Abstract
Background
Osteoporosis parallels aging and functional mechanical unloading (e.g., space flight and bed rest), jeopardizing mineral density, microstructure, and integrity of bone and leading to an increased risk of fracture. A way to combat this deterioration is to harness the sensitivity of bone to mechanical signals.
Objective
This study evaluates the longitudinal effect of a dynamic mechanical loading through the heel on human bone in vivo during 90-day bed rest, monitored by quantitative ultrasound (QUS) imaging and dual-energy X-ray absorptiometry (DXA) in localized regions of interests, i.e., calcaneus.
Methods
A total of 29 bed rest individuals were evaluated (11 control and 18 treatment) with a brief (10-minute) daily low-intensity (0.3g), high-frequency (30Hz) dynamic mechanical stimulation countermeasure through vibrational inhibition bone erosion (VIBE). Both QUS and DXA detected longitudinal bone density and quality changes.
Results
Ultrasound velocity (UV) decreased in the control group and increased in the group treated with low-intensity loading. The UV increased by 1.9% and 1.6% at 60- and 90-day bed rest (p=0.01) in VIBE over control groups. A trend was found in broadband ultrasound attenuation (BUA), with a VIBE benefit of 1.8% at day 60 and 0.5% at day 90 in comparison with control (p=0.5). Bone mineral density (BMD) assessed by DXA decreased -4.50% for control individuals and -2.18% for VIBE individuals, showing a moderate effect of the mechanical intervention (p=0.19). Significant correlations between QUS and DXA were observed, with a combined BUA and UV vs. BMD: r2=0.70.
Conclusion
These results indicated that low-intensity, high-frequency loading has the potential to mitigate regional bone loss induced by long-term bed rest and that QUS imaging may be able to assess the subtle changes in bone alteration.
Translational potential of this article
Quantitative ultrasound has shown the efficacy of noninvasively assessing bone mass and structural properties in cadaver and isolated trabecular bone samples. While its ability in measuring in vivo bone quality and density is still unclear, a scanning confocal ultrasound imaging is developed and can perform an instant assessment for the subtle changes of such bone loss. This ultrasound imaging modality can potentially be used in the clinical assessment of bone mass. Moreover, physical stimulation has shown the ability to prevent bone loss induced by functional disuse and estrogen deficiency in animal models. However, its treatment capability is unclear. This study has shown that low-magnitude mechanical signals, introduced using low-intensity vibration (LIV), can mitigate regional bone loss caused by functional disuse. Thus localized mechanical treatment, and the quantitative ultrasound imaging have shown translational potential to noninvasively attenuate bone loss, and assess bone mass in the clinic, e.g., in an extreme condition such as long-term space mission, and long-term bedrest such as in case of spinal cord injury.
Keywords: Bed rest, Bone remodelling, Confocal acoustic navigation, Osteoporosis and osteopenia, Ultrasound imaging, Whole-body vibration
Introduction
Musculoskeletal deterioration and its associated complications, i.e., disuse osteopenia and muscle atrophy, are significant threats for astronauts and increase the risk of fractures during a long-term space mission, such as staying in the space station, and during the trip to Mars. Accumulated data have demonstrated that space flight, particularly in long-term missions, has detrimental effects on the musculoskeletal system. Results from short-term space mission (2–12 weeks) indicated that space flight with microgravity alters calcium metabolism and bone mineral density (BMD) in several hundreds of men and women who have flown in space. With plans for extraorbital human space exploration, such as human being flight to Mars through the extended manned vehicle with 18–36 months of duration, the risk and challenge to the musculoskeletal system will be tremendous, and so little progress has been made in understanding the significance of the problem. There is an almost complete lack of onboard measurements for assessing longitudinal musculoskeletal loss and associated evaluation of countermeasure outcomes.
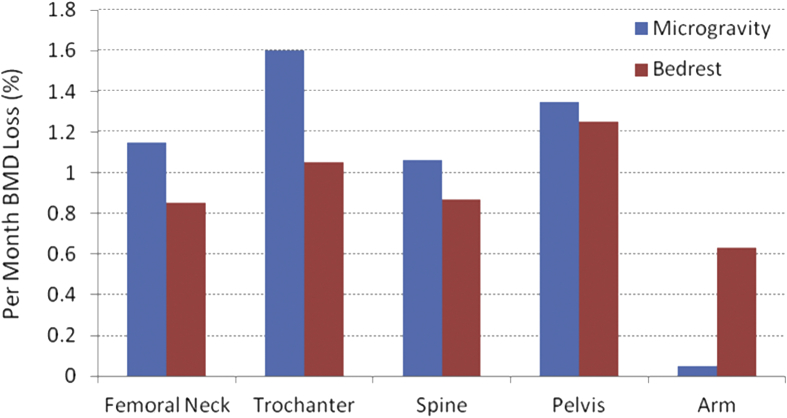
Osteoporosis and osteopenia are reductions in bone mass or density that lead to deteriorated and fragile bones, with diminishing of both the structure and strength of bone, each critical in defining the ability of the bone to resist fracture [66]. On average, the magnitude and rate of the loss is staggering; astronauts lose bone mineral in the lower appendicular skeleton at a rate approaching 2% per month [39], [40], [41], [43], [72]. Although osteopenia can affect the whole body, complications often occur predominantly at specific sites of the skeleton with great load-bearing demands. The greatest BMD losses have been observed in the skeleton of the lower body, i.e., in pelvic bones (−11.99 ± 1.22%) and in the femoral neck (−8.17 ± 1.24%), whereas there was no apparent decay found in the skull region [39], [41], [72] (Figure 1). Moreover, it is apparent that the full recovery of bone mass may never occur [17], [18], [37], [38], [65], [75], [78], potentiating skeletal complications later in the astronaut's life [37]. Similar results were found in bed rest studies [1]. In a −6-degree head-down tilt 7-day bed rest model for microgravity, it was observed that there was a decreased bone formation rate in the iliac crest [2] (Figure 1). Bone loss and the resulting decrease in bone strength is a serious health threat and a principal physiological hurdle to man's extended presence in space. Assuming a 2.5-year return trip to Mars, half of an astronaut's bone density may vanish, severely jeopardizing his/her health and well-being. The progressive adaptation of the human biological system for short- and long-term space flight remains largely unknown, i.e., current exercise countermeasure protocols cannot sufficiently prevent bone loss [19]. Part of the reason is extremely difficult to monitor continuous adaptive decay of bone loss during the space flight.
Figure 1.
Short-term (<6 months) space mission and bed rest induced the loss of bone mineral density, averaged per month loss. Data adapted from reference LeBlanc et al., 2000, 2007 [40], [43]. BMD = bone mineral density.
Bone tissue rapidly accommodates changes in its functional environment to ensure that sufficient skeletal mass is appropriately placed to withstand the regions of functional activity, an attribute described as Wolff's law [82], [83]. This adaptive capability of musculoskeletal tissues suggests that biophysical stimuli may be able to provide a site-specific, exogenous treatment for controlling bone mass, and the effect of a mechanical influence on bone morphology has become a basic tenet of bone physiology [48], [77]. Absence of functional loading results in the loss of bone mass [20], [62], [70], [79], and exercise or increased activity results in increased bone mass [25], [31]. Similarly, increasing exercise of musculoskeletal tissues can significantly increase blood flow, oxygen and exchange fluid in muscle [32], [59], [61], [63]. Less-intensive, high-frequency mechanical vibration has demonstrated to be effective in functional disuse animal models and early-stage osteoporosis, as well as osteopenia, which is related to specific mechanical parameters, e.g., strain, cycle number, shear strain and strain energy [7], [29], [44], [45], [53], [63], [71]. In a 60-day human bed rest study, it was demonstrated that resistive mechanical vibration stimuli could retard bone loss in the weight-bearing skeleton [14], [73], [80]. It has been shown that low-magnitude (e.g., 0.2–0.3 g), high-frequency mechanical loading has anabolic effects in human skeleton and maintaining bone mass and quality (including bone information of density and structural and mechanical strength parameters) [15], [44], [50], [53], [71], [81]. Mechanical loading–induced bone loss recovery not only showed efficacy using low-intensity of stimulation but also demonstrated significance in higher intensity of exercise. In a 56-day bed rest study, resistive vibration exercises have shown to be effective in preventing bone loss and muscle atrophy [4], [5], [67]. These results suggest that the mechanical loading as the countermeasure applied in the skeleton is effective to prevent bone losses from the tibia. This underlines the importance of mechanical usage for the maintenance of the human skeleton.
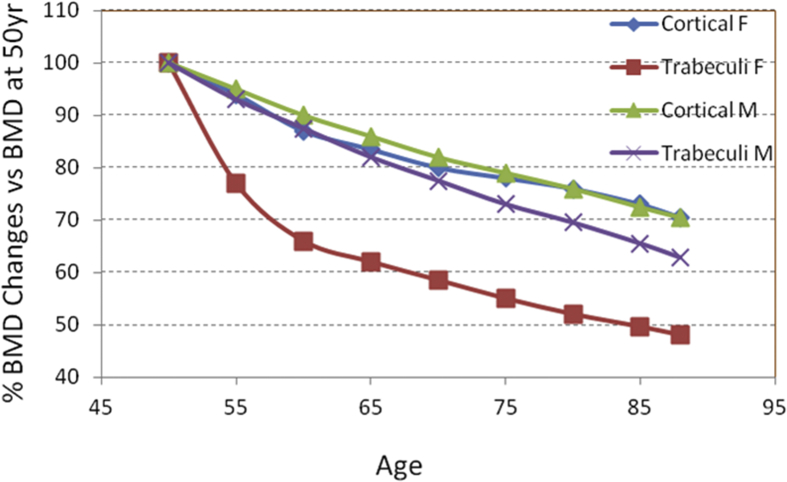
Osteoporotic bone loss can be measured through commonly used clinical modality, i.e., using areal bone mineral density (aBMD) assessed by dual-energy X-ray absorptiometry (DXA), e.g., estimating bone loss with ageing, based on which longitudinal studies with multiple population over 25 years have revealed that losses of BMD are sensitive to age in both women and men (Figure 2). DXA is currently a standard technique used because of its relative precision (∼2%) and whole-body/multisite imaging ability (spine, hip, wrist and total skeleton), which provides BMD based on the percentage of X-ray energy passing through bone and other tissue mass and detected radiation attenuation [9]. The measurements obtained are an averaged mineral density of the whole bone tissue and do not provide information about the integrity of the trabecular architecture or about the mechanical properties of the composing trabeculae. Thus, the density, structure and strength of bone, whether in normal or osteopenic states, remain largely unknown (i.e., it is extremely difficult to monitor the strength and ductility in vivo). An improved diagnostic tool is needed to evaluate both the quantity and quality of bone, which will help in early detection and therefore the possible prevention and treatment of this disease.
Figure 2.
Age-related BMD loss in both cortical and trabecular bones in women and men. Longitudinal data were assessed using DXA. Data are adapted from Khosla A, and Riggs BL, 2005 [28]. BMD = bone mineral density; DXA = dual-energy X-ray absorptiometry.
Quantitative ultrasound (QUS) with improved imaging methodology has been introduced [13], [35] to evaluate bone status for osteoporosis in a manner that is nonionizing, easy to use, repeatable and noninvasive [8], [12], [16]. Ample data are suggesting that QUS can provide both bone density and structure information [11], [51], [52], [54], [55], [58] and predict fracture risk [3], [21], [24], [27]. A recent development by this laboratory has shown that QUS scanning can predict both structural and strength properties of trabecular bone and estimate the alteration of trabecular orientation index [46], [47], [60]. Introduction of QUS in space flight may provide for an understanding of the adaptive decay of bone mass and bone strength during space flight without introducing radiation and, with prompt treatment, will reduce fracture risk during missions and upon return to normal gravity [10], [13], [60], [64], [76].
The objective of this study was to evaluate the hypothesis that confocal scanning QUS can reveal the progressive bone loss and predict bone density and structure in disuse osteopenia through a long duration bed rest study (90-day) that simulated microgravity. The aims include (1) to assess bone status at heel region using QUS scanning and to compare it to X-ray image and (2) to evaluate the effect of low-intensity mechanical vibration to mitigate disuse bone loss. The capability of QUS measurement of bone quantity changes during the bed rest period was evaluated by correlating QUS parameter change to DXA-measured BMD, the current standard for aBMD measure. These measurements will provide insight into the utilization of QUS for monitoring the bone quantity and quality change altered by functional disuse. Human bed rest is an established ground-based model to simulate microgravity and evaluate preventive measures for the detrimental effects of space flight [43], [57]. Both control and loading groups were evaluated, in which the mechanically stimulated group was subject to a low-intensity whole-body vibration [vibrational inhibition of bone erosion (VIBE)] [26], [53], [68], [69], [71], [81].
Methods
Participants and measurement schedule
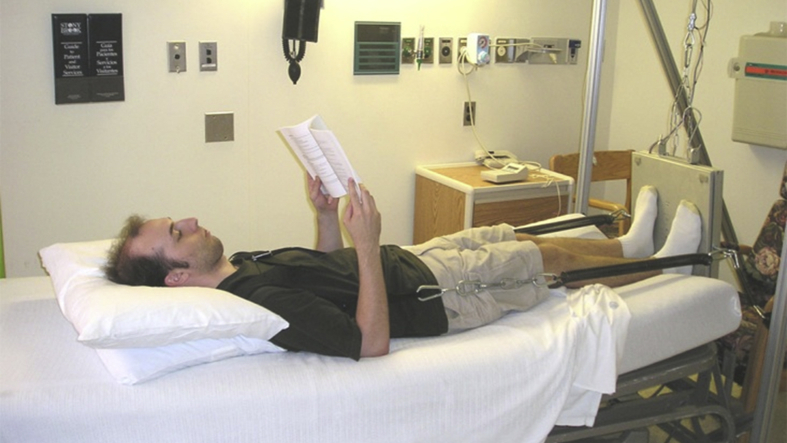
This study was approved by both the Stony Brook University Institutional Review Board and the University of Texas Medical Branch at Galveston Institutional Review Board. While this is part of NASA's bed rest project conducted at the University of Texas Medical Branch at Galveston, the participants who meet the general health requirements (nonosteopenic, nondrug, nonsmoking, including both genders) were recruited through the recruitment team and screening, e.g., public advertisement. Informed written consent was obtained from all volunteers. A total of 29 volunteers (aged 25–48 years, 17 male and 12 female) participated in the bed rest study, which includes 10–15 days normal activity before bed rest, 90-day bed rest and 14-day recovery period. The volunteers were recruited in an on-going pattern and rotated through bed rest unit by keeping approximately 5 volunteers in the bed rest unit at the same time. Participants were screened to have normal bone mineral density, normal physical activity level and no diseases or medication that affects bone metabolism. Female participants included in this study were screened to check for normal menstrual cycles. Participants were randomly assigned into two groups, 11 individuals in the control group and 18 individuals in the low-intensity vibration countermeasure–treated experimental group. Both the groups were subjected to functional disuse with a 6-degree head-down tilt for 90 days during which no physical exercise was allowed (Figure 3). Stretching twice each day and massage therapy every other day during bed rest were provided to the participants, who were monitored 24 h/day by cameras. Daily vital signs including blood pressure, heart rate, body temperature, respiratory rate and body weight, as well as fluid intake and output, were monitored during the study. Standard diet was provided based on NASA space flight nutritional requirements, including caloric intake of 35.7 kcal/kg body weight, fluid intake of 28.5 mL/kg body weight and carbohydrate:fat:protein ratio 55:30:15. All food had to be consumed. Caloric intake was adjusted to maintain weight within 5% of the baseline.
Figure 3.
Daily mechanical loading was applied to the bed rest individuals with a 6-degree head-down tilt for 90 days during which no physical exercise was allowed. The low-magnitude, high-frequency stimulation was applied to the individuals while in a supine position using a vest and a bungee spring system, which loaded the individual horizontally to the vibration platform on mobile support positioned at the foot of the bed. The mechanical vibration was set at 0.3 g, 30 Hz and 10 min per day.
The VIBE group was given a daily low-intensity vibration (LIV) treatment (10 min/day, 30 Hz, 0.3 g) during the bed rest period. LIV was applied to the individuals while in a supine position using a vest and spring system, which loaded the individual to the vibration platform on mobile support positioned at the foot at the bed (Figure 3). Spring loading was adjusted to match 60% of the participants’ body weight. This loading achieved a vibration magnitude at the hip which measured 80% of the magnitude seen in an individual standing on the plate.
QUS measurements were performed using the scanning confocal acoustic diagnostic navigation (SCAN) system developed in our laboratory. Measurements were scheduled at three time points: before bed rest, 60 days into bed rest and 90 days into bed rest. DXA measurements were performed using the same schedule. The SCAN system measured bone mass changes at the calcaneus on both control and experimental groups, which were compared with changes in bone mass as measured by DXA at heel, spine and hip.
QUS measurement
QUS measurement was performed on individuals’ right foot using the SCAN system while they still maintained the 6-degree tilt. The technical aspect of the QUS parameter calculation has been published before [84]. The device measures the overall ultrasound attenuation (ATT; dB), the broadband ultrasound attenuation (BUA; dB/MHz) and the ultrasound velocity (UV; m/s) to generate ATT, BUA and UV images. Measurements were performed over an area measuring 38 mm × 38 mm, at 0.5 mm resolution.
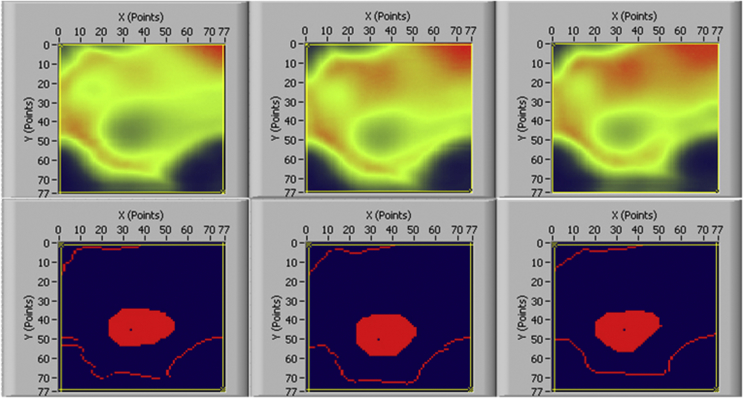
An automatic irregular region of interest (ROI) selection method was used in this study for QUS measurement (Figure 7). The technical detail of this method was published in a separate study [85]. The rationale of using the automatic irregular ROI selection is based on our previous study [85] and the study by Damilakis et al. [12], which showed that automatic irregular ROI selection can adapt to the anatomy of the calcaneus and has better clinical performance than regular shaped ROI as used in clinical devices. Also, the irregular ROI was automatically selected by the computer, eliminating the possible human error. The same ROI can be selected and applied to UV and BUA images generated at different time points to calculate UV and BUA values for each individual. Figure 4 showed the ROI selected from before bed rest, at Day 60 and Day 90 of QUS measurement using this method for a bed rest individual. This automatic ROI technique could solve the issue of longitudinal stability of the ROI location, which is important in clinical situations, especially for assessing individual changes over time [36]. A 500-pixel ROI was determined using the automatic ROI technique and used for the calculation of BUA and UV results.
Figure 7.
SCAN system–determined BMD change (mean ± standard error) from 90-day bed rest showed a similar pattern to DXA-determined BMD change. BMD = bone mineral density; DXA = dual-energy X-ray absorptiometry; SCAN = scanning confocal acoustic diagnostic navigation; VIBE = vibrational inhibition of bone erosion.
Figure 4.
QUS attenuation images and obtained irregular ROI by automatic procedures at baseline, day 60 and day 90 for bed rest individuals. QUS = quantitative ultrasound; ROI = region of interest.
The short-term precision of the bed rest study was calculated using QUS data collected at the pre bed rest time point. Three repetitive scans were conducted for each individual. The root mean square of individuals’ standard deviations was used to calculate the short-term QUS precision.
Bed rest study requires individuals to stay in bed, maintaining 6-degree head-down tilt while doing the QUS measurement. The SCAN system was designed to take the measurement at the edge of the bed with the individual's leg dangling over the bed and the foot resting on a foot measurement stage. A foot stopper was designed to facilitate repositioning of the foot perpendicular to the ultrasound pathway in subsequent scans, and restraining straps were used to immobilize the heel and lower leg. Before each measurement, alcohol swabs were used to clean the individual's foot before positioning the foot into the SCAN system.
Three scans were performed for each individual at each bed rest time point. In between each scan, the individual's lower leg was taken out of the measurement position and repositioned to check repeatability. Two phantoms reflected the heal bone density were used to calibrate the measurement system. Phantom calibration scans were performed before the measurements, and the phantom result was checked over bed rest period to verify system stability.
DXA measurement
aBMD was measured at baseline, 60 and 90 days into bed rest using a DXA (Hologic, MA) with individuals transferred to the DXA room on a 6-degree tilt transport gurney. All the BMD tests were conducted using the same DXA machine. Measurements were analyzed in the following regions: heel, whole body and hip (femoral neck and trochanter) (coefficient of variation = 2.0%). For the BMD analysis at the heel, the back part of the calcaneus, corresponding to where the QUS ROI was selected, was chosen to calculate calcaneus BMD. All the DXA scans and data collections were performed by an experienced technician to ensure data quality and eliminate interobserver variability. QUS parameters and DXA-determined BMD were evaluated through multiple correlations.
Statistics
A general linear model was used to perform the analysis of variance for UV, BUA and BMD. The model was constructed with sources of variance such as treatment groups (control and VIBE groups), time of measurement points (pre bed rest, 60 days and 90 days into bed rest), treatment groups with the interaction of time and time points with the interaction of individuals nested in different groups. The individuals were randomly assigned to either control or VIBE group or treated as a random factor in the general linear model. P values smaller than 0.05 were considered to be statistically significant. The analysis was performed using Minitab, a statistical analysis software package (Minitab, Inc., State College, PA, USA).
Results
The demographic profile of the participants was listed in Table 1. There was no difference between control and VIBE group regarding age, weight and height. There were seven more male individuals in the VIBE group. The baseline measurement using QUS and DXA (Table 2) did not show any value differences in BUA and UV measurement, as well as BMD at heel, femoral neck and whole-body regions.
Table 1.
Demographic profiles.
| Group | Gender | Race | Age (years) | Height (cm) | Weight (kg) |
|---|---|---|---|---|---|
| Control (n = 11) | 5 male 6 female |
6 white 5 black |
34.7 ± 7.9 (26–48) | 171 ± 11 | 74 ± 18 |
| VIBE (n = 18) | 12 male 6 female |
7 white 7 black 4 Latino |
35.6 ± 7.1 (25–47) | 172 ± 7 | 75 ± 9 |
VIBE = vibrational inhibition of bone erosion.
Table 2.
Baseline measure for different participant groups.
| Group | QUS |
DXA |
|||
|---|---|---|---|---|---|
| UV (m/s) | BUA (dB/MHz) | Heel BMD | FN BMD | WB BMD | |
| Control (n = 11) | 1547 ± 27 | 93 ± 20 | 0.65 ± 0.11 | 0.86 ± 0.11 | 1.19 ± 0.11 |
| VIBE (n = 18) | 1559 ± 33 | 93 ± 19 | 0.66 ± 0.11 | 0.87 ± 0.12 | 1.19 ± 0.10 |
BMD = bone mineral density; BUA = broadband ultrasound attenuation; DXA = dual-energy X-ray absorptiometry; FN BMD = DXA BMD at femoral neck region; Heel BMD = DXA BMD at heel region; UV = ultrasound velocity; VIBE = vibrational inhibition of bone erosion; WB BMD = averaged DXA BMD.
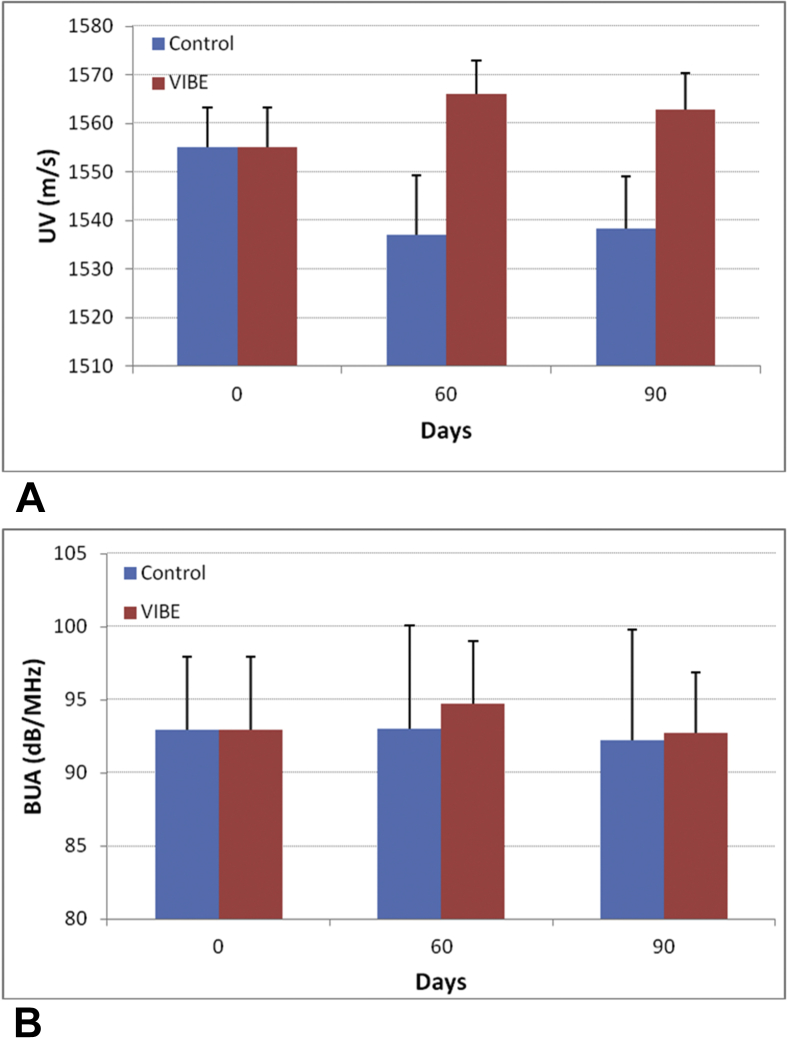
The short-term in vivo precision calculated from pre bed rest data showed a coefficient of variation of 0.41% for BUA and 1.97% for UV. Quantitative calculation of the UV values showed that UV decreased from 1555 ± 8 m/s (n = 29, average ± standard error) to 1537 ± 12 m/s (−1.2%) at Day 60 and to 1538 ± 11 m/s (−1.1%) at Day 90 for control individuals. For the VIBE individuals, UV increased from 1555 ± 8 m/s to 1566 ± 7 m/s (0.72%) at Day 60 and decreased to 1562 ± 8 m/s (0.5%) at Day 90 (Figure 5A). The mean UV percentage change between control and VIBE groups was 1.9% for 60 days and 1.6% for 90 days. A significant difference was found for UV between control and VIBE group (p = 0.01) with the interaction of time, as well as between individuals within each group (p < 0.001). No significant difference was found between Day 60 and Day 90 (p = 0.5).
Figure 5.
SCAN system shows the change in UV (mean ± Standard Error) and BUA (mean ± standard error) from baseline to 60 days and 90 days, for 11 control individuals and 18 VIBE individuals. BUA = broadband ultrasound attenuation; SCAN = scanning confocal acoustic diagnostic navigation; UV = ultrasound velocity; VIBE = vibrational inhibition of bone erosion.
A similar trend was observed for BUA values. BUA changed from 93.0 ± 5.0 dB/MHz (n = 29, average ± standard error) to 93.0 ± 7.1 dB/MHz (0.03%) at Day 60 and to 92.3 ± 7.6 dB/MHz (−0.7%) at Day 90 for control individuals; for VIBE individuals, BUA increased from 93.0 ± 5.0 dB/MHz to 94.7 ± 4.4 dB/MHz (1.9%) at Day 60 and fell to 92.7 ± 4.2 dB/MHz (−0.2%) at Day 90 as indicated in Figure 5B. The overall present change between VIBE and control groups was 1.8% at Day 60 and 0.5% at Day 90. Analysis of variance test showed no significant differences between control and VIBE (p = 0.51) and between time points (p = 0.7). A significant difference was found between individuals within each group (P < 0.001).
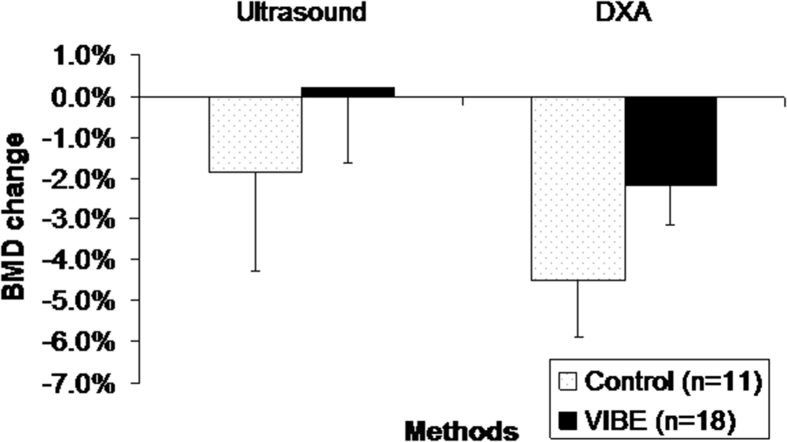
DXA-measured heel BMD showed a decrease from 0.657 ± 0.033 g/cm2 (n = 29, average ± standard error) to 0.637 ± 0.035 g/cm2 (−2.5%) at Day 60 and 0.626 ± 0.035 g/cm2 (−4.2%) at Day 90 for control group. For VIBE group, heel BMD decreased from 0.657 ± 0.025 g/cm2 to 0.655 ± 0.025 g/cm2 (−0.4%) at Day 60 and 0.647 ± 0.026 g/cm2 (−1.7%) at Day 90. The mean BMD percentage change for the control group was −4.50%, which was essentially twice as high as the −2.18% heel BMD loss for VIBE individuals. The difference between control and VIBE group with the interaction of time was moderately different but not significant (p = 0.199). The difference between individuals (p < 0.001) within each group and between time points (p < 0.001) was significant.
While the trend of increased BMD loss values was observed in the heel of control individuals compared to VIBE individuals, no significant BMD change was found at other skeletal sites between control and loaded groups, such as femoral neck (control −1.56% vs. VIBE -2.45%, p = 0.29) and whole-body region (control −0.91% vs. VIBE -1.09%, p = 0.73).
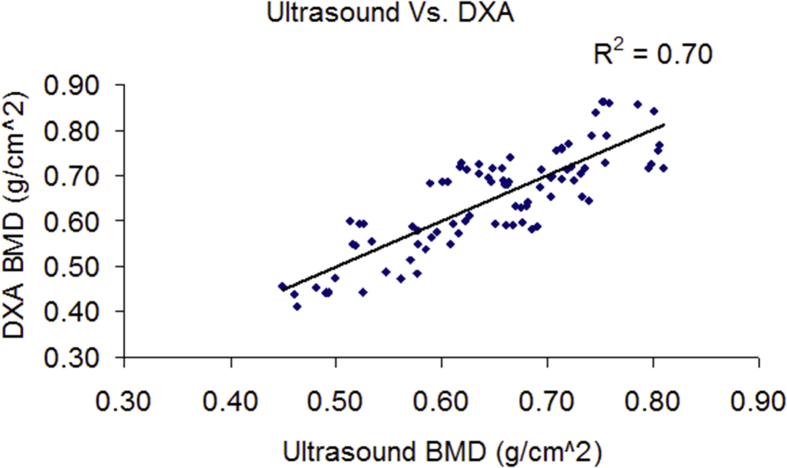
Correlation tests between SCAN QUS–measured UV and BUA and DXA-measured BMD at the calcaneus site, pooled all the data points together, rendered r2 = 0.69 between BUA and DXA heel BMD and r2 = 0.54 between UV and BMD. Combined BUA and UV using a linear regression model further increases the r2 to 0.70 between QUS and DXA as shown in Figure 6. Combined BUA and UV showed that a mean percentage change for the control group was −1.84% and 0.18% for VIBE group. The mean BMD percentage change as measured by DXA was −4.5% for the control group and −2.18% for the VIBE group (Figure 7).
Figure 6.
SCAN system–determined BMD showed a high correlation (r2 = 0.70) with DXA-measured BMD. BMD = bone mineral density; DXA = dual-energy X-ray absorptiometry; SCAN = scanning confocal acoustic diagnostic navigation.
Discussion
Osteopenia and osteoporosis are systemic skeletal diseases, and long-term bed rest resulted in bone loss all across the body. The calcaneus QUS is one of the primary measurements of assessing change in bone quantity and quality. Bone loss at the heel has been investigated in previous bed rest studies and before and after space flights by DXA technique. It was indicated that bone loss at the calcaneus occurs at a rate of 1.6–3.7% per month during extended space flight (2 + months) [33], [43], [49]. In bed rest studies, bone loss averages 1.73–2.75% per month with individuals in bed for four months [6], [38], [43], [74]. The rate of bone loss from the calcaneus tended to be smaller than the loss observed in bed rest studies because the heel is subject to ground reaction forces during in-flight treadmill exercises. However, bone loss at calcaneus could be astonishing in individual astronauts [42], [43]. Previous three-manned Skylab missions reported by LeBlanc et al. [43] showed that bone loss in calcaneus reached −3.5% per month (−7.4% in 59-day flight) for one crew member and −2.7% (−7.9% in 84-day flight) and −1.5% (−4.5% in 84-day flight) per month for other two crew members. In this study, calcaneus BMD loss was averaged approximately −1.5% per month with large individual differences (ranging from a gain of 0.22%/month to a loss of 6.54%/month). Given the significant different response as individuals subject to the microgravity environment and the relatively large percentage bone loss at human heel, SCAN system can be used as an effective tool to monitor individual bone quantity change with reasonable precision in a microgravity environment.
Ultrasonic imaging with UV and BUA changes during bed rest period showed the increase of both values from before bed rest to Day 60 into the bed rest and returned to pre–bed rest level at day 90 into the bed rest for VIBE subjects. This suggested the improvement/maintenance of bone quality and quantity for bed rest individuals due to VIBE treatment. For control individuals, both BUA and UV decreased from baseline to Day 60 and plateaued from Day 60 to Day 90, suggesting a fast adaptation of bone tissue to disuse environment in the first 60 days with little or no change during the last month of bed rest. Overall, the different response to bed rest between VIBE and control individuals was significant (p < 0.05) as indicated by UV measurement. While DXA-measured heel BMD did not show a significant difference (p = 0.199) between control and VIBE group, the mean percentage bone loss in the control group is 2% more than that in the VIBE group. These results indicate a positive effect of high-frequency, low-magnitude vibration as a mean to counter bone loss in bed rest environment.
The reduced BMD loss at the calcaneus site seen in VIBE individuals (VIBE −2.18% vs. control −4.50%) was not evident at other skeletal sites such as the femoral neck or across the entire skeleton (whole body). This may be because the vibration countermeasure was directly applied at the human foot. Compared to other skeletal sites, calcaneus was subject to stronger vibration forces than the hip and spine, which experienced less vibration due to the natural damping system introduced by the human body. Further studies are needed to explore the most effective way to introduce VIBE countermeasure to the appendicular and axial skeleton, such as changing intensity (increasing g-force) and duration (10–20 min) or incorporating refractory periods to enhance the receptivity of the skeleton [56].
In combination with some gravitational loading, low-intensity, high-frequency mechanical loading has been shown to promote bone mass in children with disabling conditions, including cerebral palsy [1], [2] and Duchenne muscular dystrophy [3] and girls with idiopathic scoliosis [4]. This is anabolic to bone and muscle in young women with osteoporosis [5], and augments bone accretion in childhood cancer survivors [6], and Crohn's disease [7]. While VIBE cannot be considered a substitute for exercise, these studies indicate that they represent salutary mechanical signals to improve clinical endpoints in individuals with limited exercise capacity [8] and may represent a means of priming responsiveness to exercise [56].
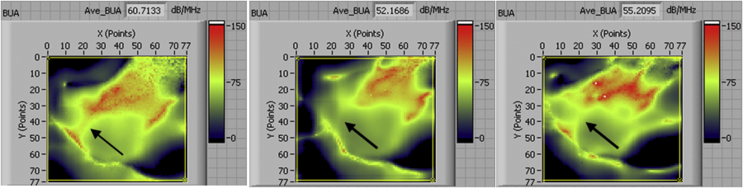
The ultrasound images from the confocal scanning can reveal the regional distribution density and strength of tested bone (Figure 8). It is capable of identifying the local acoustic feature change of bone, which is important for a longitudinal study such as bed rest study because bone loss may happen first at some specific sites and can only be discovered from the spatially distributed data such as the images generated from the confocal acoustic scanning technique. This technique can make an ultrasound to be more accurate, straightforward and efficient in the detection of bone loss. Overall, a significant correlation between SCAN and DXA was observed at dedicated sites, e.g., calcaneus, in bed rest (r2 = 0.70), suggesting that BMD loss is one of the major contributors for bone alterations in the skeleton during unloading. However, it should be noted that this study is mainly focused on longitudinal changes of bone through 90-day functional disuse.
Figure 8.
SCAN system shows the BUA images measured at baseline, 60 days and 90 days (from left to right) for one control individual. Arrow indicates regional bone property changes due to bed rest. BUA = broadband ultrasound attenuation; SCAN = scanning confocal acoustic diagnostic navigation.
For a longitudinal follow-up, the ability of QUS to distinguish between trabecular bone, cortical bone and soft tissue changes was a concern. Overlying soft tissues and cortical shell have been shown to influence the results of UV [30], [85] and BUA [34], [84] measurements, especially if variations in tissue thickness and composition occur. In a separate study, our laboratory introduced a newly developed three-dimensional surface topology mapping technique, which could potentially quantify the soft tissue thickness and hence enhance the measurement accuracy [85]. Also, experiment and model simulation has been introduced to quantify the influence of cortical shell on the BUA measurement [34], [84]. With the newly developed technique, QUS could be a useful tool for the monitoring of longitudinal changes in bone for both astronaut and ground-based operation. It seems clear that the changes in BMD are much more heterogeneous in bed rest than the skeletal changes occurring in metabolic bone disease, such as osteoporosis: Measurement of changes occurring at one site may not be used to predict changes at other sites during bed rest. Although SCAN system does show advantages as a portable, imaging-based, nonradiation and easy-to-use modality, it can only be applied at the peripheral skeletal region at the current stage. While osteoporosis-induced bone losses are demonstrated systematically, the consequences of bone loss–induced fracture often occurred at the critical skeletal sites, such as the hip and spine. Although the longitudinal monitoring for the individual bone mass change in the peripheral skeletal regions may be feasible, further study for bone assessment at the more critical sites, e.g., hip and spine, is needed.
It is noted that although QUS and DXA data correlated well with r2 = 0.7 between BUA and BMD, QUS data showed an increase for VIBE group whereas DXA showed a decrease. While QUS result can largely be explained by DXA, e.g. BMD, it has been shown that QUS result report additional bone density information using microCT measurement, e.g. related to trabecular space (Tb.Sp), trabecular thickness (Tb,Th.) and structure model index (SMI), in addition to BMD. In addition, DXA is an accurate measurement of total BMD, which is dominated by cortical BMD, but the physics of QUS should address a combination of both cortical and trabecular BMD, which is potentially a better measure of bone density and structural properties.
Limitations of this study include the modest and uneven sample size (n = 11 for control and n = 18 for VIBE group), concerning limited resources and costs of such human study. Human bed rest studies have provided unique analogue simulation of microgravity environment and insights into the physiology of non–weight-bearing and deconditioning associated with space flight. Several of previous studies reported bone and muscle alterations after 30–60 days of bed rest and showed efficacy and significance [14], [22], [23], [73]. Although a total of 29 individuals were considered a small number, the data have shown the efficacy of bone alteration between simulated microgravity and mechanical stimulation using both DXA and QUS in the heel region. The statistical power of the study could be further improved if the study can be extended to address the aforementioned concerns. Despite the sample size and distribution as limited by the study protocol, there are more than 260 ultrasound scans performed during the study. The promising results as presented in this study will warrant further investigation of QUS in bed rest, as well as in the microgravity environment. In addition, further development of QUS in the critical skeletal sites, such as the hip and spine, would provide significant assessments in fracture risk of the skeleton.
Conclusion
QUS and DXA demonstrated significant correlation for BMD at the calcaneus region to monitor the longitudinal bone loss and the effect of LIV as a means to prevent bone loss in the skeleton. The confocal scanning ultrasound imaging is capable for bone mass assessment in the ROI, which may be used to predict the alteration of bone loss in the long-term bed rest simulation and potentially future space mission.
Conflict of interest
YXQ has patents and patents pending as it related to the ultrasound diagnosis of bone. CTR has patents and patents pending as it relates to the use of mechanical signals to treat musculoskeletal disorders. He is also a founder of Marodyne and BTT-Health.
Acknowledgments
This work was kindly supported by the National Space Biomedical Research Institute (TD00405 & SMST01603) through NASA Cooperative Agreement NCC 9-58, and NASA. The authors are grateful for the DXA measurement for the bedrest subjects performed by the Johnson Space Center led UT Medical Center at Galveston bedrest facility. The authors would like to thank Ben Adler at Stony Brook University, UTMB nursing staff, and Wyle Lab engineers for their helps in schedule arrangement, equipment setting, discussion comments, and technical support.
References
- 1.Arnaud S.B., Harper J.S., Navidi M. Mineral distribution in rat skeletons after exposure to a microgravity model. J Grav Physiol. 1995;2:115–116. [PubMed] [Google Scholar]
- 2.Arnaud S.B., Sherrard D.J., Maloney N., Whalen R.T., Fung P. Effects of 1-week head-down tilt bed rest on bone-formation and the calcium endocrine system. Aviat Space Environ Med. 1992;63:14–20. [PubMed] [Google Scholar]
- 3.Bauer D.C., Gluer C.C., Cauley J.A., Vogt T.M., Ensrud K.E., Genant H.K. Broadband ultrasound attenuation predicts fractures strongly and independently of densitometry in older women. A prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 3-24-1997;157:629–634. [PubMed] [Google Scholar]
- 4.Belavy D.L., Miokovic T., Armbrecht G., Rittweger J., Felsenberg D. Resistive vibration exercise reduces lower limb muscle atrophy during 56-day bed-rest. J Musculoskelet Neuronal Interact. 2009;9:225–235. [PubMed] [Google Scholar]
- 5.Belavy D.L., Ohshima H., Rittweger J., Felsenberg D. High-intensity flywheel exercise and recovery of atrophy after 90 days bed–rest. BMJ Open.Sport Exerc Med. 2017;3:e000196. doi: 10.1136/bmjsem-2016-000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bloomfield S.A. Changes in musculoskeletal structure and function with prolonged bed rest. Med Sci Sports Exerc. 1997;29:197–206. doi: 10.1097/00005768-199702000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Busa B., Miller L.M., Rubin C.T., Qin Y.X., Judex S. Rapid establishment of chemical and mechanical properties during lamellar bone formation. Calcif Tissue Int. 2005;77:386–394. doi: 10.1007/s00223-005-0148-y. [DOI] [PubMed] [Google Scholar]
- 8.Cepollaro C., Gonnelli S., Montagnani A., Caffarelli C., Cadirni A., Martini S. In vivo performance evaluation of the Achilles Insight QUS device. J Clin Densitom. 2005;8:341–346. doi: 10.1385/jcd:8:3:341. [DOI] [PubMed] [Google Scholar]
- 9.Chesnut C.H.I. 1993. Non-invasive methods for bone mass measurement; pp. 77–87. [Google Scholar]
- 10.Collet P., Uebelhart D., Vico L., Moro L., Hartmann D., Roth M. Effects of 1- and 6-month spaceflight on bone mass and biochemistry in two humans. Bone. 1997;20:547–551. doi: 10.1016/s8756-3282(97)00052-5. [DOI] [PubMed] [Google Scholar]
- 11.Cortet B., Boutry N., Dubois P., Legroux-Gerot I., Cotten A., Marchandise X. Does quantitative ultrasound of bone reflect more bone mineral density than bone microarchitecture? Calcif Tissue Int. 2004;74:60–67. doi: 10.1007/s00223-002-2113-3. [DOI] [PubMed] [Google Scholar]
- 12.Damilakis J., Papadokostakis G., Perisinakis K., Maris T.G., Karantanas A.H. Hip fracture discrimination by the Achilles Insight QUS imaging device. Eur J Radiol. 2007;63:59–62. doi: 10.1016/j.ejrad.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 13.Defontaine M., Bonneau S., Padilla F., Gomez M.A., Nasser Eddin M., Laugier P. 2D arrays device for calcaneus bone transmission: an alternative technological solution using crossed beam forming. Ultrasonics. 2004;42:745–752. doi: 10.1016/j.ultras.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 14.DiVasta A.D., Feldman H.A., Rubin C.T., Gallagher J.S., Stokes N., Kiel D.P. The ability of low-magnitude mechanical signals to normalize bone turnover in adolescents hospitalized for anorexia nervosa. Osteoporos Int. 2017;28:1255–1263. doi: 10.1007/s00198-016-3851-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilsanz V., Wren T.A., Sanchez M., Dorey F., Judex S., Rubin C. Low-level, high-frequency mechanical signals enhance musculoskeletal development of young women with low BMD. J Bone Miner Res. 2006;21:1464–1474. doi: 10.1359/jbmr.060612. [DOI] [PubMed] [Google Scholar]
- 16.Gomez M.A., Defontaine M., Giraudeau B., Camus E., Colin L., Laugier P. Vivo performance of a matrix-based quantitative ultrasound imaging device dedicated to calcaneus investigation. Ultrasound Med Biol. 2002;28:1285–1293. doi: 10.1016/s0301-5629(02)00616-6. [DOI] [PubMed] [Google Scholar]
- 17.Goode A. Musculoskeletal change during spaceflight: a new view of an old problem. Br J Sports Med. 1999;33:154. [PubMed] [Google Scholar]
- 18.Goode A.W., Rambaut P.C. The skeleton in space. Nature. 9-19-1985;317:204–205. doi: 10.1038/317204a0. [DOI] [PubMed] [Google Scholar]
- 19.Grigoriev A.I., Oganov V.S., Bakulin A.V., Poliakov V.V., Voronin L.I., Morgun V.V. Clinical and psychological evaluation of bone changes among astronauts after long term space flights (Russian) Aviakosmicheskaia I Ekologicheskaia Meditsina. 1998;32:21–25. [PubMed] [Google Scholar]
- 20.Gross T.S., Edwards J.L., McLeod K.J., Rubin C.T. Strain gradients correlate with sites of periosteal bone formation. J Bone Miner Res. 1997;12:982–988. doi: 10.1359/jbmr.1997.12.6.982. [DOI] [PubMed] [Google Scholar]
- 21.Hans D., Dargent-Molina P., Schott A.M., Sebert J.L., Cormier C., Kotzki P.O. Ultrasonographic heel measurements to predict hip fracture in elderly women: the EPIDOS prospective study. Lancet. 8-24-1996;348:511–514. doi: 10.1016/s0140-6736(95)11456-4. [DOI] [PubMed] [Google Scholar]
- 22.Hargens A.R., Vico L. Long-duration bed rest as an analog to microgravity. J Appl Physiol (1985.) 4-15-2016;120:891–903. doi: 10.1152/japplphysiol.00935.2015. [DOI] [PubMed] [Google Scholar]
- 23.Holt J.A., Macias B.R., Schneider S.M., Watenpaugh D.E., Lee S.M., Chang D.G. WISE 2005: aerobic and resistive countermeasures prevent paraspinal muscle deconditioning during 60-day bed rest in women. J Appl Physiol (1985.) 5-15-2016;120:1215–1222. doi: 10.1152/japplphysiol.00532.2015. [DOI] [PubMed] [Google Scholar]
- 24.Huopio J., Kroger H., Honkanen R., Jurvelin J., Saarikoski S., Alhava E. Calcaneal ultrasound predicts early postmenopausal fractures as well as axial BMD. A prospective study of 422 women. Osteoporos Int. 2004;15:190–195. doi: 10.1007/s00198-003-1534-9. [DOI] [PubMed] [Google Scholar]
- 25.Jones H.H., Priest J.D., Hayes W.C., Tichenor C.C., Nagel D.A. Humeral hypertrophy in response to exercise. J.Bone Joint Surg Am. 1977;59:204–208. [PubMed] [Google Scholar]
- 26.Judex S., Boyd S., Qin Y.X., Turner S., Ye K., Muller R. Adaptations of trabecular bone to low magnitude vibrations result in more uniform stress and strain under load. Ann Biomed Eng. 2003;31:12–20. doi: 10.1114/1.1535414. [DOI] [PubMed] [Google Scholar]
- 27.Khaw K.T., Reeve J., Luben R., Bingham S., Welch A., Wareham N. Prediction of total and hip fracture risk in men and women by quantitative ultrasound of the calcaneus: EPIC-Norfolk prospective population study. Lancet. 1-17-2004;363:197–202. doi: 10.1016/S0140-6736(03)15325-1. [DOI] [PubMed] [Google Scholar]
- 28.Khosla S., Riggs B.L. Pathophysiology of age-related bone loss and osteoporosis. Endocrinol Metab Clin North Am. 2005;34:1015–1030. doi: 10.1016/j.ecl.2005.07.009. xi. [DOI] [PubMed] [Google Scholar]
- 29.Kiel D.P., Hannan M.T., Barton B.A., Bouxsein M.L., Sisson E., Lang T. Low-magnitude mechanical stimulation to improve bone density in persons of advanced age: a randomized, placebo-controlled trial. J Bone Miner Res. 2015;30:1319–1328. doi: 10.1002/jbmr.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kotzki P.O., Buyck D., Hans D., Thomas E., Bonnel F., Favier F. Influence of fat on ultrasound measurements of the os calcis. Calcif Tissue Int. 1994;54:91–95. doi: 10.1007/BF00296057. [DOI] [PubMed] [Google Scholar]
- 31.Krolner B., Toft B., Pors N.S., Tondevold E. Physical exercise as prophylaxis against involutional vertebral bone loss: a controlled trial. Clin Sci (Lond) 1983;64:541–546. doi: 10.1042/cs0640541. [DOI] [PubMed] [Google Scholar]
- 32.Lam H., Qin Y.X. The effects of frequency-dependent dynamic muscle stimulation on inhibition of trabecular bone loss in a disuse model. Bone. 2008;43:1093–1100. doi: 10.1016/j.bone.2008.07.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lang T., LeBlanc A., Evans H., Lu Y., Genant H., Yu A. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J Bone Miner Res. 2004;19:1006–1012. doi: 10.1359/JBMR.040307. [DOI] [PubMed] [Google Scholar]
- 34.Langton C.M., Subhan M. Computer and experimental simulation of a cortical end-plate phase cancellation artefact in the measurement of BUA at the calcaneus. Physiol Meas. 2001;22:581–587. doi: 10.1088/0967-3334/22/3/314. [DOI] [PubMed] [Google Scholar]
- 35.Laugier P., Fournier B., Berger G. Ultrasound parametric imaging of the calcaneus: in vivo results with a new device. Calcif Tissue Int. 1996;58:326–331. doi: 10.1007/BF02509380. [DOI] [PubMed] [Google Scholar]
- 36.Laugier P., Novikov V., Elmann-Larsen B., Berger G. Quantitative ultrasound imaging of the calcaneus: precision and variations during a 120-Day bed rest. Calcif Tissue Int. 2000;66:16–21. doi: 10.1007/s002230050005. [DOI] [PubMed] [Google Scholar]
- 37.LeBlanc A., Schneider V. Can the adult skeleton recover lost bone? Exp Gerontol. 1991;26:189–201. doi: 10.1016/0531-5565(91)90011-a. [DOI] [PubMed] [Google Scholar]
- 38.LeBlanc A., Schneider V., Evans H., Engelbretson D., Krebs J. Bone mineral loss and recovery after 17 Weeks of bed rest. J Bone Min Res. 1990;5:843–850. doi: 10.1002/jbmr.5650050807. [DOI] [PubMed] [Google Scholar]
- 39.LeBlanc A., Schneider V., Shackelford L. Bone mineral and lean tissue loss after long duration spaceflight. Trans Am Soc Bone Min Res. 1996;11S:567. [PubMed] [Google Scholar]
- 40.LeBlanc A., Schneider V., Shackelford L., West S., Oganov V., Bakulin A. Bone mineral and lean tissue loss after long duration space flight. J Musculoskelet Neuronal Interact. 2000;1:157–160. [PubMed] [Google Scholar]
- 41.LeBlanc A., Shackelford L., Feiveson A., Oganov V. vol. 1. 1999. p. 17. (Bone loss in space: shuttle/mir experience and bed-rest counter measure program). [Google Scholar]
- 42.LeBlanc A., Shackelford L., Schneider V. Future human bone research in space. Bone. 1998;22:113S–116S. doi: 10.1016/s8756-3282(98)00013-1. [DOI] [PubMed] [Google Scholar]
- 43.LeBlanc A.D., Spector E.R., Evans H.J., Sibonga J.D. Skeletal responses to space flight and the bed rest analog: a review. J Musculoskelet Neuronal Interact. 2007;7:33–47. [PubMed] [Google Scholar]
- 44.Leonard M.B., Shults J., Long J., Baldassano R.N., Brown J.K., Hommel K. Effect of low magnitude mechanical stimuli on bone density and structure in pediatric crohn's disease: a randomized placebo controlled trial. J Bone Miner Res. 2016 Jun;31(6):1177–1188. doi: 10.1002/jbmr.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leung K.S., Li C.Y., Tse Y.K., Choy T.K., Leung P.C., Hung V.W. Effects of 18-month low-magnitude high-frequency vibration on fall rate and fracture risks in 710 community elderly--a cluster-randomized controlled trial. Osteoporos Int. 2014;25:1785–1795. doi: 10.1007/s00198-014-2693-6. [DOI] [PubMed] [Google Scholar]
- 46.Lin L., Cheng J., Lin W., Qin Y.-X. Prediction of trabecular bone principal structural orientation using quantitative ultrasound scanning. J Biomech. 2012;45:1790–1795. doi: 10.1016/j.jbiomech.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin L., Lin W., Qin Y.X. Enhanced correlation between quantitative ultrasound and structural and mechanical properties of bone using combined transmission-reflection measurement. J Acoust Soc Am. 2015;137:1144–1152. doi: 10.1121/1.4906830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin R.B., Burr D.B. 1989. Structure, function and adaptation of compact bone. New York. [Google Scholar]
- 49.McCarthy I., Goodship A., Herzog R., Oganov V., Stussi E., Vahlensieck M. Investigation of bone changes in microgravity during long and short duration space flight: comparison of techniques. Eur J Clin Invest. 2000;30:1044–1054. doi: 10.1046/j.1365-2362.2000.00719.x. [DOI] [PubMed] [Google Scholar]
- 50.Mogil R.J., Kaste S.C., Ferry R.J., Jr., Hudson M.M., Mulrooney D.A., Howell C.R. Effect of low-magnitude, high-frequency mechanical stimulation on BMD among young childhood cancer survivors: a randomized clinical trial. JAMA Oncol. 7-1-2016;2:908–914. doi: 10.1001/jamaoncol.2015.6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nicholson P.H., Muller R., Cheng X.G., Ruegsegger P., Van der P.G., Dequeker J. Quantitative ultrasound and trabecular architecture in the human calcaneus. J Bone Miner Res. 2001;16:1886–1892. doi: 10.1359/jbmr.2001.16.10.1886. [DOI] [PubMed] [Google Scholar]
- 52.Njeh C.F., Fuerst T., Diessel E., Genant H.K. Is quantitative ultrasound dependent on bone structure? A reflection. Osteoporos Int. 2001;12:1–15. doi: 10.1007/PL00020939. [DOI] [PubMed] [Google Scholar]
- 53.Ozcivici E., Luu Y.K., Adler B., Qin Y.X., Rubin J., Judex S. Mechanical signals as anabolic agents in bone. Nat Rev Rheumatol. 2010;6:50–59. doi: 10.1038/nrrheum.2009.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Padilla F., Jenson F., Bousson V., Peyrin F., Laugier P. Relationships of trabecular bone structure with quantitative ultrasound parameters: in vitro study on human proximal femur using transmission and backscatter measurements. Bone. 2008;42:1193–1202. doi: 10.1016/j.bone.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 55.Padilla F., Laugier P. Recent developments in trabecular bone characterization using ultrasound. Curr Osteoporos Rep. 2005;3:64–69. doi: 10.1007/s11914-005-0006-x. [DOI] [PubMed] [Google Scholar]
- 56.Patel V.S., Chan M.E., Pagnotti G.M., Frechette D.M., Rubin J., Rubin C.T. Incorporating refractory period in mechanical stimulation mitigates obesity-induced adipose tissue dysfunction in adult mice. Obesity (Silver Spring) 2017 Oct;25(10):1745–1753. doi: 10.1002/oby.21958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pavy-Le Traon A., Heer M., Narici M.V., Rittweger J., Vernikos J. From space to Earth: advances in human physiology from 20 years of bed rest studies (1986-2006) Eur J Appl Physiol. 2007;101:143–194. doi: 10.1007/s00421-007-0474-z. [DOI] [PubMed] [Google Scholar]
- 58.Qin Y., Xia Y., Lin W., Gruber B., Judex S., Rubin C. Quantitative prediction of bone density and strength in human calcanei using a scanning confocal acoustic diagnostic system. J Bone Miner Res J Bone Miner Res. 2005;20 S230-S230. [Google Scholar]
- 59.Qin Y.X., Lam H., Ferreri S., Rubin C. Dynamic skeletal muscle stimulation and its potential in bone adaptation. J Musculoskelet Neuronal Interact. 2010;10:12–24. [PMC free article] [PubMed] [Google Scholar]
- 60.Qin Y.X., Lin W., Mittra E., Xia Y., Cheng J., Judex S. Prediction of trabecular bone qualitative properties using scanning quantitative ultrasound. Acta Astronaut. 2013;92:79–88. doi: 10.1016/j.actaastro.2012.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qin Y.X., Lin W., Rubin C.T. Load-induced bone fluid flow pathway as definded by in-vivo intramedullary pressure and streaming potentials measurements. Ann Biomed Eng. 2002;30:693–702. doi: 10.1114/1.1483863. [DOI] [PubMed] [Google Scholar]
- 62.Qin Y.X., Otter M.W., Rubin C.T., McLeod K.J. The influence of intramedullary hydrostatic pressure on transcortical fluid flow patterns in bone. Trans Ortho Res Soc. 1997;22:885. [Google Scholar]
- 63.Qin Y.X., Rubin C.T., McLeod K.J. Nonlinear dependence of loading intensity and cycle number in the maintenance of bone mass and morphology. J Orthop Res. 1998;16:482–489. doi: 10.1002/jor.1100160414. [DOI] [PubMed] [Google Scholar]
- 64.Qin Y.X., Xia Y., Lin W., Cheng J., Muir J., Rubin C. Longitudinal assessment of human bone quality using scanning confocal quantitative ultrasound. J Acoust Soc Am. 2008;123:3638. [Google Scholar]
- 65.Rambaut P., Goode A. Skeletal changes during space flight. Lancet. 1985;2:1050–1052. doi: 10.1016/s0140-6736(85)90916-x. [DOI] [PubMed] [Google Scholar]
- 66.Riggs B.L., Melton L.J., III. The worldwide problem of osteoporosis: insights afforded by epidemiology. Bone. 1995;17:505S–511S. doi: 10.1016/8756-3282(95)00258-4. [DOI] [PubMed] [Google Scholar]
- 67.Rittweger J., Beller G., Armbrecht G., Mulder E., Buehring B., Gast U. Prevention of bone loss during 56 days of strict bed rest by side-alternating resistive vibration exercise. Bone. 2010;46:137–147. doi: 10.1016/j.bone.2009.08.051. [DOI] [PubMed] [Google Scholar]
- 68.Rubin C., Recker R., Cullen D., Ryaby J., McCabe J., McLeod K. Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: a clinical trial assessing compliance, efficacy, and safety. J Bone Miner Res. 2004;19:343–351. doi: 10.1359/JBMR.0301251. [DOI] [PubMed] [Google Scholar]
- 69.Rubin C., Turner A.S., Bain S., Mallinckrodt C., McLeod K. Anabolism. Low mechanical signals strengthen long bones. Nature. 2001;412:603–604. doi: 10.1038/35088122. [DOI] [PubMed] [Google Scholar]
- 70.Rubin C.T., Lanyon L.E. Regulation of bone formation by applied dynamic loads. J Bone Joint Surg [Am.] 1984;66:397–402. [PubMed] [Google Scholar]
- 71.Rubin C.T., Seeherman H., Qin Y.X., Gross T.S. The mechanical consequences of load bearing in the equine third metacarpal across speed and gait: the nonuniform distributions of normal strain, shear strain, and strain energy density. FASEB J. 2013;27:1887–1894. doi: 10.1096/fj.12-216804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ruff C., Beck T., Newman D., Oden M., Shaffner G., LeBlanc A. vol. 1. 1999. pp. 86–87. (Skeletal consequences of reduced gravity environments). [Google Scholar]
- 73.Salanova M., Gambara G., Moriggi M., Vasso M., Ungethuem U., Belavy D.L. Vibration mechanosignals superimposed to resistive exercise result in baseline skeletal muscle transcriptome profiles following chronic disuse in bed rest. Sci Rep. 11-24-2015;5:17027. doi: 10.1038/srep17027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shackelford L.C., LeBlanc A.D., Driscoll T.B., Evans H.J., Rianon N.J., Smith S.M. Resistance exercise as a countermeasure to disuse-induced bone loss. J Appl Physiol. 2004;97:119–129. doi: 10.1152/japplphysiol.00741.2003. [DOI] [PubMed] [Google Scholar]
- 75.Shackleford L., LeBlanc A., Feiveson A., Oganov V. Bone loss in space: shuttle/Mir experience and bed rest countermeasure program. 1st Biennial Space Biomed Inv Workshop. 1999;1:86–87. [Google Scholar]
- 76.Sone T., Imai Y., Tomomitsu T., Fukunaga M. Calcaneus as a site for the assessment of bone mass. Bone. 1998;22:155S–157S. doi: 10.1016/s8756-3282(98)00004-0. [DOI] [PubMed] [Google Scholar]
- 77.Stokes I.A. Analysis of symmetry of vertebral body loading consequent to lateral spinal curvature. Spine. 11-1-1997;22:2495–2503. doi: 10.1097/00007632-199711010-00006. [DOI] [PubMed] [Google Scholar]
- 78.Tilton F., Degioanni J., Schneider V. Long term follow up of SkyLab Bone Demineralization. Aviat Space Environ Med. 1980;51:1209–1213. [PubMed] [Google Scholar]
- 79.Turner C.H. Site-specific skeletal effects of exercise: importance of interstitial fluid pressure. Bone. 1999;24:161–162. doi: 10.1016/s8756-3282(98)00184-7. [DOI] [PubMed] [Google Scholar]
- 80.Wang H., Wan Y., Tam K.F., Ling S., Bai Y., Deng Y. Resistive vibration exercise retards bone loss in weight-bearing skeletons during 60 days bed rest. Osteoporos Int. 2012;23:2169–2178. doi: 10.1007/s00198-011-1839-z. [DOI] [PubMed] [Google Scholar]
- 81.Ward K., Alsop C., Caulton J., Rubin C., Adams J., Mughal Z. Low magnitude mechanical loading is osteogenic in children with disabling conditions. J Bone Miner Res. 2004;19:360–369. doi: 10.1359/JBMR.040129. [DOI] [PubMed] [Google Scholar]
- 82.Wolff J. 1892. Das Gesetz Der Transformation Der Knochen. Berlin. [Google Scholar]
- 83.Wolff J. 1986. The Law of bone remodeling. Berlin. [Google Scholar]
- 84.Xia Y., Lin W., Qin Y. The influence of cortical end-plate on broadband ultrasound attenuation measurements at the human calcaneus using scanning confocal ultrasound. J Acoust Soc Am. 2005;118:1801–1807. doi: 10.1121/1.1979428. [DOI] [PubMed] [Google Scholar]
- 85.Xia Y., Lin W., Qin Y.X. Bone surface topology mapping and its role in trabecular bone quality assessment using scanning confocal ultrasound. Osteoporos Int. 2007;18:905–913. doi: 10.1007/s00198-007-0324-1. [DOI] [PubMed] [Google Scholar]