Abstract
Fear conditioning is an associative learning process by which organisms learn to avoid environmental stimuli that are predictive of aversive outcomes. Fear extinction learning is a process by which avoidance of fear-conditioned stimuli is attenuated when the environmental stimuli is no longer predictive of the aversive outcome. Aberrant fear conditioning and extinction learning are key elements in the development of several anxiety disorders. The 129S1 inbred strain of mice is used as an animal model for maladaptive fear learning because this strain has been shown to generalize fear to other nonaversive stimuli and is less capable of extinguishing fear responses relative to other mouse strains, such as the C57BL/6. Here we report new environmental manipulations that enhance fear and extinction learning, including the ability to discriminate between an aversively paired tone and a neutral tone, in both the 129S1 and C57BL/6 strains of mice. Specifically, we show that discontinuous (“pipped”) tone stimuli significantly enhance within-session extinction learning and the discrimination between neutral and aversively paired stimuli in both strains. Furthermore, we find that extinction training in novel contexts significantly enhances the consolidation and recall of extinction learning for both strains. Cumulatively, these results underscore how environmental changes can be leveraged to ameliorate maladaptive learning in animal models and may advance cognitive and behavioral therapeutic strategies.
Keywords: anxiety, extinction learning, fear learning, novelty-facilitated extinction, strain differences, post-traumatic stress disorder (PTSD), 129S1, C57BL/6
1. INTRODUCTION
Increased probability of survival is associated with an organism’s ability to learn when neutral environmental stimuli become predictive of an aversive outcome as is the ability to diminish a fearful response when stimuli no longer predict the aversive outcome (fear extinction). Fear becomes maladaptive when the response to fear stimuli is overgeneralized to other stimuli or when fear responses persist after extinction training. In humans, maladaptive fear or deficits in extinction learning are core features of generalized anxiety disorder (GAD) and post-traumatic stress disorder (PTSD).1,2
Rodent models of fear learning (ie, fear conditioning) and fear extinction learning have enriched our understanding of genes and neural circuits involved in the expression of GAD and PTSD symptoms (reviewed in Fenster et al1). The Pavlovian model of fear conditioning in rodents requires the pairing of a neutral stimulus, such as a tone (conditioned stimulus, CS), with an inherently aversive stimulus (unconditioned stimulus, US), such as a footshock.3 Following one or more CS-US pairings, rodents will typically display a fearful response to the CS alone (conditioned response). Mice respond to fearful stimuli by displaying either passive (freezing) or active (flight) avoidance behavior. Extinction learning is the process by which avoidance of fearful stimuli is diminished through repeated presentation of the CS in absence of the US. Extinction learning involves both forming new associations4–11 and degradation of the originally learned CS-US pairing.12–16 In humans, extinction learning is a main component of exposure therapy, which is often used in the treatment of PTSD. While this treatment is effective for some, it has very limited success for up to 50% of the population,17 suggesting that further research on extinction-based therapies is needed to improve and individualize treatment options.
Advancements in research show that the propensity for developing PTSD in humans is heritable.18 Twin studies estimate the heritability of PTSD to range between 40% and 50%.18–20. In mice, distinct inbred wild-type strains display markedly different fear conditioning and extinction abilities.21 For example, the 129S1 strain is capable of learning and consolidating fear conditioning, but exhibit little to no fear extinction learning or reduced freezing even after mass exposure to an unpaired CS.22,23 However, 129S1 mice do exhibit some extinction learning when exposed to fear conditioning protocols using a weak footshock, yet they are unable to recall learned extinction when tested 24 hours later.23 Moreover, 129S1 mice also display greater fear generalization to contexts that are similar but are nonconditioned.24,25 In contrast, C57BL/6 mice have repeatedly been shown to exhibit robust fear extinction learning, as evidenced by a significant decrease in freezing behavior after exposure to an unpaired CS.25–27
Efforts to elucidate the differences in extinction learning between 129S1 and C57BL/6 mice have led to a greater understanding of neurophysiological and genetic substrates responsible for the expression and suppression of fear. For example, studies have shown that 129S1 mice have a downregulated hypothalamic-pituitary-adrenal axis24; reduced neural activation in the infralimbic subregion of the medial prefrontal cortex,27 a brain region critical for modulating extinction learning28; enlarged dendritic arbors in basolateral amygdala (BLA) neurons24; and a greater fraction of parvalbumin-positive inhibitory interneurons surrounded by perineuronal nets in the BLA.29 The extinction learning deficits in the 129S1 can be ameliorated by dietary zinc restriction,30 deep brain stimulation of the nucleus accumbens,23 pharmacological treatment with the α2-adrenoreceptor antagonist, yohimbine, the selective serotonin reuptake inhibitor, fluoxetine, or the dopamine precursor, L-dopa.24,27,31 Interestingly, a recent study used linkage crosses between 129S1 and C57BL/6 mice combined with quantitative trait loci analysis to identify a panel of genes in the amygdala that show expression differences that correlate to the extinction learning deficits.29 Of the genes identified, the expression of Ppid, a gene that is functionally coupled to the glucocorticoid receptor, was found to reduce extinction learning deficits in 129S1 mice when its expression was increased.29 These data suggest that certain therapeutic interventions can ameliorate the extinction learning deficits that may be genetically encoded in neural circuits of the 129S1 mice.
In the current study, therefore, our research sought to find behavioral approaches that could similarly minimize the extinction deficits in 129S1 mice. We found that the use of discontinuous (“pipped”) tones ameliorated the extinction learning deficits in 129S1 mice and enhanced the ability of both 129S1 and C57BL/6 mice to discriminate between aversively paired stimuli (CS+) and unpaired or neutral stimuli (CS−). Pipped tones have been commonly used in in vivo neuro-physiological studies because they lead to greater neural activation compared with continuous tones5,32,33 and enhance stimulus discrimination.34,35 In addition, we found extinction learning and recall were enhanced when unpaired CS+ presentation (without footshock) occurred in a daily novel context (novelty-facilitated extinction). These two environmental manipulations diminished the 129S1 extinction deficits (ie, fear generalization and fear extinction) to more closely resemble the learning phenotype of the C57BL/6.
2 |. MATERIALS AND METHODS
2.1 |. Mice
All mice were obtained from commercial vendors or bred in our colony using naïve mice from the same vendors. Mice were either male or female 129S1/SvImJ obtained from Jackson Laboratories (Stock # 002448; Bar Harbor, Maine), or C57BL/6NTac obtained from Taconic Farms (Model # B6NTac; Hudson, New York City), referred to hereafter as 129S1 and C57BL/6, respectively.
Experiments were conducted using mice aged 9 to 15 weeks at the time of testing with approximately equal number of males and females (males = 97, females = 90). Mice were housed by sex (2 to 5 per cage) in microisolation cages in a temperature-controlled (22°C) vivarium with a 14-hour/10-hour light/dark cycle for a minimum of 4 weeks prior to behavior studies. Mice were provided with ad libitum food and water. All experiments were conducted during the light phase. Prior to fear conditioning, mice were randomly assigned to two groups: US+ (footshocked) and US-(no footshock). Mice in the US+ group received a paired tone (CS+) and a footshock (US+) during fear conditioning. Mice in the US-group were exposed to the same tones but did not receive the paired footshock (US−). The number of mice used in each experiment is given in the figure legends. The Institutional Animal Care and Use Committee of the University of Michigan approved all experiments and guidelines for animal care set by the National Institutes of Health.
2.2 |. Fear conditioning apparatus
The basic system was as previously described.36–39 Briefly, fear conditioning experiments were conducted in a 27 × 27 × 11 cm chamber with clear acrylic backs and doors, aluminum sides, stainless steel rod floors (rods were spaced 1/8 inches apart), stainless steel drop pans (Med Associates), and overhead lights set at 4 lx. Footshocks were administered through the rods via solid state shock scramblers and electronic constant current shock sources controlled by Actimetrics Freezeframe version 4 software (Wilmette, Illinois) run on a desktop PC. Actimetrics Freezeframe version 4 software was also used to record behavior using individual cameras mounted above each chamber. Chamber details were altered to create different experimental contexts in some experiments (as indicated in figure legends).
2.2.1 |. Fear conditioning
Mice were fear conditioned in context A for one training session. Context A was assembled using the conditioning chamber described above with the addition of light blue curtains covering the clear acrylic doors, and the clear acrylic backs exposed to the beige walls of the room. In context A, floor pans and rods were cleaned using 75% ethanol, and floor pans received four sprays of 75% ethanol to provide a distinct background odor. Mice were individually placed in the conditioning chambers and exposed to context A for 120 seconds prior to any tone presentation. After the pretone period, mice received five CS+/US+ pairings that consisted of a 25-second, 50-dB, 4.0-kHz tone that was either continuous or discontinuous (“pipped”). For the pipped tones, each pip lasted for 200 ms and was presented at 1 Hz (ie, 25 total pips). For the US+ groups, both types of tones coterminated with a 2-second, 0.6-mA footshock. In addition, mice were exposed to five unpaired 25-second, 50-dB, white noise continuous or pipped tones (CS-). The order of CS+ and CS− presentations and the interstimulus interval (ISI) were pseudorandomized (ISI duration was between 20 and 180 seconds). There was a 120-second no-stimulus consolidation period (post-tone period) after the final CS+/US pairing before mice were returned to their home cage.
For initial experiments (Figure 1), the tone frequencies of CS+ and CS− were counterbalanced. No differences were found in overall freezing or discrimination when assigning 4 kHz or white noise frequencies to the CS+ (data not graphed, main effect of tone frequency: F1,37 = 0.45; P = .51). Therefore, for the remaining experiments (Figures 2–4), the 4-kHz tone was assigned to the CS+, while the CS− was composed of white noise.
FIGURE 1.
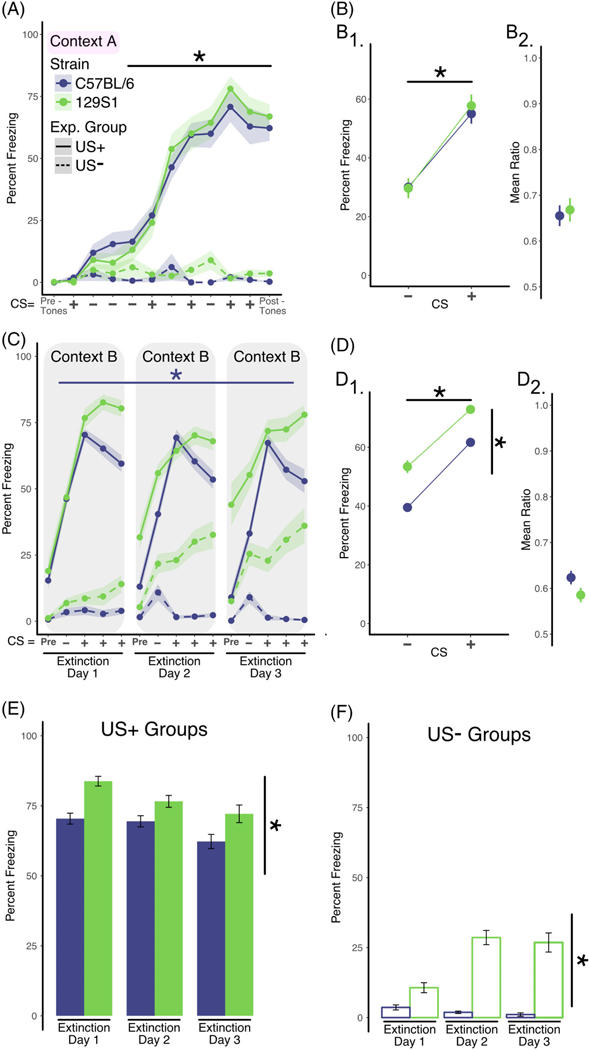
Comparisons between 129S1 and C57BL/6 on discriminative fear conditioning and extinction learning. A, Analysis of training data. Two separate groups of mice were used in this experiment. Mice in the US+ group (solid lines, C57BL/6; n = 20 [11 males; 9 females], 129S1; n = 17 [8 males; 9 females]) were exposed to auditory stimuli that consisted of white noise (CS−; white noise, 25 seconds) or 4-kHz pure tone (25 seconds) that co-terminated with a foot shock (CS+). Mice in the US-group (dashed lines, C57BL/6 n = 8,129S1 n = 8) received the identical auditory stimuli but did not receive the foot shocks. Mice in the US+ group exhibited increased freezing across the training session (repeated measures ANOVA; effect of training, P < .001) independent of strain. In the absence of shock, mice in the US-group did not exhibit significant levels of freezing. B, Analysis of discrimination learning during training. The mean percent freezing averaged across all CS+ presentations during training was significantly greater in both strains when compared with the mean freezing in response to the CS− presentations (B1, two-factor ANOVA; effect of CS, P < .001), and there was no difference in the discrimination ratios for the two strains (B2). C, Analysis of within-session extinction learning. Twenty-four hours following training, mice in both groups were exposed to four CS− stimuli followed by 12 CS+ presentations (displayed in bins of four stimuli) in a new context (context B) for 3 days (extinction days 1–3). Repeated exposure of the C57BL/6 mice to the CS+ tone in the US+ group resulted in significantly less freezing within extinction sessions shown by repeated measures ANOVA, where data are collapsed across days of training (effect of bin, P < .001). Conversely, repeated exposure to the CS+ tone in the absence of shock in the 129S1 mice failed to reduce freezing within the extinction sessions. D, Analysis of tone discrimination during extinction. Mice in both strains exhibited significantly more freezing when exposed to the CS+ tone (two-factor ANOVA; effect of CS, P < .001) and significant differences in freezing by strain (effect of strain, P < .0001) (D1). Similarly, there was no significant difference between the C57BL/6 and 129S1 strains when the discrimination ratio was calculated (D2). E, Analysis of extinction across days (US+ Group). Freezing across extinction days was analyzed by calculating the mean percent freezing for the three binned CS+ presentations for each of the three extinction days (solid line in panel C). While both C57BL/6 and 129S1 mice exhibited modest reductions in freezing across extinction days, this effect was not significant, although 129S1 mice freeze more as compared to the C57BL/6 (two-factor ANOVA; effect of strain, P < .001). F, Analysis of freezing in the US-group across days. Freezing in the US-group was calculated each day as the mean percent freezing measured during the CS+ tone presentations (dashed line in panel C). Somewhat surprisingly, the 129S1 mice exhibited increased “freezing” across the 3 days of re-exposure to the CS+ tone compared with the C57BL/6 strain (two-factor ANOVA; effect of strain, P < .0001), in spite of the fact that they never received the foot shock. Data shown as sample mean with shaded region or bars representing the SE of the mean. Asterisk (*) denotes statistical significance for main effect of training (horizontal line) or strain (vertical line). Data are presented as mean ± SEM
FIGURE 2.
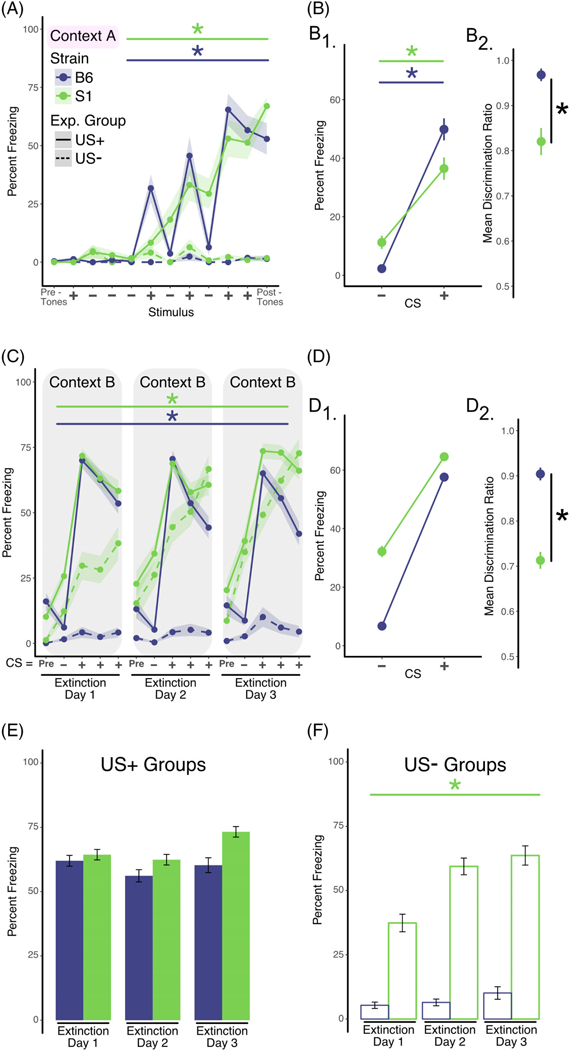
Pipped tone CS presentations ameliorate extinction deficits in 129S1 mice and enhance discrimination learning for both C57BL/6 and 129S1 mice. A, Analysis of training data. Two separate groups of mice were used in this experiment. Mice in the US+ group (solid lines, C57BL/6 n = 13 [7 males; 6 females], 129S1 n = 18 [10 males; 8 females]) were exposed to discontinuous or pipped auditory stimuli that consisted of white noise (CS−; 200-ms white noise pips, delivered at 1 Hz for 25 seconds) or 4-kHz pure tone (200-ms pips, delivered at 1 Hz for 25 seconds) that coterminated with a foot shock (CS+). Mice in the US− group (dashed lines, C57BL/6 n = 8, 129S1 n = 8) received the identical auditory stimuli but did not receive the foot shocks. Mice in the US+ group exhibited increased freezing across the training session (repeated measures ANOVA; effect of training, P < .001) independent of strain. In the absence of shock, mice in the US− group did not exhibit significant levels of freezing. B, Analysis of discrimination learning during training. The mean percent freezing averaged across all CS+ presentations during training was significantly greater in both strains when compared with the mean freezing in response to the CS− presentations (B1, two-factor ANOVA; effect of CS, P < .001). However, calculation of the discrimination ratio showed that C57BL/6 discriminates CS+ from CS− better than 129S1 mice (B2, unpaired t test, P < .001). C, Analysis of within-session extinction learning. Twenty-four hours following training, mice in both groups were exposed to four CS− stimuli followed by 12 CS+ presentations (displayed in bins of four stimuli) in a new context (Context B) for 3 days (extinction days 1–3). Repeated exposure to the CS+ tone in the US+ group resulted in significantly less freezing within extinction sessions for both strains (using separate repeated measures ANOVA per strain; effect of bin, P < .05). D, Analysis of tone discrimination during extinction. Mice in both strains exhibited significantly more freezing when exposed to the CS+ tone (repeated measures ANOVA; effect of training, D1, P < .001). However, the C57BL/6 showed significantly better freezing discrimination to the CS+ (unpaired t test, P < .0001) than 129S1 strain when the discrimination ratio was calculated (D2). E, Analysis of extinction across days (US+ Group). Freezing across extinction days was analyzed by calculating the mean percent freezing for the three binned CS+ presentations for each of the three extinction days (solid line in panel C). No statistically significant reductions in freezing were observed for either C57BL/6 or 129S1 mice. F, Analysis of freezing in the US− group across days. Freezing in the US− group was calculated each day as the mean percent freezing measured during the CS+ tone presentations (dashed line in panel C). The 129S1 mice exhibited increased “freezing” across the 3 days of re-exposure to the CS+ tone compared with the C57BL/6 strain (repeated measures ANOVA; effect of “extinction” day, P < .0001), in spite of the fact that they never received the foot shock. Data shown as sample mean with shaded region or bars representing the SE of the mean. Asterisk (*) denotes statistical significance for main effect of training (horizontal line) or strain (vertical line) and a dagger (†) for significant interaction between main effects. Data are presented as mean ± SEM
FIGURE 4.
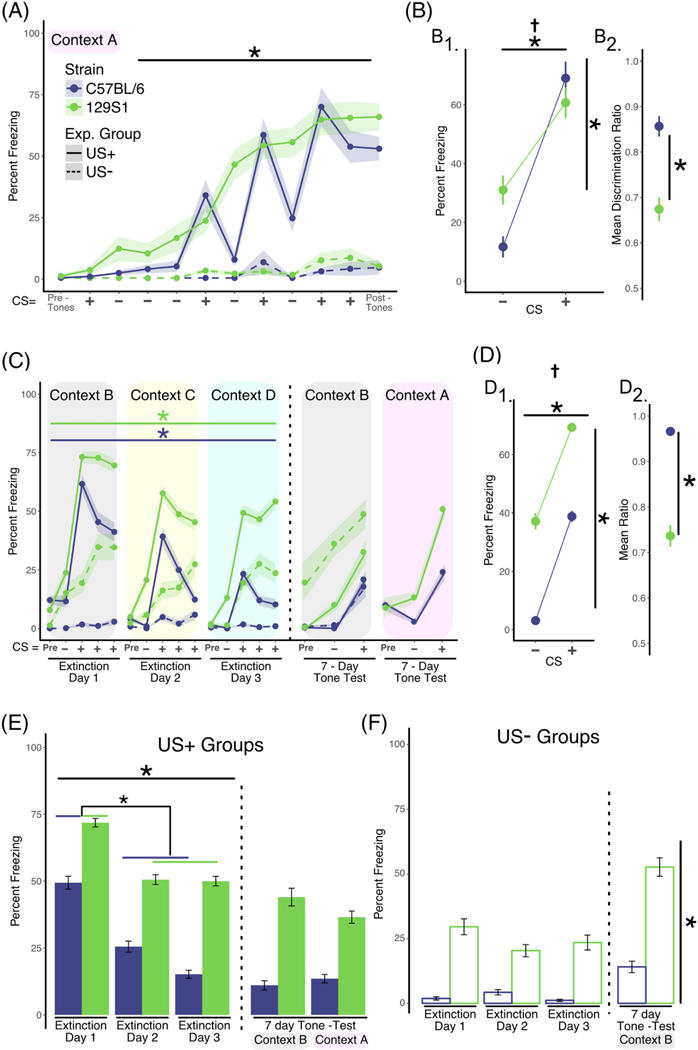
Presentation of unpaired CS+ tones in novel contexts during extinction training enhances the consolidation of extinction learning across training sessions. A, Analysis of training data. Two separate groups of mice were used in this experiment. Mice in the US+ group (solid lines, C57BL/6 n = 17 [10 males; 7 females], 129S1 n = 22 [11 males; 11 females]) were exposed to discontinuous or pipped auditory stimuli that consisted of white noise (CS−; 200-ms white noise pips, delivered at 1 Hz for 25 seconds) or 4-kHz pure tone (200-ms pips, delivered at 1 Hz for 25 seconds), which coterminated with a foot shock (CS+). Mice in the US− group (dashed lines, C57BL/6 n = 8, 129S1 n = 8) received the identical auditory stimuli but did not receive the foot shocks. Mice in the US+ group exhibited increased freezing across the training session (repeated measures ANOVA; effect of training, P < .001) independent of strain. In the absence of shock, mice in the US− group did not exhibit significant levels of freezing. B, Analysis of discrimination learning during training. The mean percent freezing averaged across all CS+ presentations during training was significantly greater in both strains when compared with the mean freezing in response to the CS− presentations (B1, 2-factor ANOVA; effect of CS, P < .001). However, calculation of the discrimination ratio showed that C57BL/6 discriminate CS+ and CS−better than 129S1 mice (B2, unpaired t test, P < .001). C, Analysis of within-session extinction learning. Twenty-four hours following training, mice in both groups were exposed to four CS− stimuli followed by 12 CS+ presentations (displayed in bins of four stimuli) in a novel context each day of extinction training (context B, C, D) for 3 days (extinction days 1–3). For both strains, repeated exposure to the CS+ tone in the US+ group resulted in significantly less freezing within extinction training sessions using separate repeated measures ANOVA per strain; effect of bin, P < .01). D, Analysis of tone discrimination during extinction. Mice in both strains exhibited significantly more freezing when exposed to the CS+ tone (D1, 2-factor ANOVA; effect of CS, P < .001). However, the C57BL/6 showed significantly better CS discrimination compared to the 129S1 strain when the discrimination ratio was calculated (D2, unpaired t test, P < .0001). E, Analysis of extinction across days (US+ Group). Freezing across extinction days was analyzed by calculating the mean percent freezing for the three binned CS+ presentations for each of the three extinction days, each in a novel context (solid line in panel C). Statistically significant reductions in freezing were observed for both C57BL/6 and 129S1 mice across training sessions (main effect of training session, P < .0001). Post-hoc analysis confirmed that there were significant differences in freezing between extinction day 1 and extinction days 2 and 3 for both strains (lower asterisk, P < .01). To determine to what extent extinction learning was retained long-term, mice in both strains were randomly segregated into two groups and freezing during CS+ and CS− presentations was measured in either context B or context A (the training context) 7 days after extinction training (data to the right of the dotted line in Figure 4E). When tested 7 days after the last day of extinction training mice in both strains exhibited freezing levels that were indistinguishable from extinction training day 3, regardless of context or strain. F, Analysis of freezing in the US− group across days. Freezing in the US− group was calculated each day as the mean percent freezing measured during the CS+ tone presentations (dashed line in panel C). The 129S1 mice exhibited increased “freezing” across the 3 days of re-exposure to the CS+ tone compared with the B6 strain (P < .0001), in spite of the fact that they never received the foot shock. Data shown as sample mean with shaded region or bars representing the SE of the mean. Asterisk (*) denotes statistical significance for main effect of training (horizontal line) or strain (vertical line) and a dagger (†) for significant interaction between main effects. Data are presented as mean ± SEM
2.2.2 |. Extinction training
After fear conditioning, mice were tested for initial fear recall and within-session fear extinction, which continued over three extinction training sessions (E1, E2 and E3), one per day, using a new context. The contexts were organized as follows: context B consisted of a smooth white acrylic sheet to hide the rod floors, a white curtain covering the clear acrylic doors, a rounded smooth white acrylic wall covering the clear acrylic back, and red light (5 lx); floor pans and rods were cleaned using 2% acetic acid and floor pans received four sprays of 2% acetic acid to provide a distinct background odor. Context C consisted of a cloth texture over a smooth acrylic sheet to hide the rod floors, a dark white and blue striped curtain covering the clear acrylic doors, a white cotton sheet covering the clear acrylic backs, and white light (6 lx) provided by floor lamps (instead of overhead lights); floor pans and rods were cleaned using N-acetylcysteine (rotten egg scent) and floor pans received four sprays of the rotten egg scent to provide a distinct background odor. Context D consisted of a gray rubber nub matt over a smooth acrylic sheet to hide the rod floors, no curtain, a blue sheet covering the clear acrylic backs, and blue lights (4 lx); floor pans and rods were cleaned using a vanilla scent and floor pans received four sprays of the vanilla scent to provide a distinct background odor. Seven days after E3, mice were tested for long-term fear recall in context A or B.
2.3 |. Open-field test
To assess differences in locomotor activity levels between strains open-field experiments were conducted as described previously.40,41 Briefly, these were carried out in a 53 × 38 × 26 cm open box with smooth white opaque acrylic walls and floor (Chemtainer, Lombard, Illinois). Illumination in the center of the box was ~38 lx. Animals were released in the center of the box and allowed to freely explore for 10 minutes. Actimetrics Limelight version 4 software, run on a desktop PC using individual cameras mounted above each open-field box, was used to record behavior and calculate total distance traveled and time spent in the center and perimeter.
2.4 |. Statistical analyses
Minimum sample sizes were predetermined using response means and variance obtained from a series of pilot experiments (a sample size of 10 per group resulted in 60% power for detecting a moderate effect [Cohen’s d = 0.65] at α = .5).42 Sample sizes for individual experiments are reported in the figure legends. To avoid skewing the data with any potential sex differences, a balanced designed was used in each experiment with approximately equal number of males and females. Fear conditioning and extinction training videos were quantified using Actimetrics Freeze Frame version 4 software, with automated detection of freezing, which was defined as 1-second bouts of nearly complete animal immobility except for the movement necessary for breathing. Percent time freezing was calculated as the time spent freezing divided by the total session time. In a subset of experiments, human observers verified the automated detection of freezing by manually scoring videos; a representative comparison between automated and human scoring is shown in Figure S1A and B. These data showed that while freezing values were slightly lower when scored by a human observer, these differences were not statistically significant (effect of scorer, F1,14) = 0.4; P = .54). Therefore, automated scoring was employed for all data analysis. Data were exported in comma-separated variable format from Freeze Frame 4, averaged across the duration of either pretone, CS by type (and binned into groups of 3 CS of the same kind), footshock (when applicable) and post-tone periods. To assess discriminative freezing between the CS+ and CS−, a discrimination ratio was calculated as freezing to CS+ divided by the sum of freezing to CS+ and CS−. Subsequent statistical data analyses and plotting were performed in R Studio43 using open-source packages44–47 and custom-written scripts. Statistical analyses employed were multifactor analysis of variance (ANOVA) for parametric data including between and/or within subjects factors. Specifically, for the analysis of within-session extinction (panel C of each figure), the data were analyzed separately by strain and collapsed across days as our results show significant trial-to-trial variability and within-session locomotor differences (see US-groups in all figures). Between-session extinction was analyzed using a two-factor (training day, strain), between-subjects ANOVA with freezing data averaged across bins for each training day. For further interrogation of statistically significant ANOVA effects, post-hoc Tukey’s t tests for multiple comparisons were carried out as indicated in text.
3 |. RESULTS
3.1 |. Fear conditioning and extinction phenotypes for C57BL/6 & 129S1 mice
We first sought to establish phenotypes for discriminative fear conditioning and extinction learning for 129S1 and C57BL/6 mice using validated behavioral protocols.5,48 A statistical analysis of fear conditioning data yielded a main effect of training (ie, CS+ presentations) for freezing (F3,105 = 43.15; P < .001) with no strain interaction, suggesting that there were no differences between 129S1 and C57BL/6 mice in associative fear learning (Figure 1A). Similarly, both strains were equally able to discriminate between CS type (CS+/CS−) as evidenced by a main effect of CS type on freezing (F1,70 = 48.79; P < .001) with no strain interaction or differences in the mean discrimination ratio (defined as freezing to CS+ divided by the sum of freezing to CS+ and CS−). All together, these findings indicate that both 129S1 and C57BL/6 displayed effective associative learning and could discriminate CS+ from CS− (Figure 1B1-B2). The US-groups were analyzed separately and showed no significant increase in freezing in response to tone presentations and no differences between strains.
To assess the ability of each strain to reduce freezing behavior when the CS+ was not paired with a footshock, we measured within-session extinction learning (Figure 1C). Within-session extinction was statistically assessed using separate one-way repeated measures ANOVA per strain. Our results show that C57BL/6 mice exhibit a statistically significant decrease in freezing as a factor of CS+ presentations (F8 132 = 4.98; P < .001), while 129S1 mice did not. In agreement with prior studies, these data show the presence of extinction learning deficits in the 129S1 strain as compared to C57BL/6 mice.25–27 Analysis of discrimination performance during extinction (Figure 1D1) shows a main effect of CS type (CS+/CS−, F1,202 = 82.5; P = .001) and a main effect of strain (F1,202 = 24.34; P = .001) on freezing. Further-more, no statistically significant differences were found in the mean discrimination ratio (Figure 1D2), supporting the conclusion that both strains of mice were capable of discriminating the CS type, although 129S1 mice freeze more significantly to both CS+ and CS− (main effect of strain, F1,202 = 24.34; P < .001).
To monitor the consolidation and recall of extinction memories, freezing between extinction training sessions was measured. For this analysis, freezing levels were averaged across all CS+ presentations for each extinction training session (Figure 1E). Our analyses showed a main effect of mouse strain (F1,60 = 9.97; P < .01), but no effect of training session that indicates that while C57BL/6 mice freeze less than 129S1, neither strain’s freezing levels significantly decreased between extinction training sessions. In contrast to these results, previous studies (Figure S2A-B) show that C57BL/6 mice exhibit greater levels of within-session extinction and efficient consolidation of extinction memories over days25–27; this discrepancy in results may be explained by the fact that these studies used a greater number of unreinforced CS+ presentations (20–30 vs 12 per extinction training session in the current study).
Interestingly, the US-groups (Figure 1C, dashed lines) showed a within-session increase in what is scored as “freezing” (F10,140 = 3.29; P < .001), but as there is no paired aversive stimulus, this phenotype must be interpreted as an indiscriminate fear response or a lack of motivated locomotion. This phenotype is particularly pronounced in the 129S1 mouse strain, which “froze” significantly more than the C57BL/6 (F1,14 = 34.91; P = .0001). To verify that automated tracking of freezing behavior was accurately assessing bouts of freezing, a subset of behavioral videos was manually scored by experimenters, and similar results were found (Figure S1A and B). To further corroborate this phenotype, we employed an open-field test to measure overall locomotor activity. Our results (Figure S3C-D) showed that 129S1 mice show significantly reduced levels of overall locomotion (C57BL/6 = 10.13 m, ± 0.44 vs 129S1 = 4.27 m, ± 0.38; t(14) = 5.21, P < .0001) and spend less time in the center of the field (C57BL/6 = 39.06%, ± 2.25 vs 129S1 = 18.19%, ± 2.98; t(14) = 1.12, P < .01). Consistent with many prior studies, these results suggest that untrained or experimentally naïve 129S1 mice display greater levels of baseline anxiety-like behavior and/or decreased levels of locomotion.49–51 In addition, our results show an increase in between-session freezing (Figure 1F, main effect of strain, F1,42 = 38.19; P < .001), whereby the 129S1 strain showed a greater level of “freezing.” This increased immobility was consistent across all extinction training days, with no effects of training session (Figure 1F).
3.2 |. Pipped tones enhance fear discrimination and within-session extinction in both C57BL/6 and 129S1 mice
In vivo neurophysiological recordings in the medial prefrontal cortex, amygdala and other brain regions most commonly utilize pipped tones rather than continuous tones because they result in less attenuation of neuronal firing.5,32,33 Tone pips have also been found to enhance stimulus discrimination during fear conditioning.34,35 Recently, high-frequency tone pips (40 Hz) were also found to entrain gamma-frequency firing patterns in medial prefrontal cortex, auditory cortex and hippocampus—this treatment led to enhancements in spatial memory in mice.52 Based on these observations, we tested the effectiveness of pipped tones for training 129S1 and C57BL/6 mice on discriminative fear conditioning and extinction learning. Similar to the results from the continuous tone experiment, using pipped tones results in a significant effect of training (ie, CS+ presentations) on freezing (F3,87 = 22.76; P < .001) with no strain interaction, suggesting that both 129S1 and C57BL/6 form an association between the CS+ and footshock to a similar degree (Figure 2A). Furthermore, there was a main effect of CS type (ie, CS+/CS−) on freezing (F1,58 = 76.51; P < .001) indicating that both strains could discriminate CS+ from CS−. Importantly, there was also a significant strain × CS type interaction (Figure 2B1, F1,58 = 7.81; P < .01), suggesting that C57BL/6 discriminate between CS+ and CS− tones to a higher degree. In agreement with this analysis, the discrimination ratio was significantly higher for C57BL/6 mice compared to 129S1 mice (Figure 2B2, t(29) = 3.977, P < .001). Additionally, a comparison of the discrimination ratios for the continuous tone protocol (Figure 1B1-B2, means = 0.66, 0.64 for 129S1 and C57BL/6, respectively) and the pipped tone protocol (Figure 11-B2, means = 0.82, 0.97 for 129S1 and C57BL/6, respectively) suggests that pipped tones enhance discrimination learning for both strains. As was the case using the continuous tone protocol, neither the 129S1 nor the C57BL/6 US-groups showed an increase in freezing over time or with tone presentation alone.
In contrast to the continuous tone experiments, the pipped tone protocol led to significant within-session extinction learning for both C57BL/6 mice (F11,77 = 3.31; P < .001) and 129S1 mice (F11,77 = 1.98; P < .05) (Figure 2C). Similarly, as in fear conditioning, both 129S1 and C57BL/6 mice were able to discriminate between the CS+ and CS− during extinction training (Figure 2D1, main effect of CS type, F1,58 = 76.51; P < .001). Furthermore, analysis of the discrimination ratio showed that C57BL/6 mice discriminate the CS+ better than 129S1 mice (Figure 2D2, 129S1 mean = 0.71 ± 0.02, C57BL/6 mean-= 0.90 ± 0.03, t(29) = 5.17, P < .0001). However, analysis of freezing levels between-session (Figure 2E), resulted in no significant effects, suggesting that using pipped CS tones did not impact the consolidation or recall of extinction memories.
Again, statistical analysis of the US-groups showed a main effect of strain (F1,42 = 71.09; P < .0001), indicating that the 129S1 mice displayed significantly higher “freezing” (although they never received a footshock) than the C57BL/6 mice. Furthermore, there was also a main effect of “training session” (F2,42 = 3.4; P < .05), indicating that there was an increase in “freezing” over training days (Figure 2F).
3.3 |. Delayed extinction training does not impact the performance of 129S1 or C57BL/6 mice
To examine the possibility that heightened fear from the fear conditioning context or fear generalization might suppress extinction learning and consolidation, we imposed a 7-day delay between fear conditioning and extinction learning. As expected, analysis of fear conditioning results showed that both 129S1 and C57BL/6 mice learned the association between the CS+ and the footshock as showed by a main effect of training (ie, CS+ presentations) on freezing (F3,42 = 11.9; P < .001; Figure 3A). In addition, both strains were able to discriminate between CS+ and CS− tones (main effect of tone on freezing, F1,26 = 63.62; P < .0001, Figure 3B1). In agreement with previous experiments (Figure 2), analysis of the discrimination index indicates that C57BL/6 exhibit better discrimination when compared to the 129S1 mice (Figure 3B2, 129S1 mean = 0.73 ± 0.04, C57BL/6 mean = 0.89 ± 0.04, t(14) = 2.9955, P < .01). The US-groups were analyzed separately and showed no significant increase in freezing over time or with tone presentations alone, and showed no differences between strains.
FIGURE 3.
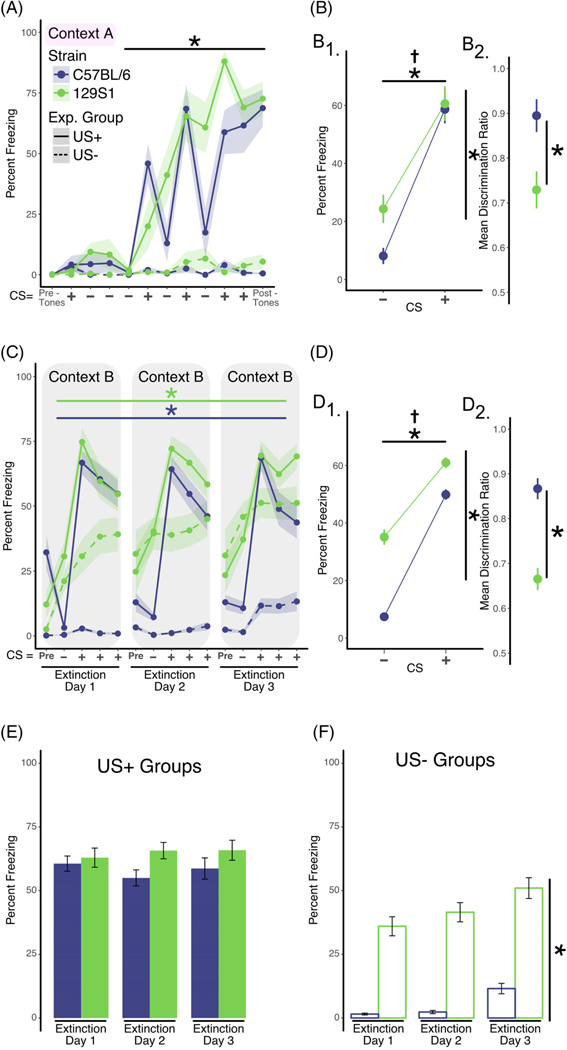
Delaying extinction training by 7 days does not impact extinction learning or discrimination of the CS+. A, Analysis of training data. Two separate groups of mice were used in this experiment. Mice in the US+ group (solid lines, C57BL/6 n = 8 [four males; four females], 129S1 n = 8 [four males; four females]) were exposed to discontinuous or pipped auditory stimuli that consisted of white noise (CS−; 200-ms white noise pips, delivered at 1 Hz for 25 seconds) or 4-kHz pure tone (200-ms pips, delivered at 1 Hz for 25 seconds) that co-terminated with a foot shock (CS+). Mice in the US-group (dashed lines, C57BL/6 n = 8,129S1 n = 8) received the identical auditory stimuli but did not receive the foot shocks. Mice in the US+ group exhibited increased freezing across the training session (repeated measures ANOVA; effect of training, P < .001) independent of strain. In the absence of shock, mice in the US-group did not exhibit significant levels of freezing. B, Analysis of discrimination learning during training. The mean percent freezing averaged across all CS+ presentations during training was significantly greater in both strains when compared with the mean freezing in response to the CS− presentations (B1, two-factor ANOVA; effect of CS, P < .0001). However, calculation of the discrimination ratio showed that C57BL/6 discriminates the CS+ and CS− better than 129S1 mice (B2, unpaired t test, P < .01). C, Analysis of within-session extinction learning. Seven days following training, mice in both groups were exposed to four CS− stimuli followed by 12 CS+ presentations (displayed in bins of four stimuli) in a new context (Context B) for 3 days (extinction days 1–3). Repeated exposure to the CS+ tone in the US+ group resulted in significant decreases in freezing within extinction sessions for both strains (using separate repeated measures ANOVA per strain; effect of bin, P < .01). D, Analysis of tone discrimination during extinction. Mice in both strains exhibited significantly more freezing when exposed to the CS+ tone (D1, two-factor ANOVA; effect of CS, P < .001). However, the C57BL/6 showed significantly better freezing discrimination to the CS+ compared with the 129S1 strain when the discrimination ratio was calculated (D2, unpaired t test; P < .0001). E, Analysis of extinction across days (US+ Group). Freezing across extinction days was analyzed by calculating the mean percent freezing for the three binned CS+ presentations for each of the three extinction days (solid line in panel C). No statistically significant reductions in freezing were observed for either C57BL/6 and 129S1 mice. F, Analysis of freezing in the US-group across days. Freezing in the US-group was calculated each day as the mean percent freezing measured during the CS+ tone presentations (dashed line in panel C). The 129S1 mice exhibited increased “freezing” across the 3 days of re-exposure to the CS+ tone compared with the C57BL/6 strain (two-factor ANOVA; effect of strain, P < .0001), in spite of the fact that they never received the foot shock. Data shown as sample mean with shaded region or bars representing the SE of the mean. Asterisk (*) denotes statistical significance for main effect of training (horizontal line) or strain (vertical line) and a dagger (†) for significant interaction between main effects. Data are presented as mean ± SEM
Similar to experiments in which extinction training was performed immediately after fear conditioning, when extinction training was delayed by 7 days both strains of mice exhibited significant within-session extinction learning (Figure 3C, C57BL/6 mice: main effect of training (F11,132 = 2.68; P < .01) and 129S1 mice: main effect of training (F11,132 = 3.15; P < .001)). Likewise, both strains were able to discriminate between the CS+ and CS− tones (main effect of tone type, F1,48 = 40.96; P < .0001) although C57BL/6 mice had enhanced discrimination relative to the 129S1 mice (129S1 mean = 0.67 ± 0.02, C57BL/6 mean = 0.87 ± 0.02, t(62) = 5.90, P < .0001; Figure 3D2). Neither 129S1 nor C57BL/6 showed a between-session reduction in freezing (Figure 3E).
The 7-day delay protocol also did not change the increase in immobility observed in the 129S1 group within-session (Figure 2C, main effect of strain, F1,42 = 33.29; P < .0001) or between-session (Figure 2F, main effect of strain, F1,28 = 48.07; P < .0001; main effect of session, F3,21 = 3.91; P < .05).
3.4 |. Exposure to novel contexts during training enhances the consolidation of extinction learning across training sessions
Our experiments modulating the parameters of a discrete CS led us to hypothesize that altering the context might also stimulate the consolidation of extinction learning. As in our previous experiments, both 129S1 and C57BL/6 mice displayed normal fear conditioning (main effect of training, F3,39 = 10.735; P < .001) with no differences between the strains (Figure 4A). Again, both strains were able to discriminate between CS+ and CS− (main effect of CS type, F1,26 = 78.04; P < .0001) with C57BL/6 mice displaying better discrimination as evidenced by a significant interaction between CS type and strain (Figure 4B1, F1,26 = 8.61; P < .01) during conditioning along with a higher discrimination ratio (Figure 4B2, 129S1 mean = 0.67 ± 0.02, C57BL/6 mean = 0.86 ± 0.03, t(13) = 4.9561, P < .001). As before, the US-groups were analyzed separately and showed no significant increase in freezing over time with presentations of tone alone and there were no differences between strains.
Using daily novel contexts for extinction training resulted in effective within-session extinction learning with both C57BL/6 mice (F11,46 = 9.49; P < .001) and 129S1 mice (F11,80 = 13.11; P < .001) displaying significant decreases in freezing. We also found main effects for CS type (F134 = 92.98; P < .0001), indicating that both strains could discriminate CS+. However, our results indicate that the 129S1 still exhibit higher freezing levels (Figure 4D1, main effect of strain [F1,34 = 93.44; P < .0001]). Analysis of the discrimination ratio confirmed that C57BL/6 mice discriminate the CS+ better than 129S1 mice (Figure 4D2, 129S1 mean = 0.66 ± 0.02, C57BL/6 mean = 0.93 ± 0.02, t(17) = 12.68, P < .0001).
Importantly, exposing mice to a new context for each extinction training day significantly enhanced consolidation and recall of tone extinction memories between sessions (Figure 4E; main effect of training session F2,111 = 20.9; P < .0001). Post-hoc analysis showed that both strains significantly reduced their level of freezing to the CS + between extinction Day 1 and Day 2 (Figure 4E, 129S1 ∆ = 21.28 ± 5.67, t(146) = 3.75, P < .01; C57BL/6 ∆ = 23.89 ± 6.45, t(146) = 3.7, P < .01). Interestingly, while both strains showed significant decreases in freezing over the course of extinction training, C57BL/6 mice froze significantly less than 129S1 during each session (main effect of mouse strain (F1,111 = 53.41; P < .0001).
Reductions in between-session freezing suggest that repeated extinction training in novel contexts enhances consolidation and/or recall of learning. To test if extinction learning was maintained long-term, we compared freezing levels from extinction Day 3 to performance during a recall test 7 days later (7-day tone test). We found no significant differences indicating that both strains exhibit long-term extinction memory. Furthermore, we tested if extinction training in novel contexts would effectively extinguish fear responses even in the conditioning context. We found no differences in freezing levels between extinction day 3 (context D) and the 7-day tone test in the conditioning context (A), indicating robust and specific extinction of the tone-footshock association.
Once again, the US-groups showed elevated immobility, particularly in the 129S1 mouse strain, which showed significantly more “freezing” than the C57BL/6 (F1,32 = 26.64; P = .0001), and there was no effect of CS+ presentation (Figure 4C, dashed lines) or training session (Figure 4F).
4 |. DISCUSSION
In this study, we report on the important role that environmental characteristics play in the capability of wild-type mice from two inbred mouse strains to discriminate between stimuli that are predictive of aversive outcomes (vs neutral outcomes) and to enhance learning and consolidation of extinction memory. Specifically, this study shows that utilizing pipped rather than continuous tones during extinction training ameliorates learning deficits in 129S1 mice and that repeated extinction training is more effective when conducted in multiple novel contexts than a single repeated context.
In agreement with prior studies, we found that 129S1 mice fear condition to tone stimuli as well as C57BL/6 mice.25–27 Our results also replicate the observation that 129S1 mice have conditioned fear extinction deficits when a continuous tone is used as a CS.25–27 Critically, we found that altering the CS from a continuous to a discontinuous tone (“pip”) significantly enhanced discrimination learning for both strains and ameliorated the within-session extinction learning deficits in the 129S1 mice. The use of tone pips as CS is not without precedent, as pipped tones have been used extensively in neurophysiological studies of fear learning and extinction5,32,33 and more recently to enhance spatial memory in mice.52 Pipped tones more closely mimic the auditory features of ultrasonic vocalizations of rodents and recruit the same brain structures during fear conditioning.53,54 The effects of pipped tones as a CS on extinction learning have not been systematically studied. However, our findings are corroborated by a comparison of a study24 in which pips were used and improved within-session extinction learning in 129S1 mice when compared to studies that used continuous tones.25–27,30 We further show that while pips improved within-session extinction, they did not impact the consolidation of extinction learning in the 129S1 or C57BL/6 strains.
Further studies are needed to elucidate the neurophysiological mechanisms of how pips improve fear discrimination and extinction learning. It is possible that pips more reliably recruit the relevant neural circuits for extinction learning than do continuous tones. Likely neurophysiological targets would include: neurons in the medial pre-frontal cortex and in the basal amygdala that have been shown to become active and phase locked to CS presentations in extinction training5,7,48; activation of the gamma-aminobutyric acid-releasing intercalated cells (ITCs)4,6,8,11; or N-methyl-D-aspartic acid receptor (NMDAR)-dependent activity in the BLA.55–57 Interestingly, recent studies suggest that recruitment of ITCs and/or NMDARs are mechanisms engaged primarily during the initial extinction session but are less engaged during subsequent training in the same environment.9,55,56 Consistent with this idea, our results showed that the utilization of pips positively influenced within-session extinction but had little to no effect on the consolidation and performance on subsequent training days. From a behavioral theory perspective, it is possible that the effects of pips may be explained by the difference in predictive value between a single continuous tone vs numerous pips. However, it is unlikely that the effectiveness of pips can be fully explained as “mass extinction training” as the mass extinction method has been shown to be ineffective for 129S1 mice.22,23
To further our studies on environmental variables that facilitate extinction learning and consolidation, we tested the possibility that delaying extinction training relative to fear conditioning would enhance extinction learning. Our hypothesis was that extinction training would be more effective with greater separation from the aversive experience. Overall, imposing a 7-day delay after fear conditioning did not appear to alter the extinction or discrimination learning phenotype relative to training on consecutive days (Figure 2). These results are consistent with rodent and human studies that suggest that remote traumatic memories are more resistant to extinction or exposure therapy.58,59
In light of our results suggesting that changing the quality of the CS was effective in modulating maladaptive fear, we tested the possibility that extinction training within a daily novel context might enhance consolidation of extinction memories. Using the novel context paradigm, we found significant enhancements in the consolidation and recall of extinction learning. More specifically, our results show that using daily novel context reduced freezing levels in the C57BL/6 to levels near those seen prior to fear conditioning, while the 129S1 performance is improved and more closely resembles C57BL/6 performance. Remarkably, we find that extinction recall is maintained for both strains even when the extinguished CS+ is presented in the original fear conditioning context (Figure 4, 7-day tone test, context A). This data suggest that the enhancement in the consolidation of extinction learning is proportional to the number of new contexts experienced. Given that extinction performance is believed to rely on the formation of new memories, extinction may be enhanced because a greater number of associations (memories) between the CS and nonaversive environments are formed. In support of this idea, a recent study in humans finds that replacing a threat stimulus with a novel stimulus facilitates extinction learning by engaging ventromedial prefrontal cortex.60From a neurophysiological perspective, one hypothesis to be tested is that using a daily novel context may retain the engagement of neural circuits underlying extinction learning that usually attenuate after the first day of training in the same context.9,55,57 These results may also have important implications for the effectiveness of cognitive-behavioral therapies based on extinction learning, such as those used to treat PTSD. It may be valuable in human subjects to increase the number of therapeutic contexts in which an unreinforced CS+ is presented and determine whether this reduces physiological fear responses as well as self-reported fear.
Finally, our experiments show that untrained 129S1 exhibit significantly decreased levels of locomotion and exploratory behavior over time compared to C57BL/6. These results are in support of previous studies showing that the 129S1 have reduced levels of locomotion and increased basal maladaptive fear behavior.49–51 This observation is very important for fear learning and memory studies in 129S1 mice as it can impact the interpretation of experiments in which the lack of movement (ie, freezing) is the primary dependent variable. Our studies are unable to fully distinguish whether increased immobility in the 129S1 is due to, or influenced by, innate fear to the experimental contexts or is a phenotype that is independent of fear or anxiety. The high level of immobility was not observed during initial training (see pretone, panel A of each figure) and increased over time, which suggests reduced attention or a lack of motivation for exploration vs a fear phenotype. In fact, while the 129S1 were previously found to exhibit only a small difference in overall mobility in a home cage-like environment (~14% lower than C57BL/6 during the light phase), they also show drastically low levels of climbing and exploring on-shelter, indicating deficits may be in the tendency to explore rather than motor.61 Thus, the increased immobility phenotype of the naive 129S1 suggests that some degree of extinction learning may be masked. Given the abundance of behavioral and neurophysiological data, the existence of extinction learning deficits in the 129S1 seem incontrovertible, which is further consistent with the data presented herein, but researchers should take note that US-controls are likely needed for full interpretation of behavioral data. The attenuated motivation for approach and exploratory behavior in the 129S1 may also be indicative of other neural circuitry deficits underlying motivation, and as such, may represent key factors that contribute to the extinction learning deficits in the 129S1. Elucidating the contributions of each of these phenotypes and the underlying neuronal circuitry is of utmost importance because the balance between approach and escape behavior is a critical component to extinction learning, which has translatable implications for the diagnosis and treatment of PTSD in humans.1
Supplementary Material
Acknowledgments
Funding information
National Institute of General Medical Sciences, Grant/Award Number: K12GM111725;National Institute on Aging, Grant/Award Number: R01AG052934
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Fenster RJ LAM L, Ressler KJ, Suh J. Brain circuit dysfunction in post-traumatic stress disorder: from mouse to man. Nat Rev Neurosci. 2018;19:535–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Milad MR, Pitman RK, Ellis CB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66(12):1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pavlov IP. Conditional Reflexes: An Investigation of the Physiological Activity of the Cerebral Cortex. Oxford, England: Oxford University Press; 1927. [Google Scholar]
- 4.Chhatwal JP, Myers KM, Ressler KJ, Davis M. Regulation of gephyrin and GABAA receptor binding within the amygdala after fear acquisition and extinction. J Neurosci. 2005;25(2):502–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herry C, Ferraguti F, Singewald N, Letzkus JJ, Ehrlich I, Lüthi A. Neuronal circuits of fear extinction. EurJ Neurosci. 2010;31(4):599–612. [DOI] [PubMed] [Google Scholar]
- 6.Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Paré D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454(7204):642–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420(6911):70–74. [DOI] [PubMed] [Google Scholar]
- 8.Amano T, Unal CT, Pare D. Synaptic correlates of fear extinction in the amygdala. Nat Neurosci. 2010;13(4):489–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.An B, Kim J, Park K, Lee S, Song S, Choi S. Amount of fear extinction changes its underlying mechanisms. Elife. 2017;6:489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi DC, Maguschak KA, Ye K, Jang SW, Myers KM, Ressler KJ. Prelimbic cortical BDNF is required for memory of learned fear but not extinction or innate fear. Proc Natl Acad Sci U S A. 2010;107(6): 2675–2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin H-C, Mao S-C, Gean P-W. Block of gamma-aminobutyric acid-A receptor insertion in the amygdala impairs extinction of conditioned fear. Biol Psychiatry. 2009;66(7):665–673. [DOI] [PubMed] [Google Scholar]
- 12.Rescorla R, Wagner A. A Theory of Pavlovian Conditioning: Variations in the Effectiveness of Reinforcement and Nonreinforcement. New York, NY: Appletone-Century-Crofts; 1972. [Google Scholar]
- 13.Dalton GL, Wang YT, Floresco SB, Phillips AG. Disruption of AMPA receptor endocytosis impairs the extinction, but not acquisition of learned fear. Neuropsychopharmacology. 2008;33(10): 2416–2426. [DOI] [PubMed] [Google Scholar]
- 14.Kim J, Lee S, Park K, et al. Amygdala depotentiation and fear extinc¬tion. Proc Natl Acad Sci US A. 2007;104(52):20955–20960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin C-H, Lee C-C, Gean P-W. Involvement of a calcineurin cascade in amygdala depotentiation and quenching of fear memory. Mol Pharmacol. 2003;63(1):44–52. [DOI] [PubMed] [Google Scholar]
- 16.Mao S-C, Chang CH, Wu CC, Orejarena MJ, Manzoni OJ, Gean PW. Inhibition of spontaneous recovery of fear by mGluR5 after prolonged extinction training. PLOS One. 2013;8(3):e59580. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Kar N Cognitive behavioral therapy for the treatment of post-traumatic stress disorder: a review. Neuropsychiatr Dis Treat. 2011;7: 167–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Logue MW, Amstadter AB, Baker DG, et al. The psychiatric genomics consortium posttraumatic stress disorder workgroup: posttraumatic stress disorder enters the age of large-scale genomic collaboration. Neuropsychopharmacology. 2015;40(10):2287–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Afifi TO, Asmundson GJG, Taylor S, Jang KL. The role of genes and environment on trauma exposure and posttraumatic stress disorder symptoms: a review of twin studies. Clin Psychol Rev. 2010;30(1): 101–112. [DOI] [PubMed] [Google Scholar]
- 20.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52(12):1048–1060. [DOI] [PubMed] [Google Scholar]
- 21.Holmes A, Singewald N. Individual differences in recovery from traumatic fear. Trends Neurosci. 2013;36(1):23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacPherson K, Whittle N, Camp M, Gunduz Cinar O, Singewald N, Holmes A. Temporal factors in the extinction of fear in inbred mouse strains differing in extinction efficacy. Biol Mood Anxiety Disord. 2013; 3(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whittle N, Schmuckermair C, Gunduz Cinar O, et al. Deep brain stim¬ulation, histone deacetylase inhibitors and glutamatergic drugs rescue resistance to fear extinction in a genetic mouse model. Neuropharmacology. 2013;64:414–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Camp MC, MacPherson KP, Lederle L, et al. Genetic strain differences in learned fear inhibition associated with variation in neuroendocrine, autonomic, and amygdala dendritic phenotypes. Neuropsychopharmacology. 2012;37(6):1534–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Temme SJ, Bell RZ, Pahumi R, Murphy GG. Comparison of inbred mouse substrains reveals segregation of maladaptive fear pheno-types. Front Behav Neurosci. 2014;8:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camp M, Norcross M, Whittle N, et al. Impaired Pavlovian fear extinction is a common phenotype across genetic lineages of the 129 inbred mouse strain. Genes Brain Behav. 2009;8(8):744–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hefner K, Whittle N, Juhasz J, et al. Impaired fear extinction learning and cortico-amygdala circuit abnormalities in a common genetic mouse strain. J Neurosci. 2008;28(32):8074–8085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giustino TF, Maren S. The role of the medial prefrontal cortex in the conditioning and extinction of fear. Front Behav Neurosci. 2015;9: 758–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gunduz Cinar O, Brockway E, Lederle L, et al. Identification of a novel gene regulating amygdala-mediated fear extinction. Mol Psychiatry. 2019;24:601–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whittle N, Hauschild M, Lubec G, Holmes A, Singewald N. Rescue of impaired fear extinction and normalization of cortico-amygdala circuit dysfunction in a genetic mouse model by dietary zinc restriction. J Neurosci. 2010;30(41):13586–13596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whittle N, Maurer V, Murphy C, et al. Enhancing dopaminergic signal¬ing and histone acetylation promotes long-term rescue of deficient fear extinction. Transl Psychiatry. 2016;6(12):e974–e974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blair HT, Sotres-Bayon F, Moita MAP, Ledoux JE. The lateral amygdala processes the value of conditioned and unconditioned aversive stimuli. Neuroscience. 2005;133(2):561–569. [DOI] [PubMed] [Google Scholar]
- 33.Quirk GJ, Armony JL, LeDoux JE. Fear conditioning enhances different temporal components of tone-evoked spike trains in auditory cortex and lateral amygdala. Neuron. 1997;19(3):613–624. [DOI] [PubMed] [Google Scholar]
- 34.Bang SJ, Allen TA, Jones LK, Boguszewski P, Brown TH. Asymmetrical stimulus generalization following differential fear conditioning. Neuro-biol Learn Mem. 2008;90(1):200–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ito W et al. Enhanced generalization of auditory conditioned fear in juvenile mice. Learn Mem. 2009;16(3):187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKinney BC, Murphy GG. The L-type voltage-gated calcium channel Cav1.3 mediates consolidation, but not extinction, of contextually conditioned fear in mice. Learn Mem. 2006;13(5):584–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKinney BC, Chow CY, Meisler MH, Murphy GG. Exaggerated emotional behavior in mice heterozygous null for the sodium channel Scn8a (Nav1.6). Genes Brain Behav. 2008a;7(6):629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McKinney BC, Sze W, White JA, Murphy GG. L-type voltage-gated calcium channels in conditioned fear: a genetic and pharmacological analysis. Learn Mem. 2008b;15(5):326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Temme SJ, Murphy GG. The L-type voltage-gated calcium channel Ca V1.2 mediates fear extinction and modulates synaptic tone in the lateral amygdala. Learn Mem. 2017;24(11):580–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krueger JN, Moore SJ, Parent R, McKinney BC, Lee A, Murphy GG. A novel mouse model of the aged brain: over-expression of the L-type voltage-gated calcium channel CaV1.3. Behav Brain Res. 2017;322: 241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perkowski JJ, Murphy GG. Deletion of the mouse homolog of KCNAB2, a gene linked to monosomy 1p36, results in associative memory impairments and amygdala hyperexcitability. J Neurosci. 2011;31(1):46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen J Statistical Power Analysis for the Behavioral Sciences. 2nd ed New York, NY: Academic Press; 1988. [Google Scholar]
- 43.R Development Core Team. R: A language and environment for statistical computing. (R version 3.5.2) 2016. https://www.R-project.org. Accessed December 20, 2018.
- 44.Nuñez JR, Anderton CR, Renslow RS. Optimizing colormaps with consideration for color vision deficiency to enable accurate interpretation of scientific data. PLOS One. 2018;13(7):e0199239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singmann H, Bolker B & Westfall J, 2015. afex: Analysis of Factorial Experiments (R package version 0.13–145). https://CRAN.R-project.org/package=afex. Accessed December 20, 2018. [Google Scholar]
- 46.Wickham H, 2010. ggplot2: Elegant Graphics for Data Analysis (Use R!). New York, NY: Springer-Verlag. [Google Scholar]
- 47.Wickham H, François R, Henry L, Müller K 2015. dplyr: A Grammar of Data Manipulation (R package version 0.7.6). https://CRAN.Rproject.org/package=dplyr. Accessed December 20, 2018. [Google Scholar]
- 48.Grewe BF, Gründemann J, Kitch LJ, et al. Neural ensemble dynamics underlying a long-term associative memory. Nature. 2017;543(7647): 670–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crawley JN, Belknap JK, Collins A, et al. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl). 1997;132(2):107–124. [DOI] [PubMed] [Google Scholar]
- 50.Dockstader CL, van der Kooy D. Mouse strain differences in opiate reward learning are explained by differences in anxiety, not reward or learning. J Neurosci. 2001;21(22):9077–9081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Võikar V, Kõks S, Vasar E, Rauvala H. Strain and gender differences in the behavior of mouse lines commonly used in transgenic studies. Physiol Behav. 2001;72(1–2):271–281. [DOI] [PubMed] [Google Scholar]
- 52.Martorell AJ, Paulson AL, Suk HJ, et al. Multi-sensory gamma stimulation ameliorates Alzheimer’s-associated pathology and improves cognition. Cell. 2019;177:256–271.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bang SJ, Brown TH. Perirhinal cortex supports acquired fear of auditory objects. Neurobiol Learn Mem. 2009;92(1):53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Furtak SC, Allen TA, Brown TH. Single-unit firing in rat perirhinal cortex caused by fear conditioning to arbitrary and ecological stimuli. J Neurosci. 2007;27(45):12277–12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laurent V, Westbrook RF. Distinct contributions of the basolateral amygdala and the medial prefrontal cortex to learning and relearning extinction of context conditioned fear. Learn Mem. 2008;15(9):657–666. [DOI] [PubMed] [Google Scholar]
- 56.Laurent V, Marchand AR, Westbrook RF. The basolateral amygdala is necessary for learning but not relearning extinction of context conditioned fear. Learn Mem. 2008;15(5):304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Laurent V, Westbrook RF. Role of the basolateral amygdala in the reinstatement and extinction of fear responses to a previously extinguished conditioned stimulus. Learn Mem. 2010;17(2): 86–96. [DOI] [PubMed] [Google Scholar]
- 58.Pitman RK, Delahanty DL. Conceptually driven pharmacologic approaches to acute trauma. CNS Spectr. 2005;10(2):99–106. [DOI] [PubMed] [Google Scholar]
- 59.Tsai L-H, Gräff J. On the resilience of remote traumatic memories against exposure therapy-mediated attenuation. EMBO Rep. 2014;15 (8):853–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dunsmoor JE, Kroes MCW, Li J, Daw ND, Simpson HB, Phelps EA. Role of human ventromedial prefrontal cortex in learning and recall of enhanced extinction. J Neurosci. 2019;2713–2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Loos M, Koopmans B, Aarts E, et al. Sheltering behavior and loco¬motor activity in 11 genetically diverse common inbred mouse strains using home-cage monitoring. PLOS One. 2014;9(9): e108563. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


