Abstract
The porous materials of SnO2@NGO composite was synthesized by thermal reduction process at 550 °C in presence ammonia and urea as catalyst. In this process, the higher electrostatic attraction between the SnO2@NGO nanoparticles were anchored via thermal reduction reaction. These synthesized SnO2@ NGO composites were confirmed by Raman, XRD, XPS, HR-TEM, and EDX results. The SnO2 nanoparticles were anchored in the NGO composite in the controlled nanometer scale proved by FE-TEM and BET analysis. The SnO2@NGO composite was used to study the electrochemical properties of CV, GCD, and EIS analysis for supercapacitor application. The electrochemical properties of SnO2@NGO exhibited the specific capacitance (~378 F/g at a current density of 4 A/g) and increasing the cycle stability up to 5000 cycles. Therefore, the electrochemical results of SnO2@NGO composite could be promising for high-performance supercapacitor applications.
Subject terms: Supercapacitors, Electrocatalysis
Introduction
The nanotechnology of graphene-based materials are widely fabricated as active electrode for supercapacitor applications in presence of various strong electrolytes. In particular, the graphitic carbon electrodes are important role in the supercapacitor device applications1–6. Graphene is 2D carbon materials that contains high thermal conductivity, electrical conductivity, carrier mobility, and lateral quantum efficiencies. The carbon based materials are great interest because of the increasing the electronic capacity, mechanical properties, and superior chemical stability for photovoltaics, supercapacitors, and fuel cells applications5,7–12. The electrochemical performance of porous carbon material as electrical double layer capacitors (EDLC) is mainly depends on porosity, electrical conductivity, its dielectric constant, and various electrolytes. Based on the properties, the hybrid materials are classified into ultra-capacitor and conventional capacitors in the electrochemical reaction and its mechanism13–17. Recently, the N-doped graphene oxides (NGO) are widely used as electrode materials for supercapacitor, sensors and batteries studied in detail18–20. The NGO-metal oxides were synthesized to improve the electrochemical properties such as rate capability by using different catalyst21,22. Already the numerous research work reported on the NGO with metal oxides fabrication via CVD and thermal annealing process for supercapacitor and batteries applications. The various nanostructured metal oxides are fabricated in the electrochemical studies for increasing the specific capacitance and cyclic stability. In particular, the tin oxide (SnO2) is an n-type semiconducting material and its excellent chemical properties allow to fabricate electrodes, which used as the candidate for supercapacitor applications23,24. The nanostructured SnO2 is the most favorable metal oxides due to its increasing the capacitance, low cost, less poisonousness, and wide band gap ~3.6 eV. The various nanostructured SnO2/carbon composite was synthesized by hydrothermal process for electrochemical supercapacitor, sensors, and solar cells25–29. The SnO2 and RuO2 mixtures are used in the electrode materials for storage properties with an excellent cyclic stability30. The SnO2/graphene composite reported the increasing electrochemical performance and cyclic stability via microwave synthesis31. The construction of Ni/SnO2 composite shows an excellent capacitance and cyclic retention reported in the literature32. The hierarchical SnO2 composite displays the specific capacitance of ~188 F/g with 2000 cycles33. In the present study, SnO2@NGO composite was synthesized and it’s electrochemical properties investigated by CV analysis. The SnO2@NGO composite was analyzed by using Raman, XRD, XPS, BET, SEM, EDS, and HR-TEM analysis. Furthermore, the SnO2@NGO composite was studied by CV, GCD, and EIS techniques with 6 M KOH electrolyte.
Results and Discussion
Structural and surface morphology of SnO2@NGO
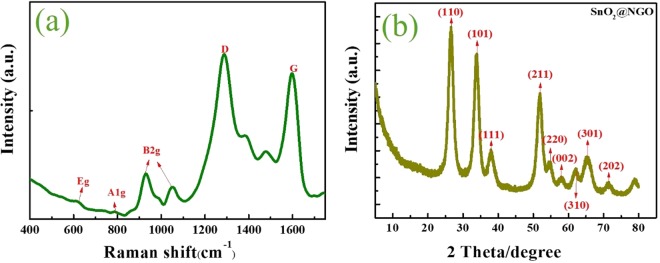
The schematic illustration of SnO2@NGO synthesis via thermal reduction process is depicted in Fig. 1. The Raman spectral analysis used to study the carbon-based materials and its defect structure. Figure 2(a) represent the Raman results of graphene oxide (GO) and NGO obtained by a thermal reduction reaction. The properties of graphene materials are represented at 1,580 cm−1 to the E2g peaks of sp2 C atoms, and D band at 1,350 cm−1, which was ascribed to the breathing modes of the A1g symmetry34. These peaks provide the information of local defects and disorder behavior of NGO by Raman spectroscopy (Fig. 2a). The Raman peaks represented that the D band at 1358 cm−1 and G bands 1597 cm−1 in the NGO structure. Moreover, the D/G intensity of NGO decreased when comparted to GO composite. The intensity change is may be due to the reduction of the NGO materials by thermal reduction process in the sp2 carbon structure. The peak position at lower wave numbers represent the different vibration modes of SnO2 nanoparticles in the NGO composite35,36.
Figure 1.
Schematic illustration of SnO2@NGO synthesis via thermal reduction process.
Figure 2.
(a) Raman, and (b) XRD of SnO2@NGO composite.
The XRD studies of GO indicated the peak at about 2θ = 10.80° with d -spacing 0.89 nm. The typical XRD peak of GO confirmed the well exfoliated carbon sheets in graphite structure has been reported previously37–39. The peak position at 2θ = (9.84° and 19.7°), corresponds to the (002) and (100) planes of GO materials. This may be due to the thermal reduction of GO to graphitic crystal structure in presence of high temperature. In addition, the diffraction pure SnO2 was studied in previous reports40–43 and SnO2@NGO diffraction peaks are represented in Fig. 2(b). The XRD of SnO2@NGO composite at 26.57°, 33.87°, 37.86°, 52.26°, 54.93°, 57.87°, 62.15°, 65.33°, and 71.28° are clearly distinguishable and corresponding planes (110), (101), (111), (211), (220), (002), (310), (301) and (202), respectively. The structure of tetragonal confirmed the PDF file no: JCPDS 41–1445. Therefore, the SnO2@NGO composite, which might be due to the exfoliation of NGO sheets at 550 °C by thermal reduction process.
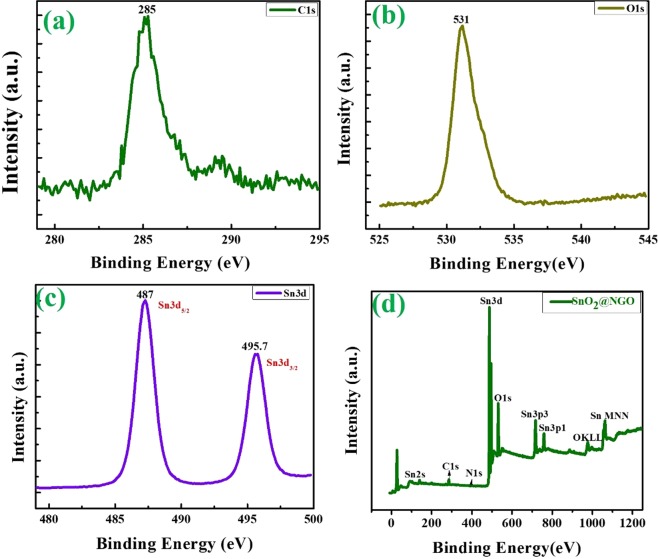
Figure 3 represent the XPS peaks of SnO2@NGO composite. The survey spectrum (Fig. 3d) indicates that C 1s (285), O 1s (532), and Sn3d (487) eV, which complete the effective adornment of SnO2 nanoparticles onto the NGO surface. The Fig. 3c of Sn3d shows the main peaks of (3d5/2) and 3d3/2 corresponds to the binding energies of 487.0 eV and 495.5 eV, respectively. The binding energy difference between Sn3d5/2 and Sn3d3/2 almost ~8.7 eV. These results confirmed the identical to the binding energies of SnO2 and compared to the previous reports44–46.
Figure 3.
XPS results of (a) C 1s (b) O 1s (c) Sn 3d and (d) survey spectrum of SnO2 @NGO composite.
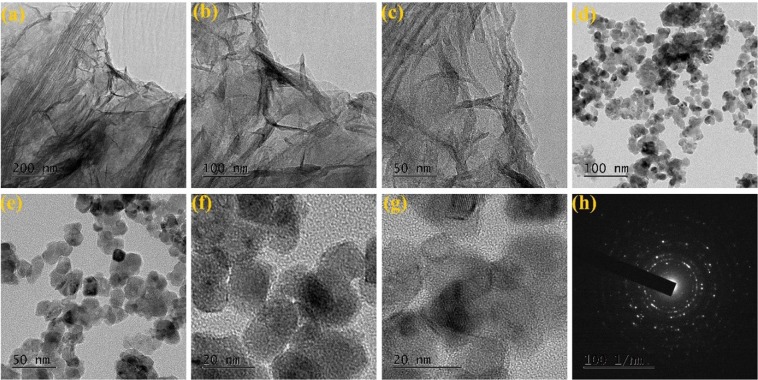
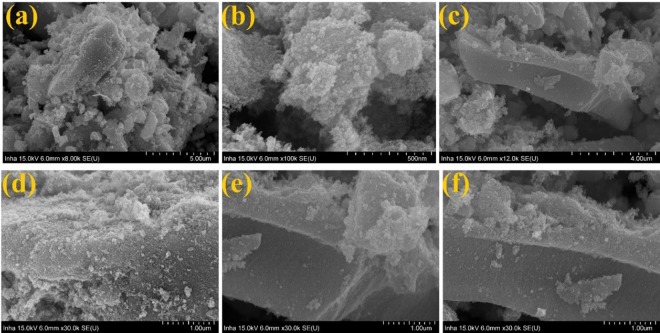
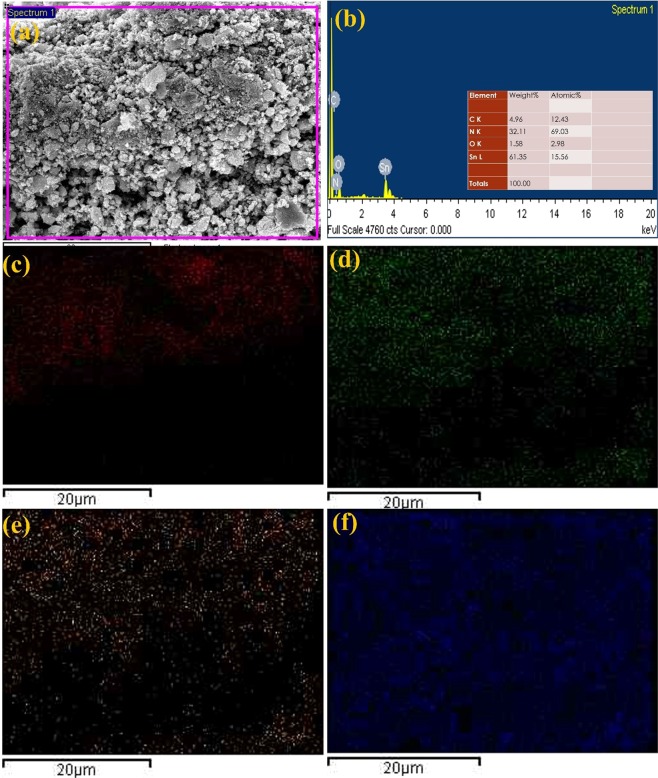
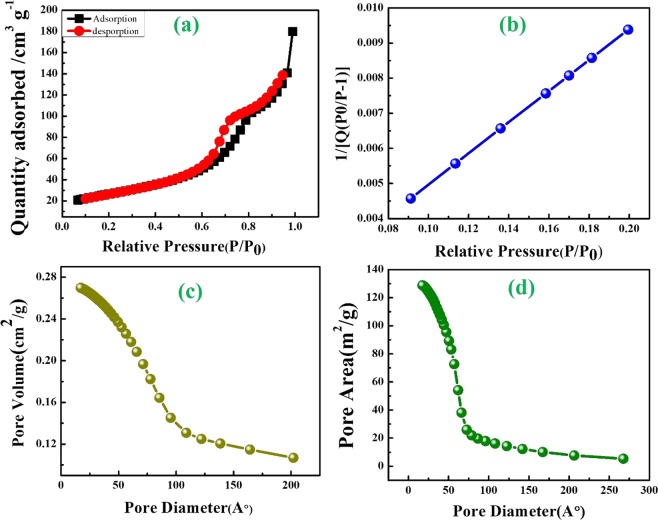
Figures 4 and 5 represent morphological behaviors of SnO2@NGO composite studied by FE-TEM analysis. The (Fig. 4a–c) morphology of shows that altered the magnifications of NGO composite. The nanocrystalline SnO2 nanoparticles was decorated on the NGO and size of the particles in the range of ~10–20 nm (Fig. 4d). The high-resolution TEM of SnO2@NGO composite (Fig. 4a–c) represent the distance of the SnO2 nanoparticles are ~0.28 nm, matching to the (110) plane of SnO2. The SAED image of the SnO2@NGO (Fig. 4h) represent that the nanocrystalline behavior and this results consistent with XRD analysis of the composite. Figure 5 shows the different magnifications of SEM, SEM-EDS results confirms the C, O, N and Sn (Fig. 6) elements of the composite. In these results the N-doping level (32 wt %) on GO was achieved by simple hydrothermal method. The BET results are depicted in the Fig. 7. The surface area of the SnO2@NGO composite of about ~180 m2/g, pore volume of 0.27 cm3/g, and pore area of ~130 m2/g. The BJH analysis results are confirmed a mean pore size of the nanoparticles are ~(10–20) nm in the SnO2@NGO composite.
Figure 4.
(a–c) TEM morphology of NGO materials, (d–g) HR- images of SnO2@NGO composite and (h) SAED pattern of composite.
Figure 5.
(a–f) SEM-Morphology of SnO2@NGO composite.
Figure 6.
(a–b) SEM-EDS and (c) C 1s (d) O 1s (e) N 1s and (f) Sn 3d morphology of SnO2@NGO composite.
Figure 7.
BET results of (a–d) SnO2@NGO composite.
Electrochemical measurements of SnO2@NGO composite
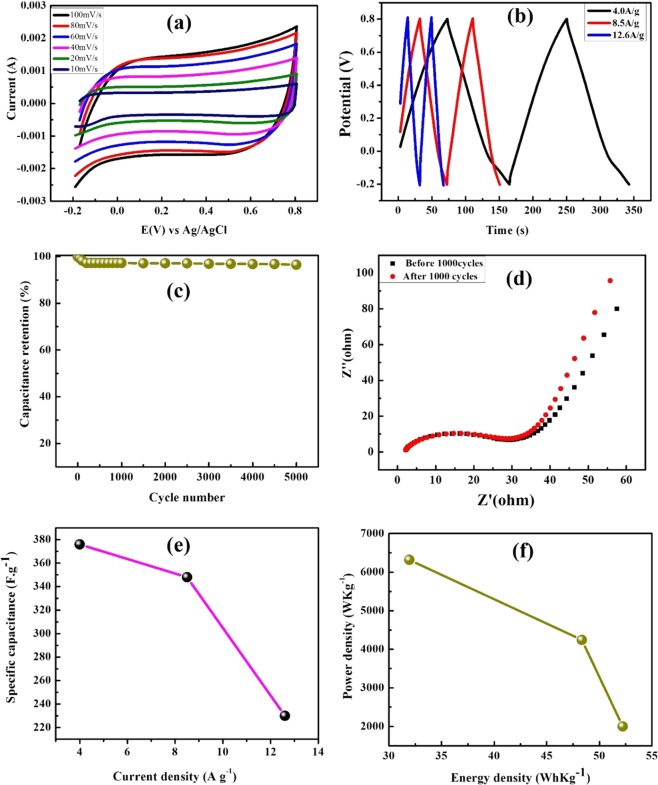
The electrochemical results of GO, RGO, NGO and SnO2 composite and its supercapacitor properties were reported in the previous studies26,47–49. The SnO2@NGO composite was studied by cyclic voltammetric analysis results are shown in Fig. 8 and Table 1. The composite results indicate the ideal capacitive nature showing the rectangular profile owing to outstanding trend for supercapacitor. The trend of CV curves of composite electrodes, rectangular properties and corresponding increase of current than that of pristine SnO2. These properties of composite shows the increasing the specific capacitance because of the combined influence from EDLC and pseudo capacitance of the composite. Figure 8a represent the CV results of SnO2@NGO composite electrodes with the scanning rate from (10–100) mV s−1 in presence of 6 M KOH aqueous electrolyte. The electrochemical properties were studied the potentials range between (−0.2 and 0.8 V), it can be seen that the increasing the capacitance behaviors between the electrode and electrolyte. The scan rates increases, the current density also increases, because of the anodic and cathodic current change towards the reversible reaction. This fast redox or reversible reactions occurring between the electro-active material/electrolyte interfaces in presence 6 M KOH. Because of the CV represent a slight alteration with certain number of functional groups react in the NGO and SnO2 nanoparticles. The specific capacitance of SnO2@NGO electrodes are intended from the CV curves. The cyclic voltammetry results are shown Fig. 8a. In this CV experiments, the scan rate increased from 10 to 100 mV s−1 and also increases the electrochemical supercapacitor properties. The enhancement of electrochemical properties of the composite is mainly denoted to the more electroactive sites via EDLC and pseudo capacitance arises in the SnO2@NGO composite. Further, the SnO2 nanoparticles at the NGO matrix successfully decorated and low internal resistance with high electrical conductivity of the composite for electrochemical reversible reaction26,47–49.
Figure 8.
(a) CVs of the SnO2@NGO composite electrodes at various scan rates of (10–100) mV s−1 in 6 M KOH. (b) Charge/discharge profiles of the composite electrode at different current densities (4, 8.5, and 12.6) A/g. (c) Variation of specific capacitance as a function of cyclic number at a current density of 4.0 A/g, (d) EIS, and (e) the effect of Energy density vs. Power density.
Table 1.
Parameters for the supercapacitor of SnO2 and SnO2@NGO electrodes reported in the literature.
| Electrode material |
Preparation method |
Capacitance (F/g) |
Cyclic stability | Ref. |
|---|---|---|---|---|
|
SnO2@C nanoparticles |
Calcination process at (500–700) °C |
37.8 F/g at 5 mV/s |
NA | 54 |
|
SO42−/SnO2 nanocomposite |
Alkaline hydrolysis |
51.95 F/g at 5 mV/s |
7.5% loss after 2,000 cycles |
55 |
|
Hydrous SnO2 Thin films composite |
Chemical Synthesis of Thin film |
25 F/g at 5 mV/s |
NA | 56 |
|
MWCNT/SnO2 composite |
Decoration by sonochemical process |
133.33 F/g at 0.5 mV/s |
10% loss after 500 cycles |
57 |
| GO/SnO2 | Spray pyrolysis |
61.7 F/g at 1 A/g |
1% loss after 106 cycles |
52 |
| SnO2/Graphene |
Microwave assisted one-pot reaction |
99.7 F/g at 5 mV/s1 |
NA | 48 |
| GNs/SnO2–MWCNTs |
Chemical method followed by calcination |
224 F/g at 10 mA |
8% loss after 6000 cycles |
58 |
|
RGO/SnO2 composite |
Microwave assisted deposition |
348 F/g at 50 m A g−1 |
2% loss after 1,000 cycles |
59 |
|
SnO 2 @NGO composite |
Thermal reduction process |
378 F/g at 4 A/g |
11% loss after 5,000 cycles |
This work |
NA-Not available.
Furthermore, the SnO2@NGO composite for charge-discharge test from the GCD curves represented in Fig. 8(b). The GCD curves of composite electrodes are increases the current density of 4, 8.5 and 12.6 A/g by using 6 M KOH solution. The GCD results evidently designates the triangular shape of the composite materials and the contribution of EDLC and pseudo capacitance properties from CV analysis. The composite electrodes are confirmed the higher discharge time than that of pristine SnO2 composite represent the increasing specific capacitance with stronger electrolytes26,47–49.
The specific capacitance values are ~378 F/g, 345 F/g, and 230 F/g at the current density of 4, 8.5, and 12.6 A/g, respectively. The Fig. (8c,d) represent the cyclic retention and corresponds to the current density (vs) its specific capacitance values. The trend of the specific capacitance values was decreased, as the current density increased 4, 8.5, and 12.6 A/g. Because of the diffusion of the electrolyte ions, depends on the morphology, surface of the materials and concentration of the SnO2 and NGO components. The electrochemical properties of the SnO2@NGO nanoparticles was compared to the previous reports for supercapacitor applications50–52.
In addition, the SnO2@NGO composite, energy density and power density results are shown in Fig. 8(e,f). The cyclic stability is the important property of the electrode for practical applications in the supercapacitors. Figure 8(c) shows the GCD analysis at a current density of 4 A/g for 5,000 cycles. After 2,000 cycles, the composite electrode maintained 89% of its original performance, which signifies of electroactive material has better cyclic stability49,53 and reversibility of the GCD process.
Furthermore, the EIS results were estimated at open circuit potential by relating the various ac voltages in the frequency range from 0.1 Hz to 100 kHz. Figure 8(d) represent the EIS result of Nyquist plots for SnO2@NGO composite, which is indicate that the real and imaginary parts of EIS results, respectively. First, the smaller semi-circular loop at higher frequencies is attributed to the Faradaic reactions in presence of the 6 M KOH electrolyte. The lower frequency region of the EIS increases due to the capacitive nature of SnO2@NGO composite. The phase angle of EIS of composite electrode was perceived to be higher than 45° and low frequencies demonstrating the electrochemical capacitive nature of the composite materials. In this regard, the charge transfer resistance (Rct) of the NGO and composite electrodes are ~36 and 38, respectively. In this lower value of Rct represent the stable electrochemical performance. The low-frequency region of the impedance analysis results are called Warburg resistance of diffusion behavior in presence of 6 M KOH with in the electrodes. The vertical slope of Warburg curves specifies the rapid development of an electric double-layer in the composite because of quick ion diffusion in presence of 6 M KOH electrolyte for supercapacitor applications49,54.
Conclusions
The porous material of SnO2@NGO composite synthesized by thermal reduction process and studied their electrochemical characteristics towards high-performance supercapacitors. The Raman, X-ray diffraction, and photoelectron spectroscopy analysis reveals the successful formation of SnO2@NGO composite. The specific capacitance of the SnO2@NGO composite displayed the capacitance ~378 F/g at a current density of 4 A/g in the 6 M KOH solution. Moreover, the composite electrode exhibited an excellent cycling stability at 4 A/g. The composite electrode maintained 89% of its original performance after 5000 cycles in presence of 6 M KOH electrolyte, there by representing the plausible applicability for energy storage applications.
Materials and Methods
Materials
The graphene oxide from graphite flakes, sulfuric acid (H2SO4,), phosphoric acid (H3PO4), potassium permanganate (KMnO4), potassium hydroxide (KOH), hydrogen peroxide (H2O2, 30%) in this experiment. The tin (IV) chloride pentahydrate (SnCl4.5H2O, 98%), ammonia solution (NH3, 30%), N-methyl-2-pyrrolidone and polytetrafluorethylene (PTFE) were chemicals received from Sigma-Aldrich, South Korea.
Graphene oxide synthesis (GO)
The graphene oxide (GO) materials were developed by Hummer’s technique in the previous reports27–29. The graphite (5 g), H2SO4 (400 mL), H3PO4 (50 mL), and KMnO4 (18 g) were mixed in the three-neck flask using a magnetic stirrer at 40 °C and continually stirred for 48 h to achieve the complete conversion from graphite. After that, the reaction mixture was changes from dark purple to greenish brown color and the calculate amount 20 mL of H2O2 was added to complete the conversion of GO. The GO was purified by using 1 M of HCl or ethanol and then purified the oven at 80 °C for 12 h.
N-Doped graphene oxide synthesis (NGO)
The calculated amount of 0.5 g of GO was distributed in the 300 mL of distilled water followed by ultra-sonication and the solution becomes the brownish GO suspension. The GO suspension and required amount of 20 mL of excess of water is added and stirred for 4 h and filtered/dried in the vacuum oven at 90 °C for 4 h. Then the calculated amount of 1 g of urea and ammonia and excess of 20 mL of ammonia was added and continuously stirred at 90 °C for 12 h. Finally, the GO product was dried in the oven at 200 °C for 12 h, and purified by using ethanol solvent.
SnO2@NGO composite synthesis
Briefly, 0.2 g of GO was distributed in 150 mL of water and sonication for 1 h to become the homogeneous solution. Then the calculated amount of 1.2 g of stannous chloride, 25% of 10 mL ammonia solution was added to the GO solution to maintain the basic medium. Afterwards, the reaction GO solution was refluxed at 200 °C for 8 h by using three neck flask with condenser. Then, the reaction becomes changed to black color product of SnO2@NGO and dried in the vacuum oven at 180 °C for 12 h. Further, the SnO2@NGO sample was calcination at 550 °C for 8 h and collected the product for further characterization.
Preparation of electrochemical analysis
The CV experiment was studied in the regular three-electrode system connected through Autolab PGSTAT302N (Metrohm, Netherlands). The SnO2@NGO composite (working electrode), carbon black, and PVDF in the stoichiometric of 75: 15: 10 and dispersed in the n-methyl 2-pyrrolidone. The resulted black paste was then covered onto a nickel wire collector and dried at 110 °C for 12 h. The mass loading of the SnO2@NGO composite is around 1.5 mg cm−2. In this experiment, platinum wire (counter electrode) and Ag/AgCl (reference electrode) were fabricated in the CV analysis. The synthesized SnO2@NGO composite and its electrochemical properties were determined by cyclic voltammetry analysis. The CV curves were documented at various scan rates (10, 20, 40, 60, 80, and 100) mV s−1 in a potential range of (−0.2 to 0.8) V. The GCD curves were acquired at various current densities (4, 8.5 and 12.6) A/g and EIS results in the frequency range of (0.1 Hz to 100 kHz). The capacitance of SnO2@NGO composite was designed from the CV Eq. (1), and GCD curves Eq. (2) were shown in the previous reports30–33.
Materials characterization
Raman studies were analyzed in the range (100 to 4000 cm−1) by using RM200 confocal Raman spectroscopy. The composite was studied the wide-angle XRD analysis documented by Rigaku Rotaflex (RU-200B) diffractometry in presence Cu Kα radiation. The morphological properties of SnO2@NGO were analyzed by using FE-SEM-4800, and JEM-2010F FE-TEM, Hitachi Japan. The SnO2@NGO composite was examined by X-ray photoelectron spectroscopy by (Thermal Fisher Scientific, USA) with Kα radiation.
Acknowledgements
This research programme fully supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF-2017R1D1A1B03028368, NRF-2017M3A9E2063256), funded by the Ministry and Institute for Information & communications Technology Promotion (IITP) grant funded by the Korea Government (MSIP) (No. R75201600050001002) and also supported under the frame work of 2017 International and Cooperation Programmed by National Research Foundation of Korea (Grant No. 2017K1A4A3013662).
Author Contributions
S.R. and H.S.K. design the project and the experiments for supercapacitor applications. S.R. carried out the experiments of SnO2@NGO composite. All authors S.R., H.M.Y., Y.J.L., G.W.H., A.K., A.S., H.-S.K., H.S.K. and J.-H.K. involved in the characterization of the SnO2@NGO composite and the discussions prominent up to the writing of the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Heung Soo Kim, Email: heungsoo@dgu.edu.
Joo-Hyung Kim, Email: joohyung.kim@inha.ac.kr.
References
- 1.Simon P, Gogotsi Y. Materials for electrochemical capacitors. Nat. Mater. 2008;7:845–854. doi: 10.1038/nmat2297. [DOI] [PubMed] [Google Scholar]
- 2.Miller J. R., Simon P. MATERIALS SCIENCE: Electrochemical Capacitors for Energy Management. Science. 2008;321(5889):651–652. doi: 10.1126/science.1158736. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Yadi, Hu Zhongai, Liang Yarong, Yang Yuying, An Ning, Li Zhimin, Wu Hongying. Growth of 3D SnO2 nanosheets on carbon cloth as a binder-free electrode for supercapacitors. Journal of Materials Chemistry A. 2015;3(29):15057–15067. doi: 10.1039/C5TA02479J. [DOI] [Google Scholar]
- 4.Dhole I. A., Navale Y. H., Pawar C. S., Navale S. T., Patil V. B. Physicochemical and supercapacitive properties of electroplated nickel oxide electrode: effect of solution molarity. Journal of Materials Science: Materials in Electronics. 2018;29(7):5675–5687. [Google Scholar]
- 5.Min J, et al. Facile synthesis of porous iron oxide/graphene hybrid nanocomposites and potential application in electrochemical energy storage. New J. Chem. 2017;41:13553–13559. doi: 10.1039/C7NJ03416D. [DOI] [Google Scholar]
- 6.Zhu S, et al. Low-Charge-Carrier-Scattering Three-Dimensional α-MnO2/β-MnO2 Networks for Ultra-High-Rate Asymmetrical Supercapacitors. ACS Appl. Energy Mater. 2019;2:1051–1059. doi: 10.1021/acsaem.8b01592. [DOI] [Google Scholar]
- 7.Maile NC, et al. Facial growth of Co(OH)2 nanoflakes on stainless steel for supercapacitors: effect of deposition potential. J. Mater. Sci. Mater. Electron. 2019;30:5555–5566. doi: 10.1007/s10854-019-00849-5. [DOI] [Google Scholar]
- 8.Ramesh S, et al. Ni(OH)2-decorated nitrogen doped MWCNT nanosheets as an efficient electrode for high performance supercapacitors. Sci. Rep. 2019;9:6034. doi: 10.1038/s41598-019-42281-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramesh S, Haldorai Y, Kim HS, Kim J-H. A nanocrystalline Co3O4 @polypyrrole/MWCNT hybrid nanocomposite for high performance electrochemical supercapacitors. RSC Adv. 2017;7:36833–36843. doi: 10.1039/C7RA06093A. [DOI] [Google Scholar]
- 10.Bhunia K, Khilari S, Pradhan D. Trimetallic PtAuNi alloy nanoparticles as an efficient electrocatalyst for the methanol electrooxidation reaction. Dalt. Trans. 2017;46:15558–15566. doi: 10.1039/C7DT02608K. [DOI] [PubMed] [Google Scholar]
- 11.Salgado S, Pu L, Maheshwari V. Targeting Chemical Morphology of Graphene Oxide for Self-Assembly and Subsequent Templating of Nanoparticles: A Composite Approaching Capacitance Limits in Graphene. J. Phys. Chem. C. 2012;116:12124–12130. doi: 10.1021/jp3023875. [DOI] [Google Scholar]
- 12.Zhu SJ, Zhang J, Ma JJ, Zhang YX, Yao KX. Rational design of coaxial mesoporous birnessite manganese dioxide/amorphous-carbon nanotubes arrays for advanced asymmetric supercapacitors. J. Power Sources. 2015;278:555–561. doi: 10.1016/j.jpowsour.2014.12.054. [DOI] [Google Scholar]
- 13.Lozano-Castelló D, et al. Influence of pore structure and surface chemistry on electric double layer capacitance in non-aqueous electrolyte. Carbon N. Y. 2003;41:1765–1775. doi: 10.1016/S0008-6223(03)00141-6. [DOI] [Google Scholar]
- 14.Gupta V, Miura N. Polyaniline/single-wall carbon nanotube (PANI/SWCNT) composites for high performance supercapacitors. Electrochim. Acta. 2006;52:1721–1726. doi: 10.1016/j.electacta.2006.01.074. [DOI] [Google Scholar]
- 15.Wang W, Hao Q, Lei W, Xia X, Wang X. Graphene/SnO2/polypyrrole ternary nanocomposites as supercapacitor electrode materials. RSC Adv. 2012;2:10268. doi: 10.1039/c2ra21292g. [DOI] [Google Scholar]
- 16.Xu J, Wang K, Zu S-Z, Han B-H, Wei Z. Hierarchical Nanocomposites of Polyaniline Nanowire Arrays on Graphene Oxide Sheets with Synergistic Effect for Energy Storage. ACS Nano. 2010;4:5019–5026. doi: 10.1021/nn1006539. [DOI] [PubMed] [Google Scholar]
- 17.Yu G, et al. Enhancing the Supercapacitor Performance of Graphene/MnO2 Nanostructured Electrodes by Conductive Wrapping. Nano Lett. 2011;11:4438–4442. doi: 10.1021/nl2026635. [DOI] [PubMed] [Google Scholar]
- 18.Ding S, Luan D, Boey FYC, Chen JS, Lou XW. (David). SnO2 nanosheets grown on graphene sheets with enhanced lithium storage properties. Chem. Commun. 2011;47:7155. doi: 10.1039/c1cc11968k. [DOI] [PubMed] [Google Scholar]
- 19.Ning G, et al. Three-dimensional hybrid materials of fish scale-like polyaniline nanosheet arrays on graphene oxide and carbon nanotube for high-performance ultracapacitors. Carbon N. Y. 2013;54:241–248. doi: 10.1016/j.carbon.2012.11.035. [DOI] [Google Scholar]
- 20.Zhao L, et al. Nitrogen-Containing Hydrothermal Carbons with Superior Performance in Supercapacitors. Adv. Mater. 2010;22:5202–5206. doi: 10.1002/adma.201002647. [DOI] [PubMed] [Google Scholar]
- 21.Zhu SJ, et al. Rational design of octahedron and nanowire CeO2@MnO2 core–shell heterostructures with outstanding rate capability for asymmetric supercapacitors. Chem. Commun. 2015;51:14840–14843. doi: 10.1039/C5CC03976B. [DOI] [PubMed] [Google Scholar]
- 22.Zhu S, et al. Structural Directed Growth of Ultrathin Parallel Birnessite on β-MnO2 for High-Performance Asymmetric Supercapacitors. ACS Nano. 2018;12:1033–1042. doi: 10.1021/acsnano.7b03431. [DOI] [PubMed] [Google Scholar]
- 23.Xu C, Sun J, Gao L. Controllable synthesis of monodisperse ultrathin SnO2 nanorods on nitrogen-doped graphene and its ultrahigh lithium storage properties. Nanoscale. 2012;4:5425. doi: 10.1039/c2nr31357j. [DOI] [PubMed] [Google Scholar]
- 24.Xu C, Sun J, Gao L. Direct growth of monodisperse SnO2 nanorods on graphene as high capacity anode materials for lithium ion batteries. J. Mater. Chem. 2012;22:975–979. doi: 10.1039/C1JM14099J. [DOI] [Google Scholar]
- 25.Yin XM, et al. One-Step Synthesis of Hierarchical SnO2 Hollow Nanostructures via Self-Assembly for High Power Lithium Ion Batteries. J. Phys. Chem. C. 2010;114:8084–8088. doi: 10.1021/jp100224x. [DOI] [Google Scholar]
- 26.Li F, et al. One-step synthesis of graphene/SnO2 nanocomposites and its application in electrochemical supercapacitors. Nanotechnology. 2009;20:455602. doi: 10.1088/0957-4484/20/45/455602. [DOI] [PubMed] [Google Scholar]
- 27.Pusawale SN, Deshmukh PR, Lokhande CD. Chemical synthesis of nanocrystalline SnO2 thin films for supercapacitor application. Appl. Surf. Sci. 2011;257:9498–9502. doi: 10.1016/j.apsusc.2011.06.043. [DOI] [Google Scholar]
- 28.Sun L, et al. Nitrogen-doped graphene with high nitrogen level via a one-step hydrothermal reaction of graphene oxide with urea for superior capacitive energy storage. RSC Adv. 2012;2:4498. doi: 10.1039/c2ra01367c. [DOI] [Google Scholar]
- 29.Ramesh S, Kim HS, Haldorai Y, Han Y-K, Kim J-H. Fabrication of nanostructured MnO2/carbon nanotube composite from 3D precursor complex for high-performance supercapacitor. Mater. Lett. 2017;196:132–136. doi: 10.1016/j.matlet.2017.03.044. [DOI] [Google Scholar]
- 30.Duraivel M, et al. Superior one-pot synthesis of a doped graphene oxide electrode for a high power density supercapacitor. New J. Chem. 2018;42:11093–11101. doi: 10.1039/C8NJ01672K. [DOI] [Google Scholar]
- 31.Selvan Ramakrishnan Kalai, Perelshtein Ilana, Perkas Nina, Gedanken Aharon. Synthesis of Hexagonal-Shaped SnO2Nanocrystals and SnO2@C Nanocomposites for Electrochemical Redox Supercapacitors. The Journal of Physical Chemistry C. 2008;112(6):1825–1830. doi: 10.1021/jp076995q. [DOI] [Google Scholar]
- 32.Chen Y-J, Zhu C-L, Xue X-Y, Shi X-L, Cao M-S. High capacity and excellent cycling stability of single-walled carbon nanotube/SnO2 core-shell structures as Li-insertion materials. Appl. Phys. Lett. 2008;92:223101. doi: 10.1063/1.2937839. [DOI] [Google Scholar]
- 33.Xia H, Wang Y, Lin J, Lu L. Hydrothermal synthesis of MnO2/CNT nanocomposite with a CNT core/porous MnO2 sheath hierarchy architecture for supercapacitors. Nanoscale Res. Lett. 2011;6:595. doi: 10.1186/1556-276X-6-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saravanakumar B, Purushothaman KK, Muralidharan G. Interconnected V2O5 Nanoporous Network for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces. 2012;4:4484–4490. doi: 10.1021/am301162p. [DOI] [PubMed] [Google Scholar]
- 35.Zhao B, et al. Bivalent tin ion assisted reduction for preparing graphene/SnO2 composite with good cyclic performance and lithium storage capacity. Electrochim. Acta. 2011;56:7340–7346. doi: 10.1016/j.electacta.2011.06.037. [DOI] [Google Scholar]
- 36.Wang S, Jiang SP, Wang X. Microwave-assisted one-pot synthesis of metal/metal oxide nanoparticles on graphene and their electrochemical applications. Electrochim. Acta. 2011;56:3338–3344. doi: 10.1016/j.electacta.2011.01.016. [DOI] [Google Scholar]
- 37.Ramesh S, Kathalingam A, Karuppasamy K, Kim H-S, Kim HS. Nanostructured CuO/Co2O4@ nitrogen doped MWCNT hybrid composite electrode for high-performance supercapacitors. Compos. Part B Eng. 2019;166:74–85. doi: 10.1016/j.compositesb.2018.11.116. [DOI] [Google Scholar]
- 38.Winter Martin, Brodd Ralph J. What Are Batteries, Fuel Cells, and Supercapacitors? Chemical Reviews. 2004;104(10):4245–4270. doi: 10.1021/cr020730k. [DOI] [PubMed] [Google Scholar]
- 39.Augustyn V, Simon P, Dunn B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014;7:1597. doi: 10.1039/c3ee44164d. [DOI] [Google Scholar]
- 40.Hwang S-W, Hyun S-H. Synthesis and characterization of tin oxide/carbon aerogel composite electrodes for electrochemical supercapacitors. J. Power Sources. 2007;172:451–459. doi: 10.1016/j.jpowsour.2007.07.061. [DOI] [Google Scholar]
- 41.Zhang LL, Zhou R, Zhao XS. Graphene-based materials as supercapacitor electrodes. J. Mater. Chem. 2010;20:5983. doi: 10.1039/c000417k. [DOI] [Google Scholar]
- 42.Lei Z, Zhang J, Zhao XS. Ultrathin MnO2 nanofibers grown on graphitic carbon spheres as high-performance asymmetric supercapacitor electrodes. J. Mater. Chem. 2012;22:153–160. doi: 10.1039/C1JM13872C. [DOI] [Google Scholar]
- 43.Algharaibeh Z, Liu X, Pickup PG. An asymmetric anthraquinone-modified carbon/ruthenium oxide supercapacitor. J. Power Sources. 2009;187:640–643. doi: 10.1016/j.jpowsour.2008.11.012. [DOI] [Google Scholar]
- 44.Yan X, Tai Z, Chen J, Xue Q. Fabrication of carbon nanofiber–polyaniline composite flexible paper for supercapacitor. Nanoscale. 2011;3:212–216. doi: 10.1039/C0NR00470G. [DOI] [PubMed] [Google Scholar]
- 45.Liu Y, et al. Enhanced electrochemical performance of hybrid SnO2@MOx (M = Ni, Co, Mn) core–shell nanostructures grown on flexible carbon fibers as the supercapacitor electrode materials. J. Mater. Chem. A. 2015;3:3676–3682. doi: 10.1039/C4TA06339B. [DOI] [Google Scholar]
- 46.Sun J, et al. 3D core/shell hierarchies of MnOOH ultrathin nanosheets grown on NiO nanosheet arrays for high-performance supercapacitors. Nano Energy. 2014;4:56–64. doi: 10.1016/j.nanoen.2013.12.006. [DOI] [Google Scholar]
- 47.Xia X, et al. Self-supported hydrothermal synthesized hollow Co3O4 nanowire arrays with high supercapacitor capacitance. J. Mater. Chem. 2011;21:9319. doi: 10.1039/c1jm10946d. [DOI] [Google Scholar]
- 48.Lim SP, Huang NM, Lim HN. Solvothermal synthesis of SnO2/graphene nanocomposites for supercapacitor application. Ceram. Int. 2013;39:6647–6655. doi: 10.1016/j.ceramint.2013.01.102. [DOI] [Google Scholar]
- 49.Zhang M, et al. Fast synthesis of SnO2/graphene composites by reducing graphene oxide with stannous ions. J. Mater. Chem. 2011;21:1673–1676. doi: 10.1039/C0JM03410J. [DOI] [Google Scholar]
- 50.He C, et al. Mosaic-Structured SnO2@C Porous Microspheres for High-Performance Supercapacitor Electrode Materials. Electrochim. Acta. 2014;142:157–166. doi: 10.1016/j.electacta.2014.07.077. [DOI] [Google Scholar]
- 51.Wang R, Xu C, Sun J, Gao L, Yao H. Solvothermal-Induced 3D Macroscopic SnO2/Nitrogen-Doped Graphene Aerogels for High Capacity and Long-Life Lithium Storage. ACS Appl. Mater. Interfaces. 2014;6:3427–3436. doi: 10.1021/am405557c. [DOI] [PubMed] [Google Scholar]
- 52.Lu T, et al. Electrochemical behaviors of graphene–ZnO and graphene–SnO2 composite films for supercapacitors. Electrochim. Acta. 2010;55:4170–4173. doi: 10.1016/j.electacta.2010.02.095. [DOI] [Google Scholar]
- 53.Wang Yong, Lee Jim Yang, Zeng Hua Chun. Polycrystalline SnO2Nanotubes Prepared via Infiltration Casting of Nanocrystallites and Their Electrochemical Application. Chemistry of Materials. 2005;17(15):3899–3903. doi: 10.1021/cm050724f. [DOI] [Google Scholar]
- 54.Cui H, Liu Y, Ren W, Wang M, Zhao Y. Large scale synthesis of highly crystallized SnO2 quantum dots at room temperature and their high electrochemical performance. Nanotechnology. 2013;24:345602. doi: 10.1088/0957-4484/24/34/345602. [DOI] [PubMed] [Google Scholar]
- 55.Gao Y, et al. SO42−/SnO2 as a new electrode for electrochemical supercapacitors. Ceram. Int. 2014;40:8925–8929. doi: 10.1016/j.ceramint.2014.01.047. [DOI] [Google Scholar]
- 56.Pusawale SN, Deshmukh PR, Lokhande CD. Chemical synthesis and characterization of hydrous tin oxide (SnO2:H2O) thin films. Bull. Mater. Sci. 2011;34:1179–1183. doi: 10.1007/s12034-011-0168-3. [DOI] [Google Scholar]
- 57.Vinoth V, et al. SnO2-decorated multiwalled carbon nanotubes and Vulcan carbon through a sonochemical approach for supercapacitor applications. Ultrason. Sonochem. 2016;29:205–212. doi: 10.1016/j.ultsonch.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 58.Rakhi RB, Alshareef HN. Enhancement of the energy storage properties of supercapacitors using graphene nanosheets dispersed with metal oxide-loaded carbon nanotubes. J. Power Sources. 2011;196:8858–8865. doi: 10.1016/j.jpowsour.2011.06.038. [DOI] [Google Scholar]
- 59.Hsieh C-T, Lee W-Y, Lee C-E, Teng H. Electrochemical Capacitors Fabricated with Tin Oxide/Graphene Oxide Nanocomposites. J. Phys. Chem. C. 2014;118:15146–15153. doi: 10.1021/jp502958w. [DOI] [Google Scholar]