Abstract
Alcohol consumption is often characterized by heavy episodic, or binge drinking, which has been on the rise. The aim of this study was to examine the neural dynamics of inhibitory control in demographically matched groups of young, healthy adults (N=61) who reported engaging in binge (BD) or light drinking patterns (LD). Electroencephalography signal was recorded during a fast-paced visual Go/NoGo paradigm probing the ability to inhibit prepotent responses. No group differences were found in task performance. BDs showed attenuated event-related theta (4–7 Hz) on inhibition trials compared to LDs, which correlated with binge episodes and alcohol consumption but not with measures of mood or disposition including impulsivity. A greater overall decrease of early beta power (15–25 Hz) in BDs may indicate deficient preparatory “inhibitory brake” before deliberate responding. The results are consistent with deficits in the inhibitory control circuitry and are suggestive of allostatic neuroadaptive changes associated with binge drinking.
Keywords: binge drinking, alcohol, EEG, theta, beta, oscillations, Go/NoGo, inhibitory control, response inhibition
Introduction
Binge drinking is defined as alcohol consumption elevating the blood alcohol concentration (BAC) levels to at least 0.08 g/dL, which usually occurs when four/five drinks are consumed by women or men, respectively, within two hours (National Institute on Alcohol Abuse and Alcoholism, 2017). However, many individuals exceed this level of intake and consume alcohol at much higher levels (Naimi et al., 2010; Terry-McElrath and Patrick, 2016). Binge pattern of excessive drinking is associated with a range of negative consequences and incurs high costs to society (Bouchery et al., 2011; Sacks et al., 2015). It represents a major public health concern given rising prevalence rates (Hingson et al., 2017), and the evidence that harmful consequences increase with hazardous drinking levels (Haber et al., 2016).
It has been proposed that binge drinking may be a precursor to alcohol use disorder (AUD) as alcohol consumption transitions from impulsivity to compulsivity (Kimbrough et al., 2017; Koob, 2013; Koob and Le Moal, 2008a). Indeed, increased alcohol consumption is associated with impaired self-control which could contribute to excessive drinking and may predict future heavy drinking and alcohol dependence (Nigg et al., 2006; Paz et al., 2018). Behavioral disinhibition is considered to be an important dimension in the development of AUD (Goldstein and Volkow, 2011; Koob and Volkow, 2010; Kwako et al., 2016; Volkow et al., 2002) and prefrontally-mediated deficits of inhibitory control and other executive functions have been found in individuals with AUD (Le Berre et al., 2017; Oscar-Berman and Marinkovic, 2004, 2007; Sullivan and Pfefferbaum, 2005). Furthermore, neuroimaging evidence indicates that acute alcohol intoxication primarily affects the prefrontal neurofunctional system subserving top-down cognitive control (Anderson et al., 2011; Kovacevic et al., 2012; Marinkovic et al., 2012a; Marinkovic et al., 2013; Rosen et al., 2016), including impairments of response inhibition (Gan et al., 2014; Kareken et al., 2013; Marinkovic et al., 2000; Nikolaou et al., 2013; Schuckit et al., 2012).
Inhibitory control relies on the ability to suppress inappropriate or unwanted actions (Aron et al., 2014; Chikazoe et al., 2007), but it also critically engages other cognitive functions including attentional control and working memory (Erika-Florence et al., 2014; Hampshire, 2015). It has been studied extensively with tasks that demand stopping or withholding dominant responses, such as a Go/NoGo task (Aron et al., 2014; Simmonds et al., 2008). This paradigm instructs participants to rapidly respond to target or “Go” stimuli (response activation), and to withhold responding to occasional “NoGo” stimuli (response inhibition) (Garavan et al., 1999). Functional magnetic resonance imaging (fMRI) studies have indicated that successful performance on the Go/NoGo task primarily recruits prefrontal regions, including the ventral and lateral prefrontal cortices, the anterior cingulate cortex (ACC), the presupplementary motor area (preSMA), and the basal ganglia among others (Aron et al., 2014; Criaud and Boulinguez, 2013; Hampshire et al., 2010; Levy and Wagner, 2011; Simmonds et al., 2008; Swick et al., 2011; Wiecki and Frank, 2013). Although fMRI is an excellent spatial mapping tool, its temporal resolution is low due to constraints imposed by neurovascular coupling (Buxton, 2002). In contrast, scalp electroencephalography (EEG) measures neural activity directly and can provide highly precise insight into the task-evoked neural activity in real time but its spatial resolution is limited due to biophysical properties of the signal (Nunez and Srinivasan, 2006).
Because of its oscillatory nature, the EEG signal can be analyzed within the relevant frequency bands during task engagement (Amzica and Lopes da Silva, 2011; Basar et al., 2001; Engel and Fries, 2010; Lundqvist et al., 2018; Pfurtscheller and Lopes da Silva, 1999). Event-related theta oscillations (4 – 7 Hz) are sensitive to cognitive effort elicited by tasks probing cognitive control and performance monitoring (Brier et al., 2010; Cavanagh and Frank, 2014; Hanslmayr et al., 2008; Kovacevic et al., 2012; Rosen et al., 2016). Studies using source-localization of the magnetoencephalography (MEG) and EEG signal have shown that the ACC and preSMA in the medial prefrontal cortex are major generators of event-related theta oscillations during such tasks (Hanslmayr et al., 2008; Kovacevic et al., 2012; Marinkovic et al., 2019; Marinkovic et al., 2012b). These observations have been confirmed with intracranial EEG recordings which have revealed that the ACC is a principal generator of the fronto-midline theta observed on the scalp (Cohen et al., 2008; Wang et al., 2005). Additional sources have been reported in the lateral prefrontal cortex (Beaton et al., 2018; Correas et al., 2018; Kovacevic et al., 2012; Marinkovic et al., 2019; Raghavachari et al., 2001; Rosen et al., 2016).
Studies manipulating acute alcohol intoxication have shown that event-related theta oscillations are attenuated by a moderate alcohol dose especially under high-conflict conditions during decision making (Beaton et al., 2018; Kovacevic et al., 2012; Marinkovic et al., 2019; Marinkovic et al., 2012b; Rosen et al., 2016). Based on their association with AUD in genetic linkage studies, theta oscillations have been suggested as an endophenotype indicating a predisposition to develop alcoholism or inhibitory-related disorders (Rangaswamy et al., 2007; Salvatore et al., 2015). However, the supporting evidence of theta involvement in inhibitory control in binge drinkers or individuals with AUD is scarce. Most of the extant studies have used an equiprobable (50:50) Go/NoGo design which biases responding strategy towards target detection at the expense of inhibitory control (Wessel, 2018). Since the Go (target) stimuli are much more salient for task performance, they evoke greater prefrontal theta when measured with MEG, which is attenuated in BDs (Correas et al., 2018). EEG studies with equiprobable Go/NoGo tasks in young BDs have reported reduced theta power in BDs to both target and nontarget stimuli (Lopez-Caneda et al., 2017). Similarly, in two EEG studies using this design, abstaining individuals with chronic AUD, and offspring of individuals with AUD who were deemed to be at high risk for developing alcoholism, showed lower event-related theta power than control participants (Kamarajan et al., 2006; Kamarajan et al., 2004; Pandey et al., 2016). However, studies using theta to examine inhibitory control in binge drinkers or in individuals with AUD with an asymmetric Go/NoGo design are lacking. To address this gap in the literature, the present study employed a fast-pace task variant with a 80:20 Go/NoGo ratio which establishes Go response dominance and engages inhibitory control on NoGo trials (Wessel, 2018). This has allowed us to test the hypothesis that binge drinking is associated with impaired inhibitory control and to examine whether this is reflected in attenuated task-dependent theta oscillations.
Furthermore, because this task requires countermanding of a prepotent tendency to respond, we examined task-dependent beta oscillations (15–25 Hz) which provide temporally precise insight into anticipatory motor engagement, response preparation, inhibition, and execution. Beta oscillations are considered to be the preferred frequency of the sensorimotor system and can serve as an index of the functional engagement of the underlying cortico-subcortical circuitry (Baker, 2007; Jenkinson and Brown, 2011; Khanna and Carmena, 2017; Kilavik et al., 2013). They are particularly sensitive to the neural activity related to movement activation and inhibition (Engel and Fries, 2010; Jenkinson and Brown, 2011) and are affected by alcohol intoxication (Marinkovic et al., 2000), but they have not been examined in the context of binge drinking. Unlike event-related theta power which increases in response to a salient stimulus, beta power is high at baseline and it decreases during anticipatory, actual, or even imagined engagement of the motor system. Following a potential brief beta increase that may be inhibitory in nature (Pogosyan et al., 2009; Swann et al., 2009), beta decrease (also termed “desynchronization”) is the principal characteristic of event-related beta power. It is easily observed during movement preparation as it presumably indicates readiness to execute a motor response (Baker, 2007; Engel and Fries, 2010; Jenkinson and Brown, 2011; Kilavik et al., 2013). The beta decrease is most dominant over the sensorimotor cortices which are the primary generators of the observed beta changes (Beaton et al., 2018). After a command to execute or inhibit a movement has been issued but before the actual response, beta power rebounds and increases above baseline levels (Cheyne et al., 2006; Kilavik et al., 2013). The beta rebound has shorter latency on NoGo trials on which there is no actual response, which can be interpreted as an active inhibition process (Khanna and Carmena, 2017; Solis-Escalante et al., 2012). These features make event-related beta oscillations well suited for tracking response preparation and execution stages, as well as post-movement adjustments of the motor system in real time (Beaton et al., 2018; Jenkinson and Brown, 2011). As the Go/NoGo task probes inhibitory control with potential relevance to self-control dysregulation which is implicated in addiction (Baler and Volkow, 2006; Leeman et al., 2012), investigating beta oscillatory activity in binge drinkers is of particular interest.
The aim of the current study was to examine the neural dynamics of inhibitory control in young adults with and without histories of binge drinking. Using a visual Go/NoGo task, the present study focused on task-dependent event-related changes in theta (4 – 7 Hz) and beta (15 – 25 Hz) oscillations in order examine the neural indices of cognitive and motor aspects of inhibitory control respectively in young adults engaging in binge drinking. We hypothesized that individuals with a history of binge drinking would exhibit impaired inhibitory control manifested in suboptimal task performance, decreased event-related theta power on NoGo trials, and alterations in the pattern of beta activity during response preparation.
Methods
Participants
Sixty-one healthy, non-smoking, right-handed individuals (M ± SD = 23.41 ± 3.4 years of age, 31 females) participated in this study. They were recruited from the local community through approved ads and postings and were queried about their alcohol and drug use and health history in a brief telephone screen interview. None of the participants reported drug or tobacco use at least one month prior to the study. They had no history of brain injury, or other neuropsychiatric or medical problems, and none were taking medications at the time of the study. In the present study, a binge episode was defined as consuming six/five or more drinks for men/women within a two hour time span. This criterion was adopted based on empirical evidence indicating that this level of drinking is more likely to result in BAC reaching or exceeding 0.08% (Lange and Voas, 2001). Based on screening information, 29 individuals were classified as binge drinkers (BD) if they reported three or more binge episodes in the past six months with at least one episode in the last month. On average, BDs reported 14.09 ± 13.6 binge episodes in the past six months (Table 1), with median = 10, mode = 20, range = 3 to 72. The high end of the range was reported by a participant who weighed the most in the entire cohort. The next highest number of reported binge episodes in the past six months was 30. The remaining 32 participants were Low Drinkers (LD), who reported no more than one binge episode in the past six months, .09 ± .3 on average. The two groups were matched on age, sex, education, ethnicity/race, and family history of alcoholism. The study’s procedures were approved by the Institutional Review Board and written informed consent was obtained from each participant prior to the experiment. Participants were monetarily compensated for their participation. The data of four additional participants in the theta analysis and three from the beta analysis were discarded due to poor data quality.
Table 1.
Participant characteristics. For each group, means ± standard deviations are included for each variable. Group comparisons are expressed as Mann-Whitney U-values or χ2 (marked with 1).
| BD (n = 29) | LD (n = 32) | Statistical Value | p | |
|---|---|---|---|---|
| Age | 23.41 ± 3.5 | 23.41 ± 3.4 | 460 | .954 |
| % Female | 51.7% | 50% | .0151 | .903 |
| White/Non-Hispanic | 65.5% | 71.9% | .0671 | .796 |
| Family history of alcoholism | 55% | 44% | .4031 | .526 |
| Undergraduate GPA | 3.13 ± .5 | 3.44 ± .4 | 277 | < .05 |
| Education years | 15.79 ± 2 | 16 ± 2 | 416 | .483 |
| In the past six months | ||||
| Binge episodes | 14.09 ± 13.6 | .09 ± .3 | 0 | < .001 |
| Blackouts | 4.66 ± 3.7 | .03 ± .2 | 2.5 | < .001 |
| Drinking days/week | 3.21 ± 1.3 | 1.66 ± .8 | 135.5 | < .001 |
| Drinks per occasion | 5.52 ± 1.5 | 1.81 ± .9 | 18.5 | < .001 |
| Drinks consumed per week | 17.72 ± 8.6 | 3.27 ± 2.3 | 27.5 | < .001 |
| Age of first drink | 17.25 ± 2.2 | 18.45 ± 2.1 | 163.5 | < .001 |
| Max. number of drinks in 24 hrs | 12.09 ± 5.7 | 4.73 ± 2.2 | 32 | < .001 |
| No. times felt drunk past month | 5.74 ± 4.8 | 2.00 ± 1.7 | 157 | < .001 |
| Consequences of alcohol (YAACQ) | 11.07 ± 5.3 | 2 ± 1.9 | 41 | < .001 |
| Alcoholism-related symptoms (SMAST) | 3.36 ± 3.3 | .56 ± .9 | 202 | < .001 |
| Drinking motives (DMQ) | 1.99 ± .4 | 1.64 ± .3 | 192 | < .001 |
| Anxiety (GAD) | 4.61 ± 5.4 | 2.38 ± 3.2 | 326.5 | .065 |
| Depression (PHQ) | 4.86 ± 5 | 2.13 ± 2 | 322.5 | .059 |
| Impulsivity (ABIS) | 2.06 ± .5 | 1.83 ± .3 | 319 | .055 |
| Sensation Seeking (BSSS) | 3.75 ± .7 | 3.39 ± .7 | 309.5 | < .05 |
| EPQ | ||||
| Neuroticism | 3.86 ± 3.52 | 3.44 ± 3.34 | 411.5 | .585 |
| Psychoticism | 2.54 ± 2.12 | 2.28 ± 1.63 | 428 | .763 |
| Extraversion | 9.43 ± 2.43 | 8.22 ± 3.5 | 371 | .248 |
| NIH Toolbox | ||||
| Working Memory | 0.76 ± 0.10 | 0.74 ± 0.11 | 400.5 | .353 |
| Dimensional Shift | 0.87 ± 0.14 | 0.92 ± 0.05 | 349 | .097 |
| Processing Speed | 0.58 ± 0.07 | 0.6 ± 0.09 | 382 | .236 |
| Episodic Memory | 0.78 ± 0.17 | 0.8 ± 0.15 | 395 | .550 |
Procedure
Participants completed a battery of questionnaires which included handedness (Oldfield, 1971) and medical history. They were asked about their alcohol drinking habits, including the frequency, quantity, and the pattern of alcohol consumption (modified from Cahalan et al., 1969), the magnitude of response to alcohol (Self-Rating of the Effects of Alcohol, SRE, Schuckit et al., 1997), the severity of their alcoholism-related symptoms (Short Michigan Alcoholism Screening Test, SMAST, Selzer et al., 1975), their motives for engaging in alcohol use (Drinking Motive Questionnaire, DMQ, Kuntsche and Kuntsche, 2009), and the consequences of their drinking (Young Adult Alcohol Consequences Questionnaire, YAACQ, Read et al., 2006). They provided a detailed report on their daily drinking during the past month (Timeline Followback, TLFB, Sobell and Sobell, 1996). Their disinhibition and impulsivity traits were assessed by an abbreviated Impulsiveness Scale (Abbreviated Impulsiveness Scale, ABIS, Coutlee et al., 2014). Participants also completed questionnaires to measure their personality (Eysenck Personality Questionnaire, EPQ, Eysenck and Eysenck, 1975), depression (Patient Health Questionnaire-9, PHQ, Kroenke and Spitzer, 2002), and anxiety (Generalized Anxiety Disorder, GAD, Spitzer et al., 2006). In addition, they completed the NIH Toolbox Cognitive Battery (Gershon et al., 2013) which included tests probing working memory, cognitive flexibility, processing speed, and episodic memory (Table 1). Participants were screened for drug use with a 12-panel urine multidrug test (Discover, American Screening Corporation) at the beginning of the recording session. They all tested negative and proceeded with the recording.
Experimental paradigm
Participants took part in a visual Go/NoGo task which probes the ability to inhibit prepotent responses. They were presented with a pseudorandomized series of ‘X’ and ‘Y’ letters and were instructed to press a button with their right index finger as quickly and as accurately as possible every time ‘X’ and ‘Y’ stimuli alternated (Go, 80% of trials) and to withhold responding when the stimuli repeated (NoGo, 20% of trials) (Garavan et al., 1999). The task comprised a total of 685 stimuli presented for 230 ms with a stimulus onset asynchrony (SOA) of 1400 ± 200 ms. A random jitter was added to each trial in 50 ms increments to mitigate timing predictability. Stimuli were presented individually in white font on a black background with the Presentation software package (Version 18.1; www.neurobs.com) within a visual angle spanning .93° (horizontal) and 0.99° (vertical). At all other times a fixation dot was presented in the middle of the screen.
Data acquisition and analysis
EEG signal was recorded with a 64-channel Brain Vision system (Brain Products GmbH, Germany) and was sampled continuously at 500 Hz. The signal was referenced online to the nose, and a bipolarly referred vertical electro-oculogram (EOG) was recorded to monitor eyeblinks and eye movements. Electrode impedance was kept below 5 kΩ.
Data Preprocessing
EEG data were analyzed using MATLAB (Mathworks, Natick, MA) routines that incorporated publicly available algorithms including FieldTrip (Oostenveld et al., 2011), and EEGLAB (Delorme and Makeig, 2004). Continuous data were band-pass filtered at 0.1 – 100 Hz, and were segmented into epochs extending from −300 to 800 ms relative to each stimulus onset. A 300 ms pad was added to the beginning and end of the epoch to account for edge artifacts resulting from the Morlet wavelet convolution (Oostenveld et al., 2011). Noisy channels were removed by visual inspection and trials with large artifacts were removed with a threshold-based rejection. The default threshold started at 100μV focusing on the posterior electrodes, but was adjusted for each participant as needed with the goal of rejecting large artifacts while keeping most trials with eyeblinks. This helped to optimize an independent component analysis (ICA) method (Delorme and Makeig, 2004) which was then used to detect and remove the eyeblink and heartbeat artifacts. Data were analyzed in the time-frequency domain by computing complex power spectrum of each trial with Morlet wavelets within the theta (4–7 Hz) and beta (15–25 Hz) bands (Beaton et al., 2018; Kovacevic et al., 2012). The wavelet results were additionally inspected for artifacts and the padding was removed. The analysis was conducted in a manner blind to group membership. Average event-related power is presented as percent signal change from the baseline (−300 to 0 ms). Analysis of the raw power in the baseline indicated that the two groups did not differ in either theta or beta bands, assuring that the observed group differences were indeed due to event-related changes in power.
Data analysis
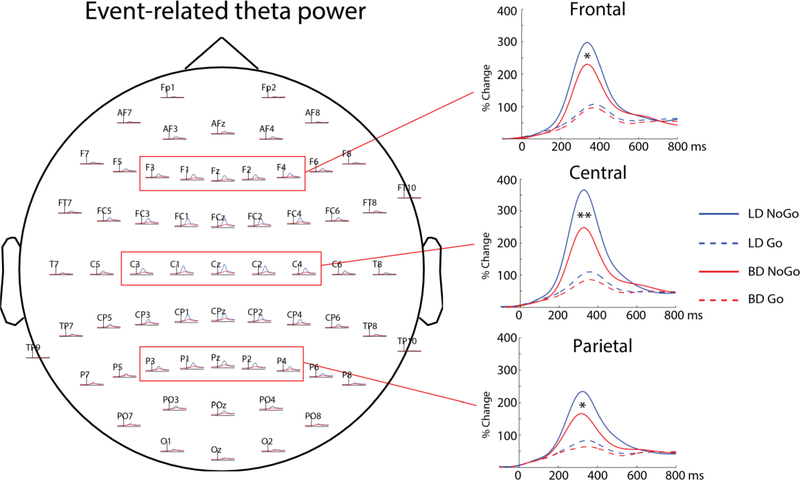
Data were analyzed for each channel which were then grouped into frontal (Fz, F1, F2, F3, F4), central (Cz, C1, C2, C3, C4) and parietal (Pz, P1, P2, P3, P4) clusters and averaged within each cluster to analyze group and condition effects on theta power (see Fig 2). For beta, only the central (Cz, C1, C2, C3, C4) electrode region was used (see Fig 3) to capture activity of the sensorimotor cortices which are the primary generators of event-related changes in beta oscillations (Baker, 2007; Beaton et al., 2018). Only trials on which responses were correctly executed (Go) and withheld (NoGo) were included in the analysis. By incorporating Go and NoGo trials in a 4:1 ratio, this task creates a prepotency to respond. As a consequence, effortful response inhibition is needed to overcome it and withhold responses on NoGo trials. This response dominance also leads to occasional premature button pressing. All responses made between −250 ms before and 200 ms after the stimulus onset were counted as premature and were excluded from the analysis.
Statistical analysis
Group differences in demographics were tested with χ2, and those in drinking habits, personality aspects, and cognitive functions were analyzed with a non-parametric Mann-Whitney U test to account for possible violations of distribution normality (Table 1). Data were analyzed with a mixed-design ANCOVA with Group as the between-subjects factor, Task Condition as the repeated measures factor, and impulsivity (Abbreviated Impulsiveness Scale, ABIS, Coutlee et al., 2014) as a covariate. Group differences between the frontal, central, and parietal clusters were additionally examined for theta. No effects of sex were observed in the initial analyses for either the behavioral or electrophysiological data so this factor was subsequently removed from the analysis. Associations between the principal EEG measures, representative drinking variables, and several dispositional indices were examined with a non-parametric Spearman’s Rho (rs) index that was calculated across the whole sample. The following EEG measures were included in the correlational analysis: theta NoGo, theta Go, and early beta averaged across both task conditions. Drinking variables comprised the number of binge episodes, maximum number of drinks in 24 hrs, average daily alcohol intake, and number of drinking days per week, all assessed over the past 6 months. Mood and personality variables included anxiety, depression, and impulsivity. A false discovery rate approach (.20) (Hochberg and Benjamini, 1990) was used to correct for multiple correlations.
Results
Behavioral measures
Performance
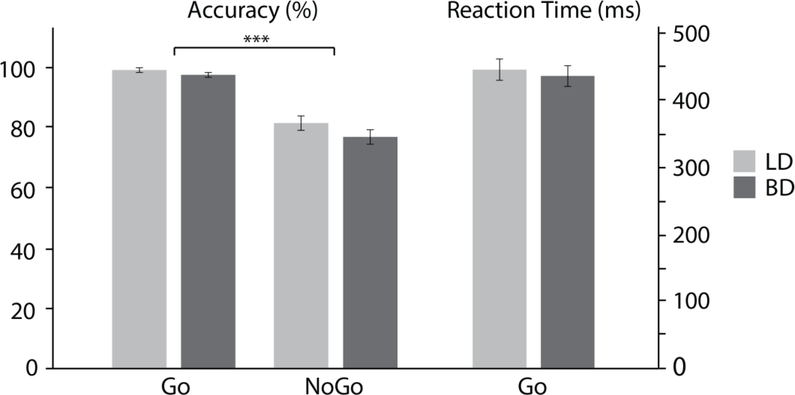
As shown in Fig 1, participants responded more accurately to Go trials (98.3% ± 3.6) than to NoGo trials (79.1% ± 12.2) resulting in a main effect of condition, F(1, 59) = 136.61, p < .001. No group differences were observed for response accuracy on either Go, F(1, 59) = .39, p = .54, or NoGo trials, F(1, 59) = .06, p = .81. The LD (447.1ms ± 88.2) and BD (441.5ms ± 77.9) groups responded with comparable speed, F(1, 59) = .07, p = .79.
Figure 1.
Accuracy and reaction times (means ± standard errors) are shown for the low drinking (LD) and binge drinking (BD) groups and for the Go and NoGo conditions.
Drinking habits, personality characteristics, and cognitive functions
Table 1. lists demographic characteristics and group differences in drinking habits, experiences and motivational dimensions, personality traits, dispositional mood measures, and cognitive functions. BDs reported more binge episodes in the previous six months than LDs, higher levels of alcohol consumption overall, they started drinking at an earlier age than LDs and experienced more negative consequences of drinking including blackouts. They expressed higher levels of social, coping, and enhancement drinking motives. BDs reported higher sensation seeking, and marginally higher levels of impulsivity, anxiety, and depression than LDs. However, the two groups did not differ on personality traits nor on cognitive tests.
Electrophysiological measures
Event-related theta power
Event-related theta power peaked at ~350 ms after stimulus onset so the effects of Group and Condition were analyzed within a time interval of 300 to 400 ms (Fig 2) to capture peak event-related changes while controlling for impulsivity. Overall, there was a main effect of Condition, as NoGo trials elicited greater event-related theta power than Go trials, F(1, 56) = 7.9, p = .007. A Group x Condition interaction, F(1, 56) = 5.7, p = .02 was due to theta attenuation for NoGo trials in the BD group, F(1, 56) = 8.27, p = .006, with group differences on Go trials not reaching significance, F(1, 56) = 1.70, p = .19. Region-specific analysis indicated that, compared to LDs, BDs had reduced theta power on NoGo trials at the frontal, F(1, 56) = 5.2, p = .03, central, F(1, 56) = 9.75, p =.003, and parietal, F(1, 56) = 5.8, p = .02, electrode regions. In contrast, group differences on Go trials did not reach significance for any electrode cluster including the frontal, F(1, 56) = .41, p = .52, central, F(1, 56) = 3.18, p =.08, and parietal, F(1, 56) = 1.87, p = .18 regions. Lower theta during response inhibition was associated with higher levels of drinking, as NoGo theta power correlated negatively with the number of reported binge episodes, rs = −.29, p = .03, daily alcohol intake, rs = −.26, p = .04, and the average number of weekly drinking days, rs = −.25, p = .05. The maximum number of drinks consumed in 24 hrs in the previous six months correlated with theta power on NoGo, rs = −.29, p = .04, and Go trials, rs = −.29, p = .04. None of the dispositional variables were related to theta, all coefficients < .07, all p-values > .6.
Figure 2.
Frontal, central, and parietal electrode clusters and group average time courses for event-related theta power averaged within each cluster. Overall, event-related theta power was greater on NoGo trials, it peaked at ~350 ms, and it was most prominent in the central region. BD participants had reduced NoGo theta power in all three regions compared to LDs. *p < .05, **p < .01
Event-related beta power
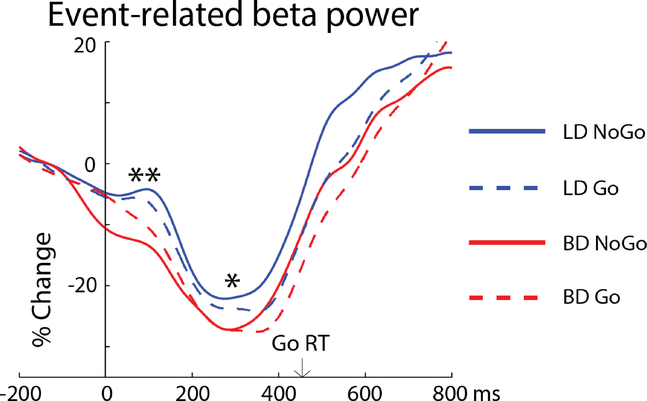
Event-related beta power is also expressed as percent signal change from baseline (Fig 3). It starts decreasing prior to stimulus onset in anticipation of making motor movement over the sensorimotor cortices. An early, transient increase in beta power during preparatory stage is visible in LDs, followed by an overall beta decrease with a nadir at ~300 ms and a rebound of beta power subsequent to issuing a motor command. A main effect of Group was observed within 50–125 ms time window, as BDs had greater beta desynchronization than LDs, F(1, 56) = 8.08, p = .006 (Fig 3). Following the early transient increase in beta power, the LD group maintained an overall higher level of beta power. This was reflected in a main effect of Group as measured at the beta nadir (250–350ms), F(1, 56) = 5.06, p = .028 which, however, correlated with the early time interval, rs = .51, p < .001. As expected, beta power rebounded earlier on inhibitory NoGo trials, which was confirmed by a main effect of Condition (500–600ms), F(1, 56) = 10.33, p = .002. No group differences were observed during the beta rebound, F(1, 56) = 0.67, p = .42.
Figure 3.
Event-related beta power time course averaged over the central electrode cluster. LD participants show an early, transient increase in beta power (50 – 125 ms). * p < .05, ** p < .01. Average Go reaction time is marked by arrow.
Discussion
The present study examined the neural dynamics of inhibitory control in young adults as a function of their drinking patterns. In the absence of differences in task performance, BD and LD groups differed on the neural indices of the engagement of cognitive control and the circuitry subserving response preparation. Event-related theta oscillations (4 – 7 Hz) were attenuated in BDs compared to LDs on trials requiring response inhibition as shown by the Group x Condition interaction, which may indicate less efficient long-range top-down integration engaged by the salient response suppression requirement. Decreased theta power on NoGo trials was associated with increased levels of binge and high-intensity drinking, and alcohol consumptions levels but not dispositional or mood measures. An early, transient increase of event-related beta power (15 – 25 Hz) was observed in LDs which is consistent with a brief “braking pause” during response preparation which may underlie deliberate decision to response or withhold responding and which immediately precedes issuance of the motor execution or inhibition commands. In contrast, BDs showed only a beta decrease which may be indicative of a deficient engagement of response inhibition mechanisms. Even though the correlations between the early beta power and drinking variables did not survive correction for multiple correlations, the lower levels of inhibition during the motor preparatory stage may be suggestive of allostatic neuroadaptive changes in neural transmission as a result of heavy episodic drinking patterns. Group differences in both theta and beta frequency bands were significant after controlling for self-reported impulsivity.
A Go/NoGo task with 80% Go trials, such as the one used in the current study, probes inhibitory control by creating a prepotency to respond (Aron et al., 2014; Garavan et al., 1999; Wessel, 2018), as participants are required to withhold responding on a minority of trials. Because theta oscillations are associated with engagement of top-down cognitive control functions (Cavanagh and Frank, 2014; Kovacevic et al., 2012; Marinkovic et al., 2019; Rosen et al., 2016; Yamanaka and Yamamoto, 2010), they are well suited to examine the cognitive processes associated with behavioral control. In the current study, NoGo trials elicited much greater event-related theta power than Go trials, as would be expected based on their salience and inhibitory demands, in addition to their low presentation frequency, and task relevance. A significant Group x Condition interaction indicated that BDs exhibited attenuated theta activity selectively on NoGo trials (Fig 2), suggesting that binge drinking may be primarily associated with impaired processes that underlie inhibitory control. This novel finding is broadly consistent with previous reports of the selective vulnerability of the top-down circuitry underlying inhibitory control to alcohol intoxication (Anderson et al., 2011; Gan et al., 2014; Kareken et al., 2013; Kovacevic et al., 2012; Marinkovic et al., 2019; Marinkovic et al., 2012a; Marinkovic et al., 2013; Nikolaou et al., 2013; Rosen et al., 2016; Schuckit et al., 2012). Though less directly pertinent to inhibitory control per se, lower theta has been reported in studies employing equiprobable Go/NoGo tasks in large groups of individuals with AUD (Kamarajan et al., 2004; Pandey et al., 2016) and in young adult binge drinkers (Correas et al., 2018; Lopez-Caneda et al., 2017).
Functional imaging studies have reported decreased activity on NoGo or Stop-signal trials in BDs which correlated with measures of alcohol intake (Ahmadi et al., 2013; Hu et al., 2016) and impulsivity (Ahmadi et al., 2013). It has been proposed that protracted heavy alcohol intake is accompanied by incremental degradation of cognitive and motivational functions and that the resulting disinhibition, as reflected in impaired self-control, plays a major role in addiction (Crews et al., 2016; Field et al., 2008; Goldstein and Volkow, 2002, 2011; Koob and Volkow, 2010; Kwako et al., 2016; Volkow et al., 2002). Systematic reviews have confirmed deficient activity in the inhibitory control network across a range of addictions (Luijten et al., 2014). This is broadly consistent with our findings of negative correlations between NoGo theta power and a range of drinking variables including the number of self-reported binge episodes and weekly drinking levels.
Previous studies have shown that impulsivity and other externalizing traits can predict future alcohol use (Finn, 2000; Littlefield et al., 2014; Regier et al., 1990; Verdejo-Garcia et al., 2008). Indeed, dysregulation of impulse control concerns the inability to resist engaging in the activity that one declares to be unwanted or even harmful. The inability to maintain inhibitory control over drinking has been considered by some researchers to be fundamental to drug abuse (Field et al., 2010; Fillmore, 2003; Finn, 2000; Jentsch and Taylor, 1999; Sher and Trull, 1994). Evidence suggests that the vulnerability to alcoholism shares a common genetic component with externalizing traits which may predispose individuals to a spectrum disorders including AUD (Begleiter and Porjesz, 1999; Dick et al., 2004; Heinz et al., 2011; Pihl et al., 1993; Schuckit et al., 2004). The current findings suggest that impulsivity as measured with ABIS (Coutlee et al., 2014) did not drive the observed group effects on event-related theta power on inhibitory (NoGo) trials which prevail when controlling for self-reported impulsivity, leading us to believe that these two systems have separate mediators. However, group differences indicating greater readiness to respond on the part of BDs emerged from the analysis of beta oscillations.
In an effort to investigate the neural characteristics of the ability to suppress a prepotent tendency to respond, we have analyzed event-related beta oscillations which are sensitive to motor preparation. Beta oscillations are thought to reflect functional interactions between the neocortex and the basal ganglia as beta power typically decreases in a lateralized and anticipatory manner during movement preparation and execution (Baker, 2007; Jenkinson and Brown, 2011; Kilavik et al., 2013) with a maximal nadir over the sensorimotor cortex (Beaton et al., 2018; Litvak et al., 2011). In the current study, LDs had an early, transient increase in the overall beta power at ~100 ms in contrast to BDs who showed only beta desynchronization. Beta increase is associated with motor inhibition (Khanna and Carmena, 2017; Pogosyan et al., 2009; Swann et al., 2009) so this brief rise is suggestive of a momentary, transient “inhibitory pause” prior to issuing the final motor command to execute the response. It has been well established that motor inhibition is subserved by the indirect pathway comprising cortical excitation of the striatum which inhibits the subthalamic-pallidal output to the thalamus and the cortex resulting in motor hypoactivity (Haynes and Haber, 2013; Lanciego et al., 2012; Zavala et al., 2015). Short latency of this transient beta increase is consistent with engagement of the cortico-subthalamic hyperdirect pathway which underlies rapid response suppression (Frank, 2006; Nambu et al., 2002; Wessel and Aron, 2017). This finding suggests that in LDs, the motor response sequence incorporates a brief inhibitory stage that may facilitate a deliberate decision to respond or to withhold responding possibly via lateral competition of alternative activations (Tunstall et al., 2002). In contrast, BDs did not exhibit this early beta increase which is consistent with their greater readiness to respond. Given that BDs regularly imbibe alcohol at higher levels and have more high-intensity drinking episodes than BDs, it is possible that the observed dysregulation of the early motor preparation phase reflects neural hyperexcitability. Indeed, we have reported findings on other neural indices indicating decreased inhibitory signaling during wakeful rest in binge drinkers (Affan et al., 2018). These observations are consistent with allostatic neuroadaptive changes (Clapp et al., 2008; Koob and Le Moal, 2005, 2008b) whereby hazardous drinking results in downregulation of inhibitory and upregulation of excitatory signaling (Finn and Crabbe, 1997; Most et al., 2014; Roberto and Varodayan, 2017; Vengeliene et al., 2008). With the majority of intrinsic and efferent fibers being GABAergic (Lanciego et al., 2012), the basal ganglia are particularly vulnerable to the effects of binge-like drinking which has been reported in animal models (Cuzon Carlson et al., 2011; Wilcox et al., 2014) and human postmortem studies (Laukkanen et al., 2013).
In the current study the BD and LD groups did not differ in task performance despite clear group differences in both event-related theta and beta bands. This finding is consistent with many other EEG studies reporting group differences on neural measures in the absence of behavioral deficits (Crego et al., 2012; Crego et al., 2009; Crego et al., 2010; Lopez-Caneda et al., 2013; Lopez-Caneda et al., 2012; Lopez-Caneda et al., 2017; Maurage et al., 2009; Petit et al., 2012). This divergence between the behavioral and direct measures of neural activity is indicative of greater EEG sensitivity to neural deficits associated with the intermittent pattern of high-level drinking. Because binge drinking has been conceptualized as a transitional stage in a cyclic process potentially leading towards compulsive intake (Kimbrough et al., 2017; Koob, 2013; Koob and Le Moal, 2008a), EEG measures could potentially serve as biomarkers signifying transition to dependence.
Despite the notable novel findings of this study, there are also limitations that should be mentioned. The study employed a relatively small sample size which precluded a well-powered investigation of possible sex differences in inhibitory control. Though novel and unique, the findings of an early beta decrease in BDs that potentially signify deficient response inhibition should be replicated in a larger cohort of binge drinkers, as well as individuals with AUD.
In conclusion, the present study used EEG and a visual Go/NoGo task to examine the neural dynamics of inhibitory control in BDs in an effort to address existing gaps in the literature. Compared to LDs, BDs showed reduced event-related theta power on NoGo trials, suggesting that binge drinking is associated with deficits in the top-down circuitry subserving inhibitory control. A unique and novel finding was an early reduction in event-related beta power in BDs, which may indicate a deficient preparatory “inhibitory brake” in these individuals which may be suggestive of allostatic neuroadaptive changes associated with binge drinking. The present study has contributed novel insights into the alterations of cognitive and motor aspects of inhibitory control in binge drinkers in the absence of performance deficits. Because binge drinking has been proposed as a transitional phase leading to chronic alcoholism, the present findings may inform future studies on heavy alcohol use. The alterations in brain signals could potentially serve as diagnostic indicators of a transition to dependence. When paired with alcohol-related cues, Go/NoGo paradigms can enhance neurofeedback-based preventive strategies focusing on inhibitory control for those at risk of developing alcoholism.
Highlights.
Binge (BDs) and light drinkers (LDs) do not differ in Go/NoGo performance
No group differences on cognition, personality; higher sensation seeking in BDs
Inhibitory NoGo trials evoke much greater event-related theta power than Go trials
Theta power is lower to NoGo trials in BDs and it correlates with drinking
Early beta decrease in BDs may indicate greater response readiness
Acknowledgements
This work was supported by start-up funds from the College of Sciences at San Diego State University and the National Institute on Alcohol Abuse and Alcoholism (R01-AA016624). The authors are grateful to Rifqi Affan, Audrey Andrews, Nicole Fong, and Morgan Slauter for assistance with data acquisition, and to Lauren Beaton, Laura Wagner, Joe Happer, and Martina Knezevic for assistance with data analysis and manuscript preparation.
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Affan RO, Huang S, Cruz SM, Holcomb LA, Nguyen E, Marinkovic K, 2018. High-intensity binge drinking is associated with alterations in spontaneous neural oscillations in young adults. Alcohol 70, 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadi A, Pearlson GD, Meda SA, Dager A, Potenza MN, Rosen R, Austad CS, Raskin SA, Fallahi CR, Tennen H, 2013. Influence of alcohol use on neural response to go/no-go task in college drinkers. Neuropsychopharmacology 38, 2197–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amzica F, Lopes da Silva FH, 2011. Cellular substrates of brain rhythms, in: Schomer D, Lopes da Silva FH (Eds.), Niedermeyer’s Electroencephalography: Basic principles, clinical applications, and related fields. Lippincott Williams & Wilkins, Philadelphia, pp. 33–63. [Google Scholar]
- Anderson BM, Stevens MC, Meda SA, Jordan K, Calhoun VD, Pearlson GD, 2011. Functional imaging of cognitive control during acute alcohol intoxication. Alcohol Clin Exp Res 35, 156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA, 2014. Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn Sci 18, 177–185. [DOI] [PubMed] [Google Scholar]
- Baker SN, 2007. Oscillatory interactions between sensorimotor cortex and the periphery. Curr Opin Neurobiol 17, 649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baler RD, Volkow ND, 2006. Drug addiction: the neurobiology of disrupted self-control. Trends Mol Med 12, 559–566. [DOI] [PubMed] [Google Scholar]
- Basar E, Basar-Eroglu C, Karakas S, Schurmann M, 2001. Gamma, alpha, delta, and theta oscillations govern cognitive processes. Int J Psychophysiol 39, 241–248. [DOI] [PubMed] [Google Scholar]
- Beaton LE, Azma S, Marinkovic K, 2018. When the brain changes its mind: Oscillatory dynamics of conflict processing and response switching in a flanker task during alcohol challenge. PLoS One 13, e0191200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, 1999. What is inherited in the predisposition toward alcoholism? A proposed model Alcohol Clin Exp Res 23, 1125–1135. [DOI] [PubMed] [Google Scholar]
- Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD, 2011. Economic costs of excessive alcohol consumption in the U.S., 2006 Am J Prev Med 41, 516–524. [DOI] [PubMed] [Google Scholar]
- Brier MR, Ferree TC, Maguire MJ, Moore P, Spence J, Tillman GD, Hart J Jr., Kraut MA, 2010. Frontal theta and alpha power and coherence changes are modulated by semantic complexity in Go/NoGo tasks. Int J Psychophysiol 78, 215–224. [DOI] [PubMed] [Google Scholar]
- Buxton RB, 2002. Introduction to Functional Magnetic Resonance Imaging. Cambridge University Press, New York, NY. [Google Scholar]
- Cahalan D, Cisin IH, Crossley HM, 1969. American drinking practices: A national study of drinking behavior and attitudes Monograph #6. Rutgers Center of Alcohol Studies, New Brunswick, NJ. [Google Scholar]
- Cavanagh JF, Frank MJ, 2014. Frontal theta as a mechanism for cognitive control. Trends Cogn Sci 18, 414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheyne D, Bakhtazad L, Gaetz W, 2006. Spatiotemporal mapping of cortical activity accompanying voluntary movements using an event-related beamforming approach. Hum Brain Mapp 27, 213–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikazoe J, Konishi S, Asari T, Jimura K, Miyashita Y, 2007. Activation of right inferior frontal gyrus during response inhibition across response modalities. J Cogn Neurosci 19, 69–80. [DOI] [PubMed] [Google Scholar]
- Clapp P, Bhave SV, Hoffman PL, 2008. How adaptation of the brain to alcohol leads to dependence: a pharmacological perspective. Alcohol Res Health 31, 310–339. [PMC free article] [PubMed] [Google Scholar]
- Cohen MX, Ridderinkhof KR, Haupt S, Elger CE, Fell J, 2008. Medial frontal cortex and response conflict: evidence from human intracranial EEG and medial frontal cortex lesion. Brain Res 1238, 127–142. [DOI] [PubMed] [Google Scholar]
- Correas A, Lopez-Caneda E, Beaton L, Rodriguez Holguin S, Garcia-Moreno LM, Anton-Toro LF, Cadaveira F, Maestu F, Marinkovic K, 2018. Decreased event-related theta power and phase-synchrony in young binge drinkers during target detection: An anatomically-constrained MEG approach. J Psychopharmacol, 269881118805498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutlee CG, Politzer CS, Hoyle RH, Huettel SA, 2014. An abbreviated impulsiveness scale constructed through confirmatory factor analysis of the Barratt Impulsiveness Scale version 11. Archives of Scientific Psychology 2, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crego A, Cadaveira F, Parada M, Corral M, Caamano-Isorna F, Rodriguez Holguin S, 2012. Increased amplitude of P3 event-related potential in young binge drinkers. Alcohol 46, 415–425. [DOI] [PubMed] [Google Scholar]
- Crego A, Holguin SR, Parada M, Mota N, Corral M, Cadaveira F, 2009. Binge drinking affects attentional and visual working memory processing in young university students. Alcohol Clin Exp Res 33, 1870–1879. [DOI] [PubMed] [Google Scholar]
- Crego A, Rodriguez-Holguin S, Parada M, Mota N, Corral M, Cadaveira F, 2010. Reduced anterior prefrontal cortex activation in young binge drinkers during a visual working memory task. Drug Alcohol Depend 109, 45–56. [DOI] [PubMed] [Google Scholar]
- Crews FT, Vetreno RP, Broadwater MA, Robinson DL, 2016. Adolescent Alcohol Exposure Persistently Impacts Adult Neurobiology and Behavior. Pharmacol Rev 68, 1074–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criaud M, Boulinguez P, 2013. Have we been asking the right questions when assessing response inhibition in go/no-go tasks with fMRI? A meta-analysis and critical review. Neurosci Biobehav Rev 37, 11–23. [DOI] [PubMed] [Google Scholar]
- Cuzon Carlson VC, Seabold GK, Helms CM, Garg N, Odagiri M, Rau AR, Daunais J, Alvarez VA, Lovinger DM, Grant KA, 2011. Synaptic and morphological neuroadaptations in the putamen associated with long-term, relapsing alcohol drinking in primates. Neuropsychopharmacology 36, 2513–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Makeig S, 2004. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics. Journal of Neuroscience Methods 134, 9–21. [DOI] [PubMed] [Google Scholar]
- Dick DM, Li TK, Edenberg HJ, Hesselbrock V, Kramer J, Kuperman S, Porjesz B, Bucholz K, Goate A, Nurnberger J, Foroud T, 2004. A genome-wide screen for genes influencing conduct disorder. Mol Psychiatry 9, 81–86. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P, 2010. Beta-band oscillations--signalling the status quo? Curr Opin Neurobiol 20, 156–165. [DOI] [PubMed] [Google Scholar]
- Erika-Florence M, Leech R, Hampshire A, 2014. A functional network perspective on response inhibition and attentional control. Nature communications 5, 4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck HJ, Eysenck SBG, 1975. Manual of the Eysenck Personality Questionnaire. Hodder & Staughton, London. [Google Scholar]
- Field M, Schoenmakers T, Wiers RW, 2008. Cognitive processes in alcohol binges: a review and research agenda. Curr Drug Abuse Rev 1, 263–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Wiers RW, Christiansen P, Fillmore MT, Verster JC, 2010. Acute alcohol effects on inhibitory control and implicit cognition: implications for loss of control over drinking. Alcohol Clin Exp Res 34, 1346–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT, 2003. Drug abuse as a problem of impaired control: current approaches and findings. Behav Cogn Neurosci Rev 2, 179–197. [DOI] [PubMed] [Google Scholar]
- Finn DA, Crabbe JC, 1997. Exploring alcohol withdrawal syndrome. Alcohol Health Res World 21, 149–156. [PMC free article] [PubMed] [Google Scholar]
- Finn P, 2000. Acute effects of alcohol on cognition and impulsive-disinhibited behavior, in: Noronha A, Eckardt M, Warren K (Eds.), Review of NIAAA’s neuroscience and behavioral research portfolio, vol. 34 US Department of health and human services, Bethesda, MD, pp. 337–356. [Google Scholar]
- Frank MJ, 2006. Hold your horses: a dynamic computational role for the subthalamic nucleus in decision making. Neural Netw 19, 1120–1136. [DOI] [PubMed] [Google Scholar]
- Gan G, Guevara A, Marxen M, Neumann M, Junger E, Kobiella A, Mennigen E, Pilhatsch M, Schwarz D, Zimmermann US, Smolka MN, 2014. Alcohol-induced impairment of inhibitory control is linked to attenuated brain responses in right fronto-temporal cortex. Biol Psychiatry 76, 698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Stein EA, 1999. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Natl Acad Sci U S A 96, 8301–8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon RC, Wagster MV, Hendrie HC, Fox NA, Cook KF, Nowinski CJ, 2013. NIH toolbox for assessment of neurological and behavioral function. Neurology 80, S2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND, 2002. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry 159, 1642–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND, 2011. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci 12, 652–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber JR, Harris-Olenak B, Burroughs T, Jacob T, 2016. Residual Effects: Young Adult Diagnostic Drinking Predicts Late-Life Health Outcomes. J Stud Alcohol Drugs 77, 859–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A, 2015. Putting the brakes on inhibitory models of frontal lobe function. NeuroImage 113, 340–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM, 2010. The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage 50, 1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanslmayr S, Pastotter B, Bauml KH, Gruber S, Wimber M, Klimesch W, 2008. The electrophysiological dynamics of interference during the Stroop task. J Cogn Neurosci 20, 215–225. [DOI] [PubMed] [Google Scholar]
- Haynes WI, Haber SN, 2013. The organization of prefrontal-subthalamic inputs in primates provides an anatomical substrate for both functional specificity and integration: implications for Basal Ganglia models and deep brain stimulation. J Neurosci 33, 4804–4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz AJ, Beck A, Meyer-Lindenberg A, Sterzer P, Heinz A, 2011. Cognitive and neurobiological mechanisms of alcohol-related aggression. Nat Rev Neurosci 12, 400–413. [DOI] [PubMed] [Google Scholar]
- Hingson RW, Zha W, White AM, 2017. Drinking Beyond the Binge Threshold: Predictors, Consequences, and Changes in the U.S. Am J Prev Med 52, 717–727. [DOI] [PubMed] [Google Scholar]
- Hochberg Y, Benjamini Y, 1990. More powerful procedures for multiple significance testing. Stat Med 9, 811–818. [DOI] [PubMed] [Google Scholar]
- Hu S, Zhang S, Chao HH, Krystal JH, Li CSR, 2016. Association of drinking problems and duration of alcohol use to inhibitory control in nondependent young adult social drinkers. Alcoholism: clinical and experimental research 40, 319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson N, Brown P, 2011. New insights into the relationship between dopamine, beta oscillations and motor function. Trends Neurosci 34, 611–618. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR, 1999. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 146, 373–390. [DOI] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones K, Chorlian D, Padmanabhapillai A, Rangaswamy M, Stimus A, Begleiter H, 2006. Event-related oscillations in offspring of alcoholics: neurocognitive disinhibition as a risk for alcoholism. Biol Psychiatry 59, 625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones KA, Choi K, Chorlian DB, Padmanabhapillai A, Rangaswamy M, Stimus AT, Begleiter H, 2004. The role of brain oscillations as functional correlates of cognitive systems: a study of frontal inhibitory control in alcoholism. Int J Psychophysiol 51, 155–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kareken DA, Dzemidzic M, Wetherill L, Eiler W 2nd, Oberlin BG, Harezlak J, Wang Y, O’Connor SJ 2013. Family history of alcoholism interacts with alcohol to affect brain regions involved in behavioral inhibition. Psychopharmacology (Berl) 228, 335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna P, Carmena JM, 2017. Beta band oscillations in motor cortex reflect neural population signals that delay movement onset. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilavik BE, Zaepffel M, Brovelli A, MacKay WA, Riehle A, 2013. The ups and downs of beta oscillations in sensorimotor cortex. Exp Neurol 245, 15–26. [DOI] [PubMed] [Google Scholar]
- Kimbrough A, Kim S, Cole M, Brennan M, George O, 2017. Intermittent Access to Ethanol Drinking Facilitates the Transition to Excessive Drinking After Chronic Intermittent Ethanol Vapor Exposure. Alcohol Clin Exp Res 41, 1502–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, 2013. Theoretical frameworks and mechanistic aspects of alcohol addiction: alcohol addiction as a reward deficit disorder. Curr Top Behav Neurosci 13, 3–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M, 2005. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci 8, 1442–1444. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M, 2008a. Addiction and the brain antireward system. Annu Rev Psychol 59, 29–53. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M, 2008b. Review. Neurobiological mechanisms for opponent motivational processes in addiction. Philos Trans R Soc Lond B Biol Sci 363, 3113–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND, 2010. Neurocircuitry of addiction. Neuropsychopharmacology 35, 217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacevic S, Azma S, Irimia A, Sherfey J, Halgren E, Marinkovic K, 2012. Theta oscillations are sensitive to both early and late conflict processing stages: effects of alcohol intoxication. PLoS One 7, e43957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, 2002. The PHQ-9: a new depression diagnostic and severity measure. Psychiatric annals 32, 509–515. [Google Scholar]
- Kuntsche E, Kuntsche S, 2009. Development and validation of the Drinking Motive Questionnaire Revised Short Form (DMQ-R SF). J Clin Child Adolesc Psychol 38, 899–908. [DOI] [PubMed] [Google Scholar]
- Kwako LE, Momenan R, Litten RZ, Koob GF, Goldman D, 2016. Addictions Neuroclinical Assessment: A Neuroscience-Based Framework for Addictive Disorders. Biol Psychiatry 80, 179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciego JL, Luquin N, Obeso JA, 2012. Functional neuroanatomy of the basal ganglia. Cold Spring Harb Perspect Med 2, a009621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange JE, Voas RB, 2001. Defining binge drinking quantities through resulting blood alcohol concentrations. Psychol Addict Behav 15, 310–316. [DOI] [PubMed] [Google Scholar]
- Laukkanen V, Storvik M, Hakkinen M, Akamine Y, Tupala E, Virkkunen M, Tiihonen J, 2013. Decreased GABA(A) benzodiazepine binding site densities in postmortem brains of Cloninger type 1 and 2 alcoholics. Alcohol 47, 103–108. [DOI] [PubMed] [Google Scholar]
- Le Berre AP, Fama R, Sullivan EV, 2017. Executive Functions, Memory, and Social Cognitive Deficits and Recovery in Chronic Alcoholism: A Critical Review to Inform Future Research. Alcohol Clin Exp Res 41, 1432–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman RF, Patock-Peckham JA, Potenza MN, 2012. Impaired control over alcohol use: An under-addressed risk factor for problem drinking in young adults? Exp Clin Psychopharmacol 20, 92–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BJ, Wagner AD, 2011. Cognitive control and right ventrolateral prefrontal cortex: reflexive reorienting, motor inhibition, and action updating. Ann N Y Acad Sci 1224, 40–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlefield AK, Stevens AK, Sher KJ, 2014. Impulsivity and Alcohol Involvement: Multiple, Distinct Constructs and Processes. Curr Addict Rep 1, 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvak V, Jha A, Eusebio A, Oostenveld R, Foltynie T, Limousin P, Zrinzo L, Hariz MI, Friston K, Brown P, 2011. Resting oscillatory cortico-subthalamic connectivity in patients with Parkinson’s disease. Brain 134, 359–374. [DOI] [PubMed] [Google Scholar]
- Lopez-Caneda E, Cadaveira F, Crego A, Doallo S, Corral M, Gomez-Suarez A, Rodriguez Holguin S, 2013. Effects of a persistent binge drinking pattern of alcohol consumption in young people: a follow-up study using event-related potentials. Alcohol Alcohol 48, 464–471. [DOI] [PubMed] [Google Scholar]
- Lopez-Caneda E, Cadaveira F, Crego A, Gomez-Suarez A, Corral M, Parada M, Caamano-Isorna F, Rodriguez Holguin S, 2012. Hyperactivation of right inferior frontal cortex in young binge drinkers during response inhibition: a follow-up study. Addiction 107, 1796–1808. [DOI] [PubMed] [Google Scholar]
- Lopez-Caneda E, Rodriguez Holguin S, Correas A, Carbia C, Gonzalez-Villar A, Maestu F, Cadaveira F, 2017. Binge drinking affects brain oscillations linked to motor inhibition and execution. J Psychopharmacol 31, 873–882. [DOI] [PubMed] [Google Scholar]
- Luijten M, Machielsen MW, Veltman DJ, Hester R, de Haan L, Franken IH, 2014. Systematic review of ERP and fMRI studies investigating inhibitory control and error processing in people with substance dependence and behavioural addictions. J Psychiatry Neurosci 39, 149–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist M, Herman P, Miller EK, 2018. Working Memory: Delay Activity, Yes! Persistent Activity? Maybe Not. J Neurosci 38, 7013–7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic K, Beaton LE, Rosen BQ, Happer JP, Wagner LC, 2019. Disruption of Frontal Lobe Neural Synchrony During Cognitive Control by Alcohol Intoxication. J Vis Exp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic K, Halgren E, Klopp J, Maltzman I, 2000. Alcohol effects on movement-related potentials: a measure of impulsivity? J Stud Alcohol 61, 24–31. [DOI] [PubMed] [Google Scholar]
- Marinkovic K, Rickenbacher E, Azma S, Artsy E, 2012a. Acute alcohol intoxication impairs top-down regulation of Stroop incongruity as revealed by blood oxygen level-dependent functional magnetic resonance imaging. Hum Brain Mapp 33, 319–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic K, Rickenbacher E, Azma S, Artsy E, Lee AK, 2013. Effects of acute alcohol intoxication on saccadic conflict and error processing. Psychopharmacology (Berl) 230, 487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic K, Rosen BQ, Cox B, Kovacevic S, 2012b. Event-related theta power during lexical-semantic retrieval and decision conflict is modulated by alcohol intoxication: Anatomically-constrained MEG. Frontiers in Psychology 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurage P, Pesenti M, Philippot P, Joassin F, Campanella S, 2009. Latent deleterious effects of binge drinking over a short period of time revealed only by electrophysiological measures. J Psychiatry Neurosci 34, 111–118. [PMC free article] [PubMed] [Google Scholar]
- Most D, Ferguson L, Harris RA, 2014. Molecular basis of alcoholism. Handb Clin Neurol 125, 89–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naimi TS, Nelson DE, Brewer RD, 2010. The intensity of binge alcohol consumption among U.S. adults. Am J Prev Med 38, 201–207. [DOI] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Takada M, 2002. Functional significance of the cortico-subthalamo-pallidal ‘hyperdirect’ pathway. Neurosci Res 43, 111–117. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism, 2017. Drinking levels defined.
- Nigg JT, Wong MM, Martel MM, Jester JM, Puttler LI, Glass JM, Adams KM, Fitzgerald HE, Zucker RA, 2006. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. J Am Acad Child Adolesc Psychiatry 45, 468–475. [DOI] [PubMed] [Google Scholar]
- Nikolaou K, Critchley H, Duka T, 2013. Alcohol affects neuronal substrates of response inhibition but not of perceptual processing of stimuli signalling a stop response. PLoS One 8, e76649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez PL, Srinivasan R, 2006. Electric fields of the brain: The neurophysics of EEG. Oxford University Press, New York. [Google Scholar]
- Oldfield RC, 1971. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9, 97–113. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM, 2011. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci 2011, 156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, Marinkovic K, 2004. Alcoholism and the brain: An overview. Alcohol Research and Health 27, 125–133. [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, Marinkovic K, 2007. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol Rev 17, 239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey AK, Kamarajan C, Manz N, Chorlian DB, Stimus A, Porjesz B, 2016. Delta, theta, and alpha event-related oscillations in alcoholics during Go/NoGo task: Neurocognitive deficits in execution, inhibition, and attention processing. Prog Neuropsychopharmacol Biol Psychiatry 65, 158–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz AL, Rosselli M, Conniff J, 2018. Identifying Inhibitory Subcomponents Associated with Changes in Binge Drinking Behavior: A 6-Month Longitudinal Design. Alcohol Clin Exp Res 42, 1815–1822. [DOI] [PubMed] [Google Scholar]
- Petit G, Kornreich C, Maurage P, Noel X, Letesson C, Verbanck P, Campanella S, 2012. Early attentional modulation by alcohol-related cues in young binge drinkers: an event-related potentials study. Clin Neurophysiol 123, 925–936. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH, 1999. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol 110, 1842–1857. [DOI] [PubMed] [Google Scholar]
- Pihl RO, Peterson JB, Lau MA, 1993. A biosocial model of the alcohol-aggression relationship. J Stud Alcohol Suppl 11, 128–139. [DOI] [PubMed] [Google Scholar]
- Pogosyan A, Gaynor LD, Eusebio A, Brown P, 2009. Boosting cortical activity at Beta-band frequencies slows movement in humans. Curr Biol 19, 1637–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavachari S, Kahana MJ, Rizzuto DS, Caplan JB, Kirschen MP, Bourgeois B, Madsen JR, Lisman JE, 2001. Gating of human theta oscillations by a working memory task. J Neurosci 21, 3175–3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangaswamy M, Jones KA, Porjesz B, Chorlian DB, Padmanabhapillai A, Kamarajan C, Kuperman S, Rohrbaugh J, O’Connor SJ, Bauer LO, Schuckit MA, Begleiter H, 2007. Delta and theta oscillations as risk markers in adolescent offspring of alcoholics. Int J Psychophysiol 63, 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read JP, Kahler CW, Strong DR, Colder CR, 2006. Development and preliminary validation of the young adult alcohol consequences questionnaire. J Stud Alcohol 67, 169–177. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK, 1990. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study [see comments]. Jama 264, 2511–2518. [PubMed] [Google Scholar]
- Roberto M, Varodayan FP, 2017. Synaptic targets: Chronic alcohol actions. Neuropharmacology 122, 85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen BQ, Padovan N, Marinkovic K, 2016. Alcohol hits you when it is hard: Intoxication, task difficulty, and theta brain oscillations. Alcohol Clin Exp Res 40, 743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks JJ, Gonzales KR, Bouchery EE, Tomedi LE, Brewer RD, 2015. 2010 National and State Costs of Excessive Alcohol Consumption. Am J Prev Med 49, e73–e79. [DOI] [PubMed] [Google Scholar]
- Salvatore JE, Gottesman II, Dick DM, 2015. Endophenotypes for Alcohol Use Disorder: An Update on the Field. Curr Addict Rep 2, 76–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Kalmijn J, 2004. The search for genes contributing to the low level of response to alcohol: patterns of findings across studies. Alcohol Clin Exp Res 28, 1449–1458. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Tipp JE, 1997. The Self-Rating of the Effects of alcohol (SRE) form as a retrospective measure of the risk for alcoholism. Addiction 92, 979–988. [PubMed] [Google Scholar]
- Schuckit MA, Tapert S, Matthews SC, Paulus MP, Tolentino NJ, Smith TL, Trim RS, Hall S, Simmons A, 2012. fMRI differences between subjects with low and high responses to alcohol during a stop signal task. Alcohol Clin Exp Res 36, 130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selzer ML, Vinokur A, Van Rooijen L, 1975. A self-administered Short Michigan Alcoholism Screening Test (SMAST). Journal of Studies on Alcohol 36, 117–126. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Trull TJ, 1994. Personality and disinhibitory psychopathology: alcoholism and antisocial personality disorder. J Abnorm Psychol 103, 92–102. [DOI] [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH, 2008. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia 46, 224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell L, Sobell M, 1996. Timeline followback users’ manual for alcohol use. Toronto, Canada: Addiction Research Foundation. [Google Scholar]
- Solis-Escalante T, Muller-Putz GR, Pfurtscheller G, Neuper C, 2012. Cue-induced beta rebound during withholding of overt and covert foot movement. Clin Neurophysiol 123, 1182–1190. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Kroenke K, Williams JB, Löwe B, 2006. A brief measure for assessing generalized anxiety disorder: the GAD-7. Archives of internal medicine 166, 1092–1097. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A, 2005. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology (Berl) 180, 583–594. [DOI] [PubMed] [Google Scholar]
- Swann N, Tandon N, Canolty R, Ellmore TM, McEvoy LK, Dreyer S, DiSano M, Aron AR, 2009. Intracranial EEG reveals a time- and frequency-specific role for the right inferior frontal gyrus and primary motor cortex in stopping initiated responses. J Neurosci 29, 12675–12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick D, Ashley V, Turken U, 2011. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. Neuroimage 56, 1655–1665. [DOI] [PubMed] [Google Scholar]
- Terry-McElrath YM, Patrick ME, 2016. Intoxication and binge and high-intensity drinking among US young adults in their mid-20s. Subst Abus 37, 597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunstall MJ, Oorschot DE, Kean A, Wickens JR, 2002. Inhibitory interactions between spiny projection neurons in the rat striatum. J Neurophysiol 88, 1263–1269. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Bilbao A, Molander A, Spanagel R, 2008. Neuropharmacology of alcohol addiction. Br J Pharmacol 154, 299–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Lawrence AJ, Clark L, 2008. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci Biobehav Rev 32, 777–810. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Goldstein RZ, 2002. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiol Learn Mem 78, 610–624. [DOI] [PubMed] [Google Scholar]
- Wang C, Ulbert I, Schomer DL, Marinkovic K, Halgren E, 2005. Responses of human anterior cingulate cortex microdomains to error detection, conflict monitoring, stimulus-response mapping, familiarity, and orienting. J Neurosci 25, 604–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel JR, 2018. Prepotent motor activity and inhibitory control demands in different variants of the go/no-go paradigm. Psychophysiology 55. [DOI] [PubMed] [Google Scholar]
- Wessel JR, Aron AR, 2017. On the Globality of Motor Suppression: Unexpected Events and Their Influence on Behavior and Cognition. Neuron 93, 259–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiecki TV, Frank MJ, 2013. A computational model of inhibitory control in frontal cortex and basal ganglia. Psychol Rev 120, 329–355. [DOI] [PubMed] [Google Scholar]
- Wilcox MV, Cuzon Carlson VC, Sherazee N, Sprow GM, Bock R, Thiele TE, Lovinger DM, Alvarez VA, 2014. Repeated binge-like ethanol drinking alters ethanol drinking patterns and depresses striatal GABAergic transmission. Neuropsychopharmacology 39, 579–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka K, Yamamoto Y, 2010. Single-trial EEG Power and Phase Dynamics Associated with Voluntary Response Inhibition. Journal of Cognitive Neuroscience 22, 714–727. [DOI] [PubMed] [Google Scholar]
- Zavala B, Zaghloul K, Brown P, 2015. The subthalamic nucleus, oscillations, and conflict. Mov Disord 30, 328–338. [DOI] [PMC free article] [PubMed] [Google Scholar]