Abstract
BACKGROUND
Hepatocellular carcinoma (HCC) appears in most of cases in patients with advanced liver disease and is currently the primary cause of death in this population. Surveillance of HCC has been proposed and recommended in clinical guidelines to obtain earlier diagnosis, but it is still controversial and is not accepted worldwide.
AIM
To review the actual evidence to support the surveillance programs in patients with cirrhosis as well as the diagnosis procedure.
METHODS
Systematic review of recent literature of surveillance (tools, interval, cost-benefit, target population) and the role of imaging diagnosis (radiological non-invasive diagnosis, optimal modality and agents) of HCC.
RESULTS
The benefits of surveillance of HCC, mainly with ultrasonography, have been assessed in several prospective and retrospective analysis, although the percentage of patients diagnosed in surveillance programs is still low. Surveillance of HCC permits diagnosis in early stages allows better access to curative treatment and increases life expectancy in patients with cirrhosis. HCC is a tumor with special radiological characteristics in computed tomography and magnetic resonance imaging, which allows highly accurate diagnosis without routine biopsy confirmation. The actual recommendation is to perform biopsy only in indeterminate nodules.
CONCLUSION
The evidence supports the recommendation of performing surveillance of HCC in patients with cirrhosis susceptible of treatment, using ultrasonography every 6 mo. The diagnosis evaluation of HCC can be established based on noninvasive imaging criteria in patients with cirrhosis.
Keywords: Surveillance, Hepatocellular carcinoma, Ultrasonography, Cirrhosis, Imaging diagnosis
Core tip: Hepatocellular carcinoma is one of the tumors with the worst prognosis and the 5-year survival is discouraging. The advantages of surveillance of hepatocellular carcinoma in patients with cirrhosis remains controversial, but the best strategy considered is to diagnose the tumor in early stage, which gives the opportunity to access better curative treatment. The current review will focus on the more recent available evidence about surveillance and diagnosis of hepatocellular carcinoma.
INTRODUCTION
Primary liver cancer is the 6th most commonly diagnosed cancer and was the 4h cause of cancer death worldwide in 2018, including hepatocellular carcinoma (75%-85%) and intrahepatic cholangiocarcinoma (10%-15%)[1]. In 2018, 841,080 new cases of hepatocellular carcinoma (HCC) were diagnosed (4.7% of all new cases of cancer) and 781,631 patients died of this disease. It is more common in men and is currently the 2nd leading cause of cancer death worldwide in men and the 6th in women[2]. According to data from the surveillance, epidemiology and end results program, the 5-year survival for liver cancer is only 18%[3].
Incidence, mean age at diagnosis, and risk factors for HCC vary regionally according to the prevalence of hepatitis B virus (HBV) and hepatitis C virus (HCV)[4,5]. However, the increasing incidence of non-alcoholic fatty liver disease (NAFLD) in high socioeconomic countries, the viral load suppression with chronic antiviral treatment of HBV, the high rates of HCV curation with the new direct-acting antiviral (DAA) therapy as well as HBV vaccination programs could change this paradigm in the next decades[6].
The improvement in cirrhotic patient care and better management of clinical complications associated with chronic liver disease in the last years has led to a sustained decrease in mortality, and currently HCC development is the most severe and life threatening complication in these patients[7-9]. Consequently, any action carried out to improve the prognosis of patients with end stage liver disease, must take into account early diagnosis of this cancer. In fact, HCC accomplishes the recommendation for surveillance programs established by the World Health Organization: it is an important health problem with high morbidity and mortality. The target population is clearly defined, the diagnosis test is easy to apply and there is a well-designed and accepted diagnosis process. Also, the diagnosis in early stages of the tumor allows access to curative treatment and a better prognosis.
MATERIALS AND METHODS
In this first part of the chapter, we review the evidence available on surveillance of HCC in patients with chronic liver disease according to the etiology and fibrosis status, the cost-effectiveness of such programs, and the impact in survival of surveillance. In the second part of the article, we review the diagnosis tools in these patients.
The review was conducted using the Preferred Reported Items for Systematic Reviews guidelines. A computer-aided systematic literature search of PubMed and Scopus databases was performed. This review has been divided into two different parts: screening and diagnosis. The development of this article was conducted by members of the multidisciplinary team for diagnosis and evaluation of HCC at our hospital (two hepatologists and three radiologists). The literature search was carried out by each author according to the part of the paper assigned to each physician. The combination of keywords in the first part were as follows: “Screening AND/OR Surveillance AND Hepatocellular Carcinoma” and for the second part: “Diagnosis AND Hepatocellular Carcinoma AND LIRADS”. In addition, the references of the more relevant studies (excluding case reports and articles in non-English languages) and specially the review and meta-analysis articles were manually searched to identify additional studies not detected in the previous selection.
In the first step, the title and abstract of each identified record was screened in order to explore the accuracy of the search and select only those really related to the topics. After this, the final list of selected articles was retrieved as full text for detailed assessment.
According to the topic assigned to each author, the most appropriate studies were selected to carry out the specific part of the review.
RESULTS
Survival advantages of surveillance diagnosis
The efficacy of any medical procedure should be based on objective data extracted from randomized and controlled studies. In the case of the hypothetical efficacy of surveillance programs in HCC, only two studies have assessed this aspect, both performed in Asia, and both in carriers of hepatitis B surface antigen (HBsAg). In the first, the screening test used was the determination of alpha-fetoprotein (AFP) every 6 mo in the study group (n = 3712) versus a control group without follow-up (n = 1869). Despite earlier diagnosis in the screening group, there were no differences in 5-year survival between the groups[10].
In the latter study, in the screening group (n = 9373), an ultrasonography (US) performed as well as an AFP have been performed every 6 mo comparing with control group without intervention (n = 9373). Despite a low adherence of 60%, it showed an improvement in survival in the screening group, achieving a reduction in mortality to approximately 37%[11].
To date, no other study carried out in this context (Asian HBsAg-carrying patients) has evaluated the profitability of screening and therefore, these data have not been able to be extrapolated to other populations (e.g., western countries), as in other causes of chronic liver disease. This could be explained because the approach of a study of these characteristics (surveillance vs no surveillance) faces the refusal of the patients to sign an informed consent that includes the possibility of being part of the control group, as has been described in an article published a few years ago[12].
Given the absence of evidence with prospective series, several studies have tried to demonstrate the effectiveness of screening indirectly. American series reported that surveillance is associated with improved early stage detection, curative treatments and survival, despite adherence rates as low as less than 20%[13,14]. A recent meta-analysis of studies published between 1990 and 2014, including abstracts presented in congresses from 2009 to 2012, identified a total of 45 original articles (most of them retrospective, n = 38) that included a total of 15,158 patients with HCC, of which 41% had been diagnosed in screening programs. In most of the studies, the surveillance test employed was a combination of US and AFP (n = 39) and was conducted in Europe (n = 13), America (n = 15), and Asia (n = 15). This meta-analysis confirmed that these patients had tumors diagnosed in earlier stages of the disease, with greater possibility of curative treatment and better survival[15].
It must be taken into account that observational studies, especially in the setting of screening tools, have important bias that can confound assessment of screening test efficacy, that include lead-time bias (apparent improving survival because of an anticipated diagnosis), that can be minimized using correction formulas and length time bias (overrepresentation of slower growing tumors), that is inherent to this kind of study.
Based on the available evidence, the last published clinical practice guidelines from AASLD and EASL recommend the conduct of surveillance in patients with liver cirrhosis, with a moderate evidence level and a strong recommendation[16,17].
A more recent meta-analysis published in 2018, which included 19511 patients from 22 studies, showed an overall real world adherence rate to HCC surveillance imaging every 6-12 mo in 52%, better than previously reported[18]. The authors observed that the prospective studies had an adherence rate of 71%, when compared with 39% of retrospective studies, suggesting that being aware of surveillance may have a positive effect on adherence rates. On the other hand, they did not identify any other factor related to HCC adherence (geographical area, etiology of liver disease, surveillance test or interval). Nevertheless, another study comparing HCC survival in Japan (with intensive national surveillance program, n = 1174) versus Hong Kong (none program, n = 1675) over similar time periods (Japan 2000–2013, Hong Kong, China 2003–2014) showed that in Japan over 75% of cases are currently detected by surveillance, whereas in Hong Kong less than 20%. Median survival was 52 mo in Japan and 17.8 mo in Hong Kong; this survival advantage persisted after allowance for lead-time bias. A total of 63% of Japanese patients had early disease at diagnosis and 63% received curative treatment versus 31.7% and 44.1%, respectively in Hong-Kong. This suggests a clear benefit of the surveillance program[19].
In United States population, (a country where surveillance remains controversial), in real world, a matched case-control study carried out in the Veterans Affairs health care system, surveillance with ultrasound (US) and AFP was not associated with decreased HCC-related mortality[20].
Table 1 summarizes the more recent, retrospective studies, published in the last years about surveillance of HCC in real life, and not included in previous metanalyses. As shown in the table, surveillance adherence remains low, both in Europe and the United States.
Table 1.
Studies about the advantages and results of surveillance in HCC
| Ref. |
Location |
Inclusion period | n | Screening (%) | Results surveillance group |
| UNI/MULTI | |||||
| Edenvik et al[21] | Sweden UNI | 2005-2012 | 616 | 22% | Better survival |
| van Meer et al[22] | Netherlands MULTI | 2005-2012 | 1074 | 27% | Smaller tumor size, earlier tumor stage, more often curative treatment and improving 1, 3, 5 years survival rates |
| Singal et al[23] | United States MULTI | 2012-2013 | 374 | 42% | Early tumor detection and improved survival |
| Mittal et al[24] | United States MULTI | 2005-2010 | 887 | 46.5% | Reduction in mortality |
| Atiq et al[25] | United States UNI | 2010-2013 | 680 | 11.5% | Early HCC |
UNI: Uni-center; MULTI: Multicenter; HCC: Hepatocellular carcinoma.
Cost-effectiveness of surveillance programs
As we have explained, HCC is a potential target for cancer surveillance as it occurs in well-defined at-risk populations, and curative therapy is possible when small tumors are diagnosed. Surveillance has been recommended by regional and national liver societies all over the world and is practiced widely. However, there is a lack of randomized controlled trials in real settings that could help to address the incidence from which the surveillance should be applied because it is the key parameter which determines the cost-effectiveness of HCC screening[26].
Most studies use decision models (Markov chain or decision tree), which usually include the full economic evaluation of HCC screening programs, a comparison between HCC techniques, and the outcome measures expressed in terms of quality adjusted life years[27-32]. In general, a screening strategy is likely to be cost-effective in every setting considered, and a semiannual surveillance has been shown to be the most cost effective timing strategy.
Discrepancies in the results exist when determining the type of technology to be used. US alone or in association with AFP technology is likely to be the most cost-effective, and the use of computed tomography (CT) shows controversial results. Screening should be implemented to detect HCC at an early stage of cirrhosis and is likely not cost-effective in advanced HCC or after liver transplantation[33].
Optimal surveillance interval
The interval between screening examinations for HCC has been established based on both the tumor growth rate and the tumor incidence in the target population and its cost-effectiveness[17]. In studies carried out on the growth rate of untreated HCC, the time of duplication of tumor size is variable depending on factors such as their degree of differentiation[34]. Currently, the recommended interval between scans for screening is 6 mo. This strategy increases the detection of small size lesions compared with the annual screening, in which curative treatments can be applied more frequently with greater patient survival, and has proved to be cost-effective[27,35]. It does not seem that shortening to 3-mo screening interval improves the detection rate of small HCC (candidates for more radical treatments) or that it has an impact on survival over screening every 6 mo[36].
Surveillance tools
HCC screening includes imaging techniques and biomarkers.
Radiological: US: It is the most used test for the screening of HCC due to its wide availability, non-invasiveness, acceptable diagnostic accuracy, and cost. In addition, US provides additional information useful for the monitoring and assessment of the cirrhotic patient such as the appearance of ascites and portal thrombosis, but has the limitation of being an operator-dependent technique. Although it is difficult to establish its sensitivity and specificity due to the heterogeneity of the studies and their limitations, a meta-analysis that included 13 studies and 3571 patients found a sensitivity and specificity of 94% for the detection of HCC[37]. However, this sensitivity is lower (63%) when it comes to lesions in early stages. Sensitivity of US can be affected by certain conditions such as obesity, the presence of ascites, or very advanced liver disease, so in some cases it may be necessary to use alternative techniques[38].
CT and magnetic resonance imaging: They are useful for the diagnosis of liver lesions, but in terms of screening tests, they are not cost-effective. Although these are more sensitive tests for the detection of lesions, especially in early stages, this greater sensitivity does not justify in most cases the increase in cost[27]. It does not seem that annual CT or magnetic resonance imaging (MRI) is preferable to US every 6 mo given the estimated doubling time of HCC. Apart from the cost, there are other disadvantages in these techniques that limit their usefulness as screening tests such as radiation, the risk of nephrotoxicity, allergic reactions by CT contrast, the availability of MRI equipment in some centers, the duration of the MR as well as the discomfort and the use of contrasts with gadolinium. However, in patients in whom US assessment is difficult and may have a low sensitivity, the benefit of using alternative techniques such as CT or MRI and as well as its periodicity should be individualized.
Biomarkers: Serum biomarkers cannot be used alone for HCC surveillance because of their relatively low sensitivity and specificity. However, combined with imaging techniques, they can increase the sensitivity although this increases false-positive results.
AFP: It is the most studied biomarker of HCC. A positive result is considered to be higher than 20 ng/mL, although with these values it has a relatively low specificity and with levels above 200 ng/mL the technique has a high specificity, but a low sensitivity. According to the results of some studies, adding the determination of AFP to the imaging controls could increase the sensitivity to an additional 6%-8% of in the detection of HCC in in early stages, at the expense of slightly decreasing the specificity[39,40]. This low yield of AFP is due to the fact that in certain chronic liver diseases, altered levels of this molecule can be observed without relation to HCC and only 10%-20% of HCC in initial stages has high values[17]. In addition, adding AFP to US significantly increases the cost of screening[27]. For all these reasons, a categorical recommendation of adding AFP to the imaging test cannot be established.
Although studies that demonstrate an increase in survival with the addition of AFP are lacking, since it could improve the detection of early lesions (and therefore susceptible to a more radical treatment) and the fact that a progressive increase in this determination in semi-annual controls may increase the suspicion of HCC, the decision to add AFP to imaging tests should be individualized[39].
Currently, the main guidelines do not establish a clear recommendation on its use. While the EASL guide does not recommend adding AFP to image screening, the AASLD guideline recommends screening with or without AFP along with the semester imaging test, so its use should be individualized[16,17].
Other biomarkers: There are other markers, des-gamma-carboxy prothrombin (DCP), the ratio of glycosylated AFP (L3 fraction) to total AFP and others, but for now they cannot be recommended as a screening technique. DCP and AFP-L3 have been associated with advanced HCC and portal vein invasion, but currently cannot be recommended as a screening technique, because none of them have been adequately studied as surveillance tests[17].
Populations
Screening should be performed in populations considered to be high risk. It is established that screening is cost-efficient in cirrhotic patients with a risk of developing HCC of 1.5% per year or more and in non-cirrhotic HBV patients with a risk of 0.2% per year or more[17]. There are populations whose risk is not clearly established, such as non-cirrhotic NASH patients or patients with HCV who have reached a sustained viral response.
Cirrhosis/advanced fibrosis: Almost 80% of HCC develop on cirrhotic livers by any etiology. The studies carried out suggest a cost-effective screening for cirrhotic patients with a risk of developing HCC of 1.5% per year or more. This risk is equal or greater in cirrhotic patients by any etiology, in which this strategy would be beneficial. There are diseases such as cirrhosis of autoimmune origin in which although in several studies the incidence of HCC is less than 1.5% per year, a meta-analysis that included more than 6,000 patients obtained an annual incidence of 1.007%, but the 95% confidence interval (CI) was up to 1.47% per year[41,42]. Table 2 shows the observed incidences of HCC according to their etiology.
Table 2.
Annual incidence of HCC in cirrhotic patients by etiology
| Ref. | Location | n | Follow-up period | Study design | Incidence |
| UNI/MULTI | |||||
| Tansel et al[42] | North America, Europe, Asia, Australia. MULTI | 6528 | Median 8 yr | Meta-analysis | 1.007% (95%CI: 0.69–1.47) |
| Fattovich et al[43] | Europe MULTI | 297 | Median 66 yr | Retrospective | 2.2% for hepatitis B virus and 2.5% for hepatitis C virus |
| Mancebo et al[44] | Spain UNI | 450 | Median 42 mo | Prospective | 2.6% |
| Shibuya et al[45] | Japan MULTI | 396 (134 stage III or IV) | Median 43 mo | Prospective | 1.5% for PBC stage III/IV |
UNI: Uni-center; MULTI: Multicenter.
In patients with advanced liver disease (Child C or Child B patients with massive ascites or deep jaundice), who are not candidates for a liver transplant, screening is not cost-effective since due to their clinical situation, they would not benefit from HCC treatment[17,46].
In the case of patients included in the waiting list for liver transplantation, screening for HCC should still be carried out even if they have a decompensated disease since the HCC can alter their position on list or exclude them in the case of exceeding the accepted criteria for transplant.
Regarding the age of the patient, there are no data to support interrupting the screening at a certain age, but this decision would be given by the patient's clinical situation, their life expectancy and comorbidities that may prevent a treatment of HCC.
In patients with fibrosis grade 3, for any etiology, screening is also recommended, although in some of these groups the benefit and its cost-effectiveness are still unclear and need further studies[17].
NAFLD: NAFLD is a cause of liver disease that is gaining prominence given the growing number of cases diagnosed, especially in industrialized countries[47]. The natural history of HCC in patients with NAFLD and its pathogenesis is not known, although some theories involving proinflammatory cytokines, lipotoxicity, certain genetic polymorphisms in genes such as PNPLA3 and MBOAT7, alterations in the microbiota, and possible increased absorption of iron have been discussed[48-50]. Although the risk of developing HCC is greater in those with a cirrhotic liver, this disease poses an increased risk of developing HCC even in non-cirrhotic patients[51]. In fact, a recent meta-analysis that included 168571 patients (13345 of them NASH) concluded that the risk of HCC in non-cirrhotic patients with NASH liver disease is 2.5 times higher than that in other etiologies[52]. These results should be interpreted with caution given the heterogeneity of the studies included in the meta-analysis and the lack of data on the degree of fibrosis. However, given the large number of patients included, it is a relevant result.
Some studies have suggested that the diagnosis is later than in other etiologies due to possible underdiagnosis of conditions that increase the risk of HCC such as cirrhosis and also due to the greater difficulty in interpreting US, because of the attenuation of the US by subcutaneous fat, and the difficulty of obtaining images of the entire liver[38,51]. Therefore, US has lower sensitivity for the detection of smaller tumors and in some series the prognosis could be worse. Although HCC may occur in patients with NAFLD in the absence of cirrhosis with a higher risk than in other etiologies, there is a lack of evidence on the cost-effectiveness of screening, which is why it is currently recommended in patients with cirrhosis or fibrosis 3 (advanced fibrosis can be diagnosed by elastography or by scoring systems like FIB-4), although it is based on expert opinions[53].
HCV: HCV is a risk factor for the development of cirrhosis and HCC. Achieving sustained viral response with interferon-based regimens has shown to be beneficial and reduce the risk of development of HCC for all degrees of fibrosis[54]. With the recent regimens based on direct-acting antivirals, sustained viral response rates are higher than with the interferon-based regimens and achieve a reduction in the risk of suffering HCC of more than 70%[55-57]. However, in some groups of patients, there is evidence to suggest that there may be a relationship between the use of DAA and early development of HCC after treatment. In these studies, risk factors have been identified, including the presence of non-characterizable nodules in cirrhotic patients prior to treatment initiation in which the response rate is 2.83 (95%CI: 1.55, 5.16) compared to those without nodules or with benign nodules[58]. This possibility makes adequate compliance in screening especially important in these patients. Other studies have also shown a higher incidence of HCC de novo in patients treated with DAA versus IFN. This may be due to the fact that treatments based on DAA are used in older patients with more advanced liver disease, so after adjusting the incidence for these risk factors, patients treated with DAA that reach sustained viral response (SVR) present no greater risk of HCC than those treated with IFN[59].
Hepatitis treatment aims to reduce the risk of developing HCC, although it does not diminish completely[60]. The risk is present especially in cirrhotic patients, although patients with fibrosis grade 3 also continue to present an increased risk, so the screening should be performed every 6 mo[57]. There are several reasons that could justify this, such as an underestimation of the degree of fibrosis due to causes such as "sampling error" in the case of biopsies due to the size of the sample. In addition, it is important to note that after reaching the sustained viral response, the diagnostic accuracy of the elastographic techniques changes and can also underestimate fibrosis, so with the current evidence these techniques cannot be recommended in patients on SVR to decide on the need for screening[61,62]. For this reason and although after the SVR it seems that there may be a reduction in fibrosis, these results should be interpreted with caution since this reduction may be overestimated by elastographic techniques and there are also a lack of data that correlates the reduction of fibrosis after SVR with a risk reduction that would allow interrupting the screening; so the current recommendation is to continue performing lifelong screening for fibrosis 3 and cirrhotic patients according to the pre-treatment fibrosis assessment despite a reduction in elastographic measures after achieving SVR[16,17]. Table 3 shows the most recent studies and meta-analysis with the incidence of de novo HCC in patients treated with DAA.
Table 3.
Annual HCC incidence in cirrhotic patients with HCV
| Ref. | Location | n | Follow-up period | Study design | Incidence |
| UNI/MULTI | |||||
| Li et al[55] | US MULTI | 17836 | Median 2719.2 d in patients treated with IFN, and 396.4 d for the ones treated with DAAs | Retrospective | Annual incidence in cirrhotic patients 2.28% treated with DAA and 2.12% in patients treated with IFN. Annual incidence in patients with no treatment of 4.531% |
| Piñero et al[57] | Latin America MULTI | 1400 | Median 16 mo | Prospective | Accumulated incidence in cirrhotic patients of 3% at 1 year and 6% at 2 yr |
| Waziry et al[59] | Europe, Asia, North America, South America MULTI | 11523 | Median 5.5 yr in patients treated with IFN and 1 yr in patients treated with DAA | Meta-analysis | Annual incidence 1.14% in patients with SVR treated with IFN and 2.96% in patients SVR treated with DAA. After adjusting for age and follow-up period, no greater risk is observed in those treated with DAA |
| Nahon et al[63] | France Multi | 1270 | Median 67.5 mo | Prospective | 2.6% in cirrhotic patients in SVR with DAA. In patients with SVR the annual incidence is 12% |
UNI: Uni-center; MULTI: Multicenter; IFN: Interferon; DAA: Direct-acting antiviral; SVR: Sustained viral response.
HBV: HBV is associated with HCC even in non-cirrhotic patients (30% of the HCCs associated with HBV occur in these patients). This is due to the ability of viral DNA to integrate into host cells and act as a mutagenic agent. Therefore, although the presence of liver cirrhosis is the major risk factor in these patients, presenting a chronic B virus infection already constitutes an increased risk to develop HCC even in the absence of cirrhosis. The incidence of HCC in non-cirrhotic HBV patients ranges from 0.1% to 0.8% per year and in cirrhotic patients from 2.2% to 4.3% per year[16,17].
In non-cirrhotic patients with HBV, the recommendation is established when the risk of developing HCC is 0.2% per year. This is because non-cirrhotic patients diagnosed with early-stage HCC are better candidates for radical treatments (such as surgery), so the cost-benefit of screening is different to cirrhotic patients in whom the liver function can limit the applicability of some treatments and therefore the annual incidence threshold to initiate screening in this group is lower[17].
The AASLD guide establishes populations at risk of HCC that require screening of cirrhotic and non-cirrhotic HBV patients with the following characteristics[16,64]: (1) High-risk HBsAg patients including African or Asian men (these ethnic groups have an increased risk of HCC older than 40 years and Asian women older than 50 years[65,66]. (2) Patients with first-degree relatives diagnosed with HCC. (3) Patients with delta virus. (4) It is difficult to establish the risk in children and adolescents, but it seems reasonable to recommend screening patients with fibrosis grade 3 or cirrhosis and patients with first-degree relatives diagnosed with HCC.
In addition, predictive models have been proposed to assess the risk of HCC such as REACH-B and PAGE-B (in Caucasian patients on antiviral treatment), based on risk factors (viral load, male sex, age, etc). These models stratify HCC risk in at least three groups: low, intermediate, and high risk groups. Patients in the PAGE-B risk class have less than the 0.2%/year risk for HCC[67,68]. Although the use of these scores has some limitations and cannot be universally applied, they can be useful to assess the need for antiviral treatment in certain cases or to assist in the decision to initiate HCC screening in patients who do not belong to the groups indicated.
Alcohol: Alcohol, due to its genotoxicity is directly related to the development of HCC, although it seems that this role is entirely due to the development of cirrhosis in patients without other etiological factors[17,69,70]. Therefore, there is currently no recommendation on screening in patients with alcohol abuse who do not have advanced fibrosis/cirrhosis. However, it is relatively frequent that alcohol is not presented as the only cause of liver disease, but as an added factor to other etiologies such as viral hepatitis. In these patients, the sum of risk factors such as the consumption of large amounts of alcohol and others such as diabetes could increase the risk of HCC[71-73]. It will be necessary in the future to identify the role that these etiological factors play in order to decide to initiate a screening program in non-cirrhotic patients.
Other etiologies: In other pathologies such as primary biliary cholangitis and autoimmune hepatitis, although the evidence is limited, screening does not seem beneficial if they do not present cirrhosis[17].
DISCUSSION
Imaging diagnosis
All current guidelines on the management of HCC accept that this tumor can only be diagnosed by means of imaging techniques if the lesion meets specific criteria and if it is a patient at risk for developing this neoplasm. If both conditions are not met, the biopsy will be necessary for the diagnosis[16,17,74].
The typical scenario is usually a lesion detected by surveillance US in a patient with liver disease, or a casual finding in imaging techniques that initially had another objective (being the liver disease previously known, or discovered at that time).
Characterization by means of imaging tests is based on the fact that HCC has specific vascular characteristics that reflect the results of the process of hepatocarcinogenesis: there is an increase in arterial supply and a decrease in portal vein branches. In a multiphasic study (CT or MRI), this means that a nodule will show greater vascularization in the arterial phase than the rest of the parenchyma, whereas in venous phases the opposite will occur, presenting lower contrast uptake than the surrounding liver[75-78]. Demonstrating this behavior in a lesion of at least 1 cm, identified in a patient at risk, is diagnostic of HCC with values of specificity and positive predictive value that approach 100%[79-82]. Sensitivity values are variable, depending on several factors, largely on the size of the lesion (for lesions between 1 and 2 cm, they are about 60%, increasing these values with lesion size)[78]. For nodule(s) < 1 cm the specificity is lower, because even benign entities as arteriovenous fistula can have the same appearance. Therefore a close follow-up with US at 4-mo intervals is recommended. If the lesion remains stable for 12 mo, can return to regular surveillance (US every 6 mo)[17].
When the previous conditions are met for the diagnosis of HCC, biopsy is not considered necessary, since it will not improve the accuracy of the imaging tests. In addition, the biopsy can have diagnostic limitations (false negatives due to error in the sample or complicated differentiation between dysplasia vs carcinoma), technical difficulties for the procedure (obesity, ascites, location of the tumor that makes access difficult) and, above all potential complications because it is an invasive technique like bleeding or dissemination of the disease[79].
Currently, the imaging techniques validated for the diagnosis of HCC are multiphasic studies using CT and MRI. This conditions a maximum demand on the image, since not only is demanded to detect a lesion, but the objective is to characterize it. Therefore, in these cases it is essential to define quality criteria related to the technique itself, so that in concluding that a lesion "does not meet HCC criteria," we can be sure that the study by image cannot reach the diagnosis. This avoids, for example, indicating a biopsy without being sure of having exhausted the non-invasive diagnostic path or ceasing to diagnose an injury that, depending on the context, can change the patient's management in a crucial way.
As for purely technical requirements, both CT and MRI are described in the LIRADS guidelines and the OPTN/UNOS has also published similar standards[83,84]. These recommendations are in line with the current availability of techniques in the vast majority of centers (for example, it is suggested as a minimum, a multidetector CT of at least eight rows of detectors, and a MRI with 1.5 Tesla).
The intravenous contrast should be administered in a suitable dose, adapted to each patient and in CT the injection rate should be high whenever possible (recommended at least 4 mL/s). Regarding the phases to be performed, three are essential in CT (late arterial, portal and delayed phase) and in MRI the precontrast phase must be added (which is optional in CT, unless previous treatments have been performed that have used radiopaque contrast agents like Lipiodol®). Lesions in MRI are intrinsically T1 hyperintense lesions, due to the presence of elements like proteins, hemoglobin degradation products, copper or melanine, in which visual determination of hyperintensity attributable to contrast enhancement can be difficult; so precontrast imaging provides a baseline against which this enhancement can be identified and allows for subtraction imaging if necessary.
MRI provides the availability of numerous additional sequences unlike CT, which can provide more information.
The review of the quality of the images in the dynamic study is essential, which are similar in CT and MRI, whose compliance ensures that the images have been obtained at the optimum moment of hepatic vascularization.
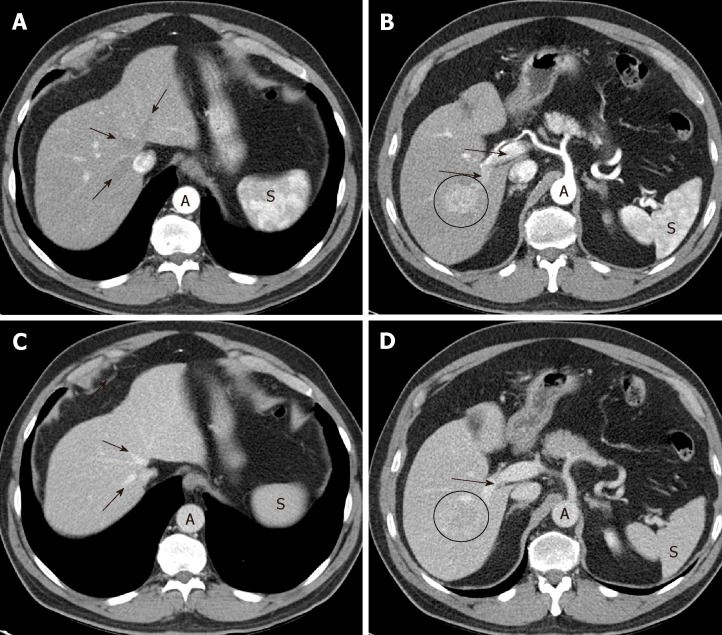
Late arterial phase: The hepatic arterial branches have to show an intense and homogeneous enhancement (which is especially guaranteed with a high flow rate of intravenous contrast injection on CT); in the portal vein the enhancement must be starting, but with non-opacified suprahepatic veins (Figure 1A,B). These three conditions indicate that the phase is adequate, allowing time for the hypervascular lesion to become opaque, but without there being a hepatic venous return. For an optimal moment of image acquisition, it is recommended to use automatic contrast detection techniques -bolus tracking or bolus test-, which adapt the phases to the cardiac output of each patient.
Figure 1.
Optimal late arterial phase and portal phase. A, B: Hepatic artery and branches are fully enhanced. Portal vein is enhanced (arrows) but hepatic veins not yet enhanced by antegrade flow (arrows). Heterogeneous spleen. Aorta of very high density; C, D: Portal phase: portal veins are fully enhanced (D: arrows). Hepatic veins are enhanced by antegrade flow (C: arrows). Liver parenchyma is at peak enhancement. Homogeneous spleen. Portal vein even denser than aorta.
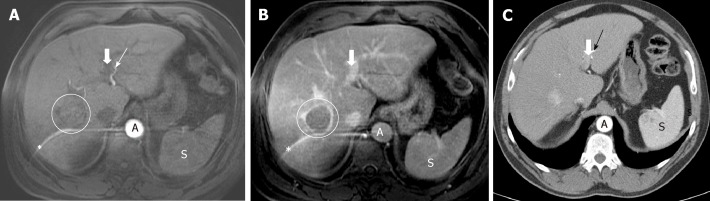
If the portal vein still does not show contrast, we are in an early arterial phase, much less sensitive to detect HCC (Figure 2). On the other hand, if the suprahepatic veins are opacified by hepatic anterograde flow (not by retrograde contrast flow from the right atrium), it will be too late of a phase. In both cases, the exploration will have lost the ability to detect a hypervascular lesion.
Figure 2.
Importance of precise late arterial phase. A: Too early arterial phase. Aorta and left hepatic artery (thin arrow) with high signal intensity, but no contrast is seen in portal vein (thick arrow). No contrast in the suspicious lesion; B: Late venous phase: washout and capsule in the lesion (but no diagnoses due to lack of hyperintensity in arterial phase due to bad technique). Note the artifact in MRI images (*); C: CT was performed in the same patient with a good late arterial phase depicting hyperattenuation of the lesion that it is now diagnostic. Contrast in left portal vein can be seen (thick arrow).
Portal phase: Maximum hepatic parenchymal and portal vein enhancement (mainly depend on an adequate dose of contrast) is given; the suprahepatic veins are opacified by anterograde flow (Figure 1C,D). In this case, what is of interest is the maximum density in the hepatic parenchyma, which will make more evident the differences between a focal lesion and the surrounding non-tumor tissue.
Late venous phase (also known as delayed or equilibrium phase): Obtained between 2 and 5 minutes after injection of the contrast, with which both the liver and the vessels will have a lower density than in the portal phase.
If the multiphasic study does not meet these quality criteria, the next step is to consider whether the study is repeated or an alternative imaging technique is needed.
Once the imaging technique is considered valid, the behavior of the lesion in the different phases is assessed to determine if it meets diagnostic criteria for HCC, which is applicable when it reaches at least 1 cm in maximum diameter. As previously stated, the behavior of HCC correlates with its vascular characteristics, and the diagnosis is based on the findings depicted in Table 4.
Table 4.
Findings for HCC diagnosis
| Vascular phase (CT/MRI) | Feature | Comments |
| Late arterial phase | Arterial phase hyperenhancement also known as "wash-in" | The lesion must be hypervascular with an enhancing part higher in attenuation or intensity than the liver, depicting a nonrim-like enhancement unequivocally greater in whole or in part of the lesion than the surrounding liver parenchyma |
| Portal phase or late venous phase | Washout | The lesion will present lower contrast uptake than the surrounding parenchyma |
| "Capsule appearance" | A ring of peripheral uptake in the lesion |
CT: Computed tomography; MRI: Magnetic resonance imaging.
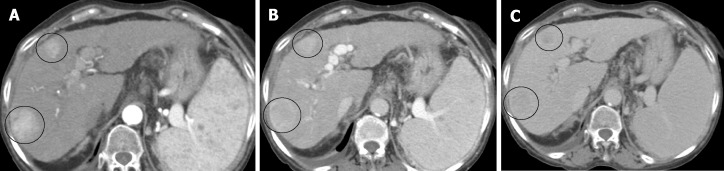
Demonstrating an arterial phase hyperenhancement (APHE) with washout in venous phases, allows the diagnosis of HCC (Figure 3). The LIRADS criteria give the presence of the "enhancing capsule" in nodules ≥ 20 mm the same value as the washout. The diagnosis of HCC is also considered as the growth of a hypervascular nodule by ≥ 50% of its diameter in ≤ 6 mo (Table 5).
Figure 3.
Importance of delayed phase. A: Late arterial phase, 2 hypervascular lesions (circles); B: Portal phase, no washout is seen. Non-diagnostic imaging findings; C: Delayed phase: Washout in both lesions (circles). Diagnosis by imaging.
Table 5.
Computed tomography/magnetic resonance imaging diagnostic table
| Nonrim-like APHE | ||
| Observation size | 10-19 mm | ≥ 20 mm |
| Enhancing "capsule" | LR-4 | LR-5 |
| Non-peripheral washout or threshold grown | LR-5 | LR-5 |
APHE: Arterial phase hyperenhancement; LR-5: LI-RADS lesions.
These are the only criteria that allow the diagnosis by image of HCC. Several "ancillary findings" are described, which can guide or increase the suspicion of HCC, but in no case establish a diagnosis, if the previously defined criteria are not met. Examples of these findings are outlined in Table 6.
Table 6.
Ancillary findings (LIRADS 2018)
| Favoring HCC in particular | Favoring malignancy in general | Favoring benignity |
| Non-enhancing "capsule" | US visibility as discrete nodule | Size stability > 2 yr |
| Nodule-in-nodule | Subthreshold growth | Size reduction |
| Mosaic architecture | Restricted diffusion | Parallels blood pool |
| Blood products in mass | Mild-moderate T2 hyperintensity | Marked T2 hyperintensity |
| Fat in mass, more than adjacent liver | Fat sparing in solid mass | Undistorted vessels |
| Iron sparing in solid mass | Iron in mass, more than liver | |
| Transitional phase hypointensity | Hepatobiliary phase isointensity | |
| Hepatobiliary phase hypointensity | ||
| Corona enhancement |
HCC: Hepatocellular carcinoma; US: Ultrasonography.
Treated lesions have special considerations at LIRADS classification, establishing 3 categories known as nonviable, equivocal or viable. Viable tissue after treatment is considered when APHE, washout or similar pretreatment enhancement is seen. Multi-disciplinary discussion for consensus management is mandatory in these patients.
Hepatospecific contrasts
Hepatobiliary agents in MRI are a type of intravenous contrast with dual properties: on one hand it has an initial extracellular distribution, so that, like the rest of contrasts, it allows to assess the vascularization of the lesion; on the other, it is captured and excreted by the hepatocyte, in a different proportion: Gadoxetate disodium has 50% uptake and hepatobiliary elimination, while in Gadobenate dimeglumine it has a 5% hepatobiliary elimination (the rest in both has a renal elimination). Its application in patients with suspected HCC is also based on the process of hepatocarcinogenesis: the evolution from regeneration nodule to HCC, there is a decrease in hepatocyte capacity for uptake and elimination of the bile duct of hepatospecific contrast due to alterations in membrane transporters[75,76,85]. Thus, when images of the liver are obtained at the moment when the peak of contrast uptake by the hepatocyte exists (after 20 min for the Gadoxetic and around 60 min for the Gadobenate), the non-tumor parenchyma should show enhancement, while most HCCs will show low signal intensity, as will any other lesion that does not have functioning hepatocytes (for example, benign lesions such as a cyst or a hemangioma, or malignant lesions, such as a metastasis).
The use of this type of contrast may increase the sensitivity and the negative predictive value, but it does not improve the specificity and is still considered an "ancillary finding" without constituting a major criteria for the diagnosis of HCC[17].
CEUS
The US contrast is based on microbubbles, and has a purely intravascular distribution, without passage to the interstitium, which results in a lesion behavior somewhat different to that seen in iodinated contrast media for CT and in extracellular gadolinium-based media for MRI[86]. This is especially important in cholangiocarcinoma, which in CEUS may show homogeneous enhancement in the arterial phase, followed by rapid washing, so that this behavior would no longer be specific for HCC in this technique[87,88]. It has been described as a differentiating factor between both entities that the HCC has an earlier enhancement and a less intense and later washing (> 60 s) than the cholangiocarcinoma[86,89].
The use is recommended only in centers with experience, being a highly operator-dependent technique, less reproducible than CT or MRI, and serves for a targeted assessment of a lesion, without allowing a study of the entire liver.
Thus, CEUS is not suitable for screening or surveillance. Rather, it is used to characterize lesion(s) identified on a screening and surveillance US or on CT/MRI. It is not recommended as a first-line imaging technique, because CT or MRI will be needed for staging, but it can be utilized when both CT and MRI are contraindicated or are inconclusive for the HCC diagnosis[17]. In this way, a CEUS LIRADS has also been developed[83].
CEUS is not only a valuable contributor to multimodality imaging for character-izing nodules in a cirrhotic liver, but may also be used, for example, to guide biopsy or treatment of lesions that are difficult to visualize with pre-contrast US, or to detect enhancement in a portal thrombus, in order to differentiate bland thrombus from neoplastic thrombosis[86]. This differentiation takes on importance due to the increased incidence of non-neoplastic portal vein thrombosis[90].
TC versus RM
There are no conclusive studies demonstrating the superiority of one technique over another for the diagnosis of HCC, although the tendency is for a slight advantage of MRI, due to greater sensitivity, especially for small lesions (< 20 mm), and the MRI provides more information for ancillary findings, in addition to the added value of being able to use an hepatospecific contrast[16,17].
The choice between CT or MRI will depend to a great extent on other variables, beyond the diagnosis such as the availability of the technique, the radiologist's experience or certain characteristics of the patient like obesity, ascites or difficulty in performing apneas that can significantly limit the quality of the MR image, with CT being a more reliable technique in these circumstances. Claustrophobia can also prevent a patient from having an MRI. As for CT, it is necessary to take into account ionizing radiation (especially in young patients and multiphasic studies, which need several series) and allergy to iodinated contrast. Renal insufficiency limits the use of contrasts, both in CT and MRI, although patients in hemodialysis can have performed a CT scan, while MRI is contraindicated in this case because of the risk of nephrogenic systemic fibrosis[16].
LI-RADS®
Standardized report and assessment by reference center and multidisciplinary committee. The introduction of LIRADS aims to achieve a standardized process that is part of the study by image of the patient at risk of developing HCC, from the technique of completion to the written report of the examination[83].
In each liver lesion detected, the size (larger diameter), the liver segment where it is located and the degree of suspicion of HCC should be described according to their behavior (Table 7). To determine the likelihood of HCC, the LI-RADS categories are suggested (from LI-RADS 1, corresponding to lesion with benign features, to LI-RADS 5, which is a lesion with diagnostic criteria for HCC). The intermediate categories (LI-RADS 2, 3 and 4) refer to an increase in the probability of HCC, but without making it possible to achieve diagnostic imaging, so that management in these cases will always depend on a multidisciplinary and individualized assessment of each patient, being able to decide on options as different as a biopsy, to perform an alternative imaging technique as well as having a more or less narrow follow-up, or even a treatment[16].
Table 7.
LI-RADS 2018 recommendations for untreated ≥ 1 cm lesions without pathologic proof in patients at high risk for HCC
| LR-NC | Cannot be categorized (image degradation, lack of key phases) |
| LR-1 | Definitely benign (e.g., cyst, hemangioma, perfusion alteration) |
| LR-2 | Probably benign (probable but no definitive LR-1 findings) |
| LR-3 | Intermediate probability of malignancy (nonmalignant and malignant entities each have moderate probability) |
| LR-4 | High probability but no certainty of HCC |
| LR-5 | Definitively HCC |
| LR-M | Probably or definitely malignant, not HCC specific (e.g., HCC not meeting LR-5 criteria, intrahepatic cholangiocarcinoma, metastases to liver) |
| LR-TIV | Tumor in vein |
HCC: Hepatocellular carcinoma.
The evaluation of imaging studies in a multidisciplinary committee and reference centers is recommended, which results in better results in diagnosis, management and even patient survival[16].
The last studies about regular surveillance of HCC in advanced liver diseases, suggest that it could be cost-beneficial in this context, although the evidence in clinical practice is still limited. US at 6-mo interval appears the most extending tool for surveillance and a CT and/or MRI are the most accepted imaging techniques for HCC diagnosis, relegating the biopsy procedure only for selected cases. Advances in HBV control viremia and HCV definitive curation is decreasing the HCC incidence. In the next decades, the high risk subgroups that will benefit from surveillance remains an important research goal in this new stage.
ARTICLE HIGHLIGHTS
Research background
Surveillance of hepatocellular carcinoma (HCC) has been proposed and recommended in clinical guidelines, in order to obtain earlier diagnosis but it is still controversial and it is not accepted worldwide.
Research motivation
Emerging populations like non-alcoholic fatty liver disease patients or hepatitis C virus (HCV) after achieving sustained viral response (SVR) are at risk of developing HCC. Should they be screened? What is the ideal screening tool attending cost-effectiveness?
Research objectives
Support the surveillance programs in patients at risk of developing HCC because of the cost-effectiveness of early diagnosis.
Research methods
Systematic review of recent literature of surveillance (tools, interval, cost-benefit, target population) and the role of imaging diagnosis (radiological non-invasive diagnosis, optimal modality and agents) of HCC.
Research results
The benefits of surveillance of HCC, mainly with ultrasonography, have been assessed in several prospective and retrospective analysis. Surveillance of HCC permits diagnosis in early stages allowing better access to curative treatment and increased life expectancy in patients at risk.
Research conclusions
The actual evidence supports the recommendation of performing surveillance of HCC in patients with cirrhosis or advanced fibrosis of any etiology susceptible of treatment, using ultrasonography every 6 mo. In some populations of non-cirrhotic hepatitis B virus patients the screening can be cost-effective. The diagnosis evaluation of HCC can be established based on noninvasive imaging criteria in patients with cirrhosis.
Research perspectives
Further studies need for evaluating the cost-effectiveness of screening in emerging populations like non-cirrhotic non-alcoholic fatty liver disease patients or HCV who achieved SVR. Utility of hepatospecific contrasts needs further evaluation.
Footnotes
Conflict-of-interest statement: No conflict of interest to declare.
PRISMA 2009 Checklist statement: This systematic review was conducted according to the PRISMA guidelines.
Manuscript source: Invited manuscript
Peer-review started: March 26, 2019
First decision: May 24, 2019
Article in press: July 27, 2019
Specialty type: Medicine, research and experimental
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Ciccone MM, Inchingolo R, Sibulesky L, Squadrito PG, Zanetto A S-Editor: Ma YJ L-Editor: Filipodia E-Editor: Zhou BX
Contributor Information
Sonia Pascual, Liver Unit, Hospital General Universitario de Alicante, Alicante 03010, Spain; Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas, CIBEREHD, Alicante 03010, Spain. pascual_son@gva.es.
Cayetano Miralles, Liver Unit, Hospital General Universitario de Alicante, Alicante 03010, Spain.
Juan M Bernabé, Radiology Department, Hospital General Universitario de Alicante, Alicante 03010, Spain.
Javier Irurzun, Radiology Department, Hospital General Universitario de Alicante, Alicante 03010, Spain.
Mariana Planells, Radiology Department, Hospital General Universitario de Alicante, Alicante 03010, Spain.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta , ZA , Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R, Dandona L, Fleming T, Forouzanfar MH, Hancock J, Hay RJ, Hunter-Merrill R, Huynh C, Hosgood HD ,Johnson CO, Jonas JB, Khubchandani J, Kumar GA, Kutz M, Lan Q, Larson HJ, Liang X, Lim SS, Lopez AD, MacIntyre MF, Marczak L, Marquez N, Mokdad AH, Pinho C, Pourmalek F, Salomon JA, Sanabria JR, Sandar L, Sartorius B, Schwartz SM, Shackelford KA, Shibuya K, Stanaway J, Steiner C, Sun J, Takahashi K, Vollset SE, Vos T, Wagner JA, Wang H, Westerman R, Zeeb H, Zoeckler L, Abd-Allah F, Ahmed MB, Alabed S, Alam NK, Aldhahri SF, Alem G, Alemayohu MA, Ali R, Al-Raddadi R, Amare A, Amoako Y, Artaman A, Asayesh H, Atnafu N, Awasthi A, Saleem HB, Barac A, Bedi N, Bensenor I, Berhane A, Bernabé E, Betsu B, Binagwaho A, Boneya D, Campos-Nonato I, Castañeda-Orjuela C, Catalá-López F, Chiang P, Chibueze C, Chitheer A, Choi JY, Cowie B, Damtew S, das Neves J, Dey S, Dharmaratne S, Dhillon P, Ding E, Driscoll T, Ekwueme D, Endries AY, Farvid M, Farzadfar F, Fernandes J, Fischer F, G/Hiwot TT, Gebru A, Gopalani S, Hailu A, Horino M, Horita N, Husseini A, Huybrechts I, Inoue M, Islami F, Jakovljevic M, James S, Javanbakht M, Jee SH, Kasaeian A, Kedir MS, Khader YS, Khang YH, Kim D, Leigh J, Linn S, Lunevicius R, El Razek HMA, Malekzadeh R, Malta DC, Marcenes W, Markos D, Melaku YA, Meles KG, Mendoza W, Mengiste DT, Meretoja TJ, Miller TR, Mohammad KA, Mohammadi A, Mohammed S, Moradi-Lakeh M, Nagel G, Nand D, Le Nguyen Q, Nolte S, Ogbo FA, Oladimeji KE, Oren E, Pa M, Park EK, Pereira DM, Plass D, Qorbani M, Radfar A, Rafay A, Rahman M, Rana SM, Søreide K, Satpathy M, Sawhney M, Sepanlou SG, Shaikh MA, She J, Shiue I, Shore HR, Shrime MG, So S, Soneji S, Stathopoulou V, Stroumpoulis K, Sufiyan MB, Sykes BL, Tabarés-Seisdedos R, Tadese F, Tedla BA, Tessema GA, Thakur JS, Tran BX, Ukwaja KN, Uzochukwu BSC, Vlassov VV, Weiderpass E, Wubshet Terefe M, Yebyo HG, Yimam HH, Yonemoto N, Younis MZ, Yu C, Zaidi Z, Zaki MES, Zenebe ZM, Murray CJL, Naghavi M.Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3:524–548. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Cancer Institute. 2019. SEER Cancer Statistics Review, 1975-2015. SEER; p. [cited 2019 Feb 3]. Available from: https://seer.cancer.gov/csr/1975_2015/index.html. [Google Scholar]
- 4.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–1273.e1. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertuccio P, Turati F, Carioli G, Rodriguez T, La Vecchia C, Malvezzi M, Negri E. Global trends and predictions in hepatocellular carcinoma mortality. J Hepatol. 2017;67:302–309. doi: 10.1016/j.jhep.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70:151–171. doi: 10.1016/j.jhep.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Bosetti C, Levi F, Lucchini F, Zatonski WA, Negri E, La Vecchia C. Worldwide mortality from cirrhosis: an update to 2002. J Hepatol. 2007;46:827–839. doi: 10.1016/j.jhep.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 8.Benvegnù L, Gios M, Boccato S, Alberti A. Natural history of compensated viral cirrhosis: a prospective study on the incidence and hierarchy of major complications. Gut. 2004;53:744–749. doi: 10.1136/gut.2003.020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sangiovanni A, Del Ninno E, Fasani P, De Fazio C, Ronchi G, Romeo R, Morabito A, De Franchis R, Colombo M. Increased survival of cirrhotic patients with a hepatocellular carcinoma detected during surveillance. Gastroenterology. 2004;126:1005–1014. doi: 10.1053/j.gastro.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 10.Chen JG, Parkin DM, Chen QG, Lu JH, Shen QJ, Zhang BC, Zhu YR. Screening for liver cancer: results of a randomised controlled trial in Qidong, China. J Med Screen. 2003;10:204–209. doi: 10.1258/096914103771773320. [DOI] [PubMed] [Google Scholar]
- 11.Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:417–422. doi: 10.1007/s00432-004-0552-0. [DOI] [PubMed] [Google Scholar]
- 12.Poustchi H, Farrell GC, Strasser SI, Lee AU, McCaughan GW, George J. Feasibility of conducting a randomized control trial for liver cancer screening: is a randomized controlled trial for liver cancer screening feasible or still needed? Hepatology. 2011;54:1998–2004. doi: 10.1002/hep.24581. [DOI] [PubMed] [Google Scholar]
- 13.Davila JA, Morgan RO, Richardson PA, Du XL, McGlynn KA, El-Serag HB. Use of surveillance for hepatocellular carcinoma among patients with cirrhosis in the United States. Hepatology. 2010;52:132–141. doi: 10.1002/hep.23615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davila JA, Henderson L, Kramer JR, Kanwal F, Richardson PA, Duan Z, El-Serag HB. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Ann Intern Med. 2011;154:85–93. doi: 10.7326/0003-4819-154-2-201101180-00006. [DOI] [PubMed] [Google Scholar]
- 15.Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med. 2014;11:e1001624. doi: 10.1371/journal.pmed.1001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 17.European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu.; European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 18.Zhao C, Jin M, Le RH, Le MH, Chen VL, Jin M, Wong GL, Wong VW, Lim YS, Chuang WL, Yu ML, Nguyen MH. Poor adherence to hepatocellular carcinoma surveillance: A systematic review and meta-analysis of a complex issue. Liver Int. 2018;38:503–514. doi: 10.1111/liv.13555. [DOI] [PubMed] [Google Scholar]
- 19.Johnson P, Berhane S, Kagebayashi C, Satomura S, Teng M, Fox R, Yeo W, Mo F, Lai P, Chan SL, Tada T, Toyoda H, Kumada T. Impact of disease stage and aetiology on survival in hepatocellular carcinoma: implications for surveillance. Br J Cancer. 2017;116:441–447. doi: 10.1038/bjc.2016.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moon AM, Weiss NS, Beste LA, Su F, Ho SB, Jin GY, Lowy E, Berry K, Ioannou GN. No Association Between Screening for Hepatocellular Carcinoma and Reduced Cancer-Related Mortality in Patients With Cirrhosis. Gastroenterology. 2018;155:1128–1139.e6. doi: 10.1053/j.gastro.2018.06.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edenvik P, Davidsdottir L, Oksanen A, Isaksson B, Hultcrantz R, Stål P. Application of hepatocellular carcinoma surveillance in a European setting. What can we learn from clinical practice? Liver Int. 2015;35:1862–1871. doi: 10.1111/liv.12764. [DOI] [PubMed] [Google Scholar]
- 22.van Meer S, de Man RA, Coenraad MJ, Sprengers D, van Nieuwkerk KM, Klümpen HJ, Jansen PL, IJzermans JN, van Oijen MG, Siersema PD, van Erpecum KJ. Surveillance for hepatocellular carcinoma is associated with increased survival: Results from a large cohort in the Netherlands. J Hepatol. 2015;63:1156–1163. doi: 10.1016/j.jhep.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 23.Singal AG, Mittal S, Yerokun OA, Ahn C, Marrero JA, Yopp AC, Parikh ND, Scaglione SJ. Hepatocellular Carcinoma Screening Associated with Early Tumor Detection and Improved Survival Among Patients with Cirrhosis in the US. Am J Med. 2017;130:1099–1106.e1. doi: 10.1016/j.amjmed.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 24.Mittal S, Kanwal F, Ying J, Chung R, Sada YH, Temple S, Davila JA, El-Serag HB. Effectiveness of surveillance for hepatocellular carcinoma in clinical practice: A United States cohort. J Hepatol. 2016;65:1148–1154. doi: 10.1016/j.jhep.2016.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atiq O, Tiro J, Yopp AC, Muffler A, Marrero JA, Parikh ND, Murphy C, McCallister K, Singal AG. An assessment of benefits and harms of hepatocellular carcinoma surveillance in patients with cirrhosis. Hepatology. 2017;65:1196–1205. doi: 10.1002/hep.28895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cucchetti A, Cescon M, Erroi V, Pinna AD. Cost-effectiveness of liver cancer screening. Best Pract Res Clin Gastroenterol. 2013;27:961–972. doi: 10.1016/j.bpg.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 27.Andersson KL, Salomon JA, Goldie SJ, Chung RT. Cost effectiveness of alternative surveillance strategies for hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2008;6:1418–1424. doi: 10.1016/j.cgh.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson Coon J, Rogers G, Hewson P, Wright D, Anderson R, Jackson S, Ryder S, Cramp M, Stein K. Surveillance of cirrhosis for hepatocellular carcinoma: a cost-utility analysis. Br J Cancer. 2008;98:1166–1175. doi: 10.1038/sj.bjc.6604301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cadier B, Bulsei J, Nahon P, Seror O, Laurent A, Rosa I, Layese R, Costentin C, Cagnot C, Durand-Zaleski I, Chevreul K ANRS CO12 CirVir and CHANGH groups. Early detection and curative treatment of hepatocellular carcinoma: A cost-effectiveness analysis in France and in the United States. Hepatology. 2017;65:1237–1248. doi: 10.1002/hep.28961. [DOI] [PubMed] [Google Scholar]
- 30.Cucchetti A, Trevisani F, Cescon M, Ercolani G, Farinati F, Poggio PD, Rapaccini G, Nolfo MA, Benvegnù L, Zoli M, Borzio F, Giannini EG, Caturelli E, Chiaramonte M, Pinna AD Italian Liver Cancer (ITA. LI.CA) Group. Cost-effectiveness of semi-annual surveillance for hepatocellular carcinoma in cirrhotic patients of the Italian Liver Cancer population. J Hepatol. 2012;56:1089–1096. doi: 10.1016/j.jhep.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka H, Iijima H, Nouso K, Aoki N, Iwai T, Takashima T, Sakai Y, Aizawa N, Iwata K, Ikeda N, Iwata Y, Enomoto H, Saito M, Imanishi H, Nishiguchi S. Cost-effectiveness analysis on the surveillance for hepatocellular carcinoma in liver cirrhosis patients using contrast-enhanced ultrasonography. Hepatol Res. 2012;42:376–384. doi: 10.1111/j.1872-034X.2011.00936.x. [DOI] [PubMed] [Google Scholar]
- 32.Chang Y, Lairson DR, Chan W, Lu SN, Aoki N. Cost-effectiveness of screening for hepatocellular carcinoma among subjects at different levels of risk. J Eval Clin Pract. 2011;17:261–267. doi: 10.1111/j.1365-2753.2010.01432.x. [DOI] [PubMed] [Google Scholar]
- 33.Ruggeri M. Hepatocellular carcinoma: cost-effectiveness of screening. A systematic review. Risk Manag Healthc Policy. 2012;5:49–54. doi: 10.2147/RMHP.S18677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barbara L, Benzi G, Gaiani S, Fusconi F, Zironi G, Siringo S, Rigamonti A, Barbara C, Grigioni W, Mazziotti A. Natural history of small untreated hepatocellular carcinoma in cirrhosis: a multivariate analysis of prognostic factors of tumor growth rate and patient survival. Hepatology. 1992;16:132–137. doi: 10.1002/hep.1840160122. [DOI] [PubMed] [Google Scholar]
- 35.Santi V, Trevisani F, Gramenzi A, Grignaschi A, Mirici-Cappa F, Del Poggio P, Di Nolfo MA, Benvegnù L, Farinati F, Zoli M, Giannini EG, Borzio F, Caturelli E, Chiaramonte M, Bernardi M Italian Liver Cancer (ITA. LI.CA) Group. Semiannual surveillance is superior to annual surveillance for the detection of early hepatocellular carcinoma and patient survival. J Hepatol. 2010;53:291–297. doi: 10.1016/j.jhep.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 36.Trinchet JC, Chaffaut C, Bourcier V, Degos F, Henrion J, Fontaine H, Roulot D, Mallat A, Hillaire S, Cales P, Ollivier I, Vinel JP, Mathurin P, Bronowicki JP, Vilgrain V, N'Kontchou G, Beaugrand M, Chevret S Groupe d'Etude et de Traitement du Carcinome Hépatocellulaire (GRETCH) Ultrasonographic surveillance of hepatocellular carcinoma in cirrhosis: a randomized trial comparing 3- and 6-month periodicities. Hepatology. 2011;54:1987–1997. doi: 10.1002/hep.24545. [DOI] [PubMed] [Google Scholar]
- 37.Singal A, Volk ML, Waljee A, Salgia R, Higgins P, Rogers MA, Marrero JA. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30:37–47. doi: 10.1111/j.1365-2036.2009.04014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simmons O, Fetzer DT, Yokoo T, Marrero JA, Yopp A, Kono Y, Parikh ND, Browning T, Singal AG. Predictors of adequate ultrasound quality for hepatocellular carcinoma surveillance in patients with cirrhosis. Aliment Pharmacol Ther. 2017;45:169–177. doi: 10.1111/apt.13841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Biselli M, Conti F, Gramenzi A, Frigerio M, Cucchetti A, Fatti G, D'Angelo M, Dall'Agata M, Giannini EG, Farinati F, Ciccarese F, Andreone P, Bernardi M, Trevisani F. A new approach to the use of α-fetoprotein as surveillance test for hepatocellular carcinoma in patients with cirrhosis. Br J Cancer. 2015;112:69–76. doi: 10.1038/bjc.2014.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tzartzeva K, Obi J, Rich NE, Parikh ND, Marrero JA, Yopp A, Waljee AK, Singal AG. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis. Gastroenterology. 2018;154:1706–1718.e1. doi: 10.1053/j.gastro.2018.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yeoman AD, Al-Chalabi T, Karani JB, Quaglia A, Devlin J, Mieli-Vergani G, Bomford A, O'Grady JG, Harrison PM, Heneghan MA. Evaluation of risk factors in the development of hepatocellular carcinoma in autoimmune hepatitis: Implications for follow-up and screening. Hepatology. 2008;48:863–870. doi: 10.1002/hep.22432. [DOI] [PubMed] [Google Scholar]
- 42.Tansel A, Katz LH, El-Serag HB, Thrift AP, Parepally M, Shakhatreh MH, Kanwal F. Incidence and Determinants of Hepatocellular Carcinoma in Autoimmune Hepatitis: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2017;15:1207–1217.e4. doi: 10.1016/j.cgh.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fattovich G, Pantalena M, Zagni I, Realdi G, Schalm SW, Christensen E European Concerted Action on Viral Hepatitis (EUROHEP) Effect of hepatitis B and C virus infections on the natural history of compensated cirrhosis: a cohort study of 297 patients. Am J Gastroenterol. 2002;97:2886–2895. doi: 10.1111/j.1572-0241.2002.07057.x. [DOI] [PubMed] [Google Scholar]
- 44.Mancebo A, González-Diéguez ML, Cadahía V, Varela M, Pérez R, Navascués CA, Sotorríos NG, Martínez M, Rodrigo L, Rodríguez M. Annual incidence of hepatocellular carcinoma among patients with alcoholic cirrhosis and identification of risk groups. Clin Gastroenterol Hepatol. 2013;11:95–101. doi: 10.1016/j.cgh.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 45.Shibuya A, Tanaka K, Miyakawa H, Shibata M, Takatori M, Sekiyama K, Hashimoto N, Amaki S, Komatsu T, Morizane T. Hepatocellular carcinoma and survival in patients with primary biliary cirrhosis. Hepatology. 2002;35:1172–1178. doi: 10.1053/jhep.2002.33157. [DOI] [PubMed] [Google Scholar]
- 46.Bruix J, Sherman M American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bellentani S. The epidemiology of non-alcoholic fatty liver disease. Liver Int. 2017;37 Suppl 1:81–84. doi: 10.1111/liv.13299. [DOI] [PubMed] [Google Scholar]
- 48.Liu YL, Patman GL, Leathart JB, Piguet AC, Burt AD, Dufour JF, Day CP, Daly AK, Reeves HL, Anstee QM. Carriage of the PNPLA3 rs738409 C >G polymorphism confers an increased risk of non-alcoholic fatty liver disease associated hepatocellular carcinoma. J Hepatol. 2014;61:75–81. doi: 10.1016/j.jhep.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 49.Donati B, Dongiovanni P, Romeo S, Meroni M, McCain M, Miele L, Petta S, Maier S, Rosso C, De Luca L, Vanni E, Grimaudo S, Romagnoli R, Colli F, Ferri F, Mancina RM, Iruzubieta P, Craxi A, Fracanzani AL, Grieco A, Corradini SG, Aghemo A, Colombo M, Soardo G, Bugianesi E, Reeves H, Anstee QM, Fargion S, Valenti L. MBOAT7 rs641738 variant and hepatocellular carcinoma in non-cirrhotic individuals. Sci Rep. 2017;7:4492. doi: 10.1038/s41598-017-04991-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Margini C, Dufour JF. The story of HCC in NAFLD: from epidemiology, across pathogenesis, to prevention and treatment. Liver Int. 2016;36:317–324. doi: 10.1111/liv.13031. [DOI] [PubMed] [Google Scholar]
- 51.Piscaglia F, Svegliati-Baroni G, Barchetti A, Pecorelli A, Marinelli S, Tiribelli C, Bellentani S HCC-NAFLD Italian Study Group. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: A multicenter prospective study. Hepatology. 2016;63:827–838. doi: 10.1002/hep.28368. [DOI] [PubMed] [Google Scholar]
- 52.Stine JG, Wentworth BJ, Zimmet A, Rinella ME, Loomba R, Caldwell SH, Argo CK. Systematic review with meta-analysis: risk of hepatocellular carcinoma in non-alcoholic steatohepatitis without cirrhosis compared to other liver diseases. Aliment Pharmacol Ther. 2018;48:696–703. doi: 10.1111/apt.14937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Younossi ZM, Otgonsuren M, Henry L, Venkatesan C, Mishra A, Erario M, Hunt S. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. 2015;62:1723–1730. doi: 10.1002/hep.28123. [DOI] [PubMed] [Google Scholar]
- 54.Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158:329–337. doi: 10.7326/0003-4819-158-5-201303050-00005. [DOI] [PubMed] [Google Scholar]
- 55.Li DK, Ren Y, Fierer DS, Rutledge S, Shaikh OS, Lo Re V, 3rd, Simon T, Abou-Samra AB, Chung RT, Butt AA. The short-term incidence of hepatocellular carcinoma is not increased after hepatitis C treatment with direct-acting antivirals: An ERCHIVES study. Hepatology. 2018;67:2244–2253. doi: 10.1002/hep.29707. [DOI] [PubMed] [Google Scholar]
- 56.Ioannou GN, Green PK, Berry K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatol. 2017 doi: 10.1016/j.jhep.2017.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Piñero F, Mendizabal M, Ridruejo E, Herz Wolff F, Ameigeiras B, Anders M, Schinoni MI, Reggiardo V, Palazzo A, Videla M, Alonso C, Santos L, Varón A, Figueroa S, Vistarini C, Adrover R, Fernández N, Perez D, Tanno F, Hernández N, Sixto M, Borzi S, Bruno A, Cocozzella D, Soza A, Descalzi V, Estepo C, Zerega A, de Araujo A, Cheinquer H, Silva M LALREAN. Treatment with direct-acting antivirals for HCV decreases but does not eliminate the risk of hepatocellular carcinoma. Liver Int. 2019;39:1033–1043. doi: 10.1111/liv.14041. [DOI] [PubMed] [Google Scholar]
- 58.Mariño Z, Darnell A, Lens S, Sapena V, Díaz A, Belmonte E, Perelló C, Calleja JL, Varela M, Rodriguez M, Rodriguez de Lope C, Llerena S, Torras X, Gallego A, Sala M, Morillas RM, Minguez B, Llaneras J, Coll S, Carrion JA, Iñarrairaegui M, Sangro B, Vilana R, Sole M, Ayuso C, Ríos J, Forns X, Bruix J, Reig M. Time association between hepatitis C therapy and hepatocellular carcinoma emergence in cirrhosis: Relevance of non-characterized nodules. J Hepatol. 2019;70:874–884. doi: 10.1016/j.jhep.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 59.Waziry R, Hajarizadeh B, Grebely J, Amin J, Law M, Danta M, George J, Dore GJ. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: A systematic review, meta-analyses, and meta-regression. J Hepatol. 2017;67:1204–1212. doi: 10.1016/j.jhep.2017.07.025. [DOI] [PubMed] [Google Scholar]
- 60.Jacobson IM, Lim JK, Fried MW. American Gastroenterological Association Institute Clinical Practice Update-Expert Review: Care of Patients Who Have Achieved a Sustained Virologic Response After Antiviral Therapy for Chronic Hepatitis C Infection. Gastroenterology. 2017;152:1578–1587. doi: 10.1053/j.gastro.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 61.D'Ambrosio R, Aghemo A, Fraquelli M, Rumi MG, Donato MF, Paradis V, Bedossa P, Colombo M. The diagnostic accuracy of Fibroscan for cirrhosis is influenced by liver morphometry in HCV patients with a sustained virological response. J Hepatol. 2013;59:251–256. doi: 10.1016/j.jhep.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 62.European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu.; European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2018. J Hepatol. 2018;69:461–511. doi: 10.1016/j.jhep.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 63.Nahon P, Layese R, Bourcier V, Cagnot C, Marcellin P, Guyader D, Pol S, Larrey D, De Lédinghen V, Ouzan D, Zoulim F, Roulot D, Tran A, Bronowicki JP, Zarski JP, Riachi G, Calès P, Péron JM, Alric L, Bourlière M, Mathurin P, Blanc JF, Abergel A, Serfaty L, Mallat A, Grangé JD, Attali P, Bacq Y, Wartelle C, Dao T, Thabut D, Pilette C, Silvain C, Christidis C, Nguyen-Khac E, Bernard-Chabert B, Zucman D, Di Martino V, Sutton A, Roudot-Thoraval F, Audureau E ANRS CO12 CirVir Group. Incidence of Hepatocellular Carcinoma After Direct Antiviral Therapy for HCV in Patients With Cirrhosis Included in Surveillance Programs. Gastroenterology. 2018;155:1436–1450.e6. doi: 10.1053/j.gastro.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 64.Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS, Jr, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560–1599. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48:335–352. doi: 10.1016/j.jhep.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 66.Chayanupatkul M, Omino R, Mittal S, Kramer JR, Richardson P, Thrift AP, El-Serag HB, Kanwal F. Hepatocellular carcinoma in the absence of cirrhosis in patients with chronic hepatitis B virus infection. J Hepatol. 2017;66:355–362. doi: 10.1016/j.jhep.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 67.Yang HI, Yuen MF, Chan HL, Han KH, Chen PJ, Kim DY, Ahn SH, Chen CJ, Wong VW, Seto WK REACH-B Working Group. Risk estimation for hepatocellular carcinoma in chronic hepatitis B (REACH-B): development and validation of a predictive score. Lancet Oncol. 2011;12:568–574. doi: 10.1016/S1470-2045(11)70077-8. [DOI] [PubMed] [Google Scholar]
- 68.Papatheodoridis G, Dalekos G, Sypsa V, Yurdaydin C, Buti M, Goulis J, Calleja JL, Chi H, Manolakopoulos S, Mangia G, Gatselis N, Keskin O, Savvidou S, de la Revilla J, Hansen BE, Vlachogiannakos I, Galanis K, Idilman R, Colombo M, Esteban R, Janssen HL, Lampertico P. PAGE-B predicts the risk of developing hepatocellular carcinoma in Caucasians with chronic hepatitis B on 5-year antiviral therapy. J Hepatol. 2016;64:800–806. doi: 10.1016/j.jhep.2015.11.035. [DOI] [PubMed] [Google Scholar]
- 69.Desai A, Sandhu S, Lai JP, Sandhu DS. Hepatocellular carcinoma in non-cirrhotic liver: A comprehensive review. World J Hepatol. 2019;11:1–18. doi: 10.4254/wjh.v11.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Trevisani F, Frigerio M, Santi V, Grignaschi A, Bernardi M. Hepatocellular carcinoma in non-cirrhotic liver: a reappraisal. Dig Liver Dis. 2010;42:341–347. doi: 10.1016/j.dld.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 71.Kuper H, Tzonou A, Kaklamani E, Hsieh CC, Lagiou P, Adami HO, Trichopoulos D, Stuver SO. Tobacco smoking, alcohol consumption and their interaction in the causation of hepatocellular carcinoma. Int J Cancer. 2000;85:498–502. [PubMed] [Google Scholar]
- 72.Kasmari AJ, Welch A, Liu G, Leslie D, McGarrity T, Riley T. Independent of Cirrhosis, Hepatocellular Carcinoma Risk Is Increased with Diabetes and Metabolic Syndrome. Am J Med. 2017;130:746.e1–746.e7. doi: 10.1016/j.amjmed.2016.12.029. [DOI] [PubMed] [Google Scholar]
- 73.Kawamura Y, Arase Y, Ikeda K, Seko Y, Imai N, Hosaka T, Kobayashi M, Saitoh S, Sezaki H, Akuta N, Suzuki F, Suzuki Y, Ohmoto Y, Amakawa K, Tsuji H, Kumada H. Large-scale long-term follow-up study of Japanese patients with non-alcoholic Fatty liver disease for the onset of hepatocellular carcinoma. Am J Gastroenterol. 2012;107:253–261. doi: 10.1038/ajg.2011.327. [DOI] [PubMed] [Google Scholar]
- 74.Vogel A, Cervantes A, Chau I, Daniele B, Llovet J, Meyer T, Nault JC, Neumann U, Ricke J, Sangro B, Schirmacher P, Verslype C, Zech CJ, Arnold D, Martinelli E ESMO Guidelines Committee. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv238–iv255. doi: 10.1093/annonc/mdy308. [DOI] [PubMed] [Google Scholar]
- 75.Choi JY, Lee JM, Sirlin CB. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part I. Development, growth, and spread: key pathologic and imaging aspects. Radiology. 2014;272:635–654. doi: 10.1148/radiol.14132361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Choi JY, Lee JM, Sirlin CB. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part II. Extracellular agents, hepatobiliary agents, and ancillary imaging features. Radiology. 2014;273:30–50. doi: 10.1148/radiol.14132362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matsui O, Kobayashi S, Sanada J, Kouda W, Ryu Y, Kozaka K, Kitao A, Nakamura K, Gabata T. Hepatocelluar nodules in liver cirrhosis: hemodynamic evaluation (angiography-assisted CT) with special reference to multi-step hepatocarcinogenesis. Abdom Imaging. 2011;36:264–272. doi: 10.1007/s00261-011-9685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ayuso C, Rimola J, Vilana R, Burrel M, Darnell A, García-Criado Á, Bianchi L, Belmonte E, Caparroz C, Barrufet M, Bruix J, Brú C. Diagnosis and staging of hepatocellular carcinoma (HCC): current guidelines. Eur J Radiol. 2018;101:72–81. doi: 10.1016/j.ejrad.2018.01.025. [DOI] [PubMed] [Google Scholar]
- 79.Forner A, Vilana R, Ayuso C, Bianchi L, Solé M, Ayuso JR, Boix L, Sala M, Varela M, Llovet JM, Brú C, Bruix J. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: Prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology. 2008;47:97–104. doi: 10.1002/hep.21966. [DOI] [PubMed] [Google Scholar]
- 80.Roberts LR, Sirlin CB, Zaiem F, Almasri J, Prokop LJ, Heimbach JK, Murad MH, Mohammed K. Imaging for the diagnosis of hepatocellular carcinoma: A systematic review and meta-analysis. Hepatology. 2018;67:401–421. doi: 10.1002/hep.29487. [DOI] [PubMed] [Google Scholar]
- 81.Bruix J, Reig M, Sherman M. Evidence-Based Diagnosis, Staging, and Treatment of Patients With Hepatocellular Carcinoma. Gastroenterology. 2016;150:835–853. doi: 10.1053/j.gastro.2015.12.041. [DOI] [PubMed] [Google Scholar]
- 82.Sangiovanni A, Manini MA, Iavarone M, Romeo R, Forzenigo LV, Fraquelli M, Massironi S, Della Corte C, Ronchi G, Rumi MG, Biondetti P, Colombo M. The diagnostic and economic impact of contrast imaging techniques in the diagnosis of small hepatocellular carcinoma in cirrhosis. Gut. 2010;59:638–644. doi: 10.1136/gut.2009.187286. [DOI] [PubMed] [Google Scholar]
- 83.American College of Radiology. Liver Imaging Reporting and DataSystem version 2018. Accessed Jul 10 2018, from : https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS/CT-MRI-LI-RADS-v2018. [Google Scholar]
- 84.Wald C, Russo MW, Heimbach JK, Hussain HK, Pomfret EA, Bruix J. New OPTN/UNOS policy for liver transplant allocation: standardization of liver imaging, diagnosis, classification, and reporting of hepatocellular carcinoma. Radiology. 2013;266:376–382. doi: 10.1148/radiol.12121698. [DOI] [PubMed] [Google Scholar]
- 85.Joo I, Lee JM. Recent Advances in the Imaging Diagnosis of Hepatocellular Carcinoma: Value of Gadoxetic Acid-Enhanced MRI. Liver Cancer. 2016;5:67–87. doi: 10.1159/000367750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wilson SR, Lyshchik A, Piscaglia F, Cosgrove D, Jang HJ, Sirlin C, Dietrich CF, Kim TK, Willmann JK, Kono Y. CEUS LI-RADS: algorithm, implementation, and key differences from CT/MRI. Abdom Radiol (NY) 2018;43:127–142. doi: 10.1007/s00261-017-1250-0. [DOI] [PubMed] [Google Scholar]
- 87.Vilana R, Forner A, Bianchi L, García-Criado A, Rimola J, de Lope CR, Reig M, Ayuso C, Brú C, Bruix J. Intrahepatic peripheral cholangiocarcinoma in cirrhosis patients may display a vascular pattern similar to hepatocellular carcinoma on contrast-enhanced ultrasound. Hepatology. 2010;51:2020–2029. doi: 10.1002/hep.23600. [DOI] [PubMed] [Google Scholar]
- 88.Galassi M, Iavarone M, Rossi S, Bota S, Vavassori S, Rosa L, Leoni S, Venerandi L, Marinelli S, Sangiovanni A, Veronese L, Fraquelli M, Granito A, Golfieri R, Colombo M, Bolondi L, Piscaglia F. Patterns of appearance and risk of misdiagnosis of intrahepatic cholangiocarcinoma in cirrhosis at contrast enhanced ultrasound. Liver Int. 2013;33:771–779. doi: 10.1111/liv.12124. [DOI] [PubMed] [Google Scholar]
- 89.Kim TK, Noh SY, Wilson SR, Kono Y, Piscaglia F, Jang HJ, Lyshchik A, Dietrich CF, Willmann JK, Vezeridis A, Sirlin CB. Contrast-enhanced ultrasound (CEUS) liver imaging reporting and data system (LI-RADS) 2017 - a review of important differences compared to the CT/MRI system. Clin Mol Hepatol. 2017;23:280–289. doi: 10.3350/cmh.2017.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zanetto A, Campello E, Spiezia L, Burra P, Simioni P, Russo FP. Cancer-Associated Thrombosis in Cirrhotic Patients with Hepatocellular Carcinoma. Cancers (Basel) 2018;10:E450. doi: 10.3390/cancers10110450. [DOI] [PMC free article] [PubMed] [Google Scholar]