Abstract
Objectives
This study sought to investigate the clinical utility and the predictive relevance of absolute rest myocardial blood flow (MBF) by cardiac magnetic resonance (CMR) in acute myocardial infarction.
Background
Microvascular obstruction (MVO) remains one of the worst prognostic factors in patients with reperfused ST-segment elevation myocardial infarction (STEMI). Clinical trials have focused on cardioprotective strategies to maintain microvascular functionality, but there is a need for a noninvasive test to determine their efficacy.
Methods
A total of 64 STEMI patients post–primary percutaneous coronary intervention underwent 3-T CMR scans acutely and at 6 months (6M). The protocol included cine function, T2-weighted edema imaging, pre-contrast T1 mapping, rest first-pass perfusion, and late gadolinium enhancement imaging. Segmental MBF, corrected for rate pressure product (MBFcor), was quantified in remote, edematous, and infarcted myocardium.
Results
Acute MBFcor was significantly reduced in infarcted myocardium compared with remote MBF (MBFinfarct 0.76 ± 0.20 ml/min/g vs. MBFremote 1.02 ± 0.21 ml/min/g, p < 0.001), but it significantly increased at 6M (MBFinfarct 0.76 ± 0.20 ml/min/g acute vs. 0.85 ± 0.22 ml/min/g at 6M, p < 0.001). On a segmental basis, acute MBFcor had incremental prognostic value for infarct size at 6M (odds of no LGE at 6M increased by 1.4:1 [p < 0.001] for each 0.1 ml/min/g increase of acute MBFcor) and functional recovery (odds of wall thickening >45% at 6M increased by 1.38:1 [p < 0.001] for each 0.1 ml/min/g increase of acute MBFcor). In subjects with coronary flow reserve >2 or index of myocardial resistance <40, acute MBF was associated with long-term functional recovery and was an independent predictor of infarct size reduction.
Conclusions
Acute MBF by CMR could represent a novel quantitative imaging biomarker of microvascular reversibility, and it could be used to identify patients who may benefit from more intensive or novel therapies.
Key Words: acute myocardial infarction, magnetic resonance, myocardial blood flow
Abbreviations and Acronyms: 6M, 6 month(s); CFR, coronary flow reserve; CMR, cardiac magnetic resonance; EF, ejection fraction; FPP, first-pass perfusion; IMH, intramyocardial hemorrhage; IMR, index of microvascular resistance; IS, infarct size; LGE, late gadolinium enhancement; LV, left ventricular; MBF, myocardial blood flow; MBFcor, myocardial blood flow corrected; MBFCULPRIT, average myocardial blood flow in the culprit territory; MI, myocardial infarction; MVO, microvascular obstruction; PPCI, primary percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction; Tmn, transit time at rest; T2W, T2-weighted; WT, wall thickening
Graphical abstract
Despite the improvement in mortality rates following the introduction of primary percutaneous coronary intervention (PPCI), the incidence of heart failure post–myocardial infarction (MI) remains persistently high. Microvascular impairment despite successful restoration of epicardial coronary artery patency is associated with poor long-term recovery and outcome 1, 2. There is a recognized lack of innovative medical therapy targeting the microcirculatory function, partly because of an insufficient understanding of the underpinning pathophysiological mechanisms (3). Invasive coronary measurements such as the index of microvascular resistance (IMR) have emerging clinical utility for patients’ stratification at the time of PPCI, with abnormal values being more likely associated with microvascular obstruction (MVO) (4).
Although standard cardiac magnetic resonance (CMR) techniques such as late gadolinium enhancement (LGE) allow anatomic volumetric quantification of MVO and intramyocardial hemorrhage (IMH) as biomarkers of severe microvascular disease (5), they do not provide any insight into microvascular function. Currently there is no noninvasive imaging method to stratify patients at risk of developing MVO or to determine the dynamic changes in microvascular impairment at the time of PPCI.
First-pass perfusion (FPP) by CMR for assessment of myocardial blood flow (MBF) (6) has been extensively used to estimate abnormal perfusion in patients with chronic artery disease (7), but a systematic investigation of MBF in acute STEMI is lacking.
By using MBF CMR, we sought to investigate: 1) the degree of microvascular impairment and its longitudinal changes in relation to the severity of ischemic injury; and 2) the extent to which the acute microvascular dysfunction predicts 6-month (6M) infarct size (IS) and myocardial functional recovery. This may help establish CMR as a clinically useful tool to determine the crucial role played by the microvascular function in the MI healing process and potentially provide novel and specific imaging biomarkers to assess the clinical efficacy of cardioprotective strategies.
Methods
Patient study group
Survivors of acutely reperfused STEMI post-PPCI were prospectively recruited between October 2010 and March 2015. The study protocol was approved by the local ethics committee, and all patients gave written informed consent. Acute clinical management reflected contemporary practice and guidelines (Supplemental Appendix).
Coronary physiology
Transit time at rest (Tmn) and after hyperemia, coronary flow reserve (CFR), and IMR measurements were performed at the time of primary percutaneous coronary intervention (PPCI) in the infarct-related artery, as previously described (8) (Supplemental Appendix).
CMR protocol
CMR was performed on a 3-T magnetic resonance scanner (either MAGNETOM TIMTrio or MAGNETOM Verio, Siemens Healthcare, Erlangen, Germany), acutely (1 to 3 days post-PPCI) and at 6M. The CMR protocol (details of the sequences can be found in the Supplemental Appendix) included functional cine imaging, tissue characterization techniques such edema T2-prepared steady-state free precession (SSFP) imaging (T2-weighted [T2W]), native shortened modified Look-Locker inversion recovery (ShMOLLI) T1 mapping, FPP at rest, and LGE. To track the first pass of a gadolinium-based contrast agent, 0.03 mmol/kg gadoterate meglumine (Dotarem, Guerbet, Villepinte, France) was injected at rest. LGE images were collected 10 to 15 min after the administration of an additional 0.1 mmol/kg of contrast agent. The inversion time was adjusted for nulling of remote normal myocardium.
Matching short-axis images covering the entire left ventricle were acquired using all techniques except for FPP, which was limited to 3 to 5 matching short-axis slices.
CMR imaging analysis
CMR analysis is described in detail in the Supplemental Appendix.
Both global and segmental analyses were performed on anonymized images using cvi42 software (Circle Cardiovascular Imaging Inc., Calgary, Canada) by 3 experienced operators (A.B., D.V., A.B.); all of the images were reviewed by an experienced CMR cardiologist (E.D.A). Apical slices affected by partial volume effects and slices where the outflow tract was visible were excluded from the study in all sequences. For segmental analyses, short-axis images were divided into 6 equiangular segments with the right ventricle–left ventricle junction as the reference point. The wall thickening (WT) analyses at follow-up were performed by an operator (A.B.) without knowledge of the baseline analyses. Functional recovery at 6M was assessed using a WT cutoff of 45% (9). Quantification of T2W edema and IS on LGE, both acutely and at 6M, was performed using a signal intensity threshold of 2 SD and 5 SD above the mean intensity of the remote reference region of interest (ROI), respectively, as previously described (10). When present, MVO and/or IHM were included in the measurements of left ventricular (LV) infarct or edema volume. Segmental tissue state was defined as remote if negative to LGE and on T2W, edematous if positive on T2W and negative to LGE, infarcted if positive to both (with a distinction between LGE 1% to 50% and LGE 51% to 100%), MVO (including segments with LGE >75% and MVO), and MVO in combination with IMH (including segments with LGE >75% with MVO and IMH) on the basis of acute LGE images. IS reduction was calculated as follows: (acute IS − 6M IS) / acute IS. Native T1 values were derived from short-axis T1 maps using in-house dedicated software MC-ROI (Interactive Data Language, version 6.1, Exelis Visual Information Solutions, Boulder, Colorado). Quantitative perfusion analysis to derive absolute MBF) (ml/min/g) was performed using an in-house MatLab software, as previously described 6, 7 (Figures 1A to 1L). MBF values were corrected for the heart rate–blood pressure product by dividing resting MBF by heart rate (in beats/min) × systolic blood pressure (mm Hg) / 10,000 (MBF corrected [MBFcor]). A per subject index of MBF (average MBF in the culprit territory [MBFCULPRIT]) was calculated averaging segmental MBFcor of the culprit coronary artery territory.
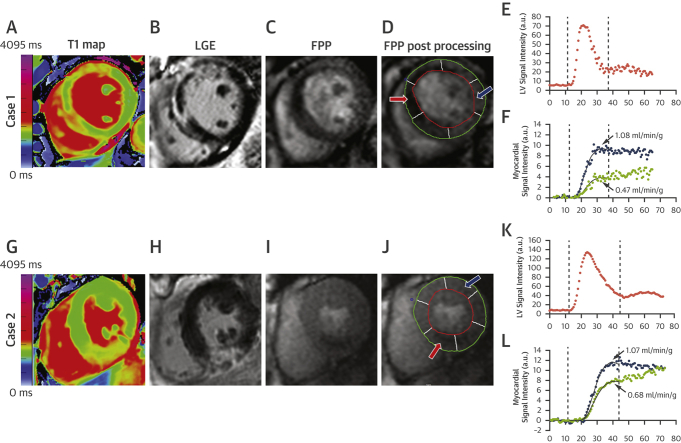
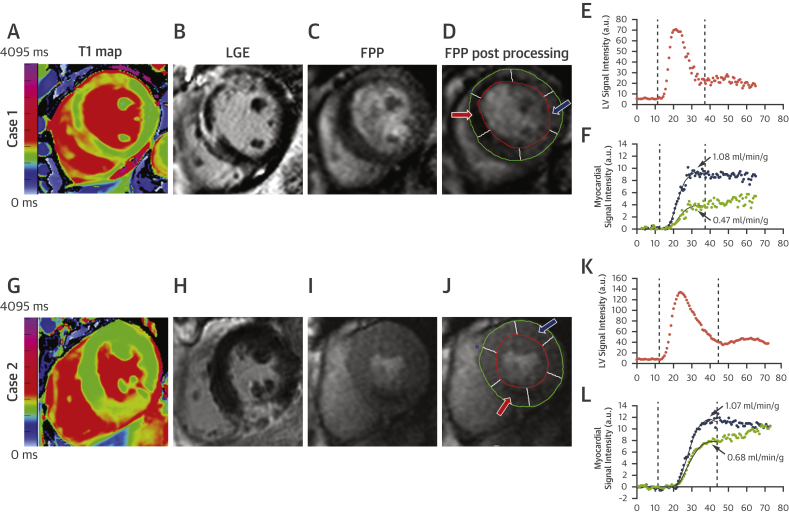
Figure 1.
Perfusion Quantification for Assessment of Resting Segmental MBF
(A to F) Case #1, anterior infarct with microvascular obstruction (MVO); (G to L) Case #2, inferior infarct with no microvascular obstruction. T1 maps and late gadolinium enhancement (LGE) images show the infarcted regions (A and B, anterior with microvascular obstruction; G and H, inferior) with hypoperfusion in the corresponding myocardium on first-pass perfusion (FPP) images (C and I). From segmental quantification (D and J), the myocardial tissue curves for myocardial blood flow (MBF) (F and L) demonstrated a reduced myocardial blood flow in the infarcted region (red arrow, red curve) compared with the remote myocardium (blue arrow, blue curve). (E and K) The arterial input function calculated in the blood pool. a.u. = arbitrary units; LV = left ventricular.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics software (version 22.0, IBM Corporation, Armonk, New York) and the R statistical environment (version 3.4.1, R Project, Vienna, Austria). Normally distributed continuous variables are expressed as mean ± SD, and not normally distributed variables are expressed as median and interquartile ranges (first quartile to third quartile). The normality of the data was assessed visually using quantile-quantile plots and by the Shapiro-Wilk test. For normally distributed variables, comparison among groups were performed with unpaired Student’s t-tests, and comparisons within each group of follow-up versus baseline used paired Student’s t-tests. For variables such as %LGE (% of LV mass) that are not normally distributed, we used Wilcoxon rank sum and signed rank tests.
The lme4 package (11) for the R statistical environment was used to build linear models with mixed effects (LME) for the analysis of segmental MBFcor acutely and over 6M follow-up (details in the Supplemental Appendix).
To investigate the predictive value of acute rest MBFcor for LGE, WT at 6M, and WT<45% at 6M, we used Generalized Additive Models for Location Scale and Shape (GAMLSS) using acute rest MBFcor, LGE and MVO as predictors (details in the Supplemental Appendix). Spearman correlation coefficients were used to assess any relationships among segmental MBFcor, WT, and LGE and MBFCULPRIT with invasive measurements and 6M myocardial functionality. Receiver-operating characteristic (ROC) analysis was performed to assess the diagnostic performance of MBFCULPRIT, LGE, and invasive measurements in the acute setting in predicting LV dysfunction (ejection fraction [EF] <50%) at 6M in patients with IMR <40 or CFR >2. Independent predictors of IS reduction from acute to 6M, in the same subgroup of patients, were assessed by adopting linear regression models. Variables with a p value <0.10 at univariable analysis were then entered into the multivariable models. The p values ≤0.05 were considered statistically significant.
Results
Of 104 patients with STEMI who consented, 40 were excluded because of claustrophobia or technical issues (n = 12), bystander cardiomyopathy (n = 6), poor-quality image (n = 10), and declined follow-up scan (n = 12). A total of 64 patients underwent acute and follow-up scan. The measurement of IMR, CFR, and Tmn was feasible in 53 of 64 patients. Clinical and demographic baseline characteristics are shown in Table 1.
Table 1.
Baseline Patient Characteristics (N = 64)
| Age, yrs | 60 ± 9 |
| Sex, male:female | 50:14 |
| Risk factors | |
| Current smoker | 18 (30) |
| Diabetes | 4 (7) |
| Dyslipidemia | 17 (28) |
| Hypertension | 22 (36) |
| Family history of CHD | 25 (41) |
| Peak troponin I (mg/ml) | 65 (41–158) |
| Pain to balloon time (min) | 179 (137–239) |
| Time from PPCI to CMR (days) | 2 (1–3) |
| Culprit coronary artery | |
| LAD | 29 (45) |
| RCA | 29 (45) |
| LCx | 6 (10) |
| No. of vessels diseased | |
| 1 | 41 (67) |
| 2 | 15 (25) |
| 3 | 5 (8) |
| TIMI flow pre-PPCI | |
| 0 | 50 (78) |
| 1 | 6 (9) |
| 2 | 7 (11) |
| 3 | 1 (2) |
| TIMI flow post-PPCI | |
| 0 | 0 (0) |
| 1 | 0 (0) |
| 2 | 4 (6) |
| 3 | 60 (94) |
| Medications during PPCI | |
| GP IIb/IIIa inhibitor | 8 (13) |
| Bivalirudin | 42 (66) |
| Heparin | 39 (61) |
| Clopidogrel | 10 (16) |
| Medications post-PPCI | |
| Beta-blockers | 61 (95) |
| ACE inhibitors | 62 (97) |
| Statins | 64 (100) |
| Aspirin | 61 (95) |
| Diuretic | 4 (8) |
| Nitrates | 53 (83) |
| Clopidogrel | 53 (83) |
| Ticagrelor | 11 (22) |
| Invasive measurements post-PPCI (n = 53) | |
| IMR | 32 (20–46) |
| CFR | 1.7 (1.3–2.3) |
| Tmn (ms) | 0.8 (0.4–1.2) |
Values are mean ± SD, n (%), or median (first to third quartile).
ACE = angiotensin-converting enzyme; CFR = coronary flow reserve; CHD = coronary heart disease; CMR = cardiac magnetic resonance; GP = glycoprotein; IMR = index of microvascular resistance; LAD = left anterior descending coronary artery; LCx = left circumflex coronary artery; PPCI = primary percutaneous coronary intervention; RCA = right coronary artery; TIMI = Thrombolysis In Myocardial Infarction; Tmn = transit time at rest.
CMR findings
The CMR findings are reported in Table 2. Acutely, all patients had positive edema, and only 1 had negative LGE; of 40 patients (63%) with MVO, 31 had also IMH. In 10 patients (i.e., 41 segments), myocardial edema persisted at 6M. Longitudinal native T1 changes relative to the degree of injury are also reported in Table 2 and Supplemental Figure 1A.
Table 2.
CMR Findings
| Acute (n = 64) | 6 Months (n = 64) | p Value | |
|---|---|---|---|
| EF (%) | 47 ± 9 | 54 ± 9 | <0.001 |
| EDV (ml) | 153 ± 41 | 164 ± 42 | 0.001 |
| ESV (ml) | 83 ± 31 | 76 ± 31 | 0.002 |
| LGE, 5 SD (LV%)∗ | 25 (14–32) | 12 (8–19) | <0.001 |
| Edema, 2 SD (LV%)∗ | 40 (33–48) | 0 (0–0) | <0.001 |
| MSI (%)∗ | 41 (31–60) | 68 (58–78) | <0.001 |
| MVO patients | 40 (63) | — | — |
| MVO (g)∗ | 0.8 (0–2.6) | — | — |
| IMH patients | 31 (48) | — | — |
| IMH (g)∗ | 0 (0–2.9) | — | — |
| MBFCULPRIT (ml/min/g) | 0.78 ± 0.14 | 0.87 ± 0.16 | <0.001 |
| Heart rate (beats/min) | 67 ± 12 | 57 ± 8 | <0.001 |
| Systolic blood pressure (mm Hg) | 113 ± 15 | 119 ± 18 | 0.002 |
| Rate pressure product (beats/min·mm Hg/1e4) | 0.75 ± 0.15 | 0.68 ± 0.15 | <0.001 |
| Segmental analysis | |||
| WT (%) | |||
| WTremote (n = 276) | 85 ± 34 | 88 ± 33 | 0.200 |
| WTedema (n = 228) | 68 ± 29 | 80 ± 30 | <0.001 |
| WTinfarct (n = 406) | 36 ± 27 | 48 ± 28 | <0.001 |
| WTMVO (n = 36) | 20 ± 17 | 32 ± 15 | 0.005 |
| WT MVO+IMH (n = 56) | 15 ± 13 | 18 ± 14 | 0.062 |
| T1 (ms) | |||
| T1remote (n = 150) | 1,190 ± 55 | 1,183 ± 46 | 0.133 |
| T1edema (n = 152) | 1,258 ± 64 | 1,197 ± 35 | <0.001 |
| T1infarct (n = 306) | 1,351 ± 86 | 1,239 ± 56 | <0.001 |
| T1MVO (n = 31) | 1,340 ± 70 | 1,281 ± 56 | <0.001 |
| T1MVO+IMH (n = 47) | 1,386 ± 82 | 1,280 ± 54 | <0.001 |
| MBFcor (ml/min/g) | |||
| MBFremote (n = 276) | 1.02 ± 0.21 | 1.08 ± 0.23 | <0.001 |
| MBFedema (n = 228) | 0.97 ± 0.18 | 1.03 ± 0.21 | <0.001 |
| MBFinfarct (n = 406) | 0.76 ± 0.20 | 0.85 ± 0.22 | <0.001 |
| MBFMVO (n = 36) | 0.69 ± 0.16 | 0.76 ± 0.16 | <0.001 |
| MBFMVO+IMH (n = 56) | 0.59 ± 0.11 | 0.62 ± 0.10 | 0.080 |
Values are mean ± SD, median (first to third quartile), or n (%).
CMR = cardiac magnetic resonance; EDV = end-diastolic volume; EF = ejection fraction; ESV = end-systolic volume; IMH = intramyocardial hemorrhage; LGE = late gadolinium enhancement; LV = left ventricle; MBFcor, myocardial blood flow corrected; MBFCULPRIT = average myocardial blood flow in the culprit territory; MSI = myocardial salvage index; MVO = microvascular obstruction; WT = wall thickening; 1e4 = 104.
Values were not normally distributed, and the p values then pertain to a paired Wilcoxon test.
Longitudinal MBFcor changes following acute MI
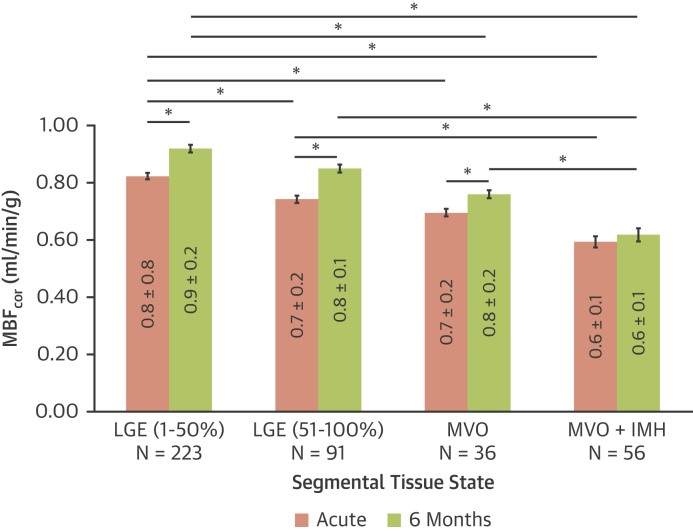
Acute MBFcor decreased with worsening ischemic injury (p < 0.001) (Table 2, Supplemental Figure 1B), with the lowest MBFcor values observed in infarcted segments with MVO and IMH. Over 6M, MBFcor improved significantly in all segment classes, including the groups of segments with LGE and/or MVO. In segments with IMH, MBFcor did not change significantly over time (p = 0.08) (Figure 2).
Figure 2.
MBFcor Changes Over 6M in Acutely Infarcted Myocardium
Myocardial blood flow corrected (MBFcor) improves significantly at 6 months (6M) in all segments, with the exception of segments with an acute presentation with microvascular obstruction (MVO) and intramyocardial hemorrhage (IMH). Bars are SEM. Mean values in the bars are reported with SD. ∗p < 0.001. LGE = late gadolinium enhancement.
Tissue state and severity of injury by T1 mapping as determinants of 6M MBFcor changes
To determine the effect of tissue state on MBFcor changes between the acute phase and 6M, we used a mixed-effects model for MBFcor, with tissue state and time point (acute, 6M), and their interaction as independent predictors. At the acute stage, edema, LGE, and LGE in combination with MVO were associated with a significant reduction of MBFcor by 0.03 ml/min/g (p = 0.05), 0.18 ml/min/g (p < 0.001), and 0.38 ml/min/g (p < 0.001), respectively (Table 3). These model predictions are in close agreement with the differences in acute MBFcor among tissue states that can be inferred from Table 2. At 6M, there was a significant, albeit small, overall improvement in MBFcor (0.04 ml/min/g, p < 0.001), independent of tissue state. 6M LGE with edema had a by 0.11 ml/min/g worse effect than acutely (p = 0.002).
Table 3.
Estimated Effect in Mixed-Effects Model for Mean Resting MBFcor, Acutely and at 6M
| Multivariable Associations | Coefficient ± SE | p Value |
|---|---|---|
| Linear mixed-effect model for MBF with tissue state as predictors | ||
| (Intercept) | 1.00 ± 0.017 | <0.001 |
| Edema, acute | −0.03 ± 0.016 | 0.053 |
| LGE | −0.18 ± 0.015 | <0.001 |
| LGE + MVO acute | −0.38 ± 0.020 | <0.001 |
| 6M | 0.04 ± 0.013 | <0.001 |
| Edema 6M | 0.00 ± 0.075 | 0.989 |
| LGE with edema 6M | −0.11 ± 0.037 | 0.002 |
| LGE 6M | −0.19 ± 0.013 | <0.001 |
| Linear mixed-effect model for MBFcor with T1 as predictor | ||
| (Intercept) | 0.90 ± 0.02 | <0.001 |
| T1 (100-ms change) | −0.09 ± 0.007 | <0.001 |
| 6M | 0.01 ± 0.01 | 0.409 |
| T1: 6M | −0.05 ± 0.016 | 0.001 |
A 100-ms change of T1 rather than a 1-s change, was used here to estimate the effect size because it is of the same order of magnitude as the T1 differences at the acute stage between remote myocardium and regions with ischemic injury.
6M = 6 months; other abbreviations as in Table 2.
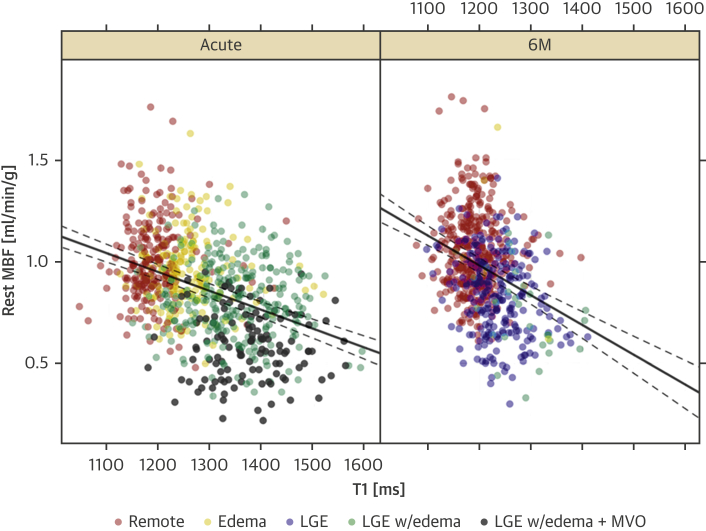
Next, we investigated the association between MBFcor and native T1 as quantitative marker of severity of injury. Acutely, a 100-ms increase in myocardial T1 was associated with a significant MBFcor reduction of 0.10 ± 0.007 ml/min/g, whereas at 6M, an increase in native T1 by 100 ms was associated with a further MBFcor reduction by 0.05 ± 0.02 ml/min/g (p = 0.001) (Table 3, Figure 3). We found a negative association between MBFcor and T1 for all segments independent of the tissue state (Figure 3). Notably, if regression coefficients for the effect of native T1 on MBF are estimated for each tissue state, the association between MBF and native T1 is significant only in the acute phase for LGE with edema, and at 6M for edema, LGE, and LGE with edema.
Figure 3.
Relationship Between MBFcor and Native T1 Over 6M
The lines show the prediction from a linear mixed effects model for myocardial blood flow corrected (MBFcor), with native T1 and time point (with interaction term included) as predictors. The model included a random intercept by patient. The dashed lines show the 95th percentile ranges for the predictions from the linear mixed effects model. If regression coefficients for the effect of native T1 on myocardial blood flow are estimated for each tissue state, the association between myocardial blood flow and native T1 is significant only in the acute phase for late gadolinium enhancement with edema, and at 6 months (6M) for edema, late gadolinium enhancement (LGE), and late gadolinium enhancement with edema. MVO = microvascular obstruction.
Relationship between MBFcor and 6M infarct size
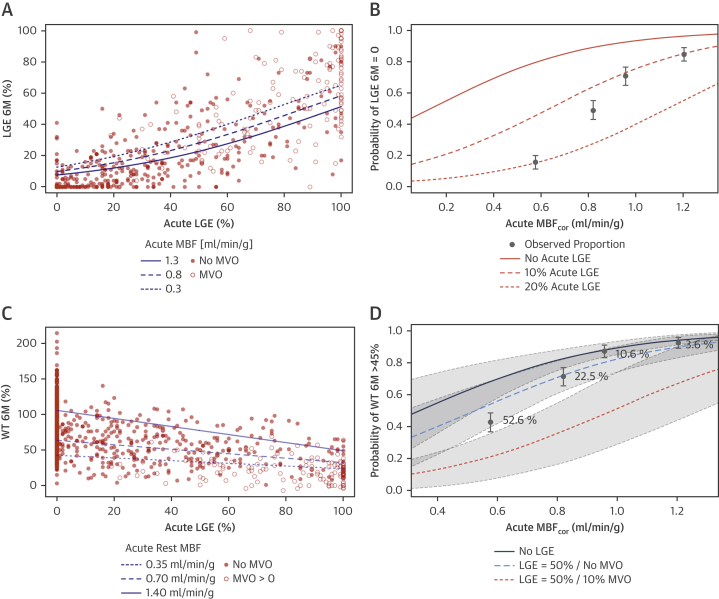
Both acutely and at 6M, MBFcor was strongly associated with LGE (Supplemental Figure 2A). Acute MBFcor had a significant effect on 6M LGE, independent of acute LGE and MVO (p = 0.013) (Table 4, Figures 4A and 4B). As an example of point estimates from the model summarized in Table 4, we note that for segments with LGE of 50% and acute MBFcor of 0.3 ml/min/g, the predicted IS at 6M (34%) was ∼6% larger than in segments with the same LGE and an acute MBFcor of 0.8 ml/min/g (28% LGE at 6M) and ∼11% larger than in segments with same LGE and an acute MBFcor of 1.3 ml/min/g (23% LGE at 6M) (Figure 4A). There was no evidence of an interaction between acute LGE and MBFcor for predicting 6M LGE.
Table 4.
Predictive Value of Acute MBFcor, LGE, and MVO for 6M LGE and WT (GAMLSS Model)
| Multivariable Associations | Coefficient ± SE | t Value | p Value |
|---|---|---|---|
| GAMLSS for LGE 6M | |||
| (Intercept) | −1.7 ± 0.209 | −8.4 | <0.001 |
| Acute LGE (%) | 0.025 ± 0.002 | 15.4 | <0.001 |
| Acute MBFcor (ml/min/g) | −0.564 ± 0.228 | −2.5 | 0.013 |
| MVO (%) | 0.017 ± 0.006 | 2.6 | 0.009 |
| GAMLSS for WT 6M | |||
| (Intercept) | 74.147 ± 1.522 | 48.71 | <0.001 |
| Acute LGE (%) | −0.377 ± 0.042 | −9.01 | <0.001 |
| Acute MBFcor (ml/min/g) (centered) | 60.066 ± 6.314 | 9.51 | <0.001 |
| MVO (%) | −0.944 ± 0.238 | −3.97 | <0.001 |
| Acute LGE: acute MBFcor | −0.360 ± 0.151 | −2.39 | 0.017 |
Figure 4.
Predictive Value of MBFcor for Final IS and 6M Functional Recovery
(A) The lines show the predictions for acute myocardial blood flow corrected (MBFcor) equal to 0.3, 0.8, and 1.3 ml/min/g. (B) The observed proportions of segments with no 6-month (6M) late gadolinium enhancement (LGE) are shown by quartile of acute myocardial blood flow corrected, with 95% confidence intervals. The lines show the prediction for no 6-month late gadolinium enhancement as a function of acute myocardial blood flow corrected and acute late gadolinium enhancement of 0%, 10%, and 20%. (C) The continuous lines show prediction for acute myocardial blood flow corrected equal to 0.35, 0.70, and 1.40 ml/min/g. (D) The observed proportions of segments with wall thickening (WT) <45% are shown by quartile of acute myocardial blood flow corrected, with 95% confidence intervals. The lines show the predictions for wall thickening <45% as a function of acute myocardial blood flow corrected, and 0% and 50% late gadolinium enhancement, and with and without microvascular obstruction in the case of 50% acute late gadolinium enhancement. IS = infarct size.
In segments without LGE at 6M, 97% had no acute LGE, whereas the remaining 3% had a median acute LGE of 10.5%, which was resorbed over 6M. Conversely, 9% of segments without acute LGE showed a median LGE of 13% at 6M. The probability of no LGE at 6M varied significantly with acute MBFcor and LGE, as shown in Figure 4B. The odds of no LGE at 6M increased by 1.4:1 for each 0.1 ml/min/g increase of acute MBFcor (95% confidence interval: 1.21 to 1.58; p < 0.001), independent of acute LGE. Each 10% increase of acute LGE decreased the odds of no LGE at 6M by 1:4.7 (p < 0.001).
Predictive value of acute MBFcor for 6M segmental functional recovery
Acute MBFcor and LGE are simultaneous, independent predictors of 6M WT (Table 4, Supplemental Figure 2B and 2C). Notably, our results show that in segments with intermediate LGE (Figure 4C), acute MBFcor can account for the relatively large variability of 6M WT independent of the level of acute LGE; segments with intermediate LGE (i.e., 50%) and MBFcor of 0.35 ml/min/g have a 15% reduction of WT 6M compared with segments with the same LGE and MBFcor of 0.7 ml/min/g, and a 28% reduction compared with segments with same LGE and normal MBFcor of 1.0 ml/min/g.
Acute MBFcor impairment decreases the likelihood of 6M functional recovery (Figure 4D), both in a univariate model that includes only MBFcor (p < 0.001) and in a multivariate model that also includes acute T1 (p = 0.001), acute LGE (p = 0.02), and MVO (p = 0.001) as additional predictors. In the multivariate model the odds of WT >45% at 6M increased by 1.38:1 (95% confidence interval: 1.29 to 1.48) for each 0.1 ml/min/g increase of acute MBFcor (Table 5).
Table 5.
Odds Ratios and 95% Confidence Intervals for Univariate and Multivariate Logistic Regression Model for WT at 6M >45% (GAMLSS), With Forest Plots for Univariate and Multivariate Regression Analysis
| OR | 2.5% Percentile for OR | 97.5% Percentile for OR | t Value | p Value | |
|---|---|---|---|---|---|
| A. Univariate model | |||||
| 0.1 ml/min/g MBFcor change | 1.793 | 1.62 | 1.98 | 11.46 | <0.001 |
| 100 ms acute T1 change | 0.282 | 0.22 | 0.36 | −10.55 | <0.001 |
| 10% LGE change | 0.654 | 0.61 | 0.70 | −13.08 | <0.001 |
| 10% MVO change | 0.026 | 0.01 | 0.06 | −8.10 | <0.001 |
| B. Multivariate model | |||||
| Intercept | 0.172 | 0.12 | 0.26 | −8.52 | <0.001 |
| 0.1 ml/min/g MBFcor change | 0.717 | 0.63 | 0.82 | −4.86 | <0.001 |
| 100 ms acute T1 change | 1.667 | 1.22 | 2.29 | 3.17 | 0.002 |
| 10% LGE change | 1.098 | 1.00 | 1.22 | 1.79 | 0.074 |
| 10% MVO change | 4.437 | 1.92 | 10.27 | 3.48 | <0.001 |
 | |||||
Additional predictive value of MBF in addition to invasive measurements for 6M EF and is reduction
Results for CFR, IMR, and Tmn post-PPCI are summarized in Table 1.
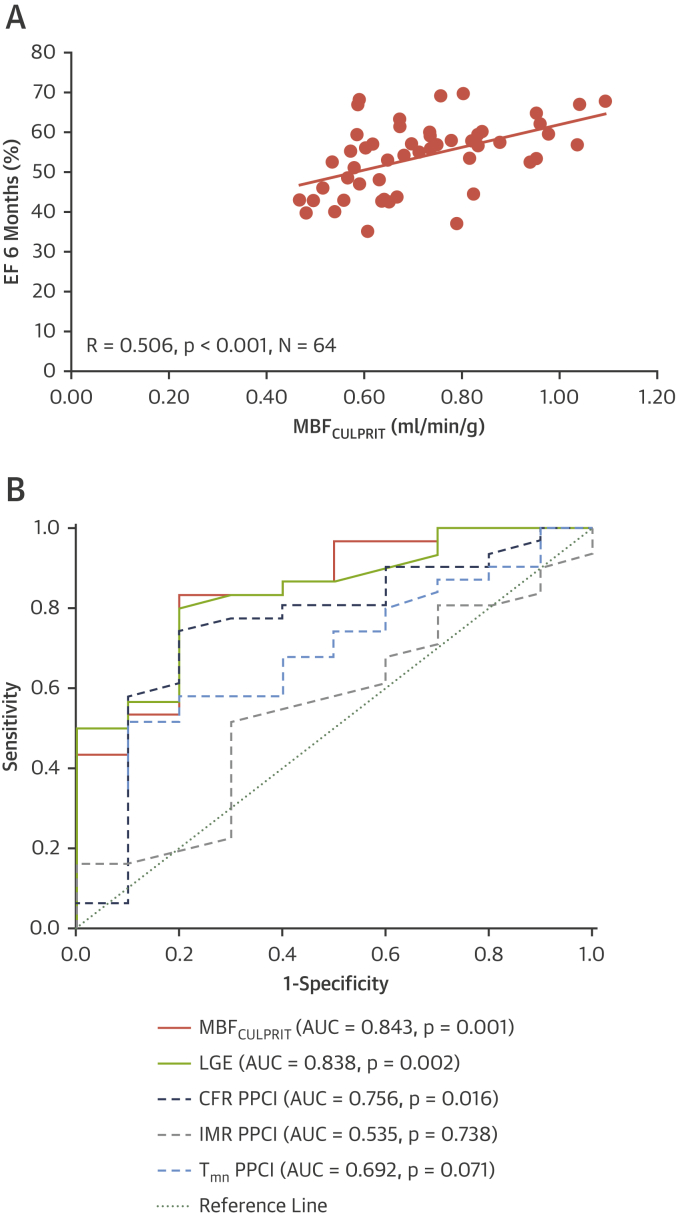
MBFCULPRIT correlated positively with 6M EF (R = 0.506, p < 0.001) (Figure 5A). When EF was simultaneously adjusted by acute LGE (p = 0.005) and acute EF (p < 0.001), the association remained significant (p = 0.02). In patients with IMR <40 or CFR >2 (N = 40), acute MBFCULPRIT and LGE were equally strong predictors of EF <50% at 6M (area under the curve: 0.843, p = 0.001 for MBFCULPRIT; and area under the curve: 0.838, p = 0.002 for LGE) (Figure 5B). MBFCULPRIT (p = 0.031) was independently associated with IS reduction (Table 6).
Figure 5.
Relationship Between Acute MBFCULPRIT and EF 6M
(A) Per patient analysis. (B) Receiver-operating characteristic curves showing the diagnostic performance of acute average myocardial blood flow in the culprit territory (MBFCULPRIT) and late gadolinium enhancement (LGE), index of microvascular resistance (IMR), coronary flow reserve (CFR), and transit time at rest (Tmn) in predicting left ventricular (LV) dysfunction (ejection fraction [EF] <50%) at 6 months (6M) in patients with index of microvascular resistance <40 or coronary flow reserve >2 at the time of primary percutaneous coronary intervention. AUC = area under the curve; PPCI = primary percutaneous coronary intervention.
Table 6.
Multivariable Associations Among Clinical Characteristics, Invasive Measurements at the Time of PPCI, and Infarct Size Reduction at 6M in Patients With IMR <40 or CFR >2
| IS Reduction From Acute to 6M |
||||
|---|---|---|---|---|
| Univariable Analysis |
Multivariable Analysis |
|||
| Estimate Coefficient | p Value | Estimate Coefficient | p Value | |
| Age (yrs) | −0.005 | 0.163 | — | — |
| Male | 0.016 | 0.861 | — | — |
| Diabetes | −0.144 | 0.266 | — | — |
| Hypertension | −0.161 | 0.026 | −0.175 | 0.012 |
| Hypercholesterolemia | −0.110 | 0.165 | — | — |
| Active smoking | 0.009 | 0.896 | — | — |
| Family history of CHD | 0.056 | 0.413 | — | — |
| Pain to balloon time (min) | 0.0001 | 0.152 | — | — |
| LAD vs. non-LAD | 0.108 | 0.116 | — | — |
| TIMI pre | −0.038 | 0.131 | — | — |
| TIMI post | 0.114 | 0.474 | — | — |
| CFR PPCI | 0.095 | 0.041 | 0.201 | 0.195 |
| IMR PPCI | −0.002 | 0.473 | — | — |
| EDV (ml) | 0.000 | 0.710 | — | — |
| ESV (ml) | 0.000 | 0.714 | — | — |
| EF (%) | 0.001 | 0.733 | — | — |
| Acute IS (%) | −0.001 | 0.673 | — | — |
| MBFCULPRIT (ml/min/g) | 0.350 | 0.071 | 0.394 | 0.031 |
| MVO (yes/no) | −0.141 | 0.037 | — | — |
| MVO (%) | −0.020 | 0.206 | 0.201 | 0.209 |
| IMH (yes/no) | −0.105 | 0.124 | — | — |
| IMH (%) | −0.008 | 0.388 | — | — |
Discussion
The microcirculation plays a crucial role in infarct healing as the main supply conduit of oxygen and nutrients and as a delivery path for pharmacological treatments. An impairment in microvascular function is associated with a poor prognosis (12). To the best of our knowledge, this is the first CMR study using absolute MBF quantification to systematically estimate the longitudinal changes in microvascular function and predict functional recovery following STEMI.
The main findings of the study are as follows: 1) the acute microvascular dysfunction is strongly associated with the extent of the ischemic injury; 2) it is reversible, depending on the severity of the acute injury; 3) acute MBFcor, LGE, and MVO are simultaneous predictors of final IS; 4) MBFcor has incremental predictive value for final IS and segmental recovery, compared with predictions that are based solely on acute LGE; and 5) in a lower-risk subgroup of patients (IMR <40 or CFR >2), acute rest MBF in the culprit territory may be superior to acute invasive measurements in predicting long-term recovery and may be an independent predictor of IS reduction. Taken together, these results suggest that quantifying myocardial blood at the acute stage leads to an improved prediction of segmental myocardial viability and wall motion at follow-up.
The lack of detailed understanding of the pathophysiological cascade leading up to irreversible microvascular damage in the context of ischemia-reperfusion injury has prevented adequate pharmacological treatment (3). Previous evidence by positron emission tomography or CMR stress testing demonstrated partial recovery of the vasomotor function over time 13, 14; however, such studies were performed in a subacute phase post-MI, and MBF was assessed in the myocardium pertaining to the culprit artery, thus leading to values likely not fully representative of the severity of injury. For the first time, our findings clearly show that the progressive deterioration of the acute microvascular function following ischemic injury is reversible even in severely damaged tissue. MBF normalization in areas with intermediate degree of ischemic insult is in line with pre-clinical evidence showing reversible endothelial damage and increased permeability of the microvessels at the time of reperfusion 15, 16. In contrast, for areas with MVO, the underlying mechanisms promoting functional recovery and infarct reduction are less clear. The infarcted myocardium, once thought to be “dead,” is actually a dynamic tissue undergoing an extensive process of remodeling, ultimately forming a core of scar, surrounded by neoangiogenesis in the infarct border zone 17, 18. No improvement in MBF was seen in infarcted areas with persistent edema at 6M. As shown recently, residual iron deposits at 6M following acute IMH resulted from severe acute microvascular impairment (19). Therefore, the correlation between persistent edema and microvascular dysfunction seems plausible and in line with putative mechanisms for edema in the presence of capillary leakage or hemorrhage.
In this study, we demonstrate for the first time the incremental role of microvascular function for final IS in segments with intermediate or lower LGE transmurality, independent of the extent of the acute LGE and MVO. The long-term prognostic value of even small amounts of LGE has been previously studied 20, 21. Our results show that the probability of having no 6M LGE is significantly affected by acute microvascular function, a finding suggesting that measures to restore microvascular function after MI may have longer-term benefits. Unexpectedly, we found that a small fraction of segments without LGE acutely were positive to LGE at 6M. There are at least 3 potential explanations for this finding: 1) a mismatch of short-axis positions imaged at different time points; 2) lower diagnostic performance of standard CMR techniques such as T2W and LGE compared with more accurate parametric maps to quantify ischemic injury; and 3) late development of necrotic tissue within an area at risk.
Finally, our study shows that in patients with preserved microvascular function as assessed by invasive measurements post-PPCI (i.e., IMR <40 or CFR >2), acute MBF estimates of microvascular function could provide a tool to stratify patients and predict LV remodeling. The predictive relevance of the changes in microvascular function early after PPCI are known (8): CMR MBF assessment could represent a valid alternative to invasive repeated measurements.
Study limitations
The results of this study should be interpreted in light of some limitations. In this study, continuous variables such as MBF and native T1 represent averages for each myocardial segment. Any estimate of an effect modification of MVO in the relationship of native T1 and MBF is likely not representative of MVO itself because MVO in most segments represented a relatively small subarea of a myocardial segment. Our IMR measurements (median 32; interquartile range: 20 to 46) are comparable to those in previous studies 4, 22; however, we did not find a significant relationship between IMR and MVO/MBFCULPRIT. There may be several reasons for this, including the small sample size and the assessment of resting perfusion rather than stress perfusion 3 days after the invasive coronary measurements (8). Further larger studies are needed to corroborate our findings.
Conclusions
Microvascular function estimated using CMR MBF has additional clinical and prognostic value beyond tissue characterization. In the context of novel potential therapeutic targets such as endothelial integrity, vascular permeability, or angiogenesis (23), MBF measurements could represent a useful noninvasive tool for risk stratification of patients with STEMI post-PPCI and to guide cardioprotective treatment.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Acute microvascular impairment is dynamic and reversible over time. Beyond EF and IS, acute microvascular function has incremental prognostic value for LV remodeling.
TRANSLATIONAL OUTLOOK: Further studies are required to determine whether adjunct therapy targeting microvascular function at the time of PPCI will improve patients’ outcomes.
Acknowledgments
The authors thank the clinical staff of the Oxford Heart Centre, the staff of the Oxford Centre for Clinical Magnetic Resonance Research (OCMR), and the Oxford Acute Vascular Imaging Centre (AVIC).
Footnotes
Dr. Dall’Armellina is currently affiliated with the Leeds Institute of Cardiovascular and Metabolic Medicine, Division of Biomedical Imaging, University of Leeds, Leeds, United Kingdom. This work is supported by the British Heart Foundation (BHF) and the Oxford National Institute for Health Research Biomedical Research Centre. Profs. Channon, Choudhury, and Neubauer acknowledge support from the Oxford British Heart Foundation Centre of Research Excellence. Dr. Dall’Armellina is a BHF Intermediate Clinical Research Fellow. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Appendix
References
- 1.de Waha S., Patel M.R., Granger C.B. Relationship between microvascular obstruction and adverse events following primary primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: an individual patient data pooled analysis from seven randomized trials. Eur Heart J. 2017;38:3502–3510. doi: 10.1093/eurheartj/ehx414. [DOI] [PubMed] [Google Scholar]
- 2.Symons R., Pontone G., Schwitter J. Long-term incremental prognostic value of cardiovascular magnetic resonance after ST-segment elevation myocardial infarction. A study of the Collaborative Registry on CMR in STEMI. J Am Coll Cardiol Img. 2018;11:813–825. doi: 10.1016/j.jcmg.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz Longacre L., Kloner R.A., Arai A.E. New horizons in cardioprotection: recommendations from the 2010 National Heart, Lung, and Blood Institute Workshop. Circulation. 2011;124:1172–1179. doi: 10.1161/CIRCULATIONAHA.111.032698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fearon W.F., Low A.F., Yong A.S. Prognostic value of the Index of Microcirculatory Resistance measured after primary percutaneous coronary intervention. Circulation. 2013;127:2436–2441. doi: 10.1161/CIRCULATIONAHA.112.000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carrick D., Haig C., Ahmed N. Comparative prognostic utility of indexes of microvascular function alone or in combination in patients with an acute ST-segment-elevation myocardial infarction. Circulation. 2016;134:1833–1847. doi: 10.1161/CIRCULATIONAHA.116.022603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jerosch-Herold M., Wilke N., Stillman A.E., Wilson R.F. Magnetic resonance quantification of the myocardial perfusion reserve with a Fermi function model for constrained deconvolution. Med Phys. 1998;25:73–84. doi: 10.1118/1.598163. [DOI] [PubMed] [Google Scholar]
- 7.Selvanayagam J.B., Jerosch-Herold M., Porto I. Resting myocardial blood flow is impaired in hibernating myocardium: a magnetic resonance study of quantitative perfusion assessment. Circulation. 2005;112:3289–3296. doi: 10.1161/CIRCULATIONAHA.105.549170. [DOI] [PubMed] [Google Scholar]
- 8.Cuculi F., Dall’Armellina E., Manlhiot C. Early change in invasive measures of microvascular function can predict myocardial recovery following PCI for ST-elevation myocardial infarction. Eur Heart J. 2014;35:1971–1980. doi: 10.1093/eurheartj/eht434. [DOI] [PubMed] [Google Scholar]
- 9.Baks T., van Geuns R.-J., Biagini E. Effects of primary angioplasty for acute myocardial infarction on early and late infarct size and left ventricular wall characteristics. J Am Coll Cardiol. 2006;47:40–44. doi: 10.1016/j.jacc.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Liu D., Borlotti A., Viliani D. CMR native T1 mapping allows differentiation of reversible versus irreversible myocardial damage in ST-segment-elevation myocardial infarction: an OxAMI study (Oxford Acute Myocardial Infarction) Circ Cardiovasc Imaging. 2017;10:e005986. doi: 10.1161/CIRCIMAGING.116.005986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bates D., Mächler M., Bolker B., Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. [Google Scholar]
- 12.Wu K.C., Zerhouni E.A., Judd R.M. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation. 1998;97:765–772. doi: 10.1161/01.cir.97.8.765. [DOI] [PubMed] [Google Scholar]
- 13.Teunissen P.F., Timmer S.A., Danad I. Coronary vasomotor function in infarcted and remote myocardium after primary percutaneous coronary intervention. Heart. 2015;101:1577–1583. doi: 10.1136/heartjnl-2015-307825. [DOI] [PubMed] [Google Scholar]
- 14.Lee B.C., Hsu H.C., Tseng W.Y. Effect of cardiac rehabilitation on angiogenic cytokines in postinfarction patients. Heart. 2009;95:1012–1018. doi: 10.1136/hrt.2008.153510. [DOI] [PubMed] [Google Scholar]
- 15.Svendsen J.H., Bjerrum P.J., Haunso S. Myocardial capillary permeability after regional ischemia and reperfusion in the in vivo canine heart. Effect of superoxide dismutase. Circ Res. 1991;68:174–184. doi: 10.1161/01.res.68.1.174. [DOI] [PubMed] [Google Scholar]
- 16.Kloner R.A., Ganote C.E., Jennings R.B. The “no-reflow” phenomenon after temporary coronary occlusion in the dog. J Clin Invest. 1974;54:1496–1508. doi: 10.1172/JCI107898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frangogiannis N.G. The mechanistic basis of infarct healing. Antioxid Redox Signal. 2006;8:1907–1939. doi: 10.1089/ars.2006.8.1907. [DOI] [PubMed] [Google Scholar]
- 18.Van Kerckhoven R., van Veghel R., Saxena P.R., Schoemaker R.G. Pharmacological therapy can increase capillary density in post-infarction remodeled rat hearts. Cardiovasc Res. 2004;61:620–629. doi: 10.1016/j.cardiores.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 19.Bulluck H., Rosmini S., Abdel-Gadir A. Residual myocardial iron following intramyocardial hemorrhage during the convalescent phase of reperfused ST-segment–elevation myocardial infarction and adverse left ventricular remodeling. Circ Cardiovasc Imaging. 2016;9:e004940. doi: 10.1161/CIRCIMAGING.116.004940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwong R.Y., Chan A.K., Brown K.A. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation. 2006;113:2733–2743. doi: 10.1161/CIRCULATIONAHA.105.570648. [DOI] [PubMed] [Google Scholar]
- 21.Kim H.W., Klem I., Shah D.J. Unrecognized non-Q-wave myocardial infarction: prevalence and prognostic significance in patients with suspected coronary disease. PLoS Med. 2009;6:e1000057. doi: 10.1371/journal.pmed.1000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cuculi F., De Maria G.L., Meier P. Impact of microvascular obstruction on the assessment of coronary flow reserve, index of microcirculatory resistance, and fractional flow reserve after ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2014;64:1894–1904. doi: 10.1016/j.jacc.2014.07.987. [DOI] [PubMed] [Google Scholar]
- 23.Hollander M.R., de Waard G.A., Konijnenberg L.S.F. Dissecting the effects of ischemia and reperfusion on the coronary microcirculation in a rat model of acute myocardial infarction. PLoS One. 2016;11:e0157233. doi: 10.1371/journal.pone.0157233. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.