Abstract
Background
Owing to the compressive nature of the neuropathy, patients with carpal tunnel syndrome (CTS) have prolonged distal motor latency (DML), sensory nerve latency (SNL), median nerve swelling and restricted median nerve mobility. The purpose of this study was to noninvasively augment carpal tunnel space using radioulnar wrist compression (RWC) and evaluate its effects on median nerve pathological properties in patients with CTS. It was hypothesized that the RWC intervention would reduce the median nerve DML, SNL and cross-sectional area (CSA) and enhance longitudinal median nerve mobility in patients. with CTS.
Methods
Eleven patients diagnosed with CTS participated in this study. A portable RWC intervention splint was developed to apply 10 N of compressive force across the wrist. Three daily sessions of RWC were performed over 4 weeks of intervention (15 min per session, 45 min per day, 7 days per week). Each 15-min session consisted of three 5-min blocks of RWC, with a 1-min rest in between consecutive blocks. Patients were evaluated at Week 0 (baseline), Week 2 (mid-intervention) and Week 4 (end of intervention). DML and SNL of the median nerve were evaluated using established nerve conduction study techniques. Median nerve CSA at the distal wrist crease was obtained by ultrasound imaging. Median nerve motion associated with finger flexion/extension was captured by dynamic ultrasound imaging and quantified using a speckle cross-correlation algorithm. Finger flexion/extension was recorded using an electrogoniometer. The slope of the regressed linear equation of median nerve displacement as a function of finger flexion angle was used to quantify nerve mobility.
Results
Patients with CTS showed significantly decreased DML (p = 0.048) and median nerve CSA (p < 0.001) and increased nerve mobility (p < 0.001) at mid-intervention compared to baseline. However, DML, CSA and mobility of the median nerve did not differ significantly between Weeks 2 and 4 (p = 0.574, 1.00 and 0.139, respectively). Median nerve SNL was not significantly affected throughout the 4-week intervention (p = 0.330 for Week 0 vs. 2; p = 1.00 for Week 2 vs. 4).
Conclusion
This study revealed that RWC intervention with 10-N force applied to the wrist in the radioulnar direction could restore impaired neurophysiological and biomechanical functions of the median nerve. The beneficial effects of RWC intervention for the median nerve were in evidence after a relatively short period of two weeks. These functional improvements could be explained by intermittent decompression of the median nerve via RWC-induced augmentation of the carpal arch.
The translational potential of this article
Biomechanically manipulating the carpal tunnel by RWC decompresses the median nerve and has the potential to become an alternative treatment for CTS.
Keywords: Carpal tunnel syndrome, Cross-sectional area, Median nerve, Mobility, Nerve conduction, Radioulnar wrist compression
Introduction
Carpal tunnel syndrome (CTS) is the most common peripheral entrapment neuropathy resulting from compression of the median nerve at the wrist, affecting approximately 4% of the US general population [1]. The pathophysiology of CTS is likely multifactorial but thought to be driven mainly by mechanical insult on the median nerve resulting from increased pressure within the carpal tunnel [2].
Electrophysiologically testing median nerve conduction has also been widely used for clinical diagnosis of CTS [3], [4]. When compared to healthy individuals, whose median nerve distal motor latency (DML) and sensory nerve latency (SNL) are generally less than 4.2 ms [5], [6] and 3.4 ms [6], [7], respectively, patients with CTS classically present with prolonged median nerve DML [8] and SNL [9].
Median nerve cross-sectional area (CSA) is commonly evaluated ultrasonographically as a means of diagnosing CTS [10], [11]. Median nerve CSA is considered abnormal when the CSA is > 9 mm2 proximal to the carpal tunnel entry [12], >10.03 mm2 in the proximal carpal tunnel [13] or > 10.5 mm2 at the level of pisiform bone [14]. In patients with CTS, increased median nerve CSA, specifically within the proximal segment of the carpal tunnel, is an indicator of pathological state of nerve swelling [15], [16], [17].
The median nerve is a mobile structure that stretches, compresses and translates in response to upper extremity motion. In healthy hands, the median nerve experiences sufficient excursion in response to hand motions to dissipate mechanical stress [18], [19]. In patients with CTS, mobility of the median nerve is commonly restricted which is indicative of nerve dysfunction [20], [21], [22]. Therapeutically, gliding exercises are used to improve impaired nerve mobility as a conservative intervention for CTS [23], [24].
Symptoms of CTS are commonly managed by conservative and/or surgical means. Conservative interventions for CTS include wrist splinting [25], nonsteroidal antiinflammatory drugs [26] and corticosteroid injection [27]; however, their effectiveness remains uncertain. Surgical treatment of CTS is performed by transecting the transverse carpal ligament. However, surgical release disrupts the anatomical, biomechanical and physiological functions of the carpal tunnel [28] and is associated with complications such as loss of grip strength, postoperative pillar pain [29] and symptom recurrence [30].
Recent studies have demonstrated a method to augment the carpal arch biomechanically as a way to decompress the median nerve [31], [32]. Geometric modelling [31] and in vitro studies [32] have showed that carpal arch width narrowing is associated with palmar bowing of the transverse carpal ligament, leading to increased arch height and area. Radioulnar wrist compression (RWC) increased the carpal arch area and lessened the flattening ratio of the median nerve in vivo [33], [34], suggesting that the median nerve had been decompressed. However, it remains unknown how RWC improves the pathomechanics and pathophysiology of the median nerve in patients with CTS.
The purpose of this study was to evaluate the effects of 4 weeks of daily RWC on median nerve DML, SNL, CSA and longitudinal mobility in patients with CTS. It was hypothesized that RWC intervention would reduce median nerve DML and SNL, decrease median nerve CSA and increase median nerve longitudinal mobility in patients with CTS.
Materials and methods
Human individuals
Eleven patients (56.2 ± 12.3 years; 4 male and 7 female) clinically diagnosed with CTS voluntarily participated in this study, and the more affected hand of each participant was evaluated. Participants were recruited based on a history of paraesthesia, pain and/or numbness in the median-innervated hand territory persisting for at least 3 months; at least one positive physical examination of Tinel's sign, Phalen's test or carpal compression test; a mean CTS severity score greater than 1.5 using the questionnaire [35]; DML larger than 4.2 ms and SNL larger than 3.4 ms [6]. Patients were excluded from participation if they had systemic diseases (i.e. rheumatoid arthritis, diabetes, fibromyalgia), a history of major injury to the hand and wrist, underwent previous surgical intervention, current night splinting treatment, musculoskeletal/neuromuscular disorders or body mass index above 30 or if they were currently pregnant. The study was approved by the institutional review board, and written informed consent was obtained from each participant before participation.
RWC intervention
A portable device was developed to noninvasively apply compressive forces across the wrist in the radioulnar direction and centred at the distal level of the carpal tunnel. Force application was achieved by air pressure through a medical balloon (5.5 × 5.5 cm) attached to the ulnar side of a thermoplastic wrist brace (DJO Global, Vista, CA, USA). Air pressure applied using a sphygmomanometer generated 140 mmHg, or 10 N, compressive force to the wrist [34]. Patients were trained to wear the portable RWC device three times daily over 4 weeks of intervention. Each bracing session included three 5-min wrist compressions followed by a 1-min rest in between.
Outcome measures
Patients were evaluated biweekly over the 4-week period, which included three data collection time points at Weeks 0 (baseline), 2 (mid-intervention) and 4 (end of intervention). At each time point, assessment of intervention outcomes included median nerve conduction (DML and SNL), median nerve CSA and median nerve mobility.
Median nerve conduction
Nerve conduction studies were performed using a NeuroMax 1002 nerve conduction system (XLTEK, Oakville, Ontario, Canada). DML and SNL were examined using a standard technique of supramaximal percutaneous stimulation. Room temperature was maintained at 25°C, and skin temperature, over 32°C. To evaluate DML, the active electrode was placed over the abductor pollicis brevis muscle, and the reference electrode was placed 4 cm above the active electrode, just distal to the metacarpophalangeal joint of the thumb. The median nerve was stimulated supramaximally at the wrist, 7 cm proximal to the active electrode, by two stainless-steel electrodes placed longitudinally over the median nerve. To evaluate SNL, the reference and active ring electrodes were placed at the distal and proximal interphalangeal joints of the index finger, respectively. Stimulator probes were placed over the median nerve 14 cm proximal to the reference electrode. DML and SNL were evaluated during the visits at Weeks 0, 2 and 4.
Median nerve CSA
Participants were instructed to lay with their forearm supinated with the wrist neutrally positioned in a thermoplastic splint shaped according to the individual's arm geometry. An ultrasound imaging system (Acuson S2000; Siemens Medical Solutions USA, Mountain View, CA, USA) with an 18L6HD linear array transducer (Siemens Medical Solutions USA, Mountain View, CA, USA) was used to assess median nerve morphology. The transducer was placed transversely at the level of the distal wrist crease and perpendicular to the long axis of the forearm at the inlet of the carpal tunnel. Ultrasound images with 0.062-mm resolution and 640 × 480 pixels image size were captured by a single investigator (Y.Y.) who was experienced in musculoskeletal ultrasound imaging of the carpal tunnel. The image depth was set as 2.5 cm, and the gain was set as 8 dB. Median nerve CSA was obtained using the ImageJ (US National Institutes of Health, Bethesda, MD, USA) multipoint selection tool to trace the median nerve within its hyperechoic border.
Median nerve mobility
Patients laid supine on a testing bed with the arm abducted 30°, the forearm supinated and the wrist in neutral. The patient's arm was secured within a thermoplastic splint shaped according to his/her arm geometry. The thumb was fixed in 45° extension, and the proximal/distal interphalangeal joints of the four fingers were fixed in an anatomical neutral position using a flat splint at the dorsum of hand. The ultrasound transducer was used to identify the axial imaging plane that clearly contained the hook of hamate and ridge of trapezium, corresponding to the distal boundary of the carpal tunnel. The tuberosities of the pisiform and the scaphoid were identified in the axial imaging plane, corresponding to the proximal boundary of the carpal tunnel. The ultrasound system setup was the same as mentioned previously. A line of radiopaque markers (0.33 mm diameter; Beekley Corporation, Bristol, CT, USA) were attached to the patient's palmar surface to define the proximal and distal boundary of the carpal tunnel under ultrasound imaging. The radiopaque markers were placed 10 mm distal and parallel to the hamate–trapezium line and 10 mm proximal and parallel to the pisiform–scaphoid line to prevent artifacts caused by shadow from the metal markers from entering the ultrasound images.
High-frequency (17 MHz) dynamic ultrasound images captured the longitudinal median nerve within the carpal tunnel as a superficial structure that is hypoechogenic compared to the speckle structure of the tendons during finger flexion/extension. The ultrasound transducer was mounted on the palmar surface after locating the plane in which the median nerve was the thickest. Angular finger motion at the metacarpophalangeal joints was recorded using an electrogoniometer attached to the back of the hand and four fingers and was synchronized to ultrasound image capture. Ultrasound frame rate for acquisition was set as 30 Hz. Cyclic finger flexion/extension was conducted between neutral (0°) and 90° flexion at a pace of 0.3 Hz, synchronized to a metronome for 1 min. The procedure was executed three times with a 3-min rest provided between consecutive trials. Three random finger flexion phases from 0° to 90° were analyzed.
Longitudinal median nerve displacement at each flexion angular position was analyzed using a custom LabVIEW (National Instruments, Austin, TX, USA) program using speckle cross-correlation algorithm [36]. Sequences of images were converted to digital format and analyzed offline frame by frame. The program defined ranges of interest (ROIs) (6.2 × 1.8 mm) at 0.62-mm increments along the midline of the median nerve and tracked each ROI frame by frame corresponding to finger motion. Individual ROIs ranged from the proximal to distal boundary of the carpal tunnel. Median nerve displacement was calculated as the mean value of individual displacements of all ROIs. Least-squares linear regression was performed for median nerve displacement as a function of finger flexion angle. The slope of the linear equation was used to quantify the mobility of the nerve. Static structures such as carpal bones were also tracked using the same methodology to eliminate error from probe or forearm movement relative to the probe during finger flexion. Any movement of bone structures was subtracted from the nerve displacement values to give the best estimation of median nerve displacement.
Statistical analysis
One-way repeated-measures analysis of variance was performed to examine the effect of RWC intervention time (Weeks 0, 2 and 4) on median nerve DML, SNL, CSA and mobility in patients with CTS. Post hoc Tukey's tests were used for pairwise comparisons, and p-values less than 0.05 were considered statistically significant. All statistical analyses were performed using SigmaStat 3.4 (Systat Software Inc., San Jose, CA, USA).
Results
Median nerve conduction
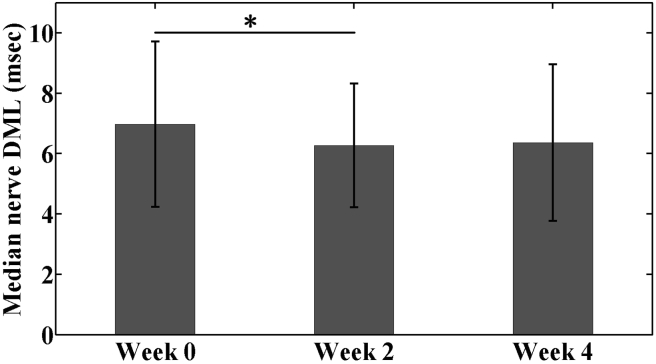
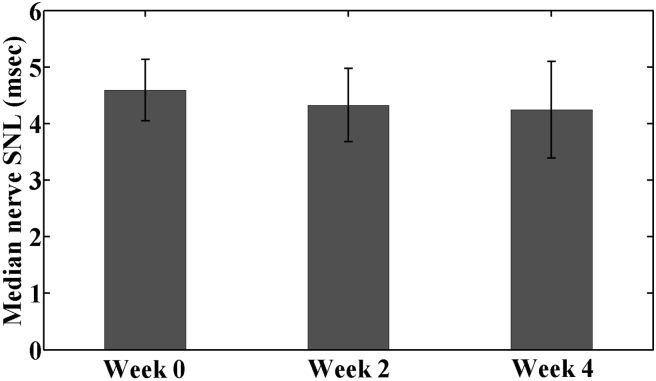
Median nerve conduction outcomes DML and SNL are shown in Figure 1, Figure 2, respectively. The median nerve DML at baseline (Week 0) was 6.98 ± 2.74 msec. After 2 weeks of RWC intervention (Week 2), median nerve DML decreased by 0.71 msec (10.2%) to 6.27 ± 2.05 msec, which was significantly lower than the baseline value (p < 0.05). By Week 4, median nerve DML increased slightly to 6.36 ± 2.59 msec, which was not statistically different from either baseline (p = 0.285) or mid-intervention (p = 0.574). SNL values were 4.59 ± 0.54, 4.33 ± 0.65 and 4.25 ± 0.86 ms at Weeks 0, 2 and 4, respectively; however, there were no significant differences among these values (Week 0 vs. 2, p = 0.330; Week 2 vs. 4, p > 0.99; Week 0 vs. 4, p = 0.469).
Figure 1.
Median nerve DML in the patients with CTS under radioulnar wrist compression intervention from baseline (Week 0) through 4-week treatment (Week 4) (*p < 0.05).
CTS = carpal tunnel syndrome; DML = distal motor latency.
Figure 2.
Median nerve SNL in the patients with CTS under radioulnar wrist compression intervention from baseline (Week 0) through 4-week treatment (Week 4).
CTS = carpal tunnel syndrome; SNL = sensory nerve latency.
Median nerve CSA
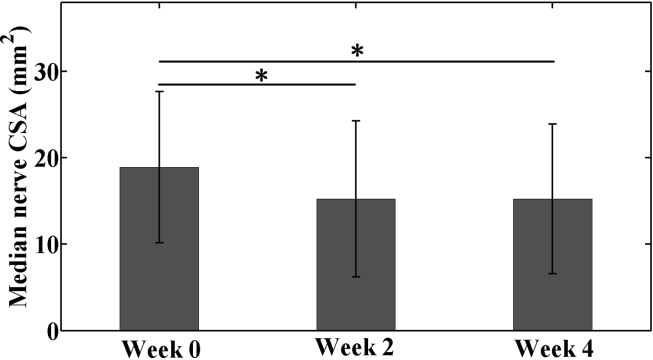
At baseline, median nerve CSA was 18.9 ± 8.7 mm2, which decreased by 19.4% to 15.2 ± 9.0 mm2 after 2 weeks of RWC intervention (p < 0.001, Fig. 3). Median nerve CSA at Week 4 was 15.3 ± 8.7 mm2, not significantly different from that at Week 2 (p > 0.99) but still significantly less than that at Week 0 (p < 0.001).
Figure 3.
Median nerve CSA in the patients with CTS under radioulnar wrist compression intervention from baseline (Week 0) through 4-week treatment (Week 4) (*p < 0.05).
CSA = cross-sectional area; CTS = carpal tunnel syndrome.
Median nerve mobility
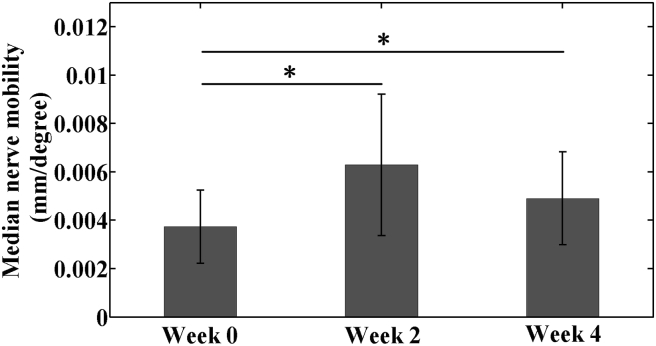
The RWC intervention significantly affected median nerve mobility (p < 0.01, Fig. 4). Mobility at Week 2 (0.0063 ± 0.0029 mm/degree) was 1.73 times that of Week 0 (0.0037 ± 0.0015 mm/degree, p < 0.001). At Week 4, mobility (0.0049 ± 0.0019 mm/degree) had significantly improved compared with baseline (p < 0.05); however, between Weeks 2 and 4, median nerve mobility did not differ significantly (p = 0.139).
Figure 4.
Median nerve longitudinal mobility in the patients with CTS under radioulnar wrist compression intervention from baseline (Week 0) through 4-week treatment (Week 4) (*p < 0.05).
CTS = carpal tunnel syndrome.
Discussion
The present study investigated the effects of RWC intervention on median nerve pathomechanical and pathophysiological parameters in patients with CTS over a period of 4 weeks. RWC intervention was applied in a relatively low dosage of 10-N force application thrice daily, totalling 45 min. We found that the median nerve responded positively during the first 2 weeks, demonstrating decreased DML and CSA and increased nerve mobility, although SNL was somewhat refractory to the intervention. The improvements were maintained over the subsequent 2 weeks of intervention, although additional gains were not obvious.
The median nerve conduction studies indicated that DML reduced by 0.71 msec after 2 weeks of daily RWC, a 10.2% decrease from the initial time point (baseline 6.98 msec). Further improvements were not observed by Week 4, but rather beneficial effects of the intervention were sustained. Our biomechanical treatment method improved patients’ DML in just 2 weeks, compared favourably to conservative splinting treatment over 6 weeks of splinting (0.71 msec vs 0.13 msec) [37]. Postoperatively, median nerve conduction studies have shown delayed recovery of sensory and motor nerve conduction, taking as long as 6–8 weeks [38]. Another study showed only slight improvement of median nerve DML during the 3-month follow-up period after carpal tunnel release surgery, and DML was still abnormal 3 months after operation [39]. In the present study, noticeable median nerve DML improvement after 2 weeks of RWC intervention is in line with previous studies investigating carpal tunnel release (6.27 msec vs about 6 msec) [39]. In our study, we found that median nerve SNL was not more refractory to RWC intervention within the 4-week period. Nerve motor fibres are less susceptible to pressure than sensory fibres [40]; thus, sensory qualities are the first affected in nerve entrapment disorders, resulting in noticeable symptoms of pain and paraesthesia by patients. It is possible that sensory fibres might also recover more slowly than motor fibres after median nerve decompression. Although DML improvement did not return to normal and no noticeable improvement of SNL was detected after RWC, a longer intervention period and a higher dosage might help gain greater benefit to improve nerve sensory and motor conduction.
Increased median nerve CSA in patients with CTS is commonly considered to be a result of oedema and fibrous tissue proliferation [41]. In the present study, morphological analysis of the median nerve over a period of 2 weeks of RWC intervention showed median nerve CSA reduced by 3.7 mm2, a 19.4% decrease from baseline (18.9 mm2). An additional 2 weeks of intervention did not reduce median nerve CSA further; however, the benefits of wrist compression on median nerve CSA were maintained relative to the baseline value. When compared to surgical intervention, RWC reduced median nerve swelling at the distal wrist crease within a shorter treatment period. One study showed that median nerve CSA reduced by 3.8 mm2 from 16.2 mm2 12 weeks after carpal tunnel release [42]. Another study showed median nerve CSA decreased by only 1.7 mm2 from 15.5 mm2 one month after release surgery, a result which was maintained during the following 5 months [43].
In patients with CTS, constraints on the median nerve from elevated carpal tunnel pressure (Lluch, 1992) or endoneurial oedema and perineurium fibrosis [44] might be factors that compromise the kinetic behaviour of the median nerve. In physiological conditions, 10 N of radioulnar compression decreased arch width by nearly 1.0 mm and increased arch height and area by approximately 0.5 and 5.5 mm2, respectively [33]. For patients with CTS, Marquardt et al [34] showed that compressive forces of 10 N applied across the wrist of patients with CTS increased arch area by 13% and reduced median nerve flattening (a measure which reflects the degree of nerve compression). In our study, 10-N RWC applied for 2 weeks enhanced median nerve longitudinal mobility, an outcome likely attributable to median nerve decompression from carpal tunnel area augmentation and pressure decrease.
Parameters strongly associated with the CTS pathophysiology, including median nerve conduction, CSA and longitudinal mobility, were evaluated in this study to determine the cumulative, long-term effects of RWC intervention. Patients enrolled in our study performed three RWC sessions each day for 4 weeks, totalling 45 min of daily wrist compression. After 2 weeks, median nerve DML, CSA and mobility all improved. These positive results might be due to intermittent decompression of the median nerve by increased carpal arch area. Between the second and fourth weeks of RWC intervention, the initial decreases observed in median nerve DML and CSA and initial increase in nerve mobility remained much the same, indicative of a sustained effect of prolonged treatment. Over time, the carpal tunnel structure might adapt to wrist compression and thus spare less space in the carpal tunnel compared to the first 2-week RWC intervention using the same force level of RWC.
The present study is an initial attempt to examine clinically relevant outcome measures of the median nerve in response to our novel RWC intervention. The study is limited in a small sample size with a relatively short period of intervention. Future studies can be designed to investigate intervention protocols with increased daily dosage or lengthened intervention periods beyond 4 weeks to allow for determination of an optimal treatment strategy. Large-scale clinical trials can also further evaluate the efficacy of RWC intervention for patients with CTS with different degrees of symptom and function severity.
In conclusion, our findings indicate that RWC intervention has demonstrated benefits for median nerve recovery and is a promising strategy to improve median nerve swelling and distal motor latency and to restore the nerve's natural kinematic behaviour in patients suffering with CTS. Although larger and longer term clinical studies are needed to prove the clinical efficacy of RWC intervention as a treatment option for CTS, this study highlights the potential of RWC intervention as a new noninvasive biomechanical strategy for CTS management.
Conflicts of interest
The authors have no conflicts of interest to disclose in relation to this article.
Acknowledgement
This study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R01AR068278. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jot.2019.01.002.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Atroshi I., Gummesson C., Johnsson R., Ornstein E., Ranstam J., Rosén I. Prevalence of carpal tunnel syndrome in a general population. J Am Med Assoc. 1999;282(2):153–158. doi: 10.1001/jama.282.2.153. [DOI] [PubMed] [Google Scholar]
- 2.Lluch A.L. Thickening of the synovium of the digital flexor tendons: cause or consequence of the carpal tunnel syndrome? J Hand Surg. 1992;17(2):209–211. doi: 10.1016/0266-7681(92)90091-f. [DOI] [PubMed] [Google Scholar]
- 3.Jablecki C.K., Andary C.M.T., So Y.T., Wilkins D.E., Williams F.H. Literature review of the usefulness of nerve conduction studies and electromyography for the evaluation of patients with carpal tunnel syndrome. Muscle Nerve. 1993;16(12):1392–1414. doi: 10.1002/mus.880161220. [DOI] [PubMed] [Google Scholar]
- 4.De Krom M.C.T.F.M., Knipschild P.G., Spaans F., Kester A.D.M. Efficacy of provocative tests for diagnosis of carpal tunnel syndrome. Lancet. 1990;335(8686):393–395. doi: 10.1016/0140-6736(90)90218-t. [DOI] [PubMed] [Google Scholar]
- 5.De Lean J. Transcarpal median sensory conduction: detection of latent abnormalities in mild carpal tunnel syndrome. Can J Neurol Sci. 1988;15(4):388–393. [PubMed] [Google Scholar]
- 6.Jackson D.A., Clifford J.C. Electrodiagnosis of mild carpal tunnel syndrome. Arch Phys Med Rehabil. 1989;70(3):199–204. [PubMed] [Google Scholar]
- 7.Kimura J. The carpal tunnel syndrome: localization of conduction abnormalities within the distal segment of the median nerve. Brain. 1979;102(3):619–635. doi: 10.1093/brain/102.3.619. [DOI] [PubMed] [Google Scholar]
- 8.Simpson J.A. Electrical signs in the diagnosis of carpal tunnel and related syndromes. J Neurol Neurosurg Psychiatry. 1956;19(4):275. doi: 10.1136/jnnp.19.4.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilliatt R.W., Sears T.A. Sensory nerve action potentials in patients with peripheral nerve lesions. J Neurol Neurosurg Psychiatry. 1958;21(2):109. doi: 10.1136/jnnp.21.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakamichi K.I., Tachibana S. Ultrasonographic measurement of median nerve cross-sectional area in idiopathic carpal tunnel syndrome: diagnostic accuracy. Muscle Nerve. 2002;26(6):798–803. doi: 10.1002/mus.10276. [DOI] [PubMed] [Google Scholar]
- 11.Kutlar N., Bayrak A.O., Bayrak İ.K., Canbaz S., Türker H. Diagnosing carpal tunnel syndrome with Doppler ultrasonography: a comparison of ultrasonographic measurements and electrophysiological severity. Neurol Res. 2017;39(2):126–132. doi: 10.1080/01616412.2016.1275455. [DOI] [PubMed] [Google Scholar]
- 12.Wong S.M., Griffith J.F., Hui A.C., Lo S.K., Fu M., Wong K.S. Carpal tunnel syndrome: diagnostic usefulness of sonography. Radiology. 2004;232(1):93–99. doi: 10.1148/radiol.2321030071. [DOI] [PubMed] [Google Scholar]
- 13.El Miedany Y.M., Aty S.A., Ashour S. Ultrasonography versus nerve conduction study in patients with carpal tunnel syndrome: substantive or complementary tests? Rheumatology. 2004;43(7):887–895. doi: 10.1093/rheumatology/keh190. [DOI] [PubMed] [Google Scholar]
- 14.Yesildag A., Kutluhan S., Sengul N., Koyuncuoglu H.R., Oyar O., Guler K. The role of ultrasonographic measurements of the median nerve in the diagnosis of carpal tunnel syndrome. Clin Radiol. 2004;59(10):910–915. doi: 10.1016/j.crad.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 15.Buchberger W., Judmaier W., Birbamer G., Lener M., Schmidauer C. Carpal tunnel syndrome: diagnosis with high-resolution sonography. Am J Roentgenol. 1992;159(4):793–798. doi: 10.2214/ajr.159.4.1529845. [DOI] [PubMed] [Google Scholar]
- 16.Moran L., Perez M., Esteban A., Bellon J., Arranz B., del Cerro M. Sonographic measurement of cross-sectional area of the median nerve in the diagnosis of carpal tunnel syndrome: correlation with nerve conduction studies. J Clin Ultrasound. 2009;37(3):125–131. doi: 10.1002/jcu.20551. [DOI] [PubMed] [Google Scholar]
- 17.Mhoon J.T., Juel V.C., Hobson-Webb L.D. Median nerve ultrasound as a screening tool in carpal tunnel syndrome: correlation of cross-sectional area measures with electrodiagnostic abnormality. Muscle Nerve. 2012;46(6):861–870. doi: 10.1002/mus.23426. [DOI] [PubMed] [Google Scholar]
- 18.Topp K.S., Boyd B.S. Structure and biomechanics of peripheral nerves: nerve responses to physical stresses and implications for physical therapist practice. Phys Ther. 2006;86(1):92–109. doi: 10.1093/ptj/86.1.92. [DOI] [PubMed] [Google Scholar]
- 19.Phillips J.B., Smit X., Zoysa N.D., Afoke A., Brown R.A. Peripheral nerves in the rat exhibit localized heterogeneity of tensile properties during limb movement. J Physiol. 2004;557(3):879–887. doi: 10.1113/jphysiol.2004.061804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liong K., Lahiri A., Lee S., Chia D., Biswas A., Lee H.P. Predominant patterns of median nerve displacement and deformation during individual finger motion in early carpal tunnel syndrome. Ultrasound Med Biol. 2014;40(8):1810–1818. doi: 10.1016/j.ultrasmedbio.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 21.Filius A., Scheltens M., Bosch H.G., van Doorn P.A., Stam H.J., Hovius S.E. Multidimensional ultrasound imaging of the wrist: changes of shape and displacement of the median nerve and tendons in carpal tunnel syndrome. J Orthop Res. 2015;33(9):1332–1340. doi: 10.1002/jor.22909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hough A.D., Moore A.P., Jones M.P. Reduced longitudinal excursion of the median nerve in carpal tunnel syndrome. Arch Phys Med Rehabil. 2007;88(5):569–576. doi: 10.1016/j.apmr.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 23.Akalin E., El Ö., Peker Ö., Senocak Ö., Tamci S., Gülbahar S. Treatment of carpal tunnel syndrome with nerve and tendon gliding exercises. Am J Phys Med Rehabil. 2002;81(2):108–113. doi: 10.1097/00002060-200202000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Coppieters M.W., Alshami A.M. Longitudinal excursion and strain in the median nerve during novel nerve gliding exercises for carpal tunnel syndrome. J Orthop Res. 2007;25(7):972–980. doi: 10.1002/jor.20310. [DOI] [PubMed] [Google Scholar]
- 25.O'Connor D., Marshall S., Massy-Westropp N. Non-surgical treatment (other than steroid injection) for carpal tunnel syndrome. Cochrane Database Syst Rev. 2003;1 doi: 10.1002/14651858.CD003219. CD003219-CD003219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang M.H., Chiang H.T., Lee S.J., Ger L.P., Lo Y.K. Oral drug of choice in carpal tunnel syndrome. Neurol. 1998;51(2):390–393. doi: 10.1212/wnl.51.2.390. [DOI] [PubMed] [Google Scholar]
- 27.Marshall S., Tardif G., Ashworth N. Local corticosteroid injection for carpal tunnel syndrome. Cochrane Database Syst Rev. 2007;2:CD001554. doi: 10.1002/14651858.CD001554.pub2. [DOI] [PubMed] [Google Scholar]
- 28.Piazzini D.B., Aprile I., Ferrara P.E., Bertolini C.A., Tonali P., Maggi L.O. A systematic review of conservative treatment of carpal tunnel syndrome. Clin Rehabil. 2007;21(4):299–314. doi: 10.1177/0269215507077294. [DOI] [PubMed] [Google Scholar]
- 29.Brooks J.J., Schiller J.R., Allen S.D., Akelman E. Biomechanical and anatomical consequences of carpal tunnel release. Clin Biomech. 2003;18(8):685–693. doi: 10.1016/s0268-0033(03)00052-4. [DOI] [PubMed] [Google Scholar]
- 30.Steyers C.M. Recurrent carpal tunnel syndrome. Hand Clin. 2002;18(2):339–345. doi: 10.1016/s0749-0712(01)00005-1. [DOI] [PubMed] [Google Scholar]
- 31.Li Z.M., Tang J., Chakan M., Kaz R. Carpal tunnel expansion by palmarly directed forces to the transverse carpal ligament. J Biomech Eng. 2009;131 doi: 10.1115/1.3148469. 081011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Z.M., Gabra J.N., Marquardt T.L., Kim D.H. Narrowing carpal arch width to increase cross-sectional area of carpal tunnel—a cadaveric study. Clin Biomech. 2013;28:402–407. doi: 10.1016/j.clinbiomech.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marquardt T.L., Gabra J.N., Li Z.M. Morphological and positional changes of the carpal arch and median nerve during wrist compression. Clin Biomech. 2015;30(3):248–253. doi: 10.1016/j.clinbiomech.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marquardt T.L., Evans P.J., Seitz W.H., Li Z.M. Carpal arch and median nerve changes during radioulnar wrist compression in carpal tunnel syndrome patients. J Orthop Res. 2016;34(7):1234–1240. doi: 10.1002/jor.23126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levine D.W., Simmons B.P., Koris M.J., Daltroy L.H., Hohl G.G., Fossel A.H. A self-administered questionnaire for the assessment of severity of symptoms and functional status in carpal tunnel syndrome. JBJS. 1993;75(11):1585–1592. doi: 10.2106/00004623-199311000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Dilley A., Greening J., Lynn B., Leary R., Morris V. The use of cross-correlation analysis between high-frequency ultrasound images to measure longitudinal median nerve movement. Ultrasound Med Biol. 2001;27(9):1211–1218. doi: 10.1016/s0301-5629(01)00413-6. [DOI] [PubMed] [Google Scholar]
- 37.Walker W.C., Metzler M., Cifu DX Swartz Z. Neutral wrist splinting in carpal tunnel syndrome: a comparison of night-only versus full-time wear instructions. Arch Phys Med Rehabil. 2000;81(4):424–429. doi: 10.1053/mr.2000.3856. [DOI] [PubMed] [Google Scholar]
- 38.Schlagenhauff R.E., Glasauer F.E. Pre-and postoperative electromyographic evaluations in the carpal tunnel syndrome. J Neurosurg. 1971;35(3):314–319. doi: 10.3171/jns.1971.35.3.0314. [DOI] [PubMed] [Google Scholar]
- 39.El-Karabaty H., Hetzel A., Galla T.J., Horch R.E., Lücking C.H., Glocker F.X. The effect of carpal tunnel release on median nerve flattening and nerve conduction. Electromyogr Clin Neurophysiol. 2005;45(4):223–227. [PubMed] [Google Scholar]
- 40.Lundborg G., Gelberman R.H., Minteer-Convery M., Lee Y.F., Hargens A.R. Median nerve compression in the carpal tunnel—functional response to experimentally induced controlled pressure. J Hand Surg. 1982;7(3):252–259. doi: 10.1016/s0363-5023(82)80175-5. [DOI] [PubMed] [Google Scholar]
- 41.Vogelin E., Nuesch E., Juni P., Reichenbach S., Eser P., Ziswiler H.R. Sonographic follow-up of patients with carpal tunnel syndrome undergoing surgical or nonsurgical treatment: prospective cohort study. J Hand Surg Am. 2010;35(9):1401–1409. doi: 10.1016/j.jhsa.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 42.Kim J.K., Koh Y.D., Kim J.O., Choi S.W. Changes in clinical symptoms, functions, and the median nerve cross-sectional area at the carpal tunnel inlet after open carpal tunnel release. Clin Orthop Surg. 2016;8(3):298–302. doi: 10.4055/cios.2016.8.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mondelli M., Filippou G., Aretini A., Frediani B., Reale F. Ultrasonography before and after surgery in carpal tunnel syndrome and relationship with clinical and electrophysiological findings. A new outcome predictor? Scand J Rheumatol. 2008;37(3):219–224. doi: 10.1080/03009740801914850. [DOI] [PubMed] [Google Scholar]
- 44.Mackinnon S.E., Dellon A.L., Hudson A.R., Hunter D.A. A primate model for chronic nerve compression. J Reconstr Microsurg. 1985;1(03):185–194. doi: 10.1055/s-2007-1007073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.