Abstract
Cognitive function declines with age throughout the animal kingdom, and increasing evidence shows that disruption of the proteasome system contributes to this deterioration. The proteasome has important roles in multiple aspects of the nervous system, including synapse function and plasticity, as well as preventing cell death and senescence. Previous studies have shown neuronal proteasome depletion and inhibition can result in neurodegeneration and cognitive deficits, but it is unclear if this pathway is a driver of neurodegeneration and cognitive decline in aging. We report that overexpression of the proteasome β5 subunit enhances proteasome assembly and function. Significantly, we go on to show that neuronal‐specific proteasome augmentation slows age‐related declines in measures of learning, memory, and circadian rhythmicity. Surprisingly, neuronal‐specific augmentation of proteasome function also produces a robust increase of lifespan in Drosophila melanogaster. Our findings appear specific to the nervous system; ubiquitous proteasome overexpression increases oxidative stress resistance but does not impact lifespan and is detrimental to some healthspan measures. These findings demonstrate a key role of the proteasome system in brain aging.
Keywords: aging, Drosophila, neurodegeneration, proteasome
1. INTRODUCTION, RESULTS, AND DISCUSSION
With age, there is a progressive decline in 26S proteasome function in the nervous system of mammals (Keller, Hanni, & Markesbery, 2000) as well as flies (Figure 1a), with a corresponding increase in 20S proteasome levels but not activity, which either declines or is unchanged (Figure 1a; Keller et al., 2000; Tonoki et al., 2009; Vernace, Arnaud, Schmidt‐Glenewinkel, & Figueiredo‐Pereira, 2007). These changes likely result from reduced capacity of the existing proteasome (Bulteau, Petropoulos, & Friguet, 2000), diminished 26S assembly (Tonoki et al., 2009; Vernace et al., 2007) and disassembly of the 26S proteasome into free 20S to compensate for reduced 20S functionality. It has been shown that proteasome depletion and inhibition in mice can mirror brain aging phenotypes, producing neurodegeneration, cognitive deficits, and formation of Lewy‐like bodies (Bedford et al., 2008; Romero‐Granados, Fontan‐Lozano, Aguilar‐Montilla, & Carrion, 2011). The goal of this study is to establish whether age‐related cognitive decline can be ameliorated by augmenting proteasome function.
Figure 1.
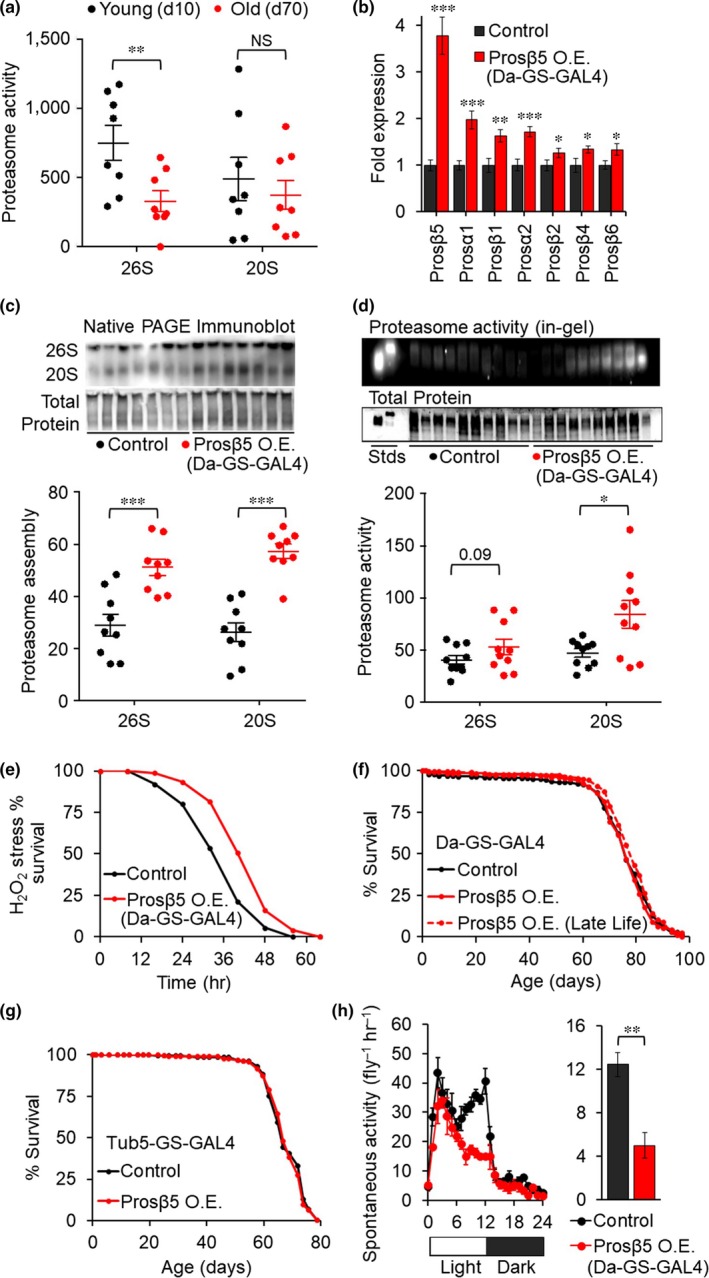
Prosβ5 drives upregulation of multiple proteasome subunits and increases proteasome function but does not extend lifespan when ubiquitously overexpressed. (a) Proteasome activity declines with age in W1118 fly heads, N = 8. (b) Overexpression of Prosβ5 through Da‐GS‐GAL4>UAS‐Prosβ5 ± 200 μM RU486 increases mRNA expression of multiple 20S core proteasome subunits in day 10 female flies, N = 8. (c) Prosβ5 overexpression increases assembly of 20S and 26S proteasome, Native PAGE immunoblot, values normalized to total protein, based on India ink stain, N = 9. (d) Prosβ5 overexpression increases 20S proteasome based on an in‐gel Suc‐LLVY‐AMC activity overlay assay, values normalized to total protein, based on Coomassie stain, N = 10. (e) Prosβ5 overexpression under control of the driver Da‐GS‐GAL4 increases oxidative stress resistance. Flies were fed 4.4 M H2O2 mixed with 5% sucrose, and survival was monitored every 8 hr, N = 75. Flies were removed from RU486 during stress assay to prevent potential differences in consumption. (f, g) Drosophila lifespan is not increased by Prosβ5 overexpression under either of the ubiquitous drivers Da‐GS‐GAL4 or Tub5‐GS‐GAL4 ± 200 μM RU486, N = 200. (h) Prosβ5 overexpression under control of the driver Da‐GS‐GAL4 reduces healthspan in flies based on spontaneous activity measures at day 50, N = 3–4 vials with 25 flies per vial. Logrank evaluations for lifespans are included in Figure S1 Whole uncropped immunoblot images are provided in Figure S2. NS p>0.05, *p<0.05, ** p<0.01, ***p<0.001. Significance is based on Students T‐test. Values are Mean ± SEM.
The size and complexity of the proteasome has made manipulating its expression a challenge. Elevating the proteasome β5 subunit increases both expression of other subunits and whole proteasome assembly in mammalian cell cultures (Chondrogianni et al., 2005; Liu et al., 2007) and Caenorhabditis elegans (Chondrogianni, Georgila, Kourtis, Tavernarakis, & Gonos, 2015). We used the same approach in Drosophila melanogaster, utilizing UAS‐Prosβ5 (fly ortholog of the β5 subunit; Staudt et al., 2005). We used the mifepristone (RU486) inducible GeneSwitchGAL4 driver system to limit gene overexpression to adulthood, thereby removing developmental artifacts and allowing experiment and control animals to be genetically identical siblings. Overexpression of Prosβ5 posteclosion increased mRNA of other core proteasome subunits (Figure 1b), enhanced proteasome assembly (Figure 1c and Figure S1) and activity (Figure 1d), and increased oxidative stress resistance (Figure 1e), independent of artifacts from RU486 treatment (Figure S2). Despite improvements to oxidative stress resistance, ubiquitous elevation of proteasome function did not impact lifespan when induced either throughout adulthood or in late life (from 40 days posteclosion) (Figure 1f,g; Figures S3 and S4). This finding was confirmed using two independent ubiquitous driver lines (Da‐GS‐GAL4 and Tub5‐GS‐GAL4). In addition, we did not observe any improvements in healthspan and instead found reduced spontaneous activity in middle‐aged flies providing some indication of toxicity (Figure 1h). Our findings conflict with a recent report that Prosβ5 under control of Da‐GS‐GAL4 extends lifespan (Nguyen et al., 2019). We note that a considerably lower dose of RU486 was employed in the study by Nguyen and colleagues (~23 μM in contrast to 200 μM in the present study). Our different results may stem from differences in levels of transgene induction; it is possible that low‐level ubiquitous proteasome overexpression is beneficial while higher levels may be detrimental.
In contrast, when Prosβ5 overexpression was limited to the nervous system (using the pan‐neuronal driver Elav‐GS‐GAL4), we observed a robust extension in median and maximum lifespan which was reproduced across four independent cohorts (Figure 2a and Figure S5). Because of concerns regarding potential off‐target effects from RU486 on Drosophila lifespan (Landis et al., 2015), we undertook parallel experiments in flies of the same genetic background, minus the UAS‐Prosβ5 transgene, and found no impact of RU486 treatment on lifespan (Figure 2b and Figure S5). Thus, we can conclude that the extension in lifespan from pan‐neuronal Prosβ5 overexpression is not an artifact of RU486 treatment. Importantly, pan‐neuronal Pr
Figure 2.
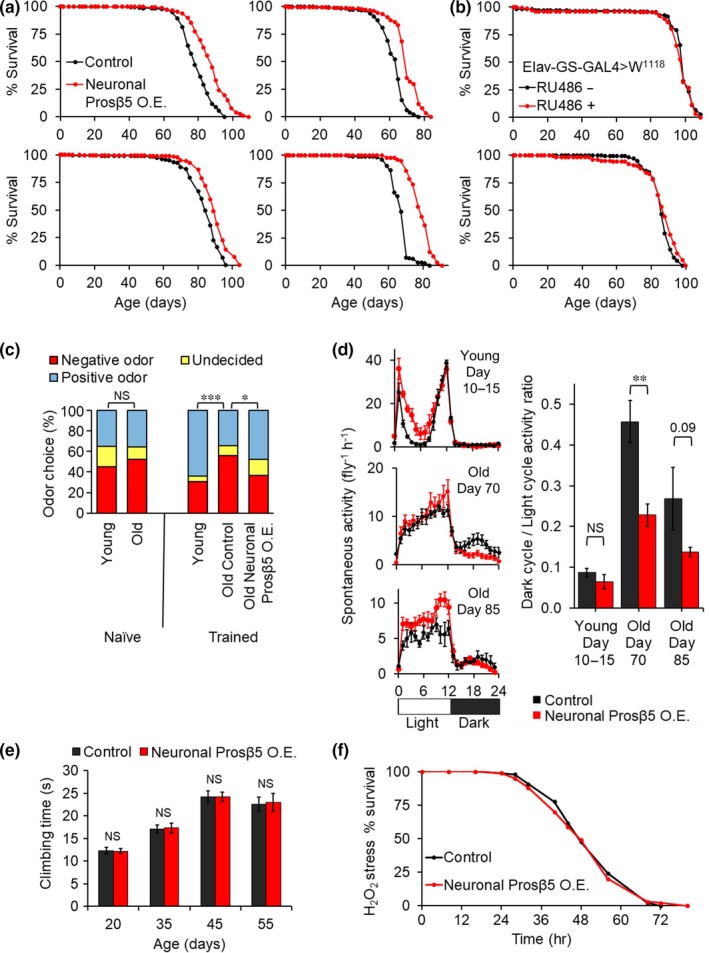
Neuronal‐specific Prosβ5 overexpression extends lifespan and reduces age‐related deficits in learning, memory, and brain function. (a) Neuronal Prosβ5 overexpression (Elav‐GS‐GAL4>UAS‐Prosβ5 ± 200 μM RU486) extends female fly lifespan. Evaluations are based on four independent lifespan assays, N = 200–250 each. (b) RU486 alone does not extend lifespan in the genetic background evaluated. Lifespan measure of Elav‐GS‐GAL4>W1118 flies ± 200 μM RU486. UAS‐Prosβ5 flies were backcrossed into the evaluated W1118 strain prior to the start of this investigation. (c) Neuronal Prosβ5 overexpression reduces age‐related cognitive deficits in olfaction aversion training. Experiments performed as in Malik and Hodge (2014) N = 150. (d) Age disrupts circadian rhythmicity. Neuronal Prosβ5 overexpression reduces declines in circadian rhythmicity, N = 125. (e) Neuronal Prosβ5 overexpression does not improve muscle function evaluated through climbing capacity. N = 100. (f) No improvement in oxidative stress resistance observed with neuronal‐specific Prosβ5 overexpression. Logrank evaluations for lifespans are included in Figure S1. NS p>0.05, *p<0.05, **p<0.01, ***p<0.001. Significance is based on Students T‐test except panel C where significance is based on Chi‐Sq test. Values are Mean ± SEM
osβ5 overexpression not only extended lifespan but also reduced age‐related cognitive deficits. We demonstrated improvements in learning and memory in aged animals using olfaction aversion training. Animals were exposed in alternation to two neutral odors (3‐octanol & 4‐methylcyclohexanol), one of which was paired with exposure to a mild electric shock. After five training rounds, animals were permitted to recover for one hour and then placed in a T‐maze with opposing odors from either side (Malik & Hodge, 2014). While young (10 days posteclosion) animals showed a significant increase in avoidance of the “negative” odor after training, old (70 days posteclosion) animals showed no increase in avoidance after training. However, Drosophila with neuronal Prosβ5 overexpression continued to show a posttraining increase in avoidance of the “negative” odor at old age (Figure 2c), demonstrating a retention of associative learning ability. In both humans and animal models, circadian rhythmicity is well‐established as correlating with and potentially contributing to age‐related cognitive decline (Antoniadis, Ko, Ralph, & McDonald, 2000). Drosophila show a defined activity distribution with high activity during the day and low activity at night. With age, this pattern becomes less defined. We found this rhythmicity deficit to be partially prevented in flies which overexpressed Prosβ5 (Figure 2d). Significantly, age‐related declines in climbing capacity were not altered in these animals. This suggests that the improvements in activity measures are independent of muscle function (Figure 2e). Additionally, the animals showed no increase in oxidative stress resistance when fed hydrogen peroxide, further supporting a neuronal‐specific role rather than whole body adaptation (Figure 2f). Furthermore improvements appear independent of impact from RU486‐induced off‐target effects. No increase in proteasome activity or behavioral changes were observed under treatment with RU486 in the absence of the psmb5 transgene (Figure S6).
Our findings demonstrate that pan‐neuronal augmentation of proteasome function can ameliorate age‐related cognitive decline, specifically in learning, memory, and circadian rhythmicity. We also show that pan‐neuronal proteasome overexpression reproducibly extends lifespan while ubiquitous proteasome overexpression did not improve lifespan and may be detrimental to healthspan. This finding underscores the importance of the nervous system and neuronal proteasome function as determinants of lifespan in Drosophila.
2. EXPERIMENTAL PROCEDURES
Details provided in supplemental files.
CONFLICT OF INTEREST
None declared.
Supporting information
ACKNOWLEDGMENTS
This research was supported by NIA/NIH R56 AG061051 (AMP), 2018 Glenn Foundation for Medical Research and AFAR Grants for Junior Faculty (AMP), Voelcker Young Investigator Award (AMP), San Antonio Nathan Shock Center Pilot Grant (AMP), San Antonio Pepper Center Rapid Response Grant (AMP), NIH/NIA T32 AG021890 (EM).
Munkácsy E, Chocron ES, Quintanilla L, Gendron CM, Pletcher SD, Pickering AM. Neuronal‐specific proteasome augmentation via Prosβ5 overexpression extends lifespan and reduces age‐related cognitive decline. Aging Cell. 2019;18:e13005 10.1111/acel.13005
REFERENCES
- Antoniadis, E. A. , Ko, C. H. , Ralph, M. R. , & McDonald, R. J. (2000). Circadian rhythms, aging and memory. Behavioral Brain Research, 111, 25–37. 10.1016/S0166-4328(00)00145-5 [DOI] [PubMed] [Google Scholar]
- Bedford, L. , Hay, D. , Devoy, A. , Paine, S. , Powe, D. G. , Seth, R. , … Mayer, R. J. (2008). Depletion of 26S proteasomes in mouse brain neurons causes neurodegeneration and Lewy‐like inclusions resembling human pale bodies. Journal of Neuroscience, 28, 8189–8198. 10.1523/JNEUROSCI.2218-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulteau, A. L. , Petropoulos, I. , & Friguet, B. (2000). Age‐related alterations of proteasome structure and function in aging epidermis. Experimental Gerontology, 35, 767–777. 10.1016/S0531-5565(00)00136-4 [DOI] [PubMed] [Google Scholar]
- Chondrogianni, N. , Georgila, K. , Kourtis, N. , Tavernarakis, N. , & Gonos, E. S. (2015). 20S proteasome activation promotes life span extension and resistance to proteotoxicity in Caenorhabditis elegans . FASEB Journal, 29, 611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chondrogianni, N. , Tzavelas, C. , Pemberton, A. J. , Nezis, I. P. , Rivett, A. J. , & Gonos, E. S. (2005). Overexpression of proteasome beta5 assembled subunit increases the amount of proteasome and confers ameliorated response to oxidative stress and higher survival rates. Journal of Biological Chemistry, 280, 11840–11850. [DOI] [PubMed] [Google Scholar]
- Keller, J. N. , Hanni, K. B. , & Markesbery, W. R. (2000). Possible involvement of proteasome inhibition in aging: Implications for oxidative stress. Mechanisms of Ageing and Development, 113, 61–70. 10.1016/S0047-6374(99)00101-3 [DOI] [PubMed] [Google Scholar]
- Landis, G. N. , Salomon, M. P. , Keroles, D. , Brookes, N. , Sekimura, T. , & Tower, J. (2015). The progesterone antagonist mifepristone/RU486 blocks the negative effect on life span caused by mating in female Drosophila . Aging (Albany NY), 7, 53–69. 10.18632/aging.100721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Liu, X. , Zhang, T. , Luna, C. , Liton, P. B. , & Gonzalez, P. (2007). Cytoprotective effects of proteasome beta5 subunit overexpression in lens epithelial cells. Molecular Vision, 13, 31–38. [PMC free article] [PubMed] [Google Scholar]
- Malik, B. R. , & Hodge, J. J. (2014). Drosophila adult olfactory shock learning. Journal of Visualized Experiments, e50107 10.3791/50107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, N. N. , Rana, A. , Goldman, C. , Moore, R. , Tai, J. , Hong, Y. , … Hur, J. H. (2019). Proteasome beta5 subunit overexpression improves proteostasis during aging and extends lifespan in Drosophila melanogaster . Scientific Reports, 9, 3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero‐Granados, R. , Fontan‐Lozano, A. , Aguilar‐Montilla, F. J. , & Carrion, A. M. (2011). Postnatal proteasome inhibition induces neurodegeneration and cognitive deficiencies in adult mice: A new model of neurodevelopment syndrome. PLoS ONE, 6, e28927 10.1371/journal.pone.0028927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudt, N. , Molitor, A. , Somogyi, K. , Mata, J. , Curado, S. , Eulenberg, K. , … Vorbrüggen, G. (2005). Gain‐of‐function screen for genes that affect Drosophila muscle pattern formation. PLoS Genetics, 1, e55 10.1371/journal.pgen.0010055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonoki, A. , Kuranaga, E. , Tomioka, T. , Hamazaki, J. , Murata, S. , Tanaka, K. , & Miura, M. (2009). Genetic evidence linking age‐dependent attenuation of the 26S proteasome with the aging process. Molecular and Cellular Biology, 29, 1095–1106. 10.1128/MCB.01227-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernace, V. A. , Arnaud, L. , Schmidt‐Glenewinkel, T. , & Figueiredo‐Pereira, M. E. (2007). Aging perturbs 26S proteasome assembly in Drosophila melanogaster . FASEB Journal, 21, 2672–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


