Abstract
It has been found that obese people have a higher proportion in suffering from osteoarthritis (OA), not only in the weight-bearing joints like knee and hip joints, even in non-weight-bearing joints such as hand joints. One of the reasons is because the large amount of adipose tissue secretes some factors, which can promote the occurrence of arthritis. As an important structure of the knee joint, the infrapatellar fat pad (IPFP) is actually a piece of adipose tissue. The aim of this review is to offer a comprehensive view of the anatomy and physiological characteristics of IPFP and its relationship with the pathological process of OA, indicating the important function of IPFP in OA. At the same time, with the development of adipose derived stem cells in the treatment of OA, owing to its special advantages, the IPFP is becoming a kind of important, minimally invasive fat stem cell source, providing a new approach for the treatment of OA. We hope that this review will offer an overview of all published data regarding the IPFP and will indicate novel directions for future research.
Keywords: Infrapatellar fat pad, Osteoarthritis, Human mesenchymal stem cells
Core tip: The infrapatellar fat pad (IPFP) is a piece of adipose tissue, however, due to its special position in the knee joint and the anatomical and physiological characteristics, IPFP plays important roles in the pathological process of osteoarthritis (OA). Meanwhile, the IPFP is becoming a kind of important, minimally invasive fat stem cell source, which can treat OA. We herein widely review the pertinent literature, summarize and analyze the IPFP related vascular and nerve supply, the dual role of IPFP, and its future application prospects.
INTRODUCTION
Traditionally speaking, what we said osteoarthritis (OA) always refer to the large joint OA, such as knee OA (KOA) and hip OA. This disease mainly affected women aged over 65, especially obese people and those with previous injuries[1]. OA can be considered as a disease that changes not just cartilage but the entire joint organ, including the subchondral bone, menisci, ligaments, periarticular muscle, capsule, and synovium[2]. Although most OA cases are caused by mechanically factors that injury the joint tissues, OA is generally recognized as a multifactorial process such as age, heredity, obesity, and nutritional factors[3,4]. Recent studies indicated the increased risk of hand OA in obese patients, leading people to focus on the systemic inflammatory mediators secreted by adipose tissue, such as cytokines, interleukins (ILs), growth factors, and adipokines in knee OA[5]. Meanwhile, it was found that weight loss can significantly reduce the knee pain in OA patients[6]. And it is worth mentioning that OA has been defined as a local inflammatory disease[7].
The infrapatellar fat pad (IPFP), which is located intra-articularly and extrasynovially in the knee joint, is abundant in adipose tissue[8]. Considering the IPFP location and the role of inflammatory mediators in the OA process, few researchers are now paying attention on the effect of IPFP in the physiopathology of OA and its new application prospect in this disease. This review is therefore to provide a brief update on the role of IPFP in the process of OA and the innovative therapeutic strategies using IPFP nowadays.
ANATOMIC AND BIOLOGICAL CHARACTERISTICS OF IPFP
The IPFP was first described by Hoffa as “Hoffas fat pad” and “Hoffas disease” in 1904[9]. It is an intra-articular and extrasynovial inclusion, covered by synovial membrane[8,10]. On the transverse section, the IPFP is located between the patellar retinacula and patellar tendon anteriorly and the trochlear surface of the femur posteriorly; on the sagittal section, it is located inferiorly to the patella and anteriorly to the femoral condyle and intercondylar notch. The IPFP is composed of adipocytes and adipose connective tissues containing collagen that is embedded in an amorphous ground substance encompassing glycosaminoglycans. It can be divided into two portions, inner and outer tissues[11]. The inner tissue is the core of the pad with hard pillow-like adipose tissue with cushioning properties, whereas the outer tissue is a soft adipose tissue surrounding the inner tissue. It was described that the inner tissue may undergo a compressive load and the outer tissue may undergo a tensional load. The IPFP has the space-filling properties in the joint cavity, which implies an essential role in joint function such as secreting synovial fluid[12], promoting lubrication[13], and shock absorption.
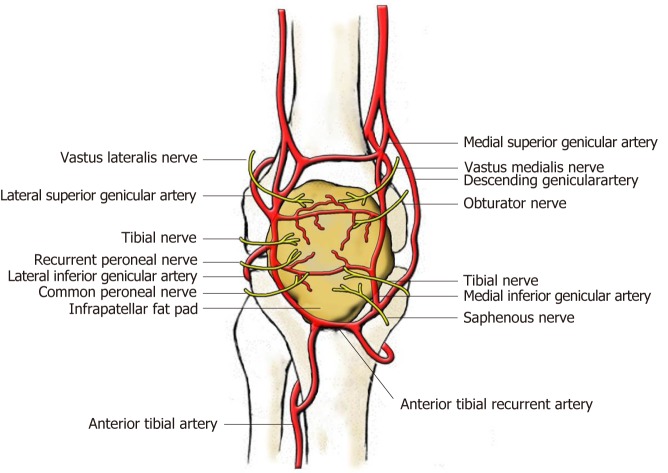
The periphery of the IPFP is highly vascularized, but the center is poorly vascularized. The blood of IPFP is supplied by two vertical arteries, which are connected by two to three horizontal arteries[14] (Figure 1). The primary blood supply originates from the synovial membrane. The IPFP is also richly innervated and contains lymphatic vessels. Bohnsack et al[15] and Witoński et al[16] found significant distribution of substance-P (SP) nerves inside the IPFP. As a neurotransmitter, SP is released from primary afferent nerve endings and exists in the central, autonomous, and peripheral nerve systems. Besides pain mediation, SP also plays an important role in chronic inflammatory conditions[17]. This neurogenous inflammation is hypothesized to attribute to anterior knee pain. As reported, the nerves of the IPFP are originating from the posterior articular branch of the posterior tibial nerve[18]. However, a study aiming at knee joint innervation described the innervations of the IPFP in detail[19]. The anteromedial portion of the IPFP is innervated by branches of the saphenous, tibial, and obturator nerves and the nerve to vastus medialis, while the anterolateral portion is supplied by branches from the nerve to vastus lateralis as well as the tibial, recurrent peroneal, and common peroneal nerves. The major component of the IPFP is adipose tissue, which can secrete proinflammatory cytokines and growth factors[20]; therefore, the IPFP could be believed to play a role in joint inflammation. As reported, the IPFP could participate in OA synovial inflammation and the severe inflammation in the IPFP was associated with severe pain in OA[21,22].
Figure 1.
Blood and nerve supply of the infrapatellar fat pad (view from the front).
DUAL ROLE OF IPFP IN KOA
The IPFP itself can not only secrete large amounts of inflammatory cytokines, adipokines, and growth factors, but also respond to the local inflammatory environment in the joint. A cross-talk between the IPFP and the joint exists (Figure 2). Many scholars believe that the IPFP plays an important role in KOA, but these results are not completely consistent[23].
Figure 2.
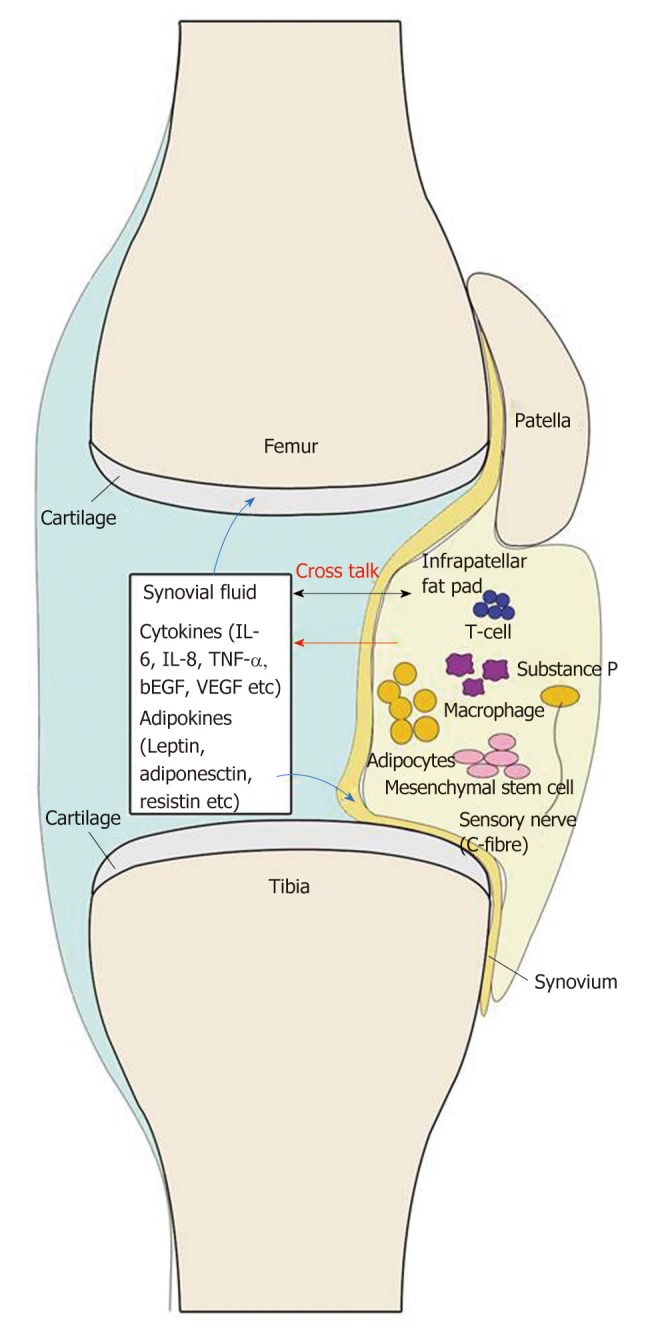
Current view of the infrapatellar fat pad and its interaction with other joint tissues. The cells in the infrapatellar fat pad (IPFP) secrete cytokines, adipokines, and other factors to the synovial fluid, and then these factors react on the cells in the IPFP, synovium, and cartilage.
Positive effect of IPFP in KOA
Since the IPFP is located between articular cartilage and bone surface, it may reduce the knee load and protect the knee joint under physiological conditions or in the early stage of KOA[24]. It has been reported that the IPFP can improve the distribution of joint fluid in joints by increasing synovial area and reduce the friction during exercise[25]. In 2014, Pan et al[24] conducted a cohort study of 1100 community populations. They found that the maximum area of female IPFP was negatively correlated with the degree of medial tibial plateau and femoral cartilage damage, and the WOMAC score of knee pain at rest through multivariate analysis. For every 1 cm2 increase in the area of the IPFP, the score of female knee pain at rest decreased by 0.86 points after 2.6 years. However, the maximum area of female IPFP had nothing to do with the degree of cartilage damage of the lateral tibial plateau and the WOMAC score when walking or going up and down stairs. The above association is not seen in men. It can be seen that the IPFP is at least associated with pain symptoms in women with KOA and has protective effects on cartilage.
Han et al[26] conducted a cross-sectional survey of 977 community populations in 2014. The result showed that the area of the IPFP was significantly positively correlated with age, height, and articular cartilage volume. However, the area of the IPFP had nothing to do with body mass index (BMI), although BMI was recognized as a risk factor for KOA. They also found that the area of the IPFP was negatively correlated with osteophytes and subchondral bone marrow edema, and these two indicators were associated with anterior knee pain and cartilage degradation, and were the most common lesions of the subchondral bone in KOA. In addition, some researchers have found that the volume of the IPFP was positively correlated with age though it was not related to the BMI of healthy people and patients with KOA, indicating that changes in body metabolism do not necessarily affect the IPFP, and that the IPFP might not be an inflammatory factor in the early stages of KOA[27]. Therefore, the IPFP may play a protective role in the early stages of KOA.
The IPFP may protect the knee joint by secreting protective biochemical factors. Some researchers have found that lipid-mediated lipoxygen A4 levels in IPFP-derived fat-regulating mediators were higher in healthy people, which can prevent cartilage degradation in the knee[28,29]. In addition, leptin secreted by the IPFP can promote the production of articular cartilage proteoglycan and type II collagen, stimulate the synthesis of insulin-like growth factor-1 and transforming growth factor-β, enhance chondrocyte proliferation, and thus protect against the pathogenesis of KOA[30-34]. What’s more, IPFP-derived mesenchymal stem cells (MSCs) have a greater chondroitin effect than bone marrow-derived MSCs, and it can also block the secretion of proinflammatory mediators in synovial and chondrocytes of OA patients[35,36]. A randomized controlled trial in 2015 also showed that mixed transplantation of the IPFP and type I collagen scaffold in the animal model of meniscus injury has better meniscus repair than simple type I collagen scaffold transplantation, which can relieve the formation of KOA to a certain extent[37].
Another important reason may be that the IPFP has the effect of relieving shock, since abnormal biomechanical load plays an important role in the occurrence and development of KOA[38]. Meanwhile, the IPFP may also increase the stability of the knee joint, like the patellar ligament, so as to prevent the occurrence of KOA.
Negative effect of IPFP in KOA
The IPFP is in close contact with the synovial layer and articular cartilage, producing cytokines locally in the joint cavity[39]. In patients with KOA, the IPFP can secrete higher levels of inflammatory factors and adipokines than subcutaneous fat, such as IL-6, lipase, adiponectin, and visfatin, which can promote the pathological process of KOA[40].
Obesity state, indeed, is described as a chronic active inflammatory condition[41], as manifested by increased levels of the inflammatory markers C-reactive protein and IL-6 in the systemic circulation of obese individuals. Obesity has been demonstrated as an important risk factor for the incidence of OA, not only because of mechanically overweight load but also metabolic effects. KOA is the most common OA[42]. Increased weight in women could elevate the risk for KOA[43], while weight loss may reduce the risk for developing symptomatic KOA among adults[44] and reducing BMI could bring healthy benefits[45]. The overload of obese people allows an easy understanding for the mechanical pathogenesis of OA. However, the metabolic factor was observed in the development of OA in non-weight-bearing joints, such as hand OA in overweight or high BMI people. Adipokines secreted by adipose tissue may be involved in the development of OA[5,46]. In 2014, Ballegaard et al[22] found that KOOS pain scores in obese patients with KOA were significantly positively correlated with magnetic resonance imaging (MRI) inflammatory signal variables in the IPFP, so inflammatory IPFP in obese patients with KOA may cause knee pain. A cross-sectional study by Cowan in 2015 also showed that patellofemoral arthritis patients had greater IPFP volume on MRI compared with healthy knees, and the volume of IPFP was also positively correlated with KOOS pain scores[47]. The reduction of joint space in patients with KOA can lead to inflammation of the IPFP, which can increase the secretion of synovial inflammatory factors on the surface of femoral condyle while causing swelling of the patellar ligament[48,49].
In addition, some studies hold the opposite view that leptin exerts a proinflammatory role, even though leptin secreted by the IPFP has a protective effect on joints. Leptin level was higher in synovial fluid and serum of OA patients compared with controls[50,51] and had a positive correlation with severity of OA[52]. An in vitro study showed that leptin could increase matrix metallopreteinase (MMP) production in human osteoarthritic cartilage and correlated with MMP-1 and MMP-3 in OA synovial fluid[53]. Leptin could also increase the gene expression of a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)-4 and -5[33] and production of NO, PGE2, IL-6, and IL-8[54]. Therefore, inflammatory IPFP may also have a negative effect in the pathogenesis of KOA.
ROLE OF IPFP IN ANTERIOR KNEE PAIN
In patients with KOA, anterior knee pain is a very important complaint of patients, and it is also a problem that patients want to solve urgently. Benjamin et al[55] and Eivazi et al[56] had proposed the conception of an “enthesis organ”, including the structures like ligament, tendon, or joint capsule attached to bone. The IPFP is one of the most important components of the enthesis organ in the anterior knee region. It is always considered as a potential pathogenic factor of knee pain, especially anterior knee pain. The IPFP pressure varies when the knee motions[57] and hyperpression would induce chronic hyperplasia of the IPFP[58]. However, the mechanical hypertrophy or swelling is not the main cause of anterior knee pain. Inflammation is the onset of any pain. The inflammatory mediators increased in the IPFP in patients with anterior knee pain[59]. On the other hand, chronic hypertrophy of the IPFP and concomitant soft tissue impingement lead to ischemia, induce the abnormal distribution of SP, and ultimately result in chronic neurogenic tissue inflammation[60,61]. Zhang et al[62] showed that the signal intensity change of the IPFP on MRI was closely related to the fluctuation of knee pain symptoms in patients with KOA. However, excision of the IPFP did not improve knee joint function, range of motion, or symptoms of anterior knee pain in patients with KOA, and the exact relationship between the IPFP and knee pain before KOA has not been elucidated[63].
FUTURE APPLICATION OF IPFP IN TISSUE ENGINEERING
Human MSCs have been identified to be multipotent and can be isolated from a large number of adult tissues such as bone marrow, adipose tissue, and umbilical cord blood. MSCs[64,65]. They have tissue-regenerative properties that exert potent immunomodulatory, antiapoptotic, antifibrotic, and anti-inflammatory effects[35,66,67]. MSCs from the IPFP have been proved to possess significant chondrogenic potential and could provide a clinically feasible source of chondroprogenitor cells[68,69]. MSCs from the IPFP are found to be therapeutic for a one-step surgical procedure to regenerate cartilage tissue[70] and improve symptoms of KOA by intra-articular injection[71]. The extraction procedure of MSCs derived from bone marrow, however, is invasive[72]. In this respect, MSCs isolated from adipose tissue is less invasive and MSCs derived from intra-articular joint tissues are more phenotypically similar to chondrocytes[73]. In the presence of chondrogenic media, fat pad-derived MSCs produce Types I, II, and VI collagen. Type VI collagen, which also presents in articular cartilage, plays an important role in the interaction between chondrocytes and the extracellular matrix[74]. Furthermore, Luo et al[69,75] demonstrated that IPFP-derived stem cells could produce a structure and spatial composition mimicking those of native articular cartilage[75]. Prabhakar et al[76] isolated progenitor cells from the IPFP, expanded, and then seeded them onto a mechanically stable biodegradable polymer film. After culturing the cells for 28 d, they found the self-assembled tissue rich in sulfated glycosaminoglycan and collagen which had the potential to be implanted into defect sites as a potential treatment for cartilage defect regeneration. Therefore, benefiting from its location, minimal invasiveness, and chondrogenic potential, the IPFP could be used for treating OA via repairing damaged cartilage.
DISCUSSION
In this review, we discuss the role of the IPFP in the disease process of KOA and its application prospect in the treatment of this disease. OA has been regarded as a degenerative joint disease with alteration of articular cartilage. Inflammation is recognized as contributing to the symptoms and progression of OA. Obesity is nowadays considered as a chronic low-grade inflammatory status which is related to the release of inflammatory mediators by adipose tissue. The IPFP is indeed a form of adipose tissue and enlarges with age[27]. Due to its close location with articular cartilage and the synovium, it can be speculated with much relationship with OA and synovitis.
Adipocytes, preadipocytes, macrophages, fibroblasts, and other cells in the IPFP release inflammatory mediators such as adipokines and cytokines growth factors that influence cartilage degeneration and synovitis. With the characteristics of minimal invasiveness and multipotentiality, MSCs are now being isolated from the IPFP to provide novel therapies for KOA.
However, further studies should be performed to investigate the precise role of the IPFP in KOA. The role of adipokines in pain should be investigated. In addition, whether adipokines exert their effect on nociceptors in joints remain to be studied. Besides the effect of mediators released by the IPFP on cartilage, tissues such as the synovium and bone marrow are also involved in KOA, so it is also pertinent to explore the effect of mediators on these tissues.
In conclusion, we consider that the IPFP plays an important role in the physiopathology of KOA. Further attention should be focused on the IPFP to explore its new application in KOA.
ACKNOWLEDGEMENTS
Many thanks to Guodong Peng for providing help of the pictures.
Footnotes
Conflict-of-interest statement: There are no conflicts of interest declared by any other author.
Manuscript source: Unsolicited manuscript
Peer-review started: January 22, 2019
First decision: May 31, 2019
Article in press: June 27, 2019
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Exbrayat JM, Yukata K S-Editor: Dou Y L-Editor: A E-Editor: Liu JH
Contributor Information
Li-Feng Jiang, Department of Orthopedics Surgery, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310000, Zhejiang Province, China.
Jing-Hua Fang, Department of Orthopedics Surgery, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310000, Zhejiang Province, China.
Li-Dong Wu, Department of Orthopedics Surgery, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310000, Zhejiang Province, China. wulidong@zju.edu.cn.
References
- 1.Marhadour T, Guellec D, Saraux A, Devauchelle-Pensec V, Jousse-Joulin S, Cornec D. [Osteoarthritis epidemiology and risk factors] Soins. 2012;(768):28–29. [PubMed] [Google Scholar]
- 2.Hunter DJ, Felson DT. Osteoarthritis. BMJ. 2006;332:639–642. doi: 10.1136/bmj.332.7542.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Felson DT. Osteoarthritis as a disease of mechanics. Osteoarthritis Cartilage. 2013;21:10–15. doi: 10.1016/j.joca.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osteoarthritis: causes and diagnosis. Harvard Men's Health Watch, 2006. Available from: https://www.health.harvard.edu/newsletter_article/Osteoarthritis_Causes_and_diagnosis.
- 5.Berenbaum F, Eymard F, Houard X. Osteoarthritis, inflammation and obesity. Curr Opin Rheumatol. 2013;25:114–118. doi: 10.1097/BOR.0b013e32835a9414. [DOI] [PubMed] [Google Scholar]
- 6.Hochberg MC. Osteoarthritis year 2012 in review: clinical. Osteoarthritis Cartilage. 2012;20:1465–1469. doi: 10.1016/j.joca.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 7.Goldring MB. The role of the chondrocyte in osteoarthritis. Arthritis Rheum-US. 2000;43:1916–1926. doi: 10.1002/1529-0131(200009)43:9<1916::AID-ANR2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 8.Swan A, Mercer S. Anatomy of the infrapatellar fat pad. New Zealand J Physiother. 2005;33:19–22. [Google Scholar]
- 9.Hoffa A. The influence of the adipose tissue with regard to the pathology of the knee joint. JAMA. 1904;43:795–0796. [Google Scholar]
- 10.Gallagher J, Tierney P, Murray P, O'Brien M. The infrapatellar fat pad: anatomy and clinical correlations. Knee Surg Sports Traumatol Arthrosc. 2005;13:268–272. doi: 10.1007/s00167-004-0592-7. [DOI] [PubMed] [Google Scholar]
- 11.Nakano T, Wang YW, Ozimek L, Sim JS. Chemical composition of the infrapatellar fat pad of swine. J Anat. 2004;204:301–306. doi: 10.1111/j.0021-8782.2004.00283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davies DV, White JE. The structure and weight of synovial fat pads. J Anat. 1961;95:30–37. [PMC free article] [PubMed] [Google Scholar]
- 13.MacConaill MA. The movements of bones and joints; the synovial fluid and its assistants. J Bone Joint Surg Br. 1950;32-B:244–252. doi: 10.1302/0301-620X.32B2.244. [DOI] [PubMed] [Google Scholar]
- 14.Kohn D, Deiler S, Rudert M. Arterial blood supply of the infrapatellar fat pad. Anatomy and clinical consequences. Arch Orthop Trauma Surg. 1995;114:72–75. doi: 10.1007/BF00422828. [DOI] [PubMed] [Google Scholar]
- 15.Bohnsack M, Meier F, Walter GF, Hurschler C, Schmolke S, Wirth CJ, Rühmann O. Distribution of substance-P nerves inside the infrapatellar fat pad and the adjacent synovial tissue: a neurohistological approach to anterior knee pain syndrome. Arch Orthop Trauma Surg. 2005;125:592–597. doi: 10.1007/s00402-005-0796-4. [DOI] [PubMed] [Google Scholar]
- 16.Witoński D, Wagrowska-Danielewicz M. Distribution of substance-P nerve fibers in the knee joint in patients with anterior knee pain syndrome. A preliminary report. Knee Surg Sports Traumatol Arthrosc. 1999;7:177–183. doi: 10.1007/s001670050144. [DOI] [PubMed] [Google Scholar]
- 17.Harrison S, Geppetti P. Substance p. Int J Biochem Cell Biol. 2001;33:555–576. doi: 10.1016/s1357-2725(01)00031-0. [DOI] [PubMed] [Google Scholar]
- 18.Saddik D, McNally EG, Richardson M. MRI of Hoffa's fat pad. Skeletal Radiol. 2004;33:433–444. doi: 10.1007/s00256-003-0724-z. [DOI] [PubMed] [Google Scholar]
- 19.GARDNER E. The innervation of the knee joint. Anat Rec. 1948;101:109–130. doi: 10.1002/ar.1091010111. [DOI] [PubMed] [Google Scholar]
- 20.Coppack SW. Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc. 2001;60:349–356. doi: 10.1079/pns2001110. [DOI] [PubMed] [Google Scholar]
- 21.Eymard F, Pigenet A, Citadelle D, Lachaniette CH, Poignard A, Benelli C, Berenbaum F, Chevalier X, Houard X. Infrapatellar fat pad induces an inflammatory and a pro-degradative phenotype in autologous fibroblast-like synoviocytes from patients with knee OA. Osteoarthr Cartila. 2014;22:S448. doi: 10.1002/art.38657. [DOI] [PubMed] [Google Scholar]
- 22.Ballegaard C, Riis RG, Bliddal H, Christensen R, Henriksen M, Bartels EM, Lohmander LS, Hunter DJ, Bouert R, Boesen M. Knee pain and inflammation in the infrapatellar fat pad estimated by conventional and dynamic contrast-enhanced magnetic resonance imaging in obese patients with osteoarthritis: A cross-sectional study. Osteoarthr Cartila. 2014:22. doi: 10.1016/j.joca.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 23.Stannus OP, Jones G, Blizzard L, Cicuttini FM, Ding C. Associations between serum levels of inflammatory markers and change in knee pain over 5 years in older adults: a prospective cohort study. Ann Rheum Dis. 2013;72:535–540. doi: 10.1136/annrheumdis-2011-201047. [DOI] [PubMed] [Google Scholar]
- 24.Pan F, Han W, Wang X, Liu Z, Jin X, Antony B, Cicuttini F, Jones G, Ding C. A longitudinal study of the association between infrapatellar fat pad maximal area and changes in knee symptoms and structure in older adults. Ann Rheum Dis. 2015;74:1818–1824. doi: 10.1136/annrheumdis-2013-205108. [DOI] [PubMed] [Google Scholar]
- 25.Ding C, Stannus O, Cicuttini F, Antony B, Jones G. Body fat is associated with increased and lean mass with decreased knee cartilage loss in older adults: a prospective cohort study. Int J Obes (Lond) 2013;37:822–827. doi: 10.1038/ijo.2012.136. [DOI] [PubMed] [Google Scholar]
- 26.Han W, Cai S, Liu Z, Jin X, Wang X, Antony B, Cao Y, Aitken D, Cicuttini F, Jones G, Ding C. Infrapatellar fat pad in the knee: is local fat good or bad for knee osteoarthritis? Arthritis Res Ther. 2014;16:R145. doi: 10.1186/ar4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chuckpaiwong B, Charles HC, Kraus VB, Guilak F, Nunley JA. Age-associated increases in the size of the infrapatellar fat pad in knee osteoarthritis as measured by 3T MRI. J Orthop Res. 2010;28:1149–1154. doi: 10.1002/jor.21125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gierman LM, Wopereis S, van El B, Verheij ER, Werff-van der Vat BJ, Bastiaansen-Jenniskens YM, van Osch GJ, Kloppenburg M, Stojanovic-Susulic V, Huizinga TW, Zuurmond AM. Metabolic profiling reveals differences in concentrations of oxylipins and fatty acids secreted by the infrapatellar fat pad of donors with end-stage osteoarthritis and normal donors. Arthritis Rheum. 2013;65:2606–2614. doi: 10.1002/art.38081. [DOI] [PubMed] [Google Scholar]
- 29.Bastiaansen-Jenniskens YM, Clockaerts S, Feijt C, Zuurmond AM, Stojanovic-Susulic V, Bridts C, de Clerck L, DeGroot J, Verhaar JA, Kloppenburg M, van Osch GJ. Infrapatellar fat pad of patients with end-stage osteoarthritis inhibits catabolic mediators in cartilage. Ann Rheum Dis. 2012;71:288–294. doi: 10.1136/ard.2011.153858. [DOI] [PubMed] [Google Scholar]
- 30.Nakajima R, Inada H, Koike T, Yamano T. Effects of leptin to cultured growth plate chondrocytes. Horm Res. 2003;60:91–98. doi: 10.1159/000071877. [DOI] [PubMed] [Google Scholar]
- 31.Dumond H, Presle N, Terlain B, Mainard D, Loeuille D, Netter P, Pottie P. Evidence for a key role of leptin in osteoarthritis. Arthritis Rheum. 2003;48:3118–3129. doi: 10.1002/art.11303. [DOI] [PubMed] [Google Scholar]
- 32.Figenschau Y, Knutsen G, Shahazeydi S, Johansen O, Sveinbjörnsson B. Human articular chondrocytes express functional leptin receptors. Biochem Biophys Res Commun. 2001;287:190–197. doi: 10.1006/bbrc.2001.5543. [DOI] [PubMed] [Google Scholar]
- 33.Bao JP, Chen WP, Feng J, Hu PF, Shi ZL, Wu LD. Leptin plays a catabolic role on articular cartilage. Mol Biol Rep. 2010;37:3265–3272. doi: 10.1007/s11033-009-9911-x. [DOI] [PubMed] [Google Scholar]
- 34.Bao JP, Jiang LF, Chen WP, Hu PF, Wu LD. Expression of vaspin in the joint and the levels in the serum and synovial fluid of patients with osteoarthritis. Int J Clin Exp Med. 2014;7:3447–3453. [PMC free article] [PubMed] [Google Scholar]
- 35.Manferdini C, Maumus M, Gabusi E, Piacentini A, Filardo G, Peyrafitte JA, Jorgensen C, Bourin P, Fleury-Cappellesso S, Facchini A, Noël D, Lisignoli G. Adipose-derived mesenchymal stem cells exert antiinflammatory effects on chondrocytes and synoviocytes from osteoarthritis patients through prostaglandin E2. Arthritis Rheum. 2013;65:1271–1281. doi: 10.1002/art.37908. [DOI] [PubMed] [Google Scholar]
- 36.English A, Jones EA, Corscadden D, Henshaw K, Chapman T, Emery P, McGonagle D. A comparative assessment of cartilage and joint fat pad as a potential source of cells for autologous therapy development in knee osteoarthritis. Rheumatology (Oxford) 2007;46:1676–1683. doi: 10.1093/rheumatology/kem217. [DOI] [PubMed] [Google Scholar]
- 37.Oda S, Otsuki S, Kurokawa Y, Hoshiyama Y, Nakajima M, Neo M. A new method for meniscus repair using type I collagen scaffold and infrapatellar fat pad. J Biomater Appl. 2015;29:1439–1448. doi: 10.1177/0885328215568984. [DOI] [PubMed] [Google Scholar]
- 38.Guilak F, Fermor B, Keefe FJ, Kraus VB, Olson SA, Pisetsky DS, Setton LA, Weinberg JB. The role of biomechanics and inflammation in cartilage injury and repair. Clin Orthop Relat Res. 2004:17–26. doi: 10.1097/01.blo.0000131233.83640.91. [DOI] [PubMed] [Google Scholar]
- 39.Ushiyama T, Chano T, Inoue K, Matsusue Y. Cytokine production in the infrapatellar fat pad: another source of cytokines in knee synovial fluids. Ann Rheum Dis. 2003;62:108–112. doi: 10.1136/ard.62.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klein-Wieringa IR, Kloppenburg M, Bastiaansen-Jenniskens YM, Yusuf E, Kwekkeboom JC, El-Bannoudi H, Nelissen RG, Zuurmond A, Stojanovic-Susulic V, Van Osch GJ, Toes RE, Ioan-Facsinay A. The infrapatellar fat pad of patients with osteoarthritis has an inflammatory phenotype. Ann Rheum Dis. 2011;70:851–857. doi: 10.1136/ard.2010.140046. [DOI] [PubMed] [Google Scholar]
- 41.Balistreri CR, Caruso C, Candore G. The role of adipose tissue and adipokines in obesity-related inflammatory diseases. Mediators Inflamm. 2010;2010:802078. doi: 10.1155/2010/802078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parmet S, Lynm C, Glass RM. JAMA patient page. Osteoarthritis of the knee. Jama-J Am Med Assoc. 2003;289:1068. doi: 10.1001/jama.289.8.1068. [DOI] [PubMed] [Google Scholar]
- 43.Felson DT, Zhang Y, Hannan MT, Naimark A, Weissman B, Aliabadi P, Levy D. Risk factors for incident radiographic knee osteoarthritis in the elderly: the Framingham Study. Arthritis Rheum-US. 1997;40:728–733. doi: 10.1002/art.1780400420. [DOI] [PubMed] [Google Scholar]
- 44.Muthuri SG, Hui M, Doherty M, Zhang W. What if we prevent obesity? Risk reduction in knee osteoarthritis estimated through a meta-analysis of observational studies. Arthritis Care Res (Hoboken) 2011;63:982–990. doi: 10.1002/acr.20464. [DOI] [PubMed] [Google Scholar]
- 45.Losina E, Walensky RP, Reichmann WM, Holt HL, Gerlovin H, Solomon DH, Jordan JM, Hunter DJ, Suter LG, Weinstein AM, Paltiel AD, Katz JN. Impact of obesity and knee osteoarthritis on morbidity and mortality in older Americans. Ann Intern Med. 2011;154:217–226. doi: 10.1059/0003-4819-154-4-201102150-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yusuf E, Nelissen RG, Ioan-Facsinay A, Stojanovic-Susulic V, DeGroot J, van Osch G, Middeldorp S, Huizinga TW, Kloppenburg M. Association between weight or body mass index and hand osteoarthritis: a systematic review. Ann Rheum Dis. 2010;69:761–765. doi: 10.1136/ard.2008.106930. [DOI] [PubMed] [Google Scholar]
- 47.Cowan SM, Hart HF, Warden SJ, Crossley KM. Infrapatellar fat pad volume is greater in individuals with patellofemoral joint osteoarthritis and associated with pain. Rheumatol Int. 2015;35:1439–1442. doi: 10.1007/s00296-015-3250-0. [DOI] [PubMed] [Google Scholar]
- 48.Schweitzer ME, Falk A, Berthoty D, Mitchell M, Resnick D. Knee effusion: normal distribution of fluid. AJR Am J Roentgenol. 1992;159:361–363. doi: 10.2214/ajr.159.2.1632356. [DOI] [PubMed] [Google Scholar]
- 49.Clements KM, Ball AD, Jones HB, Brinckmann S, Read SJ, Murray F. Cellular and histopathological changes in the infrapatellar fat pad in the monoiodoacetate model of osteoarthritis pain. Osteoarthritis Cartilage. 2009;17:805–812. doi: 10.1016/j.joca.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 50.Beekhuizen M, Gierman LM, van Spil WE, Van Osch GJ, Huizinga TW, Saris DB, Creemers LB, Zuurmond AM. An explorative study comparing levels of soluble mediators in control and osteoarthritic synovial fluid. Osteoarthritis Cartilage. 2013;21:918–922. doi: 10.1016/j.joca.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 51.de Boer TN, van Spil WE, Huisman AM, Polak AA, Bijlsma JW, Lafeber FP, Mastbergen SC. Serum adipokines in osteoarthritis; comparison with controls and relationship with local parameters of synovial inflammation and cartilage damage. Osteoarthritis Cartilage. 2012;20:846–853. doi: 10.1016/j.joca.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 52.Staikos C, Ververidis A, Drosos G, Manolopoulos VG, Verettas DA, Tavridou A. The association of adipokine levels in plasma and synovial fluid with the severity of knee osteoarthritis. Rheumatology (Oxford) 2013;52:1077–1083. doi: 10.1093/rheumatology/kes422. [DOI] [PubMed] [Google Scholar]
- 53.Koskinen A, Vuolteenaho K, Nieminen R, Moilanen T, Moilanen E. Leptin enhances MMP-1, MMP-3 and MMP-13 production in human osteoarthritic cartilage and correlates with MMP-1 and MMP-3 in synovial fluid from OA patients. Clin Exp Rheumatol. 2011;29:57–64. [PubMed] [Google Scholar]
- 54.Vuolteenaho K, Koskinen A, Kukkonen M, Nieminen R, Päivärinta U, Moilanen T, Moilanen E. Leptin enhances synthesis of proinflammatory mediators in human osteoarthritic cartilage--mediator role of NO in leptin-induced PGE2, IL-6, and IL-8 production. Mediators Inflamm. 2009;2009:345838. doi: 10.1155/2009/345838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Benjamin M, Moriggl B, Brenner E, Emery P, McGonagle D, Redman S. The "enthesis organ" concept: why enthesopathies may not present as focal insertional disorders. Arthritis Rheum. 2004;50:3306–3313. doi: 10.1002/art.20566. [DOI] [PubMed] [Google Scholar]
- 56.Eivazi MG, Selfe J. Infrapatellar fat pad lesions: theoretical considerations and practical implications. Physical Therapy Reviews. 2008;13:11–16. [Google Scholar]
- 57.Bohnsack M, Hurschler C, Demirtas T, Rühmann O, Stukenborg-Colsman C, Wirth CJ. Infrapatellar fat pad pressure and volume changes of the anterior compartment during knee motion: possible clinical consequences to the anterior knee pain syndrome. Knee Surg Sports Traumatol Arthrosc. 2005;13:135–141. doi: 10.1007/s00167-004-0561-1. [DOI] [PubMed] [Google Scholar]
- 58.Bohnsack M, Klages P, Hurschler C, Halcour A, Wilharm A, Ostermeier S, Rühmann O, Wirth CJ. Influence of an infrapatellar fat pad edema on patellofemoral biomechanics and knee kinematics: a possible relation to the anterior knee pain syndrome. Arch Orthop Trauma Surg. 2009;129:1025–1030. doi: 10.1007/s00402-006-0237-z. [DOI] [PubMed] [Google Scholar]
- 59.Witoński D, Wągrowska-Danilewicz M, Kęska R, Raczyńska-Witońska G, Stasikowska-Kanicka O. Increased interleukin 6 and tumour necrosis factor α expression in the infrapatellar fat pad of the knee joint with the anterior knee pain syndrome: a preliminary report. Pol J Pathol. 2010;61:213–218. [PubMed] [Google Scholar]
- 60.Malcangio M, Bowery NG. Peptide autoreceptors: does an autoreceptor for substance P exist? Trends Pharmacol Sci. 1999;20:405–407. doi: 10.1016/S0165-6147(99)01388-7. [DOI] [PubMed] [Google Scholar]
- 61.Woolf CJ, Allchorne A, Safieh-Garabedian B, Poole S. Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumour necrosis factor alpha. Br J Pharmacol. 1997;121:417–424. doi: 10.1038/sj.bjp.0701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Y, Nevitt M, Niu J, Lewis C, Torner J, Guermazi A, Roemer F, McCulloch C, Felson DT. Fluctuation of knee pain and changes in bone marrow lesions, effusions, and synovitis on magnetic resonance imaging. Arthritis Rheum. 2011;63:691–699. doi: 10.1002/art.30148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Beeck A, Clockaerts S, Somville J, Van Heeswijk JH, Van Glabbeek F, Bos PK, Reijman M. Does infrapatellar fat pad resection in total knee arthroplasty impair clinical outcome? A systematic review. Knee. 2013;20:226–231. doi: 10.1016/j.knee.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 64.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 65.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 66.Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9:11–15. doi: 10.1016/j.stem.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol Ther. 2012;20:14–20. doi: 10.1038/mt.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Buckley CT, Vinardell T, Kelly DJ. Oxygen tension differentially regulates the functional properties of cartilaginous tissues engineered from infrapatellar fat pad derived MSCs and articular chondrocytes. Osteoarthritis Cartilage. 2010;18:1345–1354. doi: 10.1016/j.joca.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 69.Luo L, Thorpe SD, Buckley CT, Kelly DJ. The effects of dynamic compression on the development of cartilage grafts engineered using bone marrow and infrapatellar fat pad derived stem cells. Biomed Mater. 2015;10:055011. doi: 10.1088/1748-6041/10/5/055011. [DOI] [PubMed] [Google Scholar]
- 70.Jurgens WJ, van Dijk A, Doulabi BZ, Niessen FB, Ritt MJ, van Milligen FJ, Helder MN. Freshly isolated stromal cells from the infrapatellar fat pad are suitable for a one-step surgical procedure to regenerate cartilage tissue. Cytotherapy. 2009;11:1052–1064. doi: 10.3109/14653240903219122. [DOI] [PubMed] [Google Scholar]
- 71.Koh YG, Jo SB, Kwon OR, Suh DS, Lee SW, Park SH, Choi YJ. Mesenchymal stem cell injections improve symptoms of knee osteoarthritis. Arthroscopy. 2013;29:748–755. doi: 10.1016/j.arthro.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 72.Im GI, Kim DY, Shin JH, Hyun CW, Cho WH. Repair of cartilage defect in the rabbit with cultured mesenchymal stem cells from bone marrow. J Bone Joint Surg Br. 2001;83:289–294. doi: 10.1302/0301-620x.83b2.10495. [DOI] [PubMed] [Google Scholar]
- 73.Segawa Y, Muneta T, Makino H, Nimura A, Mochizuki T, Ju YJ, Ezura Y, Umezawa A, Sekiya I. Mesenchymal stem cells derived from synovium, meniscus, anterior cruciate ligament, and articular chondrocytes share similar gene expression profiles. J Orthop Res. 2009;27:435–441. doi: 10.1002/jor.20786. [DOI] [PubMed] [Google Scholar]
- 74.Wickham MQ, Erickson GR, Gimble JM, Vail TP, Guilak F. Multipotent stromal cells derived from the infrapatellar fat pad of the knee. Clin Orthop Relat Res. 2003:196–212. doi: 10.1097/01.blo.0000072467.53786.ca. [DOI] [PubMed] [Google Scholar]
- 75.Luo L, O'Reilly AR, Thorpe SD, Buckley CT, Kelly DJ. Engineering zonal cartilaginous tissue by modulating oxygen levels and mechanical cues through the depth of infrapatellar fat pad stem cell laden hydrogels. J Tissue Eng Regen Med. 2017;11:2613–2628. doi: 10.1002/term.2162. [DOI] [PubMed] [Google Scholar]
- 76.Prabhakar A, Lynch AP, Ahearne M. Self-Assembled Infrapatellar Fat-Pad Progenitor Cells on a Poly-ε-Caprolactone Film For Cartilage Regeneration. Artif Organs. 2016;40:376–384. doi: 10.1111/aor.12565. [DOI] [PubMed] [Google Scholar]