Abstract
Context: We sought to describe our experience with the Hybrid Assistive Limb® (HAL®) for active knee extension and voluntary ambulation with remaining muscle activity in a patient with complete paraplegia after spinal cord injury.
Findings: A 30-year-old man with complete paraplegia used the HAL® for 1 month (10 sessions) using his remaining muscle activity, including hip flexor and upper limb activity. Electromyography was used to evaluate muscle activity of the gluteus maximus, tensor fascia lata, quadriceps femoris, and hamstring muscles in synchronization with the Vicon motion capture system. A HAL® session included a knee extension session with the hip flexor and voluntary gait with upper limb activity. After using the HAL® for one month, the patient’s manual muscle hip flexor scores improved from 1/5 to 2/5 for the right and from 2/5 to 3/5 for the left knee, and from 0/5 to 1/5 for the extension of both knees.
Conclusion/clinical relevance: Knee extension sessions with HAL®, and hip flexor and upper-limb-triggered HAL® ambulation seem a safe and feasible option in a patient with complete paraplegia due to spinal cord injury.
Keywords: Chronic spinal cord injury, Complete paraplegia, Gait analysis, Hybrid Assistive Limb®, Rehabilitation, Upper limb activity
Introduction
Patients with complete paraplegia after spinal cord injury (SCI) are unable to stand or walk on their own. These patients tend to develop osteoporosis, muscle spasms, and joint contracture. However, standing exercises have been shown to decrease decubitus ulcers, osteoporosis, hip joint flexion and adduction deformities, and improve the performance of the cardiovascular and digestive systems in these patients.1
Robotic devices have recently been used in the clinical setting for patients with chronic SCI. Exoskeleton robotic devices with a treadmill, such as the Lokomat2 (Hocoma, Switzerland) and LOPES,3 and powered exoskeleton devices such as ReWalk (Robotics, Israel),4 have angular sensors in the joints and pelvis as well as foot force pressure sensors. However, they have no sensors to detect the neuromuscular activation of wearers.
The Hybrid Assistive Limb® (HAL®; Cyberdyne Inc, Ibaraki, Japan) is a wearable robot suit that assists the wearer in voluntary control of the knee joint and hip joint motion by detecting signals from force/pressure sensors in the shoes and very weak bioelectric signals on the skin surface. Power units on both sides of the hip and knee joints consist of angular sensors and actuators, and the control system comprises cybernic voluntary control (CVC) mode and cybernic autonomous control (CAC) subsystem.5 The HAL® suit has a hybrid control system that includes the CVC and CAC modes. The CVC mode supports the patient’s voluntary motion by providing assistive torque to each joint according to the voluntary muscle activity. The CAC mode can move the patient’s leg using signals from the force-pressure sensors.
Gait training with the HAL® has been reported to improve gait ability in patients with chronic stroke,6–8 chronic SCI,8–12 and postoperative patients with thoracic ossification of the posterior longitudinal ligament.13–15 We previously reported that the CAC mode can be used in patients with C4 complete quadriplegia without detectable bioelectric signals in the lower extremities.11 In addition, we previously reported the use of CVC mode of HAL® for single joint in a patient with complete C4 quadriplegia to restore elbow joint flexion using remaining trapezius muscle activation. We found that the voluntary motion provided by HAL® for the paralyzed joint using the remaining muscle contraction might have activated the paralyzed muscle.16
We consider that providing motion assist and realizing closer to natural form of movement in accordance with detected voluntary neuromuscular activities using the HAL CVC mode may be effective to improve motor function. We hypothesized that voluntary knee extension and ambulation are possible with the HAL® CVC mode using the remaining muscle activity as the trigger for voluntary motion in patients with chronic SCI and complete paraplegia.
Here, we describe the effects of HAL® using a trigger of upper limb motion, in restoring active knee extension and voluntary ambulation in a patient with complete paraplegia caused by chronic SCI. This study was conducted with the approval of the Ethics Committee of the Tsukuba University Faculty of Medicine (H26-22).
Case report
Patient
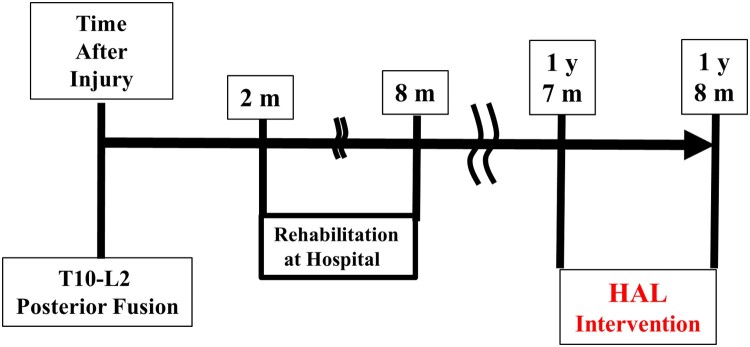
A 30-year-old man with complete paraplegia due to chronic SCI presented to our hospital to undergo the HAL intervention 1 year and 7 months post-injury. He had no other appreciable disease. His clinical course is summarized in Figure 1.
Figure 1.
Summary of the patient’s clinical course. Y, years; M, months; HAL®, Hybrid Assistive Limb®.
He sustained a burst fracture of the twelfth thoracic vertebra and was diagnosed with SCI. He underwent posterior fusion with an instrumented rod at the T10-L2 level at an emergency hospital on the same day, and was transferred to a rehabilitation hospital 2 month after the injury. When he was admitted to the hospital, he had complete paraplegia with grade A on the American Spinal Cord Injury Association (ASIA) impairment scale (AIS),17 0 points on the International Standards for Neurological and Functional Classification of Spinal Cord Injury (ISNCSCI) lower extremity motor score (LEMS), and T11 motor and sensory neurological level. After 6 months of in-hospital rehabilitation, he gained the ability to perform daily activities on his own with the help of a wheelchair. His neurological findings were AIS grade A and 2 points in LEMS.
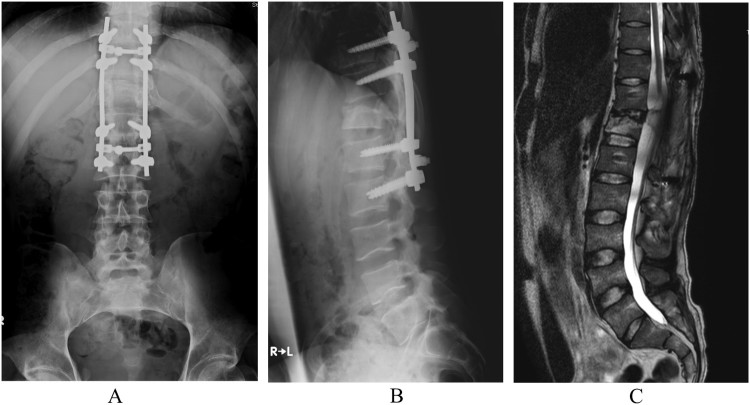
During his first visit to our hospital, the patient’s hip flexor manual muscle testing (MMT) scores were 1/5 on the right and 2/5 on the left. Adductor MMT scores was 1/5 on the right and 2/5 on the left, because he could not flex his left hip against gravity to cover full range of the joint motion, and could not flex his right hip fully in gravity eliminated position. The patient was able to move his right leg inward but not upward, and there was no muscle contraction below either hip flexors or adductors (see Video 1). He was able to ambulate with a walking aid and two therapists’ full assistance, and had sensory loss 3 cm below the umbilicus, including the perianal region. His neurological findings were AIS grade A, 3 points in LEMS, and neurological sensory T11 on both sides. Articular contracture was absent, and no urinary bladder or bowel function remained. A plain radiograph of the thoracic spine showed a wedge-shaped deformity of the twelfth vertebra and posterior fusion with an instrumented rod at the T10-L2 level (Figs. 2A and 2B). Magnetic resonance imaging before the intervention showed disruption of the spinal cord at the Th11 level at T2WI (Fig. 2C). Neurological findings improved in both hip flexors however, but knee extensor activity remained unchanged. We intended to use the HAL® to perform active knee extension and voluntary ambulation. Gait with HAL is based on the user’s voluntary muscle activation and passive support with an overhead harness.
Figure 2.
(A) AP view, (B) Lateral view of plain radiograph of the thoracic spine posterior fusion with an instrumented rod at the T10-L2 level. Wedge shaped deformity of the twelfth vertebra. (C) Magnetic resonance imaging showed disruption of the spinal cord at the level of Th11 at T2WI.
HAL® intervention
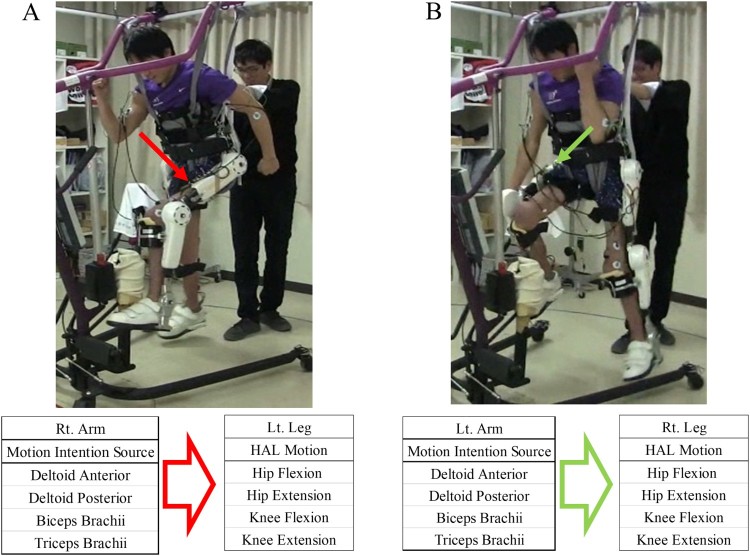
The patient underwent 10 HAL® sessions over the course of 1 month. We defined each locomotion opportunity with the HAL® as a HAL® session and 10 HAL® sessions as the HAL® intervention. No additional therapies were implemented during the HAL® intervention. We evaluated the patient’s muscle activity using a Trigno™ Lab Wireless electromyography (EMG) System (Delsys, Inc., Boston, MA, USA) before the intervention. Although hip flexor activity was observed in both hips, no activation of either knee extensor was seen on the EMG. We designed the HAL® intervention based on the patient’s remaining muscle activity with two goals in mind. The first, was to regain active knee extension and the second, to perform voluntary gait with HAL®. HAL® session consisted of two parts: (1) a session for active knee extension using hip flexor activation; and (2) a session for voluntary gait using his upper limb activation. The latter comprised motion intention from the deltoid anterior (DA) and deltoid posterior (DP) for contralateral hip flexion and extension, and motion intention from the biceps and triceps brachii for contralateral knee flexion and extension. We named the “upper limb triggered HAL” session the “UT HAL” method (Fig. 3).
Figure 3.
Upper limb triggered HAL method. Motion intention from the deltoid anterior and deltoid posterior for contralateral hip flexion and extension, and motion intention from the biceps and triceps brachii for contralateral knee flexion and extension. (A) Left leg flexion triggered by right arm flexion. (B) Right leg flexion triggered by left arm flexion.
A typical HAL® session lasted 90 minutes, including the time required to attach and detach the device (15 and 10 minutes, respectively). The evaluation before and after each HAL® session lasted 20 minutes and included: active hip flexion, five times; active knee extension, five times; and active combined motion of hip flexion and knee extension such as kicking motion, five times. The remaining time was allocated as follows: approximately 15 minutes for knee extension exercise and approximately 30 minutes for walking with the HAL® (approximately 10 minutes including periods of rest). A physiatrist was present in case of an emergency, a therapist and two assistants took the HAL® suit on and off, and an engineer implemented the gait analysis. For safety reasons, a walking device (All-in-One Walking Trainer, Ropox A/S, Naestved, Denmark) with a harness was used to provide body weight support.
Assessments
Assessments were performed before and after each HAL® session. A surface EMG System was used to evaluate muscle activity of the tensor fasciae lata (TFL) for hip flexion, femoral quadriceps (Quad) for knee extension, medial hamstrings (Ham) for knee flexion, and gluteus maximum (Gmax) for hip extension on both sides. Each muscle activity was evaluated using EMG, collected at 2000Hz, and filtered with a 30–400-Hz bandwidth passing filter using scripts on MATLAB 8.2 (Mathworks, Natick, MA, USA). Motion capture (Vicon MX with 16 T20S cameras, Vicon, Oxford, UK) was used to evaluate foot motion in synchronization with EMG. Following the Vicon plug-in gait marker set, auto-reflective markers were placed on the feet, head of the second metatarsal bone for the toe, lateral malleolus for the ankle, and posterior peak for the calcaneus of the heel. The swing phase and stance phase within a gait cycle were extracted according to the movement trajectory of the markers. Heel strikes were detected as the lower peaks of the height of the heel markers, and toe lifts were detected at the lower peaks of the toe markers. The swing phase started with a toe lift and ended with the succeeding heel strike on the same side. The stance phase started with a heel strike and ended with the succeeding toe lift.
Results
Surface EMG before the intervention showed no apparent activation in either Quad (Figs. 4 and 5). Knee extension could not be performed using HAL® by placing the electrodes on both Quads. Therefore, the electrodes for knee extension were placed on both TFLs.
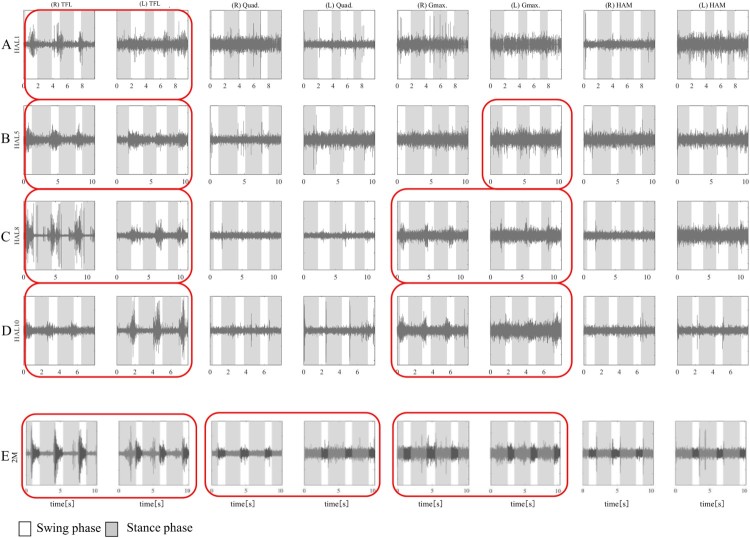
Figure 4.
Muscle activity before, during, and after each HAL® knee extension session: HAL 1st (A), 5th (B), 8th (C), and 10th session (D), and a follow-up evaluation performed 2 months after the intervention (E). Each session includes active hip flexion, five times; active knee extension, five times; and active combined motion of hip flexion and knee extension such as kicking motion, five times. TFL, tensor fasciae lata; Quad, femoral quadriceps; (R), Right; (L), Left; Pre, before; Post, after. Red borders show muscle activation according to the knee extension phase.
Figure 5.
Muscle activity during each HAL® gait session: HAL 1st (A), 5th (B), 8th (C), and 10th session (D), and a follow-up evaluation 2 months after the intervention (E). TFL, tensor fasciae lata; Quad, femoral quadriceps; Gmax, gluteus maximum; Ham, hamstrings; (R), Right; (L), Left; Pre, before; Post, after. Red borders show muscle activation according to gait cycle.
During the knee extension session of the first HAL® session, both TFLs, especially in the right side, were activated during the knee extension phase more than before the HAL session, however neither Quad was activated before, during, and after the HAL session (Fig. 4A). During the voluntary gait session using upper limb muscle activation, there was phase-dependent activation of both TFL, right side dominantly, in swing phases, while there was no phase-dependent activation of muscles other than TFLs (Fig. 4A). Before the fifth session, the left Quad was slightly activated during the knee extension phase (Fig. 4B). Therefore, we put the HAL® electrode for knee extension on both Quads for the knee extension session. During the fifth HAL® session, each knee could extend triggered by each Quad during knee extension session, however we did not observe activation of both Quads via electromyography (Fig. 4B). During the voluntary gait session with upper limb triggered HAL, both TFLs were periodically activated in the swing phase, and left Gmax in sync with the stance phase (Fig. 5B).
In the fifth session, there was left Quad activation in the knee extension session and left Gmax activation in the upper limb triggered HAL gait session. Based on these observations, we put hip and knee electrodes in the recommended position on both TFLs for hip flexion, both Gmax muscles for hip extension, both Quads for knee extension, and both hamstrings for knee flexion during the latter five sessions.
Figure 4C shows muscle activation during the eighth knee extension session. Before the session, there was activation in both Quads during knee extension, especially in the left side periodically. During the HAL® gait session, the TFL was periodically activated according to the gait cycle, and we also observed periodic Gmax contraction during swing phase (Fig. 5C).
Figure 4D shows muscle activation during the last knee session. Before and during the session, there was periodical activation in the left Quad and during the HAL knee extension session, the right Quad was also activated periodically. After the HAL® session, the left Quad was more periodically activated than before. During the HAL gait session, the TFL was activated in a phase-dependent manner in sync with the gait cycle (Fig. 5A-D). Gmax was activated periodically during stance for the left side in an earlier session using upper-limb triggered HAL (Fig. 5B), and during swing for the both sides in the later sessions using lower-limb triggered HAL (Fig. 5C-D). In addition, after the HAL® session, the patient was able to ambulate with a walking device and a little support from one therapist (See Video 2).
We evaluated the patient 2 months post-intervention (Figs 4E and 5E). Fig 4E shows the evaluation of knee extension without HAL. Muscle activation of both Quads are observed. Fig 5E shows the gait evaluation with a walking device and a little assistance from a therapist. There was muscle activation in both TFL, Quads, and Gmax. The patient did not receive any treatment after HAL intervention.
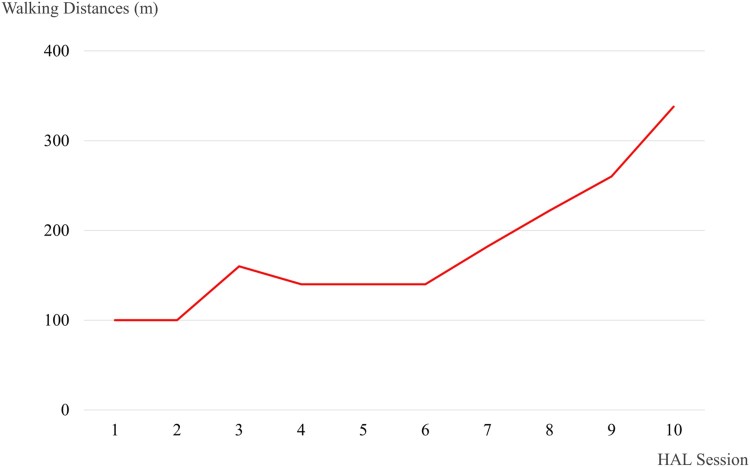
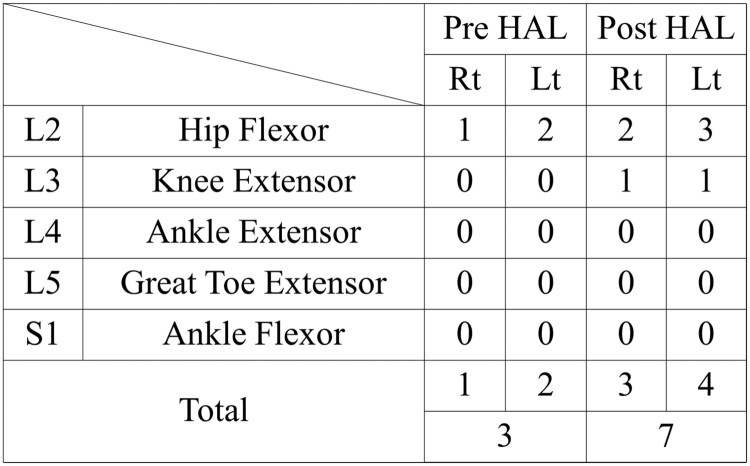
Walking distance increased from 100 m (1st session) to 338 m (10th session) (Fig. 6). No adverse events associated with the HAL® intervention were observed. After the intervention, the patients’ hip flexor MMT score improved from 2/5 to 3/5 on the left and 1/5 to 2/5 on the right. Similarly, knee extensor MMT scores improved from 0/5 to 1/5, and LEMS improved from 4 to 7 (Fig. 7).
Figure 6.
Walking distance in each HAL session.
Figure 7.
Summary of the International Standards for Neurological and Functional Classification of Spinal Cord Injury lower extremity motor scores before and after the Hybrid Assistive Limb® (HAL®) intervention. Rt, right; Lt, left; Pre, before; Post, after.
Discussion
In this study, the patient started voluntary motion for joints associated with the paralyzed muscles using remaining muscle activation. Since then, he was able to move paralyzed joints with preferable muscle activation. His remaining muscle activation in the TFL and upper limb might have motivated paralyzed muscles in the hip and knee flexor and extensor in sync with the gait cycle. The patient’s gait was based on his own voluntary muscle activation; therefore, we describe the gait as voluntary ambulation. Although the gait was assisted by the overhead harness and a therapist, all aspects of the gait, including walking speed, timing to swing and land, step length, and magnitude of joint motions, were voluntarily controlled via the patient’s residual voluntary muscle activity.
The HAL® has been reported to be a feasible tool for some types of neuromuscular disorders,6––15 and improve ambulation in patients with chronic SCI.8––11 However, conventional methods using the CVC mode of HAL® is not applicable in patients with complete paraplegia and difficulty detecting bioelectrical signals.
Our patient was able to extend the knee with his remaining muscle activation in the hip flexor and paralyzed knee extensor. With adequate HAL® assistance, he was also able to walk with upper extremity activity as well as paralyzed hip and knee flexor and extensor.
We focused on the structural analogy and symmetric motion of upper extremity muscle activation during gait between the shoulder and hip. The lower extremities and contralateral upper extremities almost move at the same time in synchrony during natural locomotion. As the shoulder flexes, the contralateral hip flexes, and the other shoulder extends simultaneously with the contralateral hip extension. Based on this contralateral relationship, we put electrodes on the biceps and triceps brachii to drive the contralateral knee flexion and extension, respectively and on the DA and DP to drive the contralateral hip flexion and extension, respectively. Previous studies reported that paralyzed lower limb muscles of patients with SCI can be activated by leg motion from a central pattern generator,18––20 and arm-leg coordination was discussed to be useful for gait assistive technology.21 We consider that synergy of the upper and contralateral limbs through voluntary gait in HAL® intervention may activate paralyzed lower limb muscles. We observed hip flexor contraction in phase dependent manner in sync with natural gait, and phase dependent left hip extensor contraction at earlier sessions using upper-limb triggered HAL. Though his hip extensor was contracted periodically in the later sessions using lower-limb triggered HAL, its phase was not in sync with natural gait. We consider that his hip and knee extensors did not contract phase-dependently, because those muscles were not strong enough to support his lower limbs during stance phase.
Our protocol included sessions for voluntary knee extension. In patients who have difficulty extending the knee, a long leg brace in the knee-locked position is usually needed during walking exercises. Therefore, it is difficult for them to train with knee extensor movement during gait exercise. Gait with the HAL® enabled our patient to perform knee extensor movement in the standing phase according to the gait cycle without knee locking.12 However, we could not observe periodic Quad contraction during HAL gait in this case. Therefore, we consider that both sessions—knee extension with hip flexor and locomotion with upper limb muscle activation—were effective in improving muscle activity.
We consider that the muscle activation acquired during voluntary ambulation using motion intention of residual activity of upper limb muscle and during voluntary knee extension using residual activity of hip flexor, may contribute to the improvement of the patient’s paralyzed muscle activation.
The operating system of HAL, using neuromuscular activities, more directly reflects user’s motion intention, compared with other exoskeleton robots such as Lokomat,2 LOPES,3 and ReWalk.4 We focused on exploiting residual voluntary muscle activation in a patient with paraplegia. Using the HAL’s motion assist function in the scheme of upper-lower limbs coordination during gait, voluntary ambulation was possible and an improvement in muscle activity was observed.
We consider that an ambulation protocol using HAL and voluntary upper limb muscle activation is feasible in patients with complete paraplegia patients and may activate paralyzed muscles. Conventional gait training using orthoses for complete paraplegia requires to lock the knee joint in extended position, as well as excessive upper limb usage for weight bearing.1 Upper limb triggered HAL enables patients with complete paraplegia to perform knee unlocked gait for long duration and distance without requiring the force from the upper limbs. Our patient performed longer distance gait as intervention progressed. We believe that this occurred because the patient became accustomed to the system/intervention.
Future perspective includes application to more severe patients such as patients with cervical cord or upper thoracic cord injuries, as well as patients who have difficulty performing gait training with conventional orthoses. Providing them with chances to do voluntary controlled gait, exploiting their residual neuromuscular activities, may contribute to a novel perspective with a broader range of gait rehabilitation in patients with spinal cord injuries.
Conclusions
In this study, we reported the safety and feasibility of knee extension sessions with HAL® and hip flexor and upper-limb-triggered HAL® ambulation in a patient with complete paraplegia caused by SCI. We observed Quad activation during voluntary knee extension, and activation of the Quad, Ham, TFL, and Gmax during HAL locomotion according to the gait cycle. HAL® enables voluntary ambulation of a patient with complete paraplegia.
Funding Statement
This study was supported by the Industrial Disease Clinical Research Grants of the Ministry of Health, Labor, and Welfare in Japan (14060101-01).
Acknowledgements
We appreciate Daisuke Yamagami MD, physiatrist of Kanagawa Rehabilitation Hospital, and Genny Kroll-Rosen, the director, and all staff of Making Strides, for information on clinical course of the patient before inclusion to the study. We also thank Mayuko Sakamaki and Yumiko Ito, Center for Innovative Medicine and Engineering (CIME), University of Tsukuba Hospital, for their excellent technical assistance.
Disclaimer statements
Authors’ contributions All authors participated in the design, execution, and analysis of these studies and have seen and approved the final version of the manuscript. Yukiyo Shimizu and Hideki Kadone participated in the study design and drafted the manuscript. Yukiyo Shimizu, Hideki Kadone and Shigeki Kubota, executed HAL intervention, performed the data. Kosaku Saotome, Akira Matsushita performed radiological evaluation. Tomoyuki Ueno, Hiroki Watanabe, Ayumu Endo, Kazue Tsurumi, Ryu Ishimoto helped to execute HAL intervention and performed the data. Yoshiyuki Sankai conceived the device. Kenji Suzuki, Tetsuya Abe, Aiki Marushima, Masao Koda, Akira Matsumura, Yasushi Hada and Masashi Yamazaki participated in the study design and helped to draft the manuscript. Masashi Yamazaki is the principal investigator of this study and participated in the design and coordination of the study. Furthermore, this manuscript has been revised by a professional editor whose first language is English.
Contributor statement A commercial party having a direct financial interest in the results of the research supporting this article has conferred or will confer a financial benefit to one or more of the authors. Yoshiyuki Sankai is CEO of Cyberdyne Inc., Ibaraki, Japan. This study was proposed by the authors. Cyberdyne was not directly involved in the study design, collection, analysis, interpretation of data, writing of the report, or the decision to submit the paper for publication. No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit on the following authors or on any organization with which these authors are associated: Yukiyo Shimizu, Hideki Kadone, Shigeki Kubota, Kenji Suzuki, Kousaku Saotome, Tomoyuki Ueno, Tetsuya Abe, Aiki Marushima, Hiroki Watanabe, Ayumu Endo, Kazue Tsurumi, Ryu Ishimoto, Akira Matsushita, Masao Koda, Akira Matsumura, Yasushi Hada, and Masashi Yamazaki.
Informed consent Written informed consent was obtained from the patient for publication of this case report and accompanying images without masking.
Ethics approval This study was conducted with approval from the Ethics Committee of the Tsukuba University Faculty of Medicine.
ORCID
Yukiyo Shimizu http://orcid.org/0000-0001-7491-4516
Kenji Suzuki http://orcid.org/0000-0003-1736-5404
Hiroki Watanabe http://orcid.org/0000-0002-4436-5437
Akira Matsushita http://orcid.org/0000-0003-2335-674X
References
- 1.Karimi MT. Evidence-based evaluation of physiological effects of standing and walking in individuals with spinal cord injury. Iran J Med Sci 2011;36(4):242–53 [PMC free article] [PubMed] [Google Scholar]
- 2.Colombo G, Joerg M, Schreier R, Dietz V.. Treadmill training of paraplegic patients using a robotic orthosis. J Rehabil Res Dev 2000;37(6):693–700 [PubMed] [Google Scholar]
- 3.Veneman JF, Kruidhof R, Hekman EE, Ekkelenkamp R, Van Asseldonk EH, van der Kooij H.. Design and evaluation of the LOPES exoskeleton robot for interactive gait rehabilitation. IEEE Trans Neural Syst Rehabil Eng 2007;15(3):379–86 doi: 10.1109/TNSRE.2007.903919 [DOI] [PubMed] [Google Scholar]
- 4.Miller LE, Zimmermann AK, Herbert WG.. Clinical effectiveness and safety of powered exoskeleton-assisted walking in patients with spinal cord injury: systematic review with meta-analysis. Med Devices (Auckl) 2016;9:455–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawamoto H, Sankai Y.. Power assist method based on Phase Sequence and muscle force condition for HAL. Advanced Robotics 2005;19(7):717–34 doi: 10.1163/1568553054455103 [DOI] [Google Scholar]
- 6.Kawamoto H, Kamibayashi K, Nakata Y, Yamawaki K, Ariyasu R, Sankai Y, et al. Pilot study of locomotion improvement using hybrid assistive limb in chronic stroke patients. BMC Neurol 2013;13:141 doi: 10.1186/1471-2377-13-141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nilsson A, Vreede KS, Haglund V, Kawamoto H, Sankai Y, Borg J.. Gait training early after stroke with a new exoskeleton--the hybrid assistive limb: a study of safety and feasibility. J Neuroeng Rehabil 2014;11:92 doi: 10.1186/1743-0003-11-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wall A, Borg J, Palmcrantz S.. Clinical application of the Hybrid Assistive Limb (HAL) for gait training-a systematic review. Front Syst Neurosci 2015;9:48 doi: 10.3389/fnsys.2015.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aach M, Cruciger O, Sczesny-Kaiser M, Hoffken O, Meindl R, Tegenthoff M, et al. Voluntary driven exoskeleton as a new tool for rehabilitation in chronic spinal cord injury: a pilot study. Spine J 2014;14(12):2847–53 doi: 10.1016/j.spinee.2014.03.042 [DOI] [PubMed] [Google Scholar]
- 10.Sczesny-Kaiser M, Hoffken O, Aach M, Cruciger O, Grasmucke D, Meindl R, et al. HAL® exoskeleton training improves walking parameters and normalizes cortical excitability in primary somatosensory cortex in spinal cord injury patients. J Neuroeng Rehabil 2015;12:68 doi: 10.1186/s12984-015-0058-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikumi A, Kubota S, Shimizu Y, Kadone H, Marushima A, Ueno T, et al. Decrease of spasticity after hybrid assistive limb® training for a patient with C4 quadriplegia due to chronic SCI. J Spinal Cord Med 2016:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimizu Y, Nakai K, Kadone H, Yamauchi S, Kubota S, Ueno T, et al. , The Hybrid Assistive Limb(R) intervention for a postoperative patient with spinal dural arteriovenous fistula and chronic spinal cord injury: a case study. J Spinal Cord Med 2017:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakakima H, Ijiri K, Matsuda F, Tominaga H, Biwa T, Yone K, et al. A newly developed robot suit hybrid assistive limb facilitated walking rehabilitation after spinal surgery for thoracic ossification of the posterior longitudinal ligament: a case report. Case Rep Orthop 2013;2013:621405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujii K, Abe T, Kubota S, Marushima A, Kawamoto H, Ueno T, et al. The voluntary driven exoskeleton Hybrid Assistive Limb (HAL) for postoperative training of thoracic oss: ification of the posterior longitudinal ligament: a case report. J Spinal Cord Med 2016:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kubota S AT, Fujii K, Marushima A, Ueno T, Haginoya A, Endo A, et al. Improvement of walking ability using Hybrid Assistive Limb training in a patient with severe thoracic myelopathy caused by ossification of the posterior longitudinal ligament - A case report. J Spine S7:003.doi: 10.4172/2165-7939.S7-003 [DOI] [Google Scholar]
- 16.Shimizu Y, Kadone H, Kubota S, Ikumi A, Abe T, Marushima A, et al. Active elbow flexion is possible in C4 quadriplegia using hybrid assistive limb (HAL®) technology: A case study. J Spinal Cord Med 2017;1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, et al. International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med 2011;34(6):535–46 doi: 10.1179/204577211X13207446293695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dietz V. Quadrupedal coordination of bipedal gait: implications for movement disorders. J Neurol 2011;258(8):1406–12 doi: 10.1007/s00415-011-6063-4 [DOI] [PubMed] [Google Scholar]
- 19.Dietz V, Colombo G, Jensen L, Baumgartner L.. Locomotor capacity of spinal cord in paraplegic patients. Ann Neurol 1995;37(5):574–82 doi: 10.1002/ana.410370506 [DOI] [PubMed] [Google Scholar]
- 20.Kawashima N, Nozaki D, Abe MO, Akai M, Nakazawa K.. Alternate leg movement amplifies locomotor-like muscle activity in spinal cord injured persons. J Neurophysiol 2005;93(2):777–85 doi: 10.1152/jn.00817.2004 [DOI] [PubMed] [Google Scholar]
- 21.La Scaleia V, Sylos-Labini F, Hoellinger T, Wang L, Cheron G, Lacquaniti F, et al. Control of leg movements driven by EMG activity of shoulder muscles. Front Hum Neurosci 2014;8:838. [DOI] [PMC free article] [PubMed] [Google Scholar]