Summary
Procalcitonin (PCT), a precursor for calcitonin, is a prohormone involved in the inflammatory processes, which has been poorly studied in the context of pregnancy. During severe inflammation, PCT derives from almost all cell types, including monocytes and parenchymal tissues, making it a good predictive and diagnostic marker of an inflammatory state with rapidly increased serum levels in inflammation or sepsis. In normal pregnancy, PCT is basally expressed at very low level by decidual cells, even if decidual macrophages, which in normal pregnancy are skewed to M2 macrophages, are resistant to lipopolysaccharide (LPS)‐induced production of PCT. As PCT increase is associated with an inflammatory state, several research groups investigated whether PCT can be considered a marker of pre‐eclampsia, a pregnancy disease characterized by systemic inflammation. The first aim of this review is to summarize what is already known about the tissues synthesizing PCT, about the stimuli that cause the increase of circulating PCT levels and how PCT acts as a proinflammatory stimulus by itself. Secondly, we will describe the role of this prohormone in normal pregnancy and in pregnancies complicated by pre‐eclampsia, highlighting the involvement of the decidual macrophages and the proinflammatory cytokine tumor necrosis factor‐α in the modulation of PCT expression in the decidual microenvironment.
Keywords: macrophages, procalcitonin, pregnancy, pre‐eclampsia, TNF‐α
Procalcitonin: general characteristics
Procalcitonin (PCT), the prohormone of calcitonin (CT), is a protein that consists of 116 amino acids and a molecular mass of approximately 14 kDa (Fig. 1), and was first described in the late 1970s 1, 2.
Figure 1.
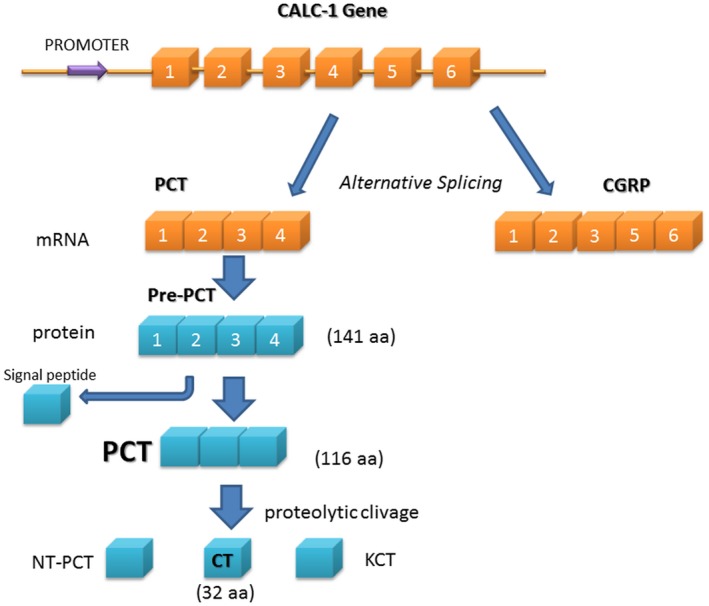
Schematic representation of the gene calcitonin‐related polypeptide alpha‐1 (CALC‐1) and procalcitonin (PCT) synthesis. CT = calcitonin; NT‐PCT = N‐terminal PCT; KCT = katacalcitonin. PCT mRNA is produced by alternative splicing of the same gene of calcitonin gene‐related peptide (CGRP). mRNA translation leads to the synthesis of a protein of 141 amino acids, named pre‐PCT, that is cleaved in PCT.
PCT is produced from the (calcitonin‐related polypeptide alpha‐1 (CALC‐1) gene located on chromosome 11 (11p15.2), containing six exons (Fig. 1). The mRNA product is known as pre‐PCT, which is further cleaved to generate the PCT (116 amino acid). Finally, this protein is cleaved into three distinct molecules: active CT (32 amino acid), katacalcitonin (21 amino acid) and N‐terminal PCT (57 amino acid) 3. Normally, the CALC‐1 gene in thyroid C cells is induced by elevated calcium level, glucocorticoid, calcitonin gene‐related peptide (CGRP), glucagon, gastrin or β‐adrenergic stimulations (Fig. 1) 3, 4; almost all the PCT formed in thyroid C cells is converted to CT, so that no PCT is released into the circulation 4. Hence, the PCT level in healthy subjects is very low (<0·05 ng/ml) 5.
PCT as a marker of sepsi or severe inflammation
During severe inflammation or sepsis the serum levels of PCT rapidly increase (>0·5–1 ng/ml) 4. For this reason, PCT is now generally accepted as a good predictive and diagnostic marker of the inflammatory process and as an additional tool to guide antibiotic prescribing 4, 6, 7, 8. The serum PCT levels rise more rapidly than C‐reactive protein (CRP) levels and peak within a very short time. Moreover, if the patient responds appropriately to the treatment, the level of PCT returns to normal range faster than CRP, which makes it a more effective biomarker for sepsis 9. During bacterial infection and sepsis, almost all the peripheral tissues have some involvement in PCT production (Fig. 2), including monocytes and parenchymal tissues, making its up‐regulation less dependent on one type of cell, tissue or organ 4, 10. Several studies have shown that the induction of PCT during infection is still abundant in the serum of infected patients with total thyroidectomy 5, 11, 12.
Figure 2.
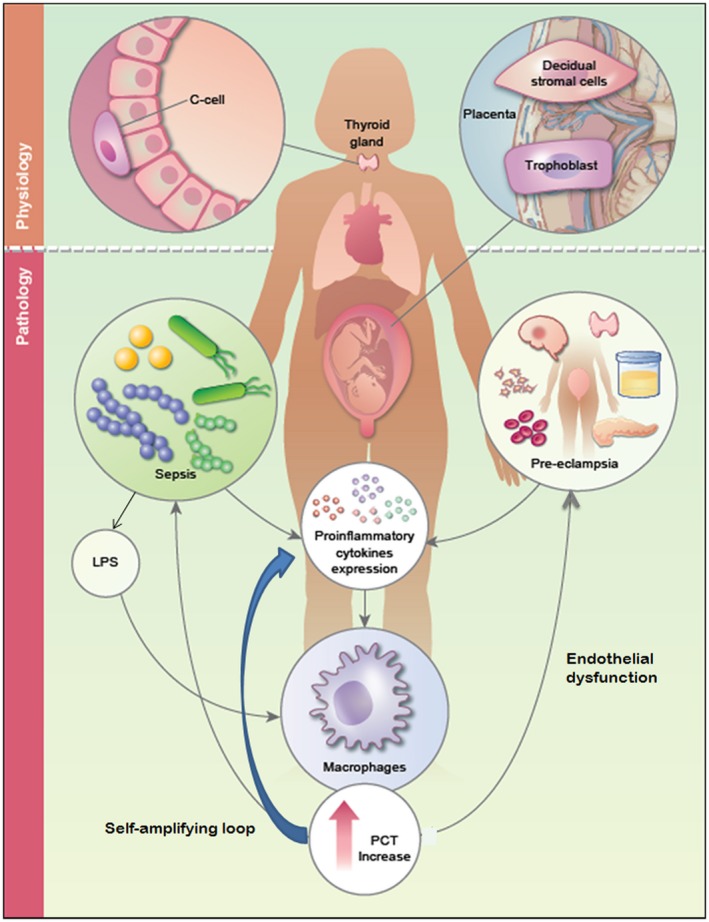
Secretion and biological function of procalcitonin (PCT) in physiological and pathological pregnancy. In healthy conditions PCT is produced mainly in thyroid C cells from the calcitonin‐related polypeptide alpha‐1 (CALC‐1) gene, but almost all the PCT formed in these cells are converted to calcitonin (CT) so that no PCT is released into the circulation. During normal pregnancy, extravillous trophoblast and decidual stromal cells start to synthesize PCT under physiological conditions, but the presence of this prohormone is destined to remain confined in the microenvironment; probably only a very little amount of this decidual PCT is able to reach the circulation contributing to the small increase of PCT serum level in pregnancy. During sepsis PCT is produced mainly by two alternative mechanisms; direct pathway induced by bacterial endotoxins [lipopolysaccharide (LPS)] or other toxic metabolite from microbes, and indirect pathway induced by various proinflammatory cytokines such as tumor necrosis factor (TNF)‐α, interleukin (IL)‐1β, IL‐6 and IL‐8. Pre‐eclampsia is a pathological condition of the pregnancy characterized by an increase of circulating proinflammatory cytokines that can induced directly an augmentation of PCT.
During inflammatory status, PCT is released as an acute‐phase reactant in response to inflammatory stimuli, especially those of bacterial origin. In these cases, PCT is produced mainly by two alternative mechanisms: the direct pathway, induced by bacterial endotoxins or other toxic metabolite from microbes (such as DNA, fimbriae or peptidoglycans) and the indirect pathway, induced by various proinflammatory cytokines such as tumor necrosis factor (TNF)‐α, interleukin (IL)‐1β, IL‐6 and IL‐8 5, 13, 14. For example, on one hand, bacteria may induce the expression of a specific transcription factor enhancer or, alternatively, displace a constitutive negative regulator of PCT expression leading to the transcription of PCT 5. On the other hand, an indirect induction of PCT by proinflammatory cytokines has also been suggested by different studies. In fact, perfusion of patients with TNF‐α or IL‐6 results in increased blood concentration of PCT 15. Similarly, intravenous administration of TNF‐α and IL‐2 leads to rapid and substantial release of PCT in cancer patients 16. Kettelhack et al. showed that isolated limb perfusion with TNF‐α leads to an induction of systemic serum PCT 17. Whang et al. confirmed the results in another experimental setting, where the injection of hamsters with TNF‐α resulted in robust PCT induction in the serum, similar to PCT elevation seen in experimental sepsis 18. Balog et al. reported that bacteria‐induced stimulation of PCT by human leukocytes (monocytes and granulocytes) was inhibited by incubation of these cells with anti‐TNF‐α monoclonal antibodies 19. In this experiment, the intracellular PCT expression was up‐regulated by an 18‐h in‐vitro stimulation with Staphylococcus aureus, known to be able to induce TNF‐α synthesis 20. Incubation of cells with S. aureus resulted in TNF‐α production by mononuclear and granulocytic cells 19, 20. Incubation of cells with S. aureus in the presence of monoclonal antibody against TNF‐α resulted in neutralization of the TNF‐α in the cell culture supernatant and failed to stimulate intracellular PCT synthesis 19. These findings demonstrate that TNF‐α is the main mediator in the S. aureus‐induced stimulation of PCT production in monocytes and granulocytes, as the effect could be almost totally abrogated when the cytokine was neutralized by anti‐TNF‐α 19.
Increased levels of sPCT are, however, attenuated by the release of IFN‐γ in response to viral infections, and non‐infectious inflammatory stimuli, such as autoimmune disease and chronic inflammatory processes, are much less pronounced, rarely exceeding 0·5 ng/ml 21, 22. Interestingly, during these processes, the mature CT peptide concentration either does not increase or is only slightly augmented 16, 23.
Several studies have shown that, in patients with sepsis, higher PCT levels are associated with a greater risk of progression to severe sepsis and septic shock, worsening the survival prognosis. Local bacterial infections and abscesses do not significantly raise PCT levels 22, 24, 25. PCT levels fall with successful treatment of severe bacterial infection and severe non‐infectious inflammatory stimuli 4. Persistent or recurrent PCT elevation in the latter setting should prompt suspicion of secondary infection.
PCT levels may also be elevated in medullary thyroid carcinoma 26 and small‐cell lung carcinoma 27, paralytic/vascular ileus exhibiting paraneoplastic production 28 and renal failure 29. PCT, although useful in bacterial sepsis, has no value in the assessment of fungal or viral infections and shows no response to intracellular microorganisms (i.e. Mycoplasma) or in local infections with no systemic response 30. Similar to CRP, clinical conditions associated with high baseline PCT levels include burns, major surgery and systemic inflammatory processes 30. PCT can also be used in the guidance of antibiotic therapy. Optimizing antibiotic therapy is important at an individual patient‐level but can also minimize emergence of antibiotic resistance 31.
The studies conducted to date are starting to clarify the pathways involved in PCT expression, but little is known about the functions that this prohormone performs at a paracrine and systemic level.
PCT biological functions
The physiological importance and regulation of PCT production are not well understood. Several hypotheses suggest that PCT may be involved in the calcium metabolism, cytokine network and modulation of nitric oxide (NO) synthesis, as well as pain‐relieving effects 32. There are no enzymes in the plasma to break down PCT. Therefore, if PCT enters the circulation it remains unchanged, with a half‐life of approximately 30 h, with no evidence of sPCT binding to any cellular receptors 32.
PCT is able to induce, in peripheral blood cells, an increase of proinflammatory cytokine levels, such as IL‐6, TNF‐α and IL‐1β, in a dose‐dependent manner 33. Despite this, and further evidence 34, 35, the PCT proinflammatory characteristics are not yet unanimously accepted.
In vascular smooth muscle cells, Hoffmann and co‐workers showed an inhibitory effect of PCT on lipopolysaccharide (LPS)‐mediated TNF‐α synthesis at the transcriptional level 36. Monneret et al. demonstrated that a simultaneous incubation of human whole blood cells with LPS and PCT led to the suppression of LPS‐induced production of TNF‐α, but the prohormone had no effect on LPS‐induced IL‐1, IL‐6 and IL‐8 37. Consequently, in this series of studies, PCT was proposed to exhibit an anti‐inflammatory effect. The explanation for this apparent contradiction could be that PCT can act as a proinflammatory modulator on the cells that have previously been primed by inflammatory cytokines or LPS 38. Thus, it appears that cellular targets of PCTs’ actions are multiple, including both leukocytes and non‐hematopoietic cells.
PCT impair the function and viability of human hepatocytes and endothelium and exert general cytotoxicity in vitro 39, 40. PCT with TNF‐α induces endothelial barrier disruption and (at concentrations of 0·02 ng/ml) reduces endothelial cell migration and in‐vitro tube formation. The mechanisms are unclear and need further investigation 39.
The synthesis of PCT by peripheral blood monocytes and tissue macrophages
The amount of macrophages that can contribute to plasmatic levels of PCT is not fully understood. There are few studies on the production of PCT by human macrophages in culture: Linscheid et al. has revealed that after 5 days of culture macrophages did not express either calcitonin or calcitonin gene‐related peptide (CGRP)‐1 mRNA after stimulation with several proinflammatory factors 41. The study demonstrated the capability of peripheral blood mononuclear cells (PBMCs) to secrete PCT only after an adherence to endothelial cells or plastic surfaces. Other studies showed the synthesis of PCT by PBMCs with contrasting results 5, due perhaps to the fact that within the PBMC population we have immune cells deriving from very different precursors. The presence of PCT has been previously observed by Oberhoffer and colleagues in freshly isolated PBMCs both at transcriptional and translational levels 42. Herget‐Rosenthal et al. described a correlation between PBMCs and PCT expression and the concentration of PCT in the blood from controls and patients with advanced chronic kidney disease 43. Moreover, Balog et al. showed that Gram‐positive bacteria have the TNF‐inducing ability to elevate the intracellular content of PCT in human monocytes 19.
Rami and co‐workers demonstrated the capability of human macrophages cultured to synthesize PCT after 7 days of culture under basal conditions; the researchers also demonstrated the incapacity of human macrophages to respond to LPS in terms of PCT expression and production when they are not polarized to M1 macrophages 44. In this article they have analysed, for the first time, the PCT expression by macrophages cultured in gravid serum, demonstrating a hampered capability to respond to LPS 44.
PCT as a marker of sepsis during pregnancy
Although PCT continues to be found increasingly useful in modern clinical practice, there are only a few published data on PCT in pregnancy 45, 46. As we have already described, PCT seems to be a useful biomarker for severe bacterial infections, and this could also be the case in the obstetrics and gynecology field, but the use of PCT as a marker of sepsis during pregnancy is controversial, as the general reference values for PCT in pregnancy have not been currently established. More data are needed to also support the use of PCT in obstetrics and gynecology 31, because reference intervals (RIs) for this prohormone, which are essential for clinical decision‐making, are lacking. The existing RIs for PCT are mainly based on general adults, not involving pregnant women. A Swiss study provides reference values for PCT during the third trimester, at delivery and in the immediate postpartum period 47. A recent Chinese study, aimed to establish reference intervals for PCT in healthy pregnant women in the Chinese population, indicated that the serum PCT levels are significantly higher in pregnant versus non‐pregnant women, and this increase is particularly evident postpartum 48. These observations can be justified by the placental production of PCT, due to the physiological synthesis by trophoblast and stromal cell of the decidua, as demonstrated by Agostinis and colleagues in a recent article 49.
Several studies have indicated that pregnant serum PCT is not relevant to predict spontaneous preterm birth 50 or for maternal bacterial infection in pregnancy 31; for instance, during chorioamnionitis PCT is more likely to be released by the fetus rather than by placental tissue 51, indicating that it cannot be a good marker for maternal infection. All these investigations concur to validate the observations that gravid condition (pregnancy hormones and immunity state) can render the main PCT producers resistant to LPS activation. Rami and co‐workers, in effect, demonstrated that macrophages cultured in gravid serum, when stimulated with LPS, significantly decrease the level of mRNA for PCT 44. This effect is due probably to progesterone, because after LPS stimulation progesterone also down‐regulates the expression on PCT in human macrophages while 17‐β‐estradiol increases and human chorionic gonadotropin has no effect 44.
As described previously, most published data about PCT in pregnancy concern the diagnostic and/or prognostic role of this prohormone in pre‐eclampsia (PE) 52, 53, 54, 55.
PCT as a biomarker of PE
Excessive inflammation at fetal–maternal interface has been associated with gestational complications such as preterm labour, intrauterine growth restriction and PE. In healthy pregnancy, immune regulatory mechanisms prevent excessive systemic inflammation; however, in PE, the regulation of immune responses is disrupted as a result of aberrant activation of innate immune cells 56, 57.
PE is a multi‐systemic disorder of human pregnancy, characterized by widespread vascular endothelial malfunction and vasospasm that occurs after 20 weeks’ gestation, and can present as late as 4–6 weeks postpartum. It is clinically defined by hypertension and proteinuria, often characterized by elevated liver enzymes, thrombocytopenia with or without pathological edema 58.
The notion that the placenta is the origin of the pathogenic mechanisms on the basis of PE is now universally accepted 58. Incomplete spiral artery remodeling is considered the initial step in the pathogenetic events leading to hypertension, proteinuria and associated maternal and fetal dysfunction.
As PE is a disorder that occurs around the 20th week of gestation, whereas placental damage begins as early as the first trimester of pregnancy, one of the major challenges in the study of this syndrome is to find a predictive marker. At present, however, there are no plasmatic factors that can be considered PE markers with a predictive value. Perhaps for this reason, numerous studies have been carried out during the last 5 years to verify if the PCT could be an ideal candidate for this role. In Table 1 we report a summary of the studies that aimed to understand if PCT is a PE marker 49, 52, 53, 54, 55, 59, 60, 61, 62, 63. In general, we can deduce that PCT plasmatic levels are increased in PE and its levels correlate with the severity of the disease, but PCT has no predictive value.
Table 1.
Summary of the studies aimed to understand if procalcitonin (PCT) is a pre‐eclampsia (PE) diagnostic, predictive and/or prognostic marker of PE
| Study | Results | Significance |
|---|---|---|
| Agostinis et al. (2018) | Diagnostic marker: yes Predictive marker: no | P < 0·005 |
| (n = 30 PE and 30 HP; predictive study; n = 13 PE and 13 HP) | ||
| Uckan and Sahin (2018) | Diagnostic marker: yes | P < 0·001 |
| (n = 30 PE and 30 HP) | ||
| Jannesari and Kazemi (2017) | Diagnostic marker: yes | P < 0·001 |
| (n =59 PE and 50 HP) | ||
| Duckworth et al. (2016) | Diagnostic marker: yes Predictive marker: no | PCT does not represent a useful diagnostic test for determining the development of PE within 14 days (P > 0·001) |
| (n = 143 PE and 280 HP) | ||
| Birdir et al. (2015) | Diagnostic marker: yes Predictive marker: no | PCT does not represent a predictive marker for PE (P > 0·001) |
| (n = 35 PE and 100 HP) | ||
| Artunc‐Ulkumen et al. (2015) | Diagnostic marker: yes Predictive marker: yes | PCT concentrations were significantly higher in PE group (P = 0·001) and levels were correlated with the severity of the PE. PCT can be used for screening test for PE due to high sensitivity (P < 0·001) |
| (n =40 PE and 40 HP) | ||
| Lucy et al. (2013) | Predictive marker: no | PCT does not represent a useful diagnostic test for determining the development of PE within 14 days (P > 0·001) |
| (n = 287 women) | ||
| Kucukgoz Gulec et al. (2012) | Diagnostic marker: yes | P < 0·001 |
| (n = 64 PE and 33 HP) | ||
| Can et al. (2011) | Diagnostic marker: yes | P < 0·001 |
| (n = 72 PE and 33 HP) | ||
| Montagnana et al. (2008) | PCT is a useful prognostic marker of the PE severity | PCT level in the severe PE group was significantly higher than in the mild PE and hypertensive groups. They concluded that rather than being a simple marker, PCT is an inflammatory mediator (such as cytokine) |
| (n = 24 PE and 12 with hypertension but without proteinuria) |
HP = healthy pregnant.
In PE, the systemic maternal inflammatory response is enhanced and characterized by a generalized intravascular inflammatory reaction. Intravascular leukocytes, clotting and complement systems activation are involved in the pathogenesis of PE 64. Several studies have reported higher levels of inflammatory cytokines in PE than those with normal pregnancies 64, 65, 66, 67. For example, IL‐6 and TNF‐α, potent inflammatory mediators associated with endothelial damage and oxidative stress, are increased in pre‐eclamptic patients, whereas no difference in IL‐1β was observed 67, 68, 69.
The increase of circulating proinflammatory cytokines in PE can be responsible for the augmentation of systemic PCT levels; furthermore, as we have previously discussed, increased PCT levels induce proinflammatory cytokine production that stimulates PCT release which, in turn, triggers the production of PCT itself, causing a positive loop of PCT secretion 5. Another role that PCT could play in PE pathogenesis is connected with its cytotoxic activity on hepatocytes and endothelium 39, 40. Indeed, it is well known that peculiar characteristics of PE are endothelial dysfunction and liver damage 70. Regarding the local role of the PCT at the fetal–maternal interface in PE, Agostinis et al. detected a strong increase in PCT mRNA expression in PE compared to normal placenta 49. Even though trophoblast and decidual stromal and endothelial cells produce PCT under physiological conditions, they cannot be considered the unique cell types responsible for the observed phenomenon. Indeed, all tissue macrophages are certainly involved in PCT production in pre‐eclamptic decidua, as demonstrated by co‐localization of CD68 immunoreactivity with PCT. It is known that PE sera contain specific factors, such as cytokines or protein aggregates 67, 71, that are able to modulate the synthesis of several proteins 72. The main proinflammatory cytokine whose levels have been shown to increase in PE patients is TNF‐α 67, 73. In particular, Agostinis and colleagues took advantage of an anti‐TNF‐α antibody, adalimumab, to hamper the activity of TNF‐α 49. Sera incubated with this antibody completely lose their ability to promote PCT up‐regulation but, unexpectedly, TNF‐α alone, used at the concentration found in PE sera, was completely ineffective while still able to increase the expression of IL‐1β 49, 73. Similar findings were observed by Balog and co‐workers, based on the observation that the anti‐TNF‐α antibody significantly decreased intracellular PCT production by leukocytes stimulated with S. aureus 19. These data together suggest that TNF‐α is necessary for the up‐regulation of PCT, but it probably requires co‐operation with additional factors still to be identified (Fig. 3).
Figure 3.
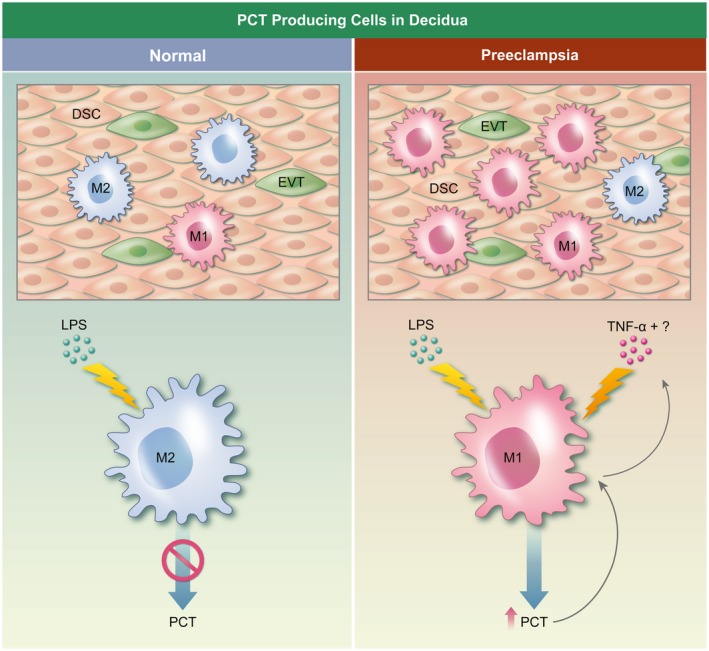
Model of procalcitonin (PCT) synthesis by decidual cells in normal conditions and in pre‐eclampsia. Decidual stromal cells (DSC), extravillous trophoblast (EVT) and macrophages (M) are able to produce PCT in decidua, but the contribution of M in pathological conditions is the most relevant (right panel), while in normal conditions pregnancy hormones block the macrophage response to lipopolysaccharide (LPS) in terms of PCT production (left panel). Tumor necrosis factor (TNF)‐α is necessary for the up‐regulation of PCT induced by pre‐eclamptic sera on macrophages, but it probably requires co‐operation with additional factors, still to be identified.
Conclusions
On the basis of the analysis of the data present in the literature we can conclude that PCT, unlike the normal non‐pregnant condition, is not relevant to predict maternal bacterial infection because pregnancy conditions (hormones and immunity state) can induce a resistance to LPS activation by the main PCT producers.
In PE, the local and the systemic increase of proinflammatory cytokines enhance PCT production by macrophages; this triggers a self‐amplifying loop, in which PCT induces an increase of the proinflammatory cytokine production by macrophages. PCT, on its own, is able to induces direct damage acting on endothelium and consequently exacerbating the pre‐eclamptic endothelial dysfunction.
Concerning the clinical and diagnostic importance of PCT, its serum levels can be considered a good diagnostic marker of PE, although PCT cannot be considered a predictive marker of PE onset. Furthermore, PCT emerges as a good prognostic marker of the severity of PE. However, further studies are necessary to confirm this observation.
Disclosure
None to declare.
Acknowledgements
The authors thank Berkan Vural for the English editing. This work was supported by grants from the Institute for Maternal and Child Health, IRCCS ‘Burlo Garofolo’, Trieste, Italy (RC 20/16, RC 23/18). Fondazione Cassa di Risparmio Trieste to R. B.
OTHER ARTICLES PUBLISHED IN THIS REVIEW SERIES
The immunology of the fetal‐placental unit comes of age. Clinical and Experimental Immunology 2019, 198: 11–14.
Embryonic extracellular vesicles as informers to the immune cells at the maternal‐fetal interface. Clinical and Experimental Immunology 2019, 198: 15–23.
The role of neutrophil activation in determining the outcome of pregnancy and modulation by hormones and/or cytokines. Clinical and Experimental Immunology 2019, 198: 24–36.
Influence of maternal microbiota during pregnancy on infant immunity. Clinical and Experimental Immunology 2019, 198: 47–56.
References
- 1. Moya F, Nieto A, R‐candela JL. Calcitonin biosynthesis: evidence for a precursor. Eur J Biochem 1975; 55:407–13. [DOI] [PubMed] [Google Scholar]
- 2. Allison J, Hall L, MacIntyre I, Craig RK. The construction and partial characterization of plasmids containing complementary DNA sequences to human calcitonin precursor polyprotein. Biochem J 1981; 199:725–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Meisner M. Pathobiochemistry and clinical use of procalcitonin. Clin Chim Acta 2002; 323:17‐29. [DOI] [PubMed] [Google Scholar]
- 4. Vijayan AL, Ravindran S, Saikant R, Lakshmi S, Kartik R. Procalcitonin: a promising diagnostic marker for sepsis and antibiotic therapy. J Intens Care 2017; 5:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Matwiyoff GN, Prahl JD, Miller RJ et al Immune regulation of procalcitonin: a biomarker and mediator of infection. Inflamm Res 2012; 61:401–9. [DOI] [PubMed] [Google Scholar]
- 6. Rhee C. Using procalcitonin to guide antibiotic therapy. Open Forum Infect Dis 2017; 4:ofw249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Soni NJ. United States Agency for Health Care Policy and Research, Blue Cross and Blue Shield Association, Technology Evaluation Center, Effective Health Care Program (US). Procalcitonin‐guided antibiotic therapy. Comparative effectiveness review no. 78. Rockville, MD: Agency for Healthcare Research and Quality, 2012:1 online resource [PDF file (various pagings)]. [Google Scholar]
- 8. Schuetz P, Christ‐Crain M, Thomann R et al Effect of procalcitonin‐based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA 2009; 302:1059–66. [DOI] [PubMed] [Google Scholar]
- 9. Standage SW, Wong HR. Biomarkers for pediatric sepsis and septic shock. Expert Rev Anti Infect Ther 2011; 9:71–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Muller B, White JC, Nylen ES, Snider RH, Becker KL, Habener JF. Ubiquitous expression of the calcitonin‐i gene in multiple tissues in response to sepsis. J Clin Endocrinol Metab 2001; 86:396–404. [DOI] [PubMed] [Google Scholar]
- 11. Bolko P, Manuszewska‐Jopek E, Michalek K, Wasko R, Jaskula M, Sowinski J. Efficacy of procalcitonin measurement in patients after total thyroidectomy due to medullary thyroid carcinoma. Arch Immunol Ther Ex 2003; 51:415–9. [PubMed] [Google Scholar]
- 12. Nishikura T. Procalcitonin (PCT) production in a thyroidectomized patient. Intens Care Med 1999; 25:1031. [DOI] [PubMed] [Google Scholar]
- 13. Meisner M. Procalcitonin: biochemie und klinische diagnostic [Procalcitonin: Biochemistry and Clinical Diagnostics]. Bremen: UNI‐MED, 2010. [Google Scholar]
- 14. Dandona P, Nix D, Wilson MF et al Procalcitonin increase after endotoxin injection in normal subjects. J Clin Endocrinol Metab 1994; 79:1605–8. [DOI] [PubMed] [Google Scholar]
- 15. Nijsten MW, Olinga P, The TH et al Procalcitonin behaves as a fast responding acute phase protein in vivo and in vitro . Crit Care Med 2000; 28:458–61. [DOI] [PubMed] [Google Scholar]
- 16. Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 1993; 341:515–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kettelhack C, Hohenberger P, Schulze G, Kilpert B, Schlag PM. Induction of systemic serum procalcitonin and cardiocirculatory reactions after isolated limb perfusion with recombinant human tumor necrosis factor‐alpha and melphalan. Crit Care Med 2000; 28:1040–6. [DOI] [PubMed] [Google Scholar]
- 18. Whang KT, Vath SD, Becker KL et al Procalcitonin and proinflammatory cytokine interactions in sepsis. Shock 2000; 14:73–8. [DOI] [PubMed] [Google Scholar]
- 19. Balog A, Ocsovszki I, Mandi Y. Flow cytometric analysis of procalcitonin expression in human monocytes and granulocytes. Immunol Lett 2002; 84:199–203. [DOI] [PubMed] [Google Scholar]
- 20. Timmerman CP, Mattsson E, Martinez‐Martinez L et al Induction of release of tumor necrosis factor from human monocytes by staphylococci and staphylococcal peptidoglycans. Infect Immun 1993; 61:4167–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Meili M, Muller B, Kulkarni P, Schutz P. Management of patients with respiratory infections in primary care: procalcitonin, C‐reactive protein or both? Expert Rev Respir Med 2015; 9:587–601. [DOI] [PubMed] [Google Scholar]
- 22. Ryu JA, Yang JH, Lee D et al Clinical usefulness of procalcitonin and C‐reactive protein as outcome predictors in critically Ill patients with severe sepsis and septic shock. PLOS ONE 2015; 10:e0138150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Snider RH Jr, Nylen ES, Becker KL. Procalcitonin and its component peptides in systemic inflammation: immunochemical characterization. J Invest Med 1997; 45:552–60. [PubMed] [Google Scholar]
- 24. Yu Y, Li XX, Jiang LX et al Procalcitonin levels in patients with positive blood culture, positive body fluid culture, sepsis, and severe sepsis: a cross‐sectional study. Infect Dis (Lond) 2016; 48:63–9. [DOI] [PubMed] [Google Scholar]
- 25. Eberhard OK, Haubitz M, Brunkhorst FM, Kliem V, Koch KM, Brunkhorst R. Usefulness of procalcitonin for differentiation between activity of systemic autoimmune disease (systemic lupus erythematosus/systemic antineutrophil cytoplasmic antibody‐associated vasculitis) and invasive bacterial infection. Arthritis Rheum 1997; 40:1250–6. [DOI] [PubMed] [Google Scholar]
- 26. Kaczka K, Mikosinski S, Fendler W, Celnik A, Pomorski L. Calcitonin and procalcitonin in patients with medullary thyroid cancer or bacterial infection. Adv Clin Exp Med 2012; 21:169–78. [PubMed] [Google Scholar]
- 27. Patout M, Salaun M, Brunel V, Bota S, Cauliez B, Thiberville L. Diagnostic and prognostic value of serum procalcitonin concentrations in primary lung cancers. Clin Biochem 2014; 47:263–7. [DOI] [PubMed] [Google Scholar]
- 28. Maruna P, Frasko R, Gurlich R. Plasma procalcitonin in patients with ileus. Relations to other inflammatory parameters. Physiol Res 2008; 57:481–6. [DOI] [PubMed] [Google Scholar]
- 29. Jeeha R, Skinner DL, De Vasconcellos K, Magula NP. Serum procalcitonin levels predict acute kidney injury in critically ill patients. Nephrology 2018; 23:1090–5. [DOI] [PubMed] [Google Scholar]
- 30. Lee H. Procalcitonin as a biomarker of infectious diseases. Korean J Intern Med 2013; 28:285–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tujula B, Kokki H, Rasanen J, Kokki M. Procalcitonin; a feasible biomarker for severe bacterial infections in obstetrics and gynecology? Acta Obstet Gynecol Scand 2018; 97:505–6. [DOI] [PubMed] [Google Scholar]
- 32. Maruna P, Nedelnikova K, Gurlich R. Physiology and genetics of procalcitonin. Physiol Res 2000; 49(Suppl 1):S57–61. [PubMed] [Google Scholar]
- 33. Liappis AP, Gibbs KW, Nylen ES et al Exogenous procalcitonin evokes a pro‐inflammatory cytokine response. Inflamm Res 2011; 60:203–7. [DOI] [PubMed] [Google Scholar]
- 34. Tavares E, Minano FJ. Immunoneutralization of the aminoprocalcitonin peptide of procalcitonin protects rats from lethal endotoxaemia: neuroendocrine and systemic studies. Clin Sci (Lond) 2010; 119:519–34. [DOI] [PubMed] [Google Scholar]
- 35. Wagner KE, Martinez JM, Vath SD et al Early immunoneutralization of calcitonin precursors attenuates the adverse physiologic response to sepsis in pigs. Crit Care Med 2002; 30:2313–21. [DOI] [PubMed] [Google Scholar]
- 36. Hoffmann G, Schobersberger W. Anti‐inflammatory procalcitonin (PTC) in a human whole blood model septic shock. Cytokine 2001; 14:127–8. [DOI] [PubMed] [Google Scholar]
- 37. Monneret G, Pachot A, Laroche B, Picollet J, Bienvenu J. Procalcitonin and calcitonin gene‐related peptide decrease LPS‐induced TNF production by human circulating blood cells. Cytokine 2000; 12:762–4. [DOI] [PubMed] [Google Scholar]
- 38. Hoffmann G, Czechowski M, Schloesser M, Schobersberger W. Procalcitonin amplifies inducible nitric oxide synthase gene expression and nitric oxide production in vascular smooth muscle cells. Crit Care Med 2002; 30:2091–5. [DOI] [PubMed] [Google Scholar]
- 39. Wagner NM, Van Aken C, Butschkau A et al Procalcitonin impairs endothelial cell function and viability. Anesth Analg 2017; 124:836–45. [DOI] [PubMed] [Google Scholar]
- 40. Sauer M, Doss S, Ehler J, Mencke T, Wagner NM. Procalcitonin impairs liver cell viability and function in vitro: a potential new mechanism of liver dysfunction and failure during sepsis? Biomed Res Int 2017; 2017:6130725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Linscheid P, Seboek D, Schaer DJ, Zulewski H, Keller U, Muller B. Expression and secretion of procalcitonin and calcitonin gene‐related peptide by adherent monocytes and by macrophage‐activated adipocytes. Crit Care Med 2004; 32:1715–21. [DOI] [PubMed] [Google Scholar]
- 42. Oberhoffer M, Stonans I, Russwurm S et al Procalcitonin expression in human peripheral blood mononuclear cells and its modulation by lipopolysaccharides and sepsis‐related cytokines in vitro . J Lab Clin Med 1999; 134:49–55. [DOI] [PubMed] [Google Scholar]
- 43. Herget‐Rosenthal S, Klein T, Marggraf G et al Modulation and source of procalcitonin in reduced renal function and renal replacement therapy. Scand J Immunol 2005; 61:180–6. [DOI] [PubMed] [Google Scholar]
- 44. Rami D, La Bianca M, Agostinis C, Zauli G, Radillo O, Bulla R. The first trimester gravid serum regulates procalcitonin expression in human macrophages skewing their phenotype in vitro . Mediators Inflamm 2014; 2014:248963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Torbe A. Maternal plasma procalcitonin concentrations in pregnancy complicated by preterm premature rupture of membranes. Mediators Inflamm 2007; 2007:35782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Herzum I, Renz H. Inflammatory markers in SIRS, sepsis and septic shock. Curr Med Chem 2008; 15:581–7. [DOI] [PubMed] [Google Scholar]
- 47. Paccolat C, Harbarth S, Courvoisier D, Irion O, de Tejada BM. Procalcitonin levels during pregnancy, delivery and postpartum. J Perinat Med 2011; 39:679–83. [DOI] [PubMed] [Google Scholar]
- 48. Hu Y, Yang M, Zhou Y, Ding Y, Xiang Z, Yu L. Establishment of reference intervals for procalcitonin in healthy pregnant women of Chinese population. Clin Biochem 2017; 50:150–4. [DOI] [PubMed] [Google Scholar]
- 49. Agostinis C, Rami D, Zacchi P et al Pre‐eclampsia affects procalcitonin production in placental tissue. Am J Reprod Immunol 2018; 79:e12823. [DOI] [PubMed] [Google Scholar]
- 50. Ducarme G, Desroys du Roure F, Le Thuaut A, Grange J, Vital M, Dimet J. Efficacy of serum procalcitonin to predict spontaneous preterm birth in women with threatened preterm labour: a prospective observational study. BMC Pregnancy Childbirth 2018; 18:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stranak Z, Feyereisl J, Korcek P, Feyereislova S, Krofta L. Procalcitonin is more likely to be released by the fetus rather than placental tissue during chorioamnionitis. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2016; 160:499–502. [DOI] [PubMed] [Google Scholar]
- 52. Artunc‐Ulkumen B, Guvenc Y, Goker A, Gozukara C. Relationship of neutrophil gelatinase‐associated lipocalin (NGAL) and procalcitonin levels with the presence and severity of the preeclampsia. J Matern Fetal Neonatal Med 2015; 28:1895–900. [DOI] [PubMed] [Google Scholar]
- 53. Kucukgoz Gulec U, Tuncay Ozgunen F, Baris Guzel A et al An analysis of C‐reactive protein, procalcitonin, and D‐dimer in pre‐eclamptic patients. Am J Reprod Immunol 2012; 68:331–7. [DOI] [PubMed] [Google Scholar]
- 54. Can M, Sancar E, Harma M, Guven B, Mungan G, Acikgoz S. Inflammatory markers in preeclamptic patients. Clin Chem Lab Med 2011; 49:1469–72. [DOI] [PubMed] [Google Scholar]
- 55. Montagnana M, Lippi G, Albiero A et al Procalcitonin values in preeclamptic women are related to severity of disease. Clin Chem Lab Med 2008; 46:1050–1. [DOI] [PubMed] [Google Scholar]
- 56. Bergstrom S. Infection‐related morbidities in the mother, fetus and neonate. J Nutr 2003; 133:1656S–S1660. [DOI] [PubMed] [Google Scholar]
- 57. Thaxton JE, Nevers TA, Sharma S. TLR‐mediated preterm birth in response to pathogenic agents. Infect Dis Obstet Gynecol 2010; 2010:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Phipps E, Prasanna D, Brima W, Jim B. Preeclampsia: updates in pathogenesis, definitions, and guidelines. Clin J Am Soc Nephrol 2016; 11:1102–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Uckan K, Sahin HG. Serum amyloid A, procalcitonin, highly sensitive C reactive protein and tumor necrosis factor alpha levels and acute inflammatory response in patients with hemolysis, elevated liver enzymes, low platelet count (HELLP) and eclampsia. J Obstet Gynaecol Res 2018; 44:440–7. [DOI] [PubMed] [Google Scholar]
- 60. Jannesari R, Kazemi E. Level of high sensitive C‐reactive protein and procalcitonin in pregnant women with mild and severe preeclampsia. Adv Biomed Res 2017; 6:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Duckworth S, Griffin M, Seed PT et al Diagnostic biomarkers in women with suspected preeclampsia in a prospective multicenter study. Obstet Gynecol 2016; 128:245–52. [DOI] [PubMed] [Google Scholar]
- 62. Birdir C, Janssen K, Stanescu AD et al Maternal serum copeptin, MR‐proANP and procalcitonin levels at 11‐13 weeks gestation in the prediction of preeclampsia. Arch Gynecol Obstet 2015; 292:1033–42. [DOI] [PubMed] [Google Scholar]
- 63. Lucy C, Suzy D, Melanie G, Paul S, Christopher R, Andrew S. OP007. PLGF in combination with other commonly utilised tests and other biomarkers for predicting need for delivery for pre‐eclampsia within 14days in women presenting prior to 35weeks' gestation. Pregnancy Hypertens 2013; 3:64–5. [DOI] [PubMed] [Google Scholar]
- 64. Redman CW, Sargent IL. Immunology of pre‐eclampsia. Am J Reprod Immunol 2010; 63:534–43. [DOI] [PubMed] [Google Scholar]
- 65. Ertas IE, Kahyaoglu S, Yilmaz B et al Association of maternal serum high sensitive C‐reactive protein level with body mass index and severity of pre‐eclampsia at third trimester. J Obstet Gynaecol Res 2010; 36:970–7. [DOI] [PubMed] [Google Scholar]
- 66. Mihu D, Costin N, Mihu CM, Blaga LD, Pop RB. C‐reactive protein, marker for evaluation of systemic inflammatory response in preeclampsia. Rev Med Chir Soc Med Nat Iasi 2008; 112:1019–25. [PubMed] [Google Scholar]
- 67. Kumar A, Begum N, Prasad S, Agarwal S, Sharma S. IL‐10, TNF‐alpha and IFN‐gamma: potential early biomarkers for preeclampsia. Cell Immunol 2013; 283:70–4. [DOI] [PubMed] [Google Scholar]
- 68. Conrad KP, Miles TM, Benyo DF. Circulating levels of immunoreactive cytokines in women with preeclampsia. Am J Reprod Immunol 1998; 40:102–11. [DOI] [PubMed] [Google Scholar]
- 69. Kupferminc MJ, Peaceman AM, Wigton TR, Rehnberg KA, Socol ML. Tumor necrosis factor‐alpha is elevated in plasma and amniotic fluid of patients with severe preeclampsia. Am J Obstet Gynecol 1994; 170:1752–7; discussion 7–9. [PubMed] [Google Scholar]
- 70. Milne F, Redman C, Walker J et al Assessing the onset of pre‐eclampsia in the hospital day unit: summary of the pre‐eclampsia guideline (PRECOG II). BMJ 2009; 339:b3129. [DOI] [PubMed] [Google Scholar]
- 71. Cheng SB, Nakashima A, Sharma S. Understanding pre‐eclampsia using Alzheimer's etiology: an intriguing viewpoint. Am J Reprod Immunol 2016; 75:372–81. [DOI] [PubMed] [Google Scholar]
- 72. Agostinis C, Tedesco F, Bulla R. Alternative functions of the complement protein C1q at embryo implantation site. J Reprod Immunol 2016; 119:74–80. [DOI] [PubMed] [Google Scholar]
- 73. Lau SY, Guild SJ, Barrett CJ et al Tumor necrosis factor‐alpha, interleukin‐6, and interleukin‐10 levels are altered in preeclampsia: a systematic review and meta‐analysis. Am J Reprod Immunol 2013; 70:412–27. [DOI] [PubMed] [Google Scholar]


