Summary
Antiviral interferons (IFN-alpha/beta) are possibly responsible for the high tolerance of bats to zoonotic viruses. Previous studies focused on the IFN system of megabats (suborder Yinpterochiroptera). We present statistically robust RNA sequencing (RNA-seq) data on transcriptomes of cells from the “microbat” Myotis daubentonii (suborder Yangochiroptera) responding at 6 and 24 h to either an IFN-inducing virus or treatment with IFN. Our data reveal genes triggered only by virus, either in both humans and Myotis (CCL4, IFNL3, CH25H), or exclusively in Myotis (STEAP4). Myotis cells also express a series of conserved IFN-stimulated genes (ISGs) and an unusually high paralog number of the antiviral ISG BST2 (tetherin) but lack several ISGs that were described for megabats (EMC2, FILIP1, IL17RC, OTOGL, SLC24A1). Also, in contrast to megabats, we detected neither different IFN-alpha subtypes nor an unusually high baseline expression of IFNs. Thus, Yangochiroptera microbats, represented by Myotis, may possess an IFN system with distinctive features.
Subject Areas: Biological Sciences, Immunity, Omics, Transcriptomics
Graphical Abstract
Highlights
-
•
Virus- and IFN-responsive transcriptomes of the microbat Myotis daubentonii
-
•
CCL4, IFNL3, CH25H, STEAP4 are IFNB-like genes triggered by virus only
-
•
Microbats encode more paralogs of BST2 (tetherin) than any other mammal
-
•
Clear differences between the IFN systems of microbats and megabats
Biological Sciences; Immunity; Omics; Transcriptomics
Introduction
Bats are major reservoirs for viral zoonotic pathogens. Virus infections that are acute and highly aggressive in humans can be persistent and avirulent in bats (Calisher et al., 2006). An important contributor of virus tolerance by bats is thought to be the antiviral type I interferon (IFN-α/β) system (Dobson, 2005). Indeed, in some bats the IFN system has peculiar features and is under strong positive selection (De La Cruz-Rivera et al., 2018, Pavlovich et al., 2018, Yan, 2015, Zhang et al., 2013, Zhou et al., 2016). Bats may thus possess a fortified IFN system that suppresses viruses down to levels of persistence (Dobson, 2005, Pavlovich et al., 2018, Wynne and Wang, 2013, Zhang et al., 2013).
Type I IFNs are a group of cytokines that encompass several subtypes, such as IFN-β, IFN-α 1 to 13, and some others (Garcin et al., 2013). Type III IFNs (IFN-λ 1, 2, 3) are similar, but unlike the systemic type I IFNs they act locally on epithelial cells (Hamming et al., 2010). IFN induction occurs by cellular sensing of viral RNA structures, resulting in the activation of IFN transcription factors, such as IRF3. The once-secreted IFNs bind to their receptor, activate the transcription factors STAT1/2 via the JAK kinases, and drive the expression of multiple IFN-stimulated genes (ISGs) with antiviral and immunomodulatory activity (Schoggins, 2014).
Prototypical ISGs respond only to IFN signaling. However, there are ISGs additionally or even exclusively activated after virus sensing. Thus, the IFN response encompasses virus-response genes (IRF dependent), IFN-specific ISGs (STAT dependent), and universal ISGs (responding to both infection and IFNs) (Schmid et al., 2010).
Bats (order Chiroptera) are classified into the suborders Yinpterochiroptera (Syn. Pteropodiformes) and Yangochiroptera (Syn. Vespertilioniformes) (Teeling et al., 2018). Members of both suborders are infected by a wide range of virus species (Calisher et al., 2006). However, despite some progress, the contribution of the IFN response to their special ability to host viruses is not yet clarified. For Yinpterochiroptera such as Pteropus alecto, vampyrus or Rousettus aegyptiacus (all family Pteropodidae, or “megabats”), several studies had measured the global transcriptional response of cells to infection with, e.g., Ebola, Marburg, Nipah, or Hendra virus (Hölzer et al., 2016, Kuzmin et al., 2017, Wynne et al., 2014) or to the IFN-inducing Newcastle Disease virus (NDV) (Glennon et al., 2015). By contrast, for the more diverse bat suborder Yangochiroptera (generically termed “microbats”) there are just two virus-induced cell transcriptomes available (Gerrard et al., 2017, Wu et al., 2013). However, the full virus response capability of bats remains uncharted because almost all cell response studies bar one (Glennon et al., 2015) with megabats and all studies with microbats employed wild-type viruses, which are weak IFN inducers. With respect to the response to IFN itself, there are expression profiles of cells from the megabat P. alecto (De La Cruz-Rivera et al., 2018, Zhang et al., 2017), and Shaw et al. recently conducted a pan-species comparison of IFN-stimulated cell transcriptomes, which included the megabat P. vampyrus and the Yangochiroptera microbat (family Vespertilionidae) Myotis lucifugus (Shaw et al., 2017).
Thus, previous studies on virus-induced or IFN-stimulated transcriptional profiles of cells overwhelmingly focused on megabats (family Pteropodidae) of the suborder Yinpterochiroptera. This suborder contains 7 bat families, whereas the Yangochiroptera, having separated around 60 million years ago, contain 14 families (Teeling et al., 2018). Yangochiroptera species host SARS-like coronaviruses (Drexler et al., 2011), rhabdoviruses and flaviviruses (Calisher et al., 2006), paramyxoviruses (Drexler et al., 2012), and bunyaviruses such as Crimean-Congo hemorrhagic fever-like virus (Muller et al., 2016), Hantavirus (Weiss et al., 2012), or Rift Valley fever virus (Calisher et al., 2006, Oelofsen and Van der Ryst, 1999). Nonetheless, for Yangochiroptera microbats there are (1) no studies on the transcriptomal cell responses to an IFN-inducing virus and (2) only one study on the IFN-stimulated gene expression profile (Shaw et al., 2017), which, however, focused on conserved ISGs. Moreover, to our knowledge a side-by-side, global comparison of early and late transcriptomes of cells in response to either virus or IFN is not available for any organism.
To fill these gaps, we devised a study for the Yangochiroptera microbat Myotis daubentonii (family Vespertilionidae). M. daubentonii is a carrier of lyssaviruses prevailing from Europe to Japan (McElhinney et al., 2018). On the phylogenetical tree, it is maximally separated from the previously studied Pteropodidae megabats (Teeling et al., 2018). We stimulated M. daubentonii cells either with a strong IFN inducer virus or with type I IFN and measured global transcription at two different time points. Our results reveal several unique features like novel virus-response genes and an elevated number of paralogs of the antiviral ISG BST2. Unlike reported for megabats (Pavlovich et al., 2018, Zhou et al., 2016) we could not detect different IFN-alpha subtypes or unusually high base levels of IFNs. Thus, besides exhibiting conserved antiviral responses and ISGs, several traits of the Yangochiroptera microbats are unique and not even observed for megabats.
Results and Discussion
Outline of the Experiments to Measure Transcriptomes
The aim of our study was to obtain the full picture of the antiviral type I IFN response of the Yangochiroptera microbat representative M. daubentonii, using a kidney cell line (MyDauNi/2c) described previously (Fuchs et al., 2017, Muller et al., 2012). IFNs have anti-proliferative activity, which is the reason why many cell lines have lost their ability to induce IFN and/or to respond to IFN over their passaging history (Hess et al., 2012). MyDauNi/2c cells, however, exhibited the typical phosphorylation of STAT1 in response to exogenously added pan-species IFN-α B/D (Figure S1A). Moreover, STAT1 phosphorylation was downmodulated by Ruxolitinib, an established inhibitor of JAK1/2-mediated IFN signaling (Stewart et al., 2014). STAT1 phosphorylation and Ruxolitinib effect were comparable with the IFN-competent human A549 (Kuri et al., 2010, Weber et al., 2015) and the R. aegytiacus megabat cell line Ro6E-J (Fuchs et al., 2017) that we used in parallel. These results demonstrate the capacity of the MyDauNi/2c cells to respond to IFN.
To elucidate the overall antiviral potency and the unimpeded ability to produce IFN in response to infection, we employed a mutant of Rift Valley fever virus that has its IFN suppressor NSs replaced by the Renilla luciferase gene (RVFVΔNSs::Renilla) (Kuri et al., 2010). RVFVΔNSs::Renilla is a strong inducer of type I IFNs, is sensitive to the antiviral action of IFNs, and able to infect cells from a wide range of vertebrates (Kuri et al., 2010). The built-in Renilla luciferase facilitates rapid and quantitative measurements of viral replication. Also, the M. daubentonii MyDauNi/2c cells could be infected with our reporter virus, permitting replication levels similar as in human A549 lung cell line or the fetal R. aegytiacus line Ro6E-J (Jordan et al., 2009) (Figure S1B). Moreover, treatment with the IFN signaling inhibitor Ruxolitinib increased RVFVΔNSs::Renilla replication, whereas IFN-α suppressed it. Although the cell lines are from different organs, these results, taken together, demonstrate that our immortalized Yangochiroptera/Vespertilionidae cell line MyDauNi/2c possesses a fully functional IFN system that is comparable with the one of human and megabat cell lines.
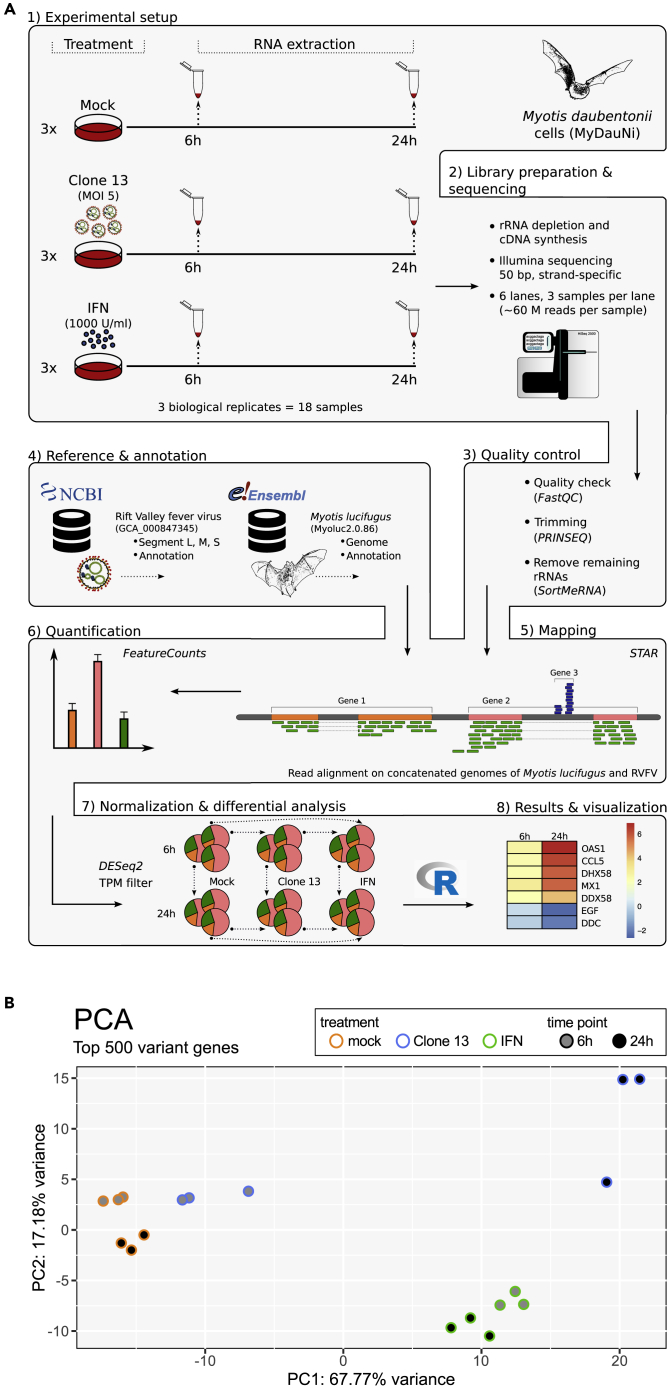
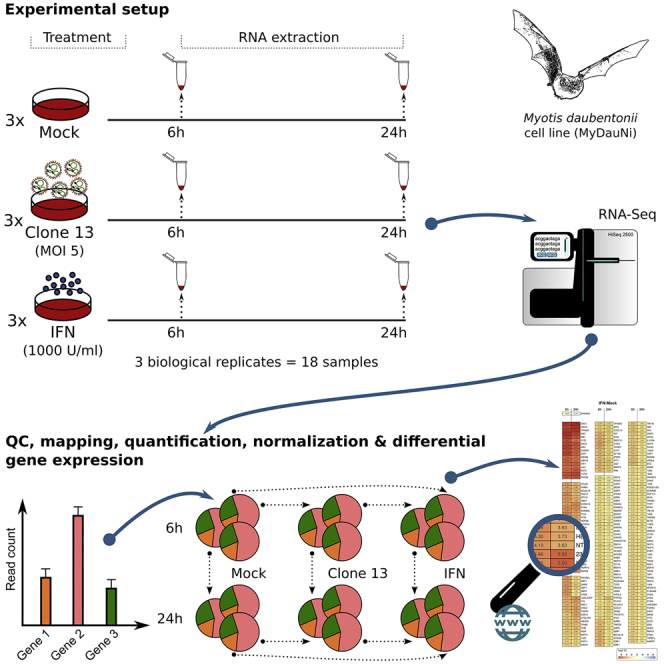
Figure 1A gives an overview of the experimental setup. The MyDauNi/2c cells were either mock treated, infected with a strong IFN inducer (RVFV NSs mutant Clone 13) (Billecocq et al., 2004), or treated with 1,000 U/ml IFN-α. Total cell RNAs were isolated at 6 and 24 h post infection, and rRNA-depleted cDNA libraries were sequenced with 60–70 million strand-specific single-end reads per sample (Table S1). Of note, all experiments were performed in three independent, biological replicates.
Figure 1.
Experiments and Analyses to measure M. daubentonii Transcriptomes in Response to Virus and IFN
(A) Workflow.
(B) Principal component analyses. The biological replicates of the transcriptomes of uninfected (mock), Clone 13-infected, and IFN-treated cells, sampled at two different time points, were analyzed and visualized by PCAGO (see text).
Around 70% of the quality-checked and trimmed reads could be uniquely mapped to the reference genome of the related M. lucifugus (Table S2), using appropriate tools (Dobin and Gingeras, 2015, Kopylova et al., 2012, Schmieder and Edwards, 2011). Based on pairwise comparisons of the mean values of the three replicates, protein-encoding genes that were induced or repressed by an absolute log2 fold change (FC) ≥ 2 and with an adjusted p ≤ 0.05 (DESeq2 (v1.16.1) [Love et al., 2014]) were defined as significantly responding to either Clone 13 infection or IFN. To test whether biological replicates cluster together, we performed various principal component analyses (PCA) using our PCAGO web service (https://doi.org/10.1101/433078). In general, and visualized by the PCA based on the top 500 variant genes (Figures 1B and S2A), we found the biological replicates to cluster together. The PCA revealed that the transcriptomes of the 6-h post Clone 13 infection samples are closest to the mock samples and that the IFN-stimulated profiles of both time points are highly similar. The highest variance in the data (PC1 with 67,77%) can be explained by the large expression differences between the mock and Clone 13 6-h samples compared with the Clone 13 and IFN-stimulated 24-h samples. At 24 h, the Clone 13-infected cells show an expression pattern that is clearly distinguishable from all other conditions. One of the Clone 13 24-h replicates clustered somewhat apart from the other two replicates (see Figure 1B), but the difference between these accounts for only 3.3% (PC2; Figure S2B) of the whole variation in the gene expression data when directly compared with the mock 24-h samples. A 2D- and a 3D-PCA-movie, visualizing the overall stable clustering and how the principal components change by adding more and more less-variant genes to the transformation, can be found in the online supplement (Videos S1 and S2). CLARK classification (Ounit et al., 2015) and Krona visualization (Ondov et al., 2011) revealed ∼1% of the reads with high similarity to the RVFV reference genome in this specific sample, compared with 3%–4% in the other two replicates (Figure S3). In addition to our reference-based analyses, we have created a comprehensive de novo transcriptome assembly for M. daubentonii by combining (Fu et al., 2012) the output of various suitable assembly tools (Bankevich et al., 2012, Grabherr et al., 2011, Liu et al., 2016, Xie et al., 2014) according to the results of Hölzer and Marz (Hölzer and Marz, 2019).
Virus-Responsive Genes
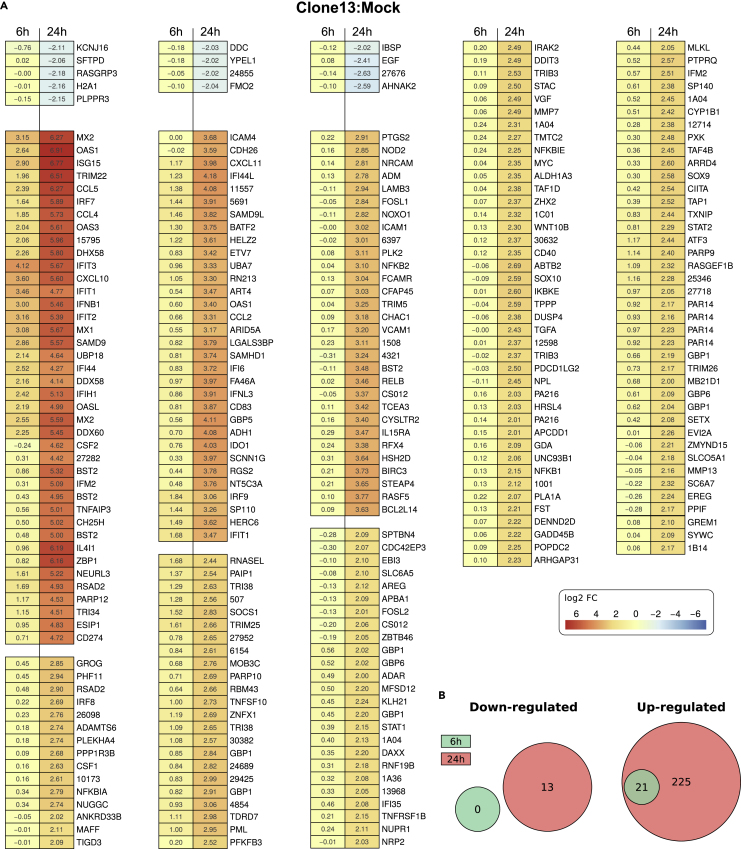
At 6 h post infection with the IFN inducer virus Clone 13, 21 genes were upregulated (Figures 2A and 2B). Among these were prototypical, IRF3-driven genes (IFNB1, CCL5, CXCL10, IFIT1 to 3, OAS1, and OASL), and others reported to be expressed by infected cells (DDX58, DDX60, DHX58, IFIH1, IFI44, ISG15) (Balogh et al., 2014, Doganay et al., 2017, Glennon et al., 2015, Grandvaux et al., 2002, McWhirter et al., 2004, Puthia et al., 2016, Schmid et al., 2010). By contrast, upregulated genes such as IRF7, MX1, MX2, OAS3, SAMD9, and USP18 are prototypical ISGs (Rusinova et al., 2013, Shaw et al., 2017) that most likely are activated by the Clone 13-induced IFN in the supernatant (see later discussion). Altogether the upregulated genes are commanding biological processes of the immediate-early innate response to viruses, as shown by GO term enrichment using the Piano package (Varemo et al., 2013) (Figure S4A, upper panel).
Figure 2.
Myotis daubentonii Genes Differentially Regulated in Response to Clone 13 Infection
(A) Heatmap of log2-fold changes in infected cells as compared with mock infected cells. The displayed genes were filtered by an absolute log2-fold change of 2 and an adjusted p value < 0.05. Genes that are similarly regulated at 6 and 24 h post infection are clustered together. Data are averages from three biological replicates. Genes missing a gene name and a function in the current Ensembl annotation of M. lucifugus were listed by their unique and abbreviated ID numbers: e.g., “ENSMLUG00000015795” was shortened to “15795.”
(B) Venn diagrams showing the numbers of significantly regulated mRNAs at the two time points of infection.
At 24 h post infection, 246 genes were upregulated and 13 genes were downregulated. All the genes from the 6-h time point are included in the 24-h-upregulated genes (see Figures 2A and 2B). Comparisons with the pan-species database of IFN-triggered transcriptomes (Shaw et al., 2017) identified conserved additional ISGs (besides the ones mentioned for the 6-h time point) such as, e.g., ADAR, BST2, various GBPs, HERC6, IFITM 2 and 3, IRF9, PARP12, PML, RSAD2, TRIM22, SOCS1, STAT1, STAT2, or ZBP1. SAMHD1, by contrast, was recently described as being IRF3 dependent in human, monkey, and porcine cells (Yang et al., 2016). Among the late virus responders were also more unusual genes such as CSF2 or NEURL3. Both are weakly IFN stimulated in Rattus norvegicus but not in any other mammalian species (Shaw et al., 2017). In M. daubentonii, CSF2 is exclusively virus inducible (see below) and NEURL3 is strongly virus as well as (weakly) IFN inducible.
The genes upregulated at this later time point were functionally more diversified than the 6-h ones, as they promote adaptive immunity, apoptosis, and also RNA polymerase II activity, besides the innate immune response (Figure S4A, lower panel). The genes downregulated at 24 h post infection were mostly involved in carbohydrate metabolism and protein transport.
All significantly up- or downregulated genes can be investigated with our Interactive Gene Observer search tool https://www.rna.uni-jena.de/supplements/mda/report.html which was was modified from the output of the Reporting Tools package (Huntley et al., 2013). Full gene tables and expression statistics for the individual biological replicates and all pairwise comparisons can be found in the electronic supplement, Figure S5. Coverage of viral genome is documented in Figure S6.
IFN-Responsive Genes
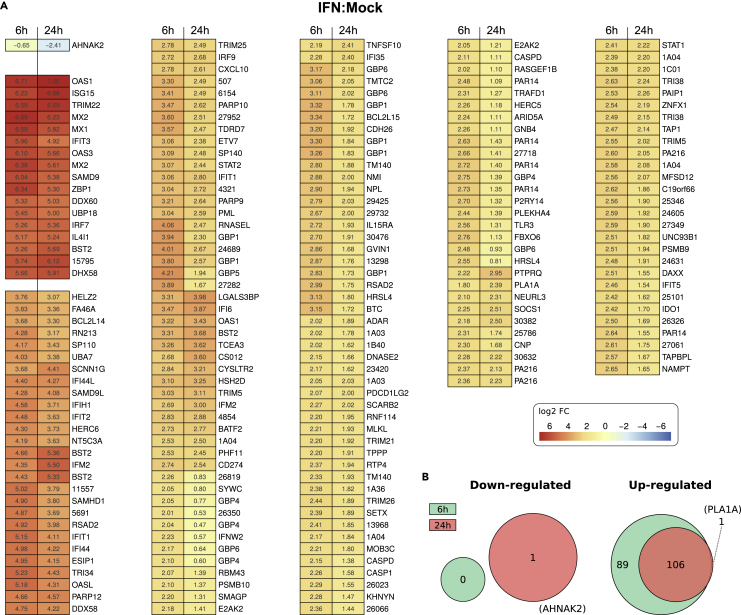
When cells were treated for 6 h with IFN, 195 genes were strongly upregulated (i.e., substantially more than after 6 h of Clone 13 infection). No gene was downregulated (Figures 3A and 3B). The IFN-regulated genes encompassed prototypical (OAS1, ISG15, Mx1, IFIT3, BST2, DHX58) but also less known ISGs (BCL2L14, RNF213, ESIP1).
Figure 3.
Myotis daubentonii Genes Differentially Regulated in Response to Type I IFN
(A) Heatmap of log2-fold changes in IFN-treated cells as compared with mock infected cells. The displayed genes were filtered as described for Figure 2. Genes that are similarly regulated at 6 and 24 h post IFN are clustered together. Data are averages from three biological replicates. Genes missing a gene name and a function in the current Ensembl annotation of M. lucifugus were listed by their unique and abbreviated ID numbers: e.g., “ENSMLUG00000015795” was shortened to “15795.”
(B) Venn diagrams showing the numbers of significantly regulated mRNAs at the two time points of IFN treatment.
At 24 h, the number of upregulated genes was decreased to 106, of which 1 gene (PLA1A) was actually unique for this late time point. Moreover, just 1 gene (AHNAK2) is significantly downregulated at 24 h. Thus, M. daubentonii cells are capable of rapidly responding to IFN, and at the 24-h time point almost half of the stimulated genes were back to base levels. This transient character of the type I IFN response is in agreement with recent studies on cells from megabat (P. alecto) (De La Cruz-Rivera et al., 2018), man (Jilg et al., 2014), and mouse (Mostafavi et al., 2016).
For the IFN-stimulated transcriptomes, predominant biological processes both at 6 and 24 h post infection encompassed antigen processing and presentation, antiviral defense, immune response, and NF-kB regulation (Figure S4B). Additionally, at 6 h there are indications of both up- and downregulation of intracellular and transmembrane transport, and at 24 h oxidoreductase activity is mostly downregulated.
Genes Uniquely Regulated by Virus Infection
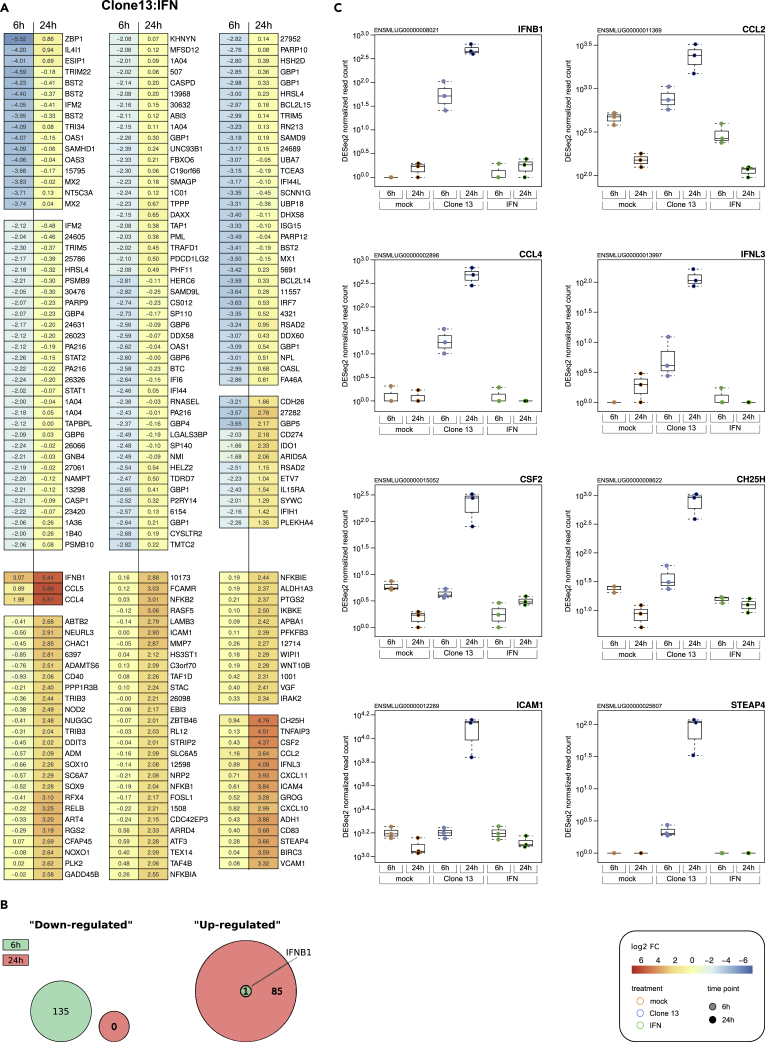
To our knowledge, a kinetic and side-by-side comparison of virus-driven versus IFN-driven host cell transcriptomes was not reported for any mammalian species so far. Therefore, beyond the bat-specific aspects this study was initiated for, we took advantage of our database to filter out genes that are uniquely regulated by either treatment. To address this issue, we compared gene expression at the 6- and 24-h time points of Clone 13 infection and IFN treatment with each other. Overall, running the two transcriptomes next to each other showed once more that at 6 h Clone 13 infection stimulated less genes (denominated as being “downregulated”) than 6-h IFN treatment (Figures 4A and 4B). We could identify some genes that are upregulated exclusively by Clone 13, most prominently the prototypical, IRF3-driven virus-response gene IFNB1. When restricting ourselves to genes that were not at all regulated by IFN but upregulated by a log2 of more than 3 by virus (i.e., stricter than the log2-fold change of 2 filter we had set for the heatmaps), a similar virus-only response behavior was exhibited by other cytokines such as, e.g., CCL2, CCL4, IFNL3 (IFN-λ3), and CSF2, and also by more untypical genes such as CH25H, ICAM1, and STEAP4. Figure 4C shows RNA-seq data as expression box plots to illustrate the exclusive virus-response behavior in M. daubentonii cells.
Figure 4.
Genes Differentially Regulated by Virus Infection versus Type I IFN
(A) Heatmap of log2-fold changes in IFN-treated cells as compared with Clone 13-infected cells. The displayed genes were filtered by an absolute log2-fold change of 2 and an adjusted p value < 0.05. Genes that are similarly regulated at 6 and 24 h post IFN are clustered together. Data are averages from three biological replicates. Genes missing a gene name and a function in the current Ensembl annotation of M. lucifugus were listed by their unique and abbreviated ID numbers: e.g., “ENSMLUG00000015795” was shortened to “15795.”
(B) Venn diagrams showing the numbers of significantly regulated mRNAs at the two time points of IFN treatment.
(C) Expression box plots of selected mRNAs that responded significantly to Clone 13 treatment but not to control and IFN-stimulated conditions. The plots show the DESeq2-normalized expression values for each condition and biological replicate. Graphs show mean values and standard deviations from the three biological replicates.
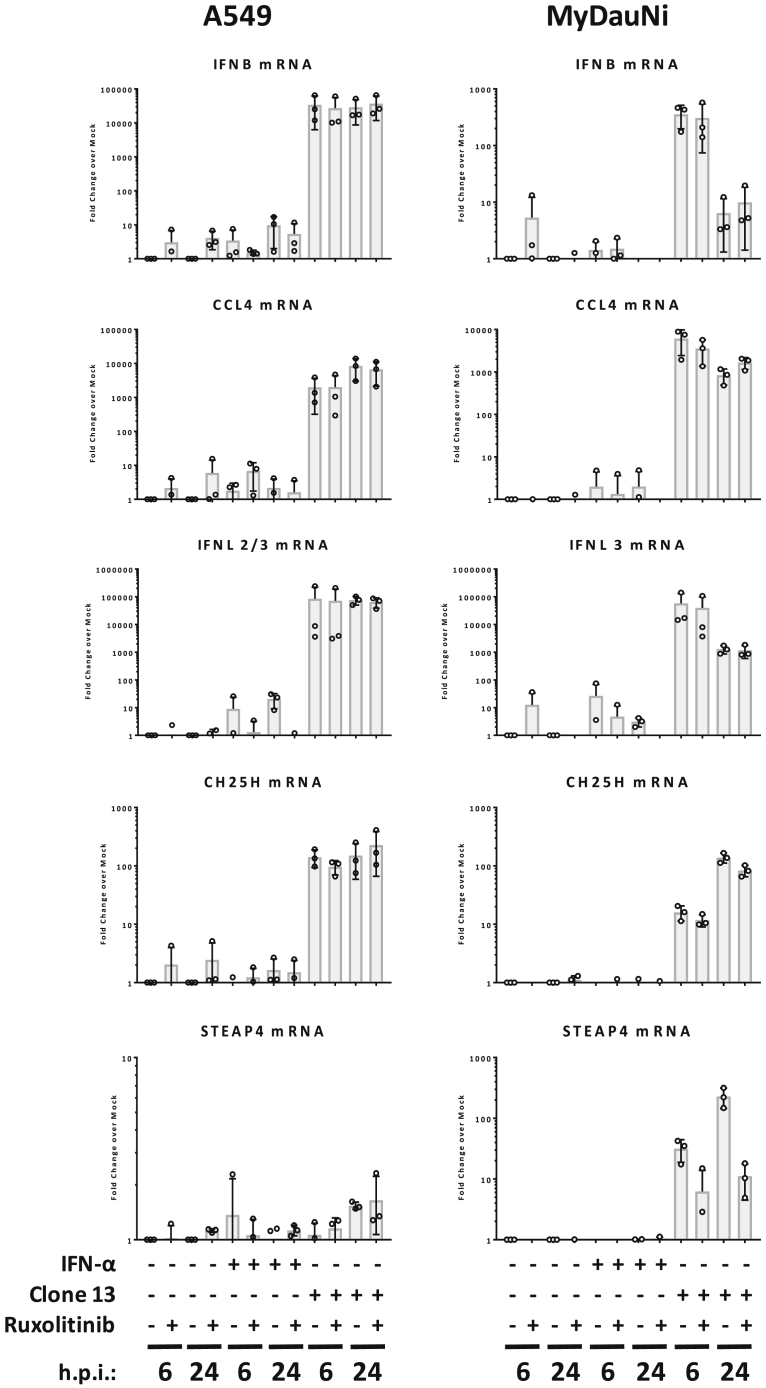
Not all these genes are known as pure virus responders. Therefore, we verified our findings by RT-qPCR for the strongest early virus responders (IFNB1, CCL4, IFNL3, CH25H, STEAP4) and compared with the human system. Although being a late responder, CH25H (cholesterol 25-hydroxylase) is included because it is a powerful, broad-spectrum virus inhibitor. Human A549 and MyDauNi cells were infected with Clone 13, treated with IFN, or left untreated, and samples were taken 6 and 24 h later. Moreover, to exclude any potential cross talk by virus-triggered IFN, we kept in parallel cells under the JAK/STAT signaling inhibitor Ruxolitinib. In both the human and the Myotis cells, Ruxolitinib expectedly inhibited STAT1 phosphorylation and upregulation of the prototypical IFN-dependent ISG Mx1 (Figure S7). The RT-qPCR data shown in Figure 5 demonstrate that IFNB1, CCL4, IFNL3, and CH25A are exclusively virus induced with no stimulation whatsoever by IFN. (Of note, a downmodulation of IFNB1 at 24 h post infection was detected by RT-qPCR but not by the less sensitive NGS [see Figure 2].) This was true for both the human and the M. daubentonii cells, indicating a conserved behavior. STEAP4 (six transmembrane epithelial antigen of prostate), by contrast, was identified as a virus-only response gene in M. daubentonii cells but unresponsive to both IFN and Clone 13 in human cells.
Figure 5.
RT-qPCR Confirmation of Virus-Only Response Genes
Human A549 and M. daubentonii MyDauNi cells, treated or not with Ruxolitinib, were incubated with 1,000 U/ml IFN-α or infected with Clone 13 (MOI 5) for 6 or 24 h, respectively. Expression of IFNB1, CCL4, IFNL2/3 (A549)/IFNL3 (MyDauNi), CH25H, and STEAP4 were monitored by RT-qPCR. The graphs show the fold induction over mock, for the respective time point, with mean values and standard deviations from three independent replicates.
Literature and database searches were done to find information about these genes that we found to behave like IFNB1. As remarked earlier, the transcriptomes published so far were from either virus-infected or from IFN-treated cells but not from both in parallel. Thus, transcriptomes as well as most studies on individual ISGs could not clearly distinguish between primary (i.e., virus dependent) and secondary (virus-induced IFN) responses to infection, thus impeding a clear distinction of gene expressions that are unique for either of these triggers. Moreover, Ruxolitinib was not used before to systematically study IFN-independent cell responses to virus infection. Hence, we had aimed to clearly differentiate true virus-response genes from conventional ISGs indirectly activated by virus-induced IFN.
The chemokine CCL4 (Macrophage inflammatory protein-β; MIP1-β), an attractant of immune cells such as macrophages or natural killer cells, is not an ISG in humans or other mammals (Shaw et al., 2017). However, CCL4 was found to be upregulated after 24 h of infection with the IFN-inducing NDV in P. vampyrus megabat cells (Glennon et al., 2015). Our results show that this occurs independently of IFN signaling. Thus, we propose CCL4 to be an apparently conserved (humans, megabats, microbats) virus response gene.
The type III interferon IFNL3 (IFNL2/3 in humans) was shown to be stimulated by IRF3 or IRF7 as well as by Sendai virus infection in human (Ank et al., 2006, Osterlund et al., 2007) and by NDV in P. vampyrus cells (Glennon et al., 2015). With regard to IFN responsiveness, there are no entries for mice, humans, and other mammals in the databases (Carrasco Pro et al., 2018, Rusinova et al., 2013, Shaw et al., 2017). Our Ruxolitinib data demonstrate that also IFNL3 is a conserved virus-response gene.
CH25H is a powerful broad-spectrum inhibitor of virus entry (Liu et al., 2013, Reboldi et al., 2014). It is not responsive to NDV infection in P. vampyrus cells (Glennon et al., 2015). CH25H is published as being weakly IFN stimulated in human (which we could not observe in our A549 cells), rat, horse, and dog; unresponsive to IFN in sheep, pigs and bats; and actually downregulated in cattle (Shaw et al., 2017). Thus, CH25H is a little conserved (and weak) ISG that we find in humans and M. daubentonii to be a novel and strong virus-response gene.
STEAP4 is a TNF-α- and IL1β-induced metalloreductase (Kralisch et al., 2009). It is IFN stimulated in humans and pigs but not in any other mammalian species (Shaw et al., 2017). Interestingly, we observed a complete non-responsiveness to any stimulant in human A549 cells, whereas it was upregulated by Clone 13 infection in M. daubentonii cells. In P. vampyrus, by contrast, it is not among the virus-activated (Glennon et al., 2015).
Thus, taken together, we mapped the global responses of M. daubentonii to both virus infection and IFN treatment. Moreover, owing to our statistically robust datasets, early and late time points of sampling, and control experiments using Ruxolitinib, we could identify genes that are like IFNB1 exclusively upregulated by virus infection, either in both humans and Myotis (CCL4, IFNL3, CH25H) or only in Myotis (STEAP4).
Specific Features of the Microbat Responses
A somewhat surprising finding was the low presence of type I IFN subtypes besides IFNB1. In fact, we could identify only a type I IFN gene with similarity to horse IFNW2 (IFN-ω2) as being modestly IFN stimulated at the 6-h time point, where it reached a log2 fold change of 2.23 and a significant p value (see Figure 3). Another detectable type I IFN subtype (although it did not reach the significance threshold) was a relative to human IFNA5. Also Shaw et al. did not list any type I IFN in their pan-mammal transcriptome study (Shaw et al., 2017), whereas studies in megabats (Pavlovich et al., 2018, Zhou et al., 2016) showed an upregulation of IFN-α- or IFN-ω-like genes upon Sendai virus infection by RT-PCR. Moreover, Zhou et al. reported high base levels of several type I IFN subtypes in the megabat P. alecto (Zhou et al., 2016), which was observed neither for the megabat R. aegyptiacus (Pavlovich et al., 2018) nor by us in M. daubentonii microbat cells.
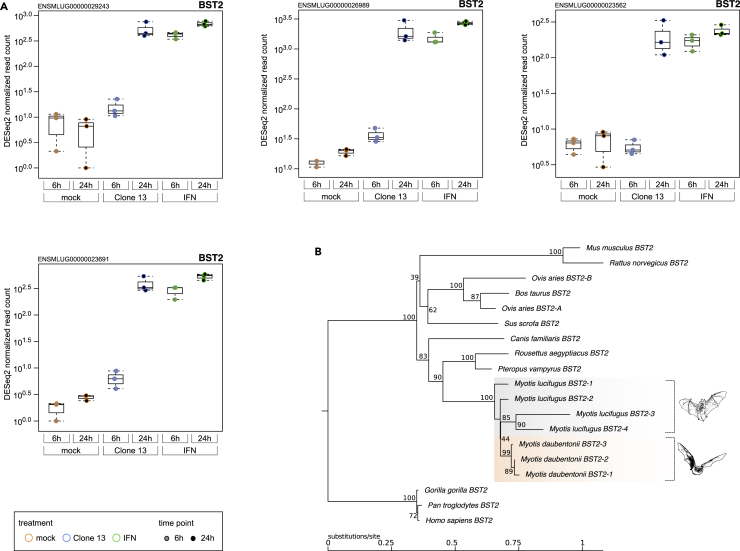
An interesting feature of our M. daubentonii data was the detection of multiple paralogs of Mx and BST2. In the genome of the related M. lucifugus we identified three and four copies of Mx and BST2, respectively. Based on the M. lucifugus reference genome, M. daubentonii significantly upregulated either all three (Mx) or all four (BST2) genes (Figure 6A). Mx (myxovirus resistance protein) is a large cytosolic GTPase with a broad antiviral spectrum (Haller et al., 2015). Almost all investigated vertebrate species express multiple paralogs (Haller et al., 2015). Bat Mx proteins (including Mx1 of M. daubentonii) were shown to confer antiviral activity (Fuchs et al., 2017). BST2 (bone marrow stromal cell antigen 2), also called Tetherin, is an antiviral transmembrane protein tethering enveloped virus particles to the plasma membrane (Douglas et al., 2010). A search in the annotated genomes of mammals revealed that Yangochiroptera/Vespertilionidae such as Myotis lucifugus, brandtii und davidii, as well as Eptesicus fuscus harbor several copies of the BST2 gene, whereas Miniopterus natalensis (Yangochiroptera/Miniopteridae) seems to have just one copy (Figure 6B and data not shown). Using our M. daubentonii RNA-seq data, we were able to detect expression of four BST2 paralogs in the genome of M. lucifugus. However, two had an identical sequence (data not shown), so we confirm three BST2 paralogs with a differing sequence in the transcriptome of M. daubentonii. Thus, Vespertilionidae microbats are unique in harboring a multitude of BST2 paralogs, since all other species (except for sheep, which have two [Varela et al., 2017]) have only one copy. Overall, our observations are that Yangochiroptera/Vespertilionidae microbats express a high number of paralogs of Mx and unexpectedly also BST2, two ISGs with a broad antiviral spectrum. This may indicate an increased capability to directly fend off virus infections.
Figure 6.
BST2 Paralogs and Phylogeny
We identified four BST2 paralogous genes in the genome of M. lucifugus that served as a reference in this study. From the de novo transcriptome assembly of the M. daubentonii RNA-seq data, we were able to distinguish only three different BST2-like mRNAs.
(A) The box plots show arithmetic means and standard deviations from the DESeq2-normalized expression values of the M. daubentonii RNA-seq reads, uniquely mapped to the M. lucifugus reference genome.
(B) Phylogenetic tree of twelve selected mammalian BST2 genes and the four and three BST2 paralogs identified in the M. lucifugus genome or M. daubentonii transcriptome, respectively.
Comparison with Other Published Innate Immunity Bat Transcriptomes
As mentioned, previous transcriptional profiling studies of bats overwhelmingly used weak IFN inducer viruses or IFN and megabat cells, and only one study so far (again in megabats [Glennon et al., 2015]) used a truly IFN-inducing virus. Moreover, a global, side-by-side comparison between virus- and IFN-stimulated transcriptomes seems not to exist for any species, not even for humans. In addition to the points raised earlier on IFN subtypes and ISGs, we compared some of our findings with the distinctive results from previous bat studies.
Glennon et al. profiled the transcriptome of cells from the megabat P. vampyrus infected for 24 h with the IFN-inducing NDV (Glennon et al., 2015). They observed activation of IFNB1 and prototypical ISGs (e.g., DDX58, IFIH1, BST2, Mx1, or ISG15) and identified several novel ISGs, most prominently MORC3 (MORC family CW-type zinc finger 3). Subsequent RT-PCR analyses showed that this novel ISG was not consistently IFN dependent in cells from other bats but was NDV induced in all bat species, including the microbat Myotis velifer incautus (Glennon et al., 2015). In the pan-species IFN response comparisons of Shaw et al., MORC3 was identified as a core ISG (Shaw et al., 2017). Also, in our M. daubentonii transcriptomes, MORC3 was upregulated by IFN and Clone 13, although only weakly (see https://www.rna.uni-jena.de/supplements/mda/report.html). Interestingly, MORC3 is known as a restriction factor of herpes viruses (Sloan et al., 2016) but a pro-viral factor for influenza virus (Ver et al., 2015).
De la Cruz Vera et al. identified for the megabat P. alecto several genes that were not previously reported as being ISGs, namely, EMC2, FILIP1, IL17RC, OTOGL, SLC10A2, SLC24A1, and RNASEL (De La Cruz-Rivera et al., 2018). Our transcriptomes show that EMC2, FILIP1, IL17RC, OTOGL, and SLC24A1/2 are not ISGs in M. daubentonii, whereas RNASEL was confirmed. In fact, RNASEL is an ISG in most of the species investigated by Shaw et al. except for humans and chicken (Shaw et al., 2017), thus bolstering the conclusions of de la Cruz Vera et al.. Thus, the microbat ISG profile is distinct from the one of megabats.
Conclusions
Bats, which comprise about 20% of all living mammal species, have recently come into the focus of virus and innate immunity research. However, the group of “bats” is very heterogeneous and encompasses both Yinpterochiroptera and Yangochiroptera, which have diverged since approximately 60 million years (Teeling et al., 2018). For Yangochiroptera microbats, in contrast to megabats of the Yinpterochiroptera suborder, the IFN system is only poorly characterized. Here, we presented the time-dependent transcriptional profiles of M. daubentonii cells infected with a strong IFN inducer virus or treated with IFN. Our data revealed virus-triggered genes (CCL4, IFNL3, CH25H, STEAP4) that are not regulated by IFN itself, as well as the presence of multiple paralogs of directly antiviral ISGs such as Mx and BST2. CCL4 and IFNL3 attract immune cells and enhance local antiviral activity, respectively, and Mx, BST2, and CH25H are broad-spectrum virus inhibitors. Although our data are derived from a single cell line and may not be entirely generalizable, our analysis indicates that the Yangochiroptera microbat IFN system may exhibit features not observed for megabats or humans (STEAP4 as virus-response gene, at least 3 BST2 paralogs). Moreover, we report the absence of a series of megabat ISGs (EMC2, FILIP1, IL17RC, OTOGL, SLC24A1) and confirm a set of ISGs as being conserved also in Yangochiroptera. Also, in contrast to findings in megabat cells, we could not detect an increased copy number or upregulation of type I IFN genes other than IFNB1, or unusually high base levels of IFNs. Moreover, based on true biological replicates we offer statistically robust RNA-seq data on a time-resolved comparison between virus-induced and IFN-stimulated transcriptional responses of an IFN competent mammalian cell line.
Limitations of the Study
Potential caveats of our paper are that (1) an immortalized Myotis daubentonii cell line was used, (2) the Myotis daubentonii cells are of kidney origin and their IFN response were compared with that of a human lung cell line, and (3) it is unknown how much the innate immune response of Myotis cells are representative for the entire suborder Yangochiroptera.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank Ivonne Görlich and Marco Groth from the Core Facility DNA sequencing of the Leibniz Institute on Aging, Fritz Lipmann Institute in Jena for their help with RNA sequencing, Bernd Schmeck for analyzing RNA integrity, and Georg Kochs and Alejandro Brun for kindly providing antibodies. Work in the F.W. laboratory is funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Projektnummer 197785619 – SFB 1021 and by SPP 1596 (grant numbers We 2616/7-1, We 2616/7-2), by the RAPID consortium of the Bundesministerium für Bildung und Forschung (BMBF, grant number 01KI1723E), and by the LOEWE-Schwerpunkt “Medical RNomics” of the Land Hessen. M.M. and M.H. are supported by the DFG SPP 1596. Work in the laboratory of C.D. was supported by the DFG grant SPP 1596 (grant number DR 772/10-2) and by the RAPID consortium of the BMBF (grant number 01KI1723A). Funding for open access charge: DFG SPP 1596 (grant number We 2616/7-2).
Author Contributions
Conceptualization, M.H., A.S., M.A.M., C.D., M.M., and F.W.; Methodology, M.H., A.S., J.W., M.A.M., C.D., M.M., and F.W.; Software, M.H. and M.M.; Validation, A.S. and F.W.; Formal Analysis, M.H. and F.W.; Investigation, M.H., A.S., J.W., and M.A.M.; Resources, C.D., M.M., and F.W.; Data Curation, M.H. and M.M.; Writing – Original Draft, F.W.; Writing – Review & Editing, M.H., A.S., M.A.M., M.M., and F.W.; Supervision, M.M. and F.W.; Project Administration, F.W.; Funding Acquisition, C.D., M.M., and F.W.
Declaration of Interests
The authors declare no competing interests.
Published: September 27, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.08.016.
Contributor Information
Manja Marz, Email: manja@uni-jena.de.
Friedemann Weber, Email: friedemann.weber@vetmed.uni-giessen.de.
Data and Code Availability
Our online search tool is available at https://www.rna.uni-jena.de/supplements/mda/report.html. The raw read data was deposited in the GEO database under accession number GEO: GSE121301. All intermediate files such as the quality-trimmed and rRNA-cleaned reads of all 18 samples, mappings, the extended genome annotation, and raw read counts were uploaded to the Open Science Framework under https://doi.org/10.17605/OSF.IO/X9KAD.
Supplemental figures and files are also found at https://www.rna.uni-jena.de/supplements/mda/.
Supplemental Information
All reads were quality checked with FASTQC and Prinseq. Ribosomal RNAs were removed by SortMeRNA. Shown are read statistics before and after trimming.
References
- Ank N., West H., Bartholdy C., Eriksson K., Thomsen A.R., Paludan S.R. Lambda interferon (IFN-lambda), a type III IFN, is induced by viruses and IFNs and displays potent antiviral activity against select virus infections in vivo. J. Virol. 2006;80:4501–4509. doi: 10.1128/JVI.80.9.4501-4509.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balogh A., Bator J., Marko L., Nemeth M., Pap M., Setalo G., Jr., Muller D.N., Csatary L.K., Szeberenyi J. Gene expression profiling in PC12 cells infected with an oncolytic Newcastle disease virus strain. Virus Res. 2014;185:10–22. doi: 10.1016/j.virusres.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Bankevich A., Nurk S., Antipov D., Gurevich A.A., Dvorkin M., Kulikov A.S., Lesin V.M., Nikolenko S.I., Pham S., Prjibelski A.D. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billecocq A., Spiegel M., Vialat P., Kohl A., Weber F., Bouloy M., Haller O. NSs protein of Rift Valley fever virus blocks interferon production by inhibiting host gene transcription. J. Virol. 2004;78:9798–9806. doi: 10.1128/JVI.78.18.9798-9806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calisher C.H., Childs J.E., Field H.E., Holmes K.V., Schountz T. Bats: important reservoir hosts of emerging viruses. Clin. Microbiol. Rev. 2006;19:531–545. doi: 10.1128/CMR.00017-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco Pro S., Dafonte Imedio A., Santoso C.S., Gan K.A., Sewell J.A., Martinez M., Sereda R., Mehta S., Fuxman Bass J.I. Global landscape of mouse and human cytokine transcriptional regulation. Nucleic Acids Res. 2018;46:9321–9337. doi: 10.1093/nar/gky787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Cruz-Rivera P.C., Kanchwala M., Liang H., Kumar A., Wang L.F., Xing C., Schoggins J.W. The IFN response in bats displays distinctive IFN-stimulated gene expression kinetics with atypical RNASEL induction. J. Immunol. 2018;200:209–217. doi: 10.4049/jimmunol.1701214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A., Gingeras T.R. Mapping RNA-seq reads with STAR. Curr. Protoc. Bioinformatics. 2015;51:11.14.1–19. doi: 10.1002/0471250953.bi1114s51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson A.P. What links bats to emerging infectious diseases? Science. 2005;310:628–629. doi: 10.1126/science.1120872. [DOI] [PubMed] [Google Scholar]
- Doganay S., Lee M.Y., Baum A., Peh J., Hwang S.Y., Yoo J.Y., Hergenrother P.J., Garcia-Sastre A., Myong S., Ha T. Single-cell analysis of early antiviral gene expression reveals a determinant of stochastic IFNB1 expression. Integr. Biol. (Camb). 2017;9:857–867. doi: 10.1039/c7ib00146k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas J.L., Gustin J.K., Viswanathan K., Mansouri M., Moses A.V., Fruh K. The great escape: viral strategies to counter BST-2/tetherin. PLoS Pathog. 2010;6:e1000913. doi: 10.1371/journal.ppat.1000913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexler J.F., Corman V.M., Muller M.A., Maganga G.D., Vallo P., Binger T., Gloza-Rausch F., Cottontail V.M., Rasche A., Yordanov S. Bats host major mammalian paramyxoviruses. Nat. Commun. 2012;3:796. doi: 10.1038/ncomms1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexler J.F., Corman V.M., Wegner T., Tateno A.F., Zerbinati R.M., Gloza-Rausch F., Seebens A., Muller M.A., Drosten C. Amplification of emerging viruses in a bat colony. Emerg. Infect. Dis. 2011;17:449–456. doi: 10.3201/eid1703.100526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L., Niu B., Zhu Z., Wu S., Li W. CD-HIT: accelerated for clustering the next-generation sequencing data. Bioinformatics. 2012;28:3150–3152. doi: 10.1093/bioinformatics/bts565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs J., Hölzer M., Schilling M., Patzina C., Schoen A., Hoenen T., Zimmer G., Marz M., Weber F., Muller M.A. Evolution and antiviral specificities of interferon-induced Mx proteins of bats against ebola, influenza, and other RNA viruses. J. Virol. 2017;91 doi: 10.1128/JVI.00361-17. e00361–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcin G., Bordat Y., Chuchana P., Monneron D., Law H.K., Piehler J., Uze G. Differential activity of type I interferon subtypes for dendritic cell differentiation. PLoS One. 2013;8:e58465. doi: 10.1371/journal.pone.0058465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrard D.L., Hawkinson A., Sherman T., Modahl C.M., Hume G., Campbell C.L., Schountz T., Frietze S. Transcriptomic signatures of tacaribe virus-infected jamaican fruit bats. mSphere. 2017;2 doi: 10.1128/mSphere.00245-17. 245–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon N.B., Jabado O., Lo M.K., Shaw M.L. Transcriptome profiling of the virus-induced innate immune response in pteropus vampyrus and its attenuation by nipah virus interferon antagonist functions. J. Virol. 2015;89:7550–7566. doi: 10.1128/JVI.00302-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabherr M.G., Haas B.J., Yassour M., Levin J.Z., Thompson D.A., Amit I., Adiconis X., Fan L., Raychowdhury R., Zeng Q.D. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011;29:644–U130. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandvaux N., Servant M.J., tenOever B., Sen G.C., Balachandran S., Barber G.N., Lin R.T., Hiscott J. Transcriptional profiling of interferon regulatory factor 3 target genes: direct involvement in the regulation of interferon-stimulated genes. J. Virol. 2002;76:5532–5539. doi: 10.1128/JVI.76.11.5532-5539.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller O., Staeheli P., Schwemmle M., Kochs G. Mx GTPases: dynamin-like antiviral machines of innate immunity. Trends Microbiol. 2015;23:154–163. doi: 10.1016/j.tim.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Hamming O.J., Gad H.H., Paludan S., Hartmann R. Lambda interferons: new cytokines with old functions. Pharmaceuticals (Basel) 2010;3:795–809. doi: 10.3390/ph3040795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess R.D., Weber F., Watson K., Schmitt S. Regulatory, biosafety and safety challenges for novel cells as substrates for human vaccines. Vaccine. 2012;30:2715–2727. doi: 10.1016/j.vaccine.2012.02.015. [DOI] [PubMed] [Google Scholar]
- Hölzer M., Krahling V., Amman F., Barth E., Bernhart S.H., Carmelo V.A., Collatz M., Doose G., Eggenhofer F., Ewald J. Differential transcriptional responses to Ebola and Marburg virus infection in bat and human cells. Sci. Rep. 2016;6:34589. doi: 10.1038/srep34589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzer M., Marz M. De novo transcriptome assembly: A comprehensive cross-species comparison of short-read RNA-Seq assemblers. Gigascience. 2019;8(5):giz039. doi: 10.1093/gigascience/giz039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntley M.A., Larson J.L., Chaivorapol C., Becker G., Lawrence M., Hackney J.A., Kaminker J.S. ReportingTools: an automated result processing and presentation toolkit for high-throughput genomic analyses. Bioinformatics. 2013;29:3220–3221. doi: 10.1093/bioinformatics/btt551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilg N., Lin W., Hong J., Schaefer E.A., Wolski D., Meixong J., Goto K., Brisac C., Chusri P., Fusco D.N. Kinetic differences in the induction of interferon stimulated genes by interferon-alpha and interleukin 28B are altered by infection with hepatitis C virus. Hepatology. 2014;59:1250–1261. doi: 10.1002/hep.26653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan I., Horn D., Oehmke S., Leendertz F.H., Sandig V. Cell lines from the Egyptian fruit bat are permissive for modified vaccinia Ankara. Virus Res. 2009;145:54–62. doi: 10.1016/j.virusres.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopylova E., Noe L., Touzet H. SortMeRNA: fast and accurate filtering of ribosomal RNAs in metatranscriptomic data. Bioinformatics. 2012;28:3211–3217. doi: 10.1093/bioinformatics/bts611. [DOI] [PubMed] [Google Scholar]
- Kralisch S., Sommer G., Weise S., Lipfert J., Lossner U., Kamprad M., Schrock K., Bluher M., Stumvoll M., Fasshauer M. Interleukin-1beta is a positive regulator of TIARP/STAMP2 gene and protein expression in adipocytes in vitro. FEBS Lett. 2009;583:1196–1200. doi: 10.1016/j.febslet.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Kuri T., Habjan M., Penski N., Weber F. Species-independent bioassay for sensitive quantification of antiviral type I interferons. Virol. J. 2010;7:50. doi: 10.1186/1743-422X-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmin I.V., Schwarz T.M., Ilinykh P.A., Jordan I., Ksiazek T.G., Sachidanandam R., Basler C.F., Bukreyev A. Innate immune responses of bat and human cells to filoviruses: commonalities and distinctions. J. Virol. 2017;91 doi: 10.1128/JVI.02471-16. e02471–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Li G., Chang Z., Yu T., Liu B., McMullen R., Chen P., Huang X. BinPacker: packing-based de novo transcriptome assembly from RNA-seq data. PLoS Comput. Biol. 2016;12:e1004772. doi: 10.1371/journal.pcbi.1004772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.Y., Aliyari R., Chikere K., Li G., Marsden M.D., Smith J.K., Pernet O., Guo H., Nusbaum R., Zack J.A. Interferon-inducible cholesterol-25-hydroxylase broadly inhibits viral entry by production of 25-hydroxycholesterol. Immunity. 2013;38:92–105. doi: 10.1016/j.immuni.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElhinney L.M., Marston D.A., Wise E.L., Freuling C.M., Bourhy H., Zanoni R., Moldal T., Kooi E.A., Neubauer-Juric A., Nokireki T. Molecular epidemiology and evolution of european bat lyssavirus 2. Int. J. Mol. Sci. 2018;19:E156. doi: 10.3390/ijms19010156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWhirter S.M., Fitzgerald K.A., Rosains J., Rowe D.C., Golenbock D.T., Maniatis T. IFN-regulatory factor 3-dependent gene expression is defective in Tbk1-deficient mouse embryonic fibroblasts. Proc. Natl. Acad. Sci. U S A. 2004;101:233–238. doi: 10.1073/pnas.2237236100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafavi S., Yoshida H., Moodley D., LeBoite H., Rothamel K., Raj T., Ye C.J., Chevrier N., Zhang S.Y., Feng T. Parsing the interferon transcriptional network and its disease associations. Cell. 2016;164:564–578. doi: 10.1016/j.cell.2015.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M.A., Devignot S., Lattwein E., Corman V.M., Maganga G.D., Gloza-Rausch F., Binger T., Vallo P., Emmerich P., Cottontail V.M. Evidence for widespread infection of African bats with Crimean-Congo hemorrhagic fever-like viruses. Sci. Rep. 2016;6:26637. doi: 10.1038/srep26637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M.A., Raj V.S., Muth D., Meyer B., Kallies S., Smits S.L., Wollny R., Bestebroer T.M., Specht S., Suliman T. Human coronavirus EMC does not require the SARS-coronavirus receptor and maintains broad replicative capability in mammalian cell lines. MBio. 2012;3 doi: 10.1128/mBio.00515-12. e00515–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelofsen M.J., Van der Ryst E. Could bats act as a reservoir hosts for Rift Valley fever virus? Onderstepoort J. Vet. Res. 1999;66:51–54. [PubMed] [Google Scholar]
- Ondov B.D., Bergman N.H., Phillippy A.M. Interactive metagenomic visualization in a Web browser. BMC Bioinformatics. 2011;12:385. doi: 10.1186/1471-2105-12-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterlund P.I., Pietilae T.E., Veckman V., Kotenko S.V., Julkunen I. IFN regulatory factor family members differentially regulate the expression of type III IFN (IFN-lambda) genes. J. Immunol. 2007;179:3434–3442. doi: 10.4049/jimmunol.179.6.3434. [DOI] [PubMed] [Google Scholar]
- Ounit R., Wanamaker S., Close T.J., Lonardi S. CLARK: fast and accurate classification of metagenomic and genomic sequences using discriminative k-mers. BMC Genomics. 2015;16:236. doi: 10.1186/s12864-015-1419-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovich S.S., Lovett S.P., Koroleva G., Guito J.C., Arnold C.E., Nagle E.R., Kulcsar K., Lee A., Thibaud-Nissen F., Hume A.J. The Egyptian rousette genome reveals unexpected features of bat antiviral immunity. Cell. 2018;173:1098–1110.e18. doi: 10.1016/j.cell.2018.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthia M., Ambite I., Cafaro C., Butler D., Huang Y., Lutay N., Rydstrom G., Gullstrand B., Swaminathan B., Nadeem A. IRF7 inhibition prevents destructive innate immunity-A target for nonantibiotic therapy of bacterial infections. Sci. Transl. Med. 2016;8:336ra359. doi: 10.1126/scitranslmed.aaf1156. [DOI] [PubMed] [Google Scholar]
- Reboldi A., Dang E.V., McDonald J.G., Liang G., Russell D.W., Cyster J.G. Inflammation. 25-Hydroxycholesterol suppresses interleukin-1-driven inflammation downstream of type I interferon. Science. 2014;345:679–684. doi: 10.1126/science.1254790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusinova I., Forster S., Yu S., Kannan A., Masse M., Cumming H., Chapman R., Hertzog P.J. Interferome v2.0: an updated database of annotated interferon-regulated genes. Nucleic Acids Res. 2013;41:D1040–D1046. doi: 10.1093/nar/gks1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid S., Mordstein M., Kochs G., Garcia-Sastre A., Tenoever B.R. Transcription factor redundancy ensures induction of the antiviral state. J. Biol. Chem. 2010;285:42013–42022. doi: 10.1074/jbc.M110.165936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieder R., Edwards R. Quality control and preprocessing of metagenomic datasets. Bioinformatics. 2011;27:863–864. doi: 10.1093/bioinformatics/btr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoggins J.W. Interferon-stimulated genes: roles in viral pathogenesis. Curr. Opin. Virol. 2014;6:40–46. doi: 10.1016/j.coviro.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw A.E., Hughes J., Gu Q., Behdenna A., Singer J.B., Dennis T., Orton R.J., Varela M., Gifford R.J., Wilson S.J. Fundamental properties of the mammalian innate immune system revealed by multispecies comparison of type I interferon responses. PLoS. Biol. 2017;15:e2004086. doi: 10.1371/journal.pbio.2004086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan E., Orr A., Everett R.D. MORC3, a component of PML nuclear bodies, has a role in restricting herpes simplex virus 1 and human cytomegalovirus. J. Virol. 2016;90:8621–8633. doi: 10.1128/JVI.00621-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C.E., Randall R.E., Adamson C.S. Inhibitors of the interferon response enhance virus replication in vitro. PLoS One. 2014;9:e112014. doi: 10.1371/journal.pone.0112014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeling E.C., Vernes S.C., Davalos L.M., Ray D.A., Gilbert M.T.P., Myers E., Bat K.C. Bat biology, genomes, and the Bat1K project: to generate chromosome-level genomes for all living bat species. Annu. Rev. Anim. Biosci. 2018;6:23–46. doi: 10.1146/annurev-animal-022516-022811. [DOI] [PubMed] [Google Scholar]
- Varela M., Piras I.M., Mullan C., Shi X., Tilston-Lunel N.L., Pinto R.M., Taggart A., Welch S.R., Neil S.J.D., Kreher F. Sensitivity to BST-2 restriction correlates with Orthobunyavirus host range. Virology. 2017;509:121–130. doi: 10.1016/j.virol.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varemo L., Nielsen J., Nookaew I. Enriching the gene set analysis of genome-wide data by incorporating directionality of gene expression and combining statistical hypotheses and methods. Nucleic Acids Res. 2013;41:4378–4391. doi: 10.1093/nar/gkt111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ver L.S., Marcos-Villar L., Landeras-Bueno S., Nieto A., Ortin J. The cellular factor NXP2/MORC3 is a positive regulator of influenza virus multiplication. J. Virol. 2015;89:10023–10030. doi: 10.1128/JVI.01530-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M., Sediri H., Felgenhauer U., Binzen I., Banfer S., Jacob R., Brunotte L., Garcia-Sastre A., Schmid-Burgk J.L., Schmidt T. Influenza virus adaptation PB2-627K modulates nucleocapsid inhibition by the pathogen sensor RIG-I. Cell Host Microbe. 2015;17:309–319. doi: 10.1016/j.chom.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S., Witkowski P.T., Auste B., Nowak K., Weber N., Fahr J., Mombouli J.V., Wolfe N.D., Drexler J.F., Drosten C. Hantavirus in bat, Sierra Leone. Emerg. Infect. Dis. 2012;18:159–161. doi: 10.3201/eid1801.111026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Zhou P., Ge X., Wang L.F., Baker M.L., Shi Z. Deep RNA sequencing reveals complex transcriptional landscape of a bat adenovirus. J. Virol. 2013;87:503–511. doi: 10.1128/JVI.02332-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne J.W., Shiell B.J., Marsh G.A., Boyd V., Harper J.A., Heesom K., Monaghan P., Zhou P., Payne J., Klein R. Proteomics informed by transcriptomics reveals Hendra virus sensitizes bat cells to TRAIL-mediated apoptosis. Genome Biol. 2014;15:532. doi: 10.1186/s13059-014-0532-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne J.W., Wang L.F. Bats and viruses: friend or foe? PLoS Pathog. 2013;9:e1003651. doi: 10.1371/journal.ppat.1003651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., Wu G., Tang J., Luo R., Patterson J., Liu S., Huang W., He G., Gu S., Li S. SOAPdenovo-Trans: de novo transcriptome assembly with short RNA-Seq reads. Bioinformatics. 2014;30:1660–1666. doi: 10.1093/bioinformatics/btu077. [DOI] [PubMed] [Google Scholar]
- Yan W.D. Going batty: studying natural reservoirs to inform drug development. Nat. Med. 2015;21:831–833. doi: 10.1038/nm0815-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Zhan Y., Zhou Y.J., Jiang Y.F., Zheng X.C., Yu L.X., Tong W., Gao F., Li L.W., Huang Q.F. Interferon regulatory factor 3 is a key regulation factor for inducing the expression of SAMHD1 in antiviral innate immunity. Sci. Rep. 2016;6:29665. doi: 10.1038/srep29665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G.J., Cowled C., Shi Z.L., Huang Z.Y., Bishop-Lilly K.A., Fang X.D., Wynne J.W., Xiong Z.Q., Baker M.L., Zhao W. Comparative analysis of bat genomes provides insight into the evolution of flight and immunity. Science. 2013;339:456–460. doi: 10.1126/science.1230835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Zeng L.P., Zhou P., Irving A.T., Li S., Shi Z.L., Wang L.F. IFNAR2-dependent gene expression profile induced by IFN-alpha in Pteropus alecto bat cells and impact of IFNAR2 knockout on virus infection. PLoS One. 2017;12:e0182866. doi: 10.1371/journal.pone.0182866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Tachedjian M., Wynne J.W., Boyd V., Cui J., Smith I., Cowled C., Ng J.H., Mok L., Michalski W.P. Contraction of the type I IFN locus and unusual constitutive expression of IFN-alpha in bats. Proc. Natl. Acad. Sci. U S A. 2016;113:2696–2701. doi: 10.1073/pnas.1518240113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
All reads were quality checked with FASTQC and Prinseq. Ribosomal RNAs were removed by SortMeRNA. Shown are read statistics before and after trimming.
Data Availability Statement
Our online search tool is available at https://www.rna.uni-jena.de/supplements/mda/report.html. The raw read data was deposited in the GEO database under accession number GEO: GSE121301. All intermediate files such as the quality-trimmed and rRNA-cleaned reads of all 18 samples, mappings, the extended genome annotation, and raw read counts were uploaded to the Open Science Framework under https://doi.org/10.17605/OSF.IO/X9KAD.
Supplemental figures and files are also found at https://www.rna.uni-jena.de/supplements/mda/.