Abstract
In acute myeloid leukemia (AML), myelodysplasia‐related changes contribute to a poor prognosis. This retrospective, propensity score‐matched study analyzed 108 newly diagnosed AML patients with features of myelodysplasia syndrome (MDS) (aged 14‐60 years) from 2014 to 2018, who received either idarubicin and cytarabine (IA) or decitabine, idarubicin and cytarabine (DAC+IA), and compared efficacy and toxicity between the two regimens. After propensity score matching, there were 54 patients in each group. The rate of complete remission (CR) was higher in the DAC+IA group than in the IA group (85.2% vs 68.5%, P = .040) after the first course, and toxicities were comparable in both groups. Multivariate analysis indicated that the combination with DAC was independent factor for CR rate after the first induction therapy (OR = 2.978, 95% CI:1.090‐8.137, P = .033). Subgroup analysis showed a CR advantage for DAC+IA (vs IA) for patients of intermediate‐high risk status according to National Comprehensive Cancer Network prognostic stratification. In conclusion, DAC+IA is therefore offered as a new induction choice for newly diagnosed AML patients with features of MDS, aged <60 years old, especially in intermediate‐high risk status.
Keywords: acute myeloid leukemia, chemotherapy, complete remission, decitabine, induction therapy, MDS features
1. INTRODUCTION
Acute myeloid leukemia (AML) is a heterogeneous disease. IA, a regimen of 3 days of idarubicin (IDA) and 7 days of cytarabine (Ara‐C), has been one of the standard 3+7 induction treatments for AML. Generally, 30% to 40% of adult patients could not achieve satisfying outcomes.1 One of the factors that contributes to its poor prognosis, AML with myelodysplasia‐related changes (AML‐MRC),2, 3 includes a history of myelodysplasia syndrome (MDS), MDS‐related cytogenetic abnormalities, and multilineage dysplasia,4 with two of these conditions not involving an MDS history and accounting for over 30% of AML cases.5 Besides, part of AML patients also had some features of MDS such as a history of more than 6 months of macrocytic anemia and low percent of blasts in bone marrow, which were easy to be ignored. Considering the characteristic abnormalities of MDS in these patients, we assume that the addition of decitabine (DAC) to AML therapy may improve its induction effectiveness as DAC, a deoxyribonucleic acid (DNA) methyltransferase inhibitor (DNMTi), has already been approved for treating MDS6, 7 and elderly patients with AML.8 In this study, we retrospectively analyzed 108 newly diagnosed AML patients between 2014 and 2018, who were treated with either IA or DAC+IA as induction therapy, to compare the curative and side effects of DAC+IA to those of standard two‐drug induction therapy (IA).
1.1. Patients and methods
1.1.1. Patients
In this study, we retrospectively analyzed newly diagnosed AML patients (excluding M3) in the department of hematology of the First Affiliated Hospital of Sun Yat‐sen University between January 2014 and December 2018 according to the 2008 World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia.9 All patients were aged 14‐60 years; and therapy‐related leukemia and previously diagnosed blood disease, such as myelodysplastic/myeloproliferative neoplasm (MDS/MPN)‐transformed leukemia, were excluded. Moreover, the patients were required to meet at least one of the following standards: (a) Anemia, leucopenia, or thrombocytopenia for over 6 months; (b) Macrocytic anemia: mean corpuscular volume (MCV) >95.0 fL according to the reference value in our hospital10; (c) Observation of marrow dyshematopoiesis; (d) 20%‐30% immature cells in bone marrow; or (e) Chromosomal karyotyping or fluorescence in situ hybridization (FISH) detected −5/del(5q), −7/del(7q), i(17q)/t(17p), −13/del(13q), del(11q), del(12p)/t(12p), idic(X)(q13), +8, or del(20q).4 All consecutive patients who met the standards and received DAC+IA or IA regimen were included without selection. Patients underwent prognostic stratification and response estimation in accordance with the 2018 National Comprehensive Cancer Network (NCCN) guidelines.11 Finally, a total of 108 patients were enrolled.
1.2. Treatment protocols and outcomes
1.2.1. Induction therapy
Patients in the DAC+IA group received DAC combined with an IA regimen as follows: Decitabine 20 mg/m2 intravenously for five consecutive days (days 1‐5), a standard dose of cytarabine (100‐200 mg/m2/d) intravenously for seven days (days 1‐7) with idarubicin (8‐10 mg/m2) intravenously daily for 3 days (days 1‐3). In the IA group, patients were treated with 8‐10 mg/m2 IDA (days 1‐3) combined with 100‐200 mg/m2/d cytarabine for days 1‐7.
Supportive care was given during treatment. Transfusions of blood products were also provided when necessary. Red blood cells (RBCs) were infused when hemoglobin was <60 g/L (6.0 g/dL) or symptoms of anemia were observed, while platelets were given to patients with platelets <20 × 109/L (20 000 mcL) and those who showed any signs of bleeding. Subcutaneous granulocyte colony‐stimulating factor (G‐CSF) was injected when neutrophils were <0.5 × 109/L (500 mcL) during the myelosuppression stage. Treatment‐related toxicities were evaluated with the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. Time to hematopoietic recovery was measured from the first day of the chemotherapy to the time when the neutrophil count was >0.5 × 109/L (500 mcL)12 or the platelet count was higher than 20 × 109/L (20 000 mcL). Only patients achieving complete remission (CR) were considered for the analysis of recovery.
1.3. Follow‐up treatment
The treatment response was evaluated around 21‐28 days after chemotherapy and used to divide the patients into CR, partial remission (PR), and no response (NR), according to the 2018 NCCN clinical practice guideline of AML. Those who achieved CR after the first course of treatment entered consolidation treatment. The others received re‐induction (the applied regimens are shown in Figure 1), with consolidation treatment started if they achieved CR. Otherwise, patients who did not achieve CR after two cycles of induction therapy were regarded as induction failure and received salvage treatment. Relapse following CR was defined based on the reappearance of leukemic blasts in the peripheral blood or a finding of more than 5% blasts in bone marrow. Overall survival (OS) was measured from the date of diagnosis until death from any cause. Progression‐free survival (PFS) was calculated from treatment initiation to death or disease progression.
Figure 1.
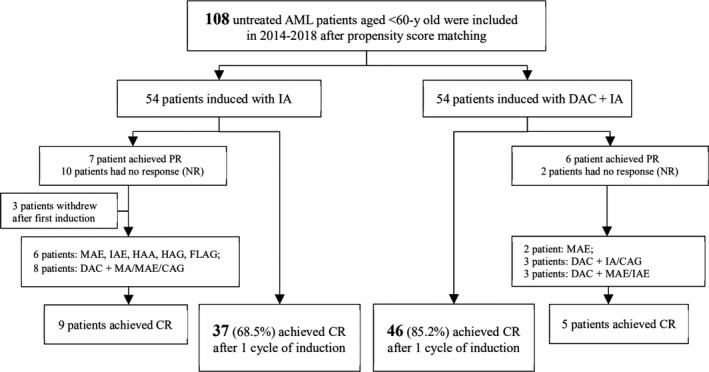
Enrollment and outcomes in patients with decitabine treatment. Abbreviations: AML, acute myeloid leukemia; CAG, Cytarabine + Aclarubicin +G‐CSF; CR, complete remission; DAC, Decitabine; FLAG, Fludarabine + Cytarabine +G‐CSF; HAA/HAG, Homoharringtonine + Cytarabine +Aclarubicin/ Granulocyte Colony‐Stimulating Factor (G‐CSF); IA, Idarubicin + Cytarabine; MAE/IAE, Mitoxantrone/ Idarubicin (MA) + Cytarabine + Etoposide); MDS, myelodysplasia syndrome; PR, partial remission
1.4. Statistical methods
We used propensity scoring to minimize bias and ensure similarities between the two treatment groups. Patients were matched with age, gender, initial white blood cell (WBC), history of symptoms, MCV, blasts in bone marrow, dysplasia, cytogenetics related to MDS, and NCCN prognostic stratification. By using a 1:1 nearest neighbor matching algorithm that pairs patients with the closest propensity scores within a defined limit (calipers of width equal to 0.20), the propensity score yielded two matched cohorts of 54 patients.
Chi‐square or Fisher's exact tests were used for the difference analysis of characteristics, CR rates, and the incidence of adverse events between two arms. The duration of cytopenia for different groups was compared by the Mann‐Whitney U test. For risk factor analysis of CR rates, the Logistic regression was used. The Kaplan‐Meier test was used to estimate OS and PFS. All reported P values are two‐sided, and P <.05 is considered statistically significant. All statistical analyses were performed using SPSS 19.0 software.
2. RESULTS
2.1. Baseline characteristics
Between January 2014 and December 2018, a total of 134 patients with a median age of 36 years old (range, 14‐60 years old) met the study inclusion criteria, with 60 and 74 treated with DAC+IA and IA regimen, respectively. Baseline characteristics for these patients (before propensity score matching) are listed in Table S1, and it did not vary significantly between the two groups.
To minimize the effects of treatment selection bias, adjustments were made using the propensity score matching method, and we identified 54 patients in each group. Patient characteristics used in the propensity score analysis are detailed in Table 1 and were well balanced between the two groups. The median age was 35.5 years (range, 14‐60) in the DAC+IA group and 35.0 years (range, 15‐59) in the IA group (P = .826). A bone marrow aspiration exam showed that dysplasia was found in 12 patients (11.1%). The results of karyotype analysis and FISH showed that 11 (10.2%) had at least one of the MDS‐related cytogenetic changes. Based on the 2018 NCCN criteria, 34 patients (31.5%) were classified as favorable‐risk, 37 (34.3%) as intermediate‐risk, and 37 (34.3%) as poor‐risk.
Table 1.
Characteristics of 108 patients after propensity score matching
| IA (n = 54) | DAC+IA (n = 54) | P value | |
|---|---|---|---|
| Age, years | |||
| Median | 35 | 35.5 | .902 |
| Range | 15‐57 | 14‐59 | |
| History, months | |||
| Median | 1.0 | 1.0 | .841 |
| Range | 0.2‐7.0 | 0.1‐6.0 | |
| WBC, ×109/L | |||
| Median | 18.25 | 25.00 | .808 |
| Range | 1.44‐361.0 | 1.27‐230.0 | |
| MCV, fL | |||
| Median | 100.6 | 100.4 | .837 |
| Range | 80‐115 | 69‐121 | |
| Blasts, % | |||
| Median | 53.5 | 56.5 | .888 |
| Range | 21.0‐94.0 | 21.0‐91.0 | |
| Gender, n(%) | |||
| Male | 30 (55.6) | 33 (61.1) | .696 |
| Female | 24 (44.4) | 21 (38.9) | |
| FAB category, n(%) | |||
| M0 | 1 (1.9) | 2 (3.7) | .751 |
| M1 | 6 (11.1) | 5 (9.3) | |
| M2 | 22 (40.7) | 19 (35.2) | |
| M4 | 6 (11.1) | 6 (11.1) | |
| M5 | 19 (35.2) | 20 (37.0) | |
| M6 | 0 (0.0) | 2 (3.7) | |
| Dysplasia, n(%) | |||
| 0 | 48 (88.9) | 48 (88.9) | 1.000 |
| 1 | 6 (11.1) | 6 (11.1) | |
| Cytogenetics related to MDS, n(%) | |||
| 0 | 49 (90.7) | 48 (88.9) | 1.000 |
| 1 | 5 (9.3) | 6 (11.1) | |
| NCCN prognostic stratification, n(%) | |||
| Favor | 17 (31.5) | 17 (31.5) | .784 |
| Intermediate | 17 (31.5) | 20 (37) | |
| Poor | 20 (37) | 17 (31.5) | |
Abbreviations: FAB, French‐American‐British classification; MCV, mean corpuscular volume; MDS, myelodysplasia syndrome; WBC, white blood cell.
2.2. Response to induction treatment
At the end of the first course of induction therapy, the DAC + IA group displayed a statistically significant increase in CR rates (85.2% vs 68.5%, P = .040; Figure 1). Among 22 patients without CR who were administered a second course of the induction, as shown in Figure 1, nine responded after chemotherapy combined with DAC and five after intense chemotherapy such as mitoxantrone/cytarabine/etoposide (MAE) and fludarabine/ cytarabine/G‐CSF (FLAG).
To further confirm the factors associated with the response of AML patients, we performed multivariate logistic regression analysis. Remarkably, CR rate was independently correlated with combinations including DAC (P = .033, OR 95% CI = 1.090 to 8.137) and NCCN risk status (Intermediate vs Favorable: P = .032, OR 95% CI: 0.032‐0.860; Poor vs Favorable: P = .019, OR 95% CI: 0.025‐0.715), which was adjusted for age and prognostic markers such as FLT3‐ITD (shown in Figure 2).
Figure 2.
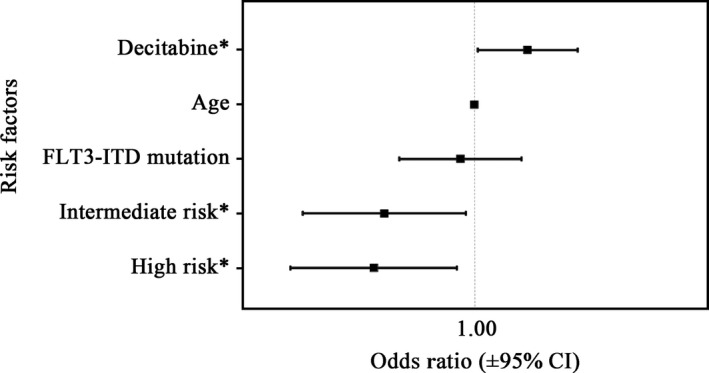
Multivariate analysis of complete remission rate. *P < .05
2.3. Subgroup analysis
In an exploratory subgroup analysis using Logistic regression (Figure 3), a CR advantage for the DAC + IA compared with the IA could be demonstrated for patients with intermediate karyotype (P = .035) or FLT3‐ITD mutations (P = .044). Combining the cytogenetics with molecular results, a significant difference between DAC + IA and IA group was revealed in the subgroup of patients with intermediate‐poor risk status (56.8% vs 81.1%, P = .027).
Figure 3.
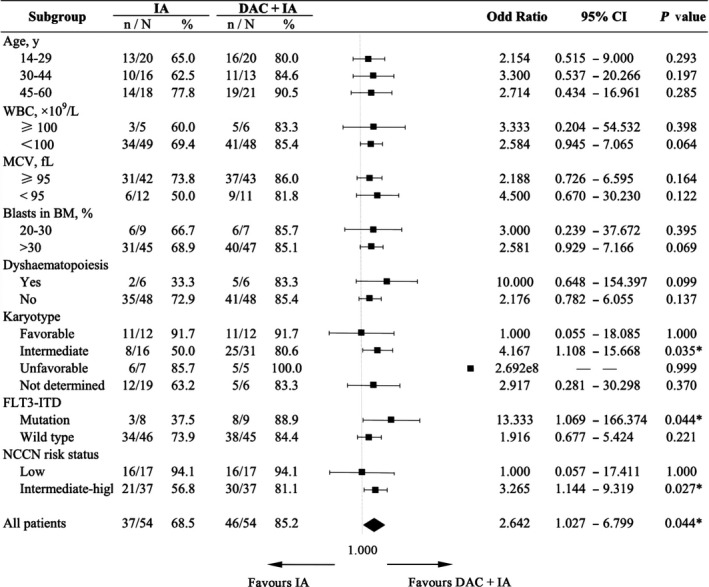
Subgroup analysis of complete remission rate after one course of induction. Abbreviations: MCV, mean corpuscular volume; BM, bone marrow. *P < .05
2.4. Treatment‐related toxicity
In particular, the combinations including DAC did not add extra treatment‐related side effects, and toxicities were comparable in both groups.
To evaluate the hematologic toxicities listed in Table 2, we analyzed 83 patients who achieved CR after the first induction chemotherapy. All patients experienced WHO Grade 4 neutropenia and thrombocytopenia. The time to neutrophil recovery ranged from 8 to 36 days in DAC+IA group (median time, 20 days), whereas in the non‐DAC group, the time ranged from 16 to 24 days (median time, 19 days). The median time to platelet recovery for both DAC+IA and non‐DAC groups was 18 days. Neither the time to neutrophil nor platelet recovery differed between the two arms. Furthermore, no significant difference was observed in the amount of infused suspension of RBC or apheresis platelets between DAC+IA and IA arm.
Table 2.
Hematologic toxicity during induction treatment
| Toxicity | IA (n = 37) | DAC+IA (n = 46) | P value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Granulocyte decreased (Grade 4) | 37 | 100 | 46 | 100 | |
| Granulocyte recovery, days | |||||
| Median | 19 | 20 | .168 | ||
| Range | 16‐24 | 8‐36 | |||
| Platelet decreased (Grade 4) | 37 | 100 | 46 | 100 | |
| Platelet recovery, days | |||||
| Median | 18 | 18 | .778 | ||
| Range | 13‐24 | 11‐26 | |||
| Platelet transfusions, U | |||||
| Median | 5 | 5 | .294 | ||
| Range | 2‐14 | 2‐13 | |||
| Anemia | |||||
| Grade 1‐2 | 0 | 0.0 | 1 | 2.2 | .610 |
| Grade 3 | 1 | 2.7 | 2 | 4.3 | |
| Grade 4 | 36 | 97.3 | 43 | 93.5 | |
| RBC transfusions, U | |||||
| Median | 6 | 6 | .796 | ||
| Range | 1.5‐24 | 0‐16 | |||
Abbreviation: RBC, red blood cells.
Nonhematologic toxicities mainly referred to infections. Pulmonary infections had the highest incidence rate. Other common infections included upper respiratory infection (URI), oral infection, and skin soft‐tissue infection. There was no obvious difference in nonhematologic toxicities between the groups (Table 3).
Table 3.
Most frequent nonhematologic toxicities
| Toxicity | IA (n = 54) | DAC+IA (n = 54) | P value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Vomiting | |||||
| Grade 0 | 49 | 90.7 | 44 | 81.5 | .334 |
| Grade 1‐2 | 4 | 7.4 | 9 | 16.7 | |
| Grade 3‐4 | 1 | 1.9 | 1 | 1.9 | |
| Diarrhea | |||||
| Grade 0 | 53 | 98.1 | 50 | 92.6 | .360a |
| Grade 1‐2 | 1 | 1.9 | 4 | 7.4 | |
| Rash | |||||
| Grade 0 | 51 | 94.4 | 50 | 92.6 | 1.000a |
| Grade 1‐2 | 3 | 5.6 | 4 | 7.4 | |
| ALT/AST increased | |||||
| Grade 0 | 50 | 92.6 | 48 | 88.9 | .507 |
| Grade 1‐2 | 4 | 7.4 | 6 | 11.1 | |
| ALP increased | |||||
| Grade 0 | 52 | 96.3 | 54 | 100 | .475a |
| Grade 1‐2 | 2 | 3.7 | 0 | 0 | |
| Blood bilirubin increased | |||||
| Grade 0 | 51 | 94.4 | 49 | 90.7 | .713a |
| Grade 1‐2 | 3 | 5.6 | 5 | 9.3 | |
| Infection sites | |||||
| Upper respiratory | 12 | 22.2 | 9 | 16.7 | .466 |
| Lung | 25 | 46.3 | 30 | 55.6 | .336 |
| Tooth/gum/lip | 14 | 25.9 | 13 | 24.1 | .824 |
| Skin soft tissue | 10 | 18.5 | 12 | 22.2 | .633 |
| Anorectal | 4 | 7.4 | 4 | 7.4 | 1.000a |
| Sepsis | 5 | 9.3 | 5 | 9.3 | 1.000 |
| Septic shock | 1 | 1.9 | 2 | 3.7 | 1.000a |
| No infections | 2 | 3.7 | 0 | 0 | .475a |
Abbreviations: ALP: alkaline phosphatase; ALT: alanine transaminase; AST, Aspartate aminotransferase.
Analyzed by Fisher's exact test.
2.5. Survival
With a median follow‐up of 6.7 months, the 1‐year probability of OS and PFS for the whole group was 86.8% (SE ± 4.3%) and 81.9% (SE ± 5.0%), respectively. No significant difference between the study arms could be demonstrated with respect to 1‐year OS (DAC+IA 91.0% vs IA 84.2%, P = .991) and PFS (DAC+IA 90.6% vs IA 76.8%, P = .826). Long‐term survival analysis requires a longer duration to follow‐up.
3. DISCUSSION
According to the WHO classification criteria for AML in 2008, AML with MRCs should either include a history of MDS or have no MDS history but be associated with MLD or an MDS‐related cytogenetic abnormality, all of which suggest a poor prognosis.
Compared with 187 cases of AML‐NOS, Xiao‐Qian Xu et al13 reported that markedly lower WBC counts and hemoglobin were observed in AML with MLD and MDS‐related cytogenetic abnormality, 36.5% of which had a complex karyotype. It is also reported that the genes with the highest frequencies of mutation in AML‐MRC were ASXL1, TP53, RUNX1, and DNMT3A, of 21%, 28%, 12%, and 9%,14, 15 which were predicted to a poor prognosis. The CR rate achieved in patients treated with the IA/DA regimen for induction chemotherapy was 63.6%, which was significantly lower than that achieved in the AML‐NOS patients (77.5%).13 The 3 + 7 regimen did not achieve satisfactory results, which was the same as the results in our study.
DAC is a DNA methylated transferase (DNMT) inhibitor when used at a low dose (5‐20 mg/m2 per dose) and is approved for MDS and elderly AML patients. In some refractory and relapsed (R/R) AML, it can be combined with a Cytarabine/Aclamycin/Granulocyte colony‐stimulating factor (CAG) regimen.16 In an open‐label phase I study performed in 2011,17 30 newly diagnosed AML patients (median age at 55) were included and treated with DAC at a dose of 20 mg/m2 per day for 3‐7 days to achieve epigenetic priming for intensive chemotherapy; this treatment was followed by daunorubicin (DNR) and Ara‐C (DA) regimen. The total response rate was 90%, with 57% (17/30) achieving CR and 33% (10/30) achieving PR. In 2015, Jiang et al18 analyzed the treatment response of the DAC‐sequential HAA regimen (Homoharringtonine+cytarabine +aclarubicin) in high‐risk or R/R AML patients aged 16 to 59 years old and found an overall CR rate after two cycles of up to 65.2%, which was better than the HAA group (41.7%). However, the use of DAC combined with chemotherapy‐induced therapy in newly diagnosed AML patients with MDS features under 60 years old has rarely been reported.
Considering that some AML patients show the characteristics of MDS, we suggest that DAC could be added to AML chemotherapy to improve its efficacy. The potential increase in side effects caused by this combination needs to be further explored. In our retrospective study, the CR rate after the first induction therapy was, therefore, significantly better than the historical control (85.2% vs 68.5%, P = .040), and there was no difference in either hematologic or nonhematologic toxicities. Multivariate analysis indicated that the combination with DAC were independent factors for CR rate after the first induction therapy.
We therefore propose that DAC plus chemotherapy is an option for initial induction treatment in AML with features of MDS, which can improve outcomes to some extent without increasing treatment‐related side effects.
Which group of patients can benefit from the combination of DAC and chemotherapy? The subgroup analysis showed that intermediate karyotype, positive FLT3‐ITD mutation, or intermediate‐poor risk stratification according to NCCN criteria may be factors that predict the CR rate.10, 19
How does DAC work in combination with chemotherapeutics? In the past, some studies conducted in vitro experiments and found that combinations including DAC improved the sensitivity of leukemia cells (HL‐60, Kasumi‐1) to conventional chemotherapy drugs, such as cytarabine, aclarubicin, and HHT, by promoting apoptosis in leukemia cells.18 For example, caspase‐3 and caspase‐9 were expressed at significantly higher levels, and the antiapoptotic protein Bcl‐xl was expressed at lower levels.20 In addition, the demethylation of DAC had definitely been observed; the expression of DNMT1, DNMT3A, DNMT3B proteins or mRNAs are lower,20 and the demethylation of Wnt/beta‐catenin pathway inhibitors was found to have anti‐leukemia effects.21 However, whether this plays an important role or is simply an accompanying effect needs to be further studied.
In summary, DAC+IA represents a new option of induction therapy for newly diagnosed AML patients with MDS features, aged <60 years old, especially in intermediate‐poor risk status.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
ACKNOWLEDGMENTS
This work was partly supported by the Natural Science Foundation of Guangdong (No. 2016A030313197), the Clinical Medical Research 5010 Program of Sun Yat‐sen University (No. 2017005) to Dr Li and the Science and Technology Planning Project of Guangdong Province (No. 2017A020215105) to Dr Tong.
Liu F, Wang H, Liu J, et al. A favorable inductive remission rate for decitabine combined with chemotherapy as a first course in <60‐year‐old acute myeloid leukemia patients with myelodysplasia syndrome features. Cancer Med. 2019;8:5108–5115. 10.1002/cam4.2418
Contributor Information
Xiuzhen Tong, Email: tongxz05@163.com.
Juan Li, Email: 13719209240@163.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Choi Y, Kim S‐D, Park Y‐H, et al. Allogeneic hematopoietic cell transplantation in acute myeloid leukemia arising from the myelodysplastic syndrome. Acta Haematol. 2015;133(2):183‐192. [DOI] [PubMed] [Google Scholar]
- 3. Jaglal MV, Duong VH, Bello CM, et al. Cladribine, cytarabine, filgrastim, and mitoxantrone (CLAG‐M) compared to standard induction in acute myeloid leukemia from myelodysplastic syndrome after azanucleoside failure. Leuk Res. 2014;38:443‐446. [DOI] [PubMed] [Google Scholar]
- 4. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391‐2405. [DOI] [PubMed] [Google Scholar]
- 5. Weinberg OK, Seetharam M, Ren L, et al. Clinical characterization of acute myeloid leukemia with myelodysplasia‐related changes as defined by the 2008 WHO classification system. Blood. 2009;113:1906‐1908. [DOI] [PubMed] [Google Scholar]
- 6. Yun S, Vincelette ND, Abraham I, Robertson KD, Fernandez-Zapico ME, Patnaik MM. Targeting epigenetic pathways in acute myeloid leukemia and myelodysplastic syndrome: a systematic review of hypomethylating agents trials. Clin Epigenetics. 2016;8:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bejar R, Lord A, Stevenson K, et al. mutations predict response to hypomethylating agents in myelodysplastic syndrome patients. Blood. 2014;124:2705‐2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Qian S, Li J, Tian T, et al. Effect of low-dose cytarabine and aclarubicin in combination with granulocyte colony-stimulating factor priming (CAG regimen) on the outcome of elderly patients with acute myeloid leukemia. Leukemia Res. 2007;31:1383-1388. [DOI] [PubMed] [Google Scholar]
- 9. Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937‐951. [DOI] [PubMed] [Google Scholar]
- 10. Li L, Nie L, Yu MH, et al. The prognostic value of a modified WPSS based on routine laboratory parameters in patients with myelodysplastic syndromes: a preliminary result. Zhonghua Xue Ye Xue Za Zhi. 2009;30:313‐317. [PubMed] [Google Scholar]
- 11. O'Donnell MR, Tallman MS, Abboud CN, Altman JK, Appelbaum FR, Bhatt VR, et al. 2018. Acute Myeloid Leukemia, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology [DB/OL]. https://www.nccn.org/professionals/physician_gls/pdf/aml.pdf. [DOI] [PubMed]
- 12. Willemze R, Suciu S, Meloni G, et al. High‐dose cytarabine in induction treatment improves the outcome of adult patients younger than age 46 years with acute myeloid leukemia: results of the EORTC‐GIMEMA AML‐12 trial. J Clin Oncol. 2014;32:219‐228. [DOI] [PubMed] [Google Scholar]
- 13. Xu X‐Q, Wang J‐M, Gao L, et al. Characteristics of acute myeloid leukemia with myelodysplasia‐related changes: A retrospective analysis in a cohort of Chinese patients. Am J Hematol. 2014;89:874‐881. [DOI] [PubMed] [Google Scholar]
- 14. Devillier R, Mansat‐De MV, Gelsi‐Boyer V, et al. Role of ASXL1 and TP53 mutations in the molecular classification and prognosis of acute myeloid leukemias with myelodysplasia‐related changes. Oncotarget. 2015;6:8388‐8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Metzeler KH, Walker A, Geyer S, et al. mutations and response to the hypomethylating agent decitabine in acute myeloid leukemia. Leukemia. 2012;26:1106‐1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ye X‐N, Zhou X‐P, Wei J‐Y, et al. Epigenetic priming with decitabine followed by low‐dose idarubicin/cytarabine has an increased anti‐leukemic effect compared to traditional chemotherapy in high‐risk myeloid neoplasms. Leuk Lymphoma. 2016;57:1311‐1318. [DOI] [PubMed] [Google Scholar]
- 17. Scandura JM, Roboz GJ, Moh M, et al. Phase 1 study of epigenetic priming with decitabine prior to standard induction chemotherapy for patients with AML. Blood. 2011;118:1472‐1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jiang X, Wang Z, Ding B, et al. The hypomethylating agent decitabine prior to chemotherapy improves the therapy efficacy in refractory/relapsed acute myeloid leukemia patients. Oncotarget. 2015;6:33612‐33622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tennant GB, Al‐Sabah AI, Burnett AK. Prognosis of myelodysplasic patients: non‐parametric multiple regression analysis of populations stratified by mean corpuscular volume and marrow myeloblast number. Br J Haematol. 2002;119:87‐96. [DOI] [PubMed] [Google Scholar]
- 20. Geng S, Yao H, Weng J, et al. Effects of the combination of decitabine and homoharringtonine in SKM‐1 and Kg‐1a cells. Leuk Res. 2016;44:17‐24. [DOI] [PubMed] [Google Scholar]
- 21. Li K, Hu C, Mei C, et al. Sequential combination of decitabine and idarubicin synergistically enhances anti‐leukemia effect followed by demethylating Wnt pathway inhibitor promoters and downregulating Wnt pathway nuclear target. J Transl Med. 2014;12:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


