Abstract
Restrictions of in-feed antibiotics use in poultry has pushed research toward finding appropriate alternatives such as Direct-Fed Microbials (DFM). In this study, previously tested Bacillus isolates (B. subtilis and B. amyloliquefaciens) were used to evaluate their therapeutic and prophylactic effects against Salmonella enterica serovar Enteritidis (S. Enteritidis) in broiler chickens. For this purpose, initial antibacterial activity of Bacillus-DFM (104 spores/g or 106 spores/g) against S. Enteritidis colonization in crop, proventriculus and intestine was investigated using an in vitro digestive model. Furthermore, to evaluate therapeutic and prophylactic effects of Bacillus-DFM (104 spores/g) against S. Enteritidis colonization, altogether 60 (n = 30/group) and 30 (n = 15/group) 1-day-old broiler chickens were randomly allocated to either DFM or control group (without Bacillus-DFM), respectively. Chickens were orally gavaged with 104 cfu of S. Enteritidis per chicken at 1-day old, and cecal tonsils (CT) and crop were collected 3 and 10 days later during the therapeutic study, whereas they were orally gavaged with 107 cfu of S. Enteritidis per chicken at 6-day-old, and CT and crop were collected 24 h later from two independent trials during the prophylactic study. Serum superoxide dismutase (SOD), FITC-d and intestinal IgA levels were reported for both chicken studies, in addition cecal microbiota analysis was performed during the therapeutic study. DFM significantly reduced S. Enteritidis concentration in the intestine compartment, and in both proventriculus and intestine compartments as compared to the control when used at 104 spores/g and 106 spores/g, respectively (p < 0.05). DFM significantly reduced FITC-d and IgA as well as SOD and IgA levels (p < 0.05) compared to the control in therapeutic and prophylactic studies, respectively. Interestingly, in the therapeutic study, there were significant differences in bacterial community structure and predicted metabolic pathways between DFM and control. Likewise, phylum Actinobacteria and the genera Bifidobacterium, Roseburia, Proteus, and cc_115 were decreased, while the genus Streptococcus was enriched significantly in the DFM group as compared to the control (MetagenomeSeq, p < 0.05). Thus, the overall results suggest that the Bacillus-DFM can reduce S. Enteritidis colonization and improve the intestinal health in chickens through mechanism(s) that might involve the modulation of gut microbiota and their metabolic pathways.
Keywords: Bacillus, broiler chickens, Salmonella Enteritidis, antimicrobial, anti-inflammatory (activity)
Introduction
Antibiotics have been widely used in animal production for decades not only for therapeutic purposes, but also as antimicrobial growth promoters (AGPs) to enhance growth rate and feed conversion efficiency (1, 2). Although the use of AGPs has a significant positive economic impact in commercial animal production systems, there is a greater concern regarding possibilities of their use in developing antimicrobial resistance (AMR) in bacterial populations. Because of this reason, the use of in-feed antibiotics has been completely banned in Europe since January 1st, 2006 (EC Regulation No. 1831/2003) and has also been restricted in several non-European countries, including Taiwan and South Korea (3). Since January 2017, medically important antibiotics to human health are no longer allowed in animal production for growth promotion or feed efficiency in the United States and require licensed veterinarian prescription to use them for prevention, control, and treatment of animal diseases (FDA's Guidance #213).
The poultry industry is the fastest growing animal industry and is expected to grow continuously as demand for meat and eggs is accelerating due to growing populations, increasing incomes and urbanization (4). However, due to ban or restrictions on AGPs, there are growing challenges for the poultry industry to cope with enteric pathogens such as Salmonella. This has created huge demand for finding alternatives to AGPs, and thus several possible alternatives such as enzymes (in), organic acids, probiotics, prebiotics, etheric oils, and immunostimulants have already been widely studied (2, 5).
Among those alternatives, probiotics or Direct-Fed Microbials (DFM), which were defined as “a live microbial feed supplement that beneficially affects the host animal by improving its intestinal microbial balance” (6), have generated significant interest during the last two decades to all sectors of animal production. The majority of microbes used as DFM are bacteria that belong to around 40 different species in 7 bacterial genera including Lactobacillus, Bifidobacterium, Propionbacterium, Enterococcus, Pediococcus, Bacillus, and Bacteroides. In addition to these bacteria, yeast (Saccharomyces cerevisiae) and molds (Aspergillus niger and Aspergillus oryzae) were also reported as DFM (7). Moreover, certain strains of Clostridium such as Clostridium butyricum MIYAIRI 588 were also used as potential probiotics (8). Unlike other bacteria whose vegetative cells are used as DFM, spores from Bacillus sps. can be used as DFM because they are more stable and heat tolerant (9–11), and thus well-suited for its application in pelleted feeds (12). Previous studies reported the ability of Bacillus spores to germinate and enumerate within the gastrointestinal tract of the poultry (13–15). In poultry, several studies have reported beneficial effects of Bacillus isolates when used as DFM on production parameters and pathogen inhibition (16–18), which might be achieved through increasing nutrient digestibility, improving intestinal morphology, balancing intestinal microbiota, and modulating immunity (19–21). Moreover, our previous studies based on the selected candidates of Bacillus sps. reported the reduction in the recovery of Salmonella Typhimurium in both chicks and poults after experimental infection in preliminary laboratory trials (22) as well as in poults during the brooding phase of commercial turkey production (12). However, the modes of action for improved performance by Bacillus species were not well-defined, and performance parameters were varied within species or strains, demanding appropriate screening and characterization of Bacillus isolates prior to commercialization (23).
Norum™ (Eco-Bio/Euxxis Bioscience LLC, Fayetteville, AR) is a Bacillus spore direct DFM culture consisting of two isolates of Bacillus amyloliquefaciens and one isolate of Bacillu subtilis which were isolated in our laboratory and screened based on in vitro enzyme production profiles and Clostridium perfringens reduction (24). In addition, these isolates were shown to reduce digesta viscoscity, bacterial translocation, improve performance, bone quality parameters, and balance intestinal microbiota in chickens raised with rye-based diets or corn distiller-dried grains with solubles (21, 25). However, the effect of dietary supplementation of Norum™ has not been evaluated in vivo in an established Salmonella challenge model until now. Thus, the objectives of this study were to evaluate the antimicrobial effects of Norum™ DFM against S. Enteritidis in an in vitro digestion model that simulates the pH and enzymatic conditions present in the crop, proventriculus, and intestine of broiler chickens, as well as the therapeutic and prophylactic effects against S. Enteritidis colonization in crop and cecal tonsil (CT), aside from its effects on intestinal health parameters, and cecal microbiota composition in broiler chickens.
Materials and Methods
Preparation of Treatments and Diets
Norum™ (Eco-Bio/Euxxis Bioscience LLC, Fayetteville, AR) is a Bacillus spore DFM culture consisting of three isolates: two Bacillus amyloliquefaciens and one Bacillu subtilis. The product contains a concentration of stable Bacillus spores (~3 × 1011 spores/g). DFM was added into the feed to obtain the experimental diet with a final concentration of 104 or 106 spores/g feed. Samples of feed containing the DFM were subjected to 100°C for 10 min to eliminate vegetative cells and validate the number of spores per gram of feed after inclusion and mixing steps. Following heat treatment, 10-fold dilutions of the feed samples were plated on TSA, letting spores in the feed sample germinate to vegetative cells after incubation at 37°C for 24 h, hence representing the number of spores present per gram of feed. The experimental diet used in this study was formulated to approximate the nutritional requirements of broiler chickens as recommended by the National Research Council (26), and adjusted to breeder's recommendations (27). No antibiotics were added to the diet (Supplementary Table 1). All animal handling procedures complied with the Institutional Animal Care and Use Committee (IACUC) at the University of Arkansas, Fayetteville (protocol #18030).
Bacterial Strain and Culture Conditions
The organism used in all experiments was a poultry isolate of Salmonella enterica serovar Enteritidis (S. Enteritidis), bacteriophage type 13A, obtained from the USDA National Veterinary Services Laboratory (Ames, IA, United State). This strain was resistant to 25 μg/mL of novobiocin (NO, catalog no.N-1628, Sigma) and was selected for resistance to 20 μg/mL of nalidixic acid (NA, catalog no.N-4382, Sigma) in our laboratory. For the present studies, 100 μL of S. Enteritidis from a frozen aliquot was added to 10 mL of tryptic soy broth (Catalog no. 22092, Sigma), incubated at 37°C for 8 h, and passed three times every 8 h to ensure that all bacteria were in log phase as previously described (28). Post-incubation, bacterial cells were washed three times with sterile 0.9% saline by centrifugation at 1,864 × g for 10 min, reconstituted in saline, quantified by densitometry with a spectrophotometer (Spectronic 20D+, Spectronic Instruments Thermo Scientific, Rochester, NY, United States), and finally diluted to an approximate concentration of 1 × 108, 4 × 104, and 4 × 107 cfu/mL. Concentrations of S. Enteritidis were further verified by serial dilution and plating on brilliant green agar (BGA, Catalog no. 70134, Sigma) with NO and NA for enumeration of actual cfu used to in the experiments.
Experiment 1. In vitro Digestion Model
In this experiment, the antimicrobial activity of two different concentrations of DFM (104 or 106 spores/g) against S. Enteritidis was determined using an in vitro digestion model described previously (24, 29) that simulates the pH and enzymatic conditions present in the crop, proventriculus, and intestine of broilers. Experiments were run in quintuplicate. Briefly, 5 g of feed with or without DFM was placed inside 50 mL polypropylene centrifuge tubes, followed by the addition of 1 ml of 1 × 108 cfu/mL S. Enteritidis suspension in each tube. Subsequently, the media and corresponding enzymes to simulate each compartment of the in vitro digestion model were added to the tubes, respecting the stirring conditions and incubation times established. Finally, in each compartment, 1 mL of sample was collected to enumerate S. Enteritidis.
Experiment 2. Effect of Therapeutic Administration of DFM on S. Enteritidis
This experiment was performed to evaluate the therapeutic effect of 104 spores/g DFM in broiler chickens infected with S. Enteritidis. Sixty 1 day-old male Cobb-Vantress broiler chickens (Fayetteville, AR, USA) were challenged with 1 × 104 S. Enteritidis cfu per bird and randomly allocated to one of two groups (n = 30 chickens/group): (1) control group challenged only with S. Enteritidis and (2) DFM group challenged with S. Enteritidis and also with 104 spores/g Norum™. On days 3 and 10 post-S. Enteritidis challenge, 15 chickens were euthanized by CO2 inhalation, and the crop and CT from 12 birds per group were aseptically collected to evaluate S. Enteritidis recovery. Blood samples were collected from the femoral vein and centrifuged (1,000 × g for 15 min) to separate the serum for the determination of fluorescein isothiocyanate-dextran (FITC-d) concentration and superoxide dismutase (SOD) activity at day 10. The concentration of FITC-d administered was calculated based on group body weight at day 9 post-S. Enteritidis challenge. Furthermore, intestinal samples for total intestinal IgA levels were also collected.
Experiment 3. Effect of Prophylactic Administration of DFM on S. Enteritidis
In this experiment, two independent trials were conducted to evaluate the prophylactic administration of 104 spores/g DFM in reducing the incidence of S. Enteritidis in broiler chickens. In each trial, 30 day-of-hatch male Cobb-Vantress broiler chickens (Fayetteville, AR, USA) were randomly allocated to one of two groups (n = 15 chickens): (1) control group challenged only with S. Enteritidis and (2) DFM group challenged with S. enteritidis and also with 104 spores/g Norum™. Chicks were placed in heated brooder batteries with a controlled age-appropriate environment and provided with their respective diet and water ad libitum. At day 6, all chickens were orally gavaged with 1 × 107 cfu of S. Enteritidis per bird. Chicks were euthanized by CO2 inhalation 24 h post-S. Enteritidis challenge, and the crop and CT from 12 birds per group were aseptically collected to evaluate S. Enteritidis recovery. Blood samples were collected from the femoral vein and centrifuged (1,000 × g for 15 min) to separate the serum for the determination of FITC-d and SOD. The concentration of FITC-d administered was calculated based on group body weight at 6 d old. Furthermore, intestinal samples for total intestinal IgA levels were also collected.
Salmonella Recovery
The crop and CT collected in experiments 2 and 3 were homogenized and diluted with saline (1:4 w/v), and 10-fold dilutions were plated on BGA with NO and NA, incubated at 37°C for 24 h to enumerate total S. Enteritidis colony forming units. Following plating to enumerate total S. Enteritidis, the crop and CT samples were enriched in double strength tetrathionate enrichment broth and further incubated at 37°C for 24 h. Enrichment samples were streaked onto Xylose Lysine Tergitol-4 (XLT-4, Catalog No. 223410, BD DifcoTM) selective media for confirmation of Salmonella presence.
Serum Determination of FITC-d Leakage
FITC-d (MW 3-5 KDa; Sigma-Aldrich Co., St. Louis, MO) was used as a marker of paracellular transport and mucosal barrier dysfunction (30, 31). In both in vivo experiments, 1 h before the chicks were euthanized by CO2 inhalation, 12 broiler chickens from each group were given an oral gavage dose of FITC-d (8.32 mg/kg of body weight) and the rest were used as controls. The concentrations of FITC-d from diluted sera (1:5 PBS) were measured fluorometrically at an excitation wavelength of 485 nm and an emission wavelength of 528 nm (Synergy HT, Multi-mode microplate reader, BioTek Instruments, Inc., VT, USA). FITC-d concentrations were reported as ng of FITC-d/mL of serum (31).
Enzyme-Linked Immunosorbent Assay for Total IgA Levels
Total IgA levels in both in vivo experiments were determined in 12 gut rinse samples each as previously described (32). A commercial indirect ELISA set was used to quantify IgA according to the manufacturer's instructions (Catalog No. E30-103, Bethyl Laboratories Inc., Montgomery, TX 77356). Ninety six-well plates (Catalog No. 439454, Nunc MaxiSorp, Thermo Fisher Scientific, Rochester, NY) were used, and samples diluted to 1:100 were measured at 450 nm using an ELISA plate reader (Synergy HT, multi-mode microplate reader, BioTek Instruments, Inc., Winooski, VT, USA). Total intestinal IgA levels obtained were multiplied by the dilution factor (100) to determine the amount of chicken IgA in the undiluted samples.
SOD Determination
SOD activity was measured in 12 serum samples per group using a commercial assay kit (item No. 706002, Cayman chemical company, Ann Arbor, Michigan, United States) following the manufacturer's instructions. The three types of SOD (Cu/Zn, Mn, and FeSOD) were determined in samples diluted to 1:5. Samples were measured at 450 nm using an ELISA plate reader (Synergy HT, multi-mode microplate reader, BioTek Instruments, Inc., Winooski, VT, USA).
Data and Statistical Analysis
Log cfu/g of S. Enteritidis, total intestinal IgA, SOD activity and serum FITC-d concentrations were subjected to analysis of variance (ANOVA) as a completely randomized design using the General Linear Models procedure of SAS (33). Significant differences among the means were determined by Duncan's multiple-range test at p < 0.05. Enrichment data were expressed as positive/total chickens (%), and the percent recovery of S. Enteritidis was compared using the Chi-Squared test of independence (34), testing all possible combinations to determine the significance (p < 0.05).
Cecal Microbiota Analysis
DNA Extraction and PCR
Six cecal samples from each group (control and DFM groups) from the therapeutic study at day 10 post-S. enteritidis challenge were used for the cecal microbiota study. DNA extraction, PCR, and library preparation were similar as described earlier (5, 35). In brief, about 200 mg of ileal content from each sample was used for genomic DNA extraction using QIAamp® fast DNA stool mini kit (Qiagen, Catalog # 51604) following manufacturer's instructions with addition incorporation of bead beating step. For bead beating, a pellet from each sample was resuspended in 1 ml inhibit Ex buffer provided with kit and transferred to 2 ml microcentrifuge tubes with screw cap (Thermofisher Scientific, Catalog # 3468) containing 0.25 ml of sterile 0.1 mm glass leads (BioSpec, Mfr # 11079101). Bead beating was performed using Bead mill 24 (Fisher Scientific) for 6 cycles where each cycle contained a run time of 0.30 s and stopping time of 0.11 s between each cycle. The V1-V3 region of 16S rRNA gene from each 10 ng genomic DNA samples was amplified by using unique barcoded universal primers as described previously (36). PCR was performed using Q5® High-Fidelity DNA Polymerase (NEB; New England Biolabs) in a final volume of 50 μl following manufacturer's instructions. The PCR condition included initial denaturation at 98 °C for 30 s followed by 30 cycles of exponential amplifications using denaturation at 98°C for 10 s, annealing at 58°C for 30 s, extension at 72°C for 30 s, and final extension at 72°C for 2 min. Amplicons were purified from 0.7% agarose gel, concentration was measured using a Qubit dsDNA broad range assay kit (Life Technologies, United States), and equal concentrations (20 ng/μl) of amplicons were pooled together. The purified pooled amplicons were sequenced using MiSeq Illumina 300 cycle paired end options at the University of California, Riverside (Riverside, CA, United States).
16S rRNA Gene Sequence Analysis
Raw sequence reads were analyzed using Quantitative Insights into Microbial Ecology, QIIME version 1.9.1 (37) at Jetstream cloud computing platform (38, 39) using the pipelines as described previously (5, 35). Paired end reads were joined together using join_paired_ends.py command of QIIME with fastq-join option (40). After joining, barcode positions were formatted using a customized Perl script, and barcodes were removed using extract_barcodes.py command of QIIME. Split_libraries_fastq.py command of QIIME was used for demultiplexing and quality filtering of joined reads. Reads having a Phred quality score <20 were discarded. The chimeric sequences were identified using USEARCH version 6.1.544 (41), and chimeric sequences along with shorter sequences (<100 bp) were excluded for downstream analysis. The OTU picking was performed using pick_open_reference_otus.py command of QIIME with uclust method (41). Taxonomy was assigned based on green genes taxonomy and reference database version 13_8 (42) with RDP classifier (43). For further statistical analysis and visual exploration, an OTU table with taxa in plain format and a metadata file were uploaded to the MicrobiomeAnalyst tool (44). Data were filtered using the following options: minimum count 4 and low count filter based on 20% prevalence in samples. Alpha diversity analysis was calculated based on Shannon Index. Data were normalized using cumulative sum scaling before any statistical comparisons (45). Significant differences in alpha diversity among different groups were calculated based on ANOVA/T-test where a significant difference level was set at p < 0.05. Beta diversity was calculated based on Weighted UniFrac distance metric (46) and statistical comparisons among groups were performed with Analysis of Similarities method (ANOSIM). To determine differentially abundant phyla and genera among different groups, a MetagenomeSeq (45) that uses zero-inflated Gaussian fit model was used, where the level of significance was set at p < 0.05. PICRUSt ver. 1.1.3 (47) was further utilized to predict the functional pathways from 16S rRNA gene sequencing data using a closed OTU table created with the Greengenes database 13.8. The statistical analysis and visualization in the third level KEGG pathways predicted by PICRUSt between two groups were performed using the Statistical Analysis of Metagenomic Profiles (STAMP ver. 2.1.3) (48).
Results
In vitro Digestive Model
The antibacterial effect of DFM at two different concentrations (104 spores/g and 106 spores/g) against S. Enteritidis colonization in crop, proventriculus, and intestine using an in vitro digestive model is shown in Table 1. When DFM was used at 104 spores/g of feed S. Enteritidis colonization in the intestinal compartment was significantly reduced (p < 0.05), while at a higher concentration (106 spores/g) S. Enteritidis colonization in both proventriculus and intestinal compartments was significantly reduced (p<0.05) as compared to the control group (Table 1). However, the antibacterial effect of DFM was more pronounced at higher dose and especially in the intestinal compartment, where it reduced the S. Enteritidis colonization by more than 7 log10 and brought it to an undetectable level.
Table 1.
Evaluation of the antibacterial activity of different DFM ratios on S. Enteritidis† in an in vitro digestive model using the plating method‡.
| Treatment | Crop | Proventriculus | Intestine |
|---|---|---|---|
| Control | 7.78 ± 0.00 | 5.03 ± 0.12 | 7.23 ± 0.00 |
| DFM (104 spores/g) | 7.78 ± 0.00 | 5.11 ± 0.03 | 5.31 ± 0.10 |
| DFM (106 spores/g) | 7.66 ± 0.01 | 4.22 ± 0.04 | 0.00 ± 0.00 |
Values within treatment columns for each treatment with different superscripts differ significantly (p < 0.05).
Each mean is represented by five observations (n = 5) ± SE.
Inoculum used 108 cfu/mL of S. Enteritidis.
Data expressed in Log10 cfu/mL.
Prophylactic Effects of DFM
Effect on S. Enteritidis CT and Crop Colonization
The prophylactic effect of DFM (104 cfu/g) on S. Enteritidis CT and crop colonization in broiler chickens is shown in Table 2. Although there were no significant differences, there were tendencies in reducing S. Enteritidis count, and its incidence in both trials and tissues of chickens in the DFM group as compared to the control group (Table 2). In trial 1, S. Enteritidis incidence was reduced by 17% in both CT and crop in DFM group as compared to the control. Similarly, in trial 2, S. Enteritidis recovery was decreased by 17 and 23%, respectively, in CT and crop in the DFM group in comparison with the control group. In addition, S. Enteritidis count was reduced by less than half log10 and more than 1 log10 in CT and crop, respectively, in both trials when comparing the DFM group with control group (Table 2).
Table 2.
Effect of prophylactic administration of DFM (104 cfu/g) on S. Enteritidis cecal tonsil (CT) and crop colonization in broiler chickens.
| Treatments | CT Log10 cfu/g | CT ± (%) | Crop Log10 cfu/g | Crop ± (%) |
|---|---|---|---|---|
| Trial 1 | ||||
| Control | 4.01 ± 0.29 | 12/12 (100%) | 2.68 ± 0.47 | 9/12 (75%) |
| DFM | 3.72 ± 0.55 | 10/12 (83%) | 2.11 ± 0.66 | 6/12 (58%) |
| Trial 2 | ||||
| Control | 3.94 ± 0.22 | 12/12 (100%) | 2.69 ± 0.48 | 9/12 (75%) |
| DFM | 3.75 ± 0.56 | 10/12 (83%) | 2.08 ± 0.64 | 5/12 (42%) |
Data expressed in Log10 cfu/g (Mean ± SE) of tissue from 12 chickens.
Chickens were orally gavaged with 107 cfu of S. Enteritidis per chicken at 6 days old, samples were collected 24 h later.
Data expressed as positive/total chickens (%).
Superoxide Dismutase (SOD) Activity, Serum FITC-d Concentration, and Total Intestinal IgA Levels
The SOD activity, serum FITC-d concentration and total intestinal IgA levels in broiler chickens with or without receiving DFM into the diet are shown in Table 3. DFM significantly reduced SOD activity and total intestinal IgA levels as compared to the control group (p < 0.05). However, no significant difference was observed with FITC-d between two groups as shown in Table 3.
Table 3.
Evaluation of Superoxide dismutase (SOD) activity, serum fluorescein isothiocyanate-dextran (FITC-d) concentration, and total intestinal IgA in broilers chickens that were fed with or without DFM in the diet.
| Treatments | SOD (U/mL) | FITC-d (μg/mL) | IgA (μg/mL) |
|---|---|---|---|
| Control | 4.50 ± 0.31 | 0.591 ± 0.055 | 14.21 ± 0.83 |
| DFM | 1.97 ± 1.85 | 0.664 ± 0.063 | 10.57 ± 0.82 |
Samples were collected 24 h post-S. Enteritidis challenge.
Data expressed Mean ± SE from 12 chickens, where different letters indicate statistical significant difference at p < 0.05.
Therapeutic Effects of DFM
Effect on S. Enteritidis CT and Crop Colonization
The therapeutic effect of DFM (104 cfu/g) on S. Enteritidis CT and crop colonization in broiler chickens is shown in Table 4. Although there were no significant differences, there were tendencies in reducing S. Enteritidis count and its incidence in both ages and tissues of chickens in DFM group as compared to the control group (Table 4). At 3-day old, the S. Enteritidis count and its incidence in CT were reduced by ~2 log10 and 25%, respectively, by DFM group as compared to the control group. In addition, at 10-d old, DFM reduced the S. Enteritidis count in CT and crop by more than 1 log10 as compared to the control group, while the incidence of S. Enteritidis was decreased by 17 and 16%, respectively (Table 4).
Table 4.
Effect of therapeutic administration of DFM (104 cfu/g) on S. Enteritidis cecal tonsil (CT) and crop colonization in broiler chickens.
| Treatments | CT Log10 cfu/g | CT ± (%) | Crop Log10 cfu/g | Crop ± (%) |
|---|---|---|---|---|
| Trial 3-d | ||||
| Control | 6.44 ± 0.15 | 12/12 (100%) | 3.18 ± 0.46 | 10/12 (83%) |
| DFM | 4.66 ± 0.82 | 9/12 (75%) | 3.05 ± 0.45 | 10/12 (83%) |
| Trial 10-d | ||||
| Control | 6.61 ± 0.21 | 12/12 (100%) | 2.93 ± 0.65 | 7/12 (58%) |
| DFM | 5.49 ± 0.76 | 10/12 (83%) | 1.78 ± 0.65 | 5/12 (42%) |
Data expressed in Log10 cfu/g (Mean ± SE) of tissue from 12 chickens.
Chickens were orally gavaged with 104 cfu of S. Enteritidis per chicken at 1 day old; samples were collected 3 and 10 days later.
Data expressed as positive/total chickens (%).
SOD Activity, Serum FITC-d Concentration, and Total Intestinal IgA Levels
The SOD activity, serum FITC-d concentration and total intestinal IgA levels in broiler chickens with or without receiving DFM into the diet at day 10 post-S. Enteritidis challenge are shown in Table 5. DFM significantly reduced FITC-d and intestinal IgA levels as compared to the control (p < 0.05). In the case of SOD activity, there was a numerical reduction in the DFM group compared to the control group; however, no significant difference was observed.
Table 5.
Evaluation of Superoxide dismutase (SOD) activity, serum fluorescein isothiocyanate-dextran (FITC-d) concentration, and total intestinal IgA in broilers chickens with or without receiving DFM into the diet at day 10 post-S. Enteritidis challenge.
| Treatments | SOD (U/mL) | FITC-d (μg/mL) | IgA (μg/mL) |
|---|---|---|---|
| Control | 10.34 ± 0.67 | 0.700 ± 0.020 | 14.34 ± 2.81 |
| DFM | 9.29 ± 0.88 | 0.531 ± 0.013 | 6.21 ± 2.31 |
Data expressed as Mean ± SE from 12 chickens, where different letters indicate statistical significant difference at p < 0.05.
Cecal Microbiota
Summarization of the OTU table resulted a total of 441,934 reads that range from 27,654 to 43,856 reads per sample. The total number of OTUs after data filtering was 1,108.
Taxonomic Assignments
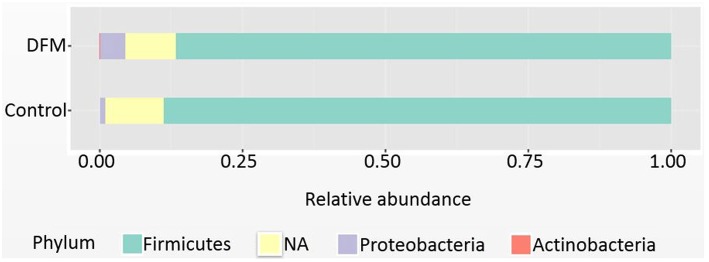
Phylum level Firmicutes were found as a predominant phylum in both groups (Control group, 88.71%; DFM group, 86.68%) followed by Proteobacteria and Actinobacteria as shown in Figure 1. Actinobacteria were significantly reduced in the DFM group as compared to the control group (p < 0.05).
Figure 1.
Relative abundance of major phyla recovered in ceca of broiler chickens at day 10 from two different treatment groups (control and DFM). NA refers to those reads that could not be assigned to any phyla.
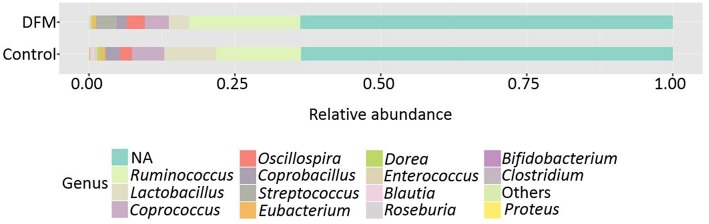
Genus level The relative abundance of different genera present in the control and DFM groups is shown in Figure 2. Ruminococcus was found as a predominant genus in both groups (Control group, 14.48%; DFM group, 19.14%), followed by Lactobacillus (Control group, 8.91%; DFM group, 3.40%), and Streptococcus (Control group, 0.15%; DFM group, 3.68%) in control and DFM, respectively.
Figure 2.
Relative abundance of major genera recovered in ceca of broiler chickens at day 10 from two different treatment groups (control and DFM). NA refers to those reads that could not be assigned to any genera. Genera having counts <100 are merged together in “Others”.
The genera Bifidobacterium (Control group, 0.094%; DFM group, not detected), Roseburia (Control group, 0.19%; DFM group, 0.035%), Proteus (Control group, 0.07%; DFM group, not detected), and cc_115 (Control group, 0.04%; DFM group, not detected) were significantly decreased, while the genus Streptococcus was significantly enriched in the DFM group as compared to the control group (MetagenomeSeq, p < 0.05). In addition, some of the notable genera such as Enterococcus, Dorea, Coprobacillus, Coprococcus, Eubacterium, and Blautia were numerically reduced in the DFM group as compared to the control group.
Microbial Diversities analysis
Alpha diversity Alpha diversity of control and DFM groups as measured by Shannon index is shown in Figure 3. The average Shannon index in the control group was 4.61 ± 0.09 (Mean ± SE) and 4.27 ± 0.22 in the case of the DFM group. However, there was no significant difference observed between both groups.
Figure 3.
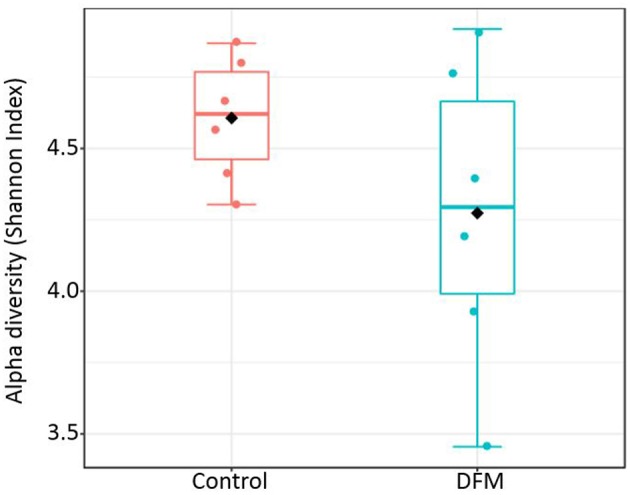
Alpha diversity of two different groups (control and DFM) as measured by Shannon Index. No significant difference was observed between them (T-test, p > 0.05). The diamond shape represents the mean value in each group.
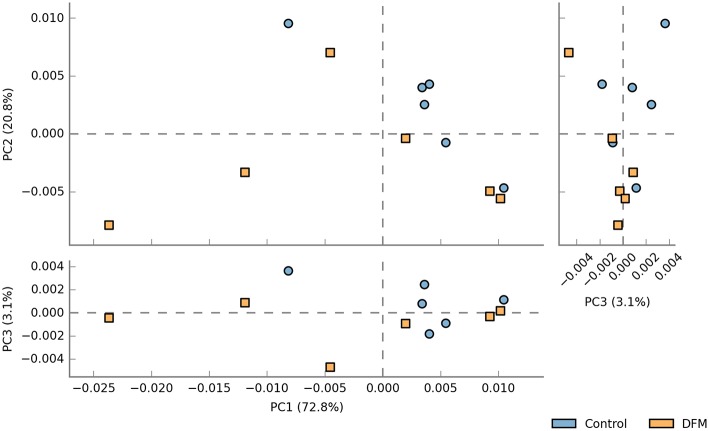
Beta diversity Beta diversity between control and DFM groups as measured by weighted UniFrac metric is illustrated in PCoA plot (Figure 4). Analysis of similarities (ANOSIM) showed significant difference in microbial community structure between the two groups (R = 0.35, p < 0.01).
Figure 4.
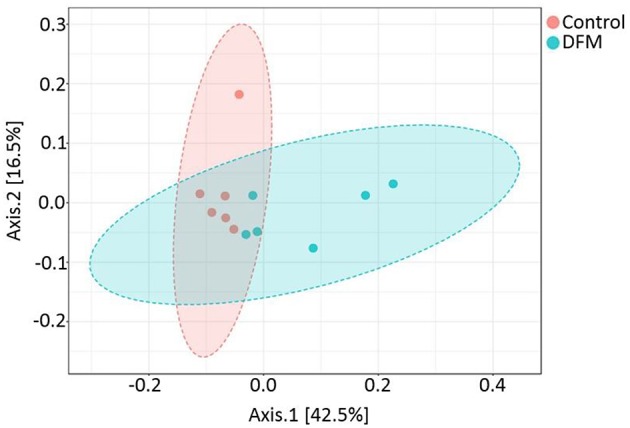
PCoA plot showing difference in microbial community structure between control and DFM groups (ANOSIM; R = 0.35 and p < 0.01).
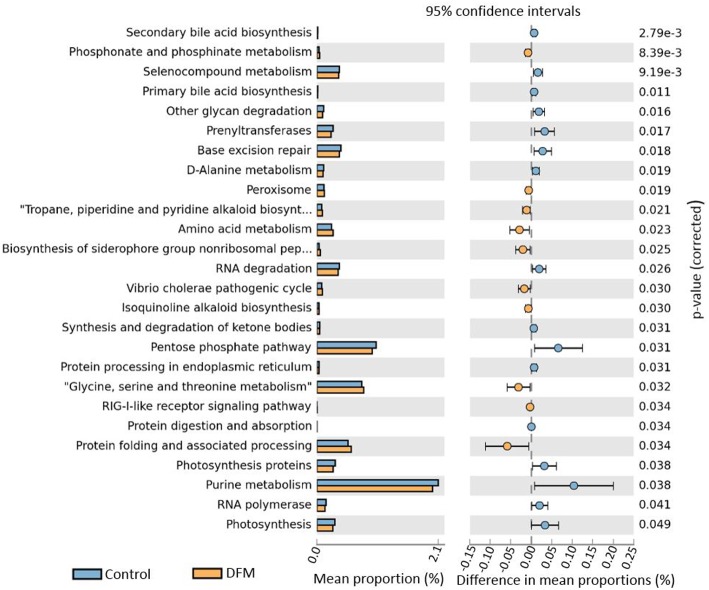
Functional potentialities of cecal bacterial community The predicted functions of cecal microbiota in the control and DFM groups by PICRUSt and their analysis by STAMP are shown in Figures 5, 6. The PCA plot shows that the third level KEGG pathways of the DFM group are relatively distinct in comparison to the control group (Figure 5). More specifically, many bacterial genes that are involved in various metabolic pathways such as bile acid synthesis (primary and secondary), carbohydrate metabolism (pentose phosphate pathway and other glycan degradation), and nucleotide metabolism (purine) were predicted to be enriched in the control group. On the other hand, bacterial genes that could involve in amino acid metabolism (Glycine, Serine, and Threonine) and alkaloid biosynthesis (isoquinoline, tropane, piperidine, and pyridine alkaloids) were predicted to be enriched in the DFM group (Figure 6).
Figure 5.
PCA plot comparing third level KEGG pathways between control and DFM groups. The third level KEGG pathways were predicted using PICRUSt followed by the generation of PCA plot using STAMP.
Figure 6.
Extended error bar plot generated by STAMP showing differential abundant third level KEGG pathways between control and DFM group. Only significant features with p < 0.05 (Welch's t-test) were included in the plot.
Discussion
Previous research reported nontyphoidal Salmonella sps., Clostridium perfringens, Campylobacter sps., and Escherichia coli as some of the most important foodborne bacterial pathogens in the United States (49). The overall health-related costs associated with foodborne illness from those pathogens was estimated to be around $51.0 and $77.7 billion based on the basic and enhanced model, respectively, as described earlier (50). Nontyphoidal Salmonella sp. was reported as a major causative agent for hospitalization and deaths of patients in the United States (49). S. Enterica serotype Enteritidis (S. Enteritidis) which emerged as an important human illness during 1980s is currently one of the most common nontyphoidal Salmonella serotypes worldwide, especially in developed countries (51). Poultry and their products (eggs and meat) are considered as one of the most important sources of S. Enteritidis infection in humans; however, S. Enteritidis was also isolated from non-poultry sources such as market hog carcass, steer and heifer carcass, cow and bull carcass, and ground beef (52–54).
Several studies have been conducted with the objective to reduce S. Enteritidis load in poultry and their products using various approaches such as antibodies, bacteriophages, probiotics, prebiotics, vaccines, and integrated farm management (55–59). In the present study, we evaluated the effects of Norum™ (DFM) to reduce S. Enteritidis colonization using both in vitro and in vivo trials in broiler chickens. Our previous study using an in vitro digestion model showed a reduction of C. perfringens by the isolates used in Norum™ in different non-corn based diets demonstrating their antibacterial property against this Gram-positive bacteria (24). The antimicrobial activity of various species of Bacillus, including B. subtilis and B. amyloliquefaciens, were studied elsewhere and found to be effective mainly against Gram-positive bacteria (60–63). In the current study, we also observed the reduction of S. Enteritidis by DFM in the intestinal compartment simulated in the model and in both proventriculus and intestinal compartments, when using 104 spores/g and 106 spores/g DFM, respectively. The Salmonella Enteritidis colonization was reduced by more than 7 log10 cfu/mL and brought to an undetectable level in the intestinal compartment when DFM was used at 106 spores/g of feed, suggesting its more noticeable antibacterial effects at a higher dose. These findings further suggest that DFM exhibits a wide range of antibacterial activities which can be effective for both Gram-positive and negative bacteria. Although the detailed mechanism is not well-understood, these antibacterial properties of DFM might be achieved not only through competitive exclusion and production of antimicrobial peptides (AMPs), but also might be indirectly through one or several beneficial effects exhibited by them including secretion of exogenous enzymes, alternation of immunity, gut microbiota, and morphology (23, 24, 64, 65). The AMPs secreted by Bacillus sps. are diverse in nature with different chemical structure (60) and include bacteriocins, glycopeptides, lipopeptides, and cyclic peptides (61).
The antibacterial activity of Bacillus isolates in Norum™ against Clostridium perfringens (24), S. Enteritidis, Escherichia coli, and Clostridium difficile (64) was evaluated earlier using an in vitro model and reported as promising DFM candidates. In addition, dietary supplementation with DFM (106 spores/g) mitigated the negative impacts of necrotic enteritis in broiler chickens using a laboratory challenge model (35). Therefore, considering that the model of necrotic enteritis is more severe than a Salmonella infection model and in vitro results, in this study, we evaluated the therapeutic and prophylactic effects of those isolates in Norum™ (104 spores/g) against S. Enteritidis CT and crop colonization in broiler chickens. Although there were no significant differences, there were tendencies in reducing S. Enteritidis count and its incidence in both ages (3 and 10 days) and tissues (CT and crop) of chickens by DFM as compared to the control during the therapeutic study. Similar tendencies were also reported in both trials during the prophylactic study. This may be due to the lower dose of Bacillus spores (104 spores/g of feed) used during the in vivo trials, because the antibacterial effect was more pronounced with higher dose compared to the lower dose as demonstrated by the in vitro digestion model (Table 1). A similar antimicrobial dose-dependent response of Bacillus-DFM against necrotic enteritis was observed earlier, where the higher dose (106 cfu/g of feed) mitigated negative impacts of NE more than the lower dose (104 cfu/g of feed) (66).
Several enteric pathogens including Salmonella sps. disrupt the intestinal tight junctions leading to the increase in gut permeability; commonly known as “leaky gut” (67, 68). Serum FITC-d increases with inflammation and is considered as a good indicator to measure enteric inflammation induced gut permeability in broiler chickens (69). The significant reduction (p < 0.05) of serum FITC-d level by DFM as compared to the control group in the therapeutic study might be due to the alleviation of negative impacts of S. Enteritidis by increasing the regulation of tight junction proteins (23, 70). Antioxidant enzymes such as SOD play a vital role to degrade superoxide anions and hydrogen peroxide produced during an inflammatory process. There was a significant (p < 0.05) and numerical increase of SOD activity in the control group of the prophylactic and therapeutic study, respectively, when compared to the group treated with DFM. The increased SOD activity in the control group could be related to the response to increased oxidative stress due to severe intestinal damage caused by S. Enteritidis, since SOD plays a key role in the reduction of oxidative stress (71). Similarly, the significant increase in IgA level (p < 0.05) in both in vivo trials might be associated with disruption of intestinal epithelium, since secretion of intestinal IgA serves as the first line of defense to protect the intestinal epithelium from enteric toxins and pathogenic microorganism, as well as to antagonize the inflammatory processes and enhance the non-specific defense mechanisms (32, 72). In contrast, the decrease of SOD activity and IgA level by DFM could be related to its anti-inflammatory and immune modulating properties to mitigate the negative impacts of S. Enteritidis, reducing the gut morphological and immunological alterations through expression of the cytoprotective proteins and modulation of various cytokines (19, 23, 73–76).
Along with the advancement in sequencing technologies, the cost of sequencing has significantly reduced recently, making microbiota studies more affordable. It is now a well-accepted fact that the gut microbiota plays a key role in health and diseases of both humans and animals, which has been reviewed elsewhere (77–80). Although detailed mechanisms are unknown, the supplementation of various alternatives to antibiotics including Bacillus-DFM can improve overall intestinal health and growth in chickens (24, 35), probably due to the modulation of the gut microbiota, which is one of the important mechanisms of action exhibited by alternatives to antibiotics in order to exert beneficial effects on the host (2, 23, 81–83). Moreover, the inclusion of Bacillus-DFM has been shown to alter the cecal (20) and ileal (21) microbiota in broiler chickens.
The cecum of the chicken harbors the greatest bacterial diversity and is an important organ for water regulation and production of short chain fatty acids (SCFA) through carbohydrate fermentation (23, 84). The ceca of young chickens are mainly dominated by the phylum Firmicutes, Proteobacteria, and Actinobacteria, whereas the relative abundance of Bacteriodetes increases with age and was detected only after 15 days in broiler chickens (85). We also reported Firmicutes as the dominant phyla in both groups followed by Proteobacteria and Actinobacteria. Actinobacteria were significantly lowered by the DFM, which could be due to the antibacterial activity of DFM against S. Enteritidis since Actinobacteria were increased in chickens infected with S. Enteritidis (5, 86). The genus Proteus and the genus cc_115 of the family Erysipelotrichaceae were significantly higher in the control group as compared to the DFM. The increased abundance of Proteus and cc_115 was associated with necrotic enteritis in broiler chickens (87). Similarly, the genus Proteus and the bacterial family Erysipelotrichaceae were found to be associated with intestinal dysbiosis in humans as reported in the DisbiomeR database (88). Thus, the increase of Proteus and cc_115 in the control might be associated with gut dysbiosis and inflammation caused by S. Enteritidis (89), whereas their decrease in the DFM group might be due to the antibacterial property of DFM. Furthermore, the increase of Bifidobacterium and Roseburia in the control group might be due to the inflammatory response, since these genera were found to have anti-inflammatory properties (90, 91). A significant increase in Bifidobacterium after S. Enteritidis inoculation was also reported earlier in chickens (92). Although some of the species of Streptococcus cause infection in poultry (93, 94) they are commensal organisms present in the GI tract of chickens and have been used as potential probiotics (95, 96) because of their ability to reduce pathogen colonization through competitive exclusion and reduction of the pH through lactic acid production (97). Thus, increase in Streptococcus by DFM in the present study may be playing a vital role in reducing the colonization and incidence of S. Enteritidis; however, a higher resolution to the strain level is needed to understand the actual effects as two strains of the same species can carry out completely opposite roles (98).
DFM not only affected the bacterial composition in the ceca of broiler chickens but also the community structure as indicated by the beta diversity analysis. However, in the case of alpha diversity, although there was numerically higher diversity in the control group, no significant difference was observed between the two groups. This may be related to one of the theories that the DFM promotes growth of the host by reducing the number and diversity of the commensal microbiota, which will allow increased nutrient utilization by intestinal epithelial cells and lower detrimental effects of microbial metabolites (99). These regulations by DFM might be achieved through changes in bacterial genes involved in various metabolic pathways (Figures 5, 6). One of the important metabolic pathways predicted to be enriched in the control group was bile acid synthesis. Bile acids are considered as important regulators of the gut microbiota and reduced levels of bile acids in the gut are associated with bacterial overgrowth and intestinal inflammation (100, 101). Enrichment of the bile acid synthesis pathway in the control group might be a response to the lower level of bile acids and inflammation caused by S. Enteritidis and other dysbiosis associated bacteria colonization in the gut. Similarly, another glycan degradation pathway was enriched in the control group, and this might be related to the response of mucinogeneis as a result of S. Enteritidis inflammation and the overgrowth of Bifidobacterium in the control group, which can degrade the host-derived glycans (102). Amino acids serve as precursors for microbial-derived SCFA such as acetate, propionate, and butyrate, which has been reviewed elsewhere (103). Meanwhile, the increase in metabolic pathways associated with the metabolism of amino acids (glycine, serine, and threonine) in the DFM group could be related to the amino acid fermenting ability of the Bacillus-DFM (104) to produce SCFA. SCFA serves as nutrients for colonocytes and other gut epithelial cells and plays a key role in shaping the gut microbiota of the host (105). Future investigation of the effects of DFM in the Salmonella challenged model by metagenomics and metabolomics analysis will reveal more functional potentialities of DFM.
In summary, the overall results of the present study suggest that the Bacillus-DFM (Norum™) can be used for the prevention and treatment of S. Enteritidis infection since it has the potential to reduce S. Enteritidis colonization and mitigate its negative effects in broiler chickens. These effects of Norum™ could be achieved through mechanism(s) that might involve the modulation of gut microbiota and their metabolic pathways. The effects of Norum™ against S. Enteritidis at a higher dose (106 spores/g) may disclose more promising results and are currently under evaluation.
Data Availability
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Ethics Statement
All animal handling procedures complied with the Institutional Animal Care and Use Committee (IACUC) at the University of Arkansas, Fayetteville (protocol #18030).
Author Contributions
BA and GT-I designed the experiments and wrote the first version of the manuscript. BA, DH-P, and BS-C performed the experiment. YK, MA, JL, and BH aided in the analysis and interpretation of the data and supervised the project. GT-I, BA, JL, and XH-V contributed to editing the final version of the manuscript. All the authors reviewed and finally approved the manuscript.
Conflict of Interest Statement
MA was employed by Eco-Bio LLC. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2019.00282/full#supplementary-material
References
- 1.Dibner JJ, Richards JD. Antibiotic growth promoters in agriculture: history and mode of action. Poult Sci. (2005) 84:634–43. 10.1093/ps/84.4.634 [DOI] [PubMed] [Google Scholar]
- 2.Huyghebaert G, Ducatelle R, Van Immerseel F. An update on alternatives to antimicrobial growth promoters for broilers. Vet J. (2011) 187:182–8. 10.1016/j.tvjl.2010.03.003 [DOI] [PubMed] [Google Scholar]
- 3.Maron DF, Smith TJS, Nachman KE. Restrictions on antimicrobial use in food animal production: an international regulatory and economic survey. Global Health. (2013) 9:48. 10.1186/1744-8603-9-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mottet A, Tempio G. Global poultry production: current state and future outlook and challenges. Worlds Poult Sci J. (2017) 73:245–56. 10.1017/S0043933917000071 [DOI] [Google Scholar]
- 5.Hernandez-Patlan D, Solis-Cruz B, Adhikari B, Pontin KP, Latorre JD, Baxter MF, et al. Evaluation of the antimicrobial and intestinal integrity properties of boric acid in broiler chickens infected with Salmonella Enteritidis: proof of concept. Res Vet Sci. (2019) 123:7–13. 10.1016/j.rvsc.2018.12.004 [DOI] [PubMed] [Google Scholar]
- 6.Fuller R. Probiotics in man and animals. J Appl Bacteriol. (1989) 66:365–78. 10.1111/j.1365-2672.1989.tb05105.x [DOI] [PubMed] [Google Scholar]
- 7.Buntyn JO, Schmidt TB, Nisbet DJ, Callaway TR. The role of direct-fed microbials in conventional livestock production. Annu Rev Anim Biosci. (2016) 4:335–55. 10.1146/annurev-animal-022114-111123 [DOI] [PubMed] [Google Scholar]
- 8.Hagihara M, Yamashita R, Matsumoto A, Mori T, Kuroki Y, Kudo H, et al. The impact of Clostridium butyricum MIYAIRI 588 on the murine gut microbiome and colonic tissue. Anaerobe. (2018) 54:8–18. 10.1016/j.anaerobe.2018.07.012 [DOI] [PubMed] [Google Scholar]
- 9.Nicholson WL. Roles of Bacillus endospores in the environment. Cell Mol Life Sci. (2002) 59:410–6. 10.1007/s00018-002-8433-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Setlow B, Atluri S, Kitchel R, Koziol-Dube K, Setlow P. Role of dipicolinic acid in resistance and stability of spores of Bacillus subtilis with or without DNA-protective alpha/beta-type small acid-soluble proteins. J Bacteriol. (2006) 188:3740–7. 10.1128/JB.00212-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moeller R, Setlow P, Reitz G, Nicholson WL. Roles of small, acid-soluble spore proteins and core water content in survival of Bacillus subtilis spores exposed to environmental solar UV radiation. Appl Environ Microbiol. (2009) 75:5202–8. 10.1128/AEM.00789-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolfenden RE, Pumford NR, Morgan MJ, Shivaramaiah S, Wolfenden AD, Pixley CM, et al. Evaluation of selected direct-fed microbial candidates on live performance and Salmonella reduction in commercial turkey brooding houses. Poult Sci. (2011) 90:2627–31. 10.3382/ps.2011-01360 [DOI] [PubMed] [Google Scholar]
- 13.Lu J, Idris U, Harmon B, Hofacre C, Maurer JJ, Lee MD. Diversity and succession of the intestinal bacterial community of the maturing broiler chicken. Appl Environ Microbiol. (2003) 69:6816–24. 10.1128/AEM.69.11.6816-6824.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barbosa TM, Serra CR, La Ragione RM, Woodward MJ, Henriques AO. Screening for Bacillus isolates in the broiler gastrointestinal tract. Appl Environ Microbiol. (2005) 71:968–78. 10.1128/AEM.71.2.968-978.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Latorre JD, Hernandez-Velasco X, Kallapura G, Menconi A, Pumford NR, Morgan MJ, et al. Evaluation of germination, distribution, and persistence of Bacillus subtilis spores through the gastrointestinal tract of chickens. Poult Sci. (2014) 93:1793–800. 10.3382/ps.2013-03809 [DOI] [PubMed] [Google Scholar]
- 16.Fritts C, Kersey J, Motl M, Kroger E, Yan F, Si J, et al. Bacillus subtilis C-3102 (Calsporin) improves live performance and microbiological status of broiler chickens. J Appl Poul Res. (2000) 9:149–55. 10.1093/japr/9.2.149 [DOI] [Google Scholar]
- 17.Vil à B, Fontgibell A, Badiola I, Esteve-Garcia E, Jiménez G, Castillo M, et al. Reduction of Salmonella enterica var. Enteritidis colonization and invasion by Bacillus cereus var. toyoi inclusion in poultry feeds. Poult Sci. (2009) 88:975–9. 10.3382/ps.2008-00483 [DOI] [PubMed] [Google Scholar]
- 18.Dersjant-Li Y, Awati A, Kromm C, Evans C. A direct fed microbial containing a combination of three-strain Bacillus sp. can be used as an alternative to feed antibiotic growth promoters in broiler production. J Appl Anim Nutr. (2014) 2:e11 10.1017/jan.2014.4 [DOI] [Google Scholar]
- 19.Lee KW, Lillehoj HS, Jang SI, Lee SH, Bautista DA, Siragusa GR. Effect of Bacillus subtilis-based direct-fed microbials on immune status in broiler chickens raised on fresh or used litter. Asian Aust J Anim Sci. (2013) 26:1592–97. 10.5713/ajas.2013.13178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lei X, Piao X, Ru Y, Zhang H, Péron A, Zhang H. Effect of Bacillus amyloliquefaciens-based direct-fed microbial on performance, nutrient utilization, intestinal morphology and cecal microflora in broiler chickens. Asian Aust J Anim Sci. (2015) 28:239–46. 10.5713/ajas.14.0330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Latorre JD, Hernandez-Velasco X, Vicente JL, Wolfenden R, Hargis BM, Tellez G. Effects of the inclusion of a Bacillus direct-fed microbial on performance parameters, bone quality, recovered gut microflora, and intestinal morphology in broilers consuming a grower diet containing corn distillers dried grains with solubles. Poult Sci. (2017) 96:2728–35. 10.3382/ps/pex082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shivaramaiah S, Pumford NR, Morgan MJ, Wolfenden RE, Wolfenden AD, Torres-Rodríguez A, et al. Evaluation of Bacillus species as potential candidates for direct-fed microbials in commercial poultry. Poult Sci. (2011) 90:1574–80. 10.3382/ps.2010-00745 [DOI] [PubMed] [Google Scholar]
- 23.Grant A, Gay CG, Lillehoj HS. Bacillus spp. as direct-fed microbial antibiotic alternatives to enhance growth, immunity, and gut health in poultry. Avian Pathol. (2018) 47:339–51. 10.1080/03079457.2018.1464117 [DOI] [PubMed] [Google Scholar]
- 24.Latorre JD, Hernandez-Velasco X, Kuttappan VA, Wolfenden RE, Vicente JL, Wolfenden AD, et al. Selection of Bacillus spp. for cellulase and xylanase production as direct-fed microbials to reduce digesta viscosity and clostridium perfringens proliferation using an in vitro digestive model in different poultry diets. Front Vet Sci. (2015) 2:25. 10.3389/fvets.2015.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Latorre JD, Hernandez-Velasco X, Bielke LR, Vicente JL, Wolfenden R, Menconi A, et al. Evaluation of a Bacillus direct-fed microbial candidate on digesta viscosity, bacterial translocation, microbiota composition and bone mineralisation in broiler chickens fed on a rye-based diet. Br Poult Sci. (2015) 56:723–32. 10.1080/00071668.2015.1101053 [DOI] [PubMed] [Google Scholar]
- 26.National Research Council (1994). Nutrient Requirements of Poultry. 9th ed Washington, DC: National Academy Press. [Google Scholar]
- 27.Cobb-Vantress Inc Cobb 500 Broiler Performance and Nutrition Supplement. Siloam Springs, AR: Cobb-Vantress (2015). Available at: https://cobbstorage.blob.core.windows.net/guides/3914ccf0-6500-11e8-9602-256ac3ce03b1
- 28.Lin J, Lee IS, Frey J, Slonczewski JL, Foster JW. Comparative analysis of extreme acid survival in Salmonella Typhimurium, Shigella flexneri, and Escherichia coli. J Bacteriol. (1995) 177:4097–104. 10.1128/jb.177.14.4097-4104.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Annett CB, Viste JR, Chirino-Trejo M, Classen HL, Middleton DM, Simko E. Necrotic enteritis:effect of barley, wheat and corn diets on proliferation of Clostridium perfringens type A. Avian Pathol. (2002) 31:598–601. 10.1080/0307945021000024544 [DOI] [PubMed] [Google Scholar]
- 30.Yan Y, Kolachala V, Dalmasso G, Nguyen H, Laroui H, Sitaraman SV, et al. Temporal and spatial analysis of clinical and molecular parameters in dextran sodium sulfate induced colitis. PLoS ONE. (2009) 4:e6073. 10.1371/journal.pone.0006073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baxter MFA, Merino-Guzman R, Latorre JD, Mahaffey BD, Yang Y, Teague KD, et al. Optimizing fluorescein isothiocyanate dextran measurement as a biomarker in a 24-h feed restriction model to induce gut permeability in broiler Chickens. Front Vet Sci. (2017) 4:56. 10.3389/fvets.2017.00056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merino-Guzmán R, Latorre JD, Delgado R, Hernandez-Velasco X, Wolfenden AD, Teague KD, et al. Comparison of total immunoglobulin A levels in different samples in Leghorn and broiler chickens. Asian Pac J Trop Biomed. (2017) 7:116–20. 10.1016/j.apjtb.2016.11.021 [DOI] [Google Scholar]
- 33.SAS Institute Inc (2002). SAS/Share: 9.4 User's Guide. 2nd ed Cary, NC: SAS Documentation. [Google Scholar]
- 34.Zar JH. Biostatistical Analysis. 2nd ed Englewood Cliffs, NJ: Prentice Hall Inc; (1984). [Google Scholar]
- 35.Hernandez-Patlan D, Solis-Cruz B, Pontin KP, Hernandez X, Merino-Guzman R, Adhikari B, et al. Impact of a Bacillus direct-fed microbial on growth performance, intestinal barrier integrity, necrotic enteritis lesions and ileal microbiota in broiler chickens using a laboratory challenge model. Front Vet Sci. (2019) 6:108. 10.3389/fvets.2019.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adhikari B, Kwon YM. Characterization of the culturable subpopulations of Lactobacillus in the chicken intestinal tract as a resource for probiotic development. Front Microbiol. (2017) 8:1389. 10.3389/fmicb.2017.01389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. (2010) 7:335–6. 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Towns J, Cockerill T, Dahan M, Foster I, Gaither K, Grimshaw A, et al. XSEDE: accelerating scientific discovery. Comput Sci Eng. (2014) 16:62–74. 10.1109/MCSE.2014.80 [DOI] [Google Scholar]
- 39.Stewart CA, Cockerill TM, Foster I, Hancock D, Merchant N, Skidmore E, et al. Jetstream: a self-provisioned, scalable science and engineering cloud environment. In: Proceedings of the 2015 XSEDE Conference: Scientific Advancements Enabled by Enhanced Cyberinfrastructure. St. Louis: ACM; (2015). 29. [Google Scholar]
- 40.Aronesty E. Ea-utils: Command-Line Tools for Processing Biological Sequencing Data. (2011). Available online at: https://github.com/ExpressionAnalysis/ea-utils
- 41.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. (2010) 26:2460–1. 10.1093/bioinformatics/btq461 [DOI] [PubMed] [Google Scholar]
- 42.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. (2006) 72:5069–72. 10.1128/AEM.03006-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naïve bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. (2007) 73:5261–7. 10.1128/AEM.00062-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dhariwal A, Chong J, Habib S, King IL, Agellon LB, Xia J. MicrobiomeAnalyst: a web-based tool for comprehensive statistical, visual and meta-analysis of microbiome data. Nucleic Acids Res. (2017) 45:W180–8. 10.1093/nar/gkx295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paulson JN, Stine OC, Bravo HC, Pop M. Differential abundance analysis for microbial marker-gene surveys. Nat Methods. (2013) 10:1200–2. 10.1038/nmeth.2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lozupone C, Lladser ME, Knights D, Stombaugh J, Knight R. UniFrac: An effective distance metric for microbial community comparison. ISME J. (2011) 5:169–72. 10.1038/ismej.2010.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Langille MGI, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. (2013) 31:814–21. 10.1038/nbt.2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parks DH, Tyson GW, Hugenholtz P, Beiko RG. STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics. (2014) 30:3123–4. 10.1093/bioinformatics/btu494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, et al. Foodborne illness acquired in the United States-major pathogens. Emerg Infect Dis. (2011) 17:7–15. 10.3201/eid1701.P11101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scharff RL. Economic burden from health losses due to foodborne illness in the United States. J Food Prot. (2012) 75:123–31. 10.4315/0362-028X.JFP-11-058 [DOI] [PubMed] [Google Scholar]
- 51.Patrick ME, Adcock PM, Gomez TM, Altekruse SF, Holland BH, Tauxe RV, et al. Salmonella Enteritidis infections, United States, 1985-1999. Emerg Infect Dis. (2004) 10:1–7. 10.3201/eid1001.020572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.White PL, Naugle AL, Jackson CR, Fedorka-Cray PJ, Rose BE, Pritchard KM, et al. Salmonella Enteritidis in meat, poultry, and pasteurized egg products regulated by the US food safety and Inspection service, 1998 through 2003. J Food Prot. (2007) 70:582–91. 10.4315/0362-028X-70.3.582 [DOI] [PubMed] [Google Scholar]
- 53.Gantois I, Ducatelle R, Pasmans F, Haesebrouck F, Gast R, Humphrey TJ, et al. Mechanisms of egg contamination by Salmonella Enteritidis. FEMS Microbiol Rev. (2009) 33:718–38. 10.1111/j.1574-6976.2008.00161.x [DOI] [PubMed] [Google Scholar]
- 54.Antunes P, Mourão J, Campos J, Peixe L. Salmonellosis: the role of poultry meat. Clin Microbiol Infect. (2016) 22:110–21. 10.1016/j.cmi.2015.12.004 [DOI] [PubMed] [Google Scholar]
- 55.Fulton R, Nersessian B, Reed W. Prevention of Salmonella Enteritidis infection in commercial ducklings by oral chicken egg-derived antibody alone or in combination with probiotics. Poult Sci. (2002) 81:34–40. 10.1093/ps/81.1.34 [DOI] [PubMed] [Google Scholar]
- 56.Fiorentin L, Vieira ND, Barioni WJr. Oral treatment with bacteriophages reduces the concentration of Salmonella Enteritidis PT4 in caecal contents of broilers. Avian Pathol. (2005) 34:258–63. 10.1080/01445340500112157 [DOI] [PubMed] [Google Scholar]
- 57.Donalson LM, McReynolds JL, Kim WK, Chalova VI, Woodward CL, Kubena LF, et al. The influence of a fructooligosaccharide prebiotic combined with alfalfa molt diets on the gastrointestinal tract fermentation, Salmonella Enteritidis infection, and intestinal shedding in laying hens. Poult Sci. (2008) 87:1253–62. 10.3382/ps.2007-00166 [DOI] [PubMed] [Google Scholar]
- 58.Trampel DW, Holder TG, Gast RK. Integrated farm management to prevent Salmonella Enteritidis contamination of eggs. J Appl Poult Res. (2014) 23:353–65. 10.3382/japr.2014-00944 [DOI] [Google Scholar]
- 59.Kilroy S, Raspoet R, Haesebrouck F, Ducatelle R, Van Immerseel F. Prevention of egg contamination by Salmonella Enteritidis after oral vaccination of laying hens with Salmonella Enteritidis Δ tolC and Δ acrABacrEFmdtABC mutants. Vet Res. (2016) 47:82 10.1186/s13567-016-0369-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cladera-Olivera F, Caron GR, Brandelli A. Bacteriocin-like substance production by Bacillus licheniformis strain P40. Lett Appl Microbiol. (2004) 38:251–6. 10.1111/j.1472-765X.2004.01478.x [DOI] [PubMed] [Google Scholar]
- 61.Baindara P, Mandal SM, Chawla N, Singh PK, Pinnaka AK, Korpole S. Characterization of two antimicrobial peptides produced by a halotolerant Bacillus subtilis strain SK.DU.4 isolated from a rhizosphere soil sample. AMB Express. (2013) 3:2. 10.1186/2191-0855-3-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kadaikunnan S, Rejiniemon T, Khaled JM, Alharbi NS, Mothana R. In vitro antibacterial, antifungal, antioxidant and functional properties of Bacillus amyloliquefaciens. Ann Clin Microbiol Antimicrob. (2015) 14:9. 10.1186/s12941-015-0069-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yilmaz M, Soran H, Beyatli Y. Antimicrobial activities of some Bacillus spp. strains isolated from the soil. Microbiol Res. (2006) 161:127–31. 10.1016/j.micres.2005.07.001 [DOI] [PubMed] [Google Scholar]
- 64.Latorre JD, Hernandez-Velasco X, Wolfenden RE, Vicente JL, Wolfenden AD, Menconi A, et al. Evaluation and selection of Bacillus species based on enzyme production, antimicrobial activity, and biofilm synthesis as direct-fed microbial candidates for poultry. Front Vet Sci. (2016) 3:95. 10.3389/fvets.2016.00095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nawawi MH, Mohamad R, Tahir PM, Saad WZ. Extracellular xylanopectinolytic enzymes by Bacillus subtilis ADI1 from EFB's Compost. Int Sch Res Notices. (2017) 2017:7831954. 10.1155/2017/7831954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tactacan G, Schmidt J, Miille M, Jimenez D. A Bacillus subtilis (QST 713) spore-based probiotic for necrotic enteritis control in broiler chickens. J Appl Poult Res. (2013) 22:825–31. 10.3382/japr.2013-00730 [DOI] [Google Scholar]
- 67.Berkes J, Viswanathan V, Savkovic S, Hecht G. Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation. Gut. (2003) 52:439–51. 10.1136/gut.52.3.439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Awad WA, Hess C, Hess M. Enteric pathogens and their toxin-induced disruption of the intestinal barrier through alteration of tight junctions in chickens. Toxins. (2017) 9:E60. 10.3390/toxins9020060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vicuña EA, Kuttappan VA, Tellez G, Hernandez-Velasco X, Seeber-Galarza R, Latorre JD, et al. Dose titration of FITC-D for optimal measurement of enteric inflammation in broiler chicks. Poult Sci. (2015) 94:1353–9. 10.3382/ps/pev111 [DOI] [PubMed] [Google Scholar]
- 70.Chichlowski M, Croom J, McBride B, Havenstein G, Koci M. Metabolic and physiological impact of probiotics or direct-fed-microbials on poultry: a brief review of current knowledge. Int J Poult Sci. (2007) 6:694–704. 10.3923/ijps.2007.694.704 [DOI] [Google Scholar]
- 71.Carillon J, Rouanet JM, Cristol JP, Brion R. Superoxide dismutase administration, a potential therapy against oxidative stress related diseases: several routes of supplementation and proposal of an original mechanism of action. Pharm Res. (2013) 30:2718–28. 10.1007/s11095-013-1113-5 [DOI] [PubMed] [Google Scholar]
- 72.Mantis NJ, Rol N, Corthésy B. Secretory IgA's complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. (2011) 4:603–11. 10.1038/mi.2011.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee KW, Lee SH, Lillehoj HS, Li GX, Jang SI, Babu US, et al. Effects of direct-fed microbials on growth performance, gut morphometry, and immune characteristics in broiler chickens. Poult Sci. (2010) 89:203–16. 10.3382/ps.2009-00418 [DOI] [PubMed] [Google Scholar]
- 74.Dersjant-Li Y, Gibbs K, Awati A, Klasing KC. The effects of enzymes and direct fed microbial combination on performance and immune response of broilers under a coccidia challenge. J Appl Anim Nutr. (2016) 4:e6 10.1017/jan.2016.2 [DOI] [Google Scholar]
- 75.Wang H, Ni X, Qing X, Liu L, Lai J, Khalique A, et al. Probiotic enhanced intestinal immunity in broilers against subclinical necrotic enteritis. Front Immunol. (2017) 8:1592. 10.3389/fimmu.2017.01592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu Y, Shao Y, Song B, Zhen W, Wang Z, Guo, et al. Effects of Bacillus coagulans supplementation on the growth performance and gut health of broiler chickens with Clostridium perfringens-induced necrotic enteritis. J. Anim. Sci. Biotechnol. (2018) 9:9. 10.1186/s40104-017-0220-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sekirov I, Russell SL, Antunes LCM, Finlay BB. Gut microbiota in health and disease. Physiol Rev. (2010) 90:859–904. 10.1152/physrev.00045.2009 [DOI] [PubMed] [Google Scholar]
- 78.Liang D, Leung RKK, Guan W, Au WW. Involvement of gut microbiome in human health and disease: brief overview, knowledge gaps and research opportunities. Gut Pathog. (2018) 10:3. 10.1186/s13099-018-0230-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Adhikari B, Kwon YM, Hargis BM, Tellez-Isaias G. Prokaryotes rule the world. In: Evrensel A, editor. Gut Microbiota. London: IntechOpen; (2018). p. 144–55. [Google Scholar]
- 80.Brugman S, Ikeda-Ohtsubo W, Braber S, Folkerts G, Pieterse CMJ, Bakker PAHM. A comparative review on microbiota manipulation: lessons from fish, plants, livestock, and human research. Front Nutr. (2018) 5:80. 10.3389/fnut.2018.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Allen HK, Trachsel J, Looft T, Casey TA. Finding alternatives to antibiotics. Ann NY Acad Sci. (2014) 1323:91–100. 10.1111/nyas.12468 [DOI] [PubMed] [Google Scholar]
- 82.Tellez G, Latorre JD. Editorial: Alternatives to antimicrobial growth promoters and their impact in gut microbiota, health and disease. Front. Vet. Sci. (2017) 4:196. 10.3389/fvets.2017.00196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim JY, Kwon YM, Kim IS, Kim JA, Yu DY, Adhikari B, et al. Effects of the brown seaweed Laminaria japonica supplementation on serum concentrations of igg, triglycerides, and cholesterol, and intestinal microbiota composition in rats. Front Nutr. (2018) 5:23. 10.3389/fnut.2018.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oakley BB, Lillehoj HS, Kogut MH, Kim WK, Maurer JJ, Pedroso A, et al. The chicken gastrointestinal microbiome. FEMS Microbiol Lett. (2014) 360:100–12. 10.1111/1574-6968.12608 [DOI] [PubMed] [Google Scholar]
- 85.Ranjitkar S, Lawley B, Tannock G, Engberg RM. Bacterial succession in the broiler gastrointestinal tract. Appl Environ Microbiol. (2016) 82:2399–410. 10.1128/AEM.02549-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mon KKZ, Saelao P, Halstead MM, Chanthavixay G, Chang H-C, Garas L, et al. Salmonella enterica serovars Enteritidis infection alters the indigenous microbiota diversity in young layer chicks. Front Vet Sci. (2015) 2:61. 10.3389/fvets.2015.00061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Latorre JD, Adhikari B, Park SH, Teague KD, Graham LE, Mahaffey BD, et al. Evaluation of the epithelial barrier function and ileal microbiome in an established necrotic enteritis challenge model in broiler chickens. Front Vet Sci. (2018) 5:199. 10.3389/fvets.2018.00199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Janssens Y, Nielandt J, Bronselaer A, Debunne N, Verbeke F, Wynendaele E, et al. Disbiome database: linking the microbiome to disease. BMC Microbiol. (2018) 18:50. 10.1186/s12866-018-1197-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Videnska P, Sisak F, Havlickova H, Faldynova M, Rychlik I. Influence of Salmonella enterica serovar Enteritidis infection on the composition of chicken cecal microbiota. BMC Vet Res. (2013) 9:140. 10.1186/1746-6148-9-140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Scott KP, Antoine JM, Midtvedt T, van Hemert S. Manipulating the gut microbiota to maintain health and treat disease. Microb Ecol Health Dis. (2015) 26:25877. 10.3402/mehd.v26.25877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.O'Callaghan A, van Sinderen D. Bifidobacteria and their role as members of the human gut microbiota. Front Microbiol. (2016) 7:925. 10.3389/fmicb.2016.00925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu L, Lin L, Zheng L, Tang H, Fan X, Xue N, et al. Cecal microbiome profile altered by Salmonella enterica, serovar Enteritidis inoculation in chicken. Gut pathogens. (2018) 10:34. 10.1186/s13099-018-0261-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chadfield MS, Bojesen AM, Christensen JP, Juul-Hansen J, Nielsen SS, Bisgaard M. Reproduction of sepsis and endocarditis by experimental infection of chickens with Streptococcus gallinaceus and Enterococcus hirae. Avian Pathol. (2005) 34:238–47. 10.1080/03079450500112252 [DOI] [PubMed] [Google Scholar]
- 94.Sekizaki T, Nishiya H, Nakajima S, Nishizono M, Kawano M, Okura M, et al. Endocarditis in chickens caused by subclinical infection of Streptococcus gallolyticus subsp. gallolyticus. Avian Dis. (2008) 52:183–6. 10.1637/8048-070307-Case [DOI] [PubMed] [Google Scholar]
- 95.Owings WJ, Reynolds DL, Hasiak RJ, Ferket PR. Influence of a dietary supplementation with Streptococcus faecium M-74 on broiler body weight, feed conversion, carcass characteristics and intestinal microbial colonization. Poult Sci. (1990) 69:1257–64. 10.3382/ps.0691257 [DOI] [PubMed] [Google Scholar]
- 96.Herrera P, O'Bryan C, Crandall P, Ricke S. Growth response of Salmonella enterica Typhimurium in co-culture with ruminal bacterium Streptococcus bovis is influenced by time of inoculation and carbohydrate substrate. Food Res Int. (2012) 45:1054–7. 10.1016/j.foodres.2011.07.001 [DOI] [Google Scholar]
- 97.Roto SM, Rubinelli PM, Ricke SC. An Introduction to the avian gut microbiota and the effects of yeast-based prebiotic-type compounds as potential feed additives. Front Vet Sci. (2015) 2:28. 10.3389/fvets.2015.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fåk F, Bäckhed F. Lactobacillus reuteri prevents diet-induced obesity, but not atherosclerosis, in a strain dependent fashion in Apoe–/– mice. PLoS ONE. (2012) 7:e46837. 10.1371/journal.pone.0046837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gadde U, Kim WH, Oh ST, Lillehoj HS. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: a review. Anim Health Res Rev. (2017) 18:26–45. 10.1017/S1466252316000207 [DOI] [PubMed] [Google Scholar]
- 100.Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS. Bile acids and the gut microbiome. Curr Opin Gastroenterol. (2014) 30:332–8. 10.1097/MOG.0000000000000057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jia W, Xie G, Jia W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat Rev Gastroenterol Hepatol. (2018) 15:111–28. 10.1038/nrgastro.2017.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zúñiga M, Monedero V, Yebra MJ. Utilization of host-derived glycans by intestinal Lactobacillus and Bifidobacterium Species. Front Microbiol. (2018) 9:1917. 10.3389/fmicb.2018.01917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lin R, Liu W, Piao M, Zhu H. A review of the relationship between the gut microbiota and amino acid metabolism. Amino Acids. (2017) 49:2083–90. 10.1007/s00726-017-2493-3 [DOI] [PubMed] [Google Scholar]
- 104.Neis E, Dejong C, Rensen S. The role of microbial amino acid metabolism in host metabolism. Nutrients. (2015) 7:2930–46. 10.3390/nu7042930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Koropatkin NM, Cameron EA, Martens EC. How glycan metabolism shapes the human gut microbiota. Nat Rev Microbiol. (2012) 10:323–35. 10.1038/nrmicro2746 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.