Abstract
Purpose:
Increasing radiation dose to the heart is associated with worse survival in stage III non-small cell lung cancer. We sought to evaluate the ability of optimized volumetric modulated arc therapy (VMAT) and intensity modulated proton therapy (IMPT) to spare cardiac substructures. We also wanted to determine how a cardiac optimization treatment planning algorithm influences dose distribution to other thoracic organs at risk (OARs).
Methods and Materials:
Cardiac substructures were retrospectively contoured for all patients with stage III non-small cell lung cancer who were treated at our institution with VMAT to 60 Gy in 2-Gy fractions. The structures included valves, atrioventricular node, coronary arteries, chambers, and great vessels. New cardiac-optimized VMAT plans were created to spare these structures while preserving planning target volume coverage and maintaining standard dose constraints to OARs. Dosimetry variables for the new cardiac-optimized VMAT plans were compared via paired t test with the original VMAT plans. IMPT plans were also created, and the cardiac-optimized VMAT plans were then similarly compared with the IMPT plans.
Results:
Twenty-six patients who were treated from July 2013 to September 2017 were included. Compared with the original VMAT plans, statistically significant improvements were demonstrated for all cardiac structures for the new cardiac-optimized VMAT plans while maintaining or improving appropriate lung, esophagus, and spinal cord constraints and planning target volume coverage goals. Compared with cardiac-optimized VMAT, IMPT demonstrated additional statistically significant improvements for some cardiac dosimetry metrics while maintaining or improving other thoracic OAR constraints.
Conclusions:
VMAT is now widely available, and high-quality VMAT plans that incorporate cardiac substructures into the optimization process can provide overall improvements in dose to OARs and, in particular, substantial sparing of critical cardiac structures. IMPT provides some incremental dosimetric improvements beyond cardiac-optimized VMAT, the clinical significance of which remains uncertain.
Introduction
In the Radiation Therapy Oncology Group (RTOG) non-small cell lung cancer (NSCLC) trial RTOG 0617, increased radiation dose to critical intrathoracic structures was posited as a possible cause of the unexpected worse survival of the dose-escalated 74-Gy arm compared with the 60-Gy arm, which subsequently remained the standard of care.1 Heart V5 (volume of heart receiving 5 Gy) and heart V30 were associated with an increased risk of death that persisted on multivariable analysis.1 Several other large, single-institutional cohorts of patients enrolled on prospective clinical trials have also demonstrated heart dose to be independently associated with cardiac events and overall survival.
Increasingly, it has become apparent that cardiac events can occur as a consequence of therapy in an early time frame relevant to patients with lung cancer, especially as survival continues to improve, as was seen most recently in the PACIFIC trial.2–5
Advanced photon radiation techniques such as intensity modulated radiation therapy (IMRT) and its rotational successor volumetric modulated arc therapy (VMAT) are now widely available, and they have the capacity to dramatically lower the cardiac dose in many locally advanced NSCLC cases compared with 3-dimensional conformal radiation therapy (3DCRT). A secondary analysis of RTOG 0617 demonstrated that IMRT was associated with lower cardiac doses and lower rates of severe pneumonitis; the authors concluded that IMRT should be used routinely instead of 3DCRT.6 Randomized phase 3 trials of IMRT versus 3DCRT were not conducted for locally advanced NSCLC treated with concurrent chemoradiation, and thus subgroup or secondary analyses remain the highest level of clinical data supporting use of IMRT; at the population level, an analysis of the National Cancer Database does endorse a survival benefit for IMRT for T3 and T4 tumors, and with now-widespread adoption of IMRT/VMAT, such trials will never occur.7
Although IMRT and VMAT offer potentially substantial improvements over 3DCRT, proton beam therapy (PBT)—and particularly intensity modulated proton therapy (IMPT)—may offer dosimetric advantages in many NSCLC cases beyond what photon-based techniques can provide. Compared with the original passive-scatter PBT technology, pencil beam IMPT allows for improved dose contouring to irregularly shaped tumors and tumors adjacent to critical organs at risk (OARs). Neutron contamination is also decreased which lowers integral dose. However, integral dose and low-dose wash are routinely superior for both passive-scatter PBT and IMPT compared with any photon-based technique.8 Randomized verification of clinical benefit to PBT is ongoing through the NRG Oncology 1308 randomized trial,9 although an analysis of the National Cancer Database shows a survival benefit for PBT relative to photons.10 Although PBT is increasing in availability because of the construction of new facilities, it still remains inaccessible for many patients compared with IMRT/VMAT.10 As such, in this study, we sought to quantify the capability of “best-case scenario” cardiac-optimized VMAT to spare the heart and cardiac substructures compared with IMPT, while maintaining target volume coverage and accepted dose constraints to other critical thoracic OARs.
Methods and Materials
We retrospectively reviewed all cases of NSCLC treated with VMAT at Winship Cancer Institute of Emory University or Winship at Emory University Hospital Midtown. We sought to maintain a patient population that received similar doses and had a similar burden of disease in the vicinity of the central thoracic structures. As such, we included in this analysis only patients with stage III disease. These patients would be expected to have relatively heavy burdens of disease in the central thorax, and thus planning their cases would have similar demands in target-volume coverage and sparing OARs. Additionally, we only included patients treated to 60 Gy in 2-Gy fractions concurrent with chemotherapy, to preclude the need for biologically effective dose conversions and equivalent dose in 2-Gy fractions calculations. For example, some patients with stage III disease, particularly in the early years of VMAT use at our center, were treated with alternative radiation regimens including dose escalation, hypofractionation in patients unfit for chemotherapy, and use of 1.8-Gy fractions, and these patients were excluded.
All patients had undergone 4-dimensional computed tomography (CT) scans, but only patients whose original radiation therapy (RT) courses were planned on a free-breathing CT scan were included. This approach was necessary because accurately visualizing intracardiac anatomy is challenging on a pixel-averaged CT scan, which is composed of the average values of each pixel from all phases of the respiratory cycle per 4-dimensional CT scan. Some patients at our institution had original RT courses planned on pixel-averaged CT scans as opposed to free-breathing CT scans, and these patients were excluded. Although image registration can be performed and intracardiac structures contoured on free-breathing CT could then be transferred to the pixel-averaged CT, this process is often not precise, particularly given the small size and winding course of several of the structures that were to be contoured, notably the coronary arteries.
In total, 26 patients treated from July 2013 to September 2017 met the inclusion criteria. Naturally, we had available all original RT plans with which these patients were treated. During this period, it was not our practice to routinely contour and include in the treatment planning algorithm many of the numerous intrathoracic and intracardiac substructures for which there has not been clear clinical relevance of sparing dose. Several thoracic and cardiac contouring atlases are available that describe techniques to contour these often-small structures.11–16 Thus, in preparation for creating new cardiac-optimized VMAT plans, for the 26 patients a single operator retrospectively contoured a comprehensive list of intrathoracic and intracardiac structures that included heart valves, chambers, the coronary arteries, the great vessels, and the atrioventricular node, using a uniform methodology.12
Our dosimetry team created new cardiac-optimized VMAT plans by incorporating the previously mentioned structures into the Eclipse treatment planning system’s plan optimizer, with the goal of maximizing sparing of the intracardiac substructures while maintaining planning target volume (PTV) coverage and standard dose constraints to routine OARs. Among the original VMAT plans, 20 plans used 6-MV photons and 6 used 10-MV photons. All but 1 of the cardiac-optimized plans used 6-MV photons. Among the original VMAT plans, 22 plans used 2 arcs (clockwise, and counterclockwise), 3 used 3 arcs, and 1 used 4 arcs because of the necessity in this case to treat upper and lower target volumes separately. Among the cardiac-optimized VMAT plans, 17 plans used 2 arcs, 8 used 3 arcs, and 4 arcs were used for the same plan that used 4 arcs originally. Additional arcs were added as necessary to improve dosimetry, with the understanding that the trade-off is increased in-room treatment time.
For proton planning, RayStation version 8A (Ray-Search Laboratories, Stockholm, Sweden) was used to generate all IMPT treatment plans using the Monte Carlo dose algorithm for optimization and final dose calculation. Each plan consisted of 2 to 3 beams that were robustly optimized with a range uncertainty of 4% to account for potential systematic errors in the CT scan to Hounsfield unit conversion. In addition, 5-mm isocenter perturbations in the left to right, anterior to posterior, and superior to inferior directions were included in the robust optimization to simulate daily setup variations and to ensure plan robustness. Nineteen patients were optimized using single-field optimization, a proton planning technique that uses each individual beam to cover the entire target volume uniformly. Cases planned with single-field optimization were generally less complex volumetrically, often with less intricate proximity to critical OARs.
Seven patients with relatively more complex target volumes were optimized using multifield optimization, a proton planning technique in which each individual beam covers only specific portions of the target and the overall contribution from all beams covers the target uniformly. Robust evaluation was performed for all treatment plans to verify plan robustness under different scenarios, which consisted of simulating a combination of range errors of 5% and 5 mm in daily setup variations. For all perturbed plans, the clinical target volume (CTV) D95 (dose to 95% of the volume) was kept within 3% of the nominal plan value.
We collected dosimetry information from the original VMAT plans, from the new cardiac-optimized VMAT plans, and from the IMPT plans. Maximum dose (Dmax) and mean dose (Dmean) were recorded for all intrathoracic and intracardiac substructures. We also recorded metrics for PTV coverage and routinely analyzed clinically relevant dose-volume relationships for standard thoracic OARs. PTV coverage metrics were not recorded for IMPT plans because the IMPT plans were prescribed to the CTV, not the PTV, which is standard IMPT planning practice. Dosimetry variables were compared between groups using paired t tests. Significance was assessed at the .05 alpha level.
Results
All patients included were in stage III according to either the American Joint Committee on Cancer (AJCC) 7th or the AJCC 8th edition; none of the patients were stage III strictly because of N3 nodal disease. All 26 patients were stage IIIA per the AJCC 7th edition. Per the AJCC 8th edition, 19 patients were stage IIIA, and 7 patients were stage IIIB. The T stage distribution was follows: 1 (5 patients), 2 (9 patients), 3 (8 patients), and 4 (4 patients). N stage distribution was 0 (2 patients);, 1 (3 patients), and 2 (21 patients). The primary tumor location was in the right upper lobe in 6 patients, the right middle lobe in 3 patients, the right lower lobe in 2 patients, the left upper lobe in 7 patients, and the left lower lobe in 8 patients.
Table 1 shows the dosimetric comparison between the original VMAT plans with which the patients were treated and the new cardiac-optimized VMAT plans that were created. Statistically significant improvements were demonstrated for the cardiac-optimized VMAT plans for essentially all dosimetry metrics (except heart Dmax, left atrium Dmax, pulmonary artery Dmax, and lung V5), including for the whole heart structure, valves, atrioventricular node, coronary arteries, chambers, and great vessels and the lungs, spinal cord, and esophagus, while PTV coverage was maintained.
Table 1.
Paired t test comparison between original VMAT plans and cardiac-optimized VMAT plans (n = 26)
| Dosimetry variable | Original VMAT plans (mean; SD) | Cardiac-optimized VMAT plans (mean; SD) | P Value |
|---|---|---|---|
| Cardiac parameters (Gy) | |||
| Heart Dmax | 63.63; 7.36 | 64.89; 3.23 | .378 |
| Heart Dmean | 16.1; 8.46 | 12.35; 7.44 | <.001* |
| Heart V5 | 64.14; 27.75 | 55.39; 28.06 | .002* |
| Heart V30 | 18.72; 14.44 | 12.47; 11.53 | <.001* |
| Heart V40 | 11.48; 10.44 | 7.87; 8.21 | <.001* |
| Heart V45 | 9.01; 8.85 | 6.48; 7.02 | <.001* |
| Heart V60 | 3.36; 3.73 | 2.72; 3.27 | <.001* |
| Aortic valve Dmax | 31.66; 17.53 | 22.9; 16.42 | <.001* |
| Aortic valve Dmean | 19.83; 12.64 | 11.42; 8.46 | <.001* |
| Mitral valve Dmax | 29.37; 21.64 | 24.56; 22.75 | .001* |
| Mitral valve Dmean | 16.73; 14.01 | 11.2; 12.25 | <.001* |
| Pulmonic valve Dmax | 35.43; 16.52 | 26.83; 18.71 | <.001* |
| Pulmonic valve Dmean | 25.12; 15.43 | 14.06; 14.53 | <.001* |
| Tricuspid valve Dmax | 16.6; 14.26 | 9.74; 10.55 | <.001* |
| Tricuspid valve Dmean | 10.34; 10.76 | 5.58; 6.88 | <.001* |
| Atrioventricular node Dmax | 20.41; 13.6 | 13.39; 12.4 | <.001* |
| Atrioventricular node Dmean | 14.00; 10.01 | 8.05; 7.14 | <.001* |
| Left main coronary artery Dmax | 38.77; 17.62 | 26.34; 18.89 | <.001* |
| Left main coronary artery Dmean | 30.15; 16.3 | 16.42; 14.12 | <.001* |
| Left anterior descending coronary artery Dmax | 34.78; 20.39 | 27.39; 21.11 | <.001* |
| Left anterior descending coronary artery Dmean | 22.59; 16.3 | 14.37; 13.85 | <.001* |
| Left circumflex coronary artery Dmax | 36.85; 23.71 | 32.58; 25.37 | <.001* |
| Left circumflex coronary artery Dmean | 26.86; 19.85 | 19.33; 16.92 | <.001* |
| Right coronary artery Dmax | 26.14; 18.72 | 18.13; 17.08 | <.001* |
| Right coronary artery Dmean | 15.74; 13.44 | 9.4; 9.03 | <.001* |
| Left atrium Dmax | 54.84; 17.19 | 51.82; 19.99 | .056 |
| Left atrium Dmean | 20.97; 11.86 | 17.53; 10.73 | <.001* |
| Left ventricle Dmax | 40.1; 22.45 | 35.71; 24.87 | <.001* |
| Left ventricle Dmean | 11.29; 10.3 | 8.34; 8.79 | <.001* |
| Right atrium Dmax | 36.08; 22.31 | 31.37; 23.68 | .004* |
| Right atrium Dmean | 13.89; 14.00 | 11.11; 12.27 | <.001* |
| Right ventricle Dmax | 33.15; 17.41 | 23.65; 17.85 | <.001* |
| Right ventricle Dmean | 12.24; 11.64 | 6.93; 6.22 | <.001* |
| Ascending aorta Dmax | 55.28; 12.49 | 50.77; 17.03 | .001* |
| Ascending aorta Dmean | 27.92; 11.81 | 20.4; 10.96 | <.001* |
| Pulmonary artery Dmax | 65.13; 4.22 | 65.21; 3.27 | .935 |
| Pulmonary artery Dmean | 37.89; 9.56 | 32.24; 10.48 | <.001* |
| Superior vena cava Dmax | 51.74; 17.46 | 47.39; 20.58 | .001* |
| Superior vena cava Dmean | 33.08; 18.83 | 29.4; 18.47 | .005* |
| Other OAR parameters (Gy) | |||
| Lungs: CTV V5 | 58.18; 9.5 | 56.56; 9.24 | .165 |
| Lungs: CTV V20 | 23.25; 6.85 | 22.38; 6.09 | .043* |
| Lungs: CTV Dmean | 14.07; 3.1 | 13.58; 2.83 | .005* |
| Spinal cord Dmax | 31.07; 8.31 | 28; 7.92 | .008* |
| Esophagus Dmean | 22.09; 7.23 | 21.22; 6.97 | .020* |
| PTV coverage parameters (Gy) | |||
| PTV Dmax | 67.23; 2.24 | 65.51; 6.32 | .160 |
| PTV minimum dose | 52.72; 4.29 | 51.18; 4.61 | .009* |
| PTV V100% | 95.38; 0.8 | 95.24; 0.61 | .196 |
Abbreviations: CTV = clinical target volume; Dmax = maximum dose; Dmean = mean dose; OAR = organs at risk; PTV = planning target volume; SD = standard deviation; VMAT = volumetric modulated arc therapy.
Significant P value.
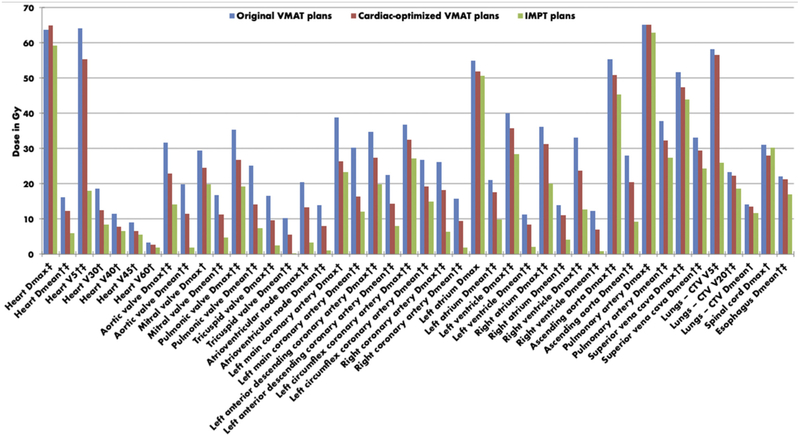
Table 2 shows the dosimetric comparison between the cardiac-optimized VMAT plans and the IMPT plans that were created. A general pattern of statistically significant improvements was also demonstrated for the IMPT compared with the cardiac-optimized VMAT plans for most dosimetric variables. Heart V30, heart V40, heart V45, heart V60, mitral valve Dmax, left main coronary artery Dmax, left atrium Dmax, lung Dmean, and spinal cord Dmax were not statistically different between plans. Figure 1 demonstrates dose differences among the 3 plans, in graphical form.
Table 2.
Paired t test comparison between cardiac-optimized VMAT plans and IMPT plans (n = 26)
| Dosimetry variable | Cardiac-optimized VMAT plans (mean; SD) | IMPT plans (mean; SD) | P Value |
|---|---|---|---|
| Cardiac parameters (Gy) | |||
| Heart Dmax | 64.89; 3.23 | 59.24; 12.56 | .038* |
| Heart Dmean | 12.35; 7.44 | 5.91; 5.3 | <.001* |
| Heart V5 | 55.39; 28.06 | 18.1; 13.68 | <.001* |
| Heart V30 | 12.47; 11.53 | 8.37; 8.47 | .077 |
| Heart V40 | 7.87; 8.21 | 6.48; 6.94 | .401 |
| Heart V45 | 6.48; 7.02 | 5.61; 6.20 | .547 |
| Heart V60 | 2.72; 3.27 | 1.85; 2.01 | .127 |
| Aortic valve Dmax | 22.9; 16.42 | 14.06; 16.66 | .007* |
| Aortic valve Dmean | 11.42; 8.46 | 1.84; 2.58 | <.001* |
| Mitral valve Dmax | 24.56; 22.75 | 19.77; 23.97 | .053 |
| Mitral valve Dmean | 11.2; 12.25 | 4.71; 7.97 | <.001* |
| Pulmonic valve Dmax | 26.83; 18.71 | 19.3; 21.78 | .003* |
| Pulmonic valve Dmean | 14.06; 14.53 | 7.41; 14.61 | <.001* |
| Tricuspid valve Dmax | 9.74; 10.55 | 2.52; 6.9 | <.001* |
| Tricuspid valve Dmean | 5.58; 6.88 | 0.29; 0.69 | <.001* |
| Atrioventricular node Dmax | 13.39; 12.4 | 3.4; 10.4 | <.001* |
| Atrioventricular node Dmean | 8.05; 7.14 | 1.16; 4.45 | <.001* |
| Left main coronary artery Dmax | 26.34; 18.89 | 23.22; 20.22 | .148 |
| Left main coronary artery Dmean | 16.42; 14.12 | 12.18; 15.67 | .028* |
| Left anterior descending coronary artery Dmax | 27.39; 21.11 | 19.87; 23.01 | <.001* |
| Left anterior descending coronary artery Dmean | 14.37; 13.85 | 8.07; 13.94 | .001* |
| Left circumflex coronary artery Dmax | 32.58; 25.37 | 27.29; 26.24 | .008* |
| Left circumflex coronary artery Dmean | 19.33; 16.92 | 14.9; 18.64 | .011* |
| Right coronary artery Dmax | 18.13; 17.08 | 6.46; 14.42 | <.001* |
| Right coronary artery Dmean | 9.40; 9.03 | 1.92; 4.97 | <.001* |
| Left atrium Dmax | 51.82; 19.99 | 50.57; 18.98 | .325 |
| Left atrium Dmean | 17.53; 10.73 | 9.83; 8.81 | <.001* |
| Left ventricle Dmax | 35.71; 24.87 | 28.33; 27.35 | <.001* |
| Left ventricle Dmean | 8.34; 8.79 | 2.03; 3.00 | <.001* |
| Right atrium Dmax | 31.37; 23.68 | 20.08; 24.29 | <.001* |
| Right atrium Dmean | 11.11; 12.27 | 4.2; 9.05 | <.001* |
| Right ventricle Dmax | 23.65; 17.85 | 12.75; 19.38 | <.001* |
| Right ventricle Dmean | 6.93; 6.22 | 0.87; 1.94 | <.001* |
| Ascending aorta Dmax | 50.77; 17.03 | 45.31; 22.83 | .005* |
| Ascending aorta Dmean | 20.40; 10.96 | 9.16; 8.49 | <.001* |
| Pulmonary artery Dmax | 65.21; 3.27 | 62.9; 0.71 | .001* |
| Pulmonary artery Dmean | 32.24; 10.48 | 27.33; 11.64 | <.001* |
| Superior vena cava Dmax | 47.39; 20.58 | 43.98; 24.06 | .023* |
| Superior vena cava Dmean | 29.4; 18.47 | 24.42; 22.31 | .002* |
| Other OAR parameters (Gy) | |||
| Lungs: CTV V5 | 56.56; 9.24 | 26.04; 9.02 | <.001* |
| Lungs: CTV V20 | 22.38; 6.09 | 18.65; 7.04 | <.001* |
| Lungs: CTV Dmean | 13.58; 2.83 | 11.69; 10.95 | .368 |
| Spinal cord Dmax | 28.00; 7.92 | 30.25; 13.02 | .224 |
| Esophagus Dmean | 21.22; 6.97 | 17.01; 7.76 | <.001* |
Abbreviations: CTV = clinical target volume; Dmax = maximum dose; Dmean = mean dose; IMPT = intensity modulated proton therapy; OAR = organs at risk; SD = standard deviation; VMAT = volumetric modulated arc therapy.
Significant P value.
Figure 1.
Median doses in Gy for original VMAT plans, cardiac-optimized VMAT plans, and IMPT plans. †Statistically significant difference between original and cardiac-optimized VMAT plans. ‡Statistically significant difference between cardiac-optimized VMAT and IMPT plans. Abbreviations: CTV = clinical target volume; Dmax = maximum dose; Dmean = mean dose; IMPT = intensity modulated proton therapy; VMAT = volumetric modulated arc therapy.
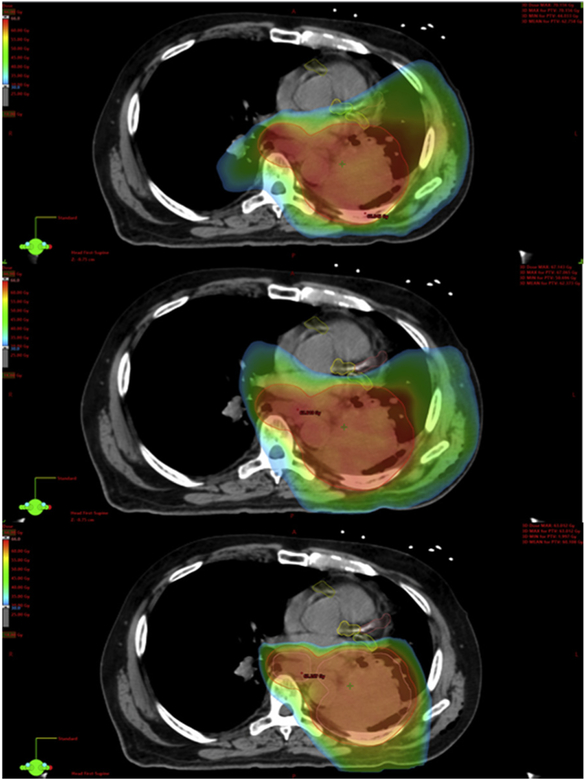
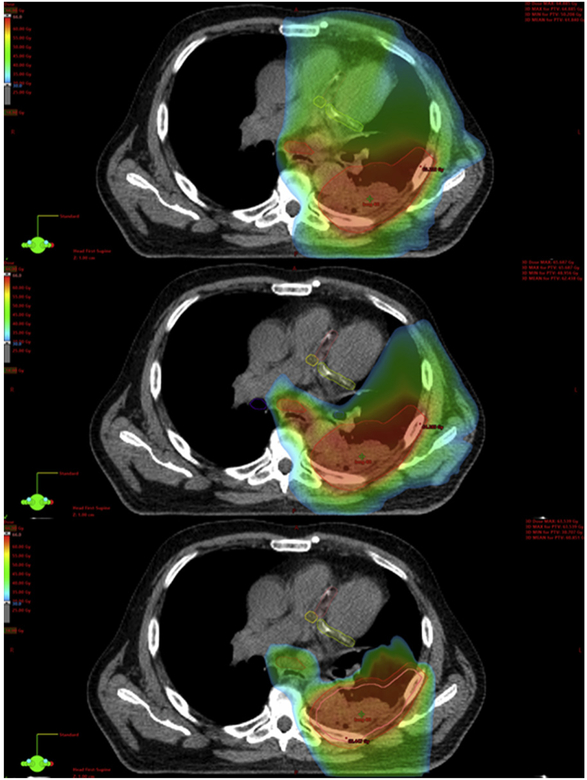
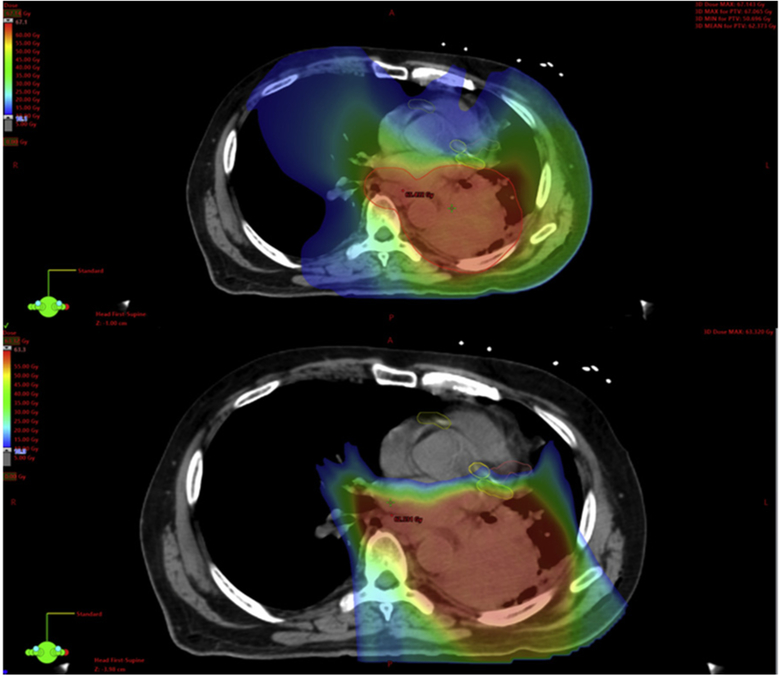
Figures 2 and 3 demonstrate example dose color wash comparisons for 2 of the cases. These color wash images use identical dose color threshold parameters and are set at identical image slices. These figures demonstrate the capacity of the cardiac-optimized VMAT plans and IMPT plans to spare higher dose levels from reaching critical intracardiac structures, most notably the coronary arteries in these images. Moving from the original VMAT plans to the cardiac-optimized VMAT plans, there are some small shifts in the distribution of the higher dose levels away from the heart and into the chest wall and adjacent thoracic musculature, which is of unclear, but likely low, clinical significance. Although the cardiac-optimized VMAT plans display notable sparing of the coronary arteries at the higher dose levels, Figure 4 demonstrates the significant underperformance of even a cardiac-optimized VMAT plan in terms of low-dose wash compared with IMPT. The IMPT plans demonstrate the characteristic capacity of IMPT for sharp dose falloff beyond the target, and low integral dose, compared with the VMAT plans.
Figure 2.
Example case 1. Original volumetric modulated arc therapy plan (top). Cardiac-optimized volumetric modulated arc therapy plan (middle). Intensity modulated proton therapy plan (bottom). Dose thresholding demonstrating the high-dose color wash: 30 Gy (blue), 45 Gy (green), 60 Gy (red).
Figure 3.
Example case 2. Original volumetric modulated arc therapy plan (top). Cardiac-optimized volumetric modulated arc therapy plan (middle). Intensity modulated proton therapy plan (bottom). Dose thresholding demonstrating the high-dose color wash: 30 Gy (blue), 45 Gy (green), 60 Gy (red).
Figure 4.
Example case 1. Cardiac-optimized volumetric modulated arc therapy plan (top). Intensity modulated proton therapy plan (bottom). Dose thresholding including low-dose color wash: 10 Gy (blue), 35 Gy (green), 60 Gy (red).
Discussion
Delineation of the full complement of cardiac substructures can be time-consuming and have some degree of a learning curve, so in most radiation oncologists’ practices they have not routinely been contoured. For example, in the most recently reported large cooperative group trial for locally advanced NSCLC, RTOG 0617, the heart, but not other intracardiac substructures, was required to be contoured and spared dose.17 Pericarditis was historically the primary fear with excess cardiac dose; however, several studies have now shown that damage to the myocardium itself and perfusion defects are realities.18,19 Marks et al demonstrated radiation volume–dependent perfusion defects in 40% of patients with left-sided breast cancer within 2 years of treatment.18 Gomez et al demonstrated real-time changes in echocardiograms and brain natriuretic peptide levels during thoracic radiation courses.19 In a Surveillance, Epidemiology, and End Results Program study of patients with small cell lung cancer conducted by our group, pericarditis was demonstrated to compose a relatively low proportion of cardiac events experienced after radiation—exceeded in frequency by heart failure and the very general diagnosis of acute heart disease. It was similar in frequency to acute myocardial infarction, cardiomyopathy, and dysrhythmia, all of which have a pathophysiology theoretically contributed to by radiation damage to the valves, conducting system, or coronary arteries.20 Therefore, it seems likely that although concrete clinical outcome advantages to sparing specific central thoracic or intracardiac substructures will remain difficult to quantify, any achievable decrease in dose for many of these structures seems likely to be preferable21 given the increasing evidence that lower dose seems to be better, without a threshold, for the whole heart structure.3,4,22
We anticipated that the new cardiac-optimized VMAT plans would improve dosimetry to intracardiac structures compared with the original VMAT plans, although we assumed this would be at the expense of dose to other thoracic OARs such as the lungs, spinal cord, and esophagus. Dosimitry to the intracardiac structures was improved as expected, but surprisingly, dosimetry to the other thoracic OARs was maintained or improved in quality; no costs to the other thoracic OARs were demonstrated in the cardiac-optimized VMAT plans. Skill and experience with VMAT planning has likely improved over time, which also could contribute to the general improvements seen with the cardiac-optimized plans. Additionally, software upgrades to Eclipse over time could have led to improvements in plan quality. Although these factors may play a small role in improved cardiac dose, the plan improvements we demonstrated go beyond these factors, with more substantial contributions from incorporation of the new structures into the optimization process. This is particularly evident when analyzing the comparison dose color washes of the different radiation plans (Figs 1 and 2) and visualizing the isodose lines for the cardiac-optimized VMAT plans nicely wrap around and avoid critical structures such as the coronary arteries, whereas the original VMAT plans are ignorant of the coronary arteries and therefore show no preference in avoiding them. Including the numerous new, small, central structures in the optimization process allows for dose painting in a manner that improves not only whole heart and cardiac substructure dose but also dose spillage into the spinal cord, esophagus, and lungs.
In some ways, the rise of IMPT parallels the rise of IMRT/VMAT: a new and expensive radiation technology becomes available that offers such potentially large dosimetric advantages that it becomes tempting for the radiation oncology community to offer routine use before proof of clinical benefit in many situations. Also, as with IMRT/VMAT, there seems to be a learning curve for IMPT, whereby our ability to deliver it has improved over time; eg, now pencil beam or spot-scanning techniques, often multifield optimized, are beginning to overtake the original passive-scatter technology. Regardless, the statistical comparison of the cardiac-optimized VMAT plans and the IMPT plans demonstrates the striking ability of the IMPT plans to deposit negligible dose to many of the intracardiac structures, even in advanced left lower lobe cases. Additionally, Figure 3 demonstrates the classic capability of IMPT to eliminate much of the low-dose wash compared with VMAT.
Of course, these advantages come with the known caveats that (1) IMPT is potentially more susceptible to tissue heterogeneities and movement of target volumes and OARs and that (2) IMPT, although increasing in availability, is still not a routine practicality for many patients. With this study, we have demonstrated that although IMPT offers potential dosimetric improvements over photon-based techniques—the clinical relevance of which remains uncertain, pending randomized trial results—high-quality, cardiac-optimized VMAT plans can be generated at most centers by incorporating the cardiac substructures into the treatment-planning process. With delineation of the numerous cardiac substructures, despite the initial learning curve and small increase in contouring time, overall plan workflow time may not necessarily be lengthened because of the natural improvements the substructures bring to the optimization process, which might preclude need for time-consuming replans to meet OAR dose constraints. We recommend routine inclusion of the cardiac substructures in the optimization process for all cases of stage III NSCLC treated with VMAT or IMPT. Finally, it is possible that dose escalation in locally advanced NSCLC might be revisited as techniques arise to improve critical OAR dosimetry metrics that seem to be closely linked to survival.
Conclusions
Radiation dose to the heart and intracardiac substructures can be substantially lowered using a cardiac-sparing optimization algorithm with VMAT without increasing radiation dose to other critical thoracic OARs and without compromising target volume coverage. Although the clinical significance of sparing these intracardiac substructures requires further investigation, it seems likely that less dose must be better. As such, we recommend routine inclusion of the cardiac substructures in the optimization process for all cases of stage III NSCLC treated with VMAT or IMPT as an effective means of improving dosimetry to OARs, especially the heart.
Sources of support:
Research reported in this publication was supported in part by the Biostatistics and Bioinformatics Shared Resource of Winship Cancer Institute of Emory University and National Institutes of Health/National Cancer Institute under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures: Dr Higgins reports the following disclosures: consultant for Astra Zeneca and Varian, advisory board for Genentech and Astra Zeneca, and funded research for RefleXion Medical. Dr Curran is group chairman and a principal investigator for NRG Oncology (previously RTOG).
References
- 1.Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16:187–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Speirs CK, DeWees TA, Rehman S, et al. Heart dose is an independent dosimetric predictor of overall survival in locally advanced non-small cell lung cancer. J Thorac Oncol. 2017;12:293–301. [DOI] [PubMed] [Google Scholar]
- 3.Wang K, Eblan MJ, Deal AM, et al. Cardiac toxicity after radiotherapy for stage III non-small-cell lung cancer: Pooled analysis of dose-escalation trials delivering 70 to 90 Gy. J Clin Oncol. 2017;35: 1387–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dess RT, Sun Y, Matuszak MM, et al. Cardiac events after radiation therapy: Combined analysis of prospective multicenter trials for locally advanced non-small-cell lung cancer. J Clin Oncol. 2017;35: 1395–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antonia SJ, Villegas A, Daniel D, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379:2342–2350. [DOI] [PubMed] [Google Scholar]
- 6.Chun SG, Hu C, Choy H, et al. Impact of intensity-modulated radiation therapy technique for locally advanced non-small-cell lung cancer: A secondary analysis of the NRG Oncology RTOG 0617 Randomized Clinical Trial. J Clin Oncol. 2017;35:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jegadeesh N, Liu Y, Gillespie T, et al. Evaluating intensity-modulated radiation therapy in locally advanced non-small-cell lung cancer: Results from the National Cancer Data Base. Clin Lung Cancer. 2016;17:398–405. [DOI] [PubMed] [Google Scholar]
- 8.Kase Y, Yamashita H, Fuji H, et al. A treatment planning comparison of passive-scattering and intensity-modulated proton therapy for typical tumor sites. J Radiat Res. 2012;53:272–280. [DOI] [PubMed] [Google Scholar]
- 9.Comparing photon therapy to proton therapy to treat patients with lung cancer. Available at: https://clinicaltrials.gov/ct2/show/NCT01993810. Accessed March 26, 2019.
- 10.Higgins KA, O’Connell K, Liu Y, et al. National Cancer Database analysis of proton versus photon radiation therapy in non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2017;97:128–137. [DOI] [PubMed] [Google Scholar]
- 11.Duane F, Aznar MC, Bartlett F, et al. A cardiac contouring atlas for radiotherapy. Radiother Oncol. 2017;122:416–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng M, Moran JM, Koelling T, et al. Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J Radiat Oncol Biol Phys. 2011;79:10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee J, Hua KL, Hsu SM, et al. Development of delineation for the left anterior descending coronary artery region in left breast cancer radiotherapy: An optimized organ at risk. Radiother Oncol. 2017; 122:423–430. [DOI] [PubMed] [Google Scholar]
- 14.Lorenzen EL, Taylor CW, Maraldo M, et al. Inter-observer variation in delineation of the heart and left anterior descending coronary artery in radiotherapy for breast cancer: A multi-centre study from Denmark and the UK. Radiother Oncol. 2013;108:254–258. [DOI] [PubMed] [Google Scholar]
- 15.Wennstig AK, Garmo H, Hallstrom P, et al. Inter-observer variation in delineating the coronary arteries as organs at risk. Radiother Oncol. 2017;122:72–78. [DOI] [PubMed] [Google Scholar]
- 16.Zhou R, Liao Z, Pan T, et al. Cardiac atlas development and validation for automatic segmentation of cardiac substructures. Radiother Oncol. 2017;122:66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radiation Therapy Oncology Group Foundation I. RTOG 0617. 2011. Updated Protocol. Available at: https://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?ptidZ387&modeZbroadcasts&page896Z5&studyZ0617. Accessed March 26, 2019.
- 18.Marks LB, Yu X, Prosnitz RG, et al. The incidence and functional consequences of RT-associated cardiac perfusion defects. Int J Radiat Oncol Biol Phys. 2005;63:214–223. [DOI] [PubMed] [Google Scholar]
- 19.Gomez DR, Yusuf SW, Munsell MF, et al. Prospective exploratory analysis of cardiac biomarkers and electrocardiogram abnormalities in patients receiving thoracic radiation therapy with high-dose heart exposure. J Thorac Oncol. 2014;9:1554–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferris MJ, Jiang R, Behera M, Ramalingam SS, et al. Radiation therapy is associated with an increased incidence of cardiac events in patients with small cell lung cancer. Int J Radiat Oncol Biol Phys. 2018;102:383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gore EM, Hu C, Bar Ad V, et al. Impact of incidental cardiac radiation on cardiopulmonary toxicity and survival for locally advanced non-small cell lung cancer: Reanalysis of NRG Oncology/RTOG 0617 with centrally contoured cardiac structures. Int J Radiat Oncol Biol Phys. 2016;96:S129–S130. [Google Scholar]
- 22.Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. [DOI] [PubMed] [Google Scholar]