Abstract
The key role of mitochondria in oxidative metabolism and redox homeostasis explains the link between mitochondrial dysfunction and the development of metabolic disorders. Mitochondria's highly dynamic nature, based on alterations in biogenesis, mitophagy, fusion and fission, allows adjusting sequential redox reactions of the electron transport chain (ETC) and dissipation of the membrane potential by ATP synthase, to different environmental cues. With reactive oxygen species being an inevitable by-product of oxidative phosphorylation (OXPHOS), alterations on mitochondrial oxidative rate with a consequent excessive load of reactive oxygen species have been traditionally associated with pathological conditions. However, reactive oxygen species have also been suggested as promoters of mitohormesis, a process in which low, non-cytotoxic concentrations of reactive oxygen species promote mitochondrial homeostasis. Therefore, signaling systems involved in the regulation of mitochondrial homeostasis are attractive candidates for drug development for metabolic diseases triggered by mitochondrial dysfunction. Reversible phosphorylation downstream the cyclic AMP (cAMP) signaling cascade and deacetylation mediated by sirtuins are recognized as major mitochondrial regulators.
Keywords: Mitochondria, ROS, Mitohormesis, Metabolic diseases, cAMP, Sirtuin
1. Introduction
Mitochondria are known for their key role in cellular energetic metabolism involving oxidative phosphorylation (OXPHOS), β-oxidation of free fatty acids and the tricarboxylic acid cycle (TCA). Also the participation of mitochondria in redox and calcium homeostasis makes mitochondrial signaling pathways regulators of many cellular functions implicated in vital processes [1-3]. Several studies have indicated that the misbalance of energy homeostasis is linked to a compromised mitochondrial function, which appears to be an early event in the development of metabolic disorders. In fact, metabolic disorders are associated with an increase in oxidative damage correlated with a deregulation in mitochondrial dynamics and decreased expression of genes involved in the control of mitochondrial biogenesis [4].
Mitochondrial homeostasis is dependent on dynamic processes such as fusion, fission and removal of irreversibly damaged mitochondria (mitophagy), preventing the accumulation of dysfunctional organelles (Fig. 1A). However, mitophagy must be counter-balanced by mitochondrial biogenesis, allowing tuning mitochondrial function to the cellular needs [5]. Indeed, signaling pathways activated by physiological stimuli such as changes in nutritional availability and energy requirements coordinate adaptive changes in oxidative metabolism mediated by a crosstalk between the nucleus and mitochondrial genomes [6]. Moreover, modification of mitochondrial proteins by reversible phosphorylation downstream of the cAMP-dependent pathway and deacetylation mediated by sirtuins (SIRTs) have emerged as major regulatory mechanisms for rapid modulations of mitochondrial homeostasis [7-9].
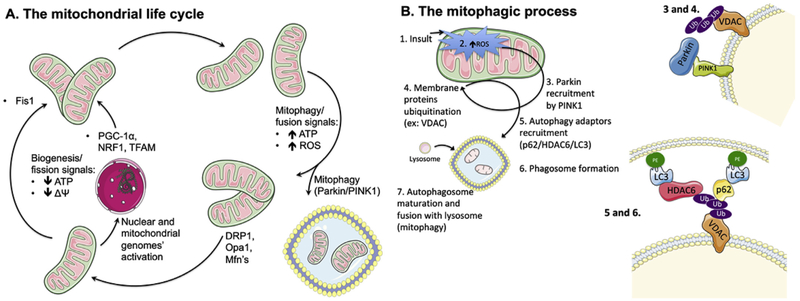
Fig. 1.
A. The mitochondrial life cycle. The mitochondrial population is a highly dynamic and fluid entity within a cell, with organelle units continually being produced or removed, depending on the cell's needs and diverse signals. One of the cell's mechanisms to cope with a decrease in cellular ATP levels, with concomitant fall in mitochondrial membrane potential (ΔΨ), is to generate more mitochondria either by fission of existing ones (which decreases membrane area per organelle, thus elevating ΔΨ) or simply producing newer ones, with resource to the genetic templates within the nucleus and the mitochondrial genome. For the biogenesis process, example of key players are PGC-1α, NRF1 and TFAM, while FIS1 is typically crucial for fission. Conversely, when ATP levels are high, oxidative stress is also elevated. As such, it becomes energetically costly to maintain numerous, unnecessary mitochondria, some of them quite damaged. As such, the cell induces either the removal of damaged mitochondria by mitophagy (which involves, for example, Parkin and PINK1, and is discussed in more detail in Fig. 1B) or fuses unnecessary mitochondria (using effectors such as DRP1, OPA1 or MFN, reducing the number of units but increasing they surface area, effectively decreasing ΔΨ and thus contributing to a lower ATP generation rate as well as decreased oxidative stress. DRP1: dynamin related protein 1, Fis1: mitochondrial fission 1 protein, Mfn: mitofusin, NRF1: nuclear respiratory factor 1, Opa1: mitochondrial dynamin-like 120 kDa protein, PINK: phosphatase and tensin homolog (PTEN)-induced kinase 1, PGC-1α: peroxisome proliferator-activated receptor gamma coactivator-1α, ROS: reactive oxygen species, TFAM: mitochondrial transcription factor A. B. Mitochondrial autophagy (mitophagy) dependency on ROS. When an insult (1) leads to elevation of mitochondrially-generated ROS levels (2), with concomitant loss of membrane potential, the membrane-bound PINK1 protein is stabilized and undergoes autophosphorylation, which triggers the recruitment of Parkin (3), a soluble E3 ubiquitin ligase. Parkin then ubiquitinates (4) other outer mitochondrial membrane proteins (such as VDAC, MFN1, and others), which is a signal for the recruitment of the autophagy adaptors p62/SQSTM1 and HDAC6 (5). These proteins anchor active LC3 units, which are themselves bound to phosphatidylethanolamine, serving as initiator point for phagosome membrane formation and maturation. Finally, the mature autophagosome is fused with hydrolytic-enzymes carrying lysosomes (7), which leads to the degradation of the lysophagosome's contents. HDAC6: histone deacetylase 6, LC3: microtubule-associated protein 1A/1B-light chain 3, MFN: mitofusin, p62/SQSTM1: p62/signaling adaptor sequestosome 1, PE: phosphatidylethanolamine, PINK1: phosphatase and tensin homolog (PTEN)-induced kinase 1, ROS: reactive oxygen species, VDAC: voltage-dependent anion channel. . (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Interestingly, mitochondrial adaptive processes may also involve signaling by reactive oxygen species (ROS). Traditionally, increased ROS formation has been associated with numerous diseases and age-related disorders [10]. With ROS being an inevitable by-product of OXPHOS, an imbalance between increased formation of reducing equivalents by the TCA cycle and the capacity of the ETC results in increased ROS formation and oxidative stress, and high concentrations of ROS cause damage to membranes, proteins, carbohydrates, and DNA [11,12]. However, conditions that induce a mild increase in mitochondrial-derived ROS such as caloric restriction (CR) and physical exercise, have a known pro-longevity effect by a process known as mitohormesis [2]. A mitohormetic response is proposed to be triggered by transient mitochondrial stress wherein low, non-cytotoxic concentrations of ROS can serve as signals to mitochondrial and antioxidant signaling pathways promoting changes in the antioxidant defense system and mitochondrial dynamics, therefore resulting in extended lifespan [2,11]. In the present review, we summarize current knowledge concerning the relationship between ROS formation and mitochondrial function, in a hormetic perspective. We also discuss how the crosstalk between cAMP and SIRTs may have a critical impact on mitochondrial homeostasis.
2. The dynamics of mitochondrial function
Mitochondrial quality control mechanisms are necessary to maintain a healthy mitochondrial network within cells, by controlling mitochondrial distribution, mass and activity [13]. As the mitochondrial DNA (mtDNA) accounts for only 13 subunits of the ETC, mitochondrial biogenesis is the process, dependent on coordinated mitochondrial and nuclear gene expression, by which cells increase mitochondrial mass and shape bioenergetic capacity [6]. Among the regulators of this process, peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) acts as a co-activator of the nuclear respiratory factors (NRFs) 1 and 2, inducing the expression of nuclear-encoded mitochondrial genes, including subunits of the ETC, mitochondrial ribosomal proteins and key mitochondrial enzymes [14,15]. Additionally, NRFs also regulate the mitochondrial transcription factor A (TFAM), which drives transcription and replication of mtDNA (Fig. 1A) [15]. Since stimulation of mitochondrial biogenesis coordinately increases mitochondrial mass and substrate oxidation, it could be expected that increased rates of electron flow through the ETC could increase ROS generation. However, it has been shown that PGC-1α buffers ROS as demonstrated by a burst in ROS when PGC-1α is downregulated during myogenesis [16] as well as the attenuation of mitochondrial disease in mouse models of disrupted cytochrome c oxidase (COX) activity [17].
Mitochondrial network organizational changes also result in crucial alterations in cellular function since it influences mitochondrial homeostasis. These events are governed by a complex molecular machinery that adapts mitochondrial function to the cell metabolic needs and thus alter calcium signaling, OXPHOS rates and ROS generation [6]. On one hand, fusion events result in a more interconnected network, allowing the exchange of DNA and metabolites between neighboring mitochondria, therefore optimizing mitochondrial function. On the other hand, fission events facilitate mitochondrial transport and the elimination of damaged organelles by mitophagy [5,13].
The selective elimination of dysfunctional or damaged mitochondria by mitophagy is also crucial in maintaining mitochondrial homeostasis and cellular metabolism since the major factor driving this process is the mitochondrial membrane potential [18]. In fact, one well-established pathway that regulates mitophagy is the impairment of PINK1 (phosphatase and tensin homolog (PTEN)-induced kinase 1) import system in depolarized mitochondria, resulting in its accumulation on the outer mitochondrial membrane (OMM) and the recruitment of Parkin, which ubiquitinates OMM proteins and triggers mitophagy (Fig. 1B) [19]. It is important to note that mitophagy is a crucial precursor to mitochondrial biogenesis, allowing the expansion of a healthy mitochondrial network, and that biogenesis must balance mitophagy for proper mitochondrial turnover [13], which is supported by the proposed link between PINK1 and regulation of mtDNA content [20]. Since mitophagy primes the elimination of dysfunctional and depolarized mitochondria for degradation, thus preventing oxidative stress, mitophagy has been suggested to be a longevity factor as shown by the extended lifespan involving mitophagy induction upon mild attenuation of mitochondrial function or by dietary restriction [13,18].
3. The dual role of mitochondrial ROS on cellular function
As a major source of ROS generated by the ETC and other mitochondrial enzymes, mitochondria act as regulators of the cellular redox state [21]. Electrons from reducing equivalents such as NADH and FADH2, generated by intermediary metabolism are transferred along a series of carriers, with increased oxidation potentials, in the four complexes of the ETC. This exergonic process is coupled to proton translocation across the inner mitochondrial membrane, generating an electrochemical gradient. The movement of protons back to the matrix through the ATP synthase drives the conversion of ADP and Pi to ATP. The leak of single electrons from the ETC results in one-electron reduction of oxygen originating mitochondrial superoxide anion [22].
The critical role of the reductive power provided by NADH/NADPH for several cellular processes, conditions of reductive stress such as when NADH is supplied to the ETC at a rate higher than the reduction of oxygen takes place, has been pointed as a first insult toward metabolic diseases [23]. Under physiological conditions, antioxidant enzymes such as superoxide dismutase (SOD) and glutathione peroxidase detoxify ROS and prevent oxidative stress. Non-enzymatic defenses such as vitamin C and phenolic compounds also act as ROS scavengers. However, conditions of decreased NAD+/NADH ratio observed in the early phases of hyperglycemic exposure [24] or faulty proteins in the ETC caused by mtDNA defects, overwhelm the endogenous antioxidant capacity. This results in oxidative damage to lipids, proteins and mtDNA with progressive mitochondrial dysfunction further boosting ROS generation and creating a vicious cycle of oxidative damage [11]. In fact, oxidative stress caused by mitochondrial dysfunction has been associated with the development of several pathologies such as diabetes, neurodegenerative and age-related disorders in which a decrease in mitochondrial content could be a quality-control process [11,12,19].
Despite the harmful side of increased ROS generation and its association with disease and aging, ROS are also closely related to human health through their impact on cellular signaling and diverse physiological processes [3]. Regarding mitochondrial function, the cellular redox state has been proposed as a regulatory signal for mitochondrial biogenesis [9,25]. In a model of high-fat induced-obesity, at a state where increased mitochondrial content is observed in skeletal muscle, the inclusion of a mitochondrial-targeted antioxidant in the diet prevented the increase in mitochondrial biogenesis [26]. This suggested that stimulation of mitochondrial biogenesis aims to prevent excessive ROS formation due to the surplus of reducing equivalents to the ETC. Also treatment of mouse embryonic cells with H2O2 has been shown to increase PGC-1α and 1β transcription [27] and in C2C12 cells, pretreatment with the antioxidant N-acetyl-L-cysteine (NAC) prevented the increase in PGC-1α mRNA induced by H2O2 [28]. In addition, by modulating stress kinases and transcription factors, ROS induce cellular antioxidant defenses that overlap with the regulation by PGC-1α and PGC-1β on the expression of catalase and SOD [29].
ROS may also confer cellular protection by regulating autophagy. In fact, autophagy is induced in conditions of increased ROS formation such as starvation, an event blocked by ROS scavengers [30]. ROS were found to modify a cysteine residue near the catalytic domain of autophagy-related protein 4, a protease that regulates autophagy [8]. Mitophagy is also triggered by ROS (Fig. 1B). Transient low concentrations of H2O2 and rotenone, an inhibitor of the ETC complex I, were shown to trigger fission-dependent mitophagy in the absence of mitochondrial dysfunction [18]. Recently it has been shown that treatment with NAC inhibits PINK1-dependent Parkin translocation to mitochondria [31] and superoxide is the major species of ROS that mediates mitophagy following Parkin translocation to mitochondria, since SOD inhibitors were able to interfere with the progression of Parkin/PINK1-mediated mitophagy [32].
4. Mitohormesis as a gatekeeper of metabolic health
The study of mitochondrial ROS as signaling agents of paramount importance for normal cellular physiology and the concept that cell exposure to mild stressors elicits an adaptive response [33], has expanded the concept of hormesis into a mitochondrial-centric view [34]. Mitohormesis describes various forms of mitochondrial stress that elicit a beneficial retrograde signaling response that includes the modulation of mitochondrial dynamics, the expression of nuclear and mitochondrial-encoded genes and genes related to the antioxidant response, stimulating mitochondrial function and increasing cellular defense mechanisms, that can be either transient metabolic and biochemical alterations or long-lasting cytoprotective mechanisms that increase stress resistance [2,35] (Fig. 2A).
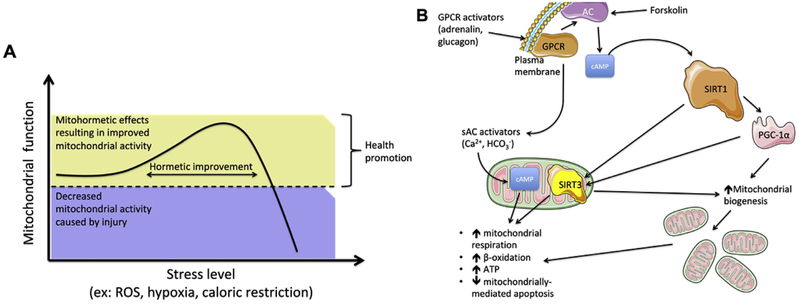
Fig. 2.
A. The mitohormetic response. ROS and other mitochondrial toxicants are well known to cause the development of a mitohormetic response, when presented at low values. In fact, a small harmful effect will boost a response of overdrive, i.e., the cell will try to elevate mitochondrial activity to combat the injury, whether by increasing mitochondrial numbers and active respiratory components, to mitophagic (removing the more damaged units) and fission (increasing the number of mitochondria, while diminishing their overall surface area per unit, thus increasing ΔΨ and ATP generation) events. However, this is a tough balancing act to pull through, for the tipping point where activity rapidly decreases can be easily traversed, resulting in the more commonly known toxic effects of ROS and other mitochondrial toxicants. B. The role of cAMP on mitochondrial metabolism and mitohormesis. cAMP signaling affects mitochondrial homeostasis and metabolism, for it can result in an increase in NAD+ and the activation of sirtuin 1 (SIRT1), which deacetylates (and thus hyperactivates) peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α), the master regulator of mitochondrial biogenesis, leading to elevated mitochondrial numbers and thus increased overall cellular mitochondrial activity. Similarly, cAMP can lead to the activation of SirT3 within the mitochondrial matrix, leading to the deacetylation of several proteins, resulting in the elevation of mitochondrial activity.
The decline of mitochondrial function with age has pointed to mitochondria as major players in the aging process [36], as major generators of ROS considered as unwanted by-products of oxidative phosphorylation. Being mitochondria a principal target for ROS, this would lead to the gradual loss of cell function overtime due to oxidative stress and accumulated damage. This theory has emerged as a rather untouchable dogma but began to be questioned by studies reporting the negative action of antioxidants [37]. For instance, antioxidants supplemented to the dietary regimen failed in delaying cardiovascular disease and even were reported to increase cancer growth [38,39]. Furthermore, the recognition of increased ROS as a mediator of skeletal muscle adaptation to exercise, associated with improved mitochondrial function [40], and the link between ROS, CR and longevity [34,35], have supported the concept of a mitohormetic adaptive response mediated by ROS (Fig. 3). Indeed, acute exercise stimulates expression of PGC1-α, resulting in increased mitochondrial content and oxidative capacity in skeletal muscle [41] and several reports have brought attention to the fact that oral administration of antioxidants prevent exercise-induced adaptation of muscle mitochondria [42]. In S. cerevisae, CR was shown to increase mitochondrial function and, inevitably, ROS formation [35]. The upregulation of genes associated with the endogenous antioxidant defence response has been proposed as a mechanism by which CR and exercise prevent age-related disorders. Nuclear factor erythroid 2-related factor-2 (Nrf2) is a transcription factor that regulates cellular redox status by promoting the antioxidant response elements (ARE) signaling, a pathway that becomes less active with aging [43]. Nrf2 also impacts mitochondrial function since Nrf2 is associated with impaired OXPHOS while activators of Nrf2 promote mitophagy and resistance to oxidative stress [44]. Recently, it has been shown that ablation of Nrf2 impairs mitohormesis in conditions of OXPHOS deficiency due to mitochondrial uncoupling [45].
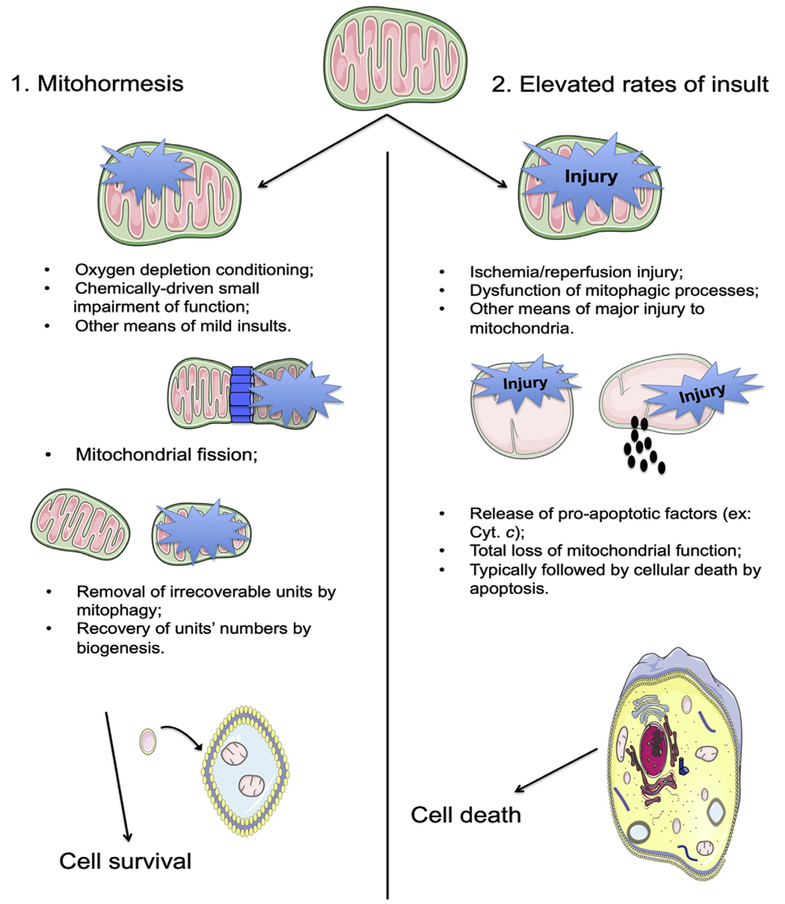
Fig. 3. Comparison between (1) mild, mitohormetic-inducing events and (2) highly injurious phenomena and respective downstream events.
A mild insult to a cell's mitochondrial population (such are, for example, small bursts of oxygen and nutrient deprivation, extremely low doses of mitochondrial inhibitors/toxicants) is a conditioning stimulus for the removal of older, less functional, more susceptible mitochondria from the population pool, allowing it to be repopulated by the generation of new, unharmed (biogenesis) mitochondria (1). However, if this insult is too prolonged or highly harmful, or if the cell has an inherent condition (for example, aged mitochondria with a lower threshold for damage or alterations in the typical players of the mitophagic event, such is the case with Parkin mutations in Parkinson's Disease) that renders it more sensitive to the insult, then ROS generation can be too elevated and widespread for the cell to repopulate the mitochondrial pool (2). In this case, the insult will normally lead to cell death, typically by apoptosis.
One of the first findings was in the nematode Caenorhabditis elegans, where reduced glucose availability causes an increase in both ROS and catalase activity, ultimately culminating in increased survival rates [46]. Several other studies have further demonstrated that many strategies promoting longevity share a common downstream: increased mitochondrial ROS. Inhibition of the mitochondrial ETC by certain mutations or inactivation of mitochondrial superoxide dismutase extends C. elegans lifespan [47]. Also low doses of rotenone, an inhibitor of mitochondrial complex I, has been shown to extend C. elegans lifespan [47] as well as to induce hormesis in primary human fibroblasts, an effect not possible in neither older cells nor with higher concentrations of rotenone [48]. Inhibition of the signaling mediated by the autophagy regulator mammalian target of rapamycin complex 1 (mTORC) and consequent induction of autophagy by caloric restriction or by pharmacological agents has also been found to promote longevity in yeast, worms, flies and mice [49].
Regulation of mitophagy and mitochondrial biogenesis by mitohormesis can mediate the positive impact of a mild induction of ROS signaling pathways. Animals with cardiac impaired mitophagy and consequent accumulation of damaged ROS-forming mitochondria develop cardiomyopathy, which can be surprisingly improved by the ROS-dependent activation of compensatory autophagic pathways of mitochondrial quality control, preventing a vicious cycle of ROS formation and mitochondrial dysfunction [50]. Also, double KO-AOX mice with a muscle-specific COX15 knockout and expressing alternative oxidases (AOXs), that bypass respiratory complexes III and IV, transferring electrons directly to oxygen, exhibit decreased ROS generation, PGC-1α signaling and lifespan [25]. Livers from adult mice in which mitochondrial superoxide dismutase 2 was depleted during embryonic development display mitochondrial adaptive responses with increased mitochondrial biogenesis and antioxidant defenses, while exhibiting decreased ROS [51].
5. Modulation of mitochondrial homeostasis by sirtuins and cAMP
Activation of signaling pathways involved in the regulation of mitochondrial homeostasis constitutes an important therapeutic target for metabolic diseases triggered by mitochondrial dysfunction. cAMP signaling and the sirtuins (SIRT1-7), a family of lysine deacylases that regulate the activity of enzymes, transcription factors and chromatin may constitute a central network at the crossroads of energy metabolism, metabolic diseases, and aging. Both these pathways converge into the regulation of mitochondrial function and ultimately maintain cellular energy homeostasis [7,9,52,53].
Sirtuins are activated by conditions of low cellular energy status that result in high NAD+ levels which SIRTs use as co-substrates to remove acyl moieties from lysines on histones and proteins [52,54]. SIRT1 is predominantly located in the nucleus and its activation stimulates mitochondrial biogenesis, thus preventing metabolic complications [55-59]. By migrating into mitochondria, SIRT 1 also deacetylates and increases SIRT3 activity [60], which localizes at the mitochondrial matrix where it deacetylates and regulates the activity of mitochondrial proteins including intermediary metabolism, fatty acid oxidation, OXPHOS and the oxidative stress response [9,61]. Resveratrol, a known SIRT1 activator that increases PGC-1α activity and prevents metabolic decline [8] has also been shown to stimulate mitophagy [62].
SIRT3 is also a known modulator of mitohormesis. In fact, several studies have demonstrated that the most famous member of the sirtuin family that localizes to mitochondria has a tremendous direct impact on mitochondrial function upon a mild stressing event. For example, when animals are subjected to caloric restriction, SIRT3 is fundamental for the tackling of oxidative stress [92,93], and its expression is upregulated by oxidative stress [94]. This is further supported by the fact that SIRT3 is paramount for the activity of the mitochondrial native manganese superoxide dismutase (MnSOD) [93] and loss of SIRT3 leads to oxidative stress, genomic mutations and elevated cancer proneness [95]. As such, it is unsurprising that SIRT3 leads to the activation of known tumor suppressing gene LKB1 [96], via a pathway that involves the activation of AMPK (strikingly, SIRT1 is also capable of activation of LKB1 [97]). The induction of SIRT3 by oxidative stress has a noticeable effect on mitochondrial metabolism [98,99]. Finally, it appears the unfolded protein response, a defense mechanism heavily involved in mitohormesis has in SIRT3 one of its critical components [100]. Given all of this, it is safe to assume that SIRT3 plays a role in the mitohormetic response.
While SIRT1 and 3 are by far the most studied sirtuin family members, in particular regarding mitochondrial function and the role of oxidative stress, it is not true that there are no known effects of the other family members. In fact, the cytosolic SIRT2 has been shown to regulate mitochondrial OXPHOS, ATP generation, mitochondrial dynamics, oxidative stress and mitochondrial biogenesis in normal and metabolic disease settings [71,72]. SIRT3 is not the only mitochondrial sirtuin, for SIRT4 and 5 are also native to mitochondria. SIRT4, thought to not be a deacetylase like SIRT1-3 but a NAD + -dependent ADP-ribosyltransferase, has a clear effect on mitochondrial quality control, metabolic regulation and ageing processes [73-75], and recent evidences also point to a rather interesting deacetylating activity of lysine residues [75]. SIRT5 is a lysine demalonylase [76] that is involved in mitochondrial dynamics regulation [77,78], ATP generation and metabolic fluxes [78,79], with impact in OXPHOS due to cardiolipin binding [80] and overall mitochondrial metabolic homeostasis [81]. Regarding SIRT6, which is a highly specific lysine deacetylase [82], it has been shown that it can protect mitochondria from ischemia/reperfusion injury by inhibition of inflammatory activity [83], from hyperglycemia through AMPK activation [84], from known mitochondrial toxicant and anticancerous agent doxorubicin [85], while also coordinating with p53 [86] and SIRT1 [87] to protect and boost mitochondrial function. Finally, SIRT 7 has also been shown to be a potent regulator of mitochondrial function, since its deletion leads to a reduction of the expression of nuclearly encoded mitochondrial genes, leading to mitochondrial dysfunction [88], while also playing key roles in metabolic regulation of mitochondrial function and biogenesis [89-91]. As such, it seems that there is a rather large overlap of functionality and targets between the different SIRT family members, which might appear to lead to some redundancy in function, but it is more probable to attribute this to a case of different effectors in various cellular compartments and with somewhat different functions that ultimately result in the same broad goals.
cAMP is a ubiquitous second messenger that in the mitochondria orchestrates mitochondrial fusion/fission, motility and mitophagy and acts as a mediator of metabolic signals regulating mitochondrial homeostasis and ROS generation [7,63]. The adenylyl cyclases (ACs) family comprises ten isoforms that are responsible for the conversion of cAMP [64]. In fact, a soluble AC (sAC) is described not only in nucleus and cytosol, but also in the mitochondria of cardiac, liver and skeletal muscle cells [65,70]. Since OXPHOS is regulated by cAMP generated inside mitochondria by sAC, the role of this isoform in the mechanisms regulating mitochondrial activity and consequently in the balance between energy storage and energy consumption have been under investigation [66]. Mitochondrial sAC, and thus the sAC/Protein Kinase A (PKA) axis, is stimulated by physiological bicarbonate concentrations, and its presence increases OXPHOS activity while limiting ROS production. In isolated mitochondria, stimulation of the mitochondrial cAMP pathway by bicarbonate has been shown to decrease the susceptibility to calcium-induced mitochondrial permeability transition, an abrupt increase in permeability of the inner membrane to solutes that uncouples OXPHOS [67]. In addition, cAMP levels have been shown to increase by activation of sAC [63]. In fasted mice, the induction of cAMP signaling enhances SIRT3 activity that deacetylates leucine-rich protein 130, resulting in increased mitochondrial transcription and improved OXPHOS efficiency [68]. Therefore, targeting sAC has been shown to improve bioenergetics defects, including COX activity, respiration and mitochondrial biogenesis [66]. Evidence also supports that the stimulation of cAMP signaling pathways with forskolin, an activator for the nine transmembrane members of the AC family, induces mitochondrially encoded genes, and thus that extra-mitochondrial cAMP signaling impacts mitochondrial function [68]. Another important link can also be established between cAMP and SIRT1. Activation of the cAMP signaling pathway has been shown to result in SIRT1 phosphorylation, increasing its deacetylase activity and stimulating fatty acid oxidation [69] (Fig. 2B). Taken together, these data support that the regulation of mitochondrial function is mediated by the activity of several AC isoforms, including cAMP effects on the activity of sirtuins [63,70]. Therefore, it is conceivable that alterations in the AC-SIRTs axis and the associated mitochondrial dysfunction could be linked to metabolic-related diseases (Fig. 4).
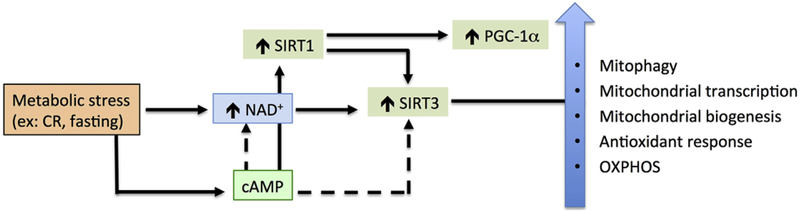
Fig. 4. cAMP-sirtuin crosstalk.
Conditions that increase NAD+ such as caloric restriction and fasting stimulate sirtuins (SIRT) 1 and 3 activities that converge into enhanced mitochondrial function, through deacetylation of mitochondrial proteins and increased mitochondrial mass. SIRT1 also deacetylates and increases SIRT3 activity, although it is unclear if this happens due to cytosolic or mitochondrial cyclic AMP (cAMP). cAMP signaling, indirectly (dashed lines) by increasing NAD+ or directly (solid lines) by phosphorylation increases sirtuin activity.
6. Conclusions and perspectives
In the last years, the risk associated with the indiscriminate use of antioxidants and the role of ROS as signaling molecules has come under the spotlight. ROS have been proposed as triggers of an adaptive response that strengthens cellular defensive mechanisms, as a strategy to maintain mitochondrial homeostasis and therefore prevent metabolic unbalance. By triggering mitophagy and biogenesis, low, non-toxic concentrations of ROS mediate the expansion of a healthy mitochondrial network able to use the reducing equivalents, generated by intermediary metabolism, thus preventing conditions of reductive stress and a vicious cycle of increased ROS generation and oxidative damage. Better knowledge about the signaling pathways that mediate mitonuclear communication and the mechanisms underlying mitohormesis may help to design therapies more effective in improving metabolic health.
Acknowledgements
This work was financed by the European Regional Development Fund, through the Centro 2020 Regional Operational Programme: project CENTRO-01-0145-FEDER-000012-HealthyAging2020, the Portugal 2020 - Operational Programme for Competitiveness and Internationalisation, and the Portuguese national funds via FCT – Fundação para a Ciência e a Tecnologia, I.P.: project POCI-01-0145-FEDER-016770, as well as by UID/NEU/04539/2013 (CNC.IBILI Consortium strategic project) and the Dr. Robert Pfleger Foundation (project mitochondrial Sirtuins of CS). JST and JAA are recipients of scholarships from FCT (SFRH/BPD/94036/2013 and PD/BD/114173/2016, respectively). DS is also supported by the Glenn Foundation for Medical Research and grants from the NIH (R37 AG028730, R01 AG019719 and R01 DK100263), and Epigenetics Seed Grant (601139_2018) from Department of Genetics, Harvard Medical School.
Abbreviations:
- AC
adenylyl cyclase
- AOX
alternative oxidase
- ARE
antioxidant response element
- cAMP
cyclic AMP
- COX
cytochrome c oxidase
- CR
caloric restriction
- DRP1
dynamin related protein 1
- ETC
electron transport chain
- mtDNA
mitochondrial DNA
- FIS1
mitochondrial fission 1 protein
- HDAC6
histone deacetylase 6
- LC3
microtubule-associated protein 1A/1B-light chain 3
- MFN
mitofusin
- mTORC
mammalian target of rapamycin complex 1
- NAC
N-acetyl-l-cysteine
- NRF
nuclear respiratory factor
- NRF-2
nuclear factor erythroid 2–related factor 2
- OMM
outer mitochondrial membrane
- OPA1
mitochondrial dynamin-like 120 kDa protein
- OXPHOS
oxidative phosphorylation
- p62/SQSTM1
p62/ signaling adaptor sequestosome 1
- PE
phosphatidylethanolamine
- PGC-1α
peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- PINK1
PTEN-induced kinase 1
- PKA
protein kinase A
- PTEN
phosphatase and tensin homolog
- ROS
reactive oxygen species
- sAC
soluble AC
- SIRT
sirtuin
- SOD
superoxide dismutase
- TCA
tricarboxylic acid cycle
- TFAM
mitochondrial transcription factor A
- VDAC
voltage-dependent anion channel
- ΔΨ
mitochondrial membrane potential
Footnotes
Author disclosure statement
D.A.S. is a founder, equity owner, advisor to, director of, consultant to, investor in and/or inventor on patents licensed to Vium, Jupiter Orphan Therapeutics, Cohbar, Galilei Biosciences, GlaxoSmithKline, OvaScience, EMD Millipore, Wellomics, Inside Tracker, Caudalie, Bayer Crop Science, Longwood Fund, Zymo Research, EdenRoc Sciences (and affiliates Arc-Bio, Dovetail Genomics, Claret Bioscience, Revere Biosensors, UpRNA and MetroBiotech, an NAD booster company, and Liberty Biosecurity). Life Biosciences (and affiliates Selphagy, Senolytic Therapeutics, Spotlight Biosciences, Iduna, Animal Biosciences, Iduna, Immetas, Continuum Biosciences, Jumpstart Fertility, an NAD booster company). DS sits on the boards of directors of both companies. DS is an inventor on a patent application filed by Mayo Clinic and Harvard Medical School that has been licensed to Elysium Health; his personal share is directed to the Sinclair lab. For more information see https://genetics.med.harvard.edu/sinclair-test/people/sinclair-other.php. The other authors declare no competing financial interest.
References
- [1].Cheng Z, Ristow M, Mitochondria and metabolic homeostasis, Antioxidants Redox Signal. 19 (2013) 240–242. [DOI] [PubMed] [Google Scholar]
- [2].Ristow M, Zarse K, How increased oxidative stress promotes longevity and metabolic health: the concept of mitochondrial hormesis (mitohormesis), Exp. Gerontol 45 (2010) 410–418. [DOI] [PubMed] [Google Scholar]
- [3].Shadel GS, Horvath TL, Mitochondrial ROS signaling in organismal homeostasis, Cell 163 (2015) 560–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bhatti JS, Bhatti GK, Reddy PH, Mitochondrial dysfunction and oxidative stress in metabolic disorders - a step towards mitochondria based therapeutic strategies, Biochim. Biophys. Acta (BBA) - Mol. Basis Dis 1863 (2017) 1066–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Liesa M, Shirihai OS, Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure, Cell Metabol. 17 (2013) 491–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yu SB, Pekkurnaz G, Mechanisms orchestrating mitochondrial dynamics for energy homeostasis, J. Mol. Biol 430 (2018) 3922–3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Di Benedetto G, Gerbino A, Lefkimmiatis K, Shaping mitochondrial dynamics: the role of cAMP signalling, Biochem. Biophys. Res. Commun 500 (2018) 65–74. [DOI] [PubMed] [Google Scholar]
- [8].Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro JS, Hubbard BP, Varela AT, Davis JG, Varamini B, Hafner A, Moaddel R, Rolo AP, Coppari R, Palmeira CM, de Cabo R, Baur JA, Sinclair DA, SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function, Cell Metabol. 15 (2012) 675–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Singh CK, Chhabra G, Ndiaye MA, Garcia-Peterson LM, Mack NJ, Ahmad N, The role of sirtuins in antioxidant and redox signaling, Antioxidants Redox Signal. 28 (2018) 643–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Stepien KM, Heaton R, Rankin S, Murphy A, Bentley J, Sexton D, Hargreaves IP, Evidence of oxidative stress and secondary mitochondrial dysfunction in metabolic and non-metabolic disorders, J. Clin. Med 6 (2017) E71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dai DF, Chiao YA, Marcinek DJ, Szeto HH, Rabinovitch PS, Mitochondrial oxidative stress in aging and healthspan, Longev. Heal 3 (6) (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].López-Lluch G, Hernández-Camacho JD, Fernández-Ayala DJM, Navas P, Mitochondrial dysfunction in metabolism and ageing: shared mechanisms and outcomes? Biogerontology 19 (6) (2018) 461–480. [DOI] [PubMed] [Google Scholar]
- [13].Palikaras K, Lionaki E, Tavernarakis N, Balancing mitochondrial biogenesis and mitophagy to maintain energy metabolism homeostasis, Cell Death Differ. 22 (2015) 1399–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Villena JA, New insights into PGC-1 coactivators: redefining their role in the regulation of mitochondrial function and beyond, FEBS J. 282 (2015) 647–672. [DOI] [PubMed] [Google Scholar]
- [15].Wenz T, Regulation of mitochondrial biogenesis and PGC-1α under cellular stress, Mitochondrion 13 (2013) 134–142. [DOI] [PubMed] [Google Scholar]
- [16].Baldelli S, Aquilano K, Ciriolo MR, PGC-1α buffers ROS-mediated removal of mitochondria during myogenesis, Cell Death Dis. 5 (2014) e1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Viscomi C, Bottani E, Civiletto G, Cerutti R, Moggio M, Fagiolari G, Schon EA, Lamperti C, Zeviani M, In vivo correction of COX deficiency by activation of the AMPK/PGC-1α Axis, Cell Metabol. 14 (2011) 80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Frank M, Duvezin-Caubet S, Koob S, Occhipinti A, Jagasia R, Petcherski A, Ruonala MO, Priault M, Salin B, Reichert AS, Mitophagy is triggered by mild oxidative stress in a mitochondrial fission dependent manner, Biochim. Biophys. Acta 1823 (2012) 2297–2310. [DOI] [PubMed] [Google Scholar]
- [19].Pickles S, Vigié P, Youle RJ, Mitophagy and quality control mechanisms in mitochondrial maintenance, Curr. Biol 28 (2018) R170–R185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tufi R, Gandhi S, de Castro IP, Lehmann S1, Angelova PR, Dinsdale D, Deas E, Plun-Favreau H, Nicotera P, Abramov AY, Willis AE, Mallucci GR, Loh SH, Martins LM, Enhancing nucleotide metabolism protects against mitochondrial dysfunction and neurodegeneration in a PINK1 model of Parkinson's disease, Nat. Cell Biol 16 (2014) 157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Murphy MP, How mitochondria produce reactive oxygen species, Biochem. J 417 (2009) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Quinlan CL, Perevoshchikova IV, Hey-Mogensen M, Orr AL, Brand MD, Sites of reactive oxygen species generation by mitochondria oxidizing different substrates, Redox Biol 1 (2013) 304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Teodoro JS, Rolo AP, Palmeira CM, The NAD ratio redox paradox: why does too much reductive power cause oxidative stress? Toxicol. Mech. Methods 23 (2013) 297–302. [DOI] [PubMed] [Google Scholar]
- [24].Teodoro JS, Gomes AP, Varela AT, Duarte FV, Rolo AP, Palmeira CM, NAD+/NADH ratio observed in the early phases of hyperglycemic exposure Uncovering the beginning of diabetes: the cellular redox status and oxidative stress as starting players in hyperglycemic damage, Mol. Cell. Biochem 376 (2013) 103–110. [DOI] [PubMed] [Google Scholar]
- [25].Dogan SA, Cerutti R, Benincá C, Brea-Calvo G, Jacobs HT, Zeviani M, Szibor M, Viscomi C, Perturbed redox signaling exacerbates a mitochondrial myopathy, Cell Metabol. (2018) 30455–30458 pii: S1550-4131(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jain SS, Paglialunga S, Vigna C, Ludzki A, Herbst EA, Lally JS, Schrauwen P, Hoeks J, Tupling AR, Bonen A, Holloway GP, High-fat diet-induced mitochondrial biogenesis is regulated by mitochondrial-derived reactive oxygen species activation of CaMKII, Diabetes 63 (2014) 1907–1913. [DOI] [PubMed] [Google Scholar]
- [27].St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jäger S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM, Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators, Cell 127 (2006) 397–408. [DOI] [PubMed] [Google Scholar]
- [28].Irrcher I, Ljubicic V, Hood DA, Interactions between ROS and AMP kinase activity in the regulation of PGC-1α transcription in skeletal muscle cells, Am. J. Physiol. Cell Physiol 296 (2009) C116–C123. [DOI] [PubMed] [Google Scholar]
- [29].Valle I, Álvarez-Barrientos A, Arza E, Lamas S, Monsalve M, PGC-1α regulates the mitochondrial antioxidant defense system in vascular endothelial cells, Cardiovasc. Res 66 (2005) 562–573. [DOI] [PubMed] [Google Scholar]
- [30].Li L, Chen Y, Gibson SB, Starvation-induced autophagy is regulated by mitochondrial reactive oxygen species leading to AMPK activation, Cell. Signal 25 (2013) 50–65. [DOI] [PubMed] [Google Scholar]
- [31].Xiao B, Goh JY, Xiao L, Xian H, Lim KL, Liou YC, Reactive oxygen species trigger Parkin/PINK1 pathway-dependent mitophagy by inducing mitochondrial recruitment of Parkin, J. Biol. Chem 292 (2017) 16697–16708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Xiao B, Deng X, Lim GGY, Xie S, Zhou ZD, Lim KL, Tan EK, Superoxide drives progression of Parkin/PINK1-dependent mitophagy following translocation of Parkin to mitochondria, Cell Death Dis. 8 (2017) e3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Calabrese EJ, Hormesis: improving predictions in the low-dose zone, EXS 101 (2012) 101–164. [DOI] [PubMed] [Google Scholar]
- [34].Sanz A, Mitochondrial reactive oxygen species: do they extend or shorten animal lifespan? Biochim. Biophys. Acta 1857 (2016) 1116–1126. [DOI] [PubMed] [Google Scholar]
- [35].Sharma PK, Agrawal V, Roy N, Mitochondria-mediated hormetic response in life span extension of calorie-restricted Saccharomyces cerevisiae, Age (Dordr) 33 (2011) 143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Harman D, The biologic clock: the mitochondria? J. Am. Geriatr. Soc 20 (1972) 145–147. [DOI] [PubMed] [Google Scholar]
- [37].Ristow M, Unraveling the truth about antioxidants: mitohormesis explains ROS-induced health benefits, Nat. Med 20 (2014) 709–711. [DOI] [PubMed] [Google Scholar]
- [38].Albanes D, Heinonen OP, Taylor PR, Virtamo J, Edwards BK, Rautalahti M, Hartman M, Palmgren J, Freedman LS, Haapakoski J, Barrett MJ, Pietinen P, Malila N, Tala E, Liippo K, Salomaa ER, Tangrea JA, Teppo L, Askin FB, Taskinen E, Erozan Y, Greenwald P, Huttunen JK, Alpha-tocopherol and beta-carotene supplements and lung cancer incidence in the alpha-tocopherol, beta-carotene cancer prevention study: effects of base-line characteristics and study compliance, J. Natl. Cancer Inst 88 (1996) 1560–1270. [DOI] [PubMed] [Google Scholar]
- [39].Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, Ross C, Arnold A, Sleight P, Probstfield J, Dagenais GR, HOPE and HOPE-TOO Trial Investigators, Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial, J. Am. Med. Assoc 293 (2005) 1338–1347. [DOI] [PubMed] [Google Scholar]
- [40].Irving BA, Lanza IR, Henderson GC, Rao RR, Spiegelman BM, Nair KS, Combined training enhances skeletal muscle mitochondrial oxidative capacity independent of age, J. Clin. Endocrinol. Metab 100 (2015) 1654–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chow LS, Greenlund LJ, Asmann YW, Short KR, McCrady SK, Levine JA, Nair KS, Impact of endurance training on murine spontaneous activity, muscle mitochondrial DNA abundance, gene transcripts, and function, J. Appl. Physiol 102 (2007) 1078–1089. [DOI] [PubMed] [Google Scholar]
- [42].Ristow M, Zarse K, Oberbach A, Klöting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Blüher M, Antioxidants prevent health-promoting effects of physical exercise in humans, Proc. Natl. Acad. Sci. U. S. A 106 (2009) 8665–8670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zhang H, Davies KJA, Forman HJ, Oxidative stress response and Nrf2 signaling in aging, Free Radic. Biol. Med 88 (2015) 314–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Holmstrom KM, Rostov RV, Dinkova-Kostova AT, The multifaceted role of Nrf2 in mitochondrial function, Curr. Opin. Toxicol 1 (2016) 80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Coleman V, Sa-Nguanmoo P, Koenig J, Schulz TJ, Grune T, Klaus S, Kipp AP, Ost M, Partial involvement of Nrf2 in skeletal muscle mitohormesis as an adaptive response to mitochondrial uncoupling, Sci. Rep 8 (2018) 2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M, Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress, Cell Metabol. 6 (2007) 280–293. [DOI] [PubMed] [Google Scholar]
- [47].Dancy BM, Sedensky MM, Morgan PG, Effects of the mitochondrial respiratory chain on longevity in C. elegans, Exp. Gerontol 56 (2014) 245–255. [DOI] [PubMed] [Google Scholar]
- [48].Marthandan S, Priebe S, Groth M, Guthke R, Platzer M, Hemmerich P, Diekmann S, Hormetic effect of rotenone in primary human fibroblasts, Immun. Ageing 12 (2015) 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Lamming DW, Inhibition of the mechanistic target of rapamycin (mTOR)-Rapamycin and beyond, Cold Spring Harb. Perspect. Med 6 (2016) a025924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Song M, Chen Y, Gong G, Murphy E, Rabinovitch PS, Dorn GW 2nd, Supersuppression of mitochondrial reactive oxygen species signaling impairs compensatory autophagy in primary mitophagic cardiomyopathy, Circ. Res 115 (2014) 348–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Cox CS, McKay SE, Holmbeck MA, Christian BE, Scortea AC, Tsay AJ, Newman LE, Shadel GS, Mitohormesis in mice via sustained basal activation of mitochondrial and antioxidant signaling, Cell Metabol. (2018) 30454–30456 S1550-4131(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Elhassan YS, Philp AA, Lavery GG, Targeting NAD+ in metabolic disease: new insights into an old molecule, J. Endocr. Soc 1 (2017) 816–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Zhang F, Zhang L, Qi Y, Xu H, Mitochondrial cAMP signaling, Cell. Mol. Life Sci 73 (2016) 4577–4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Sauve AA, Celic I, Avalos J, Deng H, Boeke JD, Schramm VL, Chemistry of gene silencing: the mechanism of NAD+-dependent deacetylation reactions, Biochemistry 40 (2001) 15456–15463. [DOI] [PubMed] [Google Scholar]
- [55].Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA, Resveratrol improves health and survival of mice on a high calorie diet, Nature 444 (2006) 337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Herranz D, Muñoz-Martin M, Cañamero M, Mulero F, Martinez-Pastor B, Fernandez-Capetillo O, Serrano M, Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer, Nat. Commun 1 (2010) 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Minor RK, Baur JA, Gomes AP, Ward TM, Csiszar A, Mercken EM, Abdelmohsen K, Shin YK, Canto C, Scheibye-Knudsen M, Krawczyk M, Irusta PM, Martín-Montalvo A, Hubbard BP, Zhang Y, Lehrmann E, White AA, Price NL, Swindell WR, Pearson KJ, Becker KG, Bohr VA, Gorospe M, Egan JM, Talan MI, Auwerx J, Westphal CH, Ellis JL, Ungvari Z, Vlasuk GP, Elliott PJ, Sinclair DA, de Cabo R, SRT1720 improves survival and healthspan of obese mice, Sci. Rep 1 (2011) 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschöp MH, Sirt1 protects against high-fat diet-induced metabolic damage, Proc. Natl. Acad. Sci. U.S.A 105 (2008) 9793–9798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Yuan Y, Cruzat VF, Newsholme P, Cheng J, Chen Y, Lu Y, Regulation of SIRT1 in aging: roles in mitochondrial function and biogenesis, Mech. Ageing Dev 155 (2016) 10–21. [DOI] [PubMed] [Google Scholar]
- [60].Kwon S, Seok S, Yau P, Li X, Kemper B, Kemper JK, Obesity and aging diminish sirtuin 1 (SIRT1)-mediated deacetylation of SIRT3, leading to hyperacetylation and decreased activity and stability of SIRT3, J. Biol. Chem 292 (2017) 17312–17323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Scher MB, Vaquero A, Reinberg D, SirT3 is a nuclear NAD+-dependent histone deacetylase that translocates to the mitochondria upon cellular stress, Genes Dev. 21 (2007) 920–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Ren X, Chen L, Xie J, Zhang Z, Dong G, Liang J, Liu L, Zhou H, Luo P, Resveratrol ameliorates mitochondrial elongation via drp1/parkin/PINK1 signaling in senescent-like cardiomyocytes, Oxid. Med. Cell Longev 2017 (2017) 4175353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Acin-Perez R, Salazar E, Kamenetsky M, Buck J, Levin LR, Manfredi G, Cyclic AMP produced inside mitochondria regulates oxidative phosphorylation, Cell Metabol. 9 (2009) 265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Kumar S, Kostin S, Flacke JP, Reusch HP, Ladilov Y, Soluble adenylyl cyclase controls mitochondria-dependent apoptosis in coronary endothelial cells, J. Biol. Chem 284 (2009) 14760–14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Zippin JH, Chen Y, Nahirney P, Kamenetsky M, Wuttke MS, Fischman DA, Levin LR, Buck J, Compartmentalization of bicarbonate-sensitive adenylyl cyclase in distinct signaling microdomains, FASEB J. 17 (2003) 82–84. [DOI] [PubMed] [Google Scholar]
- [66].Acin-Perez R, Salazar E, Brosel S, Yang H, Schon EA, Manfredi G, Modulation of mitochondrial protein phosphorylation by soluble adenylyl cyclase ameliorates cytochrome oxidase defects. EMBO Mol. Med 1 (2009) 392–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Wang Z, Liu D, Varin A, Nicolas V, Courilleau D, Mateo P, Caubere C, Rouet P, Gomez AM, Vandecasteele G, Fischmeister R, Brenner C, A cardiac mitochondrial cAMP signaling pathway regulates calcium accumulation, permeability transition and cell death, Cell Death Dis. 7 (2016) e2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Liu L, Nam M, Fan W, Akie TE, Hoaglin DC, Gao G, Keaney JF Jr., Cooper MP, Nutrient sensing by the mitochondrial transcription machinery dictates oxidative phosphorylation, J. Clin. Investig 124 (2014) 768–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Gerhart-Hines Z, Dominy JE Jr., Blättler SM, Jedrychowski MP, Banks AS, Lim JH, Chim H, Gygi SP, Puigserver P, The cAMP/PKA pathway rapidly activates SIRT1 to promote fatty acid oxidation independently of changes in NAD(+), Mol. Cell 44 (2011) 851–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Acin-Perez R, Russwurm M, Günnewig K, Gertz M, Zoidl G, Ramos L, Buck J, Levin LR, Rassow J, Manfredi G, Steegborn C, A phosphodiesterase 2A isoform localized to mitochondria regulates respiration, J. Biol. Chem 286 (2011) 30423–30432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Liu G, Park SH, Imbesi M, Nathan WJ, Zou X, Zhu Y, Jiang H, Parisiadou L, Gius D, Loss of NAD-dependent protein deacetylase Sirtuin-2 alters mitochondrial protein acetylation and dysregulates mitophagy, Antioxidants Redox Signal. 26 (15) (2017) 849–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Lemos V, de Oliveira RM, Naia L, Szegö É, Ramos E, Pinho S, Magro F, Cavadas C, Rego AC, Costa V, Outeiro TF, Gomes P, The NAD+-dependent deacetylase SIRT2 attenuates oxidative stress and mitochondrial dysfunction and improves insulin sensitivity in hepatocytes, Hum. Mol. Genet 26 (21) (2017) 4105–4117. [DOI] [PubMed] [Google Scholar]
- [73].Lang A, Piekorz RP, Novel role of the SIRT4-OPA1 axis in mitochondrial quality control, Cell Stress 2 (1) (2017) 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Parik S, Tewary S, Ayyub C, Kolthur-Seetharam U, Loss of mitochondrial SIRT4 shortens lifespan and leads to a decline in physical activity, J. Biosci 43 (2) (2018) 243–247. [PubMed] [Google Scholar]
- [75].Anderson KA, Huynh FK, Fisher-Wellman K, Stuart JD, Peterson BS, Douros JD, Wagner GR, Thompson JW, Madsen AS, Green MF, Sivley RM, Ilkayeva OR, Stevens RD, Backos DS, Capra JA, Olsen CA, Campbell JE, Muoio DM, Grimsrud PA, Hirschey MD, SIRT4 is a lysine deacetylase that controls leucine metabolism and insulin secretion, Cell Metabol. 25 (2017) 838–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Peng C, Lu Z, Xie Z, Cheng Z, Chen Y, Tan M, Luo H, Zhang Y, He W, Yang K, Zwaans BM, Tishkoof D, Ho L, Lombard D, He TC, Dai J, Verdin E, Ye Y, Zhao Y, The first identification of lysine malonylation substrates and its regulatory enzyme, Mol. Cell. Proteom 10 (12) (2011) M111.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Guedouari H, Daigle T, Scorrano L, Hebert-Chatelain E, Sirtuin 5 protects mitochondria from fragmentation and degradation during starvation, Biochim. Biophys. Acta Mol. Cell Res 1864 (1) (2017) 169–176. [DOI] [PubMed] [Google Scholar]
- [78].Zhang M, Wu J, Sun R, Tao X, Wang X, Kang Q, Wang H, Zhang L, Liu P, Zhang J, Xia Y, Zhao Y, Yang Y, Xiong Y, Guan K-L, Zou Y, Ye D, SIRT5 deficiency suppresses mitochondrial ATP production and promotes AMPK activation in response to energy stress, PLoS One 14 (2) (2019) e0211796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Nishida Y, Rardin MJ, Carrico C, He W, Sahu AK, Gut P, Najjar R, Fitch M, Hellerstein M, Gibson BW, Verdin E, SIRT5 regulates both cytosolic and mitochondrial protein malonylation with glycolysis as a major target, Mol. Cell 59 (2) (2015) 321–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Zhang Y, Bharathi SS, Rardin MJ, Lu J, Maringer KV, Sims-Lucas S, Prochownik EV, Gibson BW, Goetzman ES, Lysine desuccinylase SIRT5 binds to cardiolipin and regulates the electron transport chain, J. Biol. Chem 292 (24) (2017) 10239–10249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Wang G, Meyer JG, Cai W, Li ME, Softie S, Kahn CR, Sirt5 plays a critical role in mitochondrial protein acylation and mitochondrial metabolic homeostasis in brown fat, Diabetes 67 (Supplement 1) (2018) 274-OR. [Google Scholar]
- [82].Michishita E, McCord RA, Berber E, Lioi M, Padilla-Nash H, Damian M, Cheung P, Kusumoto R, Kawahara TL, Barrett JC, Chang HY, Bohr VA, Ried T, Gozani O, Chua KF, SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin, Nature 452 (7186) (2008) 492–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Cheng M-Y, Cheng Y-W, Yan J, Hu X-Q, Zhang H, Wang Z-R, Yin Q, Cheng W, SIRT6 suppresses mitochondrial defects and cell death via the NF-kB pathway in myocardial hypoxia/reoxygenation induced injury, Am. J. Transl. Res 8 (11) (2016) 5005–5015. [PMC free article] [PubMed] [Google Scholar]
- [84].Fan Y, Yang Q, Yang Y, Gao Z, Ma Y, Zhang L, Liang W, Ding G, Sirt6 suppresses high glucose-induced mitochondrial dysfunction and apoptosis in podocytes through AMPK activation, Int. J. Biol. Sci 15 (3) (2019) 701–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Pillai V, Samant S, Kanwal A, Gupta MP, The nuclear deacetylase Sirt6 protects mitochondria and mitigates chemotherapy-induced cardiomyocyte senescence, Circulation 138 (2018) A14800. [Google Scholar]
- [86].Li M, Hou T, Gao T, Lu X, Yang Q, Zhu Q, Li Z, Liu C, Mu G, Liu G, Bao Y, Wen H, Wang L, Wang H, Zhao Y, Gu W, Yang Y, Zhu W-G, p53 cooperates with SIRT6 to regulate cardiolipin de novo biosynthesis, Cell Death Dis. 9 (2018) 941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Liu TF, Vachharajani VT, Yoza BK, McCall CE, NAD+-dependent sirtuin 1 and 6 proteins coordinate a switch from glucose to fatty acid oxidation during the acute inflammatory response, J. Biol. Chem 287 (31) (2012) 25758–25769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Ryu D, Jo YS, Sasso GL, Stein S, Zhang H, Perino A, Lee JU, Zeviani M, Romand R, Hottiger MO, Schoonjans K, Auwerx J, A SIRT7-dependent acetylation switch of GABPß1 controls mitochondrial function, Cell Metabol. 20 (5) (2014) 856–869. [DOI] [PubMed] [Google Scholar]
- [89].Yan WW, Liang YL, Zhang QX, Wang D, Lei MZ, Qu J, He XH, Lei QY, Wang YP, Arginine methylation of SIRT7 couples glucose sensing with mitochondrial biogenesis, EMBO Rep. 19 (12) (2018) e46377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Wronska A, Lawniczak A, Wierzbicki PM, Kmiec Z, Age-related changes in Sirtuin 7 expression in calorie-restricted and refed rats, Gerontology 62 (2016) 304–310. [DOI] [PubMed] [Google Scholar]
- [91].Fang J, Ianni A, Smolka C, Vakhrusheva O, Nolte H, Krüger M, Wietelmann A, Simonet NG, Adrian-Segarra JM, Vaquero A, Braun T, Bober E, Sirt7 promotes adipogenesis in the mouse by inhibiting autocatalytic activation of Sirt1, Proc Nat Acad Sci USA 114 (40) (2017) E8352–E8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, Prolla TA, Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction, Cell 143 (5) (2010) 802–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Qiu X, Brown K, Hirschey MD, Verdin E, Chen D, Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation, Cell Metabol. 12 (6) (2010) 662–667. [DOI] [PubMed] [Google Scholar]
- [94].Hirschey MD, Shimazu T, Goetzman E, Jing Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD, Li Y, Saha AK, Ruderman NB, Bain JR, Newgard CB, Farese RV Jr., Alt FW, Kahn CR, Verdin D, SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation, Nature 464 (7285) (2010) 121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Kim HS, Patel K, Muldoon-Jacobs K, Bisht KS, Aykin-Burns N, Pennington JD, van der Meer R, Nguyen P, Savage J, Owens KM, Vassilopoulos A, Ozden O, Park SH, Singh KK, Abdulkadir SA, Spitz DR, Deng CX, Gius D, SIRT3 is a mitochondria-localized tumor suppressor required for maintenance of mitochondrial integrity and metabolism during stress, Cancer Cell 17 (1) (2010) 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Pillai VB, Sundaresan NR, Kim G, Gupta M, Rajamohan SB, Pullai JB, Samant S, Ravindra PV, Isbatan A, Gupta MP, Exogenous NAD blocks cardiac hypertrophic response via activation of the SIRT3-LKB1-AMP-activated kinase pathway, J. Biol. Chem 285 (2010) 3133–3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Lan F, Cacicedo JM, Ruderman N, Ido Y, SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1: possible role in AMP-activated protein kinase activation, J. Biol. Chem 283 (2008) 27628–27635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Jing E, Emanuelli B, Hirschey MD, Boucher J, Lee KY, Lombard D, Verdin EM, Kahn CR, Sirtuin-3 (Sirt3) regulates skeletal muscle metabolism and insulin signaling via altered mitochondrial oxidation and reactive oxygen species production, Proc Nat Acad Sci USA 108 (35) (2011) 14608–14613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Zhang B, Cui S, Bai X, Zhuo L, Sun X, Hong Q, Fu B, Wang J, Chen X, SIRT3 overexpression antagonizes high glucose accelerated cellular senescence in human diploid fibroblasts via the SIRT3-FOXO1 signaling pathway, Age 35 (6) (2013) 2237–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Yi H-S, Chang JY, Shong M, The mitochondrial unfolded protein response and mitohormesis: a perspective on metabolic diseases, J. Mol. Endocrinol 61 (5) (2018) R91–R105. [DOI] [PMC free article] [PubMed] [Google Scholar]