Abstract
Advances in chemotherapy and radiotherapy have greatly improved cancer survival, but their side effects can sometimes be more dangerous than the cancer itself. Understanding these risks is especially important when the consequences are as life-threatening as heart failure. Melphalan and fludarabine are drugs used in many chemotherapy regimens and are not usually associated with cardiotoxicity. In this report we present a patient that developed acute heart failure followed by persistent heart failure after conditioning with these drugs following a bone marrow transplant.
<Learning objective: The chemotherapeutic agents melphalan and fludarabine used individually are rarely associated with cardiotoxicity. However, this case report of myocarditis and persistent heart failure, adds to the growing literature suggesting an exponential rise in cardiac complications when these agents are used in combination. Awareness of this association is important to consider when choosing and monitoring a chemotherapy regimen.>
Keywords: Cardiotoxicity, Melphalan, Fludarabine, Heart failure
Introduction
Advances in chemotherapy and radiotherapy have had the greatest impact on cancer survival, but their combined side effects have led patients to deal with serious organic complications as a result of their treatment. In attempting to treat the cancer, many times anti-cancer therapies cause complications that need treatment themselves. Frequently, use of these agents in combination will result in the addition of toxicities and can exponentially increase their side-effect profile. Even whilst these drugs are used within therapeutic dose ranges they can cause toxicities. They need not be dosed excessively in order to cause complications. The most common side effects of anticancer chemotherapeutic agents (CCAs) are nausea, vomiting, hair loss, stomatitis, and leukopenia [1].
Another major toxicity seen with some agents is cardiotoxicity. Although cardiotoxicity is less common than the gastrointestinal disturbances, it can occur particularly with the use of the anthracycline agents doxorubicin and daunorubicin [2].
In addition to the anthracyclines, two other CCAs have been implicated in causing cardiotoxicity: melphalan and fludarabine. Melphalan is an alkylating agent that has been reported to cause paroxysmal arrhythmias and fludarabine is a purine analog that has rarely been associated with cardiac dysfunction [3]. These therapies have been used in combination to treat multiple myeloma, chronic lymphocytic leukemia (CLL), chronic myeloid leukemia, acute lymphoblastic leukemia, non-Hodgkin lymphoma, acute myeloblastic leukemia, and paroxysmal nocturnal hemoglobinemia and have been well tolerated. Individually these agents do not typically cause cardiotoxicity however in combination the risk increases resulting in a greater potential for heart failure. We present a case of myocarditis with persistent systolic heart failure associated with these medications.
Case report
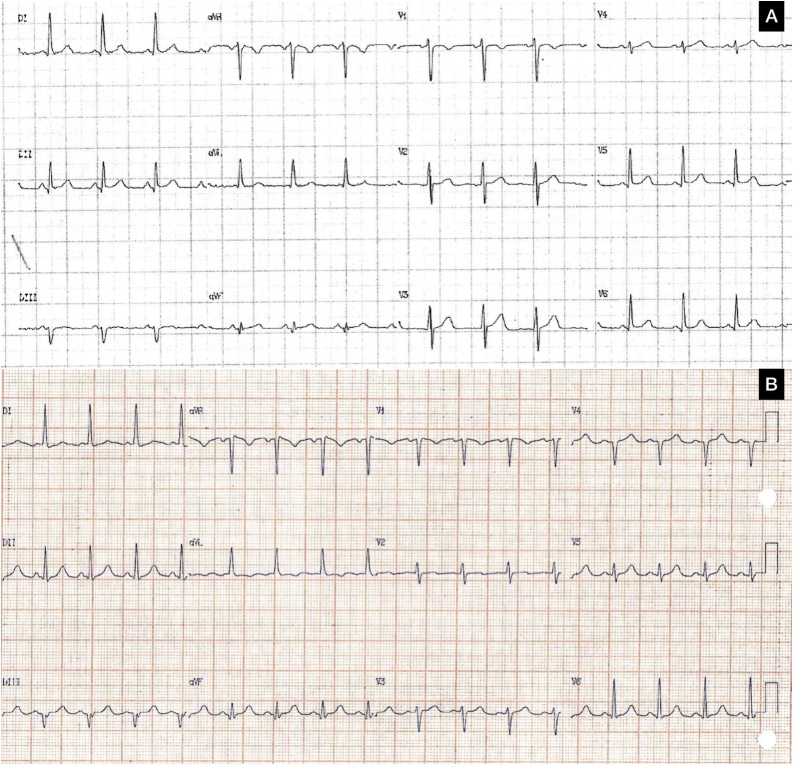
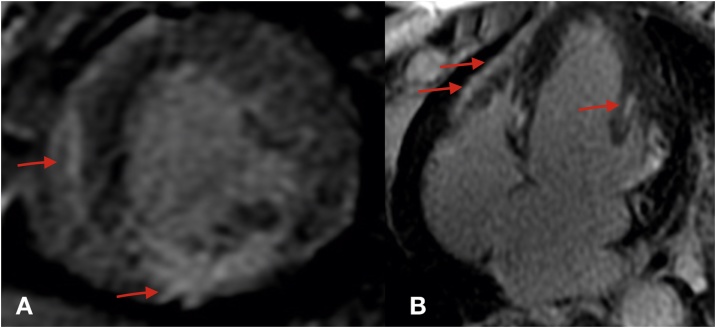
A 48-year old woman with an 8-year history of refractory mycosis fungoides underwent chemotherapy and an allogenic bone-marrow transplant. Peripheral blood was the source of stem cells. Graft-versus-host disease prophylaxis was performed with methotrexate and cyclosporine. Low-intensity pre-treatment was performed with fludarabine 30 mg/m2 and melphalan 70 mg/m2. Baseline echocardiogram showed a normal left ventricle function. The patient was asymptomatic until the fourth day of chemotherapy induction when she presented with pulmonary edema. Cardiac biomarkers (creatine kinase-MB and troponin I), thyroid-stimulating hormone (TSH), and electrolytes were within normal limits. Electrocardiography (ECG) after chemotherapy showed slower R wave progression in precordial leads compared with previously (Fig. 1). Chest X-ray showed new cardiac area enlargement and pulmonary congestion. Echocardiography showed an ejection fraction of 45%. She continued her usual chemotherapy but furosemide, captopril, and spironolactone were added. An echocardiogram was repeated 3 days later and showed an even further reduced ejection fraction of 23%. Cardiac biomarkers, TSH, and electrolytes were within normal limits. The patient’s cardiac magnetic resonance imaging (MRI) was compatible with myocarditis (Fig. 2). Metoprolol was added to her medication regimen after initial management. She had good bone marrow recovery, however one month later new skin lesions were consistent with disease relapse. This patient’s ejection fraction after 6 months was still low even with optimal heart failure treatment (Table 1).
Fig. 1.
Electrocardiogram (ECG). Before (A) and after chemotherapy (B). ECG after showed slower R wave progression in precordial leads.
Fig. 2.
Cardiac magnetic resonance imaging. Short-axis (A) and four-chambers (B) view showing a pattern of subepicardical and midwall contrast enhancement in the inferior, anterolateral, and inferoseptal wall. See red arrows.
Table 1.
Echocardiogram parameters. Measurement points of the left ventricle (LV) were obtained by ultrasonic cardiography.
| Left ventricle (mm)a | Left atrium (mm) | Pulmonary artery pressure (mmHg)b | Left ventricular ejection fraction (%)c | Mitral valve | |
|---|---|---|---|---|---|
| Baseline | 51 × 37 | 38 | 15 | 53 | Normal |
| 4th day post bone marrow transplant | 61 × 47 | 47 | 20 | 45 | Moderate insufficiency |
| 1 month after | 64 × 52 | 43 | 37 | 37 | Moderate insufficiency |
| 6 months later | 59 × 50 | 37 | 34 | 32 | Mild insufficiency |
LV diastolic and systolic diameter were measured from two-dimensional images in the parasternal long-axis view at the level of the mitral valve chordae.
Systolic pulmonary artery pressure per modified Bernoulli equation using tricuspid regurgitation.
Left ventricular ejection fraction by Simpson’s method.
Discussion
The unfortunate side effect of many anti-cancer chemotherapeutic therapies is the associated variety of toxicities which therefore limits their use. Cardiotoxicity thus compromises the effectiveness of CCAs. This issue is particularly problematic as the number of cancer survivors is increasing and higher doses of these agents both individually and in combination, therefore with additive cardiotoxic effects, are being utilized [4].
Drug-induced myocarditis can occur acutely, sub-acutely, chronically, or as a late sequela. Cardiac events that occur as a result of chemotherapy include mild blood pressure changes, thrombosis, ECG changes, arrhythmias, myocarditis, pericarditis, myocardial infarction, cardiomyopathy, cardiac failure (of the left ventricle), and congestive heart failure. Our patient had a normal baseline cardiac function but developed acute heart failure during the bone marrow transplant conditioning. Normal serum markers of inflammation do not exclude an acute myocardial inflammatory process [5]. ECG has low sensitivity but is widely available and thus used for risk stratification rather than as a screening tool for suspected myocarditis. A more valuable tool used in diagnosis of myocarditis is cardiovascular MRI which can demonstrate acute myocardial inflammation [5]. While endomyocardial biopsy is the gold standard in diagnosis, this patient’s laboratory findings and imaging in the context of her clinical picture make myocarditis the likely diagnosis.
Melphalan is an L-phenylalanine mustard that is the most commonly used alkylating agent used in multiple myeloma and can be used in palliative treatment of carcinomas of the ovaries and breast [6]. Expected side effects include bone marrow suppression, hypersensitivity reactions, nausea, vomiting, diarrhea, oral ulcers, and pulmonary toxicity.
Fludarabine phosphate is a fluorinated derivative of the nucleotide analog vidarabine and is used to treat low-grade leukemias and lymphomas and is also used to enhance graft acceptance in transplantation [7]. Fludarabine can cause a variety of neurologic and pulmonary toxicities.
Although cardiac dysfunction is well described with other alkylating agents such as cyclophosphamide, single-agent melphalan has not been shown to affect cardiac contractility in prospective studies [8]. Fludarabine has also only been rarely associated with cardiac dysfunction, with a single report of non-fatal congestive heart failure in 2 of 27 patients treated for CLL [9]. Ritchie et al. described three cases of severe heart failure in patients treated with the present association, however they had partial or complete recovery after 6 months [3].
Our patient developed acute heart failure during the conditioning with melphalan and fludarabine, which itself would be a rare side effect of those medications. More unusual in this case is the persistent systolic heart failure 6 months after the transplant even with using guideline-based therapy for heart failure. This cardiotoxicity could be due to the redundancy in signaling pathways that are activated by the use of these CCAs [10]. CCAs are designed to have direct effects that target the adverse proliferation of cancer cells while also inadvertently affecting cardiomyocytes and endothelial cells. The indirect targeting of these cells leads to the loss of their protective effects. For example, some nonanthracycline chemotherapeutic drugs can dysregulate endothelial nitric oxide synthase and induce protein kinase C resulting in coronary spasms and endothelium-independent vasoconstriction. Others can affect the coagulation cascade and predispose to thrombosis, exacerbate atrial fibrillation, or induce mitochondrial dysfunction [10]. Even though many mechanisms for cardiotoxicity have been described, we cannot affirm with certainty how melphalan and fludarabine act in the heart, and we believe the myocarditis could be caused by an indirect effect in light of the negative cardiac biomarkers.
Unfortunately, the therapy-induced cardiotoxicity of anticancer agents has limited their use to some extent and therefore necessitated the need for more effective risk prediction and more successful prevention techniques. Some methods to decrease the cardiotoxic effects include decreasing the dosage used, increasing the infusion time, and using liposomal encapsulated forms [2].
The treatment for chemotherapy-induced heart failure is not clear. Steroid use is still controversial in patients with myocarditis and there is no strong evidence to support their use in chemotherapy-induced cardiomyopathy, therefore we decided to not use. Standard heart failure guidelines include beta-blockers, diuretics, angiotensin-converting enzyme inhibitors (ACEIs), or angiotensin-II receptor blockers (ARBs) that should be used in accordance with the New York Heart Association functional class [5].
Studies are underway to evaluate the use of beta-blockers, ACEIs and ARBs, and statins to prevent the cardiotoxic effects of anticancer therapy. Predicting the pre-treatment risk for adverse cardiac events, early detection of these events, and prevention and treatment of cardiotoxicity are essential to the continued use of chemotherapies.
Conflict of interest
The authors have declared that no competing interests exist.
Funding
No financial support needed.
References
- 1.Plenderleith I. Treating the treatment: toxicity of cancer chemotherapy. Can Fam Physician. 1990;36:1827–1830. [PMC free article] [PubMed] [Google Scholar]
- 2.Bhave M., Shah A.N., Akhter N., Rosen S.T. An update on the risk prediction and prevention of anticancer therapy-induced cardiotoxicity. Curr Opin Oncol. 2014;26:590–599. doi: 10.1097/CCO.0000000000000132. [DOI] [PubMed] [Google Scholar]
- 3.Ritchie D.S., Seymour J.F., Roberts A.W., Szer J., Grigg A.P. Acute left ventricular failure following melphalan and fludarabine conditioning. Bone Marrow Transplant. 2001;28:101–103. doi: 10.1038/sj.bmt.1703098. [DOI] [PubMed] [Google Scholar]
- 4.Shakir D.K., Rasul K.I. Chemotherapy induced cardiomyopathy: pathogenesis, monitoring and management. J Clin Med Res. 2009;1:8–12. doi: 10.4021/jocmr2009.02.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kindermann I., Barth C., Mahfoud F., Ukena C., Lenski M., Yilmaz A. Update on myocarditis. JACC. 2012;59:779–792. doi: 10.1016/j.jacc.2011.09.074. [DOI] [PubMed] [Google Scholar]
- 6.Giralt S., Aleman A., Anagnostopoulos A., Weber D., Khouri I., Anderlini P. Fludarabine/melphalan conditioning for allogeneic transplantation in patients with multiple myeloma. Bone Marrow Transplant. 2002;30:367–373. doi: 10.1038/sj.bmt.1703652. [DOI] [PubMed] [Google Scholar]
- 7.Mak T.W., Saunders M.E. Transplantation. In: Mak T.W., Saunders M.E., editors. The immune response: basic and clinical principles. Elsevier Academic; New York: 2006. pp. 873–921. [Google Scholar]
- 8.Ghielmini M., Zappa F., Menafoglio A., Caoduro L., Pampallona S., Gallino A. The high-dose sequential (Milan) chemotherapy/PBSC transplantation regimen for patients with lymphoma is not cardiotoxic. Ann Oncol. 1999;10:533–537. doi: 10.1023/a:1026434732031. [DOI] [PubMed] [Google Scholar]
- 9.Spriano M., Clavio M., Carrara P., Canepa L., Miglino M., Perri I. Fludarabine in untreated and previously treated B-CLL patients: a report on efficacy and toxicity. Haematologica. 1994;79:2118–2124. [PubMed] [Google Scholar]
- 10.Madedd C., Deidda M., Piras A., Caddedu C., Demrtas L., Puzoni M. Pathophysiology of cardiotoxicity induced by nonanthracycline chemotherapy. J Cardiovasc Med. 2016;17:12–18. doi: 10.2459/JCM.0000000000000376. [DOI] [PubMed] [Google Scholar]