Abstract
Aims:
Increasing evidence suggests that metabolism affects brain physiology. Here we examine the effect of GLP-1 on simple visual-evoked fMRI responses in cortical areas.
Material and methods:
Lean (n=10) and non-diabetic obese (n=10) subjects received exenatide (a GLP-1 agonist) or saline infusion and fMRI responses to visual stimuli (food and nonfood images) were recorded. We analyzed the effect of exenatide on fMRI signals across the cortical surface with special reference to the visual areas. We evaluated the effects of exenatide on the raw fMRI signal and on the fMRI signal change during visual stimulation (vs. rest).
Results:
In line with previous studies, we find that exenatide eliminates the preference for food (over nonfood) images present under saline infusion in high-level visual cortex (temporal pole). In addition, we find that exenatide (vs. saline) also modulates the response of early visual areas, enhancing responses to both food and nonfood images in several extra-striate occipital areas, similarly in obese and lean participants. Unexpectedly, exenatide increased fMRI raw signals (signal intensity during rest periods without stimulation) in a large occipital region, which were negatively correlated to BMI.
Conclusions:
In both lean and obese individuals, exenatide affects neural processing in visual cortex, both in early visual areas and in higher order areas. This effect may contribute to the known effect of GLP1-analogues on food related behavior.
Keywords: exenatide, fMRI, obesity, visual cortex
Introduction
Mounting evidence shows that metabolic state can affect neuronal function and plasticity, influencing brain physiology and ultimately producing an impact on cognitive processes, emotions, and behavior [1–2]. The interplay between nutrition and brain health has long been established by the effect of specific diet alterations on neuro-psychological responses [3–5]. For example, western diets have been associated with impaired cognitive functions, anxiety, anhedonic behavior, and to higher incidence of emotional disorders [6–9]. Obesity [10] and type 2 diabetes (T2DM) [11] are associated with altered food-reward mechanisms and eating behavior, involving multiple pathways that include signaling from the periphery. Hormones secreted by the adipose tissue and gut convey information on the nutritional status of the body to the brain, modulating the central regulation of food intake [12]. Amongst these signals, GLP-1 has attracted much attention, mainly because of the use of GLP-1 receptor agonists (GLP-1RA) in clinical practice and the associated body weight reduction. GLP-1 is secreted after food ingestion by the enteroendocrine L cells located in the distal jejunum and ileum [13]. Besides stimulating insulin secretion and inhibiting glucagon release, GLP-1 reduces food intake and enhances satiety by modulating brain mechanisms that control eating behavior [14]. We recently tested the influence of exenatide, a GLP-1 agonist, on brain metabolism [15], and observed a marked increase of cerebral glucose uptake within few minutes after exenatide infusion. Moreover, we recently analyzed the effect of GLP-1 on hypothalamic responses to food-cues, measured as fMRI responses to visual stimuli containing food or non-food images. We found that exenatide reduced the preference for food-cues in the hypothalamus, and more so in obese than in lean observers [16]. Consistent with a central satiating effect of GLP-1, van Bloemendaal et al. [17] reported a decreased preference for food pictures in the insula, amygdala, putamen, and orbitofrontal cortex following short-term administration of a GLP-1RA in obese individuals. After Roux-En-Y Gastric Bypass (RYGB), obese T2DM subjects show increased activation in the fusiform gyrus, somatosensory cortex, and frontal middle gyrus along with a marked increase in plasma GLP-1 level [18–19]. Although some of these studies have employed visual images as food cues, most have focused on brain areas (gustation, reward and, executive functions), which respond differentially to food versus non-food stimuli [20–22] and no study has examined the modulation of the early sensory response to visual images (irrespective of their food/non-food content) in cortical visual areas.
Vision plays an important role in food selection in humans [23–24]. The sight of food elicits a variety of reactions ranging from anticipatory responses to food ingestion - i.e. the cephalic phase of insulin release and heart rate changes [25–26] - to a cascade of cognitive processes, involving memory and emotion [27] as well as executive function, e.g. resisting the temptation of palatable food [28–29]. Thus, a change of responsivity in early visual areas could trigger a cascade of effects potentially impacting food appreciation and food related behaviors. To test this hypothesis, here we ask whether exenatide is able to influence the basic physiology of early visual areas, irrespectively of the content of visual images (food related or otherwise).
To address this question, a new analysis based on a novel approach has been performed on data from a previous study [16]. First, rather than focusing on deep-brain structures involved in feeding behavior (e.g. hypothalamus) we examine the effects of exenatide over the entire cortical surface. Second, our analysis is not limited to assessing the effects of exenatide on the preference for food cues that many brain areas display, but it allows us to assess the effects of exenatide on cortical activity, irrespective of stimulus type – an aspect that has not been addressed in the prior study, or any previous work.
Materials and Methods
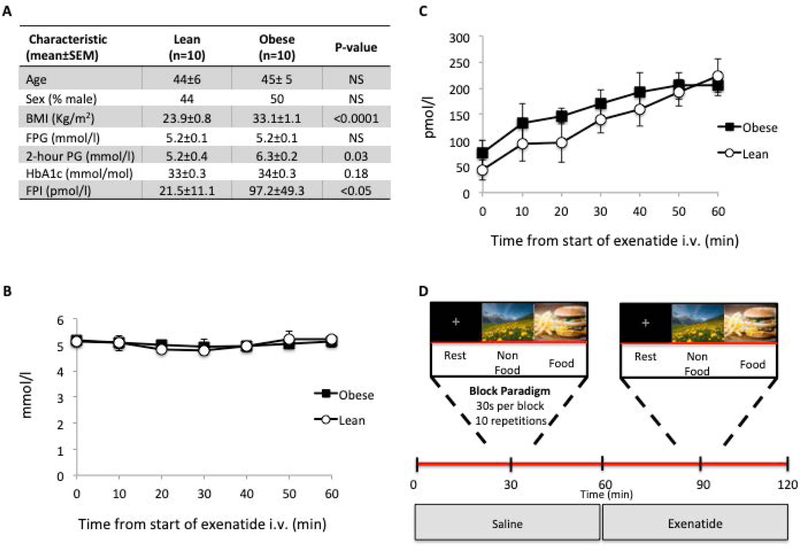
The study population included 10 lean participants and 10 obese non-diabetic participants, matched for age, gender, and fasting plasma glucose concentration. Participants received a 75 g OGTT 5–14 days before MRI session for appropriate classification of glucose regulation. Obese subjects had higher BMI and fasting plasma insulin concentration (Figure 1A).
Figure 1.
A: Anthropometric and metabolic differences between lean and obese subjects (p-values computed with a nonparametric Mann-Whitney test).
B-C: mean plasma glucose (B) and insulin (C) levels during exenatide intravenous infusion; differences across time-points and subject groups (lean and obese) were analyzed with an ANOVA for repeated measures.
D: Stimulus design
The protocol was approved by the Institutional Review Board of the University of Texas and all study procedures were conducted in accordance with the guidelines of the Declaration of Helsinki. Written informed consent was obtained from all participants before entering the study.
All data were collected in a single session starting at 8:00 A.M. after overnight fast. MR acquisitions started after cannulation of two peripheral veins for blood sample drawing and infusion of dextrose/saline/exenatide. Two consecutive (saline and exenatide) primed (exenatide 0.3 µg) continuous (0.05 µg/min) infusions (60 min each) were performed and two brain fMRI datasets acquired starting 30 min after the beginning of each infusion. During exenatide infusion, plasma glucose levels were measured at 5 min interval and a variable dextrose (20%) intravenous infusion was administered to 3 lean subjects and 2 obese subjects to maintain plasma glucose concentration constant at the fasting level (Fig. 1B). Concomitantly, plasma insulin concentration increased to a similar extent in lean and obese individuals (Fig. 1C). During fMRI acquisitions, three types of visual stimuli were presented in blocks of 30s: rest, food images, and nonfood images (Fig. 1D). During rest blocks, the screen was uniformly black with a fixation cross. Food images were popular, high-calorie-content foods. Nonfood images were outdoor landscape scenes. Stimulus-presentation time was synchronized with image repetition time (TR) at 3 s per image (each block was composed of 10 images); to avoid habituation, individual pictures did not repeat within or between acquisition blocks. The order of the three blocks was fixed (rest-food-nonfood) and the sequence was repeated ten times, with one rest block added at the end (yielding a total fMRI duration of 310 time points, about 15 minutes).
MR Image Acquisition and analysis
All image data were acquired with a 3-Tesla, research-dedicated Siemens TIM/TRIO (Erlangen,Germany). A T1-weighted anatomical MRI was obtained at the beginning of each session (TR = 2,200 ms, echo time = 2.72 ms, inversion time = 766 ms, flip angle = 138, and resolution of 1 × 1 × 1 mm). Following the infusions (saline and exenatide), two whole-brain T2* Blood Oxygen Level Dependent effect (BOLD) fMRI volumes were acquired using Echo Planar Imaging (EPI) at TR = 3,000 ms, echo time = 30 ms, an in-plane spatial resolution of 1.7 × 1.7 mm, as 3-mm-thick sagittal slices. Anatomical data were processed using the FMRIB Software Library (FSL) toolkit (http://www.fmrib.ox.ac.uk/fsl/) to correct for spatial inhomogeneity and to perform non-linear noise reduction. Brain surfaces were reconstructed and inflated from the T1 images using the FreeSurfer toolkit (http://surfer.nmr.mgh.harvard.edu/)[30–32].
Individual whole brain (right & left hemisphere) surface maps were then registered to a common FreeSurfer template surface, pseudo-hemisphere (fsaverage_sym) using the FreeSurfer spherical registration system [31]. With minimal metric distortion, this approach matches morphologically homologous cortical areas based on cortical folding patterns, then resamples individual results to a standard (fsaverage_sym) surface. The resulting pseudo-hemisphere surface representation is composed of a network of vertices that align at a sub-voxel resolution to the volumetric anatomy both across subjects, and between hemispheres. BOLD data were pre-processed using the Statistical Parametric Mapping (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) toolkit, which consisted of sync interpolation in time to correct for the slice acquisition sequence and motion correction with a six-parameter least squares rigid body realignment routine using the first functional image as a reference. The transformation matrix required to align each BOLD acquisition to the relevant participant’s anatomy was computed using FSL-FLIRT with 6 degrees of freedom. Individual voxel time courses were analyzed with a non-standard approach, motivated by the preliminary observation that exenatide affected the raw fMRI signal intensity across a large region of the cortex (mainly occipital, where our analyses are focused). In order to control for this shift, we started by quantifying, for each cortical vertex, the raw fMRI signal during the rest blocks (which is unaffected by visual stimulation). Next we normalized the fMRI time-course by this value to obtain the percentage signal change in the food and non-food blocks, relative to the rest blocks. This yields three parameters (rest, food, non-food) per acquisition. Note that standard practice would have required using the average fMRI signal across the whole time-course as normalization constant, to which no physiological meaning is attached. In this case, we used the average fMRI in the rest blocks alone (because, in our stimulation protocol, visual stimulation blocks are twice as frequent as rest blocks, implying that the average fMRI signal across the whole time-course actually incorporates a component of the visual response). Moreover, while the absolute fMRI signal is set by the MR settings and specifications, we argue that the comparison of this value across consecutive acquisitions (performed without moving the patient in/out the scanner, with identical settings) and across brain regions (i.e. regional variations of the parameter) does have a physiological meaning, and reflects the modulations of non-visual factors – e.g., exenatide. Volume maps of the three parameters were obtained for each BOLD acquisition (i.e. separately for the saline and exenatide conditions in each participant). The results were registered to the participant’s anatomy in FreeSurfer and projected to the individual surface after smoothing with a 5mm FWHM Gaussian kernel. Finally, data from all subjects and runs were projected to the fsaverage_sym surface and corresponding vertices of the two hemispheres were averaged. Thus, after averaging across hemispheres, each vertex of this surface has 20 (subjects) x 2 (conditions: saline/exenatide) values of our three parameters: resting BOLD, food responses and non-food responses. These were analyzed with an ANOVA for repeated measures, with between factor “Obesity” (lean/obese) and within factors “Condition” (saline/exenatide) and “Image type” (food/non-food), followed by post-hoc t-tests (two-sample t-tests investigating the differences between obese and lean subjects; paired t-tests investigating the differences between saline and exenatide, or between food and non-food pictures; one-sample t-tests to identify voxels with significant visual responses, i.e. non-zero percent signal change in response to any image, food or non-food; in all cases, p-values are two-tailed). In this way, each vertex of the fsaverage_sym pseudo-hemisphere is assigned with a F or t value, which are shown on its inflated and flattened view of the cortical surface (see Figure 2), after thresholding to show values with p<0.001 uncorrected. Note that p-values are not corrected for multiple comparisons, because the purpose of these maps is simply to reveal the topographical distribution of signal modulations over the cortical surface – rather than testing the statistical significance of the effects, which is addressed with a different ROI-based analysis appraoach. For the ROI-based approach, we assessed the statistical effects of the above factors by analyzing average data from vertices within pre-selected regions of interest: early visual areas V1-V2 (from the Van Essen parcellation atlas) [33], ventral stream visual areas (fusiform gyrus and temporal pole, as defined in the Destrieux 2009 Atlas [34] and a large lateral occipital region defined manually to include the complex of lateral occipital areas encompassing LO and V8 (from [33]), all shown in Figure 2A. The required atlases were fit to the fsaverage_sym surface; for each ROI, we averaged values of resting BOLD and percent signal change across all fsaverage_sym vertices; finally, we run the appropriate statistics on the ROI averages. For all ROI-based statistics, we report exact p-values.
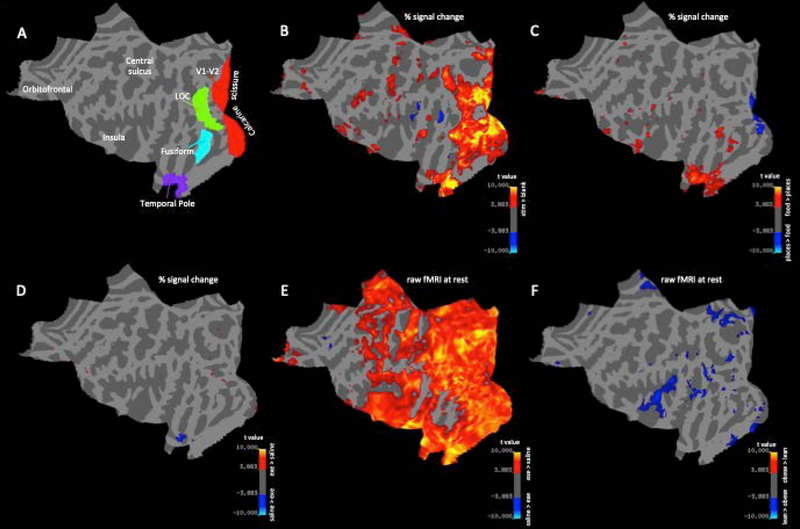
Figure 2.
statistical parametric mapping approach showing the topographical distribution of the effects of image type, exenatide and obesity on percent signal change (B-D) and the raw fMRI signal at rest (E-F) over the cortical surface. (A) flattened view of cortical template used to align all subjects and hemispheres, with anatomical markers and colored areas representing the regions of interest used for Figure 3–4. (B) map of responses to visual stimulation, averaged across all conditions; (C) map of modulation of visual responses to food vs. non-food stimuli; (D) map of modulation of visual responses during exenatide vs. saline infusion; (E) map of modulation of the raw fMRI signal at rest with exenatide; (F) map of significant modulation of the raw fMRI signal at rest in obese vs. lean participants. B-F maps are thresholded at two-tailed p<0.001 uncorrected.
Results
Effects of Exenatide and Body Weight on Visual Stimuli
We analyzed fMRI data from a previous study [16] specifically testing the effect of exenatide on visual responses measured over the cortical surface of lean and obese subjects.
Fig. 2A represents the alignment of all participants to a template cortical map with pre-defined regions of interest that allows for morphing the pattern of sulci and gyri to match a standard atlas and achieve an excellent inter-individual alignment of all the main cortical areas.
In Figure 2B, the map is color-coded to show visual responses by averaging the percent signal change in response to food and non-food pictures, with or without exenatide and pooling obese and lean subjects. As expected, responses (defined using a threshold two-tailed p-value of 0.001, uncorrected) were primarily located in the occipital cortex and in the portion of the temporal lobe hosting the ventral visual pathway. Figure 2C shows the results of paired t-tests comparing activity in response to food vs. non-food pictures (averaged across exenatide and saline experiments and pooled for subject groups). Fusiform, temporal and limbic areas displayed preference for food pictures over non-food images (at the same threshold of two-tailed p < 0.001). The opposite was seen in early visual cortex V1 and V2, where a preference for nonfood pictures was apparent, likely related to differences in the low-level visual statistics of the two image sets.
Having established these basic patterns of responses, we determined the effects of the variables of interest: i.e. exenatide infusion, obesity, and their interactions. Figures 2D show the results of paired t-tests comparing visual responses during exenatide vs. saline (irrespective of image type). The temporal pole showed a decrease of response to visual stimuli with exenatide, whereas the opposite was seen in several small cortical patches located in extra-striate occipital areas. No cluster of vertices consistently showed an effect of body weight or an interaction between body weight and exenatide (maps not shown).
To gain better insight into the trends shown in Figure 2, we proceed with a ROI-based approach (Figure 3), focusing on four predefined ROIs placed at key levels of the visual hierarchy. In early and mid-level visual areas (V1-V2 and fusiform gyrus), there was no significant effect of either exenatide or obesity image type (all main effects and interaction terms: F(1,18) < 0.3, p > 0.24). In V1-V2 there was a (non-significant) trend towards stronger responses to non-food images; the opposite preference (for food images) was observed in the fusiform gyrus (main effect of within subject factor food/non-food image: F(1,18) = 7.26, p = 0.015). Exenatide had a strong and selective effect on responses to food images in the temporal pole – the preferred stimulus for this area – leaving responses to non-food images unaffected (ANOVA for repeated measures, main effect of within subject factor exenatide: F(1,18) = 6.95, p = 0.017, main effect of within subject factor stimulus type: F(1,18) = 18.70, p < 0.001, interaction of exenatide x stimulus type: F(1,18) = 4.80, p = 0.042; all other main effects and interaction terms: F(1,18) < 2.24, p > 0.15). In contrast, exenatide increased responses in the lateral-occipital ROI, irrespectively of stimulus type (ANOVA for repeated measures, main effect of within subject factor exenatide: F(1,18) = 6.26, p = 0.022, all other main effects and interaction terms: F(1,18) < 1.62, p > 0.28).
Figure 3.
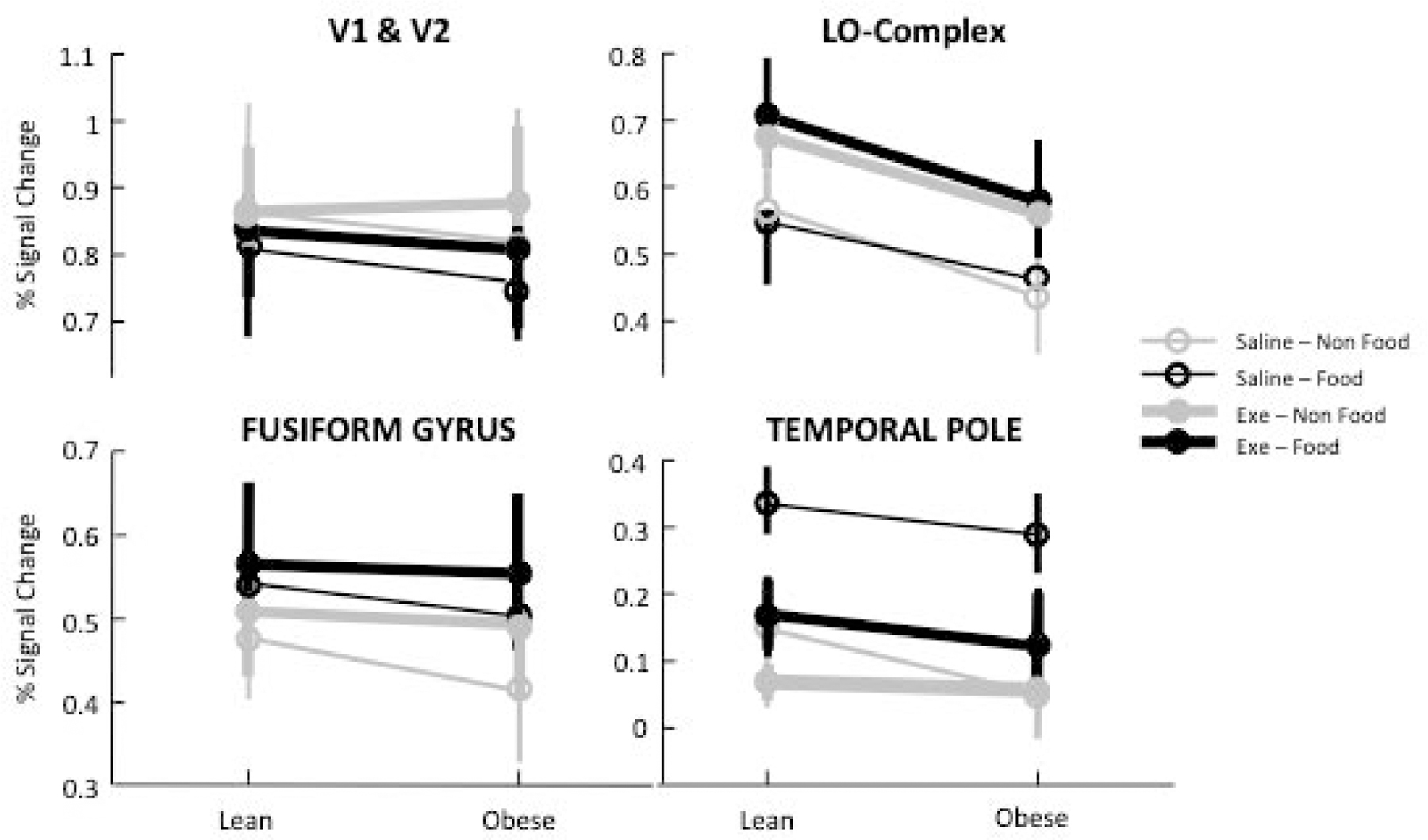
ROI-based approach showing the effects of image type, exenatide and obesity on percent signal change in the four regions of interest defined in Figure 2A.
Effects of Exenatide and Body Weight on BOLD Signal at Rest
The standard approach in fMRI limits analyses to BOLD responses, defined as percent signal changes relative to rest periods, with no consideration for the average BOLD signal at rest. This signal depends primarily on the MR acquisition settings; however, EPI contrast (i.e. MR contrast used for fMRI) has been reported to be affected by physiological parameters such such as vasodilation and cerebral blood flow [35]. We reasoned that these physiological parameters might well co-vary with obesity and exenatide and we adopted the same statistical approach used for Figures 2B–D and Figure 3 to analyze the effects of obesity and exenatide on the raw fMRI signal during periods of rest (no visual stimulus). Figure 2E shows that resting BOLD was higher during exenatide infusion than during saline infusion (each vertex in the map reports a t-value obtained with a paired-t-test comparing resting BOLD with exenatide and saline in all 20 participants; threshold for significance is set to p < 0.001 uncorrected). Although the difference is significant across a large cortical territory, it may still be regarded as regional as it clearly spares large territories in the frontal cortex. Figure 2F shows that resting BOLD was also lower in obese compared to lean subjects (the map shows two-sample t-test comparing the resting BOLD at each vertex between obese and lean subjects, at two-tailed p < 0.001 uncorrected). This difference was more localized, primarily involving the occipital and insular lobes. The two effects, obesity and exenatide, appear to be independent (no vertex showing a significant interaction, map not shown). Similarly, in the ROI analysis (Figure 4), we found a significant effect of exenatide in all regions of interest (all F(1,18) > 37, all p < 0.001), a significant effect of obesity in all but temporal pole region (V1-V2: F(1,18) = 9.5, p = 0.006; LOC: F(1,18), p = 0.002; Fusiform Gyrus: F(1,18) = 5.02, p = 0.032; Temporal Pole: F(1,18) = 0.09, p = 0.76), and no interaction between the two (all F < 0.9, all p > 0.3). Moreover, Figure 5 shows that the raw fMRI signal at rest was negatively correlated with BMI (hence lower in obese participants, who have BMI >= 30) and increased with exenatide. The slope of the linear fit was similar for data acquired during saline and exenatide infusion (R > 0.5 and p < 0.05 in both cases); only the intercepts differ markedly (1209 a.u. for saline and 1273 a.u. for exenatide) accounting for an increase of about 6% of raw fMRI signal at rest with exenatide, independent of BMI. Open circles show the average BOLD signal for the lean and obese group (different abscissa), following saline or exenatide infusion (gray and black, respectively). BOLD signal under saline is lower in obese than in lean subjects (post-hoc two-sample t-test: t(18) = 3.2804, p < 0.01). This is still true if we compare BOLD signals in obese individuals under exenatide with BOLD signals in lean individuals under saline (post-hoc two-sample t-test: t(18) = 4.4187, p < 0.001).
Figure 4.
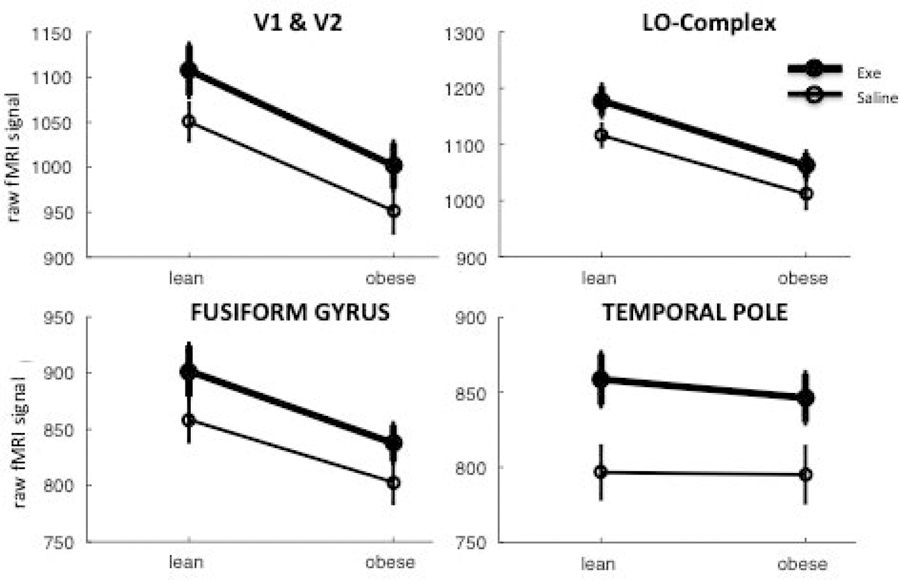
ROI-based approach showing the effects of exenatide and obesity on the raw fMRI signal at rest.
Figure 5.
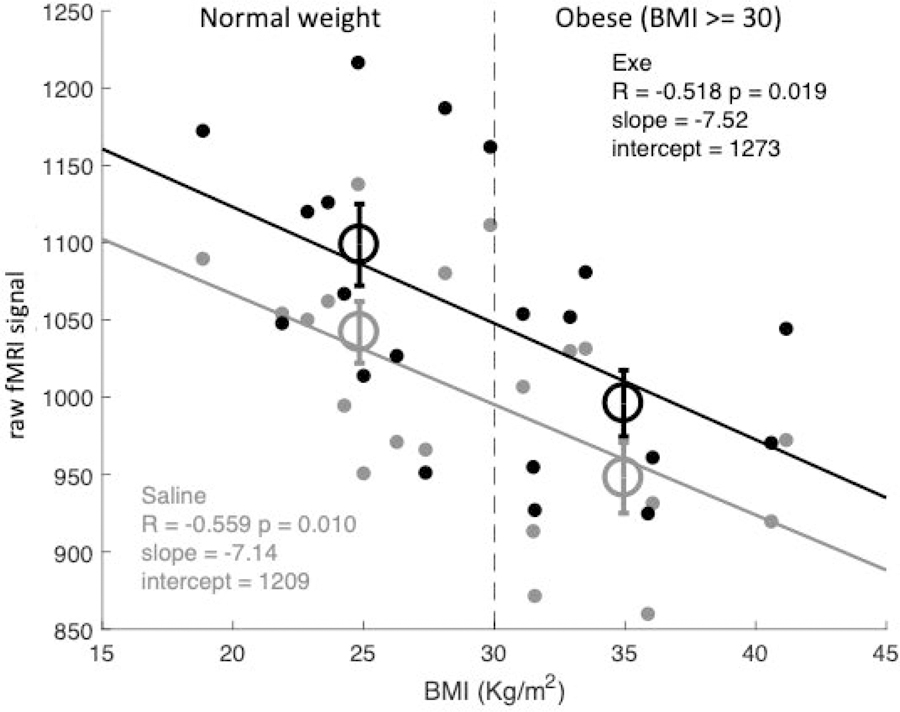
Raw fMRI signal at rest plotted against each subject’s BMI, separately for the saline and exenatide conditions (implying two data-points per participant, gray and black respectively). The vertical dashed line shows the cut-off used to define the dichotomic factor obesity (BMI >= 30). Open circles give the average BOLD signal in lean and obese individuals (different abscissas) in the saline and exenatide conditions (gray and black respectively). Text insets give Pearson’s correlation coefficients (R) with associated p-values, and the parameters of the linear fit through the data, separately for the two conditions (gray and black text for the saline and exenatide conditions).
Discussion
This study goes beyond our previous work, which showed that exenatide affects the preference for food over nonfood pictures in deep brain structures including the hypothalamus [16], and demonstrates regionally specific effects of exenatide on cortical BOLD signals.
We observed a stimulus-selective effect of exenatide in the temporal pole, a region involved in high-level visual processing and semantic interpretation of visual images. During saline infusion, this region showed strong responses to food images and no responses to nonfood images. During exenatide infusion, this food-response was nearly abolished. This interaction is not explained by differences in the low-level characteristics of the two image sets as no exenatide and image interaction was detected in areas V1-V2. Together with earlier findings of decreased reactivity to food stimuli in subcortical areas after exenatide infusion [16], our results support the notion that GLP-1 profoundly affects brain function, interfering not only with the homeostatic regulation of appetite in subcortical regions but also with the cortical sensory response to food-related stimuli.
In addition, we found a non-selective enhancement of BOLD responses to both food and non-food visual stimuli in several extrastriate occipital areas. This provides preliminary evidence that metabolic challenges might affect activity in sensory processing areas, at a relatively low-level in the hierarchy of the visual areas, and with an acute and very specific metabolic effect of GLP1 in central systems involved in the regulation of insulin secretion and satiety. Our technique (fMRI) does not allow to determine whether the observed effects have functional, physiological correlates – e.g. whether the increased BOLD signal is paralleled by enhanced visual evoked potentials; or if, on the contrary, these response changes reflect changes in the hemodynamic coupling that generates fMRI signal. Notably, in these same areas, exenatide also induces a large increase of the raw fMRI signal at rest, a variable that is not commonly analyzed in fMRI studies. The absolute value of this parameter has no physiological meaning, as it mainly depends on the MR acquisition setting. However, we found regional variations of this value across consecutive acquisitions under identical settings. Specifically, during exenatide infusion, there was an increase of fMRI signal at rest that extends over a large cortical territory including the occipital lobe and sparing the frontal cortex. The signal co-varied with BMI and it was significantly lower in obese subjects.
Previous studies have focused on brain responses to visual food cues in areas involved in appetite and reward processing (insula, amygdala, orbitofrontal cortex and striatum) demonstrating that obese individual, as compared to lean subjects, exhibit increased brain activation in bilateral insula, right amygdala and orbitofrontal cortex [36]. Conversely, lean individuals have been shown to have greater activation of visual cortex in response to food cues than obese subjects [37]. However, Cornier et al did not fully explore the cortical pathways underlying visual evoked BOLD signal change within the cortex.
It is difficult to determine the physiologic basis of this finding. Based on the few studies that have analyzed the raw fMRI parameter in relation to physiological variables, we propose that this change might reflect a modulation of regional cerebral blood flow (rCBF), since this has been linked to the fMRI signal at rest [38] and it is known to co-vary with BMI [39]. In particular, the impaired vasodilation previously reported in obese subjects [39–40] could explain the reduction of CBF. Impaired vasodilation may also be the result of an inflammatory state, as it occurs in obesity and associated metabolic perturbations [41–43]. GLP-1 could ameliorate this dysfunction by affecting the inflammatory pathways and by modulating blood flow [44] through vasodilation [45], systolic blood pressure and sympathetic activity [46]. In this view, obesity would be associated with a decline in CBF that could be rescued by GLP-1 receptor activation. Moreover, given that CBF is closely coupled with glucose utilization in resting human brain [47], the effect of exenatide on the raw fMRI BOLD signal could also reflect increased cerebral glucose uptake induced by exenatide as previously shown by ourselves [15]. Finally, the effect of exenatide on beta-cell function should be taken into account given the profound effect on brain metabolism and neural function of insulin [48].
We recognize that the sample size of our study is small, but, in spite of that, it was sufficient to reveal strong and reliable effects of exenatide. Notably, other recent studies with even smaller groups of diabetic patients, did manage to highlight metabolic effects on brain activity [49]. We also acknowledge that the stimulus set employed for visual stimulation, chosen for a previous study with slightly different aims, may not be completely balanced in terms of low-level visual statistics. This may have resulted in a differential activation of early visual areas V1-V2. Nonetheless, this does not invalidate our primary observations as they were made in areas representing the semantic content of the images, irrespective of their simple visual features. We also did not attempt to manipulate two key variables that modulate the response to food pictures: the energy content of the depicted food and the hunger state of the individual. Behavioral studies have shown that these factors influence eating behavior, e.g., people have a higher preference for energy-rich foods [50] and foods are rated as more pleasant when people are hungry. We found that food responses in the temporal pole were selectively reduced by exenatide infusion. It remains to be determined whether this interaction could be potentiated and/or extended to other cortical areas by manipulating food’s energy content and the hunger state of the participants.
In conclusion, the present study demonstrates that exenatide modulates BOLD visually evoked responses in cortical regions encoding the content of visual stimuli – food or non-food related. In addition, exenatide and body weight affect the intensity of the raw fMRI signal at rest, which we speculatively associate with variations of CBF and/or glucose metabolism. We believe that our study expands the literature available suggesting an important impact of body weight on food cues brain responses, which is characterized by a complex modulation of multiple brain areas involved in visual stimuli processing that can be further acutely modulated by GLP-1 receptor activation.
Acknowledgments.
The authors thank Sandra Martinez, RN, for her excellent care of the patients during the study and Lorrie Albarado and Shannon Balmer, from the University of Texas Health Science Center, for their expert secretarial assistance in preparation of the manuscript.
Funding. This study was partly supported by Qatar Foundation grant NPRP 4–248-3–076 and National Institutes of Health grant ROI DK097554–01 to M.A.-G. Support of P.B. additionally came from the European Research Council, under the grant Ecsplain. Exenatide was provided by AstraZeneca.
Footnotes
Data availability: The data that support the findings of this study are available on request from the corresponding author (G.D.).
Duality of Interest: R.D. is on the advisory boards of AstraZeneca, Novo Nordisk, Janssen, Intarcia, and Boehringer Ingelheim; receives research support from Bristol-Myers Squibb, Boehringer Ingelheim, Takeda, and AstraZeneca; and is on the speaker’s bureaus of Novo Nordisk and AstraZeneca. No other potential conflicts of interest relevant to this article were reported. S.D.P. has received research support from AstraZeneca, Boheringer Ingelheim, Novartis Pharmaceuticals, and has served in advisory boards of Abbott, AstraZeneca, Boheringer Ingelheim, Glaxo SmithKline, Mundipharma, Novo Nordisk, Servier laboratoires, sanofy, Takeda.
References
- 1.Agrawal R, et al. , Deterioration of plasticity and metabolic homeostasis in the brain of the UCD-T2DM rat model of naturally occurring type-2 diabetes. Biochim Biophys Acta, 2014. 1842(9): p. 1313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mattson MP, et al. , Intermittent metabolic switching, neuroplasticity and brain health. Nat Rev Neurosci, 2018. 19(2): p. 63–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Canevelli M, et al. , Nutrition and Dementia: Evidence for Preventive Approaches? Nutrients, 2016. 8(3): p. 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dadhania VP, et al. , Nutraceuticals against Neurodegeneration: A Mechanistic Insight. Curr Neuropharmacol, 2016. 14(6): p. 627–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Augustin K, et al. , Mechanisms of action for the medium-chain triglyceride ketogenic diet in neurological and metabolic disorders. Lancet Neurol, 2018. 17(1): p. 84–93. [DOI] [PubMed] [Google Scholar]
- 6.Pagoto SL, et al. , Association of post-traumatic stress disorder and obesity in a nationally representative sample. Obesity (Silver Spring), 2012. 20(1): p. 200–5. [DOI] [PubMed] [Google Scholar]
- 7.Dixon JB, et al. , Severely obese people with diabetes experience impaired emotional well-being associated with socioeconomic disadvantage: results from diabetes MILES - Australia. Diabetes Res Clin Pract, 2013. 101(2): p. 131–40. [DOI] [PubMed] [Google Scholar]
- 8.Francis H and Stevenson R, The longer-term impacts of Western diet on human cognition and the brain. Appetite, 2013. 63: p. 119–28. [DOI] [PubMed] [Google Scholar]
- 9.Dutheil S, et al. , High-Fat Diet Induced Anxiety and Anhedonia: Impact on Brain Homeostasis and Inflammation. Neuropsychopharmacology, 2016. 41(7): p. 1874–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carnell S, et al. , Neuroimaging and obesity: current knowledge and future directions. Obes Rev, 2012. 13(1): p. 43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chechlacz M, et al. , Diabetes dietary management alters responses to food pictures in brain regions associated with motivation and emotion: a functional magnetic resonance imaging study. Diabetologia, 2009. 52(3): p. 524–33. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz MW, et al. , Central nervous system control of food intake. Nature, 2000. 404(6778): p. 661–71. [DOI] [PubMed] [Google Scholar]
- 13.Eissele R, et al. , Glucagon-like peptide-1 cells in the gastrointestinal tract and pancreas of rat, pig and man. Eur J Clin Invest, 1992. 22(4): p. 283–91. [DOI] [PubMed] [Google Scholar]
- 14.Turton MD, et al. , A role for glucagon-like peptide-1 in the central regulation of feeding. Nature, 1996. 379(6560): p. 69–72. [DOI] [PubMed] [Google Scholar]
- 15.Daniele G, et al. , Exenatide Regulates Cerebral Glucose Metabolism in Brain Areas Associated With Glucose Homeostasis and Reward System. Diabetes, 2015. 64(10): p. 3406–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eldor R, et al. , Discordance Between Central (Brain) and Pancreatic Action of Exenatide in Lean and Obese Subjects. Diabetes Care, 2016. 39(10): p. 1804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Bloemendaal L, et al. , Effects of glucagon-like peptide 1 on appetite and body weight: focus on the CNS. J Endocrinol, 2014. 221(1): p. T1–16. [DOI] [PubMed] [Google Scholar]
- 18.Manning S, Pucci A, and Batterham RL, Roux-en-Y gastric bypass: effects on feeding behavior and underlying mechanisms. J Clin Invest, 2015. 125(3): p. 939–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frank S, et al. , Neuronal Food Reward Activity in Patients With Type 2 Diabetes With Improved Glycemic Control After Bariatric Surgery. Diabetes Care, 2016. 39(8): p. 1311–7. [DOI] [PubMed] [Google Scholar]
- 20.Rothemund Y, et al. , Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage, 2007. 37(2): p. 410–21. [DOI] [PubMed] [Google Scholar]
- 21.Stoeckel LE, et al. , Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage, 2008. 41(2): p. 636–47. [DOI] [PubMed] [Google Scholar]
- 22.Scharmuller W, et al. , Appetite regulation during food cue exposure: a comparison of normal-weight and obese women. Neurosci Lett, 2012. 518(2): p. 106–10. [DOI] [PubMed] [Google Scholar]
- 23.Linne Y, et al. , Vision and eating behavior. Obes Res, 2002. 10(2): p. 92–5. [DOI] [PubMed] [Google Scholar]
- 24.Laska M, Freist P, and Krause S, Which senses play a role in nonhuman primate food selection? A comparison between squirrel monkeys and spider monkeys. Am J Primatol, 2007. 69(3): p. 282–94. [DOI] [PubMed] [Google Scholar]
- 25.Drobes DJ, et al. , Food deprivation and emotional reactions to food cues: implications for eating disorders. Biol Psychol, 2001. 57(1–3): p. 153–77. [DOI] [PubMed] [Google Scholar]
- 26.Wallner A, et al. , Evolution of cardiovascular risk factors among 18-year-old males in Austria between 1986 and 2005. Wien Klin Wochenschr, 2010. 122(5–6): p. 152–8. [DOI] [PubMed] [Google Scholar]
- 27.Berthoud HR and Morrison C, The brain, appetite, and obesity. Annu Rev Psychol, 2008. 59: p. 55–92. [DOI] [PubMed] [Google Scholar]
- 28.van den Bos R and de Ridder D, Evolved to satisfy our immediate needs: self-control and the rewarding properties of food. Appetite, 2006. 47(1): p. 24–9. [DOI] [PubMed] [Google Scholar]
- 29.Kroese FM, Evers C, and De Ridder DT, How chocolate keeps you slim. The effect of food temptations on weight watching goal importance, intentions, and eating behavior. Appetite, 2009. 53(3): p. 430–3. [DOI] [PubMed] [Google Scholar]
- 30.Dale AM, Fischl B, and Sereno MI, Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage, 1999. 9(2): p. 179–94. [DOI] [PubMed] [Google Scholar]
- 31.Fischl B and Dale AM, Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A, 2000. 97(20): p. 11050–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salat DH, et al. , Thinning of the cerebral cortex in aging. Cereb Cortex, 2004. 14(7): p. 721–30. [DOI] [PubMed] [Google Scholar]
- 33.Van Essen DC, A Population-Average, Landmark- and Surface-based (PALS) atlas of human cerebral cortex. Neuroimage, 2005. 28(3): p. 635–62. [DOI] [PubMed] [Google Scholar]
- 34.Destrieux C, et al. , Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage, 2010. 53(1): p. 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olson IR, et al. , Using perfusion fMRI to measure continuous changes in neural activity with learning. Brain Cogn, 2006. 60(3): p. 262–71. [DOI] [PubMed] [Google Scholar]
- 36.van Bloemendaal L et al. GLP-1 receptor activation modulates appetite- and reward-related brain areas in humans. Diabetes 2014. December;63(12):4186–96 [DOI] [PubMed] [Google Scholar]
- 37.Cornier MA et al. The effects of overfeeding on the neuronal response to visual food cues in thin and reduced-obese individuals. PLoS One 2009. July 28;4(7):e6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown GG, et al. , BOLD and perfusion response to finger-thumb apposition after acetazolamide administration: differential relationship to global perfusion. J Cereb Blood Flow Metab, 2003. 23(7): p. 829–37 [DOI] [PubMed] [Google Scholar]
- 39.Selim M, et al. , The effects of body mass index on cerebral blood flow velocity. Clin Auton Res, 2008. 18(6): p. 331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stapleton PA, et al. , Obesity and vascular dysfunction. Pathophysiology, 2008. 15(2): p. 79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Milanski M, et al. , Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci, 2009. 29(2): p. 359–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arruda AP, et al. , Hypothalamic actions of tumor necrosis factor alpha provide the thermogenic core for the wastage syndrome in cachexia. Endocrinology, 2010. 151(2): p. 683–94. [DOI] [PubMed] [Google Scholar]
- 43.Thaler JP, et al. , Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest, 2012. 122(1): p. 153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chaudhuri A, et al. , Exenatide exerts a potent antiinflammatory effect. J Clin Endocrinol Metab, 2012. 97(1): p. 198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koska J, et al. , Exenatide Protects Against Glucose- and Lipid-Induced Endothelial Dysfunction: Evidence for Direct Vasodilation Effect of GLP-1 Receptor Agonists in Humans. Diabetes, 2015. 64(7): p. 2624–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakatani Y, et al. , Effects of GLP-1 Receptor Agonists on Heart Rate and the Autonomic Nervous System Using Holter Electrocardiography and Power Spectrum Analysis of Heart Rate Variability. Diabetes Care, 2016. 39(2): p. e22–3. [DOI] [PubMed] [Google Scholar]
- 47.Vaishnavi SN, et al. , Regional aerobic glycolysis in the human brain. Proc Natl Acad Sci U S A, 2010. 107(41): p. 17757–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kullmann S, et al. , Brain Insulin Resistance at the Crossroads of Metabolic and Cognitive Disorders in Humans. Physiol Rev, 2016. 96(4): p. 1169–209. [DOI] [PubMed] [Google Scholar]
- 49.Farr OM and Mantzoros CS, Obese individuals with type 2 diabetes demonstrate decreased activation of the salience-related insula and increased activation of the emotion/salience-related amygdala to visual food cues compared to non-obese individuals with diabetes: A preliminary study. Diabetes Obes Metab, 2018. [DOI] [PMC free article] [PubMed]
- 50.Drewnowski A and Greenwood MR, Cream and sugar: human preferences for high-fat foods. Physiol Behav, 1983. 30(4): p. 629–33. [DOI] [PubMed] [Google Scholar]