Abstract
We investigated psychological stress response in the brain regions involved in emotion-motor-executive control in psychogenic non-epileptic seizures (PNES). 12 PNES patients and 12 healthy controls (HCs) underwent stress task and resting state functional MRI (fMRI), mood and quality of life (QOL) assessments, and measurements of salivary cortisol, alpha-amylase, and heart rate. Group differences were assessed, and we correlated beta values from a priori selected brain regions showing stress task fMRI group differences with other stress response measures. We also used the regions showing stress task fMRI group differences as seeds for resting state functional connectivity (rs-FC) analysis. Mood and QOL were worse in PNES versus HCs. Physiological and assessment measures were similar except ‘Planful Problem Solving’ coping that was greater for HCs (p = .043). Perceived stress associated negatively with heart rate change (rs = −0.74, p = .0063). There was stress fMRI hyporeactivity in left/right amygdala and left hippocampus in PNES versus HCs (corrected p < .05). PNES exhibited a positive association between alpha-amylase change and right amygdala activation (rs = 0.71, p = .010). PNES versus HCs exhibited greater right amygdala rs-FC to left precentral and inferior/middle frontal gyri (corrected p < .05). Our findings of fMRI hyporeactivity to psychological stress, along with greater emotion-motor-executive control network rs-FC in PNES when compared to HCs suggest a dysregulation in stress response circuitry in PNES.
Keywords: Psychogenic non-epileptic seizures (PNES), fMRI, Cortisol, Alpha-amylase, Psychological stress, Emotion
Abbreviations: AA, salivary alpha-amylase; BAI, Beck Anxiety Inventory; BDI-II, Beck Depression Inventory-II; CMT, control math task; CORT, salivary cortisol; dAA, percent change in alpha-amylase; dCORT, percent change in cortisol; dHR, change in heart rate; DSSQ, Dundee Stress State Questionnaire; fMRI, functional magnetic resonance imaging; FND, functional neurological disorders; IQR, interquartile range; HCs, healthy controls; HPA-axis, hypothalamic pituitary adrenal axis; HR, heart rate; PANAS, Positive Affect and Negative Affect Schedule; PNES, psychogenic non-epileptic seizures; POMS, Profile of Mood States; PSS, Perceived Stress Scale; QOL, quality of life; ROIs, regions of interest; rs-FC, resting state functional connectivity; rs-fMRI, resting state functional magnetic resonance imaging; SF-36, Short Form-36; SMT, stress math task; SNS, sympathetic nervous system; STAI-t, trait- related State-Trait Anxiety Inventory; STAI-s, state-related State-Trait Anxiety Inventory; TMD, total mood disturbance (from POMS); UAB, University of Alabama at Birmingham; WCQ, Ways of Coping Questionnaire
Highlights
-
•
HPA-axis and autonomic system activation to acute stress are similar in PNES and HC.
-
•
PNES vs. HC show hyporeactivity to psychological stress in emotion-control regions.
-
•
PNES vs. HC show stronger emotion-motor/executive control functional connectivity.
-
•
Level of perceived stress modulates heart rate response to psychological stress.
-
•
Alpha-amylase and amygdala stress reactivity are positively associated in PNES.
1. Introduction
In a typical physiological stress response, the brain sends signals to the hypothalamus, which coordinates a fast response between the sympathetic nervous system (SNS) and the adrenal glands. This is often referred to as the “fight or flight” response with the slower response mediated through the hypothalamic pituitary adrenal axis (HPA-axis). Both systems utilize positive and negative feedback mechanisms to restore the system back to a functional and stable state and maintain homeostasis (Ulrich-Lai and Herman, 2009). In psychogenic non-epileptic seizures (PNES), the abnormal physiological responses to psychological stress are thought to be the basis of their etiology (Stone et al., 2011). To date, few studies investigated HPA-axis activity in PNES (Bakvis et al., 2009; Bakvis et al., 2010; Tunca et al., 2000; Tunca et al., 1996). Compared to HCs, baseline morning serum cortisol did not significantly differ from patients with PNES (Tunca et al., 2000) or from conversion disorder patients (20 of 25 patients had PNES) (Tunca et al., 1996). One study did not find differences between PNES and HCs in salivary cortisol related to acute psychological stress (Bakvis et al., 2009). Further there were no group differences in stress-induced salivary cortisol levels or the cortisol awakening response, but there were elevated diurnal cortisol levels in PNES compared to HCs (Bakvis et al., 2010). Taken together, these studies suggest that while patients with PNES may display elevated levels of diurnal cortisol, they have similar HPA-axis reactivity to acute psychological stress compared to HCs. However, it is unknown whether this similarity extends to the neural correlates of psychological and emotional stress responses in PNES.
Alpha-amylase, an enzyme found in saliva, is considered a surrogate marker of SNS activity and also shows a rise in levels following stress (Granger et al., 2007). Stressful conditions such as written examinations and exposure to extreme temperatures (Chatterton Jr et al., 1996), as well as viewing emotionally negative images (van Stegeren et al., 2006) or playing a stressful video game (Skosnik et al., 2000) have all induced large increases in salivary alpha-amylase. The Trier Social Stress Test (Kirschbaum et al., 1993), an experimental paradigm designed to induce moderate levels of psychosocial stress and shown to increase cortisol levels, also elicited large pre- to post-stress increases in alpha-amylase levels (Gordis et al., 2006; Nater et al., 2006; Nater et al., 2005). One study investigated basal diurnal levels of alpha-amylase between PNES and HCs and found no group differences (Bakvis et al., 2010), but it is unclear if a similar alpha-amylase response would be observed to psychological and emotional stressors.
Functional neurological disorders (FND) including PNES are thought to be a network disorder with clinical symptomatology and phenotypic expression dependent on which node of the network is involved in the generation and maintenance of the disorder (Szaflarski and LaFrance, 2018). Functional MRI (fMRI) studies have consistently shown resting state functional connectivity (rs-FC) for emotion-regulation and motor-control regions to be stronger in patients with PNES (Ding et al., 2013; Szaflarski et al., 2018; van der Kruijs et al., 2012) and in FND (Diez et al., 2019; Morris et al., 2017; Wegrzyk et al., 2018) compared to HCs. Less consistent findings have been shown in the differential fMRI response to emotional/negative stimuli between HCs and persons with conversion disorder/FND including PNES. One study showed enhanced amygdala response to fearful vs. neutral faces compared to happy vs. neutral in HCs, but no difference in FND, suggesting a dampened amygdala response to negative emotional stimuli in FND (Voon et al., 2010), while others showed conversion disorder patients had increased amygdala, frontal and motor activation to emotionally negative face stimuli relative to HCs (Aybek et al., 2015). Another study showed differential activation to negatively valenced emotional faces (e.g. sad, fearful, neutral) in a number of regions, including increased precentral and postcentral gyrus activation to neutral faces and decreased prefrontal activation to sad, fearful and neutral faces in PNES compared to HCs (Szaflarski et al., 2018). Individuals with functional movement disorder (i.e. functional dystonia and functional tremor) also differed from HCs and those with primary organic movement disorder in their responses to negatively emotional stimuli in a number of prefrontal and motor-control brain regions (Espay et al., 2018a, Espay et al., 2018, Espay et al., 2019). Further, patients with functional tremor showed increased task-based functional connectivity between left amygdala and left middle frontal gyrus for intensely negative emotional stimuli (Espay et al., 2018). Others showed that compared to controls, conversion disorder/FND patients exhibited increased amygdala activation with simultaneous sensorimotor and emotional stimulation (Hassa et al., 2017) and when they received negative feedback during instrumental avoidance learning (Morris et al., 2017). Additionally, recall of traumatic and stressful life events also elicited increased amygdala activation in conversion disorder patients (Aybek et al., 2014; Kanaan et al., 2007).
Despite the growing fMRI literature in PNES and FNDs, no imaging studies to date have specifically examined the neural underpinnings of psychological stress response in patients with PNES. Thus, we aimed to investigate how patients with PNES respond to acute psychological and emotional stress in a priori defined brain regions of interest (ROIs) involved in emotion processing (insula, hippocampus and amygdala), motor function (precentral gyrus and postcentral gyrus), and emotion/executive control (anterior cingulate and inferior frontal cortex) that have been indicated as vital nodes in the FND/PNES network (Aybek et al., 2014; Ding et al., 2013; Espay et al., 2018a, Espay et al., 2018, Espay et al., 2019; Hassa et al., 2017; Morris et al., 2017; Szaflarski et al., 2018; Szaflarski and LaFrance, 2018; van der Kruijs et al., 2012; Voon et al., 2010) and overlap with regions involved in the fMRI response to psychological stress (Dedovic et al., 2009; Goodman et al., 2019; Pruessner et al., 2008). Based on these previous studies, we hypothesized that compared to HCs, patients with PNES would exhibit similar physiological responses to acute psychological stress, differential fMRI response to acute psychological and emotional stress in these a priori brain regions of interest, and stronger rs-FC between emotion-regulation and motor-control regions.
2. Material and methods
2.1. Participants
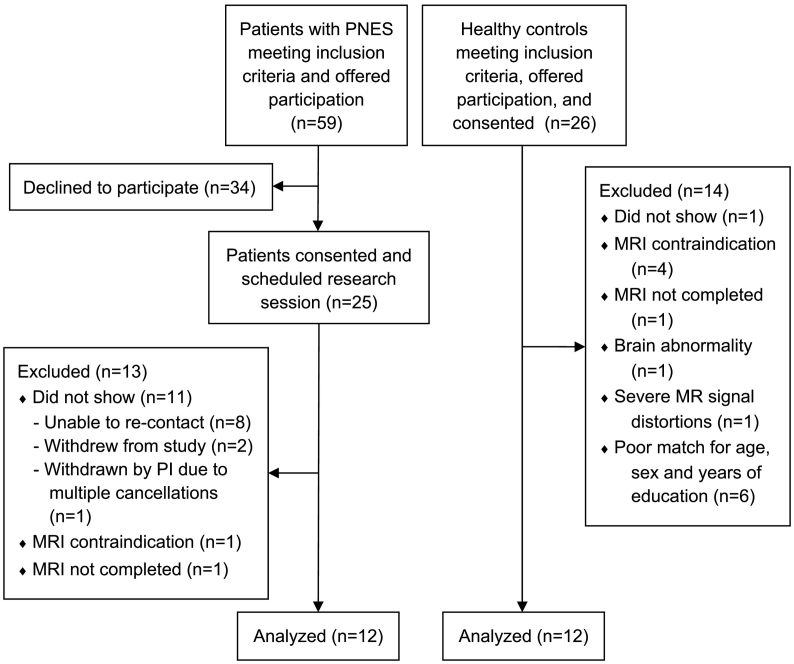
From August 2013 to April 2016, fifty-nine consecutive patients who met study criteria were approached prospectively for participation from the University of Alabama at Birmingham (UAB) Epilepsy Monitoring Unit after video-EEG monitoring confirmed definite diagnosis of PNES. Inclusion criteria for adult patients (≥18 years old) were normal brain MRI and presence of major motor symptoms (Griffith et al., 2007). Patients with mixed diagnosis combining epileptic seizures and PNES, and patients with typical and spontaneous PNES not documented during video-EEG monitoring were not approached for participation. Twenty-five patients provided written informed consent (Fig. 1). Twelve patients completed all study procedures and were included in the analyses; 12 age-/sex-/education-matched HCs were included from a group of 26 individuals recruited from UAB and surrounding Birmingham metro region (Fig. 1; Table 1). At the time of study participation, patients with PNES were not taking antiepileptic drugs. Eleven patients with PNES and 12 HCs participated in a separate portion of the study, which examined brain's responses to emotional faces task (Szaflarski et al., 2018). Individuals had no suicidal ideation in the previous year, were physically healthy, fluent in English language, and had no contraindications to receiving MRI/fMRI at 3 T. HCs had no self-reported history of psychiatric or neurological illness. Of the 12 patients with PNES, psychiatric history was not available for one patient, three had no comorbid psychiatric diagnoses, one carried a diagnosis of posttraumatic stress disorder (PTSD), and seven reported having depression and/or anxiety. Urine pregnancy tests prior to MRI were negative for female participants.
Fig. 1.
CONSORT flow diagram outlining recruitment of the 12 patients with psychogenic non-epileptic seizures (PNES) and 12 age-/sex-/education-matched healthy control participants.
Table 1.
Demographic, clinical, assessment, and behavioral variables for the 12 patients with psychogenic non-epileptic seizures (PNES) and 12 healthy controls (HCs).
| PNES | HCs | p-value | ||
|---|---|---|---|---|
| Age | 44.5 (20.5) | 35.5 (15.0) | 0.47 | |
| Sex, female | 11 (92) | 11 (92) | 1.0 | |
| Education, years | 14.5 (3.0) | 15.0 (5.3) | 0.43 | |
| Age of illness onset | 36.5 (17.5) | – | – | |
| Illness duration, years | 2 (2.75) | – | – | |
| Number of seizures in past 3 months | 24 (27.8) | – | – | |
| Assessments | ||||
| Profile of mood states total mood disturbance | 80.0 (85.0) | 6.0 (32.0) | 0.012 | |
| Beck Depression Inventory-II (BDI-II) | 28 (24.5) | 3.0 (7.0) | 0.0006 | |
| Beck Anxiety Inventory (BAI) | 15.0 (18.5) | 4.5 (5.0) | 0.0008 | |
| Trait-related Anxiety Inventory | 50.0 (20.0) | 30.5 (14.5) | 0.0055 | |
| 14-Item Perceived Stress Scale (PSS-14) | 34.5 (19.0) | 19.0 (6.0) | 0.0042 | |
| Short Form-36 (SF-36) | Physical functioning | 42.5 (27.5) | 95.0 (60.0) | 0.048 |
| Limitations due to physical health | 0.0 (25.0) | 100.0 (12.5) | 0.0001 | |
| Limitations due to emotional problems | 16.7 (50.0) | 100.0 (33.3) | 0.0019 | |
| Energy/Fatigue | 33.3 (32.5) | 70.0 (28.3) | 0.0065 | |
| Emotional well being | 52.0 (30.0) | 74.0 (18.0) | 0.0041 | |
| Social functioning | 31.3 (37.5) | 93.8 (31.3) | 0.0001 | |
| Pain | 38.8 (55.0) | 78.8 (28.8) | 0.029 | |
| General health | 32.5 (42.4) | 85.0 (10.0) | <0.0001 | |
| Ways of coping questionnaire | Confrontative coping | 3.5 (5.5) | 5.0 (3.5) | 0.98 |
| Distancing | 6.5 (10.0) | 5.5 (4.0) | 0.84 | |
| Self-controlling | 10.0 (8.0) | 11.5 (10.0) | 0.20 | |
| Seeking social support | 7.0 (8.0) | 7.0 (9.0) | 0.93 | |
| Accepting responsibility | 5.0 (4.5) | 4.0 (3.5) | 0.45 | |
| Escape-avoidance | 6.0 (7.0) | 5.0 (6.5) | 0.31 | |
| Planful problem solving | 8.5 (6.0) | 13.0 (9.0) | 0.043 | |
| Positive reappraisal | 4.5 (7.0) | 8.5 (10.0) | 0.16 | |
| Behavioral task performance | ||||
| Control math task | Math Accuracy, % correct | 89.7 (20.6) | 92.6 (11.8) | 0.36 |
| Math Response Time, msec | 3095 (700) | 2356 (822) | 0.12 | |
| Tone Accuracy, % correct | 100.0 (6.3) | 100.0 (0.0) | 0.28 | |
| Tone Response Time, msec | 1298 (548) | 912 (240) | 0.12 | |
| Stress math task | Math Accuracy, % correct | 43.7 (15.1) | 50.8 (14.3) | 0.35 |
| Math Response Time, msec | 3314 (679) | 3438 (272) | 0.56 | |
| Tone Accuracy, % correct | 100.0 (0.0) | 100.0 (0.0) | 1.0 | |
| Tone Response Time, msec | 953 (410) | 838 (200) | 0.30 | |
Data reported as median (inter-quartile range) except for sex, which is reported as frequency (percentage); msec = milliseconds.
This study was approved by the UAB Institutional Review Board with guidance on the use of deception regarding psychological stress exposure and remuneration for participation described below (Sloan and Hull, 2006). All study procedures were carried out in accordance with the Declaration of Helsinki ethics principles. Participants provided written informed consent before study participation. The informed consent document did not contain details of deception. However, we debriefed all participants at the end of the study to explain the rationale for use of deception, and they were given the opportunity to ask questions regarding the nature of the study. All participants were remunerated the full amount irrespective of their performance or what was told to them during the study session.
2.2. Assessments
Before the MRI, participants were administered the SF-36 health-related quality of life assessment (Ware Jr and Sherbourne, 1992), the 14-item Perceived Stress Scale (PSS) (Cohen et al., 1983), the Beck Depression Inventory-II (BDI-II) (Beck et al., 1996), the Beck Anxiety Inventory (BAI) (Beck and Steer, 1990), the Profile of Mood States (POMS) to calculate total mood disturbance (TMD) score reflecting transient mood state (Szaflarski et al., 2003), and the State-Trait Anxiety Inventory to measure levels of general (STAI-t) and state-related (STAI-s) anxiety (Spielberger et al., 1970). The POMS consists of subscales for various mood constructs (tension/anxiety, depression/dejection, anger/hostility, vigor/activity, fatigue/inertia, and confusion/bewilderment); thus, the TMD score provides a summary measure of mood state that includes constructs also captured with the BDI-II, BAI and STAI. The STAI-s, the Positive Affect and Negative Affect Schedule (PANAS) (Watson et al., 1988), and Dundee Stress State Questionnaire (DSSQ short form assessing dimensions of task engagement, distress and worry) (Matthews et al., 2013) were administered before and after the MRI. Participants also completed the revised Ways of Coping Questionnaire (WCQ) after MRI to evaluate coping strategies after just having a potentially stressful experience (Folkman and Lazarus, 1985).
2.3. Induction of psychological stress
We utilized a stress-induction paradigm that includes the component of social evaluative threat (provided by a combination of the circumstances of being monitored for performance, the high expectations set prior to the start of the task, the uncertainty of actual performance, and the negative feedback given) to induce moderate levels of psychological stress (Allendorfer et al., 2014; Dedovic et al., 2005). Event-related fMRI tasks were programmed using E-prime, version 1.1 (Psychology Software Tools, Inc.), and have been previously used in patients with epilepsy and HCs (Allendorfer et al., 2014). During fMRI, participants performed a low-stress control math task (CMT) with simple subtraction problems and positive feedback (e.g., “This task seems to be fairly easy for you.”). After the CMT, participants were given instructions for the stress math task (SMT) and told their performance would be evaluated. They now had 3 answers to choose from with an unknown variable time for responses to count. They were told they needed to achieve a certain percentage of correct answers based on their age and level of education in order for their data to be used and to be remunerated the full amount for participation (at the end of the study, participants were debriefed and told they would be receiving the full amount). Participants then performed the SMT during fMRI, which had more difficult subtraction and negative feedback designed to induce moderate levels of psychological stress (e.g., “You are not responding quickly enough for your answers to be counted.”) (Allendorfer et al., 2014). Auditory feedback was pre-recorded and provided at 8 instances during each task regardless of actual performance and intended to provide a low-stress environment during the CMT and a more stressful environment during the SMT. During both tasks, participants heard 8 times a train of tones with visual instructions to press “1” or “2” on the response box. This tone condition allowed us to monitor attentiveness during CMT and SMT, independent of math performance. Prior to MRI, participants performed a practice task that included simple subtraction problems and 2 instances of the tone condition to ensure they understood the task instructions.
2.4. Physiological measures
Heart rate was recorded following the 8 CMT and SMT feedback messages. At 8 time points, participants provided 1 mL saliva samples: at 60, 45, and 30 min prior to MRI, immediately after MRI, and at 15, 30, 45, and 60 min after MRI. Samples were immediately placed on ice then transferred to a − 20 °C freezer for storage. Salivary cortisol and alpha-amylase concentrations were assayed at the UAB Metabolism Core Lab using a standard kit (Salimetrics, LLC) to assess HPA-axis and sympathetic nervous system activation, respectively (Petrakova et al., 2015).
2.5. Neuroimaging
Neuroimaging was performed using a 3.0 T Siemens Allegra scanner. Prior to anatomical and task fMRI scans, 132 resting state fMRI (rs-fMRI) volumes were acquired with the participant's eyes open while viewing a black screen during gradient-echo EPI T2*-weighted imaging (TR/TE 3000/23 msec, FOV 24.0 × 24.0 × 11.5 cm, matrix 128 × 128, flip angle 84°, 2.5 mm isotropic). A high-resolution T1-weighted anatomical brain scan (TR/TE 2300/2.17 msec, FOV 25.6 × 25.6 × 19.2 cm, matrix 256 × 256, flip angle 9°, 1 mm isotropic) was acquired, followed by EPI T2*-weighted fMRI scans (TR/TE 3000/23 msec, FOV 25.6 × 25.6 × 13.8 cm, matrix 128 × 128, flip angle 84°, voxel size 2 × 2 × 3 mm) while participants performed the CMT and SMT. Forty-six oblique 3 mm slices 20° transverse-to-coronal from AC-PC line to minimize signal distortions from sinuses were positioned for fMRI while participants performed the CMT and SMT (Deichmann et al., 2003).
2.6. Statistical analysis
The Wilcoxon rank sum test and Fisher's Exact test were performed, when appropriate, to compare demographic, assessment and performance variables between groups. Repeated measures analysis of variance was performed for each assessment taken pre- and post-stress induction to examine main effects of group and time and their interactions.
Consistent with our previous study and due to known increase in anticipatory stress response prior to MRI (Allendorfer et al., 2014; Gossett et al., 2018) we regarded the last sample (1 h after stress fMRI completion) as the recovery baseline and calculated percent change in both cortisol (dCORT) and alpha-amylase (dAA) from the sample immediately post-stress to the last sample as measures of HPA-axis activation and SNS activation, respectively. We performed Wilcoxon rank sum test (2-tailed) for change in heart rate (dHR), dCORT and dAA measures to investigate group differences in responsiveness to acute psychological stress. Spearman correlation, which is more robust against the effects of outliers in a small sample, evaluated the association between physiological measures (dHR, dCORT and dAA) and PSS. Statistical analyses were performed using SAS (Statistical Analysis System version 9.3, Cary, NC), with p < .05 considered significant. For PSS associations with physiological measures, p < .017 was considered significant after Bonferroni correction for multiple comparisons.
2.7. Neuroimaging data analysis
AFNI was used to analyze and visualize the task fMRI data (Cox, 1996). Anatomical and fMRI scans were aligned, and a co-registration algorithm applied to correct for head motion (Cox and Jesmanowicz, 1999). Non- brain voxels were removed, and anatomical and fMRI scans were normalized into Montreal Neurologic Institute (MNI) space, and fMRI scans resampled to 2 × 2 × 2 mm3 voxel resolution followed by spatial smoothing to effective smoothness of 6 mm Gaussian full width half-maximum (FWHM).
We performed single-subject statistical modeling of blood‑oxygen-level-dependent (BOLD) fMRI response to math problems, feedback, and tone events. For each subject, we extracted event times used for fMRI data analyses from E-prime behavioral data. CMT and SMT math problems and tones were modeled as separate events using a canonical hemodynamic response function, and positive and negative feedback were modeled as short blocks to include entire duration of 6 s and 8.5 s, respectively. The fMRI response to acute psychological stress was assessed using two contrasts: negative versus positive auditory feedback, and hard versus easy math. This is consistent with previous studies utilizing negative versus positive feedback and/or difficult versus easy mental arithmetic in assessing fMRI stress response (Dedovic et al., 2005, Dedovic et al., 2009; Goodman et al., 2016, Goodman et al., 2019). Beta-weight values for the ideal waveform of each event and contrast were determined using linear regression with the 3dREMLfit program. Single-subject modeling also covaried for motion-correction parameters and signal drift.
Group differences in neural response to acute stress were investigated using 3dttest++ to perform a two-sample t-test with the two contrasts. Due to the potential for confounding effects of mood, we included TMD from POMS as a covariate. The POMS depression subscale was previously shown to be highly correlated with BDI-II in patients with seizure disorders (Griffith et al., 2005), and, in our study, TMD and BDI-II were strongly associated in PNES (rs = 0.95; p < .0001) and HCs (rs = 0.81; p = .0013). In each group, TMD was also positively associated with scores on the BAI (rs = 0.95; p < .0001 in PNES; rs = 0.58; p = .049 in HC) and the STAI-t (rs = 0.65; p = .021 in PNES; rs = 0.77; p = .0035 in HC). Thus, we controlled for the measure reflecting overall mood state rather than solely depression and/or anxiety scores in our analyses. To determine statistical thresholding parameters, the spatial autocorrelation function (ACF) in the 3dFWHx program was used to estimate noise smoothness and then fit to a mixed model, which was then used to generate noise random fields, estimate the probability of false-positive clusters, and determine the cluster threshold for different voxelwise thresholds using the 3dClustSim program (Cox et al., 2017). For fMRI group maps, we focused on seven brain ROIs involved in emotion processing (insula, hippocampus and amygdala), motor function (precentral gyrus and postcentral gyrus) and emotion/executive function (anterior cingulate and inferior frontal cortex), and used a small volume correction based on a priori hypotheses for group activation differences in these regions that have been indicated as network nodes in the FND/PNES network (Ding et al., 2013; Espay et al., 2018a, Espay et al., 2018, Espay et al., 2019; Hassa et al., 2017; Morris et al., 2017; Szaflarski et al., 2018; Szaflarski and LaFrance, 2018; van der Kruijs et al., 2012; Voon et al., 2010). Cluster thresholds for voxelwise p = .005 were calculated using 10,000 Monte Carlo simulations for each anatomically-defined set of bilateral brain regions to achieve activation clusters significant at corrected p < .05. The results of these simulations yielded a critical cluster extent volume threshold of 168 mm3 for the insula, 152 mm3 for the hippocampus, 80 mm3 for the amygdala, 224 mm3 for the precentral gyrus, 240 mm3 for the postcentral gyrus, 320 mm3 for the inferior frontal gyrus, and 192 mm3 for the anterior cingulate. Beta-weight values from regions showing significant group differences in fMRI response to acute psychological stress were extracted. These beta-weight values were used in Spearman correlation analyses with dHR, dCORT, dAA, and PSS to assess relationships between neural stress reactivity and other measures of stress reactivity and stress perception, respectively, with p < .0125 considered significant after Bonferroni correction for multiple comparisons.
SPM12 (Welcome Trust Center for Neuroimaging, London, UK) was used to analyze rs-fMRI data using standard procedures (i.e. slice timing correction, co-registration of brain volumes using rigid-body motion transforms, anatomical-fMRI spatial alignment, and spatial normalization to 2 × 2 × 2mm3 voxels in MNI space using unified segmentation algorithm) (Ashburner and Friston, 2005). For each subject, nuisance regression was conducted using the 6 motion parameters and their first derivatives to remove potential sources of noise. This was followed by a step-wise data scrubbing procedure (Power et al., 2012), and time points with severe signal artifacts (i.e. due to motion) were first interpolated prior to bandpass filtering (0.01 < f < 0.08 Hz). Then, a principal component analysis was performed to extract components of white matter and cerebral spinal fluid that were used as regressors in a second nuisance regression (Behzadi et al., 2007), followed by 8 mm FWHM spatial smoothing. Regions showing significant task fMRI group differences in neural response to acute psychological stress were used as functional seed regions in rs-FC analysis. Functional connectivity maps were produced by extracting the mean time series for each seed region and performing Pearson correlation with the time series of all other brain voxels. Fisher Z-transformation was then applied to every correlation coefficient value, and group statistical analysis performed. Group differences in rs-FC of these task fMRI-defined seed regions were assessed, with results significant at topological FDR-corrected p < .05 (voxelwise p = .001).
3. Results
3.1. Demographic, clinical, assessment and behavioral data
Table 1 summarizes demographic, clinical, assessment, and behavioral variables for both groups. PNES and HC groups were similar in age, sex, years of education, and behavioral performance on the CMT and SMT (Table 1). Despite low math accuracy on the SMT, both groups achieved 100% tone accuracy, indicating attentiveness during the task. Median (IQR) for age of onset of PNES was 36.5 (17.5), for illness duration was 2 (2.75) years, and for number of seizures in past 3 months was 24 (27.8). PNES group endorsed significantly more negative mood than HC group on the POMS, BDI-II, BAI and STAI-t, and scored worse than HC group on the PSS-14 and SF-36. WCQ scores were similar except HCs scored higher on “Planful Problem Solving”.
There were no significant group-by-time interactions for the PANAS, STAI-s, and DSSQ (all p > .05). There were main effects of group (F(1) = 7.90, p = .0102) and time (F(1) = 13.16, p = .0015) for positive affect, and a main effect of group only (F(1) = 5.46, p = .029) for negative affect (Fig. 2A–B). There were main effects of group (F(1) = 14.82, p = .0009) and time (F(1) = 16.50, p = .0005) for STAI-s (Fig. 2C) and for DSSQ distress subscale (F(1) = 12.64, p = .0018 and F(1) = 41.71, p < .0001, respectively; Fig. 2D).
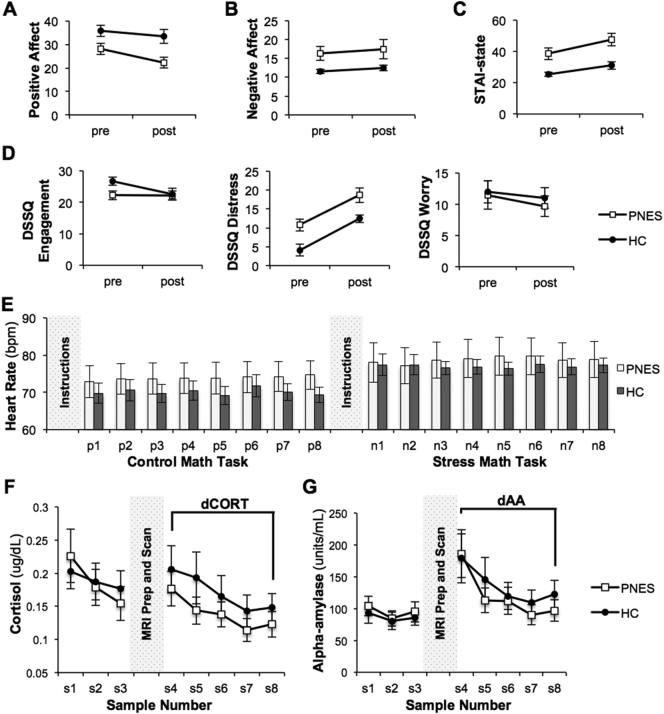
Fig. 2.
Pre- and post-stress performance on assessments and physiological measures of stress reactivity during the study session for the 12 patients with psychogenic non-epileptic seizures (PNES) and 12 healthy control (HC) participants. PNES and HC groups exhibit parallel trajectories for (A) positive affect, (B) negative affect, (C) state-related STAI scores, and (D) the distress subscale of the DSSQ. Graphs indicate mean +/− SEM at each time point for each assessment. (E) Heart rate was recorded following each of the 8 positive feedback messages (p1-p8) during the Control Math Task, and following each of the 8 negative feedback messages (n1-n8) during the Stress Math Task. Change in average heart rate between tasks was calculated for each group. (F) Salivary cortisol levels and (G) alpha-amylase levels are shown for samples collected at 60, 45 and 30 min before MRI preparation and scanning (s1, s2, and s3, respectively) and then immediately after completion of stress fMRI (s4) and another 4 samples at 15-min intervals (s5-s8). We regarded cortisol and alpha-amylase levels at s8 to be the recovery baseline in order to calculate percent change in cortisol reduction (dCORT) and alpha-amylase reduction (dAA) from the sample immediately post-stress (s4). Graphs indicate mean +/− SEM at each time point for each measure.
3.2. Physiological measures
Change in heart rate was similar between groups (Fig. 2E): median dHR (IQR) was 2.6 (6.3) bpm for PNES and 3.4 (10.8) bpm for HCs (p = .58). Changes in cortisol and alpha-amylase levels were similar between groups (Fig. 2F–G): median dCORT (IQR) was 61.5 (60.7) for PNES and 34.1 (94.3) for HCs (p = .64); median dAA (IQR) was 68.2 (153.5) for PNES and 43.3 (131.5) for HCs (p = .75). Results of Spearman correlation analysis indicated that with increased PSS, there was significant decrease in dHR (rs = −0.74, p = .0063) to psychological stress in PNES but not in HCs (rs = 0.10, p = .75). PSS was not significantly associated with dCORT or dAA in PNES (rs = −0.47, p = .12 and rs = −0.45, p = .14, respectively). In HCs, PSS was also not significantly associated with dCORT or dAA (rs = 0.44, p = .16 and rs = 0.09, p = .78, respectively).
3.3. Neuroimaging
In the seven a priori selected ROIs, patients with PNES and HCs did not significantly differ in their fMRI stress response to negative versus positive auditory feedback whether or not controlling for the mood states (TMD). However, independent of the mood states, in the fMRI stress response to hard versus easy math, PNES showed hyporeactivity compared to HCs in the left and right amygdala and the left hippocampus (Table 2; Fig. 3B1). Graphing the mean beta-weight values showed an opposite response between groups for each significant cluster (Fig. 3B2). Spearman correlation analysis indicated a significant positive association in PNES only between fMRI stress response in the right amygdala and dAA (rs = 0.71, p = .010; Fig. 3B3). There were no other significant associations detected between fMRI stress response and PSS or other physiological measures in either group (all p > .0125).
Table 2.
Location and extent of brain regions in which patients with psychogenic non-epileptic seizures (PNES) compared to healthy controls (HCs) exhibited (A) decreased stress task fMRI response and (B) increased resting state functional connectivity with the right amygdala seed.
| Brain Regions | Peak MNI (x, y, z) | Peak t-value | Cluster Extent (mm3) | |
|---|---|---|---|---|
| A. Stress task fMRI response | ||||
| L. amygdala | –18, –2, –12 | –3.93 | 200 | |
| PNES < HCs | R. amygdala | 18, 0, –14 | –4.28 | 136 |
| L. hippocampus | –22, –20, –12 | –5.69 | 184 | |
| B. Resting state functional connectivity of Right Amygdala seed | ||||
| L. precentral gyrus | –44, –12, 58 | 5.52 | 1256 | |
| PNES > HCs | L. inferior/middle frontal gyrus | –36, 14, 34 | 5.46 | 1448 |
Fig. 3.
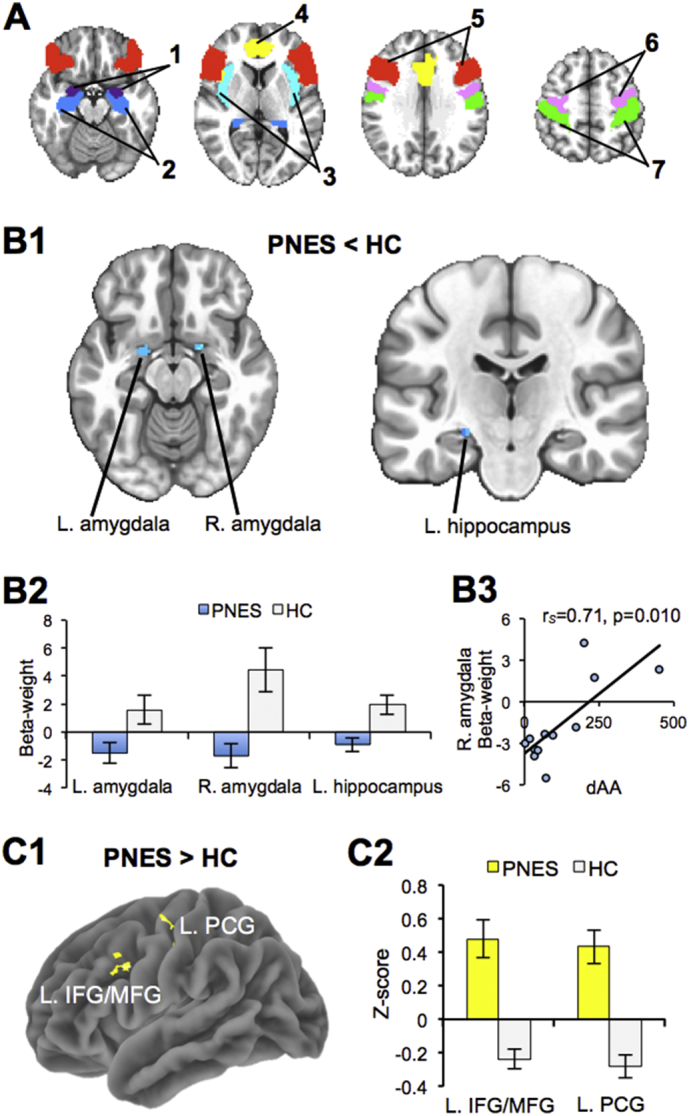
Statistical maps of significant clusters showing PNES and HC group differences in stress-related task activation and resting state functional connectivity. (A) We defined seven a priori regions of interest: (1) amygdala, (2) hippocampus, and (3) insula involved in emotion; (4) anterior cingulate and (5) inferior frontal gyrus involved in executive function; (6) precentral gyrus and (7) postcentral gyrus involved in motor control. (B1) Compared to HC, PNES exhibited decreased response (in blue) to stress fMRI (hard versus easy math) in the bilateral amygdala and left hippocampus (corrected p < .05). (B2) The bar graph below of beta-weight values (mean +/− SEM) for each significant region illustrates an opposite response between groups. (B3) The scatter plot illustrates the significant association between stress task fMRI response in the right amygdala and stress-related change in alpha-amylase (dAA) in patients with PNES. (C1) Compared to HC, PNES exhibited increased resting state functional connectivity (corrected p < .05) between the right amygdala seed region to both the left inferior/middle frontal gyrus (L. IFG/MFG) and the left precentral gyrus (L. PCG). (C2) To the right, the bar graph of z-scores (mean +/− SEM) for each region illustrates that the group differences in functional connectivity are due to an opposing pattern of connectivity. L. = left hemisphere; R. = right hemisphere.
These 3 task fMRI-defined brain regions (i.e., the left and right amygdala and left hippocampus) were used as rs-fMRI seed regions and showed significantly increased right amygdala rs-FC in PNES compared to HCs to both the left precentral gyrus and left inferior/middle frontal gyrus (corrected p < .05; Table 2; Fig. 3C1). Graphing the mean z-scores showed an opposite pattern in rs-FC between groups (increased in PNES and decreased in HC) for the right amygdala to both the left precentral gyrus and left inferior/middle frontal gyrus (Fig. 3C2).
4. Discussion
We examined the biochemical and neuroimaging biomarkers of stress response in PNES focusing on the brain regions specific to the emotion-motor-executive control. Further, we showed group differences in a priori defined regions of interest in their resting state functional connectivity. Finally, we assessed group differences in demographic, assessment, and stress reactivity variables, and associations between stress measures. These main results are discussed in the context of existing literature and future research implications.
4.1. Stress response assessments and coping styles
The analysis of stress response assessments indicates that patients with PNES have decreased positive affect, and increased negative affect, state-related anxiety, and task-related distress compared to age-/sex-/education-matched HCs. This is despite the fact that fMRI task performance was the same for both groups and despite the fact that the stress-induction paradigm elicited similar mood and behavioral effects in both groups (i.e. similar direction of change). Both groups also had similar coping styles differing only in “Planful Problem Solving,” which was less utilized by PNES. This problem-focused coping style involves efforts to eliminate or change the source of stress (Folkman et al., 1986), which may be one of the pathophysiological mechanisms of PNES development and maintenance. This is similar to task-oriented coping, which is more effective than the emotion-focused coping that was previously found to be more utilized by PNES patients with greater psychopathology (Myers et al., 2013). Thus, our results indicate that the coping strategies are different between PNES and HCs and, as such, they need to be further explored as possible targets for behavioral interventions aiming at improving control of PNES (LaFrance Jr et al., 2014).
4.2. Physiological correlates of psychological stress response and perceived stress
As hypothesized based on previous stress response studies in PNES (Bakvis et al., 2009; Bakvis et al., 2010; Tunca et al., 2000; Tunca et al., 1996), we observed similar physiological responses to acute psychological stress including change in heart rate, cortisol and alpha-amylase between PNES and HCs. Our findings are also consistent with a study in patients with motor FND that found similar cortisol and alpha-amylase response to the TSST as HCs (Apazoglou et al., 2017). However, we found levels of perceived stress to be differentially associated with physiological stress response in each group. Patients with PNES with higher levels of perceived stress exhibited smaller stress-related changes in heart rate during stress induction (rs = −0.74); this relationship was not seen in HCs. Given the positive and negative feedback loops in stress response systems which work to maintain homeostasis (Ulrich-Lai and Herman, 2009), a typical pattern of increased heart rate with acute stress is expected. However, a pattern in PNES showing overall dampening of physiological response in those who exhibit elevated levels of perceived stress may indicate stress system dysregulation. Future investigation into such relationships is warranted.
4.3. Neural correlates of psychological stress response, functional connectivity, and relationships with physiological measures and perceived stress
Of the seven a priori defined brain regions of interest involved in emotion, motor, and executive control (Aybek et al., 2014; Szaflarski et al., 2018; Szaflarski and LaFrance, 2018; van der Kruijs et al., 2012; Voon et al., 2010), we observed fMRI stress hyporeactivity in the left and right amygdala and left hippocampus. This supports our hypothesis of their differential involvement in fMRI response to acute psychological stress in PNES versus HC. These differences were independent of the mood states (TMD) suggesting that mood variability may not exert a strong influence in these results. The increased activation observed in HCs is consistent with previous fMRI studies in HCs which showed increased hippocampus activity with hard math versus control math during a stress-induction task (Dedovic et al., 2009), increased amygdala response with threat processing (Wood et al., 2014) and social evaluation (Miedl et al., 2016), and both increased amygdala and hippocampus response with fear conditioning (Harnett et al., 2016). Our findings are also consistent with a previous study suggesting a dampened amygdala response to negative emotional stimuli in patients with conversion disorder compared to HCs (Voon et al., 2010). Therefore, the opposite responses observed in hippocampus and amygdala of patients with PNES compared to HCs is supportive of hyporeactivity to acute emotional and psychological stress in these emotion-processing regions. Interestingly, we also observed a significant positive relationship between right amygdala and alpha-amylase responses to stress in PNES (rs = 0.71). An increase in alpha-amylase levels with stress indicates SNS activation (Apazoglou et al., 2017; Petrakova et al., 2015). Since amygdala stress activation is overall decreased in PNES compared to HCs, this result suggests a potential coupling between SNS hypo-responsiveness and hypo-activation of the right amygdala in response to acute psychological stress in patients with PNES. This is also consistent with our result of dampened heart rate response in patients with PNES who exhibit elevated levels of perceived stress.
Using the fMRI task-defined left hippocampus and left and right amygdala ROIs as seeds in rs-FC analysis showed significant differences in right amygdala functional connectivity to the left precentral and the left inferior/middle frontal gyri that was increased in PNES compared to HCs. The observed involvement of right amygdala is consistent with other structural and functional connectivity studies of patients with PNES/FNDs that investigated amygdala connectivity (Diez et al., 2019; Hernando et al., 2015; Morris et al., 2017; Voon et al., 2010; Wegrzyk et al., 2018). However, in our study, greater rs-FC between emotion (amygdala) and motor control regions (precentral gyrus) in PNES suggests potential underlying pathophysiology of PNES that present with major motor symptoms, which was characteristic of our sample (all participants had major motor events). Our findings are also consistent with previous work outlining increased cortical thickness in the motor and premotor regions in PNES compared to controls (Labate et al., 2012) and suggest that stronger rs-FC between emotion-regulation and motor-control regions in PNES/FND allows for the manifestation of involuntary motor symptoms by overriding executive control regions (Ding et al., 2013; van der Kruijs et al., 2012).
We also found greater rs-FC in PNES than HCs between the right amygdala and left inferior/middle frontal gyri. This finding is consistent with previous studies showing that compared to HCs, patients with FND had increased amygdala rs-FC with the dorsolateral prefrontal cortex (Morris et al., 2017) and patients with functional tremor had increased task-related functional connectivity between left amygdala and left middle frontal gyrus for an intense emotion task (Espay et al., 2018). The presence of stronger connectivity between emotion (amygdala) and executive control regions (inferior/middle frontal gyri) in PNES/FND provides support for the underlying pathophysiology being the lack of ability to inhibit motor and other behaviors. The similar findings observed in patients with functional movement disorders and FND in general indicate that the findings may not be specific to PNES but rather a feature of all FNDs (Szaflarski and LaFrance, 2018). Thus, our results provide additional evidence for a functional basis for dysregulation in the emotion-motor-executive control network in PNES/FND.
4.4. Study limitations, future directions and conclusion
There are study limitations that must be considered, and we provide recommendations for future work. One limitation is a relatively small sample size, which is prevalent in PNES research due to a number of factors. By recruiting patients from the Epilepsy Monitoring Unit, we encountered obstacles including patients living too far to participate or patients being unapproachable due to upset feelings about their diagnosis of PNES. Also, many patients did not have established medical care with our center, which likely contributed to the relatively low (56%) return rate even though patients were initially interested, provided written informed consent and scheduled research sessions. While requiring considerably more work and resources to implement, future studies should consider a multi-center design to increase study sample size. Functional connectivity studies suggest that variable brain network disruption may lead to variable presentation of PNES (Szaflarski et al., 2018; Szaflarski and LaFrance, 2018; van der Kruijs et al., 2012; Voon et al., 2010), but larger studies are needed to adequately address this. Larger future studies should also consider recruitment of an epilepsy population or other appropriate clinical population as control groups, as they may provide additional information regarding differences in stress systems and related responses (McSweeney et al., 2017; Szaflarski et al., 2018). Not only can additional control groups improve our understanding of the neurobiology of different seizure disorders, it may help identify potential targets for therapeutic strategies resulting in better overall treatment responses.
Other limitations include the timing of saliva samples collection, the lack of a structured clinical interview, potential medication effects, and the different assessments administered. Saliva sampling was limited to the hour before and after MRI, thus registering only short-term changes from acute psychological stress. While we found similar and consistent with previous studies stress-related cortisol response between PNES and HCs, measurement of diurnal cortisol may be more informative for identifying pathophysiological mechanisms related to HPA-axis. A recent study highlighted the effect of chronic stress in altering basal HPA-axis activity while leaving cortisol response to acute psychological stress intact (Berger et al., 2017). Compared to HCs, patients with PNES have been found to exhibit elevated diurnal levels of cortisol (Bakvis et al., 2010). Thus, it would be interesting to assess how these relate to neuroimaging measures in future studies. Furthermore, although self-report and chart review can provide some information regarding psychiatric comorbities, future studies should consider use of a structured clinical interview to more fully capture the psychiatric profile of study participants. Future studies should also consider potential medication effects. While the PNES group was not taking antiepileptic drugs at the time of study participation, we did not collect information regarding other medications, which may have potential confounding effects that we are unable to account for in this study. Finally, there are other assessments that could have been administered in order to further characterize the PNES and HC groups. However, we focused on stress-related assessments to complement the study's primary aim of assessing physiological and fMRI responses to acute psychological stress in PNES in brain regions specific to the emotion-motor-executive control network.
Despite limitations, we consider our investigation to be a valuable intermediary to future studies of psychological stress reactivity in PNES. Our comprehensive approach of utilizing stress-related self-report assessments, in addition to physiological and neuroimaging measures of stress response allowed for an initial investigation of the interplay of these different factors.
Funding
This work was supported by the Epilepsy Center and Civitan International Research Center at the University of Alabama at Birmingham, United States.
Disclosures
JBA is a consultant for LivaNova, Inc. and has served as a guest editor for Clinical Therapeutics. JPS has received funding from NIH, NSF, Shor Foundation for Epilepsy Research, EFA, Department of Defense, UCB Biosciences, NeuroPace Inc., FDA, AES, SAGE Therapeutics Inc., Greenwich Biosciences Inc., Serina Therapeutics Inc., and Eisai, Inc., is on the Consulting/Advisory Boards for SAGE Therapeutics Inc., Greenwich Biosciences Inc., NeuroPace, Inc., Upsher-Smith Laboratories, Inc., Medical Association of the State of AL, Serina Therapeutics Inc., LivaNova Inc., Lundbeck, and Elite Medical Experts LLC, and serves as an editorial board member for Epilepsy & Behavior, Journal of Epileptology (associate editor), Journal of Medical Science, Epilepsy Currents (contributing editor), and Folia Medica Copernicana. The remaining authors have no conflicts of interest.
Acknowledgements
The study was supported by the UAB Epilepsy Center and the UAB Civitan International Research Center. The data were presented in part at the 2015 Organization for Human Brain Mapping Meeting, the 2015 American Epilepsy Society Meeting, and the 2018 American Academy of Neurology Meeting. We thank Drs. Helen Barkan and Lawrence Ver Hoef for referring patients to our study.
References
- Allendorfer J.B., Heyse H., Mendoza L., Nelson E.B., Eliassen J.C., Storrs J.M., Szaflarski J.P. Physiologic and cortical response to acute psychosocial stress in left temporal lobe epilepsy - a pilot cross-sectional fMRI study. Epilepsy Behav. 2014;36C:115–123. doi: 10.1016/j.yebeh.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Apazoglou K., Mazzola V., Wegrzyk J., Frasca Polara G., Aybek S. Biological and perceived stress in motor functional neurological disorders. Psychoneuroendocrinology. 2017;85:142–150. doi: 10.1016/j.psyneuen.2017.08.023. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Aybek S., Nicholson T.R., Zelaya F., O’Daly O.G., Craig T.J., David A.S., Kanaan R.A. Neural correlates of recall of life events in conversion disorder. JAMA Psychiatry. 2014;71:52–60. doi: 10.1001/jamapsychiatry.2013.2842. [DOI] [PubMed] [Google Scholar]
- Aybek S., Nicholson T.R., O’Daly O., Zelaya F., Kanaan R.A., David A.S. Emotion-motion interactions in conversion disorder: an FMRI study. PLoS One. 2015;10 doi: 10.1371/journal.pone.0123273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakvis P., Roelofs K., Kuyk J., Edelbroek P.M., Swinkels W.A., Spinhoven P. Trauma, stress, and preconscious threat processing in patients with psychogenic nonepileptic seizures. Epilepsia. 2009;50:1001–1011. doi: 10.1111/j.1528-1167.2008.01862.x. [DOI] [PubMed] [Google Scholar]
- Bakvis P., Spinhoven P., Giltay E.J., Kuyk J., Edelbroek P.M., Zitman F.G., Roelofs K. Basal hypercortisolism and trauma in patients with psychogenic nonepileptic seizures. Epilepsia. 2010;51:752–759. doi: 10.1111/j.1528-1167.2009.02394.x. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A. Psychological Corporation; San Antonio, TX: 1990. Manual for the Beck Anxiety Inventory. [Google Scholar]
- Beck A.T., Steer R.A., Brown G.K. Psychological Corporation; San Antonio, TX: 1996. Manual for Beck Depression Inventory-II. [Google Scholar]
- Behzadi Y., Restom K., Liau J., Liu T.T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M., Leicht A., Slatcher A., Kraeuter A.K., Ketheesan S., Larkins S., Sarnyai Z. Cortisol awakening response and acute stress reactivity in first nations people. Sci. Rep. 2017;7 doi: 10.1038/srep41760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterton R.T., Jr., Vogelsong K.M., Lu Y.C., Ellman A.B., Hudgens G.A. Salivary alpha-amylase as a measure of endogenous adrenergic activity. Clin. Physiol. 1996;16:433–448. doi: 10.1111/j.1475-097x.1996.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Cohen S., Kamarck T., Mermelstein R. A global measure of perceived stress. J. Health Soc. Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox R.W., Jesmanowicz A. Real-time 3D image registration for functional MRI. Magn. Reson. Med. 1999;42:1014–1018. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Cox R.W., Chen G., Glen D.R., Reynolds R.C., Taylor P.A. FMRI clustering in AFNI: false-positive rates redux. Brain Connect. 2017;7:152–171. doi: 10.1089/brain.2016.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedovic K., Renwick R., Mahani N.K., Engert V., Lupien S.J., Pruessner J.C. The Montreal imaging stress task: using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. J. Psychiatry Neurosci. 2005;30:319–325. [PMC free article] [PubMed] [Google Scholar]
- Dedovic K., Rexroth M., Wolff E., Duchesne A., Scherling C., Beaudry T., Lue S.D., Lord C., Engert V., Pruessner J.C. Neural correlates of processing stressful information: an event-related fMRI study. Brain Res. 2009;1293:49–60. doi: 10.1016/j.brainres.2009.06.044. [DOI] [PubMed] [Google Scholar]
- Deichmann R., Gottfried J.A., Hutton C., Turner R. Optimized EPI for fMRI studies of the orbitofrontal cortex. Neuroimage. 2003;19:430–441. doi: 10.1016/s1053-8119(03)00073-9. [DOI] [PubMed] [Google Scholar]
- Diez I., Ortiz-Teran L., Williams B., Jalilianhasanpour R., Ospina J.P., Dickerson B.C., Keshavan M.S., LaFrance W.C., Jr., Sepulcre J., Perez D.L. Corticolimbic fast-tracking: enhanced multimodal integration in functional neurological disorder. J. Neurol. Neurosurg. Psychiatry. 2019;90:929–938. doi: 10.1136/jnnp-2018-319657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J.R., An D., Liao W., Li J., Wu G.R., Xu Q., Long Z., Gong Q., Zhou D., Sporns O., Chen H. Altered functional and structural connectivity networks in psychogenic non-epileptic seizures. PLoS One. 2013;8 doi: 10.1371/journal.pone.0063850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espay A.J., Maloney T., Vannest J., Norris M.M., Eliassen J.C., Neefus E., Allendorfer J.B., Chen R., Szaflarski J.P. Dysfunction in emotion processing underlies functional (psychogenic) dystonia. Mov. Disord. 2018;33:136–145. doi: 10.1002/mds.27217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espay A.J., Maloney T., Vannest J., Norris M.M., Eliassen J.C., Neefus E., Allendorfer J.B., Lang A.E., Szaflarski J.P. Impaired emotion processing in functional (psychogenic) tremor: a functional magnetic resonance imaging study. Neuroimage Clin. 2018;17:179–187. doi: 10.1016/j.nicl.2017.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espay A.J., Ries R., Maloney T., Vannest J., Neefus E., Dwivedi A., Allendorfer J., Wulsin L.R., LaFrance W.C., Jr., Lang A.E., Szaflarski J.P. Clinical and neural responses to cognitive behavioral therapy for functional tremor. Neurology. 2019 doi: 10.1212/WNL.0000000000008442. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman S., Lazarus R.S. If it changes it must be a process: study of emotion and coping during three stages of a college examination. J. Pers. Soc. Psychol. 1985;48:150–170. doi: 10.1037//0022-3514.48.1.150. [DOI] [PubMed] [Google Scholar]
- Folkman S., Lazarus R.S., Dunkel-Schetter C., DeLongis A., Gruen R.J. Dynamics of a stressful encounter: cognitive appraisal, coping, and encounter outcomes. J. Pers. Soc. Psychol. 1986;50:992–1003. doi: 10.1037//0022-3514.50.5.992. [DOI] [PubMed] [Google Scholar]
- Goodman A.M., Wheelock M.D., Harnett N.G., Mrug S., Granger D.A., Knight D.C. The hippocampal response to psychosocial stress varies with salivary uric acid level. Neuroscience. 2016;339:396–401. doi: 10.1016/j.neuroscience.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman A.M., Allendorfer J.B., Heyse H., Szaflarski B.A., Eliassen J.C., Nelson E.B., Storrs J.M., Szaflarski J.P. Neural response to stress and perceived stress differ in patients with left temporal lobe epilepsy. Hum. Brain Mapp. 2019;40:3415–3430. doi: 10.1002/hbm.24606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordis E.B., Granger D.A., Susman E.J., Trickett P.K. Asymmetry between salivary cortisol and alpha-amylase reactivity to stress: relation to aggressive behavior in adolescents. Psychoneuroendocrinology. 2006;31:976–987. doi: 10.1016/j.psyneuen.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Gossett E.W., Wheelock M.D., Goodman A.M., Orem T.R., Harnett N.G., Wood K.H., Mrug S., Granger D.A., Knight D.C. Anticipatory stress associated with functional magnetic resonance imaging: implications for psychosocial stress research. Int. J. Psychophysiol. 2018;125:35–41. doi: 10.1016/j.ijpsycho.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger D.A., Kivlighan K.T., el-Sheikh M., Gordis E.B., Stroud L.R. Salivary alpha-amylase in biobehavioral research: recent developments and applications. Ann. N. Y. Acad. Sci. 2007;1098:122–144. doi: 10.1196/annals.1384.008. [DOI] [PubMed] [Google Scholar]
- Griffith N.M., Szaflarski J.P., Szaflarski M., Kent G.P., Schefft B.K., Howe S.R., Privitera M.D. Measuring depressive symptoms among treatment-resistant seizure disorder patients: POMS depression scale as an alternative to the BDI-II. Epilepsy Behav. 2005;7:266–272. doi: 10.1016/j.yebeh.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Griffith N.M., Szaflarski J.P., Schefft B.K., Isaradisaikul D., Meckler J.M., McNally K.A., Privitera M.D. Relationship between semiology of psychogenic nonepileptic seizures and Minnesota multiphasic personality inventory profile. Epilepsy Behav. 2007;11:105–111. doi: 10.1016/j.yebeh.2007.04.021. [DOI] [PubMed] [Google Scholar]
- Harnett N.G., Shumen J.R., Wagle P.A., Wood K.H., Wheelock M.D., Banos J.H., Knight D.C. Neural mechanisms of human temporal fear conditioning. Neurobiol. Learn. Mem. 2016;136:97–104. doi: 10.1016/j.nlm.2016.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassa T., Sebastian A., Liepert J., Weiller C., Schmidt R., Tuscher O. Symptom-specific amygdala hyperactivity modulates motor control network in conversion disorder. Neuroimage Clin. 2017;15:143–150. doi: 10.1016/j.nicl.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernando K.A., Szaflarski J.P., Ver Hoef L.W., Lee S., Allendorfer J.B. Uncinate fasciculus connectivity in patients with psychogenic nonepileptic seizures: a preliminary diffusion tensor tractography study. Epilepsy Behav. 2015;45:68–73. doi: 10.1016/j.yebeh.2015.02.022. [DOI] [PubMed] [Google Scholar]
- Kanaan R.A., Craig T.K., Wessely S.C., David A.S. Imaging repressed memories in motor conversion disorder. Psychosom. Med. 2007;69:202–205. doi: 10.1097/PSY.0b013e31802e4297. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C., Pirke K.M., Hellhammer D.H. The ‘Trier Social Stress Test’—a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Labate A., Cerasa A., Mula M., Mumoli L., Gioia M.C., Aguglia U., Quattrone A., Gambardella A. Neuroanatomic correlates of psychogenic nonepileptic seizures: a cortical thickness and VBM study. Epilepsia. 2012;53:377–385. doi: 10.1111/j.1528-1167.2011.03347.x. [DOI] [PubMed] [Google Scholar]
- LaFrance W.C., Jr., Baird G.L., Barry J.J., Blum A.S., Frank Webb A., Keitner G.I., Machan J.T., Miller I., Szaflarski J.P., Consortium N.E.S.T.T. Multicenter pilot treatment trial for psychogenic nonepileptic seizures: a randomized clinical trial. JAMA Psychiatry. 2014;71:997–1005. doi: 10.1001/jamapsychiatry.2014.817. [DOI] [PubMed] [Google Scholar]
- Matthews G., Szalma J., Panganiban A.R., Neubauer C., Warm J.S. Profiling task stress with the Dundee Stress State Questionnaire. In: Cavalcanti L., Azevedo S., editors. Psychology of Stress. Nova Science Publishers, Inc; 2013. pp. 49–91. [Google Scholar]
- McSweeney M., Reuber M., Levita L. Neuroimaging studies in patients with psychogenic non-epileptic seizures: a systematic meta-review. Neuroimage Clin. 2017;16:210–221. doi: 10.1016/j.nicl.2017.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miedl S.F., Blechert J., Klackl J., Wiggert N., Reichenberger J., Derntl B., Wilhelm F.H. Criticism hurts everybody, praise only some: common and specific neural responses to approving and disapproving social-evaluative videos. Neuroimage. 2016;132:138–147. doi: 10.1016/j.neuroimage.2016.02.027. [DOI] [PubMed] [Google Scholar]
- Morris L.S., To B., Baek K., Chang-Webb Y.C., Mitchell S., Strelchuk D., Mikheenko Y., Phillips W., Zandi M., Jenaway A., Walsh C., Voon V. Disrupted avoidance learning in functional neurological disorder: implications for harm avoidance theories. Neuroimage Clin. 2017;16:286–294. doi: 10.1016/j.nicl.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers L., Fleming M., Lancman M., Perrine K., Lancman M. Stress coping strategies in patients with psychogenic non-epileptic seizures and how they relate to trauma symptoms, alexithymia, anger and mood. Seizure. 2013;22:634–639. doi: 10.1016/j.seizure.2013.04.018. [DOI] [PubMed] [Google Scholar]
- Nater U.M., Rohleder N., Gaab J., Berger S., Jud A., Kirschbaum C., Ehlert U. Human salivary alpha-amylase reactivity in a psychosocial stress paradigm. Int. J. Psychophysiol. 2005;55:333–342. doi: 10.1016/j.ijpsycho.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Nater U.M., La Marca R., Florin L., Moses A., Langhans W., Koller M.M., Ehlert U. Stress-induced changes in human salivary alpha-amylase activity—associations with adrenergic activity. Psychoneuroendocrinology. 2006;31:49–58. doi: 10.1016/j.psyneuen.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Petrakova L., Doering B.K., Vits S., Engler H., Rief W., Schedlowski M., Grigoleit J.S. Psychosocial stress increases salivary alpha-amylase activity independently from plasma noradrenaline levels. PLoS One. 2015;10 doi: 10.1371/journal.pone.0134561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner J.C., Dedovic K., Khalili-Mahani N., Engert V., Pruessner M., Buss C., Renwick R., Dagher A., Meaney M.J., Lupien S. Deactivation of the limbic system during acute psychosocial stress: evidence from positron emission tomography and functional magnetic resonance imaging studies. Biol. Psychiatry. 2008;63:234–240. doi: 10.1016/j.biopsych.2007.04.041. [DOI] [PubMed] [Google Scholar]
- Skosnik P.D., Chatterton R.T., Jr., Swisher T., Park S. Modulation of attentional inhibition by norepinephrine and cortisol after psychological stress. Int. J. Psychophysiol. 2000;36:59–68. doi: 10.1016/s0167-8760(99)00100-2. [DOI] [PubMed] [Google Scholar]
- Sloan L., Hull J. Institutional review board management and function. In: Bankert E.A., Amdur R.J., editors. Deception of Research Subjects. 2nd ed. Jones and Bartlett Publishers; Sudbury, Massachusetts: 2006. pp. 210–215. [Google Scholar]
- Spielberger C.D., Gorsuch R.L., Lushene R.E. Consulting Psychologists Press; Palo Alto, CA: 1970. The State-Trait Anxiety Inventory. [Google Scholar]
- Stone J., LaFrance W.C., Jr., Brown R., Spiegel D., Levenson J.L., Sharpe M. Conversion disorder: current problems and potential solutions for DSM-5. J. Psychosom. Res. 2011;71:369–376. doi: 10.1016/j.jpsychores.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Szaflarski J.P., LaFrance W.C., Jr. Psychogenic Nonepileptic Seizures (PNES) as a network disorder - evidence from neuroimaging of functional (psychogenic) neurological disorders. Epilepsy Curr. 2018;18:211–216. doi: 10.5698/1535-7597.18.4.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski J.P., Szaflarski M., Hughes C., Ficker D.M., Cahill W.T., Privitera M.D. Psychopathology and quality of life: psychogenic non-epileptic seizures versus epilepsy. Med. Sci. Monit. 2003;9:CR113–118. [PubMed] [Google Scholar]
- Szaflarski J.P., Allendorfer J.B., Nenert R., LaFrance W.C., Jr., Barkan H.I., DeWolfe J., Pati S., Thomas A.E., Ver Hoef L. Facial emotion processing in patients with seizure disorders. Epilepsy Behav. 2018;79:193–204. doi: 10.1016/j.yebeh.2017.12.004. [DOI] [PubMed] [Google Scholar]
- Tunca Z., Fidaner H., Cimilli C., Kaya N., Biber B., Yesil S., Ozerdem A. Is conversion disorder biologically related with depression?: a DST study. Biol. Psychiatry. 1996;39:216–219. doi: 10.1016/0006-3223(95)00474-2. [DOI] [PubMed] [Google Scholar]
- Tunca Z., Ergene U., Fidaner H., Cimilli C., Ozerdem A., Alkin T., Aslan B.U. Reevaluation of serum cortisol in conversion disorder with seizure (pseudoseizure) Psychosomatics. 2000;41:152–153. doi: 10.1176/appi.psy.41.2.152. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai Y.M., Herman J.P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kruijs S.J., Bodde N.M., Vaessen M.J., Lazeron R.H., Vonck K., Boon P., Hofman P.A., Backes W.H., Aldenkamp A.P., Jansen J.F. Functional connectivity of dissociation in patients with psychogenic non-epileptic seizures. J. Neurol. Neurosurg. Psychiatry. 2012;83:239–247. doi: 10.1136/jnnp-2011-300776. [DOI] [PubMed] [Google Scholar]
- van Stegeren A., Rohleder N., Everaerd W., Wolf O.T. Salivary alpha amylase as marker for adrenergic activity during stress: effect of betablockade. Psychoneuroendocrinology. 2006;31:137–141. doi: 10.1016/j.psyneuen.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Voon V., Brezing C., Gallea C., Ameli R., Roelofs K., LaFrance W.C., Jr., Hallett M. Emotional stimuli and motor conversion disorder. Brain. 2010;133:1526–1536. doi: 10.1093/brain/awq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware J.E., Jr., Sherbourne C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care. 1992;30:473–483. [PubMed] [Google Scholar]
- Watson D., Clark L.A., Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J. Pers. Soc. Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wegrzyk J., Kebets V., Richiardi J., Galli S., de Ville D.V., Aybek S. Identifying motor functional neurological disorder using resting-state functional connectivity. Neuroimage Clin. 2018;17:163–168. doi: 10.1016/j.nicl.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood K.H., Ver Hoef L.W., Knight D.C. The amygdala mediates the emotional modulation of threat-elicited skin conductance response. Emotion. 2014;14:693–700. doi: 10.1037/a0036636. [DOI] [PMC free article] [PubMed] [Google Scholar]