Abstract
Objectives
Specific challenge tests (SICs) are considered reference tests for allergic occupational diseases diagnosis. However, in numerous cases, SICs cannot be carried out in the diagnosis of allergy to latex due to the risk of generalized reactions. The aim of the study was to evaluate the usefulness of sIgE determination to recombinant latex allergens in diagnostics of occupational respiratory allergy.
Materials and Methods
The study group comprised 44 healthcare workers (HCW) suspected of suffering from occupational respiratory allergy to latex (they underwent a physical examination, skin‐prick tests (SPTs) to common and latex allergens, spirometry and SIC) and 17 controls not occupationally exposed to latex, with a positive sIgE against latex. Each serum was tested for allergen‐specific IgE to aeroallergens, latex, eight recombinant latex allergens and CCD‐markers.
Results
Specific IgE against Hev b5, 6.01, and 6.02 were significantly more frequently detected in HCWs and their mean serum levels were higher compared with the control group. In 26 HCWs with occupational asthma (OA), sensitization to Hev b5, Hev b6.01, Hev b6.02 was significantly more frequent than in 18 HCWs with work‐exacerbated asthma (WEA); they had positive results SPT to latex significantly more frequently in comparison with subjects with WEA.
Conclusions
Test for recombinant latex allergens is much more accurate in recognition of latex allergy than test for latex extract, which seems to produce false‐positive results in patients with pollen allergy. The measurements of sIgE against recombinant latex allergens Hev b 6.01, 6.02, 5, and 8 are useful in differentiating OA from WEA.
Keywords: airway allergy, diagnostic methods, healthcare workers, latex allergy, occupational allergy, recombinant latex allergens
1. INTRODUCTION
Natural rubber latex gloves have been in use in a healthcare setting since 1980. The average prevalence of latex allergy and sensitization worldwide remains 9.7% and 12.4% among healthcare workers, 7.2% and 30.4% among susceptible patients, and 4.3% and 2.1% among general population.1 After an intervention designed to reduce latex allergen exposure from gloves the prevalence of latex allergy among healthcare workers (HCWs) is falling in many countries; however, the use of other latex‐containing products (such as urinary catheters, oxygen facemasks, endotracheal tubes, laryngeal airways) is still popular in medical practice.2, 3
Due to potential anaphylactic reaction manifestation of latex allergy, in some cases the diagnosis of latex allergy should be based on a clinical history of latex allergy and positive laboratory tests. Skin prick tests (SPTs) with latex allergens are reported to have sensitivity of 67%‐89% and a specificity of 92%‐96%.4 Generally, the diagnostic sensitivity and specificity of SPTs evaluation is less than that of specific IgE (sIgE).3 In vitro tests with the improved natural latex extract spiced with the recombinant Hev b5 b (Hevea brasiliensis) latex allergen have sensitivity of 90% and specificity of 98.3%.3 They are highly sensitive in demonstrating sensitization in exposed patients; however, the tests do not differentiate between true latex allergy and cross‐reactivity, and sometimes produce false negative or false‐positive results.3, 5, 6
Specific challenge tests (bronchial, nasal, conjunctival) are considered as reference tests for allergic occupational diseases diagnosis due to their high sensitivity and specificity.7 For example, a nasal provocation test with latex allergens has a sensitivity of 96% and specificity of 100%.8 Moreover, the conjunctival allergen challenge test is reported to be the most sensitive diagnostic method in latex eye allergy (92% sensitivity and 100% specificity), while the sensitivity of skin prick test with latex is 84% and of sIgE—88%.9
On the other hand, some data show that serum sIgE against latex are often found in individuals without overt latex allergy.6, 10 According to other authors, as far as latex allergy is concerned, results of sIgE test should be used only for screening purposes, but not for reliable diagnosis of occupational latex allergy in HCWs. The determination of specific IgE to latex in vitro explains little in terms of clinical relevance, so it is difficult to distinguish between clinically significant latex allergy and asymptomatic sensitization.11
Generally, recognition of occupational respiratory allergy is based on a positive result of specific inhalation challenge tests (SICs).7 Although, in numerous cases, SICs cannot be carried out in the diagnosis of allergy to latex due to the risk of generalized reactions.12 Nevertheless, diagnosis of occupational allergy needs to use additional reliable, objective methods with high specificity and sensitivity to be able to establish a certain diagnosis.
Application of recombinant allergens in the diagnostics of allergy, including occupational one, is more and more prevalent. Currently, 15 latex allergens Hev b (Hevea brasiliensis allergens)—Hev b1 to Hev b15—have been described and denominated by the WHO International Union of Immunological Societies Allergen Nomenclature Committee (http://www.allergen.org). Most of them may be used for in vitro diagnosis.10, 11, 12 By using an appropriate panel of recombinant latex allergens, cross‐reactivity can be excluded and/or specific sensitization can be confirmed, without the necessity of the SICs performance. Moreover, in vitro tests with recombinant latex allergens could be advised in patients with a potential risk of generalized reactions.12
The aim of the study was to assess the profile of recombinant latex allergens among HCWs to evaluate the value of recombinant IgE in determination of occupational allergic disease and hypersensitivity to latex among healthcare workers with work‐related respiratory symptoms.
2. MATERIAL AND METHODS
The study group comprised 44 healthcare workers with the sIgE against natural rubber latex present in serum. They were selected from 107 HCWs diagnosed due to a suspicion of occupational respiratory allergy to latex.
The control group consisted of 17 atopic subjects without clinical manifestation of latex allergy and not occupationally exposed to latex (data from the questionnaire) but with sIgE against natural rubber latex present in serum.
The exclusion criterion for the study was the continuous use of antihistamines, oral corticosteroids, and antidepressants.
2.1. Clinical symptoms
The questionnaire, performed by a physician among all patients, included questions on ocular, skin, and respiratory (rhinitis, cough, wheezing, shortness of breath, chest tightness) symptoms, especially those due to latex, family history of atopy, medication use, tobacco smoking habit, and exposure to pet allergens at home.
2.2. Spirometry
Spirometry using the Jaeger Master Scope Spirometer (VIASYS HealthCare, Germany) was performed in all the subjects in accordance with the American Thoracic Society (ATS) and European Respiratory Society (ERS) guidelines.13
2.3. Skin prick test
SPTs were performed with the following aeroallergens: Dermatophagoides pteronyssinus, Dermatophagoides farinae, Acarus siro, Thyrophagus putrescentiae, Lepidoglyphus destructor, mixed feathers, grass pollens, tree pollens, weeds, molds, dog, cat (Allergopharma, Reinbek, Germany). Histamine (10 mg/mL) (Allergopharma, Reinbeck, Germany), and glycerosaline solution was used as a positive and a negative control, respectively.
Latex skin prick tests were performed using Alyostal 903 Latex (Stallergenes, France) among 36 HCWs (only in the patients with a negative history of generalized allergic reactions) and among 17 individuals from the control group. Histamine (10 mg/mL) (Stallergenes, France) and phenolated glycerosaline solution were used as positive and negative controls, respectively. The largest wheal diameter was assessed after 15 min. A wheal diameter of ≥3 mm and equal to or greater than half of that formed by histamine was defined as positive, indicating sensitization.4
2.4. In vitro tests
Samples of the serum were collected, secured, and stored at −70°C for further study. All sera were analyzed for total IgE and specific IgE against common aeroallergens: mixed grass and tree pollens (gx1, tx1, tx9), latex (k82), recombinant latex allergens (rHev b1, rHev b3, rHev b5, rHev 6.01, rHev 6.02, rHev b8, rHev b9, rHev b11), cross‐reactive carbohydrate determinant (CCD)‐markers [Ro214—MUXF3 (sugar‐epitope from Bromelain) and HRP (Ro400—horse radish peroxidase)] and profiling rBet v2 (t216—Birch, Betulaverrucos). Maltose‐binding protein (MBP as fusion component) was used as a negative control.
Specific IgE antibodies and total IgE were measured in sera by the use of the ImmunoCAP 100 System (Thermo Fisher Scientific, Phadia, Uppsala, Sweden). Specific IgE values equal to or greater than 0.35 kUA/L were considered positive. Total IgE levels higher than 100 kU/L were considered elevated.
2.5. SIC with latex
In 40 of 44 HCWs (with sIgE to latex in serum) a specific challenge test with latex allergens was conducted using a standardized protocol in a special challenge room (room space 6 m2 with temperature 22‐25°C). The patient was handling and shaking powdered latex gloves starting with two gloves. After each 5 minutes the next pair of gloves was given to the patient, who continued handling and shaking them for 60 min or until the symptoms were observed. The SIC was monitored for functional tests. The day before, there was a “control” day consisting in exposing the subject to a “control” substance (vinyl gloves) for 60 min and monitoring the functional parameters in a similar way as in the case of a “challenge” day. The positive SIC reaction was evaluated according to a previously described protocol and international recommendations.7, 14
2.6. Diagnostic criteria
The patients were considered to have respiratory allergic disease to latex if they had developed clinical manifestations (respiratory symptoms—cough, dyspnea, rhinitis) following contact with latex, and if they had a positive SPT results with latex and/or specific IgE antibodies to latex in serum.
Occupational asthma (OA) to latex among the HCWs was diagnosed when the SIC was positive.7, 15 Recognition of OA in the patients with contraindications to SIC was based on a positive work‐related history of latex allergy manifestation, the presence of positive SPT results and/or sIgE to latex, positive bronchodilator test [increase in FEV1 (forced expiratory volume in 1 second) and/or FVC (forced vital capacity) ≥12% and ≥ 200 ml increase compared with baseline], having an asthma attack or use of asthma medications in the past 12 months [Henneberger et al, 2011]. The subjects with worsening of their asthma symptoms at work with a negative SIC were defined as work‐exacerbated asthma (WEA).16
In our study, SIC test was carry out in the 40 (90.9%) HCWs with a negative history of anaphylaxis and without spirometric contraindication to performed the SIC. The SIC with latex allergens was not performed in three subjects due to anaphylactic reaction in history and in one case due to low spirometric values. Twenty‐two subjects showed a positive SIC response in terms of changes in FEV1, while 18 subjects showed a negative SIC response. In four cases the OA due to latex was recognized based on clinical history and proved sensitization to latex, WEA was recognized in 18 subjects in negative results of SIC with latex allergens.
2.7. Statistics
Statistical analyses were performed using Statistica 8. Continuous variables were expressed as mean values ± standard deviations, while the nominal variables as numbers and percentages. The Chi‐square test (or Fisher's exact test) was used to compare the HCWs and the control group as well as the HCWs with OA to latex and work‐exacerbated respiratory symptoms. A P‐value of <0.05 was considered as significant.
3. RESULTS
The study group consisted of nine (20.5%) males and 35 (79.6%) females, while the control group consisted of eight (47.1%) males and nine (52.9%) females. The mean age was 43.6 ± 8.8 years in the study group and 30.8 ± 11.3 years in the control one. The study population characteristics are shown in Table 1. A positive history of atopy was found in 10 (22.7%) HCWs and in six (35%) controls. The results of SPTs to common allergens, latex, the total IgE and sIgE to latex levels in serum are presented in Table 2.
Table 1.
Characteristics of the study population: the HCWs and the control group
|
Analyzed parameter [N (%)] |
Study group of HCWs N = 44 |
Control group N = 17 (100%) |
||
|---|---|---|---|---|
|
With OA N = 26 (100%) |
With WEA N = 18 (100%) |
All N = 44 (100%) |
||
| Age (y) (mean ± SD) | 42.54 ± 7.5 | 41.67 ± 9.6 | 43.6 ± 8.77 | 30.8 ± 11.32 |
| Sex | ||||
| Men | 2 (7.7%) | 7 (38.9%)a | 9 (20.5%) | 8 (47.1%) |
| Women | 24 (92.3%) | 11 (61.1%)a | 35 (79.6%) | 9 (52.9%) |
| Smoking status | ||||
| Current smoker | 3 (11.5%) | 1 (5.6%) | 4 (9.1%) | 2 (11.8%) |
| Ex‐smoker | 9 (34.6%) | 2 (11.1%) | 11 (25.0%) | 2 (11.8%) |
| Non‐smoker | 14 (53.9%) | 15 (83.3%) | 29 (65.9%) | 13 (76.5%) |
| Family history of atopy | 9 (34.6%)a | 1 (5.6%) | 10 (22.7%) | 6 (35.3%) |
| Animals at home | 3 (11.5%) | 3 (16.7%) | 6 (13.6%) | 2 (11.8%) |
| Symptoms associated with exposure to latex | ||||
| At least one symptom from the respiratory tract | 26 (100%) | 18 (100%) | 44 (100%) | 0 |
| Cough | 19 (73.1%) | 12 (66.7%) | 31 (70.5%) | 0 |
| Dyspnea | 22 (84.6%) | 6 (33.3%) | 28 (63.6%) | 0 |
| Nasal symptoms | 22 (84.6%) | 4 (22.2%) | 26 (59.1%) | 0 |
| Eye symptoms | 15 (57.7%) | 1 (5.6%) | 16 (36.4%) | 0 |
| Skin symptoms | 14 (53.9%) | 7 (38.9%) | 21 (47.7%) | 0 |
| Anaphylaxis | 3 (11.5%) | 0 | 3 (6.8%) | 0 |
Abbreviations: OA, occupational asthma; due to latex occupational allergy; HCWs, health‐care workers; WEA, work‐exacerbated asthma.
P < 0.05; The Chi‐square test (or Fisher's exact test) was used to compare the HCWs and the control group as well as the HCWs with OA to latex and work‐exacerbated respiratory symptoms.
Table 2.
The results of skin prick tests (SPTs) to common allergens, latex, and evaluation of the total IgE in the HCWs with occupational asthma to latex (OA), in the subjects with work‐exacerbated asthma (WEA) and in the control group
| SPT results [N (%)] |
Study group of HCWs N = 44 |
Control group N = 17 (100%) |
||
|---|---|---|---|---|
|
With OA N = 26 (100%) |
With WEA N = 18 (100%) |
All N = 44 (100%) |
||
| At least one SPT positive to a common allergen | 17 (65.4%)a | 5 (27.8%) | 22 (50.0%) | 9 (52.9%) |
| SPT positive to | ||||
| Mixed feathers | 2 (7.7%) | 0 | 2 (4.6%) | 1 (5.9%) |
| Grass pollens | 7 (26.9%) | 1 (5.6%) | 8 (18.2%) | 8 (47.1%)a |
| Mixed tree pollens I* | 7 (26.9%) | 0) | 7 (15.9%) | 6 (35.3%) |
| Mixed tree pollens II** | 7 (26.9%) | 1 (5.6%) | 8 (18.2%) | 6 (35.3%) |
| Mixed moulds I* | 0 | 0 | 0 | 2 (11.7%) |
| Mixed moulds II** | 1 (3.9%) | 0 | 1 (2.3%) | 1 (5.9%) |
| Dermatophagoides pteronyssinus | 8 (30.8%) | 4 (22.2%) | 12 (27.3%) | 3 (17.7%) |
| Dermatophagoides farinae | 7 (26.9%) | 3 (16.7%) | 10 (22.7%) | 1 (5.9%) |
| Weeds | 6 (23.1%) | 2 (11.1%) | 8 (18.2%) | 5 (29.4%) |
| Acarus siro | 6 (23.1%) | 2 (11.1%) | 8 (18.2%) | 0 |
| Lepidoglyphus destructor | 7 (26.9%) | 3 (16.7%) | 10 (22.7%) | 1 (5.9%) |
| T putrescientiae | 6 (23.1%) | 2 (11.1%) | 8 (18.2%) | 0 |
| Cat dander | 2 (7.7%) | 0 | 2 (4.6%) | 0 |
| Dog dander | 1 (3.9%) | 0 | 1 (2.3%) | 0 |
| Positive SPT results to latex | 24 (92.3%)a | 12 (66.7%) | 36 (81.8%) | 0 |
|
Total IgE level (kU/L) (mean ± SD) Total IgE > 100 kU/L |
187.94 ± 235.2 11 (42.3%) |
148.22 ± 117.7 10 (55.6%) |
162.1 ± 189.9 21 (47.7%) |
315.5 ± 216.4 14 (82.4%)a |
| sIgE to latex range (kUA/L) | ||||
| 0.35‐0.7 | 5 (19.2%) | 6 (33.3%) | 11 (25.0%) | 4 (23.5%) |
| 0.71‐3.5 | 11 (42.3%) | 8 (44.4%) | 19 (43.2%) | 12 (70.6%) |
| 3.51‐17.5 | 8 (30.8%) | 4 (22.2%) | 12 (27.3%) | 1 (5.9%) |
| 17.5‐100 | 2 (7.7%) | 0 | 2 (4.6%) | 0 |
| ≥100 | 0 | 0 | 0 | 0 |
Abbreviations: HCWs, health‐care workers; OA, occupational asthma, due to latex occupational allergy; SPT, skin prick test; WEA, work‐exacerbated asthma.
Mixed tree pollens I*:( alder, hazel poplar, elm, willow), mixed tree pollens I**(birch, beech, oak, plane‐tree), mixed moulds I* (Alternaria tenuis, Botrytis cinerea, Cladosporium. herbarum, Curvularia lunata, Helminthosporium halodes, Fusarium moniliforme), mixed moulds II** (Aspergillus fumigatus, Mucor mucedo, Penicillium notatum, Pullularia pullulans, Rhizopus nigricans, Serpula lacrymans), mixed feathers (hen, goose, duck), SPT with latex was caried out only in patients with a negative history of generalized allergic reactions.
P<0.05; The Chi‐square test (or Fisher's exact test) was used to compare the HCWs and the control group as well as the HCWs with OA to latex and work‐exacerbated respiratory symptoms.
Specific IgE against Hev b5, 6.01, 6.02 were detected significantly more frequently in the study population and their mean serum levels were higher compared to the control group (P < 0.05), (Figure 1).
Figure 1.

Mean level of specific IgE levels to recombinant latex allergens in the study group and in the control group. *P < 0.05; The Chi‐square test (or Fisher’s exact test) was used to compare all HCWs and the control group. Abbreviations: HCWs, health‐care workers; rHev b, recombinant Hevea brasiliensis allergen; sIgE, specific IgE antibody
Among the HCWs with positive sIgE to latex, six subjects were sensitized to Hev b8 (four monosensitized), while in the control group 7 individuals were sensitized to Hev b8 (five monosensitized) and all of them were allergic to pollen allergens.
Positive SPT results to latex were found in 36 (81.8%) of all the subjected HCW. In our study, sensitization to Hev b5, Hev b6.01, Hev b6.02, and positive results of SPT to latex were reported significantly more frequent in the subjected HCWs with OA than in the subjected HCWs with WEA (Table 3.) However, in the control group the sIgE to Hev b8 was found statistically more frequent than in the HCWs. Thirteen patients (76%) from the control group were atopic based on the positive results of the Phadiatop test (Figure 2). Profilin sensitization to rBet v2 and Hev b8 was significantly more frequent in the control group in comparison with the corresponding parameters in the study group of HCWs. Sensitization towards CCD markers: HRP and MUXF3 in the controls was higher, but it did not reach the significance level.
Table 3.
The number of subjects with positive sIgE antibody to recombinant allergens in the HCWs with occupational asthma to latex (OA), in the subjects with work‐exacerbated asthma (WEA)
| Recombinant allergens |
Study group of HCWs N = 44 |
Control group N = 17 (100%) |
||
|---|---|---|---|---|
|
With OA N = 26 (100%) |
With WEA N = 18 (100%) |
All N = 44 (100%) |
||
| Hev b1 | 2 (7.7%) | 0 | 2 (4.6%) | 0 |
| Hev b3 | 1 (3.9%) | 2 (11.1%) | 3 (6.8%) | 2 (11.8%) |
| Hev b5 | 14 (53.9%)a | 1 (5.6%) | 15 (34.1%)a | 0 |
| Hev b6.01 | 16 (61.5%)a | 4 (22.2%) | 20 (45.5%)a | 1 (5.9%) |
| Hev b6.02 | 15 (57.7%)a | 3 (16.7%) | 18 (40.9%)a | 1 (5.9%) |
| Hev b8 | 3 (11.5%) | 3 (16.7%) | 6 (13.6%) | 7 (41.2%)a |
| Hev b9 | 0 | 1 (5.6%) | 1 (2.3%) | 0 |
| Hev b11 | 4 (15.4%) | 1 (5.6%) | 5 (11.4%) | 0 |
| MUXF3(CCD‐ marker) | 2 (7.7%) | 1 (5.6%) | 3 (6.8%) | 4 (23.5%) |
| HRP(CCD‐ marker) | 2 (7.7%) | 3 (16.7%) | 5 (11.4%) | 5 (29.4%) |
Abbreviation: CCD, cross‐reactive carbohydrate determinant; HCWs, health‐care workers; Hev b, recombinant Hevea brasiliensis allergen; HRP, horse radish peroxidase; MUXF3, sugar‐epitope from Bromelain; OA, occupational asthma, due to latex occupational allergy; WEA, work‐exacerbated asthma.
P < 0.05; The Chi‐square test (or Fisher's exact test) was used to compare the HCWs and the control group as well as the HCWs with OA to latex and work‐exacerbated respiratory symptoms.
Figure 2.
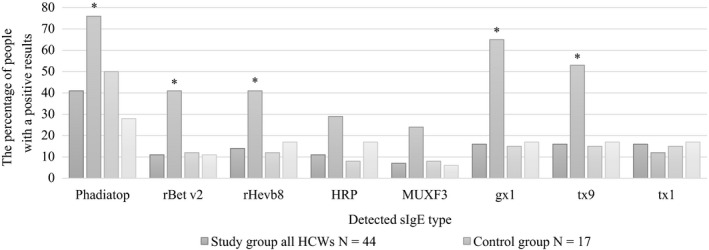
Comparison of IgE antibody analysis in the HCWs with occupational asthma to latex (OA) and in the subjects with work‐exacerbated asthma (WEA) and in the control group. *P < 0.05; The Chi‐square test (or Fisher’s exact test) was used to compare the HCWs and the control group as well as the HCWs with OA to latex and work‐exacerbated respiratory symptoms. Abbreviations: due to latex occupational allergy; gx1, mixed grass (Dactylis glomerata, Festuca elatior, Lolium perenne, Phleum pratense, Poa pratensis) tx9‐ mixed tree pollens (Alnus incana, Betula verrucosa, Corylus avellana, Quercus alba, Salix caprea) tx1‐ mixed tree pollens (Acer negundo, Betula verrucosa, Querbus alba, Ulmus americana, Juglans californica); HCWs, health‐care workers; HRP, horse radish peroxidase; MUXF3, sugar‐epitope from Bromelain; OA, occupational asthma; Phadiatop, a multiaeroallergen screen test; rBetv2, profilin Birch, Betula verrucosa); rHev b8, recombinant Hevea brasiliensis allergen; sIgE, specific IgE antibody; WEA, work‐exacerbated asthma
4. DISCUSSION
HCWs are usually sensitized to Hev b2, Hev b5, Hev b6.02, and Hev b13 allergens.6, 11 Sastre et al17 have evaluated the profile of sensitization to natural and recombinant natural rubber latex allergens among HCWs and presented that Hev b6.01 and Hev b5 were major allergens in this population. German authors have concluded that Hev b 2, 5, 6.01, and 13 are the major allergens for HCW and spina bifida patients, while the Hev b1 has been identified as a major allergen only for spina bifida subjects. It was proposed that this allergen panel should be present in the standardized latex extract in vitro and in vivo diagnostics.11 Similarly, Kurup et al have shown that the combination of Hev b2,5,6, 13 had detected over 80% HCWs with latex allergy.6 Yagami et al18 have revealed that HCWs with occupational allergy have been sensitized to Hev b6.02 and Garnier et al have shown that Hev b6.01, 6.02, 2, and 5 were the major allergens in the population suspected of being allergic to latex. Furthermore, they have concluded that Hev b5 was particularly useful in the case where clinical symptoms and SPT result were discordant.19
In our population of HCWs the most common recombinant allergens identified were: Hev b5, Hev b6.01, Hev b6.02. We found sIgE to Hev b5 in over 34.1% of the HCWs, while sIgE to Hev b6.01 in 45.5% and sIgE to Hev b6.02 in 40.9% of the cases. Our results are in agreement with outcomes of other researchers, but the frequency of sIgE detected was lower than the one determined in other studies that have found rates of sIgE to Hev b5, 6.01, 6.02 to be between 50%‐85%.6, 11, 19 Similarly, Ott et al have revealed that the prevalence of sIgE antibodies against Hev b6.02 was 52%, Hev b5%‐50%, Hev b8%‐15% in patients suffering from an immediate type of natural rubber latex allergy.20 In contrast, in the same study only 6% of sera samples from control individuals were Hev b6.01, 6.02 sIgE positive. In the control group, Hev b8 (41.2%) was the most common allergen yielding positive results.
In our study the HCWs diagnosed as sensitive to Hev b5, Hev b6.01, Hev b6.02 had positive SPT results to latex and clinically significant latex allergy. This data showed good concordance between both tests and confirmed the results obtained by other researches.21
Moreover, in the subjects with OA the sIgE to Hev b5, Hev b6.01, Hev b6.02, were found statistically more frequently than in the subjects with WEA. A similar correlation has been observed by Vandenplas et al.12 Although sensitization to Hev b5, Hev b6.01, Hev b6.02 was more frequent in the subjects with OA than in those with WEA, determinations of sIgE to latex in serum did not allow accurate differentiation of OA from WEA. However, Vandenplas et al have revealed that high levels of sIgE against rHev b5 combined with the presence of Hev b6.01 or 6.02 are the most efficient predictors of a bronchial response to natural rubber latex in sensitized patient. What is more, subjects with a positive SIC with latex showed a significantly higher rate of sIgE reactivity to Hev b5, 6.01, 6.02, and 11 than those with a negative SIC, which strongly supports a diagnosis of latex‐induced OA12 as shown in Table 3. Nevertheless, SIC tests are still the most reliable tests used to distinguish OA from WEA.16 On the other hand, in the patients with contraindications for SICs with latex allergens alternative diagnostic methods such as immunologic tests with recombinant latex allergens are necessary.
An important issue in latex allergy is cross‐reactivity with other allergens. It has been proved that in atopic individuals, especially those sensitized to pollen, fruit and insect venoms false‐positive results of serological tests to latex may be found.5, 11 The major cause for false‐positive latex sIgE results seems to be sensitization to profilin (Hev b8) and CCD. The Hev b8 antigen is related to cross‐reactivity to exotic fruit and pollens.19 Hence, the clinical relevance of Hev b8 (latex profilin) is still unclear. Some reports have shown that patients monosensitized to latex profiling (Hev b8) may undergo exposure to latex without any consequences.22, 23 In our study 41.2% (7/17) of the control subjects had positive sIgE to Hev b8, but they had negative results of SPTs to latex and no clinical symptoms due to latex exposure. Five of these cases were monosensitized to Hev b8 and all of them had positive sIgE to birch pollen profilin (rBet v2), grass pollens (gx1), and tree pollens (tx9), moreover they had positive SPTs results to grass pollen. Generally, 65% (11/17) of our control individuals had positive sIgE to grass pollens and 53% (9/17) to tree pollens, which is in concordance with the other studies, reporting that sensitization to Hev b8 is common among subjects with positive sIgE to grass and/or tree pollen, and thus, implying sensitization to cross‐reactive plant panallergens.20, 24
In our study group of HCWs, 13.6% (6/44) of the cases had positive results to Hev b8. Four of them were monosensitized to Hev b8, one patient additionally had a positive result to Hev b5, 6.01, 6.02, and another one had sIgE to Hev b8 and Hev b3. In five subjects positive results sIgE to rBet v2 profilin were found. Two subjects monosensitized to Hev b8 had positive result to rBet v2 and negative SIC to latex (the subjects with WEA). We suppose that Hev b8 has been related to pollen sensitization. Ganglberger et al have tested the sera of 50 HCWs; 12 patients sensitized to Hev b8 and all Hev b8 simultaneously showed allergic symptoms to pollen or plant foods.25 The other study performed in 10 individuals with the presence of sIgE to latex and positive results of SPT with latex extracts has revealed that none of them had presented IgE reactivity to Hev b8, and that nine of them had positive glove‐wearing test. In contrast, seven patients monosensitized to Hev b8 had negative glove‐wearing test and all of them had sIgE to profilins rBet v2 and rPhl p12 (timothy grass pollen profiling).22 Our findings are consistent with other studies and confirm that the presence of sIgE for recombinant latex allergens in serum allows differentiation between clinical latex allergy and cross‐sensitization to pollen allergens. These data suggest that profilin sensitization determines false‐positive results in vitro diagnostic tests, as there is a high homology between latex profilin and birch pollen profiling.26
Also CCDs can yield a false‐positive result of sIgE to latex.10, 27 Many plant allergens are glycoproteins, for example, latex allergen—Hev b2. It is still a matter under discussion if sIgE to CCD plays a role in the diagnosis of occupational allergy.24, 28 According to Wiszniewska et al, the presence of sIgE to CCD in serum was not associated with occupational respiratory allergy.29
In our study there were 11.4% (5/44) of the HCWs with positive results of sIgE to HRP (horseradish peroxidase—CCD) and 6.8% (3/44) with sIgE to MUXF3 (CCD markers). Similar observations have been described by other research, for example, German authors have shown that 8.3% of HCWs with latex allergy had positive sIgE to CCDs.11 According to the authors, CCDs were only of minor relevance in patients with clinical relevant latex allergy. Another study has revealed that some sera of latex‐allergic patients contained sIgE to bromelain or sIgE to HRP.24 Further studies in a group of CCD‐positive patients with venom allergy have confirmed that CCD‐dependent reactivity with latex allergens is not associated with clinical hypersensitivity.27
Quirce et al have shown that CCDs may interfere in the diagnosis of occupational allergy giving false‐positive results sIgE in serum and recognition of these components is significant.28 Also Ebo et al have suggested including a cocktail of CCD markers, which would allow discrimination between clinically relevant and irrelevant sIgE to latex.24 According to other authors, a solution to the problem is to use non‐glycosylated recombinant latex allergens in the applied tests.11
In our study, among control individuals with sIgE to latex, five subjects (29.4%) were positive to HRP and four (23.5%) were positive to MUXF3. All of them had sIgE to grass and/or tree pollens and they did not show any symptoms upon contact with latex. This is in concordance with previous reports describing sensitization to pollen and latex.24, 30 Ebo et al have reported that 3/22 (14%) of sera from latex‐sensitized individuals were positive for bromelain and 8/20 (40%) for HRP.24 Other studies have reported that patients with positive results to CCD markers show sensitivity to pollens, food, latex, insect venoms, but do not show clinical hypersensitivity to the majority of these allergens.30
The limitation of our study is that we could not measure Hev b2 and Hev b13 allergens, as at the time of the study those allergens were not available for in vitro diagnosis. The number of examined subjects is relatively low; however, the prevalence of latex allergy has decreased significantly during the last decade.
In conclusion, allergy profile among HCWs and in the control individuals without latex‐induced symptoms, but with positive sIgE against latex is different. The results indicate that Hev b 6.01, 6.02, and 5 were prevalent in the HCWs comparing to the control group, furthermore, in the HCWs with latex occupational respiratory allergy Hev b5, 6.01, 6.02 were more prevalent than in the subjects with work‐exacerbated asthma.
Evaluation of sIgE reactivity to individual latex allergens can detect sensitization to panallergens and can facilitate the final diagnosis of latex allergy. Our results indicate that the ImmunoCAP test for recombinant latex allergens is much more accurate in recognition of true occupational latex allergy than the ImmunoCAP test for latex extract spiked with Hev b5, which seems to produce some false‐positive results in patients with pollen allergy and does not differentiate between genuine allergy and cross‐reactivity. Moreover, the measurements of sIgE against recombinant latex allergens Hev b6.01, 6.02, and 5 are useful in differentiating OA from WEA.
In the HCWs with positive sIgE to latex and negative/not available SPT to latex the ImmunoCAP tests containing recombinant latex allergens Hev b5, 6.01, 6.02, 8 and CCD markers should be performed to distinguish allergy and cross‐reactivity.
DISCLOSURE
Approval of the research protocol: The Regional Bioethical Committee approved the study protocol (approval decision number 8/2012). Informed consent: All of the participants gave their informed consent prior to the commencement of the study. Registry and the registration no. of the study/trial: This study was on internal grant number 11.3/2012. Animal Studies: N/A. Conflict of interest: The authors declare no conflict of interest.
AUTHOR CONTRIBUTION
Ewa Nowakowska‐Świrta, MSc was involved in concept, collecting material, and interpretation of results. Marta Wiszniewska, PhD was involved in concept, statistical analysis, and research methodology. Prof. Jolanta Walusiak‐Skorupa, MD, PhD was involved in concept, interpretation of results, and research methodology.
Nowakowska‐Świrta E, Wiszniewska M, Walusiak‐Skorupa J. Allergen‐specific IgE to recombinant latex allergens in occupational allergy diagnostics. J Occup Health. 2019;61:378–386. 10.1002/1348-9585.12064
REFERENCES
- 1. Wu M, McIntosh J, Liu J. Current prevalence rate of latex allergy: why it remains a problem? J Occup Health. 2016;58(2):138‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vandenplas O, Raulf M. Occupational latex allergy: the current state of affairs. Curr Allergy Asthma Rep. 2017;17(3):14. [DOI] [PubMed] [Google Scholar]
- 3. Raulf M. Latex allergy. Pediatr Allergy Immunol. 2016;27(suppl 23):165‐170. [Google Scholar]
- 4. van Kampen V, de Blay F, Folletti I, et al. EAACI position paper: skin prick testing in the diagnosis of occupational type I allergies. Allergy. 2013;68(5):580‐584. [DOI] [PubMed] [Google Scholar]
- 5. Ünsel M, Mete N, Ardeniz Ö, Sin A, Gülbahar O, Kokuludağ A. Diagnostic value of specific IgE analysis in latex allergy. Int Arch Allergy Immunol. 2012;158(3):281‐287. [DOI] [PubMed] [Google Scholar]
- 6. Kurup VP, Sussman GL, Yeang HY, et al. Specific IgE response to purified and recombinant allergens in latex allergy. Clin Mol Allergy. 2005;3(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vandenplas O, Suojalehto H, Aasen TB, et al. Specific inhalation challenge in the diagnosis of occupational asthma: consensus statement. Eur Respir J. 2014;43(6):1573‐1587. [DOI] [PubMed] [Google Scholar]
- 8. Ünsel M, Mete N, Ardeniz Ö, et al. The importance of nasal provocation test in the diagnosis of natural rubber latex allergy. Allergy. 2009;64(6):862‐867. [DOI] [PubMed] [Google Scholar]
- 9. Chelminska M, Niedoszytko M, Jassem E. Clinical value of conjunctival allergen challenge in diagnosing allergic conjunctivitis related to latex. Int Arch Allergy Immunol. 2011;154(2):149‐154. [DOI] [PubMed] [Google Scholar]
- 10. Mari A, Scala E, D’Ambrosio C, Breiteneder H, Wagner S. Latex allergy within a cohort of not‐at‐risk subjects with respiratory symptoms: prevalence of latex sensitization and assessment of diagnostic tools. Int Arch Allergy Immunol. 2007;143(2):135‐143. [DOI] [PubMed] [Google Scholar]
- 11. Raulf‐Heimsoth M, Rihs H‐P, Rozynek P, et al. Quantitative analysis of immunoglobulin E reactivity profiles in patients allergic or sensitized to natural rubber latex (Hevea brasiliensis). Clin Exp Allergy. 2007;37(11):1657‐1667. [DOI] [PubMed] [Google Scholar]
- 12. Vandenplas O, Froidure A, Meurer U, et al. The role of allergen components for the diagnosis of latex‐induced occupational asthma. Allergy. 2016;71(6):840‐849. [DOI] [PubMed] [Google Scholar]
- 13. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319‐338. [DOI] [PubMed] [Google Scholar]
- 14. Wiszniewska M, Nowakowska‐Świrta E, Pałczyński C, Walusiak‐Skorupa J. Diagnosing of bakers' respiratory allergy: Is specific inhalation challenge test essential? Allergy Asthma Proc. 2011;32(2):111‐118. [DOI] [PubMed] [Google Scholar]
- 15. Tarlo S, Lemiere C. Occupational asthma. N Engl J Med. 2014;370(7):640‐649. [DOI] [PubMed] [Google Scholar]
- 16. Henneberger PK, Redlich CA, Callahan DB, et al. An official American Thoracic Society Statement: work‐exacerbated asthma. Am J Respir Crit Care Med. 2011;184(3):368‐378. [DOI] [PubMed] [Google Scholar]
- 17. Sastre J, Raulf‐Heimsoth M, Fernandez‐Nieto M, et al. Profile of sensitization to individual latex allergens among health care workers allergic to natural rubber latex. J Allergy Clin Immun. 2004;113(2):S76‐S77. [Google Scholar]
- 18. Yagami A, Suzuki K, Saito H, Matsunaga K. Hev b 6.02 Is the most important allergen in Health Care Workers sensitized occupationally by natural rubber latex gloves. Allergol Int. 2009;58(3):347‐355. [DOI] [PubMed] [Google Scholar]
- 19. Garnier L, Selman L, Rouzaire P, et al. Molecular allergens in the diagnosis of latex allergy. Eur Ann Allergy Clin Immunol. 2012;44(2):73‐79. [PubMed] [Google Scholar]
- 20. Ott H, Schroder C, Raulf‐Heimsoth M, et al. Microarrays of recombinant hevea brasiliensis proteins: a novel tool for the component‐resolved diagnosis of natural rubber latex allergy. J Investig Allergol Clin Immunol. 2010;20(2):129‐138. [PubMed] [Google Scholar]
- 21. Schuler S, Ferrari G, Schmid‐Grendelmeier P, Harr T. Microarray‐based component‐resolved diagnosis of latex allergy: isolated IgE‐mediated sensitization to latex profilin Hev b8 may act as confounder. Clin Transl Allergy. 2013;3(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Quercia O, Stefanini GF, Scardovi A, et al. Patients monosensitised to Hev b 8 (Hevea brasiliensis latex profiling) may safely undergo major surgery in a normal (non‐latex safe) environment. Eur Ann Allergy Clin Immunol. 2009;41(4):112‐116. [PubMed] [Google Scholar]
- 23. Antonicelli L, Micucci C, Mistrello G, et al. Improving latex‐allergy diagnosis: the clinical role of Hev b8‐specific IgE. Allergy. 2008;63(5):620‐621. [DOI] [PubMed] [Google Scholar]
- 24. Ebo DG, Hagendorens MM, De Knop KJ, et al. Component‐resolved diagnosis from latex allergy by microarray. Clin Exp Allergy. 2010;40(2):348‐358. [DOI] [PubMed] [Google Scholar]
- 25. Ganglberger E, Radauer C, Wagner S, et al. Hev b 8, the Hevea brasiliensis latex profilin, is a cross‐reactive allergen of latex, plant foods and pollen. Int Arch Allergy Immunol. 2001;125(3):216‐227. [DOI] [PubMed] [Google Scholar]
- 26. Hauser M, Roulias A, Ferreira F, Egger M. Panallergens and their impact on the allergic patient. Allergy Asthma Clin Immunol. 2010;6(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mahler V, Gutgesell C, Valenta R, Fuchs T. Natural rubber latex and hymenoptera venoms share immunoglobinE‐epitopes accounting for cross‐reactive carbohydrate determinants. Clin Exp Allergy. 2006;36(11):1446‐1456. [DOI] [PubMed] [Google Scholar]
- 28. Quirce S, Salcedo G. The role of cross‐reactive carbohydrate determinants in the diagnosis of occupational allergy. Clin Exp Allergy. 2010;40(7):962‐964. [DOI] [PubMed] [Google Scholar]
- 29. Wiszniewska M, Zgorzelska‐Kowalik J, Nowakowska‐Świrta E, Walusiak‐Skorupa J. Identification of cross‐reactive carbohydrate determinants in subjects reporting work‐related respiratory symptoms. Int J Occup Med Environ Health. 2015;28(1):90‐101. [DOI] [PubMed] [Google Scholar]
- 30. Ebo DG, Hagendorens MM, Bridts CH, De clerck LS, Stevens WJ. Sensitization to cross‐reactive carbohydrate determinants and the ubiquitous protein profilin: mimickers of allergy. Clin Exp Allergy. 2004;34(1):137‐144. [DOI] [PubMed] [Google Scholar]


