Abstract
The ART program in low- and middle-income countries (LMIC) like India, follows a public health approach with a standardized regimen for all people living with HIV (PLHIV). Based on the evidence from high-income countries (HIC), the risk of an enhanced, and accentuated onset of premature-aging or age-related diseases has been observed in PLHIV. However, very limited data is available on residual inflammation and immune activation in the populations who are on first-generation anti-HIV drugs like zidovudine and lamivudine that have more toxic side effects. Therefore, the aim of the present study was to evaluate the levels of systemic inflammation and understand the risk of age-associated diseases in PLHIV on long-term suppressive ART using a large number of biomarkers of inflammation and immune activation. Blood samples were obtained from therapy naïve PLHIV (Pre-ART, n = 43), PLHIV on ART for >5 years (ART, n = 53), and HIV-negative healthy controls (HIVNC, n = 41). Samples were analyzed for 92 markers of inflammation, sCD14, sCD163, and telomere length. Several statistical tests were performed to compare the groups under study. Multivariate linear regression was used to investigate the associations. Despite a median duration of 8 years of successful ART, sCD14 (p < 0.001) and sCD163 (p = 0.04) levels continued to be significantly elevated in ART group as compared to HIVNC. Eleven inflammatory markers, including 4E-BP1, ADA, CCL23, CD5, CD8A, CST5, MMP1, NT3, SLAMF1, TRAIL, and TRANCE, were found to be significantly different (p < 0.05) between the groups. Many of these markers are associated with age-related co-morbidities including cardiovascular disease, neurocognitive decline and some of these markers are being reported for the first time in the context of HIV-induced inflammation. Linear regression analysis showed a significant negative association between HIV-1-positivity and telomere length (p < 0.0001). In ART-group CXCL1 (p = 0.048) and TGF-α (p = 0.026) showed a significant association with the increased telomere length and IL-10RA was significantly associated with decreased telomere length (p = 0.042). This observation warrants further mechanistic studies to generate evidence to highlight the need for enhanced treatment monitoring and special interventions in HIV-infected individuals.
Keywords: long term antiretroviral therapy, LMIC (lower middle income country), inflammation markers, HIV, India
Introduction
The most remarkable achievement in the battle against the Human Immunodeficiency Virus (HIV) is the discovery of very efficient, well-tolerated combinational antiretroviral therapy (ART) that has transformed the deadly viral infection into a chronic, manageable disease. In the absence of a cure, HIV-infection requires lifelong treatment. Though treatment successfully controls HIV-replication, and prevents opportunistic infections, HIV-infected persons on long term ART suffer from some maladies that are typically associated with human aging (1). The effect of HIV-associated inflammation and immune activation on the premature onset of immunosenescence despite effective viral suppression are thought to be the primary reasons for early aging in people living with HIV (PLHIV) (2).
Chronic, low-grade systemic inflammation resulting from an increased pro-inflammatory state contributes to the progressive pathophysiological changes associated with aging. This process of increase in pro-inflammation followed by a chronic inflammatory state is termed as “inflamm-aging” and is a significant risk factor for morbidity and mortality in the elderly people (3). The inflammatory environment triggers the development of several age-related non-infectious comorbidities (NICMs) (1). In HIV infection, it is believed that viral persistence in a rare population of long-lived, latently infected cells despite successful ART contributes to the chronic inflammatory state (1).
Unlike in high-income countries (HIC), the ART program in low- and middle-income countries (LMIC) like India, follows a public health approach with a standardized regimen for all PLHIV. As of December 2016, nearly 1 million PLHIV were receiving free ART through the government program in the country (4). The recommended first-line ART regimen includes one non-nucleoside reverse transcriptase inhibitor (NNRTI), either nevirapine (NVP) or efavirenz (EFV), in the backbone of two nucleoside reverse transcriptase inhibitors (NRTI), either zidovudine (AZT), or tenofovir (TDF), and lamivudine (3TC) (5). Although perfect adherence to treatment remains a challenge, the reasonably good response to first-line therapy indicates the overall success of the ART program in the country (6). Thus, with the expansion of the ART program and its consequences in PLHIV, the burden of age-related non-AIDS diseases are likely to increase. Since the environment has an enormous impact on age and age-related diseases, and the genetic determinants of aging may vary across populations, studies conducted in HICs might not apply to the LMICs (7).
The present study attempted to evaluate HIV-associated inflammation and immune activation and the contribution to inflamm-aging in PLHIV on long-term combination ART (cART) by assessing markers of inflammation as well as aging, including a panel of 92 inflammatory markers, two well-characterized immune activation markers (sCD14 and sCD163), and telomere length. The study provides useful insights into the role of inflammation and aging in HIV-1 infected individuals despite successful ART.
Materials and Methods
Study Design and Participants
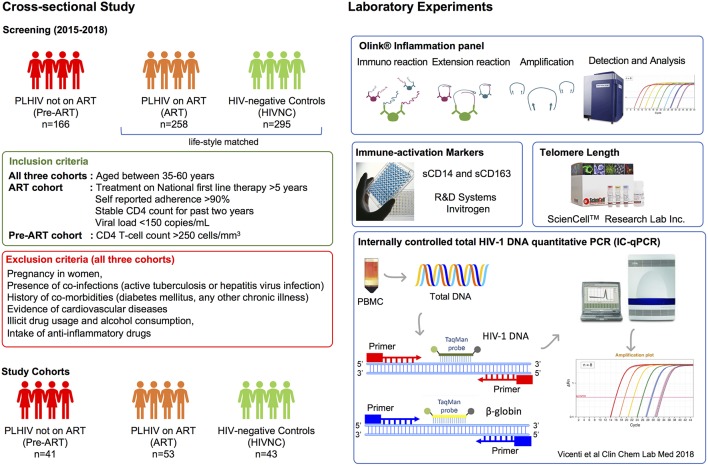
The study included three groups of individuals: (i) treatment-naïve PLHIV with viremia and moderate CD4 count (Pre-ART herein) (ii) PLHIV of age between 35 and 60 years on ART for more than 5 years with suppressed viremia on national first-line ART and >90% adherence to treatment (ART herein), and (iii) life-style, age, and gender-matched (with ART group) HIV-1 negative healthy individuals free of any kind of chronic illness (HIVNC herein). The HIV-1 positive cohort was recruited from a tertiary care ART Center at the Government Hospital for Thoracic Medicine (GHTM), Chennai, India, at the time of routine standard-of-care hospital visit. Exclusion criteria were pregnancy in women, Immune Reconstitution Inflammatory Syndrome (IRIS), presence of co-infections like active tuberculosis or hepatitis virus infection, history of co-morbidities like diabetes mellitus, obesity, evidence of cardiovascular disease or any chronic illness, illicit drug usage, alcohol consumption, and intake of anti-inflammatory drugs in the past 1 month. For the ART group, we screened 258 individuals and recruited 55 persons who matched the above criteria for the study. After screening 166 individuals for the pre-ART group, 41 who met the eligibility criteria were recruited for the study. Plasma viral load was measured using the Abbott RealTime HIV-1 viral load assay (Abbott, US). Two individuals in the ART group had viral load >150 copies/mL (4,000 and 1,800 copies/mL, respectively) and had to be excluded from the study. We screened 295 healthy individuals in and around Chennai, India, and identified 43 lifestyle, age, and gender-matched individuals for the HIVNC group.
The overall study design is presented in Figure 1. After first-time counseling and obtaining informed consent to participate in the study, 15 mL of venous blood was collected from each study participant.
Figure 1.
Flow diagram of study design and experimental plan. 424 HIV-1 positive individuals and 295 HIV-1 negative healthy controls were screened. Following defined inclusion and exclusion criteria, 43 healthy controls, and 53 HIV positive ART-experienced subjects and 41 ART-naïve HIV-1 positive subjects were recruited for the study. The methodology used for the study is also presented.
Proteomic Profiling of the Soluble Factors in Plasma
Plasma samples from the Pre-ART, ART, and HIVNC groups were subjected to soluble proteome analysis using the Olink® Inflammation Panel that includes 92 inflammation-related soluble factors (Olink Bioscience AB, Uppsala, Sweden) (8). Of the 92 proteins, only 75 were detectable in >50% of the samples, and therefore our analysis was restricted to these proteins alone. Two Pre-ART samples and one HIVNC sample did not pass the quality control and had to be excluded from our analysis. We also measured two widely reported biomarkers of immune activation, sCD14 (Human CD14 Quantikine ELISA Kit R&D Systems, UK) and sCD163 (Thermo Scientific™ Pierce™ Human CD163 Kit, Thermo Scientific, USA) in plasma.
Telomere Length in Peripheral Blood Mononuclear Cells (PBMC)
As a molecular biomarker of “biological aging,” the leucocyte telomere length was measured in PLHIV on ART and HIV-negative controls. Genomic DNA was extracted from PBMC using the QIAamp DNA Mini Kit (Qiagen, Germany) and telomere length was measured using the Absolute Human Telomere Length Quantification qPCRAssay Kit (AHTLQ; ScienCell Research Laboratories, US) following manufacturer's instructions. All samples were tested in triplicate.
Total HIV-1 DNA Quantification Using IC-qPCR Marker for HIV-1 Reservoir Level
To quantify total HIV-1 DNA from PBMC, internally controlled qPCR (IC-qPCR) was performed as described previously (9). IC-qPCR was performed in duplicate using 500 ng template DNA and Takara Premix Ex Taq™ (Probe qPCR) (Takara, Japan). Primers targeting HIV-1 LTR and Beta-globulin were used. Total HIV-1 DNA copy numbers were calculated based on the linear equation of the 10-fold Beta-globulin standard curve derived from Jurkat cells and the 10-fold pNL4-3 plasmid standard curve, diluted in 50 ng/μL of Jurkat DNA to mimic clinical samples and normalized to obtain HIV-1 DNA copies per million PBMC.
Statistical Analysis and Data Visualization
Based on the normality of the data Mann Whitney U-test, Spearman correlation and one-way analysis of variance (ANOVA) were performed to investigate the difference in mean levels of protein expression (NPx) in the different groups under study. Multivariate linear regression for the outcome of telomere length was used to investigate the association between HIV status and HIV treatment duration adjusting for chronological age as well as other disease and patient characteristics. These same models were used to investigate evidence of mediation of the HIV-telomere relationship by soluble biomarkers. Feature ranking process using Random Forest algorithm was executed using R package randomForest. A heatmap was generated to visualize the clustering of samples based on protein expression using gplots v3.0.1 in R. Similarity in protein expression within the groups was visualized using a principal component analysis using PCAtools. All other analysis was performed using base R. P-values are not corrected for multiple comparisons, although false discovery rate was used in some of the biomarker discovery analyses.
Ethical Clearances
The study was approved by the Institutional Ethics Committee of the National Institute for Research in Tuberculosis (NIRT IEC No: 2015023) and Institutional Review Board of the Government Hospital for Thoracic Medicine (GHTM-27102015) Chennai, India. All the study participants gave written informed consent. Patient identities were anonymized and delinked before analysis.
Results
Patient Characteristics
Cohort characteristics are presented in Table 1. All three cohorts were gender-matched. There were 51, 43, and 51% of females in the Pre-ART, ART, and HIVNC, respectively. The median age of ART, Pre-ART, and HIVNC groups were 45, 40, and 46 years, respectively. In the ART group, the median (IQR) duration of treatment was 8 years (6–10 years). As per the inclusion criteria, all the patients had >90% of self-reported adherence which was further confirmed by viral load <150 copies/mL and very low-level HIV-1 reservoir with median (25th−75th) 2.87 (2.62–3.18) log10 copies/106 PBMC. Within the ART group, 57% (30/53) were on zidovudine, lamivudine, and nevirapine (ZDV/3TC/NVP) regimen and the remaining 43% (23/53) were on tenofovir, lamivudine, and efavirenz (TDF/3TC/EFV) regimen. All individuals were initiated on antiretroviral treatment in the chronic phase of the disease with a median (25–75th) CD4 count of 186 (100–280) cells/μL, as per the National guidelines for eligibility to ART existing then.
Table 1.
Patients' demographic and clinical parameters.
| Parameter | Treatment naïve (Pre-ART) | Treatment experienced (ART) | HIV-negative control (HIVNC) | P-values |
|---|---|---|---|---|
| N | 41 | 53 | 43 | ND |
| Gender, Female, N (%) | 21 (51) | 23 (43) | 22 (51) | 0.6734# |
| At sampling | ||||
| Age in years, median (IQR) | 40 (37–43) | 45 (42–49) | 46 (40–54) | <0.0001* |
| CD4 count (cells/μL); median (IQR) | 367 (251–578) | 667 (476–797) | NA | <0.0001§ |
| CD8 count (cells/μL); median (IQR) | 1,138 (872–1,625) | 772 (337–1,092) | NA | <0.0001§ |
| CD4:CD8 ratio, median (IQR) | 0.329 (0.1,863–0.529) | 0.76 (0.575–1.013) | NA | <0.0001§ |
| Viral Load, Log10 copies/mL, mean (SD) | 4.4943 (0.9036) | 2.14 | NA | <0.0001§ |
| Years on treatment, median (IQR) | NA | 8 (6–10) | NA | ND |
| Treatment Regimen, n (%) | ||||
| ZDV+3TC+NVP | NA | 30 (57%) | NA | ND |
| TDF+3TC+EFV | 23 (43%) | |||
| CD4 Count at treatment initiation (cells/μL), median (IQR) | NA | 186 (100–280) | NA | ND |
| HIV-1 Reservoir by total DNA count, log10 copies/mL/106 PBMC, Median (IQR) | ND | 2.87 (2.62–3.18) | ND | ND |
NA, Not available; ND, Not Done;
Kruskal-Wallis test,
χ2 test,
Mann-Whitney test.
ZDV, zidovudine; 3TC, lamivudine; NVP, nevirapine; TDF, tenofovir and EFV, efavirenz; PBMC, Peripheral blood mononuclear cells.
Soluble Markers of Immune Activation in Plasma
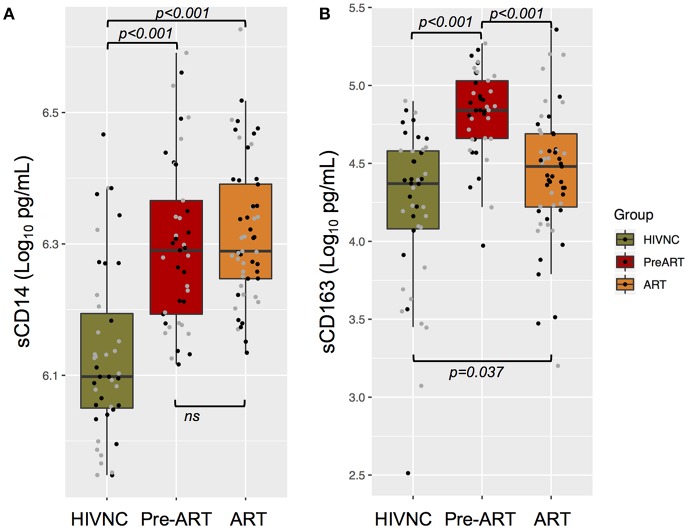
Soluble markers of immune activation, sCD14, and sCD163, were measured in plasma samples of the three study groups. PLHIV had significantly higher levels of sCD14 (Figure 2A) and sCD163 (Figure 2B) in plasma as compared to HIVNC (p < 0.001, Mann Whitney U-Test). Interestingly, despite prolonged suppressive ART (median duration of 8 years of cART), there was no significant decline in the levels of sCD14 in PLHIV. Levels of sCD163 were significantly lower in the ART group as compared to the Pre-ART group (30,014 pg/mL vs. 68,192 pg/mL, p < 0.001, Mann Whitney U Test), but were significantly higher as compared to the HIVNC group (p = 0.04, Mann Whitney U-Test). We did not find any significant correlation between duration of cART and sCD14 (Spearman r: 0.163; p = 0.2432) or sCD163 (Spearman r: 0.154; p = 0.2720) levels in the ART group.
Figure 2.
Plasma immune activation markers. Soluble CD14 (A) and CD163 (B) in plasma of the three groups of individuals were measured using ELISA.
Soluble Markers of Inflammation in Plasma
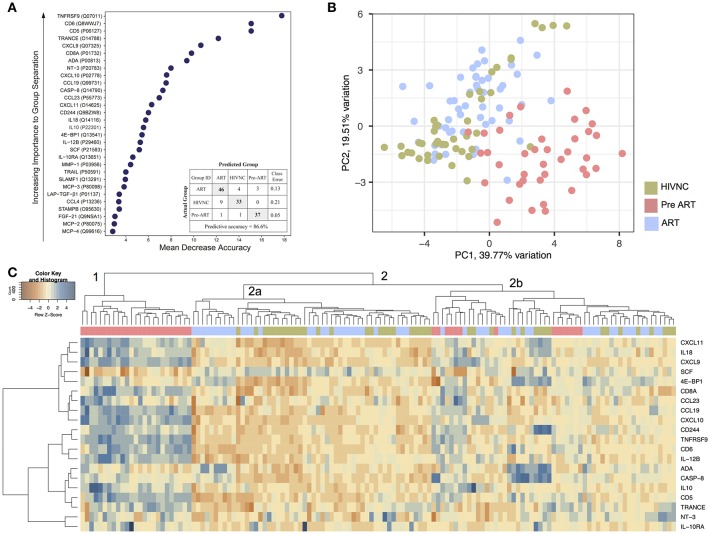
To identify candidate biomarkers for future study, we looked for biomarkers that differed significantly between the groups by Random Forest (RF) analysis (Figure 3A). The proteins with the most significant differences were TNFRSF9, sCD6, sCD5, TRANCE, and CXCL9. The supervised principal component analysis (PCA) based on the top 30 proteins that were identified as in RF showed that 82% (32/39) of Pre-ART samples grouped together (Figure 3B). In HIVNC, 74% (31/43) grouped together while in the ART group, 94% (50/53). Three ART patients grouped together closely with the Pre-ART group. Hierarchical clustering analysis (HCA) with top 20 proteins, revealed a large cluster of 25 Pre-ART samples (cluster 1) along with several small clusters of ART and HIVNC samples together (cluster 2a). Five protein clusters were also identified with a group-specific distinct profile (Figure 3C).
Figure 3.
Plasma inflammation markers. (A) The random forest (RF) analysis of soluble factors resulted in predictive accuracies of 86.6% for HIVNC, Pre-ART, and ART. The soluble factors importance plots display the top 30 metabolites, which most strongly contribute to the groups' separation for HIVNC, Pre-ART, and ART. (B) Principal component analysis using PCAtool indicating grouping in different disease categories. (C) Hierarchical clustering analysis of ANOVA of top 20 differentially expressed proteins with false discovery rate (FDR) <0.001.
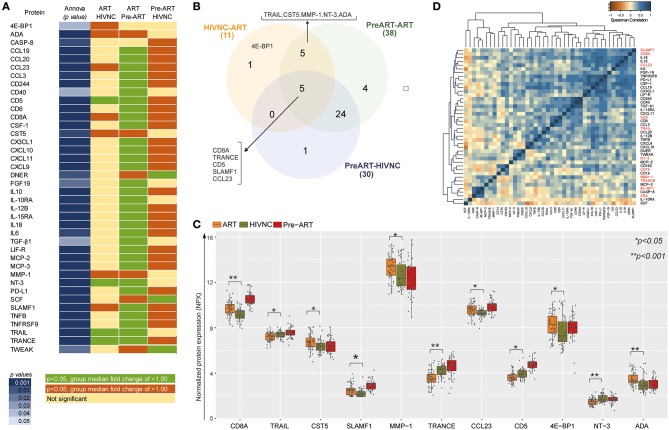
Among the 75 proteins, 40 showed a significant difference (p < 0.05, Tukey HSD) between at least one of the groups compared (ART vs. Pre-ART, Pre-ART vs. HIVNC and ART vs. HIVNC; Figure 4A). Levels of 30 inflammatory proteins were found to be significantly different between the Pre-ART and HIVNC groups, 38 proteins were significantly different between the Pre-ART and ART groups, and 11 proteins were significantly different (p < 0.01, Tukey HSD) between the HIVNC and ART groups (Figure 4B). Out of 11 proteins, 4E-BP1 was found to differ significantly only between the ART and HIVNC groups, and not between any of the other groups. Five proteins were different between all the three groups (CD8A, TRANCE, sCD5, SLAMF1, and CCL23; Figure 4B). Of the proteins that showed a significant difference between the ART and HIVNC groups, levels of soluble NT3, CD5, TRAIL, and TRANCE were lower, but levels of ADA, MMP1, CST5, and 4E-BP1 were higher when compared to both HIVNC and Pre-ART groups. On the other hand, CD8A, SLAMF1, and CCL23 were higher in the ART group as compared to the HIVNC group (Figure 4C). CD8A, SLAMF1, and CCL23 also strongly correlated with each other and with other interleukins (IL18, IL10, and IL6) in the ART group (Figure 4D). Interestingly in the ART group, 20 proteins were correlated with sCD163, but only three proteins CCL23, CCL3, and IL6 along with sCD163 correlated with sCD14 (Supplementary Figure 1).
Figure 4.
Comparative analysis of the proteins that differed significantly between groups. (A) Proteins that have statistically significant difference (p < 0.05, Tukey HSD) (in dark blue shade) between at least one of the groups compared (ART vs. Pre-ART, Pre-ART vs. HIVNC and ART vs. HIVNC). (B) Venn diagram showing significantly different protein in the study group. The sum of the numbers in each large circle represents the total number of differentially expressed proteins in plasma in the different groups (HIVNC vs. ART, Pre-ART vs.ART, and Pre-ART vs. HIVNC). The overlapping part of the circles represents significantly different proteins in the indicated groups. (C) Comparative analysis of 11 soluble markers that are significantly different between ART and HIVNC. (D) Heatmap and clustering of significantly different proteins on their protein-protein pair relation by Spearman-correlation in ART patients. *p < 0.05, **p < 0.001.
Telomere Length
Telomere length was analyzed only in two groups (HIVNC and ART) as PBMC were not available for the Pre-ART group. Linear regression analysis, after adjusting for chronological age, and gender showed a significant negative association of HIV-1 positive status on telomere length (−2.84, 95%CI, −4.012, −1.67, p < 0.0001) with the ART group having significantly shorter telomeres. However, no significant association was observed between duration of treatment and telomere length after adjusting for age and markers of disease progression within the ART group. Several of the biomarkers considered were significantly associated with telomere length after adjustment for age, HIV status, and gender; these include CXCL1 and CD40 which are both weakly positively associated with telomere length, MMP-10, and CX3CL1, which are positively associated with telomere length. OSM (Oncostatin M) is negatively associated with telomere length. The results are outlined in detail in Supplementary Table 1. Within the ART group, after adjusting for age, gender, duration of treatment, CD4 count at initiation, CD8/CD4 ratio, and sCD14, we observed CXCL1 and TGF-α to have a significant association with increased telomere length (0.2905, 95%CI: 0.0029,0.5780 p = 0.048) and (0.7865, 95%CI: 0.1003, 1.4727, p = 0.026), respectively. We also found that IL-10RA was significantly associated with decreased telomere length (−1.79, 95%CI: −3.51, −0.07 p = 0.042).
Discussion
This study examined a cohort of PLHIV on long-term successful cART from India and found that despite a median duration of 8 years of suppressive cART, several soluble inflammatory markers that were found to be elevated in HIV-1 infected untreated subjects, were also significantly elevated in the ART group as compared to the HIVNC group. This study found higher levels of the inflammatory protein CST5, as compared to HIVNC, in addition to higher levels of 4E-BP1, SLAMF1, CCL23, and lower levels of NT3 proteins, for the first time in PLHIV on long term cART. This is the first study from India in a standardized public health setting with standard first-line cART which observed that despite successful long-term cART, persistent immune activation, and residual inflammation exist in PLHIV who are therefore at higher risk of inflamm-aging and age-related diseases as these biomarkers were previously shown to be associated with several age-related diseases (summarized in Table 2).
Table 2.
Summary of statistically different proteins between ART and HIVNC and their role in disease pathogenesis and biomarkers of age-associated diseases.
| Protein | Function | Described in age-related diseases | References |
|---|---|---|---|
| 4E-BP1 (Q13541) | Mediator of the regulation of protein translation through the MAP kinase and mTORC1 pathways. | Hepatocellular carcinoma (HCC), cancer | (10) |
| Adenosine Deaminase (ADA) (P00813) | Plays an important role in purine metabolism and enhances CD4+ T-cell differentiation and proliferation. Enhance HIV-1 effector responses by restoring T lymphocyte function |
Cardiovascular diseases, stroke, acute lymphoblastic leukemia | (11, 12) |
| CCL23 (P55773) | Shows chemotactic activity for monocytes, resting T-lymphocytes, and neutrophils | Rheumatoid arthritis, atopic dermatitis, atherosclerosis | (13) |
| CD5 (P06127) | May act as a receptor in regulating T-cell proliferation. | Rheumatoid arthritis | (14) |
| CD8A (P01732) | Plays an essential role in the immune response against both external and internal stimuli Marker of CD8 T-cell activation in HIV-infection. |
Gastric cancer, asthama | (15, 16) |
| Cystatin D (CST5) (P28325) | May play a role in controlling proteolytic activity during inflammatory processes | Traumatic brain injury | (17) |
| Matrix metalloproteinase-1 (MMP-1) (P03956) | Cleavage of several types of collagens, interacts and cleaves the secreted HIV-1 Tat protein, leading to a decrease in neuronal Tat's mediated neurotoxicity | Arthritis, ulcerative colitis, coronary atherosclerosis, cancer | (18–22) |
| Neurotrophin-3 (NT-3) (P20783) | Promoting the survival and differentiation of neurons as well as neurogenesis | Neuro-cognitive impairment, multiple sclerosis, coronary atherosclerosis | (23–25) |
| SLAMF1 (Q13291) | Role in controlling innate and adaptive immune responses | Rheumatoid arthritis | (26) |
| TRAIL (P50591) | An immune effector protein which induces apoptotic cell death of cancerous or infected cells. Involve in HIV-1 pathogenesis by inducing CD4+ T-cell death |
Rheumatoid arthritis, atherosclerosis, psoriasis, Alzheimer disease. | (27) |
| TRANCE (RANKL) (O14788) | Plays important roles in dendritic cell maturation and survival, regulation of T cell-dependent immune response, and stimulation of osteoclastogenesis | Rheumatoid arthritis, psoriatic arthritis, inflammatory bowel disease, non-traumatic fracture | (28, 29) |
The uniport id is given along with the protein name.
It has been reported that certain age-related NICMs like diabetes mellitus, cardiovascular disease, cancer, bone fracture, and renal failure, are more common among HIV-1 infected individuals as compared to the general population. Exposure to prolonged cART along with low-grade inflammation and persistent immune activation seen in HIV-infection is thought to be the cause for the increased risk of NICMs (2, 30). Chronic inflammatory conditions and persistent immune activation are believed to be the major drivers of aging physiology. Inflammatory biomarkers of aging and their association with co-morbid diseases have been studied well in many elderly populations. Proteins like 4E-BP1 and the association of mTOR with aging and age-related diseases (31, 32), has already been documented. However, there are no studies that have examined the response of plasma 4E-BP1 to ART. We found higher levels of 4E-BP1 in the ART group as compared to both HIVNC and the Pre-ART group, suggesting that this marker may be associated with duration of HIV infection and/or exposure to anti-retroviral drugs and could serve as potential biomarkers of immune-aging related to HIV and long-term ART.
In the case of well-studied immune activation markers, the ART group had significantly higher levels of sCD14 like that seen in the Pre-ART group. Contradictory results have been reported from different studies on the effect of cART on sCD14 (33–35) due to a variety of contributors (36, 37). On the other hand, sCD163, a marker of vascular inflammation (38) and neurocognitive impairment (39), was higher in PLHIV than in healthy controls, which is in contrast to previous reports (40) but in line with other studies which showed that sCD163 was higher compared to the HIV-negative controls within 2 years of therapy (41, 42). The novelty of our study was to explore a large panel of inflammatory markers and we showed significantly higher levels of MMP1 (43), ADA (44), CD8A (16), SLAMF1 (45), and CCL23 (46) in PLHIV on long term ART. Many of these markers have been reported to be linked with early stages of various age-related diseases, including atherosclerosis, arthritis, cardiovascular diseases (Table 2).
sCD5 and TRAIL have been reported as biomarkers of inflammation and related diseases in both HIV-infected as well as uninfected individuals (47–49). We found lower levels of sCD5 and TRAIL in the ART group as compared to the HIVNC group. Lower plasma and cerebrospinal fluid levels of TRAIL were observed in early Alzheimer Disease (AD) (50). The TRAIL-mediated apoptosis also plays an essential role in the clearance of virus-infected cells. An earlier study proposed to use TRAIL agonist in patients with prolonged viral suppression to eradicate the latent HIV-1 reservoir in CD4+ T cells and macrophages (51). The lower level of TRAIL in patients with long term ART could hinder the clearance of the latent HIV-1 reservoir and dampen immune responses to other viral infections including influenza and cytomegalovirus (27). CD5, a negative regulator of antigen receptor signal transduction in lymphocytes (52), is reported to stimulate the production of the anti-inflammatory cytokine IL-10 by B-cells (53). Low levels of sCD5 in HIV-infected individuals, associated with the existing pro-inflammatory state, could promote the development of age-related cancers and other diseases in these individuals. An earlier study conducted in a Swedish cohort reported normalization of sCD5 to a healthy state upon anti-HIV treatment (54), which is in contrast to our findings. However, the Swedish cohort was on two-decades-long successful therapy and was initiated on ART in the early stages of HIV-infection, unlike our cohort which was started on ART only in stage 2 or 3 of the disease as per national guidelines and policies available then.
TRANCE/RANKL, an essential regulator of bone metabolism, was found to be lower in the ART group than in HIVNC. There is existing literature to show that HIV-infected individuals treated with NRTIs and NNRTIs have lower than normal levels of circulating RANKL (55). Lower levels of TRANCE have been reported as an independent predictor of non-traumatic fracture, indicating its effect on osteoclastogenesis (29). Though Cystatin D (CST5) has not been well-studied in HIV, our study showed altered levels of this protein in HIV-1 infected individuals. CST5 is ultra-early inflammatory marker of traumatic brain injury (56). To the best of our knowledge, this is the first study reporting elevated levels of CST5 in HIV infection.
NT3, another inflammatory marker, is reported to be strongly associated with neurocognitive impairment in PLHIV (24, 25) and MMP1 with senescence-associated conditions (57, 58). Higher levels of these two proteins could be favoring age-associated changes and premature aging in HIV-infected individuals with prolonged exposure to cART. The decreased expression of NT3 was also observed in colorectal cancer (59), while increased levels of plasma MMP1 have been associated with coronary atherosclerosis (20) and cancers (21, 22) providing further evidence to support the role of these proteins in disease development during aging.
Higher levels of CCL23 and SLAMF1, which are involved in monocyte activation, in the ART group could contribute to the sustained release of sCD14 (60). Further, continued immune activation could be due to several other mechanisms including, sustained HIV-replication in reservoir sites like lymph nodes and gastrointestinal tract (61), low levels of HIV and its proteins (62, 63), altered gut microbiota in PLHIV (64), and different ART regimens (65).
Telomere length (TL) is a marker of replicative senescence and is a well-known predictor of health outcome in aging populations. Chronic inflammation can potentially contribute to age-related diseases through increased production of reactive oxygen species that damage telomeres and lead to cellular and immunological senescence (66). Several studies have noticed an association between TL and age-related diseases (67). In HIV infection, immune activation, and expanded proliferation of leukocyte subsets immediately after infection, along with uncontrolled viremia have been associated with a rapid decrease in TL (68), followed by increase in TL with ART initiation. However, telomere induced replicative senescence with long term ART has also been reported (69). Our study also found shorter telomeres in PLHIV on ART for >5 years and noticed a significant association between TL and inflammatory markers like CXCL1, TGF-α, and IL10RA. Telomere length shortening has been well-reported in several inflammatory conditions and related diseases like cancer. Zadka et al. (70) showed a positive correlation between IL10RA expression and disease pathogenesis in colorectal cancer (70).
Our study has some limitations that merit comment. First, the HIV-infected population selected for this study were from the best pool of successfully treated individuals (free of co-infections and co-morbidities), through the Indian National ART program. The findings may not generalize to the general population of treated HIV-1 positive individuals. Second, due to the lack of earlier studies in this setting, the design of our study, and limited sample size, the conclusions drawn are limited to associations with modest significance. Also, many statistical tests were run, but only the most significant results have been highlighted, for this reason, the results should be considered hypothesis generating. Third, the patients were not monitored virologically as a standard of care, and we only have virological data at the time of sampling. Any potential viral blips which are most unlikely reflected by the patients CD4 history could have introduced bias in the expression of inflammatory markers. However, among the few studies involving inflammatory markers in virally suppressed populations from LMICs (71, 72), our study is the first comprehensive study on markers associated with inflammation and aging, that compared a large panel of inflammatory markers in PLHIV on long term suppressive ART from India, which uses a standardized public health approach for HIV-treatment monitoring. Finally, the telomere length comparison between the ART group and the HIVNC could not be adjusted for health factors or disease progression markers, such as CD4 as these were not measured on HIVNC. As Telomere length was taken as the average for PBMCs, the difference in CD4 and CD8 cells percentages may have contributed to the magnitude of the difference; further studies investigating difference based on cell type are needed.
In conclusion, we observed several soluble inflammatory biomarkers that differed significantly between PLHIV and healthy individuals, despite 8 years of successful therapy- Interestingly, several of these biomarkers have been previously shown to be associated with inflammatory conditions like cancer, cardiovascular, neurological and skeletal diseases.
Put together, these data suggest that HIV-1 infected individuals, even those on long-term successful ART, may be at higher risk of developing inflammatory diseases leading to inflamm-aging. The low-grade chronic inflammation is a major risk factor for the development of many age-associated diseases and to some extent morbidity and mortality in elderly people even in the general population. Therefore clinical and nutritional intervention or use of anti-inflammatory drugs may potentially be considered in this population that can reduce the burden of excessive immune activation and inflammation. Besides, we found a significant difference in telomere length between subjects on long-term ART and HIVNC subjects, even after adjusting for age; this warrants further investigation.
Data Availability
The datasets generated for this study are available on request to the corresponding author.
Author Contributions
HB, SS, NM, MS, and NC performed the laboratory experiments. AA and EG performed bioinformatics and statistical analysis. UN and AA made the figures. AP, RS, VS, and ST recruited study subjects and provided the clinical data. AP and PN provided the clinical interpretation. UN and LH conceived and designed the study. UN wrote the first draft of the paper reviewed by EG, HB, MS, and LH. All the authors approved the final version of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
HB acknowledges support from the HIV Research Trust, UK supported in part by ViiV Health Care, Council of Scientific and Industrial Research (CSIR), India. Authors would like to thank technical support received from Dr. Ponnuraj CP, Mrs. Gomathi, Mr. Kannan Muthuramalingam, and Mr. Sathya Murthi. This manuscript has been released as a Pre-Print at bioRxiv (73).
Footnotes
Funding. This work was supported by grants from the Swedish Research Council Establishment Grant [2017-01330 (UN), 2017-01898 (EG)], Swedish Research Council (Interdisciplinary-2018-06156) (UN) Swedish Physicians Against AIDS Foundation (FOb20170004) (UN), and Jeanssons Stiftelser (JS2016–0185) (UN) and Indian Council of Medical Research, India (LH).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2019.01965/full#supplementary-material
References
- 1.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. (2011) 62:141–55. 10.1146/annurev-med-042909-093756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sokoya T, Steel HC, Nieuwoudt M, Rossouw TM. HIV as a cause of immune activation and immunosenescence. Mediators Inflamm. (2017) 2017:6825493. 10.1155/2017/6825493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, et al. Inflamm-aging. an evolutionary perspective on immunosenescence. Ann N Y Acad Sci. (2000) 908:244–54. 10.1111/j.1749-6632.2000.tb06651.x [DOI] [PubMed] [Google Scholar]
- 4.Unaids (2016). Country Report: India. Available online at: http://www.unaids.org/en/regionscountries/countries/india/ (accessed July 10, 2018).
- 5.NACO (2013). Antiretroviral Therapy Guidelines for HIV-Infected Adults and Adolescents. New Delhi: Department of Aids Control, Ministry of Health and Family Welfare, Government of India. [Google Scholar]
- 6.Neogi U, Heylen E, Shet A, Chandy S, Shamsunder R, Sonnerborg A, et al. Long-term efficacy of first line antiretroviral therapy in Indian HIV-1 infected patients: a longitudinal cohort study. PLoS ONE. (2013) 8:e55421. 10.1371/journal.pone.0055421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffman JM, Lyu Y, Pletcher SD, Promislow DEL. Proteomics and metabolomics in ageing research: from biomarkers to systems biology. Essays Biochem. (2017) 61:379–88. 10.1042/EBC20160083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Assarsson E, Lundberg M, Holmquist G, Bjorkesten J, Thorsen SB, Ekman D, et al. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS ONE. (2014) 9:e95192. 10.1371/journal.pone.0095192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vicenti I, Meini G, Saladini F, Giannini A, Boccuto A, Schiaroli E, et al. Development of an internally controlled quantitative PCR to measure total cell-associated HIV-1 DNA in blood. Clin Chem Lab Med. (2018) 56:e75–7. 10.1515/cclm-2017-0587 [DOI] [PubMed] [Google Scholar]
- 10.Yanagiya A, Suyama E, Adachi H, Svitkin YV, Aza-Blanc P, Imataka H, et al. Translational homeostasis via the mRNA cap-binding protein, eIF4E. Mol Cell. (2012) 46:847–58. 10.1016/j.molcel.2012.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez-Navio JM, Casanova V, Pacheco R, Naval-Macabuhay I, Climent N, Garcia F, et al. Adenosine deaminase potentiates the generation of effector, memory, and regulatory CD4+ T cells. J Leukoc Biol. (2011) 89:127–36. 10.1189/jlb.1009696 [DOI] [PubMed] [Google Scholar]
- 12.Naval-Macabuhay I, Casanova V, Navarro G, Garcia F, Leon A, Miralles L, et al. Adenosine deaminase regulates treg expression in autologous T cell-dendritic cell cocultures from patients infected with HIV-1. J Leukoc Biol. (2016) 99:349–59. 10.1189/jlb.3A1214-580RR [DOI] [PubMed] [Google Scholar]
- 13.Berahovich RD, Miao Z, Wang Y, Premack B, Howard MC, Schall TJ. Proteolytic activation of alternative CCR1 ligands in inflammation. J Immunol. (2005) 174:7341–51. 10.4049/jimmunol.174.11.7341 [DOI] [PubMed] [Google Scholar]
- 14.Brown MH, Lacey E. A ligand for CD5 is CD5. J Immunol. (2010) 185:6068–74. 10.4049/jimmunol.0903823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibbings DJ, Marcet-Palacios M, Sekar Y, Ng MC, Befus AD. CD8 alpha is expressed by human monocytes and enhances Fc gamma R-dependent responses. BMC Immunol. (2007) 8:12. 10.1186/1471-2172-8-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishanian P, Hofmann B, Wang Y, Jackson AL, Detels R, Fahey JL. Serum soluble CD8 molecule is a marker of CD8 T-cell activation in HIV-1 disease. AIDS. (1991) 5:805–12. 10.1097/00002030-199107000-00003 [DOI] [PubMed] [Google Scholar]
- 17.Kopitar-Jerala N. Innate immune response in brain, NF-kappa B signaling and cystatins. Front Mol Neurosci. (2015) 8:73. 10.3389/fnmol.2015.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desrochers PE, Jeffrey JJ, Weiss SJ. Interstitial collagenase (matrix metalloproteinase-1) expresses serpinase activity. J Clin Invest. (1991) 87:2258–65. 10.1172/JCI115262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rumbaugh J, Turchan-Cholewo J, Galey D, St. Hillaire C, Anderson C, Conant K, et al. Interaction of HIV Tat and matrix metalloproteinase in HIV neuropathogenesis: a new host defense mechanism. FASEB J. (2006) 20:1736–8. 10.1096/fj.05-5619fje [DOI] [PubMed] [Google Scholar]
- 20.Castillo L, Rohatgi A, Ayers CR, Owens AW, Das SR, Khera A, et al. Associations of four circulating chemokines with multiple atherosclerosis phenotypes in a large population-based sample: results from the dallas heart study. J Interferon Cytokine Res. (2010) 30:339–47. 10.1089/jir.2009.0045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warnecke-Eberz U, Metzger R, Holscher AH, Drebber U, Bollschweiler E. Diagnostic marker signature for esophageal cancer from transcriptome analysis. Tumour Biol. (2016) 37:6349–58. 10.1007/s13277-015-4400-4 [DOI] [PubMed] [Google Scholar]
- 22.Huang Z, Yang Q, Huang Z. Identification of critical genes and five prognostic biomarkers associated with colorectal cancer. Med Sci Monit. (2018) 24:4625–33. 10.12659/MSM.907224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bothwell M. Recent advances in understanding neurotrophin signaling. F1000Res. (2016) 5. 10.12688/f1000research.8434.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bergman E, Ulfhake B, Fundin BT. Regulation of NGF-family ligands and receptors in adulthood and senescence: correlation to degenerative and regenerative changes in cutaneous innervation. Eur J Neurosci. (2000) 12:2694–706. 10.1046/j.1460-9568.2000.00149.x [DOI] [PubMed] [Google Scholar]
- 25.Meeker RB, Poulton W, Markovic-Plese S, Hall C, Robertson K. Protein changes in CSF of HIV-infected patients: evidence for loss of neuroprotection. J Neurovirol. (2011) 17:258–73. 10.1007/s13365-011-0034-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Punnonen J, Cocks BG, Carballido JM, Bennett B, Peterson D, Aversa G, et al. Soluble and membrane-bound forms of signaling lymphocytic activation molecule (SLAM) induce proliferation and Ig synthesis by activated human B lymphocytes. J Exp Med. (1997) 185:993–1004. 10.1084/jem.185.6.993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cummins N, Badley A. The TRAIL to viral pathogenesis: the good, the bad and the ugly. Curr Mol Med. (2009) 9:495–505. 10.2174/156652409788167078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mueller CG, Hess E. Emerging functions of RANKL in lymphoid tissues. Front Immunol. (2012) 3:261. 10.3389/fimmu.2012.00261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schett G, Kiechl S, Redlich K, Oberhollenzer F, Weger S, Egger G, et al. Soluble RANKL and risk of nontraumatic fracture. JAMA. (2004) 291:1108–13. 10.1001/jama.291.9.1108 [DOI] [PubMed] [Google Scholar]
- 30.Guaraldi G, Orlando G, Zona S, Menozzi M, Carli F, Garlassi E, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis. (2011) 53:1120–6. 10.1093/cid/cir627 [DOI] [PubMed] [Google Scholar]
- 31.Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. (2013) 493:338–45. 10.1038/nature11861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oka SI, Hirata T, Suzuki W, Naito D, Chen Y, Chin A, et al. Thioredoxin-1 maintains mechanistic target of rapamycin (mTOR) function during oxidative stress in cardiomyocytes. J Biol Chem. (2017) 292:18988–9000. 10.1074/jbc.M117.807735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. (2011) 203:780–90. 10.1093/infdis/jiq118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hattab S, Guiguet M, Carcelain G, Fourati S, Guihot A, Autran B, et al. Soluble biomarkers of immune activation and inflammation in HIV infection: impact of 2 years of effective first-line combination antiretroviral therapy. HIV Med. (2015) 16:553–62. 10.1111/hiv.12257 [DOI] [PubMed] [Google Scholar]
- 35.Van Den Dries L, Claassen MAA, Groothuismink ZMA, Van Gorp E, Boonstra A. Immune activation in prolonged cART-suppressed HIV patients is comparable to that of healthy controls. Virology. (2017) 509:133–9. 10.1016/j.virol.2017.06.014 [DOI] [PubMed] [Google Scholar]
- 36.Mudd JC, Brenchley JM. Gut mucosal barrier dysfunction, microbial dysbiosis, and their role in HIV-1 disease progression. J Infect Dis. (2016) 214(Suppl 2):S58–66. 10.1093/infdis/jiw258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dandona L, Dandona R, Kumar GA, Shukla DK, Paul VK, Balakrishnan K, et al. Nations within a nation: variations in epidemiological transition across the states of India, 1990-2016 in the global burden of disease study. Lancet. (2017) 390:2437–60. 10.1016/S0140-6736(17)32804-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Subramanian S, Tawakol A, Burdo TH, Abbara S, Wei J, Vijayakumar J, et al. Arterial inflammation in patients with HIV. JAMA. (2012) 308:379–86. 10.1001/jama.2012.6698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burdo TH, Weiffenbach A, Woods SP, Letendre S, Ellis RJ, Williams KC. Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. AIDS. (2013) 27:1387–95. 10.1097/QAD.0b013e32836010bd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castley A, Berry C, French M, Fernandez S, Krueger R, Nolan D. Elevated plasma soluble CD14 and skewed CD16+ monocyte distribution persist despite normalisation of soluble CD163 and CXCL10 by effective HIV therapy: a changing paradigm for routine HIV laboratory monitoring? PLoS ONE. (2014) 9:e115226. 10.1371/journal.pone.0115226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams JC, Zhang X, Karki M, Chi YY, Wallet SM, Rudy BJ, et al. Soluble CD14, CD163, and CD27 biomarkers distinguish ART-suppressed youth living with HIV from healthy controls. J Leukoc Biol. (2018) 103:671–80. 10.1002/JLB.3A0717-294RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kroeze S, Wit FW, Rossouw TM, Steel HC, Kityo CM, Siwale M, et al. Plasma biomarkers of HIV-related systemic inflammation and immune activation in sub-Saharan Africa before and during suppressive antiretroviral therapy. J Infect Dis. (2019) 220:1029–33. 10.1093/infdis/jiz252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang WC, Sala-Newby GB, Susana A, Johnson JL, Newby AC. Classical macrophage activation up-regulates several matrix metalloproteinases through mitogen activated protein kinases and nuclear factor-kappaB. PLoS ONE. (2012) 7:e42507. 10.1371/journal.pone.0042507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Climent N, Martinez-Navio JM, Gil C, Garcia F, Rovira C, Hurtado C, et al. Adenosine deaminase enhances T-cell response elicited by dendritic cells loaded with inactivated HIV. Immunol Cell Biol. (2009) 87:634–9. 10.1038/icb.2009.53 [DOI] [PubMed] [Google Scholar]
- 45.Farina C, Theil D, Semlinger B, Hohlfeld R, Meinl E. Distinct responses of monocytes to Toll-like receptor ligands and inflammatory cytokines. Int Immunol. (2004) 16:799–809. 10.1093/intimm/dxh083 [DOI] [PubMed] [Google Scholar]
- 46.Nardelli B, Tiffany HL, Bong GW, Yourey PA, Morahan DK, Li Y, et al. Characterization of the signal transduction pathway activated in human monocytes and dendritic cells by MPIF-1, a specific ligand for CC chemokine receptor 1. J Immunol. (1999) 162:435–44. [PubMed] [Google Scholar]
- 47.Herbeuval JP, Boasso A, Grivel JC, Hardy AW, Anderson SA, Dolan MJ, et al. TNF-related apoptosis-inducing ligand (TRAIL) in HIV-1-infected patients and its in vitro production by antigen-presenting cells. Blood. (2005) 105:2458–64. 10.1182/blood-2004-08-3058 [DOI] [PubMed] [Google Scholar]
- 48.Balestrieri E, Grelli S, Matteucci C, Minutolo A, D'ettorre G, Di Sora F, et al. Apoptosis-associated gene expression in HIV-infected patients in response to successful antiretroviral therapy. J Med Virol. (2007) 79:111–7. 10.1002/jmv.20768 [DOI] [PubMed] [Google Scholar]
- 49.Volpato S, Ferrucci L, Secchiero P, Corallini F, Zuliani G, Fellin R, et al. Association of tumor necrosis factor-related apoptosis-inducing ligand with total and cardiovascular mortality in older adults. Atherosclerosis. (2011) 215:452–8. 10.1016/j.atherosclerosis.2010.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu YY, Hsu JL, Wang HC, Wu SJ, Hong CJ, Cheng IH. Alterations of the neuroinflammatory markers IL-6 and TRAIL in Alzheimer's disease. Dement Geriatr Cogn Dis Extra. (2015) 5:424–34. 10.1159/000439214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lum JJ, Pilon AA, Sanchez-Dardon J, Phenix BN, Kim JE, Mihowich J, et al. Induction of cell death in human immunodeficiency virus-infected macrophages and resting memory CD4 T cells by TRAIL/Apo2l. J Virol. (2001) 75:11128–36. 10.1128/JVI.75.22.11128-11136.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perez-Villar JJ, Whitney GS, Bowen MA, Hewgill DH, Aruffo AA, Kanner SB. CD5 negatively regulates the T-cell antigen receptor signal transduction pathway: involvement of SH2-containing phosphotyrosine phosphatase SHP-1. Mol Cell Biol. (1999) 19:2903–12. 10.1128/MCB.19.4.2903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gary-Gouy H, Harriague J, Bismuth G, Platzer C, Schmitt C, Dalloul AH. Human CD5 promotes B-cell survival through stimulation of autocrine IL-10 production. Blood. (2002) 100:4537–43. 10.1182/blood-2002-05-1525 [DOI] [PubMed] [Google Scholar]
- 54.Sperk M, Zhang W, Nowak P, Neogi U. Plasma soluble factor following two decades prolonged suppressive antiretroviral therapy in HIV-1-positive males: a cross-sectional study. Medicine. (2018) 97:e9759. 10.1097/MD.0000000000009759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mora S, Zamproni I, Cafarelli L, Giacomet V, Erba P, Zuccotti G, et al. Alterations in circulating osteoimmune factors may be responsible for high bone resorption rate in HIV-infected children and adolescents. AIDS. (2007) 21:1129–35. 10.1097/QAD.0b013e32810c8ccf [DOI] [PubMed] [Google Scholar]
- 56.Hill LJ, Di Pietro V, Hazeldine J, Davies D, Toman E, Logan A, et al. Cystatin D (CST5): an ultra-early inflammatory biomarker of traumatic brain injury. Sci Rep. (2017) 7:5002. 10.1038/s41598-017-04722-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Benanti JA, Williams DK, Robinson KL, Ozer HL, Galloway DA. Induction of extracellular matrix-remodeling genes by the senescence-associated protein APA-1. Mol Cell Biol. (2002) 22:7385–97. 10.1128/MCB.22.21.7385-7397.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lei LT, Chen JB, Zhao YL, Yang SP, He L. Resveratrol attenuates senescence of adipose-derived mesenchymal stem cells and restores their paracrine effects on promoting insulin secretion of INS-1 cells through Pim-1. Eur Rev Med Pharmacol Sci. (2016) 20:1203–13. [PubMed] [Google Scholar]
- 59.Genevois AL, Ichim G, Coissieux MM, Lambert MP, Lavial F, Goldschneider D, et al. Dependence receptor TrkC is a putative colon cancer tumor suppressor. Proc Natl Acad Sci USA. (2013) 110:3017–22. 10.1073/pnas.1212333110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Theil D, Farina C, Meinl E. Differential expression of CD150 (SLAM) on monocytes and macrophages in chronic inflammatory contexts: abundant in Crohn's disease, but not in multiple sclerosis. J Clin Pathol. (2005) 58:110–1. 10.1136/jcp.2004.019323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chun TW, Nickle DC, Justement JS, Large D, Semerjian A, Curlin ME, et al. HIV-infected individuals receiving effective antiviral therapy for extended periods of time continually replenish their viral reservoir. J Clin Invest. (2005) 115:3250–5. 10.1172/JCI26197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meier A, Alter G, Frahm N, Sidhu H, Li B, Bagchi A, et al. MyD88-dependent immune activation mediated by human immunodeficiency virus type 1-encoded toll-like receptor ligands. J Virol. (2007) 81:8180–91. 10.1128/JVI.00421-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anand AR, Rachel G, Parthasarathy D. HIV proteins and endothelial dysfunction: implications in cardiovascular disease. Front Cardiovasc Med. (2018) 5:185. 10.3389/fcvm.2018.00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sessa L, Reddel S, Manno E, Quagliariello A, Cotugno N, Del Chierico F, et al. Distinct gut microbiota profile in antiretroviral therapy-treated perinatally HIV-infected patients associated with cardiac and inflammatory biomarkers. AIDS. (2019) 33:1001–11. 10.1097/QAD.0000000000002131 [DOI] [PubMed] [Google Scholar]
- 65.Hileman CO, Funderburg NT. Inflammation, immune activation, and antiretroviral therapy in HIV. Curr HIV/AIDS Rep. (2017) 14:93–100. 10.1007/s11904-017-0356-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jurk D, Wilson C, Passos JF, Oakley F, Correia-Melo C, Greaves L, et al. Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nat Commun. (2014) 2:4172. 10.1038/ncomms5172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rizvi S, Raza ST, Mahdi F. Telomere length variations in aging and age-related diseases. Curr Aging Sci. (2014) 7:161–7. 10.2174/1874609808666150122153151 [DOI] [PubMed] [Google Scholar]
- 68.Pathai S, Lawn SD, Gilbert CE, Mcguinness D, Mcglynn L, Weiss HA, et al. Accelerated biological ageing in HIV-infected individuals in South Africa: a case-control study. AIDS. (2013) 27:2375–84. 10.1097/QAD.0b013e328363bf7f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cobos Jimenez V, Wit FW, Joerink M, Maurer I, Harskamp AM, Schouten J, et al. T-Cell activation independently associates with immune senescence in HIV-infected recipients of long-term antiretroviral treatment. J Infect Dis. (2016) 214:216–25. 10.1093/infdis/jiw146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zadka L, Kulus MJ, Kurnol K, Piotrowska A, Glatzel-Plucinska N, Jurek T, et al. The expression of IL10RA in colorectal cancer and its correlation with the proliferation index and the clinical stage of the disease. Cytokine. (2018) 110:116–25. 10.1016/j.cyto.2018.04.030 [DOI] [PubMed] [Google Scholar]
- 71.Margolick JB, Bream JH, Martinez-Maza O, Lopez J, Li X, Phair JP, et al. Frailty and circulating markers of inflammation in HIV+ and HIV- men in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr. (2017) 74:407–17. 10.1097/QAI.0000000000001261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Margolick JB, Bream JH, Nilles TL, Li H, Langan SJ, Deng S, et al. Relationship between T-cell responses to CMV, markers of inflammation, and frailty in HIV-uninfected and HIV-infected men in the multicenter AIDS cohort study. J Infect Dis. (2018) 218:249–58. 10.1093/infdis/jiy005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Babu H, Ambikan AT, Gabriel EE, Akusaravi SS, Palaniapan AN, Sundaraj V, et al. Systemic inflammation and risk of age-associated diseases in people living with HIV on long term suppressive antiretroviral therapy. bioRxiv. (2018) 418012 10.1101/418012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.