Abstract
The intestinal epithelium does not function in isolation, but interacts with many components including the Enteric Nervous System (ENS). Understanding ENS and intestinal epithelium interactions requires multidisciplinary approaches to uncover cells involved, mechanisms used, and the ultimate influence on intestinal physiology. This review is intended to serve as a reference for epithelial biologists interested in studying these interactions. With this in mind, this review aims to summarize the basic anatomy of the epithelium and ENS, mechanisms by which they interact, and techniques used to study these interactions. We highlight in vitro, ex vivo and in vivo techniques. Additionally, ENS influence on epithelial proliferation and gene expression within stem and differentiated cells as well as gastrointestinal cancer are discussed.
Abbreviations used in this paper: 5-HT, 5-hydroxytryptamine; 5-HT3R, 5-hydroxytryptamine 3 receptor; ACh, acetylcholine; AITC, allyl isothicyanate; CPI, crypt proliferation index; EEC, enteroendocrine cell; ENS, enteric nervous system; GI, gastrointestinal; HIO, human intestinal organoid; ISC, intestinal stem cell; Lgr5, leucine-rich repeat–containing G protein–coupled receptor; NE, norepinephrine; NGF, nerve growth factor; SI, small intestine; TA, transit-amplifying
Summary.
Interactions between the enteric nervous system and intestinal epithelium are thought to play a vital role in intestinal homeostasis. In this review intended for epithelial researchers, we highlight basic anatomy, techniques, and recent findings of this growing field.
Proper intestinal function results from interactions between multiple components including the epithelium, microbiota, immune cells, and the Enteric Nervous System (ENS). Increasing numbers of studies suggest interactions between epithelial and ENS components influence intestinal homeostasis. As these interactions include topics from both epithelial biology and neuroscience, mechanistic understanding of this communication will require a multidisciplinary approach. This review serves as a brief introduction to the field of epithelial-ENS interactions for epithelial researchers, highlights common approaches used to reveal these interactions, and describes evidence for dynamic interactions between the epithelium and ENS.
Anatomy of the Intestinal Epithelium and ENS
Intestinal Epithelium
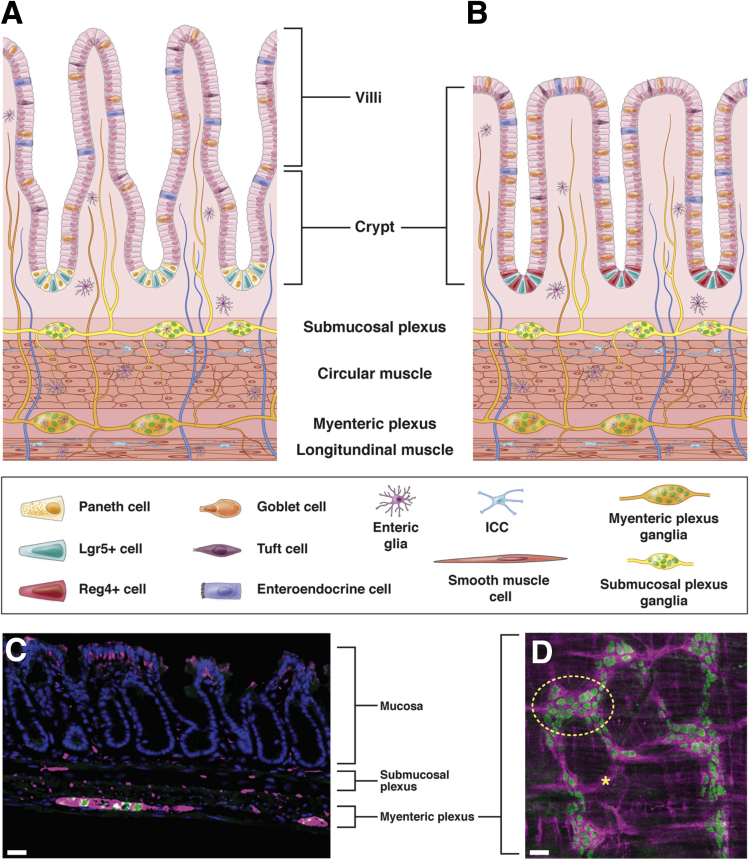
The intestinal epithelium is a single layer, columnar epithelium responsible for absorption of nutrients and exclusion of pathogens. Throughout the small intestine (SI) and colon, with the exception of the most proximal colon, intestinal stem cells (ISCs) reside in the crypt base (Figure 1A and B). ISCs express the Wnt-signaling effector and G protein–coupled receptor, leucine-rich repeat–containing G protein–coupled receptor (Lgr5).1 Reserve stem cells, which proliferate less than Lgr5+ ISCs do, express proteins Bmi-1, Tert, or Hopx and can give rise to the highly proliferative Lgr5+ population during times of stress or dysfunction.2 Paneth cells are support cells for the SI ISCs and reside within the crypt base.3 Although the epithelium turns over once per week, ISCs and Paneth cells in the crypt base are longer lived.3, 4
Figure 1.
Anatomy of intestinal epithelium, ENS, and intestinal muscle layers. (A) Cartoon depiction of SI epithelium depicting villi with differentiated cell types and crypts with progenitor cell niche located above the ENS and musculature of intestine. (B) Cartoon depicting of colonic epithelium depicting differentiated cells and progenitors within crypts located above the ENS and musculature of intestine. (A, B) Mucosal glia located within mucosa, intraganglionic glia are located adjacent to neuronal cell bodies within ganglia, and intramuscular glia are located along nerves within muscle layers. Yellow nerves represent intrinsic nerves from submucosal plexus, orange nerves represent intrinsic nerves from myenteric plexus, and blue nerves represent extrinsic innervation into intestine. The legend is located below the graphic. (C) Immunofluorescent image of colonic epithelium. Epithelial cells (DAPI, blue) are located above submucosal and myenteric neurons. Neuronal nuclei marked with HuC/D (green) and neuronal projections marked with PGP9.5 (magenta). Note that the staining at the top of the crypts is background staining. (D) Longitudinal view of myenteric plexus depicting enteric neuron morphology using same markers as described in panel C. Circle represents one ganglia (yellow dashed circle). (C, D) Scale bar = 50 um. ICC, interstitial cells of Cajal.
ISCs divide once per day to give rise to themselves and daughter cells, which make up the transit-amplifying (TA) zone. These daughter cells divide to produce differentiated epithelial cells. Within the proliferative TA cells, tightly controlled gene programs direct the differentiation of functional cell types of the epithelium.5, 6 The majority of SI differentiated cells are absorptive in nature, while others are secretory, such as goblet cells, or specialized signaling cells, such as enteroendocrine cells (EECs) and tuft cells.7, 8 In contrast to the SI, colonic crypts are enriched in secretory goblet cells, while absorptive colonocytes compose much of the remaining epithelium. Within the colon, EECs and tuft cells, present in fewer numbers, are responsible for hormonal signaling. Across the SI and colon, regulation of ISC proliferation and TA cell gene expression ensures the epithelium contains appropriate numbers and types of differentiated cells for proper intestinal function.
Enteric Nervous System
A brief overview of ENS anatomy will be provided here as it has been reviewed extensively.9, 10, 11, 12 Along the intestines, the mammalian ENS contains 2 layers of interconnected neurons and glia, called plexuses. The myenteric plexus, located between the longitudinal and circular muscle layers, coordinates motor movements of the gut (Figure 1A–D). The submucosal plexus, located above the circular muscle layer closest to the mucosa, regulates secretory activity (Figure 1A–C). Within both plexuses, neuronal cell bodies reside in groups called ganglia (circle, Figure 1D), which are surrounded by glia. Neuronal projections connect individual ganglia to each other, as well as to nonneuronal targets, including the epithelium. Although not discussed further here, other important cell types reside in proximity to the ENS including muscle cells, stromal cells, interstitial cells of Cajal, and immune cells.13, 14, 15
The intestine contains 2 discrete classes of neuronal innervation. First, nerves from neurons and glia with cell bodies within the intestine are termed intrinsic innervation.9 Second, extrinsic innervation arises from neurons with cell bodies outside the intestine. Extrinsic innervation includes neurons of the parasympathetic and sympathetic branches of the peripheral nervous system, dorsal root ganglia, and nodose ganglia.16, 17, 18 Many, but not all, extrinsic nerves enter the intestine through the vagus nerve. In this review, the influence of both extrinsic and intrinsic nerves on the epithelium is discussed.
Techniques for Studying Interactions Between the ENS and Epithelium
Interactions between the ENS and epithelium likely play a role in homeostatic regulation of intestinal function. Much progress has been made, yet the field will benefit from increased mechanistic understandings of these interactions. It will be necessary to apply techniques from both neuroscience and epithelial fields to reveal identities of cellular players, mechanisms of communication, and impacts on intestinal function.
Communication Mechanisms
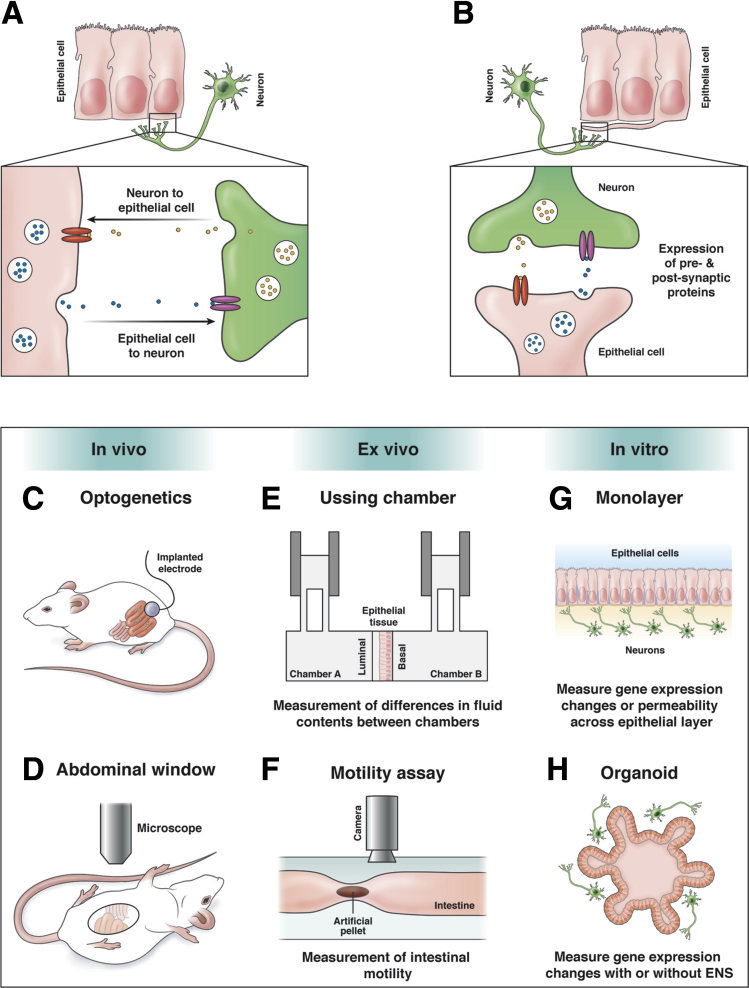
Intercellular communication between the epithelium and ENS likely occurs where the cells are in close proximity. Known intercellular epithelial cell communication mechanisms include soluble molecule secretion, extracellular vesicle release, and juxtacrine signaling.19, 20, 21 Furthermore, intercellular neuronal communication occurs primarily through synaptic release of contents from vesicles, although evidence for extracellular vesicles in neuronal communication increases.22, 23 Conventionally, communication between the epithelium and ENS is thought to occur through paracrine signaling mechanisms, whereby molecules diffuse from one cell to bind receptors on a nearby cell (Figure 2A). Remarkably, recent evidence demonstrated direct synaptic connections between neurons and epithelial cells (Figure 2B). Using scanning electron microscopy, researchers observed synapses between enteric nerves and cellular projections from EECs, termed neuropods.24 These connections permit transfer of the rabies virus, thus verifying their synaptic nature.25 Interestingly, synapses have been demonstrated only between epithelial cells and extrinsic nerves, and it remains unknown if neuropods also connect the intrinsic ENS and epithelium. As discussed subsequently, some functions are understood, yet the full extent of these synaptic contacts and their impact on epithelial-ENS communication remains unknown.
Figure 2.
Mechanisms of epithelial-ENS interactions and current techniques. (A) Paracrine model of neuron-epithelial cell communication. Diffusion of molecules between intestinal epithelial cells and enteric neurons may result in communication in both directions. Release of molecules (yellow or blue circles) from one cell can bind to receptors (red or magenta ovals) on the signal-receiving cell resulting in downstream signaling. (B) Depiction of direct synaptic connections between neurons and epithelial neuropod cells. Expression of synaptic proteins between the neuron and neuropod cell allows for direct communication between the two cells. As with diffusion, expression of hormones or neurotransmitters (yellow or blue circles) from the presynaptic cell binds to receptors (red or magenta ovals) on the opposing postsynaptic cell. Expression of pre- and postsynaptic proteins has been shown with this model. (C–H) Approaches used to study epithelial-ENS interactions using in vitro, ex vivo, or in vivo techniques: (C) optogenetics to manipulate ENS activity in vivo; (D) abdominal window to visualize intestinal activity in vivo in anesthetized animals; and (E) Ussing chamber to study secretion, signaling, or barrier function with ENS cultured on basal side. (The Ussing chamber can be an ex vivo or in vitro technique depending on type of epithelial tissue or cell monolayer used); (F) intestinal motility assay measures propagation of artificial fecal pellet in ex vivo setting; (G) co-culture of monolayers; or (H) organoids allowing for measurement of in vitro gene expression changes or barrier permeability with or without ENS influence on epithelium.
Unraveling the mechanisms of epithelial and ENS interactions requires identification of the cells, receptors, and ligands involved, as well as the location and timing of the interactions. Molecularly diverse epithelial cells and neurons can be characterized with antibody labeling to identify cell type, neurotransmitter expression, and presence of receptors for candidate neurotransmitters.26, 27, 28 As neuron-epithelial cell communication likely requires close association, neuronal axon position identifies candidate cells with which a neuron may interact. Single-cell labeling techniques are required to link axons with corresponding cell bodies because the dense interganglionic axon tracts preclude visualization of individual axons (asterisk, Figure 1D).29, 30, 31 Information about the timing and pattern of activities of both epithelial and ENS cells contributes to understanding mechanisms. Neuronal activity begins with electrical depolarization and subsequent influx of calcium ions. This calcium influx leads to axonal release of neurotransmitter, through which neurons communicate. EECs also exhibit calcium transients after ligand binding and upon release of hormones and neurotransmitters.32, 33 Future studies likely will increase numbers of known synaptic connections between epithelial cells and the ENS. Measurement of cellular activity, identification of cell type, and location of these interactions will improve understanding of epithelial-ENS communication.
Functional Assays
To study effects of interactions between the epithelium and ENS, in vivo, ex vivo, and in vitro techniques provide options to balance experimental accessibility with biological relevance. In vivo approaches visualize tissue dynamics within the living animal with difficult accessibility and high biological relevance. Ex vivo approaches provide accessibility and relevance via assessment of functional changes within intact organ tissue outside of the animal. In vitro approaches sacrifice some biological relevance, in exchange for high accessibility in manipulation of individual system components outside the organism. In the following paragraphs, we discuss application of these 3 categories of techniques to epithelial-ENS interactions.
In Vivo
Despite the challenges, investigators have devised innovative strategies to manipulate and observe both epithelial and ENS function in vivo. Optogenetic techniques successfully manipulate ENS activity in awake animals (Figure 2C).34, 35 Rakhilin et al34 demonstrate abdominal windows are suitable to visualize SI neuron activity in anesthetized mice (Figure 2D). Furthermore, observation of in vivo epithelium can be achieved with application of internal endomicroscopy, as well as multiphoton microscopy on exposed loops of SI in anesthetized animals.36, 37, 38 These pioneering technical advances are critical to moving the field forward, but have yet to be applied to epithelial-ENS interaction studies.
Ex Vivo
Ex vivo approaches have been more commonly used in epithelial-ENS studies. Researchers use ex vivo tissue in an Ussing chamber to study barrier function and measure electrolyte movement across the epithelium (Figure 2E).39 Video recordings of artificial fecal pellet propagation through the ex vivo intestine allow quantification of spatial and temporal dynamics of peristalsis, which may rely on epithelial-ENS communication (Figure 2F).40, 41 Importantly, many ex vivo approaches sever extrinsic innervation during removal of the intestines from the animal. As intact connections between the extrinsic nerves and spinal cord are likely important, some dissections techniques take care to maintain connections within the spinal cord. This allows for ex vivo study of the dialogue among the epithelium, ENS, and extrinsic neurons.35
In Vitro
As in vitro approaches provide accessibility to generate and manipulate cells of interest, many sources of epithelium and ENS are used successfully. Two-dimensional epithelial monolayers, from immortalized cell lines or differentiated cells, lack in vivo morphology, yet the physical separation of epithelial cells from neurons facilitates the study of secretion, ion movement or barrier function (Figure 2G).42, 43, 44, 45 Organoids, another source of epithelium, can be generated from single crypts or ISCs and can be cultured with or without an ENS component. Organoids contain most major epithelial cell types arranged in 3-dimensional morphology similar to that in vivo (Figure 2H).46, 47 Similar to epithelial cells, the source of ENS cells within in vitro systems varies. Common approaches include dissociated cells from adult ENS or immortalized cultures of neurons or glial cells.43, 48 As the ENS develops from migrating neural crest cells that generate all ENS cell types, directed differentiation of embryonic stem cells into a functional ENS has also been successful.49, 50
Recent breakthroughs in human intestinal organoid (HIO) culture techniques have expanded culture studies. Workman et al50 differentiated human embryonic stem cells to generate ENS and epithelial sources independently for subsequent co-culture. Interestingly, after 4 weeks of in vitro growth, the resulting ENS harbored morphology similar to a developmentally young ENS. In an effort to mature the ENS, researchers transplanted organoid or ENS tissue into kidney capsules of mice for 6–10 weeks. This in vivo growth shapes ganglia morphology more similarly to that of an adult, and generates an ENS capable of electrical activity. Unfortunately, as epithelial gene expression was not tested between the in vitro and in vivo conditions, it is unknown if the epithelial cells mature similarly to the neuronal counterparts during in vivo growth.
In vitro approaches aim to model in vivo environments and cell types, yet care must be taken to validate experimental conditions and outcomes. Although the HIO method, with subsequent in vivo growth, critically advances in vitro techniques, limitations remain. Notably, nitric oxide neurons are present only in transplanted HIOs, suggesting in vitro growth conditions lack an important requirement for their development. Acetylcholine (ACh)-positive neurons, which may impact ISC dynamics and neuronal activity, are seen within in vitro cultures, but not after transplantation. Furthermore, neuropod connections between neurons and epithelial cells are absent under in vitro growth conditions, indicating a lack of cues required for their formation. Importantly, these data highlight both temporal and environmental conditions as critical components for modeling epithelial-ENS interactions in vitro.
Interactions Between the ENS and Intestinal Epithelium
Influence of Neurons on Epithelial Gene Expression
As organoids develop in the absence of an ENS, it is possible to study the influence of the ENS on epithelial gene expression. Within in vitro HIO-ENS co-cultures, epithelial proliferation increases compared with HIOs alone.50 Genes involved in gastrointestinal (GI) development change, including an increase in epidermal growth factor signaling and a decrease in the transforming growth factor beta pathway. Additionally, genes important for absorptive, goblet, and Paneth cell lineage specification are decreased. However, tuft cell and EEC transcripts show higher expression, suggesting an increase in these populations. These results indicate the ENS can affect cellular differentiation, which is critical for representation of all relevant epithelial cell types in culture, but may also influence epithelial physiology and function in vivo.51, 52 In another study, co-culture of a monolayer of organoid-grown differentiated cells with dissociated adult mouse ENS cells, shows no changes in epithelial proliferation.43 However, the epithelial cell density increases by 40% and transforming growth factor beta expression also increases. Furthermore, epithelial cells increased chromogranin A and zonula occludens-1 transcript expression, suggesting increased EEC populations and epithelial barrier function, respectively.43 These data imply that epithelial physiology may ultimately benefit from the presence of the ENS, as decreased barrier integrity can negatively impact epithelial physiology.
While co-culture studies have moved the field forward, the variety in sources of cell types can produce varying results. The above in vitro data report conflicting impacts of the ENS on intestinal epithelium. The monolayer co-culture experiments revealed insignificant changes in epithelial proliferation, whereas HIO proliferation increased. Both findings suggest the ENS impacts epithelial lineage composition and gene expression. Differences in technical approaches may explain these discrepancies, such as the developmental age of the cells. The monolayer experiments used cells from adult mice, whereas the HIO experiments used in vitro grew differentiated cells from human embryonic stem cells, suggesting that developmental age of the cells influences the ultimate effect. In the future, establishing optimized standard protocols will be critical for comparing data from different studies.
Neurotransmitters and Epithelial Proliferation
Tight regulation of epithelial proliferation is vital for intestinal homeostasis. Research to link neurotransmitters to this process is ongoing and mechanisms remain mysterious. Serotonin (5-hydroxytryptamine [5-HT]) has been implicated in many aspects of intestinal physiology, including regulation of epithelial proliferation. Upregulation of 5-HT signaling in the SI epithelium increases crypt proliferation index (CPI), crypt depth, and villus height.53 Although the epithelium also releases 5-HT, the causative source of 5-HT is of neuronal origin. Interestingly, as serotonergic innervation is absent from the epithelium, the authors explored alternative mechanisms. Many cholinergic neurons innervate the epithelium and release ACh to stimulate mucosal secretion.54 Indeed, inhibition of ACh with simultaneous upregulation of 5-HT signaling returns CPI to wild type levels, suggesting that 5-HT effects on epithelial proliferation occur through a cholinergic neuronal intermediate.53
The search for mechanisms of cholinergic control of epithelial dynamics reveals a complicated picture. Gross et al53 report no effect of cholinergic signaling on CPI unless in a background of increased 5-HT, as pharmacological inhibition of ACh signaling alone yields wild type CPI. In another study, increased ACh signaling in cultured gastric organoids increases epithelial growth in an ENS-dependent manner, which suggests a proliferative role for ACh.55 Further evidence demonstrates an inhibitory influence of ACh on proliferation, as whole-animal knockout of the M2, M3, or M5 ACh receptors increases SI proliferation.56 Last, ACh treatment of SI organoids decreases cyclinD1 transcript expression, corroborating an inhibitory role for ACh in epithelial proliferation.57 Interestingly, Lgr5+ stem cells express the M3 receptor, motivating further studies of ACh influence on stem cell behavior.58 Clearly, ACh impacts epithelial proliferation, yet the exact mechanisms, sources, and conditions remain to be determined.
Another regulator of epithelial proliferation is extrinsic innervation of the intestine. Elimination of vagal extrinsic input to the intestine via vagotomy results in altered epithelial proliferation.59 Norepinephrine (NE) is an attractive candidate to mediate this process as extrinsic sympathetic nerves, rather than intrinsic nerves, utilize NE within the intestine.60, 61 ISCs express the NE receptor, Adra2α, and application of NE to SI organoids decreases proliferation.57 As with ACh signaling, much remains unknown, yet it continues as an avenue for future research of possible influence of extrinsic ENS control over epithelial proliferation. The peripheral nervous system may also influence the functional properties of epithelial cells.62 Caco-2 cells, engineered to express Adra2α, increase peptide absorption when exposed to NE.63 Together, these neurotransmitter studies, as well as the expression of neurotransmitter receptors within ISCs, will motivate future studies to explore the mechanisms by which the ENS influences epithelial proliferation dynamics.
Enteroendocrine Cells
EECs comprise only 1% of the epithelial population, yet are essential to intestinal physiology.64 EECs respond to chemical, and possibly mechanical, stimuli within the lumen and secrete hormones and neurotransmitters in response.65, 66, 67, 68 Increasing lines of evidence highlight vital roles for connections between EECs and neurons in intestinal function, including communication to the central nervous system about luminal contents and intestinal motility.
In response to luminal contents, EECs signal to the brain. This is thought to occur primarily through slow-acting hormones, such as cholecystkinin.69, 70 Recent work demonstrates a faster mechanism for message relay: synapses between EEC neuropods and extrinsic sensory afferent nerve fibers. In response to luminal sugar, EECs release glutamate into the synapse with vagal nodose neurons, increasing neuronal activity. This synaptic response conveys information to the brain faster than cholecystkinin.25 The full extent of the roles of EEC neuropods in nutrient sensing and gut-brain communication remains to be determined. Yet, EECs detect and respond to a wide variety of chemicals such as allyl isothicyanate (AITC), found in mustard and wasabi; NE, a neurotransmitter; and isovalerate, a microbial product.33 As the epithelium is completely replenished over the course of 1 week, if EEC-neuron connections were crucial for proper intestinal function, newly born EECs must re-establish connections with neurons frequently. While this may suggest a dynamic sensory network that is constantly reconnecting, there are reports of long-lived EECs, surviving for up to 60 days, which make longer lasting connections possible.24
EEC connections to neurons can influence both intestinal contractions and detection of mechanical sensation. Using strips of intestinal tissue, Nozawa et. al. demonstrated exogenously supplied AITC causes muscle contractions. These contractions occur in a 5-HT3 receptor (5-HT3R)–dependent manner. Thus, in response to AITC, EECs release 5-HT, which stimulates 5-HT3R–expressing neurons to initiate contractions.71 The precise role of epithelial 5-HT in control of contractile activity remains an active area of research.72, 73, 74, 75 As the identity of the 5-HT3R–expressing nerves was not characterized in this study, it remains unknown if EECs communicate directly or indirectly with intrinsic neurons. In addition, neuronal and microbial products may also modulate EEC response to mechanical stimuli. Using ex vivo colonic preparations with intact sensory neurons, Bellono et al33 tested if EEC detection of luminal chemicals can modulate neuronal sensation of mechanical stimuli within the epithelium. Indeed, when EECs are exposed to either NE or isovalerate, activity within extrinsic sensory neurons increases. This increase is larger than the normally observed responses to identical mechanical stimuli in the absence of EEC activation.33 Although this was found to be dependent on 5HT3R, the precise mechanism remains to be determined, but suggests a role for EECs in intestinal hypersensitivity.
Tuft Cells
Tuft cells are a recently described population of chemosensory cells within the epithelium thought to communicate with the ENS.8, 76 Surprisingly, tuft cells are lost within 1 week from organoid cultures. However, this is prevented either by co-culture with neurons, or by increasing cholinergic signaling.55 Interestingly, culturing organoids with neurons increases both organoid formation efficiency and size, yet this is lost upon ablation of tuft cells.55 This indicates tuft cells are necessary for the ENS to convey its growth benefits to the epithelium. Surprisingly, the same study shows in vivo ablation of tuft cells prevents mice from recovering from chemically induced injury.55 Tuft cells may influence epithelial dynamics during recovery from injury, although it remains to be determined if this effect is mediated through the ENS. Furthermore, tuft cells express choline acetyltransferase, the enzyme required to synthesize ACh. While it is unknown whether synaptic vesicle release occurs within tuft cells, it is exciting to speculate that tuft cells may also communicate with the ENS and epithelium via cholinergic signaling.77
Enteric Glia and the Epithelial Barrier
Interactions between enteric glia and epithelial cells have been suspected to regulate barrier function, although recent data challenge this notion. Initial work to ablate glia in vivo using ganciclovir-induced cell death, caused severe inflammation, decreased barrier integrity, and eventual SI carcinomas.78 However, in vivo glial ablation without ganciclovir shows no gross changes to barrier integrity. Despite this, ablation of glia decreases intestinal transit time in male mice, suggesting a role in intestinal motility, but not in barrier function.79 In vitro work demonstrates an influence of glia on epithelial cell monolayer barrier function. Paracrine signaling from cultured glial cells upregulates adherens junctions and extracellular matrix proteins within epithelial cells, and glial-derived S-nitrosoglutathione decreases epithelial barrier permeability.42, 44, 80, 81 Furthermore, manipulation of glial activity in ex vivo whole-colon preparations showed passive paracellular permeability is independent of glial activity, but active transepithelial permeability increases upon glial activation.39 Thus, in these data, glia affect active epithelial electrolyte transport, but not passive barrier function. These seemingly conflicting results bring to light differences among in vitro, ex vivo, and in vivo approaches and variations in experimental techniques. With the development of standardized techniques for manipulating cell populations in conditions that best mimic intestinal environments, it is likely that any glial influence on the epithelium will be clarified.
Cancer: Bidirectional Signaling Between ENS and Epithelial Tumor Cells
In recent years, many groups have examined the role of neurons and neurotransmitters in GI tumor initiation, maintenance, and metastasis. In human patients, dense innervation within tumors correlates with decreased long-term patient survival, suggesting that innervation may contribute to tumor growth and metastasis. Furthermore, severance of vagal nerve innervation of the intestine, via vagotomy procedures, correlates with decreased gastric tumor formation in mouse cancer models and humans.82, 83 In mouse gastric and colorectal tumor models, ACh from extrinsic innervation activates the M3 ACh receptor on Lgr5+ ISCs and increases Wnt signaling.58, 84 Interestingly, cholinergic stimulation of tumor cells induces nerve growth factor (NGF) expression, which causes increased tumor innervation in a tropomyosin receptor kinase–dependent manner.84 Remarkably, the ACh-NGF axis is sufficient to promote tumor initiation. In mice engineered to overexpress NGF within the epithelium, gastric tumors formed spontaneously, and colonic tumors formed at a higher rate after a chemical assault than controls.84 Interestingly, tuft cells play a role in initial cholinergic signaling and tumor formation. Tuft cells increase in number during early stages of tumorigenesis and are required for tumor formation, epithelial NGF expression, and tumor innervation in numerous cancer models.84 Furthermore, in vitro work demonstrated colorectal cancer cells adhere and migrate along enteric neuron projections via N-cadherin attachments, suggesting another mechanism by which tumor innervation complicates tumor dynamics.85 Clearly, the presence of the ENS impacts tumor initiation and progression, and this represents an intriguing area for future research in GI tumors.
Future Directions
In this review, we have highlighted important discoveries in intestinal biology regarding epithelial-ENS interactions. Much of the data yield an incomplete picture of the breadth and depth of these interactions. The field is poised to benefit from integration of neuroscience and epithelial techniques. Application of in vivo tools to modulate ENS activity, while probing the effects on the epithelium, likely will increase mechanistic understanding of the complex intestinal environment. Single cell sequencing will facilitate the development of tools to target subpopulations of ENS and epithelial cells. Additionally, uncovering anatomical structures and synaptic connections that make interactions possible are critical for progress. Although beyond the scope of this review, understanding and targeting ENS components of intestinal disease, such as inflammation or Hirschsprung’s disease, is an avenue of future research. Patients with Hirschsprung’s disease, characterized by a complete absence or malformation of a subset of the ENS, suffer from epithelial inflammation even after removal of the aganglionic intestine. It is exciting to explore if restoring functional epithelial-ENS interactions to aganglionic regions would return intestinal physiology to normal.86 Future studies undoubtedly will reveal the extent to which interactions between these 2 systems contribute to both intestinal homeostasis and disease.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding The work was supported by the University of Oregon Vice President for Research and Innovation’s Incubating Interdisciplinary Initiatives Award.
References
- 1.Barker N., van Es J.H., Kuipers J., Kujala P., van den Born M., Cozijnsen M., Haegebarth A., Korving J., Begthel H., Peters P.J., Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 2.Tian H., Biehs B., Warming S., Leong K.G., Rangell L., Klein O.D., de Sauvage F.J. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255–259. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sato T., van Es J.H., Snippert H.J., Stange D.E., Vries R.G., van den Born M., Barker N., Shroyer N.F., van de Wetering M., Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roth S., Franken P., Sacchetti A., Kremer A., Anderson K., Sansom O. Fodde R Paneth cells in intestinal homeostasis and tissue injury. PLoS One. 2012;7:e38965. doi: 10.1371/journal.pone.0038965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fre S., Huyghe M., Mourikis P., Robine S., Louvard D., Artavanis-Tsakonas S. Notch signals control the fate of immature progenitor cells in the intestine. Nature. 2005;435:964–968. doi: 10.1038/nature03589. [DOI] [PubMed] [Google Scholar]
- 6.Yang Q., Bermingham N.A., Finegold M.J., Zoghbi H.Y. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294:2155–2158. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- 7.Latorre R., Sternini C., De Giorgio R., Greenwood-Van Meerveld B. Enteroendocrine cells: a review of their role in brain-gut communication. Neurogastroenterol Motil. 2016;28:620–630. doi: 10.1111/nmo.12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Middelhoff M., Westphalen C.B., Hayakawa Y., Yan K.S., Gershon M.D., Wang T.C., Quante M. Dclk1-expressing tuft cells: critical modulators of the intestinal niche? Am J Physiol Gastrointest Liver Physiol. 2017;313:G285–G299. doi: 10.1152/ajpgi.00073.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furness J.B. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9:286–294. doi: 10.1038/nrgastro.2012.32. [DOI] [PubMed] [Google Scholar]
- 10.Poole D.P., Furness J.B. Enteric nervous system structure and neurochemistry related to function and neuropathology. Physiol Gastrointest Tract. 2012:557–581. [Google Scholar]
- 11.Gulbransen B.D., Sharkey K.A. Novel functional roles for enteric glia in the gastrointestinal tract. Nat Rev Gastroenterol Hepatol. 2012;9:625–632. doi: 10.1038/nrgastro.2012.138. [DOI] [PubMed] [Google Scholar]
- 12.Costa M., Brookes S.J., Hennig G.W. Anatomy and physiology of the enteric nervous system. Gut. 2000;47(suppl 4):iv15–iv19. doi: 10.1136/gut.47.suppl_4.iv15. discussion iv26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kondo J., Powell A.E., Wang Y., Musser M.A., Southard-Smith E.M., Franklin J.L., Coffey R.J. LRIG1 regulates ontogeny of smooth muscle−derived subsets of interstitial cells of Cajal in mice. Gastroenterology. 2015;149:407–419.e8. doi: 10.1053/j.gastro.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Owens B.M.J., Simmons A. Intestinal stromal cells in mucosal immunity and homeostasis. Mucosal Immunol. 2013;6:224–234. doi: 10.1038/mi.2012.125. [DOI] [PubMed] [Google Scholar]
- 15.Sailaja B.S., He X.C., Li L. The regulatory niche of intestinal stem cells. J Physiol. 2016;594:4827–4836. doi: 10.1113/JP271931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brookes S.J.H., Spencer N.J., Costa M., Zagorodnyuk V.P. Extrinsic primary afferent signalling in the gut. Nat Rev Gastroenterol Hepatol. 2013;10:286–296. doi: 10.1038/nrgastro.2013.29. [DOI] [PubMed] [Google Scholar]
- 17.Blackshaw L.A., Brookes S.J.H., Grundy D., Schemann M. Sensory Transmission in the gastrointestinal tract. Neurogastroenterol Motil. 2007;19:1–19. doi: 10.1111/j.1365-2982.2006.00871.x. [DOI] [PubMed] [Google Scholar]
- 18.Uesaka T., Young H.M., Pachnis V., Enomoto H. Development of the intrinsic and extrinsic innervation of the gut. Dev Biol. 2016;417:158–167. doi: 10.1016/j.ydbio.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 19.Singh B., Coffey R.J. Trafficking of epidermal growth factor receptor ligands in polarized epithelial cells. Annu Rev Physiol. 2014;76:275–300. doi: 10.1146/annurev-physiol-021113-170406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farin H.F., Jordens I., Mosa M.H., Basak O., Korving J., Tauriello D.V.F., de Punder K., Angers S., Peters P.J., Maurice M.M., Clevers H. Visualization of a short-range Wnt gradient in the intestinal stem-cell niche. Nature. 2016;530:340–343. doi: 10.1038/nature16937. [DOI] [PubMed] [Google Scholar]
- 21.van Niel G., D’Angelo G., Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 22.Zappulli V., Friis K.P., Fitzpatrick Z., Maguire C.A., Breakefield X.O. Extracellular vesicles and intercellular communication within the nervous system. J Clin Invest. 2016;126:1198–1207. doi: 10.1172/JCI81134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Budnik V., Ruiz-Cañada C., Wendler F. Extracellular vesicles round off communication in the nervous system. Nat Rev Neurosci. 2016;17:160–172. doi: 10.1038/nrn.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bohórquez D.V., Shahid R.A., Erdmann A., Kreger A.M., Wang Y., Calakos N., Wang F., Liddle R.A. Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J Clin Invest. 2015;125:782–786. doi: 10.1172/JCI78361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaelberer M.M., Buchanan K.L., Klein M.E., Barth B.B., Montoya M.M., Shen X., Bohórquez D.V. A gut-brain neural circuit for nutrient sensory transduction. Science. 2018;361:eaat5236. doi: 10.1126/science.aat5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Flier L.G., Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 27.Qu Z.-D., Thacker M., Castelucci P., Bagyánszki M., Epstein M.L., Furness J.B. Immunohistochemical analysis of neuron types in the mouse small intestine. Cell Tissue Res. 2008;334:147–161. doi: 10.1007/s00441-008-0684-7. [DOI] [PubMed] [Google Scholar]
- 28.Furness J.B. Blackwell; Oxford, England: 2006. The Enteric Nervous System. [Google Scholar]
- 29.Lasrado R., Boesmans W., Kleinjung J., Pin C., Bell D., Bhaw L., McCallum S., Zong H., Luo L., Clevers H., Vanden Berghe P., Pachnis V. Lineage-dependent spatial and functional organization of the mammalian enteric nervous system. Science. 2017;356:722–726. doi: 10.1126/science.aam7511. [DOI] [PubMed] [Google Scholar]
- 30.Boesmans W., Lasrado R., Vanden Berghe P., Pachnis V. Heterogeneity and phenotypic plasticity of glial cells in the mammalian enteric nervous system. Glia. 2015;63:229–241. doi: 10.1002/glia.22746. [DOI] [PubMed] [Google Scholar]
- 31.Wattchow D.A., Brookes S.J.H., Costa M. The morphology and projections of retrogradely labeled myenteric neurons in the human intestine. Gastroenterology. 1995;109:866–875. doi: 10.1016/0016-5085(95)90396-8. [DOI] [PubMed] [Google Scholar]
- 32.Liou A.P., Sei Y., Zhao X., Feng J., Lu X., Thomas C., Pechhold S., Raybould H.E., Wank S.A. The extracellular calcium-sensing receptor is required for cholecystokinin secretion in response to l -phenylalanine in acutely isolated intestinal I cells. Am J Physiol Liver Physiol. 2011;300:G538–G546. doi: 10.1152/ajpgi.00342.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bellono N.W., Bayrer J.R., Leitch D.B., Castro J., Zhang C., O’Donnell T.A., Brierley S.M., Ingraham H.A., Julius D. Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell. 2017;170:185–198.e16. doi: 10.1016/j.cell.2017.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rakhilin N., Barth B., Choi J., Muñoz N.L., Kulkarni S., Jones J.S., Small D.M., Cheng Y.-T., Cao Y., LaVinka C., Kan E., Dong X., Spencer M., Pasricha P., Nishimura N., Shen X. Simultaneous optical and electrical in vivo analysis of the enteric nervous system. Nat Commun. 2016;7:11800. doi: 10.1038/ncomms11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Makadia P.A., Najjar S.A., Saloman J.L., Adelman P., Feng B., Margiotta J.F., Albers K.M., Davis B.M. Optogenetic activation of colon epithelium of the mouse produces high-frequency bursting in extrinsic colon afferents and engages visceromotor responses. J Neurosci. 2018;38:5788–5798. doi: 10.1523/JNEUROSCI.0837-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orzekowsky-Schroeder R., Klinger A., Martensen B., Blessenohl M., Gebert A., Vogel A., Hüttmann G. In vivo spectral imaging of different cell types in the small intestine by two-photon excited autofluorescence. J Biomed Opt. 2011;16:116025. doi: 10.1117/1.3655587. [DOI] [PubMed] [Google Scholar]
- 37.Bao H., Boussioutas A., Reynolds J., Russell S., Gu M. Imaging of goblet cells as a marker for intestinal metaplasia of the stomach by one-photon and two-photon fluorescence endomicroscopy. J Biomed Opt. 2009;14:064031. doi: 10.1117/1.3269681. [DOI] [PubMed] [Google Scholar]
- 38.Goto K., Kato G., Kawahara I., Luo Y., Obata K., Misawa H., Ishikawa T., Kuniyasu H., Nabekura J., Takaki M. In vivo imaging of enteric neurogenesis in the deep tissue of mouse small intestine. PLoS One. 2013;8:e54814. doi: 10.1371/journal.pone.0054814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grubišić V., Gulbransen B.D. Enteric glial activity regulates secretomotor function in the mouse colon but does not acutely affect gut permeability. J Physiol. 2017;595:3409–3424. doi: 10.1113/JP273492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoffman J.M., Brooks E.M., Mawe G.M. Gastrointestinal motility monitor (GIMM) J Vis Exp. 2010;46:2435. doi: 10.3791/2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swaminathan M., Hill-Yardin E., Ellis M., Zygorodimos M., Johnston L.A., Gwynne R.M., Bornstein J.C. Video imaging and spatiotemporal maps to analyze gastrointestinal motility in mice. J Vis Exp. 2016;108:e53828. doi: 10.3791/53828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Savidge T.C., Newman P., Pothoulakis C., Ruhl A., Neunlist M., Bourreille A., Hurst R., Sofroniew M.V. Enteric glia regulate intestinal barrier function and inflammation via release of S-nitrosoglutathione. Gastroenterology. 2007;132:1344–1358. doi: 10.1053/j.gastro.2007.01.051. [DOI] [PubMed] [Google Scholar]
- 43.Puzan M., Hosic S., Ghio C., Koppes A. Enteric nervous system regulation of intestinal stem cell differentiation and epithelial monolayer function. Sci Rep. 2018;8:6313. doi: 10.1038/s41598-018-24768-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Landeghem L., Mahé M.M., Teusan R., Léger J., Guisle I., Houlgatte R., Neunlist M. Regulation of intestinal epithelial cells transcriptome by enteric glial cells: impact on intestinal epithelial barrier functions. BMC Genomics. 2009;10:507. doi: 10.1186/1471-2164-10-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neunlist M., Aubert P., Bonnaud S., Van Landeghem L., Coron E., Wedel T., Naveilhan P., Ruhl A., Lardeux B., Savidge T., Paris F., Galmiche J.P. Enteric glia inhibit intestinal epithelial cell proliferation partly through a TGF-beta1-dependent pathway. Am J Physiol Liver Physiol. 2007;292:G231–G241. doi: 10.1152/ajpgi.00276.2005. [DOI] [PubMed] [Google Scholar]
- 46.Sato T., Vries R.G., Snippert H.J., van de Wetering M., Barker N., Stange D.E., van Es J.H., Abo A., Kujala P., Peters P.J., Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 47.Sato T. Clevers H Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science. 2013;340:1190–1194. doi: 10.1126/science.1234852. [DOI] [PubMed] [Google Scholar]
- 48.Anitha M., Joseph I., Ding X., Torre E.R., Sawchuk M.A., Mwangi S., Hochman S., Sitaraman S.V., Anania F., Srinivasan S. Characterization of fetal and postnatal enteric neuronal cell lines with improvement in intestinal neural function. Gastroenterology. 2008;134:1424–1435. doi: 10.1053/j.gastro.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nagy N., Goldstein A.M. Enteric nervous system development: a crest cell’s journey from neural tube to colon. Semin Cell Dev Biol. 2017;66:94–106. doi: 10.1016/j.semcdb.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Workman M.J., Mahe M.M., Trisno S., Poling H.M., Watson C.L., Sundaram N., Chang C.-F., Schiesser J., Aubert P., Stanley E.G., Elefanty A.G., Miyaoka Y., Mandegar M.A., Conklin B.R., Neunlist M., Brugmann S.A., Helmrath M.A., Wells J.M. Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat Med. 2017;23:49–59. doi: 10.1038/nm.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Howitt M.R., Lavoie S., Michaud M., Blum A.M., Tran S.V., Weinstock J.V., Gallini C.A., Redding K., Margolskee R.F., Osborne L.C., Artis D., Garrett W.S. Tuft Cells, Taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science. 2016;351:1329–1333. doi: 10.1126/science.aaf1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Worthington J.J. The intestinal immunoendocrine axis: novel cross-talk between enteroendocrine cells and the immune system during infection and inflammatory disease. Biochem Soc Trans. 2015;43:727. doi: 10.1042/BST20150090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gross E.R., Gershon M.D., Margolis K.G., Gertsberg Z.V., Cowles R.A., Cowles R.A. Neuronal serotonin regulates growth of the intestinal mucosa in mice. Gastroenterology. 2012;143:408–417.e2. doi: 10.1053/j.gastro.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Specian R.D., Neutra M.R. Mechanism of rapid mucus secretion in goblet cells stimulated by acetylcholine. J Cell Biol. 1980;85:626–640. doi: 10.1083/jcb.85.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Westphalen C.B., Asfaha S., Hayakawa Y., Takemoto Y., Lukin D.J., Nuber A.H., Brandtner A., Setlik W., Remotti H., Muley A., Chen X., May R., Houchen C.W., Fox J.G., Gershon M.D., Qante M., Wang T.C. Long-lived intestinal tuft cells serve as colon cancer–initiating cells. J Clin Invest. 2014;124:1283–1295. doi: 10.1172/JCI73434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Greig C.J., Cowles R.A. Muscarinic acetylcholine receptors participate in small intestinal mucosal homeostasis. J Pediatr Surg. 2017;52:1031–1034. doi: 10.1016/j.jpedsurg.2017.03.037. [DOI] [PubMed] [Google Scholar]
- 57.Davis E.A., Zhou W., Dailey M.J. Evidence for a direct effect of the autonomic nervous system on intestinal epithelial stem cell proliferation. Physiol Rep. 2018;6:e13745. doi: 10.14814/phy2.13745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao C.-M., Hayakawa Y., Kodama Y., Muthupalani S., Westphalen C.B., Andersen G.T., Flatberg A., Johannessen H., Friedman R.A., Renz B.W., Sandvik A.K., Beisvag V., Tomita H., Hara A., Quante M., Li Z., Gershon M.D., Kaneko K., Fox J.G., Wang T.C., Chen D. Denervation suppresses gastric tumorigenesis. Sci Transl Med. 2014;6:250ra115. doi: 10.1126/scitranslmed.3009569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lachat J.-J., Goncalves R.P. Influence of autonomic denervation upon the kinetics of the ileal epithelium of the rat. Cell Tissue Res. 1978;192:285–297. doi: 10.1007/BF00220746. [DOI] [PubMed] [Google Scholar]
- 60.Li Z., Caron M.G., Blakely R.D., Margolis K.G., Gershon M.D. Dependence of serotonergic and other nonadrenergic enteric neurons on norepinephrine transporter expression. J Neurosci. 2010;30:16730–16740. doi: 10.1523/JNEUROSCI.2276-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nezami B.G., Srinivasan S. Enteric nervous system in the small intestine: pathophysiology and clinical implications. Curr Gastroenterol Rep. 2010;12:358–365. doi: 10.1007/s11894-010-0129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mittal R., Debs L.H., Patel A.P., Nguyen D., Patel K., O’Connor G., Grati M., Mittal J., Yan D., Eshraghi A.A., Deo S.K., Daunert S., Liu X.Z. Neurotransmitters: the critical modulators regulating gut-brain axis. J Cell Physiol. 2017;232:2359–2372. doi: 10.1002/jcp.25518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Berlioz F., Maoret J.J., Paris H., Laburthe M., Farinotti R., Rozé C. Alpha(2)-adrenergic receptors stimulate oligopeptide transport in a human intestinal cell line. J Pharmacol Exp Ther. 2000;294:466–472. [PubMed] [Google Scholar]
- 64.Sternini C., Anselmi L., Rozengurt E. Enteroendocrine cells: a site of “taste” in gastrointestinal chemosensing. Curr Opin Endocrinol Diabetes Obes. 2008;15:73–78. doi: 10.1097/MED.0b013e3282f43a73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rindi G., Leiter A.B., Kopin A.S., Bordi C., Solcia E. The “normal” endocrine cell of the gut: changing concepts and new evidences. Ann N Y Acad Sci. 2004;1014:1–12. doi: 10.1196/annals.1294.001. [DOI] [PubMed] [Google Scholar]
- 66.Wang F., Knutson K., Alcaino C., Linden D.R., Gibbons S.J., Kashyap P., Grover M., Oeckler R., Gottlieb P.A., Li H.J., Leter A.B., Farrugia G., Beyder A. Mechanosensitive ion channel Piezo2 is important for enterochromaffin cell response to mechanical forces. J Physiol. 2017;595:79–91. doi: 10.1113/JP272718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alcaino C., Knutson K.R., Treichel A.J., Yildiz G., Strege P.R., Linden D.R., Li J.H., Leiter A.B., Szurszewski J.H., Farrugia G., Beyder A. A population of gut epithelial enterochromaffin cells is mechanosensitive and requires Piezo2 to convert force into serotonin release. Proc Natl Acad Sci U S A. 2018;115:E7632–E7641. doi: 10.1073/pnas.1804938115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beyder A In pursuit of the epithelial mechanosensitivity mechanisms. Front Endocrinol. 2019;9:804. doi: 10.3389/fendo.2018.00804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Konturek S.J., Konturek J.W., Pawlik T., Brzozowski T. Brain-gut axis and its role in the control of food intake. J Physiol Pharmacol. 2004;55:137–154. [PubMed] [Google Scholar]
- 70.Small C.J., Bloom S.R. Gut hormones and the control of appetite. Trends Endocrinol Metab. 2004;15:259–263. doi: 10.1016/j.tem.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 71.Nozawa K., Kawabata-Shoda E., Doihara H., Kojima R., Okada H., Mochizuki S., Sano Y., Inamura K., Matsushime H., Koizumi T., Yokoyama T., Ito H. TRPA1 regulates gastrointestinal motility through serotonin release from enterochromaffin cells. Proc Natl Acad Sci U S A. 2009;106:3408–3413. doi: 10.1073/pnas.0805323106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Capasso R., Aviello G., Romano B., Borrelli F., De Petrocellis L., Di Marzo V., Izzo A.A. Modulation of mouse gastrointestinal motility by allyl isothiocyanate, a constituent of cruciferous vegetables (Brassicaceae): evidence for TRPA1-independent effects. Br J Pharmacol. 2012;165:1966–1977. doi: 10.1111/j.1476-5381.2011.01703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Heredia D.J., Gershon M.D., Koh S.D., Corrigan R.D., Okamoto T., Smith T.K. Important role of mucosal serotonin in colonic propulsion and peristaltic reflexes: in vitro analyses in mice lacking tryptophan hydroxylase 1. J Physiol. 2013;591:5939–5957. doi: 10.1113/jphysiol.2013.256230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Spencer N.J., Sia T.C., Brookes S.J., Costa M., Keating D.J. CrossTalk opposing view: 5-HT is not necessary for peristalsis. J Physiol. 2015;593:3229–3231. doi: 10.1113/JP270183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smith T.K., Gershon M.D. CrossTalk proposal: 5-HT is necessary for peristalsis. J Physiol. 2015;593:3225–3227. doi: 10.1113/JP270182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gerbe F., van Es J.H., Makrini L., Brulin B., Mellitzer G., Robine S., Romagnolo B., Shroyer N.F., Bourgaux J.-F., Pignodel C., Clevers H., Jay P. Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J Cell Biol. 2011;192:767–780. doi: 10.1083/jcb.201010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schütz B., Jurastow I., Bader S., Ringer C., von Engelhardt J., Chubanov V., Gudermann T., Diener M., Kummer W., Krasteva-Christ G., Weihe E. Chemical coding and chemosensory properties of cholinergic brush cells in the mouse gastrointestinal and biliary tract. Front Physiol. 2015;6:87. doi: 10.3389/fphys.2015.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bush T.G., Savidge T.C., Freeman T.C., Cox H.J., Campbell E.A., Mucke L., Johnson M.H., Sofroniew M.V. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell. 1998;93:189–201. doi: 10.1016/s0092-8674(00)81571-8. [DOI] [PubMed] [Google Scholar]
- 79.Rao M., Rastelli D., Dong L., Chiu S., Setlik W., Gershon M.D., Corfas G. Enteric glia regulate gastrointestinal motility but are not required for maintenance of the epithelium in mice. Gastroenterology. 2017;153:1068–1081.e7. doi: 10.1053/j.gastro.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Van Landeghem L., Chevalier J., Mahé M.M., Wedel T., Urvil P., Derkinderen P., Savidge T., Neunlist M. Enteric glia promote intestinal mucosal healing via activation of focal adhesion kinase and release of ProEGF. Am J Physiol Liver Physiol. 2011;300:G976–G987. doi: 10.1152/ajpgi.00427.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ruhl A., Trotter J., Stremmel W. Isolation of enteric glia and establishment of transformed enteroglial cell lines from the myenteric plexus of adult rat. Neurogastroenterol Motil. 2001;13:95–106. doi: 10.1046/j.1365-2982.2001.00246.x. [DOI] [PubMed] [Google Scholar]
- 82.Bahmanyar S., Ye W., Dickman P.W., Nyrén O. Long-term risk of gastric cancer by subsite in operated and unoperated patients hospitalized for peptic ulcer. Am J Gastroenterol. 2007;102:1185–1191. doi: 10.1111/j.1572-0241.2007.01161.x. [DOI] [PubMed] [Google Scholar]
- 83.Lundegardh G., Ekbom A., McLaughlin J.K. Gastric cancer risk after vagotomy. Gut. 1994;35:946–949. doi: 10.1136/gut.35.7.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hayakawa Y., Sakitani K., Konishi M., Asfaha S., Niikura R., Tomita H., Renz B.W., Tailor Y., Macchini M., Middelhoff M., Jiang Z., Tanaka T., Dubeykovskaya Z.A., Kim W., Chen X., Urbanska A.M., Nagar K., Westphalen C.B., Quante M., Lin C.S., Gershon M.D., Hara A., Zhao C.M., Chen D., Worthley D.L., Koike K., Wang T.C. Nerve growth factor promotes gastric tumorigenesis through aberrant cholinergic signaling. Cancer Cell. 2017;31:21–34. doi: 10.1016/j.ccell.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Duchalais E., Guilluy C., Nedellec S., Touvron M., Bessard A., Touchefeu Y., Bossard C., Boudin H., Louarn G., Neunlist M., Van Landeghem L. Colorectal cancer cells adhere to and migrate along the neurons of the enteric nervous system. Cell Mol Gastroenterol Hepatol. 2018;5:31–49. doi: 10.1016/j.jcmgh.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Heuckeroth R.O. Hirschsprung disease — integrating basic science and clinical medicine to improve outcomes. Nat Rev Gastroenterol Hepatol. 2018;15:152–167. doi: 10.1038/nrgastro.2017.149. [DOI] [PubMed] [Google Scholar]