Summary
Background
One of the possible causes of dissatisfaction reported by many patients after total knee replacement (TKR) is the lack of agreement between component size and bone structure. To avoid this complication and facilitate the procedure, preoperative planning with digitized templates is recommended. Surgical navigation indicates the best position and the most adequate size of arthroplasty and may therefore replace preoperative radiographic measurement. The objective of the study was to check agreement between the sizes of TKR components measured before surgery with digitized templates, the size recommended by the navigation and sizes actually implanted.
Methods
In 103 patients scheduled for TKR, preoperative full-limb radiography was performed to measure the mechanical and anatomical axes of the limb, femur and tibia. The most adequate size of the femoral and tibial components was planned by superimposing digitized templates. The size recommended in navigation and the size of the finally implanted components were also recorded.
Results
A high level of agreement was found between the sizes of femoral and tibial components measured by X-rays and in navigation (0.750 and 0.772, respectively) (intraclass correlation and Cronbach's alpha). Agreement between the sizes recommended by X-rays and navigation and those finally implanted was 0.886 for the femur and 0.891 for the tibia. Agreement levels were not different in cases with prior deformities of limb axis.
Conclusions
The high level of agreement found in component sizes between radiographic measurement with digitized templates and navigation suggests that preoperative X-ray measurement is not needed when navigation is used for placement of implants during TKR.
The translational potential of this article
Computer-assisted surgery may avoid preoperative measurement with templates in TKR.
Keywords: Component size, Digital templating, Navigation, Total knee replacement
Introduction
Despite the good results achieved with total knee replacement (TKR), expectations in terms of absence of pain and full return to prior activity are not met in a high proportion of patients (up to 20%), with the resultant high dissatisfaction rate [1]. Literature has analyzed the potential causes of these deficient results, but they have not been clearly defined, and the issue is still unresolved [2].
Incongruity between the sizes of prosthetic components and bone structure has been considered a potential cause of pain and limitation of postoperative mobility. Anteroposterior oversizing of the tibial tray [3], [4] or presence of a medial overhang, seen in 20% of cases in some series [5], is associated with pain, decreased mobility, and loss of function after implantation [6].
Preoperative radiographic planning is currently considered indispensable for an adequate procedure and to achieve better clinical and functional results after TKR [7]. This planning allows for measurement of the mechanical and anatomic axes of the leg, indicates implant size and best position, analyzes bone deformities and deficiencies, anticipates gestures in response to certain situations and foresees technical modifications not contemplated in standard surgery. Planning facilitates implant procedure on the one hand, whereas on the other hand, it provides adequate control of surgical armamentarium, facilitates nursing care and may even be considered a measure of protection in the event of a lawsuit. Manual templates were used for planning some years ago, but they have been replaced by digitized templates and callipers, which have increased convenience for the surgeon and accuracy and represent a good source of information [8].
In TKR, computer-assisted surgery (CAS) has been shown to be of value for achieving a better femorotibial mechanical axis and to decrease outliers as compared with the standard procedure [9], [10], an advantage that is even more attractive when faced with major deformities [11]. CAS also provides a study of ligament balance and an analysis of gap in flexion and extension, ensures correction of bone sections in terms of thickness and direction and provides a guide on the gestures required for adequate placement of arthroplasty [12]. After collection of bony landmarks in femur and tibia and surface mapping, it also allows for finding the size of the individual components and helps obtain the best position of arthroplasty in the coronal, axial and lateral planes.
The aim of our study was to first check agreement between the sizes of the femoral and tibial components measured before surgery with digitized templates and the size recommended by the CAS. These two measurements will be compared with the sizes actually implanted, finding the agreement between the three measurements. The second objective was to analyze whether agreement between these measurements is altered depending on the prior mechanical axis of the limb, that is, if the results are modified when femorotibial deformities exist. To sum up, the aim of this study is to ascertain whether use of navigation makes preoperative measurement with templates unnecessary. If so, this could suggest that obtaining the size with bone surface mapping and collection of landmarks of certain anatomical structures in navigation is a valid procedure and avoids the need for other types of measurements.
Material and methods
This study is a prospective, nonrandomized study. The same TKR model (Apex; OMNIlife Science, Massachusetts, USA) was implanted in 103 cases with the help of surgical navigation. Mean patient age was 72.12 years [standard deviation (SD), 9.692]; age ranged from 41 to 88 years. Mean body mass index was 31.731 (SD, 5.748). There were 71 female and 32 male patients, and 11 cases were bilateral. As preoperative workup, all patients underwent lateral X-rays of the knee and frontal full-limb radiography in a standing position including a metallic calliper of known diameter located close to the knee, which allows for adequate sizing of bone structure. From the picture archiving and communication system of the hospital (Impax 6.3.1. 2813; Agfa Healthcare N.U. Montsel, Belgium), images were loaded into the surgical planning software (Agfa Orthopaedics Tools v 2.06). This tool was used to first calculate the anatomical and mechanical axes of femur and tibia and then the anatomical and mechanical axes of the limb. Optionally, the software itself estimates the osteotomies and corrections needed to obtain a knee with normal alignment. Finally, using the digital templates supplied by the implant manufacturer, which are stored in a template file, the adequate size of the femoral and tibial components was calculated by superimposing them on the anteroposterior and lateral knee views (Figure 1). Axis angulation, planning performed and sizes selected were all recorded and stored and thus ready to be exported. Measurements were performed by two of the authors, who are widely experienced in the use of this planning system. All TKRs were cemented; the posterior stabilized knee was used in 23 cases, whereas an ultracongruent polyethylene insert was used in another 22 cases.
Figure 1.
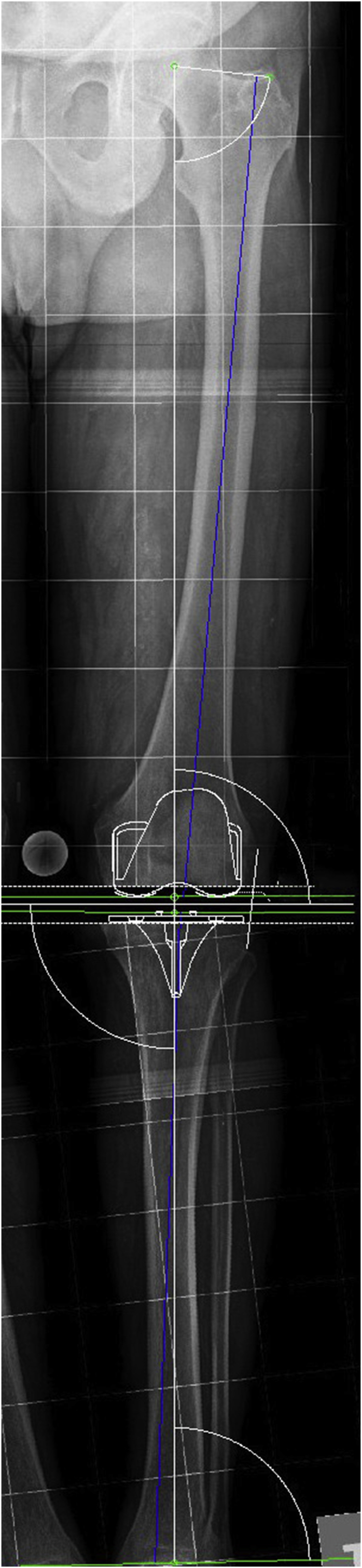
Measurement of mechanical axis of the limb and implant sizes in preoperative X-rays.
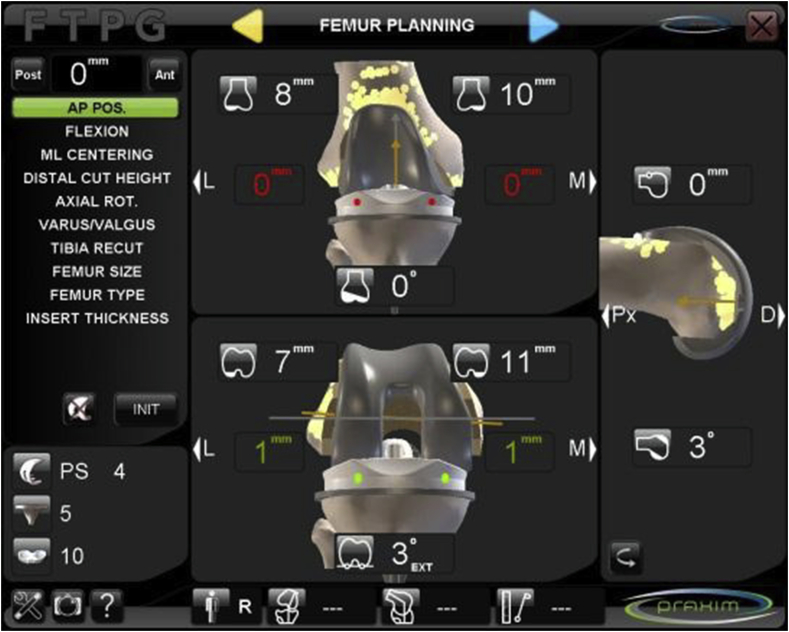
The same image-free navigation system was used in all cases (Nanostation; Total Knee Surgetics, Praxim, S.A. La Tronche, France). During the surgical procedure, the system collects different types of information and recommends use of definitive implants of the adequate size (Figure 2). Before navigation was started, femoral and tibial osteophytes were resected. At surgery, the size of femoral and tibial component that covered, but did not exceed, the resected bone plane completely was implanted, and the size finally implanted was recorded in the electronic clinical history of the patient. All patients received information about the study and signed a specific informed consent. Approval for this study was granted by the regional ethics committee [Regional Ethics Committee of Asturias, Spain (PI12/01098)].
Figure 2.
Screen of the navigation system showing the recommended sizes.
Data were statistically processed using agreement analysis. The intraclass correlation coefficient of average measures (Cronbach's alpha) with its 95% confidence interval was used. IBM SPSS Statistics v21 and Medcalc 15.2 were used for statistical analysis (IBM Corporation, Armonk, New York, EE.UU.).
Results
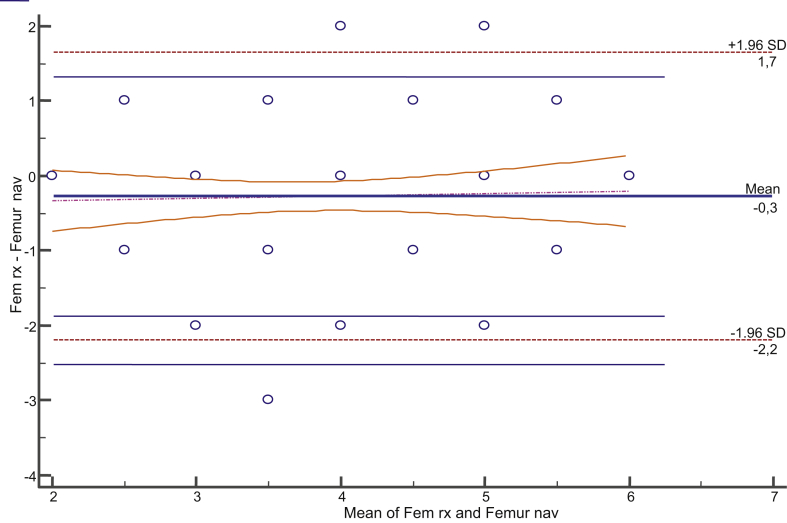
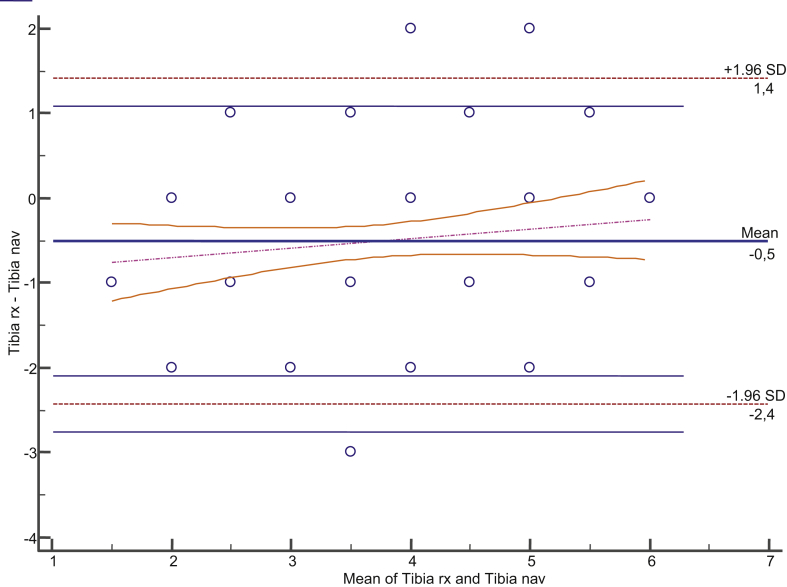
Mean prior mechanical femorotibial angle of the limb was 3.375° (SD, 12.751; range, −26° to +20.1°). This angle ranged from ≤4° and ≥4° in 11 cases only. Valgus angulation was found in 29 cases, and varus angulation, in 63. Varus angulation was considered as positive, and valgus angulation, as negative. Agreement between the sizes of the femoral component measured in radiography and navigation was 0.750 (0.631–0.831) (Figure 3). Agreement between the sizes measured in full-limb radiography and navigation and those actually implanted was 0.886 (0.842–0.919). As regards the tibial component, agreement between the sizes measured in radiography and navigation was 0.772 (0.663–0.846) (Figure 4). Agreement between the sizes measured in radiography and navigation and those actually implanted was 0.886 (0.842–0.919) for the femoral component and 0.891 (0.849–0.923) for the tibial implant (Table 1). Agreement between the polyethylene thickness measured in navigation and the one actually used was 0.332 (0.130–0.548). Agreement of measures was also studied taking into account the prior radiographic deformity, and no differences related to variation of such deformity were seen (Table 2).
Figure 3.
Bland–Altman plot. Agreement between femur X-ray size and navigation.
Figure 4.
Bland–Altman plot. Agreement between tibia X-ray size and navigation.
Table 1.
Agreement between implant size measurements.
| Component | Agreement | Mean | 95% CI lower limit | 95% CI upper limit |
|---|---|---|---|---|
| Femur | X-ray navigation | 0.750 | 0.631 | 0.831 |
| X-ray implanted size | 0.868 | 0.806 | 0.911 | |
| X-ray navigation implanted size | 0.886 | 0.842 | 0.919 | |
| Tibia | X-ray navigation | 0.772 | 0.663 | 0.846 |
| X-ray implanted size | 0.871 | 0.809 | 0.912 | |
| X-ray navigation implanted size | 0.891 | 0.849 | 0.923 |
CI = confidence interval.
Table 2.
Agreement between sizes measured using X-rays, navigation and implant placed based on prior deformity.
| Component | Deformity | Mean | 95% CI lower limit | 95% CI upper limit |
|---|---|---|---|---|
| Femur | Valgus (≤−4°) | 0.874 | 0.769 | 0.936 |
| Normal (−3° a +3°) | 0.795 | 0.236 | 0.962 | |
| Varus (≥4°) | 0.895 | 0.840 | 0.933 | |
| Tibia | Valgus (≤−4°) | 0.919 | 0.852 | 0.959 |
| Normal (−3° a +3°) | 0.780 | 0.180 | 0.959 | |
| Varus (≥4°) | 0.884 | 0.824 | 0.926 |
CI = confidence interval.
Discussion
Prior studies have shown a good correlation between preoperative radiographic measurement and the information provided by navigation in TKRs. Both the mechanical limb axis and rotation of prosthetic components show a high level of agreement between radiographic studies and information provided by navigation [13]. These findings suggest that when CAS is used, some preoperative or postoperative radiographic studies are not essential. Our study was also intended to ascertain whether navigation may also avoid measurement of prosthesis size with templates in preoperative radiographic planning. If a good level of agreement exists between measures provided by X-rays and navigation, this could also confirm that data collection with navigation allows adequate TKR size and orientation to be achieved.
Adequate placement of prosthetic components is an essential premise to achieve good results with TKR [14]. As regards size, however, it is known that oversizing of the tibial tray is very common (up to 32% in some series) and leads to a poorer clinical outcome, less postoperative mobility and persistence of pain [15]. This poorer outcome is maintained over time until at least five years after surgery [5], [16]. Oversizing at femoral level also causes clinical changes [17]. By contrast, undersizing of the component may cause a lesion of the anterior femoral cortex with a risk of notching [18].
As a result of these findings, several technical modifications in TKR design have been recommended, including asymmetry of tibial trays and increase in size with variable widths, as well as differentiation of sizes by patient sex [19]. In an attempt to perform individualized surgery and predefine the right size of components, use of individualized cutting templates has been recommended. Some studies have shown that with this procedure, the previously determined size was modified at surgery in almost 80% of femurs and more than 50% of tibias [20], which prevents adequate preoperative planning [21]. A recent meta-analysis of 21 randomized clinical trials [22] comparing standard surgery and surgery with individualized cutting templates showed no advantages of this procedure in operating time, implant positioning, final limb axis or clinical outcome.
Measurement of adequate size of TKR with templates at preoperative planning is a widely recommended procedure [8]. Measurement was traditionally made by superimposing knee X-rays on precalibrated acetate templates. Availability problems were common, measurement was subjective, the size chosen was not recorded in the patient's clinical history, oversizing or undersizing errors would occur because X-ray magnification was often unknown and, in addition, X-ray images are no longer represented on photographic plates. For some authors, there was a marked discrepancy between the size measured with these acetate templates and the size required by the bone [23]. Discrepancies were up to 50% in the femur and up to 70% in the tibia [6]. However, the method was considered to be valid, and security and accuracy improved if an approximate size was accepted [24], [25].
Digitized templates that can be used in any computer in which the appropriate software has been installed have been available for some years now. This is a fast procedure that allows for planning and simulation options, requires no physical X-rays or templates, may be integrated into the hospital picture archiving and communication system, includes help software, allows for image storage and export and may be used as legal documentation or for research. This digital procedure is considered to provide more accurate measures and to be more reproducible and simple as compared with the manual procedure, especially at tibial level. It may be stated that templates of this type provide good reliability for both femur and tibia when callipers and models specific for each implant are used. According to literature reports (Table 3), accuracy in the femur ranges from 42.5% to 83%, and if a greater or smaller size is considered, accuracy ranges from 92% to 100%. Accuracy in the tibia ranges from 48% to 90%, increasing to values of 88%–100%. Accuracy may be considered to be close to 60% in both components but increases to 97% when a greater or smaller size is added. Because physical X-rays are not currently available, it is assumed that this digital measurement system will be the one finally accepted.
Table 3.
Component size accuracy in different series.
| Author | TKR | Femur accuracy (%) | Femur accuracy ±1 (%) | Tibia accuracy (%) | Tibia accuracy ±1 (%) |
|---|---|---|---|---|---|
| Miller and Purtill [28] | 25 | 52.0 | 100 | 48.0 | 96.0 |
| Trickett et al [27] | 40 | 48.0 | 98.0 | 55.0 | 100 |
| Kniesel et al [31] | 46 | 42.5 | 97.0 | 71.0 | 98.0 |
| Hsu et al [30] | 48 | 58.0 | 96.0 | 50.0 | 88.0 |
| Specht et al [26] | 50 | 48.0 | 92.0 | 52.0 | 94.0 |
| The et al [33] | 65 | 55.0 | 92.0 | 52.0 | 94.0 |
| McLawhorn et al [34] | 76 | 66.0 | 99.0 | 66.0 | 97.0 |
| Hsu el al [32] | 82 | 83.0 | 100 | 90.0 | 100 |
| Peek et al [8] | 92 | 71.0 | 100 | 60.0 | 100 |
| Levine et al [29] | 176 | 69.0 | 100 | 63.0 | 97.0 |
| Mean | 59.2 | 97.4 | 60.7 | 96.4 |
TKR = total knee replacement.
Preoperative measurements may also be performed by computed tomography. It has recently been reported that 3D measurement with computed tomography is not more reliable than measurement with digitized templates in standard X-rays [35]. A significant correlation has also been reported between anthropometric data of the patient (sex, weight and height) and size of bone structure and, thus, adequate implant size. These data have not been linearly shown, but it has been found that if measurement with digitized templates in the preoperative study is added to these data, agreement reaches 99% if a greater or smaller size is accepted [36].
CAS allows for preoperative measurement of bone surfaces and, thus, for recommending the best size of arthroplasty. In image-free navigation systems, similar to the one used in our study, size is derived from bony landmarks collected and extensive mapping of joint surfaces. It is advised to first resect osteophytes and to have sufficient, adequately collected landmarks. In our series, agreement between sizes measured in X-rays and navigation was 0.750 and 0.772 for the femur and the tibia, respectively, and agreement increased when the size of the implant actually implanted was added. In this case, agreement was 0.886 for the femur and 0.891 for the tibia. If we consider excellent agreement values > 0.81 and acceptable and moderate agreement values ranging from 0.61 to 0.80 and those from 0.41 to 0.60, respectively [37], our results represent excellent agreement between the size determined in planning and navigation.
Coronal deformity of the limb does not appear to modify accuracy between sizes measured in X-rays and the size of implanted components [23]. This study supports this statement as agreement levels did not change depending on the preoperative mechanical axis.
Literature on the agreement between the size recommended by the CAS system and the size actually implanted is scarce. Some authors found no good relationship between both. For Benjamín [38], in 60% of cases, the size of the implanted femoral component was smaller than that recommended by the navigation system. This author concluded that navigation does not replace other measurement systems using templates. The reason for this discrepancy may be due to the use of navigation systems other than the one used by us that collect other landmarks, do not map surfaces, do not take into account true femoral anatomy and do not allow the position of the component to be moved forward or backward in planning. The findings in other studies [39], [40] warning of the frequency of anterior femoral notching and secondarily on sizing of the femoral component after CAS may be due to these same causes. This complication did not occur in any case in our series, maybe because, unlike with other TKR models, two widths per size are available with the TKR model used.
There is no prior literature relating the two measurements taken in our study and the size actually implanted. As noted, there are studies of agreement comparing digitized template sizes and final sizes and studies relating sizes determined by planning with templates and with navigation, but no study relates both. Thus, no comparison may be made with other studies.
Our study has its limitations. There is a risk of erroneously measuring the size of bone structure in digitized X-rays. Anatomical landmarks may also be collected incorrectly in navigation. The authors are widely experienced in both template measurement and CAS. Our study was mainly conducted on knees with prior deformities, which may have influenced the outcome. On the other hand, the size considered most adequate, just covering the cut surface, was actually implanted. It cannot be stated that there were no errors in this aspect. Our findings were made with a specific navigation system, and different results may possibly have been achieved using other systems.
Based on the high level of agreement found between component sizes proposed in the radiography and with navigation and the size actually implanted, we conclude that CAS may avoid the need for preoperative measurement with templates in TKR. A good level of agreement between the size determined with navigation and the size of the implanted prosthesis allows preoperative measurement with digitized templates to be obviated.
Conflict of interest
The authors have no conflicts of interest to be declared.
Ethical approval
All procedures in studies involving human participants were performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Funding
This work was supported by the National Institute of Health of Spain (Instituto de Salud Carlos III, Ministry of Health, Spain) using FEDER funds (grant number PI12/01098).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jot.2018.10.006.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Carr A.J., Robertsson O., Graves S., Price A.J., Arden N.K., Judge A. Knee replacement. Lancet. 2012;379:1331–1340. doi: 10.1016/S0140-6736(11)60752-6. [DOI] [PubMed] [Google Scholar]
- 2.Bourne R.B., Chesworth B.M., Davis A.M., Mohamed N.N., Charron K.D. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;48:57–63. doi: 10.1007/s11999-009-1119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McArthur J., Makrides P., Thangarajah T., Brooks S. Tibial component overhang in total knee replacement: incidence and functional outcomes. Acta Orthop Belg. 2012;78:199–202. [PubMed] [Google Scholar]
- 4.Bonnin M.P., Schmidt A., Basiglini L., Bossard N. Dantony E Mediolateral oversizing influences pain, function, and flexion after TKA. Knee Surg Sports Traumatol Arthrosc. 2013;21:2314–2324. doi: 10.1007/s00167-013-2443-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nielsen C.S., Nebergall A., Huddleston J., Kallemose T., Malchau H., Troelsen A. Medial overhang of the tibial component is associated with higher risk of inferior KOOS pain score after knee replacement. J Artrhoplasty. 2018;33:1394–1398. doi: 10.1016/j.arth.2017.12.027. [DOI] [PubMed] [Google Scholar]
- 6.Bonnin M.P., Saffarini M., Shepherd D., Bossard N., Dantony E. Oversizing the tibial component in TKAs: incidence, consequences and risk factors. Knee Surg Sports Traumatol Arthrosc. 2016;24:2532–2540. doi: 10.1007/s00167-015-3512-0. [DOI] [PubMed] [Google Scholar]
- 7.Tanzer M., Makhdom A.M. Preoperative planning in primary total knee arthroplasty. J Am Acad Orthop Surg. 2016;24:220–230. doi: 10.5435/JAAOS-D-14-00332. [DOI] [PubMed] [Google Scholar]
- 8.Peek A.C., Bloch B., Auld J. How useful is templating for total knee replacement component sizing? Knee. 2012;19:266–269. doi: 10.1016/j.knee.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Mason J.B., Fehring T.K., Estok R., Banel D., Fahrbach K. Meta-analysis of alignment outcomes in computer-assisted total knee arthroplasty surgery. J Arthroplasty. 2007;22:1097–1106. doi: 10.1016/j.arth.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Hetaimish B.M., Khan M.M., Simunovic N., Al-Harbi H.H., Bhandari M., Zalzal P.K. Meta-analysis of navigation vs conventional total knee arthroplasty. J Arthroplasty. 2012;27:1177–1182. doi: 10.1016/j.arth.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 11.Hernández-Vaquero D., Suarez-Vazquez A., Sandoval-Garcia M.A., Noriega-Fernandez A. Computer assistance increases precision of component placement in total knee arthroplasty with articular deformity. Clin Orthop Relat Res. 2010;468:1237–1241. doi: 10.1007/s11999-009-1175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haaker R.G., Stockheim M., Kamp M., Proff G., Breitenfelder J., Ottersbach A. Computer-assisted navigation increases precision of component placement in total knee arthroplasty. Clin Orthop Relat Res. 2005;433:152–159. doi: 10.1097/01.blo.0000150564.31880.c4. [DOI] [PubMed] [Google Scholar]
- 13.Hernández-Vaquero D., Noriega-Fernandez A., Suarez-Vazquez A., Roncero-Gonzalez S., Sierra-Pereira A.A., Gil-Martinez L. Frontal alignment in total knee arthroplasty. Comparative study between radiographic measurement and surgical navigation. Rev Española Cirugía Ortopédica Traumatol. 2017;61:313–318. doi: 10.1016/j.recot.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Lotke P.A., Ecker M.L. Influence of positioning of prosthesis in total knee replacement. J Bone Joint Surg Am. 1977;59:77–79. [PubMed] [Google Scholar]
- 15.Martin S., Saurez A., Ismaily S., Ashfaq K., Noble P., Incavo S.J. Maximizing tibial coverage is detrimental to proper rotational alignment. Clin Orthop Relat Res. 2014;472:121–125. doi: 10.1007/s11999-013-3047-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heylen S., Foubert K., Van Haver A., Nicolai P. Effect of femoro-tibial component size mismatch on outcome in primary total knee replacement. Knee. 2016;23:532–534. doi: 10.1016/j.knee.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Mahoney O.M., Kinsey T. Overhang of the femoral component in total knee arthroplasty: risk factors and clinical consequences. J Bone Joint Surg Am. 2010;92:1115–1121. doi: 10.2106/JBJS.H.00434. [DOI] [PubMed] [Google Scholar]
- 18.Zalzal P., Backstein D., Gross A.E., Papini M. Notching of the anterior femoral cortex during total knee arthroplasty characteristics that increase local stresses. J Arthroplasty. 2006;21:737–743. doi: 10.1016/j.arth.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 19.Barrett W.P. The need for gender-specific prostheses in TKA: does size make a difference? Orthopedics. 2006;29:S53–S55. [PubMed] [Google Scholar]
- 20.Stronach B.M., Pelt C.E., Erickson J., Peters C.L. Patient-specific total knee arthroplasty required frequent surgeon-directed changes. Clin Orthop Relat Res. 2013;471:169–174. doi: 10.1007/s11999-012-2573-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Leeuwen J.A.M.J., Snorrason F., Röhrl S.M. No radiological and clinical advantages with patient-specific positioning guides in total knee replacement. Acta Orthop. 2018;89:89–94. doi: 10.1080/17453674.2017.1393732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huijbregts H.J.T.A.M., Khan R.J.K., Sorensen E., Fick D.P. Patient-specific instrumentation does not improve radiographic alignment or clinical outcomes after total knee arthroplasty. Acta Orthop. 2016;87:386–394. doi: 10.1080/17453674.2016.1193799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ooka N.H.M., Campos A.L.S., da Fonseca V.M., Rodrigues L.E.O., Filho E.B., Franco J.S. Pre-operative templating for knee arthroplasty shows low accuracy with standard X-rays. Int Orthop. 2018;42:1275–1282. doi: 10.1007/s00264-018-3764-7. [DOI] [PubMed] [Google Scholar]
- 24.Aslam N., Lo S., Nagarajah K., Pasapula C., Akmal M. Reliability of preoperative templating in total knee arthroplasty. Acta Orthop Belg. 2004;70:560–564. [PubMed] [Google Scholar]
- 25.Hernández-Vaquero D., Abat F., Sarasquete J., Monllau J.C. Reliability of preoperative measurement with standardized templating in total knee arthroplasty. World J Orthoped. 2013;4:287–290. doi: 10.5312/wjo.v4.i4.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Specht L.M., Levitz S., Iorio R., Healy W.L., Tilzey J.F. A comparison of acetate vs digital templating for total knee arthroplasty. Clin Orthop Relat Res. 2007;464:179–183. [PubMed] [Google Scholar]
- 27.Trickett R.W., Hodgson P., Forster M.C., Robertson A. The reliability and accuracy of digital templating in total knee replacement. J Bone Joint Surg Br. 2009;91:903–906. doi: 10.1302/0301-620X.91B7.21476. [DOI] [PubMed] [Google Scholar]
- 28.Miller A.G., Purtill J.J. Accuracy of digital templating in total knee arthroplasty. Am J Orthop (Belle Mead NJ) 2012;41:510–512. [PubMed] [Google Scholar]
- 29.Levine B., Fabi D., Deirmengian C. Digital templating in primary total hip and knee arthroplasty. Orthopedics. 2010;33:797. doi: 10.3928/01477447-20100924-04. [DOI] [PubMed] [Google Scholar]
- 30.Hsu A.R., Gross C.E., Bhatia S., Levine B.R. Template-directed instrumentation in total knee arthroplasty: cost saving analysis. Orthopedics. 2012;35:e1596–e1600. doi: 10.3928/01477447-20121023-15. [DOI] [PubMed] [Google Scholar]
- 31.Kniesel B., Konstantinidis L., Hirschmuller A., Südkamp N., Helwig P. Digital templating in total knee and hip replacement: an analysis of planning accuracy. Int Orthop. 2014;38:733–739. doi: 10.1007/s00264-013-2157-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsu A.R., Kim J.D., Bhatia S., Levine B.R. Effect of training level on accuracy of digital templating in primary total hip and knee arthroplasty. Orthopedics. 2012;35:179–183. doi: 10.3928/01477447-20120123-15. [DOI] [PubMed] [Google Scholar]
- 33.The B., Diercks R.L., van Ooijen P.M., van Horn J.R. Comparison of analog and digital preoperative planning in total hip and knee arthroplasties: a prospective study of 173 hips and 65 total knees. Acta Orthop. 2005;76:78–84. doi: 10.1080/00016470510030364. [DOI] [PubMed] [Google Scholar]
- 34.McLawhorn A.S., Carroll K.M., Blevins J.L., DeNegre S.T., Mayman D.J., Jerabek S.A. Template-directed instrumentation reduces cost and improves efficiency for total knee arthroplasty: an economic decision analysis and pilot study. J Arthroplasty. 2015;30:1699–1704. doi: 10.1016/j.arth.2015.04.043. [DOI] [PubMed] [Google Scholar]
- 35.Miura M., Hagiwara S., Nakamura J., Wako Y., Kawarai Y., Ohtori S. Inter-observer and intra-observer reliability of CT-based three-dimensional preoperative planning for primary total knee arthroplasty. J Arthroplasty. 2018;33:1572–1578. doi: 10.1016/j.arth.2017.12.035. [DOI] [PubMed] [Google Scholar]
- 36.Sershon R.A., Courtney P.M., Rosenthal B.D., Sporer S.M., Levine B.R. Can demographic variables accurately predict component sizing in primary total knee arthroplasty? J Arthroplasty. 2017;32:3004–3008. doi: 10.1016/j.arth.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 37.Landis J.R., Koch G.G. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 38.Benjamin J. Determining femoral component position using CAS and measured resection. Clin Orthop Relat Res. 2008;466:2745–2750. doi: 10.1007/s11999-008-0426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee J.H., Wang S.I. Risk of anterior femoral notching in navigated total knee arthroplasty. Clin Orthop Surg. 2015;7:217–224. doi: 10.4055/cios.2015.7.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Minoda Y., Watanabe K., Iwaki H., Takahashi S., Fukui M., Nakamura H. Theoretical risk of anterior femoral cortex notching in total knee arthroplasty using a navigation system. J Arthroplasty. 2013;28:1533–1537. doi: 10.1016/j.arth.2013.02.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.