Abstract
In the era of rituximab, the International Prognostic Index (IPI) has been inefficient in initial risk stratification for patients with R‐CHOP‐treated diffuse large B‐cell lymphoma (DLBCL). To estimate the predictive values of PET/CT quantitative parameters and three prognostic models consisting of baseline and interim parameters for three‐year progression‐free survival (PFS), we conducted an analysis of 85 patients in China with DLBCL underwent baseline and interim PET/CT scans and treated at the Department of Hematology of Peking University Third Hospital from November 2012 to November 2017. The PET/CT parameters, viz. the baseline and interim values of standardized uptake value (SUVmax), total metabolic tumor volume (TMTV), and total lesion glycolysis (TLG), and their rates of change, were analyzed by a receiver operating characteristics curve, Kaplan‐Meier analysis, and log‐rank test. Besides, the National Comprehensive Cancer Network International Prognostic Index (NCCN‐IPI) was also included in the multivariate Cox hazards model. Owing to the strong correlation between TMTV and TLG at baseline and interim (Pearson's correlation coefficient, r = 0.823, P‐value = 0.000, and 0.988, P‐value = 0.000, respectively), only TLG was included in the multivariate Cox hazards model, where TLG0 > 1036.61 g and %ΔSUVmax < 86.02% showed predictive value independently (HR = 10.42, 95% CI 2.35‐46.30, P = 0.002, and HR = 4.86, 95% CI 1.27‐18.54, P = 0.021, respectively). Replacing TLG in the equation, TMTV0 and TMTV1 both showed significantly predictive abilities like TLG (HR = 8.22, 95% CI 1.86‐32.24, P = 0.005, and HR = 2.96, 95% CI 1.16‐7.54, P = 0.023, respectively). After dichotomy, NCCN‐IPI also gave a significant performance (P = 0.035 and P = 0.010, respectively, in TLG and TMTV models). The baseline variables, that is, TMTV0, TLG0 and dichotomized NCCN‐IPI, and the interim variables TMTV1 and %ΔSUVmax, presented independent prognostic value for PFS. In prognostic model 2 (TLG0 + %ΔSUVmax), the group with TLG0 > 1036.61 g and %ΔSUVmax < 86.02% recognized 19 (82.6%) of the relapse or progression events, which showed the best screening ability among three models consisting of baseline and interim PET/CT parameters.
Keywords: diffuse large B‐cell lymphoma, metabolic tumor volume, prognostic model, total lesion glycolysis
1. INTRODUCTION
Diffuse large B‐cell lymphoma (DLBCL) is the most prevalent type of non‐Hodgkin lymphoma. Although the addition of rituximab to a CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone)‐like regimen (R‐CHOP) has improved DLBCL outcomes significantly, over 25% of patients treated with R‐CHOP unfortunately experience treatment failure.1 The early recognition of patients with a poor prognosis and the tailoring of their curative remediation plan are undoubtedly key interventions. For the past 20 years, the International Prognostic Index (IPI) has been the basis for initial risk stratification for patients with CHOP‐treated DLBCL, facilitating treatment selection and prognosis evaluation. However, the advent of rituximab reduced the prognostic ability of the IPI. In 2013,2 National Comprehensive Cancer Network International Prognostic Index (NCCN‐IPI), an enhanced IPI, was recommended to discriminate the high‐risk group, which was also demonstrated in eastern ethnic populations.3, 4 However, after evaluating the NCCN‐IPI in 284 Japanese patients with R‐CHOP‐treated DLBCL, Nakaya et al5 concluded that this index did not reflect progression‐free survival (PFS) in their cohort. Adams and Kwee6 thought that patients with a high‐risk NCCN‐IPI still had quite a high PFS rate of 40‐60%. Another effective method was to evaluate quantitative parameters derived from F18‐fluorodeoxyglucose positron emission tomography‐computed tomography (18F‐FDG PET/CT). PET/CT has been introduced into the guidelines of the National Comprehensive Cancer Network because of its capabilities of accurately revealing the stages of cancers and monitoring the effects of therapies. Among several parameters of PET/CT, standardized uptake value (SUVmax) was the most common for quantifying tracer uptake. SUV‐related quantitative measures, such as total metabolic tumor volume (TMTV) and total lesion glycolysis (TLG), which can assess the baseline and interim tumor burden, have gained increasing importance for therapy response monitoring and prognostic assessment.7 However, interpretations of these parameters are still controversial. Owing to the unstable manifestations of assessments and prognostic values reported in many studies,8, 9, 10, 11, 12, 13 the interests of researchers have gradually moved toward the TMTV and TLG parameters. Interim PET/CT parameters have demonstrated prognostic value in Hodgkin lymphoma, and several studies are testing the response‐adapted treatment regimens. If these interim parameters have the same role in DLBCL, we would also be able to try clinical trials of response‐adapted treatment regimens for DLBCL. Thus, more studies of the quantitative parameters are needed to assess their ability in discriminating high‐risk patients, and to compare the superiority of PET/CT and the NCCN‐IPI.
This study sought to retrospectively analyze the association between relapsed/refractory disease and the clinical characteristics, NCCN‐IPI, and PET/CT‐related quantitative parameters (baseline and interim SUVmax, TMTV, and TLG), and to explore new prognostic models that combines baseline and interim parameters for discriminating high‐risk patients efficiently.
2. MATERIALS AND METHODS
2.1. Subjects
A retrospective study of 85 consecutive patients newly histologically diagnosed with DLBCL was performed. All of them had undergone a baseline PET/CT scan before initial R‐CHOP or R‐CHOP‐like therapy and an interim PET/CT scan after 2‐4 cycles of chemotherapy at the Department of Hematology of Peking University Third Hospital from November 2012 to November 2017. Inclusion criteria were as follows: (a) age ≥18 years; (b) histologically confirmed DLBCL; (c) treated with R‐CHOP or R‐CHOP‐like chemotherapy; (d) completed baseline and interim PET/CT scans; and (e) with complete clinical information. The following were the exclusion criteria: (a) presence of concurrent acute or chronic infections; (b) malignant tumor history; and (c) lactating or pregnant. The Medical Research Ethics Committee of Peking University Third Hospital approved the study procedures. Informed consents were obtained from all of the patients, who were informed that the study would be conducted anonymously and their privacy would thus be respected.
The clinical information consisted of the patient's age, gender, B symptom, Ann Arbor stage, Eastern Cooperative Oncology Group performance status (ECOG PS), lactate dehydrogenase (LDH) ratio, extranodal disease, NCCN‐IPI score and risk groups, baseline and interim quantitative parameters (SUVmax, TMTV, and TLG), therapeutic regimen, follow‐up time, current status, and PFS. PFS was defined as the first date of documentation of a new lesion or enlargement of a previous lesion, or death from the disease.14 Based on The Lugano Classification,15 progressive metabolic disease was defined as PET/CT score 4 or 5 with an increase in intensity of uptake from baseline and/or new FDG‐avid foci consistent with lymphoma at interim PET/CT assessment. The NCCN‐IPI score used a maximum of eight points for the categorized age (41‐60 years, 1 point;61‐75 years, 2 points; >75 years, 3 points) and LDH ratio (1‐3 times, 1 point; >3 times, 2 points) at the upper limit of normal, in addition to extranodal disease involvement in major organs (bone marrow, central nervous system, liver/gastrointestinal tract, or lung), Ann Arbor stage III/IV, and ECOG PS (≥2), each carrying 1 point. PFS was defined as lymphoma progression or death as a result of any cause measured from the time point of entry into the study.
2.2. 18F‐FDG PET/CT
All the data were acquired and processed with the Siemens 52‐cycles Biograph 64 PET/CT scanner and MedEx PET/CT central imaging and information system, respectively. 18F‐FDG was supplied by the Institute of Isotope, China Institute of Atomic Energy Sciences. Before FDG injection, patients rested for at least 6 hours without parenteral nutrition and the serum glucose level was decreased to the normal levels (typically 4‐7 mmol/L). After injection of the 0.10‐0.15 mCi/kg 18F‐FDG, the patients rested for 60 minutes before the PET/CT scan. The PET images were collected by scanning 5‐7 bed positions (2.0‐minute acquisition time per bed position), covering the region from the base of the skull through to the upper thigh. PET images were reconstructed with TrueX algorithm, Iterations 4, Subsets 16, Zoom 2.7, FWHM 4.0. The final images were evaluated with the MedEx PET/CT central imaging and information system. The baseline PET/CT scan was obtained before initial R‐CHOP treatment, and the interim scan was completed after 2‐4 chemotherapy cycles.
2.3. Image evaluation
The evaluations of both the baseline and interim images were completed by two senior nuclear medicine radiologists, respectively, using the MedEx PET/CT central imaging and information system. The general definition of a positive (abnormal) PET finding (using visual assessment) as being a focal or diffuse FDG uptake above background in a location incompatible with normal anatomy/physiology seems to be appropriate in the majority of cases. However, the following exceptions were noted16, 17: (a) mild and diffusely increased FDG uptake at the site of moderate‐sized or large residual masses (ie ≥2 cm in diameter), with an intensity no more than that of the mediastinal blood pool (MBP), was considered negative for the presence of residual lymphoma, whereas diffuse or focal uptake exceeding that of the MBP was considered indicative of lymphoma; (b) new lung nodules ≥1.5 cm in patients with no evidence of pulmonary lymphoma before therapy were considered suggestive of lymphoma if their uptake exceeded that of the MBP, whereas the degree of uptake was unreliable for assessment for nodules <1.5 cm owing to partial volume averaging; and (c) clearly increased (multi)focal bone (marrow) uptake was considered positive for lymphoma, whereas diffusely increased FDG uptake in the bone marrow at 2‐3 weeks after chemotherapy was not misinterpreted as diffuse lymphomatous marrow involvement.
With the fixed threshold method (41% of focal lesion SUVmax), we delineated the region of interest around the focus lesions. The system semi‐automatically collected, processed, and output the SUVmax, SUVmean, and TMTV data. Whole‐body TLG was calculated as Σ(SUVmean × MTV). Baseline PET/CT parameters were recorded as SUVmax0, TMTV0, and TLG0, following which the interim parameters, difference, and difference ratio were recorded, respectively, as SUVmax1, TMTV1, and TLG1; ΔSUVmax, ΔTMTV, and ΔTLG; and %ΔSUVmax, %ΔTMTV, and %ΔTLG.
2.4. Statistical analysis
The NCCN‐IPI scores were categorized into four risk groups: low (0‐1), low‐intermediate (2‐3), high‐intermediate (4‐5), and high (6‐8). The baseline and interim parameters were combined for prognosis analysis. Descriptive analysis and chi‐squared tests were applied for the clinical information and their relationship with PFS. A receiver operating characteristic (ROC) curve was used to determine the optimal cutoff values of SUVmax, TMTV, and TLG for 3‐year % PFS, where the cutoff values of these parameters with AUC > 0.7 were determined by the Youden index (maximum sum of sensitivity and specificity). Survival analysis was completed with a Kaplan‐Meier (K‐M) survival analysis, and differences between groups were analyzed with the log‐rank test. Independent predictive variables were determined with univariate and multivariate Cox regression analyses by the method of Forward LR Pearson's correlation coefficient analysis was used for the bivariate correlation analysis of two likely correlated parameters (eg TMTV and TLG). All analyses were conducted using SPSS 19.0 (IBM, Armonk, NY, USA). A two‐sided P‐value of less than 0.05 was considered significant.
3. RESULTS
3.1. Clinical characteristics
The age and three‐year PFS distribution for all the patients are summarized in Table 1. There were a total of 85 enrolled patients (46 men, 39 women, age 55.4 ± 16 years). The median follow‐up time was 34 months (range: 7‐76 months). In total, 62 (72.9%) patients survived for 3 years without disease progression or relapse or death from the disease. Twenty‐three (27.1%) encountered disease progression or relapse at a median of 14.6 months. Patients with a B symptom, higher Ann Arbor stage, higher LDH ratio, extranodal disease involvement in major organs, and higher NCCN‐IPI risk showed a significantly lower three‐year PFS.
Table 1.
Clinical information and three‐year PFS for the study participants
| Characteristic | Total (%) | 3‐Year PFS, N (%) | Chi‐square value | P‐Value | |
|---|---|---|---|---|---|
| Age, years | >18 to ≤40 | 15 (17.6) | 13 (86.7%) | 6.335 | 0.096 |
| >41 to ≤60 | 36 (42.4) | 29 (80.6%) | |||
| >61 to ≤75 | 26 (30.6) | 16 (61.5%) | |||
| >75 | 8 (9.4) | 4 (50.0%) | |||
| Gender | Male | 46 (54.1) | 32 (69.6%) | 0.579 | 0.447 |
| Female | 39 (45.9) | 30 (76.9%) | |||
| B symptoms | No | 62 (72.9) | 49 (79.0%) | 4.307 | 0.038 |
| Yes | 23 (27.1) | 13 (56.5%) | |||
| Ann Arbor stage | I/II | 32 (37.6) | 29 (90.6%) | 8.131 | 0.004 |
| III/IV | 53 (62.4) | 33 (62.3%) | |||
| ECOG PS | 0‐1 | 71 (83.5) | 54 (76.1%) | 2.119 | 0.145 |
| ≥2 | 14 (16.5) | 8 (57.1%) | |||
| LDH ratio | ≤1 | 44 (51.8) | 37 (84.1%) | 6.187 | 0.045 |
| >1 to ≤3 | 37 (43.5) | 22 (59.5%) | |||
| >3 | 4 (4.7) | 3 (75.0%) | |||
| Extranodal diseasea | No | 31 (36.5) | 28 (90.3%) | 7.469 | 0.006 |
| Yes | 54 (63.5) | 34 (63.0%) | |||
| NCCN‐IPI risk groups | Low (0‐1) | 13 (15.3) | 12 (92.3%) | 18.270 | 0.000 |
| Low‐intermediate (2‐3) | 36 (42.4) | 31 (86.1%) | |||
| High‐intermediate (4‐5) | 25 (29.4) | 16 (64.0%) | |||
| High (6‐8) | 11 (12.9) | 3 (27.3%) | |||
| Total | 85 (100) | 62 (72.9%) | |||
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; LDH, lactate dehydrogenase, NCCN‐IPI, National Comprehensive Cancer Network International Prognostic Index; PFS, progression‐free survival.
Disease in the bone marrow, CNS, liver/gastrointestinal tract, or lung.
3.2. ROC analysis of quantitative PET/CT parameters
Table 2 summarizes the baseline, interim, difference between them, and difference ratio of the PET/CT quantitative parameters. Parameters with AUCs < 0.7, that is, SUVmax0, ΔSUVmax, ΔTMTV, and ΔTLG, were excluded. Then, the cutoff values of the others were calculated by the maximum Youden index. The results of them were as follows: TMTV0 (AUC 0.745; cutoff value 80.74 cm3; sensitivity/specificity 91.3%/56.5%), TLG0 (0.738; 1036.61 g; 91.3%/56.5%), SUVmax1 (0.751; 3.85; 69.6%/74.2%), TMTV1 (0.735; 4.32 cm3; 60.9%/87.1%), TLG1 (0.737; 14.07 g; 60.9%/85.5%), %ΔSUVmax (0.715; 86.02%; 87%/51.6%), %ΔTMTV (0.723; 99.22%; 60.9%/85.5%), and %ΔTLG (0.727; 99.86%; 60.9%/83.9%). TMTV0 and TLG0 had the best performance in terms of the sensitivity of disease progression or relapse. The %ΔSUVmax showed the best sensitivity among the interim parameters. To avoid mistaken eliminations of parameters, we also tested the dichotomized ΔTMTV and ΔTLG for PFS owing to their AUCs being nearly 0.7, but got unsatisfactory results.
Table 2.
Receiver operating characteristic curve analysis and three‐year PFS from related parameters
| Variables | AUC | Cutoff value | Sensitivity (%) | Specificity (%) | Three‐year PFS (above vs. below cutoffs) (%) |
|---|---|---|---|---|---|
| SUVmax0 | 0.573 | – | – | – | – |
| TMTV0 | 0.745 | 80.74 | 91.3 | 56.5 | 56.3 vs. 94.6 |
| TLG0 | 0.738 | 1036.61 | 91.3 | 56.5 | 56.3 vs. 94.6 |
| SUVmax1 | 0.751 | 3.85 | 69.6 | 74.2 | 50.0 vs. 86.8 |
| TMTV1 | 0.735 | 4.32 | 60.9 | 87.1 | 36.4 vs. 85.7 |
| TLG1 | 0.737 | 14.07 | 60.9 | 85.5 | 39.1 vs. 85.5 |
| %ΔSUVmax | 0.715 | 86.02 | 87 | 51.6 | 91.4 vs. 60.0 |
| %ΔTMTV | 0.723 | 99.22 | 60.9 | 85.5 | 85.5 vs. 39.1 |
| %ΔTLG | 0.727 | 99.86 | 60.9 | 83.9 | 85.2 vs. 41.7 |
| ΔSUVmax | 0.446 | – | – | – | – |
| ΔTMTV | 0.689 | – | – | – | – |
| ΔTLG | 0.683 | – | – | – | – |
| NCCN‐IPI (>3 vs. ≤3) | 52.8 vs. 87.8 |
AUC, area under the receiver operating characteristic curve; MTV, metabolic tumor volume; NCCN‐IPI, National Comprehensive Cancer Network International Prognostic Index; PFS, progression‐free survival; SUVmax, maximum standardized uptake value; TLG, total lesion glycolysis. The subscripts 0 and 1 represent baseline and interim measures, respectively.
3.3. Kaplan‐Meier survival curve and Cox regression analysis
As both of the baseline and interim TMTV and TLG values showed a strong correlation (Pearson's correlation coefficient, r = 0.823 and 0.988, respectively, and both of P‐value = 0.000) due to the similar calculation mode, TLG reflected the tumor metabolic intensity in addition to the tumor volume, which could possibly be a better estimate of tumor burden compared with the TMTVs. Consequently, only TLGs were included in the K‐M and multivariate Cox regression analysis. In Data S1 section, we can also find those results after TMTV replacing TLG. Based on cutoff values derived from the ROC curve analysis, these dichotomized quantitative variables showed significantly separated survival curves by K‐M analysis (Figure 1). All of the higher groups of TLG0, SUVmax1, and TLG1 presented significantly poorer PFS. Patients with %ΔSUVmax, %ΔTMTV, and %ΔTLG less than the cutoff values got the same poor PFS. In the K‐M analysis of the NCCN‐IPI risk groups, great differences were presented between the low‐ and high‐risk groups, especially for the high‐intermediate and high‐risk groups, but the survival curves for the low‐ and low‐intermediate risk groups almost converged.
Figure 1.
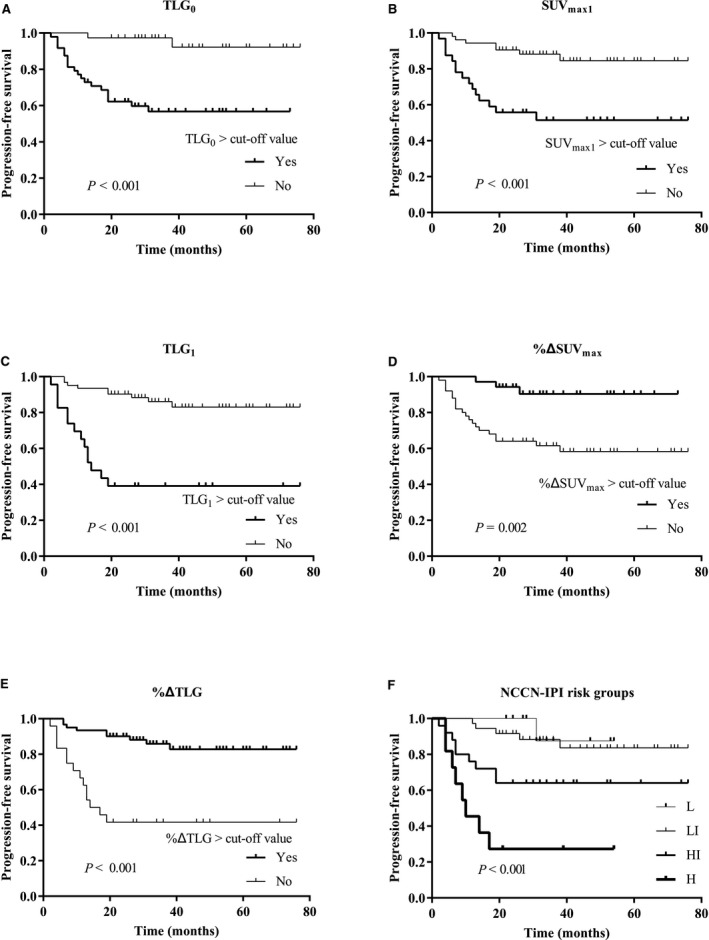
Kaplan‐Meier survival analysis of dichotomized quantitative parameters of PET/CT and NCCN‐IPI risk groups. H, high‐risk group; HI, high‐intermediate risk group; L, low‐risk group; LI, low‐intermediate risk group; NCCN‐IPI, National Comprehensive Cancer Network International Prognostic Index; SUVmax, maximum standardized uptake value; TLG, total lesion glycolysis. The subscripts 0 and 1 represent baseline and interim measures, respectively
The NCCN‐IPI, TMTV0, TLG0, SUVmax1, TMTV1, TLG1, and %ΔSUVmax all showed significantly predictive values for PFS in the univariate Cox analysis. Due to the strong correlation between TMTV and TLG, only TLGs were included in the multivariate Cox regression analysis. Besides, due to the same correlation between TLGs (or TMTVs) and %ΔTLG(or %ΔTMTV), %ΔTLG and %ΔTMTV were also excluded. Because of the better performance of %ΔSUVmax in sensitivity derived from ROC analysis, SUVmax1 strongly correlated with %ΔSUVmax was also excluded.
Eventually, NCCN‐IPI, TLG0, TLG1, and %ΔSUVmax were entered into the multivariate COX regression analysis, but only TLG0 and %ΔSUVmax showed predictive value independently (hazard ratio, HR = 10.42, 95% confidence interval, CI 2.35‐46.30, P‐value = 0.002, and HR = 4.86, 95% CI 1.27‐18.54, P = 0.021, respectively). We also conducted another multivariate model analysis by replacing the TLGs with the TMTVs, where TMTV0 and TMTV1 both showed significantly predictive abilities (Table S1). Although the NCCN‐IPI scores of the four risk groups (low‐, low‐intermediate, high‐intermediate, and high‐risk groups) were initially included in the multivariate analysis, they failed when entered into the model equation (Table 3). However, we also tried analyzing a multivariate regression equation model using the dichotomized NCCN‐IPIs (divided into two groups of low/low‐intermediate and high‐intermediate/high‐risk groups) and concluded that TLG0, TLG1, and the dichotomized NCCN‐IPIs showed predictive value independently (HR = 5.839, 95% CI 1.20‐28.44, P‐value = 0.029; HR = 3.082, 95% CI 1.15‐8.25, P = 0.025; HR = 3.00, 95% CI 1.08‐8.33, P = 0.035, respectively) (Table S2), whereas that of %ΔSUVmax in this model showed a trend for significance as an independent predictor of PFS (HR = 3.80, 95% CI 0.98‐14.77, P‐value = 0.054). TMTVs also presented similar results after replacing TLGs (Table S2).
Table 3.
Risk factors of clinical and quantitative PET/CT parameters for PFS analyzed by univariate and multivariate Cox regression analyses
| Covariate | Univariate analyses | Multivariate analyses | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P‐Value | HR | 95% CI | P‐Value | |
| NCCN‐IPI risk groups | – | – | 0.000 | – | – | – |
| Low | – | – | – | – | – | – |
| Low‐intermediate | 1.79 | 0.21, 15.36 | 0.59 | – | – | – |
| High‐intermediate | 5.72 | 0.73, 45.2 | 0.098 | – | – | – |
| High | 17.49 | 2.17, 140.99 | 0.007 | – | – | – |
| TMTV0 (>80.74) | 10.32 | 2.42, 44.084 | 0.002 | – | – | – |
| TLG0 (>1036.61) | 10.39 | 2.43, 44.39 | 0.002 | 10.42 | 2.35, 46.30 | 0.002 |
| TMTV1 (>4.32) | 6.93 | 2.97, 16.17 | 0.000 | – | – | – |
| TLG1 (>14.07) | 6.25 | 2.68, 14.56 | 0.000 | 2.39 | 0.93, 6.16 | 0.072 |
| %ΔSUVmax (<86.02) | 5.60 | 1.66, 18.88 | 0.005 | 4.86 | 1.27, 18.54 | 0.021 |
CI, confidence interval; HR, hazards ratio; MTV, metabolic tumor volume; NCCN‐IPI, National Comprehensive Cancer Network International Prognostic Index; PFS, progression‐free survival; SUVmax, maximum standardized uptake value; TLG, total lesion glycolysis. The subscripts 0 and 1 represent baseline and interim measures, respectively. The MTV variables were not included in the multivariate model owing to their correlation with the TLG variables.
3.4. Predictive models
On the basis of the ROC analysis and the multivariate Cox model (Tables 2 and 3), three models consisting of both baseline and interim parameters (viz. model 1[TLG0 + TLG1], model 2[TLG0 + %ΔSUVmax], and model 3[NCCN‐IPI + %ΔSUVmax]) were built and analyzed by K‐M analysis and log‐rank test (Table 4). As a result, in model 2, the group with TLG0 > 1036.6 and %ΔSUVmax < 86.02% recognized 19 (82.6%) of the relapse or progression events, whereas only four events were picked out by the other three risk groups (Table 4). The three‐year PFS of this group was 32.1%, whereas all these of the other three groups were more than 90%. In model 1, the double‐positive group predicted a lower three‐year PFS of 27.8% and picked out 13 of 23 patients with relapse or progression, whereas 10 of them were still omitted in the other three risk groups.
Table 4.
Results of three prognostic models derived by Kaplan‐Meier survival analysis
| Model | Baseline variables | Interim variables | n | Three‐year PFS, N (%) | P‐Valuea |
|---|---|---|---|---|---|
| Model 1 | TLG0 ≤ 1036.6 | TLG1 ≥ 14.068 | 32 | 31 (96.90) | 0.157 |
| TLG1 > 14.068 | 5 | 4 (80.00) | |||
| Total | 37 | 35 (94.60) | |||
| TLG0 > 1036.6 | TLG1 ≤ 14.068 | 30 | 22 (73.30) | 0.000 | |
| TLG1 > 14.068 | 18 | 5 (27.80) | |||
| Total | 48 | 27 (56.30) | |||
| Model 2 | TLG0 ≤ 1036.6 | ΔSUVmax ≥ 86.02% | 15 | 14 (93.3) | 0.547 |
| ΔSUVmax < 86.02% | 22 | 21 (95.5) | |||
| Total | 37 | 35 (94.6) | |||
| TLG0 > 1036.6 | ΔSUVmax ≥ 86.02% | 20 | 18 (90.0) | 0.000 | |
| ΔSUVmax < 86.02% | 28 | 9 (32.1) | |||
| Total | 48 | 27 (56.5) | |||
| Model 3 | NCCN‐IPI ≤ 3 | ΔSUVmax ≥ 86.02% | 23 | 22 (95.70) | 0.198 |
| ΔSUVmax < 86.02% | 26 | 21 (80.80) | |||
| Total | 49 | 43 (87.80) | |||
| NCCN‐IPI > 3 | ΔSUVmax ≥ 86.02% | 12 | 10 (83.30) | 0.009 | |
| ΔSUVmax < 86.02% | 24 | 9 (37.50) | |||
| Total | 36 | 19 (52.80) | |||
| Total | Total | 85 | 62 (72.90) |
NCCN‐IPI, National Comprehensive Cancer Network International Prognostic Index; PFS, progression‐free survival; SUVmax, maximum standardized uptake value; TLG, total lesion glycolysis (subscripts 0 and 1 represent baseline and interim measures, respectively).
Log‐rank test.
4. DISCUSSION
In the rituximab era, the risk stratification value of the IPI score has become weaker with increase in the curative rate, especially for the high‐intermediate and high‐risk groups.2 Therefore, more impactful prognostic tools are urgently needed. Herein, we mainly discuss the IPI‐related score system and PET/CT‐related parameters.
Some studies had revised the IPI score by adding new clinical prognostic factor(s),18 regrouping the original IPI score,2, 19 or specifically focusing on elderly patients (E‐IPI).20 The NCCN‐IPI was the most ideal one. By readjusting the age, LDH ratio, and extranodal disease, the NCCN‐IPI showed a better discrimination of patient outcomes (both overall survival and PFS) compared with the original IPI. However, some studies concluded that the NCCN‐IPI was not useful or that a PFS of 40‐60% still remained for the high‐risk group.2, 3, 4, 5, 6 In our study, K‐M survival analysis showed distinct differences among the four risk groups except the low and low‐intermediate groups. In the multivariate Cox regression analysis, the NCCN‐IPI scores categorized into four risk groups showed no significance, but the dichotomized NCCN‐IPI scores (low‐ and low‐intermediate vs. high‐ and high‐intermediate risk groups) significantly predicted PFS independently. The NCCN‐IPI is an optimal predictive tool owing to its convenience and repeatability. It can also be combined with interim parameters (ie SUVmax1, TMTV1, and TLG1, or their variance ratios) to form a screening model for high‐risk patients.
On the other hand, some studies focused on filtering PET/CT‐related quantitative parameters for discriminating patients with a poor prognosis, and attempted some response‐adapted clinical trials. Baseline and interim (after 2‐4 cycles of chemotherapy) parameters (ie SUVmax, TMTV, TLG, and their variance ratios) were studied, but obtained some controversial results.9, 10, 11, 12, 13
Because of its convenience and repeatability, SUVmax has become the most commonly used PET/CT parameter, but its prognostic value also has not reached a consensus.9, 10, 11, 12, 13 According to mainstream opinions,9, 10 high baseline and interim SUVmax measures indicate a poor outcome, reflecting a high proliferation of the tumor. In contrast, Gallicchio et al11 found that a higher SUVmax0 was associated with better PFS. This also denied the predictive significance of TMTV0 and TLG0. Those authors surmised that patients with a high baseline metabolic activity usually respond right away to chemotherapy. Adams et al12 also argued that baseline SUVmax, TMTV, and TLG values had no predictive significance. In that study, the median values of SUVmax0, TMTV0, and TLG0 were used as the cutoff values, respectively, rather than the results of ROC curve analysis. In our study, SUVmax0 showed no significance and the interim SUVmax measure (%ΔSUVmax < 86.02) was statistically significant in the univariate Cox analysis, and %ΔSUVmax entered the multivariate model and presented predictive value independently in the multivariate model. The baseline SUVmax represented the metabolic and proliferative status, while the interim SUVmax‐related parameters could assess the chemotherapeutic response and interim proliferative status of the tumor.
However, the SUVmax representing only one‐pixel point of the lesion could not reflect the condition of the whole lesion. In particular, for low‐uptake lesions, their uptake values were often overestimated as a result of background noise.21 In contrast, SUVmean made up for the shortcomings of SUVmax. Consequently, the volume parameter TLG, derived from SUVmean and TMTV, may perform better in predicting the metabolic activity of the total lesion.
TLG and TMTV, which require a three‐dimensional delineation or segmentation of FDG‐avid lesions from PET/CT, could give a better evaluation of the tumor burden for patient risk stratification. Their volumes are usually measured by several different SUVmax thresholds: a fixed 41% SUVmax threshold; an absolute threshold (>2.5 is commonly used); a method of liver SUVmean plus 2 standard deviations (SDs) as a marginal threshold; and a visually adjusted variable SUVmax threshold. On the basis of the recommendation of the European Association of Nuclear Medicine guidelines7 and the research by Meignan et al22 we chose the fixed 41% SUVmax threshold owing to its better reproducibility and interobserver agreement. Because of the different patient ethnicities and measurement methods, the optimal cutoffs for TMTV0 and TLG0 varied from 70 to 850.3 cm3 8, 13, 23, 24, 25, 26, 27 (TMTV0) and 826.5 to 4758 g 8, 13, 26, 27 (TLG0). Our optimal cutoffs for TMTV0and TLG0 were 80.74 cm3 and 1036.6 g, respectively, which were similar to the 70 cm3 and 826.5 g cutoff values used in the study of Zhou et al13 about Chinese patients. Although the 41% threshold method may give lower results compared with the other methods, the TMTV0 and TLG0 of our study showed significant prognostic value independently.
However, the fixed 41% SUVmax threshold does not always result in useful tumor definitions owing to noise, tracer uptake in homogeneities in the tumor and background, and sometimes a low tumor/background ratio. In our study, the optimal cutoffs of TMTV1 and TLG1 were skewed toward very low values (4.32 cm3 and 14.07 g, respectively); in particular, the cutoffs of %ΔTMTV and %ΔTLG were nearly 100% (99.22% and 99.86%, respectively), which were similar to those reported by Mikhaeel et al8 The excessive percentages restricted the clinical value of %ΔTMTV and %ΔTLG. Because of the low interim SUVmax measures, the fixed 41% SUVmax threshold method may cause underestimated values when delineating VOIs. Although TMTV1 and TLG1 showed significant value for predicting PFS in the Cox regression analysis, we surmise that they could have better performance if the liver SUVmean plus 2SDs or absolute threshold (SUV > 2.5) methods were used instead to delineate the interim tumor lesions. More studies are needed to confirm if this is true.
Several studies13, 28, 29, 30 have reported TLG as being a more powerful predictor of outcomes for patients with DLBCL. Zhou et al,13 Esfahani et al,28 and Ceriani et al29 surmised that TLG was the only independent predictor, rather than TMTV and SUVmax. Whereas other four studies8, 24, 25, 31 analyzing baseline TMTV only concluded that TMTV was the independent predictor for PFS (Table S4). Xie et al analyzed both of baseline TMTV and TLG and concluded that both were independent predictors. However, in our study, we found a strong correlation between TMTV and TLG, meaning that when TMTV and TLG were both included into the model equation, the more powerful one would kick out the other from the regression model. In the former studies mentioned above, the P‐values of their univariate analyses for TMTV and TLG were usually less than 0.001, and they had very similar K‐M survival curve results, respectively. However, all of those studies forcibly combined TMTV and TLG into the Cox model equation, which may lead to the mistaken elimination of TMTV or TLG. Consequently, we tried not to incorporate the correlated variables into the Cox regression analysis model equation simultaneously.
We found that the baseline parameters TMTV0 and TLG0 had 91.3% sensitivity, respectively (Table 2), which could help discriminate the majority of patients with poor outcomes. The interim parameters TMTV1 and TLG1 showed 87.1% and 85.5% specificity, respectively, helping to distinguish even more patients with a high risk of relapse or progression from those with baseline high risk. The %ΔSUVmax (derived from baseline and interim data with 87% sensitivity) could also re‐discriminate patients with a high relapse risk after 2‐4 cycles of R‐CHOP chemotherapy. Although TLG0, SUVmax1, TLG1, %ΔSUVmax, %ΔTLG, and NCCN‐IPI all showed significance in the K‐M survival analysis, only TLG0, TLG1, and %ΔSUVmax were entered into the multivariate model, where upon only TLG0 and %ΔSUVmax demonstrated predictive value independently. We combined the baseline and interim variables into three prognostic models. Model 2, consisting of TLG0 and %ΔSUVmax, was superior in screening high‐risk patients, where the group of TLG0 > 1036.6 cm3 and %ΔSUVmax < 86.02% picked out 19 (82.6%) of the relapse or progression events, whereas only four events were omitted in the other three groups. Model 2 showed a better prognostic ability than model 1 (TLG0 + TLG1) and model 3 (dichotomized NCCN‐IPI + %ΔSUVmax). Patients with low baseline TLG results received a three‐year PFS of approximately 94.6% and had no relationship with %ΔSUVmax. For patients with high baseline results, whether %ΔSUVmax was below or above 86.2% would predict their outcomes (three‐year PFS: 32.1% vs. 90.0%).
Adams and Kwee6 reviewed and conducted a meta‐analysis of nine studies on the prognostic value of interim PET/CT in R‐CHOP‐treated DLBCL. They found that the prognostic value was homogeneously suboptimal across the studies, and it was not consistently proven to surpass the prognostic potential of the IPI. There is a lack of studies comparing interim PET/CT parameters with the newly developed NCCN‐IPI. Our study compared PET/CT parameters with NCCN‐IPI by using Cox regression analysis. The NCCN‐IPI scores categorized into four risk groups showed no significance in the multivariate regression model and were therefore considered unusable for this model (Table 3). However, the dichotomized NCCN‐IPIs were entered into the model and showed independent predictive value (HR = 3.0, 95% CI 1.08‐8.33, P‐value = 0.035, in model of TLG; HR = 3.61, 95% CI 1.36‐9.56, P‐value = 0.01, in model of TMTV), similar to TLG and TMTV. Thus, we combined the dichotomized NCCN‐IPI with ΔSUVmax into model 3, but the result was not as good as that obtained with model 2.
From the results of our research, the baseline variables, that is, TMTV0, TLG0 and dichotomized NCCN‐IPI, and the interim variables TMTV1 and %ΔSUVmax, presented independent prognostic value for PFS, and the model consisting of the baseline and interim parameters (model 2 [TLG0 + %ΔSUVmax]) also presented superior screening ability. The repeatability and effectiveness of our results still need to be validated by more studies. However, unifying the various delineating methods must be a priority, which need more studies to make a consensus about the method of threshold.
5. CONCLUSIONS
The results of our study showed the independent prognostic abilities of TLG0 and ΔSUVmax. When replacing TLG with TMTV measures, TMTV0 and TMTV1 also showed independent prognostic value in the multivariate Cox regression model. Dichotomized NCCN‐IPI also got the same result. Model 2 comprising TLG0 and %ΔSUVmax picked out 19 (82.6%) of the relapse or progression events, demonstrating that a model combining baseline and interim parameters can be a powerful prognostic tool. Generally speaking, the baseline quantitative parameters of PET/CT (TMTV0 and TLG0) had the best predictive ability. The %ΔSUVmax could also help with further screening. However, the method of a fixed 41% threshold was not satisfactory owing to a lower lesion/background ratio of SUVmax in delineating and calculating the interim volume parameters (ie TMTV1 and TLG1), which may cause these values to be underestimated. Consequently, we thought that maybe the methods of liver SUVmean plus 2SDs or absolute threshold (SUV > 2.5) could be used for detecting interim volume parameters after the method of a fixed 41% threshold has delineated and calculated the baseline volume parameters. Whether these tools could be used for driving response‐adapted therapy still needs further validation in clinical trials.
CONFLICT OF INTEREST
None of the authors have any conflict of interest to disclose.
Supporting information
ACKNOWLEDGMENTS
We thank the two nuclear medicine radiologists from Peking University Third Hospital for their work in acquiring the parameter data, and all the patients who allowed us to carry out this research.
Zhang Y‐Y, Song L, Zhao M‐X, Hu K. A better prediction of progression‐free survival in diffuse large B‐cell lymphoma by a prognostic model consisting of baseline TLG and %ΔSUVmax . Cancer Med. 2019;8:5137–5147. 10.1002/cam4.2284
REFERENCES
- 1. Le Gouill S, Casasnovas RO. Interim PET‐driven strategy in de novo diffuse large B‐cell lymphoma: do we trust the driver? Blood. 2017;129:3059‐3070. [DOI] [PubMed] [Google Scholar]
- 2. Zhou Z, Sehn LH, Rademaker AW, et al. An enhanced international prognostic index (NCCN‐IPI) for patients with diffuse large B‐cell lymphoma treated in the rituximab era. Blood. 2014;123:837‐842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang C‐E, Chen Y‐Y, Lu C‐H, Chen P‐T, Lee K‐D, Chen C‐C. Validation of an enhanced international prognostic index (NCCN‐IPI) in an Asian cohort of patients with diffuse large B cell lymphoma. Ann Hematol. 2015;94:1063‐1065. [DOI] [PubMed] [Google Scholar]
- 4. Yang Y, Wang L, Ma Y, Han T, Huang M. The enhanced international prognostic index for diffuse large B‐cell lymphoma. Am J Med Sci. 2017;353:459‐465. [DOI] [PubMed] [Google Scholar]
- 5. Nakaya A, Fujita S, Satake A, et al. Enhanced international prognostic index in Japanese patients with diffuse large B‐cell lymphoma. Leuk Res Rep. 2016;6:24‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Adams HJ, Kwee TC. Prognostic value of interim FDG‐PET in R‐CHOP‐treated diffuse large B‐cell lymphoma: systematic review and meta‐analysis. Crit Rev Oncol Hematol. 2016;106:55‐63. [DOI] [PubMed] [Google Scholar]
- 7. Boellaard R, Delgado‐Bolton R, Oyen W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328‐354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mikhaeel NG, Smith D, Dunn JT, et al. Combination of baseline metabolic tumour volume and early response on PET/CT improves progression‐free survival prediction in DLBCL. Eur J Nucl Med Mol Imaging. 2016;43:1209‐1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Park S, Moon SH, Park LC, et al. The impact of baseline and interim PET/CT parameters on clinical outcome in patients with diffuse large B cell lymphoma. Am J Hematol. 2012;87:937‐940. [DOI] [PubMed] [Google Scholar]
- 10. Chihara D, Oki Y, Onoda H, et al. High maximum standard uptake value (SUVmax) on PET scan is associated with shorter survival in patients with diffuse large B cell lymphoma. Int J Hematol. 2011;93:502‐508. [DOI] [PubMed] [Google Scholar]
- 11. Gallicchio R, Mansueto G, Simeon V, et al. F‐18 FDG PET/CT quantization parameters as predictors of outcome in patients with diffuse large B‐cell lymphoma. Eur J Haematol. 2014;92:382‐389. [DOI] [PubMed] [Google Scholar]
- 12. Adams H, de Klerk J, Fijnheer R, et al. Prognostic superiority of the national comprehensive cancer network international prognostic index over pretreatment whole‐body volumetric‐metabolic FDG‐PET/CT metrics in diffuse large B‐cell lymphoma. Eur J Haematol. 2015;94:532‐539. [DOI] [PubMed] [Google Scholar]
- 13. Zhou M, Chen Y, Huang H, Zhou X, Liu J, Huang G Prognostic value of total lesion glycolysis of baseline 18F‐fluorodeoxyglucose positron emission tomography/computed tomography in diffuse large B‐cell lymphoma. Oncotarget. 2016;7:83544‐83553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579‐586. [DOI] [PubMed] [Google Scholar]
- 15. Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non‐Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059‐3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Juweid ME, Stroobants S, Hoekstra OS, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007;25:571‐578. [DOI] [PubMed] [Google Scholar]
- 17. Barrington SF, Mikhaeel NG, Kostakoglu L, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol. 2014;32(27):3048–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cox MC, Nofroni I, Ruco L, et al. Low absolute lymphocyte count is a poor prognostic factor in diffuse‐large‐B‐cell‐lymphoma. Leuk Lymphoma. 2008;49:1745‐1751. [DOI] [PubMed] [Google Scholar]
- 19. Sehn LH, Berry B, Chhanabhai M, et al. The revised international prognostic index (R‐IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B‐cell lymphoma treated with R‐CHOP. Blood. 2007;109:1857‐1861. [DOI] [PubMed] [Google Scholar]
- 20. Advani RH, Chen H, Habermann TM, et al. Comparison of conventional prognostic indices in patients older than 60 years with diffuse large B‐cell lymphoma treated with R‐CHOP in the US Intergroup Study (ECOG 4494, CALGB 9793): consideration of age greater than 70 years in an elderly prognostic index (E‐IPI). Br J Haematol. 2010;151:143‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Akamatsu G, Ikari Y, Nishida H, et al. Influence of statistical fluctuation on reproducibility and accuracy of SUVmax and SUVpeak: a phantom study. J Nucl Med Technol. 2015;43:222‐226. [DOI] [PubMed] [Google Scholar]
- 22. Meignan M, Sasanelli M, Casasnovas RO, et al. Metabolic tumour volumes measured at staging in lymphoma: methodological evaluation on phantom experiments and patients. Eur J Nucl Med Mol Imaging. 2014;41:1113‐1122. [DOI] [PubMed] [Google Scholar]
- 23. Song M‐K, Chung J‐S, Shin H‐J, et al. Clinical significance of metabolic tumor volume by PET/CT in stages II and III of diffuse large B cell lymphoma without extranodal site involvement. Ann Hematol. 2012;91:697‐703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sasanelli M, Meignan M, Haioun C, et al. Pretherapy metabolic tumour volume is an independent predictor of outcome in patients with diffuse large B‐cell lymphoma. Eur J Nucl Med Mol Imaging. 2014;41:2017‐2022. [DOI] [PubMed] [Google Scholar]
- 25. Casasnovas RO, Sasanelli M, Berriolo‐Riedinger A, et al. Baseline metabolic tumor volume is predictive of patient outcome in diffuse large B cell lymphoma. Blood. 2012;120:1598. [Google Scholar]
- 26. Cottereau A‐S, Lanic H, Mareschal S, et al. Molecular profile and FDG‐PET/CT total metabolic tumor volume improve risk classification at diagnosis for patients with diffuse large B‐cell lymphoma. Clin Cancer Res. 2016;22:3801‐3809. [DOI] [PubMed] [Google Scholar]
- 27. Xie M, Zhai W, Cheng S, Zhang H, Xie Y, He W. Predictive value of F‐18 FDG PET/CT quantization parameters for progression‐free survival in patients with diffuse large B‐cell lymphoma. Hematology. 2016;21:99‐105. [DOI] [PubMed] [Google Scholar]
- 28. Esfahani SA, Heidari P, Halpern EF, Hochberg EP, Palmer EL, Mahmood U. Baseline total lesion glycolysis measured with (18)F‐FDG PET/CT as a predictor of progression‐free survival in diffuse large B‐cell lymphoma: a pilot study. Am J Nucl Med Mol Imaging. 2013;3:272‐281. [PMC free article] [PubMed] [Google Scholar]
- 29. Ceriani L, Martelli M, Zinzani PL, et al. Utility of baseline 18FDG‐PET/CT functional parameters in defining prognosis of primary mediastinal (thymic) large B‐cell lymphoma. Blood. 2015;126:950‐956. [DOI] [PubMed] [Google Scholar]
- 30. Kim TM, Paeng JC, Chun IK, et al. Total lesion glycolysis in positron emission tomography is a better predictor of outcome than the international prognostic index for patients with diffuse large B cell lymphoma. Cancer. 2013;119:1195‐1202. [DOI] [PubMed] [Google Scholar]
- 31. Shagera QA, Cheon GJ, Koh Y, et al. Prognostic value of metabolic tumour volume on baseline (18)F‐FDG PET/CT in addition to NCCN‐IPI in patients with diffuse large B‐cell lymphoma: further stratification of the group with a high‐risk NCCN‐IPI. Eur J Nucl Med Mol Imaging. 2019. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


