1. Introduction
Over 20 antiseizure drugs (ASDs) for the symptomatic management of the seizures of epilepsy have been brought to the clinic on the basis of efficacy in rodent acute and chronic seizure models. Nonetheless, over 30% of patients remain pharmacoresistant [1]. Many ASDs also carry significant adverse effects liability that can severely limit clinical use, particularly in pediatric and elderly patients. Preclinical models that better represent the patients who are still in need of effective therapies should thus be prioritized in initial ASD identification to advance potentially better tolerated, mechanistically-novel therapies.
2. Approach to drug development for symptomatic seizures
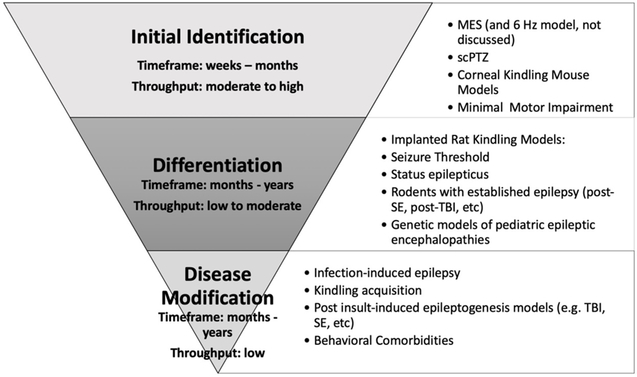
The wealth of acute and chronic seizure models available today makes the selection of the most appropriate approach to identify a new ASD challenging and, at times, even overwhelming (Figure 1). Further, ASD development has historically been performed in two phases: Identification and Differentiation, with a primary emphasis on phenotype-based (e.g. seizures), instead of target-based, drug development. For the purposes of this editorial, we will primarily focus on moderate- to high-throughput identification in a phenotype-based drug screening program; i.e. models that use behavioral seizures as the primary outcome measure to demonstrate anticonvulsant efficacy of an investigational agent. The majority of approved ASDs are effective in two models of acute (i.e. single event) seizure [2]: maximal electroshock (MES) and subcutaneous pentylenetetrazol tests (scPTZ; Table 1). These models are relatively easy to implement and amenable to moderate- to high-throughput screening of large libraries of compounds in a short timeframe. As a result, the MES and scPTZ models are positioned earlier in the development process than the resource-intensive models with spontaneous recurrent seizures (SRS; Figure 1). Kindling models with chronic evoked seizures have also been incredibly useful to identify the remaining currently available mechanistically-distinct compounds (e.g. levetiracetam [3]), as well as inform on the potential for adverse effects liability in an epileptic substrate (i.e. D-CPPene [4]), which has certainly benefited patients with epilepsy (Table 2). Nonetheless, these models have only identified therapies that treat a single symptom: seizures. Despite more than 20 clinically-available ASDs, the percentage of drug-resistant patients has remained unchanged; the approach to ASD development clearly must be revised.
Figure 1.
Approach to the identification and differentiation of promising antiseizure drugs.
Table 1.
Common preclinical models of acute and chronic seizure in use for early ASD development, as well as innovative models of pharmacoresistant chronic seizures.
| Animal Model | Acute vs. Chroni c Seizur es |
Induction Protocol |
Seizure Endpoints |
Pharmacoresi stant Model? |
Translational Relevance |
ASD Identified to Validate the Model |
Effective ASDs at Non-Motor Impairing Doses |
|---|---|---|---|---|---|---|---|
| Maximal Electroshock (MES) | Acute | 60 Hz 0.2 second alternating 50 mA current (mouse) or 150 mA (rat) | Tonic hindlimb extension seizure | No | Model for generalized tonic-clonic seizures; highly reproducible | PHT | PHT a; CBZ a; LTG f; VPA a,f; PB a; FBM a; GBP a,f; TPM f; EZG c; CBD d |
| Subcutaneous Pentylenetetrazol (s.c. PTZ) | Acute | Subcutaneous injection of chemoconvulsant pentylenetetrazol at dose known to elicit seizure in >97% of rodent strain (85 mg/kg in male CF-1 mice from Charles River) | Clonic spasms | No | Some translational relevance to absence seizures | ESM | ESM a; VPA a,f; BZD a; EZG f; FBM a; GBP a; PB a; TGB f; VGB f; CBD d |
| Corneal Kindled Mouse (50/60 Hz; traditional) | Chronic | 50/60 Hz 3 mA 3 second stimulus delivered to anesthetized corneas | Presence of Racine stage seizures and/or mean seizure score | No | Model of chronic secondarily generalized focal seizures with clear behavioral and neuropathological deficits | Potentially LEV | PHT a; CBZ a; LTG f; LEV f; TGB f; EZG c,f; VGB f; GBP a; PB a,e; FBM a; VPA a,c,e,f; BDZ a,c,e,f; CBD d |
| 6 Hz Corneal Kindled Mouse b | Chronic | 6 Hz 3 sec stimulation of various current intensity (strain-dependent) delivered to anesthetized corneas for 2-4 weeks | Forelimb clonus | Yes | Model of pharmacoresistant chronic secondarily generalized focal seizures | To be determined | CLZ b; VPA b; CBZ b |
| Lamotrigine-Resistant Corneal Kindled Mouse e | Chronic | 60 Hz 3 mA 3 second stimulus delivered to anesthetized corneas; mice receive lamotrigine pretreatment during 3-4 week kindling acquisition period | Presence of Racine stage seizures and/or mean seizure score | Yes | Model of pharmacoresistant chronic secondarily generalized focal seizures | To be determined | VPA e; DZP e; LEV e |
Based on data available within the NINDS PANAChE database, accessed 04/2019.
Based on Leclercq et al, Epilepsy Research 2014
Based on Barker-Haliski et al, Neurochemical Research. 2017
Based on Patra et al, Epilepsia 2019
Based on Koneval et al, Epilepsia 2018
Based on Rowley and White, Epilepsy Research 2010
Table 2.
Comparative efficacy of prototype ASDs in electrically kindled rodent models of chronic seizures.
| Antiseizure Drug | Amygdala- kindled rat* |
Hippocampal kindled rat |
Corneal kindled mouse (60 Hz) |
Corneal kindled mouse (6 Hz) |
|---|---|---|---|---|
| Benzodiazepines | + | + A | + A, B, C | + D |
| Brivaracetam | + | ND | ND | ND |
| Cannabidiol | ND | ND | + E | ND |
| Carbamazepine | + | + F | + F | + D |
| Eslicarbazepine acetate |
+ | ND | + G | ND |
| Ethosuximide | NE | NE A | NE A, F | ND |
| Felbamate | + | + | + A, E | ND |
| Gabapentin | + | NEA | + A | ND |
| Lacosamide | + | + G | ND | ND |
| Lamotrigine | + | + F | + C, F | ND |
| Levetiracetam | + | 50% effectiveF | + C, F | + D |
| Oxcarbazepine | + | ND | ND | ND |
| Perampanel | ND | + | ND | ND |
| Phenobarbital | + | + | + C | ND |
| Phenytoin | NE | + | + F | ND |
| Pregabalin | + | + I | ND | ND |
| Retigabine (ezogabine) |
+ | ND F | + C | ND |
| Tiagabine | + | + F | + F | ND |
| Topiramate | + | NE F | 50% effective F | ND |
| Valproate | + | + F | + B, C, F | + D |
| Vigabatrin | + | ND F | + F, J | ND |
| Zonisamide | + | ND | ND | ND |
+ indicates compounds that are effective in the kindling model. NE indicates “not effective”. ND indicates “no published data”. Bold text indicates compounds that are ineffective in the kindling model. Italic text indicates compounds that demonstrate a pharmacological profile different from the clinically validated amygdala-kindled rat.
Efficacy data of amygdala-kindled rats adapted from data presented in Table 2 of Klein et al, Epilepsia 2017 [18], with permission of John Wiley & Sons.
from NINDS PANAChE database (https://panache.ninds.nih.gov), accessed June 2019.
from Barker-Haliski et al, Neurochemical Research 2017
from Koneval et al, Epilepsia 2018
from Leclercq et al, Epilepsy Research 2014
from Patra et al, Epilepsia 2019
from Rowley and White, Epilepsy Research 2010
from Potschka et al, Epilepsy Research 2014
from Stohr et al, Epilepsy Research 2007
from Vartanian et al, Epilepsy Research 2006
from Klitgaard and Matagne, Epilepsy Research 1998
ASD identification tailored to specific patient groups may rapidly advance more mechanistically-novel pharmacotherapies for epilepsy, while we await the identification and validation of additional models. Effort is ongoing to address pharmacoresistant epilepsy with more late-stage differentiation models [5], but there is still opportunity to integrate models with pharmacoresistant chronic seizures even earlier into the ASD identification process. Until a new compound is brought forth to the clinic solely on the basis of efficacy in a preclinical model that is not MES, scPTZ, or kindling, the field will fail to validate any other preclinical model. Improved understanding of the processes and molecular targets underlying specific epilepsy syndromes clearly suggests that a new model may one day be clinically-validated. However, this validation is likely still years to decades away, while some revisions to current practice can better address the outstanding needs of patients today.
3. Expert Opinion
3.1. Pharmacoresistant chronic seizure models should be prioritized
Chronic seizure models offer the most etiologically-relevant platform on which to accurately replicate clinical epilepsy and are thus deserving of more use earlier in ASD identification. Unlike the acute MES/scPTZ tests that are most often conducted in young adult, neurologically-intact rodents, chronic seizure models reproduce the neuropathology and behavioral comorbidities associated with epilepsy. Chronic seizure models may also identify promising compounds that exert an anticonvulsant effect through novel mechanisms (e.g. inflammation [6]). Refinements in computational power have certainly improved our ability to implement preclinical models with SRS detected by EEG, but the fact remains that initial identification of treatments for symptomatic seizures – i.e. ASDs – must rely on models suited to moderate- to high-throughput testing to accommodate large libraries of compounds. Models with SRS detected by EEG frankly cannot support lead selection and optimization necessary to advance the most promising compounds and as such, implies that more “low-tech” models will still comprise a large proportion of the initial phases of ASD development (Figure 1). Pharmacoresistant chronic seizure models that are amenable to moderate- to high-throughput screening should be further prioritized for ASD identification.
The 50/60 Hz corneal kindled mouse (CKM) is a well-established model of kindling suitable to moderate-throughput drug screening [7]. Unlike the amygdala-kindled rat, CKM have minimal technical requirements to quickly generate ample numbers of animals to screen large libraries of compounds [7,8]. CKM demonstrate a pharmacological profile similar to amygdala-kindled rats, and may, in fact, more closely align with amygdala-kindled rats than previously reported alignment with hippocampal-kindled rats [8] on the basis of documented sensitivity to gabapentin, levetiracetam, and topiramate (Table 2). While the amygdala-kindled rat was validated with the identification of levetiracetam [9], the 50/60 Hz CKM represents a model of chronic seizures that exhibits behavioral [10] and neuropathological [11] changes of epilepsy, and is suitable for moderate-throughput screening unlike the amygdala-kindled rat. However much like the amygdala-kindled rat, CKM are not innately pharmacoresistant [8]. Modifications have produced a 6 Hz corneal kindling protocol that may be more pharmacoresistant [12], albeit the published pharmacological profile of this model is quite limited (Table 1). More recently, a lamotrigine-resistant CKM has demonstrated a broad and highly pharmacoresistant profile, including resistance to retigabine and carbamazepine (Table 1 [10]). Specifically, this model is elicited by the chronic administration of lamotrigine early in the epileptogenesis process, similar to a patient receiving lamotrigine monotherapy upon epilepsy diagnosis. Seizures are still evoked in the presence of lamotrigine, which mimics poor seizure control in man, and the animals subsequently develop a highly-drug resistant kindled seizure. The lamotrigine-resistant CKM [10], instead of naïve CKM, should thus be positioned in parallel to frontline validated acute seizure tests for ASD Identification. Addressing drug-resistant epilepsy earliest carries the potential to advance ASDs with pharmacological profiles distinct from current standards-of-care.
3.2. Models for ASD identification should align with clinical need.
Historical ASD development has also not adequately addressed the needs of all patients with epilepsy primarily because development is often conducted in neurologically-intact male rodents equivalent to a 20- to 40-year-old human adult [2]. Better representation of patient demographics in preclinical models is thus an underexploited strategy that can be deployed today to identify impactful ASDs. The very young (pediatric patients) and very old (geriatric patients) themselves require more effective and better tolerated therapies. Use of established seizure models in rodents harboring specific genotypes associated with age-related neurological disorders could identify beneficial therapies. Further, such an approach would shift our primary reliance on phenotype-based screening to a hybridized target-based drug screening platform, which may even then identify therapies that are broadly applicable to other epilepsy syndromes, in general.
Much effort is currently dedicated to the identification of therapies for pediatric epileptic encephalopathies. These syndromes are devastating but incredibly rare; e.g. Dravet’s syndrome occurs in an estimated 1:15,700 live births [13]. Nonetheless, numerous preclinical models of Scn1a+/− variants (the gene implicated in Dravet’s syndrome) have been developed to replicate the age-dependent clinical course of Dravet’s syndrome, but these preclinical models are not clinically-validated. There has yet to be an ASD brought forth on the basis of efficacy in a Scn1a+/− model that was otherwise missed in an established seizure model in adult rodents. As an example, cannabidiol (CBD) gained FDA approval for Dravet’s syndrome in 2018. While CBD attenuates the incidence of SRS in Scn1a+/− mice [14], CBD also demonstrates broad antiseizure efficacy in the MES, scPTZ, and kindling models in adult, wild-type rodents [15]. This one example suggests that an investigational compound that is intended for Dravet’s syndrome may just as easily advance to clinic on the basis of efficacy in the MES or kindling models in wild-type rodents at significant cost-savings. Until one of these pediatric genetic epilepsy models alone identifies a compound that is effective in patients with epilepsy, these models will remain late-stage differentiation tools that are primarily useful to increase confidence in an otherwise promising ASD.
At the opposite end of life, the prevalence of epilepsy is 1.5% among patients 65 or older; twice as frequent as in younger adults [16]. Patients with the most common neurological disorder of aging, Alzheimer’s disease (AD), are also increasingly appreciated to experience nonconvulsive focal seizures [17]. As a result of the near exclusive use of adult, neurologically-intact rodents for historical ASD development, our preclinical understanding of the efficacy and tolerability of ASDs to guide the management of seizures in elderly patients, and those with AD, is under informed. There is now compelling justification to extend ASD development into aged preclinical models because of the combined recognition that seizures arise in AD and the worldwide growth of individuals living well-beyond their 70’s.
Very little effort is presently devoted to ASD development in aged rodents. Fortunately, the available rodent models that are often used for ASD identification (MES, kindling) can also be readily applied to aged rodent models of aging disorders (e.g. AD). Seizure threshold increases in an age-dependent manner; adjusting the electroconvulsive stimulus for the MES test in aged rodents in merely requires modifications of the stimulation intensity. Further, unlike many pediatric epilepsies that arise due to genetic variants, age-related epilepsy is more frequently a result of factors that do not arise due to ion-channel variants, which may also provide valuable insight into the mechanisms underlying ictogenesis and epileptogenesis. Application of well-established seizure models to aged rodents with appropriate genetic mutations associated with aging disorders (e.g. AD) could even identify therapeutic targets or treatments that may carry broad applicability to other seizure disorders, in general. Elderly patients with epilepsy represent the fastest growing population with this condition; any effort to implement aged rodents for ASD development would meaningfully impact the pharmacotherapeutic management of seizures in the elderly. Altogether, more strategic integration of appropriately-aged preclinical seizure and epilepsy models into ASD development practices thus represents an untapped opportunity to advance more beneficial therapies for differentiation studies.
ASD development practice should increasingly emphasize special populations, including pharmacoresistant patients, and the very young and very old. Lamotrigine-resistant CKM is a frontline screening model that exhibits the pharmacological profile and throughput capacity to support screening of large compound libraries in an etiologically-relevant preclinical model of pharmacoresistant chronic seizures. There is also significant opportunity to apply the standard models that have been instrumental to the historical identification of ASDs to aged rodents. It should be clear that this editorial has, however, only discussed strategies to improve the initial identification of potential ASDs (Figure 1), nor has it addressed the need for disease-modifying agents. This is not to underscore the value of EEG monitoring of SRS in the late-stage differentiation of promising agents. Differentiation studies improve confidence that an agent may ultimately be efficacious for the symptomatic treatment of seizures in the patient with epilepsy, but no SRS model has yet to be clinically-validated and these models are not currently suited to moderate- to high-throughput ASD identification. As a result, use of the MES, scPTZ, or kindling models in selected genetic models of age-related neurological conditions represents a presently untapped opportunity for the field to integrate more etiologically-relevant models earlier into the ASD development process, which may also ultimately inform on the processes underlying ictogenesis and epileptogenesis, in general.
Acknowledgments
Funding:
The author declares funding from the University of Washington Department of Pharmacy, the University of Washington Royalty Research Fund and the National Center for Advancing Translational Sciences (KL2 TR002317).
Footnotes
Declaration of Interest:
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed
Reviewer Disclosures:
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- 1.Chen Z, Brodie MJ, Liew D, et al. Treatment Outcomes in Patients With Newly Diagnosed Epilepsy Treated With Established and New Antiepileptic Drugs: A 30-Year Longitudinal Cohort Study. JAMA Neurol. 2018. March 1;75(3):279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker-Haliski ML, White HS. Antiepileptic drug development and experimental models In: Wyllie E, editor. Wyllie’s treatment of epilepsy: Principles and practice: Sixth edition. 6th ed. Philadelphia: Wolters Kluwer Health; 2015. p. 516–521. [Google Scholar]
- 3.Klitgaard H, Matagne A, Gobert J, et al. Evidence for a unique profile of levetiracetam in rodent models of seizures and epilepsy. Eur J Pharmacol. 1998. July 24;353(2–3):191–206. [DOI] [PubMed] [Google Scholar]
- 4.Potschka H, Loscher W. Corneal kindling in mice: behavioral and pharmacological differences to conventional kindling. Epilepsy research. 1999;37(2):109–20.* This manuscript details the pharmacological sensitivity of corneal kindled mice and highlights the utility of this mouse kindling model for ASD development, including the greater sensitivity of kindled rodents to adverse effects with investigational drugs.
- 5.Kehne JH, Klein BD, Raeissi S, et al. The National Institute of Neurological Disorders and Stroke (NINDS) Epilepsy Therapy Screening Program (ETSP). Neurochemical research. 2017. May 02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vezzani A, Friedman A, Dingledine RJ. The role of inflammation in epileptogenesis. Neuropharmacology. 2013. June;69:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matagne A, Klitgaard H. Validation of corneally kindled mice: a sensitive screening model for partial epilepsy in man. Epilepsy research. 1998. June;31(1):59–71. [DOI] [PubMed] [Google Scholar]
- 8.Rowley NM, White HS. Comparative anticonvulsant efficacy in the corneal kindled mouse model of partial epilepsy: Correlation with other seizure and epilepsy models. Epilepsy research. 2010. December;92(2–3):163–9. [DOI] [PubMed] [Google Scholar]
- 9.Klitgaard H Levetiracetam: the preclinical profile of a new class of antiepileptic drugs? Epilepsia. 2001;42 Suppl 4:13–8.** This manuscript describes the pharmacological profile of levetiracetam and provides insight into how a compound with a novel mechamism of action may still be identified in a kindling model, despite inefficacy in the MES or scPTZ models.
- 10.Koneval Z, Knox KM, White HS, et al. Lamotrigine-resistant corneal-kindled mice: A model of pharmacoresistant partial epilepsy for moderate-throughput drug discovery. Epilepsia. 2018. May 11.** This manuscript details a novel, highly drug-resistant chronic seizure model that is well-suited to moderate-throughput ASD screening in parallel with the frontline use of the MES or scPTZ tests for ASD identification.
- 11.Loewen JL, Barker-Haliski ML, Dahle EJ, et al. Neuronal Injury, Gliosis, and Glial Proliferation in Two Models of Temporal Lobe Epilepsy. Journal of neuropathology and experimental neurology. 2016. April;75(4):366–78.* This manuscript demonstrates that corneal kindled mice exhibit neuroinflammation within hippocampal structures at time of enrollment for ASD development studies.
- 12.Albertini G, Walrave L, Demuyser T, et al. 6 Hz corneal kindling in mice triggers neurobehavioral comorbidities accompanied by relevant changes in c-Fos immunoreactivity throughout the brain. Epilepsia. 2017. November 20. [DOI] [PubMed] [Google Scholar]
- 13.Krueger J, Berg AT. Incidence of Dravet Syndrome in a US Population. Pediatr Neurol Briefs. 2015. December;29(12):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaplan JS, Stella N, Catterall WA, et al. Cannabidiol attenuates seizures and social deficits in a mouse model of Dravet syndrome. Proc Natl Acad Sci U S A. 2017. October 17;114(42):11229–11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patra PH, Barker-Haliski M, White HS, et al. Cannabidiol reduces seizures and associated behavioral comorbidities in a range of animal seizure and epilepsy models. Epilepsia. 2018. December 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leppik IE. Treatment of Epilepsy in the Elderly. Epilepsy currents / American Epilepsy Society. 2001. November;1(2):46–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lam AD, Deck G, Goldman A, et al. Silent hippocampal seizures and spikes identified by foramen ovale electrodes in Alzheimer’s disease. Nat Med. 2017. June;23(6):678–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klein P, Dingledine R, Aronica E et al. Commonalities in epileptogenic processes from different acute brain insults: Do they translate? Epilepsia. 2018. January;59(1):37–66. doi: 10.1111/epi.13965. Epub 2017 Dec 15. [DOI] [PMC free article] [PubMed] [Google Scholar]