SUMMARY
The study assessed the antimicrobial and antioxidant activities of commonly used and commercially available essential oils as an alternative to synthetic preservatives. The plant sources were as follows: lavender (Lavandula angustifolia), tea tree (Melaleuca alternifolia), bergamot (Citrus bergamia) and peppermint (Mentha piperita). The antioxidant activity of essential oils was tested by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2´-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) methods. The microdilution broth susceptibility assay revealed that lavender and bergamot essential oils were more efficient in inhibiting the bacterial growth than other tested oils, with the minimum inhibitory concentration of 5 μg/mL. This study also reports the successful implementation of an electrostatic extrusion technique for encapsulating essential oils into alginate beads, which enables the essential oils to maintain their free radical scavenging ability over time.
Key words: alginate, essential oils, high volatility, natural preservatives
INTRODUCTION
Essential oils are complex mixtures of volatile chemical compounds. Each of these substances has its own strong odour and can be found in different parts of a plant, such as roots, flowers, leaves, stems, fruits and seeds (1). Recently, Rehman et al. (2) have determined essential oils as the final terpenoid products that are produced by the specific enzymes known as terpene synthases. Also, essential oils are known as secondary metabolites of plants that are formed in plant cells as part of their defence mechanism. These substances have vital functions in allowing the plant to adapt to environmental conditions, protect itself against microorganisms and pests, and attract insects for pollination (3). Many studies (1–4) have reported a therapeutic effect of essential oils regarding their anticancer, antiviral, antibacterial, antifungal and antioxidant properties. The value of essential oils also reflects in their ability to prolong shelf-life and maintain characteristics of food products (3).
Parke and Lewis (5) reported the formation of carcinogenic nitrosamines from nitrites used in meat processing, and the possible rodent carcinogenicity of butylated hydroxyanisole used as a preservative for edible oils, fats and fried foods. Another study conducted by Gultekin and Doguc (6) revealed a likely possibility of allergic reactions to benzoates, which are commonly used as food preservatives. Many consumers today are concerned about possible harmful effects of synthetic preservatives on human health. Consequently, the food industry is changing according to customer demand. This provides an opportunity for developing alternatives to artificial preservatives, extending the applicability of essential oils.
The Lavandula genus (lavender) consists of approx. 20 species. Lavender is a well-known plant, used not only in cosmetics but also in food and pharmaceutical industry, mainly due to its pleasant odour and calming effect (1). The essential oil of the Melaleuca alternifolia (tea tree) possesses many biological activities, particularly antimicrobial, antioxidant, anti-inflammatory and analgesic properties (4). Citrus bergamia (bergamot) has been known for centuries for its aromatic properties. The essential oil extracted from its pericarp is used in perfumery as well as in traditional medicine (7). Mentha piperita (peppermint) is commonly used as a food and beverage flavouring, cosmetic fragrance, perfume additive, etc. In traditional medicine, peppermint is used for its diuretic, antiseptic, stomachic and carminative properties (8).
The external factors (e.g. light, oxygen, temperature) have a negative effect on the bioactivity of essential oils. This problem can be overcome by encapsulation that allows achieving higher stability than bulk oil (9). Essential oils can be encapsulated by various techniques, such as molecular inclusion, coacervation, spray drying, emulsification, ionic gelation or emulsion extrusion (10). Different types of proteins, polysaccharides, lipids and synthetic materials have been used to encapsulate essential oils or their components.
Nowadays, the application of essential oils in the modern food preservation industry is limited. This might be due to a variation of their properties caused by differences in their chemical compositions. Therefore, more research is needed to confirm the use of a particular essential oil in the food industry. Moreover, several studies have reported difficulties handling essential oils because of their unstable nature, i.e. volatility and rapid degradation under standard conditions (3, 4, 9, 10). Therefore, maintaining biological activity of essential oils over time presents a major challenge for the food industry. In this regard, the aim of this study is to evaluate the chemical composition, antioxidant potential and antimicrobial activity of lavender (Lavandula angustifolia), tea tree (Melaleuca alternifolia), bergamot (Citrus bergamia) and peppermint (Mentha piperita) essential oils. In addition to that, encapsulation into alginate beads by using electrostatic extrusion technique was performed. The ability of encapsulated essential oils to retain their antioxidant potential was tested after storage for 12 months.
MATERIALS AND METHODS
Essential oils
Essential oils of lavender (Lavandula angustifolia), tea tree (Melaleuca alternifolia), bergamot (Citrus bergamia) and peppermint (Mentha piperita) used in this study were purchased from Aura Cacia (Norway, IA, USA).
Gas chromatography/mass spectrometry analysis
Chemical composition of each essential oil was determined by GC/MS spectroscopy. The GC/MS analysis was conducted with HP G 1800C Series II GCD analytical system (Hewlett-Packard, Palo Alto, CA, USA) equipped with HP-5MS capillary column (30 m×0.25 mm i.d., film thickness 0.25 µm). Helium (1.0 mL/min) was used as carrier gas. The injector and transfer line temperatures were set to 260 °C. The mass spectra were obtained in the range m/z=40 to 450 atomic mass units (amu) and the electron ionization at 70 eV. A volume of 1 µL of diluted sample (20 µL in 2 mL EtOH) was injected in split mode (1:10). The identification of the constituents was performed by comparing their mass spectra and retention indices (RIs) with those reported in the literature (11, 12).
DPPH free radical scavenging activity
Relatively stable organic radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) has been widely used in the determination of antioxidant activity of single compounds, as well as of complex mixtures. The assay is based on the reduction of alcoholic DPPH solutions in the presence of a hydrogen donating antioxidant (13). The reduction of DPPH is followed by a decrease in its absorbance. According to the previously described procedure (14), an aliquot of each sample (0.2 mL) was mixed with 2.8 mL of DPPH ethanolic solution (Sigma-Aldrich Chemie, Merck, Steinheim, Germany). Ethanol (Vrenje Spiritana, Belgrade, Serbia) was used as a blank, while ethanol with DPPH solution was used as a control. Free radical scavenging activity was determined by measuring the absorbance of the solution using UV-Vis double beam spectrophotometer (HALO DB-20; Dynamica GmbH, Salzburg-Mayrwies, Austria) at 525 nm after 40 min of reaction at room temperature in the dark. Trolox (Sigma-Aldrich Chemie, Merck) was used as a standard and the results were expressed in mmol Trolox equivalents per litre of essential oil (mmol/L). The antioxidant potential of the non-encapsulated essential oils was measured before and after the storage. To evaluate the stability of the samples, the antioxidant activity of the essential oils released in water was determined after storage for 12 months in PET containers.
ABTS radical scavenging capacity
The assay is based on the reduction of 2,2´-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) cation radical. It was carried out according to the procedure described by Re et al. (15) with slight modifications. Briefly, ABTS radical cations were generated by reacting aqueous ABTS (Sigma-Aldrich Chemie, Merck) with potassium persulfate (Centrohem, Belgrade, Serbia) and maintaining the mixture in the dark at room temperature for at least 12 h before use. Afterwards, the absorbance of the ABTS solution was set to (0.70±0.02) at 734 nm by adding phosphate buffer. The volume of 30 μL of appropriately diluted oil samples was mixed with 3 mL of ABTS solution. After reaction for 10 min, the absorbance was measured at 734 nm using a UV-Vis double beam spectrophotometer (HALO DB-20; Dynamica GmbH). The antioxidant activity of the non-encapsulated essential oil was determined before and after storage for 12 months. Additionally, antioxidant activity of the alginate beads was determined after storage for 12 months in order to assess the effect of encapsulation on retaining the biological properties of essential oil. All measurements were done in triplicate. Trolox was used as a standard and the results were expressed in mmol Trolox equivalents per litre of essential oil.
Antimicrobial activity
Microdilution broth susceptibility assay (16) with slight changes was used to examine the antimicrobial properties of the essential oils. Stock solutions of essential oils were prepared in 10% dimethylsulfoxide (DMSO; Centrohem) at the concentration of 20 μg/mL. The tested concentrations in the microtiter plates were in the range of 0.078–10 μg/mL. The microorganisms used for the assay were Gram-positive bacterium Staphylococcus aureus ATCC 25923 and Gram-negative bacterium Salmonella Typhimurium ATCC 14028. Bacterial suspensions were grown overnight at 37 °C in Mueller Hinton broth (HiMedia, Mumbai, India). The final concentration of the bacteria used for inoculation was standardized to approx. 105 CFU/mL. Resazurin sodium salt (6.75 mg/mL in distilled water; Sigma-Aldrich, Merck, St. Louis, MO, USA), an indicator of the bacterial growth, was added to the bacterial suspensions and 50 µL of the solution were then placed in the wells containing the sample dilutions. As a positive control, only the bacterial suspension was used. The wells containing only the serial sample dilutions without microorganism were used as a negative control. After the inoculation, the microtiter plates were incubated at 37 °C for 24 h. The minimum inhibitory concentration (MIC) was defined as the lowest concentration of a sample (µg/mL) at which no visible growth was detected. To determine the minimum bactericidal concentration (MBC), the dilutions representing the already established MIC were subcultured on the surface of an appropriate Mueller Hinton Agar base (HiMedia) and incubated at 37 °C for 24 h. If no bacterial growth was detected, the tested concentration of the sample was considered to be the MBC.
Encapsulation
Alginates have been broadly applied as carriers in various fields such as the biomedical, environmental, industrial and food industries because of their non-toxic, biodegradable, and biocompatible properties (9, 10). Alginate beads loaded with essential oils were obtained using emulsion extrusion method (9). Solution of 2% (m/V) sodium alginate was prepared by dissolving sodium alginate (Carl Roth, Karlsruhe, Germany) in distilled water under magnetic stirring at 300 rpm (Isolab Laborgeräte GmbH, Eschau, Germany). The mass fraction of the essential oil in the alginate solution was 4%. Alginate/essential oil emulsions were stabilized using homogenizer (Ultra-Turrax® T25; Ika-Labortechnik, Staufen, Germany) under vigorous mixing at 10 000 rpm for 5 min. The alginate/essential oil emulsions were extruded through 1.1-mm blunt stainless steel needle using a syringe pump (model 11; Harvard Apparatus, Cambridge, MA, USA) under a constant flow rate of 40 mL/h. The spherical droplets were obtained by the action of electrostatic force and ion exchange. Electrostatic potential (6 kV) was formed by electrostatic encapsulation system (VAR V1 Nisco Encapsulation Unit, Zürich, Switzerland). The beads were left in the calcium chloride solution (Acros Organics, Morris Plains, NJ, USA) for 60 min to finalise their formation. Finally, the beads were washed in distilled water (30 mL per 0.6 g) and stored at ambient temperature ((23±2) °C) for 12 months.
Freeze drying
The primary difficulty in removing water from the beads by vapourization is to maintain the essential oil that is more volatile than water. Several studies have been performed recently in preventing oil loss during drying (9, 10, 17). Freeze drying is one of the most appropriate and innovative ways to dehydrate alginate/essential oil beads. The compact tabletop unit (Alpha 1-4 LSCplus; Martin Christ Gefriertrocknungsanlagen GmbH, Osterode am Harz, Germany) was used for freeze drying of specimens. The drying programme was as follows: –25 °C at the pressure of 7.6 Pa for 22 h and then 20 °C at the pressure of 7.6 Pa for 2 h. The dried samples were kept at 25 °C and the same pressure for 2 h, and then stored in tightly closed vials.
Morphological analysis
The dimensions and shape of the beads were estimated by SMZ18 stereo zoom microscope (Nikon, Coventry, UK), equipped with a camera (SHR Plan Apo 1x WD60; Nikon). The diameter was determined as an average of the largest (dmax) and smallest dimensions (dmin) of the beads. The deformation of the spherical shape was calculated using sphericity factor (SF) according to the following equation (9):
| SF=(dmax-dmin)/(dmax+dmin) /1/ |
The reduction in the bead size after drying was expressed by shrinkage factor (kSF(drying)) as follows:
| kSF(drying)=(dc-dc(dry))/dc /2/ |
where dc is the diameter of the wet beads and dc(dry) is the diameter of the freeze-dried beads.
Statistical evaluation
The analyses were done in triplicates and expressed as mean value±standard deviation. Statistical significance was calculated by one-way ANOVA test, and p≤0.05 was considered to indicate statistically significant results.
RESULTS AND DISCUSSION
GC-MS analysis of essential oils
According to the data presented in Table 1, tea tree essential oil had a high content of terpenes such as terpinen-4-ol (44.72%), γ-terpinene (19.47%) and α-terpinene (7.84%). The experimental results are in accordance with those reported by Hart et al. (18) and Ninomiya et al. (19), who also determined terpinen-4-ol (more than 40%) as the main compound of tea tree oil. Similarities between lavender and bergamot essential oil can be observed through the predominant presence of linalool (35.68 and 22.93%, respectively) and linalool acetate (42.25 and 57.51%, respectively). Shellie et al. (20) reported linalool and linalool acetate contents in lavender oil in the range 25–45 and 25–38%, respectively, while Furneri et al. (21) reported 5–13 and 20–27%, respectively, in bergamot essential oil. Two major compounds of peppermint oil were isomenthol (49.30%) and menthyl acetate (5.63%).
Table 1. Compounds identified in tea tree (Melaleuca alternifolia), lavender (Lavandula angustifolia), bergamot (Citrus bergamia) and peppermint (Mentha piperita) essential oils.
| Compound | RI | w/% | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tea tree | Lavender | Bergamot | Peppermint | |||||||
| α-thujene | 924.9 | 0.86 | 0.16 | – | 0.83 | |||||
| α-pinene | 930.3 | 2.57 | – | 0.15 | 1.15 | |||||
| camphene | 944.5 | – | 0.13 | – | – | |||||
| β-pinene | 972.9 | 0.70 | – | 0.78 | – | |||||
| sabinene | 973.2 | – | 0.14 | – | 0.51 | |||||
| myrcene | 992.5 | 0.75 | 0.19 | 0.37 | – | |||||
| α-phellandrene | 1003.7 | 0.33 | 0.26 | – | – | |||||
| n.i. | 1011.1 | – | – | – | 0.17 | |||||
| α-terpinene | 1015.5 | 7.84 | 0.75 | – | – | |||||
| p-cymene | 1024.4 | 5.15 | 0.21 | 1.03 | 0.19 | |||||
| β-phellandrene | 1027.4 | 1.54 | – | – | – | |||||
| limonene | 1028.4 | – | – | 6.67 | – | |||||
| 1,8-cineole | 1028.6 | 3.15 | 1.88 | – | 1.71 | |||||
| sylvestrene | 1029.0 | – | 0.67 | – | – | |||||
| γ-terpinene | 1058.1 | 19.47 | 0.15 | 1.39 | 0.13 | |||||
|
Artemisia ketone |
1063.8 | – | 0.21 | – | 0.34 | |||||
| cis-sabinene hydrate | 1070.2 | – | 0.10 | – | 0.87 | |||||
| trans-sabinene hydrate | 1102.0 | – | – | – | 0.11 | |||||
| cis-linalool oxide | 1073.2 | – | 0.65 | 0.26 | – | |||||
| terpinolene | 1087.7 | 3.21 | – | – | – | |||||
| trans-linalool oxide | 1089.8 | – | 0.61 | 0.23 | – | |||||
| linalool | 1105.0 | – | 35.68 | 22.93 | 0.38 | |||||
| 1-octen-3-yl acetate | 1116.5 | – | 0.66 | – | – | |||||
| n.i. | 1121.3 | – | – | – | 0.08 | |||||
| camphor | 1141.8 | – | 0.64 | – | – | |||||
| menthofuran | 1162.8 | – | – | – | 7.16 | |||||
| menthol | 1166.8 | – | – | – | 3.39 | |||||
| borneol | 1167.4 | – | 1.75 | – | – | |||||
| isomenthol | 1176.6 | – | – | – | 49.30 | |||||
| terpinen-4-ol | 1178.5 | 44.72 | 3.94 | 0.49 | – | |||||
| neoisomenthol | 1184.6 | – | – | – | 0.99 | |||||
| α-terpineol | 1194.6 | 3.11 | 0.44 | 0.33 | – | |||||
| hexyl isovalerate | 1245.1 | – | 0.32 | – | – | |||||
| pulegone | 1240.9 | – | – | – | 1.57 | |||||
| ascaridole | 1241.0 | – | – | 0.24 | – | |||||
| linalool acetate | 1258.6 | 0.24 | 42.25 | 57.51 | – | |||||
| n.i. | 1258.7 | – | – | 0.33 | – | |||||
| piperitone | 1259.6 | – | – | – | 0.29 | |||||
| n.i. | 1264.6 | – | – | 0.41 | – | |||||
| neomenthyl acetate | 1276.8 | – | – | – | 0.23 | |||||
| lavandulyl acetate | 1294.8 | – | 1.72 | – | – | |||||
| menthyl acetate | 1295.4 | – | – | – | 5.63 | |||||
| isomenthyl acetate | 1309.4 | – | – | – | 0.17 | |||||
| hexyl tiglate | 1337.9 | – | 0.17 | – | – | |||||
| n.i. | 1348.2 | – | 0.20 | – | – | |||||
| n.i. | 1356.4 | – | 0.28 | – | – | |||||
| n.i. | 1362.2 | – | 0.34 | – | – | |||||
| terpinyl acetate | 1351.6 | – | – | 0.71 | – | |||||
| neryl acetate | 1369.3 | – | 0.52 | 1.87 | – | |||||
| Compound | RI | w/% | ||||||||
| Tea tree | Lavender | Bergamot | Peppermint | |||||||
| β-bourbonene | 1381.6 | – | – | – | 0.29 | |||||
| geranyl acetate | 1388.6 | – | 0.85 | 0.72 | – | |||||
| n.i. | 1405.2 | – | – | 0.62 | – | |||||
| sesquithujene | 1405.7 | – | 0.12 | – | – | |||||
| α-gurjunene | 1406.3 | 0.32 | – | – | – | |||||
| trans- caryophyllene | 1416.4 | 0.35 | 0.91 | 0.47 | 1.79 | |||||
| α-trans- bergamotene | 1434.6 | – | – | 0.39 | – | |||||
| aromadendrene | 1435.5 | 1.25 | – | – | – | |||||
| α-humulene | 1451.3 | 0.50 | 0.13 | – | – | |||||
| alloaromaden- -drene |
1458.2 | 0.30 | – | – | – | |||||
| trans-β- farnesene | 1459.1 | – | 0.04 | – | – | |||||
|
trans-cadina- -1(6),4-diene |
1472.4 | 1.31 | – | – | – | |||||
|
cis-muurola- -4(14),5-diene |
1479.8 | – | 0.11 | – | – | |||||
| germacrene D | 1479.8 | – | – | – | 1.00 | |||||
| valencene | 1493.2 | 1.51 | – | – | – | |||||
| β-bisabolene | 1510.7 | – | – | 2.08 | – | |||||
| lavandulyl isovalerate | 1512.6 | – | 0.42 | – | – | |||||
| γ-cadinene | 1513.2 | – | 0.21 | – | – | |||||
| d-cadinene | 1523.1 | 0.44 | – | – | – | |||||
|
trans-cadina- -1,4-diene |
1531.9 | 0.39 | – | – | – | |||||
| caryophyllene oxide | 1580.9 | – | 1.31 | – | – | |||||
| Sum of identified | 100.00 | 98.30 | 98.64 | 99.72 | ||||||
RI=retention index relative to n-alkanes on HP-5 capillary column, n.i.=not identified
Free radical scavenging activity of essential oil
All the examined essential oils were able to reduce DPPH and ABTS free radicals before and after storage (Table 2). Comparison of the values determined by DPPH assay for fresh essential oil showed that peppermint essential oil had the highest value of (23.3±1.0) mmol/L, followed by lavender, bergamot and tea tree essential oils with the minimum value of (7.6±0.1) mmol/L. The similarity in free radical scavenging ability between lavender and bergamot essential oils might be associated with their similar GC-MS profiles (Table 1).
Table 2. Free radical scavenging capacities of the essential oils measured by the DPPH and ABTS methods.
| Plant source | Fresh | After 12 months of storage |
||
|---|---|---|---|---|
|
c(DPPH)*/ (mmol/L) |
c(ABTS)((mmol/L) |
c(DPPH)*/ (mmol/L) |
c(ABTS)/(mmol/L) | |
| Lavender | (18.9±0.2)a | (48.6±0.7) a | (11.9±0.2)a | (22.3±0.3)a |
| Bergamot | (17.0±0.4)a | (99.0±1.0)b | (9.6±0.9)a | (62.8±0.3)b |
| Tea tree | (7.6±1.0)b | (168.7±1.4)c | (5.8±0.2)b | (120.2±0.8)c |
| Peppermint | (23.3±1.0)c | (190.9±2.5)d | (16.5±0.4)c | (141.6±0.8)d |
Expressed as Trolox equivalent per litre of the essential oil solution. Values are expressed as mean±standard deviation (N=3). Mean values with a different letter in superscript within the same column are significantly different from each other (Tukey-Kramer post hoc test, p<0.05)
After storage for 12 months, DPPH-scavenging ability of tea tree and peppermint essential oils (Table 2) decreased by 23.6 and 29.1%, respectively. On the other hand, lavender and bergamot essential oils were less stable during storage with the DPPH values reduced by 37.2 and 43.4%, respectively. Tea tree essential oil was more effective in neutralizing ABTS radical cations ((168.7±1.4) mmol/L) than DPPH. Among the other samples, peppermint essential oil had the strongest ABTS free radical scavenging activity ((190.9±2.5) mmol/L), which is in agreement with the results obtained by DPPH method. Bergamot showed intermediate ((99.0±0.9) mmol/L), and lavender showed the weakest ((48.6±0.7) mmol/L) ABTS free radical scavenging activity. However, there was a notable drop in the ABTS radical scavenging ability of lavender essential oil (54.0%) after 12 months of storage compared to the fresh sample. The ABTS radical scavenging ability of bergamot, tea tree and peppermint essential oils also decreased by 36.6, 28.7 and 25.9%, respectively.
It should be pointed out that the data variation for antioxidant capacity of essential oils was observable depending on the applied assay. This might be the result of specific interactions of essential oil components with either DPPH or ABTS free radicals. Olszowy and Dawidowicz (22) had the same observations. They reported that variations in the antioxidant potency of the essential oils result from the presence of compounds with conjugated double bonds, which serve as donors of hydrogen/electron, and also from their total content in the essential oils.
Free radical scavenging activity of the essential oils released from the beads
In the present study, the experiments were also conducted with water, which was used as a medium for storage of alginate beads containing essential oils for 12 months at 25 °C. Results obtained by DPPH test showed that encapsulation into alginate beads resulted in preserving the antioxidant capacity of peppermint essential oil by 72%, while other essential oils maintained the antioxidant activity in the range from 40 to 50% (Table 2).
The results obtained by the ABTS radical scavenging ability test were as follows: peppermint essential oil beads retained 80% antioxidant capacity after 12 months of storage, lavender essential oil beads 54%, and bergamot and tea tree essential oil beads 49 and 33%, respectively (Table 2).
Overall, it was observed that encapsulation had a positive effect on maintaining free radical scavenging ability of the essential oils. They partially retained their antioxidant properties even after 12 months of storage under light exposure and at ambient temperature. It is assumed that essential oil beads will likely be more resistant to food processing regimes than non-encapsulated essential oil.
Antimicrobial activity of tested oils
Among foodborne pathogens, Staphylococcus aureus and Salmonella Typhimurium are known as species resistant to antibiotics (23). The antibacterial activity of essential oils against both of these strains and a range of other microorganisms was confirmed in previous studies (1, 4, 7, 11, 23). Variations in the reported inhibitory concentrations of oils are probably due to the differences in their chemical composition. In addition to that, the ratio of the primary active constituents also varies from one plant to another. Besides, the synergistic effect of the components, ecological factors and techniques used for obtaining essential oils also have a significant impact on the final results (4, 22).
Assessment of MIC and MBC values of the essential oils against S. aureus and S. Typhimurium was conducted at the concentration range of 0.078–10 μg/mL. It was observed that the concentration of 10 μg/mL inhibited the bacterial growth. The MIC values shown in Table 3 reveal that lavender and bergamot essential oils at 5 μg/mL were more efficient in inhibiting the bacterial growth than other tested oils. In addition to their antibacterial properties, lavender and bergamot essential oils have shown strong antimicrobial activity against other microorganisms such as yeasts and fungi (1, 21), which can be attributed to the major components or synergy among the major and some minor components presented in their GC-MS profiles (Table 1). This confirms the previously published findings where linalool and linalool acetate were the main antimicrobials and antioxidative compounds detected in lavender and bergamot essential oils (24, 25). On the other hand, the concentration higher than 10 μg/mL is required to achieve a bactericidal effect of tea tree, peppermint and bergamot essential oils. According to Işcan et al. (26), menthol was found to be responsible for the antimicrobial activity of peppermint essential oil (bioautography assay). However, it was also reported that the dominant substances responsible for antimicrobial activity of tea tree oil were terpinene-4-ol and terpinene (27). Nevertheless, the results of antimicrobial assay revealed that despite the large variation in the main substances, each of the studied essential oils possesses antibacterial activity. Therefore, they can be recommended as a potential source of active ingredients for food, cosmetic or pharmaceutical industry. According to previous research data obtained on essential oil nanoparticles (9), it is expected that encapsulation of essential oils enhances their antimicrobial activity. The mechanism of antibacterial activity is still not fully understood. Nevertheless, it is suggested that the structural integrity of cell membrane is disturbed by the accumulation of the bioactive components present in the essential oil, which can influence the cell metabolism causing cell death. According to the published data (24, 25), the antimicrobial mechanism of action differs with the type of essential oil or the microbial strain.
Table 3. Antimicrobial activity of essential oils.
| Plant source | Salmonella Typhimurium | Staphylococcus aureus | ||
|---|---|---|---|---|
| MIC/(µg/mL) | MBC/(µg/mL) | MIC/(µg/mL) | MBC/(µg/mL) | |
| Lavender | 5 | 10 | 5 | 10 |
| Bergamot | 5 | >10 | 5 | 10 |
| Tea tree | 10 | 10 | 10 | >10 |
| Peppermint | 10 | 10 | 10 | >10 |
MIC=minimum inhibitory concentration, MBC=minimum bactericidal concentration
Morphological characteristics of alginate/essential oil beads
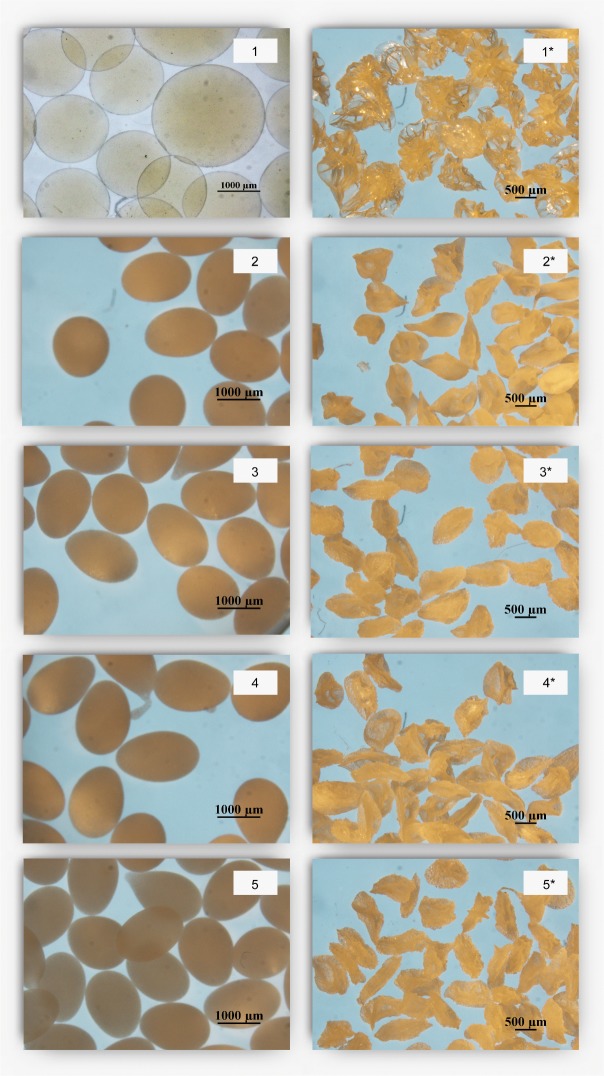
The shape and surface morphology of the alginate beads containing essential oils before and after freeze drying was analysed to establish the influence of essential oils on bead morphological characteristics. Before extrusion, alginate and essential oils were adequately mixed, resulting in stable emulsions. Sphericity factor (SF) was used to determine the deviation from the spherical shape of the beads: the zero value indicates a perfect sphere, while higher values of SF indicate more deformities in the bead shape. SFs for wet and dried samples are shown in Table 4. Regardless of the type of the used essential oil, all of the obtained wet beads had a similar, oval shape resembling droplets, which is quite distinct from an ideal spherical shape (Fig. 1, images 1–5). The formation of the beads with an elongated shape was observed in former studies (28–30). This effect might be attributed to the speed of the emulsion flow, the system voltage, the solution properties or the diameter of the needle used for droplet formation. Sublimation of water from the hydrogel matrix resulted in the irregularly shaped beads with the SF higher than 0.2. Fig. 1 (images 1*–5*) shows the effect of the freeze drying on the outer structure of the formed beads. Apparently, essential oil acts as a filler preventing the beads from fracturing upon freeze drying, which, in turn, can be seen on the empty beads. Due to the increase in the volume of water upon crystallization, freeze drying of hydrogels often leads to the formation of a porous surface structure and cracks. Recent studies have revealed that adding a flavouring ingredient can prevent such results (9, 17). Overall, the results showed that essential oils can be entrapped in alginate matrix by electrostatic extrusion technique. Moreover, using a simple two-component system (alginate/essential oil) was sufficient enough for the formation of beads, thus making the use of emulsifiers and stabilizers unnecessary.
Table 4. Morphological characteristics of the beads.
| Sample | d/mm | SF | kSF(drying) | ||
|---|---|---|---|---|---|
| Wet | Dried | Wet | Dried | ||
| Blank | 2.00±0.04 | 1. 4±0.2 | 0.233 | 0.391 | 0.302 |
| Lavender | 1.51±0.07 | 0.9±0.2 | 0.241 | 0.521 | 0.397 |
| Bergamot | 1.6±0.9 | 1.2±0.1 | 0.294 | 0.407 | 0.367 |
| Tea tree | 1.58±0.08 | 1. 1±0.1 | 0.238 | 0.541 | 0.331 |
| Peppermint | 1.46±0.09 | 1.0±0.1 | 0.225 | 0.402 | 0.348 |
SF=sphericity factor of the beads before and after freeze drying, kSF(drying)=shrinkage factor of the beads after freeze drying. Values are expressed as mean±standard deviation (N=15)
Fig. 1.
Alginate/essential oil beads before (1=blank, 2=lavender, 3=bergamot, 4=tea tree, 5=peppermint) and after (1*–5* respectively) freeze drying
CONCLUSIONS
Today a variety of methods are available to protect commercial food and feed products from spoilage and contamination, each of them with its advantages and disadvantages. This study provides information about different properties of lavender, peppermint, bergamot and tea tree essential oils, which may find an application in the modern food preservation industry. GC-MS analysis revealed that lavender and bergamot essential oils have similar chemical compositions, with linalool and linalool acetate as the main components. These components are of great importance as they have been widely used in food and cosmetic industries as a fragrance component. Additionally, lavender and bergamot essential oils were more efficient in inhibiting the growth of Salmonella Typhimurium and Staphylococcus aureus pathogenic bacteria (MIC values of 5 μg/mL) than the other tested essential oils. On the other hand, the results of DPPH and ABTS assays indicated that among the essential oils tested in this study, lavender and peppermint essential oils exhibited the highest antioxidant capacity. Still, it must be pointed out that the results of a single assay can provide only limited knowledge of the antioxidant properties of essential oils. Therefore, the combination of the two methods applied in this study was effective for assessing the antioxidant activity of the essential oils. As it was mentioned before, the GC-MS analysis revealed that essential oils have complex and unique chemical compositions which can explain the variation in their antimicrobial and antioxidant properties. The notable drop was observed in DPPH and ABTS radical scavenging activities of all essential oils tested after 12 months of storage. Nevertheless, the study demonstrated that the encapsulation of essential oils into alginate using electrostatic extrusion technique was efficient for maintaining their free radical scavenging ability. The morphology of the obtained beads was assumed to be unaffected by the oil used, offering advantages for developing food products with adjustable characteristics and desired properties.
ACKNOWLEDGEMENTS
This research was supported by the Saint Petersburg State Institute of Technology (Technical University), Russia, and the III Project of the Ministry of Education, Science and Technological Development, Serbia, no. 46010. Authors are grateful to Prof. Radenko Radošević (University of Belgrade, Serbia) for assistance in the microscopic analysis.
Footnotes
CONFLICT OF INTEREST: The authors declare no conflict of interest.
REFERENCES
- 1.Andrys D, Kulpa D, Grzeszczuk M, Bihun M, Dobrowolska A. Antioxidant and antimicrobial activities of Lavandula angustifolia Mill. field-grown and propagated in vitro. Folia Hortic. 2017;29(2):161–80. 10.1515/fhort-2017-0016 [DOI] [Google Scholar]
- 2.Rehman R, Hanif MA, Mushtaq Z, Al-Sadi AM. Biosynthesis of essential oils in aromatic plants: A review. Food Rev Int. 2016;32(2):117–60. 10.1080/87559129.2015.1057841 [DOI] [Google Scholar]
- 3.Hyldgaard M, Mygind T, Meyer RL. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front Microbiol. 2012;3:12. 10.3389/fmicb.2012.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baldissera MD, Da Silva AS, Oliveira CB, Vaucher RA, Santos RCV, Duarte T, et al. Effect of tea tree oil (Melaleuca alternifolia) on the longevity and immune response of rats infected by Trypanosoma evansi. Res Vet Sci. 2014;96(3):501–6. 10.1016/j.rvsc.2014.03.013 [DOI] [PubMed] [Google Scholar]
- 5.Parke DV, Lewis DF. Safety aspects of food preservatives. Food Addit Contam. 1992;9(5):561–77. 10.1080/02652039209374110 [DOI] [PubMed] [Google Scholar]
- 6.Gultekin F, Doguc DK. Allergic and immunologic reactions to food additives. Clin Rev Allergy Immunol. 2013;45(1):6–29. 10.1007/s12016-012-8300-8 [DOI] [PubMed] [Google Scholar]
- 7.Babish JG, Dahlberg CJ, Ou JJ, Keller WJ, Gao W, Kaadige MR, et al. Synergistic in vitro antioxidant activity and observational clinical trial of F105, a phytochemical formulation including Citrus bergamia, in subjects with moderate cardiometabolic risk factors. Can J Physiol Pharmacol. 2016;94(12):1257–66. 10.1139/cjpp-2016-0062 [DOI] [PubMed] [Google Scholar]
- 8.Vaka SRK, Murthy SN. Enhancement of nose-brain delivery of therapeutic agents for treating neurodegenerative diseases using peppermint oil. Pharmazie. 2010;65(9):690–2. [PMC free article] [PubMed] [Google Scholar]
- 9.Lević S, Pajić Lijaković I, Đorđević V, Rac V, Rakić V, Šolević Knudsen T, et al. Characterization of sodium alginate/d-limonene emulsions and respective calcium alginate/d-limonene beads produced by electrostatic extrusion. Food Hydrocoll. 2015;45(1):111–23. 10.1016/j.foodhyd.2014.10.001 [DOI] [Google Scholar]
- 10.Kalušević A, Lević S, Đorđević V, Beatović D, Jelačić S, Bugarski B, et al. Encapsulation of basil (Ocimum basilicum) essential oil. Proceedings of the 6th European Congress on Food: CEFood Congress; 2012 May 23–26; Novi Sad, Serbia: Institute of Food Technology, Novi Sad, Serbia; 2012. pp. 1087–92. [Google Scholar]
- 11.König WA, Joulain D, Hochmuth DH. Terpenoids and related constituents of essential oils. Library of MassFinder 4, Hamburg, Germany; 2019. Available from: https://massfinder.com/wiki/Terpenoids_Library.
- 12.Adams RP. Identification of essential oil components by gas chromatography/mass spectrometry. Carol Stream, IL, USA: Allured Publishing Corporation; 2007. [Google Scholar]
- 13.Hara K, Someya T, Sano K, Sagane Y, Watanabe T, Wijesekara RGS. Antioxidant activities of traditional plants in Sri Lanka by DPPH free radical-scavenging assay. Data Brief. 2018;17(1):870–5. 10.1016/j.dib.2018.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salevic A, Kaluševic A, Levic S, Bugarski B, Nedovic V. Effect of extraction conditions on phenolic compounds from blackberry leaves extracts. In: FOODBALT 2017: 11th Baltic conference on food science and technology ‘Food science and technology in a changing world’; 2017 April 27–28; Jelgava, Latvia: Latvia University of Agriculture; 2017. pp. 40–7. https://doi.org/ 10.22616/foodbalt.2017.041 [DOI] [Google Scholar]
- 15.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M. Rice- -Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26(9-10):1231–7. 10.1016/S0891-5849(98)00315-3 [DOI] [PubMed] [Google Scholar]
- 16.Klančnik A, Piskernik S, Jeršek B, Smole Možina S. Evaluation of diffusion and dilution methods to determine the antibacterial activity of plant extracts. J Microbiol Methods. 2010;81(2):121–6. 10.1016/j.mimet.2010.02.004 [DOI] [PubMed] [Google Scholar]
- 17.Holkem AT, Raddatz GC, Lorenzioni Nunes G, Cichoski AJ, Jacob-Lopes E, Grosso CRF, et al. Development and characterization of alginate microcapsules containing Bifidobacterium BB-12 produced by emulsification/internal gelation followed by freeze drying. Lebensm Wiss Technol. 2016;71:302–8. 10.1016/j.lwt.2016.04.012 [DOI] [Google Scholar]
- 18.Hart PH, Brand C, Carson CF, Riley TV, Prager RH, Finlay-Jones JJ. Terpinen-4-ol, the main component of the essential oil of Melaleuca alternifolia (tea tree oil), suppresses inflammatory mediator production by activated human monocytes. Inflamm Res. 2000;49(11):619–26. 10.1007/s000110050639 [DOI] [PubMed] [Google Scholar]
- 19.Ninomiya K, Maruyama N, Inoue S, Ishibashi H, Takizawa T, Oshima H, et al. The essential oil of Melaleuca alternifolia (tea tree oil) and its main component, terpinen-4-ol protect mice from experimental oral candidiasis. Biol Pharm Bull. 2012;35(6):861–5. 10.1248/bpb.35.861 [DOI] [PubMed] [Google Scholar]
- 20.Shellie R, Mondello L, Marriott P, Dugo G. Characterisation of lavender essential oils by using gas chromatography-mass spectrometry with correlation of linear retention indices and comparison with comprehensive two-dimensional gas chromatography. J Chromatogr A. 2002;970(1-2):225–34. 10.1016/S0021-9673(02)00653-2 [DOI] [PubMed] [Google Scholar]
- 21.Furneri PM, Mondello L, Mandalari G, Paolino D, Dugo P, Garozzo A, et al. In vitro antimycoplasmal activity of Citrus bergamia essential oil and its major components. Eur J Med Chem. 2012;52(1):66–9. 10.1016/j.ejmech.2012.03.005 [DOI] [PubMed] [Google Scholar]
- 22.Olszowy M, Dawidowicz AL. Essential oils as antioxidants: Their evaluation by DPPH, ABTS, FRAP, CUPRAC, and β-carotene bleaching methods. Monatsh Chem. 2016;147(12):2083–91. 10.1007/s00706-016-1837-0 [DOI] [Google Scholar]
- 23.Mattazi N, Farah A, Fadil M, Chraibi M, Benbrahim KF. Essential oils analysis and antibacterial activity of the leaves of Rosmarinus officinalis, Salvia officinalis and Mentha piperita cultivated in Agadir (Morocco). Int J Pharm Pharm Sci. 2015;7(9):73–9. [Google Scholar]
- 24.Fisher K, Phillips CA. The effect of lemon, orange and bergamot essential oils and their components on the survival of Campylobacter jejuni, Escherichia coli O157, Listeria monocytogenes, Bacillus cereus and Staphylococcus aureus in vitro and in food systems. J Appl Microbiol. 2006;101(6):1232–40. 10.1111/j.1365-2672.2006.03035.x [DOI] [PubMed] [Google Scholar]
- 25.Mandalari G, Bennett RN, Bisignano G, Trombetta D, Saija A, Faulds CB, et al. Antimicrobial activity of flavonoids extracted from bergamot (Citrus bergamia Risso) peel, a byproduct of the essential oil industry. J Appl Microbiol. 2007; 103(6):056–64. https://doi.org/ 10.1111/j.1365-2672.2007.03456.x [DOI] [PubMed]
- 26.Işcan G, Kirimer N, Kürkcüoğlu M, Başer KH, Demirci F. Antimicrobial screening of Mentha piperita essential oils. J Agric Food Chem. 2002;50(14):3943–6. 10.1021/jf011476k [DOI] [PubMed] [Google Scholar]
- 27.Carson CF, Hammer KA, Riley TV. Melaleuca alternifolia (tea tree) oil: A review of antimicrobial and other medicinal properties. Clin Microbiol Rev. 2006;19(1):50–62. 10.1128/CMR.19.1.50-62.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Oliveira EF, Paula HCB, de Paula RCM. Alginate/cashew gum nanoparticles for essential oil encapsulation. Colloids Surf B Biointerfaces. 2014;113:146–51. 10.1016/j.colsurfb.2013.08.038 [DOI] [PubMed] [Google Scholar]
- 29.Hosseini SF, Zandi M, Rezaei M, Farahmandghavi F. Two- -step method for encapsulation of oregano essential oil in chitosan nanoparticles: Preparation, characterization and in vitro release study. Carbohydr Polym. 2013;95(1):50–6. 10.1016/j.carbpol.2013.02.031 [DOI] [PubMed] [Google Scholar]
- 30.Majeed H, Bian YY, Ali B, Jamil A, Majeed U, Khan QF, et al. Essential oil encapsulations: Uses, procedures, and trends. Rsc Adv. 2015;5(72):58449–63. 10.1039/C5RA06556A [DOI] [Google Scholar]