Abstract
BACKGROUND
In China, hepatitis B virus-related acute-on-chronic liver failure (HBV-ACLF) is the most common liver failure characterized by serious clinical syndromes of liver decompensation with a very high mortality. Bacterial and/or fungal infections are the most common complications that are associated with high short-term mortality. Bacterial translocation from the intestine, impaired hepatic clearance, and immune paralysis of circulating immune cells are thought to contribute to infectious complications in liver failure. The control of bacterial and fungal infections is the key to improving HBV-ACLF outcomes. Active prevention, early diagnosis, and timely treatment of bacterial and fungal infections are essential for treating HBV-ACLF.
AIM
To investigate the frequency and role of bacterial and fungal infections in patients with HBV-ACLF.
METHODS
Patients with HBV-ACLF hospitalized at Taihe Hospital, Hubei University of Medicine from January 2014 to December 2017 were retrospectively enrolled. Patient-related information was retrieved from the hospital case database, including general information, blood biochemistry, complications, etc. According to the occurrence of secondary infection or not, the patients were divided into an infection group and a non-infection group. The sites, types, and incidences of bacterial and fungal infections and the influence of infections on the prognosis of HBV-ACLF were statistically analyzed. The risk factors for infections were assessed by unconditional logistic regression.
RESULTS
There were 174 cases of HBV-ACLF that met the enrollment criteria, of which 114 (65.52%) were diagnosed with infectious complications. Infections occurred in the abdominal cavity (87 cases), respiratory tract (51 cases), urinary tract (18 cases), and biliary tract (10 cases). Patients with infectious complications had a significantly higher 28-d mortality (70.18%, 80/114) than those without (40.00%, 24/60) (70.18% vs 40.00%, P < 0.05). And patients with infectious complications had a much higher incidence of non-infectious complications (54.39%, 62/114) (54.39% vs 15.00%, P < 0.05), leading to an extremely high 28-d mortality of 88.71% (55/62) (P < 0.05). The grade of liver failure, period of hospital stay ≥ 30 d, age ≥ 45 years, and percentage of neutrophils > 70% were identified as risk factors for infectious complications.
CONCLUSION
The high incidence of infectious complications in patients with HBV-ACLF is associated with severity and deterioration of the disease and may contribute to the extremely high mortality of these patients.
Keywords: Hepatitis B, Acute-on-chronic liver failure, Bacterial infection, Fungal infection, Prognosis
Core tip: Hepatitis B virus-related acute-on-chronic liver failure (HBV-ACLF) is the most common type of liver failure with a high mortality and complications. Bacterial and/or fungal infections are the most common complications of liver failure. The aim of this study was to investigate the frequency and role of bacterial and fungal infections in patients with HBV-ACLF. A total of 174 patients with HBV-ACLF were retrospectively analyzed. Patients with infectious complications had a significantly higher mortality (70.18%) than those without (40.00%, 24/60). In conclusion, infections can significantly increase the mortality rate of liver failure.
INTRODUCTION
Liver failure is a severe form of liver damage caused by a variety of factors, manifested as destroyed liver anatomical structure, function failure, and occurrence of serious clinical syndromes of liver decompensation, with mortality rates as high as 50%-80%[1]. In China, the most common cause of liver failure is hepatitis virus infections, among which hepatitis B virus (HBV) is the most prevalent, and acute-on-chronic liver failure (ACLF) is the major clinical subtype[2]. The mechanism of ACLF is very complex and far from elucidated, although the “three-strike” theory is widely accepted[3]. HBV-related ACLF (HBV-ACLF) is often complicated by hepatic encephalopathy, hepatorenal syndrome, gastrointestinal hemorrhage, and bacterial and/or fungal infections[4], among which bacterial and/or fungal infections are the most common complications of liver failure. The rate of bacterial and fungal infections in liver failure patients can be as high as 81.2%[5]. Bacterial translocation from the intestine, impaired hepatic clearance, and immune paralysis of circulating immune cells are thought to contribute to infectious complications in liver failure[6].
Bacterial or fungal infections and liver failure are mutually causative and interactive. Infection-induced sepsis is a common cause of ACLF. Due to impaired immunity and hypoproteinemia, patients with middle- or late-stage liver failure are susceptible to bacterial and fungal infections[7]. Thus, bacterial and/or fungal infections are a trigger as well as a complication of liver failure. Since infections play such an important role in the occurrence, development, and prognosis of liver failure, the prevention, early diagnosis, and treatment of infections are indispensable in the management of liver failure. Preventing and controlling bacterial and/or fungal infections is extremely challenging due to the increasing incidence of antibiotic resistance and the diversification of multidrug-resistant bacteria[8].
In this study, 203 patients with HBV-ACLF were retrospectively analyzed. The aim of the study was to investigate the relationship between bacterial and/or fungal infections and prognosis. The characteristics of infection sites, infection types, and factors related to the development of infections in liver failure were also investigated.
MATERIALS AND METHODS
Patients
This retrospective study enrolled HBV-ACLF patients admitted to the Department of Infectious Diseases, Taihe Hospital, Shiyan, China, from January 2014 to December 2017. Diagnostic criteria for chronic hepatitis B were based on the Guideline of Prevention and Treatment for Chronic Hepatitis B (2015 Update)[9]. ACLF was defined according to Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the Study of the Liver 2014[10]. Diagnosis of infections was made in accordance with the CDC definitions for nosocomial infections, 1988[11]. The diagnostic criteria for fungal infection were based on the EORTC/MSG study[12]. Sepsis and septic shock were defined according to The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3)[13]. The exclusion of patients was based on the following criteria: (1) Co-infections with hepatitis A virus (HAV), hepatitis C virus (HCV), hepatitis D virus, hepatitis E virus (HEV), or human immunodeficiency virus; and (2) Liver failure complicated by alcoholic, drug-induced, or autoimmune liver diseases. This study met the ethical requirements and was approved by the Ethical Committee of Taihe Hospital.
Data collection
General information and clinical data were collected from the patient database of Taihe Hospital, including (1) Age and gender; (2) Biochemical indicators: G test, GM test, blood lactic acid, serum procalcitonin, C-reactive protein, erythrocyte sedimentation rate, alanine aminotransferase (ALT), aspartate aminotransferase (AST), glutamyl transferase, total bilirubin (TBIL), albumin (ALB), and creatinine; (3) White blood cell (WBC) count, percentage of neutrophils, and platelet count; (4) Coagulation function indicators: prothrombin activity, prothrombin time (PT), standardized international ratio; and (5) Complications of HBV-ACLF: bacterial and fungal infections, hepatic encephalopathy, hepatorenal syndrome, and gastrointestinal hemorrhage. Patients who left the hospital in an extremely critical condition or died were considered as dead.
Definitions related to infections and HBV-ACLF
Spontaneous bacterial peritonitis was defined as polymorphonuclear cell count in ascitic fluid ≥ 250/mm3, with no other known cause of infection. Infectious diarrhea was consistent with one of the following: acute diarrhea, fecal routine microscopic examination showing the presence of white blood cells (≥ 10/high power field); acute diarrhea, with fever, nausea, vomiting, abdominal pain, etc.; acute diarrhea occurring more than three times a day for two consecutive days or more than five times a day; pathogen-related evidence can be detected. Urinary tract infection was defined as abnormal urinary sediment (> 10 leukocytes/field) with positive urine Gram stain or culture in a symptomatic patient. Soft and skin tissue infections were considered when the skin had purulent secretions, pustules, furuncles, etc.; the patient had local pain or tenderness, local redness, or fever, and the pathogen was positive. Lower respiratory tract infections were considered when imaging findings suggested that pulmonary infiltrative lesions were associated with at least one respiratory symptoms, such as cough, sputum, dyspnea, and chest pain, with at least one finding on auscultation (rales or crepitations), or one sign of infection (core body temperature > 38 °C or < 36 °C with shivering or leukocyte count > 10000/mm3 or < 4000/mm3) in the absence of antibiotic use. Spontaneous bacteremia was defined as positive blood cultures without a known source of infection. Unproven infection was considered when fever and leukocytosis required antibiotic treatment without any identifiable source[5,8,11].
Diagnostic criteria for fungal infections were the following. Invasive candidiasis was defined as isolation of Candida spp in one or more blood cultures (candidaemia) or from normally sterile body fluids. Invasive aspergillosis was defined as detection of Aspergillus by direct examination and/or culture of respiratory samples in the presence of radiological imaging compatible with lung infection[12].
Sepsis was defined as life-threatening organ dysfunction caused by a dysregulated host response to infection. Septic shock was defined as a subset of sepsis in which particularly profound circulatory, cellular, and metabolic abnormalities were associated with a greater risk of mortality than with sepsis alone[13].
Score models and ACLF grade
Model for End-Stage Liver Disease (MELD) score, MELD-sodium (MELD-Na) score, integrated MELD (iMELD) score, Child-Turcotte-Pugh (CTP) score, albumin-bilirubin (ALBI) score, and the ACLF grading system were used as described in the previous literature[2,14]. CTP score is the cumulative result of the scores for five items (ascites, hepatic encephalopathy, TBIL, Alb, and PT extension time), with 1-3 points for each item and a maximum of 15 points. The equation for MELD score was as follows: 3.8 LN [TBIL (mg/dL)] + 11.2 LN (INR) + 9.6 LN [Cr [mg/dL)] + 6.4 × cause (0 for cholestatic or alcoholic liver diseases and 1 for all others). The equation for MELD-Na score was as follows: MELD + 1.59 × (135 - Na), wherein Na is 135 mmol/L if Na > 135 mmol/L and 120 mmol/L if Na < 120 mmol/L. The equation for iMELD score is as follows: MELD + (0.3 × Age) - (0.7 × Na) + 100. The equation for ALBI score is as follows: [log10TBIL (μmol/L) × 0.66] + [Alb (g/L) × -0.085].
ACLF grade 1 (ACLF-1) was defined as the presence of renal single organ failure, nervous system failure with renal damage, or other single organ failure with renal/neural damage; ACLF grade 2 (ACLF-2) as the presence of two organ failures; ACLF grade 3 (ACLF-3) as the presence of three organ failures and above[1].
Statistical analysis
Data were analyzed using SPSS 23.0 statistical software (Chicago, IL, United States). Normally distributed variables are expressed the mean ± standard deviation and were compared between two groups using the t-test. Non-normally distributed variables are presented as medians (interquartile range), and comparisons between two groups were performed using the Mann-Whitney U test. Count data are described as rates or percentages, and the χ2 test or Fisher’s exact probability test were applied for group comparisons. Unconditional logistic regression was used to analyze infection-related factors. The area under the receiver operating characteristic curve was used to assess the predictive power of the factors for the incidence of infections, and the cut-off value of the continuous variable was calculated. P < 0.05 was considered statistically significant.
RESULTS
Patient inclusion
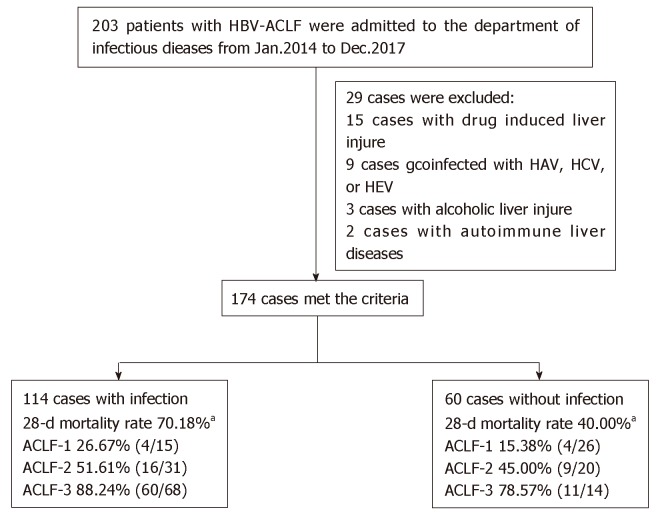
Two hundred and three patients with HBV-ACLF met the inclusion criteria, and 29 patients were excluded based on the exclusion criteria (15 patients with HAV, HCV, or HEV infection; 9 with drug-induced liver disease; 3 with alcoholic liver disease; and 2 with autoimmune liver disease). Thus, 174 patients were finally included, including 114 with infectious complications and 60 without (Figure 1). Almost all infections were nosocomial infections (84.21%, 96/114), and few patients presented infections on admission (15.79%, 18/114).
Figure 1.
The recruitment and inclusion of patients with hepatitis B virus-related acute-on-chronic liver failure. aP < 0.05 compared with those without infections. HBV-ACLF: Hepatitis B virus-related acute-on-chronic liver failure; HAV: Hepatitis A virus; HCV: Hepatitis C virus; HEV: Hepatitis E virus; ACLF: Acute-on-chronic liver failure.
Baseline characteristics
Among the 174 HBV-ACLF patients, there were 138 males and 36 females, with an average age of 47.44 ± 11.49 years (range, 22-76 years). A total of 181 bacterial or fungal infection events occurred in the 114 patients with infections. The ACLF patients with infectious complications were dominated by female and elderly patients compared with those without infectious complications (P < 0.05) (Table 1). All 114 patients with infections met the diagnostic criteria for sepsis, among whom 10 (8.77%, 10/114) presented septic shock.
Table 1.
Baseline characteristics of hepatitis B virus-related acute-on-chronic liver failure patients with and without infections
| Total (n = 174) | Infection (n = 114) | No infection (n = 60) | t/χ2/z | P-value | |
| Age (yr) | 47.44 ± 11.49 | 49.28 ± 11.32 | 43.95 ± 11.10 | 2.972 | 0.003 |
| Male, n (%) | 138 (79.31) | 82 (71.93) | 56 (93.33) | 10.975 | 0.001 |
| Hepatic encephalopathy | 25 (14.37) | 23 (20.18) | 2 (3.33) | 9.063 | 0.002 |
| Hepatorenal syndrome | 11 (6.32) | 10 (8.77) | 1 (1.67) | 3.351 | 0.100 |
| Gastrointestinal hemorrhage | 11 (6.32) | 10 (8.77) | 1 (1.67) | 3.351 | 0.100 |
| ACLF-1 | 41 (23.56) | 15 (13.16) | 26 (43.33) | 19.874 | 0.000 |
| ACLF-2 | 51 (29.31) | 31 (27.19) | 20 (33.33) | 0.715 | 0.398 |
| ACLF-3 | 82 (47.13) | 68 (59.65) | 14 (23.33) | 20.806 | 0.000 |
| INR | 2.09 (1.07) | 2.215 (1.24) | 1.835 (0.695) | -3.193 | 0.001 |
| ALB (g/L) | 32.38 ± 5.31 | 31.42 ± 4.63 | 34.197 ± 6.06 | -3.110 | 0.002 |
| Cr (μmol/L) | 55.65 (35.425) | 57.20 (41.175) | 55.00 (25.525) | -1.604 | 0.109 |
| Na (mmol/L) | 138.20 (6.52) | 137.25 (7.95) | 139.1 (5.325) | -2.752 | 0.006 |
| WBC (G/L) | 5.375 (3.623) | 6.32 (4.07) | 4.64 (3.085) | -3.655 | 0.000 |
| NE% | 69.85 (16.55) | 72.95 (13.875) | 63.15 (12.95) | -4.300 | 0.000 |
| ALT (U/L) | 379.50 (842.25) | 305.5 (608.75) | 793.5 (867.5) | -4.050 | 0.000 |
| AST (U/L) | 260.50 (591) | 224.5 (342.5) | 599.0 (858.25) | -4.001 | 0.000 |
| TBIL (μmol/L) | 260.16 ± 128.63 | 277.2 ± 134.7 | 227.8 ± 110.13 | 2.599 | 0.010 |
| PT (S) | 23.30 (11.325) | 25.20 (14.3) | 21.15 (7.15) | -2.956 | 0.003 |
| MELD | 20.58 ± 8.52 | 22.33 ± 8.92 | 17.25 ± 6.58 | 4.267 | 0.000 |
| MELD-Na | 22.51 ± 10.44 | 25.09 ± 11.14 | 17.6 ± 6.7 | 5.525 | 0.000 |
| iMELD | 38.81 ± 10.87 | 41.74 ± 11.38 | 33.23 ± 7.05 | 6.076 | 0.000 |
| ALBI | -1.205 ± 0.53 | -1.103 ± 0.45 | -1.399 ± 0.613 | 3.293 | 0.001 |
| CTP | 11.00 (2) | 12.00 (2) | 10.00 (2.75) | -4.896 | 0.000 |
TBIL: Total bilirubin; PT: Prothrombin time; WBC: White blood cells; NE%: Percentage of neutrophils; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; ALB: Albumin. INR: Standardized international ratio; Cr: Creatinine; Na: Sodium; CTP: Child-Turcotte-Pugh; MELD: Model for End-Stage Liver Disease; MELD-Na: MELD-sodium; iMELD: Integrated MELD; ALBI: Albumin-bilirubin; ACLF-1: Acute-on-chronic liver failure grade 1; ACLF-2: Acute-on-chronic liver failure grade 2; ACLF-3: Acute-on-chronic liver failure grade 3.
Sample culture and pathogen identification
A total of 188 samples were collected and cultured, among which 39 were positive. The positive rates for bacterial or fungal culture in ascites, sputum, blood, urine, and throat swabs were 4.76% (3/63), 52.27% (23/44), 7.69% (3/39), 21.05% (4/19), and 50% (5/10), respectively. The 10 stool samples and 2 bone marrow samples were all negative, while the only secretion sample was positive (Table 2).
Table 2.
Positivity rate among 188 cultured samples
| Samples | Positive samples | Positive rate (%) | |
| Ascites | 63 | 3 | 4.76 |
| Sputum | 44 | 23 | 52.27 |
| Blood | 39 | 3 | 7.69 |
| Urine | 19 | 4 | 21.05 |
| Throat swab | 10 | 5 | 50 |
| Stool | 10 | 0 | 0 |
| Bone marrow | 2 | 0 | 0 |
| Other secretion | 1 | 1 | 100 |
| Total | 188 | 39 | 20.74 |
A total of 41 strains were isolated from the 188 samples, with 21 bacteria (8 Gram-positive, 13 Gram-negative) and 20 fungi. There were 14 (66.67%, 14/21) strains of Enterobacter and Enterococcus out of the 21 bacteria, among which Enterobacter was the most frequently detected (52.38%, 11/21) and dominant among the Gram-negative bacteria (84.62%, 11/13). Among the 21 bacteria strains, multidrug resistant bacteria were isolated from 11 (52.38%) samples. The 20 fungi identified included 8 yeast-like fungi, 3 Candida albicans, 3 Candida tropicalis, 4 Candida parapsilosis, 1 Aspergillus fumigatus, and 1 Candida lusitaniae (Table 3).
Table 3.
Distribution of bacteria and fungi and source of specimens
| Total | constituent ratio (%) |
Source of specimens (n = 41) |
||||||
| Ascites | Sputum | Blood | Urine | Throat swab | Other secretion | |||
| Escherichia coli | 3 | 7.32 | 2 | 0 | 0 | 1 | 0 | 0 |
| Enterococcus faecium | 2 | 4.88 | 1 | 0 | 0 | 1 | 0 | 0 |
| Klebsiella pneumoniae | 4 | 9.76 | 0 | 4 | 0 | 0 | 0 | 0 |
| Acinetobacter baumannii | 2 | 4.88 | 0 | 2 | 0 | 0 | 0 | 0 |
| Enterobacter aerogenes | 3 | 7.32 | 0 | 2 | 0 | 0 | 1 | 0 |
| Staphylococcus aureus | 2 | 4.88 | 0 | 1 | 1 | 0 | 0 | 0 |
| Klebsiella ozaenae | 1 | 2.44 | 0 | 1 | 0 | 0 | 0 | 0 |
| Enterococcus faecalis | 1 | 2.44 | 0 | 0 | 1 | 0 | 0 | 0 |
| Streptococcus haemolyticus | 1 | 2.44 | 0 | 0 | 1 | 0 | 0 | 0 |
| Streptococcus agalactiae | 1 | 2.44 | 0 | 0 | 0 | 1 | 0 | 0 |
| Staphylococcus haemolyticus | 1 | 2.44 | 0 | 0 | 0 | 0 | 0 | 1 |
| yeast-like fungi | 8 | 19.51 | 0 | 6 | 0 | 1 | 1 | 0 |
| Candida | 11 | 26.83 | 0 | 8 | 0 | 0 | 3 | 0 |
| Aspergillus fumigatus | 1 | 2.44 | 0 | 1 | 0 | 0 | 0 | 0 |
Infection sites
There were 181 infections among the 114 HBV-ACLF patients with infections, among whom 64 (56.14%) presented one infection site and 50 (43.86%) patients had two or more infection sites. Among the patients with two or more infection sites, 37 (32.46%) were infected at two sites, 9 (7.89%) at three, and 4 (3.51%) at four. The most common infection site was the abdominal cavity (87 cases), followed by the respiratory tract (51 cases), urinary tract (18 cases), biliary tract (10 cases), intestinal tract (4 cases), oral cavity (4 cases), blood (3 cases), skin and soft tissue (3 cases), and venous catheter site (1 case).
HBV-ACLF patients with infectious complications show a high incidence of non-infectious complications
Among the 174 HBV-ACLF patients, 71 (40.8%, 71/174) presented non-infectious complications (hepatic encephalopathy, hepatorenal syndrome, and gastrointestinal hemorrhage), with a total of 58 (58.59%) hepatic encephalopathy cases, 26 (26.26%) hepatorenal syndrome cases, and 15 (15.15%) gastrointestinal hemorrhage cases. There were significantly higher frequencies of non-infectious complications in HBV-ACLF patients with infections than in those without, including hepatic encephalopathy (56.82% vs 13.33%, P < 0.001), hepatorenal syndrome (27.27% vs 18.18%, P < 0.01), and gastrointestinal hemorrhage (15.91% vs 9.09%, P < 0.05) (Table 1).
Risk factors for infectious complications in patients with HBV-ACLF
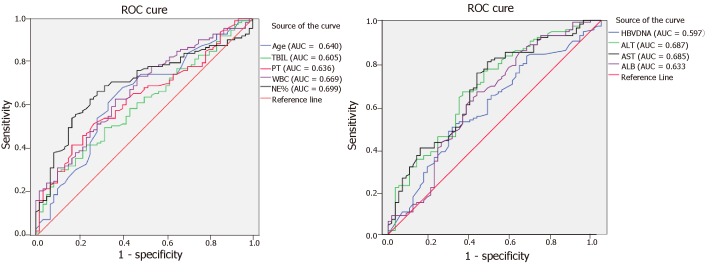
Among the 174 HBV-ACLF patients, 114 presented infections. Various indexes, such as gender, age, serum HBV DNA load, severity of liver failure, ALT, AST, TBIL, ALB, period of hospital stay, PT, WBC, and neutrophil percentage, were analyzed to determine their roles in infectious complications. The cut-off values for the associated indexes were established by unconditional logistic regression, which showed that age ≥ 45 years, middle- and late-stage of ACLF, AST < 538.5 U/L, ALT < 493.5 U/L, TBIL ≥ 348.35 mol/L, WBC > 10 G/L, neutrophil percentage > 70%, hospital stay ≥ 30 d, ALB ≤ 33.1 g/L, and PT ≥ 27.55 s were all risk factors for bacterial and/or fungal infections (Figure 2, Table 4).
Figure 2.
Receiver operating characteristic curve for assessing risk factors for bacterial and/or fungal infections in patients with hepatitis B virus-related acute-on-chronic liver failure. ROC: Receiver operating characteristic; HBV-ACLF: Hepatitis B virus-related acute-on-chronic liver failure; TBIL: Total bilirubin; PT: Prothrombin time; WBC: White blood cell; NE%: Percentage of neutrophils; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; ALB: Albumin.
Table 4.
Risk factors for the development of bacterial and/or fungal infections
| Total | Secondary infection n (%) | χ2 | OR (95%CI) | P-value | ||
| Gender | Female | 36 | 32 (88.89) | 9.263 | 5.46 (1.83, 16.31) | 0.002 |
| Male | 138 | 82 (59.42) | ||||
| Age (yr) | ≥ 45 | 107 | 80 (74.77) | 10.214 | 2.88 (1.51, 5.49) | 0.001 |
| < 45 | 67 | 34 (50.75) | ||||
| HBVDNA (IU/mL) | < 4.575 × 105 | 79 | 59 (74.68) | 6.281 | 2.32 (1.20, 4.46) | 0.012 |
| ≥ 4.575 × 105 | 95 | 55 (57.89) | ||||
| Stage of liver failure | Middle and late | 131 | 102 (77.9) | 30.459 | 9.09 (4.15, 19.89) | 0.000 |
| Early | 43 | 12 (27.91) | ||||
| ACLF grade | ACLF-1 | 41 | 15 (36.59) | 18.117 | 5.05 (2.40, 10.64) | 0.000 |
| ACLF-(2, 3) | 133 | 99 (74.44) | ||||
| ALT (U/L) | < 493.5 | 97 | 75 (78.95) | 15.273 | 3.71 (1.92, 7.17) | 0.000 |
| ≥ 493.5 | 77 | 39 (50.65) | ||||
| AST (U/L) | <538.5 | 120 | 92 (76.67) | 19.847 | 4.78 (2.40, 9.51) | 0.000 |
| ≥ 538.5 | 54 | 22 (40.74) | ||||
| TBIL (µmol/mL) | ≥ 348.35 | 40 | 34 (85) | 7.925 | 3.83 (1.50, 9.73) | 0.005 |
| < 348.35 | 134 | 80 (59.7) | ||||
| WBC (G/L) | ≥ 10 | 24 | 24 (100) | 7.099 | 15.73 (2.07, 119.45) | 0.008 |
| < 10 | 150 | 90 (60) | ||||
| NE% | > 70 | 86 | 71 (82.56) | 20.283 | 4.95 (2.47, 9.94) | 0.000 |
| ≤ 70 | 88 | 43 (48.86) | ||||
| Hospital stay (d) | ≥ 30 | 48 | 40 (83.33) | 9.312 | 1.45 (0.66, 3.17) | 0.003 |
| < 30 | 126 | 74 (58.73) | ||||
| ALB (g/L) | ≤ 33.1 | 101 | 76 (75.25) | 9.812 | 2.80 (1.47, 5.33) | 0.002 |
| > 33.1 | 73 | 38 (52.05) | ||||
| PT (s) | ≥ 27.55 | 58 | 48 (82.76) | 10.685 | 3.64 (1.68, 7.89) | 0.001 |
| < 27.55 | 116 | 66 (56.89) |
TBIL: Total bilirubin; PT: Prothrombin time; WBC: White blood cells; NE%: Percentage of neutrophils; ALT: Alanine aminotransferase; ALB: Albumin. AST: Aspartate aminotransferase; ACLF grade: Acute-on-chronic liver failure grade; ACLF-1: Acute-on-chronic liver failure grade 1; ACLF-(2, 3): Acute-on-chronic liver failure grades 2 and 3.
Infectious complications accelerate the progression of HBV-ACLF
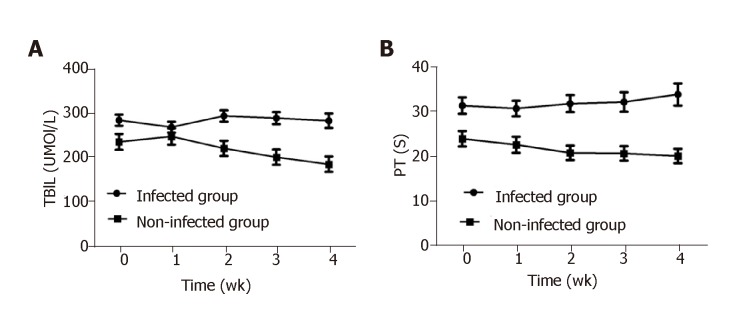
In patients with infections, TBIL was much higher at admission than that in patients without infections, and the elevated TBIL was sustained during the hospitalization without any obvious reduction. TBIL in patients without infections was mildly increased at week 1 of hospitalization and steadily decreased from week 2 to week 4 or at discharge. The difference in TBIL between the above two groups was statistically significant at admission, week 2, and week 4 (P < 0.05) (Figure 3A). Similarly, PT in patients with infections was much higher at admission than in patients without infections (P < 0.05) and remained at a high-level during hospitalization, while PT in patients without infections gradually decreased. PT differed significantly between patients with and without infections at admission, week 1, week 2, and week 4 (P < 0.05, Figure 3B).
Figure 3.
Kinetics of serum total bilirubin and prothrombin time in hepatitis B virus-related acute-on-chronic liver failure patients during hospital stay. A and B: Serum total bilirubin (A) and prothrombin time (B) in hepatitis B virus-related acute-on-chronic liver failure patients at admission, week 1, week 2, and week 4. TBIL: Total bilirubin; PT: Prothrombin time.
Infectious complications lead to extremely critical situations and a high mortality in patients with HBV-ACLF
The scores of MELD, MELD-Na, iMELD, CTP, and ALBI were all much higher for patients with infections than for patients without (P < 0.01) (Table 1). Further investigation showed that the higher the scores of MELD, MELD-Na, iMELD, CTP, and ALBI, the higher the incidence of infections (P < 0.05) (Table 5). A comparison of HBV-ACLF levels between patients with or without infections yielded the following results: ACLF-1 13.16% vs 43.33%, ACLF-2 27.19% vs 33.33%, and ACLF-3 59.65% vs 23.33%, which implied that ACLF patients with infections presented higher ACLF grades than those without.
Table 5.
Correlation of grades of acute-on-chronic liver failure and liver failure scores with bacterial and/or fungal infections
| Total, n | Infection, n(%) | No infection n (%) | Corelation coefficient | Sig | χ2 | P-value | |
| ACLF grade | 174 | 114 | 60 | 0.365 | 0.000 | 26.697 | 0.000 |
| ACLF-1 | 41 | 15 (36.59) | 26 (63.41) | ||||
| ACLF-2 | 51 | 31 (60.78) | 20 (39.22) | ||||
| ACLF-3 | 82 | 68 (82.93) | 14 (17.07) | ||||
| MELD | 174 | 114 | 60 | 0.285 | 0.001 | 15.421 | 0.001 |
| < 20 | 87 | 46 (52.87) | 41 (47.13) | ||||
| 20-29 | 62 | 45 (72.58) | 17 (27.42) | ||||
| 30-39 | 21 | 19 (90.48) | 2 (9.52) | ||||
| ≥ 40 | 4 | 4 (100) | 0 | ||||
| MELD-Na | 174 | 114 | 60 | 0.294 | 0.001 | 16.475 | 0.001 |
| < 25 | 121 | 69 (57.02) | 52 (42.98) | ||||
| 25-34 | 31 | 23 (74.19) | 8 (25.81) | ||||
| 35-44 | 17 | 17 (100) | 0 | ||||
| ≥ 45 | 5 | 5 (100) | 0 | ||||
| iMELD | 174 | 114 | 60 | 0.368 | 0.000 | 27.181 | 0.000 |
| < 30 | 32 | 15 (46.88) | 17 (53.12) | ||||
| 30-39 | 74 | 39 (52.7) | 35 (47.3) | ||||
| 40-49 | 41 | 34 (82.93) | 7 (17.07) | ||||
| 50-59 | 20 | 19 (95) | 1 (5) | ||||
| ≥ 60 | 7 | 7 (100) | 0 | ||||
| ALBI | 174 | 114 | 60 | 0.215 | 0.015 | 8.454 | 0.015 |
| ≤ -2.60 | 4 | 1 (25) | 3 (75) | ||||
| -2.60~-1.39 | 49 | 26 (53.06) | 23 (46.94) | ||||
| > -1.39 | 121 | 87 (71.9) | 34 (28.1) | ||||
| CTP | 174 | 114 | 60 | 0.257 | 0.000 | 12.301 | 0.000 |
| 5-9 | 33 | 13 (39.4) | 20 (60.6) | ||||
| ≥ 10 | 141 | 101 (71.63) | 40 (28.37) |
CTP: Child-Turcotte-Pugh; MELD: Model for End-Stage Liver Disease; MELD-Na: MELD-sodium; iMELD: Integrated MELD; ALBI: Albumin-bilirubin; ACLF grade: Acute-on-chronic liver failure grade; ACLF-1: Acute-on-chronic liver failure grade 1; ACLF-2: Acute-on-chronic liver failure grade 2; ACLF-3: Acute-on-chronic liver failure grade 3.
There were 104 (59.77%) deaths among the 174 HBV-ACLF patients. The mortality among patients with infections was markedly increased (70.18%, 80/114) compared with those without (40%, 24/60) (P < 0.05). Two or more infection sites further raised the mortality compared with one infection site (80% vs 62.5%, P < 0.05). Among all 174 patients, 71 had non-infectious complications, with a mortality of 88.73% (63/71). Among the 114 patients with infections, 62 had non-infectious complications, with a mortality of 88.71% (55/62), which was remarkably higher than that in infected patients without non-infectious complications (48.09%, 25/52) (P < 0.05). The mortality of the 114 sepsis patients was 70.18% (80/114), among whom 10 septic shock patients were all dead (100%).
DISCUSSION
This study showed that among HBV-ACLF patients, the incidences of bacterial and fungal infection and in-hospital 28-d mortality were 65.5% and 59.77%, respectively. The mortality of patients with infections was significantly higher than that of patients without (70.18% vs 40%). Infections at multiple sites markedly raised mortality compared with an infection at a single site. The above findings are consistent with previous reports[15-17]. In this study, the most common infection site was the abdominal cavity, followed by the respiratory tract, urinary system, and biliary tract, which is basically in accordance with results from other studies[18-20]. Thus, surveillance for signs of infection in the abdominal cavity, lungs, and urinary system and repeated ascites tests, urine tests, and chest imaging examinations are necessary for early identification of bacterial or fungal infections.
Among the 188 samples, only 20.94% were positive by culture, which implied that it is very hard to characterize the pathogen of infection in HBV-ACLF patients. Thus, the timing of sampling and bedside culture is essential to improve the detection rate. Among the positive samples, Gram-negative bacteria accounted for 61% of all detected bacteria and were mostly Enterobacter, which was associated with the bacterial translocation from the intestine[21-23]. Thus, antibiotics with a high sensitivity for Enterobacter can be applied for the empiric therapy. The finding that 11 of 21 (52.38%) bacterial strains were multidrug-resistant is noteworthy since multidrug-resistant bacteria are difficult to control and lead to a high mortality. Candida was the most frequently isolated fungus among the 20 fungal samples, followed by yeast-like fungi. Aspergillus fumigatus was isolated in one sample. The lung lesion of this specific patient progressed rapidly, complicated by the occurrences of respiratory failure and hepatic encephalopathy in the short term, and the patient died after 24 d of hospitalization. According to the related literature[24-26], secondary fungal infection is often induced by “double infection”, with nosocomial infection as the most common acquisition type, which leads to an extremely critical situation and high mortality. Risk factors for secondary fungal infection include long-term use of broad-spectrum antibiotics, long hospital stays, use of glucocorticoids, and invasive procedures[27,28]. Immune function failure in the middle and late stages of liver failure is another important factor for secondary fungal infection. Thus, oral care and regular screening for fungi in sputum, throat swabs, urine, and stools are very important for patients with ACLF, and timely intervention should be applied when early signs of fungal infection are evidenced.
The susceptibility of HBV-ACLF patients to bacterial and fungal infections is complex and involves multiple risk factors. Based on the results of this study, age ≥ 45 years, advanced stages of ACLF, AST < 538.5 U/L, ALT < 493.5 U/L, TBIL ≥ 348.35 µmol/L, WBC > 10 G/L, neutrophil percentage > 70%, hospital stay ≥ 30 d, ALB ≤ 33.1 g/L, and PT ≥ 27.55 s were identified as risk factors related to infection, consistent with previous reports[29,30]. Other risk factors mentioned in the literature include invasive procedures, use of antibacterial drugs, and comorbid illness (e.g., diabetes)[31,32]. In this study, the incidence of non-infectious complications as well as mortality were significantly increased in ACLF patients with infections compared with those without, indicating that effective control of bacterial and fungal infections may improve the outcomes of HBV-ACLF.
Various score models have been proposed to predict the prognosis of liver failure[14,33]. In this study, MELD, MELD-Na, iMELD, CTP, and ALBI scores were all markedly higher in HBV-ACLF patients with infections than in their counterparts without infections. Increasing scores were correlated with increasing infection incidence and high mortality, providing more evidence that bacterial and fungal infections will accelerate the progression of HBV-ACLF and lead to a poor prognosis.
The levels of TBIL and PT were mildly decreased in HBV-ACLF patients with infections at week 1, supposedly due to the comprehensive measures implemented, such as an artificial liver support system[34,35] and antiviral treatment. The levels of TBIL and PT remained high or even increased thereafter until week 4. By contrast, in the patients without infections, TBIL and PT were mildly elevated at the first week. Most of these patients were in the early stage of ACLF when admitted, and the elevation of TBIL and PT reflected the progression of ACLF. After that, the levels of TBIL and PT steadily declined. These results support the notion that bacterial or fungal infections will significantly prolong the hospital stay.
In summary, HBV-ACLF patients are susceptible to bacterial and fungal infections characterized by multiplicity of infection sites and diversity of pathogens, dominated by the Gram-negative bacillus Enterobacter. Once complicated with bacterial and/or fungal infections, HBV-ACLF patients have a high incidence of non-infectious complications, resulting in an advanced grade of ACLF and high mortality. Thus, the control of bacterial and fungal infections is pivotal for improving the outcomes of HBV-ACLF, and active prevention, early diagnosis, and timely treatment of bacterial and fungal infections are indispensable for the treatment of HBV-ACLF. As a retrospective single-center study with a relatively small sample size, the profiling of bacterial and fungal infections might be incomplete, and there may be bias in the frequencies of infectious complications and related mortality of HBV-ACLF. A prospective, multi-center, cohort study is needed to further characterize bacterial and fungal infections in HBV-ACLF patients.
ARTICLE HIGHLIGHTS
Research background
Bacterial and/or fungal infections are a trigger as well as a complication of liver failure, since patients with middle- or late-stage liver failure are susceptible to bacterial and fungal infections, and infection-induced sepsis is a common cause of acute-on-chronic liver failure (ACLF), while the risk factors which predispose to infections are not clear.
Research motivation
Infections are important causes of mortality in liver failure. However, the type of infection, the site of infections, predictors of infection, and their impact on outcomes in patients with hepatitis B virus-related ACLF (HBV-ACLF) are not fully elucidated. Establishing a model for predicting secondary infections in liver failure may be vital for clinical management of HBV-ACLF.
Research objectives
To investigate the influence of secondary infections on the progression of the disease and the related factors of secondary infections in patients with HBV-ACLF, and to elucidate the relationship between the infections in HBV-ACLF and the prognosis of the disease.
Research methods
Patients with HBV-ACLF at Taihe Hospital of Hubei University of Medicine from January 2014 to December 2017 were retrospectively enrolled. General information and clinical data were collected from the patient database of Taihe Hospital. The infection sites, complications, infection types, and infection rate and the influence of infections on the prognosis of HBV-ACLF were analyzed. SPSS23.0 software was used for statistical analyses. Unconditional logistic regression was used to analyze infection-related factors. The area under the receiver operating characteristic curve was used to assess the predictive power of the factors for the incidence of infections.
Research results
HBV-ACLF was susceptible to secondary infections, which were characterized by multiple sites and multiple strains. The pathogens of bacterial infection were mostly from Enterobacter, and the detection rate of pathogens was low. Patients with infectious complications had a significantly higher 28-d mortality (70.18%) than those without (40.00%, 24/60), and patients with infectious complications had a much higher incidence of non-infectious complications (54.39%, 62/114), leading to an extremely high mortality of 88.71% (55/62). The grade of liver failure, period of hospital stay ≥ 30 d, age ≥ 45 years, and percentage of neutrophils > 70% were identified as risk factors for infection complications.
Research conclusions
The high incidence of infection complications in patients with HBV-ACLF is associated with the severity and deterioration of the disease and may contribute to the extremely high mortality of these patients. Prevention of the occurrence of infections and early diagnosis and timely treatment of infections are indispensable for the treatment of HBV-ACLF.
Research perspectives
As a retrospective study, there are limitations like relatively small number of cases and imperfect follow-up data. Especially, the long-term survival rate and related biochemical indicators are not well tracked. In the future, prospective, multi-center, large-sample cohort studies are needed.
ACKNOWLEDGEMENTS
The authors would like to thank Mrs. Xue-Qin Qin and Ms. Yuan-Yuan Chen from the Department of Infectious Diseases, Taihe Hospital, for their help in collecting the data of patients and Dr. Jing Wang from Hubei University of Medicine for her help with statistical analysis.
Footnotes
Institutional review board statement: This study was reviewed and approved by the Ethics Committee of Shiyan Taihe Hospital.
Informed consent statement: Patients were not required to give informed consent to the study because the analysis used anonymous clinical data that were obtained after each patient agreed to treatment by written consent.
Conflict-of-interest statement: All authors declare no conflicts of interest related to this article.
Data sharing statement: No additional data are available.
Manuscript source: Unsolicited manuscript
Peer-review started: April 22, 2019
First decision: May 9, 2019
Article in press: July 20, 2019
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Blackadar C, Lopez J S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Zhou BX
Contributor Information
Chen Wang, Department of Infectious Diseases, Taihe Hospital, Hubei University of Medicine, Shiyan 442000, Hubei Province, China.
De-Qiang Ma, Department of Infectious Diseases, Taihe Hospital, Hubei University of Medicine, Shiyan 442000, Hubei Province, China.
Sen Luo, Department of Infectious Diseases, Taihe Hospital, Hubei University of Medicine, Shiyan 442000, Hubei Province, China.
Chuan-Min Wang, Department of Infectious Diseases, Taihe Hospital, Hubei University of Medicine, Shiyan 442000, Hubei Province, China.
De-Ping Ding, Department of Infectious Diseases, Taihe Hospital, Hubei University of Medicine, Shiyan 442000, Hubei Province, China.
You-You Tian, Department of Infectious Diseases, Taihe Hospital, Hubei University of Medicine, Shiyan 442000, Hubei Province, China.
Kang-Jian Ao, Department of Infectious Diseases, Taihe Hospital, Hubei University of Medicine, Shiyan 442000, Hubei Province, China.
Yin-Hua Zhang, Department of Infectious Diseases, Taihe Hospital, Hubei University of Medicine, Shiyan 442000, Hubei Province, China.
Yue Chen, Department of Infectious Diseases, Taihe Hospital, Hubei University of Medicine, Shiyan 442000, Hubei Province, China.
Zhong-Ji Meng, Department of Infectious Diseases, Taihe Hospital, Hubei University of Medicine, Shiyan 442000, Hubei Province, China; Institute of Biomedical Research, Taihe Hospital, Hubei University of Medicine, Shiyan 442000, Hubei Province, China. zhongji.meng@163.com.
References
- 1.Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, Gerbes A, Wendon J, Alessandria C, Laleman W, Zeuzem S, Trebicka J, Bernardi M, Arroyo V CANONIC Study Investigators of the EASL–CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426–1437, 1437.e1-1437.e9. doi: 10.1053/j.gastro.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 2.Gu WY, Xu BY, Zheng X, Chen J, Wang XB, Huang Y, Gao YH, Meng ZJ, Qian ZP, Liu F, Lu XB, Shang J, Li H, Wang SY, Sun X, Li H. Acute-on-Chronic Liver Failure in China: Rationale for Developing a Patient Registry and Baseline Characteristics. Am J Epidemiol. 2018;187:1829–1839. doi: 10.1093/aje/kwy083. [DOI] [PubMed] [Google Scholar]
- 3.Blasco-Algora S, Masegosa-Ataz J, Gutiérrez-García ML, Alonso-López S, Fernández-Rodríguez CM. Acute-on-chronic liver failure: Pathogenesis, prognostic factors and management. World J Gastroenterol. 2015;21:12125–12140. doi: 10.3748/wjg.v21.i42.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rahimi RS, Rockey DC. End-stage liver disease complications. Curr Opin Gastroenterol. 2013;29:257–263. doi: 10.1097/MOG.0b013e32835f43b0. [DOI] [PubMed] [Google Scholar]
- 5.Cai J, Zhang M, Han T, Jiang HQ. Characteristics of infection and its impact on short-term outcome in patients with acute-on-chronic liver failure. Medicine (Baltimore) 2017;96:e8057. doi: 10.1097/MD.0000000000008057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selvapatt N, Singanayagam A, Wendon J, Antoniades CG. Understanding infection susceptibility in patients with acute-on-chronic liver failure. Intensive Care Med. 2014;40:1363–1366. doi: 10.1007/s00134-014-3349-x. [DOI] [PubMed] [Google Scholar]
- 7.Bernsmeier C, Singanayagam A, Patel VC, Wendon J, Antoniades CG. Immunotherapy in the treatment and prevention of infection in acute-on-chronic liver failure. Immunotherapy. 2015;7:641–654. doi: 10.2217/imt.15.27. [DOI] [PubMed] [Google Scholar]
- 8.Shalimar, Rout G, Jadaun SS, Ranjan G, Kedia S, Gunjan D, Nayak B, Acharya SK, Kumar A, Kapil A. Prevalence, predictors and impact of bacterial infection in acute on chronic liver failure patients. Dig Liver Dis. 2018;50:1225–1231. doi: 10.1016/j.dld.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Hou J, Wang G, Wang F, Cheng J, Ren H, Zhuang H, Sun J, Li L, Li J, Meng Q, Zhao J, Duan Z, Jia J, Tang H, Sheng J, Peng J, Lu F, Xie Q, Wei L Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association. Guideline of Prevention and Treatment for Chronic Hepatitis B (2015 Update) J Clin Transl Hepatol. 2017;5:297–318. doi: 10.14218/JCTH.2016.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarin SK, Kedarisetty CK, Abbas Z, Amarapurkar D, Bihari C, Chan AC, Chawla YK, Dokmeci AK, Garg H, Ghazinyan H, Hamid S, Kim DJ, Komolmit P, Lata S, Lee GH, Lesmana LA, Mahtab M, Maiwall R, Moreau R, Ning Q, Pamecha V, Payawal DA, Rastogi A, Rahman S, Rela M, Saraya A, Samuel D, Saraswat V, Shah S, Shiha G, Sharma BC, Sharma MK, Sharma K, Butt AS, Tan SS, Vashishtha C, Wani ZA, Yuen MF, Yokosuka O APASL ACLF Working Party. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL) 2014. Hepatol Int. 2014;8:453–471. doi: 10.1007/s12072-014-9580-2. [DOI] [PubMed] [Google Scholar]
- 11.CDC definitions for nosocomial infections, 1988. Am Rev Respir Dis. 1989;139:1058–1059. doi: 10.1164/ajrccm/139.4.1058. [DOI] [PubMed] [Google Scholar]
- 12.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Muñoz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR, Zaoutis T, Bennett JE European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group; National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lei Q, Ao K, Zhang Y, Ma D, Ding D, Ke C, Chen Y, Luo J, Meng Z. Prognostic factors of the short-term outcomes of patients with hepatitis B virus-associated acute-on-chronic liver failure. Clinics (Sao Paulo) 2017;72:686–692. doi: 10.6061/clinics/2017(11)07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garg H, Kumar A, Garg V, Sharma P, Sharma BC, Sarin SK. Clinical profile and predictors of mortality in patients of acute-on-chronic liver failure. Dig Liver Dis. 2012;44:166–171. doi: 10.1016/j.dld.2011.08.029. [DOI] [PubMed] [Google Scholar]
- 16.Fernández J, Acevedo J, Wiest R, Gustot T, Amoros A, Deulofeu C, Reverter E, Martínez J, Saliba F, Jalan R, Welzel T, Pavesi M, Hernández-Tejero M, Ginès P, Arroyo V European Foundation for the Study of Chronic Liver Failure. Bacterial and fungal infections in acute-on-chronic liver failure: prevalence, characteristics and impact on prognosis. Gut. 2018;67:1870–1880. doi: 10.1136/gutjnl-2017-314240. [DOI] [PubMed] [Google Scholar]
- 17.Yang L, Wu T, Li J, Li J. Bacterial Infections in Acute-on-Chronic Liver Failure. Semin Liver Dis. 2018;38:121–133. doi: 10.1055/s-0038-1657751. [DOI] [PubMed] [Google Scholar]
- 18.Leber B, Spindelboeck W, Stadlbauer V. Infectious complications of acute and chronic liver disease. Semin Respir Crit Care Med. 2012;33:80–95. doi: 10.1055/s-0032-1301737. [DOI] [PubMed] [Google Scholar]
- 19.Bajaj JS, O'Leary JG, Wong F, Reddy KR, Kamath PS. Bacterial infections in end-stage liver disease: current challenges and future directions. Gut. 2012;61:1219–1225. doi: 10.1136/gutjnl-2012-302339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piano S, Brocca A, Mareso S, Angeli P. Infections complicating cirrhosis. Liver Int. 2018;38 Suppl 1:126–133. doi: 10.1111/liv.13645. [DOI] [PubMed] [Google Scholar]
- 21.Tilg H, Cani PD, Mayer EA. Gut microbiome and liver diseases. Gut. 2016;65:2035–2044. doi: 10.1136/gutjnl-2016-312729. [DOI] [PubMed] [Google Scholar]
- 22.Miyake Y, Yamamoto K. Role of gut microbiota in liver diseases. Hepatol Res. 2013;43:139–146. doi: 10.1111/j.1872-034X.2012.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarin SK, Choudhury A. Acute-on-chronic liver failure: terminology, mechanisms and management. Nat Rev Gastroenterol Hepatol. 2016;13:131–149. doi: 10.1038/nrgastro.2015.219. [DOI] [PubMed] [Google Scholar]
- 24.Zhang L, Liu ZY. [End-stage liver disease and invasive fungal infection] Zhonghua Gan Zang Bing Za Zhi. 2018;26:13–16. doi: 10.3760/cma.j.issn.1007-3418.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Lin LN, Zhu Y, Che FB, Gu JL, Chen JH. Invasive fungal infections secondary to acute-on-chronic liver failure: a retrospective study. Mycoses. 2013;56:429–433. doi: 10.1111/myc.12044. [DOI] [PubMed] [Google Scholar]
- 26.Hassan EA, Abd El-Rehim AS, Hassany SM, Ahmed AO, Elsherbiny NM, Mohammed MH. Fungal infection in patients with end-stage liver disease: low frequency or low index of suspicion. Int J Infect Dis. 2014;23:69–74. doi: 10.1016/j.ijid.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 27.Verma N, Singh S, Taneja S, Duseja A, Singh V, Dhiman RK, Chakrabarti A, Chawla YK. Invasive fungal infections amongst patients with acute-on-chronic liver failure at high risk for fungal infections. Liver Int. 2019;39:503–513. doi: 10.1111/liv.13981. [DOI] [PubMed] [Google Scholar]
- 28.Ma Y, Chen H, Zhang X. [Clinical study on the risk factors of severe hepatitis with nosocomial fungal infection] Zhonghua Gan Zang Bing Za Zhi. 2015;23:376–377. [PubMed] [Google Scholar]
- 29.Nanchal RS, Ahmad S. Infections in Liver Disease. Crit Care Clin. 2016;32:411–424. doi: 10.1016/j.ccc.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 30.Albillos A, Martínez J. Prognostic value of bacterial infection in acute and chronic liver failure. Liver Int. 2016;36:1090–1092. doi: 10.1111/liv.13141. [DOI] [PubMed] [Google Scholar]
- 31.Shalimar, Kedia S, Sharma H, Vasudevan S, Sonika U, Upadhyaya AD, Acharya SK. Predictors of infection in viral-hepatitis related acute liver failure. Scand J Gastroenterol. 2017;52:1413–1419. doi: 10.1080/00365521.2017.1374449. [DOI] [PubMed] [Google Scholar]
- 32.Bruns T, Zimmermann HW, Stallmach A. Risk factors and outcome of bacterial infections in cirrhosis. World J Gastroenterol. 2014;20:2542–2554. doi: 10.3748/wjg.v20.i10.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lei Q, Zhang Y, Ke C, Yan C, Huang P, Shen H, Lei H, Chen Y, Luo J, Meng Z. Value of the albumin-bilirubin score in the evaluation of hepatitis B virus-related acute-on-chronic liver failure, liver cirrhosis, and hepatocellular carcinoma. Exp Ther Med. 2018;15:3074–3079. doi: 10.3892/etm.2018.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piechota M, Piechota A, Misztal M, Bernas S, Pietraszek-Grzywaczewska I. An evaluation of the usefulness of extracorporeal liver support techniques in patients with severe liver dysfunction. Arch Med Sci. 2019;15:99–112. doi: 10.5114/aoms.2017.67998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piechota M, Piechota A. An Evaluation of the Usefulness of Extracorporeal Liver Support Techniques in Patients Hospitalized in the ICU for Severe Liver Dysfunction Secondary to Alcoholic Liver Disease. Hepat Mon. 2016;16:e34127. doi: 10.5812/hepatmon.34127. [DOI] [PMC free article] [PubMed] [Google Scholar]