Abstract
We set out to examine the relations between prenatal exposure to the natural disaster Superstorm Sandy, maternal depression, and offspring electrodermal activity (EDA). EDA was measured via skin conductance response (SCR) magnitude in 198 children (M = 42.54 months, SD = 12.76) during a startle paradigm. In keeping with prior research, we expected prenatal depression to be associated with hyporeactive EDA and prenatal stress to be associated with hyperreactive EDA. SCR magnitude was lower in children prenatally exposed to depression alone, when compared to Superstorm Sandy, and controls. SCR magnitude of children prenatally exposed to both maternal depression and the storm was lower than that of all other groups. Our results emphasize the influence of maternal prenatal mental health, support targeted risk assessment for children who experienced an adverse prenatal environment, and highlight the need for a deeper understanding of the interactions between maternal mood and stress on the developing child.
Keywords: electrodermal activity, stress, depression, natural disasters
1. Introduction
The accelerated pace of development in offspring (i.e., a fetus) during gestation makes the prenatal period particularly susceptible to disruption stemming from environmental insults (Barker, 1998; Drake, Tang, & Nyirenda, 2007). Cortisol, a hormone produced by the maternal hypothalamo-pituitary-adrenal (HPA) axis, is secreted in response to stressors that she experiences and in association with psychological conditions, including depression (Field et al., 2004; C. A. Sandman, Wadhwa, Chicz-DeMet, Porto, & Garite, 1999). In utero maternal cortisol can cross the placenta to the fetal compartment and may activate a cascade of epigenetic, hormonal, and immune changes affecting the development of the central and peripheral nervous systems (Chrousos, 1992; Glover, 2011; Van den Bergh, Mulder, Mennes, & Glover, 2005; Wadhwa, Sandman, & Garite, 2001; W. Zhang et al., 2018). Acute prenatal maternal stress (PNMS) that pregnant women experience, such as natural disasters, and/or mental health issues including depression may therefore inadvertently increase their offspring's risk for suboptimal development. The aim of this study is to evaluate one aspect of sympathetic nervous system (SNS) functioning via skin conductance in the offspring of women who endured a natural disaster, Superstorm Sandy, and/or depression during the index pregnancy.
Experimental manipulations of PNMS in animals have demonstrated that gestational stress levels are associated with altered offspring HPA axis function, brain development, and stress response (Ehrlich & Rainnie, 2015; Langley-Evans, 2007; Van den Hove et al., 2014). Human studies are often restricted to relying on normative stress levels during pregnancy (e.g., common stressful life events), which may lack acute negative valence, when examining associations between PNMS and offspring emotion dysregulation, difficult temperament, and altered stress response (Austin, Leader, & Reilly, 2005; Baibazarova et al., 2013; Buthmann et al., 2018; Davis et al., 2007; O’Connor, Heron, Golding, Beveridge, & Glover, 2002; Zhu et al., 2014). Quasi-experimental studies of natural disasters during pregnancy have afforded researchers with the opportunity to evaluate offspring exposed to the acute stressor quasi-randomly while controlling for other potential confounders, although results have been mixed. For example, exposure to the Quebec Ice Storm of 1998 was associated with behavioral and cognitive aberrations (King, Dancause, Turcotte-Tremblay, Veru, & Laplante, 2012; King & Laplante, 2005) among affected offspring. Subsequent studies focused on devastating floods found associations between the 2008 Iowa Flood and altered offspring cortisol stress response (Yong Ping et al., 2015), whereas exposure to the 2011 Queensland Flood was associated with maladaptive interpersonal skills in early childhood (Simcock et al., 2017). Further, in utero exposure to Hurricane Katrina (2005) was not associated with measures of offspring temperament (Tees et al., 2010), whereas a study of the repercussions of Superstorm Sandy found increased temperament problems over the first two years of life among exposed offspring (Nomura et al., 2019; Wei Zhang, Finik, et al., 2018). Few studies have prospectively collected objective biological measures or followed the index cohort through early childhood to decipher the long-term consequences of PNMS.
Kopala-Sibley and colleagues have already demonstrated that postnatal exposure to Superstorm Sandy was associated with heightened depression and anxiety symptoms in children who already exhibited negative emotional traits (Kopala-Sibley, Danzig, et al., 2016; Kopala-Sibley, Kotov, et al., 2016). The same group also demonstrated that reactivity at the neural level to negative-valence images became more exaggerated following exposure to this storm in the postnatal period (Kessel et al., 2018). These findings strengthen the idea that prenatal exposure to the same storm may lead to meaningful vulnerabilities as children develop.
A US Preventive Services Task Force estimates that one in seven women experience a depressive disorder during pregnancy (Curry et al., 2019). The fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5; American Psychiatric Association, 2013) now includes a peripartum onset specifier, noting that half of commonly known postpartum episodes begin during pregnancy. Researchers have identified increased risks among offspring of women who experience PNMD, many of which are theorized to be consequences of elevated maternal cortisol levels and dysregulated immune function. Davis and colleagues found that PNMD was associated with higher maternal cortisol, higher neonatal cortisol, poor habituation, lower vagal tone, and higher negative reactivity at two months of age (Davis et al., 2007, 2004). Similarly, additional studies have found associations between PNMD symptoms and poor infant temperament profiles (McGrath, Records, & Rice, 2008; Nomura et al., 2019; Wei Zhang, Finik, et al., 2018) and antisocial behaviors at 16-17 years of age (Hay, Pawlby, Waters, Perra, & Sharp, 2010).
Few studies have specifically examined offspring outcomes of PNMS in conjunction with PNMD. Stress is often used as a broad term in research and is applied to identify normative strains, role conflicts, chronic strains, daily hassles, natural or manmade disasters, and psychological symptoms and disorders, such as anxiety and depression (Glover, 2014). In studies focused on the latter, Austin and colleagues found that prenatal anxiety and its interaction with postnatal maternal depression, but not PNMD independently or number of stressful life events predicted poor infant temperament (Austin, Hadzi-Pavlovic, Leader, Saint, & Parker, 2005). More recently Nomura and colleagues looked to elucidate the effects of PNMD and a natural disaster, Superstorm Sandy. They found that PNMD was associated with infant temperament profiles characterized by high negative affect and low emotion regulation, and the interaction of PNMD and Superstorm Sandy exposure exacerbated infant temperament problems (Nomura et al., 2019).
A common gap in studies within this field is availability of objective (i.e., biological) measures of 'stress' with its association to child outcomes past infancy/toddlerhood. The aim of this study is to evaluate the relations between PNMS and PNMD in association with offspring autonomic nervous system (ANS) functioning in early childhood as captured by one measure of the sympathetic nervous system, skin conductance response (SCR) magnitude. One prior study has examined offspring ANS function in relation to PNMS and PNMD exposure. van Dijk and colleagues found no relation between PNMD or measures of psychosocial stressors and cardiac measures of the sympathetic and parasympathetic nervous system (SNS and PNS, respectively) in children 5-6 years old at rest (van Dijk, van Eijsden, Stronks, Gemke, & Vrijkotte, 2012). The present study investigates associations between PNMD, PNMS in the form a specific stressful event (i.e., Superstorm Sandy), and one aspect of SNS reactivity via SCR magnitude to startling stimuli in preschool aged children.
The HPA axis and ANS both respond to stressful stimuli and researchers have identified dysfunction in both systems in association with emotion dysregulation and psychopathology (Chen, Raine, Glenn, & Granger, 2015; Dieleman et al., 2015). Furthermore, corticotrophin releasing hormone (which stimulates cortisol production via adrenocorticotropic hormone) that stems from the central nucleus of the amygdala is known to activate noradrenaline-producing neurons in the locus coeruleus (Curtis, Bello, Connolly, & Valentino, 2002). Noradrenaline drives the SNS and the “fight or flight” response to stressors. Electrodermal activity (EDA), also referred to as skin conductance, is considered a measure of SNS that is recorded as the amount of eccrine sweat gland production (Kandel, Schwartz, Jessell, Siegelbaum, & Hudspeth, 2014). It is important to note that no one measure can fully represent the function of the entire SNS (Jänig & Häbler, 2000), but that EDA is largely separate from parasympathetic modification. EDA hyperactivity has been associated with fearfulness and inhibition (Fowles, Kochanska, & Murray, 2000; Scarpa, Raine, Venables, & Mednick, 1997), whereas hypoactivity has been associated with aggression (Gao, Tuvblad, Schell, Baker, & Raine, 2015), attention deficit hyperactivity disorder (ADHD) and oppositional defiant disorder (ODD; Crowell et al., 2006), and depression (Sarchiapone et al., 2018).
Data for this study are drawn from a prospective birth cohort study following a diverse group of pregnant women exposed to Superstorm Sandy, which impacted the New York metropolitan area in October 2012 (Finik and Nomura, 2017). The storm killed 53 people in New York and 117 total (Centers for Disease Control and Prevention, 2013). Nearly 8 million customers were without electricity in the northeastern U.S., gasoline was scarce, and public transportation systems sustained damage that continues to be under repair (CNN Library, n.d.). Information regarding maternal storm exposure was collected prospectively via self-report. Maternal mental health was collected at the second trimester, and offspring ANS function was evaluated at the preschool age.
We aim to address three major questions. First, we surveyed the electrodermal function of children prenatally exposed to an acute, specific, non-normative stressful event, i.e., Superstorm Sandy. Second, we observed the electrodermal function of offspring as a function of exposure to PNMD in early childhood. Finally, we investigated the nature of the interplay between PNMS and PNMD on offspring electrodermal function by examining the interaction term between PNMS and PNMD. Following reports that PNMS is associated with more fearfulness (e.g., Austin et al., 2005) and stress response (e.g., Davis et al., 2007), we hypothesized that children prenatally exposed to Superstorm Sandy are more electrodermally reactive to startling stimuli as indicated by greater SCR magnitude. Following research connecting depression with hypoactive electrodermal activity (Sarchiapone et al., 2018), we further hypothesized that children exposed to PNMD have lower SCR magnitude to startling stimuli. Our third aim is exploratory and we therefore made no hypotheses regarding the electrodermal pattern of children exposed to both PNMS and PNMD.
2. Methods
2.1. Participants
Participants for this study were drawn from an ongoing prospective birth cohort study. Recruitment took place at the Ob/Gyn clinics at Mount Sinai Hospital, located in East Harlem roughly one mile from the East River, which surged and flooded a major highway during Superstorm Sandy. Recruitment was expanded to New York Presbyterian Queens Hospital in 2012, which serves Queens and western Long Island. It is located approximately two and half miles from Flushing Bay, which surged and caused flooding during Superstorm Sandy, and fifteen miles from the Rockaway Beach area that sustained severe damage from the storm. HIV infection, maternal psychosis, maternal age < 15 years, or life-threatening medical complications of the fetus constituted criteria for exclusion. The institutional review boards at the Icahn School of Medicine at Mount Sinai, New York Presbyterian Queens Hospital, and City University of New York approved all study procedures to ensure compliance with The Code of Ethics of the World Medical Association and all participants gave written informed consent.
Briefly, the relevant study procedures are as follows. Women were recruited and provided informed consent during their second trimester. Information regarding mothers' mental, physical, and socioemotional health was collected following consent. Superstorm Sandy experience was recorded prospectively or at recruitment, depending on the timing of recruitment in relation to the storm. At 3-4 years postpartum the mother and child were invited to CUNY Queens College for a comprehensive follow-up assessment. Further information can be found elsewhere (Finik & Nomura, 2017). Psychophysiological data were recorded during a startle probe paradigm described in detail below.
Of the 329 children who completed a follow up assessment, 104 had EDA data that was not usable due to excess artefact in the raw signal or technical malfunction. A further 29 children were missing maternal mental health data, yielding N = 196. Younger children were more likely to have data with artefact or technical malfunction (age in months of children with artefact M = 38.75, SD = 13.95; children without artefact M = 42.85, SD = 12.43, p = .013). No other significant differences were found with respect to child gender, in utero storm exposure, prevalence of maternal depression, or skin conductance response magnitude (all p > .52). The attrition rate from infant assessments to postnatal follow up was 32%. See Finik and Nomura (2017) for details.
2.2. Procedure
Children were seated next to their mothers and asked to remain still while passively watching the computer monitor in front of them for the duration of the experiment. The computer displayed a relaxing, child-friendly nature scene depicting panda bears in a zoo. The experiment room was kept at 71.6-75.2 °F, as recommended (Braithwaite, Watson, Jones, & Rowe, 2013). The door to the room was closed with an unoccupied room and another door between the experiment room and a communal hallway to minimize noise. A three minute baseline period was followed by a one minute period of six 90 dB auditory stimuli one second in duration with varying inter-stimulus intervals of 6-12 seconds was presented with E-Prime 2.0.
2.3. Measures
2.3.1. Electrodermal activity.
Two pre-gelled disposable Ag/AgCl electrodes were placed on the left side of the left foot of the children with .05 molar NaCl gel to improve the conduction of the signal added as needed. A BioNomadix Wireless EDA transmitter connected to the electrodes via snap connection and transmitted the EDA signal at 2000 Hz to a Biopac MP150 acquisition system and a computer running Acqknowledge 4.4 software (Biopac, Goleta, CA). To prevent distress unrelated to the stimuli, the child’s mother was present in me room. 60 Hz and 1 Hz noise were filtered from the signal. A skin conductance response (SCR) specific to a stimulus was defined as an increase in signal as compared to the period prior to stimulus presentation of at least 0.02 μS occurring 1-7 seconds after stimulus presentation. This threshold was set following the recommendations from Braithwaite et al. (2013).
2.3.2. Superstorm Sandy exposure.
Acute PNMS related to Superstorm Sandy was determined based on timing of the index pregnancy (0 = not pregnant during the storm, 1 = pregnant during the storm). The storm impacted the metropolitan New York area (October 29, 2012). Based on this calculation, 44.3% of the sample were exposed to the storm in utero (n = 87) and 55.6% (n = 109) were not. Timing of index pregnancy was not available for 2 cases (.006% of the sample).
2.3.3. Maternal depression.
PNMD was measured with the Edinburgh Postnatal Depression Scale (EPDS) (Masaeli, Kheirabadi, Maracy, & Akbaripour, 2012; Murray & Carothers, 1990), a widely used self-report inventory of prenatal depression, assessed during the second trimester. Questions about mood in the past seven days were responded to on a four-point Likert scale ranging from “yes, all the time,” “yes, most of the time,” “no, not very often,” and “no, not at all.” The items were summed and the recommended cutoff score of 12 was used to determine presence of major depression based on depressive symptoms. This inventory has acceptable reliability (Cronbach’s α = .74 during pregnancy, Cronbach’s α = .79 in postpartum), satisfactory sensitivity (79%) and specificity (85%). Within the sample 22% (n = 44) met this threshold. The high portion of participants who met the threshold compared to the prevalence of depression in pregnant women in the population (6%) may be due to people with depression being more likely to join the study, either for mental health or financial resources.
2.3.4. Race/Ethnicity.
Maternal ethnicity was collected via self-report. Mothers were asked to identify their race/ethnicity as one of the following: White Hispanic, Black Hispanic, Mixed Hispanic, Black (non-Hispanic), White (non-Hispanic), Asian, or Other.
2.3.5. Confounders.
Following a review of the literature, a directed acyclic graph (DAG) was diagrammed to represent the theorized causal pathway between acute PNMS, PNMD and offspring ANS function (as represented by EDA measures) (Textor, van der Zander, Gilthorpe, Liśkiewicz, & Ellison, 2016). Of the measured variables available to the present analysis, maternal anxiety, trauma, and ethnicity were identified as confounders, which have been strongly associated with prenatal stress and child neurodevelopment in previous research, which required adjustment in order capture the total effect of acute PNMS and PNMD on childhood EDA. These potential confounders were measured as follows.
The State-Trait Anxiety Inventory (STAI) (Spielberger, 1983) measures current “state anxiety” and typical “trait anxiety.” The state and trait subscales are each assessed by 20 self-report statements that describes participants on a 4-point Likert scale ranging from 1 “not at all” to 4 “very much so.” This was administered during the second trimester or at the time of enrollment into the study. Anxiety was determined based on presence of "state anxiety" or "trait anxiety" according to the suggested thresholds of a sum score of 40 or greater (Dennis, Coghlan, & Vigod, 2013). Cronbach’s alpha was .90 for state anxiety and .90 for train anxiety, indicating excellent reliability.
The Posttraumatic Diagnostic Scale (PDS) (Foa, Cashman, Jaycox, & Perry, 1997) is a self-report measure used to determine severity of symptoms related to posttraumatic stress disorder (PTSD) using 49 items related to the DSM-IV criteria for PTSD. When a traumatic event is reported, symptom frequency is reported on a scale of 0 (Not at all or only one time) to 3 (5 or more times a week/almost always). This was administered following the storm or at the time of enrollment into the study. A sum score was used as a measure of prior trauma before pregnancy. Cronbach’s alpha for the scale was .93, indicating excellent reliability.
2.4. Analytical plan
The raw signal for each subject was visually inspected to determine whether or not the recording should be excluded due to artefact or technical malfunction (n = 104). Independent samples t-tests indicated that there were no differences between data with artefact and data without artefact with respect to child gender, in utero storm exposure status, prevalence of maternal depression, or SCR magnitude (all p > .52). Younger children were, however, more likely to have data with artefact or technical malfunction (age in months of children with artefact M = 38.75, SD = 13.95; children without artefact M = 42.85, SD = 12.43, p = .013). Electrodermal reactivity to the startling stimuli was quantified as the SCR magnitude specific to the stimuli. As per AcqKnowledge 4.4 software, the SCR magnitude is calculated based on the amplitude of each specific SCR and the SCR frequency, or percentage of the 6 startle probes that elicited a response.
Storm severity, as measured by the PDS, was weakly correlated with state anxiety (r = .20), trait anxiety, (r = .22), and maternal depression (r = .27); while maternal anxiety was moderately correlated with maternal depression (r = .38). The variance inflation factor (VIF) did not exceed 2.0 for the final model, indicating minimal model distortion due to multicollinearity. There were no significant differences between participants based on the OB/GYN clinic of recruitment with respect to SCR magnitude, baseline SCR frequency, storm severity, depression, or anxiety (p > .26). Further, there were no significant differences among prenatal exposure groups and baseline SCR frequency [F(3, 191) = .83, p = .5].
All statistical tests specified an alpha level of .05 and were performed in SAS version 9.4 (SAS Institute, Cary, NC). Descriptive statistics for the sample were obtained. Raw SCR magnitude values were transformed via square root to reduce the positive skew, a method commonly used in studies using EDA (Braithwaite et al., 2013). Before transformation, kurtosis and skew were 8.9 and 2.65, respectively. After transformation, kurtosis and skew were 0.81 and 0.88, respectively. To address missing data, multiple imputation was used for key variables (SCR magnitude = 31.3%, prenatal depression = 12.5%, postnatal depression = 26.1%, anxiety = 16.1%, trauma = 16.7%). Multiple imputation procedures followed those outlined by Liu and De using the fully conditional specification (FCS) method in SAS (Liu & De, 2015). Results of the multivariable models with and without imputation indicated no notable differences in estimates or significance, thus only results from the imputed models are reported here.
Following a review of the literature, a Directed Acyclic Graph (DAG) was made to represent the theorized causal pathway between PNMS, PNMD and offspring ANS function (as represented by EDA measures) (Textor et al., 2016). Of the measured variables available to the present analysis, maternal anxiety, trauma, and ethnicity were identified as confounders requiring adjustment in order to capture the direct effect of PNMS and PNMD on childhood EDA. Maternal education and marital status, proxies for socioeconomic status in women who are pregnant or new mothers, were identified as intermediate variables along the theoretical causal pathway from Superstorm Sandy experience, maternal depression, and childhood physiological reactivity. These variables were therefore not included to reduce the likelihood of overfitting and bias via unnecessary adjustment.
Intrafamilial correlation due to multiple children within one family (n = 30) was evaluated and the clustering effect was adjusted as follows. First, an empty model (Model 1) was carried out to compute the interclass correlation coefficient (ICC) (Shrout & Fleiss, 1979) due to clustering at the family level. Results of the empty model indicated that 24.9% (ICC = 0.249) of the variation in SCR magnitude was due to between family differences. As this represents a substantial amount of clustering, a random intercept for family was included in all models. Subsequently, univariable models predicting SCR magnitude were carried out with acute PNMS (Model 2) and PNMD (Model 3) as predictors. Finally multivariable models predicting SCR magnitude (unadjusted and unadjusted, Model 4 -5) were carried out. The model specifications can be found below:
To explore the potential interaction effect of PNMD*PNMD on SCR magnitude, post hoc analyses were conducted using Tukey's HSD test to compare means of SCR magnitude across group status (no prenatal exposure = 0, PNMD only = 1, PNMS only = 2, both PNMD and PNMS = 3).
3. Results
Table 1 displays descriptive statistics for the sample stratified by PNMD and PNMS. Of the sample 42.3% (n = 85) were not exposed to acute PNMS or PNMD in utero. 35.2% (n = 69) were exposed to acute PNMS but not PNMD in utero, 13.3% (n = 26) were exposed to PNMD but not acute PNMS, and 9.2% (n = 18) were exposed to both acute PNMS and PNMD.
Table 1.
Descriptive statistics
| Full Sample | No Exposure | PNMS Only | PNMD Only | PNMS + PNMD | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N = 198 | N = 85, 42.3% | N = 69, 35.2% | N = 26, 13.3% | N = 18, 18% | ||||||
| Child Characteristics | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Age (months) | 42.54 | 12.76 | 47.19 | 11.23 | 36.71 | 12.14 | 48.86 | 10.55 | 33.80 | 11.06 |
| % Female | n = 102 | 51.5% | n = 41 | 48.2% | n = 39 | 56.5% | n = 14 | 53.8% | n = 8 | 44.4% |
| Ethnicity | N | % | N | % | N | % | N | % | N | % |
| Asian | 20 | 10.1 | 10 | 11.8 | 7 | 10.1 | 1 | 3.8 | 2 | 11.1 |
| Black | 58 | 29.3 | 27 | 31.8 | 13 | 18.8 | 14 | 53.9 | 4 | 22.2 |
| Hispanic | 86 | 43.4 | 34 | 40.0 | 37 | 53.6 | 8 | 30.8 | 7 | 38.9 |
| White | 30 | 15.2 | 14 | 16.5 | 10 | 14.5 | 2 | 7.7 | 4 | 22.2 |
| Other | 4 | 2.0 | 0 | 0 | 2 | 2.9 | 1 | 3.8 | 1 | 5.6 |
| Marital Status | N | % | N | % | N | % | N | % | N | % |
| Married | 78 | 39.4 | 29 | 34.1 | 33 | 47.8 | 8 | 30.8 | 8 | 44.4 |
| Common-law | 12 | 6.1 | 4 | 4.7 | 8 | 11.6 | 0 | 0 | 0 | 0 |
| Never Married | 106 | 53.5 | 51 | 60.0 | 28 | 40.6 | 18 | 69.2 | 9 | 50.0 |
| Widowed | 1 | 0.5 | 1 | 1.2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Divorced/Separated | 1 | 0.5 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 5.6 |
| Maternal Education | N | % | N | % | N | % | N | % | N | % |
| Primary School | 5 | 2.5 | 0 | 0 | 3 | 4.3 | 1 | 3.8 | 1 | 5.6 |
| Some High School | 29 | 14.6 | 16 | 18.8 | 4 | 5.8 | 6 | 23.1 | 3 | 16.7 |
| High School/GED | 39 | 19.7 | 17 | 20.0 | 16 | 23.2 | 6 | 23.1 | 0 | 0 |
| Some College | 57 | 28.8 | 26 | 30.6 | 20 | 29.0 | 5 | 19.2 | 6 | 33.3 |
| Associate's Degree | 14 | 7.1 | 5 | 5.9 | 4 | 5.8 | 3 | 11.5 | 2 | 11.1 |
| Bachelor's Degree | 26 | 13.1 | 11 | 12.9 | 10 | 14.5 | 3 | 11.5 | 2 | 11.1 |
| Graduate Degree | 28 | 14.1 | 10 | 11.8 | 12 | 17.4 | 2 | 7.7 | 4 | 22.2 |
| Mental Health | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Prior Trauma Score | 4.82 | 9.1 | 3.6 | 6.9 | 5.14 | 9.8 | 10.00 | 13.7 | 2.29 | 4.9 |
| State Anxiety | 37.93 | 11.8 | 34.8 | 10.7 | 34.78 | 8.7 | 46.48 | 12.2 | 52.47 | 10.6 |
| Trait Anxiety | 38.42 | 10.6 | 35.0 | 8.8 | 34.74 | 7.7 | 49.35 | 9.8 | 52.65 | 6.1 |
| Psychophysiology | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Raw SCR Magnitude | 0.40 | 0.5 | 0.41 | 0.5 | 0.5 | 0.7 | 0.23 | 0.3 | 0.21 | 0.2 |
| SCR Frequency | 50.09 | 27.1 | 54.42 | 28.6 | 48.79 | 27.0 | 43.59 | 22.6 | 44.44 | 24.3 |
Note: SCR = Skin Conductance Response.
3.1. Linear Mixed Effects Models
Results of Models 1-5 can be found in Table 2. The initial intercept only model (Model 1) indicated that a substantial amount of the variation in SCR magnitude was explained by between family differences (29%). Results from our univariable models (2, 3) indicate that acute PNMS did not predict SCR magnitude (BPNMS = 0.01, p > .05), whereas PNMD did significantly predict SCR magnitude (BPNMS = −0.14, p < .001).
Table 2.
Summary of Linear Mixed Effects Models
| Model 1a | Model 2b | Model 3c | Model 4d | Model 5e | |
|---|---|---|---|---|---|
| Intercept | 0.50 (0.01)*** | 0.50 (0.01)*** | 0.53 (0.01)*** | 0.41 (0.30)*** | 0.19 (0.04)*** |
| Storm Exposure | -------- | 0.01 (0.02) | -------- | −0.03 (0.04) | −0.01 (0.04) |
| Depression | -------- | -------- | −0.14 (0.02)*** | 0.12 (0.03)** | 0.17 (0.04)** |
| Storm*Depression | -------- | -------- | -------- | 0.03 (0.04) | 0.01 (0.04) |
| Postnatal Depression | -------- | -------- | -------- | -------- | −0.06 (0.03)* |
| Trauma | -------- | -------- | -------- | -------- | 0.02 (0.04) |
| Anxiety | -------- | -------- | -------- | -------- | 0.08 (0.02)*** |
| Ethnicity | -------- | -------- | -------- | -------- | 0.04 (0.01) |
Note:
p < .05;
p **< .01;
p ***< .001
Number outside parentheses reflects unstandardized beta, number in parentheses reflects standard error.
Empty Model, ICC = 0.21.
Superstorm Sandy exposure.
Prenatal maternal depression.
Superstorm Sandy x Prenatal depression.
Model 4 adjusted for postnatal depression, ethnicity, anxiety, and other trauma.
Acute PNMS remained non-significant and PNMD remained a significant predictor in our unadjusted multivariable model (4), while the interaction of the two was non-significant (BPNMS = −0.03, p > 0.05; BPNMS = 0.12, p = .005; BPNMSxPNMD = 0.03, p > 0.05). No changes in significance were noted following adjustment for confounders (maternal anxiety, trauma and race/ethnicity) in Model 5 (BPNMS = −0.004, p > 0.05; BPNMD = 0.17, p = .002; Badjusted = 0.01, p > 0.05).
3.2. Tukey Post Hoc Analysis
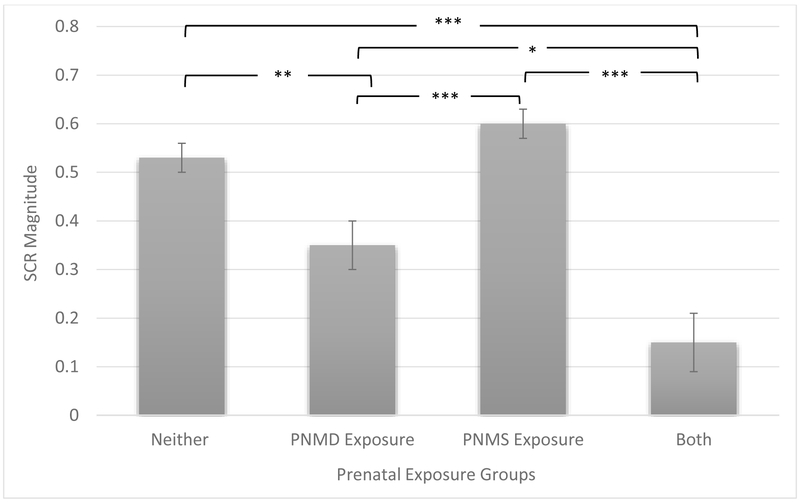
Tukey's post hoc analysis further indicated that SCR magnitude of children exposed to both PNMD and acute PNMS was lower than in children exposed to neither in utero (p = .002), PNMD only (p = .046), and acute PNMS only (p = .002). SCR magnitude was also lower in children exposed to PNMD only as compared to children exposed to neither in utero (p = .008) and children exposed to acute PNMS only (p = .002). Reported p-values have been adjusted for multiple comparison using the Benjamini-Hochberg procedure with a 5% false discovery rate. Full results are available in Table 3. The mean and standard error per group are represented in Figure 1.
Table 3.
Post hoc contrasts linear mixed effects model (Tukey)
| (I) Group | (J) Group | Mean Difference (I-J) |
SE | t-ratio | p value | (95% CI) | Cohen’s d |
|---|---|---|---|---|---|---|---|
| PNMD | 0.18 | 0.06 | 3.30 | 0.0081 | (0.04 - 0.33) | 0.09 | |
| Control | PNMS | −0.07 | 0.03 | −1.96 | 0.2051 | (−0.15 - 0.02) | 0.05 |
| Both | 0.38 | 0.06 | 5.98 | 0.0002 | (0.22 - 0.55) | 0.16 | |
| Control | −0.18 | 0.06 | 3.30 | 0.0081 | (0.04 - 0.33) | 0.09 | |
| PNMD | PNMS | −0.25 | 0.06 | −4.28 | 0.0002 | (−0.40 - −0.10) | 0.11 |
| Both | 0.20 | 0.07 | 2.67 | 0.0457 | (0.01 - 0.39) | 0.07 | |
| Control | 0.07 | 0.03 | −1.96 | 0.2051 | (−0.15 - 0.02) | 0.05 | |
| PNMS | PNMD | 0.25 | 0.06 | −4.28 | 0.0002 | (−0.40 - −0.10) | 0.11 |
| Both | 0.45 | 0.06 | 7.10 | 0.0002 | (0.28 - 0.61) | 0.19 | |
| Control | −0.38 | 0.06 | 5.98 | 0.0002 | (0.22 - 0.55) | 0.16 | |
| Both | PNMD | −0.20 | 0.07 | 2.67 | 0.0457 | (0.01 - 0.39) | 0.07 |
| PNMS | −0.45 | 0.06 | 7.10 | 0.0002 | (0.28 - 0.61) | 0.19 |
Note: SE = standard error, CI = confidence interval.
All significance values were corrected for multiple testing using the Benjamini-Hochberg procedure with a 5% false discovery rate.
PNMS = Prenatal Maternal Stress related to Superstorm Sandy.
PNMD = Prenatal Maternal Depression.
Figure 1. Skin Conductance Response Magnitude by Prenatal Exposure Groups.
Note: SCR = Skin Conductance Response. SCR Magnitude values are normalized by square root transformation. Bars reflect the standard error of the mean.
*p < .05, **p < .01, ***p < .001.
4. Conclusions
This study provides novel insight into offspring exposed to PNMD separately and in conjunction with acute PNMS. Our aim was to explore the dynamics of the associations between maternal prenatal stress, depression, and offspring autonomic function. Contrary to our initial hypotheses, in utero exposure to Superstorm Sandy was not related to SCR magnitude. PNMD exposure was negatively related to one measure of SNS reactivity such that SCR magnitude to startling stimuli was lower than that of children not exposed to PNMD. Post hoc analyses revealed that those exposed to PNMD only and those exposed to both PNMD and acute PNMS had lower SCR magnitude than those only exposed to acute PNMS. Lastly, despite finding no interaction between PNMS and PNMD, those exposed to both were less reactive than those exposed to PNMD only, suggesting acute stress may exacerbate the impact of PNMD. Although van Dijk and colleagues found no correlations between PNMD, psychosocial stress, and cardiac autonomic function at rest (van Dijk et al., 2012), evaluation of the impact of one specific acute prenatal stressor in conjunction with PNMD on offspring SCR magnitude in response to startling stimuli was previously uninvestigated. Further, van Dijk and colleagues used a cardiac measure of autonomic function, which may have been influenced by the parasympathetic nervous system.
The nonsignificant findings of acute prenatal stress and enhanced offspring reactivity were unexpected, given the literature documenting this relation (Austin, Hadzi-Pavlovic, et al., 2005; Baibazarova et al., 2013; Davis et al., 2007). Our colleagues have previously reported that Superstorm Sandy exposure was associated with higher emotional reactivity over the course of the first two years of life (Wei Zhang, Rajendran, et al., 2018). It is possible that the association between acute prenatal stress and skin conductance response was nonsignificant because of directional fractionation of the autonomic nervous system (Lacey, 1967), individual differences in responsivity, or postnatal factors that may mask the effect, such as mother-child bond or socioeconomic status. Importantly, prior research has identified sex and timing of stress effects, such that early gestation PNMS exposure correlated with aberrant stress response in male offspring (Mueller & Bale, 2008; Curt A. Sandman, Davis, Buss, & Glynn, 2012). Had we examined these factors, we may have uncovered an association between PNMS and electrodermal reactivity. Future research would do well to consider these factors.
Our findings regarding PNMD confirm our initial hypothesis that children exposed to PNMD were less electrodermally reactive to startling stimuli, potentially suggesting a blunted SNS response. We found low SCR magnitude in PNMD exposed children, as compared to acute PNMS-only exposed children, consistent with our initial hypothesis. The SCR magnitude was even lower for children exposed to both acute PNMS and PNMD. Several mechanisms may underlie this finding. First, it is possible that longer term exposure to depression than to an acute stressor such as a natural disaster may result in greater alteration in fetal development, which may in turn contribute to the blunted SNS reactivity observed. Second, it is also possible that blunted electrodermal reactivity has a genetic component related to depression that may be heritable from the mother, since depression is associated with low electrodermal activity. A third mechanism may lie in exposure to excess cortisol, increased inflammation, or decreased oxytocin influencing epigenetic mechanisms, which may result in disruptions in development that lead to blunted SCR magnitude (e.g., HPA axis dysregulation). Although no prenatal measure of cortisol is available, support for this third mechanism comes from work by our colleagues’ finding that placental gene expression of three genes known to modulate cortisol levels moderated the relationship between PNMD and increased negative affect at six months of age (Wei Zhang, Finik, et al., 2018).
These findings should be interpreted cautiously in light of several limitations. First, one or more measures of cortisol, oxytocin, or inflammation throughout pregnancy would have shed light on an underlying mechanism. Without these data, it is impossible to describe a mechanism underlying our results. While we were unable in the present study to examine the specific trimester(s) acute PNMS and PNMD were experienced, this line of inquiry could have helped clarify critical windows of susceptibility to this aspect of SNS dysregulation. Our measure of SNS reactivity is limited, in that electrodermal activity only represents one measure of the SNS, and directional fractionation of the autonomic nervous system and individual differences in reactivity may lead to variation in skin conductance responses (Jänig & Häbler, 2000; Lacey, 1967). We therefore urge caution in generalizing our findings to other measures of SNS function. Moreover, we quantified skin conductance responses as a 0.02 μS amplitude increase as compared to the period immediately preceding the stimulus, and does not reflect the skin conductance level, which is likely to have been much lower during baseline. Further, we were unable to examine potential paternal contribution in this analysis, as half of all participating mothers were single and did not provide information regarding the father of their child. In addition, a majority of measures used in the present analysis consisted of self-report questionnaires (maternal depression, anxiety, trauma history) which are subject to bias. Lastly, it is possible that natural disasters are fundamentally different from other forms of prenatal stress (e.g., financial or interpersonal difficulties) because of media coverage, sustained damage, etc. We also quantified storm exposure based on whether or not the child was in utero or not at the time, and were not able to further examine our results according to level of storm severity experienced. We did not examine which aspects of the storm experience (e.g., power loss, home evacuation, injury) most strongly correlated with electrodermal activity, nor did we examine the variability of the magnitude of stress experienced. Our measure of PNMS may therefore have limited generalizability to other prenatal stressors.
Acknowledging these limitations, these results extend the literature on PNMS, PNMD, and child development in a prospective longitudinal study. We used a more objective measure of stress reactivity than parent or observer temperament ratings, as is commonly used in the literature. We also differentiated between acute stress and depression, which may impact underlying biological systems across different time scales (i.e., short term versus long term). Further, we adjusted our statistical model for anxiety, prior trauma, and race/ethnicity in order to parse out the total effect of acute PNMS and PNMD on SCR magnitude.
Moving forward, researchers should aim to fill the gaps that still remain in our understanding of the links between prenatal maternal mental health and offspring development, in order to identify and intervene prior to the onset of burdensome developmental conditions. We have uncovered an association between PNMD and blunted SCR magnitude to startling stimuli, but further research is needed to elucidate potential underlying mechanisms. Fetal exposure to excess maternal cortisol, inflammation, or other chemical messengers may alter gene expression and impact neurological development, particularly for children exposed to two or more conditions. Analysis of timing of acute PNMS or PNMD exposure and degree of cortisol exposure, inflammation, epigenetic profiles, and early life brain structure may shed light on underlying mechanisms. Moreover, postnatal factors that may mitigate or aggravate these associations such as parental bond, socioeconomic status, and the natural environment should be examined.
The US Preventive Services Task Force on Interventions to Prevent Perinatal Depression recently recommended that doctors refer pregnant women for counseling to prevent depression onset if at least one risk factor is present, including history of depression or abuse, presence of depression or anxiety symptoms, low socioeconomic status, or lack of social support (Curry et al., 2019). Our findings underscore the importance of this recommendation, and point to the occurrence of a natural disaster or similar acute stressor as additional factors for consideration. Recent hurricanes in the United States, Puerto Rico, and Japan, and reports that extreme weather events will increase in frequency and scale, underscore the need to understand the relation between climate change and mental health (Grinsted, Moore, & Jevrejeva, 2013; Khan, 2017; McCarthy & Intergovernmental Panel on Climate Change, 2001). The Rockefeller Foundation-Lancet Commission on planetary health highlights the association between climate-related environmental changes and increased anxiety and depression symptoms termed “solastalgia” that have been identified by several studies (Warsini, Mills, & Usher, 2014; Whitmee et al., 2015). Thus far, however, transmission of this phenomenon to future generations has been largely unstudied. Moreover, pregnant women may be particularly susceptible to harmful mental health consequences from natural disasters in the context of the increased risk for minor depression in the pregnant population as compared to the non-pregnant population (Ashley, Harper, Arms-Chavez, & LoBello, 2016).
Highlights.
Prenatal Superstorm Sandy exposure is not linked to child electrodermal reactivity.
Prenatal maternal depression exposure is linked to low electrodermal reactivity.
Reactivity is lower for children exposed to both conditions than all groups.
Acknowledgments
Funding: This work was supported by the National Institutes of Health [R01MH1027929-01].
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). American Psychiatric Pub. [Google Scholar]
- Ashley JM, Harper BD, Arms-Chavez CJ, & LoBello SG (2016). Estimated prevalence of antenatal depression in the US population. Archives of Women’s Mental Health, 19(2), 395–400. 10.1007/s00737-015-0593-1 [DOI] [PubMed] [Google Scholar]
- Austin M-P, Hadzi-Pavlovic D, Leader L, Saint K, & Parker G (2005). Maternal trait anxiety, depression and life event stress in pregnancy: relationships with infant temperament. Early Human Development, 81(2), 183–190. 10.1016/j.earlhumdev.2004.07.001 [DOI] [PubMed] [Google Scholar]
- Austin M-P, Leader LR, & Reilly N (2005). Prenatal stress, the hypothalamic–pituitary–adrenal axis, and fetal and infant neurobehaviour. Early Human Development, 81(11), 917–926. 10.1016/j.earlhumdev.2005.07.005 [DOI] [PubMed] [Google Scholar]
- Baibazarova E, van de Beek C, Cohen-Kettenis PT, Buitelaar J, Shelton KH, & van Goozen SHM (2013). Influence of prenatal maternal stress, maternal plasma cortisol and cortisol in the amniotic fluid on birth outcomes and child temperament at 3 months. Psychonenroendocrinology, 38(6), 907–915. 10.1016/j.psyneuen.2012.09.015 [DOI] [PubMed] [Google Scholar]
- Barker DJP (1998). In utero programming of chronic disease. Clinical Science, 95(2), 115 10.1042/CS19980019 [DOI] [PubMed] [Google Scholar]
- Braithwaite JJ, Watson D, Jones R, & Rowe M (2013). A guide for analysing electrodermal activity (EDA) & skin conductance responses (SCRs) for psychological experiments. Psychophysiology, 49, 1017–1034. [Google Scholar]
- Buthmann J, Ham J, Davey K, Finik J, Dana K, Pehme P, … Nomura Y (2018). Infant temperament: repercussions of Superstorm Sandy-related maternal stress. Child Psychiatry & Human Development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2013). Deaths associated with Hurricane Sandy-October-November 2012. MMWR. Morbidity and Mortality Weekly Report, 6(20), 393–397. [PMC free article] [PubMed] [Google Scholar]
- Chen FR, Raine A, Glenn AL, & Granger DA (2015). Hypothalamic pituitary adrenal activity and autonomic nervous system arousal predict developmental trajectories of children’s comorbid behavior problems. Developmental Psychobiology, 55(3), 393–405. 10.1002/dev.21379 [DOI] [PubMed] [Google Scholar]
- Chrousos GP (1992). The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA: The Journal of the American Medical Association, 267(9), 1244–1252. 10.1001/jama.267.9.1244 [DOI] [PubMed] [Google Scholar]
- CNN Library, (n.d.). Hurricane Sandy Fast Facts. Retrieved March 13, 2018, from CNN website https://www.cnn.com/2013/07/13/world/americas/hurricane-sandy-fast-facts/index.html
- Crowell SE, Beauchaine TP, Gatzke-Kopp L, Sylvers P, Mead H, & Chipman-Chacon J (2006). Autonomic correlates of attention-deficit/hyperactivity disorder and oppositional defiant disorder in preschool children. Journal of Abnormal Psychology, 115(1), 174–178. 10.1037/0021-843X.115.1.174 [DOI] [PubMed] [Google Scholar]
- Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, Davidson KW, … Wong JB (2019). Interventions to Prevent Perinatal Depression: US Preventive Services Task Force Recommendation Statement. JAMA, 321(6), 580–587. 10.1001/jama.2019.0007 [DOI] [PubMed] [Google Scholar]
- Curtis AL, Bello NT, Connolly KR, & Valentino RJ (2002). Corticotropin-Releasing Factor Neurones of the Central Nucleus of the Amygdala Mediate Locus Coeruleus Activation by Cardiovascular Stress. Journal of Neuroendocrinology, 14(8), 667–682. 10.1046/j.1365-2826.2002.00821.x [DOI] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Schetter CD, Hobel C, Chicz-Demet A, & Sandman CA (2007). Prenatal Exposure to Maternal Depression and Cortisol Influences Infant Temperament. Journal of the American Academy of Child & Adolescent Psychiatry, 46(6), 737–746. 10.1097/chi.0b013e318047b775 [DOI] [PubMed] [Google Scholar]
- Davis EP, Snidman N, Wadhwa PD, Glynn LM, Schetter CD, & Sandman CA (2004). Prenatal Maternal Anxiety and Depression Predict Negative Behavioral Reactivity in Infancy. Infancy, 6(3), 319–331. 10.1207/s15327078in0603_1 [DOI] [Google Scholar]
- Dennis C-L, Coghlan M, & Vigod S (2013). Can we identify mothers at-risk for postpartum anxiety in the immediate postpartum period using the State-Trait Anxiety Inventory? Journal of Affective Disorders, 150(3), 1217–1220. 10.1016/j.jad.2013.05.049 [DOI] [PubMed] [Google Scholar]
- Dieleman GC, Huizink AC, Tulen JHM, Utens EMWJ, Creemers HE, van der Ende J, & Verhulst FC (2015). Alterations in HPA-axis and autonomic nervous system functioning in childhood anxiety disorders point to a chronic stress hypothesis. Psychoneuroendocrinology, 51, 135–150. 10.1016/j.psyneuen.2014.09.002 [DOI] [PubMed] [Google Scholar]
- Drake AJ, Tang JI, & Nyirenda MJ (2007). Mechanisms underlying the role of glucocorticoids in the early life programming of adult disease. Clinical Science, 113(5), 219–232. 10.1042/CS20070107 [DOI] [PubMed] [Google Scholar]
- Ehrlich DE, & Rainnie DG (2015). Prenatal Stress Alters the Development of Socioemotional Behavior and Amygdala Neuron Excitability in Rats. Neuropsychopharmacology, 40(9), 2135–2145. 10.1038/npp.2015.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field T, Diego M, Dieter J, Hernandez-Reif M, Schanberg S, Kuhn C, … Bendell D (2004). Prenatal depression effects on the fetus and the newborn. Infant Behavior and Development, 27(2), 216–229. 10.1016/j.infbeh.2003.09.010 [DOI] [Google Scholar]
- Finik J, & Nomura Y (2017). Cohort Profile: Stress in Pregnancy (SIP) Study. International Journal of Epidemiology, 46(5), 1388–1388k. 10.1093/ije/dyw264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa EB, Cashman L, Jaycox L, & Perry K (1997). The validation of a self-report measure of posttraumatic stress disorder: The Posttraumatic Diagnostic Scale. Psychological Assessment, 9(4), 445–451. 10.1037/1040-3590.9.4.445 [DOI] [Google Scholar]
- Fowles DC, Kochanska G, & Murray K (2000). Electrodermal activity and temperament in preschool children. 11. [PubMed] [Google Scholar]
- Gao Y, Tuvblad C, Schell A, Baker L, & Raine A (2015). Skin conductance fear conditioning impairments and aggression: A longitudinal study. Psychophysiology, 52(2), 288–295. 10.1111/psyp.12322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover V (2011). Annual Research Review: Prenatal stress and the origins of psychopathology: an evolutionary perspective: Prenatal stress and the origins of psychopathology. Journal of Child Psychology and Psychiatry, 52(4), 356–367. 10.1111/j.1469-7610.2011.02371.x [DOI] [PubMed] [Google Scholar]
- Glover V (2014). Maternal depression, anxiety and stress during pregnancy and child outcome; what needs to be done. Best Practice & Research Clinical Obstetrics & Gynaecology, 25(1), 25–35. 10.1016/j.bpobgyn.2013.08.017 [DOI] [PubMed] [Google Scholar]
- Grinsted A, Moore JC, & Jevrejeva S (2013). Projected Atlantic hurricane surge threat from rising temperatures. Proceedings of the National Academy of Sciences, 110(14), 5369–5373. 10.1073/pnas.1209980110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay DF, Pawlby S, Waters CS, Perra O, & Sharp D (2010). Mothers’ Antenatal Depression and Their Children’s Antisocial Outcomes. Child Development, 81(1), 149–165. [DOI] [PubMed] [Google Scholar]
- Jänig W, & Häbler HJ (2000). Specificity in the organization of the autonomic nervous system: a basis for precise neural regulation of homeostatic and protective body functions. Progress in Brain Research, 122, 351–367. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM, Siegelbaum SA, & Hudspeth AJ (2014). Principles of neural science (5th ed.). New York, NY: McGraw-Hill, Health Professions Division. [Google Scholar]
- Kessel EM, Nelson BD, Kujawa A, Hajcak G, Kotov R, Bromet E, … Klein DN (2018). Hurricane Sandy exposure alters the development of neural reactivity to negative stimuli in children. Child Development, 89(2), 339–348. 10.1111/cdev.12691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A (2017, September 10). Fires, droughts and hurricanes: What’s the link between climate change and natural disasters? Los Angeles Times; Retrieved from http://www.latimes.com/science/sciencenow/la-sci-sn-climate-change-natural-disasters-20170907-htmlstory.html [Google Scholar]
- King S, Dancause K, Turcotte-Tremblay A-M, Veru F, & Laplante DP (2012). Using Natural Disasters to Study the Effects of Prenatal Maternal Stress on Child Health and Development: Natural Disasters and Prenatal Maternal Stress. Birth Defects Research Part C: Embryo Today: Reviews, 96(4), 273–288. 10.1002/bdrc.21026 [DOI] [PubMed] [Google Scholar]
- King S, & Laplante DP (2005). The effects of prenatal maternal stress on children’s cognitive development: Project Ice Storm. Stress, 8(1), 35–45. 10.1080/10253890500108391 [DOI] [PubMed] [Google Scholar]
- Kopala-Sibley DC, Danzig AP, Kotov R, Bromet E, Carlson G, Olino TM, … Klein DN (2016). Negative emotionality and its facets moderate the effects of exposure to Hurricane Sandy on children’s postdisaster depression and anxiety symptoms. Journal of Abnormal Psychology, 125(4), 471–481. 10.1037/abn0000152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopala-Sibley DC, Kotov R, Bromet E, Carlson G, Danzig AP, Black SR, & Klein DN (2016). Personality diatheses and Hurricane Sandy: Effects on post-disaster depression. Psychological Medicine, 46(4), 865–875. 10.1017/S0033291715002378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey J (1967). Somatic response patterning and stress: Some revisions of activation theory. Psychological Stress: Issues in Research, 14–37. [Google Scholar]
- Langley-Evans SC (2007). Metabolic programming in pregnancy: studies in animal models. Genes & Nutrition, 2(1), 33–38. 10.1007/s12263-007-0005-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, & De A (2015). Multiple Imputation by Fully Conditional Specification for Dealing with Missing Data in a Large Epidemiologic Study. International Journal of Statistics in Medical Research, 4(3), 287–295. 10.6000/1929-6029.2015.04.03.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaeli N, Kheirabadi GR, Maracy MR, & Akbaripour S (2012). Psychometric Properties and Diagnostic Accuracy of the Edinburgh Postnatal Depression Scale in a Sample of Iranian Women. Iranian Journal of Medical Sciences, 37(1), 32–38. [PMC free article] [PubMed] [Google Scholar]
- McCarthy JJ, & Intergovernmental Panel on Climate Change (Eds.). (2001). Climate change 2001: impacts, adaptation, and vulnerability: contribution of Working Group II to the third assessment report of the Intergovernmental Panel on Climate Change. Cambridge, UK: ; New York: Cambridge University Press. [Google Scholar]
- McGrath JM, Records K, & Rice M (2008). Maternal depression and infant temperament characteristics. Infant Behavior and Development, 31(1), 71–80. 10.1016/j.infbeh.2007.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller BR, & Bale TL (2008). Sex-Specific Programming of Offspring Emotionality after Stress Early in Pregnancy. Journal of Neuroscience, 28(36), 9055–9065. 10.1523/JNEUROSCI.1424-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray L, & Carothers AD (1990). The validation of the Edinburgh Post-natal Depression Scale on a community sample. The British Journal of Psychiatry: The Journal of Mental Science, 157, 288–290. [DOI] [PubMed] [Google Scholar]
- Nomura Y, Davey K, Pehme P, Finik J, Glover V, Zhang W, … Ham J (2019).Influence of in-utero exposure to maternal depression and natural disaster-related stress on infant temperament at 6 months: The children of Superstorm Sandy. Infant Mental Health Journal. 10.1002/imhj.21766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor TG, Heron J, Golding J, Beveridge M, & Glover V (2002). Maternal antenatal anxiety and children’s behavioural/emotional problems at 4 years. Report from the Avon Longitudinal Study of Parents and Children. The British Journal of Psychiatry: The Journal of Mental Science, 180, 502–508. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Wadhwa PD, Chicz-DeMet A, Porto M, & Garite TJ (1999). Maternal corticotropin-releasing hormone and habituation in the human fetus. Developmental Psychobiology, 34(3), 163–173. [DOI] [PubMed] [Google Scholar]
- Sandman Curt A., Davis EP, Buss C, & Glynn LM (2012). Exposure to Prenatal Psychobiological Stress Exerts Programming Influences on the Mother and Her Fetus. Neuroendocrinology, 95(1), 8–21. 10.1159/000327017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarchiapone M, Gramaglia C, Iosue M, Carli V, Mandelli L, Serretti A, … Zeppegno P (2018). The association between electrodermal activity (EDA), depression and suicidal behaviour: A systematic review and narrative synthesis. BMC Psychiatry, 18, 1–27. 10.1186/s12888-017-1551-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpa A, Raine A, Venables PH, & Mednick SA (1997). Heart rate and skin conductance in behaviorally inhibited Mauritian children. Journal of Abnormal Psychology, 106(2), 182–190. [DOI] [PubMed] [Google Scholar]
- Shrout PE, & Fleiss JL (1979). Intraclass Correlations: Uses in Assessing Rater Reliability. 9. [DOI] [PubMed] [Google Scholar]
- Simcock G, Kildea S, Elgbeili G, Laplante DP, Cobham V, & King S (2017). Prenatal maternal stress shapes children’s theory of mind: the QF2011 Queensland Flood Study. Journal of Developmental Origins of Health and Disease, 5(4), 483–492. https://doi. org/10.1017/S2040174417000186 [DOI] [PubMed] [Google Scholar]
- Spielberger CD (1983). Manual for the State-Trait Anxiety Inventory STAI. Palo Alto, CA: Mind Garden. [Google Scholar]
- Tees MT, Harville EW, Xiong X, Buekens P, Pridjian G, & Elkind-Hirsch K (2010). Hurricane Katrina-Related Maternal Stress, Maternal Mental Health, and Early Infant Temperament. Maternal and Child Health Journal, 14(4), 511–518. 10.1007/s10995-009-0486-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Textor J, van der Zander B, Gilthorpe MS, Liśkiewicz M, & Ellison GT (2016). Robust causal inference using directed acyclic graphs: the R package ‘dagitty’. International Journal of Epidemiology, 45(6), 1887–1894. 10.1093/ije/dyw341 [DOI] [PubMed] [Google Scholar]
- Van den Bergh BRH, Mulder EJH, Mennes M, & Glover V (2005). Antenatal maternal anxiety and stress and the neurobehavioural development of the fetus and child: links and possible mechanisms. A review. Neuroscience & Biobehavioral Reviews, 29(2), 237–258. 10.1016/j.neubiorev.2004.10.007 [DOI] [PubMed] [Google Scholar]
- Van den Hove DLA, Leibold NK, Strackx E, Martinez-Claros M, Lesch KP, Steinbusch HWM, … Prickaerts J (2014). Prenatal stress and subsequent exposure to chronic mild stress in rats; interdependent effects on emotional behavior and the serotonergic system. European Neuropsychopharmacology, 24(4), 595–607. 10.1016/j.euroneuro.2013.09.006 [DOI] [PubMed] [Google Scholar]
- van Dijk AE, van Eijsden M, Stronks K, Gemke RJBJ, & Vrijkotte TGM (2012). Prenatal Stress and Balance of the Child’s Cardiac Autonomic Nervous System at Age 5-6 Years. PLoS ONE, 7(1), e30413 10.1371/journal.pone.0030413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa PD, Sandman CA, & Garite TJ (2001). The neurobiology of stress in human pregnancy: implications for prematurity and development of the fetal central nervous system. Progress in Brain Research, 133, 131–142. [DOI] [PubMed] [Google Scholar]
- Warsini S, Mills J, & Usher K (2014). Solastalgia: Living With the Environmental Damage Caused By Natural Disasters. Prehospital and Disaster Medicine; Cambridge, 29(1), 87–90. 10.1017/S1049023X13009266 [DOI] [PubMed] [Google Scholar]
- Whitmee S, Haines A, Beyrer C, Boltz F, Capon AG, de Souza Dias BF, … Yach D (2015). Safeguarding human health in the Anthropocene epoch: report of The Rockefeller Foundation-Lancet Commission on planetary health. The Lancet, 386(10007), 1973–2028. 10.1016/S0140-6736(15)60901-1 [DOI] [PubMed] [Google Scholar]
- Yong Ping E, Laplante DP, Elgbeili G, Hillerer KM, Brunet A, O’Hara MW, & King S (2015). Prenatal maternal stress predicts stress reactivity at 2½ years of age: The Iowa Flood Study. Psychoneuroendocrinology, 56, 62–78. 10.1016/j.psyneuen.2015.02.015 [DOI] [PubMed] [Google Scholar]
- Zhang W, Li Q, Deyssenroth M, Lambertini L, Finik J, Ham J, … Nomura Y (2018). Timing of prenatal exposure to trauma and altered placental expressions of hypothalamic-pituitary-adrenal axis genes and genes driving neurodevelopment. Journal of Neuroendocrinology, 30(4), el2581 10.1111/jne.12581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Wei, Finik J, Dana K, Glover V, Ham J, & Nomura Y (2018). Prenatal Depression and Infant Temperament: The Moderating Role of Placental Gene Expression. Infancy, 23(2), 211–231. 10.1111/infa.12215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Wei, Rajendran K, Ham J, Finik J, Buthmann J, Davey K, … Nomura Y (2018). Prenatal exposure to disaster-related traumatic stress and developmental trajectories of temperament in early childhood: Superstorm Sandy pregnancy study. Journal of Affective Disorders, 234, 335–345. 10.1016/j.jad.2018.02.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu P, Sun M-S, Hao J-H, Chen Y-J, Jiang X-M, Tao R-X, … Tao F-B (2014). Does prenatal maternal stress impair cognitive development and alter temperament characteristics in toddlers with healthy birth outcomes? Developmental Medicine & Child Neurology, 56(3), 283–289. 10.1111/dmcn.12378 [DOI] [PubMed] [Google Scholar]