eandi CM, Blodi B. Idiopathic macular telangiectasia. Arch Ophthalmol. 2006;124(4):450–460. [DOI] [PubMed] [Google Scholar]
- 49.Charbel Issa P, van der Veen RL, Stijfs A, Holz FG, Scholl HP, Berendschot TT. Quantification of reduced macular pigment optical density in the central retina in macular telangiectasia type 2. Exp Eye Res. 2009;89(1):25–31. [DOI] [PubMed] [Google Scholar]
- 50.Bayon-Porras RM, Pascual-Camps I, Plaza-Laguardia C, Gallego-Pinazo R. Idiopathic macular telangiectasia type 2: Prevalence and a morphometric and phenotypic study. Arch Soc Esp Oftalmol. 2018;93(3):105–112. [DOI] [PubMed] [Google Scholar]
- 51.Klein R, Blodi BA, Meuer SM, Myers CE, Chew EY, Klein BE. The prevalence of macular telangiectasia type 2 in the Beaver Dam eye study. Am J Ophthalmol. 2010;150(1):55–62 e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aung KZ, Wickremasinghe SS, Makeyeva G, Robman L, Guymer RH. The prevalence estimates of macular telangiectasia type 2: the Melbourne Collaborative Cohort Study. Retina. 2010;30(3):473–478. [DOI] [PubMed] [Google Scholar]
- 53.Sallo FB, Leung I, Mathenge W, et al. The prevalence of type 2 idiopathic macular telangiectasia in two African populations. Ophthalmic Epidemiol. 2012;19(4):185–189. [DOI] [PubMed] [Google Scholar]
- 54.Heeren TF, Clemons T, Scholl HP, Bird AC, Holz FG, Charbel Issa P. Progression of vision loss in macular telangiectasia type 2. Invest Ophthalmol Vis Sci. 2015;56(6):3905–3912. [DOI] [PubMed] [Google Scholar]
- 55.Ting DSW, Cheung CY, Lim G, et al. Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. JAMA. 2017;318(22):2211–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burlina PM, Joshi N, Pacheco KD, Freund DE, Kong J, Bressler NM. Use of deep learning for detailed severity characterization and estimation of 5-year risk among patients with age-related macular degeneration. JAMA Ophthalmol. 2018;136(12):1359–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grassmann F, Mengelkamp J, Brandl C, et al. A deep learning algorithm for prediction of Age-Related Eye Disease Study Severity Scale for age-related macular degeneration from color fundus photography. Ophthalmology. 2018. [DOI] [PubMed] [Google Scholar]
- 58.Vaziri K, Moshfeghi DM, Moshfeghi AA. Feasibility of telemedicine in detecting diabetic retinopathy and age-related macular degeneration. Semin Ophthalmol. 2015;30(2):81–95. [DOI] [PubMed] [Google Scholar]
- 59.Powner MB, Gillies MC, Zhu M, Vevis K, Hunyor AP, Fruttiger M. Loss of Muller’s cells and photoreceptors in macular telangiectasia type 2. Ophthalmology. 2013;120(11):2344–2352. [DOI] [PubMed] [Google Scholar]
- 60.dell’Omo R, Virgili G, Rizzo S, et al. Role of lamellar hole-associated epiretinal proliferation in lamellar macular holes. Am J Ophthalmol. 2017;175:16–29. [DOI] [PubMed] [Google Scholar]
- 61.Obana A, Sasano H, Okazaki S, Otsuki Y, Seto T, Gohto Y. Evidence of carotenoid in surgically removed lamellar hole-associated epiretinal proliferation. Invest Ophthalmol Vis Sci. 2017;58(12):5157–5163. [DOI] [PubMed] [Google Scholar]
- 62.Sauer L, Gensure RH, Hammer M, Bernstein PS. Fluorescence lifetime imaging ophthalmoscopy: a novel way to assess macular telangiectasia type 2. Ophthalmology Retina. 2018;2(6):587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Müller S, Issa PC, Heeren TFC, Thiele S, Holz FG, Herrmann P. Macular pigment distribution as prognostic marker for disease progression in macular telangiectasia type 2. Am J Ophthalmol. 2018;194:163–169. [DOI] [PubMed] [Google Scholar]