Abstract
Human papillomavirus (HPV) infection which continues to be the most common sexually transmitted disease, has been identified as a major risk factor for cervical cancer. Therefore, it is very important to understand and grasp the distribution of HPV in Chinese population, and make the foundation for the development of cervical cancer vaccine in China. An extensive search strategy was conducted in multiple literature databases. All retrieved studies were screened by October 31, 2018. The prevalence of HPV infection was analyzed using random effects model. A total of 68 studies satisfied the inclusion criteria for our study. The national overall prevalence of HPV infection was 15.54% (95% CI: 13.83%‐17.24%). we also performed subgroup analysis by age, geographic location, level of economic development, HPV assay method, and type of HPV infection. The top 5 common HPV types detected in general population, were HPV 16 (3.52%, 95% CI: 3.18%‐3.86%), 52 (2.20%, 95% CI: 1.93%‐2.46%), 58 (2.10%, 95% CI: 1.88%‐2.32%), 18 (1.20%, 95% CI: 1.05%‐1.35%), and 33 (1.02%, 95% CI: 0.89%‐1.14%). Except for the higher prevalence of HPV infection in 2009 and 2010, the prevalence of HPV infection in other years changed little, ranged from 13.2% to 17.4%. HPV type in Chinese women was quite distinctive. HPV infection played a critical role in the occurrence of cervical cancer, understanding the distribution of HPV type and performing the HPV type testing had important clinical value for colposcopy referral and increasing the detection rate. Therefore, our findings could provide evidence for cervical cancer screening and vaccine, in order to reduce the burden of cervical cancer.
Keywords: China, distribution, general population, HPV
1. INTRODUCTION
Cervical cancer is one of the most common malignancies among women worldwide. According to Global Cancer Statistics in 2012, an estimated 527 600 new cases and 265 700 deaths were found in women worldwide; cervical cancer is recognized as the fourth most common cancer and the fourth leading cause of cancer‐related death in women and have been a major public health problem.1The burden of cervical cancer in developing countries is much higher than that in developed countries. The incidence of cervical cancer was not the top 10 in developed countries, but the second in the developing countries. The mortality was the ninth in the developed countries, and the third in developing countries.1 Based on data in 2011‐2015 from USA, the number of new cases was 7.4 per 100 000 women per year, and the number of deaths was 2.3 per 100 000 women per year. In terms of the latest National Cancer Statistics in China, the number of new cases was 10.08 per 100 000 women per year, and the number of deaths was 2.98 per 100 000 women per year.2
Human papillomavirus (HPV) infection continues to be the most common sexually transmitted disease, has been identified as a major risk factor for cervical cancer. Eighty per cent of people will get an HPV infection in their life. HPV infection continues to cause significant burden on women. Most HPV infections are asymptomatic, transient, and will clear within 1‐2 years, but those that persist can progress to precancer or cancer.3 Many epidemiologic studies have found that nearly 100% of patients with cervical cancer have HPV infection.4, 5, 6 Sexually transmitted HPV types is usually identified as two categories: (a) High‐risk HPV, which mainly include HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68, are strongly associated with cervical cancer. Two of these, HPV 16 and 18, are responsible for most HPV‐caused cancers. (b) Low‐risk HPV, which mainly include HPV 6, 11, 30, 42, 43, 44, and 61, and HPV 6 and 11 cause 90% of all genital warts. HPV testing is more sensitive than cytology in the screening of cervical cancer.
As the largest developing country in the world, China has about 130 000 new cases of cervical cancer each year, accounting for about 1/4 of new cases in the world. If effective measures are not taken, it is estimated that the number of new cases of cervical cancer will increase to 187 000 by 2050.7 China has actively promoted the screening and prevention of cervical cancer in recent years, but the incidence and mortality of cervical cancer have remained high, and the age distribution becomes younger. Currently, the vaccines for cervical cancer are mainly targeted at tetravalent vaccine (HPV 16, 18, 6, and 11) and bivalent vaccine (HPV 16 and 18), based on the data from HPV epidemiological survey in Europe or the United States.8 It needs to be considered whether the vaccines are suitable for Chinese population. It is very important to understand and grasp the distribution of HPV in Chinese general population, which provides scientific basis for prevention and treatment of cervical cancer. In 2018, Zhou HL and Xu HH et al respectively published a systematic review on prevalence and distribution of HPV in patients, which has invasive carcinoma of cervix (ICC), high‐grade squamous intraepithelial lesion (HSIL), or low‐grade squamous intraepithelial lesion (LSIL).8, 9 These studies mainly studied the prevalence and distribution of HPV in patients, but did not study on general population; the results might be influenced by selection bias, which cannot indeed reflect the real situation of HPV infection in general population. Therefore, we perform this study to integrate the findings of 68 population‐based studies. The aim was to explore that (a) temporal trends and geographical patterns in the HPV infection epidemic and (b) the differences between china and foreign countries.
2. METHODS
2.1. Search strategy
An extensive search strategy was conducted in multiple literature databases by October 31, 2018. The literature databases included PubMed, Web of Science, Chinese Scientific Journals Full text Database (CQVIP), China National Knowledge Infrastructure (CNKI), and Wanfang Data. We set the following key words: (a) “Papillomaviridae” and free word “China” were used for PubMed and Web of Science; (b) “Papillomaviridae” was set as subject heading (including title, abstract, and keyword) to search Chinese database. We also reviewed reference lists from all the relevant original research and review studies to identify additional possibly eligible studies. This study was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement issued in 2009.10
2.2. Study selection
We used Endnote® (version X6; Thomson Reuters, Inc, Philadelphia, PA) bibliographic software to create an electronic library, which collected all the retrieved studies from literature databases. After deleting the duplicated studies by the software identification, 2 independent authors (Bo Zhu, Tingting Zuo) performed title/abstract screening and full text screening in turn. When any disagreements happened, 3 independent authors (Bo Zhu, Tingting Zuo, and Mengdan Li) reached agreement by discussion.
The inclusion and exclusion criteria were as follows: (a) the subjects of studies should come from the general population, excluded the studies that contained outpatients and/or patients; (b) for the detection of HPV infection, both high‐risk and low‐risk types must be included; (c) the methodology of HPV detection should be explicitly stated; (d) the study contained the prevalence of HPV infection, or provided the available data to calculate the estimates; (e) when 2 studies were found to be from the same population, the latest or maximum sample size article was included.
2.3. Quality assessment
The methodological quality and validity of the included studies were assessed by an existing tool, which was modified to assess risk of bias in prevalence studies.11 Ten aspects in the tool were used to evaluate the quality of the included study, which was shown in supplementary Data S1. The included studies were assessed a score of 0 (high risk) or 1 (low risk) for each aspect. The score of study quality ranged from 0‐10. The study with 8‐10 points was regarded as “high quality”, the study with 4‐7 points was regarded as “moderate quality”, and otherwise, the study was regarded as “low quality”. The quality assessment tool is shown in supplementary Data S1.
2.4. Data extraction
The included studies were independently read in detail by two reviewers (Bo Zhu and Tingting Zuo). Two reviewers used a fixed table to extract the information, which included the following: first author and year of publication, sample size, age, study period, region classification, and province.
2.5. Statistical analysis
Stata software package (Version 12.0; Stata Corp., College Station, TX) was used in the pooled analysis. The random effects model (DerSimonian‐Laird) was used to pool the study‐specific estimates to obtain an overall summary estimate of the prevalence of HPV infection. In order to fully reflect the distribution of HPV infection in China, we performed subgroup analysis by age, geographic location, the level of economic development, HPV assay method, and type of HPV infection. In geographic location, studies were categorized into 1 of 6 geographical locations according to the traditional Chinese administrative regions: East China, Northeast, North China, South China, Central China, Northwest, and Southwest. In economic development level, studies were categorized as 3 groups by per capita GDP in 2016, which included low, moderate, and high level. HPV assay method mainly contained PCR & hybridization, real‐time PCR, and sequencing PCR. Type of HPV infection included high risk HPV or low risk HPV, single infection or multiple infection, and the top 10 HPV infection subtypes.
3. RESULTS
3.1. Search results
A total of 45 907 studies were found through searching the database (MEDLINE: 2005, Web of Science: 1312, CQVIP: 10903, CNKI: 8845 and Wanfang: 22842). First, the Endnote® bibliographic software was used to identify the duplicated studies, 34 736 studies were removed. Second, 9381 studies were removed by reading title or abstracts, which included the studies related to HPV and cancers, reviews, and genetic polymorphisms. Third, 1598 studies were removed by reading full text. In the remaining 192 papers, a total of 94 studies contained outpatients and/or patients, 16 studies did not provide sufficient data to calculate the prevalence of HPV, and 14 studies were from the same population. Finally, the remaining 68 population‐based studies (English studies: 34; Chinese studies: 34) satisfied the inclusion criteria for our study. A flow chart of the screening process is shown in Figure 1.
Figure 1.
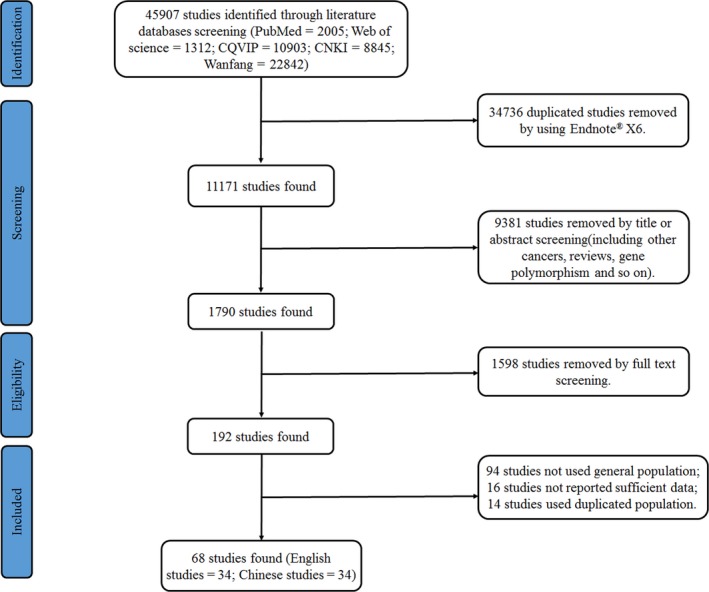
The flow chart of screening process in our meta‐analysis
3.2. The characteristics of the included studies
In the included studies, we found there were 31 provinces which reported the prevalence of HPV infection in general population, except for Gansu, Jilin, and Guizhou. The number of studies reported in 10 provinces was over 3. A total of 1705956 people were included in our study, the number of people in each province ranged from 1008 to 714 235. The prevalence of HPV infection ranged from 6.2% (Hong Kong) to 31.3% (Hainan). The characteristic of the included studies was in Table 1. Figure 2 showed the number of studies, the sample size, and the prevalence on HPV infection in each province.
Table 1.
The characteristic of the included studies
| Author | Year of publication | Number of subjects | Age | Study period | The definition of HPV POSITIVITY | The specimen type | HPV assay method | Region classification | Province | City | The quality score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Jin, LM | 2018 | 116441 | 21‐78 | 2013‐2016 | HPV DNA | Exfoliated cells | Sequencing PCR | city | Shanghai | 9 | |
| Zhang, C | 2018 | 33562 | 11‐96 | 2016.1‐2016.12 | HPV DNA | Exfoliated cells | PCR & hybridization | city | Shanghai | 9 | |
| Wang, X | 2018 | 5655 | 17‐68 | 2014.4‐2015.12 | HPV DNA | Exfoliated cells | PCR & hybridization | city | Inner Mongolia | 9 | |
| Dong, L | 2017 | 1997 | 35‐45 | 1999.6 | HPV DNA | Exfoliated cells | PCR & hybridization | rural | Shanxi | Xianghua | 9 |
| Zhou, X. H | 2017 | 2452 | 20‐80 | 2013.3‐2013.12 | HPV DNA | Exfoliated cells | PCR & hybridization | city | Shanghai | 9 | |
| Xu, H. H | 2017 | 37967 | 15‐90 | 2012.12‐2016.2 | HPV DNA | Exfoliated cells | Real‐time PCR | city | Zhejiang | Taizhou | 8 |
| Zhong, TY | 2017 | 714235 | 16‐77 | 2010.8‐2015.12 | HPV DNA | Exfoliated cells | PCR & hybridization | city | Jiangxi | Nanchang, Jiu‐jiang, Yingtan, Jingdezhen, Shangrao, Yichun, Xinyu, Pingxiang,Fuzhou, Ji'an, and Ganzhou | 9 |
| Wu, X | 2017 | 2300 | 18‐45 | 2013.3‐2013.7 | HPV DNA | Exfoliated cells | PCR & hybridization | city | Guangxi | Liuzhou | 9 |
| Abulizi, G. | 2017 | 6000 | 21‐60 | HPV DNA | Exfoliated cells | Real‐time PCR | rural | Xinjiang | Uyghur | 9 | |
| Zhang, J | 2017 | 58650 | 13‐81 | 2012.6‐2016.5 | HPV DNA | Exfoliated cells | PCR & hybridization | city | Sichuan | Chengdu | 8 |
| Qi, ZW | 2017 | 4250 | 18‐69 | 2013.1‐2016.1 | HPV DNA | Exfoliated cells | PCR & hybridization | city and rural | Hebei | Chengde | 8 |
| Shen, XP | 2017 | 20000 | 35‐64 | 2015 | HPV DNA | Exfoliated cells | Real‐time PCR | rural | Ningxia | 7 | |
| Zhang Xiaohong | 2017 | 38408 | Not reported | 2012.3‐2015.12 | HPV DNA | Exfoliated cells | PCR & hybridization | city and rural | Shaanxi | 7 | |
| Yang, DM | 2017 | 9001 | 22‐84 | 2016.1‐2016.12 | HPV DNA | Exfoliated cells | PCR & hybridization | city | Hunan | Changsha | 8 |
| Jin, DC | 2017 | 50502 | 18‐50 | 2015.1‐2015.12 | HPV DNA | Exfoliated cells | Real‐time PCR | city | Henan | Zhengzhou | 8 |
| Baloch, Z | 2016 | 17898 | 18‐93 | 2013.10‐2015.2 | HPV DNA | Exfoliated cells | Sequencing PCR | city and rural | Yunnan | 9 | |
| Gu, Y | 2016 | 10501 | 39‐55 | 2014.1‐2015.5 | HPV DNA | Exfoliated cells | PCR & hybridization | city | Shanghai | 9 | |
| Liu, F | 2016 | 2187 | 25‐65 | 2007‐2009 | HPV DNA | Exfoliated cells | Sequencing PCR | rural | Henan | anyang | 9 |
| Wei, F | 2016 | 2378 | 18‐55 | 2014.5‐2014.7 | HPV DNA | Exfoliated cells | Real‐time PCR | city | Guangxi | Liuzhou | 8 |
| Zeng, XX | 2016 | 8284 | 18‐65 | 2012.1‐2012.12 | HPV DNA | Exfoliated cells | Sequencing PCR | city | Guangdong | Heyuan | 9 |
| Niyazi, M | 2016 | 883 | 15‐54 | 2006.9 | HPV DNA | Exfoliated cells | PCR & hybridization | city | Xinjiang | Hetian | 8 |
| Zhang, Y | 2016 | 3000 | 30‐59 | 2015.3‐2015.6 | HPV DNA | Exfoliated cells | PCR & hybridization | city | Guangdong | Shenzhen | 8 |
| Que, M | 2016 | 21078 | 35‐64 | 2014 | HPV DNA | Exfoliated cells | PCR & hybridization | rural | Anhui | 8 | |
| Ji,HY | 2016 | 5892 | 18‐80 | 2013.10‐2015.10 | HPV DNA | Exfoliated cells | PCR & hybridization | city and rural | Qinghai | 9 | |
| Long, Xin | 2016 | 29580 | 16‐81 | 2010.11‐2015.9 | HPV DNA | Exfoliated cells | PCR & hybridization | city and rural | Chongqing | 9 | |
| Wang, JX | 2015 | 191829 | 35‐65 | 2013 | HPV DNA | Exfoliated cells | PCR & (mass‐spectrometric detection) MSD | city | Jiangsu | Wuxi | 8 |
| Mijit, F | 2015 | 4500 | 20‐69 | 2008.6‐2008.9 | HPV DNA | Exfoliated cells | PCR & hybridization | city | Xinjiang | Hetian | 8 |
| Zhao, Y | 2015 | 6273 | 17‐59 | 2004.5‐2007.4 | HPV DNA | Exfoliated cells | PCR & hybridization | city and rural | — | Beijing, Shanghai, Shenzhen, Shenyang, Shanxi, Henan, Xinjiang | 10 |
| Chen, X | 2015 | 2000 | 21‐65 | 2013.4‐2013.10 | HPV DNA | Exfoliated cells | Sequencing PCR | city | Tianjin | 8 | |
| Hong, H | 2015 | 1373 | 22‐64 | 2012.4‐2012.6 | HPV DNA | Exfoliated cells | PCR & hybridization | rural | Zhejiang | Ningbo | 8 |
| Yang, L | 2015 | 9,460 | Not reported | Not reported | HPV DNA | Exfoliated cells | Not reported | city | Shandong | Weihai | 8 |
| Zhao, Q | 2015 | 24817 | 18‐80 | 2012.7‐2013.7 | HPV DNA | Exfoliated cells | PCR & hybridization | city | Hunan | 9 | |
| Li, XL | 2015 | 1008 | 23‐78 | 2014.8‐2014.10 | HPV DNA | Exfoliated cells | PCR & hybridization | city | Hubei | Yichang | 9 |
| Chen, MH | 2014 | 11231 | 25‐74 | 2012.1‐2012.12 | HPV DNA | Exfoliated cells | Sequencing PCR | city | Jiangsu | Wuxi | 8 |
| Jing, L | 2014 | 78355 | 18‐75 | 2011.5‐2012.11 | HPV DNA | Exfoliated cells | Real‐time PCR | city | Guangdong | 9 | |
| Zhu, MH | 2014 | 7796 | 18‐70 | 2012.3‐2012.7 | HPV DNA | Exfoliated cells | PCR & hybridization | city | Guangdong | Huizhou | 8 |
| Pei, YY | 2014 | 1944 | 20‐67 | Before 2014 | HPV DNA | Exfoliated cells | PCR & hybridization | city | Guangdong | Shenzhen | 7 |
| Yang, L | 2013 | 1759 | 24‐77 | 2010.6‐2010.10 | HPV DNA | Exfoliated cells | Real‐time PCR | city | Heilongjiang | Daqing | 8 |
| Wu, EQ | 2013 | 4,215 | 17‐54 | 2006.5‐2007.4 | HPV DNA | Exfoliated cells | PCR & hybridization | city and rural | — | Beijing, Shanghai, Shanxi, Henan, and Xinjiang | 10 |
| Zhang, R | 2013 | 10000 | 17‐89 | 2011.3‐2011.5 | HPV DNA | Exfoliated cells | PCR & hybridization | rural | Shanghai | Fengxian | 9 |
| Wu, XQ | 2013 | 4228 | 19‐59 | 2011.11‐2012.4 | HPV DNA | Exfoliated cells | PCR & hybridization | city | Guangdong | Guangzhou | 8 |
| Weng, ZC | 2013 | 1029 | Not reported | 2010.10‐2012.5 | HPV DNA | Exfoliated cells | PCR & hybridization | city | Hainan | Haikou | 7 |
| Wu, D | 2013 | 2050 | 15‐91 | 2010.1‐2012.11 | HPV DNA | Exfoliated cells | PCR & hybridization | city | Beijing | 8 | |
| Wang, S | 2012 | 24041 | 18‐60 | 2007‐2010 | HPV DNA | Exfoliated cells | PCR & hybridization | city | Liaoning | 9 | |
| Wang, Y | 2012 | 3052 | 20‐79 | 2011.3‐2012.3 | HPV DNA | Exfoliated cells | PCR & hybridization | city | Fujian | 8 | |
| ZhangJing | 2012 | 3685 | 29‐67 | 2010.7‐2011.6 | HPV DNA | Exfoliated cells | PCR & hybridization | rural | Shaanxi | 8 | |
| Chen, JC | 2012 | 3399 | 26‐59 | 2010.1‐2011.4 | HPV DNA | Exfoliated cells | PCR & hybridization | city | Guangdong | Shantou | 8 |
| Wang, YY | 2012 | 9683 | Not reported | 2006.4‐2010.4 | HPV DNA | Exfoliated cells | PCR & hybridization | city | Guangdong | Shenzhen | 7 |
| Zhao, R | 2011 | 5552 | 25‐54 | 2006.9‐2008.12 | HPV DNA | Exfoliated cells | PCR & hybridization | city and rural | Beijing | 9 | |
| Liu, SS | 2011 | 1570 | Not reported | 2007.1‐2008.4 | HPV DNA | Exfoliated cells | PCR & hybridization | city | Hong Kong | 9 | |
| Ablimit, T | 2011 | 400 | 26‐65 | 2008.7‐2008.9 | HPV DNA | Exfoliated cells | PCR & hybridization | rural | Xinjiang | Hetian | 7 |
| Liu, JX | 2011 | 4874 | 24‐55 | 2008.7‐2010.12 | HPV DNA | Exfoliated cells | PCR & hybridization | city and rural | Guangxi | 8 | |
| Chen HC | 2011 | 10602 | 30‐65 | 1991‐1992 | HPV DNA | Exfoliated cells | PCR & hybridization | city | Taiwan | 9 | |
| Yip, Y. C | 2010 | 1600 | 20‐60 | 2008.5‐2008.8 | HPV DNA | Exfoliated cells | PCR & hybridization | city | Macao | 8 | |
| Ye, J | 2010 | 4987 | 20‐79 | 2007.11‐2008.8 | HPV DNA | Exfoliated cells | PCR & hybridization | rural | Zhejiang | 9 | |
| Wu, D | 2010 | 2338 | 20‐70 | 2008.5‐2009.5 | HPV DNA | Exfoliated cells | PCR & hybridization | city | Fujian | Fuzhou | 9 |
| Jiang, Y | 2010 | 4911 | 20‐69 | 2008.4‐2008.9 | HPV DNA | Exfoliated cells | PCR & hybridization | city and rural | Fujian | Xiamen | 8 |
| Zhouyuan | 2010 | 20905 | 14‐84 | 2008.10‐2009.3 | HPV DNA | Exfoliated cells | PCR & hybridization | city and rural | Zhejiang | 9 | |
| Jin, Q | 2009 | 3036 | 19‐65 | 2007.8 | HPV DNA | Exfoliated cells | PCR & hybridization | city | Xizang | Lhasa, Shigatse, Nagqu | 9 |
| Li, N | 2008 | 2374 | 15‐59 | 2004.5‐2006.9 | HPV DNA | Exfoliated cells | PCR & hybridization | city and rural | Shanxi, Guangdong, Liaoning | 10 | |
| Chao A | 2008 | 10014 | 30‐ | 2004.6‐2005.6 | HPV DNA | Exfoliated cells | PCR & hybridization | city | Taiwan | 9 | |
| Zhang, X | 2007 | 702 | 19‐59 | 2006.8‐2006.9 | HPV DNA | Exfoliated cells | PCR & hybridization | city | Liaoning | Shenyang | 9 |
| Dai, M | 2006 | 662 | 15‐59 | 2004.3‐2004.4 | HPV DNA | Exfoliated cells | PCR & hybridization | rural | Shanxi | 9 | |
| Li, LK | 2006 | 685 | 15‐59 | 2005.6‐2005.7 | HPV DNA | Exfoliated cells | PCR & hybridization | city | Liaoning | Shenyang | 9 |
| Shi, JF | 2006 | 1137 | 15‐59 | Before 2006 | HPV DNA | Exfoliated cells | PCR & hybridization | city | Guangdong | Shenzhen | 9 |
| Wu, KH | 2004 | 500 | 21‐45 | 1999.7‐2001.6 | HPV DNA | Exfoliated cells | PCR & hybridization | city | Guangdong | Guangzhou | 8 |
| Chen Feng | 2004 | 3233 | 30‐50 | 2002 | HPV DNA | Exfoliated cells | PCR & hybridization | rural | Shanxi | 8 | |
| Shen Yanhong | 2003 | 9683 | 30‐50 | Before 2003 | HPV DNA | Exfoliated cells | PCR & hybridization | rural | Shanxi | 8 |
Because the number of references was too much, the authors made the references of the included studies in Supplementary Data S2.
Figure 2.
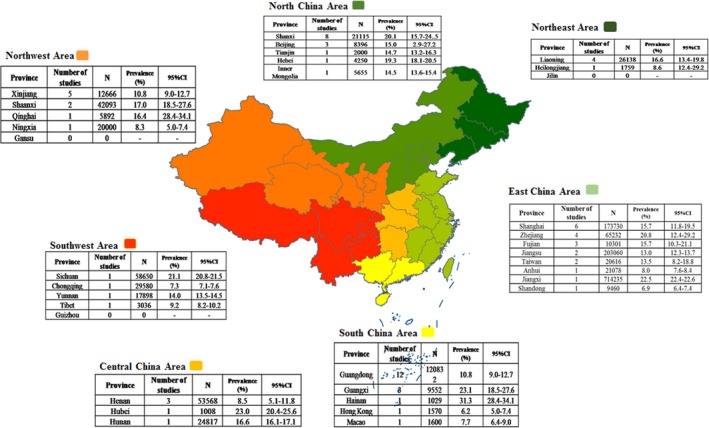
The number of studies, the sample size, and the prevalence on Human papillomavirus (HPV) infection in each province of China
We assessed the quality of included studies using the modified quality assessment tool. The quality scores of the included studies were shown in Table 1. The quality scores ranged from 7 to 11 and 10. Only 6 studies were assessed as moderate quality, and all other studies were assessed as high quality.
3.3. The infection of HPV in Chinese women
In the included studies, there were 71 databases that reported the overall prevalence of HPV infection, the national overall prevalence of HPV infection was 15.54% (95% CI: 13.83%‐17.24%); there were 50 databases that reported HPV infection in urban, the prevalence of HPV infection was 14.86% (95% CI: 12.84%‐16.88%), and there were 21 databases that reported HPV infection in rural, the prevalence of low risk HPV infection was 13.70% (95% CI: 11.18%‐16.22%).
According to the traditional geographical regions of China, we have divided China into seven regions, which included East China, North China, Central China, South China, Southwest, Northwest, and Northeast, the prevalence of HPV infection were 15.99% (95% CI: 12.84%,‐19.13%), 18.43% (95% CI: 13.39%‐23.46%), 12.96% (95% CI: 8.44%‐17.48%), 13.51% (95% CI: 11.34%‐15.68%), 12.92% (95% CI: 5.31%‐20.53%), 12.57% (95% CI: 8.49%‐16.65%), and 19.85% (95% CI: 3.46%‐36.23%), respectively. According to the level of GDP per capita in 2016, China was categorized into 3 groups, namely, low, middle, and high, the prevalence of HPV infection were 16.72%(95% CI: 13.81%‐19.62%), 18.01%(95% CI: 12.67%‐23.35%), and 13.41%(95% CI: 11.74%‐15.09%). According to HPV assay method, the prevalence of HPV infection was 16.55% by PCR and hybridization and the prevalence of HPV infection was 11.73% by sequencing or real‐time PCR.
There were 66 databases that reported the prevalence of high risk HPV infection, the prevalence of high risk HPV infection was 11.93% (95% CI: 10.93%‐12.92%), and there were 46 databases that reported the prevalence of low risk HPV infection, the prevalence of low risk HPV infection was 2.78% (95% CI: 2.35%‐3.20%); there were 35 databases that reported the prevalence of single HPV infection, the prevalence of single HPV infection was 11.00% (95% CI: 9.32%‐12.68%), and there were 48 databases that reported the prevalence of multiple HPV infection, the prevalence of multiple HPV infection was 3.44% (95% CI: 2.69%‐4.18%), the multiple HPV infection included dual infection (2.30%, 95% CI: 2.01%‐2.58%), triple infection (0.51%, 95% CI: 0.42%‐0.60%), fourfold infection (0.11%, 95% CI: 0.07%‐0.15%), and fivefold infection (0.04%, 95% CI: 0.01%‐0.07%), respectively. In the detection of the HPV subtype, we found that HPV16, 52, 58, 18, and 33 are the top 5 subtypes, and the prevalence of the top 5 subtypes was 3.52% (95% CI: 3.18%‐3.86%), 2.20% (95% CI: 1.93%‐2.46%), 2.10% (95% CI: 1.88%‐2.32%), 1.20% (95% CI: 1.05%‐1.35%), 1.02% (95% CI: 0.89%‐1.14%).
We further stratified the analysis by age, found that there were 12 databases which reported the prevalence of high risk HPV infection by age. Because of different age groups in databases, 12 databases were divided into 3 groups for analysis. In group 1, the prevalence of HPV infection in <25, 55‐59, 60‐65 age groups were higher with 13.31%, 14.93%, and 16.33%, respectively, and that in 25‐29, 30‐34 age groups were lower with 9.97% and 9.50%, respectively. In group 2, the prevalence of HPV infection in <25, >65 age groups were higher with 19.21% and 13.33%, respectively, and that in 25‐34, 55‐64 age groups were lower with 9.95% and 9.84%, respectively. In group 3, the prevalence of HPV infection in <25, >60 age groups were higher with 33.37% and 26.03%, respectively, and that in 31‐40, 41‐50 age groups were lower with 13.94% and 13.86%, respectively.
In the included studies, the year of the epidemiological investigation ranged from 1991 to 2016. There was only one study that reported 1991, 1992, 1999, 2000, 2001, 2002, and 2003, the number of studies which reported other years were at least 4 or more. Therefore, we pooled 7 datasets that reported 1991, 1992, 1999, 2000, 2001, 2002, and 2003, to get more stable result. Except for the higher prevalence of HPV infection in 2009 and 2010, the prevalence of HPV infection in other years changed little, ranged from 13.2% to 17.4%. Figure 3 showed the time trend of HPV infection between 2003 and 2016.
Figure 3.
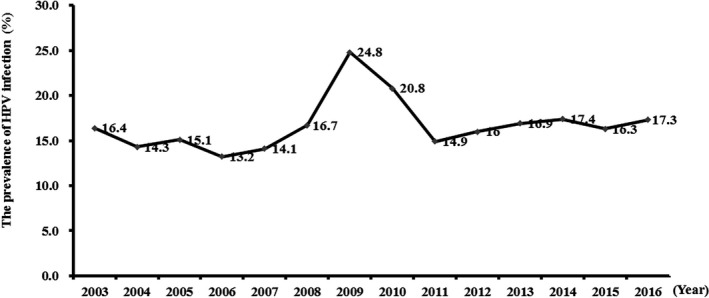
The time trend of Human papillomavirus (HPV) infection between 2003 and 2016
4. DISCUSSION
The prevalence of HPV infection varied geographically, and the prevalence of cervical infection with human papillomavirus (HPV) in women also varies greatly worldwide. In order to understand the situation of HPV infection in Chinese women accurately, we conducted a pooled analysis of 68 population‐based studies, which contained 1 705 956 subjects. As far as we know, this is the most comprehensive and extensive, HPV‐related study in China. The national overall prevalence of HPV infection was 15.54% (95% CI: 13.83%‐17.24%), which ranged from 6.2% (Hong Kong) to 31.3% (Hainan) and varied by region. This may be due to the large Chinese population and its territories. Meanwhile, age, economic conditions, cultural habits, and population migrations might also influence factors.12 Therefore, we further carried out subgroup analysis by different regions and economic levels. The results from our study indicated that the prevalence of HPV infection in urban was similar to that in rural. A high burden of HPV infection was found in East, North, and Northeast region of China. The higher prevalence of HPV infection was found in middle and high economic level. The prevalence of high risk HPV infection, the prevalence of high risk HPV infection (11.93%) was as 4 times as high as that of low risk HPV infection (2.78%). We also analyzed the HPV infection epidemiological trend between 2003 and 2016, and found that the prevalence of HPV infection were higher in 2009 and 2010, the prevalence of HPV infection in other years changed little, ranged from 13.2% to 17.4%.
The prevalence of single infection was 11.00% (95% CI: 9.32‐%‐12.68%) while the prevalence of multiple infection was 3.44% (95% CI: 2.69%‐4.18%). In the multiple HPV infection individuals, the frequencies of 5, 4, 3, and 2 genotypes were 0.04%, 0.11%, 0.51%, and 2.30%, respectively. We found that the main form of HPV infection was single infection, and multiple HPV infections were less common, the results were consistent with previous studies. Some studies had suggested that multiple HPV infections could potentially have competitive and/or cooperative interactions between HPV genotypes. Multiple HPV infections would increase the duration of infection, and patients with multiple high viral loads showed a fourfold to sixfold increased risk of cervical precancerous cytological lesions compared with patients with single high viral loads. There was an association between infection with multiple HPV types and an increased risk of cervical cancer. The mechanisms and potential oncogenic effects of multiple infections warrant further investigation, which could be useful in the development of HPV prophylactic vaccines.
In 2009, on the evidence of epidemiological and biological research, International Cancer Research Institute (IARC) defined 12 HPV types as HPV 16, 18, 31, 33, 35,39, 45, 51, 52, 58, and 59 as carcinogenic HPV (I carcinogen), and defined HPV 68 as a potentially carcinogenic HPV (2A carcinogen). HPV 26, 53, 66, 67, 67, 82, etc, which may be carcinogenic, were defined as Carcinogenic HPV (2B carcinogen).The carcinogenic HPV clustered in 4 species groups of the alpha genus, which included HPV 51 in species 5, HPV 56 in species 6, HPV 18, 39, 45, and 59 in species 7 and HPV 16, 31, 33, 35, 52, and 58 in species 9.13 Based on WHO worldwide data, the 5 most frequent HPV types in the general female population are HPV 16, 18, 31, 58, and 52. HPV 16, 18, 58, 31, and 33 are the most common in America, while the 5 most frequent HPV types in Europe were HPV 16, 18, 31, 33, and 58.14 In our study, HPV 16, 52, 58, 18, and 33 are the top 5 HPV infection subtypes in China, the distribution of which was different from other regions in the world. We also found that except HPV 18, HPV 16, 52, 58, and 33 was in species 9 of the alpha genus. HPV 16 and HPV 18 are well known as oncogenic genotypes and account for about 70% of invasive cervical cancers (ICC) worldwide. Though more variation in the ranking of individual types was in different countries, HPV 33, 45, 31, 58, 52, and 35 make up the rest of the top 8.15 For the Chinese women, Bao YP, et al found that in high‐grade squamous intraepithelial lesions (HSIL) and low‐grade squamous intraepithelial lesions (LSIL) samples, HPV 16, 58, 52, 18, and 33 were the most commonly detected types after adjusting for confounding factors (geographic area, classification of cervical disease status, and HPV testing method).16Zhou HL, et al found that in invasive carcinoma of cervix (ICC), the top 5 common HPV types were HPV 16, 18, 58, 52, and 33 in descending order of frequency. The present prophylactic vaccine for cervical cancer is developed based on the results of the HPV epidemiological survey abroad. In China, the quadrivalent vaccine (HPV 16, 18, 6, and 11) and the bivalent vaccine (HPV 16 and 18) were licensed. In our study, the top 5 common HPV types were HPV 16, 18, 58, 52, and 33 in Chinese general population. Therefore, the quadrivalent and bivalent vaccines were unable to cover the common HPV infection types in China, could not provide effective protection for cervical cancer.
In age group, we found that the prevalence of HPV infection in <20 and <25 age groups were high, then there was a distinct decline. When the age reached 60 or even older, the prevalence of HPV infection had increased again. The high prevalence of HPV infection in younger women may be related to unhealthy sexual habits such as premature sexual life, excessive frequency, and excessive sexual partners.17 Although the prevalence of HPV infection in young women is high, most of HPV infection may be cleared automatically within 1‐2 years, so the prevalence of HPV infection would be reduced. The immune ability declined with age in old women, especially in the premenopausal and postmenopausal women, the ability in eliminating previous and new infections weakened, so the high prevalence of HPV infection was also in older women.18
There were several limitations in our meta‐analysis. First, because our study aims to reflect the HPV prevalence in general population. In the process of detecting HPV for cervical cancer screening, cervical high‐grade lesions, and cancer might be screened out, we should analyze the prevalence of HPV in cervical high‐grade lesions and cancer. But, in process of collecting the included studies, we found that only a few studies reported the number of cervical high‐grade lesions and cancer, fewer studies reported the prevalence of HPV in cervical high‐grade lesions and cancer was too small, most of studies did not report the prevalence of HPV infection in cervical high‐grade lesions and cancer. We could not analyze the prevalence of HPV infection in cervical high‐grade lesions and cancer. Second, different detection methods might have different effects on the detection rate of HPV infection, and might affect the prevalence of HPV infection. Therefore, we performed subgroup analysis by the HPV assay method, the prevalence of HPV infection was 16.55% by PCR & hybridization, and the prevalence of HPV infection was 11.73% by sequencing or real‐time PCR. The HPV assay method might be the heterogeneity source of the prevalence of HPV infection, it deserved our attention in the follow‐up studies. Third, in our study, we had considered the effects of many factors on the prevalence of HPV infection in our study. Because our included studies did not report standardized rate, when we merged the prevalence of HPV infection, we just only focused on the description of the results, could not compare.
In conclusion, HPV type in Chinese women was quite distinctive. HPV infection played a critical role in the occurrence of cervical cancer, which affected by geographical region, economic conditions, cultural habits, and population migrations. In the screening process of cervical cancer, we should pay attention to mental stress, destruction of the cervical tissue by invasive operation and economic burden caused by over diagnosis and treatment, when improving the sensitivity of screening and reducing the missed diagnosis rate were emphasized. Understanding the distribution of HPV type and performing the HPV type testing had important clinical value for colposcopy referral and increasing the detection rate. Therefore, our findings could provide evidence for cervical cancer screening and vaccine, in order to reduce the burden of cervical cancer.
CONFLICT OF INTEREST
None declared.
5.
Table 2.
The pooled prevalence of Human papillomavirus (HPV) infection in Chinese women
| Number of databases | Number of Subjects | Prevalence (%) | 95% CI | |
|---|---|---|---|---|
| Overall | 71 | 1705956 | 15.54 | 13.83‐17.24 |
| Subgroup Analysis | ||||
| Areas | ||||
| Urban | 50 | 1488733 | 14.86 | 12.84‐16.88 |
| Rural | 21 | 113946 | 13.70 | 11.18‐16.22 |
| East China | 18 | 1197096 | 15.99 | 12.84‐19.13 |
| North China | 13 | 35761 | 18.43 | 13.39‐23.46 |
| Central China | 5 | 79393 | 12.96 | 8.44‐17.48 |
| South China | 20 | 155199 | 13.51 | 11.34‐15.68 |
| Southwest | 4 | 109164 | 12.92 | 5.31‐20.53 |
| Northwest | 9 | 80651 | 12.57 | 8.49‐16.65 |
| Northeast | 6 | 33552 | 19.85 | 3.46‐36.23 |
| GDP | ||||
| High | 36 | 631416 | 13.41 | 11.74‐15.09 |
| Middle | 15 | 178795 | 18.01 | 12.67‐23.35 |
| Low | 22 | 859989 | 13.48 | 11.57‐15.39 |
| HPV Assay Method | ||||
| PCR & hybridization | 57 | 1167563 | 16.55 | 14.53‐18.56 |
| Sequencing or real‐time PCR | 12 | 337104 | 11.73 | 9.34‐14.11 |
| HPV types | ||||
| High risk HPV | 66 | 1426787 | 11.93 | 10.93‐12.92 |
| Low risk HPV | 46 | 1176995 | 2.78 | 2.35‐3.20 |
| HPV infection types | ||||
| Single infection | 35 | 1424052 | 11.00 | 9.32‐12.68 |
| Multiple infection | 48 | 1419715 | 3.44 | 2.69‐4.18 |
| Dual infection | 14 | 445316 | 2.30 | 2.01‐2.58 |
| Triple infection | 13 | 442280 | 0.51 | 0.42‐0.60 |
| Fourfold infection | 9 | 406238 | 0.11 | 0.07‐0.15 |
| Fivefold infection | 5 | 204499 | 0.04 | 0.01‐0.07 |
| The top 10 subtypes | ||||
| HPV 16 | 68 | 1604203 | 3.52 | 3.18‐3.86 |
| HPV52 | 65 | 1574238 | 2.20 | 1.93‐2.46 |
| HPV58 | 65 | 1579921 | 2.10 | 1.88‐2.32 |
| HPV18 | 60 | 1566134 | 1.20 | 1.05‐1.35 |
| HPV33 | 54 | 1524449 | 1.02 | 0.89‐1.14 |
| HPV31 | 52 | 1467424 | 0.84 | 0.74‐0.95 |
| HPV51 | 50 | 1487714 | 0.72 | 0.64‐0.81 |
| HPV 56 | 49 | 1415424 | 0.66 | 0.57‐0.74 |
| HPV6 | 48 | 1500726 | 0.63 | 0.48‐0.78 |
| HPV11 | 45 | 1506233 | 0.56 | 0.43‐0.69 |
Table 3.
The pooled prevalence of Human papillomavirus (HPV) infection by age group
| Age group | Number of databases | Number of Subjects | Prevalence (%) | 95% CI |
|---|---|---|---|---|
| Group 1 | ||||
| <25 | 4 | 3273 | 13.31 | 7.33‐19.28 |
| 25‐29 | 4 | 13619 | 9.97 | 6.89‐13.04 |
| 30‐34 | 4 | 22629 | 9.50 | 6.53‐12.47 |
| 35‐39 | 4 | 27780 | 10.38 | 7.66‐13.10 |
| 40‐44 | 4 | 30320 | 10.58 | 7.97‐13.19 |
| 45‐49 | 4 | 26892 | 10.24 | 8.19‐12.29 |
| 50‐54 | 4 | 32050 | 12.29 | 10.45‐14.13 |
| 55‐59 | 2 | 31302 | 14.93 | 7.96‐21.90 |
| 60‐65 | 2 | 10953 | 16.33 | 3.01‐29.65 |
| 66‐70 | 1 | 5302 | 10.37 | 9.55‐11.19 |
| Group 2 | ||||
| <25 | 3 | 876 | 19.21 | 10.62‐27.79 |
| 25‐34 | 3 | 6463 | 9.95 | 3.46‐16.43 |
| 35‐44 | 3 | 8782 | 12.54 | 7.26‐17.82 |
| 45‐54 | 2 | 1900 | 12.03 | 3.14‐20.92 |
| 55‐64 | 2 | 270 | 9.84 | 2.51‐17.18 |
| >65 | 1 | 30 | 13.33 | 1.17‐25.49 |
| Group 3 | ||||
| <20 | 2 | 1732 | 33.37 | 17.67‐49.07 |
| 21‐30 | 5 | 31403 | 14.65 | 8.40‐20.89 |
| 31‐40 | 5 | 48829 | 13.94 | 8.03‐19.85 |
| 41‐50 | 5 | 52141 | 13.86 | 7.58‐20.14 |
| 51‐60 | 4 | 21496 | 18.89 | 10.74‐27.03 |
| >60 | 2 | 2342 | 26.03 | 24.26‐27.81 |
Supporting information
ACKNOWLEDGMENTS
This work was supported by National key research and development program (2016YFC1303001).
Zhu B, Liu Y, Zuo T, et al. The prevalence, trends, and geographical distribution of human papillomavirus infection in China: The pooled analysis of 1.7 million women. Cancer Med. 2019;8:5373–5385. 10.1002/cam4.2017
Bo Zhu and Yunyong Liu contributed equally to this work and should be considered co‐first authors.
REFERENCES
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87‐108. [DOI] [PubMed] [Google Scholar]
- 2. Chen WQ, Sun KX, Zheng RS, et al. Report of cancer incidence and morality in different areas of China, 2014. China Cancer. 2018;27:1‐14. [Google Scholar]
- 3. Centers for Disease Control and Prevention (CDC) . Human papillomavirus‐associated cancers ‐ United States, 2004–2008. MMWR Morb Mortal Wkly Rep. 2012;61:258–261. [PubMed] [Google Scholar]
- 4. Cogliano V, Baan R, Straif K, Grosse Y, Secretan B, Ghissassi FE. Carcinogenicity of human papillomaviruses. Lancet Oncol. 2005;6:204. [DOI] [PubMed] [Google Scholar]
- 5. Ronco G, Dillner J, Elfström KM, et al. Efficacy of HPV‐based screening for prevention of invasive cervical cancer: follow‐up of four European randomised controlled trials. Lancet (London, England). 2014;383:524–532. [DOI] [PubMed] [Google Scholar]
- 6. C Kitchener H, Canfell K, Gilham C, et al. The clinical effectiveness and cost‐effectiveness of primary human papillomavirus cervical screening in England: extended follow‐up of the ARTISTIC randomised trial cohort through three screening rounds. Health Technol Assess. 2014;18:1–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. [DOI] [PubMed] [Google Scholar]
- 8. Zhou H‐L, Zhang W, Zhang C‐J, et al. Prevalence and distribution of human papillomavirus genotypes in Chinese women between 1991 and 2016: A systematic review. J Infect. 2018;76:522–528. [DOI] [PubMed] [Google Scholar]
- 9. Xu HH, Wang K, Feng XJ, et al. Prevalence of human papillomavirus genotypes and relative risk of cervical cancer in China: a systematic review and meta‐analysis. Oncotarget. 2018;9:15386–15397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Panic N, Leoncini E, de Belvis G, et al. Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta‐analysis (PRISMA) statement on the quality of published systematic review and meta‐analyses. PLoS ONE. 2013;8:e83138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hoy D, Brooks P, Woolf A, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65:934–939. [DOI] [PubMed] [Google Scholar]
- 12. Kliucinskas M, Nadisauskiene RJ, Minkauskiene M. Prevalence and risk factors of HPV infection among high‐risk rural and urban Lithuanian women. Gynecol Obstet Invest. 2006;62:173–180. [DOI] [PubMed] [Google Scholar]
- 13. Bouvard V, Baan R, Straif K, et al. A review of human carcinogens–Part B: biological agents. Lancet Oncol. 2009;10:321–322. [DOI] [PubMed] [Google Scholar]
- 14. Castellsague X. HPV and cervical cancer in the 2007 report. Vaccine. 2007;25(Suppl 3):C1‐230. [DOI] [PubMed] [Google Scholar]
- 15. Li Ni, Franceschi S, Howell‐Jones R, Snijders P, Clifford GM. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: variation by geographical region, histological type and year of publication. Int J Cancer. 2011;128:927–935. [DOI] [PubMed] [Google Scholar]
- 16. Bao Yp, Li N, Smith Js, Qiao Yl. Human papillomavirus type‐distribution in the cervix of Chinese women: a meta‐analysis. Int J STD AIDS. 2008;19:106–111. [DOI] [PubMed] [Google Scholar]
- 17. Alder S, Perinetti C, Mints M, et al. Acceptance of human papillomavirus (HPV) vaccination among young women in a country with a high prevalence of HPV infection. Int J Oncol. 2013;43:1310–1318. [DOI] [PubMed] [Google Scholar]
- 18. Carozzi Fm, Ocello C, Burroni E, et al. Effectiveness of HPV vaccination in women reaching screening age in Italy. J Clin Virol. 2016;84:74–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


