Abstract
We present a paroxysmal atrial fibrillation (AF) patient who had frequent AF events originating from a persistent left superior vena cava (PLSVC) with an unsuccessful catheter ablation using a non-irrigated radiofrequency catheter and was successfully cured by a successful PLSVC isolation using a second-generation cryoballoon catheter (28-mm, second-generation cryoballoon, Arctic Front Advance, Medtronic, Minneapolis, MN, USA).
<Learning objective: This is the first case report illustrating a successful ablation of a persistent left superior vena cava in a patient with drug-resistant atrial fibrillation (AF) using a second-generation cryoballoon (CB) with a good outcome. By choosing an appropriate position and attaching the CB fixedly, the CB ablation was able to cure the AF smoothly without any complications including left phrenic nerve palsy or left coronary artery stenosis. An intracardiac electrocardiogram and 3-dimensional mapping system could detect conducted ectopic beats initiating AF.>
Keywords: Persistent left superior vena cava, Atrial fibrillation, Cryoballoon ablation, Persistent left superior vena cava isolation, No complication
Introduction
Catheter ablation is an established therapy for drug-resistant paroxysmal atrial fibrillation (AF). Cryoballoon (CB) ablation is also an effective therapy for AF originating from the pulmonary veins (PVs) [1]. In addition, the second-generation CB catheter is more effective than the first-generation [2]. It is necessary to avoid phrenic nerve (PN) injury (PNI) during CB ablation. PNI complications can occur with CB ablation of the right-sided PVs, but rarely develop with left-sided PVs [3], [4]. However, PNI can be prevented with low output stimulation from each subclavian vein [5].
In general, it is not easy to cure AF from non-PV origins by ablation such as from the posterior left atrium and persistent left superior vena cava (PLSVC). With the first-generation CB catheter, there was a report that a PLSVC isolation (PLSVC-I) was performed [6], but to the best of our knowledge, there have still been no reports that have evaluated the second-generation balloon for a PLSVC-I.
Case report
A 55-year-old Japanese woman was admitted to our institution, who had symptomatic, drug-refractory paroxysmal AF, and hoped for a radical cure by ablation. She had a history of hypertension and dyslipidemia. Structural heart disease was not detected by echocardiography, but a PLSVC was observed in the echocardiography before the ablation. In the first session, she underwent a PV isolation (PVI) and cavotricuspid isthmus ablation under conscious sedation with propofol and dexmedetomidine using a non-irrigated tip catheter. Radiofrequency current was delivered with an 8-mm-tip non-irrigated ablation catheter (Ablaze; Japan Lifeline, Inc., Tokyo, Japan). In addition, a PLSVC (maximum diameter 30.2 mm) isolation (PLSVC-I) was also performed using the same catheter because AF was repeatedly confirmed to be induced from the PLSVC using high dose isoproterenol. However, we could not completely perform the PLSVC-I using a non-irrigated radiofrequency catheter (maximum power 35 W, maximum duration 30 s). Since she had palpitations due to frequent AF attacks under beta-blockers and antiarrhythmic drugs after the 1st session, we performed a 2nd session using a second-generation CB catheter four years after the 1st session.
A 6-Fr 8-pole or 4-pole 3-site mapping catheter (BeeAT; Japan Lifeline Co. Ltd.) was inserted through the right jugular vein and positioned in the coronary sinus for pacing, recording, and internal cardioversion. Two 5-Fr circular mapping catheters (Libero; Japan Lifeline Co. Ltd. or AFocus II; Abbott/St. Jude Medical, Minneapolis, MN, USA) through 2 long sheaths, were placed in the PVs and PLSVC, respectively. We confirmed reconnections from 3 PVs excluding the right superior PV. Radiofrequency current was delivered with a 4-mm irrigated tip ablation catheter (FlexAbility™; Abbott) through another long sheath. A few attempts were successfully performed for the PVI under the guidance of an EnSite Precision™ Cardiac mapping System (Abbott) with a 3-dimensional geometric reconstruction of the left atrium, PVs, and PLSVC from the multidetector computed tomography images. Furthermore, AF was reproducibly initiated by an atrial premature contraction originating from the PLSVC during an isoproterenol infusion at 15 μg/min before the ablation (Fig. 1). Since we failed the PLSVC-I procedure with a conventional catheter previously, we chose a CB catheter this time.
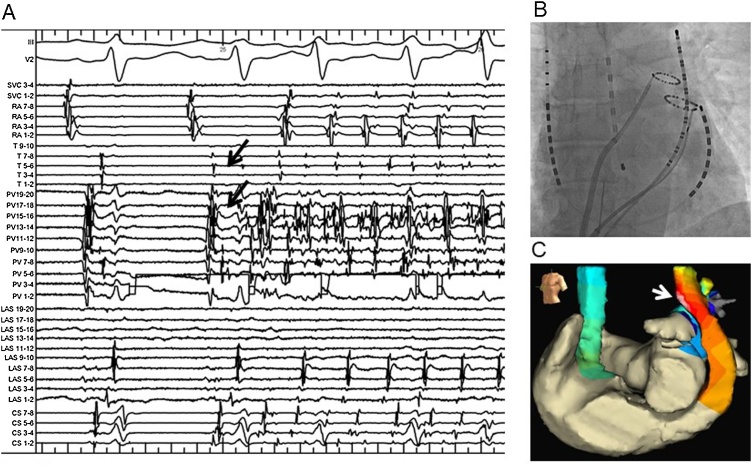
Fig. 1.
Recording of an intracardiac electrocardiogram identifying an APC originating from the PLSVC. (A) Reproducible AF initiated by an APC (arrows) originating from the PLSVC was identified during an isoproterenol infusion at 15 μg/min before the ablation. (B) A large size ring catheter and 20-pole catheter are placed within the distal PLSVC and another small size ring catheter is placed in the LSPV. A temperature monitoring catheter is placed in the esophagus. (C) An activation map is displayed on the three-dimensional reconstructed geometries of the LA, PLSVC, and RA on the EnSite™ NavX™ mapping system in the left anterior oblique projection before the ablation. The distal PLSVC anterior wall was the earliest activation site at the onset of AF (white arrow).
AF, atrial fibrillation; APC, atrial premature contraction; PLSVC, persistent left superior vena cava; LSPV, left superior pulmonary vein; LA, left atrium; RA, right atrium.
A spiral mapping catheter (Achieve, Medtronic, Minneapolis, MN, USA) was placed in the PLSVC and the pacing threshold of the left-sided PN was measured before the CB ablation. During freezing, the PN was paced at maximum output (max, 10.0 V for 1 ms). A complete occlusion by the CB catheter was confirmed with a contrast injection in the PLSVC (Fig. 2). The first and second freezing attempts were terminated due to an insufficient temperature drop because of decreased PN capture (231 and 32 s, minimum temperature of −48, 43 ℃). By moving the CB catheter slightly downward, the pacing threshold and contact of the CB catheter improved. We smoothly performed a successful PLSVC isolation without any complications (240 s, minimum temperature of −56 ℃). Dissociated spikes were also observed in the PLSVC after the PLSVC-I (Fig. 3). The PN function was never damaged by the PLSVC ablation (total three applications, 513 s). Finally, AF was no longer inducible after the elimination of the PLSVC potentials. We did not perform any additional ablation. No complications occurred such as left PNI and/or coronary spasms. In addition, we finally confirmed the absence of PLSVC stenosis using PLSVC venography. Over one year had passed from the last ablation, and no AF has been confirmed with frequent Holter electrocardiograms. In addition, confirmation of the absence of stenosis of the re-isolated veins and coronary arteries was performed by computed tomographic angiography.
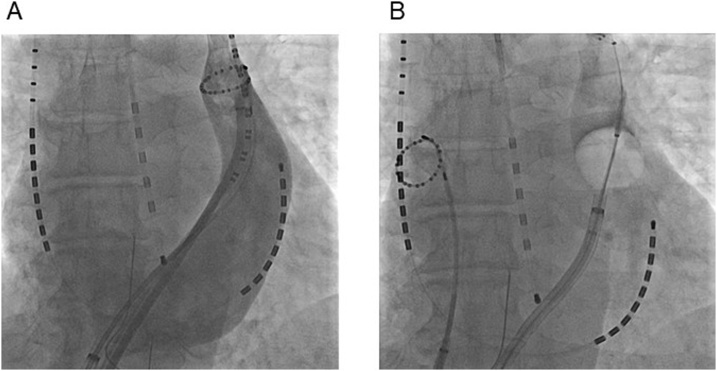
Fig. 2.
The cryoballoon catheter in the PLSVC. (A) Selective venography of the PLSVC in the anterior posterior projection. A ring catheter and 20-pole catheter are placed within the distal PLSVC. (B) The cryoballoon catheter is positioned at the level of the mid-PLSVC. We visualized the pooling of contrast agent at the successful ablation site.
PLSVC, persistent left superior vena cava.
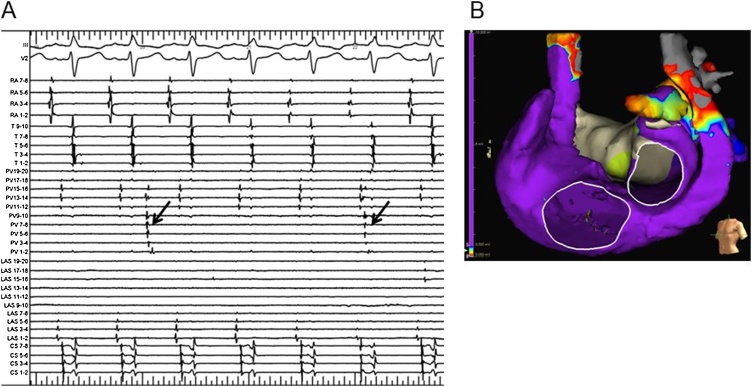
Fig. 3.
Recording of the intracardiac electrocardiogram and voltage map after ablation. (A) Dissociated spikes (arrows) were observed in the PLSVC after the PLSVC isolation using the 28-mm cryoballoon. AF was not induced at all after the PLSVC isolation. (B) After the PLSVC isolation, a voltage map was displayed on the three-dimensional reconstructed geometries of the LA, PLSVC, and RA on the EnSite™ NavX™ mapping system in the left anterior oblique projection during sinus rhythm. The distal PLSVC exhibited low voltage areas.
AF, atrial fibrillation; PLSVC, persistent left superior vena cava; LA, left atrium; RA, right atrium.
Discussion
A PLSVC is the most common congenital malformation of the thoracic venous return and is present in 0.3–0.5% of individuals in the general population [7]. Despite the rare malformations, there is a high probability of observing patients with a PLSVC when ablating AF.
In 2004, Hsu et al. first reported the demonstration of AF originating from a PLSVC and the electrical isolation of that area [8]. In fact, four patients (0.5%) had PLSVCs among 844 AF patients and three (75%) of the four patients developed AF from a PLSVC origin in our institute. We performed a successful ablation in all except for this case.
In clinical practice, some cases undergo a challenging ablation. Wissner et al. [6] demonstrated that it was not easy to isolate the PLSVC. This patient had an AF recurrence after the first ablation partly due to the failure of the PLSVC isolation. We believe that one of the causes of the unsuccessful ablation was that we used a non-irrigated catheter, but some cases in which the treatment was difficult existed due to the size and natural shape of the PLSVC. However, the association of the size of the PLSVC and PLSVC-I success rate are still unclear. Remarkably, it is important to know which part of the PLSVC is best to isolate it. They have already experienced two cases with a first-generation CB ablation of the PLSVC, but they could not achieve an effective CB ablation to eliminate the AF [6].
Furthermore, we could perform a successful ablation without any complications using the advice from past reports [4], [5]. In fact, the CB ablation was immediately discontinued using a double-stop technique to avoid PN palsy [9].
In this case, we could repeatably confirm AF originating from the same site of the PLSVC, and, as a result, this patient was successfully ablated by CB ablation. In addition, the success was related to the AF origin being on the distal side, which was smaller than the diameter of the balloon. From our experience in this case, we believe that CB ablation at a high position in the PLSVC might cause PNI. Further, we were able to prove that with the Ensite NavX and intracardiac electrocardiograms.
Therefore, in order to avoid injury to the left PN and left coronary artery, we suggest performing an effective PLSVC isolation using a CB as one of the treatment strategies. It is a matter of course, that it is necessary to gain experience with CB ablation and understand the shape of the PLSVC in individual cases.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Kuck K.H., Brugada J., Fürnkranz A., Metzner A., Ouyang F., Chun K.R. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. 2016;374:2235–2245. doi: 10.1056/NEJMoa1602014. [DOI] [PubMed] [Google Scholar]
- 2.Greiss H., Berkowitsch A., Wojcik M., Zaltsberg S., Pajitnev D., Deubner N. The impact of left atrial surface area and the second generation cryoballoon on clinical outcome of atrial fibrillation cryoablation. Pacing Clin Electrophysiol. 2015;38:815–824. doi: 10.1111/pace.12637. [DOI] [PubMed] [Google Scholar]
- 3.Ghosh J., Singarayar S., Kabunga P., McGuire M.A. Subclavian vein pacing and venous pressure waveform measurement for phrenic nerve monitoring during cryoballoon ablation of atrial fibrillation. Europace. 2015;17:884–890. doi: 10.1093/europace/euu341. [DOI] [PubMed] [Google Scholar]
- 4.Andrade J.G., Khairy P., Guerra P.G., Deyell M.W., Rivard L., Macle L. Efficacy and safety of cryoballoon ablation for atrial fibrillation: a systematic review of published studies. Heart Rhythm. 2011;8:1444–1451. doi: 10.1016/j.hrthm.2011.03.050. [DOI] [PubMed] [Google Scholar]
- 5.Okishige K., Aoyagi H., Kawaguchi N., Katoh N., Yamashita M., Nakamura T. Novel method for earlier detection of phrenic nerve injury during cryoballoon applications for electrical isolation of pulmonary veins in patients with atrial fibrillation. Heart Rhythm. 2016;13:1810–1816. doi: 10.1016/j.hrthm.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Wissner E., Tilz R., Konstantinidou M., Metzner A., Schmidt B., Chun K.R. Catheter ablation of atrial fibrillation in patients with persistent left superior vena cava is associated with major intraprocedural complications. Heart Rhythm. 2010;7:1755–1760. doi: 10.1016/j.hrthm.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Tyrak K.W., Holda J., Holda M.K., Koziej M., Piatek K., Klimek-Piotrowska W. Persistent left superior vena cava. Cardiovasc J Afr. 2017;28:e1–4. doi: 10.5830/CVJA-2016-084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu L.F., Jaïs P., Keane D., Wharton J.M., Deisenhofer I., Hocini M. Atrial fibrillation originating from persistent left superior vena cava. Circulation. 2004;109:828–832. doi: 10.1161/01.CIR.0000116753.56467.BC. [DOI] [PubMed] [Google Scholar]
- 9.Gooch C.L., Weimer L.H. The electrodiagnosis of neuropathy: basic principles and common pitfalls. Neurol Clin. 2007;25:1–28. doi: 10.1016/j.ncl.2007.01.011. [DOI] [PubMed] [Google Scholar]