Abstract
Background
Intravenous acetaminophen (IV APAP) is an option in multimodal postoperative analgesia. Prior trials focus on hip and knee arthroplasties, whereas large-scale data on utilization and effectiveness in shoulder arthroplasties are lacking.
Methods
Data on 67,494 (452 hospitals) partial/total shoulder arthroplasties were extracted from the Premier claims database (2011–2016). Patients were categorized by receipt and dosage of IV APAP. Multilevel models measured associations between IV APAP and opioid utilization (in oral morphine equivalents), length/cost of stay and opioid-related complications. Effect estimates (adjusted % change) with 95% confidence intervals (CIs) are reported.
Results
IV APAP was used in 17.7% (n = 11,949) of patients with an increasing utilization trend. Most patients received only one dose on the day of surgery (69.5%; n = 8308). When adjusting for relevant covariates, IV APAP was not associated with meaningful effects on outcomes. Specifically, its use (versus no use) was not associated with decreased (but rather somewhat increased) opioid utilization: + 5.4% (CI 3.6–7.1%; P < 0.05).
Conclusion
In this first large-scale study that assesses IV APAP in shoulder arthroplasties, IV APAP use was not associated with decreased opioid utilization or the length/cost of stay. These results do not support routine use of IV APAP in this cohort, especially given its high cost.
The translational potential for this article
Multimodal pain control to assist in reducing the opioid pain medications are seen as a route to improved postoperative patient outcomes, better pain control and expedited hospital discharge. Acetaminophen plays a significant role in these protocols in many institutions, but it is not established if this expensive IV formulation is superior to the oral formulation. This study evaluates the use and effectiveness of IV acetaminophen following shoulder arthroplasty at a large number of institutions.
Keywords: Intravenous acetaminophen, Multimodal pain regimen, Opioid use, Postoperative pain control, Shoulder arthroplasty, Shoulder surgery
Introduction
Inadequate pain control after surgery may contribute to postoperative complications such as a myocardial infarction, impaired wound healing and inadequate respiratory effort [1]. Historically, opioid medications have been a mainstay of postoperative pain control, but they have many deleterious side effects, such as drowsiness, postoperative nausea and vomiting, ileus, respiratory depression, bladder dysfunction in the short term and the risk of addiction in the longer term [2]. For these reasons, multimodal pain regimens including acetaminophen have become increasingly popular across many surgical subspecialities, including orthopaedic surgery and particularly lower extremity joint arthroplasties.
The current practice guidelines and recommendations of the American Academy of Orthopaedic Surgeons, American Society of Anesthesiologists, Joint Commission, American College of Critical Care Medicine, American Pain Society support the use of multimodal analgesia MMA [3], [4], [5], [6], [7], [8]. Multimodal regimens use multiple medications and nondrug interventions to target the central and peripheral nervous system at different sites in the pain pathway, providing additive or synergistic effects in comparison to the use of a single agent [8]. The goal of this approach is to improve pain control and reduce side effects by decreasing dosing requirements of opioids [9].
Acetaminophen has been effectively used in combination with opioids for its analgesic effects [10], [11]. The intravenous formulation of acetaminophen (IV APAP) was approved in the United States in 2010 for management of mild-to-moderate pain, moderate-to-severe pain with adjunctive analgesics and reduction of fever. It allows for the use of acetaminophen in postoperative patients who cannot take oral formulations due to nausea, vomiting, recovery from gastrointestinal surgery or impaired drug absorption. In addition, the intravenous form provides quicker and higher peak plasma and cerebrospinal fluid drug concentrations than oral or rectal dosing [12]. IV APAP has been shown in many studies to be both safe and effective as an adjunct in multimodal analgesia for a wide variety of surgical procedures, particularly total joint arthroplasty [13], [14], [15], [16]. Despite IV APAP's widespread use in total knee and hip arthroplasty, its use has not been widely studied in the shoulder arthroplasty population. The use of IV APAP as an adjunct in multimodal analgesia has increased as the demand for shoulder arthroplasty grows along pressures to both reduce inpatient hospital stays and decrease opioid usage.
The purpose of this study was to identify (1) the prevalence of IV APAP use in a large number of hemiarthroplasty, total shoulder arthroplasty (TSA) and reverse shoulder arthroplasty procedures, (2) the effectiveness of IV APAP use in pain control in the postoperative period and (3) associated complications. We hypothesized that IV APAP use in TSA would reduce the utilization of opioids, decrease in-hospital complications, shorten hospital length of stay (LOS) and lower the in-hospital cost.
Materials and methods
Data source and study design
Administrative claims data were collected from the Premier Healthcare Database (Premier Healthcare Solutions, Inc., Charlotte, NC) that contains data on approximately one in five US hospital discharges [17]. Records include International Classification of Disease-9th revision (ICD-9) codes and complete inpatient billing items. The Mount Sinai Hospital Institutional Review Board approved the use of the Health Insurance Portability and Accountability Act (HIPAA)-compliant anonymized data for this study (project #14-00647).
Study sample
The study sample was defined using ICD-9 codes for TSA, including reverse total shoulder (ICD-9 81.80, 81.88) and partial shoulder arthroplasty (ICD-9 81.81) for the period of 2011–2016. Exclusion criteria were unknown gender (n = 35), unknown discharge status (n = 226), nonelective procedure (n = 15,040), outpatient procedure (n = 3790), hospitals that performed <30 shoulder arthroplasties (n = 1831) to ensure sufficient sample size per hospital, no billing for opioids (n = 4007, as it is one of the main study outcomes) and opioid utilization greater than the 95th percentile (n = 3552, to exclude outliers) [18].
Study variables
The main effect of interest was utilization of IV APAP (extracted from medication billing data) categorized by the number of doses (1 or 2+) and day of utilization (day of surgery or days after: day 0, day 1 and day 2+). The main outcomes included opioid utilization, length and cost of hospital stay, opioid-related adverse drug events during the inpatient stay (as previously reported): respiratory, gastrointestinal, genitourinary, central nervous system and “other” complications and naloxone use (as a marker of an opioid-related complication) [19]. Complications were defined using ICD-9 codes depicted in Appendix I. “Other” complications include postoperative bradycardia, rash or itching, drugs causing adverse effects and fall from bed. The use of opioids was defined by using billing data that were then converted into oral morphine equivalents (OMEs) using the Lexicomp “opioid agonist conversion” and GlobalRPH “opioid analgesic converter” [20], [21], [22]. The main assumption is that billing for opioids is a fair proxy for administration of opioids. However, we recognize that billing for opioids does not equate to actual administration of opioids. As the mismatch between billing and actual administration is likely equal among treatment groups (i.e. those that received IV APAP versus those that did not), the effect of this limitation should be minimal. Moreover, this variable has been used in several publications by our study group after an initial validation study demonstrating agreement between opioids billed in the Premier data set and institutional electronic medical record pharmacy data. Therefore, for this study, we assume that billing for opioids appropriately standardizes for opioid consumption between two large population samples.
Patient demographics included age, gender and race/ethnicity (white, black, Hispanic and others). Health care–related characteristics were insurance type (commercial, Medicaid, Medicare, uninsured or unknown), hospital location (rural or urban), hospital size (<300, 300–499 and ≥500 beds), hospital teaching status and mean per-hospital annual number of shoulder arthroplasties. Procedure-related variables included the year of procedure and type of procedure (partial, total and reverse shoulder arthroplasty), whereas analgesia-related variables included the use of a peripheral nerve block, use of nonopioid analgesics [gabapentin/pregabalin, cyclooxygenase-2 (cox-2) inhibitors, other nonsteroidal antiinflammatory drugs (NSAIDs) and ketamine] and the use of patient-controlled analgesia (PCA). Comorbidity-related variables included the Quan adaptation of the Charlson-Deyo Comorbidity Index and specific variables associated with opioid utilization including substance use/abuse (including smoking), pain conditions and psychiatric comorbidities [23], [24].
Statistical analysis
The Chi-square test, t test and Mann–Whitney rank-sum test were used for categorical and continuous variables in the univariable analyses. In addition, we assessed trends in opioid utilization before and after the introduction of IV APAP to the US market. Multilevel multivariable models assessed the association between IV APAP use and outcomes while adjusting for covariates deemed to be clinically significant or those were significant at the P < 0.15 level in univariable analyses [25]. Multilevel modelling accounted for the correlation of patients within a hospital (i.e. patients “nested” within each hospital). This type of model will fit one regression line for each hospital, using all the patients from that hospital as it is not uncommon for patients within the same hospital to receive similar care [26], [27]. Included hospitals had sufficient patients (n > 30) per hospital, which is the recommended sample size to help reduce bias while performing a multilevel model [18]. Each model was assessed using the area under the receiver-operating characteristic curve (c-statistic), which is an indication of how a model discriminates between levels of the outcome; >0.7 is an indication of a model with good discriminatory power. Odds ratios (ORs) and 95% confidence intervals (CIs) are reported for binary outcomes [25]. Continuous outcomes (opioid utilization and length and cost of hospital stay) were log-transformed because of the skewness of the data; these outcomes are reported as percent change with 95% CI [28], [29].
All analyses were performed using SAS 9.4 (Cary, NC, USA). In SAS, the PROC GLIMMIX was used for the multilevel multivariable regression models [27].
Sensitivity analysis
To assess robustness of the current study findings, we additionally performed two sensitivity analyses. First, we compared the effect estimates for IV APAP use to estimates from control nonopioid analgesic, COX-2 inhibitors as we would theoretically expect both to be associated with decreased opioid utilization.
The second sensitivity analysis applied calculated propensity scores (i.e. the propensity to receive IV APAP). Propensity score analysis is used to reduce selection bias in nonrandomized studies by equating patients based on observed baseline characteristics [30], [31]. The covariates used to estimate the propensity score were age, annual shoulder arthroplasty volume, hospital size, Deyo Index, gender, race, year of procedure, payor, hospital location, hospital teaching status, use of peripheral nerve block, type of procedure, use of nonopioid analgesics (gabapentin/pregabalin, COX-2 inhibitors, NSAIDs, and ketamine), use of PCA, comorbidity-related variables for substance use/abuse (including smoking), pain conditions, and psychiatric comorbidities [24]. The propensity score was then used in two analysis strategies: (1) covariate adjustment and (2) matching. In the covariate adjustment approach, the propensity score was used as a covariate (next to IV APAP) in a set of regression models. In the matching approach, the propensity score was used to match treated to untreated patients (i.e. matching patients with similar propensity to receive IV APAP). Here, we matched IV APAP patients with three patients who did not receive IV APAP. We measured the covariate balance between the groups by comparing standardized differences on the original study sample and the matched sample (as previously described) and found standardized differences <10% in the matched cohort, generally accepted as acceptable balance [31], [32].
Results
Overall, 67,494 shoulder arthroplasty patients were included from 452 hospitals; IV APAP was used in 17.7% (n = 11,949) of patients.
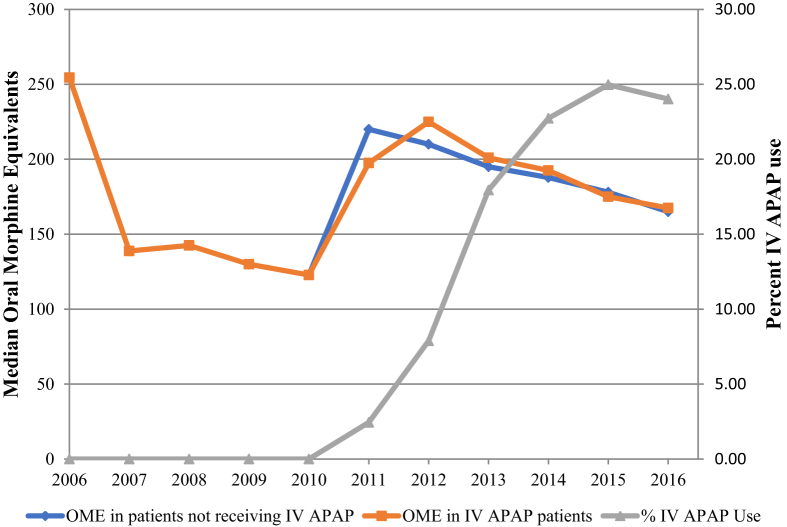
Table 1 shows characteristics of the study population by IV APAP use. The majority of patients receiving IV APAP received the medication on Day 0 (the day of surgery) and only received a single dose (69.5% n = 8308). Although most comparisons are statistically significant, particularly higher IV APAP use was seen in rural hospitals and in those with ≥500 beds and a lower annual shoulder arthroplasty volume. Moreover, IV APAP use was also higher in patients receiving other nonopioid analgesics. Interestingly, the use of IV APAP increased sharply from 3.4% (n = 292) in 2011 to 21.8% (n = 2870) in 2016 (Fig. 1; these represent row percentages, not column percentages as depicted in Table 1). The overall median opioid utilization (in OME) showed a decreasing trend with no differences in patients receiving IV APAP versus those who did not (Fig. 1).
Table 1.
Characteristics of the study population by IV APAP use. *Continuous variables median and interquartile range reported, instead of N and %, respectively.**Chi-square test for categorical variables, t test for continuous variables.
| Study population characteristics | IV APAP Use |
P** | |||
|---|---|---|---|---|---|
| Yes (n = 11,949) |
No (n = 55,545) |
||||
| n | % | n | % | ||
| Dose and day of Use | |||||
| Day 0 | |||||
| 0 | 421 | 3.5 | — | — | |
| 1 | 8308 | 69.5 | — | — | |
| 2+ | 3220 | 26.9 | — | — | |
| Day 1 | |||||
| 0 | 8670 | 72.6 | — | — | |
| 1 | 1582 | 13.2 | — | — | |
| 2+ | 1697 | 14.2 | — | — | |
| Day 2+ | |||||
| 0 | 11,510 | 96.3 | — | — | |
| 1 | 236 | 2.0 | — | — | |
| 2+ | 203 | 1.7 | — | — | |
| Patient demographics | |||||
| Median Age* | 70 | 63–76 | 70 | 63–76 | 0.7795 |
| Gender | 0.0228 | ||||
| Female | 6720 | 56.2 | 31,869 | 57.4 | |
| Male | 5229 | 43.8 | 23,676 | 42.6 | |
| Race/Ethnicity | <0.0001 | ||||
| White | 10,528 | 88.1 | 47,166 | 84.9 | |
| Black | 572 | 4.8 | 2454 | 4.4 | |
| Hispanic | 1 | 0.0 | 45 | 0.1 | |
| Other | 848 | 7.1 | 5880 | 10.6 | |
| Health care related | |||||
| Insurance type | 0.0010 | ||||
| Commercial | 2698 | 22.6 | 12,225 | 22.0 | |
| Medicaid | 343 | 2.9 | 1299 | 2.3 | |
| Medicare | 8341 | 69.8 | 39,165 | 70.5 | |
| Uninsured | 54 | 0.5 | 221 | 0.4 | |
| Unknown | 513 | 4.3 | 2635 | 4.7 | |
| Hospital location | 0.0052 | ||||
| Rural | 1233 | 10.3 | 5270 | 9.5 | |
| Urban | 10,716 | 89.7 | 50,275 | 90.5 | |
| Hospital size | <0.0001 | ||||
| <300 beds | 4756 | 39.8 | 23,477 | 42.3 | |
| 300–499 beds | 3886 | 32.5 | 18,172 | 32.7 | |
| ≥500 beds | 3307 | 27.7 | 13,896 | 25.0 | |
| Hospital teaching status | |||||
| Nonteaching | 7277 | 60.9 | 33,903 | 61.0 | 0.7814 |
| Teaching | 4672 | 39.1 | 21,642 | 39.0 | |
| Median# of annual shoulder arthroplasties per hospital* | 46 | 31–81 | 52 | 31–83 | <0.0001 |
| Procedure related | |||||
| Year of procedure | <0.0001 | ||||
| 2011 | 292 | 2.4 | 8180 | 14.7 | |
| 2012 | 943 | 7.9 | 8896 | 16.0 | |
| 2013 | 2144 | 17.9 | 8637 | 15.6 | |
| 2014 | 2716 | 22.7 | 8935 | 16.1 | |
| 2015 | 2984 | 25.0 | 10,596 | 19.1 | |
| 2016 | 2870 | 24.0 | 10,301 | 18.5 | |
| Type of procedure | <0.0001 | ||||
| Total shoulder arthroplasty | 5244 | 43.9 | 25,327 | 45.6 | |
| Reverse shoulder arthroplasty | 5534 | 46.3 | 23,807 | 42.9 | |
| Partial shoulder arthroplasty | 1171 | 9.8 | 6411 | 11.5 | |
| Analgesia related | |||||
| Peripheral nerve block | 1988 | 16.6 | 12,801 | 23.0 | <0.0001 |
| NSAIDs | 4665 | 39.0 | 17,931 | 32.3 | <0.0001 |
| Cox-2 inhibitors | 3260 | 27.3 | 10,835 | 19.5 | <0.0001 |
| Ketamine | 418 | 3.5 | 1293 | 2.3 | <0.0001 |
| Gabapentinoids | 3434 | 28.7 | 11,893 | 21.4 | <0.0001 |
| Patient-controlled analgesia | 840 | 7.0 | 5986 | 10.8 | <0.0001 |
| Comorbidity related | |||||
| Charlson-Deyo Comorbidity Index (categorized) | 0.0075 | ||||
| 0 | 7901 | 66.1 | 37,479 | 67.5 | |
| 1 | 2777 | 23.2 | 12,143 | 21.9 | |
| 2 | 802 | 6.7 | 3657 | 6.6 | |
| 2+ | 469 | 3.9 | 2266 | 4.1 | |
| History of substance use/abuse | 1143 | 9.6 | 5264 | 9.5 | 0.7642 |
| Pain conditions | 2381 | 19.9 | 10,501 | 18.9 | 0.0100 |
| Psychiatric comorbidities | 2765 | 23.1 | 12,401 | 22.3 | 0.0531 |
Cox-2 = cyclooxygenase-2; IV APAP = intravenous acetaminophen; NSAIDs = nonsteroidal antiinflammatory drugs.
Figure 1.
The use of IV APAP increased sharply between 2011 and 2016. The overall median opioid utilization (in OME) showed a decreasing trend with no differences in patients receiving IV APAP versus those who did not.
IV APAP = intravenous acetaminophen; OME = oral morphine equivalent.
Table 2 shows the outcome variables by IV APAP use. While generally small differences between groups were observed, patients receiving IV APAP (versus those who did not) had higher total costs of hospitalization (median $18,425 vs. $17,938, P < 0.0001).
Table 2.
Outcome variables by IV APAP use.
| Study outcomes | IV APAP Use |
Pb | |||
|---|---|---|---|---|---|
| Yes (n = 11,949) |
No (n = 55,545) |
||||
| n | % | n | % | ||
| Resource utilization | |||||
| Oral morphine equivalentsa | 226 | 111–295 | 233 | 113–310 | <0.0001 |
| Length of hospital staya | 2 | 1–2 | 2 | 1–2 | <0.0001 |
| Cost of hospitalizationa | $18,425 | $13,495–$21,514 | $17,938 | $13,146–$21,058 | <0.0001 |
| Opioid-related adverse effects | |||||
| Respiratory | 197 | 1.6 | 1075 | 1.9 | 0.0366 |
| Gastrointestinal | 165 | 1.4 | 720 | 1.3 | 0.4607 |
| Central nervous system | 72 | 0.6 | 348 | 0.6 | 0.7626 |
| Genitourinary system | 347 | 2.9 | 1187 | 2.1 | <0.0001 |
| Other | 70 | 0.6 | 427 | 0.8 | 0.0339 |
| Use of naloxone | 95 | 0.8 | 552 | 1.0 | 0.0431 |
IV APAP = intravenous acetaminophen.
Continuous variables median and interquartile range reported, instead of N and %, respectively
Chi-square test for categorical variables, t test for continuous variables.
After adjusting for relevant covariates (Table 3), IV APAP (compared with no IV APAP use) was not associated with decreased effects for resource utilization outcomes, but rather somewhat increased (but clinically nonsignificant) effects; for opioid utilization, this was +5.4% (CI, 3.6–7.1%, P < 0.0001). No significant effects were seen for opioid-related adverse effects.
Table 3.
After adjusting for relevant covariates, IV APAP (vs. no IV APAP) is not associated with significantly decreased (but rather somewhat increased) effects for resource utilization outcomes; this did not apply to opioid-related adverse effects (*P < 0.05,**% change).
| IV APAP Use |
|
|---|---|
| [Reference = no IV APAP] | |
| Resource utilization | |
| Oral morphine equivalents** | 5.4% (3.6%; 7.1%)* |
| Length of hospital stay** | 2.7% (0.6%; 4.8%)* |
| Cost of hospitalization** | 2.2% (1.4%; 2.9%)* |
| Opioid-related adverse effects | |
| Respiratory | 0.97 (0.79; 1.19) |
| Gastrointestinal | 1.13 (0.91; 1.42) |
| Central nervous system | 1.11 (0.82; 1.51) |
| Genitourinary system | 1.12 (0.95; 1.33) |
| Other | 0.85 (0.62; 1.17) |
| Use of naloxone | 0.88 (0.67; 1.19) |
IV APAP = intravenous acetaminophen.
Sensitivity analyses
Table 4 describes the association between a nonopioid analgesic, COX-2 inhibitors and study outcomes. In comparison with IV APAP, COX-2 usage demonstrated expected effect estimates and was associated with a reduction in opioid utilization: −6.1% (95% CI, 7.5−4.6%; P < 0.05). In addition, COX-2 usage showed lower odds of the opioid-related adverse effects, although only “other” complications reached statistical significance (P < 0.05).
Table 4.
The association between a nonopioid analgesic, COX-2 inhibitors and study outcomes (*P < 0.05, **% change).
| Study outcomes | Cox-2 inhibitor use |
|---|---|
| [Reference = no Cox-2] | |
| Resource utilization | |
| Oral morphine equivalents** | −6.1% (−7.5%; −4.6%)* |
| Length of hospital stay** | −6.4% (−8.1%; −4.6%)* |
| Cost of hospitalization** | −1.2% (−1.9%; −0.5%)* |
| Opioid-related adverse effects | |
| Respiratory | 0.91 (0.76; 1.10) |
| Gastrointestinal | 0.84 (0.67; 1.06) |
| Central nervous system | 0.96 (0.71; 1.29) |
| Genitourinary system | 0.94 (0.80; 1.11) |
| Other | 0.71 (0.52; 0.97)* |
| Use of naloxone | 0.91 (0.71; 1.17) |
Cox-2 = cyclooxygenase-2.
The propensity score matching approach reduced our cohort from n = 67,494 to n = 11,560, corresponding to 17% of the original cohort. Both the matched and the covariate adjustment analysis corroborated findings from our main analysis: IV APAP was not associated with decreased (but rather somewhat increased) opioid utilization, cost/length of hospitalization and opioid-related complications.
Discussion
In this retrospective cohort study utilizing a hospital administrative claims database, we assessed the use of IV APAP and its effectiveness in pain management in the postoperative period after shoulder arthroplasty. This is the first study examining the use of IV APAP, a new and exciting medication with a rapid increase in use, on patients undergoing shoulder surgery in the literature. Our hypothesis was that IV APAP use in shoulder arthroplasty would reduce the utilization of opioids, decrease in-hospital complications, shorten hospital LOS and lower the in-hospital cost. However, our results show overall usage of IV APAP increased sharply between 2011 and 2016 in shoulder arthroplasty, although there was no association with any significant meaningful effects on opioid utilization, LOS, inpatient cost or opioid-related outcomes.
Since its introduction and the Food and Drug Administration approval in 2010, IV APAP has seen a rapid increase in use across various surgical subspecialities. Outside of orthopaedics, it has been shown to significantly reduce time to ambulation in patients undergoing bowel surgery and lower length of hospital stay in patients undergoing laparoscopic colon resection [33], [34]. In paediatric spine surgery, IV APAP has also shown improved pain control postoperatively. Olbrecht et al. demonstrated that IV APAP use hastened oral intake in adolescent patients and had a significant opioid-sparing effect associated with shorter LOS [35]. However, in a similar cohort, use of IV APAP in the 24-hour postoperative period demonstrated fewer time points with a visual analogue scale (VAS) greater than 6 but did not reduce oxycodone consumption.
IV APAP has shown comparable outcomes in orthopaedic surgery, even becoming standard in multimodal pain regimens after hip and knee arthroplasty. Sinatra et al., in a randomized, double-blinded, placebo-controlled trial, showed that IV APAP 1,000 mg of q6h had significantly improved pain responses after total knee (TKA) or hip (THA) arthroplasties than those who received placebo [36]. A more recent expanded analysis of this group confirmed statistically significant reductions in pain intensity levels over the initial 24 h postoperatively, as well as greater time to need rescue opioid dosing (3.9/2.1 h for THA/TKA and 0.8 h for placebo) [14]. In a combination of two previously unpublished double-blind, randomized, placebo-controlled clinical trials, IV APAP had a greater efficacy than placebo at pain intensity at up to 3 h from administration; patients who received IV APAP required less than half the amount of rescue medication after THA [15].
However, other studies have not shown such strong results. Raiff et al. examined the efficacy of a single dose of IV APAP intraoperatively during THA and TKA. They found no difference in the OMEs received in the 24-h postoperative period and no difference in the length of Post-Anesthesia Care Unit (PACU) stay [37]. In a cohort of patients receiving at least one dose of IV APAP versus none after undergoing TKA, Nwagbologu found no differences in mean morphine equivalents at 24 or 48 h postoperatively, opioid-related side effects or LOS [38]. Kelly et al. similarly found no difference in the daily morphine equivalent use or LOS between IV APAP and control groups of patients undergoing TKA and even saw a nonsignificant trend towards increased opioid use in the IV APAP group [16]. Our data showed a similar small but statistically (while not clinically) significant increase in opioid utilization. One area of orthopaedic surgery with more positive outcomes with IV APAP was in a cohort of geriatric hip fractures. Patients who received IV APAP had a significantly shorter mean LOS, lower mean pain score, lower mean narcotic usage, lower rate of missed physical therapy sessions and higher likelihood of discharge home [39].
One issue of critical importance in the widespread adoption of IV APAP is cost. IV APAP is currently approximately $45 per 1-g vial (average wholesale price, not taking into account rebates or discounts), whereas the oral formulation of APAP is about $0.05–0.10 per dose. Several large hospital systems across the country noted a large increase in costs in relation to this medicine and removed it from their formulary [40]. Several large database studies have shown that patients receiving IV APAP in comparison to opioids alone after orthopaedic surgery was associated with shorter LOS and decreased hospitalization costs, but these studies were performed by and/or with funding from the company with exclusive licence to IV APAP in the United States [41], [42], [43]. Placebo-controlled studies have not shown such a reduction in LOS [16], [38]. In fact, our data showed a slightly higher total cost of hospitalization. In a blinded, placebo-controlled comparison of IV and oral APAP for pain control after unilateral TKA under spinal anaesthesia, PACU pain scores were similar in oral and IV groups, as was total opioid consumption at 6 and 24 h, with no differences in secondary outcomes, including time to PACU discharge and time to breakthrough pain [44]. Politi et al. also showed no difference between IV and oral APAP when used within a multimodal perioperative pain regimen. Patients received 1-g dose preoperatively and then every 6 h for the first 24 h postoperatively. There was no difference in hydromorphone equivalents given at any specific time interval and no difference in 24-hour average VAS pain scores [45]. As many total shoulder arthroplasty orthopaedic patients are able to tolerate medication by mouth immediately after surgery, these studies raise the question of whether the high-priced IV APAP has any benefits over the much less-expensive oral formulation in this patient population.
Limitations
There are several limitations to this study. First, this analysis utilized data from the Premier Perspective Database, which represents about 20% of hospital episodes in the U.S. but is limited to billing claims. While billing practices may vary between hospitals, this variation should be independent of the association we are trying to assess with this analysis (i.e. an association between IV APAP and opioid use), thus minimizing its effect. Importantly, billed codes for medications do not necessarily equate to administration; however, they do represent intention of ordering by providers. One of the main factors that may influence this contrast is the use of PCA pumps, which may lead to minimal utilization of a billed quantity of medication. However, we adjusted for this mismatch by involving PCA use in our analysis. Second, detailed clinical data such as preoperative opioid use, VAS pain scores, single shot or catheter used for nerve blocks or the use of long-/short-acting opioids are not available in the database and could therefore not be assessed. Finally, IV APAP use was overall low; most patients received only one dose on the day of surgery, which may not be the most effective use. There is no consensus in the literature of an optimal use in different study populations, and ideal dosing amount and frequency in general surgery patients may not work well in orthopaedic patients. Additional studies are needed to better elucidate the most effective way to use this medication.
Conclusion
In conclusion, this is the first study examining the use of IV APAP, a new medication with rapidly increasing utilization, after shoulder arthroplasty (hemiarthroplasty, total and reverse shoulder arthroplasty). In this retrospective cohort study utilizing a large hospital administrative claims database, we assessed the use of IV APAP and its effectiveness in pain management in the postoperative period. Our results show that overall usage of IV APAP trended upwards between 2011 and 2016. However, IV APAP use was not associated with any significant meaningful improvement in opioid utilization, LOS, hospital cost or opioid-related outcomes. Future studies on the optimal dosing and administration of this medicine are warranted to determine its value in orthopaedic patients.
Conflict of interest
The author(s) have no conflicts of interest relevant to this article.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Appendix I. Opioid-related adverse effects
| Adverse event | ICD-9 diagnosis code |
|---|---|
| Respiratory | |
| Bradypnoea | 786.09 acute |
| Pulmonary insufficiency after surgery and trauma | 518.5 acute |
| Respiratory complications | 997.3 acute |
| Asphyxia | 799.01 |
| Hypoxaemia | 799.02 |
| Gastrointestinal | |
| Constipation | 564.09 |
| Constipation, narcotic induced | E937.9 acute |
| Dizziness or vertigo | 386.2 acute |
| Dry mouth | 527.7 acute |
| Ileus, postoperative | 997.4 acute |
| Paralytic ileus | 560.1 |
| Nausea or vomiting | 787.01 acute |
| Nausea or vomiting after gastrointestinal surgery | 564.3 acute |
| Central nervous system | |
| Cerebral hypoxia | 997.01 |
| Nervousness | 799.2 chronic/acute |
| Delirium | 780.09 acute |
| Confusion, postoperative | 293.9 acute |
| Confusion classified otherwise | 293 acute |
| Altered mental status | 780.97 acute |
| Genitourinary system | |
| Urinary retention | 788.2 acute |
| Oliguria | 997.5 acute/relatedness |
| Other | |
| Bradycardia, postoperative | 997.1 acute/relatedness |
| Rash or itching | 698.9 acute/relatedness |
| Drugs causing adverse effects with therapeutic use | E935.2 acute/relatedness |
| Fall from bed | E884.4 |
Adopted from: Kessler ER, Shah M, Gruschkus SK, Raju A. Cost and quality implications of opioid-based postsurgical pain control using administrative claims data from a large health system: opioid-related adverse events and their impact on clinical and economic outcomes. Pharmacotherapy. 2013; 33:383–391.
References
- 1.Smith H.S. Perioperative intravenous acetaminophen and NSAIDs. Pain Med (Malden, Mass) 2011;12(6):961–981. doi: 10.1111/j.1526-4637.2011.01141.x. [eng] [DOI] [PubMed] [Google Scholar]
- 2.Dahl J.B., Rosenberg J., Dirkes W.E., Mogensen T., Kehlet H. Prevention of postoperative pain by balanced analgesia. Br J Anaesth. 1990;64(4):518–520. doi: 10.1093/bja/64.4.518. [eng] [DOI] [PubMed] [Google Scholar]
- 3.Moucha C.S., Weiser M.C., Levin E.J. Current strategies in anesthesia and analgesia for total knee arthroplasty. J Am Acad Orthop Surg. 2016;24(2):60–73. doi: 10.5435/JAAOS-D-14-00259. [eng] [DOI] [PubMed] [Google Scholar]
- 4.Brox W.T., Roberts K.C., Taksali S., Wright D.G., Wixted J.J., Tubb C.C. The American Academy of Orthopaedic Surgeons evidence-based guideline on management of hip fractures in the elderly. J Bone J Surg Am. 2015;97(14):1196–1199. doi: 10.2106/JBJS.O.00229. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Management ASoATFoAP Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116(2):248–273. doi: 10.1097/ALN.0b013e31823c1030. [eng] [DOI] [PubMed] [Google Scholar]
- 6.Commission J. Safe use of opioids in hospitals. Sentinel Event Alert. 2012;(49):1–5. [eng] [PubMed] [Google Scholar]
- 7.Barr J., Fraser G.L., Puntillo K., Ely E.W., Gelinas C., Dasta J.F. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263–306. doi: 10.1097/CCM.0b013e3182783b72. [eng] [DOI] [PubMed] [Google Scholar]
- 8.Chou R., Gordon D.B., de Leon-Casasola O.A., Rosenberg J.M., Bickler S., Brennan T. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists' Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain: Off J Am Pain Soc. 2016;17(2):131–157. doi: 10.1016/j.jpain.2015.12.008. [eng] [DOI] [PubMed] [Google Scholar]
- 9.Elia N., Lysakowski C., Tramer M.R. Does multimodal analgesia with acetaminophen, nonsteroidal antiinflammatory drugs, or selective cyclooxygenase-2 inhibitors and patient-controlled analgesia morphine offer advantages over morphine alone? Meta-analyses of randomized trials. Anesthesiology. 2005;103(6):1296–1304. doi: 10.1097/00000542-200512000-00025. [eng] [DOI] [PubMed] [Google Scholar]
- 10.Yeh Y.C., Reddy P. Clinical and economic evidence for intravenous acetaminophen. Pharmacotherapy. 2012;32(6):559–579. doi: 10.1002/j.1875-9114.2011.01085.x. [eng] [DOI] [PubMed] [Google Scholar]
- 11.Toms L., McQuay H.J., Derry S., Moore R.A. Single dose oral paracetamol (acetaminophen) for postoperative pain in adults. Cochrane Database Syst Rev. 2008;(4) doi: 10.1002/14651858.CD004602.pub2. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singla N.K., Parulan C., Samson R., Hutchinson J., Bushnell R., Beja E.G. Plasma and cerebrospinal fluid pharmacokinetic parameters after single-dose administration of intravenous, oral, or rectal acetaminophen. Pain Pract: Off J World Inst Pain. 2012;12(7):523–532. doi: 10.1111/j.1533-2500.2012.00556.x. [eng] [DOI] [PubMed] [Google Scholar]
- 13.Zhou T.J., Tang J., White P.F. Propacetamol versus ketorolac for treatment of acute postoperative pain after total hip or knee replacement. Anesth Analg. 2001;92(6):1569–1575. doi: 10.1097/00000539-200106000-00044. [eng] [DOI] [PubMed] [Google Scholar]
- 14.Sinatra R.S., Jahr J.S., Reynolds L., Groudine S.B., Royal M.A., Breitmeyer J.B. Intravenous acetaminophen for pain after major orthopedic surgery: an expanded analysis. Pain Pract: Off J World Inst Pain. 2012;12(5):357–365. doi: 10.1111/j.1533-2500.2011.00514.x. [eng] [DOI] [PubMed] [Google Scholar]
- 15.Singla N.K., Hale M.E., Davis J.C., Bekker A., Gimbel J., Jahr J. IV acetaminophen: efficacy of a single dose for postoperative pain after hip arthroplasty: subset data analysis of 2 unpublished randomized clinical trials. Am J Therapeut. 2015;22(1):2–10. doi: 10.1097/MJT.0000000000000026. [eng] [DOI] [PubMed] [Google Scholar]
- 16.Kelly J.S., Opsha Y., Costello J., Schiller D., Hola E.T. Opioid use in knee arthroplasty after receiving intravenous acetaminophen. Pharmacotherapy. 2014;34(Suppl 1):22s–26s. doi: 10.1002/phar.1518. [eng] [DOI] [PubMed] [Google Scholar]
- 17.Makadia R., Ryan P.B. Transforming the premier perspective hospital database into the observational medical outcomes partnership (OMOP) common data model. EGEMS (Washington, DC) 2014;2(1):1110. doi: 10.13063/2327-9214.1110. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moineddin R., Matheson F.I., Glazier R.H. A simulation study of sample size for multilevel logistic regression models. BMC Med Res Methodol. 2007;7:34. doi: 10.1186/1471-2288-7-34. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kessler E.R., Shah M., Gruschkus S.K., Raju A. Cost and quality implications of opioid-based postsurgical pain control using administrative claims data from a large health system: opioid-related adverse events and their impact on clinical and economic outcomes. Pharmacotherapy. 2013;33(4):383–391. doi: 10.1002/phar.1223. [eng] [DOI] [PubMed] [Google Scholar]
- 20.Center for Medicare and Medicaid Services . 2017. Opioid oral morphine milligram equivalent (MME) conversion factors.https://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/Opioid-Morphine-EQ-Conversion-Factors-Aug-2017.pdf Available at: Accessed August 28, 2018. [Google Scholar]
- 21.Lexicomp . 2016. Opioid agonist conversion.http://online.lexi.com/lco/action/calc/calculator/70050 Available at: Accessed September 19, 2016. [Google Scholar]
- 22.GlobalRPH . 2016. Opioid analgesic converter.http://globalrph.com/narcoticonv.htm Available at: Accessed 9/19/2016. [Google Scholar]
- 23.Quan H., Sundararajan V., Halfon P., Fong A., Burnand B., Luthi J.C. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [eng] [DOI] [PubMed] [Google Scholar]
- 24.Ladha K.S., Patorno E., Huybrechts K.F., Liu J., Rathmell J.P., Bateman B.T. Variations in the use of perioperative multimodal analgesic therapy. Anesthesiology. 2016;124(4):837–845. doi: 10.1097/ALN.0000000000001034. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hosmer D.W.L.S. 2nd ed. John Wiley & Sons, Inc.; Hoboken, NJ: 2005. Applied logistic regression. [Google Scholar]
- 26.Twisk J.W.R. Cambridge University Press; Cambridge, UK: 2006. Applied multilevel analysis: a practical guide. [Google Scholar]
- 27.Witte J.S., Greenland S., Kim L.L., Arab L. Multilevel modeling in epidemiology with GLIMMIX. Epidemiology (Cambridge, Mass) 2000;11(6):684–688. doi: 10.1097/00001648-200011000-00012. [eng] [DOI] [PubMed] [Google Scholar]
- 28.Moran J.L., Solomon P.J. A review of statistical estimators for risk-adjusted length of stay: analysis of the Australian and New Zealand Intensive Care Adult Patient Data-Base, 2008-2009. BMC Med Res Methodol. 2012;12:68. doi: 10.1186/1471-2288-12-68. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rascati K.L., Smith M.J., Neilands T. Dealing with skewed data: an example using asthma-related costs of medicaid clients. Clin Therapeut. 2001;23(3):481–498. doi: 10.1016/s0149-2918(01)80052-7. [eng] [DOI] [PubMed] [Google Scholar]
- 30.Rosenbaum P.R., Rubin D.B. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. [Google Scholar]
- 31.Austin P.C. An introduction to propensity score methods for reducing the effects of Confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Austin P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–3107. doi: 10.1002/sim.3697. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zafar N., Davies R., Greenslade G.L., Dixon A.R. The evolution of analgesia in an 'accelerated' recovery programme for resectional laparoscopic colorectal surgery with anastomosis. Colorectal Dis : Off J Assoc Coloproctol Great Br Ireland. 2010;12(2):119–124. doi: 10.1111/j.1463-1318.2009.01768.x. [eng] [DOI] [PubMed] [Google Scholar]
- 34.Ziolkowski K., Kaufman J., Jambunathan J., Berge J., Menet L., Chappy S. The clinical use of intravenous acetaminophen postoperatively on patients who have undergone bowel surgery. AORN J. 2015;102(5) doi: 10.1016/j.aorn.2015.09.011. 515.e1-15.e10. [eng] [DOI] [PubMed] [Google Scholar]
- 35.Olbrecht V.A., Ding L., Spruance K., Hossain M., Sadhasivam S., Chidambaran V. Intravenous acetaminophen reduces length of stay via mediation of postoperative opioid consumption following posterior spinal fusion in a pediatric cohort. Clin J Pain. 2018 Jul;34(7):593–599. doi: 10.1097/AJP.0000000000000576. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sinatra R.S., Jahr J.S., Reynolds L.W., Viscusi E.R., Groudine S.B., Payen-Champenois C. Efficacy and safety of single and repeated administration of 1 gram intravenous acetaminophen injection (paracetamol) for pain management after major orthopedic surgery. Anesthesiology. 2005;102(4):822–831. doi: 10.1097/00000542-200504000-00019. [eng] [DOI] [PubMed] [Google Scholar]
- 37.Raiff D., Vaughan C., McGee A. Impact of intraoperative acetaminophen administration on postoperative opioid consumption in patients undergoing hip or knee replacement. Hosp Pharm. 2014;49(11):1022–1032. doi: 10.1310/hpj4911-1022. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nwagbologu N., Sarangarm P., D'Angio R. Effect of intravenous acetaminophen on postoperative opioid consumption in adult orthopedic surgery patients. Hosp Pharm. 2016;51(9):730–737. doi: 10.1310/hpj5109-730. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bollinger A.J., Butler P.D., Nies M.S., Sietsema D.L., Jones C.B., Endres T.J. Is scheduled intravenous acetaminophen effective in the pain management protocol of geriatric hip fractures? Geriatr Orthop Surg Rehabil. 2015;6(3):202–208. doi: 10.1177/2151458515588560. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brennan Z. States News Service; 2017. Senator seeks more info from Mallinckrodt on price increases of acetaminophen injection.http://www.highbeam.com/doc/1G1-492869019.html?refid=easy_hf Available at: [Google Scholar]
- 41.Maiese B.A., Pham A.T., Shah M.V., Eaddy M.T., Lunacsek O.E., Wan G.J. Hospitalization costs for patients undergoing orthopedic surgery treated with intravenous acetaminophen (IV-APAP) plus other IV analgesics or IV opioid monotherapy for postoperative pain. Adv Ther. 2017;34(2):421–435. doi: 10.1007/s12325-016-0449-8. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hansen R.N., Pham A., Strassels S.A., Balaban S., Wan G.J. Comparative analysis of length of stay and inpatient costs for orthopedic surgery patients treated with IV acetaminophen and IV opioids vs. IV opioids alone for post-operative pain. Adv Ther. 2016;33(9):1635–1645. doi: 10.1007/s12325-016-0368-8. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shaffer E.E., Pham A., Woldman R.L., Spiegelman A., Strassels S.A., Wan G.J. Estimating the effect of intravenous acetaminophen for postoperative pain management on length of stay and inpatient hospital costs. Adv Ther. 2017;33(12):2211–2228. doi: 10.1007/s12325-016-0438-y. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O'Neal J.B., Freiberg A.A., Yelle M.D., Jiang Y., Zhang C., Gu Y. Intravenous vs oral acetaminophen as an adjunct to multimodal analgesia after total knee arthroplasty: a prospective, randomized, double-blind clinical trial. J Arthroplasty. 2017;32(10):3029–3033. doi: 10.1016/j.arth.2017.05.019. [eng] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Politi J.R., Davis R.L., 2nd, Matrka A.K. Randomized prospective trial comparing the use of intravenous versus oral acetaminophen in total joint arthroplasty. J Arthroplasty. 2017;32(4):1125–1127. doi: 10.1016/j.arth.2016.10.018. [eng] [DOI] [PubMed] [Google Scholar]